Abstract
Phosducin (Phd) and Phd-like proteins (PhLPs) selectively bind guanine nucleotide protein (G protein) βγ subunits (Gβγ), while Phd-like orphan proteins (PhLOPs) lack the major functional domain for the binding of Gβγ. A retina- and pineal gland-specific transcription factor, cone-rod homeobox (CRX), was identified by a yeast two-hybrid screen using PhLOP1 as the bait. Direct protein-protein interactions between Phd or PhLOP1 and CRX were demonstrated using a β-galactosidase quantitative assay in the yeast two-hybrid system and were confirmed by an in vitro binding assay and a glutathione S-transferase (GST) pull-down assay. To determine if the interaction with Phd or PhLOP1 affected CRX transactivation, a 120-bp interphotoreceptor retinoid binding protein (IRBP) promoter-luciferase reporter construct containing a CRX consensus element (GATTAA) was cotransfected into either COS-7 or retinoblastoma Weri-Rb-1 cells with expression constructs for CRX and either Phd or PhLOP1. Phd and PhLOP1 inhibited the transcriptional activation activity of CRX by 50% during transient cotransfection in COS-7 cells and by 70% in Weri-Rb-1 cells and COS-7 cells stably transfected with CRX. Phd inhibited CRX transactivation in a dose-dependent manner. Whereas Phd is a cytoplasmic phosphoprotein, coexpression of Phd with CRX results in Phd being localized both in the cytoplasm and nucleus. By contrast, PhLOP1 is found in the nucleus even without CRX coexpression. To address the physiological relevance of these potential protein interacting partners, we identified immunoreactive proteins for Phd and CRX in retinal cytosolic and nuclear fractions. Immunohistochemical analysis of bovine retinas reveals colocalization of Phd isoforms with CRX predominantly in the inner segment of cone cells, with additional costaining in the outer nuclear layer and the synaptic region. Our findings demonstrate that both Phd and PhLOP1 interact directly with CRX and that each diminishes the transactivation activity of CRX on the IRBP promoter. A domain that interacts with CRX is found in the carboxyl terminus of the Phd isoforms. Phd antibody-immunoreactive peptides are seen in light-adapted mouse retinal cytosolic and nuclear extracts. Neither Phd nor PhLOP1 affected CRX binding to its consensus DNA element in electrophoretic mobility shift assays. A model that illustrates separate functional roles for interactions between Phd and either SUG1 or CRX is proposed. The model suggests further a mechanism by which Phd isoforms could inhibit CRX transcriptional activation.
Phosducin (Phd) is an acidic phosphoprotein (30), abundantly expressed in retinal photoreceptors and pinealocytes (11, 33, 36) but ubiquitously distributed among other tissues (14, 15). Previous work clearly established that retinal Phd plays a role in the guanine nucleotide protein (G protein) signaling pathway by competing with G protein α subunits (Gα) for binding with the Gβγ complex (30, 65). The efficacy of Phd binding to Gβγ is determined, in part, by its phosphorylation state at serine 73 (29). The balance between phosphorylation by cyclic AMP-dependent protein kinase A and dephosphorylation by protein phosphatase 2A results in an increase of phosphorylated Phd during darkness and its decrease upon exposure to light (4, 9, 25, 26, 29, 63, 65). The dephosphorylated form of Phd favors the binding of Gβγ, which, in light, prevents receptor-mediated Gα reactivation (32, 65) and blocks interactions between Gβγ and its effectors (25, 26, 41, 64).
Phd and its isoforms represent a superfamily of proteins, and some members of the family are unable to interact with Gβγ. The Phd-like proteins (PhLPs) that bind Gβγ, including PhLPL and PhLPS, which are induced by ethanol treatment of a neuronal-glial cell culture, are structurally and potentially functionally similar to Phd (39, 53, 60). Recently, it was demonstrated that PhLPL interacts with SUG1 (3), a potential transcriptional mediator and a subunit of the 26S proteasome complex. Three PhLPs other than PhLPL and PhLPS were identified by our laboratory from human retina (14). The coding sequence for PhLP1 is identical to that for Phd, and PhLP1 has an additional 36-amino-acid (aa) domain at its amino (N) terminus and binds Gβγ with an affinity that is comparable to that of Phd. Two isoforms, Phd-like orphan proteins (PhLOPs) PhLOP1 and PhLOP2, failed to bind Gβγ. PhLOP1 lacks the first 52 N-terminal residues of Phd, but it contains the complete carboxyl (C) terminus of Phd. PhLOP2 has only a limited amino acid sequence homology to Phd, although its nucleotide sequence has significant homology to that of Phd (14).
To define proteins that interact with the PhLOPs, PhLOP1 was used as the bait in a yeast two-hybrid screen (67). Two genes encoding proteins that interact with Phd isoforms were identified from a bovine retinal cDNA library: the gene encoding the bovine orthologue of yeast SUG1, named bovine SUG1 (accession no. AF069053), and the gene encoding the bovine orthologue of human and mouse cone-rod homeobox (CRX), a retina-specific transcription factor named bovine CRX (bCRX; accession no. AF154123). CRX is a member of the Otd/Otx homeobox gene family, encoding pair-like homeodomain transcription factors that are involved in the development and regulation of the anterior head structure and sensory organs (10, 19, 20). In adult mammals, CRX is exclusively expressed in retinal photoreceptors, both cone and rod cells, and pinealocytes (10, 19, 20). In the retina, CRX binds to a conserved consensus site (TAATCC/A) in the upstream promoter regions of several photoreceptor-specific genes including genes for opsins, interphotoreceptor retinoid-binding protein (IRBP), β-phosphodiesterase, and arrestin (10, 20). CRX also regulates photoreceptor differentiation and survival (19, 20, 59). In the pineal gland, CRX binds to and transactivates the pineal regulatory element (TAATC/T) in the upstream region of the gene for the pineal gland night-specific ATPase and other genes encoding the rate-limiting enzymes for melatonin synthesis: serotonin N-acetyltransferase (NAT) and hydroxyindole-O-methyltransferase (35). Pineal CRX mRNA shows a daily oscillation that may contribute to the circadian expression of pineally expressed genes (35). In contrast, retinal CRX mRNA is reported not to oscillate significantly during a light-dark cycle. Still, the mRNA of retinal NAT exhibits a daily rhythm (52), suggesting that CRX is regulated in the retina through posttranslational regulatory mechanisms.
As an initial step to address the impact of Phd isoforms on CRX's function in retina-specific gene activation, we examined the effect that Phd isoforms have on CRX transcriptional activation in vitro. The potential for in vivo interactions of Phd isoforms and CRX in cell cultures and retina is also described, and a model is presented.
MATERIALS AND METHODS
Plasmid construction.
The complete coding sequences for human retinal Phd, PhLOP1, and different deletion mutants of PhLOP1 were cloned into the pBD-GAL4 phagemid vector (Stratagene, La Jolla, Calif.) downstream of the GAL4 DNA binding domain (BD) between its EcoRI and PstI restriction endonuclease sites as described previously (67). Glutathione S-transferase (GST) fusion proteins of Phd and PhLOP1 were made with the pGEX-3X vector (Pharmacia Biotech Inc., Piscataway, N.J.), as described previously (14, 64, 67). To create an amino-terminally six-histidine-tagged bCRX (6xHis-bCRX) bacterial expression construct, the bCRX coding region was amplified by PCR from the cDNA clone encoding full-length bCRX in the pGAD10 vector (Clontech Laboratories, Inc., Palo Alto, Calif.), which was isolated from the bovine retinal cDNA library screen. BamHI and EcoRI sites (underlined) were introduced into the following +5′ sense and the −3′ antisense bCRX primers, respectively: +5′ bCRX (1–20) BamHI (5′-CGGGATCC/ATG/ATG/GCG/TAT/ATG/AAC/CC-3′ [sense]) and −3′ bCRX (897–877) EcoRI (5′-CCGAATTC/CTA/CAA/GAT/CTG/AAA/CTT/CCA-3′ [antisense]).
The PCR fragment was digested with BamHI and EcoRI and ligated in-frame into pTrcHisA vector (Invitrogen Corporation, San Diego, Calif.). Plasmid 120 IRBP/LUC (pIRBP) containing the human IRBP promoter fragment −123 to +18 was generated by inserting the IRBP promoter fragment (−123 to +18) digested from the pNB21 plasmid (kindly provided by Nicoletta Bobola) (8) into the HindIII site of the pRL-null vector (Promega, Madison, Wis.) upstream of the Renilla luciferase reporter gene (7). Mammalian expression constructs were made with the pcDNA3 vector (Invitrogen). The complete coding regions of human Phd and PhLOP1 were amplified by PCR from the original clones in λMAX vector obtained from a human retina cDNA library (14) with the following primers: +5′ Phd (1–18) BamHI (5′-CCGGATCC/ATG/GAA/GAA/GCC/AAA/AGC-3′ [sense]), +5′ PhLOP1 (1–18) BamHI (5′-CCGGATCC/ATG/TCT/TCT/CCT/CAG/AGT-3′ [sense]), and −3′ Phd/PhLOP1 PstI, EcoRI, BamHI (5′-GCCGGATCCGAATTCTGCAG/TCA/TTC/AAC/ATC/TTC/ TTC-3′ [antisense for both Phd and PhLOP1]).
The PCR fragments were digested with BamHI and EcoRI and ligated into the pcDNA3 vector. To create the pcDNA3-bCRX construct, the cDNA insert including the full-length coding region of bCRX was digested with EcoRI from the pGAD10 vector obtained from the yeast two-hybrid screen and ligated into the pcDNA3 vector. All the cDNA constructs were completely sequenced from both +5′ and −3′ directions using the ABI PRISM genetic analyzer, model 310 (Perkin-Elmer, Foster City, Calif.), to confirm the correct reading frame and the complete nucleotide sequence.
Yeast two-hybrid system.
The yeast reporter host strain Saccharomyces cerevisiae CG-1945 used for the two-hybrid screen was described previously (67). The other strain, Y190 (MATa ura3-52 his3-200 lys2-801 ade2-101 trp1-901 leu2-3,112 gal4Δ gal80Δ cyhr2 LYS2::GAL1UAS-HIS3TATA-HIS3, URA3::GAL1UAS-GAL1TATA-LacZ) (Clontech) was used for the liquid β-galactosidase (β-Gal) assay. The yeast cells were grown in yeast extract-peptone-dextrose or appropriate selection medium to maintain plasmids. Yeast transformation was done by the lithium acetate method using the YEASTMAKER yeast transformation system (Clontech). PhLOP1 in the pBD-GAL4 vector was used as a bait to screen a bovine retina cDNA library in yeast expression vector pGAD10, as described previously (67). Qualitative and quantitative β-Gal assays were performed as described previously (67), except that yeast strain Y190 was utilized for the quantitative assay.
Generation and affinity purification of anti-bCRX polyclonal antisera.
Rabbit antisera against the peptide of bCRX (aa 279 to 292) (CTYNPHDPLDYKDQS) were made by Zymed Laboratories Inc. (South San Francisco, Calif.) according to their PolyQuik polyclonal peptide antibody protocol. The peptide conjugate was injected into a rabbit, and sera from bleeds at 4, 8, and 12 weeks (the follow-up program of the PolyQuik polyclonal peptide antibody protocol) of the rabbit were affinity purified against the peptide with the SulfoLink kit (Pierce, Rockford, Ill.) according to the manufacturer's instruction.
Affinity purification of recombinant proteins and in vitro binding assay.
Fusion proteins GST-Phd and GST-PhLOP1, and a GST control were expressed in Escherichia coli strain DH5α (GIBCO BRL, Gaithersburg, Md.) induced with isopropyl-β-d-thiogalactopyranoside (IPTG) and purified as previously described (14). The six-His tagged bCRX protein was also expressed in E. coli strain DH5α induced with IPTG at a final concentration of 0.1 mM for 4 h at 37°C after the optical density at 600 nm (OD600) reached 0.8. The six-His-tagged protein was purified with Ni-nitrilotriacetic acid (NTA) resin (Qiagen Inc., Santa Clarita, Calif.) under denaturing conditions with 8 M urea. After being washed, Ni-NTA resin with 6xHis-bCRX attached was incubated in phosphate-buffered saline (PBS) at room temperature for 30 min for the protein to renature (67). The renatured protein was used directly for the in vitro binding assay without being eluted from the resin (58). To purify the native 6xHis-bCRX protein for the electrophoretic mobility shift assay (EMSA), the culture was induced with 0.1 mM IPTG for 30 min at 37°C after the OD600 reached 0.8, and 6xHis-bCRX was purified with Ni-NTA resin under nondenaturing conditions by following the manufacturer's instructions. After being washed, 6xHis-bCRX was eluted with 1× elution buffer (1 M imidazole, 0.5 M NaCl, 20 mM Tris-HCl, pH 7.9). Purified 6xHis-bCRX was buffer exchanged into PBS using Centricon 10 concentrators (Millipore, Bedford, Mass.).
The in vitro binding assay was performed as described previously (67). The bound proteins were detected with either anti-GST monoclonal (1:1,000; Pharmacia Biotech Inc.), anti-Phd monoclonal (1D6; 1:1,000; kindly provided by H. Dua and L. Donoso), or anti-bCRX polyclonal (1:1,000; Zymed Laboratories Inc.) antibodies and the appropriate secondary antibodies using an ECL kit (Amersham, Arlington Heights, Ill.).
GST pull-down assay.
Bovine eyes were obtained from a local slaughterhouse. Mouse eyes were from C57BL/6J mice (Jackson Laboratories). Retinas were dissected immediately, and the cytosolic and nuclear extracts of retinas were prepared as described previously (16) with a few modifications. The nuclei were washed three times with buffer 1 before disruption in the same buffer by sonication. The cytosolic and nuclear fractions were then centrifuged at 11,000 × g for 10 min in a microcentrifuge, after which the pellets were removed. The fractions were then used for immunoblot analysis and in the GST pull-down assay.
In the GST pull-down assay, GST fusion proteins that had been previously attached to glutathione-Sepharose 4B beads (50-μl bed volume) were suspended in 100 μl of PBS–900 μl of bovine retinal nuclear extract in buffer 1 and gently rotated at 4°C overnight. The beads were washed four times with 1 ml of PBS, stripped in 240 μl of 1× sodium dodecyl sulfate (SDS) sample buffer, and boiled for 5 min before extracted proteins were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblot analysis, as previously described (14).
Cell culture and transient transfection.
Tissue culture media and supplements, except GlutaMAX (GIBCO BRL), were obtained from Irvine Scientific (Santa Ana, Calif.). COS-7 cells (American Type Culture Collection, Manassas, Va.) were maintained in Dulbecco's modified Eagle's medium as described previously (12). Weri-Rb-1 retinoblastoma cells (American Type Culture Collection) were maintained in suspension culture in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM GlutaMAX, 100 U of penicillin/ml, and 100 μg of streptomycin/ml.
Transient transfections were performed in six-well plates using Superfect transfection reagent (Qiagen) by following the manufacturer's instructions. Each transfection mixture contained 1 μg of reporter construct (pIRBP), 0.4 μg of each expression construct (pcDNA3-bCRX and pcDNA3-Phd or pcDNA3-PhLOP1), and 0.2 μg of pGL3-P plasmid (Promega), which contains a firefly luciferase reporter gene under the control of the simian virus 40 basic promoter (internal control for transfection efficiency). The pcDNA3 empty vector was used when necessary to equate the total amount of DNA for each sample. The transfected cells were incubated for 44 h before being harvested for the luciferase reporter assay.
Establishment of bCRX stably transfected cell lines.
pcDNA3-bCRX plasmid (2 μg) was transfected into COS-7 cells as described for transient transfection. Two days after transfection, the transfected cells were replated at a 1:10 ratio into 60-mm-diameter dishes and were subjected to selection with 500 μg of Geneticin (G418; GIBCO BRL)/ml. Medium was changed every 2 days. After 2 weeks of selection, the resultant resistant cell pool was plated at 1, 5, and 10 cells per well into 96-well tissue culture plates in G418-containing medium. After 1 to 2 weeks of incubation at 37°C and 5% CO2, the cells were checked under an inverted microscope to choose the wells with only one colony, which came from one cell. These clones of stably transfected cells were amplified in G418 selective medium and were screened for CRX mRNA and protein expression using Northern and immunoblot analyses, respectively.
Northern and immunoblot analyses.
The COS-7 cells stably transfected with bCRX were grown in 60-mm-diameter dishes and were harvested for total RNA isolation by removing the medium and immediately lysing the cells with 1 ml of the RNA STAT-60 reagents (TEL-TEST, INC.) per dish. Total cellular RNA was isolated by following the manufacturer's instruction. Total RNA (10 μg) was resolved on a 1.5% agarose gel containing 2.5 M formaldehyde and transferred to a Hybond-N+ nucleic acid transfer membrane (Amersham). The membrane was hybridized with a [α-32P]dCTP-labeled randomly primed bCRX cDNA probe generated from EcoRI digestion of the bCRX clone containing the full-length coding region in the pGAD10 vector obtained from the yeast two-hybrid screen. The membrane was hybridized at 65°C in Rapid-hyb hybridization buffer (Amersham) for 2 h, washed under high-stringency conditions, and exposed to a phosphorimager screen (Molecular Dynamics, Sunnyvale, Calif.) as described previously (12). Subsequently, the membrane was stripped and hybridized with a radiolabeled cDNA probe for actin to assess equal loading and transfer efficiency.
For immunoblot analysis, the cells grown in 60-mm-diameter dishes were washed 3 times with PBS, scraped in 500 μl of PBS, and sonicated 10 times for 1 s each on ice. Protein concentrations in whole-cell homogenates were determined with protein assay reagent (Bio-Rad Laboratories). Equal amounts of proteins were utilized for electrophoresis and blotting by previously published protocols (14). The immobilized proteins were detected with anti-bCRX peptide polyclonal antibodies (1:1,000) (Zymed) and goat anti-rabbit secondary antibodies (1:10,000) (Bio-Rad Laboratories) using an ECL kit.
Cotransfection of COS-7 cells stably transfected with bCRX.
COS-7 clone D4 cells stably transfected with bCRX, which had the highest CRX protein expression, were plated at 105/ml into six-well plates in G418 selective medium 20 h before transfection. One microgram of reporter construct (pIRBP), 0.8 μg of Phd or PhLOP1 expression construct, and 0.2 μg of pGL3-P plasmid were used with 12 μl of Superfect reagent in a total of 60 μl of serum-free medium (7). Medium was exchanged for G418 selective medium 3 h after transfection, and the cells were incubated for an additional 41 h (total of 44 h after transfection) before being harvested for the luciferase reporter assay.
Luciferase reporter assay.
The transfected cells were harvested and both firefly and Renilla luciferase activities were assayed with 20 μl of cell lysate, using the Dual-Luciferase reporter assay system (Promega) and the TD-20/20 luminometer (Turner Designs, Sunnyvale, Calif.). Renilla luciferase activity was normalized to the firefly luciferase activity of the same sample.
Immunocytochemistry and confocal microscopy.
Transiently transfected COS-7 cells were replated into eight-well chamber slides (Becton Dickinson Labware, Franklin Lakes, N.J.) at 2 × 105 cells/ml 24 h after transfection and incubated for an additional 24 h. Media were removed, and the cells were fixed with 4% paraformaldehyde for 15 min. After fixation, the cells were washed three times with PBS and incubated in blocking buffer (3% bovine serum albumin, 5% normal goat serum, 0.2% Triton X-100 in PBS) for 1 h at room temperature.
Eyecups were prepared with fresh bovine eyes, immersed in 4% paraformaldehyde for 2 days at 4°C, washed with PBS, and subjected to increasing concentrations of sucrose (12, 15, and 18% in PBS). The retinas, including the pigment epithelia, were dissected, divided, and frozen. Frozen retinas were sectioned at 7 μm on a Leica JUNG CM 3000. The sections were heated for 20 min in preheated 0.1% sodium citrate buffer (pH 6.0), cooled at room temperature, and then rinsed with PBS and blocked as described above for the transfected COS-7 cells.
After the blocking buffer was removed, the cells or retinal sections were incubated with the appropriate primary antibody or a mixture of antibodies containing the anti-Phd monoclonal (1D6; 1:500) and the anti-bCRX polyclonal (1:1,000) antibodies overnight at 4°C. Following the washing steps, cells or sections were reacted with a secondary antibody mixture containing a Dichloro triazinyl amino fluorescein-conjugated goat anti-mouse secondary antibody (1:100) (Chemicon International, Inc., Temecula, Calif.) and a Cy3-conjugated goat anti-rabbit secondary antibody (1:100) (Chemicon International, Inc.) for 1 h at room temperature. After three washes with PBS, the slides were mounted with Vectashield mounting medium for fluorescence (Vector) and photographed with a confocal microscope (Carl Zeiss, Inc., Thornwood, N.Y.). The digitized images were processed and analyzed using Adobe Photoshop.
EMSA.
Nuclear extracts of Weri-Rb-1 cells were prepared as previously described (17). Recombinant 6xHis-bCRX was purified under nondenaturing conditions as described above. EMSA was performed with 2 μg of nuclear extract or 50 ng of purified 6xHis-bCRX in a binding mixture containing 20 mM Tris-HCl (pH 7.5), 0.35 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 10% glycerol, 1 μg of poly(dI-dC), and 10 fmol of labeled probe in a total volume of 20 μl, as described previously (8). The incubation lasted for 10 min at room temperature, and then either the anti-bCRX antibody or the anti-His monoclonal antibody (Clontech) was added and the mixture was incubated for an additional 10 min. To test if Phd and PhLOP1 affect the DNA binding ability of CRX, a purified GST-Phd or GST-PhLOP1 fusion protein was added to the reaction mixture and the mixture was incubated for 10 min before the labeled probe was added. The tubes were incubated for an additional 10 min before loading. The double-stranded oligonucleotide probe (5′-GGGCTTGAATTAGACAGGATTAAAGGCT-3′; upper strand) contained both Ret-1/PCE-1 (AATTAG in murine IRBP [underlined]) and the CRX-binding element (GATTAA in murine IRBP [underlined]) (6). The double-stranded A oligonucleotide, containing only the CRX binding site (5′-AGACAGGATTAAAGGCTTACTG-3′; upper strand) (7) was used as a specific competitor.
Statistical analyses.
All the data sets in Fig. 1, 4, and 5 were analyzed using one-way analysis of variance.
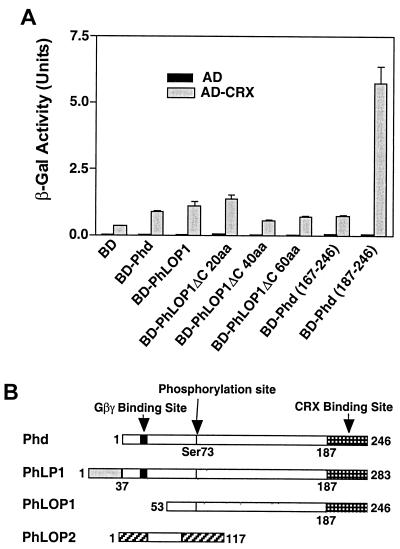
FIG. 1.
(A) Quantitative analysis of the interaction between Phd, PhLOP1, truncated PhLOP1, and bCRX. Either AD-bCRX or the AD vector was cotransformed with the indicated BD fusion constructs into the yeast reporter strain Y190. The transformants were processed for the β-Gal activity assay. Data are means ± standard errors of two independent experiments done in triplicate. The β-Gal activity is expressed in standard units (1,000 × OD420/time [minutes] × OD600). (B) Schematic alignment of the amino acid sequences of the Phd isoforms. Open bars, identical sequences among different isoforms; solid bars, Gβγ BD (TGPKGVINDWR) (64). Domains unique to either PhLP1 or PhLOP2 are shown by bars with different patterns. Arrows identify the Gβγ BD, the phosphorylation site (Ser73), and the CRX BD.
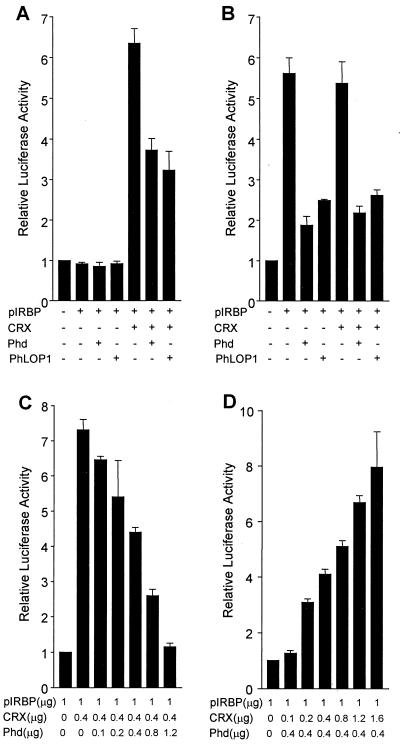
FIG. 4.
Down-regulation of CRX-mediated transactivation by Phd and PhLOP1 in transiently cotransfected COS-7 or retinoblastoma Weri-Rb-1 cells. COS-7 (A) and Weri-Rb-1 cells (B) were cotransfected with 1 μg of the reporter construct pIRBP and expression plasmids for bCRX (0.4 μg) and/or Phd or PhLOP1 (0.4 μg). The promoterless pRL-null vector was cotransfected with the pcDNA3 vector as a control for basal luciferase activity of the cells, and its luciferase activity was set at 1 (left bar). pGL3-P control plasmid (0.2 μg) was added to each sample as an internal control for transfection efficiency. Total amounts of DNA were adjusted with the pcDNA3 vector. Values are means ± standard errors of results from three or four independent experiments performed in duplicate. (C and D) COS-7 cells were cotransfected with the indicated amount of each plasmid and with 0.2 μg of pGL3-P control plasmid. Total amounts of DNA were adjusted with the pcDNA3 vector. Values are means ± standard errors of the means of results from three independent experiments performed in duplicate.
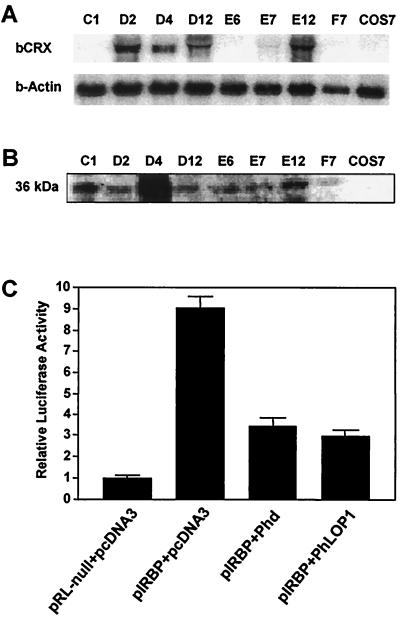
FIG. 5.
Down-regulation of CRX-mediated transactivation by Phd and PhLOP1 in COS-7 cells stably transfected with bCRX. (A) Total RNA (10 μg) from each clone and the untransfected COS-7 cells were subjected to Northern blot analysis with an [α-32P]dCTP-labeled bCRX cDNA probe. (B) Equal amounts of proteins from each clone of cells stably transfected with bCRX and untransfected COS-7 cells were immunoblotted and detected with an anti-bCRX antibody. (C) COS-7 clone D4 cells stably transfected with bCRX were cotransfected with 1 μg of the reporter construct pIRBP (pRL null for control), 0.8 μg of expression plasmids for Phd or PhLOP1, and 0.2 μg of pGL3-P control plasmid. Total amounts of DNA were adjusted with the pcDNA3 vector. The luciferase activity of the control was set at 1. Values are means ± standard errors of the means of results from four independent experiments performed in duplicate.
RESULTS
Interaction of Phd and PhLOP1 with bCRX in yeast.
In order to identify interacting partners for Phd isoforms, we screened a bovine retinal cDNA MATCHMAKER yeast two-hybrid library (Clontech), using full-length PhLOP1 as the bait (67). Of 68 clones selected by growth on His− plates, 7 clones were confirmed to specifically interact with PhLOP1 with more-stringent criteria (67). Five of these clones were cDNA for SUG1, a subunit of the 26S proteosome, which is also a potential transcriptional mediator (67). The other two clones were cDNA for CRX, a recently characterized retina-specific Otd/Otx-like paired-homeodomain transcription factor regulating photoreceptor differentiation and gene expression (10, 19, 20, 59). One contained the complete coding region of bCRX and a 5′-noncoding region, which encodes an additional 28 aa and which serves as a linker between the GAL4 DNA BD and the bCRX protein (accession no. AF154123). The other CRX cDNA contained the identical 5′-noncoding region and the coding sequence for the amino-terminal 178 aa of the bCRX protein, which includes the homeodomain (aa 39 to 98), suggesting that the essential domain for PhLOP1 interaction is within this region.
To determine if Phd can also interact with CRX and to determine the CRX-interacting domain of PhLOP1, the GAL4 DNA BD fusion of either Phd, PhLOP1, or different deletion mutants of PhLOP1 was cotransformed with either the GAL4 transcriptional activation domain (AD) vector or AD-bCRX to the yeast reporter strain Y190. A β-Gal quantitative assay was used to estimate the strength of interaction. None of the BD hybrid proteins activated reporter expression when cotransformed with the AD vector (Fig. 1A), while both Phd and PhLOP1 activated reporter expression above the control level (BD plus AD-bCRX) (P < 0.01) when cotransformed with AD-bCRX (Fig. 1A). The measurement of the β-Gal activity suggests that the last 60 aa at the carboxyl termini of Phd and PhLOP1 (Phd aa 187 to 246) had the strongest interaction with CRX. The strength of the interaction with CRX decreased significantly when up to 40 aa were truncated from the C terminus of PhLOP1 (ΔC 40aa) (P < 0.01), indicating that the C termini of Phd and PhLOP1 are the sites for CRX interaction (Fig. 1).
Direct association of Phd and PhLOP1 with CRX in vitro.
In vivo interactions in yeast can occur by either direct protein-protein interaction or indirectly through intermediary factors. To test whether or not the Phd isoforms could bind the CRX protein directly, we purified GST fusion proteins of Phd and PhLOP1 and 6xHis-bCRX for an in vitro binding assay. Ni-NTA beads, with or without immobilized 6xHis-bCRX, were incubated with GST-Phd, GST-PhLOP1, or a GST control. After extensive washing, bound proteins were eluted with 1 M imidazole. Aliquots of the proteins from the supernatant, washes, and eluate were analyzed by immunoblotting with appropriate antibodies to identify GST, Phd and PhLOP1, and CRX. Our results demonstrated that GST-Phd and GST-PhLOP1 were retained by 6xHis-bCRX-bound Ni-NTA beads but that the GST control was not retained (Fig. 2A and B). No proteins were retained by Ni-NTA beads themselves without the 6xHis-bCRX (data not shown). These data confirmed the specificity of the direct protein-protein interaction between Phd or PhLOP1 and CRX. Figure 2C shows that equal amounts of 6xHis-bCRX protein were used for each sample.
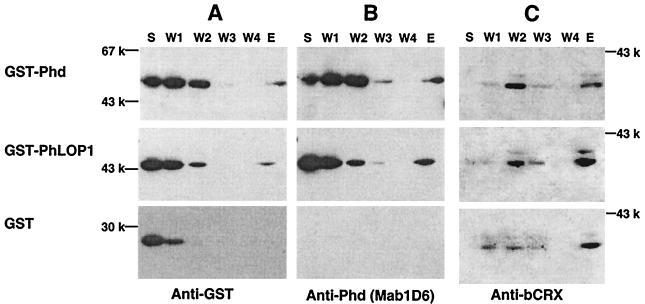
FIG. 2.
In vitro protein-protein interaction between Phd or PhLOP1 and CRX. GST-Phd and GST-PhLOP1 fusion proteins and a GST control were incubated with 6xHis-bCRX attached to Ni-NTA resin. After being washed four times, the bound proteins were eluted. The supernatant (S), washes (W1 to W4), and eluate (E) were subjected to immunoblot analysis, sequentially, with anti-GST monoclonal (A), anti-Phd monoclonal (B), or anti-bCRX polyclonal (C) antibodies.
The protein expression of CRX and Phd in mouse retina upon adaptation to light and darkness.
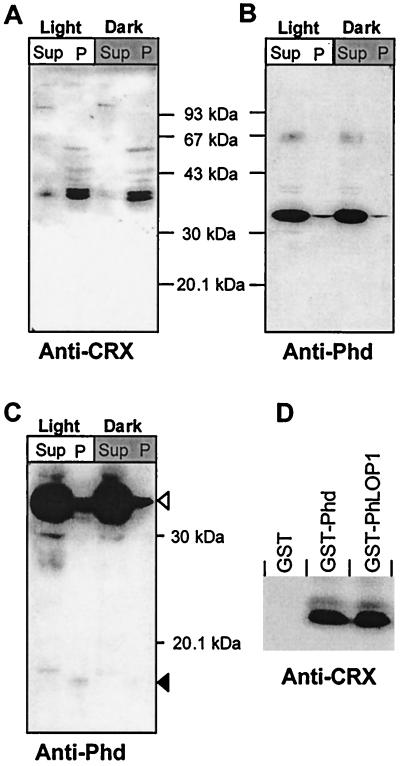
The mRNA for CRX is abundantly expressed throughout the 24-h diurnal cycle with peak levels at 0200 being threefold greater than levels at 1600 in the pineal gland (35). However, CRX mRNA levels in the retina do not appear to mirror the changes in the pineal gland during the light-dark cycle (52). CRX proteins have been reported to separate into a doublet on SDS-PAGE and immunoblot analysis, with an apparent molecular mass of ∼39 kDa (10). A doublet of ∼36 and 38 kDa was identified with our affinity-purified anti-CRX in retina and pineal gland, but these proteins were absent in 10 other adult mouse tissues (data not shown). In mouse retina, CRX is localized predominantly in the nucleus but it is also detectable in the cytosolic fraction. Indeed, more CRX is found in the cytoplasm of light-adapted retinas than in cytoplasm of those adapted to darkness. The total amount of CRX protein remained constant after 2 h of dark adaptation (Fig. 3A), which is consistent with the published data on rat retinal CRX mRNA during a 24-h light-dark cycle (52). In contrast, Phd resides mostly in the cytoplasm, but a detectable amount is found in the nuclear fraction. Again the total amount of Phd protein does not change significantly after dark adaptation for 2 h (Fig. 3B). Earlier studies suggest that Phd isoforms are targeted for degradation by the 26S proteasomal system (3, 67). A Phd antibody-immunoreactive peptide, presumably a degradation product of Phd, is seen in both the cytoplasm and the nucleus in fractions from light-adapted retinas but not in dark-adapted retinas upon prolonged exposure of immunoblots (Fig. 3C). Surprisingly a slightly lower-molecular-weight peptide is observed in the nuclear fraction than in the cytosolic fraction.
FIG. 3.
GST pull-down assay of retinal CRX by GST-Phd or -PhLOP1. (A and B) Immunoblot analysis of Phd and CRX expression in light- and dark-adapted mouse retinal cytosolic (Sup) and nuclear (P) extracts. (C) The same as panel B with a longer exposure. Open arrowhead, full-length Phd; solid arrowhead, Phd antibody immunoreactive peptides in light-adapted retinal extract but not in dark-adapted retinal extracts. (D) GST fusion protein attached to glutathione-Sepharose 4B beads, which were incubated with bovine retinal nuclear extract overnight at 4°C. After extensive washing, the bound proteins were subjected to immunoblot analysis with anti-bCRX affinity-purified antibody.
Pull-down of CRX but not OTX2 by GST-Phd and GST-PhLOP1.
The direct interaction of Phd and PhLOP1 with CRX was further investigated by GST pull-down experiments. As shown in Fig. 3D, retinal bCRX protein was retained selectively by either Phd- or PhLOP1-GST fusion proteins. Gβ was also retained by the GST-Phd fusion proteins but not by GST-PhLOP1 or GST alone (data not shown), verifying previously published results (14). Since the Gβ binding site and the CRX binding site on Phd and PhLOP1 do not overlap, it is possible that Phd binds CRX either alone or in combination with the Gβγ complex at the same time. OTX2, another member of the homeodomain-containing transcription factors, which is highly homologous to CRX and which is also expressed in adult retinas (7, 18), was not retained by GST-Phd or GST-PhLOP1 (data not shown), suggesting that the interaction between Phd or PhLOP1 and CRX is very specific. Recent studies suggest that OTX2 and CRX have different affinities for different DNA binding elements (N. Bobola et al., personal communiation), implying that, although similar in their homeodomains, these two transcription factors might have different functions as well as different regulatory mechanisms in the retina.
Phd and PhLOP1 inhibit CRX transactivation in COS-7 and retinoblastoma Weri-Rb-1 cells.
CRX binds to and transactivates many photoreceptor-specific genes including the IRBP gene (6, 7, 10), leading us to the obvious question of the possible effect that Phd isoforms would have on CRX's transactivation activity. We first studied the effect of Phd and PhLOP1 on CRX-driven transcription by transient cotransfection in COS-7 cells, utilizing a 120-bp IRBP promoter-luciferase reporter construct with a well-characterized CRX binding element (6, 7). The promoterless pRL-null vector was cotransfected with the empty pcDNA3 vector as a control for basal Renilla luciferase activity of the cells (Fig. 4A, bar 1 [numbering from left]). As predicted, the IRBP promoter does not have any activity in COS-7 cells without CRX (bar 2). When expressed alone, neither Phd (bar 3) nor PhLOP1 (bar 4) activated the IRBP promoter, while CRX (bar 5) transactivated the promoter six- to sevenfold. Cotransfection of either Phd (bar 6) or PhLOP1 (bar 7) with CRX inhibited the transactivation activity of CRX on the IRBP promoter by ∼50% (P < 0.01). To confirm that this inhibitory effect is the result of the specific protein-protein interaction between Phd or PhLOP1 and CRX, we did a control experiment with the cytomegalovirus (CMV) promoter driving the Renilla luciferase reporter gene. The CMV promoter itself had very high activity in COS-7 cells. However, neither CRX nor Phd or PhLOP1 had any effect on the CMV promoter activity (data not shown), indicating that the inhibitory effect of Phd and PhLOP1 is neither through the basal transcriptional machinery nor through direct inhibition of the Renilla luciferase activity.
To test a physiologically relevant retinal cell line, we repeated the cotransfection experiment with retinoblastoma cell line Weri-Rb-1. As demonstrated here and previously shown (7, 18), CRX is highly expressed in Weri-Rb-1 cells and the IRBP promoter is activated by endogenous CRX in the cells, so cotransfection of the CRX expression construct did not further enhance the promoter activation, which is probably saturated. Both Phd and PhLOP1 inhibited the promoter transactivation by ∼70% (P < 0.01) with or without CRX cotransfection (Fig. 4B). Cotransfection of increasing amounts of plasmid DNA expressing Phd resulted in a dose-dependent inhibition of CRX transactivation in COS-7 cells, with 100% inhibition when Phd DNA amounts were increased threefold over amounts of CRX DNA (Fig. 4C). This inhibitory effect by large amounts of Phd was confirmed to be caused by the specific interaction between Phd and CRX because the same amounts of Phd did not affect the CMV promoter activity in a similar cotransfection study, run in parallel, with the CMV promoter driving the Renilla luciferase reporter gene (data not shown). In contrast, when the Phd DNA amount was fixed, increasing amounts of CRX DNA overrode the inhibition by Phd (Fig. 4D).
The transient transfection efficiency for a single DNA construct is about 20 to 30% in COS-7 cells with Superfect transfection reagent, as determined with the enhanced green fluorescent protein DNA in the pEGFP-C2 vector (Clontech) (data not shown). The percent efficiency for cotransfection of pIRBP with two expression constructs, one for CRX and one for Phd, would be even lower, suggesting that the low efficiency in cotransfection may explain, in part, the low inhibitory effect (50% in COS-7) of Phd and PhLOP1 on the transactivation activity of CRX in transiently cotransfected cells. If the inhibitory effect is through direct protein-protein interaction between Phd or PhLOP1 and CRX, the two interacting proteins must be expressed in the same cells as those that carry the pIRBP reporter construct for the inhibitory effect to occur. If one of the three constructs was stably transfected, only two constructs would be needed to be cotransfected. The ratio of the doubly transfected cells would be higher than that of the triply transfected cells in the transient cotransfection experiment. As a result, a stronger inhibition was predicted. To test this, we did a selection for COS-7 cells stably transfected with bCRX and obtained eight clones of stably transfected cells (clones C1, D2, D4, D12, E6, E7, E12, and F7). Clone D2 had the highest level of CRX mRNA (Fig. 5A), but clone D4 had the highest CRX protein expression (Fig. 5B). The expression level of CRX in D4 was ∼30% of that in adult mouse retinas by immunoblot analysis (data not shown). We chose COS-7 clone D4 stably transfected with bCRX for cotransfection of either the Phd or PhLOP1 expression construct with the pIRBP reporter plasmid. As shown by Fig. 5C, both Phd and PhLOP1 inhibited the transactivation activity of stably expressed bCRX by about 70% (P < 0.01), which is comparable to the effect in Weri-Rb-1 cells (Fig. 4B). These results suggest that CRX alone can activate the 120-bp IRBP promoter and that Phd and PhLOP1 can diminish the promoter transactivation by CRX.
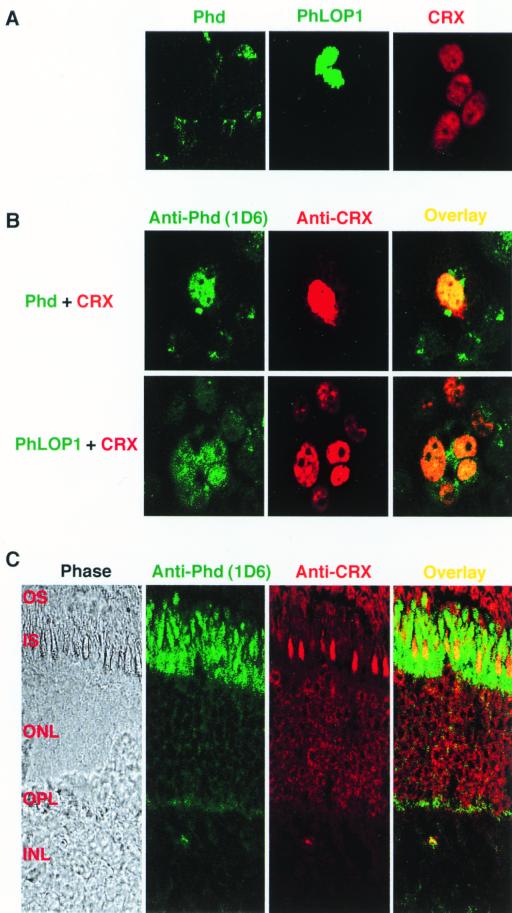
Colocalization of Phd or PhLOP1 and CRX in cotransfected COS-7 cells and retinal photoreceptors.
Phd is reported to be a soluble cytoplasmic protein, while CRX is a nuclear transcription factor. How and where in the cell does the interaction between Phd and CRX happen? To answer this question, we did an immunocytochemical localization study with transiently transfected COS-7 cells. As shown by Fig. 6A, CRX was localized to the nuclei of the transfected cells while Phd was localized to the cytoplasm. PhLOP1, which lacks the N-terminal domain of Phd, was localized to the nucleus (Fig. 6A). When Phd was cotransfected with CRX, colocalization was seen both in the nucleus and the cytoplasm, while PhLOP1 was predominantly colocalized with CRX to the nuclei of the cotransfected cells (Fig. 6B).
FIG. 6.
Immunocytochemical localization of Phd, PhLOP1, and CRX in transiently transfected COS-7 cells and bovine retina. (A and B) COS-7 cells were transfected or cotransfected with the indicated expression plasmids and were processed for immunocytochemistry as described in Materials and Methods. The anti-Phd monoclonal and anti-bCRX polyclonal antibodies were mixed and used as primary antibodies for all the samples. (C) Bovine retinal frozen sections were stained with a mixture of anti-Phd monoclonal and anti-bCRX polyclonal antibodies and appropriate secondary antibodies and imaged with a confocal microscope. OS, outer segment; IS, inner segment; ONL, outer nuclear layer; OPL, outer plaxiform layer; INL, inner nuclear layer.
In bovine retinal sections, Phd antibody immunoreactivity was highest in the inner-segment layer but was evident throughout the retinal layers, while CRX was localized mainly in the outer nuclear layer. Colocalization in the inner segment of cone photoreceptor cells and more weakly in the outer nuclear layer and the synaptic region was demonstrated (Fig. 6C).
Neither Phd nor PhLOP1 affected the DNA binding ability of CRX in vitro.
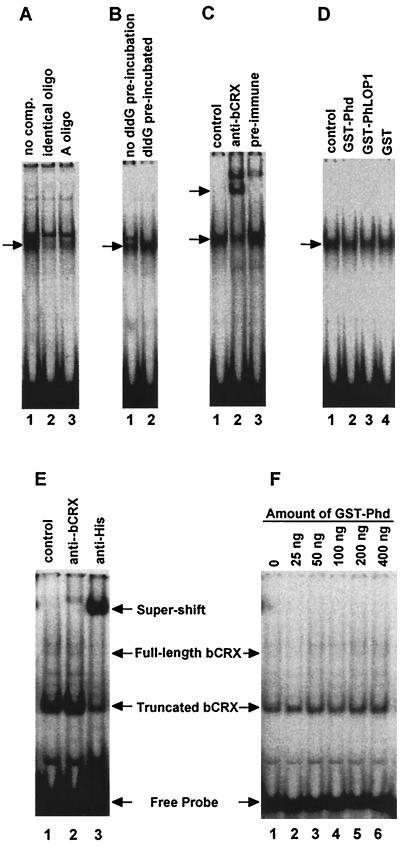
To understand if Phd and PhLOP1 directly affect the DNA binding affinity of CRX, we did EMSAs with a [γ-32P]ATP end-labeled probe containing both the Ret-1/PCE-1 site and the CRX binding site from the IRBP promoter (6). CRX expression is higher in Weri-Rb-1 cells than in adult mouse retinas, while Phd is below detection in the cell line by immunoblot analysis (data not shown). We first used nuclear extracts from Weri-Rb-1 cells to test whether exogenous Phd or PhLOP1 could affect CRX binding to its consensus DNA element. As shown in Fig. 7, there was a shifted band in the nuclear extract of Weri-Rb-1 cells; the anti-bCRX antiserum supershifted at least 50% of the band. The remaining part of the band that was not supershifted by the specific antibody might be the OTX2-probe complex since both CRX and OTX2 exist in Weri-Rb-1 cells and both bind to the same cis element (7). In addition, Erx, another retina-specific homeodomain-containing transcription factor that binds to the Ret-1/PCE-1 site of the opsin promoter (37) may exist in Weri-Rb-1 cell nuclear extract. Neither GST-Phd nor GST-PhLOP1 affected the DNA binding affinity of CRX in nuclear extracts (Fig. 7D). The failure of Phd and PhLOP1 to inhibit CRX from binding to the DNA element in vitro may be the result of other proteins interacting with Phd and PhLP in the nuclear extract. Because the binding domain for the Gβγ complex does not overlap with the binding domain for CRX, the Gβγ complex may not affect Phd interaction with CRX; however, SUG1 and CRX binding domains do overlap and this may compromise Phd's interaction with CRX since SUG1 is present both in the cytosol and the nucleus (40, 62).
FIG. 7.
EMSA. Equal amounts of a [γ-32P]ATP end-labeled oligonucleotide probe containing both the Ret-1/PCE-1 site and the CRX binding site were incubated with either 2 μg of Weri-Rb-1 cell nuclear extract (A to D) or 50 ng of purified 6xHis-bCRX protein (E and F). Arrows identify specifically shifted or supershifted bands. (A) Lane 1, no competitor; lane 2, 100-fold molar excess of the nonlabeled identical oligonucleotide; lane 3, 100-fold molar excess of the nonlabeled A oligonucleotide. (B) Lane 1, the labeled probe was mixed with poly(dI-dC) before the nuclear extract was added to the reaction mixture; lane 2, poly(dI-dG) was preincubated with the nuclear extract for 10 min before the labeled probe was added. (C) Lane 1, no serum; lane 2, anti-bCRX serum (1:20 final dilution); lane 3, preimmune serum (1:20 final dilution). (D) Fifty nanograms of GST-Phd (lane 2), GST-PhLOP1 (lane 3), or GST (lane 4) purified protein was incubated with 2 μg of Weri-Rb-1 cell nuclear extract before the labeled probe was added. For the control (lane 1), an equal volume of PBS was added to the binding mixture instead of the GST fusion proteins. (E) Lane 1, control; lane 2, affinity-purified anti-bCRX (1:100 final dilution); lane 3, anti-His monoclonal antibody (1:100 final dilution). (F) 6xHis-bCRX (50 ng) was preincubated with increasing amounts of GST-Phd for 10 min before 0.5 fmol of labeled probe (∼5,500 cpm) was added.
To exclude the possibility that other Phd-interacting proteins in the nuclear extract might interfere with the interaction between Phd or PhLOP1 and CRX, we purified the 6xHis-bCRX recombinant protein under nondenaturing conditions and repeated the EMSA. As shown in Fig. 7E, most of the purified protein is in a truncated form which contains the homeodomain because the six-His tag is at the N terminus and the homeodomain is also near the N terminus. This is confirmed by a supershift of the complex formed by the truncated recombinant protein with an anti-His monoclonal antibody (Fig. 7E, lane 3) but not with the anti-bCRX antibody (Fig. 7E, lane 2), which recognizes the C terminus of the protein. This truncated 6xHis-bCRX protein has an apparent molecular mass of ∼30 kDa, compared to ∼40 kDa for the full-length 6xHis-bCRX, on an immunoblot visualized with anti-His monoclonal antibodies and antimouse secondary antibodies. The anti-bCRX antibody did not recognize the truncated form, but it recognized the full-length 6xHis-bCRX protein on the same immunoblot (data not shown). The anti-bCRX antibody formed a weaker supershifted band because it only supershifts the complex formed by the full-length 6xHis-bCRX (Fig. 7E, lane 2). Then we tested increasing amounts of GST-Phd with less probe (0.5 fmol; 5,500 cpm in each reaction) to see if it could inhibit CRX-DNA binding. With up to 400 ng GST-Phd (eight times the amount of 6xHis-bCRX in the reaction), we did not see any effect of Phd on CRX-DNA binding (Fig. 7F).
DISCUSSION
Phd was identified first in the retina as a specialized Gβγ binding protein of photoreceptors (28), but additional observations now suggest that Phd is found widely within tissues of the body and, in fact, may be a common component of many G protein-coupled receptor systems (15). Likewise, the ability of Phd to bind Gβγ subunits is well documented, but there is a growing literature that suggests that Phd may have other functional capabilities beyond that associated with Gβγ binding and modulation of amplification by the phototransduction cascade.
Phd may serve a variety of G protein signaling pathways in other tissues, but, in retinal photoreceptor cells, such diversity in signaling pathways has yet to be demonstrated. Since the α subunit of transducin (Tα) and Phd compete for the same Gβγ (Tβγ) pool, it is reasonable to anticipate that Tα and Phd would appear together during retinal development. The pattern of appearance and change of Phd, Tβ, and Tα during retinal development in the mouse shows that both Phd and Tβ are demonstrable nearly a week earlier than Tα is detectable (2, 31). This disparity is seen again in knockout mice. In hemizygous rhodopsin knockout mice, Phd is elevated by 50% while other phototransduction proteins, including Tα and Tβ, remain at normal levels. In addition, in young homozygous rhodopsin knockout mice lacking the photoreceptor outer segments, Phd protein levels are normal but other phototransduction proteins are significantly reduced (34). Under appropriate developmental and knockout conditions, Phd is shown as an entity that is available to serve functions other than modulation of the phototransduction cascade.
We have identified three members of the Phd superfamily. PhLP1 contains a consensus Gβγ-binding domain and it bound Gβγ, whereas PhLOP1 and PhLOP2 lacked the Gβγ consensus domain and failed to bind Gβγ (14). Still, Phd, PhLP1, and PhLOP1 have extensive homology including identical amino acid sequences at the C termini (Fig. 1B). Using PhLOP1 as bait in a yeast two-hybrid system, SUG1 and CRX were identified as potential functional partners. Characterization of SUG1 interactions with Phd isoforms has been published by us (67) and independently by Barhite and coworkers (3).
One of the CRX clones that were identified in the two-hybrid screen encoded only 178 aa, representing the N terminus of CRX, so CRX-PhLOP1 interactions must occur within the N-terminal 178 aa of CRX. The clone contains the homeodomain (aa 39 to 98) that binds DNA. EMSA results, however, do not support the assumption that the interaction domain of CRX with Phd or PhLOP1 overlaps with its DNA BD because the presence of GST-Phd or -PhLOP1 did not affect the DNA binding affinity of CRX. Since both Phd and PhLOP1 interact with CRX and only Phd binds Gβγ, the binding of Gβγ by Phd does not appear to restrict its interactions with CRX.
Within the C termini of Phd, PhLP1, and PhLOP1 lies a domain that interacts with CRX (Fig. 1B). It was demonstrated that a 60-aa peptide from the C termini of the Phd isoforms is sufficient to interact with CRX (Fig. 1A). A site for interaction of Phd isoforms with SUG1 is also near the C terminus and overlaps with the CRX BD. However, SUG1 interactions with Phd isoforms require additional interactive sites, some of which are near the N terminus (67). These observations would predict that Phd could interact with Gβγ and still interact with SUG1 or CRX. Interactions between Phd and SUG1 are much stronger than those with CRX (data not shown), suggesting that Phd preferentially binds SUG1. Indeed, SUG1 and CRX binding sites overlap, implying that the Phd isoforms probably can interact with only one of the two proteins at the same time.
Our findings demonstrate that both Phd and PhLOP1 inhibit, during transient cotransfection and in cells stably transfected with CRX, the transactivation activity of CRX on the short IRBP promoter. In order to inhibit CRX transactivation, Phd or PhLOP1 must enter the nucleus to interact with CRX or intercept CRX in the cytoplasm before it enters the nucleus. Phd is usually a cytoplasmic protein, but Phd immunoreactivity is found in the nucleus when the protein is coexpressed with CRX. PhLOP1 is localized predominantly in the nucleus, with or without CRX cotransfection, suggesting that the Phd N terminus contains signals, perhaps the site binding Gβγ, that prevent translocation of Phd from the cytoplasm to the nucleus. Analyses of retinal cytoplasmic and nuclear fractions reveal Phd antibody immunoreactivity with small peptides, possibly degraded products of Phd isoforms, suggesting that Phd may be subjected to partial proteolysis before entrance into the nucleus (Fig. 3C).
Protein degradation, as a component of signaling pathways, is receiving increasing attention. For example, the nuclear factor κB (NF-κB) of the immune system is kept inactive in the cytoplasm by an inhibitory factor, I-κB, and NF-κB is activated when I-κB is degraded by proteolysis through the 26S proteasome system (47, 50, 57). Exposure of dark-adapted animals to light deactivates, in the pineal gland, a β-adrenergic pathway that triggers a rapid decrease in N-acetyltransferase (NAT) activity and a concomitant increase in NAT protein degradation through the proteasomal system (22). In another example, the entrainment by light of the Drosophila melanogaster circadian clock is mediated by an ubiquitin-proteasome-dependent degradation of the timeless (TIM) protein, a clock gene product (42).
Negative regulation of the transactivation activity of transcription factors through direct protein-protein interactions between two different families of proteins has been reported for the AP1 family of transcription factors and the nuclear hormone receptors (27, 43, 54–56). Functional antagonism between the retinoic acid receptor and either the viral transactivator BZLF1 or the oncoprotein Myb has also been reported to be mediated by physical interaction between the two transcription factors (48, 49). Similar inhibitory interaction by intrafamily or cross-family heterodimerization of two transcription factors has also been reported for homeodomain-containing transcription factors (5, 66).
Transcriptional repressors in many circumstances are as important as activators in the regulation of gene expression (1, 23, 24, 61). Repressors act by a variety of mechanisms, including direct interactions with the basal transcriptional machinery or activators, thereby blocking their activity, and competition for cis-regulating elements leading to exclusion of activators from the promoter. Another mechanism is the recruitment of corepressors that form bridges between repressors and their targets. Phd isoforms act as transcriptional repressors through specific interaction with the retina-specific transcriptional activator CRX. EMSA results suggest that Phd isoforms do not affect CRX binding to its consensus element, implying that the mechanism of transcriptional inhibition by Phd of CRX is further downstream of the cascade and probably involves blocking the interaction of CRX with either its coactivators or the basal transcriptional machinery.
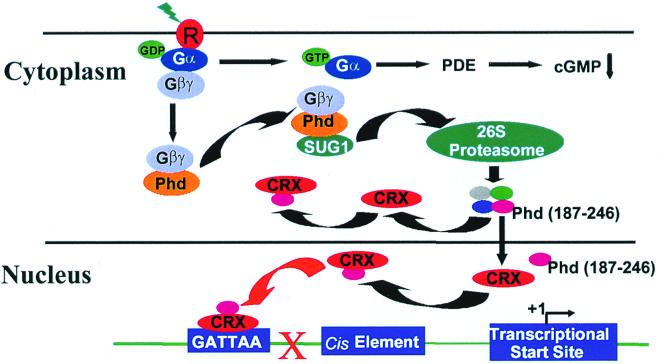
A conceptional model that consolidates the numerous observations that relate to a role for Phd in the regulation of CRX transactivation activity is proposed (Fig. 8). The focus of the model is on interactions between Phd, SUG1, and CRX. It proposes that SUG1 acts to guide Phd to the 26S proteasome where it is degraded into peptides. The 26S proteasome degrades ubiquitinated proteins, and ubiquitin-dependent proteolysis of both Phd (M. Obin, X. Zhu, and C. M. Craft, unpublished observations) and Gβ (44–46) has been demonstrated. Once bound with SUG1, Phd, alone or with Gβγ, enters the proteasome within the cytoplasm and there Phd is fragmented into smaller peptides. Peptides containing the C-terminal fragments of Phd or PhLOP1 are then free to interact with CRX, either in the cytoplasm or within the nucleus. The peptide complex interferes with CRX interactions with either its coactivators or the basal transcriptional machinery, thus inhibiting CRX-mediated gene transactivation. In summary, this working model sketches a series of molecular events that links components of the phototransduction pathway with light-initiated changes in transcriptional regulation within the photoreceptor cells of the retina.
FIG. 8.
Proposed model for the interaction of Phd with SUG1 and CRX. Light-activated rhodopsin initiates a GDP-GTP exchange on Gα with release of Gβγ. The released Gβγ complex is then bound by Phd. Free Phd or the Phd-Gβγ complex then interacts with SUG1 and is targeted to the 26S proteasome for degradation. The degraded products, the peptides, are released by the proteasome. The C-terminal peptide of Phd interacts with CRX either in the cytoplasm or in the nucleus, preventing CRX from activating the retinally expressed genes that contain one or more CRX consensus binding elements in their promoter region and that are normally controlled by CRX. cGMP, cyclic GMP; PDE, cAMP-phosphodiesterase.
Genes encoding NAT, hydroxyindole-O-methyltransferase, and pineal gland night-specific ATPase (35) are examples of retina- and pineal gland-specific genes that are down-regulated in light and up-regulated in darkness and which fit our proposed model. Other retina- and pineal gland-specific genes, such as the gene encoding zebra fish IRBP (51) and the rodent rod arrestin (13, 38), whose expression pattern changes in the opposite way during the light-dark cycle probably are not exclusively controlled by CRX in vivo, although they also have CRX binding sites in their promoter regions. OTX2 might contribute in the regulation of these genes, since OTX2 binds to the same DNA element in vitro and activates the short IRBP promoter that contains a single CRX binding site to the same extent as CRX in transient cotransfection (7). A recent report on the CRX knockout mouse phenotype showed that not all the genes that contain CRX binding sites in their promoter regions are adversely affected by disrupting the CRX gene (21), suggesting that OTX2 or other retinal or pineal transcription factors are necessary in retina- and pineal gland-specific expression of those genes.
ACKNOWLEDGMENTS
We gratefully acknowledge Aimin Li and Bruce Brown for excellent technical support, Nicoletta Bobola for the IRBP promoter, Larry A. Donoso and H. Dua for the Phd monoclonal antibody, and Wolfgang Baehr for the bovine retina yeast expression library. In addition, we thank Richard N. Lolley for critical discussions throughout this project, for editorial support, and for experimental suggestions.
These studies were supported, in part, by grants EY00395 (C. M. Craft and R. N. Lolley) and EY03042 from the Core Vision Research Center (Doheny Eye Institute), by grants from the L. K. Whittier Foundation (C.M.C.) and the Neurogenetic Analysis Core (Hans-Jürgen Fülle), and by a Howard Hughes Medical Institute Research Resources Grant (C.M.C.). C.M.C. is the Mary D. Allen Professor for Vision Research, Doheny Eye Institute.
ADDENDUM IN PROOF
Since acceptance of this paper, we have shown that a short region of phosducin can activate transcription directly without the need to partner with CRX for inhibition (X. Zhu and C. M. Craft, Biochem. Biophys. Res. Commun. 270:504–509, 2000).
Footnotes
Dedicated to Mary D. Allen for her generous support of vision research and to the memory of Richard N. Lolley, who died on 3 April 2000.
REFERENCES
- 1.Ashraf S I, Ip Y T. Transcriptional control: repression by local chromatin modification. Curr Biol. 1998;8:R683–R686. doi: 10.1016/s0960-9822(98)70435-x. [DOI] [PubMed] [Google Scholar]
- 2.Babila T, Schaad N C, Simonds W F, Shinohara T, Klein D C. Development of MEKA (phosducin), G beta, G gamma and S-antigen in the rat pineal gland and retina. Brain Res. 1992;585:141–148. doi: 10.1016/0006-8993(92)91199-o. [DOI] [PubMed] [Google Scholar]
- 3.Barhite S, Thibault C, Miles M F. Phosducin-like protein (PhLP), a regulator of G beta gamma function, interacts with the proteasomal protein SUG1. Biochim Biophys Acta. 1998;1402:95–101. doi: 10.1016/s0167-4889(97)00141-9. [DOI] [PubMed] [Google Scholar]
- 4.Bauer P H, Muller S, Puzicha M, Pippig S, Obermaier B, Helmreich E J, Lohse M J. Phosducin is a protein kinase A-regulated G-protein regulator. Nature. 1992;358:73–76. doi: 10.1038/358073a0. [DOI] [PubMed] [Google Scholar]
- 5.Bendall A J, Rincon-Limas D E, Botas J, Abate-Shen C. Protein complex formation between Msx1 and Lhx2 homeoproteins is incompatible with DNA binding activity. Differentiation. 1998;63:151–157. doi: 10.1046/j.1432-0436.1998.6330151.x. [DOI] [PubMed] [Google Scholar]
- 6.Boatright J H, Borst D E, Peoples J W, Bruno J, Edwards C L, Si J S, Nickerson J M. A major cis activator of the IRBP gene contains CRX-binding and Ret-1/PCE-I elements. Mol Vis. 1997;3:15. [PubMed] [Google Scholar]
- 7.Bobola N, Briata P, Ilengo C, Rosatto N, Craft C M, Corte G, Ravazzolo R. OTX2 homeodomain protein binds a DNA element necessary for interphotoreceptor retinoid binding protein gene expression. Mech Dev. 1999;82:165–169. doi: 10.1016/s0925-4773(98)00162-2. [DOI] [PubMed] [Google Scholar]
- 8.Bobola N, Hirsch E, Albini A, Altruda F, Noonan D, Ravazzolo R. A single cis-acting element in a short promoter segment of the gene encoding the interphotoreceptor retinoid-binding protein confers tissue-specific expression. J Biol Chem. 1995;270:1289–1294. doi: 10.1074/jbc.270.3.1289. [DOI] [PubMed] [Google Scholar]
- 9.Chen F, Lee R H. Phosducin and betagamma-transducin interaction I: effects of post-translational modifications. Biochem Biophys Res Commun. 1997;233:370–374. doi: 10.1006/bbrc.1997.6460. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Wang Q L, Nie Z, Sun H, Lennon G, Copeland N G, Gilbert D J, Jenkins N A, Zack D J. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron. 1997;19:1017–1030. doi: 10.1016/s0896-6273(00)80394-3. [DOI] [PubMed] [Google Scholar]
- 11.Craft C M, Lolley R N, Seldin M F, Lee R H. Rat pineal gland phosducin: cDNA isolation, nucleotide sequence, and chromosomal assignment in the mouse. Genomics. 1991;10:400–409. doi: 10.1016/0888-7543(91)90325-9. [DOI] [PubMed] [Google Scholar]
- 12.Craft C M, Murage J, Brown B, Zhan-Poe X. Bovine arylalkylamine N-acetyltransferase activity correlated with mRNA expression in pineal and retina. Brain Res Mol Brain Res. 1999;65:44–51. doi: 10.1016/s0169-328x(98)00336-2. [DOI] [PubMed] [Google Scholar]
- 13.Craft C M, Whitmore D H, Donoso L A. Differential expression of mRNA and protein encoding retinal and pineal S-antigen during the light/dark cycle. J Neurochem. 1990;55:1461–1473. doi: 10.1111/j.1471-4159.1990.tb04927.x. [DOI] [PubMed] [Google Scholar]
- 14.Craft C M, Xu J, Slepak V Z, Zhan-Poe X, Zhu X, Brown B, Lolley R N. PhLPs and PhLOPs in the phosducin family of G beta gamma binding proteins. Biochemistry. 1998;37:15758–15772. doi: 10.1021/bi980921a. [DOI] [PubMed] [Google Scholar]
- 15.Danner S, Lohse M J. Phosducin is a ubiquitous G-protein regulator. Proc Natl Acad Sci USA. 1996;93:10145–10150. doi: 10.1073/pnas.93.19.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deryckere F, Gannon F. A one-hour minipreparation technique for extraction of DNA-binding proteins from animal tissues. BioTechniques. 1994;16:405. [PubMed] [Google Scholar]
- 17.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong S L, Fong W B. Elements regulating the transcription of human interstitial retinoid-binding protein (IRBP) gene in cultured retinoblastoma cells. Curr Eye Res. 1999;18:283–291. doi: 10.1076/ceyr.18.4.283.5360. [DOI] [PubMed] [Google Scholar]
- 19.Freund C L, Gregory-Evans C Y, Furukawa T, Papaioannou M, Looser J, Ploder L, Bellingham J, Ng D, Herbrick J A, Duncan A, Scherer S W, Tsui L C, Loutradis-Anagnostou A, Jacobson S G, Cepko C L, Bhattacharya S S, McInnes R R. Cone-rod dystrophy due to mutations in a novel photoreceptor-specific homeobox gene (CRX) essential for maintenance of the photoreceptor. Cell. 1997;91:543–553. doi: 10.1016/s0092-8674(00)80440-7. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa T, Morrow E M, Cepko C L. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- 21.Furukawa T, Morrow E M, Li T, Davis F C, Cepko C L. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet. 1999;23:466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
- 22.Gastel J A, Roseboom P H, Rinaldi P A, Weller J L, Klein D C. Melatonin production: proteasomal proteolysis in serotonin N-acetyltransferase regulation. Science. 1998;279:1358–1360. doi: 10.1126/science.279.5355.1358. [DOI] [PubMed] [Google Scholar]
- 23.Gray S, Levine M. Transcriptional repression in development. Curr Opin Cell Biol. 1996;8:358–364. doi: 10.1016/s0955-0674(96)80010-x. [DOI] [PubMed] [Google Scholar]
- 24.Hanna-Rose W, Hansen U. Active repression mechanisms of eukaryotic transcription repressors. Trends Genet. 1996;12:229–234. doi: 10.1016/0168-9525(96)10022-6. [DOI] [PubMed] [Google Scholar]
- 25.Hawes B E, Touhara K, Kurose H, Lefkowitz R J, Inglese J. Determination of the G beta gamma-binding domain of phosducin. A regulatable modulator of G beta gamma signaling. J Biol Chem. 1994;269:29825–29830. [PubMed] [Google Scholar]
- 26.Hekman M, Bauer P H, Sohlemann P, Lohse M J. Phosducin inhibits receptor phosphorylation by the beta-adrenergic receptor kinase in a PKA-regulated manner. FEBS Lett. 1994;343:120–124. doi: 10.1016/0014-5793(94)80302-1. [DOI] [PubMed] [Google Scholar]
- 27.Jonat C, Rahmsdorf H J, Park K K, Cato A C, Gebel S, Ponta H, Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- 28.Lee R H, Brown B M, Lolley R N. Light-induced dephosphorylation of a 33K protein in rod outer segments of rat retina. Biochemistry. 1984;23:1972–1977. doi: 10.1021/bi00304a014. [DOI] [PubMed] [Google Scholar]
- 29.Lee R H, Brown B M, Lolley R N. Protein kinase A phosphorylates retinal phosducin on serine 73 in situ. J Biol Chem. 1990;265:15860–15866. [PubMed] [Google Scholar]
- 30.Lee R H, Lieberman B S, Lolley R N. A novel complex from bovine visual cells of a 33,000-dalton phosphoprotein with beta- and gamma-transducin: purification and subunit structure. Biochemistry. 1987;26:3983–3990. doi: 10.1021/bi00387a036. [DOI] [PubMed] [Google Scholar]
- 31.Lee R H, Lieberman B S, Lolley R N. Retinal accumulation of the phosducin/T beta gamma and transducin complexes in developing normal mice and in mice and dogs with inherited retinal degeneration. Exp Eye Res. 1990;51:325–333. doi: 10.1016/0014-4835(90)90029-t. [DOI] [PubMed] [Google Scholar]
- 32.Lee R H, Ting T D, Lieberman B S, Tobias D E, Lolley R N, Ho Y K. Regulation of retinal cGMP cascade by phosducin in bovine rod photoreceptor cells. Interaction of phosducin and transducin. J Biol Chem. 1992;267:25104–25112. [PubMed] [Google Scholar]
- 33.Lee R H, Whelan J P, Lolley R N, McGinnis J F. The photoreceptor-specific 33 kDa phosphoprotein of mammalian retina: generation of monospecific antibodies and localization by immunocytochemistry. Exp Eye Res. 1988;46:829–840. doi: 10.1016/s0014-4835(88)80035-6. [DOI] [PubMed] [Google Scholar]
- 34.Lem J, Krasnoperova N V, Calvert P D, Kosaras B, Cameron D A, Nicolo M, Makino C L, Sidman R L. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc Natl Acad Sci USA. 1999;96:736–741. doi: 10.1073/pnas.96.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Chen S, Wang Q, Zack D J, Snyder S H, Borjigin J. A pineal regulatory element (PIRE) mediates transactivation by the pineal/retina-specific transcription factor CRX. Proc Natl Acad Sci USA. 1998;95:1876–1881. doi: 10.1073/pnas.95.4.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lolley R N, Craft C M, Lee R H. Photoreceptors of the retina and pinealocytes of the pineal gland share common components of signal transduction. Neurochem Res. 1992;17:81–89. doi: 10.1007/BF00966868. [DOI] [PubMed] [Google Scholar]
- 37.Martinez J A, Barnstable C J. Erx, a novel retina-specific homeodomain transcription factor, can interact with Ret 1/PCEI sites. Biochem Biophys Res Commun. 1998;250:175–180. doi: 10.1006/bbrc.1998.9261. [DOI] [PubMed] [Google Scholar]
- 38.McGinnis J F, Austin B J, Stepanik P L, Lerious V. Light-dependent regulation of the transcriptional activity of the mammalian gene for arrestin. J Neurosci Res. 1994;38:479–482. doi: 10.1002/jnr.490380414. [DOI] [PubMed] [Google Scholar]
- 39.Miles M F, Barhite S, Sganga M, Elliott M. Phosducin-like protein: an ethanol-responsive potential modulator of guanine nucleotide-binding protein function. Proc Natl Acad Sci USA. 1993;90:10831–10835. doi: 10.1073/pnas.90.22.10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mounkes L C, Fuller M T. The DUG gene of Drosophila melanogaster encodes a structural and functional homolog of the S. cerevisiae SUG1 predicted ATPase associated with the 26S proteasome. Gene. 1998;206:165–174. doi: 10.1016/s0378-1119(97)00564-7. [DOI] [PubMed] [Google Scholar]
- 41.Muller S, Straub A, Schroder S, Bauer P H, Lohse M J. Interactions of phosducin with defined G protein beta gamma-subunits. J Biol Chem. 1996;271:11781–11786. doi: 10.1074/jbc.271.20.11781. [DOI] [PubMed] [Google Scholar]
- 42.Naidoo N, Song W, Hunter-Ensor M, Sehgal A. A role for the proteasome in the light response of the timeless clock protein. Science. 1999;285:1737–1741. doi: 10.1126/science.285.5434.1737. [DOI] [PubMed] [Google Scholar]
- 43.Nicholson R C, Mader S, Nagpal S, Leid M, Rochette-Egly C, Chambon P. Negative regulation of the rat stromelysin gene promoter by retinoic acid is mediated by an AP1 binding site. EMBO J. 1990;9:4443–4454. doi: 10.1002/j.1460-2075.1990.tb07895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obin M, Nowell T, Taylor A. A comparison of ubiquitin-dependent proteolysis of rod outer segment proteins in reticulocyte lysate and a retinal pigment epithelial cell line. Curr Eye Res. 1995;14:751–760. doi: 10.3109/02713689508995796. [DOI] [PubMed] [Google Scholar]
- 45.Obin M, Nowell T, Taylor A. The photoreceptor G-protein transducin (Gt) is a substrate for ubiquitin-dependent proteolysis. Biochem Biophys Res Commun. 1994;200:1169–1176. doi: 10.1006/bbrc.1994.1574. [DOI] [PubMed] [Google Scholar]
- 46.Obin M S, Jahngen-Hodge J, Nowell T, Taylor A. Ubiquitinylation and ubiquitin-dependent proteolysis in vertebrate photoreceptors (rod outer segments). Evidence for ubiquitinylation of Gt and rhodopsin. J Biol Chem. 1996;271:14473–14484. doi: 10.1074/jbc.271.24.14473. [DOI] [PubMed] [Google Scholar]
- 47.Orian A, Whiteside S, Israel A, Stancovski I, Schwartz A L, Ciechanover A. Ubiquitin-mediated processing of NF-kappa B transcriptional activator precursor p105. Reconstitution of a cell-free system and identification of the ubiquitin-carrier protein, E2, and a novel ubiquitin-protein ligase, E3, involved in conjugation. J Biol Chem. 1995;270:21707–21714. doi: 10.1074/jbc.270.37.21707. [DOI] [PubMed] [Google Scholar]
- 48.Pfitzner E, Becker P, Rolke A, Schule R. Functional antagonism between the retinoic acid receptor and the viral transactivator BZLF1 is mediated by protein-protein interactions. Proc Natl Acad Sci USA. 1995;92:12265–12269. doi: 10.1073/pnas.92.26.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfitzner E, Kirfel J, Becker P, Rolke A, Schule R. Physical interaction between retinoic acid receptor and the oncoprotein myb inhibits retinoic acid-dependent transactivation. Proc Natl Acad Sci USA. 1998;95:5539–5544. doi: 10.1073/pnas.95.10.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piette J, Piret B, Bonizzi G, Schoonbroodt S, Merville M P, Legrand-Poels S, Bours V. Multiple redox regulation in NF-kappaB transcription factor activation. Biol Chem. 1997;378:1237–1245. [PubMed] [Google Scholar]
- 51.Rajendran R R, van Niel E E, Stenkamp D L, Cunningham L L, Raymond P A, Gonzalez-Fernandez F. Zebrafish interphotoreceptor retinoid-binding protein: differential circadian expression among cone subtypes. J Exp Biol. 1996;199:2775–2787. doi: 10.1242/jeb.199.12.2775. [DOI] [PubMed] [Google Scholar]
- 52.Sakamoto K, Oishi K, Okada T, Onuma Y, Yokoyama K, Sugimoto K, Ishida N. Molecular cloning of the cone-rod homeobox gene (Crx) from the rat and its temporal expression pattern in the retina under a daily light-dark cycle. Neurosci Lett. 1999;261:101–104. doi: 10.1016/s0304-3940(99)00008-7. [DOI] [PubMed] [Google Scholar]
- 53.Schroder S, Lohse M J. Inhibition of G-protein betagamma-subunit functions by phosducin-like protein. Proc Natl Acad Sci USA. 1996;93:2100–2104. doi: 10.1073/pnas.93.5.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schule R, Evans R M. Functional antagonism between oncoprotein c-Jun and steroid hormone receptors. Cold Spring Harbor Symp Quant Biol. 1991;56:119–127. doi: 10.1101/sqb.1991.056.01.016. [DOI] [PubMed] [Google Scholar]
- 55.Schule R, Rangarajan P, Kliewer S, Ransone L J, Bolado J, Yang N, Verma I M, Evans R M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990;62:1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- 56.Schule R, Rangarajan P, Yang N, Kliewer S, Ransone L J, Bolado J, Verma I M, Evans R M. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci USA. 1991;88:6092–6096. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sears C, Olesen J, Rubin D, Finley D, Maniatis T. NF-kappa B p105 processing via the ubiquitin-proteasome pathway. J Biol Chem. 1998;273:1409–1419. doi: 10.1074/jbc.273.3.1409. [DOI] [PubMed] [Google Scholar]
- 58.Sinha D, Bakhshi M, Vora R. Ligand binding assays with recombinant proteins refolded on an affinity matrix. BioTechniques. 1994;17:509–514. [PubMed] [Google Scholar]
- 59.Swain P K, Chen S, Wang Q L, Affatigato L M, Coats C L, Brady K D, Fishman G A, Jacobson S G, Swaroop A, Stone E, Sieving P A, Zack D J. Mutations in the cone-rod homeobox gene are associated with the cone-rod dystrophy photoreceptor degeneration. Neuron. 1997;19:1329–1336. doi: 10.1016/s0896-6273(00)80423-7. [DOI] [PubMed] [Google Scholar]
- 60.Thibault C, Sganga M W, Miles M F. Interaction of phosducin-like protein with G protein betagamma subunits. J Biol Chem. 1997;272:12253–12256. doi: 10.1074/jbc.272.19.12253. [DOI] [PubMed] [Google Scholar]
- 61.Torchia J, Glass C, Rosenfeld M G. Co-activators and co-repressors in the integration of transcriptional responses. Curr Opin Cell Biol. 1998;10:373–383. doi: 10.1016/s0955-0674(98)80014-8. [DOI] [PubMed] [Google Scholar]
- 62.Wang W, Chevray P M, Nathans D. Mammalian Sug1 and c-Fos in the nuclear 26S proteasome. Proc Natl Acad Sci USA. 1996;93:8236–8240. doi: 10.1073/pnas.93.16.8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Willardson B M, Wilkins J F, Yoshida T, Bitensky M W. Regulation of phosducin phosphorylation in retinal rods by Ca2+/calmodulin-dependent adenylyl cyclase. Proc Natl Acad Sci USA. 1996;93:1475–1479. doi: 10.1073/pnas.93.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu J, Wu D, Slepak V Z, Simon M I. The N terminus of phosducin is involved in binding of beta gamma subunits of G protein. Proc Natl Acad Sci USA. 1995;92:2086–2090. doi: 10.1073/pnas.92.6.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshida T, Willardson B M, Wilkins J F, Jensen G J, Thornton B D, Bitensky M W. The phosphorylation state of phosducin determines its ability to block transducin subunit interactions and inhibit transducin binding to activated rhodopsin. J Biol Chem. 1994;269:24050–24057. [PubMed] [Google Scholar]
- 66.Zhang H, Hu G, Wang H, Sciavolino P, Iler N, Shen M M, Abate-Shen C. Heterodimerization of Msx and Dlx homeoproteins results in functional antagonism. Mol Cell Biol. 1997;17:2920–2932. doi: 10.1128/mcb.17.5.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu X, Craft C M. Interaction of phosducin and phosducin isoforms with a 26S proteasomal subunit, SUG1. Mol Vis. 1998;4:13. [PubMed] [Google Scholar]