Abstract
Background
We aimed to improve the assessment of the drug activity in meningioma clinical trials based on the study of the 3D volume growth rate (3DVGR) in a series of aggressive meningiomas. We secondarily aimed to correlate 3DVGR study with patient outcome.
Methods
We performed a post hoc analysis based on volume data and 3DVGR extracted from CEVOREM study including 18 patients with 32 recurrent high-grade meningiomas and treated with everolimus and octreotide. The joint latent class model was used to classify tumor 3DVGR undertreatment.
Results
Class 1 includes lesions responding to treatment with decrease in volume in the first 3 months, and then a stabilization thereafter (9.5% of tumors) (mean pretreatment 3DVGR = 6.13%/month; mean undertreatment 3DVGR = −18.7%/month within 3 first months and −0.14%/month after the 3 first months). Class 2 includes lesions considered as stable or with a slight increase in volume undertreatment (65.5%) (mean pretreatment 3DVGR = 6.09%/month; undertreatment 3DVGR = −0.09% within the first 3 months). Class 3 includes lesions without 3DVGR decrease (25%) (mean pretreatment 3DVGR = 46.9%/month; mean undertreatment 3DVGR = 19.2%/month within the first 3 months). Patients with class 3 lesions had a significantly worse progression-free survival (PFS) rate than class 1 and 2 ones.
Conclusions
Tumor 3DVGR could be helpful to detect early signal of drugs antitumoral activity or nonactivity. This volume response classification could help in the assessment of drug activity in tumors with mostly volume stabilization and rare response as aggressive meningiomas even with a low number of patients in complement to 6 months PFS.
Keywords: clinical trial, drug activity, meningioma, outcome, tumor growth rate
Key Points.
1. 3D volume growth rate calculation in high-grade meningiomas.
2. Improvement of assessment of drug activity in meningioma clinical trial.
3. Classification of the volumetric response undertreatment (everolimus and octreotide).
Importance of the Study.
Meningioma clinical trials are rare, mostly with small sample size and heterogeneous patient population, particularly in regard to tumor kinetic, limiting the value of progression-free survival (PFS). Moreover, responses as defined by Response Assessment in Neuro-oncology (RANO) to current chemotherapy are rare, and so, not appropriated to assess treatment effect. However, tumor volume stabilization or tumor growth slowdown should be considered as beneficial for the patient. We demonstrate that 3D volume growth rate (3DVGR) can be used to assess drug activity based on data issued from a meningioma clinical trial we recently achieved. 3DVGR is more sensible than classical 6-month PFS (PFS6) for signal of activity and appear relevant despite study limitation which includes a limited number of patients and a heterogeneous population. We propose to classify the response in different patterns depending on the 3DVGR: response, stabilization, growth slowdown, and persistent growth, which could be helpful to assess drug activity in future meningioma clinical trials.
Meningioma clinical trials testing medical therapies are rare. They mostly include a limited number of patients. The outcome and progression-free survival (PFS) results of these clinical trials are particularly variable even for the same drug, leading to a difficult interpretation and sometimes hazardous comparison.1–4 These clinical trials pose many problems such as the heterogeneity of the included patients or the lack of reliable control. The 6-month PFS (PFS6) rate is the most consensual and used criteria nowadays. Recently, the Response Assessment in Neuro-oncology (RANO) criteria group recommended the assessment of PFS6 as the principal criterion for meningioma clinical trials.5,6 However, drug response following RANO criteria remains rare in the specific population of meningioma tumors. Further, RANO criteria do not consider the tumor aggressiveness, the WHO grade, and the tumor growth rate. Therefore, for example, a tumor with a 2D surface growth rate at 3% per month will not be considered as progressing at 6 months regardless of drug activity. Most of the meningioma clinical trials are single arm, nonrandomized trials without control. To facilitate the comparison of results, Kaley et al proposed a PFS6 interest rate on the basis of multiple clinical trials in the literature. Recently, the European Organization for Research and Treatment of Cancer trial proposed a randomized clinical trial within the control arm of different drugs on the basis of local standard of care, but the lack of a reliable and homogeneous control is a consistent limiting factor.7
In parallel, the recent development of volume measurement software and MRI millimetric T1 gadolinium sequences facilitate a reliable and time-limited volume measurement. Time course volume collection allows precise measurement of the volume growth rate. To date, the 2D surface growth rate has been mainly used, but recent studies suggested that the interest and reliability of the 3D volume growth rate (3DVGR) study improve the sensibility threshold for tumor volume change and improves the precision of tumor delineation due to frequent asymmetrical meningioma configurations with various extensions.5,6,8–10
In this study, we aimed to improve the assessment of the drug activity in meningioma clinical trials on the basis of the study of the tumor volume and the 3D growth rate in a series of aggressive recurrent meningiomas. Secondarily, we aimed to correlate the 3DVGR study with patient outcome.
Materials and Methods
Patient Selection and Follow-up
Twenty patients with aggressive and recurrent meningiomas were included in phase 2 CEVOREM clinical trial combining mTOR inhibitor everolimus and somatostatin agonist octreotide as previously reported (NCT02333565).11 The trial was approved by appropriate local ethics committee and a written consent was provided by each patient before inclusion.
The minimum required 2D surface (per RANO) growth rate for inclusion was 10% per 6 months. Everolimus was administered orally at a dose of 10 mg/day, and octreotide was administered through intramuscular injection at a dose of 30 mg/month. Treatment was discontinued at progression or in case of nonacceptable adverse effects. Two patients with incomplete data of volume were excluded from the analysis. Two patients treated in less than 2 months because of toxicity were included in the study. Millimetric T1 gadolinium MRI scans were conducted before inclusion, at inclusion, and then every 3 months until progression. An independent central review of the MRIs was conducted with two reviewers and one adjudicator using Iplannet, Brain Lab software. For each lesion, a mean of the two measures (one per reviewer) was performed. The two patients with incomplete volume data collection were excluded from this volumetric study. Two pretreatment MRIs were required to assess pretreatment 3DVGR. Then, after inclusion, MRI was performed every 3 months until progression. Millimetric T1-weighted sequences with gadolinium were requested. The volume of the different lesions was measured at pretreatment and then collected longitudinally from treatment initiation (baseline time: t = 0) until the occurrence of progression or censoring. The tumor volumes, which were collected after the occurrence of a progression, were ignored. Progression was evaluated by investigators based on the RANO criteria. We conducted a post hoc analysis on the basis of volume data and 3DVGR.
Molecular Analyses
DNA was extracted from formalin-fixed paraffin-embedded tissue sections of meningiomas. The coding exons and exon–intron boundaries of NF2 gene was sequenced using the Custom QIAseq targeted DNA Panel (Qiagen) following the manufacturer’s instructions.
Statistical Analyses
Statistical analyses were conducted using the SOPHiA RADIOMICS platform.
All the lesions were considered as independent in the analyses (even for an individual with several lesions).
We classified the temporal dynamics of undertreatment tumor volumes; then, we compared the pretreatment and undertreatment growth rates. We investigated the impact of pretreatment dynamics of tumor volumes on their undertreatment dynamics. We also investigated the association between longitudinal profiles of tumor volumes and PFS time (Supplementary Figure 1).
Preinclusion–inclusion growth rates were compared with 3-month inclusion and 3-month progression or censured growth rates using Wilcoxon paired t test; P < .05 was considered as significant.
The correlation between profiles of undertreatment growth rates and risk of progression was evaluated by the model.
In the present study, log() refers to the natural logarithm transformation.
Pretreatment Tumor Growth Rates
We assumed that the pretreatment volumetric evolution of each lesion was exponential (ie, linear evolution of the pretreatment log-volumes).12,13 The pretreatment tumor growth rates (TGRpre-ttt), which referred to a percent increase in tumor volume during 1 month, were thus defined using the formula:
where V−t and V0 are the volumes (in cubic centimeters) measured at pretreatment and at baseline, respectively, and t is the delay (in months) between these two visits.
Model Formulation
We used a joint latent class mixed model14 to specify and classify the temporal trajectories of meningioma volumes while considering potential informative censorship due to the occurrence of progression. The study population was thus considered heterogeneous and decomposed into G homogeneous latent (unobserved) classes. The correlation between the evolution of the repeated tumor volumes and the risk of progression was explained by the latent classes.
The model assumed a Gaussian distribution of the longitudinal marker. However, the distribution of the volumes in the population was log-normal (as shown in Supplementary Figure 2). After log-transformation of the volumes, the Gaussian assumption of the marker was verified.
The joint latent class model was decomposed into three submodels, namely (i) a class membership submodel, which defines the probability that the lesion belongs to each latent class; (ii) a longitudinal submodel, which defines the evolution of the tumor log-volume over time given the latent class; and (iii) a proportional hazard submodel, which defines the individual hazard of progression over time given the latent class.
To determine the specification of the model, we firstly tried to adjust the class membership model (probability to belong to each latent class) on the following covariates: age (<55 years vs ≥55 years), gender (female vs male), WHO grade (I–II vs III), previous surgery (one vs multiple). Based on a backward stepwise procedure according to BIC (Bayesian information criteria, criteria which emphasizes the observed likelihood while penalizing the model complexity), the best model did not select any of these potential prognostic factors. Then, several specifications of the longitudinal volumetric trajectory were investigated in order to fit at best the log-volumetric observations: linear evolution, linear evolution with change of slope at several time points (t = 2, 3, …, 9 months), quadratic and cubic evolution over time of the log-volumes. Joint models were estimated by considering one, two, three, or four latent class(es).
The models were estimated based on the maximum likelihood approach using the lcmm package (v. 1.9.2) of the R software (v. 4.0.1).
According to the results showed in the Supplementary Table 1 and the minimization of the BIC, the best model assumed three latent classes and a linear evolution of the log-volumes, with change of slope at 3 months after treatment initiation.
Each lesion was thus assumed to follow an exponential trajectory over time, with a change of tumor growth after 3 months:
where Vt is the tumor volume (in cubic centimeters) at time t (t > 0, t in months), V0 is the tumor volume at baseline, and TGt < 3 and is the tumor growth of the lesion in the first 3 months, whereas TGt ≥ 3 is the tumor growth afterward. 𝕀{∙} is the indicator function, which equals 1 when the condition is verified, and 0 otherwise.
Our joint latent class mixed model was finally specified as follows: (i) the class membership submodel was adjusted on the discretized pretreatment TGR variable (discretized as ≤5% vs >5% per month to validate the linearity assumptions of the model). (ii) The longitudinal submodel was adjusted on the following variables: linear slope between 0 (treatment initiation) and 3 months and another after 3 months (both were specific to each latent class and estimated by the model); discretized pretreatment TGR variable (≤5% vs >5%) with class-specific effects; interaction between the discretized pretreatment TGR variable and the two slopes of evolution described above; class-specific intercept and random intercept. (iii) The proportional hazard submodel involved a class-specific baseline hazard (Weibull form).
Results
Population Description
The present study involved 32 growing lesions from 18 patients (17 individuals with recurrent high-grade meningiomas and one patient with recurrent refractory WHO grade I meningioma). Twelve patients harbored one lesion; one patient, two lesions; two patients, three lesions; and three patients, four lesions. Table 1 and Supplementary Table 2 summarize patient and tumor characteristics. Supplementary Figure 3 shows that the pretreatment median GR was 5% per month. Multiple lesion characteristics are summarized in Supplementary Table 3.
Table 1.
Patient Characteristics
| Characteristics | (n = 18) |
|---|---|
| Age (years), median (range) | 55 (30–73) |
| Gender F/M | 10/8 |
| Patient with NF2 germline mutation | 4 |
| Patients with multiple growing locations | 6 |
| Number of growing tumors | 32 |
| KPS, n (%) | |
| 90–100 | 4 (22) |
| 70–80 | 11 (61) |
| <70 | 3 (17) |
| Previous surgery, n (%) | |
| One | 2 (11) |
| Multiple | 16 (89) |
| Previous radiotherapy or radiosurgery, n (%) | |
| None | 1 (6) |
| One | 8 (44) |
| Multiple | 9 (50) |
| Previous chemotherapy, n (%) | |
| Yes | 4 (22) |
| No | 14 (78) |
| WHO grade, n (%) | |
| WHO grade I | 1 (5%) |
| WHO grade II | 10 (55%) |
| WHO grade III | 7 (40%) |
Abbreviation: KPS, Karnofsky Performance Status.
Determination of Three Different 3DVGR Drug Response Classes
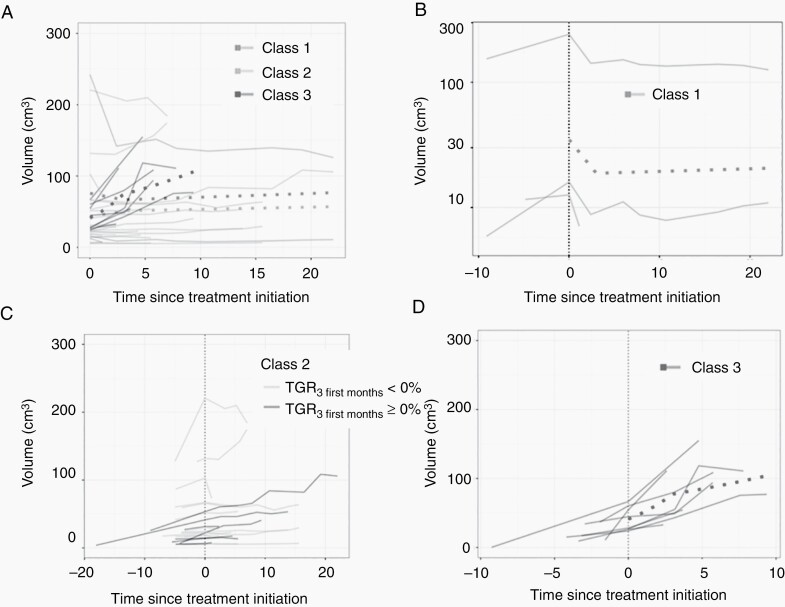
The results of the joint latent class mixed models were summarized considering the one, two, three, or four latent class(es) (Supplementary Table 4). The log-likelihood, the number of parameters, the AIC, the BIC, the sample size-adjusted BIC, and the posterior probability to belong in one latent class are displayed for each model. According to BIC analysis, the best model identified three significantly different latent classes, that is, the population was decomposed into three distinct profiles of tumor volumetric trajectories and hazard of event; and identified a linear evolution of the log-volumes, with change of slope at 3 months after treatment initiation. Using this joint model, we estimated the P-value of the score test for the conditional independence assumption of the longitudinal and survival data given the latent class structure .918. The longitudinal evolutions of tumor volumes were found as statistically different among the three classes (Supplementary Table 5). The model determined a significant variation of the TGR at 3 months postoperative. Figure 1 depicts the different volume growth curves, and Table 2 summarizes the data. Comparing classes 1, 2, and 3, PFS was significantly different (Figure 2A).
Fig. 1.
(A) Pretreatment and undertreatment growth curves of the different tumors depending on the class (1, green line; 2, blue line; and 3, redline). Dotted curves represented the mean growth curve for each class. (B) Pretreatment and undertreatment growth curves of class 1 meningioma with volume decrease then tumor stabilization. The dotted curve represented the mean growth curve of class 1 tumors. (C) Pretreatment and undertreatment growth curves of class 2 meningioma with volume stabilization or slow growth. The dotted curve represented the mean growth curve of class 2 tumors. Growth curves of the tumor with 3DVGR < 0% are represented in blue, and growth curves of the tumor with 3DVGR ≥ 0% are represented in gray. (D) Pretreatment and undertreatment growth curves of class 3 meningioma with high-volume increase. The dotted curve represented the mean growth curve of class 3 tumors. Abbreviations: 3DVGR, 3D volume growth rate; TGR, tumor growth rate.
Table 2.
Tumor Mean and Median 3DVGR Depending on the Class (1, 2, and 3) and the Period: Pretreatment, During the First 3 Months Undertreatment, and After 3 Months Undertreatment
| Class 1 | Class 2 | Class 3 | Overall Population | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | P | Mean | SD | Median | P | Mean | SD | Median | P | Mean | SD | Median | P | |
| Pre-ttt TGR | 6.13 | 5.01 | 5.08 | 6.09 | 7.28 | 3 | 46.89 | 58.8 | 23.15 | 16.30 | 33.75 | 5.10 | ||||
| Under-ttt TGR (within 3 months) | −18.7 | 3.36 | −17.81 | .003 | −0.09 | 1.34 | −1.2 | .000 | 19.16 | 2.73 | 19.16 | .75 | 2.97 | 10.99 | 0.33 | .000 |
| Under-ttt TGR (after the 3 first months) | −0.14 | 0.58 | 0.42 | .003 | 1.81 | 0.43 | 1.24 | .000 | 13.46 | 2.08 | 13.46 | .084 | 4.51 | 5.33 | 1.96 | .000 |
Abbreviations: 3DVGR, 3D volume growth rate; TGR, tumor growth rate; ttt, treatment.
Estimated P-values from the Wilcoxon ranked tests when comparing the pretreatment and undertreatment (in the first 3 months and after 3 months of treatment) 3DVGR depending on the class (1, 2, and 3).
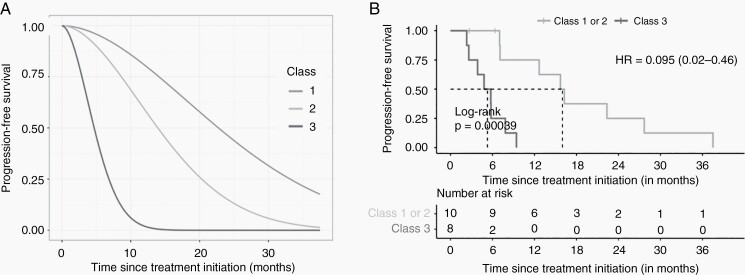
Fig. 2.
(A) Predicted progression-free survival (PFS) probabilities in the three latent classes of the estimated joint latent class mixed model. The three curves were estimated by the model and represent the mean probabilities of being free of recurrence over time in each latent class. (B) PFS depending on the class.
Class 1 (decrease then stable) includes the lesions responding to treatment with a decrease in volume in the first 3 months and a stabilization thereafter, n = 3/32 (9.5% of tumors). The mean pretreatment GR was 6.13% per month and the mean undertreatment GR was −18.7% per month within the first 3 months and −0.14% per month afterward (Table 2).
Class 2 (stable/slight increase) includes the lesions considered as stable or with a slight increase in volume undertreatment, n = 21/32 (65.5% of tumors). Pretreatment GR mean was 6.09% per month. Undertreatment GR was at −0.09% within the first 3 months and at 1.81% per month afterward. We investigated the split of this class into two groups, namely, the lesions with an undertreatment TGR ≤ 0% (stable; n = 14/32; 43.5% of tumors) and >0% (slight increase; n = 7/32; 22% of tumors) in the first 3 months after treatment initiation.
Class 3 (high increase) includes the lesions that do not respond to treatment: the volumes continue to strongly increase after treatment initiation (n = 8/32; 25% of tumors). Pretreatment GR mean was at 46.9% per month, and the undertreatment TGR mean was at 19.2% per month within the first 3 months and at 13.5% per month afterward.
Preinclusion and Undertreatment 3DVGR Comparison
Eighteen patients with 32 growing tumors were included for this post hoc analysis. Table 2 represents the mean and median 3DVGR. A significant decrease in the preinclusion–inclusion 3DVGR was observed comparing 3DVGR between 3 months of inclusion and after 3 months in classes 1 and 2 (Table 2). No significant difference in pretreatment and undertreatment GR was observed in class 3 (high-volume increase undertreatment). Both patients who were treated less than 2 months because drug toxicity was classified in class 3.
Correlation With PFS
We investigated the PFS in each latent class. The retained class for each patient was the class with the most aggressive lesion (class 3 > class 2 > class 1). Progression was evaluated by the investigator and defined according to the RANO criteria (25% of surface increase considering the largest tumor diameter and evaluated by the investigator). Significant differences were found comparing PFS curves for classes 1, 2, and 3 (Figure 2A). The class 3 lesions with strong volumetric growth undertreatment had a significantly worse PFS than those in classes 1 and 2 classified together (Figure 2A and B).
Different Patterns of Response in the Same Patient
Six patients harbored multiple meningiomas. NF2 mutation or loss of heterozygosity was found in the tumors of these six patients. Four patients were harboring type 2 neurofibromatosis. The pattern of response of the multiple meningiomas (classes 1, 2, and 3) was variable in 4/6 patients.
The Preinclusion Growth Rate Impacts the Treatment Response
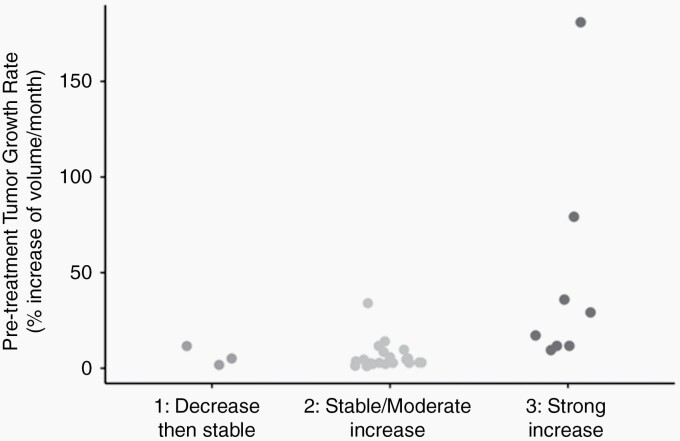
We analyzed the correlation between the preinclusion growth rate and the treatment response. Based on the median growth rate from the cohort, tumor preinclusion growth rates were separated as a low preinclusion growth rate (≤5% per month) (n = 15) and a high preinclusion growth rate (>5% per month) (n = 17). All the tumors with a low preinclusion growth rate respond as class 1 or 2 (Figure 3). Lesions with a high pretreatment TGR (>5%) were classified in classes 1 and 2 in 9/17 cases and in class 3 in 8/17 cases.
Fig. 3.
Classification of pretreatment growth rate depending on drug response classes.
We also analyzed the impact of the baseline volume on the pretreatment growth rate and the response classification. No correlation was observed even for the smallest and largest lesions (Supplementary Figure 4A and B).
Correlation study of the following parameters: patient age (<55 years vs ≥55 years), gender (female vs male), WHO grade (I–II vs III), lesion number (one vs multiple), previous surgery (one vs multiple) with the response classification was performed. No association was found as statistically significant (ie, with estimated P-value < .05), but the limited number of patients should be considered (Supplementary Tables 6 and 7; Supplementary Figure 5).
Most tumors were NF2-mutated so that NF2 mutation was not discriminating for response prediction.
Discussion
A Real Necessity to Improve the Assessment of Meningioma Clinical Trials
The PFS6 is the most consensual marker of drug efficacy in meningiomas nowadays and remains the most used criterium providing a comparison of study results.5,10,15 But in meningiomas, response to drugs according to the RANO criteria is rare. The PFS6 does not consider the growth rate, tumor aggressiveness, and the WHO grade. Then, the PFS6 is adapted to the most aggressive meningiomas but is less relevant in intermediately aggressive meningiomas as WHO grade II recurrent meningiomas. For instance, the RANO working group recommends for clinical trial a preinclusion surface GR at 15% per 6 months; however, theoretically, this meningioma will not be considered as progressive following the RANO criteria at 6 months. By contrast, with the same case, if the volume analysis concludes to class 3 (no change in GR undertreatment), the drug will be considered as without activity in this patient. The surface that results from the product of the longer diameter with its longer perpendicular as the RANO criteria recommendation remains to be of interest in evaluating the PFS6 and hence the clinical benefit for patients. However, the development of multiple-volume auto-segmentation software greatly facilitates volume assessment, which appears to be the most accurate for drug activity evaluation. To our opinion, 3DVGR evaluation is very complementary to PFS6 and could provide a more precise assessment of the drug activity than could PFS6 by providing for each patient his/her control corresponding to the pretreatment GR. Moreover, each patient has her/his control comparing pretreatment versus undertreatment 3DVGR, which is more sensible and could highlight drug positive effect even with a low number of patients, as in most aggressive meningioma clinical trials.
Proposal for a Response Pattern Classification
The three proposed classes in the present study were mathematically established and could not be directly applicable for clinics and patients. The mathematical analysis aims to retrospectively discriminate different patterns of response but did not establish a threshold to classify patients in clinical practice. However, translation of the different patterns to clinics seems feasible and relatively simple. Given the importance of PFS evaluation at 6 months in the specific population of recurrent high-grade meningiomas, the determination of volume assessment at 6 months seems also relevant. We translated 3DVGR/months in 3DVGR/6 months. Literature data suggest that an error margin on volume measurement of 10% is acceptable.6 The first pattern class corresponds to a decrease in volume (≥25% of the pretreatment volume at 6 months). The second class corresponds to volume stabilization or slight increase/growth rate slowdown. The third class corresponds to a persistent growth rate (Table 3).
Table 3.
Proposal of a Response Pattern Classification at 6 months
| Pattern | Volume Response Undertreatment | Pretreatment GR Range | Undertreatment GR Range |
Clinical Impact |
|---|---|---|---|---|
| 1 | Decrease | ≥+10%/ 6 months |
Volume decrease ≥ –25%/6 months |
Clinical benefit |
| 2A | Stabilization | ≥+10%/6 months | Volume decrease ≤–25%/6 months and GR ≤ +10%/6 months |
Clinical benefit |
| 2B | Slight increase with GR slowdown |
>+30%/6 months | +10% ≤ GR ≤ +30%/6 months | Likely clinical benefit |
| 3A | Persistent slight increase | ≥+10%/6 months <+30%/6 months |
≥+10%/6 months <+30%/6 months |
No or modest clinical interest |
| 3B | Persistent high increase | ≥+30%/6 months | >+30%/6 months | No or modest clinical interest |
Abbreviation: GR, growth rate.
For class 1, the mean volume decrease was approximately −6% per month during the first 3 months for this clinical trial combining everolimus and octreotide. In the RANO group, they considered that, for a spheric tumor, a decrease in the surface of 25% corresponds to a decrease in volume of 65%.6 To our opinion, a decrease in volume of at least 25% is limited/but sufficient to be of interest for the detection of activity signal. The RANO group recently introduced the notion of partial response for meningioma, corresponding to a decrease in 25% of the surface, which was exceptionally observed in the present study as in most meningioma clinical trials.
We proposed to divide class 2 into two classes: class 2A, volume stabilization, and class 2B, slight growth. For class 2A, tumor volume stabilization could be defined by a stable volume (GR ≤ 0% per month or ≤10% per 6 months). For class 2B, 3DVGR slowdown could be defined by a 3DVGR comprised between 0% and 5% per month or between 10% and 30% per 6 months in patients with pretreatment 3DVGR > 5% per month.
Class 3 corresponds to persistent tumor growth undertreatment. Class 3 could also be divided in two classes. Class 3A includes tumors with pretreatment and undertreatment slight increase (3DVGR ≥ +10%/6 months and <30%/6 months). Class 3B includes tumors with pretreatment and undertreatment high 3DVGR (≥30%/6 months). Class 3 corresponds to the lack or the insufficiency of drug activity.
Management of Multiple Meningiomas
In this study, six patients presented multiple lesions with heterogeneous pretreatment 3DVGR and also heterogeneous response pattern in 4/6 cases (Supplementary Table 3). To assess the response, for the outcome, the most aggressive lesion should be considered. In contrast, for drugs activity analysis, we suggest to consider the response of the different lesions. To improve the patient clinical management, a differentiated analysis could be proposed to adapt the therapy to each lesion.
Activity Study of the Combination of Everolimus and Octreotide in Aggressive Meningiomas
We previously demonstrated an in vitro antiproliferative activity of the combination of octreotide and everolimus but also demonstrated the lack of induced apoptosis.16 Mathematical volume analysis confirmed the clinical antiproliferative activity of the drugs combination: 65.5% of tumor 3DVGR stabilization or slowdown. Conversely, a volume decrease of more than 25% at 3 months after treatment initiation was observed in only 13% of cases and did not achieve the criteria of complete or partial response established by the RANO group.5 This volumetric analysis also confirmed the positive results of octreotide and everolimus combination in aggressive meningiomas.
Interestingly, we observed different patterns of response in the same patients bearing NF2-mutated tumor or type 2 neurofibromatosis, suggesting that NF2 mutation is likely not a reliable marker of octreotide and everolimus combination efficacy. This is according to previous studies, showing various growth patterns of meningioma growth in the same NF2 patient.17
By contrast, we observed an impact of the preinclusion growth rate on the response pattern. A low preinclusion growth rate defined as ≤5% per month seems a favorable marker of efficacy of the drugs combination. Conversely, everolimus and octreotide combination efficacy in tumors with the highest preinclusion growth rates (≥20% per month) seems poor and has limited clinical interest. In the present study, tumor volume at baseline does not appear to impact the 3DVGR evolution and the response classification.
The Potential Impact of the Study Results on Patient Clinical Management
Tumor GR could be helpful to detect early signals of drug antitumoral activity or in contrast of nonactivity.12,18
Class 1 (decrease in volume), 2A (volume stabilization), and 2B (slight volume increase/GR slowdown) provide a benefit for the patient. In case-by-case analysis, a treatment continuation despite progression could be relevant. For instance, in the case of one progressive lesion and many other controlled lesions under medical therapy, it could be of interest to specifically treat the growing lesion and to continue the medical therapy for the other lesions. Moreover, in the case of pattern 2B, with a growth rate slowdown of the tumor and no other therapeutic alternative, the treatment could be continued despite progression.
By contrast, in the case of pattern 3 after 3 months of treatment, the nonactivity of drugs could lead to shift to another treatment.
Limitations of 3DVGR Assessment
The volume measurement is clearly related to MRI quality. Millimetric MRIs are requested. In cases with invasive and poor-limited meningioma, the volume measurement could be challenging and should be done by the same physician with the same software during the same volume analysis session to improve the reproducibility. In these specific cases, software with automatic segmentation is not sufficiently precise nowadays, and their measurement analyses could lack reproducibility. The threshold of growth or volume decrease at 10%/6 months could be challenging in these specific cases.
Benign meningioma growth was reported to be linear or exponential.19,20 In our experience, aggressive meningioma growth is mostly linear, particularly in a short- or mild-term follow-up. Nevertheless, in case of spontaneous slowing of 3DVGR, comparison of pretreatment and undertreatment 3DVGR could be biased
In cases with an initial infracentimetric tumoral lesion, 3DVGR could be extremely high, leading to complex analysis and a probably out-of-interest 3DVGR. The use of cc/month unit may be an alternative accurate option.
Conclusions
Thanks to statistical model and BIC criteria, we identified three distinct classes of the evolution of meningioma lesions under the combination everolimus and octreotide: class 1 with volume decrease within the first 3 months and stabilization thereafter; class 2 with a volume stabilization or slight increase in volume undertreatment; class 3: the lesions with a strong volumetric growth undertreatment. PFS was significantly better for classes 1 and 2 than for class 3. Classes 1 and 2 represent 75% of all the treated tumors.
For high-grade meningiomas clinical trials, we suggest the determination of the preinclusion and undertreatment growth rates to classify the response in three different patterns (class 1: decrease in volume; class 2A: volume stabilization; class 2B: growth rate slowdown; and class 3: persistent slight [3A] and high [3B] growth), which could help in the evaluation of drug activity even with a low number of patients and in complement with 6 months PFS. This volume response classification seems particularly relevant for the evaluation of drug activity in tumors with mostly volume stabilization and rare response as aggressive meningiomas or for the assessment of therapies with mostly antiproliferative activity.
Supplementary Material
Funding
The study was supported by Institut National du Cancer (Inca, France). Drugs were provided by Novartis Pharma, France. Novartis partly supports translational researches and imaging central review.
Conflict of interest statement. None declared.
Authorship statement. Experimental design: T.G., O.C., T.C., L.F., J.S., its implementation A.B., D.F.G., G.M., or analysis: H.P., M.P., T.G., D.A. and interpretation of the data: T.G., O.C., T.C., M.K., M.S., E.T., H.D.
References
- 1. Nunes FP, Merker VL, Jennings D, et al. Bevacizumab treatment for meningiomas in NF2: a retrospective analysis of 15 patients. PLoS One. 2013;8(3):e59941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nayak L, Iwamoto FM, Rudnick JD, et al. Atypical and anaplastic meningiomas treated with bevacizumab. J Neurooncol. 2012;109(1):187–193. [DOI] [PubMed] [Google Scholar]
- 3. Alanin MC, Klausen C, Caye-Thomasen P, et al. Effect of bevacizumab on intracranial meningiomas in patients with neurofibromatosis type 2 - a retrospective case series. Int J Neurosci. 2016;126(11):1002–1006. [DOI] [PubMed] [Google Scholar]
- 4. Lou E, Sumrall AL, Turner S, et al. Bevacizumab therapy for adults with recurrent/progressive meningioma: a retrospective series. J Neurooncol. 2012;109(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang RY, Bi WL, Weller M, et al. Proposed response assessment and endpoints for meningioma clinical trials: report from the Response Assessment in Neuro-Oncology Working Group. Neuro Oncol. 2019;21(1):26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang RY, Unadkat P, Bi WL, et al. Response assessment of meningioma: 1D, 2D, and volumetric criteria for treatment response and tumor progression. Neuro Oncol. 2019;21(2):234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Preusser M, Silvani A, Rhun EL, et al. Trabectedin for recurrent WHO grade II or III meningioma: a randomized phase II study of the EORTC Brain Tumor Group (EORTC-1320-BTG). J Clin Oncol. 2019;37(15_suppl):2007–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peyre M, Zanello M, Mokhtari K, et al. Patterns of relapse and growth kinetics of surgery- and radiation-refractory meningiomas. J Neurooncol. 2015;123(1):151–160. [DOI] [PubMed] [Google Scholar]
- 9. Furtner J, Schöpf V, Seystahl K, et al. Kinetics of tumor size and peritumoral brain edema before, during, and after systemic therapy in recurrent WHO grade II or III meningioma. Neuro Oncol. 2016;18(3):401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383–e391. [DOI] [PubMed] [Google Scholar]
- 11. Graillon T, Sanson M, Campello C, et al. Everolimus and octreotide for patients with recurrent meningioma: results from the phase II CEVOREM Trial. Clin Cancer Res. 2020;26(3):552–557. [DOI] [PubMed] [Google Scholar]
- 12. Ferté C, Fernandez M, Hollebecque A, et al. Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials. Clin Cancer Res. 2014;20(1):246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Talkington A, Durrett R. Estimating tumor growth rates in vivo. Bull Math Biol. 2015;77(10):1934–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Proust-Lima C, Séne M, Taylor JM, Jacqmin-Gadda H. Joint latent class models for longitudinal and time-to-event data: a review. Stat Methods Med Res. 2014;23(1):74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaley T, Barani I, Chamberlain M, et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol. 2014;16(6):829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graillon T, Defilles C, Mohamed A, et al. Combined treatment by octreotide and everolimus: octreotide enhances inhibitory effect of everolimus in aggressive meningiomas. J Neurooncol. 2015;124(1):33–43. [DOI] [PubMed] [Google Scholar]
- 17. Abi Jaoude S, Peyre M, Degos V, Goutagny S, Parfait B, Kalamarides M. Validation of a scoring system to evaluate the risk of rapid growth of intracranial meningiomas in neurofibromatosis type 2 patients. J Neurosurg. 2020:1–9. [DOI] [PubMed] [Google Scholar]
- 18. Ferté C, Koscielny S, Albiges L, et al. Tumor growth rate provides useful information to evaluate sorafenib and everolimus treatment in metastatic renal cell carcinoma patients: an integrated analysis of the TARGET and RECORD phase 3 trial data. Eur Urol. 2014;65(4):713–720. [DOI] [PubMed] [Google Scholar]
- 19. Oya S, Kim SH, Sade B, Lee JH. The natural history of intracranial meningiomas. J Neurosurg. 2011;114(5):1250–1256. [DOI] [PubMed] [Google Scholar]
- 20. Hashiba T, Hashimoto N, Izumoto S, et al. Serial volumetric assessment of the natural history and growth pattern of incidentally discovered meningiomas. J Neurosurg. 2009;110(4):675–684. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.