Abstract
This study aimed to investigate the efficacy of whole-body vibration training (WBVT) on blood brain-derived neurotrophic factor (BDNF) levels and determine the clinical and functional outcomes in patients with fibromyalgia syndrome (FMS). Thirty-two women with FMS were randomized into an intervention group (IG), receiving 6 weeks of WBVT, or a control group (CG) with no intervention. The outcomes at the baseline and follow-up in both groups included blood BDNF levels, sit-to-stand test (STS), 6-minute walk test (6MWT), Fibromyalgia Impact Questionnaire (FIQ), Pittsburgh Sleep Quality Index (PSQI), Beck Depression Inventory (BDI), and visual analogue scale (VAS). WBVT resulted in a group-by-time interaction effect. Thus, after the intervention time, the IG had increased blood BDNF levels (p=0.045), a higher number of repetitions on the STS test (p=0.011), and increased walking distance on the 6MWT (p=0.010), compared to CG. Moreover, there was a reduction in the scores of the FIQ (p=0.001), the PSQI (p=0.001), the BDI (p=0.017), and pain assessed using VAS (p=0.008) in IG. The results demonstrate that WBVT promotes an increase in blood BDNF levels, with concomitant improvement in lower limb muscle strength, aerobic capacity, clinical symptoms, and quality of life in women with FMS. This trial is registered with Brazilian Clinical Trials Registry (REBEC; RBR-38nbbx) (https://ensaiosclinicos.gov.br/rg/RBR-38nbbx).
1. Introduction
Fibromyalgia syndrome (FMS) is characterized by chronic widespread pain, neuroinflammation [1], nociception-driven amplification of neural signalling (central sensitization) [2], systemic low-grade inflammation, and muscle dysfunction [3–5]. These central and peripheral changes often occur concomitantly with clinical symptoms, such as depression [6] and sleep disturbance [7], which may compromise the biological rhythms. Consequently, it may also lead to a decrease in physical function, modifying the quality of life [3].
Brain-derived neurotrophic factor (BDNF) is a key molecule involved in plastic changes related to central and peripheral plasticity, neuroinflammation, pain, and other clinical symptoms in pathological conditions including FMS [1, 8]. Of note, FMS has a vastly negative impact on clinical and functional aspects, and current literature points to physical exercise and cognitive-behavioral therapy as the nonpharmacological choice interventions [9]. Once some interventions like exercise enhance the expression of BDNF in normal and pathological conditions, it is crucial to evaluate the efficacy of complementary therapeutic interventions on BDNF levels in FMS.
Unfortunately, evidence-supporting therapies for the management of FMS are limited to small trials of low methodological quality [10]. Mascarenhas et al., in a recent review, found moderate-quality evidence of a positive association between pain-reduction exercises and improved quality of life. The authors suggested that further high-quality trials might increase certainty with respect to the effectiveness of exercises in FMS [10].
Concerning exercises on the vibrating platform, a review work pointed out that whole-body vibration (WBV) exercises are safe, viable, and well tolerated by patients with chronic conditions including FMS and are less tiring and time-consuming than a standard exercise protocol [11]. In this regard, despite works showing WBV in fatigue and pain [9, 12], quality of life [9, 13–15], balance [9, 15–17], muscle strength [14], inflammatory status [4], and parameters of oxidative balance [18] in FMS patients, the works differed in aspects such as the vibratory stimulus (vertical or alternating), the vibratory protocol (frequency, amplitude, time of sets, rest interval, and duration), and time points (immediate or training) [19, 20]. Moreover, because FMS presents a negative influence on several outcomes (clinical, functional, and physiological) [10], there remains a gap regarding the evaluation of primary and secondary outcomes representing different aspects of the syndrome. In addition, the results of the works often present low effect size estimates, emphasizing the need for new studies with an experimental design and internal control ensuring methodological quality to fulfill the gaps that still exist [10]. Of note, the majority of previous works investigated the additional effects of the intervention combining whole-body vibration training (WBVT) with an associated exercise program, resulting in a gap regarding the stand-alone intervention effect [9]. Finally, as far as we know, no previous study evaluated the effects of WBVT on blood BDNF levels and crucial clinical (e.g., sleep quality and depression screening) symptoms associated with the biological rhythms in women with FMS. Therefore, our work aims to investigate the efficacy of WBVT on blood BDNF levels, clinical and functional outcomes, and quality of life in women with FMS. We hypothesize that stand-alone WBVT can promote changes in blood BDNF levels while improving lower limb muscle strength, aerobic capacity, clinical symptoms, sleep quality, and depressive symptoms.
2. Materials and Methods
2.1. Study Design
This was a randomized controlled clinical trial with concealed allocation and assessor blinding for score counts, developed at Universidade Federal dos Vales do Jequitinhonha e Mucuri (Federal University of the Jequitinhonha and Mucuri Valleys) (Diamantina/Minas Gerais, Brazil). This is a convenience sample. The recruitment of the patients was from health centers in the local community between June 2017 and June 2018. Randomization was performed using individual allocation code numbers placed within opaque, sealed envelopes, without contact with the participants.
The study was approved by the local ethics committee (identification number: 2.057.949) and registered on the Brazilian Clinical Trials Registry (REBEC; RBR-38nbbx). This study follows checklists for randomized and controlled clinical trials—Consolidated Standards of Reporting Trials (CONSORT) and Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT).
2.2. Study Population
The inclusion criteria were being female, aged 50 to 60 years, from the perimenopause period onwards, and with a diagnosis of FMS confirmed by a rheumatologist. In addition, the patients also needed to be non-smokers and non-consumers of alcohol and be physically inactive, not having participated in any exercise programs during the previous 24 months. We highlight that the patients that self-reported physical activity levels lower than those recommended by the American College of Sports Medicine (ACSM) were classified as sedentary and were included [21].
The exclusion criteria were the presence of any concomitant disease that could be exacerbated by physical activity; inflammatory diseases; patients in psychiatric follow-up; patients performing physical activity more than twice a week; patients who displayed any of the possible contraindications for WBV stimulus, such as acute hernia, orthopedic and prosthetic lesions, metabolic or neuromuscular diseases, epilepsy, or stroke [22]; and patients taking oral or topical immunosuppressive medication (corticosteroids) [4]. The same requirements were applied to both groups.
Potential participants were screened to verify eligibility before baseline assessment and randomization. Only one researcher assigned the allocation of all the patients into the groups. All patients were asked which medications they were taking regularly (Table 1) and were randomly allocated to the intervention group (IG), who underwent WBVT, or the control group (CG), who did not receive the intervention. The IG patients underwent familiarization with the WBV stimulus and were informed as to the correct positioning and method of performing the exercise. CG patients received weekly phone calls from researcher and were instructed to maintain their routine of daily living activities. Follow-up occurred for the assessment of possible complications or changes in the daily routine, such as the presence of exacerbations, or the beginning of physical therapy or regular physical activity.
Table 1.
Medications of the patients.
| Medication | CG (n = 15) | IG (n = 17) |
|---|---|---|
| Gastric distress | 1 (6.6%) | 2 (11.7%) |
| Analgesics | 2 (13.3%) | 7 (41.1%) |
| Anticonvulsant | 2 (13.3%) | 4 (23.5%) |
| Antidepressants | 10 (66.6%) | 6 (35.2%) |
| Antidiabetic | 5 (33.3%) | 3 (17.6%) |
| Antihypertensive | 9 (60%) | 5 (29.4%) |
| Anxiolytics | 2 (13.3%) | 0 (0.0%) |
| Antirheumatic | 1 (6.6%) | 1 (5.8%) |
| Neuropathic pain | 2 (13.3%) | 2 (11.7%) |
| Hypercholesterolemia | 2 (13.3%) | 4 (23.5%) |
| TSH suppression | 2 (13.3%) | 2 (11.7%) |
| Muscle relaxants | 2 (13.3%) | 2 (11.7%) |
The numbers represent the number and percentage of participants in the group who use the medication. CG: control group (n = 15). IG: intervention group (n = 17).
Both groups carried out baseline evaluations and follow-up after six weeks. In the IG, the second evaluation was performed 48 hours after the final intervention to minimize the residual effects of the final exercise session [12]. The evaluations always followed the same order. The participants came to the laboratory at 7 am having fasted for at least 8 hours and without using the medication they normally take in the morning. Moreover, all patients abstained from medication for at least 12 hours before evaluation to minimize possible acute effects. They were instructed to bring the medication to the laboratory. Soon after blood collection had been carried out, a standardized snack was offered, and the medication was ingested. Initially, personal and sociodemographic data were collected on the evaluation form, in addition to complete medical history, medications in use, and information on living habits. Blood collection was performed by a specialized professional and questionnaires were applied. Subsequently, functional tests and anthropometric assessments were performed.
2.3. Intervention
The IG performed WBVT three times per week on alternate days, for 6 weeks. The training protocol consisted of dynamic squatting, i.e., flexing lower limb joints during squatting for 3 seconds up, followed by 3 seconds down, on a synchronic vibrating platform (FitVibe® Excel Pro, GymnaUniphy, Belgium). The number of each set increased progressively over the 6 weeks. Thus, the patients performed sets of 6 to 8 squats. During each squat, the examiner instructed the patient to perform a semicomplete knee extension, i.e., up (angle 10°) for 3 seconds, followed by 3 seconds of knee bend, i.e., down (angle 60°). Although each squat required 6 seconds, because each position change (up/down-down/up) required 1 second, the total time of each squat was 8 seconds. Between sets, the patient was instructed to rest for 30 seconds on the vibratory platform turned off. Of note, each training day lasted around 180 to 624 seconds (i.e., 3 minutes to 10.6 minutes). To minimize resonant catastrophe, the participants were also instructed to remain with their feet on the platform and their spine, arms, and head in the instructed position (simulating the motion of sitting in a chair) [23]. The damping effect of different footwear was avoided through patients performing the exercises barefoot [24]. In addition, to ensure that each of the lower limbs received the same amount of vibration stimulus, a predetermined distance between the feet was set, with 14 cm to the right and 14 cm to the left of the platform vibration centre [25].
The mechanical stimulation parameters of the vibration consisted of the following: frequency of 35–40 Hz, amplitude of 4 mm, and acceleration gravity ranging from 2.78 to 3.26 g (Table 2). The training protocol was adapted from previous studies by our research group [4, 22, 26, 27]. A physical therapist monitored all the training sessions, including pain intensity and perceived exertion (RPE) before and after each intervention session. Of note, although pain and RPE were assessed during the exercise sessions (study internal control), we did not find any difference between subjects before and after the sessions while the intervention progressed.
Table 2.
Whole-body vibration training load program (adapted from [22]).
| Weeks | Vibration parameters (intervention group) | Total time per set (sec) | Number of repetitions per set | Total number of sets | Rest time between sets (seconds) | ||
|---|---|---|---|---|---|---|---|
| Frequency (Hz) | Amplitude (mm) | Acceleration (G) | |||||
| 1 | 35 | 4 | 2.78 | 16 | 5 | 6 | 30 |
| 2 | 35 | 4 | 2.78 | 24 | 8 | 7 | 30 |
| 3 | 35 | 4 | 2.78 | 32 | 10 | 8 | 30 |
| 4 | 40 | 4 | 3.26 | 35 | 11 | 8 | 30 |
| 5 | 40 | 4 | 3.26 | 40 | 13 | 8 | 30 |
| 6 | 40 | 4 | 3.26 | 48 | 16 | 8 | 30 |
Hz: hertz; mm: millimetres; G: gravity acceleration.
2.4. Outcome Measures
2.4.1. Primary Outcomes: Measurement of Blood BDNF Levels
Peripheral blood samples were collected aseptically by puncturing the median cubital vein. Blood was collected with the patient at rest for at least 10 minutes. The blood sample was then centrifuged twice at 3000 rpm for 10 minutes. The plasma was kept frozen at −80 °C until blood analyses. Blood BDNF levels were measured using a conventional sandwich enzyme-linked immunosorbent assay kit (DuoSet, R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions. The detection limit was 5.0 pg/mL for the kit.
2.4.2. Secondary Outcomes: Lower Limb Muscle Strength, Aerobic Capacity, Quality of Life, and Clinical Symptoms
The sit-to-stand test (STS) was used to assess lower limb muscle strength [28], and aerobic capacity was assessed using the 6-minute walk test (6MWT) [29, 30]. The Brazilian version of the Fibromyalgia Impact Questionnaire (FIQ) was used to assess health status, functional capacity, and main symptoms of FMS [31]. Quality of sleep, depression, and pain intensity were assessed using the Pittsburgh Sleep Quality Index (PSQI) [32], the Beck Depression Inventory [33], and the visual analogue scale (VAS) [34], respectively. The assessor was previously trained, and the intra-assessor reliability was greater than 80% for all outcomes. In addition, the same blinded assessor collected all the secondary outcomes [4].
2.5. Statistical Analysis
The data were reported as mean and 95% confidence interval (CI). For each outcome variable, we used a two-way repeated measure ANOVA to compare the main effects over time (time effect) and the interaction (group × time interactions). Post hoc analyses were evaluated using the Scheffé test. The level of statistical significance was set to p < 0.05. Effect size (eta squared: ƞ2) < 0.25 represented small effect, between 0.25 and 0.4 represented moderate effect, and >0.4 represented large effect [35]. The statistical power was also determined. As there were no missing data, intention-to-treat analyses were not performed.
The sample size to produce a significant effect was estimated at 15 volunteers per group, considering the comparison between groups for the blood BDNF levels (primary outcome), with an effect size of 1.1, statistical power of 80%, and alpha error of 5% [26].
3. Results
In total, 71 patients were screened for eligibility. Of these, 36 did not meet the inclusion criteria and 3 refused to participate. Thus, 32 women with FMS participated in the study and were randomized into two groups, that is, 17 patients in the IG and 15 in the CG (Figure 1).
Figure 1.
CONSORT flow diagram for the study.
At the baseline, there were no significant differences between the groups in patient characteristics. Patients from the CG and the IG were overweight according to the body mass index (BMI) classification (29.79 + 3.10 and 29.88 + 4.59 kg/m2, respectively) (Table 3).
Table 3.
Demographic, clinical, and anthropometric characteristics at baseline.
| Outcome | CG (n = 15) | IG (n = 17) | p value |
|---|---|---|---|
| Age (years) | 54 (50–58) | 56 (53–59) | 0.426 |
| Time since diagnosis (years) | 7.67 (5.73–9.61) | 9.53 (7.46–11.60) | 0.183 |
| Weight (kg) | 71.35 (66.79–75.91) | 72.09 (65.64–78.52) | 0.849 |
| Height (m) | 1.56 (1.53–1.59) | 1.56 (1.54–1.58) | 0.839 |
| BMI (kg/m2) | 29.79 (28.07–31.51) | 29.88 (27.52–32.24) | 0.953 |
Values are means (95% confidence interval). CG: control group (n = 15). IG: intervention group (n = 17). BMI: body mass index. p value: unpaired t-test.
There was no statistical difference between the groups regarding the blood BDNF levels or clinical and functional outcomes at baseline (Table 4).
Table 4.
Biomarker levels and clinical and functional outcomes at baseline.
| Outcome | CG (n = 15) | IG (n = 17) | p value |
|---|---|---|---|
| Biomarker(ng/mL) | |||
| BDNF | 2.39 (1.82–2.96) | 2.35 (1.59–3.11) | 0.922 |
|
| |||
| Functional outcomes | |||
| 6MWT, m | 427.27 (398.96–455.58) | 447.35 (418.78–476.74) | 0.292 |
| STS, rep. | 8.27 (6.71–9.93) | 8.00 (6.48–9.52) | 0.976 |
|
| |||
| Clinical outcomes | |||
| FIQ, score | 66.68 (59.27–74.09) | 72.53 (64.21–80.85) | 0.277 |
| Pain, VAS | 5.77 (4.55–6.99) | 7.14 (6.24–8.04) | 0.081 |
| PSQI, score | 13.33 (11.61–15.05) | 12.18 (9.78–14.58) | 0.422 |
| BDI, score | 25.20 (19.34–31.06) | 21.65 (16.43–26.87) | 0.341 |
Values are means (95% confidence interval). CG: control group (n = 15). IG: intervention group (n = 17). BDNF, brain-derived neurotrophic factor; 6MWT, six-minute walk test; STS, sit-to-stand test; rep: repetition; FIQ, Fibromyalgia Impact Questionnaire; VAS, visual analogue scale; PSQI, Pittsburgh Sleep Quality Index; BDI, Beck Depression Inventory. p value: unpaired t-test (all variables showed normal distribution).
There was an interaction effect (group-by-time) after the WBVT (Table 5 and Figure 2). IG patients showed increased blood BDNF levels (p=0.045), a higher number of repetitions on the STS test (p=0.011), and increased walking distance on the 6MWT (p=0.010) compared to the CG. There was also a decrease in scores on the FIQ (p=0.001), the PSQI (p=0.001), and the BDI (p=0.017) questionnaires and in the perception of pain assessed using VAS (p=0.008).
Table 5.
Effects of 6 weeks of intervention and change from baseline on biomarker levels, functional outcomes, and clinical outcomes in control and whole-body vibration training groups.
| Outcome | CG (week 6) | Δ (change from baseline) | IG (week 6) | Δ (change from baseline) | Interaction p value | Partial η2 | Power |
|---|---|---|---|---|---|---|---|
| Biomarker(ng/mL) | |||||||
| BDNF | 1.80 (1.42 to 2.19) | −0.58 (−0.39 to −0.77) | 2.58 (1.83 to 3.34) | 0.23 (0.03 to 0.43) | 0.045 | 0.81 | 0.99 |
|
| |||||||
| Functional outcomes | |||||||
| 6MWT, m | 415.40 (396.63 to 434.17) | −11.87 (−30.80 to 7.06) | 470.35 (445.68 to 495.02) | 22.59 (3.68 to 41.50) | 0.010 | 0.88 | 1.0 |
| STS, rep. | 8.67 (7.22 to 10.12) | 0.40 (−0.22 to 1.02) | 10.41 (9.16 to 11.66) | 2.41 (1.04 to 3.78) | 0.011 | 0.88 | 1.0 |
|
| |||||||
| Clinical outcomes | |||||||
| FIQ, score | 68.79 (62.23 to 75.35) | 2.12 (−4.45 to 8.69) | 45.12 (34.99 to 55.25) | −27.41 (−34.81 to 20.01) | 0.001 | 0.97 | 1.0 |
| Pain, VAS | 5.91 (4.78 to 7.04) | 0.15 (−1.44 to 1.74) | 3.46 (2.06 to 4.86) | 3.46 (2.06 to 4.86) | 0.008 | 0.93 | 1.0 |
| PSQI, score | 14.13 (12.37 to 15.89) | 0.80 (−0.64 to 2.24) | 7.18 (5.18 to 9.18) | −5.00 (−7.04 to 2.96) | 0.001 | 0.38 | 0.34 |
| BDI, score | 25.20 (19.40 to 31.00) | 0 (−1.26 to 1.26) | 14.76 (9.10 to 20.42) | −6.88 (−12.17 to −1.59) | 0.017 | 0.86 | 1.0 |
Values are means (95% confidence interval). CG: control group (n = 15). IG: intervention group (n = 17). BDNF: brain-derived neurotrophic factor; 6MWT: six-minute walk test; STS: sit-to-stand test; rep.: repetition; FIQ: Fibromyalgia Impact Questionnaire; VAS: visual analog scale; PSQI: Pittsburgh Sleep Quality Index; BDI: Beck Depression Inventory. Interaction, group x time. Eta partial, η2.
Figure 2.
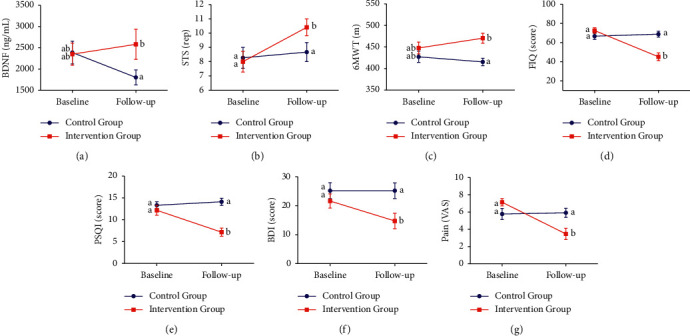
Group x time effect of blood BDNF level, clinical and functional symptoms at baseline and 6-week follow-up. Blue represents the control group (CG, n = 15), and red represents the intervention group (IG, n = 17). The results are presented as mean ± SEM. (a) Blood brain-derived neurotrophic factor (BDNF) levels. (b) Sit-to-stand test (STS), rep (repetitions). (c) Six-minute walk test (6MWT). (d) Fibromyalgia Impact Questionnaire (FIQ). (e) Sleep quality: Pittsburgh Sleep Quality Index (PSQI). (f) Depression screening: Beck Depression Inventory (BDI). (g) Pain: visual analogue scale (VAS). Means followed by different symbols (∗) represent significance within group (time effect). Means followed by different lowercase letters (a, b) represent significance between groups (group × time interactions) (Scheffé test, p < 0.05).
4. Discussion
To the best of our knowledge, this study is the first to evaluate the WBVT effect on blood BDNF levels and symptoms associated to the biological rhythms (e.g., sleep quality and depression screening) in women with FMS. The major findings demonstrated the following: (i) increased blood BDNF levels; (ii) improved lower limb muscle strength and aerobic capacity; (iii) improved clinical symptoms, i.e., sleep quality, depression symptoms, pain; and (iv) improved quality of life after a 6-week intervention of WBVT.
FMS symptoms are often related to physical inactivity, which in turn contributes and reinforces the impact of FMS, compromising physical activity, limiting daily function, and leading to progressive deconditioning with further reductions in physical capacity in FMS patients [36, 37]. Therefore, the results of improved physical function and exercise capacity after WBVT are clinically important evidencing the positive stand-alone effect of WBVT in physical aspects of FMS patients. In addition, the FIQ score reduction is of great clinical relevance demonstrating that stand-alone WBVT also promotes improvement in health status, functional capacity, and the main FMS symptoms, i.e., pain and fatigue, thereby reducing the impact of the disease and promoting an improvement in quality of life [38].
The literature demonstrates that plasma-free BDNF represents only the free (unbound) portion. This small fraction of unbound BDNF represents the bioavailable pool free to associate with TrkB or p75 receptors [39]. Thus, the WBVT probably promoted an increase in the BDNF portion while also reducing pain. Thus, we believe that the increase in blood BDNF levels (which represents the free unbound portion) seems to indicate a chronic adaptation inherent to training, influencing the pain defense mechanism. In addition, we believe that there was the maintenance of BDNF levels to the circulating pool, including musculoskeletal tissue cells. This hypothesis is in line with studies that have demonstrated that BDNF is a protein acting in autocrine and/or paracrine signalling within skeletal muscle. The expression increases through muscle contraction to enhance fat oxidation in an AMPK-dependent manner [40, 41].
Because BDNF plays an important role in the pathophysiology of the stress response and the pathogenesis of stress-associated mood disorders and its restoration may represent a critical mechanism underlying the antidepressant therapeutic effects, many investigators have focused on BDNF as a probable biomarker in depression. This is because the protein can cross the blood-brain barrier and circulating BDNF was correspondingly associated with cortical BDNF levels [42, 43]. However, it is still unknown whether BDNF alters sleep quality [44].
Previous studies indicated a possible link between improvement in subjective sleep outcomes and pain reduction in patients with FMS [34, 45]. Of note, the role of sleep quality in controlling pain and the pathophysiology of FMS suggests the need to develop alternatives to improve sleep quality. Some hypotheses try to explain the mode of action of WBVT in pain reduction. For example, activation of A-fibers produced during vibration can compete with central and peripheral nociceptive activity in the dorsal horn of the spinal cord, resulting in reduced second-order nociceptive activity with a consequent decrease in pain perception [23].
Although our experimental design did not focus on investigating associations and disease impact on different outcomes that affect biological rhythms in FMS patients, previous works suggested an association between the variables investigated in our work in FMS patients [36, 46]. Our statistical analysis does not allow causal inferences about the relationships between our outcome variables. Thus, we cannot make the claim that the BDNF is responsible for any change in other outcome variables given our study design and analysis.
However, it is worthwhile to highlight the large effect size (greater than 0.8) for most the outcomes, and only the sleep quality showed a moderate effect size (0.38) [35]. With this respect, the size of our sample was adequate to estimate the effect size. Thus, our analysis showed the short-term effects of WBVT in FMS were also clinically important in addition to being statistically significant for the outcomes and instruments assessed.
Thus, we suggested as a perspective to carry out further studies investigating the associations between BDNF plasma levels and clinical and functional outcomes including mediation/moderation analyses to confirm the hypothesis.
We emphasize that our study is at the forefront because no previous study investigated WBVT as a stand-alone intervention to FMS patients considering outcomes that together modify biological rhythms.
5. Strengths and Limitations
We highlight as strength the methodological quality because our work obtained a score of 8 in 10 points in the Physiotherapy Evidence Database Scale (PEDro scale) [47]. It is noteworthy that the items not reached in the total score (blinding of the subjects and therapist who administered the therapy) do not apply to studies with WBVT.
Although patients in both groups were instructed to abstain from using medication for 12 hours before experimental procedures, the possible chronic effect of medication intake on blood BDNF levels cannot be excluded, especially that of antidepressants [48]. However, surprisingly, the use of antidepressants was more common in the CG.
Another limitation was that we only analysed blood BDNF levels as biomarker. Thus, despite the contribution of blood BDNF levels to the circulating total, they are considerably lower than those of serum, so this small fraction of unbound BDNF represents a bioavailable pool that is free to associate with TrkB or p75 receptors [39, 49]. Despite this, we observed group vs time interactions in BDNF levels.
The level of general social interaction may also account for the results, as the control group did not experience these social exchanges. However, the patients of the control group received weekly phone calls to minimize this aspect. Moreover, the fact that the level of physical activity was self-reported by the patients may also constitute a limitation of the study.
Finally, our results cannot be generalized to other populations (e.g., different gender and age groups), as blood BDNF levels seem to interact with age and menstrual cycle.
6. Conclusions
Our data together are clinically relevant demonstrating the efficacy of WBVT maintaining blood BDNF levels with concomitant improvement in aspects related to biological rhythm (i.e., quality of sleep and depression symptoms). In addition, our data reinforced the WBVT effectiveness in reducing pain intensity and improving lower limb muscle strength, aerobic capacity, and quality of life in women with FMS.
Acknowledgments
We would like to thank Universidade Federal dos Vales do Jequitinhonha e Mucuri (UFVJM) for institutional support and Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council for Scientific and Technological Development) (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Coordination for the Improvement of Higher Education Personnel) (CAPES), and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Research Support Foundation for the State of Minas Gerais) (FAPEMIG) for support and grants.
Data Availability
The study data are with the researchers and can be provided when necessary.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Theoharides T. C., Tsilioni I., Bawazeer M. Mast cells, neuroinflammation and pain in fibromyalgia syndrome. Frontiers in Cellular Neuroscience . 2019;13:p. 353. doi: 10.3389/fncel.2019.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolf C. J. Central sensitization: implications for the diagnosis and treatment of pain. Pain . 2011;152(3):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alciati A., Atzeni F., Grassi M., et al. Features of mood associated with high body weight in females with fibromyalgia. Comprehensive Psychiatry . 2018;80:57–64. doi: 10.1016/j.comppsych.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro V., Mendonça V., Souza A., et al. Inflammatory biomarkers responses after acute whole body vibration in fibromyalgia. Brazilian Journal of Medical and Biological Research . 2018;51 doi: 10.1590/1414-431x20176775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jablochkova A., Bäckryd E., Kosek E., et al. Unaltered low nerve growth factor and high brain-derived neurotrophic factor levels in plasma from patients with fibromyalgia after a 15-week progressive resistance exercise. Journal of Rehabilitation Medicine . 2019;51(10):779–787. doi: 10.2340/16501977-2593. [DOI] [PubMed] [Google Scholar]
- 6.Nugraha B., Karst M., Engeli S., Gutenbrunner C. Brain-derived neurotrophic factor and exercise in fibromyalgia syndrome patients: a mini review. Rheumatology International . 2012;32(9):2593–2599. doi: 10.1007/s00296-011-2348-2. [DOI] [PubMed] [Google Scholar]
- 7.Nijs J., Loggia M. L., Polli A., et al. Sleep disturbances and severe stress as glial activators: key targets for treating central sensitization in chronic pain patients? Expert Opinion on Therapeutic Targets . 2017;21(8):817–826. doi: 10.1080/14728222.2017.1353603. [DOI] [PubMed] [Google Scholar]
- 8.Bjersing J. L., Erlandsson M., Bokarewa M. I., Mannerkorpi K. Exercise and obesity in fibromyalgia: beneficial roles of IGF-1 and resistin? Arthritis Research & Therapy . 2013;15(1):p. R34. doi: 10.1186/ar4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mingorance JA, Montoya P, Miranda JGV, Riquelme I. The Therapeutic Effects of Whole-Body Vibration in Patients With Fibromyalgia. A Randomized Controlled Trial. Frontiers in Neurology . 2021;12:p. 853. doi: 10.3389/fneur.2021.658383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mascarenhas R. O., Souza M. B., Oliveira M. X., et al. Association of therapies with reduced pain and improved quality of life in patients with fibromyalgia: a systematic review and meta-analysis. JAMA Internal Medicine . 2020;118 doi: 10.1001/jamainternmed.2020.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stania M., Juras G., Słomka K., Chmielewska D., Król P. The application of whole-body vibration in physiotherapy - A narrative review. Acta Physiologica Hungarica . 2016;103(2):133–145. doi: 10.1556/036.103.2016.2.1. [DOI] [PubMed] [Google Scholar]
- 12.Alentorn-Geli E., Padilla J., Moras G., Haro C. L., Fernández-Solà J. Six weeks of whole-body vibration exercise improves pain and fatigue in women with fibromyalgia. The Journal of Alternative and Complementary Medicine . 2008;14(8):975–981. doi: 10.1089/acm.2008.0050. [DOI] [PubMed] [Google Scholar]
- 13.Olivares P. R., Gusi N., Parraca J. A., Adsuar J. C., Del Pozo-Cruz B. Tilting whole body vibration improves quality of life in women with fibromyalgia: a randomized controlled trial. The Journal of Alternative and Complementary Medicine . 2011;17(8):723–728. doi: 10.1089/acm.2010.0296. [DOI] [PubMed] [Google Scholar]
- 14.Sañudo Corrales FdB, Hoyo Lora Md, Carrasco Páez L., McVeigh JG, Corral Pernia JA, Cabeza Ruiz R. The effect of a 6-week exercise programme and whole body vibration on strength and quality of life in women with fibromyalgia: a randomised study. Clinical and Experimental Rheumatology . 2010;24:5–10. [PubMed] [Google Scholar]
- 15.Sañudo B., Carrasco L., Hoyo M., Oliva-Pascual-Vaca C, Rodríguez-Blanco C. Changes in body balance and functional performance following whole-body vibration training in patients with fibromyalgia syndrome: A randomized controlled trial. Journal of rehabilitation medicine . 2013;45(7):678–684. doi: 10.2340/16501977-1174. [DOI] [PubMed] [Google Scholar]
- 16.Adsuar J, Del Pozo-Cruz B, Parraca J, Olivares P, Gusi N. The single-leg stance static balance in women with fibromyalgia: A randomized controlled trial. The Journal of sports medicine and physical fitness . 2012;52:85–91. [PubMed] [Google Scholar]
- 17.Sañudo B., de Hoyo M., Carrasco L., Rodríguez-Blanco C., Oliva-Pascual-Vaca Á., McVeigh J. G. Effect of whole-body vibration exercise on balance in women with fibromyalgia syndrome: a randomized controlled trial. The Journal of Alternative and Complementary Medicine . 2012;18(2):158–164. doi: 10.1089/acm.2010.0881. [DOI] [PubMed] [Google Scholar]
- 18.Santos J, Mendonça V, Ribeiro V, Tossige-Gomes R, Fonseca S, Prates A. Does whole body vibration exercise improve oxidative stress markers in women with fibromyalgia? Brazilian Journal of Medical and Biological Research . 2019;52 doi: 10.1590/1414-431x20198688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bidonde J., Busch A. J., van der Spuy I., Tupper S., Kim S. Y., Boden C. Whole body vibration exercise training for fibromyalgia. Cochrane Database of Systematic Reviews . 2017;9 doi: 10.1002/14651858.cd011755.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong Y., Wang W., Zheng J., Chen S., Qiao J., Wang X. Whole body vibration exercise for chronic musculoskeletal pain: a systematic review and meta-analysis of randomized controlled trials. Archives of Physical Medicine and Rehabilitation . 2019;100(11):2167–2178. doi: 10.1016/j.apmr.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 21.WHO. Guidelines on Physical Activity and Sedentary Behaviour . Geneva, Switzerland: WHO; 2020. [PubMed] [Google Scholar]
- 22.Avelar N. C. P., Simão A. P., Tossige-Gomes R., et al. The effect of adding whole-body vibration to squat training on the functional performance and self-report of disease status in elderly patients with knee osteoarthritis: a randomized, controlled clinical study. The Journal of Alternative and Complementary Medicine . 2011;17(12):1149–1155. doi: 10.1089/acm.2010.0782. [DOI] [PubMed] [Google Scholar]
- 23.Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. European Journal of Applied Physiology . 2010;108(5):877–904. doi: 10.1007/s00421-009-1303-3. [DOI] [PubMed] [Google Scholar]
- 24.Marín P. J., Bunker D., Rhea M. R., Ayllón F. N. Neuromuscular activity during whole-body vibration of different amplitudes and footwear conditions: implications for prescription of vibratory stimulation. Journal of Strength and Conditioning Research . 2009;23(8):2311–2316. doi: 10.1519/jsc.0b013e3181b8d637. [DOI] [PubMed] [Google Scholar]
- 25.Simão A. P., Avelar N. C., Tossige-Gomes R., et al. Functional performance and inflammatory cytokines after squat exercises and whole-body vibration in elderly individuals with knee osteoarthritis. Archives of Physical Medicine and Rehabilitation . 2012;93(10):1692–1700. doi: 10.1016/j.apmr.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Simão AP, Mendonça VA, Avelar NCP, et al. Whole Body Vibration Training on Muscle Strength and Brain-Derived Neurotrophic Factor Levels in Elderly Woman with Knee Osteoarthritis: A Randomized Clinical Trial Study. Frontiers in physiology . 2019;10:p. 756. doi: 10.3389/fphys.2019.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neves C. D. C., Lacerda A. C. R., Lage V. K. S., et al. Whole body vibration training increases physical measures and quality of life without altering inflammatory-oxidative biomarkers in patients with moderate COPD. Journal of Applied Physiology . 2018;125(2):520–528. doi: 10.1152/japplphysiol.01037.2017. [DOI] [PubMed] [Google Scholar]
- 28.Collado-Mateo D., Adsuar J. C., Dominguez-Muñoz F. J., Olivares P. R., Gusi N. Impact of fibromyalgia in the sit-to-stand-to-sit performance compared with healthy controls. PM&R . 2017;9(6):588–595. doi: 10.1016/j.pmrj.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 29.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. American Journal of Respiratory and Critical Care Medicine . 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 30.Mannerkorpi K., Svantesson U., Carlsson J., Ekdahl C. Tests of functional limitations in fibromyalgia syndrome: a reliability study. Arthritis & Rheumatism . 1999;12(3):193–199. doi: 10.1002/1529-0131(199906)12:3<193::aid-art6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 31.Marques A. P., Santos A. M. B., Assumpção A., Matsutani L. A., Lage L. V., Pereira C. A. B. Validação da versão bra-sileira do Fibromyalgia Impact Questionnaire (FIQ) Revista Brasileira de Reumatologia . 2006;46:24–31. doi: 10.1590/s0482-50042006000100006. [DOI] [Google Scholar]
- 32.Bertolazi A. N., Fagondes S. C., Hoff L. S., et al. Validation of the Brazilian Portuguese version of the Pittsburgh sleep quality index. Sleep Medicine . 2011;12(1):70–75. doi: 10.1016/j.sleep.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 33.Gomes-Oliveira M. H., Gorenstein C., Neto F. L., Andrade L. H., Wang Y. P. Validation of the Brazilian Portuguese version of the Beck Depression Inventory-II in a community sample. Revista Brasileira de Psiquiatria . 2012;34(4):389–394. doi: 10.1016/j.rbp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Bigatti S. M., Cronan T. A. A comparison of pain measures used with patients with fibromyalgia. Journal of Nursing Measurement . 2002;10(1):5–14. doi: 10.1891/jnum.10.1.5.52550. [DOI] [PubMed] [Google Scholar]
- 35.Cohen J. Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educational and Psychological Measurement . 1973;33(1):107–112. doi: 10.1177/001316447303300111. [DOI] [Google Scholar]
- 36.Assumpção A, Sauer JF, Mango PC, Pascual Marques A. Physical function interfering with pain and symptoms in fibromyalgia patients. Clinical and experimental rheumatology . 2010;28(6):S57–63. [PubMed] [Google Scholar]
- 37.Gaudreault N., Boulay P. Cardiorespiratory fitness among adults with fibromyalgia. Breathe . 2018;14(2):e25–e33. doi: 10.1183/20734735.019717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams DA, Arnold LM. Measures Applied to the Assessment of Fibromyalgia: Fibromyalgia Impact Questionnaire (FIQ), Brief Pain Inventory (BPI), the Multidimensional Fatigue Inventory (MFI-20), the MOS Sleep Scale, and the Multiple Ability Self-Report Questionnaire (MASQ; cognitive dysfunction) Arthritis Care & Research . 2011;63(11):p. S86. doi: 10.1002/acr.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh J. J., Tschakovsky M. E. Exercise and circulating BDNF: mechanisms of release and implications for the design of exercise interventions. Applied Physiology, Nutrition, and Metabolism . 2018;43(11):1095–1104. doi: 10.1139/apnm-2018-0192. [DOI] [PubMed] [Google Scholar]
- 40.Bogaerts A., Delecluse C., Claessens A. L., Coudyzer W., Boonen S., Verschueren S. M. P. Impact of whole-body vibration training versus fitness training on muscle strength and muscle mass in older men: a 1-year randomized controlled trial. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences . 2007;62(6):630–635. doi: 10.1093/gerona/62.6.630. [DOI] [PubMed] [Google Scholar]
- 41.Krabbe K. S., Mortensen E. L., Avlund K., et al. Brain-Derived Neurotrophic Factor Predicts Mortality Risk in Older Women. Journal of the American Geriatrics Society . 2009;57(8):1447–1452. doi: 10.1111/j.1532-5415.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- 42.Schmitt K., Holsboer-Trachsler E., Eckert A. BDNF in sleep, insomnia, and sleep deprivation. Annals of Medicine . 2016;48(1-2):42–51. doi: 10.3109/07853890.2015.1131327. [DOI] [PubMed] [Google Scholar]
- 43.Karege F., Perret G., Bondolfi G., Schwald M., Bertschy G., Aubry J.-M. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Research . 2002;109(2):143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 44.Uchida S., Shioda K., Morita Y., Kubota C., Ganeko M., Takeda N. Exercise effects on sleep physiology. Frontiers in Neurology . 2012;3:p. 48. doi: 10.3389/fneur.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Çetin B., Güleç H., Toktaş H. E., Ulutaş Ö., Yılmaz S. G., İsbir T. Objective measures of sleep in fibromyalgia syndrome: Relationship to clinical, psychiatric, and immunological variables. Psychiatry Research . 2018;263:125–129. doi: 10.1016/j.psychres.2018.02.057. [DOI] [PubMed] [Google Scholar]
- 46.Häuser W. Fibromyalgia syndrome: Basic knowledge, diagnosis and treatment. Medizinische Monatsschrift fur Pharmazeuten . 2016;39(12):504–511. [PubMed] [Google Scholar]
- 47.Blobaum P. Physiotherapy evidence database (PEDro) Journal of the Medical Library Association . 2006;94(4):p. 477. [Google Scholar]
- 48.Sumpton J. E., Moulin D. E. Fibromyalgia: presentation and management with a focus on pharmacological treatment. Pain Research and Management . 2008;13(6):477–483. doi: 10.1155/2008/959036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zagrebelsky M., Korte M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology . 2014;76:628–638. doi: 10.1016/j.neuropharm.2013.05.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study data are with the researchers and can be provided when necessary.


