Abstract
Among the plethora of debilitating neurological disorders of COVID-19 syndrome in survivors, the scope of SARS-CoV-2-induced dysautonomia (DNS) is yet to be understood, though the implications are enormous. Herein, we present an inclusive mini-review of SARS-CoV-2-induced DNS and its associated complications. Although, the direct link between Covid-19 and DSN is still speculative, the hypothetical links are thought to be either a direct neuronal injury of the autonomic pathway or a para/post-infectious immune-induced mechanism. SARS-CoV-2 infection-induced stress may activate the sympathetic nervous system (SNS) leading to neuro-hormonal stimulation and activation of pro-inflammatory cytokines with further development of sympathetic storm. Sympathetic over-activation in Covid-19 is correlated with increase in capillary pulmonary leakage, alveolar damage, and development of acute respiratory distress syndrome. Furthermore, SARS-CoV-2 can spread through pulmonary mechanoreceptors and chemoreceptors to medullary respiratory center in a retrograde manner resulting in sudden respiratory failure. Taken together, DSN in Covid-19 is developed due to sympathetic storm and inhibition of Parasympathetic nervous system-mediated anti-inflammatory effect with development of cytokine storm. Therefore, sympathetic and cytokine storms together with activation of Renin-Angiotensin-System are the chief final pathway involved in the development of DSN in Covid-19.
Keywords: neurodegenerative diseases, dysautonomia, covid-19, sympathetic storm, cytokine storm
1. Introduction
Dysautonomia (DSN) is a neurological disorder caused by dysfunction of autonomic nervous system (ANS) that affect the functions of heart, bladder, sweat gland, pupils, intestines, and other autonomic functions (Chakraborty et al., 2020). DSN is of two types, the primary type, which is also called inherited or neurological type while the secondary type is acquired due to various diseases including diabetes mellitus, autoimmune disease, toxicity, and alcoholism (Axelrod, 2004). DSN could be part of neuropathic disorders, which are somatic, sensory, or mixed in different neurological disorders as in multiple system atrophy, Parkinson disease, Lewy body dementia, autonomic failure, autonomic gangliopathy, and orthostatic tachycardia syndrome (Shoenfeld et al., 2020). Both sympathetic nervous system (SNS) and parasympathetic nervous system (PSNS) are affected in DSN leading to sympathetic storm and abnormal autonomic response such as abnormal sweating, exercise intolerance, insomnia, resting tachycardia, postural hypotension, fatigue, urinary and bowel dysfunctions (Martínez-Lavín, 2015; Murta et al., 2020).
DSN is mainly diagnosed by the measurement of heart rate (HR) and blood pressure (BP) at lying position and three minute at standing position, however autonomic response reflexes such as sudomotor response and thermoregulatory sweat test are more diagnostic (Sealey & Lui, 2004).
The associated comorbidities of coronavirus disease 2019 (COVID-19), such as hypertension, chronic lung disease, kidney diseases, heart failure, diabetes mellitus, and obesity are linked with increased sympathetic nerve activity (Carnagarin et al., 2019; Díaz et al., 2020). Furthermore, as part of its extra-pulmonary manifestations, COVID-19 infection may also heighten the sympathetic discharge through emotional distress, changes in blood gases, immune/inflammatory factors or angiotensin-converting enzyme (ACE)1/ACE2 imbalance (Gurwitz, 2020; Porzionato et al., 2020; Zhang et al., 2020). In both cases, the heightened sympathetic stimulation in COVID-19 patients may be classified into a short or long term “Post-COVID-19” DSN (Porzionato et al., 2020). Post-COVID-19 DSN is not well understood aspect of present COVID-19 pandemic, with clinical features that overlay and overlap with autonomic and motor-sensory symptoms. Initial and early systematic analysis of DSN in COVID-19 is mostly missing and may provide early insight into the spectrum and range of this condition (Shouman et al., 2021).
Also, several studies and reviews have reported the neuroinvasive nature of SARS-CoV-2 that further results in neurological complications including DSN (Leonardi et al., 2020; Paniz-Mondolfi et al., 2020; Rogers et al., 2020; Troyer et al., 2020), but the main link in SARS-CoV-2-induced DSN is still speculative. Deconditioning in COVID-19 patients was initially thought as a mechanism for the associated DSN (Dani et al., 2021), other reports argued against the thought (Goodman et al., 2021a), suggesting a direct neuronal injury of autonomic pathway or indirect immune-mediated mechanisms . Therefore, in this literature review we present an inclusive perspective of SARS-CoV-2-induced DNS and its associated complications.
2. Methods
We performed an extensive literature search covering the period up to September 2021 using the MEDLINE/PubMed electronic database with the following search strategy: key words “SARS-CoV-2”. When we used the key words “SARS-CoV-2 AND neurodegenerative disease” a total of 515 papers were found. A further use of “SARS-CoV-2 AND Dysautonomia”, gave a total of 41 papers while “COVID-19 AND Dysautonomia”, gave a total of 57 papers. The literatures identified were divided into clinical trials: 2, Case reports: 32, Commentaries: 9 and Reviews: 14. English-language original publications and letters to editors between January 2020 and July 2021 were selected for the study; also review papers’ references and textbook chapters were consulted especially for “viral infection and Dysautonomia”
3. Results
3.1. Viral Infection and Dysautonomia
It has been reported that autonomic dysfunction or DSN is associated with various viral infections including human immune deficiency virus (HIV), herpes viruses, enterovirus 71, flavivirus and human T-lymphotropic virus. Manifestations of viral infections-induced-DSN are of wide-spectrum such as loss of heart rate variability in HIV, hypersalivation, photophobia, dyspnea, and piloerection in rabies (Carod-Artal, 2018). Osztovits et al., confirmed the association between chronic hepatitis C virus and development of autonomic dysfunction (Osztovits et al., 2009). There is a strong association between early respiratory syncytial virus infection and loss of heart rate variability and baroreflex sensitivity that cause cardiopulmonary complications in the neonates (Stock et al., 2010). Previous experimental report demonstrated that West Nile virus could cause autonomic dysfunctions including gastrointestinal, respiratory and cardiovascular complications (Wang et al., 2011). Indeed, Epstein-Barr virus (EBV) infection may lead to DSN through alteration of cerebral autoregulation by high nitric oxide release with subsequent ANS dysfunction(Sternberg, 2012).
In DSN, both SNS and PSNS neurotransmitters are involved, however, selective cholinergic DSN has been observed in infection with EBV due to the development of auto-antibodies that affect muscarinic cholinergic receptor with progression of post-ganglionic DSN (Palma et al., 2020). Complex regional pain syndrome and DSN have been reported in a 14 year female due to selective adrenergic DSN caused by auto-antibodies against β-2 adrenoceptors (Hendrickson et al., 2016).
Notably, the level of ANS control is regulated by hypothalamus, cerebral cortex, reticular formation and limbic system, therefore, viral infections that cause meningitis and/encephalitis may disturb central autonomic dysfunction leading to DSN (Silverman et al., 2005). In some neurotropic viruses, involvement of peripheral autonomic neurons adjacent to the affected viscera may affect autonomic circuits and reflexes leading to autonomic dysfunction (Feng et al., 2020). Therefore, both central and peripheral parts of ANS are affected during severe viral infections, suggesting a causal relationship between viral infection and autonomic dysfunction with subsequent development of DSN (Smeraski et al., 2004). These verdicts suggest that viral infections could cause DNS through dysregulation of central and peripheral circuit of ANS.
3.2. COVID-19 and Dysautonomia
The coronavirus shares a commons feature of neuroinvasion. Great similarities have been seen between SARS-CoV and SARS-CoV-2 (Khatoon et al., 2020). These neurotrophic viruses have multiple routes including direct and indirect pathway (Khatoon et al., 2020). In the direct mechanism, SARS-CoV-2 reaches the ANS by employing the retrograde axonal transport via the olfactory nerve (Li et al., 2020), ACE2 in brainstem (Baig et al., 2020), systemic blood circulation (Baig et al., 2020), immune injury (Wu & Yang, 2020), and neuronal pathways (McGavern & Kang, 2011), while the virus may indirectly invade the ANS via the enteric nervous system (ENS) and its sympathetic afferent neurons by infecting the gastrointestinal tract (Toljan, 2020).
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) usually dominate the clinical presentation of Covid-19, but recently it is clear that this pandemic affects a wide variety of organs through development of DSN (Lo, 2021). SARS-CoV-2 infection-induced stress may activate SNS leading to neuro-hormonal stimulation and activation of pro-inflammatory cytokines with development of features of sympathetic storm leading to sweating, tachycardia, and tremor (Porzionato et al., 2020). Sympathetic overstimulation and/or storm in Covid-19 occurs due to different factors including hypoxia, emotional factors, immunological/pro-inflammatory factors and high angiotensin II level that results from the imbalance between angiotensin converting enzyme 2 (ACE2) and angiotensin converting enzyme (ACE) axis (Porzionato et al., 2020). The down-regulation of ACE2 by SARS-CoV-2 is known to induce overactivation of AngII, which increase sympathetic stimulation and outflow, through the central pathway at subfornical and area postrema, and/or the peripheral pathway through activation of carotid bodies (Karahan et al., 2021). Additionally, the down-regulation of ACE2 by SARS-CoV-2 in the solitary tract nucleus due to neuro-invasive effect of SARS-CoV-2 may further increase AngII and sympathetic stimulation (Dey et al., 2021). Furthermore, hypercytokinemia mainly IL-6 is associated with sympathetic stimulation and worsening of Covid-19 severity (Mastitskaya et al., 2021).
3.2.1. COVID-19 And sympathetic stimulation
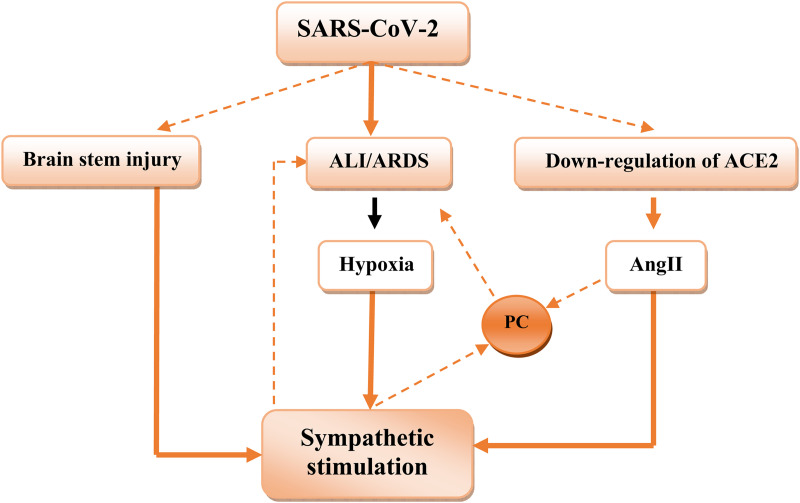
It has been reported that peripheral hypoxic chemo-sensitive pathway is a common way for sympathetic over-activation in various cardio-pulmonary disorders (Porzionato et al., 2013). Sympathetic over-activation in Covid-19 is correlated with more tissue injury, complications, and mortality. It increases capillary pulmonary leakage, alveolar damage, and development of ARDS in Covid-19 (Matsushita et al., 2020). Marvar et al., illustrated that sympathetic stimulation leads to vascular inflammation and T cells activation in mice (Marvar et al., 2010). Therefore, sympathetic ablation prevents immune activation and release of pro-inflammatory cytokines in AngII-induced hypertension in murine model (Xiao et al., 2015). In contrast, the activation of PSNS is associated with the inhibition of the release of tumor necrosis factor-alpha (TNF-α) from activated macrophages (Huston et al., 2006). Ylikoski et al., reported significant dysfunction of PSNS with dominant effect of SNS due to brain stem involvement (Ylikoski et al., 2020). Thus, imbalance of SNS/PSNS axis of ANS may affect release of pro-inflammatory cytokines and immune-inflammatory response in Covid-19. In this context, high circulating catecholamine levels may reflect sympathetic-mediated neutrophilia and T cell dysfunction in Covid-19 due to sympathetic storm (Tomar et al., 2020) ( Figure 1 ).
Figure 1.
Sympathetic stimulation in covid-19: brain injury by SARS-CoV-2 leads to direct stimulation of sympathetic outflow, SARS-CoV-2-induced acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) cause to hypoxia, which leads to sympathetic stimulation. Downregulation of ACE2 by SARS-CoV-2 increase angiotensin II (AngII), which activate release of pro-inflammatory cytokines (PC), which cause further ALI andARDS.
Moreover, in Covid-19 patients with underlying comorbidities that are characterized by high sympathetic activity such as diabetes mellitus and hypertension, in combination with hyperinflammation and hypoxia may increase life-threatening complications such as arrhythmia, cardiac arrest, and acute myocardial infarction (Al-kuraishy et al., c 2021). Development of Covid-19 severity is linked with sympathoexcitation or sympathetic storm and vagal suppression could contribute into fatal Covid-19 complications like cytokine storm (Díaz et al., 2020). Thus, it is proposed that vagal and PSNS stimulation might be useful in Covid-19 patients through modulation of sympathoexcitation and inhibition release of pro-inflammatory cytokines (Del Rio et al., 2020). Yang et al., illustrated that cholinergic agonists suppress inflammation through inhibition of endocytosis and inflammatory signals such as high mobility group protein 1 (HMGB1) (Yang et al., 2019a). In addition, molecular docking study observed that nicotinic acetylcholine receptor (nAChR) may be a potential binding receptor for SARS-CoV-2 (Alexandris et al., 2021). Thus, inhibition of nAChR by SARS-CoV-2 may lead to the inhibition of PSNS and exaggeration of SNS with subsequent progression of cytokine storm as a consequence of the inhibition of vagal anti-inflammatory mediated by diminution of nAChR activity (Alexandris et al., 2021). Aside, α-1 and β-receptor antagonists have beneficial effects in Covid-19 through reduction of sympathetic stimulation and development of cytokine storm (Konig et al., 2020; Vasanthakumar, 2020). Therefore, in addition to reducing sympathetic stimulation, adrenergic receptors antagonists, β-receptor antagonists also inhibit the interaction between SARS-CoV-2 and receptor binding sites of ACE2 and CD147 (Vasanthakumar, 2020).
3.2.2. COVID-19 And postural orthostatic tachycardia syndrome
The nucleus of solitary tract also receives sensory information from mechanoreceptors and chemoreceptor of respiratory tract, while efferent neurons from nucleus of solitary tract control cardio-pulmonary response during stressful conditions (Cornejo et al., 2018). Invasion of these tracts by SARS-CoV-2 may cause respiratory failure and sick sinus syndrome (Li et al., 2020). Also, the cardiac nods receive autonomic stimuli from both SNS and PSNS, which is dysregulated under the control of disturbed nucleus of the solitary tract by SARS-CoV-2 invasion, leading to arrhythmia or asystole (Powell et al., 2021). Furthermore, SARS-CoV-2 can spread through pulmonary mechanoreceptors and chemoreceptors to the medullary respiratory center in a retrograde manner; this may explain the sudden respiratory failure in Covid-19 patients (Prabhakar et al., 2020). The average time for SARS-CoV-2 to reach the respiratory center is eight days in 88% Covid-19 patients that are presented with neurological manifestations; this may explain the development of respiratory failure at this time of SARS-CoV-2 infection (Mao et al., 2020). Blishteyn et al., retrospectively studied 20 Covid-19 patients that were presented with DSN and other neurological disorders. 85% of the patients had persistent autonomic dysfunction such as postural orthostatic tachycardia syndrome (POTS) suggesting post-Covid-19 autonomic dysfunction (Schofield et al., 2014). POTS is characterized by persistent increment in heart rate ≥ 30 beat/minutes of head-up tilt or at standing position with palpitation, chest pain, exercise and orthostatic intolerances (Goldstein, 2021). Other symptoms of POTS including gastrointestinal disorders, headache, chronic pain and sleep disturbances. Moreover, POTS and post-COVID-19 squealae manifest a multi-disciplinary syndrome and multi-systemic effects (Goldstein, 2021). Miglis et al., reported the first case of COVID-9-induced POTS in women, characterized by hyperactivity and restlessness on day 7 of acute COVID-19. On day 24 she developed adrenergic storm characterized by tremor and exaggerated restlessness. Besides, on day 45 she developed recurrent facial flushing, non-pruritic hives and dermatographia. Investigations illustrated normal noradrenalin level at supine position with elevation of noradrenalin level at upright position (Miglis et al., 2020).
It has been reported that POTS is associated with autoimmunity disorders and high autoimmunity biomarkers such as anti-phospholipid and antinuclear antibodies have been confirmed with this autonomic disorder (Blitshteyn, 2015). Similarly, autoantibodies against acetylcholine, adrenergic β, nicotinic ganglionic and G-protein coupled receptors have been observed in POTS (Li et al., 2014). Into the bargain, post-acute Covid-19 known as long COVID-19 may cause orthostatic intolerance syndrome due to direct SARS-CoV-2 invasion or immune mediated interruption of ANS (Dani et al., 2021).
Different mechanisms are attributed to the pathophysiology of POTS including hypovolemia due to nocturnal sweating, and excessive nauseas and vomiting with subsequent SNS overactivation and sympathetic outflow that collectively called Grinch heart (Fu et al., 2010). Similarly, SARS-CoV-2 could destroy extra-cardiac postganglionic SNS neurons, which increase outflow of SNS in a similar manner of autonomic neuropathy (González-Hermosillo et al., 2021). Likewise, SARS-CoV-2 may induce brain fog due to invasion of brain stem and alteration of sympathetic outflow and cerebral perfusion (Stefano et al., 2021) . Besides, immune mediated autoimmunity and autoantibodies are associated with development of POTS due to molecular mimicry between viral proteins and human neurons (Goldstein, 2021).
As well, Hinduja et al., observational study involved fifty patients COVID-19 showed that 26% of patients had sweat dysfunction as measured by sudoscan test (electrochemical skin conductance) with motor-sensory and autonomic dysfunction (Hinduja et al., 2021). This preliminary and introductory study suggests that COVID-19 should be screened and assessed for risk of DSN.
Furthermore, Moreno-Escobar et al., retrospective study revealed that COVID-19 patients with transvers myelitis are associated with DSN are highly respond to the combined treatment with bromocriptine and methylprednisolone (Moreno-Escobar et al., 2021). In general COVID-19 may associate with different neurological manifestations like meningoencephalitis, stroke, and Guillian-Barre syndrome (Madaan et al., 2021).
Indeed, a retrospective study comprised 27 COVID-19 patients that developed DSN between 0 to 122 days following acute COVID-19. The COVID-19 patients developed orthostatic headache (22%), lightheadedness (93%), hyperhidrosis (11%), syncope (11%), and burning pain (11%) with sudomotor and cardiovagal dysfunctions in 36% and 27% respectively (Shouman et al., 2021). However, most of autonomic dysfunction following COVID-19 is mild, though exacerbation of preexisting hemodynamic instabilities might be the underlying cause of severe DSN in COVID-19.
Moreover, a case series involved six COVID-19 patients developed DSN following six weeks from acute COVID-19. The patients experienced sudomotor abnormality, activity intolerance and orthostatic hypotension with significant POTS (Goodman et al., 2021). It has been reported that DSN is not limited to the critically COVID-19 patients, since DSN may be presented as an initial symptoms in the absence of acute respiratory symptoms in a formerly healthy adult women (Abdelnabi et al., 2021).
Involvement of ANS expressed by DSN could summative most of reported COVID-19-induced neurological manifestations. So, DSN might be occurring secondary to COVID-19, which also called long COVID-19 (Barizien et al., 2021). In a cohort study comprise 12 COVID-19 patients with fatigue and 15 COVID-19 patients without fatigue compared with 12 healthy subjects illustrated that patients with long COVID-19 experienced DSN characterized by heart rate variability, which is more common in COVID-19 patients with fatigue (Barizien et al., 2021; Becker, 2021). This finding proposed that long COVID-19 is associated with persistence symptoms such as hypoxia and fatigue due to developed DSN (Crook et al., 2021).
From these findings it is unclear to find the precise association between Covid-19 and DSN, though direct SARS-CoV-2 effect though para-infectious or post-infectious immune-induced mechanisms might be the possible ways for this association (Ellul et al., 2020). Hence, the presentation of persistent symptoms such as headache, postural tachycardia, chest tightness, myalgia and fatigue in recovered Covid-19 patients refereed to long-haul Covid-19 or Covid-19-mediated DSN (Shaw et al., 2019).
3.2.3. COVID-19, neuropilin-1 and dysautonomia
Of interest, neuropilin-1 (NRP-1), which is signaling protein, was shown to be an entry factor for SARS-CoV-2 and increase its infectivity (Mayi et al., 2021). NRP-1 acts as a surface receptor and involved in immune function, axonal guidance, and tumor progression (Yang et al., 2019b). NRP-1 is highly expressed in olfactory neurons and participates into transmission of SARS-CoV-2 to the brain stem and other parts of CNS (Kyrou et al., 2021). As well, NRP-1 is upregulated in diabetes mellitus, which might explain the COVID-19 severity in diabetic patients (Mourad et al., 2021). It has been shown that NRP-1 is regarded as an immune checkpoint of T cells leading to exaggerated immune response in COVID-19 (Saleki et al., 2021). It has been shown, that sympathetic and other ANS preganglionic neurons required class 3 semaphorin (SEMA3) signaling via NRP-1 to target adrenal medulla and control autonomic stress response (Lumb et al., 2018). NRP-1 acts as a receptor for SEMA3, and both of them are engaged with sympathetic activation (Chen et al., 1998). Therefore, activation of NRP-1 during SARS-CoV-2 infection might be a potential mechanism of sympathetic storm in COVID-19 (Singh et al., 2021).
It has been suggested that NRP-1 may enhance entry of SARS-CoV-2 into the brain through olfactory neurons with subsequent development of neurological dysfunctions including seizure, confusion, stroke and autonomic dysfunction (Davies et al., 2020). Overexpression of NRP-1 in the CNS during SARS-CoV-2 infection contributes to the inhibition of anti-inflammatory Fas ligand with subsequent neuroinflammation and ANS dysfunction (Singh et al., 2021). Taken together, targeting of NRP-1 may reduce SARS-CoV-2 infectivity and neurological disorders in in particular DSN in COVID-19 (Liu et al., 2021).
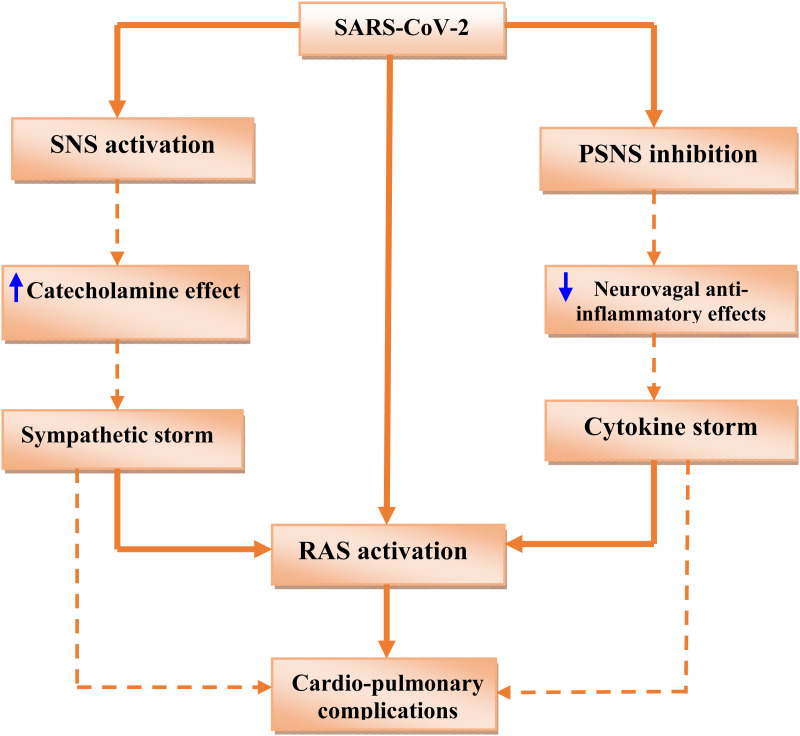
Taken together, Covid-19-induced DSN is mainly related to the activation of SNS and the inhibition of PSNS (sympathetic storm) that induce cardiopulmonary complications and propagation of SARS-CoV-2 infection with advancement of associated inflammatory reactions through upregulation of ACE2 and RAS activity ( Figure 2 ).
Figure 2.
Covid-19-induced dysautonomia (DSN):S ARS-CoV-2 infection leads to activation of sympathetic nervous system (SNS) with increasing circulating catecholamine and development of sympathetic storm. Also, SARS-CoV-2 infection inhibits parasympathetic nervous system (PSNS) with inhibition of neurovagal anti-inflammatory effects and development of cytokine storm. Both of sympathetic storm and cytokine storm activate renin-angiotensin system (RAS) with development of cardiopulmonary complications.
Molecular Mechanism of Dysautonomia in COVID-19
Regarding the molecular mechanism of DSN, it has been observed that familial DSN is caused by mutation in the IKBKAP gene, which encodes IKAP/Help1 protein. This mutation leads to reduction in the synthesis of IKAP/Help1 protein in central and peripheral neurons with development of DSN (Boone et al., 2010) . In addition, modulation of IKBKAP gene by small molecules may affect expression of IKAP/Help1 protein; thereby DNS could be attenuated (Ajiro et al., 2021).
In heart failure there is a noradrenalin spillover and sympathetic activation with parasympathetic inhibition due to alteration the expression of N-type calcium channel with enhancement of cardiac excitability (Lara et al., 2010). The development of DNS may affect the response to the different drugs, in a study involved 595 patients with DNS there is abnormal response and high adverse effects to the anti-cholinergic, cholinergic and anti-convulsant drugs (Perl et al., 2021).
In Covid-19, IKBKAP gene is downregulated due to inflammatory changes, and Zexie herbal medicine ameliorates COVID-19-complications mainly DSN through upregulation of IKBKAP gene (Dai et al., 2021). Similarly, N-type calcium channels are upregulated in SARS-CoV-2 infection leading to autonomic dysfunction and cardiac conductive abnormality. So, calcium channel blockers could be effective in the management of COVID-19-induced DSN (Mahgoub et al., 2021). Furthermore, Reddy and colleague reported the first case of POTS following mRNA COVID-19 vaccination in a 42 years old man (Reddy et al., 2021) suggesting that development of neutralizing antibodies following COVID-19 vaccination may trigger autoimmune reactions and development of DSN. Though, the underlying molecular mechanism of COVID-19 and COVID-19 vaccination is poorly understood. Therefore, experimental, in vitro and exvivo studies are warranted in this regards.
4. Conclusion
DSN in Covid-19 is defined as over activity of sympathetic component of ANS with a wide range of clinical presentations. DSN in Covid-19 is mainly acute; however chronic type can be seen in recovered post-covid-19 patients. DSN in Covid-19 leads to hemodynamic instability in severely affected Covid-19 patients secondary to baroreflex failure caused by SARS-CoV-2 infection. DSN in Covid-19 is developed due to sympathetic storm and inhibition of PSNS-mediated anti-inflammatory effect with development of cytokine storm. Therefore, sympathetic and cytokine storms together with the activation of RAS are the chief final pathways involved in the development of DSN in Covid-19. It is unclear to find the precise association between Covid-19 and DSN, though direct SARS-CoV-2 effect or para-infectious or post-infectious immune-induced mechanisms might be the possible ways for this association. Clinicians in Covid-19 era must be aware that early and prompt diagnosis of this clinical spectrum is essential for correct management and recovery. In the foreseeable future, harnessing of medical research in this clinical field through clinical trials and prospective studies are reasonable to confirm the link between Covid-19 and development of DSN.
Abbreviations
- SARS-CoV-2
Severe Acute Respiratory Syndrome Corona Virus 2
- Covid-19
The Coronavirus disease 2019
- DSN
Dysautonomia
- S-DNS
SARS-CoV-2-induced Dysautonomia
- SNS
Sympathetic nervous system
- PSNS
Parasympathetic nervous system
- ACE2
Sngiotensin converting enzyme 2
- POTS
Postural orthostatic tachycardia syndrome
Footnotes
Data Availability: All data supporting the findings of this study are available within the article
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Gideon Ampoma. Gyebi https://orcid.org/0000-0002-1945-1739
References
- Abdelnabi M., Eshak N., Almaghraby A. (2021). COVID-19 Associated dysautonomia: Not limited to critically Ill! response to: Dysautonomia: An overlooked neurological manifestation in a critically Ill COVID-19 patient. The American Journal of the Medical Sciences, 21, 00192–0. 10.1016/j.amjms.2021.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajiro M., Awaya T., Kim Y. J., Iida K., Denawa M., Tanaka N., Kurosawa R., Matsushima S., Shibata S., Sakamoto T. (2021). Therapeutic manipulation of IKBKAP mis-splicing with a small molecule to cure familial dysautonomia. Nature Communications, 12, 1–12. 10.1038/s41467-021-24705-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandris N., Lagoumintzis G., Chasapis C. T., Leonidas D. D., Papadopoulos G. E., Tzartos S. J., Tsatsakis A., Eliopoulos E., Poulas K., Farsalinos K. (2021). Nicotinic cholinergic system and COVID-19: In silico evaluation of nicotinic acetylcholine receptor agonists as potential therapeutic interventions. Toxicology Reports, 8, 73–83. 10.1016/j.toxrep.2020.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod F. B. (2004). Familial dysautonomia. Muscle & Nerve, 29(3), 352–363. 10.1002/mus.10499. [DOI] [PubMed] [Google Scholar]
- Baig A. M., Khaleeq A., Ali U., Syeda H. (2020). Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS chemical Neuroscience, 11(7), 995–998. 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- Barizien N., Le Guen M., Russel S., Touche P., Huang F., Vallée A. (2021). Clinical characterization of dysautonomia in long COVID-19 patients. Scientific Reports, 11(1), 1–7. 10.1038/s41598-021-93546-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R. C. (2021). Autonomic dysfunction in SARS-COV-2 infection acute and long-term implications COVID-19 editor's Page series. Journal of Thrombosis and Thrombolysis, 1–16. 10.1007/s11239-021-02549-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitshteyn S. (2015). Autoimmune markers and autoimmune disorders in patients with postural tachycardia syndrome (POTS). Lupus, 24(13), 1364–1369. 10.1177/0961203315587566 [DOI] [PubMed] [Google Scholar]
- Boone N., Loriod B., Bergon A., Sbai O., Formisano-Tréziny C., Gabert J., Khrestchatisky M., Nguyen C., Féron F., Axelrod F. B. (2010). Olfactory stem cells, a new cellular model for studying molecular mechanisms underlying familial dysautonomia. PLoS one, 5(12), e15590. 10.1371/journal.pone.0015590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnagarin R., Lambert G. W., Kiuchi M. G., Nolde J. M., Matthews V. B., Eikelis N., Lambert E. A., Schlaich M. P. (2019). Effects of sympathetic modulation in metabolic disease. Ann NY Acad Sci, 1454(1), 80–89. 10.1111/nyas.14217 [DOI] [PubMed] [Google Scholar]
- Carod-Artal F. J. (2018). Infectious diseases causing autonomic dysfunction. Clinical Autonomic Research, 28(1), 67–81. 10.1007/s10286-017-0452-4 [DOI] [PubMed] [Google Scholar]
- Chakraborty T., Kramer C. L., Wijdicks E. F., Rabinstein A. A. (2020). Dysautonomia in guillain–barré syndrome: Prevalence, clinical spectrum, and outcomes. Neurocritical Care, 32(1), 113–120. 10.1007/s12028-019-00781-w [DOI] [PubMed] [Google Scholar]
- Chen H., He Z., Bagri A., Tessier-Lavigne M. (1998). Semaphorin–neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron, 21(6), 1283–1290. 10.1016/s0896-6273(00)80648-0 [DOI] [PubMed] [Google Scholar]
- Cornejo M. P., De Francesco P. N., Romero G. G., Portiansky E. L., Zigman J. M., Reynaldo M., Perello M. (2018). Ghrelin receptor signaling targets segregated clusters of neurons within the nucleus of the solitary tract. Brain Structure and Function, 223(7), 3133–3147. 10.1007/s00429-018-1682-5 [DOI] [PubMed] [Google Scholar]
- Crook H, Raza S, Nowell J, Young M, Edison P. (2021). Long covid—mechanisms, risk factors, and management. bmj, 374(n1648). 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- Dai Y, Qiang W, Gui Y, Tan X, Pei T, Lin K, Cai S, Sun L, Ning G, Wang J. (2021) A large-scale transcriptional study reveals inhibition of COVID-19 related cytokine storm by traditional Chinese medicines. Science bulletin, 66(9), 884–888. 10.1016/j.scib.2021.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani M., Dirksen A., Taraborrelli P., Torocastro M., Panagopoulos D., Sutton R., Lim P. B. (2021). Autonomic dysfunction in ‘long COVID’: Rationale, physiology and management strategies. Clinical Medicine, 21(1), e63–e67. 10.7861/clinmed.2020-0896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Randeva H. S., Chatha K., Hall M., Spandidos D. A., Karteris E., Kyrou I. (2020). Neuropilin-1 as a new potential SARS-CoV-2 infection mediator implicated in the neurologic features and central nervous system involvement of COVID-19. Molecular Medicine Reports, 22(5), 4221–4226. 10.3892/mmr.2020.11510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio R., Marcus N. J., Inestrosa N. C. (2020). Potential role of autonomic dysfunction in covid-19 morbidity and mortality. Frontiers in Physiology, 11(1248). ecollection 10.3389/fphys.2020.561749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey J., Alam M. T., Chandra S., Gupta J., Ray U., Srivastava A. K., Tripathi P. P. (2021). Neuroinvasion of SARS-CoV-2 may play a role in the breakdown of the respiratory center of the brain. Journal of Medical Virology, 93(3), 1296–1303. 10.1002/jmv.26521 [DOI] [PubMed] [Google Scholar]
- Díaz H. S., Toledo C., Andrade D. C., Marcus N. J., Del Rio R. (2020). Neuroinflammation in heart failure: New insights for an old disease. The Journal of Physiology, 598(1), 33–59. 10.1113/JP278864 [DOI] [PubMed] [Google Scholar]
- Ellul M. A., Benjamin L., Singh B., Lant S., Michael B. D., Easton A., Kneen R., Defres S., Sejvar J., Solomon T. (2020). Neurological associations of COVID-19. The Lancet Neurology, 19(9), 767–783. 10.1016/S1474-4422(20)30221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M., Xiang B., Fan L., Wang Q., Xu W., Xiang H. (2020). Interrogating autonomic peripheral nervous system neurons with viruses–A literature review. Journal of Neuroscience Methods, 346, 108958. 10.1016/j.jneumeth.2020.108958 [DOI] [PubMed] [Google Scholar]
- Fu Q., VanGundy T. B., Galbreath M. M., Shibata S., Jain M., Hastings J. L., Bhella P. S., Levine B. D. (2010). Cardiac origins of the postural orthostatic tachycardia syndrome. Journal of the American College of Cardiology, 55(25), 2858–2868. 10.1016/j.jacc.2010.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. S. (2021). The possible association between COVID-19 and postural tachycardia syndrome. Heart Rhythm, 18(4), 508–509. 10.1016/j.hrthm.2020.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Hermosillo J. A., Martínez-López J. P., Carrillo-Lampón S. A., Ruiz-Ojeda D., Herrera-Ramírez S., Amezcua-Guerra L. M., MdR M.-A. (2021). Post-Acute COVID-19 symptoms, a potential link with myalgic encephalomyelitis/chronic fatigue syndrome: A 6-month survey in a Mexican cohort. Brain Sciences, 11(6), 760–766. 10.3390/brainsci11060760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman B. P., Khoury J. A., Blair J. E., Grill M. F. (2021a). COVID-19 dysautonomia. Frontiers in Neurology, 12(624968), 543. 10.3389/fneur.2021.624968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D. (2020). Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Development Research, 81(5), 537–540. 10.1002/ddr.21656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson J. E., Hendrickson E. T., Gehrie E. A., Sidhu D., Wallukat G., Schimke I., Tormey C. A. (2016). Complex regional pain syndrome and dysautonomia in a 14-year-old girl responsive to therapeutic plasma exchange. Journal of Clinical Apheresis, 31(4), 368–374. 10.1002/jca.21407 [DOI] [PubMed] [Google Scholar]
- Hinduja A., Moutairou A., Calvet J.-H. (2021). Sudomotor dysfunction in patients recovered from COVID-19. Neurophysiologie Clinique, 51(2), 193–196. 10.1016/j.neucli.2021.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston J. M., Ochani M., Rosas-Ballina M., Liao H., Ochani K., Pavlov V. A., Gallowitsch-Puerta M., Ashok M., Czura C. J., Foxwell B. (2006). Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. The Journal of Experimental Medicine, 203(7), 1623–1628. 10.1084/jem.20052362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karahan M., Demirtaş A. A., Hazar L., Erdem S., Ava S., Dursun M. E., Keklikçi U. (2021). Autonomic dysfunction detection by an automatic pupillometer as a non-invasive test in patients recovered from COVID-19. Graefe's Archive for Clinical and Experimental Ophthalmology, 257(9), 2821–2826. 10.1007/s00417-021-05209-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatoon F., Prasad K., Kumar V. (2020). Neurological manifestations of COVID-19: Available evidences and a new paradigm. Journal of Neurovirology, 26(5), 619–630. 10.1007/s13365-020-00895-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig M. F., Powell M., Staedtke V., Bai R.-Y., Thomas D. L., Fischer N., Huq S., Khalafallah A. M., Koenecke A., Xiong R. (2020). Preventing cytokine storm syndrome in COVID-19 using α-1 adrenergic receptor antagonists. The Journal of Clinical Investigation, 130(7), 3345–3347. 10.1172/JCI139642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrou I., Randeva H. S., Spandidos D. A., Karteris E. (2021). Not only ACE2—the quest for additional host cell mediators of SARS-CoV-2 infection: Neuropilin-1 (NRP1) as a novel SARS-CoV-2 host cell entry mediator implicated in COVID-19. Signal transduction and targeted therapy, 168(6), 1–3. 10.1038/s41392-020-00460-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara A., Damasceno D. D., Pires R., Gros R., Gomes E. R., Gavioli M., Lima R. F., Guimaraes D., Lima P., Bueno Jr C. R. (2010). Dysautonomia due to reduced cholinergic neurotransmission causes cardiac remodeling and heart failure. Molecular and Cellular Biology, 30(7), 1746–1756. 10.1128/MCB.00996-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi M., Padovani A., McArthur J. C. (2020). Neurological manifestations associated with COVID-19: A review and a call for action. Journal of Neurology, 267(6), 1573–1576. 10.1007/s00415-020-09896-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Yu X., Liles C., Khan M., Vanderlinde-Wood M., Galloway A., Zillner C., Benbrook A., Reim S., Collier D. (2014). Autoimmune basis for postural tachycardia syndrome. Journal of the American Heart Association, 3(1), e000755. 10.1161/JAHA.113.000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. C., Bai W. Z., Hashikawa T. (2020). The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. Journal of Medical Virology, 92(6), 552–555. 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.-D., Zhong L.-P., He J., Zhao Y.-X. (2021). Targeting neuropilin-1 interactions is a promising anti-tumor strategy. Chinese Medical Journal, 134(6), 508–517. 10.1097/CM9.0000000000001200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Y. L. (2021). COVID-19, fatigue, and dysautonomia. Journal of Medical Virology, 93,(3), 1213–1213. 10.1002/jmv.26552 [DOI] [PubMed] [Google Scholar]
- Lumb R., Tata M., Xu X., Joyce A., Marchant C., Harvey N., Ruhrberg C., Schwarz Q. (2018). Neuropilins guide preganglionic sympathetic axons and chromaffin cell precursors to establish the adrenal medulla. Development (Cambridge, England), 145(21). 10.1242/dev.162552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaan P., Singanamalla B., Saini L. (2021 ). Neurological Manifestations of COVID-19 in Children: Time to Be More Vigilant . Pediatr Neurol, 115(28). 10.1016/j.pediatrneurol.2020.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahgoub S., El-Sayed M.-I. K., El-Shehry M. F., Awad S. M., Mansour Y. E., Fatahala S. S. (2021). Synthesis of novel calcium channel blockers with ACE2 inhibition and dual antihypertensive/anti-inflammatory effects: A possible therapeutic tool for COVID-19 . Bioorg Chem ., 116(105272). 10.1016/j.bioorg.2021.105272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA neurology, 77(6), 683–690. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Lavín M. (2015). Hypothesis: Human papillomavirus vaccination syndrome—small fiber neuropathy and dysautonomia could be its underlying pathogenesis. Clinical Rheumatology, 34(7), 1165–1169. 10.1007/s10067-015-2969-z [DOI] [PubMed] [Google Scholar]
- Marvar P. J., Thabet S. R., Guzik T. J., Lob H. E., McCann L. A., Weyand C., Gordon F. J., Harrison D. G. (2010). Novelty and significance. Circulation Research, 107(2), 263–270. 10.1161/CIRCRESAHA.110.217299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastitskaya S., Thompson N., Holder D. (2021). Selective vagus nerve stimulation as a therapeutic approach for the treatment of ARDS: A rationale for neuro-immunomodulation in COVID-19 disease. Frontiers in Neuroscience, 15(667036). eCollection 2021 10.3389/fnins.2021.667036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., Marchandot B., Jesel L., Ohlmann P., Morel O. (2020). Impact of COVID-19 on the cardiovascular system: A review. Journal of Clinical Medicine, 9(5), 1407. 10.3390/jcm9051407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayi B. S., Leibowitz J. A., Woods A. T., Ammon K. A., Liu A. E., Raja A. (2021). The role of neuropilin-1 in COVID-19. PLoS Pathogens, 17(1), e1009153. 10.1371/journal.ppat.1009153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern D. B., Kang S. S. (2011). Illuminating viral infections in the nervous system. Nature Reviews Immunology, 11(5), 318–329. 10.1038/nri2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglis M. G., Prieto T., Shaik R., Muppidi S., Sinn D.-I., Jaradeh S. (2020). A case report of postural tachycardia syndrome after COVID-19. Clinical Autonomic Research, 30(5), 449–451. 10.1007/s10286-020-00727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Escobar M. C., Kataria S., Khan E., Subedi R., Tandon M., Peshwe K., Kramer J., Niaze F., Sriwastava S. (2021). Acute transverse myelitis with dysautonomia following SARS-CoV-2 infection: A case report and review of literature. Journal of Neuroimmunology, 353(577523). 10.1016/j.jneuroim.2021.577523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad D., Azar N. S., Azar S. T. (2021). Diabetic nephropathy and COVID-19: The potential role of immune actors. International Journal of Molecular Sciences, 22(15), 7762. 10.3390/ijms22157762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murta V., Villarreal A., Ramos A. J. (2020). Severe acute respiratory syndrome coronavirus 2 impact on the central nervous system: Are astrocytes and microglia main players or merely bystanders? ASN neuro, 12, 1759091420954960. 10.1177/1759091420954960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osztovits J., Horváth T., Abonyi M., Tóth T., Visnyei Z., Bekö G., Csák T., Lakatos P. L., Littvay L., Fehér J. (2009). Chronic hepatitis C virus infection associated with autonomic dysfunction. Liver International, 29(10), 1473–1478. 10.1111/j.1478-3231.2009.02075.x. [DOI] [PubMed] [Google Scholar]
- Palma J. A., Gupta A., Sierra S., Gomes I., Balgobin B., Norcliffe-Kaufmann L., Devi L. A., Kaufmann H. (2020). Autoantibodies blocking M3 muscarinic receptors cause postganglionic cholinergic dysautonomia. Annals of Neurology, 88(6), 1237–1243. 10.1002/ana.25882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R. E., Reidy J., Lednicky J., Sordillo E. M., Fowkes M. (2020). Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Journal of Medical Virology, 92(7), 699–702. 10.1002/jmv.25915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl L., Hakimian D., Maayan C., Rekhtman D., Fried E., Salmon-Divon M., Sapozhnikov D. M., Cheishvili D. (2021). Uncommon side effects of common drugs in patients with familial dysautonomia. Pharmacoepidemiology and Drug Safety. 10.1002/pds.5326 [DOI] [PubMed] [Google Scholar]
- Porzionato A., Emmi A., Barbon S., Boscolo-Berto R., Stecco C., Stocco E., Macchi V., De Caro R. (2020). Sympathetic activation: A potential link between comorbidities and COVID-19. The FEBS Journal, 287(17), 3681–3688. 10.1111/febs.15481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porzionato A., Macchi V., De Caro R. (2013). Role of the carotid body in obesity-related sympathoactivation. Hypertension, 61(6), e57–e57. 10.1161/HYPERTENSIONAHA.113.01248 [DOI] [PubMed] [Google Scholar]
- Powell M., Ward B., Dickson R., Patrick C. (2021). Prehospital Sinus node dysfunction and asystole in a previously healthy patient With COVID-19. Prehospital Emergency Care, 1–4. 10.1080/10903127.2021.1924325 [DOI] [PubMed] [Google Scholar]
- Prabhakar H., Mahajan C., Kapoor I. (2020). COVID-19 and neuroinvasion. Anesthesia and Analgesia, 131(2), e91–e92. 10.1213/ANE.0000000000004918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S., Reddy S., Arora M. (2021). A case of postural orthostatic tachycardia syndrome secondary to the messenger RNA COVID-19 vaccine. Cureus, 13(5), e14837. 10.7759/cureus.14837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. P., Chesney E., Oliver D., Pollak T. A., McGuire P., Fusar-Poli P., Zandi M. S., Lewis G., David A. S. (2020). Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. The Lancet Psychiatry, 7(7), 611–627. 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleki K., Banazadeh M., Miri N. S., Azadmehr A. (2021). Triangle of cytokine storm, central nervous system involvement, and viral infection in COVID-19: The role of sFasL and neuropilin-1. Reviews in the Neurosciences. 10.1515/revneuro-2021-0047 [DOI] [PubMed] [Google Scholar]
- Schofield J., Blitshteyn S., Shoenfeld Y., Hughes G. (2014). Postural tachycardia syndrome (POTS) and other autonomic disorders in antiphospholipid (hughes) syndrome (APS). Lupus, 23(7), 697–702. 10.1177/0961203314524468 [DOI] [PubMed] [Google Scholar]
- Sealey B., Lui K. (2004). Diagnosis and management of vasovagal syncope and dysautonomia. AACN Advanced Critical Care, 15(3), 462–477. 10.1097/00044067-200407000-00012 [DOI] [PubMed] [Google Scholar]
- Shaw B., Stiles L., Bourne K., Green E., Shibao C., Okamoto L., Garland E., Gamboa A., Diedrich A., Raj V. (2019). The face of postural tachycardia syndrome–insights from a large cross-sectional online community-based survey. Journal of Internal Medicine, 286(4), 438–448. 10.1111/joim.12895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y., Ryabkova V. A., Scheibenbogen C., Brinth L., Martinez-Lavin M., Ikeda S., Heidecke H., Watad A., Bragazzi N. L., Chapman J. (2020). Complex syndromes of chronic pain, fatigue and cognitive impairment linked to autoimmune dysautonomia and small fiber neuropathy. Clinical Immunology, 214, 108384. 10.1016/j.clim.2020.108384 [DOI] [PubMed] [Google Scholar]
- Shouman K., Vanichkachorn G., Cheshire W. P., Suarez M. D., Shelly S., Lamotte G. J., Sandroni P., Benarroch E. E., Berini S. E., Cutsforth-Gregory J. K. (2021). Autonomic dysfunction following COVID-19 infection: An early experience. Clinical Autonomic Research, 31(3), 385–394. 10.1007/s10286-021-00803-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M. N., Pearce B. D., Biron C. A., Miller A. H. (2005). Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunology, 18(1), 41–78. 10.1089/vim.2005.18.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Kumar N., Kumar A., Kumar A., Shaju A. R. (2021). Sympathetic storm or cytokine storm: A diagnostic dilemma in patient of traumatic brain injury with COVID 19. Acta Neurologica Taiwanica, 30(2), 78–80. [PubMed] [Google Scholar]
- Smeraski C. A., Sollars P. J., Ogilvie M. D., Enquist L. W., Pickard G. E. (2004). Suprachiasmatic nucleus input to autonomic circuits identified by retrograde transsynaptic transport of pseudorabies virus from the eye. Journal of Comparative Neurology, 471(3), 298–313. 10.1002/cne.20030 [DOI] [PubMed] [Google Scholar]
- Stefano G. B., Ptacek R., Ptackova H., Martin A., Kream R. M. (2021). Selective neuronal mitochondrial targeting in SARS-CoV-2 infection affects cognitive processes to induce ‘brain fog’and results in behavioral changes that favor viral survival. Medical science monitor: international medical journal of experimental and clinical research, 27, e930886–e930881. 10.12659/MSM.930886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg Z. (2012). Autonomic dysfunction: A unifying multiple sclerosis theory, linking chronic cerebrospinal venous insufficiency, vitamin D3, and epstein-barr virus. Autoimmunity Reviews, 12(2), 250–259. 10.1016/j.autrev.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Stock C., Teyssier G., Pichot V., Goffaux P., Barthelemy J.-C., Patural H. (2010). Autonomic dysfunction with early respiratory syncytial virus-related infection. Autonomic Neuroscience, 156(1-2), 90–95. 10.1016/j.autneu.2010.03.012 [DOI] [PubMed] [Google Scholar]
- Toljan K. (2020). Letter to the editor regarding the viewpoint “evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host–virus interaction, and proposed neurotropic mechanism”. ACS chemical Neuroscience, 11(8), 1192–1194. 10.1021/acschemneuro.0c00174 [DOI] [PubMed] [Google Scholar]
- Tomar B., Anders H.-J., Desai J., Mulay S. R. (2020). Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells, 9(6), 1383. 10.3390/cells9061383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer E. A., Kohn J. N., Hong S. (2020). Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behavior, and Immunity, 87(1), 34–39. 10.1016/j.bbi.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthakumar N. (2020). Beta-adrenergic blockers as a potential treatment for COVID-19 patients. BioEssays, 42(11), 2000094. 10.1002/bies.202000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Siddharthan V., Hall J. O., Morrey J. D. (2011). Autonomic nervous dysfunction in hamsters infected with west Nile virus. PLoS One, 6(5), e19575. 10.1371/journal.pone.0019575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Yang X. O. (2020). TH17 Responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor fedratinib. Journal of microbiology. Immunology and Infection, 53(3), 368–370. 10.1016/j.jmii.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Kirabo A., Wu J., Saleh M. A., Zhu L., Wang F., Takahashi T., Loperena R., Foss J. D., Mernaugh R. L. (2015). Renal denervation prevents immune cell activation and renal inflammation in angiotensin II–induced hypertension. Circulation Research, 117(6), 547–557. 10.1161/CIRCRESAHA.115.306010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Liu H., Zeng Q., Imperato G. H., Addorisio M. E., Li J., He M., Cheng K. F., Al-Abed Y., Harris H. E. (2019a). Inhibition of HMGB1/RAGE-mediated endocytosis by HMGB1 antagonist box A, anti-HMGB1 antibodies, and cholinergic agonists suppresses inflammation. Molecular Medicine, 25(5), 1–13. 10.1186/s10020-019-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. G., Wen R. T., Qi K., Li J., Zheng G. X., Wang Y. F., Hong Y. G., Zhang Y. M. (2019b). The neuropilin-1 ligand, Sema3A, acts as a tumor suppressor in the pathogenesis of acute leukemia. The Anatomical Record, 302(7), 1127–1135. 10.1002/ar.24016 [DOI] [PubMed] [Google Scholar]
- Ylikoski J., Markkanen M., Mäkitie A. (2020). Pathophysiology of the COVID-19 - entry to the CNS through the nose 140(10), 886–889. 10.1002/ar.24016 [DOI] [PubMed] [Google Scholar]
- Zhang H., Penninger J. M., Li Y., Zhong N., Slutsky A. S. (2020). Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Medicine, 46(4), 586–590. 10.1007/s00134-020-05985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]