ABSTRACT
Anthrax disease is caused by infection with the bacteria Bacillus anthracis which, if left untreated, can result in fatal bacteremia and toxemia. Current treatment for infection requires prolonged administration of antibiotics. Despite this, inhalational and gastrointestinal anthrax still result in lethal disease. By identifying key metabolic steps that B. anthracis uses to grow in host-like environments, new targets for antibacterial strategies can be identified. Here, we report that the ilvD gene, which encodes dihydroxyacid dehydratase in the putative pathway for synthesizing branched chain amino acids, is necessary for B. anthracis to synthesize isoleucine de novo in an otherwise limiting microenvironment. We observed that ΔilvD B. anthracis cannot grow in media lacking isoleucine, but growth is restored when exogenous isoleucine is added. In addition, ΔilvD bacilli are unable to utilize human hemoglobin or serum albumin to overcome isoleucine auxotrophy, but can when provided with the murine forms. This species-specific effect is due to the lack of isoleucine in human hemoglobin. Furthermore, even when supplemented with physiological levels of human serum albumin, apotransferrin, fibrinogen, and IgG, the ilvD knockout strain grew poorly relative to nonsupplemented wild type. In addition, comparisons upon infecting humanized mice suggest that murine hemoglobin is a key source of isoleucine for both WT and ΔilvD bacilli. Further growth comparisons in murine and human blood show that the auxotrophy is detrimental for growth in human blood, not murine. This report identifies ilvD as necessary for isoleucine production in B. anthracis, and that it plays a key role in allowing the bacilli to effectively grow in isoleucine poor hosts.
IMPORTANCE Anthrax disease, caused by B. anthracis, can cause lethal bacteremia and toxemia, even following treatment with antibiotics. This report identifies the ilvD gene, which encodes a dihydroxyacid dehydratase, as necessary for B. anthracis to synthesize the amino acid isoleucine in a nutrient-limiting environment, such as its mammalian host. The use of this strain further demonstrated a unique species-dependent utilization of hemoglobin as an exogenous source of extracellular isoleucine. By identifying mechanisms that B. anthracis uses to grow in host-like environments, new targets for therapeutic intervention are revealed.
KEYWORDS: anthrax, bacillus, isoleucine, amino acid dependency, auxotrophy, nutrient acquisition
INTRODUCTION
Anthrax is a deadly disease caused by Bacillus anthracis, a Gram-positive, encapsulated, and spore-forming bacteria (1, 2). These bacteria can infect a host through multiple routes. The most common of these is cutaneous infection, though infections through gastrointestinal and inhalational routes present with higher mortality (3). Cutaneous infections exhibit a characteristic black eschar that, if untreated, results in 20% mortality (1). Gastrointestinal and inhalational infections’ early symptoms are more generic, with gastrointestinal infections causing fever, nausea, and diarrhea (1, 3, 4). Inhalational infection first presents with fever, headache, and dry cough (1–4). Due to the commonplace nature of these symptoms, early diagnosis of anthrax is difficult, which can allow progression to lethal bacteremia. Treatment for anthrax disease requires a 60-day regimen of ciprofloxacin or doxycycline antibiotics (1). However, even with intervention, the inhalational and gastrointestinal forms of infection still result in 50% and 40% mortality, respectively (1, 3). Sepsis is one of the hallmarks of these late stage B. anthracis infections, during which the bacilli multiply up to 1 × 108 CFU/ml in blood (5).
In order to multiply to such a degree during infection, the bacilli must scavenge nutrients from their host environment. Iron and heme-iron uptake systems, and their influence on proliferative growth in mammalian hosts, have been studied in B. anthracis (6–10). Less studied in this pathogen is the role of acquisition of other nutrients, such as branched chain amino acids (BCAAs) in growth and infection (11–14). These three amino acids (valine, leucine, and isoleucine) play major roles in protein synthesis. In addition to being used for anabolism, BCAAs act as metabolic intermediates for both vitamins and branched chain fatty acids, the predominant form of fatty acid in Gram-positive bacterial membranes (15, 16). Importantly, BCAAs act as direct cofactors for CodY, a global transcriptional regulator (17–19). In B. anthracis, CodY is known to regulate multiple virulence genes, including the critical virulence mechanisms of toxin production and heme acquisition (20–22). As has been previously observed, controlling BCAA synthesis and availability can thus manipulate bacterial virulence by way of CodY activity (19).
Previous research has established that B. anthracis is auxotrophic for valine in a medium designed to simulate the chemical composition of blood (23). To obtain necessary valine, B. anthracis digests hemoglobin (23, 24). An analysis of human hemoglobin’s amino acid composition shows that, while it is a rich source of both leucine and valine, it contains no isoleucine residues. This leads to the hypothesis that B. anthracis must either synthesize this amino acid de novo or acquire it from other exogenous sources in Homo sapiens. Prior bioinformatics analysis of sequenced strains suggests B. anthracis synthesizes all three BCAAs via a branched pathway that, in places, utilizes the same enzymes to transform separate substrate precursors into their respective BCAA products (25). By knocking out the dihydroxyacid dehydratase gene ilvD of this BCAA synthesis pathway, it may be possible to halt biosynthesis, induce isoleucine auxotrophy, and in turn reduce or prevent the mutant bacteria’s growth in blood.
Here, we examined the effect of BCAA omission on the growth of B. anthracis and find that a mutant lacking ilvD cannot grow in the absence of isoleucine. Interestingly, this growth phenotype extends to several media that have been supplemented with known and abundant serum proteins, as well as whole human blood media. The findings are species specific, because murine proteins, especially hemoglobin, complement the mutant growth, permitting virulence during murine intranasal anthrax infection. B. anthracis is capable of infecting a wide range of hosts thanks to its ability to modulate metabolic processes in response to differing host environments. This report shows that, when compensating for environmental isoleucine deficiency, this ability is solely dependent upon the dihydroxyacid dehydratase enzyme.
RESULTS
A B. anthracis isoleucine auxotrophy.
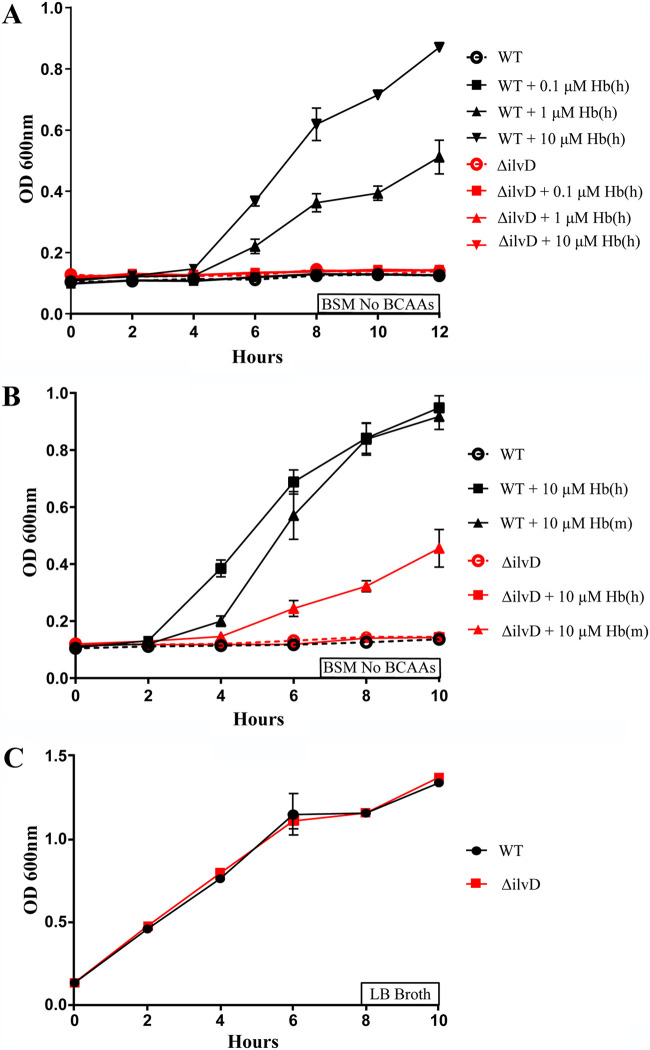
We sought to understand how B. anthracis may utilize host amino acids during infection. Hemoglobin is one of the more prominent components of blood, both in concentration and function, and has served as a source of the critical metal iron for numerous bacterial pathogens (9, 10). Using a medium designed to mimic the chemical composition of blood, termed blood serum mimic (BSM), we previously observed that this pathogen grew in the presence of hemoglobin, but that its growth was not dependent on heme-iron bound to hemoglobin. Instead, bacilli were degrading hemoglobin, which released valine to fuel their growth (23). We wondered if other branched chain amino acids, much like valine, may also stimulate the growth of B. anthracis and thus generated BSM that is free of all three BCAAs: leucine, isoleucine, and valine (BSM No BCAAs). Indeed, when wild-type B. anthracis is seeded in BSM lacking these amino acids, there is no growth (Fig. 1A, black circle). Hypothesizing that hemoglobin may be a source of these missing amino acids, we tested if growth of bacilli could be rescued with the addition of this protein. While a low concentration of hemoglobin (0.1 μM) (Fig. 1A, black square) was insufficient to restore growth, the conditions with hemoglobin at 1 and 10 μM were seen to stimulate replication in a dose-dependent manner (Fig. 1A, black triangles). This second range of 1 to 10 μM remains far less than the physiological concentration of human hemoglobin, approximately 130 to 175 g/liter, or 2.1 to 2.7 mM (28). These data suggest that hemoglobin may therefore be a rich source of isoleucine and leucine, in addition to valine.
FIG 1.
The effect of ΔilvD mutation on growth of bacilli on hemoglobin. (A) Growth curve of WT and ΔilvD B. anthracis in BSM No BCAAs, supplemented with a gradient of Hb(h): 0.1 to 10 μM. (B) WT and ΔilvD B. anthracis in BSM No BCAAs, each supplemented with 10 μM either Hb(h) or Hb(m). (C) Growth curve comparing WT and ΔilvD B. anthracis in LB broth. Error bars on all graphs represent the standard deviations in optical density, at 600 nm, between replicates at each time point.
To investigate this finding, we sought to disable the bacilli’s ability to synthesize both of these amino acids by generating a targeted deletion in the gene ilvD, which encodes dihydroxyacid dehydratase. This enzyme catalyzes the conversion of (R)-2,3-dihydroxy-3-methylpentanoate to (S)-3-methyl-2-oxopentanoate, the immediate precursor to l-isoleucine (25, 29). In addition, this same enzyme putatively catalyzes (R)-2-3-dihydroxy-3-methylbutanoate to form 2-oxoisovalerate, a precursor for both l-valine and l-leucine (25). Indeed, when the B. anthracis mutant strain lacking ΔilvD was seeded in this environment, it failed to replicate on human hemoglobin, regardless of dose (Fig. 1A, red lines). Interestingly, when this experiment was repeated with hemoglobin from a murine source, the mutant bacilli exhibited growth, albeit at lower levels than wild-type (Fig. 1B). We sought to explain this dichotomy given the similarity of human and murine hemoglobin to each other. One notable difference was that while murine hemoglobin contained 12 isoleucine residues, the human version does not contain a single isoleucine residue (Table 1). Interestingly, when either wild-type or the mutant strain were grown in LB, their growth rates were nearly identical (Fig. 1C), which suggested that the auxotrophy observed on human hemoglobin was not due to a general defect in growth. Instead, it likely originated from a specific nutrient that was lacking under these conditions. Since one version of hemoglobin contained isoleucine and the other did not, we hypothesized that bacilli were breaking down murine hemoglobin and using its isoleucine to overcome a synthesis auxotrophy.
TABLE 1.
Blood proteins and isoleucine composition
| No. of Ile residues |
[Ile] (mM) |
||||||
|---|---|---|---|---|---|---|---|
| Protein | g/mol | g/L in blood | Molarity (mM) | Human | Mouse | Human | Mouse |
| Hemoglobin | 64,458 | 130–175 (28) | 2.1–2.7 | 0 | 12 | 0 | 25.2–32.4 |
| Albumin | 66,500 | 35–55 (28) | 0.52–0.83 | 9 | 11 | 4.7–7.5 | 5.7–9.1 |
| IgG | 150,000 | 7–15 (53) | 0.05–0.10 | Variable | Variable | Variable | Variable |
| Transferrin | 78,000 | 1.9–3.3 (54) | 0.024–0.042 | 16 | 23 | 0.38–0.67 | 0.55–0.99 |
| Fibrinogen | 340,000 | 1.0–4.0 (53) | 0.003–0.012 | 124 | 150 | 0.37–1.5 | 0.45–1.8 |
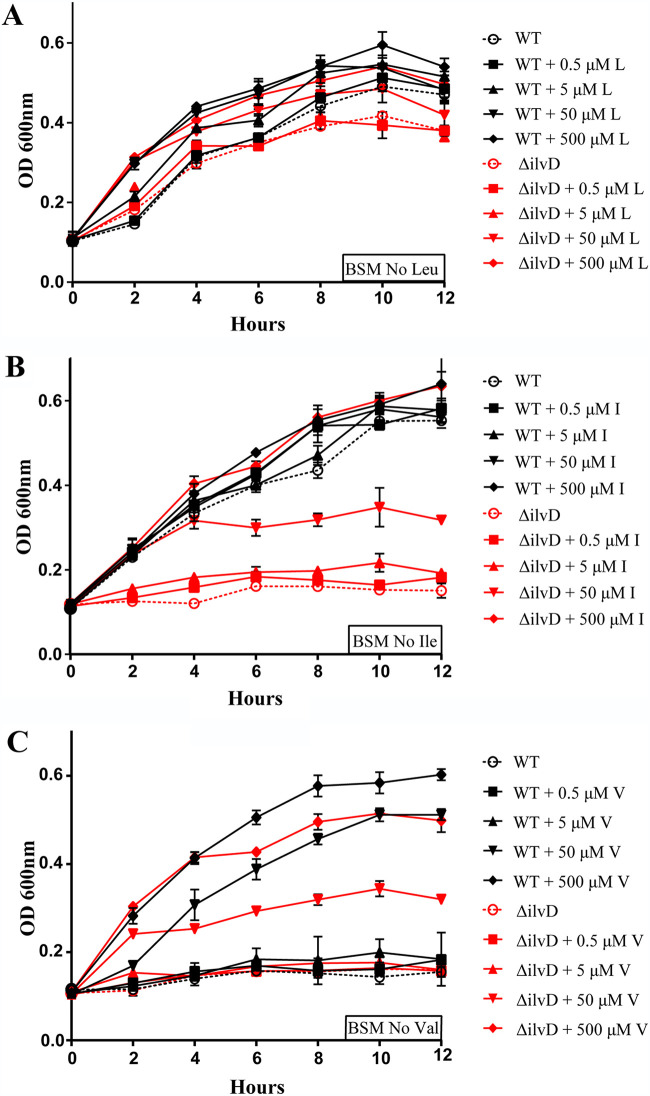
To test this hypothesis, we first needed to confirm the specific role of ilvD in the synthesis of branched chain amino acids for growing bacilli. To do this, we generated BSM lacking each of the three branched chain amino acids. When wild-type or mutant bacilli were grown in BSM lacking leucine, little difference in growth between the two strains was noted, suggesting each were able to synthesize leucine (Fig. 2A). Furthermore, the addition of leucine from 0.5 to 500 μM did not change the growth pattern between each strain. In contrast, when the mutant bacilli were grown in BSM lacking isoleucine, they failed to double in optical density. Only with 50 μM (half WT growth) and 500 μM (equal WT growth) exogenous isoleucine added to the mutant was any growth observed (Fig. 2B), whereas the addition of isoleucine had no effect on the wild-type strain. When this experiment was repeated with valine, wild type bacilli were unable to grow in BSM that lacked valine, needing 50 and 500 μM exogenous valine to replicate (Fig. 2C). A similar effect was seen for the ilvD mutant strain. Collectively, this data indicates that an ilvD knockout mutation induces an isoleucine auxotrophy, but does not significantly influence the growth patterns of WT B. anthracis when either leucine or valine are titrated into the media.
FIG 2.
Examination of the role of ilvD in isoleucine dependency. (A) Growth curve of WT and ΔilvD B. anthracis in BSM No Leu, supplemented with a 10× gradient of Leu: 0.5 to 500 μM. (B) Growth curve of WT and ΔilvD B. anthracis in BSM No Ile, supplemented with a 10× gradient of Ile: 0.5 to 500 μM. (C) Growth curve of WT and ΔilvD B. anthracis in BSM No Val, supplemented with a 10× gradient of Val: 0.5 to 500 μM. Error bars on all graphs represent the standard deviations in optical density, at 600 nm, between replicates at each time point.
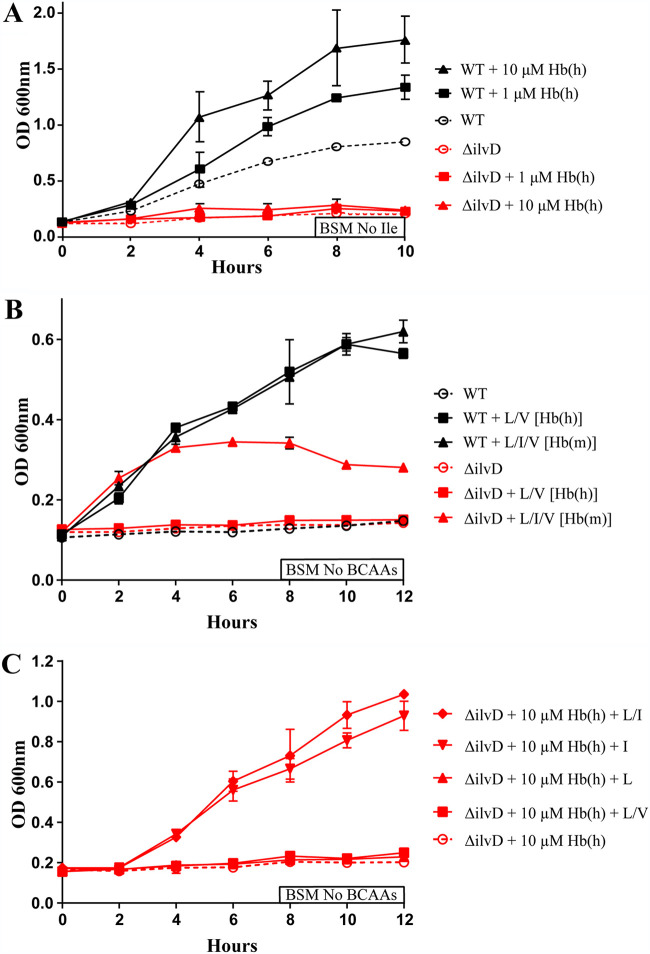
The ΔilvD mutation impacts growth on hemoglobin in a species-specific manner.
With the observation that the ΔilvD mutation prevented growth on hemoglobin, the question was raised as to whether this effect was specific to human derived hemoglobin, which lacks isoleucine, compared with mouse hemoglobin, which contains 12 isoleucine moieties. Because of this variation, we hypothesized that the results of Fig. 1A was caused solely by an isoleucine auxotrophy. As human hemoglobin [Hb(h)] does not contain any isoleucine residues, this protein cannot rescue an isoleucine specific auxotrophy. We tested this in Fig. 3A, where we again compared growth of WT and ΔilvD bacilli with the addition of Hb(h), but now in BSM medium lacking only isoleucine. Here, we see once again that WT bacteria grow in a dose-dependent manner with the addition of Hb(h), whereas ΔilvD bacilli are unable to grow even with the addition of 10 μM Hb(h). Once we established that ΔilvD could not grow on Hb(h) in an environment due to the absence of isoleucine, we next determined if the rescued growth seen in Fig. 1B was specifically due to the difference in BCAA content between Hb(h) and mouse hemoglobin [Hb(m)]. This was tested in Fig. 3B, where we supplied WT and ΔilvD bacilli with valine, leucine, and/or isoleucine at molar equivalency to 10 μM Hb(h) or Hb(m). As expected, ΔilvD mutant bacilli grew only in the condition with Hb(m) BCAAs. Furthermore, the growth patterns agree with the results in Fig. 1B, giving confidence that the rescued growth is primarily due to the difference in BCAA makeup between Hb(h) and Hb(m). Finally, we tested whether it was the addition of isoleucine, or another BCAA, that allowed ΔilvD to grow on Hb(m) instead of Hb(h). To test this, we grew ΔilvD on 10 μM Hb(h) in BSM with no BCAAs and then supplied the bacteria with 3 mM total BCAAs in different combinations. This growth curve, seen in Fig. 3C, shows that ΔilvD growth on Hb(h) is only rescued in two conditions: addition of 3 mM isoleucine, and addition of 1.5 mM each leucine and isoleucine. With this experiment, it is clear that ΔilvD mutant bacilli are only capable of growing on Hb(h) when exogenous isoleucine is present.
FIG 3.
The relationship between hemoglobin and BCAAs for the growth of ΔilvD B. anthracis. (A) Growth curve of WT and ΔilvD B. anthracis in BSM No Ile, supplemented with a gradient of Hb(h): 1 to 10 μM. (B) WT and ΔilvD B. anthracis in BSM No BCAAs, supplemented with BCAAs in molar equivalency to 10 μM either Hb(h) or Hb(m). (C) ΔilvD B. anthracis in BSM No BCAAs, supplemented with 10 μM Hb(h) and 3 mM BCAAs divided equally between I, L, L and V, or L and I. Error bars on all graphs represent the standard deviations in optical density, at 600 nm, between replicates at each time point.
The ΔilvD mutation affects growth on multiple blood proteins.
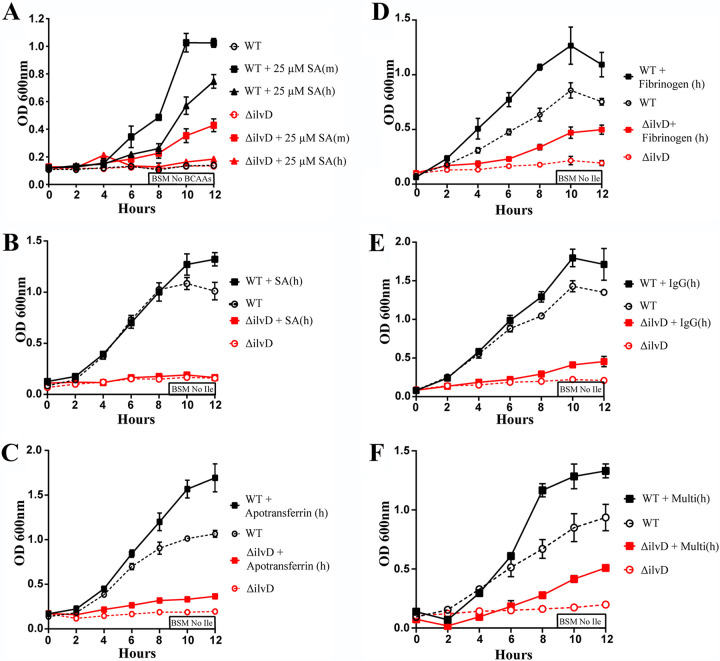
In addition to hemoglobin, there are many other proteins present in the blood which could serve as isoleucine sources for bacteria. The most common of these is serum albumin (SA). Similar to hemoglobin, the amino acid composition of serum albumin varies depending on the species of origin (Table 1). While both mouse serum albumin [SA(m)] and human serum albumin [SA(h)] do contain isoleucine residues, SA(m) contains approximately two more isoleucine residues per molecule than SA(h). In Fig. 4A, we tested whether either form of SA was capable of rescuing ΔilvD growth. Bacilli were grown in BSM lacking BCAAs, supplemented with 25 μM SA(h) or SA(m). Mirroring the hemoglobin dependent growth pattern seen in Fig. 1B, ΔilvD bacilli grew only on SA(m) supplement, while WT bacilli grew with the addition of either SA(h) or SA(m). Curious of the marked difference in ΔilvD growth despite the isoleucine present in SA(h), we repeated the experiment utilizing BSM No Ile and much higher, physiological levels of human serum albumin, 660 μM. However, despite the increased amount of protein added to solution, ΔilvD still failed to grow on SA(h), demonstrating that ΔilvD bacilli cannot utilize SA(h) as a source of isoleucine (Fig. 4B). Further experimentation examined the ability of WT and ΔilvD to grow on physiological levels of other human serum proteins: 35 μM apotransferrin (Fig. 4C), 8.5 μM fibrinogen (Fig. 4D), and 85 μM IgG (Fig. 4E). In each of these growth curves, we observed that WT B. anthracis growth was enhanced by the addition, while ΔilvD growth barely increased once supplemented. Notably, the rescued ΔilvD growth never matched growth observed in nonsupplemented WT bacillus conditions. Upon observing the partially rescued ΔilvD growth in each of these single-protein supplement experiments, we hypothesized that while physiological levels of single proteins may be insufficient to fully rescue ΔilvD growth, supplementing with a combination of all the proteins may rescue growth. To test this, we performed a growth curve supplementing the BSM No Ile with physiological levels of multiple human proteins: human serum albumin, IgG, apotransferrin, and fibrinogen, or Multi(h) (Fig. 4F). We observed that even physiological levels of all of these proteins at once was insufficient to fully rescue ΔilvD growth. These growth curves demonstrate that, while human serum albumin, apotransferrin, fibrinogen, and IgG do contain isoleucine residues, neither physiological levels of the proteins individually, nor in combination, are enough to fully rescue ΔilvD growth compared with nonsupplemented WT.
FIG 4.
Role of serum proteins in WT and ΔilvD B. anthracis growth. (A) Growth curve of WT B. anthracis Sterne in BSM No BCAAs, supplemented with 25 μM SA(h) or SA(m). (B) Growth curve of WT and ΔilvD B. anthracis in BSM No Ile, supplemented 660 μM SA(h). (C) Growth curve of WT and ΔilvD B. anthracis in BSM No Ile, supplemented with 35 μM apotransferrin(h). (D) Growth curve of WT and ΔilvD B. anthracis in BSM No Ile, supplemented with 8.5 μM fibrinogen(h). (E) Growth curve of WT and ΔilvD B. anthracis in BSM No Ile, supplemented with 85 μM IgG(h). (F) Growth curve of WT and ΔilvD B. anthracis in BSM No Ile, supplemented with Multi(h), i.e., 660 μM SA(h), 35 μM apotransferrin(h), 8.5 μM fibrinogen(h), and 85 μM IgG(h). Error bars on all graphs represent the standard deviations in optical density, at 600 nm, between replicates at each time point.
Role of isoleucine during anthrax disease.
Following the observations in Fig. 4A and F, we hypothesized that ΔilvD bacilli would be forced to acquire isoleucine from an exogenous source during infection. Given that mouse hemoglobin harbors 12 such residues, this might mean the mutant strain can execute virulence and initiate fulminant anthrax, if bacilli can liberate isoleucine from Hb. We first infected A/J mice with 3 × 106 WT or ΔilvD B. anthracis spores via intranasal challenge. This mouse model is widely used to simulate anthrax for the Sterne strain and here represents the most relevant model for virulence assessment (30). As hypothesized, both the wild-type and ΔilvD strains were equally virulent, the difference in survival not being significant (Fig. 5A) (P = 0.21). Upon death of the hosts, organs were collected to determine CFU per gram, where it was observed that infected A/J mouse organs yielded approximately 5 to 8 log10 CFU/g, with again no statistical difference observed in any organ between treatment groups (Fig. 5B). Reasoning that the model may show enhanced sensitivity to anthrax and thus mask a difference between the two strains, we next compared WT and ΔilvD infection outcomes in C57BL/6 mice, a murine strain partially resistant to anthrax due to their fully functional immune system (note: C57BL/6 mice could not be infected via intranasal infection for this reason and produce a robust infection; thus, they were infected via subcutaneous (s.c.) injection with 1.3 × 108 spores). Once again, we observed that the bacterial strains were equally capable of lethal infection in C57BL/6 mice, with all infected mice dying by day 3 (Fig. 5C). While the bacterial loads from this infection were lower, ranging from 0 to 6 log10 CFU/g, which supports the general resistance of this strain to Sterne bacilli, there remained no statistically significant difference between the infected groups in bacterial burden (Fig. 5D). With these infections, we can confidently say that exogenous sources of isoleucine are sufficient to support in vivo infection of mouse models with either WT or mutant bacilli.
FIG 5.
The virulence of WT and ΔilvD in multiple murine models. (A) Survival of A/J mice infected intranasally with 3 × 106 WT (n = 11) or ΔilvD (n = 12) spores. (B) Box and whisker plot of CFU per gram in heart, lung, spleen, kidneys, and liver of A/J mice. (C) Survival curve of C57BL/6 mice infected s.c. with 1.3 × 108 WT or ΔilvD spores (n = 10). (D) Box and whisker plot of CFU per gram in heart, lung, spleen, kidneys, and liver of C57BL/6 mice. (E) Survival curve of Townes [C57BL/6+Hb(h)] mice infected s.c. with 1.3 × 108 WT or ΔilvD spores (n = 10). Compared with survival data of C57BL/6 mice infected with WT spores. (F) Box and whisker plot of CFU per gram in heart, lung, spleen, kidneys, and liver of Townes [C57BL/6+Hb(h)] mice, compared with CFU per gram data of C57BL/6 mice infected with WT spores. Significance of survival was determined using Gehan-Breslow-Wilcoxon comparison. P values of CFU per gram were determined by Sidak’s multiple comparisons test.
As we believe that the primary source of exogenous isoleucine in these infections is Hb(m), which contains 12 isoleucines, we hypothesized that a murine host humanized for human hemoglobin (which contains no isoleucine) may be resistant to anthrax disease. To test this idea, we repeated the above infection parameters in the Townes murine strain which express human hemoglobin alpha, beta, and gamma subunits. As these mice are derived from a C57BL/6 lineage, we infected with the same dose and route as in Fig. 5C: 1.3 × 108 spores via s.c. injection. Interestingly, and consistent with an important role of hemoglobin in nutrient uptake, wild-type bacilli caused greater mortality and a higher bacterial burden in the C57BL/6 line with murine hemoglobin than the C57BL/6 Townes line with humanized hemoglobin (Fig. 5E and F). Indeed, 40% of Townes mice survived the infection outright, compared to 0% of C57BL/6 mice infected with WT spores (P < 0.0001). Townes mice overall had approximately 0 to 3 log10 CFU/g in all organs, compared to approximately 2 to 6 log10 CFU/g in C57BL/6 WT-infected organs with a statistically significant reduction in the bacterial load of four organs in the Townes background: heart, lungs, kidneys, and liver. When the experiment was repeated in the Townes background using the bacterial strain unable to synthesize endogenous isoleucine, there was a reduction in virulence compared to wild- type, but it was not statistically significant. Taken together, these data suggest that the substitution of murine hemoglobin with human hemoglobin, which cannot serve as an isoleucine source, has a rather significant effect on the ability of B. anthracis to induce complete anthrax disease. It further indicates, since the ilvD mutant is still able to cause disease but at a reduced level, that B. anthracis must be able to acquire isoleucine from sources other than hemoglobin, possibly other blood proteins such as that shown in Fig. 4.
WT and ΔilvD growth differences are observed in human, not mouse, heat inactivated plasma blood (HIPB).
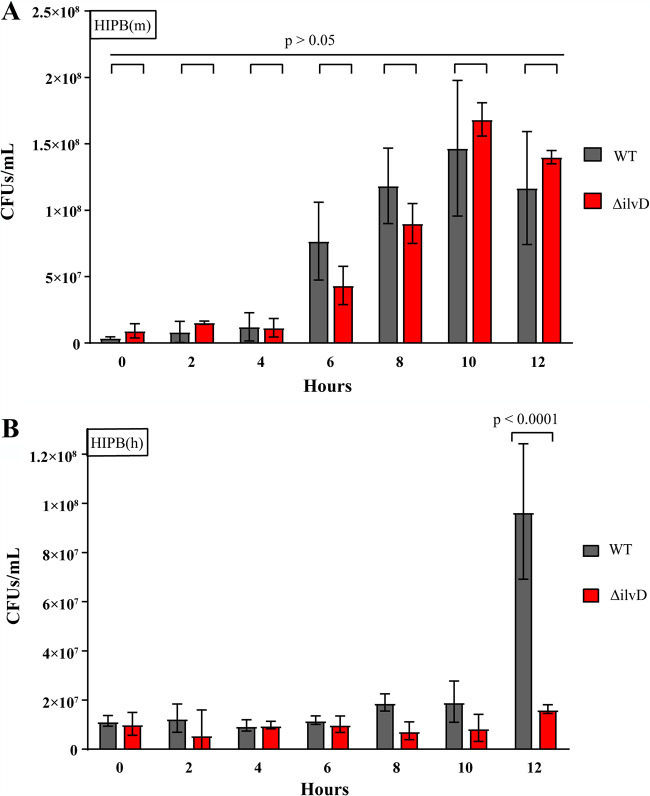
To this point, we have observed in vitro that the growth of mutant and WT bacilli depended upon the species source of serum proteins. In vivo, we observed that exchanging murine hemoglobin for humanized hemoglobin resulted in significantly decreased mortality and bacterial loads following infection with either strain of bacillus. Despite the reduced overall virulence in mice with humanized hemoglobin, there was no significant difference between mice infected with WT or ΔilvD bacilli, although the ΔilvD bacilli did show a trend toward attenuation. We hypothesize that the mutant was still capable of causing anthrax disease in this instance because it was scavenging sufficient isoleucine from other serum proteins. We have already seen that SA(m) is capable of supporting ΔilvD growth, while SA(h) is not. In addition, we have seen that many prominent human proteins are not capable of supporting ΔilvD bacterial growth when supplied at physiologic levels. To test the idea that human blood will not support the growth of the ΔilvD strain, we grew WT and ΔilvD bacilli directly in heat inactivated plasma blood (HIPB) from a murine (m) or human (h) source. In Fig. 6A, we seeded WT and ΔilvD bacilli in HIPB(m) from a BALB/c murine source. Here, we observed that CFU/ml of both WT and ΔilvD increased dramatically from hour 6 and onward, but that there was no difference in levels at the final time point. Indeed, bacterial concentrations increased similarly between the experimental groups at all time points observed (P > 0.05). This agrees with our expectation that murine serum proteins are capable of fully supporting ΔilvD bacilli in addition to WT bacilli.
FIG 6.
Growth of WT and ΔilvD in murine or human blood. HIPB medium was inoculated with 1 × 107 CFU/ml WT or ΔilvD bacilli. From each time point, aliquots of each replicate tube were serially diluted and plated in triplicate. Triplicate serial dilution plates were averaged to determine the CFU per milliliter of each replicate. (A) HIPB(m) was inoculated with WT or ΔilvD bacilli and measured over 12 h. No significant difference was observed at any time point (P > 0.05). (B) HIPB(h) was inoculated with WT and ΔilvD bacilli and measured over 12 h. No significant difference was observed between groups from 0-h through 10-h time points (P > 0.05). Significantly different CFU per milliliter counts between groups were observed at the 12-h time point (P < 0.0001). Significance was calculated using Sidak’s multiple comparisons test. Error bars represent standard deviation between replicate average CFU counts at each time point.
In contrast, we observe in Fig. 6B that the WT bacilli grew much better than the ΔilvD strain at 12 h, which was statistically significant (P < 0.0001). Comparing growth just between hour 10 and 12, the average CFU per milliliter of WT bacilli increased by 5× (1.9 × 107 to 9.6 × 107), while average ΔilvD bacterial levels merely doubled (8.6 × 106 to 1.6 × 107). This observed contrast between growth patterns in HIPB(h) and HIPB(m) agree with previous experiments, namely, that the makeup of human and murine blood, in terms of their isoleucine content, dictate whether or not growth is dependent on an exogenous source of this BCAA or not.
DISCUSSION
In this study, we record a novel observation, namely, that a knockout mutation in the ilvD gene of B. anthracis prevents the bacteria from growing on human hemoglobin. This effect is due to an inability to synthesize isoleucine in the absence of this branched chain amino acid in the globin mainframe, an effect not observed for the murine version, which contains isoleucine. This observed growth comparison also applied to human serum albumin, fibrinogen, IgG, and apotransferrin and was transferable to equivalent experiments using human, but not murine, blood. Finally, while both strains of bacillus remained virulent in murine strain strains, disease was attenuated in mice expressing humanized hemoglobin, suggesting the anthrax disease proceeds via the utilization of host-derived isoleucine.
This series of experiments would not be possible without the evolutionary quirk that resulted in human hemoglobin completely lacking isoleucine. However, that begs the question of why this key protein would lack such an essential amino acid. There are a few different reasons why this could be. The first is due to evolutionary pressure. A preliminary examination of hemoglobin subunit sequences from rhesus macaques, gorillas, and chimpanzees reveals that they all lack isoleucine in their hemoglobin. Like humans, all of these are Old World primates. It is therefore possible that this evolved as a strategy against a prevalent threat in the Old World environment, that pressure possibly being infection. One likely source of this pressure is Plasmodium falciparum, a blood parasite that causes malaria and is endemic to the Old World regions where these species originate. During infection, the malaria parasite is known to degrade and metabolize hemoglobin (31). What makes this a likely source of evolutionary pressure is the recent finding that parasite multiplication is dependent upon access to isoleucine (32). Therefore, it is possible that malarial disease would be attenuated in organisms whose hemoglobin contained less isoleucine. Whether there was another pressure placed on loss of isoleucine by members of the Bacillus genus is interesting to contemplate.
The second reason is due to biochemistry. All three BCAAs perform similar roles in the structure of a protein, their long hydrophobic R groups often forming the lipophilic core of such molecules. Despite this, there are slight differences between them. Leucine is a common component of alpha helices (33). However, valine and isoleucine possess additional methyl groups that cause steric hindrance, thus relegating them to beta-sheet structures (34). The seemingly slight difference of this methyl group placement could result in minor, yet impactful, changes in biochemical properties. The functional unit of the hemoglobin molecule is the porphyrin ring, which binds the Fe(II) ligand, which allows oxygen uptake. Though the porphyrin ring itself contains no amino acids, it is bound in place by a small number of amino acids that were identified to possess key structural properties, designated as mechanically sensitive (35). Between each pair of alpha and beta subunits, human hemoglobin utilizes 19 amino acids, 12 of which are either Val or Leu (35). It is unlikely that isoleucine is excluded from all of these key BCAA binding residues purely through happenstance. To understand why this may be, we can look at myoglobin, which also utilizes a porphyrin ring. Here, experiments have been performed that mutated individual BCAAs that bound the porphyrin into other BCAAs to determine mechanistic effects (36–39). Carver et al. observed that mutating Val68 into an isoleucine dramatically increased the free energies of the ligated state and the inner kinetic barrier, which resulted from steric hindrance from the Ile R-group (38). Experimentation by Ishikawa et al. focused on mutation of a single isoleucine residue that helped bind the porphyrin ring in myoglobin, Ile107. It was observed that substituting this Ile for other BCAAs caused differences in the free energy of the ligand binding intermediate state, as well as the free energy of the activation state (37). Though the effects of these mutations are admittedly dependent upon the position of the amino acid within the molecule, it does indicate that the single methyl group on the isoleucine side chain could influence the biochemical properties of the porphyrin ring binding pocket enough to warrant its absence.
A tempting direction for future studies is to capitalize on this inducible auxotrophy for pharmaceutical intervention. The ilvD gene and its protein product, dihydroxy-acid dehydratase, perform a function not found in humans, and protein-protein BLAST showed no human proteins share significant homology with dihydroxy-acid dehydratase reference protein (WP_000137358.1). This makes it unlikely that a pharmaceutical targeting the bacterial protein would induce off-target effects in H. sapiens. As evidenced by the growth curve experiments in this study, loss of this protein function prevents growth on human hemoglobin and significantly reduces growth on human blood media. This follows a strategy known as nutritional immunity, a method of combating pathogen growth by limiting access to essential nutrients. Previous investigations into nutritional immunity have primarily focused on trace minerals, such as iron, zinc, and manganese (40). Targeting dihydroxy-acid dehydratase would coincide with a newly developing approach of artificially inducing auxotrophies to create nutritional deficits in bacteria and fungi (41–44). However, while our results were promising when utilizing tightly controlled medias in growth curves, we failed to see significant attenuation in our in vivo mouse models. This was anticipated when utilizing the A/J and C57BL/6 murine models, as mouse hemoglobin is a rich source of isoleucine to supplement growth despite the auxotrophy. In addition, when we used the Townes strain, which removes hemoglobin as a source of isoleucine due to mutations for humanized hemoglobin, we saw significant attenuation of disease in mice infected with both the WT and ΔilvD strains. However, we did not observe significant difference between survival of the WT and mutant bacilli infected Townes model. It is likely that growth was supported by the mouse serum albumin in spite of the humanized hemoglobin, which we show is able to support ΔilvD growth. Overall, it is an important reminder that, while hemoglobin may be a dominant proteolytic target, it is far from the only protein that B. anthracis could scavenge amino acids from. It is doubtful that B. anthracis growth could be halted via complete isolation from all potential sources of isoleucine in vivo.
While it may not be possible to attenuate bacterial growth in vivo by artificially inducing an isoleucine auxotrophy, this ilvD KO could be used to allow control of intracellular isoleucine concentrations. In the past, isoleucine auxotrophy in Staphylococcus aureus has been mimicked by the use of mupirocin (45). However, this induced auxotrophy represents an opportunity to establish confident controls, without concern for drug dosages, in order to study bacterial metabolism in an absence of intracellular isoleucine synthesized de novo. In addition to protein structures and bacterial cell walls, BCAAs act as important regulators of CodY (17–19, 46). CodY is a master regulator in many species of Gram-positive genera, including clostridia, Staphylococcus, Streptococcus, Listeria, and Bacillus (46). This protein binds to a “CodY-box” sequence (AATT TTCWGAAAATT) upstream of many genes, repressing activation to directly regulate many proteins in B. anthracis, including virulence regulator AtxA, sporulation genes, toxin-producing operons pagA, lef, and Cya, and finally the BCAA synthesis operons, including the gene ilvD (20, 22, 46). While CodY is itself regulated by a complex combination of factors, among the most prominent are BCAAs (17, 46–48). These amino acids are capable of directly binding the CodY protein to enhance binding and repress unnecessary synthesis proteins (17). Among the BCAAs, Ile has the most powerful effect on CodY, overriding signals from other sources to induce CodY activation and keep virulence factors repressed (18, 19). It may therefore be possible to utilize the ilvD pathway for quorum quenching agents (QQA) (20, 49–51). These QQA are antivirulence agents that are neither bacteriostatic nor bactericidal, but function to indirectly reduce bacterial virulence.
However, it is unlikely that this strategy will succeed by halting this ilvD pathway. On the contrary, it is likely that, by removing generation of intracellular Ile, we deactivated CodY. This could have begun a cascade, causing the activation of multiple virulence genes as the bacterium was struggling to upregulate BCAA biosynthetic pathways. This may have had the effect of forcing the bacilli into a state of physiological stress. Similar effects have been observed in S. aureus, where the virulence factor agr was prematurely activated following derepression from CodY (52). This presents three broad outcomes for isoleucine auxotrophic bacteria during infection. If there is no isoleucine present, the bacteria will starve. If there is plentiful isoleucine, then the bacteria may achieve high enough levels of Ile to sustain growth and satiate CodY, resulting in normal growth. However, if given low levels, then perhaps the bacteria would be trapped in physiological stress and have greater overall virulence factor output than either normal bacilli or mutant bacilli in an isoleucine rich environment. This final option offers an additional explanation as to why Townes mice infected with ΔilvD mutants had such similar survival curves to those infected with WT bacteria, despite removal of hemoglobin as a source of ile. The mutant bacteria may have had increased virulence factor output that increased mortality and compensated for the environmental detriment. Further investigation into genetic expressions as a reaction to environmental nutrient levels is needed.
While investigations into the full scope of CodY and BCAA metabolism are ongoing, single gene knockout mutants that induce powerful phenotypes, such as Ile auxotrophy, are invaluable for studies addressing the extent of these metabolic interactions. Prior experimentation conducted in isoleucine limited environments were unable to account for intracellular levels of this key BCAA synthesized de novo. With this mutation preventing such complicating factors, it is hopeful that this will assist future studies in elucidating phenotypes induced by the far-reaching effects of these broad metabolic regulators.
MATERIALS AND METHODS
Bacterial strains and spore preparation.
This study used the nonencapsulated B. anthracis Sterne strain 34F2, as it is vulnerable to the complement system and thus can be used in biosafety level 2. B. anthracis spores were prepared and stored as previously described (26). B. anthracis Sterne strain 34F2 was used as the wild-type parent strain (WT), from which the ΔilvD mutant was generated.
Generation of B. anthracis ΔilvD mutant.
Escherichia coli (DH5α) K1077 were used for the cloning and amplification of ilvD (BAS1717). The demethylated plasmid DNA containing ilvD flanking regions was electroporated into B. anthracis. Wild-type B. anthracis strain Sterne (34F2) was used to generate an ilvD deletion via allelic replacement using temperature-sensitive plasmid PLM4 as previously described (27). Presence of the ilvD plasmid insert was confirmed through diagnostic digest and sequencing. Transformed bacilli were tested for antibiotic resistance, and DNA sequencing was performed to verify the presence or absence of wild-type and mutant ilvD (BAS1717) DNA sequences.
Growth media.
Bacteria were grown in LB or 4× blood serum mimic (BSM), formulated as previously described (23). This medium contains a representative concentration of amino acids, salts, and sugars to imitate adult human blood. However, it does not contain cells, proteins, hormones, or immune factors. Throughout this study, blood serum mimic was formulated with the either exclusion of all BCAAs, or the individual exclusion of valine (V), leucine (L), or isoleucine (I). Supplements to the BSM media included the following serum proteins: hemoglobin (Hb), serum albumin (SA), apotransferrin, fibrinogen, and IgG. In addition, these proteins may have come from human (h) or mouse (m) sources. In growth curves where the effects of supplements and BCAA combinations were tested, BCAAs were added to a total of 3 mM (for example, 1 mM each L, I, and V; 1.5 mM each L and I, or 3 mM L). One growth curve used equivalent BCAA levels of 10 μM Hb(h): 620 μM V, 720 μM L, and 0 μM I; or Hb(m): 480 μM V, 680 μM L, and 120 μM I. Finally, growth curves were performed in heat inactivated plasma blood (HIPB) generated from either human (h) or BALB/c mouse (m) whole blood sources.
Growth curve assays.
Prior to every growth curve assay, the appropriate spore strains were cultured overnight in LB broth while shaking at 37°C. Vegetative cells were collected via centrifugation and washed four times with the assay culture medium. Cells were added to culture conditions to create a starting OD of 0.1. A minimum of three replicates were made per condition. Cultures were incubated at 37°C while shaking. Optical density was measured every 2 h for 8 to 12 h. Growth curves in blood media could not be observed by this method due to opacity. Instead, blood cultures were inoculated with 1 × 107 CFU of bacilli per ml and then incubated at 37°C while shaking. Bacterial growth was measured by making three sets of serial dilutions per replicate at each time point. Serial dilutions were grown on LB plates overnight to calculate the CFU per milliliter per tube per time point.
Murine strains.
Three murine strains were used in the course of this experiment: A/J mice, C57BL/6 mice, and Townes mice. A/J mice lack the C5a component of complement, making them susceptible to intranasal infection with B. anthracis Sterne strain. Further experimentation used C57BL/6 mice and Townes mice strain 013071 [genotype: homozygous for Hbb <tm3(HBG1, HBB)Tow>, homozygous for Hba<tm1(HBA)Tow], which possess mutations for humanized hemoglobin. As Townes mice are derived from C57BL/6 lineage, experiments performed on C57BL/6 serve as controls for Townes mice. All mice were obtained from Jackson Labs.
Mouse infection model.
Throughout this study, all mice were female and 6 to 8 weeks old at the time of infection. A/J mice were infected intranasally with 10× the calculated 50% lethal dose (previously calculated as 3.1 × 105 spores) of spores from either the WT (n = 11) or the ΔilvD B. anthracis Sterne strain (n = 12), suspended in 50 μl of 1× PBS. In contrast, C57BL/6 and Townes mice are resistant to infection with B. anthracis Sterne strain and instead were infected via s.c. injection with 1.3 × 108 bacterial spores suspended in 50 μl sterile saline (n = 10). All infected mice were monitored for 10 to 12 days. When mice became moribund or at the end of the study, they were euthanized and necropsied to collect the heart, lungs, liver, kidneys, and spleen. Organs were weighed, homogenized, and plated to determine the number of CFU per gram. Morbidity was determined through observation of multiple features, including posture, activity level, squinting, staggered movement, coat appearance, edema, and hyperpnea. Animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals and with approval by the Institutional Animal Care and Use Committee (protocol no. AN-5177) at Baylor College of Medicine.
ACKNOWLEDGMENTS
This work was supported by grants AI097167, AI146481, AI125778, and AI09465 from the National Institutes of Health Allergy and Infectious Diseases Division (NIAID).
We have no conflict of interest to declare.
Contributor Information
Anthony Maresso, Email: maresso@bcm.edu.
Michael J. Federle, University of Illinois at Chicago
REFERENCES
- 1.Johns Hopkins Center for Health Security. 2014. Bacillus anthracis (anthrax) Johns Hopkins Center for Health Security, Baltimore, MD. https://www.centerforhealthsecurity.org/resources/fact-sheets/pdfs/anthrax.pdf.
- 2.Office of Laboratory Security. 2011. Pathogen safety data sheets: infectious substances—Bacillus anthracis. Government of Canada. https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/bacillus-anthracis-material-safety-data-sheets-msds.html. [Google Scholar]
- 3.CDC. 2017. Anthrax. https://www.cdc.gov/anthrax/index.html.
- 4.Bouzianas DG. 2009. Medical countermeasures to protect humans from anthrax bioterrorism. Trends Microbiol 17:522–528. 10.1016/j.tim.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Dixon T, Meselson M, Guillemin J, Hanna P. 1999. Anthrax. N Engl J Med 341:815–826. 10.1056/NEJM199909093411107. [DOI] [PubMed] [Google Scholar]
- 6.Balderas MA, Nobles CL, Honsa ES, Alicki ER, Maresso AW. 2012. Hal is a Bacillus anthracis heme acquisition protein. J Bacteriol 194:5513–5521. 10.1128/JB.00685-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honsa ES, Fabian M, Cardenas AM, Olson JS, Maresso AW. 2011. The five near-iron transporter (NEAT) domain anthrax hemophore, IsdX2, scavenges heme from hemoglobin and transfers heme to the surface protein IsdC. J Biol Chem 286:33652–33660. 10.1074/jbc.M111.241687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maresso AW, Garufi G, Schneewind O. 2008. Bacillus anthracis secretes proteins that mediate heme acquisition from hemoglobin. PLoS Pathog 4:e1000132. 10.1371/journal.ppat.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nobles CL, Maresso AW. 2011. The theft of host heme by Gram-positive pathogenic bacteria. Metallomics 3:788–796. 10.1039/c1mt00047k. [DOI] [PubMed] [Google Scholar]
- 10.Fabian M, Solomaha E, Olson JS, Maresso AW. 2009. Heme transfer to the bacterial cell envelope occurs via a secreted hemophore in the gram-positive pathogen bacillus anthracis. J Biol Chem 284:32138–32146. 10.1074/jbc.M109.040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolar SL, Ibarra JA, Rivera FE, Mootz JM, Davenport JE, Stevens SM, Horswill AR, Shaw LN. 2013. Extracellular proteases are key mediators of Staphylococcus aureus virulence via the global modulation of virulence-determinant stability. Microbiologyopen 2:18–34. 10.1002/mbo3.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehman K, Nuxoll AS, Yamada KJ, Kielian T, Carson SD, Fey PD. 2019. Protease-mediated growth of Staphylococcus aureus on host proteins is opp3 dependent. mBio 10:e02553–18. 10.1128/mBio.02553-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parlindungan E, May BK, Jones OAH. 2019. Metabolic insights into the effects of nutrient stress on Lactobacillus plantarum B21. Front Mol Biosci 6:1–11. 10.3389/fmolb.2019.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valenzuela-Miranda D, Gallardo-Escárate C. 2018. Dual RNA-Seq uncovers metabolic amino acids dependency of the intracellular bacterium Piscirickettsia salmonis infecting Atlantic salmon. Front Microbiol 9:1–11. 10.3389/fmicb.2018.02877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webb ME, Smith AG, Abell C. 2004. Biosynthesis of pantothenate. Nat Prod Rep 21:695–721. 10.1039/b316419p. [DOI] [PubMed] [Google Scholar]
- 16.Gong D, Tang M, Liu S, Li Q. 2017. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Adv Prod Eng Manag 12:51–61. [Google Scholar]
- 17.Shivers RP, Sonenshein AL. 2004. Activation of the Bacillus subtilis global regulator CodY by direct interaction with branched-chain amino acids. Mol Microbiol 53:599–611. 10.1111/j.1365-2958.2004.04135.x. [DOI] [PubMed] [Google Scholar]
- 18.Guédon E, Serror P, Ehrlich SD, Renault P, Delorme C. 2001. Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol Microbiol 40:1227–1239. 10.1046/j.1365-2958.2001.02470.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser JC, King AN, Grigg JC, Sheldon JR, Edgell DR, Murphy MEP, Brinsmade SR, Heinrichs DE. 2018. Repression of branched-chain amino acid synthesis in Staphylococcus aureus is mediated by isoleucine via CodY, and by a leucine-rich attenuator peptide. PLoS Genet 14:e1007159-30. 10.1371/journal.pgen.1007159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Schaik W, Château A, Dillies M-A, Coppée J-Y, Sonenshein AL, Fouet A. 2009. The global regulator CodY regulates toxin gene expression in Bacillus anthracis and is required for full virulence. Infect Immun 77:4437–4445. 10.1128/IAI.00716-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Château A, Schaik W, Six A, Aucher W, Fouet A. 2011. CodY regulation is required for full virulence and heme iron acquisition in Bacillus anthracis. FASEB J 25:4445–4456. 10.1096/fj.11-188912. [DOI] [PubMed] [Google Scholar]
- 22.Château A, van Schaik W, Joseph P, Handke LD, McBride SM, Smeets FMH, Sonenshein AL, Fouet A. 2013. Identification of CodY targets in Bacillus anthracis by genome-wide in vitro binding analysis. J Bacteriol 195:1204–1213. 10.1128/JB.02041-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terwilliger A, Swick MC, Pflughoeft KJ, Pomerantsev A, Lyons CR, Koehler TM, Maresso A. 2015. Bacillus anthracis overcomes an amino acid auxotrophy by cleaving host serum proteins. J Bacteriol 197:2400–2411. 10.1128/JB.00073-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terwilliger A, Maresso AW, Baylor O. 2016. Iron and zinc exploitation during bacterial pathogenesis. Metallomics 7:1541–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanehisa Laboratories. 2017. Valine, leucine, and isoleucine biosynthesis. https://www.genome.jp/kegg-bin/show_pathway?bat00290+BAS1307.
- 26.Jelinski J, Terwilliger A, Green S, Maresso A. 2020. Progress towards the development of a NEAT vaccine for anthrax II: immunogen specificity and alum effectiveness in an inhalational model. Infect Immun 88:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark J, Terwilliger A, Nguyen C, Green S, Nobles C, Maresso A. 2019. Heme catabolism in the causative agent of anthrax. Mol Microbiol 112:515–531. 10.1111/mmi.14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le T, Bhushan V, Rao DA. 2008. First aid for the USMLE Step 1. McGraw-Hill Professional Publishing. [Google Scholar]
- 29.Lopatovskaya KV, Seliverstov AV, Lyubetsky VA. 2010. Attenuation regulation of the amino acid and aminoacyl-tRNA biosynthesis operons in bacteria: a comparative genomic analysis. Mol Biol 44:128–139. 10.1134/S0026893310010164. [DOI] [PubMed] [Google Scholar]
- 30.Welkos SL, Keener TJ, Gibbs PH. 1986. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect Immun 51:795–800. 10.1128/iai.51.3.795-800.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loria P, Miller S, Foley M, Tilley L. 1999. Inhibition of the peroxidative degradation of haem as the basis of action of chloroquine and other quinoline antimalarials. Biochemistry J 339:363–370. 10.1042/bj3390363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Istvan ES, Gluzman IY, Gross J, Goldberg DE. 2006. Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systems. Proc Natl Acad Sci USA 103:8840–8845. 10.1073/pnas.0601876103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou PY, Fasman GD. 1973. Structural and functional role of leucine residues in proteins. J Mol Biol 74:263–281. 10.1016/0022-2836(73)90372-0. [DOI] [PubMed] [Google Scholar]
- 34.Lyu PC, Sherman JC, Chen A, Kallenbach NR. 1991. α-helix stabilization by natural and unnatural amino acids with alkyl side chains. Proc Natl Acad Sci USA 88:5317–5320. 10.1073/pnas.88.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bocahut A, Bernad S, Sebban P, Sacquin-Mora S. 2011. Frontier residues lining globin internal cavities present specific mechanical properties. J Am Chem Soc 133:8753–8761. 10.1021/ja202587a. [DOI] [PubMed] [Google Scholar]
- 36.Dantsker D, Roche C, Samuni U, Blouin G, Olson JS, Friedman JM. 2005. The position 68(E11) side chain in myoglobin regulates ligand capture, bond formation with heme iron, and internal movement into the xenon cavities. J Biol Chem 280:38740–38755. 10.1074/jbc.M506333200. [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa H, Uchida T, Takahashi S, Ishimori K, Morishima I. 2001. Ligand migration human myoglobin: steric effects of isoleucine 107(G8) on O2 and CO binding. Biophys J 80:1507–1517. 10.1016/S0006-3495(01)76123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carver TE, Rohlfs RJ, Olson JS, Gibson QH, Blackmore RS, Springer BA, Sligar SG. 1990. Analysis of the kinetic barriers for ligand binding to sperm whale myoglobin using site-directed mutagenesis and laser photolysis techniques. J Biol Chem 265:20007–20020. 10.1016/S0021-9258(17)45475-5. [DOI] [PubMed] [Google Scholar]
- 39.Quillin ML, Li T, Olson JS, Phillips GN, Dou Y, Ikeda-Saito M, Regan R, Carlson M, Gibson QH, Li H. 1995. Structural and functional effects of apolar mutations of the distal valine in myoglobin. J Mol Biol 245:416–436. 10.1006/jmbi.1994.0034. [DOI] [PubMed] [Google Scholar]
- 40.Hennigar SR, McClung JP. 2016. Nutritional immunity: starving pathogens of trace minerals. Am J Lifestyle Med 10:170–173. 10.1177/1559827616629117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atkins T, Prior RG, Mack K, Russell P, Nelson M, Oyston PCF, Dougan G, Titball RW. 2002. A mutant of Burkholderia pseudomallei, auxotrophic in the branched chain amino acid biosynthetic pathway, is attenuated and protective in a murine model of melioidosis. Infect Immun 70:5290–5294. 10.1128/IAI.70.9.5290-5294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jastrzębowska K, Gabriel I. 2015. Inhibitors of amino acids biosynthesis as antifungal agents. Amino Acids 47:227–249. 10.1007/s00726-014-1873-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Awasthy D, Gaonkar S, Shandil RK, Yadav R, Bharath S, Marcel N, Subbulakshmi V, Sharma U. 2009. Inactivation of the ilvB1 gene in mycobacterium tuberculosis leads to branched-chain amino acid auxotrophy and attenuation of virulence in mice. Microbiology (Reading) 155:2978–2987. 10.1099/mic.0.029884-0. [DOI] [PubMed] [Google Scholar]
- 44.Kim G-L, Lee S, Luong TT, Nguyen CT, Park S-S, Pyo S, Rhee D-K. 2017. Effect of decreased BCAA synthesis through disruption of ilvC gene on the virulence of Streptococcus pneumoniae. Arch Pharm Res 40:921–932. 10.1007/s12272-017-0931-0. [DOI] [PubMed] [Google Scholar]
- 45.Reiss S, Pané-Farré J, Fuchs S, François P, Liebeke M, Schrenzel J, Lindequist U, Lalk M, Wolz C, Hecker M, Engelmann S. 2012. Global analysis of the Staphylococcus aureus response to mupirocin. Antimicrob Agents Chemother 56:787–804. 10.1128/AAC.05363-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stenz L, Francois P, Whiteson K, Wolz C, Linder P, Schrenzel J. 2011. The CodY pleiotropic repressor controls virulence in gram-positive pathogens. FEMS Immunol Med Microbiol 62:123–139. 10.1111/j.1574-695X.2011.00812.x. [DOI] [PubMed] [Google Scholar]
- 47.Brinsmade SR. 2017. CodY, a master integrator of metabolism and virulence in Gram-positive bacteria. Curr Genet 63:417–425. 10.1007/s00294-016-0656-5. [DOI] [PubMed] [Google Scholar]
- 48.Tojo S, Satomura T, Morisaki K, Deutscher J, Hirooka K, Fujita Y. 2005. Elaborate transcription regulation of the Bacillus subtilis ilv-leu operon involved in the biosynthesis of branched-chain amino acids through global regulators of CcpA, CodY and TnrA. Mol Microbiol 56:1560–1573. 10.1111/j.1365-2958.2005.04635.x. [DOI] [PubMed] [Google Scholar]
- 49.Defoirdt T. 2018. Quorum-sensing systems as targets for antivirulence therapy. Trends Microbiol 26:313–328. 10.1016/j.tim.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Huedo P, Coves X, Daura X, Gibert I, Yero D. 2018. Quorum sensing signaling and quenching in the multidrug-resistant pathogen Stenotrophomonas maltophilia. Front Cell Infect Microbiol 8:122. 10.3389/fcimb.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang K, Zhang XH. 2014. Quorum quenching agents: resources for antivirulence therapy. Mar Drugs 12:3245–3282. 10.3390/md12063245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pohl K, Francois P, Stenz L, Schlink F, Geiger T, Herbert S, Goerke C, Schrenzel J, Wolz C. 2009. CodY in Staphylococcus aureus: a regulatory link between metabolism and virulence gene expression. J Bacteriol 191:2953–2963. 10.1128/JB.01492-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McPherson R, Pincus M. 2017. Henry’s clinical diagnosis and management by laboratory methods. Elsevier Health Sciences. [Google Scholar]
- 54.Helander A. 1999. Absolute or relative measurement of carbohydrate-deficient transferrin in serum? Experiences with three immunological assays. Clin Chem 45:131–135. 10.1093/clinchem/45.1.131. [DOI] [PubMed] [Google Scholar]