Abstract
Although protein crosslinking is often linked with aging as well as some age-related diseases, very few molecular details are available on the nature of the amino acids involved, or mechanisms that are responsible for crosslinking. Recent research has shown that several amino acids are able to generate reactive intermediates that ultimately lead to covalent crosslinking through multiple non-enzymatic mechanisms. This information has been derived from proteomic investigations on aged human lenses and the mechanisms of crosslinking, in each case, have been elucidated using model peptides. Residues involved in spontaneous protein-protein crosslinking include aspartic acid, asparagine, cysteine, lysine, phosphoserine, phosphothreonine, glutamic acid and glutamine. It has become clear, therefore, that several amino acids can act as potential sites for crosslinking in the long-lived proteins that are present in aged individuals. Moreover, the lens has been an invaluable model tissue and source of crosslinked proteins from which to determine crosslinking mechanisms that may lead to crosslinking in other human tissues.
Introduction
The human lens contains some of the oldest proteins in the body with proteins in the lens nucleus being formed before birth and lasting until death. These long-lived proteins (LLPs); therefore, have decades to undergo spontaneous chemistry based on the functional groups of the twenty naturally occurring amino acids. One such chemical reaction that occurs is crosslinking, i.e. the formation of new covalent bonds between proteins and other proteins or metabolites. In aged tissues, covalent crosslinking of polypeptides is not uncommon. Because crosslinking of proteins is intrinsically difficult to study, historically a common approach has been to look for covalent crosslinks that have already been discovered. Classic ones in this category are the isopeptide bond formed from Gln residues by glutaminase, or di-tyrosine that can form by treatment of proteins with metals and oxidants. This approach, of course is unlikely reveal whether novel crosslinks are present. As elaborated elsewhere in this review, a more challenging task is to ask “what covalent crosslinks are present?”. An even more challenging quest is to be quantitative i.e. try to apportion the total crosslinking present to individual well-defined reactions. In a heterogeneous biological system such as the lens, this is a demanding task, but as outlined herein, significant progress is being made.
Fundamentally, there are two types of protein crosslinking processes in humans. One can be termed structural and functional, where typically enzymatic mechanisms have evolved to establish crosslinks that benefit the organism. The other category of protein crosslinking can be considered spontaneous (non-enzymatic), deleterious, and dysfunctional. In this review, the first category, which involves crosslinking as a process that is crucial for the function of the final polypeptide, will be covered only very briefly.
Structural/functional crosslinking
There are several well-studied examples of these protein crosslinks that are essential for protein function and they are enzyme-mediated.
i. Disulfide bonds
The most widespread structural/functional type of crosslinking is disulfide crosslinking (Wilkinson and Gilbert, 2004), where two cysteine residues are oxidised in order to “lock in” a particular protein fold or conformation. Disulfide bonds can be intramolecular or intermolecular. This type of directed crosslink is mostly employed to facilitate protein structure and hence function. Although disulfide bonds can form spontaneously during protein synthesis, often specific chaperones, e.g. protein disulfide isomerase, are required to form proper disulfide crosslinks and to prevent protein misfolding (Wilkinson and Gilbert, 2004).
ii. Elastin and Collagen
In other cases, the introduction of covalent crosslinks is used to provide mechanical stability for the tissue in which the protein is located. Examples of such crosslinks include desmosine and isodesmosine in elastin (Thomas et al., 1963). The aromatic ring in both of these molecules, is formed from lysine residues via the action of the enzyme lysyl oxidase which converts the ɛ-amino side chain into an aldehyde (Akagawa and Suyama, 2000). This aldehyde then condenses with other side chain amino groups with the result that four lysine residues of tropoelastin are covalently linked in mature elastin fibres. Elastin is a long-lived protein (LLP) whose main function is to allow stretched organs or tissues to resume their original shape. Such multiple site crosslinking may enhance the ability of this major connective tissue component to remain functional over a lifetime. Elastin in the heart is there for life, and in a 70 year-old heart it has recoiled through more than 2 billion beats.
The most abundant protein in the human body is collagen and, like elastin, it is another example of a LLP. In collagen, similar lysine-derived crosslinks provide strength by linking individual collagen fibrils. Collagen contains high levels of an unusual amino acid derivative, hydroxylysine, and this modified amino acid participates in covalent crosslinking mediated by lysyl oxidases (Gaar et al., 2020). In addition, unmodified lysine residues in collagen can crosslink with methionine to form sulfilimine crosslinks that, interestingly, require the enzyme peroxidasin, bromine, and hydrogen peroxide to create the sulfilimine bond (Vanacore et al., 2009). The stiffness of the collagenous lens capsule is likely due to this sulfilimine crosslink (Dyksterhuis et al., 2011). Until recently it was widely believed that the age-related increase in tissue stiffness and loss of elasticity, was due to increased levels of this covalent crosslinking. A recent careful investigation has shown this not to be the case. In contrast to expectations, levels of the major crosslink, hydroxy-lysino-norleucine (formed after borohydride reduction) in rat tail tendon actually decreased significantly with age (Stammers et al., 2020).
iii. Ubiquitination and transglutaminase
One of the most important, and widespread, protein crosslinking processes in the body involves the covalent attachment of an 8.6kDa protein, ubiquitin. This highly conserved small protein is expressed in all eukaryotic cells. Ubiquitination of cellular proteins, targets them for degradation by the proteasome (Schrader et al., 2009) but it can also alter protein interactions and signaling within the cell. In the lens, ubiquitination plays an important role in lens development and in organelle degradation (Rowan et al., 2017; Shang and Taylor, 2004). Crosslinking can take place in several ways. Generally, lysine residues on the substrate protein are bound to the C-terminal carboxyl group of ubiquitin via an isopeptide bond, although serine, cysteine and threonine residues may also be covalently linked. Polyubiquitination can also occur (Sadowski et al., 2012). These processes are carefully regulated by a three step mechanism that involves activating enzymes (E1), conjugating enzymes (E2), and ligating enzymes (E3) (Schrader et al., 2009).
Isopeptide crosslinks involving glutamine and lysine residues can also be formed by transglutaminases. Typically transglutaminase catalysis results in crosslinked and insoluble protein aggregates. Probably the best known example involves Factor XIIIa, a transglutaminase that catalyzes the formation of isopeptide bonds between fibrins and is necessary for the formation of blood clots (Takahashi et al., 1986). Transglutaminase has been found in many ocular tissues and is believed to be important for establishing stabilized structures of connective tissues of the eye (Raghunath, 1999 #117}. Interestingly, lens transglutaminase has been indirectly implicated in opacification in an in vivo rabbit cataract model (Lentini et al., 2011).
A related group of small peptide tags are referred to as SUMO (Small Ubiquitin like Modifier) proteins. SUMOylation is involved in the regulation of transcription, nuclear-cytosolic transport, DNA repair and apoptosis. Although closely related in mechanism to ubiquitination, SUMOylation does not target substrate proteins for degradation. For a review see Hay et al. (Hay, 2005). In the eye, sumoylation of the transcription factor Pax6 is important for proper differentiation of lens and retina cells (Yan et al., 2010).
iv. Other crosslinking modifications of proteins
Numerous cases are known where covalent bonds are formed between a protein and some other chemical moiety for example, phosphate, fatty acids (Dietrich and Ungermann, 2004; Schey et al., 2010) or carbohydrates. In some instances, such as the binding of sugars or ascorbate, crosslinked proteins can also be produced (Nagaraj et al., 2012). It appears that such carbohydrate-mediated protein-protein crosslinking occurs mainly through the formation of diketone intermediates (Monnier, 1990). One interesting example of chemically-induced protein-protein crosslinking is found in insect cocoons (Butenandt et al., 1957). The caterpillars of moths and butterflies (order Lepidoptera) use tanning of secreted silk strands to strengthen and colour their cocoons prior to metamorphosis. Tanning and crosslinking of this insect protein is achieved via the secretion of reactive o-diphenols or o-aminophenols from a separate gland. In the presence of oxygen these aromatic chemicals oxidise to yield highly reactive o-quinones or o-aminoquinones (Manthey et al., 1990). The exudation of such chemicals onto the white silk strands, leads to covalent binding to amino groups of the silk fibroin and results in yellow or brown coloration of the cocoon. It also induces protein-protein crosslinking (Manthey et al., 1992).
Deleterious crosslinking
The main focus of this review will be on crosslinks that form spontaneously as a result of protein aging and are, for the most part, deleterious to protein function.
i. Of moths and men
There appears to be parallels between the above Lepidoptera scenario for tanning cocoons and the chemical reactions that take place in human lenses with the onset of nuclear cataract. In the case of human lenses the orange/brown coloration which is used to classify the progression of age-related nuclear cataract (Pirie, 1968) appears to be due mostly to the covalent binding of the endogenous aminophenol, 3-hydroxykynurenine (Korlimbinis and Truscott, 2006). 3-Hydroxykynurenine is a UV filter compound that is made in the human lens from tryptophan (Bova et al., 1999). In the presence of air, 3-hydroxykynurenine autoxidises to its o-aminoquinone form and this highly reactive species then binds to proteins. This process can be readily observed in the test tube when proteins, such as lens crystallins, are incubated in the presence of 3-hydroxykynurenine (Aquilina et al., 1999).
One of the outcomes of this process is protein-protein crosslinking. Although this was technically a very challenging task, analytical procedures were developed to show that indeed 3-hydroxykynurenine is bound covalently to proteins in the human lens(Korlimbinis and Truscott, 2006). This study found that all human lenses above the age of 50 have measurable levels of 3-hydroxykynurenine bound to crystallins. Human lenses past middle age are therefore “primed” to become both coloured and crosslinked if the environment becomes oxidative. Indeed, a large amount of protein-protein crosslinking is associated with the progression of age-related nuclear cataract (Dilley and Pirie, 1974). It is therefore likely that human age-related nuclear cataract and the tanning of cocoons share aspects of the same basic chemistry.
ii. Old proteins and young proteins: experimental traps for young players
There are a number of examples where a particular protein when isolated from aged tissues behaves quite differently from the same protein purified from the organs or tissues of young people. This fact alone must be recognized if the aim is to study aging changes to a particular protein. Using the lens as an example, where outer cortical fiber cells contain newly synthesized protein and inner nuclear fiber cells contain proteins as old as the organism, it is a simple process to isolate α-crystallin from the lenses of young animals using gel filtration. α-Crystallin elutes as a distinct peak that is separate from the other major crystallins: β-crystallin and γ-crystallin. Using the same purification strategy on old lenses as a starting point to track aging changes to α-crystallin will lead to misleading results because, by middle age, very little α-crystallin elutes in this same position by gel filtration. Much of aged α-crystallin has been ensnared into larger aggregates, or has become insoluble, due to its chaperone action in binding to other lens proteins as they denatured (Roy and Spector, 1976). In addition, a significant proportion of α-crystallin has been truncated (Grey and Schey, 2009). Another complication is that crosslinking or racemization of α-crystallin, could cause it to elute at a position different from that of the originally synthesized polypeptide. Some modified β-crystallins and γ-crystallins may co-elute at the same position as “young” α-crystallin. This presents a research conundrum that applies to other fractionation procedures as well.
As a result of these many uncontrollable factors and the complexity of age-related protein modifications, if the objective of the research is to track changes to one crystallin, a reasonable approach is to use the same dissected region (e.g. using a trephine) from a young and an old lens as a starting point. That way, in the absence of any purification steps, all of the various forms of the protein, in this example α-crystallin, will be present and a more meaningful ‘old versus young’ comparison using proteomics will be the outcome. This ‘non-fractionation approach’ may also be the most appropriate one for examining protein changes within other organs and tissues from aged animals.
As an aside, similar factors may be problematic if immunological techniques for protein purification are employed when comparing protein changes within young and old tissues. This is because age-related posttranslational modifications can impair the binding of antibodies to the old protein. A clear illustration of this can be seen in the case of γS-crystallin from human lenses of different ages. In lenses past teenage years, western blotting with three separate antibodies showed little immunoreactive monomeric γS-crystallin, despite the fact that modified forms of the crystallin are certainly present (Friedrich et al., 2012). This method also revealed the large degree of covalent crosslinking of γS-crystallin that is apparent in human lenses from the first decade onwards. Indeed, early work of Hanson et al., showed disulfide crosslinks in cysteine rich γ-crystallins, the abundance of some were age dependent (Hanson et al., 1998). A similar intramolecular disulfide bond was enriched in insoluble αA-crystallin (Lund et al., 1996).
iii. Old proteins are everywhere
It has been recognised only recently that many proteins in the human body are long-lived (Lynnerup et al., 2008; Toyama et al., 2019; Toyama et al., 2013). Over time, these long-lived proteins (LLPs) degrade (D’Angelo et al., 2009; Truscott et al., 2016). The major processes seem to involve spontaneous reactions and include racemisation (Geiger and Clarke, 1987), isomerisation (Geiger and Clarke, 1987), truncation (Robertson et al., 2008; Wang et al., 2019), deamidation (Lampi et al., 2014; Wilmarth et al., 2006) and crosslinking. The last of these five categories is the subject of this review although it in important to note that these modifications do not happen in isolation and that lens proteins exist with combinations of such modifications. It is also worth noting that some modifications of old proteins are catalysed by enzymes. One example is arginine deiminase (Zhou et al., 2018)
iv. Old proteins are heterogeneous
Newly synthesized proteins are typically homogeneous and run as tight bands on gels. In contrast, this is often not the case for some polypeptides from older tissues and this feature can be readily observed, when proteins from aged tissues are examined by gel electrophoresis. A common observation is that old LLPs electrophorese as high molecular weight aggregates that migrate as streaks or smears on SDS PAGE gels. For example, this is true for lens proteins (Friedrich et al., 2012) and Tau from brain (Watanabe et al., 1999). This smearing pattern reflects the fact that LLPs from organs or tissues of older individuals are heterogeneous and may be crosslinked. Oxidation of LLPs can also lead to heterogeneity due principally to oxidation of sulfur-containing amino acids. In an oxidative environment, disulfide bonds can form adventitiously because antioxidants and their enzymatic partners are in short supply, or the flux of oxidants overwhelms the cell’s antioxidant defenses. This situation occurs in the human nuclear cataract lens (Anderson et al., 1979; Truscott and Augusteyn, 1977) where more than 90% of protein thiol groups are oxidized into disulfide bonds in advanced cataract (Truscott and Augusteyn, 1977). By contrast the loss of protein thiols in lens crystallins as a function of age is relatively minor (Truscott and Augusteyn, 1977). The result of an oxidative environment is the formation of protein-protein (PSSP) and protein-mixed (e.g. glutathione-protein, PSSG) disulfide bond formation (Lou, 2003). It has been shown that glutathiolation can lead to the degradation of lens proteins (Zetterberg et al., 2006). Although not the topic of this review, there are a number of enzyme-based antioxidant defence systems based around glutathione, vitamin E and ascorbate whose main function is to keep proteins in their reduced form. A reducing environment within the cell also acts to minimise the modification of LLPs by reactive metabolites such as diketones or quinones (Manthey et al., 1990). When this reducing environment is altered in cells, oxidative processes can lead to protein crosslinking (Grune et al., 2001).
v. Protein-protein crosslinks: blind search or targeted search?
Until it became recognized that crosslinking of proteins can arise from the spontaneous breakdown of Asp/Asn (Friedrich et al., 2018), Cys, phosphoSer, phosphoThr (Linetsky and LeGrand, 2005; Wang et al., 2014) or Glu/Gln residues (Friedrich et al., 2021), protein-protein crosslinking was thought to form via di-tyrosine (di-Tyr). di-Tyr crosslinks have been known for many years and arise under oxidative conditions from Tyr residues via a radical mechanism (Giulivi et al., 2003). Thus, di-Try became a “conventional target” in searches for protein crosslinks. An advantage to searching for this particular crosslink is that it is fluorescent, it survives acid hydrolysis and is also easily recognized using mass spectrometry.
One example of such a targeted search paradigm for this di-Tyr protein-protein crosslink was published for Aβ (Al-Hilaly et al., 2013). It had been recognised from gel electrophoresis studies that a large proportion (>30%) of Aβ in plaque from human AD brains is comprised of crosslinked Aβ (Roher et al., 1996). A search for di-Tyr in Aβ did indeed find some of this compound. However, since this study was targeted and also was not quantitative, the question remained: is di-Tyr responsible for most of the Aβ crosslinking in humans, or does the identification of di-Tyr simply represent the detection of a trace component because of the sensitivity of the search technique? This is an important issue for understanding the principal molecular mechanisms that are responsible for crosslinking in each particular instance. Knowledge of mechanisms and locations of protein crosslinks can also reveal aspects of the in vivo structure of the protein substrate, for example exposed regions, as well as the environment to which the original protein has been exposed. In the case of Aβ such information is also important for understanding factors that impinge upon the binding of potential therapeutic antibodies for Alzheimer disease treatment. The example of Aβ serves to emphasize how important agnostic or untargeted analysis can be. A subsequent comprehensive proteomic analysis using the “blind” approach revealed that the major crosslink of Aβ is not in fact di-Tyr but consists of a heterogeneous population of crosslinked dimers, one form of which is crosslinked between the N-terminal amino group of Asp1 and the carboxyl side chain of Glu22 (Brinkmalm et al., 2019). The identification of other novel Aβ crosslinks in human plaques requires further experiments. It is noteworthy that similar considerations may apply to the crosslinking of α-synuclein in Parkinson’s disease, where a targeted search showed di-Tyr to be present (Al-Hilaly et al., 2016). As with AD, it will be of interest to determine if di-Tyr is really the major crosslink present in the Lewy bodies of the second most common neurodegenerative disease.
One interesting by-product of the blind approach is that it has revealed that carbohydrate-mediated crosslinking in the lens appears to be quantitatively a minor process. This is surprising because human lens proteins simmer at 35°C in ~1mM solutions of both glucose and ascorbate. Since this incubation can occur for decades, it would appear to be ideal conditions to promote covalent binding and yet searches do not detect such crosslinks, or only as trace components. This finding is in agreement with quantitative studies on AGE compounds which have found very low levels of compounds such as carboxymethyl Lys. Surprisingly, levels of this compound were not elevated in diabetes (Lyons et al., 1991).
In summary, conclusions reached about protein crosslinking can be influenced by the choice of the experimental approach used for detection. This factor is often overlooked in experimental design. If the objective is to discover the major processes involved in crosslinking, then an agnostic or blind search paradigm is the most likely strategy to uncover novel mechanisms of protein-protein crosslinking. This has been the revelation of recent human lens studies which have uncovered a number of novel crosslinks and crosslinking mechanisms.
vi. Protein-protein Crosslinks arise due to inherent instability of certain amino acids
Due to the vast chemical reaction space that can potentially lead to protein crosslinks, the identification of the structure of crosslinks is intrinsically difficult and some of the techniques employed will be discussed later in this review. Another complicating factor is that there may be a more than one type of crosslink present in an aged protein, and even if only one specific amino acid is responsible, there will likely be multiple sites for this amino acid within the sequence of the protein. Slight modifications to the chemical structure of a primary crosslink, such as found in collagen, will mean that the crosslinks may be heterogeneous, and this will compound the analytical results. Another potential source of difficulty is that protein crosslinks will often be present together with a number of other age-related PTMs such as racemisation, deamidation, glycation and truncation (Hains and Truscott, 2010; Hooi and Truscott, 2011; Korlimbinis et al., 2009; Srivastava and Srivastava, 2003). Each of these modifications will affect the structures of tryptic peptides formed in the digestion step of a standard proteomic analysis and will therefore lead to a diverse range of crosslinked tryptic peptides. Despite these issues, our understanding of age-related protein crosslinking has proceeded significantly in the past decade and certain amino acids such as Cys, Ser, Thr, Asp/Asn, Glu/Gln and Lys appear to play dominant roles in crosslink formation.
Modelling spontaneous protein-protein crosslink formation
Unlike enzymatic protein-protein crosslinking processes, the use of conventional modelling techniques involving cells or animal models to study spontaneous protein crosslinking mechanisms is unfeasible because these processes are slow, often taking years to accumulate in appreciable amounts. This long timeframe generally limits modelling of these spontaneous processes to recombinant proteins or small synthetic peptides. In order to produce large enough quantities for analysis, extended incubations of either a protein or peptide, with a potential crosslinking partner are necessary. In the case of protein crosslinking, the partner molecule typically contains a free amino or sulfhydryl group, such as Lys or Cys, while the reaction is monitored for crosslink formation by liquid chromatography and/or mass spectrometry.
i. Protein models
While the use of recombinant proteins may seem an obvious choice to model spontaneous crosslinking mechanisms, several factors limit their use. Firstly, spontaneous reactions in proteins may occur more slowly than in unstructured peptides due to structural factors and protein-protein interactions. This can be addressed by increasing the incubation temperature above 37°C, however, this can lead to protein denaturation and precipitation (Velasco et al., 1997). Secondly, crosslinking will not be the only reaction that occurs. Long term maintenance of protein solubility may be another consideration. Other processes such as truncation (Wang et al., 2019), deamidation, racemization and isomerization (Geiger and Clarke, 1987) will occur throughout the protein complicating subsequent proteomic analysis. These factors limit the use of recombinant, or purified, proteins in studies of spontaneous processes such as crosslinking.
ii. Peptide models
In practice, we have found it to be more informative and productive to use small peptides to model sites where protein crosslinking has been detected in biological systems. These peptides typically contain three to five canonical amino acids corresponding to the crosslink site, and these can be bookended with UV absorbing amino acids such as Tyr or Phe to aid in HPLC detection. To increase peptide stability and reduce unintended reactions, the N-terminus is typically blocked by acetylation. The overall result is a less complex sample that allows easier monitoring of the peptide incubations by HPLC and mass spectrometry. Importantly, peptide mixtures can be incubated at elevated temperatures to speed the formation of spontaneous processes. This option would not be possible using a protein due to thermal denaturation and possible irreversible aggregation. One limiting factor that should be recognised is that the use of peptides does not provide information on the effect of the local environment, or protein folding, on these crosslinking processes.
Monitoring of crosslink formation generally involves the incubation of the peptide of interest with, or without, the crosslinking partner. It is often advantageous to use a small binding partner as it reduces the challenges involved in structurally characterizing often large protein-protein crosslinks. In the case of a Lys crosslink, N-Acetyl-Lys would appear to be an obvious choice, but in practice, a crosslink formed with this molecule sometimes does not allow sufficient separation by HPLC for easy identification and/or monitoring of the rate of crosslink formation. A small di- or tripeptide containing a Lys residue does not suffer this limitation but complicates the analysis due to possible degradation processes taking place within this partner peptide. Experience has shown that it is preferable to use a surrogate molecule, such as phenylethylamine, to mimic the side chain amino group of Lys (Friedrich et al., 2018; Friedrich et al., 2019; Wang et al., 2019). Its aromatic structure also aids in UV detection and alters the elution time of the crosslink sufficiently to allow isolation by HPLC.
The rate of crosslinking can be further examined using peptides, in ways that are not readily available in protein models. For example, neighboring amino acids and pH may have an impact on the rate of crosslink formation and pathways of spontaneous chemical processes. While amino acid sequence can be altered in recombinant proteins, production of recombinant proteins can be time-consuming and the amino acid substitutions may affect solubility and structure. By comparison, small peptide mimics can be readily synthesized with a series of substitutions of neighboring amino acids in order to observe their influence on crosslinking (Friedrich et al., 2017; Friedrich et al., 2019). The success of this approach was highlighted in a study into the crosslinking of βA4-crystallin in the human lens, where Cys 5 was found to be significantly crosslinked with GSH by age 20 (Wang and Schey, 2018). By sequentially substituting amino acids in Ac-TLQCTK, which corresponds to the site of the Cys crosslink in βA4 crystallin, the importance of the Lys residue two residues away from the Cys, was revealed. The side chain amino group of Lys accelerated crosslink formation by nucleophilic attack on the Cys 5 cystine leading to a reactive dehydroalanine (DHA) intermediate. Ultimately the DHA formed protein-protein crosslinks with Lys and Cys residues (Friedrich et al., 2017).
Similarly, peptide models have been helpful in the identification of protein-protein crosslinking that may result from two or more linked spontaneous processes. This is exemplified by protein-protein crosslinking observed between C-terminally truncated Asn or Asp residues with Lys (Friedrich et al., 2019; Wang et al., 2019). Through the use of peptide models and isolation of intermediate products, it was found that spontaneous cleavage next to Asp lead to the generation of a C-terminal Asp anhydride. This anhydride intermediate could then either react with water to form a C-terminal Asp, or with a nucleophile, such as a free amino group to form a covalent crosslink (Wang et al., 2019). In the case of Asn, cleavage results in the formation of C-terminal cyclic succinimide. This succinimide was not itself the reactive intermediate, but it hydrolysed to Asp and Asp-amide. The latter readily deamidated via an anhydride and in this way crosslinking at a terminal Asn could occur (Friedrich et al., 2019).
While the use of peptide models has been important in the understanding of spontaneous process such as crosslinking, it is important to note their limitations, for example test tube experiments cannot fully recapitulate the conditions present in the cell. Peptide models primarily provide information on the effect of conditions and neighbouring amino acids on the reactions. On the other hand, recombinant proteins may recapitulate folds but can have multiple conformations or can be incorrectly folded (Baneyx and Mujacic, 2004), a feature that can be further compounded if amino acid substitutions are made. Another key limitation to the use of whole proteins is the need to perform digestion, typically enzymatic, after the modifications have occurred. Conditions of digestion could influence the products that result. Other considerations relate to amino acid sequence. For example, a high percentage of hydrophobic amino acid may result in protein insolubility and it is difficult to study the modification of insoluble proteins for example cytoskeletal components. Many of these concerns can be minimised by using peptide models which allow researchers to alter conditions such as buffer, salt concentration, pH or solvents in order to more thoroughly characterise the reactions.
Overall, the use of small peptide mimics, in conjunction with proteomics of tissues such as the lens, has proven to be an extremely fruitful approach which has yielded a large amount of information on the variety of mechanisms of protein crosslink formation.
Methods for studying protein-protein crosslinking
i. Gel electrophoresis
Multiple techniques were employed in the early study of protein-protein crosslinking in aged lenses. Gel electrophoresis was the most widely used technique for visualizing protein-protein crosslinking (Garadi et al., 1987; Harrington et al., 2004; Padgaonkar et al., 1989; Srivastava et al., 2008; Srivastava et al., 2004; Takemoto and Hansen, 1982). Two-dimensional diagonal electrophoresis was especially useful for studying reversible protein-protein crosslinking through the formation of disulfide bonds (Garadi et al., 1987; Padgaonkar et al., 1989; Takemoto and Hansen, 1982). For two-dimensional diagonal electrophoresis, samples were sequentially resolved in two gel systems containing the same concentrations of polyacrylamide. Protein disulfide bonds were reduced between the first-and second-dimension separation. Therefore, proteins with no intermolecular disulfide bonds will migrate with the same relative mobility in both gel systems and will appear on the diagonal and disulfide linked proteins will “off-diagonal” in the second dimensional gel after the reduction of the disulfide bonds. Other methods such as size exclusion chromatography (SEC) have been used to separate the high molecular weight (HMW) complexes (Dilley and Pirie, 1974; Harrington et al., 2004).
ii. Acid hydrolysis
Protein-protein crosslinking was also studied at the amino acid level. In this method, lens proteins were hydrolyzed by 6 M HCl at 110°C and the samples were then further separated by HPLC and analyzed by mass spectrometry (Linetsky et al., 2004; Nagaraj et al., 1991). This method has been used to study dehydroalanine-mediated protein-protein crosslinking (Linetsky et al., 2004) and advanced glycation end products (AGE) (Glomb and Pfahler, 2001; Nagaraj et al., 1991; Smuda et al., 2015). Some structures formed during crosslinking are not stable under acid hydrolysis conditions, and in these cases, enzymatic hydrolysis can be performed using a combination of multiple proteolytic enzymes (proteinase K, Pronase E, aminopeptidase and carboxypeptidase Y) (Glomb and Pfahler, 2001). Acid hydrolysis and enzymatic hydrolysis reduce the heterogenous nature of the crosslinks, therefore these methods aid in detecting and quantifying protein-protein crosslinks. However, when proteins are hydrolyzed, information related to the specific proteins and residues involved in crosslinking is lost.
iii. Mass spectrometry-based crosslinked peptide identification
Mass spectrometry has been used for studying protein-protein crosslinking together with gel electrophoresis (Srivastava et al., 2004) and acid hydrolysis (Linetsky et al., 2004; Smuda et al., 2015). Until recently, the high resolution, mass accuracy, and sensitivity offered by mass spectrometry with ongoing improvements in crosslinked peptide searching algorithms renders possible the direct analysis of residues involved in crosslinking (Sinz, 2010). A general workflow follows a bottom-up proteomic approach and is illustrated in Figure 1. In a typical experiment to identify protein-protein crosslinking, proteins are digested to peptides by a site-specific enzyme such as trypsin or endoproteinase Lys-C. The resulting peptides are separated and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Subsequent database searching with specialized software (discussed below) identifies crosslinked peptides. As an optional step, enrichment of crosslinked peptides can be performed before LC-MS analysis. This method has been widely used for chemically crosslinked samples to obtain a low-resolution characterization of protein complexes and sites of protein-protein interaction (Barysz and Malmstrom, 2018; Yu and Huang, 2018). Recently, this strategy has been used for characterizing protein-protein crosslinks formed in aged human lenses and multiple crosslinking mechanisms have been detected (discussed below) (Friedrich et al., 2018; Friedrich et al., 2019; Wang et al., 2019; Wang et al., 2014). By identifying crosslinked peptides, this method reveals proteins, residues and chemistries involved in the crosslinking process.
Figure 1:
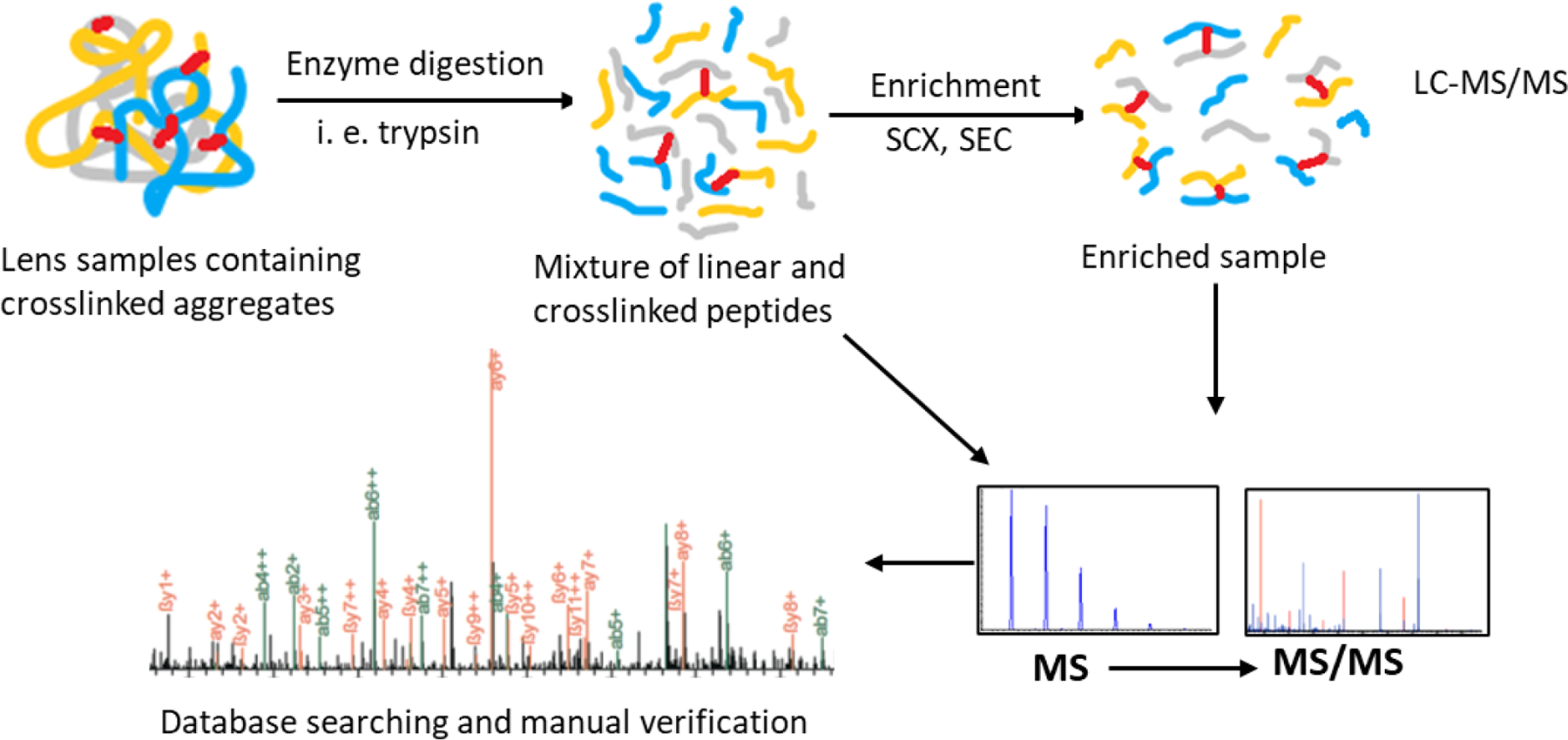
Outline of mass spectrometry-based crosslinked peptide identification
LC-MS/MS analysis of crosslinked peptides requires high-resolution tandem mass spectrometers to accurately decipher sites and structures of protein crosslinks. Both precursor and fragment ions are normally detected in an Orbitrap analyzer. Instrument methods can be modified to improve identification of crosslinked peptides. For example, crosslinked tryptic peptides typically have at least four positively charged residues (two N-terminal amino groups and two positively charged C-termini) and often carry more changes than linear peptides. Based on the prevalence of more highly charged peptides, data-dependent enrichment of tandem mass spectra from crosslinked peptides can be accomplished by high-charge-state driven acquisition (Singh et al., 2008). Low-charge-state species (i. e. +1 and +2) are avoided for fragmentation to save instrument time for more informative higher charge state ions. Different fragmentation methods can also be chosen to obtain complementary results. Higher-energy C-trap dissociation (HCD) is often used and it has been reported to provide the highest number of identified cross-linked peptides (Kolbowski et al., 2017). Electron transfer dissociation (ETD) using supplemental activation with HCD (EThcD) was found to give the best sequence coverage for high-charged crosslinked peptides (Kolbowski et al., 2017). HCD has been used for crosslinked peptide identification in human lenses (Friedrich et al., 2018; Friedrich et al., 2019; Wang et al., 2019). Other dissociation methods have not been tested to identify protein-protein crosslinking in human lenses.
iv. Enrichment of Crosslinked Proteins and Peptides
A majority of protein-protein crosslinks formed in the lens are formed non-enzymatically and occur through very slow reactions. For crosslinking to occur, residues involved in the crosslink must be in close proximity, therefore protein conformation changes during aging could result in crosslinks occurring between different residues. In addition, multiple crosslinking mechanisms could lead to heterogeneous crosslinked species. Age-related modifications to proteins add further complexities to the samples. Therefore, tryptic peptides of a human lens sample constitute an extremely complex mixture with crosslinked peptides far less abundant compared with linear peptides. Even for intentional chemically crosslinked samples used to examine protein-protein interactions, the low abundance of crosslinked peptides is one of the major challenges (Barysz and Malmstrom, 2018; Sinz, 2010; Yu and Huang, 2018). It is no exaggeration to compare crosslinked peptide identification in human lenses with looking for “a needle in a haystack”. Therefore, enrichment for crosslinked proteins or peptides is essential for detecting low abundance crosslinked peptides.
It is believed that protein-protein crosslinking in aged human lenses is associated with protein aggregation, insolubilization, and cataract formation (Dilley and Pirie, 1974; Linetsky et al., 2004; Nagaraj et al., 2012). A certain degree of enrichment can be achieved at the protein level. The insoluble protein fraction from aged cataract lenses may provide a better chance to detect protein-protein crosslinks due to increased accumulation of crosslinked proteins in these fractions (Wang et al., 2014). High molecular weight complexes isolated by size exclusion chromatography can also be used as a means to enrich for crosslinked proteins (Fu et al., 1984; Srivastava et al., 2008).
Using intentionally chemically crosslinked samples, considerable efforts have been made to enrich for crosslinked peptides (Fritzsche et al., 2012; Kang et al., 2009; Leitner et al., 2012; Sohn et al., 2012). Since crosslinked peptides contain two linear peptides, they are typically larger than linear peptides. One enrichment strategy includes separation of peptide mixtures using size exclusion chromatography (SEC) and collecting higher molecular weight peptides for further analysis (Leitner et al., 2012). Additionally, crosslinked peptides will exist in higher charge states (≥4+) at acidic pH due to containing more basic residues and larger sizes (Fritzsche et al., 2012). Utilizing this fact, strong cation exchange (SCX) is another approach for enriching crosslinked peptides (Fritzsche et al., 2012). SCX enrichment has been used for identification of crosslinked peptides in human lenses (Friedrich et al., 2019; Wang et al., 2014). Both SEC and SCX enrichment are crude enrichment methods based on the average properties of crosslinked peptides and enrichment is limited. Missed-cleaved linear peptides and histidine containing peptides have additional basic residues that also exhibit increased retention on SCX resins. In addition, crosslinked peptides could be small when enzyme cleavage sites are close to crosslinked residues and this type of crosslinked peptide can be lost during SEC enrichment. Digestion by different proteases before SEC enrichment may provide complementary results (Leitner et al., 2012).
v. Software and Database Searching
For crosslinked peptide identification, the search algorithms need to pair all peptides that contain a residue that is capable of crosslinking, therefore, the search space is exponentially increased (Yu and Huang, 2018). Such a large search space results in a high risk of random matches and an estimation of the correct false discovery rate (FDR) for crosslinked peptides identification is difficult (Fischer and Rappsilber, 2017). Often, initial searches are made against a limited protein database, e.g. consisting of only major lens proteins, to reduce search time and reduce the number of false positive identifications. Manual validation of tandem mass spectra is generally required for correct identification of crosslinked peptides. Using high measured mass accuracy on both precursor ions and fragment ions is very important for decreasing false identification (Scherl et al., 2008). A large number of algorithms have been developed for searching candidate cross-linked peptides and a detailed list can be found in the review paper by Yu and Huang (Yu and Huang, 2018). These algorithms are mainly designed for searching crosslinked peptides in intentionally chemically crosslinked samples and some of them are developed toward specific crosslinkers (Chakrabarty et al., 2020), therefore, they cannot be used for searching crosslinked peptides in lens samples. Two approaches have been used for identifying crosslinked peptides in the lens. One approach is to treat crosslinked peptides as linear peptides bearing a large modification. Several spectral alignment algorithms have been developed to allow for unrestricted PTM searches (or Blind searches) such as TagRecon blind search mode (Dasari et al., 2010), MODa (Na et al., 2012) and MaxQuant dependent peptide search (Cox et al., 2011). These programs are generally used for identifying unexpected modifications and they can also help for identifying one of the peptides that is involved in crosslinking. De novo sequencing can then be used to generate a sequence tag for the second peptide involved in crosslinking. This sequence tag can be used for searching potential candidates for the second peptide in the database. The change in mass of the parent ion can be used to predict the potential crosslinking chemistry. This method is inefficient and often the tandem mass spectra do not have enough fragments to provide a sequence tag that is long enough for identifying the second peptide. However, this strategy lead to the original identification of crosslinked peptides in human lens (Wang et al., 2014) and it is essential for identification of new protein-protein crosslinking mechanisms (Wang et al., 2019). Once a crosslinking mechanism is suspected, crosslinked peptides can be searched by a second approach that uses crosslink searching software focused on specific known chemistry. For example, data analysis based on the first approach unexpectedly detected irreversible GSH modifications leading to a search for dehydroalanine-mediated protein-protein crosslinks in human lenses by MassMatrix (Xu et al., 2008) and multiple crosslinked peptides were detected (Wang et al., 2014). Later, a similar approach revealed a new crosslinking mechanism between Lys residues and N-terminal amines with C-terminal Asp residues (Wang et al., 2019). Additional crosslinked peptide identification by StavroX (Gotze et al., 2012) demonstrated crosslinking between Lys residues and newly formed C-terminal Asp residues after protein truncation that commonly occurs in human lens proteins (Wang et al., 2019). Recently, pLink2 was reported to outperform many major searching tools and can handle multiple data files together to search against a large database (Chen et al., 2019), so pLink2 was used for recent crosslinked peptide identification in human lenses (Friedrich et al., 2019). Other searching tools can also be used such as Kojak (Hoopmann et al., 2015) and SIM-XL (Lima et al., 2015), but these searching algorithms have not been applied to study protein-protein crosslinks in human lenses. It is important to emphasize again that crosslink searching software can only identify potential crosslinked peptides, hence manual verification is essential for correct identifications. With fast improvement in searching algorithms, crosslinked peptide identification is anticipated to become a routine analysis with enhanced sensitivity and improved accuracy. Thus, we anticipate that protein-protein crosslinking in human lenses will be better understood.
Mechanisms of Lens Protein Crosslinking
Using the knowledge of protein stability and the discovery technique of LC-MS/MS, we have discovered five mechanisms of spontaneous, potentially deleterious protein-protein crosslinking in the lens that fall into two broad categories: cyclic intermediates and alkene intermediates. To be sure, advances in mass spectrometry sensitivity have improved our ability to detect low abundance crosslinks in the presence of highly abundant lens proteins.
i. Crosslinks via cyclic intermediates
Cyclic intermediates have historically been invoked to explain the mechanism of deamidation; a major posttranslational modification on lens proteins (Hains and Truscott, 2010; Wilmarth et al.). Hydrolysis of the cyclic succinimide intermediate formed by Asn residues results in deamidation and isomerization to produce L-Asp, D-Asp, L-isoAsp, and D-isoAsp (Geiger and Clarke, 1987). The analogous cyclic glutarimide is the predicted intermediate in Gln deamidation. Importantly, both succinimide and glutarimide intermediates can react with available nucleophiles, such as Lys residues, to form protein-protein crosslinks (Friedrich et al., 2018; Friedrich et al., 2021) (Figure 2). Such crosslinks have been identified in lens proteins and, given the prevalence of deamidation, a number of such crosslinks are likely to be present in aged human lenses.
Figure 2.
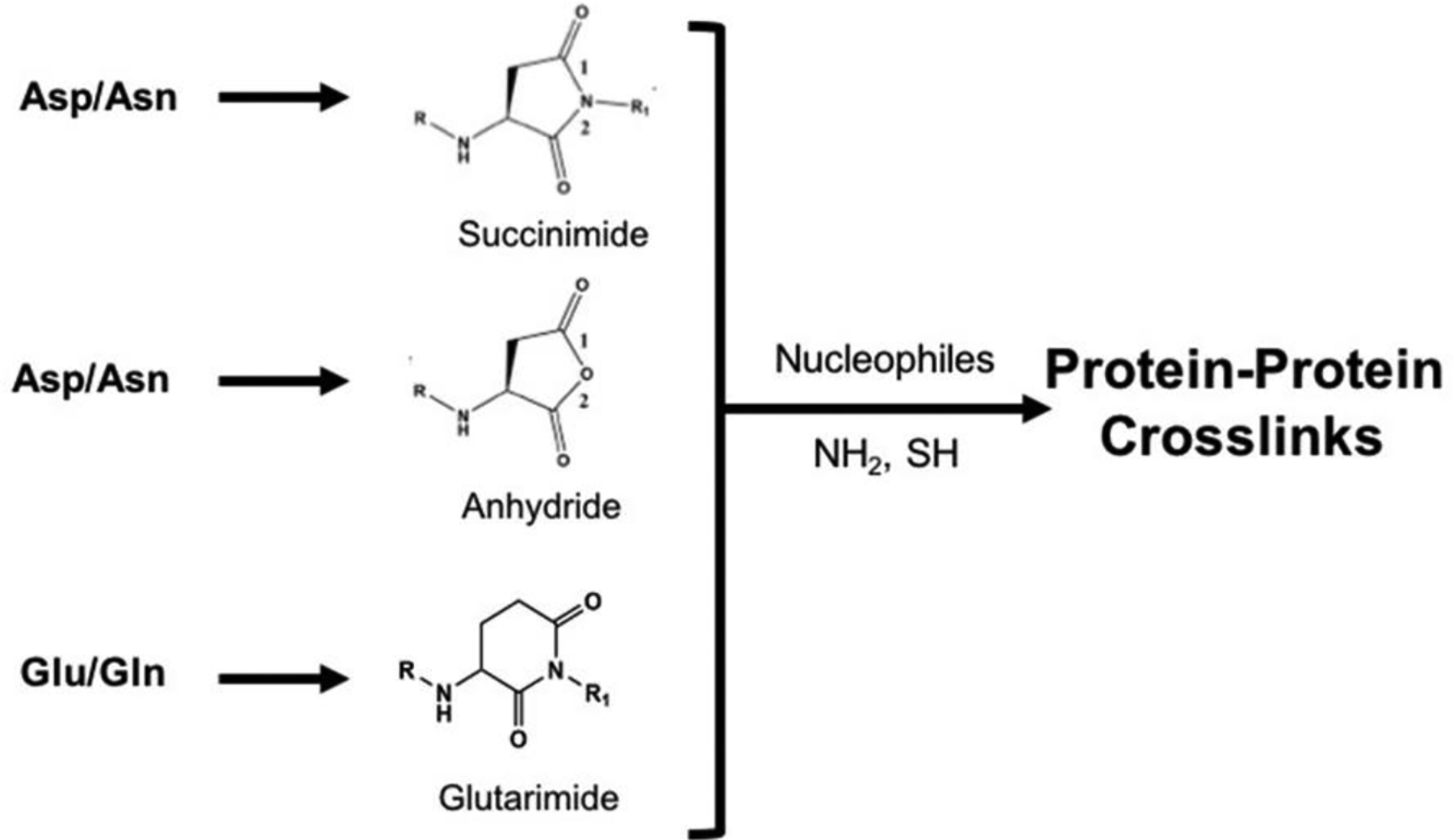
Formation of protein crosslinks via cyclic intermediates.
Other reactive cyclic structures are also capable of being formed in proteins including anhydrides (Figure 2). Upon protein truncation at Asn or Asp, a common finding in lens proteins (Grey and Schey, 2009), is cyclization of the C-terminal residue to form an anhydride which may precede crosslinking to nucleophiles such as Lys residues or protein N-terminal amines (Friedrich et al., 2019; Wang et al., 2019). Interestingly, in the case of C-terminal Asn, a cyclic succinimide is likely formed, but model peptide studies indicate that this succinimide is not itself reactive. Subsequent deamidation and anhydride formation is required for crosslinking to occur (Friedrich et al., 2019). It is important to note that structural changes in proteins such as cyclization require some flexibility and we suspect that most crosslinks, as is the case with deamidation (Clarke, 1987; Hooi et al., 2012; Rivers et al., 2008), occur in unstructured, flexible regions of lens proteins. As mentioned above, model peptide studies were critical to elucidate these crosslinking mechanisms and small peptides afford the structural flexibility to facilitate such studies.
ii. Crosslinks via alkenyl intermediates
A second general mechanism leading to abundant lens protein crosslinks is through a dehydroalanine (DHA) intermediate (Figure 3). Loss of phosphoric acid from phosphoserine (or water from Ser) may lead to the formation of DHA. Analogous loss of phosphoric acid or water from phosphothreonine or Thr residues leads to dehydrobutyrine (DHB) formation. Loss of H2S from Cys residues and asymmetric cleavage of Cystine (loss of -S-SR) can also result in DHA formation. Although DHA is relatively stable in the absence of nucleophiles, as evidenced by its detection in lens proteins (Srivastava et al., 2004), over time this intermediate can react with Cys and Lys residues to form irreversible lanthionine and lysinoalanine crosslinks (Linetsky et al., 2004). Such crosslinks were predicted by early studies of Linetsky et al. (Linetsky et al., 2004) based on acid hydrolysis of lens proteins. Advances in mass spectrometry have led to the identification of more than 90 specific lens protein products that result from DHA chemistry. Importantly, some of these products are quite abundant (Wang and Schey, 2018) and have been shown to increase in cataract lenses (Linetsky et al., 2004; Linetsky and LeGrand, 2005). Moreover, the extraordinarily high abundance of glutathione (GSH) in the lens predicts two likely outcomes: 1) that DHA formed from oxidized glutathione (GSSG) (Younis et al., 2008) will react with nucleophilic amino acid side chains and 2) reduced GSH will react with DHA present on lens proteins. DHA formed from oxidized glutathione will react with lysine, for example, to form a modification with a mass shift of +144.0535 Da corresponding to addition of (DHA)Gly to the peptide after facile loss of γGlu (Friedrich et al., 2018). Glutathione reaction with DHA on a serine/threonine residue will form a thioether linked modification with a mass shift of 289.0733 Da for intact GSH crosslinks, 160.0307 Da for CysGly crosslinks, and 103.0092 Da for Cys crosslinks; the latter two modifications being likely degradation products of the GSH crosslink. The same modifications on cysteine residues will cause a mass shift of 273.0961 Da for GSH thioether modification, 144.0535 Da for CysGly crosslinks and 87.0320 Da for Cys crosslinks.
Figure 3.
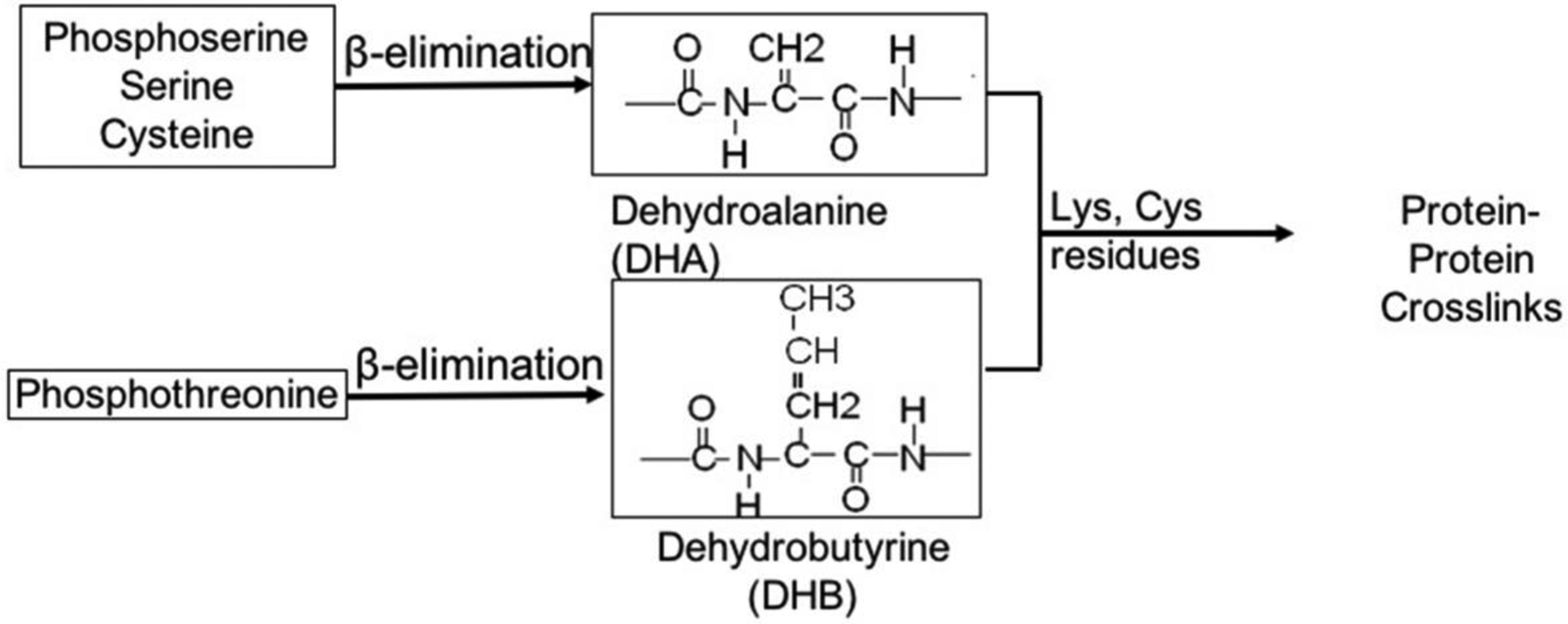
Protein-protein crosslinking mediated by DHA and DHB. Crosslinking mediated by DHA formed from serines or DHB formed from threonines results in loss of a molecule of H2O (ΔM=−18.01057). Crosslinking mediated by DHA formed from cysteines results in loss of a molecule of H2S (ΔM=−33.98772).
Conclusions and Future Directions
There is clear evidence that many age-related crosslinks are present in aged human lenses and are also likely to be found in other LLPs throughout the body. Advances in detection technologies such as mass spectrometry have allowed multiple specific crosslinking mechanisms to be elucidated. New advances in enrichment strategies and data MS/MS interpretation algorithms will likely lead to the discovery of new protein crosslinks.
An important question, and challenge, remains regarding the abundance of the many specific forms of crosslinked lens proteins as a function of age. Although some relative quantitation has been done, without crosslinked standards, absolute abundance determination remains a significant challenge. Further, without the knowledge of abundance, the relative importance of each specific crosslink remains difficult to determine.
Since most of the identified crosslinks are irreversible except under the harshest chemical conditions, a strategy to treat protein aggregation must focus on prevention rather than reversing such crosslinks. Only during the early stages of non-covalent protein aggregation would de-aggregation strategies be most likely to be effective. This is an important point to communicate given the number of LLPs in the body and the number of diseases where protein aggregation of LLPs is believed to play an etiological role, e.g. Alzheimer’s disease. As the human population ages, elucidation of protein crosslinking chemistries will only become more important for determining future therapeutic strategies for age-related diseases.
Highlights.
Review of crosslinking chemistry in proteins
Summary of enzymatic and non-enzymatic crosslinking mechanisms
Discussion of methods to detect protein crosslinks
Acknowledgements
The work reviewed in this paper was supported by R01 EY013462 (KLS), R01 EY024258(KLS), P30 EY008126, R01 EY013570(RJWT) and by the Mass Spectrometry Research Center at Vanderbilt University. The authors would also like to thank the honorees of this special issue for their contributions to lens research, for stimulating discussion, for their guidance and advice, and, most importantly, their friendship over the course of our careers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akagawa M and Suyama K, 2000. Mechanism of formation of elastin crosslinks. Connect Tissue Res. 41, 131–141. 10.3109/03008200009067665 [DOI] [PubMed] [Google Scholar]
- Al-Hilaly YK, Biasetti L, Blakeman BJ, Pollack SJ, Zibaee S, Abdul-Sada A, Thorpe JR, Xue WF and Serpell LC, 2016. The involvement of dityrosine crosslinking in alpha-synuclein assembly and deposition in Lewy Bodies in Parkinson’s disease. Sci Rep. 6, 39171. 10.1038/srep39171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hilaly YK, Williams TL, Stewart-Parker M, Ford L, Skaria E, Cole M, Bucher WG, Morris KL, Sada AA, Thorpe JR and Serpell LC, 2013. A central role for dityrosine crosslinking of Amyloid-beta in Alzheimer’s disease. Acta Neuropathol Commun. 1, 83. 10.1186/2051-5960-1-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EI, Wright DD and Spector A, 1979. The state of sulfhydryl groups in normal and cataractous human lens proteins. II. Cortical and nuclear regions. Exp Eye Res. 29, 233–243. 10.1016/0014-4835(79)90004-6 [DOI] [PubMed] [Google Scholar]
- Aquilina JA, Carver JA and Truscott RJ, 1999. Elucidation of a novel polypeptide cross-link involving 3-hydroxykynurenine. Biochemistry. 38, 11455–11464. 10.1021/bi990458h [DOI] [PubMed] [Google Scholar]
- Baneyx F and Mujacic M, 2004. Recombinant protein folding and misfolding in Escherichia coli. Nat Biotechnol. 22, 1399–1408. 10.1038/nbt1029 [DOI] [PubMed] [Google Scholar]
- Barysz HM and Malmstrom J, 2018. Development of Large-scale Cross-linking Mass Spectrometry. Mol Cell Proteomics. 17, 1055–1066. 10.1074/mcp.R116.061663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bova LM, Wood AM, Jamie JF and Truscott RJ, 1999. UV filter compounds in human lenses: the origin of 4-(2-amino-3-hydroxyphenyl)-4-oxobutanoic acid O-beta-D-glucoside. Invest Ophthalmol Vis Sci. 40, 3237–3244. [PubMed] [Google Scholar]
- Brinkmalm G, Hong W, Wang Z, Liu W, O’malley TT, Sun X, Frosch MP, Selkoe DJ, Portelius E, Zetterberg H, Blennow K and Walsh DM, 2019. Identification of neurotoxic cross-linked amyloid-beta dimers in the Alzheimer’s brain. Brain. 142, 1441–1457. 10.1093/brain/awz066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenandt A, Keck J and Neubert G, 1957. Über Ommochrome VIII. Modell-Versuche zur Konstitution der Ommochrome: Über Oxydationsprodukte der 3-Hydroxy-Anthranilsäure. Justus Liebigs Annalen der Chemie. 602, 61–72. 10.1002/jlac.19576020106 [DOI] [Google Scholar]
- Chakrabarty JK, Sadananda SC, Bhat A, Naik AJ, Ostwal DV and Chowdhury SM, 2020. High Confidence Identification of Cross-Linked Peptides by an Enrichment-Based Dual Cleavable Cross-Linking Technology and Data Analysis tool Cleave-XL. J Am Soc Mass Spectrom. 31, 173–182. 10.1021/jasms.9b00111 [DOI] [PubMed] [Google Scholar]
- Chen ZL, Meng JM, Cao Y, Yin JL, Fang RQ, Fan SB, Liu C, Zeng WF, Ding YH, Tan D, Wu L, Zhou WJ, Chi H, Sun RX, Dong MQ and He SM, 2019. A high-speed search engine pLink 2 with systematic evaluation for proteome-scale identification of cross-linked peptides. Nat Commun. 10, 3404. 10.1038/s41467-019-11337-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S, 1987. Propensity for spontaneous succinimide formation from aspartyl and asparaginyl residues in cellular proteins. Int J Pept Protein Res. 30, 808–821. 10.1111/j.1399-3011.1987.tb03390.x [DOI] [PubMed] [Google Scholar]
- Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV and Mann M, 2011. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res. 10, 1794–1805. 10.1021/pr101065j [DOI] [PubMed] [Google Scholar]
- D’angelo MA, Raices M, Panowski SH and Hetzer MW, 2009. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 136, 284–295. 10.1016/j.cell.2008.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S, Chambers MC, Slebos RJ, Zimmerman LJ, Ham AJ and Tabb DL, 2010. TagRecon: high-throughput mutation identification through sequence tagging. J Proteome Res. 9, 1716–1726. 10.1021/pr900850m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich LE and Ungermann C, 2004. On the mechanism of protein palmitoylation. EMBO Rep. 5, 1053–1057. 10.1038/sj.embor.7400277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilley KJ and Pirie A, 1974. Changes to the proteins of the human lens nucleus in cataract. Exp Eye Res. 19, 59–72. 10.1016/0014-4835(74)90073-6 [DOI] [PubMed] [Google Scholar]
- Dyksterhuis LB, White JF, Hickey M, Kirby N, Mudie S, Hawley A, Vashi A, Nigro J, Werkmeister JA and Ramshaw JA, 2011. Impact of heparan sulfate chains and sulfur-mediated bonds on the mechanical properties of bovine lens capsule. Biophys J. 100, 2077–2083. 10.1016/j.bpj.2011.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer L and Rappsilber J, 2017. Quirks of Error Estimation in Cross-Linking/Mass Spectrometry. Anal Chem. 89, 3829–3833. 10.1021/acs.analchem.6b03745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MG, Lam J and Truscott RJ, 2012. Degradation of an old human protein: age-dependent cleavage of gammaS-crystallin generates a peptide that binds to cell membranes. J Biol Chem. 287, 39012–39020. 10.1074/jbc.M112.391565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MG, Wang Z, Oakley AJ, Schey KL and Truscott RJW, 2017. Hotspots of age-related protein degradation: the importance of neighboring residues for the formation of non-disulfide crosslinks derived from cysteine. Biochem J. 474, 2475–2487. 10.1042/BCJ20170268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MG, Wang Z, Schey KL and Truscott RJW, 2018. DehydroalanylGly, a new post translational modification resulting from the breakdown of glutathione. Biochim Biophys Acta Gen Subj. 1862, 907–913. 10.1016/j.bbagen.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MG, Wang Z, Schey KL and Truscott RJW, 2018. Spontaneous cross-linking of proteins at aspartate and asparagine residues is mediated via a succinimide intermediate. Biochem J. 475, 3189–3200. 10.1042/BCJ20180529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MG, Wang Z, Schey KL and Truscott RJW, 2019. Mechanism of protein cleavage at asparagine leading to protein-protein cross-links. Biochem J. 476, 3817–3834. 10.1042/BCJ20190743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MG, Wang Z, Schey KL and Truscott RJW, 2021. Spontaneous protein-protein crosslinking at glutamine and glutamic acid residues in long-lived proteins. Biochem J. 478, 327–339. 10.1042/BCJ20200798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsche R, Ihling CH, Gotze M and Sinz A, 2012. Optimizing the enrichment of cross-linked products for mass spectrometric protein analysis. Rapid Commun Mass Spectrom. 26, 653–658. 10.1002/rcm.6150 [DOI] [PubMed] [Google Scholar]
- Fu SC, Su SW, Wagner BJ and Hart R, 1984. Characterization of lens proteins. IV. Analysis of soluble high molecular weight protein aggregates in human lenses. Exp Eye Res. 38, 485–495. 10.1016/0014-4835(84)90126-x [DOI] [PubMed] [Google Scholar]
- Gaar J, Naffa R and Brimble M, 2020. Enzymatic and non-enzymatic crosslinks found in collagen and elastin and their chemical synthesis. Organic Chemistry Frontiers. 7, 2789–2814. [Google Scholar]
- Garadi R, Giblin FJ and Reddy VN, 1987. Disulfide-linked membrane proteins in X-ray-induced cataract. Ophthalmic Res. 19, 141–149. 10.1159/000265486 [DOI] [PubMed] [Google Scholar]
- Geiger T and Clarke S, 1987. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J Biol Chem. 262, 785–794. [PubMed] [Google Scholar]
- Giulivi C, Traaseth NJ and Davies KJ, 2003. Tyrosine oxidation products: analysis and biological relevance. Amino Acids. 25, 227–232. 10.1007/s00726-003-0013-0 [DOI] [PubMed] [Google Scholar]
- Glomb MA and Pfahler C, 2001. Amides are novel protein modifications formed by physiological sugars. J Biol Chem. 276, 41638–41647. 10.1074/jbc.M103557200 [DOI] [PubMed] [Google Scholar]
- Gotze M, Pettelkau J, Schaks S, Bosse K, Ihling CH, Krauth F, Fritzsche R, Kuhn U and Sinz A, 2012. StavroX--a software for analyzing crosslinked products in protein interaction studies. J Am Soc Mass Spectrom. 23, 76–87. 10.1007/s13361-011-0261-2 [DOI] [PubMed] [Google Scholar]
- Grey AC and Schey KL, 2009. Age-related changes in the spatial distribution of human lens alpha-crystallin products by MALDI imaging mass spectrometry. Invest Ophthalmol Vis Sci. 50, 4319–4329. 10.1167/iovs.09-3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grune T, Shringarpure R, Sitte N and Davies K, 2001. Age-related changes in protein oxidation and proteolysis in mammalian cells. J Gerontol A Biol Sci Med Sci. 56, B459–467. 10.1093/gerona/56.11.b459 [DOI] [PubMed] [Google Scholar]
- Hains PG and Truscott RJ, 2010. Age-dependent deamidation of lifelong proteins in the human lens. Invest Ophthalmol Vis Sci. 51, 3107–3114. 10.1167/iovs.09-4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SR, Smith DL and Smith JB, 1998. Deamidation and disulfide bonding in human lens gamma-crystallins. Exp Eye Res. 67, 301–312. 10.1006/exer.1998.0530 [DOI] [PubMed] [Google Scholar]
- Harrington V, Mccall S, Huynh S, Srivastava K and Srivastava OP, 2004. Crystallins in water soluble-high molecular weight protein fractions and water insoluble protein fractions in aging and cataractous human lenses. Mol Vis. 10, 476–489. [PubMed] [Google Scholar]
- Hay RT, 2005. SUMO: a history of modification. Mol Cell. 18, 1–12. 10.1016/j.molcel.2005.03.012 [DOI] [PubMed] [Google Scholar]
- Hooi MY, Raftery MJ and Truscott RJ, 2012. Age-dependent deamidation of glutamine residues in human gammaS crystallin: deamidation and unstructured regions. Protein Sci. 21, 1074–1079. 10.1002/pro.2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooi MY and Truscott RJ, 2011. Racemisation and human cataract. D-Ser, D-Asp/Asn and D-Thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses. Age (Dordr). 33, 131–141. 10.1007/s11357-010-9171-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopmann MR, Zelter A, Johnson RS, Riffle M, Maccoss MJ, Davis TN and Moritz RL, 2015. Kojak: efficient analysis of chemically cross-linked protein complexes. J Proteome Res. 14, 2190–2198. 10.1021/pr501321h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Mou L, Lanman J, Velu S, Brouillette WJ and Prevelige PE Jr., 2009. Synthesis of biotin-tagged chemical cross-linkers and their applications for mass spectrometry. Rapid Commun Mass Spectrom. 23, 1719–1726. 10.1002/rcm.4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbowski L, Mendes ML and Rappsilber J, 2017. Optimizing the Parameters Governing the Fragmentation of Cross-Linked Peptides in a Tribrid Mass Spectrometer. Anal Chem. 89, 5311–5318. 10.1021/acs.analchem.6b04935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korlimbinis A, Berry Y, Thibault D, Schey KL and Truscott RJ, 2009. Protein aging: truncation of aquaporin 0 in human lens regions is a continuous age-dependent process. Exp Eye Res. 88, 966–973. 10.1016/j.exer.2008.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korlimbinis A and Truscott RJ, 2006. Identification of 3-hydroxykynurenine bound to proteins in the human lens. A possible role in age-related nuclear cataract. Biochemistry. 45, 1950–1960. 10.1021/bi051744y [DOI] [PubMed] [Google Scholar]
- Lampi KJ, Wilmarth PA, Murray MR and David LL, 2014. Lens beta-crystallins: the role of deamidation and related modifications in aging and cataract. Prog Biophys Mol Biol. 115, 21–31. 10.1016/j.pbiomolbio.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner A, Reischl R, Walzthoeni T, Herzog F, Bohn S, Forster F and Aebersold R, 2012. Expanding the chemical cross-linking toolbox by the use of multiple proteases and enrichment by size exclusion chromatography. Mol Cell Proteomics. 11, M111 014126. 10.1074/mcp.M111.014126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentini A, Tabolacci C, Mattioli P, Provenzano B and Beninati S, 2011. Spermidine delays eye lens opacification in vitro by suppressing transglutaminase-catalyzed crystallin cross-linking. Protein J. 30, 109–114. 10.1007/s10930-011-9311-7 [DOI] [PubMed] [Google Scholar]
- Lima DB, De Lima TB, Balbuena TS, Neves-Ferreira AGC, Barbosa VC, Gozzo FC and Carvalho PC, 2015. SIM-XL: A powerful and user-friendly tool for peptide cross-linking analysis. J Proteomics. 129, 51–55. 10.1016/j.jprot.2015.01.013 [DOI] [PubMed] [Google Scholar]
- Linetsky M, Hill JM, Legrand RD and Hu F, 2004. Dehydroalanine crosslinks in human lens. Exp Eye Res. 79, 499–512. 10.1016/j.exer.2004.06.026 [DOI] [PubMed] [Google Scholar]
- Linetsky M and Legrand RD, 2005. Glutathionylation of lens proteins through the formation of thioether bond. Mol Cell Biochem. 272, 133–144. 10.1007/s11010-005-6908-1 [DOI] [PubMed] [Google Scholar]
- Lou MF, 2003. Redox regulation in the lens. Prog Retin Eye Res. 22, 657–682. 10.1016/s1350-9462(03)00050-8 [DOI] [PubMed] [Google Scholar]
- Lund AL, Smith JB and Smith DL, 1996. Modifications of the water-insoluble human lens alpha-crystallins. Exp Eye Res. 63, 661–672. 10.1006/exer.1996.0160 [DOI] [PubMed] [Google Scholar]
- Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C and Heinemeier J, 2008. Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS One. 3, e1529. 10.1371/journal.pone.0001529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons TJ, Silvestri G, Dunn JA, Dyer DG and Baynes JW, 1991. Role of glycation in modification of lens crystallins in diabetic and nondiabetic senile cataracts. Diabetes. 40, 1010–1015. 10.2337/diab.40.8.1010 [DOI] [PubMed] [Google Scholar]
- Manthey MK, Pyne SG and Truscott RJ, 1990. Mechanism of reaction of 3-hydroxyanthranilic acid with molecular oxygen. Biochim Biophys Acta. 1034, 207–212. 10.1016/0304-4165(90)90078-b [DOI] [PubMed] [Google Scholar]
- Manthey MK, Pyne SG and Truscott RJ, 1992. Involvement of tyrosine residues in the tanning of proteins by 3-hydroxyanthranilic acid. Proc Natl Acad Sci U S A. 89, 1954–1957. 10.1073/pnas.89.5.1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier VM, 1990. Nonenzymatic glycosylation, the Maillard reaction and the aging process. J Gerontol. 45, B105–111. 10.1093/geronj/45.4.b105 [DOI] [PubMed] [Google Scholar]
- Na S, Bandeira N and Paek E, 2012. Fast multi-blind modification search through tandem mass spectrometry. Mol Cell Proteomics. 11, M111 010199. 10.1074/mcp.M111.010199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj RH, Linetsky M and Stitt AW, 2012. The pathogenic role of Maillard reaction in the aging eye. Amino Acids. 42, 1205–1220. 10.1007/s00726-010-0778-x [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Sell DR, Prabhakaram M, Ortwerth BJ and Monnier VM, 1991. High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc Natl Acad Sci U S A. 88, 10257–10261. 10.1073/pnas.88.22.10257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgaonkar V, Giblin FJ and Reddy VN, 1989. Disulfide cross-linking of urea-insoluble proteins in rabbit lenses treated with hyperbaric oxygen. Exp Eye Res. 49, 887–899. 10.1016/s0014-4835(89)80047-8 [DOI] [PubMed] [Google Scholar]
- Pirie A, 1968. Color and solubility of the proteins of human cataracts. Invest Ophthalmol. 7, 634–650. [PubMed] [Google Scholar]
- Rivers J, Mcdonald L, Edwards IJ and Beynon RJ, 2008. Asparagine deamidation and the role of higher order protein structure. J Proteome Res. 7, 921–927. 10.1021/pr070425l [DOI] [PubMed] [Google Scholar]
- Robertson LJ, David LL, Riviere MA, Wilmarth PA, Muir MS and Morton JD, 2008. Susceptibility of ovine lens crystallins to proteolytic cleavage during formation of hereditary cataract. Invest Ophthalmol Vis Sci. 49, 1016–1022. 10.1167/iovs.07-0792 [DOI] [PubMed] [Google Scholar]
- Roher AE, Chaney MO, Kuo YM, Webster SD, Stine WB, Haverkamp LJ, Woods AS, Cotter RJ, Tuohy JM, Krafft GA, Bonnell BS and Emmerling MR, 1996. Morphology and toxicity of Abeta-(1–42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer’s disease. J Biol Chem. 271, 20631–20635. 10.1074/jbc.271.34.20631 [DOI] [PubMed] [Google Scholar]
- Rowan S, Chang ML, Reznikov N and Taylor A, 2017. Disassembly of the lens fiber cell nucleus to create a clear lens: The p27 descent. Exp Eye Res. 156, 72–78. 10.1016/j.exer.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy D and Spector A, 1976. Absence of low-molecular-weight alpha crystallin in nuclear region of old human lenses. Proc Natl Acad Sci U S A. 73, 3484–3487. 10.1073/pnas.73.10.3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski M, Suryadinata R, Tan AR, Roesley SN and Sarcevic B, 2012. Protein monoubiquitination and polyubiquitination generate structural diversity to control distinct biological processes. IUBMB Life. 64, 136–142. 10.1002/iub.589 [DOI] [PubMed] [Google Scholar]
- Scherl A, Shaffer SA, Taylor GK, Hernandez P, Appel RD, Binz PA and Goodlett DR, 2008. On the benefits of acquiring peptide fragment ions at high measured mass accuracy. J Am Soc Mass Spectrom. 19, 891–901. 10.1016/j.jasms.2008.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schey KL, Gutierrez DB, Wang Z, Wei J and Grey AC, 2010. Novel fatty acid acylation of lens integral membrane protein aquaporin-0. Biochemistry. 49, 9858–9865. 10.1021/bi101415w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader EK, Harstad KG and Matouschek A, 2009. Targeting proteins for degradation. Nat Chem Biol. 5, 815–822. 10.1038/nchembio.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang F and Taylor A, 2004. Function of the ubiquitin proteolytic pathway in the eye. Exp Eye Res. 78, 1–14. 10.1016/j.exer.2003.10.003 [DOI] [PubMed] [Google Scholar]
- Singh P, Shaffer SA, Scherl A, Holman C, Pfuetzner RA, Larson Freeman TJ, Miller SI, Hernandez P, Appel RD and Goodlett DR, 2008. Characterization of protein cross-links via mass spectrometry and an open-modification search strategy. Anal Chem. 80, 8799–8806. 10.1021/ac801646f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinz A, 2010. Investigation of protein-protein interactions in living cells by chemical crosslinking and mass spectrometry. Anal Bioanal Chem. 397, 3433–3440. 10.1007/s00216-009-3405-5 [DOI] [PubMed] [Google Scholar]
- Smuda M, Henning C, Raghavan CT, Johar K, Vasavada AR, Nagaraj RH and Glomb MA, 2015. Comprehensive analysis of maillard protein modifications in human lenses: effect of age and cataract. Biochemistry. 54, 2500–2507. 10.1021/bi5013194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn CH, Agnew HD, Lee JE, Sweredoski MJ, Graham RL, Smith GT, Hess S, Czerwieniec G, Loo JA, Heath JR, Deshaies RJ and Beauchamp JL, 2012. Designer reagents for mass spectrometry-based proteomics: clickable cross-linkers for elucidation of protein structures and interactions. Anal Chem. 84, 2662–2669. 10.1021/ac202637n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava K, Chaves JM, Srivastava OP and Kirk M, 2008. Multi-crystallin complexes exist in the water-soluble high molecular weight protein fractions of aging normal and cataractous human lenses. Exp Eye Res. 87, 356–366. 10.1016/j.exer.2008.07.001 [DOI] [PubMed] [Google Scholar]
- Srivastava OP, Kirk MC and Srivastava K, 2004. Characterization of covalent multimers of crystallins in aging human lenses. J Biol Chem. 279, 10901–10909. 10.1074/jbc.M308884200 [DOI] [PubMed] [Google Scholar]
- Srivastava OP and Srivastava K, 2003. BetaB2-crystallin undergoes extensive truncation during aging in human lenses. Biochem Biophys Res Commun. 301, 44–49. 10.1016/s0006-291x(02)02975-3 [DOI] [PubMed] [Google Scholar]
- Stammers M, Niewczas IS, Segonds-Pichon A and Clark J, 2020. Mechanical stretching changes crosslinking and glycation levels in the collagen of mouse tail tendon. J Biol Chem. 295, 10572–10580. 10.1074/jbc.RA119.012067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Takahashi Y and Putnam FW, 1986. Primary structure of blood coagulation factor XIIIa (fibrinoligase, transglutaminase) from human placenta. Proc Natl Acad Sci U S A. 83, 8019–8023. 10.1073/pnas.83.21.8019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto LJ and Hansen JS, 1982. Intermolecular disulfide bonding of lens membrane proteins during human cataractogenesis. Invest Ophthalmol Vis Sci. 22, 336–342. [PubMed] [Google Scholar]
- Thomas J, Elsden DF and Partridge SM, 1963. Partial Structure of Two Major Degradation Products from the Cross-Linkages in Elastin. Nature. 200, 651–652. 10.1038/200651a0 [DOI] [PubMed] [Google Scholar]
- Toyama BH, Arrojo EDR, Lev-Ram V, Ramachandra R, Deerinck TJ, Lechene C, Ellisman MH and Hetzer MW, 2019. Visualization of long-lived proteins reveals age mosaicism within nuclei of postmitotic cells. J Cell Biol. 218, 433–444. 10.1083/jcb.201809123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama BH, Savas JN, Park SK, Harris MS, Ingolia NT, Yates JR 3rd and Hetzer MW, 2013. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 154, 971–982. 10.1016/j.cell.2013.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott RJ and Augusteyn RC, 1977. The state of sulphydryl groups in normal and cataractous human lenses. Exp Eye Res. 25, 139–148. 10.1016/0014-4835(77)90126-9 [DOI] [PubMed] [Google Scholar]
- Truscott RJW, Schey KL and Friedrich MG, 2016. Old Proteins in Man: A Field in its Infancy. Trends Biochem Sci. 41, 654–664. 10.1016/j.tibs.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacore R, Ham AJ, Voehler M, Sanders CR, Conrads TP, Veenstra TD, Sharpless KB, Dawson PE and Hudson BG, 2009. A sulfilimine bond identified in collagen IV. Science. 325, 1230–1234. 10.1126/science.1176811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco PT, Lukas TJ, Murthy SN, Duglas-Tabor Y, Garland DL and Lorand L, 1997. Hierarchy of lens proteins requiring protection against heat-induced precipitation by the alpha crystallin chaperone. Exp Eye Res. 65, 497–505. 10.1006/exer.1997.0358 [DOI] [PubMed] [Google Scholar]
- Wang Z, Friedrich MG, Truscott RJW and Schey KL, 2019. Cleavage C-terminal to Asp leads to covalent crosslinking of long-lived human proteins. Biochim Biophys Acta Proteins Proteom. 1867, 831–839. 10.1016/j.bbapap.2019.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Lyons B, Truscott RJ and Schey KL, 2014. Human protein aging: modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates. Aging Cell. 13, 226–234. 10.1111/acel.12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z and Schey KL, 2018. Quantification of thioether-linked glutathione modifications in human lens proteins. Exp Eye Res. 175, 83–89. 10.1016/j.exer.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Takio K and Ihara Y, 1999. Deamidation and isoaspartate formation in smeared tau in paired helical filaments. Unusual properties of the microtubule-binding domain of tau. J Biol Chem. 274, 7368–7378. 10.1074/jbc.274.11.7368 [DOI] [PubMed] [Google Scholar]
- Wilkinson B and Gilbert HF, 2004. Protein disulfide isomerase. Biochim Biophys Acta. 1699, 35–44. 10.1016/j.bbapap.2004.02.017 [DOI] [PubMed] [Google Scholar]
- Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, Pevzner PA and David LL, 2006. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J Proteome Res. 5, 2554–2566. 10.1021/pr050473a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Zhang L and Freitas MA, 2008. Identification and characterization of disulfide bonds in proteins and peptides from tandem MS data by use of the MassMatrix MS/MS search engine. J Proteome Res. 7, 138–144. 10.1021/pr070363z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Gong L, Deng M, Zhang L, Sun S, Liu J, Ma H, Yuan D, Chen PC, Hu X, Liu J, Qin J, Xiao L, Huang XQ, Zhang J and Li DW, 2010. Sumoylation activates the transcriptional activity of Pax-6, an important transcription factor for eye and brain development. Proc Natl Acad Sci U S A. 107, 21034–21039. 10.1073/pnas.1007866107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis IR, Elliott M, Peer CJ, Cooper AJ, Pinto JT, Konat GW, Kraszpulski M, Petros WP and Callery PS, 2008. Dehydroalanine analog of glutathione: an electrophilic busulfan metabolite that binds to human glutathione S-transferase A1–1. J Pharmacol Exp Ther. 327, 770–776. 10.1124/jpet.108.142208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C and Huang L, 2018. Cross-Linking Mass Spectrometry: An Emerging Technology for Interactomics and Structural Biology. Anal Chem. 90, 144–165. 10.1021/acs.analchem.7b04431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg M, Zhang X, Taylor A, Liu B, Liang JJ and Shang F, 2006. Glutathiolation enhances the degradation of gammaC-crystallin in lens and reticulocyte lysates, partially via the ubiquitin-proteasome pathway. Invest Ophthalmol Vis Sci. 47, 3467–3473. 10.1167/iovs.05-1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Mittereder N and Sims GP, 2018. Perspective on Protein Arginine Deiminase Activity-Bicarbonate Is a pH-Independent Regulator of Citrullination. Front Immunol. 9, 34. 10.3389/fimmu.2018.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]


