Abstract
We have identified a new gene encoding the G protein α subunit, gna-3, from the filamentous fungus Neurospora crassa. The predicted amino acid sequence of GNA-3 is most similar to the Gα proteins MOD-D, MAGA, and CPG-2 from the saprophytic fungus Podospora anserina and the pathogenic fungi Magnaporthe grisea and Cryphonectria parasitica, respectively. Deletion of gna-3 leads to shorter aerial hyphae and premature, dense conidiation during growth on solid medium or in standing liquid cultures and to inappropriate conidiation in submerged culture. The conidiation and aerial hypha defects of the Δgna-3 strain are similar to those of a previously characterized adenylyl cyclase mutant, cr-1. Supplementation with cyclic AMP (cAMP) restores wild-type morphology to Δgna-3 strains in standing liquid cultures. Solid medium augmented with exogenous cAMP suppresses the premature conidiation defect, but aerial hypha formation is still reduced. Submerged-culture conidiation is refractory to cAMP but is suppressed by peptone. In addition, Δgna-3 submerged cultures express the glucose-repressible gene, qa-2, to levels greatly exceeding those observed in the wild type under carbon-starved conditions. Δgna-3 strains exhibit reduced fertility in homozygous crosses during the sexual cycle; exogenous cAMP has no effect on this phenotype. Intracellular steady-state cAMP levels of Δgna-3 strains are decreased 90% relative to the wild type under a variety of growth conditions. Reduced intracellular cAMP levels in the Δgna-3 strain correlate with lower adenylyl cyclase activity and protein levels. These results demonstrate that GNA-3 modulates conidiation and adenylyl cyclase levels in N. crassa.
G-protein-coupled receptors (GPCRs) are a family of seven transmembrane helix receptors that bind ligands such as neurotransmitters, pheromones, and odorants. GPCRs are associated with heterotrimeric G proteins, consisting of α, β, and γ subunits (for a review, see reference 82). In the inactive state, the heterotrimer is docked at the receptor and GDP is bound to the Gα subunit. Binding of a ligand activates the receptor, leading to the exchange of GDP for GTP on Gα and the subsequent dissociation of Gα-GTP from the Gβγ moiety. Gα-GTP and the Gβγ dimer can activate downstream effectors, such as enzymes and ion channels, to produce a response to the signal (for a review, see reference 7). The response is terminated and the cycle is completed with hydrolysis of GTP by Gα. The GDP-bound Gα protein reassociates with Gβγ, and the heterotrimer is then able to bind to the receptor to await the next cycle of activation (for reviews, see references 10 and 20).
Neurospora crassa is a filamentous fungus that has a complex life cycle due to its ability to produce both asexual and sexual spores (for a review, see reference 71). Grown with adequate nitrogen, N. crassa remains in the asexual cycle and extends basal hyphae to form a complex, intertwined network called a mycelium. Asexual spores, or conidia, are produced by two pathways: macroconidiation and microconidiation. Both types of conidiation require an air-water interface and therefore are repressed in submerged culture. Macroconidiation is initiated by the production of specialized aerial hyphae above the surface of the agar. Conidiophores are formed at the tips of aerial hyphae and give rise to chains of multinucleate macroconidia. Microconidiation differs from macroconidiation in that uninucleate spores form inside the basal hyphae and burst through the hyphal wall upon maturation. In response to nitrogen-limiting conditions, N. crassa enters the sexual cycle by forming female reproductive structures called protoperithecia. Fertilization occurs when the nucleus from a conidium of the opposite mating type is taken up by a specialized hypha (trichogyne) emanating from the female structure. Subsequently, meiosis and sexual spore (ascospore) maturation occurs within the fertilized structure (perithecium).
Genes encoding two Gα subunits, gna-1 and gna-2, have been identified in N. crassa (79). The Δgna-1 strain has a decreased growth rate and reduced mycelial mass during vegetative growth, defects which are more pronounced under hyperosmotic conditions (32). In the sexual cycle, the Δgna-1 strain is female-sterile and forms aberrant perithecia after fertilization (5, 32). The Δgna-2 strain does not possess any phenotypes; however, Δgna-1 Δgna-2 strains are more impaired in Δgna-1 defects (5). Both Δgna-1 and Δgna-1 Δgna-2 strains have decreased intracellular steady-state cyclic AMP (cAMP) levels and adenylyl cyclase activity (33). Results of immunoinhibition experiments suggest that GNA-1 directly interacts with the adenylyl cyclase enzyme to regulate its activity (33).
Studies of Gα subunits in several saprophytic and pathogenic fungi have revealed roles for these proteins in the regulation of virulence, cAMP levels, and asexual and sexual development (for reviews, see references 8 and 38). Mutation of the Gα subunit gene mod-D in the saprophyte Podospora anserina results in impaired aerial hypha and mycelial development, defects which are repressed by exogenous cAMP (49). The P. anserina adenylyl cyclase gene, PaAC, has been cloned as a partial suppressor of the mod-D mutation (48). The Δmod-D strain is also defective in vegetative incompatibility through a cAMP-independent pathway (49). Deletion of any of the three Magnaporthe grisea Gα genes, magA, magB, or magC, reduces fertility. Perithecia fail to form after fertilization in the ΔmagB strain, and the ΔmagA and ΔmagC strains do not produce mature asci (44). Deletion of either magA or magC does not affect the pathogenicity of the organism; however, the ΔmagB strain displays reduced virulence. The ΔmagB strains have decreased appressorium formation, which is suppressed by exogenous cAMP. Two genes encoding Gα subunits, cpg-1 and cpg-2, have been cloned from Cryphonectria parasitica (24). The Δcpg-1 strains have reduced conidiation and 2.5-fold-higher cAMP levels, are female-sterile in heterozygous crosses, and are avirulent. The Δcpg-2 strain has only half the wild-type level of cAMP and is virulent (24). Four Gα genes have been cloned from Ustilago maydis, but only deletion of gpa3 produces an obvious phenotype. The Δgpa-3 mutants have elongated cells and are unable to respond to pheromone or to form the dikaryotic mycelium after fertilization (63). Both the cell morphology and mating defects are suppressed by exogenous cAMP (39). Deletion of gpa3 or of the catalytic subunit of the cAMP-dependent protein kinase gene, adr1, results in avirulence, suggesting that both participate in a cAMP signaling pathway (22, 63). In Ustilago hordei, loss of the Gα gene, fil1, impairs dimorphic switching between the sporulation and filamentous growth habits. This defect is overcome by supplementation with exogenous cAMP (43). Cryptococcus neoformans strains with the Gα gene, gpa1, deleted are mating deficient and avirulent. All mutant phenotypes in the Δgpa1 strain are suppressed by cAMP supplementation (2, 3).
Here we report identification of a new Gα gene from N. crassa, gna-3. The predicted protein sequence of GNA-3 is most similar to that of P. anserina MOD-D, M. grisea MAGA, and C. parasitica CPG-2. Phenotypes of Δgna-3 and cr-1 (an adenylyl cyclase mutant) strains were analyzed during the asexual and sexual cycles. We measured the levels of cAMP, adenylyl cyclase activity and protein, and transcripts for several genes in Δgna-3, cr-1, and wild-type strains. Our results revealed roles for gna-3 in the regulation of conidiation and the level of adenylyl cyclase protein in N. crassa.
MATERIALS AND METHODS
Strains, media and growth conditions.
Wild-type strains 74-OR23-1A (abbreviated 74A; FGSC 987) and 74-OR23-1a (abbreviated 74a; FGSC 988) and the cr-1 B123A (FGSC 4008) mutant were acquired from the Fungal Genetics Stock Center (FGSC) at the University of Kansas Medical Center, Kansas City, Kans. Strains were cultured on either Vogel's minimal medium (VM) for vegetative growth or synthetic crossing medium (SCM) to induce the sexual cycle (19). Standing VM liquid cultures were supplemented with 1 mM cAMP (Sigma) or 2% (wt/vol) Bacto Peptone (Difco Laboratories, Detroit, Mich.) where indicated. For examination of hyperosmotic sensitivity, strains were grown on solid VM supplemented with 0.75 M NaCl, 0.75 M KCl, or 1.5 M sorbitol.
Isolation and sequencing of the gna-3 gene.
Degenerate primers based on conserved regions of Gα proteins (79) were used in PCR with genomic DNA from a Δgna-1 Δgna-2 N. crassa strain (5). The resultant 250-bp gna-3 PCR product was subcloned into plasmid pBluescript II SK(+), yielding plasmid pGNA3. The pGNA3 insert was labeled (32) and used as a probe to screen the N. crassa λ-BARGEM7 genomic lambda library (59). A total of 4 positive plaques were obtained from 40,000 total screened. After conversion to double-stranded plasmids (59), the four clones were subjected to Southern analysis (32) using the pGNA3 insert as a probe. One of the clones (4-3b) was chosen for further analysis, since it contained the gna-3 amino acid coding region centered in an approximately 6.7-kb insert. A 4.35-kb region of the 4-3b insert extending from the extreme amino terminus to the third BamHI site (see Fig. 1; site B3) was sequenced (Core Sequencing Facility, Department of Microbiology and Molecular Genetics, University of Texas—Houston Medical School). A full-length gna-3 cDNA was isolated by screening an N. crassa cDNA library made using RNA from SCM-grown cultures (55). The NsiI-XhoI fragment corresponding to the amino acid coding region was used as a probe. Sequencing was performed as described for the genomic clone. All sequence data were analyzed using Seqed (Applied Biosystems version 1.0.3) and the GCG program package (GCG Inc., Madison, Wis.).
FIG. 1.
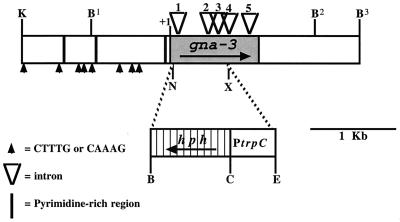
Gene structure of gna-3 from N. crassa. The shaded areas indicate the amino acid coding regions of gna-3 and hph, and arrows within these areas signify the direction of transcription. The translational initiation codon for gna-3 is indicated by +1. Intron positions (1, +125 to +183; 2, +533 to +619; 3, +749 to +809; 4, +939 to +994; 5, +1234 to +1293) are indicated by open triangles. Bars and arrows within the upstream region indicate pyrimidine-rich regions and consensus sites for binding by SRY family HMG box proteins, respectively. The dashed line at the NsiI and XhoI sites illustrate the region of gna-3 that was replaced by hph. Restriction sites: K, KpnI; B, BamHI; C, ClaI; E, EcoRV; X, XhoI, and N, NsiI.
gna-3 gene replacement mutation and complementation in trans.
A gna-3 gene replacement plasmid (pSR20) was made that contains genomic DNA extending from the extreme 5′ end of clone 4-3b to the KpnI site. Within this fragment, the region between the NsiI and XhoI sites was replaced by the EcoRV-BamHI fragment containing the bacterial hygromycin resistance gene (hph) from plasmid pCSN44 (72) (see Fig. 1). The pSR20 construct was linearized, and 2 μg was electroporated into 8-day-old conidia of 74A, as previously described (30, 80). Electroporation products were plated using regeneration agar (14) on sorbose medium containing 200 μg of hygromycin B per ml (32, 72). Genomic DNA from hygromycin-resistant strains was digested with EcoRI and subjected to Southern analysis (32) using a 1.8-kb KpnI-EcoRI fragment that lies outside of the genomic region in pSR20 as a probe. Heterokaryotic transformants containing nuclei with the gene replacement were crossed to wild-type strain 74a in order to isolate homokaryotic progeny. The homokaryotic Δgna-3 strains isolated were designated 2a1, 2a3, 2a17, 31c1, and 31c2 (all are MATA). A MATa Δgna-3 strain was obtained by crossing 31c2 to 74a and is designated 43c2.
A 1-μg portion of the original 4-3b clone was reintroduced into 31c2 8-day-old conidia by electroporation to construct a complemented Δgna-3 strain (80). The 4-3b vector also contains the bar gene, encoding resistance to glufosinate (AgrEvo, Wilmington, Del.) (58). Electroporation products were plated using regeneration agar (14) on nitrogen-free sorbose medium plates containing 220 μg of glufosinate per ml, 0.5% proline, and 2% sucrose (58). Glufosinate-resistant transformants were checked for single integration events by using Southern analysis (32). Heterokaryotic transformants containing nuclei with the bar gene were purified to homokaryotic strains by sequential plating and selection. This rescued strain is designated Δgna-3 + gna-3+.
Northern and RT-PCR analysis.
The tissues used for RNA extraction were as follows. For reverse transcriptase PCR (RT-PCR) and cr-1 expression analysis, conidia were inoculated at 3.6 × 106 conidia/ml in VM and cultured at 30°C in the dark for 16 h with shaking at 200 rpm. For analysis of con-10 and qa-2 expression, conidia were inoculated at 5.5 × 105 conidia/ml and cultured at 30°C in the dark for 16 h with shaking at 200 rpm. For con-10 analysis, VM was the medium. For qa-2 expression studies, conidia were inoculated into VM and cultured for 16 h, at which time the mycelia were washed with sterile water and transferred to VM, VM plus 0.1% (vol/vol) quinic acid, or VM minus sucrose plus 0.1% (vol/vol) quinic acid (50). Total RNA was isolated as previously described (68). For Northern analysis, 30 μg of total RNA was electrophoresed and transferred (78) and the membrane was hybridized as described for Southern analysis (32). cr-1 was detected using a 1.25-kb SalI-PstI fragment from the region containing the catalytic domain (36). A 200-bp EcoRI-BamHI fragment (from pBW100) (64) was used as a probe for the con-10 gene, and a 237-bp SmaI-SphI fragment (pQA2-1088) (obtained from B. Tyler, University of California, Davis, Calif.) was used as a probe for the qa-2 gene (26, 50). A cosmid containing an rRNA gene (obtained from D. E. Ebbole, Texas A&M University) or the cox-5 gene (from pSRCOX-5) (68) was used as the loading control probe.
RT-PCR was performed using 1 μg of total RNA with the Access RT-PCR system (Promega Corp., Madison, Wis.) as recommended by the manufacturer. Primers were designed to overlap the NsiI and XhoI sites within the gna-3 coding region. A total of 15 PCR cycles were performed; low cycle numbers have previously been demonstrated to result in linear amplification (25). Reaction products were electrophoresed on an agarose gel, blotted to nylon membranes, and subjected to Southern analysis (32). The 0.9-kb NsiI-XhoI fragment of gna-3 was used as a probe.
Western analysis.
Submerged VM cultures were inoculated with 3.6 × 106 conidia/ml and grown for 16 h at 30°C with shaking at 200 rpm. Plasma membranes were isolated as described previously (79), and protein was quantitated using the Bradford protein assay (Bio-Rad, Hercules, Calif.). Samples containing 30 μg of total protein were subjected to Western analysis (32). Primary antibodies against GNA-1, GNA-2, and GNB-1 were used at dilutions of 1:400, 1:200, and 1:5,000 (5, 32, 33), respectively. Detection was as described previously (32).
Western analysis of the CR-1 protein was performed using particulate fractions (30 μg of protein) isolated as described below for adenylyl cyclase assays. Generation of an antibody to the 52,362-Da amino-terminal portion of the CR-1 protein will be described in detail elsewhere (F. D. Ivey, A. M. Kays, and K. A. Borkovich, unpublished observations). For Western analysis, a 1:20,000 dilution of the CR-1 primary antibody was used and a goat anti-rabbit immunoglobulin G (heavy plus light chain) horseradish peroxidase conjugate (Bio-Rad) was used at 1:10,000 dilution for the secondary antibody. Detection was performed using the enhanced chemiluminescence method (Amersham Pharmacia Biotech, Little Chalfont, England), as described by the manufacturer. A duplicate gel was electrophoresed and fixed, stained with Coomassie brilliant blue, and destained to indicate relative protein amounts as described previously (69).
Phenotypic analysis.
Apical extension rates were determined by measuring colony diameters at various times as previously described (32). For mating assays, strains were grown on SCM, with or without 2.5 mM cAMP, for 6 days at room temperature in constant light to induce the production of protoperithecia. Protoperithecia were fertilized using conidial suspensions from strains grown on VM. Cultures were incubated at room temperature for 7 days in constant light and then viewed and photographed using a SZH10 research stereoscope and a PM-20 exposure control unit (Olympus, Lake Success, N.Y.). Submerged cultures were inoculated using 5 × 105 conidia/ml and cultured at 30°C for 16 h with shaking at 200 rpm in the dark (50). Samples were viewed using an Axioskop epifluorescence microscope (Zeiss, Thornwood, N.Y.), and images were digitally photographed using a DEI-750 color charge-coupled device camera (Optronics, Goleta, Calif.).
Measurement of intracellular steady-state cAMP levels and adenylyl cyclase activity.
Samples from 16-h VM submerged cultures (grown as described above) or VM (in constant light for 3 days at room temperature) or SCM (in constant light for 6 days at room temperature) cellophane-overlaid plates were ground in liquid nitrogen and extracted in 6% trichloroacetic acid. Samples were centrifuged at 20,229 × g for 30 min at 4°C. cAMP in the supernatant was purified and quantitated as described previously (11, 33). Protein in the pellet was solubilized using 0.5% sodium dodecyl sulfate in 0.1 M NaOH and quantitated using the bicinchoninic acid protein assay reagent (Pierce).
For measurement of adenylyl cyclase activity, cultures were inoculated at 3.6 × 106 conidia/ml and grown at 30°C for 16 h with shaking at 200 rpm. The mycelia were collected and ground in liquid nitrogen (33, 66, 67), and the particulate fraction was isolated by centrifugation. It was empirically determined that centrifugation for 45 min at 4°C at 170,000 × g resulted in the removal of all activity from the soluble fraction to the pellet. Total protein in the pellet was measured using the Bradford assay. Adenylyl cyclase activity was measured using 200 μg of total protein, with [α-32P]ATP (ICN, Irvine, Calif.) as the substrate. Basal activity was determined by preincubation with 100 μM guanosine 5′-O-2-thiodiphosphate (GDP-βS; Amersham Pharmacia, Piscataway, NJ) (75). For GTP-stimulated conditions, reaction mixtures were preincubated with 100 μM 5′ guanylylimidodiphosphate (GppNHp) (Sigma, St. Louis, Mo.). Reactions were performed and the resulting [α-32P]cAMP was purified and quantitated as described previously (33, 66, 67).
Nucleotide sequence accession number.
The accession number of the gna-3 genomic sequence is AF281862.
RESULTS
Identification of the N. crassa gna-3 gene.
Previously, degenerate oligonucleotide primers designed to amplify conserved regions of mammalian Gα genes were used to isolate PCR products corresponding to gna-1 (approximately 200 bp) and gna-2 (approximately 250 bp) from N. crassa (79). A third N. crassa Gα gene, gna-3, was identified as an approximately 250-bp product present in PCR products containing genomic DNA from the Δgna-1 Δgna-2 strain (data not shown). The gna-2 and gna-3 products are larger than that of gna-1 due to the presence of an intron whose position is conserved in mammalian Gαi and Gαs family genes (Fig. 1) (79).
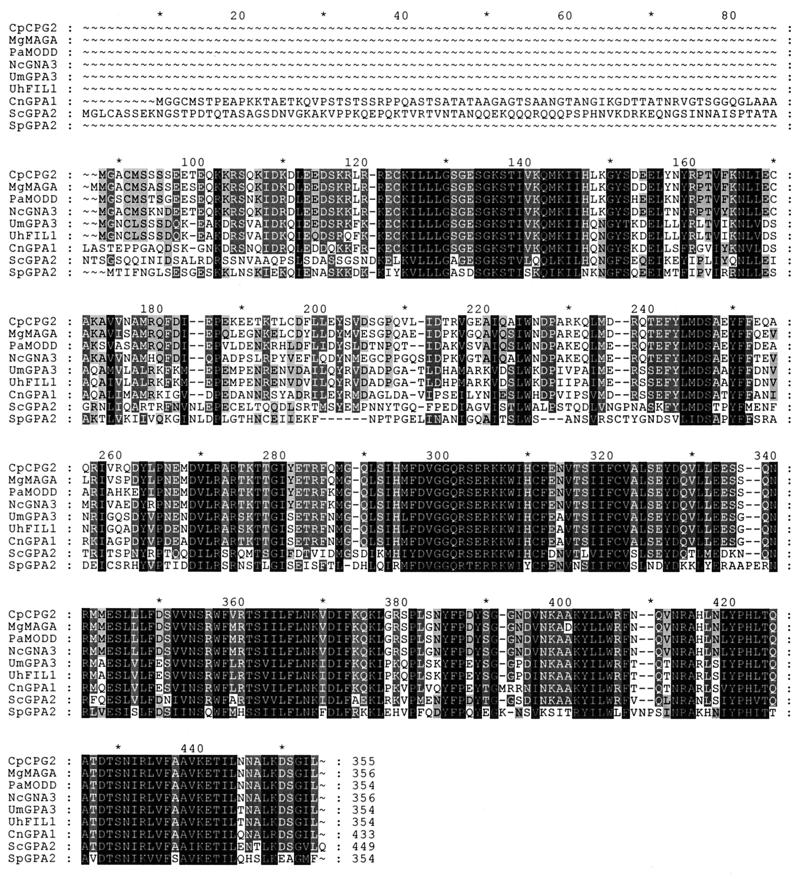
The gna-3 PCR product was used as a probe to screen a N. crassa BARGEM-7 λ genomic library (59). A hybridizing clone was isolated, and a 4.35-kb region containing the gna-3 coding sequence from the 5′ end to the third BamHI site (B3) was sequenced (Fig. 1). The predicted amino acid coding sequence of gna-3 has the highest identity to Gα proteins from several filamentous fungi (Fig. 2). These include M. grisea MAGA (86.8%), P. anserina MOD-D (86.7%), C. parasitica CPG2 (86.5%), U. maydis Gpa3 (68.9%), U. hordei Fil1 (68.4%), and C. neoformans Gpa1 (65.1%). GNA-3 exhibits less identity to Gα proteins from the yeasts Saccharomyces cerevisiae (Gpa2; 51.3%) and Schizosaccharomyces pombe (Gpa2; 47.1%).
FIG. 2.
Amino acid sequence alignment of Gα proteins homologous to GNA-3 from filamentous fungi and yeasts. Alignment was performed using the GeneDoc program. Dark and light shading indicate identical and similar amino acids, respectively. Abbreviations and accession numbers: C. parasitica (Cp) CPG2, L32177; M. grisea (Mg) MAGA, AF011340; P. anserina (Pa) MODD, AF038122; N. crassa (Nc) GNA3, AF281862; U. maydis (Um) GPA3, U85777; U. hordei (Uh) Fil1, U76672; C. neoformans (Cn) GPA1, U09372; S. cerevisiae (Sc) GPA2, S50478; and S. pombe (Sp) GPA2, 2832746.
The region upstream of the gna-3 coding region contains three pyrimidine-rich regions (Fig. 1); such motifs participate in transcriptional regulation in fungi (29). Several consensus sites (CTTTG or CAAAG) for binding by SRY family HMG-box proteins were also identified in the upstream region of gna-3 (6). The genomic gna-3 sequence contains five introns within the predicted amino acid coding region (12). Sequence analysis of a full-length gna-3 cDNA verified the positions of all introns (data not shown). Interestingly, all five intron positions are conserved between N. crassa gna-3 and C. parasitica cpg2 (reference 15 and data not shown).
Deletion of gna-3 by targeted gene replacement.
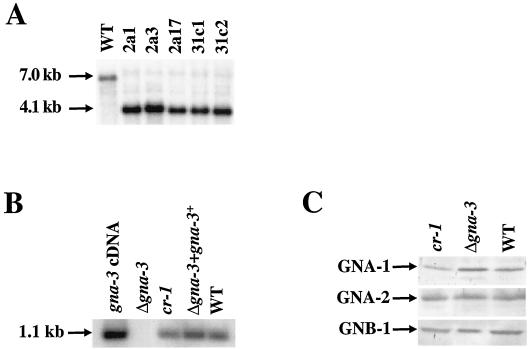
A Δgna-3 mutant was isolated after electroporation of a wild-type strain with a construct in which the gna-3 amino acid coding region was replaced by the bacterial hygromycin B resistance gene (hph+; Fig. 1). Genomic DNA from hygromycin-resistant transformants was subjected to Southern analysis following digestion with EcoRI (Fig. 3A). In this way, heterokaryotic strains containing both wild-type (corresponding to a 7.0-kb fragment) and Δgna-3 (4.1-kb fragment) nuclei were identified. Homokaryotic Δgna-3 strains were obtained by crossing heterokaryotic primary transformants to the wild type, with selection for growth of progeny on hygromycin B. Replacement of the gene in all nuclei was confirmed using Southern analysis (Fig. 3A).
FIG. 3.
Southern and Western analyses. (A) Southern analysis. DNA from the wild type (WT, strain 74A) and from the indicated Δgna-3 homokaryotic strains was digested with EcoRI and subjected to Southern analysis using a KpnI-EcoRI fragment of gna-3 as a probe. The sizes of hybridizing fragments are indicated by arrows on the left. (B) Quantitative RT-PCR. Reaction mixtures contained total RNA from the wild-type strain, 74A (WT) or from Δgna-3 (strain 31c2), Δgna-3 + gna-3+, or cr-1 strains or a plasmid containing the gna-3 cDNA (positive control). Southern analysis was performed using the NsiI-XhoI region of the gna-3 amino acid and coding region as a probe. The position of the 1.1-kp hybridizing RT-PCR product is shown by the arrow. (C) Western analysis. Samples containing 30 μg of plasma membrane protein were subjected to Western analysis using antiserum directed against GNA-1, GNA-2, or GNB-1, as indicated on the left. Strains are the same as in panel B.
To verify that any phenotypes of the Δgna-3 strain were due to the deletion of the gene, the gna-3 genomic clone was reintroduced by electroporation. This rescued strain was selected by conferral of resistance to glufosinate from the Streptomyces bar gene under the control of the Aspergillus nidulans promoter trpC (58). Homokaryons were purified from initial heterokaryotic strains by repetitive plating and selection of colonies resistant to hygromycin and glufosinate. Single gna-3 integration events in Δgna-3 + gna-3+ strains were confirmed by Southern analysis (data not shown).
Due to the comigration of the gna-3 transcript with rRNA and the low levels of gna-3 transcript present, Northern analysis was not an efficient means of examining the levels of the gna-3 transcript (data not shown). Therefore, quantitative RT-PCR was used to demonstrate the presence of the gna-3 mRNA transcript in tissues from submerged cultures. The results demonstrate that wild-type and Δgna-3 + gna-3+ strains possess comparable levels of the 1.1-kb RT-PCR product, as predicted (Fig. 3B). The adenylyl cyclase mutant, cr-1, has comparable levels of the gna-3 product. The Δgna-3 mutant lacked the product, consistent with loss of the gene in this strain.
Deletion of the gna-1 or gna-2 Gα genes does not affect the expression of other identified G proteins in N. crassa (5, 33). In contrast, deletion of the Gβ subunit gene, cpgb-1, in C. parasitica results in decreased levels of the CPG-1 Gα protein (34). To assess whether deletion of gna-3 influences the levels of known G proteins in N. crassa, expression of the Gα subunits, GNA-1 and GNA-2, and the Gβ subunit, GNB-1, was examined using Western analysis (Fig. 3C) (5, 32, 33). The levels of these three G proteins are the same in wild-type and Δgna-3 strains, indicating that the loss of gna-3, like that of gna-1 or gna-2, does not influence the levels of other G-protein subunits in N. crassa. Analysis of these G proteins in cr-1 mutants showed that the amounts of GNA-2 and GNB-1 were normal while GNA-1 levels were slightly lower than in wild type (Fig. 3C).
Δgna-3 strains have reduced fertility during homozygous crosses.
The Gα proteins GNA-1 and GNA-2 contribute to female fertility in N. crassa, and their homologues have also been shown to regulate sexual fertility in filamentous fungi (5, 8, 32, 38). Therefore, the Δgna-3 strains were examined for defects during the sexual cycle. When cultured under nitrogen-limiting conditions (SCM), Δgna-3 strains produced protoperithecia in amounts similar to those produced by wild-type and Δgna-3 + gna-3+ strains and were fertile when used as males or females in heterozygous crosses (data not shown). Perithecial formation was observed 2 days after fertilization and ascospores were produced within 11 days in both wild-type and Δgna-3 strains (data not shown).
The behavior of Δgna-3 strains was also studied during homozygous crosses. Abundant mature perithecia were observed for wild-type and Δgna-3 + gna-3+ strains (Fig. 4). However, the Δgna-3 strains produced smaller perithecia that lacked beaks. In addition, a significant fraction of the Δgna-3 perithecia were embedded in the agar. Ascospores from Δgna-3 homozygous crosses were not observed for a minimum of 14 days, and the number of spores produced was significantly reduced relative to that produced by the wild type (data not shown). The few spores present included black, tan, and white spores, of which only the black spores were viable (data not shown). All black spores carried the Δgna-3 mutation (data not shown). The addition of cAMP to the media of the male and/or female Δgna-3 strains had no effect on the number or viability of spores produced in the homozygous crosses (data not shown). The lack of response to cAMP suggests that this stage in the sexual cycle is mediated through a cAMP-independent pathway.
FIG. 4.
Sexual-cycle phenotypes. Protoperithecia from strains 74A and Δgna-3 + gna-3+ (MATA) were fertilized with a 74a conidial suspension, while those from strain Δgna-3 (strain 31c2, MATA) were fertilized with a Δgna-3 (strain 43c2, MATa) conidial suspension. Perithecia were photographed 7 days after fertilization. Arrows indicate perithecia embedded in the agar.
Hyperosmotic sensitivity.
Deletion of gna-1 results in decreased growth rate and mycelial mass during vegetative growth; these properties are more prominent on hyperosmotic media (32). The Δgna-1 Δgna-2 strain is more impaired in this trait, indicating overlapping functions (5). To determine whether GNA-3 contributes to osmosensitivity, the apical extension rate of Δgna-3 strains was compared to that of the wild type on minimal medium (VM) plates with and without the hyperosmotic agents 0.75 M NaCl, 0.75 M KCl, or 1.5 M sorbitol (data not shown). Relative to the wild type, the growth rate of Δgna-3 strains was 78 to 83% on VM, 69 to 74% on VM-NaCl, and 69 to 73% on VM-KCl. The Δgna-3 strain was more sensitive to 1.5 M sorbitol, with growth rates of 57 to 61% of that of the wild type. Therefore, deletion of gna-3 has only a slight effect on apical extension rates on VM hyperosmotic media, with the greatest effect observed during growth on sorbitol. This is in contrast to Δgna-1 strains, which display much greater and almost equal sensitivity to NaCl, KCl, and sorbitol (32).
Asexual growth and development of Δgna-3 mutants.
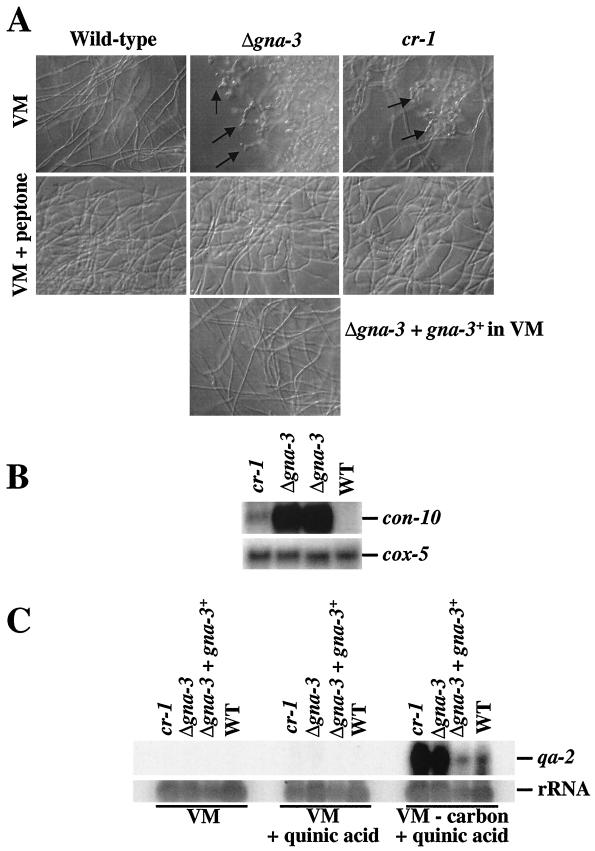
During vegetative growth on solid medium, aerial hyphae grow up from the basal hyphae and produce conidiophores, which terminally differentiate at the tips to yield macroconidia. In contrast to wild-type strains, Δgna-3 mutants produce short aerial hyphae and conidiate prematurely, yielding a dense conidiation pattern on VM plates and in VM standing liquid cultures. Ectopic insertion of gna-3 complements the defects in aerial hypha formation and conidiation (data not shown).
Dense premature conidiation and shorter aerial hyphae are also phenotypes of the N. crassa cr-1 mutant (73). When cultured on solid medium, cr-1 strains produce abundant conidia with no aerial hyphae and have very low apical extension rates. Previous work has demonstrated that the cr-1 strain contains no intracellular cAMP and lacks adenylyl cyclase activity (66, 67, 73, 74). In addition, the cr-1 mutation can be complemented using a gene encoding a predicted adenylyl cyclase from N. crassa that has been demonstrated to map to the cr-1 locus (54).
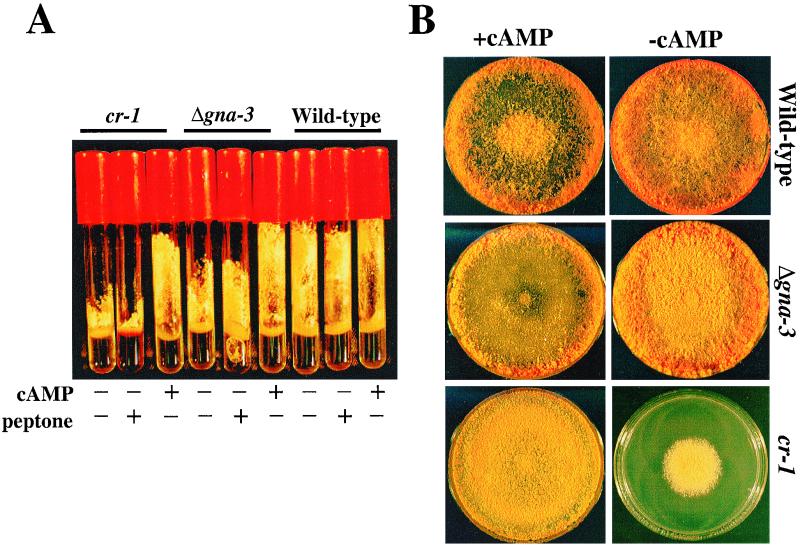
The cr-1 aerial hyphae and premature conidiation defects were previously shown to be corrected by exogenous cAMP (74). Based on the similar conidiation phenotypes, the ability of the Δgna-3 strains to respond to cAMP was examined. Δgna-3 aerial hyphae were taller than those from the cr-1 strain but shorter than wild-type aerial hyphae in standing liquid cultures (Fig. 5A). After supplementation with 1 mM cAMP, both the cr-1 and Δgna-3 strain aerial hyphae were restored to a cAMP-enhanced wild-type height. Previous work has demonstrated that supplementation with rich nutrients, such as peptone, inhibits inappropriate conidiation of some mutant strains in submerged cultures (50). To determine whether peptone has a general inhibitory effect on conidiation, peptone supplementation in standing liquid cultures was tested for wild-type, cr-1, and Δgna-3 strains. The results indicate that the premature conidiation and aerial hypha height of the cr-1 and Δgna-3 strains were unaffected by supplementation with peptone in standing liquid cultures (Fig. 5A).
FIG. 5.
Analysis of standing liquid and solid medium cultures. (A) Standing liquid cultures. Tubes contain liquid VM with no additions or with added 2 mM cAMP or 2% (wt/vol) peptone, as indicated. The Δgna-3 strain is 31c2. Cultures were incubated at 30°C for 3 days in the dark followed by 4 days at room temperature in the light. (B) Growth on solid medium. Plate cultures on VM with or without 2.5 mM cAMP were photographed after incubation at room temperature for 4 days in the light. The Δgna-3 strain is the same as in panel A.
Previous work has shown that exogenous cAMP converts the conidiation morphology and apical extension rate of cr-1 to those of wild-type strains on solid medium (74). Therefore, strains were observed after growth on solid medium in the absence and presence of cAMP (Fig. 5B). On solid VM, the wild-type strain produced tall, abundant aerial hyphae. The cr-1 strain grew colonially, and both cr-1 and Δgna-3 strains had few aerial hyphae and exhibited dense, premature conidiation across the surface of the plate. The addition of cAMP had no effect on the apical extension rate of the wild-type strain but slightly inhibited aerial hypha growth. cr-1 strains had apical extension rates similar to those of the wild type, and premature conidiation was suppressed in both the cr-1 and Δgna-3 strains. However, exogenous cAMP did not restore aerial hypha height in the two mutant strains. The results suggest a divergence in the aerial hypha formation pathway under different culture conditions, in that the short aerial hypha defect of cr-1 and Δgna-3 strains could be corrected by cAMP in standing liquid cultures but not on solid medium. However, this hypothesis is tentative, in that aerial hypha height and amount were inhibited by cAMP addition in wild-type strains.
Δgna-3 strains conidiate in submerged culture.
Given an air-water interface, wild-type N. crassa strains produce conidiophores; conversely, conidiation is normally repressed in liquid submerged cultures. However, carbon or nitrogen starvation induces wild-type strains to conidiate in submerged culture (18, 28, 50, 61, 77). To explore another possible role for gna-3 during conidiation, we analyzed submerged-culture growth morphology. We found that 16-h submerged cultures of wild-type strains produced long, slender hyphae that intertwined to form a mycelium with no conidiophores (Fig. 6A). In contrast, both the Δgna-3 and cr-1 strains formed conidiophores in submerged culture, with a greater amount being formed in Δgna-3 strains. Supplementation with cAMP did not affect the submerged-culture conidiation phenotype of Δgna-3 and cr-1 strains (data not shown). The wild-type strain grown in submerged cultures containing peptone produced swollen hyphae but was otherwise unaffected (Fig. 5A). The Δgna-3 strain containing the ectopic copy of gna-3 did not conidiate in submerged culture and responded to cAMP and peptone supplementation in a manner similar to the wild-type strain (Fig. 5A and data not shown). Although swelling of the hyphae was observed, peptone addition repressed conidiation in both the Δgna-3 and cr-1 strains.
FIG. 6.
Submerged-culture conidiation. (A) Morphology. Cultures were grown in liquid VM with or without peptone at 30°C for 16 h in the dark with shaking. Arrows indicate conidiophores. The Δgna-3 strain is 31c2. (B) Analysis of con-10 expression. Samples containing 20 μg of total RNA were subjected to Northern analysis, using the con-10 gene as a probe. The cox-5 gene probe was used as a loading control. The Δgna-3 strain is the same as in panel A. (C) Analysis of qa-2 expression. Samples containing 25 μg of total RNA isolated from strains cultured under the indicated conditions were analyzed on Northern blots by hybridization with the qa-2 gene. The blot was hybridized with an rRNA gene to check the relative amount of RNA in each lane. The Δgna-3 strain is the same as in panel A. WT, wild type.
Submerged-culture conidiation in the cr-1 and Δgna-3 strains was further evaluated using Northern analysis to determine the levels of a conidiation-specific gene, con-10 (50, 64). Similar to previous observations, the con-10 transcript was not detected in the wild-type strain grown in submerged culture (Fig. 6B) (50). Consistent with the observed submerged-culture morphology, the con-10 transcript was produced in cr-1 and Δgna-3 strains, with significantly higher levels being found in the Δgna-3 mutant (Fig. 6B).
Mutation of the rco-3 gene results in submerged-culture conidiation in N. crassa (50). The predicted RCO-3 protein is most similar to the S. cerevisiae glucose transporter Hxt1p (42, 50). Δrco-3 mutants express carbon-repressible genes in high-carbon medium and have decreased high- and low-affinity glucose transport (50). Since gna-3, cr-1, and rco-3 mutants exhibit submerged-culture conidiation, we explored a role for GNA-3 and CR-1 in carbon sensing. Northern analysis was performed to probe the levels of the carbon-repressible gene qa-2 under various growth conditions (Fig. 6C) (26). Expression of qa-2 was not detected in VM cultures from any strain (Fig. 6C). The mRNA was barely detectable in all strains in VM supplemented with quinic acid. The qa-2 gene was expressed in the wild-type strain in the presence of the inducer quinic acid during carbon starvation. Interestingly, qa-2 transcript levels were greatly elevated over those observed for the wild type in cr-1 and Δgna-3 strains under the same conditions. The levels of qa-2 mRNA in cr-1 and Δgna-3 strains were similar, suggesting that cAMP negatively influences the expression of qa-2 during carbon starvation in submerged culture.
Δgna-3 strains have decreased steady-state intracellular cAMP levels and adenylyl cyclase activity.
Previous analysis of cr-1 mutants has shown that exogenous cAMP suppresses phenotypic defects and that these strains contain no detectable adenylyl cyclase activity (66, 67, 73, 74). Based on the similar morphological phenotypes and response to cAMP observed in cr-1 and Δgna-3 strains, intracellular steady-state cAMP levels of wild-type, Δgna-3, and cr-1 strains were measured under various growth conditions.
Intracellular steady-state cAMP levels of the Δgna-3 strains 2a3 and 31c2 were reduced to 9.40% ± 2.46% and 9.30% ± 1.63% on VM and 11.4% ± 1.03% and 10.4% ± 1.83% on SCM solid plates, respectively, compared to the wild type (Table 1). Furthermore, the levels of cAMP in 16-h submerged cultures of 2a3 and 31c2 strains were 25.6% ± 7.08% and 37.8% ± 2.88% those of the wild type. Therefore, the loss of gna-3 results in significantly reduced intracellular cAMP levels under all growth conditions. Consistent with previous results, the cr-1 strain contained no detectable intracellular cAMP when cultured on VM or SCM plates or in submerged culture (reference 74 and data not shown).
TABLE 1.
Steady-state intracellular cAMP levels
| Strain | Genotype | Intracellular cAMP level (pmol/mg of protein)a (% of wild type) for:
|
||
|---|---|---|---|---|
| 16-h germlings | VM plates | SCM plates | ||
| 2a3 | Δgna-3 | 1.01 ± 0.29 (25.6 ± 7.08) | 0.86 ± 0.23 (9.40 ± 2.46) | 1.23 ± 0.10 (11.4 ± 1.03) |
| 31c2 | Δgna-3 | 1.53 ± 0.11 (37.8 ± 2.88) | 0.85 ± 0.15 (9.30 ± 1.63) | 1.01 ± 0.12 (10.4 ± 1.83) |
| 74A | Wild type | 3.96 ± 0.72 (100) | 9.13 ± 1.12 (100) | 9.87 ± 1.36 (100) |
Errors are indicated as the standard error of the mean. Values are calculated using data from five independent experiments.
To determine whether the decreased intracellular cAMP levels resulted from the loss of direct or indirect stimulation of adenylyl cyclase by GNA-3, the activity of the enzyme was measured in total membrane fractions from N. crassa. Enzyme activity was measured using either Mg2+-ATP or Mn2+-ATP as substrates. To utilize Mg2+-ATP under both basal and GTP-stimulated conditions, adenylyl cyclase requires the presence of a Gα subunit (66, 67). Therefore, results from assays containing Mg2+-ATP give a measure of the level of G-protein-stimulated adenylyl cyclase activity. Activity with Mn2+-ATP as the substrate is independent of G proteins and is a measure of the total amount of active adenylyl cyclase enzyme present (66, 67). Whole-cell extracts and total-membrane fractions derived from these extracts were found to have similar levels of total activity for both wild-type and Δgna-3 strains (data not shown).
In assay mixtures containing Mg2+-ATP, adenylyl cyclase specific activity in the Δgna-3 strain was reduced to 34.8% ± 3.72% and 31.4% ± 0.22% of wild-type levels under basal and GTP-stimulated conditions, respectively (Table 2). Comparably, the level of Mn2+-ATP-dependent activity was 31.1% ± 0.57% of the wild type in the Δgna-3 strains (Table 2). When the level of Mg2+-ATP dependent adenylyl cyclase activity was corrected for the amount of Mn2+-ATP activity, the relative specific activities of the Δgna-3 and wild-type strains were similar under both basal and GTP-stimulated conditions. This result suggests that the decreased cAMP levels and adenylyl cyclase activity of gna-3 strains are due to lower levels of enzyme rather than to loss of a Gα required for stimulation, as observed in Δgna-1 strains (33).
TABLE 2.
Adenylyl cyclase activity
| Strain | Mg2+ activitya (mean ± SEM)
|
% of wild-type Mg2+ activity
|
Fold stimulationd | Mn2+ activitya | % of wild-type Mn2+ activity | Relative sp acte
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Basalb | GTP stimulatedc | Basal | GTP stimulated | Basal | GTP stimulated | ||||
| Δgna-3f | 1.32 ± 0.14 | 12.0 ± 0.09 | 34.8 ± 3.72 | 31.4 ± 0.22 | 9.80 ± 0.46 | 131 ± 2.40 | 31.0 ± 0.57 | 4.51 ± 0.15 | 9.14 ± 0.10 |
| Wild type | 3.79 ± 0.06 | 38.2 ± 3.37 | 100 | 100 | 9.49 ± 1.33 | 422 ± 45.8 | 100 | 3.53 ± 1.07 | 9.25 ± 1.80 |
Units are picomoles of cAMP per minute per milligram of protein.
Determined using the nonhydrolyzable analogue GDP-βS.
Determined using the nonhydrolyzable analogue GppNHp.
Calculated by dividing the GTP-stimulated activity by the basal activity.
Determined by dividing the Mg2+ activity by the Mn2+ activity and multiplying by 100.
Δgna-3 strain 31c2.
Δgna-3 strains have normal cr-1 transcript levels but reduced CR-1 protein levels.
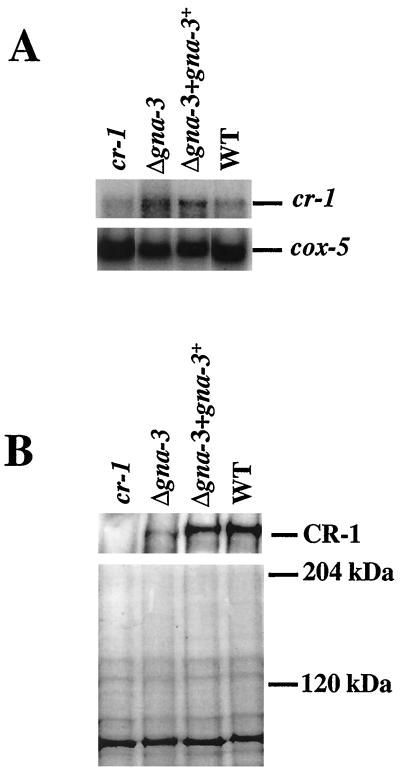
The mechanism by which GNA-3 regulates the expression of adenylyl cyclase was explored by measuring the amount of cr-1 transcript and protein present in Δgna-3 strains. Although different amounts of RNA were present, Northern analysis indicated that the 7.0-kb cr-1 mRNA could be detected in all strains (Fig. 7A). Production of a cr-1 transcript has previously been observed for the cr-1 B123A allele (36, 54). Significantly, total RNA and cr-1 mRNA levels were similar for the Δgna-3 and wild-type strains. Observance of wild-type levels of the cr-1 transcript in Δgna-3 strains suggests that GNA-3 does not regulate the transcription of adenylyl cyclase.
FIG. 7.
Analysis of cr-1 transcript and protein levels. (A) Northern analysis. Samples containing 20 μg of total RNA were analyzed for the presence of the cr-1 transcript (top) using a gene fragment from the catalytic domain of cr-1 as a probe. Hybridization with cox-5 (bottom) was performed to determine the relative amount of RNA in each lane. The Δgna-3 strain is 31c2. (B) Western analysis. Samples containing 30 μg of total membrane protein from 16-h germlings were subjected to Western analysis using the CR-1 antibody (top). The position of the major immunoreactive species is shown on the right. The Δgna-3 strain is the same as in panel A. A duplicate gel was Coomassie stained to check protein loading (bottom). The sizes of molecular mass standards are indicated on the right. WT, wild type.
Since there is no evidence that GNA-3 regulates adenylyl cyclase at the level of transcription, a possible effect on protein levels was examined. A specific antibody against the approximately 60-kDa amino-terminal portion of the CR-1 protein was generated in rabbits (Ivey et al., unpublished). No cross-reacting species was detected using the preimmune serum during Western analysis (data not shown). A reactive species slightly larger than 200 kDa was detected in the wild-type and Δgna-3 + gna-3+ strains by using immune serum (Fig. 7B); this protein was not present in the cr-1 mutant, consistent with the absence of adenylyl cyclase activity in this strain. Identical results were observed for whole-cell extract and particulate fractions (data not shown). A duplicate gel was Coomassie stained to verify equal loading of protein samples (Fig. 7B).
Deletion of gna-3 resulted in a significant decrease in the amount of the CR-1 protein (Fig. 7B). The consistency between the levels of Mn2+-ATP-dependent adenylyl cyclase activity and CR-1 protein detected during Western analysis indicates that GNA-3 mediates adenylyl cyclase activity by regulating the amount of CR-1 protein. The normal transcript levels observed in Δgna-3 mutants suggest that this regulation occurs posttranscriptionally. Such an effect could occur by GNA-3 regulating translation or turnover of the CR-1 protein in N. crassa.
DISCUSSION
We have investigated the importance of heterotrimeric G-protein-mediated signal transduction systems in the response of N. crassa to environmental cues. The Gα protein GNA-1 has been shown to participate in mating and stress response pathways and to overlap functionally with a second Gα, GNA-2 (5, 32). Analysis of adenylyl cyclase activity supports the hypothesis that GNA-1 is a direct positive regulator of this enzyme in N. crassa (33). Here we have reported the identification and functional analysis of a third N. crassa Gα subunit gene, gna-3. One wild-type allele of gna-3 is necessary for successful completion of the sexual cycle. GNA-3 negatively regulates conidiation through a cAMP-dependent pathway in standing liquid cultures and on solid medium. Relative to the wild type, both cr-1 and Δgna-3 strains have greatly elevated levels of a glucose-repressible gene when starved for carbon. Examination of a role for GNA-3 in adenylyl cyclase regulation demonstrates that GNA-3 influences cAMP levels by posttranscriptionally controlling the amount of adenylyl cyclase protein.
Deletion of gna-1 results in the production of very small aberrant perithecia, which rarely produce ascospores (32). Loss of gna-2 does not independently affect mating; however, the Δgna-1 Δgna-2 strain displays a more severe defect in female fertility with complete absence of perithecia and ascospores (5). The defect in female fertility caused by the deletion of gna-1 suggests that GNA-1 may participate in the N. crassa pheromone response pathway by coupling to a pheromone receptor. Results of studies of gna-2 support a minor role for this Gα in pheromone sensing. In contrast, Δgna-3 strain perithecia are smaller than wild-type perithecia, lack beaks, are often embedded in the agar, and produce few viable ascospores during homozygous crosses. The differences between gna-1, gna-2, and gna-3 mutants suggest that various aspects of mating, such as fertilization, perithecial formation, and production of sexual spores, are independently regulated.
Differential regulation of the sexual cycle by Gα subunits is also observed in M. grisea and C. parasitica. Regulation of mating is similar in M. grisea and N. crassa in that the gna-1 homologue, magB, and the gna-3 homologue, magA, regulate similar aspects of mating (44). The gna-2 homologue, magC, appears to have overlapping functions with magA rather than magB, in that both the ΔmagA and ΔmagC mutants display the same defects. Homologues of gna-1 (cpg-1) and gna-3 (cpg-2) have been identified in C. parasitica, and only the deletion of cpg-1 impairs sexual development (24). Phenotypic similarities resulting from mutation of these Gα proteins suggest that a common mode of regulation exists for monitoring the sexual cycle in these related filamentous fungi.
The loss of gna-3 leads to decreased aerial hypha height and premature conidiation in standing liquid cultures as well as to inappropriate conidiation in submerged cultures. These results suggest a role for GNA-3 as a crucial negative regulator of conidiation in response to specific environmental and/or growth cues. One activating signal for conidiation is blue light (57); however, Δgna-3 strains grown in standing liquid cultures or on solid medium prematurely initiate conidiation even in the dark (data not shown). A similar phenotype is observed in the adenylyl cyclase mutant, cr-1 (73). Exogenous cAMP suppresses the premature and dense conidiation defect of both Δgna-3 and cr-1 strains in standing liquid and plate cultures. These findings suggest that under these growth conditions, conidiation is mediated by a cAMP-dependent mechanism.
Submerged-culture conidiation is induced by carbon starvation in N. crassa (18, 28, 50, 61, 77). The submerged conidiation phenotype of cr-1 and Δgna-3 strains is similar to that observed in rco-3 mutants (50). Despite the high sequence similarity of RCO-3 to a S. cerevisiae glucose transporter, RCO-3 has been proposed to act as a glucose sensor because both high- and low-affinity transport are affected in Δrco-3 strains (50). The abundant submerged conidiation of the Δrco-3 strains, like that observed for the cr-1 and Δgna-3 strains, is suppressed by peptone. However, unlike Δgna-3 strains, Δrco-3 does not display conidiation defects on solid medium and is sorbose resistant (reference 50 and data not shown). The qa-2 gene is expressed in Δrco-3 to levels comparable to those in carbon-starved wild-type cultures, independent of the carbon status. Expression of qa-2 is significantly higher in cr-1 and Δgna-3 strains than in the wild type in submerged culture under carbon-limiting conditions. The higher levels of qa-2 expression is negatively influenced by cAMP levels during carbon starvation. This is the first time that cAMP levels have been shown to affect the transcription of qa-2. Future work investigating the relationships between rco-3, gna-3, and cr-1 will facilitate the elucidation of carbon sensing in N. crassa.
In the filamentous fungus A. nidulans, induction of conidiophore development under normal growth conditions requires exposure to air and can be induced by transferring cultures to media lacking either carbon or nitrogen (4, 70). Deletion or dominant interfering mutations of the Gα subunit gene, fadA, or a loss-of-function mutation in a regulator of G protein signaling gene, flbA, results in inappropriate submerged-culture conidiation (30, 84). Both fadA mutations suppress the flbA mutation, indicating that FlbA negatively regulates the activity of FadA (84). When cultures of the ΔflbA mutant are transferred to media lacking a carbon or nitrogen source, conidiation does not occur, suggesting that FlbA is necessary in the response to carbon and nitrogen starvation (41). Although FadA is more similar to N. crassa GNA-1 than to GNA-3, the observed phenotypes of fadA mutants suggest that G-protein-mediated regulation of conidiation is a common theme in filamentous fungi.
Nutrient limitation induces morphogenesis in many species and is mediated by G proteins in other fungi, including S. cerevisiae. Diploid S. cerevisiae cells switch from budding to pseudohyphal growth during nitrogen starvation (for reviews, see references 9, 62, and 76). Deletion of either GPA2 or RAS2 reduces pseudohyphal development; loss of both genes results in a severe growth defect with an additive effect on filamentation (40, 45). Studies have shown that the high-affinity ammonium permease, Mep2p, is required for pseudohyphal growth; however, alternative nitrogen sources are still capable of being transported into the cell by other permeases (46). A GPCR, Gpr1p, has been identified by yeast two-hybrid analysis using Gpa2p as bait, indicating that these two proteins interact (83, 85). The Δgpr1/Δgpr1 diploid is defective in pseudohyphal growth. Transcription of GPR1 is induced in response to nitrogen starvation, and Gpr1p is subsequently required for sensing the presence of fermentable sugars, leading to increased cAMP levels (37, 47). Both the Δmep2/Δmep2 and the Δgpr1/Δgpr1 switch defects are suppressed in the presence of dominant-active GPA2 or RAS2 mutations or exogenous cAMP (46, 47, 85). Studies with the Δgpa2/Δgpa2 strain have shown that Gpa2p is necessary for glucose-induced accumulation of cAMP (17). Increased levels of cAMP activate the cAMP-dependent protein kinase (PKA) catalytic subunit, Tpk2p, which is a positive regulator of filamentous growth (60, 65).
The different effects of exogenous cAMP during sexual and vegetative development in Δgna-3 strains indicate that GNA-3 participates in both cAMP-dependent and -independent pathways. Likewise, P. anserina MOD-D functions in cAMP-dependent and -independent pathways (48, 49). Similar dual functions have also been observed with Gpa2p in S. cerevisiae. As mentioned above, Gpa2p is required for glucose-induced increases in cAMP. Recently, an interaction between GTP-bound Gpa2p and the meiosis/sporulation protein kinase, Ime2p, has been shown to inhibit Ime2p kinase activity (21).
Loss of cpg-2 or magA does not affect pathogenesis, but deletion of gpa3, gpa1, and fil1 renders the respective organism avirulent. The intracellular cAMP levels are altered or the mutant phenotype is corrected by exogenous cAMP in many cases, predicting that these Gα proteins control cAMP levels, presumably by directly regulating adenylyl cyclase activity (8, 15, 16, 38, 63). However, the activity and/or protein levels of adenylyl cyclase have not been investigated in these various organisms. Previous studies from our laboratory show that GNA-1 is a direct, positive regulator of adenylyl cyclase activity in N. crassa. Deletion of GNA-1 results in an 85% decrease in Mg2+-ATP-dependent adenylyl cyclase activity, and addition of a GNA-1 antibody specifically inhibits this activity in wild-type cells (33). The work presented here demonstrates that GNA-3 is not a direct stimulator of adenylyl cyclase but, instead, regulates the amount of the enzyme present in the organism. Therefore, two different G proteins, GNA-1 and GNA-3, coordinate the amount of positive stimulation and the level of the enzyme, respectively, leading to dual regulation of adenylyl cyclase in N. crassa.
This study demonstrates that GNA-3 posttranscriptionally regulates the level of adenylyl cyclase to mediate vegetative development in N. crassa. The high level of similarity in amino acid sequence and function suggests that Gα proteins homologous to GNA-3 may share a comparable mode of adenylyl cyclase regulation in filamentous fungi. This system of regulation could be compared to what is known about adenylyl cyclase in diverse fungal species. In S. cerevisiae, adenylyl cyclase activation requires Gpa2p and Ras2p and binding of the cytosolic cyclase-associated protein (CAP) to the C-terminal region of the adenylyl cyclase protein, Cyr1p (23, 81). cyr1 mutants arrest at the G1 phase of the cell cycle in the absence of exogenous cAMP. In contrast, adenylyl cyclase is dispensable in the fission yeast S. pombe (51–53). CAP is required for adenylyl cyclase activity, and mutation of gpa2 results in decreased intracellular cAMP levels and a failure to produce cAMP in response to glucose (31, 35). Genetic studies with S. pombe have shown that Gpa2 acts upstream of adenylyl cyclase; however, direct regulation of adenylyl cyclase by Gpa2 has not been demonstrated (56, 76). The amino acid sequences encoded by the M. grisea and U. maydis adenylyl cyclase genes, MAC1 and uac1, respectively, are most similar to CR-1 from N. crassa (1, 27). Deletion of MAC1 has a dramatic effect on conidiation, growth rate, sexual development, and appressorium formation, and MAC1 can complement the N. crassa cr-1 mutation (1). The Δuac1 mutant is defective in dimorphic switching and maintains constitutive filamentous growth (27). In contrast to S. cerevisiae, adenylyl cyclase is not essential for cell viability in N. crassa, M. grisea, U. maydis, and S. pombe, suggesting a divergence in the physiological role of cAMP between budding yeast and other fungi (1, 27, 51–53, 66, 73). Based on the current model for regulation of adenylyl cyclase in all of these fungal systems, we predict that Ras and possibly CAP will be important in the regulation of adenylyl cyclase in filamentous fungi.
Our data support PKA as a central component in numerous developmental pathways in N. crassa (Fig. 8). The PKA regulatory subunit, mcb, was previously identified and shown to regulate cell polarity during vegetative hyphal growth (13). The catalytic subunit of PKA has recently been isolated, and a deletion mutant has been constructed (M. Plamann, personal communication). Deletion of the catalytic subunit results in a strain with phenotypes identical to those of the cr-1 strain. The common phenotypes observed for this mutant and the cr-1 and Δgna-3 strains suggest that PKA is responsible for positively regulating the extension of basal hyphae and the formation of aerial hyphae while negatively regulating conidiation. Our work further indicates that a cAMP-dependent pathway facilitates glucose-repressible gene expression under carbon-limiting conditions. RCO-3 has been previously demonstrated to negatively mediate submerged-culture conidiation and glucose-regulated gene expression (50). At present, possible links between RCO-3 and GNA-3 pathways have not been determined.
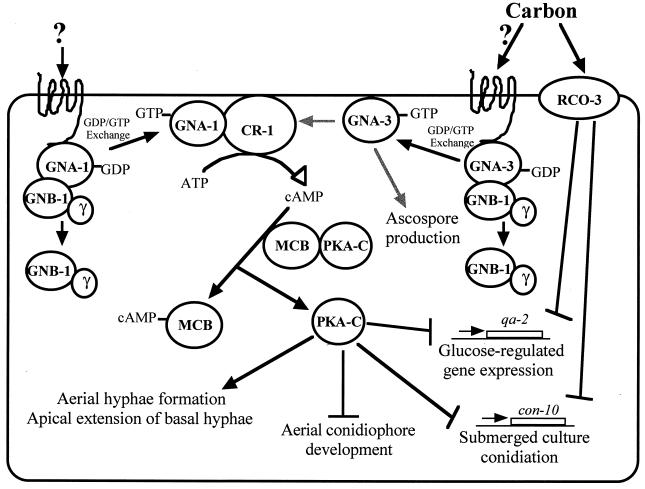
FIG. 8.
Model for action of GNA-3 in N. crassa. Control of adenylyl cyclase protein levels by GNA-3 and GTP-dependent stimulation by GNA-1 results in regulation of intracellular cAMP levels. Binding of cAMP to PKA results in dissociation of the MCB (regulatory) and PKA-C (catalytic) subunits. Activated PKA-C positively regulates aerial hypha formation and apical extension of basal hyphae and negatively regulates aerial and submerged conidiation and glucose-regulated gene expression. GNA-3 also regulates ascospore production through a cAMP-independent pathway. RCO-3 has previously been shown to negatively influence submerged conidiation and glucose-regulated gene expression (50). A Gβ protein, GNB-1, has been identified in N. crassa, and its function is currently being investigated (Q. Yang, S. I. Poole, and K. A. Borkovich, unpublished data). Gray arrows indicate steps that may involve intermediate components. Question marks indicate unknown ligands for GPCRs coupled to GNA-1 and GNA-3.
Much work has been done to elucidate roles for Gα subunits in virulence in pathogenic fungi. Most of these studies have specifically addressed the correlation of reduced pathogenicity with the loss of a Gα protein. Deletion of Gα subunits leads to morphological and mating defects that compromise the ability of the fungus to invade and infect its host. Frequently, pathogenicity is restored by supplementing the mutant with exogenous cAMP, suggesting regulation of adenylyl cyclase activity as a role for Gα subunits related to GNA-3 (8, 38). Understanding the events that occur downstream of these heterotrimeric Gα proteins could lead to the construction of an appropriate pharmacological agent to disrupt the ability of pathogenic fungi to cause infection. The high degree of sequence and functional conservation between GNA-3 homologues suggests that concepts elucidated during studies in N. crassa will be widely applicable to understanding virulence in filamentous pathogens.
ACKNOWLEDGMENTS
We thank Michael Plamann for communicating results prior to publication, Douglas Ivey for advice on adenylyl cyclase assays, Thomas Vida for help with photography; Edward Pieters at AgrEvo for glufosinate, and Ming Hang Zhang for isolation of a gna-3 cDNA clone. We acknowledge Alicia Dombroski, Daniel Ebbole, Dale Hereld, Gloria Turner, and Malcolm Winkler for many helpful discussions.
This work was supported by Public Health Service grant GM-48626 from the National Institutes of Health (to K.A.B.).
REFERENCES
- 1.Adachi K, Hamer J E. Divergent cAMP signaling pathways regulate growth and pathogenesis in the rice blast fungus Magnaporthe grisea. Plant Cell. 1998;10:1361–1373. doi: 10.1105/tpc.10.8.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alspaugh J A, Cavallo L M, Perfect J R, Heitman J. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol Microbiol. 2000;36:352–365. doi: 10.1046/j.1365-2958.2000.01852.x. [DOI] [PubMed] [Google Scholar]
- 3.Alspaugh J A, Perfect J R, Heitman J. Cryptococcus neoformans mating and virulence are regulated by the G-protein α subunit GPA1 and cAMP. Genes Dev. 1997;11:3206–3217. doi: 10.1101/gad.11.23.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelrod D E. Kinetics of differentiation of conidiophores and conidia by colonies of Aspergillus nidulans. J Gen Microbiol. 1972;73:181–184. doi: 10.1099/00221287-73-1-181. [DOI] [PubMed] [Google Scholar]
- 5.Baasiri R A, Lu X, Rowley P S, Turner G E, Borkovich K A. Overlapping functions for two G protein α subunits in Neurospora crassa. Genetics. 1997;147:137–145. doi: 10.1093/genetics/147.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baxevannis A D, Landsman D. The HMG-1 box protein family: classification and functional relationships. Nucleic Acids Res. 1995;23:1604–1613. doi: 10.1093/nar/23.9.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for βγ dimers as well as α subunits. Cell. 1992;71:1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- 8.Bölker M. Sex and crime: Heterotrimeric G proteins in fungal mating and pathogenesis. Fungal Genet Biol. 1998;225:143–156. doi: 10.1006/fgbi.1998.1102. [DOI] [PubMed] [Google Scholar]
- 9.Borges-Walmsley M I, Walmsley A R. cAMP signalling in pathogenic fungi: control of dimorphic switching and pathogenicity. Trends Microbiol. 2000;8:133–141. doi: 10.1016/s0966-842x(00)01698-x. [DOI] [PubMed] [Google Scholar]
- 10.Borkovich K A. Signal transduction pathways and heterotrimeric G proteins. In: Brambl R, Marzluf G A, editors. The Mycota III: biochemistry and molecular biology. Berlin, Germany: Springer-Verlag KG; 1996. pp. 211–233. [Google Scholar]
- 11.Brown B L, Albano J D M, Ekins R P, Spherzi A M. A simple and sensitive saturation assay method for the measurement of adenosine 3′: 5′-cyclic monophosphate. Biochem J. 1971;121:561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruchez J J P, Eberle J, Russo V E A. Regulatory sequences involved in the translation of Neurospora crassa mRNA: Kozak sequences and stop codons. Fungal Genet Newsl. 1993;40:85–88. [Google Scholar]
- 13.Bruno K S, Aramayo R, Minke P F, Metzenberg R L, Plamann M. Loss of growth polarity and mislocalization of septa in a Neurospora mutant altered in the regulatory subunit of cAMP-dependent protein kinase. EMBO J. 1996;15:5772–5782. [PMC free article] [PubMed] [Google Scholar]
- 14.Case M E, Geever R F, Asch D K. Use of gene replacement transformation to elucidate gene function in the qa gene cluster of Neurospora crassa. Genetics. 1992;130:729–736. doi: 10.1093/genetics/130.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen B, Gao S, Choi G H, Nuss D L. Extensive alteration of fungal gene transcript accumulation and elevation of G-protein-regulated cAMP levels by a virulence-attenuating hypovirus. Proc Natl Acad Sci USA. 1996;93:7996–8000. doi: 10.1073/pnas.93.15.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi G H, Chen B, Nuss D L. Virus-mediated or transgenic suppression of a G-protein α subunit and attenuation of fungal virulence. Proc Natl Acad Sci USA. 1995;92:305–309. doi: 10.1073/pnas.92.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colombo S, Ma P, Cauwenberg L, Winderickx J, Crauwels M, Teunissen A, Nauwelaers D, de Winde J H, Gorwa M-F, Colavizza D, Thevelein J M. Involvement of distinct G-proteins, Gpa2 and Ras, in glucose- and intracellular acidification-induced cAMP signalling in the yeast Saccharomyces cerevisiae. EMBO J. 1998;17:3326–3341. doi: 10.1093/emboj/17.12.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortat M, Turian G. Conidiation of Neurospora crassa in submerged culture without mycelial phase. Arch Microbiol. 1974;95:305–309. doi: 10.1007/BF02451771. [DOI] [PubMed] [Google Scholar]
- 19.Davis R H, De Serres F J. Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 1970;17:79–143. [Google Scholar]
- 20.Dessauer C W, Posner B A, Gilman A G. Visualizing signal transduction: receptors, G-proteins, and adenylate cyclases. Clin Sci. 1996;91:527–537. doi: 10.1042/cs0910527. [DOI] [PubMed] [Google Scholar]
- 21.Donzeau M, Bandlow W. The yeast trimeric guanine nucleotide-binding protein α subunit, Gpa2p, controls the meiosis-specific kinase Ime2p activity in response to nutrients. Mol Cell Biol. 1999;19:6110–6119. doi: 10.1128/mcb.19.9.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dürrenberger F, Wong K, Kronstad J W. Identification of a cAMP-dependent protein kinase catalytic subunit required for virulence and morphogenesis in Ustilago maydis. Proc Natl Acad Sci USA. 1998;95:5684–5689. doi: 10.1073/pnas.95.10.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Field J, Vojtek A, Ballester R, Bolger G, Colicelli J, Ferguson K, Gerst J, Kataoka T, Michaeli T, Powers S, Rigg M, Rodgers L, Wieland I, Wheland B, Wigler M. Cloning and characterization of CAP, the S. cerevisiae gene encoding the 70 kd adenylyl cyclase-associated protein. Cell. 1990;61:319–327. doi: 10.1016/0092-8674(90)90812-s. [DOI] [PubMed] [Google Scholar]
- 24.Gao S, Nuss D L. Distinct roles for two G protein α subunits in fungal virulence, morphology, and reproduction revealed by targeted gene disruption. Proc Natl Acad Sci USA. 1996;93:14122–14127. doi: 10.1073/pnas.93.24.14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gause W C, Adamovicz J. Use of PCR to quantitate relative differences in gene expression. In: Dieffenbach C W, Dveksler G S, editors. PCR primer: a laboratory manual. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 293–311. [Google Scholar]
- 26.Geever R F, Huiet L, Baum J A, Tyler B M, Patel V B, Rutledge B J, Case M E, Giles N H. DNA sequence, organization and regulation of the qa gene cluster of Neurospora crassa. J Mol Biol. 1989;207:15–34. doi: 10.1016/0022-2836(89)90438-5. [DOI] [PubMed] [Google Scholar]
- 27.Gold S, Duncan G, Barrett K, Kronstad J. cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 1994;8:2805–2816. doi: 10.1101/gad.8.23.2805. [DOI] [PubMed] [Google Scholar]
- 28.Guignard R, Grange F, Turian G. Microcycle conidiation induced by partial nitrogen deprivation in Neurospora crassa. Can J Microbiol. 1974;30:1210–1215. [Google Scholar]
- 29.Gurr S J, Unkles S E, Kinghorn J R. The structure and organization of nuclear genes of filamentous fungi. In: Kinghorn J R, editor. Gene structure in eukaryotic microbes. Oxford, United Kingdom: IRL Press Ltd.; 1987. pp. 93–139. [Google Scholar]
- 30.Hicks J K, Yu J H, Keller N P, Adams T H. Aspergillus sporulation and mycotoxin production both require inactivation of the FadA Gα protein-dependent signaling pathway. EMBO J. 1997;16:4916–4923. doi: 10.1093/emboj/16.16.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isshiki T, Mochizuki N, Maeda T, Yamamoto M. Characterization of a fission yeast gene, gpa2, that encodes a Gα subunit involved in the monitoring of nutrition. Genes Dev. 1992;6:2455–2462. doi: 10.1101/gad.6.12b.2455. [DOI] [PubMed] [Google Scholar]
- 32.Ivey F D, Hodge P N, Turner G E, Borkovich K A. The Gαi homologue gna-1 controls multiple differentiation pathways in Neurospora crassa. Mol Biol Cell. 1996;7:1283–1297. doi: 10.1091/mbc.7.8.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivey F D, Yang Q, Borkovich K A. Positive regulation of adenylyl cyclase activity by a Gαi homolog in Neurospora crassa. Fungal Genet Biol. 1999;26:48–61. doi: 10.1006/fgbi.1998.1101. [DOI] [PubMed] [Google Scholar]
- 34.Kasahara S, Wang P, Nuss D L. Identification of bdm-1, a gene involved in G protein β-subunit function and α-subunit accumulation. Proc Natl Acad Sci USA. 2000;97:412–417. doi: 10.1073/pnas.97.1.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawamukai M, Gerst J, Field J, Riggs M, Rodgers L, Wigler M, Young D. Genetic and biochemical analysis of the adenylyl cyclase-associated protein, cap, in Schizosaccharomyces pombe. Mol Biol Cell. 1992;3:167–180. doi: 10.1091/mbc.3.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kore-eda S, Murayama T, Uno I. Isolation and characterization of the adenylate cyclase structural gene of Neurospora crassa. Jpn J Genet. 1991;66:317–334. doi: 10.1266/jjg.66.317. [DOI] [PubMed] [Google Scholar]
- 37.Kraakman L, Lemaire K, Ma P, Teunissen A W, Donaton M C, Van Dijck P, Winderickx J, de Winde J H, Thevelein J M. A Saccharomyces cerevisiae G-protein coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose. Mol Microbiol. 1999;32:1002–1012. doi: 10.1046/j.1365-2958.1999.01413.x. [DOI] [PubMed] [Google Scholar]
- 38.Kronstad J, DeMaria A D, Funnell D, Laidlaw R D, Lee N, deSa M M, Ramesh M. Signaling via cAMP in fungi: interconnections with mitogen-activated protein kinase pathways. Arch Microbiol. 1998;170:395–404. doi: 10.1007/s002030050659. [DOI] [PubMed] [Google Scholar]
- 39.Krüger J, Loubradou G, Regenfelder E, Hartmann A, Kahmann R. Crosstalk between cAMP and pheromone signalling pathways in Ustilago maydis. Mol Gen Genet. 1998;260:193–198. doi: 10.1007/s004380050885. [DOI] [PubMed] [Google Scholar]
- 40.Kübler E, Mösch H-U, Rupp S, Lisanti M P. Gpa2p, a G-protein α-subunit, regulates growth and pseudohyphal development in Saccharomyces cerevisiae via a cAMP-dependent mechanism. J Biol Chem. 1997;272:20321–20323. doi: 10.1074/jbc.272.33.20321. [DOI] [PubMed] [Google Scholar]
- 41.Lee B N, Adams T H. fluG and fluA function interdependently to initiate conidiophore development in Aspergillus nidulans through brlAβ activation. EMBO J. 1996;15:299–309. [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis D A, Bisson L F. The HXT1 gene product of Saccharomyces cerevisiae is a new member of the family of hexose transporters. Mol Cell Biol. 1991;11:3804–3813. doi: 10.1128/mcb.11.7.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lichter A, Mills D. Fil1, a G-protein α-subunit that acts upstream of cAMP and is essential for dimorphic switching in haploid cells of Ustilago hordei. Mol Gen Genet. 1997;256:426–435. doi: 10.1007/s004380050586. [DOI] [PubMed] [Google Scholar]
- 44.Liu S, Dean R A. G protein α subunit genes control growth, development, and pathogenicity of Magnaporthe grisea. Mol Plant-Microbe Interact. 1997;10:1075–1086. doi: 10.1094/MPMI.1997.10.9.1075. [DOI] [PubMed] [Google Scholar]
- 45.Lorenz M C, Heitman J. Yeast pseudohyphal growth is regulated by GPA2, a G protein α homolog. EMBO J. 1997;16:7008–7018. doi: 10.1093/emboj/16.23.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lorenz M C, Heitman J. The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae. EMBO J. 1998;17:1236–1247. doi: 10.1093/emboj/17.5.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lorenz M C, Pan X, Harashima T, Cardenas M E, Xue Y, Hirsch J P, Heitman J. The G protein-coupled receptor Gpr1 is a nutrient sensor that regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Genetics. 2000;154:609–622. doi: 10.1093/genetics/154.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loubradou G, Begueret J, Turcq B. An additional copy of the adenylate cyclase-encoding gene relieves developmental defects produced by a mutation in a vegetative incompatibility-controlling gene in Podospora anserina. Gene. 1996;170:119–123. doi: 10.1016/0378-1119(95)00847-0. [DOI] [PubMed] [Google Scholar]
- 49.Loubradou G, Begueret J, Turcq B. MOD-D, a Gα subunit of the fungus Podospora anserina, is involved in both regulation of development and vegetative incompatibility. Genetics. 1999;152:519–528. doi: 10.1093/genetics/152.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madi L, McBride S A, Bailey L A, Ebbole D J. rco-3, a gene involved in glucose transport and conidiation in Neurospora crassa. Genetics. 1997;146:499–508. doi: 10.1093/genetics/146.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maeda T, Watanabe Y, Kunitomo H, Yamamoto M. Adenylyl cyclase is dispensable for vegetative cell growth in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1990;87:7814–7818. doi: 10.1073/pnas.87.20.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsumoto K, Uno I, Oshima Y, Ishikawa T. Isolation and characterization of yeast mutants deficient in adenylate cyclase and cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1982;79:2355–2359. doi: 10.1073/pnas.79.7.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsumoto K, Uno I, Ishikawa T. Identification of the structural gene and nonsense alleles for adenylate cyclase in Saccharomyces cerevisiae. J Bacteriol. 1984;157:277–282. doi: 10.1128/jb.157.1.277-282.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murayama T, Fujisawa Y, Okano Y. A suppressor mutation which suppresses adenylyl cyclase mutations in Neurospora crassa. Exp Mycol. 1995;19:320–323. doi: 10.1006/emyc.1995.1039. [DOI] [PubMed] [Google Scholar]
- 55.Nelson M A, Kang S, Braun E L, Crawford M E, Dolan P L, Leonard P M, Mitchell J, Armijo A M, Beran L, Blueyes E, Cushing T, Errett A, Fleharty M, Gorman M, Judson K, Miller R, Ortega J, Pavlova I, Perea J, Todisco S, Trujillo R, Valentine J, Wells A, Werner Washburne M, Natvig D O. Expressed sequence from conidial, mycelial and sexual stages of Neurospora crassa. Fungal Genet Biol. 1997;21:348–363. doi: 10.1006/fgbi.1997.0986. [DOI] [PubMed] [Google Scholar]
- 56.Nocero M, Isshiki T, Yamamoto M, Hoffman C S. Glucose repression of fbp1 transcription in Schizosaccharomyces pombe is partially regulated by adenylate cyclase activation by a G protein α subunit encoded by gpa2 (git8) Genetics. 1994;138:39–45. doi: 10.1093/genetics/138.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paietta J, Sargent M L. Photoreception in Neurospora crassa: correlation of reduced light sensitivity with flavin deficiency. Proc Natl Acad Sci USA. 1981;78:5573–5577. doi: 10.1073/pnas.78.9.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pall M L. The use of Ignite (Fasta; glufosinate; phosphinothricin) to select transformants of bar containing plasmids in Neurospora crassa. Fungal Genet Newsl. 1993;40:58–59. [Google Scholar]
- 59.Pall M L, Brunelli J P. New plasmid and λ/plasmid hybrid vectors and a Neurospora crassa genomic library containing the bar selectable marker and the Cre/lox site-specific recombination system for use in filamentous fungi. Fungal Genet Newsl. 1994;41:63–64. [Google Scholar]
- 60.Pan X, Heitman J. Cyclic AMP-dependent protein kinase regulates pseudophyphal differentiation in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:4874–4887. doi: 10.1128/mcb.19.7.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plesofsky-Vig N, Light D, Brambl R. Paedogenetic conidiation in Neurospora crassa. Exp Mycol. 1983;7:283–286. [Google Scholar]
- 62.Posas F, Takekawa M, Saito H. Signal transduction by MAP kinase cascades in budding yeast. Curr Opin Microbiol. 1998;1:175–182. doi: 10.1016/s1369-5274(98)80008-8. [DOI] [PubMed] [Google Scholar]
- 63.Regenfelder E, Spelling T, Hartmann A, Lauenstein S, Bölker M, Kahmann R. G proteins in Ustilago maydis: transmission of multiple signals? EMBO J. 1997;16:1934–1942. doi: 10.1093/emboj/16.8.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roberts A N, Berlin V, Hager K M, Yanofsky C. Molecular analysis of a Neurospora crassa gene expressed during conidiation. Mol Cell Biol. 1988;8:2411–2418. doi: 10.1128/mcb.8.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robertson L S, Fink G R. The three yeast A kinases have specific signaling functions in pseudohyphal growth. Proc Natl Acad Sci USA. 1998;95:13783–13787. doi: 10.1073/pnas.95.23.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenberg G B, Pall M L. Reconstitution of adenylate cyclase in Neurospora from two components of the enzyme. Arch Biochem Biophys. 1983;221:254–260. doi: 10.1016/0003-9861(83)90142-x. [DOI] [PubMed] [Google Scholar]
- 67.Rosenberg G B, Pall M L. Characterization of an ATP-Mg2+-dependent guanine nucleotide-stimulated adenylate cyclase from Neurospora crassa. Arch Biochem Biophys. 1983;221:243–253. doi: 10.1016/0003-9861(83)90141-8. [DOI] [PubMed] [Google Scholar]
- 68.Sachs M S, Yanofsky C. Developmental expression of genes involved in conidiation and amino acid biosynthesis in Neurospora crassa. Dev Biol. 1991;148:117–128. doi: 10.1016/0012-1606(91)90322-t. [DOI] [PubMed] [Google Scholar]
- 69.Sambrook J, Fritsch E F, Maniatis T. Detection and analysis of proteins expressed from cloned genes. In: Nolan C, editor. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 18.1–18.88. [Google Scholar]
- 70.Skromne I, Sanchez O, Aguirre J. Starvation stress modulates the expression of the Aspergillus nidulans brlA regulatory gene. Microbiology. 1995;141:21–28. doi: 10.1099/00221287-141-1-21. [DOI] [PubMed] [Google Scholar]
- 71.Springer M L. Genetic control of fungal differentiation: the three sporulation pathways of Neurospora crassa. Bioessays. 1993;15:365–374. doi: 10.1002/bies.950150602. [DOI] [PubMed] [Google Scholar]
- 72.Staben C, Jensen B, Singer M, Pollock J, Schechtman M, Kinsey J, Selker E. Use of a bacterial hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet Newsl. 1989;36:79–81. [Google Scholar]
- 73.Terenzi H F, Flawiá M M, Torres H N. A Neurospora crassa morphological mutant showing reduced adenylate cyclase activity. Biochem Biophys Res Commun. 1974;58:990–996. doi: 10.1016/s0006-291x(74)80241-x. [DOI] [PubMed] [Google Scholar]
- 74.Terenzi H F, Flawia M M, Tellez-Iñon M T, Torres H N. The control of Neurospora crassa morphology by cyclic adenosine 3′,5′-monophosphate and dibutyryl cyclic adenosine 3′,5′-monophosphate. J Bacteriol. 1976;126:91–99. doi: 10.1128/jb.126.1.91-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Theibert A, Devreotes P N. Surface receptor-mediated activation of adenylate cyclase in Dictyostelium. J Biol Chem. 1986;261:15121–15125. [PubMed] [Google Scholar]
- 76.Thevelein J M, de Winde J H. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1999;33:904–918. doi: 10.1046/j.1365-2958.1999.01538.x. [DOI] [PubMed] [Google Scholar]
- 77.Ton That T T, Turian G. Ultrastructural study of microcyclic macroconidiation in Neurospora crassa. Arch Microbiol. 1978;116:279–288. doi: 10.1007/BF00417852. [DOI] [PubMed] [Google Scholar]
- 78.Tsui H-C T, Pease A J, Koehler T M, Winkler M E. Detection and quantitation of RNA transcribed from bacterial chromosomes and plasmids. In: Adolph K W, editor. Methods in molecular genetics. San Diego, Calif: Academic Press, Inc.; 1994. pp. 197–200. [Google Scholar]
- 79.Turner G E, Borkovich K A. Identification of a G protein α subunit from Neurospora crassa that is a member of the Gi family. J Biol Chem. 1993;268:14805–14811. [PubMed] [Google Scholar]
- 80.Vann D C. Electroporation-based transformation of freshly harvested conidia of Neurospora crassa. Fungal Genet Newsl. 1995;42A:53. [Google Scholar]
- 81.Wang J, Suzuki N, Nishida Y, Kataoka T. Analysis of the function of the 70-kilodalton cyclase-associated protein (CAP) by using mutants of adenylyl cyclase defective in CAP binding. Mol Cell Biol. 1993;13:4087–4097. doi: 10.1128/mcb.13.7.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wess J. G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J. 1997;11:346–354. [PubMed] [Google Scholar]
- 83.Xue Y, Batlle M, Hirsch J P. GPR1 encodes a putative G protein-coupled receptor that associates with the Gpa2p Gα subunit and functions in a Ras-independent pathway. EMBO J. 1998;17:1996–2007. doi: 10.1093/emboj/17.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu J H, Wieser J, Adams T H. The Aspergillus FlbA RGS domain protein antagonizes G protein signaling to block proliferation and allow development. EMBO J. 1996;15:5184–5190. [PMC free article] [PubMed] [Google Scholar]
- 85.Yun C W, Tamaki H, Nakayama R, Yamamoto K, Kumagai H. G-protein coupled receptor from yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1997;240:287–292. doi: 10.1006/bbrc.1997.7649. [DOI] [PubMed] [Google Scholar]