Abstract
Background:
Activated phosphoinositide 3-kinase delta syndrome (APDS) is a combined immunodeficiency with a heterogeneous phenotype considered reversible by allogeneic hematopoietic cell transplantation (HCT).
Objectives:
This study sought to characterize HCT outcomes in APDS.
Methods:
Retrospective data were collected on 57 patients with APDS1/2 (median age, 13 years; range, 2–66 years) who underwent HCT.
Results:
Pre-HCT comorbidities such as lung, gastrointestinal, and liver pathology were common, with hematologic malignancy in 26%. With median follow-up of 2.3 years, 2-year overall and graft failure–free survival probabilities were 86% and 68%, respectively, and did not differ significantly by APDS1 versus APDS2, donor type, or conditioning intensity. The 2-year cumulative incidence of graft failure following first HCT was 17% overall but 42% if mammalian target of rapamycin inhibitor(s) (mTORi) were used in the first year post-HCT, compared with 9% without mTORi. Similarly, 2-year cumulative incidence of unplanned donor cell infusion was overall 28%, but 65% in the context of mTORi receipt and 23% without. Phenotype reversal occurred in 96% of evaluable patients, of whom 17% had mixed chimerism. Vulnerability to renal complications continued post-HCT, adding new insights into potential nonimmunologic roles of phosphoinositide 3-kinase not correctable through HCT.
Conclusions:
Graft failure, graft instability, and poor graft function requiring unplanned donor cell infusion were major barriers to successful HCT. Post-HCT mTORi use may confer an advantage to residual host cells, promoting graft instability. Longer-term post-HCT follow-up of more patients is needed to elucidate the kinetics of immune reconstitution and donor chimerism, establish approaches that reduce graft instability, and assess the completeness of phenotype reversal over time.
Keywords: Primary immunodeficiency, activated phosphoinositide 3-kinase delta syndrome, lymphoproliferation, allogeneic hematopoietic cell transplantation, graft failure, mTOR inhibitor, serotherapy
Activated phosphoinositide 3-kinase (PI3K) delta syndrome (APDS) was first described in 2013, with heterozygous mutations in PI3Kδ catalytic p110δ (PIK3CD) or regulatory p85α (PIK3R1) subunits leading to APDS1 and APDS2, respectively.1,2 PI3Ks are expressed in various hematopoietic cells and have important roles in Tand B lymphocyte homeostasis. The heterogeneous immunological phenotype of APDS may include reduced naive T cells, increased senescent CD8+ T cells, increased transitional B cells, decreased class-switched memory B cells, and dysgammaglobulinemia.1–6 Clinical manifestations vary, including, among others, recurrent sinopulmonary infections, enteropathy, autoimmunity, nonneoplastic lymphoproliferation, lymphoma, and impaired EBV, cytomegalovirus (CMV), and varicella-zoster virus control.4,5,7
Supportive care may include antimicrobials and/or immunoglobulin replacement. Corticosteroids, rituximab, and splenectomy may attenuate autoimmune and lymphoproliferative disease manifestations.3,8 The mammalian target of rapamycin (mTOR) activated downstream of PI3K has a significant role in the regulation of immune responses and therefore mTOR inhibitor(s) (mTORi; rapamycin/sirolimus or everolimus) can ameliorate the severity of nonneoplastic lymphoproliferative disease and restore natural killer cell function.9,10 Selective PI3K-delta inhibitors are of interest as a more targeted treatment option and are under continued clinical investigation,11 but the ability of these therapies to prevent the development of life-and organ-threatening complications for the life span of affected individuals remains to be seen. Allogeneic hematopoietic cell transplantation (HCT) offers a potential immunologic cure for patients with APDS1/2;12,13 however, questions remain regarding the optimal timing, intensity, and approach to HCT for APDS1/2, as well as the donor chimerism needed to durably achieve engraftment of donor cells and to prevent immunopathological manifestations of disease.14 Herein, we present the largest international retrospective study of HCT outcomes of patients with APDS1/2 to date.
METHODS
Data collection
We conducted an international case series study of the clinical outcomes of patients with APDS1/2 undergoing HCT. Each participating site obtained approval to contribute deidentified data as per institutional requirements, in accordance with the Declaration of Helsinki, prior to contributing data. Data transfer agreements were put in place when deemed required. The APDS European Society for Immunodeficiencies registry and European Society for Blood and Marrow Transplantation Inborn Errors Working Party were queried to identify European contributors.
Criteria for inclusion were (1) pathogenic germline PIK3CD or PIK3R1 mutation and (2) receipt of HCT for APDS1/2 disease manifestations. A deidentified data query form was completed by participating physicians for each patient.
Data captured included patient demographics; pre-HCT disease manifestations and comorbidities; HCT-comorbidity index (HCT-CI) score;15 prior therapies; HCT approach including conditioning drugs and intensity, graft source, dose, manipulation, and donor demographics; graft-versus-host disease (GVHD) prophylaxis approach; HCT complications; engraftment and chimerism kinetics; immune reconstitution; degree of post-HCT phenotype reversal; follow-up duration; and survival outcomes.
Endpoint definitions and captured complications are detailed in Table E1 in this article’s Online Repository at www.jacionline.org.
Data were locked for analysis on July 13, 2020.
Statistical analyses
Descriptive statistics were used for patient and HCT characteristics. Conditioning intensity was categorized as myeloablative conditioning (MAC), reduced-toxicity MAC (RT-MAC, regimens using treosulfan as an alkylator), reduced-intensity conditioning (RIC), and nonmyeloablative conditioning based on published consensus definitions and the treating center’s intent.16,17
Survival curves were constructed for overall survival (OS) and graft failure (GF)-free survival (GFFS) using the Kaplan-Meier method and compared using the log-rank test.
Cumulative incidence curves were constructed using the method of Fine and Gray and compared using K-sample tests.18 The cumulative incidences of transplant-related mortality, GF, and subsequent unplanned donor cell infusion (DCI) were determined (competing risk: death), as well as CD4+ T-cell recovery >200 cells/μL (competing risks: death, GF). Cumulative incidence curves were constructed for subgroups based on conditioning intensity, use of serotherapy, HLA match, and use of mTORi post-HCT.
Survival curves, Kruskal-Wallis, and Fisher exact tests were generated using GraphPad Prism, version 8.4.3 (Graph-Pad Software, La Jolla, Calif; www.graphpad.com). Cumulative incidence curves were generated using R program, version 3.6.1 (R Core Development Team, Vienna, Austria; www.r-project.org). Results were considered statistically significant if 2-tailed P < .05.
RESULTS
Fifty-seven pediatric and adult patients with APDS who received HCT (43 with PIK3CD, 14 with PIK3R1 mutations) were included, all with confirmed pathogenic mutations and 20 with previously detailed HCT courses.1,2,12,13,19–29 Patient and disease characteristics are shown in Table I. All patients had clinical indications for HCT, and none were transplanted preemptively based on genetic diagnosis alone k(see Table E2 in this article’s Online Repository at www.jacionline.org). For 45 patients (79%), diagnosis had been genetically confirmed by the time of first HCT. Many had significant organ pathology entering first HCT, with median HCT-CI score of 2. History of immune cytopenias was reported in 33% of patients and hematologic malignancy in 26%. Most patients had received immunomodulating therapies before proceeding to HCT, including mTORi in 49%, rituximab in 26%, and PI3K inhibitor in 7%. Immunoglobulin therapy for immunomodulation or replacement occurred in 86% of patients prior to HCT. Active disease at time of HCT was common, including infection (51%), hepatosplenomegaly (51%), immune cytopenias (18%), or hematological malignancy (11%).
TABLE I.
Patient baseline characteristics
| Patients (n = 57) | |
|---|---|
| Demographics | |
| Male | 35 (61) |
| Age at time of first HCT (y) | 13 (2–66) |
| Genetic defect* | |
| Known at time of first HCT | 45 (79) |
| Familial (confirmed) | 10 (18) |
| PIK3CD gain of function, heterozygous | 43 (75) |
| c.3061G>A, p.E1021K | 33 |
| Other | 10† |
| PIK3R1 splice site mutation, heterozygous | 14 (25) |
| c.1425+1 G>A | 10 |
| Other | 4† |
| Clinical history and phenotype | |
| Noninfectious lung pathology | 38 (67) |
| Gastrointestinal pathology | 32 (57) |
| History of immune cytopenias | 19 (33) |
| Hematological malignancy | 15 (26) |
| B-cell lymphoma | 13 (23)ठ|
| Multiple myeloma | 1 (2) |
| Hepatosplenic CD8+ T-cell lymphoma | 1 (2) |
| Liver pathology | 11 (19) |
| Known nodular regenerative hyperplasia or portal hypertension | 7 (12) |
| Renal pathology | 8 (14) |
| Cardiac pathology | 4 (7) |
| Prior therapies | |
| Immunoglobulin infusions | 49 (86) |
| mTORi | 28 (49) |
| Rituximab | 23 (40) |
| Nonmalignant indication (lymphoproliferation, autoimmunity) | 15 (26) |
| As part of multidrug chemotherapy for malignancy | 8 (14) |
| Splenectomy | 10 (18) |
| PI3K inhibitor | 4 (7) |
| Clinical status at HCT | |
| HCT-CI score at first HCT (n = 48)§ | 2 (0–7) |
| Pediatric only (n = 37) | 2 (0–7) |
| Adult only (n = 11) | 4 (1–6) |
| HCT-CI score ≥3 | 21 (44) |
| Pediatric only (n = 37), HCT-CI score ≥3 | 12 (32) |
| Adult only (n = 11), HCT-CI score ≥3 | 9 (82) |
| Karnofsky/Lansky performance status at first HCT (n = 44)(%) | 90 (30–100) |
| Hematological malignancy status at HCT | |
| In complete remission at HCT | 10 (18) |
| In partial remission or active at HCT | 6 (11)|| |
| Hepatosplenomegaly | 29 (51) |
| Active infection | 29 (51) |
| Active immune cytopenias | 10 (18) |
Values are n, n (%), or median (range).
Pathogenicity was confirmed for all mutations.
Other PIK3CD mutations: c.1573G>A, p.E525K (n = 3); c.1002C>A, p.N334K (n = 2); c.1246T>C, p.C416R (n = 2); c.3074A>G, p.E1025G (n = 1); c.1574A>C p.E5 25A (n = 1); c.371 G>A, p.G124D (n = 1). Other PIK3R1 mutations: c.1425+1 G>T (n = 2); n = 1 each: c.1422_1 425+1 delCCAG, c.1425+1 G>C.
B-cell lymphoma details: diffuse large B-cell lymphoma (n = 7, 1 patient with 2 separate lymphomas; 5 EBV-positive, 2 EBV-negative); Hodgkin lymphoma (n = 6; 2 EBV-positive, 2 EBV-negative, EBV data not available for 2), marginal zone lymphoma (n = 2; 1 EBV-positive, 1 EBV-negative).
Lung function results, FEV1, and diffusing capacity of carbon monoxide (DLCO), were available in 8 of 37 pediatric patients, FEV1 only in 17, DLCO only in 1, and not performed in 11, due to patient inability or center practices. Thus, HCT-CI scores in pediatric patients may be underestimated. Of 11 adults, FEV1 and DLCO were available for 9, and FEV1 only for 2.
One patient had 2 B-cell lymphomas prior to HCT, EBV-positive nodular sclerosing classical Hodgkin lymphoma in CR1 at time of HCT, and EBV-positive marginal zone lymphoma active and untreated prior to HCT.
Donor, graft, and HCT platform characteristics for 66 HCTs performed are detailed in Tables II and III. Unrelated donors were the most frequent donor source (62%), with unmanipulated bone marrow as the most frequent graft source (48%). Matched sibling donors (MSDs) were used in 11% of HCTs; these recipients also had a lower median HCT-CI score, 1, and younger median age, 9 years, as compared to recipients of grafts from other donor types, although these differences did not reach statistical significance. Most HCTs were either RIC (n = 35, 53%) or MAC (n = 24, 36%). The median age of patients receiving RIC versus MAC/RT-MAC at time of first HCT was the same, 13 years, but age range was wider for recipients of RIC, who also had higher median HCT-CI score (RIC 3, MAC 2, RT-MAC 0, P = .05). Most HCTs were performed using serotherapy (n = 55, 83%). Adults were more likely than children to have hematologic malignancy as a HCT indication (67% vs 16%, P =.001), but age at first HCT did not correlate with survival.
TABLE II.
Donor and graft characteristics
| HCTs (n = 66) | |
|---|---|
| Graft source | |
| Bone marrow | 32 (48) |
| PBSCs | 31 (47) |
| Single umbilical cord | 3 (5) |
| Graft dose by graft type, TNC × 108 cells/kg | |
| Bone marrow, unmanipulated (n = 29) | 3.47 (0.86–9.67) |
| PBSCs, unmanipulated (n = 21) | 12.1 (4–40.7) |
| PBSCs, manipulated (n = 7) | 8 (0.092–13.3) |
| Single umbilical cord (n = 3) | 0.499 (0.34–0.5) |
| Donor source | |
| Unrelated (non-cord) | 41 (62) |
| HLA-10/10 | 29 (44) |
| HLA-8/8 | 36 (55) |
| Other: HLA-7/8 (n = 3), HLA-5/8 (n = 1), HLA 6/6 (n = 1) | 5 (8) |
| HLA-haploidentical | 15 (23) |
| HLA-matched sibling | 7 (11) |
| Female donor into male recipient* | 16 (28) |
| Donor age, y* | 27 (4–58) |
| ABO* | |
| Matched | 29 (50) |
| Minor mismatch | 15 (26) |
| Major mismatch | 12 (21) |
| Major and minor mismatch | 2 (3) |
| CMV serostatus D/R* | |
| D+/R+ | 23 (40) |
| D+/R unknown | 13 (22) |
| D+/R− | 1 (2) |
| D−/R+ | 8 (14) |
| D−/R− | 7 (12) |
| D−/R unknown | 6 (10) |
D/R, Donor/recipient; TNC, total nucleated cells.
Values are n (%) or median (range).
Number of HCTs for which data were available: donor sex, ABO compatibility, and CMV serostatus (n = 58); donor age (n = 53).
TABLE III.
Transplant platform characteristics, by donor type
| Total HCTs (n = 66) | MSD HCTs (n = 7) | 8/8 MUD HCTs (n = 36) | Haplo HCTs (n = 15) | Other HCTs (n = 8) | |
|---|---|---|---|---|---|
| Conditioning intensity | |||||
| MAC | 24 (36) | 7 (100) | 11 (31) | 5 (33) | 1 (13) |
| RT-MAC | 6 (9) | 0 | 6 (17) | 0 | 0 |
| RIC | 35 (53) | 0 | 19 (53) | 9 (60) | 7 (88) |
| Nonmyeloablative conditioning | 1 (2) | 0 | 0 | 1 (7) | 0 |
| Serotherapy use | 55 (83) | 5 (71) | 34 (84) | 11 (73) | 5 (63) |
| Antithymocyte globulin | 33 (50) | 4 (57) | 18 (50) | 8 (53) | 3 (38) |
| Rabbit | 29 (44) | 4 (57) | 15 (42) | 7 (47) | 3 (38) |
| Horse | 4 (6) | 0 | 3 (8) | 1 (7) | 0 |
| Alemtuzumab | 22 (33) | 1 (14) | 16 (44) | 3 (20) | 2 (25) |
| Proximal timing (administered day −8 or closer to HCT) | 15 (23) | 1 (14) | 12 (33) | 0 | 2 (25) |
| Intermediate timing (administered between days −16 and −9) | 7 (11) | 0 | 4 (11) | 3 (20) | 0 |
| Total body irradiation (total dose, 2–4 Gy) | 12 (18) | 0 | 6 (17) | 3 (20) | 3 (38) |
| GVHD prophylaxis | |||||
| Calcineurin inhibitor-based | 48 (73) | 7 (100) | 29 (81) | 5 (3) | 7 (88) |
| Posttransplantation cyclophosphamide-based | 13 (20) | 0 | 5 (14) | 8 (53) | 0 |
| Graft manipulation | 8 (13) | 0 | 4 (11) | 3 (20) | 1 (13) |
| α/b T-cell/CD19+ depletion, with or without CD45RA− add-back | 6 (9) | 0 | 3 (8) | 2 (13) | 1 (13) |
| α/b T-cell depletion | 1 (2) | 0 | 0 | 1 (7) | 0 |
| CD34+ positive selection | 1 (2) | 0 | 1 (3) | 0 | 0 |
| No pharmacologic prophylaxis apart from serotherapy | 3 (5) | 0 | 0 | 2 (13) | 1 (13) |
| Other*/incomplete information | 2 (3) | 0 | 2 (6) | 0 | 0 |
Values are n or n (%).
In addition to rabbit antithymocyte globulin and graft manipulation, patient received abatacept and mycophenolate mofetil early post-HCT, followed by methotrexate.
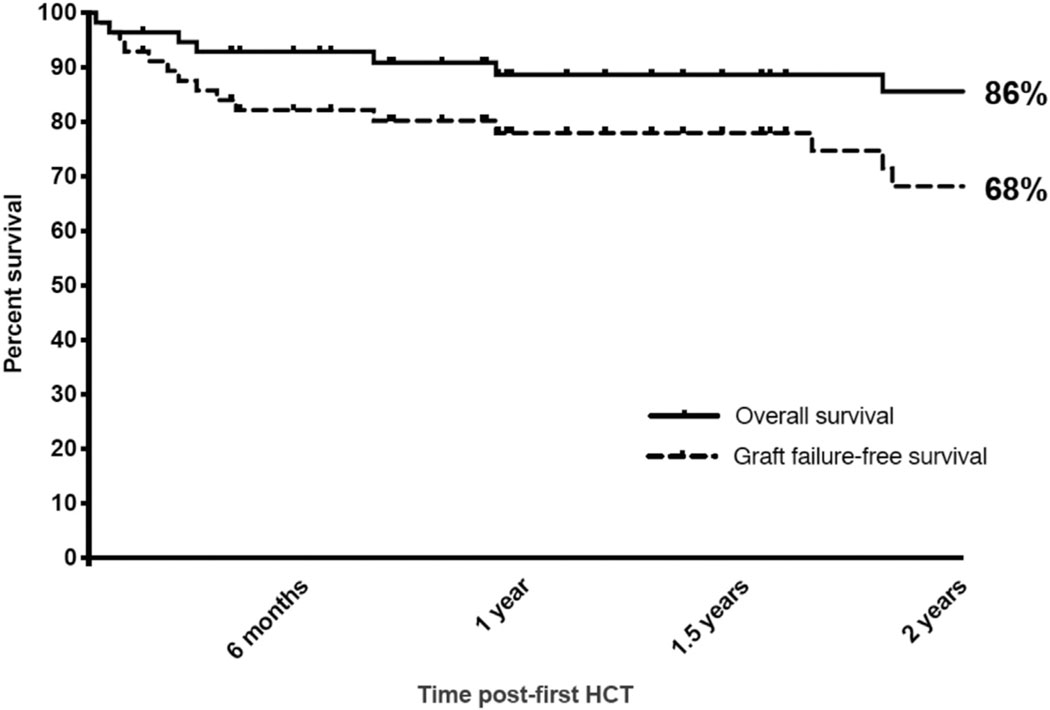
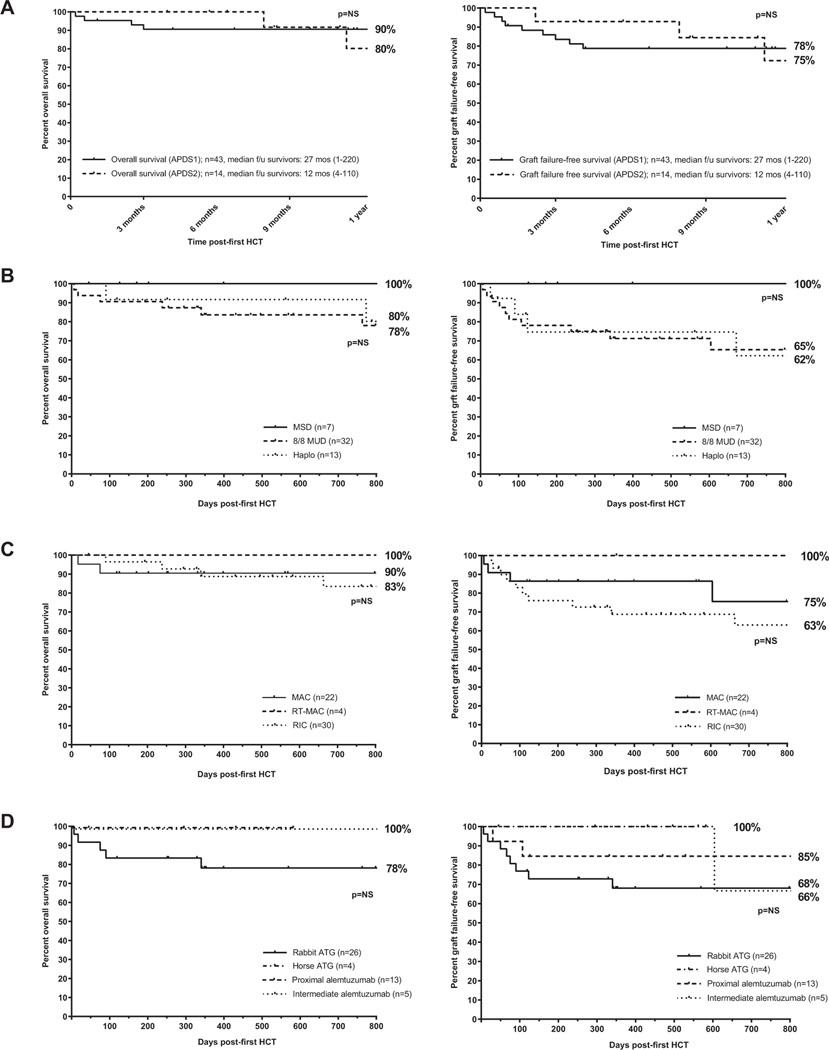
The median follow-up was 27 months overall by the reverse Kaplan-Meier method and 26.3 months (range, 1.5–220.6 months) from first HCT in surviving patients. The 2-year probabilities of OS and GFFS from first HCT were 86% and 68%, respectively (Fig 1). Differences in OS or GFFS were not statistically significant by underlying diagnosis (APDS1 vs APDS2), conditioning intensity, serotherapy choice, or donor source (Fig 2). Subcohorts with 2-year OS and GFFS probabilities of 100% included recipients of RT-MAC (n = 4) or MSD (n = 7), albeit limited by small numbers; in addition, 1 very late GF (day +1862) occurred in 1 RT-MAC HCT. Transplant-related mortality occurred due to late sepsis beyond 6 months post-HCT (n = 3), early post-HCT preengraftment infections (n = 2), viral infection (n = 1), sinusoidal obstructive syndrome (SOS, n = 1), and multiorgan failure in the setting of poor graft function (n = 1); 63% of deaths occurred in the first 100 days post-HCT. Outcomes by number of patients transplanted at individual centers are shown in Table E3 in this article’s Online Repository at www.jacionline.org.
FIG 1.
Kaplan-Meier survival curves depicting OS and GFFS for all patients (n = 57) with median follow-up of 27 months overall by the reverse Kaplan-Meier method and 26.3 months (range, 1.5–220.6 months) from first HCT in survivors.
FIG 2.
Kaplan-Meier survival curves depicting OS and GFFS by underlying diagnosis (A), donor type (smallest subgroup of mismatched unrelated donor and cord excluded, n = 5) (B), conditioning intensity (smallest subgroup of nonmyeloablative/immunosuppression only conditioning excluded, n = 1) (C), or serotherapy choice (of note, follow up is shorter for smaller subgroups of horse antithymocyte globulin [ATG] and intermediate timing alemtuzumab) (D). NS, Not significant.
Neutrophil engraftment occurred at median 16 days (range, 11–127 days) and platelet engraftment at median 21 days (range, 7–162 days). GF or graft instability and requirement for DCI occurred frequently, with 8 patients (14%) requiring a second HCT and 1 patient requiring a third HCT (Table IV). A total of 43 subsequent DCIs were administered to 18 patients (32%), including 24 donor lymphocyte infusions and 10 stem cell boosts. Mixed chimerism was the most common indication for intervention (n = 20), followed by poor graft function (n = 10).
TABLE IV.
Engraftment and subsequent unplanned cell infusions
| First HCT, patients (n = 57) | Second HCT, patients (n = 8) | Third HCT, patients (n = 1) | |
|---|---|---|---|
| Engraftment | |||
| Primary graft failure | 2 (4) | 0 | 0 |
| Secondary graft failure | 7 (12) | 1 (13) | 0 |
| Unstable chimerism or threatened graft failure (not progressing to graft failure) | 4 (7) | 2 (25) | 0 |
| Poor graft function | 9 (16) | 3 (38) | 0 |
| Subsequent unplanned cell infusion, no. of patients* | 18 (32) | 4 (50) | 0 |
| Donor lymphocyte infusion† | 7 (12) | 2 (25) | 0 |
| Repeat HCT‡ | 8 (14) | 1 (13) | 0 |
| Peripheral blood stem cell boost§ | 5 (9) | 1 (13) | 0 |
Values are n or n (%).
A total of 43 subsequent unplanned cell infusions were administered.
A total of 24 unplanned donor lymphocyte infusions were administered, for mixed chimerism (n = 18), viral infection (n = 2), lymphoma relapse (n = 2), poor graft function (n = 1), promoting immune reconstitution (n = 1).
Indications for repeat HCT included graft failure (n = 6), mixed chimerism (n = 2), and lymphoma relapse (n = 1).
A total of 10 stem cell boosts were administered, for poor graft function (n = 9) and to promote immune reconstitution (n = 1, included CD3+ add back).
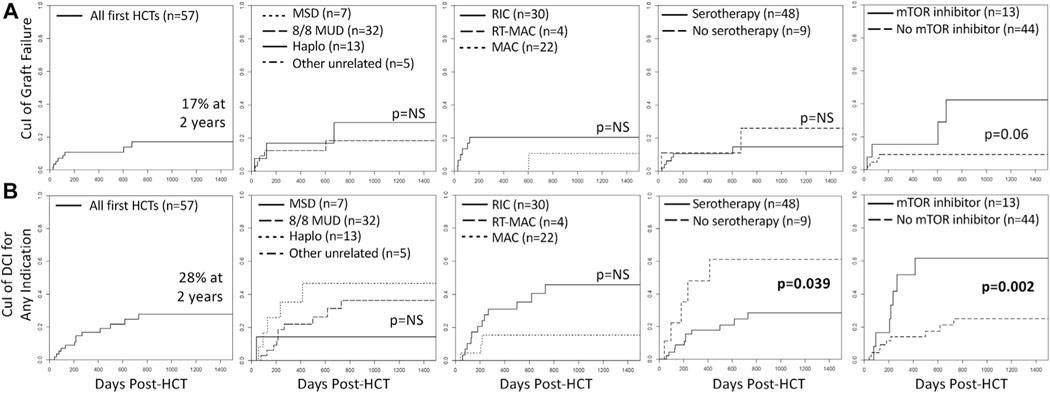
By competing risk analysis, the estimated probability of GF following first HCT was 10% (95% CI, 4%−44%) at 1 year and 17% (95% CI, 8%−30%) at 2 and 3 years (Fig 3). However, when mTORi was used within the first year after HCT (in 13 patients), it rose from 15% (95% CI, 2%−40%) at 1 year to 42% (95% CI, 9%−73%) at 2 and 3 years, compared with a stable and lower incidence of 9% (95% CI, 3%−20%) at 1, 2, and 3 years without mTORi use, P = .06, approaching statistical significance. More strikingly, the estimated probability of DCI, overall 17% (95% CI, 8%−28%) at 1 year and 28% (95% CI, 15%−42%) at 2 and 3 years, was 55% (95% CI, 23%−79%) at 1 year and 65% (95% CI, 27%−86%) at 2 and 3 years when mTORi was used, compared with 12% (95% CI, 4%−24%) at 1 year and 23% (95% CI, 10%−39%) at 2 and 3 years without mTORi use, P = .002. The cumulative incidence of DCI, but not GF, also differed by use of serotherapy, where serotherapy-free regimens had 1- and 3-year estimated probabilities of DCI of 48% (95% CI, 12%−78%) and 61% (95% CI, 17%−87%), compared with 18% (95% CI, 8%−30%) and 28% (95% CI, 15%−44%) with serotherapy-containing regimens, P = .039. The cumulative incidence of GF or DCI did not differ by conditioning intensity or HLA match/relatedness.
FIG 3.
Cumulative incidence (CuI) of graft failure after first HCT (A) and subsequent DCI for any indication after first HCT (B), overall and by donor type, conditioning intensity (excludes nonmyeloablative immunosuppression-only conditioning, n = 1), serotherapy use during conditioning, and mTORi use within the first year after HCT. No graft failure was observed with MSD or other unrelated donor (mismatched, n = 4; cord, n = 1). One patient had graft failure 1862 days post-RT-MAC-HCT requiring retransplantation (not depicted).
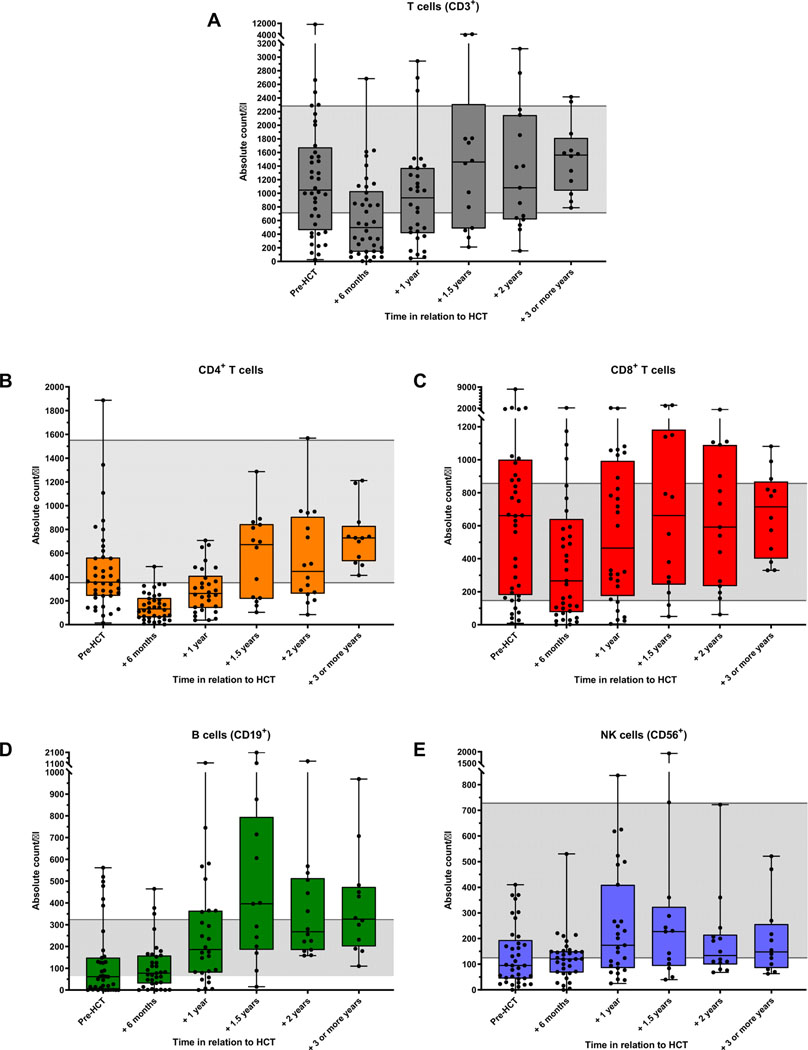
Total T cells and CD4+ T cells were slower to recover than CD8+ T cells, B cells, and natural killer cells were, with median total T cells within normal range at 1-year post-HCTand median CD4+ T cells within normal range at 1.5 years, whereas median counts for other lymphocyte subsets largely recovered by 6 months (Fig 4). Persistent profound T-cell lymphopenia (total T cells range, 46–155 cells/μL) was reported in 5 patients (17% of those with available data) at 10 to 12 months post-HCT; 2 were receiving systemic therapy for GVHD. At 1 year, the estimated probability of CD4+ T-cell count recovery above 200 cells/μL was 41% (95% CI, 27%−54%) and did not differ significantly by conditioning intensity or donor type. CD4+ T-cell recovery during the first year post-HCT was similar for serotherapy-containing and serotherapy-free regimens, although serotherapy-free HCT numbers were small and thus limited post-HCT lymphocyte subset data analyses for this group (see Fig E1 in this article’s Online Repository at www.jacionline.org).
FIG 4.
Lymphocyte subset counts pre-HCT and reconstitution post-HCT in total T cells (A), CD4+ T cells (B), CD8+ T cells (C), B cells (D), and natural killer (NK) cells (E). Only patients with data for ≥1 post-HCT time point are included. One patient is represented twice, at time of first HCT and at time of subsequent HCT 5 years later. Gray shading represents normal adult reference ranges.
Acute GVHD was reported in 22 patients (39%), 5 post-DCI; maximum grade 3 acute GVHD occurred in 4 patients (7%), 3 post-DCI. Chronic GVHD occurred in 9 patients (16%). The estimated probability of grades 3 and 4 acute GVHD at 1 year was 29% (95% CI, 3%−64%) with MSD, compared with 14% (95% CI, 2%−38%) for HLA haploidentical, 0% for matched unrelated donors (MUDs), and 0% for umbilical cord blood, P = .018. The cumulative incidence of grades 2 to 4 acute GVHD did not differ significantly by donor type, conditioning intensity, or serotherapy use, nor did the cumulative incidence of grades 3 and 4 acute GVHD differ by conditioning intensity or serotherapy use. (See Fig E2 in this article’s Online Repository at www.jacionline.org)
Regimen-related and infectious post-HCT complications are summarized in Table V. SOS developed in 3 patients, all recipients of RIC regimens, including 1 patient with a history of pre-HCT EBV-related SOS who developed SOS anew, 1 patient had known prior liver pathology and likely multifactorial liver injury in the setting of sepsis but met Baltimore criteria,30 and 1 with no known baseline liver pathology. Interestingly, renal failure requiring dialysis was observed in 6 patients (11%), of whom 3 had known immune-mediated renal pathology prior to HCT. Additionally, 1 patient developed papillary renal cell carcinoma 1-year post-HCT, in complete remission postcryoablation at last follow-up. Infectious complications were notable for EBV in blood requiring therapy in 11% of patients, CMV and adenovirus organ involvement in 7% and 2%, respectively, and lower respiratory tract viral infection other than CMV or adenovirus in 14%. CMV disease (1 fatal) and EBV-posttransplantation lymphoproliferative disease occurred exclusively in patients who received proximal serotherapy (within 8 days of HCT), with the exception of 1 case of primary CMV infection (seronegative donor and recipient) after serotherapy-free conditioning. One patient developed renal failure requiring ongoing hemodialysis due to biopsy-proven, BK polyomavirus-associated nephropathy; BK nephropathy may have predated HCT, as this patient had renal insufficiency at baseline in the presence of BK viremia, but no pre-HCT renal biopsy was performed.
TABLE V.
Outcomes for all patients
| Outcome | Patients (n = 57) |
|---|---|
| Transplant-related mortality | 8 (14) |
| Infection* | 6 (11) |
| Organ toxicity (regimen-related) | 2 (4) |
| Acute GVHD | 22 (39) |
| Grade 2–4 | 13 (23) |
| Grade 3–4 | 4 (7) |
| Chronic GVHD† | 9 (16) |
| Mild | 3 (11) |
| Moderate | 1 (2) |
| Severe | 2 (4) |
| Organ toxicities | |
| Renal failure requiring dialysis‡ | 6 (11) |
| Sinusoidal obstructive syndrome§ | 3 (5) |
| Congestive heart failure | 3 (5) |
| ARDS | 3 (5) |
| Respiratory failure requiring ECMO§ | 2 (4) |
| DAH, IPS, BO, or COP | 0 |
| Infectious complications | |
| CMV infection requiring treatment; CMV disease | 26 (46); 4 (7) |
| EBV in blood requiring therapy; EBV-PTLD | 6 (11); 3 (5) |
| Adenoviremia requiring treatment; adenovirus with organ involvement | 4 (7); 1 (2) |
| HHV-6 in blood requiring treatment; HHV-6 encephalitis | 5 (9); 0 |
| BK virus-associated hemorrhagic cystitis; biopsy-proven BK nephropathy | 10 (18); 1 (2) |
| Lower respiratory viral infection other than CMV or adenovirus | 8 (14) |
| HSV requiring treatment | 6 (11) |
| VZV requiring treatment | 3 (5) |
| Bacteremia, with or without sepsis; sepsis | 19 (33); 9 (16) |
| Other significant bacterial infection | 10 (18) |
| Fungal infection requiring systemic treatment | 3 (5) |
| Pneumocystis jiroveci pneumonia | 1 (2) |
| Toxoplasmosis reactivation | 0 |
ARDS, Acute respiratory distress syndrome; BO, bronchiolitis obliterans; COP, cryptogenic organizing pneumonia; DAH, diffuse alveolar hemorrhage; ECMO, extracorporeal membranous oxygenation; HHV-6, human herpesvirus-6; HSV, herpes simplex virus; IPS, idiopathic pneumonia syndrome; PTLD, post-transplantation lymphoproliferative disease; VZV, varicella zoster virus.
Values are n or n (%).
Attributed to Pseudomonas aeruginosa (n = 3, at days +6, +238, +340), Rhizomucor pusillus (n = 1, day +17), sepsis in asplenic patient (n = 1, day +663), and CMV disease (n = 1, day +75). Only the last patient had active GVHD (grade 2, skin only) requiring systemic corticosteroids, diagnosed a week before death; 1 other patient had grade 2, skin only GVHD that developed following donor lymphocyte infusion for lymphoma relapse, treated with calcineurin inhibitor alone, but died of multidrug-resistant Pseudomonas.
Chronic skin GVHD without available data on severity reported for 3 patients. One patient had probable ocular-only chronic GVHD, not diagnostic of GVHD per 2014 consensus criteria and not included above.
Occurred in the setting of severe infection (n = 5), SOS, and thrombotic microangiopathy (n = 1). Three patients had significant known preexisting renal pathology. Two patients had concurrent respiratory failure requiring ECMO; outcomes included transplant-related mortality (n = 4) and chronic kidney disease (n = 2, focal segmental glomerulosclerosis on immunosuppression; BK-associated nephropathy requiring hemodialysis).
Developed following RIC in all 3 patients, 2 of whom had known prior known liver pathology (n = 2). The remaining patient, without prior known liver pathology, received 16 mg/kg total of busulfan, targeting area under the curve of 60 mg · h/L.
Of evaluable, engrafted survivors (n = 47), 45 (96%) are alive and well with phenotype reversal, 8 (17%) in the setting of mixed chimerism in either whole blood or myeloid and/or CD3+ compartments (Table VI). One patient has continued disease manifestations (immune thrombocytopenia, hypogammaglobulinemia) over 2 years post-HCT in the setting of mixed donor chimerism (85% myeloid, 58% CD3+) despite having received 9 DCIs, albeit with improvement of the disseminated Mycoplasma orale infection that prompted HCT. Another has resolution of recurrent respiratory infections, enteropathy, CMV and EBV infection, and immunoglobulin replacement requirement but had alphaherpesvirus infections 2 years post-HCT despite prophylaxis, in the context of continued mixed chimerism (26% myeloid, 34% CD3+). Four other patients have significant ongoing complications related to GVHD or chronic kidney disease in the setting of 100% donor chimerism and resolution of underlying reversible disease manifestations. Of 46 engrafted survivors with available data, 83% are off immunoglobulin replacement as of last follow-up. Of 8 patients remaining on immunoglobulin replacement, 6 have <2 years follow-up so may still have evolving humoral reconstitution.
TABLE VI.
Outcomes of engrafted survivors
| Outcome | Patients (n = 48) |
|---|---|
| Alive and well with phenotype reversal | 41 (85) |
| Full donor chimerism (>95%)* | 33 (69) |
| Mixed CD3+ donor chimerism only (<95%)† | 3 (6) |
| Mixed donor chimerism in both compartments (<95%)‡ | 3 (6) |
| Other§ | 2 (6) |
| Alive with phenotype reversal but significant ongoing complications|| | 4 (8) |
| Other | 3 (6) |
| Partial phenotype reversal, mixed donor chimerism¶ | 2 (4) |
| Too early to evaluate phenotype reversal (<100 days post-HCT) | 1 (2) |
Values are n (%).
Whole blood or myeloid donor chimerism >95%; CD3+ chimerism, if available (n = 14), was >95%.
Whole blood or myeloid donor chimerism >95%; CD3+ chimerism <95% (9.2%, 40%, 93%).
Whole blood or myeloid donor chimerism <95% (range, 52%−82%) and CD3+ himerism <95% (range, 67.5%−84%).
Whole blood donor chimerism <95% (n = 1, 94%) and full CD3+ donor chimerism but mixed myeloid and whole blood donor chimerism (n = 1, 96%, 86%, 90%, respectively).
Ongoing complications include GVHD (n = 2) and chronic kidney disease (n = 2). All have 100% donor chimerism.
One patient has resolution of recurrent respiratory infections and enteropathy with negative EBV, CMV, and adenovirus in blood, but recent ocular HSV and VZV despite prophylaxis 2 years post-HCT; last donor chimerism: 35% whole blood, 26% myeloid, 34% CD3+, 17% CD19+. The other patient has improvement of disseminated Mycoplasma orale infection but continued immune thrombocytopenia and hypogammaglobulinemia 2.8 years post-HCT; last donor chimerism: 85% myeloid, 58% CD3+, 99% CD19+, 82% natural killer.
No clear correlation was noted between OS and donor chimerism. Infectious deaths occurred in the setting of full donor chimerism (n = 2, at days +75 and +340), preengraftment (n = 2, at days +6 and +17), split donor chimerism and profound lymphopenia (n = 1 at day +238; 0.06% CD3+, 99.6% myeloid chimerism at day +141), and mixed donor chimerism (n = 1 at day +663 and attributed to prior splenectomy; prior to death had phenotype reversal despite 50% whole blood chimerism at day +515). Whole blood, myeloid, and CD3+ donor chimerism trends expectedly showed a trend of more frequent mixed chimerism in patients who received RIC, but not all patients with mixed chimerism across compartments required intervention (see Fig E3 in this article’s Online Repository at www.jacionline.org). Of note, the ability to analyze chimerism trends and differences between platforms was limited by intercenter variability in the type of chimerism study and frequency of assessment, as well as by lymphopenia hindering the ability to assess CD3+ chimerism early post-HCT.
DISCUSSION
Since the original description of APDS in 2013,1,2 increased understanding of this disease has prompted interest in earlier access to definitive treatment such as HCT, necessitating improved awareness of factors that might optimize the approach to HCT. Prior reports have confirmed that HCT is potentially curative for patients with APDS1/2, but those reports have also highlighted barriers to achieving better success rates in HCT for this disease, including high risk of graft instability, significant comorbidities, and poorly controlled infections, autoimmunity, and lymphoproliferation pre-HCT.12,13 Herein, we further characterized the international experience with the largest cohort of patients with APDS1/2 who have received transplants to date and examined the relative contribution of HCT-related factors such as donor type, conditioning regimen, and post-HCT therapies to HCT outcomes in these patients.
Whereas MSDs are typically the preferred donor type in practice, no statistically significant differences in outcomes were noted based on donor type. Importantly, particularly in an autosomal dominant disease, unaffected MSD options may be particularly scarce.31 Our findings suggest that having only MUD or HLA-haploidentical donor options should not dissuade or delay HCT for patients with APDS1/2 in need. Similarly equivalent outcomes between MUD and HLA-haploidentical HCTs for nonmalignant diseases and/or lymphoma have been reported in both TCRαβ+/CD19+ depletion-based and in posttransplantation cyclophosphamide-based platforms.32–41
Conditioning intensity, with historical preference for myeloablation in hard-to-engraft diseases, does not appear to be a key factor in the outcomes reported here. The high risk of graft failure or need for DCI was a consistent finding, with no statistically significant differences in the probabilities of OS, GFFS, GF, or need for DCI based on conditioning intensity. The 100% 2-year GFFS in the RT-MAC subgroup may suggest particular promise with this approach, but the outcome estimates for this subgroup are limited by small numbers, and more patient outcomes data are required to draw conclusions. Given the activated, dysregulated immune function that predominates this disease, it is not surprising that the use of serotherapy and thus the intensity of host lymphodepletion, rather than myeloablation, contributed to the risk of graft instability and need for DCIs in this study. The importance of robust host lymphodepletion was also demonstrated through the relationship between post-HCT mTORi use and the risk of graft instability or need for DCIs. It is known that mTORi ameliorate the function and survival of lymphocytes from patients with APDS by reducing T-cell senescence, increasing naive T-cell percentage, and normalizing IL-2–mediated lymphoproliferation.2 Therefore, mTORi use early post-HCT may actually provide an undesirable survival advantage to residual host lymphocytes, thus mediating graft instability. Whether these results are applicable to other clinical HCT situations, such as somatic PI3K mutations in patients with lymphoma without underlying primary immunodeficiency diagnosis or in patients with non-APDS immunodeficiency with disease pathophysiology that includes mTOR pathway activation, merits further investigation. For patients with APDS1/2, progress toward improving HCT outcomes may come from work related to tailoring serotherapy dosing, optimizing lymphodepletion over myeloablation, avoidance of post-HCT mTORi until there is confidence that residual host lymphocytes are eradicated, and optimizing the immune dysregulation pre-HCT as best possible.
In comparing the clinical manifestations pre-HCT in our cohort and in the cohort described by Coulter et al,3 in which 91% of patients did not proceed to HCT, patients in our cohort who all ultimately proceeded to HCT had a greater baseline frequency of enteropathy (57% vs 25%), immune cytopenias (33% vs 17%), and hematologic malignancy (26% vs 13%), suggesting that these manifestations may be particularly refractory to standard therapy and may justify sooner consideration of HCT once identified. Many patients in our cohort entered HCT with active, uncontrolled manifestations of immune dysregulation. Optimizing pre-HCT disease status with disease-attenuating agents such as mTORi, rituximab, and PI3K inhibitors, as well as moving patients to HCT before significant organ dysfunction develops, might reduce some of the struggles with graft stability and failure while also affording utilization of lower toxicity approaches to successful HCT. Baseline liver pathology was observed frequently, and SOS developed even after RIC. Poor pulmonary function pre-HCT was also frequent, and severe post-HCT respiratory complications such as acute respiratory distress syndrome or extracorporeal membrane oxygenation requirement were seen in 9% of patients. The long-term impact of these transplant-related organ toxicities on morbidity and longevity is of concern. Thus, the choice of conditioning intensity for these patients must be closely guided by underlying comorbidities; full lung function evaluations including spirometry and diffusion capacity of carbon monoxide whenever possible, in children as well as adults, along with a low threshold for detailed liver evaluation prior to HCT, may help inform these decisions.
The incidence of pre-HCT renal pathology and post-HCT renal complications was also notable in this cohort, with 2 cases of severe chronic kidney disease long term. One of these patients developed post-HCT focal segmental glomerulosclerosis, a feature reported pre-HCT in other patients with APDS, in the setting of full donor chimerism. It has been shown that hyperactivated PI3K-Akt signaling within kidney podocytes sensitizes them to injury and apoptosis42 and has also been linked to renal tissue hyperproliferation and renal cell carcinoma, a complication observed post-HCT in 1 patient in this cohort.43,44 These findings suggest that patients with APDS may remain at risk of renal complications post-HCT even if the hematopoietic system is fully donor and may merit closer observation in this regard, both due to possible increased vulnerability to renal insults early post-HCT and for chronic complications long term.
The minimum donor chimerism necessary for phenotype reversal remains to be defined, but it is notable that phenotype reversal has been observed in the setting of stable mixed chimerism in some patients. Regardless, given the risk of graft loss or instability, close monitoring of donor chimerism, including CD3+ donor chimerism when possible, is necessary to identify declining donor chimerism early enough to intervene and should continue long term even after full donor chimerism is established, as illustrated by secondary GF and return of disease manifestations 5 years post-RT-MAC MUD HCT in a patient who ultimately required 3 HCTs.
Identifying the HCT approach associated with the most desirable immune reconstitution profile was not feasible based on the data available and given the heterogeneity of platforms, donors, and graft sources used in this cohort, along with the numerous DCIs administered for various indications. In terms of infectious complications, CMV and EBV requiring treatment, which are particularly problematic pre-HCT in patients with APDS, occurred with similar frequency as would be expected in a general HCT recipient population.45,46 There was a subset of patients in this cohort with prolonged, profound lymphopenia and future studies should aim to characterize whether such occurrences are related to the HCT approach/complications or might be inherent, nonhematopoietic disease features for a subset of patients with APDS1/2. Immune reconstitution may be affected by serotherapy use, but serotherapy-free HCTs were few in this cohort, thus not providing ample comparison to serotherapy-containing approaches. Given the probable importance of serotherapy in the conditioning of these patients as well as the tendency for patients with APDS1/2 to enter HCT with poor viral control and/or virus-associated malignancy, fine-tuning serotherapy exposure to the graft is future work of utmost importance when striving to improve immune reconstitution and avoid viral complications, while also optimally preventing GVHD.47
In conclusion, this large international investigation of HCT outcomes for patients with APDS1/2 has yielded important insights into the breadth and severity of pre-HCT comorbidities, ongoing limitations in disease optimization pre-HCT, the incidence of major HCT complications, and key factors that may affect graft stability and loss. Use of mTORi post-HCT appears to be detrimental to graft stability and should be avoided in the setting of clinically significant mixed or split donor chimerism. Longer detailed follow-up of graft stability, late toxicities, immune reconstitution, and phenotype reversal is needed to further inform the optimal timing of and approach to HCT for patients with APDS1/2.
Supplementary Material
Acknowledgments
We thank the European Society for Immunodeficiencies Registry Working Party, the European Reference Network (ERN-RITA), and the Primary Immune Deficiency Treatment Consortium. This research was funded in part from the Intramural Program of the National Cancer Institute, National Institutes of Health.
This research was funded in part from the Intramural Program of the National Cancer Institute, National Institutes of Health. The funding source had no involvement in study design; collection, analysis, and interpretation of data; writing of the report; or in the decision to submit the article for publication.
Abbreviations used
- APDS
Activated phosphoinositide 3-kinase delta syndrome
- CMV
Cytomegalovirus
- DCI
Unplanned donor cell infusion
- GF
Graft failure
- GFFS
Graft failure–free survival
- GVHD
Graft-versus-host disease
- HCT
Hematopoietic cell transplantation
- HCT-CI
HCT comorbidity index
- MAC
Myeloablative conditioning
- MSD
Matched sibling donor
- mTOR
Mammalian target of rapamycin
- mTORi
mTOR inhibitor
- MUD
Matched unrelated donor
- OS
Overall survival
- PI3K
Phosphoinositide 3-kinase
- RIC
Reduced intensity conditioning
- RT-MAC
Reduced-toxicity myeloablative conditioning
- SOS
Sinusoidal obstructive syndrome
Footnotes
Clinical implications: HCT for patients with APDS reverses phenotype but is associated with high incidence of graft instability, regardless of conditioning intensity or donor type, which is increased by post-HCT mTORi use.
Disclosure of potential conflict of interest: C. C. Dvorak has consulted for Omeros Corp and Alexion, Inc. P. Soler-Palacin has received personal fees from UCB Pharma. S. Jolles has received support from Health and Care Research Wales, CSL Behring, Takeda, LFB, Biotest, Binding Site, Sanofi, GlaxoSmithKline, UCB Pharma, Grifols, BPL SOBI, Weatherden, Zarodex, Pharming, and Octapharma for projects, advisory boards, meetings, studies, speaking, Data Safety and Monitoring Boards, and clinical trials. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Angulo I, Vadas O, Garcon F, Banham-Hall E, Plagnol V, Leahy TR, et al. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science 2013;342:866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, et al. Dominant-activating gerμLine mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nat Immunol 2014;15:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coulter TI, Chandra A, Bacon CM, Babar J, Curtis J, Screaton N, et al. Clinical spectrum and features of activated phosphoinositide 3-kinase delta syndrome: a large patient cohort study. J Allergy Clin Immunol 2017;139:597–606.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elkaim E, Neven B, Bruneau J, Mitsui-Sekinaka K, Stanislas A, Heurtier L, et al. Clinical and immunologic phenotype associated with activated phosphoinositide 3-kinase delta syndrome 2: a cohort study. J Allergy Clin Immunol 2016;138: 210–8.e9. [DOI] [PubMed] [Google Scholar]
- 5.Lucas CL, Chandra A, Nejentsev S, Condliffe AM, Okkenhaug K. PI3Kdelta and primary immunodeficiencies. Nat Rev Immunol 2016;16:702–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards ESJ, Bier J, Cole TS, Wong M, Hsu P, Berglund LJ, et al. Activating PIK3CD mutations impair human cytotoxic lymphocyte differentiation and function and EBV immunity. J Allergy Clin Immunol 2019;143:276–91.e6. [DOI] [PubMed] [Google Scholar]
- 7.Jamee M, Moniri S, Zaki-Dizaji M, Olbrich P, Yazdani R, Jadidi-Niaragh F, et al. Clinical, immunological, and genetic features in patients with activated PI3Kdelta syndrome (APDS): a systematic review. Clin Rev Allergy Immunol 2020;59: 323–33. [DOI] [PubMed] [Google Scholar]
- 8.Elgizouli M, Lowe DM, Speckmann C, Schubert D, Hulsdunker J, Eskandarian Z, et al. Activating PI3Kδelta mutations in a cohort of 669 patients with primary immunodeficiency. Clin Exp Immunol 2016;183:221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maccari ME, Abolhassani H, Aghamohammadi A, Aiuti A, Aleinikova O, Bangs C, et al. Disease evolution and response to rapamycin in activated phosphoinositide 3-kinase delta syndrome: the European Society for Immunodeficiencies-Activated Phosphoinositide 3-Kinase delta Syndrome Registry. Front Immunol 2018;9:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz-Garcia R, Vargas-Hernandez A, Chinn IK, Angelo LS, Cao TN, Coban-Akdemir Z, et al. Mutations in PI3K110delta cause impaired natural killer cell function partially rescued by rapamycin treatment. J Allergy Clin Immunol 2018;142: 605–17.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao VK, Webster S, Dalm V, Sediva A, van Hagen PM, Holland S, et al. Effective “activated PI3Kdelta syndrome”-targeted therapy with the PI3Kdelta inhibitor leniolisib. Blood 2017;130:2307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nademi Z, Slatter MA, Dvorak CC, Neven B, Fischer A, Suarez F, et al. Hematopoietic stem cell transplant in patients with activated PI3K delta syndrome. J Allergy Clin Immunol 2017;139:1046–9. [DOI] [PubMed] [Google Scholar]
- 13.Okano T, Imai K, Tsujita Y, Mitsuiki N, Yoshida K, Kamae C, et al. Hematopoietic stem cell transplantation for progressive combined immunodeficiency and lymphoproliferation in patients with activated phosphatidylinositol-3-OH kinase delta syndrome type 1. J Allergy Clin Immunol 2019;143:266–75. [DOI] [PubMed] [Google Scholar]
- 14.Notarangelo LD. Hematopoietic stem cell transplantation for activated phosphoinositide 3-kinase delta syndrome: Who, when, and how? J Allergy Clin Immunol 2019;143:91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorror ΜL, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009;15:1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carreras E, Dufour C, Mohty M, Kroger N. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. 7th ed. New York (NY): Springer; 2019. [PubMed] [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 19.Dimitrova D, Gea-Banacloche J, Steinberg SM, Sadler JL, Hicks SN, Carroll E, et al. Prospective study of a novel, radiation-free, reduced-intensity bone marrow transplantation platform for primary immunodeficiency diseases. Biol Blood Marrow Transplant 2020;26:94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ewertowska M, Grzesk E, Urbanczyk A, Dabrowska A, Babol-Pokora K, Lecka M, et al. Activated phosphoinositide 3-kinase delta syndrome 1 and 2 (APDS 1 and APDS 2): similarities and differences based on clinical presentation in two boys. Allergy Asthma Clin Immunol 2020;16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox TA, Chakraverty R, Burns S, Carpenter B, Thomson K, Lowe D, et al. Successful outcome following allogeneic hematopoietic stem cell transplantation in adults with primary immunodeficiency. Blood 2018;131:917–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong CR, Lee S, Hong KT, Choi JY, Shin HY, Choi M, et al. Successful haploidentical transplantation with post-transplant cyclophosphamide for activated phosphoinositide 3-kinase delta syndrome. J Allergy Clin Immunol Pract 2019;7: 1034–7.e1. [DOI] [PubMed] [Google Scholar]
- 23.Crank MC, Grossman JK, Moir S, Pittaluga S, Buckner CM, Kardava L, et al. Mutations in PIK3CD can cause hyper IgM syndrome (HIGM) associated with increased cancer susceptibility. J Clin Immunol 2014;34:272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas CL, Zhang Y, Venida A, Wang Y, Hughes J, McElwee J, et al. Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. J Exp Med 2014;211: 2537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Wang W, Liu L, Hou J, Ying W, Hui X, et al. Report of a Chinese cohort with activated phosphoinositide 3-kinase delta syndrome. J Clin Immunol 2018;38: 854–63. [DOI] [PubMed] [Google Scholar]
- 26.Tsujita Y, Mitsui-Sekinaka K, Imai K, Yeh TW, Mitsuiki N, Asano T, et al. Phosphatase and tensin homolog (PTEN) mutation can cause activated phosphatidylinositol 3-kinase delta syndrome-like immunodeficiency. J Allergy Clin Immunol 2016;138:1672–80.e10. [DOI] [PubMed] [Google Scholar]
- 27.Takeda AJ, Zhang Y, Dornan GL, Siempelkamp BD, Jenkins ΜL, Matthews HF, et al. Novel PIK3CD mutations affecting N-terminal residues of p110delta cause activated PI3Kdelta syndrome (APDS) in humans. J Allergy Clin Immunol 2017; 140:1152–6.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deau MC, Heurtier L, Frange P, Suarez F, Bole-Feysot C, Nitschke P, et al. A human immunodeficiency caused by mutations in the PIK3R1 gene. J Clin Invest 2014;124:3923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ClinVar accession: VCV000827733.1. ClinVar [Internet]. Available at: https://www.ncbi.nlm.nih.gov/clinvar/variation/827733/. Accessed April 10, 2021.
- 30.Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 1987;44:778–83. [DOI] [PubMed] [Google Scholar]
- 31.Acevedo MJ, Wilder JS, Adams S, Davis J, Kelly C, Hilligoss D, et al. Outcomes of related and unrelated donor searches among patients with primary immunodeficiency diseases referred for allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2019;25:1666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dreger P, Sureda A, Ahn KW, Eapen M, Litovich C, Finel H, et al. PTCy-based haploidentical vs matched related or unrelated donor reduced-intensity conditioning transplant for DLBCL. Blood Adv 2019;3:360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burroughs LM, O’Donnell PV, Sandmaier BM, Storer BE, Luznik L, Symons HJ, et al. Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 2008;14:1279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rastogi N, Katewa S, Thakkar D, Kohli S, Nivargi S, Yadav SP. Reduced-toxicity alternate-donor stem cell transplantation with posttransplant cyclophosphamide for primary immunodeficiency disorders. Pediatr Blood Cancer 2018;65(1):e26783. [DOI] [PubMed] [Google Scholar]
- 35.Brettig T, Smart J, Choo S, Mechinaud F, Mitchell R, Raj TS, et al. Use of TCR alpha(+)beta(+)/CD19(+)-depleted haploidentical hematopoietic stem cell transplant is a viable option in patients with primary immune deficiency without matched sibling donor. J Clin Immunol 2019;39:505–11. [DOI] [PubMed] [Google Scholar]
- 36.Laberko A, Sultanova E, Gutovskaya E, Shipitsina I, Shelikhova L, Kurnikova E, et al. Mismatched related vs matched unrelated donors in TCRalphabeta/CD19-depleted HSCT for primary immunodeficiencies. Blood 2019;134:1755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertaina A, Merli P, Rutella S, Pagliara D, Bernardo ME, Masetti R, et al. HLA-haploidentical stem cell transplantation after removal of alphabeta1 T and B cells in children with nonmalignant disorders. Blood 2014;124:822–6. [DOI] [PubMed] [Google Scholar]
- 38.Klein OR, Chen AR, Gamper C, Loeb D, Zambidis E, Llosa N, et al. Alternative-donor hematopoietic stem cell transplantation with post-transplantation cyclophosphamide for nonmalignant disorders. Biol Blood Marrow Transplant 2016;22: 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanate AS, Mussetti A, Kharfan-Dabaja MA, Ahn KW, DiGilio A, Beitinjaneh A, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood 2016;127:938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed S, Kanakry JA, Ahn KW, Litovich C, Abdel-Azim H, Aljurf M, et al. Lower graft-versus-host disease and relapse risk in post-transplant cyclophosphamide-based haploidentical versus matched sibling donor reduced-intensity conditioning transplant for Hodgkin lymphoma. Biol Blood Marrow Transplant 2019; 25:1859–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah RM, Elfeky R, Nademi Z, Qasim W, Amrolia P, Chiesa R, et al. T-cell receptor alphabeta(+) and CD19(+) cell-depleted haploidentical and mismatched hematopoietic stem cell transplantation in primary immune deficiency. J Allergy Clin Immunol 2018;141:1417–26.e1. [DOI] [PubMed] [Google Scholar]
- 42.Garner KL, Betin VMS, Pinto V, Graham M, Abgueguen E, Barnes M, et al. Enhanced insulin receptor, but not PI3K, signalling protects podocytes from ER stress. Sci Rep 2018;8:3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Santis MC, Sala V, Martini M, Ferrero GB, Hirsch E. PI3K signaling in tissue hyper-proliferation: from overgrowth syndromes to kidney cysts. Cancers (Basel) 2017;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo H, German P, Bai S, Barnes S, Guo W, Qi X, et al. The PI3K/AKT pathway and renal cell carcinoma. J Genet Genomics 2015;42:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melendez-Munoz R, Marchalik R, Jerussi T, Dimitrova D, Nussenblatt V, Beri A, et al. Cytomegalovirus infection incidence and risk factors across diverse hematopoietic cell transplantation platforms using a standardized monitoring and treatment approach: a comprehensive evaluation from a single institution. Biol Blood Marrow Transplant 2019;25:577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ljungman P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis 2019;19: e260–72. [DOI] [PubMed] [Google Scholar]
- 47.de Koning C, Nierkens S, Boelens JJ. Strategies before, during, and after hematopoietic cell transplantation to improve T-cell immune reconstitution. Blood 2016; 128:2607–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.