Abstract
Ionizing radiation (IR) exposure causes mammalian cells to undergo p53-dependent cell cycle arrest and/or apoptosis. The in vivo role of DNA-dependent protein kinase (DNA-PK) in the transduction of the DNA damage signal to p53 remains unresolved. To determine the relationship between DNA-PK and p53, we studied the cell cycle and apoptotic responses to IR in mice deficient in DNA-PK. Using the slip mouse, which harbors an inactivating mutation of the DNA-PK catalytic subunit (DNA-PKcs), we demonstrated not only that these DNA-PKcs null mutants were highly radiosensitive but also that upon IR treatment, p53 accumulated in their cultured cells and tissue. Induced p53 was transcriptionally active and mediated the induction of p21 and Bax in slip cells. Examination of the thymic cell cycle response to IR treatment indicated that the slip G1/S-phase cell cycle checkpoint function was intact. We further show that slip mice exhibited a higher level of spontaneous thymic apoptosis as well as a more robust apoptotic response to IR than wild-type mice. Together, these data demonstrate that the p53-mediated response to DNA damage is intact in cells devoid of DNA-PK activity and suggest that other kinases, such as the product of the gene (ATM) mutated in ataxia telangiectasia, are better candidates for regulating IR-induced phosphorylation and accumulation of p53.
DNA-dependent protein kinase (DNA-PK) is a multiprotein complex composed of a heterodimeric (Ku70 and Ku86) DNA binding component as well as the 460-kDa DNA-PK catalytic subunit (DNA-PKcs) (27). Molecular analysis of the DNA-PK components has revealed that DNA-PK is not only important for the effective rearrangement of the antigen receptor molecules in lymphoid cells but also intimately involved in the cellular response to DNA damage (14, 45, 47). Current evidence suggests that in response to single- and double-stranded DNA breaks generated by ionizing radiation (IR) or as intermediates in the V(D)J recombination process, the Ku70-Ku86 heterodimer binds the broken ends of the DNA in a sequence-independent manner, followed by the binding and activation of DNA-PKcs (23, 44). Once activated, DNA-PKcs acts as a serine/threonine kinase, but its in vivo substrates have remained elusive. In vitro, DNA-PKcs has been shown to phosphorylate a variety of proteins, including several transcription factors, the RNA polymerase II holoenzyme, the Ku components, and itself, suggesting a role for DNA-PK in the regulation of gene expression (1, 31). In vitro, DNA-PKcs also phosphorylates the p53 protein, a critical molecule in a signal transduction pathway bringing about cell cycle arrest and/or apoptosis in response to DNA damage (35, 37). DNA-PKcs phosphorylates p53 at serines 15 and 37, and peptides derived from the amino-terminal portion of p53 are routinely used as substrates in an assay to detect DNA-PKcs protein kinase activity (36, 39). Whether DNA-PKcs phosphorylates p53 and induces its accumulation in response to DNA damage in vivo, however, continues to be controversial. Studies with the scid mouse have lent insight into the role of DNA-PKcs in DNA damage response and V(D)J recombination (9, 36, 38, 42, 47). The scid mouse harbors a point mutation in the DNA-PKcs gene, resulting in an 83-amino-acid truncation that leaves the DNA-PKcs kinase region intact (2, 7). Detectable levels of DNA-PKcs are still present in the scid mouse, and several studies have demonstrated that when scid mice or cells derived from those mice are exposed to IR, p53 is appropriately induced and is functional (21, 24, 39, 41). Recently, however, Woo and colleagues have shown that in the established scid cell line SCGR11 and in the human glioma cell line MO59J, where DNA-PK activity is undetectable, DNA-PK is necessary for the activation of p53 (48).
DNA sequence analysis of the large DNA-PKcs revealed its membership in a family of proteins related to the phosphatidylinositol 3-kinase (PI3-K) protein superfamily (25). PI3-K-related members function as protein kinases and include several other proteins demonstrated to be involved in responding to DNA damage or in cell cycle checkpoint function, such as ATM, ATR, tel 1, and Rad3 (45). The inactivation of one member of this family, Atm, brings about a defective thymic G1/S-phase cell cycle checkpoint function in response to IR, due to its inability to induce p53 (5).
In this study, the role of DNA-PK in the transduction of signals from damaged DNA to p53 was addressed in vivo by using the slip mouse (8, 28), one of several DNA-PKcs null mutant mice recently generated (22, 46). The slip mouse, an insertional mutant in which the 5′ portion of the DNA-PKcs gene has been disrupted, represents an important tool in studying the role of DNA-PK in the p53 response to IR.
MATERIALS AND METHODS
Mice.
All mice were housed in a pathogen-free environment. slip mice were generated on an FVB/N background as previously described (28). Wild-type FVB/N mice were purchased from Charles River, Frederick, Md. Both males and females were used in the described studies at 4 to 6 weeks of age. Irradiated mice were subjected to whole-body exposure to gamma irradiation from a 137Cs source (4.6 Gy/min) and were sacrificed at specified intervals after exposure. When determining the sensitivity of slip mice to IR, groups of 15 slip, scid, and wild-type FVB/N mice were used in each study. All mouse work was performed in accordance with the guidelines established by the National Institutes of Health.
Cells and Western blotting.
Mouse embryo fibroblasts (MEFs) were prepared from isolated 14-day-old FVB/N or slip embryos, which were briefly treated with 0.25% trypsin and 1 mM EDTA, with periodic pipetting to disperse cells. MEFs were plated in Dulbecco's modified Eagle's medium containing 15% fetal bovine serum, 100 mM l-glutamine, and 5× penicillin and streptomycin antibiotic. Whole-cell protein extracts were prepared by lysing the cells in a buffer containing 10 mM Tris (pH 8.0), 10% glycerol, 1 mM EDTA, 1 mM dithiothreitol, 400 mM NaCl, and 1% NP-40, plus complete proteinase inhibitors (Boehringer Mannheim). Protein concentrations were determined using the Bio-Rad protein assay. Typically, 50 μg of whole-cell extract was used in Western blotting, except for the DNA-PKcs Western blots, where 200 μg of extract was used. For Western blotting, whole-cell extracts were resolved on Tris-glycine precast polyacrylamide gels (Novex) and transferred to 0.2 μM nitrocellulose. Blocked membranes were incubated with either anti-DNA-PKcs (Ab-4; NeoMarkers), anti-p53 (FL-393; Santa Cruz Biotech), anti-p21 (C-19; Santa Cruz Biotech), or anti-Bax (D21; Santa Cruz Biotech) primary antibodies and were subsequently developed using Western Breeze (Novex) according to the manufacturer's protocol. Actin was visualized by incubation of the membranes with antiactin (I-19; Santa Cruz Biotech) primary antibody, followed by washing in phosphate-buffered saline–Tween 20 (0.1%), incubation with anti-goat secondary antibody (Amersham), and development with Super Signal (Pierce). Quantitation of the p53, p21, and Bax proteins visualized by Western blotting was carried out by densitometric analysis.
TUNEL assay and immunostaining.
Apoptotic analysis was carried out both by morphological assessments and by using a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay. Cells in both thymus and gut tissue sections stained with hematoxylin and eosin (H&E) were scored for evidence of chromatin condensation and cell fragmentation as well as cell shrinkage. Cytological analysis was followed by TUNEL assays, which were carried out using Oncor's Apoptag system according to the manufacturer's recommendation. Briefly, DNA of irradiated and unirradiated FVB/N and slip tissue sections was end labeled with digoxigenin-dUTP and visualized by peroxidase-conjugated antidigoxigenin antibody. Quantitation of apoptotic bodies was performed on three to five labeled tissue sections for either untreated or irradiated FVB/N and slip mice. Here, apoptotic bodies staining brown were scored and averaged from five independent counts obtained within 1-mm2 microscopic (200×) fields of each section.
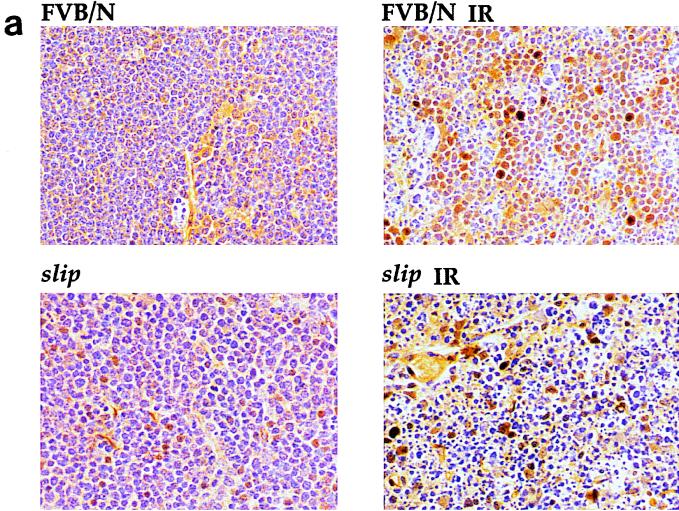
Tissue for immunostaining was fixed in at least 20 volumes of 10% buffered formalin, paraffin embedded, and sectioned. All tissues were sectioned and initially stained with H&E for histopathological analyses. Detection of p53-expressing cells in slip and FVB/N tissue (see Fig. 4) was performed using the AB-7 antibody (Oncogene Science). Here, untransfected cells as well as cells expressing p53 from a transfected construct were included as controls for p53 immunostaining. p53-positive nuclei were quantitated by scoring brown-staining nuclei and averaging the nuclear counts obtained from five 1-mm2 microscopic fields (200×) per section for a total of five mice for each category of untreated and treated slip and mice.
FIG. 4.
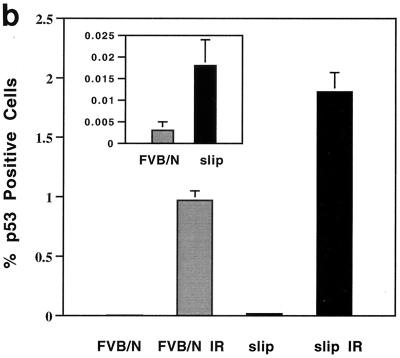
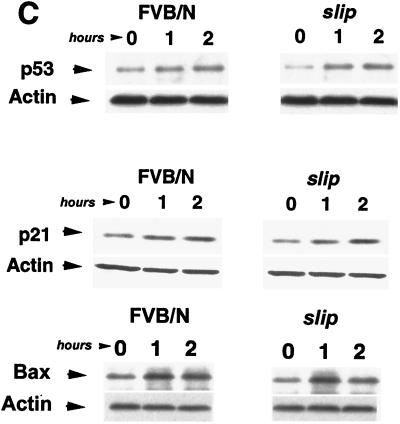
slip p53 levels are induced in vivo as well as in cultured cells in response to irradiation. (a) Induction of p53 expression was analyzed in vivo by subjecting slip and FVB/N mice to 10 Gy of IR, dissecting the thymus and gut after 4 h, and performing immunohistochemistry using an anti-p53 antibody. The criterion for p53 positivity was brown staining of nuclei in the thymuses of untreated (FVB/N and slip) and irradiated (slip IR and FVB/N IR) mice, shown here at a ×68 magnification. (b) Graph representing the percentage of living cells expressing p53 (plus the standard error) in untreated thymuses (slip and FVB/N) and those treated with 10 Gy (slip IR and FVB/N IR). The percentage of live cells expressing p53 in untreated and slip thymuses was replotted on a different scale (inset). The number of living cells here was determined by counting morphologically intact H&E-stained nuclei within five 1-mm2 20× fields in each of five thymuses per category. (c) Western blot analysis of p53, p21, and Bax was performed using 50 μg each of whole-cell protein extracts prepared from FVB/N and slip MEF cells which were either unirradiated (0) or incubated for 1 or 2 h following IR treatment. Using densitometry, a two- to threefold induction of p53, p21, and Bax was detected by 2 h posttreatment in wild-type and slip MEFs. Equal loading of protein extracts was confirmed using an antiactin antibody as a control.
DNA laddering was carried out using a DNA laddering kit (Trevigen). Six hours following 10-Gy irradiation, FVB/N and slip mice were sacrificed, and isolated thymuses were frozen and used to make high-molecular-weight DNA. Fragmented DNA was resolved on 1.5% agarose and detected by ethidium bromide.
Cell cycle analysis.
Analysis of the G1/S-phase cell cycle checkpoint was carried out both in vivo and in cultured cells. For in vivo analysis, slip and FVB/N mice were exposed to 10 Gy and immediately injected with 1 to 2 ml of bromodeoxyuridine (BrdU)-containing cell proliferation reagent (Amersham Pharmacia Biotech) per g of body weight. One to two hours post-IR, mice were euthanized, the thymuses were removed, and the thymocytes were flushed with phosphate-buffered saline. Cells were fixed in 70% ethanol for 30 min at room temperature, washed, and treated with 2 N HCl for 10 to 20 min. Acid neutralization was carried out in 100 mM Na2B4O7, pH 8.5, and cells were then treated with fluorescein isothiocyanate-conjugated anti-BrdU (Becton Dickinson) for 30 min, washed, and resuspended in 10 μg of propidium iodide solution/ml. After 30 min, the cells were analyzed by two-dimensional flow cytometry (FACScan; Becton Dickinson). Flow cytometry scatter plots were generated as increasing fluorescein isothiocyanate fluorescence on the y axis versus increasing fluorescence of propidium iodide on the x axis. S-phase cells were then gated and quantitated. G1/S-phase checkpoint analysis was also carried out on cultured cells, which were first irradiated and then immediately pulsed for 1 h with a cell proliferation reagent as described by the manufacturer. Cells were then harvested, fixed in 70% ethanol, and treated as described above for thymocytes.
Immunohistochemical analysis of cell proliferation was also performed on the thymuses of slip mice exposed to IR. Groups of five each of slip and wild-type mice were first irradiated with 10 Gy, and at 1 to 2 h posttreatment, together with untreated groups, were pulsed with cell proliferation reagent. Mice were then euthanatized, and the thymuses were removed, fixed in 10% buffered formalin, and immunostained with an anti-BrdU antibody purchased from Dako.
RESULTS
slip mice are deficient in DNA-PKcs protein production.
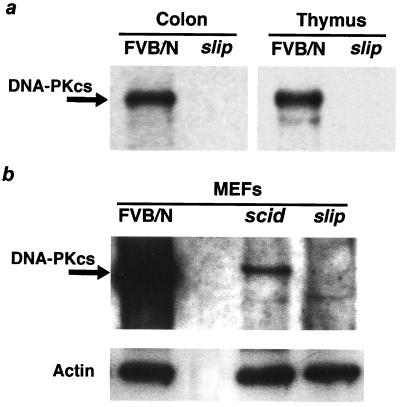
The slip mouse is a mutant generated by the integration of transgene DNA at a single site into intronic sequences between exons 7 and 8 of the DNA-PKcs gene. Members of our group previously demonstrated by reverse transcriptase PCR that DNA-PKcs transcripts are not expressed in the slip mouse (28). DNA-PKcs expression in slip mice was further assessed using Western blotting of whole-cell extracts from MEFs and tissues. An antibody cocktail that recognized epitopes at the amino and carboxy termini as well as the middle portion of DNA-PKcs was employed. The Western blot shown in Fig. 1a demonstrates that DNA-PKcs was expressed at high levels in both the colons and thymuses of FVB/N mice but was undetectable in extracts of the slip colons and thymuses. This was confirmed when slip and FVB/N MEFs were analyzed by Western blotting; Fig. 1b shows that DNA-PKcs expression was high in FVB/N MEFs, low in scid MEFs, and undetectable in slip MEFs.
FIG. 1.
DNA-PKcs is undetectable in slip mice. (a) Whole-cell extracts (200 μg) prepared from the thymuses and colons of FVB/N and slip mice were analyzed for the presence of DNA-PKcs (460 kDa) by Western blotting. Equal loading of protein in each lane was determined by using Ponceau S staining of the nitrocellulose membrane prior to antibody incubation (data not shown). (b) Whole-cell extracts of FVB/N, scid, and slip MEFs were used to confirm that slip mice lack any detectable levels of DNA-PKcs, which is present in both FVB/N and scid cells. Here, equal loading of protein extracts was monitored by antiactin antibody detection.
slip mice are highly sensitive to the effects of IR.
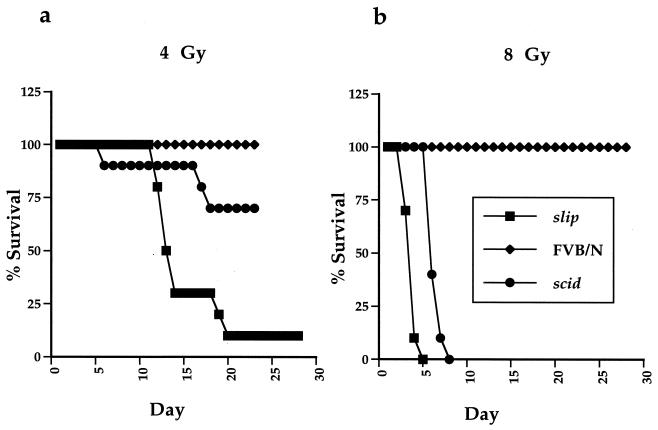
Cellular recovery from IR-induced DNA damage has been shown to require DNA-PKcs (14, 47). To determine whether this requirement was evident in slip mice, 5- to 6-week-old mice were exposed to 4 or 8 Gy of gamma irradiation and carefully monitored for 28 days (Fig. 2), at which point the experiment was terminated. At both 4 and 8 Gy, all FVB/N mice survived for the duration of the experiment. Unlike the wild-type mice, however, slip mice were extremely sensitive to gamma irradiation. At 4 Gy, 90% of the slip mice died between days 12 and 19 (Fig. 2a), while at 8 Gy, 100% died between 3 and 5 days after treatment (Fig. 2b). Although radiosensitive, scid mice were more resistant to gamma irradiation than slip animals (Fig. 2). These results demonstrate that slip mice are extremely radiation sensitive and suggest that the low levels of DNA-PKcs present in scid mice may confer some radioprotection relative to that in the DNA-PKcs null slip mice. However, the difference in the genetic backgrounds of scid and slip mice could also contribute to the apparent disparity observed between slip and scid mouse sensitivity to gamma irradiation.
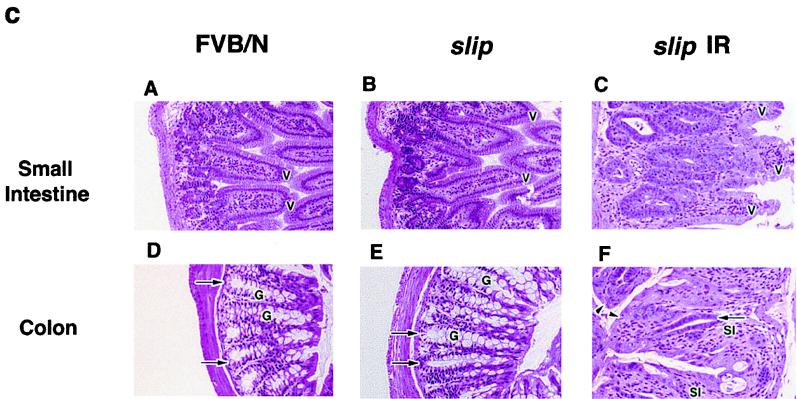
FIG. 2.
slip mice are highly sensitive to the effects of IR. Groups of FVB/N, scid, and slip mice were irradiated at 4 Gy (a) and 8 Gy (b) and monitored for 28 days. The percentage of surviving mice is plotted against the number of days post-IR treatment. (c) Small intestine (H&E, ×56) and colon (H&E, ×68) sections from untreated FVB/N and slip mice and irradiated (10 Gy) slip mice (slip IR). In untreated small intestine sections, normal crypts and villi (V) are evident, while irradiated slip small intestines show marked hyperplastic crypts and shortened, blunted, fused villi (V). In untreated colon sections, normal crypts (arrows) and goblet cells (G) are evident, while irradiated slip colons show diffuse goblet cell depletion, crypt cell hyperplasia (arrows), subacute inflammation (SI) in the lamina propria, and piling up and karyomegaly of the surface epithelium (arrowheads). Irradiated FVB/N small intestine and colon sections appear essentially like their untreated counterparts except for mild crypt cell hyperplasia (data not shown).
To determine whether slip mice exhibited any evidence of radiation injury, control and mutant mice were irradiated with 8 Gy and sacrificed between 2 and 4 days post-IR. Histological studies showed that the majority of tissues from both groups of mice, including that from the brain, lungs, liver, skin, muscle, heart, bone, and kidneys, retained a normal morphology throughout the course of the study. By day 2, however, slip mice displayed clear gastrointestinal abnormalities, including inflammation, not seen in control wild-type mice. At days 3 and 4 posttreatment, the slip mouse lesions became more severe. In the small intestine, villi were shortened, blunted, and fused; crypts were markedly hyperplastic. In the colon, there was diffuse goblet cell depletion, ablation of some crypts, moderate to marked hyperplasia of remaining crypts, and piling up and karyomegaly of the surface epithelium (Fig. 2c). These types of gut lesions can interfere with the absorption of water and electrolytes and likely contributed to slip mouse morbidity. Intestines of similarly irradiated wild-type FVB/N mice were histologically normal, except for mild crypt cell hyperplasia in the small intestines (data not shown). These results demonstrate that slip mice are highly sensitive to the effects of IR and suggest that the severe gut lesions observed in irradiated slip mice contributed to their accelerated mortality.
G1/S-phase cell cycle checkpoint is not defective in slip mouse cells.
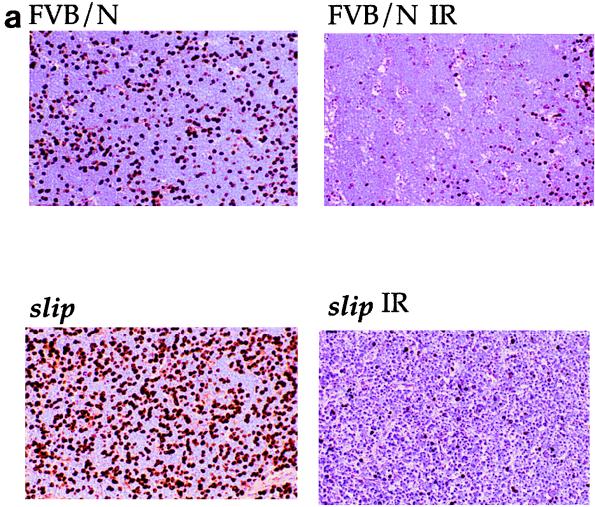
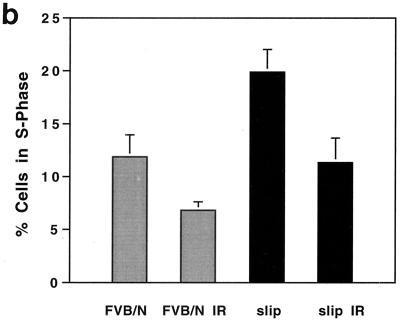
The in vivo cell cycle response of DNA-PKcs null mice to IR was determined by both immunostaining and flow cytometry. Immunostaining of thymus sections from irradiated and untreated slip and wild-type mice was used to analyze BrdU incorporation and showed that upon IR exposure, the number of BrdU-positive cells in both wild-type and slip thymuses was reduced (Fig. 3a). For flow cytometry, slip and wild-type mice were exposed to 10 Gy and injected immediately with BrdU, and after 1 to 2 h, the mice were euthanatized and thymic cells were isolated and analyzed. A comparison of untreated slip and wild-type mice showed a significantly higher number of BrdU-positive cells among slip thymic cells (P = 0.02) than among those derived from FVB/N mice (Fig. 3). This result indicates that slip thymic cells demonstrate enhanced proliferation as early as 5 to 6 weeks of age. This is not surprising, since slip mice rapidly develop and eventually die from thymic lymphoblastic lymphomas between 5 and 6 months of age. When subjected to IR treatment, both wild-type mice and slip mice exhibited a 42% decrease in the number of cells in S phase, indicative of activation of the G1/S-phase cell cycle checkpoint (Fig. 3). To confirm these in vivo data, G1/S-phase cell cycle checkpoint analysis was performed on slip MEFs, showing a 35% reduction in S-phase cells 1 h after exposure to IR (data not shown). In all, these results suggest that in vivo, DNA-PKcs is not required in the activation of the G1/S-phase cell cycle checkpoint in response to DNA damage.
FIG. 3.
G1/S-phase cell cycle checkpoint in response to IR in slip mice. slip and wild-type mice were treated with 10 Gy, pulsed with BrdU, and analyzed for its incorporation by immunohistochemistry (a) and flow cytometry (b). (a) Representative thin sections (magnification, ×68) of thymuses from unirradiated and irradiated FVB/N and slip mice, immunostained for BrdU incorporation. (b) Graph showing the mean percentage of the total thymocytes (plus the standard error) in S phase of the cell cycle demonstrates BrdU incorporation for untreated and irradiated wild-type and slip mice. Each mean value was generated from S-phase values for 5 to 10 mice. Paired t test analysis revealed a significant difference between slip and irradiated slip (slip IR) samples (P < 0.02).
p53 accumulates in irradiated slip mice and leads to p21 and Bax induction.
Cell cycle progression in the normal thymus is mediated by p53 (4, 5, 10). To determine whether p53 accumulates in response to DNA damage in DNA-PKcs null cells, slip and FVB/N mice were exposed to 10 Gy of IR and the thymuses were immunostained for p53 (Fig. 4a). Nuclei staining positive for p53 were scored microscopically, and Fig. 4b shows that for untreated mice, p53-positive nuclei, even though low in number, were significantly more numerous in slip than in wild-type thymuses (P = 0.026). Notably, with 10 Gy of IR, p53 was significantly induced in both slip and FVB/N mice (Fig. 4b).
Induction of p53 was also analyzed by Western blotting. Both slip and FVB/N MEFs were treated with 10 Gy of IR and harvested at hourly intervals posttreatment. Figure 4c shows that p53 protein induction occurred in wild-type and slip MEFs by 1 h post-IR and in both cases reached levels of two- to threefold by 2 h post-IR.
To determine if the p53 accumulating in irradiated slip cells was functional, we monitored levels of the p53 transcriptionally induced proteins p21 (16, 17) and Bax (39) following radiation. MEFs from control and slip mice were treated with 10 Gy of IR, and protein extracts were prepared at various times thereafter. Western blot analysis using whole-cell protein extracts demonstrated that at 10 Gy, the induction of p21 in slip fibroblasts was similar to that observed in FVB/N fibroblasts, initiating within 1 h and increasing twofold by 2 h posttreatment (Fig. 4c). Similarly, Bax was also induced two- to threefold over the course of 2 h post-IR treatment in both slip and FVB/N MEFs (Fig. 4c).
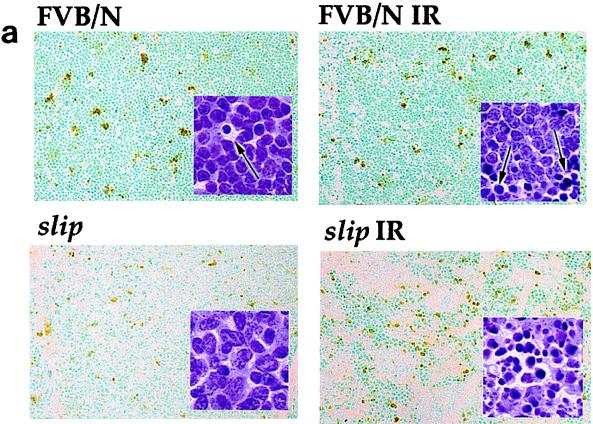
Irradiation-induced apoptosis occurs in slip mice.
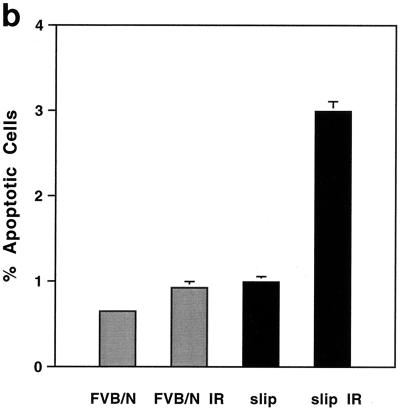
The p53 protein can control several cellular pathways, including the induction of apoptosis in response to DNA damage in the thymus (5, 12, 15, 37). Determination of whether IR-induced, p53-mediated apoptosis can occur in the absence of detectable DNA-PKcs in slip mice was based on the results from cytomorphological determination and TUNEL as well as DNA laddering assays. Representative thymus sections from untreated and irradiated slip and FVB/N mice were stained with H&E and scrutinized for the appearance of apoptosis (cytoplasmic shrinkage and karyorrhexis). As expected, unirradiated, wild-type thymuses had few apoptotic cells, but their numbers dramatically increased in response to irradiation. Figure 5 shows that in unirradiated slip thymuses, the numbers of apoptotic cells were generally higher than in untreated FVB/N thymuses. As with wild-type animals, irradiation of slip mice induced a dramatic increase in the number of apoptotic cells (Fig. 5). Quantitation of the TUNEL assay results showed that in untreated mice, slip thymuses contained a significantly higher number of apoptotic nuclei than wild-type thymuses (P = 0.002); moreover, within 1 h post-IR treatment, apoptosis had dramatically increased in slip (P < 0.0001) compared to wild-type (P = 0.08) thymuses (Fig. 5b).
FIG. 5.
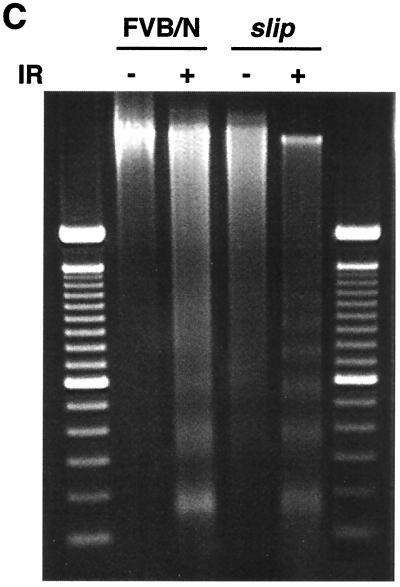
IR-induced apoptosis occurs in the absence of DNA-PKcs. (a) TUNEL assay performed on thymuses from untreated wild-type (FVB/N) and slip mice as well as mice irradiated with 10 Gy (FVB/N IR and slip IR). Set into each panel is the corresponding H&E (×136) stain showing examples of apoptotic cells (black arrows). (b) Quantitation of the TUNEL assay results showing the percentage of TUNEL-positive cells in the thymus with no treatment (slip and FVB/N) and at 1 h after treatment with 10 Gy (slip IR and FVB/N IR). (c) DNA laddering in FVB/N and slip thymuses with (+) and without (−) irradiation. Samples were flanked on the gel by 100-bp markers.
DNA laddering studies with irradiated mice confirmed the apoptotic response of slip cells to IR. As shown in Fig. 5c, there was no evidence of laddering in DNA isolated from cells of unirradiated wild-type mice, and levels were low but detectable in untreated slip animals. However, DNA laddering dramatically increased in DNA isolated from both FVB/N and slip mice exposed to 10 Gy of IR. Collectively, the apoptotic staining, the DNA laddering, and the Bax induction observed in these studies indicate that p53-mediated, IR-induced apoptosis can occur in the absence of DNA-PK.
DISCUSSION
During their lifetime, mammalian cells are continually exposed to agents capable of introducing double-stranded breaks of genomic DNA. These breaks can be caused by normal endogenous cell processes, such as V(D)J recombination, necessary for the creation of immunological diversity, or by exposure to free-radical intermediates generated from the body's normal metabolic processes, routine diagnostic X-rays, or anticancer therapy. It is crucial that cellular repair of these breaks be both timely and accurate, since the consequences of unrepaired or improperly repaired DNA can lead to genomic instability and inevitably to the development of cancer. Understanding the process by which cells repair DNA double-stranded breaks, as well as the molecules involved in signaling and eliciting a cellular response to such damage, is of fundamental importance.
In this study, we addressed the issue of whether DNA-PK is a requisite component of the p53-dependent DNA damage response pathway in vivo. Previous studies aimed at understanding the in vivo role of DNA-PKcs in p53 DNA damage response have used the scid mouse, whose residual DNA-PK activity can confound interpretation of any p53 induction data. Here, we attempted to resolve this issue by exploiting the slip mouse, which harbors a null mutation in DNA-PKcs (28). We show that in mice devoid of DNA-PKcs, the p53 protein accumulates in response to IR treatment, is functional, and is capable of inducing p21. Moreover, the G1/S cell cycle checkpoint is intact in slip cells in vivo. These results strongly support in vitro studies performed with MEFs isolated from DNA-PKcs−/− mice (10, 29) but not those of Woo et al. (48), who reported that p53 cannot bind DNA following IR in two cell lines with DNA-PKcs deficiency. The results from the latter study could be accounted for, at least in one cell line, by the presence of a mutation in the DNA binding region of p53 (3, 10).
We demonstrate for the first time that in response to IR, p53 can induce Bax, and subsequently apoptosis, in the complete absence of DNA-PK activity in vivo. Previously, p53-mediated apoptosis was demonstrated in scid mice, which possess a mutationally crippled version of DNA-PKcs (24, 30, 39). Interestingly, our slip mice experienced a more robust apoptotic response to IR than did FVB/N mice. Moreover, unirradiated slip mice also demonstrated a significantly higher level of apoptosis than their wild-type counterparts. One relatively trivial explanation for this comes from the observation that p53 is actually more abundant in the slip than in the wild-type thymus, both before and following IR. IR-mediated elevation in p53 has also been described for scid mice and DNA-PKcs−/− MEFs (10, 24). It has been suggested that this p53 accumulation is the result of the persistence of DNA double-stranded breaks in the absence of DNA-PKcs and the consequent activation of ATM (10); if so, it is noteworthy that p53 can be still acutely induced even in the context of such chronic baseline DNA damage. A more intriguing possibility accounting for the observed apoptotic enhancement in slip thymocytes is that although DNA-PK activity is clearly not required for p53-mediated apoptosis, DNA-PKcs may nonetheless participate in negatively regulating apoptosis in the thymus.
The work presented here places the role of DNA-PK as the direct activator of p53 in vivo in doubt and suggests that other molecules in the cell may be responsible for regulating p53 activation in response to DNA damage. One such molecule is the p53 regulator protein MDM2, which is responsible for p53 degradation as well as for its transportation out of the nucleus. The MDM2 protein is itself transcriptionally activated by p53, and phosphorylation of the p53 serine-15 residue blocks the p53-MDM2 interaction. Therefore, stress signals from DNA damage could conceivably target MDM2 and prevent its interaction with p53. Currently, the best candidate as the direct p53 activator in response to DNA damage is the ATM gene product. ATM, like DNA-PKcs, is a member of the PI3-K superfamily, and ATM−/− cells display a lack of p53 accumulation and abnormal G1/S-phase cell cycle checkpoints in response to irradiation, suggesting that ATM acts upstream of p53 in an IR-induced signal transduction pathway. Recently, Banin et al. (4) and Canman et al. (11) convincingly demonstrated that in response to DNA-damaging agents, ATM phosphorylates serine-15 on p53. This study, together with the fact that there is reduced p53 serine-15 phosphorylation in IR-treated ataxia telangiectasia cells, suggests a role for ATM as the kinase directly responsible for p53 induction upon IR exposure.
ACKNOWLEDGMENTS
We thank M. Gottesman and H. Morse III for critical reading of the manuscript.
This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. N01-C0-56000.
ADDENDUM IN PROOF
While the manuscript was in press, a paper by Wang and colleagues was published (Proc. Natl. Acad. Sci. USA 97:1584–1588, 2000) demonstrating that in another DNA-PKcs null mutant, IR induction of Bax expression and apoptosis in thymocytes is significantly reduced. The results of a comparable experiment carried out on our slip mice exposed to 10 Gy of IR for 10 h demonstrated that the slip thymuses consisted only of naked stroma, thymic epithelium viable-appearing vessels, clusters of neutrophils, and much cell debris. Variations in genetic background between the two DNA-PKcs null mice used in these studies likely contribute to the differences observed in the apoptotic repsonse to DNA damage.
REFERENCES
- 1.Anderson C W, Lees-Miller S P. The nuclear serine/threonine protein kinase DNA-PK. Crit Rev Eukaryot Gene Expr. 1992;2:283–314. [PubMed] [Google Scholar]
- 2.Araki R, Fujimori A, Hamatani K, Mita K, Saito T, Mori M, Fukumura R, Morimyo M, Muto M, Itoh M, Tatsumi K, Abe M. Nonsense mutation at Tyr-4046 in the DNA-dependent protein kinase catalytic subunit of severe combined immune deficiency mice. Proc Natl Acad Sci USA. 1997;94:2438–2443. doi: 10.1073/pnas.94.6.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araki R, Fukumura R, Fujimori A, Taya Y, Shiloh Y, Kurimasa A, Burma S, Li G C, Chen D J, Sato K, Hoki Y, Tatsumi K, Abe M. Enhanced phosphorylation of p53 serine 18 following DNA damage in DNA-dependent protein kinase catalytic subunit-deficient cells. Cancer Res. 1999;59:3543–3546. [PubMed] [Google Scholar]
- 4.Banin S, Moyal L, Shieh S, Taya Y, Anderson C W, Chessa L, Smorodinsky N I, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 5.Barlow C, Brown K D, Deng C-X, Tagle D A, Wynshaw-Boris A. Atm selectively regulates distinct p53-dependent cell-cycle checkpoint and apoptotic pathways. Nat Genet. 1997;17:453–456. doi: 10.1038/ng1297-453. [DOI] [PubMed] [Google Scholar]
- 6.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley J N, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 7.Blunt T, Gell D, Fox M, Taccioli G E, Lehmann A R, Jackson S P, Jeggo P A. Identification of a nonsense mutation in the carboxyl-terminal region of DNA-dependent protein kinase catalytic subunit in the scid mouse. Proc Natl Acad Sci USA. 1996;93:10285–10290. doi: 10.1073/pnas.93.19.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogue M A, Jhappan C, Roth D B. Analysis of variable (diversity) joining recombination in DNA dependent protein kinase (DNA-PK)-deficient mice reveals DNA-PK-independent pathways for both signal and coding joint formation. Proc Natl Acad Sci USA. 1998;22:15559–15564. doi: 10.1073/pnas.95.26.15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosma G C, Custer R P, Bosma M J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 10.Burma S, Kurimasa A, Xie G, Taya Y, Araki R, Abe M, Crissman H A, Ouyang H, Li G C, Chen D J. DNA-dependent protein kinase-independent activation of p53 in response to DNA damage. J Biol Chem. 1999;274:17139–17143. doi: 10.1074/jbc.274.24.17139. [DOI] [PubMed] [Google Scholar]
- 11.Canman C E, Lim D S, Cimprich K A, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan M B, Siliciano J D. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 12.Clarke A R, Purdie C A, Harrison D J, Morris R G, Bird C C, Hooper M L, Wyllie A H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 13.Critchlow S E, Jackson S P. DNA end-joining: from yeast to man. Trends Biochem Sci. 1998;23:394–398. doi: 10.1016/s0968-0004(98)01284-5. [DOI] [PubMed] [Google Scholar]
- 14.Danska J S, Guidos C J. Essential and perilous: V(D)J recombination and DNA damage checkpoints in lymphocyte precursors. Semin Immunol. 1997;9:199–206. doi: 10.1006/smim.1997.0072. [DOI] [PubMed] [Google Scholar]
- 15.Danska J S, Holland D P, Mariathasan S, Williams K M, Guidos C J. Biochemical and genetic defects in the DNA-dependent protein kinase in murine scid lymphocytes. Mol Cell Biol. 1996;16:5507–5517. doi: 10.1128/mcb.16.10.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 17.el-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 18.Elson A, Wang Y, Daugherty C J, Morton C C, Zhou F, Campos-Torres J, Leder P. Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc Natl Acad Sci USA. 1996;93:13084–13089. doi: 10.1073/pnas.93.23.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finnie N J, Gottlieb T M, Blunt T, Jeggo P A, Jackson S P. DNA-dependent protein kinase activity is absent in xrs-6 cells: implications for site-specific recombination and DNA double-strand break repair. Proc Natl Acad Sci USA. 1995;92:320–324. doi: 10.1073/pnas.92.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiscella M, Ullrich S J, Zambrano N, Shields M T, Lin D, Lees-Miller S P, Anderson C W, Mercer W E, Appella E. Mutation of the serine 15 phosphorylation site of human p53 reduces the ability of p53 to inhibit cell cycle progression. Oncogene. 1993;8:1519–1528. [PubMed] [Google Scholar]
- 21.Fried L M, Koumenis C, Peterson S R, Green S L, van Zijl P, Allalunis-Turner J, Chen D J, Fishel R, Giaccia A J, Brown J M, Kirchgessner C U. The DNA damage response in DNA-dependent protein kinase-deficient scid mouse cells: replication protein A hyperphosphorylation and p53 induction. Proc Natl Acad Sci USA. 1996;93:13825–13830. doi: 10.1073/pnas.93.24.13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao Y, Chaudhuri J, Zhu C, Davidson L, Weaver D T, Alt F W. A targeted DNA-PKcs-null mutation reveals DNA-PK-independent functions for KU in V(D)J recombination. Immunity. 1998;9:367–376. doi: 10.1016/s1074-7613(00)80619-6. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb T M, Jackson S P. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993;72:131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- 24.Gurley K E, Kemp C J. p53 induction, cell cycle checkpoints, and apoptosis in DNAPK-deficient scid mice. Carcinogenesis. 1996;17:2537–2542. doi: 10.1093/carcin/17.12.2537. [DOI] [PubMed] [Google Scholar]
- 25.Hartley K O, Gell D, Smith G C, Zhang H, Divecha N, Connelly M A, Admon A, Lees-Miller S P, Anderson C W, Jackson S P. DNA-dependent protein kinase catalytic subunit: a relative of phosphatidylinositol 3-kinase and the ataxia telangiectasia gene product. Cell. 1995;82:849–856. doi: 10.1016/0092-8674(95)90482-4. [DOI] [PubMed] [Google Scholar]
- 26.Huang L C, Clarkin K C, Wahl G M. p53-dependent cell cycle arrests are preserved in DNA-activated protein kinase-deficient mouse fibroblasts. Cancer Res. 1996;56:2940–2944. [PubMed] [Google Scholar]
- 27.Jeggo P A. DNA-PK: at the cross-roads of biochemistry and genetics. Mutat Res. 1997;384:1–14. doi: 10.1016/s0921-8777(97)00009-8. [DOI] [PubMed] [Google Scholar]
- 28.Jhappan C, Morse III H C, Fleischmann R D, Gottesman M M, Merlino G. DNA-PKcs: a T-cell tumour suppressor encoded at the mouse scid locus. Nat Genet. 1997;17:483–486. doi: 10.1038/ng1297-483. [DOI] [PubMed] [Google Scholar]
- 29.Jimenez G S, Bryntesson F, Torres-Arzayus M I, Priestley A, Beeche M, Saito S, Sakaguchi K, Apella E, Jeggo P A, Taccioli G E, Wahl G M, Hubank M. DNA-dependent protein kinase is not required for the p53-dependent response to DNA damage. Nature. 1999;400:81–83. doi: 10.1038/21913. [DOI] [PubMed] [Google Scholar]
- 30.Kemp C J, Vo K, Gurley K E. Resistance to skin tumorigenesis in DNAPK-deficient SCID mice is not due to immunodeficiency but results from hypersensitivity to TPA-induced apoptosis. Carcinogenesis. 1999;11:2051–2056. doi: 10.1093/carcin/20.11.2051. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn A, Gottlieb T M, Jackson S P, Grummt I. DNA-dependent protein kinase: a potent inhibitor of transcription by RNA polymerase I. Genes Dev. 1995;9:193–203. doi: 10.1101/gad.9.2.193. [DOI] [PubMed] [Google Scholar]
- 32.Lane D. Awakening angels. Nature. 1998;394:616–617. doi: 10.1038/29166. [DOI] [PubMed] [Google Scholar]
- 33.Lees-Miller S P, Sakaguchi K, Ullrich S J, Appella E, Anderson C W. Human DNA-activated protein kinase phosphorylates serines 15 and 37 in the amino-terminal transactivation domain of human p53. Mol Cell Biol. 1992;12:5041–5049. doi: 10.1128/mcb.12.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin S, Bucci T J, Cohen S M, Fix A S, Hardisty J F, LeGrand E K, Maronpot R P, Trump B F. The nomenclature of cell death: recommendations of an ad hoc committee of the Society of Toxicologic Pathologists. Toxicol Pathol. 1999;27:484–490. doi: 10.1177/019262339902700419. [DOI] [PubMed] [Google Scholar]
- 35.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 36.Lieber M R. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988;55:7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- 37.Lowe S W, Schmitt E M, Smith S W, Osborne B A, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 38.Malynn B A, Blackwell T K, Fulop G M, Rathbun G A, Furley A J, Ferrier P, Heinke L B, Phillips R A, Yancopoulos G D, Alt F W. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell. 1988;54:453–460. doi: 10.1016/0092-8674(88)90066-9. [DOI] [PubMed] [Google Scholar]
- 39.Miyashita T, Reed J C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 40.Prives C. Signaling to p53: breaking the MDM2-p53 circuit. Cell. 1998;95:5–8. doi: 10.1016/s0092-8674(00)81774-2. [DOI] [PubMed] [Google Scholar]
- 41.Rathmell W K, Kaufmann W K, Hurt J C, Byrd L L, Chu G. DNA-dependent protein kinase is not required for accumulation of p53 or cell cycle arrest after DNA damage. Cancer Res. 1997;57:68–74. [PubMed] [Google Scholar]
- 42.Roth D B, Menetski J P, Nakajima P B, Bosma M J, Gellert M. V(D)J recombination: broken DNA molecules with covalently sealed (hairpin) coding ends in scid mouse thymocytes. Cell. 1992;70:983–991. doi: 10.1016/0092-8674(92)90248-b. [DOI] [PubMed] [Google Scholar]
- 43.Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle D A, Smith S, Uziel T, Sfez S, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 44.Suwa A, Hirakata M, Takeda Y, Jesch S A, Mimori T, Hardin J A. DNA-dependent protein kinase (Ku protein-p350 complex) assembles on double-stranded DNA. Proc Natl Acad Sci USA. 1994;91:6904–6908. doi: 10.1073/pnas.91.15.6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szumiel I. Monitoring and signaling of radiation-induced damage in mammalian cells. Radiat Res. 1998;150:92–101. [PubMed] [Google Scholar]
- 46.Taccioli G E, Amatucci A G, Beamish H J, Gell D, Xiang X H, Torres Arzayus M I, Priestley A, Jackson S P, Marshak Rothstein A, Jeggo P A, Herrera V L. Targeted disruption of the catalytic subunit of the DNA-PK gene in mice confers severe combined immunodeficiency and radiosensitivity. Immunity. 1998;9:355–366. doi: 10.1016/s1074-7613(00)80618-4. [DOI] [PubMed] [Google Scholar]
- 47.Weaver D T. Regulation and repair of double-strand DNA breaks. Crit Rev Eukaryot Gene Expr. 1996;6:345–375. doi: 10.1615/critreveukargeneexpr.v6.i4.20. [DOI] [PubMed] [Google Scholar]
- 48.Woo R A, McLure K G, Lees-Miller S P, Rancourt D E, Lee P W. DNA-dependent protein kinase acts upstream of p53 in response to DNA damage. Nature. 1998;394:700–704. doi: 10.1038/29343. [DOI] [PubMed] [Google Scholar]