Abstract
Affective neuroscience research using electrocortical event-related potentials has provided valuable insights on alterations in emotion processing in internalizing disorders. However, internalizing disorders are accompanied by additional impairments in social cognition and functioning, and most extant research examines neural responses to broad categories of emotional scenes or faces presented irrespective of context. Examining neural reactivity specifically to interpersonal emotional scenes may more precisely capture and disentangle processes involved in depression and social anxiety, two highly comorbid forms of psychopathology. The current study validated a novel set of positive and threatening interpersonal emotional stimuli in a sample of emerging adults (N = 114) who completed a modified emotional interrupt paradigm while electroencephalogram and behavioral data were recorded. Participant ratings of valence and arousal supported the validity of the emotional images. Consistent with prior research, sustained neurophysiological processing indexed by the late positive potential (LPP) was observed for interpersonal emotional images, especially positive, compared to neutral images. Elevated LPP reactivity to both positive and threatening interpersonal images moderated the effects of chronic interpersonal stress on social anxiety symptoms, such that enhanced LPP reactivity in conjunction with higher levels of chronic interpersonal stress was associated with elevated social anxiety symptoms. These results were unique to social anxiety symptoms and not symptoms of depression, suggesting sustained neural processing of interpersonal stimuli may differentiate social anxiety from depression. Future research on emotional reactivity specifically within the interpersonal domain is needed to inform our understanding of developmental pathways to internalizing psychopathology.
Introduction
Many forms of psychopathology are accompanied by impairments in social cognition and functioning (Kennedy & Adolphs, 2012). Epidemiological research on adults reveals prevalence rates of 9.5% for depressive disorders and 18.1% for anxiety disorders (Kessler et al., 2005), with the risk of developing a subsequent comorbid anxiety or depressive disorder between 13.6–46.9% across fifteen years (McGrath et al., 2020). Though internalizing disorders share phenotypic manifestations of social impairment, such as social withdrawal, they are characterized by distinct cognitive processing biases (Armstrong & Olatunji, 2012; Gotlib et al., 2004; Mathews & MacLeod, 2005) and neurological patterns of emotional reactivity (MacNamara et al., 2016; 2017). While anxiety is typically associated with heightened attention towards threatening emotional faces (Bar-Haim et al., 2007), depression corresponds with reduced orienting towards positive stimuli and sustained attention for dysphoric stimuli (Kellough et al., 2008; Lazarov et al., 2018; Peckham, McHugh, and Otto, 2010). Furthermore, dual-process models of vulnerability (Beevers, 2005) distinguish between early, automatic attentional biases typically evidenced in anxiety (Bar-Haim et al., 2007) and alterations in later elaborative processing of emotional information more common in depression (Gotlib & Joorman, 2010).
The high temporal resolution afforded by electroencephalography (EEG) is optimally suited for studying the dynamics of interpersonal emotional processing biases. Event-related potential (ERP) research enables the simultaneous assessment and disentanglement of initial and later stages of processing in response to discrete stimuli. Of particular relevance, the late positive potential (LPP) is an ERP component characterized by a sustained positive deflection beginning around 300ms post stimulus onset that is heightened for emotional stimuli compared to neutral, indexing the sustained processing of motivationally salient stimuli (Schupp et al., 2000; Hajcak, MacNamara, and Olvet, 2010). The LPP is evidenced to be a reliable, stable indicator of individual differences in emotion processing across development (Bondy et al., 2018; Cassidy, Robertson, & O’Connell, 2012; Pegg et al., 2019a). Furthermore, research indicates that the LPP is sensitive to the content, valence, arousal, and class of stimuli (Olofsson et al., 2008; Thom et al., 2014; Weinberg & Hajcak, 2010). Specifically, social aspects of stimuli have been shown to be particularly salient, such that among neutral images, those containing people or faces elicited larger LPPs than those without people (Ferri, Weinberg, & Hajcak, 2012; Weinberg & Hajcak, 2010). There is additional evidence that emotional scenes yield larger LPP amplitudes relative to emotional faces presented out of context (Kujawa, Klein, & Hajcak, 2012; Thom et al., 2014). Studies have demonstrated that the LPP is modulated by altering the salience of a stimulus through manipulations of attention allocation and cognitive interpretation (Dunning & Hajcak, 2009; Foti & Hajcak, 2008; MacNamara, Ochsner & Hajcak, 2011; Moser et al., 2014).
Within internalizing disorders, the literature evidences heterogeneous patterns of LPP reactivity. Both dimensional and diagnostic approaches indicate reductions in LPP magnitude in depression (Foti et al., 2010; Kujawa et al., 2015), whereas anxiety, especially social anxiety, is associated with an enhanced LPP (MacNamara et al., 2019; MacNamara, Kotov, & Hajcak, 2016; Moser et al., 2008). However, these general trends can be further qualified based on specific stimulus attributes. Recent research suggests that though depressed individuals typically show blunted reactivity to normative, validated stimuli, depression is associated with heightened LPP reactivity to affective self-referent stimuli (Benau et al., 2019; Speed et al., 2016). Furthermore, within the broader category of unpleasant or negative stimuli, anxiety appears to be associated with enhanced neural reactivity for threatening or angry stimuli (Kujawa et al., 2015; Moser et al., 2008; Mueller et al., 2009), with higher reactivity for socially threatening stimuli compared to physically threatening stimuli (Goldin et al., 2009). Enhanced neural reactivity to angry faces in particular has been associated with better treatment response for anxious youth (Bunford et al., 2017). The majority of the reviewed studies use either broad groups of stimuli from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 1997), often combining across depictions of violence, aggressive animals, accidents, natural disasters, and mutilation scenes, or isolated facial expressions presented out of context. While these studies have provided valuable insights, the generalizability of these types of stimuli to quotidian social processes is limited, and research demonstrates aberrations in social processes are fundamental to the emergence of psychopathology (Kennedy & Adolphs, 2012). This highlights the importance of considering stimuli specificity, both regarding emotional valence and the social context. Understanding patterns of attentional allocation and emotional reactivity in commonly-encountered social contexts could help elucidate the underlying mechanisms contributing to the onset and maintenance of internalizing disorders.
However, the etiologic pathways for the development of internalizing symptoms are complex, and intrinsic endophenotypic mechanisms are not insulated or independent from extrinsic environmental influences (Cuthbert, 2014). Though it is well established that stress, especially interpersonal stress, is a potent predictor of depression and social anxiety (Starr et al., 2014; Uliaszek et al., 2010; Vrshek-Shallhorn et al., 2015), the vulnerability factors that predispose some individuals to be more susceptible to the effects of interpersonal stress remain unclear. Other ERP research demonstrates the association between a reduced reward positivity, a neural marker of reward responsiveness, and depression was moderated by exposure to stressful life events (Goldstein et al., 2020). There is some evidence that amygdala reactivity to threatening stimuli in conjunction with exposure to acute stressful events may be a candidate neural biomarker of stress-vulnerability (McLaughlin et al., 2014; Swartz et al., 2015). Source localization research has correlated the scalp recorded LPP with blood-oxygen-level-dependent (BOLD) activation in the amygdala as well as prefrontal regions and the visual cortices (Bunford et al., 2018; Liu et al., 2012). Furthermore, there is evidence that the neural processes indexed by the LPP prospectively predict the onset of internalizing symptoms in children, in combination with parent-reported stress exposure (Kujawa et al., 2016), potentially reflecting a vulnerability to psychopathology. However, the methods for measuring stress varied and these studies used broadly threatening and emotional images. In order to better understand vulnerability to interpersonal stress specifically, research using carefully selected interpersonal stimuli is needed.
Emerging adulthood is characterized by increased salience of peer and potential partner relationships (Nelson et al., 2016), and is a high-risk developmental period for the emergence of psychopathology, particularly mood and anxiety disorders (Kessler et al., 2007; Kessler, Berglund, et al., 2005). Therefore, the present study aimed to extend prior literature on the divergent patterns of emotional reactivity in depression and social anxiety by developing and testing a novel set of threatening and positive interpersonal images selected for relevance to adolescents and emerging adults to enhance ecological validity. First, participant ratings of valence and arousal were examined to validate the novel set of interpersonal emotional images. Then, differences in behavior and neurophysiological reactivity indexed by the LPP based on emotional condition were evaluated. Finally, in order to test the relationship between individual differences in interpersonal emotion processing and internalizing psychopathologies, we tested associations with symptoms of social anxiety and depression, both as main effects and in combination with chronic interpersonal stress. This was done to test the extent to which underlying vulnerabilities reflected by these neural markers may enhance risk for internalizing symptoms in combination with interpersonal stress, an established risk factor for both depression and social anxiety (Starr et al., 2014; Uliaszek et al., 2010; Vrshek-Shallhorn et al., 2015). We expected LPP amplitudes would be enhanced for both positive and threatening interpersonal images compared to neutral images. Furthermore, we anticipated that social anxiety symptoms would be associated with heightened LPP amplitudes and depressive symptoms with blunted LPP amplitudes to both threatening and pleasant interpersonal scenes, and that these aberrant patterns of reactivity would compound the effects of chronic interpersonal stress on internalizing symptoms.
Method
Participants
Participants (N=130) were undergraduate students recruited as part of a study of emotional and social functioning in emerging adults. During a single lab session, participants completed a series of self-report questionnaires followed by computer-based tasks presented in a counterbalanced order while EEG was continuously recorded (see Pegg & Kujawa, 2020 for our prior work using a monetary reward task with a subset of this sample). Participants were 18–22 years old (M = 19.32, SD = 1.15) and were 67.7% female, 10.1% Hispanic/Latino, 50.8% White/Caucasian, 25.4% Asian, 14.6% Black/African American, 0.8% American Indian/Alaskan Native, and 8.5% multiracial. Ethnicity was assessed separately from race. Participants with significant hearing or visual impairments or who were not fluent in English were excluded. Of included participants, 4 did not complete the interpersonal emotional interrupt task, 11 were excluded for having fewer than 15 artifact-free correct trials per condition in the interpersonal emotional interrupt task (described below), and 1 participant had missing data due to an EEG recording issue, resulting in an analyzed sample of 114 participants. The Institutional Review Board at Vanderbilt University approved this study, and informed consent was obtained from all participants.
Measures
Interpersonal Emotional Interrupt Task
While EEG was recorded, participants completed a novel interpersonal emotion task analogous to the emotional interrupt paradigm, which has been shown to reliably elicit the LPP in prior research (Kujawa et al., 2012; Nelson et al., 2015; Pegg et al., 2019a). Stimuli for this task were specifically selected to be relevant to the social experiences of adolescents and emerging adults, and consisted of 15 threatening interpersonal images (e.g. bullying by peers, young people arguing with parents or friends), 15 pleasant interpersonal images (e.g. friends laughing, young happy couples), and 15 non-social neutral images (e.g. nature and city scenes). Stimuli were primarily obtained through stock image sites but three images in the neutral condition were derived from the Open Affective Standardized Image Set (OASIS; Kurdi, Lozano, & Banaji, 2017)1. 80 images were initially selected based on face validity and preliminary ratings of valence and arousal were obtained by research staff who were not informed of the study details following the same procedures as participants. The final set of 45 images were determined based on these initial ratings and consistency with specifically selected interpersonal themes. Trials consisted of a fixation cross (+) presented for 800ms, followed by the random presentation of an image for 1000ms, a target arrow (< or >) for 150ms, and the same image presented for an additional 400ms (Figure 1). In order to ensure attention to the task and as a behavioral measure of emotional interference with performance, participants were instructed to click the right or left mouse button in concordance with the direction of the target arrow on each trial. Responses prior to 150ms or after 2150ms target presentation were considered errors. Only correct trials were included in analysis. Inter-trial intervals varied randomly from 1500ms to 2000ms. After completing 6 practice trials, participants completed two blocks of the task, with each image presented once in each block, yielding a total of 90 trials. Considering prior research on the psychometric properties of the LPP indicates a minimum of 12 artifact free trials are needed to obtain a stable LPP difference score, while 8 trials are sufficient for a stable LPP in a single condition (Moran, Jendrusina, & Moser, 2013), the inclusion of 12 images presented twice (for a total of 30 trials per condition) is sufficient for reliably eliciting the LPP. Images were presented across the full screen with participants seated approximately 18 inches away from the monitor.
Figure 1.
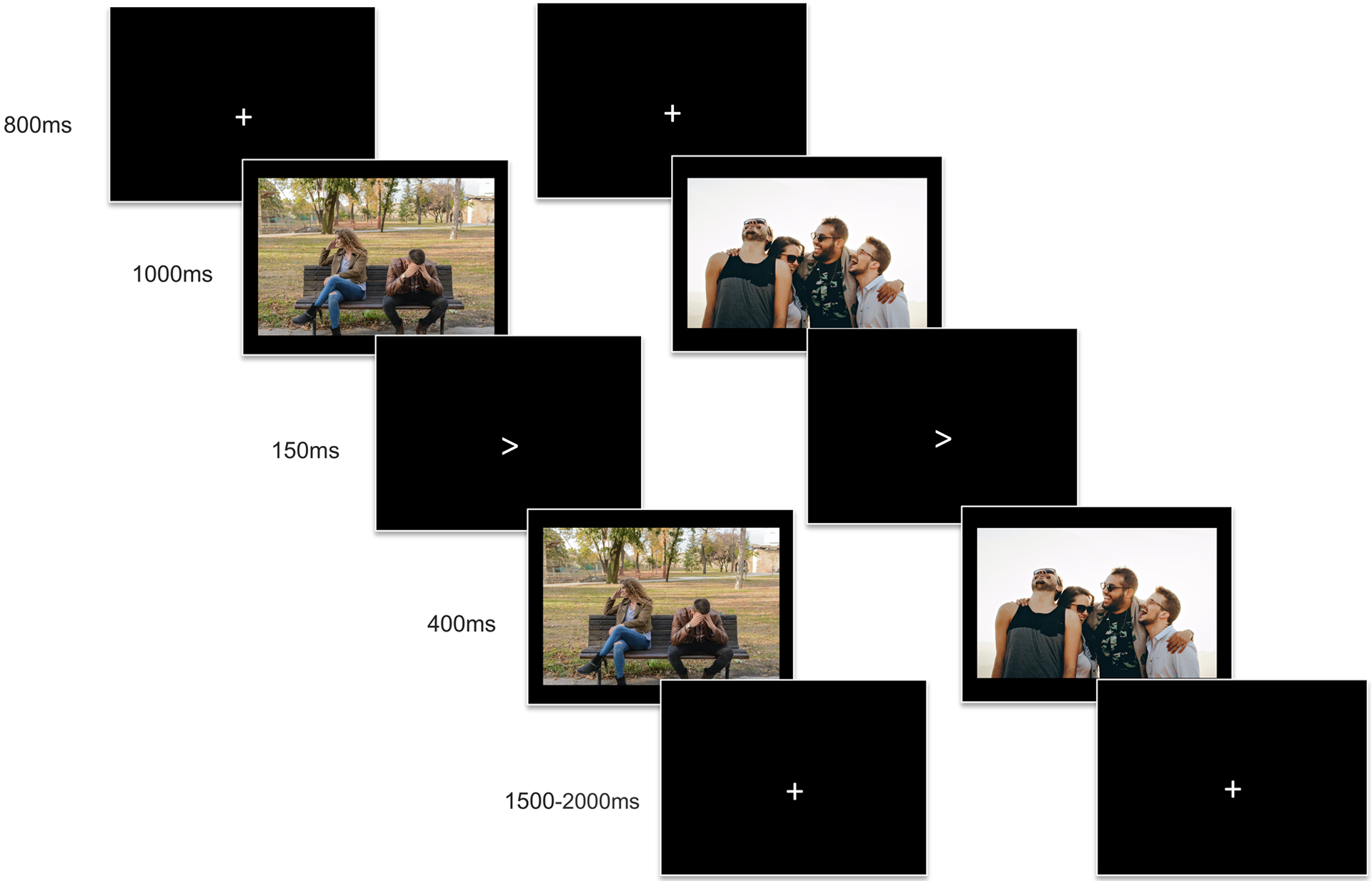
Trial sequences for the interpersonal emotional interrupt task with an example threatening image (left) and positive image (right).
In order to further validate the images, participants completed valence and arousal ratings for each of the stimuli in the task at the conclusion of the assessment using the self-assessment manikin (SAM) rating scales based on the procedures for validating the International Affective Picture System (Lang, Bradley, & Cuthbert, 2005). Valence was assessed on a scale from 1 (happy) to 9 (unhappy) while arousal was rated on a scale from 1 (aroused) to 9 (unaroused; see Table S1 and Figure S1 in the Supplementary Information for a description and average valence/arousal for each image and the SAM rating scale). Average ratings were reverse coded prior to analysis to be consistent with the IAPS procedures, which used the scales from 1 (unpleasant) to 9 (highly pleasant) for valence and 1 (nonarousing) to 9 (highly arousing) for arousal.
Questionnaires
Chronic interpersonal stress was assessed through the stressor items from the Responses to Stress Questionnaire- Interpersonal Stress version (RSQ; Connor-Smith & Compas, 2002). The initial 9 items list potential stressors (i.e. “Having conflict with a good friend,” “Frequent arguments with a partner/spouse”), which participants rated on a scale from 1 (not at all) to 4 (very) for how stressful each experience was in the prior six months. A mean composite score for chronic interpersonal stress was computed by averaging the endorsed interpersonal stressor items (M = 1.66, SD = 0.52). The internal consistency for the interpersonal stressor items was α = .77.
To measure current depressive and anxious symptoms, the 64-item Inventory of Depression and Anxiety Symptoms (IDAS; Watson et al., 2007) was administered. Participants rated the extent to which they have experienced each item in the previous two weeks on a scale from 1 (not at all) to 5 (extremely). The IDAS contains two broad scales, general depression and dysphoria, as well as ten symptom specific scales, including suicidality, lassitude, insomnia, appetite loss, appetite gain, ill temper, well-being, panic, social anxiety, and traumatic intrusions. Consistent with our prior work differentiating neural processes underlying depression and social anxiety, the present study examined the dysphoria and social anxiety scales (Pegg et al., 2019b). The means and internal consistencies of symptoms in the present sample (M = 21.58, SD = 6.77, α = .86 for dysphoria; M = 9.75, SD = 4.07, α = .83 for social anxiety) were comparable to those evidenced in previous research on college students and young adults (M = 20.43, SD = 7.68, α = .89 for dysphoria; M = 9.60, SD = 4.14, α = .82 for social anxiety; Watson et al., 2007). Based on the balanced clinical cutoffs for the IDAS (Stasik-O’Brien et al., 2019), 27.3% of participants endorsed clinical levels of social anxiety symptoms and 17.8% endorsed clinical levels of dysphoria symptoms.
EEG Data Collection and Processing
EEG data were continuously recorded using a 64-channel actiCHamp system from BrainProducts (Munich, Germany). A single scalp electrode (Cz) was used as the reference for the online data acquisition, and data were re-referenced offline to the linked mastoid recordings (TP9 and TP10). Electrooculogram was recorded using facial electrodes placed 1 cm to the outer corner of each eye, as well as 1 cm above and below the right eye and referenced to an electrode placed on the back of the neck of the participant, per the BrainProducts bipolar-to-auxiliary adapter design. Data were collected at a sampling rate of 1000 Hz. Offline processing was completed using BrainVision Analyzer (BVA) software (BrainProducts, Munich, Germany). Data were band pass filtered from .01 to 30 Hz. Trials were segmented from 200ms prior to stimulus onset to 1000ms after onset. Semi-automated procedures were used for initial artifact rejection and eye blink correction, identifying voltage steps of more than 50 microvolts per ms between sampling points, voltage differences greater than 175 microvolts within a trial, and lowest allowed activity of .50 microvolts within 100ms intervals. Visual inspection was subsequently employed to identify and remove additional artifacts. Trials were baseline corrected to 200ms prior to stimuli onset. Interpolation with the signal from surrounding electrodes was used to resolve faulty recordings at single electrodes. Consistent with research on the requisite number of trials for obtaining a stable LPP (Moran, Jendrusina & Moser, 2013), included participants had a minimum of 12 artifact free trials per condition. Participants had an average of 27.18 (SD = 3.39) trials for the neutral condition, 27.24 (SD = 3.23) for the threatening condition, and 27.46 (SD = 3.14) for the positive condition at POz. The LPP was scored as the average activity within the commonly used time window of 400–1000 ms (Foti & Hajcak, 2008; Stange et al., 2017; Weinberg & Hajcak, 2010) at a pooling of occipitoparietal sites (POz, PO3, PO4; Codispoti, De Cesarei, & Ferrari, 2012; Kujawa et al., 2015; Van Dongen, Van Strien, & Dijkstra, 2016), which is consistent both with prior literature and the maximal distributions observed in the grand average data (Figure 2). Average LPPs within this time window and pooling were computed for each condition and participant, and these values were exported from BVA for further statistical evaluation. There is considerable variability in the scoring of the LPP across studies, depending on stimulus presentation duration. Given the potential for distinct patterns of results to emerge at earlier and later stages of processing, exploratory analyses also examined the LPP split into relatively earlier (400–700 ms) and later LPP (700–1000 ms) windows (see Supplementary Information; Auerbach et al., 2015; Schupp et al., 2004). Split-half reliability for the LPP component ranged from acceptable to good (Spearman-Brown coefficients: positive = .83, threatening = .75, neutral = .79).
Figure 2.
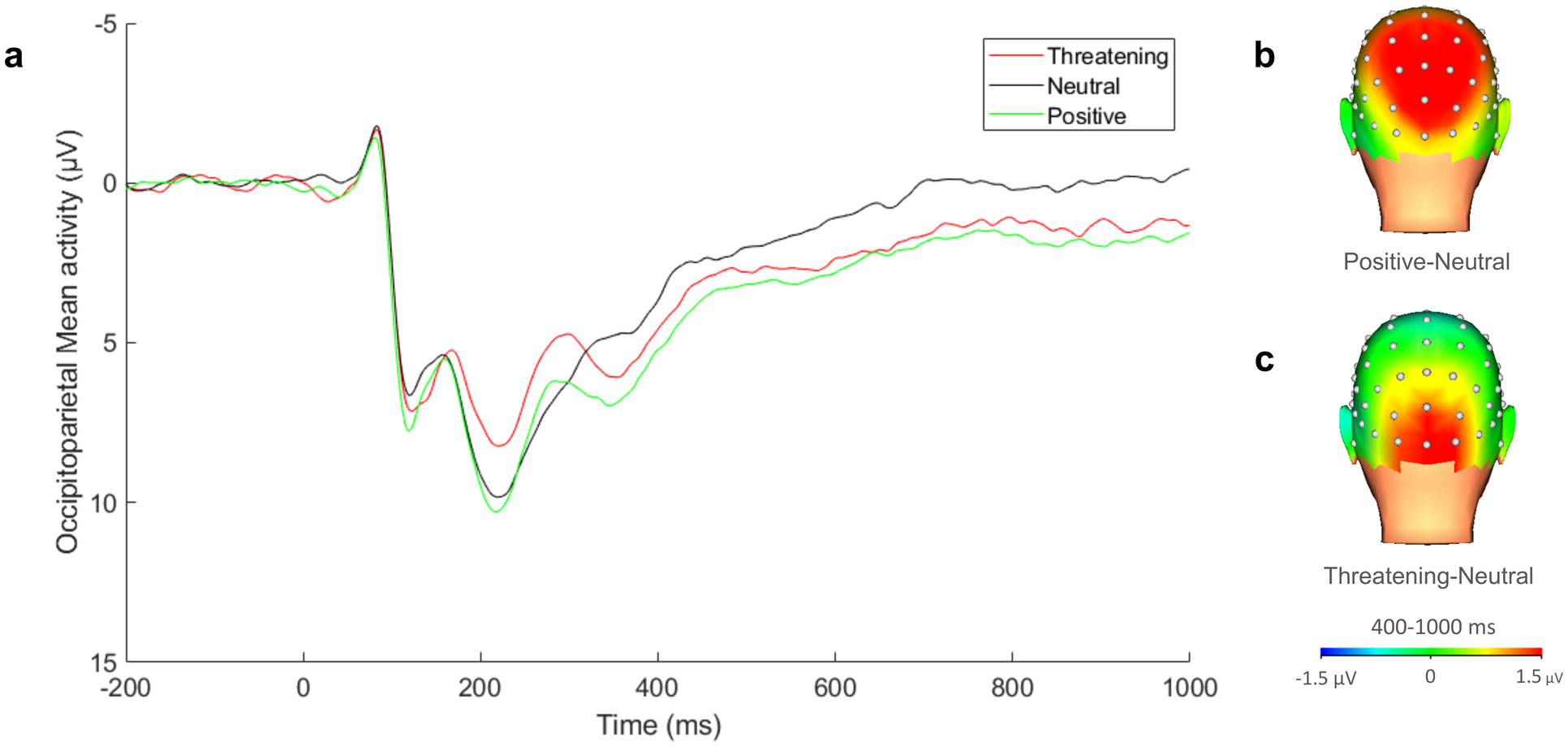
Grand average ERP waveform for the LPP across POz, PO3, and PO4. Scalp distributions reflect the interpersonal emotional condition minus neutral.
Data Analysis
Repeated-measures ANOVAs were conducted to examine differences in subjective valence and arousal ratings as well as on behavioral performance (reaction time [RT] and accuracy) across conditions. Similarly, a repeated-measures ANOVA was performed to determine the effect of condition on the LPP. Where assumptions of sphericity were violated, the Greenhouse-Geisser correction was applied.
Next, a series of hierarchical linear regressions were conducted in SPSS to assess the main effects of LPP amplitude and chronic interpersonal stress, as well as the interactive effect in the prediction of depressive and social anxiety symptoms. In all models, LPP magnitudes for the neutral condition were entered first to control for individual differences in baseline reactivity and isolate the effect of the emotional condition on the LPP. Next, the mean-centered LPP amplitudes for each emotional condition and mean-centered levels of chronic interpersonal stress were entered separately, and finally, the interaction between the LPP for each condition and stress were entered. Interaction terms were calculated by taking the product of the mean-centered variables. Simple slopes at the mean and one standard deviation above and below the mean were subsequently examined for significant interactions, as well as the region of significance using the Johnson-Neyman technique (Johnson & Neyman, 1936) in the PROCESS macro for SPSS version 3.4 (Hayes, 2017). False discovery rate (FDR) corrections using the Benjamini-Hochberg method (Benjamini & Hochberg, 1995) were applied to the critical p values in all regression models to minimize the possibility of Type I errors resulting from multiple comparisons.
Results
The descriptive statistics and bivariate correlations between age, gender, the LPP and clinical symptom measures are presented in Table 1. The means and standard deviations for self-reported stimuli ratings and behavioral performance across each emotional condition are provided in Table 2.
Table 1.
Descriptive statistics and bivariate correlations for clinical and neural measures
| M (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | Social Anxiety Symptoms | 9.71 (4.07) | - | ||||||
| 2 | Depressive Symptoms | 21.58 (6.77) | 0.45** | ||||||
| 3 | Interpersonal Stress | 1.65 (0.52) | 0.35** | 0.55** | |||||
| 4 | LPP Positive Images | 2.41 (4.38) | 0.17 | −0.03 | −0.03 | ||||
| 5 | LPP Threatening Images | 1.98 (4.39) | 0.13 | −0.03 | −0.10 | 0.82** | |||
| 6 | LPP Neutral Images | 0.79 (4.76) | 0.06 | −0.11 | −0.12 | 0.73** | 0.71** | ||
| 7 | Age | 19.32 (1.15) | −0.24* | 0.04 | 0.09 | −0.18 | −0.17 | −0.07 | |
| 8 | Gender | - | 0.18 | 0.13 | 0.00 | −0.08 | −0.06 | −0.12 | −0.09 |
p < .05,
p < .01
Table 2.
Self-report ratings and behavioral performance for the interpersonal emotion interrupt task
| Threatening | Positive | Neutral | |
|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |
| Valence | 2.41 (0.88) | 7.46 (1.02) | 6.11 (0.75) |
| Arousal | 6.06 (1.34) | 5.58 (1.31) | 3.89 (1.37) |
| Reaction Time (ms) | 298.82 (130.59) | 295.75 (125.04) | 293.57 (123.77) |
| Accuracy (%) | 92.58 (7.56) | 93.01 (7.00) | 92.21 (7.69) |
Valence and arousal results
Valence ratings significantly differed by emotion condition, F(1.42, 124) = 936.28, p < .001, ηp2 = .88, such that positive images were rated as more pleasant than both neutral, F(1, 124) = 241.79, p < .001, ηp2 = .66, and threatening images, F(1, 124) = 1085.36, p < .001, ηp2 = .90. Threatening images were also rated as less pleasant than neutral, F(1, 124) = 1075.15, p < .001, ηp2 = .90. Similarly, there was a significant effect of condition on arousal ratings, F(1.71, 124) = 120.52, p < .001, ηp2 = .50. Both positive, F(1, 124) = 190.11, p < .001, ηp2 = .61, and threatening images, F(1, 124) = 156.35, p < .001, ηp2 = .56, were rated as more arousing than neutral images. Furthermore, threatening images were rated as more arousing than positive images, F(1, 124) = 11.76, p = .001, ηp2 = .09.
Behavioral results
The effects of emotion condition on reaction time and accuracy were not significant (ps > .09).
LPP results
The emotion condition effect on LPP magnitude was significant, F(2, 114) = 15.81, p < .001, ηp2 = .12, such that the LPP was enhanced for threatening images compared to neutral, F(1, 114) = 13.30, p < .001, ηp2 = .11, and for positive images compared to neutral, F(1, 114) = 26.67, p < .001, ηp2 = .19. The LPP was not significantly enhanced for positive images compared to threatening images, F(1, 114) = 2.93, p= .09, ηp2= .03.
Associations with symptoms of social anxiety and depression
Results of the hierarchical linear regression analyses testing the main effects of LPP, chronic interpersonal stress, and their interaction on symptoms of social anxiety and depression are presented in Tables 3 and 4. There was a significant main effect of chronic interpersonal stress on both dysphoria (B = .54–.55, t = 6.71–6.81, ps < .001) and social anxiety symptoms (B = .35–.37, t = 3.95–4.11, ps < .001). There were no main effects of LPP on either social anxiety or depressive symptoms (ps > .06). There were significant interactions between chronic interpersonal stress and the LPP to both threatening (B = .24, t = 2.60, FDR-corrected p = .02) and positive images (B = .24, t = 2.54, FDR-corrected p = .02) on social anxiety symptoms, but not symptoms of dysphoria2. The variance inflation factors for the regression models were between 1.01–2.18 while tolerance values ranged from .46–.99, indicating minimal multicollinearity among the predictors. Results from regression models using a residual scoring approach to quantifying the LPP are presented in the Supplementary Information.
Table 3.
Regression models testing the main and interactive effects of the LPP and chronic interpersonal stress in the prediction of social anxiety symptoms
| ΔR2 | b(SE) | B | ΔR2 | b (SE) | B | |||
|---|---|---|---|---|---|---|---|---|
| Step 1: | LPP Neutral | 0.02 | −0.04 (0.12) | −0.05 | LPP Neutral | 0.04 | −0.10 (0.12) | −0.12 |
| LPP Threat | 0.16 (0.12) | 0.18 | LPP Positive | 0.24 (0.13)^ | 0.26^ | |||
| Step 2: | LPP Neutral | 0.13*** | −0.02 (0.11) | −0.02 | LPP Neutral | 0.12*** | −0.04 (0.11) | −0.05 |
| LPP Threat | 0.17 (0.12) | 0.19 | LPP Positive | 0.20 (0.12) | 0.22 | |||
| Interpersonal Stress | 2.86 (0.70)*** | 0.37*** | Interpersonal Stress | 2.74 (0.69)*** | 0.35*** | |||
| Step 3: | LPP Neutral | 0.05* | 0.02 (0.11) | 0.03 | LPP Neutral | 0.05* | 0.01 (0.11) | 0.01 |
| LPP Threat | 0.14 (0.11) | 0.16 | LPP Positive | 0.17 (0.12) | 0.18 | |||
| Interpersonal Stress | 3.59 (0.73)*** | 0.46*** | Interpersonal Stress | 3.48 (0.74)*** | 0.45*** | |||
| LPP × Stress | 0.42 (0.16)* | 0.24* | LPP × Stress | 0.42 (0.17)** | 0.24** | |||
| Full Model: | R2 = .20 | R2 = .21 |
p < .10,
p < .05,
p < .01,
p <.001
Note: p-values shown are prior to FDR-correction. B = standardized coefficients; b = unstandardized coefficients.
Table 4.
Regression models testing the main and interactive effects of the LPP and chronic interpersonal stress in the prediction of depressive symptoms
| ΔR2 | b (SE) | B | ΔR2 | b (SE) | B | |||
|---|---|---|---|---|---|---|---|---|
| Step 1: | LPP Neutral | 0.02 | −0.24 (0.19) | −0.17 | LPP Neutral | 0.02 | −0.28 (0.20) | −0.20 |
| LPP Threat | 0.14 (0.21) | 0.09 | LPP Positive | 0.19 (0.22) | 0.13 | |||
| Step 2: | LPP Neutral | 0.29*** | −0.17 (0.16) | −.12 | LPP Neutral | 0.29*** | −0.13 (0.17) | −0.09 |
| LPP Threat | 0.17 (0.17) | 0.11 | LPP Positive | 0.09 (0.18) | 0.06 | |||
| Interpersonal Stress | 7.11 (1.04)*** | 0.55*** | Interpersonal Stress | 7.04 (1.05)*** | 0.54*** | |||
| Step 3: | LPP Neutral | 0.00 | −0.17 (0.16) | −.12 | LPP Neutral | 0.00 | −0.13 (0.17) | 0.01 |
| LPP Threat | 0.17 (0.17) | 0.11 | LPP Positive | 0.09 (0.18) | 0.16 | |||
| Interpersonal Stress | 7.07 (1.14)*** | 0.55*** | Interpersonal Stress | 7.04 (1.15)*** | 0.43*** | |||
| LPP × Stress | −.02 (0.25) | −.01 | LPP × Stress | −.00 (0.26) | 0.00 | |||
| Full Model: | R2 = .31 | R2 = .31 |
p < .05,
p < .01,
p <.001
Note: p-values shown are prior to FDR-correction. B = standardized coefficients; b = unstandardized coefficients.
These interactions were probed by examining the simple slopes at the mean and one standard deviation above and below the mean values for chronic interpersonal stress (Figure 3). The relationship between the LPP to positive interpersonal stimuli and social anxiety symptoms was positive and significant only at one standard deviation above the mean for chronic interpersonal stress (b = .39, t = 2.82, p = .006), but not at the mean (b = .17, SE = .12, t = 1.46, p = .15) or one standard deviation below the mean level of stress (b = −.05, SE = .15, t = −.30, p = .77). The Johnson-Neyman test indicated that the effect of the LPP to positive interpersonal stimuli on social anxiety symptoms becomes significant when levels of chronic interpersonal stress are .29 SD above the mean or higher (score of 1.81 or higher on RSQ stressor items; range = 1.00 – 3.25).
Figure 3.
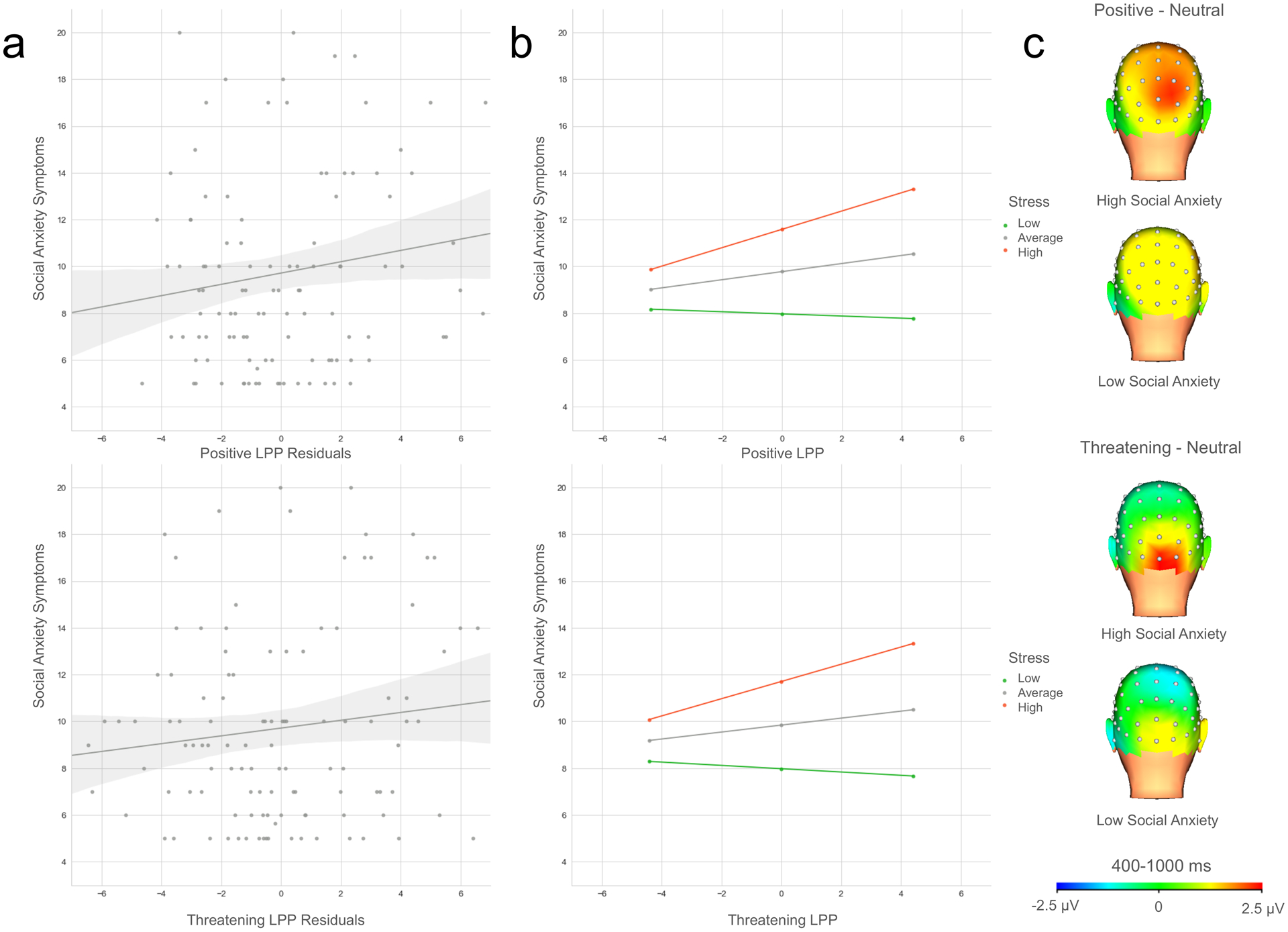
a) Scatterplots depicting the overall association between LPP residuals to positive (top) and threatening (bottom) images and social anxiety symptom levels. b) Plots of the simple slopes for the interaction effect between LPP to positive (top) and threatening (bottom) stimuli at low, average, and high levels of chronic interpersonal stress (note: low, average, and high represent −1 SD, mean, and +1 SD). c) Scalp distributions depicting the LPP for participants above the Johnson-Neyman region of significance threshold for stress with high and low levels of social anxiety (note: the median split for high and low social anxiety symptom levels was used for illustrative purposes only; the primary analyses used social anxiety symptoms as a continuous variable).
A comparable interaction effect was observed for LPP reactivity to threatening interpersonal stimuli. The association between the LPP to threatening images and social anxiety symptoms was positive and significant at one standard deviation above the mean for chronic interpersonal stress (b = .37, SE = .14, t = 2.72, p = .008), but neither at the mean (b = .15, SE = .11, t = 1.31, p = .19) or one standard deviation below the mean level of stress (b = −.07, SE = .15, t = −.48, p = .63). The Johnson-Neyman test indicated that the effect of the LPP to threatening interpersonal stimuli on social anxiety becomes significant when levels of chronic interpersonal stress are .36 SD above the mean or higher (score of 1.85 or higher on RSQ stressor items; range = 1.00 – 3.25). For the interactive effects between both the LPP to positive and stress and threatening stimuli and stress, 33.94% of the participants had levels of chronic interpersonal stress above the Johnson-Neyman region of significance scores.
The full regression models for LPP to threatening and positive stimuli accounted for approximately twenty percent of the variability in social anxiety symptoms (R2 = .20 and R2 = .21, respectively), with the interaction between the LPP and chronic interpersonal stress significantly contributing five percent unique variance (ΔR2 = .05, F(1, 107) = 6.76, p = .01 and ΔR2 = .05, F(1, 107) = 6.46, p = .01, respectively).
In order to assess the robustness of the interaction effect between chronic interpersonal stress and the LPP to positive and threatening images, additional post hoc models co-varying depressive symptoms were tested. This is imperative given the high comorbidity and overlap between depressive and social anxiety symptoms demonstrated in extant research (Kessler & Wang, 2008; Kotov et al., 2017; Lilienfeld & Treadway, 2016), and the significant correlation between these internalizing domains in the present sample (r = .45, p < .001). The interactions between chronic interpersonal stress and LPP reactivity to threatening and positive stimuli on social anxiety symptoms remained significant when co-varying symptoms of dysphoria (B = .25, t = 2.78, FDR-corrected p = .03 and B = .24, t = 2.68, FDR-corrected p = .02, respectively). Finally, because age was significantly associated with social anxiety symptoms (r = −.24, p = .01) and gender had a trend level association with social anxiety symptoms (r = .18, p = .06), two additional post hoc models co-varying these demographic factors in addition to depressive symptom levels were tested. Again, the interactions between chronic interpersonal stress and LPP reactivity to threatening and positive stimuli on social anxiety symptoms remained significant (B = .23, t = 2.68, FDR-corrected p = .02 and B = .24, t = 2.83, FDR-corrected p = .05, respectively).
Discussion
The present study tested a novel set of interpersonal emotional stimuli relevant to emerging adults at the self-report, behavioral, and neurophysiological levels in order to fill a critical gap in the literature and further clarify common versus distinct patterns of interpersonal emotion processing within internalizing disorders. The subjective ratings for valence and arousal validated the selected stimuli in the expected directions, with positive images rated as more pleasant than neutral images, and threatening images rated as least pleasant. Threatening images were rated as most arousing, followed by positive images, and neutral images rated least arousing. However, contrary to expectations our data did not reveal significant interference on RT or accuracy based on interpersonal emotional condition. The LPP component, a reliable indicator of individual differences in emotion processing, was significantly modulated by interpersonal emotional condition consistent with the LPP literature using other validated stimulus sets, providing further support for this novel stimulus set at the neurophysiological level. Specifically, both threatening and positive interpersonal images elicited an enhanced occipitoparietal LPP compared to neutral images, further validating this stimuli set. Finally, results revealed a significant interaction between the LPP to interpersonal emotional images and chronic interpersonal stress, such that enhanced LPP amplitudes in conjunction with elevated stress were associated with higher levels of social anxiety symptoms, but not depressive symptoms.
Interpersonal emotional images and behavioral measures
We expected threatening and positive interpersonal images would result in reduced accuracy and increased reaction times compared to the neutral image condition. However, we did not observe any impact of condition on participants’ behavioral performance, although we did observe the expected modulation of subjective ratings of the images. Developmental research evidences reduced RTs and increased accuracy on emotion interrupt paradigms with age (Pegg et al., 2019a), thus as cognitive control improves, behavioral interference effects decrease, which may partially account for the lack of an effect of interpersonal emotional condition on accuracy. Though some research on adults found slowed RTs and decreased accuracy for emotional conditions compared to neutral (Weinberg & Hajcak, 2011), other research had null behavioral effects depending on the type of stimuli used (Kujawa, Klein, & Hajcak, 2012). A study using both IAPS images and emotional faces evidenced behavioral interference for the IAPS images but not for emotional faces (Kujawa, Klein, & Hajcak, 2012), suggesting highly arousing or shocking stimuli may impair behavioral performance whereas more subtle and naturalistic stimuli comparable to those used in the current study may not. Further, the lack of an effect of emotion processing on behavioral performance and low reliability of RTs supports the utility of ERP measures of emotion processing, capturing alterations in emotion processing that may not otherwise be apparent using traditional behavioral approaches.
Interpersonal emotional images and ERP measures
In accordance with our expectations, neurophysiological reactivity indexed by the LPP to interpersonal stimuli in the present study was enhanced for both interpersonal emotional conditions compared with the neutral condition. This is also consistent with prior research assessing the LPP in response to more established stimuli sets (Kujawa, Klein, & Hajcak, 2012; Schupp et al., 2000; Weinberg & Hajcak, 2010). Interestingly, we observed a broader distribution across the scalp for the LPP to positive interpersonal stimuli compared with threatening stimuli, which diverges from extant research suggesting the LPP is sensitive primarily to arousal rather than valence (Olofsson et al., 2008; Hajcak, MacNamara & Olvet, 2010). Based on theories of changes in social orientation across development which characterize emerging adulthood by an increase in social affiliation towards peers and potential partners (Nelson, Jarcho, & Guyer, 2016) and the interpersonal specificity of the chosen stimuli, it is possible that the salience and selective attention for positive interpersonal stimuli is heightened in this developmental population. Further, evidence from source localization research suggests differing neural circuits are involved in generating the scalp-recorded LPP for positively compared with negatively valenced stimuli, such that the medial prefrontal cortex and nucleus accumbens are activated solely for pleasant images (Liu et al., 2012). This selective recruitment of cortical and subcortical regions may contribute to the relatively widespread distribution of the LPP across the scalp in response to positive interpersonal images observed in the present study.
Interpersonal emotional images: Relevance to social anxiety and depression
We hypothesized that under high levels of stress, relative enhancements in LPP amplitudes would be associated with higher social anxiety symptoms, while reduced LPPs would be associated with elevated depressive symptoms. However, our results revealed that enhanced LPPs to both threatening and positive interpersonal images in combination with high levels of chronic interpersonal stress predicted higher social anxiety symptoms, while no LPP alterations were observed in association with depressive symptoms. These results demonstrate the sensitivity of the LPP to individual differences in interpersonal emotion processing with potential clinical utility. The moderating influence suggests sustained attentional processing of social information indexed by elevated LPP amplitudes compound with high levels of chronic interpersonal stress to confer increased risk uniquely for social anxiety, rather than depression. These findings suggest the LPP is a useful neurophysiological measure that can be elicited in response to range of salient images relevant to specific forms of psychopathology and clinical questions. The interactive effect between the LPP and chronic interpersonal stress also highlights the importance of examining multiple interacting factors contributing to psychopathology.
The specificity of this interaction effect to social anxiety symptoms is consistent with the existing literature theorizing early, automatic biases for threatening stimuli are associated with anxiety (Bar-Haim et al., 2007; Mathews & MacLeod, 2005). The present findings extend prior research by demonstrating biased processing of positive interpersonal stimuli in combination with high interpersonal stress is additionally associated with risk for social anxiety. In contrast to expectations based on evidence of blunted LPPs in depression (Foti et al., 2010; MacNamara, Kotov & Hajcak, 2016; Weinberg, Perlman & Kotov, 2016), we did not find any associations between the LPP and symptoms of dysphoria. The dual-process model of cognitive vulnerability suggests anxiety is associated with early, automatic processing biases whereas depression tends to be associated with later, maladaptive elaborative processing (Beevers, 2005). Though the ERP literature typically refers to the LPP as an indicator of relatively later stages of processing, ERP components emerge at the temporal scale of milliseconds. Therefore, it is likely that the LPP component using the current task design reflects relatively early processing biases rather than elaborative processing, which may emerge across several seconds. This notion is supported by eye-tracking research showing the association between dysphoria and reduced processing of pleasant stimuli did not emerge during initial stages of attention (Sears et al., 2016). Additionally, given that recent research has shown depression is characterized by increased processing for self-relevant stimuli (Benau et al., 2019; Speed et al., 2016), it is possible the null findings for dysphoria were due to a reduced salience for stimuli depicting non-relevant others. Finally, a growing body of literature suggests depression is associated with disruptions in positive valence systems, particularly for reductions in reward processing (Admon & Pizzagalli, 2015; Kujawa, Hajcak & Klein, 2019; Weinberg, Pearlman, & Kotov, 2016), thus ERP tasks targeting social reward-processing may elicit more robust associations with depressive symptomatology (Kujawa, Hajcak, Klein, 2019; Pegg et al., 2019b). The preliminary evidence in our study suggests sustained attentional processing of social information indexed by elevated LPP amplitudes compound with high levels of chronic interpersonal stress to confer risk uniquely for social anxiety, rather than depression.
Our results also revealed a significant main effect of chronic interpersonal stress in all models, such that higher levels of stress corresponded with higher levels of both social anxiety symptoms and depressive symptoms. This is consistent with the existing literature on the role of stress in the etiology and maintenance of internalizing disorders (Hammen, 2016; Starr et al., 2014; Uliaszek et al., 2012; Vrshek-Shallhorn et al., 2015). The null finding for a main effect of the LPP on internalizing symptoms but significant interactive effect suggests the possibility that neurophysiological markers of emotion processing may represent trait-like vulnerability factors that predispose some individuals to increased risk for internalizing symptoms in combination with stress. This theory is supported by research demonstrating neural reactivity to emotional content prospectively predicted the development of internalizing symptoms in response to an acute stressor (Kujawa et al., 2016). However, since the present data are cross-sectional this distinction is unable to be tested in our sample. Future longitudinal research is needed to disentangle whether the LPP could be a biomarker of stress-vulnerability, as well as whether this interaction effect emerges consistently across development. These results additionally warrant replication in clinical samples using objective measures of stress exposure to more precisely assess this vulnerability-stress theory (Harkness & Monroe, 2016; Monroe & Simons, 1991).
Limitations
Several limitations to the present study need to be addressed. First, the cross-sectional design inhibits interpretations regarding the direction of the observed effects, warranting future longitudinal research to clarify whether the LPP to interpersonally relevant images could reflect an early emerging vulnerability to psychopathology or sensitivity to stress. Based on prior research demonstrating individuals experiencing depression and anxiety are prone to generating more dependent stressors (Uliaszek et al., 2012) it is possible that participants with higher levels of social anxiety experience more dependent interpersonal stressors. Additionally, the self-report measurement of stress used in the present study conflates the subjective interpretations of stress exposure and severity, and participants with high internalizing symptom levels may perceive interpersonal stressors as more severe. Additional research is needed to test these associations using established, objective measures of stress (Harkness & Monroe, 2016). It is also worth noting that although a portion of participants did report experiencing clinical levels of social anxiety and depressive symptoms, these proportions were relatively small, potentially contributing to the null findings in the depression models. Future research in clinical samples is needed. Finally, the control condition for the interpersonal threat task used non-social neutral images rather than neutral social images, given evidence that the presence of faces in neutral images enhances the LPP (Ferri, Weinberg, & Hajcak, 2012). Yet, this limits comparisons between neutral and emotional interpersonal stimuli, and future research is needed to develop and validate neutral interpersonal images.
Conclusion
Considering the high rate of comorbidity between depression and anxiety as well as overlapping phenotypic impairments in social functioning, distinguishing between shared versus distinct mechanisms is imperative. Alterations in the processing of interpersonal emotional stimuli evidenced in the present study suggest the LPP as a candidate mechanism. A similar pattern of results may emerge using validated stimuli sets, such as the IAPS, but the stimuli selected for the present study are more theoretically linked to the processes that involved in the emergence of both depression and anxiety. The present results have implications for preventive interventions, suggesting enhanced LPPs to interpersonal emotional stimuli may help identify those at heightened risk for social anxiety, even at subclinical symptom levels. However, future neuroimaging and neurophysiological research focusing on more narrowly defined stimuli in order to target specific emotional processes of interest and relevance is needed.
Supplementary Material
Acknowledgments
The authors would like to thank all of the study members that contributed to data collection, especially Haley Green, Michael West, and Emilia Cárdenas.
Funding sources
This work was supported in part by UL1 TR000445 from NCATS/NIH. SP was supported by NIH/NIMH T32 -MH18921 during completion of this work.
Footnotes
Open Practices Statement
The task images and valence and arousal ratings are available upon request from the corresponding author. None of the experiments were preregistered.
The full image set for the interpersonal emotional interrupt task is available by contacting the corresponding author.
Results were comparable using the IDAS general depression subscale (ps = .61–.68).
This is a post-peer-review, pre-copyedit version of an article published in Cognitive, Affective, & Behavioral Neuroscience. The final authenticated version is available online at: http://dx.doi.org/10.3758/s13415-021-00925-6
References
- Admon R., & Pizzagalli D. (2015). Dysfunctional reward processing in depression. Current Opinion in Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong T, & Olatunji B (2012). Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clinical Psychology Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach RP, Stanton CH, Proudfit GH, & Pizzagalli DA (2015). Self-referential processing in depressed adolescents: A high-density event-related potential study. Journal of Abnormal Psychology, 124(2), 233–245. 10.1037/abn0000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg M, & Van Ijzendoorn M (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. [DOI] [PubMed] [Google Scholar]
- Beevers C (2005). Cognitive vulnerability to depression: A dual process model. Clinical Psychology Review. [DOI] [PubMed] [Google Scholar]
- Benau E, Hill K, Atchley R, O’Hare A, Gibson L, Hajcak G, et al. (2019). Increased neural sensitivity to self-relevant stimuli in major depressive disorder. Psychophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: series B (Methodological), 57(1), 289–300. [Google Scholar]
- Bondy E, Stewart J, Hajcak G, Weinberg A, Tarlow N, Mittal V, et al. (2018). Emotion processing in female youth: Testing the stability of the late positive potential. Psychophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunford N, Kujawa A, Fitzgerald KD, Monk CS, & Phan KL (2018). Convergence of BOLD and ERP measures of neural reactivity to emotional faces in children and adolescents with and without anxiety disorders. Biological Psychology, 134, 9–19. [DOI] [PubMed] [Google Scholar]
- Bunford N, Kujawa A, Fitzgerald KD, Swain JE, Hanna GL, Koschmann E, … & Phan KL (2017). Neural reactivity to angry faces predicts treatment response in pediatric anxiety. Journal of Abnormal Child Psychology, 45(2), 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy S, Robertson I, & O’Connell R (2012). Retest reliability of event-related potentials: Evidence from a variety of paradigms. Psychophysiology. [DOI] [PubMed] [Google Scholar]
- Codispoti M, De Cesarei A, & Ferrari V (2012). The influence of color on emotional perception of natural scenes. Psychophysiology, 49(1), 11–16. [DOI] [PubMed] [Google Scholar]
- Connor-Smith JK, & Compas BE (2002). Vulnerability to social stress: Coping as a mediator or moderator of sociotropy and symptoms of anxiety and depression. Cognitive Therapy and Research, 26(1), 39–55. [Google Scholar]
- Cuthbert BN (2014). Translating intermediate phenotypes to psychopathology: The NIMH Research Domain Criteria. Psychophysiology, 51(12), 1205–1206. [DOI] [PubMed] [Google Scholar]
- Dunning J, & Hajcak G (2009). See no evil: Directing visual attention within unpleasant images modulates the electrocortical response. Psychophysiology. [DOI] [PubMed] [Google Scholar]
- Ferri J, Weinberg A, & Hajcak G (2012). I see people: The presence of human faces impacts the processing of complex emotional stimuli. Social Neuroscience. [DOI] [PubMed] [Google Scholar]
- Foti D, & Hajcak G (2008). Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience. [DOI] [PubMed] [Google Scholar]
- Foti D, Olvet DM, Klein DN, & Hajcak G (2010). Reduced electrocortical response to threatening faces in major depressive disorder. Depression and Anxiety, 27(9), 813–820. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, & Gross JJ (2009). Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry, 66(2), 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BL, Kessel EM, Kujawa A, Finsaas MC, Davila J, Hajcak G, & Klein DN (2020). Stressful life events moderate the effect of neural reward responsiveness in childhood on depressive symptoms in adolescence. Psychological medicine, 50(9), 1548–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I, & Joormann J (2010). Cognition and Depression: Current Status and Future Directions. Annual Review of Clinical Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I, Krasnoperova E, Yue D, & Joormann J (2004). Attentional Biases for Negative Interpersonal Stimuli in Clinical Depression. Journal of Abnormal Psychology. [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, & Olvet DM (2010). Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental Neuropsychology, 35(2), 129–155. [DOI] [PubMed] [Google Scholar]
- Hammen C (2016). Depression and stressful environments: identifying gaps in conceptualization and measurement. Anxiety, Stress and Coping. [DOI] [PubMed] [Google Scholar]
- Harkness K, & Monroe S (2016). The assessment and measurement of adult life stress: Basic premises, operational principles, and design requirements. Journal of Abnormal Psychology. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2017). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford publications. [Google Scholar]
- Johnson PO, & Neyman J (1936). Tests of certain linear hypotheses and their application to some educational problems. Statistical Research Memoirs,1,57–93. [Google Scholar]
- Kellough J, Beevers C, Ellis A, & Wells T (2008). Time course of selective attention in clinically depressed young adults: An eye tracking study. Behaviour Research and Therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D, & Adolphs R (2012). The social brain in psychiatric and neurological disorders. Trends in Cognitive Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Merikangas KR, & Wang PS (2007). Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annu. Rev. Clin. Psychol, 3, 137–158. [DOI] [PubMed] [Google Scholar]
- Kessler RC, & Wang PS (2008). The descriptive epidemiology of commonly occurring mental disorders in the United States. Annu. Rev. Public Health, 29, 115–129. [DOI] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, … & Eaton NR (2017). The Hierarchical Taxonomy of Psychopathology (HiTOP): a dimensional alternative to traditional nosologies. Journal of Abnormal Psychology, 126(4), 454. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Danzig AP, Black SR, Bromet EJ, Carlson GA, … & Klein DN (2016). Neural reactivity to emotional stimuli prospectively predicts the impact of a natural disaster on psychiatric symptoms in children. Biological Psychiatry, 80(5), 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, & Klein D (2019). Reduced reward responsiveness moderates the effect of maternal depression on depressive symptoms in offspring: evidence across levels of analysis. Journal of Child Psychology and Psychiatry and Allied Disciplines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein D, & Hajcak G (2012). Electrocortical reactivity to emotional images and faces in middle childhood to early adolescence. Developmental Cognitive Neuroscience, 2 (4), 458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, MacNamara A, Fitzgerald KD, Monk CS, & Phan KL (2015). Enhanced neural reactivity to threatening faces in anxious youth: evidence from event-related potentials. Journal of Abnormal Child Psychology, 43(8), 1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdi B, Lozano S, & Banaji MR (2017). Introducing the open affective standardized image set (OASIS). Behavior Research Methods, 49(2), 457–470. [DOI] [PubMed] [Google Scholar]
- Lang P, Bradley M, & Cuthbert B (1997). International affective picture system (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention. [Google Scholar]
- Lang PJ, Bradley M, & Cuthbert B (2005). International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical Report A-6. University of Florida. Gainesville, FL. [Google Scholar]
- Lazarov A, Ben-Zion Z, Shamai D, Pine D, & Bar-Haim Y (2018). Free viewing of sad and happy faces in depression: A potential target for attention bias modification. Journal of Affective Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld SO, & Treadway MT (2016). Clashing diagnostic approaches: DSM-ICD versus RDoC. Annual Review of Clinical Psychology, 12, 435–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Huang H, McGinnis-Deweese M, Keil A, & Ding M (2012). Neural substrate of the late positive potential in emotional processing. Journal of Neuroscience, 32(42), 14563–14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Jackson T, Fitzgerald J, Hajcak G, & Phan K (2019). Working Memory Load and Negative Picture Processing: Neural and Behavioral Associations With Panic, Social Anxiety, and Positive Affect. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Klumpp H, Kennedy AE, Langenecker SA, & Phan KL (2017). Transdiagnostic neural correlates of affective face processing in anxiety and depression. Depression and Anxiety, 34(7), 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Kotov R, & Hajcak G (2016). Diagnostic and Symptom-Based Predictors of Emotional Processing in Generalized Anxiety Disorder and Major Depressive Disorder: An Event-Related Potential Study. Cognitive Therapy and Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Ochsner K, & Hajcak G (2011). Previously reappraised: The lasting effect of description type on picture-elicited electrocortical activity. Social Cognitive and Affective Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, & MacLeod C (2005). Cognitive Vulnerability to Emotional Disorders. Annual Review of Clinical Psychology. [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Lim CCW, Plana-Ripoll O, Holtz Y, Agerbo E, Momen NC, … & Al-Hamzawi A (2020). Comorbidity within mental disorders: a comprehensive analysis based on 145 990 survey respondents from 27 countries. Epidemiology and psychiatric sciences, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, & Sheridan MA (2014). Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depression and anxiety, 31(10), 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe S, & Simons A (1991). Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychological Bulletin. [DOI] [PubMed] [Google Scholar]
- Moran TP, Jendrusina AA, & Moser JS (2013). The psychometric properties of the late positive potential during emotion processing and regulation. Brain Research, 1516, 66–75. [DOI] [PubMed] [Google Scholar]
- Moser J, Hartwig R, Moran T, Jendrusina A, & Kross E (2014). Neural markers of positive reappraisal and their associations with trait reappraisal and worry. Journal of Abnormal Psychology. [DOI] [PubMed] [Google Scholar]
- Moser J, Huppert J, Duval E, & Simons R (2008). Face processing biases in social anxiety: An electrophysiological study. Biological Psychology. [DOI] [PubMed] [Google Scholar]
- Mueller E, Hofmann S, Santesso D, Meuret A, Bitran S, & Pizzagalli D (2009). Electrophysiological evidence of attentional biases in social anxiety disorder. Psychological Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Jarcho JM, & Guyer AE (2016). Social re-orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience, 17, 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Perlman G, Hajcak G, Klein DN, & Kotov R (2015). Familial risk for distress and fear disorders and emotional reactivity in adolescence: An event-related potential investigation. Psychological Medicine, 45(12), 2545–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson J, Nordin S, Sequeira H, & Polich J (2008). Affective picture processing: An integrative review of ERP findings. Biological Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham A, McHugh R, & Otto M (2010). A meta-analysis of the magnitude of biased attention in depression. Depression and Anxiety. [DOI] [PubMed] [Google Scholar]
- Pegg S, Dickey L, Mumper E, Kessel E, Klein D, & Kujawa A (2019a). Stability and change in emotional processing across development: A 6-year longitudinal investigation using event-related potentials. Psychophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg S, Ethridge P, Shields GS, Slavich GM, Weinberg A, & Kujawa A (2019b). Blunted Social Reward Responsiveness Moderates the Effect of Lifetime Social Stress Exposure on Depressive Symptoms. Frontiers in Behavioral Neuroscience, 13, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg S, & Kujawa A (2020). The effects of a brief motivation manipulation on reward responsiveness: A multi-method study with implications for depression. International Journal of Psychophysiology, 150, 100–107. [DOI] [PubMed] [Google Scholar]
- Schupp H, Cuthbert B, Bradley M, Cacioppo J, Tiffany I, & Lang P (2000). Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Hillman CH, Hamm AO, & Lang PJ (2004). Brain processes in emotional perception: Motivated attention. Cognition and Emotion. 10.1080/02699930341000239 [DOI] [Google Scholar]
- Sears C, Thomas C, Lehuquet J, & Johnson J (2010). Attentional biases in dysphoria: An eye-tracking study of the allocation and disengagement of attention. Cognition and Emotion, 24 (8), 1349–1368. [Google Scholar]
- Speed B, Nelson B, Auerbach R, Klein D, & Hajcak G (2016). Depression Risk and Electrocortical Reactivity During Self-Referential Emotional Processing in 8 to 14 Year-Old Girls. Journal of Abnormal Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange J, MacNamara A, Barnas O, Kennedy A, Hajcak G, Phan K, et al. (2017). Neural markers of attention to aversive pictures predict response to cognitive behavioral therapy in anxiety and depression. Biological Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr L, Hammen C, Connolly N, & Brennan P (2014). Does relational dysfunction mediate the association between anxiety disorders and later depression? Testing an interpersonal model of comorbidity. Depression and Anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasik-O’Brien SM, Brock RL, Chmielewski M, Naragon-Gainey K, Koffel E, McDade-Montez E, O’Hara MW, & Watson D (2019). Clinical Utility of the Inventory of Depression and Anxiety Symptoms (IDAS). Assessment. 10.1177/1073191118790036 [DOI] [PubMed] [Google Scholar]
- Swartz JR, Williamson DE, & Hariri AR (2015). Developmental change in amygdala reactivity during adolescence: effects of family history of depression and stressful life events. American Journal of Psychiatry, 172(3), 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom N, Knight J, Dishman R, Sabatinelli D, Johnson DC, & Clementz B. (2014). Emotional scenes elicit more pronounced self-reported emotional experience and greater EPN and LPP modulation when compared to emotional faces. Cognitive, Affective, & Behavioral Neuroscience, 14(2), 849–860. [DOI] [PubMed] [Google Scholar]
- Uliaszek A, Zinbarg R, Mineka S, Craske M, Griffith J, Sutton J, et al. (2012). A longitudinal examination of stress generation in depressive and anxiety disorders. Journal of Abnormal Psychology. [DOI] [PubMed] [Google Scholar]
- Uliaszek A, Zinbarg R, Mineka S, Craske M, Sutton J, Griffith J, et al. (2010). The role of neuroticism and extraversion in the stress-anxiety and stress-depression relationships. Anxiety, Stress and Coping, 23 (4), 363–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen NN, Van Strien JW, & Dijkstra K (2016). Implicit emotion regulation in the context of viewing artworks: ERP evidence in response to pleasant and unpleasant pictures. Brain and Cognition, 107, 48–54. [DOI] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Stroud C, Mineka S, Hammen C, Zinbarg R, Wolitzky-Taylor K, et al. (2015). Chronic and episodic interpersonal stress as statistically unique predictors of depression in two samples of emerging adults. Journal of Abnormal Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, … & Stuart S (2007). Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychological Assessment, 19(3), 253. [DOI] [PubMed] [Google Scholar]
- Weinberg A, & Hajcak G (2010). Beyond Good and Evil: The Time-Course of Neural Activity Elicited by Specific Picture Content. Emotion. [DOI] [PubMed] [Google Scholar]
- Weinberg A, & Hajcak G (2011). The late positive potential predicts subsequent interference with target processing. Journal of Cognitive Neuroscience. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Perlman G, Kotov R, & Hajcak G (2016). Depression and reduced neural response to emotional images: Distinction from anxiety, and importance of symptom dimensions and age of onset. Journal of Abnormal Psychology, 125 (1), 26–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


