ABSTRACT
Copper is an essential micronutrient that also exerts toxic effects at high concentrations. This review summarizes the current state of knowledge on copper handling and homeostasis systems in Escherichia coli and Salmonella enterica. We describe the mechanisms by which transcriptional regulators, efflux pumps, detoxification enzymes, metallochaperones, and ancillary copper response systems orchestrate cellular response to copper stress. E. coli and S. enterica are important pathogens of humans and animals. We discuss the critical role of copper during killing of these pathogens by macrophages and in nutritional immunity at the bacterial-pathogen–host interface. In closing, we identify opportunities to advance our understanding of the biological roles of copper in these model enteric bacterial pathogens.
KEYWORDS: copper, E. coli, S. enterica, uropathogenic E. coli, copper homeostasis, copper toxicity, Enterobacteriaceae, Escherichia coli, Salmonella enterica, virulence
INTRODUCTION
Copper is an indispensable micronutrient required for a number of biological processes in organisms from all three domains of life (1). Bioavailability of copper increased around two billion years ago during the Great Oxygenation Event due to the enhanced solubility of Cu(II) ions (2). Copper is a transition metal that participates in redox reactions by cycling between cuprous [Cu(I)] and cupric [Cu(II)] oxidation states. Additionally, copper is at the top of the Irving-Williams series, resulting in the thermodynamically favorable mismetalation of metalloenzymes, particularly iron- and zinc-containing enzymes, with copper. The combination of redox-cycling properties and mismetalation of essential enzymes renders copper toxic when found in excess. Escherichia coli and Salmonella enterica are Gram-negative bacteria with significant impact on human and animal health globally. These bacteria are exposed to copper in the environment and also within mammalian hosts. Toxicity of copper is mitigated in bacterial cells by buffering, efflux, and detoxification systems to limit levels of bioavailable and total copper. Here, we discuss the mechanisms involved in copper homeostasis in E. coli and S. enterica and the role of copper during interaction between hosts and these bacterial pathogens.
Bacterial cuproenzymes
Bioinformatic analysis reveals that 72% of bacteria appear to utilize copper, primarily as a cofactor in enzymes (3). Around 0.35% of proteins in E. coli have been predicted to be copper-containing proteins (4). The bacterial cuproproteome is relatively small and is largely restricted to aerobic species (3). Cytochrome oxidases are the predominant group of cuproenzymes, followed by nitrite/nitric oxide/nitrous oxide reductases, copper-zinc superoxide dismutase, multicopper oxidase, NADH dehydrogenase, and amine oxidase in E. coli and S. enterica (5). These diverse groups of enzymes are involved in functions such as generation of proton gradient, denitrification, combating superoxide, oxidation of more toxic Cu(I) to less toxic Cu(II) ions, oxidation of NADH, and utilization of amines as a carbon and nitrogen source. Notably, these proteins are localized in the inner membrane and/or periplasm, with no known instances of cuproenzymes in the cytoplasm of E. coli and S. enterica.
Copper acquisition
Bacteria with a higher physiological requirement for copper utilize a cuprophore (methanobactin) or a copper-specific transporter (CtaA, a P-type ATPase, or CcoA, a major facilitator superfamily protein) to meet the cellular demand for copper (6–9). Copper-specific import systems have not been identified in E. coli and S. enterica, suggesting that these bacteria require only relatively low levels of copper. Import via porins and unidentified transporters and uptake of Cu(II)-yersiniabactin complexes have been reported as possible means of copper acquisition in E. coli (10–13). Yersiniabactin is a siderophore produced by multiple Gram-negative pathogens, including a number of uropathogenic E. coli strains (14). There are many gaps in the current understanding of the uptake and trafficking of copper prior to metalation of cuproenzymes in bacteria, and this is the subject of a recent review (15). An E. coli cell is estimated to contain 1.7 × 105 copper atoms (16). Zeptomolar (10−21 M) sensitivity of the copper-activated transcription factor CueR (17), however, suggests that most of this copper is localized outside the cytoplasm in E. coli and that copper is buffered to limit its bioavailability in the cytoplasm and periplasm. Bacteria rely on copper export and buffering systems to maintain an infinitesimally low level of bioavailable copper in the cytoplasm. Glutathione (GSH) and other thiols can act as low-affinity buffers for copper in the cytoplasm (18). E. coli and S. enterica utilize multiple metallochaperones, discussed below, to minimize levels of bioavailable copper. When copper is not exported or buffered, the periplasmic compartment, delimited by the inner and outer membranes in Gram-negative bacteria, is the primary site of not only copper utilization but also copper-inflicted damage.
Copper toxicity via generation of ROS
Historically, copper has been implicated in damaging macromolecules by participating in a Fenton/Haber-Weiss reaction to generate hydroxyl radicals (16, 19–23). Reactive oxygen species (ROS) such as superoxide and hydrogen peroxide are produced as metabolic by-products during respiration. During copper toxicity, Cu(II) ions are reduced by superoxide to Cu(I) and oxygen. Cu(I) is then oxidized to regenerate Cu(II) during nonenzymatic disproportionation of hydrogen peroxide, resulting in the production of hydroxyl radicals and hydroxide ions. The cyclic nature of these reactions generates a steady stream of the extremely reactive hydroxyl radicals, which then inflict oxidative damage on macromolecules in their vicinity. Since the periplasm is the most oxygenated and copper-replete cellular compartment in Gram-negative bacteria, multiple mechanisms discussed below are utilized to sequester copper and export it to the exterior.
Mismetalation of proteins by copper
Conversely, ROS-induced toxicity contradicts the observations on increased copper toxicity under anaerobic conditions, compared to aerobic environments (24–26). Disruption of iron-sulfur clusters has emerged as a major molecular target for copper toxicity by imposing conditional auxotrophy of the branched-chain amino acids glutamine and glutamate in E. coli (24, 27). Macomber and Imlay published a paradigm-shifting study on the destruction of iron-sulfur clusters in dehydratases involved in the biosynthesis of branched-chain amino acids (isoleucine, leucine, and valine) by copper (24). Djoko et al. have reported exacerbation of copper stress in E. coli mutants lacking the copper efflux pump CopA and rescue of the copper-induced growth defect by supplementing glutamate and glutamine (27). Copper induces intracellular scarcity of glutamate in E. coli by decreasing the activity of glutamate oxoglutarate aminotransferase, an enzyme containing iron-sulfur clusters (27). While iron-sulfur clusters are the subject of investigations on copper-mediated mismetalation, it is worth noting that copper has the highest relative ligand affinity compared to other transition metals. The preferential metalation of copper is due to its location in the Irving-Williams series, and proteins containing iron, manganese, cobalt, nickel, and zinc as cofactors are all potential targets of mismetalation by copper (28). Given the similarities in copper homeostasis and overall biology, it is not unreasonable to speculate that copper disrupts Fe-S clusters in S. enterica, a hypothesis that awaits experimental investigation. In addition to overt toxicity, copper also affects multiple facets of cellular biology and metabolism in E. coli and S. enterica, which is beyond the scope of this review and was recently reviewed by Giachino and Waldron (29). In summary, copper exerts toxic effects via ROS-dependent and -independent mechanisms.
COPPER RESPONSE AND DEFENSE MECHANISMS
Bacteria mitigate the toxic effects of copper by utilizing copper-binding buffers, transcriptional regulators, efflux pumps, oxidases, and metallochaperones. Early studies in Enterococcus hirae, a Gram-positive bacterium, were instrumental in generating a framework for understanding copper homeostasis in bacteria (30, 31). Broadly conserved and lineage-specific copper homeostasis systems have been identified in numerous bacteria, including pathogens (5, 29, 32). Here, we discuss, compare, and contrast the mechanisms utilized by E. coli (Fig. 1) and S. enterica (Fig. 2) to combat toxicity and ultimately restore the homeostasis of copper in the cytoplasm and periplasm (Table 1).
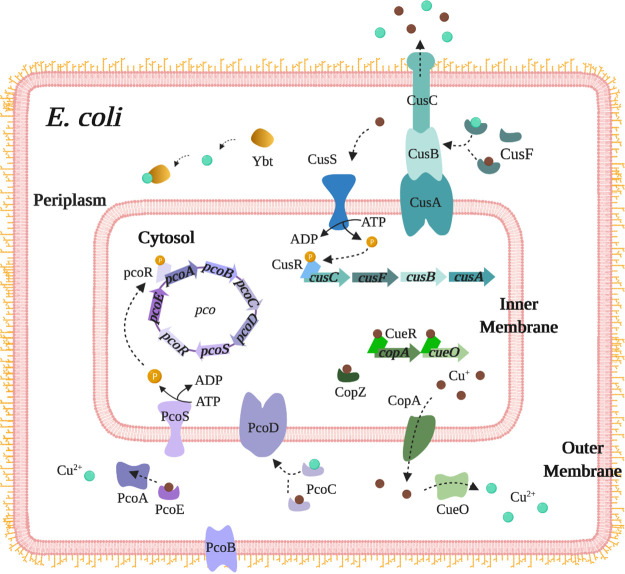
FIG 1.
Overview of the copper detoxification systems in E. coli. The CueR regulon (green), composed of CopA and CueO, plays a central role in handling excess copper in E. coli. Cu(I)-bound CueR is the transcriptional activator for copA and cueO genes. CopA is an inner-membrane ATPase that pumps Cu(I) from the cytoplasm into the periplasm. CueO is a periplasmic multicopper oxidase that converts the more toxic Cu(I) to the less toxic Cu(II). Another mechanism for copper detoxification is the Cus system (blue), comprising the transmembrane efflux pump, CusCBA. The periplasmic copper chaperone CusF escorts Cu(I) and Cu(II) to the CusCBA complex. CusS hydrolyzes ATP upon sensing copper to phosphorylate CusR and activates transcription of the cusCFBA operon. The plasmid-borne pco operon is found only in selected strains of E. coli and is regulated by the PcoSR two-component system. The siderophore yersiniabactin binds to sequester Cu(II), thereby preventing it from reducing to the more toxic Cu(I).
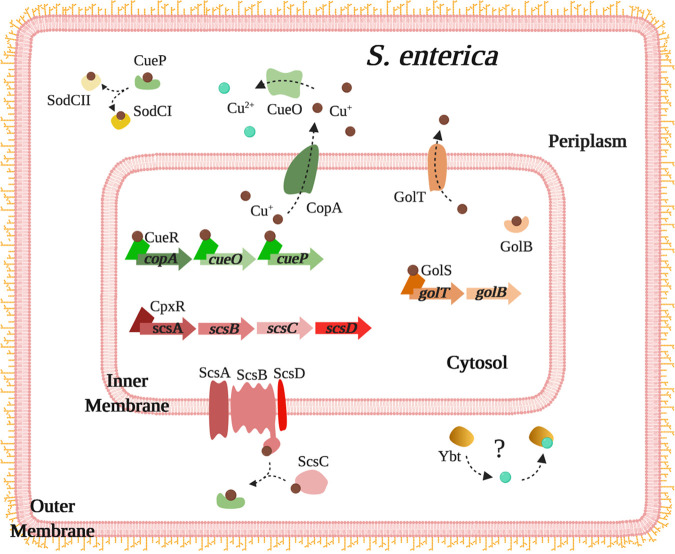
FIG 2.
Overview of the copper detoxification systems in S. enterica. The CueR system of S. enterica is identical to that of E. coli, with the exception of an additional periplasmic copper chaperone, CueP. CueP delivers copper to superoxide dismutases, SodCI and SodCII. The Gol system, transcriptionally regulated by the sensor GolS, includes the GolT inner-membrane Cu(I) efflux pump and the cytoplasmic chaperone GolB. ScsB and ScsC proteins from the disulfide reductase system bind and deliver copper to CueP. Some S. enterica strains encode Cus and Pco systems, identical to that of E. coli (not depicted here; see Fig. 1). The siderophore yersiniabactin is also produced by S. enterica, but its role in copper resistance remains to determined.
TABLE 1.
Effectors of copper homeostasis in E. coli and S. enterica
| Gene | Protein | Function |
|---|---|---|
| cueR | CueR | Copper-responsive transcriptional activator |
| copA | CopA | Inner membrane ATPase for efflux of cytoplasmic copper |
| copA | CopZ | Cytoplasmic chaperone |
| cueO/cuiD | CueO/CuiD | Periplasmic multicopper oxidase |
| cueP | CueP | Periplasmic binding protein |
| cusS | CusS | Copper-responsive inner membrane kinase of CusR |
| cusR | CusR | Transcriptional activator |
| cusA | CusA | Inner membrane antiporter |
| cusB | CusB | Periplasmic adapter |
| cusC | CusC | Outer membrane channel |
| cusF | CusF | Periplasmic chaperone |
| golS | GolS | Gold/copper-responsive transcriptional activator |
| golT | GolT | Inner membrane ATPase for efflux of cytoplasmic copper |
| golB | GolB | Cytoplasmic chaperone |
| cutC | MicL (sRNAa) | Post-transcriptional regulator of copper sensitivity |
Small non-coding regulatory RNA.
The copper-sensing transcriptional regulator CueR.
CueR is a copper-responsive transcriptional regulator belonging to the MerR family (17, 33–35). CueR proteins from E. coli and S. enterica are highly conserved, with 89.6% identity. The E. coli CueR regulon is composed of copA and cueO genes, encoding a copper-specific inner membrane ATPase and a periplasmic copper-dependent multicopper oxidase, respectively. The CueR regulon in S. enterica includes cueP, encoding a periplasmic copper-binding protein, in addition to copA and cueO (36). copA and cueR genes are separated by the ybaS and ybaT genes, which are involved in the glutamate-dependent acid tolerance system in E. coli (27). In S. enterica, copA and cueR are located adjacently in the genome and divergently transcribed (37). Despite variation in genetic organization, functions of CueR and CopA are essentially identical in these bacteria. CueR exists as a dimer and coordinates one Cu(I) ion per monomer in E. coli (17). The dimer binds its target promoter whether bound to copper or not, but only holo-CueR activates the transcription of genes under its control by unwinding the DNA (38). CueR binds to an inverted-repeat containing consensus sequence (CCTTCCNNNNNNGGAAGG) known as the CueR box in the promoter region of copA and cueO genes in E. coli (39). CueR also activates the transcription of cueO, copA, and cueP genes during copper stress in S. enterica (40).
The copper efflux pump CopA.
The primary mechanism of defense against cytoplasmic copper in E. coli and S. enterica is efflux by the inner membrane P1B-type ATPase, CopA, which pumps Cu(I) ions from the cytoplasm into the periplasm (37, 41). Specifically, these organisms encode a CopA1-type of ATPase that demonstrates a higher rate of copper efflux from the cytoplasm to the periplasm, compared to CopA2-type pumps. CopA proteins from E. coli and S. enterica exhibit 92.7% identity. A BLAST search reveals that CopA is highly conserved in multiple biomedically important genera in the family Enterobacteriaceae, including Citrobacter, Enterobacter, Klebsiella, and Shigella. One of only two known chromosomally encoded soft-metal-translocating ATPases in the E. coli genome, CopA shares no overlapping specificity with ZntA, which translocates Zn(II), Cd(II), and Pb(II) (41). CopA is also important for delivery of copper ions to the copper-zinc-containing periplasmic superoxide dismutase SodCII in S. enterica (42). Osman et al. demonstrated an accumulation of inactive apo-SodCII in the periplasm of S. enterica mutants lacking both CopA and the gold/copper transporter GolT, but not in single mutants, indicating that one of these proteins must be present for delivery of copper to metalate and thereby activate SodCII (42). CopA is also involved in copper resistance in a closely related enteric bacterium, Klebsiella pneumoniae (43). Once in the periplasm, Cu(I) ions could be oxidized to Cu(II) ions, sequestered by chaperones, incorporated into cuproenzymes, and/or exported across the outer membrane.
The copper chaperone CopZ in E. coli.
Recent reports reveal that CopZ is an intracellular copper chaperone in E. coli that is, surprisingly, encoded by copA (44, 45). The full-length copA transcript leads to the production of CopZ by a programmed ribosomal frameshifting mechanism, in addition to translation to CopA. A truncated version of the copA transcript is also generated in E. coli and translated to CopZ. This is a remarkable mechanism of gene optimization where a single gene codes for both an efflux pump and a metallochaperone. Sequence alignment and homology analysis reveals that the nucleotides responsible for ribosomal frameshifting (CCCAAAG) resulting in production of CopZ production in E. coli share 100% homology with those in S. enterica serovar Typhimurium strain SL1344. While this observation suggests the presence of a conserved mechanism, whether CopZ is produced and utilized as a copper chaperone in S. enterica remains to be validated experimentally.
The GolST-B system in S. enterica.
The Gol system is an additional copper efflux system that is encoded in the chromosome of S. enterica, but not in E. coli (46). As the name indicates, this system was originally identified for its induction of expression by gold (46). Cupric-GolS, a gold/copper-responsive transcriptional regulator, induces the expression of golST and golB loci (40). GolS, like CueR, belongs to the MerR family of transcriptional regulators and exhibits a comparable level of affinity for copper (42, 47). Unlike CueR, which senses only copper, GolS responds to the bioavailable levels of both copper and gold in the cytoplasm. The golT gene encodes a CopA-like inner membrane P1B-type ATPase. GolT and CopA restore wild-type levels of copper resistance in mutants lacking both efflux pumps, indicating a redundant role (48). In the absence of functional CopA, GolT confers copper resistance by efficiently effluxing copper ions from the cytoplasm to the periplasm (37). Efflux of Cu(I) by GolT plays a critical role in metalation of virulence-associated periplasmic superoxide dismutase, SodCII, analogous to the role of CopA in metalating SodCII (42). The golB gene encodes a cytoplasmic gold/copper chaperone with a function reminiscent of CopZ in E. coli.
The periplasmic multicopper oxidase CueO.
CueO, also sometimes known as CuiD in S. enterica, is a periplasmic copper-dependent multicopper oxidase that oxidizes Cu(I) to its less toxic Cu(II) state, thereby limiting copper-mediated damage to the bacterial cell (49–51). Apo-CueO is secreted via the twin-arginine translocation pathway, and copper is incorporated into the active site within the periplasm of E. coli (52). CueO is an exception among proteins transported by the twin-arginine translocation pathway that typically secretes folded holo-proteins. Additionally, CueO oxidizes enterobactin and 2,3-dihydrobenzoic acid (an intermediate in the enterobactin biosynthetic pathway) to preempt reduction of Cu(II) ions to more toxic Cu(I) ions by these siderophores (53). This process decreases the efficiency of enterobactin-mediated iron uptake during copper stress, as indicated by overaccumulation of iron in an E. coli mutant lacking CueO (51). CueO plays a significant role in combating periplasmic copper stress in both E. coli and S. enterica under aerobic conditions.
The periplasmic copper metallochaperones CueP and CusF.
CueP binds Cu(I) ions delivered by CopA and GolT with high affinity (10−15 M) and is an integral effector of protection from copper toxicity in S. enterica (36, 48, 54). In comparison, CusF, found in the periplasm of E. coli, has a lower affinity for Cu(I) at 1.8 × 10−7 M than CueP (55). It is interesting that CusF from E. coli, which uses the CusCBA efflux system to decrease periplasmic copper bioavailability, has a lower affinity for Cu(I) than CueP from S. enterica, which lacks a known periplasmic copper efflux mechanism. An accumulation of inactive apo-SodCII is observed in an S. enterica mutant lacking cueP, indicating a critical role for CueP in the metalation of SodCII (42). In contrast to CueP, which is regulated by CueR (a cytoplasmic copper sensor), CusF, regulated by CusS (a periplasmic copper sensor), does not appear to play a role in the metalation of SodC in uropathogenic E. coli (UPEC) (56). Expression of CueP is coordinately regulated by both CueR, which detects free cytosolic copper, and CpxR/CpxA, which monitors envelope stress (36). CueP has been proposed as a partial functional equivalent of the Cus transenvelope copper efflux system found in E. coli (26). E. coli possesses the periplasmic copper chaperone CusF (57), which is discussed below in conjunction with other members of the Cus system.
Periplasmic disulfide reductase ScsC/B pathway in S. enterica.
The suppressor of copper sensitivity (scs) operon was originally identified, and characterized for its role in protection from copper toxicity in S. enterica (58–60). ScsA, ScsB, and ScsD are inner membrane proteins, whereas ScsC is a soluble periplasmic protein (54). Transcription of the scs locus is upregulated by copper in a CpxAR-dependent mechanism (59). ScsA conveys resistance against hydrogen peroxide and does not appear to affect sensitivity to copper (61). Loss of scsB imparts copper sensitivity of a magnitude equivalent to that seen in a mutant lacking scsBCD genes, indicating its eminent role in copper resistance among Scs proteins (59). Binding of Cu(I) ions by ScsB and ScsC with high affinity (∼10−14 M) and delivery to periplasmic copper chaperone CueP are the mechanistic basis of Scs-mediated resistance to copper (54).
The transenvelope copper efflux system CusCBA and CusF.
E. coli minimizes periplasmic copper levels by utilizing the Cus copper efflux complex, which is controlled by a copper-responsive two-component regulatory system (49, 62). The Cus system is comprised of the structural proteins CusCFBA, and the two-component system CusRS encoded by the divergently transcribed cusCFBA and cusRS operons (49, 63). The cus system contributes to reinstating copper homeostasis under low-oxygen conditions and also during exposure to near-lethal levels of copper (64). CusR is responsive to both copper and silver ions, and the Cus system also confers protection from silver toxicity (65, 66). Upon phosphorylation by CusS during copper stress, CusR binds to the inverted repeat AAAATGACAANNTTGTCATTTT, located upstream of cusCFBA genes (39). Transcription of Cus structural genes is also activated by the hydrogen peroxide-responsive two-component regulatory system HprRS, and copper induces the expression of hprRS via CusRS (39, 67). The CusCBA structural proteins assemble to form a tripartite, transenvelope copper transporter complex, and CusF is a soluble periplasmic Cu(I) chaperone. CusA is an inner membrane proton-substrate antiporter, driven by the proton motive force, containing multiple metal binding sites (63, 64). CusB is a periplasmic adapter protein which links CusA with CusC (64). CusC is an outer membrane factor possessing no known metal binding sites (64). Together, these proteins form a channel between the inner and outer membranes, traversing the periplasm (64). The individual components exist in a disassembled form to minimize the impact on plasticity and dynamic function of the periplasm in the absence of toxic levels of copper or silver ions (68). CusF delivers Cu(I) ions expelled by CopA to the CusCBA complex for export from the cell (57, 63). Recently, the cus locus was identified in an integrative conjugative element in antibiotic-resistant strains of S. enterica (69). In summary, CusCBA, aided by CusF, has the unique role of transporting periplasmic copper to the extracellular space.
The plasmid-borne copper resistance system Pco.
Plasmid-encoded pco operon provides an additional layer of copper tolerance to the chromosomal cue, gol, and cus systems in E. coli and S. enterica (70, 71). Protein products of the pco operon include soluble periplasmic proteins (PcoA, PcoC, and PcoE), an inner membrane copper pump (PcoD), and an outer membrane copper transporter (PcoB) (72). Djoko et al. demonstrated that PcoA and PcoC interact to oxidize Cu(I) ions to Cu(II) ions and that they might also interact with PcoB to export copper (72). PcoC also plays an integral role in supplying copper for proper assembly of PcoA (72). The CusRS two-component system also induces expression of the plasmid-borne pcoE gene in E. coli (62). Other pco genes are responsive to the cognate pco-specific two-component regulatory system, PcoSR, and are not responsive to activation by CusRS (62, 72). The pco locus is typically found across diverse E. coli isolates, particularly those originating from swine, due to the use of high levels of copper in animal feed (73). Recently, E. coli harboring the pco operon was isolated in Europe from the urine of patients with urinary tract infection (74). This finding underscores the urgent need for responsible use of trace elements in food animal production to mitigate the spread of metal resistance elements, which are often coselected with genetic determinants of antibiotic resistance.
Siderophore-mediated protection from copper.
Bacteria elaborate high-affinity, iron-chelating molecules known as siderophores to acquire iron (14). Various siderophores are elaborated by E. coli and S. enterica strains and may include enterobactin, salmochelin (glycosylated enterobactin), aerobactin, and/or yersiniabactin (12, 75). Enterobactin is produced by both commensal and pathogenic E. coli strains, while salmochelin, aerobactin, and/or yersiniabactin are utilized by pathogenic strains (14). In contrast, S. enterica strains produce at least enterobactin and salmochelin, often in conjunction with other siderophores (12). In addition to its canonical role as a siderophore, yersiniabactin produced by UPEC also binds to copper and other metals, including cobalt, chromium, gallium, and nickel (76, 77). In the presence of high levels of bioavailable copper in the milieu, yersiniabactin facilitates copper resistance by binding and sequestering Cu(II) ions, thereby preventing its reduction to the more toxic Cu(I) ions (78). Oxidized products of enterobactin and 2,3-dihydroxybenzoic acid generated by CueO also act as a sink to sequester copper in E. coli (53). Enterobactin also conveys protection from copper toxicity in S. enterica, comparable to its role in E. coli (40). In summary, enterobactin and yersiniabactin confer added protection from copper toxicity in E. coli and S. enterica.
The sigma E-regulated small RNA MicL and membrane lipoprotein Lpp in E. coli.
CutC has been proposed to be a cytoplasmic copper-binding protein in E. coli, and a mutant lacking cutC is more sensitive to copper than its parental strain (79). It is now known that copper tolerance previously attributed to CutC is actually conferred by MicL, a σE-dependent small regulatory RNA, encoded within the cutC gene (80). The transcriptional response to maintain homeostasis during membrane stress is governed by σE (81). Independently, loss of σE itself also renders E. coli more sensitive to the toxic effects of copper (13). MicL represses the translation of Lpp, an outer membrane lipoprotein, which is also the most abundant protein in E. coli (80). The mutant lacking cutC also lacks micL and produces high levels of Lpp, leading to increased copper sensitivity (79, 80). Enhanced resistance to copper in a mutant lacking lpp, compared to the parental strain, further supports the link between Lpp levels, response to copper, and extracytoplasmic stress (80). In summary, the σE-MicL regulatory pathway plays an important role in protection from copper toxicity by decreasing the levels of Lpp in E. coli.
Periplasmic disulfide reductase DsbC in E. coli.
Disulfide bond formation in the periplasm is critical for structural and functional integrity of many periplasmic and secreted proteins in Gram-negative bacteria. Copper triggers the formation of erroneous disulfide bonds in periplasmic and secreted proteins in E. coli (82). Consequently, disulfide isomerase/reductase DsbC is required to resolve such nonnative disulfide bonds introduced by copper. E. coli mutants lacking DsbC exhibit higher sensitivity to copper than the parental wild-type strain, revealing the impact of copper stress on the structure of extracytoplasmic proteins (82). Whether DsbC interacts directly with copper needs to be tested, in light of the recent findings in S. enterica showing that ScsC and ScsB proteins involved in disulfide reduction bind copper (54).
Glutathione in E. coli.
Balance between the reduced (GSH) and oxidized (glutathione disulfide [GSSG]) forms of glutathione (l-γ-glutamyl-l-cysteine-glycine) plays a critical role in maintaining a reductive milieu in the cytoplasm. Glutathione is involved in mitigating sensitivity to copper in mutants that are defective in copper efflux (83–85). Failure to produce glutathione, however, has no impact on copper sensitivity in wild-type E. coli cells, suggesting a limited role for glutathione in combating copper toxicity.
Functional genomic analysis of copper stress in E. coli and S. enterica.
DNA microarray studies have revealed remarkable similarities in the copper-responsive transcriptional profiles of E. coli (39, 86) and S. enterica (40). As expected, the known copper efflux and detoxification genes copA, cueO, and cusCFBA, regulated by CueR and CusR, are upregulated in E. coli during growth in copper-supplemented media. In addition, CpxAR (the key regulatory system for transcription of genes involved in maintaining cell envelope homeostasis), YedVW (renamed HprRS, a two-component system that senses and responds to hydrogen peroxide stress), and SoxS (the master regulator of transcriptional response to superoxide) are also activated during copper stress in E. coli. Exposure to high levels of copper also induces the expression of genes encoded in the enterobactin operon, suggesting an increase in cellular demand for iron during copper stress (86). High CueO activity during copper stress also disrupts enterobactin-dependent uptake of iron in UPEC. A mutant lacking CueO accumulates iron at a higher level than the parental strain and, as a result, exhibits increased virulence in the murine model of urinary tract infection (UTI) (51). Genes under the regulatory control of CpxAR are also highly expressed in copper-intoxicated S. enterica (40). Additionally, copper triggers the expression of the sitABCD ferrous iron/manganese ABC transporter and enterobactin biosynthetic genes. Utilizing a mutant deficient in enterobactin production, Pontel et al. demonstrated a protective role for enterobactin against copper toxicity in S. enterica (40). While soxS is induced by copper stress in S. enterica, none of the genes from the SoxS regulon were identified as copper-responsive genes. Taken together, these studies highlight the extensive cross talk between regulation of copper homeostasis and other critical cellular processes, such as maintenance of envelope homeostasis, management of iron metabolism, and response to reactive oxygen species.
COPPER AT THE HOST-PATHOGEN INTERFACE
Overview of pathogenic E. coli-mammalian host interaction.
Commensal E. coli resides in the gut of many mammals, including humans (87). Pathogenic E. coli strains are endowed with virulence and fitness genes that appear to be acquired by an ancestral commensal E. coli strain by horizontal gene transfer (88). These virulence and fitness factors promote successful colonization, induction of infection and inflammation, and subversion of the host immune system. Pathogenic E. coli strains can infect the gut or extraintestinal sites, such as the urinary tract, bloodstream, systemic sites, mammary glands, and central nervous system (88). Intestinal pathogenic E. coli strains are further divided into various pathotypes, such as enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), enterohemorrhagic E. coli (EHEC), and attaching and invading E. coli (AIEC) (87, 88). These pathotypes have a unique assortment of virulence factors that induce distinct pathological changes in the host. Intestinal pathogenic E. coli organisms are transmitted by the fecal-oral route or as food- or waterborne pathogens. Depending on the pathotype, the clinical symptoms can be self-limiting secretory diarrhea, severe inflammatory diarrhea, dysentery, or potentially fatal hemolytic-uremic syndrome.
Extraintestinal pathogenic E. coli strains exhibit a commensal-like lifestyle in the intestine but cause pathological changes at other sites, such as the urinary tract, bloodstream, central nervous system, and mammary glands (87). Uropathogenic E. coli (UPEC) is the predominant cause of urinary tract infection in humans and is among the most common bacterial infections in humans globally. UPEC is also a major etiological agent of bloodstream infections in humans and is a significant cause of mortality due to urosepsis in the elderly. Studies from various groups have revealed a role for copper during host-UPEC interaction in the urinary tract (78, 89), and this is discussed below. Extraintestinal pathogenic E. coli that encodes a K1 type of capsular polysaccharide is a causative agent of human neonatal meningitis. In addition to human infections, E. coli is a pathogen of immense importance in veterinary medicine and food animal production.
Regardless of the pathotype, E. coli is primarily found as an extracellular pathogen during interaction with mammalian hosts. A notable exception is the intracellular reservoir formation by UPEC in the urothelial cells lining the urinary bladder (90). The extracellular lifestyle of E. coli is an important contrast to that of S. enterica, whose intracellular lifestyle is a defining feature of its interaction with mammalian hosts.
A primer for mammalian-host–S. enterica interaction.
S. enterica is an extremely common foodborne bacterial pathogen (91). S. enterica serovars Typhi and Typhimurium cause typhoid fever and gastroenteritis in humans, respectively (92). Typhoid fever is a result of systemic dissemination of Salmonella. The most common outcomes of salmonellosis in industrialized countries are nontyphoidal gastroenteritis and diarrhea in humans (91). However, invasive nontyphoidal Salmonella strains are emerging as a major public health problem, especially in Africa (93). Salmonella is also an important pathogen impacting the health of a broad range of animals throughout the world. Both serovars of S. enterica invade the enteric epithelium via M cells to reach the lamina propria. Nontyphoidal S. enterica organisms are phagocytosed by macrophages and neutrophils, but the phagocytes are killed by the virulence factors elaborated by this pathogen, including those delivered through the type III secretion system. These events culminate in acute enteritis, leading to the clinical presentation of inflammatory diarrhea (94). On the other hand, upon reaching the lamina propria, typhoidal S. enterica undergoes a prolonged replicative phase in macrophages and utilizes this protected intracellular niche to disseminate throughout the body (95). Following a brief period of gastrointestinal symptoms, typhoid is marked by prolonged fever, reflecting sustained bacteremia. Both serovars of S. enterica spend a significant amount of time within the host as an intracellular pathogen. Within host cells, Salmonella proliferates within vacuoles known as Salmonella-containing vacuoles (96). By utilizing virulence factors, including type III secretion system-delivered effectors, Salmonella modifies the pathogen-containing vacuole to establish a replicative niche (97, 98). S. enterica serovar Typhimurium induces a typhoid fever-like systemic infection in mice and has served as an experimental model to understand the pathogenesis and host response during systemic salmonellosis.
A brief introduction to copper homeostasis in mammals.
Roles of key proteins involved in copper homeostasis is depicted in Fig. 3 (99, 100). Copper is absorbed in the small intestine via CTR1 transporter in the enterocytes (101). Enterocytes utilize ATP7A, a P-type ATPase, to traffic copper to the trans-Golgi network and also to export copper into the bloodstream (102). Mutations in the Atp7a gene result in severe systemic copper deficiency, known as Menkes disease (103). Hepatocytes also import copper from circulation via Ctr1 (104). ATP7B, another P-type ATPase closely related to ATP7A, is used to mobilize copper to the trans-Golgi network in the hepatocytes to produce ceruloplasmin (104, 105). Ceruloplasmin is the predominant reservoir of circulating copper in mammals (105, 106). Ceruloplasmin is a 132-kDa glycoprotein with six copper atoms that is produced and secreted into circulation by the liver (105). During copper excess, hepatocytes excrete copper into bile by mobilizing ATP7B to the biliary network (107). Mutations that disrupt the function of ATP7B result in hepatic copper overload, leading to the hepatolenticular degeneration observed in patients with Wilson’s disease (108). CTR2 is another protein that indirectly affects copper uptake by cells indirectly by modulating the activity of Ctr1 (109). CTR1, CTR2, ATP7A, and ceruloplasmin are expressed in many cell types, including macrophages.
FIG 3.
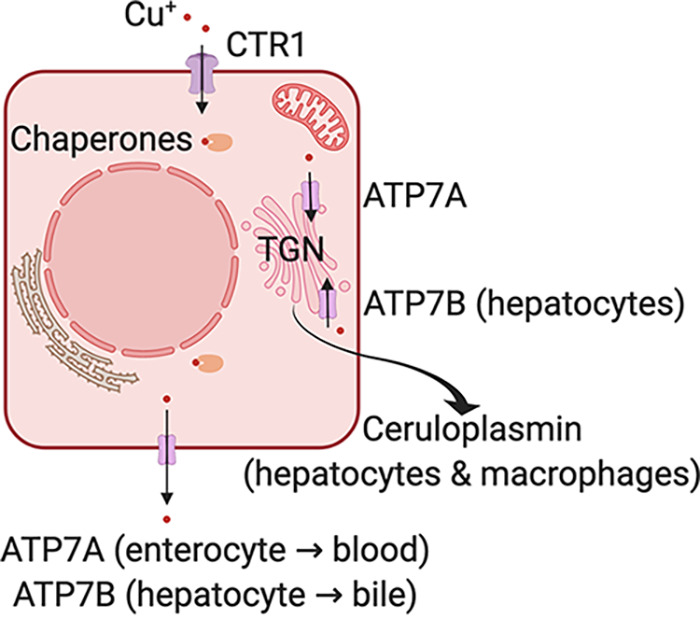
Key players involved in copper homeostasis in mammals. Copper (Cu+) is imported into the cytoplasm via the CTR1 transporter and is directed to organelles by metallochaperones. The P-type ATPases ATP7A and ATP7B transport copper into the trans-Golgi network (TGN). These proteins are also essential for copper absorption in the gut (ATP7A) and biliary excretion of excess copper in the liver (ATP7B). Hepatocytes are the source of circulating ceruloplasmin, and macrophages secrete ceruloplasmin in response to bacterial ligands.
Copper and nutritional immunity.
The antimicrobial activity of copper is weaponized by the host immune system during infection. Levels of bioavailable and ceruloplasmin-bound copper increase during the acute-phase reaction triggered by infection and inflammation (110). Manipulating nutrient availability to curtail pathogen growth during infection is known as nutritional immunity and is an integral part of the innate immune response (111). Bacterial pathogens, however, have multiple mechanisms to detect and overcome this nutrient depletion strategy, including the well-characterized pathways for acquiring iron by secreting siderophores (112, 113). Mammalian hosts actively limit the availability of iron, zinc, and manganese during infection, with a simultaneous increase in copper availability, suggesting an antimicrobial role for copper at the host-pathogen interface (113–122). Researchers working on multiple host-pathogen interaction systems have garnered evidence that indicates attempts by the host to poison invading bacteria and fungi through toxic concentrations of copper and zinc (123–127). Hypoferremia and hypercupremia are synchronously observed during infection, highlighting the opposite effects of these transition metals on pathogen growth in vivo (110, 128).
Ceruloplasmin-driven hypercupremia of infection.
Ceruloplasmin is a multicopper ferroxidase that oxidizes Fe (II) ions to Fe (III) ions, which can then bind transferrin. Ferrous iron (Fe2) is exported by ferroportin, but only ferric iron (Fe3) binds to transferrin, making multicopper ferroxidases a vital link in iron transport within mammalian hosts (129). In addition to the ceruloplasmin secreted from the liver, a membrane-anchored form of ceruloplasmin is also found in many tissues (130). Ceruloplasmin is produced as a major acute-phase reactant during host response to infection and inflammation in humans and nonhuman primates (110, 131, 132). In contrast, ceruloplasmin is a minor acute-phase reactant in mice (133). Thus, hypercupremia of infection is a result of increased hepatic secretion of ceruloplasmin.
Amoebas use copper to kill E. coli.
The role of copper as a key determinant of the outcome of interaction between bacteria and their protozoal predators serves as an evolutionary framework to understand the antibacterial role of copper during interaction between phagocytes of vertebrate hosts and bacterial pathogens. Bacteria utilize copper efflux pumps to resist digestion within vacuoles when foraged by amoebas. Expression of copper transporters is upregulated in the social amoeba Dictyostelium discoideum subsequent to engulfment of E. coli, resulting in increased levels of intracellular copper (134). E. coli mutants lacking CopA were defective in limiting predation by amoebas, as was evident from their poor survival within amoebas relative to the parental strain (134). This study highlights the selective pressure imposed on bacteria to maintain and acquire copper efflux/detoxification systems to protect themselves from protozoa.
Role of copper during microbial infections.
Copper is involved in optimal protection from a diverse set of bacterial pathogens, both Gram positive and negative, and fungal pathogens, in addition to E. coli and S. enterica. A Pseudomonas aeruginosa mutant lacking the CueA copper efflux pump is attenuated in colonizing mouse spleens (135). Acinetobacter baumannii mutants lacking a transcriptional regulator of copper efflux (CusR) and a putative efflux pump (CopD) are attenuated during pneumonia in the mouse model (136). A CopA mutant strain of Neisseria gonorrhoeae displays poor survival in human cervical epithelial cells (137). Streptococcus pneumoniae mutants deficient in the CopA copper efflux pump and the CupA copper chaperone are attenuated in a murine model of pneumonia (138, 139). Cryptococcus neoformans utilizes copper detoxification machinery to successfully colonize the lungs in a mouse model (140). In summary, copper is involved in innate protection against a broad range of pathogens.
Antibacterial effect of copper against E. coli and S. enterica in macrophages.
Early insights into the role of copper in antimicrobial function of macrophages were gleaned from studies conducted in copper-depleted rats (141). Macrophages from copper-depleted rats exhibit decreased capacity for respiratory burst and poor killing of Candida albicans compared to controls with adequate copper levels. Wagner et al. utilized X-ray microprobe technology for precise determination of concentration of selected elements, including copper, inside the phagosomes of mouse macrophages (142). Copper was found at significantly higher levels inside Mycobacterium avium-containing phagosomes from macrophages stimulated with gamma interferon before or after infection relative to unstimulated controls. This direct evidence of copper accumulation within phagosomes was followed by a comprehensive study that shed light on copper-dependent killing of E. coli in a murine macrophage-like (RAW264.7) cell line (143). White et al. demonstrated that RAW 264.7 macrophages stimulated with proinflammatory molecules (gamma interferon [IFN-γ] and lipopolysaccharide [LPS]) import higher levels of copper by using the CTR1 copper transporter (143). Copper is then trafficked into the phagolysosome by the ATP7A copper transporter. An E. coli mutant lacking CopA is killed more effectively by RAW 264.7 cells in 1 to 2 h postinfection than the parental wild-type strain in an ATP7A-dependent manner (143).
In the same vein, S. enterica mutants lacking both CopA and GolT pumps, but not single mutants, exhibit poor survival within RAW 264.7 cells at 12 and 24 h postinfection (48). Infection of primary bone marrow-derived murine macrophages with S. enterica results in increased abundance in transcripts of copper transporters (ctr1, ctr2, and atp7a), and ceruloplasmin (125). Chelation of copper promotes survival of S. enterica in mouse macrophages. However, copper accumulates in distinct compartments within infected macrophages and does not colocalize with Salmonella-containing vacuoles. Copper appears to limit S. enterica survival by participating in slower killing mechanisms, such as nitrosative stress, and by limiting iron availability within macrophages, in contrast to its role in faster, oxidative-burst-dependent killing of E. coli (125, 143). Ladomersky et al. demonstrated that ATP7A-dependent trafficking of copper plays a role in killing of S. enterica in primary murine macrophages isolated from ATP7ALysMcre and control mice (144). ATP7ALysMcre-transgenic mice lack ATP7A in cells of myeloid lineage, which includes macrophages and neutrophils. Furthermore, the copper transporters CopA and GolT provide protection from macrophage killing only in wild-type macrophages, indicating an antagonistic use of copper by the host and efflux pumps by the pathogen (144).
Copper deficiency in humans is associated with reduced neutrophil function and results in significantly compromised bactericidal activity (126, 145). Copper plays a critical role in the differentiation of a human myelocyte cell line (HL-60) into neutrophil-like cells in vitro (146). However, the mechanisms of copper uptake and trafficking to the phagolysosome and its contribution to the bactericidal activity in neutrophils remain to be determined.
Copper modulates the function of macrophages.
Separate from its overt antimicrobial function, copper is also involved in modulating the function of macrophages. Inflammasomes are protein complexes composed of sensor proteins (in the AIM2 or NLR family of proteins) that detect various host- and pathogen-derived signals (Fig. 4) (147). These sensors are coupled with an adaptor molecule and cysteine proteases (caspase 1 or 3). Activation of inflammasomes results in caspases processing key cytokines into active forms (interleukin 1β [IL-1β] and IL-18) and a unique form of inflammatory cell death (pyroptosis) (147). IL-1β is a potent proinflammatory cytokine that is active at both local and systemic levels. Intracellular copper activates canonical NLRP3-dependent inflammasome in murine and human macrophages (Fig. 4) by altering redox homeostasis (148). As a corollary, decreased bioavailability of copper dampens the outcome of both caspase-1-dependent inflammasome activation and LPS-induced shock in mice (148). This effect is specific for NLRP3, since copper does not appear to affect the activation of noncanonical NLRP1-, NLRC4-, and AIM-2-dependent inflammasomes (148).
FIG 4.
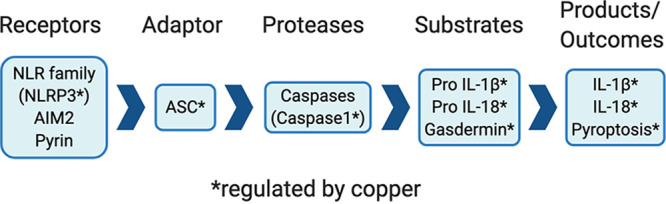
Copper regulates inflammasome activation. Copper is required for signaling via NLRP3 receptor protein to engage the ASC adaptor. This leads to activation of caspase I, which cleaves precursors of substrates to yield active IL-1β and IL-18 (proinflammatory cytokines). Gasdermin triggers an inflammatory form of cell death, known as pyroptosis.
Regulation of mast cell activity by copper.
Mast cells are derived from the myeloid lineage of hematopoietic cells and play important roles in hypersensitivity reactions. The involvement of mast cells in the host response to bacterial infections is being increasingly appreciated (149, 150). A key protein released by activated mast cells is tryptase, a serine protease. Tryptase is required for effective control of bacterial growth in vivo due to its role in promoting recruitment of neutrophils to the site of infection (151). By regulating the expression of tryptase gene, copper exerts an outsized influence on the phenotypes exhibited by mast cells (152). Mast cells play a key role in the exfoliation of urothelial cells in the urinary bladder during UTI (153). However, the influence of copper on mast cells in the urinary tract remains to be explored. These emerging roles of copper as a modulator of inflammasome activation in macrophages and mast cell function require further investigation to fully comprehend their scope and impact.
UTI in patients with Menkes disease.
The importance of copper in protection against pathogen colonization is illustrated by the increased susceptibility to infection observed in patients with inborn errors in copper metabolism. Menkes disease is an X-linked lethal genetic disease associated with mutations in the atp7A gene, resulting in systemic copper deficiency (103). There has been limited success in restoring copper homeostasis with existing therapeutic approaches, with a promising candidate in the pipeline (103, 154). Historical clinical reports reveal that recurrent and fulminant UTI is a major cause of mortality in patients with Menkes disease (103, 155). Typically, affected individuals die by 3 years of age, but a milder form, occipital horn syndrome (OHS), is often not diagnosed until 5 to 10 years of age (103). The primary clinical features are related to connective tissue disorder, but these patients are particularly susceptible to UTI, and recurrent UTI is often the first sign which brings the child to the attention of medical professionals (103). This increased susceptibility to UTI in patients with systemic copper deficiency suggests an important role for copper in limiting bacterial colonization. In a murine model of UTI, a UPEC mutant lacking the Cus copper efflux system is attenuated during infection (156). In addition to clinical findings, multiple lines of evidence from experimental studies, discussed below, indicate an important and novel biological role for copper in protection against UTI.
Urinary copper mobilization during infection.
A transcriptome sequencing (RNA-seq) study revealed that the cus genes were significantly expressed by UPEC during clinical UTI, compared to culture in healthy human urine (156). Strong induction of expression of cus genes, but not copA and cueO, is not surprising, because UPEC experiences not only copper toxicity but also hypoxia during clinical UTI in patients (156). Genes encoding CopA and CueO were upregulated ∼4-fold in clinical UTI samples but did not meet the threshold for differential expression used in that study (156). Human urinary copper content, determined by inductively coupled plasma mass spectrometry, is significantly elevated during UTI caused by UPEC, K. pneumoniae, and Proteus mirabilis (89, 156). Independently, Chaturvedi et al. reported detection of copper-yersiniabactin complexes in UTI urine samples (78). Ceruloplasmin, the primary carrier of circulating copper, is found at higher levels in urine from UTI patients and is positively correlated with urinary copper content (89). The reductive milieu of the urine favors formation and maintenance of highly bactericidal Cu(I) ions (89). Urinary copper levels, however, do not increase during UPEC-induced UTI in the mouse model. Interestingly, urine from healthy mice contains inherently high levels of copper, in contrast to that from humans and nonhuman primates (89). UTI caused by UPEC in the nonhuman primate model (Chlorocebus aethiops [vervet monkey]) faithfully emulates clinical features of UTI in humans, including copper mobilization. Furthermore, supplementation and deficiency of copper result in decreased and enhanced UPEC colonization during UTI, respectively (89, 156). An early (24 to 48 h postinoculation) increase in renal and urinary copper content followed by a decrease (72 h postinoculation) has been reported during systemic infection with Candida albicans (157). It is unlikely that kidneys are copper depleted later during infection because of increased urinary excretion, since serum levels of copper remain elevated during infection, unless abscess formation and associated hemodynamic changes interfere with renal copper uptake during renal colonization by C. albicans (157). However, the effect of systemic bacterial infection on renal and urinary copper content remains to be investigated. Collectively, these findings indicate that copper is a protective innate immune effector mobilized to urine as a conserved host response to UTI caused by Gram-negative bacterial and fungal pathogens.
Yersiniabactin uptake genes are involved in the virulence of uropathogenic E. coli.
UPEC strains are more likely to carry yersiniabactin-biosynthetic genes than other pathotypes and commensal strains of E. coli (158, 159). Yersiniabactin production is associated with higher copper resistance among UPEC isolates, since it binds and shuttles copper (78). In addition to sequestering copper, Cu(II)-yersiniabactin complexes emulate superoxide dismutase activity to mitigate superoxide stress in UPEC within murine macrophages (160). Loss of FyuA, the outer membrane receptor for uptake of yersiniabactin-metal complexes, results in attenuation of UPEC in the mouse urinary tract (161). The genes ybtP and ybtQ encode ABC transporters localized on the inner membrane that play an essential role in the uptake of yersiniabactin (162). In a murine model of cystitis, ybtP and ybtQ genes contribute to the virulence of a prototypical UPEC strain, UTI89 (163). Since the mutants lacking fyuA, ybtP, and ybtQ are competent to produce and utilize enterobactin and salmochelin as siderophores during infection, decreased virulence could not be attributed to the loss of iron uptake alone. These observations suggest that a non-iron uptake function of yersiniabactin might play a role in attenuation observed in vivo. The intracellular copper and iron contents of these mutants and parental wild-type strains should be evaluated to distinguish whether yersiniabactin-mediated protection from copper stress or yersiniabactin-directed acquisition of iron is critical during infection. Taken together, these studies indicate complex siderophore-dependent and -independent roles for yersiniabactin in the virulence of UPEC.
Role of host-derived copper in limiting S. enterica colonization.
The oral gavage route of inoculation more closely emulates the natural course of colonization, infection, and systemic dissemination of S. enterica serovar Typhimurium than intraperitoneal inoculation in murine models. Achard et al. reported that CueO plays an important role in the virulence of S. enterica (strain SL1344) in a mouse model of intestinal colonization and systemic dissemination following oral gavage (164). There was an ∼100-fold decrease in the recovery of a cueO mutant from spleens and livers compared to the parental wild-type strain. A double mutant lacking CopA and GolT exhibited an ∼2-fold decrease in fitness during spleen and liver colonization, compared to the parental strain, in a mouse model of systemic salmonellosis (strain SL1344) initiated by intraperitoneal infection (144). However, oral inoculation of a CopA GolT double mutant (strain SL1344) does not result in a detectable decrease in colonization of spleen and liver (48). Another study utilizing intraperitoneal route of infection also did not identify a role of CopA, GolT, and CueP in the fitness of S. enterica (strain 14028) in various wild-type strains of mice (165). CopA- and GolT-dependent intramacrophage survival in S. enterica exhibited strain-specific differences, with a clear role in strain SL1344 but not in strain 14028. These genes were found to contribute to the fitness of S. enterica during systemic infection triggered by a higher-dose inoculation (2 × 104 CFU/mouse), but the loss of fitness was mitigated when a smaller inoculum (5 × 102 CFU/mouse) was utilized. These conflicting pieces of evidence suggest a model system-specific role for copper at the host-Salmonella interface. Investigations of the survival of Salmonella within cells derived from different lineages in spleen and liver could help reconcile the differences observed in these studies. Key points on the roles of copper at the host-pathogen interface are presented below and summarized in Table 2.
TABLE 2.
Key points
| No. | Observation |
|---|---|
| 1 | Effectors of nutritional immunity trigger concurrent hypoferremia and hypercupremia during infection. |
| 2 | Copper is co-opted by the innate immune system for protection against several bacterial pathogens. |
| 3 | Macrophages import copper via CTR1 during infection, and ATP7A pumps copper into the phagosome to kill to E. coli and S. enterica. |
| 4 | Copper is mobilized to urine during UTI in humans, and is recapitulated in a non-human primate model of UTI. |
| 5 | Uropathogenic E. coli overcome copper toxicity in vivo by utilizing Cus efflux system and yersiniabactin. |
| 6 | CueO plays an important role in the virulence of S. enterica in a murine model of infection. |
| 7 | CopA/GolT in S. enterica exhibit bacterial strain and inoculum dose-dependent variation in contribution to fitness during infection. |
Copper-based therapeutics.
Humans have harnessed the antimicrobial action of copper since early civilizations (115, 119, 166). Storage of water in copper vessels has been reported to inactivate E. coli (167, 168). Copper surfaces function synergistically with common disinfectants (169) and are increasingly being used to minimize microbial contamination in health care settings, and mechanisms of copper intoxication in bacteria exposed to metallic copper are beginning to be unraveled (166, 170, 171). There is a keen interest in exploiting the antimicrobial role of copper in the innate immune response through development of novel copper-based therapeutics (22). This is primarily driven by the grim outlook on the already high and continuously increasing levels of resistance to antibiotics that pose a threat to global public health. Extended-spectrum β-lactamase-producing bacteria, which are often multidrug resistant, are particularly concerning and lead to increased reliance on carbapenems as an antibiotic of last resort (172). Copper inhibits metallo-β-lactamase to rescue the sensitivity to carbapenems in resistant E. coli (173). Furthermore, use of copper-pyrithione complexes significantly decreases the concentration of copper required to revive carbapenem sensitivity. Another copper-dependent approach to selectively target β-lactamase-producing E. coli also utilizes pyrithione (174). In this case, pyrithione is released from a complex with cephalosporin only as a product of catalysis by β-lactamase. It shows promising activity against resistant strains in a copper-dependent manner. Copper-dependent killing or growth inhibition has been demonstrated for Mycobacterium tuberculosis, S. aureus, N. gonorrhoeae, and Mycoplasma species (175–178). These reports set the stage for productive inquiry on copper-dependent killing of E. coli and S. enterica, in addition to other pathogens, and the use of copper as an adjunct or alternative therapeutic to antibiotics.
Concerningly, there is evidence for coselection of resistance elements against both antibiotics and heavy metals, including copper (179–181). Bacteria may encounter the selective pressure of copper in a variety of settings, including agriculture, livestock operations, and health care facilities (182–185). The recent recovery of urinary E. coli isolates harboring the pco operon is of particular concern, as these isolates often produce extended-spectrum β-lactamases (74). Understanding the biological and evolutionary basis of coselection for metal and antibiotic resistance is critical for further exploitation of metal toxicity for therapeutic purposes.
Outstanding questions.
The essentiality of copper in biological processes and the mechanisms mitigating the toxic effects of excess copper have been subjected to extensive investigation. On the other hand, the process through which bacteria acquire copper remains alarmingly understudied and presents an opportunity to devise new ways to exploit the antimicrobial use of copper by augmenting its import. Although the copper detoxification systems of E. coli and S. enterica have been extensively studied, the copper response systems of K. pneumoniae, other members of Enterobacteriaceae, and closely related pathogens such as Proteus mirabilis remain ripe for investigation based on insights gleaned from studies on these model enteric bacteria. While the role of copper during UTI is an active area of investigation, the impact of copper during enteric and systemic infection by E. coli remains to be investigated. Antimicrobial and growth inhibitory functions of copper have attracted the most attention from researchers. Understanding the effects of copper on expression of virulence factors is yet another critical area that needs to be elucidated. Mechanisms of delivery of host-derived copper to sites of infection also remain to be determined. Ceruloplasmin appears to be the source of copper in urine during UTI (89). Several questions on the cellular origin of ceruloplasmin, the signals that activate its expression, and its secretion/translocation into the urine remain to be explored. Mounting evidence for copper as an innate immune effector represents an exciting direction for potential research on host-directed therapy against bacterial infections.
In summary, review of copper homeostatic systems and their role at host-pathogen interface in E. coli and S. enterica has revealed opportunities to advance our understanding of the biological roles of copper to fully harness its antimicrobial activity. Researchers working on these topics have generated valuable genetic and biochemical tools and animal models that have set the stage for productive inquiry in the years to come.
ACKNOWLEDGMENTS
We thank members of the Subash lab for helpful comments. We apologize to researchers whose work could not be cited due to space constraints.
The Subash lab is supported by funds from Texas A&M University, Texas A&M Agrilife Research, and awards DK114224 and AI135645 from the National Institutes of Health (S.S.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conceptualization S.S.; Writing A.H., K.C.-H., and S.S.
We have no conflicts of interest to declare.
REFERENCES
- 1.Festa RA, Thiele DJ. 2011. Copper: an essential metal in biology. Curr Biol 21:R877–R883. doi: 10.1016/j.cub.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boal AK, Rosenzweig AC. 2009. Structural biology of copper trafficking. Chem Rev 109:4760–4779. doi: 10.1021/cr900104z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridge PG, Zhang Y, Gladyshev VN. 2008. Comparative genomic analyses of copper transporters and cuproproteomes reveal evolutionary dynamics of copper utilization and its link to oxygen. PLoS One 3:e1378. doi: 10.1371/journal.pone.0001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreini C, Banci L, Bertini I, Rosato A. 2008. Occurrence of copper proteins through the three domains of life: a bioinformatic approach. J Proteome Res 7:209–216. doi: 10.1021/pr070480u. [DOI] [PubMed] [Google Scholar]
- 5.Arguello JM, Raimunda D, Padilla-Benavides T. 2013. Mechanisms of copper homeostasis in bacteria. Front Cell Infect Microbiol 3:73. doi: 10.3389/fcimb.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenney GE, Dassama LMK, Pandelia ME, Gizzi AS, Martinie RJ, Gao P, DeHart CJ, Schachner LF, Skinner OS, Ro SY, Zhu X, Sadek M, Thomas PM, Almo SC, Bollinger JM, Jr, Krebs C, Kelleher NL, Rosenzweig AC. 2018. The biosynthesis of methanobactin. Science 359:1411–1416. doi: 10.1126/science.aap9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tottey S, Rich PR, Rondet SA, Robinson NJ. 2001. Two Menkes-type ATPases supply copper for photosynthesis in Synechocystis PCC 6803. J Biol Chem 276:19999–20004. doi: 10.1074/jbc.M011243200. [DOI] [PubMed] [Google Scholar]
- 8.Raimunda D, Gonzalez-Guerrero M, Leeber BW, III, Arguello JM. 2011. The transport mechanism of bacterial Cu+-ATPases: distinct efflux rates adapted to different function. Biometals 24:467–475. doi: 10.1007/s10534-010-9404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khalfaoui-Hassani B, Wu H, Blaby-Haas CE, Zhang Y, Sandri F, Verissimo AF, Koch HG, Daldal F. 2018. Widespread distribution and functional specificity of the copper importer CcoA: distinct Cu uptake routes for bacterial cytochrome c oxidases. mBio 9:e00065-18. doi: 10.1128/mBio.00065-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutkenhaus JF. 1977. Role of a major outer membrane protein in Escherichia coli. J Bacteriol 131:631–637. doi: 10.1128/JB.131.2.631-637.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh EI, Robinson AE, Bandara N, Rogers BE, Henderson JP. 2017. Copper import in Escherichia coli by the yersiniabactin metallophore system. Nat Chem Biol 13:1016–1021. doi: 10.1038/nchembio.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porcheron G, Garenaux A, Proulx J, Sabri M, Dozois CM. 2013. Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front Cell Infect Microbiol 3:90. doi: 10.3389/fcimb.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egler M, Grosse C, Grass G, Nies DH. 2005. Role of the extracytoplasmic function protein family sigma factor RpoE in metal resistance of Escherichia coli. J Bacteriol 187:2297–2307. doi: 10.1128/JB.187.7.2297-2307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subashchandrabose S, Mobley HL. 2015. Back to the metal age: battle for metals at the host-pathogen interface during urinary tract infection. Metallomics 7:935–942. doi: 10.1039/C4MT00329B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart LJ, Thaqi D, Kobe B, McEwan AG, Waldron KJ, Djoko KY. 2019. Handling of nutrient copper in the bacterial envelope. Metallomics 11:50–63. doi: 10.1039/C8MT00218E. [DOI] [PubMed] [Google Scholar]
- 16.Nies DH, Herzberg M. 2013. A fresh view of the cell biology of copper in enterobacteria. Mol Microbiol 87:447–454. doi: 10.1111/mmi.12123. [DOI] [PubMed] [Google Scholar]
- 17.Changela A, Chen K, Xue Y, Holschen J, Outten CE, O'Halloran TV, Mondragon A. 2003. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science 301:1383–1387. doi: 10.1126/science.1085950. [DOI] [PubMed] [Google Scholar]
- 18.Morgan MT, Nguyen LAH, Hancock HL, Fahrni CJ. 2017. Glutathione limits aquacopper(I) to sub-femtomolar concentrations through cooperative assembly of a tetranuclear cluster. J Biol Chem 292:21558–21567. doi: 10.1074/jbc.M117.817452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura T, Nishioka H. 1997. Intracellular generation of superoxide by copper sulphate in Escherichia coli. Mutat Res 389:237–242. doi: 10.1016/s1383-5718(96)00153-2. [DOI] [PubMed] [Google Scholar]
- 20.Imlay JA. 2013. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liochev SI, Fridovich I. 2002. The Haber-Weiss cycle—70 years later: an alternative view. Redox Rep 7:55–57. Author reply, 59–60. doi: 10.1179/135100002125000190. [DOI] [PubMed] [Google Scholar]
- 22.Dalecki AG, Crawford CL, Wolschendorf F. 2017. Copper and antibiotics: discovery, modes of action, and opportunities for medicinal applications. Adv Microb Physiol 70:193–260. doi: 10.1016/bs.ampbs.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Lemire JA, Harrison JJ, Turner RJ. 2013. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol 11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 24.Macomber L, Imlay JA. 2009. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A 106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beswick PH, Hall GH, Hook AJ, Little K, McBrien DC, Lott KA. 1976. Copper toxicity: evidence for the conversion of cupric to cuprous copper in vivo under anaerobic conditions. Chem Biol Interact 14:347–356. doi: 10.1016/0009-2797(76)90113-7. [DOI] [PubMed] [Google Scholar]
- 26.Pontel LB, Soncini FC. 2009. Alternative periplasmic copper-resistance mechanisms in Gram negative bacteria. Mol Microbiol 73:212–225. doi: 10.1111/j.1365-2958.2009.06763.x. [DOI] [PubMed] [Google Scholar]
- 27.Djoko KY, Phan MD, Peters KM, Walker MJ, Schembri MA, McEwan AG. 2017. Interplay between tolerance mechanisms to copper and acid stress in Escherichia coli. Proc Natl Acad Sci U S A 114:6818–6823. doi: 10.1073/pnas.1620232114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irving H, Williams RJP. 1953. 637. The stability of transition-metal complexes. J Chem Soc 3192. doi: 10.1039/jr9530003192. [DOI] [Google Scholar]
- 29.Giachino A, Waldron KJ. 2020. Copper tolerance in bacteria requires the activation of multiple accessory pathways. Mol Microbiol 114:377–390. doi: 10.1111/mmi.14522. [DOI] [PubMed] [Google Scholar]
- 30.Odermatt A, Suter H, Krapf R, Solioz M. 1993. Primary structure of two P-type ATPases involved in copper homeostasis in Enterococcus hirae. J Biol Chem 268:12775–12779. doi: 10.1016/S0021-9258(18)31455-8. [DOI] [PubMed] [Google Scholar]
- 31.Solioz M, Stoyanov JV. 2003. Copper homeostasis in Enterococcus hirae. FEMS Microbiol Rev 27:183–195. doi: 10.1016/S0168-6445(03)00053-6. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez-Montes G, Arguello JM, Valderrama B. 2012. Evolution and diversity of periplasmic proteins involved in copper homeostasis in gamma proteobacteria. BMC Microbiol 12:249. doi: 10.1186/1471-2180-12-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoyanov JV, Hobman JL, Brown NL. 2001. CueR (YbbI) of Escherichia coli is a MerR family regulator controlling expression of the copper exporter CopA. Mol Microbiol 39:502–511. doi: 10.1046/j.1365-2958.2001.02264.x. [DOI] [PubMed] [Google Scholar]
- 34.Outten FW, Outten CE, Hale J, O'Halloran TV. 2000. Transcriptional activation of an Escherichia coli copper efflux regulon by the chromosomal MerR homologue, cueR. J Biol Chem 275:31024–31029. doi: 10.1074/jbc.M006508200. [DOI] [PubMed] [Google Scholar]
- 35.Petersen C, Moller LB. 2000. Control of copper homeostasis in Escherichia coli by a P-type ATPase, CopA, and a MerR-like transcriptional activator, CopR. Gene 261:289–298. doi: 10.1016/S0378-1119(00)00509-6. [DOI] [PubMed] [Google Scholar]
- 36.Pezza A, Pontel LB, Lopez C, Soncini FC. 2016. Compartment and signal-specific codependence in the transcriptional control of Salmonella periplasmic copper homeostasis. Proc Natl Acad Sci U S A 113:11573–11578. doi: 10.1073/pnas.1603192113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espariz M, Checa SK, Audero ME, Pontel LB, Soncini FC. 2007. Dissecting the Salmonella response to copper. Microbiology (Reading) 153:2989–2997. doi: 10.1099/mic.0.2007/006536-0. [DOI] [PubMed] [Google Scholar]
- 38.Andoy NM, Sarkar SK, Wang Q, Panda D, Benitez JJ, Kalininskiy A, Chen P. 2009. Single-molecule study of metalloregulator CueR-DNA interactions using engineered Holliday junctions. Biophys J 97:844–852. doi: 10.1016/j.bpj.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto K, Ishihama A. 2005. Transcriptional response of Escherichia coli to external copper. Mol Microbiol 56:215–227. doi: 10.1111/j.1365-2958.2005.04532.x. [DOI] [PubMed] [Google Scholar]
- 40.Pontel LB, Scampoli NL, Porwollik S, Checa SK, McClelland M, Soncini FC. 2014. Identification of a Salmonella ancillary copper detoxification mechanism by a comparative analysis of the genome-wide transcriptional response to copper and zinc excess. Microbiology (Reading) 160:1659–1669. doi: 10.1099/mic.0.080473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rensing C, Fan B, Sharma R, Mitra B, Rosen BP. 2000. CopA: an Escherichia coli Cu(I)-translocating P-type ATPase. Proc Natl Acad Sci U S A 97:652–656. doi: 10.1073/pnas.97.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osman D, Patterson CJ, Bailey K, Fisher K, Robinson NJ, Rigby SE, Cavet JS. 2013. The copper supply pathway to a Salmonella Cu,Zn-superoxide dismutase (SodCII) involves P(1B)-type ATPase copper efflux and periplasmic CueP. Mol Microbiol 87:466–477. doi: 10.1111/mmi.12107. [DOI] [PubMed] [Google Scholar]
- 43.Bachman MA, Breen P, Deornellas V, Mu Q, Zhao L, Wu W, Cavalcoli JD, Mobley HL. 2015. Genome-wide identification of Klebsiella pneumoniae fitness genes during lung infection. mBio 6:e00775-15. doi: 10.1128/mBio.00775-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drees SL, Klinkert B, Helling S, Beyer DF, Marcus K, Narberhaus F, Lubben M. 2017. One gene, two proteins: coordinated production of a copper chaperone by differential transcript formation and translational frameshifting in Escherichia coli. Mol Microbiol 106:635–645. doi: 10.1111/mmi.13841. [DOI] [PubMed] [Google Scholar]
- 45.Meydan S, Klepacki D, Karthikeyan S, Margus T, Thomas P, Jones JE, Khan Y, Briggs J, Dinman JD, Vazquez-Laslop N, Mankin AS. 2017. Programmed ribosomal frameshifting generates a copper transporter and a copper chaperone from the same gene. Mol Cell 65:207–219. doi: 10.1016/j.molcel.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Checa SK, Espariz M, Audero ME, Botta PE, Spinelli SV, Soncini FC. 2007. Bacterial sensing of and resistance to gold salts. Mol Microbiol 63:1307–1318. doi: 10.1111/j.1365-2958.2007.05590.x. [DOI] [PubMed] [Google Scholar]
- 47.Pontel LB, Audero ME, Espariz M, Checa SK, Soncini FC. 2007. GolS controls the response to gold by the hierarchical induction of Salmonella-specific genes that include a CBA efflux-coding operon. Mol Microbiol 66:814–825. doi: 10.1111/j.1365-2958.2007.05963.x. [DOI] [PubMed] [Google Scholar]
- 48.Osman D, Waldron KJ, Denton H, Taylor CM, Grant AJ, Mastroeni P, Robinson NJ, Cavet JS. 2010. Copper homeostasis in Salmonella is atypical and copper-CueP is a major periplasmic metal complex. J Biol Chem 285:25259–25268. doi: 10.1074/jbc.M110.145953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grass G, Rensing C. 2001. Genes involved in copper homeostasis in Escherichia coli. J Bacteriol 183:2145–2147. doi: 10.1128/JB.183.6.2145-2147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh SK, Grass G, Rensing C, Montfort WR. 2004. Cuprous oxidase activity of CueO from Escherichia coli. J Bacteriol 186:7815–7817. doi: 10.1128/JB.186.22.7815-7817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tree JJ, Ulett GC, Ong CL, Trott DJ, McEwan AG, Schembri MA. 2008. Trade-off between iron uptake and protection against oxidative stress: deletion of cueO promotes uropathogenic Escherichia coli virulence in a mouse model of urinary tract infection. J Bacteriol 190:6909–6912. doi: 10.1128/JB.00451-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stolle P, Hou B, Bruser T. 2016. The Tat substrate CueO is transported in an incomplete folding state. J Biol Chem 291:13520–13528. doi: 10.1074/jbc.M116.729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grass G, Thakali K, Klebba PE, Thieme D, Muller A, Wildner GF, Rensing C. 2004. Linkage between catecholate siderophores and the multicopper oxidase CueO in Escherichia coli. J Bacteriol 186:5826–5833. doi: 10.1128/JB.186.17.5826-5833.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Subedi P, Paxman JJ, Wang G, Ukuwela AA, Xiao Z, Heras B. 2019. The Scs disulfide reductase system cooperates with the metallochaperone CueP in Salmonella copper resistance. J Biol Chem 294:15876–15888. doi: 10.1074/jbc.RA119.010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kittleson JT, Loftin IR, Hausrath AC, Engelhardt KP, Rensing C, McEvoy MM. 2006. Periplasmic metal-resistance protein CusF exhibits high affinity and specificity for both CuI and AgI. Biochemistry 45:11096–11102. doi: 10.1021/bi0612622. [DOI] [PubMed] [Google Scholar]
- 56.Saenkham P, Ritter M, Donati GL, Subashchandrabose S. 2020. Copper primes adaptation of uropathogenic Escherichia coli to superoxide stress by activating superoxide dismutases. PLoS Pathog 16:e1008856. doi: 10.1371/journal.ppat.1008856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Padilla-Benavides T, George Thompson AM, McEvoy MM, Arguello JM. 2014. Mechanism of ATPase-mediated Cu+ export and delivery to periplasmic chaperones: the interaction of Escherichia coli CopA and CusF. J Biol Chem 289:20492–20501. doi: 10.1074/jbc.M114.577668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta SD, Wu HC, Rick PD. 1997. A Salmonella typhimurium genetic locus which confers copper tolerance on copper-sensitive mutants of Escherichia coli. J Bacteriol 179:4977–4984. doi: 10.1128/jb.179.16.4977-4984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopez C, Checa SK, Soncini FC. 2018. CpxR/CpxA controls scsABCD transcription to counteract copper and oxidative stress in Salmonella enterica serovar Typhimurium. J Bacteriol 200:e00126-18. doi: 10.1128/JB.00126-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shepherd M, Heras B, Achard ME, King GJ, Argente MP, Kurth F, Taylor SL, Howard MJ, King NP, Schembri MA, McEwan AG. 2013. Structural and functional characterization of ScsC, a periplasmic thioredoxin-like protein from Salmonella enterica serovar Typhimurium. Antioxid Redox Signal 19:1494–1506. doi: 10.1089/ars.2012.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anwar N, Sem XH, Rhen M. 2013. Oxidoreductases that act as conditional virulence suppressors in Salmonella enterica serovar Typhimurium. PLoS One 8:e64948. doi: 10.1371/journal.pone.0064948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munson GP, Lam DL, Outten FW, O'Halloran TV. 2000. Identification of a copper-responsive two-component system on the chromosome of Escherichia coli K-12. J Bacteriol 182:5864–5871. doi: 10.1128/jb.182.20.5864-5871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chacon KN, Mealman TD, McEvoy MM, Blackburn NJ. 2014. Tracking metal ions through a Cu/Ag efflux pump assigns the functional roles of the periplasmic proteins. Proc Natl Acad Sci U S A 111:15373–15378. doi: 10.1073/pnas.1411475111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Outten FW, Huffman DL, Hale JA, O'Halloran TV. 2001. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J Biol Chem 276:30670–30677. doi: 10.1074/jbc.M104122200. [DOI] [PubMed] [Google Scholar]
- 65.Franke S, Grass G, Rensing C, Nies DH. 2003. Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli. J Bacteriol 185:3804–3812. doi: 10.1128/jb.185.13.3804-3812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gudipaty SA, McEvoy MM. 2014. The histidine kinase CusS senses silver ions through direct binding by its sensor domain. Biochim Biophys Acta 1844:1656–1661. doi: 10.1016/j.bbapap.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Urano H, Yoshida M, Ogawa A, Yamamoto K, Ishihama A, Ogasawara H. 2017. Cross-regulation between two common ancestral response regulators, HprR and CusR, in Escherichia coli. Microbiology (Reading) 163:243–252. doi: 10.1099/mic.0.000410. [DOI] [PubMed] [Google Scholar]
- 68.Santiago AG, Chen TY, Genova LA, Jung W, George Thompson AM, McEvoy MM, Chen P. 2017. Adaptor protein mediates dynamic pump assembly for bacterial metal efflux. Proc Natl Acad Sci U S A 114:6694–6699. doi: 10.1073/pnas.1704729114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arai N, Sekizuka T, Tamamura Y, Kusumoto M, Hinenoya A, Yamasaki S, Iwata T, Watanabe-Yanai A, Kuroda M, Akiba M. 2019. Salmonella genomic island 3 is an integrative and conjugative element and contributes to copper and arsenic tolerance of Salmonella enterica. Antimicrob Agents Chemother 63:e00429-19. doi: 10.1128/AAC.00429-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Billman-Jacobe H, Liu Y, Haites R, Weaver T, Robinson L, Marenda M, Dyall-Smith M. 2018. pSTM6-275, a conjugative IncHI2 plasmid of Salmonella enterica that confers antibiotic and heavy-metal resistance under changing physiological conditions. Antimicrob Agents Chemother 62:e02357-17. doi: 10.1128/AAC.02357-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brown NL, Barrett SR, Camakaris J, Lee BT, Rouch DA. 1995. Molecular genetics and transport analysis of the copper-resistance determinant (pco) from Escherichia coli plasmid pRJ1004. Mol Microbiol 17:1153–1166. doi: 10.1111/j.1365-2958.1995.mmi_17061153.x. [DOI] [PubMed] [Google Scholar]
- 72.Djoko KY, Xiao Z, Wedd AG. 2008. Copper resistance in E. coli: the multicopper oxidase PcoA catalyzes oxidation of copper(I) in Cu(I)Cu(II)-PcoC. Chembiochem 9:1579–1582. doi: 10.1002/cbic.200800100. [DOI] [PubMed] [Google Scholar]
- 73.Chalmers G, Rozas KM, Amachawadi RG, Scott HM, Norman KN, Nagaraja TG, Tokach MD, Boerlin P. 2018. Distribution of the pco gene cluster and associated genetic determinants among swine Escherichia coli from a controlled feeding trial. Genes 9:504. doi: 10.3390/genes9100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sutterlin S, Tellez-Castillo CJ, Anselem L, Yin H, Bray JE, Maiden MCJ. 2018. Heavy metal susceptibility of Escherichia coli isolated from urine samples from Sweden, Germany, and Spain. Antimicrob Agents Chemother 62:e00209-18. doi: 10.1128/AAC.00209-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garenaux A, Caza M, Dozois CM. 2011. The ins and outs of siderophore mediated iron uptake by extra-intestinal pathogenic Escherichia coli. Vet Microbiol 153:89–98. doi: 10.1016/j.vetmic.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 76.Koh EI, Hung CS, Parker KS, Crowley JR, Giblin DE, Henderson JP. 2015. Metal selectivity by the virulence-associated yersiniabactin metallophore system. Metallomics 7:1011–1022. doi: 10.1039/c4mt00341a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robinson AE, Lowe JE, Koh EI, Henderson JP. 2018. Uropathogenic enterobacteria use the yersiniabactin metallophore system to acquire nickel. J Biol Chem 293:14953–14961. doi: 10.1074/jbc.RA118.004483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chaturvedi KS, Hung CS, Crowley JR, Stapleton AE, Henderson JP. 2012. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat Chem Biol 8:731–736. doi: 10.1038/nchembio.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gupta SD, Lee BT, Camakaris J, Wu HC. 1995. Identification of cutC and cutF (nlpE) genes involved in copper tolerance in Escherichia coli. J Bacteriol 177:4207–4215. doi: 10.1128/jb.177.15.4207-4215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo MS, Updegrove TB, Gogol EB, Shabalina SA, Gross CA, Storz G. 2014. MicL, a new sigmaE-dependent sRNA, combats envelope stress by repressing synthesis of Lpp, the major outer membrane lipoprotein. Genes Dev 28:1620–1634. doi: 10.1101/gad.243485.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mecsas J, Rouviere PE, Erickson JW, Donohue TJ, Gross CA. 1993. The activity of sigma E, an Escherichia coli heat-inducible sigma-factor, is modulated by expression of outer membrane proteins. Genes Dev 7:2618–2628. doi: 10.1101/gad.7.12b.2618. [DOI] [PubMed] [Google Scholar]
- 82.Hiniker A, Collet JF, Bardwell JC. 2005. Copper stress causes an in vivo requirement for the Escherichia coli disulfide isomerase DsbC. J Biol Chem 280:33785–33791. doi: 10.1074/jbc.M505742200. [DOI] [PubMed] [Google Scholar]
- 83.Helbig K, Bleuel C, Krauss GJ, Nies DH. 2008. Glutathione and transition-metal homeostasis in Escherichia coli. J Bacteriol 190:5431–5438. doi: 10.1128/JB.00271-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Große C, Schleuder G, Schmole C, Nies DH. 2014. Survival of Escherichia coli cells on solid copper surfaces is increased by glutathione. Appl Environ Microbiol 80:7071–7078. doi: 10.1128/AEM.02842-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Macomber L, Rensing C, Imlay JA. 2007. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J Bacteriol 189:1616–1626. doi: 10.1128/JB.01357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kershaw CJ, Brown NL, Constantinidou C, Patel MD, Hobman JL. 2005. The expression profile of Escherichia coli K-12 in response to minimal, optimal and excess copper concentrations. Microbiology (Reading) 151:1187–1198. doi: 10.1099/mic.0.27650-0. [DOI] [PubMed] [Google Scholar]
- 87.Riley LW. 2020. Distinguishing pathovars from nonpathovars: Escherichia coli. Microbiol Spectr 8:AME-0014-2020. doi: 10.1128/microbiolspec.AME-0014-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaper JB, Nataro JP, Mobley HL. 2004. Pathogenic Escherichia coli. Nat Rev Microbiol 2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 89.Hyre AN, Kavanagh K, Kock ND, Donati GL, Subashchandrabose S. 2017. Copper is a host effector mobilized to urine during urinary tract infection to impair bacterial colonization. Infect Immun 85:e01041-16. doi: 10.1128/IAI.01041-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. 2015. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Besser JM. 2018. Salmonella epidemiology: a whirlwind of change. Food Microbiol 71:55–59. doi: 10.1016/j.fm.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 92.Hiyoshi H, Tiffany CR, Bronner DN, Baumler AJ. 2018. Typhoidal Salmonella serovars: ecological opportunity and the evolution of a new pathovar. FEMS Microbiol Rev 42:527–541. doi: 10.1093/femsre/fuy024. [DOI] [PubMed] [Google Scholar]
- 93.Haselbeck AH, Panzner U, Im J, Baker S, Meyer CG, Marks F. 2017. Current perspectives on invasive nontyphoidal Salmonella disease. Curr Opin Infect Dis 30:498–503. doi: 10.1097/QCO.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anderson CJ, Kendall MM. 2017. Salmonella enterica serovar Typhimurium strategies for host adaptation. Front Microbiol 8:1983. doi: 10.3389/fmicb.2017.01983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vazquez-Torres A, Fang FC. 2001. Salmonella evasion of the NADPH phagocyte oxidase. Microbes Infect 3:1313–1320. doi: 10.1016/s1286-4579(01)01492-7. [DOI] [PubMed] [Google Scholar]
- 96.Liss V, Hensel M. 2015. Take the tube: remodelling of the endosomal system by intracellular Salmonella enterica. Cell Microbiol 17:639–647. doi: 10.1111/cmi.12441. [DOI] [PubMed] [Google Scholar]
- 97.Hensel M, Shea JE, Gleeson C, Jones MD, Dalton E, Holden DW. 1995. Simultaneous identification of bacterial virulence genes by negative selection. Science 269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 98.Galan JE, Curtiss R, 3rd. 1989. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A 86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang X, Garrick MD, Collins JF. 2019. Animal models of normal and disturbed iron and copper metabolism. J Nutr 149:2085–2100. doi: 10.1093/jn/nxz172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Linder MC. 2020. Copper homeostasis in mammals, with emphasis on secretion and excretion. A review. Int J Mol Sci 21:4932. doi: 10.3390/ijms21144932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nose Y, Kim BE, Thiele DJ. 2006. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab 4:235–244. doi: 10.1016/j.cmet.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 102.Wang Y, Zhu S, Hodgkinson V, Prohaska JR, Weisman GA, Gitlin JD, Petris MJ. 2012. Maternofetal and neonatal copper requirements revealed by enterocyte-specific deletion of the Menkes disease protein. Am J Physiol Gastrointest Liver Physiol 303:G1236–G1244. doi: 10.1152/ajpgi.00339.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tumer Z, Moller LB. 2010. Menkes disease. Eur J Hum Genet 18:511–518. doi: 10.1038/ejhg.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ralle M, Huster D, Vogt S, Schirrmeister W, Burkhead JL, Capps TR, Gray L, Lai B, Maryon E, Lutsenko S. 2010. Wilson disease at a single cell level: intracellular copper trafficking activates compartment-specific responses in hepatocytes. J Biol Chem 285:30875–30883. doi: 10.1074/jbc.M110.114447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hellman NE, Gitlin JD. 2002. Ceruloplasmin metabolism and function. Annu Rev Nutr 22:439–458. doi: 10.1146/annurev.nutr.22.012502.114457. [DOI] [PubMed] [Google Scholar]
- 106.Linder MC. 2016. Ceruloplasmin and other copper binding components of blood plasma and their functions: an update. Metallomics 8:887–905. doi: 10.1039/c6mt00103c. [DOI] [PubMed] [Google Scholar]
- 107.Hasan NM, Gupta A, Polishchuk E, Yu CH, Polishchuk R, Dmitriev OY, Lutsenko S. 2012. Molecular events initiating exit of a copper-transporting ATPase ATP7B from the trans-Golgi network. J Biol Chem 287:36041–36050. doi: 10.1074/jbc.M112.370403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Medici V, Rossaro L, Sturniolo GC. 2007. Wilson disease—a practical approach to diagnosis, treatment and follow-up. Dig Liver Dis 39:601–609. doi: 10.1016/j.dld.2006.12.095. [DOI] [PubMed] [Google Scholar]
- 109.Logeman BL, Wood LK, Lee J, Thiele DJ. 2017. Gene duplication and neo-functionalization in the evolutionary and functional divergence of the metazoan copper transporters Ctr1 and Ctr2. J Biol Chem 292:11531–11546. doi: 10.1074/jbc.M117.793356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Markowitz H, Gubler CJ, Mahoney JP, Cartwright GE, Wintrobe MM. 1955. Studies on copper metabolism. XIV. Copper, ceruloplasmin and oxidase activity in sera of normal human subjects, pregnant women, and patients with infection, hepatolenticular degeneration and the nephrotic syndrome. J Clin Invest 34:1498–1508. doi: 10.1172/JCI103201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weinberg ED. 1975. Nutritional immunity. Host's attempt to withold iron from microbial invaders. JAMA 231:39–41. doi: 10.1001/jama.1975.03240130021018. [DOI] [PubMed] [Google Scholar]
- 112.Cassat JE, Skaar EP. 2013. Iron in infection and immunity. Cell Host Microbe 13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Skaar EP. 2010. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog 6:e1000949. doi: 10.1371/journal.ppat.1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stafford SL, Bokil NJ, Achard ME, Kapetanovic R, Schembri MA, McEwan AG, Sweet MJ. 2013. Metal ions in macrophage antimicrobial pathways: emerging roles for zinc and copper. Biosci Rep 33:e00049. doi: 10.1042/BSR20130014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chaturvedi KS, Henderson JP. 2014. Pathogenic adaptations to host-derived antibacterial copper. Front Cell Infect Microbiol 4:3. doi: 10.3389/fcimb.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Festa RA, Thiele DJ. 2012. Copper at the front line of the host-pathogen battle. PLoS Pathog 8:e1002887. doi: 10.1371/journal.ppat.1002887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Garcia-Santamarina S, Thiele DJ. 2015. Copper at the fungal pathogen-host axis. J Biol Chem 290:18945–18953. doi: 10.1074/jbc.R115.649129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hodgkinson V, Petris MJ. 2012. Copper homeostasis at the host-pathogen interface. J Biol Chem 287:13549–13555. doi: 10.1074/jbc.R111.316406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ladomersky E, Petris MJ. 2015. Copper tolerance and virulence in bacteria. Metallomics 7:957–964. doi: 10.1039/c4mt00327f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Samanovic MI, Ding C, Thiele DJ, Darwin KH. 2012. Copper in microbial pathogenesis: meddling with the metal. Cell Host Microbe 11:106–115. doi: 10.1016/j.chom.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fu Y, Chang FM, Giedroc DP. 2014. Copper transport and trafficking at the host-bacterial pathogen interface. Acc Chem Res 47:3605–3613. doi: 10.1021/ar500300n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kehl-Fie TE, Skaar EP. 2010. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol 14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stocks CJ, Phan MD, Achard MES, Nhu NTK, Condon ND, Gawthorne JA, Lo AW, Peters KM, McEwan AG, Kapetanovic R, Schembri MA, Sweet MJ. 2019. Uropathogenic Escherichia coli employs both evasion and resistance to subvert innate immune-mediated zinc toxicity for dissemination. Proc Natl Acad Sci U S A 116:6341–6350. doi: 10.1073/pnas.1820870116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kapetanovic R, Bokil NJ, Achard ME, Ong CL, Peters KM, Stocks CJ, Phan MD, Monteleone M, Schroder K, Irvine KM, Saunders BM, Walker MJ, Stacey KJ, McEwan AG, Schembri MA, Sweet MJ. 2016. Salmonella employs multiple mechanisms to subvert the TLR-inducible zinc-mediated antimicrobial response of human macrophages. FASEB J 30:1901–1912. doi: 10.1096/fj.201500061. [DOI] [PubMed] [Google Scholar]
- 125.Achard ME, Stafford SL, Bokil NJ, Chartres J, Bernhardt PV, Schembri MA, Sweet MJ, McEwan AG. 2012. Copper redistribution in murine macrophages in response to Salmonella infection. Biochem J 444:51–57. doi: 10.1042/BJ20112180. [DOI] [PubMed] [Google Scholar]
- 126.Djoko KY, Ong CL, Walker MJ, McEwan AG. 2015. The role of copper and zinc toxicity in innate immune defense against bacterial pathogens. J Biol Chem 290:18954–18961. doi: 10.1074/jbc.R115.647099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Besold AN, Culbertson EM, Culotta VC. 2016. The Yin and Yang of copper during infection. J Biol Inorg Chem 21:137–144. doi: 10.1007/s00775-016-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ganz T. 2018. Iron and infection. Int J Hematol 107:7–15. doi: 10.1007/s12185-017-2366-2. [DOI] [PubMed] [Google Scholar]
- 129.Vashchenko G, MacGillivray RT. 2013. Multi-copper oxidases and human iron metabolism. Nutrients 5:2289–2313. doi: 10.3390/nu5072289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mostad EJ, Prohaska JR. 2011. Glycosylphosphatidylinositol-linked ceruloplasmin is expressed in multiple rodent organs and is lower following dietary copper deficiency. Exp Biol Med (Maywood) 236:298–308. doi: 10.1258/ebm.2010.010256. [DOI] [PubMed] [Google Scholar]
- 131.Gabay C, Kushner I. 1999. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 132.Berendt RF, Knutsen GL, Powanda MC. 1978. Nonhuman primate model for the study of respiratory Klebsiella pneumoniae infection. Infect Immun 22:275–281. doi: 10.1128/IAI.22.1.275-281.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wait R, Chiesa G, Parolini C, Miller I, Begum S, Brambilla D, Galluccio L, Ballerio R, Eberini I, Gianazza E. 2005. Reference maps of mouse serum acute-phase proteins: changes with LPS-induced inflammation and apolipoprotein A-I and A-II transgenes. Proteomics 5:4245–4253. doi: 10.1002/pmic.200401292. [DOI] [PubMed] [Google Scholar]
- 134.Hao X, Luthje F, Ronn R, German NA, Li X, Huang F, Kisaka J, Huffman D, Alwathnani HA, Zhu YG, Rensing C. 2016. A role for copper in protozoan grazing—two billion years selecting for bacterial copper resistance. Mol Microbiol 102:628–641. doi: 10.1111/mmi.13483. [DOI] [PubMed] [Google Scholar]
- 135.Schwan WR, Warrener P, Keunz E, Stover CK, Folger KR. 2005. Mutations in the cueA gene encoding a copper homeostasis P-type ATPase reduce the pathogenicity of Pseudomonas aeruginosa in mice. Int J Med Microbiol 295:237–242. doi: 10.1016/j.ijmm.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 136.Williams CL, Neu HM, Alamneh YA, Reddinger RM, Jacobs AC, Singh S, Abu-Taleb R, Michel SLJ, Zurawski DV, Merrell DS. 2020. Characterization of Acinetobacter baumannii copper resistance reveals a role in virulence. Front Microbiol 11:16. doi: 10.3389/fmicb.2020.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Djoko KY, Franiek JA, Edwards JL, Falsetta ML, Kidd SP, Potter AJ, Chen NH, Apicella MA, Jennings MP, McEwan AG. 2012. Phenotypic characterization of a copA mutant of Neisseria gonorrhoeae identifies a link between copper and nitrosative stress. Infect Immun 80:1065–1071. doi: 10.1128/IAI.06163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Johnson MD, Kehl-Fie TE, Klein R, Kelly J, Burnham C, Mann B, Rosch JW. 2015. Role of copper efflux in pneumococcal pathogenesis and resistance to macrophage-mediated immune clearance. Infect Immun 83:1684–1694. doi: 10.1128/IAI.03015-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shafeeq S, Yesilkaya H, Kloosterman TG, Narayanan G, Wandel M, Andrew PW, Kuipers OP, Morrissey JA. 2011. The cop operon is required for copper homeostasis and contributes to virulence in Streptococcus pneumoniae. Mol Microbiol 81:1255–1270. doi: 10.1111/j.1365-2958.2011.07758.x. [DOI] [PubMed] [Google Scholar]
- 140.Ding C, Festa RA, Chen YL, Espart A, Palacios O, Espin J, Capdevila M, Atrian S, Heitman J, Thiele DJ. 2013. Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell Host Microbe 13:265–276. doi: 10.1016/j.chom.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Babu U, Failla ML. 1990. Respiratory burst and candidacidal activity of peritoneal macrophages are impaired in copper-deficient rats. J Nutr 120:1692–1699. doi: 10.1093/jn/120.12.1692. [DOI] [PubMed] [Google Scholar]
- 142.Wagner D, Maser J, Lai B, Cai Z, Barry CE, 3rd, Honer Zu Bentrup K, Russell DG, Bermudez LE. 2005. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell's endosomal system. J Immunol 174:1491–1500. doi: 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]
- 143.White C, Lee J, Kambe T, Fritsche K, Petris MJ. 2009. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem 284:33949–33956. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ladomersky E, Khan A, Shanbhag V, Cavet JS, Chan J, Weisman GA, Petris MJ. 2017. Host and pathogen copper-transporting P-type ATPases function antagonistically during Salmonella infection. Infect Immun 85:e00351-17. doi: 10.1128/IAI.00351-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Heresi G, Castillo-Durán C, Muñoz C, Arévalo M, Schlesinger L. 1985. Phagocytosis and immunoglobulin levels in hypocrupremic infants. Nutrition Res 5:1327–1334. doi: 10.1016/S0271-5317(85)80043-9. [DOI] [Google Scholar]
- 146.Bae B, Percival SS. 1993. Retinoic acid-induced HL-60 cell differentiation is augmented by copper supplementation. J Nutr 123:997–1002. doi: 10.1093/jn/123.6.997. [DOI] [PubMed] [Google Scholar]
- 147.Rathinam VAK, Chan FK. 2018. Inflammasome, inflammation, and tissue homeostasis. Trends Mol Med 24:304–318. doi: 10.1016/j.molmed.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Deigendesch N, Zychlinsky A, Meissner F. 2018. Copper regulates the canonical NLRP3 inflammasome. J Immunol 200:1607–1617. doi: 10.4049/jimmunol.1700712. [DOI] [PubMed] [Google Scholar]
- 149.Johnzon CF, Ronnberg E, Pejler G. 2016. The role of mast cells in bacterial infection. Am J Pathol 186:4–14. doi: 10.1016/j.ajpath.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 150.Malaviya R, Ikeda T, Ross E, Abraham SN. 1996. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 151.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, Adachi R. 2007. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem 282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 152.Hu Frisk JM, Kjellen L, Kaler SG, Pejler G, Ohrvik H. 2017. Copper regulates maturation and expression of an MITF:tryptase axis in mast cells. J Immunol 199:4132–4141. doi: 10.4049/jimmunol.1700786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Choi HW, Bowen SE, Miao Y, Chan CY, Miao EA, Abrink M, Moeser AJ, Abraham SN. 2016. Loss of bladder epithelium induced by cytolytic mast cell granules. Immunity 45:1258–1269. doi: 10.1016/j.immuni.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guthrie LM, Soma S, Yuan S, Silva A, Zulkifli M, Snavely TC, Greene HF, Nunez E, Lynch B, De Ville C, Shanbhag V, Lopez FR, Acharya A, Petris MJ, Kim BE, Gohil VM, Sacchettini JC. 2020. Elesclomol alleviates Menkes pathology and mortality by escorting Cu to cuproenzymes in mice. Science 368:620–625. doi: 10.1126/science.aaz8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wheeler EM, Roberts PF. 1976. Menkes's steely hair syndrome. Arch Dis Child 51:269–274. doi: 10.1136/adc.51.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Subashchandrabose S, Hazen TH, Brumbaugh AR, Himpsl SD, Smith SN, Ernst RD, Rasko DA, Mobley HL. 2014. Host-specific induction of Escherichia coli fitness genes during human urinary tract infection. Proc Natl Acad Sci U S A 111:18327–18332. doi: 10.1073/pnas.1415959112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li CX, Gleason JE, Zhang SX, Bruno VM, Cormack BP, Culotta VC. 2015. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proc Natl Acad Sci U S A 112:E5336–E5342. doi: 10.1073/pnas.1513447112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Spurbeck RR, Dinh PC, Jr, Walk ST, Stapleton AE, Hooton TM, Nolan LK, Kim KS, Johnson JR, Mobley HL. 2012. Escherichia coli isolates that carry vat, fyuA, chuA, and yfcV efficiently colonize the urinary tract. Infect Immun 80:4115–4122. doi: 10.1128/IAI.00752-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Henderson JP, Crowley JR, Pinkner JS, Walker JN, Tsukayama P, Stamm WE, Hooton TM, Hultgren SJ. 2009. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog 5:e1000305. doi: 10.1371/journal.ppat.1000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chaturvedi KS, Hung CS, Giblin DE, Urushidani S, Austin AM, Dinauer MC, Henderson JP. 2014. Cupric yersiniabactin is a virulence-associated superoxide dismutase mimic. ACS Chem Biol 9:551–561. doi: 10.1021/cb400658k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brumbaugh AR, Smith SN, Subashchandrabose S, Himpsl SD, Hazen TH, Rasko DA, Mobley HL. 2015. Blocking yersiniabactin import attenuates extraintestinal pathogenic Escherichia coli in cystitis and pyelonephritis and represents a novel target to prevent urinary tract infection. Infect Immun 83:1443–1450. doi: 10.1128/IAI.02904-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fetherston JD, Bertolino VJ, Perry RD. 1999. YbtP and YbtQ: two ABC transporters required for iron uptake in Yersinia pestis. Mol Microbiol 32:289–299. doi: 10.1046/j.1365-2958.1999.01348.x. [DOI] [PubMed] [Google Scholar]
- 163.Koh EI, Hung CS, Henderson JP. 2016. The yersiniabactin-associated ATP binding cassette proteins YbtP and YbtQ enhance Escherichia coli fitness during high-titer cystitis. Infect Immun 84:1312–1319. doi: 10.1128/IAI.01211-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Achard ME, Tree JJ, Holden JA, Simpfendorfer KR, Wijburg OL, Strugnell RA, Schembri MA, Sweet MJ, Jennings MP, McEwan AG. 2010. The multi-copper-ion oxidase CueO of Salmonella enterica serovar Typhimurium is required for systemic virulence. Infect Immun 78:2312–2319. doi: 10.1128/IAI.01208-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fenlon LA, Slauch JM. 2017. Cytoplasmic copper detoxification in Salmonella can contribute to SodC metalation but is dispensable during systemic infection. J Bacteriol 199:e00437-17. doi: 10.1128/JB.00437-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Grass G, Rensing C, Solioz M. 2011. Metallic copper as an antimicrobial surface. Appl Environ Microbiol 77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sharan R, Chhibber S, Attri S, Reed RH. 2010. Inactivation and sub-lethal injury of Escherichia coli in a copper water storage vessel: effect of inorganic and organic constituents. Antonie Van Leeuwenhoek 98:103–115. doi: 10.1007/s10482-010-9435-3. [DOI] [PubMed] [Google Scholar]
- 168.Sharan R, Chhibber S, Attri S, Reed RH. 2010. Inactivation and injury of Escherichia coli in a copper water storage vessel: effects of temperature and pH. Antonie Van Leeuwenhoek 97:91–97. doi: 10.1007/s10482-009-9395-7. [DOI] [PubMed] [Google Scholar]
- 169.Steinhauer K, Meyer S, Pfannebecker J, Teckemeyer K, Ockenfeld K, Weber K, Becker B. 2018. Antimicrobial efficacy and compatibility of solid copper alloys with chemical disinfectants. PLoS One 13:e0200748. doi: 10.1371/journal.pone.0200748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Casey AL, Adams D, Karpanen TJ, Lambert PA, Cookson BD, Nightingale P, Miruszenko L, Shillam R, Christian P, Elliott TS. 2010. Role of copper in reducing hospital environment contamination. J Hosp Infect 74:72–77. doi: 10.1016/j.jhin.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 171.Vincent M, Duval RE, Hartemann P, Engels-Deutsch M. 2018. Contact killing and antimicrobial properties of copper. J Appl Microbiol 124:1032–1046. doi: 10.1111/jam.13681. [DOI] [PubMed] [Google Scholar]
- 172.Mazzariol A, Bazaj A, Cornaglia G. 2017. Multi-drug-resistant Gram-negative bacteria causing urinary tract infections: a review. J Chemother 29:2–9. doi: 10.1080/1120009X.2017.1380395. [DOI] [PubMed] [Google Scholar]
- 173.Djoko KY, Achard MES, Phan MD, Lo AW, Miraula M, Prombhul S, Hancock SJ, Peters KM, Sidjabat HE, Harris PN, Mitic N, Walsh TR, Anderson GJ, Shafer WM, Paterson DL, Schenk G, McEwan AG, Schembri MA. 2017. Copper ions and coordination complexes as novel carbapenem adjuvants. Antimicrob Agents Chemother 62:e02280-17. doi: 10.1128/AAC.02280-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zaengle-Barone JM, Jackson AC, Besse DM, Becken B, Arshad M, Seed PC, Franz KJ. 2018. Copper influences the antibacterial outcomes of a beta-lactamase-activated prochelator against drug-resistant bacteria. ACS Infect Dis 4:1019–1029. doi: 10.1021/acsinfecdis.8b00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Totten AH, Crawford CL, Dalecki AG, Xiao L, Wolschendorf F, Atkinson TP. 2019. Differential susceptibility of Mycoplasma and Ureaplasma species to compound-enhanced copper toxicity. Front Microbiol 10:1720. doi: 10.3389/fmicb.2019.01720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dalecki AG, Haeili M, Shah S, Speer A, Niederweis M, Kutsch O, Wolschendorf F. 2015. Disulfiram and copper ions kill Mycobacterium tuberculosis in a synergistic manner. Antimicrob Agents Chemother 59:4835–4844. doi: 10.1128/AAC.00692-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Crawford CL, Dalecki AG, Narmore WT, Hoff J, Hargett AA, Renfrow MB, Zhang M, Kalubowilage M, Bossmann SH, Queern SL, Lapi SE, Hunter RN, Bao D, Augelli-Szafran CE, Kutsch O, Wolschendorf F. 2019. Pyrazolopyrimidinones, a novel class of copper-dependent bactericidal antibiotics against multi-drug resistant S. aureus. Metallomics 11:784–798. doi: 10.1039/c8mt00316e. [DOI] [PubMed] [Google Scholar]
- 178.Djoko KY, Goytia MM, Donnelly PS, Schembri MA, Shafer WM, McEwan AG. 2015. Copper(II)-bis(thiosemicarbazonato) complexes as antibacterial agents: insights into their mode of action and potential as therapeutics. Antimicrob Agents Chemother 59:6444–6453. doi: 10.1128/AAC.01289-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gullberg E, Albrecht LM, Karlsson C, Sandegren L, Andersson DI. 2014. Selection of a multidrug resistance plasmid by sublethal levels of antibiotics and heavy metals. mBio 5:e01918-14. doi: 10.1128/mBio.01918-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sutterlin S, Edquist P, Sandegren L, Adler M, Tangden T, Drobni M, Olsen B, Melhus A. 2014. Silver resistance genes are overrepresented among Escherichia coli isolates with CTX-M production. Appl Environ Microbiol 80:6863–6869. doi: 10.1128/AEM.01803-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sandegren L, Linkevicius M, Lytsy B, Melhus A, Andersson DI. 2012. Transfer of an Escherichia coli ST131 multiresistance cassette has created a Klebsiella pneumoniae-specific plasmid associated with a major nosocomial outbreak. J Antimicrob Chemother 67:74–83. doi: 10.1093/jac/dkr405. [DOI] [PubMed] [Google Scholar]
- 182.Silver S. 2003. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev 27:341–353. doi: 10.1016/S0168-6445(03)00047-0. [DOI] [PubMed] [Google Scholar]
- 183.Hobman JL, Crossman LC. 2015. Bacterial antimicrobial metal ion resistance. J Med Microbiol 64:471–497. doi: 10.1099/jmm.0.023036-0. [DOI] [PubMed] [Google Scholar]
- 184.Argudin MA, Lauzat B, Kraushaar B, Alba P, Agerso Y, Cavaco L, Butaye P, Porrero MC, Battisti A, Tenhagen BA, Fetsch A, Guerra B. 2016. Heavy metal and disinfectant resistance genes among livestock-associated methicillin-resistant Staphylococcus aureus isolates. Vet Microbiol 191:88–95. doi: 10.1016/j.vetmic.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 185.Yazdankhah S, Rudi K, Bernhoft A. 2014. Zinc and copper in animal feed—development of resistance and co-resistance to antimicrobial agents in bacteria of animal origin. Microb Ecol Health Dis 25:1598041. doi: 10.3402/mehd.v25.25862. [DOI] [PMC free article] [PubMed] [Google Scholar]