Abstract
Background
Schizophrenia is a serious mental illness that affects more than 21 million people worldwide. Both genetics and the environment play a role in its etiology and pathogenesis. Symptoms of schizophrenia are mainly categorized into positive, negative, and cognitive. One major approach to identify and understand these diverse symptoms in humans has been to study behavioral phenotypes in a range of animal models of schizophrenia.
Objective
We aimed to provide a comprehensive review of the behavioral tasks commonly used for measuring schizophrenia-like behaviors in rodents together with an update of the recent study findings.
Methods
Articles describing phenotypes of schizophrenia-like behaviors in various animal models were collected through a literature search in Google Scholar, PubMed, Web of Science, and Scopus, with a focus on advances over the last 10 years.
Results
Numerous studies have used a range of animal models and behavioral paradigms of schizophrenia to develop antipsychotic drugs for improved therapeutics. In establishing animal models of schizophrenia, the candidate models were evaluated for schizophrenia-like behaviors using several behavioral tasks for positive, negative, and cognitive symptoms designed to verify human symptoms of schizophrenia. Such validated animal models were provided as rapid preclinical avenues for drug testing and mechanistic studies.
Conclusion
Based on the most recent advances in the field, it is apparent that a myriad of behavior tests are needed to confirm and evaluate the congruency of animal models with the numerous behaviors and clinical signs exhibited by patients with schizophrenia.
Keywords: Schizophrenia, symptom, behavioral task, animal model, genetic, environment
1. INTRODUCTION
Schizophrenia, a serious mental illness with a lifetime worldwide prevalence of 0.4% [1], affects more than 21 million people [2]. Schizophrenia is a complex neuropsychiatric disorder whose etiology and pathogenesis have been attributed to both genetics and the environment [3]. Symptoms ordinarily begin between the late teens and early thirties [1]. Symptoms of schizophrenia have been typically categorized into three clusters: positive, negative, and cognitive [4, 5]. Positive symptoms involve hallucinations (false perceptions), delusions (abnormal beliefs), disorganized thinking, and experiences that are not characteristic of a normal mental
state [5-9]. Negative symptoms comprise social withdrawal, lack of motivation, impoverished speech, emotional blunting, and abnormalities in social interaction, which are all signs representing deficits in normal social functions [7-10]. Lastly, cognitive symptoms include impairments in working memory, attention, and executive function [5, 7-13]. Currently, antipsychotic drugs are mainly effective against positive symptoms, showing little therapeutic success in mitigating negative and cognitive symptoms [9, 10, 13, 14]. Even then, existing antipsychotics against positive symptoms have limited efficacy and have harmful side-effects [15].
To date, the etiology of schizophrenia remains unclear. Furthermore, there are no biological markers for the diagnosis of schizophrenia, and patient diagnosis is only based on an established set of clinical symptoms [16, 17]. In addition, medication selection and assessment of treatment, prognosis, and life functioning of people with schizophrenia are primarily focused on clinical signs [18, 19]. Hence, the confirmation of these clinical symptoms in human patients is of the utmost importance. Various animal models are needed to aid in the identification of these diverse symptoms, which may be achieved through more rapid monitoring of disease progression than is feasible in humans [4]. Nonetheless, it is difficult to fully reproduce symptoms of schizophrenia in experimental animals [8]. Although there are individual variances in the overall brain anatomy, the gross anatomy of the brain, including long-range neural projections as well as many of the neuronal and molecular pathways underlying brain function, is evolutionarily conserved among rodents and humans [7, 20]. Moreover, there remain commonalities in behavioral abnormalities between rodents and humans, with conserved circuitry [20]. Animal models of schizophrenia should fulfill the three main criteria of face, construct, and predictive validity for the disorder [21]. Face validity represents how well the animal model mimics the symptoms of schizophrenia in human patients; construct validity describes the conformity of the model’s pathophysiology and etiology with those proposed in human schizophrenia; and predictive validity indicates the expected response to established and novel therapeutics [4, 5, 21, 22]. Consequently, animal models of schizophrenia should be devised based on construct validity and evaluated according to both face and predictive validities [5]. In particular, face and predictive validities are confirmed by subjecting the model animals to various behavioral tasks [23]. Preclinical and clinical studies have thus established similar behavioral tasks for laboratory animals and human patients with the primary objective of correctly studying homologous actions in both organisms [7]. Within the past 10 years, multiple review papers have examined the ways in which schizophrenia is constructed and modeled in animals [4, 21, 24-37]. In contrast, to the authors’ knowledge, no review papers have attempted to elucidate the various behavioral tasks employed to evaluate these animal models in the same encompassing scope and detail. The primary goal of this review paper was to fill this gap by providing a comprehensive but concise account of how to confirm schizophrenia-like behavior in animal models.
2. BEHAVIORAL TASKS MEASURING POSITIVE SYMPTOMS OF SCHIZOPHRENIA
Schizophrenia's key positive symptoms include paranoia, visions, disorganized perception, and traits that are uniquely human. A subgroup of patients with schizophrenia also shows psychomotor agitation, which includes hyperactivity or increased stereotypic movements [38, 39]. Due to its face validity and simple quantification compared to other positive symptoms, the measurement of locomotor activity has been extensively used to elucidate the positive symptom of schizophrenia in preclinical fields [23]. However, this symptom is not exclusive to schizophrenia, as patients with several neuropsychiatric disorders such as attention deficit disorder and bipolar disorder also exhibit psychomotor agitation [20, 40]. The involvement of dopamine in movement control is well known, and psychomotor agitation in schizophrenia could be caused by hyperdopaminergic activity. Thus, enhanced dopaminergic activity in rodents results in hyperlocomotion, including greater horizontal movement, rearing, and a range of stereotyped behaviors [23]. Furthermore, patients with schizophrenia show a deficit in sensorimotor gating, which is widely accepted as an endophenotype of schizophrenia [41]. The impairment causes oversensitivity to sensory stimulation resulting in cognitive fragmentation and cognitive disorders [42]. The deficit in sensorimotor gating is thus considered to be an “interface of psychosis and cognition” rather than a simple positive symptom [43]. Here, we describe the behavioral tasks most commonly used to measure positive symptoms, namely the open field test for evaluating locomotor activity and prepulse inhibition (PPI) for evaluating sensorimotor gating.
2.1. Open Field Test
Locomotor activity describes the ambulatory movement of a species in a given environment. Usually, it is measured by using the open field test, which tests subjects by placing them in a confined space and subsequently determining the distance they traveled and time they spent traveling over the total duration of the experiment [40]. This can be conveniently measured using automated photocell cages or scored by observation. More complex methodology includes measurement of the ethological range of natural behaviors and qualitative analysis of behavior patterns and perseverative features [44]. In mouse models, a typical method of locomotor activity measurement entails the placement of the animal in a rectangular acrylic arena, with transparent walls, that is completely novel to the animal. Mice are allowed to freely explore the area for a set amount of time while their movements are manually or automatically recorded [44].
In various established models of schizophrenia, animals display increased locomotor activity, either at the baseline level or in response to a novel environment. Table 1 lists recent articles on locomotor activity in several types of animal models, including developmental, genetic, and pharmacological models. In the developmental model, maternal immune activation (MIA) by the inflammatory agent polyinosinic-polycytidylic acid (poly(I:C)) during pregnancy [45] and perinatal levodopa (L-dopa) treatment in juvenile female mice [46] induced hyperlocomotion in the offspring. Various genetic models of schizophrenia have also shown different locomotion results depending on which gene is manipulated. Most genetically induced animal models of schizophrenia display an increase in locomotor activity in various conditions. Such conditions include Sterol regulatory element-binding protein 1c (SREBP1c) knockout (KO) mice [47], G protein-coupled receptor 88 (GPR88) KO mice [48], dystrobrevin binding protein 1 (Dtnbp1) deficiency mice [49], knockdown of metabotropic glutamate receptor 5 (mGluR 5) mice [50], N-methyl-D-aspartate (NMDA) receptor ablation mice [51], brain-specific collapsin response mediator protein 2 (CRMP2) KO mice [52], lq21.1 hemizygous microdeletion (hemizygotic Df(h1q21)/+) mice + amphetamine [53], dopamine transporter (DAT) KO mice [54], and glial glutamate and aspartate transporter (GLAST) KO mice [55]. However, no effect on locomotor activity was observed in animal models with reduced solute carrier family 1 member 1 (SLC1A1) expression [56], growth arrest-specific 7 (GAS7) deficiency mice [57] (however, this model has decreased sensorimotor gating, as measured by PPI), type III neuregulin-1 (NRG1) +/− male mice from mutant fathers [58] (however, this model has increased sociability in the three-chamber test), and selective knockdown mice of phospholipase C-β1 (PLC-β1) in the medial prefrontal cortex (mPFC) [59]. Pharmacologically, several drugs, including amphetamine and MK-801 (NMDA receptor (NMDAR) antagonist) increase locomotor activity but can inversely lead to a decrease at higher doses due to sedative and anesthetic effects [23]. Recently, Catha edulis forsk (khat plant) extract [60], hM4D-mediated inhibition of glutamic acid decarboxylase 65 (GAD65) neurons in the ventral hippocampus (vHPC) [61], and phencyclidine (PCP) + Δ9-tetrahydrocannabinol (Δ9-THC) treatment [62] were also shown to induce schizophrenia-like hyperlocomotion behavior.
Table 1. Recent studies using behavioral paradigms that measure positive symptoms of schizophrenia in rodent models.
| Paradigm | Animal Model | Experimental Manipulation | Results | References |
|---|---|---|---|---|
| Open field test | Developmental | Poly(I:C)-induced MIA mouse offspring | ↑ increased locomotion | [45] |
| Perinatal L-Dopa treatment in female juvenile mice | ↑ increased locomotion | [46] | ||
| Genetic | SREBP1c KO mice | ↑ increased locomotion | [47] | |
| GPR88 KO mice | ↑ increased locomotion | [48] | ||
| Dtnbp1 deficient mice | ↑ increased locomotion | [49] | ||
| mGlu5 KO mice | ↑ increased locomotion | [50] | ||
| NMDA receptor ablated mice | ↑ increased locomotion | [51] | ||
| Brain-specific CRMP2 KO mice | ↑ increased locomotion | [52] | ||
| Df(h1q21)/+ mice (1q21.1 hemizygous microdeletion) | ↑ increased locomotion | [53] | ||
| DAT KO mice | ↑ increased locomotion | [54] | ||
| GLAST KO mice | ↑ increased locomotion | [55] | ||
| Reduced SLC1A1 expression in mice | NE | [56] | ||
| GAS7 deficient mice | NE | [57] | ||
| Type III NRG1+/− male mice from the mutant fathers | NE | [58] | ||
| Selective mPFC PLC-β1 knockdown mice | NE | [59] | ||
| Pharmacological | Amphetamine (1 and 5 mg/kg) or MK-801 (0.01 and 0.05 mg/kg),Higher doses of amphetamine (25 mg/kg) or MK-801 (0.25 mg/kg) administration in mice | ↑ increased locomotionNE | [23] | |
| Administration of Catha edulis forsk extract in mice | ↑ increased locomotion | [60] | ||
| hM4D-mediated inhibition of GAD65 interneurons in the mouse vHPC | ↑ increased locomotion | [61] | ||
| PCP + Δ9-THC treated mice | ↑ increased locomotion | [62] | ||
| Prepulse inhibition | Developmental | Poly(I:C)-induced MIA mouse offspring | ↓ decreased inhibition | [70] |
| Genetic | SREBP1c KO mice | ↓ decreased inhibition | [47] | |
| NAc-TMEM mice | ↓ decreased inhibition | [80] | ||
| GPR88 KO mice | ↓ decreased inhibition | [48] | ||
| Dtnbp1 deficient mice | ↓ decreased inhibition | [49] | ||
| Df(h1q21)/+ mice (1q21.1 hemizygous microdeletion + amphetamine) | ↓ decreased inhibition | [53] | ||
| Chakragati mice | ↓ decreased inhibition | [72] | ||
| mGlu5 KO mice | ↓ decreased inhibition | [50] | ||
| GAS7 deficient mice | ↓ decreased inhibition | [57] | ||
| DAT KO mice | ↓ decreased inhibition | [54] | ||
| Nlgn2 R215H KI homozygous mice | ↑ increased inhibition | [73] | ||
| Selective mPFC PLC-β1 knockdown mice | NE | [59] | ||
| Type III NRG1+/−□male mice from mutant fathers | NE | [58] | ||
| Reelin-deficient + corticosterone-treated mice | NE | [71] | ||
| Pharmacological | hM4D-mediated inhibition of parvalbumin interneurons in the mouse vHPC | ↓ decreased inhibition | [61] | |
| PCP+ Δ9-THC treated mice | ↓ decreased inhibition | [62] | ||
| hM4D-mediated inhibition of GAD65 interneurons in the mouse vHPC | NE | [61] |
Abbreviations: CRMP2 = collapsin response mediator protein 2; DAT = dopamine transporter; Dtnbp1 = dystrobrevin binding protein; GAD65 = glutamic acid decarboxylase 65; GAS7 = growth arrest-specific 7; GLAST = glial glutamate and aspartate transporter; GPR88 = G protein-coupled receptor 88; KO = knockout; L-dopa = levodopa; mGluR5 = metabotropic glutamate receptor 5 = MIA, maternal immune activation; mPFC = medial prefrontal cortex; NAc-TMEM mice = Tmem168 vector was injected into the NAc of C57BL/6J mice; Nlgn2 R215H = missense mutation R215H of neuroligin 2; NMDA = N-methyl-D-aspartate; NRG1 = neuregulin-1; PCP = phencyclidine; PLC-β1 = phospholipase C-β1; poly(I:C) = polyinosinic-polycytidylic acid; SLC1A1 = Solute Carrier Family 1 Member 1; SREBP1c = sterol regulatory element-binding protein 1c; vHPC = ventral hippocampus; Δ9-THC = Δ9-tetrahydrocannabinol; NE = no effect.
In most of these recent studies, the consensus finding was an increase in locomotor activity. This hyperlocomotion phenotype was generated through varied means, mostly genetic manipulations and environmental alterations, or the interaction between the two. Genetic manipulations have mainly focused on the alteration of genes that were previously implicated as risk factors for schizophrenia in human patients [47-49, 52, 56, 59] or shown to play a role in NMDAR dysfunction [50, 51]. Additionally, some studies have also suggested the involvement of alterations in the dopaminergic [53, 54] and γ-aminobutyric acid (GABA)ergic systems [61] in inducing schizophrenia-linked hyperlocomotion. The locomotor phenotype of rodent models of schizophrenia has been further elucidated by previous review papers [40, 63, 64] where other mechanisms are discussed in detail.
2.2. Prepulse Inhibition
As a common measure of sensorimotor gating, PPI is extensively accepted as one of the most important parameters in assessing the genetic base of schizophrenia [65] . PPI is the level of startle reflex reduction when a non-startling (weak initial) acoustic stimulus is presented prior to a startling (strong) stimulus [42, 66, 67]. In brief, PPI can be measured by recording the animal response in the presence or absence of a weaker, non-startling prepulse that precedes the startling pulse by a short delay. One of the primary advantages of PPI measurement is its translatability between mice and humans, as it is one of the few tests that are essentially conserved across all vertebrate species [41]. PPI results are reproducible and convenient to measure, as most set-ups are automatic. Furthermore, experiments do not require prior subject training or any special preparation [40, 68]. Although there is general agreement in the literature regarding the protocols and equipment used, it is important to note that some factors may confound the results of PPI such as environment, age, sex, inter-stimulus intervals, stimulus modalities, treatment doses, and animal strains [23, 69].
The various factors found to be associated with deficiency in sensorimotor gating include the developmental stage [70], stress [71], as well as risk factors of various types [48] including genetic risk factors previously established in patients with schizophrenia [53, 71-73]. Previous reviews have extensively summarized and discussed the use of PPI in animal models of schizophrenia in terms of genetic [23, 41, 65, 74-77] and pharmacological [78, 79] manipulations. Many recent studies have also clarified schizophrenia-like behaviors by measuring the deficiency of sensorimotor gating through PPI in several types of animal models (Table 1).
A recent report has found that a developmentally-induced animal model of BALB/c mouse offspring by poly(I:C) maternal injection exhibited impaired PPI [70]. Moreover, most recent studies involving genetically induced animal models of schizophrenia have revealed a deficit in PPI. The animal models included SREBP-1c KO mice [47], C57BL/6J mice with adeno-associated virus Tmem168 vector injected into the nucleus accumbens (NAc-TMEM mice) [80], GPR88 KO mice [48], Dtnbp1 deficient mice [49], lq21.1 hemizygous microdeletion (hemizygotic Df(h1q21)/+ mice) plus amphetamine [53], chakragati mice [72], mGlu5 KO mice [50], GAS7-deficient mice [57], and DAT KO mice [54]. However, some genetically manipulated models have revealed an increase in PPI or no effect. These models include missense mutation R215H of neuroligin 2 (Nlgn2 R215H) knock-in (KI) homozygous mice [73], selective knockdown mice of phospholipase C-β1 (PLC-β1) in the medial prefrontal cortex (mPFC) [59], type III neuregulin-1 (NRG1) +/− male mice from fathers with the mutation (however, this model shows increased sociability in the three-chamber test) [58], and reelin-deficient and corticosterone-treated mice (however, this model shows increased sociability in the three-chamber test and decreased spontaneous alternation in the Y-maze) [71]. Pharmacologically, hM4D-mediated inhibition of parvalbumin neurons in vHPC [61] and phencyclidine (PCP) + Δ9-THC treatment [62] also resulted in decreased PPI in mice. However, some pharmacological animal models of schizophrenia show no change in PPI, such as mice with hM4D-mediated inhibition of GAD65 interneurons in the vHPC (however, this model shows increased locomotor activity and decreased spontaneous alternation in the Y-maze) [61].
Table 1 shows that various developmental, genetic, or pharmacological animal models of schizophrenia that exhibit positive symptoms, including hyperlocomotion and deficiency of sensorimotor gating. In general, within the past 10 years, most studies of animal models utilizing the locomotor activity and PPI paradigms have employed genetic followed by pharmacological and developmental models. Numerous studies attempting to characterize their animal models of schizophrenia primarily measure locomotor activity and sensorimotor gaiting using the open field test and PPI, respectively (Fig. 1), to prove the presence of positive symptoms or congruent behaviors [42, 75, 81]. Particularly, PPI is considered a gold standard for measuring schizophrenia-like impairment in animal models [82]. Hence, among the symptoms of schizophrenia, behavioral tests measuring positive symptoms have been well described. The neural basis for these has been well reported through the development of different animal models and subsequent drug trials.
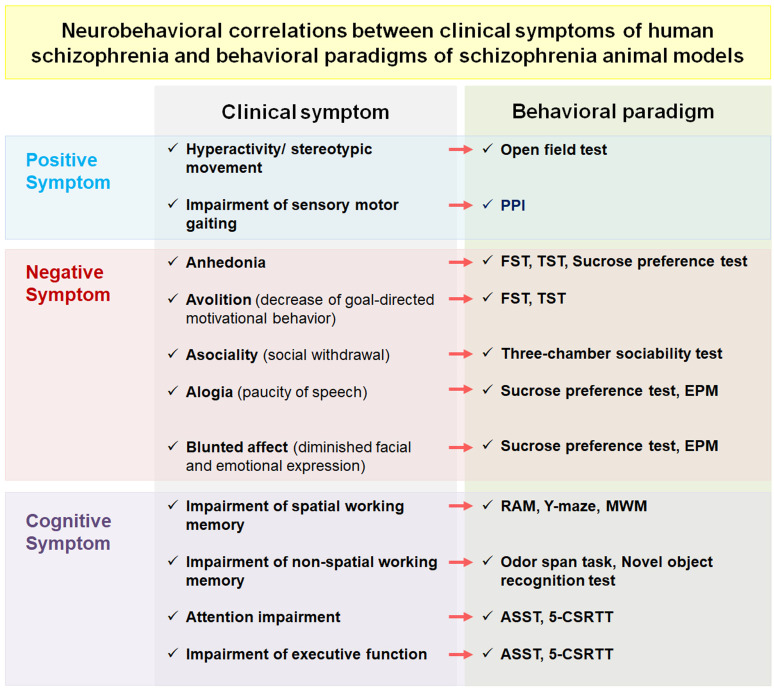
Fig. (1).
Schematic flow diagram of neurobehavioral correlations between clinical symptoms of human patients with schizophrenia and behavioral paradigms of schizophrenia animal models. Symptoms of schizophrenia in human patients have been translated into behavioral versions in various animal models to elucidate the etiology and precise mechanisms of schizophrenia. Abbreviations: PPI, prepulse inhibition test; FST, forced swim test; TST, tail suspension test; EPM, elevated plus maze; RAM, radial arm maze; MWM, Morris water maze; ASST, attentional set-shifting task; 5-CSRTT, 5-choice serial reaction time task. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3. BEHAVIORAL TASKS MEASURING NEGATIVE SYMPTOMS OF SCHIZOPHRENIA
Negative symptoms, also known as defect symptoms of schizophrenia, are characterized by a reduction in normal functioning, which leads to disability and reduced quality of life [83]. These symptoms are generally considered to reflect features such as anhedonia (inability to experience pleasure from positive stimuli), avolition (decreased goal-directed motivational behavior), asociality (social withdrawal), alogia (paucity of speech), and blunted affect (diminished facial and emotional expression) [84-87]. Although the mechanisms underlying the negative symptoms are complex and not well elucidated, recent studies have suggested the hypofunction of NMDA, more specifically, the reduced activation of the NMDAR subtypes as a mechanism implicated in the pathophysiology of negative symptoms [88, 89]. NMDA hypofunction of cortical interneurons could lead to the activation of GABAergic interneurons in the ventral tegmental area [90]. The increased interneuron activity could decrease adequate dopamine release in the PFC by inhibiting stimulation of the mesocortical dopaminergic pathway, and could thus contribute to the negative symptoms [91]. Because most therapeutic drugs for schizophrenia are dopaminergic antagonists, they are mainly effective against positive symptoms, only showing low efficacy against negative symptoms [5, 92, 93]. Therefore, cautiously proven animal models combined with suitable evaluations of negative symptoms are key to the development of new therapeutic strategies for these symptoms [94]. Certain negative symptoms, such as alogia, however, are virtually impossible to reproduce in laboratory animals; thus, such behavior may be uniquely human [83]. In contrast, at least theoretically, anhedonia, avolition, and asociality may be experienced by both humans and animals and can therefore be replicated in a mouse model [84]. Here, we describe several common tasks to assess negative symptoms in animal models of schizophrenia, such as the forced swim test (FST), tail suspension test (TST), sucrose preference test, three-chamber sociality test, and elevated plus maze (EPM).
3.1. Forced Swim Test
Swimming, or the lack thereof, in the FST is one of the most well-known animal behavioral tasks utilized for assessing the effect of potential anti-depressant drugs [95]. Trained observers measure the immobility time of subject animals in a transparent acrylic vessel containing water over a period of several minutes. The animal is considered immobile when it remains floating only performing the movements necessary to stay afloat and retain its head above water [96]. The FST and other behavioral paradigms used to evaluate depression-like behaviors have also been applied to assess negative symptoms of schizophrenia, including anhedonia and avolition [84].
Many studies have utilized the FST in past years, and numerous factors have been associated with increased immobility in animal models of schizophrenia. Such factors include prenatal MIA [97], genetic factors that were found to be related to schizophrenia in human patients, such as transcription factors [98], other schizophrenia-associated genes [99], receptor-associated proteins [100], compromised glutathione synthesis [101], reduced brain serotonin levels [102], deregulated monoaminergic signaling [103], and prefrontal cortex dysfunction [104]. As shown in Table 1, recent studies using animal models of schizophrenia have shown various results with the FST. Developmentally, lipopolysaccharide (LPS)-treated Naval Medical Research Institute (NMRI) mouse offspring also exhibited increased immobility time [97]. A general increase in immobility time was observed in genetically induced animal models, including Engrailed-2 (En2) KO mice [98], prostaglandin E2 type 4 (EP4) receptor-associated protein (EPRAP) KO mice [100], neuronal calcium sensor-1 (NCS-1) KO mice [105], neurotensin (NT) KO mice [104], and heterozygous reeler mice with corticosterone [99]. However, several genetic schizophrenic models have shown decreased immobility time in the FST, namely GRIA1 KO mice [106], anaplastic lymphoma kinase (ALK) KO homozygous mice [103], glutamate-cysteine ligase modifier (GCLM) KO mice [101], DBA/2J mice [102], and receptor protein tyrosine phosphatase gamma (PTPRG) KO mice [107]. Lastly, Grin1(Rgsc174)/Grin1+ mice, a heterozygous mutant strain with a non-synonymous mutation of the C to T transition in exon 18 of the coding ionotropic glutamate receptor NMDA1 (Grin1) gene, revealed no effect on immobility time [108]. Pharmacologically induced models that exhibited an increase in immobility time include ketamine [109, 110], MK-801 [111, 112], and PCP [113], which are all non-competitive NMDAR antagonists. Overall, taking into consideration the 10-year span of the review article, most animal studies utilizing the FST employed mainly genetic followed by pharmacological and developmental models.
Consequently, previous studies have evaluated and discussed in detail the specific factors usually affecting the FST and providing justification for its common use in measuring negative symptoms of schizophrenia, whereas others have argued about its “validity” as a measure of depression-like behavior [114]. Yet, other studies have explored the validity of this test through the use of several antidepressants [115] but have also discussed its ability to measure psychomotor retardation [116].
3.2. Tail Suspension Test
Similar to the fst, the tst is used as an evaluation paradigm for depression-like behavior [117] and anhedonia and avolition, which are negative symptoms of schizophrenia in mice [84]. Mice are suspended by their tails with tape in such a way that they cannot escape or hang on nearby surfaces [118]. The principal response of animals is to struggle, but this is followed by periods of immobility when mice are dangling passively and entirely stationary [119]. Previous reviews have evaluated this test in animal models of depression [120], detailed the methodology [118], and evaluated the effects of several antidepressants and opioids in this assay [121].
Recently, in some developmental animal models of schizophrenia, increased immobility time was observed in LPS-treated NMRI mouse offspring [97] and prenatal LPS-exposed mice [122]. Genetically, increased immobility time was observed in SREBP1c KO mice [47], forebrain-specific conditional neural cell adhesion molecule (NCAM)-deficient mice [123], male membrane-bound catechol-O-methyltransferase (MB-COMT)-deficient mice [124], NCS-1 KO mice [105], and NT KO mice [104]. Conversely, decreased immobility was observed in GRIA1 KO [106] and DBA/2J mice [102]. However, no effect was seen in Grin1(Rgsc174)/Grin1+ mice [108]. Pharmacologically, increased immobility was seen in mice treated with a subchronic ketamine regimen (5 days, 10 mg/kg/day, intraperitoneal) [110], whereas no effect was observed in mice with chronic administration of ketamine (10 days, 100 mg/kg/day, intraperitoneal) [119] and subchronic PCP administration (10 days, 10 mg/kg/day, subcutaneous) (however, this model shows decreased spontaneous alternation in the Y-maze and decreased working memory in the novel object test) [125]. Collectively, there was a general increase in immobility time in the schizophrenia-related animal models discussed above (Table 2). As with the other behavioral assays for negative symptoms, genetic risk factor alterations were the prime cause of the increase in immobility time followed by pharmacological and developmental alterations.
Table 2. Recent studies using behavioral paradigms that measure negative symptoms of schizophrenia in rodent models.
| Paradigm | Animal Model | Experimental Manipulation | Results | References |
|---|---|---|---|---|
| Forced swim test | Developmental | LPS-treated NMRI mouse offspring | ↑ increased immobility | [97] |
| Genetic | En2 null mutant mice | ↑ increased immobility | [98] | |
| EPRAP KO mice | ↑ increased immobility | [100] | ||
| NCS-1 KO mice | ↑ increased immobility | [105] | ||
| NT KO mice | ↑ increased immobility | [104] | ||
| Heterozygous reeler mice + corticosterone | ↑ increased immobility | [99] | ||
| GRIA1 KO mice | ↓ decreased immobility | [106] | ||
| ALK KO homozygous mice | ↓ decreased immobility | [103] | ||
| GCLM KO mice | ↓ decreased immobility | [101] | ||
| DBA/2J mice | ↓ decreased immobility | [102] | ||
| PTPRG KO mice | ↓ decreased immobility | [107] | ||
| Grin1(Rgsc174)/Grin1+ mice | NE | [108] | ||
| Pharmacological | Chronic administration of ketamine in mice | ↑ increased immobility | [109] | |
| Subchronic treatment with ketamine in mice | ↑ increased immobility | [110] | ||
| 13-day treatment with MK-801 in mice | ↑ increased immobility | [112] | ||
| 15-day treatment with MK-801 in mice | ↑ increased immobility | [111] | ||
| PCP treatment in mice | ↑ increased immobility | [113] | ||
| Tail suspension test | Developmental | LPS-treated NMRI mouse offspring | ↑ increased immobility | [97] |
| Prenatal LPS-exposed mice | ↑ increased immobility | [122] | ||
| Genetic | SREBP1c KO mice | ↑ increased immobility | [47] | |
| Forebrain-specific NCAM-deficient mice | ↑ increased immobility | [123] | ||
| Male MB-COMT deficient mice | ↑ increased immobility | [124] | ||
| NCS-1 KO mice | ↑ increased immobility | [105] | ||
| NT KO mice | ↑ increased immobility | [104] | ||
| GRIA1 KO mice | ↓ decreased immobility | [106] | ||
| DBA/2J mice | ↓ decreased immobility | [102] | ||
| Grin1(Rgsc174)/Grin1+ mice | NE | [108] | ||
| Pharmacological | Subchronic treatment with ketamine in mice | ↑ increased immobility | [110] | |
| Chronic administration of ketamine in mice | NE | [119] | ||
| Subchronic PCP administration in mice | NE | [125] | ||
| Sucrose preference test | Developmental | Viral mimic Poly(I:C)-induced MIA mouse offspring | ↓ decreased preference | [129] |
| Perinatal L-Dopa treatment in male juvenile mice | ↓ decreased preference | [46] | ||
| Genetic | CD2 KO mice | ↓ decreased preference | [130] | |
| G72/G30 Tg mice | ↓ decreased preference | [131] | ||
| Grin1(ΔPV) mice + MK-801 | ↓ decreased preference | [132] | ||
| Grik4 KO mice | ↑ increased preference | [133] | ||
| Ahi1 +/- KO mice + chronic unpredictable stress | NE | [134] | ||
| GluA1 KO mice | NE | [135] | ||
| NRG1 mutant mice + stress | NE | [136] | ||
| Pharmacological | JNJ chronic administration in mice | ↓ decreased preference | [137] | |
| Three-chamber sociality test | Genetic | Brain-specific CRMP 2 knockout (cKO) mice | ↓ decreased social interaction | [52] |
| Pcm1+/− mice | ↓ decreased social interaction | [140] | ||
| SREBP1c KO mice | ↓ decreased social interaction | [47] | ||
| CPB-K mice | ↓ decreased social interaction | [141] | ||
| Glu-CB1(-/-) female mice | ↓ decreased social interaction | [142] | ||
| Reelin deficiency + corticosterone in male mice | ↓ decreased social interaction | [71] | ||
| Sarm1 knockdown mice | ↓ decreased social interaction | [143] | ||
| V1aR KO mice | ↓ decreased social interaction | [144] | ||
| Type III NRG1+/- male mice from mutant fathers | ↑ increased social interaction | [58] | ||
| Selective knockdown of PLC-β1 in mPFC of male mice | NE | [59] | ||
| NRG1 mutant mice | NE | [146] | ||
| Pharmacological | Ketamine treatment in mice | ↓ decreased social interaction | [145] | |
| PCP treatment in mice | ↓ decreased social interaction | [113] | ||
| Administration of Catha edulis forsk extract | ↓ decreased social interaction | [60] | ||
| Elevated plus maze | Genetic | Nlgn2 R215H knock-in homozygous mice | ↑ increased anxiety-like behavior | [73] |
| Heterozygous Ank3 KO mice | ↑ increased anxiety-like behavior | [152] | ||
| Female DISC1(D453G) mice | ↑ increased anxiety-like behavior | [153] | ||
| Kynurenine 3-monooxygenase-deficient mice | ↑ increased anxiety-like behavior | [154] | ||
| NAc-TMEM mice | ↑ increased anxiety-like behavior | [80] | ||
| FFAR1-/- female mice | ↓ decreased anxiety-like behavior | [155] | ||
| Forebrain knockout of DNMT1 in mice | ↓ decreased anxiety-like behavior | [156] | ||
| CRMP2 deficient mice | ↓ decreased anxiety-like behavior | [157] | ||
| Mutant DISC1 male mice | NE | [158] | ||
| Pcm1(+/-) mice | NE | [140] | ||
| Type III NRG1+/- male mice from mutant fathers | NE | [58] |
Abbreviations: Ahi1 = Abelson helper integration site 1; ALK = anaplastic lymphoma kinase; Ank3 = ankyrin 3; CD2 = cyclin-D2; CRMP2 = collapsin response mediator protein 2; DISC1 = disrupted-in-schizophrenia 1; DNMT1, DNA methyltransferase 1; En2 = engrailed-2; EPRAP = EP4 receptor-associated protein; FFAR1 = free fatty acid receptor 1; GCLM = glutamate-cysteine ligase modifier; GLAST = glial glutamate and aspartate transporter; GluA1 = glutamate A1; Glu-CB1 = cannabinoid receptor type 1 in glutamergic neurons; GRIA1 = glutamate ionotropic receptor AMPA type subunit 1; Grik4 = glutamate receptor, ionotropic, kainate 4; Grin1 = glutamate ionotropic receptor NMDA type subunit 1; JNJ = JNJ-28871063; KO = knockout; L-dopa = levodopa; LPS = lipopolysaccharide; MB-COMT = membrane-bound catechol-O-methyltransferase; MIA = maternal immune activation; mPFC = medial prefrontal cortex; NAc-TMEM mice = Tmem168 vector injected into the NAc of C57BL/6J mice; NCAM = neural cell adhesion molecule; NCS-1 = neuronal calcium sensor; Nlgn2 R215H = missense mutation R215H of neuroligin 2; NMRI = Naval Medical Research Institute; NRG1 = neuregulin-1; NT = neurotensin; Pcm1 = pericentriolar material 1; PCP = phencyclidine; PLC-β1 = phospholipase C-β1; poly(I:C) = polyinosinic-polycytidylic acid; PTPRG = receptor protein tyrosine phosphatase gamma; SARM1 = sterile alpha and TIR motif-containing 1; SREBP1c = sterol regulatory element-binding protein 1c; Tg = transgenic; V1AR = vasopressin receptor 1A; NE = no effect.
3.3. Sucrose Preference Test
The sucrose preference test is a compensation dependent test that aims to assess anhedonia, i.e., the failure to feel pleasure, depression, and anxiety [5, 126]. Rodents have been shown to naturally prefer consuming sweet food and sucrose solutions when given a free choice between sucrose solution and plain water [127]. However, when showing signs of stress and depression, rodents fail to show a preference for sweetened water to regular water [127]. The rodents are routinely given the choice of two drinking bottles, one containing plain drinking water and the other containing a sucrose solution. The intake of water and sucrose solutions is then assessed regularly, and sucrose preference is determined as the proportion of the intake of sucrose over the total volume of both sucrose and plain water consumption [126, 128].
Recent studies involving animal models of schizophrenia have shown various responses to this test (Table 2). Decreases in sucrose preference were seen in prenatal immune activated mice using viral mimic poly(I:C) [129], perinatal levodopa (L-dopa) treatment in juvenile male [46], cyclin-D2 (CD2) KO mice [130], G72/G30 transgenic (Tg) mice [131], and parvalbumin interneuron-specific NMDAR 1 (NR1) KO mice (Grin1(ΔPV) mice) with MK-801 treatment [132]. Conversely, an increased preference for sucrose was exhibited in glutamate ionotropic receptor, kainate type subunit 4 KO mice [133]. Meanwhile, no effect was shown in Abelson helper integration site 1 +/− KO mice coupled with chronic unpredictable stress [134], GluA1 KO mice [135], and NRG1 +/− KO mice with repeated psychosocial stress [136]. Pharmacologically, a decreased sucrose preference was seen in adolescent mice chronically treated with JNJ-28871063 (JNJ), a pan-ErbB kinase inhibitor [137].
Recent studies employing this task have reported a general decrease in sucrose preference in animal models related to schizophrenia. The factors that have been identified to lead to this decrease in sucrose preference include increased dopamine levels [46], prenatal immune activation [129], hippocampal parvalbumin-interneuron dysfunction [130], candidate genes for schizophrenia [131], the functional deficit in NMDARs on parvalbumin (PV)-positive interneurons (PV-NMDARs) [132], and pharmacological blocking of the ErbB signaling pathway [137]. An increase in sucrose preference was reported by studies testing novel antidepressants in animal models of schizophrenia [138] and models with dysregulation of glutamatergic signaling [133]. Interestingly, some studies involving schizophrenia models have not reported changes in sucrose preference, including studies employing schizophrenia-related gene mutation [134], glutamergic dysfunction [135], and gene-environment interaction models of schizophrenia [136].
3.4. Three-chamber Sociability Test
Reduced social interaction, lower desire to engage in social communication, and socio-cognitive function deficiencies are among the negative symptoms of schizophrenia related to asociality [85]. While the concepts underlying social interaction measurement in humans and rodents are similar, the measured social interaction contents differ. Thus, social behavior in humans involves a wide range of cognitive structures classified as agonistic, romantic, and affiliate, while sensory modalities in rodents are expressed as visual, olfactory, and auditory in nature [85]. The three-chamber sociability test is a commonly used method to measure sociability function in neuropsychiatric and neurological animal models, including those of schizophrenia [139]. The apparatus consists of three communicating chambers, with both end chambers having wire cups. Under each wire cup, a stranger mouse and inanimate novel object, or a familiar and stranger mouse may be placed. Social interaction is measured as the number and duration of interactions with the stranger mouse and object, or a familiar and stranger mouse. Interactions include time spent in each chamber, sniffing the stranger mouse or novel object, and entries into each chamber as a locomotor control [6].
Most animal models of schizophrenia exhibit a decrease in social interaction (Table 2). Genetically, animal models including CRMP2 KO mice [52], pericentriolar material 1 +/− (Pcm1 +/−) mice [140], SREBP-1c KO mice [47], CPB-K strain mice [141], deletion of cannabinoid receptor type 1 on cortical glutamatergic neurons of female mice [142], reelin-deficient mice treated with corticosterone [71], sterile alpha and TIR motif-containing 1 protein knockdown mice [143], and vasopressin receptor 1A KO mice [144] have shown a decrease in social interaction. Similarly, pharmacologically induced animal models using ketamine treatment [145], PCP [113], and Catha edulis forsk administration [60] have also displayed a decrease in social interaction. Conversely, increased social interaction behavior was found in genetically induced-type III NRG1 +/− males mice from mutant fathers [58]. Meanwhile, no effect in social interaction behavior was found in mice with heterozygous NRG1 mutation [146] and selective knockdown of PLC-β1 in the mPFC [59].
Previous reviews have discussed this paradigm in detail, discussing the methodology [92, 139] and possible strain differences [147]. Moreover, novel assays measuring sociability in mice have also been proposed with the aim of producing an ethologically valid version of the behavioral paradigm [148]. Recent studies have come to the same general conclusion of decreased sociability in rodent models of schizophrenia. The factors causing these impairments in social interaction include the deletion of schizophrenia risk genes [52, 58, 59, 144, 146], including microtubule anchoring proteins [140], innate immunity-related protein alterations [143] in dopaminergic and serotonergic parameters such as neuron number, neuron density, and volume in subregions [141]; cannabinoid receptor deletion [142], stress [71], amygdala dysfunction related to NMDAR-mediated hypofunction [145], and plant extracts with psychoactive components similar to amphetamine [60].
3.5. Elevated Plus Maze
It is challenging to model decreased facial and emotional gestures as negative symptoms of schizophrenia in rodents. Therefore, researchers have reported anti-anxiety behavior in tasks and consider this a measure of blunted affect [84]. The EPM task is seen as the gold standard for measuring rodent anxiety. The test is based on rodents' natural dislike for open and high areas, and their innate spontaneous exploratory behavior in new environments [149-151]. The experimental apparatus consists of open and closed arms that perpendicularly cross each other and share a common center area at their midway. Generally, mice are placed in the center area at the beginning of the trial and thereafter allowed to move freely between areas. The ratios of the frequency and time spent on the open arms versus the closed arms reflect space-induced anxiety in mice [150, 151]. In contrast to other behavioral assays that measure anxiety through the use of noxious stimuli (e.g., electrical stimulation, food/water scarcity, loud noises, interaction with predator odors) and typically triggered programmed reactions, this task relies on the mice's inherent desire to explore new conditions and their propensity toward dim, enclosed areas, normal height, and open-space terror [150].
Changes in anxiety in the EPM in recent studies have been observed mainly in genetic models of schizophrenia (Table 2). Animal models used in recent studies that presented increased anxiety-like behavior was detected in several genetic models, including Nlgn2 R215H KI mice [73], heterozygous ankyrin G-deficient mice [152], missense mutated female mice of D465G in disrupted-in-schizophrenia 1 (DISC1) gene [153], kynurenine 3-monooxygenase-deficient mice [154], and NAc-TMEM mice [80]. Conversely, animal models exhibiting anxiolytic behavior include free fatty acid receptor 1 −/− female mice [155], forebrain KO mice of DNA methyltransferase 1 [156], and CRMP2-deficient mice [157]. Conversely, no effect on anxiety-like behavior was seen in mutant DISC1 male mice [158], Pcm1 +/− mice (however, this model has decreased sociability in the three-chamber test) [140], and type III NRG1 +/− male mice from mutant fathers (however, this model shows increased sociability in the three-chamber test) [58].
Previous reviews have described the methodology [159] of this behavioral assay in detail with the additional assessment of ethological parameters [150, 160]. Others have argued that the fear of heights plays the main role in creating the phobic anxiety state measured in the behavior assay [161]. Yet, another group has recently adapted this task to measure anxiety-like behavior in fish [162]. Recent studies have found that manipulations in schizophrenia risk-related genes involved in neuronal morphogenesis [157], neuronal development, and maintenance of neuronal function [155] and DNA methylation catalytic enzymes [156] have resulted in anxiolytic behavior, as measured by the EPM. In contrast, risk factors causing anxiety include previously reported genetic mutations in patients with schizophrenia [80, 152, 153] and postsynaptic adhesion proteins [73].
Table 2 shows that various developmental, genetic, or pharmacological animal models of schizophrenia that exhibit negative symptoms, including anhedonia, avolition, blunted affect and asociality. Overall, considering the 10-year scope of the review article, most animal studies utilizing the FST and TST have mainly employed genetic followed by pharmacological and developmental models. Conversely, studies utilizing the sucrose preference test were predominantly genetic, followed by studies employing developmental and pharmacological models. In contrast, the three-chamber sociability test has been predominantly utilized for genetic models followed by developmental models. Finally, the EPM has been solely utilized for genetic animal models of schizophrenia. For the negative symptoms of schizophrenia, many tasks have been designed to attempt to replicate the deficiency in normal functioning usually found in patients with schizophrenia [5, 83, 163] (Fig. 1). Among various behavioral paradigms, the FST, TST, and sucrose preference test can be selected for measurement of anhedonia. The FST and TST can be also used for confirming avolition. Additionally, the sucrose preference test and EPM and three-chamber sociability test can be used as measures of blunted affect and asociality, respectively. To confirm phenotypes of anhedonia and blunted affect, the FST and TST and EPM, respectively, have been extensively used as gold standards. Although the sucrose preference test is also widely applied, there is a limitation of the task measuring the behavioral response to a reward because the consumed sucrose levels can be affected by the interest in reward. Variability in the results of behavioral assays falling under this category has been reported in recent years. Although various developmental, genetic, and pharmacological animal models of schizophrenia have presented negative symptoms, there have also been some opposite results. Possibly, the low face validity of animal models for the negative symptoms of schizophrenia might be due to the difficulty in interpreting the results due to various confounding elements. As such, there is a continuous need for better assessments that can lead to improved reliability, validity, and characterization of models for negative symptoms.
4. BEHAVIORAL TASKS MEASURING COGNITIVE SYMPTOMS OF SCHIZOPHRENIA
According to some reports, almost 98% of people with schizophrenia experience cognitive impairment, which is one of the main symptoms of schizophrenia [164]. The cognitive symptoms of schizophrenia reflect deficiencies in different cognitive capacities such as information processing, abstract categorization, executive function, cognitive flexibility, attention, memory, and visual processing [165, 166]. For adequate cognitive control, the coordination of multiple brain regions, including the PFC, medial frontal cortex, and parietal regions, is required [167]. Among them, the PFC is considered to serve a primary role in combining different types of incoming information sourced from various brain regions and supplying top-down processing to coordinate behaviors because it extensively interconnects with sensory, motor, and subcortical brain regions [167]. However, in patients with schizophrenia, occurrence of various structural or molecular defects, such as neurodevelopmental abnormality, changes in synaptogenesis and neuroplasticity, alteration in neuronal maturation, and imbalance of neurotransmitters, has been confirmed in the relevant brain regions [165]. Although the precise mechanisms inducing cognitive symptoms are unclear, the suggested hypothesis is similar to that for negative symptoms. Hypofunctional NMDARs on cortical GABAergic interneurons could lead to an imbalance of neuronal excitation/inhibition and reduced cortical γ-oscillation, associated with the cognitive impairment seen in schizophrenia [168, 169]. Cognitive symptoms are resistant to therapeutics and greatly influence patient routine functioning, therefore providing the best predictor of a patient’s functional status [8, 20, 42, 170].
Meanwhile, the National Institute of Mental Health in the United States of America has defined seven functional areas that are important in the diagnosis of schizophrenia. Such functional areas include attention/vigilance, working memory, logic and problem solving, speed of thinking, visual and memory learning, verbal and memory performance, and social cognition [171]. For use in preclinical studies, specific rodent tasks have been designed in each of these fields to recapitulate the cognitive processes underlying the effective performance of homologous tasks in humans [8, 42]. While parallel tasks between rodents and humans use different sensory modalities, temporal schedules, rewards, and motor requirements, both types require PFC function [42]. In this section, we described several common rodent tasks used to study cognitive symptoms of schizophrenia in all seven proposed cognitive domains, except the previously described one, i.e., social cognition.
4.1. Maze Tests, for Spatial Working Memory
Working memory, one of the key memory functions, is the ability to quickly form memories from unique events and thus discriminate against fresh valid information from older and already invalid memorized evidence [172]. Working memory is dependent on the integrity of prefrontal cortical function and is important for human reasoning and judgment [173]. In patients with schizophrenia, working memory is commonly impaired, which can be measured by clinical working-memory tasks [174]. Preclinical research on rodents employs both spatial and non-spatial working-memory tasks (Table 3). Spatial working-memory tasks include the radial arm maze (RAM), T-maze or Y-maze alternation tasks, Morris water maze (MWM), radial arm water maze, Barnes circular maze, and spatial span task [175-177]. Non-spatial working-memory tasks include the delayed match to sample, delayed non-match to sample, delayed stimulus discrimination task, and odor span task [42, 178]. Performance deficiency in these tasks may signify not only impairments in working memory, but also decreased behavioral adaptability or heightened persistence, which can also be observed in patients with schizophrenia [20, 179] .
Table 3. Recent studies using behavioral paradigms that measure cognitive symptoms of schizophrenia in rodent models.
| Paradigm | Animal Model | Experimental Manipulation | Results | References |
|---|---|---|---|---|
| RAM | Genetic | Alpha-CaMKII+/- mice | ↓ decreased working memory | [181] |
| Grin1(Rgsc174)/Grin1+ mice | ↓ decreased working memory | [108] | ||
| HRM + dizocilpine | ↓ decreased working memory | [182] | ||
| Homer1 KO mice | ↓ decreased working memory | [183] | ||
| Ywhae (+/-) mice | ↓ decreased working memory | [184] | ||
| NRG1 TM HET mice | NE | [185] | ||
| α7-nAChR KO mice | NE | [186] | ||
| Pharmacological | Chronic PCP administration in mice | NE | [187] | |
| Y-maze/ T-maze | Genetic | mGlu5 KO mice | ↓ decreased spontaneous alternation | [50] |
| Inhibition of GAD65 neurons in mice | ↓ decreased spontaneous alternation | [61] | ||
| Corticosterone-treated HRM | ↓ decreased spatial learning and memory | [71] | ||
| Pharmacological | Subchronic PCP administration in mice | ↓ decreased spontaneous alternation | [125] | |
| MWM | Developmental | Neonatal lesioning of the vHPC in mice | NE | [201] |
| Genetic | Dys1B (+/+) mice | ↓ decreased spatial learning and memory | [197] | |
| mGlu5 KO mice | ↓ decreased spatial learning and memory | [50] | ||
| Nlgn2 R215H knock-in homozygous mice | ↓ decreased spatial learning and memory | [73] | ||
| NRG1 HET mice | ↓ decreased spatial learning and memory | [198] | ||
| Intraflagellar Transport 88 (Ift88) gene KO mice | ↓ decreased spatial learning and memory | [199] | ||
| ZnT3 KO mice | NE | [202] | ||
| Pharmacological | Administration of Catha edulis forsk extract in mice | ↓ decreased spatial learning and memory | [60] | |
| MK-801 treatment in mice | ↓ decreased spatial learning and memory | [200] | ||
| PCP + Δ9-THC treatment in mice | ↓ decreased spatial learning and memory | [62] | ||
| Novel object recognition | Developmental | MIA in mice | ↓ decreased performance | [208] |
| Genetic | Ift88 gene KO mice | ↓ decreased performance | [199] | |
| Null mutation in pallid and dysbindin in mice | ↓ decreased performance | [209] | ||
| Spp homozygous mutant mice | ↓ decreased performance | [210] | ||
| Alpha7-nAChR KO mice | ↓ decreased performance | [186] | ||
| Pharmacological | Antagonism of the muscarinic acetylcholine system in mice | ↓ decreased performance | [212] | |
| L-Methionine in mice | ↓ decreased performance | [211] | ||
| PCP + Δ9-THC in mice | ↓ decreased performance | [62] | ||
| Subchronic PCP administration in mice | ↓ decreased performance | [113, 125] | ||
| Odor span task | Genetic | α7-nAChR KO mice | ↓ decreased working memory | [213] |
| Pharmacological | Subchronic treatment with ketamine (10 mg/kg and 30 mg/kg) rats | ↓ decreased performance | [216] | |
| Attentional set-shifting task | Developmental | Neonatal treatment with nuclear progesterone receptor antagonist in rats | ↓ decreased adult performance | [228] |
| Postnatal administration (PND, 7, 9, & 11) of ketamine 30 mg/kg in mice | ↓ decreased performance | [230] | ||
| Pharmacological | Cuprizone administration in rats | ↓ the specific decrease in the ability to shift between perceptual dimensions | [229] | |
| Sertindole (2.5 mg/kg) administration in mice | prevented ketamine-induced cognitive inflexibility | [231] | ||
| Neurotoxic damage to orbitofrontal and medial prefrontal cortical areas in mice | ↓ decreased affective and attentional sets | [232] | ||
| 5-choice serial reaction task | Developmental | Gestational exposure to high-fat diet in mice | ↑ increased impulsivity | [238] |
| Gestational exposure to a low-protein diet in mice | ↑ marked inattention | [238] | ||
| Genetic | Alpha7-nAChR KO mice | ↑ increased omission levels | [213] | |
| Increased NRG3 gene expression in mice mPFC | ↑ increased impulsivity | [239] | ||
| Map2k7 +/- mice | ↑ increased attentional deficit | [240] | ||
| Df(h15q13)/+ mice | ↓ decreased accuracy | [241] | ||
| Pharmacological | Chronic oral, but not acute injections of nicotine in mice | ↓ decrease phencyclidine-induced impulsivity | [242] |
Abbreviations: 5-HT7 = 5-hydroxytryptamine 7, serotonin 7; CaMKII = calmodulin-dependent protein kinase II; Dys1B = dysbindin-1B; GAD65 = glutamic acid decarboxylase 65; Grin1 = glutamate ionotropic receptor NMDA type subunit 1; HRM = heterozygous reeler mice; Ift88 = intraflagellar transport 88; KO = knockout; Map2k7 = mitogen-activated protein kinase kinase 7; mGluR = metabotropic glutamate receptor 5; MIA = maternal immune activation; mPFC = medial prefrontal cortex; Nlgn2 R215H = missense mutation R215H of neuroligin 2; NRG1 = neuregulin-1; NRG3 = neuregulin-3; PCP = phencyclidine; vHPC = ventral hippocampus; ZnT3 = zinc transporter 3; α7-nAChR = alpha7-nicotinic acetylcholine receptor; Δ9-THC = Δ9-tetrahydrocannabinol; NE = no effect.
RAM is one of the best known spatial memory measures for different species including rodents, birds, and even humans [42]. RAM usually involves an eight-armed, octagonal central chamber. Each arm is baited, and the animal is required to enter each arm and receive a reward. The spatial working memory of the subject is generally measured by the number of baited arms reached prior to the re-entry of an already explored arm. If the subject does not re-enter an arm that was previously visited, the maximum number of eight is reached [180], although this may vary depending on the experimental setup and the numbers of bait and reward arms. Most of the recent studies employing this behavioral assay have shown a cognitive deficit in various animal models, including alpha-CaMKII +/− mice [181], Grin1(Rgsc174)/Grin1+ mice [108], heterozygous reeler mice [182], Homer1-KO mice [183], and heterozygous KO mice of YWHAE, a gene encoding 14-3-3 epsilon[184]. Conversely, no effect has
been observed in RAM studies using heterozygous transmembrane domain Nrg1 mutant mice [185], alpha7-nicotinic acetylcholine receptor (α7-nAChR) KO mice (however, this model shows decreased working memory, as tested in the novel object recognition task) [186], and mice with chronic PCP administration [187]. Previous reviews have discussed the methodology and factors affecting the results of the RAM [180, 188, 189]. More recent studies have found various factors causing a deficit in working memory as measured by the RAM, including deficiency in schizophrenia candidate genes involved in synaptic plasticity and cognitive function-related factors [108, 181-184]. However, some schizophrenia risk factors such as manipulations in receptor expression [186], and genes [185], as well as drugs used to elicit symptoms of schizophrenia [187] in animal models did not cause performance deficiencies in the RAM.
The Y-maze is commonly used to measure both general activity and spatial working memory, as it is based on the natural tendency of rodents to alternate non-reinforced choices in the Y-maze on consecutive chances [190]. In brief, the subject is placed in one of the arm compartments and allowed to move freely to other arm compartments for several minutes, while the sequences of arm compartment entries are being recorded. An alternation is defined as an entry into the three designated arm compartments consecutively [190, 191]. Recent studies involving the use of the Y-maze have found that several animal models of schizophrenia displayed impaired spontaneous alternation and thus deficit in working memory. These models include mGlu5 KO mice [50], hM4D-treated mice for GAD65 neuron inhibition [61], heterozygous reelin-deficient mice with corticosterone treatment [71], and mice with subchronic PCP administration [125]. Previous reports have detailed the methodology and apparatus needed for this behavior assay [192-195]. Recent studies involving animal models of schizophrenia implicated genetic risk factors as the main cause of impairment in this task.
First described by Morris [177], the MWM has been used to evaluate spatial working memory and visual learning and memory abilities in rodents. The subject is arbitrarily positioned in a circular labyrinth arena filled with opaque water with a small hidden platform accessible to avoid the water. The experiment lasts for several days, with several trials conducted each day. After the training trials, a probe trial is performed without the hidden platform to evaluate reference memory and perseverance. The interval between the training and probe trials may vary from a few hours to a few months. A “reversal” variant of the task involves training the subject thoroughly in one quadrant with the hidden platform and moving the platform to a different quadrant in subsequent trials to assess the erasure of original spatial memory and re-learning [196]. Most recent studies involving the use of this behavioral task have shown impaired spatial working memory and visual learning and memory in genetic or chemically-induced schizophrenia animal models, including dysbindin-1B (+/+) mice [197], mGlu5 KO mice [50], Nlgn2 R215H KI homozygous mice [73], heterozygous mice deleted of NRG1 [198], conditional deletion of the intraflagellar transport 88 gene in mice [199], subchronic Catha edulis forsk extract or mice orally treated with ketamine [60], MK-801 treated mice [200], and PCP + Δ9-THC treated mice [62]. Meanwhile, neonatal lesioning of the vHPC [201] and female zinc transporter 3 KO mice [202] did not show any difference in spatial learning and memory. Previous reviews have detailed the methodology and factors affecting results in the MWM [203-205]. Such factors include the type of apparatus and training procedure [203], environmental variables [205], and measuring indices [204]. Most factors leading to the expected decrease in spatial learning and memory in animal models of schizophrenia include genetic risk factors implicated in human patients [197, 198], abnormalities in glutamatergic signaling [50], postsynaptic adhesion protein [73], disrupted ciliogenesis in the cortex and hippocampus [199], administration of plant extracts [60], and drugs blocking the NMDAR channels [62, 200]. Conversely, recent studies involving developmental manipulations in neonatal mice [201] and deletion of neuronal synaptic vesicle transporter [202] reported no changes in spatial learning and memory as measured by the MWM task.
4.2. Novel Object Recognition Test, for Visual Learning and Memory
Also known as spontaneous object recognition or visual pair-comparison test, this test is widely used as a visual learning and memory test in rats and mice [206]. This behavioral activity requires a brief exposure of the animal to two items. One of the items is first presented to the animal placed in a chamber, which the subject will visit again after a break that can vary from a few minutes to a few days. At this point, the subject is introduced to the initial object and to a second, new object. Rodents have the tendency to explore the new object more than the familiar one; therefore, no rule learning or pre-training is usually required prior to the performance of this behavioral task [206, 207].
Recent studies employing this behavior analysis have revealed cognitive impairment in various developmental, pharmacological, and genetic rodent models of schizophrenia (Table 3) including mice subjected to MIA by LPS on embryonic day 8 (E8) [208], conditional deletion of the intraflagellar transport 88 gene in mice [199], mice with null mutation in the pallidin or dysbindin gene, both of which comprise the biogenesis of lysosome-related organelles complex 1 [209]; dysbindin salt and pepper mice, which possess a single point mutation on the Dtnbp1 gene [210]; mice treated with the muscarinic acetylcholine system antagonist, scopolamine (2 mg/kg, intraperitoneal), l-methionine treated mice [211]; PCP + Δ9-THC treated mice [62], mice treated subchronically with PCP [113, 125], and alpha7-nicotinic acetylcholine receptor (α7-nAChR) KO mice [186]. Recent studies using animal models of schizophrenia have found that various factors could lead to decreased visual learning and memory. These factors include neonatal manipulations via MIA [208], neuronal cilia disruption [199], mutations in biogenesis of lysosome-related organelle proteins [209], schizophrenia risk-related genes [210], psychostimulant administration [212], methylation modulation [211], cannabis potentiation of phencyclidine administration [62], and NMDAR blockage or hypofunction [113, 125].
4.3. Odor Span Task, for Non-spatial Working Memory
The task of odor span is designed to assess the contribution of the hippocampus to non-spatial working memory [176]. This behavioral task has been used with both rats [176] and mice [213]. Briefly, the task requires the animal to dig a food reward in a scented bowl. Afterward, two bowls are presented to the animal-one bowl containing the familiar scent from the previous task, and another one with a novel scent. The bowl is baited with a novel smell aiming for the animal to ignore the previously baited bowl and search only in the fresh scented bowl. The animal is then presented with three scented bowls-the first two bowls previously presented to the animal, and a third, novel one. Again, the animal should remember the first two scents and therefore avoid digging in those bowls in favor of the bowl with the novel scent. The number of different scents can be increased up to 24, and the subject must always remember the previously encountered bowls and thus only commence digging in the novel bowl. The bowls are always arranged in a random order to ensure that the subject cannot use any visual signals when selecting the novel odor. The number of odors that the subject is able to successfully remember before making its first mistake (digging in an old scent) is considered the “span” of the trial. To date, few studies have employed the odor span task in rodent models of schizophrenia [213-215] (Table 3). Subchronic treatment of ketamine (10 mg/kg and 30 mg/kg daily for 5 consecutive days) induced a substantial deficit of odor span detection [216]. In contrast, α7-nAChR KO mice exhibited impaired olfactory working memory, as shown by the higher number of sessions required to attain the span length criteria in training trials, and reduced span length and lower span completion rates in test trials [213].
The odor span task may also serve to test the verbal learning and memory domains. Although there is no absolute homolog of verbal information between humans and rodents, the main principle in the animal models of psychiatric disorders is to maximally reenact a subset of processes damaged by the diseases. As odor-based interactions between rodents are important in various communication types [217], perhaps olfactory data comprise the closest analogue for rodents to verbal information [42]. If the controversy surrounding this concept can be overcome, this task could be classified under the verbal learning and memory domains [42]. In the past 10 years, animal experiments that have utilized the odor span task have employed genetic and pharmacological models.
4.4. Attentional Set-shifting Task, for Reasoning and Problem Solving
The attentional set-shifting task (ASST) is a method developed for use with animal models to measure cognitive flexibility. ASST was first described by Birrell and Brown [218] but it was later revised for mice [219]. For the animal to discriminate relevant from unnecessary prompts, an attentional set is formed whereby the subject acquires that a set of rules may be applied to complex stimuli [218]. The ASST necessitates that the subject inhibit previously learned responses that are unsuitable to the present conditions [220]. Patients with schizophrenia often have poor set-shifting capabilities, which translates to poor executive functioning [221-223]. This symptom is generally called executive inflexibility or a diminished ability to shift focus [223, 224]. In ASST, a cognitive set is formed by creating a reinforced rule in a subject. For example, in certain conditions, a relevant cue such as a digging medium should be remembered by the subject, while an irrelevant cue such as an odor should be ignored. Such correlation is then strengthened by subsequent activities where the recipient must remember the specific shape of the digging medium and the irrelevant cue, such as odor varies. The relevance of the shape of the digging medium is reinforced by food rewards. Cognitive versatility could be tested using a reversal or extradimensional move. In the reversal shift, the previously irrelevant cue is now set to be relevant (previous “relevant” digging medium to another type of digging medium, e.g., paper to woodchips), which tests the subject’s capability to ignore the positive stimuli from the first set of experiments, and thus the animal’s flexibility in maintaining the previously formed attentional set. While in the extradimensional shift, the animal’s cognitive flexibility is once again challenged by changing the previously learned attentional set by adding another dimension and shifting the relevance. For example, if the previous relevant cue was the digging medium, now it will be some type of odor (e.g., the relevant cue is transferred from the paper medium to a cinnamon odor). Failure to change choices from the previously learned attentional set in either test suggests a deficit in cognitive flexibility [225-227]. Recent studies utilizing this behavioral assay have found general impairment in cognitive flexibility, as illustrated by impaired reversal learning and extradimensional set-shifting, in animal models of schizophrenia (Table 3). Such models include mice undergoing neonatal treatment with nuclear progesterone receptor antagonist [228], adolescent rats that were administered cuprizone, a copper chelator known to cause demyelination [229]; ketamine (30 mg/kg)-treated mice on postnatal days 7, 9, and 11 [230]; mice subjected to administration of ketamine (10 or 20 mg/kg) [231], and mice with NMDA-induced neurotoxic damage to orbitofrontal and medial prefrontal cortical areas [232]. Factors leading to impairment in problem solving and reasoning, as measured by the ASST, include disruption of the mesocortical dopamine pathway [228], abnormalities in brain white matter and oligodendroglia [229], dysfunctions in the GABAergic system by the blockade of NMDARs [230], and neurotoxic damage to the orbitofrontal and medial prefrontal cortical areas [232]. Conversely, the administration of NMDAR 2B (NR2B) subunit-selective antagonists improves the performance of animal models in the ASST [231]. Previous reviews have detailed the methodology [219, 233] as well as species-specific variations [225, 234] involved in this behavioral test. Collectively, within the 10-year scope of the study, most animal experiments utilizing the ASST employed pharmacological followed by developmental models.
4.5. 5-choice Serial Reaction Time Task, for Attention/Vigilance and Speed of Processing
The 5-choice serial reaction time task (5-CSRTT) was designed to measure visuospatial attentional processes and motor impulsivity in animals [235]. The task has begun to clarify the basic neural circuitry and neuromodulation of executive and cognitive prefrontal functions [236] and to clarify disrupted neuropsychological mechanisms in attentional dysfunctions including schizophrenia, Alzheimer and Parkinson’s diseases, aging, and attention deficit/hyperactivity disorder, as an analogue of the human continuous performance task [42, 237]. Briefly, in the 5-CSRTT chamber, there are at least five holes, which can be illuminated and laden with food for reward on the opposite side. The rodents are required to perceive a pseudo-randomly illuminated hole among the five holes and to provide a nose-poke response in the correct aperture to obtain the food reward. The attentional capacity and speed of response are assessed as the percentage of responses to the right location and the mean latency to respond correctly after detecting the light stimulus, respectively. Additionally, premature responses made before the stimulus presentation are interpreted as impulsive behavior [237].
Recent application of the 5-CSRTT to mouse models of schizophrenia has found general attentional impairment and impulsivity (Table 3). Increased impulsivity was observed in male mice raised from female mice exposed to a high-fat diet during gestation [238], while marked inattention was found in the offspring of female mice fed a low-protein diet during gestation [238]. Moreover mice exhibiting significantly higher omission levels include α7-nAChR KO mice [213], mice with overexpressed neuregulin-3 gene in the mPFC [239], mice haploin sufficient for dual specificity mitogen-activated protein kinase kinase 7 gene, encoding MKK7 [240]; and mice with 15q13.3 microdeletion (Df[h15q13]/+) [241], while significant increase in attentional impairment, through premature or perseverative responding, was found in neonatal and adult mice treated with PCP [242] and mice with 5-HT7 receptor downregulation in the mPFC. Factors causing impairments in attention and processing speed include manipulations in maternal diet and subsequent gestational growth disturbances in the offspring [238], schizophrenia-associated genetic risk factors [213, 241], overexpression of genes regulating impulsivity [239], and impairment of genes strongly linked to synaptic plasticity and neocortex development [240]. Previous reviews have detailed and discussed the variations in methodology involved in the 5-CSRTT [243-246]. They have also reviewed the strengths of this task, which include its high translational value and adaptability to task modifications [243, 245], and the various factors, such as sedation, motivation deficits, and locomotor impairments [247], which can affect results. In general, within the past 10-years, most animal experiments utilizing the 5-CSRTT employed developmental followed by genetic and pharmacological models.
Table 3 shows that various developmental, genetic, or pharmacological animal models of schizophrenia exhibit cognitive symptoms, including spatial and non-spatial working memory and impairment of attention and executive function. In general, within the 10 year scope of the study, most recent animal experiments utilizing the RAM maze test have employed genetic followed by pharmacological models, while the Y-maze test has employed genetic followed by pharmacological models, and the MWM test has been primarily used in studies employing genetic followed by pharmacological and developmental models. Conversely, the novel object test has been mostly used with pharmacological followed by genetic and finally developmental models. In contrast, the odor span task has been primarily only employed for both genetic and pharmacological models of schizophrenia, while the ASST paradigm has been predominantly utilized by both pharmacological and developmental models. Finally, most animal experiments utilizing the 5-CSRTT have employed developmental followed by genetic and pharmacological models. Behavioral tasks measuring cognitive symptoms have been developed to attempt to elucidate each of the cognitive domains affected in patients with schizophrenia, with a focus on (spatial, non-spatial, and visual) memory and attention [8, 130, 163, 164, 167, 171] (Fig. 1). Most recent studies of animal models of schizophrenia induced by genetic, pharmacological, and developmental applications have reported impairment in performing these tasks. For spatial and non-spatial working-memory tests, maze tests, including the RAM, Y-maze, and MWM, and the odor span task can be respectively selected. Among the spatial working-memory tests, the MWM is more realistic and complex than the other maze tests and is thus considered the gold standard, although less stressful tasks including the RAM and Y-maze are also widely applied. The MWM and novel object recognition test can be applied for visual learning and memory testing. Additionally, attention and more complex executive function can be assessed using the ASST and 5-CSRTT paradigms. Researchers need to be cautious that alterations in behavioral output could also be induced by non-cognitive elements such as olfaction, vision, anxiety, and locomotor activity.
Based on the most recent advances in the field, it is apparent that a myriad of behavioral tests is needed to confirm and evaluate the congruency of animal models with the numerous behaviors and clinical signs exhibited by patients with schizophrenia. Animal models have been developed to mimic schizophrenia-associated behaviors and serve as preclinical models in drug research. As such, some behavioral tests used to measure schizophrenia-associated behaviors in human patients have been translated into animal versions to allow further confirmation of preclinical trials and better comprehension of the underlying neural basis of the disorders [248, 249]. These animal behavioral tasks are key to filling the gap between preclinical and clinical research [250, 251]. Intrinsically, deciding which behavioral task best describes an animal model is challenging. While, in principle, an exhaustive battery of tasks seems best to fully comprehend and reliably characterize an animal model, this approach, if ever possible, would require excessive time and resources [251]. Thus, researchers are faced with the task of selecting the appropriate behavioral assays that can best describe their animal model. There are no established rules to help guide the selection of the appropriate behavioral tasks. Moreover, suitable behavioral tasks may vary depending on the researchers’ goal.
5. CONCLUSION
At present, most analyses concerning the modeling of schizophrenia in animals have only focused on the identification of the different means through which scientists are manipulating, generating, and developing these animal models; however, a gap remains to be filled in the narrative scrutinizing the behavioral tasks being extensively employed in the quest to characterize these models. This review provided an overview of the behavioral tasks currently used to assess the validity of the available animal models of schizophrenia and the recent findings of studies that have employed these behavioral paradigms.
In assessing positive symptoms in animal models of schizophrenia, measurement of locomotor activity, followed by the measurement of PPI, remain the standard behavioral tasks that have been employed by studies in recent years, while the FST, followed by the EPM, TST, three-chamber sociality test, and sucrose preference test were the behavioral paradigms most utilized in detecting negative symptoms of schizophrenia. Lastly, novel object recognition, followed by the MWM, RAM, 5-CSRTT, ASST, Y-maze, or T-maze, and finally the odor span task, were the behavioral tasks preferred by the latest studies that evaluated the cognitive symptoms of schizophrenia in their respective animal models.
Collectively, these tests reproduce dysfunctions found in patients with schizophrenia and may reveal meaningful theoretical and neurobiological correlations between preclinical and clinical findings. Fundamentally, the formulation and application of these behavioral tasks allow the advancement of research using animal models, which, in turn, furthers the elucidation of schizophrenia’s etiology and exact mechanisms.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- 5-HT7
5-hydroxytryptamine 7, serotonin 7
- Ahi1 +/- KO
Abelson helper integration site 1 +/− KO
- ALK
Anaplastic Lymphoma Kinase
- Ank3
Ankyrin 3
- CaMKII
Calmodulin-dependent protein kinase II
- CD2
Cyclin-D2
- CRMP2
Collapsin Response Mediator Protein 2
- CUB
for Complement C1r/C1s, Uegf, Bmp1
- DAT
Dopamine Transporter
- DISC1
Disrupted-in-Schizophrenia 1
- DNMT1
DNA Methyltransferase 1
- Dtnbp1
Dystrobrevin Binding Protein 1
- Dys1B
Dysbindin-1B
- En2
Engrailed-2
- EPRAP
EP4 Receptor-associated Protein
- FFAR1
Free Fatty Acid Receptor 1
- GAD65
Glutamic Acid Decarboxylase 65
- GAS7
Growth Arrest-specific 7
- GCLM
Glutamate-cysteine Ligase Modifier
- GLAST
Glial Glutamate and Aspartate Transporter
- GluA1
Glutamate A1
- Glu-CB1
cannabinoid receptor type 1 in glutamergic neurons
- GPR88
G Protein-Coupled Receptor 88
- GRIA1
Glutamate Ionotropic Receptor AMPA type subunit 1
- Grik4
Glutamate receptor, ionotropic, kainate 4
- Grin1
Glutamate Ionotropic Receptor NMDA Type Subunit 1
- HRM
Heterozygous Reeler Mice
- Ift88
Intraflagellar Transport 88
- JNJ
JNJ-28871063
- KO
Knockout
- L-dopa
Levodopa
- LPS
Lipopolysaccharide
- Map2k7
Mitogen-activated protein kinase kinase 7
- MB-COMT
Membrane-bound catechol-O-methyltransferase
- mGlu5
metabotropic glutamate receptor 5
- MIA
Maternal Immune Activation
- mPFC
medial prefrontal cortex
- NAc-TMEM mice
Tmem168 vector was injected into the NAc of mice
- NCAM
Neural Cell Adhesion Molecule
- NCS-1
Neuronal Calcium Sensor
- Nlgn2 R215H
missense mutation R215H of neuroligin 2
- NMDA
N-methyl-D-aspartate
- NMRI
Naval Medical Research Institute
- NRG1
Neuregulin-1
- NRG3
Neuregulin-3
- NT
Neurotensin
- Pcm1
Pericentriolar material 1
- PCP
Phencyclidine
- PLC-β1
Phospholipase C-β1
- poly(I:C)
polyinosinic-polycytidylic acid
- PTPRG
receptor protein tyrosine phosphatase gamma
- SARM1
Sterile Alpha and TIR Motif-Containing 1
- SLC1A1
Solute Carrier Family 1 Member 1
- SREBP1c
Sterol Regulatory Element-binding Protein 1c
- Tg
Transgenic
- V1AR
Vasopressin Receptor 1A
- vHC
ventral Hippocampus
- vHPC
entral hippocampus
- ZnT3
Zinc transporter 3
- α7-nAChR
alpha7-nicotinic acetylcholine receptor
- Δ9-THC
Δ9-tetrahydrocannabinol
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The study was funded by National Research Foundation (NRF) of Korea grant funded by the Korean Government (NRF-2020R1A4A1019395).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest, financial or otherwise. The present manuscript has not been previously published and is not under consideration for publication elsewhere.
REFERENCES
- 1.Saha S., Chant D., Welham J., McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2(5):e141. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Disease G.B.D., Injury I., Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee P.R., Brady D.L., Shapiro R.A., Dorsa D.M., Koenig J.I. Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychopharmacology. 2005;30(10):1883–1894. doi: 10.1038/sj.npp.1300722. [DOI] [PubMed] [Google Scholar]
- 4.Winship I.R., Dursun S.M., Baker G.B., Balista P.A., Kandratavicius L., Maia-de-Oliveira J.P., Hallak J., Howland J.G. An Overview of Animal Models Related to Schizophrenia. Can. J. Psychiatry. 2018:1–13. doi: 10.1177/0706743718773728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyamoto Y., Nitta A. Behavioral phenotypes for negative symptoms in animal models of schizophrenia. J. Pharmacol. Sci. 2014;126(4):310–320. doi: 10.1254/jphs.14R02CR. [DOI] [PubMed] [Google Scholar]
- 6.Onaolapo A.Y., Aina O.A., Onaolapo O.J. Melatonin attenuates behavioural deficits and reduces brain oxidative stress in a rodent model of schizophrenia. Biomed. Pharmacother. 2017;92:373–383. doi: 10.1016/j.biopha.2017.05.094. [DOI] [PubMed] [Google Scholar]
- 7.Canetta S., Kellendonk C. Can we use mice to study schizophrenia? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018;373(1742):20170032. doi: 10.1098/rstb.2017.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellendonk C., Simpson E.H., Kandel E.R. Modeling cognitive endophenotypes of schizophrenia in mice. Trends Neurosci. 2009;32(6):347–358. doi: 10.1016/j.tins.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson E.H., Kellendonk C., Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65(5):585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nestler E.J., Hyman S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010;13(10):1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volk D.W., Lewis D.A. GABA Targets for the Treatment of Cognitive Dysfunction in Schizophrenia. Curr. Neuropharmacol. 2005;3(1):45–62. doi: 10.2174/1570159052773396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribeiro-Santos A., Lucio Teixeira A., Salgado J.V. Evidence for an immune role on cognition in schizophrenia: a systematic review. Curr. Neuropharmacol. 2014;12(3):273–280. doi: 10.2174/1570159X1203140511160832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cirillo M.A., Seidman L.J. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol. Rev. 2003;13(2):43–77. doi: 10.1023/A:1023870821631. [DOI] [PubMed] [Google Scholar]
- 14.Terry A.V., Jr Role of the central cholinergic system in the therapeutics of schizophrenia. Curr. Neuropharmacol. 2008;6(3):286–292. doi: 10.2174/157015908785777247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J.P., Malhotra A.K. Pharmacogenetics and antipsychotics: therapeutic efficacy and side effects prediction. Expert Opin. Drug Metab. Toxicol. 2011;7(1):9–37. doi: 10.1517/17425255.2011.532787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Astrachan B.M., Harrow M., Adler D., Brauer L., Schwartz A., Schwartz C., Tucker G. A checklist for the diagnosis of schizophrenia. Br. J. Psychiatry. 1972;121(564):529–539. doi: 10.1192/bjp.121.5.529. [DOI] [PubMed] [Google Scholar]
- 17.Andreasen N.C., Grove W.M. Thought, language, and communication in schizophrenia: diagnosis and prognosis. Schizophr. Bull. 1986;12(3):348–359. doi: 10.1093/schbul/12.3.348. [DOI] [PubMed] [Google Scholar]
- 18.Galuppi A., Turola M.C., Nanni M.G., Mazzoni P., Grassi L. Schizophrenia and quality of life: how important are symptoms and functioning? Int. J. Ment. Health Syst. 2010;4:31. doi: 10.1186/1752-4458-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel K.R., Cherian J., Gohil K., Atkinson D. Schizophrenia: overview and treatment options. P&T. 2014;39(9):638–645. [PMC free article] [PubMed] [Google Scholar]
- 20.Powell C.M., Miyakawa T. Schizophrenia-relevant behavioral testing in rodent models: a uniquely human disorder? Biol. Psychiatry. 2006;59(12):1198–1207. doi: 10.1016/j.biopsych.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones C.A., Watson D.J., Fone K.C. Animal models of schizophrenia. Br. J. Pharmacol. 2011;164(4):1162–1194. doi: 10.1111/j.1476-5381.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagan J.J., Jones D.N.C. Predicting drug efficacy for cognitive deficits in schizophrenia. Schizophr. Bull. 2005;31(4):830–853. doi: 10.1093/schbul/sbi058. [DOI] [PubMed] [Google Scholar]
- 23.van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr. Bull. 2010;36(2):246–270. doi: 10.1093/schbul/sbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forsingdal A., Jørgensen T.N., Olsen L., Werge T., Didriksen M., Nielsen J. Can Animal Models of Copy Number Variants That Predispose to Schizophrenia Elucidate Underlying Biology? Biol. Psychiatry. 2019;85(1):13–24. doi: 10.1016/j.biopsych.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Swerdlow N.R., Light G.A. Animal Models of Deficient Sensorimotor Gating in Schizophrenia: Are They Still Relevant? Curr. Top. Behav. Neurosci. 2016;28:305–325. doi: 10.1007/7854_2015_5012. [DOI] [PubMed] [Google Scholar]
- 26.Kanyuch N., Anderson S. Animal Models of Developmental Neuropathology in Schizophrenia. Schizophr. Bull. 2017;43(6):1172–1175. doi: 10.1093/schbul/sbx116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayhan Y., McFarland R., Pletnikov M.V. Animal models of gene-environment interaction in schizophrenia: A dimensional perspective. Prog. Neurobiol. 2016;136:1–27. doi: 10.1016/j.pneurobio.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratajczak P., Wozniak A., Nowakowska E. Animal models of schizophrenia: developmental preparation in rats. Acta Neurobiol. Exp. (Warsz.) 2013;73(4):472–484. doi: 10.55782/ane-2013-1953. [DOI] [PubMed] [Google Scholar]
- 29.Cadinu D., Grayson B., Podda G., Harte M.K., Doostdar N., Neill J.C. NMDA receptor antagonist rodent models for cognition in schizophrenia and identification of novel drug treatments, an update. Neuropharmacology. 2018;142:41–62. doi: 10.1016/j.neuropharm.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 30.Yanagi M., Southcott S., Lister J., Tamminga C.A. Animal models of schizophrenia emphasizing construct validity. Prog. Mol. Biol. Transl. Sci. 2012;105:411–444. doi: 10.1016/B978-0-12-394596-9.00012-3. [DOI] [PubMed] [Google Scholar]
- 31.Mouri A., Nagai T., Ibi D., Yamada K. Animal models of schizophrenia for molecular and pharmacological intervention and potential candidate molecules. Neurobiol. Dis. 2013;53:61–74. doi: 10.1016/j.nbd.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 32.Bondi C., Matthews M., Moghaddam B. Glutamatergic animal models of schizophrenia. Curr. Pharm. Des. 2012;18(12):1593–1604. doi: 10.2174/138161212799958576. [DOI] [PubMed] [Google Scholar]
- 33.Burrows E.L., Hannan A.J. Cognitive endophenotypes, gene-environment interactions and experience-dependent plasticity in animal models of schizophrenia. Biol. Psychol. 2016;116:82–89. doi: 10.1016/j.biopsycho.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Lu L., Mamiya T., Koseki T., Mouri A., Nabeshima T. Genetic animal models of schizophrenia related with the hypothesis of abnormal neurodevelopment. Biol. Pharm. Bull. 2011;34(9):1358–1363. doi: 10.1248/bpb.34.1358. [DOI] [PubMed] [Google Scholar]
- 35.Steullet P., Cabungcal J.H., Coyle J., Didriksen M., Gill K., Grace A.A., Hensch T.K., LaMantia A.S., Lindemann L., Maynard T.M., Meyer U., Morishita H., O’Donnell P., Puhl M., Cuenod M., Do K.Q. Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol. Psychiatry. 2017;22(7):936–943. doi: 10.1038/mp.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feifel D., Shilling P.D. Promise and pitfalls of animal models of schizophrenia. Curr. Psychiatry Rep. 2010;12(4):327–334. doi: 10.1007/s11920-010-0122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazar N.L., Neufeld R.W., Cain D.P. Contribution of nonprimate animal models in understanding the etiology of schizophrenia. J. Psychiatry Neurosci. 2011;36(4):E5–E29. doi: 10.1503/jpn.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsson H., Rydén E., Boman M., Långström N., Lichtenstein P., Landén M. Risk of bipolar disorder and schizophrenia in relatives of people with attention-deficit hyperactivity disorder. Br. J. Psychiatry. 2013;203(2):103–106. doi: 10.1192/bjp.bp.112.120808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamshere M.L., Stergiakouli E., Langley K., Martin J., Holmans P., Kent L., Owen M.J., Gill M., Thapar A., O’Donovan M., Craddock N. Shared polygenic contribution between childhood attention-deficit hyperactivity disorder and adult schizophrenia. Br. J. Psychiatry. 2013;203(2):107–111. doi: 10.1192/bjp.bp.112.117432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amann L.C., Gandal M.J., Halene T.B., Ehrlichman R.S., White S.L., McCarren H.S., Siegel S.J. Mouse behavioral endophenotypes for schizophrenia. Brain Res. Bull. 2010;83(3-4):147–161. doi: 10.1016/j.brainresbull.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Geyer M.A., McIlwain K.L., Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol. Psychiatry. 2002;7(10):1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- 42.Young J.W., Powell S.B., Risbrough V., Marston H.M., Geyer M.A. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol. Ther. 2009;122(2):150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desbonnet L., Waddington J.L., O’Tuathaigh C.M. Mutant models for genes associated with schizophrenia. Biochem. Soc. Trans. 2009;37(Pt 1):308–312. doi: 10.1042/BST0370308. [DOI] [PubMed] [Google Scholar]
- 44.Walsh R.N., Cummins R.A. The Open-Field Test: a critical review. Psychol. Bull. 1976;83(3):482–504. doi: 10.1037/0033-2909.83.3.482. [DOI] [PubMed] [Google Scholar]
- 45.da Silveira V.T., Medeiros D.C., Ropke J., Guidine P.A., Rezende G.H., Moraes M.F.D., Mendes E.M.A.M., Macedo D., Moreira F.A., de Oliveira A.C.P. Effects of early or late prenatal immune activation in mice on behavioral and neuroanatomical abnormalities relevant to schizophrenia in the adulthood. Int. J. Dev. Neurosci. 2017;58:1–8. doi: 10.1016/j.ijdevneu.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 46.de Matos L.O., Reis A.L.A.L., Guerra L.T.L., Guarnieri L.O., Moraes M.A., Aquino N.S.S., Szawka R.E., Pereira G.S., Souza B.R. l-Dopa treatment during perinatal development leads to different behavioral alterations in female vs. male juvenile Swiss mice. Pharmacol. Biochem. Behav. 2018;173:1–14. doi: 10.1016/j.pbb.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Lee S., Kang S., Ang M.J., Kim J., Kim J.C., Kim S.H., Jeon T.I., Jung C., Im S.S., Moon C. Deficiency of sterol regulatory element-binding protein-1c induces schizophrenia-like behavior in mice. Genes Brain Behav. 2019;18(4):e12540. doi: 10.1111/gbb.12540. [DOI] [PubMed] [Google Scholar]
- 48.Meirsman A.C., de Kerchove d’Exaerde A., Kieffer B.L., Ouagazzal A.M. GPR88 in A2A receptor-expressing neurons modulates locomotor response to dopamine agonists but not sensorimotor gating. Eur. J. Neurosci. 2017;46(4):2026–2034. doi: 10.1111/ejn.13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhardwaj S.K., Stojkovic K., Kiessling S., Srivastava L.K., Cermakian N. Constant light uncovers behavioral effects of a mutation in the schizophrenia risk gene Dtnbp1 in mice. Behav. Brain Res. 2015;284:58–68. doi: 10.1016/j.bbr.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 50.Burrows E.L., McOmish C.E., Buret L.S., Van den Buuse M., Hannan A.J. Environmental enrichment ameliorates behavioral impairments modeling schizophrenia in mice lacking metabotropic glutamate receptor 5. Neuropsychopharmacology. 2015;40(8):1947–1956. doi: 10.1038/npp.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yasuda K., Hayashi Y., Yoshida T., Kashiwagi M., Nakagawa N., Michikawa T., Tanaka M., Ando R., Huang A., Hosoya T., McHugh T.J., Kuwahara M., Itohara S. Schizophrenia-like phenotypes in mice with NMDA receptor ablation in intralaminar thalamic nucleus cells and gene therapy-based reversal in adults. Transl. Psychiatry. 2017;7(2):e1047. doi: 10.1038/tp.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang H., Kang E., Wang Y., Yang C., Yu H., Wang Q., Chen Z., Zhang C., Christian K.M., Song H., Ming G.L., Xu Z. Brain-specific Crmp2 deletion leads to neuronal development deficits and behavioural impairments in mice. Nat. Commun. 2016;7:11773. doi: 10.1038/ncomms11773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen J., Fejgin K., Sotty F., Nielsen V., Mørk A., Christoffersen C.T., Yavich L., Lauridsen J.B., Clausen D., Larsen P.H., Egebjerg J., Werge T.M., Kallunki P., Christensen K.V., Didriksen M. A mouse model of the schizophrenia-associated 1q21.1 microdeletion syndrome exhibits altered mesolimbic dopamine transmission. Transl. Psychiatry. 2017;7(11):1261–1261. doi: 10.1038/s41398-017-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong P., Sze Y., Chang C.C.R., Lee J., Zhang X. Pregnenolone sulfate normalizes schizophrenia-like behaviors in dopamine transporter knockout mice through the AKT/GSK3β pathway. Transl. Psychiatry. 2015;5:e528. doi: 10.1038/tp.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karlsson R-M., Tanaka K., Saksida L.M., Bussey T.J., Heilig M., Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2009;34(6):1578–1589. doi: 10.1038/npp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Afshari P., Yao W-D., Middleton F.A. Reduced Slc1a1 expression is associated with neuroinflammation and impaired sensorimotor gating and cognitive performance in mice: Implications for schizophrenia. PLoS One. 2017;12(9):e0183854. doi: 10.1371/journal.pone.0183854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang T., Hong J., Di T., Chen L. MPTP impairs dopamine D1 receptor-mediated survival of newborn neurons in ventral hippocampus to cause depressive-like behaviors in adult mice. Front. Mol. Neurosci. 2016;9:101. doi: 10.3389/fnmol.2016.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shang K., Talmage D.A., Karl T. Parent-of-origin effects on schizophrenia-relevant behaviours of type III neuregulin 1 mutant mice. Behav. Brain Res. 2017;332:250–258. doi: 10.1016/j.bbr.2017.05.057. [DOI] [PubMed] [Google Scholar]
- 59.Kim S.W., Seo M., Kim D.S., Kang M., Kim Y.S., Koh H.Y., Shin H.S. Knockdown of phospholipase C-β1 in the medial prefrontal cortex of male mice impairs working memory among multiple schizophrenia endophenotypes. J. Psychiatry Neurosci. 2015;40(2):78–88. doi: 10.1503/jpn.130285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bogale T., Engidawork E., Yisma E. Subchronic oral administration of crude khat extract (Catha edulis forsk) induces schizophernic-like symptoms in mice. BMC Complement. Altern. Med. 2016;16(1):153. doi: 10.1186/s12906-016-1145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen R., Morrissey M.D., Mahadevan V., Cajanding J.D., Woodin M.A., Yeomans J.S., Takehara-Nishiuchi K., Kim J.C. Parvalbumin and GAD65 interneuron inhibition in the ventral hippocampus induces distinct behavioral deficits relevant to schizophrenia. J. Neurosci. 2014;34(45):14948–14960. doi: 10.1523/JNEUROSCI.2204-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodríguez G., Neugebauer N.M., Yao K.L., Meltzer H.Y., Csernansky J.G., Dong H. Δ9-tetrahydrocannabinol (Δ9-THC) administration after neonatal exposure to phencyclidine potentiates schizophrenia-related behavioral phenotypes in mice. Pharmacol. Biochem. Behav. 2017;159:6–11. doi: 10.1016/j.pbb.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 63.Beninger R.J. The role of dopamine in locomotor activity and learning. Brain Res. 1983;287(2):173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- 64.Young J.W., Minassian A., Geyer M.A. Locomotor profiling from rodents to the clinic and back again. Curr. Top. Behav. Neurosci. 2016;28:287–303. doi: 10.1007/7854_2015_5015. [DOI] [PubMed] [Google Scholar]
- 65.Powell S.B., Zhou X., Geyer M.A. Prepulse inhibition and genetic mouse models of schizophrenia. Behav. Brain Res. 2009;204(2):282–294. doi: 10.1016/j.bbr.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoffman H.S., Ison J.R. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol. Rev. 1980;87(2):175–189. doi: 10.1037/0033-295X.87.2.175. [DOI] [PubMed] [Google Scholar]
- 67.Graham F.K. Presidential Address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12(3):238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 68.Clapcote S.J., Lipina T.V., Millar J.K., Mackie S., Christie S., Ogawa F., Lerch J.P., Trimble K., Uchiyama M., Sakuraba Y., Kaneda H., Shiroishi T., Houslay M.D., Henkelman R.M., Sled J.G., Gondo Y., Porteous D.J., Roder J.C. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54(3):387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 69.Shoji H., Miyakawa T. Relationships between the acoustic startle response and prepulse inhibition in C57BL/6J mice: a large-scale meta-analytic study. Mol. Brain. 2018;11(1):42–42. doi: 10.1186/s13041-018-0382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eßlinger M., Wachholz S., Manitz M-P., Plümper J., Sommer R., Juckel G., Friebe A. Schizophrenia associated sensory gating deficits develop after adolescent microglia activation. Brain Behav. Immun. 2016;58:99–106. doi: 10.1016/j.bbi.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 71.Schroeder A., Buret L., Hill R.A., van den Buuse M. Gene-environment interaction of reelin and stress in cognitive behaviours in mice: Implications for schizophrenia. Behav. Brain Res. 2015;287:304–314. doi: 10.1016/j.bbr.2015.03.063. [DOI] [PubMed] [Google Scholar]
- 72.Young J.W., Ratty A., Dawe G.S., Geyer M.A. Altered exploration and sensorimotor gating of the chakragati mouse model of schizophrenia. Behav. Neurosci. 2014;128(4):460–467. doi: 10.1037/a0036425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen C-H., Lee P-W., Liao H-M., Chang P-K. Neuroligin 2 R215H Mutant Mice Manifest Anxiety, Increased Prepulse Inhibition, and Impaired Spatial Learning and Memory. Front. Psychiatry. 2017;8:257. doi: 10.3389/fpsyt.2017.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Powell S.B., Weber M., Geyer M.A. Genetic models of sensorimotor gating: relevance to neuropsychiatric disorders. Curr. Top. Behav. Neurosci. 2012;12:251–318. doi: 10.1007/7854_2011_195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swerdlow N.R., Weber M., Qu Y., Light G.A., Braff D.L. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl.) 2008;199(3):331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khan A., Powell S.B. Sensorimotor gating deficits in “two-hit” models of schizophrenia risk factors. Schizophr. Res. 2018;198:68–83. doi: 10.1016/j.schres.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van den Buuse M., Garner B., Koch M. Neurodevelopmental animal models of schizophrenia: effects on prepulse inhibition. Curr. Mol. Med. 2003;3(5):459–471. doi: 10.2174/1566524033479627. [DOI] [PubMed] [Google Scholar]
- 78.Peres F.F., Levin R., Almeida V., Zuardi A.W., Hallak J.E., Crippa J.A., Abilio V.C. Cannabidiol, among Other Cannabinoid Drugs, Modulates Prepulse Inhibition of Startle in the SHR Animal Model: Implications for Schizophrenia Pharmacotherapy. Front. Pharmacol. 2016;7:303. doi: 10.3389/fphar.2016.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu Z., Yang Z., Zhang M., Bao X., Han F., Li L. The role of N-methyl-D-aspartate receptors and metabotropic glutamate receptor 5 in the prepulse inhibition paradigms for studying schizophrenia: pharmacology, neurodevelopment, and genetics. Behav. Pharmacol. 2018;29(1):13–27. doi: 10.1097/FBP.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 80.Fu K., Miyamoto Y., Sumi K., Saika E., Muramatsu S.I., Uno K., Nitta A. Overexpression of transmembrane protein 168 in the mouse nucleus accumbens induces anxiety and sensorimotor gating deficit. PLoS One. 2017;12(12):e0189006. doi: 10.1371/journal.pone.0189006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walther S., Strik W. Motor symptoms and schizophrenia. Neuropsychobiology. 2012;66(2):77–92. doi: 10.1159/000339456. [DOI] [PubMed] [Google Scholar]
- 82.Dickerson D.D., Bilkey D.K. Aberrant neural synchrony in the maternal immune activation model: using translatable measures to explore targeted interventions. Front. Behav. Neurosci. 2013;7:217. doi: 10.3389/fnbeh.2013.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ellenbroek B.A., Cools A.R. Animal models for the negative symptoms of schizophrenia. Behav. Pharmacol. 2000;11(3-4):223–233. doi: 10.1097/00008877-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 84.O’Tuathaigh C.M., Kirby B.P., Moran P.M., Waddington J.L. Mutant mouse models: genotype-phenotype relationships to negative symptoms in schizophrenia. Schizophr. Bull. 2010;36(2):271–288. doi: 10.1093/schbul/sbp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Tuathaigh C.M.P., Desbonnet L., Waddington J.L. Genetically modified mice related to schizophrenia and other psychoses: seeking phenotypic insights into the pathobiology and treatment of negative symptoms. Eur. Neuropsychopharmacol. 2014;24(5):800–821. doi: 10.1016/j.euroneuro.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 86.Kirkpatrick B., Fenton W.S., Carpenter W.T., Jr, Marder S.R. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr. Bull. 2006;32(2):214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee J.S., Jung S., Park I.H., Kim J-J. Neural Basis of Anhedonia and Amotivation in Patients with Schizophrenia: The Role of Reward System. Curr. Neuropharmacol. 2015;13(6):750–759. doi: 10.2174/1570159X13666150612230333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Idris N. Atypical antipsychotic prevents and reverses negative symptoms in models of schizophrenia. Int. Neuropsychiatr. Dis. J. 2017;9(3):1–10. doi: 10.9734/INDJ/2017/34161. [DOI] [Google Scholar]
- 89.Lau C.G., Zukin R.S. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 2007;8(6):413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 90.Balu D.T. The NMDA Receptor and Schizophrenia: From Pathophysiology to Treatment. Adv. Pharmacol. 2016;76:351–382. doi: 10.1016/bs.apha.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ellaithy A., Younkin J., González-Maeso J., Logothetis D.E. Positive allosteric modulators of metabotropic glutamate 2 receptors in schizophrenia treatment. Trends Neurosci. 2015;38(8):506–516. doi: 10.1016/j.tins.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hanks A.N., Dlugolenski K., Hughes Z.A., Seymour P.A., Majchrzak M.J. Pharmacological disruption of mouse social approach behavior: relevance to negative symptoms of schizophrenia. Behav. Brain Res. 2013;252:405–414. doi: 10.1016/j.bbr.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 93.Barnes S.A., Der-Avakian A., Young J.W. Preclinical Models to Investigate Mechanisms of Negative Symptoms in Schizophrenia. Schizophr. Bull. 2017;43(4):706–711. doi: 10.1093/schbul/sbx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sahin C., Doostdar N., Neill J.C. Towards the development of improved tests for negative symptoms of schizophrenia in a validated animal model. Behav. Brain Res. 2016;312:93–101. doi: 10.1016/j.bbr.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 95.Nomura S., Shimizu J., Kinjo M., Kametani H., Nakazawa T. A new behavioral test for antidepressant drugs. Eur. J. Pharmacol. 1982;83(3-4):171–175. doi: 10.1016/0014-2999(82)90248-5. [DOI] [PubMed] [Google Scholar]
- 96.Neves G., Borsoi M., Antonio C.B., Pranke M.A., Betti A.H., Rates S.M.K. Is Forced Swimming Immobility a Good Endpoint for Modeling Negative Symptoms of Schizophrenia? - Study of Sub-Anesthetic Ketamine Repeated Administration Effects. An. Acad. Bras. Cienc. 2017;89(3):1655–1669. doi: 10.1590/0001-3765201720160844. [DOI] [PubMed] [Google Scholar]
- 97.Babri S., Doosti M-H., Salari A-A. Strain-dependent effects of prenatal maternal immune activation on anxiety- and depression-like behaviors in offspring. Brain Behav. Immun. 2014;37:164–176. doi: 10.1016/j.bbi.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 98.Brielmaier J., Senerth J.M., Silverman J.L., Matteson P.G., Millonig J.H., DiCicco-Bloom E., Crawley J.N. Chronic desipramine treatment rescues depression-related, social and cognitive deficits in Engrailed-2 knockout mice. Genes Brain Behav. 2014;13(3):286–298. doi: 10.1111/gbb.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Notaras M.J., Vivian B., Wilson C., van den Buuse M. Interaction of reelin and stress on immobility in the forced swim test but not dopamine-mediated locomotor hyperactivity or prepulse inhibition disruption: Relevance to psychotic and mood disorders. Schizophr. Res. 2020;215:485–492. doi: 10.1016/j.schres.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 100.Fujikawa R., Higuchi S., Ikedo T., Nagata M., Hayashi K., Yang T., Miyata T., Yokode M., Minami M. Behavioral abnormalities and reduced norepinephrine in EP4 receptor-associated protein (EPRAP)-deficient mice. Biochem. Biophys. Res. Commun. 2017;486(2):584–588. doi: 10.1016/j.bbrc.2017.03.095. [DOI] [PubMed] [Google Scholar]
- 101.Kulak A., Cuenod M., Do K.Q. Behavioral phenotyping of glutathione-deficient mice: relevance to schizophrenia and bipolar disorder. Behav. Brain Res. 2012;226(2):563–570. doi: 10.1016/j.bbr.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 102.Popova N.K., Naumenko V.S., Tibeikina M.A., Kulikov A.V. Serotonin transporter, 5-HT1A receptor, and behavior in DBA/2J mice in comparison with four inbred mouse strains. J. Neurosci. Res. 2009;87(16):3649–3657. doi: 10.1002/jnr.22155. [DOI] [PubMed] [Google Scholar]
- 103.Bilsland J.G., Wheeldon A., Mead A., Znamenskiy P., Almond S., Waters K.A., Thakur M., Beaumont V., Bonnert T.P., Heavens R., Whiting P., McAllister G., Munoz-Sanjuan I. Behavioral and neurochemical alterations in mice deficient in anaplastic lymphoma kinase suggest therapeutic potential for psychiatric indications. Neuropsychopharmacology. 2008;33(3):685–700. doi: 10.1038/sj.npp.1301446. [DOI] [PubMed] [Google Scholar]
- 104.Li Z., Boules M., Williams K., Gordillo A., Li S., Richelson E. Similarities in the behavior and molecular deficits in the frontal cortex between the neurotensin receptor subtype 1 knockout mice and chronic phencyclidine-treated mice: relevance to schizophrenia. Neurobiol. Dis. 2010;40(2):467–477. doi: 10.1016/j.nbd.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Rezende V.B., Rosa D.V., Comim C.M., Magno L.A.V., Rodrigues A.L.S., Vidigal P., Jeromin A., Quevedo J., Romano-Silva M.A. NCS-1 deficiency causes anxiety and depressive-like behavior with impaired non-aversive memory in mice. Physiol. Behav. 2014;130:91–98. doi: 10.1016/j.physbeh.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 106.Maksimovic M., Vekovischeva O.Y., Aitta-aho T., Korpi E.R. Chronic treatment with mood-stabilizers attenuates abnormal hyperlocomotion of GluA1-subunit deficient mice. PLoS One. 2014;9(6):e100188. doi: 10.1371/journal.pone.0100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cressant A., Dubreuil V., Kong J., Kranz T.M., Lazarini F., Launay J-M., Callebert J., Sap J., Malaspina D., Granon S., Harroch S. Loss-of-function of PTPR γ and ζ, observed in sporadic schizophrenia, causes brain region-specific deregulation of monoamine levels and altered behavior in mice. Psychopharmacology (Berl.) 2017;234(4):575–587. doi: 10.1007/s00213-016-4490-8. [DOI] [PubMed] [Google Scholar]
- 108.Umemori J., Takao K., Koshimizu H., Hattori S., Furuse T., Wakana S., Miyakawa T. ENU-mutagenesis mice with a non-synonymous mutation in Grin1 exhibit abnormal anxiety-like behaviors, impaired fear memory, and decreased acoustic startle response. BMC Res. Notes. 2013;6:203. doi: 10.1186/1756-0500-6-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chatterjee M., Ganguly S., Srivastava M., Palit G. Effect of ‘chronic’ versus ‘acute’ ketamine administration and its ‘withdrawal’ effect on behavioural alterations in mice: implications for experimental psychosis. Behav. Brain Res. 2011;216(1):247–254. doi: 10.1016/j.bbr.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 110.Suárez-Santiago J.E., Briones-Aranda A., Espinosa-Raya J., Picazo O. Agonist E-6837 and antagonist SB-271046 of 5-HT6 receptors both reverse the depressive-like effect induced in mice by subchronic ketamine administration. Behav. Pharmacol. 2017;28(7):582–585. doi: 10.1097/FBP.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 111.Langen B., Dost R., Egerland U., Stange H., Hoefgen N. Effect of PDE10A inhibitors on MK-801-induced immobility in the forced swim test. Psychopharmacology (Berl.) 2012;221(2):249–259. doi: 10.1007/s00213-011-2567-y. [DOI] [PubMed] [Google Scholar]
- 112.Woźniak M., Cieślik P., Marciniak M., Lenda T., Pilc A., Wieronska J.M. Neurochemical changes underlying schizophrenia-related behavior in a modified forced swim test in mice. Pharmacol. Biochem. Behav. 2018;172:50–58. doi: 10.1016/j.pbb.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 113.Shin E.J., Kim J.M., Nguyen X.K.T., Nguyen T.T.L., Lee S.Y., Jung J.H., Kim M.J., Whang W.K., Yamada K., Nabeshima T., Kim H.C. Effects of gastrodia elata bl on phencyclidine-induced schizophrenia-like psychosis in mice. Curr. Neuropharmacol. 2011;9(1):247–250. doi: 10.2174/157015911795017263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Commons K.G., Cholanians A.B., Babb J.A., Ehlinger D.G. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem. Neurosci. 2017;8(5):955–960. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kara N.Z., Stukalin Y., Einat H. Revisiting the validity of the mouse forced swim test: Systematic review and meta-analysis of the effects of prototypic antidepressants. Neurosci. Biobehav. Rev. 2018;84:1–11. doi: 10.1016/j.neubiorev.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 116.Unal G., Canbeyli R. Psychomotor retardation in depression: A critical measure of the forced swim test. Behav. Brain Res. 2019;372:112047. doi: 10.1016/j.bbr.2019.112047. [DOI] [PubMed] [Google Scholar]
- 117.Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl.) 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 118.Can A., Dao D.T., Terrillion C.E., Piantadosi S.C., Bhat S., Gould T.D. The tail suspension test. J. Vis. Exp. 2012;(59):e3769. doi: 10.3791/3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chatterjee M., Jaiswal M., Palit G. Comparative evaluation of forced swim test and tail suspension test as models of negative symptom of schizophrenia in rodents. ISRN Psychiatry. 2012;2012:595141. doi: 10.5402/2012/595141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yan H.C., Cao X., Das M., Zhu X.H., Gao T.M. Behavioral animal models of depression. Neurosci. Bull. 2010;26(4):327–337. doi: 10.1007/s12264-010-0323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berrocoso E., Ikeda K., Sora I., Uhl G.R., Sánchez-Blázquez P., Mico J.A. Active behaviours produced by antidepressants and opioids in the mouse tail suspension test. Int. J. Neuropsychopharmacol. 2013;16(1):151–162. doi: 10.1017/S1461145711001842. [DOI] [PubMed] [Google Scholar]
- 122.Al-Amin M.M., Sultana R., Sultana S., Rahman M.M., Reza H.M. Astaxanthin ameliorates prenatal LPS-exposed behavioral deficits and oxidative stress in adult offspring. BMC Neurosci. 2016;17(1):11. doi: 10.1186/s12868-016-0245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bisaz R., Sandi C. Vulnerability of conditional NCAM-deficient mice to develop stress-induced behavioral alterations. Stress. 2012;15(2):195–206. doi: 10.3109/10253890.2011.608226. [DOI] [PubMed] [Google Scholar]
- 124.Tammimaki A., Aonurm-Helm A., Zhang F.P., Poutanen M., Duran-Torres G., Garcia-Horsman A., Mannisto P.T. Generation of membrane-bound catechol-O-methyl transferase deficient mice with disctinct sex dependent behavioral phenotype. J. Physiol. Pharmaol. (6):827–842. [PubMed] [Google Scholar]
- 125.Castañé A., Santana N., Artigas F. PCP-based mice models of schizophrenia: differential behavioral, neurochemical and cellular effects of acute and subchronic treatments. Psychopharmacology (Berl.) 2015;232(21-22):4085–4097. doi: 10.1007/s00213-015-3946-6. [DOI] [PubMed] [Google Scholar]
- 126.Serchov T., van Calker D., Biber K. Sucrose Preference Test to Measure Anhedonic Behaviour in Mice. Bio Protoc. 2016;6(19):e1958. doi: 10.21769/BioProtoc.1958. [DOI] [Google Scholar]
- 127.Liu M-Y., Yin C-Y., Zhu L-J., Zhu X-H., Xu C., Luo C-X., Chen H., Zhu D-Y., Zhou Q-G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018;13(7):1686–1698. doi: 10.1038/s41596-018-0011-z. [DOI] [PubMed] [Google Scholar]
- 128.Brenes Sáenz J.C., Villagra O.R., Fornaguera Trías J. Factor analysis of Forced Swimming test, Sucrose Preference test and Open Field test on enriched, social and isolated reared rats. Behav. Brain Res. 2006;169(1):57–65. doi: 10.1016/j.bbr.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 129.Labouesse M.A., Langhans W., Meyer U. Abnormal context-reward associations in an immune-mediated neurodevelopmental mouse model with relevance to schizophrenia. Transl. Psychiatry. 2015;5:e637. doi: 10.1038/tp.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grimm C.M., Aksamaz S., Schulz S., Teutsch J., Sicinski P., Liss B., Kätzel D. Schizophrenia-related cognitive dysfunction in the Cyclin-D2 knockout mouse model of ventral hippocampal hyperactivity. Transl. Psychiatry. 2018;8(1):212. doi: 10.1038/s41398-018-0268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cheng L., Hattori E., Nakajima A., Woehrle N.S., Opal M.D., Zhang C., Grennan K., Dulawa S.C., Tang Y.P., Gershon E.S., Liu C. Expression of the G72/G30 gene in transgenic mice induces behavioral changes. Mol. Psychiatry. 2014;19(2):175–183. doi: 10.1038/mp.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bygrave A.M., Masiulis S., Nicholson E., Berkemann M., Barkus C., Sprengel R., Harrison P.J., Kullmann D.M., Bannerman D.M., Kätzel D. Knockout of NMDA-receptors from parvalbumin interneurons sensitizes to schizophrenia-related deficits induced by MK-801. Transl. Psychiatry. 2016;6:e778. doi: 10.1038/tp.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Catches J.S., Xu J., Contractor A. Genetic ablation of the GluK4 kainate receptor subunit causes anxiolytic and antidepressant-like behavior in mice. Behav. Brain Res. 2012;228(2):406–414. doi: 10.1016/j.bbr.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wolf G., Lifschytz T., Ben-Ari H., Tatarskyy P., Merzel T.K., Lotan A., Lerer B. Effect of chronic unpredictable stress on mice with developmental under-expression of the Ahi1 gene: behavioral manifestations and neurobiological correlates. Transl. Psychiatry. 2018;8(1):124. doi: 10.1038/s41398-018-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barkus C., Feyder M., Graybeal C., Wright T., Wiedholz L., Izquierdo A., Kiselycznyk C., Schmitt W., Sanderson D.J., Rawlins J.N.P., Saksida L.M., Bussey T.J., Sprengel R., Bannerman D., Holmes A. Do GluA1 knockout mice exhibit behavioral abnormalities relevant to the negative or cognitive symptoms of schizophrenia and schizoaffective disorder? Neuropharmacology. 2012;62(3):1263–1272. doi: 10.1016/j.neuropharm.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Desbonnet L., O’Tuathaigh C., Clarke G., O’Leary C., Petit E., Clarke N., Tighe O., Lai D., Harvey R., Cryan J.F., Dinan T.G., Waddington J.L. Phenotypic effects of repeated psychosocial stress during adolescence in mice mutant for the schizophrenia risk gene neuregulin-1: a putative model of gene × environment interaction. Brain Behav. Immun. 2012;26(4):660–671. doi: 10.1016/j.bbi.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 137.Golani I., Tadmor H., Buonanno A., Kremer I., Shamir A. Disruption of the ErbB signaling in adolescence increases striatal dopamine levels and affects learning and hedonic-like behavior in the adult mouse. Eur. Neuropsychopharmacol. 2014;24(11):1808–1818. doi: 10.1016/j.euroneuro.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 138.Liu Y., Lan N., Ren J., Wu Y., Wang S.T., Huang X-F., Yu Y. Orientin improves depression-like behavior and BDNF in chronic stressed mice. Mol. Nutr. Food Res. 2015;59(6):1130–1142. doi: 10.1002/mnfr.201400753. [DOI] [PubMed] [Google Scholar]
- 139.Kaidanovich-Beilin O., Lipina T., Vukobradovic I., Roder J., Woodgett J.R. Assessment of social interaction behaviors. J. Vis. Exp. 2011;(48):e2473. doi: 10.3791/2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zoubovsky S., Oh E.C., Cash-Padgett T., Johnson A.W., Hou Z., Mori S., Gallagher M., Katsanis N., Sawa A., Jaaro-Peled H. Neuroanatomical and behavioral deficits in mice haploinsufficient for Pericentriolar material 1 (Pcm1). Neurosci. Res. 2015;98:45–49. doi: 10.1016/j.neures.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Panther P., Nullmeier S., Dobrowolny H., Schwegler H., Wolf R. CPB-K mice a mouse model of schizophrenia? Differences in dopaminergic, serotonergic and behavioral markers compared to BALB/cJ mice. Behav. Brain Res. 2012;230(1):215–228. doi: 10.1016/j.bbr.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 142.Terzian A.L.B., Micale V., Wotjak C.T. Cannabinoid receptor type 1 receptors on GABAergic vs. glutamatergic neurons differentially gate sex-dependent social interest in mice. Eur. J. Neurosci. 2014;40(1):2293–2298. doi: 10.1111/ejn.12561. [DOI] [PubMed] [Google Scholar]
- 143.Lin C-W., Hsueh Y-P. Sarm1, a neuronal inflammatory regulator, controls social interaction, associative memory and cognitive flexibility in mice. Brain Behav. Immun. 2014;37:142–151. doi: 10.1016/j.bbi.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 144.Egashira N., Tanoue A., Matsuda T., Koushi E., Harada S., Takano Y., Tsujimoto G., Mishima K., Iwasaki K., Fujiwara M. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav. Brain Res. 2007;178(1):123–127. doi: 10.1016/j.bbr.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 145.Mihara T., Mensah-Brown K., Sobota R., Lin R., Featherstone R., Siegel S.J. Amygdala activity associated with social choice in mice. Behav. Brain Res. 2017;332:84–89. doi: 10.1016/j.bbr.2017.04.040. [DOI] [PubMed] [Google Scholar]
- 146.Schneider S., Götz K., Birchmeier C., Schwegler H., Roskoden T. Neuregulin-1 mutant mice indicate motor and sensory deficits, indeed few references for schizophrenia endophenotype model. Behav. Brain Res. 2017;322(Pt A):177–185. doi: 10.1016/j.bbr.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 147.Sankoorikal G.M., Kaercher K.A., Boon C.J., Lee J.K., Brodkin E.S. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol. Psychiatry. 2006;59(5):415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 148.Bove M., Ike K., Eldering A., Buwalda B., de Boer S.F., Morgese M.G., Schiavone S., Cuomo V., Trabace L., Kas M.J.H. The Visible Burrow System: A behavioral paradigm to assess sociability and social withdrawal in BTBR and C57BL/6J mice strains. Behav. Brain Res. 2018;344:9–19. doi: 10.1016/j.bbr.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 149.Estanislau C., Morato S. Behavior ontogeny in the elevated plus-maze: prenatal stress effects. Int. J. Dev. Neurosci. 2006;24(4):255–262. doi: 10.1016/j.ijdevneu.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 150.Walf A.A., Frye C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007;2(2):322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Komada M., Takao K., Miyakawa T. Elevated plus maze for mice. J. Vis. Exp. 2008;(22):e1088. doi: 10.3791/1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van der Werf I.M., Van Dam D., Missault S., Yalcin B., De Deyn P.P., Vandeweyer G., Kooy R.F. Behavioural characterization of AnkyrinG deficient mice, a model for ANK3 related disorders. Behav. Brain Res. 2017;328:218–226. doi: 10.1016/j.bbr.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 153.Dachtler J., Elliott C., Rodgers R.J., Baillie G.S., Clapcote S.J. Missense mutation in DISC1 C-terminal coiled-coil has GSK3β signaling and sex-dependent behavioral effects in mice. Sci. Rep. 2016;6:18748. doi: 10.1038/srep18748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Erhardt S., Pocivavsek A., Repici M., Liu X-C., Imbeault S., Maddison D.C., Thomas M.A.R., Smalley J.L., Larsson M.K., Muchowski P.J., Giorgini F., Schwarcz R. Adaptive and Behavioral Changes in Kynurenine 3-Monooxygenase Knockout Mice: Relevance to Psychotic Disorders. Biol. Psychiatry. 2017;82(10):756–765. doi: 10.1016/j.biopsych.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Aizawa F., Ogaki Y., Kyoya N., Nishinaka T., Nakamoto K., Kurihara T., Hirasawa A., Miyata A., Tokuyama S. The Deletion of GPR40/FFAR1 Signaling Damages Maternal Care and Emotional Function in Female Mice. Biol. Pharm. Bull. 2017;40(8):1255–1259. doi: 10.1248/bpb.b17-00082. [DOI] [PubMed] [Google Scholar]
- 156.Morris M.J., Na E.S., Autry A.E., Monteggia L.M. Impact of DNMT1 and DNMT3a forebrain knockout on depressive- and anxiety like behavior in mice. Neurobiol. Learn. Mem. 2016;135:139–145. doi: 10.1016/j.nlm.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nakamura H., Yamashita N., Kimura A., Kimura Y., Hirano H., Makihara H., Kawamoto Y., Jitsuki-Takahashi A., Yonezaki K., Takase K., Miyazaki T., Nakamura F., Tanaka F., Goshima Y. Comprehensive behavioral study and proteomic analyses of CRMP2-deficient mice. Genes Cells. 2016;21(10):1059–1079. doi: 10.1111/gtc.12403. [DOI] [PubMed] [Google Scholar]
- 158.Shevelkin A.V., Terrillion C.E., Abazyan B.N., Kajstura T.J., Jouroukhin Y.A., Rudow G.L., Troncoso J.C., Linden D.J., Pletnikov M.V. Expression of mutant DISC1 in Purkinje cells increases their spontaneous activity and impairs cognitive and social behaviors in mice. Neurobiol. Dis. 2017;103:144–153. doi: 10.1016/j.nbd.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kraeuter A.K., Guest P.C., Sarnyai Z. The Elevated Plus Maze Test for Measuring Anxiety-Like Behavior in Rodents. Methods Mol. Biol. 2019;1916:69–74. doi: 10.1007/978-1-4939-8994-2_4. [DOI] [PubMed] [Google Scholar]
- 160.Rodgers R.J., Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 1997;21(6):801–810. doi: 10.1016/S0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 161.File S.E. The interplay of learning and anxiety in the elevated plus-maze. Behav. Brain Res. 1993;58(1-2):199–202. doi: 10.1016/0166-4328(93)90103-W. [DOI] [PubMed] [Google Scholar]
- 162.Hope B.V., Hamilton T.J., Hurd P.L. Submerged plus maze: A novel test for studying anxiety-like behaviour in fish. Behav. Brain Res. 2019;362:332–337. doi: 10.1016/j.bbr.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 163.Neill J.C., Barnes S., Cook S., Grayson B., Idris N.F., McLean S.L., Snigdha S., Rajagopal L., Harte M.K. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol. Ther. 2010;128(3):419–432. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 164.Keefe R.S., Eesley C.E., Poe M.P. Defining a cognitive function decrement in schizophrenia. Biol. Psychiatry. 2005;57(6):688–691. doi: 10.1016/j.biopsych.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 165.Tripathi A., Kar S.K., Shukla R. Cognitive Deficits in Schizophrenia: Understanding the Biological Correlates and Remediation Strategies. Clin. Psychopharmacol. Neurosci. 2018;16(1):7–17. doi: 10.9758/cpn.2018.16.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Center for Mental Health Services, National Institute of Mental Health (U.S.). Mental Health: A Report of the Surgeon General. Rockville, Md. : Department of Health and Human Services, U.S., Public Health Service ; Pittsburgh, PA : For sale by the Supt. of Docs. 1999 [Google Scholar]
- 167.Lesh T.A., Niendam T.A., Minzenberg M.J., Carter C.S. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36(1):316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jackson M.E., Homayoun H., Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc. Natl. Acad. Sci. USA. 2004;101(22):8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lisman J.E., Coyle J.T., Green R.W., Javitt D.C., Benes F.M., Heckers S., Grace A.A. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31(5):234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Green M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 171.Green M.F., Nuechterlein K.H., Gold J.M., Barch D.M., Cohen J., Essock S., Fenton W.S., Frese F., Goldberg T.E., Heaton R.K., Keefe R.S., Kern R.S., Kraemer H., Stover E., Weinberger D.R., Zalcman S., Marder S.R. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol. Psychiatry. 2004;56(5):301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 172.Zeng H., Chattarji S., Barbarosie M., Rondi-Reig L., Philpot B.D., Miyakawa T., Bear M.F., Tonegawa S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107(5):617–629. doi: 10.1016/S0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- 173.D’Esposito M., Postle B.R. The cognitive neuroscience of working memory. Annu. Rev. Psychol. 2015;66:115–142. doi: 10.1146/annurev-psych-010814-015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Berberian A.A., Trevisan B.T., Moriyama T.S., Montiel J.M., Oliveira J.A., Seabra A.G. Working memory assessment in schizophrenia and its correlation with executive functions ability. Br. J. Psychiatry. 2009;31(3):219–226. doi: 10.1590/S1516-44462009000300007. [DOI] [PubMed] [Google Scholar]
- 175.Diamond D.M., Park C.R., Heman K.L., Rose G.M. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9(5):542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 176.Dudchenko P.A., Wood E.R., Eichenbaum H. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. J. Neurosci. 2000;20(8):2964–2977. doi: 10.1523/JNEUROSCI.20-08-02964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 178.Dudchenko P.A. An overview of the tasks used to test working memory in rodents. Neurosci. Biobehav. Rev. 2004;28(7):699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 179.Crider A. Perseveration in schizophrenia. Schizophr. Bull. 1997;23(1):63–74. doi: 10.1093/schbul/23.1.63. [DOI] [PubMed] [Google Scholar]
- 180.Olton D.S. The radial arm maze as a tool in behavioral pharmacology. Physiol. Behav. 1987;40(6):793–797. doi: 10.1016/0031-9384(87)90286-1. [DOI] [PubMed] [Google Scholar]
- 181.Matsuo N., Yamasaki N., Ohira K., Takao K., Toyama K., Eguchi M., Yamaguchi S., Miyakawa T. Neural activity changes underlying the working memory deficit in alpha-CaMKII heterozygous knockout mice. Front. Behav. Neurosci. 2009;3:20–20. doi: 10.3389/neuro.08.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Carboni G., Tueting P., Tremolizzo L., Sugaya I., Davis J., Costa E., Guidotti A. Enhanced dizocilpine efficacy in heterozygous reeler mice relates to GABA turnover downregulation. Neuropharmacology. 2004;46(8):1070–1081. doi: 10.1016/j.neuropharm.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 183.Szumlinski K.K., Lominac K.D., Kleschen M.J., Oleson E.B., Dehoff M.H., Schwarz M.K., Seeburg P.H., Worley P.F., Kalivas P.W. Behavioral and neurochemical phenotyping of Homer1 mutant mice: possible relevance to schizophrenia. Genes Brain Behav. 2005;4(5):273–288. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- 184.Ikeda M., Hikita T., Taya S., Uraguchi-Asaki J., Toyo-oka K., Wynshaw-Boris A., Ujike H., Inada T., Takao K., Miyakawa T., Ozaki N., Kaibuchi K., Iwata N. Identification of YWHAE, a gene encoding 14-3-3epsilon, as a possible susceptibility gene for schizophrenia. Hum. Mol. Genet. 2008;17(20):3212–3222. doi: 10.1093/hmg/ddn217. [DOI] [PubMed] [Google Scholar]
- 185.Duffy L., Cappas E., Lai D., Boucher A.A., Karl T. Cognition in transmembrane domain neuregulin 1 mutant mice. Neuroscience. 2010;170(3):800–807. doi: 10.1016/j.neuroscience.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 186.Young J.W., Meves J.M., Tarantino I.S., Caldwell S., Geyer M.A. Delayed procedural learning in α7-nicotinic acetylcholine receptor knockout mice. Genes Brain Behav. 2011;10(7):720–733. doi: 10.1111/j.1601-183X.2011.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li Z., Kim C.H., Ichikawa J., Meltzer H.Y. Effect of repeated administration of phencyclidine on spatial performance in an eight-arm radial maze with delay in rats and mice. Pharmacol. Biochem. Behav. 2003;75(2):335–340. doi: 10.1016/S0091-3057(03)00085-6. [DOI] [PubMed] [Google Scholar]
- 188.Hodges H. Maze procedures: the radial-arm and water maze compared. Brain Res. Cogn. Brain Res. 1996;3(3-4):167–181. doi: 10.1016/0926-6410(96)00004-3. [DOI] [PubMed] [Google Scholar]
- 189.Walsh T.J., Chrobak J.J. The use of the radial arm maze in neurotoxicology. Physiol. Behav. 1987;40(6):799–803. doi: 10.1016/0031-9384(87)90287-3. [DOI] [PubMed] [Google Scholar]
- 190.Gerlai R. A new continuous alternation task in T-maze detects hippocampal dysfunction in mice. A strain comparison and lesion study. Behav. Brain Res. 1998;95(1):91–101. doi: 10.1016/S0166-4328(97)00214-3. [DOI] [PubMed] [Google Scholar]
- 191.Crusio W.E., Bertholet J.Y., Schwegler H. No correlations between spatial and non-spatial reference memory in a T-maze task and hippocampal mossy fibre distribution in the mouse. Behav. Brain Res. 1990;41(3):251–259. doi: 10.1016/0166-4328(90)90112-R. [DOI] [PubMed] [Google Scholar]
- 192.Prieur E.A.K., Jadavji N.M. Assessing Spatial Working Memory Using the Spontaneous Alternation Y-maze Test in Aged Male Mice. Bio Protoc. 2019;9(3):e3162. doi: 10.21769/BioProtoc.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Deacon R.M.J., Rawlins J.N.P. T-maze alternation in the rodent. Nat. Protoc. 2006;1(1):7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- 194.Wenk G.L. Assessment of Spatial Memory Using the T Maze. Curr. Protoc. Neurosci. 1998;4(1):8.5B.1–8.5A.7. doi: 10.1002/0471142301.ns0805bs04. [DOI] [PubMed] [Google Scholar]
- 195.Kraeuter A.K., Guest P.C., Sarnyai Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol. Biol. 2019;1916:105–111. doi: 10.1007/978-1-4939-8994-2_10. [DOI] [PubMed] [Google Scholar]
- 196.Terry A.V., Jr . In: Methods of Behavior Analysis in Neuroscience. Buccafusco J.J., editor. CRC Press/Taylor & Francis, Taylor & Francis Group, LLC.: Boca Raton (FL); 2009. Frontiers in Neuroscience: Spatial Navigation (Water Maze) Tasks. [PubMed] [Google Scholar]
- 197.Yang W., Zhu C., Shen Y., Xu Q. The pathogenic mechanism of dysbindin-1B toxic aggregation: BLOC-1 and intercellular vesicle trafficking. Neuroscience. 2016;333:78–91. doi: 10.1016/j.neuroscience.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 198.Clarke D.J., Sarkissian L., Todd S.M., Suraev A.S., Bahceci D., Brzozowska N., Arnold J.C. Nrg1 deficiency modulates the behavioural effects of prenatal stress in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;88:86–95. doi: 10.1016/j.pnpbp.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 199.Berbari N.F., Malarkey E.B., Yazdi S.M.Z.R., McNair A.D., Kippe J.M., Croyle M.J., Kraft T.W., Yoder B.K. Hippocampal and cortical primary cilia are required for aversive memory in mice. PLoS One. 2014;9(9):e106576–e106576. doi: 10.1371/journal.pone.0106576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ning H., Cao D., Wang H., Kang B., Xie S., Meng Y. Effects of haloperidol, olanzapine, ziprasidone, and PHA-543613 on spatial learning and memory in the Morris water maze test in naïve and MK-801-treated mice. Brain Behav. 2017;7(8):e00764. doi: 10.1002/brb3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Naert A., Gantois I., Laeremans A., Vreysen S., Van den Bergh G., Arckens L., Callaerts-Vegh Z., D’Hooge R. Behavioural alterations relevant to developmental brain disorders in mice with neonatally induced ventral hippocampal lesions. Brain Res. Bull. 2013;94:71–81. doi: 10.1016/j.brainresbull.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 202.Thackray S.E., McAllister B.B., Dyck R.H. Behavioral characterization of female zinc transporter 3 (ZnT3) knockout mice. Behav. Brain Res. 2017;321:36–49. doi: 10.1016/j.bbr.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 203.D’Hooge R., De Deyn P.P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Brain Res. Rev. 2001;36(1):60–90. doi: 10.1016/S0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 204.Tomás Pereira I., Burwell R.D. Using the spatial learning index to evaluate performance on the water maze. Behav. Neurosci. 2015;129(4):533–539. doi: 10.1037/bne0000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Brandeis R., Brandys Y., Yehuda S. The use of the Morris Water Maze in the study of memory and learning. Int. J. Neurosci. 1989;48(1-2):29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- 206.Clark R.E., Martin S.J. Interrogating rodents regarding their object and spatial memory. Curr. Opin. Neurobiol. 2005;15(5):593–598. doi: 10.1016/j.conb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 207.Dere E., Huston J.P., De Souza Silva M.A. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci. Biobehav. Rev. 2007;31(5):673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 208.Coyle P., Tran N., Fung J.N., Summers B.L., Rofe A.M. Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav. Brain Res. 2009;197(1):210–218. doi: 10.1016/j.bbr.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 209.Spiegel S., Chiu A., James A.S., Jentsch J.D., Karlsgodt K.H. Recognition deficits in mice carrying mutations of genes encoding BLOC-1 subunits pallidin or dysbindin. Genes Brain Behav. 2015;14(8):618–624. doi: 10.1111/gbb.12240. [DOI] [PubMed] [Google Scholar]
- 210.Chang E.H., Fernando K., Yeung L.W.E., Barbari K., Chandon T.S., Malhotra A.K. Single point mutation on the gene encoding dysbindin results in recognition deficits. Genes Brain Behav. 2018;17(5):e12449. doi: 10.1111/gbb.12449. [DOI] [PubMed] [Google Scholar]
- 211.Wang L., Alachkar A., Sanathara N., Belluzzi J.D., Wang Z., Civelli O. A Methionine-Induced Animal Model of Schizophrenia: Face and Predictive Validity. Int. J. Neuropsychopharmacol. 2015;18(12):pyv054. doi: 10.1093/ijnp/pyv054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Botton P.H., Costa M.S., Ardais A.P., Mioranzza S., Souza D.O., da Rocha J.B., Porciúncula L.O. Caffeine prevents disruption of memory consolidation in the inhibitory avoidance and novel object recognition tasks by scopolamine in adult mice. Behav. Brain Res. 2010;214(2):254–259. doi: 10.1016/j.bbr.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 213.Young J.W., Kerr L.E., Kelly J.S., Marston H.M., Spratt C., Finlayson K., Sharkey J. The odour span task: a novel paradigm for assessing working memory in mice. Neuropharmacology. 2007;52(2):634–645. doi: 10.1016/j.neuropharm.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 214.Branch C.L., Galizio M., Bruce K. What-Where-When Memory in the Rodent Odor Span Task. Learn. Motiv. 2014;47:18–29. doi: 10.1016/j.lmot.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Galizio M., April B., Deal M., Hawkey A., Panoz-Brown D., Prichard A., Bruce K. Behavioral pharmacology of the odor span task: Effects of flunitrazepam, ketamine, methamphetamine and methylphenidate. J. Exp. Anal. Behav. 2016;106(3):173–194. doi: 10.1002/jeab.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rushforth S.L., Steckler T., Shoaib M. Nicotine improves working memory span capacity in rats following sub-chronic ketamine exposure. Neuropsychopharmacology. 2011;36(13):2774–2781. doi: 10.1038/npp.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Arakawa H., Blanchard D.C., Arakawa K., Dunlap C., Blanchard R.J. Scent marking behavior as an odorant communication in mice. Neurosci. Biobehav. Rev. 2008;32(7):1236–1248. doi: 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Birrell J.M., Brown V.J. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 2000;20(11):4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tait D.S., Chase E.A., Brown V.J. Attentional set-shifting in rodents: a review of behavioural methods and pharmacological results. Curr. Pharm. Des. 2014;20(31):5046–5059. doi: 10.2174/1381612819666131216115802. [DOI] [PubMed] [Google Scholar]
- 220.Barense M.D., Fox M.T., Baxter M.G. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn. Mem. 2002;9(4):191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ceaser A.E., Goldberg T.E., Egan M.F., McMahon R.P., Weinberger D.R., Gold J.M. Set-shifting ability and schizophrenia: a marker of clinical illness or an intermediate phenotype? Biol. Psychiatry. 2008;64(9):782–788. doi: 10.1016/j.biopsych.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Garner J.P., Thogerson C.M., Würbel H., Murray J.D., Mench J.A. Animal neuropsychology: validation of the Intra-Dimensional Extra-Dimensional set shifting task for mice. Behav. Brain Res. 2006;173(1):53–61. doi: 10.1016/j.bbr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 223.Laurent V., Podhorna J. Subchronic phencyclidine treatment impairs performance of C57BL/6 mice in the attentional set-shifting task. Behav. Pharmacol. 2004;15(2):141–148. doi: 10.1097/00008877-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 224.Scheggia D., Papaleo F. An Operant Intra-/Extra-dimensional Set-shift Task for Mice. J. Vis. Exp. 2016;(107):e53503. doi: 10.3791/53503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Heisler J.M., Morales J., Donegan J.J., Jett J.D., Redus L., O’Connor J.C. The attentional set shifting task: a measure of cognitive flexibility in mice. J. Vis. Exp. 2015;(96):e51944. doi: 10.3791/51944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Glickstein S.B., Desteno D.A., Hof P.R., Schmauss C. Mice lacking dopamine D2 and D3 receptors exhibit differential activation of prefrontal cortical neurons during tasks requiring attention. Cereb. Cortex. 2005;15(7):1016–1024. doi: 10.1093/cercor/bhh202. [DOI] [PubMed] [Google Scholar]
- 227.Fox M.T., Barense M.D., Baxter M.G. Perceptual attentional set-shifting is impaired in rats with neurotoxic lesions of posterior parietal cortex. J. Neurosci. 2003;23(2):676–681. doi: 10.1523/JNEUROSCI.23-02-00676.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Willing J., Wagner C.K. Progesterone receptor expression in the developing mesocortical dopamine pathway: importance for complex cognitive behavior in adulthood. Neuroendocrinology. 2016;103(3-4):207–222. doi: 10.1159/000434725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gregg J.R., Herring N.R., Naydenov A.V., Hanlin R.P., Konradi C. Downregulation of oligodendrocyte transcripts is associated with impaired prefrontal cortex function in rats. Schizophr. Res. 2009;113(2-3):277–287. doi: 10.1016/j.schres.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jeevakumar V., Driskill C., Paine A., Sobhanian M., Vakil H., Morris B., Ramos J., Kroener S. Ketamine administration during the second postnatal week induces enduring schizophrenia-like behavioral symptoms and reduces parvalbumin expression in the medial prefrontal cortex of adult mice. Behav. Brain Res. 2015;282:165–175. doi: 10.1016/j.bbr.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 231.Kos T., Nikiforuk A., Rafa D., Popik P. The effects of NMDA receptor antagonists on attentional set-shifting task performance in mice. Psychopharmacology (Berl.) 2011;214(4):911–921. doi: 10.1007/s00213-010-2102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bissonette G.B., Martins G.J., Franz T.M., Harper E.S., Schoenbaum G., Powell E.M. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J. Neurosci. 2008;28(44):11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Brown V.J., Tait D.S. Attentional Set-Shifting Across Species. Curr. Top. Behav. Neurosci. 2016;28:363–395. doi: 10.1007/7854_2015_5002. [DOI] [PubMed] [Google Scholar]
- 234.Popik P., Nikiforuk A. Attentional Set-Shifting Paradigm in the Rat. Curr. Protoc. Neurosci. 2015;72. doi: 10.1002/0471142301.ns0951s72. [DOI] [PubMed] [Google Scholar]
- 235.Robbins T.W. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl.) 2002;163(3-4):362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 236.Dalley J.W., Cardinal R.N., Robbins T.W. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav. Rev. 2004;28(7):771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 237.Bari A., Dalley J.W., Robbins T.W. The application of the 5- choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat. Protoc. 2008;3(5):759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- 238.Grissom N.M., Herdt C.T., Desilets J., Lidsky-Everson J., Reyes T.M. Dissociable deficits of executive function caused by gestational adversity are linked to specific transcriptional changes in the prefrontal cortex. Neuropsychopharmacology. 2015;40(6):1353–1363. doi: 10.1038/npp.2014.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Loos M., Mueller T., Gouwenberg Y., Wijnands R., van der Loo R.J., Birchmeier C., Smit A.B., Spijker S. Neuregulin-3 in the mouse medial prefrontal cortex regulates impulsive action. Biol. Psychiatry. 2014;76(8):648–655. doi: 10.1016/j.biopsych.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 240.Openshaw R.L., Thomson D.M., Penninger J.M., Pratt J.A., Morris B.J. Mice haploinsufficient for Map2k7, a gene involved in neurodevelopment and risk for schizophrenia, show impaired attention, a vigilance decrement deficit and unstable cognitive processing in an attentional task: impact of minocycline. Psychopharmacology (Berl.) 2017;234(2):293–305. doi: 10.1007/s00213-016-4463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Nilsson S.R.O., Celada P., Fejgin K., Thelin J., Nielsen J., Santana N., Heath C.J., Larsen P.H., Nielsen V., Kent B.A., Saksida L.M., Stensbøl T.B., Robbins T.W., Bastlund J.F., Bussey T.J., Artigas F., Didriksen M. A mouse model of the 15q13.3 microdeletion syndrome shows prefrontal neurophysiological dysfunctions and attentional impairment. Psychopharmacology (Berl.) 2016;233(11):2151–2163. doi: 10.1007/s00213-016-4265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Scott D., Taylor J.R. Chronic nicotine attenuates phencyclidine-induced impulsivity in a mouse serial reaction time task. Behav. Brain Res. 2014;259:164–173. doi: 10.1016/j.bbr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Higgins G.A., Silenieks L.B. Rodent test of attention and impulsivity: the 5-choice serial reaction time task. Curr. Protoc. Pharmacol. 2017;78:5.49.1–5.49. doi: 10.1002/cpph.27. [DOI] [PubMed] [Google Scholar]
- 244.Remmelink E., Chau U., Smit A.B., Verhage M., Loos M. A one-week 5-choice serial reaction time task to measure impulsivity and attention in adult and adolescent mice. Sci. Rep. 2017;7:42519. doi: 10.1038/srep42519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Huang H., Guadagna S., Mereu M., Ciampoli M., Pruzzo G., Ballard T., Papaleo F. A schizophrenia relevant 5-Choice Serial Reaction Time Task for mice assessing broad monitoring, distractibility and impulsivity. Psychopharmacology (Berl.) 2017;234(13):2047–2062. doi: 10.1007/s00213-017-4611-z. [DOI] [PubMed] [Google Scholar]
- 246.Fizet J., Cassel J.C., Kelche C., Meunier H. A review of the 5-Choice Serial Reaction Time (5-CSRT) task in different vertebrate models. Neurosci. Biobehav. Rev. 2016;71:135–153. doi: 10.1016/j.neubiorev.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 247.Asinof S.K., Paine T.A. The 5-choice serial reaction time task: a task of attention and impulse control for rodents. J. Vis. Exp. 2014;(90):e51574. doi: 10.3791/51574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bale T.L., Abel T., Akil H., Carlezon W.A., Jr, Moghaddam B., Nestler E.J., Ressler K.J., Thompson S.M. The critical importance of basic animal research for neuropsychiatric disorders. Neuropsychopharmacology. 2019;44(8):1349–1353. doi: 10.1038/s41386-019-0405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Garner J.P. The significance of meaning: why do over 90% of behavioral neuroscience results fail to translate to humans, and what can we do to fix it? ILAR J. 2014;55(3):438–456. doi: 10.1093/ilar/ilu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hvoslef-Eide M., Mar A.C., Nilsson S.R., Alsiö J., Heath C.J., Saksida L.M., Robbins T.W., Bussey T.J. The NEWMEDS rodent touchscreen test battery for cognition relevant to schizophrenia. Psychopharmacology (Berl.) 2015;232(21-22):3853–3872. doi: 10.1007/s00213-015-4007-x. [DOI] [PubMed] [Google Scholar]
- 251.Yee B.K., Singer P. A conceptual and practical guide to the behavioural evaluation of animal models of the symptomatology and therapy of schizophrenia. Cell Tissue Res. 2013;354(1):221–246. doi: 10.1007/s00441-013-1611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]