Abstract
Objectives: Peripheral skeletal muscle dysfunction is an important extrapulmonary manifestation of chronic obstructive pulmonary disease (COPD) that can be counteracted by exercise training. This study aimed to review the effect of three major exercise training modalities, which are used in pulmonary rehabilitation to improve on skeletal muscle mass, function, and exercise capacity in COPD.
Methods: PubMed, Embase, EBSCO, Web of Science, and the PEDro database were searched on April 25, 2020. Only randomized controlled studies published in English evaluating the effects of exercise interventions on peripheral skeletal muscle mass, strength, and exercise capacity in stable COPD patients were included. The quality of included studies was evaluated using the PEDro scale. The mean difference (MD) or the standardized mean difference (SMD) with 95% CI was calculated to summarize the results. Subgroup meta-analysis was used to investigate the effects of different exercise training modalities and different outcome measures. The Grading of Recommendations Assessment, Development, and Evaluation guidelines were used to rate evidence quality.
Results: A total of 30 randomized controlled trials involving 1,317 participants were included. Data from trials investigating endurance exercise (EE), resistance exercise (RE), and combined aerobic and resistance exercise (CE) were pooled into a meta-analysis, and the differences compared with the non-exercising COPD control were improvement in the muscle strength and exercise capacity in stable COPD patients. Subgroup meta-analysis for different exercise training modalities showed that RE significantly improved muscle strength (SMD = 0.6, 95% CI 0.35–0.84, I2 = 61%), EE and CE significantly increased VO2peak (EE: MD = 3.5, 95% CI 1.1–5.91, I2 = 92%; CE: MD = 1.66, 95% CI 0.22–3.1, I2 = 1%). Subgroup meta-analysis for different outcome measures showed that only isotonic strength was improved after exercise interventions (SMD = 0.89, 95% CI 0.51–1.26, I2 = 71%).
Conclusion: Moderate evidence supports that exercise training in stable COPD patients has meaningful and beneficial effects on peripheral skeletal muscle strength and exercise capacity. Peripheral skeletal muscle shows a higher response to RE, and the isotonic test is relatively sensitive in reflecting muscle strength changes. The proportion of aerobic and resistance exercise components in a combined exercise program still needs exploration.
Systematic Review Registration: The review was registered with the PROSPERO: (The website is https://www.crd.york.ac.uk/PROSPERO/, and the ID is CRD42020164868).
Keywords: chronic obstructive pulmonary disease, exercise training, meta-analysis, skeletal muscle dysfunction, exercise capacity
Introduction
Chronic obstructive pulmonary disease (COPD) is a common disease characterized by persistent respiratory symptoms and expiratory flow limitation (1). Furthermore, many patients with COPD experience systematic symptoms, including impaired cardiopulmonary and skeletal muscle function (2, 3). Skeletal muscle dysfunction is one of the significant systemic manifestations of COPD, characterized by the loss of muscle mass, a transition of the fiber type proportion, a decrease in the capillary to fiber ratio, and muscle strength and endurance (4, 5). In most patients with COPD, the observed decrease in muscle strength is proportional to muscle mass loss, suggesting that the onset of skeletal muscle dysfunction is caused by paralleled chronic inactivity and muscle deconditioning rather than myopathy (6). The existence of dyspnea in COPD decreases physical activity, and the decrease in physical activity induces and accelerates skeletal muscle dysfunction, worsening the dyspnea in patients, forming a vicious cycle that causes further deconditioning on COPD (7). Recently, lower limb muscle function has been associated with exercise capacity in COPD (8). Previous studies have confirmed that skeletal muscle dysfunction is an additional important contributor to COPD exercise restriction and function impairments (9, 10), and it is closely related to the quality of life, readmission rate, and mortality (11, 12).
Pulmonary rehabilitation is a comprehensive management program designed for COPD and has significant clinical effects in improving dyspnea, quality of life, and exercise capacity (1). As the cornerstone of pulmonary rehabilitation, exercise training can effectively reverse or at least stabilize the loss of skeletal muscle mass and strength in patients with COPD, and it is considered currently the most effective non-pharmaceutical intervention for COPD skeletal muscle dysfunction (13). The American Thoracic Society/European Respiratory Society (ATS/ERS) statement provided a short overview of the effects of exercise interventions on the muscle function and mass in COPD, showing that exercise interventions can improve the morphology and function of COPD skeletal muscle (12), but the included literatures are extensive and heterogeneous. Another international guideline described and analyzed the effects of different exercise modalities in COPD skeletal muscle dysfunction and provided a GRADE scale for evidence quality (4). In 2018, a review included 70 English language literature to be analyzed and concluded that exercise intervention could improve COPD skeletal muscle strength, endurance, and mass, despite the fact that intervention programs and outcome measures were heterogeneous (14). Therefore, although previous international guidelines and recent reviews have consistently concluded that exercise training improves COPD skeletal muscle dysfunction, it is still difficult to clarify the degree of real benefit due to the diversity and heterogeneity of exercise intervention programs and outcome measures. Previous meta-analysis of exercise in COPD explored the effects of resistance exercise (RE) on exercise capacity (15), endurance exercise (EE) vs. RE (16), and combined aerobic and resistance exercise (CE) vs. EE on lower limb muscle strength and exercise capacity (17). However, these studies focused on the effects of single exercise modality or the compared effects of two exercise modalities. There is still a lack of comprehensive quantitative effect of exercise on peripheral skeletal muscle mass, strength, and exercise capacity in COPD.
In this systematic review and meta-analysis, the effects of exercise interventions on peripheral skeletal muscle mass, strength, and exercise capacity in COPD were determined. The characteristics of different exercise modalities were further discussed to provide a theoretical reference for developing a targeted COPD exercise rehabilitation program.
Methods
Search Strategy and Selection Criteria
This systematic review and meta-analysis was registered (PROSPERO registration number: CRD42020164868) and conducted according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) recommendations (18). According to the principle of population intervention comparison outcomes, the inclusion criteria were as follows: (a) participants diagnosed with stable COPD, and without gender and age restrictions; (b) EE and or RE was used for intervention; (c) a comparable control group applied with other treatments, including health education and sham training; (d) outcomes including skeletal muscle mass related parameters (body mass index, BMI; fat-free mass index, FFM; and cross-sectional area, CSA), strength-related parameters (isometric, isotonic, and isokinetic strength), endurance exercise capacity (6-min walking distance, 6MWD), and peak exercise capacity (peak oxygen consumption, VO2peak); and (e) randomized controlled study published in English. The exclusion criteria were as follows: (a) the immediate response to a single exercise test or exercise session was studied; (b) the follow-up effects of previous exercise program were studied; (c) traditional Chinese exercise and yoga were used for interventions; (d) animal trials, observational trials, expert opinions, literature reviews, comments, and letters were involved; (e) regular exercise programs were utilized in control groups (e.g., breath training, ≥twice a week); and (f) data could not be extracted.
Electronic searches of PubMed, Embase, EBSCO, Web of Science, and PEDro database were conducted from inception to April 25, 2020 using Medical Subject Headings (MeSH) terms and free-text keywords. In addition to the PEDro database, the following search terms were used: (COPD OR chronic obstructive pulmonary disease OR chronic obstructive lung disease OR chronic obstructive airway disease) AND (exercise OR exercise training OR rehabilitation OR pulmonary rehabilitation OR aerobic exercise OR endurance exercise OR resistance exercise OR strength training OR combined exercise) AND (muscle OR skeletal muscle). Search filters were applied, including article type (randomized controlled trials), species (humans), and language (English). In the PEDro database, the search terms were as follows: topic (chronic respiratory disease), method (clinical trial), therapy (fitness training), and abstract and title (COPD). Searches were supplemented by reviewing the reference lists of the included studies, previous review, meta-analysis, and guidelines.
To determine the eligibility of identified studies, two investigators independently conducted the process of study selection. Cohen's kappa was used to quantify the interrater agreement. Discrepancies of opinion between authors about study eligibility were resolved through discussions with a third investigator.
Data Analysis
Two investigators independently extracted data on study design, sample characteristics, intervention programs, and effects of exercise from included studies. Discrepancies were resolved through discussions with a third investigator. The studies were described in terms of study design (sample size, and PEDro score), sample characteristics (age, sex, FEV1%pred for forced expiratory volume in 1 s, and BMI), intervention programs (site, exercise modality, intensity, frequency, and duration), effects of exercise (outcome measures and change data), and adherence to the program. For trials with more than one exercise intervention group, the effects of each exercise intervention were evaluated. For trials with more than one outcome measures, the data of each outcome measures was included and analyzed. For trials with multiple time points, only the pre-intervention and post-intervention outcomes were extracted.
Predetermined primary outcomes included skeletal muscle mass (BMI, FFM, and CSA), strength (isometric, isotonic, and isokinetic strength), endurance exercise capacity (6MWD), and peak exercise capacity (VO2peak). Secondary outcomes were attrition rate and severe adverse events. The change in mean and SD were calculated for each outcome and used to estimate the effects of the exercise. Summary measures for continuous outcomes were mean difference (MD) or standard mean difference (SMD) with 95% CI, and odds ratio (OR) with 95% CI for the attrition rate.
Review Manager (version 5.3) provided by Cochrane was used for meta-analysis. Random-effects model was used for analyzing. The I2 statistic, representing the percentage of variation across studies due to heterogeneity, was used to assess heterogeneity between studies. Planned subgroup analyses were conducted in terms of exercise modalities (EE, RE, and CE) and outcome measures (isometric, isotonic, and isokinetic strength test). Sensitivity analyses were performed to check the heterogeneity source based on the intervention program and characteristics of the participants when subgroup analysis could not determine the source of substantial heterogeneity. Visual inspection of funnel plots and Egger's test were undertaken in Stata (version 15) to assess publication bias. Trim and fill method was used when there was a publication bias. The methodological quality of randomized controlled trial (RCTs) was assessed using the physiotherapy evidence database (PEDro) scale. When available, the PEDro rating and score were obtained from the PEDro database. Otherwise, two investigators independently rated and scored the publications; discrepancies were resolved through discussions with a third investigator. The PEDro scale includes 11 items with 10 scores, and a higher score means better quality (19). It should be noted that the eligibility criteria item does not contribute to the total score. PEDro scale 9–10 was considered high quality, 6–8 was generally high quality, 4–5 was moderate quality, and <4 was low quality. The quality of evidence was assessed according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) recommendations (limitation of study design, inconsistency, indirectness, imprecision, and publication bias) (20).
Results
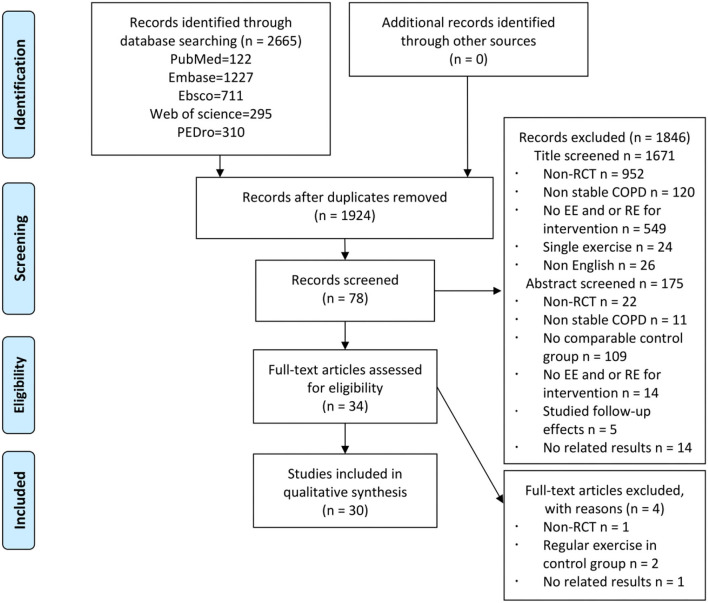
A total of 2,665 records were identified, and 30 RCTs were included in the quantitative analysis (Figure 1). A strong agreement was observed with respect to the interrater reliability of study selection (kappa = 0.89, P < 0.001). The PEDro scale of all included studies is 5.7 ± 1.4 (Supplementary Table S1), and the characteristics of participants of each included study are reported in Table 1. A total of 1,317 participants with stable COPD (age range from 46 to 79.8 years) were included, and 675 (51%) participants accepted exercise intervention. According to the criteria of Global strategy for the diagnosis, management, and prevention of COPD (GOLD), majority of the participants showed moderate to severe airflow restriction (30% ≤ FEV1%pred ≤ 80%), and four studies did not provide the baseline data of FEV1%pred (27, 30, 38, 41). Most participants were normal to overweight (BMI: 18.5–29.9 kg/cm2), while five studies did not provide this data (27, 38, 39, 42, 49). In addition, exercise intervention programs of all the included studies are presented in Table 2. Most trials were conducted in a hospital, at home, or at both the places, while six studies did not report a place (31, 35, 38, 40, 45, 50). Most studies applied exercise program duration ranges from 6 to 12 weeks, while some studies applied 14 weeks (22), 16 weeks (47), and 24 weeks (38). EE was mainly performed in the form of treadmill, cycling, or walking with a moderate to vigorous exercise intensity (Borg 4–6, even exhaustion, despite indexes used to assess were various) for two to three sessions per week. RE was mainly performed on weight machines, free weights, and elastic bands through the movements of the upper and lower limbs. One study performed RE only through the upper limbs (35) and three studies conducted RE only through the lower limbs (31, 33, 34). Exercise intensity of RE ranged from 50 to 85% 1-repetition maximum (1RM) or Borg 4–6, and exercise frequency was two to three sessions per week. The performance of CE was consistent with EE and RE. The exercise intensity of EE was Borg 4–6, while the exercise intensity of RE was often unclear. The characteristics of muscle strength testing relative to the variety of muscle strength testing methods and programs are summarized in Table 3.
Figure 1.
Study flow diagram. COPD, chronic obstructive pulmonary disease; EE, endurance exercise; RCT, randomized controlled trial; RE, resistance exercise.
Table 1.
Characteristics of included studies.
| Author, Country | I/C sample size/Male% | Age | FEV1%pred | BMI | Outcome | Attrition number* | PEDro | ||
|---|---|---|---|---|---|---|---|---|---|
| Mass | Strength | Exercise capacity | |||||||
| Endurance exercise | |||||||||
| Alcazar et al. (21), Spain | 14(79%)/ 15(87%) |
77.7 ± 7.9/ 79.8 ± 6.4 |
47.4 ± 18.1/ 58.7 ± 15.2 |
28.8 ± 3/ 32.5 ± 5.9 |
Mid-thigh CSA | Leg press | 6MWD VO2peak |
5/1* | 4 |
| Barakat et al. (22), France | 40(85%)/ 40(83%) |
63.7 ± 11.9/ 65.9 ± 10.3 |
41.9 ± 2.6/ 43.33 ± 3.6 |
24.2 ± 6.4/ 25.6 ± 4.3 |
/ | / | 6MWD | 5/4* | 6 |
| Borghi-silva et al. (23), USA | 20(65%)/ 14(86%) |
67 ± 10/ 67 ± 10 |
33 ± 9/ 35 ± 11 |
25 ± 4/ 24 ± 5 |
/ | / | 6MWD VO2peak |
0/6 | 5 |
| Borghi-silva et al. (24), USA | 10(70%)/ 10(50%) |
67 ± 7/ 66 ± 10 |
32 ± 11/ 35 ± 12 |
23.4 ± 4.4/ 27.2 ± 4.7 |
/ | / | 6MWD VO2peak |
7/5 | 5 |
| de Souto Araujo et al. (25), Brazil | 13(62%)/ 11(73%) |
56.9 ± 7.9/ 71.1 ± 10.1 |
39.2 ± 11.4/ 45.1 ± 12.6 |
30 ± 10.1/ 24.4 ± 6.7 |
/ | / | 6MWD | 1/3 | 4 |
| Gallo-Silva et al. (26), Brazil | 10/9 | 66.3 ± 6.5/ 66.5 ± 9.5 |
47.9 ± 20.5/ 47.8 ± 26.2 |
23.2 ± 2.6/ 25.7 ± 6.1 |
/ | / | 6MWD | 2/3 | 6 |
| Mehri et al. (27), Iran | 20(55%)/ 18(39%) |
52.1 ± 10.7/ 52.17 ± 11.6 |
/ |
/ | / | / | VO2peak | 0/0 | 5 |
| Petersen et al. (28), Denmark | 9(22%)/ 10(40%) |
67 ± 3/ 66 ± 3 |
33 ± 5/ 30 ± 4 |
23 ± 2/ 26 ± 2 |
/ | / | VO2 max | 0/4 | 5 |
| Pradella et al. (29), Brazil | 29(79%)/ 15(87%) |
62.4 ± 10.7/ 65.3 ± 8 |
43.9 ± 16.2/ 54 ± 22.2 |
25.2 ± 5/ 26.7 ± 5.3 |
/ | / | 6MWD | 3/3 | 5 |
| Wiyono et al. (30), Indonesia | 27(93%)/ 29(96%) |
64.3 ± 6.3/ 67.2 ± 4.5 |
/ |
19.7 ± 8.5/ 20.2 ± 6.4 |
/ | / | 6MWD VO2 max |
3/1 | 5 |
| Resistance exercise | |||||||||
| Casaburi et al. (31), USA | 12 (100%)/ 12(100%) |
68.9 ± 9.8/ 67.7 ± 8.7 |
35.9 ± 9.2/ 38.6 ± 12.1 |
27.57/ 26.31 |
Total lean mass | Leg press | VO2peak | 1/1 | 5 |
| Clark et al. (32), Scotland | 26(58%)/ 17(59%) |
51 ± 10/ 46 ± 11 |
76 ± 23/ 79 ± 23 |
26 ± 4/ 26 ± 4 |
BMI | Quadriceps | VO2 max | 5 | |
| Chen et al. (33), China | 25(88%)/ 22(68%) |
69.04 ± 8.07/ 64.95 ± 11.59 |
54.49 ± 23.62/ 54.93 ± 25.58 |
23.86 ± 3.9/ 24.15 ± 3.93 |
/ | Quadriceps | 6MWD | 4/4 | 6 |
| Hoff et al. (34), USA | 6(67%)/ 6(67%) |
62.8 ± 1.4/ 60.6 ± 3 |
32.9 ± 3.3/ 39.5 ± 6.4 |
26.27/ 26.65 |
BMI | Leg press | VO2peak | 0/0 | 6 |
| Janaudis-Ferreira et al. (35), Canada | 17(53%)/ 19(37%) |
67 ± 11/ 67 ± 11 |
37.8 ± 16.2/ 32.5 ± 14.1 |
27.9 ± 7.9/ 25.7 ± 8.2 |
/ | Biceps Triceps Anterior Middle deltoids |
/ | 4/1* | 9 |
| Nyberg et al. (36), Sweden | 22(55%)/ 22(50%) |
69 ± 5/ 68 ± 6 |
59 ± 11/ 55 ± 15 |
26 ± 4/ 25 ± 5 |
/ | Shoulder flexion Knee extension |
6MWD | 2/2* | 8 |
| O'shea et al. (37), Australia | 27/27 | 66.9 ± 7/ 68.4 ± 9.9 |
49 ± 25/ 52 ± 22 |
25.5 ± 5.1/ 27.8 ± 7.9 |
/ | Knee extension Hip abduction Shoulder horizontal flexion Shoulder flexion |
6MWD | 7/3* | 7 |
| Thabitha et al. (38), India | 30 | / | / | / | / | / | 6MWD VO2peak |
/ | 4 |
| Simpson et al. (39), Canada | 14(35%)/ 14(71%) |
73 ± 4.8/ 70 ± 5.7 |
39.5 ± 18.96/ 39.2 ± 21.39 |
/ | / | Elbow flexion Quadriceps Leg press |
6MWD VO2 max |
3/3 | 6 |
| Zambom-Ferraresi et al. (40), Spain | 14(100%)/ 8(100%) |
68 ± 7/ 69 ± 5 |
48 ± 12/ 39.7 ± 5 |
28.5 ± 3.9/ 25.7 ± 4.6 |
/ | Leg press Chest press |
6MWD VO2peak |
1/1 | 7 |
| Combined exercise | |||||||||
| Cameron-Tucker et al. (41), Australia | 43(53%)/ 41(54%) |
64.5 ± 9.3/ 67.1 ± 9.41 |
/ | 28.4 ± 7.63/ 29.7 ± 6.5 |
/ | / | 6MWD | 5/10* | 6 |
| Emery et al. (42), USA | 30(50%)/ 24(42%) |
65.4 ± 6.4/ 67.4 ± 5.9 |
43 ± 18/ 43 ± 18 |
/ | / | / | VO2 max | 4/2* | 6 |
| Lahham et al. (43), Australia | 29(59%)/ 29(59%) |
68 ± 9/ 67 ± 10 |
90 ± 8/ 92 ± 7 |
28 ± 4.5/ 28 ± 4.3 |
/ | / | 6MWD | 3/4* | 8 |
| Mendes et al. (44), Brazil | 23(83%)/ 29(66%) |
71.3 ± 6.7/ 70.8 ± 8.7 |
51.5 ± 23.9/ 41.4 ± 18.4 |
23.5 ± 4.2/ 24.6 ± 6.3 |
/ | / | 6MWD | 23/0 | 4 |
| Nakamura et al. (45), Japan | 10/ 10 |
69 ± 8.7/ 69.9 ± 7.1 |
53.2 ± 15.1/ 48.2 ± 20.1 |
21.9 ± 3.5/ 21.6 ± 3 |
/ | HGF | 6MWD VO2peak |
/ | 5 |
| Tsai et al. (46), Australia | 19(63%)/ 17(35%) |
78 ± 3/ 75 ± 9 |
60 ± 23/ 68 ± 19 |
28 ± 4/ 28 ± 5 |
/ | / | 6MWD | 1/0 | 8 |
| van Wetering et al. (47), Netherlands | 102(71%)/ 97(71%) |
65.9 ± 8.8 / 67.2 ± 8.9 |
58 ± 17/ 60 ± 15 |
26.1 ± 4.4/ 27.3 ± 4.7 |
BMI FFMI |
HGF Quadriceps |
6MWD | 15/9* | 7 |
| Wadell et al. (48), Canada | 17(53%)/ 24(54%) |
68 ± 6/ 66 ± 7 |
48 ± 12/ 48 ± 19 |
26.7 ± 4.9/ 28.9 ± 4.3 |
/ | Knee extension | 6MWD | 3/ 4* |
6 |
| Wadell et al. (49), Sweden | 15(33%)/ 13(54%) |
65 ± 7/ 63 ± 7 |
53 ± 12/ 49 ± 12 |
/ | / | / | VO2peak | 1/ 1* |
6 |
| Weiner et al. (50), Israel | 18/ 5 |
63.2 ± 2.3/ 60.1 ± 2.8 |
35 ± 2.2/ 36 ± 1.9 |
23.84/ 24.84 |
/ | / | 6MWD | 1/1* | 5 |
| Zambom-Ferraresi et al. (40), Spain | 14(100%)/ 8(100%) |
68 ± 7/ 69 ± 5 |
44.3 ± 11.9/ 39.7 ± 5 |
29.3 ± 6.4/ 25.7 ± 4.6 |
/ | Leg press Chest press |
6MWD VO2peak |
2/1 | 7 |
6MWD, 6-min walking distance; HGF, Handgrip force; I/C, Intervention group/Control group; RM, Repetition maximum; VO2, Oxygen uptake.
/Not accessible;
Attrition number is included in the sample size.
Table 2.
Characteristics of intervention protocols.
| Author, Country | Setting | Intervention contents | Intervention intensity | Intervention duration/frequency | Control group |
|---|---|---|---|---|---|
| Endurance exercise | |||||
| Alcazar et al. (21), Spain | Outpatient | First 3 weeks: HIIT (5 sets of 90 s at light intensity plus 30 s at heavy intensity) + power training (2–3 sets of 8–12 reps) Week 4–12: HIIT (10stes) + 3sets of 8reps with the optimal load |
First 3 weeks: HIIT (heavy-80%Wpeak, light-40% Wpeak) + power training (50–60% 1RM) Week 4–12: HIIT (augmented) + power training (optimal load) | 2 sessions/week, 12 weeks | Usual care |
| Barakat et al. (22), France | Outpatient | 30 min cycling + 30 min aerobic activity (5 min warm-up, 10 min aerobic activity, 15 min cool-down) | Cycle: 80%VO2 max | 3 sessions/week, 14 weeks | Routine outpatient attendance |
| Borghi-Silva et al. (23), USA | Outpatient | 30 min stretching + treadmill ambulation | 70% of the maximal speed | 3 sessions/week, 6 weeks | Usual care |
| Borghi-Silva et al. (24), USA | Outpatient | 5 min warm-up + 30 min treadmill | 70% of the peak speed/Borg 4 | 3 sessions/week, 12 weeks | Respiratory therapy, 1session/week |
| de Sauto Araujo et al. (25), Brazil | Outpatient | 15 min callisthenic activities + 30 min unsupported upper limb exercise using weights + 30 min bicycle + 15 min cool-down | Upper: 50% of the maximum load; Lower: Borg 5 | 3 sessions/week, 8 weeks | No exercise |
| Gallo-Silva et al. (26), Brazil | Laboratory | 60 min water aerobic interval exercise (10 min warm-up, 20–40min aerobic exercise, 10 min cool-down) | Borg 4–6 | 3 sessions/week, 8 weeks | Usual care |
| Mehri et al. (27), Iran | Outpatient | Treadmill exercise training with gradually increased speed | Exhaustion | 2 sessions/week, 8 weeks | No exercise |
| Petersen et al. (28), Denmark | Outpatient | Walking with 85% maximal speed + progressive ergometer cycling | Exhaustion | 2 sessions/week, 7 weeks | Usual daily activities |
| Pradella et al. (29), Brazil | Home | 40 min walking + 15 min stair exercise + arm exercise with 1 kg load (3 sets of 30 movements) | Walking: 60–70% HRmax | 3 sessions/week, 8 weeks | No exercise |
| Wiyono et al. (30), Indonesia | Outpatient | 5 min cycling, and gradually increased for 5 min/week | / | 3 sessions/week, 6 weeks | Routine outpatient attendance |
| Resistance exercise | |||||
| Casaburi et al. (31), USA | / | First 4 weeks: 3 sets of 12 reps; Week 5–10: 4 sets of 8–10 reps (seated leg press, seated leg curl, seated leg extension, standing calf raise, seated ankle dorsiflexion) |
First 4 weeks: 60% 1RM Week 5–10: 80% 1RM | 3 sessions/week, 10 weeks | No exercise |
| Clark et al. (32), Scotland | Outpatient | 3 sets of 10 reps weight exercises (bench press/triceps, body squat/quadriceps, squat calf/medial and lateral gastrocnemiius soleus, latissimus/latissimus dorsi/arm curls/biceps, leg press/quadriceps hamstrings gluteals, knee flexion/quadriceps, hamstrings) |
70% maximal load | 2 sessions/week, 12 weeks | Usual daily activities |
| Chen et al. (33), China | Home | 20–30min, 8–12 reps Thera-band exercise (straight-leg lifting, prone hip extension, thigh abduction, posterior muscle group exercises, anterior muscle group exercises, and standing calf raise) |
Borg 5 | 3 sessions/week, 12 weeks | No exercise |
| Hoff et al. (34), USA | Laboratory | 4 sets of 5 reps concentric contraction of quadriceps | 85–90% 1RM | 3 sessions/week, 8 weeks | Normal daily living |
| Janaudis-Ferreira et al. (35), Canada | / | 10–12RM using free weights and a multistation gym (biceps brachii, triceps brachii, pectoralis major and minor, latissimus dorsi, deltoids, rhomoboids) |
10–12RM | 3 sessions/week, 6 weeks | Upper limb flexibility and stretching exercises |
| Nyberg et al. (36), Sweden | Outpatient | 40 min, 2 sets of 25 reps Thera-band exercise (Latissimus row/chest press/leg extension/shoulder flexion/leg curl/elbow flexion/heel-raise/step up) |
Borg 4 | 3 sessions/week, 8 weeks | No exercise |
| O'shea et al. (37), Australia | 1 hospital + 2 home | 3 sets of 8–12 reps Thera-band exercise (hip abduction in standing, simulated lifting, SST, seated row, lunges, chest press) |
12RM and gradually increased | 3 sessions/week, 12 weeks | No exercise |
| Thabitha et al. (38), India | / | 15–30min, 1–3 sets of 10 reps using multi exerciser (chest pull-lattismus dorsi, butterfly-pectoralis major muscle, neck press-triceps brachii and deltoid, leg flexion-biceps femoris and gastronemious, leg extension) | 10RM and increased by 10% | 2 sessions/day, 3 days/week, 24 weeks | No exercise |
| Simpson et al. (39), Canada | Outpatient | 3 sets of 10 reps single limb weight lifting exercise (arm curl/leg extension/leg press) | 50–85% 1RM | 3 sessions/week, 8 weeks | No exercise |
| Zambom-Ferraresi et al. (40), Spain | / | 90 min, 3–4 sets of 6–12 reps (chest press, seated row, shoulder press, leg press, knee extension and flexion) | 50–70% 1RM | 2 sessions/week, 12 weeks | Habitual physical activity |
| Combined exercise | |||||
| Cameron-Tucker et al. (41), Australia | Outpatient | 1 h combine exercises, individualized for each participant | RPE 3–5 | 1 sessions/week, 6 weeks | No exercise |
| Emery et al. (42), USA | Outpatient | First 5 weeks: 45 min combine exercises on Nautilus equipment; Week 6–10: 60–90 min |
/ | First 5 weeks: every-day; Week 6–10: 3 sessions/week |
No exercise |
| Lahham et al. (43), Australia | Home | Aerobic: 80% of walking speed from 6MWD + 30 min whole-body exercise; Resistance: using equipment available at home (stairs and sealed water bottles) |
/ | 5 sessions/week, 8 weeks | No exercise |
| Mendes et al. (44), Brazil | Outpatient | Aerobic: 30 min treadmill walking; Resistance: 10 reps (hand weight, elbow flexion, elbow abduction, shoulder abduction, shoulder flexion, hip flexion, knee extension) |
Aerobic: 60–80% HRmax Resistance: 50% 1RM with an increase of 0.5 kg every 2 weeks | 3 sessions/week, 12 weeks | No exercise |
| Nakamura et al. (45), Japan | / | Aerobic: 20 min walking; Resistance: 30 min, 3 sets of 10 reps using self-weight or elastic bands (push-ups, leg squats, sit-ups, back extension) |
Aerobic: Borg 3–5 | 12 weeks | No exercise |
| Tsai et al. (46), Australia | Home | Aerobic: 15–20 min cycling + 15–20 min walking Resistance: 3 sets of 10 reps SST and squats exercise |
Cycle: 60–80% Wpeak Walk: 80% of best 6MWD or Aerobic: Borg 3–4 | 3 sessions/week, 8 weeks | Usual care |
| van Wetering et al. (47), Netherlands | Community | Aerobic: 30 min cycling/walking Resistance: 4 specific exercises for upper and lower limbs |
/ | 2 sessions/week, 16 weeks | Usual care |
| Wadell et al. (48), Canada | Outpatient | 2.5 h combine exercise | Moderate intensity | 3 sessions/week, 8 weeks | Usual care |
| Wadell et al. (49), Sweden | Outpatient | 45 min, (4 min aerobic, 3 min leg resistance, 4 min aerobic, 3 min arm resistance, 4 min aerobic, 3 min torso resistance) | 80–100%HR peak or Borg 5 or RPE 15 | 3 sessions/week, 12 weeks | No exercise |
| Weiner et al. (50), Israel | / | Aerobic: 30 min cycling; Resistance: 15 min rowing with low resistance +15 min resistance exercises for limbs and abdominal muscles |
Aerobic: 50% Wmax | 3 sessions/week, 6 weeks | Sham training |
| Zambom-Ferraresi et al. (40), Spain | / | Aerobic: 20–35 min cycle Resistance: 90 min, 3–4 sets of 6–12 reps (chest press, seated row, shoulder press, leg press, knee extension and flexion) |
Aerobic: 40–85% Wmax Resistance: 50–70% 1RM | 2 sessions/week for each exercise types, 12 weeks | Habitual physical activity |
6MWD, 6-min walking distance; HIIT, high intensity interval training; HGF, Handgrip force; HR, Heart rate; reps, repetitions; RM, Repetition maximum; SST, sit to stand; Wmax, Maximal work rate; VO2, Oxygen uptake.
/Not accessible.
Table 3.
Characteristics of skeletal muscle strength tests.
| Type | Author, Country | Outcomes | Methods | Apparatus | Site |
|---|---|---|---|---|---|
| Isometric test | Alcazar et al. (21), Spain | Leg press (N) | Evaluate two legs performance, test for at least 4s | Force plate | Lower limb |
| Chen et al. (33), China | Quadriceps (Nm) | Evaluate the maximal strength of dominant leg | Computerized dynamometer | Lower limb | |
| Janaudis-Ferreira et al. (35), Canada | Biceps (kg) Triceps (kg) Anterior (kg) Middle deltoids (kg) |
Evaluate the dominant side by Micro FET2, the average of the highest 3 measures were used for analysis | Hand-held dynamometer | Upper limb | |
| Nakamura et al. (45), Japan | HGF (kg) | Evaluate the dominant side | Hand-grip dynamometer | Upper limb | |
| Wadell et al. (48), Canada | Knee extension (kg) | Fixed dynamometer | Lower limb | ||
| van Wetering et al. (47), Netherlands | HGF (kg) Quadriceps (Nm) |
Unknown device | Upper limb Lower limb | ||
| Isotonic test | Casaburi et al. (31), USA | Leg press (kg) | Evaluate two legs performance by 1RM test | Pneumatic device | Lower limb |
| Clark et al. (32), Scotland | Quadriceps (kg) | 1RM test | Multigym | ||
| Hoff et al. (34), USA | Leg press (kg) | 1RM test | Force platform | ||
| O'shea et al. (37), Australia | Knee extension (kg) Hip abduction (kg) Shoulder horizontal flexion (kg) Shoulder flexion (kg) |
Averaged across right and left limbs were used for analysis | Hand-held dynamometry | Lower limb Lower limb Upper limb Upper limb | |
| Simpson et al. (39), Canada | Elbow flexion (kg) Quadriceps (kg) Leg press (kg) |
Unilateral 1RM test | Unknown device | Upper limb Lower limb | |
| Zambom-Ferraresi et al. (40), Spain | Leg press (kg) Chest press (kg) |
1RM test | Force plate | Lower limb Upper limb | |
| Isokinetic test | Chen et al. (33), China | Quadriceps (Nm) | Evaluate the maximal strength of dominant leg | Computerized dynamometer | Lower limb |
| Nyberg et al. (36), Sweden | Shoulder flexion (Nm) Knee extension (Nm) |
The highest of 5 maximal contractions was used for analysis | Computerized dynamometer | Upper limb Lower limb |
Kg, Kilogram; HGF, Handgrip force; N, Newton; RM, Repetition maximum.
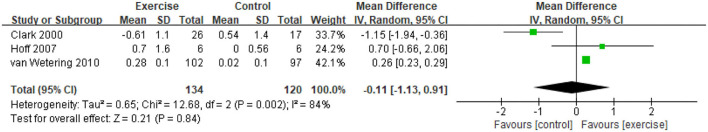
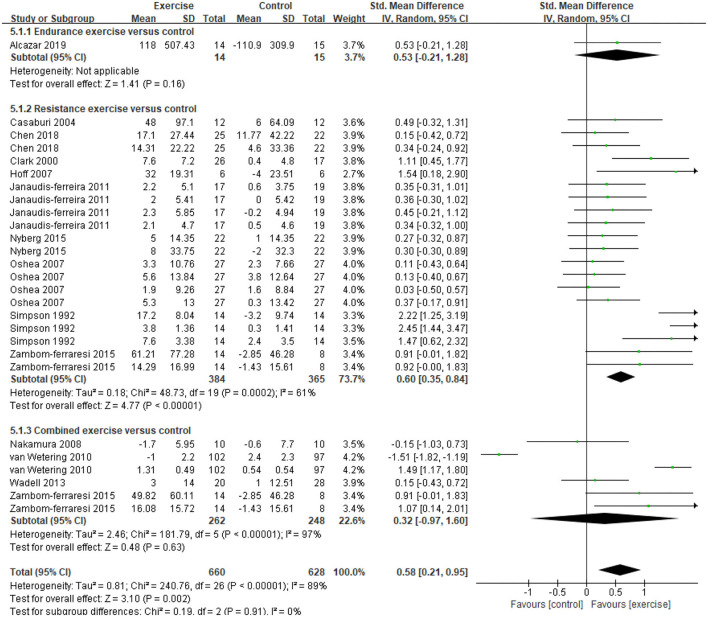
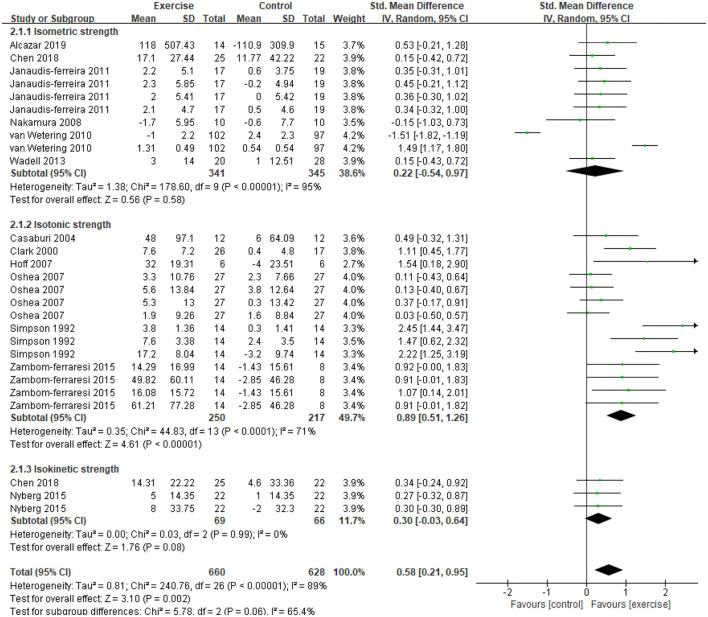
Five studies (21, 31, 32, 34, 47) provided data on skeletal muscle mass, assessed by mid-thigh CSA, BMI, FFMI, and total lean mass. In the meta-analysis, the estimated results showed that exercise intervention did not have a significant effect on changes in BMI (MD = −0.11, 95% CI: 1.13–0.91, I2 = 84%, Figure 2). Considering the high heterogeneity detected, we excluded studies with PEDro <6, and found a significant improvement in BMI (MD = 0.26, 95% CI 0.23–0.29, I2 = 0%). In addition, a CE program significantly improved FFMI (P = 0.01) (47), an EE program significantly improved the mid-thigh CSA (+4.5%, P < 0.05) of elderly patients with COPD (age: 77.7 ± 7.9 years old) (21), an RE program only found an increasing trend in the total lean mass (31). A total of 13 studies (21, 31–37, 39, 40, 45, 47, 48) with 27 data on skeletal muscle strength were provided, demonstrating a significant improvement after exercise intervention (SMD = 0.58, 95% CI 0.21–0.95, I2 = 89%). Considering the high heterogeneity detected, we first excluded studies with PEDro <6, and found a consistent result with high heterogeneity (SMD = 0.62, 95% CI 0.19–1.05, I2 = 91%). Then, we only pooled data in kilograms unit, and found a consistent result (MD = 0.78, 95% CI 0.64–0.92, I2 = 0%) besides the isometric strength test. Finally, subgroup analysis for different exercise modalities (Figure 3), muscle strength measures (Figure 4), and upper or lower limbs muscle strength found that RE provided significant benefits (SMD = 0.6, 95% CI 0.35–0.84, I2 = 61%), isometric strength significantly improved (SMD = 0.89, 95% CI 0.51–1.26, I2 =71%), and both upper and lower limbs muscle strength significantly improved (SMD = 0.78, 95% CI 0.4–1.17, I2 = 79%; SMD = 0.67, 95% CI 0.12–1.22, I2 = 91%).
Figure 2.
Pooled effect of exercise on BMI in patients with COPD. BMI, body mass index (kg/m2); CI, confidence interval; COPD, chronic obstructive pulmonary disease; SD, standard deviation.
Figure 3.
Effects of three types of exercise on skeletal muscle strength in patients with COPD. CI, confidence interval; COPD, chronic obstructive pulmonary disease; SD, standard deviation.
Figure 4.
Effects of exercise on skeletal muscle strength evaluated by three types of measurements in patients with COPD. CI, confidence interval; COPD, chronic obstructive pulmonary disease; SD, standard deviation.
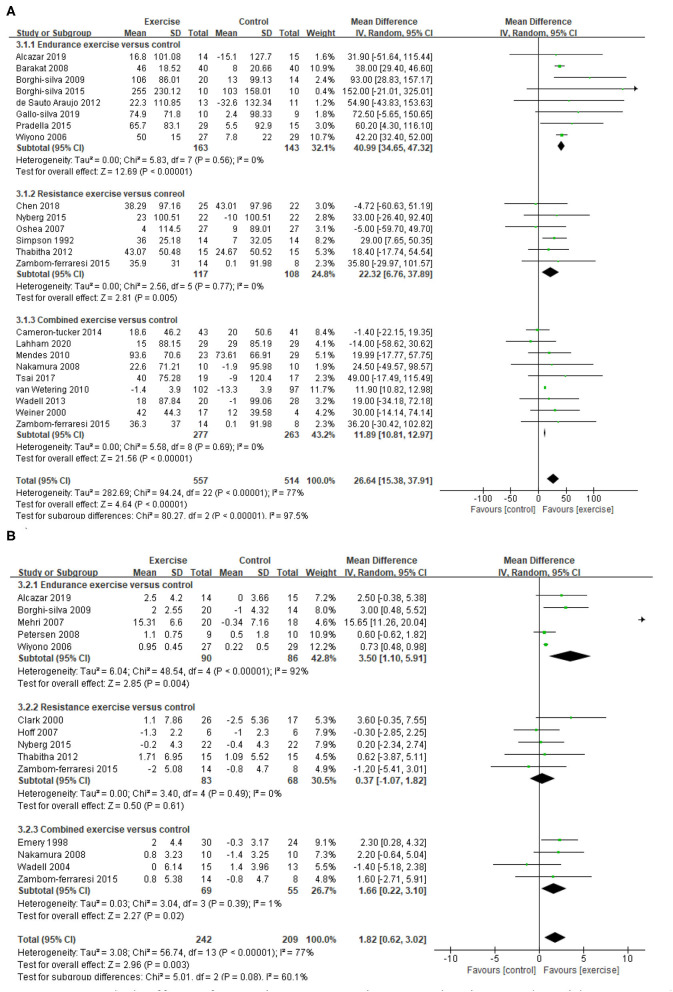
A total of 22 studies (21–26, 29, 30, 33, 36–41, 43–48, 50) provided data on endurance exercise capacity, demonstrating a significant improvement in 6MWD after exercise intervention (MD = 26.64, 95% CI 15.38–37.91, I2 = 77%). Subgroup analysis for different exercise modalities showed a consistent result, namely that all EE, RE, and CE can improve 6MWD significantly (EE: MD = 40.99, 95% CI 34.65–47.32, I2 = 0%; RE: MD = 22.32, 95% CI 6.76–37.89, I2 = 0%; CE: MD=11.89, 95% CI 10.81–12.97, I2 = 0%, Figure 5). A total of 13 studies (21, 23, 27, 28, 30, 32, 34, 36, 38, 40, 42, 45, 49) provided data on the peak exercise capacity, demonstrating a significant improvement in VO2peak after exercise intervention (MD = 1.82, 95% CI 0.62–3.02, I2 =77%). Subgroup analysis for different exercise modalities showed that EE and CE can improve VO2peak significantly (EE: MD = 3.5, 95% CI 1.1–5.91, I2 =92%; CE: MD = 1.66, 95% CI 0.22–3.1, I2 =1%, Figure 5). Considering that the methodological quality of included studies in EE was relatively low (PEDro <6), the results need to be carefully considered.
Figure 5.
Pooled effect of exercise on exercise capacity in people with COPD. (A) 6MWD, (B) VO2peak. 6MWD, 6-min walking distance (m); CI, confidence interval; COPD, chronic obstructive pulmonary disease; SD, standard deviation; VO2peak, peak oxygen consumption (ml/kg/min).
There was no difference of attrition number between exercise and control group (OR = 1.12, 95% CI 0.75–1.67, I2 = 15%, Supplementary Figure S1). The reasons for attrition in the exercise and control groups were similar (Supplementary Table S2).
Funnel plots are presented in Supplementary Figure S2. The results of Egger's test showed a significant publication bias in the results of skeletal muscle strength and 6MWD (P = 0.031 and P = 0.018, respectively). Then, the trim and fill method was used to adjust the impact of publication bias, and the results showed 0 missing studies for skeletal muscle strength results, and five missing studies for 6MWD results were merged to diminish the publication bias (Supplementary Figure S3). The certainty of the evidence for endurance and peak exercise capacity was deemed moderate, for skeletal muscle strength was deemed low, and for BMI was deemed very low (Table 4).
Table 4.
Grading of recommendations assessment, development, and evaluation summary of findings.
| Certainty Assessment | No. of Patients | Effect | Certainty | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | No. of Studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | EG | CG | Relative (95% CI) | Absolute (95% CI) | |
| BMI | 3 | Randomized trials | Seriousa | Seriousb | Not serious | Seriousc | None | 134 | 120 | – | MD 0.11 lower (1.13 lower to 0.91 higher) | ⊕◯◯◯ Very low |
| Skeletal muscle strength | 13 | Randomized trials | Seriousa | Seriousb | Not serious | Not serious | None | 660 | 628 | – | SMD 3.48 higher (1.81 to 5.15 higher) | ⊕⊕◯◯ Low |
| 6MWD | 22 | Randomized trials | Seriousa | Not serious | Not serious | Not serious | None | 557 | 514 | – | MD 12.76 higher (11.69 to 13.82 higher) | ⊕⊕⊕◯ Moderate |
| VO2peak | 13 | Randomized trials | Seriousa | Not serious | Not serious | Not serious | None | 242 | 209 | – | MD 1.82 higher (0.62 to 3.02 higher) | ⊕⊕⊕◯ Moderate |
Most of the studies are without allocation concealment, subject blinded and intention-to-treatment analysis.
There was a substantial heterogeneity among the three studies according to the heterogeneity test.
Only three studies were included in the analysis, and the sample size was relatively low.
Discussion
This systematic review and meta-analysis confirmed that regular exercise intervention for more than 6 weeks can effectively improve peripheral skeletal muscle strength and exercise capacity of patients with stable COPD. Furthermore, the greatest improvement in peripheral skeletal muscle strength appears in RE, the greatest improvement in endurance exercise capacity (6MWD: 40.99 m) appears in EE, and both EE and CE can significantly improve the peak exercise capacity.
In a previous study, skeletal muscle wasting could occur in the early COPD stages (51), and different exercise modalities could effectively improve lower limb muscle mass in COPD (14). However, in this study, exercise significantly improved the BMI of patients with COPD after excluding studies with PEDro <6. Through the analysis of literature characteristics, we proposed that exercise improved the BMI of patients with COPD unrelated to exercise modalities, but it was more affected by age and FEV1%pred. That is, the younger the age and better FEV1%pred, the lower the potential for improvement by exercise intervention. A recent meta-analysis of clinical trials has found a negative correlation between the BMI and decline of FEV1 in patients with COPD (52). Age, severity of COPD, and dyspnea degree are closely and clinically related to the loss of skeletal muscle mass and the decline of muscle function in patients with COPD (51). The results from the above-mentioned cross-sectional trials supported the speculation, but the factors that modulated the effects of exercise in COPD skeletal muscle mass still need to be explored due to the small data size in this study. Furthermore, BMI is affected by adipose and connective tissues in the body and may inadequately reflect muscle mass changes. Previous studies have found that RE can significantly improve lower limb lean muscle mass, increase the CSA of the rectus femoris and quadriceps, and decrease the density of muscle fiber (which indicate increased muscle mass per unit area) in COPD (53, 54), but have no effects on the proportion of muscle fiber type and the CSA of different muscle fiber types (an increasing trend only be found in type IIx fibers) (54). Another trial compared the effects of EE and RE on quadriceps muscle morphology and found no significant change in proportion and CSA of type I fibers, intermediate fibers, type IIx fibers, and capillarization (expressed as capillary-to-fiber ratio capillary density) after both exercise modalities, while the proportion of type IIa fibers significantly decreases after EE (55). Consistent with the present study results, both EE and RE have a beneficial effect on the peripheral skeletal muscle mass of patients with COPD, and EE seems to bring more changes in the aerobic metabolism phenotype. The exercise intervention mechanism to improve COPD skeletal muscle mass may be related to inhibiting the level of systemic inflammation, promoting skeletal muscle protein synthesis, muscle hypertrophy and regeneration, and improving the skeletal muscle metabolic enzyme activity (56).
Although there was a high heterogeneity in the methods and programs used to assess muscle strength, the results of this study still confirmed the significant positive effect of exercise on improving peripheral skeletal muscle strength in stable COPD. Subgroup analysis for different exercise modalities found that RE showed significant effects. We speculated that RE was designed for specific muscle groups that have less pressure on ventilation load and can effectively improve neuromuscular adaptation (57). Previous studies hypothesized that high-intensity whole/local body EE is sufficient to induce changes in the morphology and function of peripheral skeletal muscles in COPD (14). In the present study, only Alcazar et al. applied a 12-week high-intensity interval training program (high intensity: 80–90% Wpeak and low intensity: 40–50% Wpeak) in stable COPD patients and found that the maximum isometric contraction strength and the force development rate of leg press significantly improved (21). Hence, the dose-response relationship between EE intensity and effect still needs to be determined. Also, there was a high heterogeneity in the pooled estimates of CE, and the heterogeneity decreased after a sensitivity analysis excluding the results from van Wetering et al., but still without reaching statistical significance. In the analysis of the literature characteristics, we found that the quadriceps muscle strength of the participants was 92–95% of the normal predicted value (47), which may lead to a small potential for improvement. However, the results are still inconsistent with speculations and previous research results, that is, CE has similar or even greater effects than EE and RE alone (16, 17, 40), which may be attributed to a variety of CE programs included in this meta-analysis. First, the proportion of EE and RE in CE programs. Most programs scheduled EE and RE in one session and two to three sessions a week, respectively, apart from the program in Zambom-Ferraresi et al. (scheduled EE in one session and RE in another session, only two sessions a week). Second, the range of exercise intensity was relatively extensive, which may play a role in maintenance but not in improvement. Therefore, in the CE program for improving COPD's skeletal muscle strength, the different proportions and intensities of EE and RE might have different effects, and it is still necessary to explore the best program.
Subgroup analysis for different muscle strength testing methods found that exercise can only significantly improve isotonic muscle strength. We speculated that the isotonic muscle strength test is more familiar to the participants and has a higher correlation with daily life than other tests (58). Considering that different strength units may be the source of heterogeneity, we pooled data units in kilograms and found that exercise significantly improved isometric muscle strength. Although the data of isokinetic muscle strength showed an increasing trend after exercise (33, 36), many studies are still needed to determine the degree of response. We also conducted subgroup analysis to determine the effects of exercise on upper limbs and lower limbs muscle strength and found that exercise can improve the muscle strength of both upper and lower limbs. Although subgroup analysis was performed, high heterogeneity still existed, and the source of heterogeneity was unclear. A standard and clinically feasible measurement program is needed to quantitatively evaluate the damage of peripheral skeletal muscle strength and the response to exercise in COPD.
Consistent with previous meta-analysis (15, 59), this study found that exercise can significantly improve 6MWD (26.64 m) in patients with COPD. However, only the EE improvement reached the minimal clinical important difference of 30 m (60), which may be attributed to EE bringing more aerobic metabolism changes and greater improvements in ventilation capacity; the relatively low proportion of EE in the CE program cannot bring significant improvement. The peak exercise capacity is often evaluated using a cardiopulmonary exercise test (CPET), which is considered the gold standard to assess the exercise capacity and closely related to COPD's prognosis (61, 62). A progressive incremental exercise protocol in a treadmill or cycle ergometer is often used for CPET, and the results can provide abundant physiological information related to exercise restriction, including the heart (e.g., heart rate, VO2peak, and oxygen pulse), lung (e.g., inspiratory capacity, gas exchange, and dynamic inflation), muscle (e.g., power and lactic acid), dyspnea (Borg), and exercise initiative (62). A Cochrane review conducted in 2015 showed that pulmonary rehabilitation (at least 4 weeks of exercise training) is beneficial in improving maximal exercise capacity (measured by Wmax) in patients with COPD, and the effect size exceeds the minimal clinically important difference (4 W) (63). Although a different outcome was used in this present study, the effect of exercise is confirmed. The comparison results of the effects of different modalities exercise showed no significant differences between RE vs. the control group (15, 64), RE vs. EE (16), and CE vs. EE (64) in improving the peak exercise capacity (VO2peak, Wpeak) of patients with COPD. It seems that a contradictory deduction might be concluded that exercise does not have a significant positive effect on peak exercise capacity of patients with COPD. Based on the primary pathophysiological mechanisms of exercise limitation in patients with COPD undergoing CPET, including ventilatory abnormalities, pulmonary gas exchange abnormalities, and skeletal muscle dysfunction (61), exercise with different modalities seems beneficial in improving peak exercise capacity in patients with COPD. Consistent with the hypothesis, this meta-analysis showed that exercise could significantly improve COPD's peak exercise capacity (1.82 ml/kg/min), and both EE and CE have positive effects.
This systematic review and meta-analysis had some limitations. First, there were flaws in methodological quality of the original studies, namely the lack of subject blinding and evaluator blinding in exercise intervention trials. Second, one of the included literatures had an apparently large sample size, which may have had an impact on the research results. Sensitivity analysis was performed to reduce the impact when high heterogeneity was found. Third, we only analyzed the effects of exercise on skeletal muscle strength and still needed to explore the effects of exercise on skeletal muscle endurance and power. Fourth, the outcomes of skeletal muscle function were not assessed comprehensively in most of the included studies, which may cause a limitation. Fifth, trial designs were heterogeneous. For high heterogeneity, we used a random-effects model and subgroup analysis to analyse the source of heterogeneity, and the results were consistent.
Conclusions
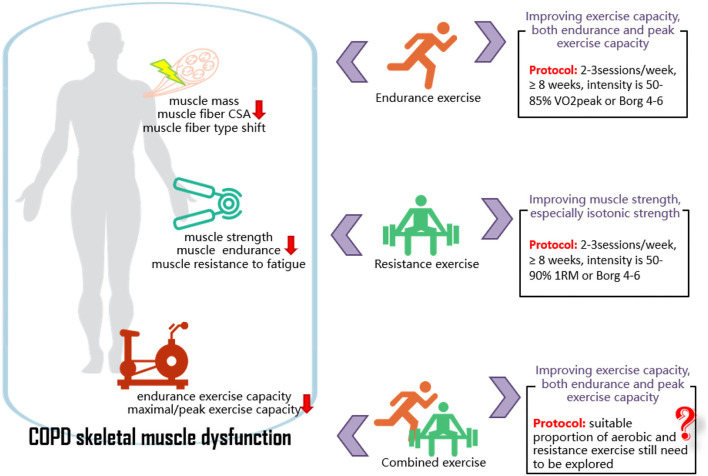
Exercise with different modalities seems effective in improving peripheral skeletal muscle strength and exercise capacity in patients with stable COPD. Specifically, EE shows a greater improvement in endurance and peak exercise capacity, and RE shows a greater improvement in peripheral skeletal muscle strength, and the isotonic test is relatively sensitive in reflecting muscle strength changes. Therefore, for patients with COPD whose exercise limitation is caused by a decreased cardiorespiratory capacity, EE might be a suitable choice. EE can be conducted in cycling, running, and walking, with an intensity of 50–85% VO2peak, 2–3 times/week, for at least 8 weeks. For patients with COPD whose exercise limitation is caused by an impaired peripheral skeletal muscle function, RE might be a preferable intervention. RE can be conducted in weight machines, free weights, and elastic bands, with an intensity of 50–90% 1RM, 2–3 times/week, for at least 8 weeks. The proportion of EE and RE in CE programs still needs to be explored and analyzed (Figure 6). High methodological quality RCTs with a large sample size are still needed to verify the present study results because of the relatively small inclusion of literature on the peripheral skeletal muscle structure and function in patients with COPD. It is also necessary to explore the effect of exercise intervention on peripheral skeletal muscle in AECOPD or patients with COPD with different severity.
Figure 6.
COPD skeletal muscle dysfunction and the effects of exercise on it. COPD, chronic obstructive pulmonary disease; CSA, cross-sectional area; RM, repetition maximum; VO2peak, peak oxygen consumption.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
XL and JX conceived of the idea for this review. JL and YW did the literature search. PL and YW collected the data. PL and JL did the quality assessment. PL did the statistical analyses and wrote the first draft of the manuscript. All authors analyzed and interpreted the data and revised and approved the final manuscript for submission.
Funding
This study was funded by the National Natural Science Foundation of China, grant numbers 81902307 and 82072551. The funder of the study played no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
Abbreviations
- 6MWD
6-min walking distance
- ATS/ERS
the American Thoracic Society/European Respiratory Society
- BMI
body mass index
- CE
combined aerobic and resistance exercise
- COPD
chronic obstructive pulmonary disease
- CPET
cardiopulmonary exercise test
- CSA
cross-sectional area
- EE
endurance exercise
- FEV1
forced expiratory volume in 1 s
- FFM
fat-free mass index
- GOLD
Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease
- GRADE
the Grading of Recommendations Assessment, Development and Evaluation
- MD
mean difference
- MeSH
medical subject headings
- OR
odds ratio
- PEDro
the Physiotherapy Evidence Database
- PRISMA
the Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- RE
resistance exercise
- SMD
standardized mean difference
- VO2peak
peak oxygen consumption.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.766841/full#supplementary-material
References
- 1.Global Initiative for Chronic Obstructive Lung Disease-GOLD . Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2019 report). Available online at: https://goldcopd.org/2019 (accessed December 5, 2018).
- 2.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. (2012) 379:1341–51. 10.1016/S0140-6736(11)60968-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. (2017) 389:1931–40. 10.1016/S0140-6736(17)31222-9 [DOI] [PubMed] [Google Scholar]
- 4.Barreiro E, Bustamante V, Cejudo P, Gáldiz JB, Gea J, de Lucas P, et al. Guidelines for the evaluation and treatment of muscle dysfunction in patients with chronic obstructive pulmonary disease. Arch Bronconeumol. (2015) 51:384–95. 10.1016/j.arbres.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 5.Rabinovich R, Vilaro J. Structural and functional changes of peripheral muscles in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. (2010) 16:123–33. 10.1097/MCP.0b013e328336438d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernard S, LeBlanc P, Whittom F, Carrier G, Jobin J, Belleau R, et al. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (1998) 158:629–34. 10.1164/ajrccm.158.2.9711023 [DOI] [PubMed] [Google Scholar]
- 7.Corhay JL, Dang DN, Van Cauwenberge H, Louis R. Pulmonary rehabilitation and COPD: providing patients a good environment for optimizing therapy. Int J Chron Obstruct Pulmon Dis. (2014) 9:27–39. 10.2147/COPD.S52012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li P, Wang Z, Lu Y, Li N, Xiao L, Su J, et al. Assessment of knee extensor and flexor function using isokinetic test in COPD: impact on exercise capacity. Int J Tuberc Lung Dis. (2020) 24:776–81. 10.5588/ijtld.19.0588 [DOI] [PubMed] [Google Scholar]
- 9.Saey D, Debigare R, LeBlanc P, Mador MJ, Cote CH, Jobin J, et al. Contractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2003) 168:425–30. 10.1164/rccm.200208-856OC [DOI] [PubMed] [Google Scholar]
- 10.Gagnon P, Saey D, Vivodtzev I, Laviolette L, Mainguy V, Milot J, et al. Impact of preinduced quadriceps fatigue on exercise response in chronic obstructive pulmonary disease and healthy subjects. J Appl Physiol (1985). (2009) 107:832–40. 10.1152/japplphysiol.91546.2008 [DOI] [PubMed] [Google Scholar]
- 11.Nyberg A, Carvalho J, Bui KL, Saey D, Maltais F. Adaptations in limb muscle function following pulmonary rehabilitation in patients with COPD—a review. Rev Port Pneumol (2006). (2016) 22:342–50. 10.1016/j.rppnen.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 12.Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigaré R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2014) 189:e15–62. 10.1164/rccm.201402-0373ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaitovich A, Barreiro E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease (COPD): what we know and can do for our patients. Am J Respir Crit Care Med. (2018) 198:175–86. 10.1164/rccm.201710-2140CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Brandt J, Spruit MA, Hansen D, Franssen FM, Derave W, Sillen MJ, et al. Changes in lower limb muscle function and muscle mass following exercise-based interventions in patients with chronic obstructive pulmonary disease: a review of the English-language literature. Chron Respir Dis. (2018) 15:182–219. 10.1177/1479972317709642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Li P, Lu Y, Wang Z, Li J, Liu X, et al. Effects of resistance training on exercise capacity in elderly patients with chronic obstructive pulmonary disease: a meta-analysis and systematic review. Aging Clin Exp Res. (2019) 32:1911–22. 10.1007/s40520-019-01339-8 [DOI] [PubMed] [Google Scholar]
- 16.Iepsen UW, Jørgensen KJ, Ringbaek T, Hansen H, Skrubbeltrang C, Lange P, et al. Systematic review of resistance training vs. endurance training in COPD. J Cardiopulm Rehabil Prev. (2015) 35:163–72. 10.1097/HCR.0000000000000105 [DOI] [PubMed] [Google Scholar]
- 17.Iepsen UW, Jørgensen KJ, Ringbæk T, Hansen H, Skrubbeltrang C, Lange P, et al. combination of resistance and endurance training increases leg muscle strength in COPD: an evidence-based recommendation based on systematic review with meta-analyses. Chron Respir Dis. (2015) 12:132–45. 10.1177/1479972315575318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman D, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. (2003) 83:713–21. 10.1093/ptj/83.8.713 [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alcazar J, Losa-Reyna J, Rodriguez-Lopez C, Navarro-Cruz R, Alfaro-Acha A, Ara I, et al. Effects of concurrent exercise training on muscle dysfunction and systemic oxidative stress in older people with COPD. Scand J Med Sci Sports. (2019) 29:1591–603. 10.1111/sms.13494 [DOI] [PubMed] [Google Scholar]
- 22.Barakat S, Michele G, George P, Nicole V, Guy A. Outpatient pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2008) 3:155–62. 10.2147/COPD.S2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borghi-Silva A, Arena R, Castello V, Simões RP, Martins LE, Catai AM, et al. Aerobic exercise training improves autonomic nervous control in patients with COPD. Respir Med. (2009) 103:1503–10. 10.1016/j.rmed.2009.04.015 [DOI] [PubMed] [Google Scholar]
- 24.Borghi-Silva A, Mendes RG, Trimer R, Oliveira CR, Fregonezi GA, Resqueti VR, et al. Potential effect of 6 vs. 12-weeks of physical training on cardiac autonomic function and exercise capacity in chronic obstructive pulmonary disease. Eur J Phys Rehabil Med. (2015) 51:211–21. Available online at: https://pubmed.ncbi.nlm.nih.gov/24594853/ [PubMed] [Google Scholar]
- 25.de Souto Araujo ZT, de Miranda Silva Nogueira PA, Cabral EE, de Paula Dos Santos L, da Silva IS, Ferreira GM. Effectiveness of low-intensity aquatic exercise on COPD: a randomized clinical trial. Respir Med. (2012) 106:1535–43. 10.1016/j.rmed.2012.06.022 [DOI] [PubMed] [Google Scholar]
- 26.Gallo-Silva B, Cerezer-Silva V, Ferreira DG, Sakabe DI, Kel-Souza LD, Bertholo VC, et al. Effects of water-based aerobic interval training in patients with COPD: a randomized controlled trial. J Cardiopulm Rehabil Prev. (2019) 39:105–11. 10.1097/HCR.0000000000000352 [DOI] [PubMed] [Google Scholar]
- 27.Mehri SN, Khoshnevis MA, Zarrehbinan F, Hafezi S, Ghasemi A, Ebadi A. Effects of treadmill exercise training on VO2 peak in chronic obstructive pulmonary disease. Natl Res Inst Tuberc Lung Dis. (2007) 6:18–24. Available online at: https://scholar.google.com/scholar_lookup?title=Effect+of+treadmill+exercise+training+on+VO2+peak+in+chronic+obstructive+pulmonary+disease+&author=SN+Mehri&author=MA+Khoshnevis&author=F+Zarrehbinan&author=S+Hafezi&author=A+Ghasemi&author=A+Ebadi&publication_year=2007&hl=en9494879 [Google Scholar]
- 28.Petersen AM, Mittendorfer B, Magkos F, Iversen M, Pedersen BK. Physical activity counteracts increased whole-body protein breakdown in chronic obstructive pulmonary disease patients. Scand J Med Sci Sports. (2008) 18:557–64. 10.1111/j.1600-0838.2007.00727.x [DOI] [PubMed] [Google Scholar]
- 29.Pradella CO, Belmonte GM, Maia MN, Delgado CS, Luise AP, Nascimento OA, et al. Home-based pulmonary rehabilitation for subjects with COPD: a randomized study. Respir Care. (2015) 60:526–32. 10.4187/respcare.02994 [DOI] [PubMed] [Google Scholar]
- 30.Wiyono WH, Riyadi J, Yunus F, Ratnawati A, Prasetyo S. The benefit of pulmonary rehabilitation against quality of life alteration and functional capacity of chronic obstructive pulmonary disease (COPD) patient assessed using St George's respiratory questionnaire (SGRQ) and 6 minutes walking distance test (6MWD). Med J Indones. (2006) 15:165–72. 10.13181/mji.v15i3.232 [DOI] [Google Scholar]
- 31.Casaburi R, Bhasin S, Cosentino L, Porszasz J, Somfay A, Lewis MI, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2004) 170:870–8. 10.1164/rccm.200305-617OC [DOI] [PubMed] [Google Scholar]
- 32.Clark C, Cochrane L, Mackay E, Paton B. Skeletal muscle strength and endurance in patients with mild COPD and the effects of weight training. Eur Respir J. (2000) 15:92–7. 10.1183/09031936.00.15109200 [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Niu M, Zhang X, Qian H, Xie A, Wang X. Effects of home-based lower limb resistance training on muscle strength and functional status in stable chronic obstructive pulmonary disease patients. J Clin Nurs. (2018) 27:e1022–e37. 10.1111/jocn.14131 [DOI] [PubMed] [Google Scholar]
- 34.Hoff J, Tjønna AE, Steinshamn S, Høydal M, Richardson RS, Helgerud J. Maximal strength training of the legs in COPD: a therapy for mechanical inefficiency. Med Sci Sports Exerc. (2007) 39:220–6. 10.1249/01.mss.0000246989.48729.39 [DOI] [PubMed] [Google Scholar]
- 35.Janaudis-Ferreira T, Hill K, Goldstein RS, Robles-Ribeiro P, Beauchamp MK, Dolmage TE, et al. Resistance arm training in patients with COPD: a randomized controlled trial. Chest. (2011) 139:151–8. 10.1378/chest.10-1292 [DOI] [PubMed] [Google Scholar]
- 36.Nyberg A, Lindstrom B, Rickenlund A, Wadell K. Low-load/high-repetition elastic band resistance training in patients with COPD: a randomized, controlled, multicenter trial. Clin Respir J. (2015) 9:278–88. 10.1111/crj.12141 [DOI] [PubMed] [Google Scholar]
- 37.O'Shea S, Taylor N, Paratz J A. predominantly home-based progressive resistance exercise program increases knee extensor strength in the short-term in people with chronic obstructive pulmonary disease: a randomised controlled trial. Aust J Physiother. (2007) 53:229–37. 10.1016/S0004-9514(07)70003-X [DOI] [PubMed] [Google Scholar]
- 38.Thabitha P, Madhavi K, Charan K. Jyothi KA. Effect of peripheral muscle strength training on exercise capacity in subjects with chronic obstructive pulmonary disease. Indian J Physiother Occup Ther. (2012) 6:91–5. Available online at: https://search.pedro.org.au/search-results/record-detail/31163 [Google Scholar]
- 39.Simpson K, Killian K, McCartney N, Stubbing DG, Jones NL. Randomised controlled trial of weightlifting exercise in patients with chronic airflow limitation. Thorax. (1992) 47:70–5. 10.1136/thx.47.2.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zambom-Ferraresi F, Cebollero P, Gorostiaga EM, Hernández M, Hueto J, Cascante J, et al. Effects of combined resistance and endurance training vs. resistance training alone on strength, exercise capacity, and quality of life in patients with COPD. J Cardiopulm Rehabil Prev. (2015) 35:446–53. 10.1097/HCR.0000000000000132 [DOI] [PubMed] [Google Scholar]
- 41.Cameron-Tucker HL, Wood-Baker R, Owen C, Joseph L, Walters EH. Chronic disease self-management and exercise in COPD as pulmonary rehabilitation: a randomized controlled trial. Int J Chron Obstruct Pulmon Dis. (2014) 9:513–23. 10.2147/COPD.S58478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emery CF, Schein RL, Hauck ER, MacIntyre NR. Psychological and cognitive outcomes of a randomized trial of exercise among patients with chronic obstructive pulmonary disease. Health Psychol. (1998) 17:232–40. 10.1037/0278-6133.17.3.232 [DOI] [PubMed] [Google Scholar]
- 43.Lahham A, McDonald CF, Moore R, Cox NS, Rawlings S, Nichols A, et al. The impact of home-based pulmonary rehabilitation on people with mild chronic obstructive pulmonary disease: a randomised controlled trial. Clin RespirJ. (2020) 14:335–44. 10.1111/crj.13138 [DOI] [PubMed] [Google Scholar]
- 44.Mendes de Oliveira JC, Studart Leitão Filho FS, Malosa Sampaio LM, Negrinho de Oliveira AC, Hirata RP, Costa D, et al. Outpatient vs home-based pulmonary rehabilitation in COPD: a randomized controlled trial. Multidiscip Respir Med. (2010) 5:401–8. 10.1186/2049-6958-5-6-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura Y, Tanaka K, Shigematsu R, Nakagaichi M, Lnoue M, Homma T. Effects of aerobic training and recreational activities in patients with chronic obstructive pulmonary disease. In J Rehabil Res. (2008) 31:275–83. 10.1097/MRR.0b013e3282fc0f81 [DOI] [PubMed] [Google Scholar]
- 46.Tsai LL, McNamara RJ, Moddel C, Alison JA, McKenzie DK, McKeough ZJ. Home-based telerehabilitation via real-time videoconferencing improves endurance exercise capacity in patients with COPD: The randomized controlled TeleR Study. Respirology. (2017) 22:699–707. 10.1111/resp.12966 [DOI] [PubMed] [Google Scholar]
- 47.van Wetering CR, Hoogendoorn M, Mol SJM., Rutten-van Mölken MPMH, Schols AM. Short- and long-term efficacy of a community-based COPD management programme in less advanced COPD: a randomised controlled trial. Thorax. (2010) 65:7–13. 10.1136/thx.2009.118620 [DOI] [PubMed] [Google Scholar]
- 48.Wadell K, Webb KA, Preston ME, Amornputtisathaporn N, Samis L, Patelli J, et al. Impact of pulmonary rehabilitation on the major dimensions of dyspnea in COPD. COPD. (2013) 10:425–35. 10.3109/15412555.2012.758696 [DOI] [PubMed] [Google Scholar]
- 49.Wadell K, Sundelin G, Henriksson-Larsén K, Lundgren R. High intensity physical group training in water—an effective training modality for patients with COPD. Respir Med. (2004) 98:428–38. 10.1016/j.rmed.2003.11.010 [DOI] [PubMed] [Google Scholar]
- 50.Weiner P, Magadle R, Berar-Yanay N, Davidovich A, Weiner M. The cumulative effect of long-acting bronchodilators, exercise, and inspiratory muscle training on the perception of dyspnea in patients with advanced COPD. Chest. (2000) 118:672–8. 10.1378/chest.118.3.672 [DOI] [PubMed] [Google Scholar]
- 51.Benz E, Trajanoska K, Lahousse L, Schoufour JD, Terzikhan N, De Roos E, et al. Sarcopenia in COPD: a systematic review and meta-analysis. Eur Respir Rev. (2019) 28:190049. 10.1183/16000617.0049-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Y, Milne S, Jaw JE, Yang CX, Xu F, Li X, et al. BMI is associated with FEV1 decline in chronic obstructive pulmonary disease: a meta-analysis of clinical trials. Respir Res. (2019) 20:236. 10.1186/s12931-019-1209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menon MK, Houchen L, Harrison S, Singh SJ, Morgan MD, Steiner MC. Ultrasound assessment of lower limb muscle mass in response to resistance training in COPD. Respir Res. (2012) 13:119. 10.1186/1465-9921-13-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewis MI, Fournier M, Storer TW, Bhasin S, Porszasz J, Ren SG, et al. Skeletal muscle adaptations to testosterone and resistance training in men with COPD. J Appl Physiol (1985). (2007) 103: 1299–310. 10.1152/japplphysiol.00150.2007 [DOI] [PubMed] [Google Scholar]
- 55.Iepsen UW, Munch GD, Rugbjerg M, Rinnov AR, Zacho M, Mortensen SP., et al. Effect of endurance vs. resistance training on quadriceps muscle dysfunction in COPD: a pilot study. Int J Chron Obstruct Pulmon Dis. (2016) 11:2659–69. 10.2147/COPD.S114351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nasis I, Kortianou EA, Clini E, Koulouris NG, Vogiatzis I. Effect of rehabilitative exercise training on peripheral muscle remodelling in patients with COPD: targeting beyond the lungs. Curr Drug Targets. (2013) 14:262–73. 10.2174/1389450111314020011 [DOI] [PubMed] [Google Scholar]
- 57.Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. (2013) 188:e13–64. 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 58.Marklund S, Bui KL, Nyberg A. Measuring and monitoring skeletal muscle function in COPD: current perspectives. Int J Chron Obstruct Pulmon Dis. (2019) 14:1825–38. 10.2147/COPD.S178948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paneroni M, Simonelli C, Vitacca M, Ambrosino N. Aerobic exercise training in very severe chronic obstructive pulmonary disease: a systematic review and meta-analysis. Am J Phys Med Rehabil. (2017) 96:541–8. 10.1097/PHM.0000000000000667 [DOI] [PubMed] [Google Scholar]
- 60.Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. (2014) 44:1428–46. 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 61.Ferrazza AM, Martolini D, Valli G, Palange P. Cardiopulmonary exercise testing in the functional and prognostic evaluation of patients with pulmonary diseases. Respiration. (2009) 77:3–17. 10.1159/000186694 [DOI] [PubMed] [Google Scholar]
- 62.Stringer W, Marciniuk D. The role of cardiopulmonary exercise testing (CPET) in pulmonary rehabilitation (PR) of chronic obstructive pulmonary disease (COPD) patients. COPD. (2018) 15:621–31. 10.1080/15412555.2018.1550476 [DOI] [PubMed] [Google Scholar]
- 63.McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2015) CD003793:1–188. 10.1002/14651858.CD003793.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liao WH, Chen JW, Chen X, Lin L, Yan HY, Zhou YQ, et al. Impact of resistance training in subjects with COPD: a systematic review and meta-analysis. Respir Care. (2015) 60:1130–45. 10.4187/respcare.03598 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.