Key Points
Question
Does the use of high-flow oxygen therapy through a nasal cannula, compared with conventional oxygen therapy, reduce requirement of intubation and time to clinical improvement among patients with severe COVID-19?
Findings
In this randomized clinical trial that included 220 patients with severe COVID-19, the rate of intubation and mechanical ventilation for those treated with high-flow oxygen therapy through a nasal cannula vs with conventional oxygen therapy was 34.3% vs 51.0%, respectively; the median time to clinical recovery was 11 days vs 14 days. Both comparisons were statistically significant.
Meaning
Among patients with severe COVID-19, treatment with high-flow oxygen therapy compared with conventional oxygen therapy reduced the likelihood of invasive mechanical ventilation and decreased time to clinical recovery.
Abstract
Importance
The effect of high-flow oxygen therapy vs conventional oxygen therapy has not been established in the setting of severe COVID-19.
Objective
To determine the effect of high-flow oxygen therapy through a nasal cannula compared with conventional oxygen therapy on need for endotracheal intubation and clinical recovery in severe COVID-19.
Design, Setting, and Participants
Randomized, open-label clinical trial conducted in emergency and intensive care units in 3 hospitals in Colombia. A total of 220 adults with respiratory distress and a ratio of partial pressure of arterial oxygen to fraction of inspired oxygen of less than 200 due to COVID-19 were randomized from August 2020 to January 2021, with last follow-up on February 10, 2021.
Interventions
Patients were randomly assigned to receive high-flow oxygen through a nasal cannula (n = 109) or conventional oxygen therapy (n = 111).
Main Outcomes and Measures
The co–primary outcomes were need for intubation and time to clinical recovery until day 28 as assessed by a 7-category ordinal scale (range, 1-7, with higher scores indicating a worse condition). Effects of treatments were calculated with a Cox proportional hazards model adjusted for hypoxemia severity, age, and comorbidities.
Results
Among 220 randomized patients, 199 were included in the analysis (median age, 60 years; n = 65 women [32.7%]). Intubation occurred in 34 (34.3%) randomized to high-flow oxygen therapy and in 51 (51.0%) randomized to conventional oxygen therapy (hazard ratio, 0.62; 95% CI, 0.39-0.96; P = .03). The median time to clinical recovery within 28 days was 11 (IQR, 9-14) days in patients randomized to high-flow oxygen therapy vs 14 (IQR, 11-19) days in those randomized to conventional oxygen therapy (hazard ratio, 1.39; 95% CI, 1.00-1.92; P = .047). Suspected bacterial pneumonia occurred in 13 patients (13.1%) randomized to high-flow oxygen and in 17 (17.0%) of those randomized to conventional oxygen therapy, while bacteremia was detected in 7 (7.1%) vs 11 (11.0%), respectively.
Conclusions and Relevance
Among patients with severe COVID-19, use of high-flow oxygen through a nasal cannula significantly decreased need for mechanical ventilation support and time to clinical recovery compared with conventional low-flow oxygen therapy.
Trial Registration
ClinicalTrials.gov Identifier: NCT04609462
This randomized trial assesses the effect of high-flow oxygen therapy through a nasal cannula vs conventional oxygen therapy on 28-day intubation rates and time to clinical recovery among patients with respiratory distress due to COVID-19.
Introduction
Infection with COVID-19, the disease caused by SARS-CoV-2, commonly results in asymptomatic or mild disease.1 Nevertheless, the number of infected patients progressing to acute hypoxemic respiratory failure might become substantially high during pandemic circumstances, thus challenging health care system capacities.2,3
Arterial hypoxemia is the leading feature of severe cases of COVID-19.4,5 Consequently, its management should rely on oxygen supplementation aiming to improve oxygenation and to assist respiratory effort throughout different support modalities. Early observational studies during the COVID-19 pandemic reported a very high mortality in patients subjected to invasive mechanical ventilation,3 while some investigators warned about early intubation and mechanical ventilation.4 Conversely, patient self-inflicted lung injury might occur during spontaneous breathing in nonintubated patients with acute respiratory failure because of high respiratory drive and potentially injurious transpulmonary pressure swings.6 Meanwhile, in agreement with both views, noninvasive respiratory support techniques could limit self-inflicted lung injury while preventing adverse events associated with intubation and mechanical ventilation.
High-flow oxygen therapy through a nasal cannula is a technique whereby a mixture of heated and humidified oxygen and air are delivered to the nose at high flow rates.7,8,9 Data suggest that high-flow oxygen therapy might decrease need for endotracheal intubation and risk of escalation of therapy in patients with acute hypoxemic respiratory failure,10,11 but with no apparent effect on mortality rates.11,12 Even though international guidelines and early observational studies proposed using high-flow oxygen therapy through a nasal cannula to initially treat patients with severe COVID-19,13,14,15 evidence supporting this is very limited. Thus, this current trial was conducted to assess the effect of high-flow oxygen therapy through a nasal cannula vs conventional oxygen therapy on need for intubation and mechanical ventilation and time to clinical recovery in patients with severe COVID-19.
Methods
Study Design and Oversight
This open-label randomized clinical trial was conducted in 3 hospitals in Colombia between August 13, 2020, and January 12, 2021, with last follow-up on February 10, 2021. The trial protocol is available in Supplement 1 and the statistical analysis plan is available in Supplement 2. The trial was designed by the steering committee (Supplement 1 and eAppendix in Supplement 3) and was approved by the ethical and biomedical research committee (EBRC) of the coordinating center (Fundación Valle del Lili; protocol number 1635; approval number 259-2020) and then by local ethical/research committees at each participating hospital. Electronic written informed consent was obtained from all patients, their next of kin, or another surrogate decision maker as appropriate. An independent data quality surveillance committee, research assistants from the coordinating center, and members of the steering committee regularly monitored all participating centers to check adherence to protocol and to evaluate the accuracy of information recorded in the electronic case report form (Supplement 1 and eAppendix in Supplement 3). An independent data and safety monitoring board (DSMB) was in charge of conducting a single interim analysis on enrollment of 50% of the sample (eAppendix in Supplement 3).
Participating patients could not be masked because of the nature of the intervention. Nevertheless, main investigators were unaware of the study group outcomes until the database was locked after the end of follow-up on February 10, 2021. An independent statistician performed all the analyses. All respiratory support devices (ie, conventional oxygen cannulas, face masks, high-flow nasal cannulas, and all consumable materials) were provided by each participating center in the context of the pandemic emergency.
Patients
Adult patients admitted to the emergency department, general ward, or intensive care unit were enrolled if they met all of the following eligibility criteria: aged 18 years or older; suspected or confirmed infection with SARS-CoV-2 (confirmation via reverse transcriptase–polymerase chain reaction test from a nasopharyngeal swab); acute respiratory failure with a ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (Pao2/Fio2) of less than 200, accompanied by clinical signs of respiratory distress (eg, use of accessory muscles and respiratory rate greater than 25/min); and less than 6 hours elapsed since fulfilling the criteria of acute respiratory failure (Supplement 1 and eAppendix in Supplement 3).
Exclusion criteria were need for immediate endotracheal intubation (see Supplement 1 for detailed prespecified intubation criteria); a partial pressure of arterial carbon dioxide greater than 55 mm Hg; pregnancy; high suspicion or confirmation of acute cardiogenic pulmonary edema; history of or current left ventricular ejection fraction of less than 45%; history of chronic heart failure (New York Heart Association class III-IV)16; clinical suspicion or confirmation of peripheral demyelinating disease; history of advanced chronic obstructive pulmonary disease (Global Initiative for Chronic Obstructive Lung Disease grade C-D)17 or hospitalization due to chronic obstructive pulmonary disease decompensation within the last year; advanced liver cirrhosis (Child-Pugh class C)18; anatomical or other conditions precluding the use of a high-flow nasal cannula; do-not-intubate or do-not-resuscitate orders; imminent death; and refusal of study participation by a patient or their next of kin.
Randomization
Eligible patients were randomly assigned in a 1:1 ratio to receive respiratory support with high-flow oxygen therapy through a nasal cannula vs conventional oxygen therapy. Randomization was centrally performed through a web-based system using computer-generated random numbers with blocks of 2 and 4, unknown to the investigators, and was stratified by study site to ensure allocation concealment. Site investigators were unaware of block size. Baseline was defined as the time of randomization.
Interventions
Randomly allocated treatments were delivered within 30 minutes after randomization. In the high-flow oxygen therapy group, respiratory support was continuously applied through large-bore binasal prongs using heated and humidified gas at an initial flow of 60 L/min and an Fio2 of 1.0. The Fio2 was subsequently adjusted to maintain pulse oxygen saturation (Spo2) values of 92% or greater. Flow rate was decreased in patients reporting discomfort due to high-flow oxygen therapy until its resolution. High-flow oxygen therapy was continuously applied until intubation or when criteria for weaning of high-flow oxygen therapy were achieved, namely, improvement in clinical signs of respiratory distress, a Pao2/Fio2 ratio higher than 200, and ability to maintain Spo2 values of 92% or greater with less than 9 L/min of conventional oxygen therapy. Patients experiencing hypoxemia after weaning from high-flow oxygen therapy recommenced high-flow oxygen therapy with a nasal cannula unless immediate intubation was necessary.
In the conventional oxygen therapy group, oxygen was applied continuously through any low-flow oxygen device or combination thereof (nasal prongs, mask with or without oxygen reservoir, Venturi mask systems). Rates of gas flow and Fio2 were adjusted to maintain Spo2 values of 92% or greater until patient intubation or recovery.
Prone position while awake was allowed in both study groups at the discretion of attending physicians. Intubation decisions for patients included in the study were based on prespecified criteria (Supplement 1 and eAppendix in Supplement 3). High-flow oxygen therapy was used during laryngoscopy and intubation only in patients randomized to the high-flow oxygen therapy group. Use of a high-flow nasal cannula was allowed after extubation in both groups according to decisions of attending physicians. Noninvasive mechanical ventilation with a face-mask interface was not used in this open-label clinical trial according to local infection control authority recommendations during the time of trial planning. The use of steroids, antibiotics, antivirals, or other antimicrobial agents was allowed at the discretion of medical attending teams.
All participants were evaluated daily from day 1 through day 28 (while remaining hospitalized) by the local study coordinators and research assistants. Trial data were recorded in the electronic case report form from randomization until day 28 (or until hospital discharge if it occurred before day 28). When hospital discharge happened before day 28, patients or family representatives were contacted via a structured telephone call to verify vital and clinical status at day 28 (Supplement 1 and eAppendix in Supplement 3).
Outcomes
The co–primary outcomes were need for intubation and time to clinical recovery within 28 days after randomization. The latter was defined as the time elapsed from randomization until the first day during the 28 days after enrollment on which a patient attained a reduction of 2 or more points from their score at randomization on a modified 7-category ordinal scale. Scores on the ordinal scale were defined as follows: 1, discharged from the hospital, resuming complete daily-life activities; 2, discharged from the hospital but with limitation of activities, home oxygen requirement, or both; 3, hospitalized in a general ward (not intensive care unit), not requiring supplemental oxygen, and no longer requiring ongoing medical care (used if hospitalization was extended for infection control reasons); 4, hospitalized in a general ward (not intensive care unit), requiring supplemental oxygen or requiring ongoing medical care (for COVID-19–related or other medical conditions); 5, hospitalized in the intensive care unit, requiring any supplemental oxygen; 6, hospitalized in the intensive care unit, requiring invasive mechanical ventilation or extracorporeal membrane oxygenation; and 7, death (eTable 1 in Supplement 3).
Eight secondary outcomes included proportion of patients requiring early intubation and mechanical ventilation; mechanical ventilation–free days within 28 days; kidney replacement therapy–free days; hospital and intensive care unit lengths of stay; overall mortality by day 28; proportion of serious adverse events; and proportion of bacterial and fungal infections. Tertiary outcomes included evolvement of oxygen flow requirement and Pao2/Fio2 ratio; time from randomization to intubation; evolvement of multiorgan and extrapulmonary organ dysfunction; relationship between the HACOR (heart rate, acidosis, consciousness, oxygenation, and respiratory rate) scale and the ROX (respiratory rate oxygenation) index and requirement of intubation; and time courses of prespecified blood markers. A detailed description of all secondary outcomes and prespecified subgroup analysis is available in the statistical analysis plan (Supplement 2).
Sample Size
Assuming an intubation rate of 60% in patients with acute respiratory hypoxemic failure undergoing conventional oxygen therapy,10 which was in agreement with data obtained from the first 75 patients with COVID-19–related severe hypoxemic respiratory failure treated in the coordinating center, it was estimated that enrollment of 196 patients would be necessary to demonstrate an absolute reduction of 20% in the rate of intubation between the high-flow oxygen therapy and conventional oxygen therapy groups with 80% power and a 2-sided α = .05. In addition, according to such initial data, 160 patients would be necessary to demonstrate a reduction of 2 days in time to recovery, with 80% power and a 2-sided α = .05. Consequently, the sample size target was 196 patients (for details, see the eAppendix in Supplement 3). Nevertheless, because of particular hospital conditions during the pandemic period, an unexpected number of patients were transferred for administrative reasons (related to health insurance) to other hospitals within 72 hours after enrollment. Due to the limitation of providing further protocolized management and the impossibility of ensuring adequate follow-up after transferring to nonparticipating hospitals, and in agreement with the recommendation stated by the Fundación Valle del Lili EBRC, these patients were excluded from the main analysis (eAppendix in Supplement 3). Consequently, because of the unexpected number of lost participants and in agreement with the EBRC recommendation, the number of randomized patients was extended to 220 participants (eAppendix in Supplement 3).
Statistical Analysis
All analyses were conducted according to the statistical analysis plan (Supplement 2) elaborated and approved by the Fundación Valle del Lili EBRC prior to locking the trial database and analyzing data. Patients were analyzed according to their randomization group, excluding those withdrawing consent. There were no missing data for the co–primary outcomes.
Continuous variables are described herein as medians and interquartile ranges and categorical variables as counts and proportions. Follow-up data on the co–primary outcomes were available for all patients included in the analysis. The effect of treatment on need for endotracheal intubation and clinical recovery was calculated with a Cox proportional hazards model adjusted for hypoxemia severity, age, and comorbidities (Supplement 1, Supplement 2, and eAppendix in Supplement 3). Results of the co–primary outcomes were not adjusted for multiplicity. The proportional hazards assumption was tested with the Grambsch and Therneau method.19 Results are reported as hazard ratios (HRs) with 95% confidence intervals and represented in Kaplan-Meier curves. No correction was made in P values and confidence intervals for the 2 primary outcomes. Analysis of treatment effect on the co–primary outcomes is also shown according to the baseline 7-category ordinal scale (ie, scores of 4 or 5 at enrollment). In addition, HRs estimated from Cox regression models for predefined subgroups (ie, age ≥60 years vs <60 years, Pao2/Fio2 ratio ≥100 vs <100, and interleukin 6 level ≥100 pg/mL vs <100 pg/mL) are provided in forest plots. We included interaction terms between each specified subgroup and the treatment groups in Cox proportional hazards models adjusted by hypoxemia severity, age, and comorbidities to calculate P values for interaction. Results of secondary outcomes were not adjusted for multiplicity; therefore, these should be considered exploratory and cannot be used to infer treatment effects.
A scheduled interim analysis was performed by the independent DSMB when the first 100 patients were enrolled. Haybittle-Peto stopping boundaries were used,20 with a threshold of P < .001 to interrupt the trial for safety and a threshold of P < .0001 to interrupt the trial for efficacy.
Post hoc sensitivity analyses to assess the treatment effect on the co–primary outcomes were repeated with similar models considering all randomized patients providing consent to use data including those who were transferred to other institutions within 72 hours after randomization. There was no loss to follow-up for co–primary outcome data among these participants up to the point at which they were transferred to nonparticipating hospitals. A post hoc sensitivity analysis was performed to evaluate the site-by-treatment effects by using a mixed-effects model with site (participating hospital) as a random effect.
A 2-tailed P < .05 was considered to be statistically significant. We used Stata software version 14.0 (StataCorp) and R software version 4.0.3 (R Foundation) for all analyses. Additional information about the planned statistical analysis is provided in Supplement 2 and the eAppendix in Supplement 3.
Results
Patients
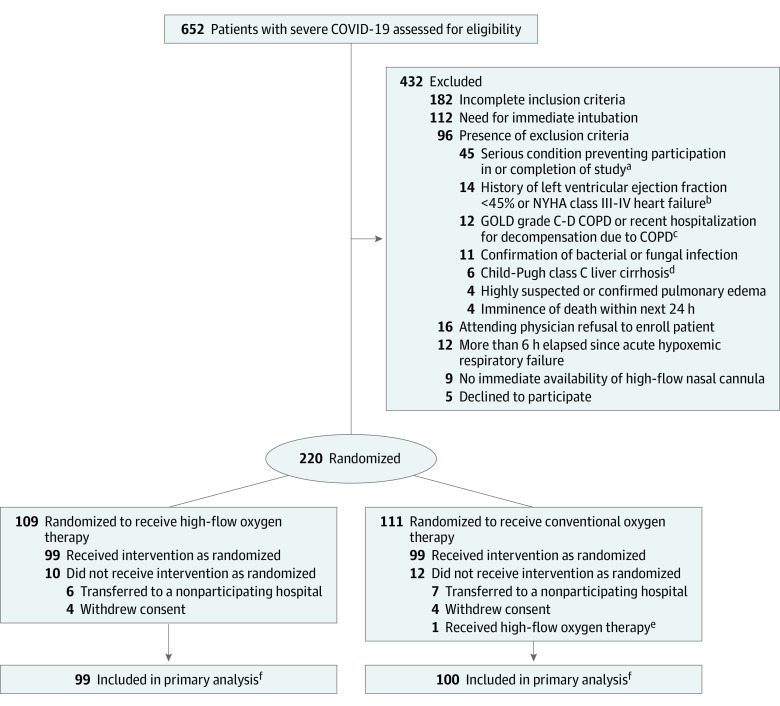
From August 2020 through January 2021, 650 patients with acute hypoxemic respiratory failure due to suspected or confirmed COVID-19 were screened; 250 were eligible for inclusion and 220 underwent randomization (Figure 1). The 28-day follow-up was completed on February 10, 2021. After exclusion of 8 patients who withdrew consent and 13 patients transferred to other hospitals (for administrative reasons) within the first 72 hours of randomization, a total of 199 were included in the analysis, with 99 randomized to high-flow oxygen therapy through a nasal cannula and 100 to conventional oxygen therapy. Baseline patient characteristics were similar between groups (Table 1; eTable 2 in Supplement 3).
Figure 1. Flow of Participants Through the Trial.
aDefined as any serious medical condition or clinical laboratory test result abnormality that, in an investigator’s judgement, could prevent safe patient participation and completion of the study. Patients for whom therapeutic limitations were anticipated or moribund patients were included in this category.
bNew York Heart Association (NYHA) class I indicates no symptoms with normal physical activity (asymptomatic); class II, mild symptoms with normal physical activity; class III, moderate symptoms with activities of daily living; and class IV, symptoms at rest.16
cCOPD indicates chronic obstructive pulmonary disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD) airflow limitations are determined by spirometry and divided into 4 grades: 1, mild (forced expiratory volume in first second of expiration [FEV1] ≥80% predicted); 2, moderate (FEV1 50%-79% predicted); 3, severe (FEV1 30%-49% predicted); and 4, very severe (FEV1 <30% predicted).17 GOLD C corresponds to patients with severe or very severe airflow limitation (spirometric grades 3 and 4) and/or 2 or more exacerbations per year and/or 1 or more hospitalized exacerbations per year, and a Modified British Medical Research Council (mMRC) questionnaire on breathlessness grade of 0 to 1 or a Chronic Obstructive Pulmonary Disease Assessment Test (CAT) score less than 10. GOLD D corresponds to patients with severe or very severe airflow limitation (spirometric grades 3 and 4) and/or 2 or more exacerbations per year and/or 1 or more hospitalized exacerbations per year, and an mMRC grade of 2 or higher or a CAT score of 10 or higher.
dChild-Pugh scores are calculated by assessing ascites (1 point for none, 2 points for slight, and 3 points for moderate), serum bilirubin level (1 point for <2.0 mg/dL, 2 points for 2.0-3.0 mg/dL, and 3 points for >3.0 mg/dL), serum albumin level (1 point for ≥3.5 g/dL, 2 points for 2.8-3.5 g/dL, and 3 points for <2.8 g/dL), prolongation of prothrombin time (1 point for <4 seconds, 2 points for 4-6 seconds, and 3 points for >6 seconds), and encephalopathy (1 point for none, 2 points for grade 1 or 2, and 3 points for grade 3 or 4). Child-Pugh class C is a total score of more than 9 points.18
eOne patient randomized to conventional oxygen therapy received high-flow oxygen therapy according to the decision of the attending physician. This patient was included in the conventional oxygen therapy group for the primary analysis.
fPatients were analyzed according to their randomization group, excluding those withdrawing consent and those transferred to nonparticipating hospitals. Because of the hospital emergency situation during the pandemic and in agreement with the recommendation by the ethical and biomedical research committee of the coordinating center, patients who were transferred to other hospitals (for health insurance–related reasons) before 72 hours had elapsed since enrollment were excluded from the primary analysis because of limitations on providing further protocolized management and impossibility of ensuring adequate follow-up. All co–primary outcome data were recorded for these patients until transfer.
Table 1. Baseline Participant Characteristics.
| Characteristics | High-flow oxygen therapy (n = 99)a,b | Conventional oxygen therapy (n = 100)a,c |
|---|---|---|
| Age, median (IQR), y | 60 (50-69) | 59 (49-67) |
| Sex, No. (%) | ||
| Female | 28 (28) | 37 (37) |
| Male | 71 (72) | 63 (63) |
| Body mass index, median (IQR)d | 28.7 (26.3-32.1) | 29.4 (26.2-33.1) |
| APACHE II score, No. (%)e | 10 (8-12) | 10 (8-12) |
| Test results for SARS-CoV-2, No. (%) | ||
| Positive RT-PCR result | 98 (99) | 100 (100) |
| RT-PCR result unavailable | 1 (1) | 0 |
| Comorbidities, No. (%) | ||
| Hypertension | 35 (35) | 44 (44) |
| Diabetes | 18 (18) | 20 (20) |
| Liver cirrhosis (Child-Pugh class A-B)f | 35 (35) | 44 (44) |
| Chronic obstructive pulmonary disease | 3 (3) | 1 (1) |
| Chronic heart failure | 3 (3) | 4 (4) |
| Chronic kidney disease | 0 | 1 (1) |
| Cancer | 1 (1) | 0 |
| Medications on hospital admission, No. (%) | ||
| Steroids | 4 (4) | 10 (10) |
| Angiotensin-converting enzyme inhibitors | 5 (4) | 8 (8) |
| Angiotensin II receptor antagonists | 24 (24) | 20 (20) |
| Statins | 7 (7) | 3 (3) |
| Time from symptom onset to randomization, median (IQR), d | 10 (7-11) | 8 (7-11) |
| Time from admission to randomization, median (IQR), d | 1 (0-1) | 1 (0-1) |
| SOFA score at randomization, median (IQR)g | 4 (3-4) | 4 (3-4) |
| Respiratory rate, median (IQR), /min | 28 (27-32) | 28 (26-31) |
| Pao2, median (IQR), mm Hg | 78 (66-97) | 73 (63-92) |
| Paco2, median (IQR), mm Hg | 32 (30-35) | 32 (30-36) |
| Pao2/Fio2 ratio, median (IQR) | 104 (85-132) | 105 (85-141) |
| Seven-category ordinal scale score at randomization, No. (%)h | ||
| Hospitalized and receiving supplemental oxygen (score of 4) | 18 (18) | 20 (20) |
| Hospitalized in ICU and receiving oxygen supplementation (score of 5) | 81 (82) | 80 (80) |
Abbreviations: Fio2, fraction of inspired oxygen; ICU, intensive care unit; Paco2, partial pressure of arterial carbon dioxide; Pao2, partial pressure of arterial oxygen; RT-PCR, reverse transcriptase–polymerase chain reaction.
Percentages may not total 100% because of rounding.
High-flow oxygen indicates therapy with high-flow oxygen through a nasal cannula.
Conventional oxygen therapy was given through a Venturi or nonrebreather face mask at a flow rate up to 15 L/min.
Calculated as weight in kilograms divided by height in meters squared.
The Acute Physiology and Chronic Health Evaluation (APACHE II) score ranges from 0 to 71; higher scores indicate greater severity of illness and risk of in-hospital death (eg, a score of 12 for a patient with pneumonia predicts an in-hospital mortality risk of 15%).
Child-Pugh scores are calculated by assessing ascites (1 point for none, 2 points for slight, and 3 points for moderate), serum bilirubin level (1 point for <2.0 mg/dL, 2 points for 2.0-3.0 mg/dL, and 3 points for >3.0 mg/dL), serum albumin level (1 point for ≥3.5 g/dL, 2 points for 2.8-3.5 g/dL, and 3 points for <2.8 g/dL), prolongation of prothrombin time (1 point for <4 seconds, 2 points for 4-6 seconds, and 3 points for >6 seconds), and encephalopathy (1 point for none, 2 points for grade 1 or 2, and 3 points for grade 3 or 4). Child-Pugh class A is a total score of 5 or 6 points and class B is a total score of 7 to 9 points.18
The Sequential Organ Failure Assessment (SOFA) score assesses the presence and intensity of respiratory, coagulation, hemodynamic, neurologic, liver, and kidney failure. Each organ is assessed on a scale from 0 (no failure) to 4 (worst possible failure); the total score ranges from 0 (no organ failure) to 24 (all organ failure).
Scores on the 7-category ordinal scale are as follows: 1, discharged from the hospital, resuming complete daily-life activities; 2, discharged from the hospital with limitation of activities, home oxygen requirement, or both; 3, hospitalized in a general ward (not ICU), not requiring supplemental oxygen, and no longer requiring ongoing medical care (used if hospitalization was extended for infection control reasons); 4, hospitalized in a general ward (not ICU), requiring supplemental oxygen or requiring ongoing medical care (for COVID-19–related or other medical conditions); 5, hospitalized in the ICU, requiring any supplemental oxygen; 6, hospitalized in the ICU, requiring invasive mechanical ventilation or extracorporeal membrane oxygenation; and 7, death. See eTable 1 in Supplement 3.
Interventions
Allocated therapies were always commenced within 30 minutes after randomization. All patients completed their treatment as randomized except 1 patient randomized to conventional oxygen therapy who underwent high-flow oxygen therapy on the decision of the attending physician. This patient was included in the conventional oxygen therapy group for the primary analysis.
High-flow oxygen therapy was continuously delivered for a total of 6 (IQR, 3-9) days in patients randomized to this therapy, and it was successfully weaned to conventional oxygen therapy by 6 (IQR, 4-7) days in 65 (65.7%) of 99 patients. Patients’ respiratory condition at 2 and 4 hours after starting randomized interventions is shown in eTable 3 in Supplement 3. Cumulative time in prone position while awake was not significantly different between groups (21 [IQR, 8-40] hours for high-flow oxygen therapy vs 18 [IQR, 8-35] hours for conventional oxygen therapy; P = .35) (eTable 4 in Supplement 3). Systemic steroids were used in 93 patients (93.9%) in the high-flow oxygen therapy group vs 92 (92.0%) of those randomized to conventional oxygen therapy. Other respiratory and pharmacological interventions are described in eTable 4 in Supplement 3.
Interim Analysis
The interim analysis was performed as planned, when the first 100 patients were enrolled. Based on this analysis, the DSMB found no reason to recommend halting the study and thus suggested continuing the trial until the planned sample size was reached.
Primary Outcomes
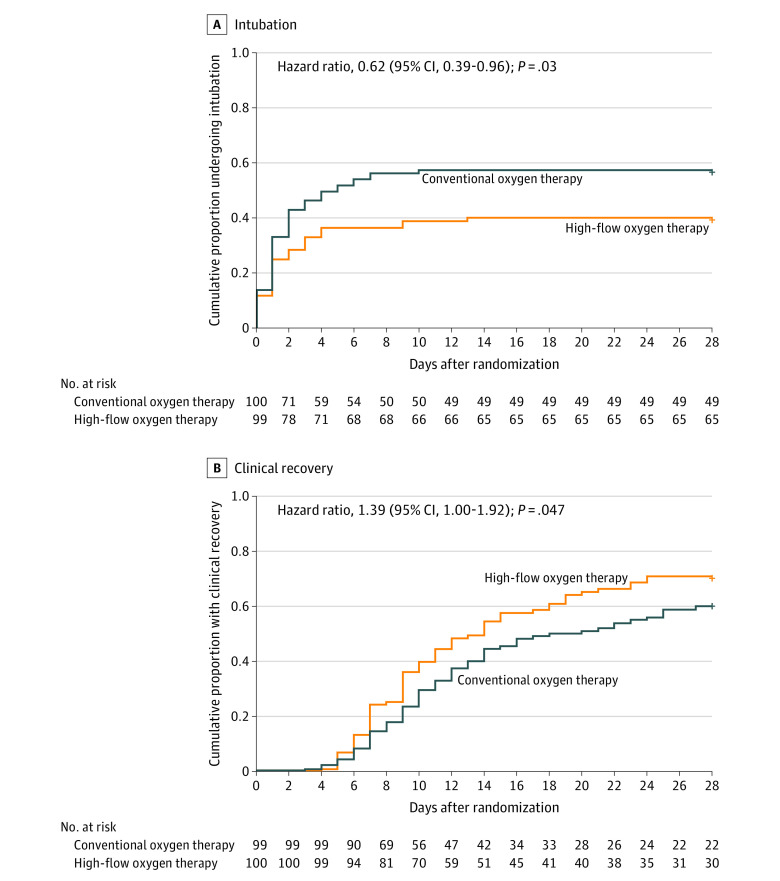
By day 28 after randomization, 34 (34.3%) of 99 patients randomized to high-flow oxygen therapy and 51 (51.0%) of 100 randomized to conventional oxygen therapy had been intubated (HR, 0.62; 95% CI, 0.39-0.96; P = .03) (Table 2 and Figure 2A). Causes of intubation were similar between groups (eTable 5 in Supplement 3). Clinical recovery occurred in 77 patients (77.8%) randomized to high-flow oxygen therapy vs 71 (71.0%) of those randomized to conventional oxygen therapy. The median time to recovery among patients in the high-flow oxygen therapy group was 11 (IQR, 9-14) days vs 14 (IQR, 11-19) days in the conventional oxygen therapy group. Patients randomized to high-flow oxygen therapy had early clinical improvement as assessed by the 7-category ordinal scale (HR, 1.39; 95% CI, 1.00-1.92; P = .047) (Table 2 and Figure 2B). The proportional hazards assumption was not violated as examined by the Grambsch and Therneau method (eTable 10 in Supplement 3).
Table 2. Primary and Secondary Outcomes.
| Outcomes | High-flow oxygen therapy (n = 99) | Conventional oxygen therapy (n = 100) | Unadjusted absolute difference (95% CI) | Effect estimate, OR or HR (95% CI)a | P value |
|---|---|---|---|---|---|
| Primary outcomes | |||||
| Intubation within 28 d, No. (%) | 34 (34.3) | 51 (51.0) | −16.7 (−30.2 to −3.1)b | HR, 0.62 (0.39-0.96)c | .03 |
| Clinical recovery within 28 d, No. (%) | 77 (77.8) | 71 (71.0) | 6.8 (−5.3 to 18.9)b | HR, 1.39 (1.00-1.92)d | .047 |
| Time to clinical recovery, median (IQR), de | 11 (9-14) | 14 (11-19) | −3.0 (−7.5 to 1.0)f | ||
| Secondary outcomes | |||||
| Intubation within 7 d, No. (%) | 31 (31.3) | 50 (50.0) | −18.7 (−32.1 to −5.3)b | HR, 0.59 (0.38-0.94)c | .03 |
| Intubation within 14 d, No. (%) | 34 (34.3) | 51 (51.0) | −16.7 (−30.2 to −3.1)b | HR, 0.63 (0.41-0.97)c | .04 |
| Ventilation-free days at day 28, median (IQR) | 28 (19-28) | 24 (14-28) | 4.5 (0.0 to 7.8)g | OR, 2.08 (1.18-3.64) | .01 |
| Kidney replacement therapy–free days, median (IQR)h | 28 (28-28) | 28 (28-28) | 0 (0 to 0)g | OR, 1.78 (0.67-4.68) | .24 |
| Length of stay, median (IQR), d | |||||
| Intensive care unit | 7 (5-13) | 9 (5-18) | −2.0 (−6.0 to −1.0)g | OR, 0.74 (0.45-1.22) | .24 |
| Hospital | 12 (9-20) | 14 (9-23) | −2.0 (−4.0 to −1.0)g | OR, 0.77 (0.47-1.25) | .29 |
| Mortality at day 14, No. (%) | 6 (6.1) | 6 (6.0) | 0.1 (−6.6 to 6.7)b | HR, 0.93 (0.29-2.93)c | .90 |
| Mortality at day 28, No. (%) | 8 (8.1) | 16 (16.0) | −7.9 (−16.9 to 1.1)b | HR, 0.49 (0.21-1.16)c | .11 |
| Serious adverse events, No. (%) | |||||
| Cardiac arrest | 2 (2.0) | 6 (6.0) | |||
| Suprasupraventricular tachycardia or ventricular arrhythmia | 3 (3.0) | 1 (1.0) | |||
| Atelectasis | 1 (1.0) | 0 | |||
| Other reported adverse events, No. (%) | |||||
| Suspected bacterial pneumonia | 13 (13.1) | 17 (17.0) | |||
| Bacteremia | 7 (7.1) | 11 (11.0) | |||
Abbreviations: HR, hazard ratio; OR, odds ratio.
Proportional odds models are adjusted for age (≥60 or <60 years), ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (Pao2/Fio2) at randomization, and comorbidities (arterial hypertension, diabetes, obesity [body mass index >30], chronic obstructive pulmonary disease, kidney failure, heart failure, and Child-Pugh class A-B liver cirrhosis). Odds ratios greater than 1 indicate benefit with use of high-flow oxygen therapy. Adjusted hazard ratios were calculated from a Cox proportional hazards model. Variables used for adjustment were the same as those listed above for odds ratios. See footnote “f” in Table 1 for Child-Pugh score and class descriptions.18
Absolute incidence differences in percentage points (percentage intubated, clinically recovered, requiring kidney replacement therapy, or who died, as appropriate).
A hazard ratio for intubation or mortality less than 1 indicates benefit with use of high-flow oxygen therapy.
A hazard ratio for clinical recovery greater than 1 indicates benefit with use of high-flow oxygen therapy. A reported lower confidence bound of 1.00 is a consequence of rounding.
Kaplan-Meier estimates of median time to clinical recovery. Time to clinical recovery was defined as the time elapsed from randomization until the first day during the 28 days after enrollment on which a patient attained a reduction of 2 or more points from their score at randomization on the modified 7-category ordinal scale (see footnote “h” in Table 1 for description of the ordinal scale).
Bootstrap 95% CI of the median difference from Kaplan-Meier estimates.
Median time-difference 95% CIs were estimated based on 5000-bootstrap resampling.
Need for kidney replacement therapy occurred in 7 patients (7.1%) randomized to high-flow oxygen therapy vs 14 (14.0%) randomized to conventional oxygen therapy (OR, 0.49; 95% CI, 0.18-1.28; P = .16).
Figure 2. Cumulative Intubation and Clinical Recovery Through Day 28 (Co–Primary Outcomes).
The effect of treatments on cumulative incidence of intubation and recovery rates was calculated with a Cox proportional hazards model adjusted for hypoxemia severity, age, and comorbidities.
Secondary Outcomes
This trial included 8 secondary outcomes. High-flow oxygen therapy was related to lower risk of intubation at days 7 and 14 (Table 2). Median ventilator-free days within the first 28 days after randomization were 28 (IQR, 19-28) days in the high-flow oxygen therapy group vs 24 (IQR, 14-28) days in the conventional oxygen therapy group (adjusted odds ratio, 0.77; 95% CI, 0.33-1.68; P = .01) (Table 2). Need for kidney replacement therapy was not significantly different between groups (Table 2). Similarly, in-hospital and intensive care unit length of stay were not significantly different between groups and neither was the proportion of adverse events (Table 2). The HR for death at day 28 was 0.49 (95% CI, 0.21-1.16; P = .11) in the high-flow oxygen therapy group compared with the conventional oxygen therapy group (Table 2). Causes of death are given in eTable 5 in Supplement 3.
Tertiary Outcomes
Time courses of Pao2/Fio2 ratio and oxygen flow in the study groups are shown in eFigures 1 and 2 in Supplement 3. Patients received a median of 22.3 (IQR, 13.2-60.7) hours of high-flow oxygen therapy vs 29.2 (IQR, 14.4-58.3) hours of conventional oxygen therapy prior to intubation (P = .69) (eTable 3 in Supplement 3). Median time spent at an Fio2 greater than 0.70 from randomization to intubation was 11.5 (IQR, 4.2-21.0) hours for high-flow oxygen therapy vs 25.8 (IQR, 13.5-46.5) hours for conventional oxygen therapy (P < .001) (eTable 3 in Supplement 3). There were no significant differences in the evolvement of multiple and extrapulmonary organ dysfunction as evaluated by the Sequential Organ Failure Assessment score during the first 7 days after randomization (eFigures 3 and 4 in Supplement 3). Time courses for predefined laboratory measures are depicted in eFigures 11-17 in Supplement 3.
Subgroup and Sensitivity Analyses
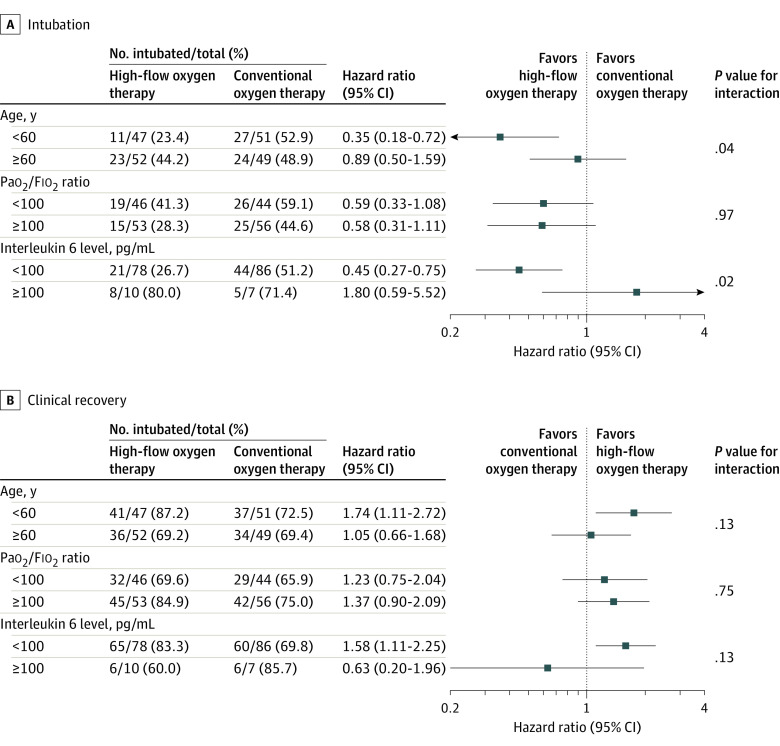
Results of the prespecified subgroup analysis are reported in Figure 3 and eFigures 5-10 in Supplement 3. There were no significant differences in treatment effect on the prespecified subgroup analyses, with the possible exceptions of age and interleukin 6 level at baseline. The effect on intubation in the high-flow oxygen therapy group may be more pronounced among patients younger than 60 years (HRs: <60 years, 0.35 [95% CI, 0.18-0.72]; ≥60 years, 0.89 [95% CI, 0.50-1.59]; P = .04 for interaction) and those with interleukin 6 levels less than 100 pg/mL (HRs: interleukin 6 <110 pg/mL, 0.45 [95% CI, 0.27-0.75]; interleukin 6 ≥110 pg/mL, 1.80 [95% CI, 0.59-5.52]; P = .02 for interaction). Nevertheless, these findings are exploratory because of lack of adjustment for multiplicity of hypothesis tests. Analyses of primary and secondary outcomes according to the baseline 7-category ordinal scale for clinical recovery as well as clinical status at 28 days are shown in eTables 6 and 7 in Supplement 3. Post hoc sensitivity analyses considering all randomized patients who provided consent to use data including those who were transferred to other institutions within 72 hours after randomization provided results similar to the main analyses (eTable 8 in Supplement 3). Similarly, post hoc sensitivity mixed-model analysis with site as a random effect was consistent with the main analysis: high-flow oxygen therapy through a nasal cannula decreased need for mechanical ventilation support (HR, 0.62; 95% CI, 0.40-0.96; P = .03) and increased the probability of clinical recovery (HR, 1.42; 95% CI, 1.03-1.97; P = .04) compared with conventional oxygen therapy (eTable 9 in Supplement 3).
Figure 3. Results of the Prespecified Subgroup Analysis on Intubation and Clinical Recovery.
The widths of the CIs were not adjusted for multiplicity and therefore cannot be used to infer treatment effects. A hazard ratio less than 1 for intubation indicates benefit with use of high-flow oxygen therapy. A hazard ratio greater than 1 for clinical recovery indicates benefit with use of high-flow oxygen therapy.
Discussion
In this open-label, multicenter randomized clinical trial among patients with acute hypoxemic respiratory failure due to severe COVID-19, the use of high-flow oxygen therapy through a nasal cannula significantly decreased the need for intubation and invasive mechanical ventilation and also led to earlier clinical recovery within 28 days.
Arterial hypoxemia is the leading feature of severe cases of SARS-CoV-2 infection.4 In general, management of acute hypoxemic respiratory failure relies on oxygen supplementation and supporting respiratory effort, and management of COVID-19–related moderate and severe hypoxemic respiratory failure should be based on similar principles. Analogous to previous studies on acute hypoxemic respiratory failure due to other etiologies,10,11,12 high-flow oxygen therapy through a nasal cannula was also able to decrease the requirement of intubation and mechanical ventilation in severe cases of COVID-19, but with no effect on mortality rates. The HiFLo-Covid trial did not use an “oxygen escalation strategy,” but instead, high-flow oxygen therapy was immediately applied in patients fulfilling inclusion criteria. In this sense, a strategy providing high-flow oxygen therapy at very early stages of respiratory failure could theoretically offer some physiological advantages including improvement of inspiratory effort, minute volume, respiratory rate, lung volumes, dynamic lung compliance, transpulmonary pressure, and lung homogeneity.21
Avoiding systematic intubation in COVID-19 could prevent complications related to invasive mechanical ventilation, sedation, delirium, and neuromuscular paralysis.21 In addition, successful prevention of intubation could optimize resources during pandemic conditions. In the current trial, both intubation and extubation procedures were performed according to well-prespecified criteria, which probably limited delays in providing protective invasive mechanical ventilation. Time elapsed from randomization to intubation and reasons for intubation were similar between study groups. Although previous studies have suggested that delay in intubation is related to increased mortality rates in patients with acute hypoxemic respiratory failure,22,23 high-flow oxygen therapy seems to be safe, as it is not associated with higher mortality rates or longer recovery times despite nonimmediate intubation in cases that eventually require it. Conversely, longer periods of high Fio2, as observed in most patients receiving conventional oxygen therapy, could have negatively influenced time to recovery such as has been suggested by experimental models in which hyperoxia was related to exacerbated lung injury.24
Patients receiving high-flow oxygen therapy showed earlier clinical recovery, although the mechanisms involved are less obvious than those implied in avoiding intubation. Very early relief of inspiratory effort could theoretically limit patient self-inflicted lung injury, which should influence clinical outcomes. Improvement of respiratory mechanics and limitation of lung injury could reduce time to clinical recovery, assuming that part of such injury might appear as a consequence of increased respiratory load that is inadequately supported during spontaneous breathing.6
Although the HiFLo-Covid trial was constructed under the assumption of physiological effects of high-flow oxygen therapy, measurements or estimations of the metabolic work of breathing, esophageal pressure monitoring, dynamic measurements of transpulmonary pressures, minute volume measurements, and estimations of nonhomogeneous distribution of tidal ventilation were not performed. Thus, the current trial evaluated only clinical outcomes, assuming that mechanisms involved in preventing the progression of lung injury, relief of respiratory effort, and improvement of gas exchange should be improved by high-flow oxygen therapy.
A recent clinical trial comparing high-flow oxygen therapy through a nasal cannula vs noninvasive ventilation through a helmet interface resulted in no significant difference in the number of days free of respiratory support within 28 days.25 In addition, the rate of endotracheal intubation was significantly lower in the noninvasive ventilation group, while the number of days free of invasive ventilation was significantly higher in the noninvasive ventilation group compared with high-flow oxygen therapy. However, these last findings should be further evaluated, as they are results of secondary outcomes.
This trial has several strengths. First, group randomization was consistently maintained until intubation and invasive mechanical ventilation support was started or until clinical recovery was reached. Second, the results are in agreement with studies of acute hypoxemic respiratory failure due to other etiologies.10,11,12 Third, this study had a well-defined protocol including prespecified criteria for endotracheal intubation and extubation, with only 1 violation regarding therapy allocation. Fourth, results were consistent even after sensitivity analysis including patients transferred to other centers due to administrative reasons within 72 hours after randomization. Fifth, sensitivity analysis evaluating the site-by-treatment effects also found similar results favoring high-flow oxygen therapy.
Limitations
This study has several limitations. First, because of its nature, this open-label trial lacked the possibility of blinding, which may affect the assessment of outcomes. Second, all participants were recruited in only 3 centers from 1 country, which restricts the generalizability of the results. Third, the trial design considered 2 co–primary end points, raising the potential for type I error. Fourth, analysis of secondary outcomes was not adjusted by multiplicity; these results should be considered exploratory. Fifth, the sample size of this trial and the number of events were relatively small, and therefore small variations in the number of events would have rendered treatment effect on the co–primary outcomes nonsignificant. Sixth, measurements or estimations for the metabolic work of breathing, transpulmonary pressures, minute volume, or estimations of nonhomogeneous distribution of tidal ventilation were not performed; thus, potential mechanisms mediating the effect of high-flow oxygen therapy through a nasal cannula on the co–primary outcomes remain theoretical. Seventh, this trial was not powered to demonstrate differences in mortality; nevertheless, the effect of high-flow oxygen therapy on need for intubation and clinical recovery could encourage its use.
Conclusions
Among patients with severe COVID-19, use of high-flow oxygen through a nasal cannula significantly decreased need for mechanical ventilation support and time to clinical recovery compared with conventional low-flow oxygen therapy.
Trial Protocol
Statistical Analysis Plan
eAppendix. Supplemental Methods
eTable 1. Seven-Point Ordinal Scale
eTable 2. Other Patient Characteristics at Randomization
eTable 3. Patient Characteristics 2-Hours and 4-Hours After the Start of Assigned Oxygenation Therapy
eTable 4. Time From Randomization to Intubation/Invasive Mechanical Ventilation Support/Additional Respiratory Support Strategies/Additional Therapies
eTable 5. Causes of Intubation at Day-7 and Causes of Death at Day-28
eTable 6. Primary and Secondary Outcomes for All Patients and According to 7-Category Ordinal Scale
eTable 7. Seven-Category Ordinal Scale at Day 28
eTable 8. Post-Hoc Sensitivity Analysis Considering All Randomized Patients Who Provided Consent to Use Data (N=212)
eTable 9. Post-Hoc Sensitivity Analysis Considering the Site-by-Treatment Effects (N=109)
eTable 10. Proportional Hazard Asssumption Test
eFigure 1. Time Course of Pao2/Fio2 Ratio for Study Groups
eFigure 2. Time Course of Oxygen Flow (L/min) for Study Groups
eFigure 3. Time Course of Multiple Organ Dysfunction According to SOFA Score for Study Groups
eFigure 4. Time Course of Extra-pulmonary Organ Dysfunction According to SOFA Score for Study Groups
eFigure 5. Kaplan Meier Estimates of Cumulative Intubation Events According to Initial Pao2/Fio2 < or ≥ 100
eFigure 7. Kaplan Meier Estimates of Cumulative Intubation Events According to Initial IL-6 < or ≥ 100 pg/mL
eFigure 8. Kaplan Meier Estimates of Clinical Recovery According to Initial Pao2/Fio2 < or ≥ 100
eFigure 9. Kaplan Meier Estimates of Clinical Recovery According to Age < or ≥ 60 Years Old
eFigure 10. Kaplan Meier Estimates of Clinical Recovery According to Initial IL-6 < or ≥ 100 pg/mL
eFigure 11. Time Course of Total Leucocyte Count for Study Groups
eFigure 12. Time Course of Neutrophil:Lymphocyte Ratio for Study Groups
eFigure 13. Time Course of Ferritin Levels for Study Groups
eFigure 14. Time Course of D-Dimer for Study Groups
eFigure 15. Time Course of Lactate Dehydrogenase Levels for Study Groups
eFigure 16. Time Course of Platelets Count for Study Groups
eFigure 17. Time Course of IL-6 for Study Groups
eReferences
Nonauthor Collaborators. HiFLo-Covid Investigators
Data Sharing Statement
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 2.Grasselli G, Zangrillo A, Zanella A, et al. ; COVID-19 Lombardy ICU Network . Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574-1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S, Hirsch JS, Narasimhan M, et al. ; Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tobin MJ. Basing respiratory management of COVID-19 on physiological principles. Am J Respir Crit Care Med. 2020;201(11):1319-1320. doi: 10.1164/rccm.202004-1076ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G, Tonetti T, Protti A, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8(12):1201-1208. doi: 10.1016/S2213-2600(20)30370-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195(4):438-442. doi: 10.1164/rccm.201605-1081CP [DOI] [PubMed] [Google Scholar]
- 7.Mauri T, Turrini C, Eronia N, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195(9):1207-1215. doi: 10.1164/rccm.201605-0916OC [DOI] [PubMed] [Google Scholar]
- 8.Piraino T. Noninvasive respiratory support in acute hypoxemic respiratory failure. Respir Care. 2019;64(6):638-646. doi: 10.4187/respcare.06735 [DOI] [PubMed] [Google Scholar]
- 9.Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth. 2011;107(6):998-1004. doi: 10.1093/bja/aer265 [DOI] [PubMed] [Google Scholar]
- 10.Frat JP, Thille AW, Mercat A, et al. ; FLORALI Study Group; REVA Network . High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185-2196. doi: 10.1056/NEJMoa1503326 [DOI] [PubMed] [Google Scholar]
- 11.Rochwerg B, Granton D, Wang DX, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45(5):563-572. doi: 10.1007/s00134-019-05590-5 [DOI] [PubMed] [Google Scholar]
- 12.Azoulay E, Lemiale V, Mokart D, et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: the HIGH randomized clinical trial. JAMA. 2018;320(20):2099-2107. doi: 10.1001/jama.2018.14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854-887. doi: 10.1007/s00134-020-06022-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demoule A, Vieillard Baron A, Darmon M, et al. High-flow nasal cannula in critically iii patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202(7):1039-1042. doi: 10.1164/rccm.202005-2007LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calligaro GL, Lalla U, Audley G, et al. The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: a multi-centre prospective observational study. EClinicalMedicine. 2020;28:100570. doi: 10.1016/j.eclinm.2020.100570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett JA, Riegel B, Bittner V, Nichols J. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart Lung. 2002;31(4):262-270. doi: 10.1067/mhl.2002.124554 [DOI] [PubMed] [Google Scholar]
- 17.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347-365. doi: 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 18.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646-649. doi: 10.1002/bjs.1800600817 [DOI] [PubMed] [Google Scholar]
- 19.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515-526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 20.Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol. 1971;44(526):793-797. doi: 10.1259/0007-1285-44-526-793 [DOI] [PubMed] [Google Scholar]
- 21.Tobin MJ, Laghi F, Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care. 2020;10(1):78. doi: 10.1186/s13613-020-00692-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrillo A, Gonzalez-Diaz G, Ferrer M, et al. Non-invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intensive Care Med. 2012;38(3):458-466. doi: 10.1007/s00134-012-2475-6 [DOI] [PubMed] [Google Scholar]
- 23.Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350(24):2452-2460. doi: 10.1056/NEJMoa032736 [DOI] [PubMed] [Google Scholar]
- 24.Sinclair SE, Altemeier WA, Matute-Bello G, Chi EY. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit Care Med. 2004;32(12):2496-2501. doi: 10.1097/01.CCM.0000148231.04642.8D [DOI] [PubMed] [Google Scholar]
- 25.Grieco DL, Menga LS, Cesarano M, et al. ; COVID-ICU Gemelli Study Group . Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA. 2021;325(17):1731-1743. doi: 10.1001/jama.2021.4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eAppendix. Supplemental Methods
eTable 1. Seven-Point Ordinal Scale
eTable 2. Other Patient Characteristics at Randomization
eTable 3. Patient Characteristics 2-Hours and 4-Hours After the Start of Assigned Oxygenation Therapy
eTable 4. Time From Randomization to Intubation/Invasive Mechanical Ventilation Support/Additional Respiratory Support Strategies/Additional Therapies
eTable 5. Causes of Intubation at Day-7 and Causes of Death at Day-28
eTable 6. Primary and Secondary Outcomes for All Patients and According to 7-Category Ordinal Scale
eTable 7. Seven-Category Ordinal Scale at Day 28
eTable 8. Post-Hoc Sensitivity Analysis Considering All Randomized Patients Who Provided Consent to Use Data (N=212)
eTable 9. Post-Hoc Sensitivity Analysis Considering the Site-by-Treatment Effects (N=109)
eTable 10. Proportional Hazard Asssumption Test
eFigure 1. Time Course of Pao2/Fio2 Ratio for Study Groups
eFigure 2. Time Course of Oxygen Flow (L/min) for Study Groups
eFigure 3. Time Course of Multiple Organ Dysfunction According to SOFA Score for Study Groups
eFigure 4. Time Course of Extra-pulmonary Organ Dysfunction According to SOFA Score for Study Groups
eFigure 5. Kaplan Meier Estimates of Cumulative Intubation Events According to Initial Pao2/Fio2 < or ≥ 100
eFigure 7. Kaplan Meier Estimates of Cumulative Intubation Events According to Initial IL-6 < or ≥ 100 pg/mL
eFigure 8. Kaplan Meier Estimates of Clinical Recovery According to Initial Pao2/Fio2 < or ≥ 100
eFigure 9. Kaplan Meier Estimates of Clinical Recovery According to Age < or ≥ 60 Years Old
eFigure 10. Kaplan Meier Estimates of Clinical Recovery According to Initial IL-6 < or ≥ 100 pg/mL
eFigure 11. Time Course of Total Leucocyte Count for Study Groups
eFigure 12. Time Course of Neutrophil:Lymphocyte Ratio for Study Groups
eFigure 13. Time Course of Ferritin Levels for Study Groups
eFigure 14. Time Course of D-Dimer for Study Groups
eFigure 15. Time Course of Lactate Dehydrogenase Levels for Study Groups
eFigure 16. Time Course of Platelets Count for Study Groups
eFigure 17. Time Course of IL-6 for Study Groups
eReferences
Nonauthor Collaborators. HiFLo-Covid Investigators
Data Sharing Statement