Abstract
Background
Taste disorders in general, and dysgeusia in particular, are relatively common disorders that may be a sign of a more complex acute or chronic medical condition. During the COVID-19 pandemic, taste disorders have found their way into the realm of general as well as specialty dentistry, with significance in screening for patients who potentially may have the virus.
Types of Studies Reviewed
The authors searched electronic databases (PubMed, Embase, Web of Science, Google Scholar) for studies focused on dysgeusia, ageusia, and other taste disorders and their relationship to local and systemic causes.
Results
The authors found pertinent literature explaining the normal physiology of taste sensation, proposals for suggested new tastes, presence of gustatory receptors in remote tissues of the body, and etiology and pathophysiology of taste disorders, in addition to the valuable knowledge gained about gustatory disorders in the context of COVID-19. Along with olfactory disorders, taste disorders are one of the earliest suggestive symptoms of COVID-19 infection.
Conclusions
Gustatory disorders are the result of local or systemic etiology or both. Newer taste sensations, such as calcium and fat tastes, have been discovered, as well as taste receptors that are remote from the oropharyngeal area. Literature published during the COVID-19 pandemic to date reinforces the significance of early detection of potential patients with COVID-19 by means of screening for recent-onset taste disorders.
Practical Implications
Timely screening and identification of potential gustatory disorders are paramount for the dental care practitioner to aid in the early diagnosis of COVID-19 and other serious systemic disorders.
Key Words: Dysgeusia, gustation, virus, taste disorders, infections, burning mouth syndrome, COVID-19, drug-induced, taste receptors, newer tastes
Abbreviation Key: BMS, Burning mouth syndrome; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SS, Sjögren syndrome
The 5 special senses described in the literature are taste, smell, vision, hearing, and equilibrium.1, 2, 3 Taste is proposed to be guiding an organism toward appropriate recognition and consumption of nutrients. It also helps the organism in the prevention of consumption of materials that are toxic or difficult to digest. In humans, 5 types of basic tastes have been identified, namely sour, salt, sweet, bitter, and umami.4 Newer tastes, such as metallic, fatty, and calcium, have also been identified.5 Each taste sensation supposedly gravitates people toward specific foods. For example, sweet-tasting foods inherently announce the carbohydrate content that then could serve as an energy source. The sensation of salt controls sodium intake and aids in the maintenance of water and electrolyte content. Although bitter is supposed to warn against poisonous food content, there are several cultures and nations in the world that savor a bitter taste in foods that are not poisonous. It would be interesting to see why bitter tastants (any chemical that elicits a taste sensation) form a substantial component of traditional medicines, such as those used in Ayurveda, the Indian medical science. Differences in taste perceptions and preferences are seen as resulting from genetic variations, personal experiences, cultural diversity, psychology, personality traits, and, of course, pathologic changes in taste sensations. The physiology of gustation is complex, involving multiple receptors, pathways, and brain centers. In our comprehensive review, we summarized the various subtopics under which the sensation of gustation is discussed and the possible mechanisms of gustatory disorders. In the context of the COVID-19 pandemic, the topic of dysgeusia assumes paramount importance, especially because it is one of the first symptoms of COVID-19 to appear. The astute and educated dental care practitioner is one of the earliest to pick up symptoms of dysgeusia.
Neuroanatomy and Physiology of Gustation
The transmission of taste signals from various sites in the oral cavity to the brain stem is believed to occur through cranial nerves, namely, facial, glossopharyngeal, and vagus.6 The location and course of the first-, second-, and third-order neurons, their corresponding ganglia and nuclei, and the cortical centers of taste have been well elucidated (Figure 1 ).7 Third-order neurons carrying the taste sensations from the thalamus also project into the orbitofrontal cortex, termed the secondary taste cortex, which is responsible for the reward value of taste.8, 9, 10 The difficulty in pinpointing a single cortical area as responsible for taste comes from the varied results that functional imaging studies have yielded in the past few decades.11, 12, 13
Figure 1.
Neuroanatomy of the taste pathway. CN: Cranial nerve. Figure courtesy of Swetha Kannan and Dr. Sita Mahalakshmi Baddireddy.
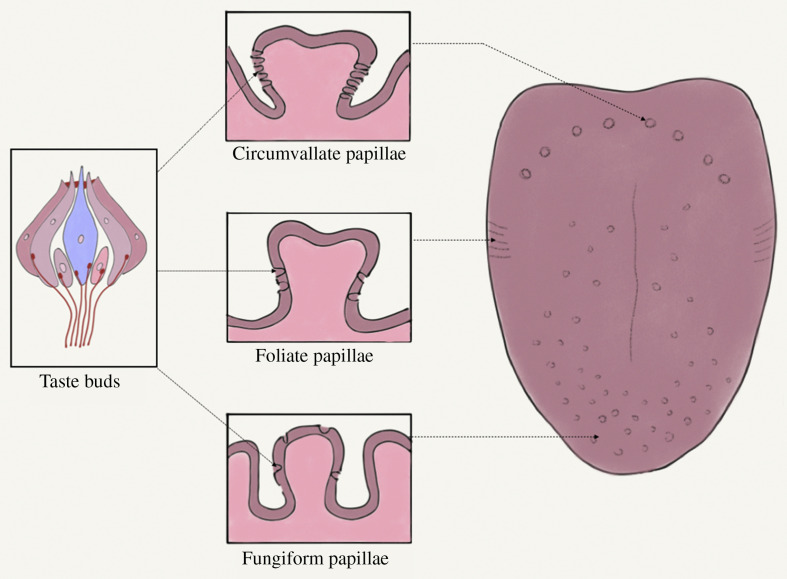
The functional unit of taste is the taste bud, located on the various tongue papillae. The relative location of the taste buds on these papillae are shown in Figure 2 . Taste buds have receptors, which are the biological transducers that convert chemical energy from tastants into electrical energy, facilitating the transmission of these taste impulses.7 , 14 , 15
Figure 2.
Location of taste buds within various papillae. Figure courtesy of Dr. Srishti Parekh.
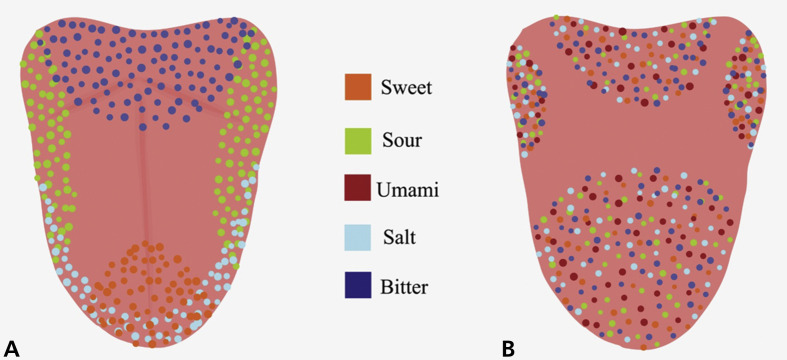
Taste buds are present on the tongue, palate, pharynx, epiglottis, and esophagus.7 Remote taste receptors have been reported in tissues from the gastrointestinal tract, bladder, brain, respiratory tract, heart, buccal mucosa, sinuses, white blood cells, bone marrow, thyroid, keratinocytes, and testicles.16, 17, 18, 19, 20, 21, 22, 23, 24 The functional specificity of taste cells has prompted researchers to divide them into the following 5 types: type I (glialike), type II (bitter, sweet, umami), type III (sour), type IV (pluripotent), and type V (marginal cells).25 , 26 The older concept of “taste mapping”—representing the tongue diagrammatically and showing the relative concentrations of specific taste sensations—has been replaced largely with the newer concept that all areas of the tongue represent the different tastes almost equally (Figure 3 ).27 A similar topographic arrangement in the cerebral cortex has also been proposed.28
Figure 3.
Anatomic distribution of specific taste receptors. A. Older concept of concentration of specific taste receptors at specific sites of the tongue (taste mapping). B. Newer concept of clusters of blends of taste receptors localizing at specific anatomic areas of the tongue. Figure courtesy of Swetha Kannan and Dr. Sita Mahalakshmi Baddireddy.
Gustation as Related to Olfaction: The Concept of Flavor
Flavor is defined as a blend of taste, smell, and touch, acting as a premonition on the safety and quality of the food being consumed.29, 30, 31 It is a perception with multiple inputs from additional sensations, including vision and hearing.32 , 33 The robust interaction between the gustatory and olfactory areas of the brain is well known.34, 35, 36, 37 There is a blend of olfaction, gustation, and texture in most of the taste perception (Figure 4 ).38 , 39 The orbitofrontal cortex, the basolateral amygdala, and the insular cortex have been reported to be associated with interactions among odor, taste, and flavor.40 , 41 Odors conditioning and enhancing specific tastes, such as saltiness, have been reported in studies during the past few decades.42 , 43
Figure 4.
Pathways of olfaction, gustation, and the concept of flavor. Figure courtesy of Dr. Srishti Parekh.
Individual Taste Sensations
The analgesic effect of sweet sensation may have a role in activating the endogenous opioid system; in addition, endocannabinoids have been reported to change taste perception by means of modulating sweet receptors.44 This might explain the preference for sweet foods in patients with chronic pain syndromes, such as burning mouth syndrome (BMS). The role of sweet sensation in regulating food intake, glucose homeostasis, and energy balance may be instrumental in the discovery of newer drugs for diseases such as diabetes and obesity.45, 46, 47 Sour taste is generally considered to be an indicator of acid (hydrogen ions). The presence of salt-sensing cells in the taste buds in fungiform papillae and their absence in the circumvallate papillae, has been confirmed.48, 49, 50 Salt sensation has a crucial role to play in the normal development of the gustatory circuits in the central nervous system.51 The role of tongue cleaning and its positive effect on the identification of salt sensation has been well known.52 , 53 Researchers have proposed that salt sensitivity in people with normal blood pressure could predict their susceptibility to hypertension.54, 55, 56
Umami (glutamic acid) occurs naturally in a variety of foods, including tomatoes, seafood, and egg yolks.57 , 58 Multiple umami receptors have been identified in rodents in the past 2 decades, and the taste is mediated through glossopharyngeal nerve.59, 60, 61, 62, 63, 64 Certain published articles refer to a positive aspect of umami taste in terms of improvement of nutritional intake, enhancement of habitual choice of healthy foods, and improvement of food flavor.65, 66, 67, 68, 69 The proposed negative effects of umami include hepatotoxicity, asthma, migraine headache, and central nervous system damage.70, 71, 72, 73 Owing to the finding that a reduction in umami taste perception may trigger loss of appetite, subsequent health issues, and reduction in the quality of life, testing for this taste has been proposed to be one of the key tools for managing the treatment of patients with dysgeusia.74
The bitter taste receptors that are present in the respiratory tract have been proposed to have crucial roles in the body’s defense mechanism against possible invading microbes.16 Their role in the possible detection of certain bacteria and in the process of bronchodilation has also been proposed and could become a novel target for therapy. Through the research of the previous 2 decades, we have come to know that approximately 30 taste receptor genes code for the bitter taste.17 , 75 Fat taste (oleogustus) is one of the newer described taste sensations and is proposed to be carried via the glossopharyngeal and chorda tympani nerves.76, 77, 78 Calcium taste has been found to help modulate calcium metabolism, intake, and homeostasis in mammals and has specific receptors coded via multiple genes.79, 80, 81, 82, 83, 84
Epidemiology of Gustatory Disorders
Prevalence rates for taste disorders have been reported as ranging from 0.6% through 20% in the literature.85, 86, 87 Researchers have found that, in general, women perceive taste better.88 Researchers from multiple studies have reported that there is a decline in taste perception with increasing age.88 , 89 There is a reported reduction in prevalence of taste dysfunctions with aging in women compared with men.85 Researchers reported a strange association of taste disorders with educational attainment, in that the higher the level of education, the lower the incidence of taste disorders.85 There was similar association reported with olfactory disorders; however, the researchers failed to provide a plausible explanation for this phenomena. Sour tasting ability was consistently found to be most affected with aging.90 Liu and colleagues85 found that Black participants in their study had a higher prevalence of taste disorders, suggesting an association between ethnicity and taste disorders.
Etiopathology of Gustatory Disorders
Taste disorders can range from dysgeusia (abnormal taste sensation) to ageusia (complete loss of taste sensation), depending on the degree of severity of the taste abnormality. The various terms used for the types of taste disorders are provided in Table 1 . The total experience of taste has olfaction as a major component.91 , 92 Most apparent gustatory dysfunctions (such as dysgeusia) are the result of impaired olfaction (for example, allergies) rather than gustation.91, 92, 93, 94 Abnormalities in transport (inability of the tastant to reach the receptor), gustatory unit (abnormality of peripheral sensory organs), and neuronal unit (such as damage to the peripheral or central nervous system) are the mechanisms of such gustatory dysfunctions.92 , 95, 96, 97 In the oral cavity, the gustatory receptors can be exposed to pathologies (such as inflammation) or artificial restorative dental materials (such as acrylic) and can thereby affect gustation.92 , 98, 99, 100
Table 1.
Taste terminology.
| TASTE TERMINOLOGY | DESCRIPTION |
|---|---|
| Ageusia | Complete loss of taste sensation38,102 |
| Aliageusia | Bad taste to normally considered “good” taste105 |
| Dysgeusia | General term used for any abnormal taste sensation104 |
| Gustation | The scientific term given for the process of taste sensation95 |
| Hypergeusia | Increased sense of taste38 |
| Hypogeusia | Diminished sense of taste38 |
| Parageusia | Altered taste sensation in the presence of a tastant103 |
| Phantogeusia | Taste sensation in the absence of a tastant103 |
| Presbygeusia | An alteration in taste sensation considered as a result of aging106 |
| Tastant | Any chemical that elicits taste sensation101 |
| Taste Agnosia | A term used when a person cannot recognize a taste sensation, independent of normalcy in intellect, sensory processing, and linguistic abilities107 |
Several genes and their associated receptors (for example, ENaC, T1R, T2R, TAS2R, and TRPV1) have been identified as coding for specific taste sensations.25 , 45 , 108, 109, 110, 111, 112, 113, 114 Conceivably, any sequence variation in these receptors and genes can cause a taste disorder. Researchers have reported that more than 50% of the drugs prescribed routinely in the United States cause some level of a taste disorder.115 The various medications that can cause dysgeusia are summarized in Table 2 . Aging is also a factor for reduction in taste sensation.92
Table 2.
Types of medications causing dysgeusia.
| TYPE OF DRUGS | REPRESENTATIVE DRUGS |
|---|---|
| Antianemic | Ferric carboxymaltose116, 117, 118 |
| Antibacterial | Ampicillin, ciprofloxacin, metronidazole116, 117, 118 |
| Anticholinergic | Atropine116, 117, 118 |
| Antidepressant | Amitriptyline, clomipramine, desipramine116, 117, 118 |
| Antiepileptic | Carbamazepine, phenytoin116, 117, 118 |
| Antifungal | Griseofulvin116, 117, 118 |
| Antihistamine and Decongestant | Chlorphenamine, loratadine116, 117, 118 |
| Antihypertensive and Cardiac Medications | Acetazolamide, amiodarone, propranolol, nitroglycerine116, 117, 118, 119, 120 |
| Anti-inflammatory | Dexamethasone116, 117, 118 |
| Antimanic | Lithium116, 117, 118 |
| Antimigraine | Dihydroergotamine, sumatriptan116, 117, 118 |
| Antineoplastic | Cisplatin, methotrexate, vincristine116, 117, 118 |
| Antiparkinsonian | Levodopa116, 117, 118 |
| Antipsychotic | Clozapine, trifluoperazine116, 117, 118 |
| Antiviral | Aciclovir, interferon116, 117, 118 |
| Anxiolytic | Alprazolam, buspirone116, 117, 118 |
| Bronchodilator | Bitolterol116, 117, 118 |
| Central Nervous System Stimulant | Amphetamine116, 117, 118 |
| Drugs for Peptic Ulcer and Gastroesophageal Reflux Disease | Esomeprazole116, 117, 118 |
| Hypnotic | Eszopiclone, zolpidem116, 117, 118 |
| Hypoglycemic | Metformin116, 117, 118 |
| Intestinal Anti-infective | Miconazole116, 117, 118 |
| Intestinal Anti-inflammatory | Budesonide116, 117, 118 |
| Lipid-Lowering | Atorvastatin116, 117, 118 |
| Muscle Relaxant | Baclofen, dantrolene116, 117, 118 |
| Pancreatic Enzyme Preparation | Pancrelipase116, 117, 118 |
| Stomatologic Preparation | Hydrogen peroxide116, 117, 118 |
| Smoking-Cessation Aids | Nicotine116, 117, 118 |
| Thyroid | Levothyroxine sodium, propylthiouracil116, 117, 118 |
Relevance in Medicine and Dentistry
As alluded to earlier, multiple cranial nerves are involved in the sensation of taste, including trigeminal, facial, glossopharyngeal, and vagus. Mediators of chemosensation of taste include, but are not limited to, the tongue (factors are health of the tongue and papillae of the tongue), saliva (quality, quantity), oral mucous membrane, nerves, and neurotransmitters.95 Consequently, any alterations in these factors can contribute to a change in taste perception. As would be expected, dentists are among the first clinicians that patients approach with taste disorders. The most common symptoms in these patients include dysgeusia, phantogeusia, or an accompanying burning sensation.121 Many dental-related factors have been hypothesized to cause taste alterations, including local trauma, medications, infections, dental restorations, and salivary gland dysfunctions.95 Radiation therapy of the head and neck area have also been found to result in dysgeusia.122 Dysgeusia is also associated with several orofacial pain conditions, such as BMS and temporomandibular disorders.123, 124, 125, 126 Researchers have also reported an association between dysgeusia and other conditions, such as Bell palsy and Guillain-Barré syndrome.127, 128, 129 The relevance of dysgeusia in the context of the COVID-19 pandemic is explained below.
Systemic conditions affecting taste
Dysgeusia has been found to be associated with several systemic medical conditions, however, the mechanisms contributing to it are diverse. Systemic diseases commonly exhibiting taste dysfunction include, but are not limited to, Sjögren syndrome (SS), chronic renal failure, end-stage liver disease, endocrine disturbances (such as diabetes mellitus and thyroid disorders), genetic disorders (familial dysautonomia), neurologic disorders, and psychiatric conditions. Alterations in taste function have also been found in pregnant and postmenopausal women.99 The conditions that are related to taste disorders etiopathologically and their proposed mechanisms are summarized in Table 3 .
Table 3.
Etiology of gustatory disorders: proposed mechanisms of action.
| DISORDER | PROPOSED MECHANISM OF ACTION |
|---|---|
| Aging and Cognition130,131 | Cognitive processes like language and memory involved in taste recognition impaired in older adults |
| Increased bitterness threshold in older adults | |
| Bacterial Infections132 | Alteration of gene expression by stimulation of Toll-like receptors |
| Cancer Treatment97 | Damage to and reduced turnover of taste receptor cells |
| Loss of connectivity between receptor cells and neurons | |
| Direct neuronal damage | |
| Chronic Kidney Disease96,133,134 | Raised salivary sodium, salivary urea, salivary bicarbonate |
| Reduced salivary zinc | |
| Uremic toxins | |
| Altered axon conduction | |
| Impaired cognitive status | |
| Chronic Rhinosinusitis135,136 | Altered functionality of T2R38 bitter taste receptor |
| Chemotherapy137 | Alteration in zinc metabolism |
| Carbonic anhydrase VI deficiency | |
| Consumption of Ethyl Alcohol14,138 | Change in taste receptor sensitivity |
| Abnormalities in micronutrient absorption | |
| Changes in saliva | |
| Alterations in taste buds | |
| COVID-19 (Severe Acute Respiratory Syndrome Coronavirus 2)14,139, 140, 141, 142, 143, 144 | Damage to the central nervous system by the virus attaching to the angiotensin-converting enzyme 2 receptors in glial cells and spinal neurons |
| Abnormal zinc homeostasis | |
| Increased proinflammatory cytokines | |
| Direct infection of cells in the tongue | |
| Consequences of obstruction of taste cells chemesthesis due to inflammation and damage to cranial nerves VII, IX, and X | |
| Diabetes Mellitus145, 146, 147 | Xerostomia secondary to diabetes mellitus |
| Diabetic neuropathy | |
| Reduction in innervation of taste buds | |
| Increased apoptosis of taste buds | |
| Lower density of the fungiform papillae | |
| Drug Induced14,99,148, 149, 150 | Hyposalivation |
| Adverse effects on taste receptors | |
| Adverse effects on taste neural pathway | |
| Abnormalities in neurotransmitter function | |
| Fungal Infection, Candida albicans99,151 | Mechanical barrier by formation of pseudomembrane due to disproportional overgrowth |
| Genetic Disorders14,152, 153, 154 | Reduced number of taste papillae |
| Changes in taste phenotypes | |
| HIV and AIDS99,155,156 | Directly affects gustatory neurons |
| Development of local lesions | |
| Xerostomia | |
| Chemosensory alterations secondary to antiretroviral therapy | |
| Neuronal degeneration secondary to HIV leukoencephalopathy | |
| Liver Failure99 | Deficiency of vitamins B and C, zinc, and copper |
| Multiple Sclerosis99,157 | Central demyelination |
| Neurodegenerative Disorders (Dementia, Alzheimer, and Others)147,158,159 | Insular atrophy |
| Secondary to effect of medications used for management | |
| Amyloid deposition in the insula and amygdala | |
| ParkinsonDisease147,160, 161, 162 | Neurodegenerative changes of the frontal operculum or orbitofrontal cortex |
| Other concomitant factors: depression, poor oral hygiene, gastrointestinal disease, and zinc deficiency | |
| Rheumatoid Arthritis163 | Neuropathy of terminal nerve fibers |
| Neuropathy secondary to rheumatoid arthritis drugs | |
| SjögrenSyndrome99 | Excessive dryness of the oral cavity and hyperviscosity of saliva |
| Stroke99,164 | Injury to the insula, pons, thalamus, midbrain, and internal capsule |
| Systemic Lupus Erythematosus165,166 | Proinflammatory cytokines |
| Inhibition of turnover of taste bud cells by inflammation | |
| Thyroid Disorder167,168 | Zinc deficiency secondary to thyroid disease |
| Reduced cognitive functions | |
| Toxins: Environmental169,170 | Chemosensory dysfunction |
|
|
|
|
| Affecting blood flow to tongue | |
| Direct interaction with saliva | |
| Heavy metals concentration in saliva producing harmful changes in taste receptors | |
| Viral Diseases14,132 | Nasal blockage; increased mucus production |
| Changes in olfaction | |
| Inflammation affecting gene expression in taste bud cells |
Dysgeusia in autoimmune disorders
There are contradicting reports on the association between reduced salivary flow and taste disorders.171, 172, 173 SS, an autoimmune disorder with associated xerostomia, has been reported to include taste disorders as well.174 The mechanisms proposed to explain dysgeusia associated with SS include systemic inflammation, interaction with genetic pathways that influence gustation, an increase in the taste threshold, and small fiber neuropathy.175 , 176 Possibly due to the fact that sweet taste is independent of salivation, there was minimal alteration of the sweet taste in patients with SS.177 , 178 Other dysgeusia-associated disorders include autoimmune encephalitis, myasthenia gravis, systemic lupus erythematosus, multiple sclerosis, and Parkinson disease.132 , 179, 180, 181, 182
Dysgeusia associated with BMS
Taste alterations accompany BMS.183, 184, 185 These include phantogeusia (bitter, metallic, burning quality).186, 187, 188, 189 The qualities of bitter and metallic that patients report as being associated with BMS are thought to be from cranial nerve IX being disinhibited subsequent to possible damage or hypofunction of the chorda tympani nerve.185 , 187 , 189 Consequently, it has been reported in the literature that patients with BMS find relief from symptoms when consuming sugary or sweet foods, which possibly stimulate a hypofunctioning chorda tympani nerve.188 , 190 , 191 There is a reported increase in taste threshold (that is, decreased taste sensitivity) of all tastes except umami.187 , 192, 193, 194 An increased fungiform papillae count has been proposed to be associated with “supertasters,” who are apparently at a higher risk of having BMS.195 , 196 Psychological factors have also been implicated in dysgeusia related to BMS.197 , 198
Dysgeusia associated with infections
Diseases such as upper respiratory infections, viral hepatitis, and oral cavity infections have been associated with an increase in the detection and identification of individual taste stimuli via taste buds, thereby causing dysgeusia.132 , 199, 200, 201, 202, 203 Inflammation has been found to disrupt normal cell turnover of taste buds, leading to dysgeusia.132 HIV infections and subsequent therapies have been reported to be associated with dysgeusia.155 , 204 , 205 Other infections reportedly associated with dysgeusia include, but are not limited to, rhinosinusitis, hepatitis E, Helicobacter pylori, Lyme disease, leprosy, syphilis, and cytomegalovirus.99 , 136 , 206 , 207
COVID-19 and Dysgeusia
Although the COVID-19 pandemic began in early 2020, the data regarding anosmia and dysgeusia in the context of the association, pathogenesis, diagnosis, and prognosis of the disease are limited.208 A change in taste sensation is one of the earliest symptoms of COVID-19 and many times precedes the actual respiratory manifestation of the disease.209, 210, 211, 212, 213 Multiple hypotheses have been proposed in an attempt to explain the mechanistic pathway of how the COVID-19 organism affects gustatory senses.209 , 214 These include, but are not limited to, damage to the central nervous system, abnormal zinc homeostasis, angiotensin-converting enzyme 2 receptor manifestation, and increased proinflammatory cytokines.14 , 139, 140, 141, 142, 143 There is a high rate of sensation recovery rate in patients with COVID-19; however, whether more permanent loss of taste sensation occurs with COVID-19 infection is still unclear.215 , 216 The higher prevalence of taste alterations associated with the pandemic has prompted organizations, such as the European Centre for Disease Prevention and Control, the American Academy of Otolaryngology–Head and Neck Surgery, and the World Health Organization, to recommend using taste alterations as a screening test and, when screening is positive, advise the clinician to test the patient for COVID-19.215 , 217 , 218 Identification of gustatory disturbances in the absence of concomitant symptoms of COVID-19 is of paramount importance to alerting physicians about the possibility of COVID-19, thereby aiding in self-isolating and testing the people affected.219
Diagnostic Testing for Gustation
Various taste-testing measures have been used, ranging from simple chairside testing to objective and measured research-level testing for gustation. In a clinical setup, a clinician may use such tastants as a dilute aqueous solution of sugar, salt, sour, and bitter, although this method is subjective and lacks sufficient sensitivity and specificity. Objective testing can be done using electrogustometry, filter paper disk method, whole mouth method, taste strip method, and e-tongue method. The electrogustometry method tests the taste sensory nerves by means of measuring the electrical taste threshold in the soft-tissue areas supplied via the chorda tympani, greater petrosal, and glossopharyngeal nerves.220 , 221 In the filter paper disk method, test disks soaked in different taste solutions are placed on selected parts of the oral cavity.220 , 221 These tastes are applied in increasing concentration grades from the lowest to a point at which the participant identifies the correct taste. In the whole mouth method, participants rinse their mouths with multiple taste solutions with various molarities and identify the taste. This method also tests for taste threshold.220 In the taste strip method, filter paper strips embedded with the 4 basic tastes are used and placed in successive graded concentrations at the center of the anterior one-third of the tongue when the tongue is protruded.220 , 222 The e-tongue method is used mostly in pharmaceutical research and uses an analytical instrument that helps interpret the data from a sensor that records electrical signals from the taste fibers.223
Effect of Taste and Smell Disorders on Nutrition
Taste and smell sensations are closely interrelated when it comes to motivation to eat, enjoyment of food, and activation of the satiety centers of the brain.224 Induction of abnormal eating habits by means of olfactory disorders has been documented in the literature.29 Although taste disorders have been reported to affect body weight, evidence is lacking to link the former to an abnormal nutritional status.224 , 225 Patients with dysgeusia do not have a substantial change in their nutrition.226 , 227 Systemic diseases, such as diabetes and hypertension, have been reported to be associated with changes in the chemosensory function.224 There have also been several researchers who have linked ageusia to weight loss and even to anorexia.228, 229, 230, 231, 232, 233, 234 Substantial taste disturbances, such as reduction in taste acuity, abnormal salt taste, and metallic taste, have been proposed to cause noncompliance with recommended renal diets in patients with chronic kidney disease, thereby culminating in nutritional deficiencies.235, 236, 237 The effect of dysgeusia on quality of life has been reported as varied in the literature, however, most researchers report considerable reductions in quality of life.238 , 239
Clinical Pearls
Taste alterations, as one of the earliest symptoms of COVID-19, can be used as a simple, early screening tool for detection of the infection. The simplest tool for testing olfaction is to have the patient respond to smelling small amounts of odorants that are easily identifiable, such as coffee, fragrant soap, or other nonpungent material. Care must be taken to ascertain that the patient is not experiences any nasal blockage, such as would occur with a common cold. In conjunction, the sense of taste can be checked easily by means of simply having the patient taste (sip and spit) dilute aqueous solutions of sugar, salt, sour, and bitter, as we mentioned above. The use of commercially available, more sophisticated kits is not usually warranted, as these techniques, such as electrogustometry, may be beyond the purview of a routine dental practice setup. Clinicians should understand that it is unlikely the patient will pick up minimal alterations of gustation unless prompted by the screening dentist. In the era of COVID-19, it may be prudent for dental clinicians to screen every patient routinely for olfactory and gustatory changes using a simple questionnaire with yes or no answers. The clinician should keep in mind that, in addition to COVID-19, several other conditions, including systemic diseases and medications, may be related etiologically to gustatory disorders. In the event that gustatory screening is positive for dysgeusia, the patient should be referred promptly for COVID-19 screening. If the patient is negative for COVID-19 and dysgeusia continues, the patient should be referred to a special senses clinic, possibly working together with the patient’s primary care physician.
Also, we should understand that taste disorders contribute considerably to reduced quality of life in patients. Screening for taste alterations can form the foundation for an early warning and detection system that a dentist can use to attempt to quell the spread and effects of COVID.
Conclusions
Gustatory disturbances are prevalent in several systemic disorders and infections, including COVID-19. Taste disorders, regardless of the etiologic factor, affect the quality of life of patients in general and of patients with COVID-19 in particular. Screening and identification of possible taste disorders should form the standard of care for dental clinicians, especially in the context of the COVID-19 pandemic. Early recognition of gustatory disorders may be crucial in identifying possible infection with the COVID-19 virus in the dental patient population. Dental clinicians must be aware of the fact that multiple factors (in addition to COVID-19) may be instrumental in the etiology and pathophysiology of dysgeusia, and a comprehensive workup of the case is needed to reach a conclusion about etiology. Knowing the short- and long-term consequences of COVID-19 infection, early screening, and identification are instrumental in potentially changing the life of a patient for the better.
Biographies
Dr. Thomas is an assistant professor, Center for Temporomandibular Disorders and Orofacial Pain, Department of Diagnostic Sciences, Rutgers School of Dental Medicine, Newark, NJ.
Dr. Chablani is an assistant professor, Department of Oral and Maxillofacial Surgery, Terna Dental College, Navi Mumbai, India.
Dr. Parekh is a periodontist in private practice, Mumbai, India.
Dr. Pichammal is a dentist in private practice, Chennai, India.
Dr. Shanmugasundaram is a professor and the head, Department of Oral Medicine and Maxillofacial Radiology, Seema Dental College and Hospital, Rishikesh, Uttarakhand, India.
Dr. Pitchumani is a postgraduate resident, Department of Periodontology, Ohio State University College of Dentistry, Columbus, OH.
Footnotes
References
- 1.Hall J.E., Hall M.E. Elsevier; 2020. Guyton and Hall Textbook of Medical Physiology. [Google Scholar]
- 2.Pomfrett C.J. Special senses. Anaesth Intensive Care Med. 2009;10(3):155–157. [Google Scholar]
- 3.Sturkie P.D. Springer; 1981. Basic Physiology. [Google Scholar]
- 4.Taruno A., Nomura K., Kusakizako T., Ma Z., Nureki O., Foskett J.K. Taste transduction and channel synapses in taste buds. Pflugers Arch. 2021;473(1):3–13. doi: 10.1007/s00424-020-02464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhari N., Roper S.D. The cell biology of taste. J Cell Biol. 2010;190(3):285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breslin P.A. An evolutionary perspective on food and human taste. Curr Biol. 2013;23(9):R409–R418. doi: 10.1016/j.cub.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witt M. Anatomy and development of the human taste system. Handb Clin Neurol. 2019;164:147–171. doi: 10.1016/B978-0-444-63855-7.00010-1. [DOI] [PubMed] [Google Scholar]
- 8.de Araujo I.E., Simon S.A. The gustatory cortex and multisensory integration. Int J Obes (Lond) 2009;33(suppl 2):S34–S43. doi: 10.1038/ijo.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rolls E.T. Functions of the orbitofrontal and pregenual cingulate cortex in taste, olfaction, appetite and emotion. Acta Physiol Hung. 2008;95(2):131–164. doi: 10.1556/APhysiol.95.2008.2.1. [DOI] [PubMed] [Google Scholar]
- 10.Rolls E.T. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55(1):11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- 11.Avery J.A., Liu A.G., Ingeholm J.E., Riddell C.D., Gotts S.J., Martin A. Taste quality representation in the human brain. J Neurosci. 2020;40(5):1042–1052. doi: 10.1523/JNEUROSCI.1751-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rolls E.T. Taste and smell processing in the brain. Handb Clin Neurol. 2019;164:97–118. doi: 10.1016/B978-0-444-63855-7.00007-1. [DOI] [PubMed] [Google Scholar]
- 13.Avery J.A., Gotts S.J., Kerr K.L., et al. Convergent gustatory and viscerosensory processing in the human dorsal mid-insula. Hum Brain Mapp. 2017;38(4):2150–2164. doi: 10.1002/hbm.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risso D., Drayna D., Morini G. Alteration, reduction and taste loss: main causes and potential implications on dietary habits. Nutrients. 2020;12(11):3284. doi: 10.3390/nu12113284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbertson T.A. The physiology of vertebrate taste reception. Curr Opin Neurobiol. 1993;3(4):532–539. doi: 10.1016/0959-4388(93)90052-z. [DOI] [PubMed] [Google Scholar]
- 16.Jeruzal-Świątecka J., Fendler W., Pietruszewska W. Clinical role of extraoral bitter taste receptors. Int J Mol Sci. 2020;21(14):5156. doi: 10.3390/ijms21145156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloxham C.J., Foster S.R., Thomas W.G. A bitter taste in your heart. Front Physiol. 2020;11:431. doi: 10.3389/fphys.2020.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Governini L., Semplici B., Pavone V., et al. Expression of taste receptor 2 subtypes in human testis and sperm. J Clin Med. 2020;9(1):264. doi: 10.3390/jcm9010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cont G., Paviotti G., Montico M., et al. TAS2R38 bitter taste genotype is associated with complementary feeding behavior in infants. Genes Nutr. 2019;14:13. doi: 10.1186/s12263-019-0640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freund J.R., Mansfield C.J., Doghramji L.J., et al. Activation of airway epithelial bitter taste receptors by Pseudomonas aeruginosa quinolones modulates calcium, cyclic-AMP, and nitric oxide signaling. J Biol Chem. 2018;293(25):9824–9840. doi: 10.1074/jbc.RA117.001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wölfle U., Elsholz F.A., Kersten A., Haarhaus B., Müller W.E., Schempp C.M. Expression and functional activity of the bitter taste receptors TAS2R1 and TAS2R38 in human keratinocytes. Skin Pharmacol Physiol. 2015;28(3):137–146. doi: 10.1159/000367631. [DOI] [PubMed] [Google Scholar]
- 22.Clark A.A., Dotson C.D., Elson A.E., et al. TAS2R bitter taste receptors regulate thyroid function. FASEB J. 2015;29(1):164–172. doi: 10.1096/fj.14-262246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lund T.C., Kobs A.J., Kramer A., et al. Bone marrow stromal and vascular smooth muscle cells have chemosensory capacity via bitter taste receptor expression. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0058945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orsmark-Pietras C., James A., Konradsen J.R., et al. Transcriptome analysis reveals upregulation of bitter taste receptors in severe asthmatics. Eur Respir J. 2013;42(1):65–78. doi: 10.1183/09031936.00077712. [DOI] [PubMed] [Google Scholar]
- 25.Kinnamon S.C., Finger T.E. Recent advances in taste transduction and signaling. F1000Res. 2019;8 doi: 10.12688/f1000research.21099.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gravina S.A., Yep G.L., Khan M. Human biology of taste. Ann Saudi Med. 2013;33(3):217–222. doi: 10.5144/0256-4947.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandrashekar J., Hoon M.A., Ryba N.J., Zuker C.S. The receptors and cells for mammalian taste. Nature. 2006;444(7117):288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 28.Avery J.A. Against gustotopic representation in the human brain: there is no Cartesian restaurant. Curr Opin Physiol. 2021;20:23–28. doi: 10.1016/j.cophys.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas D.C., Baddireddy S.M., Kohli D. Anosmia: a review in the context of coronavirus disease 2019 and orofacial pain. JADA. 2020;151(9):696–702. doi: 10.1016/j.adaj.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallowell E.S., Parikh R., Veldhuizen M.G., Marks L.E. Flavor identification and intensity: effects of stimulus context. Chem Senses. 2016;41(3):249–259. doi: 10.1093/chemse/bjv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson R.J. Flavor binding: its nature and cause. Psychol Bull. 2014;140(2):487–510. doi: 10.1037/a0033473. [DOI] [PubMed] [Google Scholar]
- 32.Auvray M., Spence C. The multisensory perception of flavor. Conscious Cogn. 2008;17(3):1016–1031. doi: 10.1016/j.concog.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Small D.M., Prescott J. Odor/taste integration and the perception of flavor. Exp Brain Res. 2005;166(3-4):345–357. doi: 10.1007/s00221-005-2376-9. [DOI] [PubMed] [Google Scholar]
- 34.Sinding C., Thibault H., Hummel T., Thomas-Danguin T. Odor-induced saltiness enhancement: insights into the brain chronometry of flavor perception. Neuroscience. 2021;452:126–137. doi: 10.1016/j.neuroscience.2020.10.029. [DOI] [PubMed] [Google Scholar]
- 35.Maier J.X. Single-neuron responses to intraoral delivery of odor solutions in primary olfactory and gustatory cortex. J Neurophysiol. 2017;117(3):1293–1304. doi: 10.1152/jn.00802.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maier J.X., Blankenship M.L., Li J.X., Katz D.B. A multisensory network for olfactory processing. Curr Biol. 2015;25(20):2642–2650. doi: 10.1016/j.cub.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maier J.X., Wachowiak M., Katz D.B. Chemosensory convergence on primary olfactory cortex. J Neurosci. 2012;32(48):17037–17047. doi: 10.1523/JNEUROSCI.3540-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hummel T., Landis B.N., Hüttenbrink K.B. Smell and taste disorders. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2011;10:Doc04. doi: 10.3205/cto000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hüttenbrink K.B. Disorders of the sense of smell and taste. Article in German. Ther Umsch. 1995;52(11):732–737. [PubMed] [Google Scholar]
- 40.Miranda M.I. Taste and odor recognition memory: the emotional flavor of life. Rev Neurosci. 2012;23(5-6):481–499. doi: 10.1515/revneuro-2012-0064. [DOI] [PubMed] [Google Scholar]
- 41.de Araujo I.E., Rolls E.T., Kringelbach M.L., McGlone F., Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 2003;18(7):2059–2068. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhou T., Feng Y., Thomas-Danguin T., Zhao M. Enhancement of saltiness perception by odorants selected from Chinese soy sauce: a gas chromatography/olfactometry-associated taste study. Food Chem. 2021;335:127664. doi: 10.1016/j.foodchem.2020.127664. [DOI] [PubMed] [Google Scholar]
- 43.Thomas-Danguin T., Guichard E., Salles C. Cross-modal interactions as a strategy to enhance salty taste and to maintain liking of low-salt food: a review. Food Funct. 2019;10(9):5269–5281. doi: 10.1039/c8fo02006j. [DOI] [PubMed] [Google Scholar]
- 44.Beauchamp G.K. Why do we like sweet taste: a bitter tale? Physiol Behav. 2016;164(Pt B):432–437. doi: 10.1016/j.physbeh.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee A.A., Owyang C. Sugars, sweet taste receptors, and brain responses. Nutrients. 2017;9(7):653. doi: 10.3390/nu9070653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kojima I., Nakagawa Y. The role of the sweet taste receptor in enteroendocrine cells and pancreatic β-cells. Diabetes Metab J. 2011;35(5):451–457. doi: 10.4093/dmj.2011.35.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakagawa Y., Nagasawa M., Yamada S., et al. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS One. 2009;4(4) doi: 10.1371/journal.pone.0005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohmoto M., Jyotaki M., Foskett J.K., Matsumoto I. Sodium-taste cells require Skn-1a for generation and share molecular features with sweet, umami, and bitter taste cells. eNeuro. 2020;7(6) doi: 10.1523/ENEURO.0385-20.2020. ENEURO.0385-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nomura K., Nakanishi M., Ishidate F., Iwata K., Taruno A. All-electrical Ca(2+)-independent signal transduction mediates attractive sodium taste in taste buds. Neuron. 2020;106(5):816–829.e6. doi: 10.1016/j.neuron.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Chandrashekar J., Kuhn C., Oka Y., et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464(7286):297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skyberg R., Sun C., Hill D.L. Selective removal of sodium salt taste disrupts the maintenance of dendritic architecture of gustatory relay neurons in the mouse nucleus of the solitary tract. eNeuro. 2020;7(5) doi: 10.1523/ENEURO.0140-20.2020. ENEURO.0140-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seerangaiyan K., Jüch F., Atefeh F., Winkel E.G. Tongue cleaning increases the perceived intensity of salty taste. J Nutr Health Aging. 2018;22(7):802–804. doi: 10.1007/s12603-018-1030-8. [DOI] [PubMed] [Google Scholar]
- 53.Quirynen M., Avontroodt P., Soers C., Zhao H., Pauwels M., van Steenberghe D. Impact of tongue cleansers on microbial load and taste. J Clin Periodontol. 2004;31(7):506–510. doi: 10.1111/j.0303-6979.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 54.Farquhar W.B., Edwards D.G., Jurkovitz C.T., Weintraub W.S. Dietary sodium and health: more than just blood pressure. J Am Coll Cardiol. 2015;65(10):1042–1050. doi: 10.1016/j.jacc.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sullivan J.M. Salt sensitivity: definition, conception, methodology, and long-term issues. Hypertension. 1991;17(1 suppl):I61–I68. doi: 10.1161/01.hyp.17.1_suppl.i61. [DOI] [PubMed] [Google Scholar]
- 56.Weinberger M.H., Fineberg N.S. Sodium and volume sensitivity of blood pressure: age and pressure change over time. Hypertension. 1991;18(1):67–71. doi: 10.1161/01.hyp.18.1.67. [DOI] [PubMed] [Google Scholar]
- 57.Hartley I.E., Liem D.G., Keast R. Umami as an ‘alimentary' taste: a new perspective on taste classification. Nutrients. 2019;11(1):182. doi: 10.3390/nu11010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurihara K. Umami the fifth basic taste: history of studies on receptor mechanisms and role as a food flavor. Biomed Res Int. 2015;2015:189402. doi: 10.1155/2015/189402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hellekant G., Schmolling J., Marambaud P., Rose-Hellekant T.A. CALHM1 deletion in mice affects glossopharyngeal taste responses, food intake, body weight, and life span. Chem Senses. 2015;40(6):373–379. doi: 10.1093/chemse/bjv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Behrens M., Meyerhof W., Hellfritsch C., Hofmann T. Sweet and umami taste: natural products, their chemosensory targets, and beyond. Angew Chem Int Ed Engl. 2011;50(10):2220–2242. doi: 10.1002/anie.201002094. [DOI] [PubMed] [Google Scholar]
- 61.Kitagawa J., Takahashi Y., Matsumoto S., Shingai T. Response properties of the pharyngeal branch of the glossopharyngeal nerve for umami taste in mice and rats. Neurosci Lett. 2007;417(1):42–45. doi: 10.1016/j.neulet.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 62.San Gabriel A., Uneyama H., Yoshie S., Torii K. Cloning and characterization of a novel mGluR1 variant from vallate papillae that functions as a receptor for L-glutamate stimuli. Chem Senses. 2005;30(suppl 1):i25–i26. doi: 10.1093/chemse/bjh095. [DOI] [PubMed] [Google Scholar]
- 63.Li X., Staszewski L., Xu H., Durick K., Zoller M., Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99(7):4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaudhari N., Landin A.M., Roper S.D. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci. 2000;3(2):113–119. doi: 10.1038/72053. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y., Zhang L., Venkitasamy C., et al. Potential effects of umami ingredients on human health: pros and cons. Crit Rev Food Sci Nutr. 2020;60(13):2294–2302. doi: 10.1080/10408398.2019.1633995. [DOI] [PubMed] [Google Scholar]
- 66.Magerowski G., Giacona G., Patriarca L., et al. Neurocognitive effects of umami: association with eating behavior and food choice. Neuropsychopharmacology. 2018;43(10):2009–2016. doi: 10.1038/s41386-018-0044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kubota M., Toda C., Nagai-Moriyama A. Relationship between umami taste acuity with sweet or bitter taste acuity and food selection in Japanese women university students. Asia Pac J Clin Nutr. 2018;27(1):107–112. doi: 10.6133/apjcn.042017.01. [DOI] [PubMed] [Google Scholar]
- 68.Qi J., Liu D.Y., Zhou G.H., Xu X.L. Characteristic flavor of traditional soup made by stewing Chinese yellow-feather chickens. J Food Sci. 2017;82(9):2031–2040. doi: 10.1111/1750-3841.13801. [DOI] [PubMed] [Google Scholar]
- 69.Dermiki M., Prescott J., Sargent L.J., Willway J., Gosney M.A., Methven L. Novel flavours paired with glutamate condition increased intake in older adults in the absence of changes in liking. Appetite. 2015;90:108–113. doi: 10.1016/j.appet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 70.Rosa S.G., Chagas P.M., Pesarico A.P., Nogueira C.W. Monosodium glutamate induced nociception and oxidative stress dependent on time of administration, age of rats and susceptibility of spinal cord and brain regions. Toxicol Appl Pharmacol. 2018;351:64–73. doi: 10.1016/j.taap.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 71.Sharmin F., Jeong B.G., Jung J., Quines V., Chun J. Cholesterol analysis of Korean eat-out foods for national food composition database. J Food Sci Technol. 2017;54(7):1837–1849. doi: 10.1007/s13197-017-2615-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimada A., Castrillon E.E., Baad-Hansen L., et al. Increased pain and muscle glutamate concentration after single ingestion of monosodium glutamate by myofascial temporomandibular disorders patients. Eur J Pain. 2016;20(9):1502–1512. doi: 10.1002/ejp.874. [DOI] [PubMed] [Google Scholar]
- 73.Yoneda J., Chin K., Torii K., Sakai R. Effects of oral monosodium glutamate in mouse models of asthma. Food Chem Toxicol. 2011;49(1):299–304. doi: 10.1016/j.fct.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 74.Satoh-Kuriwada S., Kawai M., Iikubo M., et al. Development of an umami taste sensitivity test and its clinical use. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0095177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Behrens M., Meyerhof W. Mammalian bitter taste perception. Results Probl Cell Differ. 2009;47:203–220. doi: 10.1007/400_2008_5. [DOI] [PubMed] [Google Scholar]
- 76.Andersen C.A., Nielsen L., Møller S., Kidmose P. Cortical response to fat taste. Chem Senses. 2020;45(4):283–291. doi: 10.1093/chemse/bjaa019. [DOI] [PubMed] [Google Scholar]
- 77.Gaillard D., Kinnamon S.C. New evidence for fat as a primary taste quality. Acta Physiol (Oxf) 2019;226(1) doi: 10.1111/apha.13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mattes R.D. Is there a fatty acid taste? Annu Rev Nutr. 2009;29:305–327. doi: 10.1146/annurev-nutr-080508-141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Y.D., Dahanukar A. Recent advances in the genetic basis of taste detection in Drosophila. Cell Mol Life Sci. 2020;77(6):1087–1101. doi: 10.1007/s00018-019-03320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gerbino A., Colella M. The different facets of extracellular calcium sensors: old and new concepts in calcium-sensing receptor signalling and pharmacology. Int J Mol Sci. 2018;19(4):999. doi: 10.3390/ijms19040999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoshida R., Ninomiya Y. Taste information derived from T1R-expressing taste cells in mice. Biochem J. 2016;473(5):525–536. doi: 10.1042/BJ20151015. [DOI] [PubMed] [Google Scholar]
- 82.Tordoff M.G., Alarcón L.K., Valmeki S., Jiang P. T1R3: a human calcium taste receptor. Sci Rep. 2012;2:496. doi: 10.1038/srep00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cherukuri C.M., McCaughey S.A., Tordoff M.G. Comparison of differences between PWD/PhJ and C57BL/6J mice in calcium solution preferences and chorda tympani nerve responses. Physiol Behav. 2011;102(5):496–502. doi: 10.1016/j.physbeh.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tordoff M.G., Shao H., Alarcón L.K., et al. Involvement of T1R3 in calcium-magnesium taste. Physiol Genomics. 2008;34(3):338–348. doi: 10.1152/physiolgenomics.90200.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu G., Zong G., Doty R.L., Sun Q. Prevalence and risk factors of taste and smell impairment in a nationwide representative sample of the US population: a cross-sectional study. BMJ Open. 2016;6(11) doi: 10.1136/bmjopen-2016-013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vennemann M.M., Hummel T., Berger K. The association between smoking and smell and taste impairment in the general population. J Neurol. 2008;255(8):1121–1126. doi: 10.1007/s00415-008-0807-9. [DOI] [PubMed] [Google Scholar]
- 87.Hoffman H.J., Ishii E.K., MacTurk R.H. Age-related changes in the prevalence of smell/taste problems among the United States adult population: results of the 1994 disability supplement to the National Health Interview Survey (NHIS) Ann N Y Acad Sci. 1998;855:716–722. doi: 10.1111/j.1749-6632.1998.tb10650.x. [DOI] [PubMed] [Google Scholar]
- 88.Barragán R., Coltell O., Portolés O., et al. Bitter, sweet, salty, sour and umami taste perception decreases with age: sex-specific analysis, modulation by genetic variants and taste-preference associations in 18 to 80 year-old subjects. Nutrients. 2018;10(10):1539. doi: 10.3390/nu10101539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rawal S., Hoffman H.J., Bainbridge K.E., Huedo-Medina T.B., Duffy V.B. Prevalence and risk factors of self-reported smell and taste alterations: results from the 2011-2012 US National Health and Nutrition Examination Survey (NHANES) Chem Senses. 2016;41(1):69–76. doi: 10.1093/chemse/bjv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boesveldt S., Lindau S.T., McClintock M.K., Hummel T., Lundstrom J.N. Gustatory and olfactory dysfunction in older adults: a national probability study. Rhinology. 2011;49(3):324–330. doi: 10.4193/rhino10.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maheswaran T., Abikshyeet P., Sitra G., Gokulanathan S., Vaithiyanadane V., Jeelani S. Gustatory dysfunction. J Pharm Bioallied Sci. 2014;6(suppl 1):S30–S33. doi: 10.4103/0975-7406.137257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang L., Gillis-Smith S., Peng Y., et al. The coding of valence and identity in the mammalian taste system. Nature. 2018;558(7708):127–131. doi: 10.1038/s41586-018-0165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rathee M., Jain P. StatPearls Publishing; 2021. Ageusia. [PubMed] [Google Scholar]
- 94.Payne T., Kronenbuerger M., Wong G. StatPearls Publishing; 2021. Gustatory Testing. [PubMed] [Google Scholar]
- 95.Mortazavi H., Shafiei S., Sadr S., Safiaghdam H. Drug-related dysgeusia: a systematic review. Oral Health Prev Dent. 2018;16(6):499–507. doi: 10.3290/j.ohpd.a41655. [DOI] [PubMed] [Google Scholar]
- 96.Henkin R.I., Levy L.M., Fordyce A. Taste and smell function in chronic disease: a review of clinical and biochemical evaluations of taste and smell dysfunction in over 5000 patients at The Taste and Smell Clinic in Washington, DC. Am J Otolaryngol. 2013;34(5):477–489. doi: 10.1016/j.amjoto.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 97.Doty R., Bromley S. In: Neurological Disorders: Course and Treatment. 2nd ed. Brandt T., Caplan L., Dichgans J., Diener H.-C., Kennard C., editors. Academic Press; 2003. Anosmia, ageusia, and other disorders of chemosensation; pp. 171–183. [Google Scholar]
- 98.Doty R., Bromley S.M. In: Encyclopedia of the Neurological Sciences. Robert B.D., Michael J.A., editors. Elsevier; 2014. Taste; pp. 394–396. [Google Scholar]
- 99.Doty R.L. Treatments for smell and taste disorders: a critical review. Handb Clin Neurol. 2019;164:455–479. doi: 10.1016/B978-0-444-63855-7.00025-3. [DOI] [PubMed] [Google Scholar]
- 100.Allis T.J., Leopold D.A. Smell and taste disorders. Facial Plast Surg Clin North Am. 2012;20(1):93–111. doi: 10.1016/j.fsc.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 101.Hunt J.D., Reiter E.R., Costanzo R.M. Etiology of subjective taste loss. Int Forum Allergy Rhinol. 2019;9(4):409–412. doi: 10.1002/alr.22263. [DOI] [PubMed] [Google Scholar]
- 102.Malaty J., Malaty I.A. Smell and taste disorders in primary care. Am Fam Physician. 2013;88(12):852–859. [PubMed] [Google Scholar]
- 103.Chen S.I., Chiang C.L., Chao C.T., Chiang C.K., Huang J.W. Gustatory dysfunction is closely associated with frailty in patients with chronic kidney disease. J Ren Nutr. 2021;31(1):49–56. doi: 10.1053/j.jrn.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 104.Epstein J.B., Villines D., Epstein G.L., Smutzer G. Oral examination findings, taste and smell testing during and following head and neck cancer therapy. Support Care Cancer. 2020;28(9):4305–4311. doi: 10.1007/s00520-019-05232-y. [DOI] [PubMed] [Google Scholar]
- 105.da Silva R.O.C., Lacerda W.F., Henn I.W., Chaiben C.L., Machado M.A.N., de Lima A.A.S. Relationship between taste perception and use of upper complete dentures. Spec Care Dentist. 2021;41(2):244–250. doi: 10.1111/scd.12559. [DOI] [PubMed] [Google Scholar]
- 106.Bromley S.M. Neurolocalization of taste disorders. Handb Clin Neurol. 2019;164:303–323. doi: 10.1016/B978-0-444-63855-7.00019-8. [DOI] [PubMed] [Google Scholar]
- 107.Murtaza B., Hichami A., Khan A.S., Ghiringhelli F., Khan N.A. Alteration in taste perception in cancer: causes and strategies of treatment. Front Physiol. 2017;8:134. doi: 10.3389/fphys.2017.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ahmad R., Dalziel J.E. G Protein-coupled receptors in taste physiology and pharmacology. Front Pharmacol. 2020;11:587664. doi: 10.3389/fphar.2020.587664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Diószegi J., Llanaj E., Ádány R. Genetic background of taste perception, taste preferences, and its nutritional implications: a systematic review. Front Genet. 2019;10:1272. doi: 10.3389/fgene.2019.01272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stańska K., Krzeski A. The umami taste: from discovery to clinical use. Otolaryngol Pol. 2016;70(4):10–15. doi: 10.5604/00306657.1199991. [DOI] [PubMed] [Google Scholar]
- 111.Bachmanov A.A., Bosak N.P., Lin C., et al. Genetics of taste receptors. Curr Pharm Des. 2014;20(16):2669–2683. doi: 10.2174/13816128113199990566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fushan A.A., Simons C.T., Slack J.P., Manichaikul A., Drayna D. Allelic polymorphism within the TAS1R3 promoter is associated with human taste sensitivity to sucrose. Curr Biol. 2009;19(15):1288–1293. doi: 10.1016/j.cub.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim U.K., Breslin P.A., Reed D., Drayna D. Genetics of human taste perception. J Dent Res. 2004;83(6):448–453. doi: 10.1177/154405910408300603. [DOI] [PubMed] [Google Scholar]
- 114.Lyall V., Heck G.L., Vinnikova A.K., et al. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol. 2004;558(Pt 1):147–159. doi: 10.1113/jphysiol.2004.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schiffman S.S. Influence of medications on taste and smell. World J Otorhinolaryngol Head Neck Surg. 2018;4(1):84–91. doi: 10.1016/j.wjorl.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Naik B.S., Shetty N., Maben E.V. Drug-induced taste disorders. Eur J Intern Med. 2010;21(3):240–243. doi: 10.1016/j.ejim.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 117.Doty R.L., Shah M., Bromley S.M. Drug-induced taste disorders. Drug Saf. 2008;31(3):199–215. doi: 10.2165/00002018-200831030-00002. [DOI] [PubMed] [Google Scholar]
- 118.Briggs E.R. Taste disturbances related to medication use. Consult Pharm. 2009;24(7):538–543. doi: 10.4140/tcp.n.2009.538. [DOI] [PubMed] [Google Scholar]
- 119.Heeringa M., van Puijenbroek E.P. Reversible dysgeusia attributed to losartan. Ann Intern Med. 1998;129(1):72. doi: 10.7326/0003-4819-129-1-199807010-00023. [DOI] [PubMed] [Google Scholar]
- 120.Chen C., Chevrot D., Contamin C., Romanet T., Allenet B., Mallaret M. Stomatitis and ageusia induced by candesartan. Article in French. Nephrologie. 2004;25(3):97–99. [PubMed] [Google Scholar]
- 121.Cowart B.J. Taste dysfunction: a practical guide for oral medicine. Oral Dis. 2011;17(1):2–6. doi: 10.1111/j.1601-0825.2010.01719.x. [DOI] [PubMed] [Google Scholar]
- 122.International Classification of Orofacial Pain (ICOP) Cephalalgia. 2020;40(2):129–221. doi: 10.1177/0333102419893823. [DOI] [PubMed] [Google Scholar]
- 123.Tait R.C., Ferguson M., Herndon C.M. Chronic orofacial pain: burning mouth syndrome and other neuropathic disorders. J Pain Manag Med. 2017;3(1):120. [PMC free article] [PubMed] [Google Scholar]
- 124.Snyder D.J., Bartoshuk L.M. Oral sensory nerve damage: causes and consequences. Rev Endocr Metab Disord. 2016;17(2):149–158. doi: 10.1007/s11154-016-9377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nixdorf D.R., John M.T., Schierz O., Bereiter D.A., Hellekant G. Self-reported severity of taste disturbances correlates with dysfunctional grade of TMD pain. J Oral Rehabil. 2009;36(11):792–800. doi: 10.1111/j.1365-2842.2009.01996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grushka M., Epstein J.B., Gorsky M. Burning mouth syndrome and other oral sensory disorders: a unifying hypothesis. Pain Res Manag. 2003;8(3):133–135. doi: 10.1155/2003/654735. [DOI] [PubMed] [Google Scholar]
- 127.De Seta D., Mancini P., Minni A., et al. Bell's palsy: symptoms preceding and accompanying the facial paresis. ScientificWorldJournal. 2014;2014:801971. doi: 10.1155/2014/801971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Park J.M., Kim M.G., Jung J., et al. Effect of age and severity of facial palsy on taste thresholds in Bell's palsy patients. J Audiol Otol. 2017;21(1):16–21. doi: 10.7874/jao.2017.21.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nakamura T., Tsukita K., Suzuki A., et al. Peculiar unpleasant dysgeusia as the sole initial symptom of Guillain-Barré syndrome. Intern Med. 2020;59(6):835–837. doi: 10.2169/internalmedicine.2417-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fukunaga A., Uematsu H., Sugimoto K. Influences of aging on taste perception and oral somatic sensation. J Gerontol A Biol Sci Med Sci. 2005;60(1):109–113. doi: 10.1093/gerona/60.1.109. [DOI] [PubMed] [Google Scholar]
- 131.Mojet J., Christ-Hazelhof E., Heidema J. Taste perception with age: generic or specific losses in threshold sensitivity to the five basic tastes? Chem Senses. 2001;26(7):845–860. doi: 10.1093/chemse/26.7.845. [DOI] [PubMed] [Google Scholar]
- 132.Wang H., Zhou M., Brand J., Huang L. Inflammation and taste disorders: mechanisms in taste buds. Ann N Y Acad Sci. 2009;1170:596–603. doi: 10.1111/j.1749-6632.2009.04480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brennan F., Stevenson J., Brown M. The pathophysiology and management of taste changes in chronic kidney disease: a review. J Ren Nutr. 2020;30(5):368–379. doi: 10.1053/j.jrn.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 134.Kim T.H., Kim Y.H., Bae N.Y., Kang S.S., Lee J.B., Kim S.B. Salty taste thresholds and preference in patients with chronic kidney disease according to disease stage: a cross-sectional study. Nutr Diet. 2018;75(1):59–64. doi: 10.1111/1747-0080.12374. [DOI] [PubMed] [Google Scholar]
- 135.Rowan N.R., Soler Z.M., Othieno F., Storck K.A., Smith T.L., Schlosser R.J. Impact of bitter taste receptor phenotype upon clinical presentation in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(9):1013–1020. doi: 10.1002/alr.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Othieno F., Schlosser R.J., Rowan N.R., et al. Taste impairment in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(7):783–789. doi: 10.1002/alr.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lyckholm L., Heddinger S.P., Parker G., et al. A randomized, placebo controlled trial of oral zinc for chemotherapy-related taste and smell disorders. J Pain Palliat Care Pharmacother. 2012;26(2):111–114. doi: 10.3109/15360288.2012.676618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Silva C.S., Dias V.R., Almeida J.A., Brazil J.M., Santos R.A., Milagres M.P. Effect of heavy consumption of alcoholic beverages on the perception of sweet and salty taste. Alcohol Alcohol. 2016;51(3):302–306. doi: 10.1093/alcalc/agv116. [DOI] [PubMed] [Google Scholar]
- 139.Bellocchio L., Bordea I.R., Ballini A., et al. Environmental issues and neurological manifestations associated with COVID-19 pandemic: new aspects of the disease? Int J Environ Res Public Health. 2020;17(21):8048. doi: 10.3390/ijerph17218049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Panda S., Mohamed A., Sikka K., et al. Otolaryngologic manifestation and long-term outcome in mild COVID-19: experience from a tertiary care centre in India. Indian J Otolaryngol Head Neck Surg. 2020;73(1):1–6. doi: 10.1007/s12070-020-02217-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eshraghi A.A., Mirsaeidi M., Davies C., Telischi F.F., Chaudhari N., Mittal R. Potential mechanisms for COVID-19 Induced anosmia and dysgeusia. Front Physiol. 2020;11:1039. doi: 10.3389/fphys.2020.01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cazzolla A.P., Lovero R., Lo Muzio L., et al. Taste and smell disorders in COVID-19 patients: role of interleukin-6. ACS Chem Neurosci. 2020;11(17):2774–2781. doi: 10.1021/acschemneuro.0c00447. [DOI] [PubMed] [Google Scholar]
- 143.Khan A.S., Hichami A., Khan N.A. Obesity and COVID-19: oro-naso-sensory perception. J Clin Med. 2020;9(7):2158. doi: 10.3390/jcm9072158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cooper K.W., Brann D.H., Farruggia M.C., et al. COVID-19 and the chemical senses: supporting players take center stage. Neuron. 2020;107(2):219–233. doi: 10.1016/j.neuron.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Verhulst M.J.L., Loos B.G., Gerdes V.E.A., Teeuw W.J. Evaluating All potential oral complications of diabetes mellitus. Front Endocrinol (Lausanne) 2019;10:56. doi: 10.3389/fendo.2019.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.De Carli L., Gambino R., Lubrano C., et al. Impaired taste sensation in type 2 diabetic patients without chronic complications: a case-control study. J Endocrinol Invest. 2018;41(7):765–772. doi: 10.1007/s40618-017-0798-4. [DOI] [PubMed] [Google Scholar]
- 147.Syed Q., Hendler K.T., Koncilja K. The impact of aging and medical status on dysgeusia. Am J Med. 2016;129(7):753.e1–753.e6. doi: 10.1016/j.amjmed.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 148.Genter M.B., Doty R.L. Toxic exposures and the senses of taste and smell. Handb Clin Neurol. 2019;164:389–408. doi: 10.1016/B978-0-444-63855-7.00022-8. [DOI] [PubMed] [Google Scholar]
- 149.Nolden A.A., Hwang L.D., Boltong A., Reed D.R. Chemosensory changes from cancer treatment and their effects on patients' food behavior: a scoping review. Nutrients. 2019;11(10):2285. doi: 10.3390/nu11102285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van Oort S., Kramer E., de Groot J.W., Visser O. Taste alterations and cancer treatment. Curr Opin Support Palliat Care. 2018;12(2):162–167. doi: 10.1097/SPC.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 151.Sakashita S., Takayama K., Nishioka K., Katoh T. Taste disorders in healthy “carriers” and “non-carriers” of Candida albicans and in patients with candidosis of the tongue. J Dermatol. 2004;31(11):890–897. doi: 10.1111/j.1346-8138.2004.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 152.Risso D.S., Mezzavilla M., Pagani L., et al. Global diversity in the TAS2R38 bitter taste receptor: revisiting a classic evolutionary PROPosal. Sci Rep. 2016;6:25506. doi: 10.1038/srep25506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Risso D., Morini G., Pagani L., et al. Genetic signature of differential sensitivity to stevioside in the Italian population. Genes Nutr. 2014;9(3):401. doi: 10.1007/s12263-014-0401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim U.K., Jorgenson E., Coon H., Leppert M.L., Risch N., Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299(5610):1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- 155.Fasunla A.J., Daniel A., Nwankwo U., Kuti K.M., Nwaorgu O.G., Akinyinka O.O. Evaluation of olfactory and gustatory function of HIV infected women. AIDS Res Treat. 2016;2016:2045383. doi: 10.1155/2016/2045383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zybina M.A., Chernichenko V.A., Lur'e-Pokrovskaia T.A., Arungazyeva V.V., Zaĭchuk A.I. Combined treatment of immobile rectal cancer with the use of preoperative intensive fractional irradiation. Article in Russian. Khirurgiia (Mosk) 1977;(10):72–76. [PubMed] [Google Scholar]
- 157.Doty R.L. Systemic diseases and disorders. Handb Clin Neurol. 2019;164:361–387. doi: 10.1016/B978-0-444-63855-7.00021-6. [DOI] [PubMed] [Google Scholar]
- 158.Philippi N., Noblet V., Hamdaoui M., et al. The insula, a grey matter of tastes: a volumetric MRI study in dementia with Lewy bodies. Alzheimers Res Ther. 2020;12(1):79. doi: 10.1186/s13195-020-00645-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Devi L., Ohno M. Genetic reductions of beta-site amyloid precursor protein-cleaving enzyme 1 and amyloid-beta ameliorate impairment of conditioned taste aversion memory in 5XFAD Alzheimer's disease model mice. Eur J Neurosci. 2010;31(1):110–118. doi: 10.1111/j.1460-9568.2009.07031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cecchini M.P., Federico A., Zanini A., et al. Olfaction and taste in Parkinson's disease: the association with mild cognitive impairment and the single cognitive domain dysfunction. J Neural Transm (Vienna) 2019;126(5):585–595. doi: 10.1007/s00702-019-01996-z. [DOI] [PubMed] [Google Scholar]
- 161.Cecchini M.P., Fasano A., Boschi F., Osculati F., Tinazzi M. Taste in Parkinson's disease. J Neurol. 2015;262(4):806–813. doi: 10.1007/s00415-014-7518-1. [DOI] [PubMed] [Google Scholar]
- 162.Shah M., Deeb J., Fernando M., et al. Abnormality of taste and smell in Parkinson's disease. Parkinsonism Relat Disord. 2009;15(3):232–237. doi: 10.1016/j.parkreldis.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 163.Steinbach S., Proft F., Schulze-Koops H., et al. Gustatory and olfactory function in rheumatoid arthritis. Scand J Rheumatol. 2011;40(3):169–177. doi: 10.3109/03009742.2010.517547. [DOI] [PubMed] [Google Scholar]
- 164.Onoda K., Ikeda M. Gustatory disturbance due to cerebrovascular disorder. Laryngoscope. 1999;109(1):123–128. doi: 10.1097/00005537-199901000-00024. [DOI] [PubMed] [Google Scholar]
- 165.Proft F., Steinbach S., Dechant C., et al. Gustatory and olfactory function in patients with granulomatosis with polyangiitis (Wegener's) Scand J Rheumatol. 2014;43(6):512–518. doi: 10.3109/03009742.2014.915056. [DOI] [PubMed] [Google Scholar]
- 166.Kim A., Feng P., Ohkuri T., et al. Defects in the peripheral taste structure and function in the MRL/lpr mouse model of autoimmune disease. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rivlin R.S., Osnos M., Rosenthal S., Henkin R.I. Abnormalities in taste preference in hypothyroid rats. Am J Physiol. 1977;232(1):E80–E84. doi: 10.1152/ajpendo.1977.232.1.E80. [DOI] [PubMed] [Google Scholar]
- 168.Baskoy K., Ay S.A., Altundag A., et al. Is there any effect on smell and taste functions with levothyroxine treatment in subclinical hypothyroidism? PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0149979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Halpern B.P. Environmental factors affecting chemoreceptors: an overview. Environ Health Perspect. 1982;44:101–105. doi: 10.1289/ehp.8244101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schiffman S.S., Nagle H.T. Effect of environmental pollutants on taste and smell. Otolaryngol Head Neck Surg. 1992;106(6):693–700. doi: 10.1177/019459989210600613. [DOI] [PubMed] [Google Scholar]
- 171.Rusthen S., Young A., Herlofson B.B., et al. Oral disorders, saliva secretion, and oral health-related quality of life in patients with primary Sjögren's syndrome. Eur J Oral Sci. 2017;125(4):265–271. doi: 10.1111/eos.12358. [DOI] [PubMed] [Google Scholar]
- 172.Poon R., Su N., Ching V., Darling M., Grushka M. Reduction in unstimulated salivary flow rate in burning mouth syndrome. Br Dent J. 2014;217(7):E14. doi: 10.1038/sj.bdj.2014.884. [DOI] [PubMed] [Google Scholar]
- 173.Mathews S.A., Kurien B.T., Scofield R.H. Oral manifestations of Sjögren's syndrome. J Dent Res. 2008;87(4):308–318. doi: 10.1177/154405910808700411. [DOI] [PubMed] [Google Scholar]
- 174.Vivino F.B., Bunya V.Y., Massaro-Giordano G., et al. Sjogren's syndrome: an update on disease pathogenesis, clinical manifestations and treatment. Clin Immunol. 2019;203:81–121. doi: 10.1016/j.clim.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 175.Šijan Gobeljić M., Milić V., Pejnović N., Damjanov N. Chemosensory dysfunction, oral disorders and oral health-related quality of life in patients with primary Sjögren's syndrome: comparative cross-sectional study. BMC Oral Health. 2020;20(1):187. doi: 10.1186/s12903-020-01169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Al-Ezzi M., Khan K., Tappuni A.R. Is the taste acuity affected by oral dryness in primary Sjögren's syndrome patients? Oral Dis. 2020;26(3):688–695. doi: 10.1111/odi.13259. [DOI] [PubMed] [Google Scholar]
- 177.Kamel U.F., Maddison P., Whitaker R. Impact of primary Sjogren's syndrome on smell and taste: effect on quality of life. Rheumatology (Oxford) 2009;48(12):1512–1514. doi: 10.1093/rheumatology/kep249. [DOI] [PubMed] [Google Scholar]
- 178.Kaneda H., Maeshima K., Goto N., Kobayakawa T., Ayabe-Kanamura S., Saito S. Decline in taste and odor discrimination abilities with age, and relationship between gustation and olfaction. Chem Senses. 2000;25(3):331–337. doi: 10.1093/chemse/25.3.331. [DOI] [PubMed] [Google Scholar]
- 179.Geran R., Uecker F.C., Prüss H., et al. Olfactory and gustatory dysfunction in patients with autoimmune encephalitis. Front Neurol. 2019;10:480. doi: 10.3389/fneur.2019.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tarakad A., Jankovic J. Anosmia and ageusia in Parkinson's disease. Int Rev Neurobiol. 2017;133:541–556. doi: 10.1016/bs.irn.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 181.Doty R.L., Tourbier I.A., Pham D.L., et al. Taste dysfunction in multiple sclerosis. J Neurol. 2016;263(4):677–688. doi: 10.1007/s00415-016-8030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chabwine J.N., Tschirren M.V., Zekeridou A., Landis B.N., Kuntzer T. Sweet taste loss in myasthenia gravis: more than a coincidence? Orphanet J Rare Dis. 2014;9:50. doi: 10.1186/1750-1172-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chimenos-Küstner E., de Luca-Monasterios F., Schemel-Suárez M., Rodríguez de Rivera-Campillo M.E., Pérez-Pérez A.M., López-López J. Burning mouth syndrome and associated factors: a case-control retrospective study. Med Clin (Barc) 2017;148(4):153–157. doi: 10.1016/j.medcli.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 184.Chimenos-Küstner E., Arcos-Guerra C., Marques-Soares M.S. Burning mouth syndrome: diagnostic and therapeutic keys. Article in Spanish. Med Clin (Barc) 2014;142(8):370–374. doi: 10.1016/j.medcli.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 185.Jääskeläinen S.K. Pathophysiology of primary burning mouth syndrome. Clin Neurophysiol. 2012;123(1):71–77. doi: 10.1016/j.clinph.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 186.Sardella A., Gualerzi A., Lodi G., Sforza C., Carrassi A., Donetti E. Morphological evaluation of tongue mucosa in burning mouth syndrome. Arch Oral Biol. 2012;57(1):94–101. doi: 10.1016/j.archoralbio.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 187.Nasri-Heir C., Gomes J., Heir G.M., et al. The role of sensory input of the chorda tympani nerve and the number of fungiform papillae in burning mouth syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(1):65–72. doi: 10.1016/j.tripleo.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 188.Eliav E., Kamran B., Schaham R., Czerninski R., Gracely R.H., Benoliel R. Evidence of chorda tympani dysfunction in patients with burning mouth syndrome. JADA. 2007;138(5):628–633. doi: 10.14219/jada.archive.2007.0234. [DOI] [PubMed] [Google Scholar]
- 189.Kveton J.F., Bartoshuk L.M. The effect of unilateral chorda tympani damage on taste. Laryngoscope. 1994;104(1 Pt 1):25–29. doi: 10.1288/00005537-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 190.Nasri-Heir C., Zagury J.G., Thomas D., Ananthan S. Burning mouth syndrome: current concepts. J Indian Prosthodont Soc. 2015;15(4):300–307. doi: 10.4103/0972-4052.171823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hirsch A.R., Ziad A., Kim A.Y., Lail N.S., Sharma S. Pilot study: alleviation of pain in burning mouth syndrome with topical sucralose. Headache. 2011;51(3):444–446. doi: 10.1111/j.1526-4610.2010.01821.x. [DOI] [PubMed] [Google Scholar]
- 192.Klasser G.D., Grushka M., Su N. Burning mouth syndrome. Oral Maxillofac Surg Clin North Am. 2016;28(3):381–396. doi: 10.1016/j.coms.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 193.Imura H., Shimada M., Yamazaki Y., Sugimoto K. Characteristic changes of saliva and taste in burning mouth syndrome patients. J Oral Pathol Med. 2016;45(3):231–236. doi: 10.1111/jop.12350. [DOI] [PubMed] [Google Scholar]
- 194.Just T., Steiner S., Pau H.W. Oral pain perception and taste in burning mouth syndrome. J Oral Pathol Med. 2010;39(1):22–27. doi: 10.1111/j.1600-0714.2009.00824.x. [DOI] [PubMed] [Google Scholar]
- 195.Khan J., Anwer M., Noboru N., Thomas D., Kalladka M. Topical application in burning mouth syndrome. J Dent Sci. 2019;14(4):352–357. doi: 10.1016/j.jds.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nasri-Heir C., Shigdar D., Alnaas D., Korczeniewska O.A., Eliav R., Heir G.M. Primary burning mouth syndrome: literature review and preliminary findings suggesting possible association with pain modulation. Quintessence Int. 2017;49(1):49–60. doi: 10.3290/j.qi.a39403. [DOI] [PubMed] [Google Scholar]
- 197.Kim M.J., Kim J., Kho H.S. Comparison of clinical characteristics between burning mouth syndrome patients with bilateral and unilateral symptoms. Int J Oral Maxillofac Surg. 2020;49(1):38–43. doi: 10.1016/j.ijom.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 198.Bergdahl M., Bergdahl J. Perceived taste disturbance in adults: prevalence and association with oral and psychological factors and medication. Clin Oral Investig. 2002;6(3):145–149. doi: 10.1007/s00784-002-0169-0. [DOI] [PubMed] [Google Scholar]
- 199.Cullen M.M., Leopold D.A. Disorders of smell and taste. Med Clin North Am. 1999;83(1):57–74. doi: 10.1016/s0025-7125(05)70087-0. [DOI] [PubMed] [Google Scholar]
- 200.Graham C.S., Graham B.G., Bartlett J.A., Heald A.E., Schiffman S.S. Taste and smell losses in HIV infected patients. Physiol Behav. 1995;58(2):287–293. doi: 10.1016/0031-9384(95)00049-o. [DOI] [PubMed] [Google Scholar]
- 201.Goodspeed R.B., Gent J.F., Catalanotto F.A. Chemosensory dysfunction. Clinical evaluation results from a taste and smell clinic. Postgrad Med. 1987;81(1):251–257. doi: 10.1080/00325481.1987.11699680. 260. [DOI] [PubMed] [Google Scholar]
- 202.Smith F.R., Henkin R.I., Dell R.B. Disordered gustatory acuity in liver disease. Gastroenterology. 1976;70(4):568–571. [PubMed] [Google Scholar]
- 203.Henkin R.I., Larson A.L., Powell R.D. Hypogeusia, dysgeusia, hyposmia, and dysosmia following influenza-like infection. Ann Otol Rhinol Laryngol. 1975;84(5 Pt 1):672–682. doi: 10.1177/000348947508400519. [DOI] [PubMed] [Google Scholar]
- 204.Fasunla A.J., Nwankwo U., Adebayo A.M., Nwaorgu O.G. Association between sex, CD4 cell counts, antiretroviral medications, and olfactory and gustatory functions of hiv-infected adults. Otolaryngol Head Neck Surg. 2018;158(1):90–99. doi: 10.1177/0194599817733664. [DOI] [PubMed] [Google Scholar]
- 205.Raja J.V., Rai P., Khan M., Banu A., Bhuthaiah S. Evaluation of gustatory function in HIV-infected subjects with and without HAART. J Oral Pathol Med. 2013;42(3):216–221. doi: 10.1111/jop.12006. [DOI] [PubMed] [Google Scholar]
- 206.Higuchi M.A., Fukae J., Tsugawa J., et al. Dysgeusia in a patient with guillain-barré syndrome associated with acute hepatitis e: a case report and literature review. Intern Med. 2015;54(12):1543–1546. doi: 10.2169/internalmedicine.54.3506. [DOI] [PubMed] [Google Scholar]
- 207.Cecchini M.P., Pellegrini C., Bassetto M.A., et al. Might Helicobacter pylori infection be associated with distortion on taste perception? Med Hypotheses. 2013;81(3):496–499. doi: 10.1016/j.mehy.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 208.Carrillo-Larco R.M., Altez-Fernandez C. Anosmia and dysgeusia in COVID-19: a systematic review. Wellcome Open Res. 2020;5:94. doi: 10.12688/wellcomeopenres.15917.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lozada-Nur F., Chainani-Wu N., Fortuna G., Sroussi H. Dysgeusia in COVID-19: possible mechanisms and implications. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130(3):344–346. doi: 10.1016/j.oooo.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Qiu C., Cui C., Hautefort C., et al. Olfactory and gustatory dysfunction as an early identifier of COVID-19 in adults and children: an international multicenter study. Otolaryngol Head Neck Surg. 2020;163(4):714–721. doi: 10.1177/0194599820934376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Melley L.E., Bress E., Polan E. Hypogeusia as the initial presenting symptom of COVID-19. BMJ Case Rep. 2020;13(5) doi: 10.1136/bcr-2020-236080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Aziz M., Perisetti A., Lee-Smith W.M., Gajendran M., Bansal P., Goyal H. Taste changes (dysgeusia) in COVID-19: a systematic review and meta-analysis. Gastroenterology. 2020;159(3):1132–1133. doi: 10.1053/j.gastro.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Roland L.T., Gurrola J.G., 2nd, Loftus P.A., Cheung S.W., Chang J.L. Smell and taste symptom-based predictive model for COVID-19 diagnosis. Int Forum Allergy Rhinol. 2020;10(7):832–838. doi: 10.1002/alr.22602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mahmoud M.M., Abuohashish H.M., Khairy D.A., Bugshan A.S., Khan A.M., Moothedath M.M. Pathogenesis of dysgeusia in COVID-19 patients: a scoping review. Eur Rev Med Pharmacol Sci. 2021;25(2):1114–1134. doi: 10.26355/eurrev_202101_24683. [DOI] [PubMed] [Google Scholar]
- 215.Kavaz E., Tahir E., Bilek H.C., Kemal Ö., Deveci A., Tanyel E.A. Clinical significance of smell and taste dysfunction and other related factors in COVID-19. Eur Arch Otorhinolaryngol. 2021;278(7):2327–2336. doi: 10.1007/s00405-020-06503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sayin İ., Yaşar K.K., Yazici Z.M. Taste and smell impairment in COVID-19: an AAO-HNS anosmia reporting tool-based comparative study. Otolaryngol Head Neck Surg. 2020;163(3):473–479. doi: 10.1177/0194599820931820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Parma V., Ohla K., Veldhuizen M.G., et al. More than smell-COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. 2020;45(7):609–622. doi: 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kowalski L.P., Sanabria A., Ridge J.A., et al. COVID-19 pandemic: effects and evidence-based recommendations for otolaryngology and head and neck surgery practice. Head Neck. 2020;42(6):1259–1267. doi: 10.1002/hed.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cetinkaya E.A. Coincidence of COVID-19 infection and smell-taste perception disorders. J Craniofac Surg. 2020;31(6):e625–e626. doi: 10.1097/SCS.0000000000006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kang M.G., Choi J.H., Kho H.S. Relationships between gustatory function tests. Oral Dis. 2020;26(4):830–837. doi: 10.1111/odi.13291. [DOI] [PubMed] [Google Scholar]
- 221.Berling K., Knutsson J., Rosenblad A., von Unge M. Evaluation of electrogustometry and the filter paper disc method for taste assessment. Acta Otolaryngol. 2011;131(5):488–493. doi: 10.3109/00016489.2010.535850. [DOI] [PubMed] [Google Scholar]
- 222.Landis B.N., Welge-Luessen A., Brämerson A., et al. “Taste strips:” a rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J Neurol. 2009;256(2):242–248. doi: 10.1007/s00415-009-0088-y. [DOI] [PubMed] [Google Scholar]
- 223.Gupta H., Sharma A., Kumar S., Roy S.K. E-tongue: a tool for taste evaluation. Recent Pat Drug Deliv Formul. 2010;4(1):82–89. doi: 10.2174/187221110789957309. [DOI] [PubMed] [Google Scholar]
- 224.Kershaw J.C., Mattes R.D. Nutrition and taste and smell dysfunction. World J Otorhinolaryngol Head Neck Surg. 2018;4(1):3–10. doi: 10.1016/j.wjorl.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Deems D.A., Doty R.L., Settle R.G., et al. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991;117(5):519–528. doi: 10.1001/archotol.1991.01870170065015. [DOI] [PubMed] [Google Scholar]
- 226.Mattes R.D., Cowart B.J. Dietary assessment of patients with chemosensory disorders. J Am Diet Assoc. 1994;94(1):50–56. doi: 10.1016/0002-8223(94)92041-9. [DOI] [PubMed] [Google Scholar]
- 227.Markley E.J., Mattes-Kulig D.A., Henkin R.I. A classification of dysgeusia. J Am Diet Assoc. 1983;83(5):578–580. [PubMed] [Google Scholar]
- 228.Noel C.A., Sugrue M., Dando R. Participants with pharmacologically impaired taste function seek out more intense, higher calorie stimuli. Appetite. 2017;117:74–81. doi: 10.1016/j.appet.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 229.Bressan V., Stevanin S., Bianchi M., Aleo G., Bagnasco A., Sasso L. The effects of swallowing disorders, dysgeusia, oral mucositis and xerostomia on nutritional status, oral intake and weight loss in head and neck cancer patients: a systematic review. Cancer Treat Rev. 2016;45:105–119. doi: 10.1016/j.ctrv.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 230.McLaughlin L. Taste dysfunction in head and neck cancer survivors. Oncol Nurs Forum. 2013;40(1):E4–E13. doi: 10.1188/13.ONF.E4-E13. [DOI] [PubMed] [Google Scholar]
- 231.Woschnagg H., Stöllberger C., Finsterer J. Loss of taste is loss of weight. Lancet. 2002;359(9309):891. doi: 10.1016/S0140-6736(02)07933-3. [DOI] [PubMed] [Google Scholar]
- 232.Erkurt E., Erkisi M., Tunali C. Supportive treatment in weight-losing cancer patients due to the additive adverse effects of radiation treatment and/or chemotherapy. J Exp Clin Cancer Res. 2000;19(4):431–439. [PubMed] [Google Scholar]
- 233.Chapman-Novakofski K., Brewer M.S., Riskowski J., Burkowski C., Winter L. Alterations in taste thresholds in men with chronic obstructive pulmonary disease. J Am Diet Assoc. 1999;99(12):1536–1541. doi: 10.1016/S0002-8223(99)00377-6. [DOI] [PubMed] [Google Scholar]
- 234.Poothullil J.M. Maintenance of weight loss using taste and smell sensations. J Womens Health. 1999;8(1):109–113. doi: 10.1089/jwh.1999.8.109. [DOI] [PubMed] [Google Scholar]
- 235.Fitzgerald C., Wiese G., Moorthi R.N., Moe S.M., Hill Gallant K., Running C.A. Characterizing dysgeusia in hemodialysis patients. Chem Senses. 2019;44(3):165–171. doi: 10.1093/chemse/bjz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.McMahon E.J., Campbell K.L., Bauer J.D. Taste perception in kidney disease and relationship to dietary sodium intake. Appetite. 2014;83:236–241. doi: 10.1016/j.appet.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 237.Lynch K.E., Lynch R., Curhan G.C., Brunelli S.M. Altered taste perception and nutritional status among hemodialysis patients. J Ren Nutr. 2013;23(4):288–295.e1. doi: 10.1053/j.jrn.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Doty R.L. Age-related deficits in taste and smell. Otolaryngol Clin North Am. 2018;51(4):815–825. doi: 10.1016/j.otc.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 239.Ponticelli E., Clari M., Frigerio S., et al. Dysgeusia and health-related quality of life of cancer patients receiving chemotherapy: a cross-sectional study. Eur J Cancer Care (Engl) 2017;26(2) doi: 10.1111/ecc.12633. [DOI] [PubMed] [Google Scholar]