Summary Statement:
Multicomponent interventions are effective in preventing postoperative delirium, and work is ongoing to determine if they can be effective in preventing other postoperative neurocognitive disorders. Interventions optimizing sleep, pain, and cognition are essential components for clinicians to include in strategies to maximize the recovery of body and mind of vulnerable patients.
Introduction
As older persons increasingly rely on surgical treatment, preventing perioperative neurocognitive disorders (PNDs) including postoperative delirium, delayed neurocognitive recovery, and postoperative neurocognitive disorder has become a priority for patients, families, and for perioperative research.1,2 Defined by an acute, fluctuating disturbance in attention and awareness, postoperative delirium occurs in up to 50% of older patients and is associated with excess hospital costs, higher risk of long-term cognitive impairment, and poor functional outcomes.3,4 Characterized by cognitive deficits in memory and executive function, delayed neurocognitive recovery (diagnosed within 30 postoperative days) and postoperative neurocognitive disorder (diagnosed within 3–12 months) were once considered mainly as research outcomes with questionable clinical impact. However, they are now recognized as key barriers to optimal functional recovery after surgery.5,6 For decades, strategies to prevent PNDs by targeting isolated perioperative interventions have produced negative or inconclusive results.7–10 Given that there are numerous potential inciting factors for PNDs it is likely that multicomponent interventions may be more effective. For example, one of the most successful evidence-based multicomponent prevention strategies is the Hospitalized Elder Life Program (HELP), which has been shown in meta-analysis to consistently prevent delirium in hospitalized older persons.11 There are 14 core interventions in HELP, highlighting the complex and myriad precipitating factors that can contribute to delirium and the challenges faced by clinicians seeking to employ a comprehensive program. The objective of this narrative review is to highlight and expand upon three key intervenable targets to consider in any multicomponent intervention designed to optimize perioperative brain health: sleep, pain, and cognition (Figure 1).
Figure 1:
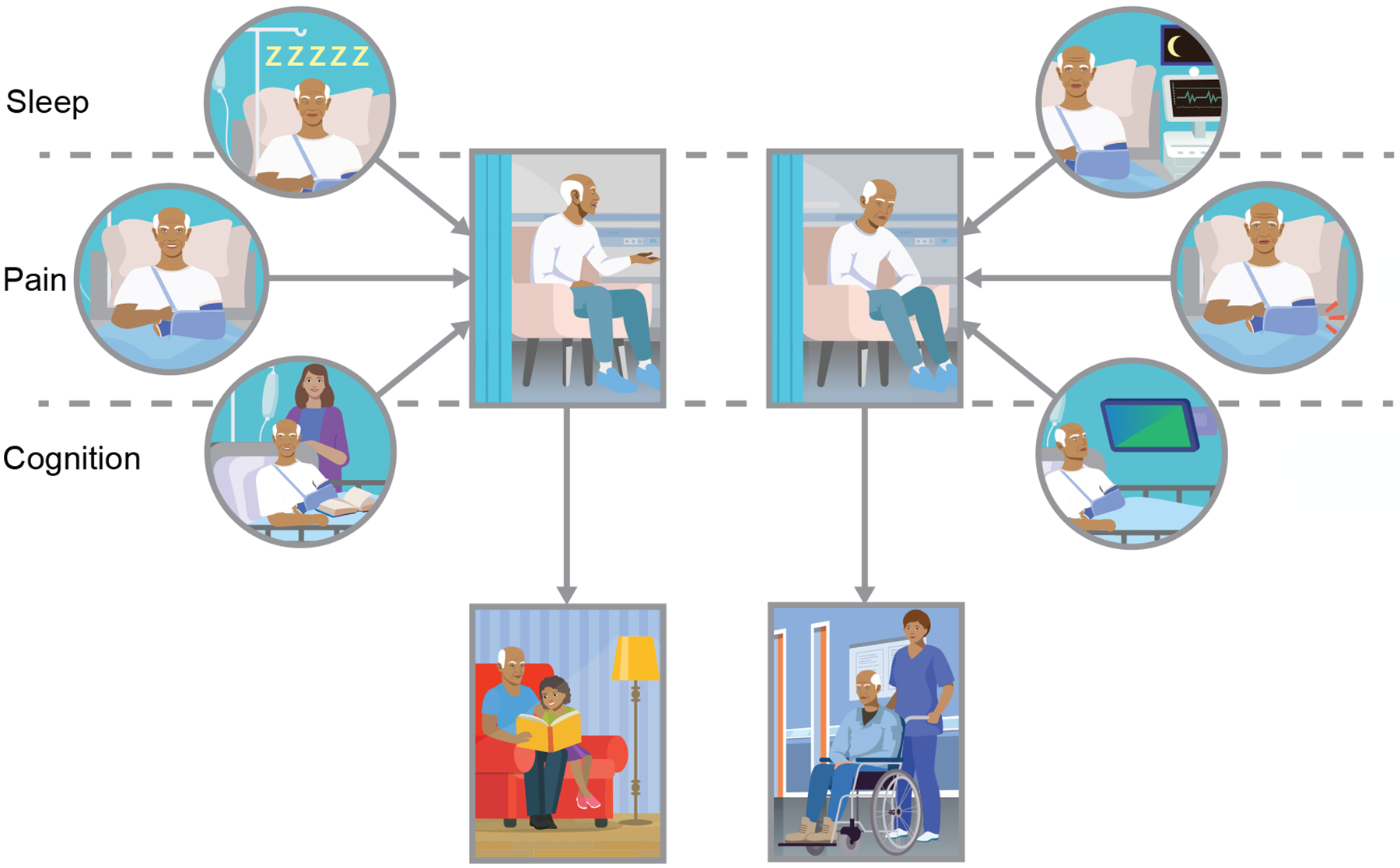
The impact of sleep, pain, and cognition on perioperative brain health and postoperative recovery. Here depicted is a patient presenting for orthopedic surgery. The scenarios depict how the patient’s sleep, pain, and cognition are well managed throughout the postoperative period (left side). The patient then remains delirium free in the hospital and returns to home at their cognitive and functional baseline (left-center panels). Conversely, if sleep, pain and cognition are poorly managed (right side), the same patient may experience delirium and/or inability to return to their cognitive and functional baseline (right-center).
Sleep
Sleep, circadian rhythms, and brain health
Sleep is a complex, naturally occurring physiological state that is critical to survival and health in animals and humans. A fundamental aspect of ensuring optimal physiological functions, including sleep, is the adherence to ~24-hour cycles known as circadian rhythms, which are thought to be ubiquitous to life on earth. If separated from our environmental and lifestyle choices, sleep-wake cycles are governed by our circadian system via the sleep-promoter hormone melatonin, which peaks during darkness.12
Sleep appears critical to optimal brain health and cognitive function, but only recently has there been increasing attention within the perioperative field.13,14 What is defined as “normal” sleep varies from individual to individual, and with age and comorbid disease. Broadly, the following five dimensions of sleep appear the most relevant to definitions and measurements of sleep health: 1) sleep duration: the total amount of sleep obtained per 24 hours, with between 7–8 hours is considered optimal for most; 2) sleep continuity or efficiency: the ease of falling asleep and returning to sleep after awakening; 3) timing of the sleep-wake cycle within the 24-hour day, 4) alertness/sleepiness: the ability to maintain attentive wakefulness; and 5) satisfaction/quality: the subjective assessment of “good” or “poor” sleep.13 All sleep “disorders” or “disturbances” can be understood via one, or commonly multiple dimensions. For example, insomnia is characterized by difficulty initiating or continuing sleep, but this often leads to lower sleep duration, irregular timings, subjective sleepiness and poor satisfaction/quality.
Unfortunately, as many as 1 in 3 will experience some form of sleep and/or circadian disturbances in our lifetime which often go unaddressed, are increasingly common worldwide, and worsen over time.15–19 Despite clinicians’ familiarity with the diurnal nature of our own sleep and behavioral cycles, and the physical and psychological toll associated with its disruption (e.g. after a busy night on-call), there remains relatively few considerations given to the impact of sleep and circadian disruption in our patients and how it may impact their brain health and overall functional recovery during the perioperative period.
Sleep disturbance and delirium
There is now increasing recognition for the potential link between sleep disturbances and perioperative neurocognitive disorders including delirium.20 Sleep/circadian disturbances are more common in older persons, and are more pronounced after critical illness and in neurodegenerative diseases such as Alzheimer’s disease, the very groups most vulnerable to PNDs.19,21–23
Sleep disruption prior to surgery has been shown to predict postoperative delirium. In an observational study of 50 adults undergoing major noncardiac surgery where sleep patterns were assessed objectively the night before surgery with a wearable actigraphy device, patients who developed postoperative delirium had significantly higher measures of preoperative sleep fragmentation including both percent time spent wake after sleep onset (WASO; mean(SD) 44(22) vs 21(20)%, p=0.012) and frequency of nightly awakenings (mean(SD) 17(9) vs 9(6), p=0.047), compared to those without delirium.24 This finding has been demonstrated in other studies, and in a recent meta-analysis of data from 12 studies and 1,199 patients where the pooled odds ratio for postoperative delirium for patients with preoperative sleep disturbances was significantly higher than for those without a preoperative sleep disturbance (OR{95%CI} 5.24{2.28,3.69}, p<0.001, I2=0%).20 Possible shared pathophysiological pathways between sleep disturbance and delirium include altered melatonin metabolism, neurotransmitter imbalance or reduced neuroprotection from key deficiencies such as Vitamin D.25–29 Undergoing major surgery with preexisting sleep disruption makes it likely these symptoms will be exacerbated during the postoperative recovery period as pain, nausea, light, noise and immobility ensue.
However, based on current evidence, one cannot conclude causation. The extent to which sleep disturbances may cause delirium or vice versa is not yet fully understood, but these two conditions may share a common neuropathology. There is some evidence that poor sleep behavior traits and circadian disruption predicts incident Alzheimer’s disease, but few large prospective studies exist for sleep and delirium.19,30 Sleep disruption and problems with rest-activity cycles are also comorbid with many conditions relevant to brain health and PNDs including heart failure and pain, and therefore these issues could potentially be a manifestation of underlying disease such as preclinical neurodegeneration.31,32 Whether disordered sleep directly increases risk of delirium, or whether it is indicative of an underlying comorbidity that increases risk may be difficult if not impossible to determine. Testing whether treatment of sleep disorders reduces delirium in controlled studies may be the best way to sort out these direct or indirect effects. How this affects the perioperative physician is also evolving. The role of sleep in the preservation of perioperative cognition is an active area of research, and it may be that sleep disruption comes to be seen as a chronic condition in need of optimization rather than reversal.
Sleep-disordered breathing, CPAP, and delirium
Sleep-disordered breathing is a complex, multisystemic disorder that warrants particular mention. In particular, the obstructive variant of sleep apnea (or OSA) is associated with obesity and increased risk for airway difficulties, adverse cardiac events, postoperative respiratory complications, and PNDs.33 In the general population, OSA is associated with reductions in cognitive reserve, increased risk for cognitive impairment and worsening executive function, and amyloid deposition in key brain regions.34–36 As many of these findings share characteristics of PNDs, a link between OSA and PNDs has also been proposed. Possible mechanisms underlying the association between OSA and delirium include abnormalities in sleep architecture leading to sleep disruption, hypoxia, vascular injury, low grade systemic inflammation, oxidative stress, and decrease in insulin growth factor‐1, as has been seen with neuronal injury and apoptosis.37
Using objective polysomnography, preoperative sleep-disordered breathing defined by a high apnea-hypopnea index was associated with a more than six-fold increased odds for postoperative delirium (OR[95%CI] 6.4[2.6–15.4], p<0.001), including patients without an existing formal OSA diagnosis.38 In a small cohort of older patients undergoing elective knee replacement, the incidence of delirium was significantly higher in patients with OSA as compared to those without OSA (8/15(53%) vs 19/95(20%), p=0.0123).39 However, a recent retrospective observational cohort study of 7792 surgical patients did not find a significant association between preoperative OSA and postoperative delirium after adjustment for perioperative confounders.40 Both OSA and postoperative delirium remain greatly under detected, and on balance of evidence their relationship still warrants close attention. For example, the Society of Anesthesia and Sleep Medicine currently recommends using preoperative screening tools such as the STOP-Bang preoperative screening for OSA, given the link with increased perioperative complications.41
Although the use of CPAP slows the deterioration of cognition, brain function and mood in non-surgical patients with OSA, thus far data from the surgical population is less conclusive.42–44 When patients who were at risk for sleep apnea were randomized in a CPAP group versus standard care, the perioperative use of CPAP did not change the incidence of postoperative delirium (12/58(21%) vs 9/56(16%), OR[95%CI] 1.36[0.52–3.54], p=0.53).45 While both preoperative and postoperative residual OSA severity as defined by apnea-hypopnea index were both significantly correlated with delirium severity in this sample, CPAP use was not found to be significantly correlated. Further studies with particular attention on CPAP adherence or the use of other respiratory adjuncts such as high-flow-nasal oxygen are ongoing and may yield positive results in the future. It remains unclear whether the best strategy to prevent postoperative delirium is to treat the OSA directly using CPAP, or to consider OSA patients at high risk and use more general delirium prevention strategies in this group. Currently, it is unknown whether OSA is a risk factor for delayed neurocognitive recovery or postoperative neurocognitive disorder. In the coming years, prospective clinical trials investigating this area will provide much needed data.46,47
Other postoperative sleep related interventions and delirium
In terms of pharmacologic interventions to prevent delirium, dexmedetomidine and melatonin have been extensively studied. For intensive care unit (ICU) patients who are mechanically ventilated, sedation with dexmedetomidine may be less likely to be associated with delirium compared to benzodiazepines or propofol.48,49 While there are inconsistent findings between studies, recent meta-analyses suggest that sedation of critically ill patients with dexmedetomidine may reduce the frequency and duration of delirium.50,51 Although these findings may primarily reflect the benefit of avoiding deliriogenic sedatives, the exact mechanism remains unclear. However, unlike all other sedatives and the commonly used anesthetics, dexmedetomidine appears most likely to preserve sleep architecture as currently inferred via electroencephalogram (EEG). In healthy volunteers, dexmedetomidine induced stage N3 non-REM sleep in a dose dependent fashion with an EEG pattern mimicking natural sleep without impairing next-day psychomotor performance.52 A low dose dexmedetomidine infusion prolonged total sleep time, increased sleep efficiency and time spent in stage N2 non-REM sleep in 76 older ICU patients.53 In a randomized, blinded, placebo-controlled trial of 700 older noncardiac surgery patients, a low dose dexmedetomidine infusion given to both ventilated and extubated patients from the time of ICU admission until 8am the morning of postoperative day one greatly reduced the risk of delirium as compared to placebo (32/350(9%) vs 79/350(23%), OR[95%CI] 0.35[0.22–0.54], p<0.0001).54 Additionally, patients in the dexmedetomidine group reported significantly better sleep quality (2[0–4] vs. 4[2–6]); 0–11 scale, lower=better sleep, median{IQR} p<0.0001). Finally, oral dexmedetomidine is now a possibility after successful testing in human subjects, however optimal dosing has yet to be established and it is not yet approved by the Food and Drug Administration. Subjects taking oral dexmedetomidine displayed both preserved sleep architecture on EEG and next-day psychomotor vigilance.55 This may open new possibilities outside of the ICU for investigation as to whether dexmedetomidine can be effective as a sleep promoting agent.
Melatonin is commonly used in the general population and in the ICU for the promotion of sleep. Given its increasing use, some understanding of its role in sleep and circadian rhythms is warranted. As mentioned above, the sleep-wake cycle is perhaps the most obvious and important behavior under intrinsic circadian output control. However, sleep-wake cycles are also affected by external cues. Of these cues, light is by far most important; others are food, sound, exercise, many of which are disrupted in sickness and hospital settings. Melatonin is the major sleep promoting hormone under circadian control. Taking its external cue from low light, its concentration peaks just before sleep initiation. Melatonin levels are measured from the saliva or serum, often in serial measurements, and are an accepted surrogate marker for our “internal time”.56 Critical care settings often involve exposure to light, noise, pain, nausea or clinical care at night which may explain the evidence for suppressed nocturnal melatonin peak secretion in ICU patients.57
Recent data suggest that delirious patients may also have reduced serum levels of melatonin.58 For this reason, melatonin supplementation has been investigated as a potential intervention to prevent delirium. In a prospective before-after trial of 500 cardiac surgery patients where prophylactic melatonin was given the night before surgery, the incidence of postoperative delirium was significantly lower in the intervention group (21/250(8.4%) vs 52/250(20.8%), p=0.001).59 While a randomized trial investigating the prophylactic use of the melatonin receptor agonist ramelteon showed some promise in preventing delirium in older medical patients (1/33, (3%) vs 11/34 (32%), ramelteon vs placebo, p=0.003), it was not shown to prevent postoperative delirium in elective cardiac surgery patients in another trial (19/59(32%) vs 22/58(38%), ramelteon vs placebo, p=0.516).60,61 Other clinical trials of melatonin or ramelteon have not demonstrated similar success, and a recent meta-analysis of 16 clinical trials concluded that evidence neither supports nor opposes the use of melatonin in the prevention of delirium of hospitalized patients.62 Trials with individually targeted timing and dosing in those who are most at risk for suppression and misalignment of melatonin secretion may yield improved results. However, this may require accounting for sleep and circadian rhythm regulation prior to the perioperative period.
Aside from postoperative delirium, the impact of melatonin levels and melatonin supplementation on other PNDs has been less extensively studied. In 97 patients aged 65–90 undergoing major orthopedic or abdominal surgery, patients with more than two-fold fluctuations in 6-sulfatoxymelatonin (6sMT), a main metabolite of melatonin, had a significantly higher incidence of delayed neurocognitive recovery as determined by a cognitive battery one week postoperatively (22/39(56%) vs 9/56 (16.7%), p<0.01).63 In contrast, a study of 36 abdominal surgery patients with a mean age of 70 found no association between abnormal 6sMT levels and the incidence of delayed neurocognitive recovery.64 In a placebo controlled trial of 139 patients older than 65 undergoing hip arthroplasty, patients given melatonin beginning the night before surgery and then for the next 5 nights had significantly higher Mini Mental Status Exam (MMSE) scores on days 1, 3 and 5, but scores between groups were similar on day 7.65 It should be noted that delayed neurocognitive recovery as defined as a predetermined decrease from baseline MMSE score was not an outcome in this trial. Significantly worse subjectively rated fatigue and sleep quality were found in the control group. In another placebo controlled trial of 54 patients undergoing breast surgery, patients administered nightly melatonin for one month preoperatively until three months after surgery did not have significantly different rates of delayed neurocognitive recovery at 2 weeks or postoperative neurocognitive disorder at 3 months, despite subjective improvements in sleep efficiency and total sleep duration.66 Due to the small sample size and resulting lack of power, as well as the substantial heterogeneity in outcome definition seen in these studies, there is a clear need for further work on the relationship between melatonin and PNDs other than delirium.
Finally, due to the overall paucity of effective sleep promoting medications, there have now been increased efforts to trial multifaceted nonpharmacologic sleep interventions to prevent ICU delirium. A before/after quality improvement project in 300 medical ICU patients implemented a bundle of environmental changes including ear plugs, eye masks and soothing music to decrease nighttime sleep disruption and promote daytime wakefulness. In the intervention group, the incidence of delirium was significantly less than in the pre-intervention group (OR[95%CI] 0.46[0.23–0.89], p=0.02).67 Taken as a whole, bundled sleep interventions may reduce the risk of delirium, but more work is needed to confirm these findings in adequately controlled trials and to pinpoint which aspect(s) of the multicomponent sleep bundles are the most effective.
Summary:
Sleep and circadian disturbances are important risk factors for the development of neurodegenerative diseases including Alzheimer’s disease, which in turn are important predisposing factors for postoperative delirium (Figure 2). To what extent sleep disturbances may cause delirium, which sleep disorders are particularly risky, or at which timepoint in the perioperative course these factors are important is unclear. Certain chronic sleep patterns may predispose to delirium, and these may in turn make patients more susceptible to acute perioperative sleep disturbances that may precipitate delirium. Additionally, postoperative delirium may cause acute de novo sleep disturbances, adding further complexity. More work is needed to untangle these relationships to derive effective interventions. While the relationship between sleep and circadian health and delirium is beginning to emerge, further work is also needed to understand the consequences of sleep disturbances and delayed neurocognitive recovery and postoperative neurocognitive disorder. Finally, there is a large degree of overlap between sleep disorders, pain, and cognition. Thus, future trials should aim to incorporate multimodal targets incorporating these components.
Figure 2:
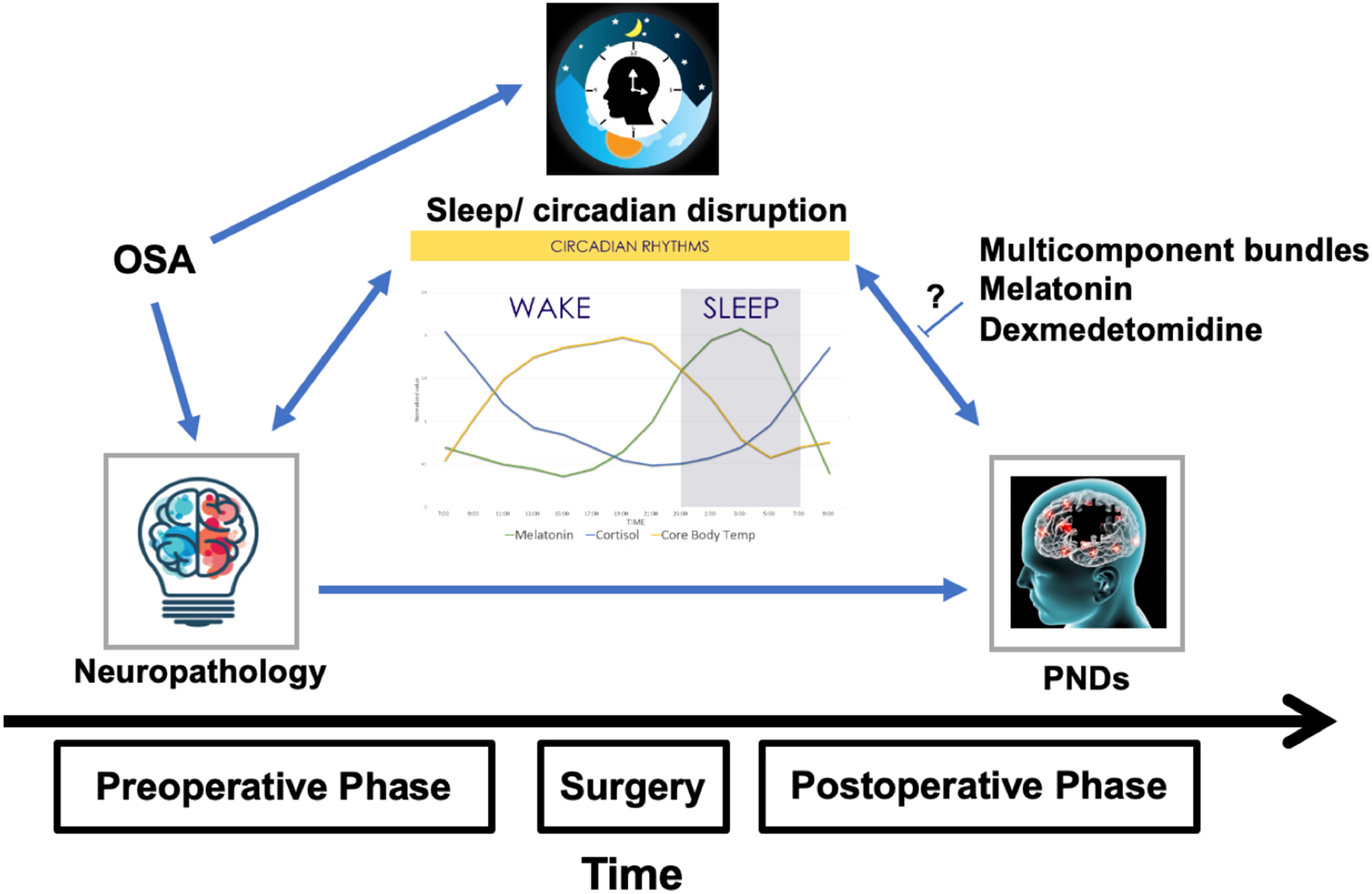
Sleep and Circadian disruption and Perioperative Neurocognitive Disorders. Preexisting sleep disorders and baseline neuropathology may contribute to sleep and circadian rhythm disruption in the perioperative period, which may then be a precipitating factor for perioperative neurocognitive disorders. OSA: Obstructive sleep apnea. PND: Perioperative neurocognitive disorders.
Pain
Pain and PNDs
The relationship between pain and PNDs, and the modification of that relationship by the treatment of pain, is incredibly complex (Figure 3). Inflammation and pain are closely biochemically linked, and many of the mediators of the body’s response to injury and inflammation in both the peripheral and central nervous system including prostaglandins, bradykinins, interleukins, and tumor necrosis factor-alpha can be elevated in painful conditions.68,69 This relationship is relevant to PNDs, as one commonly proposed mechanistic framework for both postoperative delirium and delayed neurocognitive recovery is through neuroinflammation.2,5,6,70,71 In this proposed mechanism, either peripheral or central nervous system injury leads to inflammatory cytokine release, endothelial activation, breakdown of the blood brain barrier and activation of microglia, potentially culminating in neuronal injury and subsequent brain dysfunction.72–74 Another interesting link between pain and cognitive dysfunction comes from knowledge that cholinergic neurons modulate pain signals, and cholinergic deficiency or anticholinergic medication use have been implicated in both pain hypersensitivity and delirium.27,75 These biochemical links between inflammation, pain, and neuronal injury or dysfunction are more easily identified in preclinical models than in clinical studies given the difficulties inherent to selecting and sampling the ideal mediators in perioperative patients. Despite this limitation, there are a number of studies supporting an association between pain and subsequent PNDs that are worthy of review.
Figure 3:
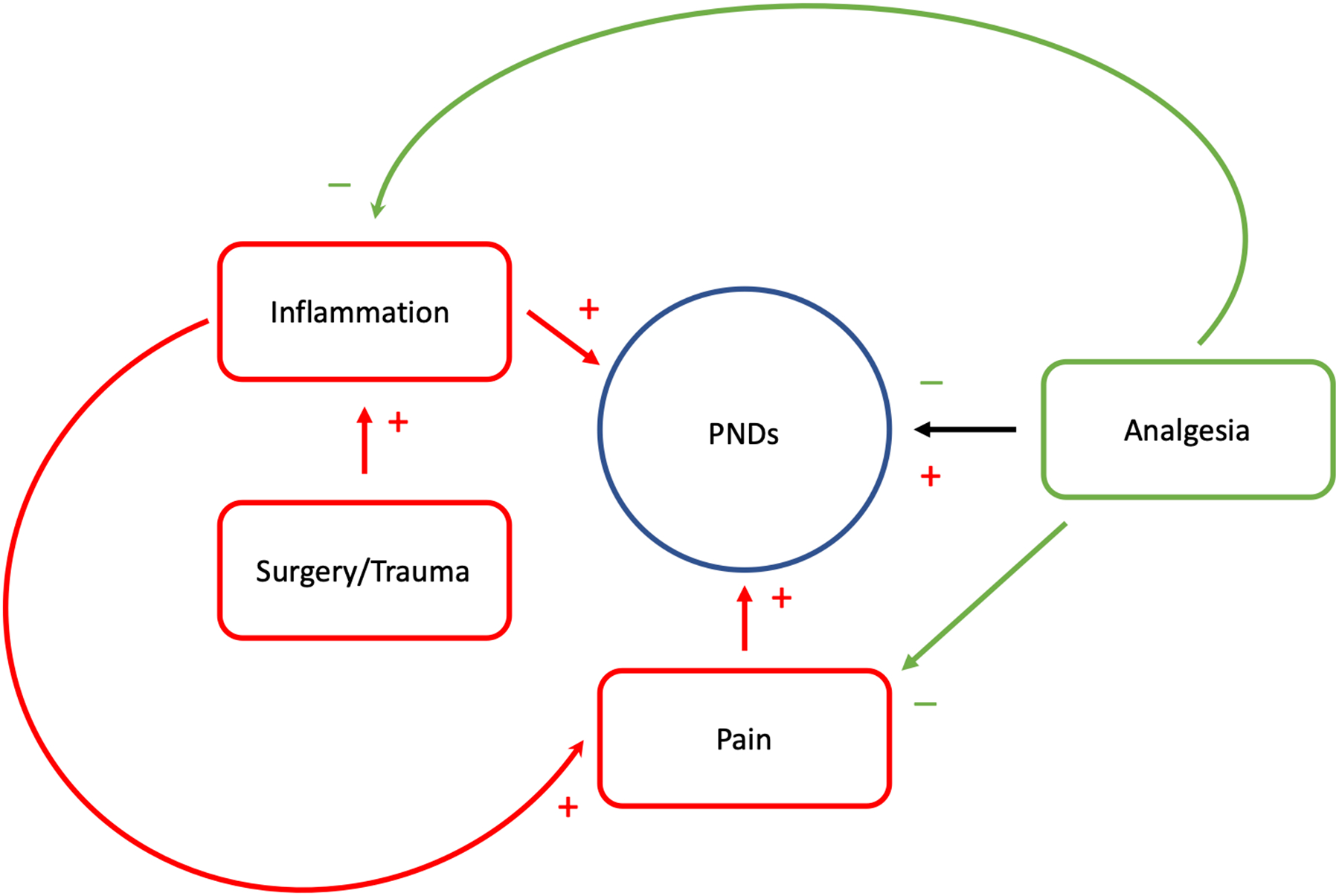
The relationships between pain, inflammation, and analgesia on the risk for perioperative neurocognitive disorders. Red lines and (+) signs signify process that may worsen other conditions. Green arrows and (−) signs indicate processes that may ameliorate or improve other conditions. PNDs: Perioperative neurocognitive disorders.
The majority of clinical studies linking pain to PNDs focus on the potential association between postoperative pain and postoperative delirium. Although this focus is reasonable given their temporal association, the presence of preoperative pain may also play a significant role and should not be overlooked. For example, in a cohort of 333 major noncardiac surgery patients 65 and older the presence of both moderate (OR[95%CI] 2.2[1.2–4.0] p<0.05) and severe (3.7[1.5–9.0], p<0.05) preoperative pain at rest were both independently predictive of postoperative delirium.76 An increase in pain scores from preoperative baseline to postoperative day one was also predictive of postoperative delirium (1.1[1.01–1.2], p<0.05). In 459 older patients undergoing elective orthopedic surgery, the severity of preoperative pain was significantly associated with an increased risk of subsequent delirium (severe pain vs none/mild pain OR[95%CI] 2.0 (1.4–3.0), p=0.013 for trend between none/mild, moderate, and severe).77 The relationship between pain and delirium may be modified by the presence of underlying depression, as subgroup analysis of patients with depression from this cohort revealed that for every one point increase on the postoperative visual analog pain scale, the risk of delirium significantly increased. Interestingly, investigations of alterations in synaptic connectivity in the prefrontal cortex suggest that changes may occur in this region for patients with depression, in chronic pain, and in disorders of executive function, suggesting a potential close neuropathological link between these conditions.78
As stated above, the preponderance of clinical evidence associating pain and PNDs involves acute postoperative pain. In a cohort of 541 older patients with hip fracture, cognitively intact patients with any episode of severe postoperative pain at rest (as defined by a score of 4 or greater on a 5 point scale) through postoperative day 3 had a nine-fold increase in the risk of subsequent delirium (RR[95%CI] 9.0[1.8–45.2], p=0.01).79 In 361 patients with a mean age of 66 undergoing major noncardiac surgery, postoperative pain at rest was significantly associated with subsequent postoperative delirium (risk ratio 1.20(1.04–1.37) per one point increase on the visual analog scale, p=0.015).80 In a similar population, patients with high levels of postoperative pain and receiving high doses of opioids had significantly higher rates of delirium, both in patients at low risk (17/34(50%) vs 35/174(20%), p=0.0004) or high risk for delirium (23/32(72%) vs 46/93(49%), p=0.031) compared to patients with lower levels of pain.81 In contrast, in 89 older patients undergoing major abdominal surgery, uncontrolled pain as defined by a pain score of greater than 5 without adequate medication administration was not found to be significantly associated with delirium (RR[95%CI] 1.0[0.7–1.4], p=0.91).82 It should be noted when discussing the evidence linking pain to PNDs that the assessment of pain can be very challenging in patients with impaired cognition, and therefore current guidelines recommend using a multisource, multidimensional approach to the assessment of pain in older persons.83 Additionally, many of the above referenced trials do not contain information as to whether chronic pain or opioid use was present preoperatively, limiting the interpretation of these results.
Pain Treatment and PNDs
The adequate diagnosis and management of pain is a core intervention in HELP as well as multicomponent ICU care guidelines such as the ABCDEF bundle, and is also recommended in consensus guidelines for reducing postoperative delirium from multiple interdisciplinary working groups.11,84–86 As discussed above, data from multiple perioperative studies suggest that the presence of preoperative or postoperative pain, or worsening severity of pain in the postoperative period is associated with subsequent delirium. In many of these studies, it is difficult to determine based on their results whether adequate treatment of pain can prevent delirium, or conversely if undertreatment of pain can precipitate delirium. As low pain scores may indicate either absence of pain or effective treatment of pain, evaluation of the impact of pain treatment on the risk of PNDs requires adjustment for either factor. This relationship is confounded even further when considering the specific medication used to treat pain, as many have been implicated as precipitating factors for delirium, especially opioids.
Opioids
Amid the enhanced awareness of PNDs and the opioid epidemic, avoidance or minimization of opioids has become an essential consideration in perioperative care. On one hand, opioids remain some of the most effective analgesics for acute pain, especially for severe painful conditions such as following trauma or surgery. On the other hand, opioid related side effects such as sedation or hallucination may precipitate, worsen, or mimic symptoms of delirium such as disorientation or hypoactive motor and cognitive function. Given these considerations, current guidelines for best practices to avoid delirium advocate for avoiding opioids, at least as first line agents.70,86 However, data suggests that simply administering less opioids may not prevent PNDs. For example, in a retrospective matched cohort study of 86 medical-surgical patients with a mean age of 80 admitted with painful conditions and with an opioid ordered, delirious patients received a significantly lower fraction of the allowed dose ordered than non-delirious patients (11/43(26.14%) vs 21/43(48.21%), p<0.001).87 In the cohort study of hip fracture patients mentioned previously where pain at rest was associated with a nine-fold increased risk of delirium, investigators found that patients administered less than 10mg of morphine equivalents per day were at significantly increased risk of delirium compared to patients receiving more than 10mg (RR[95% CI] 5.4[2.4–12.3], p<0.001).79 In 236 patients older than 65 undergoing hip fracture repair, opioid consumption during the first 3 postoperative days was not different between patients with and without delirium (mean(SD) 0.66(0.82) vs 0.49(0.59) mg/kg morphine equivalents, p=0.176), but patients with delirium did have significantly higher pain scores (mean(SD) 2.6(1.9) vs 1.7(1.8), p=0.007).88 Although the higher risk of delirium with lower opioid doses does not directly imply that those patients’ pain was less well treated, data from these cohorts suggests that increasing opioid dose is not associated with an increased risk of postoperative delirium, at least in the context of acute pain. Given the clinical and societal importance of limiting opioid use, it is reasonable to employ multimodal efforts to control pain before resorting to opioids.89 Ideally effective analgesia can be accomplished while limiting opioids, but in the event that opioid sparing techniques such as anti-inflammatory agents and regional, neuraxial, or local analgesia are not successful, residual untreated pain may have more of an effect on delirium than further limiting opioid use.
In terms of specific opioids, tramadol and meperidine have been linked to an increased risk of delirium, but there is limited data on the differential impact of the agents more typically used in the perioperative period such as fentanyl or hydromorphone.90 The mode of opioid administration may be more relevant. In the cohort study of 333 patients undergoing major noncardiac surgery mentioned previously, patients who only received oral opioid analgesics were found to have a significantly reduced risk of postoperative delirium compared to patients treated with intravenous opioids (OR[95%CI] 0.4[0.2–0.7], p<0.05).81 Additionally, a prospective cohort study of 225 patients older than 65 years undergoing non-cardiac surgery found that patients who were treated with oral opioids alone had a significantly reduced odds of delayed neurocognitive recovery as assessed by a three test battery as opposed to those treated with intravenous opioids via a patient controlled system (OR[95%CI] 0.22[0.06–0.80], p=0.02). This finding came after controlling for a number of patient and surgery specific confounders including pre- and postoperative pain levels.91
Non-Opioid Analgesics
The pathophysiological overlap between inflammation, pain, and neuronal injury makes analgesics with anti-inflammatory effects attractive candidates to prevent delirium in patients with acute postoperative pain. In a randomized trial of 620 older patients undergoing elective total joint arthroplasty, scheduled parecoxib (a selective COX2 inhibitor) for 3 days led to a significant reduction in the incidence of postoperative delirium compared to placebo (19/310(6.2%) vs 34/310(11%), p=0.031).92 The parecoxib group also had significantly less delayed neurocognitive recovery, defined by a decrease of more than 2 points on the Mini Mental Status Exam (MMSE) from baseline, than placebo controls on postoperative days 1, 3 and 5 (day 5: 28/310(8.7%) vs 53/310(19.4%), p<0.001). It should be noted that the MMSE is limited in its utility as a test for detecting cognitive dysfunction and is not recommended for this purpose by the PND nomenclature working group.1 Although acetaminophen is not considered an anti-inflammatory, it shares some characteristics of nonsteroidal anti-inflammatory drugs including action on the cyclooxygenase pathway and putative blockade of central nervous system prostaglandin production.93 A randomized, placebo-controlled factorial trial in 121 cardiac surgery patients older than 60 years found that scheduled intravenous acetaminophen for the first 48 hours postoperatively significantly lowered the incidence of delirium as compared to placebo (6/60(10%) vs 17/60(28%), p=0.01).94 Patients receiving acetaminophen also had significantly reduced delirium duration (median 1 vs 2 days, p=0.03). The rates of delayed neurocognitive recovery at discharge were not different between groups.
In both the parecoxib and acetaminophen trials, there were either clinically insignificant differences or no difference found between groups in opioid equivalents administered and in postoperative pain scores, suggesting that neither opioid sparing nor superior pain control were the main drivers of the results. It is possible that this finding was instead related to the prevention of neuroinflammation, however this hypothesis will have to be confirmed in subsequent studies as neither trial included biomarker analyses. Additionally, the possibility that the effect of these interventions occurred through reducing neuroinflammation should be taken into context with the negative findings of multiple clinical trials investigating the intraoperative use of different drugs with both anti-inflammatory and analgesic properties including intravenous lidocaine, magnesium, and steroids to prevent PNDs after cardiac surgery.95–99
Gabapentin is another non-opioid analgesic that has been investigated as an intervention to minimize perioperative opioid use. In a large double-blind placebo controlled trial involving 697 patients with a mean age of 72 years undergoing non cardiac surgery, the administration of 900mg of gabapentin preoperatively and for the first three postoperative days did result in a small but significant reduction in the amount of morphine equivalents on the first postoperative day (median(IQR) 6.7(1.3, 20.0) vs 6.7(2.7, 24.8)mg, p=0.04).100 However, there were no differences between the gabapentin and placebo groups in the primary outcome of postoperative delirium (84/350(24%) vs 72/347(20.8%), p=0.30). Commonly, especially in enhanced recovery pathways, multimodal and opioid sparing analgesia protocols will combine multiple agents. Data for this approach is limited, but one prospective study of a fast track protocol for 220 older patients undergoing major joint replacement found no cases of postoperative delirium employing a multicomponent strategy including standardized anesthetic and postoperative analgesic protocols utilizing various combinations of paracetamol, gabapentin, tramadol, celecoxib, and ibuprofen across four different centers.101
Ketamine is another commonly used opioid-sparing analgesic. Like opioids, ketamine has potential psychotropic effects such as hallucinations, nightmares, or psychosis that are undesirable in patients at risk for PNDs.102 These effects may be dose dependent, therefore trials evaluating ketamine’s effectiveness in reducing opioid consumption and PNDs focus on low dose interventions. In a three arm randomized active- and placebo-controlled trial of 56 adult patients undergoing major open abdominal surgery, the administration of low dose (0.25 mg/kg bolus and 0.125 mg/kg/h infusion) and minimal dose (no bolus, 0.015 mg/kg/h infusion) ketamine during the anesthetic and the following 48 hours resulted in lower postoperative opioid consumption as compared to placebo (mean(SD) 42.7(13.4) vs 40.2(13.5) vs (72.7(15.3) mg piritramide, p<0.0001).103 However, patients in the low dose group had significantly higher Intensive Care Delirium Screening Checklist (ICDSC) scores than both the minimal dose and placebo groups (median(IQR) 2(1,3) vs 1(0,1) and 0(0,1), respectively, p=0.007). In 58 patients older than 55 years of age undergoing cardiac surgery with cardiopulmonary bypass, patients randomized to receive an intravenous bolus of 0.5mg ketamine had significantly lower rates of postoperative delirium than placebo controls (1/29(3%) vs 9/29(31%), p=0.01).104 In a different study in the same population by the same investigators, they found that the same intervention could possibly reduce the incidence of delayed cognitive recovery at one week postoperatively, as defined by a two standard deviation decrease on assessments of memory and executive functions, as compared to placebo (7/26(27%) vs 21/26(81%), p<0.001) after adjusting for training effects using assessment data from concurrent non-surgical controls.105
The Prevention of Delirium and Complications Associated with Surgical Treatments (PODCAST) trial sought to more definitively investigate whether the prophylactic intraoperative administration of ketamine could prevent postoperative delirium, also using a three-armed design.9 In the trial, 672 patients older than 60 years undergoing major cardiac and non-cardiac surgery were randomized to either low (0.5mg/kg) or high dose (1.0mg/kg) ketamine boluses or placebo given in the time between induction and surgical incision. There was no difference in the incidence of postoperative delirium during the first three postoperative days between patients who received any dose of ketamine as compared to placebo (88/450(19.45%) vs 44/222(19.82%), p=0.92). There was also no significant difference found in delirium rates across all three groups (40/227(17.65%) vs 47/223(21.3%) vs 44/222(19.82%) in low dose, high dose, and placebo groups, respectively. p=0.80). Furthermore, no significant differences among groups were found with respect to time to delirium onset, severity, or duration of delirium. Postoperative opioid consumption was not significantly different between the three groups at any time point. Lastly, more patients in the ketamine groups reported experiencing hallucinations (45/227(20%) vs 62/223(28%) vs 40/222(18%) in low dose, high dose, and placebo groups respectively, p=0.01) and nightmares (27/227(12%) vs 34/223(15%) vs 18/222(8%), p=0.03). Therefore the results of the PODCAST trial should give providers caution when considering intraoperative ketamine as a means to either reduce the risk of delirium or postoperative opioid consumption, as neither high or low dose regimens were effective for these outcomes and there was evidence of significant harm from ketamine with regards to its psychotropic effects.
Regional or Neuraxial Analgesia
A successful regional nerve block may be very effective for postoperative pain control when placed in an appropriate candidate. If this effective analgesia can be obtained while also sparing the use of opioids, then it is theoretically possible that regional nerve blocks can prevent delirium in multiple ways. The best available data for this approach comes from studies in orthopedic surgery patients. In 207 patients at intermediate or high risk of delirium undergoing hip fracture surgery who were randomized to undergo a fascia iliaca or sham block administered on admission and repeated every 24 hours until delirium occurrence or discharge, the use of the fascia iliaca block resulted in a significantly reduced incidence of delirium (11/102(10.78%) vs 25/105(23.8%), RR[95%CI] 0.45[0.23–0.87]).106 Additionally, patients who received fascia iliaca blocks experienced lower delirium severity (mean(SD) 14.34(3.6) vs 18.61(3.4) DRSR-98 score, p<0.001), and shorter delirium duration (mean(SD) 5.22(4.28) vs 10.97(7.16) days, p<0.001). For patients undergoing total knee arthroplasty, a cohort study of 85 patients demonstrated that analgesia via a femoral nerve catheter in addition to patient-controlled analgesia (PCA) was associated with lower rates of postoperative delirium as compared to PCA alone (7/28(25%) vs 31/51(61%), p=0.002).107 After controlling for preoperative cognitive function, the odds of postoperative delirium were significantly higher in the PCA group than patients who received a femoral nerve catheter in addition to their PCA (OR[95%CI] 7.02[2.06–23.97], p=0.002). The use of intraoperative spinal anesthesia as an alternative to general anesthesia has been proposed to reduce anesthetic exposure for patients at risk for postoperative delirium. Interestingly, this may not always be the case, as patients under spinal anesthesia with supplemental monitored anesthesia care may still receive high dose of intravenous sedatives.108 This makes the interpretation of trials investigating the benefit of neuraxial anesthetics on postoperative delirium challenging. A large randomized controlled trial is currently underway specifically examining whether spinal anesthesia or general anesthesia is superior for older patients undergoing hip fracture surgery, with postoperative delirium as a secondary outcome.109
For operations on the thorax or abdomen, analgesia via the use of an epidural catheter can be very effective, albeit with the inherent risk of hypotension.110 In a trial of 70 patients older than 70 years of age randomized to either combined general and epidural anesthesia followed by patient controlled epidural analgesia (PCEA) with bupivacaine and sufentanil compared to general anesthesia and PCA alone, the use of PCEA did not significantly reduce the incidence of delirium (8/31(26%) vs 8/33(24%, p>0.05).111 A higher proportion of patients in the PCA group demonstrated poor scores on the Abbreviated Mental Test than the PCEA group on postoperative day 4 (number of patients with scores ≦ 8, 9, or 10, 5/11/17 vs 1/5/25, p<0.05) and postoperative day 5 (5/13/15 vs 1/7/23, p<0.05). In a secondary analysis of the PODCAST trial, the investigators found that patients who received postoperative epidural analgesia did not have a significantly reduced odds of postoperative delirium within the first three postoperative days compared to those without an epidural after adjusting for several confounders including age and type of procedure (aOR[95%CI] 0.65[0.32–1.35], p=0.247).112 A post hoc analysis was performed in which patients treated with an epidural were less likely to experience any episode of delirium during the study follow up, after adjustment for the same confounders (aOR[95%CI] 0.36[0.17–0.78], p=0.009). As postoperative delirium is often defined by any single episode of delirium, and this analysis was conducted post hoc, it is unclear how impactful this finding is.
Finally, the use of epidural analgesia has been included in studies evaluating the effectiveness of enhanced recovery pathways for colonic surgery. In one trial, 240 open colorectal surgery patients older than 70 years were randomized to a fast-track protocol (consisting of preoperative dietary, hydration, and bowel preparation interventions, thoracic epidural anesthesia and postoperative PCEA with ropivacaine only, and postoperative mobilization and dietary interventions) or traditional care (notably consisting of general anesthesia and postoperative fentanyl).113 Patients in the fast-track group had a significantly lower incidence of postoperative delirium within the first five days (4/117(3.4%) vs 15/116(12.9%), p=0.008).
Summary:
Severe or uncontrolled preoperative or postoperative pain, and increased levels of pain from the preoperative to postoperative period are all associated with postoperative delirium. Proper diagnosis and adequate treatment of pain remains a key component of preventative strategies for postoperative delirium. While multimodal analgesic strategies including non-opioid analgesics and regional or neuraxial analgesia have demonstrated success in effectively controlling pain and potentially reducing opioid requirements, at this time the quality of evidence underlying any one analgesic approach to prevent delirium is low. Evidence is stronger, however, that undertreatment of pain is more of a significant risk factor for postoperative delirium than treatment with potentially deliriogenic medications. There is little data available on the relationship between pain, pain treatment, and delayed neurocognitive recovery or postoperative neurocognitive disorder.
Cognition
A disturbance in cognition, either temporarily or in the longer-term, is a defining feature of postoperative delirium, delayed neurocognitive recovery, and postoperative neurocognitive disorder.1 An emerging component of perioperative care now consists of perioperative multicomponent strategies to enhance recovery. In this context, preoperative optimization and the goal of the best possible functional recovery for surgical patients increasingly includes measures taken to protect perioperative cognitive function.114 Thus, the detection of PNDs and evaluation of strategies employed to prevent them relies on a thorough understanding of the cognitive areas affected in the perioperative period, the development of validated instruments to measure perioperative cognition, and the current state of evidence for strategies to improve postoperative cognitive function.
Baseline Cognitive Performance and PNDs
The degree of preexisting organ dysfunction is an important risk factor for many types of postoperative complications.115–117 The same can be said for brain function, as poor baseline cognition is a strong predictor of future cognitive dysfunction. Although there is continuing debate as to which cognitive test or battery of tests is best suited for the perioperative period, there is strong evidence that patient performance on a preoperative cognitive test can predict PNDs. Screening tests such as the Mini-Cog and MMSE which were originally designed to detect mild cognitive impairment or dementia have been used to evaluate perioperative cognitive function. In two longitudinal cohort studies of surgical patients older than 65 years, investigators found that a preoperative Mini-Cog score indicative of moderate cognitive dysfunction (≤ 3 or ≤ 2) was associated with a significantly higher risk of postoperative delirium (OR[95%CI] 2.4[1.2, 4.9], p=0.015 and 4.5[1.3, 15.7], p=0.017, respectively) and more days with postoperative delirium (mean(SD) 4(6) vs 1(2) days, p=0.012).118,119 In 425 older hip fracture surgery patients, those with a MMSE score of less than 24 had a significantly increased incidence of postoperative delirium (76/141(54%) vs. 73/284(26%), p≤0.001).120 In a similar population, a higher preoperative MMSE score was associated with a lower incidence of postoperative delirium (OR[95%CI] 0.67(0.52–0.86, p=0.002).121
In addition to tests of global cognitive function, poor performance on targeted tests of executive function can also predict postoperative delirium in older patients undergoing major noncardiac surgery (OR[95%CI] 1.23[1.06–1.43], p<0.01 for a three-test composite and log mean ratio[95%CI] 1.27 [1.11–1.46], p<0.01 for Color Trials 2).122,123 Preoperative test performance is also associated with persistent cognitive deficits, as older hip arthroplasty patients who performed below 2 standard deviations on at least 2 of 7 neuropsychological tests had higher incidences of both delayed neurocognitive recovery at 7 days (23/91(25.3%) vs 26/195(13.3%), p=0.012) and of postoperative neurocognitive disorder at 3 months (13/87(14.9%) vs 14/197(7.1%), p=0.039) and 12 months (5/83(9.4%) vs 2/188(1.1%), p<0.001).124 In a cohort of 566 older surgical patients, lower preoperative scores on an 11 test cognitive battery was identified as the dominant risk factor for postoperative delirium after adjustment for other established predictors (RR[95%CI] 2.0[1.5–2.5] for each 0.5 SD decrease, p<0.05).125 Lastly, preoperative test performance may also help identify patients at risk for long term cognitive decline, as a higher preoperative MMSE score was associated with a lower risk of dementia 5 years after cardiac surgery (OR[95%CI] 0.68[0.54–0.84], p<0.001).126 Based in part on the findings of these studies, the Perioperative Neurotoxicity Working Group recommends evaluating baseline cognition using a screening test in patients older than 65 or who are at otherwise high risk for PNDs.127 They do not recommend one screening test in particular, however, as more work is needed to assess the predictive power and clinical utility of these screening tests in perioperative patients.
Cognitive Reserve
Cognitive reserve can be described as resiliency of an individual’s cognitive processes in the face of injury. In contrast to cognitive performance assessed at one point in time, cognitive reserve is characterized by the accumulation or loss of cognitive abilities over the lifespan. Differences in cognitive reserve have been theorized to explain observed differences between patients in phenotypes or degrees of impairment seen after similar pathologic findings of neurologic injury such as stroke or Alzheimer’s disease, and the concept can be applied to PNDs (Figure 4).128 Cognitive reserve is typically described in terms of years of education attained, occupational complexity, and cognitive lifestyle behaviors. There is some evidence to suggest that differences in cognitive reserve may predict PNDs. In two cohort studies of hospitalized older patients, each year of education obtained was associated with a significantly decreased risk of delirium (OR[95%CI] 0.91[0.87–0.95], p<0.01 and 0.76[0.62–0.95], p=0.016, respectively).129,130 Low educational attainment was also found to be a strong predictor of postoperative delirium in older patients after hip fracture surgery or hip replacement (OR[95%CI] 3.59[1.14–11.25], p<0.05).131 However, a large cohort study of similar patients did not find an association between years of education or multiple other markers of cognitive reserve and postoperative delirium.132 It should be noted that this cohort exhibited a high median years of education (15 years), suggesting that this effect may not be as evident in highly educated populations.
Figure 4:
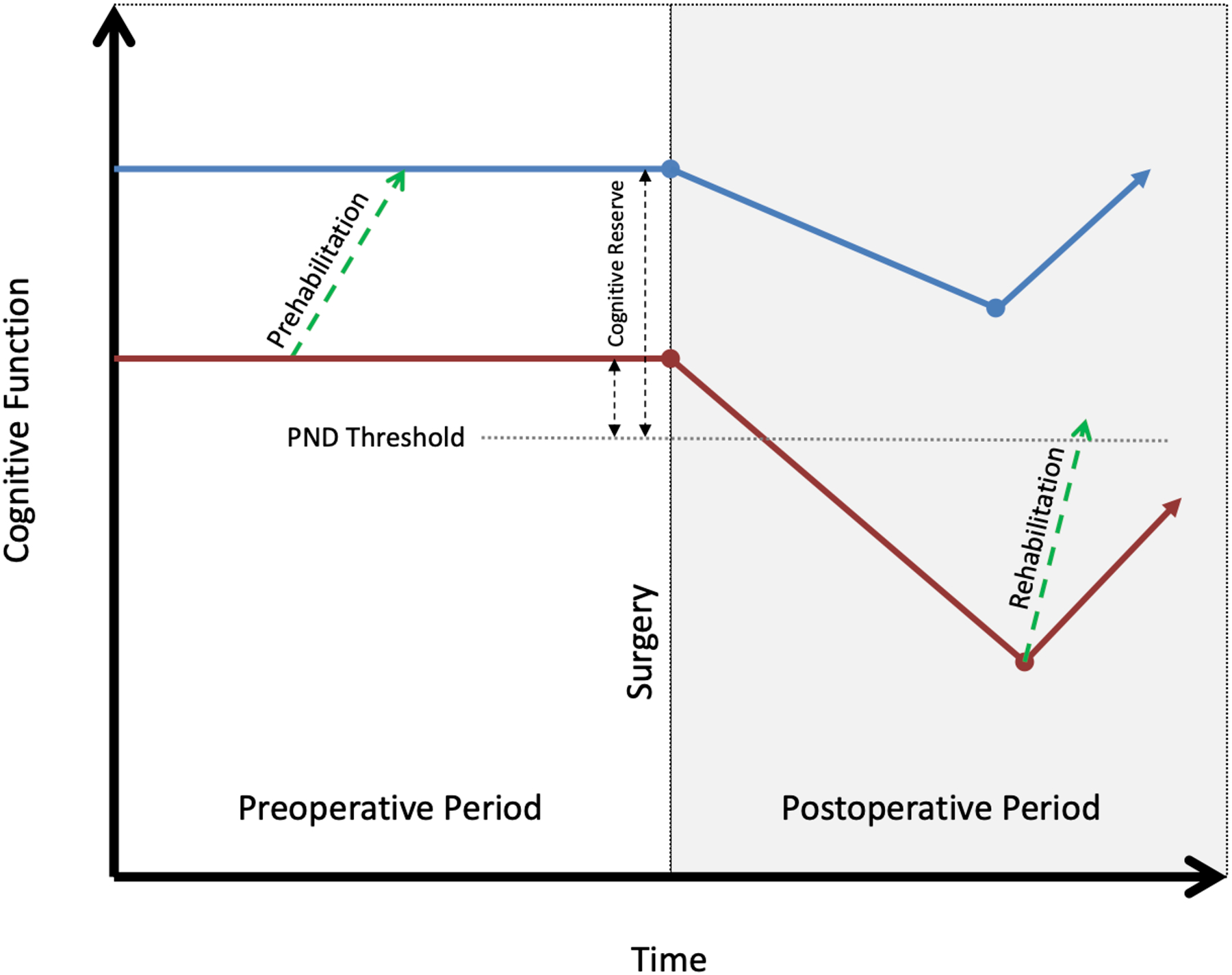
Cognitive trajectories and perioperative neurocognitive disorders. Depicted are a patient with high baseline cognitive reserve (blue line) and a patient with low baseline cognitive reserve (red line). Both patients experience an event in the perioperative period leading to a decrease in cognitive function, but only the patient with low baseline reserve may manifest symptoms. The green dashed lines represent the theoretical mechanism through which cognitive interventions in the pre – and postoperative phases may influence cognitive reserve and either prevent or aid recovery from PNDs. Adapted from Stern, Neurobiologia 2009 (reference # 128). PND: Perioperative neurocognitive disorder.
Educational attainment may also predict long term postoperative cognitive function. In a large longitudinal cohort of older patients undergoing major non-cardiac surgery, educational achievement of high school or higher was associated with a significantly lower risk of delayed neurocognitive recovery at one week (OR[95%CI] 0.6[0.4–0.9], p=0.002), but not for postoperative neurocognitive disorder at 3 months.133 In another large prospective cohort of major non-cardiac surgery patients of which 355 were older than 60, patients with postoperative neurocognitive disorder at 3 months had slightly fewer mean years of education (mean(SD) 13.2(2.4) 13.7(2.8) years, p=0.013).134 Less well characterized than educational level is the relationship between baseline cognitive lifestyle behaviors and PNDs. In a cohort of 141 patients with a mean age of 71 years, greater participation in preoperative cognitive lifestyle behaviors including reading books, using email, or playing computer games was found to be protective against delirium after elective orthopedic surgery (increase of one activity per week, OR[95%CI] 0.92[0.86–0.98], p=0.006).121
Prehabilitation to Prevent PNDs
Prehabilitation refers to the attempt to optimize preoperative modifiable risk factors to improve functional outcomes after surgery, often focusing on preoperative physical, nutritional, and psychological health. Of these three domains, physical prehabilitation has been the most studied. Although there are inconsistent results among trials and uncertainty with regards to whether physical prehabilitation can reduce postoperative complications, multiple clinical trials in abdominal and orthopedic surgery have demonstrated improved postoperative physical capacity in patients who participated in home based exercise regimens as compared to usual care.135 Nutritional prehabilitation, consisting mainly of nutritional supplements and dietary counseling, may potentially accelerate the return to preoperative functional capacity in colorectal surgery when added to a physical prehabilitation program.136 Both physical deconditioning and poor nutritional status are elements of frailty, which has been shown in retrospective studies to be strongly associated with postoperative delirium and possibly postoperative neurocognitive disorder.137,138 Such elements may be modifiable. In a six armed randomized trial of 246 older pre-frail or frail adults, physical, cognitive, nutritional, or combined intervention training were all shown to reduce future frailty scores to a significantly larger degree than a usual care control.139 If physical and nutritional prehabilitation can improve postoperative functional outcomes and reverse frailty in older persons, it is then possible that these interventions may prevent PNDs. Data from clinical trials evaluating this potential effect are limited, however. In a single center before and after unblinded study of 627 patients undergoing major abdominal surgery, the incidence of postoperative delirium was found to be significantly lower in patients who underwent a multicomponent intervention to improve physical and nutritional health and reduce frailty factors as compared to patients who did not receive this intervention (22/267(8.2%) vs 42/360(11.7%), adjusted OR[95%CI] 0.56[0.32–0.98], p=0.043).140
Bolstered by the theory that increasing cognitive reserve can protect against neurologic injury and experimental data suggesting that neurogenesis and neuroplasticity still occur in later life, numerous investigators have evaluated whether cognitive exercise can improve cognitive performance in older persons.141–144 Perhaps the most notable example is the ACTIVE trial, which demonstrated that 10 hours of computerized cognitive training led to sustained improvements in processing speed over the following 10 years.145 In perioperative research, attempts have been made to apply this technique to prevent PNDs. In a randomized trial of 141 abdominal surgery patients older than 60, 3 hours of supervised memory exercises 1–4 weeks prior to surgery significantly reduced the incidence of delayed neurocognitive recovery at one week compared to usual care (11/69 (15.9%) vs 26/72 (36.1%), p=0.007).146 In contrast to its success in older adults in the general population, computerized cognitive training appears to be less feasible in older surgical patients.147 Although a small feasibility trial of 45 older cardiac surgery patients noted high degrees of patient interest and enjoyment with a computerized cognitive prehabilitation program, there were low rates of adherence (39% during the preoperative period) and no effect on the incidence of postoperative delirium or delayed cognitive recovery was found.148 In a recent randomized trial of 251 patients older than 60 undergoing major noncardiac nonneurologic surgery, preoperative computerized cognitive exercise did not significantly reduce the risk of postoperative delirium as compared to usual care in the primary analysis. However, a post-hoc per-protocol analysis excluding patients who never used the cognitive exercise program revealed a significantly reduced incidence of postoperative delirium favoring the cognitive exercise group (16/121(13.2%) vs 29/126(23%), p=0.04).149 It should be noted that for this trial and the feasibility trial which used the same cognitive exercise platform, the median length of time spent training was around 4.5 hours, which falls short of “recommended dose” of 10 hours of cognitive exercise in the ACTIVE trial. Further investigation is necessary to determine whether adherence to cognitive prehabilitation can be improved, and whether cognitive prehabilitation can reduce delirium and/or other PNDs in an adequately powered trial.
Postoperative Cognitive Training
Postoperative cognitive training may also prevent PNDs. A randomized trial of 47 patients with a mean age of 65 years after lung transplantation demonstrated greater score improvements at 12 weeks on the Forward Digit Span (mean(SD) 0.93(1.09) vs 0.04(0.52), p=0.004) and Verbal Fluency tests (mean(SD) 1.32(1.82) vs 0.1(1.53), p=0.033) with the use of a computerized program for 8 weeks after surgery.150 Among 46 lung transplant recipients with a mean age of 66 years, the use of 8 weeks of computerized cognitive training started 4 weeks after surgery resulted in significantly higher scores on the digit span forward test (mean(SD) 0.93(1.09) vs 0.04(0.52), p=0.0044) and verbal fluency (mean(SD) 1.32(1.82) vs 0.10(1.53), p=0.0331).150 It should be noted, however, that these mean differences are less than the standard deviation for the group, which is a common benchmark used in previous studies to define PNDs. The best known multi-componential intervention to prevent hospital delirium, HELP, includes the provision of cognitively stimulating activities at least three times daily as a core intervention.151 In a before-after study of 179 consecutive abdominal surgery patients older than 65 where a modified version of HELP was implemented that focused only on nutrition, mobilization, and cognitively stimulating activities including discussing current events or word games, the delirium rate was significantly reduced in the intervention group (0/102 (0%) vs 13/77 (16.7%), p<0.001).152 A similar approach employing daily cognitively stimulating conversation and word games was employed in a randomized pilot trial in 50 older hip or knee arthroplasty patients. The investigators found that patients in the intervention group had a significantly lower incidence of delayed neurocognitive recovery as defined by a decrease of 2 points or more from the baseline MMSE score as compared to usual care controls (3/25(12%) vs 11/25(44%), p=0.012).153
Summary:
Poor baseline cognition, defined by either poor preoperative performance on screening tests of cognitive function or decreased markers of cognitive reserve, is strongly associated with PNDs. As such, the routine use of a validated screening test in the preoperative period has been recommended to help identify at-risk patients. While physical and nutritional prehabilitation may improve postoperative functional capacity and reverse frailty in non-operative patients, more investigation is necessary to determine whether these interventions can prevent PNDs. Cognitive prehabilitation has been shown to reduce delirium incidence in one clinical trial, but further studies are needed to replicate this finding, to determine the optimal training regimen, to improve adherence, and to investigate whether the technique can prevent delayed cognitive recovery and/or postoperative neurocognitive disorder. Postoperative cognitive exercise may improve postoperative cognition and prevent postoperative delirium, but high-quality evidence from adequately powered clinical trials is needed to better determine these effects.
Conclusion
The increasing awareness of the long-term negative consequences of perioperative neurocognitive disorders on functional outcomes after surgery has led to the development of multicomponent interventions to optimize postoperative brain health. Decades of perioperative research targeting isolated intraoperative interventions focusing on altering the exposure to anesthesia or surgery have not yet been able to identify a singular intervention to successfully prevent PNDs. Given the multiple predisposing and precipitating risk factors for postoperative delirium and the incomplete overlap with risk factors for delayed neurocognitive recovery and postoperative neurocognitive disorder, it is likely that a multicomponent approach encompassing all phases of the perioperative period (preoperative, intraoperative, and postoperative) may be more effective. Going forward, critically needed perioperative research into three targets for optimal perioperative brain health: sleep, pain and cognition will enable providers to better identify high risk patients and confidently employ interventions into well-defined care plans (Table 1). When this can be achieved, perioperative medicine may reach its goal of an ideal recovery for both the body and mind of surgical patients.
Table 1:
Summary of Evidence and Future Directions
| Summary | Questions for Future Research |
|---|---|
| Sleep | |
| Chronic sleep disorders including obstructive sleep apnea are associated with future delirium | Can improving preoperative sleep quality reduce delirium risk? Can increasing perioperative adherence to Continuous Positive Airway Pressure (CPAP) prevent delirium? |
| Perioperative sleep disruption is a precipitating factor for delirium | Can restoring circadian rhythm, either naturally or with the use of melatonin, prevent delirium? Can preferential use of dexmedetomidine prevent postoperative delirium in patients requiring postoperative sedation? |
| Pain | |
| Preoperative pain is a risk factor for postoperative delirium | Can optimization of preoperative pain lower the risk of postoperative delirium? |
| Poorly treated postoperative pain can precipitate postoperative delirium | Do multisource tools for pain assessment identify patients at risk for delirium? Does the use of multimodal analgesia prevent postoperative delirium? |
| Little evidence exists describing the association between postoperative pain and longer term PNDs | Is poorly controlled pain associated with delayed neurocognitive recovery? Does chronic postoperative pain increase the risk of postoperative neurocognitive disorder? |
| Cognition | |
| Poor preoperative cognitive function is one the of the strongest predictors of PNDs | Which cognitive screening test(s) best predict PNDs? Does preoperative cognitive trajectory predict PNDs? |
| Perioperative cognitive exercise may prevent postoperative delirium | Can cognitive prehabilitation prevent PNDs? Which type of cognitive exercise is best suited to prevent PNDs? What is the most effective dose? |
Acknowledgements:
The authors would like to acknowledge Lisa Graf from the BIDMC Media Services Department for her work on Figure 1.
Funding Statement:
Drs. O’Gara, Marcantonio and Subramaniam are funded by R01AG06554 from the NIH/NIA (PI: Subramaniam). Dr. Gao is funded by R03AG067985 from the NIH/NIA and a Mentored Research Training Grant from the Foundation for Anesthesia, Education and Research. Dr. O’Gara is funded by the Binational Industrial Research and Development Foundation. Dr. Marcantonio is also funded by the following relevant grants from the National Institute on Aging: K24AG035075, P01AG031720.
Footnotes
Conflicts of Interest:
Dr. O’Gara receives consulting income from Sedana Medical for work unrelated to this review. He and the other authors declare no competing interests.
References
- 1.Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, Oh ES, Crosby G, Berger M, Eckenhoff RG: Recommendations for the Nomenclature of Cognitive Change Associated with Anaesthesia and Surgery-2018. Anesthesiology 2018; 129: 872–879 [DOI] [PubMed] [Google Scholar]
- 2.Mahanna-Gabrielli E, Schenning KJ, Eriksson LI, Browndyke JN, Wright CB, Evered L, Scott DA, Wang NY, Brown CH, Oh E, Purdon P, Inouye S, Berger M, Whittington RA, Deiner S: State of the clinical science of perioperative brain health: report from the American Society of Anesthesiologists Brain Health Initiative Summit 2018. British Journal of Anaesthesia 2019; 123: 464–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sprung J, Roberts RO, Weingarten TN, Nunes Cavalcante A, Knopman DS, Petersen RC, Hanson AC, Schroeder DR, Warner DO: Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth 2017; 119: 316–323 [DOI] [PubMed] [Google Scholar]
- 4.Marcantonio ER: Delirium in Hospitalized Older Adults. New England Journal of Medicine 2017; 377: 1456–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evered LA, Silbert BS: Postoperative Cognitive Dysfunction and Noncardiac Surgery. Anesth Analg 2018; 127: 496–505 [DOI] [PubMed] [Google Scholar]
- 6.Berger M, Terrando N, Smith SK, Browndyke JN, Newman MF, Mathew JP: Neurocognitive Function after Cardiac Surgery: From Phenotypes to Mechanisms. Anesthesiology 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen LS, Johnson T, Kuipers HM, Kristensen D, Siersma VD, Vila P, Jolles J, Papaioannou A, Abildstrom H, Silverstein JH, Bonal JA, Raeder J, Nielsen IK, Korttila K, Munoz L, Dodds C, Hanning CD, Moller JT: Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand 2003; 47: 260–6 [DOI] [PubMed] [Google Scholar]
- 8.Radtke FM, Franck M, Lendner J, Krüger S, Wernecke KD, Spies CD: Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. BJA: British Journal of Anaesthesia 2013; 110: i98–i105 [DOI] [PubMed] [Google Scholar]
- 9.Avidan MS, Maybrier HR, Abdallah AB, Jacobsohn E, Vlisides PE, Pryor KO, Veselis RA, Grocott HP, Emmert DA, Rogers EM, Downey RJ, Yulico H, Noh GJ, Lee YH, Waszynski CM, Arya VK, Pagel PS, Hudetz JA, Muench MR, Fritz BA, Waberski W, Inouye SK, Mashour GA: Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet 2017; 390: 267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shroyer AL, Grover FL, Hattler B, Collins JF, McDonald GO, Kozora E, Lucke JC, Baltz JH, Novitzky D: On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med 2009; 361: 1827–37 [DOI] [PubMed] [Google Scholar]
- 11.Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK: Hospital Elder Life Program: Systematic Review and Meta-analysis of Effectiveness. Am J Geriatr Psychiatry 2018; 26: 1015–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czeisler CA, Gooley JJ: Sleep and circadian rhythms in humans. Cold Spring Harb Symp Quant Biol 2007; 72: 579–97 [DOI] [PubMed] [Google Scholar]
- 13.Buysse DJ: Sleep health: can we define it? Does it matter? Sleep 2014; 37: 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaffe K, Falvey CM, Hoang T: Connections between sleep and cognition in older adults. Lancet Neurol 2014; 13: 1017–28 [DOI] [PubMed] [Google Scholar]
- 15.Bhaskar S, Hemavathy D, Prasad S: Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. Journal of Family Medicine and Primary Care 2016; 5: 780–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stranges S, Tigbe W, Gómez-Olivé FX, Thorogood M, Kandala NB: Sleep problems: an emerging global epidemic? Findings from the INDEPTH WHO-SAGE study among more than 40,000 older adults from 8 countries across Africa and Asia. Sleep 2012; 35: 1173–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrie JE, Kumari M, Salo P, Singh-Manoux A, Kivimäki M: Sleep epidemiology--a rapidly growing field. Int J Epidemiol 2011; 40: 1431–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medic G, Wille M, Hemels ME: Short- and long-term health consequences of sleep disruption. Nat Sci Sleep 2017; 9: 151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li P, Gao L, Gaba A, Yu L, Cui L, Fan W, Lim ASP, Bennett DA, Buchman AS, Hu K: Circadian disturbances in Alzheimer’s disease progression: a prospective observational cohort study of community-based older adults. The Lancet Healthy Longevity 2020; 1: e96–e105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fadayomi AB, Ibala R, Bilotta F, Westover MB, Akeju O: A Systematic Review and Meta-Analysis Examining the Impact of Sleep Disturbance on Postoperative Delirium. Crit Care Med 2018; 46: e1204–e1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKenna H, van der Horst GTJ, Reiss I, Martin D: Clinical chronobiology: a timely consideration in critical care medicine. Crit Care 2018; 22: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu K, Li P, Gao L: Sleep, rest-activity rhythms and aging: a complex web in Alzheimer’s disease? Neurobiol Aging 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leng Y, Musiek ES, Hu K, Cappuccio FP, Yaffe K: Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol 2019; 18: 307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung JM, Sands LP, Newman S, Meckler G, Xie Y, Gay C, Lee K: Preoperative Sleep Disruption and Postoperative Delirium. J Clin Sleep Med 2015; 11: 907–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dessap AM, Roche-Campo F, Launay JM, Charles-Nelson A, Katsahian S, Brun-Buisson C, Brochard L: Delirium and Circadian Rhythm of Melatonin During Weaning From Mechanical Ventilation: An Ancillary Study of a Weaning Trial. Chest 2015; 148: 1231–1241 [DOI] [PubMed] [Google Scholar]
- 26.Campbell AM, Axon DR, Martin JR, Slack MK, Mollon L, Lee JK: Melatonin for the prevention of postoperative delirium in older adults: a systematic review and meta-analysis. BMC Geriatr 2019; 19: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hshieh TT, Fong TG, Marcantonio ER, Inouye SK: Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci 2008; 63: 764–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowman K, Jones L, Pilling LC, Delgado J, Kuchel GA, Ferrucci L, Fortinsky RH, Melzer D: Vitamin D levels and risk of delirium: A mendelian randomization study in the UK Biobank. Neurology 2019; 92: e1387–e1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilling LC, Jones LC, Masoli JAH, Delgado J, Atkins JL, Bowden J, Fortinsky RH, Kuchel GA, Melzer D: Low Vitamin D Levels and Risk of Incident Delirium in 351,000 Older UK Biobank Participants. J Am Geriatr Soc 2021; 69: 365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao L, Li P, Cui L, Wong PM, Johnson-Akeju O, Lane J, Saxena R, Scheer F, Hu K: Sleep disturbance and incident Alzheimer’s disease: A UK Biobank study of 502,538 middle-aged to older participants. Alzheimer’s & Dementia 2020; 16: e044575 [Google Scholar]
- 31.Gao L, Lim ASP, Wong PM, Gaba A, Cui L, Yu L, Buchman AS, Bennett DA, Hu K, Li P: Fragmentation of Rest/Activity Patterns in Community-Based Elderly Individuals Predicts Incident Heart Failure. Nat Sci Sleep 2020; 12: 299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finan PH, Goodin BR, Smith MT: The association of sleep and pain: an update and a path forward. The journal of pain : official journal of the American Pain Society 2013; 14: 1539–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Practice Guidelines for the Perioperative Management of Patients with Obstructive Sleep ApneaAn Updated Report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology 2014; 120: 268–286 [DOI] [PubMed] [Google Scholar]
- 34.Alchanatis M, Zias N, Deligiorgis N, Amfilochiou A, Dionellis G, Orphanidou D: Sleep apnea-related cognitive deficits and intelligence: an implication of cognitive reserve theory. Journal of Sleep Research 2005; 14: 69–75 [DOI] [PubMed] [Google Scholar]
- 35.Leng Y, McEvoy CT, Allen IE, Yaffe K: Association of Sleep-Disordered Breathing With Cognitive Function and Risk of Cognitive Impairment: A Systematic Review and Meta-analysis. JAMA Neurology 2017; 74: 1237–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.André C, Rehel S, Kuhn E, Landeau B, Moulinet I, Touron E, Ourry V, Le Du G, Mézenge F, Tomadesso C, de Flores R, Bejanin A, Sherif S, Delcroix N, Manrique A, Abbas A, Marchant NL, Lutz A, Klimecki OM, Collette F, Arenaza-Urquijo EM, Poisnel G, Vivien D, Bertran F, de la Sayette V, Chételat G, Rauchs G: Association of Sleep-Disordered Breathing With Alzheimer Disease Biomarkers in Community-Dwelling Older Adults: A Secondary Analysis of a Randomized Clinical Trial. JAMA Neurol 2020; 77: 716–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirrakhimov AE, Brewbaker CL, Krystal AD, Kwatra MM: Obstructive sleep apnea and delirium: exploring possible mechanisms. Sleep Breath 2014; 18: 19–29 [DOI] [PubMed] [Google Scholar]
- 38.Roggenbach J, Klamann M, von Haken R, Bruckner T, Karck M, Hofer S: Sleep-disordered breathing is a risk factor for delirium after cardiac surgery: a prospective cohort study. Critical Care 2014; 18: 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flink BJ, Rivelli SK, Cox EA, White WD, Falcone G, Vail TP, Young CC, Bolognesi MP, Krystal AD, Trzepacz PT, Moon RE, Kwatra MM: Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology 2012; 116: 788–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King CR, Fritz BA, Escallier K, Ju Y-ES, Lin N, McKinnon S, Avidan MS, Palanca BJ: Association Between Preoperative Obstructive Sleep Apnea and Preoperative Positive Airway Pressure With Postoperative Intensive Care Unit Delirium. JAMA Network Open 2020; 3: e203125–e203125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung F, Memtsoudis SG, Ramachandran SK, Nagappa M, Opperer M, Cozowicz C, Patrawala S, Lam D, Kumar A, Joshi GP, Fleetham J, Ayas N, Collop N, Doufas AG, Eikermann M, Englesakis M, Gali B, Gay P, Hernandez AV, Kaw R, Kezirian EJ, Malhotra A, Mokhlesi B, Parthasarathy S, Stierer T, Wappler F, Hillman DR, Auckley D: Society of Anesthesia and Sleep Medicine Guidelines on Preoperative Screening and Assessment of Adult Patients With Obstructive Sleep Apnea. Anesth Analg 2016; 123: 452–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooke JR, Ayalon L, Palmer BW, Loredo JS, Corey-Bloom J, Natarajan L, Liu L, Ancoli-Israel S: Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: a preliminary study. J Clin Sleep Med 2009; 5: 305–9 [PMC free article] [PubMed] [Google Scholar]
- 43.Dalmases M, Solé-Padullés C, Torres M, Embid C, Nuñez MD, Martínez-Garcia M, Farré R, Bargalló N, Bartrés-Faz D, Montserrat JM: Effect of CPAP on Cognition, Brain Function, and Structure Among Elderly Patients With OSA: A Randomized Pilot Study. Chest 2015; 148: 1214–1223 [DOI] [PubMed] [Google Scholar]
- 44.Crawford-Achour E, Dauphinot V, Martin MS, Tardy M, Gonthier R, Barthelemy JC, Roche F: Protective Effect of Long-Term CPAP Therapy on Cognitive Performance in Elderly Patients with Severe OSA: The PROOF Study. J Clin Sleep Med 2015; 11: 519–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nadler JW, Evans JL, Fang E, Preud’Homme XA, Daughtry RL, Chapman JB, Bolognesi MP, Attarian DE, Wellman SS, Krystal AD: A randomised trial of peri-operative positive airway pressure for postoperative delirium in patients at risk for obstructive sleep apnoea after regional anaesthesia with sedation or general anaesthesia for joint arthroplasty. Anaesthesia 2017; 72: 729–736 [DOI] [PubMed] [Google Scholar]
- 46.Berger M, Oyeyemi D, Olurinde MO, Whitson HE, Weinhold KJ, Woldorff MG, Lipsitz LA, Moretti E, Giattino CM, Roberts KC, Zhou J, Bunning T, Ferrandino M, Scheri RP, Cooter M, Chan C, Cabeza R, Browndyke JN, Murdoch DM, Devinney MJ, Shaw LM, Cohen HJ, Mathew JP: The INTUIT Study: Investigating Neuroinflammation Underlying Postoperative Cognitive Dysfunction. J Am Geriatr Soc 2019; 67: 794–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vacas S: Blood Brain Barrier Dysfunction and Postoperative Neurocognitive Disorders, 2020
- 48.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, Stiles RA, Dittus RS, Bernard GR, Ely EW: Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. Jama 2007; 298: 2644–53 [DOI] [PubMed] [Google Scholar]
- 49.Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, Bratty JR, Takala J, Dexmedetomidine for Long-Term Sedation Investigators ft: Dexmedetomidine vs Midazolam or Propofol for Sedation During Prolonged Mechanical Ventilation: Two Randomized Controlled Trials. JAMA 2012; 307: 1151–1160 [DOI] [PubMed] [Google Scholar]
- 50.Zeng H, Li Z, He J, Fu W: Dexmedetomidine for the prevention of postoperative delirium in elderly patients undergoing noncardiac surgery: A meta-analysis of randomized controlled trials. PLoS One 2019; 14: e0218088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng KT, Shubash CJ, Chong JS: The effect of dexmedetomidine on delirium and agitation in patients in intensive care: systematic review and meta-analysis with trial sequential analysis. Anaesthesia 2019; 74: 380–392 [DOI] [PubMed] [Google Scholar]
- 52.Akeju O, Hobbs LE, Gao L, Burns SM, Pavone KJ, Plummer GS, Walsh EC, Houle TT, Kim S-E, Bianchi MT, Ellenbogen JM, Brown EN: Dexmedetomidine promotes biomimetic non-rapid eye movement stage 3 sleep in humans: A pilot study. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 2018; 129: 69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu XH, Cui F, Zhang C, Meng ZT, Wang DX, Ma J, Wang GF, Zhu SN, Ma D: Low-dose Dexmedetomidine Improves Sleep Quality Pattern in Elderly Patients after Noncardiac Surgery in the Intensive Care Unit: A Pilot Randomized Controlled Trial. Anesthesiology 2016; 125: 979–991 [DOI] [PubMed] [Google Scholar]
- 54.Su X, Meng ZT, Wu XH, Cui F, Li HL, Wang DX, Zhu X, Zhu SN, Maze M, Ma D: Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016; 388: 1893–1902 [DOI] [PubMed] [Google Scholar]
- 55.Chamadia S, Hobbs L, Marota S, Ibala R, Hahm E, Gitlin J, Mekonnen J, Ethridge B, Colon KM, Sheppard KS, Manoach DS, DiBiasio A, Nguyen S, Pedemonte JC, Akeju O: Oral Dexmedetomidine Promotes Non-rapid Eye Movement Stage 2 Sleep in Humans. Anesthesiology 2020; 133: 1234–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cajochen C, Kräuchi K, Wirz-Justice A: Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol 2003; 15: 432–7 [DOI] [PubMed] [Google Scholar]
- 57.Olofsson K, Alling C, Lundberg D, Malmros C: Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand 2004; 48: 679–84 [DOI] [PubMed] [Google Scholar]
- 58.Yoshitaka S, Egi M, Morimatsu H, Kanazawa T, Toda Y, Morita K: Perioperative plasma melatonin concentration in postoperative critically ill patients: its association with delirium. J Crit Care 2013; 28: 236–42 [DOI] [PubMed] [Google Scholar]
- 59.Artemiou P, Bily B, Bilecova-Rabajdova M, Sabol F, Torok P, Kolarcik P, Kolesar A: Melatonin treatment in the prevention of postoperative delirium in cardiac surgery patients. Kardiochir Torakochirurgia Pol 2015; 12: 126–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hatta K, Kishi Y, Wada K, Takeuchi T, Odawara T, Usui C, Nakamura H, Group ftD-J: Preventive Effects of Ramelteon on Delirium: A Randomized Placebo-Controlled Trial. JAMA Psychiatry 2014; 71: 397–403 [DOI] [PubMed] [Google Scholar]
- 61.Jaiswal SJ, Vyas AD, Heisel AJ, Ackula H, Aggarwal A, Kim NH, Kerr KM, Madani M, Pretorius V, Auger WR, Fernandes TM, Malhotra A, Owens RL: Ramelteon for Prevention of Postoperative Delirium: A Randomized Controlled Trial in Patients Undergoing Elective Pulmonary Thromboendarterectomy*. Critical Care Medicine 2019; 47: 1751–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ng KT, Teoh WY, Khor AJ: The effect of melatonin on delirium in hospitalised patients: A systematic review and meta-analyses with trial sequential analysis. J Clin Anesth 2020; 59: 74–81 [DOI] [PubMed] [Google Scholar]
- 63.Wu Y, Wang J, Wu A, Yue Y: Do fluctuations in endogenous melatonin levels predict the occurrence of postoperative cognitive dysfunction (POCD)? International Journal of Neuroscience 2014; 124: 787–791 [DOI] [PubMed] [Google Scholar]
- 64.Gögenur I, Middleton B, Burgdorf S, Rasmussen LS, Skene DJ, Rosenberg J: Impact of sleep and circadian disturbances in urinary 6-sulphatoxymelatonin levels, on cognitive function after major surgery. J Pineal Res 2007; 43: 179–84 [DOI] [PubMed] [Google Scholar]
- 65.Fan Y, Yuan L, Ji M, Yang J, Gao D: The effect of melatonin on early postoperative cognitive decline in elderly patients undergoing hip arthroplasty: A randomized controlled trial. Journal of Clinical Anesthesia 2017; 39: 77–81 [DOI] [PubMed] [Google Scholar]
- 66.Hansen MV, Madsen MT, Andersen LT, Hageman I, Rasmussen LS, Bokmand S, Rosenberg J, Gögenur I: Effect of Melatonin on Cognitive Function and Sleep in relation to Breast Cancer Surgery: A Randomized, Double-Blind, Placebo-Controlled Trial. International Journal of Breast Cancer 2014; 2014: 416531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamdar BB, King LM, Collop NA, Sakamuri S, Colantuoni E, Neufeld KJ, Bienvenu OJ, Rowden AM, Touradji P, Brower RG, Needham DM: The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical ICU. Critical care medicine 2013; 41: 800–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McMahon SB, Cafferty WB, Marchand F: Immune and glial cell factors as pain mediators and modulators. Exp Neurol 2005; 192: 444–62 [DOI] [PubMed] [Google Scholar]
- 69.Campbell JN, Meyer RA: Mechanisms of neuropathic pain. Neuron 2006; 52: 77–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inouye SK, Westendorp RG, Saczynski JS: Delirium in elderly people. Lancet 2014; 383: 911–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hughes CG, Patel MB, Pandharipande PP: Pathophysiology of acute brain dysfunction: what’s the cause of all this confusion? Curr Opin Crit Care 2012; 18: 518–26 [DOI] [PubMed] [Google Scholar]
- 72.MacLullich AM, Edelshain BT, Hall RJ, de Vries A, Howie SE, Pearson A, Middleton SD, Gillies F, Armstrong IR, White TO, Cunningham C, de Rooij SE, van Munster BC: Cerebrospinal fluid interleukin-8 levels are higher in people with hip fracture with perioperative delirium than in controls. J Am Geriatr Soc 2011; 59: 1151–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW: From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jalleh R, Koh K, Choi B, Liu E, Maddison J, Hutchinson MR: Role of microglia and toll-like receptor 4 in the pathophysiology of delirium. Med Hypotheses 2012; 79: 735–9 [DOI] [PubMed] [Google Scholar]
- 75.Naser PV, Kuner R: Molecular, Cellular and Circuit Basis of Cholinergic Modulation of Pain. Neuroscience 2018; 387: 135–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vaurio LE, Sands LP, Wang Y, Mullen EA, Leung JM: Postoperative delirium: the importance of pain and pain management. Anesth Analg 2006; 102: 1267–73 [DOI] [PubMed] [Google Scholar]
- 77.Kosar CM, Tabloski PA, Travison TG, Jones RN, Schmitt EM, Puelle MR, Inloes JB, Saczynski JS, Marcantonio ER, Meagher D, Reid MC, Inouye SK: Effect of Preoperative Pain and Depressive Symptoms on the Development of Postoperative Delirium. Lancet Psychiatry 2014; 1: 431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ong W-Y, Stohler CS, Herr DR: Role of the Prefrontal Cortex in Pain Processing. Molecular neurobiology 2019; 56: 1137–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morrison RS, Magaziner J, Gilbert M, Koval KJ, McLaughlin MA, Orosz G, Strauss E, Siu AL: Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci 2003; 58: 76–81 [DOI] [PubMed] [Google Scholar]
- 80.Lynch EP, Lazor MA, Gellis JE, Orav J, Goldman L, Marcantonio ER: The impact of postoperative pain on the development of postoperative delirium. Anesth Analg 1998; 86: 781–5 [DOI] [PubMed] [Google Scholar]
- 81.Leung JM, Sands LP, Lim E, Tsai TL, Kinjo S: Does preoperative risk for delirium moderate the effects of postoperative pain and opiate use on postoperative delirium? Am J Geriatr Psychiatry 2013; 21: 946–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ganai S, Lee KF, Merrill A, Lee MH, Bellantonio S, Brennan M, Lindenauer P: Adverse Outcomes of Geriatric Patients Undergoing Abdominal Surgery Who Are at High Risk for Delirium. Archives of Surgery 2007; 142: 1072–1078 [DOI] [PubMed] [Google Scholar]
- 83.Schofield P: The Assessment of Pain in Older People: UK National Guidelines. Age and Ageing 2018; 47: i1–i22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marra A, Ely EW, Pandharipande PP, Patel MB: The ABCDEF Bundle in Critical Care. Critical care clinics 2017; 33: 225–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hughes CG, Boncyk CS, Culley DJ, Fleisher LA, Leung JM, McDonagh DL, Gan TJ, McEvoy MD, Miller TE, Perioperative Quality Initiative W: American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Postoperative Delirium Prevention. Anesthesia and analgesia 2020; 130: 1572–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015; 63: 142–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robinson S, Vollmer C: Undermedication for pain and precipitation of delirium. Medsurg Nurs 2010; 19: 79–83; quiz 84 [PubMed] [Google Scholar]
- 88.Sieber FE, Mears S, Lee H, Gottschalk A: Postoperative opioid consumption and its relationship to cognitive function in older adults with hip fracture. J Am Geriatr Soc 2011; 59: 2256–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koepke EJ, Manning EL, Miller TE, Ganesh A, Williams DGA, Manning MW: The rising tide of opioid use and abuse: the role of the anesthesiologist. Perioperative medicine (London, England) 2018; 7: 16–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Swart LM, van der Zanden V, Spies PE, de Rooij SE, van Munster BC: The Comparative Risk of Delirium with Different Opioids: A Systematic Review. Drugs Aging 2017; 34: 437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y, Sands LP, Vaurio L, Mullen EA, Leung JM: The Effects of Postoperative Pain and Its Management on Postoperative Cognitive Dysfunction. The American Journal of Geriatric Psychiatry 2007; 15: 50–59 [DOI] [PubMed] [Google Scholar]
- 92.Mu DL, Zhang DZ, Wang DX, Wang G, Li CJ, Meng ZT, Li YW, Liu C, Li XY: Parecoxib Supplementation to Morphine Analgesia Decreases Incidence of Delirium in Elderly Patients After Hip or Knee Replacement Surgery: A Randomized Controlled Trial. Anesth Analg 2017; 124: 1992–2000 [DOI] [PubMed] [Google Scholar]
- 93.Flower RJ, Vane JR: Inhibition of prostaglandin synthetase in brain explains the anti-pyretic activity of paracetamol (4-acetamidophenol). Nature 1972; 240: 410–1 [DOI] [PubMed] [Google Scholar]
- 94.Subramaniam B, Shankar P, Shaefi S, Mueller A, O’Gara B, Banner-Goodspeed V, Gallagher J, Gasangwa D, Patxot M, Packiasabapathy S, Mathur P, Eikermann M, Talmor D, Marcantonio ER: Effect of Intravenous Acetaminophen vs Placebo Combined With Propofol or Dexmedetomidine on Postoperative Delirium Among Older Patients Following Cardiac Surgery. JAMA 2019; 321: 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mathew JP, Mackensen GB, Phillips-Bute B, Grocott HP, Glower DD, Laskowitz DT, Blumenthal JA, Newman MF: Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke 2009; 40: 880–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mathew JP, White WD, Schinderle DB, Podgoreanu MV, Berger M, Milano CA, Laskowitz DT, Stafford-Smith M, Blumenthal JA, Newman MF, Mathew JP, Blumenthal JA, Fontes MA, Kertai MD, Lombard FW, Mathew JP, McDonagh DL, Monk TG, Newman MF, Podgoreanu MV, Stafford-Smith M, Swaminathan M, Warner DS, Funk BL, Balajonda N, Hall RL, Bisanar T, Clemmons KL, Li YJ, Pecora G, Phillips-Bute B, Toulgoat-Dubois Y, Waweru P, White WD, Babyak MA, Blumenthal JA, Browndyke JN, Welsh-Bohmer KA, Mark DB, Sketch MH, Bennett ER, Graffagnino C, Laskowitz DT, Strittmatter WJ, Alexander S, Collins K, Smigla G, Shearer I, Berry MF, D’Amico TA, Daneshmand MA, Davis RD, Gaca JG, Glower DD, Harpole RD, Hughes GC, Jaquiss RDB, Lin SS, Lodge AJ, Milano CA, Onaitis MW, Schroeder JN, Smith PK, Tong BC: Intraoperative Magnesium Administration Does Not Improve Neurocognitive Function After Cardiac Surgery. 2013; 44: 3407–3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Royse CF, Saager L, Whitlock R, Ou-Young J, Royse A, Vincent J, Devereaux PJ, Kurz A, Awais A, Panjasawatwong K, Sessler DI: Impact of Methylprednisolone on Postoperative Quality of Recovery and Delirium in the Steroids in Cardiac Surgery Trial: A Randomized, Double-blind, Placebo-controlled Substudy. Anesthesiology 2017; 126: 223–233 [DOI] [PubMed] [Google Scholar]
- 98.Whitlock RP, Devereaux PJ, Teoh KH, Lamy A, Vincent J, Pogue J, Paparella D, Sessler DI, Karthikeyan G, Villar JC, Zuo Y, Avezum Á, Quantz M, Tagarakis GI, Shah PJ, Abbasi SH, Zheng H, Pettit S, Chrolavicius S, Yusuf S: Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Lancet 2015; 386: 1243–1253 [DOI] [PubMed] [Google Scholar]
- 99.Sauër AM, Slooter AJ, Veldhuijzen DS, van Eijk MM, Devlin JW, van Dijk D: Intraoperative dexamethasone and delirium after cardiac surgery: a randomized clinical trial. Anesth Analg 2014; 119: 1046–52 [DOI] [PubMed] [Google Scholar]
- 100.Leung JM, Sands LP, Chen N, Ames C, Berven S, Bozic K, Burch S, Chou D, Covinsky K, Deviren V, Kinjo S, Kramer JH, Ries M, Tay B, Vail T, Weinstein P, Chang S, Meckler G, Newman S, Tsai T, Voss V, Youngblom E: Perioperative Gabapentin Does Not Reduce Postoperative Delirium in Older Surgical Patients: A Randomized Clinical Trial. Anesthesiology 2017; 127: 633–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krenk L, Rasmussen LS, Hansen TB, Bogø S, Søballe K, Kehlet H: Delirium after fast-track hip and knee arthroplasty. Br J Anaesth 2012; 108: 607–11 [DOI] [PubMed] [Google Scholar]
- 102.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr., Charney DS: Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 1994; 51: 199–214 [DOI] [PubMed] [Google Scholar]
- 103.Bornemann-Cimenti H, Wejbora M, Michaeli K, Edler A, Sandner-Kiesling A: The effects of minimal-dose versus low-dose S-ketamine on opioid consumption, hyperalgesia, and postoperative delirium: a triple-blinded, randomized, active- and placebo-controlled clinical trial. Minerva Anestesiol 2016; 82: 1069–1076 [PubMed] [Google Scholar]
- 104.Hudetz JA, Patterson KM, Iqbal Z, Gandhi SD, Byrne AJ, Hudetz AG, Warltier DC, Pagel PS: Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2009; 23: 651–7 [DOI] [PubMed] [Google Scholar]
- 105.Hudetz JA, Iqbal Z, Gandhi SD, Patterson KM, Byrne AJ, Hudetz AG, Pagel PS, Warltier DC: Ketamine attenuates post-operative cognitive dysfunction after cardiac surgery. Acta Anaesthesiol Scand 2009; 53: 864–72 [DOI] [PubMed] [Google Scholar]
- 106.Mouzopoulos G, Vasiliadis G, Lasanianos N, Nikolaras G, Morakis E, Kaminaris M: Fascia iliaca block prophylaxis for hip fracture patients at risk for delirium: a randomized placebo-controlled study. J Orthop Traumatol 2009; 10: 127–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kinjo S, Lim E, Sands LP, Bozic KJ, Leung JM: Does using a femoral nerve block for total knee replacement decrease postoperative delirium? BMC Anesthesiol 2012; 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sieber FE, Gottshalk A, Zakriya KJ, Mears SC, Lee H: General anesthesia occurs frequently in elderly patients during propofol-based sedation and spinal anesthesia. Journal of Clinical Anesthesia 2010; 22: 179–183 [DOI] [PubMed] [Google Scholar]
- 109.Neuman MD, Ellenberg SS, Sieber FE, Magaziner JS, Feng R, Carson JL: Regional versus General Anesthesia for Promoting Independence after Hip Fracture (REGAIN): protocol for a pragmatic, international multicentre trial. BMJ Open 2016; 6: e013473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hughes MJ, Ventham NT, McNally S, Harrison E, Wigmore S: Analgesia After Open Abdominal Surgery in the Setting of Enhanced Recovery Surgery: A Systematic Review and Meta-analysis. JAMA Surgery 2014; 149: 1224–1230 [DOI] [PubMed] [Google Scholar]
- 111.Mann C, Pouzeratte Y, Boccara G, Peccoux C, Vergne C, Brunat G, Domergue J, Millat B, Colson P: Comparison of intravenous or epidural patient-controlled analgesia in the elderly after major abdominal surgery. Anesthesiology 2000; 92: 433–41 [DOI] [PubMed] [Google Scholar]
- 112.Vlisides PE, Thompson A, Kunkler BS, Maybrier HR, Avidan MS, Mashour GA: Perioperative Epidural Use and Risk of Delirium in Surgical Patients: A Secondary Analysis of the PODCAST Trial. Anesth Analg 2019; 128: 944–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jia Y, Jin G, Guo S, Gu B, Jin Z, Gao X, Li Z: Fast-track surgery decreases the incidence of postoperative delirium and other complications in elderly patients with colorectal carcinoma. Langenbecks Arch Surg 2014; 399: 77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Engelman DT, Ben Ali W, Williams JB, Perrault LP, Reddy VS, Arora RC, Roselli EE, Khoynezhad A, Gerdisch M, Levy JH, Lobdell K, Fletcher N, Kirsch M, Nelson G, Engelman RM, Gregory AJ, Boyle EM: Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA Surgery 2019; 154: 755–766 [DOI] [PubMed] [Google Scholar]
- 115.Sprung J, Abdelmalak B, Gottlieb A, Mayhew C, Hammel J, Levy Pavel J, O’Hara P, Hertzer Norman R: Analysis of Risk Factors for Myocardial Infarction and Cardiac Mortality after Major Vascular Surgery. Anesthesiology 2000; 93: 129–140 [DOI] [PubMed] [Google Scholar]
- 116.Sharifpour M, Moore LE, Shanks AM, Didier TJ, Kheterpal S, Mashour GA: Incidence, Predictors, and Outcomes of Perioperative Stroke in Noncarotid Major Vascular Surgery. Anesthesia & Analgesia 2013; 116: 424–434 [DOI] [PubMed] [Google Scholar]
- 117.Bell S, Dekker FW, Vadiveloo T, Marwick C, Deshmukh H, Donnan PT, Van Diepen M: Risk of postoperative acute kidney injury in patients undergoing orthopaedic surgery—development and validation of a risk score and effect of acute kidney injury on survival: observational cohort study. BMJ 2015; 351: h5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Robinson TN, Wu DS, Pointer LF, Dunn CL, Moss M: Preoperative cognitive dysfunction is related to adverse postoperative outcomes in the elderly. J Am Coll Surg 2012; 215: 12–7; discussion 17–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Culley DJ, Flaherty D, Fahey MC, Rudolph JL, Javedan H, Huang CC, Wright J, Bader AM, Hyman BT, Blacker D, Crosby G: Poor Performance on a Preoperative Cognitive Screening Test Predicts Postoperative Complications in Older Orthopedic Surgical Patients. Anesthesiology 2017; 127: 765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee HB, Mears SC, Rosenberg PB, Leoutsakos JM, Gottschalk A, Sieber FE: Predisposing factors for postoperative delirium after hip fracture repair in individuals with and without dementia. J Am Geriatr Soc 2011; 59: 2306–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tow A, Holtzer R, Wang C, Sharan A, Kim SJ, Gladstein A, Blum Y, Verghese J: Cognitive Reserve and Postoperative Delirium in Older Adults. J Am Geriatr Soc 2016; 64: 1341–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Smith PJ, Attix DK, Weldon BC, Greene NH, Monk TG: Executive function and depression as independent risk factors for postoperative delirium. Anesthesiology 2009; 110: 781–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cunningham EL, Mawhinney T, Beverland D, O’Brien S, McAuley DF, Cairns R, Passmore P, McGuinness B: Observational cohort study examining apolipoprotein E status and preoperative neuropsychological performance as predictors of post-operative delirium in an older elective arthroplasty population. Age Ageing 2017; 46: 779–786 [DOI] [PubMed] [Google Scholar]
- 124.Silbert B, Evered L, Scott DA, McMahon S, Choong P, Ames D, Maruff P, Jamrozik K: Preexisting cognitive impairment is associated with postoperative cognitive dysfunction after hip joint replacement surgery. Anesthesiology 2015; 122: 1224–34 [DOI] [PubMed] [Google Scholar]
- 125.Jones RN, Marcantonio ER, Saczynski JS, Tommet D, Gross AL, Travison TG, Alsop DC, Schmitt EM, Fong TG, Cizginer S, Shafi MM, Pascual-Leone A, Inouye SK: Preoperative Cognitive Performance Dominates Risk for Delirium Among Older Adults. J Geriatr Psychiatry Neurol 2016; 29: 320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lingehall HC, Smulter NS, Lindahl E, Lindkvist M, Engström KG, Gustafson YG, Olofsson B: Preoperative Cognitive Performance and Postoperative Delirium Are Independently Associated With Future Dementia in Older People Who Have Undergone Cardiac Surgery: A Longitudinal Cohort Study. Crit Care Med 2017; 45: 1295–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Berger M, Schenning KJ, Brown CHt, Deiner SG, Whittington RA, Eckenhoff RG, Angst MS, Avramescu S, Bekker A, Brzezinski M, Crosby G, Culley DJ, Eckenhoff M, Eriksson LI, Evered L, Ibinson J, Kline RP, Kofke A, Ma D, Mathew JP, Maze M, Orser BA, Price CC, Scott DA, Silbert B, Su D, Terrando N, Wang DS, Wei H, Xie Z, Zuo Z: Best Practices for Postoperative Brain Health: Recommendations From the Fifth International Perioperative Neurotoxicity Working Group. Anesth Analg 2018; 127: 1406–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stern Y: Cognitive reserve. Neuropsychologia 2009; 47: 2015–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jones RN, Yang FM, Zhang Y, Kiely DK, Marcantonio ER, Inouye SK: Does educational attainment contribute to risk for delirium? A potential role for cognitive reserve. J Gerontol A Biol Sci Med Sci 2006; 61: 1307–11 [DOI] [PubMed] [Google Scholar]
- 130.Martins S, Paiva JA, Simões MR, Fernandes L: Delirium in elderly patients: association with educational attainment. Acta Neuropsychiatr 2017; 29: 95–101 [DOI] [PubMed] [Google Scholar]
- 131.Galanakis P, Bickel H, Gradinger R, Von Gumppenberg S, Förstl H: Acute confusional state in the elderly following hip surgery: incidence, risk factors and complications. Int J Geriatr Psychiatry 2001; 16: 349–55 [DOI] [PubMed] [Google Scholar]
- 132.Saczynski JS, Inouye SK, Kosar C, Tommet D, Marcantonio ER, Fong T, Hshieh T, Vasunilashorn S, Metzger ED, Schmitt E, Alsop DC, Jones RN: Cognitive and Brain Reserve and the Risk of Postoperative Delirium in Older Patients. Lancet Psychiatry 2014; 1: 437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JEW, Gravenstein JS: Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. The Lancet 1998; 351: 857–861 [DOI] [PubMed] [Google Scholar]
- 134.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS: Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008; 108: 18–30 [DOI] [PubMed] [Google Scholar]
- 135.Borrell-Vega J, Esparza Gutierrez AG, Humeidan ML: Multimodal Prehabilitation Programs for Older Surgical Patients. Anesthesiol Clin 2019; 37: 437–452 [DOI] [PubMed] [Google Scholar]
- 136.Gillis C, Buhler K, Bresee L, Carli F, Gramlich L, Culos-Reed N, Sajobi TT, Fenton TR: Effects of Nutritional Prehabilitation, With and Without Exercise, on Outcomes of Patients Who Undergo Colorectal Surgery: A Systematic Review and Meta-analysis. Gastroenterology 2018; 155: 391–410.e4 [DOI] [PubMed] [Google Scholar]
- 137.Mahanna-Gabrielli E, Zhang K, Sieber FE, Lin HM, Liu X, Sewell M, Deiner SG, Boockvar KS: Frailty Is Associated With Postoperative Delirium But Not With Postoperative Cognitive Decline in Older Noncardiac Surgery Patients. Anesth Analg 2020; 130: 1516–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nomura Y, Nakano M, Bush B, Tian J, Yamaguchi A, Walston J, Hasan R, Zehr K, Mandal K, LaFlam A, Neufeld KJ, Kamath V, Hogue CW, Brown CHt: Observational Study Examining the Association of Baseline Frailty and Postcardiac Surgery Delirium and Cognitive Change. Anesth Analg 2019; 129: 507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ng TP, Feng L, Nyunt MSZ, Feng L, Niti M, Tan BY, Chan G, Khoo SA, Chan SM, Yap P, Yap KB: Nutritional, Physical, Cognitive, and Combination Interventions and Frailty Reversal Among Older Adults: A Randomized Controlled Trial. The American Journal of Medicine 2015; 128: 1225–1236.e1 [DOI] [PubMed] [Google Scholar]
- 140.Janssen TL, Steyerberg EW, Langenberg JCM, de Lepper C, Wielders D, Seerden TCJ, de Lange DC, Wijsman JH, Ho GH, Gobardhan PD, van Alphen R, van der Laan L: Multimodal prehabilitation to reduce the incidence of delirium and other adverse events in elderly patients undergoing elective major abdominal surgery: An uncontrolled before-and-after study. PLoS One 2019; 14: e0218152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Valenzuela M, Sachdev P: Can cognitive exercise prevent the onset of dementia? Systematic review of randomized clinical trials with longitudinal follow-up. Am J Geriatr Psychiatry 2009; 17: 179–87 [DOI] [PubMed] [Google Scholar]
- 142.Ball K, Berch DB, Helmers KF, et al. : Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA 2002; 288: 2271–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M: A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385: 2255–63 [DOI] [PubMed] [Google Scholar]
- 144.Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, Joyce NM, Boniske T, Atkins SM, Merzenich MM: Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci U S A 2006; 103: 12523–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rebok GW, Ball K, Guey LT, Jones RN, Kim H-Y, King JW, Marsiske M, Morris JN, Tennstedt SL, Unverzagt FW, Willis SL: Ten-Year Effects of the ACTIVE Cognitive Training Trial on Cognition and Everyday Functioning in Older Adults. Journal of the American Geriatrics Society 2014; 62: 16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Saleh AJ, Tang GX, Hadi SM, Yan L, Chen MH, Duan KM, Tong J, Ouyang W: Preoperative cognitive intervention reduces cognitive dysfunction in elderly patients after gastrointestinal surgery: a randomized controlled trial. Med Sci Monit 2015; 21: 798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vlisides PE, Das AR, Thompson AM, Kunkler B, Zierau M, Cantley MJ, McKinney AM, Giordani B: Home-based Cognitive Prehabilitation in Older Surgical Patients: A Feasibility Study. J Neurosurg Anesthesiol 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.O’Gara BP, Mueller A, Gasangwa DVI, Patxot M, Shaefi S, Khabbaz K, Banner-Goodspeed V, Pascal-Leone A, Marcantonio ER, Subramaniam B: Prevention of Early Postoperative Decline: A Randomized, Controlled Feasibility Trial of Perioperative Cognitive Training. Anesth Analg 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Humeidan ML, Reyes J-PC, Mavarez-Martinez A, Roeth C, Nguyen CM, Sheridan E, Zuleta-Alarcon A, Otey A, Abdel-Rasoul M, Bergese SD: Effect of Cognitive Prehabilitation on the Incidence of Postoperative Delirium Among Older Adults Undergoing Major Noncardiac Surgery: The Neurobics Randomized Clinical Trial. JAMA Surgery 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Song Y, Cui X, Zhang Y, Gao H, Cai Q, Mu Z: Home-Based Computerized Cognitive Training for Postoperative Cognitive Dysfunction After Lung Transplantation in Elderly Population: A Randomized Controlled Trial. J Nerv Ment Dis 2019; 207: 693–699 [DOI] [PubMed] [Google Scholar]
- 151.Inouye SK, Bogardus ST Jr., Baker DI, Leo-Summers L, Cooney LM Jr.: The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J Am Geriatr Soc 2000; 48: 1697–706 [DOI] [PubMed] [Google Scholar]
- 152.Chen CC, Lin MT, Tien YW, Yen CJ, Huang GH, Inouye SK: Modified hospital elder life program: effects on abdominal surgery patients. J Am Coll Surg 2011; 213: 245–52 [DOI] [PubMed] [Google Scholar]
- 153.Cheng CM, Chiu MJ, Wang JH, Liu HC, Shyu YI, Huang GH, Chen CC: Cognitive stimulation during hospitalization improves global cognition of older Taiwanese undergoing elective total knee and hip replacement surgery. J Adv Nurs 2012; 68: 1322–9 [DOI] [PubMed] [Google Scholar]


