Abstract
Background and Purpose:
Brain cavernous angiomas with symptomatic hemorrhage (CASH) have a high risk of neurological disability from recurrent bleeding. Systematic assessment of baseline features and multisite validation of novel MRI biomarkers are needed to optimize clinical trial design aimed at novel pharmacotherapies in CASH.
Methods:
This prospective, multicenter, observational cohort study included adults with unresected, adjudicated brain CASH within the prior year. Six U.S. sites screened and enrolled patients starting August 2018. Baseline demographics, clinical and imaging features, functional status (modified Rankin Scale, mRS, and NIH Stroke Scale, NIHSS), and patient quality of life (QoL) outcomes (PROMIS-29 and EuroQol EQ-5D) were summarized using descriptive statistics. PROMIS-29 scores standardized against a reference population (mean 50, standard deviation 10) and one sample t-test was performed for each domain. A subgroup underwent harmonized MRI assessment of lesional iron content with quantitative susceptibility mapping (QSM) and vascular permeability with dynamic contrast enhanced quantitative perfusion (DCEQP).
Results:
As of May 2020, 849 patients were screened and 110 CASH cases enrolled (13% prevalence of trial eligible cases). The average age at consent was 46±16 years, 53% were female, 41% were familial, and 43% were brainstem lesions. At enrollment, ≥90% of the cohort had independent functional outcome (mRS≤2 and NIHSS<5). However, perceived health problems affecting QoL were reported in >30% of patients (EQ-5D). Patients had significantly worse PROMIS-29 scores for anxiety (p=0.007), but better depression (p=0.002) and social satisfaction scores (p=0.012) compared to the general reference population. Mean baseline QSM and permeability of CASH lesion was 0.45 ± 0.17 ppm and 0.39 ± 0.31 mL/100g/min, respectively, which were similar to historical CASH cases and consistent across sites.
Discussion:
These baseline features will aid investigators in patient stratification and determining the most appropriate outcome measures for clinical trials of emerging pharmacotherapies in CASH.
MeSH Keywords: Cavernous angioma, Cerebral cavernous malformation, Intracranial hemorrhage, Clinical trial, Biomarkers, Quality of Life
Subject Terms: Biomarkers, Epidemiology, Magnetic Resonance Imaging (MRI), Quality and Outcomes, Cerebrovascular Malformations, Intracranial Hemorrhage
INTRODUCTION
Cavernous angiomas (CA) of the brain are clusters of blood-filled capillary spaces (“caverns”), lined by endothelium, but lacking mature vessel wall elements. CA can occur sporadically as a solitary brain lesion, often associated with a developmental venous anomaly, or in a familial form with multiple brain lesions due to mutations in KRIT1/CCM1, CCM2, or PDCD10/CCM3. CA may be an incidental finding or patients may present with clinical symptoms (headache, focal neurologic deficit, seizure) with or without hemorrhage.
CASH (cavernous angioma with symptomatic hemorrhage) is defined as evidence of new lesional bleeding or hemorrhagic growth (≥3mm) on magnetic resonance imaging (MRI) in association with directly attributable symptoms.1 CASH patients are at high risk for recurrent bleeding or focal neurological deficit within 5 years (42%, 95% CI: 27–58).2–4 While lesions in the brainstem or deep brain locations are more likely to be associated with symptomatic hemorrhage than lesions in other locations, they also pose the greatest risks with surgical treatment.2 Thus, patients with CASH would be ideal candidates for potential drug therapies aimed at preventing rebleeding, and mitigating further disability and treatment-related morbidity.
While prior studies have reported on natural history of CAs and outcomes, none have prospectively assessed multiple cohorts across institutions with standardized data collection, including clinical and imaging characteristics, and patient-reported quality of life (QoL) assessments. Prevalence of trial eligible CASH cases has never been estimated. In addition, advanced MRI techniques have not been previously validated across sites to potentially serve as surrogate biomarker endpoints for the risk of recurrent hemorrhage. In particular, derived continuous variables from quantitative susceptibility mapping (QSM) and dynamic contrast enhanced quantitative perfusion (DCEQP) measure lesional iron content and vascular permeability, respectively, and have been correlated with lesional hemorrhage recurrence.5–7 Both have been suggested as monitoring biomarkers in clinical trials of emerging pharmacotherapies in CA.8, 9
Thus, the aims of the CASH Trial Readiness (TR) project are three-fold: (1) to estimate prevalence and baseline characteristics of CASH patients screened at multiple sites; (2) to assess the feasibility, accuracy, precision and reproducibility of MRI biomarker measurements at multiple sites; and (3) to assess recurrent clinical events and changes in MRI biomarkers and functional status over time. The study is ongoing with open enrollment until November 2022 (clinicaltrials.gov NCT03652181).10 Herein we present results related to the first aim.
METHODS
Study design and population
This is a prospective, multicenter, observational study of patients with CASH. Patients were enrolled into the CASH TR project starting in August 2018. Details of the trial design were previously published.10 This multicenter study was approved by Johns Hopkins University as the single institutional review board of record, and all patients provided informed consent to participate. Data that support the findings herein are available from the corresponding author upon reasonable request and approval by the CASH TR steering committee.
Briefly, patients were eligible for the study if: (a) ≥18 years of age; (b) diagnosed with a brain CA (single or multiple); (c) symptomatic hemorrhage within the past year; and (d) no prior treatment of the symptomatic lesion. Treatment of other lesions is not an exclusion. A hemorrhagic cavernoma is defined as acute or subacute bleeding on CT or MRI, new FLAIR signal on MRI, and or lesion expansion in any diameter by >=3mm on comparable T1-weighted or T2-weighted sequences. Patients were excluded for the following reasons: (a) the symptomatic lesion was in the spinal cord; (b) prior brain irradiation; (c) planned treatment of CASH lesion; and (d) inability to verify symptomatic hemorrhage with clinical and imaging review. Additionally, those eligible for imaging follow-up studies were excluded if there was contraindication for gadolinium contrast, if subject was pregnant or breastfeeding, or if subject was deemed unable or unlikely to return for follow-up visits (e.g., homeless or incarcerated persons).
Five recognized Centers of Excellence in CA care and research by the Angioma Alliance, patient advocacy group, and one pending site were selected as enrollment sites for the CASH TR project: University of Chicago (UoC), Mayo Clinic – Rochester, Barrow Neurological Institute (BNI), University of Utah, University of New Mexico (UNM), and University of California – San Francisco (UCSF). Recruiting sites kept screening logs of all CA patients seen at their centers, and enrolled eligible cases into the study after site activation. Patients could enroll in the screening and clinical assessment (SCA) portion of the study at all six sites (target enrollment of 200 CASH cases) or enroll in the follow-up biomarker validation (FUBV) study at four sites (UoC, Mayo, UNM, or UCSF) with a target enrollment of 120 CASH cases. Patients in both SCA and FUBV studies completed baseline clinical, radiographic, functional assessments, and patient-reported outcome assessment at an in-person visit. Those enrolled in the FUBV study also completed research imaging for MRI biomarkers (described below) at baseline and at annual follow-up visit for 2 years.
Clinical Data Collection
Common data elements and definitions were discussed among all sites and agreed upon during the TR startup phase. The dictionary of data elements collected and definitions is included in online Supplemental Material. Demographic, CCM form (familial/sporadic) and genotype (if available), medical and family history, and medication use at time of study enrollment was collected from medical records. The date and type of symptoms leading to CA diagnosis and CASH presentation were also reviewed.
Functional and Patient-Reported Outcomes
Functional outcome and patient-reported outcome measures were administered and included the modified Rankin Scale (mRS) score, NIH Stroke Scale (NIHSS), Patient-Reported Outcomes Measurement Information System (PROMIS-29), and EuroQol (EQ)-5D scale. mRS and NIHSS were assessed by certified healthcare providers whereas responses to PROMIS-29 and EQ-5D scales were provided by patients. mRS is a simple global measure of disability with higher scores reflecting increasing disability; scores range from 0 (no symptoms) to 5 (severe disability) and 6 (death).11, 12 NIHSS has values ranging from 0 to 42, with stroke severity categorized as mild (0–4), moderate (5–14), severe (15–24), and very severe (≥25).13, 14 EQ-5D includes an overall visual analog scale of health (0=poor to 100=best), and specific questions covering mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.15, 16 PROMIS-29 version 2.0 is a generic health-related QoL survey assessing 7 domains (depression, anxiety, physical function, pain interference, fatigue, sleep disturbance, and ability to participate in social roles and activities).17, 18 Each domain contains 4 questions ranked using a 5-point Likert scale, and one 11-point rating scale for pain intensity.
Imaging Features
Baseline clinical MRI scan was reviewed by each site investigator, uploaded, and adjudicated by a central imaging core laboratory. Time from symptom onset to first MRI diagnosing the CASH lesion was recorded. The location and size of the CASH lesion was ascertained on standard T2 images. Multiplicity was noted and lesion counts were performed based on hemosiderin sensitive sequences (categorized as 1, 2–100, >100). Presence of an associated developmental venous anomaly was also recorded.
MR Biomarker Data (FUBV cases only)
The FUBV study included additional assessments of mean lesional QSM (ppm) and DCEQP (Ki, mL/100g/min) on MRI, according to harmonized protocols5–7 and multisite phantom validations as previously published.19 MR biomarker derivations (mean lesional QSM and DCEQP) were calculated by the central imaging core laboratory from raw images uploaded by sites. Baseline results are presented herein for FUBV enrolled cases. These are referenced against historical CASH cases enrolled at the UoC between November 2012 and April 2018, where the techniques were adapted to CA imaging prior to the launch of the CASH TR project.
Statistical Analysis
Data were analyzed for consecutively enrolled patients into SCA and FUBV studies between August 2018 to May 2020. Descriptive statistics (i.e., frequencies, proportions, mean ± standard deviation (SD), medians, and interquartile ranges (IQR)) are reported for screened and enrolled cases. Site-specific enrollment rates are calculated as total number of eligible cases enrolled divided by total months enrolling (i.e., date since first consented case). Prevalence of CASH is calculated as number of eligible cases enrolled divided by the total number of CA cases from screening logs. Baseline clinical and imaging characteristics for CASH eligible cases are summarized both for the entire sample and by recruitment site.
PROMIS-29 domain scores were converted to T-scores, standardized to a reference population (mean 50, SD 10), and oriented so that higher scores are worse. Using guidelines for interpreting standardized effect sizes, a difference between groups of 2 points (two-tenths of a SD) indicates a small effect size, 5 points (0.5 SD) indicates a medium effect size, and 8 points (0.8 SD) indicates a large effect size.20 One-sample t-tests were used to compare whether mean T-scores for each domain differed significantly from 50 (p<0.05). Associations between PROMIS-29 and mRS scores were evaluated using linear regression analyses. Multivariable linear regression was performed to test whether pre-specified subgroups (brainstem vs. other location or familial/multifocal vs. sporadic/solitary case) were associated with PROMIS-29 domain scores, adjusting for age, sex, and log-transformed time from hemorrhage to assessment as outcomes can improve with longer time intervals. EQ-5D and NIHSS scores were compared with mRS using Spearman’s rank correlation.
RESULTS
Screening and Enrollment
As of May 2020, the CASH TR project has screened 849 CA cases across six sites. There was a greater percentage of sporadic cases (60%) and females (58%), who were predominantly non-Hispanic White race/ethnicity (90%). The mean (SD) age at time of screening was 46 ± 18 years. There was variation seen across sites with respect to demographic characteristics, especially for age, Hispanic ethnicity, and familial cases that was attributable to geographic location and referral patterns of recruiting sites (Supplementary Table I).
Of the 849 cases screened, there were 110 eligible cases, resulting in a CASH prevalence of 13%. The main reasons for study exclusion were either no symptomatic hemorrhage in the past year (72%) or prior/planned surgery of CASH lesion (14%). Additional reasons for exclusion are summarized in Table 1; these criteria are not mutually exclusive, i.e., subjects could be excluded based on meeting multiple criteria.
Table 1.
CASH screening log of cavernoma cases excluded for various reasons for: (A) Screening and Clinical Assessment (SCA), and (B) Follow-up and Biomarker Validation (FUBV) studies, by recruitment site and overall between August 2018 – March 2020.
| A) SCA study | |||||||
|---|---|---|---|---|---|---|---|
| BNI | Mayo | UNM | Utah | UCSF | UoC | Overall | |
| Exclusion criteria* | n=144 | n=235 | n=140 | n=68 | n=73 | n=189 | n=849 |
| <18 years old | 2% | 2% | 16% | 0% | 4% | 7% | 6% |
| No brain CA diagnosis | 2% | 5% | 1% | 13% | 0% | 0% | 3% |
| No symptomatic hemorrhage in past year | 67% | 69% | 89% | 70% | 70% | 70% | 72% |
| Unable to provide consent | 5% | 2% | 1% | 9% | 6% | 2% | 3% |
| Lesion located in spine | 3% | 11% | 1% | 0% | 1% | 1% | 4% |
| Prior brain irradiation | 3% | 8% | 4% | 1% | 4% | 2% | 4% |
| Verification of symptomatic hemorrhage impossible | 1% | 2% | 0% | 1% | 6% | 0% | 1% |
| Prior/planned surgery of CA | 29% | 12% | 5% | 9% | 35% | 5% | 14% |
| B) FUBV study | |||||
|---|---|---|---|---|---|
| Mayo | UNM | UCSF | UofC | Overall | |
| Exclusion Criteria* | n=218 | n=139 | n=1 | n=184 | n=542 |
| Unwilling/unable to do MRI | 0% | 0% | 0% | 2% | 1% |
| Pregnant/breastfeeding | 0% | 1% | 0% | 2% | 1% |
| Unlikely/unwilling to return for follow-up | 14% | 1% | 0% | 1% | 6% |
Percentages for criterion are not mutually exclusive. BNI: Barrow Neurological Institute, Mayo: Mayo Clinic Rochester, UNM: University of New Mexico, Utah: University of Utah, UCSF: University of California San Francisco, UofC: University of Chicago, CA: cavernous angioma
Eligible cases were enrolled at an average rate of 5.8 cases per month, with site-specific enrollment varying from 0.4 to 2.5 cases per month. The average time from symptom onset to enrollment was 4.4 ± 3.3 months; median time was 3.6 months (IQR: 1.8 – 6.3). At time of symptomatic hemorrhage leading to enrollment into CASH, the most common symptoms were focal neurological deficits (59%) and headaches (40%).
Baseline Clinical Characteristics
Baseline characteristics of the 110 enrolled CASH patients are summarized overall and by recruitment site in Table 2. The mean age at consent was 46 ± 16 years, 53% were female, 41% were familial, 90% were Caucasian, and 19% were of Hispanic ethnicity. Variability was again observed in demographic characteristics across sites similar to screened cases. Of the cohort, 29% had history of hypertension, 5% had diabetes, 9% had sleep apnea, 50% were current alcohol drinkers, 9% current tobacco smokers, and 12% were current recreational drug users, the majority of which were marijuana users. Overall, 45% of the cohort was taking Vitamin D supplements, 22% were on statins, 7% were on propranolol, and 6% were on antithrombotics (anticoagulation and/or antiplatelet agents).
Table 2.
Distribution of demographic and clinical characteristics of enrolled CASH cases by recruitment site and overall between August 2018 – March 2020.
| Characteristic* | BNI | Mayo | UNM | Utah | UCSF | UoC | Overall |
|---|---|---|---|---|---|---|---|
| Number enrolled | 13 | 20 | 9 | 10 | 5 | 53 | 110 |
| Months of enrollment | 19.3 | 20.8 | 20.6 | 19.3 | 7.6 | 20.9 | 20.9 |
| Age at enrollment (years) | 56 ± 14 | 49 ± 17 | 50 ± 14 | 52 ± 15 | 60 ± 9 | 39 ± 14 | 46 ± 16 |
| Female sex | 4 (31%) | 8 (40%) | 6 (67%) | 7 (70%) | 3 (60%) | 30 (57%) | 58 (53%) |
| White race | 12 (92%) | 19 (95%) | 7/8 (88%) | 9 (90%) | 1/2 (50%) | 46/52 (88%) | 94/105 (90%) |
| Hispanic ethnicity | 1 (8%) | 0 | 7/8 (88%) | 1 (10%) | 2 (40%) | 9 (17%) | 20/108 (19%) |
| Familial/multiple lesions | 5 (38%) | 5 (25%) | 9 (100%) | 5 (50%) | 2/4 (50%) | 19 (36%) | 45/109 (41%) |
| History of hypertension | 6 (46%) | 6 (30%) | 2 (22%) | 3 (30%) | 0 | 15 (28%) | 32 (29%) |
| History of diabetes | 2 (15%) | 0 | 0/8 (0%) | 2 (20%) | 0 | 2 (4%) | 6/109 (6%) |
| History of sleep apnea | 1/4 (25%) | 1 (5%) | 0/8 (0%) | 0 | 0 | 6/42 (14%) | 8/89 (9%) |
| Current alcohol drinker | 5 (38%) | 12 (60%) | 5 (56%) | 4 (40%) | 1 (20%) | 27 (51%) | 54 (49%) |
| Current tobacco smoker | 0 | 0 | 1 (11%) | 3 (30%) | 0 | 5 (9%) | 9 (8%) |
| Current recreational drug user | 0 | 0 | 4 (44%) | 1 (10%) | 0 | 7 (13%) | 12 (11%) |
| Body Mass Index (kg/m2) | 28.7 ± 4.6 | 27.4 ± 4.3 | 28.2 ± 4.4 | 25.4 ± 3.6 | 27.1 ± 4.8 | 26.5 ± 5.4 | 27.0 ± 4.9 |
Values are presented as mean ± standard deviation, n (%), or n/total.
Baseline Functional and QoL Outcomes
At enrollment, over 90% of the cohort had independent functional outcome as indicated by mRS≤2 and NIHSS<5 (mild) (Table 3). Only four patients (4%) had severe disability as indicated by mRS of 4 or 5. The median NIHSS was 0 (range 0 – 15); 6% were considered moderate to severe. NIHSS was moderately correlated with mRS (Spearman’s ρ=0.54, p<0.001). In contrast to mRS and NIHSS, a large percentage of patients reported experiencing current health problems on the EQ-5D questionnaire (Table 3). A total of 40% reported experiencing moderate to extreme anxiety or depression, 41% had moderate to severe pain, 30% had some mobility problems, 11% had some problems washing or dressing themselves, and 38% had problems performing usual activities. Despite these problems, the median EuroQoL health rating was 80/100 (range 29 – 100). Four of five EQ-5D domains were significantly correlated with mRS, including usual activities (Spearman’s ρ=0.54, p<0.001), mobility (ρ=0.53, p<0.001), self-care (ρ=0.44, p<0.001), and pain/discomfort (ρ=0.28, p=0.003); anxiety/depression was not associated with mRS (ρ=0.06, p=0.530).
Table 3.
Distribution of outcome measures assessed at baseline in CASH patients.
| Scale | Assessment | N (%) |
|---|---|---|
| Modified Rankin Scale | 0 (No symptoms) | 23 (21%) |
| 1 (No significant disability despite symptoms) | 43 (39%) | |
| 2 (Slight disability) | 34 (31%) | |
| 3 (Moderate disability) | 6 (5%) | |
| 4 (Moderately severe disability) | 3 (3%) | |
| 5 (Severe disability) | 1 (1%) | |
| NIH Stroke Scale | 0 – 4 (Mild) | 104 (95%) |
| 5 – 14 (Moderate) | 5 (5%) | |
| 15 – 24 (Severe) | 1 (1%) | |
| ≥ 25 (Very severe) | 0 | |
| EQ-5D Anxiety/Depression | I am not anxious or depressed | 66 (60%) |
| I am moderately anxious or depressed | 41 (37%) | |
| I am extremely anxious or depressed | 3 (3%) | |
| EQ-5D Pain/Discomfort | I have no pain or discomfort | 65 (59%) |
| I have moderate pain or discomfort | 41 (37%) | |
| I have extreme pain or discomfort | 4 (4%) | |
| EQ-5D Mobility | I have no problems in walking about | 77 (70%) |
| I have some problems in walking about | 33 (30%) | |
| I am confined to bed | 0 | |
| EQ-5D Self-care | I have no problems with self care | 98 (89%) |
| I have some problems washing or dressing myself | 12 (11%) | |
| I am unable to wash or dress myself | 0 | |
| EQ-5D Usual activities | I have no problems with performing my usual activities | 68 (62%) |
| I have some problems with performing my usual activities | 41 (37%) | |
| I am unable to perform my usual activities | 1 (1%) |
On PROMIS-29, CASH patients reported significantly less depression (47.4, 95% CI: 45.8 – 49.0, p=0.002), higher social satisfaction (47.7, 95% CI: 45.9 – 49.5, p=0.012), and worse anxiety scores (52.5, 95% CI: 50.7 – 54.3, p=0.007) compared to a general reference population (Figure 1); these represent small to medium effect sizes. Other domains were not significantly different than the reference population. Higher mRS scores were associated with worse scores for several PROMIS-29 domains, including physical functioning (5.5 per mRS point increase, 95% CI: 4.2 – 6.7, p<0.001), social participation (4.1, 95% CI: 2.5 – 5.7, p<0.001), fatigue (3.0, 95% CI: 1.3 – 4.7, p=0.001), pain (2.8, 95% CI: 1.0 – 4.6, p=0.002), and depression (2.1, 95% CI: 0.6 – 3.6, p=0.006).
Figure 1. Baseline PROMIS-29 domain scores for 110 enrolled patients in the CASH TR Project.
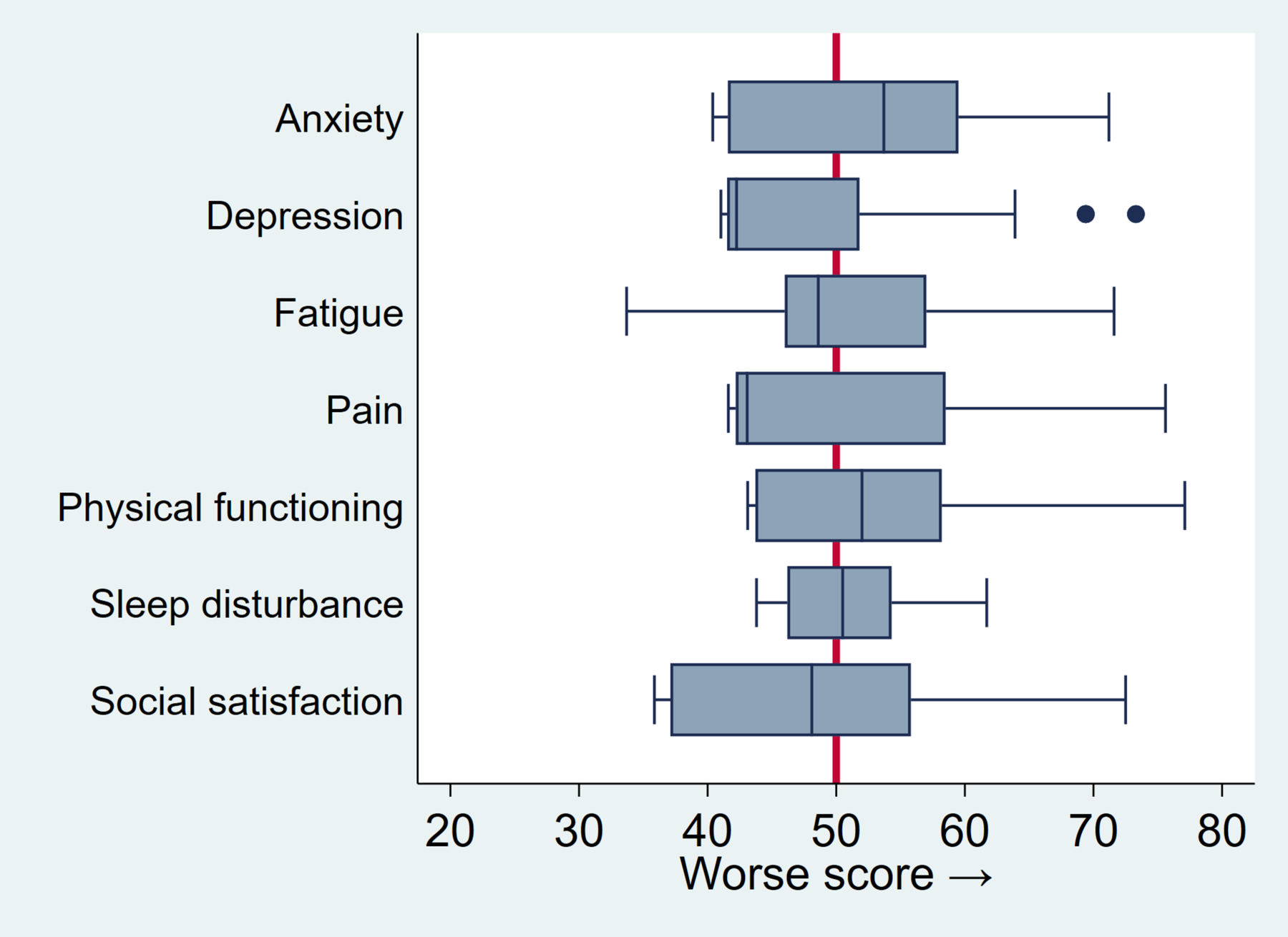
Box plots represent the distribution of standardized T-scores. The box indicates the interquartile range, blue vertical line is the median, whiskers represent lower and upper quartiles, and dots indicate outliers. Red vertical line at 50 indicates mean T-score of reference population (SD 10). Scores are oriented so that higher scores are worse across domains.
Sub-group analyses revealed no domain differences by familial form or brainstem location (data not shown). Females reported worse anxiety (3.5, 95% CI: 0.1 – 7.1, p=0.049) and social satisfaction scores (3.7, 95% CI: 0.1 – 7.3, p=0.045) than males. Increasing age was associated with worse physical functioning (1.2 points worse per decade, 95% CI: 0.2 – 2.3, p=0.018) as expected. Each doubling of time elapsed from hemorrhage date was associated with small improvements in anxiety scores (−1.05 points, p=0.03) and depression scores (−1.05 points, p=0.016), but was not associated with other domains, NIHSS, or EQ-5D.
Baseline Imaging Features
Among patients with susceptibility-weighted imaging (65%), 55% of cases had a single lesion, 30% had between 2–100 lesions, and 15% had >100 lesions. The median number of lesions ≥5mm in maximum diameter on axial T2 imaging was 1 (IQR=1 – 3, maximum=100). The plurality of CASH lesions were located in the brainstem (43%), followed by temporal lobe (12%), thalamus (11%) frontal lobe (10%), cerebellum (9%), parietal lobe (4%), occipital lobe (3%), and other locations (8%). Representative images of CASH lesions are shown in Figure 2, illustrating variability of CASH-eligible lesions.
Figure 2. Magnetic resonance image thumbnails of 73 cases enrolled in CASH TR Project.

T2 images of CASH lesion in 73 consecutively enrolled FUBV cases through May 2020. Images obtained at enrollment, cropped to the region highlighting the CASH lesion at similar scale (bar = 12.8 cm). A diagnostic CASH event must have occurred within the prior 12 months (images of diagnostic hemorrhage not shown).
Baseline Biomarker Imaging (FUBV only)
In FUBV enrolled cases at baseline, the mean lesional QSM was 0.455 ± 0.66 ppm (median=0.452, IQR=0.336 – 0.515, minimum=0.119, maximum=1.024), and mean lesional permeability was 0.391 ± 0.311 mL/100 g/ min (median=0.30, IQR=0.163 – 0.554, minimum=0.019, maximum=1.705). Values were consistent across enrolling sites, and remarkably similar to those obtained from historical CASH cases (Figure 3).
Figure 3. Baseline mean lesional QSM (A) and DCEQP Permeability Index Ki (B) obtained at enrollment in CASH TR FUBV Study.
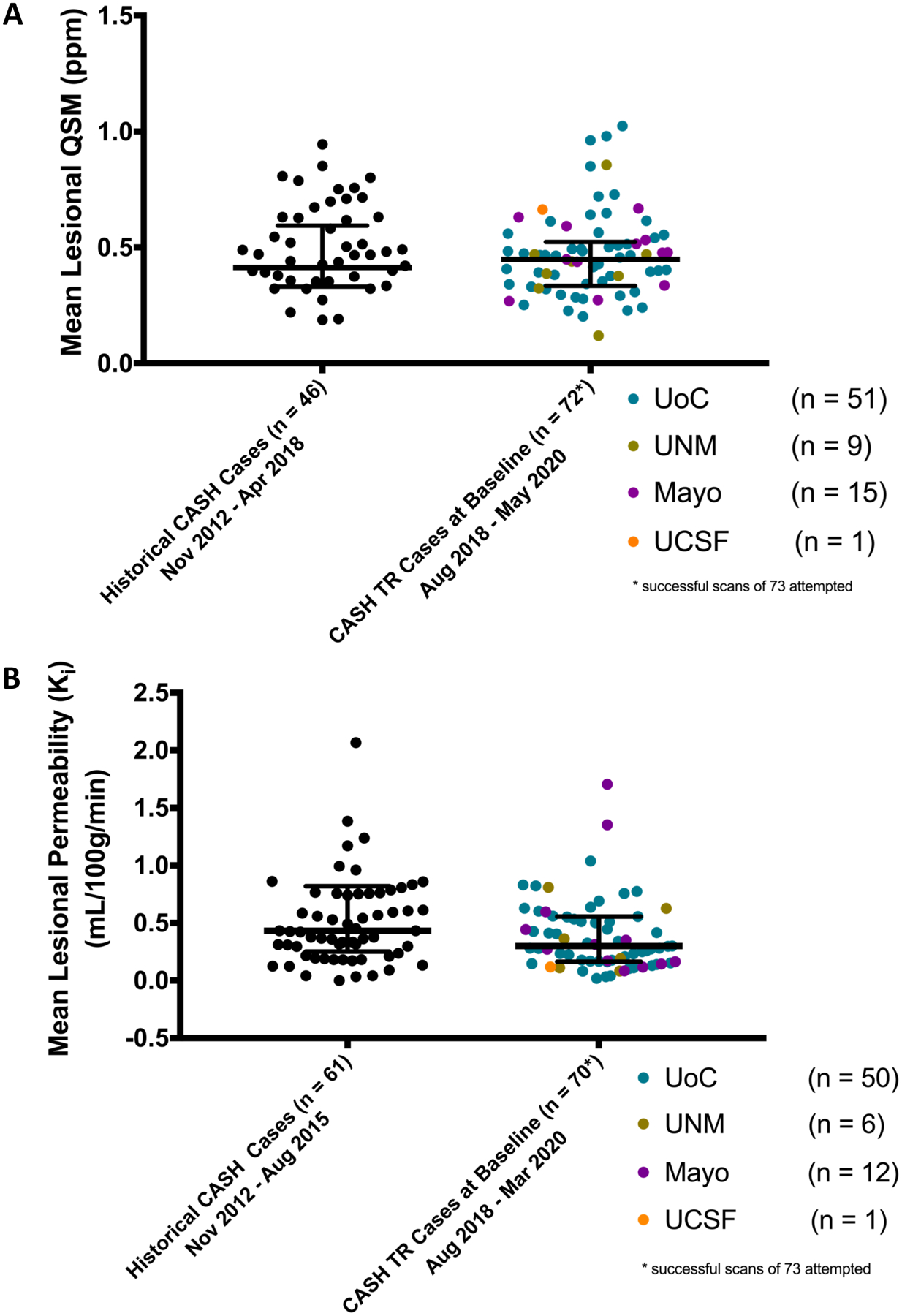
QSM data is shown from 72 cases and DCEQP data from 70 cases among 73 cases enrolled in CASH TR FUBV through May 2020. These represent successfully executed and analyzed data in 98.6% and 95.9% of cases for QSM and DCEQP, respectively. Data points are color coded for participating sites. For reference, historical pilot data is shown from 46 satisfactorily completed QSM and 61 satisfactorily completed DCEQP on CASH cases at the University of Chicago prior to initiation of the CASH TR project.
DISCUSSION
We provide a detailed summary of the first 110 cases enrolled in a multicenter TR study, thereby describing the prevalence and baseline clinical, radiographic, and functional outcome characteristics of CASH patients. This subgroup of patients was previously identified as having characteristics most suitable for enrolling into future clinical trials given the expected high risk of recurrent hemorrhage and baseline treatment equipoise. However, knowledge regarding variability in clinical characteristics, functional and patient-reported outcome, as well as feasibility of recruitment across multiple sites including harmonized data collection and MRI biomarkers was previously lacking. We observed a prevalence of 13% for CASH trial eligible patients among prospectively screened CA cases across six U.S. sites, consistent with prior estimates of 10% to 25%.10 Thus, the study is on track for achieving target enrollment of 200 SCA cases at an average rate of 5 cases enrolled per month.
Choosing a primary endpoint for a treatment trial of CASH cases requires careful consideration of the baseline data. The goal of any treatment for CASH is to prevent recurrent hemorrhage, which would be the obvious endpoint for a clinical trial. However, if one estimates a possible treatment effect of a 25% reduction in hemorrhage rate with 90% power and one-sided alpha of 0.025, a clinical trial with an average of 1.5 years of follow-up and 15% hemorrhage rate in controls would require 1,161 patients per group.10 The overall CASH prevalence of 13% in this study confirms the need for alternative or surrogate outcome measures for efficacy trials in CA.
Functional outcome measures are commonly used as endpoints in stroke clinical trials or as risk stratifiers for study inclusion. The mRS is the most commonly used outcome,21 but has known limitations including interobserver variability, insensitivity to slight changes, a heavy focus on independence in ambulation, and a lack of assessment of other important and common stroke-related disabilities, such as communication and cognition.11, 22 The NIHSS has also been used as an endpoint or patient selection tool in acute stroke trials, but suffers from ceiling effects and a strong focus on left hemispheric deficits with little accounting for language or cognition and no accounting for quality of life.23, 24 Indeed, in our study, we found that >90% of patients enrolled were functionally independent (mRS≤2 and NIHSS<5) at baseline despite ongoing disability and symptomatic complaints. Thus, there is likely little utility to these functional measures as a primary outcome in a CASH clinical trial as usage could underestimate a true treatment effect.
Patient-reported outcomes revealed more variability and differences among CASH patients, and may identify deficits in patients classified as having minimal to no disability based on functional outcome measures.25 PROMIS domains most affected in CASH patients were depression, anxiety, and social satisfaction compared to a general population, and worse scores on 5 of the 7 domains correlated with higher mRS scores. Interestingly, two CASH features representing increased severity (familial form and brainstem location) were not associated with any differences in PROMIS domains. We also found that all health domains were affected on EQ-5D, with greater than 11% (self-care) to as high as 41% (pain) reporting moderate to severe problems. Approximately 40% of our cohort reported moderate to severe anxiety/depression; however, these two items are grouped together on EQ-5D making it difficult to tease apart effects. Prior studies using patient-reported outcomes in CA have found similar results. In one study of 49 untreated, Brazilian CA patients, 33% reported anxiety/depression on EQ-5D.26 In another study of patients with brainstem CA, more than half of functionally independent patients (mRS 0–2) had at least one abnormal domain on PROMIS-29.27 Fatigue and anxiety were commonly abnormal domains. Thus, anxiety, depression, or pain may be of particular concern for CASH cases with psychosocial or psychiatric implications for care after diagnosis, including mental health screening and referrals. Ongoing follow up in the FUBV portion of this study will help determine if patient-reported QoL assessments track with changes in clinical symptoms over time, further clarifying suitability of QoL as potential endpoints for trials.
Across sites, there was considerable variability in the number of familial versus sporadic form of CA. Although there was no significant impact of this baseline feature on variability of PROMIS domains, this will be an important factor going forward to determine if any candidate medications preferentially effect familial versus sporadic forms. Most of the CASH lesions were located in the deep, subcortical regions or in the brainstem. This is not unexpected due to current practice guidelines suggesting observation after first bleed from a deep location due to the risk of morbidity with surgery. It is also important to note that almost half of CASH cases were taking relevant medications, such as Vitamin D, statins, proponalol, or anti-thrombotics, at the time of study enrollment, which may affect biomarker measurements. Future therapeutic trials may need to either exclude individuals taking these medications or require patients to stop taking these current medications before enrolling into trials testing drug efficacy. Both atorvastatin (clinicaltrials.gov: NCT02603328) and proponalol (NCT03589014) are two medications already in early trials as a potential therapy for CA.
MRI biomarkers hold promise because of greater sensitivity to hemorrhagic lesional activity than clinical events due to their ability to detect subclinical change.8 Both QSM and DCEQP are already deployed in an ongoing, single site, proof-of-concept trial of atorvastatin versus placebo in CASH patients.9 We report greater than 90% successful acquisition of mean lesional QSM and DCEQP permeability results at multiple sites following harmonized protocols, rigorous phantom validations, and image adjudication and assessment by a central core imaging laboratory.19 Baseline results of CASH cases were in similar range as cases enrolled at different sites, and prior pilot data used for biomarker validations. This is encouraging with regard to using these techniques as monitoring biomarkers in multisite clinical trials. Follow-up of these biomarkers after one and two years in the same FUBV cases is currently ongoing.
Limitations
Our observational study included recruiting sites with multidisciplinary care teams that follow current CA clinical care guidelines.2 Thus, prevalence of CASH cases at these sites may not be representative of other academic or community hospitals. However, a CASH prevalence of 13% is in line with other reports and represents empirical data on actual enrollment across 6 U.S. sites using a common protocol and demonstrates feasibility of patient recruitment into future clinical trials. This study should not be perceived as a natural history study given an exclusion criteria included planned surgery; however, our CASH cases and characteristics should be representative of cases seen at similar centers that would be eligible for enrollment into a future trial, which was the main goal of this study. Approximately 25% of cases were recruited between 6–12 months after symptomatic hemorrhage event, which can affect patient-reported outcome and imaging features. Although a number of baseline imaging features and outcomes of CASH cases were reported, future planned analyses of FUBV enrolled patients will reveal whether these measures change over time and provide estimates of the magnitude of change both within and between persons, taking into account timing of symptomatic hemorrhage in the year prior to enrollment. The primary reasons for study exclusion were either no symptomatic hemorrhage in the past year or prior/planned surgery of CASH lesion. Additionally, some FUBV patients who live far away from enrollment sites may have chosen to not participate in the study considering the burden of having to come back for multiple MRI visits. If a medical treatment option was available, then enrollment would likely increase as patients considering surgery may elect to delay invasive treatment and be more likely to travel.
SUMMARY
We report the first multicenter harmonized baseline data of CASH patients. These data will help investigators design future clinical trials, determine number of sites required to reach enrollment goals, and anticipate potential pitfalls for screening and enrollment. The ongoing CASH TR project will further elucidate the utility of patient-reported outcomes and MRI biomarkers as surrogate endpoints for recurrent hemorrhage in future clinical trials.
Supplementary Material
Acknowledgments
The authors would like to thank additional investigators and research personnel at enrollment sites who assisted with patient referrals and approvals, and patients for participating in this study.
Sources of Funding:
NIH grant U01NS104157
Conflicts of Interests/Disclosure(s):
HK, JJM, JL, DFH, and IA report other grants from NIH/NINDS during the conduct of the study. HK and IA report personal fees from Recursion Pharmaceuticals outside the submitted work. IA reports grants from StrideBio, Inc. outside the submitted work. JJM reports personal fees from AHA Stroke Associate Editor outside the submitted work. JL reports grants from GE Healthcare outside the submitted work. DFH reports personal fees from Neurotrope, personal fees from Portola Pharmaceuticals, and personal fees from medico-legal consulting outside the submitted work. NM reports other from Johns Hopkins University during the conduct of the study.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- CA
cavernous angioma
- CASH
cavernous angioma with symptomatic hemorrhage
- TR
trial readiness
- QSM
quantitative susceptibility mapping
- DCEQP
dynamic contrast enhanced quantitative perfusion
- UoC
University of Chicago
- UCSF
University of California, San Francisco
- UNM
University of New Mexico
- BNI
Barrow Neurological Institute
- SCA
screening and clinical assessment
- FUBV
follow-up and biomarker validation
REFERENCES
- 1.Al-Shahi Salman R, Berg MJ, Morrison L, Awad IA. Hemorrhage from cerebral cavernous malformations: Definition and reporting standards. Stroke. 2008;39:3222–3230 [DOI] [PubMed] [Google Scholar]
- 2.Akers A, Al-Shahi Salman R, Awad IA, Dahlem K, Flemming K, Hart B, Kim H, Jusue-Torres I, Kondziolka D, Lee C, et al. Synopsis of guidelines for the clinical management of cerebral cavernous malformations: Consensus recommendations based on systematic literature review by the angioma alliance scientific advisory board clinical experts panel. Neurosurgery. 2017;80:665–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horne MA, Flemming KD, Su IC, Stapf C, Jeon JP, Li D, Maxwell SS, White P, Christianson TJ, Agid R, et al. Clinical course of untreated cerebral cavernous malformations: A meta-analysis of individual patient data. Lancet Neurol. 2016;15:166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Shahi Salman R, Hall JM, Horne MA, Moultrie F, Josephson CB, Bhattacharya JJ, Counsell CE, Murray GD, Papanastassiou V, Ritchie V, et al. Untreated clinical course of cerebral cavernous malformations: A prospective, population-based cohort study. Lancet Neurol. 2012;11:217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan H, Liu T, Wu Y, Thacker J, Shenkar R, Mikati AG, Shi C, Dykstra C, Wang Y, Prasad PV, et al. Evaluation of iron content in human cerebral cavernous malformation using quantitative susceptibility mapping. Invest Radiol. 2014;49:498–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan H, Zhang L, Mikati AG, Girard R, Khanna O, Fam MD, Liu T, Wang Y, Edelman RR, Christoforidis G, et al. Quantitative susceptibility mapping in cerebral cavernous malformations: Clinical correlations. AJNR Am J Neuroradiol. 2016;37:1209–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girard R, Fam MD, Zeineddine HA, Tan H, Mikati AG, Shi C, Jesselson M, Shenkar R, Wu M, Cao Y, et al. Vascular permeability and iron deposition biomarkers in longitudinal follow-up of cerebral cavernous malformations. J Neurosurg. 2017;127:102–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeineddine HA, Girard R, Cao Y, Hobson N, Fam MD, Stadnik A, Tan H, Shen J, Chaudagar K, Shenkar R, et al. Quantitative susceptibility mapping as a monitoring biomarker in cerebral cavernous malformations with recent hemorrhage. J Magn Reson Imaging. 2018;47:1133–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polster SP, Stadnik A, Akers AL, Cao Y, Christoforidis GA, Fam MD, Flemming KD, Girard R, Hobson N, Koenig JI, et al. Atorvastatin treatment of cavernous angiomas with symptomatic hemorrhage exploratory proof of concept (at cash epoc) trial. Neurosurgery. 2019;85:843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polster SP, Cao Y, Carroll T, Flemming K, Girard R, Hanley D, Hobson N, Kim H, Koenig J, Koskimaki J, et al. Trial readiness in cavernous angiomas with symptomatic hemorrhage (cash). Neurosurgery. 2019;84:954–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banks JL, Marotta CA. Outcomes validity and reliability of the modified rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke. 2007;38:1091–1096 [DOI] [PubMed] [Google Scholar]
- 12.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607 [DOI] [PubMed] [Google Scholar]
- 13.Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, Haley EC, Grotta J, Marler J. Improved reliability of the nih stroke scale using video training. Ninds tpa stroke study group. Stroke. 1994;25:2220–2226 [DOI] [PubMed] [Google Scholar]
- 14.Brott T, Adams HP Jr., Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke. 1989;20:864–870 [DOI] [PubMed] [Google Scholar]
- 15.Rabin R, de Charro F. Eq-5d: A measure of health status from the euroqol group. Ann Med. 2001;33:337–343 [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Health & Human Services. U.S. Valuation of the euroqol eq-5 health states. January 2012. Agency for Healthcare Research and Quality, Rockville, MD. [Google Scholar]
- 17.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M, et al. The patient-reported outcomes measurement information system (promis): Progress of an nih roadmap cooperative group during its first two years. Med Care. 2007;45:S3–S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilkonis PA, Yu L, Dodds NE, Johnston KL, Maihoefer CC, Lawrence SM. Validation of the depression item bank from the patient-reported outcomes measurement information system (promis) in a three-month observational study. J Psychiatr Res. 2014;56:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hobson N, Polster SP, Cao Y, Flemming K, Shu Y, Huston J, Gerrard CY, Selwyn R, Mabray M, Zafar A, et al. Phantom validation of quantitative susceptibility and dynamic contrast-enhanced permeability mr sequences across instruments and sites. J Magn Reson Imaging. 2020;51:1192–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan GM, Feinn R. Using effect size-or why the p value is not enough. J Grad Med Educ. 2012;4:279–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn TJ, Dawson J, Walters MR, Lees KR. Functional outcome measures in contemporary stroke trials. Int J Stroke. 2009;4:200–205 [DOI] [PubMed] [Google Scholar]
- 22.New PW, Buchbinder R. Critical appraisal and review of the rankin scale and its derivatives. Neuroepidemiology. 2006;26:4–15 [DOI] [PubMed] [Google Scholar]
- 23.DeGraba TJ, Hallenbeck JM, Pettigrew KD, Dutka AJ, Kelly BJ. Progression in acute stroke: Value of the initial nih stroke scale score on patient stratification in future trials. Stroke. 1999;30:1208–1212 [DOI] [PubMed] [Google Scholar]
- 24.Pickard AS, Johnson JA, Feeny DH. Responsiveness of generic health-related quality of life measures in stroke. Qual Life Res. 2005;14:207–219 [DOI] [PubMed] [Google Scholar]
- 25.Stewart JC, Cramer SC. Patient-reported measures provide unique insights into motor function after stroke. Stroke. 2013;44:1111–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bicalho VC, Bergmann A, Domingues F, Frossard JT, de Souza J. Cerebral cavernous malformations: Patient-reported outcome validates conservative management. Cerebrovasc Dis. 2017;44:313–319 [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, Lanzino G, Flemming KD. Affected health domains in patients with brainstem cavernous malformations. Acta Neurochir (Wien). 2019;161:2521–2526 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


