Abstract
Background:
Consortium for Food Allergy Research investigators previously reported 52-week outcomes from a randomized controlled trial of peanut epicutaneous immunotherapy, observing modest and statistically significant induction of desensitization, highest in children ages 4 to 11 years.
Objective:
We sought to evaluate changes in efficacy, safety, and mechanistic parameters following extended open-label peanut epicutaneous immunotherapy.
Methods:
Peanut-allergic participants (4–25 years) received 52 weeks of placebo (PLB), Viaskin Peanut 100 μg (VP100) or 250 μg (VP250), and then crossed over to VP250 for PLB (PLB-VP250) and VP100 (VP100-VP250) participants and continued treatment for VP250 participants (total = 130 weeks of active epicutaneous immunotherapy). Efficacy was assessed by double-blind, placebo-controlled food challenge (5044 mg peanut protein), and adherence, safety, and mechanistic parameters were evaluated.
Results:
At week 130, desensitization success was achieved in 1 of 20 (5%) PLB-VP250, 5 of 24 (20.8%) VP100-VP250, and 9 of 25 (36%) VP250 participants, with median successfully consumed dose change from baseline of 11.5 mg, 141.5 mg, and 400 mg, respectively. Median age (years) for week 130 desensitization success was 6.2 years (interquartile range, 5.2–9.1) versus 9.4 years (interquartile range, 7.6–12.8) for failures (P < .001). Adherence was 96%. Adverse reactions were predominantly local patch-site reactions. Significant increases in peanut- and Ara h2–specific IgG4 observed at week 52 persisted to week 130. By a post hoc analysis, there were no statistically significant increases from week 52 to week 130 in either desensitization success or successfully consumed dose.
Conclusions:
Extended treatment with VP250 was well tolerated, and desensitization observed at week 52 persisted between weeks 52 and 130. Treatment success was observed predominantly in younger participants, with younger age at initiation of active therapy an important predictor of success.
Keywords: Peanut allergy, food allergy, epicutaneous immunotherapy, desensitization, IgE, IgG4, follow-up
Peanut allergy is a prevalent, typically lifelong, and potentially life-threatening disorder estimated to affect up to 1.5% of US children.1–4 Development of safe, effective, and well-tolerated therapies targeting IgE-mediated peanut allergy has potential to mitigate the adverse medical, psychosocial, and economic impacts of this disorder.5–9
Epicutaneous immunotherapy (EPIT) is an immunotherapeutic approach that has demonstrated relevant immunomodulation in preclinical food allergy models10,11 and favorable safety, tolerability, and efficacy outcomes in peanut allergy clinical trials.12–14 Previous multicenter, randomized, placebo-controlled trials have published results after 1 year of peanut EPIT.12,13 A phase IIb trial (Viaskin Peanut Efficacy and Safety [VIPES]) enrolled 221 peanut-allergic individuals (ages 6–55 years) in 22 centers in the United States and Europe, for a 1-year treatment comparison of placebo (PLB) versus peanut EPIT, concluding that in this dose-ranging study, the 250 μg Viaskin Peanut (VP250; DBV Technologies, Montrouge, France) patch resulted in a meaningful increase in reaction threshold compared with PLB.13 Effects were observed primarily in the 6- to 11-year-old age group. In a phase 3, double-blind, placebo-controlled trial (Peanut EPIT Efficacy and Safety Study [PEPITES]) conducted at 31 sites in 5 countries, 356 peanut-allergic children age 4 to 11 years without a history of severe anaphylaxis and who developed objective symptoms to less than or equal to 300 mg peanut protein during the screening double-blind, placebo-controlled food challenge (DBPCFC) were randomized 2:1 to receive VP250 or PLB patch.12 The primary end point of the study was the proportion of treatment responders at the 12-month DBPCFC. Response rates were 35.6% in the active group and 13.6% in the PLB group (P < .0001), for a between-group difference of 21.7% (95% CI, 12.4%−29.8%); however, because the predefined clinical relevance criterion required that the lower bound of this between-group difference be above 15%, this criterion was not met. In the recently published PEPITES open-label extension study (PEOPLE), investigators reported durable clinical benefit in children age 4 to 11 years. Following 1 year of peanut EPIT (DBV712 250 μg), participants received an additional 2 years of peanut EPIT, with favorable safety and tolerability profiles, including high compliance and low discontinuation rates, which enabled long-term study participation.15
The Consortium for Food Allergy Research group of investigators previously reported results from a multicenter, double-blind, placebo-controlled trial evaluating the safety and efficacy of Viaskin Peanut in children and young adults age 4 to 25 years.16 In brief, the study was designed and powered to compare treatment outcomes after 52 weeks of blinded, placebo-controlled treatment in children and young adults age 4 to 25 years. The primary study results indicated that peanut EPIT was associated with statistically significant desensitization and immunologic changes when the 100-μg and 250-μg doses (VP100 and VP250, respectively) were compared with PLB EPIT, with the highest response noted in children age 4 to 11 years. After week 52, participants crossed over to or continued treatment with VP250 in an open-label manner for up to 130 total weeks of active treatment. Here, the investigators report outcomes of that extended active therapy with VP250 by assessing changes in clinical efficacy, safety, and mechanistic parameters. Of note, conclusions among 4- to 11-year-old children are limited because the original study was neither designed nor powered to specifically evaluate this cohort. However, the findings from this long-term, multicenter trial of peanut EPIT expand current understanding and provide relevant insights regarding therapeutic application of peanut EPIT in food-allergic individuals.
METHODS
Study design
The current study reports the open-label portion of this trial through 130 weeks of EPIT, extending results of a previously published study through 52 weeks of blinded therapy with Viaskin Peanut.16 Participants were initially randomized (1:1:1) to receive blinded treatment for 52 weeks using patches of PLB, 100 μg Viaskin Peanut (VP100) or VP250. After week 52, participants were unblinded and PLB and VP100 groups crossed over to receive VP250 for a total duration of 130 weeks of active therapy. PLB participants were treated with VP250 for 130 weeks (PLB-VP250); VP100 participants received VP100 through week 52 and then VP250 from week 52 to week 130 (VP100-VP250); VP250 participants received 130 weeks of VP250 (VP250). Efficacy outcomes were assessed by DBPCFC at weeks 52 and 130. The overall study primary end point was previously reported and included a definition for treatment success (ie, desensitization success) at week 52 as passing a 5044-mg DBPCFC or achieving a 10-fold increase in successfully consumed dose (SCD) from baseline to week 52 DBPCFC.16 SCD is the cumulative dose before elicitation of symptoms during DBPCFC. For this open-label extension study, desensitization success was a study secondary end point that was defined per protocol on the basis of SCD compared with baseline DBPCFC as follows: (1) SCD greater than or equal to 444 mg if baseline SCD is 0 to 44 mg; (2) SCD greater than or equal to 10 times the baseline SCD if baseline SCD is more than 44 mg to less than 444 mg; and (3) SCD equal to 5044 mg if baseline SCD is greater than or equal to 444 mg to less than 1044 mg. For PLB-VP250 participants, the week 52 DBPCFC following PLB therapy was used as baseline, and week 52 and week 130 DBPCFCs were performed during active treatment with VP250.
Study population
Subjects were 4 to 25 years at enrollment, from 5 Consortium for Food Allergy Research US sites with inclusion and exclusion criteria previously reported.16 The study was approved by each site’s institutional review board, and written consent/assent was obtained. The study was conducted under a Food and Drug Administration investigational new drug application and monitored by an independent data and safety monitoring board convened by the National Institute of Allergy and Infectious Diseases.
Study product and EPIT dosing protocol
The Viaskin Peanut patch was supplied by DBV Technologies, with dosing and application protocol performed as previously reported.16
Adherence and safety assessments
Study product adherence was monitored throughout the study using dosing logs that were reviewed by study personnel at each visit. These logs were used to record missed doses, doses removed prematurely, or doses associated with adverse symptoms. Participants were also instructed to return all used and unused patches at each visit.
Safety assessments were continued during the extension phase, as previously described.16 Assessments included grading of patch-site reactions in addition to recording and grading of symptoms extending outside of the patch site and any systemic reactions. Adverse events, serious adverse events, and accidental exposures to peanut were reported throughout the study.
Oral food challenges
At study entry, a DBPCFC was conducted to a cumulative amount of 1044 mg of peanut protein administered in doses every 15 minutes by using a modified PRACTALL protocol.16 The DBPCFC was repeated at weeks 52 and 130 using a higher cumulative dose of 5044 mg of peanut protein.
Skin prick tests
Skin prick tests using the GREERPick device with peanut extract (Greer Laboratories, Lenoir, NC) and saline and histamine controls were performed at baseline and weeks 24, 52, 78, 104, and 130, as previously described.16
Assessment of immunologic markers/mechanisms
Peanut-specific IgE and IgG4 levels were measured in serum at baseline and weeks 12, 24, 52, 78, 104, and 130 using ImmunoCAP 250 (Thermo Fisher Scientific, Waltham, Mass). Basophil activation was determined by CD63 expression in response to stimulation of whole blood with peanut extract and measured by flow cytometry.16
Statistical methods
The design and primary results of the study were previously described.16 The focus of this analysis was to describe long-term outcomes on active treatment. Active treatment analyses describe participants from the start of active treatment (study week 52 for PLB-VP250 and postbaseline for the other groups). Participants not initiating active treatment were excluded from analyses.
Summary statistics included means and SDs or medians and interquartile ranges (IQRs) for continuous variables and proportions for categorical variables. Prespecified secondary end points included the proportion of participants and 95% CI with week 130 desensitization success, those with a week 130 SCD of greater than or equal to 1044, and those with a week 130 SCD of 5044 mg. Prespecified exploratory analyses included change in SCD from baseline to week 130 as well as univariate and treatment-adjusted logistic regression models that were fit to evaluate predictors of desensitization success. Differences in proportions between treatment groups were assessed using a 2-sided Barnard exact test, and differences in the change in SCD between treatment groups were assessed using the Wilcoxon rank-sum test; these statistical tests were not prespecified.
Using prespecified linear mixed models with a Toeplitz covariance structure fit via proc mixed in SAS, repeated-measures modeling of mechanistic data (immunologic, activated basophil, and T-cell studies) was performed where treatment group, visit, baseline value, and a treatment by visit interaction were included. Because baseline value was included as a covariate in the model, the baseline visit was not included in the data used in the analysis. The interaction was included to assess whether differences between treatment groups changed over time. If the interaction term was significant, contrasts were used to compare treatment groups at each visit; otherwise, the interaction term was dropped from the model and contrasts were used to compare treatment groups across all visits. A log10 transformation for IgE and IgG4 was used for repeated-measures modeling as well as logistic regression of baseline predictors of week 130 desensitization success.
Mechanistic outcomes were also compared between week 130 desensitization successes and failures using Wilcoxon rank-sum tests at individual time points and prespecified repeated-measure models with a Toeplitz covariance structure comparing successes to failures during the period on active treatment using similar models to those used to compare treatment group.
Post hoc exploratory analyses were performed assessing changes in desensitization and SCD between the week 52 and week 130 DBPCFCs. Overall and within-group changes were described and compared using McNemar test for proportions and the signed rank test for change in SCD. In addition, based on results of the primary analysis and other publications,12,13 results are described and evaluated separately for the 4- to 11-year-old subgroup.
For mechanistic outcomes, a prespecified P value of .01 was considered statistically significant to control for multiple comparisons; all other outcomes were assessed at the .05 level. Analyses were performed with SAS (version 9.4; SAS Institute, Cary, NC).
RESULTS
Study population
Seventy-five subjects were enrolled initially and randomized 1:1:1 into 3 treatment groups (PLB, VP100, VP250), with 74 subjects initiating 52 weeks of blinded therapy, as previously described.16 After the blinded week 52 DBPCFC end point, PLB participants received a total of 130 weeks of open-label active treatment with VP250, VP100 participants crossed over to VP250 for 78 weeks (a total of 130 weeks of active therapy), and VP250 subjects continued to receive VP250 for a total of 130 weeks of treatment (see Fig E1 in this article’s Online Repository at www.jacionline.org). Of the 74 initial participants, 59 (79.7%) completed 130 weeks of active treatment: 18 of 25 (72%) in the PLB-VP250 group, 18 of 24 (75%) in the VP100-VP250 group, and 23 of 25 (92%) in the VP250 group. Of the 15 participants who did not complete 130 weeks, 5 participants randomized to PLB did not initiate active therapy and are excluded from the analyses below. Of the remaining 10 participants, 4 participants withdrew before week 52 (1 PLB-VP250 participant because of persistent pruritus at the patch site, 1 VP100-VP250 participant because of a severe [grade 4] patch-site reaction, and 2 VP100-VP250 participants because of unrelated syncope/illness), and 6 participants withdrew between weeks 52 and 130 of active treatment for reasons unrelated to dosing reactions (3 because of participant decision, 2 because of relocation, and 1 was lost to follow-up).
Week 130 desensitization outcomes
Table I summarizes the week 130 desensitization outcomes in all participants, by treatment group and by age group. Overall, 15 of 69 (21.7%) participants initiating active EPIT met predefined desensitization success criteria at the week 130 DBPCFC; 1 of 20 (5.0%) was a PLB-VP250 participant, compared with 5 of 24 (20.8%) VP100-VP250 and 9 of 25 (36.0%) VP250 participants (PLB-VP250 vs VP250 P = .015; PLB-VP250 vs VP100-VP250 P = .16; VP100-VP250 vs VP250 P = .26) (Table I).
TABLE I.
DBPCFC results by treatment group and age
| All participants | ||||||||
|---|---|---|---|---|---|---|---|---|
| PLB-VP250 (N = 20) | VP100-VP250 (N = 24) | VP250 (N = 25) | Total (N = 69) | |||||
| n (%) | 95% exact CI | n (%) | 95% exact CI | n (%) | 95% exact CI | n (%) | 95% exact CI | |
| Week 52 desensitization success | 3 (15.0) | 3.2–37.9 | 3 (12.5) | 2.7–32.4 | 5 (20.0) | 6.8–40.7 | 11 (15.9) | 8.2–26.7 |
| SCD ≥444 mg, baseline SCD = 0 to 44 mg | 2 (10.0) | 2 (8.3) | 5 (20.0) | 9 (13.0) | ||||
| SCD ≥10 times baseline SCD of >44 to <444 mg | 1 (5.0) | 1 (4.2) | 0 | 2 (2.9) | ||||
| Week 130 desensitization success | 1 (5.0) | 0.1–24.9 | 5 (20.8) | 7.1–42.2 | 9 (36.0) | 18.0–57.5 | 15 (21.7) | 12.7–33.3 |
| SCD ≥444 mg, baseline SCD = 0 to 44 mg | 1 (5.0) | 5 (20.8) | 9 (36.0) | 15 (21.7) | ||||
| 4–11-y-old participants | ||||||||
| PLB-VP250 (N = 16) | VP100-VP250 (N = 17) | VP250 (N = 18) | Total (N = 51) | |||||
| n (%) | 95% exact CI | n (%) | 95% exact CI | n (%) | 95% exact CI | n (%) | 95% exact CI | |
| Week 52 desensitization success | 3 (18.8) | 4.0–45.6 | 2 (11.8) | 1.5–36.4 | 5 (27.8) | 9.7–53.5 | 10 (19.6) | 9.8–33.1 |
| SCD ≥444 mg, baseline SCD = 0 to 44 mg | 2 (12.5) | 1 (5.9) | 5 (27.8) | 8 (15.7) | ||||
| SCD ≥10 times baseline SCD of >44 to <444 mg | 1 (6.3) | 1 (5.9) | 0 | 2 (3.9) | ||||
| Week 130 desensitization success | 1 (6.3) | 0.2–30.2 | 4 (23.5) | 6.8–49.9 | 9 (50.0) | 26.0–74.0 | 14 (27.5) | 15.9–41.7 |
| SCD ≥444 mg, baseline SCD = 0 to 44 mg | 1 (6.3) | 4 (23.5) | 9 (50.0) | 14 (27.5) | ||||
In comparison, when evaluating desensitization success at week 52 by the definition used in the current study, 11 of 69 (15.9%) met the definition including 3 of 20 (15.0%) PLB-VP250, 3 of 24 (12.5%) VP100-VP250, and 5 of 25 (20.0%) VP250 participants. Interestingly, 3 participants in the PLB-VP250 group were desensitization successes at week 52, but only 1 met criteria for success at week 130.
Desensitization success was observed predominantly among participants age 4 to 11 years (with the exception of 1 participant in the VP100-VP250 group) at active EPIT initiation, with 14 of 51 (27.5%) achieving desensitization success at week 130 and 10 of 51 (19.6%) at week 52 (Table I). Of note, all week 130 desensitization successes occurred among participants who had the lowest peanut threshold triggering symptoms at baseline, that is, as a change in baseline SCD from 0 to 44 mg to greater than or equal to 444 mg at week 130. No participant was able to consume successfully a cumulative dose of 5044 mg peanut protein at week 130 (data not shown).
A post hoc analysis of change in desensitization successes from week 52 to week 130 was not statistically significant overall (P = .29) or within treatment groups (PLB-VP250 P = .32; VP100-VP250 P = .32; VP250 P = .10). Likewise, there was no statistically significant change in desensitization success from week 52 to week 130 in the 4- to 11-year-old age group (P = .29), yet younger children treated with VP250 demonstrated a higher desensitization success rate (9 of 18 [50%]) at week 130 than at week 52 (5 of 18 [27.8%]) (P = .10). However, the study was not powered to assess changes in desensitization success either among all participants or in this subset for the post hoc analysis.
Differences in SCD during DBPCFC among treatment groups
Fig 1, A and B, and Table II summarize SCD outcomes by treatment group for all participants. At week 130, the median SCD for all participants was 144 mg (IQR, 44–444) but differed significantly between treatment groups (P = .003). As seen in Figure 1, A, week 130 median SCD was significantly higher in the VP250 group (444 mg; IQR, 144–1044; P = .002) and the VP100-VP250 group (144 mg; IQR, 144–444; P = .015) compared with the PLB-VP250 group (44 mg; IQR, 14–144) (Table II). Similar results were observed for the median change in SCD from baseline to week 130; median change in SCD was 130 mg (IQR, 3–444) for all participants and significantly higher in the VP250 group (400 mg; IQR,100–1040; P = .002) and the VP100-VP250 group (141.5 mg; IQR, 30–444; P = .02) compared with the PLB-VP250 group (11.5 mg; IQR, −3 to 40) (Table II and Fig 1). Change in SCD from baseline to week 130 was also predominantly due to changes in the 4- to 11-year-old subgroup (n = 48; median, 130 mg; IQR, 13,443.5) versus more than 11 years old (n = 11; median, 100 mg; IQR, −40 to 1040) (see Fig E2, A, in this article’s Online Repository at www.jacionline.org). Despite the fact that all groups received active therapy for 130 weeks, and the fact that the PLB-VP250 group received the same dose and duration of treatment as the VP250 group, only the VP250 group had a potentially clinically meaningful desensitization.
FIG 1.
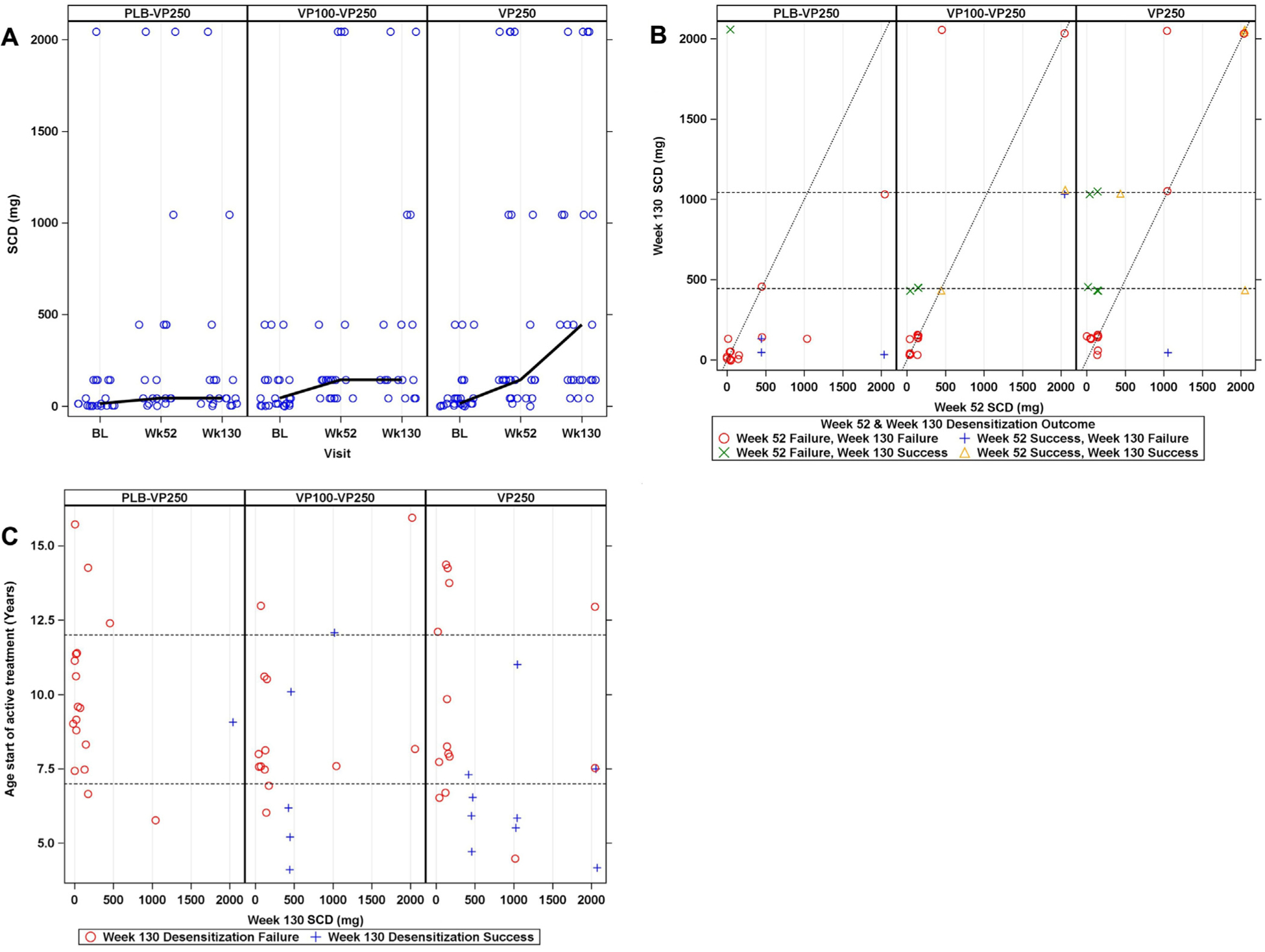
A, SCD from baseline to week 130 oral food challenge by treatment group in all participants. B, SCD at week 52 (x-axis) vs week 130 (y-axis) by treatment group and desensitization success and failure. Values to the left of the diagonal line represent participants whose SCD at week 130 is higher than at week 52. C, SCD at week 130 by age at start of therapy and desensitization success and failure (dashed lines indicate ages 7 [lower line] and 12 years [upper line]). For PLB-VP250 participants, the week 52 DBPCFC following PLB therapy was used as baseline.
TABLE II.
DBPCFC SCD (mg) and change from baseline by treatment group (all participants)
| Time point | PLB-VP250 (N = 20) | VP100-VP250 cross-over (N = 24) | VP250 (N = 25) | Total (N = 69) | P value* |
|---|---|---|---|---|---|
| Baseline,† N | 20 | 24 | 25 | 69 | .585 |
| Median (IQR) | 14.0 (4.0 to 144.0) | 44.0 (4.0 to 144.0) | 14.0 (14.0 to 44.0) | 14.0 (4.0 to 144.0) | |
| (Min, Max) | (1.0, 2044.0) | (0.0, 444.0) | (0.0, 444.0) | (0.0, 2044.0) | |
| Week 52, N | 19 | 21 | 25 | 65 | .476 |
| Median (IQR) | 44.0 (44.0 to 444.0) | 144.0 (44.0 to 144.0) | 144.0 (44.0 to 1044.0) | 144.0 (44.0 to 444.0) | |
| (Min, Max) | (1.0, 2044.0) | (44.0, 2044.0) | (0.0, 2044.0) | (0.0, 2044.0) | |
| Week 130, N | 18 | 18 | 23 | 59 | .003 |
| Median (IQR) | 44.0 (14.0 to 144.0) | 144.0 (144.0 to 444.0) | 444.0 (144.0 to 1044.0) | 144.0 (44.0 to 444.0) | |
| (Min, Max) | (1.0, 2044.0) | (44.0, 2044.0) | (44.0, 2044.0) | (1.0, 2044.0) | |
| Change from baseline at week 52,‡ N | 19 | 21 | 25 | 65 | .535 |
| Median (IQR) | 40.0 (0.0 to 300.0) | 43.0 (0.0 to 140.0) | 130.0 (30.0 to 600.0) | 100.0 (0.0 to 300.0) | |
| (Min, Max) | (0.0, 1900.0) | (−300.0, 2040.0) | (−300.0, 2040.0) | (−300.0, 2040.0) | |
| Change from baseline at week 130,§ N | 18 | 18 | 23 | 59 | .003 |
| Median (IQR) | 11.5 (−3.0 to 40.0) | 141.5 (30.0 to 444.0) | 400.0 (100.0 to 1040.0) | 130.0 (3.0 to 444.0) | |
| (Min, Max) | (−1000.0, 2000.0) | (−100.0, 1600.0) | (−300.0, 2040.0) | (−1000.0, 2040.0) | |
| Change from week 52 at week 130,∥ N | 18 | 18 | 23 | 59 | .018 |
| Median (IQR) | −35.0 (−300.0 to 0.0) | 0.0 (0.0 to 100.0) | 0.0 (0.0 to 300.0) | 0.0 (−100.0 to 100.0) | |
| (Min, Max) | (−2000.0, 2000.0) | (−1000.0, 1600.0) | (−1600.0, 1000.0) | (−2000.0, 2000.0) |
The Kruskal-Wallis test was used for comparisons.
For PLB-VP250 subjects, the week 52 value was used as baseline.
Wilcoxon pairwise test results for change from baseline at week 52 are P = .305 for PLB-VP250 vs VP250, P = .989 for PLB-VP250 vs VP100-VP250, and P = .410 for VP250 vs VP100-VP250.
Wilcoxon pairwise test results for change from baseline at week 130 are P = .002 for PLB-VP250 vs VP250, P = .020 for PLB-VP250 vs VP100-VP250, and P = .397 for VP250 vs VP100-VP250.
Wilcoxon pairwise test results for change from week 52 at week 130 are P = .013 for PLB-VP250 vs VP250, P = .051 for PLB-VP250 vs VP100-VP250, and P = .384 for VP250 vs VP100-VP250.
A post hoc analysis of the change in SCD between week 52 and week 130 was not statistically significant overall, with a median change of 0 mg (IQR, −100 to 100; P = .82) for all participants; within each treatment group, none showed a statistically significant increase, with a median change of 0 mg (IQR, 0 to 300; P = .15) in the VP250 group, 0 mg (IQR, 0 to 100; P = .66) in the VP100-VP250 group, and −35 mg (IQR, −300 to 0; P = .044) in the PLB-VP250 group (Table II; Fig 1, A and B).
In further analyses, individual participants were assessed by treatment group and desensitization success and failure to gain a better understanding of the impact of EPIT on individual treatment response (Table III and Fig 1, B). Figure 1, B, plots the week 130 (y-axis) versus week 52 (x-axis) SCD outcomes in all participants (see Fig E2, B, for the 4−11-year-old age group). Values to the left of the diagonal line represent participants whose SCD at week 130 is higher than at week 52, and those to the right of the line had a higher SCD at week 52 than at week 130. In the VP250 group, 11 subjects had a higher SCD and 5 transitioned from desensitization failure at week 52 to desensitization success at week 130, in contrast to the PLB-VP250 group, which had 11 participants with a lower SCD at week 130, and to the VP100–VP250 group showing a mixed response (5 higher, 3 lower). Four participants (2 VP100–VP250 and 2 VP250) who were defined as week 130 desensitization “failures” achieved 2044 mg SCD at the week 52 DBPCFC but failed to meet the predefined criteria for desensitization success at week 130, which would require passing the 5044 mg DBPCFC. These 4 participants clearly achieved clinically meaningful desensitization at weeks 52 and 130 when compared with baseline. However, only 14 of 69 (20.3%) participants achieved an SCD of greater than or equal to 1044 mg (PLB-VP250 = 2 of 20 [10.0%], VP100-VP250 = 4 of 24 [16.7%], VP250 = 8 of 25 [32%]) and only 23 of 69 (33.3%) achieved an SCD of greater than or equal to 444 mg (Fig 1, B) at week 130.
TABLE III.
Desensitization outcome at week 52 and week 130 by treatment group and age
| All participants | ||||
|---|---|---|---|---|
| Week 52 and week 130 desensitization outcome | PLB-VP250 (N = 18) | VP100-VP250 (N = 18) | VP250 (N = 23) | Total (N = 59) |
| n (%) | n (%) | n (%) | n (%) | |
| Week 52 success, Week 130 success | 0 (0.0) | 2 (11.1) | 4 (17.4) | 6 (10.2) |
| Week 52 success, Week 130 failure | 3 (16.7) | 1 (5.6) | 1 (4.3) | 5 (8.5) |
| Week 52 failure, Week 130 success | 1 (5.6) | 3 (16.7) | 5 (21.7) | 9 (15.3) |
| Week 52 failure, Week 130 failure | 14 (77.8) | 12 (66.7) | 13 (56.5) | 39 (66.1) |
| 4–11-y-old participants | ||||
| Week 52 and week 130 desensitization outcome | PLB-VP250 (N = 15) | VP100-VP250 (N = 15) | VP250 (N = 18) | Total (N = 48) |
| n (%) | n (%) | n (%) | n (%) | |
| Week 52 success, Week 130 success | 0 (0.0) | 1 (6.7) | 4 (22.2) | 5 (10.4) |
| Week 52 success, Week 130 failure | 3 (20.0) | 1 (6.7) | 1 (5.6) | 5 (10.4) |
| Week 52 failure, Week 130 success | 1 (6.7) | 3 (20.0) | 5 (27.8) | 9 (18.8) |
| Week 52 failure, Week 130 failure | 11 (73.3) | 10 (66.7) | 8 (44.4) | 29 (60.4) |
Factors associated with week 130 desensitization success
Analyses of baseline predictors of week 130 desensitization success were performed on all demographic, clinical, and immunologic characteristics (Table IV). Younger age at initiation of active treatment was the only factor with a statistically significant odds ratio in both univariate (odds ratio, 0.63; 95% CI, 0.46–0.85; P = .003) and treatment-adjusted models (odds ratio, 0.67; 95% CI, 0.50–0.90; P = .007). When Age × Treatment interaction was added to the treatment-adjusted model, this interaction was not significant (P = .76). Fig 1, C, shows week 130 SCD by age at start of active treatment for each treatment group by week 130 desensitization success. For simplicity, desensitization success in this figure is shown only at week 130 rather than at both weeks 52 and 130. It is noteworthy that 9 of 15 (60%) week 130 desensitization successes were ages 7 years or younger at the start of active treatment. The proportion of participants ages 7 years and younger was observed to be lowest in the PLB-VP250 group (2 of 20 [10%]) compared with the VP100-VP250 (5 of 24 [21%]) and VP250 (9 of 25 [36%]) groups. Note also that in the VP250 group, of 9 participants who were 7 years or younger, 6 of 9 were successfully desensitized.
TABLE IV.
Baseline* predictors of week 130 desensitization success
| Predictor | n/N (%) | Univariate | Multivariate (treatment-adjusted) | ||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| Age at start of active treatment (y)† | 69/69 (100.0) | 0.63 (0.46–0.85) | .0030 | 0.67 (0.50–0.90) | .007 |
| Sex | |||||
| Male (reference) | 42/69 (60.9) | 1 | — | 1 | — |
| Female | 27/69 (39.1) | 0.73 (0.22–2.42) | .6039 | 0.75 (0.21–2.63) | .649 |
| Race | |||||
| White (reference) | 59/69 (85.5) | 1 | — | 1 | — |
| Nonwhite | 10/69 (14.5) | 2.91 (0.70–12.09) | .1418 | 3.92 (0.78–19.81) | .098 |
| Asthma | |||||
| No (reference) | 33/69 (47.8) | 1 | — | 1 | — |
| Yes | 36/69 (52.2) | 0.53 (0.17–1.71) | .2899 | 0.39 (0.11–1.37) | .143 |
| Allergic rhinitis | |||||
| No (reference) | 14/69 (20.3) | 1 | — | 1 | — |
| Yes | 55/69 (79.7) | 1.86 (0.37–9.39) | .4542 | 2.32 (0.43–12.46) | .326 |
| Atopic dermatitis | |||||
| None (reference) | 46/69 (66.7) | 1 | — | 1 | — |
| Mild/moderate/severe | 23/69 (33.3) | 1.00 (0.30–3.37) | 1.0000 | 0.98 (0.27–3.52) | .975 |
| Atopic dermatitis score | 69/69 (100.0) | 1.00 (0.78–1.30) | .9725 | 1.00 (0.76–1.31) | 1.000 |
| Log10 peanut IgE | 69/69 (100.0) | 0.89 (0.37–2.15) | .7964 | 0.88 (0.35–2.23) | .789 |
| Log10 Ara h1 IgE | 69/69 (100.0) | 0.82 (0.55–1.23) | .3395 | 0.86 (0.56–1.31) | .480 |
| Log10 Ara h2 IgE | 69/69 (100.0) | 0.86 (0.39–1.87) | .6993 | 0.81 (0.36–1.81) | .601 |
| Log10 Ara h3 IgE | 69/69 (100.0) | 0.75 (0.46–1.24) | .2603 | 0.76 (0.44–1.30) | .309 |
| Log10 total IgE | 69/69 (100.0) | 2.07 (0.54–7.99) | .2890 | 2.12 (0.52–8.70) | .295 |
| Peanut IgG4 | 69/69 (100.0) | 0.64 (0.22–1.81) | .3968 | 0.81 (0.25–2.62) | .727 |
| Log10 Ara h1 IgG4 | 69/69 (100.0) | 0.59 (0.28–1.27) | .1783 | 0.61 (0.26–1.45) | .263 |
| Log10 Ara h2 IgG4 | 69/69 (100.0) | 0.54 (0.20–1.48) | .2340 | 0.50 (0.16–1.63) | .253 |
| Log10 Ara h3 IgG4 | 69/69 (100.0) | 0.41 (0.16–1.06) | .0661 | 0.48 (0.18–1.31) | .153 |
| Peanut %IgE | 69/69 (100.0) | 0.97 (0.92–1.01) | .1674 | 0.97 (0.92–1.02) | .189 |
| Ratio IgG4/IgE | 69/69 (100.0) | 0.82 (0.34–1.98) | .6572 | 0.99 (0.39–2.53) | .990 |
| Peanut SPT score (mm) | 69/69 (100.0) | 1.02 (0.94–1.12) | .5934 | 1.04 (0.94–1.14) | .446 |
SPT, Skin prick test.
Baseline values used for PLB-VP250 subjects are from week 52.
Median (IQR) age at start of active treatment was 6.2 (5.2–9.1) y in week 130 desensitization successes and 9.4 (7.6–12.8) y in failures (P = .0005).
The median age of participants achieving desensitization success at week 130 was 6.2 years (IQR, 5.2–9.1) versus 9.4 years (IQR, 7.6–12.8) for treatment failures (P < .001). Of note, the median age at start of active therapy was observed to be different between treatment groups, though no pairwise comparisons of age differences between treatment groups were statistically significant (PLB-VP250: 9.4 years, IQR, 8.3–11.4; VP100–VP250: 8.4 years, IQR, 7.5–12.1; VP250: 7.7 years, IQR, 6.5–12.1), potentially contributing to treatment differences observed between the PLB-VP250 and VP250 groups. Note that the PLB-VP250 participants were already 1 year older than the other participants at the start of active therapy, having been treated with PLB for 1 year.
Safety and adherence
Table V summarizes dosing symptoms by dose, subject, and percentage of doses per subject during 130 weeks of active peanut EPIT in each treatment group. All participants experienced dose-related symptoms. Patch-site reactions were common, occurring in 77.6% of PLB-VP250 doses, 72.3% of VP100-VP250 doses, and 73.9% of VP250 doses, with most dosing symptoms being mild and less than grade 2. There were very few non–patch-site reactions; these comprised 0.2% or fewer of doses for each treatment group, and all but 1 were mild. No systemic reactions requiring treatment with epinephrine were observed. Adherence to treatment remained excellent, with 96% of expected doses administered throughout the dosing period.
TABLE V.
Dosing symptoms on active therapy
| Patch-site reactions | Non-patch-site symptoms | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any reaction | Any patch-site reaction | Grade 2 patch-site reaction | Grade 3 patch-site reaction | Grade 4 patch-site reaction | Reaction extended past patch site | Non–patch-site reaction | Mild symptoms | Moderate symptoms | Severe symptoms | Symptoms >8 h | Treated | ||||||||||||||
| Dosing symptoms by dose | |||||||||||||||||||||||||
| Treatment group | No. of doses | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % |
| PLB-VP250 | 16,592 | 12,878 | 77.6 | 12,875 | 77.6 | 2,887 | 17.4 | 8 | 0.0 | 0 | 0.0 | 3,281 | 19.77 | 17 | 0.1 | 17 | 0.1 | 0 | 0.00 | 0 | 0.00 | 11,515 | 69.4 | 2,145 | 12.9 |
| VP100-VP250 | 18,509 | 13,382 | 72.3 | 13,379 | 72.3 | 2,694 | 14.6 | 9 | 0.0 | 1 | 0.0 | 952 | 5.14 | 22 | 0.1 | 21 | 0.1 | 1 | 0.01 | 0 | 0.00 | 10,835 | 58.5 | 4,852 | 26.2 |
| VP250 | 21,707 | 16,047 | 73.9 | 16,044 | 73.9 | 4,500 | 20.7 | 12 | 0.1 | 0 | 0.0 | 2,597 | 11.96 | 35 | 0.2 | 35 | 0.2 | 0 | 0.00 | 0 | 0.00 | 14,738 | 67.9 | 3,904 | 18.0 |
| Dosing symptoms by subject | |||||||||||||||||||||||||
| Treatment group | No. of subjects | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % |
| PLB-VP250 | 20 | 20 | 100.0 | 20 | 100.0 | 16 | 80.0 | 4 | 20.0 | 0 | 0.0 | 17 | 85.00 | 6 | 30.0 | 6 | 30.0 | 0 | 0.00 | 0 | 0.00 | 18 | 90.0 | 17 | 85.0 |
| VP100-VP250 | 24 | 24 | 100.0 | 24 | 100.0 | 22 | 91.7 | 5 | 20.8 | 1 | 4.2 | 22 | 91.67 | 8 | 33.3 | 7 | 29.2 | 1 | 4.17 | 0 | 0.00 | 22 | 91.7 | 23 | 95.8 |
| VP250 | 25 | 25 | 100.0 | 25 | 100.0 | 25 | 100.0 | 7 | 28.0 | 0 | 0.0 | 25 | 100.00 | 7 | 28.0 | 7 | 28.0 | 0 | 0.00 | 0 | 0.00 | 25 | 100.0 | 25 | 100.0 |
| Dosing symptoms by percent of doses per subject | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | No. of doses | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) |
| PLB-VP250 | 865.5 (827.5–896) | 92.6 (70.2–97.4) | 92.4 (70.2–97.4) | 4.6 (0.1–27.1) | 0 (0–0) | 0 (0–0) | 2.1 (0.5–22.4) | 0 (0–0.2) | 0 (0–0.2) | 0 (0–0) | 0 (0–0) | 81.1 (51.1–92.9) | 1.4 (0.1–18.9) |
| VP100-VP250 | 892.5 (828–900.5) | 89.4 (57.8–97.4) | 89.4 (57.8–97.4) | 4.3 (1.1–15.2) | 0 (0–0) | 0 (0–0) | 1.7 (0.8–3.7) | 0 (0–0.1) | 0 (0–0.1) | 0 (0–0) | 0 (0–0) | 53.3 (19.3–88.4) | 10 (0.3–40.9) |
| VP250 | 893 (860–907) | 95.3 (63.2–98.3) | 95.3 (63.2–98.3) | 3.8 (0.8–27.6) | 0 (0–0.1) | 0 (0–0) | 3.9 (0.7–23.8) | 0 (0–0.1) | 0 (0–0.1) | 0 (0–0) | 0 (0–0) | 90.1 (21.5–97.3) | 9.2 (1.9–18.6) |
Immunologic outcomes
Immunoglobulin data.
Fig 2 presents peanut- and Ara h2–specific IgE and IgG4 data by treatment group over time. Peanut-specific IgE had a significant interaction between treatment and visit (P < .001) (Fig 2, A); pairwise comparisons at week 52 showed that the PLB-VP250 group had statistically significantly higher peanut IgE compared with the VP100-VP250 group (P = .001) and the VP250 group (P = .008) holding baseline peanut IgE constant, whereas at week 130 the VP250 group had statistically significantly higher peanut IgE than the PLB-VP250 (P = .002) and the VP100-VP250 (P = .008) groups. No statistically significant differences in Ara h2–specific IgE were observed between treatment groups over time (Fig 2, B). Peanut- and Ara h2–specific IgG4 increases were observed in all groups over time, with the largest increase noted in the VP250 group (Fig 2, C and D). When assessing treatment effects over time, significant increases were observed between treatment groups for Ara h2–specific IgG4 levels (P = .002) (Fig 2, D), with peanut-specific IgG4 approaching significance (P = .012) (Fig 2, C). The PLB-VP250 group (P = .005) and the VP100-VP250 group (P = .002) had lower Ara h2–specific IgG4 levels over time when compared with the VP250 group. Repeated-measures analysis did not find any immunoglobulin parameters that were different over time between week 130 desensitization successes and failures (see Fig E3, A–D, in this article’s Online Repository at www.jacionline.org).
FIG 2.
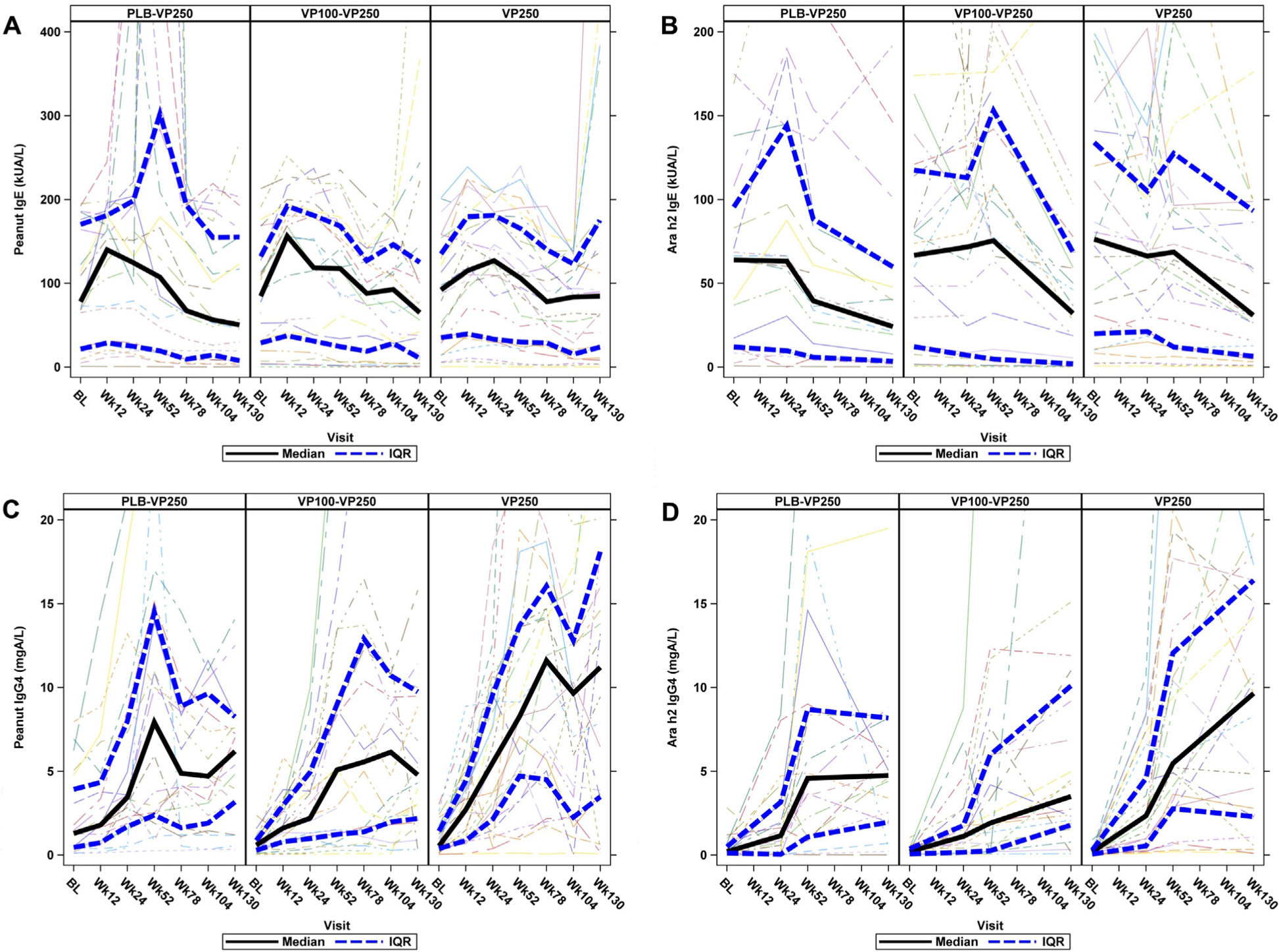
Immune mechanistic parameters from baseline through week 130 by treatment group. A, Peanut-specific IgE. At week 52, the PLB-VP250 group had higher values compared with VP100-VP250 (P = .001) and VP250 (P = .008) groups; at week 130, the VP250 group had higher values compared with PLB-VP250 (P = .002) and VP100–VP250 (P = .008) groups. B, Ara h2 IgE. C, Peanut IgG4. D, Ara h2 IgG4. Overall, the VP250 group had higher values compared with the PLB-VP250 group (P = .005); the VP250 group also had higher values when compared with the VP100–VP250 group (P = .002). A solid black line represents the median, and blue hatched lines represent the upper and lower quartiles. kUA/L, Kilounits of antibody per liter.
Skin prick test.
Peanut skin prick tests were carried out throughout the study with no significant changes noted over time in any treatment group (see Fig E4, A, in this article’s Online Repository at www.jacionline.org). Median change from baseline to week 130 included the following: PLB-VP250 −0.5 mm (IQR, −6.5 to 3.0), VP100-VP250 −4.0 mm (IQR, −7.5 to 1.0), and VP250 −5.75 mm (IQR, −7.5 to 0.50). Median change from baseline to week 130 was −6.0 mm (IQR, −8.0 to −2.0) for desensitization successes and −1.0 mm (IQR, −7.25 to 2.25) for failures (P = .089; Fig E4, B).
Basophil activation.
Effects of EPIT on in vitro basophil activation are shown in Fig 3. Compared with baseline, decreases in the percentages of activated CD63+ basophils were seen at all time points, in all treatment groups, following stimulation with 0.01 μg peanut protein. Although the percent CD63+ basophils decreased at the 0.01-μg dose, no statistically significant differences were observed between treatment groups for any of the stimulant doses over time. Likewise, change from baseline to week 130 in basophil activation was not significantly different when comparing desensitization successes and failures (see Fig E5 in this article’s Online Repository at www.jacionline.org), implying that basophil activation changes are not a reliable predictor of desensitization success.
FIG 3.
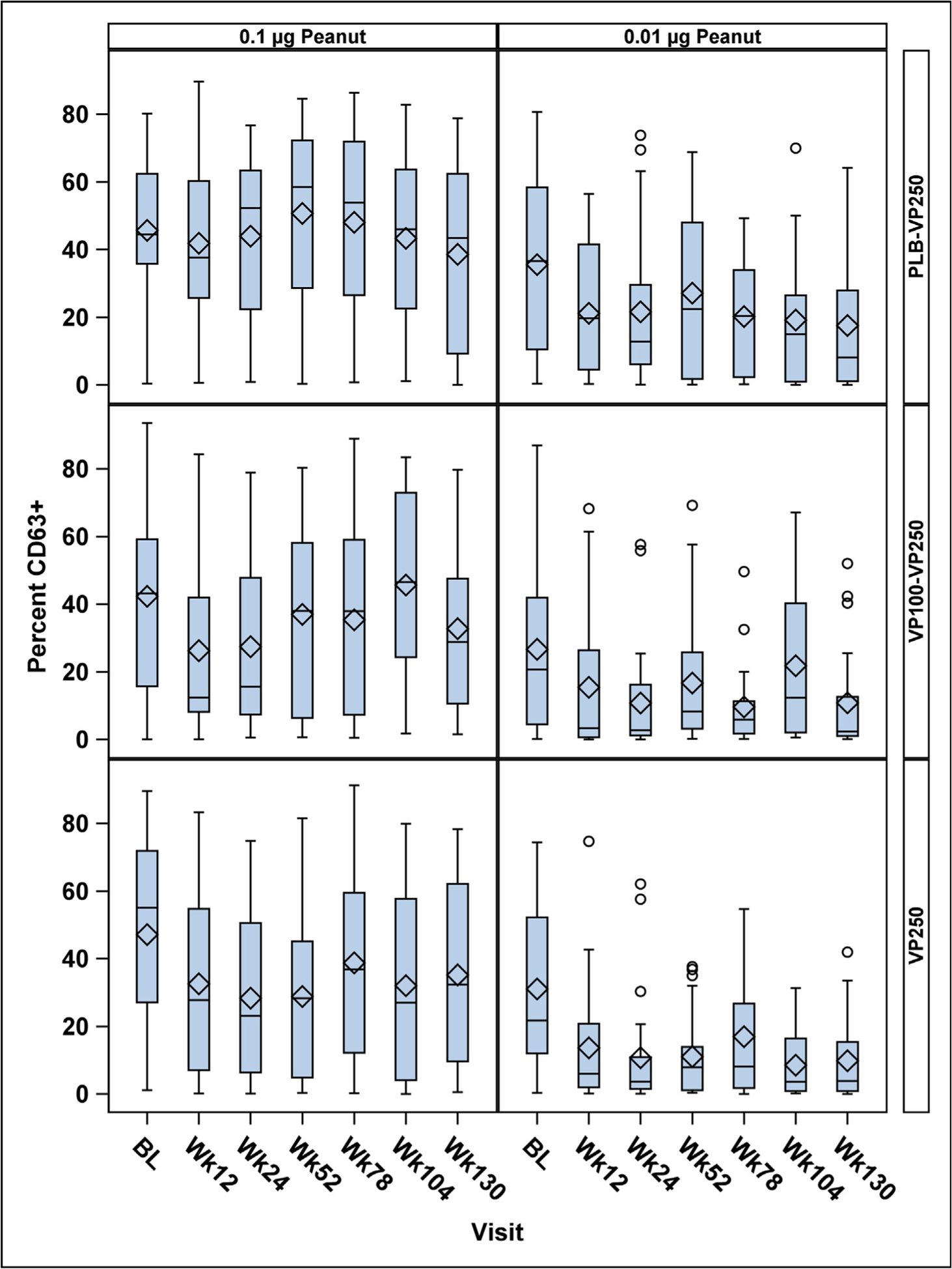
Effect of peanut EPIT on basophil activation (measured by percent CD63+ basophils) over time by treatment group. Top row, PLB-VP250 group; middle row, VP100-VP250 group; bottom row, VP250 group. Cells were stimulated with 0.1 μg/mL peanut extract (column 1) and 0.01 μg/mL peanut extract (column 2). A decrease in the percentage of CD63+ basophils over time was observed only following stimulation with 0.01 μg/mL peanut extract, and statistically significant differences between treatment groups over time were not observed. These are box plots, with the lines inside each box representing the median and the diamonds representing the mean.
DISCUSSION
Substantial progress toward development of safe, effective, and well-tolerated therapies for peanut allergy has resulted in multiple approaches currently in the therapeutic pipeline.9 Peanut EPIT is an approach that has shown promise as a therapeutic option for food allergy in both preclinical models and clinical trials, with reports of favorable safety, tolerability, and efficacy outcomes following 52 weeks of therapy.12,13,16 In the newly published report from the PEPITES open-label extension trial (PEOPLE) in children age 4 to 11 years, investigators reported continued clinical benefit during 2 additional years of peanut EPIT, associated with high compliance and low discontinuation rates, supporting an overall favorable benefit-to-risk profile for peanut EPIT.15 We report on the open-label treatment extension of a randomized, placebo-controlled, multicenter trial of peanut EPIT,16 in which the Consortium for Food Allergy Research Study Group investigated the impact of extended active treatment for 130 weeks with Viaskin Peanut on clinical, safety, and immunologic outcomes in a cohort of peanut-allergic subjects age 4 to 25 years at enrollment.
Findings reinforce the favorable safety and tolerability profile of peanut EPIT previously reported and support the concept that prolonged EPIT induces immunologic changes. On the basis of predefined criteria for treatment success, more participants randomized to the VP250 group from the outset of treatment reached some level of desensitization success when compared with VP100-VP250 and PLB-VP250 groups. In fact, desensitization successes were reached in participants who had the lowest peanut threshold triggering symptoms (ie, median DBPCFC SCD 444 mg in the VP250 group) and predominantly among 4- to 11-year-old children. A post hoc analysis did not show a statistically significant difference between weeks 52 and 130 for change in desensitization success. Furthermore, there was no statistically significant increase in SCD from week 52 to week 130. When individual participants were considered, there were 4 participants who were able to successfully consume a total of 2044 mg of peanut protein at week 130 but did not meet “desensitization success” criteria. These 4 participants were part of a group of 14 (20.3%) trial participants who attained an SCD of 1044 mg or higher and of 23 (33.3%) who attained an SCD of 444 mg or higher during the 130-week DBPCFC. It can be argued that some of these participants attained a clinically meaningful change in peanut reactive threshold (ie, desensitization) during the study, although this effect is represented in only a minority of the total study population.
Although the trial was not specifically designed or powered to examine both treatment effects and impact of age on therapeutic outcomes, age at initiation of therapy was the only pretreatment factor with a statistically significant association with week 130 desensitization success. The observation that 50% of 4- to 11-year-old children treated with VP250 in this study achieved desensitization success at week 130 compared with only 27.8% at week 52 (Table I) is interesting and suggests a need for further study in this age group. These findings are consistent with previous observations at week 52 demonstrating an increase in the desensitization success rate in younger participants.13 The potential benefit in younger peanut-allergic children has also been seen in other published EPIT trials, particularly in the VIPES trial (ages 6–55 years), which showed a higher differential in response rate in VP250-treated participants compared with PLB among 6- to 11-year-olds (34.2%) compared with the entire study population (25%). These findings were confirmed in the PEPITES trial (ages 4–11 years), which showed that a greater percentage of active versus PLB-treated subjects had an increase in peanut eliciting dose (treatment difference of 21.7%).12,13
The benefit of treatment with VP250 in the current study may be most applicable in protecting a subset of individuals from accidental exposures to peanut (ie, desensitization). Indeed, Baumert et al17 reported that increasing the preimmunotherapy reaction threshold dose from 100 mg or less peanut protein to postimmunotherapy thresholds of 300 mg and 1000 mg resulted in clinically relevant risk reduction for peanut- allergic individuals.17 When evaluating findings from the current study in the context of Baumert et al’s criteria, 33.3% of those on active EPITachieved a cumulative peanut protein dose (SCD) of greater than or equal to 444 mg (ie, ~300-mg single dose) and 20.3% achieved an SCD of greater than or equal to 1044 mg (ie, ~600-mg single dose).
With multiple therapeutic approaches currently in various stages of development, including the recent approval of Palforzia (peanut oral immunotherapy [OIT], Aimmune Therapeutics, Brisbane, Calif) by the Food and Drug Administration, it is important to consider the findings from this trial in the context of other treatment options, such as peanut OIT and sublingual immunotherapy (SLIT). Peanut OIT has demonstrated robust desensitization outcomes, with 67% of those on peanut OIT in the PALISADE trial achieving a 600-mg single peanut protein dose (1044 cumulative dose) and 76% achieving a 300-mg single dose (444 mg cumulative dose) after 12 months of treatment with 300 mg daily maintenance dosing.18 Peanut OIT can be associated with risk of potentially intolerable, treatment-limiting side effects in some individuals.18,19 Peanut SLIT has an overall favorable safety, tolerability, and efficacy profile that, particularly in pediatric populations, compares favorably to OIT.20–22 In a recently published peanut SLIT trial in children age 1 to 11 years, the median clinical reactivity threshold was 1750 mg of peanut protein after 3 to 5 years of therapy, with no more than mild treatment-related adverse events reported.20 Peanut EPIT is less potent in inducing desensitization compared with SLIT and OIT, yet its excellent safety profile and the high level of adherence by participants warrants further study. As additional licensed therapeutic options for peanut allergy proceed toward broader clinical application, the long-term sustainability of therapy and the impact of treatment burden on adherence and efficacy will be important considerations that require additional investigation.
A surprising finding in this study was the variability in treatment success among groups, especially between the PLB-VP250 and VP250 groups. Both groups received therapy with VP250 for 130 weeks. Thus, the difference in treatment response observed in the PLB-VP250 crossover group at 130 weeks is an interesting and unexpected outcome. A higher proportion of participants in the VP250 group were younger than 7 years when starting therapy, a likely contributing factor to the success of the group. Therefore, one possible explanation for the reduced treatment response in the PLB-VP250 group could be older age at start of active therapy when compared with the VP250 group, though the median difference was only 1.7 years. However, the differences at week 130 should be interpreted cautiously and in the context of the limitations of the study, including small sample size and wide age range of participants. This limits the ability to perform subgroup analyses based on specific age cutoffs, in particular a small number of participants younger than 7 years. It is interesting to consider whether 52 weeks of PLB patch treatment without antigen delivery (or at lower doses such as VP100) could have caused changes in the skin barrier, skin microbiome, and/or immune responses. This could alter immune signaling through Langerhans cells or other immune pathways, resulting in nonbeneficial immunomodulation or skewing of the immune response that is not reversible with subsequent application of active VP250 patch. Repeated patch application to the specified skin locations may unroof the upper portion of the stratum corneum, which could create a more favorable environment for colonization with Staphylococcus aureus, which has been shown to trigger peanut allergy and type 2 immune responses.23,24 It is also unexpected to note that the VP100-VP250 group had a weak efficacy response even though that group received 78 weeks of VP250 after 52 weeks of VP100. These findings suggest that early treatment with the optimal dose has the best chance of success. Further study is warranted to better understand this outcome. These results may be evaluable during open-label extension phases of study that are ongoing following the PEPITES trial.12,13
Adherence to therapy remained excellent during the open-label extension of treatment with VP250, with 96% of expected doses administered. Safety and tolerability were favorable in this extension study. All participants experienced some type of dosing reaction while on active VP250; however, patch-site reactions were typically mild and treated with antihistamines or topical steroids, reinforcing the need for continued safety surveillance in all treated patients.
Immunologic changes were observed during extended active treatment with VP250 including significant increases in peanut-and Ara h2–specific IgG4 over time, findings that are consistent with other forms of effective immunotherapy under investigation for peanut allergy. However, there was no statistically significant association between any of these individual parameters and treatment outcome, suggesting that no tested immunologic marker was a clear predictor of treatment success in this analysis. Further investigation of immune mechanistic and skin barrier dysfunction in younger age groups is warranted. This may help to better define and/or predict treatment responders.
There are several limitations to this study. The study was originally designed to have sufficient power to detect differences in week 52 EPIT outcomes as compared with PLB and was not formally powered for either week 130 outcomes or changes from week 52 to week 130. This raises the possibility that this study overestimated or underestimated the clinical impact of EPIT and limits the ability to detect a statistically significant change in desensitization, especially across the broad age range of participants in the current study. Ongoing studies with larger sample sizes may more fully define response to treatment. The current study was originally designed and powered to assess treatment outcomes among children and young adults, age 4 to 25 years, with a primary end point at week 52 and secondary end point at week 130. Our previously published week 52 data suggested that peanut EPIT was more effective in children than in young adults.16 However, it was not possible to redesign the ongoing study to increase the number of children in the 4- to 11-year age group to enhance the power of data involving children. Although the data suggest a possible age-related effect on outcomes, the study was not originally designed to detect an age effect and findings must be interpreted in this context, although other studies conducted concurrently with this trial demonstrated the impact of age on treatment outcomes and informed the design of a subsequently published trial (PEPITES trial) examining outcomes in children age 4 to 11 years.12,13 Although there was evidence of altered immune responses in some of the treatment groups, this study was not designed to predict treatment response through analysis of potential biomarkers; in addition, the role of other potential modifying factors such as the impact of the skin microbiome, barrier function, or lipid profile on treatment outcomes bears further investigation.23,24
Conclusions
Extended treatment with VP250 for 130 weeks was safe, well tolerated, and induced desensitization at week 52, which persisted between weeks 52 and 130. Persistent immunomodulatory changes were noted during extended VP250 treatment but did not differentiate treatment successes from failures. Treatment response was observed predominantly in younger children (ages 4–11 years) receiving VP250, suggesting that intervention in early life may improve therapeutic outcomes; however, further study in larger trials is warranted. Continued investigation is critical to inform careful selection of candidates for peanut EPIT to maximize efficacy, minimize risk, and develop strategies for future clinical application.
Supplementary Material
Key messages.
VP250 induced desensitization at week 52, which persisted between weeks 52 and 130, with excellent safety, tolerability, and adherence.
Younger age (4–11 years) at initiation of therapy is associated with desensitization success.
Increased peanut- and Ara h2–specific IgG4 were observed. No immune parameters predicted treatment outcomes.
An unexpected low efficacy rate in PLB-cross-over participants requires additional study.
Acknowledgments
This study is registered with ClinicalTrials.gov with ID NCT01904604 and was supported by the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) (grant nos. U19AI066738, U01AI066560, and UM2AI130836). The project was also supported by the University of Arkansas for Medical Sciences (grant no. UM1AI130781), the University of North Carolina (grant no. UM1AI30936), the Mount Sinai University (grant no. UM1AI130570), National Jewish Health (grant no. UM1AI130780), and Johns Hopkins University School of Medicine (grant no. UM1AI30838), which were supported by the NIH-NIAID, and by the University of Arkansas for Medical Sciences (grant no. UL1TR003107), the University of North Carolina (grant no. UL1TR001111), the Mount Sinai University (grant no. UL1TR000067), National Jewish Health (grant no. UL1TR002535), and Johns Hopkins University School of Medicine (grant no. UL1TR000424), which were supported by the National Center for Research Resources (NCRR), a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or the NIH. Support for this trial was also provided by DBV Technologies, Inc (Montrouge, France), through funds provided to the Consortium for Food Allergy Research. Protocol development, study conduct, data analysis, and manuscript development were conducted independently of DBV.
The following persons provided physician oversight, study coordination, and support: D. Bailey, C. Bronchick, J. Fishman, D. Fitzgerald, D. Fleischer, J. French, E. Gibson, M. Groetch, D. Hamilton, P. Hauk, L. Herlihy, S. House, S. Leung, A. Liu, K. Mudd, R. Pesek, J. Ross, J. Slinkard, P. Steele, L. Talarico, and M. Taylor. We thank A. Grishin, M. Mishoe, G. Grishina, and J. Grabowska at the Icahn School of Medicine at Mount Sinai for their contributions to mechanistic studies. We thank the staff of the clinical research units at each participating center and the Statistical and Clinical Coordinating Center and Krisy Peyton, the SACCC Project Manager. We thank J. Poyser, Project Manager for the Consortium for Food Allergy Research (CoFAR) Program (National Institutes of Health [NIH]/National Institute of Allergy and Infectious Diseases [NIAID]). The study was designed by the investigators of the CoFAR, with Dr Jones as study chair. The data were gathered by the investigators, and analyzed by the Statistical and Clinical Coordinating Center at Emmes. The manuscript was written collaboratively by Dr Scurlock and reviewed and edited by the authors. The decision and approval to publish was made by the authors, as investigators in CoFAR, Emmes, and the NIH/NIAID leadership.
Disclosure of potential conflict of interest:
A. M. Scurlock reports grant support to her institution from the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID), Immune Tolerance Network, Aimmune Therapeutics, DBV Technologies, Astellas, Regeneron, Genentech, and Food Allergy Research and Education (FARE) and clinical medical advisory board membership with DBV Technologies. A. W. Burks reports being a minority stock holder in Allertein and Mastcell pharmaceuticals; scientific advisory board membership with Aimmune Therapeutics, Consortia TX, Inc, Intrommune Therapeutics, and Prota Therapeutics; consultancy for DBV Technologies, N-fold LLC, Aravax, Genentech, and Hycor Biomedical; grant support to his institution from the NIH/NIAID, NIH/National Center for Complementary and Integrative Health, Johns Hopkins/NIH, FARE, and the Wallace Research Foundation, in addition to royalty payments from UpToDate and royalties paid from the following US patents: #7879977, #6835824, #6486311, #6441142, #5973121, and #5558869. S. H. Sicherer reports grants from NIH/NIAID and royalty payments from UpToDate, the American Academy of Allergy, Asthma & Immunology, and Johns Hopkins University Press. D. Y. M. Leung receives grant support to his institution from the NIH/NIAID and serves on the Data Safety Monitoring Committee for Aimmune Therapeutics. E. H. Kim reports clinical medical advisory board membership with DBV Technologies; consultancy with Aimmune Therapeutics, DBV Technologies, AllerGenis, Allakos, Ukko, and Vibrant America; and grant support to his institution from the NIH/NIAID, NIH/NCCIH, FARE, and the Wallace Research Foundation. A. K. Henning, P. Dawson, and R. W. Lindblad are employed by Emmes, which received grant support from the Division of Allergy, Immunology and Transplantation (DAIT)/NIH/NIAID. M. C. Berin reports scientific advisory board membership with Prota Therapeutics. H. A. Sampson reports being a part-time employee of DBV Technologies; receiving consultant fees from N-Fold Therapeutics, and royalties for various textbooks; holding stock options in DBV Technologies and N-FOLD; receives grant support to his institution from the Immune Tolerance Network and NIH/NIAID; and serves as an unpaid Board of Directors member and advisor to AllerGenis. R. A. Wood reports royalty payments from UpToDate and grant support to his institution from the NIH/NIAID, Aimmune Therapeutics, DBV Technologies, Astellas, Regeneron, HAL-Allergy, and Sanofi. S. M. Jones reports research advisory board membership with FARE and consultancy and scientific advisory board membership with Aimmune Therapeutics; received grant support to her institution from the NIH/NIAID, Immune Tolerance Network, Aimmune Therapeutics, DBV Technologies, Astellas, Regeneron, Genentech, and FARE; and has performed CSR review/preparation for DBV Technologies on behalf of Emmes. The rest of the authors declare that they have no relevant conflicts of interest.
Abbreviations used
- DBPCFC
Double-blind, placebo-controlled food challenge
- EPIT
Epicutaneous immunotherapy
- IQR
Interquartile range
- OIT
Oral immunotherapy
- PEPITES
Peanut EPIT Efficacy and Safety Study
- PLB
Placebo
- PLB-VP250
Placebo cross over to VP250 active treatment group
- SCD
Successfully consumed dose
- SLIT
Sublingual immunotherapy
- VP250
Viaskin Peanut 250 μg
- VP100
Viaskin Peanut 100 μg
- VP100-VP250
VP100 cross over to VP250 active treatment group
REFERENCES
- 1.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011;128:e9–17. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol 2010;125:1322–6. [DOI] [PubMed] [Google Scholar]
- 3.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010;126:S1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics 2009;124:1549–55. [DOI] [PubMed] [Google Scholar]
- 5.Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA Pediatr 2013;167: 1026–31. [DOI] [PubMed] [Google Scholar]
- 6.Bollinger ME, Dahlquist LM, Mudd K, Sonntag C, Dillinger L, McKenna K. The impact of food allergy on the daily activities of children and their families. Ann Allergy Asthma Immunol 2006;96:415–21. [DOI] [PubMed] [Google Scholar]
- 7.Stensgaard A, Bindslev-Jensen C, Nielsen D, Munch M, DunnGalvin A. Quality of life in childhood, adolescence and adult food allergy: patient and parent perspectives. Clin Exp Allergy 2017;47:530–9. [DOI] [PubMed] [Google Scholar]
- 8.Bilaver LA, Chadha AS, Doshi P, O’Dwyer L, Gupta RS. Economic burden of food allergy: a systematic review. Ann Allergy Asthma Immunol 2019;122: 373–80.e1. [DOI] [PubMed] [Google Scholar]
- 9.Vickery BP, Ebisawa M, Shreffler WG, Wood RA. Current and future treatment of peanut allergy. J Allergy Clin Immunol Pract 2019;7:357–65. [DOI] [PubMed] [Google Scholar]
- 10.Mondoulet L, Dioszeghy V, Ligouis M, Dhelft V, Dupont C, Benhamou P-H. Epicutaneous immunotherapy on intact skin using a new delivery system in a murine model of allergy. Clin Exp Allergy 2010;40:659–67. [DOI] [PubMed] [Google Scholar]
- 11.Mondoulet L, Dioszeghy V, Puteaux E, Ligouis M, Dhelft V, Letourneur F, et al. Intact skin and not stripped skin is crucial for the safety and efficacy of peanut epicutaneous immunotherapy (EPIT) in mice. Clin Transl Allergy 2012; 2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleischer DM, Greenhawt M, Sussman G, Bégin P, Nowak-Wegrzyn A, Petroni D, et al. Effect of epicutaneous immunotherapy vs placebo on reaction to peanut protein ingestion among children with peanut allergy: the PEPITES randomized clinical trial. JAMA 2019;321:946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampson HA, Shreffler WG, Yang WH, Sussman GL, Brown-Whitehorn TF, Nadeau KC, et al. Effect of varying doses of epicutaneous immunotherapy vs placebo on reaction to peanut protein exposure among patients with peanut sensitivity: a randomized clinical trial. JAMA 2017;318:1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones SM, Agbotounou WK, Fleischer DM, Burks W, Pesek RD, Harris MW, et al. Safety of epicutaneous immunotherapy for the treatment of peanut allergy: a phase 1 study using viaskin patch. J Allergy Clin Immunol 2016;137:1258–61. [DOI] [PubMed] [Google Scholar]
- 15.Fleischer DM, Shreffler WG, Campbell DE, Green TD, Anvari S, Assa’ad A, et al. Long-term, open-label extension study of the efficacy and safety of epicutaneous immunotherapy for peanut allergy in children: PEOPLE 3-year results. J Allergy Clin Immunol 2020;146:863–74. [DOI] [PubMed] [Google Scholar]
- 16.Jones SM, Sicherer SH, Burks AW, Leung DY, Lindblad RW, Dawson P, et al. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J Allergy Clin Immunol 2017;139:1242–52.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumert JL, Taylor SL, Koppelman SJ. Quantitative assessment of the safety benefits associated with increasing clinical peanut thresholds through immunotherapy. J Allergy Clin Immunol Pract 2018;6:457–65. [DOI] [PubMed] [Google Scholar]
- 18.PALISADE Group of Clinical Investigators, Vickery BP, Vereda A, Casale TB, Beyer K, du Toit G, Hourihane JO, et al. AR101 Oral immunotherapy for peanut allergy. N Engl J Med 2018;379:1991–2001. [DOI] [PubMed] [Google Scholar]
- 19.Virkud YV, Burks AW, Steele PH, Edwards LJ, Berglund JP, Jones SM, et al. Novel baseline predictors of adverse events during oral immunotherapy in children with peanut allergy. J Allergy Clin Immunol 2017;139:882–8.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim EH, Yang L, Ye P, Guo R, Li Q, Kulis MD, et al. Long-term sublingual immunotherapy for peanut allergy in children: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol 2019;144:1320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleischer DM, Burks AW, Vickery BP, Scurlock AM, Wood RA, Jones SM, et al. Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol 2013;131:119–27.e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burks AW, Wood RA, Jones SM, Sicherer SH, Fleischer DM, Scurlock AM, et al. Sublingual immunotherapy for peanut allergy: long-term follow-up of a randomized multicenter trial. J Allergy Clin Immunol 2015;135:1240–8.e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a TH2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol 2010;126:976–84.e1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho S-H, Strickland I, Boguniewicz M, Leung DYM. Fibronectin and fibrinogen contribute to the enhanced binding of Staphylococcus aureus to atopic skin. J Allergy Clin Immunol 2001;108:269–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


