Abstract
Purpose of review
The aim of this review is to evaluate biological, life history, environmental, and lifestyle factors and exposures that cause variability in menstrual cycle length (MCL).
Recent findings
Recent literature has detailed a number of factors that influence MCL, with particular emphasis placed on novel environmental exposures, such as air pollution and endocrine disrupting chemicals.
Summary
MCL varies widely in response to intrinsic and extrinsic inputs and is a useful predictor of reproductive health and fecundability.
Video abstract
Keywords: endocrine disrupting chemicals, environmental exposures, fecundability, female reproductive health, menstrual cycle length
INTRODUCTION
Menstrual cycle length (MCL) is a relevant indicator of cycle regularity and reproductive health [1]. MCL is sensitive to inputs from the environment and varies within and between individuals [2], though a length between 24 and 38 days is considered normal [3]. Several factors cause MCL variability, including ovarian biomarkers [4] and lifestyle-based exposures [5]. Environmental exposures, such as air pollutants and endocrine disrupting chemicals (EDCs) pose a unique threat, as many are ubiquitous, persistent, and detrimental to reproductive health and MCL [6,7]. This review aims to discuss recent literature evaluating MCL variation in response to common and novel variables in modern life. We will also discuss MCL as an indicator or predictor of outcomes, such as endometriosis, polycystic ovary syndrome (PCOS), age at menopause, and fecundability.
Box 1.
no caption available
METHODS
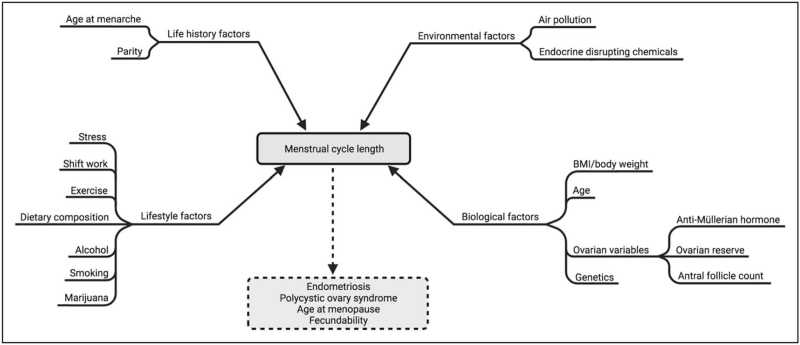
A literature search was conducted using PubMed to identify studies evaluating MCL and a number of biological, life history, environmental, and lifestyle exposures (shown as inputs in Fig. 1). Factors were identified using clinical and professional knowledge of prior literature. Novel exposures included air pollution and EDCs. Outputs (Fig. 1) were not included as search terms.
FIGURE 1.
Menstrual cycle length inputs and outputs. Several biological, environmental, lifestyle, and life history inputs (solid lines) that are known to affect MCL were used as search terms. Output conditions (dotted lines), of which MCL was considered an indicator or predictor, were not included as search terms. Created with BioRender.com. MCL, menstrual cycle length.
Search strategies are reported in Supplemental Table 1 (see Table, Supplemental Digital Content 2, which lists queries used in the literature search). Searches used the terms ‘menstrual cycle length’ and ‘cycle length’ with one of the input variables in Fig. 1, using MeSH and non-MeSH terms. Search strategies excluded articles discussing MCL in populations with polycystic ovarian syndrome (PCOS). A limited number of relevant studies were published between 2020 and 2021, so inclusion criteria were expanded to include those with a publication date from 2016 to 2021. Most searches excluded studies not performed with human participants, though five necessitated the removal of this filter because of erroneous exclusion of relevant articles.
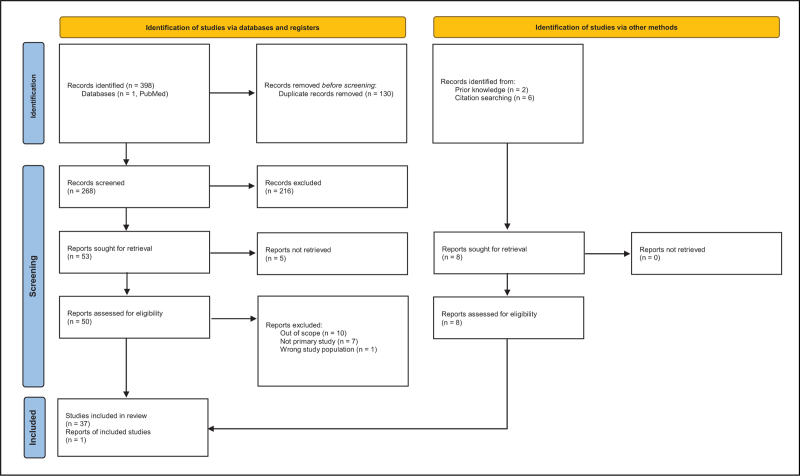
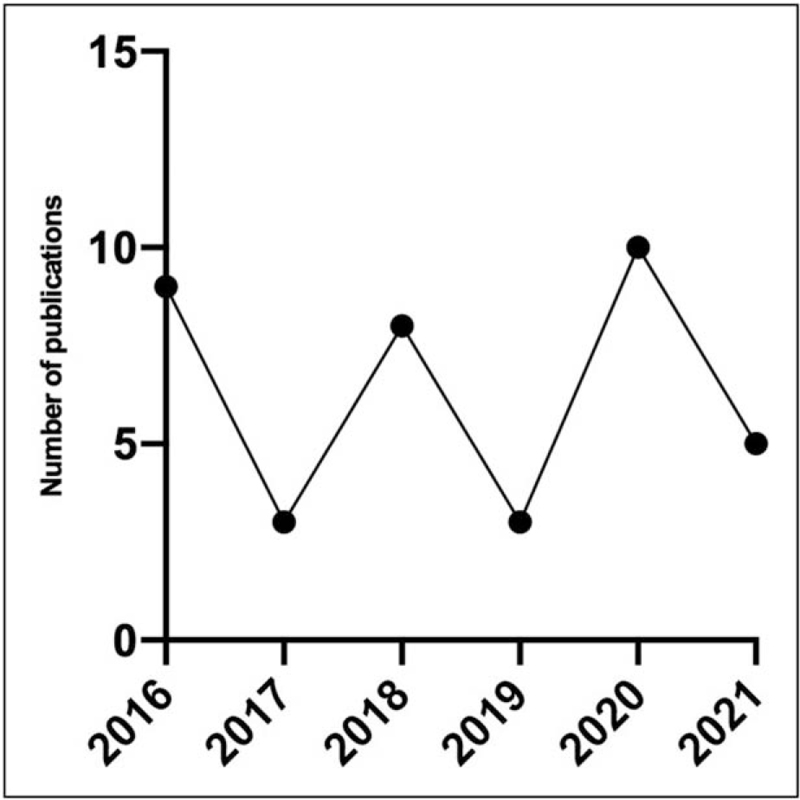
The searches yielded 268 results after duplicates were removed. Six additional articles were identified via citation searching and two were identified from knowledge of existing literature. Articles were excluded if they were not in English, were not primary studies, or were conducted in individuals with a reproductive disorder. Articles were excluded if they did not evaluate the association between MCL and variables included in the search strategies, or if they were not conducted using human participants. One article using a nonhuman primate model to study the effect of marijuana use on MCL was included because of its relevance to the scope of the review and the lack of literature in humans. The screening process (Fig. 2) yielded a total of 38 relevant studies. The distribution of relevant studies by publication date is shown in Fig. 3.
FIGURE 2.
PRISMA flow diagram describing the selection of articles included in this review. Data from [8]. For more information, visit http://www.prisma-statement.org/.
FIGURE 3.
Number of included articles published between 2016 and 2021. The number of relevant articles yielded by a PubMed literature search for associations between MCL and a number of variables and exposures from 2016 to 2021. MCL, menstrual cycle length.
REVIEW
Intrinsic factors affecting MCL are presented first and include the following biological and life history factors of age, BMI and body weight, ovarian variables, genetics, age at menarche, and parity and breastfeeding. Extrinsic factors consist of the following environmental and lifestyle exposures: air pollution, EDCs, shift work, exercise, alcohol intake, smoking, and marijuana use.
Age
The way in which MCL and cycle length variability changes across the reproductive lifespan is well documented. Although most studies demonstrate a decrease in MCL during an individual's 30s and 40s [4,9,10,11▪,12▪], MCL increases as reproductive senescence approaches and shifts in an age-dependent manner [12▪]. Follicular phase length also decreases with increasing age [9,11▪], though results are inconsistent regarding whether luteal phase length changes appreciably. Others report that MCL variability changes with age. Notably, variation of 1.5–4.5 days is more common in individuals over 35 years [13▪▪] and mean MCL variation is highest (3.1 days) at age 45 years [9]. Previously reported findings indicate that age-related decreases in MCL are because of earlier dominant follicle selection and folliculogenesis [14], whereas irregular cycles in older individuals can be attributed to variability in the occurrence and timing of ovulation as they near the menopausal transition [2].
BMI and body weight
Grieger and Norman [13▪▪] found that longer MCLs (≥36 days) were more frequently associated with a BMI of 35–50 kg/m2, though a large difference was only seen in individuals with a BMI greater than 50 kg/m2. Though not all participants reported BMI, the proportion of overweight or obese individuals within the study population was similar to the general public. Others have found similar associations between higher body fat percentages (38.7–53.5%) and cycle lengths greater than 32 days [relative risk ratio (RRR) = 2.63; 95% confidence interval (CI) 1.21–5.69] [15▪]. Over 70% of individuals experiencing longer MCLs had abdominal obesity in this study. Thus, the authors suggest that adipose tissue's endocrine functions play a role in modulating or altering hypothalamic–pituitary–ovarian signaling, leading to altered menstrual cycles [15▪].
However, results on this topic are inconsistent. Bull et al.[9] found no association between BMI and MCL, though they were limited by the exclusion of nonovulatory cycles and a population not reflective of global obesity rates. Similar results were reported in a prospective cross-sectional study of healthy women [mean BMI = 22.4 kg/m2, standard deviation (SD) = 4] [4]. In other studies, a shorter mean MCL is associated with higher BMI [10]. Tayebi et al.[16] found a significant association between BMI and MCL (P = 0.006) in school-aged students (ages 9–18) but students with a BMI more than 30 were underrepresented in the sample. Inconsistent associations between BMI and MCL variability (the definition of which varies by study) have also been reported. Higher BMI is associated with increased MCL variability and irregularity (despite not being associated with short or long MCL) in two studies [9,17▪] and decreased MCL variability in another [13▪▪]. Roman Lay et al.[15▪] propose that hormonal markers, such as sex hormone-binding globulin (SHBG), estrone (E1), and insulin may be partially mediating the interaction between measurements of weight and MCL. The underlying inconsistencies are likely because of cohort characteristics, study population, the assumption of ovulatory cycles for all episodes of bleeding, and varying definitions of MCL variability and irregularity.
Ovarian variables
Ovarian characteristics, such as anti-Müllerian hormone (AMH), ovarian volume, and antral follicle count (AFC) are associated with MCL. AMH, in particular, is the primary ovarian predictor of MCL and has a strong positive correlation with cycle length [4,18▪,19]. Zhu et al.[4] propose that AMH elongates follicular phase lengths by suppressing FSH-stimulated estradiol production from the antral follicles during folliculogenesis. A trend of increasing MCL was also seen with diminishing AMH, which is inversely correlated with increasing age [20]. Ovarian reserve and AFC are also both associated with AMH and MCL, though not to the same degree as AMH alone with MCL [4]. This association is likely because of the secretion of AMH by antral follicles and a proportional relationship between ovarian reserve and AMH.
Genetics
Three studies evaluate the genetic determinants of MCL. One reported that a polymorphism in the FSHB promoter (rs10835638; c.-211G>T) lowers follicle-stimulating hormone (FSH) levels and is associated with longer MCL [21]. As a threshold of FSH must be met for follicular recruitment, establishment of the dominant follicle, and ovulation to occur, the authors propose an association between the genetic determinants of FSH levels, ovulation, and parity, such that decreased FSH levels in individuals with the polymorphism undergo ovulation less frequently, and therefore, have lower fecundability [21]. A genome-wide association study subsequently confirmed the association between the FSHB locus and MCL and highlighted four other loci of importance: NR5A2, DOCK5/GNRH1, IGF2, AND PGR[22]. These loci are involved in steroidogenesis, FSH/luteinizing hormone (LH) release, folliculogenesis, and progesterone signaling, respectively. Although a third study found the FSHB promoter polymorphism to be associated with significantly higher serum concentrations of FSH and LH, no significant association was found between the polymorphism and MCL [23]. However, the authors note their study was underpowered to assess this association.
Age at menarche
Whitcomb et al.[19] demonstrated that cycle lengths in individuals aged 18–22 years increased with later age at menarche. Conversely, data from a preconception cohort indicates that MCL is longer in individuals who reach menarche at a younger age [24]. Others have found no appreciable association between the two [4]. Conflicting results may be because of differences in study populations (Nurses’ Health Study II [19] vs. preconception pregnancy planners [24] vs. healthy individuals recruited from a single site [4]), age at which MCL was evaluated (18–22 [19] vs. 21–45 years [4,24], and potential misclassification of MCL and age at menarche because of reliance upon patient self-report.
Parity and breastfeeding
Parity may be related to shorter MCL [11▪,24], though this association is sometimes weak [19] or nonexistent [4]. Additionally, breastfeeding appears to affect MCL. Najmabadi and colleagues [11▪] report that individuals experienced shorter mean MCL (29.6 vs. 31.0 days), shorter follicular phases (18.5 vs. 19.1 days), and shorter luteal phases (11.0 vs. 11.7 days) when partially breastfeeding. Models used were stratified by age and parity but this study was limited by homogenous cohorts and lacked data regarding metabolic variables and lifestyle behaviors. Conversely, short MCL is associated with a shorter duration of breastfeeding in a model stratified by age [19].
Air pollution
Mahalingaiah et al.[7] found that individuals exposed to total suspended particulate in air have slightly increased odds of cycle irregularity and increased time to cycle regularity after menarche. Furthermore, sulfur dioxide and particulate matter smaller than 10 μmol/l (PM10) are associated with decreased luteal phase length [25]. In a separate study, levels of nitrogen dioxide (NO2) and particulate matter smaller than 2. 5 μmol/l are associated with increased follicular phase length, though neither NO2 nor PM10 are associated with increased luteal phase length [26▪]. These results indicate that exposure to particulates released by fuel combustion may alter HPO signaling via endocrine disruption, thereby affecting MCL, possibly through lengthened follicular phases or luteal phase deficiency [6].
Endocrine disrupting chemicals
Studies have reported concerning associations between EDC exposure and MCL. Notably, many are limited by small sample size. Three prospective cohort studies reported variability in MCL following exposure to perfluoroalkyl substances (PFAS) [10,27,28]. Though Singer et al.[27] found no association between PFAS concentrations and MCL, subgroup analyses linked decreased perfluoroheptane sulfonate and perfluorooctane sulfonate (PFOS) levels to short MCL in parous individuals, and increased perfluorononanoic acid (PFNA) and perfluoroundecanoic acid levels to long MCL in individuals who had used oral contraceptives in the previous year. Interestingly, higher perfluorooctanoic acid (PFOA) concentrations are associated with decreased MCL in one study [10] but increased levels of PFOA, PFNA, perfluorohexane sulfonate, and PFOS are associated with MCL more than 35 days in another [28].
The impact of organohalogen exposure on MCL has likewise been of interest. A prospective cohort study showed no significant association between prenatal exposure to persistent organochlorine pollutants or polychlorinated biphenyls and MCL but did find that other aspects of reproductive health were impacted [29]. Recently, a study demonstrated that long and irregular cycles were common in Latinx child and adolescent farmworkers exposed to pesticides, including pyrethroids, organochlorines, and organophosphates [30▪]. The study detected pesticide exposure using wristbands but the results are limited as wristbands were worn for 1 day. Conversely, increasing concentrations of persistent organohalogens and elements, particularly polybrominated diphenyl ethers, cadmium, and selenium, are associated with decreasing MCL, whereas increased MCL is associated with higher concentrations of copper [31].
Remaining studies evaluate MCL following exposure to several other EDCs. A prospective cohort study reported increasing average MCL with increased exposure to polybrominated biphenyls (PBBs), though this was not statistically significant [32]. Moreover, a study linked shorter MCL and higher urinary concentrations of parabens, which are used as preservatives in personal care products and demonstrate estrogenic activity [33]. A similar association was reported between shorter luteal phases and higher urinary concentrations of phthalates and bisphenol A in a prospective cohort study, though no associations were found with follicular phase length [34]. Conversely, in a cross-sectional study assessing exposure to n-hexane, a volatile organic compound, 79% of exposed individuals demonstrated MCL more than 35 days vs. 20% in the control group (P = 0.007) [35▪]. Finally, a prospective cohort study evaluated the effect of dietary phytoestrogens and found that, though phytoestrogens were not associated with MCL, they may be associated with cycle regularity [36].
Stress
Whether a relationship exists between stress and MCL remains unclear, likely in part because studies rely on self-reports of perceived stress levels. Of note, physical stress from exercise or caloric restriction is not included in this conceptualization of stress. Nonetheless, among those that are still menstruating and not affected by stress-induced hypothalamic amenorrea, increased perceived stress (noted on a questionnaire) is associated with shorter MCL [13▪▪] and increased MCL irregularity [37]. The latter result is supported by Phelan et al.[38▪], who assessed how stress associated with the COVID-19 pandemic impacted menstrual characteristics. Cycle length, however, was not found to change significantly before and during the pandemic [38▪], and a separate study similarly reported no significant relationship between MCL and perceived stress [4].
Shift work
Although one study found no significant association between average hours of sleep, shift work, and MCL [4], others indicate that MCL is impacted by disrupted circadian rhythm [5,39,40▪]. The frequency of night shifts is associated with shortened MCL and shift work schedules are associated with increased likelihood of cycle irregularity in a study containing both cross-sectional and nested case–control components [39]. Particularly concerning is that these changes had not recovered 2 years later [39]. Another cross-sectional study similarly found that rotating shifts were associated with MCL irregularity [40▪]. Sleeping for fewer than 6 h per night is also significantly associated with short MCL (OR = 3.7; 95% CI 1.1–12.7) and nonsignificantly associated with long MCL (OR = 1.7, 95% CI 0.8–3.7) in a prospective cross-sectional study, leading the authors to suggest a causal association between insufficient sleep and metabolic abnormalities [5].
Exercise
Three studies evaluated MCL and exercise frequency but did not differentiate between types of exercise. Of these, two found that exercise frequency did not significantly change MCL [4,5]. The third showed that individuals with short cycles were more likely to report no regular exercise than those with normal or long MCL [13▪▪]. One prospective cohort study utilized the frequency of different exercise types to assess metabolic expenditure, concluding that individuals with shorter cycles had higher metabolic equivalent task-hours per week than individuals with MCLs between 26 and 31 days [19].
Dietary composition
Two studies evaluated the influence of diet on MCL within the review period. A cross-sectional study found that individuals with a low adherence to a Mediterranean diet had longer MCL than those whose regular diet more closely resembled a Mediterranean diet (P < 0.01) [41▪]. A second study reported moderate differences in MCL associated with dietary factors, including dietary percentage of vegetable protein, vitamin D, energy, and dairy [19].
Alcohol
Most studies show no association [4,13▪▪] or a weak association [19] between MCL and alcohol consumption. One cross-sectional study found a positive correlation between the daily quantity of alcohol consumed and MCL in individuals aged 18–35 (r = 0.119, P = 0.038) [41▪]. Inconsistencies in results may be because of differences in how alcohol intake is assessed (e.g. intake frequency [4,13▪▪] vs. quantity consumed per day [19,41▪]), sample recruitment (e.g. single site [4,41▪] vs. national [19] vs. global [13▪▪]), reliance upon self-report for MCL and alcohol consumption, and whether participants using oral contraceptives were included [41▪] or not [4,13▪▪,19].
Smoking
Two recent studies found no association between MCL and smoking status measured by active smoking (yes/no) [4] and smoking regularity (regularly/sometimes/do not smoke) [13▪▪]. Conversely, three studies report that smoking, including pack-years [20], and active smoking status [10,17▪], was associated with shorter MCL (<25 days). Again, variation in how smoking status was assessed (smoking regularity [13▪▪], number of cigarettes per day [17▪,19], active smoking [4], and blood concentration of nicotine biomarkers [10]) may explain the contrasting results.
Marijuana
One study evaluated how marijuana use affects MCL in humans, and a second study using a nonhuman primate model was identified through outside knowledge. A randomized controlled trial found individuals co-using marijuana and tobacco experience a significantly shorter luteal phase [11.4 days ± 2.2 (SD)] than participants who only use tobacco [16.8 days ± 11.3 (SD); P = 0.002] [42]. However, the authors reported no differences in follicular phase length or overall MCL, and conclusions were limited by lack of information regarding the frequency and quantity of marijuana used and combined use of tobacco and marijuana. Conversely, average MCL increased in a dose-dependent manner (4 days for each mg/7 kg/day of tetrahydrocannabinol (THC)) (95% CI 1.4–6.6 days; P = .002) in rhesus macaques given chronic, heavy doses of THC edibles [43▪]. Though only one blood sample was taken at each dose increase, the frequency and quantity of THC ingested were closely controlled.
DISCUSSION
These studies are evidence of the extent to which MCL is sensitive to internal biological factors and external exposures. The purpose of this review is to provide an update to factors, which have been the subject of publications in recent years, including novel exposures, such as air pollution and EDCs. Literature published prior to the review period has documented MCL varying in response to factors not discussed here, such as caffeine intake [44], oral contraceptive use and cessation [45], miscarriages [45], and race and ethnicity [46]. Studies identified within this review serve to further document the response of MCL to everyday life. Of particular importance are those which evaluate the association between MCL and novel environmental and lifestyle exposures. It is likely that, as individuals experience increased cumulative exposures to air pollution and EDCs and increased availability and accessibility of marijuana, MCL disturbances may become more common.
Furthermore, MCL serves as an indicator of general and reproductive health. MCL has previously been associated with differences in reported menstrual cycle symptom patterns [47], postmenopausal fracture risk [48], and mental illness [49]. In the context of reproductive health, MCL is an indicator of cumulative hormone exposure [50] and a predictor of age at menopause [19]. Indeed, individuals who experience cycle lengths less than 25 days at ages 18–22 years are at a higher risk of early menopause, suggesting accelerated oocyte depletion as the cause [19]. MCL can also be assessed as both a risk factor and a symptom of gynecologic disease. For example, short MCL increases an individual's risk for endometriosis because of more frequent exposure to retrograde menstruation [51]. MCL is also associated with PCOS, such that longer MCLs are considered a symptom of the disorder [52] and the ovarian variables that are significantly associated with MCL are strongly linked to the pathophysiology of PCOS [4].
One of the most impactful applications of the presented information pertains to fecundability. Intervals of 27–29 [24], 30–31 [53], and 32–33 days [54] have the highest fecundability among MCLs within the normal range within a population of women of childbearing age or those attempting conception. Moreover, MCL is strongly correlated with successful in-vitro fertilization treatments [55]. These findings highlight the value of using MCL as a predictor of fecundability in a clinical context.
Notably, there are cautions to be taken when assessing studies on MCL, many of which rely on self-reporting to determine menstrual characteristics. Moreover, comparing data between studies is complicated by inconsistent definitions of normal, short, or long MCLs (e.g. long MCL being ≥32 days [15▪] vs. >45 days [30▪]) and MCL variability. Researchers have previously proposed that some factors affect MCL more dramatically in individuals predisposed to shorter or longer cycles, therefore, complicating the ability to draw broad conclusions about how a given factor affects an entire population [56].
CONCLUSION
This review demonstrates how MCL varies in response to a number of biological, life history, environmental, and lifestyle factors in a way that impacts reproductive health. With the rise in cycle tracking apps, it is likely that population-level data will become available as large datasets that are used to obtain broader MCL patterns. Further research will elucidate the physiological mechanisms underlying these changes and further our understanding of topics that are new (like the genetic determinants of MCL) or underrepresented in research (like the effect of marijuana on reproductive health).
Acknowledgements
We would like to thank Dr. James F. A. Traniello (Boston University) for his mentorship and guidance.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.ACOG Committee Opinion No. 651. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol 2015; 126:e143–e146. [DOI] [PubMed] [Google Scholar]
- 2.Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. J Obstet Gynecol Neonatal Nurs 2006; 35:376–384. [DOI] [PubMed] [Google Scholar]
- 3.Fraser IS, Critchley HO, Munro MG, Broder M. Can we achieve international agreement on terminologies and definitions used to describe abnormalities of menstrual bleeding? Hum Reprod 2007; 22:635–643. [DOI] [PubMed] [Google Scholar]
- 4.Zhu R, Lee BH, Huang Z, et al. Antimüllerian hormone, antral follicle count and ovarian volume predict menstrual cycle length in healthy women. Clin Endocrinol (Oxf) 2016; 84:870–877. [DOI] [PubMed] [Google Scholar]
- 5.Lim AJ, Huang Z, Chua SE, et al. Sleep duration, exercise, shift work and polycystic ovarian syndrome-related outcomes in a healthy population: a cross-sectional study. PLoS One 2016; 11:e0167048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammer KC, Veiga A, Mahalingaiah S. Environmental toxicant exposure and menstrual cycle length. Curr Opin Endocrinol Diabetes Obes 2020; 27:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahalingaiah S, Missmer SE, Cheng JJ, et al. Perimenarchal air pollution exposure and menstrual disorders. Hum Reprod 2018; 33:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bull JR, Rowland SP, Scherwitzl EB, et al. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digit Med 2019; 2:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lum KJ, Sundaram R, Barr DB, et al. Perfluoroalkyl chemicals, menstrual cycle length, and fecundity: findings from a prospective pregnancy study. Epidemiology 2017; 28:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11▪.Najmabadi S, Schliep KC, Simonsen SE, et al. Menstrual bleeding, cycle length, and follicular and luteal phase lengths in women without known subfertility: a pooled analysis of three cohorts. Paediatr Perinat Epidemiol 2020; 34:318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights within-woman variability in the phases of the menstrual cycle, as well as the impact of age and parity on menstrual cycle length.
- 12▪.Tatsumi T, Sampei M, Saito K, et al. Age-dependent and seasonal changes in menstrual cycle length and body temperature based on big data. Obstet Gynecol 2020; 136:666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study analyzes data from six million menstrual cycles entered into a reproductive health app by women living in Japan. Results support previously established relationships between age and menstrual cycle length, though no association was found between cycle length and seasonality.
- 13▪▪.Grieger JA, Norman RJ. Menstrual cycle length and patterns in a global cohort of women using a mobile phone app: retrospective cohort study. J Med Internet Res 2020; 22:e17109. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study utilizes data from 1.5 million menstrual tracking app users to characterize global patterns in menstrual cycle length. Age, BMI, stress, and exercise were found to impact cycle length and variability, whereas alcohol consumption and smoking status were not.
- 14.Klein NA, Harper AJ, Houmard BS, et al. Is the short follicular phase in older women secondary to advanced or accelerated dominant follicle development? J Clin Endocrinol Metab 2002; 87:5746–5750. [DOI] [PubMed] [Google Scholar]
- 15▪.Roman Lay AA, Pereira A, Garmendia Miguel ML. Association between obesity with pattern and length of menstrual cycle: the role of metabolic and hormonal markers. Eur J Obstet Gynecol Reprod Biol 2021; 260:225–231. [DOI] [PubMed] [Google Scholar]; This study demonstrates an association between high body fat percentile and longer menstrual cycle lengths. The authors further propose that a mechanism involving sex hormone-binding globulin, estrone, and insulin partially underlies this relationship.
- 16.Tayebi N, Yazdanpanahi Z, Yektatalab S, et al. The relationship between body mass index (BMI) and menstrual disorders at different ages of menarche and sex hormones. J Natl Med Assoc 2018; 110:440–447. [DOI] [PubMed] [Google Scholar]
- 17▪.Wang YX, Shan Z, Arvizu M, et al. Associations of menstrual cycle characteristics across the reproductive life span and lifestyle factors with risk of type 2 diabetes. JAMA Netw Open 2020; 3:e2027928. [DOI] [PMC free article] [PubMed] [Google Scholar]; Though the primary focus of this study is the association between menstrual cycle characteristics and risk of type 2 diabetes, it found associations between cycle length irregularity and high BMI, as well as short cycle length and active smoking status.
- 18▪.Harris BS, Steiner AZ, Jukic AM. Ovarian reserve biomarkers and menstrual cycle length in a prospective cohort study. J Clin Endocrinol Metab 2021; 106:e3748–e3759. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights the role of anti-Müllerian hormone as the primary ovarian predictor of menstrual cycle length.
- 19.Whitcomb BW, Purdue-Smithe A, Hankinson SE, et al. Menstrual cycle characteristics in adolescence and early adulthood are associated with risk of early natural menopause. J Clin Endocrinol Metab 2018; 103:3909–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan HL, Bhatti S, Suhail S, et al. Antral follicle count (AFC) and serum anti-Müllerian hormone (AMH) are the predictors of natural fecundability have similar trends irrespective of fertility status and menstrual characteristics among fertile and infertile women below the age of 40 years. Reprod Biol Endocrinol 2019; 17:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruth KS, Beaumont RN, Tyrrell J, et al. Genetic evidence that lower circulating FSH levels lengthen menstrual cycle, increase age at menopause and impact female reproductive health. Hum Reprod 2016; 31:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laisk T, Kukuškina V, Palmer D, et al. Large-scale meta-analysis highlights the hypothalamic-pituitary-gonadal axis in the genetic regulation of menstrual cycle length. Hum Mol Genet 2018; 27:4323–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rull K, Grigorova M, Ehrenberg A, et al. FSHB -211 G>T is a major genetic modulator of reproductive physiology and health in childbearing age women. Hum Reprod 2018; 33:954–966. [DOI] [PubMed] [Google Scholar]
- 24.Wesselink AK, Wise LA, Hatch EE, et al. Menstrual cycle characteristics and fecundability in a North American preconception cohort. Ann Epidemiol 2016; 26:482–487.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merklinger-Gruchala A, Jasienska G, Kapiszewska M. Effect of air pollution on menstrual cycle length-a prognostic factor of women's reproductive health. Int J Environ Res Public Health 2017; 14:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26▪.Giorgis-Allemand L, Thalabard JC, Rosetta L, et al. Can atmospheric pollutants influence menstrual cycle function? Environ Pollut 2020; 257:113605. [DOI] [PubMed] [Google Scholar]; This article evaluates the impact of atmospheric pollutants on MCL, notably demonstrating increased follicular phase length with exposure to combustion particulates.
- 27.Singer AB, Whitworth KW, Haug LS, et al. Menstrual cycle characteristics as determinants of plasma concentrations of perfluoroalkyl substances (PFASs) in the Norwegian Mother and Child Cohort (MoBa study). Environ Res 2018; 166:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou W, Zhang L, Tong C, et al. Plasma perfluoroalkyl and polyfluoroalkyl substances concentration and menstrual cycle characteristics in preconception women. Environ Health Perspect 2017; 125:067012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kristensen SL, Ramlau-Hansen CH, Ernst E, et al. Prenatal exposure to persistent organochlorine pollutants and female reproductive function in young adulthood. Environ Int 2016; 92-93:366–372. [DOI] [PubMed] [Google Scholar]
- 30▪.Varnell RR, Arnold TJ, Quandt SA, et al. Menstrual cycle patterns and irregularities in hired Latinx child farmworkers. J Occup Environ Med 2021; 63:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study analyzes the effect of pesticide exposure on the menstrual cycle in Latinx child and adolescent farmworkers and found increased cycle irregularities in those predominantly exposed to pyrethroids, organochlorines, and organophosphates.
- 31.Wainman BC, Kesner JS, Martin ID, et al. Menstrual cycle perturbation by organohalogens and elements in the Cree of James Bay, Canada. Chemosphere 2016; 149:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howards PP, Terrell ML, Jacobson MH, et al. Polybrominated biphenyl exposure and menstrual cycle function. Epidemiology 2019; 30:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishihama Y, Yoshinaga J, Iida A, et al. Association between paraben exposure and menstrual cycle in female university students in Japan. Reprod Toxicol 2016; 63:107–113. [DOI] [PubMed] [Google Scholar]
- 34.Jukic AM, Calafat AM, McConnaughey DR, et al. Urinary concentrations of phthalate metabolites and bisphenol A and associations with follicular-phase length, luteal-phase length, fecundability, and early pregnancy loss. Environ Health Perspect 2016; 124:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35▪.Ruiz-García L, Figueroa-Vega N, Malacara JM, et al. Possible role of n-hexane as an endocrine disruptor in occupationally exposed women at reproductive age. Toxicol Lett 2020; 330:73–79. [DOI] [PubMed] [Google Scholar]; This study examines the association between n-hexane exposure and increased menstrual cycle length in Mexican women working in a leather shoe factory.
- 36.Levine LD, Kim K, Purdue-Smithe A, et al. Urinary phytoestrogens and relationship to menstrual cycle length and variability among healthy, eumenorrheic women. J Endocr Soc 2020; 4:bvz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nillni YI, Wesselink AK, Hatch EE, et al. Mental health, psychotropic medication use, and menstrual cycle characteristics. Clin Epidemiol 2018; 10:1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38▪.Phelan N, Behan LA, Owens L. The impact of the COVID-19 pandemic on women's reproductive health. Front Endocrinol (Lausanne) 2021; 12:642755. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the impact of stress related to the COVID-19 pandemic on female reproductive health. Though no association was found between stress and menstrual cycle length, this article depicts a relevant and understudied topic during the ongoing pandemic.
- 39.Wang Y, Gu F, Deng M, et al. Rotating shift work and menstrual characteristics in a cohort of Chinese nurses. BMC Womens Health 2016; 16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40▪.Mayama M, Umazume T, Watari H, et al. Frequency of night shift and menstrual cycle characteristics in Japanese nurses working under two or three rotating shifts. J Occup Health 2020; 62:e12180. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights the association between night shift work and menstrual cycle irregularity in Japanese nurses.
- 41▪.Onieva-Zafra MD, Fernández-Martínez E, Abreu-Sánchez A, et al. Relationship between diet, menstrual pain and other menstrual characteristics among Spanish students. Nutrients 2020; 12:1759. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examines the impact of diet and alcohol consumption on menstrual pain and cycle characteristics and found that longer menstrual cycle lengths were associated with increased alcohol intake and nonadherence to a Mediterranean diet.
- 42.Lammert S, Harrison K, Tosun N, Allen S. Menstrual cycle in women who co-use marijuana and tobacco. J Addict Med 2018; 12:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43▪.Ryan KS, Mahalingaiah S, Campbell LR, et al. The effects of delta-9-tetrahydrocannabinol exposure on female menstrual cyclicity and reproductive health in rhesus macaques. F&S Science 2021; 2:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study utilizes a nonhuman primate model to demonstrate the association between chronic heavy THC use and increased menstrual cycle length using a translationally relevant dose and vehicle of administration.
- 44.Hahn KA, Wise LA, Riis AH, et al. Correlates of menstrual cycle characteristics among nulliparous Danish women. Clin Epidemiol 2013; 5:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jukic AM, Weinberg CR, Baird DD, Wilcox AJ. Lifestyle and reproductive factors associated with follicular phase length. J Womens Health (Larchmt) 2007; 16:1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Gold EB, Lasley BL, Johnson WO. Factors affecting menstrual cycle characteristics. Am J Epidemiol 2004; 160:131–140. [DOI] [PubMed] [Google Scholar]
- 47.Li K, Urteaga I, Wiggins CH, et al. Characterizing physiological and symptomatic variation in menstrual cycles using self-tracked mobile-health data. NPJ Digit Med 2020; 3:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooper GS, Sandler DP. Long-term effects of reproductive-age menstrual cycle patterns on peri- and postmenopausal fracture risk. Am J Epidemiol 1997; 145:804–809. [DOI] [PubMed] [Google Scholar]
- 49.Barron ML, Flick LH, Cook CA, et al. Associations between psychiatric disorders and menstrual cycle characteristics. Arch Psychiatr Nurs 2008; 22:254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mumford SL, Steiner AZ, Pollack AZ, et al. The utility of menstrual cycle length as an indicator of cumulative hormonal exposure. J Clin Endocrinol Metab 2012; 97:E1871–E1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei M, Cheng Y, Bu H, et al. Length of menstrual cycle and risk of endometriosis: a meta-analysis of 11 case-control studies. Medicine (Baltimore) 2016; 95:e2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev 2016; 37:467–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Small CM, Manatunga AK, Klein M, et al. Menstrual cycle characteristics: associations with fertility and spontaneous abortion. Epidemiology 2006; 17:52–60. [DOI] [PubMed] [Google Scholar]
- 54.Wise LA, Mikkelsen EM, Rothman KJ, et al. A prospective cohort study of menstrual characteristics and time to pregnancy. Am J Epidemiol 2011; 174:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brodin T, Bergh T, Berglund L, et al. Menstrual cycle length is an age-independent marker of female fertility: results from 6271 treatment cycles of in vitro fertilization. Fertil Steril 2008; 90:1656–1661. [DOI] [PubMed] [Google Scholar]
- 56.Harlow SD, Matanoski GM. The association between weight, physical activity, and stress and variation in the length of the menstrual cycle. Am J Epidemiol 1991; 133:38–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.