Abstract
CD40, a tumor necrosis factor (TNF) receptor (TNFR) family member, conveys signals regulating diverse cellular responses, ranging from proliferation and differentiation to growth suppression and cell death. The ability of CD40 to mediate apoptosis in carcinoma cells is intriguing given the fact that the CD40 cytoplasmic C terminus lacks a death domain homology with the cytotoxic members of the TNFR superfamily, such as Fas, TNFR1, and TNF-related apoptosis-inducing ligand (TRAIL) receptors. In this study, we have probed the mechanism by which CD40 transduces death signals. Using a trimeric recombinant soluble CD40 ligand to activate CD40, we have found that this phenomenon critically depends on the membrane proximal domain (amino acids 216 to 239) but not the TNFR-associated factor-interacting PXQXT motif in the CD40 cytoplasmic tail. CD40-mediated cytotoxicity is blocked by caspase inhibitors, such as zVAD-fmk and crmA, and involves activation of caspase 8 and caspase 3. Interestingly, CD40 ligation was found to induce functional Fas ligand, TRAIL (Apo-2L) and TNF in apoptosis-susceptible carcinoma cells and to up-regulate expression of Fas. These findings identify a novel proapoptotic mechanism which is induced by CD40 in carcinoma cells and depends on the endogenous production of cytotoxic cytokines and autocrine or paracrine induction of cell death.
CD40, a member of the tumour necrosis factor (TNF) receptor (TNFR) superfamily, is expressed on a plethora of different cell types, including B cells, macrophages, dendritic cells, endothelial cells, and fibroblasts, and this widespread expression is likely to account for the central role of CD40 in the regulation of humoral immunity and host defense (54). Studies from our and other laboratories have shown that CD40 is also expressed in normal basal epithelial cells in stratified squamous epithelium and in a number of carcinomas, including ovarian, nasopharyngeal, bladder, and breast, where its precise role remains elusive (15, 55, 74, 75). The ligand for CD40 (CD40L) (gp39 or CD154) is a 39-kDa type II integral membrane protein with homology to TNF which can be induced on T cells following their activation via the T-cell receptor (54). CD40L expression has also been reported in B cells, monocytes, and NK cells, and a soluble form of this molecule has been detected in the serum of patients with hematological malignancies (73).
The central role of CD40-CD40L interactions in orchestrating immune responses is emphasized by studies of mice lacking CD40 or CD40L. In these knockout animals, thymus-dependent responses to foreign antigens, such as immunoglobulin production, isotype switching, and somatic hypermutation are impaired (39, 72). A similar phenotype (HIGMX) is observed in patients with hyperimmunoglobulin M syndrome, a genetic disease which results from mutations in the CD40L gene (6). Interestingly, HIGMX individuals also appear to be prone to development of tumors of the pancreas and liver (30). Our recent work also implicates the CD40 pathway in hepatocyte death during liver allograft rejection through a cooperative interaction with Fas, another member of the TNFR superfamily (1).
In vitro studies have shown that while CD40 ligation provides an antiapoptotic and proliferative signal for normal resting B cells (26), CD40 stimulation in lymphoblastoid and Burkitt's lymphoma cells induces growth inhibition (2, 22). CD40 ligation in carcinoma cell lines also results in growth inhibition and sensitizes these cells to apoptosis induced by a variety of agents, including TNF-α, anti-Fas, and cytotoxic drugs (15). Furthermore, when exogenously expressed, CD40 has been shown to transduce apoptotic signals in certain cell lines of epithelial or mesenchymal origin (31), but the mechanism of this phenomenon is unknown. In agreement with these in vitro findings, a recombinant soluble form of CD40L has been found to inhibit the growth of breast carcinoma cells in xeno-transplanted SCID mice (32), an observation which underlines the potential therapeutic use of CD40L for the treatment of carcinomas. In addition to its growth-regulatory properties, CD40 ligation in cell lines of epithelial or B-cell origin induces homotypic cell adhesion, up-regulation of various cell surface markers, and cytokine production (2, 11, 18, 25).
The signalling pathways that are activated by CD40 stimulation and thereby control its diverse effects on cellular phenotype have been the subject of intense investigation. While the cytoplasmic C terminus of CD40 lacks intrinsic kinase activity, adapter proteins of the TNFR-associated factor (TRAF) family, most notably TRAF2 and TRAF6, appear to mediate the activation of CD40 signalling cascades such as the cJun N-terminal kinase (JNK) and NF-κB (53, 58, 66). A TRAF2- and TRAF6-dependent extracellular signal-regulated protein kinase (ERK) mitogen-activated protein kinase signal is induced by CD40 ligation in cells of epithelial but not of B-cell origin (37, 61). Other pathways activated by CD40 stimulation include the JAK3-STAT3 (29) and phosphatidyl inositol 3-kinase–Akt (57), which may contribute to the antiapoptotic properties conferred by CD40L in B cells. Further insight into the differential activation and integration of these signals is required to explain the diverse phenotypic consequences of CD40-CD40L interactions in different cell types.
In this study, we have probed the mechanism by which CD40 transduces death signals in carcinoma cells. We have identified the membrane-proximal domain of CD40 as being important for apoptosis induction and shown that this phenomenon occurs through a crmA-sensitive, caspase-dependent pathway involving activation of cytotoxic ligands of the TNF family.
MATERIALS AND METHODS
DNA constructs.
The pcDNA3-based CD40 expression vector pc-CD40 has been previously described (19). A PXQXT254→PXQXA mutation was generated from pc-CD40 using the Quick Change site-directed mutagenesis kit of Stratagene and mutated primers 5′-GCTCCAGTGCAGGAGGCTTTACATGGATGCC-3′ and its complementary primer. To generate CD40 deletion mutant Δ(216-239), CD40 amino acids (aa) 1 to 215 were PCR amplified using a forward primer with an artificial HindIII site upstream from the start codon (5′-CTGGTCTAAGCTTGCCATGGTTC-3′) and a reverse primer with an artificial Asp718 site (5′-GCTTCTTGGTACCCTT TTTGATAAAG-3′) and introduced into HindIII/Asp718-digested pcDNA3. CD40 aa 240 to 278 were then PCR amplified using a forward primer with an Asp718 site (5′-GGAGATCAATGGTACCGACGATC-3′) and a reverse primer with a NotI site downstream from the CD40 stop codon (5′-ACCCACCGCCGGCGGAGTGA-3′). This PCR fragment was digested with Asp718 and NotI and inserted in frame in CD40 aa 1 to 215 in pcDNA3 to give pc-CD40Δ[216-239]. The presence of the above mutations was verified by sequencing.
Cell culture and treatment.
Carcinoma cell line MG75 was generated from an epithelial ovarian solid tumor, and MG79 was established from tumor cells present in the ascitic fluid of a patient with adenocarcinoma of the ovary (24) (kindly provided by M. Gilligan, Institute for Cancer Studies, University of Birmingham, Birmingham, United Kingdom). These carcinoma cell lines together with HeLa cervical and A2780 ovarian carcinoma cells were maintained in RPMI supplemented with 10% fetal calf serum. To generate stable HeLa clones expressing wild-type or mutated CD40, 2 × 106 cells were electroporated (125 μF, 450 V) with 10 μg of plasmid DNA in 0.8 ml of ice-cold phosphate-buffered saline, and subsequent selection was performed in growth medium supplemented with 600 to 650 μg of G418 (Geneticin; Gibco BRL) per ml.
For induction of apoptosis, carcinoma cell lines were plated on a 96-well plate at 8,000 cells per well in 0.2 ml of complete medium in triplicate. The following day cells were treated for 6 h with 1 μg of trimeric recombinant soluble (rsCD40L) (52) (kindly provided by Immunex Corporation, Seattle, Wash.) per ml or with 1 μg of a soluble CD40L with enhancer cross-linking antibody (Alexis Corporation) per ml and then cocultured with cycloheximide (CHX) (Sigma) for an additional 24 h time period. Alternatively, cells were treated with CH11 anti-Fas monoclonal antibody (MAb) (Immunotech), soluble Fas ligand (FasL) (Alexis Corporation) or TNF-related apoptosis-inducing ligand (TRAIL) with enhancer (Alexis Corporation). In these experiments, HeLa cells were treated with 50 μg of CHX per ml as previously described (31), MG75 and MG79 cells were exposed to 8 μg of CHX per ml, and A2780 cells were exposed to 10 to 15 μg of CHX per ml. Under these conditions, CHX caused very limited or no toxicity in these cell lines. For neutralization experiments, cells were pretreated for 1 h with anti-CD40L MAb (Ancell Corporation), NOK1 anti-FasL MAb (Pharmingen), TRAILR1:Fc (R&D Systems), neutralizing anti-TNF-α (R&D Systems), or neutralizing anti-interleukin 6 (IL-6) antibody (R&D Systems) and then cocultured with rsCD40L and CHX as described above. Purified OX34 anti-CD2 MAb was kindly provided by M. Rowe, University of Cardiff, United Kingdom. For apoptosis inhibition experiments using caspase inhibitors, cells were pretreated for 30 min with 50 μM zVAD-fmk (Calbiochem) in 0.1 ml of complete medium before addition of rsCD40L and CHX; final concentration of zVAD was then 25 μM and was maintained throughout the assay. The cell-permeating and irreversible caspase 3 and caspase 8 specific inhibitors zDEVD-fmk and zIETD-fmk, respectively, were purchased from Calbiochem.
Apoptosis assays.
Following treatment, cells were trypsinized and fixed in 1% paraformaldehyde. Cell suspensions were stained with propidium iodide (5 μg/ml), and apoptotic cells were identified on the basis of nuclear condensation and degradation and counted. A minimum of 300 cells were counted in each experiment, and assessments were performed independently by two of the authors (A.G.E. and C.D.). In some experiments, apoptosis was determined using electrophoretic analysis of DNA fragmentation (17) or in situ terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assay (Promega), according to the manufacturer's instructions.
Coimmunoprecipitations.
Human kidney embryonic 293 cells were plated on 100-cm2 dishes and allowed to adhere overnight. The following day cells were transfected with 8 μg of CD40A or Δ(216-239) expression vectors in the presence of pCMV-TRAF2 (14), Flag-tagged pME-TRAF6 (66), or control vectors using calcium phosphate. Thirty-six hours later, the cells were lysed in 0.5 ml of lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 3% glycerol, 1.5 mM EDTA, 0.5% NP-40) supplemented with protease inhibitors, and 500 μg of cell lysates was incubated overnight at 4°C with 2 μg of EA5 anti-CD40 MAb (Ancell). Following a 2-h incubation with protein G-Sepharose beads, immunoprecipitates were resolved by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis and immunoblotted for TRAF2 (C20; Santa Cruz Biotechnology), TRAF6 (M2 anti-Flag; Kodak), and CD40 (C20; Santa Cruz Biotechnology) accordingly.
JNK in vitro kinase assays.
Following stimulation, cells were washed twice in ice-cold phosphate-buffered saline and lysed in 1 ml of kinase lysis buffer (20 mM Tris [pH 7.6], 0.5% Triton X-100, 250 mM NaCl, 3 mM EGTA, 3 mM EDTA, 2 mM sodium vanadate, aprotinin (10 μg/ml), leupeptin (10 μg/ml), and 1 mM dithiothreitol) for 20 min on ice. JNK was immunoprecipitated from 200 μg of total protein extracts using 5 μl of anti-JNK1 antibody (Santa Cruz Biotechnology) for endogenous JNK and 0.8 μg of anti-hemagglutinin MAb (Boehringer/Roche) for transfected hemagglutinin-tagged JNK. In vitro kinase assays were subsequently performed as previously described (16, 19).
Generation of crmA recombinant adenovirus.
The crmA cDNA was PCR amplified from a crmA expression vector (kindly provided by Chris Gregory, University of Nottingham, Nottingham, United Kingdom) using primers with artificial BglII sites: 5′-CAAAATAGATCTCCATGGATATCTTC-3′ (crmA forward) and 5′-GA ATGAGATCTAATTAGTTGTTG-3′ (crmA reverse). The PCR product was digested with BglII, cloned in BamHI-digested pMC3 adenovirus transfer vector (4), and sequenced. A recombinant crmA adenovirus was generated by methods first described by McGrory and coworkers (49). Briefly, recombinant virus was obtained following cotransfection in 293 cells of pMC3/crmA and pJM17 (containing the entire Ad5dl309 genome). As a result of homologous recombination between these two plasmids, crmA under the control of the cytomegalovirus immediate-early promoter was inserted into the adenovirus genome in place of E1 gene sequences. Progeny virus was taken through several rounds of plaque purification before expansion of viral stocks.
Caspase 3 and caspase 8 activity assays.
Caspase 3 activity in cell extracts from carcinoma cells exposed to various agents was determined using the caspase 3 cellular activity colorimetric assay kit from Calbiochem. Briefly, 106 cells were lysed in 30 μl of a lysis buffer containing 50 mM HEPES (pH 7.4), 1 mM dithiothreitol, 0.1 mM EDTA, and 0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), supplemented with 0.1% NP-40 (Sigma). Protein concentration was determined using the Bio-Rad protein assay kit and 30 μg of total cell lysates were then incubated with 200 μM caspase 3 substrate Ac-DEVD-pNA in assay buffer at 37°C for 5 h. Absorbance at 405 nm was determined on a microtiter plate reader. Recombinant caspase 3 (30 U) was used as a positive control. For inhibition studies, cell lysates were preincubated for 15 min with the caspase inhibitors Ac-DEVD-CHO and zVAD-fmk at final concentrations of 0.1 μM before the addition of caspase 3 substrate. Activation of caspase 8 was detected by immunoblot analysis. After the indicated treatment, cells were lysed in JNK kinase lysis buffer supplemented with protease inhibitors, and 100 μg of cell lysates was resolved by sodium dodecyl sulfate–15% polyacrylamide gel electrophoresis. Immunoblotting was performed with an anti-caspase 8 antibody (C20; Santa Cruz Biotechnology) followed by enhanced chemiluminescence (ECL) (Amersham).
Reverse transcription (RT)-PCR.
cDNA synthesis from 3 μg of total RNA was performed as previously described (16). Primers used were as follows. For FasL, the forward primer was 5′-GGTCCATGCCTCTGGAATGG-3′, the reverse primer was 5′-CACATCTGCCCAGTAGTGCA-3′, and the probe was 5′-ATGAGGAACTCTAAGTATCC-3′. For TRAIL (Apo-2L [56]), the forward primer was 5′-AGACCTGCGTGCTGATCGT-3′, the reverse primer was 5′-GACCAGTTCACCATTCCTC-3′, and the probe was 5′-GTAGCAGCTCACATAACT-3′. For CD40L, the forward primer was 5′-AGAATCCTCAAATTGCGGC-3′, the reverse primer was 5′-TGTGGGTATTTGCAGCTCTG-3′, and the probe was 5′-ATGCCCAAGTCACCTTCTGT-3′. Conditions for 38 cycles of PCR amplification were as follows: for FasL, 94°C for 45 s, 54°C for 50 s, and 72°C for 50 s; for TRAIL, 94°C for 40 s, 48°C for 40 s, and 72°C for 50 s; for CD40L, 94°C for 45 s, 49°C for 60 s, and 72°C for 60 s. TNF-α primers and PCR conditions have been previously described (27). The oligonucleotide used as a TNF-α-specific probe was 5′-TGAGGCCAAGCCCTGGTAT-3′. FasL, TRAIL, CD40L, and TNF-α products (25 μl) and positive controls for FasL and CD40L (5 μl) were analyzed on a 1.5% gel before transfer onto a nylon membrane (Hybond N+; Amersham) and hybridization. For PCR amplification of CD40, primers 5′-ATGGTTCGTCTGCCTCTG-3′ (CD40-forward) and 5′-TCACTGTCTCTCCTGCACTGA-3′ (CD40-reverse), which amplify the entire CD40 cDNA, were used. Conditions for 30 cycles of PCR amplification were 94°C for 40 s, 51°C for 45 s, and 72°C for 60 s. Products (5 μl) were analyzed on a 0.8% agarose gel.
RESULTS
rsCD40L induces apoptosis in carcinoma cells.
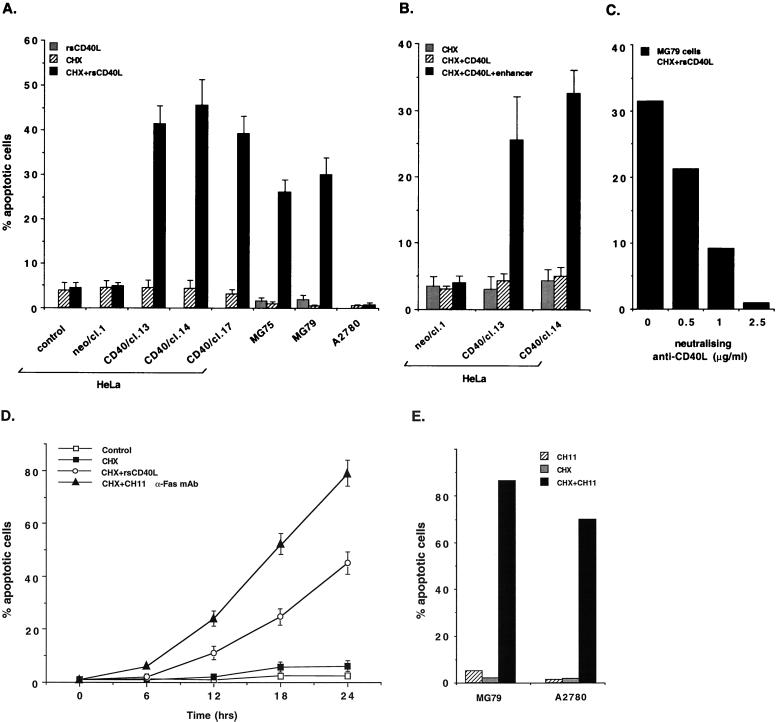
Previous work has demonstrated that membrane-anchored CD40L induces apoptosis in CD40-transfected HeLa cells when de novo protein synthesis is inhibited (31). To assess the effects of CD40 engagement on carcinoma cell death we have used an rsCD40L molecule in which the extracellular domain of CD40L is linked to an isoleucine zipper trimerization motif (52). This soluble trimeric molecule mimics membrane-bound CD40L function and retains full biological activity in resting B cells. HeLa clones stably expressing CD40 (Table 1) were treated for 6 h with 1 μg of rsCD40L ml and then cocultured with the protein synthesis inhibitor CHX for an additional 24 h time period. Cell death was quantitated using propidium iodide staining and fluorescence microscopy. These experiments demonstrated that 40 to 50% of HeLa/CD40 cells undergo apoptosis following treatment with rsCD40L and CHX (Fig. 1A). The ability of CD40 ligation to transduce apoptotic signals in CD40-transfected HeLa cells was verified by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling and electrophoretic DNA fragmentation assays (data not shown). Induction of cell death, although to a lesser degree, was also observed using similar concentrations of an alternative source of monomeric rsCD40L coupled with a cross-linking antibody which allows dimerization of soluble ligand monomers (Fig. 1B).
TABLE 1.
CD40 and Fas expression in carcinoma cell linesa
| Cell line | Expression (MFI) of:
|
||
|---|---|---|---|
| CD40 | Fas
|
||
| Control | rsCD40L treated | ||
| HeLa | 0.2 | 1.5 | 1.5 |
| HeLa/neo clone 1 | 0.2 | 1.7 | 1.7 |
| HeLa/CD40 clone 13 | 13.2 | 1.6 | 3.7 |
| HeLa/CD40 clone 14 | 22.8 | 1.7 | 3.9 |
| HeLa/CD40 clone 17 | 11.5 | NDb | ND |
| HeLa/CD40A clone 1 | 16 | 1.6 | 3.3 |
| HeLa/CD40A clone 17 | 24.8 | 1.7 | 3.4 |
| HeLa/CD40Δ(216-239) clone 8 | 13.5 | 1.3 | 1.4 |
| HeLa/CD40Δ(216-239) clone 9 | 2.4 | ND | ND |
| HeLa/CD40Δ(216-239) clone 10 | 16.5 | 1.2 | 1.4 |
| MG75 | 1.6 | 0.6 | 1.3 |
| MG79 | 17.2 | 1.6 | 2.3 |
| A2780 | 20.1 | 1.3 | 1.4 |
CD40 and Fas expression in carcinoma cell lines was determined by flow cytometry using the anti-CD40 MAb G28.5 and anti-Fas MAb UB2, respectively. The mean fluorescence intensities (MFI) from a representative experiment are shown. Only background staining (MFI < 0.3) was observed using an isotype control antibody (data not shown).
ND, not determined.
FIG. 1.
Recombinant soluble forms of CD40L induce apoptosis in carcinoma cells. (A) An rsCD40L molecule in which the extracellular domain of CD40L is linked to an isoleucine zipper trimerization motif induces cell death in carcinoma cells. The effect of CD40 ligation on survival was assessed by propidium iodide staining and fluorescence microscopy, and data are depicted as percentages of apoptotic cells (y axis) relative to untreated controls. Mean values ± standard deviations (error bars) from at least three independent experiments are shown. cl., clone. (B) CD40 oligomerization is required for effective activation of death signals in susceptible carcinoma cell lines. CD40-transfected HeLa cells were treated for 6 h with a monomeric form of CD40L (1 μg/ml; Alexis Corporation) in the presence or absence of an enhancer cross-linking molecule and then cocultured with CHX for an additional 24 h time period before apoptotic cells were counted. CD40L induced cell death only in the presence of the cross-linking antibody. No cytotoxic effect was noted upon treatment with the enhancer molecule alone (data not shown). Error bars, standard deviations. (C) Specificity of rsCD40L-induced cell death. MG79 ovarian carcinoma cells were pretreated with neutralizing anti-CD40L antibody and then exposed to rsCD40L (1 μg/ml) and CHX (8 μg/ml) treatment, as described in Materials and Methods. Apoptotic cells were counted, and mean values from two independent experiments are shown. (D) CD40 engagement induces a delayed and reduced apoptotic response compared to Fas. HeLa/CD40 clone 14 cells were treated for 6 h with rsCD40L (1 μg/ml) or CH11 (10 ng/ml) anti-Fas MAb and then cocultured with CHX for various times (0, 6, 12, 18, or 24 h). Apoptotic cells were counted and mean values ± standard deviations (error bars) from three independent experiments are shown. (E) Fas induces high levels of apoptosis in carcinoma cells which respond (MG79 ovarian carcinoma) or are resistant (A2780 ovarian carcinoma) to CD40-mediated cytotoxicity. Data shown are representative of two independent experiments.
This phenomenon was not restricted to carcinoma cells expressing exogenous CD40, as MG75 and MG79 ovarian tumor cell lines which naturally express CD40 (Table 1) also responded to rsCD40L treatment by induction of apoptosis (Fig. 1A). This effect on viability was specific for CD40, as a neutralizing anti-CD40L MAb blocked rsCD40L-induced apoptosis in MG79 cells (Fig. 1C). However, CD40-positive A2780 ovarian carcinoma cells were resistant to CD40L-induced cell death even when high amounts of recombinant ligand (5 μg/ml) were used (Fig. 1A). These experiments demonstrate that exposure of some but not all carcinoma cell lines to soluble trimeric CD40L induces apoptosis when de novo protein synthesis is inhibited, a phenomenon which is reminiscent of the effects of anti-Fas treatment on carcinoma cell survival. Indeed, treatment of HeLa/CD40 clone 14 cells with low concentrations (10 ng/ml) of CH11 anti-Fas MAb in the presence of CHX induced cell death in a time-dependent manner, but anti-Fas alone had no effect (Fig. 1D and data not shown). This apoptotic response was more potent and rapid compared to that following CD40 ligation. In addition, treatment of this HeLa/CD40 clone with 1 μg of soluble FasL per ml and 50 μg of CHX per ml for 18 h induced approximately 90% cell death, which is considerably higher than the effect of rsCD40L at the same time point (data not shown). This was not particular to CD40-transfected HeLa cells, as MG79 ovarian carcinomas were also more sensitive to Fas-induced cytotoxicity (Fig. 1E). Furthermore, A2780 cells, which do not respond to CD40 ligation by apoptosis, were found to be susceptible to anti-Fas treatment (Fig. 1E). Thus, CD40 transduces a delayed and reduced apoptotic response in carcinoma cells compared to Fas.
A membrane-proximal domain but not a PXQXT motif in the CD40 cytoplasmic tail is critical for CD40L-induced apoptosis in carcinoma cells.
To identify the CD40 domains responsible for apoptosis induction, we have generated two mutations in the cytoplasmic tail of CD40, which are known to abrogate TRAF binding and influence signalling and phenotypic changes. Thus, using site-directed mutagenesis, a Thr254→Ala mutation (PXQXT→PXQXA) was introduced in the cytoplasmic tail of CD40 (CD40A). This point mutation is known to abrogate CD40 interaction with TRAF2 and TRAF3 but not TRAF6 (66) and represses at least some of the phenotypic consequences of CD40 stimulation, such as growth inhibition, induction of homotypic cell adhesion, and up-regulation of the costimulatory molecule B7.1 (11, 25, 33). In addition, we have constructed a CD40 deletion mutant lacking aa 216 to 239 [CD40Δ(216-239)], which are critical for interaction with TRAF6 but not TRAF2 (35, 66).
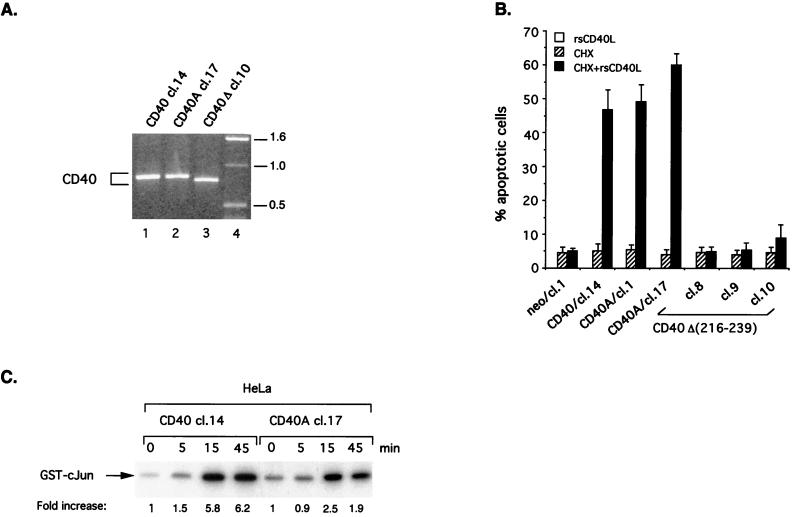
HeLa cells stably transfected with a cytomegalovirus promoter-driven CD40A or CD40Δ(216-239) expression vector were obtained [HeLa/CD40A clones 1 and 17 and HeLa/CD40Δ(216-239) clones 8, 9, and 10, respectively], and expression was verified using flow cytometry (Table 1) and RT-PCR (Fig. 2A). PCR-amplified CD40 cDNA from HeLa/CD40Δ(216-239) cells demonstrated a slightly higher electrophoretic mobility than the wild type or CD40A transfected clones, consistent with the presence of a 23-aa deletion in its CD40 cytoplasmic tail (Fig. 2A, lane 3). The effect of rsCD40L and CHX treatment on the viability of HeLa/CD40A and HeLa/CD40Δ(216-239) cells was assessed. Interestingly, it was found that HeLa/CD40A clones responded to CD40L-induced cytotoxicity to a similar degree as wild-type CD40-transfected cells (Fig. 2B). However, exposure of HeLa/CD40Δ(216-239) clone 8, 9, or 10 to rsCD40L and CHX failed to induce cell death, suggesting that the TRAF6-interacting domain of CD40 is critical for apoptosis induction (Fig. 2B).
FIG. 2.
A membrane-proximal domain but not a PXQXT motif in the CD40 cytoplasmic tail is critical for CD40L-induced apoptosis in carcinoma cells. (A) Expression of CD40 in representative CD40 and mutated CD40-transfected HeLa clones was verified by RT-PCR. PCR-amplified CD40 cDNA from HeLa/CD40 clone (cl.) 14 (lane 1), HeLa/CD40A cl. 17 (lane 2) and HeLa/CD40Δ(216-239) cl. 10 (lane 3) is shown. The higher electrophoretic mobility of the HeLa/CD40Δ(216-239) PCR product (lane 3) is consistent with the presence of a 23-aa deletion in the cytoplasmic tail of CD40. Lane 4 contains a molecular weight marker (labeled [in thousands] at right). (B) HeLa/CD40A but not HeLa/CD40Δ(216-239) cells are susceptible to CD40-mediated cell death. Cells were treated as described in the legend to Fig. 1A, and the percentage of apoptotic cells relative to untreated controls is depicted in histogram form. The percentage of apoptotic HeLa/CD40 cl. 14 cells is shown for comparison. Data are the mean values ± standard deviations (error bars) from three independent experiments. (C) HeLa/CD40A cells demonstrate decreased and delayed induction of JNK compared to wild-type CD40-expressing HeLa cells in response to CD40 stimulation. HeLa transfectants were treated with rsCD40L (1 μg/ml) for various time points (0, 5, 15, or 45 min), and cell lysates were subjected to immune complex kinase assays using glutathione S-transferase–cJun (aa 1 to 89) as substrate. Relative kinase activities were determined on a phosphorimager. Three independent experiments were performed and gave similar results.
To demonstrate that the Thr254→Ala mutated CD40 is functionally defective, the effects of CD40 engagement on JNK activation in CD40A versus CD40-transfected HeLa cells were examined. For this purpose, two clones which express similar levels of CD40 (HeLa/CD40 clone 14 and HeLa/CD40A clone 17 [Table 1]) were selected. Following CD40 stimulation, cell lysates from these cultures were immunoprecipitated with an anti-JNK1 antibody and assayed for kinase activity using glutathione S-transferase–cJun (aa 1 to 79) as substrate. CD40 ligation was found to induce a delayed and reduced JNK activation in HeLa/CD40A compared to HeLa/CD40 cells (Fig. 2C), in agreement with an important role for TRAF2 in CD40-mediated JNK induction (44, 53). Furthermore, transient expression of an N-terminally deleted dominant-negative TRAF6 inhibited CD40 and CD40A but not CD40Δ(216-239)-mediated JNK activation in HeLa cells, verifying that this CD40 membrane-proximal deletion mutant is functionally inactive with respect to TRAF6 signalling (data not shown). In addition, coimmunoprecipitation experiments in human embryonic kidney 293 cells verified the ability of CD40A to interact with TRAF6 but not TRAF2, while CD40Δ(216-239) was found to strongly associate with TRAF2 but not TRAF6 (data not shown). We therefore conclude that the membrane-proximal domain but not the PXQXT motif of the CD40 cytoplasmic tail is critical for CD40L-induced cell death in carcinoma cells.
CD40 ligation in apoptosis-susceptible carcinoma cells leads to caspase activation.
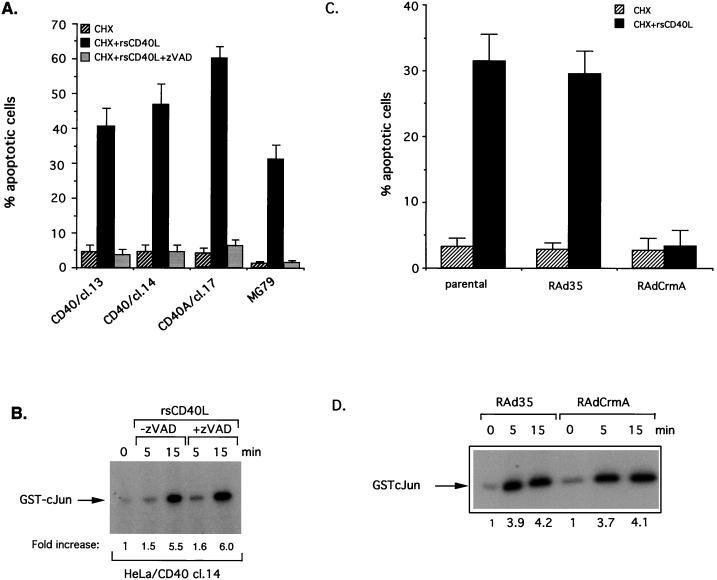
To probe the mechanism of CD40L-induced apoptosis, CD40-expressing carcinoma cells were exposed to rsCD40L and CHX in the presence of zVAD-fmk, a broad-spectrum caspase inhibitor. zVAD-fmk is known to inhibit anti-Fas or TNF-induced activation of caspases 1, 3, and 8, thereby preventing Fas and TNFR-mediated cytotoxicity (63). Interestingly, zVAD-fmk was also found to diminish CD40L-induced cell death in HeLa/CD40 cells as well as in HeLa/CD40A and MG79 cells (Fig. 3A). However, treatment of HeLa/CD40 clone 14 cells with this caspase inhibitor did not influence their ability to engage the JNK pathway in response to CD40 stimulation, suggesting that JNK is not critical for CD40-mediated apoptosis (Fig. 3B).
FIG. 3.
Caspase inhibitors block CD40-induced apoptotic but not JNK signals. (A) zVAD-fmk suppresses CD40-mediated apoptosis in HeLa/CD40, HeLa/CD40A, and MG79 cells. Cells were pretreated with zVAD-fmk for 30 min and then exposed to rsCD40L for 6 h before addition of CHX. The percentage of apoptotic cells relative to untreated controls was evaluated 24 h later. Data are the mean values ± standard deviations (error bars) from three independent experiments. cl., clone. (B) Exposure of CD40-expressing cells to concentrations of zVAD-fmk which block apoptosis does not interfere with CD40-mediated JNK activation. HeLa/CD40 cl. 14 cells were pretreated for 30 min with 25 μM zVAD-fmk or left untreated and then stimulated with rsCD40L (1 μg/ml) for various time intervals before being analyzed for endogenous JNK activity. (C) CrmA expression suppresses CD40-mediated apoptosis in MG79 ovarian carcinoma cells. Cells infected with RAd-CrmA or RAd35 control virus at a multiplicity of infection of 100 were treated with rsCD40L and CHX for 24 h as described in the legend to Fig. 1A, and the percentage of apoptotic cells relative to untreated controls was evaluated. Mean values ± standard deviations (error bars) from three independent experiments are shown. (D) CrmA does not influence JNK signalling. MG79 cells infected with RAd-CrmA or RAd35 control virus at a multiplicity of infection of 100 were treated with rsCD40L (1 μg/ml) for various time intervals and then analyzed for endogenous JNK using GST-cJun(1-89) as a substrate. Relative kinase activities are shown. Values along bottom indicate fold increase of MG79.
The cowpox virus-encoded protein crmA is a potent inhibitor of proapoptotic and proinflammatory caspases and also blocks anti-Fas and TNF-induced apoptosis (65). To determine if crmA also suppresses CD40-induced cell death, we constructed a crmA-expressing recombinant adenovirus (RAd-CrmA) and used it to infect MG79 ovarian carcinoma cells. RAd-CrmA infected MG79 cells maintained viability when challenged with rsCD40L in the presence of CHX, but infection with control virus delivering lacZ (RAd35 [70]) did not inhibit CD40L-induced cell death in these cells (Fig. 3C). Importantly, although crmA expression had a pronounced effect on viability, it did not influence the ability of CD40L to induce JNK activation in MG79 cells (Fig. 3D). We therefore conclude that caspase inhibitors block CD40-induced apoptotic but not JNK signals.
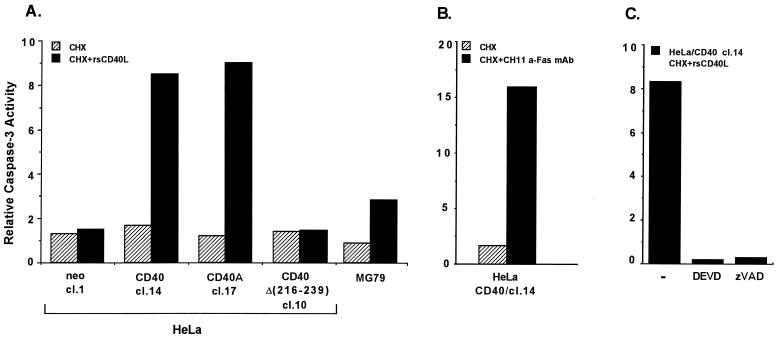
To verify that caspase activation occurs during CD40L-induced cell death, we used a colorimetric assay for the measurement of caspase 3 activity. Lysates from rsCD40L- and CHX-treated HeLa/CD40 clone 14, HeLa/CD40A clone 17 and MG79 cells demonstrated a three- to ninefold increase in enzymatic activity compared to untreated controls, which correlated with the extent of apoptosis in these cells (Fig. 4A). Treatment with rsCD40L alone had no effect, while CHX induced marginal increases in enzymatic activity (Fig. 4A and data not shown). Exposure of vector-transfected HeLa (HeLa/neo clone 1) or HeLa/CD40Δ(216-239) clone 10 cells to rsCD40L and CHX did not induce caspase 3 activity above background, in agreement with their inability to apoptose in response to CD40 stimulation (Fig. 4A). As a positive control, treatment of HeLa/CD40 clone 14 cells with 10 ng/ml CH11 anti-Fas MAb in the presence of CHX induced a 16-fold induction in caspase 3 activity (Fig. 4B), consistent with the ability of Fas to confer more potent apoptotic signals than CD40 in this cell line (Fig. 1D). These measurements were specific for caspase 3, as peptide inhibitors such as zVAD-fmk and the more selective Ac-DEVD-CHO blocked caspase 3 activity in lysates from rsCD40L- and CHX-treated HeLa/CD40 cells (Fig. 4C).
FIG. 4.
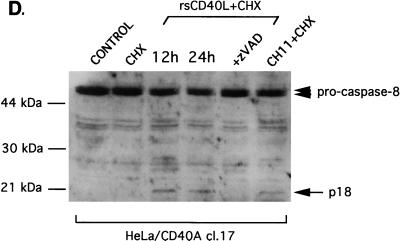
CD40 ligation mediates caspase activation in apoptosis-susceptible carcinoma cells. (A) Caspase 3 activity is induced in response to rsCD40L and CHX treatment and correlates with CD40-mediated induction of cell death in carcinoma cell lines. Active caspase 3 in lysates from rsCD40L and CHX-treated cells was measured using a colorimetric caspase 3 activity kit and Ac-DEVD-pNA as the substrate. Values shown represent the relative increase in caspase 3 activity compared to that in untreated cultures, given the arbitrary value of 1, and are representative of three independent experiments. cl., clone. (B) As a positive control, treatment of HeLa/CD40 cl. 14 cells with CH11 anti-Fas MAb (10 ng/ml) and CHX (50 μg/ml) induced robust caspase 3 activity. (C) Specificity of CD40-mediated caspase 3 activity. Lysates from rsCD40L and CHX-treated HeLa/CD40 cl. 14 cells were incubated with 0.1 μM Ac-DEVD-CHO or zVAD-fmk before being analyzed for caspase 3 activity. (D) Treatment of HeLa/CD40A cl. 17 cells with rsCD40L and CHX for 12 or 24 h (lanes 3 and 4, respectively) results in caspase 8 activation, as determined by the decrease in pro-caspase 8 levels (marked with arrowheads) and the appearance of the p18 cleaved, active form (arrow) in immunoblot analysis. As a negative control, untreated cultures or cells exposed to CHX alone did not demonstrate caspase 8 activity (lanes 1 and 2), while the p18 active form was detected in lysates from cells treated for 12 h with anti-Fas (10 ng/ml) in the presence of CHX (lane 6). Pretreatment with 50 μM zVAD-fmk suppressed CD40-mediated caspase 8 activation (lane 5).
We then investigated the effects of CD40 ligation on the activity of caspase 8, which functions upstream of caspase 3 in the Fas death pathway. Immunoblot analysis using an antibody specific for the active, cleaved form of caspase 8 (p18) demonstrated that treatment of HeLa/CD40A clone 17 cells with rsCD40L and CHX induced a reduction in pro-caspase 8 levels and formation of the fast-migrating p18 cleaved form (Fig. 4D, lanes 3 and 4). This caspase 8 activity was absent in lysates from CHX-treated cultures and was diminished in the presence of zVAD-fmk (Fig. 4D, lanes 2 and 5 respectively). The contribution of caspase 3 and caspase 8 activation to CD40-mediated apoptosis was verified by the ability of peptide inhibitors which specifically target these caspases, such as zDEVD-fmk and zIETD-fmk, respectively, to inhibit cell death in response to CD40L and CHX treatment (data not shown). Overall, these data provide the first demonstration that CD40-mediated apoptosis involves a crmA-sensitive, caspase 3- and caspase 8-dependent pathway.
CD40 stimulation induces the expression of Fas and cytotoxic ligands of the TNF superfamily in apoptosis-susceptible carcinoma cells.
The similarities between Fas and CD40-activated cell death pathways, coupled with the lack of a death domain sequence in the cytoplasmic tail of CD40, suggest that CD40-mediated apoptosis occurs through an indirect mechanism which may involve the Fas pathway. To examine this possibility, the effects of CD40 ligation on Fas expression in carcinoma cells were first assessed using flow cytometry. MG75, MG79, and HeLa/CD40 cells treated for 24 h with rsCD40L (1 μg/ml) demonstrated a small but significant and consistent increase in Fas cell surface expression (Table 1). Significant levels of Fas induction were also observed in HeLa cells carrying a Thr254→Ala mutation in the cytoplasmic tail of CD40 but not in HeLa/CD40Δ(216-239) cells (Table 1). However, treatment of HeLa/CD40Δ(216-239) cells with rsCD40L induced the expression of the cell surface marker ICAM1 (CD54, data not shown), suggesting that deletion of the membrane-proximal domain of CD40 may affect some but not all the phenotypic effects of CD40 stimulation. In addition, A2780 cells, which do not respond to CD40 stimulation by apoptosis, failed to induce Fas expression even when prolonged incubations with rsCD40L (36 or 48 h) were performed (Table 1 and data not shown).
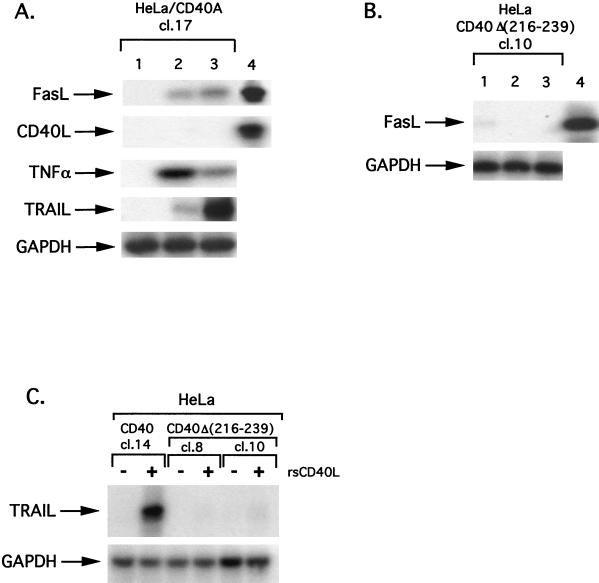
We then investigated the effects of CD40 ligation on FasL expression. For this purpose, RNA isolated from control untreated HeLa/CD40A clone 17 or from cells exposed to rsCD40L (1 μg/ml) for 2 or 12 h was subjected to semiquantitative RT-PCR using primers specific for FasL. RNA expression levels were normalized to those of GAPDH. As shown in Fig. 5A, CD40 ligation induced an increase in FasL expression levels in these cells, which was evident at 2 h and sustained at 12 h of rsCD40L treatment. Importantly, CD40 ligation was also able to induce the expression of other cytotoxic members of the TNF superfamily, such as TNF and TRAIL (Apo-2L). Strong induction of TNF RNA was observed as early as 2 h following CD40 stimulation, in agreement with a recent report (27), and levels then declined. The kinetics of TRAIL up-regulation was relatively slow, with only low levels of induction observed at 2 h and higher levels at 12 h of treatment (Fig. 5A). However, unlike the effects of rsCD40L on FasL, TNF, and TRAIL expression, CD40 engagement did not affect CD40L RNA, confirming the specificity of the observed phenomena (Fig. 5A).
FIG. 5.
CD40 ligation induces expression of Fas, FasL, TRAIL, and TNF in apoptosis-susceptible carcinoma cell lines. (A) CD40 ligation induces the expression of cytotoxic members of the TNF superfamily in apoptosis-susceptible carcinoma cells. RNA was isolated from HeLa/CD40A clone (cl.) 17 cells treated with rsCD40L (1 μg/ml) for 0, 2, or 12 h (lanes 1 to 3, respectively) and subjected to RT-PCR analysis for FasL, CD40L, TNF, or TRAIL (Apo-2L) expression, as described in Materials and Methods. Hybridization signals were normalized using GAPDH as the control. As a positive control for FasL and CD40L expression, RNA from RAd-FasL-infected MG79 cells (Knox et al., unpublished data) or from mouse L cells transfected with human CD40L was used (lane 4). Data are representative of at least three independent experiments. (B) HeLa/CD40Δ(216-239) cells fail to activate FasL RNA in response to CD40 ligation. RNA isolated from a representative HeLa/CD40Δ(216-239) clone treated with rsCD40L (1 μg/ml) for 0, 2, or 12 h (lanes 1 to 3, respectively) was subjected to RT-PCR analysis for expression of FasL or GAPDH. Lane 4 is a positive control for FasL expression as described above. (C) TRAIL is induced in CD40- but not CD40Δ(216-239)-transfected HeLa cells. HeLa/CD40 cl. 14, HeLa/CD40Δ(216-239) cl. 8, or HeLa/CD40Δ(216-239) cl. 10 cells were exposed to rsCD40L (1 μg/ml) for 12 h (lanes 2, 4, and 6, respectively) or left untreated (lanes 1, 3, and 5, respectively) before being analyzed for TRAIL or GAPDH expression. Two independent experiments were performed and gave similar results.
Interestingly, exposure of HeLa/CD40Δ(216-239) clone 10 or A2780 cells to rsCD40L failed to induce FasL expression (Fig. 5B and data not shown). In addition, while significant up-regulation of TRAIL RNA was observed in HeLa clones carrying wild-type or Thr254→Ala mutated CD40, CD40 ligation in HeLa/CD40Δ(216-239) clone 8 or clone 10 cells had no effect (Fig. 5C). We therefore conclude that CD40 stimulation induces the expression of Fas and cytotoxic ligands of the TNF superfamily in apoptosis-susceptible carcinoma cells.
Involvement of Fas or FasL and other cytotoxic ligands in CD40-mediated cell death in carcinoma cells.
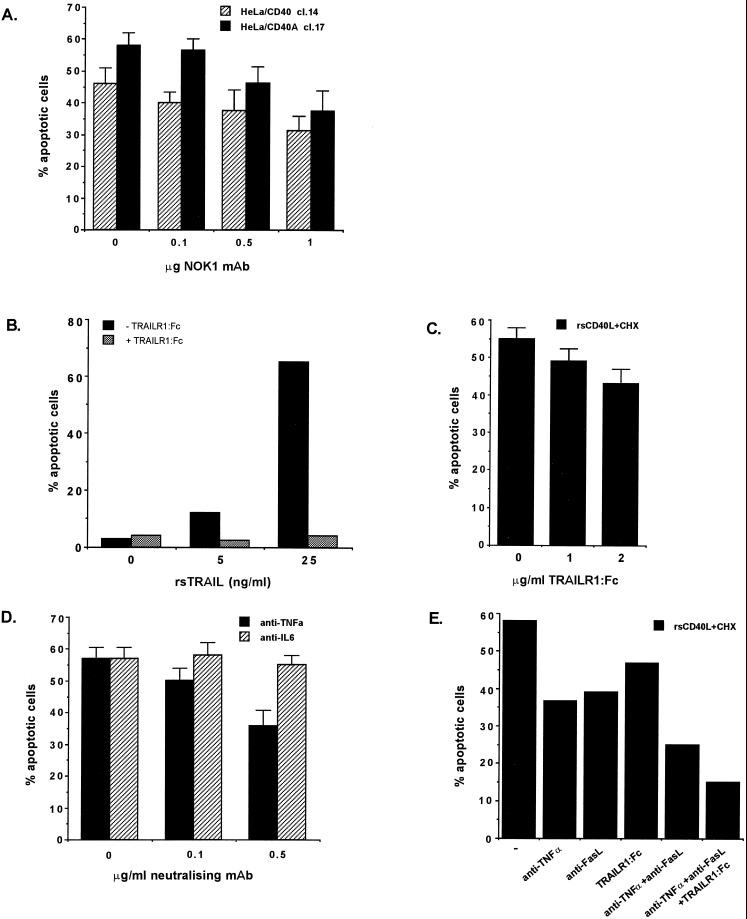
To demonstrate that the observed CD40-mediated induction of Fas and FasL expression is functional and contributes to CD40L-induced apoptosis, HeLa/CD40 clone 14 cells were incubated with the neutralizing anti-FasL MAb NOK1 in the presence of rsCD40L and then exposed to CHX before cell death was assessed. It was found that NOK1 was able to suppress CD40-mediated apoptosis by approximately 30%, and similar results were obtained with the HeLa/CD40A clone 17 cell line (Fig. 6A). Treatment with OX34 anti-CD2 isotype control antibody had no effect, confirming the specificity of the observed phenomenon (data not shown).
FIG. 6.
Inhibition of CD40-mediated FasL, TRAIL, and TNF production suppresses CD40L-induced apoptosis. (A) The neutralizing anti-FasL MAb NOK1 partially inhibits apoptosis induced by CD40L and CHX treatment of HeLa/CD40 clone (cl.) 14 and HeLa/CD40A cl. 17 cells. The percentage of apoptotic cells relative to untreated controls (mean values ± standard deviations [error bars]) from three independent experiments is shown. (B) Soluble recombinant TRAIL induces apoptosis in HeLa/CD40A cl. 17 cells when protein synthesis is inhibited. Cells were treated for 6 h with TRAIL (5 or 25 ng/ml) with enhancer in the presence (+) or absence (−) of a neutralizing TRAILR1:Fc hybrid and then incubated for 24 h in the presence of CHX, before being analyzed for cell death. Data are representative of two independent experiments. (C) A soluble TRAILR1:Fc hybrid confers only a small inhibitory effect on CD40-mediated apoptosis. HeLa/CD40A cl. 17 cells were pretreated with TRAILR1:Fc for 1 h and rsCD40L (1 μg/ml) was then added for 6 additional h. Following CHX treatment, the percentage of apoptotic cells was determined. Mean values ± standard deviations (error bars) from three independent experiments are shown. (D) A neutralizing anti-TNF but not an anti-IL-6 MAb partially inhibit CD40-mediated cell death in HeLa/CD40A cl. 17 cells. Mean values ± standard deviations (error bars) from three independent experiments are shown. Neutralizing anti-TNF MAb was also able to inhibit CD40L-induced cytotoxicity in HeLa/CD40 cl. 14 cells (data not shown). (E) Simultaneous inhibition of FasL, TNF, and TRAIL significantly suppresses CD40-mediated cell death. HeLa/CD40A cl. 17 cells were pretreated with NOK1 (1 μg/ml), anti-TNF-α (0.5 μg/ml), and TRAILR1:Fc (2 μg/ml) for 1 h, and cells were then incubated with rsCD40L (1 μg/ml) for 6 additional hours. Following CHX treatment, the percentage of apoptotic cells was determined. Data shown are representative of three independent experiments.
As TRAIL is also induced in apoptosis-susceptible carcinoma cells following CD40 stimulation, we first examined whether CD40-expressing HeLa cells are responsive to the cytotoxic activity of this ligand. We have found that treatment of HeLa/CD40A clone 17 cells with soluble TRAIL, coupled with a cross-linking reagent (Alexis Corporation), induced a concentration-dependent apoptotic effect (Fig. 6B). This was specific for TRAIL, as it was inactivated in the presence of TRAILR1:Fc, which acts as a soluble decoy receptor. We then examined the ability of the TRAILR1:Fc hybrid molecule to neutralize the proapoptotic ability of CD40 ligation and found that treatment of HeLa/CD40A clone 17 cells with TRAILR1:Fc had only a small but consistent inhibitory effect (15 to 20%) on CD40L-induced cell death (Fig. 6C), a phenomenon which could be probably attributed to the slower kinetics of TRAIL induction compared to those of TNF or FasL. Recent work has demonstrated that neutralizing anti-TNF antibodies inhibit CD40- as well as TNFR2- and CD30-mediated cell death (27). We have verified the ability of neutralizing anti-TNF to partially inhibit CD40L-induced apoptosis (Fig. 6D) and in addition, we have found that this effect can be augmented by anti-FasL MAb treatment. Thus, while NOK1 or anti-TNF alone induced only a partial (30 to 40%) decrease in CD40-mediated apoptosis, combination treatment inhibited this effect by approximately 60 to 65% and was further enhanced in the presence of TRAILR1:Fc (Fig. 6E). These data verify that even in the presence of CHX, CD40 ligation is able to induce up-regulation of cell surface expression of cytotoxic ligands. This may be accounted for by translation of these molecules during the 6-h CD40L incubation period prior to CHX treatment and/or by the presence of preexisting pools of the cytotoxic ligands.
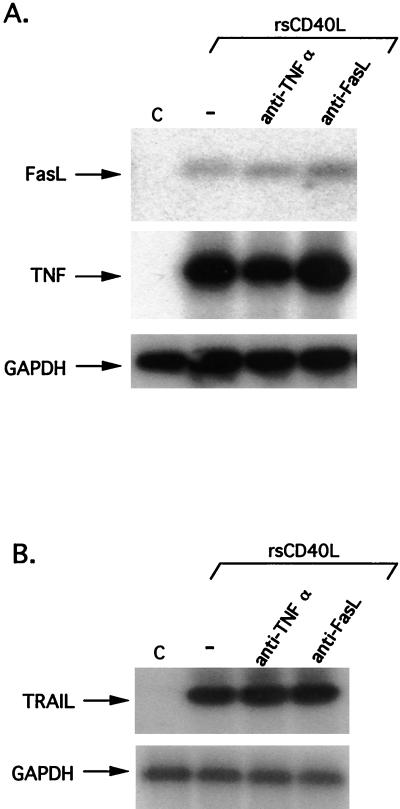
FasL and TNF up-regulation occurs rapidly upon CD40 ligation, suggesting direct transcriptional control. To verify that the ability of CD40 to regulate the expression of FasL and TNF-α is not the result of an autocrine or paracrine cascade involving these ligands and their receptors, HeLa/CD40A clone 17 cells were treated for 2 h with rsCD40L (1 μg/ml) in the presence or absence of NOK1 (1 μg/ml) or anti-TNFα antibody (0.5 μg/ml), and extracted RNA was subjected to RT-PCR for FasL, TNF, or GAPDH expression. As shown in Fig. 7A, inhibition of FasL did not influence the ability of CD40 ligation to activate TNF RNA, and conversely, neutralization of TNF had no effect on CD40-mediated induction of FasL. Furthermore, when CD40-expressing HeLa cells were treated for 12 h with rsCD40L in the presence of anti-FasL or anti-TNF antibody, induction of TRAIL also remained unaffected (Fig. 7B). Therefore, CD40 ligation can independently induce the expression of FasL, TNF, and TRAIL. This observation is further reinforced by the inability of TNF treatment to up-regulate FasL or TRAIL expression. Indeed, when HeLa/CD40 clone 14 or HeLa/CD40A clone 17 cells were exposed for 2 or 12 h to 80 ng of recombinant human TNF-α per ml, TNF mRNA was rapidly activated, in agreement with a recent report (27), but no induction of FasL or TRAIL expression was observed (data not shown).
FIG. 7.
CD40 ligation independently induces the expression of FasL, TNF, and TRAIL. (A) HeLa/CD40A clone 17 cells were pretreated for 1 h with anti-TNF-α (0.5 μg/ml) (lane 3) or NOK1 (1 μg/ml) (lane 4) or were left untreated (lane 2) and then were incubated for 2 h with rsCD40L (1 μg/ml) (lanes 2 to 4) in the presence of the neutralizing reagents, before being analyzed for FasL, TNF, or GAPDH RNA levels by RT-PCR. (B) HeLa/CD40A clone 17 cells were pretreated with neutralizing reagents for 1 h as described above and then exposed to rsCD40L (1 μg/ml) for 12 h before being analyzed for TRAIL or GAPDH RNA levels by RT-PCR.
DISCUSSION
TNFR family members convey signals leading to the regulation of diverse cellular responses, ranging from proliferation and differentiation to growth suppression and apoptosis (12, 46, 64). Among these receptors, TNFR1, Fas, TRAIL-R1, TRAIL-R2, and DR3 share death domain homology in their cytoplasmic tails, through which they transduce apoptotic signals. Paradoxically, other members of the TNFR superfamily which lack the death domain in their cytoplasmic regions, such as TNFR2, CD30, and CD40, have also been reported to suppress growth and survival in a number of carcinoma cell lines (15, 27, 31, 67, 75).
In this study, we have investigated the mechanism by which CD40 induces cell death in carcinomas. For this purpose, we have exposed human carcinoma cell lines to recombinant soluble forms of CD40L and found that induction of cell death depends on the oligomerization status of CD40L. Thus, treatment with CD40L monomers had no effect on survival, but apoptosis was induced following antibody-induced monomer cross-linking, which leads to ligand dimerization. Cell death was even more pronounced following treatment with trimeric rsCD40L (Fig. 1A and B), a phenomenon which probably reflects differences in the efficacy of these molecules to aggregate CD40 (20). Consistent with our findings, previous studies, including the recent identification of the crystal structure of the CD40-TRAF2 complex, have emphasized the significance of ligand-mediated trimerization for efficient CD40 signalling (7, 20, 50). While oligomerization of CD40 is necessary for transduction of signals which activate the cell death machinery in carcinoma cells, its execution also requires inhibition of protein synthesis by CHX, in common with the effects of anti-Fas or TNF treatment in carcinoma cell lines (9, 51). It is possible that CHX blocks the production of protective antiapoptotic proteins, thereby unmasking the cytotoxic potential of CD40 activation. Indeed, the interactions of CD40 with its ligand have been shown to induce the transcriptional up-regulation of a number of negative regulators of cell death, such as Bcl-xL, Bfl1, and A20 (10, 42, 62).
The requirement of protein synthesis inhibition for efficient killing also unveils a mechanism by which tumor cells, through the activation of antiapoptotic programs such as the reported constitutive activation of phosphatidyl inositol 3-kinase–Akt and Bcl-2 overexpression in a subset of ovarian tumors (8, 17), may escape CD40-mediated cytotoxicity. While the ability of these pathways to block CD40-mediated cell death remains to be verified, previous work has implicated Bcl-2, Bcl-xL, and Akt in suppression of TNF- and Fas-induced apoptosis in certain cell types (5, 36, 40, 63). Furthermore, CD40 is absent in a proportion of tumors of the breast and cervix as well as in a number of tumor cell lines, such as the cervical HeLa and ME180, the MCF7 breast, and the 2780CP ovarian carcinoma cell lines, suggesting possible selection for CD40-negative cells (15, 32; Eliopoulos and Young, unpublished observations). An alternative mechanism of resistance to CD40-mediated cell death may occur through disruption of CD40L-induced signals. This is exemplified by the inability of A2780 ovarian carcinoma cells to respond to CD40-mediated cell death (Fig. 1A). In these cells, NF-κB but not JNK activation in response to CD40 stimulation appears to be impaired (N. J. Gallagher, A. G. Eliopoulos, et al., unpublished data). This is not peculiar to A2780 cells, as CD40 ligation fails to induce NF-κB-dependent transcription in Hodgkin's cell lines (71) and similar deficiencies have been identified in a mouse pre-B-cell line (13). While NF-κB has been implicated in the generation of protective responses against TNF- and drug-induced cell death (68, 69), its contribution as a proapoptotic signal has also been noted (3). Indeed, anticancer drugs are known to induce FasL, as well as Fas expression, through a mechanism which critically involves activation of NF-κB (38). In addition, inhibition of NF-κB by a constitutively active IκBα has been recently shown to suppress phorbol myristate acetate- and ionomycin-induced FasL expression and apoptosis in Jurkat T cells (45), and NF-κB is a positive regulator of serum withdrawal-induced apoptosis in 293 cells (28). Interestingly, while A2780 cells do not undergo apoptosis in response to CD40 ligation, we have previously shown that their long-term exposure to CD40L in the absence of CHX leads to growth inhibition (15). This phenomenon is reminiscent of the antiproliferative properties of CD40 ligation in Burkitt's lymphoma cell lines, in the absence of an effect on viability (2). Therefore, CD40 engagement in tumor cells may activate two distinct pathways, leading to inhibition of proliferation or induction of cell death. The signalling cascades which regulate these CD40 pathways will be an interesting area for future studies.
Mutational analysis of the CD40 cytoplasmic tail demonstrated that the TRAF2- and TRAF3-interacting PXQXT motif, a major CD40 signalling effector site, is not critical for induction of apoptosis but death signals are transduced through its membrane-proximal domain. This region binds TRAF6, which is a known regulator of NF-κB, JNK, and ERK signals by CD40 (35, 37). While a role for TRAF6 in modulating cell death has not been described, TRAF6 but not TRAF2 or TRAF3 interacts with the NF-κB and apoptosis-inducing protein RIP2 (48). The contribution of TRAF6 and RIP2 to CD40-mediated cytotoxicity is currently under investigation. Intriguingly, CD40 signals generated from its membrane-proximal, TRAF6-interacting domain appear to be qualitatively different from those engaged by the PXQXT motif. Thus, ERK activation by the membrane-proximal region is Ras independent, whereas that by the PXQXT is Ras dependent (37), and recent evidence suggests differential regulation of NF-κB by these two CD40 domains (66). Furthermore, JAK or STAT signalling is engaged exclusively from the membrane-proximal region (29), and our work provides further evidence for differential signalling emanating from these two domains.
The ability of CD40 ligation to confer a reduced and delayed apoptotic response compared to Fas stimulation, coupled with previous reports demonstrating a synergistic role for CD40 in anti-Fas-induced cytotoxicity (15, 23, 59), prompted us to investigate the possibility that CD40-mediated apoptosis in carcinoma cells occurs indirectly via a mechanism involving Fas and/or its ligand. Three pieces of evidence corroborate this hypothesis. Firstly, we have shown that CD40L-induced cell death occurs through a crmA-sensitive, caspase-dependent pathway (Fig. 3 and 4), in keeping with the ability of Fas to engage a proapoptotic cascade that leads to caspase activation and is suppressed by crmA (63, 65). Furthermore, we have found that CD40 ligation induces the expression of Fas and FasL in apoptosis-susceptible cell lines (Fig. 5). Finally, we have demonstrated that CD40L-induced apoptosis in carcinoma cell lines is partially inhibited by reagents which neutralize FasL (Fig. 6).
The inability of neutralizing anti-FasL antibodies to completely abolish CD40-mediated cytotoxicity implies the contribution of additional death signals. Indeed, exposure of HeLa/CD40 cells to rsCD40L was also found to mediate transcriptional activation of other cytotoxic members of the TNF family, such as TRAIL (Apo-2L) and TNF, although with different kinetics. Consequently, we have found that a neutralizing anti-TNF antibody (27) or a soluble TRAILR1:Fc partially protects against CD40-mediated cell death and that combination treatment with these neutralizing reagents has an additive effect on the survival of CD40L-treated cells. Therefore, CD40 ligation may induce apoptosis in susceptible carcinoma cell lines via an indirect pathway targeting more than one cytotoxic ligand of the TNF family. Whether CD40 ligation regulates the redistribution of intracellular cytoplasmic pools of these cytotoxic ligands, in addition to their de novo transcription and translation remains to be elucidated. Interestingly, our preliminary results indicate the presence of a preexisting cytoplasmic FasL pool in HeLa/CD40 cells which is significantly enriched following treatment with rsCD40L. This is consistent with previous studies in human carcinoma cells, monocytes, and T cells demonstrating the presence of high intracellular levels of FasL or TRAIL which rapidly translocate to the cell surface in response to various stimuli (41, 47). These observations may explain the ability of neutralizing FasL or TRAIL to suppress CD40L-induced apoptosis in carcinoma cells even in the absence of de novo protein synthesis.
The function of many TNF/TNFR family members appears to be tightly controlled in vivo partly through regulation of their expression. For example, Fas and CD40 are widely expressed, but their ligands are restricted to activated T cells and sites of immune privilege. Conversely, TWEAK is expressed in a number of tissues, but its receptor, DR3, is found only in lymphoid cells. The restricted expression of CD40L in vivo, coupled with its antiproliferative and preapoptotic properties when applied as a soluble form, makes it a suitable candidate for tumour therapy. This is supported by the ability of CD40 ligation alone to reduce growth and survival in early-passage ovarian carcinoma cells cultured in vitro (our unpublished observations) and by a recent study demonstrating significant breast tumor regression and apoptosis in xeno-transplanted SCID mice treated with rsCD40L (32). While the mechanism of CD40-mediated carcinoma cell death in vivo is currently unknown, it is likely to involve activation of FasL and/or other death receptor ligands. In addition to regulating CD40-mediated cytotoxicity, these ligands and/or their receptors are also important in apoptosis induced by a broad spectrum of stimuli, including chemotherapy, radiation, ectopic c-myc expression, and anoikis (21, 34, 60), further emphasizing their extensive and central role in programmed cell death.
ACKNOWLEDGMENTS
We are grateful to Immunex Corporation for the gift of soluble trimeric CD40L and to Elliot Kieff and Jun-Ichiro Inoue for providing us with plasmids.
This work was generously supported by the Cancer Research Campaign, United Kingdom, grant SP2091/0501. A.G.E. is a Medical Research Council (United Kingdom) research fellow.
REFERENCES
- 1.Afford S C, Randhawa S, Eliopoulos A G, Hubscher S G, Young L S, Adams D H. CD40 activation induces apoptosis in cultured human hepatocytes via induction of cell surface Fas ligand expression and amplifies Fas-mediated hepatocyte death during allograft rejection. J Exp Med. 1999;189:441–446. doi: 10.1084/jem.189.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker M P, Eliopoulos A G, Young L S, Armitage R J, Gregory C D, Gordon J. Prolonged phenotypic, functional and molecular changes in group I Burkitt lymphoma cells on short term exposure to CD40 ligand. Blood. 1998;92:2830–2843. [PubMed] [Google Scholar]
- 3.Barkett M, Gilmore T D. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- 4.Blake N, Lee S, Redchenko I, Thomas W, Steven N, Leese A, Steigerwald-Mullen P, Kurilla M G, Frappier L, Rickinson A B. Human CD8(+) T cell responses to EBV EBNA1: HLA class I presentation of the (Gly-Ala)-containing protein requires exogenous processing. Immunity. 1997;7:791–802. doi: 10.1016/s1074-7613(00)80397-0. [DOI] [PubMed] [Google Scholar]
- 5.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a forkead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 6.Callard R E, Armitage R J, Fanslow W C, Spriggs M K. CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol Today. 1993;14:559–564. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- 7.Cheng G, Baltimore D. TANK, a co-inducer with TRAF2 of TNF- and CD40L-mediated NF-κB activation. Genes Dev. 1996;10:963–973. doi: 10.1101/gad.10.8.963. [DOI] [PubMed] [Google Scholar]
- 8.Cheng J Q, Godwin A K, Ballacosa A, Taguchi T, Franke T F, Hamilton T C, Tsichlis P N, Testa J R. Akt2, a putative oncogene encoding a member of a subfamily of protein serine threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci USA. 1992;89:9267–9271. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinnaiyan A M, O'Rourk K, Tewari M, Dixit V M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 10.Choi M S K, Boise L H, Gottschalk A R, Quintans J, Thompson C B, Klaus G G B. The role of Bcl-x(L) in CD40-mediated rescue from anti-Mu-induced apoptosis in WEHI-231 B-cell lymphoma cells. Eur J Immunol. 1995;25:1352–1357. doi: 10.1002/eji.1830250533. [DOI] [PubMed] [Google Scholar]
- 11.Clark E A, Shu G. Association between IL-6 and CD40 signalling: IL-6 induces phosphorylation of CD40 receptors. J Immunol. 1990;145:1400–1406. [PubMed] [Google Scholar]
- 12.Cleveland J L, Ihle J N. Contenders in FasL/TNF death signalling. Cell. 1995;81:479–482. doi: 10.1016/0092-8674(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 13.Courtois G, Whiteside S T, Sibley C H, Israel A. Characterization of a mutant cell line that does not activate NF-κB in response to multiple stimuli. Mol Cell Biol. 1997;17:1441–1449. doi: 10.1128/mcb.17.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devergne O, Hatzivassiliou E, Izumi K M, Kaye K M, Kleijner M, Kieff E, Mosialos G. TRAF1, TRAF2 and TRAF3 effect NF-κB activation by an Epstein-Barr Virus LMP1 domain important for B lymphocyte transformation. Mol Cell Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eliopoulos A G, Dawson C W, Mosialos G, Floettmann J E, Rowe M, Armitage R J, Dawson J, Zapata J M, Kerr D J, Wakelam M J O, Reed J C, Kieff E, Young L S. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr virus-encoded LMP1: involvement of TRAF3 as a common mediator. Oncogene. 1996;13:2243–2254. [PubMed] [Google Scholar]
- 16.Eliopoulos A G, Gallagher N J, Blake S M S, Dawson C W, Young L S. Activation of the p38 MAPK pathway by Epstein-Barr virus encoded latent membrane protein 1 (LMP1) co-regulates interleukin-6 and interleukin-8 production. J Biol Chem. 1999;274:16085–16096. doi: 10.1074/jbc.274.23.16085. [DOI] [PubMed] [Google Scholar]
- 17.Eliopoulos A G, Kerr D J, Herod J, Hodgkin L, Krajewski S, Reed J C, Young L S. The control of apoptosis and drug resistance in ovarian cancer: influence of p53 and bcl-2. Oncogene. 1995;11:1217–1228. [PubMed] [Google Scholar]
- 18.Eliopoulos A G, Stack M, Dawson C W, Kaye K M, Hodgkin L, Sihota S, Rowe M, Young L S. Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-κB pathway involving TNF receptor-associated factors. Oncogene. 1997;14:2899–2916. doi: 10.1038/sj.onc.1201258. [DOI] [PubMed] [Google Scholar]
- 19.Eliopoulos A G, Young L S. Activation of the cJun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) Oncogene. 1998;16:1731–1742. doi: 10.1038/sj.onc.1201694. [DOI] [PubMed] [Google Scholar]
- 20.Fanslow W C, Srinivasan S P, Gibson R M G, Spriggs M K, Armitage R J. Structural characteristics of CD40 ligand that determine biological function. Semin Immunol. 1994;6:267–278. doi: 10.1006/smim.1994.1035. [DOI] [PubMed] [Google Scholar]
- 21.Friesen C, Herr I, Krammer P H, Debatin K M. Involvement of the CD95 (Apo-1/Fas) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nat Med. 1996;2:574–577. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- 22.Funakoshi S, Longo D L, Beckwith M, Conley D K, Tsarfaty G, Tsarfaty I, Armitage R J, Fanslow W C, Spriggs M K, Murphy W J. Inhibition of B-cell lymphoma growth by CD40 stimulation. Blood. 1994;83:2787–2794. [PubMed] [Google Scholar]
- 23.Garrone P, Neidhardt E-M, Garcia E, Galibert L, van Kooten C, Banchereau J. Fas ligation induces apoptosis of CD40-activated human B lymphocytes. J Exp Med. 1995;182:1265–1273. doi: 10.1084/jem.182.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilligan M G, Knox P, Weedon S, Barton R, Kerr D J, Searle P, Young L S. Adenoviral delivery of B7-1 (CD80) increases the immunogenicity of human ovarian and cervical carcinoma cells. Gene Ther. 1998;5:965–974. doi: 10.1038/sj.gt.3300672. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein M D, Watts T H. Identification of distinct domains in CD40 involved in B7.1 induction or growth inhibition. J Immunol. 1996;157:2837–2843. [PubMed] [Google Scholar]
- 26.Gordon J. CD40 and its ligand: central players in B lymphocyte survival, growth and differentiation. Blood Rev. 1995;9:53–56. doi: 10.1016/0268-960x(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 27.Grell M, Zimmermann G, Gottfried E, Chen C-M, Grunwald U, Huang D C S, Lee Y-H W, Durkop H, Engelmann H, Scheurich P, Wajant H, Strasser A. Induction of cell death by tumour necrosis factor (TNF) receptor 2, CD40 and CD30: a role for TNF-R1 activation by endogenous membrane-anchored TNF. EMBO J. 1999;18:3034–3043. doi: 10.1093/emboj/18.11.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimm S, Bauer M K A, Baeuerle P A, Schulze-Osthoff K. Bcl-2 down-regulates the activity of transcription factor NF-κB induced upon apoptosis. J Cell Biol. 1996;134:13–23. doi: 10.1083/jcb.134.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanissian S H, Geha R S. JAK3 is associated with CD40 and is critical for CD40 induction of gene expression in B cells. Immunity. 1997;6:379–387. doi: 10.1016/s1074-7613(00)80281-2. [DOI] [PubMed] [Google Scholar]
- 30.Hayward A R, Levy J, Facchetti F, Notarangelo L, Ochs H D, Etzioni A, Bonnefoy J-Y, Cosyns M, Weinberg A. Cholangiopathy and tumours of the pancreas, liver and biliary tree in boys with X-linked immunodeficiency with hyper-IgM. J Immunol. 1997;158:977–983. [PubMed] [Google Scholar]
- 31.Hess S, Engelmann H. A novel function of CD40: induction of cell death in transformed cells. J Exp Med. 1996;183:159–167. doi: 10.1084/jem.183.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirano A, Longo D L, Taub D D, Ferris D K, Young L S, Eliopoulos A G, Agathanggelou A, Cullen N, Macartney J, Fanslow W C, Murphy W J. Inhibition of human breast carcinoma growth by a soluble recombinant human CD40 ligand. Blood. 1999;93:2999–3007. [PubMed] [Google Scholar]
- 33.Hostager B S, Hsing Y, Harms D E, Bishop G A. Different CD40-mediated signalling events require distinct CD40 structural features. J Immunol. 1996;157:1047–1053. [PubMed] [Google Scholar]
- 34.Hueber A-O, Zornig M, Lyon D, Suda T, Nagata S, Evan G I. Requirement for the CD95 receptor-ligand pathway in c-Myc-induced apoptosis. Science. 1997;278:1305–1309. doi: 10.1126/science.278.5341.1305. [DOI] [PubMed] [Google Scholar]
- 35.Ishida T, Mizushima S, Azuma S, Kobayashi N, Tojo T, Suzuki K, Aizawa S, Watanabe T, Mosialos G, Kieff E, Yamamoto T, Inoue J. Identification of TRAF6, a novel tumour necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem. 1996;271:28745–28748. doi: 10.1074/jbc.271.46.28745. [DOI] [PubMed] [Google Scholar]
- 36.Jaattela M, Benedict M, Tewari M, Shayman J A, Dixit V M. Bcl-x and bcl-2 inhibit TNF and Fas-induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene. 1995;10:2297–2305. [PubMed] [Google Scholar]
- 37.Kashiwada M, Shirakata Y, Inoue J-I, Nakano H, Okazaki K, Okumura K, Yamamoto T, Nagaoka H, Takemori T. Tumor necrosis factor receptor-associated factor 6 (TRAF6) stimulates extracellular signal-regulated kinase (ERK) activity in CD40 signalling along a Ras-independent pathway. J Exp Med. 1998;187:237–244. doi: 10.1084/jem.187.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green D R. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-κB and AP-1. Mol Cell. 1998;1:543–551. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- 39.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40 deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 40.Kawahara A, Kobayashi T, Nagata S. Inhibition of Fas-induced apoptosis by Bcl-2. Oncogene. 1998;17:2549–2554. doi: 10.1038/sj.onc.1202192. [DOI] [PubMed] [Google Scholar]
- 41.Kiener P A, Davis P M, Rankin B M, Klebanoff S J, Ledbetter J A, Starling G C, Liles W C. Human monocytic cells contain high levels of intracellular Fas ligand: rapid release following cellular activation. J Immunol. 1997;159:1594–1598. [PubMed] [Google Scholar]
- 42.Lee H H, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-κB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signalling in B lymphocytes. Proc Natl Acad Sci USA. 1999;96:9136–9141. doi: 10.1073/pnas.96.16.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee H H, Dempsey P W, Parks T P, Zhu X, Baltimore D, Cheng G. Specificities of CD40 signaling: involvement of TRAF2 in CD40-induced NF-κB activation and intercellular adhesion molecule-1 up-regulation. Proc Natl Acad Sci USA. 1999;96:1421–1426. doi: 10.1073/pnas.96.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S Y, Reichlin A, Santana A, Sokol K A, Nussenzweig M C, Choi Y. TRAF2 is essential for JNK but not NF-κB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7:703–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- 45.Lin B, Williams-Skipp C, Tao Y, Schleicher M S, Cano L L, Duke R C, Scheinman R I. NF-κB functions as both a proapoptotic and antiapoptotic regulatory factor within a single cell type. Cell Death Differ. 1999;6:570–582. doi: 10.1038/sj.cdd.4400528. [DOI] [PubMed] [Google Scholar]
- 46.Mapara M Y, Bargou R, Zugck C, Dohner H, Ustaoglou F, Jonker R R, Krammer P H, Dorken B. APO-1 mediated apoptosis or proliferation in human chronic B-lymphocytic leukemia—correlation with bcl-2 oncogene expression. Eur J Immunol. 1993;23:702–708. doi: 10.1002/eji.1830230320. [DOI] [PubMed] [Google Scholar]
- 47.Martinez-Lorenzo M J, Alava M A, Gamen S, Kim K J, Chuntharapai A, Pineiro A, Naval J, Anel A. Involvement of Apo2 ligand/TRAIL in activation induced death in Jurkat and human peripheral blood T cells. Eur J Immunol. 1998;28:2714–2725. doi: 10.1002/(SICI)1521-4141(199809)28:09<2714::AID-IMMU2714>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 48.McCarthy J V, Ni J, Dixit V M. RIP2 is a novel NF-κB-activating and cell death-inducing kinase. J Biol Chem. 1998;273:16968–16975. doi: 10.1074/jbc.273.27.16968. [DOI] [PubMed] [Google Scholar]
- 49.McGrory W J, Bautista D S, Graham F L. A simple technique for the rescue of early region-1 mutations into infectious human adenovirus type-5. Virol. 1988;163:614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- 50.McWhirter S M, Pullen S S, Holton J M, Crute J J, Kehry M R, Alber T. Crystallographic analysis of CD40 recognition and signaling by human TRAF2. Proc Natl Acad Sci USA. 1999;96:8408–8413. doi: 10.1073/pnas.96.15.8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miura M, Friedlander R M, Yuan J. Tumor necrosis factor-induced apoptosis is mediated by a crmA-sensitive cell death pathway. Proc Natl Acad Sci USA. 1995;92:8318–8322. doi: 10.1073/pnas.92.18.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris A E, Remmele R L, Klinke R, Macduff B M, Fanslow W C, Armitage R J. Incorporation of an isoleucine zipper motif enhances the biological activity of soluble CD40L (CD154) J Biol Chem. 1999;274:418–423. doi: 10.1074/jbc.274.1.418. [DOI] [PubMed] [Google Scholar]
- 53.Natoli G, Costanzo A, Ianni A, Templeton D J, Woodgett J R, Balsano C, Levrerop M. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science. 1997;275:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 54.Noelle R J. CD40 and its ligand in host defense. Immunity. 1996;4:415–419. doi: 10.1016/s1074-7613(00)80408-2. [DOI] [PubMed] [Google Scholar]
- 55.Peguet-Navarro J, Dalbiez-Gauthier C, Moulon C, Berthier O, Reano A, Gaucherand M, Banchereau J, Rousset F, Schmitt D. CD40 ligation of human keratinocytes inhibits their proliferation and induces their differentiation. J Immunol. 1997;158:144–152. [PubMed] [Google Scholar]
- 56.Pitti R M, Marsters S A, Ruppert S, Donahue C J, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumour necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 57.Ren C L, Morio T, Fu S F, Geha R S. Signal transduction via CD40 involves activation of lyn kinase and phosphatidylinositol-3-kinase and phosphorylation of phospholipase Cγ2. J Exp Med. 1994;179:673–680. doi: 10.1084/jem.179.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothe M, Sarma V, Dixit V M, Goeddel D V. TRAF2-mediated activation of NF-κB by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 59.Rothstein T L, Wang J K M, Panka D J, Foote L C, Wang Z, Stanger B, Cui H, Ju S, Marshak-Rothstein A. Protection against Fas-dependent Th-1-mediated apoptosis by antigen receptor engagement in B cells. Nature (London) 1995;374:163–165. doi: 10.1038/374163a0. [DOI] [PubMed] [Google Scholar]
- 60.Rytomaa M, Martins L M, Downward J. Involvement of FADD and caspase-8 signalling in detachment-induced apoptosis. Curr Biol. 1999;9:1043–1046. doi: 10.1016/s0960-9822(99)80454-0. [DOI] [PubMed] [Google Scholar]
- 61.Sakata N, Patel H R, Terada N, Aruffo A, Johnson G L, Gelfand E W. Selective activation of c-Jun kinase-activated protein kinase by CD40 on human B cells. J Biol Chem. 1995;270:30823–30828. doi: 10.1074/jbc.270.51.30823. [DOI] [PubMed] [Google Scholar]
- 62.Sarma V, Lin Z, Clark L, Rust B M, Tewari M, Noelle R J, Dixit V M. Activation of the B-cell surface receptor CD40 induces A20, a novel zinc-finger protein that inhibits apoptosis. J Biol Chem. 1995;270:12343–12346. doi: 10.1074/jbc.270.21.12343. [DOI] [PubMed] [Google Scholar]
- 63.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K-M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith C A, Farrah T, Goodwin R G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 65.Tewari M, Dixit V M. Fas- and tumour necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 66.Tsukamoto N, Kobayashi N, Azuma S, Yamamoto T, Inoue J-I. Two differently regulated nuclear factor κB activation pathways triggered by the cytoplasmic tail of CD40. Proc Natl Acad Sci USA. 1999;96:1234–1239. doi: 10.1073/pnas.96.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.VanArsdale T L, VanArsdale S L, Force W R, Walter B N, Mosialos G, Kieff E, Reed J C, Ware C F. Lymphotoxin-b receptor signalling complex: role of tumour necrosis factor receptor-associated factor 3 recruitment in cell death and activation of nuclear factor κB. Proc Natl Acad Sci USA. 1997;94:2460–2465. doi: 10.1073/pnas.94.6.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang C-Y, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 69.Wang C-Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S. NF-κB anti-apoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP1 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 70.Wilkinson G W G, Akrigg A. Constitutive and enhanced expression from the CMV major IE promoter in a defective adenovirus vector. Nucleic Acids Res. 1992;20:2233–2239. doi: 10.1093/nar/20.9.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wood K M, Roff M, Hay R T. Defective IkBa in Hodgkin cell lines with constitutively active NF-κB. Oncogene. 1998;16:2131–2139. doi: 10.1038/sj.onc.1201735. [DOI] [PubMed] [Google Scholar]
- 72.Xu J, Foy T M, Laman J D, Elliot E A, Dun J J, Waldscmidt T J, Elsemore J, Noelle R J, Flavell R A. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 73.Younes A, Snell V, Consoli U, Clodi K, Zhao S, Palmer J L, Thomas E K, Armitage R J, Andreff M. Elevated levels of biologically active soluble CD40 ligand in the serum of patients with chronic lymphocytic leukaemia. Br J Haematol. 1998;100:135–141. doi: 10.1046/j.1365-2141.1998.00522.x. [DOI] [PubMed] [Google Scholar]
- 74.Young L S, Dawson C W, Brown K W, Rickinson A B. Identification of a human epithelial cell surface protein sharing an epitope with C3d/Epstein-Barr virus receptor molecule of B lymphocytes. Int J Cancer. 1989;43:786–794. doi: 10.1002/ijc.2910430508. [DOI] [PubMed] [Google Scholar]
- 75.Young L S, Eliopoulos A G, Gallagher N J, Dawson C W. CD40 and epithelial cells: across the great divide. Immunol Today. 1998;19:502–505. doi: 10.1016/s0167-5699(98)01340-1. [DOI] [PubMed] [Google Scholar]