ABSTRACT
Plants that experience a lack of sufficient irrigation undergo hydric stress, which causes the modification of their mechanical properties. These changes include a complex network of chemical and physical signals that interact between plant-plant and plant-environment systems in a mechanism that is still not well understood, and that differs among species. This mechanical response implies different levels of vibration when the plant experiences structural modifications from self-hydraulic adjustments of flux exchange at specific frequencies, with these carrying behavioral information. To measure these signals, highly sensitive instrumentation that allows the decoding of displacement velocity and displacement of plants, which is possible through calibrated equipment such as 3D scanning laser vibrometers, is necessary. Laser vibrometry technology allows for noninvasive measurements in real-time. Physiological changes could reasonably affect the biomechanical condition of plants in terms of the frequency (hertz) and intensity of the plant’s vibration. In this research, it is proposed that the frequency changes of a plant’s vibration are related to the plant’s hydric condition and that these frequency vibrations have the ecological potential to communicate water changes and levels of hydric stress. The peak of the velocity of plant displacements was found to vary from 0.079 to 1.74 mm/s, and natural frequencies (hertz) range is between 1.8 and 2.6 Hz for plants with low hydric stress (LHS), between 1.3 and 1.6 Hz for plants with medium hydric stress (MHS), and between 6.7 and 7.8 Hz for plants with high hydric stress. These values could act as preliminary references for water management using noninvasive techniques and, knowledge of the range of natural frequencies of hydric stress risk in chili pepper crops can be applied in precision agriculture practices.
KEYWORDS: Vibration measurement, hydric stress, natural frequency, Capsicum annuum, biophysics behavior
1. Introduction
Plant stress is part of the plant lifecycle, but it also leads to crop losses of around 75% due to environmental stresses.1 One of the main factors that affect the survival of a plant is drought, which appears when the water supply to the roots is limited or the loss of water through transpiration is very high. The severity of the damage caused by drought is unpredictable due to changes in rainfall patterns, the variable moisture-holding capacity of soil, and possible increase of water losses through evapotranspiration.2 In this regard, in a situation of global water scarcity, population growth, and climate change, conserving water as much as possible and assuring crop production is a research interest from various perspectives, such as plant physiology and biophysics.3
Hence, the structural changes generated by physiological adaptations have a direct impact on the biophysical properties of plants. Also, the understanding of biomechanical phenomena follows the ‘speaking plant approach’ (SPA), which proposes that to obtain optimal crop cultivation conditions, knowledge of the physiological status of living plants is needed. In this case, it is considered that information on the velocities of the displacement of plants can indicate details of hydric condition and that these velocities can be measured in a noninvasive way by using high-resolution vibrometers.4
Therefore, the interest in the small changes and cues that plants produce to communicate its levels of hydric stress has increased, mainly to further understand the signals that help the plant to adapt to a lack of water. Consequently, limited or reduced irrigation could be applied. These cues come from small movements that could potentially generate perceptible vibroacoustic events in plants and serve as plant–plant biomechanical signaling. These events have gained attention for its potential to carry information about the plant condition.5
In this way, all living and not living objects have resonant frequencies and are associated with their natural frequencies. The natural frequencies of an object depend on its mass, stiffness, and structure. Those frequencies are the ones where the object oscillates with the highest amplitude after an external mechanical stimulus that perturbs its initial position.6 The first natural frequency of an object is called the fundamental frequency. In the case of living objects, such as plants, their mass and stiffness define their natural frequencies, and those are affected by the physiological status that, are partially by its hydromechanical status.
A wide range of technology and instruments have been used to sense water-related biophysical signals in order to detect specific unknown changes in plants. X-ray laser and image processing is an example; however, it remains in the preliminary stages due to a lack of use in the field of water-related biophysical signals detection. Another example is terahertz quantum cascade lasers (THzQCL), which consist of high radiation that could detect a water deficit in Vitis vinifera plants. This study used pictures of leaves and found a linear law to relate leaf water mass to leaf optical depth in terahertz and the projected area with 95% accuracy.7 Also, vibration signals have been previously explored in other species and organs. For example, the predominant peak response of the natural frequency of a tomato (Lycopersicon esculentum) plant’s leaf was 5 Hz, which was calculated by applying an excitation force to the stem and using a laser vibrometer.8,9 To measure vibration signals in alive tissue like plants correctly, it is necessary to have adequate sensors with high sensitivity and instruments that are able to measure very small displacements, such as laser doppler vibrometers.8–10
For instance, a laser doppler (Polytec PSV-3D – B&K) has a wide bandwidth from 0 to 100 kHz, a frequency resolution of 2 Hz, and the ability to measure displacements in the order of femtometers. In this regard, the understanding of how plants vibrate could represent additional cues to integrate the physiological, biochemical, and molecular responses linked to biomechanics that still need to be researched. Furthermore, if plants with certain levels of hydric stress vibrate differently and have frequencies that could indicate particular stress, such as lack of water, that could be measured using highly sensitive sensors, this could indicate that frequency patterns are related to physiological conditions. Thus, the setting of these frequencies could vary according to changes in mass and stiffness, which adjust depending on the structural water content.
In addition, Capsicum annuumis a plant type of interest due to its agricultural importance around the world and the fact that it is relevant to Mexican agriculture, where its production has increased by 25% in the last decade.11 This corresponds with the high commercial value of pepper fruits, which is because of their nutrient and phytochemicals content, mainly capsaicinoids. Moreover, the pepper plant’s vulnerability to a deficit of irrigation is a problem that needs to be solved to ensure agriculture water management (AWM) that can maintain crop yield, quality, and quantity under hydric stress conditions in order to overcome economic, social, and foodsafety problems in Mexico.3 The aim of this research was to estimate the frequency patterns of the velocity of displacement in whole Capsicum annuum plants and to associate these with three different levels of hydric stress, according to its matric potential. The association of novel physical features with hydric stress could be useful as vibration phenomena could be used for indicating water stress in plants.
2. Materials and methods
2.1. Plant samples
Capsicum annum plants var. Jalapeño were germinated for 6 days and transplanted after 30 days. The soil substrate used was PEAT MOSS (Sunshine Mix 3, ImpulsoraAgroquímica del Sureste S.A. de C.V., Mexico), a commercial mix of sandy-loam soil adjusted to field capacity. The germination was carried during 3 days of darkness, at an average temperature of 25°C and 53% of relative humidity (HR%). The pH range of irrigation water was maintained in a range of 6.5–7.3. The characteristics of the soil shown corresponded to the catalog number #1001 SUNAGRO-HORTICULTURE, QC CANADA. The total porosity was 84.1%, aeration porosity 1%, water easily available 33.8%, a reserve water 14%, total water available 48%, water-holding capacity 69.5%, and apparent density 0.107 g/cm.3
2.2. Establishment of hydric stress conditions
First, samples after 3 months of transplanting at their first pre-anthesis stages were maintained under irrigation at field capacity with 500 mL. Then, the hydric stress was established by a number of days of non-irrigation. The water limitation was done to set upon three treatments of hydric stress such as Low: LHS; Medium: MHS and High: HHS. The Capsicum annuum plants were adjusted stopping its watering for periods of 3–5, 5–9, and more than 10 days, respectively. Similar geometric characteristics of samples were considered and their matric potential was monitored (WATERMARK-Item # 6450WD). The values of each group are shown in Table 1.
Table 1.
Hydric stress classification of Capsicum annuum.
| Hydric stress level | Low Hydric Stress (LHS) | Medium Hydric Stress (MHS) | High Hydric Stress (HHS) |
|---|---|---|---|
| Non irrigation days | 3 a 5 | 5 a 9 | > 10 |
| Hydric potential (MPa) | −0.2 a − 0.8 | −0.81 a − 1.49 | > −1.5 |
| Relative water loss (RWC) (%) | 5 a 15 | 15 a 30 | > 50 |
| Matric potential (Centibar/kPa) | 0 a 9 | 10 a 20 | > 20 |
| Chlorophyll content | 427 ± 17 | 220.00 ± 22.2 | 130.12 ± 17.2 |
LHS: Low hydric stress, MHS: medium hydric stress, HHS: high hydric stress. The water potential is given in the theoretical values, according to the classification by (Hsiao, 1973).12 The classification of the types of water stress refers to the experimental comparisons of matric potential (Centibar/kPa) and the adjustment to the theoretical scale of water content (Flexas and Medrano, 2002).13 Chlorophyll units are expressed as (µmol·m−2)
2.3. Set-up for vibration monitoring with head-laser Doppler-Polytec 3D scanning laser (PSV-3D) and single point (Brüel&Kjaer)
The velocity of vibrational displacement from different hydric stress plants was detected throughout an output signal of the two laser vibrometers. The Polytec 3D scanning laser vibrometer motion sensor (PSV-3D) and a single point laser fiber-coupled sensor Head OFV-534 (Brüel&Kjaer, Germany), this second instrument was used as a reference point. Both laser location was set at 1.5 m of distance away from the plant (see Figure 1- experimental set-up). The 3D scan was positioned in the same axis of the plant sample, and the single point one was positioned at 90° of the measurement axes. The reference (single-point laser) was pointed to the lowest part of the plant and the 3D laser, to top points, approximately in the geometric center of the main stem. During measurement, the laser was directly above the reference point. A stiff and highly damped optical breadboard in conjunction with compliant supports was used to suppress ground-borne vibration. Measurements were done at times of day in which ground-borne vibration was not perceptible. The background noise was maintained under 6 dB while the vibrometer was tested recording signals from a piece of wood and metal surfaces.
Figure 1.

Doppler laser measurement from Capsicum annuum plants. Experimental setup (a) hydric stress was set before putting the samples into the chamber. (b) The anechoic chamber worked with a background noise output according to the ISO 26101–6 ± 0.5 dB for all points of the chamber. (c) The signal recording was obtained from 2 lasers. One 3D scanning laser and one single point laser as reference. (d) The massive signal acquisition was transduced using a controller adapted to the B&K Germany Labshop Software for data processing.
The instrument sensibility was 5 mm per each volt, and the measurements were performed approximately on the geometric center of the system (10 cm above the substrate layer). A reflective tape was placed in the stem (signal point) to increase the signal quality. Each plant was considered as a complete system to consider soil substrate, plant (stem and leaves) and roots. Signals were taken inside an anechoic chamber, with an airflow velocity of 0 m/s, 22–25°C, and 40–42.5% of relative humidity (HR) (HM34-Vaisala, US). The average illumination during the 2 hours of measurement was 0.930 w/m2. A time of 20 min was considered for plant stabilization before start the measurements. The high robustness from signals comes from an average of values obtained after 600 signals These signals were took every 120 seconds. Each signal recording had a duration of 200 ms with an average value of 50 measurements per point each second (point/second).
2.3.1. Experiment 1 – natural frequency
This consists of applying a mechanical stimulation by pushing approximately the geometric center of the plant stem, as it was previously done in leaves by Sano et al., 2015.14 However,instead of stimuli one leaf, stem was pushed. This practice contemplates the mechanical principle statement of no matter the force with which it is pushed after mechanical stimulation, the subsequent oscillation frequency is always the same and it was measured with the two abovementioned lasers. The average of plant heights was of 57 cm (the longitude considered for the natural frequency calculated by the formula). The single point laser was used as a reference to discard noise signals from the ground, air vibrations, and electrical interference from the instrument were diminished according to metrology standards recommended by the Physic Department – Acoustics and vibration laboratory from the National Center of Metrology CENAM.
2.3.1.1. The equation of natural frequency for theoretical calculation
The natural frequency [fn] estimation of a cantilever beam with uniform load (w) per unit of length, proposed by Young, Budynas, & Sadegh, 200215 (see equation 1) was used to calculate the fn of plants. The values utilized were those from dehydrated cell walls and its tensile modules between 1.9 and 4.9 Mpa (Kn), also, several constant values were replaced in the formula as Kn, referring a constant where n refers to the mode of vibration; I, the area moment of inertia; l, the length of the shaft; E the modulus of elasticity and w: Uniform load
| (1) |
2.3.2. Experiment 2 – vibrational analyze of low frequencies from C. annuum plants
A vibrational behavior was measure in a bandwidth from 0 to 50 Hz from stable plants (without pushing mechanical stimulus) to discard additional effects from external mechanical forces and recognize the vibrational pattern from C. annuum plants in a stable position.
2.4. Chlorophyll content measurements
The chlorophyll measurements were conducted using leaf tissue. The data were recorded from the same group of plants before sampling, using a SPAD-502 meter (Konica-Minolta, Japan). Three independent SPAD measurements were made per hydric stress treatment. To transform the SPAD values into chlorophyll content (µmol·m−2) and recalculated according the method used by Richardson et al., 2002.16 Results were calculated using the formula: (SPAD value ^ 0,264). The data is expressed as (µmol·m−2).
3. Statistics
Samples were randomized among the three hydric stress levels (N = 3). For the spectrogram preparation, the vibration data with frequencies below 0.8 Hz and above 20 kHz were removed. The statistical analyses were done using the comparison of three means by the Tukey test from JMP 11.
4. Results and discussion
4.1. Capsicum annuum plants under three levels of hydric stress
Initially, the classification of irrigation conditions was settled by stopping the irrigation in order to identify vibrational waves according to the range of hydric stress levels. Thus, the initial soil state was normalized at 75% of field capacity before stopping the regular irrigation and creating the experimental conditions needed to perform measurements.
Then, additional parameters, such as the hydric potential (MPa) and chlorophyll content, were measured (seen Table 1) to characterize the hydric stress levels of plants into low, medium, and high (LHS, MHS, and HHS, respectively). The matric potential of each hydric stress level was established as a range of 0 to 9 centibars/kPa for LHS, 10 to 20 centibars/kPa for MHS, and >20 centibars/kPa for HHS. The chlorophyll content was 427 µmol·m,−2 220 µmol·m,−2 and 130 µmol·m−2 for LHS, MHS, and HHS, respectively. Establishing these ranges was necessary for the classification of the hydric status to provide an additional physiological classification linked to the vibrational frequency pattern.
In the present study, the vibration terminology we will refer that the fundamental frequency as simply the lowest natural frequency. Thus, in physics and mechanical concepts, the fundamental frequency is defined as the lowest possible frequency, among all the natural frequencies, as the one found of our vibrating plant samples. Not because it is a unique one but because it’s the one first detected after the mechanical stimuli applied.17
It is possible that other natural frequencies can be multiples or harmonics of the fundamental frequency.18 The natural frequency is dependent on the mass and rigidity, which are water-dependent and define a physiological condition in vegetative materials such as plants. Following the physical fundament, the water content affects the mass and rigidity of the biological structures. The presence of water in plants is a consequence of the dynamic interactions that are dependent on evapotranspiration rates, relative humidity, temperature, and irrigation in crops.
Therefore, it is possible to observe a change in the vibration modes of the plant because its mass is not a fixed mass like that of other non-living materials. Instead, the water content in plants changes the mass of them. The vibrations of plants could be considered to be behaving according to its mass/weight and rigidity in a “water-depending” manner that strictly depends on the environmental interactions19 Therefore, the water movement can liberate energy in wave formations that propagate rapidly, sending real-time information that is quickly analyzed by plants at very low intensities and long distances.19
4.2. Capsicum annuum set up and signal detection spectra
The transduced signal was obtained from frequency versus volts and the displacement oscillation in time domain from Capsicum annuum is shown in Figure 1. The moisture status of a plant affects its cell membranes and is linked to biochemical alterations which are followed by mechanical events, such as alterations of its geometry, stiffnesses, and mass.20,21 Other biophysical factors, such as the strain that is connected to the soil system, can also vary according to hydraulic function.22 All of these interactions converge and influence the ability of plants to perform environmental sensing perception and survival. Consequently, it affects food production and influences the development of AWM.23
From a biophysical point of view, the content of water of a plant could modify an essential principle of resonance vibrations that relates to the periodic movements of a system. In plants, movements under different conditions indicate that there is a link between the water content and oscillating behavior. The natural frequency refers to the number of waves or vibrations, with the highest amplitude, that took place during a period of time where the sample oscillated without any driving or damping force.24
The spectral pattern of free motion of the plants under different hydric stress could be associated with the water content of the samples.6,25 Thus far, some studies have proposed that plants can sense and selectively respond to vibrations and resonant acoustic-free oscillations from an ecological and bioacoustics perspective but lacking a mechanical explanation.26,27
4.3. Vibrational amplitudes changes between hydric stress levels
The amplitudes show a displacement range with a velocity of 0.079 to 1.74 mm/s between samples. Concerning replicates, the samples with lower hydric stress presented significant variations between displacement amplitudes at a lower frequency as can be seen in Figure 2. The natural frequency of the ones with higher hydric stress showed smaller velocity amplitudes as can be seen in the graph in Figure 2c on the righthand side. A clear inverse tendency between vibration velocities amplitudes at their corresponding natural frequency and the water content in plants is evident in pepper plants (Figure 3).
Figure 2.
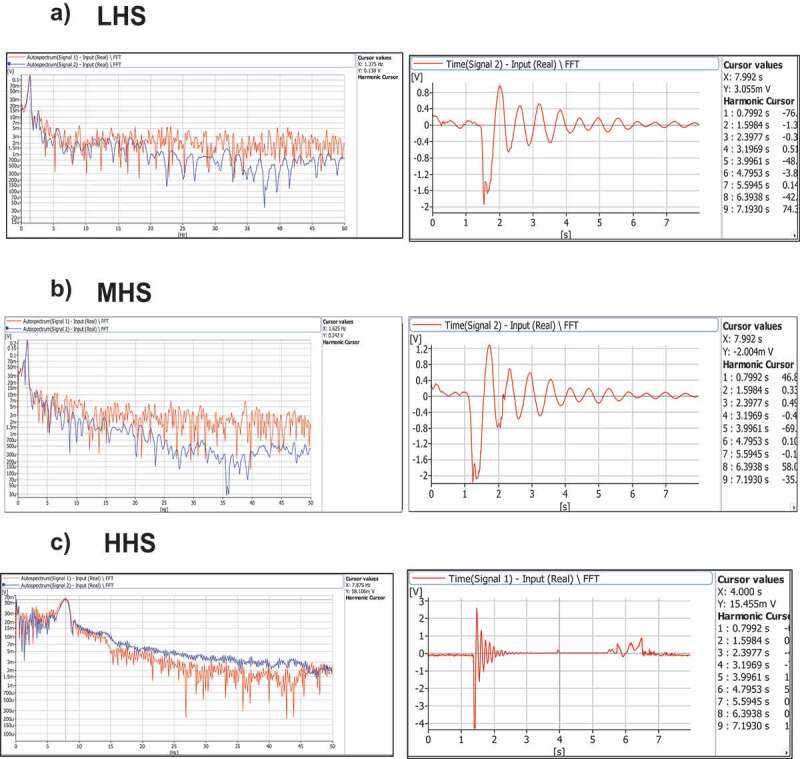
Vibration frequency and time domain spectra from Capsicum annuum. from the experimental result of displacement velocity from the stem of C. annuum plants using a doppler laser displacement sensor (see Figure 2). The displacement velocity of LHS, MHS, and HHS waveforms corresponding to the frequency domain using Fast Fourier transforms (right graphs) and its respective time-domain obtained from plants (left graphs) (a, b, and c). The red signal is the one obtained from 3D multi-scan laser. The blue line corresponds to a second signal from the second vibrometer (one single point) that was used as a reference point to avoid the possible source of noise.
Figure 3.

Velocity of displacement amplitudes from C. annum plants at their natural frequency. The bars represent amplitudes of velocity displacement at the first natural frequency of the low, medium, and high levels of hydric stressed plants (LHS, MHS, and HHS) respectively.
The difference in amplitude in the results could explain why when the water content of a vegetal material is higher, the vegetative tissues tend to be soft and, consequently, tend to vibrate at a higher amplitude and a lower frequency with a reduced amount of damping. Hence, the natural frequency of plants subjected to hydric stress conditions tends to higher and they tend to have increased damping, which means that the amplitude of displacement diminishes faster in time compared to well-irrigated plants or plants with low hydric stress. It is essential to mention that the amplitude observed could vary slightly according to the magnitude of the stimulus applied to the plant. However, when the stimulus was applied to the plant, an attempt was made to always use the same force. Nonetheless, as mentioned before, the natural frequency of objects does not depend on the amplitude of the stimulus.
According to previous studies, only a few selective acoustic waves could have a favorable effect on some species, for instance when applied to plants, certain ones have been shown to induce drought and disease tolerance in nearby plants or in parts of the same plant.12,28,29 What can be inferred from this information is that, in those circumstances, a plant could not only perceive vibrations but also develop them into some resonance phenomena when getting mechanically stimulated at resonant frequencies. Consequently, plants could release enlightening information through vibration itself as a mechanical signaling process in order to communicate its condition.
For example, some acoustic emissions (AEs) are caused by the vibration of some parts of a plant. During hydric stress, plants struggle with cavitation, where air bubbles form and explode inside the xylem, causing vibrations. This can be distinguished using recording devices attached to the plant’s xylem; however, the recording of this kind of signal is still a developing technology that has reached its technical limits due to the fact that the contact sensors used to measure cavitation or any other type of physical acoustic signal do not work on small species. This is because a regular sensor would have to be installed inside the stem, but it would not fulfill the mass-weight proportion needed to obtain the correct measurements and avoid noise, as the standard rule is that samples must be 10 times heavier than the sensor. In addition, most other techniques are destructive.13,30,31 Nevertheless, the external application of AEs has had a special effect on the defense response mechanism of plants.12,32 Despite this, direct contact cannot exhibit the extent to which these sound vibrations could be recognized from a distance.33,34
5. Natural frequency of vibrations from plants with hydric stress
Overall, the physical properties that define the natural frequencies of objects apply to the plant kingdom due to the fact that vegetal structures are subject to physical laws and they behave as ‘stationary systems’ but they constantly modify their small structures in response to highly dynamic environments. The particularities of the vegetative tissue that remains during the plant’s dynamic response and the damping can be seen in Figure 2.
Capsicum annuumemit vibrations at natural frequencies via which the plants, like a mechanical system, maintain particular movements. The results from the vibrometry recording show that the natural frequency of the vibrations is between 1.875 and 7.86 Hz. The smaller frequency corresponds to plants with LHS and a frequency of up to 6.87 Hz was found in plants with HHS (see Table 2). It is relevant to mention that these frequency values do not depend on the magnitude of the stimuli.
Table 2.
Natural frequency of C. annuum according to hydric stress.
| Hydric condition | ENF interval (Hertz) | TNFH | |
|---|---|---|---|
| LHS | 1.875 | 2.625 | 2.12–4.58 |
| MHS | 1.375 | 1.625 | Non calculated |
| HHS | 6.75 | 7.875 | 0.93–1.48 |
ENF: Experimental natural oscillation frequency from mechanical stimulus; TNF: Theoretical natural frequency was calculated with experimental values of the geometrical length of 570 mm, stem diameter of 10 mm, a mass of 0.8–1 kg and literature parameters of *Modulus of elasticity of hydrated and dehydrated cells.30,31
After both experiments, the first natural frequency generated by Capsicum annuum plants oscillated with the amplitudes, ranging from 1 to 9 Hz depending on the water status of the sample. These results are similar to the bandwidth reported in previous studies performed on other species such as tobacco (Nicotiana benthamian), wheat (Triticum aestivum L.), and poplar (Populus sp.), as well as organs such as leaves and trichomes.8,10,35
Then, it is possible to say that environmental variations cause plants to adapt their structure in order to face hostile conditions. The underlying response integrates mechanical inputs requiring immediate modifications that will, therefore, adjust the first natural frequency of vibration and other vibrations from the whole plant system.
For example, when comparing the reported values from the dehydrated cell walls and its tensile modules between 1.9 and 4.9 Mpa and replacing it in the equation afterward (1), the theoretical natural frequency of vibration obtained presents values from 0.93 to 1.48 Hz for dehydrated plants. Meanwhile, a theoretical natural frequency from 2.12 to 4.58 Hz was found for the well-irrigated plants with elastic modulus of 0.08 to 0.374 Mpa. The calculations indicate that the elasticity modules reported for some of the plant cells do not provide enough detail to generalize the frequency behavior of vegetal tissue’s vibrations. In addition, the calculations do not present the same results as the experimental data. Also, the results suggest that natural frequencies are unique for each species and its performance changes significantly compared to the recalculated values from the formulas used for the analytical data.
Recent tissue findings have raised the question of which cells participate in the emission and detection of vibrations, how they function, and how they initiate their responses. A previous report has established that the vibration modes for leaf trichomes can present modes of vibration with frequencies close to 10 Hz, which suggests that the lowest natural frequency of the vibrations of the trichrome strongly depends on the mechanics of the leaf. It also proposes that these organs serve as mechanical/acoustic antennae or sensors that could be one of the first mechanical vibration receptors at a wide bandwidth, including the higher frequencies.36
Table 2 shows the vibrational frequency signals measured from theCapsicum annuum. The interval found in the experimental natural oscillation (ENF) was in a range of 1.8 to 7.8 Hz for LHS and HHS, respectively. The table also shows the natural frequencies that were calculated, with the elastic values reported for other types of plant cell tissue studies (TNFH) ranging from 0.93 to 4.58 Hz, showing an opposite tendency, with the lowest frequency being the result calculated for HHS and the highest for LHS. The natural frequencies of the reported values of the elasticity modules from the dehydrated cells were used to recalculate the values from the non-experimental data. The comparison with the experimental results varies considerably.
The data used for the comparison (Table 2) suggest that the existing models for calculating plants’ natural frequency miss the additional structural characteristics of plants related to their hydric status and that this changes the result. These characteristics seem to behave in a specific way depending on the specie and the growing conditions. Further, the complexity of vibration patterns corresponds to the geometrical heterogeneity due to the particularity of the samples, in this case, Capsicum plants. Primarily, the relationship between geometrical heterogeneity should be considered when comparing the vibration patterns produced by other species.
Literature reports that plants and their substructures, have not only stiffness but also inertia, meaning that they are susceptible to all dynamic mechanical effects such as elastic wave propagation and vibration.37
Giving the fact, the mode of vibration refers to a particular shape of free motion that can oscillate in time, eventually dying out. The vibrational mode has a wave-shape in space and time (the modal shape), its frequency of oscillation, and how it decays in time (the damping). Vibrational modes are observed when the system, in this case, the plant, is left free to vibrate after an initial perturbation. Plus, the wave dissipation occurring inside the lignin structures of the stem, in the case of the well-irrigated plants, depends on the chemical composition of the material. In this manner, structures with more water content will vibrate at a lower frequency (see Figure 2) because the water tends to smooth the tissues causing that plant to behave as a “low pass filter” and tend to vibrate with more intensity at lower frequencies. Therefore, more porous structures or “softer” surfaces attenuate a low-frequency signal in a lower degree than less porous surfaces or “harder” tissue.37 As a result, the mechanical damping of vibration signals of well-irrigated plants is lower than the damping of drought-stressed plants.
With respect to Figure 2, graph C showing HHS shows that the reference behaves differently in terms of measurement. This is because the plants lost the support at their base provided by the soil substrate (inside the pod). Otherwise, it would have not been possible to detect the natural frequency of their vibration from a fixed place, as is the case in the first experiment, when the plants receive a mechanical stimulus (push).
The second experiment provides information on how plants vibrate within a more extended bandwidth (0 to 50 Hz) without mechanical stimuli.The vibrations show multiple small peaks and there is no significant change in vibration velocity between samples. However, this information suggests that living tissue emits active vibration. Another variable that could help to measure hydric stress in plants is the time of damping decay, which in this study, displayed a different behavior for each type of hydric stress. Thus, the time of damping decay in the curve of the signal detected could potentially be used to measure drought stress in Capsicum annuum plants.
It is uncommon for a biomechanical perspective to be taken into account when trying to identify the risk of hydric stress in a crop, such as Capsicum annuum’s. Thus, a lack of association exists between the fundamental modes of vibration of whole plants and hydric stress. The plant can behave as a hydrological-dependent damped harmonic oscillator, which implies that information from the whole system’s vibration could help to determine and predict further hydraulic and mechanic changes relating its status regarding water content. For instance, studies performed in trees have shown that their vibrational response partially depends on their natural frequency damping ratio. In addition, the results of this study show that the samples with HHS have a higher natural frequency that is ~2.55 to ~7.87 times higher, exhibiting an opposite effect to research by Sano et al. (2013).8
The authors mention the inverse behavior of this phenomenon in the vibration of leaves. In the results found here, the frequency intervals of the first natural frequency of plant vibration is modified depending on the physiological condition of the whole plant being analyzed. It is worth noting that in the mentioned study, the authors only evaluate the leaf tissue whereas this work evaluates the plant sample as a complete system. Nevertheless, leaf and stem organs present different modes of vibrations, presumably due to the chemical composition that affects their biomechanics.
In an earlier study, the same researchers calculated and assumed a relationship between the natural frequency of leaves and a change in leaf weight using a linear model that showed that R= 0.98; thus, demonstrating a direct connection between the frequency and weight decrease.8However, none of these tendencies could be replicated in this study’s experiments.
Although, similar low frequencies were found in Arabidopsis thaliana and Populus tremaplants in a previous study using a high-speed camera (Vosskuhler HCC-1000BGE). In the study, the authors applied a sinusoidal excitation with a slow sweep at a frequency of 2 Hz intervals at 30-second intervals and report that the modes of vibration for all plants are within a range of 0 to 36 Hz.21
One of the factors that influence the level of a signal propagating between two points is the spatial arrangement between the source and receiver.38 Thus, this could partially explain the fact that in some cases, plants can sense the behavior through biomechanical signals such as vibrations or acoustic cues emitted by insects. In another study focused on the vibration phenotyping of plants, the authors analyzed the vibration of 48 plant shots. A mean frequency of 1.09 Hz was identified for a total of more than 4000 data points in tobacco plants. According to these authors, water stress decreases the first natural frequency of vibration by 15%.20 The natural frequency of 5 Hz that the mentioned authors’ report can change in line with the results presented here depending on the water condition of the sample. It can vary in more than two states and when it comes to the hydric stress level, it can be classified as low or high.
Nevertheless, the significance of the bandwidth ranges of the natural frequencies of vibrational signals is to be able to add possible target interactions that plants may utilize for communicating in order to prepare them for changing environmental scenarios, such as water scarcity.39,40 It is an example of one of the full range of exquisite behaviors that have revealed unsuspected cognitive capacities in plants as non-neural but evidently signaling, communicating organisms.41
Although this study found an opposite trend regarding the HHS vibrational signal (Figure 2) and the highest displacement amplitude and water stress in plants, according to the results presented here, this seems to be directly related to the Capsicum plants’ irrigation deficit.
Furthermore, the hydraulic grade of plants is highly variable depending on the species, organs, and other factors such as age, phenological stage, and soil substrate condition. All of these can impact the dynamics of plant vibration and all plants rely on the interaction of water and the cell wall to vibrate in a specific pattern (see Figure 2).
Knowing how physiology affects a plant’s movements is essential to gain an understanding of how plants change, but mainly, it is essential for understanding the way in which they reply to mechanical and environmental stimuli, plus the additional biological functions.33,42,43 The modes reported so far as vibration signals from Capsicum annuum that were generated after a mechanical impulse (touch) (Figure 2) show that hydric condition is not considered as a constant that can replace theoretical models for different plant species. It shows that there is a need to acknowledge the previous plant’s physiological condition.14
5.1. Vibrational analysis from stable and hydric stressed plants
Regarding the second experiment, the results reveal that a plant’s water condition drastically disturbs the spectrum of its vibration signal. The samples with LHS presented vibrational peaks at frequencies of 4 and 7.9 Hz. Meanwhile, the samples with HHS showed three pronounced frequency peaks at 2.9, 3.2, and 5.15 Hz. Interestingly, the same sample was measured 20 minutes after irrigation had begun and a similar pattern was detected, with the peaks suffering a frequency displacement of 38.7, 36.8, and 38.2 Hz.
This work has detected that Capsicum annuum plants could present additional natural frequencies other than the first natural one (Table 1). These signals could be the harmonics of the first natural one (Figure 4) or they could not be. However, since plants exposed to drought experience cavitation events, identifying if additional signals found could be related to air bubbles in the xylem that are causing vibrations represents an opportunity to further explore the biomechanical functioning of chili pepper plants and to predict their tolerant dynamic to drought in terms of signals.30,44,45
Figure 4.

Vibrational patterns from C. annuum stems under different hydric stress without stimulus. The graphic shown in Figure 4, represents signals of vibration velocity of plants in the frequency domain in a bandwidth from 0 to 50 Hertz without mechanical stimulus: LHS: Low hydric stress; MHS: medium hydric stress; HHS: High hydric stress; HHS + S: signal measured 20 minutes after applying 100 mL of water in a HHS sample.
According to the classical concepts of dynamics and vibrations from Thomson, 201846 There are two general classes of vibrations – free and forced. Free vibration takes place when a system oscillates under the action of forces inherent in the system itself and when external impressed forces are absent. The system under free vibration will vibrate at one or more of its natural frequencies, which are properties of the dynamical system established by its mass and stiffness distribution. Vibration that takes place under the excitation of external forces is called forced vibration. When the excitation is oscillatory, the system is forced to vibrate at the excitation frequency. If the frequency of excitation coincides with one of the natural frequencies of the system, a condition of resonance is encountered, and large oscillations may result.46 Therefore, in this study was considered plants as vibrating systems, in a free and forced way. Regarding this, they are all subject to damping at specie-specific-physiological condition degree, finding that vibration theory sustains that energy is dissipated by friction and other resistances, that, in the case of plants, depending on their physiological status.
Regarding the abovementioned results, conditions such as hydric stress or even drought change the vibration pattern of plants and it can occur over different frequencies, similar to what the literature mentions, in a broad bandwidth of the generation of sounds from hyper audible to high-frequency ranges (UAEs).45 In addition, the frequency range on which a specific species of plant can generate any ‘emission’ wave as a consequence of its vibration could be related to a known physiological condition of interest to achieve better management of crops. Consequently, this work could help to elucidate the behavior of whole plant vibrations associated with drought.
The frequencies of free oscillations in plants are expected to get low. Mainly, the frequencies of vibration signals at the plant scale and organ scale are often found in small orders (1 Hz or 10 Hz) depending on the size of the plant, and, according to this work, this also depends on physiological state, such as water scarcity. However, due to the complex architecture of plants, they could have a multitude of modes. A plant could have 104 leaves and it will have vibration modes for each and every leaf at very similar frequencies.37 Moreover, plants’ dynamics and vibrations are worth learning because they can be used as a physiological indicator to know the health status of plants in the future.
The massive data from phenomena like the vibrations of plants could soon help as trait cues that could transform or add to predicting models that could employ learning methods such as Support Vector Machine (SVM), K-means, or Neural Networks to classify, detect, or predict stress in plants.47
Additionally, more data can be obtained if more experiments are accomplished in order to characterize the vibration modes of the chili plant under particular stresses, climatic conditions, and phenological stages, for instance. This data can be used when using machine learning methods to try to model the vibration modes of plants when they are under a specific form of stress and this can be used to discover physiological changes in plants through their vibrations. Moreover, the natural frequencies established as a consequence of hydric stress levels could be considered in further research to establish ‘stimuli waves’ for playback treatments. These signals could have ecological applications, principally to obtain agronomical benefits,48 similar to those obtained when plants were found having active thigmomorphogenic responses.49,50
Thus, the results of this work can be used if the intention is to increase the resonant responses in the crop using any bioacoustics or vibrational tool to enhance specific plant responses, principally those that increments the nutraceutical or biotechnological benefits of plants, perhaps those reported by touching or mechanical stimulus.51
Finally, since physiological, biochemical, and molecular modifications take place during self-adaptation and even though a preliminary explanation of how plants can have a sensitivity reception of external signals generated from vibration sources at specific frequencies exists, there is still a gap in the knowledge regarding the reason why they can respond to these stimuli. However, mechanical stimuli activate its metabolism, promotes division, and enhances the activity of protective enzymes and endogenous hormones, increasing the contents of soluble sugars, proteins, and levels of transcription. Thus, it could be inferred that these fluctuations are also involved in biomechanical signaling responses.52,53
5. Conclusion
The natural frequency of plants’ vibration is one of the biophysical cues that are affected by environmental contexts such as water scarcity. The vibrational waves of a plant change its pattern slightly depending on the plant’s hydric condition and consequently, modifies specific biomechanics behavior. Hence, physical signals such as plant vibrations have a high potential to enable the deciphering of biophysical responses to water scarcity and the survival of plants, but different variables need to be considered through theoretical modeling. Thus, as far as it is known, no other preliminary ‘frequency pattern’ of vibration signal for whole pepper plants has been done before. This work’s measurements show the particularities of damping curves and the natural frequencies of the vibrations of plants under different levels of hydric stress, which drastically changes the tendency. This has also not been previously analyzed or at least not using high sensitivity equipment such as scanning lasers and an anechoic chamber, using an experimental set-up with metrology considerations. The use of interferometry in biological areas such as plant physiology is an opportunity to achieve less invasive detection of and obtain precise information on the early detection of vigor and stress tolerance levels in Capsicum annum plants using high-resolution equipment. Finally, the complexity that exists in the intervals of vibration signals emitted by plants at different levels of irrigation indicates the preservation of the degrees of freedom that correspond to a high variability characteristic of biological models. Accordingly, when mathematical equations are applied to determine the natural vibration modes of plants, the answers are limited. This maybe due to the fact that the mathematical equations that have been developed to explain this phenomenon do not consider several important variables, such as the plant’s water contents. In addition, the functional biology of the stem and the whole plant characteristics are critical factors that change the first natural frequency and amplitude in the vibrational signaling of displacement velocity as physiological and biomechanical responses.
Acknowledgments
Author Laura Caicedo-López was supported with a grant from the Consejo Nacional de Ciencia y Tecnología (CONACYT-Mexico) [number 71429] and the Autonomous University of Querétaro [number 255309]. The authors thank the National Metrology Center of Mexico (CENAM) for providing calibrated equipment to perform traceable measurements. We also thank the project FOFI-UAQ 2018 with register number FIN201902
Funding Statement
This work was supported by the Universidad Autónoma de Querétaro, FOFI UAQ 2018 [FIN201902].
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to impact the text and data reported in this paper.
Geolocation information
The study area of this research was https://www.google.com/maps/@20.7049186,-100.2616979,17z
References
- 1.Ghosh D, Lin Q, Xu J, Hellmann HA.. Editorial: how plants deal with stress: exploration through proteome investigation. Front Plant Sci [Internet]. 2017;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, et al. Crop production under drought and heat stress: plant responses and management options. Front Plant Sci. 2017;8:1–16. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mardani S, Tabatabaei SH, Pessarakli M, Zareabyaneh H.. Physiological responses of pepper plant (Capsicum annuum L.). To Drought Stress J Plant Nutr. 2017;40:1453–1464. doi: 10.1080/01904167.2016.1269342. [DOI] [Google Scholar]
- 4.Nishina H. Development of speaking plant approach technique for intelligent greenhouse. Agric Agric Sci Procedia. 2015;3:13. [Google Scholar]
- 5.Khait I, Obolski U, Yovel Y, Hadany L.. Sound perception in plants. Seminars in Cell & Developmental Biology. 2019;92:134-138 doi: 10.1016/j.semcdb.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Bidhendi AJ, Geitmann A. Methods to quantify primary plant cell wall mechanics. J Exp Bot. 2019;70(14):3615–3648. doi: 10.1093/jxb/erz281. [DOI] [PubMed] [Google Scholar]
- 7.Baldacci L, Pagano M, Masini L, Toncelli A, Carelli G, Storchi P, Tredicucci A. Non ‑ invasive absolute measurement of leaf water content using terahertz quantum cascade lasers. Plant Methods. 2017;13:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sano M, Sugimoto T, Hosoya H, Ohaba M, Shibusawa S. Basic study on estimating water stress of a plant using vibration measurement of leaf. Jpn J Appl Phys. 2013;52(7S):07HC13. doi: 10.7567/JJAP.52.07HC13. [DOI] [Google Scholar]
- 9.Sano M, Nakagawa Y, Sugimoto T, Shirakawa T, Yamagishi K, Sugihara T, Ohaba M, Shibusawa S. Estimation of water stress of plant by vibration measurement of leaf using acoustic radiation force. Acoust Sci Technol. 2015;36(3):248–253. doi: 10.1250/ast.36.248. [DOI] [Google Scholar]
- 10.Magal C, Schöller M, Tautz J, Casas J. The role of leaf structure in vibration propagation. J Acoust Soc Am. 2000;108(5):2412–2418. doi: 10.1121/1.1286098. [DOI] [PubMed] [Google Scholar]
- 11.Rosales RE. Mexican farmers picking up the slack in chile cultivation. Agweb.com [Internet].
- 12.Choi B, Ghosh R, Gururani MA, Shanmugam G, Jeon J, Kim J, Park SC, Jeong MJ, Han KH, Bae DW, et al. Positive regulatory role of sound vibration treatment in Arabidopsis thaliana against Botrytis cinerea infection. Sci Rep. 2017;7(1):1–14. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Roo L, Vergeynst L, De Baerdemaeker N, Steppe K. Acoustic emissions to measure drought-induced cavitation in plants. Appl Sci [Internet]. 2016;6(3):71. doi: 10.3390/app6030071. [DOI] [Google Scholar]
- 14.Sano M, Nakagawa Y, Sugimoto T, Shirakawa T, Yamagishi K, Sugihara T, Ohaba M, Shibusawa S. Estimation of water stress of plant by vibration measurement of leaf using acoustic radiation force. Acoustical Science and Technolog. 2015;3:248–253. [Google Scholar]
- 15.Young WC, Budynas RG, Sadegh AM. Roark’s formulas for stress and strain. New York (NY): McGraw-Hill; 2002. [Google Scholar]
- 16.Richardson AD, Duigan SP, Berlyn GP. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002;153(1):185–194. doi: 10.1046/j.0028-646X.2001.00289.x. [DOI] [Google Scholar]
- 17.Fatemi M, Manduca A, Greenleaf JF. Imaging elastic properties of biological tissues by low-frequency harmonic vibration. Proc IEEE. 2003;91(10):1503–1518. doi: 10.1109/JPROC.2003.817865. [DOI] [Google Scholar]
- 18.Gray J William Kingdon Clifford. Princet Companion to Math; 2010, p. 780.
- 19.Gagliano M, Grimonprez M, Depczynski M, Renton M. Tuned in: plant roots use sound to locate water. Oecologia. 2017;184(1):151–160. doi: 10.1007/s00442-017-3862-z. [DOI] [PubMed] [Google Scholar]
- 20.de Langre E, Penalver O, Hemon P, Frachisse J-M, Bogeat-Triboulot M-B, Niez B, Badel E, Moulia B. Nondestructive and fast vibration phenotyping of plants. Plant Phenomics. 2019;2019:6379693. doi: 10.34133/2019/6379693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Der Loughian C, Tadrist L, Allain J, Diener J, Moulia B, De Langre E. Comptes rendus mecanique measuring local and global vibration modes in model plants. Comptes Rendus Mec [Internet]. 2014;342(1):1–7. doi: 10.1016/j.crme.2013.10.010. [DOI] [Google Scholar]
- 22.Robinson DA, Hopmans JW, Filipovic V, Van Der Ploeg M, Lebron I, SB J, Reinsch S, Jarvis N, Tuller M. Global environmental changes impact soil hydraulic functions through biophysical feedbacks. Glob Chang Biol. 2019;1895–1904. [DOI] [PubMed] [Google Scholar]
- 23.Bjornlund V, Bjornlund H. Understanding agricultural water management in a historical context using a socioeconomic and biophysical framework. Agric Water Manag [Internet]. 2019;213:454–467. doi: 10.1016/j.agwat.2018.10.037. [DOI] [Google Scholar]
- 24.Bhatt P. Maximum marks maximum knowledge in physics. New Delhi:Allied Publishers; 2010. [Google Scholar]
- 25.Båth M. Introduction to seismology. Berlin, Birkhäuser:Cambridge university press; 1975. [Google Scholar]
- 26.Appel HM, Cocroft RB. Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia. 2014;175(4):1257–1266. doi: 10.1007/s00442-014-2995-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gagliano M, Mancuso S, Robert D. Towards understanding plant bioacoustics. Trends Plant Sci [Internet]. 2012;17(6):323–325. doi: 10.1016/j.tplants.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Jeong MJ, Il CJ, Park SH, Kim KH, Lee SK, Kwon TR, Park SC, Siddiqui ZS. Sound frequencies induce drought tolerance in rice plant. Pakistan J Bot. 2014;46:2015–2020. [Google Scholar]
- 29.López-Ribera I, Vicient CM. Drought tolerance induced by sound in Arabidopsis plants. Plant Signal Behav [Internet]. 2017;2324(10):e1368938. doi: 10.1080/15592324.2017.1368938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vergeynst LL, Sause MGR, Hamstad MA, Steppe K. Deciphering acoustic emission signals in drought stressed branches: the missing link between source and sensor. Front Plant Sci [Internet]. 2015;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epila J, De Baerdemaeker NJF, Vergeynst LL, Maes WH, Beeckman H, Steppe K. Capacitive water release and internal leaf water relocation delay drought-induced cavitation in African Maesopsis eminii. Tree Physiol. 2017;37(4):481–490. doi: 10.1093/treephys/tpw128. [DOI] [PubMed] [Google Scholar]
- 32.Body MJA, Neer WC, Vore C, Lin C, Vu DC, Schultz JC, Cocroft RB, Appel HM, Traw MB. Caterpillar chewing vibrations cause changes in plant hormones and volatile emissions in arabidopsis thaliana. Front. Plant Sci. 2019;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dumais J, Forterre Y. “Vegetable Dynamicks”: the role of water in plant movements. Annu Rev Fluid Mech. 2012;44(1):453–478. doi: 10.1146/annurev-fluid-120710-101200. [DOI] [Google Scholar]
- 34.Zweifel R, Zeugin F. Ultrasonic acoustic emissions in drought‐stressed trees–more than signals from cavitation. New Phytol. 2008;179(4):1070–1079. doi: 10.1111/j.1469-8137.2008.02521.x. [DOI] [PubMed] [Google Scholar]
- 35.Chen C, Zhang B, Huang Q, Fu X, Liu RH. Microwave-assisted extraction of polysaccharides from Moringa oleifera Lam. leaves: characterization and hypoglycemic activity. Ind Crops Prod. 2017;100:1–11. [Google Scholar]
- 36.Liu S, Jiao J, Lu TJ, Xu F, Pickard BG, Genin GM. Article arabidopsis leaf trichomes as acoustic antennae. Biophysj [Internet]. 2017;113(9):2068–2076. doi: 10.1016/j.bpj.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Langre E. Plant vibrations at all scales: A review. J Exp Bot. 2019;70(14):3521–3531. doi: 10.1093/jxb/erz209. [DOI] [PubMed] [Google Scholar]
- 38.Forrest TG. From sender to receiver: propagation and environmental effects on acoustic signals. Am Zool. 1994;34(6):644–654. doi: 10.1093/icb/34.6.644. [DOI] [Google Scholar]
- 39.Gagliano M, Renton M. Love thy neighbour: facilitation through an alternative signalling modality in plants. BMC Ecol. 2013;13:19. doi: 10.1186/1472-6785-13-19 1 13 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gagliano M. Green symphonies: A call for studies on acoustic communication in plants. Behav Ecol. 2013;24(4):789–796. doi: 10.1093/beheco/ars206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parise AG, Gagliano M, Souza GM. Extended cognition in plants: is it possible? Plant Signal Behav [Internet]. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forterre Y. Slow, fast and furious: understanding the physics of plant movements. J Exp Bot. 2013;64(15):4745–4760. doi: 10.1093/jxb/ert230. [DOI] [PubMed] [Google Scholar]
- 43.Guo Q, Dai E, Han X, Xie S, Chao E, Chen Z. Fast nastic motion of plants and bioinspired structures. J R Soc Interface. 2015;12(110):20150598. doi: 10.1098/rsif.2015.0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tyree MT, Sperry JS. Vulnerability of xylem to cavitation and embolism. Annu Rev Plant Physiol Plant Mol Biol. 1989;40(1):19–36. doi: 10.1146/annurev.pp.40.060189.000315. [DOI] [Google Scholar]
- 45.Cochard H, Badel E, Herbette S, Delzon S, Choat B, Jansen S. Methods for measuring plant vulnerability to cavitation: A critical review. J Exp Bot. 2013;64(15):4779–4791. doi: 10.1093/jxb/ert193. [DOI] [PubMed] [Google Scholar]
- 46.Thomson W. Theory of vibration with applications. Boca Raton (Florida):CrC Press; 2018. [Google Scholar]
- 47.Kamilaris A, Prenafeta-Boldú FX. Deep learning in agriculture: A survey. Comput Electron Agric. 2018;147:70–90. doi: 10.1016/j.compag.2018.02.016. [DOI] [Google Scholar]
- 48.Fernandez-Jaramillo AA, Duarte-Galvan C, Garcia-Mier L, Jimenez-Garcia SN, Contreras-Medina LM. Effects of acoustic waves on plants: an agricultural, ecological, molecular and biochemical perspective. Sci Hortic (Amsterdam). 2018;235:340–348. doi: 10.1016/j.scienta.2018.02.060. [DOI] [Google Scholar]
- 49.Niez B, Dlouha J, Moulia B, Badel E. Water-stressed or not, the mechanical acclimation is a priority requirement for trees. Trees [Internet]. 2019;33(1):279–291. doi: 10.1007/s00468-018-1776-y. [DOI] [Google Scholar]
- 50.Gardiner B, Berry P, Review: MB. Wind impacts on plant growth, mechanics and damage. Plant Sci. 2016;245:94–118. doi: 10.1016/j.plantsci.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Li Z-G GM. Mechanical stimulation-induced cross-adaptation in plants: an overview. J Plant Biol. 2011;54(6):358. doi: 10.1007/s12374-011-9178-3. [DOI] [Google Scholar]
- 52.Hassanien RHE, Hou TZ, Li YF, Li BM. Advances in Effects of Sound Waves on Plants. J Integr Agric. 2014;13(2):335–348. doi: 10.1016/S2095-3119(13)60492-X. [DOI] [Google Scholar]
- 53.Jung J, Kim S-K, Kim JY, Jeong M-J, Ryu C-M. Beyond chemical triggers: evidence for sound-evoked physiological reactions in plants. Front Plant Sci [Internet]. 2018;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


