Abstract
Background
Gintonin-enriched fraction (GEF) is a new non-saponin component glycolipoprotein isolated from ginseng root. This study examined the effect of GEF on age-related sarcopenia in old C57BL/6J mice.
Methods
Young (3–6 months) and old (20–24 months) C57BL/6J mice received oral GEF (50 mg/kg/day or 150 mg/kg/day) daily for 5 weeks. During the oral administration period, body weight and grip strength were measured weekly. After sacrifice, muscles from the hindlimb were excised and used for hematoxylin and eosin staining and western blotting to determine the effects of GEF on sarcopenia. The thymus was photographed to compare size, and flow cytometry was performed to examine the effect of GEF on immune homeostasis in the thymus and spleen. Blood samples were collected, and the concentrations of pro-inflammatory cytokines and IGF-1 were measured.
Results
GEF caused a significant increase in muscle strength, mass, and fiber size in old mice. GEF restored age-related disruption of immune homeostasis by maintaining T cell compartments and regulating inflammatory biomarkers. Thus, GEF reduced common low-grade chronic inflammatory parameters, which are the main cause of muscle loss.
Conclusion
GEF maintained immune homeostasis and inhibited markers of chronic inflammation, resulting in anti-sarcopenia effects in aged C57BL/6J mice. Thus, GEF is a potential therapeutic agent that slows sarcopenia in the elderly.
Keywords: Ginseng, Gintonin-enriched fraction, Sarcopenia, Immune homeostasis, Immunosenescence
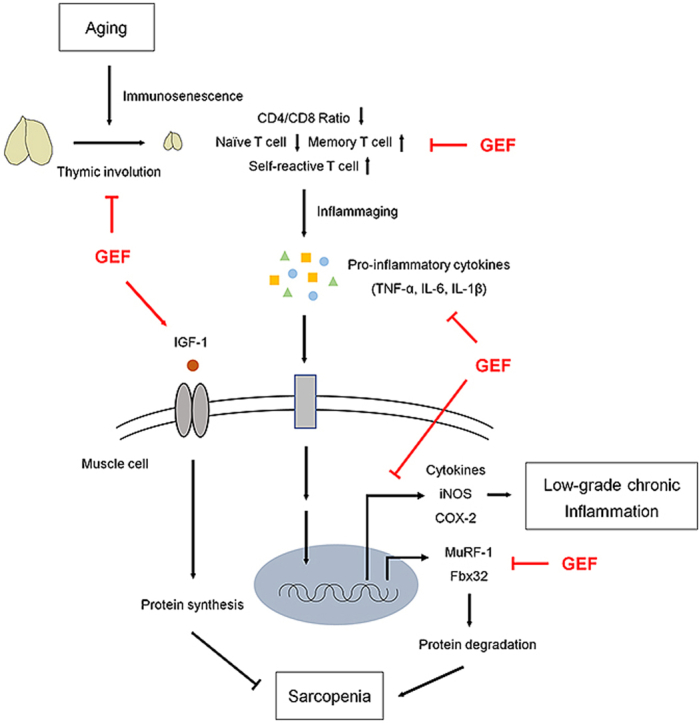
Graphical abstract
1. Introduction
Aging is associated with changes that result in loss of physiological integrity and reduce both health and quality of life. The aging population is increasing worldwide; therefore, maintaining a healthy life-style and improving quality of life is important. Almost all organs deteriorate with age, and skeletal muscle is no exception. A major factor that reduces quality of life in older people is progressive loss of skeletal muscle mass, strength, and functionality, collectively described as age-related sarcopenia. Older people who suffer from sarcopenia are at risk for physical disability, weakness, injuries, and (eventually) increased mortality [[1], [2], [3]]. Conventional interventions have been attempted to improve age-related sarcopenia, but no underlying treatments have been developed. For example, exercise is the primary strategy prescribed to prevent or treat sarcopenia [4], but the efficacy of this approach can be limited because many elderly people cannot perform physical activity at the required level. Given the severity of sarcopenia in an aging population, and the resulting high health care prices, the lack of therapeutic intervention emphasizes the need to develop effective and fundamentally safe treatments for the elderly.
Muscle metabolism is strictly controlled by balanced fluctuations in muscle protein synthesis and breakdown [5]. This balance is skewed in the elderly in that the rate of muscle protein synthesis does not match that of muscle protein breakdown, leading to a gradual reduction in the mass and strength of skeletal muscles. There are many causes of sarcopenia, such as hormonal alterations, decreased nutrient intake, and neuronal changes [[6], [7], [8]]; however, the role played by age-related immune dysregulation on muscle protein metabolism has attracted much attention.
Immunosenescence, defined as age-related impairment of the immune system, is characterized by thymic involution, accumulation of senescent T cells, development of chronic inflammatory disorders, and defective maintenance of immune cells, all of which break immune homeostasis [[9], [10], [11], [12]]. Immunosenescence is caused by a lack of immune repertoire diversity, caused by decreased production of naïve immune cells and accumulation of memory immune cells [9]. Therefore, immunosenescence is associated with the function of the thymus, the site of maturation for thymic lymphocytes or T cells. The thymus undergoes age-related atrophy, a process termed thymic involution [13]. Thymic involution results in structural degeneration and functional impairment, which together decrease the output of naïve T cells significantly, ultimately resulting in collapse of T cell homeostasis and accumulation of self-reactive T cells [13,14]. Thus, the immune response of an elderly person to new antigens is weaker than that of younger persons; it is also more responsive to self-antigens, resulting in low-grade chronic inflammation. Although general inflammatory responses are maintained by the innate immune system, the collapse of the adaptive immune system due to aging is more damaging to the health of the elderly. Recent studies report that age-related thymic involution is closely associated with low-grade chronic inflammation, called ‘inflammaging’, which could be a major cause of sarcopenia [9,15]. In low-grade chronic inflammation state, tumor necrosis factor alpha(TNF-α), interleukin (IL)-6, and IL-1β, which represent pro-inflammatory cytokines, play major role in accelerating the rate of protein degradation [16,17]. This indicates that chronic inflammation caused by broken immune homeostasis is a key process that accelerates progression of sarcopenia [[18], [19], [20]]. Thus, reversing or slowing the decline in age-related immune homeostasis, and inhibiting inflammaging, is an important approach to treating sarcopenia.
Korean ginseng (Panax ginseng Meyer, Araliaceae) is a traditional herb used in Asia to increase physical work capacity, immunity, and recovery from fatigue, and to treat inflammation [[21], [22], [23]]. Korean ginseng contains various bioactive components, including saponins (ginsenosides), non-saponin components, polysaccharides, phenolic ingredients, and alkaloids [24]. While many treatment effects have been reported for ginsenoside, the effectiveness of non-saponin components, which account for most of the ginseng, is unclear. In addition, preparation of ginsenosides is complicated and the separation process is expensive. Therefore, researchers have become interested in the efficacy of non-saponin components, which are easier to obtain. Recently, a new non-saponin fraction called “Gintonin-enriched fraction (GEF)” was isolated from ginseng [[25], [26], [27]]. Gintonin is a glycolipoprotein composed mainly of proteins containing hydrophobic and acidic amino acids, glucose as an important carbohydrate component, and lysophosphatidic acid (LPA) [26]. In particular, recent studies have shown that one of the main components of GEF, ginseng major latex-like protein151 (GLP151), is an important molecule for the medicinal effect of GEF [28]. Research has shown GEF to be effective in many ways; however, it is unclear whether it improves age-related sarcopenia. Thus, we examined the effects of GEF on age-related sarcopenia in old C57BL/6J mice aged 20–24-months (mo).
2. Materials and methods
2.1. Preparation of GEF
GEF was prepared as described previously [26]. Briefly, small pieces (>3 mm) of 4-year-old Korean White ginseng (Korea Ginseng Cooperation, Daejon, Korea) were refluxed eight times for 8 h at 80°C for with 70% edible ethanol. The ethanolic extracts were concentrated, dissolved in distilled water at a ratio of 1-10, and stored in a cold chamber at 4°C for 24 h. After centrifugation, the precipitate was lyophilized, freeze-dried, and this fraction was designated as GEF [27].
2.2. Reagents
GEF was supplied by the Ginsentology Research Laboratory of Konkuk University (Seoul, Korea). Antibodies specific for myoblast determination protein 1 (MyoD), F-box protein (Fbx32/atrogin), muscle ring finger 1 (MuRF1), and tumor necrosis factor-α (TNF-α) were purchased from Abcam (Abcam, Cambridge, UK). Antibodies specific for myocyte enhancer factor-2 (MEF-2), cyclooxygenase-2 (COX-2), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Antibodies specific for inducible nitric oxide synthase (iNOS) and interleukin 1 beta (IL-1β) were purchased from Cell Signaling Technology (Danvers, MA, USA). FITC anti-mouse CD3ε, PE anti-mouse CD45, PerCP/Cyanine5.5 anti-mouse CD4, Brilliant Violet421 anti-mouse CD8, APC anti-mouse CD62L, and Alexa Fluor700 anti-mouse CD44 were purchased from BioLegend (San Diego, CA, USA).
2.3. Animals and treatments
Female C57BL/6J mice (aged 3–6 or 20–24 mo) were purchased from the Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea) and housed at 20 ± 3°C in a room maintained under a 12 h/12 h light-dark cycle. Before oral administration of GEF, mice were adapted to the experimental facility for 1 week. Mice aged 3–6 mo (young mice; YM) were randomized into two groups (n = 10 per group) as follows: (I) YM Ctrl and (II) YM GEF 150. Mice aged 20–24 mo (old mice; OM) were randomized into three groups (n = 10 per group) as follows: (I) OM Ctrl, (II) OM GEF 50, and (III) OM GEF 150. GEF was dissolved in distilled water. Mice received oral GEF (50 mg/kg/day or 150 mg/kg/day) daily for 5 weeks. Daily dosage of GEF was established referring to several studies demonstrating the bioactive effectiveness of GEF [22,29,30], and derived from the human dose (0.25 g/60 kg/day and 0.75 g/60 kg/day) in mathematical table, as described [31]. During the oral administration period, body weight and grip strength were measured weekly. After 6 weeks (5 weeks of oral administration plus the 1 week adaptation period), mice were finally anesthetized using CO2 and blood samples were obtained via cardiac puncture. After sacrifice, thymus, spleen, and hindlimb muscles (gastrocnemius and quadriceps) were excised, weighed, and collected.
2.4. Ethics statement
All animals were humanely treated according to the standards outlined in the “Guide for the Care and Use of Laboratory Animals” arranged by the National Academy of Science and published by the National Institutes of Health. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC 200156) of CHA University (Seongnam, Kyunggi, Korea).
2.5. Grip strength test
All-limb grip strength was measured using a Chatillon Force Measurement System (Columbus Instrument, Columbus, OH, USA). Mice were put on a narrow bar and the tail was pulled slowly and gently 3–5 times. The highest tension at the point the mice let go of the bar was recorded.
2.6. Histological analysis
Gastrocnemius and quadriceps muscle samples were fixed in 4% paraformaldehyde, embedded in paraffin, cut into 10 μm sections, and stained with hematoxylin and eosin. All sections were imaged under a Nikon E600 microscope (Nikon, Tokyo, Japan). The cross sectional area of the muscle fiber was calculated using ImageJ software (Bethesda, MD, USA).
2.7. Western blot analysis
Gastrocnemius and quadriceps muscle samples were homogenized, and then lysed for 20 min in lysis buffer (iNtRON Biotechnology, Seoul, Korea) containing protease and phosphatase inhibitors. Lysate protein concentrations were evaluated in a BCA protein assay (Pierce, Rockford, IL, USA) using BSA as a standard. Equal amounts of proteins were separated by SDS-PAGE gels and transferred to Immun-Blot PVDF membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked for 1 h in 5% skim milk and then washed in Tris-buffered saline (TBS) containing 0.05% Tween-20 (TBS-T). The membranes were incubated overnight at 4°C with indicated primary antibodies. After a washing step, the membranes were blocked for 1 h in 5% skim milk containing peroxidase-conjugated anti-rabbit or anti-mouse. Signals were detected by EZ-Western Lumi Femto (DoGenBio, Seoul, Korea) and visualized using a LAS-4000 apparatus (GE Healthcare Life Sciences, Marlborough, MA, USA). Proteins band intensities on each blot were quantified by densitometric assessment using ImageJ software (Bethesda, MD, USA).
2.8. Flow cytometry analysis
Spleen and thymus were homogenized and separated into single cells by passage through a 40-μm nylon mesh strainer. The single cell suspensions were collected, and red blood cells were lysed in ACK lysis buffer (Lonza, Basel, Switzerland). The single cells were stained on ice for 20 min with the specific anti-mouse antibodies, and then washed with PBS. Flow cytometry was performed using CytoFlex (Beckman Coulter, CA, USA) and data were analyzed using FlowJo software (Ashland, OR, USA).
2.9. Serum analysis
Blood samples were obtained from each mouse by cardiac puncture at the time of euthanasia and centrifuged at 800 × g for 15 min at 4°C. Serum was collected and stored at −80°C. The concentrations of TNF-α, IL-6, and IL-1β in serum were evaluated using a Mouse Th17 Magnetic Bead Panel (Merck Millipore, MA, USA). The concentration of insulin-like growth factor (IGF)-1 in serum was measured using an IGF-1 ELISA kit (Thermo Fisher Scientific, MA, USA). All analysis were performed in accordance with the manufacturer's instructions. Serum cytokine concentrations were assessed using a Luminex 100 (Luminex, Austin, TX, USA). All samples were assayed in duplicate.
2.10. Statistical analysis
All data are expressed as the mean ± SEM. Statistical comparisons were evaluated using one-way analysis of variance (ANOVA) followed by Tukey's multiple range tests. All analyses were performed using SPSS (IBM, Armonk, NY, USA). A value of p < 0.05 was considered statistically significant and values with different letters are significantly different (a > b > c > d).
3. Results
3.1. Effect of GEF on muscle strength, mass, and fiber size in old mice
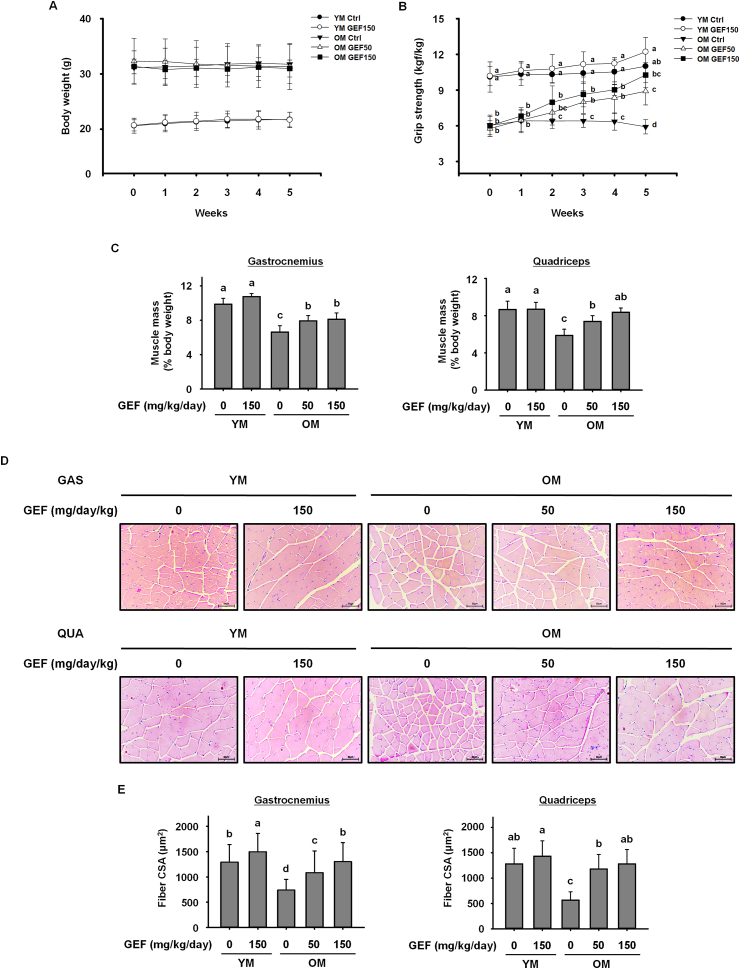
Age-related sarcopenia is characterized by progressive loss of muscle mass and strength [4]. During the administration period, we measured body weight and grip strength once a week. As shown in Fig. 1A, GEF had no effect on the body weight of old or young mice over the 5 weeks; however, there was a significant difference in body weight of young mice and old mice. Thus, measurements of grip strength and muscle weight were normalizing to body weight. The grip strength of old control mice was significantly weaker than that of young mice; also, the grip strength of old mice declined further throughout the experimental period. However, treatment with GEF increased the grip strength of old mice gradually over the 5 weeks (Fig. 1B). After 5 weeks of GEF administration, the weight of the gastrocnemius and quadriceps muscles in control old mice indicated that aging caused serious loss of skeletal muscle; however, this was improved by GEF administration (Fig. 1C). Furthermore, histological analysis of the gastrocnemius and quadriceps muscles showed that the cross-sectional area (CSA) in control old mice was lower than that in young mice; however, GEF increased the CSA in old mice (Fig. 1D and E). Taken together, the data suggest that GEF administration over a period of 5 weeks ameliorates severe sarcopenia, and increases muscle strength and muscle fiber size, in old mice.
Fig. 1.
Gintonin-enriched fraction (GEF) increases muscle strength, muscle mass, and muscle fiber size in old mice (OM). (A) Weekly body weight measurements and (B) grip strength of mice treated with GEF for 5 weeks. (C) Mass of the gastrocnemius (left) and quadriceps (right) muscles. Muscle mass was normalized to body weight on the final day of the experiment. (D) Histological images of hematoxylin and eosin-stained sections of gastrocnemius (GAS, top) and quadriceps (QUA, bottom) muscles. (E) Quantification of the cross-sectional area (CSA) of muscle fibers. The CSA of each muscle fiber was measured using the Image J program. Statistical significance was decided using one-way ANOVA, followed by Tukey's post-hoc test. Values with different letters are significantly different; p < 0.05 (a > b > c > d).
3.2. Effect of GEF on expression of proteins involved in sarcopenia in old mice
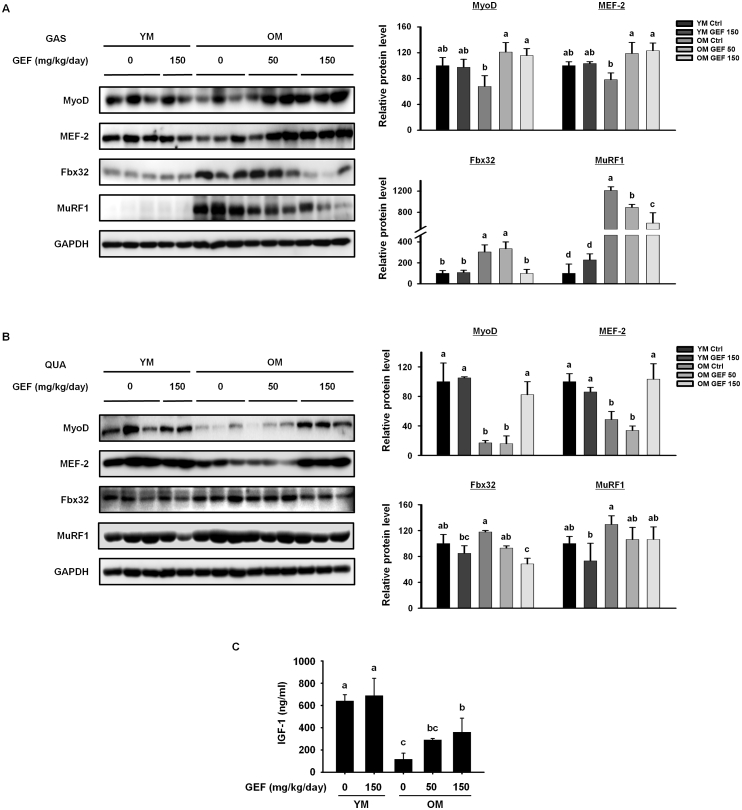
Next, we evaluated the effect of GEF on regulatory proteins involved in the muscle protein synthesis and degradation. Expression of regulatory factors involved in muscle protein synthesis (MyoD and MEF-2) or degradation (Fbx32 and MuRF1) in the gastrocnemius and quadriceps muscles was assessed (Fig. 2A and B). Compared with the YM Ctrl and YM GEF 150 groups, the OM Ctrl group showed significant decreases in MyoD and MEF-2, but increases in Fbx32 and MuRF1, in both muscles. However, after GEF administration, old mice showed increased expression of MyoD and MEF-2 and decreased expression of Fbx32 and MuRF1 in both muscles. These results indicate that GEF induces muscle protein synthesis and inhibits muscle protein degradation in old mice. Next, we measured IGF-1 levels in serum; IGF-1 is associated with protein synthesis and myogenesis (Fig. 2C). Serum levels of IGF-1 in the OM Ctrl group were lower than those in the all of YM group; however, GEF increased levels in old mice in a dose-dependent manner. Taken together, the data demonstrate that GEF contributes to muscle growth and suppresses muscle loss in old mice.
Fig. 2.
GEF regulates expression of proteins relevant to sarcopenia in old mice. Expression levels of proteins involved in muscle protein synthesis (MyoD and MEF-2) or degradation (Fbx32 and MuRF1) was measured in (A) the GAS and (B) the QUA muscle by western blotting. GAPDH served as a control. (C) IGF-1 levels in serum. Statistical significance was decided using one-way ANOVA, followed by Tukey's post-hoc test. Values with different letters are significantly different; p < 0.05 (a > b > c > d).
3.3. Effect of GEF on age-associated thymic involution and imbalance of T cell populations in old mice
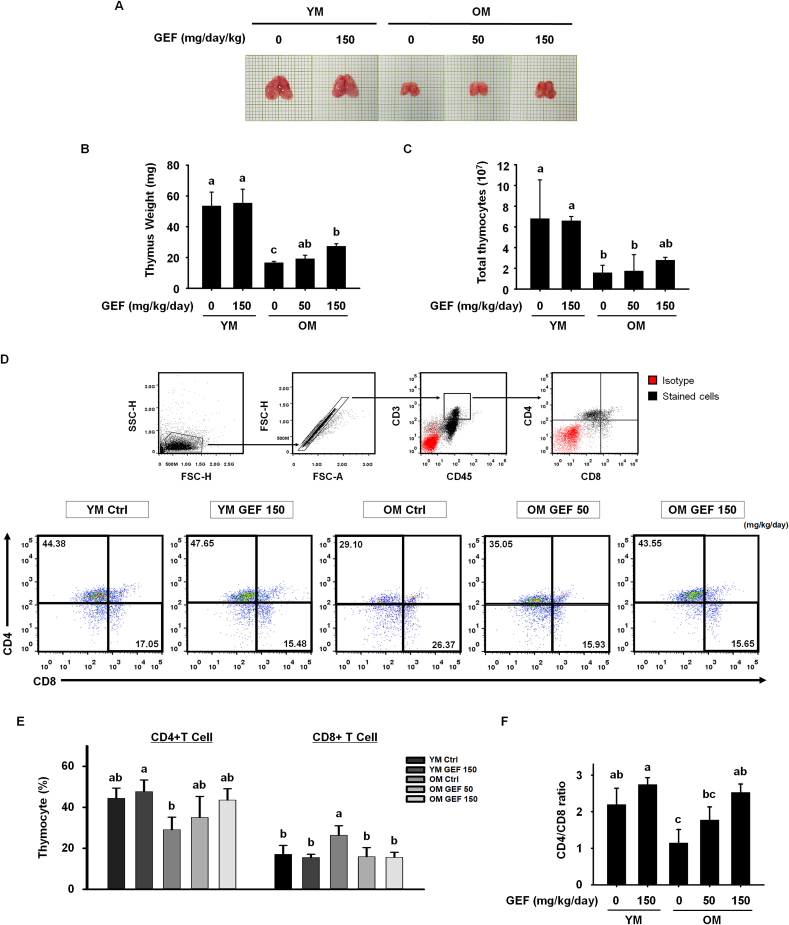
In addition to age-related loss of muscle mass and function, aging causes many changes to the immune system; indeed, these two processes are closely related. In particular, the dystrophic thymus caused by aging aggravates muscular dystrophy by altering central immune tolerance [32]. Therefore, we investigated the protective effects of GEF on immunosenescence in old mice. First, we examined the effect of GEF on thymic involution, which is a representative characteristics of immunosenescence. The size and weight of the thymuses from the OM Ctrl group were much smaller than those from the YM Ctrl and YM GEF 150 group; however, both were increased by GEF in a dose-dependent manner (Fig. 3A and B). These changes in thymus size and weight correlated with thymocyte numbers. GEF increased the numbers of thymocytes in old mice (Fig. 3C). Because the thymus is the critical primary organ in which T cells mature, we examined the effect of GEF on thymic T cell subsets using flow cytometry (Fig. 3D and E). Helper and cytotoxic T cells exhibit characteristic surface expression patterns: CD45+CD3+CD4+CD8- and CD45+CD3+CD4−CD8+, respectively. Interestingly, we observed that the percentage of CD4+ T cells in the thymus of OM Ctrl mice was lower than that in YM Ctrl group. By contrast, the proportion of CD8+ T cells was higher in the OM Ctrl group compared with YM Ctrl and YM GEF 150 group. However, the proportion of each T cell subtype in old mice receiving GEF recovered to levels observed in young mice. Based on these data, we calculated the CD4/CD8 ratio (Fig. 3F) and found that the CD4/CD8 ratio in old mice returned to normal range after GEF administration, indicating enhanced immunity. These results suggest that GEF rescues age-related disruption of immune homeostasis.
Fig. 3.
GEF inhibits age-associated thymic involution and restores the imbalance in the T cell compartment of the thymus in old mice. (A) Representative photographs of thymuses isolated from each group of mice. (B) Thymus weight. (C) Number of total thymocytes. (D) Gating strategy used for analysis of T cell subsets. Red dots represent the isotype control and black dots represent cells stained with specific antibodies (top). Representative dot plots of CD4+ and CD8+ T cells (bottom). Percentages are shown in the quadrants.(E) Bar graph showing the mean % of CD4+ and CD8+ T cells within the CD3+CD45+ leukocyte population within thymocytes. (F) Ratio of CD4/CD8 single-positive cells in the thymus. Statistical significance was decided using one-way ANOVA, followed by Tukey's post-hoc test. Values with different letters are significantly different; p < 0.05 (a > b > c > d).
3.4. Effect of GEF on secondary T cell development and maintenance of immune homeostasis in old mice
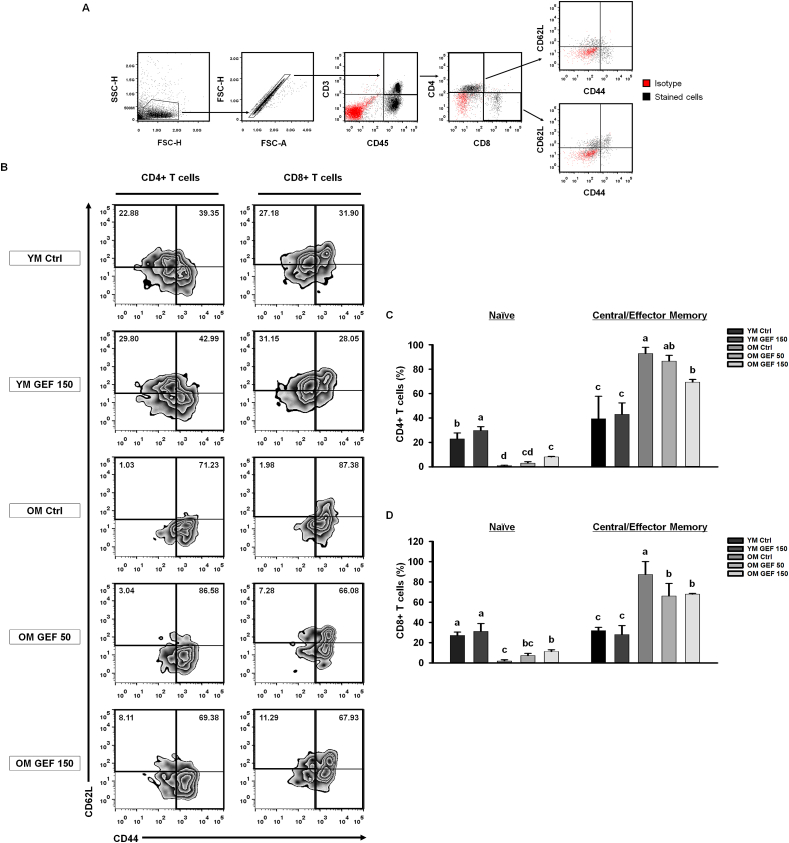
Following age-related thymic involution, maturation of T cells is blocked, and fewer mature T cells are exported to secondary lymphoid organs; this decrease in naïve T cells, coupled with an increase in central/effector memory T cells, results in imbalances in the immune system [13,33,34]. To demonstrate the effect of GEF on age-related shifts in T cell phenotype, we examined the proportions of naïve and central/effector memory T cells in the CD4+ T cell and CD8+ T cell populations, respectively, by flow cytometry (Fig. 4A). Naïve T cell populations were defined as showing low expression of CD44, while differential expression of CD62L in the high CD44 expression quadrant identified central memory T cells or effector memory T cells. The proportion of naïve CD4+ T cells (CD45+CD3+CD4+CD44loCD62Lhi) in the OM Ctrl group was significantly lower than that in the YM group; however, administration of GEF led to a slight increase this proportion. The pool of central/effector memory CD4+ T cells (CD45+CD3+CD4+CD44hi) in the OM Ctrl group was greater than that in the YM group; however, this was reduced by GEF in a dose-dependent manner (Fig. 4B). Naïve CD8+ T cells (CD45+CD3+CD8+CD44loCD62Lhi) and central/effector memory CD8+ T cells (CD45+CD3+CD8+CD44hi) showed the same trend as CD4+ T cells (Fig. 4C). Thus, GEF intensifies responses to new antigens by increasing the number of naïve T cells, while simultaneously decreasing the number of memory T cells, which have the potential to secrete pro-inflammatory cytokines. Taken together, these GEF-mediated changes maintain immune homeostasis in old mice.
Fig. 4.
GEF increases the numbers of naïve T cells and decreases those of memory T cells in old mice. (A) Gating strategy used for analysis of T cells subsets. Red dots represent isotype controls and black dots represent cells stained with specific antibodies. (B) Representative dot plots of naïve (CD44loCD62Lhi) and central/effector memory (CD44hi) T cells in splenocytes. Bar graph showing mean % of subpopulations of CD4+ (C) and CD8+ (D) T cells. Statistical significance was decided using one-way ANOVA, followed by Tukey's post-hoc test. Values with different letters are significantly different; p < 0.05 (a > b > c > d).
3.5. GEF suppresses inflammatory responses that cause sarcopenia in old mice
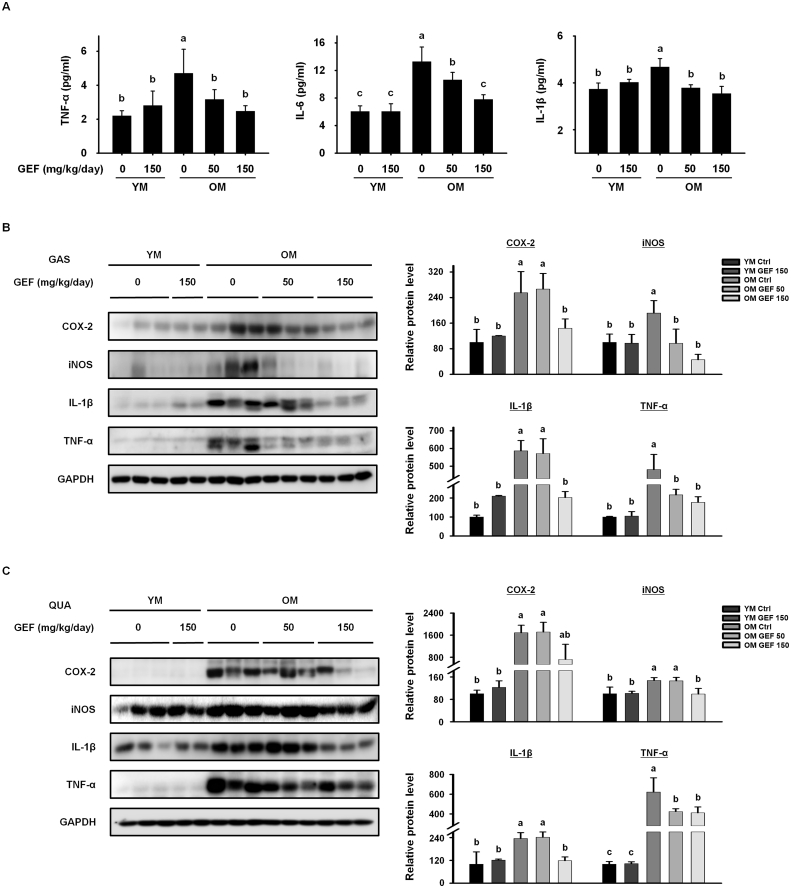
Age-related disruption of immune homeostasis causes low level chronic inflammation, which is a main driver of sarcopenia [20]. Therefore, we investigated the effect of GEF on inflammatory markers in serum, gastrocnemius, and quadriceps muscle tissue. Levels of pro-inflammatory cytokines TNF-α, IL-6, and IL-1β were significantly higher in serum from OM Ctrl mice than in mice from the YM group, indicating that OM Ctrl mice were in a low-grade chronic inflammation state (Fig. 5A). However, GEF treatment led to a significant reduction in the serum levels of all three pro-inflammatory cytokines in old mice. As pro-inflammatory cytokines circulating in the blood reach the muscle cell membrane, inflammation is induced and expression of COX-2 and iNOS is elevated. In both the gastrocnemius and quadriceps muscles, expression of COX-2 and iNOS protein in the OM Ctrl group was higher than that in the YM group; however, expression of both inflammatory markers fell significantly after GEF treatment (Fig. 5B and C). Consistent with this, expression of IL-1β and TNF-α, which is increased by aging, in both muscles fell after GEF treatment (Fig. 5B and C). Overall, these data suggest that GEF suppresses age-induced chronic inflammation and restores immunity in old mice.
Fig. 5.
GEF suppresses age-related inflammatory responses. (A) Pro-inflammatory cytokine levels in serum. Expression of proteins involved in inflammation in the (B) GAS and (C) QUA muscles were measured by western blotting. GAPDH served as a control. Statistical significance was decieded using one-way ANOVA, followed by Tukey's post-hoc test. Values with different letters are significantly different; p < 0.05 (a > b > c > d).
4. Discussion
Sarcopenia is characterized by progressive age-related loss of skeletal muscle mass and muscular dysfunction, including reduced grip strength [2]. Sarcopenia is a representative disease of the elderly population, and a major reason for a deteriorating quality of life. Therefore, inhibiting development of sarcopenia in elderly populations worldwide with increase the number of healthy life-years. Since sarcopenia is a disease caused by aging regardless of gender, we used naturally aged female mice alone to verify the effectiveness of GEF on sarcopenia. Despite the fact that many aging-associated studies used only aged female mice, further studies have to be conducted to verify the effectiveness of GEF in aged male mice also. In this study, we used C57BL/6J mice aged 20–24 mo because a mouse age of 3–6 mo is equivalent to a human age of 20–30 years, and a mouse age of 20–24 mo is equivalent to > 65 years in humans [35]. Therefore, C57BL/6J mice aged 20–24 mo were used to study sarcopenia and the results were compared with those in young mice.
We observed that grip strength, muscle mass and muscle fiber size were drastically lower in the OM Ctrl group than in the YM group. Conversely, daily treatment with GEF for 5 weeks restored all of these parameters to levels observed in young mice. Thus, GEF has the potential to improve sarcopenia in old mice.
Muscle mass is determined by an elaborate balance between muscle protein synthesis and degradation [36,37]. During aging, the rate of muscle proteolysis exceeds that of muscle protein synthesis, leading to loss of muscle mass and sarcopenia over time. Recent studies have suggested several pathways, such as PI3K-Akt or mTOR pathway, as a mechanism for muscle protein metabolism [[38], [39], [40]]. Further studies are needed to identify which molecules and mechanisms are targeted by GEF to increase protein synthesis. Here, we examined genes directly related to muscle regeneration to investigate whether GEF recovers skeletal muscle in old mice.
Recovery of skeletal muscle is accompanied by activation of protein synthesis (myogenesis) and a reduction in proteolytic activity (preventing muscle loss) [41]. Regulation of skeletal myogenesis is associated with myogenic regulatory factors such as MyoD and MEF-2 [42,43]. These muscle-specific proteins Fbx32 and MuRF1 play important roles in the ubiquitin proteasome pathway, which is associated with progression of muscle loss [44]. Here, we show that GEF increased expression of MyoD and MEF-2 and reduced expression of Fbx32 and MuRF1 in the gastrocnemius and quadriceps muscles of old mice, suggesting that GEF not only stimulates myogenesis, but also inhibits progressive muscle loss in old mice. In addition, GEF significantly increased expression of IGF-1, which is associated with reduced visceral fat, increased muscle mass, and bone concentration [45,46], in old mice.
Increasing evidence shows that age-related immunological dysregulation and chronic inflammation are responsible for development of sarcopenia [19,20,47]. Therefore, we investigated the protective effects of GEF on immunosenescence in old mice. One of the main characteristics of immunosenescence is thymic involution and the subsequent accumulation of senescent T cells. Thymic involution is characterized by abnormal thymic structure, reduced thymus size and weight, and a decreased number of total thymocytes. We observed that while thymic involution was advanced in old mice, it was reversed by GEF. The T cell compartment undergoes striking age-associated remodeling; a declining CD4/CD8 ratio is an indicator of immunosenescence [[48], [49], [50]]. Compared with CD4+ T cells, we found that the CD8+ T cell population was increased in old mice, thereby reducing the CD4/CD8 ratio. This increase in CD8+ T cells may contribute to inflammation because they secrete pro-inflammatory cytokines, which break immune homeostasis [51,52]. However, GEF maintained immune homeostasis in old mice by restoring the balance to the T cell compartments, which a CD4/CD8 ratio similar to that in young mice. With aging, the atrophied thymus loses the ability to establish central tolerance, resulting in increased numbers of self-reactive T cells that escape to the periphery, leading to inflammation [9]. Coupled with this, the T cell immune repertoire shrinks, with a reduced proportion of naïve T cells and an increased proportion of memory T cells [14,48]. Since the decline in naïve T cells due to aging correlates with a failure to maintain immune homeostasis and a reduced ability to respond to new infections, maintaining an appropriate number of naïve T cells and suppressing excessive increases in the number of memory T cells that cause chronic inflammation is important. Flow cytometry analysis showed that administration of GEF to old mice increased thymic output of naïve T cells, which was accompanied by decreased accumulation of memory T cells. These results suggest that treating old mice with GEF recovers immune homeostasis to levels observed in young mice, thereby effectively inhibiting immunosenescence.
Age-related thymic involution, the resulting increase in the proportion of CD8 T cells, and the increase in self-reactive T cell numbers lead to low-grade chronic inflammation, which is a major cause of accelerating sarcopenia [32,47,51,53]. A nonspecific state of chronic inflammation accompanied by aging is indicated by increased blood concentrations of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β [54]. When these pro-inflammatory cytokines reach the muscle, inflammation is encouraged, which triggers muscle atrophy by mediating muscle catabolism [[15], [16], [17], [18],38,55,56]. Indeed, population-based data show that blood concentrations of IL-6 and TNF-α are elevated in sarcopenic elderly persons [[57], [58], [59]]. Consistent with previous data, we observed that the serum levels of all three pro-inflammatory cytokines in OM Ctrl group were higher than in YM Ctrl group with a healthy immune state, indicating that old mice were in a low-grade chronic inflammation state. However, GEF exhibited strong anti-inflammatory activity in old mice. The inflammatory cascade caused by pro-inflammatory cytokines further stimulates inflammatory cytokine-secreting cells and increases the expression of certain inflammatory biomarkers such as COX-2 and iNOS. Increasing these factors induces muscle catabolism, leading to a decrease in muscle mass and strength. GEF downregulated expression of COX-2 and iNOS, in aged gastrocnemius and quadriceps muscles. This strong anti-inflammatory effect of GEF is thought to dampen low-grade chronic inflammation in muscles, thereby inhibiting sarcopenia.
In summary, GEF not only increased muscle strength, muscle mass, and muscle fiber size, but also stimulated muscle protein synthesis, in old mice. We focused on immunosenescence, which is the main cause of age-related sarcopenia. GEF suppressed age-related thymic involution and improved the balance between T cell compartments. Furthermore, we revealed that GEF treatment increased sensitivity to new antigens by elevating the number of naïve T cells and reducing the number of memory T cells. In addition, GEF reduced expression of pro-inflammatory biomarkers, thereby preventing muscle inflammation.
In conclusion, we proposed that GEF inhibits severe progression of sarcopenia by blocking several processes that lead to chronic inflammation, and by maintaining immune homeostasis. Collectively, the data show that GEF can be administered as a natural food supplement that inhibits sarcopenia in the elderly by maintaining immune homeostasis.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
This work was partially supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1A2C2006180), and by a grant from the Korean Society of Ginseng, funded by the Korean Ginseng Corporation.
Contributor Information
Hyun-Ji Oh, Email: guswl264@naver.com.
Heegu Jin, Email: heegu94@hanmail.net.
Seung-Yeol Nah, Email: synah@konkuk.ac.kr.
Boo-Yong Lee, Email: bylee@cha.ac.kr.
References
- 1.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visser M., Schaap L.A. Consequences of sarcopenia. Clin Geriatr Med. 2011;27(3):387–399. doi: 10.1016/j.cger.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Visser M. Obesity, sarcopenia and their functional consequences in old age. Proc Nutr Soc. 2011;70(1):114–118. doi: 10.1017/S0029665110003939. [DOI] [PubMed] [Google Scholar]
- 4.Ziaaldini M.M., Marzetti E., Picca A., Murlasits Z. Biochemical pathways of sarcopenia and their modulation by physical exercise: a narrative review. Front Med (Lausanne). 2017;4:167. doi: 10.3389/fmed.2017.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonaldo P., Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech. 2013;6(1):25–39. doi: 10.1242/dmm.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frontera W.R., Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015;96(3):183–195. doi: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- 7.Robinson S., Cooper C., Aihie Sayer A. Nutrition and sarcopenia: a review of the evidence and implications for preventive strategies. J Aging Res. 2012;2012:510801. doi: 10.1155/2012/510801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon Y.N., Yoon S.S. Sarcopenia: neurological point of view. J Bone Metab. 2017;24(2):83–89. doi: 10.11005/jbm.2017.24.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas R., Wang W., Su D.M. Contributions of age-related thymic involution to immunosenescence and inflammaging. Immun Ageing. 2020;17:2. doi: 10.1186/s12979-020-0173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu W., Rao S. Mechanisms underlying T cell immunosenescence: aging and cytomegalovirus infection. Front Microbiol. 2016;7:2111. doi: 10.3389/fmicb.2016.02111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franceschi C., Bonafe M., Valensin S., Olivieri F., De Luca M., Ottaviani E. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 12.Chei S., Oh H.J., Lee K., Jin H., Lee J.Y., Lee B.Y. Dysfunction of B Cell leading to failure of immunoglobulin response is ameliorated by dietary silk peptide in 14-month-old C57BL/6 mice. Front Nutr. 2020;7:583186. doi: 10.3389/fnut.2020.583186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer D.B. The effect of age on thymic function. Front Immunol. 2013;4:316. doi: 10.3389/fimmu.2013.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen S.S., Kim J.S., Weksler M.E. Effect of age on thymic development, T cell immunity, and helper T cell function. Rev Physiol Biochem Pharmacol. 1999;139:123–139. doi: 10.1007/BFb0033650. [DOI] [PubMed] [Google Scholar]
- 15.Dalle S., Rossmeislova L., Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol. 2017;8:1045. doi: 10.3389/fphys.2017.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curcio F., Ferro G., Basile C., Liguori I., Parrella P., Pirozzi F. Biomarkers in sarcopenia: a multifactorial approach. Exp Gerontol. 2016;85:1–8. doi: 10.1016/j.exger.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Meng S.J., Yu L.J. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010;11(4):1509–1526. doi: 10.3390/ijms11041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelke C., Dziewas R., Minnerup J., Meuth S.G., Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. 2019;49:381–388. doi: 10.1016/j.ebiom.2019.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bano G., Trevisan C., Carraro S., Solmi M., Luchini C., Stubbs B. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. 2017;96:10–15. doi: 10.1016/j.maturitas.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Chhetri J.K., de Souto Barreto P., Fougere B., Rolland Y., Vellas B., Cesari M. Chronic inflammation and sarcopenia: a regenerative cell therapy perspective. Exp Gerontol. 2018;103:115–123. doi: 10.1016/j.exger.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 21.Cho H.J., Choi S.H., Kim H.J., Lee B.H., Rhim H., Kim H.C. Bioactive lipids in gintonin-enriched fraction from ginseng. J Ginseng Res. 2019;43(2):209–217. doi: 10.1016/j.jgr.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chei S., Song J.H., Oh H.J., Lee K., Jin H., Choi S.H. Gintonin-enriched fraction suppresses heat stress-induced inflammation through LPA receptor. Molecules. 2020;25(5) doi: 10.3390/molecules25051019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chei S., Oh H.J., Jang H., Lee K., Jin H., Choi Y. Korean red ginseng suppresses the expression of oxidative stress response and NLRP3 inflammasome genes in aged C57BL/6 mouse ovaries. Foods. 2020;9(4) doi: 10.3390/foods9040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L. Characterization of Korean red ginseng (panax ginseng meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39(4):384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang S.H., Shin E.J., Shin T.J., Lee B.H., Choi S.H., Kang J. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, attenuates Alzheimer's disease-related neuropathies: involvement of non-amyloidogenic processing. J Alzheimers Dis. 2012;31(1):207–223. doi: 10.3233/JAD-2012-120439. [DOI] [PubMed] [Google Scholar]
- 26.Pyo M.K., Choi S.H., Shin T.J., Hwang S.H., Lee B.H., Kang J. A simple method for the preparation of crude gintonin from ginseng root, stem, and leaf. J Ginseng Res. 2011;35(2):209–218. doi: 10.5142/jgr.2011.35.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi S.H., Jung S.W., Kim H.S., Kim H.J., Lee B.H., Kim J.Y. A brief method for preparation of gintonin-enriched fraction from ginseng. J Ginseng Res. 2015;39(4):398–405. doi: 10.1016/j.jgr.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi S.H., Hong M.K., Kim H.J., Ryoo N., Rhim H., Nah S.Y. Structure of ginseng major latex-like protein 151 and its proposed lysophosphatidic acid-binding mechanism. Acta Crystallogr D Biol Crystallogr. 2015;71(Pt 5):1039–1050. doi: 10.1107/S139900471500259X. [DOI] [PubMed] [Google Scholar]
- 29.Lee B.H., Kim H.K., Jang M., Kim H.J., Choi S.H., Hwang S.H. Effects of gintonin-enriched fraction in an atopic dermatitis animal model: involvement of autotaxin regulation. Biol Pharm Bull. 2017;40(7):1063–1070. doi: 10.1248/bpb.b17-00124. [DOI] [PubMed] [Google Scholar]
- 30.Choi J.H., Jang M., Oh S., Nah S.Y., Cho I.H. Multi-target protective effects of gintonin in 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Mediated model of Parkinson's disease via lysophosphatidic acid receptors. Front Pharmacol. 2018;9:515. doi: 10.3389/fphar.2018.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farini A., Sitzia C., Villa C., Cassani B., Tripodi L., Legato M. Defective dystrophic thymus determines degenerative changes in skeletal muscle. Nat Commun. 2021;12(1):2099. doi: 10.1038/s41467-021-22305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bektas A., Schurman S.H., Sen R., Ferrucci L. Human T cell immunosenescence and inflammation in aging. J Leukoc Biol. 2017;102(4):977–988. doi: 10.1189/jlb.3RI0716-335R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H.X., Pan W., Zheng L., Zhong X.P., Tan L., Liang Z. Thymic epithelial cells contribute to thymopoiesis and T cell development. Front Immunol. 2019;10:3099. doi: 10.3389/fimmu.2019.03099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dutta S., Sengupta P. Men and mice: relating their ages. Life Sci. 2016;152:244–248. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 36.Anthony T.G. Mechanisms of protein balance in skeletal muscle. Domest Anim Endocrinol. 2016;56(Suppl):S23–S32. doi: 10.1016/j.domaniend.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tipton K.D., Hamilton D.L., Gallagher I.J. Assessing the role of muscle protein breakdown in response to nutrition and exercise in humans. Sports Med. 2018;48(Suppl 1):53–64. doi: 10.1007/s40279-017-0845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim C., Hwang J.K. The 5,7-dimethoxyflavone suppresses sarcopenia by regulating protein turnover and mitochondria biogenesis-related pathways. Nutrients. 2020;12(4) doi: 10.3390/nu12041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mauricio S.F., de Vasconcelos Generoso S., Leandro Marciano Vieira E., Xiao J., Prado C.M., Gonzalez M.C. Relationship between sarcopenia and mTOR pathway in patients with colorectal cancer: preliminary report. Nutr Cancer. 2019;71(1):172–177. doi: 10.1080/01635581.2018.1540716. [DOI] [PubMed] [Google Scholar]
- 40.Yoon M.S. mTOR as a key regulator in maintaining skeletal muscle mass. Front Physiol. 2017;8:788. doi: 10.3389/fphys.2017.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andre L.M., Ausems C.R.M., Wansink D.G., Wieringa B. Abnormalities in skeletal muscle myogenesis, growth, and regeneration in myotonic dystrophy. Front Neurol. 2018;9:368. doi: 10.3389/fneur.2018.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charge S.B., Rudnicki M.A. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 43.Lu J., McKinsey T.A., Zhang C.L., Olson E.N. Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol Cell. 2000;6(2):233–244. doi: 10.1016/s1097-2765(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 44.Abrigo J., Rivera J.C., Aravena J., Cabrera D., Simon F., Ezquer F. High fat diet-induced skeletal muscle wasting is decreased by mesenchymal stem cells administration: implications on oxidative stress, ubiquitin proteasome pathway activation, and myonuclear apoptosis. Oxid Med Cell Longev. 2016;2016:9047821. doi: 10.1155/2016/9047821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee C., Jeong H., Lee H., Hong M., Park S.Y., Bae H. Magnolol attenuates cisplatin-induced muscle wasting by M2c macrophage activation. Front Immunol. 2020;11:77. doi: 10.3389/fimmu.2020.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adams G.R. Insulin-like growth factor in muscle growth and its potential abuse by athletes. Br J Sports Med. 2000;34(6):412–413. doi: 10.1136/bjsm.34.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beyer I., Mets T., Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15(1):12–22. doi: 10.1097/MCO.0b013e32834dd297. [DOI] [PubMed] [Google Scholar]
- 48.Haynes L., Maue A.C. Effects of aging on T cell function. Curr Opin Immunol. 2009;21(4):414–417. doi: 10.1016/j.coi.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bredenkamp N., Nowell C.S., Blackburn C.C. Regeneration of the aged thymus by a single transcription factor. Development. 2014;141(8):1627–1637. doi: 10.1242/dev.103614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castilho J.L., Shepherd B.E., Koethe J., Turner M., Bebawy S., Logan J. CD4+/CD8+ ratio, age, and risk of serious noncommunicable diseases in HIV-infected adults on antiretroviral therapy. AIDS. 2016;30(6):899–908. doi: 10.1097/QAD.0000000000001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macaulay R., Akbar A.N., Henson S.M. The role of the T cell in age-related inflammation. Age (Dordr) 2013;35(3):563–572. doi: 10.1007/s11357-012-9381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moro-Garcia M.A., Mayo J.C., Sainz R.M., Alonso-Arias R. Influence of inflammation in the process of T lymphocyte differentiation: proliferative, metabolic, and oxidative changes. Front Immunol. 2018;9:339. doi: 10.3389/fimmu.2018.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coder B.D., Wang H., Ruan L., Su D.M. Thymic involution perturbs negative selection leading to autoreactive T cells that induce chronic inflammation. J Immunol. 2015;194(12):5825–5837. doi: 10.4049/jimmunol.1500082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bruunsgaard H., Pedersen M., Pedersen B.K. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8(3):131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Rong Y.D., Bian A.L., Hu H.Y., Ma Y., Zhou X.Z. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018;18(1):308. doi: 10.1186/s12877-018-1007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J., Leung K.S., Chow S.K., Cheung W.H. Inflammation and age-associated skeletal muscle deterioration (sarcopaenia) J Orthop Translat. 2017;10:94–101. doi: 10.1016/j.jot.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bruunsgaard H., Skinhoj P., Pedersen A.N., Schroll M., Pedersen B.K. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol. 2000;121(2):255–260. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferrucci L., Corsi A., Lauretani F., Bandinelli S., Bartali B., Taub D.D. The origins of age-related proinflammatory state. Blood. 2005;105(6):2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bruunsgaard H., Andersen-Ranberg K., Jeune B., Pedersen A.N., Skinhoj P., Pedersen B.K. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54(7):M357–M364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]