Supplemental Digital Content is available in the text.
Keywords: Air pollution, Birth weight, Critical time interval, Fine particulate matter, Maternal exposures, Metropolis-Hastings method, Nitrogen dioxide, Pregnancy cohort
Abstract
Background:
Maternal prenatal exposure to air pollution has been associated with adverse birth outcomes. However, previous studies focused on a priori time intervals such as trimesters reported inconsistent associations.
Objectives:
We investigated time-varying vulnerability of birth weight to fine particulate matter (PM2.5) and nitrogen dioxide (NO2) using flexible time intervals.
Methods:
We analyzed 1,300 live, full-term births from Maternal–Infant Research on Environmental Chemicals, a Canadian prospective pregnancy cohort spanning 10 cities (2008–2011). Daily PM2.5 and NO2 concentrations were estimated from ground-level monitoring, satellite models, and land-use regression, and assigned to participants from pre-pregnancy through delivery. We developed a flexible two-stage modeling method—using a Bayesian Metropolis–Hastings algorithm and empirical density threshold—to identify time-dependent vulnerability to air pollution without specifying exposure periods a priori. This approach identified critical windows with varying lengths (2–363 days) and critical windows that fell within, or straddled, predetermined time periods (i.e., trimesters). We adjusted the models for detailed infant and maternal covariates.
Results:
Critical windows associated with reduced birth weight were identified during mid- to late-pregnancy for both PM2.5 and NO2: –6 g (95% credible interval: –11, –1 g) and –5 g (–10, –0.1 g) per µg/m3 PM2.5 during gestational days 91–139 and 249–272, respectively; and –3 g (–5, –1 g) per ppb NO2 during days 55–145.
Discussion:
We used a novel, flexible selection method to identify critical windows when maternal exposures to air pollution were associated with decrements in birth weight. Our results suggest that air pollution impacts on fetal development may not be adequately captured by trimester-based analyses.
Exposure to ambient air pollution has been associated with a variety of adverse birth outcomes including preterm birth, decreased and low birth weight, growth restriction, and neonatal mortality.1–7 Adverse birth outcomes have consistently ranked in the top ten global causes for disability-adjusted life years,8–10 with low birth weight, small for gestational age, and preterm birth linked to increased risk of infant mortality as well as developmental, cardiovascular, and respiratory health problems in childhood and adulthood.4,11–14
Time-dependent vulnerability to air pollution persists as a critical research priority that needs to be addressed to elucidate the full impact of air pollution on fetal development and birth outcomes.1,3,6,15–20 Literature reviews have cited the need for examining progressively smaller critical time windows during pregnancy for air pollution exposure.7,21 Most previous studies have examined relatively large, predetermined exposure periods—for example, total-pregnancy, trimester-specific, or first and last month of pregnancy average.7,21 The primary limitation with a priori selection of critical time periods is that this approach may not identify true critical exposure windows that straddle two predetermined periods selected for analysis. Trimester-based or weekly analyses also assume that all critical windows are the same duration. Finally, comparing trimester averages can lead to biased estimates; for example, when estimating the association between a trimester average exposure and the outcome without controlling for other trimesters, seasonal variations in air pollution can act as unmeasured confounders.22
Recent studies linking air pollution with birth outcomes have used distributed lag nonlinear models to examine smaller time intervals. This method addresses some of the limitations associated with trimester average exposures,22 assuming a nonlinear shape via. smoothing over the lags. However, the models are highly sensitive to the specification of lag distribution—specifically, degrees of freedom (df) and smoothing function23,24—and selection of df and smoothing method can introduce bias due to mis-specification.25
To address these issues, we developed a novel two-stage modeling method26—using a Bayesian Metropolis–Hastings (M-H) algorithm coupled with an empirical density threshold—to identify critical time windows without relying on predetermined time intervals (e.g., trimesters) or distributional assumptions (e.g., distributed lag nonlinear model time lags). This approach allowed us to identify critical periods with varying lengths (i.e., 2 or 200 days) and critical periods that fell within, or straddled, predetermined time periods such as trimesters. We applied this flexible method to identify critical windows from pre-pregnancy through delivery when maternal exposures to ambient air pollution (PM2.5, NO2) disproportionately affected fetal growth and development (birth weight) in a Canadian prospective pregnancy cohort spanning 10 cities.
MATERIALS AND METHODS
Study Population
The Maternal–Infant Research on Environmental Chemicals (MIREC) Study,27 a Canadian prospective pregnancy cohort, provided data for women in 10 cities from 2008 to 2011. Participants were recruited during the first trimester of pregnancy and provided information on: demographics, socioeconomic status, behavior, residential location, reproductive history, and maternal/infant clinical data. We obtained approval to conduct the research from Research Ethics Boards at Health Canada and Hospital Sainte Justine, as well as the MIREC biobank.
Starting with 1983 MIREC participants, analyses were restricted to live, singleton, full-term births because stillbirth (n = 80), nonsingleton (n = 49), and preterm (n = 116) births are strongly associated with low birth weight. To reduce potential exposure misclassification in temporal data, participants with >25% of their daily concentrations missing during the study period were excluded from PM2.5 (n = 184) and NO2 (n = 228) analyses. We also excluded participants with missing residential (n = 50), BMI (n = 64), income (n = 62), education (n = 2), infant sex (n = 1), and alcohol consumption during pregnancy (n = 1) data. Finally, to reduce spatial exposure misclassification, we excluded participants living in large (>20 × 20 km) forward sortation areas (see the section on Air Pollution) from PM2.5 (n = 40) and NO2 (n = 38) analyses. A total of 1,334 and 1,305 mother-baby pairs were analyzed for PM2.5 and NO2, respectively. Exclusions are illustrated in eFigure A1 in eAppendix; http://links.lww.com/EDE/B865.
We applied an advanced method to impute missing daily air pollution concentrations. We did not apply imputation to the small number of missing covariates due to the nature of the missing data—specifically, daily air pollution data have periodic structure characterized by a weekly pattern, while the other covariates were socioeconomic or health-related variables without specific patterns.
Birth Weight
The objective of this study was to identify specific periods when maternal exposures to ambient air pollution may disproportionately affect birth weight; therefore, we modeled birth weight as a continuous variable rather than a binary variable based on low birth weight.
Air Pollution
For each participant, we generated air pollution concentrations for each day spanning from pre-pregnancy (day –90) through delivery. We estimated the beginning of pregnancy (day 0) based on last menstrual period and early ultrasound (see eAppendix; http://links.lww.com/EDE/B865 for details). We estimated daily concentrations of PM2.5 and NO2 by applying the relative daily variation observed at ground-level monitoring sites under Canada’s National Air Pollution Surveillance (NAPS) program to long-term, spatially refined concentrations from land-use regression28 and satellite-derived models.29 This hybrid approach30 combined the enhanced spatial scale of land-use regression and satellite-derived data with daily temporal resolution provided by NAPS measurements. Estimation of daily and long-term PM2.5 and NO2 data is described in eAppendix (eFigure A2 and A3; http://links.lww.com/EDE/B865).
Daily concentrations were missing for 1.5% (PM2.5) and 1.2% (NO2) of the study period, mainly due to missing hourly NAPS data (Table 1). We imputed missing daily values using a multistage approach that combined classical prediction techniques with phase- and frequency-fitting tools via. the multitaper method; see eAppendix for further details (eFigure A4 (a) and (b); http://links.lww.com/EDE/B865).31,32 We limited imputation to participants with at least 75% of daily air pollution data during the study period (pre-pregnancy through pregnancy). We excluded participants with less than 75% of daily data.
TABLE 1.
Descriptive Statistics for Daily PM2.5 and NO2
| PM2.5 (µg/m3) | NO2 (ppb) | |||
|---|---|---|---|---|
| All Daily Values (n = 1,334 Participants) | Unimputed Only (n = 1,324 Participants) | All Daily Values (n = 1,305 Participants) | Unimputed Only (n = 1,096 Participants) | |
| Nonmissing daily concentrations: N (%)a | 481,450 (99%) | 473,289 (99%) | 471,682 (100%) | 392,913 (99%) |
| Missing daily concentrations: n (% missing)b | 2,792 (1%) | 7,323 (2%) | 2,033 (0%) | 4,935 (1%) |
| Mean | 8.8 | 8.8 | 16.4 | 18.5 |
| Standard deviation (SD) | 7.6 | 7.6 | 13.7 | 13.7 |
| Median | 6.6 | 6.7 | 12.6 | 15.9 |
| Min | <0.001 | 0.05 | <0.001 | <0.001 |
| Max | 226.3 | 226.3 | 119.4 | 118.1 |
| Coefficient of variationc | 0.86 | 0.86 | 0.84 | 0.74 |
aN = number of MIREC participants × sum of all nonmissing gestational + pre-pregnancy days for each participant (i.e., for PM2.5, N = 1,334 participants × a maximum of 363 days per participant).
b% nonmissing = nonmissing values/maximum possible daily values × 100 (i.e., for PM2.5, the maximum % nonmissing = 481,450/484,242 × 100).
cCoefficient of variation (CV) = standard deviation/mean.
We linked daily PM2.5 and NO2 concentrations to each participant based on residential location (forward sortation area) at birth. Forward sortation areas consist of the first three characters of the Canadian postal code and can range from small sizes in urban areas (<2 × 2 km), to large sizes in rural areas (>40 × 40 km). We excluded participants living in large forward sortation areas (>20 × 20 km) from the analyses to reduce potential exposure misclassification (eTable A1; http://links.lww.com/EDE/B865). See eAppendix; http://links.lww.com/EDE/B865 for further description.
We estimated associations between air pollution and birth weight based on average exposure for each randomly selected time interval (the selection method is described further in the next section). We generated daily concentrations during each time interval using a log-normal distribution based on the estimated daily concentrations and number of days within the interval. This approach is more appropriate for assigning average exposure to intervals with varying lengths and is more robust to missing data, because the variance of the exposure depends on the number of nonmissing days.26 Briefly, the average exposure for a time interval with a small number of days has a larger variance than that of a wider interval with a greater number of days. Using a log-normal distribution rather than calculating the average exposure as the mean or median concentration for the nonmissing days addresses this issue.
Statistical Analyses
We employed a data-driven time interval selection method which examined flexible (versus predetermined) exposure periods. eFigure A5; http://links.lww.com/EDE/B865 outlines the selection process in five steps, which are described in detail below. All statistical analyses and computations were conducted using R 3.5.333 and SAS 9.4.34
(1) Generate random time intervals: We labeled each day based on the beginning of pregnancy (day 0). Time intervals were identified through random selection of start-end day pairs between day –90 (90 days before pregnancy) and delivery, using a Bayesian Metropolis-Hastings (M-H) algorithm.25 Three chains with 100,000 iterations and a burn-in of 1,000 iterations returned 300,000 intervals, which comprised the randomly generated start-end day pairs selected through the comparisons in step 2. This approach facilitated identification of multiple critical periods if more than one interval met the inclusion threshold and was not limited to uniform-length time intervals; specifically, time intervals ranged from 2 to 363 days.
(2) Select time intervals with better fit: For each time interval (the random start–end day pairs generated in step 1), we estimated the likelihood for a multiple linear regression model [1] relating birth weight with air pollution exposure during the selected time interval. We then compared each interval with subsequent time intervals based on their estimated likelihoods. Time intervals with better likelihood were retained, and those with lower likelihood were discarded. This process was repeated until we obtained the 300,000 intervals summarized in step 1.
[Model 1] birth weight ~ exposure (average exposure during the selected time interval) + gestational age at birth (days) + infant sex + smoking + education + marital status + income + parity + ethnicity + alcohol use + season of birth + maternal age + maternal pre-pregnancy BMI + error (where the error term has a normal distribution with mean zero).
Covariate specification is described in a directed acyclic graph for the direct effect of ambient air pollution exposure on birth weight (eFigure A6; http://links.lww.com/EDE/B865). There were 10 potential confounders and ancestors of the outcome (gestational age and infant sex), which were causally linked with the outcome but not the exposure. Air pollution exposure was the only time-varying variable; the 12 covariates were fixed across exposure intervals.
(3) Calculate empirical density based on selected time intervals: The M-H algorithm35 in steps 1 to 2 allowed for the selection of overlapping time intervals. Therefore, we proposed a new approach to identify potential critical periods. We calculated the selection density for each day within the study period based on the frequency with which that day occurred in the time intervals selected in steps 1 and 2. Higher frequency resulted in higher density, indicating critical exposure timing. This new approach for generating an empirical density for the study period is a simplification of the bivariate normal distribution assumption for the start-end intervals.26
(4) Identify potential critical exposure periods: Each day during the study period was ranked from the lowest to highest based on the density calculated in step 3. Those days with higher density demonstrated better fit for model 1, and thus higher rank, while those days with low density reflected poorer fit and lower rank. We introduced thresholds to determine which days appeared frequently enough to be considered important. We identified days that fell in the 75th percentile (i.e., the top 25%) as critical exposure periods.
(5) Estimate associations between air pollution and birth weight: We fit model [1] using noninformative priors (mean zero and variance 100) to estimate the change in birth weight per unit change in air pollution exposure during the critical time periods identified in step 4. We set up three chains with 10,000 samples for each chain, which resulted in 30,000 samples for the posterior distribution of each parameter. We reported 95% credible intervals in this article.
Sensitivity Analyses
We conducted sensitivity analyses to (1) examine the implications of using alternative thresholds (90th and 50th percentiles of selection density) to identify critical exposure periods; (2) examine the influence of imputed concentrations on identification of critical exposure periods; (3) compare associations between air pollution and birth weight for randomly selected versus predetermined exposure periods including pre-pregnancy (T0), individual trimesters (T1, T2, T3), total-pregnancy (T4, T1–T3), and pregnancy plus pre-pregnancy (T5, T0–T3); (4) compare our flexible selection method with distributed lag nonlinear models; and (5) examine the influence of excluding large forward sortation areas. See eAppendix; http://links.lww.com/EDE/B865 for additional details.
RESULTS
Selected Participants and Study Time Span
As described earlier, our analyses included 1,334 participants for PM2.5 and 1,305 for NO2. Because the number of women delivering after day 272 was limited, we examined the period from day –90 to 272 to maintain at least 1,000 participants for each day.
Maternal and Infant Characteristics
Table 2 summarizes maternal and infant characteristics for participants included in PM2.5 and NO2 analyses, versus all participants with live, singleton births. Most participants were married or in long-term partnerships (>1 year), and were highly educated, with high household incomes. Few participants smoked (4.3%) or consumed alcohol (20.2%) during pregnancy. However, 42% of pregnant women in this study were ≥35 years old, compared with 19.4% of pregnant women ≥35 years in the general population.36
TABLE 2.
Descriptive Statistics for Maternal and Infant Characteristics
| PM2.5 (n = 1,334)a | NO2 (n = 1,305)a | MIREC Live, Singleton Births (N = 1,854)b | ||||
|---|---|---|---|---|---|---|
| Maternal characteristics | ||||||
| Age (total N = 1,854) | n | % | n | % | n | % |
| <20 | 2 | 0.1 | 2 | 0.2 | 51 | 3 |
| 20–24 | 50 | 4 | 47 | 4 | 81 | 4 |
| 25–29 | 243 | 18 | 220 | 17 | 341 | 18 |
| 30–34 | 479 | 36 | 461 | 35 | 638 | 34 |
| ≥35 | 560 | 42 | 575 | 44 | 743 | 40 |
| Parity (previous live birth) (total N = 1,854) | 728 | 55 | 732 | 56 | 1,045 | 56 |
| Alcohol during pregnancy (total N = 1,805) | 269 | 20 | 258 | 20 | 343 | 19 |
| Smoked during pregnancy (total N = 1,805) | 57 | 4 | 57 | 94 | 5 | |
| Household income (>$50,000) (total N = 1,778) | 1,152 | 86 | 1,086 | 83 | 1,466 | 82 |
| Education (university degree) (total N = 1,804) | 1,234 | 93 | 1,203 | 92 | 1,647 | 91 |
| Married or long-term partner (total N = 1,854) | 1,277 | 96 | 1,250 | 96 | 1,767 | 95 |
| Ethnicity (White) (total N = 1,854) | 1,152 | 86 | 1,115 | 85 | 1,548 | 86 |
| Pre-pregnancy BMI | ||||||
| Underweight (total N = 1,716) | 33 | 3 | 32 | 3 | 49 | 3 |
| Normal (total N = 1,716) | 824 | 62 | 809 | 62 | 1,041 | 61 |
| Overweight (total N = 1,716) | 289 | 22 | 282 | 22 | 369 | 22 |
| Obese (total N = 1,716) | 188 | 14 | 182 | 14 | 257 | 1 |
| Infant characteristics | ||||||
| n | % | n | % | n | % | |
| Infant sex (girl) (total N = 1,853) | 623 | 47 | 616 | 47 | 873 | 47 |
| Birth season (warm: April–September) (total N = 1,854) | 694 | 52 | 697 | 53 | 960 | 52 |
| mean (SD) | range | mean (SD) | Range | mean (SD) | Range | |
| Gestational age (weeks) (total N = 1,854) | 39 (1.3) | 37–42 | 39 (1.2) | 37–42 | 39 (1.8) | 21–42 |
| Birth weight (g) (total N = 1,852) | 3,508 (453) | 1,765–5,620 | 3,510 (452) | 1,765–5,620 | 3,452 (532) | 651–5,620 |
aPreterm excluded (n = 116).
bPreterm included.
Maternal and infant characteristics were comparable for participants included in PM2.5 and NO2 analyses (Table 1). Birth weight had a symmetric distribution with mean 3,511 g and SD 458 g among participants (n = 1,541) without missing air pollution data (not shown). The mean birth weight was slightly higher for participants included in PM2.5 and NO2 analyses compared with the full cohort due to the exclusion of nonsingleton and preterm birth, but similar among participants in the PM2.5 and NO2 analyses. Other maternal and infant characteristics were similar between participants included and excluded from the analyses, providing assurance that differential exclusion based on data availability introduced little bias.
Air Pollution Concentrations
Table 1 summarizes daily ambient PM2.5 and NO2 concentrations. Mean daily concentrations were 8.8 µg/m3 (SD = 7.6) for PM2.5 and 16.4 ppb (SD = 13.7) for NO2. The distribution of daily concentrations was similar for PM2.5, with or without imputed data, whereas mean concentrations without imputed data were lower, with slightly less variation for NO2 (based on the coefficient of variation, a ratio of SD to mean). This suggests that imputed data provided more daily variation, which could better support the detection of critical exposure periods. Graphical summaries are provided for daily concentrations for T0–T3 (eFigures A7 to A10; http://links.lww.com/EDE/B865).
Critical Exposure Periods
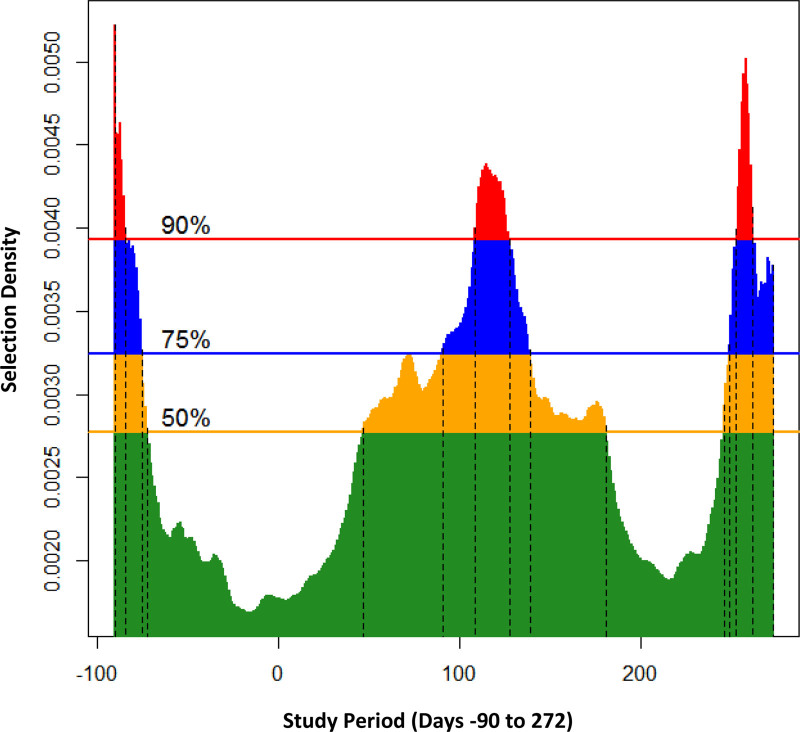
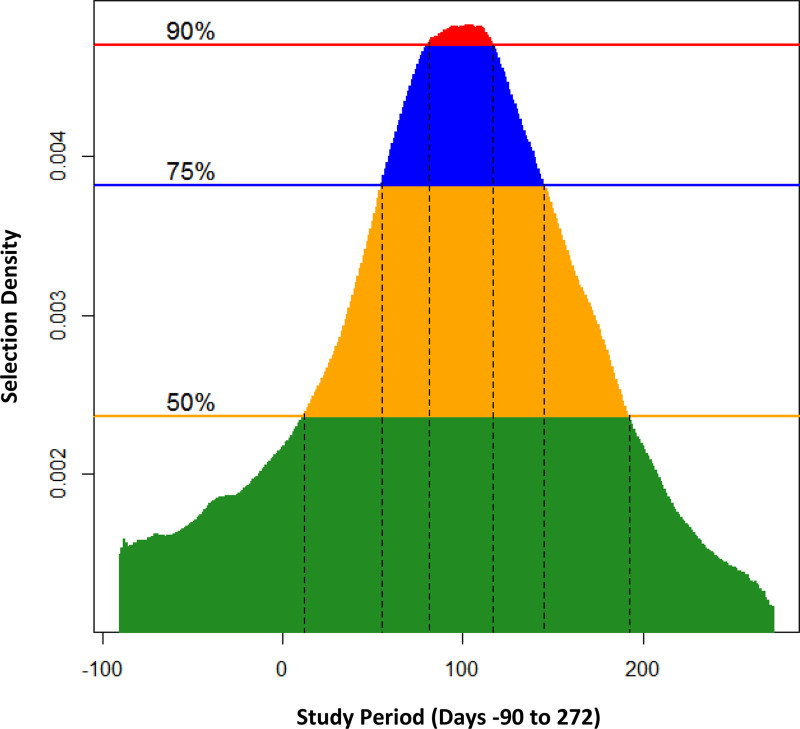
We identified three critical periods for PM2.5 based on the 75th percentile of the selection density (Figure 1) during: pre-pregnancy (days –90 to –75), mid-pregnancy (days 91–139), and late-pregnancy (days 249–272). For NO2, we identified only one critical period during mid-pregnancy (days 55–145) (Figure 2).
FIGURE 1.
Critical periods for PM2.5 (n = 1,334) at selected density levels: (A) 50% (yellow): (–90, –72), (47, 181), and (246, 272); (B) 75% (blue): (–90, –75), (91, 139), and (249, 272); and (C) 90% (red): (–90, –84), (109, 128), and (253, 262). The vertical dotted lines indicate the start-end pair days of critical periods.
FIGURE 2.
Critical periods for NO2 (n = 1,305) at selected density levels: (A) 50% (yellow): (12, 192); (B) 75% (blue): (55, 145); and (C) 90% (red): (81, 117). The vertical dotted lines indicate the start-end pair days of critical periods.
Associations Between Air Pollution and Birth Weight
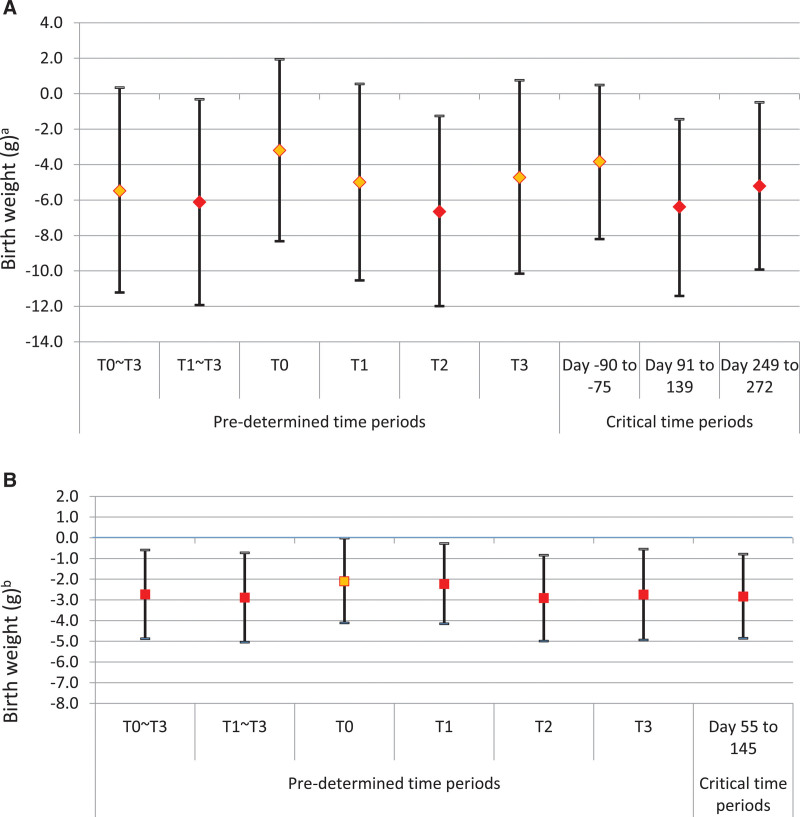
Maternal exposures to air pollution were associated with decreased birth weight (Figure 3A,B; eTables A2 to A3; http://links.lww.com/EDE/B865). PM2.5 was associated with reductions in birth weight of –6 g (–11 g, –1 g) and –5 g (–10 g, –0.1 g) per 1 µg/m3 increase in PM2.5 during mid-pregnancy (days 91–139) and late-pregnancy (days 249–272), respectively. NO2 during mid-pregnancy (days 55–145) was associated with –3 g (–5 g, –1 g) reduction in birth weight per 1 ppb increase in NO2.
FIGURE 3.
A, Associations between PM2.5 and birth weight with 95% credible intervals (per 1 µg/m3 PM2.5): six fixed time intervals (left) and three critical time intervals identified by random selection method (right). Pre-pregnancy (T0); trimester 1 (T1); trimester 2 (T2); trimester 3 (T3); pregnancy (T1–T3); and Pregnancy + Pre-pregnancy (T0–T3). aThe Y-axis indicates the reduced birth weight in gram per 1 unit change in PM2.5. B, Associations between NO2 and birth weight with 95% credible intervals (per 1 ppb NO2): six fixed time intervals (left) and one critical time interval identified by random selection method (right). Pre-pregnancy (T0); Trimester 1 (T1); Trimester 2 (T2); Trimester 3 (T3); Pregnancy (T1–T3); and Pregnancy + Pre-pregnancy (T0–T3). bThe Y-axis indicates the reduced birth weight in gram per 1 unit change in NO2.
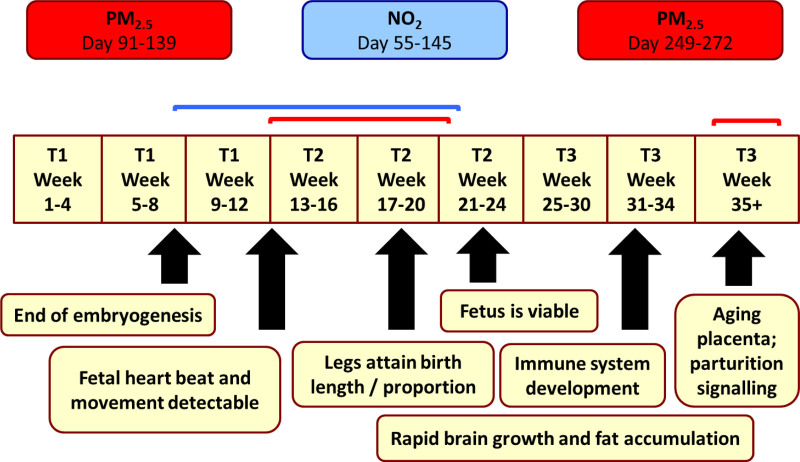
Figure 4 shows a timeline of critical exposure periods for PM2.5 and NO2 by gestational age, with corresponding milestones in fetal development. Associations between the 12 covariates and birth weight are summarized in eTable A4; http://links.lww.com/EDE/B865.
FIGURE 4.
A timeline of critical periods for reduced birth weight associated with PM2.5 and NO2 during pregnancy.
Sensitivity Analysis Results
(1) Selection thresholds: Critical periods identified for PM2.5 were more robust to selection threshold—i.e., 50th or 90th versus 75th percentile—compared with NO2 (Figures 1 and 2, eTables A2 to A3; http://links.lww.com/EDE/B865). The analyses identified several time-varying critical periods for PM2.5, which were similar across selection thresholds, whereas NO2 had just one critical period, which was narrower when we applied more conservative selection thresholds.
(2) Imputed data: Inclusion of imputed data had minimal influence on associations between air pollution and birth weight for both PM2.5 and NO2 (eFigures A11 to A12; http://links.lww.com/EDE/B865). Associations between PM2.5 and birth weight were nearly identical for models including and excluding imputed data. For NO2, associations were similar but slightly weaker in magnitude for models excluding imputed data, because there were 209 fewer participants and less variation in NO2 concentrations when imputed data were excluded.
(3) Randomly selected versus predetermined exposure periods: Estimates of association for PM2.5 and NO2 during critical periods identified in this study were comparable in magnitude with effect estimates for predetermined time periods, specifically, T1–T5 (Figure 6A,B). However, the critical periods identified by the flexible selection method did not align with the fixed trimesters; specifically, the mid-pregnancy critical period for PM2.5 (days 91–139) did not align perfectly with T2 (days 91–174). For NO2, exposures across predetermined time periods were consistently associated with reduced birth weight, while the random selection method identified a single critical period from late-T1 to mid-T2.
(4) Bayesian random selection method versus distributed-lag nonlinear models: For distributed lag models, we defined lag0 as the first day of the study period (day –90) and used Akaike information criterion to choose the best fit. Among the 19 values (df = 2 to 20), df = 4 was optimal for PM2.5 and df = 2 for NO2 (eTable A5; http://links.lww.com/EDE/B865). eFigure A13; http://links.lww.com/EDE/B865 displays the lag distributions along with the critical periods obtained by the Bayesian random selection method. For PM2.5 and NO2, distributed lag nonlinear models and Bayesian methods returned consistent results on the number of inflection points (knots) but at different locations (lags).
(5) Large forward sortation areas: Due to the small number of participants living in large forward sortation areas, the inclusion of large forward sortation areas did not change the critical periods identified in the analyses, or the associations between birth weight and exposure during those critical periods (eTable A6; http://links.lww.com/EDE/B865, eFigures A14 to A15; http://links.lww.com/EDE/B865; http://links.lww.com/EDE/B865).
DISCUSSION
We investigated time-varying vulnerability of birth weight to maternal air pollution exposure using a novel, flexible selection method to identify critical exposure periods. Maternal exposures to both PM2.5 and NO2 during pregnancy were associated with decreased birth weight in the MIREC cohort. PM2.5 displayed stronger time-dependent variation compared with NO2 (Figure 4A,B), with two critical exposure periods during mid-pregnancy and late-pregnancy. NO2 associations varied less by the timing of exposure, with a single critical exposure period during mid-pregnancy.
Time-Dependent Associations Between Air Pollution and Birth Outcomes
Recent reviews and meta-analyses have examined critical exposure periods for air pollution and birth outcomes during pregnancy. However, results varied significantly between studies, possibly due to lack of consistency in methods—particularly exposure estimation, time periods, analytical approach, and model specification—as well as variation between pollutant species, regional PM composition, birth outcomes, and study populations.1,3,6,37,38 Despite differences in methodologies, overall associations and critical periods identified in the current study were generally consistent with previous research.
In a meta-analysis of 32 studies, PM2.5 exposures in T2 and T3 were associated with reduced birth weight and low birth weight,39 which was consistent with the mid- and late-pregnancy critical periods we observed for PM2.5. Other reviews and meta-analyses found that effect estimates relating air pollution in late-pregnancy with low birth weight and preterm birth were more precise than effect estimates for early-, mid- or total-pregnancy.3,7 However, results from individual studies varied.2,7
While most studies focused on trimester-level exposure, a few examined shorter time intervals for PM2.5 and preterm birth. Warren et al.40 used a flexible model to identify critical periods in weeks 4–22. Rappazzo et al.41 found associations in early- and late-pregnancy. Acute PM2.5 exposure in late-pregnancy was also associated with preterm birth.42,43 The literature for NO2 and birth outcomes is more limited, providing little basis for evaluating the mid-pregnancy critical period we observed for NO2.
Mechanisms for Time-dependent Associations
Several mechanisms have been posited to explain air pollution-induced impacts on fetal growth and preterm birth, including oxidative stress, inflammation, blood viscosity/coagulation, hemodynamic responses/endothelial function, and endocrine disruption.1,4,20,21,44–46 Through these mechanisms, PM2.5 and NO2 can induce morphological changes that disrupt the flow of oxygen and nutrients across the placenta, resulting in reduced fetal growth; or triggering preterm birth.1,4,21,42–46 Mechanistic studies of air pollution-induced fetal impacts are limited with respect to timing of exposure. The critical exposure period we observed in late-pregnancy may be associated with decreased birth weight through induction of early delivery due to inflammation and oxidative stress, while the critical periods in early- to mid-pregnancy may be associated with placental disruption.
Pollutant-specific Associations
Critical exposure periods differed between PM2.5 and NO2 in this study. We identified two relatively narrow critical exposure periods for PM2.5 and one wider critical period for NO2. Associations between PM2.5 and birth weight were also slightly greater in magnitude compared with NO2.
The disparate critical periods identified for PM2.5 and NO2 may reflect differences in the chemical properties of the pollutants—for example, their relative oxidative potential and absorption—or different mechanisms of action. We also observed greater temporal variation in PM2.5, but greater spatial heterogeneity in NO2, which could impact their critical exposure periods. Differences in exposure assessment methodologies could also impact identification of critical windows. Previous studies have primarily relied on ground level monitoring data,47 which provides limited information regarding spatial variability, and may be poorly suited to characterizing variation in NO2 exposure. In contrast, we relied on a combination of temporally and spatially resolved air pollution data to estimate daily exposure.
Although methodologic differences limit direct comparison, pollutant-specific results in the current study are generally consistent with results of trimester-based analyses. Previous studies of PM2.5 suggest stronger effects in early- and late-pregnancy, while exposure to NO2 at multiple stages during pregnancy have been linked to adverse birth outcomes.1,20,48 Further, Klepac et al.7 reported that effect estimates for air pollution and preterm birth were more consistent for exposures in T3 for PM2.5, and T2 for NO2.
Sensitivity Analyses
Our findings were robust to alternative thresholds for density selection, imputed exposure data, and the inclusion/exclusion of large forward sortation areas. While our method identified critical exposure windows that did not directly align with predetermined exposure periods, the magnitudes of association estimated by flexible versus fixed exposure periods were comparable for both pollutants. This was expected, because our method is able to examine finer time periods.
We compared critical periods identified using our Bayesian random selection approach with distributed lag nonlinear models. Distributed lag nonlinear models returned comparable time periods for PM2.5 but not NO2 (see eAppendix; http://links.lww.com/EDE/B865 for further discussion). For both pollutants, the flexible selection method identified narrower critical periods, while distributed lag nonlinear models suggested wider time periods smoothly changing over daily lags. Smoother changes in exposure timing may be more realistic than fluctuating changes across pregnancy, but distributed lag nonlinear models are not as flexible as our method. Distributed lag nonlinear models need to specify a smoother, as well as the number and location of internal knots, a priori, which is problematic when no prior information is available. In contrast, our method provides a truly agnostic, flexible approach for identifying critical exposure windows, which is why our method returned narrower time windows than distributed lag nonlinear models.
Limitations
We classified exposure based on residential locations obtained at birth. The lack of detailed residential history during pregnancy could result in potential exposure misclassification; however, the moving rate was low (7.7%) in this study. Also, previous studies which compared exposure classification based on a single location versus detailed residential history reported that absence of residential history had a minimal impact on estimated associations between air pollution and birth outcomes,47–49 even for short (weekly) exposure windows.50 We also linked air pollution estimates to participants using forward sortation areas, that vary in size, which may have introduced exposure misclassification despite our exclusion of participants residing in the largest forward sortation areas.
Our models accounted for detailed sociodemographic and behavioral factors associated with adverse birth outcomes. However, there may be model misspecification, missing or mis-specified confounders, or unmeasured confounding due to historical or cumulative exposures, which could have resulted in unmeasured bias in our estimated associations.
The study population had, on average, higher income and education levels, but lower smoking rates compared with the general population, which may limit the generalizability of our results.
Future Work
We restricted analyses to live, full-term, singleton births, which may have underestimated time-dependent impacts of air pollution on fetal development by excluding the most vulnerable pregnancies. Future analyses are needed to identify critical windows for preterm birth and jointly examine associations between air pollution, preterm birth, and fetal growth, as well as miscarriage and subfertility.
Multipollutant models would help to elucidate potential mechanisms and critical time periods for pollution-mediated decreases in birth weight. Improved accuracy and coverage of daily pollutant concentrations would support identification of critical exposure periods. Replicating these results in a larger, population-based cohort would also be useful. Finally, this approach could be adapted to identify critical periods for childhood diseases such as asthma, autism, and attention-deficit hyperactivity disorder.
CONCLUSIONS
Our results highlight the importance of using flexible selection methods to identify critical exposure windows during gestation, and provide further evidence that exposure to PM2.5 and NO2 during pregnancy is associated with decreased birth weight. Our method identified critical time periods during for PM2.5 and mid-pregnancy for NO2, when maternal exposure to air pollution was associated with decreased birth weight. In contrast with conventional methods, our approach was not limited to predetermined exposure periods such as weeks, months, or trimesters, but rather was able to capture any critical time periods (≥2 days) within the prenatal period. Once replicated, these results could raise awareness of critical time windows for exposure to air pollution and support protective guidelines and interventions for pregnant women.
ACKNOWLEDGMENTS
We would like to thank the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Approval to conduct the research described in this paper was obtained from the Research Ethics Boards of Health Canada and Hospital Sainte Justine, as well as the MIREC biobank.
Supplementary Material
Footnotes
The MIREC Study was funded by Health Canada’s Chemicals Management Plan, the Canadian Institutes of Health Research (grant no. MOP—81285), and the Ontario Ministry of Environment. The analyses reported in the paper were funded by Health Canada’s Canada’s Clean Air Regulatory Agenda
The authors report no conflicts of interest.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
Data are accessible through the MIREC Biobank, with restrictions. https://www.mirec-canada.ca/en/biobank/.
REFERENCES
- 1.Shah PS, Balkhair T; Knowledge Synthesis Group on Determinants of Preterm/LBW births. Air pollution and birth outcomes: a systematic review. Environ Int. 2011;37:498–516. [DOI] [PubMed] [Google Scholar]
- 2.Sapkota A, Chelikowsky AP, Nachman KE, Cohen AJ, Ritz B. Exposure to particulate matter and adverse birth outcomes: a comprehensive review and meta-analysis. Air Qual Atmos Health 2012;5:369–381. [Google Scholar]
- 3.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–111. [DOI] [PubMed] [Google Scholar]
- 4.Feng S, Gao D, Liao F, Zhou F, Wang X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol Environ Saf. 2016;128:67–74. [DOI] [PubMed] [Google Scholar]
- 5.Zhang K, Lu Y, Zhao H, et al. Association between atmospheric particulate matter and adverse pregnancy outcomes in the population. Int J Clin Exp Med. 2016;9:20594–20604. [Google Scholar]
- 6.Li X, Huang S, Jiao A, et al. Association between ambient fine particulate matter and preterm birth or term low birth weight: an updated systematic review and meta-analysis. Environ Pollut. 2017;227:596–605. [DOI] [PubMed] [Google Scholar]
- 7.Klepac P, Locatelli I, Korošec S, Künzli N, Kukec A. Ambient air pollution and pregnancy outcomes: a comprehensive review and identification of environmental public health challenges. Environ Res. 2018;167:144–159. [DOI] [PubMed] [Google Scholar]
- 8.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. [DOI] [PubMed] [Google Scholar]
- 9.World Heath Organization (WHO). The Global Burden of Disease: 2004 Update. 2004. Available at: www.who.int/healthinfo/global_burden_disease/2004_report_update/en/index.html. Accessed July 9, 2020.
- 10.GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioral, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease. Lancet. 2016;388:1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd DR, Genuis SJ. The environmental burden of disease in Canada: respiratory disease, cardiovascular disease, cancer, and congenital affliction. Environ Res. 2008;106:240–249. [DOI] [PubMed] [Google Scholar]
- 12.Bush A. Asthma research: the real action is in children. Paediatr Respir Rev. 2005;6:101–110. [DOI] [PubMed] [Google Scholar]
- 13.Salvi S. Health effects of ambient air pollution in children. Paediatr Respir Rev. 2007;8:275–280. [DOI] [PubMed] [Google Scholar]
- 14.Farmer SA, Nelin TD, Falvo MJ, Wold LE. Ambient and household air pollution: complex triggers of disease. Am J Physiol Heart Circ Physiol. 2014;307:H467–H476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacasaña M, Esplugues A, Ballester F. Exposure to ambient air pollution and prenatal and early childhood health effects. Eur J Epidemiol. 2005;20:183–199. [DOI] [PubMed] [Google Scholar]
- 16.Srám RJ, Binková B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. 2005;113:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett JR. Rocking the cradle. phthalate exposure in NICU infants. Environ Health Perspect. 2005; 113. doi: 10.1289/ehp.113-A614 [Google Scholar]
- 18.Mattison DR. Environmental exposures and development. Curr Opin Pediatr. 2010;22:208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miranda ML, Maxson P, Edwards S. Environmental contributions to disparities in pregnancy outcomes. Epidemiol Rev. 2009;31:67–83. [DOI] [PubMed] [Google Scholar]
- 20.Smarr MM, Vadillo-Ortega F, Castillo-Castrejon M, O’Neill MS. The use of ultrasound measurements in environmental epidemiological studies of air pollution and fetal growth. Curr Opin Pediatr. 2013;25:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slama R, Darrow L, Parker J, et al. Meeting report: atmospheric pollution and human reproduction. Environ Health Perspect. 2008;116:791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson A, Chiu YM, Hsu HL, Wright RO, Wright RJ, Coull BA. Potential for bias when estimating critical windows for air pollution in children’s health. Am J Epidemiol. 2017;186:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gasparrini A. Modeling exposure-lag-response associations with distributed lag non-linear models. Stat Med. 2014;33:881–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasparrini A. Distributed lag linear and non-linear models in R: the package dlnm. J Stat Softw. 2011;43:1–20. [PMC free article] [PubMed] [Google Scholar]
- 25.Rushworth A. Bayesian Distributed Lag Models. 2018. arXiv preprint arXiv:1801.06670. Available at: https://alastairrushworth.github.io/papers/bayesian_dlms.pdf. Accessed January 18, 2021.
- 26.Roberts EM, English PB. Bayesian modeling of time-dependent vulnerability to environmental hazards: an example using autism and pesticide data. Stat Med. 2013;32:2308–2319. [DOI] [PubMed] [Google Scholar]
- 27.Arbuckle TE, Fraser WD, Fisher M, et al. Cohort profile: the maternal-infant research on environmental chemicals research platform. Paediatr Perinat Epidemiol. 2013;27:415–425. [DOI] [PubMed] [Google Scholar]
- 28.Hystad P, Setton E, Cervantes A, et al. Creating national air pollution models for population exposure assessment in Canada. Environ Health Perspect. 2011;119:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Donkelaar A, Martin RV, Li C, Burnett RT. Regional estimates of chemical composition of fine particulate matter using a combined geoscience-statistical method with information from satellites, models, and monitors. Environ Sci Technol. 2019;53:2595–2611. [DOI] [PubMed] [Google Scholar]
- 30.Johnson M, Macneill M, Grgicak-Mannion A, et al. Development of temporally refined land-use regression models predicting daily household-level air pollution in a panel study of lung function among asthmatic children. J Expo Sci Environ Epidemiol. 2013;23:259–267. [DOI] [PubMed] [Google Scholar]
- 31.Thomson DJ. Spectrum estimation and harmonic analysis. Proc IEEE. 1982;70:1055–1096. [Google Scholar]
- 32.Rahim KJ, Burr WS, Thomson DJ. Appendix A: Multitaper R Package in “Applications of Multitaper Spectral Analysis to Nonstationary Data”, PhD diss., 2014. Queen’s University. pp. 149–183. Available at: http://hdl.handle.net/1974/12584. Accessed January 18, 2021. [Google Scholar]
- 33.R Core Team. R: A Language and Environment for Statistical Computing. 2019. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/. Accessed October 12, 2021. [Google Scholar]
- 34.SAS Institute Inc. SAS® 9.4 Statements: Reference. Cary, NC: SAS Institute Inc; 2013. [Google Scholar]
- 35.Gelman A. Parameterization and bayesian modeling. J Am Stat Assoc. 2004;99:537–545. [Google Scholar]
- 36.Statistics Canada. Canadian Vital Statistics, Births Database, 2001 to 2016, Survey 3231 and Demography Division, Demographic Estimates Program, Figure 6. 2018. Available at: https://www150.statcan.gc.ca/n1/pub/91-209-x/2018001/article/54956-eng.htm. Accessed October 12, 2021. [Google Scholar]
- 37.Nieuwenhuijsen MJ, Dadvand P, Grellier J, Martinez D, Vrijheid M. Environmental risk factors of pregnancy outcomes: a summary of recent meta-analyses of epidemiological studies. Environ Health. 2013;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavigne É, Burnett RT, Stieb DM, et al. Fine particulate air pollution and adverse birth outcomes: effect modification by regional nonvolatile oxidative potential. Environ Health Perspect. 2018;126:077012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun X, Luo X, Zhao C, et al. The associations between birth weight and exposure to fine particulate matter (PM2.5) and its chemical constituents during pregnancy: a meta-analysis. Environ Pollut. 2016;211:38–47. [DOI] [PubMed] [Google Scholar]
- 40.Warren J, Fuentes M, Herring A, Langlois P. Spatial-temporal modeling of the association between air pollution exposure and preterm birth: identifying critical windows of exposure. Biometrics. 2012;68:1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rappazzo KM, Daniels JL, Messer LC, Poole C, Lobdell DT. Exposure to fine particulate matter during pregnancy and risk of preterm birth among women in New Jersey, Ohio, and Pennsylvania, 2000-2005. Environ Health Perspect. 2014;122:992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu WY, Yu ZB, Qiu HY, Wang JB, Chen XY, Chen K. Association between ambient air pollutants and preterm birth in Ningbo, China: a time-series study. BMC Pediatr. 2018;18:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guan T, Xue T, Gao S, et al. Acute and chronic effects of ambient fine particulate matter on preterm births in Beijing, China: a time-series model. Sci Total Environ. 2019;650(pt 2):1671–1677. [DOI] [PubMed] [Google Scholar]
- 44.Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect. 2006;114:1636–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Backes CH, Nelin T, Gorr MW, Wold LE. Early life exposure to air pollution: how bad is it? Toxicol Lett. 2013;216:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stieb DM, Lavigne E, Chen L, Pinault L, Gasparrini A, Tjepkema M. Air pollution in the week prior to delivery and preterm birth in 24 Canadian cities: a time to event analysis. Environ Health. 2019;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pereira G, Bracken MB, Bell ML. Particulate air pollution, fetal growth and gestational length: the influence of residential mobility in pregnancy. Environ Res. 2016;147:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng T, Zhang J, Sommer K, et al. Effects of environmental exposures on fetal and childhood growth trajectories. Ann Glob Health. 2016;82:41–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen L, Bell EM, Caton AR, Druschel CM, Lin S. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environ Res. 2010;110:162–168. [DOI] [PubMed] [Google Scholar]
- 50.Warren JL, Son JY, Pereira G, Leaderer BP, Bell ML. Investigating the impact of maternal residential mobility on identifying critical windows of susceptibility to ambient air pollution during pregnancy. Am J Epidemiol. 2018;187:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.