Abstract
Ubiquitination of the plasma membrane-localized yeast a-factor receptor (Ste3p) triggers a rapid, ligand-independent endocytosis leading to its vacuolar degradation. This report identifies two mutants that block uptake by blocking ubiquitination, these being mutant either for the ankyrin repeat protein Akr1p or for the redundant type I casein kinases Yck1p and Yck2p. While no obvious defect was seen for wild-type Ste3p phosphorylation in akr1 or yck mutant backgrounds, examination of the Δ320-413 Ste3p deletion mutant phosphorylation did reveal a clear defect in both mutants. The Δ320-413 deletion removes 18 Ser-Thr residues (possible YCK-independent phosphorylation sites) yet retains the 15 Ser-Thr residues of the Ste3p PEST-like ubiquitination-endocytosis signal. Two other phenotypes link akr1 and yck mutants: both are defective in phosphorylation of wild-type α-factor receptor, and while both are defective for Ste3p constitutive internalization, both remain partially competent for the Ste3p ligand-dependent uptake mode. Yck1p-Yck2p may be the function responsible in phosphorylation of the PEST-like ubiquitination-endocytosis signal. Akr1p appears to function in localizing Yck1p-Yck2p to the plasma membrane, a localization that depends on prenylation of C-terminal dicysteinyl motifs. In akr1Δ cells, Yck2p is mislocalized, showing a diffuse cytoplasmic localization identical to that seen for a Yck2p mutant that lacks the C-terminal Cys-Cys, indicating a likely Akr1p requirement for the lipid modification of Yck2p, for prenylation, or possibly for palmitoylation.
Ubiquitin plays a central role in the endocytic uptake of a number of plasma membrane proteins in the yeast Saccharomyces cerevisiae (14). With its attachment to the cytosolic domains of the plasma membrane substrate, ubiquitin provides the sorting determinant that initiates uptake. This uptake mechanism has been best characterized for yeast, but evidence suggests that both ubiquitin and ubiquitination enzymes participate in the internalization of a variety of mammalian plasma membrane proteins as well (5).
Ubiquitin-dependent endocytosis appears to differ from the well-characterized clathrin-mediated uptake of mammalian cells. For clathrin-mediated uptake, substrates for uptake are recognized and sequestered within clathrin-coated pits through the binding of short peptidyl signals to the AP-2 adaptin complex (13, 24, 41). For ubiquitin-dependent uptake in yeast, the attached ubiquitin moiety provides the recognition determinant (14). Furthermore, while the key players of clathrin-mediated uptake are present in yeast (i.e. clathrin heavy and light chains, adaptin subunits, and dynamin homologues), a definitive demonstration of a role for any of these proteins in endocytosis has remained elusive. Strains with all of the genes encoding the adaptin subunits deleted remain fully competent for ubiquitin-dependent uptake (18). For clathrin, the situation is less clear. Mutation and/or deletion of the unique clathrin heavy- or light-chain genes have partial effects on ubiquitin-dependent endocytosis, slowing but not abolishing uptake (29, 45). The clathrin involvement, therefore, is either partial or indirect: uptake, at least in part, must be clathrin independent.
A number of mutations that do abolish uptake have been identified in yeast. The largest class of these endocytosis-defective mutants are those in which the block is exerted through derangement of the actin cytoskeleton. In addition to mutations in the actin structural protein, endocytosis-defective mutants identify a variety of other actin-associated proteins, including End3p, End4p, Sac6p, Vrp1p, and Pan1p (51). Other required proteins include the type I myosins Myo3p and Myo5p, which are expected to interact with actin, and calmodulin, a known regulator of nonmuscle myosins. Although the molecular nature of the actin role in endocytosis remains uncertain, it seems likely to function in the mechanical aspect of the internalization process. Indeed, a recent report for rat mast cells indicates that actin polymerization may provide the force that drives the newly formed pinocytotic vesicles away from the plasma membrane (25).
Given the central role for ubiquitin modification in yeast endocytosis, it follows that some of the enzymes that catalyze this modification are required participants in the uptake process. Generally, protein ubiquitination involves a sequential transfer of the ubiquitin moiety from one class of ubiquitination enzyme to another, from E1 to E2 to E3 and to the substrate protein. The diversity of E2 and E3 enzymes catalyzing this process is thought to reflect the diversity of the substrates within the cell that ultimately receive ubiquitin modification. For a variety of endocytic substrates, the E2 component appears to be the redundant triad Ubc1p, Ubc4p, and Ubc5p (14). Most often implicated as the E3 component for this process is hect domain-containing protein Rsp5p (14). The human homologue of Rsp5p, NEDD4, is required for the endocytic down regulation of the renal ENaC sodium channel (44).
Ubiquitin's role in endocytosis differs from its role in proteasomal turnover. First, the degradation associated with ubiquitin-dependent endocytosis is mediated by the vacuolar proteases, not by the proteasome. Second, the two processes differ in terms of the extent of ubiquitination required: while a multiubiquitin chain composed of four or more ubiquitin moieties is required for proteasomal recognition (17), monoubiquitination suffices to trigger endocytic uptake (34, 47).
The two yeast pheromone receptors, the α- and the a-factor receptors, have been well studied as model substrates for ubiquitin-dependent endocytosis. These two G protein-coupled receptors mediate the pheromonal communication that precedes the sexual conjugation of the two yeast haploid mating types, the MATa and MATα cells. For the α-factor receptor (Ste2p), challenge with its ligand, the peptide α-factor, results in ubiquitination of Ste2p and its subsequent internalization to the vacuole (yeast lysosome), where it is degraded by the resident proteases (15, 40). The most prominent form of endocytosis associated with the a-factor receptor (Ste3p) is a constitutive or ligand-independent uptake which also delivers receptor to the vacuole for degradation (7). Ste3p constitutive endocytosis is rapid, and consequently, Ste3p is a short-lived protein, with a half-life (t1/2) of only 15 min in cells growing at 30°C.
The signal for the rapid constitutive endocytosis of Ste3p is an extended 58-residue-long PEST-like sequence located at the C-terminal end of the receptor's 183-residue regulatory C-terminal cytoplasmic tail domain (CTD) (36). Consistent with its PEST-like nature, the Ste3p endocytosis signal functions primarily as a ubiquitination signal, with the three lysine residues that map within this sequence serving redundantly as the sites for ubiquitin attachment (34). Using the receptor-ubiquitin fusion methodology developed originally for Ste2p (47), we have shown that the translational fusion of a single ubiquitin moiety in place of the Ste3p PEST-like sequence can functionally substitute for this signal in endocytosis, indicating that the Ste3p PEST-like constitutive endocytosis signal functions solely in ubiquitination, both specifying ubiquitination and serving as the site for ubiquitin attachment (34).
While genetic analyses have identified a large number of functions that participate in the initial plasma membrane uptake step, essentially nothing is known about how these proteins collaborate to effect uptake. The endocytic phenotypes of these mutants are identical: all block uptake, trapping the endocytic substrate at the cell surface. Of particular interest are the early-acting endocytic functions that must prepare the endocytic substrate for ubiquitination. The present work identifies two functions which act before the ubiquitination step in the Ste3p constitutive endocytosis pathway: the redundant type I casein kinases Yck1p-Yck2p and the ankyrin repeat protein Akr1p. Yck1p-Yck2p appear to be required for phosphorylation of the Ste3p PEST-like sequence, likely activating it as a signal for ubiquitination. Akr1p, on the other hand, is required for the proper localization of the kinases to the cell surface. Instead of localizing to the plasma membrane, in akr1Δ cells, Yck2p localizes diffusely throughout the cytoplasm, showing a mutant localization similar to that seen with mutation of the Yck2p C-terminal dicysteinyl prenylation site.
MATERIALS AND METHODS
Plasmids.
pND1092, which is GAL1-(3xHA)YCK2 carried on the CEN/ARS/URA3 vector plasmid pRS316 (43), was constructed by conventional methods from the wild-type YCK2 plasmid pL2.3 (33). A fragment containing the GAL1,10 promoter together with the RNA start and translational start plus three iterations of the hemagglutinin (HA) epitope was fused in frame to the second codon of the YCK2 open reading frame (ORF). pND1113 is identical to pND1092 except that the final two codons of the YCK2 ORF have been mutated from the Cys-Cys dicodon to a Ser-Ser dicodon.
Strains.
Two different genetic backgrounds, isogenic either with NDY341 (35) or with LRB759 (28), were used in this work (Table 1). The NDY341 isogenic strain set derives from either the wild-type parent strain RH144-3D or the temperature-sensitive end4-1 mutant version RH268-1C (30). The founders for LRB759 background strains are the wild-type LRB759 and the temperature-sensitive yck1-1::ura3− yck2-2ts derivative LRB757 (28). All other strains were constructed by standard gene replacement technologies from these four starting strains.
TABLE 1.
Yeast strains
| Strain | Genotype | Reference or source |
|---|---|---|
| NDY341 | MATα GAL1-STE3 ura3 leu2 his4 bar1-1 | 35 |
| NDY342a | MATα GAL1-STE3 end4-1 | 35 |
| NDY343a | MATα ste3Δ::LEU2 | 36 |
| NDY344a | MATα ste3Δ::LEU2 end4-1 | 36 |
| NDY356a | MATα GAL1-STE3 pep4Δ | 35 |
| NDY788a | MATα GAL1-STE3 akr1Δ::LEU2 | This work |
| NDY990a | MATα GAL1-STE3-Ubiquitin(7K→R, G76A) | 34 |
| NDY991a | MATα GAL1-STE3-Ubiquitin(7K→R, G76A) end4-1 | This work |
| NDY992a | MATα GAL1-STE3-Ubiquitin(7K→R, G76A) pep4Δ | This work |
| NDY1011a | MATα GAL1-STE3-Ubiquitin(7K→R, G76A) akr1Δ::LEU2 | This work |
| NDY1040a | MATα GAL1-STE3 vrp1Δ::URA3 | This work |
| NDY1042a | MATα GAL1-STE3-Ubiquitin(7K→R, G76A) vrp1Δ::URA3 | This work |
| NDY1046a | MATα GAL1-STE3 end3Δ::G418r | This work |
| NDY1076a | MATα GAL1-STE3-Ubiquitin(7K→R, G76A) end3Δ::G418r | This work |
| LRB758 | MATa ura3-52 leu2 his3 | 28 |
| LRB756b | MATa yck1-1::ura3− yck2-2ts | 28 |
| LRB757b | MATα STE3 yck1-1::ura3− yck2-2ts | 28 |
| LRB759b | MATα STE3 ura3-52 leu2 his3 | 28 |
| NDY547b | MATα ste3Δ::LEU2 | This work |
| NDY548b | MATα ste3Δ::LEU2 yck1-1::ura3− yck2-2ts | This work |
| NDY662b | MATα GAL1-STE3Δ365 yck1-1::ura3− yck2-2ts | This work |
| NDY877b | MATα GAL1-STE3 | This work |
| NDY913b | MATα GAL1-STE3 yck1-1::ura3− yck2-2ts | This work |
| NDY1012b | MATα GAL1-STE3-Ubiquitin(7K→R, G76A) | This work |
| NDY1037b | MATα STE3 akr1Δ::LEU2 | This work |
| NDY1039b | MATα GAL1-STE3Δ320-413 | This work |
| NDY1045b | MATα GAL1-STE3Δ320-413 yck1-1::ura3− yck2-2ts | This work |
| NDY1061b | MATα GAL1-STE3Δ320-413 akr1Δ::LEU2 | This work |
| NDY1070b | MATα GAL1-STE3Δ320-413 end3Δ::G418r | This work |
| NDY1072b | MATα GAL1-STE3Δ365 | This work |
| NDY1074b | MATα GAL1-STE3-Ubiquitin(7K→R, G76A) end3Δ::G418r | This work |
| NDY1080b | MATα GAL1-STE3 pep4Δ | This work |
| NDY1083b | MATα GAL1-STE3 akr1Δ::LEU2 | This work |
| NDY1085b | MATα GAL1-STE3-Ubiquitin(7K→R, G76A) akr1Δ::LEU2 | This work |
| NDY1090b | MATα GAL1-STE3-Ubiquitin(7K→R, G76A) yck1-1::ura3− yck2-2ts | This work |
| NDY1117b | MATα GAL1-STE3Δ365 end3Δ::G418r | This work |
| NDY1118b | MATα GAL1-STE3 end3Δ::G418r | This work |
| NDY1215b | MATaakr1Δ::LEU2 | This work |
| NDY1216b | MATα GAL1-STE3Δ365 akr1Δ::LEU2 | This work |
| NDY1218b | MATα GAL1-STE3-Ubiquitin(7K→R, G76A) akr1Δ::LEU2 pep4Δ | This work |
| NDY1223b | MATα GAL1-STE3-Ubiquitin(7K→R, G76A) yck1-1::ura3− yck2-2ts pep4Δ | This work |
| NDY414 | MATα HIS3::STE3 ura3 leu2 ade2-1oc ade1 his6 trp1am | 8 |
Isogenic to NDY341.
Isogenic to LRB758.
(i) STE3 alleles.
The constructed strains have one of six different alleles present at the STE3 locus: either wild-type STE3, ste3Δ::LEU2, GAL1-STE3, GAL1-STE3Δ365, GAL1-STE3Δ320-413, or GAL1-STE3-Ubiquitin(7K→R, G76A). The first step in the introduction of the different GAL1-driven STE3 alleles was the replacement of wild-type STE3 by ste3Δ::LEU2. For the ste3Δ::LEU2 versions of LRB759 and LRB757, i.e., NDY547 and NDY548, the ste3Δ::LEU2 allele from the plasmid pSL2165 was introduced into the STE3 strains via the two-step gene replacement strategy previously described (36). GAL1-STE3 was introduced into the chromosome using the GAL1-STE3-integrating plasmid pSL1904 (34) via the two-step gene replacement (36). The integrating plasmids used for introducing the GAL1-STE3Δ365, GAL1-STE3Δ320-413, and GAL1-STE3-Ubiquitin(7K→R, G76A) alleles are identical to pSL1904 except for the deleted or fused sequences. The STE3Δ365 allele corresponds to an in-frame deletion of Ste3p CTD residues 365 through 468 (effectively, a C-terminal truncation mutant, as the wild-type Ste3p sequence naturally terminates with residue 470) (7). The GAL1-STE3Δ320-413 allele, an in-frame deletion of codons 320 to 413, was constructed by joining a SalI site created at codons 320 and 321 to an XhoI site created at codons 413 and 414 (36). The GAL1-STE3-Ubiquitin(7K→R, G76A) allele has the C-terminal 72 codons of STE3 replaced by a mutant ubiquitin gene in which the seven ubiquitin Lys codons are replaced by Arg codons and in which the C-terminal ubiquitin Gly codon is changed to an Ala codon (34).
(ii) Disruption of endocytic functions.
Three genes required for endocytosis, AKR1, END3, and VRP1, were disrupted in a variety of different strains. For the AKR1 disruption, an akr1Δ::LEU2 allele was constructed on the URA3 integrating plasmid vector pRS306 (43). The LEU2 fragment replaces AKR1 codons 13 through 735 (the SalI-to-HindIII interval) of the 764-codon-long AKR1 ORF. Replacement of wild-type AKR1 by akr1Δ::LEU2 utilized the previously described two-step gene replacement strategy (36). For the vrp1Δ::URA3 disruption allele (27), URA3 replaces a complete deletion of the VRP1 coding sequence. Ura3+ gene replacements of chromosomal VRP1+ (37) were screened by PCR analysis of genomic DNA derived from the isolates. The end3Δ::G418r disruption allele was generated by PCR (11, 49). An upstream 60-mer oligonucleotide and a downstream 69-mer oligonucleotide were used. Nineteen bases at the 3′ end of the upstream oligonucleotide and 22 bases at the 3′ end of the downstream oligonucleotide served to prime PCR synthesis of the G418 marker from the plasmid template, pFA-kanMX2 (49). Forty-one and 47 bases at the 5′ ends of the upstream and downstream oligonucleotides, respectively, were derived from the sequences immediately upstream or downstream of the END3 coding sequence. The resulting PCR product, therefore, has 41 and 47 bp of the END3 homologous sequences surrounding the G418r selectable marker. G418r yeast colonies were selected from transformed cells (11), and bona fide disruptants were culled from the G418r transformants via PCR analysis of genomic DNA extracted from the isolates.
Immunoblot analyses.
Turnover of Ste3p and Ste3-ubiquitin (Ste3-Ub) was assessed in the different end mutant backgrounds via a nonradioactive pulse-chase protocol (34) in which a “pulse” of receptor synthesis induced with the addition of 2% galactose to exponential cultures of GAL1-STE3 or of GAL1-STE3-Ub cells in YP-raffinose (2%) medium is followed by a “chase” in which continued synthesis is repressed with the addition of 3% glucose. Culture aliquots removed at various times during the glucose chase period were then subjected to an additional incubation with energy poisons as described previously (34). Briefly, culture aliquots collected by centrifugation were resuspended in 1.4 M sorbitol–25 mM Tris-Cl (pH 7.5)–10 mM sodium azide–10 mM potassium fluoride–2 mM MgCl2. Following a 90-min incubation at 37°C, glass bead protein extracts were prepared as previously described (7). We have found the pretreatment with energy poisons prior to extract preparation to be of particular benefit for the analysis of Ste3-Ub protein turnover; the energy poison incubation apparently releases the Ste3-Ub protein from complexes which otherwise result in an extremely heterogeneous electrophoretic mobility (34). For other experiments, not involving the turnover of Ste3p or Ste3-Ub, the energy poisoning step was eliminated and glass bead extracts were directly prepared as previously described (7). The affinity-purified rabbit polyclonal antibodies used for detecting Ste3p were prepared as previously described (36).
Phosphatase treatment.
For assessments of Ste3p ubiquitination and of Ste3Δ320-413p phosphorylation, protein extracts were treated with potato acid phosphatase (Boehringer Mannheim Corp., Indianapolis, Ind.) at a final concentration of 0.06 U/ml prior to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis as described previously (35). Phosphatase treatment of in vivo-labeled protein extracts was done prior to the Ste3p immunoprecipitation as described previously (8).
Pulse-labeling and immune precipitation.
Cells were pulse-labeled for 10 min with a mixture of [35S]methionine and [35S]cysteine and then chased with excess cold amino acids for an additional 10 min as described previously (35). Pulse-labeled cells were then treated for 15 min with a-factor or were mock treated using a one-sixth volume of the 20-fold-concentrated pheromone preparations as described previously (35). Cells were then collected by centrifugation, extracts were prepared via glass bead disruption, and the labeled Ste3p was immune precipitated as previously described (35).
Ligand-dependent endocytosis.
To induce internalization of Ste3Δ365p, cultures were treated with a half volume of a cell-free filtrate prepared from a saturated culture of EG123 cells transformed with the a-factor overproduction plasmid pKK16 (22). Mock a-factor preparations were obtained from the isogenic mfa1::LEU2 mfa2::URA3 strain SM1229 (26). Internalization was monitored via our standard whole-cell protease-shaving regimen. The treatment of intact cells with pronase (Calbiochem-Novabiochem Corp., La Jolla, Calif.) and the subsequent cell extraction protocol were described previously (7). As a control for the maintenance of cell integrity, the inaccessibility to the extracellular proteases of cytoplasmic phosphoglycerol kinase (Pgk1p) was routinely assayed by Western blotting with a Pgk1p-specific antiserum (provided by Tom Stevens, University of Oregon).
Indirect immunofluorescence.
A 2-h period of expression of the epitope-tagged Yck2p was induced from cells carrying either the GAL1-(3xHA)YCK2 plasmid (pND1092) or the GAL1-(3xHA)YCK2(CC→SS) plasmid (pND1113) with the addition of 2% galactose to cultures growing exponentially in YP-raffinose (2%) medium. Cells were fixed, spheroplasted, and otherwise prepared for indirect immunofluorescence as described previously (7) except that the overall time of fixation was reduced from 16 to 3 h. Detection of the 3xHA epitope-tagged Yck2 proteins utilized a 1-h incubation with 1:3,000-diluted HA.11 monoclonal antibody (MAb) (Berkeley Antibody Co., Berkeley, Calif.) followed by a 1-h incubation with 1:500 diluted Cy3-conjugated donkey anti-mouse immunoglobulin G secondary antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, Pa.). Images were captured using a Eclipse 600 (Nikon Inc., Melville, N.Y.) equipped with a Micromax CCD camera (Roper Scientific Princeton Instruments Inc., Trenton, N.J.).
RESULTS
Akr1p and Yck1p-Yck2p act before Ste3p ubiquitination.
To identify endocytic functions that act early in endocytosis, we have used the ubiquitination event as a point of demarcation for dividing early from late functions. Five endocytosis-defective mutants were used for this analysis: end3Δ, end4-1, vrp1Δ, akr1Δ mutants and a yck1Δ yck2ts double mutant. Features of two of these mutants, the akr1Δ mutant and the yck1Δ yck2ts double mutant, suggested possible early action points. The yck1Δ yck2ts double mutant is defective for Ste2p phosphorylation as well as for Ste2p ubiquitination and endocytosis, leading Hicke et al. (16) to suggest that the YCK-dependent phosphorylation of Ste2p may be a prerequisite for ubiquitination and consequent endocytosis. Furthermore, the same yck mutant also has been shown to have a block in Ste3p constitutive uptake (28). For Akr1p, two findings suggest a possible early action point for this function. First, AKR1 was identified as a two-hybrid interactor with the Ste3p CTD (10). As the PEST-like constitutive endocytosis signal is contained within the CTD, Akr1p might participate in the initial recognition of this signal or in the presentation of the signal to the ubiquitination machinery. In addition, Akr1p also shows a differential involvement in the two Ste3p uptake modes: while it is required for constitutive endocytosis, it is dispensable for the mechanistically distinct ligand-dependent mode. As the signals which direct these two uptake modes differ (7, 36, 46), Akr1p may participate in the initial steps which select Ste3p for constitutive uptake. The other three functions, namely, END3, SLA2 (END4), and VRP1 (END5), all have demonstrated involvements with the actin cytoskeleton (51).
We have first confirmed that all five functions are required for the rapid, constitutive endocytosis and turnover of Ste3p. As noted above, previous work has shown that end4-1, akr1Δ, and yck1Δ yck2ts mutants are defective for Ste3p constitutive turnover (10, 28, 35); we have confirmed this and have shown in addition that end3Δ and vrp1Δ mutants also are defective (data not shown). Furthermore, in all five of these mutants, Ste3p accumulates at the cell surface (data not shown), indicating that the blockade to turnover is imposed at the cell surface internalization step of endocytosis.
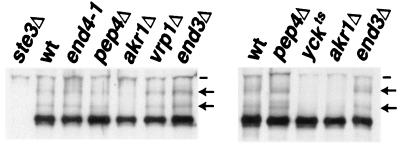
As a first step towards ordering the action points of these five endocytic functions, we have examined the mutants for effects on Ste3p ubiquitination (Fig. 1). In extracts derived from wild-type or pep4Δ cells, approximately 20% of the receptor protein is isolated as ubiquitinated species, with roughly equal proportions distributed between the presumptive mono- and diubiquitinated forms (35). For three of the mutants, the end4-1, end3Δ, and vrp1Δ mutants, Ste3p ubiquitination appears to be uncompromised: receptors from these cells are indistinguishable from receptor isolated from either wild-type or pep4Δ cells in terms of the presentation of the two ubiquitinated Ste3p species (Fig. 1). In sharp contrast are receptors isolated from the yck and akr1 mutants: Ste3p ubiquitination appears to be fully blocked in these mutants (Fig. 1). A simple interpretation is that yck and akr1 block endocytosis early, at a step prior to ubiquitination, while the other three mutations block late, at downstream, postubiquitination steps.
FIG. 1.
Effects of the endocytosis-defective mutants on Ste3p constitutive ubiquitination. Receptor ubiquitination was assessed in wild-type (wt) and mutant MATα GAL1-STE3 strains of two different genetic backgrounds. Strains isogenic to the wild-type strain NDY341 (left panel) include the temperature-sensitive end4-1 mutant (NDY342), as well as pep4Δ (NDY356), akr1Δ (NDY788), vrp1Δ (NDY1040), and end3Δ (NDY1046) mutants. In addition, the isogenic ste3Δ strain NDY343 was used as control for the specificity of Ste3p antibodies. Strains isogenic to NDY877 (wild-type MATα GAL1-STE3) (right panel) include the yck1Δ yck2ts double mutant (NDY913) and pep4Δ (NDY1080), akr1Δ (NDY1083), and end3Δ (NDY1118) strains. Protein extracts were prepared from cell cultures, following a 2-h Ste3p expression period, induced with galactose addition to log-phase cultures growing in raffinose medium. Fifteen minutes before cells were collected for extract preparation, cultures of cells isogenic to NDY341 (left panel) or to NDY877 (right panel) were shifted from 25°C to either 37 or 30°C, respectively. Protein extracts were treated with potato acid phosphatase (see Materials and Methods) to remove heterogeneity in gel migration which results from the heterogeneous phosphorylation of Ste3p and then subjected to SDS-PAGE and Western blotting with Ste3p-specific antibodies. Arrows indicate the positions of the mono- and diubiquitinated receptors; dashes indicate the positions of proteins that cross-react with the Ste3p antibodies.
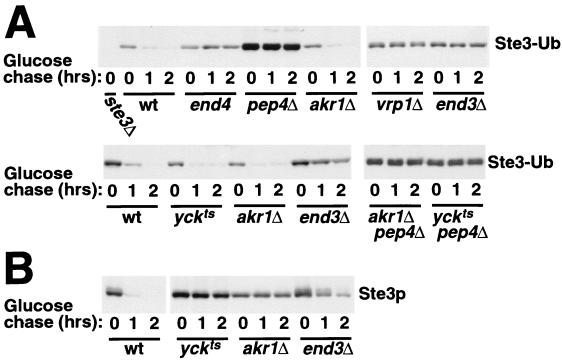
As a further test of the action points of these mutants, we have examined the participation of these functions in the uptake of an Ste3-Ub fusion protein. We thought that having ubiquitin preattached to the receptor as a translational fusion might allow the early endocytic functions that are normally required for Ste3p ubiquitination to be bypassed. The Ste3-Ub fusion used for this analysis has the 7K→R ubiquitin fused to the C terminus of a truncated Ste3p lacking its C-terminal 71 residues (including the 58-residue-long, C-terminal PEST-like endocytosis-ubiquitination signal) (34). The 7K→R ubiquitin has all seven of its lysine residues replaced by arginines and thus is incapable of nucleating the formation of a multiubiquitin chain. Previous analysis demonstrated that this Ste3-Ub fusion turns over rapidly in wild-type cells but does not turn over in either end4 or pep4 mutants, indicating that Ste3-Ub turnover depends on the endocytosis to the vacuole (34). Figure 2A shows that Ste3-Ub turnover also is blocked in the end3Δ and vrp1Δ cells. Thus, Ste3-Ub turnover, like wild-type Ste3p turnover, depends on the three actin-associated endocytic functions END3, END4, and VRP1. The akr1 and yck mutants stand in sharp contrast: neither appears to retard Ste3-Ub turnover (Fig. 2A). The Ste3-Ub turnover seen for akr1 and yck cells is PEP4 dependent (Fig. 2A), indicating that it retains the typical characteristic of endocytic transport to the vacuole. In parallel, we have also monitored the turnover of wild-type Ste3p in akr1, yck, and end3 cells (Fig. 2B); as has been shown previously (10, 28), turnover of wild-type Ste3p is virtually blocked in both akr1 and yck cells. Thus, the translational preattachment of ubiquitin confers upon the receptor the capacity to bypass the Akr1p and Yck1p-Yck2p requirements for endocytosis. Once the receptor has been ubiquitinated, Akr1p and Yck1p-Yck2p become dispensable for endocytosis. Taken together with the above Ste3p ubiquitination result (Fig. 1), we conclude that Akr1p and the Yck1p-Yck2p kinases are required early in the Ste3p constitutive endocytosis pathway, at a step prior to the ubiquitination event, likely functioning to promote this ubiquitination. Conversely, action points for End3p, End4p, and Vrp1p map downstream of the ubiquitination event. These actin-associated functions may be required for the recognition of the ubiquitinated substrate or, alternatively, for the physical uptake of the ubiquitinated substrate into the cell.
FIG. 2.
Turnover of an Ste3-Ub fusion protein in the endocytosis-defective mutants. A 2-h period of GAL1-driven expression of Ste3-Ub (A) or Ste3p (B) was induced in wild-type (wt) or end mutant yeast cultures with galactose addition and terminated with glucose addition. Fifteen minutes prior to glucose addition, cultures were shifted from 25 to 37°C. Coincident with the glucose addition (0-h time point) and at the indicated glucose chase time points, culture aliquots were removed and treated with energy poisons (see Materials and Methods). Extracts were then prepared, and the loss of the Ste3 antigen was monitored by Western blotting with Ste3p-specific antibodies. (A) Ste3-Ub turnover. Wild-type and end mutant MATα GAL1-STE3-UB strains from two different strain backgrounds were used. Strains in the end4-1 background (top panel) included wild-type (NDY990), end4-1 (NDY991), pep4Δ (NDY992), akr1Δ (NDY1011), vrp1Δ (NDY1042), and end3Δ (NDY1076) strains. NDY343 (ste3Δ) was included as control for the specificity of Ste3p antibodies. Strains in the yck1Δ yck2ts background (bottom panel) included wild-type (NDY1012), end3Δ (NDY1074), yck1Δ yck2ts (NDY1090), akr1Δ (NDY1085), akr1Δ pep4Δ (NDY1218), and yck1Δ yck2ts pep4Δ (NDY1223) strains. The high level of Ste3-Ub protein apparent in NDY992 cells (pep4Δ) likely reflects the augmented growth rate of this strain relative to the isogenic wild-type strain seen in raffinose- and/or galactose-containing culture media, not to the pep4 turnover blockade: the end mutants in which turnover is blocked (e.g., end3, end4, vrp1, yckts, and akr1 mutants) do not have similar levels of Ste3-Ub protein over accumulation. (B) Ste3p turnover in wild-type and end mutant cells. Four isogenic MATα GAL1-STE3 strains were used for this analysis, i.e., wild-type (NDY877), yck1Δ yck2ts (NDY913), akr1Δ (NDY1083), and end3Δ (NDY1118) strains.
Participation of the YCKs and AKR1 in Ste3p phosphorylation.
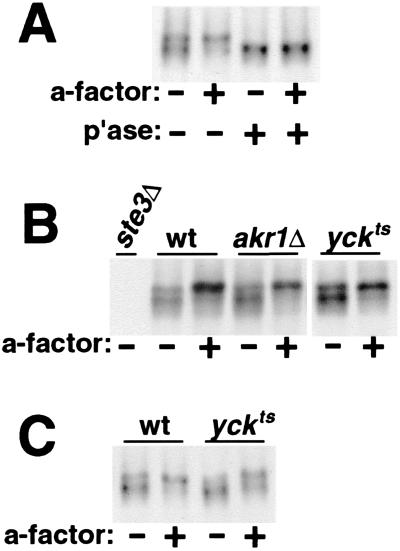
Ste3p is subject to a constitutive level of phosphorylation, which increases when cells are stimulated by pheromone (35). Much of this phosphorylation maps to the Ser- and Thr-rich regulatory CTD (8). Isolated from resting cells unstimulated by pheromone, Ste3p displays a heterogeneous gel mobility consistent with a heterogeneous constitutive phosphorylation (Fig. 3A). When isolated from a-factor-treated cells, receptor gel mobility is slowed, consistent with increased phosphorylation. Both the constitutive and ligand-induced phosphorylations collapse to a single, faster-migrating species with phosphatase treatment (Fig. 3A).
FIG. 3.
Effects of akr1 and yck mutations on Ste3p phosphorylation. The constitutive and a-factor-induced phosphorylation of Ste3p was monitored by assessing the effects of a-factor treatment on the gel mobility of newly synthesized Ste3p. MATα cells were pulse-labeled for 10 min with [35S]methionine-cysteine, chased for 10 min with excess cold amino acids, and then treated for an additional 15 min with a-factor or mock treated in parallel. This protocol allows the labeled Ste3p to be maximally available at the cell surface for binding the a-factor ligand. Protein extracts were prepared from the cells, and the Ste3p purified by immune precipitation was subjected to SDS-PAGE and autoradiography. (A) Constitutive and ligand-induced phosphorylation of Ste3p. Extracts from wild-type MATα cells (NDY414), treated with or without a-factor pheromone, were either digested with potato acid phosphatase (p'ase) or mock digested (no phosphatase added). The NDY414 strain used for this experiment has Ste3p expressed from the HIS3 promoter instead of from its natural promoter. The level of Ste3p expression from the HIS3 promoter is increased two- to threefold relative to the basal-level expression from its natural promoter. The increased expression results in no obvious alteration to the profile of phosphorylated species apparent by this pulse-chase analysis (compare with panels B and C; Ste3p for these panels is expressed from its natural endogenous promoter). (B) Effects of the akr1 and yck mutations on Ste3p phosphorylation for cells growing at 30°C. Wild-type (wt) MATα cells (LRB759) as well as isogenic akr1Δ (NDY1037) and yck1Δ yck2ts (LRB757) cells were cultured at 30°C and then labeled and treated with a-factor as described above. (C) Effects of the yck mutations on Ste3p phosphorylation for cells growing at 37°C. Wild-type (LRB759) and yck1Δ yck2ts mutant (LRB757) cells cultured at 25°C were shifted to 37°C 10 min prior to the start of pulse-labeling.
Yck1p and Yck2p are required for Ste2p phosphorylation: in yck1Δ yck2ts cells isogenic to mutants used in the present study, a striking block to both the constitutive and α-factor-induced levels of receptor phosphorylation was seen (16). We were interested to see if early AKR1- or YCK-dependent steps in Ste3p endocytosis might involve phosphorylation of the receptor. In Fig. 3B, we have examined the constitutive and ligand-induced phosphorylation of Ste3p in wild-type, akr1Δ, and yck1Δ yck2ts cells growing at 30°C. While 30°C is permissive for yck1Δ yck2ts cell growth, Ste3p constitutive endocytosis was found to be strongly impaired at this temperature (data not shown); indeed, severe effects on Ste3p constitutive endocytosis in yck1Δ yck2ts cells even at 25°C were reported (28). Yet despite these effects on Ste3p endocytosis, we can discern no effect on either constitutive or ligand-dependent phosphorylation in either akr1 or yckts mutant cells (Fig. 3B). We have also tested Ste3p phosphorylation in yckts cells at 37°C (nonpermissive for growth) (Fig. 3C). At this elevated temperature, some slight impairment of phosphorylation is apparent for Ste3p isolated both from the a-factor-treated and untreated cells. These subtle effects of the yck1Δ yck2ts mutation on Ste3p phosphorylation stand in marked contrast to the strong impairment seen for Ste2p in the isogenic MATa yck1Δ yck2ts background (see Fig. 5) (16). This difference between the two yeast pheromone receptors perhaps is not surprising given the recent description of the signaling circuit that couples a-factor binding to the feedback phosphorylation of the receptor (8). Here too, striking differences for the two pheromone receptors were found for the regulation of phosphorylation, implying that distinct kinase systems, at least in part, may be acting upon the two receptors (8).
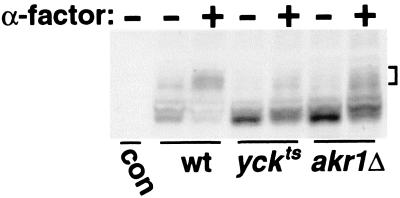
FIG. 5.
Akr1p is required together with Yck1p-Yck2p for the α-factor-induced phosphorylation of Ste2p. Cultures of three isogenic MATa strains, i.e., wild-type (wt) (LRB758), yck1Δ yck2ts (LRB756), and akr1Δ (NDY1215) strains, growing in rich medium at 25°C were shifted to 37°C for 15 min and then either treated with 10−6 M α-factor (+) for 10 min or mock treated in parallel (−). Extracts were prepared and analyzed by Western blotting with a Ste2p-specific antiserum (provided by James Konopka, SUNY at Stony Brook). Extracts prepared from the isogenic MATα strain LRB759, which does not express Ste2p, were included as a control for the specificity of the antiserum (con). The brackets at right indicate the positions of the hyperphosphorylated Ste2p species present following α-factor treatment.
While Yck1p and Yck2p clearly do not constitute the sole or even the major kinase system acting upon Ste3p (Fig. 3B and C), they may nonetheless still participate in Ste3p phosphorylation, perhaps acting specifically for the phosphorylation of the Ste3p PEST-like sequence. A YCK-dependent phosphorylation of this sequence could activate it as a signal for ubiquitination, thereby accounting for the strong impairment of Ste3p constitutive endocytosis seen in the yck mutant (28). Many of the PEST sequences that direct proteosome-mediated turnover are activated through phosphorylation (17, 31). Indeed, the Ste3p PEST-like sequence with its Ser-Thr residues embedded within an acidic residue context provides an attractive target for type I casein kinases (9). To focus analysis more directly upon the PEST-like sequence, we have constructed an in-frame deletion within Ste3p. Δ320-413 deletes 94 residues from the 183-residue-long Ste3p CTD, including 18 potential Ser-Thr phosphorylation sites. The PEST-like sequence together with its 15 potential Ser-Thr residues are retained. The CTD is largely dispensable for the gross functioning of the receptor (6), and the STE3Δ320-413 allele complements ste3 null alleles for mating (data not shown). Furthermore, as this mutant retains the C-terminal PEST-like sequence (residues 413 to 470), it remains subject to rapid, YCK- and AKR1- dependent constitutive endocytosis (data not shown).
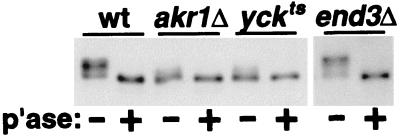
In Fig. 4, we have compared the phosphorylation of the Δ320-413 receptor in wild-type cells to its phosphorylation in end3Δ, akr1Δ, or yck1Δ yck2ts cells. Phosphorylation was assessed by examining the effects of phosphatase treatment on Ste3Δ320-413p gel migration. Clear phosphorylation of Ste3Δ320-413p is apparent in both wild-type and end3Δ cells (Fig. 4); for both, phosphatase treatment results in a striking change in receptor mobility. On the other hand, migration of the Δ320-413 receptor isolated either from akr1Δ cells or from yck1Δ yck2ts cells is not dramatically affected by phosphatase treatment (Fig. 4). Thus, with the Δ320-413 receptor as the substrate, a phosphorylation defect in yck and akr1 cells is unmasked, suggesting that the YCKs and Akr1p may indeed collaborate to phosphorylate and activate the Ste3p PEST-like signal.
FIG. 4.
Effects of akr1 and yck mutations on the phosphorylation of Ste3Δ320-413p. MATα GAL1-STE3Δ320-413 cells, either wild-type (wt) (NDY1039), akr1Δ (NDY1061), yck1Δ yck2ts (NDY1045), or end3Δ (NDY1070), growing in raffinose medium at 25°C were shifted to 30°C 1 h prior to the initiation of galactose-induced expression. Following a 90-min period of galactose-induced expression, cell extracts were prepared and were treated with phosphatase (p'ase) (+) or mock treated (−). Samples were then analyzed by SDS-PAGE and Western blotting with Ste3p-specific antibodies.
AKR1 is required for Ste2p phosphorylation.
Yck1p and Yck2p are required for both the constitutive and α-factor-induced phosphorylation of Ste2p, the α-factor pheromone receptor (16). Given the common phenotypes noted above for akr1 and yck mutants, we wondered if Akr1p might also participate. Extracts prepared from either α-factor-treated or untreated wild-type MATa cells and isogenic akr1Δ and yck1Δ yck2ts mutants were analyzed by Western blotting as previously described (16). A 10-min α-factor treatment of wild-type cells results in a striking change in Ste2p gel mobility; the bulk of the receptor population shifts to a more slowly migrating cluster (Fig. 5). We have previously shown through phosphatase treatment that this Ste2p mobility shift is primarily due to induced phosphorylation; the ubiquitinated Ste2p species induced with this pheromone treatment are less prominent and migrate to higher-molecular-weight positions (8). Consistent with the previous report (16), this induced phosphorylation is largely blocked in yck1Δ yck2ts cells (Fig. 5); only a small fraction of the receptor population undergoes a demonstrable shift upon α-factor treatment. Compared to wild-type cells, akr1Δ cells also are clearly defective for the α-factor-induced phosphorylation (Fig. 5). We note, however, that the phosphorylation defect manifested by these akr1Δ cells is somewhat less severe than that found for the isogenic yckts strain (Fig. 5). Nonetheless, for both mutants, the bulk of the receptor protein is not subject to a major shift in gel mobility, indicating that in both mutants the α-factor induced phosphorylation of Ste2p is strongly impaired. Thus, we conclude that Akr1p is required together with Yck1p-Yck2p for this phosphorylation.
Ste3p ligand-dependent endocytosis in yck and akr1 cells.
It has been reported that while being required for the constitutive endocytosis of Ste3p, Akr1p is dispensable for the alternative, Ste3p ligand-dependent uptake mode (10). We were interested to see if the yckts mutant might have the same phenotype as the akr1 mutant in this regard as well. The ligand-dependent mode of Ste3p uptake has been observed mainly under conditions which disable rapid constitutive endocytosis (7, 10). For instance, while the Δ365 truncated Ste3p mutant which lacks the PEST-like endocytosis signal is blocked for constitutive endocytosis, it remains capable of a ligand-induced endocytosis, which is apparent when Ste3Δ365p-expressing cells are exposed to the a-factor ligand (7). Unlike the constitutive uptake mode, which is coupled to rapid degradation of the receptor in the vacuole, our recent evidence indicates that the Ste3p ligand-dependent pathway links to two distinct receptor fates: while some of the internalized receptor is subject to a slow PEP4-dependent degradation, the bulk of the internalized receptor recycles back to the cell surface (L. Chen and N. G. Davis, submitted for publication).
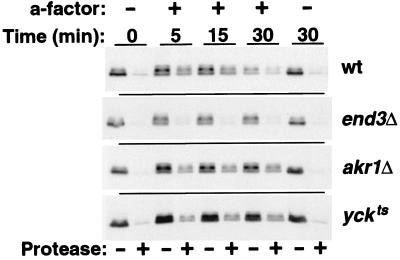
In Fig. 6, we have examined the ligand-induced uptake of the Δ365 truncated Ste3p in wild-type, akr1Δ, yck1Δ yck2ts, and end3Δ cells. Following the challenge of the cells with a-factor, Ste3Δ365p internalization was monitored via a protease-shaving protocol in which intact cells are treated with proteases. Receptor which localizes to the cell surface is susceptible to digestion by the added, extracellular proteases, while receptor which localizes to intracellular compartments (e.g., endosomes or vacuoles) is protected from digestion (7, 35). At the outset, prior to a-factor addition, receptor in all four cell backgrounds localizes to the cell surface, as is evident from its protease susceptibility (0-min time point in Fig. 6). In order to maximize the potential yck defect, cells were shifted to 37°C (nonpermissive for yck1Δ yck2ts strain growth) prior to a-factor addition. This elevated temperature results in several changes to Ste3Δ365p ligand-induced endocytosis in the wild-type background relative to the usual 30°C condition (34). Most obvious is an increase in uptake kinetics: rather than an uptake t1/2 of 30 to 40 min at 30°C (34; Chen and Davis, submitted), at 37°C the bulk of Ste3Δ365p is found to internalize over the first 5 min of a-factor treatment (Fig. 6). In addition, the Ste3Δ365p turnover rate also is dramatically increased at 37°C (Fig. 6); over 50% of the receptor protein appears to be degraded with the first 30 min of a-factor treatment, while at 30°C under identical a-factor treatment and cell growth conditions, the t1/2 for Ste3Δ365p turnover was found to be 90 min (Chen and Davis, submitted). Nonetheless, the overall trend is the same at the two temperatures: a-factor induces Ste3Δ365p internalization and ultimately its vacuolar degradation (Fig. 6). In end3Δ cells, neither feature of ligand-dependent uptake is evident; the Δ365 receptor remains surface localized and there is no a-factor-induced turnover (Fig. 6). As previously reported (10), akr1Δ cells remain competent for substantial ligand-dependent uptake (Fig. 6), with approximately 40% of the receptor protein being internalized with the initial 5 min of pheromone treatment. However, in contrast to the previous report (10), endocytosis is clearly perturbed in akr1 cells: the fraction of the receptor population internalized over the initial 5-min period is reduced, and turnover is largely abolished (Fig. 6). Furthermore, following the initial ligand-induced uptake of approximately 40% of the receptor protein over the first 5 min, internalization comes to a halt in akr1Δ cells, with no additional net internalization over the subsequent 25 min of a-factor treatment. (This difference from the previous report [10] may reflect the use of different strain backgrounds or different experimental temperatures [37 versus 30°C]). The yck1Δ yck2ts cells behave identically to the akr1Δ cells; again a rapid plateau is achieved, with approximately 40% of the Δ365 receptor being rapidly internalized, but then this plateau persists, with a failure to turn over (Fig. 6). Thus, the two endocytic functions that were distinguished for constitutive endocytosis as being early acting, i.e., Akr1p and Yck1p-Yck2p, are again clearly differentiated from the actin-associated function End3p in terms of effects on ligand-dependent endocytosis.
FIG. 6.
Akr1p and Yck1p-Yck2p remain partially competent for Ste3p ligand-induced endocytosis. The a-factor-induced internalization of the Δ365 Ste3p mutant was assessed by monitoring its changing availability to added, extracellular proteases. Uptake of the Δ365 mutant was followed in four isogenic MATα GAL1-STE3Δ365 strains, i.e., wild-type (wt) (NDY1072), end3Δ (NDY1117), akr1Δ (NDY1216), and yck1Δ yck2ts (NDY662) strains. Thirty minutes following a 90-min period of galactose-induced Ste3Δ365p expression (terminated by glucose addition), cultures were shifted from 25 to 37°C (nonpermissive for the yckts mutant). Following an additional 15 min at 37°C, cultures were treated with a-factor or mock treated. At the indicated times following the start of the pheromone treatment, culture aliquots were removed and the intact cells were digested with proteases (see Materials and Methods). Extracts prepared from these cells were analyzed by Western blotting with Ste3p-specific antibodies.
As we discuss below (see Discussion), the differences noted in akr1 and yck mutants for a-factor-dependent endocytosis may reflect a participation of these functions not at the uptake step of this pathway but rather at the downstream endocytic trafficking decisions which control sorting to the vacuole (and degradation) versus recycling back to the cell surface.
AKR1 is required for the cell surface localization of Yck2p.
The common phenotypes of akr1Δ and yck1Δ yck2ts mutants suggest the possibility of functional collaboration. Akr1p could act together with the YCKs as a subunit of the active kinase complex. Alternatively, Akr1p might bind to the PEST-like sequence of Ste3p newly delivered to the plasma membrane and serve to present this signal to the YCKs for phosphorylation. A third possibility, tested below, is that Akr1p is required for proper localization of the YCKs to the plasma membrane. Plasma membrane localization of Yck1p and Yck2p depends upon prenylation of C-terminal dicysteine sequences (48).
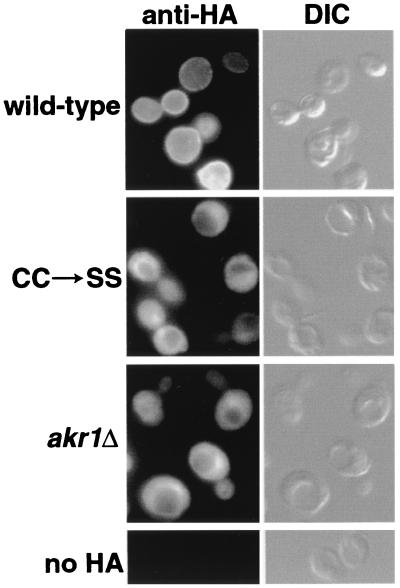
We have examined the localization of an HA epitope-tagged Yck2p by indirect immunofluorescence in wild-type and akr1Δ cells (Fig. 7). The tagged Yck2p functionally complements the yck1Δ yck2ts mutant, allowing growth at 37°C. We have also examined the localization of a mutant Yck2p that has its C-terminal Cys-Cys prenylation motif replaced by Ser-Ser (CC→SS). In wild-type cells, Yck2p localizes primarily to the cell surface (Fig. 7). Consistent with previous reports (32, 48), surface localization of Yck2p depends on prenylation: instead of localizing to the plasma membrane, the CC→SS mutant shows a diffuse, vacuole-excluded cytoplasmic localization. In some cells, the mutant Yck2p concentrates within a region adjacent to the vacuole. This area of mutant Yck2p concentration coincided with the nucleus identified by DAPI (4′,6′-diamidino-2-phenylindole) staining (data not shown), suggesting that CC→SS Yck2p may concentrate either within the nucleus or within perinuclear portions of the endoplasmic reticulum.
FIG. 7.
Akr1p is required for localization of Yck2p to the plasma membrane. The wild-type strain (NDY877) or the isogenic akr1Δ strain (NDY1083) transformed by one of two plasmids carrying GAL1-driven YCK2 constructs N-terminally tagged by the 3xHA epitope (either pND1092, which carries the tagged wild-type Yck2p, or pND1113, which carries the equivalently tagged Yck2p mutant which has its C-terminal Cys-Cys prenylation motif replaced by Ser-Ser [CC→SS]) was used. Following a 2-h period of galactose-induced expression, cells were removed from culture and fixed, and localization of the wild-type or mutant tagged Yck2p was probed in an indirect immunofluorescence protocol using the mouse HA.11 MAb directed against the HA epitope, followed by Cy3-conjugated secondary antibody directed against mouse immunoglobulin-G. As a control for the cross-reaction of the HA.11 MAb, NDY877 cells not carrying an HA-tagged construct were fixed and processed in parallel (no HA). A Nomarski (differential interference contrast [DIC]) image of each cell grouping is shown just to the right of the fluorescent image (anti-HA).
Like the cis-acting CC→SS mutation, the trans-acting akr1Δ mutation also abolishes Yck2p surface localization (Fig. 7). Indeed, localization of the tagged wild-type Yck2p in akr1Δ cells is strikingly similar to that seen for the CC→SS mutant: a diffuse cytoplasmic localization, excluded from the vacuole but often concentrating within the perinuclear region. We conclude that Akr1p function is required for proper localization of Yck2p (and likely Yck1p as well) to the plasma membrane.
The localization of the HA-tagged Yck2p differs from the surface localization reported previously for a green fluorescent protein (GFP)-Yck2p fusion. While the GFP-Yck2p fusion displayed a cell cycle-dependent localization to sites of polarized cell growth (32), the HA-tagged Yck2p was found to distribute fairly homogeneously around the cell surface (Fig. 7). This is likely not an artifactual consequence of the epitope tagging, since both the GFP and HA epitope tag are fused at the same Yck2p N-terminal site. Rather, we feel that this difference is more likely a consequence of expression; while GFP-Yck2p was expressed from the YCK2 promoter, our HA-Yck2p was overexpressed from the GAL1 promoter.
DISCUSSION
Akr1p and the YCKs act early for Ste3p constitutive endocytosis.
Two functions have been identified as acting early, prior to the ubiquitination event, for the constitutive uptake of Ste3p: Akr1p and the redundant casein kinase pair Yck1p-Yck2p. In addition to being defective for receptor ubiquitination, mutants with mutations in these two functions also were found to be defective for the phosphorylation of Ste3Δ320-413p. While having much of the Ste3p CTD together with the majority of the potential Ser-Thr CTD phosphorylation sites deleted, the Δ320-413 receptor mutant retains a functional PEST-like endocytosis signal together with its 15 potential Ser-Thr phosphorylation sites. As both uptake and phosphorylation of Ste3Δ320-413p require Akr1p and Yck1p-Yck2p function, we suggest, as has been previously suggested for the α-factor pheromone receptor (16) and the uracil permease (23), that phosphorylation likely is a prerequisite for ubiquitination of Ste3p and thus also for its constitutive endocytosis. Unlike the case for Ste2p, which shows a more complete dependence of receptor phosphorylation on the YCKs (16), our results suggest for Ste3p that the YCKs may be devoted specifically to phosphorylation of the PEST-like sequence. While Yck1p and Yck2p may act directly in phosphoryl activation of the PEST-like signal, the Akr1p requirement appears to be for proper localization of the kinases to the plasma membrane. In akr1 mutants, the YCKs are mislocalized to the cytoplasm and thus fail to efficiently find their plasma membrane-localized receptor substrate.
The activation of PEST sequences through phosphorylation is a common theme in the ubiquitin-dependent proteosomal pathway (17, 31), and thus it is not surprising to find it utilized again here for ubiquitin-dependent endocytosis. Furthermore, while we presently cannot provide evidence of direct phosphorylation of the Ste3p PEST-like sequence by the Yck1p and Yck2p kinases, this sequence, with its abundance of acidic residues, is expected to be an attractive substrate for type I casein kinases (9).
Common phenotypes of akr1 and yck mutants.
In addition to early action in Ste3p constitutive endocytosis, other phenotypes link Akr1p and Yck1p-Yck2p. Both are required for Ste2p phosphorylation (Fig. 5). Indeed, the Akr1p requirement for Ste2p phosphorylation likely accounts for the reported Akr1p requirement for Ste2p endocytosis as well (10, 16). In addition, mutations in the two functions show similar effects on the ligand-dependent endocytosis of Ste3Δ365p: both mutants remain partially competent for uptake, and for both, uptake appears to be decoupled from turnover (Fig. 6). In both mutants following a-factor challenge, a period of initially rapid ligand-dependent internalization is followed by a period in which continued receptor internalization is curtailed; a plateau to receptor internalization is achieved, with approximately 40% of the receptor internalized and the remaining 60% localized to the cell surface. In wild-type cells growing at 30°C (rather than the 37°C utilized for Fig. 6) an internalization plateau strikingly similar to that observed in the akr1 and yck mutant backgrounds (Fig. 6) was seen (Chen and Davis, submitted). Such plateaus are easily explained within the context of receptor recycling, reflecting an equilibrium where continued internalization of the receptor is balanced by the rate of its recycling return to the cell surface; at this equilibrium point, net internalization halts. In wild-type cells, two fates are coupled to Ste3p ligand-dependent uptake: while the bulk of internalized receptor recycles back to the cell surface, some receptor is delivered to the vacuole for turnover (Chen and Davis, submitted). For the akr1 and yck mutants, turnover appears to be blocked (Fig. 6), suggesting a defect specific for the degradative arm of this uptake pathway. Thus, while YCK-dependent phosphorylation may not be required for triggering the ligand-dependent uptake of Ste3p, Yck1p-Yck2p phosphorylation of the receptor or of other endocytic components may function at a downstream trafficking event to augment endosome-to-vacuole transport of the receptor.
We have seen that the participation of Akr1p in endocytosis may be understood in terms of its requirement for proper Yck localization. Might other aspects of the akr1 phenotypes be explained in this way? Both akr1 and yck mutants perturb normal yeast cell morphogenesis; cells are often enlarged with elongated buds and are sometimes multinucleate (20, 33). Consistent with a morphogenetic role, a GFP-Yck2p fusion protein shows a changing localization during the cell cycle, localizing to polarized sites of cell surface growth and cytokinesis (32). Given the Akr1p requirement for cell surface localization of Yck2p (Fig. 7), we expect this polarized plasma membrane distribution of Yck2p to be largely abolished in akr1Δ cells.
Do other functions act together with Akr1p to localize Yck1p and Yck2p? One candidate identified as a two-hybrid interactor of Akr1p is Gcs1p (20). gcs1Δ cells show morphogenetic effects similar to those observed in akr1 and yck cells. Furthermore, the cold sensitivity of the gcs1Δ mutant is suppressed by multicopy YCK1 or YCK2 (50); overproduction of the kinases might partially compensate for defective Yck localization, making more kinase activity available to the plasma membrane. It will be interesting to see if gcs1 mutants, like akr1 mutants, act to perturb Yck localization and/or function.
Role of Akr1p in YCK localization.
How does Akr1p support the plasma membrane localization of the YCKs? The plasma membrane localization of Yck1p and Yck2p depends upon a C-terminal Cys-Cys prenylation signal (32, 48). The Yck2p mislocalization in akr1Δ cells, we found, mirrored the diffuse cytoplasmic localization seen for the prenylation defective CC→SS Yck2p mutant, suggesting that Akr1p may function in Yck2p prenylation. However, the enzymatic system responsible for protein prenylation has been extensively characterized (52), and Akr1p has yet to turn up as a component.
Prenylation of C-terminal CC motifs has been studied to date only for members of the Rab G protein family. For these, the two cysteines are both modified with geranylgeranyl addition (diGG) via a prenylation reaction catalyzed by the devoted Rab geranylgeranyl transferase (GGTase) acting together with the Rab escort protein (REP) (52). In yeast, Bet2p and Bet4p comprise the α and β components of the Rab GGTase, and Mrs6p is the REP homologue (19). Rab prenylation differs in its requirements from the prenylation of C-terminal CAAX motifs (farnesylation or geranylgeranylation mediated by the farnesyl transferase or the type I GGTase, respectively). For CAAX prenylation, no accessory escort protein is required and the key substrate recognition element appears to be the CAAX site itself. For the Rab prenylation, recognition of the folded Rab structure is key (38, 39, 42), and the C-terminal CC by itself does not suffice as a prenylation signal (21). REP, which shares sequence identity with another Rab-interacting protein, Rab GDI, binds tightly to unmodified, monoGG-modified, and diGG-modified Rab proteins (2) and appears to provide the specificity component for directing the Rab GGTase to the Rab protein substrate (1). Given this Rab specificity, Rab GGTase-REP may not be the system responsible for Yck1p-Yck2p prenylation. Thus, while the C-terminal CC clearly is required for the plasma membrane localization of Yck2p (32, 48), the nature of its lipid modification remains an open question.
AKR1 encodes an 86-kDa protein with five predicted membrane-spanning domains, six ankyrin repeats mapping to an N-terminal hydrophilic domain, and a DHHC cysteine-rich zinc finger-like domain (3) located between predicted transmembrane domains. The DHHC homology is conserved through an evolutionarily diverse family of membrane proteins of unknown function from fungi, plants, and mammals (3). Perhaps significantly, another member of this DHHC family, yeast Erf2p, has been identified as participating in the palmitoylation and plasma membrane localization of Ras2p (3). For some yeast and mammalian Ras proteins, palmitoylation of cysteinyl residues adjacent or proximal to the CAAX motif is required together with the added farnesyl group both for sustained membrane association and for normal plasma membrane localization (4, 12). Unlike that of prenylation, the enzymology of palmitoylation remains quite poorly understood. It will be interesting to see if and how DHHC proteins participate in this lipid modification reaction. Future work will examine the Yck2p lipid modifications. Is Yck2p, like the Rab proteins, modified by diGG, or is one of the two C-terminal cysteines modified by palmitate? Is Akr1p function required for these modifications?
ACKNOWLEDGMENTS
We thank Peter Pryciak and Duane Jenness for insights; Lucy Robinson, Alan Bender, Jamie Konopka, and Charlie Boone for plasmids, strains, and antiserum; Jeff Loeb for help with the microscopy, and finally Linyi Chen and Amy Roth for input and support throughout the course of this work.
This work was supported by a grant from the National Science Foundation (MCB 95-06839).
REFERENCES
- 1.Anant J S, Desnoyers L, Machius M, Demeler B, Hansen J C, Westover K D, Deisenhofer J, Seabra M C. Mechanism of Rab geranylgeranylation: formation of the catalytic ternary complex. Biochemistry. 1998;37:12559–12568. doi: 10.1021/bi980881a. [DOI] [PubMed] [Google Scholar]
- 2.Andres D A, Seabra M C, Brown M S, Armstrong S A, Smeland T E, Cremers F P, Goldstein J L. cDNA cloning of component A of Rab geranylgeranyl transferase and demonstration of its role as a Rab escort protein. Cell. 1993;73:1091–1099. doi: 10.1016/0092-8674(93)90639-8. [DOI] [PubMed] [Google Scholar]
- 3.Bartels D J, Mitchell D A, Dong X, Deschenes R J. Erf2, a novel gene product that affects the localization and palmitoylation of Ras2 in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:6775–6787. doi: 10.1128/mcb.19.10.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya S, Chen L, Broach J R, Powers S. Ras membrane targeting is essential for glucose signaling but not for viability in yeast. Proc Natl Acad Sci USA. 1995;92:2984–2988. doi: 10.1073/pnas.92.7.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonifacino J S, Weissman A M. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu Rev Cell Dev Biol. 1998;14:19–57. doi: 10.1146/annurev.cellbio.14.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boone C, Davis N G, Sprague G F., Jr Mutations that alter the third cytoplasmic loop of the a-factor receptor lead to a constitutive and hypersensitive phenotype. Proc Natl Acad Sci USA. 1993;90:9921–9925. doi: 10.1073/pnas.90.21.9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis N G, Horecka J L, Sprague G F., Jr Cis- and trans-acting functions required for endocytosis of the yeast pheromone receptors. J Cell Biol. 1993;122:53–65. doi: 10.1083/jcb.122.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng Y, Davis N G. Feedback phosphorylation of the yeast a-factor receptor requires activation of the downstream signaling pathway from G protein through mitogen-activated protein kinase. Mol Cell Biol. 2000;20:563–574. doi: 10.1128/mcb.20.2.563-574.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flotow H, Roach P J. Role of acidic residues as substrate determinants for casein kinase I. J Biol Chem. 1991;266:3724–3727. [PubMed] [Google Scholar]
- 10.Givan S A, Sprague G F., Jr The ankyrin repeat-containing protein Akr1p is required for the endocytosis of yeast pheromone receptors. Mol Biol Cell. 1997;8:1317–1327. doi: 10.1091/mbc.8.7.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guldener U, Heck S, Fielder T, Beinhauer J, Hegemann J H. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996;24:2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hancock J F, Paterson H, Marshall C J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- 13.Heilker R, Spiess M, Crottet P. Recognition of sorting signals by clathrin adaptors. Bioessays. 1999;21:558–567. doi: 10.1002/(SICI)1521-1878(199907)21:7<558::AID-BIES4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 14.Hicke L. Gettin' down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol. 1999;9:107–112. doi: 10.1016/s0962-8924(98)01491-3. [DOI] [PubMed] [Google Scholar]
- 15.Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- 16.Hicke L, Zanolari B, Riezman H. Cytoplasmic tail phosphorylation of the α-factor receptor is required for its ubiquitination and internalization. J Cell Biol. 1998;141:349–58. doi: 10.1083/jcb.141.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- 18.Huang K M, D'Hondt K, Riezman H, Lemmon S K. Clathrin functions in the absence of heterotetrameric adaptors and AP180-related proteins in yeast. EMBO J. 1999;18:3897–3908. doi: 10.1093/emboj/18.14.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Y, Ferro-Novick S. Identification of yeast component A: reconstitution of the geranylgeranyltransferase that modifies Ypt1p and Sec4p. Proc Natl Acad Sci USA. 1994;91:4377–4381. doi: 10.1073/pnas.91.10.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kao L R, Peterson J, Ji R, Bender L, Bender A. Interactions between the ankyrin repeat-containing protein Akr1p and the pheromone response pathway in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:168–178. doi: 10.1128/mcb.16.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khosravi-Far R, Clark G J, Abe K, Cox A D, McLain T, Lutz R J, Sinensky M, Der C J. Ras (CXXX) and Rab (CC/CXC) prenylation signal sequences are unique and functionally distinct. J Biol Chem. 1992;267:24363–24368. [PubMed] [Google Scholar]
- 22.Kuchler K, Sterne R E, Thorner J. Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J. 1989;8:3973–3984. doi: 10.1002/j.1460-2075.1989.tb08580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchal C, Haguenauer-Tsapis R, Urban-Grimal D. A PEST-like sequence mediates phosphorylation and efficient ubiquitination of yeast uracil permease. Mol Cell Biol. 1998;18:314–321. doi: 10.1128/mcb.18.1.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 25.Merrifield C J, Moss S E, Ballestrem C, Imhof B A, Giese G, Wunderlich I, Almers W. Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nat Cell Biol. 1999;1:72–74. doi: 10.1038/9048. [DOI] [PubMed] [Google Scholar]
- 26.Michaelis S, Herskowitz I. The a-factor pheromone of Saccharomyces cerevisiae is essential for mating. Mol Cell Biol. 1988;8:1309–1318. doi: 10.1128/mcb.8.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naqvi S N, Zahn R, Mitchell D A, Stevenson B J, Munn A L. The WASp homologue Las17p functions with the WIP homologue End5p/verprolin and is essential for endocytosis in yeast. Curr Biol. 1998;8:959–962. doi: 10.1016/s0960-9822(98)70396-3. [DOI] [PubMed] [Google Scholar]
- 28.Panek H R, Stepp J D, Engle H M, Marks K M, Tan P K, Lemmon S K, Robinson L C. Suppressors of YCK-encoded yeast casein kinase 1 deficiency define the four subunits of a novel clathrin AP-like complex. EMBO J. 1997;16:4194–4204. doi: 10.1093/emboj/16.14.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payne G S, Baker D, van Tuinen E, Schekman R. Protein transport to the vacuole and receptor-mediated endocytosis by clathrin heavy chain-deficient yeast. J Cell Biol. 1988;106:1453–1461. doi: 10.1083/jcb.106.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raths S, Rohrer J, Crausaz F, Riezman H. end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J Cell Biol. 1993;120:55–65. doi: 10.1083/jcb.120.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rechsteiner M, Rogers S W. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 32.Robinson L C, Bradley C, Bryan J D, Jerome A, Kweon Y, Panek H R. The Yck2 yeast casein kinase 1 isoform shows cell cycle-specific localization to sites of polarized growth and is required for proper septin organization. Mol Biol Cell. 1999;10:1077–1092. doi: 10.1091/mbc.10.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson L C, Menold M M, Garrett S, Culbertson M R. Casein kinase I-like protein kinases encoded by YCK1 and YCK2 are required for yeast morphogenesis. Mol Cell Biol. 1993;13:2870–2881. doi: 10.1128/mcb.13.5.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth A F, Davis N G. Ubiquitination of the PEST-like endocytosis signal of the yeast a-factor receptor. J Biol Chem. 2000;275:8143–8153. doi: 10.1074/jbc.275.11.8143. [DOI] [PubMed] [Google Scholar]
- 35.Roth A F, Davis N G. Ubiquitination of the yeast a-factor receptor. J Cell Biol. 1996;134:661–674. doi: 10.1083/jcb.134.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth A F, Sullivan D M, Davis N G. A large PEST-like sequence directs the ubiquitination, endocytosis, and vacuolar degradation of the yeast a-factor receptor. J Cell Biol. 1998;142:949–961. doi: 10.1083/jcb.142.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothstein R J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- 38.Sanford J C, Pan Y, Wessling-Resnick M. Prenylation of Rab5 is dependent on guanine nucleotide binding. J Biol Chem. 1993;268:23773–23776. [PubMed] [Google Scholar]
- 39.Sanford J C, Pan Y, Wessling-Resnick M. Properties of Rab5 N-terminal domain dictate prenylation of C-terminal cysteines. Mol Biol Cell. 1995;6:71–85. doi: 10.1091/mbc.6.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schandel K A, Jenness D D. Direct evidence for ligand-induced internalization of the yeast alpha-factor pheromone receptor. Mol Cell Biol. 1994;14:7245–7255. doi: 10.1128/mcb.14.11.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmid S L. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 42.Seabra M C. Nucleotide dependence of Rab geranylgeranylation. Rab escort protein interacts preferentially with GDP-bound Rab. J Biol Chem. 1996;271:14398–14404. doi: 10.1074/jbc.271.24.14398. [DOI] [PubMed] [Google Scholar]
- 43.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J. 1996;15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- 45.Tan P K, Davis N G, Sprague G F, Payne G S. Clathrin facilitates the internalization of seven transmembrane segment receptors for mating pheromones in yeast. J Cell Biol. 1993;123:1707–1716. doi: 10.1083/jcb.123.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan P K, Howard J P, Payne G S. The sequence NPFXD defines a new class of endocytosis signal in Saccharomyces cerevisiae. J Cell Biol. 1996;135:1789–1800. doi: 10.1083/jcb.135.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terrell J, Shih S, Dunn R, Hicke L. A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol Cell. 1998;1:193–202. doi: 10.1016/s1097-2765(00)80020-9. [DOI] [PubMed] [Google Scholar]
- 48.Vancura A, Sessler A, Leichus B, Kuret J. A prenylation motif is required for plasma membrane localization and biochemical function of casein kinase I in budding yeast. J Biol Chem. 1994;269:19271–19278. [PubMed] [Google Scholar]
- 49.Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Hoekstra M F, DeMaggio A J, Dhillon N, Vancura A, Kuret J, Johnston G C, Singer R A. Prenylated isoforms of yeast casein kinase I, including the novel Yck3p, suppress the gcs1 blockage of cell proliferation from stationary phase. Mol Cell Biol. 1996;16:5375–5385. doi: 10.1128/mcb.16.10.5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wendland B, Emr S D, Riezman H. Protein traffic in the yeast endocytic and vacuolar protein sorting pathways. Curr Opin Cell Biol. 1998;10:513–522. doi: 10.1016/s0955-0674(98)80067-7. [DOI] [PubMed] [Google Scholar]
- 52.Zhang F L, Casey P J. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]