Abstract
Introduction
SARS-CoV-2 infection frequently causes neurological symptoms. Cognitive alterations are among the most frequent symptoms, and may persist beyond the acute phase of infection.
Methods
We conducted a narrative review of the literature.
Results
Hospitalised patients, and especially critically ill patients, are at greater risk of developing cognitive symptoms. Post–COVID-19 cognitive symptoms, unlike those associated with other viral illnesses, have been observed in patients with mild infection, and present some atypical features. Cognitive symptoms may last longer in COVID-19 than in other infectious processes, and more frequently affect young people. Post–COVID-19 cognitive symptoms share common features with those described in chronic fatigue syndrome, including a similar profile with affective symptoms. Brief screening tests for cognitive impairment present suboptimal diagnostic performance, and standardised criteria are needed to ensure correct diagnosis.
Post–COVID-19 cognitive impairment can have a significant impact on the patient's quality of life and functional independence, regardless of other post–COVID-19 symptoms. Currently, no specific treatments have been approved for post–COVID-19 cognitive impairment, although cognitive stimulation may be useful in some patients.
Conclusions
Post–COVID-19 cognitive symptoms are common and are often associated with other systemic symptoms. Neuropsychological evaluation may be useful for diagnosis and to quantify their severity and long-term prognosis. Detailed, and individualised assessment of cognitive impairment may enable the design of treatment plans.
Keywords: Brain fog, Cognitive symptoms, COVID-19, SARS-CoV-2
Abstract
Introducción
La infección por SARS-Cov2 con frecuencia causa síntomas neurológicos. Los síntomas cognitivos se encuentran entre los síntomas más frecuentes y pueden persistir más allá de la fase aguda de la infección.
Metodología
Revisión narrativa de la literatura.
Resultados
El riesgo de padecer síntomas cognitivos es mayor en pacientes hospitalizados, especialmente en pacientes críticos. Los síntomas cognitivos post-COVID, a diferencia de los que aparecen en otros cuadros virales, se han observado en pacientes con infección leve y presentan algunos rasgos atípicos. La duración de la sintomatología cognitiva puede ser superior a otros procesos infecciosos y afectar con mayor frecuencia a personas jóvenes. Los síntomas cognitivos post-COVID comparten rasgos comunes con los descritos en el síndrome de la fatiga crónica, incluyendo un perfil similar de síntomas afectivos asociados. Los tests rápidos de cribado de deterioro cognitivo tienen un rendimiento diagnóstico subóptimo y son necesarios criterios estandarizados para un correcto diagnóstico.
El deterioro cognitivo post-COVID puede ocasionar un impacto significativo en la calidad de vida y en la autonomía funcional del paciente, de forma independiente al resto de síntomas post-COVID. En la actualidad no existen tratamientos específicos aprobados para el deterioro cognitivo post-COVID, aunque la estimulación cognitiva puede ser útil en algunos pacientes.
Conclusiones
Los síntomas cognitivos post-COVID son frecuentes, y con asiduidad se asocian a otros síntomas sistémicos. Una evaluación neuropsicológica puede ser de utilidad para el diagnóstico y para cuantificar su severidad y pronóstico a largo plazo. Una caracterización detallada e individualizada del deterioro cognitivo permite establecer medidas de tratamiento.
Palabras clave: Niebla cerebral, Síntomas cognitivos, COVID-19
Introduction
SARS-CoV-2 infection can be associated with a wide range of neurological complications, including encephalitis, cerebrovascular events, delirium, and neuromuscular symptoms. (1) The neurological complications reported in the early months of 2020 were highly variable neurological symptoms, observed in patients who were hospitalised, mainly in intensive care units (ICU), due to severe COVID-19.
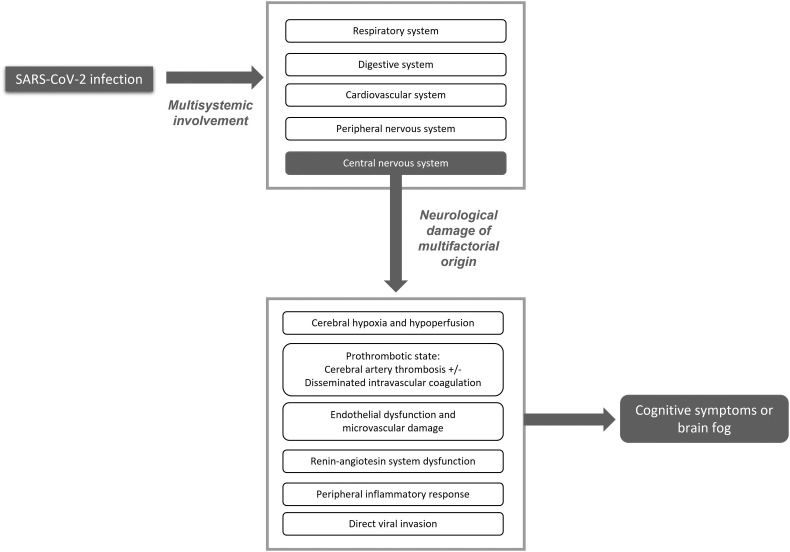
Several mechanisms of neurological damage have been proposed, including direct viral invasion of the central nervous system (CNS), and others not related with direct viral invasion such as injury of the vascular endothelium, severe peripheral inflammation, and a prothrombotic state (Fig. 1 ). (2,3) SARS-CoV-2 has been shown to invade the CNS through the olfactory mucosa (4) and olfactory nerve fibres, as well as via the haematogenous and lymphatic pathways. (3,5,6) The SARS-CoV-2 spike protein binds to the angiotensin-converting enzyme 2 (ACE2) receptor, which is abundantly expressed in neurons, astrocytes, and oligodendrocytes, enabling the virus to enter cells, inducing mitochondrial dysfunction. (7,8) Although SARS-CoV-2 viral proteins have been detected in the lower cranial nerves and brainstem neurons, their presence or absence does not seem to be correlated with symptom severity; in fact, autopsy studies of brain tissue more frequently detect inflammatory changes than presence of the virus itself. (9) Furthermore, the peripheral inflammatory activity observed in the context of SARS-CoV-2 infection translates into a marked increase in cytokine levels in the blood, causing cytokine-mediated tissue damage in several organs, including the nervous system.
Fig. 1.
SARS-CoV-2 infection causes multisystem involvement. Neurological damage results from a combination of different indirect mechanisms, rather than exclusively by direct viral infection. Patients frequently present cognitive symptoms either in isolation or combined with other neurological symptoms.
As the pandemic has progressed, neurological sequelae have been found not to be present exclusively in hospitalised patients with severe COVID-19, but also in patients with milder forms of the disease, who may also present such persistent neurological symptoms as headache, anosmia, ageusia, and cognitive, mood, and behavioural disorders. A recent study has shown that up to one-third of patients present neurological or psychiatric symptoms within 6 months of diagnosis of COVID-19. (10)
Cognitive symptoms were recognised as a typical symptom of COVID-19 later in the course of the pandemic, gaining more importance in 2021, when numerous patients began to present these sequelae. (11,12) These alterations may have an impact on functional independence, performance at work, (13) and quality of life. (14) Early and accurate diagnosis of these symptoms is therefore essential.
This review focuses on post–COVID-19 cognitive impairment, describing its characteristics, biological correlates, and treatment.
Material and methods
We performed a literature search of the MEDLINE database to locate articles published until 1 October 2021, using the following search strategy: ((SARS-Cov2) OR (COVID) OR (COVID19)) AND ((cognitive symptoms) OR (brain fog) OR (cognitive impairment)). We included original articles, case series, systematic reviews, and meta-analyses written in English, Spanish, or French, and whose abstracts could be accessed on MEDLINE. The search yielded a total of 1053 articles. After reading the titles and abstracts, we read the full texts of 154 articles that addressed the presence of cognitive symptoms during acute infection or after COVID-19, and/or the cognitive impact of SARS-CoV-2 infection on patients with a prior diagnosis of cognitive impairment of neurodegenerative origin. We subsequently performed a critical analysis of the content to restrict the number of studies included in our review. We excluded case reports and case series when other articles with a higher level of evidence provided the same information. Reviews and original articles were only included if they provided specific information on cognitive symptoms rather than generic information on these alterations within the spectrum of post–COVID-19 neurological symptoms.
Brain fog and cognitive impairment
The generic term “brain fog” has been used to refer to a series of mild cognitive symptoms of varying aetiology. This term is not exclusive to SARS-CoV-2 infection, and has previously been used in the context of other post-infectious processes and/or such other conditions as chronic fatigue syndrome. During the COVID-19 pandemic, this term has been used to describe difficulty concentrating, memory problems, and sometimes confusion, (15,16) hypersensitivity to light and sound, and tinnitus. (17) Together with fatigue, cognitive symptoms frequently persist beyond 4 weeks after SARS-CoV-2 infection. They often copresent with mood disorders such as anxiety, emotional lability, and dysphoria. Post–COVID-19 cognitive symptoms resemble those associated with chronic fatigue syndrome. (18) No correlation has been observed between sex and likelihood of developing cognitive symptoms after COVID-19; however, women do present a greater risk of developing fatigue. (16) Fatigue and affective, anxious, and depressive symptoms may influence patients’ perception of their own cognitive performance. (18,19) Presence of these symptoms may also have a negative impact on patients’ quality of life and socioeconomic and employment status.
Hospitalised patients with severe COVID-19
Most patients with severe COVID-19 present severe respiratory distress. The presence of cognitive impairment in patients with respiratory distress of all causes is well described in the literature. (20,21) The prevalence of cognitive impairment after respiratory distress at the time of hospital discharge ranges from 70% to 100%, and may reach 80% at 1 year and 20% at 5 years; and it is also associated with functional limitations. Respiratory distress may have a wide range of causes, and the pathogenic factors involved in cognitive impairment may vary depending on the aetiology, with the most relevant factors being hypoxia, inflammation, delirium, hypoperfusion, and blood–brain barrier disruption. (21) In these cases, special emphasis should be placed on modifiable risk factors, seeking to achieve good control with sedatives and mechanical ventilation, to prevent haemodynamic instability, and to provide early treatment for delirium. In the case of COVID-19, delirium may appear during the acute phase in up to 30% of hospitalised patients, and is more frequent in critically ill patients. (22) The higher frequency of delirium during acute infection may be linked to direct viral invasion of the CNS, inflammatory changes, systemic hypoperfusion, mechanical ventilation and prolonged immobilisation requiring sedoanalgesia, and greater social isolation. (23) It is well known that the duration of delirium in patients with respiratory distress or shock is a major factor in the subsequent development of long-lasting cognitive symptoms. (24)
Most of the published case series of cognitive impairment after COVID-19 describe patients requiring hospitalisation or even ICU admission due to severe COVID-19. (25) These series use different cognitive assessment tools, ranging from surveys to telephone assessments, which makes it difficult to determine the true prevalence of cognitive impairment after COVID-19 in this population. The time of symptom assessment also varies greatly between studies, with some case series performing these evaluations during the acute phase, when patients were admitted to hospital. In any case, cognitive symptoms are frequent in the case series published, even affecting 100% of patients in some series. (25) Patients requiring oxygen therapy or ICU admission have been reported to present more severe impairment. (26) Up to 69% of patients with COVID-19 admitted to the ICU present agitation at the time of extubation, and 65% of these present confusional symptoms after extubation and withdrawal of neuromuscular blocking agents; this rate is higher than those reported for patients with acute respiratory distress of other causes. (27) A recent study analysing the electronic medical histories of a large number of individuals confirms that the frequency of dementia within 6 months of acute COVID-19 is higher compared toother respiratory infections, both in hospitalised and non-hospitalised patients. (10) In that study, 2.6% of patients were diagnosed with dementia within 6 months of acute COVID-19, rising to 4.7% in those presenting COVID-19–related encephalopathy.
Patients admitted to the ICU due to acute respiratory distress are more likely to present post-traumatic stress syndrome, anxiety, and depressive symptoms (34%, 34%, and 29%, respectively); these symptoms, in turn, have a direct impact on cognitive function. (28,29) Psychotic symptoms seem not to be more prevalent in patients with SARS-CoV-2 infection than in patients with other severe viral infections. In summary, the prevalence of cognitive impairment is high among patients with severe COVID-19 and negatively affects their functional status and job performance. In fact, up to one-third of patients with COVID-19 requiring mechanical ventilation at the ICU have not returned to active work at 1 year after admission. (24)
Patients with mild COVID-19
In 80%–85% of cases, SARS-CoV-2 infection is asymptomatic or presents with mild flu-like symptoms. Although hospitalised patients, and especially those admitted to the ICU, are at greater risk of presenting cognitive symptoms, they may also present in patients with mild COVID-19 and even in initially asymptomatic individuals. (16)
In recent months, cases have been reported of persistent executive dysfunction and, to a lesser extent, memory dysfunction (for at least 3 months after acute infection) in young patients with mild COVID-19 not requiring hospital admission and presenting no other comorbidities. (30,31) Several studies have compared post–COVID-19 cognitive symptoms with other post-viral syndromes (eg, after Epstein-Barr virus infection). SARS-CoV-2 infection presents some peculiar characteristics, since the presence of cognitive symptoms does not have a clear temporal relationship with such other symptoms as affective disorders and fatigue, or with their severity. In patients with SARS-CoV-2 infection, cognitive symptoms may persist for longer than the remaining symptoms, and their progression is independent. (32)
A recent study including data from over 3700 online surveys evaluated the prevalence of post–COVID-19 cognitive symptoms and their progression. The survey was distributed to young patients aged 30–60 years, half of whom had presented mild COVID-19. Brain fog was reported by 85.1% of participants, starting the first week after acute infection and peaking at 3 months. Symptoms persisted for 7 months in 55.5% of participants. A total of 86.2% reported poorer work performance. (33) Likewise, a population-based study including cognitively healthy adults older than 40 years reported poorer scores on the Montreal Cognitive Assessment (MoCA) after mild COVID-19. (34)
In summary, unlike other post-infectious syndromes, post–COVID-19 cognitive symptoms are more frequent in patients with history of mild COVID-19, and may persist for long periods of time, negatively impacting functional status and work performance.
Profile of post–COVID-19 cognitive impairment
The available evidence on post–COVID-19 cognitive impairment is heterogeneous due to differences in inclusion criteria, time and methodology of assessment, and whether a control group was included (many studies did not include a control group). A systematic review of 33 studies analysing the cognitive and psychiatric sequelae of COVID-19 concluded that the neuropsychological evaluation of these patients should be standardised and emphasises the need for longitudinal studies. (35) The most frequent profile of post–COVID-19 cognitive impairment involves executive function and may be detected from 4 weeks after diagnosis (subacute or convalescent phase) and even after 12 weeks (persistent symptomatology). (36) Most published studies use cognitive screening tests (eg, the MoCA or Mini–Mental State Examination), administered either in person (37., 38., 39.) or by telephone; (40) and some include the Continuous Performance Test (a computerised assessment of sustained attention). (41) The studies included in the review used both objective assessments (26) and self-reported data. (42) These studies have identified attention and planning difficulties, decreased information processing speed, and deficits in short-term memory, abstraction, and orientation; (32) other alterations include anomia and difficulty understanding spoken and written language, with the latter problem being more frequent among patients who required ICU admission. (43) Language problems may negatively impact patients’ social interaction and return to work. Studies conducting massive online surveys also report executive function as the most severely impaired cognitive domain in these patients, although up to 50.5% of patients report memory problems at 7 months after acute infection. (33) Impaired visuospatial function is also reported in these patients, although the evidence is less robust (Table 1 ). (36) One limitation of some of these studies is that they administer cognitive screening tests to young patients, although no normative data are available for that age range. (32)
Table 1.
Profile of cognitive, mood, and behavioural symptoms in patients with post–COVID-19 cognitive impairment (the most frequent symptoms are marked with asterisks).
| Cognitive symptoms |
| Global cognitive function Concentration* Memory* Working memory* Verbal episodic memory Visual memory Executive function* Sustained attention* Information processing speed* Visuospatial processing Language |
| Affective symptoms |
| Anxiety* Depression* Post-traumatic stress disorder |
| Behavioural symptoms |
| Irritability Sleep disorders |
| Other symptoms |
| Fatigue* |
In addition to cognitive symptoms, COVID-19 survivors have been found to present significantly higher rates of psychiatric sequelae. (10) That same study reports that history of mental disorders is a risk factor for COVID-19 factors (1.65 times greater for all age groups and both sexes), probably linked to lifestyles and independently of known physicalrisk. (10) The risk of developing depression and cognitive impairment has been linked to poor functional status (as measured with the Post–COVID-19 Functional Status Scale) at 6 weeks from hospital discharge after COVID-19. (13)
Correlation between cognitive symptoms and biomarkers
Several studies have quantified biomarkers of neurological damage in patients with COVID-19. Some authors have found that patients with COVID-19 admitted to the ICU show elevated levels of glial fibrillary acidic protein and neurofilament light protein (NfL) in the blood, (44,45) and that high NfL levels are associated with poorer prognosis. (44,46,47) A recent study including 142 hospitalised patients found that even patients with no neurological symptoms presented high NfL levels, and that high levels were associated with poor outcomes. (47) Another study reported elevated plasma levels of IL-4 and markers of neuronal damage in neuronal-enriched extracellular vesicles in patients with neurological symptoms. (48) However, few studies have analysed biomarkers in patients undergoing cognitive assessment. Another recent study including 29 patients with mild COVID-19 found no association between cognitive function and plasma concentrations of different cytokines, although patients with COVID-19 did present executive dysfunction. (41)
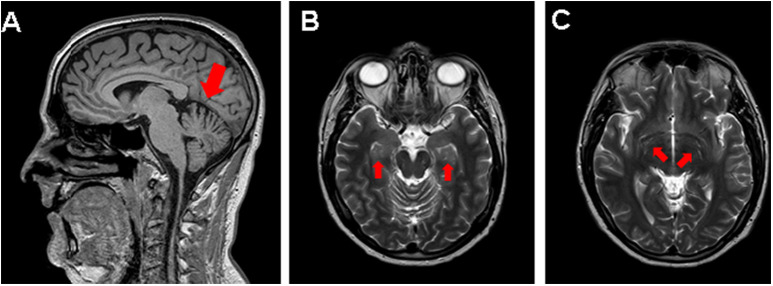
Few studies include neuroimaging assessment of patients with post–COVID-19 cognitive impairment. MRI scans of COVID-19 patients after hospital discharge have detected such findings as increased signal intensity and increased mean diffusivity in the thalamus, as well as periventricular white matter hyperintensity. (38) In our experience, dilation of perivascular spaces in the hippocampus and basal ganglia is occasionally observed in these patients (Fig. 2 ). These findings may correspond to perivascular inflammation in histopathological studies. (49)
Fig. 2.
MRI scan of a young patient with post–COVID-19 cognitive impairment. A) Mild loss of cerebellar volume. B) and C) Prominent perivascular spaces in the bilateral hippocampus (B) and basal ganglia (C).
Brain FDG-PET studies of patients with COVID-19 and cognitive symptoms have revealed a pattern of hypometabolism in the olfactory gyrus, limbic structures, hippocampus, brainstem, and cerebellum. (50) In a recent study including 15 hospitalised patients with neurological symptoms, 10 presented neuroimaging alterations, with a pattern of predominantly frontoparietal hypometabolism, which was correlated with performance in the MoCA. (51) In a 6-month longitudinal study, neuroimaging findings improved over the follow-up period, although some of these patients presented residual hypometabolism. (51)
Treatment
Establishing the most appropriate treatment requires correct identification of cognitive symptoms and evaluation of their severity. Experience with medium- and long-term management of post–COVID-19 cognitive symptoms is very limited. The recommendations currently available are the same as those issued for other entities associated with similar profiles of cognitive impairment, such as chronic fatigue syndrome. Multidisciplinary management of the non-pharmacological treatment seems reasonable for these patients. Treatment should be tailored to each patient’s needs; however, when cognitive alterations cause significant functional impact, treatment may include cognitive stimulation, language rehabilitation (particularly in patients undergoing prolonged orotracheal intubation), and occupational therapy combined with physical therapy or graded exercise therapy. (52) Gradual incorporation or adaptation to work should also be considered in some cases, as this may improve the work integration of these patients. To date, no pharmacological treatment has been approved for post–COVID-19 cognitive symptoms. Likewise, no studies have specifically addressed the use of micronutrients or dietary supplements, although a recent study suggests that the natural flavonoid luteolin may be beneficial due to its anti-inflammatory and neuroprotective properties. (53) The impact of vaccination against COVID-19 on such long-term post–COVID-19 symptoms as cognitive alterations is still unknown. Likewise, pharmacological treatment of affective symptoms should be complemented with non-pharmacological treatments, which may provide significant benefits for cognition, functional status, and quality of life. Management of these patients may occasionally require the participation of clinical psychologists and psychiatrists.
Impact of the COVID-19 pandemic on patients with dementia
Individuals with such neurodegenerative dementias as Alzheimer disease are at particularly high risk of developing severe COVID-19. Mortality in this patient group is considerably higher, in part due to their older age, presence of comorbidities, and ineligibility for ventilatory support due to their functional status prior to SARS-CoV-2 infection. COVID-19 survivors have been observed to present cognitive and functional worsening, with severity varying according to the severity of the infection. Greater apathy, agitation, and aberrant motor behaviour have been observed in individuals with mild cognitive impairment and Alzheimer's disease dementia in the context of the COVID-19 pandemic, regardless of whether they actually presented SARS-CoV-2 infection. (54) Even individuals who were not infected with the virus presented cognitive and functional decline, probably due to social isolation and the inability to attend centres providing cognitive stimulation. (25,55) This has had a major negative impact on these patients’ global quality of life.
SARS-CoV-2 infection as a possible risk factor for neurodegenerative disease
In addition to causing cognitive impairment during the acute and subacute phases, or exacerbating existing cognitive impairment, it has been suggested that SARS-CoV-2 infection may be a risk factor for the development of neurodegenerative diseases in healthy individuals. (56)
The cerebral hypoperfusion observed in the context of SARS-CoV-2 infection may accelerate amyloid-β aggregation and deposition, (3) and the aggregation and abnormal folding of such other proteins as tau, TDP-43, and α-synuclein. (7) It has also been suggested that the virus may induce functional inhibition of acetylcholine receptors and promote dysregulation of the excitatory-inhibitory balance, which may be explained by the expression of ACE2 in glutamatergic and GABAergic neurons. (8) It has been postulated that retrograde or anterograde synaptic and axonal transport of SARS-CoV-2 may result in slow, diffuse spread throughout the entire CNS, months or even years after infection. (8) However, insufficient data are available to confirm this hypothesis. Medium- and long-term follow-up of individuals with history of SARS-CoV-2 infection should include longitudinal neuropsychological assessments and analysis of multimodal biomarkers, which would help us to determine whether the infection increases the risk of developing neurodegenerative diseases.
Conclusions and future lines of research
The current evidence suggests that cognitive sequelae are frequent after COVID-19, even in mild cases not requiring hospitalisation or ICU admission. These cognitive alterations include executive dysfunction and, to a lesser extent, memory impairment, which progressively improves in most cases. No specific treatment has been established for these patients, although tailored cognitive stimulation is recommended when symptoms cause functional limitations.
Long-term follow-up studies should be conducted to determine the prevalence of post–COVID-19 cognitive impairment, its impact on public healthcare systems, and the resources needed to treat these patients. This information will enable us to quantify the global impact of these sequelae from a clinical, economic, and work-related perspective. (57)
Several international studies currently underway aim to provide large-scale clinical and biomarker data. Cooperation will enable high-quality research, involving scientists from multiple fields. (58) Some of these studies, such as those led by Yale University (COVID Mind Study) and the American Association of Alzheimer Disease, include imaging and biofluid markers. This information will be crucial in determining the extent of post–SARS-CoV-2 cognitive impairment and its prognosis in the long term.
Acknowledgements
Miren Altuna is the recipient of a Río Hortega grant from the Carlos III Institute (CM19/00066).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neurop.2021.10.005.
Appendix A. Supplementary data
Supplementary material
References
- 1.Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol [Internet] 2020 Aug 1;77(8):1018–1027. doi: 10.1001/jamaneurol.2020.2065. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon T. Neurological infection with SARS-CoV-2 — the story so far. Nat Rev Neurol [Internet] 2021;17(2):65–66. doi: 10.1038/s41582-020-00453-w. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miners S., Kehoe P.G., Love S. Cognitive impact of COVID-19: looking beyond the short term. Alzheimers Res Ther. 2020 Dec;12(1):170. doi: 10.1186/s13195-020-00744-w. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021 Feb;24(2):168–175. doi: 10.1038/s41593-020-00758-5. Available from. [DOI] [PubMed] [Google Scholar]
- 5.Chen X., Laurent S., Onur O.A., Kleineberg N.N., Fink G.R., Schweitzer F., et al. A systematic review of neurological symptoms and complications of COVID-19. J Neurol. 2021 Feb;268(2):392–402. doi: 10.1007/s00415-020-10067-3. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Sarraj S., Troakes C., Hanley B., Osborn M., Richardson M.P., Hotopf M., et al. Invited review: the spectrum of neuropathology in COVID-19. Neuropathol Appl Neurobiol. 2021 Feb;47(1):3–16. doi: 10.1111/nan.12667. Available from. [DOI] [PubMed] [Google Scholar]
- 7.Fotuhi M., Mian A., Meysami S., Raji C.A. Neurobiology of COVID-19. J Alzheimers Dis. 2020;76(1):3–19. doi: 10.3233/JAD-200581. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang F., Kream R.M., Stefano G.B. Long-term respiratory and neurological sequelae of COVID-19. Med Sci Monit Int Med J Exp Clin Res. 2020 Nov;26:e928996. doi: 10.12659/MSM.928996. 10.12659/MSM.928996 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020 Nov;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. The Lancet Psychiatry. 2021 May;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lleó A., Alcolea D. The cognitive aftermath of COVID-19. Brain Commun [Internet] 2020 Jul 1;2(2) doi: 10.1093/braincomms/fcaa072. Available from. [DOI] [Google Scholar]
- 12.Ritchie K., Chan D., Watermeyer T. The cognitive consequences of the COVID-19 epidemic: collateral damage? Brain Commun. 2020;2(2):fcaa069. doi: 10.1093/braincomms/fcaa069. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Graaf M.A., Antoni M.L., Ter Kuile M.M., Arbous M.S., Duinisveld A.J.F., Feltkamp M.C.W., et al. Short-term outpatient follow-up of COVID-19 patients: a multidisciplinary approach. EClinicalMedicine. 2021 Feb;32:100731. doi: 10.1016/j.eclinm.2021.100731. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend L., Dowds J., O’Brien K., Sheill G., Dyer A.H., O’Kelly B., et al. Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc. 2021 Jun;18(6):997–1003. doi: 10.1513/AnnalsATS.202009-1175OC. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefano G.B., Ptacek R., Ptackova H., Martin A., Kream R.M. Selective neuronal mitochondrial targeting in SARS-CoV-2 infection affects cognitive processes to induce “brain fog” and results in behavioral changes that favor viral survival. Med Sci Monit: Int Med J Exp Clin Res. 2021;Vol. 27:e930886. doi: 10.12659/MSM.930886. 10.12659/MSM.930886 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg M., Maralakunte M., Garg S., Dhooria S., Sehgal I., Bhalla A.S., et al. The conundrum of “long-COVID-19”: a narrative review. Int J Gen Med. 2021;14:2491–2506. doi: 10.2147/IJGM.S316708. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moghimi N., Di Napoli M., Biller J., Siegler J.E., Shekhar R., McCullough L.D., et al. The neurological manifestations of post-acute sequelae of SARS-CoV-2 infection. Curr Neurol Neurosci Rep. 2021 Jun;21(9):44. doi: 10.1007/s11910-021-01130-1. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham E.L., Clark J.R., Orban Z.S., Lim P.H., Szymanski A.L., Taylor C., et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann Clin Transl Neurol. 2021 May;8(5):1073–1085. doi: 10.1002/acn3.51350. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrigues E., Janvier P., Kherabi Y., Le Bot A., Hamon A., Gouze H., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;Vol. 81:e4–e6. doi: 10.1016/j.jinf.2020.08.029. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herridge M.S., Moss M., Hough C.L., Hopkins R.O., Rice T.W., Bienvenu O.J., et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016 May;42(5):725–738. doi: 10.1007/s00134-016-4321-8. Available from. [DOI] [PubMed] [Google Scholar]
- 21.Sasannejad C., Ely E.W., Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019 Nov;23(1):352. doi: 10.1186/s13054-019-2626-z. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura Z.M., Nash R.P., Laughon S.L., Rosenstein D.L. Neuropsychiatric complications of COVID-19. Curr Psychiatry Rep. 2021 Mar;23(5):25. doi: 10.1007/s11920-021-01237-9. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotfis K., Williams Roberson S., Wilson J.E., Dabrowski W., Pun B.T., Ely E.W. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care. 2020 Apr;24(1):176. doi: 10.1186/s13054-020-02882-x. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosey M.M., Needham D.M. Survivorship after COVID-19 ICU stay. Nat Rev Dis Prim. 2020 Jul;6(1):60. doi: 10.1038/s41572-020-0201-1. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alonso-Lana S., Marquié M., Ruiz A., Boada M. Cognitive and neuropsychiatric manifestations of COVID-19 and effects on elderly individuals with dementia. Front Aging Neurosci. 2020;12:588872. doi: 10.3389/fnagi.2020.588872. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almeria M., Cejudo J.C., Sotoca J., Deus J., Krupinski J. Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain, Behav Immun - Heal. 2020 Dec;9:100163. doi: 10.1016/j.bbih.2020.100163. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., et al. Neurologic features in severe SARS-CoV-2 infection. New Engl J Med. 2020;Vol. 382:2268–2270. doi: 10.1056/NEJMc2008597. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikayin S., Rabiee A., Hashem M.D., Huang M., Bienvenu O.J., Turnbull A.E., et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2016;43:23–29. doi: 10.1016/j.genhosppsych.2016.08.005. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabiee A., Nikayin S., Hashem M.D., Huang M., Dinglas V.D., Bienvenu O.J., et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016 Sep;44(9):1744–1753. doi: 10.1097/CCM.0000000000001811. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hellmuth J., Barnett T.A., Asken B.M., Kelly J.D., Torres L., Stephens M.L., et al. Persistent COVID-19-associated neurocognitive symptoms in non-hospitalized patients. J Neurovirol. 2021 Feb;27(1):191–195. doi: 10.1007/s13365-021-00954-4. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blomberg B., Mohn K.G.-I., Brokstad K.A., Zhou F., Linchausen D.W., Hansen B.-A., et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021 Sep;27(9):1607–1613. doi: 10.1038/s41591-021-01433-3. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woo M.S., Malsy J., Pöttgen J., Seddiq Zai S., Ufer F., Hadjilaou A., et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020;2(2):fcaa205. doi: 10.1093/braincomms/fcaa205. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis H.E., Assaf G.S., McCorkell L., Wei H., Low R.J., Re’em Y., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021 Jul;101019 doi: 10.1016/j.eclinm.2021.101019. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Brutto O.H., Wu S., Mera R.M., Costa A.F., Recalde B.Y., Issa N.P. Cognitive decline among individuals with history of mild symptomatic SARS-CoV-2 infection: a longitudinal prospective study nested to a population cohort. Eur J Neurol. 2021 Oct;28(10):3245–3253. doi: 10.1111/ene.14775. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanderlind W.M., Rabinovitz B.B., Miao I.Y., Oberlin L.E., Bueno-Castellano C., Fridman C., et al. A systematic review of neuropsychological and psychiatric sequalae of COVID-19: implications for treatment. Curr Opin Psychiatry. 2021 Jul;34(4):420–433. doi: 10.1097/YCO.0000000000000713. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daroische R., Hemminghyth M.S., Eilertsen T.H., Breitve M.H., Chwiszczuk L.J. Cognitive impairment after COVID-19—a review on objective test data [Internet] Front Neurol. 2021;Vol. 12:1238. doi: 10.3389/fneur.2021.699582. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pistarini C., Fiabane E., Houdayer E., Vassallo C., Manera M.R., Alemanno F. Cognitive and emotional disturbances due to COVID-19: an exploratory study in the rehabilitation setting [Internet] Front Neurol. 2021;Vol. 12:500. doi: 10.3389/fneur.2021.643646. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raman B., Cassar M.P., Tunnicliffe E.M., Filippini N., Griffanti L., Alfaro-Almagro F., et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021 Jan;31:100683. doi: 10.1016/j.eclinm.2020.100683. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Lorenzo R., Conte C., Lanzani C., Benedetti F., Roveri L., Mazza M.G., et al. Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS One [Internet] 2020 Oct 14;15(10):e0239570. doi: 10.1371/journal.pone.0239570. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baig A.M. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci Therap. 2020;Vol. 26:499–501. doi: 10.1111/cns.13372. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou H., Lu S., Chen J., Wei N., Wang D., Lyu H., et al. The landscape of cognitive function in recovered COVID-19 patients. J Psychiatr Res. 2020 Oct;129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weerahandi H., Hochman K.A., Simon E., Blaum C., Chodosh J., Duan E., et al. Post-discharge health status and symptoms in patients with severe COVID-19. J Gen Intern Med [Internet] 2021;36(3):738–745. doi: 10.1007/s11606-020-06338-4. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.RA E. Potential for cognitive communication impairment in COVID-19 survivors: a call to action for speech-language pathologists. Am J Speech-Language Pathol [Internet] 2020 Nov 12;29(4):1821–1832. doi: 10.1044/2020_AJSLP-20-00147. Available from. [DOI] [PubMed] [Google Scholar]
- 44.Sutter R., Hert L., De Marchis G.M., Twerenbold R., Kappos L., Naegelin Y., et al. Serum neurofilament light chain levels in the intensive care unit: comparison between severely ill patients with and without coronavirus disease 2019. Ann Neurol. 2021 Mar;89(3):610–616. doi: 10.1002/ana.26004. Available from. [DOI] [PubMed] [Google Scholar]
- 45.Cooper J., Stukas S., Hoiland R.L., Fergusson N.A., Thiara S., Foster D., et al. Quantification of neurological blood-based biomarkers in critically ill patients with coronavirus disease 2019. Crit Care Explor. 2020 Oct;2(10):e0238. doi: 10.1097/CCE.0000000000000238. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aamodt A.H., Høgestøl E.A., Popperud T.H., Holter J.C., Dyrhol-Riise A.M., Tonby K., et al. Blood neurofilament light concentration at admittance: a potential prognostic marker in COVID-19. J Neurol. 2021 Mar:1–10. doi: 10.1007/s00415-021-10517-6. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prudencio M., Erben Y., Marquez C.P., Jansen-West K.R., Franco-Mesa C., Heckman M.G., et al. Serum neurofilament light protein correlates with unfavorable clinical outcomes in hospitalized patients with COVID-19. Sci Transl Med. 2021 Jul;13(602) doi: 10.1126/scitranslmed.abi7643. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun B., Tang N., Peluso M.J., Iyer N.S., Torres L., Donatelli J.L., et al. Characterization and biomarker analyses of post-COVID-19 complications and neurological manifestations. Cells. 2021 Feb;10(2) doi: 10.3390/cells10020386. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee M.-H., Perl D.P., Nair G., Li W., Maric D., Murray H., et al. Microvascular injury in the brains of patients with covid-19. N Engl J Med [Internet] 2020 Dec 30;384(5):481–483. doi: 10.1056/NEJMc2033369. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guedj E., Million M., Dudouet P., Tissot-Dupont H., Bregeon F., Cammilleri S., et al. (18)F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Eur J Nucl Med Mol Imaging. 2021 Feb;48(2):592–595. doi: 10.1007/s00259-020-04973-x. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blazhenets G., Schroeter N., Bormann T., Thurow J., Wagner D., Frings L., et al. Slow but evident recovery from neocortical dysfunction and cognitive impairment in a series of chronic COVID-19 patients. J Nucl Med. 2021 Jul;62(7):910–915. doi: 10.2967/jnumed.121.262128. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Humphreys H., Kilby L., Kudiersky N., Copeland R. Long COVID and the role of physical activity: a qualitative study. BMJ Open. 2021 Mar;11(3):e047632. doi: 10.1136/bmjopen-2020-047632. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theoharides T.C., Cholevas C., Polyzoidis K., Politis A. Long-COVID syndrome-associated brain fog and chemofog: luteolin to the rescue. Biofactors. 2021 Mar;47(2):232–241. doi: 10.1002/biof.1726. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lara B., Carnes A., Dakterzada F., Benitez I., Piñol-Ripoll G. Neuropsychiatric symptoms and quality of life in Spanish patients with Alzheimer’s disease during the COVID-19 lockdown. Eur J Neurol. 2020 Sep;27(9):1744–1747. doi: 10.1111/ene.14339. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iodice F., Cassano V., Rossini P.M. Direct and indirect neurological, cognitive, and behavioral effects of COVID-19 on the healthy elderly, mild-cognitive-impairment, and Alzheimer’s disease populations. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2021 Feb;42(2):455–465. doi: 10.1007/s10072-020-04902-8. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heneka M.T., Golenbock D., Latz E., Morgan D., Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res Ther. 2020 Jun;12(1):69. doi: 10.1186/s13195-020-00640-3. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carod-Artal F.J. Post-COVID-19 syndrome: epidemiology, diagnostic criteria and pathogenic mechanisms involved. Rev Neurol. 2021 Jun;72(11):384–396. doi: 10.33588/rn.7211.2021230. 10.33588/rn.7211.2021230 Available from. [DOI] [PubMed] [Google Scholar]
- 58.Hall P.A., Sheeran P., Fong G.T., Cheah C.S.L., Oremus M., Liu-Ambrose T., et al. Biobehavioral aspects of the COVID-19 pandemic: a review. Psychosom Med. 2021 May;83(4):309–321. doi: 10.1097/PSY.0000000000000932. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material