Abstract
Pain is a multidimensional experience with sensory-discriminative, affective-motivational, and cognitive-evaluative components. Pain aversiveness is one principal cause of suffering for patients with chronic pain, motivating research and drug development efforts to investigate and modulate neural activity in the brain’s circuits encoding pain unpleasantness. Here, we review progress in understanding the organization of emotion, motivation, cognition, and descending modulation circuits for pain perception. We describe the molecularly defined neuron types that collectively shape pain multidimensionality and its aversive quality. We also review how pharmacological, stimulation, neurofeedback, surgical, and cognitive-behavioral interventions alter activity in these circuits to relieve chronic pain.
INTRODUCTION
Chronic pain conditions are leading causes of disability and suffering
Chronic pain affects about 20% of the human population worldwide (1). Although chronic pain conditions do not directly cause death, they are major sources of disability and suffering. The Global Burden of Disease Study 2019 revealed that chronic low back pain was the single greatest cause of years lived with disability (YLDs) worldwide and that several other chronic pain conditions contribute as major sources of YLDs, including neck pain, migraine, osteoarthritis, other musculoskeletal disorders, and medication overuse headache (2, 3). Furthermore, for patients affected by intractable conditions, the emotional burden associated with the prospect of living with daily pain and suffering can lead to mental disorders (4) and even suicide (5). In fact, chronic pain is considered both a symptom and a primary disease that brings about other illnesses such as depression (6, 7). Because of this immense medical, economic, and social burden, achieving a better understanding of pain biology to develop targeted, novel, safe, and effective treatments has become a worldwide priority.
Pain mechanisms and treatment: peripheral divergence and central convergence
Most research and drug development efforts to discover effective analgesics focus on peripheral nervous system (PNS) and spinal mechanisms and targets. This strategy is motivated by the relative simplicity of this approach, given the pharmacological challenges associated with efficiently engaging brain targets without generating side effects, and the early description of a PNS cell type dedicated to generating pain, the primary afferent nociceptor (8). Successful identification of molecules either selectively expressed by nociceptors or that influence their function has been the main driver of analgesic drug development in recent decades (9), such as for ion channel transducers of the transient receptor potential channel family (TRP channels) (10, 11). The recent resolution of dorsal root ganglion (DRG) neuron transcriptomes by RNA sequencing (12–16) and the discovery of additional potential drug targets, including in other DRG neuron types compared to nociceptors (for example, mechanosensory DRG neurons), as suggested by the role of the ion channel Piezo2 in mechanical allodynia (pain in response to light touch) (17, 18), bring hope for the development of additional pain treatments targeting the PNS.
A complementary approach aims to identify analgesic targets that could directly act on the main concern of individuals living with pain—pain unpleasantness, suffering, and loss of control—by leveraging the latest knowledge of pain brain circuits. Indeed, peripheral nociception’s cellular and molecular mechanisms are diverse and complex, corresponding to the function of the PNS to precisely detect, for each individual organ, a multitude of threatening environmental stimuli and/or internal dysfunctions. Notably, RNA sequencing studies also revealed dozens of DRG neuron types capable of generating pain (12–16); these studies and others have found that the mechanisms of function and molecular repertoires of these cells are dynamic and evolve considerably in an injury- and disease-specific manner as chronic pain develops. Considering a few common types of chronic pain conditions such as low back pain, osteoarthritic pain, migraine, cancer pain, or neuropathic pain (which on its own represents a broadly diverse group of conditions with diverse symptoms such as spontaneous pain and allodynia) illustrates that, for each condition, unique peripheral biological processes engage one or several distinct classes of molecularly defined primary afferent neurons to cause pain. Thus, the treatment of certain pain types by targeting nociceptors could prove exceptionally challenging, with each pain condition requiring specific research and drug development efforts, and the difficulty to faithfully model in animals some of the most prevalent human chronic pain conditions such as low back pain. Further complicating the targeting of peripheral biological processes for individual pain conditions is the growing recognition that these conditions frequently co-occur. This phenomenon, referred to as chronic overlapping pain conditions (COPCs), includes painful conditions such as temporomandibular disorder (TMD), fibromyalgia (FM), irritable bowel syndrome (IBS), vulvodynia, myalgic encephalomyelitis/chronic fatigue syndrome, interstitial cystitis/painful bladder syndrome, endometriosis, chronic tension-type headache, migraine headache, and chronic low back pain (19, 20). Therefore, overcoming the emergent peripheral divergence of chronic pain mechanisms and COPCs represents an exciting challenge for the pain field (Fig. 1).
Fig. 1. Peripheral divergence and central convergence in pain mechanisms.
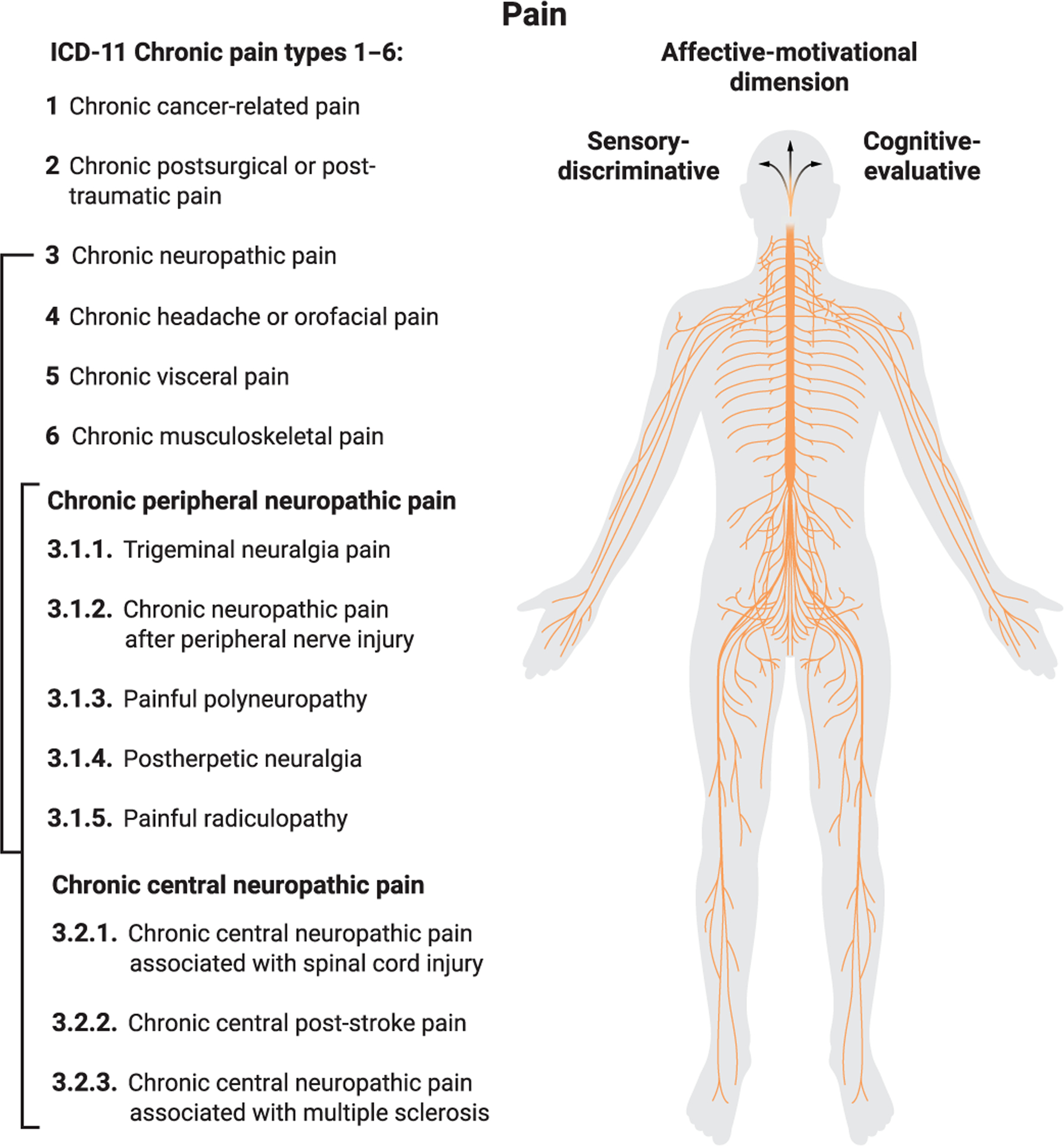
The recent International Classification of Diseases (ICD-11) adopted by the World Health Organization describes chronic pain both as a primary disease and as a symptom of other illnesses, and divides it into six main categories. This figure illustrates the multitude of pain types (using neuropathic pain subtypes as an example) that can originate from various organs and tissues of the human body. In each case, nociception is initiated through a variety of complex cellular and molecular mechanisms. Acting on the common brain mechanisms that generate pain unpleasantness raises the possibility of treating chronic pain suffering across all pain categories at once.
In contrast, after neurons of the trigeminal and spinal anterior/dorsal horn (DH) across all segmental levels process and transmit these diverse peripheral nociceptive signals to the brain, the brain’s emotional circuits generate the unpleasant quality of pain across acute and chronic pain types, including COPCs. Thus, this convergent mechanistic organization of pain brain circuits, combined with the development of preclinical assays to interrogate the affective-motivational dimension of pain, provides an opportunity to develop treatments capable of limiting pain suffering and improving the quality of life of broad patient populations, regardless of their primary condition. In this review, we discuss the neural circuits that generate the emotional responses and negative affect during pain perception, and the therapeutic approaches that target these circuits to relieve pain suffering.
THE NEURAL BASIS OF PAIN
Pain multidimensional perceptions and behaviors
Pain is both a sensory and emotional experience. Philosophers have long debated how pain relates to the perception of noxious stimuli. Some argued that pain is the representation of a noxious object/event (representationalist approach, as for vision when we see an object), whereas others characterized pain as a feeling or experience with subjective properties (qualia) that are not necessarily related to that object/event (as is the case for referred pain) (21, 22).
To reconcile these views, pain can be described as a complex multidimensional experience that includes sensory-discriminative, affective-motivational, and cognitive-evaluative components (23, 24). Pain multidimensionality integrates (i) the somatosensory perception of the noxious object/event’s features (such as location, temperature, and pressure), (ii) the encoding, within emotional and motivational circuits, of negative affect and the drive to halt the unpleasant percept, and (iii) an evaluation and modulation of pain experience by cognitive circuits. All three components are necessary to optimally select actions that limit exposure to noxious stimuli and pain experience.
As previously discussed for the field of emotions (4, 25, 26), understanding and treating pain affect require operational definitions that enable mechanistic studies. Pain includes both pre-cognitive physiological and behavioral responses (for example, withdrawal reflex and increase in heart and breathing rates) and cognitive processing of nociceptive information that leads to pain perception and affect; both are important for people living with chronic pain and can be defined and studied in animal models of pain (Fig. 2). First, primary afferent nociceptors [and, in the case of allodynia, non-nociceptive afferents (27)] engage motor and autonomic spinal/brainstem circuits to produce fast reflex responses, including withdrawal reflexes (Fig. 2A) (28). These stereotyped nocifensive responses, which persist in decerebrated animals (29, 30), limit exposure to noxious stimuli and injury while nociceptive information is transmitted to and processed in the forebrain (31–33). The multidimensional pain perception is then generated and enables the selection of more complex adaptive behaviors. Specific behaviors are chosen from a panoply of possible nocifensive responses based on the features of the noxious event (sensory-discriminative component) and the expectation—derived from recalling previous experiences and an understanding of the context that led to and accompanies pain perception—that this action is the most likely to relieve pain unpleasantness and promote positive outcomes and survival (for example, attending, cooling down and putting a bandage on the affected body part in response to a mild burn injury; Fig. 2A). During this process, changes in pain perception and its context are continuously monitored and evaluated (cognitive-evaluative dimension). If the selected nocifensive behavior fails to relieve pain unpleasantness, another nocifensive behavior is selected (for example, planning a doctor visit to treat burn pain; Fig. 2A). In fact, in high-order species, conflicting needs can lead individuals not to engage in adaptive behaviors and instead to endure pain, when considering this action beneficial to achieve superiorly important or longer-term goals, at least when the pain condition is perceived as benign (for example, deciding not to go to the doctor and instead prioritizing participation in a work activity).
Fig. 2. Categorization of reflexive versus affective-motivational nocifensive behaviors to interrogate pain affect in rodents.
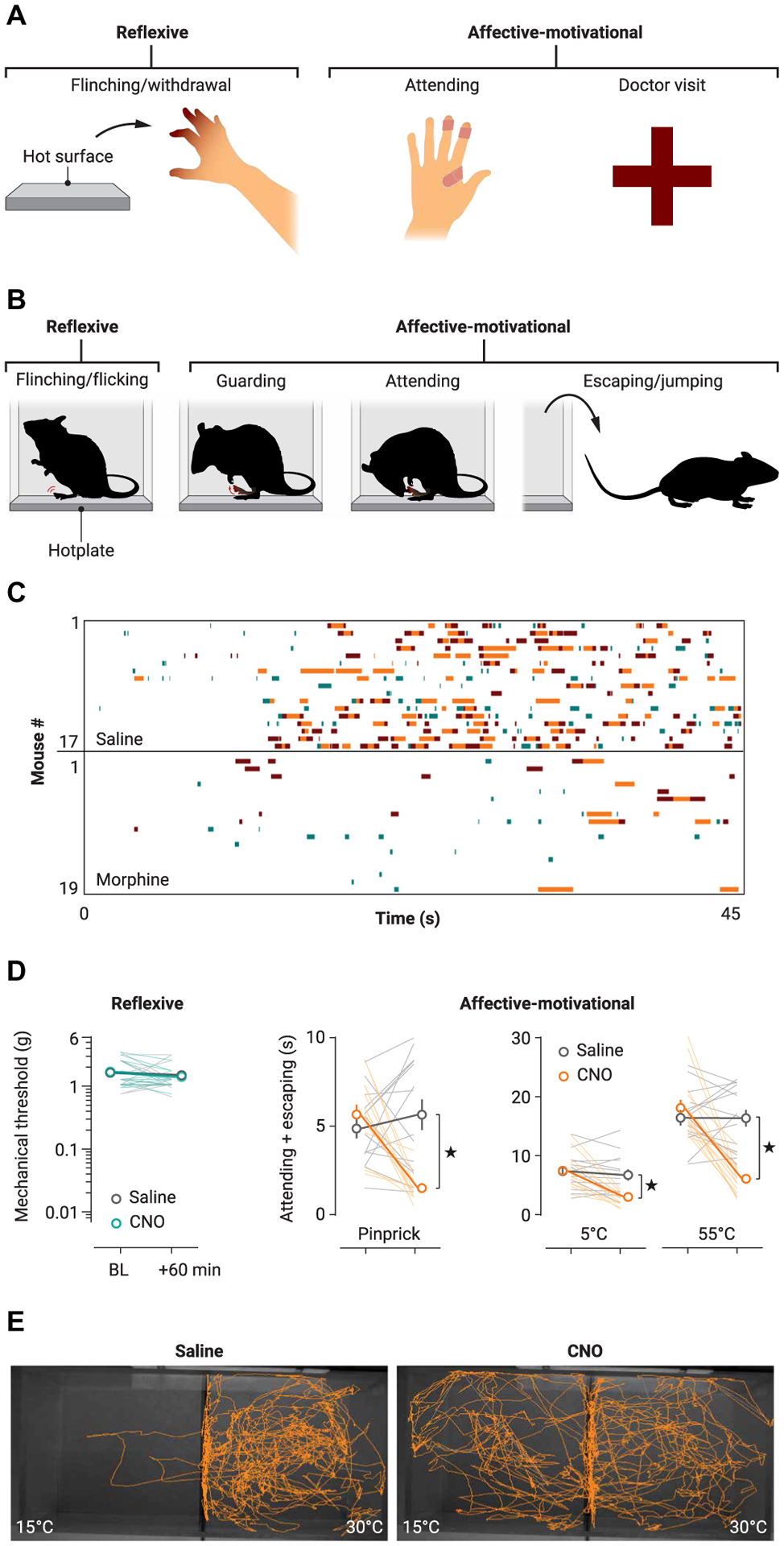
(A) Examples of human responses to noxious stimulation, which include reflexive and affective-motivational behaviors. (B) Mouse responses to noxious stimulation, such as with the hotplate test, also include reflexive and affective-motivational behaviors like protective responses (such as guarding and licking of an affected paw) and escape seeking (for example, rearing and jumping). (C) Raster plots showing the nocifensive behavioral responses of individual mice in the hotplate assay and the reduction in both reflexive (green) and affective-motivational (orange, brown) pain behaviors after morphine administration. (D) In contrast to the effect of morphine (C), inhibition of nociceptive BLA neurons with hM4Di after injection of clozapine-N-oxide (CNO) reduces affective-motivational pain behaviors in the hotplate assay, but not reflexive withdrawal. (E) In a two-plate preference assay, CNO also decreases nerve injury-induced aversion to innocuous cool stimuli in the setting of neuropathic allodynia. Adapted from (37–39).
The temporal logic of nociceptive behavior organization during acute pain perception, with pain-limiting reflexive behaviors exhibited first, followed by reflective and voluntary nocifensive behaviors, is largely conserved between humans and rodents (Fig. 2, A and B). These behaviors can be studied in detail in mice experiencing pain during the hotplate test, if this assay is used to comprehensively analyze mouse behavior (Fig. 2, B to D), rather than scoring only the latency for the first nocifensive reflex. In an opioid pharmacology study that took advantage of single-, double-, and triple-knockout mice for opioid receptor subtypes, after opioid agonist administration intracerebroventricularly, only mu opioid receptor (μ or MOP receptor), but not delta opioid receptor (δ or DOP receptor), activation suppressed reflexive nocifensive withdrawal from noxious heat; however, activation of either μ or δ could result in antinociception if paw licking and jumping on the hotplate was measured to evaluate pain perception (34). Given the known differential expression of δ and μ receptors in the brain’s pain pathways (35, 36), these results suggested that distinct circuits (and molecules in these circuits) control different nocifensive behaviors during the hotplate pain experience. By annotating video recordings of mice exposed to noxious stimuli, raster plots can be generated to categorize and quantify the rapid and stereotyped reflexive paw withdrawal and flicks/flinches, versus the delayed, reflective, voluntary, and more variable behaviors aimed to minimize pain unpleasantness, which include attending to the affected paw (such as lifting, guarding, licking, and biting) and escape behaviors (searching for an escape route via exploration, rearing, and jumping; Fig. 2B) (37–39). Thus, each mouse displays a unique sequence of attending and escape behaviors (Fig. 2C), indicating that this method can also be used to study the mechanisms that underlie the idiosyncrasies of both the experience of pain and the expected efficacy of individual actions to provide pain relief. Given this variability, attending and escape behaviors can be grouped and labeled as affective-motivational behaviors (Fig. 2D). This categorization, which can be automated using deep learning approaches such as DeepLabCut or MoSeq, described elsewhere (40–42), complements other approaches such as conditioned place preference or avoidance paradigms (43, 44), grimace scoring (45, 46), and wheel running monitoring (47) to provide a more complete description of pain experience in nonverbal animals, which, combined with rigorous experimental design (48), may better predict the clinical efficacy of treatments than when relying solely on reflexive behavior–based measurements (49).
Brain circuits for pain experience (Fig. 3A)
Fig. 3. Pain emotional and cognitive networks and treatments that can ameliorate chronic pain affect.
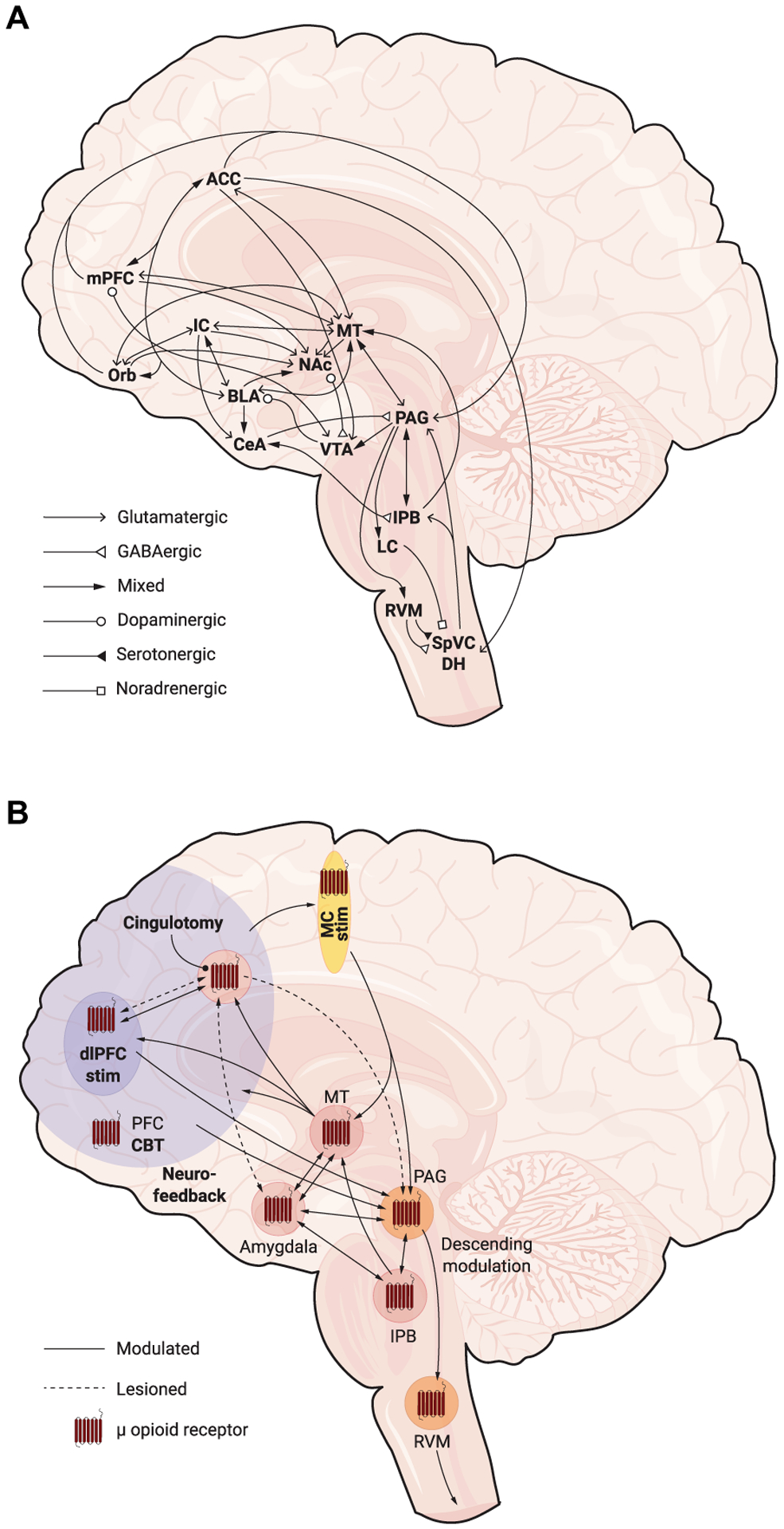
(A) Primary afferent neurons synapse onto second-order neurons in the spinal dorsal horn (DH) or the trigeminal nucleus caudalis (SpVC). These neurons, in turn, project to the lateral parabrachial nucleus (lPB) and the periaqueductal gray (PAG), which then connect with the anterior cingulate, insular, and prefrontal cortices, medial thalamus, amygdala, nucleus accumbens, and hypothalamus to generate and modulate pain experience. Note, mixed arrows indicate glutamatergic and GABAergic pathways. (B) Prevalent treatments for pain commonly use opioid receptor signaling to induce a prominent action on pain affect circuits. Investigative treatments include motor cortex stimulation (MC stim), dlPFC stimulation (dlPFC stim), neurofeedback, and cognitive behavior therapy (CBT) that act on frontal cortex circuits to modulate pain. In severe cases of intractable pain, cingulotomy reduces chronic pain. Frontal cortex modulation is hypothesized to relieve pain through descending pain control in the PAG, but notable connections to the medial and intralaminar thalamus (MT) and to the parabrachial nucleus could also play a role. ACC, anterior cingulate cortex; BLA, basolateral amygdala; CeA, central amygdala; IC, insular cortex; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; Orb, orbitofrontal cortex; RVM, rostromedial ventral medulla; VTA, ventral tegmental area.
Neuroimaging and neurophysiological studies in humans have shown that noxious stimuli elicit neural activation and connectivity patterns within and between numerous brain areas, including the somatosensory cortex, insular cortex (IC), various regions of the prefrontal cortex (PFC), anterior cingulate cortex (ACC), thalamus, periaqueductal gray (PAG), and cerebellum (50–52). Additional regions, including the basal ganglia, parabrachial complex, posterior cingulate, amygdala, hypothalamus, and supplementary motor area, show less consistent and more context-dependent responses to noxious stimuli. Earlier studies demonstrated a relatively consistent noxious stimuli–evoked response in some of these structures that correlated with the perceived intensity of pain, leading to the hypothesis of a specific network for pain perception, the “pain neuromatrix” (50, 51). More recent evidence has refuted this hypothesis by challenging the notion that pain can be uniquely associated with a specific pattern of activated brain regions (53, 54). Instead, it seems that pain perception engages brain regions that tend to coalesce in networks associated with the multidimensional components of pain experience and broader functionality related to multisensory integration, emotion regulation, general cognitive and attention processing, self-referential processing, and other functions (55, 56). At the same time, a growing number of studies have used multivariate pattern analysis tools to capture, even within the same brain regions, fine-grained differential activation patterns between the distinct components or modalities of pain experience, in healthy individuals versus patients with chronic pain (57–60). These studies have produced interesting findings; for example, although the amygdala is thought to critically contribute to the affective component of pain experience, these experiments found no specific role for this brain region in the encoding of thermal pain (58). Together, these findings suggest that the experience of pain involves numerous interconnected brain structures working together, whereas more domain-general features of the underlying experience may have distinct neural coding through more specific pathways. Pain research in animals offers unique opportunities because it allows characterization (for example, genes and proteins expressed, electrophysiological properties, and connectivity) and causal determination of the function of individual neurons in some of the regions described above. We describe here some of the rodent studies exemplifying the utility of this approach. Nevertheless, we should acknowledge the debate regarding the degree of neurophysiological and anatomical congruence between the rodent and human brain. For example, there are inconsistencies regarding the function and anatomy of the PFC subregions and thalamic nuclei across species (61–63).
In rodents, as is the case in primates, DH nociceptive projection neurons, which comprise distinct populations located predominantly in lamina I and, to a lesser extent, in deeper DH laminae [there are also, in fact, a number of nociceptive projection neurons in the intermediate and ventral horn that remain understudied (64–66)], directly transmit nociceptive information to several brain regions in the medulla [nucleus of the solitary tract (NTS), inferior olive, and reticular formation], pons [parabrachial nucleus (PB) and reticular formation], midbrain (PAG, superior colliculus, and reticular formation), and forebrain (thalamus), with the two most thoroughly studied outputs being the PB and thalamus. Activity in these ascending pathways elicits sensory-discriminative and affective-motivational pain perceptions and the array of autonomic physiological responses (for example, increase in breathing rate and grimace) and nocifensive voluntary behaviors (such as attending and escape) that characterize pain experience in most mammals. Regarding the sensory-discriminative aspect of pain, we recommend other readings that describe the function in the representation and discrimination of noxious stimuli of the lateral thalamus [ventral posteromedial (VPM), ventral posterolateral (VPL), and posterior (Po) nuclei], zona incerta (ZI), primary and secondary somatosensory cortices (S1 and S2), PFC, and posterior insular cortex (pIC) (67–69). In this translational review, we describe recent advances regarding the organization of brain circuits that shape the affective-motivational and cognitive-evaluative dimensions of pain.
Transmission of nociceptive information to the forebrain: spino-parabrachial-amygdalar and spino-thalamo-cortical circuits
Parabrachial nucleus
The PB receives diverse interoceptive and exteroceptive sensory information and plays a vital role in generating a wide array of autonomic responses, such as for pain, respiration, or thermoregulation (70–72). The lateral PB (lPB) has long been known to receive inputs from nociceptive projection neurons of the contra- and ipsilateral spinal cord (SC) DH and spinal trigeminal nucleus caudalis (SpVC; the neuroanatomical name for the trigeminal DH) and, to a lesser extent, from neurons located in deeper spinal laminae (73–75). Recently, the identification of marker genes that define distinct populations of spino-parabrachial and lPB neurons enabled detailed studies of lPB connectivity and function in pain. DH projection neuron populations characterized by the expression of distinct molecular markers [Tac1, Tac1r, Gpr83, or Phox2a (31, 76–78)] differentially innervate the external, dorsal, and superior (or internal) subdivisions of the lPB (lPBe,d,s/l), with the lPBe additionally receiving inputs from Trpv1+ trigeminal ganglion nociceptors (79, 80). Ablation of Tac1+ DH projection neurons, which innervate the lPBs/i, abolishes paw licking and conditioned avoidance, but not reflexive nocifensive behaviors, in response to sustained noxious stimulation. Optogenetic stimulation in lPB of axon terminals either from Tac1+ (78), Tacr1+, or Gpr83+ (31) DH projection neurons or from Trpv1+ nociceptors (80) drove acute and conditioned avoidance. The nocifensive responses engaged after activation of these different pathways are, however, distinct; for example, stimulation of Gpr83+ lPB inputs induced forward locomotion, whereas stimulation of Tacr1+ lPB inputs caused backward locomotion and jumping (31). Interestingly, in the setting of facial pain induced by capsaicin injection, optogenetic silencing of Trpv1+ nociceptor axon terminals in the lPB not only produced preference for the light-paired compartment in a real-time place preference assay but also diminished brisk head withdrawal after stimulation with a von Frey filament, suggesting an action both on pain affect and on reflexive withdrawal, presumably through descending control of nociception in the trigeminal DH (80). However, as is typical for optogenetically or chemogenetically driven place aversion or preference experiments in the pain field, although avoidance or preference indicates the aversive versus rewarding quality of the manipulation, whether the percept that motivates the animal’s avoidance or preference behavior resembles experiencing authentic pain or analgesia, respectively, requires further clarification. This question can be resolved by comparing neural dynamics (37). Remarkably, low-intensity optogenetic stimulation of Gpr83+ DH projection neurons, which predominantly receive input from primary afferent mechanosensory neurons and not Trpv1+ nociceptors, can also, in contrast to the Tac1r+ projection neuron population, promote place preference, suggesting a dual function in generating rewarding or aversive somatosensory experiences (31). This result also illustrates the importance of mimicking physiological firing patterns in optogenetic sufficiency experiments. Together, these studies support the idea that the lPB nociceptive circuits are essential for the expression of pain precognitive emotional physiological responses and behaviors. Furthermore, PB neurons integrate competitive signals that modulate pain, such as hunger, which inhibits nociception through inputs from hypothalamic agouti-related protein (Agrp+)-expressing neurons and neuropeptide Y signaling in the PB (81).
Amygdala
Although the amygdala is prominently considered a key brain region involved in emotional experiences, research has shown that it plays a broader role, including processing and coding the biological value of various types of salient stimuli (82–85). Basic pain research on the amygdala first identified nociceptive neurons in the central amygdala (CeA), a predominantly gamma-aminobutyric acid–ergic (GABAergic) nucleus, and examined their physiological properties and connectivity, including with the lPB (86–90). These CeA GABAergic neurons include a distinct ensemble of neurons that are activated by general anesthesia and inhibit pain (91). Recent studies have begun to investigate distinct populations of lPB neurons, defined by expression of Calca, Tac1, Nts1, Pdyn, Sst, and/or Tac1r (29, 70–72, 92–95). Together, these studies suggest that lPB neurons that receive monosynaptic input from DH projection neurons transmit nociceptive information to the lateral subdivisions of the CeA (CeL) [and the laterocapsular subdivision (CeCL), often referred to as the “nociceptive amygdala”] through two populations of Slc17a6+ (VGLUT2+) neurons: (i) Calca+ Slc17a6+ lPBe neurons, via Pdyn+ lPB neurons (92, 93), and (ii) intralaminar (ILN) and midline thalamic (MThal) neurons, via Tac1r+ lPB neurons (94, 95). In addition to the CeA, ILN, and MThal, these molecularly defined populations of lPB neurons differentially project to the bed nucleus of the stria terminalis (BNST), ventromedial hypothalamus (VMH) and lateral hypothalamus/parasubthalamic nucleus, lateral and ventrolateral PAG (lPAG and vlPAG), superior colliculus, MThal, medial PFC (mPFC), and insular cortex (IC) (29, 93–96). Functionally, silencing Calca+ lPBe-to-CeA neurons with the light chain of tetanus toxin (TetTox) inhibited footshock-induced immediate locomotor response and nocifensive jump response in the hotplate test, without altering the latency for reflexive withdrawal from noxious heat or the force of mechanical stimuli necessary to elicit a withdrawal reflex (92). These findings indicate the necessity of Calca+ lPBe-to-CeA neurons for innate escape behaviors after noxious stimulation, consistent with the essential function of the lPB for a variety of interoceptive and exteroceptive autonomic responses to threat (71). However, another study comparing the behavioral effects resulting from optogenetic stimulation of distinct lPB outputs found that activation of the lPBe-to-CeA pathway caused no substantial movement, whereas activation of either lPBd-to-VMH or lPBd-to-PAG neurons increased locomotion and jumping (93). Both studies provide evidence that the lPB-to-CeA circuit is necessary for aversive memories, albeit by manipulating different populations of neurons using dissimilar protocols: Calca+ lPBe-to-CeA neurons in a footshock-based fear conditioning assay (92) or Pdyn+ lPBd neurons in an intraplantar formalin-induced conditioned place avoidance assay (93). Tac1r+ lPB neurons receive ipsi- and contralateral monosynaptic inputs from DH projection neurons and are activated in response to noxious stimuli (95). Chemogenetic activation of Tac1r+ lPB neurons, which can disynaptically relay nociceptive information to the CeA, facilitated jumping in the hotplate test (95), as well as escape responses and nocifensive behaviors (for example, licking) in response to tail clip and after intraplantar injection of the TRPA1 agonist allyl isothiocyanate (AITC) (95) or formalin (94). Formalin-induced flinching (94) and the latency of the first nocifensive response on the hotplate (95) remained unaffected. Silencing of Tac1r+ lPBs almost completely eliminated licking induced by tail clip or AITC (95). Tac1+ lPB neurons include a subset of Calca+ lPBe-to-CeA neurons, as well as a different population of neurons that project to the medullary reticular formation region (MdD), which contains forelimb premotor neurons (29). Remarkably, in the hotplate test, either chemogenetic or optogenetic stimulation of Tac1+ lPB neurons resulted in immediate and repetitive jumping behavior and decreased licking. Complex CeA microcircuits, composed of multiple molecularly defined populations (such as Sst+, Pkcd+, and Crh+) with distinct connectivity and functions (97–101), process nociceptive information, which is transmitted from the CeL to the medial subdivision (CeM), the major output region of the CeA, and then to brainstem structures such as the PAG (99, 102) that mediate defensive behaviors. Physiological studies have demonstrated that calcitonin gene-related peptide (CGRP), which is encoded by Calca, facilitates N-methyl-d-aspartate (NMDA) receptor-mediated glutamatergic transmission at these lPB-to-CeL synapses (103), which show postsynaptic neuron type–specific (Som+ versus Crh+) alterations in synaptic transmission after sciatic nerve injury (SNI) (104). It is worth noting that Pdyn+, Sst+, and/or Crh+ GABAergic CeA neurons project back to the lPB. This inhibitory pathway normally inhibits nocifensive behaviors; however, CeA-to-lPB inhibitory inputs are reduced after infraorbital nerve injury (105). A systematic comparison between the different lPB and CeA outputs, using the same silencing/activating tools and behavioral assays to interrogate distinct aspects of the pain experience, could further clarify the contributions of the lPBe-to-CeA and other lPB output circuits to pain. Optogenetic manipulation of the CeA and connected descending circuits in the lPAG and downstream reticular formation motor networks [the dorsal and ventral medullary reticular formation (MdD and MdV), sometimes called the magnocellular reticular nucleus (Mc)] can produce freezing/immobility and/or flight behaviors in the absence of noxious stimulus or conditioning (106). In the same study, the authors reported that optogenetic activation of Slc17a6+ (VGLUT2+) lPAG neurons increased withdrawal latency in the tail immersion test. In another study, photostimulation of Pdyn+/Penk+/Slc17a6+ lPB neurons that project to the hypothalamus preoptic area (POA) could induce hypothermia, aversion, and suppression of locomotion (107). Disentangling effects on movement from those on nociception and pain experience may not be trivial. If changes in reflexive nocifensive responses after lPB, CeA, and lPAG neuron manipulations result from descending inhibition of nociception in the DH [presumably via the rostral ventromedial medulla (RVM)], one would expect to observe an antinociceptive effect that reduces not only withdrawal reflexes but also affective-motivational pain behaviors. Crucially, the maladaptive nocifensive responses observed when manipulating activity in lPB and CeA circuits (such as immediate and repetitive jumping upon Tac1+ lPB neuron activation in the hotplate assay or absence of jumping when silencing Calca+ lPBe-to-CeA neurons) illustrate the critical control function of cortical and subcortical structures. In these optogenetic and chemogenetic experiments, cortical and subcortical cognitive inputs are shunted during pain experience, resulting in failure to compute a wealth of information necessary to conceive plans (understanding current context, recalling memories from previous painful experiences, and formulating expectations) to select, among a wide variety of choices, the antinociceptive behaviors that are most likely to succeed, attempt them, and, in case of failure, adjust by selecting other behaviors (expectation violation and reformulation). Together, these results support the idea that lPB and CeA circuits mediate the expression of autonomic physiological effects and behaviors in response to noxious stimuli through connections with brainstem and hypothalamic effectors.
To be useful as a learning signal, the negative valence of acute (nociceptive) pain must be contextualized. Only then can an animal learn and thereby improve its ability to both avoid and respond to noxious stimuli in a context-specific manner to halt pain. For patients with chronic pain, the contextualization and constant evaluation of pain affect through cognitive circuits seem to drive emotional suffering and pain catastrophizing. Catastrophizing reflects maladaptive cognitions in response to actual or anticipated pain and has been associated with poor and deteriorating outcomes for people with chronic pain (108–110). A recent systematic review (111) in both healthy individuals and patients with chronic pain indicates that the brain regions most commonly linked to pain catastrophizing are those consistently active during pain processing and associated with the multidimensionality of pain, including the somatosensory cortex, thalamus, IC, ACC, and medial and dorsolateral PFC (dlPFC). The amygdala was also shown to play a role, although to a lesser extent. In healthy participants, during moderate pain, catastrophizing was negatively associated with neural activation in the amygdala (112). Compared to healthy controls, patients with chronic pain exhibited greater connectivity between the amygdala and a network of regions involved in cognitive processing, which was strongest in patients with the highest tendency to catastrophize (113). Moreover, patients showed decreased basolateral amygdala (BLA) connectivity to a network of regions involved in self-referential compared to healthy controls. Combatting this deleterious process is a major therapeutic goal. The BLA, unlike the CeA, is densely connected with cortical and subcortical cognitive circuits that process and contextualize affective information. Rodent studies have established that the BLA contains predominantly Slc17a7+ (VGLUT1+) pyramidal neurons that project to the CeA and the striatum, particularly to the nucleus accumbens (NAc) (98). Over the course of evolution, the size of the BLA versus the CeA within the amygdaloid complex markedly increased (84), evincing the critical importance of the BLA in human emotions and presumably in pain affect. However, considerably fewer rodent mechanistic studies have interrogated the contribution of BLA neurons to pain experience. Although footshock has been used extensively in the learning and memory field to investigate BLA function, the representation in the BLA of footshock and that of purely noxious stimuli considerably differ (37), presumably because the footshock generates activity in non-nociceptive primary afferents (such as mechanoreceptors and proprioceptors), producing an experience that is unquestionably aversive for the animal, but that does not precisely mimic pain experience. On the other hand, patient H.M., who had a temporal lobectomy that ablated most of the amygdala, including the BLA, but preserved the centromedial nucleus, could detect thermal noxious stimuli and report their intensity, but neither characterized them as painful nor showed motivation to avoid them (114, 115). Recently, in vivo optical recordings of about 17,000 neurons in freely behaving mice encountering noxious stimuli, combined with the chemogenetic manipulation of BLA neurons active during pain, enabled the identification of a distinct neural ensemble of CamkIIa+ Rspo2+ BLA neurons that specifically encodes the negative affective valence of noxious stimuli across pain modalities (heat, cold, and mechanical) and is necessary for the behavioral manifestation of pain affect (37). Inhibition of this nociceptive ensemble using genetic tagging in TRAP mice and Gi/o protein–coupled DREADDs (hM4Di) alleviated pain affective-motivational behaviors (attending and escape) without altering withdrawal reflexes, anxiety, or reward. Moreover, functional studies of this nociceptive ensemble revealed a causal neural basis for allodynia. Specifically, after peripheral nerve injury, innocuous stimuli begin to activate this nociceptive ensemble to drive dysfunctional perceptual changes associated with neuropathic pain, including aversion to light mechanical and cool stimuli, as reported in patients. Interestingly, this recoding phenomenon resembles that which occurs during fear conditioning, when the representation of the conditioned stimulus (CS) becomes more similar to that of the unconditioned stimulus (US) (116), suggesting that pain chronification and associative learning share common BLA mechanisms, consistent with the view that aspects of the chronic pain disease state result from maladaptive plasticity in learning circuits. Neuroanatomical and electrophysiological studies have revealed the extensive connectivity of this nociceptive ensemble, including monosynaptic inputs from cortical areas such as the ACC and IC, MThal and hypothalamus, and projections to numerous regions such as the ACC, CeA, and NAc (117). In these pathways, altered activity in the BLA during chronic pain, including in arthritis pain models, results in enhanced feedforward inhibition both of mPFC pyramidal neurons, impairing decision-making, and of CeA and intercalated cell (ITC) masses (101, 118), which are small clusters of tightly packed GABAergic neurons that receive BLA inputs and synapse onto CeA neurons.
Thalamus
In human functional magnetic resonance imaging (fMRI) studies, the thalamus is one of the brain regions most consistently activated by painful stimuli (119). The ILN and MThal, the latter of which includes the mediodorsal (MD) thalamus, are the major thalamic nuclei involved in pain affect and cognitive-evaluative processing of pain (120). As part of the dorsal thalamus, the ILN and MThal regions are composed almost entirely of Slc17a6+ (VGLUT2+) glutamatergic, excitatory neurons and are modulated by inhibitory neurons in the thalamic reticular nucleus and ZI. This region of the thalamus receives a confluence of nociceptive, arousal, and visceral information, notably not only from the Tacr1+ neurons in the lPB (94), the NTS (121), and the PAG, but also from brainstem arousal nuclei like the pedunculopontine nucleus, locus coeruleus, and various parts of the reticular formation (122, 123), as well as sparse inputs directly from the SC DH and SpVC (124). These diverse ascending signals are integrated with forebrain thalamo-amygdalar, thalamo-striatal, and thalamo-cortical loops (125–127). The ILN and MThal are composed of many small nuclei including the central medial (CM), parafascicular (Pf), central lateral (CL), reunions (Re), and submedius (Sm) (119). Sequencing data and axon morphology stratify the ILN and MThal neurons into two classes: The ILN and MThal nuclei excluding MD show similar RNA profiles, whereas the MD thalamus more closely resembles so-called higher-order processing thalamic nuclei like the posterior (Po) thalamus for somatosensation and the lateral posterior (LP) thalamus for vision (128). Each of the small nuclei in the ILN and MThal has distinct connections with the PFC (122, 123, 129, 130), and some are known to play a specific role in processing pain affect (126, 131, 132). One well-studied example shows that the Sm nucleus, through its prime prefrontal partner, the ventrolateral orbitofrontal cortex (Orb), engages the vlPAG descending pain control circuits using opioid peptides, serotonin, dopamine, and glutamate (131). Furthermore, separate modulation of MD pathways to either the BLA or ACC was found to inversely modulate pain-related aversion (127). As a final example, a recent study showed that, when the CM nucleus is lesioned before nerve injury, mechanical hyperalgesia failed to develop, and revealed that the CM receives vlPAG inputs and sends outputs to excitatory neurons in the BLA that could mediate this effect (132). Although there is evidence for the specific roles of the ILN and MThal in acute and chronic pain, more emphasis must be placed on specific pathways to fully dissect the role of the thalamus in pain affect, particularly circuits connecting the ILN, BLA, and cortical hubs for pain affect such as the ACC and IC, which themselves have dense reciprocal connections with the BLA (133, 134).
Cortical circuits involved in the affective-motivational and cognitive-evaluative dimensions of pain
The insular, anterior cingulate, and prefrontal cortices play important roles in mediating the cortical components of the affective-motivational and cognitive-evaluative aspects of pain experience. Human imaging studies performed during acute pain have specifically identified that the IC-to-PFC pathway is activated by discrimination of pain intensity, whereas the dlPFC is activated during spatial discrimination of pain (135).
The IC is one of the brain regions most consistently activated in fMRI during pain (56, 136) and while observing others in pain (137), and is the only cortical region that can be stimulated to induce pain experience (138). The anterior IC (aIC) and pIC receive visceral and nociceptive information through reciprocal connections with the PB, NTS, and ILN/MThal (139) and integrate this information with sensory and cognitive cues to generate internal and emotional states (140). The IC is thought to serve as a bridge for the exchange of pain affective and sensory-discriminative signals through reciprocal connections between the pIC, which connects to S1, S2, and lateral thalamus, and the aIC, which connects with the Orb, NAc, and ILN/MThal (139). Optogenetic inhibition of CamkIIa+ neurons in the pIC of mice and transcranial magnetic stimulation (TMS) of the pIC in humans lead to enhanced a decrease in capsaicin-induced mechanical hypersensitivity and increased heat pain thresholds, respectively (141, 142). Lesions of the pIC, but not of the ACC, prevent long-term mechanical hypersensitivity in sciatic nerve–injured mice (143). Together, these studies suggest that the pIC modulates the sensory-discriminative component of pain (141, 142). In contrast, the aIC is thought to be important for pain affect and for its relief, including via μ opioid receptors (144, 145). Injections of morphine into the aIC resulted in reduced nocifensive behaviors after hindpaw formalin injection (144).
Although the IC reciprocally connects to the BLA, these inputs display topographical patterns. The aIC preferentially targets excitatory outputs to the anterior BLA, the region preferentially associated with positive-valence neurons (146). In contrast, the pIC sends dense excitatory outputs to the posterior BLA, which is thought to be involved in negative valence processing. The entirety of the IC also sends excitatory projections to the CeA, which can drive descending circuits that mediate nocifensive behaviors. How these pathways encode pain affect and aversion during painful situations remains unexplored; however, conditioned taste aversion assays have implicated the necessity of the IC-to-amygdalar pathways (133, 147). Activation of IC-to-BLA projection neurons during a pleasurable consumption (saccharin) induced aversion to an otherwise positive cue (133). These studies suggest an important role for these reciprocal IC-amygdalar connections in generating the negative valence of pain (148).
The ACC contributes to numerous functions related to cognition (such as attention or learning), socio-emotional processes (like reward or empathy), and somatosensation, and although it is undoubtedly engaged during pain, there remains an ongoing debate as to the precise nature of its contribution (149–151). The ACC described here is distinct from the more caudal midcingulate cortex (MCC); these cingulate regions contribute differently to nocifensive behaviors (142, 152–154). In humans with ACC cingulotomies and animal models involving lesions to the ACC, pain aversiveness is often diminished, with minimal impacts on executive, cognitive, or motor functions (155, 156); however, this decrease in pain affect may be disorder- and/or context-specific, as shown by a case study in which a patient with schizoaffective disorder reported increased pain after cingulotomy (157). Structural changes have been observed in layer 2/3 (L2/3) of the ACC after induction of chronic pain in rodent models [recently reviewed here (69, 158)]. After the development of chronic pain in mice with SNI, L5 pyramidal neurons in the ACC have increased dendritic integration due to a decrease in hyperpolarization-activated cyclic nucleotide-regulated (HCN) channels, which is reversed by the serotonergic agonist 5-HT7 (159). A second study found HCN channel dysfunction in L2/3 pyramidal neurons in the ACC and mPFC developed after SNI in rats (160), further suggesting HCN channel function in the ACC changes during chronic pain.
Optogenetic activation of pyramidal neurons in the rodent ACC increases pain-related aversive behaviors. Optogenetic stimulation of pyramidal CamkIIa+ ACC neurons abolishes ketamine-induced reductions in aversion to a pinprick-paired chamber in a conditioned place preference assay (161). Optogenetic inhibition of ACC neurons in rats with either chronic constriction of the trigeminal nerve or SNI resulted in a reduction of cold hypersensitivity, similar to what is observed after ACC lesion in rodents or cingulotomy in humans (162, 163).
Bidirectional modulation of the ACC in the context of chronic pain can induce or abolish negative pain affect, resulting in secondary effects on mood, such as anxiodepressive phenotypes similar to those observed in patients with chronic pain. Lesions of the ACC abolish anxiodepressive-like behaviors in mice with SNI, including immobility during the forced swimming test and aberrant grooming behavior observed after splash (143). Conversely, optogenetic activation of predominantly Thy1+ pyramidal neurons in L2/3 and L5 of the ACC induces anxiodepressive phenotypes in healthy mice, consistent with nerve-injured mice (143). Slice electrophysiology studies revealed presynaptic and postsynaptic long-term potentiation mechanisms in the ACC that have been associated with chronic pain and comorbid anxiety (164).
The ACC input and output circuits regulating pain affect are being explored in rodents using electrophysiology, calcium imaging, and manipulation of subcircuits with opto- and chemogenetics. Experiments examining the relationship between the ACC and MD thalamus show that noxious stimulus-evoked activity in acute and chronic pain states transmits through the MD thalamus before reaching the ACC and that lesioning the MD thalamus abolishes aberrant spiking in the ACC (165). This study reported that the MD thalamus inputs to ACC L2/3 are responsible for transmitting aberrant spiking activity to L5 neurons that, in turn, project to the BLA and dorsolateral PAG (dlPAG) as well as back to the MD thalamus (127, 165, 166). Optogenetic activation of the ACC-to-MD pathway was mildly aversive, as evidenced by a slight avoidance of the side paired with optogenetic stimulation in a place preference assay (127). In contrast, optogenetic activation of the ACC-to-BLA pathway reduces SNI-associated aversion for the optogenetic stimulation-paired chamber (127). fMRI studies in humans show ACC activation during pain or pain relief, as well as when observing another human in pain (167, 168). A meta-analysis of fMRI during pain empathy consistently observed activation of the posterior ACC/anterior MCC border region and aIC and hypothesized an instrumental role for these two regions in empathy (137). Recent studies have shown that rodents likewise respond to social contagion with prosocial behaviors (169). Mice observing other mice with an acute inflammatory injury have decreased nocifensive thresholds; furthermore, this social transfer of pain is dependent on a pathway from the ACC to the NAc (170).
Although both the human and rodent PFC are similarly involved in decision-making, identification of reward, and executive functions, the rodent PFC differs in important ways from the human PFC. The most functionally analogous rodent structure to the human dlPFC lies within the rodent mPFC. Furthermore, the entire rodent PFC is agranular, whereas in humans, the mPFC, dlPFC, and most of Orb are granular (in other words, the mPFC and Orb lack a cortical L4 in rodents) (171–173). Although rodents might lack complex abstract thought, they show affective-motivational and cognitive-evaluative behaviors in response to painful stimuli not altogether dissimilar from those of humans (attending to injury, avoidance, etc.) (Fig. 2, A and B).
The mPFC, composed of the infralimbic (IL) and prelimbic (PL) cortical regions, and Orb are particularly well studied for their roles in Pavlovian and instrumental conditioning (174, 175), both of which are driven by reward or punishment (for example, pain relief or pain). As previously discussed, the Orb receives input from the Sm nucleus of the ILN and receives notable inputs from the IC and ACC that create associations between pain and environmental cues conveyed from secondary somatosensory cortex or other higher-order sensory cortices (176). The Orb responds to a diverse set of nociceptive stimuli (cutaneous, visceral, and thermal) and can act on descending pain control through its direct output to the vlPAG (131).
The mPFC plays a key role in generating complex associations using working and long-term memory. A gradient has been observed from the ACC ventrally through the PL and IL that demonstrates the importance of the more dorsal ACC (dACC) and PL for memory retrieval, whereas the ventral IL is important for working memory (177). Mice with SNI exhibit altered mPFC-to-hippocampus oscillation patterns and decreased working memory (178). The PL and IL regions change distinctively during chronic pain. The PL had no change in density of FOS protein (an immediate early gene that reports recent neural activity) in mice observing a cagemate in pain; however, there was an increase in FOS expression after observing a stranger in pain (179). Acute blockade of the glucocorticoid stress response in the PL induces a social transfer of pain for stranger mice similar to that for cagemates, whereas injection of corticosterone in the PL reduces the social transfer of pain for cagemates (179). Inputs to the PL region from the ventral tegmental area (VTA) release dopamine, which induces antinociception in a mouse model of chronic pain by activating PL-to-dlPAG neurons (180). Bilateral lesions of the PL, but not IL, result in heat hypersensitivity and anxiety-like behaviors (181). Optogenetic inhibition of PL pyramidal CamkIIa+ neurons induces anxiety-like behaviors, suggesting that the PL is involved in the regulation of social context and anxiety related to pain (181). The IL tends to show less distinctive changes during acute or chronic pain; however, BDNF protein decreases in the IL after peripheral inflammatory injury, and infusion of BDNF in the IL reverses inflammatory hypersensitivity (182). Further discussion of the distinct roles of the mPFC in chronic pain can be found elsewhere (183).
The PFC and ACC play critical roles in modulating pain experience based on the expectation of pain or pain relief. In humans, this effect is often associated with the expectation of treatment. Human fMRI and positron emission tomography scans have paved the way to understanding the brain circuits underlying this phenomenon. Across the entire brain, fMRI studies have associated placebo analgesia, a phenomenon in which pain perception is shaped by expectation, with correlated activity in the PFC, ACC, hippocampus, PAG, pons, and cerebellum (184–188). Placebo analgesia in humans has recently been reviewed (135). Recent and ongoing work in rodents has used operant conditioning, which allows more precise circuit dissections to understand the precise pathways that mediate placebo or nocebo effects. For example, pairing opioids or aspirin with a CS cue showed that rodents can anticipate analgesia (189). Further work is needed to fully establish rodent models of placebo analgesia to take full advantage of the genetic and circuit dissection tools available.
PFC outputs to the PAG are believed to play a critical role in modulating pain by activating the descending pain control pathways from the PAG to the RVM and are discussed later in this review. Together, the PFC consolidates pain affective information and sensory features, evaluates motivational factors, and computes a course of action, effected through motor circuits, to halt or choose to endure pain.
Midbrain circuits for reward and aversion, and the motivation-decision model of pain
Pain is aversive, whereas pain relief is rewarding. The motivation to avoid pain and seek pain relief is generated through dopaminergic VTA and substantia nigra compacta (SNc) outputs, particularly to the NAc (mesolimbic dopaminergic system) (190). Human fMRI studies have revealed the involvement of the VTA and NAc both during pain and when anticipating pain or its relief, as well as altered functionality during chronic pain (191–197), consistent with the dual function of the VTA-to-NAc pathway in processing both rewarding and aversive stimuli. Rodent studies have shown NAc responses analogous to those in humans during pain onset and offset (198) and have enabled investigation of the anatomy and function of discrete VTA and NAc cell types and circuits in aversion and reward (190, 199–203). For example, in a mouse model of nerve injury–induced neuropathic pain, increased excitability of NAc indirect pathway medium spiny projection neurons (MSNs) increased mechanical allodynia (204). In a rat model of migraine, vlPAG inputs to the VTA are required to generate conditioned place avoidance (205, 206). Remarkably, dysfunction in the mesolimbic dopaminergic system during chronic pain also involves non-neuronal cells, including activated microglia in the VTA that can alter dopamine release in the NAc (207). In addition, the decreased motivational drive that can accompany chronic pain has been associated with galanin receptor 1–induced depression of excitatory synaptic transmission in NAc indirect pathway MSNs (208). Inhibition of κ opioid receptor signaling in the NAc using the selective antagonist NorBNI or chemogenetic inhibition of NAc dynorphin-expressing (Pdyn+) MSNs restored normal motivation in a model of chronic inflammatory pain (209). Note that alongside this mesolimbic dopaminergic pathway, which mediates learning and anticipation of pain, the mesocortical dopamine system entrains the relative reward value (190), both systems defining the aversiveness of the situation and urgency to respond during pain. Crucially, pain aversiveness is often perceived in the context of other conflicting goals; cortical inputs to the NAc resolve these motivational conflicts and implement action decisions based on predictions (210, 211). Glutamatergic projections from the ACC, IL, and PL regions to the NAc and VTA regulate approach-avoidance behaviors (212–214). Chemogenetic inhibition and optogenetic excitation of the IL-to-NAc pathway revealed an essential role for determining the approach-avoid balance in response to a pain-predictive cue (212). Pairing chemogenetic inhibition of either the ACC-to-NAc and ACC-to-VTA (214) or PL-to-NAc (213) projections with a chamber in a conditioned place paradigm led to chamber preference in chronic injury rats, but not controls. The importance of reward circuits and motivation in the context of pain has been thoroughly reviewed elsewhere (192, 210, 211, 215, 216).
Descending circuits for pain modulation
Activity in forebrain and midbrain circuits can profoundly influence nociception at the spinal level through direct cortico-spinal connections or medullary relays (217–222). For example, ACC neuron axon terminals, which can be observed in the SC DH, facilitate spinal excitatory transmission and behavioral hypersensitivity (223). Furthermore, neurons of the somatosensory cortex also innervate the DH, control tactile sensitivity, and contribute to tactile allodynia during neuropathic pain (224). The PAG critically contributes to descending pain modulation by integrating forebrain and midbrain inputs and, through neurons located predominantly in its lateral and ventral quadrants (vlPAG), by engaging distinct populations of RVM neurons that project to the DH and facilitate or inhibit nociception. Three populations of nociceptive RVM neurons have been defined: (i) On-cells show a burst in firing rate before a nociceptive withdrawal reflex and facilitate pain; (ii) off-cells fire tonically, pause during withdrawal reflexes, and inhibit pain; and (iii) neutral cells show no alteration in firing pattern during a nociceptive reflex, and their role remains less well understood (218, 219, 225). Recent studies have begun to elucidate the molecular identity of some of these RVM-to-SC neurons, their connectivity, and modulatory function in distinct pain modalities (218, 226). A population of dual GABAergic and enkephalinergic (Penk+) RVM-to-SC neurons reduces behavioral sensitivity to both heat and mechanical stimuli (227). In contrast, another population of GABAergic, but Penk-negative, RVM-to-SC neurons facilitates mechanical pain by inhibiting spinal GABAergic and enkephalinergic (Penk+) neurons that normally presynaptically inhibit mechanosensitive primary afferent DRG neurons via GABAA and opioid receptors located on their central terminals (228). These RVM-to-SC neurons express the μ opioid receptor and represent a class of RVM on-cells. Alternatively, RVM neurons can modulate nociception by synapsing directly onto the central terminals of nociceptors and controlling their release of glutamate. Thus, RVM-to-SC serotonergic neurons release serotonin (5-HT) onto 5-HT3A- and TRPV1-expressing nociceptors, which sensitizes TRPV1 and causes hyperalgesia (229). In addition to GABA and 5-HT, another neurotransmitter, noradrenaline (NA) from the locus coeruleus (LC), critically contributes to descending pain modulation. Remarkably, activation of LC neurons that project to the SC inhibits nociception and can relieve neuropathic pain, whereas activating forebrain-projecting LC neurons increases spontaneous pain (230). This engagement of LC neurons for descending antinociception may depend on phospholipase Cβ4 (PLCβ4) signaling in PAG-to-LC neurons (231). These descending pain control systems show considerable sexual dimorphism (232), as well as modulation by additional antinociceptive and pronociceptive endogenous molecules and drugs such as hormones, neuropeptides, cannabinoids, and nicotine (233–237). Last, note that the vlPAG also contains ascending pain modulatory neurons; a recent study described a class of vlPAG/dorsal raphe nucleus dopamine antinociceptive neurons that project to the BNST (238). Remarkably, this cell population shows sexual dimorphism; its optogenetic activation inhibited nocifensive behaviors resulting from inflammatory pain in male, but not female, mice.
MANIPULATING THE BRAIN’S AFFECTIVE PATHWAYS TO PROVIDE PAIN RELIEF (FIG. 3B)
Pharmacology (opioids)
Long-term opioid use is associated with harmful side effects, as well as risk of misuse, abuse, and opioid use disorder (239). However, clinical experience suggests that, in a subgroup of patients with chronic pain, stable doses of opioids can provide durable pain relief with limited side effects. This section focuses on opioids because long-standing evidence indicates a direct action on affective-motivational and cognitive-evaluative networks (240, 241). Furthermore, the identification of the μ opioid receptor as the molecular target of clinical opioids like morphine (242) has enabled detailed mechanistic studies of neurotransmission modulation by opioids (243–245), including in affective circuits. Both clinical and rodent studies support the idea that opioids preferentially decrease the affective component of pain (246–248). For example, moderate activation of μ opioid receptors with a low dose of the biased and partial agonist PZM21 reduced affective-motivational nocifensive behaviors, without altering reflexive withdrawal from noxious stimuli in rodents (39). These features separate opioids from other common analgesic drugs that limit the production of pronociceptive mediators [for example, non-steroidal anti-inflammatory drugs (NSAIDs) (249)] or affect the function of primary afferent nociceptors (including sodium channel or calcium channel blockers such as lidocaine and ziconotide, respectively) or for which the molecular and circuit mechanisms of action remain unclear (such as gabapentinoids, anticonvulsants, and antidepressants). For example, activation of the gabapentin receptor α2δ-1, in addition to its effects on ion channels (250, 251), can promote excitatory synaptogenesis in response to thrombospondin released by astrocytes in the SC DH (252, 253). Gabapentin blocks this synaptogenesis mechanism, which may contribute to central sensitization during chronic neuropathic pain. However, it remains unclear whether gabapentinoids also influence remodeling of brain synapses of the pain affect circuitry via the same mechanisms to produce pain relief (254).
At the circuit level, μ opioid receptor distribution is prominent in emotional and cognitive brain circuits (255). The study of these opioidergic circuits has been facilitated by the generation of mutant mouse lines in which individual opioid receptor or peptide genes have been either targeted to express fluorescent receptors or DNA recombinases or flanked by loxP sites for conditional deletion experiments (117, 228, 256–262). Remarkably, μ opioid receptor–expressing neurons are found in lPB, ILN/MThal, and PAG, the three major output regions by which nociceptive DH projection neurons connect with emotional and cognitive circuits (35, 36, 257, 263, 264).
In the lPB, μ opioid receptors are expressed by Calca+ lPBe neurons, in which μ receptor activation decreases glutamate release onto CeL neurons. In the dorsomedial/midline thalamus (dMT), μ receptors are present in thalamo-cortical (ACC), thalamo-striatal [dorsomedial striatum (DMS)], and thalamo-amygdalar (CeL and BA) circuits. In the dMT, including the paraventricular (PVT) and paratenial (PT) thalamic nuclei, μ receptor activation decreases glutamatergic transmission between dMT neurons and basal amygdala (BA) and CeL amygdala neurons, resulting in an overall reduction in feedforward activation of CeM neurons (265). μ receptors are also expressed by several classes of amygdalar neurons: by some BLA neurons (266) and, more abundantly, by ITC masses and populations of CeA GABAergic neurons, including Cck+ neurons and neurons that project to the PAG, in which μ receptors regulate both the flow of information within the amygdaloid complex through G protein-coupled inwardly rectifying K+ (GIRK)-mediated hyperpolarization and the release of GABA in downstream targets (36, 267–270). Note that μ receptor expression in the PVT might also mediate the expression of opioid withdrawal symptoms and aversive memory through a PVT-to-NAc circuit (271). A recent study demonstrated that μ opioid receptor+ dMT neurons project to the dorsomedial, rather than the ventral, region of the striatum, where they synapse onto MSNs that receive convergent, μ opioid receptor–negative [although, see also (272)] input from ACC pyramidal neurons. Interestingly, these μ opioid receptor+ thalamo-striatal neurons also project back onto L5 ACC pyramidal neurons, and μ opioid receptor agonists can presynaptically decrease glutamate release onto both DMS MSNs and L5 ACC pyramidal neurons. The latter synaptic mechanism of function of μ opioid receptors may contribute to the antinociceptive effect of intracerebral ACC morphine injections on the affective component of pain, without influencing withdrawal reflexes (247, 273). Because glutamatergic thalamic μ opioid receptor+ neurons predominantly express VGLUT2, these synaptic mechanisms could underlie the reduced opioid antinociception in mice with a conditional deletion of μ receptors in Slc17a6+ neurons (274). However, aside from regulating excitability and transmitter release, including via several forms of synaptic plasticity in multiple types of pyramidal neurons, such as in the mPFC and insula (126, 272), μ receptors are also thought to be expressed by multiple populations of GABAergic cortical interneurons such as Lamp5+, Sst+, Vip+, and Pvalb+ neurons (275). Precise genetic strategies may be required to resolve the contribution of these distinct populations of cortical μ opioid receptor-expressing neurons to opioid analgesia. Although intracerebral injection of μ opioid receptor agonist into the vlPAG has long been known to produce antinociception (276), the identity and connectivity of μ opioid receptor–expressing neurons in the vlPAG remain less well understood (218). We know, however, that these vlPAG neurons regulate the activity of several classes of spinally projecting neurons in the RVM, including μ receptor–expressing on-cells (217–222, 227, 228, 277). Identifying the precise contribution to these different processes of the molecularly and pharmacologically diverse types of receptors activated by morphine-like opioids represents an exciting challenge (278–282).
Note that the expression patterns of δ and κ opioid receptors in pain circuits profoundly differ from that of μ receptors (36, 255, 257, 259, 283, 284), consistent with the divergent properties of their selective agonists. For example, in the cortex, δ receptors are predominantly expressed by Pvalb+ (PV) inhibitory interneurons rather than by thalamic μ-expressing glutamatergic inputs to the ACC, where δ enhances the glutamatergic, excitatory input from the MThal to the pyramidal neurons in the ACC by disinhibiting local feed-forward inhibition mediated by Pvalb+ interneurons (126). Note that these PV inhibitory interneurons represent the class of cortical and hippocampal neurons that abundantly coexpresses δ and μ receptors, a rare feature in the nervous system (36, 257, 275, 283–285). In the amygdala, δ receptors are predominantly found in the BLA, on the soma and axon terminals (258), in contrast to μ receptor distribution in ITC and CeA neurons. κ receptors are also present in affective and valence circuits, but generally in different cell types compared to μ, consistent with the diverging properties of their selective agonists (209, 286, 287). Last, although μ opioid receptors are expressed by nociceptors and spinal projection neurons (36, 283), conditional deletion studies suggest that μ receptors in nociceptors are dispensable for morphine analgesia [(38); however, see also (288)].
Stimulation
The first documented use of stimulation intended specifically to alleviate chronic pain was performed in the 1960s, targeting deep brain electrical stimulation (DBS) to the thalamus (185). In the 1980s, TMS was developed as an alternative to electrical stimulation (ES) (289). TMS uses magnetic induction to generate macroscopic electrical currents in the brain (289). A shift from invasive to non-invasive forms of stimulation like TMS has made stimulation and modulation of brain circuits available to a broader patient group. Today, TMS and transcranial direct current stimulation (tDCS) are the most commonly used methods for noninvasive modulation of brain circuits to alleviate chronic pain (290).
How do TMS, ES, and tDCS work?
ES and TMS drive action potential firing by exciting neurons and passing axons and backfiring input terminals at the site of stimulation (291). In contrast, tDCS is less temporally and spatially specific than ES and TMS and acts by hyperpolarizing the resting membrane potential, making the anode region more excitable and the cathode less (292). Studies in human subjects and animal models both show that high-frequency stimulus trains excite the target more efficiently than low-frequency trains (293–297). Theta burst stimulation, a TMS protocol commonly used for cortical stimulation, uses three pulse bursts delivered at high frequency (for example, 50 Hz), repeated every 200 ms (5 Hz) (293). Theta burst protocols are a compromise aimed to capture the advantages of high-frequency stimulation while limiting the risk of inducing seizures (294, 298). Examination of fMRI interleaved between TMS pulse trains shows a stimulus target volume of 5 to 10 cm3 (299). In vivo voltage dye imaging in cat cortex found a progressive rise in excitation at the targeted region throughout a 10-Hz electrical stimulus train (300), supporting previous slice electrophysiology studies that had similarly concluded that high-frequency stimulation in cortical tissue preferentially activates excitatory neurons (301). Furthermore, a recent calcium imaging study in mice showed that excitatory neurons in L2/3 activate in response to specific stimulation frequencies, similar to visual cortical neurons tuned to a specific direction of drifting grating stimuli (296). Although our understanding of the biophysics underlying brain stimulation is evolving, many important questions remain to be explored.
MCS for pain relief and as a model for understanding TMS and tDCS
The motor cortex (MC) is a common target of studies attempting to determine the neurobiology of cortical stimulation. MC stimulation (MCS) results in motor activity that enables confirmation of correct targeting. The muscle end-plate potential (MEP), which can be performed in humans and in animal models, enables examination of resting motor threshold, amplitude of stimulus-evoked responses, and long-term changes in muscle tone (299, 302). Performing MCS with MEP as the readout reveals long-term changes in MC excitability after stimulation (303, 304).
MCS was first used to reduce chronic pain in 1991, when Tsubokawa and colleagues (305) implanted electrodes into the primary MC of patients with refractory central pain. Nine of 12 patients described their pain relief as good or excellent on the days when stimulation occurred, and 8 of these patients continued the therapy with reduced chronic pain after 1 year of treatment (305). In the intervening 30 years, hundreds of patients have received MCS. A meta-analysis across studies reported that 64% of patients experienced a favorable response after MCS and 45 to 75% of patients reported a decrease of at least 5 points on a 0 to 10 visual analog scale (VAS) of pain intensity (297). Furthermore, case studies applying MCS to patients with severe, otherwise untreatable, pain showed remarkable pain relief (295, 305, 306).
The mechanisms underlying MCS efficacy remain poorly understood. fMRI imaging in human subjects identified MCS-induced hotspots of activity in descending pain control regions that correlated with suppression of acute secondary hyperalgesia (307). Intracranial injection of GABAergic or glycinergic antagonists into the PAG of SNI rats before MCS prevented MCS-induced antinociception, providing further evidence for the involvement of descending pain control (308). In contrast, MCS increased the density of FOS+ neurons in the ACC and BLA, and lesions of the aIC enhanced MCS-induced antinociception in a chronic pain rat model (309–311), together suggesting that pain affective circuits are involved in MCS analgesia as well.
Noninvasive targeting of pain-related brain regions
In addition to MC, noninvasive brain stimulation has been targeted to many pain-related brain areas with the intention of reducing chronic pain, including the ACC, IC, somatosensory cortex, and dlPFC, with varying degrees of success (312). Stimulation of S1 and/or S2 has been found to modulate perception of sensory features of pain without providing the clinically necessary reduction in pain affect (313–315). In contrast, a study using noninvasive stimulation of the ACC or IC in patients with central chronic pain found that neither target evoked a measurable effect on chronic pain scores in patients, although the ACC stimulation decreased anxiety and the IC stimulation increased heat pain thresholds (141). The most promising alternative, to the MC for noninvasive stimulation is the dlPFC, the stimulation of which reduces acute pain in healthy volunteers and decreases chronic pain scores in patients (290, 316–318).
DBS for chronic pain relief
Many regions critical for pain processing are difficult to effectively stimulate noninvasively. The ACC, VP thalamus, and PAG have all been identified as promising DBS targets for reducing chronic pain; the literature for these methods has recently been reviewed (319).
Neurofeedback
Given the crucial role of the brain in the experience of pain and its modulation, researchers have hypothesized that direct manipulation of one or more brain regions could enhance pain modulatory systems and thereby reduce the underlying central nervous system (CNS) abnormalities associated with chronic pain. In addition to the pharmacological, direct stimulation (TMS, DBS, and tDCS), and surgical techniques discussed in this review, researchers have developed neurofeedback techniques that teach individuals to self-regulate brain functionality. Neurofeedback is a noninvasive therapy that directly targets brain activity and/or connectivity patterns and uses either electroencephalograph (EEG) recordings or fMRI signals to provide individuals with real-time visual and/or auditory feedback reflective of the targeted brain functionality (320, 321).
EEG neurofeedback is used more frequently than fMRI because of its greater accessibility and lower cost. Typically, EEG neurofeedback targets a change in a specific oscillatory bandwidth, most often the alpha band (8 to 13 Hz) (322). In contrast to EEG neurofeedback, fMRI neurofeedback measures and feeds back information from specific brain regions or networks using fMRI’s higher spatial resolution. The lower temporal resolution of fMRI seems to benefit the learning of self-regulating brain functionality.
An example from one of the earliest studies of fMRI neurofeedback fed back brain signals from the dACC (323). In healthy volunteers given an evoked thermal stimulus, neurofeedback training led to increased control over brain activity and an associated increase in control over pain intensity. In a single training session, patients with chronic pain noted reduced pain that correlated with the degree of brain control over the dACC. Similarly, Guan et al. (324) modulated the rostral ACC (rACC) in a group of patients with postherpetic neuralgia (PHN). Patients learned to modulate their rACC signal and their pain perception. Using an fMRI-to-EEG amygdala fingerprint, Goldway et al. (325) conducted a neurofeedback trial in which they taught patients with FM to modulate their own amygdalar activity using a single EEG channel. Patients demonstrated improvements in objective measures of sleep and follow-up improvements in pain, demonstrating the benefit of this approach combining fMRI and EEG neurofeedback (325).
More recently, Zhang et al. (326) illustrated the potential of implicit learning strategies to modulate pain. Specifically, they used real-time decoded fMRI signals from the IC integrated into a closed-loop feedback control system and found that decoding the brain patterns without the participant’s volitional control leads to adaptive changes in the brain. These results demonstrate the need to account for these adaptive changes in the design of future systems intended to direct brain control. Although neurofeedback using fMRI and EEG is a promising avenue for therapeutic interventions, researchers must still identify the optimal brain targets, patterns, frequency bands, and networks for manipulation; demonstrate that neurofeedback training leads to learning; ensure that neurofeedback leads to measurable changes in behavior (examples include pain relief, coping, pain catastrophizing, and fear avoidance); and develop appropriate controls and clinical trial designs (327, 328). For additional information on neurofeedback in the context of pain, we direct the reader to the following reviews (320, 321, 329).
Cognitive behavioral therapy
Cognitive behavioral therapy (CBT) is a psychotherapeutic treatment encompassing a set of techniques and approaches, ranging from structured psychotherapies to self-help materials, that helps individuals learn to identify and change destructive and/or disturbing thought patterns that may negatively influence behavior and emotions (330–333). Key processes for pain management include relaxation training, cognitive restructuring, and exposure techniques. In addition to pain, CBT is used to treat a wide variety of mental health conditions including addiction (334, 335), anxiety (336, 337), depression (338, 339), and personality disorders (340). It has also proved helpful for patients with chronic pain (340, 341).
Although we refer here and below to CBT (and its neural correlates) as a singular therapy, it represents a family of psychological treatments that has evolved over time. The first generation of CBT applied learning principles intended to change overt behavior. Classic CBT (second generation) was introduced in the late 1970s and focuses on the role of maladaptive thought processes in emotion, behavior, and pain. More recently, a third generation of CBT places more emphasis on themes such as acceptance, mindfulness, values, metacognition, and interpersonal relationships, giving rise to therapies such as acceptance and commitment therapy, mindfulness-based cognitive therapy, and several others. This section will focus on classic CBT and review its neural correlates.
CBT draws on cognitive and behavioral strategies to improve pain-related functioning and help patients cope with pain (341). After CBT treatment, patients with chronic pain report reduced pain, distress, nocebo hyperalgesia, and pain catastrophizing, as well as improvement of their daily functioning (342–344). CBT-induced pain relief is highly variable between patients, and the improvement correlates strongly with the patient’s attitude: Distressed patients who see their pain as an uncontrollable and highly negative life event benefit less, whereas patients with low perceived disability and high orientation toward self-management during CBT treatment benefit more (342, 343). These observations support the hypothesis that the outcomes of multidisciplinary pain treatment correlate with the individual patient’s cognitions and coping responses (343).
Although CBT continues to be widely used for pain management, the neural mechanisms that mediate analgesia during CBT remain unclear. Human functional neuroimaging studies dominate CBT research related to pain perception. Given the relatively limited literature investigating brain activation changes during CBT treatment in patients with pain, it is helpful to first understand how CBT impacts an individual’s psychological state to affect pain processing. Studies examining the effects of distraction on pain processing found that pain-evoked activity in several cortical areas, like the S1, IC, and ACC, is stronger when an individual focuses on pain than when distracted (345–347). Neuroimaging studies evaluating the effects of emotional states on pain processing found that negative emotional states alter pain-evoked cortical activation in several brain regions, but most consistently in the ACC (345, 347, 348). Placebo administration has been shown to increase activity in the ACC, PAG, and cerebellum but decrease activity in the S1 and IC (349–351).
Studies have demonstrated that, for individuals with chronic pain, CBT generates both functional and structural changes in the brain. One of the first studies on chronic pain found that CBT treatment increased pain-evoked activity in the lateral PFC, which subsequently increased its connectivity with the thalamus, compared to controls (352). This lateral PFC region contributes to semantic processing and cognitive control, both of which are associated with exposure and cognitive restructuring therapies. Similarly, large brain networks involved in sensorimotor, self-referential, and cognitive control show altered connectivity patterns in patients with chronic pain compared to controls, which return to baseline after CBT (353–355). These connectivity changes also occurred in healthy controls when receiving CBT-based training to cope with evoked pain (356). In another study in patients with chronic pain, CBT led to increased gray matter volume in multiple regions associated with pain processing, such as the dlPFC, ACC, and S1, some of which correlated with decreased pain catastrophizing (357).
A few studies have shown evidence that CBT generally affects neural function in pain networks. Biofeedback relaxation activates the ACC, basal ganglia, S1, inferior parietal cortex, and cerebellar vermis (358). Similarly, progressive muscle relaxation, one type of CBT, gradually decreases activity in the superior frontal gyrus (SFG), inferior frontal gyrus (IFG), and posterior cingulate cortex (PCC) (359), suggesting that CBT may activate endogenous pain descending modulatory systems. Last, the efficiency of CBT for the treatment of other mental diseases such as anxiety and depression (68, 360) may suggest that the nociceptive neurons within CBT-responsive cognitive and emotional circuits are polymodal neurons that control other functions beyond pain, such as attention and mood.
Finally, researchers have identified that different cognitive strategies to modulate pain evoke distinct brain activity patterns. For example, during focused attention, brain activity localizes to the pre- and postcentral gyrus (the primary motor and somatosensory cortices, respectively), middle occipital gyrus, and inferior parietal lobe, whereas reappraisal of the pain (imaging the painful stimulus alternating between harmful or nonharmful) engaged the thalamus, amygdala, ventral lateral PFC, MCC, and parahippocampal gyrus (361). The postcentral gyrus was the only area that overlapped in activation during both strategies. In a more recent study, researchers investigated three distinct pain modulation strategies: (i) non-imaginal distraction by counting backward in steps of seven, (ii) imaginal distraction by imagining a safe place, and (iii) reinterpretation of the pain valence (reappraisal) (362). They also identified strategy-dependent activations. Reappraisal and the imaginal distraction (safe place) primarily engaged the anterior insula, whereas the non-imaginal distraction task activated primarily the central operculum. The tasks involving distraction from pain (counting and safe place) modulated activity in the PCC. Together, these findings and others suggest that combining specific strategies with targeted brain stimulation or neurofeedback enhances treatment efficacy.
Surgery
First used in 1962, cingulotomy (lesioning of the ACC or the cingulum bundle white matter) has long been an option for decreasing chronic pain unpleasantness in patients who fail to respond to other interventions (185, 363). In this initial study, 16 patients suffering from debilitating chronic pain were selected for unilateral or bilateral cingulotomies (363). The authors classified pain relief as poor, fair, good, or excellent, finding that 12 of the 16 patients experienced good or excellent pain relief and 11 of 16 showed decreases in comorbid psychiatric disorders (363). The authors further noted that pain relief was observed immediately after the cingulotomy was performed, while the patient was still in the operating room (363). Although use of this technique has decreased in recent years in favor of nondestructive alternatives, a recent meta-analysis comparing data from 11 articles that included 224 patients concluded that, across all studies, more than 60% of patients reported substantial pain relief at least 1 year after the intervention (364). In this meta-analysis, the few side effects noted included, in <5% of patients, transient postoperative confusion and/or seizures (364). However, a case study of a patient with schizoaffective disorder found that cingulotomy increased pain, the opposite of the expected effect (157). Last, note that a number of other surgical procedures are used to treat pain (365).
CONCLUSION
Although pharmacotherapy, brain stimulation, neurofeedback, CBT, and surgical protocols used to treat pain continue to improve, research regarding the brain circuits and neuron types that mediate pain affect in animal models is revealing a wealth of candidate molecular targets to develop innovative analgesic drugs that could selectively dampen the unpleasantness of pain, without altering nociception in the circuits that underlie other necessary aspects of pain experience, such as withdrawal reflexes and the sensory-discriminative dimension of pain.
Cell type–specific multiomics is revolutionizing our understanding of neuronal diversity by revealing the molecular content of individual neurons within circuits. In the pain field, single-cell/nucleus RNA sequencing (366) can now generate, from each of a subject’s nociceptive neurons, comprehensive catalogs of expressed genes that encode proteins with inhibitory functions and potential analgesic capabilities, enabling precision pain medicine. Similarly, although this review focuses on circuits, molecules in non-neuronal cells such as microglia could be targeted for the treatment of pain (367, 368). Optically recording the activity of molecularly defined nociceptive neurons in freely moving mice experiencing chronic pain and administered with candidate analgesics can be used as a screening approach that relies on the normalization of both affective-motivational behaviors and pathological neural codes associated with pain chronification in the amygdala (37) and connected regions including the ACC, IC, ILN, MThal, and lPB (148). For example, with agonists of neuromodulatory receptors such as inhibitory Gi/o protein-coupled receptors (Gi/o GPCRs), individualized drug dosage could reduce patients’ pain unpleasantness while preserving both withdrawal reflexes and the sensory-discriminative dimension of pain. Such pain asymbolia-like treatments would not only rescue the well-being and function of patients with chronic pain, but also maintain sufficient nociceptive functions necessary to sense and withdraw from noxious stimuli unrelated to their chronic pain condition, a substantial challenge when targeting primary afferent nociceptors or spinal networks and their ascending circuits. As a proof of principle, expressing and activating Gi/o protein-coupled DREADDs (hM4Di) (369) in BLA nociceptive neurons of mice alleviated pain affective-motivational behaviors across pain modalities (acute heat, cold and mechanical pain, and chronic neuropathic pain) without altering withdrawal reflexes, anxiety, or reward (37); one would expect that activating Gi/o GPCRs natively expressed in these neurons to have the same effect. Alternatively, by resolving the molecular repertoires both of the μ opioid receptor–expressing neuron types that modulate emotional and cognitive pain circuits to dampen pain affect during opioid analgesia and of the μ opioid receptor–expressing neuron types responsible for deleterious effects such as addiction and opioid-induced respiratory depression, researchers could potentially develop better opioid therapies that mimic the effect of morphine on nociceptive neurons and/or adjuvant therapeutics that oppose deleterious opioid signaling in reward and breathing circuits.
In conclusion, these neural circuit discoveries and translational endeavors, supported by outstanding efforts such as the National Institutes of Health (NIH) Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) (370) and Helping to End Addiction Long-term (HEAL) (371, 372) Initiatives and its Preclinical Screening Platform for Pain (PSPP) (373), provide an unprecedented opportunity to end the dual public health crises of chronic pain and opioid use disorders. To fulfill this goal, we will need to use these discoveries to develop better biomarkers to facilitate the development of non-addictive pain therapies. Objective biomarkers can indicate that a therapeutic intervention has reached its central target, predict the response to the therapy, enhance the quality of the clinical trial by allowing clustering of patients by presumed responsiveness, and improve monitoring of safety and efficacy over time. Frameworks for developing and validating neuroimaging-based biomarkers and composite biomarkers have been put forward (374, 375). Programs like the NIH HEAL Initiative are stimulating considerable research efforts to advance the development and translation of biomarkers to yield targeted, safe, and effective therapies for pain.
Acknowledgments:
We thank J. Blair for editing the manuscript. We regret that we could not cite numerous important studies given the reference number limit and broad scope of this review article. We encourage readers to also consider the references in the articles we could cite.
Funding:
The authors’ research on the circuits for pain and its treatment is supported by NIH grants (S.M.) R61NS11865, R01NS109450, K24NS126781, (N.M.L.) F32DE030003, (G.S.) R01DA044481, R01NS106301, and R21DA049241. G.S. is a New York Stem Cell Foundation - Robertson Investigator.
Footnotes
Competing interests: G.S. is a cofounder of Epiodyne, a drug discovery company, an inventor on a patent application related to imaging of neural dynamics to discover analgesics, and a member of the NIH PSPP Preclinical Screening Platform for Pain External Consulting Board.
REFERENCES AND NOTES
- 1.Global Burden of Disease Study 2013 Collaborators, Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 386, 743–800 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rice ASC, Smith BH, Blyth FM, Pain and the global burden of disease. Pain 157, 791–796 (2016). [DOI] [PubMed] [Google Scholar]
- 3.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilam G, Gross JJ, Wager TD, Keefe FJ, Mackey SC, What is the relationship between pain and emotion? Bridging constructs and communities. Neuron 107, 17–21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calati R, Laglaoui Bakhiyi C, Artero S, Ilgen M, Courtet P, The impact of physical pain on suicidal thoughts and behaviors: Meta-analyses. J. Psychiatr. Res 71, 16–32 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand’homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang S-J, Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 160, 19–27 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education, Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research (National Academies Press, 2011). [PubMed] [Google Scholar]
- 8.Sherrington SCS, The Integrative Action of the Nervous System (Yale Univ. Press, 1906). [Google Scholar]
- 9.Woolf CJ, Capturing novel non-opioid pain targets. Biol. Psychiatry 87, 74–81 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patapoutian A, Tate S, Woolf CJ, Transient receptor potential channels: Targeting pain at the source. Nat. Rev. Drug Discov 8, 55–68 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basbaum AI, Bautista DM, Scherrer G, Julius D, Cellular and molecular mechanisms of pain. Cell 139, 267–284 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma N, Flaherty K, Lezgiyeva K, Wagner DE, Klein AM, Ginty DD, The emergence of transcriptional identity in somatosensory neurons. Nature 577, 392–398 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renthal W, Tochitsky I, Yang L, Cheng Y-C, Li E, Kawaguchi R, Geschwind DH, Woolf CJ, Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal injury. Neuron 108, 128–144.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray P, Torck A, Quigley L, Wangzhou A, Neiman M, Rao C, Lam T, Kim J-Y, Kim TH, Zhang MQ, Dussor G, Price TJ, Comparative transcriptome profiling of the human and mouse dorsal root ganglia: An RNA-seq-based resource for pain and sensory neuroscience research. Pain 159, 1325–1345 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.North RY, Li Y, Ray P, Rhines LD, Tatsui CE, Rao G, Johansson CA, Zhang H, Kim YH, Zhang B, Dussor G, Kim TH, Price TJ, Dougherty PM, Electrophysiological and transcriptomic correlates of neuropathic pain in human dorsal root ganglion neurons. Brain 142, 1215–1226 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P, Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci 18, 145–153 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Murthy SE, Loud MC, Daou I, Marshall KL, Schwaller F, Kühnemund J, Francisco AG, Keenan WT, Dubin AE, Lewin GR, Patapoutian A, The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci. Transl. Med 10, eaat9897 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szczot M, Liljencrantz J, Ghitani N, Barik A, Lam R, Thompson JH, Bharucha-Goebel D, Saade D, Necaise A, Donkervoort S, Foley AR, Gordon T, Case L, Bushnell MC, Bönnemann CG, Chesler AT, PIEZO2 mediates injury-induced tactile pain in mice and humans. Sci. Transl. Med 10, eaat9892 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barad MJ, Sturgeon JA, Hong J, Aggarwal AK, Mackey SC, Characterization of chronic overlapping pain conditions in patients with chronic migraine: A CHOIR study. Headache 61, 872–881 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD, Overlapping chronic pain conditions: Implications for diagnosis and classification. J. Pain 17, T93–T107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auvray M, Myin E, Spence C, The sensory-discriminative and affective-motivational aspects of pain. Neurosci. Biobehav. Rev 34, 214–223 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Craig AD, How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci 3, 655–666 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Melzack R, Casey KL, Sensory, motivational, and central control determinants of pain: A new conceptual model, in The Skin Senses, Kenshalo DR, Ed. (Charles C Thomas Publisher, 1968), pp. 423–439. [Google Scholar]
- 24.Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song X-J, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K, The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 161, 1976–1982 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeDoux JE, What emotions might be like in other animals. Curr. Biol 31, R824–R829 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Anderson DJ, Adolphs R, A framework for studying emotions across species. Cell 157, 187–200 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peirs C, Williams S-PG, Zhao X, Arokiaraj CM, Ferreira DW, Noh M-C, Smith KM, Halder P, Corrigan KA, Gedeon JY, Lee SJ, Gatto G, Chi D, Ross SE, Goulding M, Seal RP, Mechanical allodynia circuitry in the dorsal horn is defined by the nature of the injury. Neuron 109, 73–90.e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gatto G, Bourane S, Ren X, Di Costanzo S, Fenton PK, Halder P, Seal RP, Goulding MD, A functional topographic map for spinal sensorimotor reflexes. Neuron 109, 91–104.e5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barik A, Thompson JH, Seltzer M, Ghitani N, Chesler AT, A brainstem-spinal circuit controlling nocifensive behavior. Neuron 100, 1491–1503.e3 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Woolf CJ, Long term alterations in the excitability of the flexion reflex produced by peripheral tissue injury in the chronic decerebrate rat. Pain 18, 325–343 (1984). [DOI] [PubMed] [Google Scholar]
- 31.Choi S, Hachisuka J, Brett MA, Magee AR, Omori Y, Iqbal N-U-A, Zhang D, DeLisle MM, Wolfson RL, Bai L, Santiago C, Gong S, Goulding M, Heintz N, Koerber HR, Ross SE, Ginty DD, Parallel ascending spinal pathways for affective touch and pain. Nature 587, 258–263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braz J, Solorzano C, Wang X, Basbaum AI, Transmitting pain and itch messages: A contemporary view of the spinal cord circuits that generate gate control. Neuron 82, 522–536 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peirs C, Seal RP, Neural circuits for pain: Recent advances and current views. Science 354, 578–584 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scherrer G, Befort K, Contet C, Becker J, Matifas A, Kieffer BL, The delta agonists DPDPE and deltorphin II recruit predominantly mu receptors to produce thermal analgesia: A parallel study of mu, delta and combinatorial opioid receptor knockout mice. Eur. J. Neurosci 19, 2239–2248 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ, Anatomy of CNS opioid receptors. Trends Neurosci. 11, 308–314 (1988). [DOI] [PubMed] [Google Scholar]
- 36.Wang D, Tawfik VL, Corder G, Low SA, François A, Basbaum AI, Scherrer G, Functional divergence of delta and mu opioid receptor organization in CNS pain circuits. Neuron 98, 90–108.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corder G, Ahanonu B, Grewe BF, Wang D, Schnitzer MJ, Scherrer G, An amygdalar neural ensemble that encodes the unpleasantness of pain. Science 363, 276–281 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, Sotoudeh C, Clark JD, Barres BA, Bohlen CJ, Scherrer G, Loss of μ opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat. Med 23, 164–173 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hübner H, Huang X-P, Sassano MF, Giguère PM, Löber S, Duan D, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, Shoichet BK, Structure-based discovery of opioid analgesics with reduced side effects. Nature 537, 185–190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiltschko AB, Tsukahara T, Zeine A, Anyoha R, Gillis WF, Markowitz JE, Peterson RE, Katon J, Johnson MJ, Datta SR, Revealing the structure of pharmacobehavioral space through motion sequencing. Nat. Neurosci 23, 1433–1443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fried NT, Chamessian A, Zylka MJ, Abdus-Saboor I, Improving pain assessment in mice and rats with advanced videography and computational approaches. Pain 161, 1420–1424 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, Bethge M, DeepLabCut: Markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci 21, 1281–1289 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Sufka KJ, Conditioned place preference paradigm: A novel approach for analgesic drug assessment against chronic pain. Pain 58, 355–366 (1994). [DOI] [PubMed] [Google Scholar]
- 44.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F, Unmasking the tonic-aversive state in neuropathic pain. Nat. Neurosci 12, 1364–1366 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuttle AH, Molinaro MJ, Jethwa JF, Sotocinal SG, Prieto JC, Styner MA, Mogil JS, Zylka MJ, A deep neural network to assess spontaneous pain from mouse facial expressions. Mol. Pain 14, 174480691876365 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AMJM, Ferrari MD, Craig KD, Mogil JS, Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 7, 447–449 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ, Inflammation-induced decrease in voluntary wheel running in mice: A nonreflexive test for evaluating inflammatory pain and analgesia. Pain 153, 876–884 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrews NA, Latrémolière A, Basbaum AI, Mogil JS, Porreca F, Rice ASC, Woolf CJ, Currie GL, Dworkin RH, Eisenach JC, Evans S, Gewandter JS, Gover TD, Handwerker H, Huang W, Iyengar S, Jensen MP, Kennedy JD, Lee N, Levine J, Lidster K, Machin I, McDermott MP, McMahon SB, Price TJ, Ross SE, Scherrer G, Seal RP, Sena ES, Silva E, Stone L, Svensson CI, Turk DC, Whiteside G, Ensuring transparency and minimization of methodologic bias in preclinical pain research: PPRECISE considerations. Pain 157, 901–909 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Negus SS, Addressing the opioid crisis: The importance of choosing translational endpoints in analgesic drug discovery. Trends Pharmacol. Sci 39, 327–330 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ingvar M, Pain and functional imaging. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci 354, 1347–1358 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moulton EA, Schmahmann JD, Becerra L, Borsook D, The cerebellum and pain: Passive integrator or active participator? Brain Res. Rev 65, 14–27 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peyron R, Laurent B, García-Larrea L, Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol. Clin 30, 263–288 (2000). [DOI] [PubMed] [Google Scholar]
- 53.Iannetti GD, Mouraux A, From the neuromatrix to the pain matrix (and back). Exp. Brain Res 205, 1–12 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Davis KD, Flor H, Greely HT, Iannetti GD, Mackey S, Ploner M, Pustilnik A, Tracey I, Treede R-D, Wager TD, Brain imaging tests for chronic pain: Medical, legal and ethical issues and recommendations. Nat. Rev. Neurol 13, 624–638 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Kucyi A, Davis KD, The dynamic pain connectome. Trends Neurosci. 38, 86–95 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Martucci KT, Mackey SC, Neuroimaging of pain: Human evidence and clinical relevance of central nervous system processes and modulation. Anesthesiology 128, 1241–1254 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo C-W, Kross E, An fMRI-based neurologic signature of physical pain. N. Engl. J. Med 368, 1388–1397 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown JE, Chatterjee N, Younger J, Mackey S, Towards a physiology-based measure of pain: Patterns of human brain activity distinguish painful from non-painful thermal stimulation. PLOS ONE 6, e24124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang M, Mouraux A, Hu L, Iannetti GD, Primary sensory cortices contain distinguishable spatial patterns of activity for each sense. Nat. Commun 4, 1979 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee J-J, Kim HJ, Čeko M, Park B.-y., Lee SA, Park H, Roy M, Kim S-G, Wager TD, Woo C-W, A neuroimaging biomarker for sustained experimental and clinical pain. Nat. Med 27, 174–182 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laubach M, Amarante LM, Swanson K, White SR, What, if anything, is rodent prefrontal cortex? eNeuro 5, ENEURO.0315–18.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Heukelum S, Mars RB, Guthrie M, Buitelaar JK, Beckmann CF, Tiesinga PHE, Vogt BA, Glennon JC, Havenith MN, Where is cingulate cortex? A cross-species view. Trends Neurosci. 43, 285–299 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Craig AD, A rat is not a monkey is not a human: Comment on Mogil (Nature Rev. Neurosci. 10, 283–294 (2009)). Nat. Rev. Neurosci 10, 466 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Hunt SP, Pini A, Evan G, Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 328, 632–634 (1987). [DOI] [PubMed] [Google Scholar]
- 65.Presley RW, Menétrey D, Levine JD, Basbaum AI, Systemic morphine suppresses noxious stimulus-evoked Fos protein-like immunoreactivity in the rat spinal cord. J. Neurosci 10, 323–335 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Menétrey D, de Pommery J, Besson JM, Electrophysiological characteristics of lumbar spinal cord neurons backfired from lateral reticular nucleus in the rat. J. Neurophysiol 52, 595–611 (1984). [DOI] [PubMed] [Google Scholar]
- 67.Kuner R, Kuner T, Cellular circuits in the brain and their modulation in acute and chronic pain. Physiol. Rev 101, 213–258 (2021). [DOI] [PubMed] [Google Scholar]
- 68.Bushnell MC, Čeko M, Low LA, Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci 14, 502–511 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan LL, Kuner R, Neocortical circuits in pain and pain relief. Nat. Rev. Neurosci 22, 458–471 (2021). [DOI] [PubMed] [Google Scholar]
- 70.Campos CA, Bowen AJ, Roman CW, Palmiter RD, Encoding of danger by parabrachial CGRP neurons. Nature 555, 617–622 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palmiter RD, The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 41, 280–293 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chiang MC, Bowen A, Schier LA, Tupone D, Uddin O, Heinricher MM, Parabrachial complex: A hub for pain and aversion. J. Neurosci 39, 8225–8230 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mehler WR, Some neurological species differences—A posteriori. Ann. N. Y. Acad. Sci 167, 424–468 (1969). [Google Scholar]
- 74.Wiberg M, Blomqvist A, The spinomesencephalic tract in the cat: Its cells of origin and termination pattern as demonstrated by the intraaxonal transport method. Brain Res. 291, 1–18 (1984). [DOI] [PubMed] [Google Scholar]
- 75.Hylden JL, Hayashi H, Bennett GJ, Dubner R, Spinal lamina I neurons projecting to the parabrachial area of the cat midbrain. Brain Res. 336, 195–198 (1985). [DOI] [PubMed] [Google Scholar]
- 76.Cameron D, Polgár E, Gutierrez-Mecinas M, Gomez-Lima M, Watanabe M, Todd AJ, The organisation of spinoparabrachial neurons in the mouse. Pain 156, 2061–2071 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roome RB, Brian Roome R, Bourojeni FB, Mona B, Rastegar-Pouyani S, Blain R, Dumouchel A, Salesse C, Thompson WS, Brookbank M, Gitton Y, Tessarollo L, Goulding M, Johnson JE, Kmita M, Chédotal A, Kania A, Phox2a defines a developmental origin of the anterolateral system in mice and humans. Cell Rep. 33, 108425 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang T, Lin S-H, Malewicz NM, Zhang Y, Zhang Y, Goulding M, LaMotte RH, Ma Q, Identifying the pathways required for coping behaviours associated with sustained pain. Nature 565, 86–90 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O’Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI, Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J. Neurosci 31, 5067–5077 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodriguez E, Sakurai K, Xu J, Chen Y, Toda K, Zhao S, Han B-X, Ryu D, Yin H, Liedtke W, Wang F, A craniofacial-specific monosynaptic circuit enables heightened affective pain. Nat. Neurosci 20, 1734–1743 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alhadeff AL, Su Z, Hernandez E, Klima ML, Phillips SZ, Holland RA, Guo C, Hantman AW, De Jonghe BC, Betley JN, A neural circuit for the suppression of pain by a competing need state. Cell 173, 140–152.e15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pignatelli M, Beyeler A, Valence coding in amygdala circuits. Curr. Opin. Behav. Sci 26, 97–106 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pessoa L, Adolphs R, Emotion processing and the amygdala: From a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat. Rev. Neurosci 11, 773–782 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Janak PH, Tye KM, From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.LeDoux J, The amygdala. Curr. Biol 17, R868–R874 (2007). [DOI] [PubMed] [Google Scholar]
- 86.Bernard JF, Peschanski M, Besson JM, A possible spino (trigemino)-ponto-amygdaloid pathway for pain. Neurosci. Lett 100, 83–88 (1989). [DOI] [PubMed] [Google Scholar]
- 87.Bernard JF, Besson JM, The spino(trigemino)pontoamygdaloid pathway: Electrophysiological evidence for an involvement in pain processes. J. Neurophysiol 63, 473–490 (1990). [DOI] [PubMed] [Google Scholar]
- 88.Carrasquillo Y, Gereau RW 4th, Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. J. Neurosci 27, 1543–1551 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Neugebauer V, Li W, Bird GC, Bhave G, Gereau IV RW, Synaptic plasticity in the amygdala in a model of arthritic pain: Differential roles of metabotropic glutamate receptors 1 and 5. J. Neurosci 23, 52–63 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neugebauer V, Li W, Processing of nociceptive mechanical and thermal information in central amygdala neurons with knee-joint input. J. Neurophysiol 87, 103–112 (2002). [DOI] [PubMed] [Google Scholar]
- 91.Hua T, Chen B, Lu D, Sakurai K, Zhao S, Han B-X, Kim J, Yin L, Chen Y, Lu J, Wang F, General anesthetics activate a potent central pain-suppression circuit in the amygdala. Nat. Neurosci 23, 854–868 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD, Elucidating an affective pain circuit that creates a threat memory. Cell 162, 363–374 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chiang MC, Nguyen EK, Canto-Bustos M, Papale AE, Oswald A-MM, Ross SE, Divergent neural pathways emanating from the lateral parabrachial nucleus mediate distinct components of the pain response. Neuron 106, 927–939.e5 (2020). [DOI] [PubMed] [Google Scholar]
- 94.Deng J, Zhou H, Lin J-K, Shen Z-X, Chen W-Z, Wang L-H, Li Q, Mu D, Wei Y-C, Xu X-H, Sun Y-G, The parabrachial nucleus directly channels spinal nociceptive signals to the intralaminar thalamic nuclei, but not the amygdala. Neuron 107, 909–923.e6 (2020). [DOI] [PubMed] [Google Scholar]
- 95.Barik A, Sathyamurthy A, Thompson J, Seltzer M, Levine A, Chesler A, A spinoparabrachial circuit defined by Tacr1 expression drives pain. eLife 10, e61135 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grady F, Peltekian L, Iverson G, Geerling JC, Direct parabrachial–cortical connectivity. Cereb. Cortex 30, 4811–4833 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilson TD, Valdivia S, Khan A, Ahn H-S, Adke AP, Gonzalez SM, Sugimura YK, Carrasquillo Y, Dual and opposing functions of the central amygdala in the modulation of pain. Cell Rep. 29, 332–346.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Veinante P, Yalcin I, Barrot M, The amygdala between sensation and affect: A role in pain. J. Mol. Psychiatry 1, 9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Neugebauer V, Amygdala pain mechanisms. Handb. Exp. Pharmacol 227, 261–284 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adke AP, Khan A, Ahn H-S, Becker JJ, Wilson TD, Valdivia S, Sugimura YK, Martinez Gonzalez S, Carrasquillo Y, Cell-type specificity of neuronal excitability and morphology in the central amygdala. eNeuro 8, ENEURO.0402–ENEU20.2020 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Neugebauer V, Mazzitelli M, Cragg B, Ji G, Navratilova E, Porreca F, Amygdala, neuropeptides, and chronic pain-related affective behaviors. Neuropharmacology 170, 108052 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li J-N, Sheets PL, The central amygdala to periaqueductal gray pathway comprises intrinsically distinct neurons differentially affected in a model of inflammatory pain. J. Physiol 596, 6289–6305 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Okutsu Y, Takahashi Y, Nagase M, Shinohara K, Ikeda R, Kato F, Potentiation of NMDA receptor-mediated synaptic transmission at the parabrachial-central amygdala synapses by CGRP in mice. Mol. Pain 13, 174480691770920 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li J-N, Sheets PL, Spared nerve injury differentially alters parabrachial monosynaptic excitatory inputs to molecularly specific neurons in distinct subregions of the central amygdala. Pain 161, 166–176 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jenne C, Morales M, Masri R, Keller A, An amygdalo-parabrachial pathway regulates pain perception and chronic pain. J. Neurosci 40, 3424–3442 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tovote P, Esposito MS, Botta P, Chaudun F, Fadok JP, Markovic M, Wolff SBE, Ramakrishnan C, Fenno L, Deisseroth K, Herry C, Arber S, Lüthi A, Midbrain circuits for defensive behaviour. Nature 534, 206–212 (2016). [DOI] [PubMed] [Google Scholar]
- 107.Norris AJ, Shaker JR, Cone AL, Ndiokho IB, Bruchas MR, Parabrachial opioidergic projections to preoptic hypothalamus mediate behavioral and physiological thermal defenses. eLife 10, e60779 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wertli MM, Burgstaller JM, Weiser S, Steurer J, Kofmehl R, Held U, Influence of catastrophizing on treatment outcome in patients with nonspecific low back pain: A systematic review. Spine 39, 263–273 (2014). [DOI] [PubMed] [Google Scholar]
- 109.Quartana PJ, Campbell CM, Edwards RR, Pain catastrophizing: A critical review. Expert. Rev. Neurother 9, 745–758 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Darnall BD, Pain psychology and pain catastrophizing in the perioperative setting: A review of impacts, interventions, and unmet needs. Hand Clin. 32, 33–39 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Galambos A, Szabó E, Nagy Z, Édes AE, Kocsel N, Juhász G, Kökönyei G, A systematic review of structural and functional MRI studies on pain catastrophizing. J. Pain Res 12, 1155–1178 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Seminowicz DA, Davis KD, Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain 120, 297–306 (2006). [DOI] [PubMed] [Google Scholar]
- 113.Jiang Y, Oathes D, Hush J, Darnall B, Charvat M, Mackey S, Etkin A, Perturbed connectivity of the amygdala and its subregions with the central executive and default mode networks in chronic pain. Pain 157, 1970–1978 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hebben N, Corkin S, Eichenbaum H, Shedlack K, Diminished ability to interpret and report internal states after bilateral medial temporal resection: Case H.M. Behav. Neurosci 99, 1031–1039 (1985). [DOI] [PubMed] [Google Scholar]
- 115.Annese J, Schenker-Ahmed NM, Bartsch H, Maechler P, Sheh C, Thomas N, Kayano J, Ghatan A, Bresler N, Frosch MP, Klaming R, Corkin S, Postmortem examination of patient HM’s brain based on histological sectioning and digital 3D reconstruction. Nat. Commun 5, 3122 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grewe BF, Gründemann J, Kitch LJ, Lecoq JA, Parker JG, Marshall JD, Larkin MC, Jercog PE, Grenier F, Li JZ, Lüthi A, Schnitzer MJ, Neural ensemble dynamics underlying a long-term associative memory. Nature 543, 670–675 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McCallum WM, Wang D, Shuster A, Berg DJ, Low SA, Scherrer G, A suite of knockin mouse lines to resolve the functional organization of opioid receptors in neural circuits, in 2019 Neuroscience Meeting Planner (Society for Neuroscience, 2019). [Google Scholar]
- 118.Thompson JM, Neugebauer V, Cortico-limbic pain mechanisms. Neurosci. Lett 702, 15–23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yen C-T, Lu P-L, Thalamus and pain. Acta Anaesthesiol. Taiwanica 51, 73–80 (2013). [DOI] [PubMed] [Google Scholar]
- 120.Melzack R, Casey KL, in The Skin Senses, Kenshalo DR, Ed. (Charles C Thomas Publisher, 1986), pp. 423–435. [Google Scholar]
- 121.Gebhart GF, Bielefeldt K, Physiology of visceral pain. Compr. Physiol 6, 1609–1633 (2016). [DOI] [PubMed] [Google Scholar]
- 122.Krout KE, Belzer RE, Loewy AD, Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J. Comp. Neurol 448, 53–101 (2002). [DOI] [PubMed] [Google Scholar]
- 123.Krout KE, Loewy AD, Parabrachial nucleus projections to midline and intralaminar thalamic nuclei of the rat. J. Comp. Neurol 428, 475–494 (2000). [DOI] [PubMed] [Google Scholar]
- 124.Gauriau C, Bernard JF, A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: The forebrain. J. Comp. Neurol 468, 24–56 (2004). [DOI] [PubMed] [Google Scholar]
- 125.Groenewegen HJ, Berendse HW, The specificity of the “nonspecific” midline and intralaminar thalamic nuclei. Trends Neurosci. 17, 52–57 (1994). [DOI] [PubMed] [Google Scholar]
- 126.Birdsong WT, Jongbloets BC, Engeln KA, Wang D, Scherrer G, Mao T, Synapse-specific opioid modulation of thalamo-cortico-striatal circuits. eLife 8, e45146 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Meda KS, Patel T, Braz JM, Malik R, Turner ML, Seifikar H, Basbaum AI, Sohal VS, Microcircuit mechanisms through which mediodorsal thalamic input to anterior cingulate cortex exacerbates pain-related aversion. Neuron 102, 944–959.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Phillips JW, Schulmann A, Hara E, Winnubst J, Liu C, Valakh V, Wang L, Shields BC, Korff W, Chandrashekar J, Lemire AL, Mensh B, Dudman JT, Nelson SB, Hantman AW, A repeated molecular architecture across thalamic pathways. Nat. Neurosci 22, 1925–1935 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kuramoto E, Pan S, Furuta T, Tanaka YR, Iwai H, Yamanaka A, Ohno S, Kaneko T, Goto T, Hioki H, Individual mediodorsal thalamic neurons project to multiple areas of the rat prefrontal cortex: A single neuron-tracing study using virus vectors. J. Comp. Neurol 525, 166–185 (2017). [DOI] [PubMed] [Google Scholar]
- 130.Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ, Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol 492, 145–177 (2005). [DOI] [PubMed] [Google Scholar]
- 131.Tang JS, Qu CL, Huo FQ, The thalamic nucleus submedius and ventrolateral orbital cortex are involved in nociceptive modulation: A novel pain modulation pathway. Prog. Neurobiol 89, 383–389 (2009). [DOI] [PubMed] [Google Scholar]
- 132.Sun Y, Wang J, Liang SH, Ge J, Lu YC, Li JN, Chen YB, Luo DS, Li H, Li YQ, Involvement of the ventrolateral periaqueductal gray matter-central medial thalamic nucleus-basolateral amygdala pathway in neuropathic pain regulation of rats. Front. Neuroanat 14, 32 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kayyal H, Yiannakas A, Chandran SK, Khamaisy M, Sharma V, Rosenblum K, Activity of insula to basolateral amygdala projecting neurons is necessary and sufficient for taste valence representation. J. Neurosci 39, 9369–9382 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Allsop SA, Wichmann R, Mills F, Burgos-Robles A, Chang CJ, Felix-Ortiz AC, Vienne A, Beyeler A, Izadmehr EM, Glober G, Cum MI, Stergiadou J, Anandalingam KK, Farris K, Namburi P, Leppla CA, Weddington JC, Nieh EH, Smith AC, Ba D, Brown EN, Tye KM, Corticoamygdala transfer of socially derived information gates observational learning. Cell 173, 1329–1342.e18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ong W-Y, Stohler CS, Herr DR, Role of the prefrontal cortex in pain processing. Mol. Neurobiol 56, 1137–1166 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K, Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 9, 463–484 (2005). [DOI] [PubMed] [Google Scholar]
- 137.Lamm C, Decety J, Singer T, Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage 54, 2492–2502 (2011). [DOI] [PubMed] [Google Scholar]
- 138.Mazzola L, Isnard J, Peyron R, Mauguire F, Stimulation of the human cortex and the experience of pain: Wilder Penfield’s observations revisited. Brain 135, 631–640 (2012). [DOI] [PubMed] [Google Scholar]
- 139.Gehrlach DA, Weiand C, Gaitanos TN, Cho E, Klein AS, Hennrich AA, Conzelmann KK, Gogolla N, A whole-brain connectivity map of mouse insular cortex. eLife 9, e55585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Picchio V, Cammisotto V, Pagano F, Carnevale R, Chimenti I, The insular cortex and the amygdala: Shared functions and interactions. Dermatol. Int, 1–15 (2012). [Google Scholar]
- 141.Galhardoni R, Da Silva VA, García-Larrea L, Dale C, Baptista AF, Barbosa LM, Bahia Menezes LM, De Siqueira SRDT, Valério F, Rosi J, De Lima Rodrigues AL, Mendes Fernandes DTR, Lorencini Selingardi PM, Marcolin MA, De Souza Duran FL, Ono CR, Lucato LT, Fernandes AMBL, Da Silva FEF, Yeng LT, Brunoni AR, Buchpiguel CA, Teixeira MJ, De Andrade DC, Insular and anterior cingulate cortex deep stimulation for central neuropathic pain disassembling the percept of pain. Neurology 92, e2165–e2175 (2019). [DOI] [PubMed] [Google Scholar]
- 142.Tan LL, Pelzer P, Heinl C, Tang W, Gangadharan V, Flor H, Sprengel R, Kuner T, Kuner R, A pathway from midcingulate cortex to posterior insula gates nociceptive hypersensitivity. Nat. Neurosci 20, 1591–1601 (2017). [DOI] [PubMed] [Google Scholar]
- 143.Barthas F, Sellmeijer J, Hugel S, Waltisperger E, Barrot M, Yalcin I, The anterior cingulate cortex is a critical hub for pain-induced depression. Biol. Psychiatry 77, 236–245 (2015). [DOI] [PubMed] [Google Scholar]
- 144.Burkey AR, Carstens E, Wenniger JJ, Tang J, Jasmin L, An opioidergic cortical antinociception triggering site in the agranular insular cortex of the rat that contributes to morphine antinociception. J. Neurosci 16, 6612–6623 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jasmin L, Granato A, Ohara PT, Rostral agranular insular cortex and pain areas of the central nervous system: A tract-tracing study in the rat. J. Comp. Neurol 468, 425–440 (2004). [DOI] [PubMed] [Google Scholar]
- 146.Kim J, Pignatelli M, Xu S, Itohara S, Tonegawa S, Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci 19, 1636–1646 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Livneh Y, Sugden AU, Madara JC, Essner RA, Flores VI, Sugden LA, Resch JM, Lowell BB, Andermann ML, Estimation of current and future physiological states in insular cortex. Neuron 105, 1094–1111.e10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Berg DJ, Chen C, Ahanonu B, Chen M, Quake S, Schnitzer MJ, Scherrer G, Resolving the molecular identity and connectivity of amygdalar neural ensembles active during pain, in 2019 Neuroscience Meeting Planner (Society for Neuroscience, 2019). [Google Scholar]
- 149.Vogt BA, Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci 6, 533–544 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wager TD, Atlas LY, Botvinick MM, Chang LJ, Coghill RC, Davis KD, Iannetti GD, Poldrack RA, Shackman AJ, Yarkoni T, Pain in the ACC? Proc. Natl. Acad. Sci. U.S.A 113, E2474–E2475 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ebitz RB, Hayden BY, Dorsal anterior cingulate: A Rorschach test for cognitive neuroscience. Nat. Neurosci 19, 1278–1279 (2016). [DOI] [PubMed] [Google Scholar]
- 152.Vogt BA, Paxinos G, Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct. Funct 219, 185–192 (2014). [DOI] [PubMed] [Google Scholar]
- 153.Vogt BA, Midcingulate cortex: Structure, connections, homologies, functions and diseases. J. Chem. Neuroanat 74, 28–46 (2016). [DOI] [PubMed] [Google Scholar]
- 154.Mussio CA, Harte SE, Borszcz GS, Regional differences within the anterior cingulate cortex in the generation versus suppression of pain affect in rats. J. Pain 21, 121–134 (2020). [DOI] [PubMed] [Google Scholar]
- 155.Fuchs PN, Peng YB, Boyette-Davis JA, Uhelski ML, The anterior cingulate cortex and pain processing. Front. Integr. Neurosci 8, 35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Strauss I, Berger A, Ben Moshe S, Arad M, Hochberg U, Gonen T, Tellem R, Double anterior stereotactic cingulotomy for intractable oncological pain. Stereotact. Funct. Neurosurg 95, 400–408 (2018). [DOI] [PubMed] [Google Scholar]
- 157.Davis KD, Hutchison WD, Lozano AM, Dostrovsky JO, Altered pain and temperature perception following cingulotomy and capsulotomy in a patient with schizoaffective disorder. Pain 59, 189–199 (1994). [DOI] [PubMed] [Google Scholar]
- 158.Bliss TVP, Collingridge GL, Kaang B-K, Zhuo M, Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat. Rev. Neurosci 17, 485–496 (2016). [DOI] [PubMed] [Google Scholar]
- 159.Santello M, Nevian T, Dysfunction of cortical dendritic integration in neuropathic pain reversed by serotoninergic neuromodulation. Neuron 86, 233–246 (2015). [DOI] [PubMed] [Google Scholar]
- 160.Matos SC, Zhang Z, Séguéla P, Peripheral neuropathy induces HCN channel dysfunction in pyramidal neurons of the medial prefrontal cortex. J. Neurosci 35, 13244–13256 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou H, Zhang Q, Martinez E, Dale J, Hu S, Zhang E, Liu K, Huang D, Yang G, Chen Z, Wang J, Ketamine reduces aversion in rodent pain models by suppressing hyperactivity of the anterior cingulate cortex. Nat. Commun 9, 3751 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Moon HC, Heo WI, Kim YJ, Lee D, Won SY, Kim HR, Ha SM, Lee YJ, Park YS, Optical inactivation of the anterior cingulate cortex modulate descending pain pathway in a rat model of trigeminal neuropathic pain created via chronic constriction injury of the infraorbital nerve. J. Pain Res 10, 2355–2364 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Elina KC, Moon HC, Islam J, Kim HK, Park YS, The effect of optogenetic inhibition of the anterior cingulate cortex in neuropathic pain following sciatic nerve injury. J. Mol. Neurosci 71, 638–650 (2021). [DOI] [PubMed] [Google Scholar]
- 164.Koga K, Descalzi G, Chen T, Ko HG, Lu J, Li S, Son J, Kim TH, Kwak C, Huganir RL, Zhao MG, Kaang BK, Collingridge GL, Zhuo M, Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron 85, 377–389 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shih HC, Yang JW, Lee CM, Shyu BC, Spontaneous cingulate high-current spikes signal normal and pathological pain states. J. Neurosci 39, 5128–5142 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yang JW, Shih HC, Shyu BC, Intracortical circuits in rat anterior cingulate cortex are activated by nociceptive inputs mediated by medial thalamus. J. Neurophysiol 96, 3409–3422 (2006). [DOI] [PubMed] [Google Scholar]
- 167.Corradi-Dell’Acqua C, Tusche A, Vuilleumier P, Singer T, Cross-modal representations of first-hand and vicarious pain, disgust and fairness in insular and cingulate cortex. Nat. Commun 7, 10904 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO, Pain-related neurons in the human cingulate cortex. Nat. Neurosci 2, 403–405 (1999). [DOI] [PubMed] [Google Scholar]
- 169.Meyza KZ, Bartal IB-A, Monfils MH, Panksepp JB, Knapska E, The roots of empathy: Through the lens of rodent models. Neurosci. Biobehav. Rev 76, 216–234 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Smith ML, Asada N, Malenka RC, Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science 371, 153–159 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brown VJ, Bowman EM, Rodent models of prefrontal cortical function. Trends Neurosci. 25, 340–343 (2002). [DOI] [PubMed] [Google Scholar]
- 172.Shiers S, Price TJ, Molecular, circuit, and anatomical changes in the prefrontal cortex in chronic pain. Pain 161, 1726–1729 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bicks LK, Koike H, Akbarian S, Morishita H, Prefrontal cortex and social cognition in mouse and man. Front. Psychol 6, 1805 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ostlund SB, Balleine BW, Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J. Neurosci 27, 4819–4825 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mulder AB, Nordquist RE, Orgüt O, Pennartz CMA, Learning-related changes in response patterns of prefrontal neurons during instrumental conditioning. Behav. Brain Res 146, 77–88 (2003). [DOI] [PubMed] [Google Scholar]
- 176.Ledoux JE, Brown R, A higher-order theory of emotional consciousness. Proc. Natl. Acad. Sci. U.S.A 114, E2016–E2025 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sigurdsson T, Duvarci S, Hippocampal-prefrontal interactions in cognition, behavior and psychiatric disease. Front. Syst. Neurosci 9, 190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cardoso-Cruz H, Lima D, Galhardo V, Impaired spatial memory performance in a rat model of neuropathic pain is associated with reduced hippocampus–prefrontal cortex connectivity. J. Neurosci 33, 2465–2480 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lidhar NK, Darvish-Ghane S, Sivaselvachandran S, Khan S, Wasif F, Turner H, Sivaselvachandran M, Fournier NM, Martin LJ, Prelimbic cortex glucocorticoid receptors regulate the stress-mediated inhibition of pain contagion in male mice. Neuropsychopharmacology 46, 1183–1193 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang S, Zhang Z, Gambeta E, Xu SC, Thomas C, Godfrey N, Chen L, M’Dahoma S, Borgland SL, Zamponi GW, Dopamine inputs from the ventral tegmental area into the medial prefrontal cortex modulate neuropathic pain-associated behaviors in mice. Cell Rep. 31, 107812 (2020). [DOI] [PubMed] [Google Scholar]
- 181.Wang GQ, Cen C, Li C, Cao S, Wang N, Zhou Z, Liu XM, Xu Y, Tian NX, Zhang Y, Wang J, Wang LP, Wang Y, Deactivation of excitatory neurons in the prelimbic cortex via Cdk5 promotes pain sensation and anxiety. Nat. Commun 6, 7660 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yue L, Ma L-Y, Cui S, Liu F-Y, Yi M, Wan Y, Brain-derived neurotrophic factor in the infralimbic cortex alleviates inflammatory pain. Neurosci. Lett 655, 7–13 (2017). [DOI] [PubMed] [Google Scholar]
- 183.Kummer KK, Mitrić M, Kalpachidou T, Kress M, The medial prefrontal cortex as a central hub for mental comorbidities associated with chronic pain. Int. J. Mol. Sci 21, 3440 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shih Y-W, Tsai H-Y, Lin F-S, Lin Y-H, Chiang C-Y, Lu Z-L, Tseng XM-T, Effects of positive and negative expectations on human pain perception engage separate but interrelated and dependently regulated cerebral mechanisms. J. Neurosci 39, 1261–1274 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Foley BS, Wall and Melzack’s Textbook of Pain, 5th Edition. Am. J. Phys. Med. Rehabil 85, 581 (2006). [Google Scholar]
- 186.Geuter S, Eippert F, Hindi Attar C, Büchel C, Cortical and subcortical responses to high and low effective placebo treatments. NeuroImage 67, 227–236 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Amanzio M, Benedetti F, Porro CA, Palermo S, Cauda F, Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum. Brain Mapp 34, 738–752 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zunhammer M, Spisák T, Wager TD, Bingel U; Placebo Imaging Consortium, Meta-analysis of neural systems underlying placebo analgesia from individual participant fMRI data. Nat. Commun 12, 1391 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Keller A, Akintola T, Colloca L, Placebo analgesia in rodents: Current and future research. Int. Rev. Neurobiol, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kim S. i., Neuroscientific model of motivational process. Front. Psychol 4, 98 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Loggia ML, Berna C, Kim J, Cahalan CM, Gollub RL, Wasan AD, Harris RE, Edwards RR, Napadow V, Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheum. 66, 203–212 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Serafini RA, Pryce KD, Zachariou V, The mesolimbic dopamine system in chronic pain and associated affective comorbidities. Biol. Psychiatry 87, 64–73 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yu S, Li W, Shen W, Edwards RR, Gollub RL, Wilson G, Park J, Ortiz A, Cao J, Gerber J, Mawla I, Chan S-T, Lee J, Wasan AD, Napadow V, Kaptchuk TJ, Rosen B, Kong J, Impaired mesocorticolimbic connectivity underlies increased pain sensitivity in chronic low back pain. NeuroImage 218, 116969 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fairhurst M, Wiech K, Dunckley P, Tracey I, Anticipatory brainstem activity predicts neural processing of pain in humans. Pain 128, 101–110 (2007). [DOI] [PubMed] [Google Scholar]
- 195.Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S, Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron 40, 1251–1257 (2003). [DOI] [PubMed] [Google Scholar]
- 196.Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D, Reward circuitry activation by noxious thermal stimuli. Neuron 32, 927–946 (2001). [DOI] [PubMed] [Google Scholar]
- 197.Forkmann K, Wiech K, Sommer T, Bingel U, Reinstatement of pain-related brain activation during the recognition of neutral images previously paired with nociceptive stimuli. Pain 156, 1501–1510 (2015). [DOI] [PubMed] [Google Scholar]
- 198.Becerra L, Navratilova E, Porreca F, Borsook D, Analogous responses in the nucleus accumbens and cingulate cortex to pain onset (aversion) and offset (relief) in rats and humans. J. Neurophysiol 110, 1221–1226 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bourdy R, Barrot M, A new control center for dopaminergic systems: Pulling the VTA by the tail. Trends Neurosci. 35, 681–690 (2012). [DOI] [PubMed] [Google Scholar]
- 200.Ferrario CR, Labouèbe G, Liu S, Nieh EH, Routh VH, Xu S, O’Connor EC, Homeostasis meets motivation in the battle to control food intake. J. Neurosci 36, 11469–11481 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Morikawa H, Paladini CA, Dynamic regulation of midbrain dopamine neuron activity: Intrinsic, synaptic, and plasticity mechanisms. Neuroscience 198, 95–111 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Morales M, Margolis EB, Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci 18, 73–85 (2017). [DOI] [PubMed] [Google Scholar]
- 203.Beier KT, Gao XJ, Xie S, DeLoach KE, Malenka RC, Luo L, Topological organization of ventral tegmental area connectivity revealed by viral-genetic dissection of input-output relations. Cell Rep. 26, 159–167.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ren W, Centeno MV, Berger S, Wu Y, Na X, Liu X, Kondapalli J, Apkarian AV, Martina M, Surmeier DJ, The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat. Neurosci 19, 220–222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Laurent RS, Martinez Damonte V, Tsuda AC, Kauer JA, Periaqueductal gray and rostromedial tegmental inhibitory afferents to VTA have distinct synaptic plasticity and opiate sensitivity. Neuron 106, 624–636.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Waung MW, Margolis EB, Charbit AR, Fields HL, A midbrain circuit that mediates headache aversiveness in rats. Cell Rep. 28, 2739–2747.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Taylor AMW, Castonguay A, Taylor AJ, Murphy NP, Ghogha A, Cook C, Xue L, Olmstead MC, De Koninck Y, Evans CJ, Cahill CM, Microglia disrupt mesolimbic reward circuitry in chronic pain. J. Neurosci 35, 8442–8450 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Schwartz N, Temkin P, Jurado S, Lim BK, Heifets BD, Polepalli JS, Malenka RC, Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science 345, 535–542 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Massaly N, Copits BA, Wilson-Poe AR, Hipólito L, Markovic T, Yoon HJ, Liu S, Walicki MC, Bhatti DL, Sirohi S, Klaas A, Walker BM, Neve R, Cahill CM, Shoghi KI, Gereau RW 4th, McCall JG, Al-Hasani R, Bruchas MR, Morón JA, Pain-induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron 102, 564–573.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fields HL, How expectations influence pain. Pain 159 (suppl. 1), S3–S10 (2018). [DOI] [PubMed] [Google Scholar]
- 211.Seymour B, Dolan RJ, Emotion, motivation, and pain, in Textbook of Pain (Elsevier, 2013), pp. 248–255. [Google Scholar]
- 212.Schwartz N, Miller C, Fields HL, Cortico-accumbens regulation of approach-avoidance behavior is modified by experience and chronic pain. Cell Rep. 19, 1522–1531 (2017). [DOI] [PubMed] [Google Scholar]
- 213.Lee M, Manders TR, Eberle SE, Su C, D’amour J, Yang R, Lin HY, Deisseroth K, Froemke RC, Wang J, Activation of corticostriatal circuitry relieves chronic neuropathic pain. J. Neurosci 35, 5247–5259 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gao SH, Shen LL, Wen HZ, Zhao YD, Chen PH, Ruan HZ, The projections from the anterior cingulate cortex to the nucleus accumbens and ventral tegmental area contribute to neuropathic pain-evoked aversion in rats. Neurobiol. Dis 140, 104862 (2020). [DOI] [PubMed] [Google Scholar]
- 215.Ziółkowska B, The role of mesostriatal dopamine system and corticostriatal glutamatergic transmission in chronic pain. Brain Sci. 11, 1311 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Taylor AMW, Becker S, Schweinhardt P, Cahill C, Mesolimbic dopamine signaling in acute and chronic pain: Implications for motivation, analgesia, and addiction. Pain 157, 1194–1198 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bannister K, Dickenson AH, The plasticity of descending controls in pain: Translational probing. J. Physiol 595, 4159–4166 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bagley EE, Ingram SL, Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology 173, 108131 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Heinricher MM, Tavares I, Leith JL, Lumb BM, Descending control of nociception: Specificity, recruitment and plasticity. Brain Res. Rev 60, 214–225 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Fields HL, Pain modulation: Expectation, opioid analgesia and virtual pain. Prog. Brain Res 122, 245–253 (2000). [DOI] [PubMed] [Google Scholar]
- 221.Lau BK, Vaughan CW, Descending modulation of pain: The GABA disinhibition hypothesis of analgesia. Curr. Opin. Neurobiol 29, 159–164 (2014). [DOI] [PubMed] [Google Scholar]
- 222.Samineni VK, Grajales-Reyes JG, Copits BA, O’Brien DE, Trigg SL, Gomez AM, Bruchas MR, Gereau RW 4th, Divergent modulation of nociception by glutamatergic and GABAergic neuronal subpopulations in the periaqueductal gray. eNeuro 4, ENEURO.0129–16.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chen T, Taniguchi W, Chen Q-Y, Tozaki-Saitoh H, Song Q, Liu R-H, Koga K, Matsuda T, Kaito-Sugimura Y, Wang J, Li Z-H, Lu Y-C, Inoue K, Tsuda M, Li Y-Q, Nakatsuka T, Zhuo M, Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nat. Commun 9, 1886 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Liu Y, Latremoliere A, Li X, Zhang Z, Chen M, Wang X, Fang C, Zhu J, Alexandre C, Gao Z, Chen B, Ding X, Zhou J-Y, Zhang Y, Chen C, Wang KH, Woolf CJ, He Z, Touch and tactile neuropathic pain sensitivity are set by corticospinal projections. Nature 561, 547–550 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Basbaum AI, Fields HL, Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci 7, 309–338 (1984). [DOI] [PubMed] [Google Scholar]
- 226.Morgan MM, Whittier KL, Hegarty DM, Aicher SA, Periaqueductal gray neurons project to spinally projecting GABAergic neurons in the rostral ventromedial medulla. Pain 140, 376–386 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhang Y, Zhao S, Rodriguez E, Takatoh J, Han B-X, Zhou X, Wang F, Identifying local and descending inputs for primary sensory neurons. J. Clin. Invest 125, 3782–3794 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.François A, Low SA, Sypek EI, Christensen AJ, Sotoudeh C, Beier KT, Ramakrishnan C, Ritola KD, Sharif-Naeini R, Deisseroth K, Delp SL, Malenka RC, Luo L, Hantman AW, Scherrer G, A brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and enkephalins. Neuron 93, 822–839.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kim YS, Chu Y, Han L, Li M, Li Z, LaVinka PC, Sun S, Tang Z, Park K, Caterina MJ, Ren K, Dubner R, Wei F, Dong X, Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 81, 873–887 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hirschberg S, Li Y, Randall A, Kremer EJ, Pickering AE, Functional dichotomy in spinal- vs prefrontal-projecting locus coeruleus modules splits descending noradrenergic analgesia from ascending aversion and anxiety in rats. eLife 6, e29808 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kim J-H, Gangadharan G, Byun J, Choi E-J, Lee CJ, Shin H-S, Yin-and-yang bifurcation of opioidergic circuits for descending analgesia at the midbrain of the mouse. Proc. Natl. Acad. Sci. U.S.A 115, 11078–11083 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Averitt DL, Eidson LN, Doyle HH, Murphy AZ, Neuronal and glial factors contributing to sex differences in opioid modulation of pain. Neuropsychopharmacology 44, 155–165 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Li M-H, Suchland KL, Ingram SL, Compensatory activation of cannabinoid CB2 receptor inhibition of GABA release in the rostral ventromedial medulla in inflammatory pain. J. Neurosci 37, 626–636 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Jareczek FJ, White SR, Hammond DL, Plasticity in brainstem mechanisms of pain modulation by nicotinic acetylcholine receptors in the rat. eNeuro 4, ENEURO.0364–16.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Umana IC, Daniele CA, Miller BA, Abburi C, Gallagher K, Brown MA, Mason P, McGehee DS, Nicotinic modulation of descending pain control circuitry. Pain 158, 1938–1950 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Silva M, Martins D, Charrua A, Piscitelli F, Tavares I, Morgado C, Di Marzo V, Endovanilloid control of pain modulation by the rostroventromedial medulla in an animal model of diabetic neuropathy. Neuropharmacology 107, 49–57 (2016). [DOI] [PubMed] [Google Scholar]
- 237.Marshall TM, Herman DS, Largent-Milnes TM, Badghisi H, Zuber K, Holt SC, Lai J, Porreca F, Vanderah TW, Activation of descending pain-facilitatory pathways from the rostral ventromedial medulla by cholecystokinin elicits release of prostaglandin-E2 in the spinal cord. Pain 153, 86–94 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yu W, Pati D, Pina MM, Schmidt KT, Boyt KM, Hunker AC, Zweifel LS, McElligott ZA, Kash TL, Periaqueductal gray/dorsal raphe dopamine neurons contribute to sex differences in pain-related behaviors. Neuron 109, 1365–1380.e5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Volkow ND, McLellan AT, Opioid abuse in chronic pain–misconceptions and mitigation strategies. N. Engl. J. Med 374, 1253–1263 (2016). [DOI] [PubMed] [Google Scholar]
- 240.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS, Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 293, 311–315 (2001). [DOI] [PubMed] [Google Scholar]
- 241.Fields HL, The doctor’s dilemma: Opiate analgesics and chronic pain. Neuron 69, 591–594 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, Tzavara E, Hanoune J, Roques BP, Kieffer BL, Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383, 819–823 (1996). [DOI] [PubMed] [Google Scholar]
- 243.Birdsong WT, Williams JT, Recent progress in opioid research from an electrophysiological perspective. Mol. Pharmacol 98, 401–409 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ, Regulation of μ-opioid receptors: Desensitization, phosphorylation, internalization, and tolerance. Pharmacol. Rev 65, 223–254 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Dang VC, Christie MJ, Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br. J. Pharmacol 165, 1704–1716 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kupers RC, Konings H, Adriaensen H, Gybels JM, Morphine differentially affects the sensory and affective pain ratings in neurogenic and idiopathic forms of pain. Pain 47, 5–12 (1991). [DOI] [PubMed] [Google Scholar]
- 247.Gomtsian L, Bannister K, Eyde N, Robles D, Dickenson AH, Porreca F, Navratilova E, Morphine effects within the rodent anterior cingulate cortex and rostral ventromedial medulla reveal separable modulation of affective and sensory qualities of acute or chronic pain. Pain 159, 2512–2521 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Navratilova E, Atcherley CW, Porreca F, Brain circuits encoding reward from pain relief. Trends Neurosci. 38, 741–750 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ, Interleukin-1β-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 410, 471–475 (2001). [DOI] [PubMed] [Google Scholar]
- 250.Li L, Chen S-R, Zhou M-H, Wang L, Li D-P, Chen H, Lee G, Jayaraman V, Pan H-L, α2δ-1 switches the phenotype of synaptic AMPA receptors by physically disrupting heteromeric subunit assembly. Cell Rep. 36, 109396 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Alles SRA, Cain SM, Snutch TP, Pregabalin as a pain therapeutic: Beyond calcium channels. Front. Cell. Neurosci 14, 83 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kim D-S, Li K-W, Boroujerdi A, Yu YP, Zhou C-Y, Deng P, Park J, Zhang X, Lee J, Corpe M, Sharp K, Steward O, Eroglu C, Barres B, Zaucke F, Xu ZC, Luo ZD, Thrombospondin-4 contributes to spinal sensitization and neuropathic pain states. J. Neurosci 32, 8977–8987 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Eroglu Ç, Allen NJ, Susman MW, O’Rourke NA, Park CY, Özkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Dolmetsch R, Garcia KC, Smith SJ, Luo ZD, Rosenthal A, Mosher DF, Barres BA, Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 139, 380–392 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wiffen PJ, Derry S, Bell RF, Rice AS, Tölle TR, Phillips T, Moore RA, Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst. Rev 6, CD007938 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Corder G, Castro DC, Bruchas MR, Scherrer G, Endogenous and exogenous opioids in pain. Annu. Rev. Neurosci 41, 453–473 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Bailly J, Del Rossi N, Runtz L, Li J-J, Park D, Scherrer G, Tanti A, Birling M-C, Darcq E, Kieffer BL, Targeting morphine-responsive neurons: Generation of a knock-in mouse line expressing Cre recombinase from the mμ-opioid receptor gene locus. eNeuro 7, ENEURO.0433–19.2020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch J-L, Koch M, Kessler P, Hentsch D, Birling M-C, Koutsourakis M, Vasseur L, Veinante P, Kieffer BL, Massotte D, A mμ–delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct. Funct 220, 677–702 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Scherrer G, Tryoen-Tóth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh J-L, Gavériaux-Ruff C, Kieffer BL, Knockin mice expressing fluorescent δ-opioid receptors uncover G protein-coupled receptor dynamics invivo. Proc. Natl. Acad. Sci. U.S.A 103, 9691–9696 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Snyder LM, Chiang MC, Loeza-Alcocer E, Omori Y, Hachisuka J, Sheahan TD, Gale JR, Adelman PC, Sypek EI, Fulton SA, Friedman RL, Wright MC, Duque MG, Lee YS, Hu Z, Huang H, Cai X, Meerschaert KA, Nagarajan V, Hirai T, Scherrer G, Kaplan DH, Porreca F, Davis BM, Gold MS, Koerber HR, Ross SE, Kappa opioid receptor distribution and function in primary afferents. Neuron 99, 1274–1288.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chen C, Willhouse AH, Huang P, Ko N, Wang Y, Xu B, Huang LHM, Kieffer B, Barbe MF, Liu-Chen L-Y, Characterization of a knock-in mouse line expressing a fusion protein of κ opioid receptor conjugated with tdTomato: 3-dimensional brain imaging via CLARITY. eNeuro 7, ENEURO.0028–20.2020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Chung PCS, Keyworth HL, Martin-Garcia E, Charbogne P, Darcq E, Bailey A, Filliol D, Matifas A, Scherrer G, Ouagazzal A-M, Gaveriaux-Ruff C, Befort K, Maldonado R, Kitchen I, Kieffer BL, A novel anxiogenic role for the delta opioid receptor expressed in GABAergic forebrain neurons. Biol. Psychiatry 77, 404–415 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Weibel R, Reiss D, Karchewski L, Gardon O, Matifas A, Filliol D, Becker JAJ, Wood JN, Kieffer BL, Gaveriaux-Ruff C, Mu opioid receptors on primary afferent nav1.8 neurons contribute to opiate-induced analgesia: Insight from conditional knockout mice. PLOS ONE 8, e74706 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R, Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J. Neurosci 15, 3328–3341 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Brunton J, Charpak S, μ-opioid peptides inhibit thalamic neurons. J. Neurosci 18, 1671–1678 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Goedecke L, Bengoetxea X, Blaesse P, Pape H-C, Jüngling K, μ-opioid receptor-mediated downregulation of midline thalamic pathways to basal and central amygdala. Sci. Rep 9, 17837 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sugita S, Tanaka E, North RA, Membrane properties and synaptic potentials of three types of neurone in rat lateral amygdala. J. Physiol 460, 705–718 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Standaert DG, Watson SJ, Houghten RA, Saper CB, Opioid peptide immunoreactivity in spinal and trigeminal dorsal horn neurons projecting to the parabrachial nucleus in the rat. J. Neurosci 6, 1220–1226 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Huang D, Grady FS, Peltekian L, Laing JJ, Geerling JC, Efferent projections of CGRP/Calca-expressing parabrachial neurons in mice. J. Comp. Neurol 529, 2911–2957 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kissiwaa SA, Patel SD, Winters BL, Bagley EE, Opioids differentially modulate two synapses important for pain processing in the amygdala. Br. J. Pharmacol 177, 420–431 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Finnegan TF, Chen S-R, Pan H-L, Effect of the μ opioid on excitatory and inhibitory synaptic inputs to periaqueductal gray-projecting neurons in the amygdala. J. Pharmacol. Exp. Ther 312, 441–448 (2005). [DOI] [PubMed] [Google Scholar]
- 271.Zhu Y, Wienecke CFR, Nachtrab G, Chen X, A thalamic input to the nucleus accumbens mediates opiate dependence. Nature 530, 219–222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Muñoz B, Haggerty DL, Atwood BK, Synapse-specific expression of mu opioid receptor long-term depression in the dorsomedial striatum. Sci. Rep 10, 7234 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, Arakawa N, Ossipov M, Fields HL, Porreca F, Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. J. Neurosci 35, 7264–7271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zhang X-Y, Dou Y-N, Yuan L, Li Q, Zhu Y-J, Wang M, Sun Y-G, Different neuronal populations mediate inflammatory pain analgesia by exogenous and endogenous opioids. eLife 9, e55289 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Smith SJ, Sümbül U, Graybuck LT, Collman F, Seshamani S, Gala R, Gliko O, Elabbady L, Miller JA, Bakken TE, Rossier J, Yao Z, Lein E, Zeng H, Tasic B, Hawrylycz M, Single-cell transcriptomic evidence for dense intracortical neuropeptide networks. eLife 8, e47889 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Fang FG, Haws CM, Drasner K, Williamson A, Fields HL, Opioid peptides (DAGO-enkephalin, dynorphin A (1--13), BAM 22P) microinjected into the rat brainstem: Comparison of their antinociceptive effect and their effect on neuronal firing in the rostral ventromedial medulla. Brain Res. 501, 116–128 (1989). [DOI] [PubMed] [Google Scholar]
- 277.Fields H, State-dependent opioid control of pain. Nat. Rev. Neurosci 5, 565–575 (2004). [DOI] [PubMed] [Google Scholar]
- 278.Convertino M, Samoshkin A, Gauthier J, Gold MS, Maixner W, Dokholyan NV, Diatchenko L, μ-Opioid receptor 6-transmembrane isoform: A potential therapeutic target for new effective opioids. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 62, 61–67 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Pasternak GW, Opioids and their receptors: Are we there yet? Neuropharmacology 76, 198–203 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.van Rijn RM, Whistler JL, Waldhoer M, Opioid-receptor-heteromer-specific trafficking and pharmacology. Curr. Opin. Pharmacol 10, 73–79 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Lemos Duarte M, Devi LA, Post-translational modifications of opioid receptors. Trends Neurosci. 43, 417–432 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Fields HL, Margolis EB, Understanding opioid reward. Trends Neurosci. 38, 217–225 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bardoni R, Tawfik VL, Wang D, François A, Solorzano C, Shuster SA, Choudhury P, Betelli C, Cassidy C, Smith K, de Nooij JC, Mennicken F, O’Donnell D, Kieffer BL, Jeffrey Woodbury C, Basbaum AI, MacDermott AB, Scherrer G, Delta opioid receptors presynaptically regulate cutaneous mechanosensory neuron input to the spinal cord dorsal horn. Neuron 81, 1443 (2014). [DOI] [PubMed] [Google Scholar]
- 284.Scherrer G, Imamachi N, Cao Y-Q, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI, Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137, 1148–1159 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Yao Z, van Velthoven CTJ, Nguyen TN, Goldy J, Sedeno-Cortes AE, Baftizadeh F, Bertagnolli D, Casper T, Chiang M, Crichton K, Ding S-L, Fong O, Garren E, Glandon A, Gouwens NW, Gray J, Graybuck LT, Hawrylycz MJ, Hirschstein D, Kroll M, Lathia K, Lee C, Levi B, McMillen D, Mok S, Pham T, Ren Q, Rimorin C, Shapovalova N, Sulc J, Sunkin SM, Tieu M, Torkelson A, Tung H, Ward K, Dee N, Smith KA, Tasic B, Zeng H, A taxonomy of transcriptomic cell types across the isocortex and hippocampal formation. Cell 184, 3222–3241.e26 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Chavkin C, Koob GF, Dynorphin, dysphoria, and dependence: The stress of addiction. Neuropsychopharmacology 41, 373–374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo C-O, Park SI, Marcinkiewcz CM, Crowley NA, Krashes MJ, Lowell BB, Kash TL, Rogers JA, Bruchas MR, Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron 87, 1063–1077 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Gaveriaux-Ruff C, Nozaki C, Nadal X, Hever XC, Weibel R, Matifas A, Reiss D, Filliol D, Nassar MA, Wood JN, Maldonado R, Kieffer BL, Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain 152, 1238–1248 (2011). [DOI] [PubMed] [Google Scholar]
- 289.Epstein CM, Physics and biophysics of TMS, in The Oxford Handbook of Transcranial Stimulation (Oxford Univ. Press, 2008). [Google Scholar]
- 290.Knotkova H, Pain: Brain Stimulation in the Treatment of Pain (Nova Science Publishers, 2010). [Google Scholar]
- 291.Histed MH, Bonin V, Clay Reid R, Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron 63, 508–522 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A, Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 1, 206–223 (2008). [DOI] [PubMed] [Google Scholar]
- 293.Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC, Theta burst stimulation of the human motor cortex. Neuron 45, 201–206 (2005). [DOI] [PubMed] [Google Scholar]
- 294.Galhardoni R, Correia GS, Araujo H, Yeng LT, Fernandes DT, Kaziyama HH, Marcolin MA, Bouhassira D, Teixeira MJ, De Andrade DC, Repetitive transcranial magnetic stimulation in chronic pain: A review of the literature. Arch. Phys. Med. Rehabil 96, S156–S172 (2015). [DOI] [PubMed] [Google Scholar]
- 295.Gaertner M, Kong JT, Scherrer KH, Foote A, Mackey S, Johnson KA, Advancing transcranial magnetic stimulation methods for complex regional pain syndrome: An open-label study of paired theta burst and high-frequency stimulation. Neuromodulation 21, 409–416 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Eles JR, Stieger KC, Kozai TDY, The temporal pattern of intracortical microstimulation pulses elicits distinct temporal and spatial recruitment of cortical neuropil and neurons. J. Neural Eng 18, 015001 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.DosSantos MF, Ferreira N, Toback RL, Carvalho AC, DaSilva AF, Potential mechanisms supporting the value of motor cortex stimulation to treat chronic pain syndromes. Front. Neurosci 10, 18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Riechert T, Stereotactic Brain Operations: Methods, Clinical Aspects, Indications (Huber, 1980). [Google Scholar]
- 299.Reijonen J, Säisänen L, Könönen M, Mohammadi A, Julkunen P, The effect of coil placement and orientation on the assessment of focal excitability in motor mapping with navigated transcranial magnetic stimulation. J. Neurosci. Methods 331, 108521 (2020). [DOI] [PubMed] [Google Scholar]
- 300.Kozyrev V, Eysel UT, Jancke D, Voltage-sensitive dye imaging of transcranial magnetic stimulation-induced intracortical dynamics. Proc. Natl. Acad. Sci. U.S.A 111, 13553–13558 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hussin AT, Boychuk JA, Brown AR, Pittman QJ, Teskey GC, Intracortical microstimulation (ICMS) activates motor cortex layer 5 pyramidal neurons mainly transsynaptically. Brain Stimul. 8, 742–750 (2015). [DOI] [PubMed] [Google Scholar]
- 302.Suzuki T, Sugawara K, Ogahara K, Higashi T, Time course of corticospinal excitability and intracortical inhibition just before muscle relaxation. Front. Hum. Neurosci 10, 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Nitsche MA, Paulus W, Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57, 1899–1901 (2001). [DOI] [PubMed] [Google Scholar]
- 304.Wu T, Sommer M, Tergau F, Paulus W, Lasting influence of repetitive transcranial magnetic stimulation on intracortical excitability in human subjects. Neurosci. Lett 287, 37–40 (2000). [DOI] [PubMed] [Google Scholar]
- 305.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S, Treatment of thalamic pain by chronic motor cortex stimulation. Pacing Clin. Electrophysiol 14, 131–134 (1991). [DOI] [PubMed] [Google Scholar]
- 306.Esfahani DR, Pisansky MT, Dafer RM, Anderson DE, Motor cortex stimulation: Functional magnetic resonance imaging-localized treatment for three sources of intractable facial pain: Report of 3 cases. J. Neurosurg 114, 189–195 (2011). [DOI] [PubMed] [Google Scholar]
- 307.Meeker TJ, Keaser ML, Khan SA, Gullapalli RP, Seminowicz DA, Greenspan JD, Non-invasive motor cortex neuromodulation reduces secondary hyperalgesia and enhances activation of the descending pain modulatory network. Front. Neurosci 13, 467 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.De Andrade EM, Martinez RCR, Pagano RL, Lopes PSS, Auada AVV, Gouveia FV, Antunes GF, Assis DV, Lebrun I, Fonoff ET, Neurochemical effects of motor cortex stimulation in the periaqueductal gray during neuropathic pain. J. Neurosurg 132, 239–251 (2020). [DOI] [PubMed] [Google Scholar]
- 309.Jung HH, Shin J, Kim J, Ahn SH, Lee SE, Koh CS, Cho JS, Kong C, Shin HC, Kim SJ, Chang JW, Rostral agranular insular cortex lesion with motor cortex stimulation enhances pain modulation effect on neuropathic pain model. Neural Plast. 2016, 3898924 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Kudo K, Takahashi T, Suzuki S, The changes of c-Fos expression by motor cortex stimulation in the deafferentation pain model. Neurol. Med. Chir 54, 537–544 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Pagano RL, Assis DV, Clara JA, Alves AS, Dale CS, Teixeira MJ, Fonoff ET, Britto LR, Transdural motor cortex stimulation reverses neuropathic pain in rats: A profile of neuronal activation. Eur. J. Pain 15, 268.e1–268.e14 (2011). [DOI] [PubMed] [Google Scholar]
- 312.Moisset X, Lanteri-Minet M, Fontaine D, Neurostimulation methods in the treatment of chronic pain. J. Neural Transm 127, 673–686 (2020). [DOI] [PubMed] [Google Scholar]
- 313.Case LK, Laubacher CM, Olausson H, Wang B, Spagnolo PA, Bushnell MC, Encoding of touch intensity but not pleasantness in human primary somatosensory cortex. J. Neurosci 36, 5850–5860 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Lockwood PL, Iannetti GD, Haggard P, Transcranial magnetic stimulation over human secondary somatosensory cortex disrupts perception of pain intensity. Cortex 49, 2201–2209 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Case LK, Laubacher CM, Richards EA, Spagnolo P, Olausson H, Bushnell MC, Inhibitory rTMS of secondary somatosensory cortex reduces intensity but not pleasantness of gentle touch. Neurosci. Lett 653, 84–91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Umezaki Y, Badran BW, DeVries WH, Moss J, Gonzales T, George MS, The efficacy of daily prefrontal repetitive transcranial magnetic stimulation (rTMS) for burning mouth syndrome (BMS): A randomized controlled single-blind study. Brain Stimul. 9, 234–242 (2016). [DOI] [PubMed] [Google Scholar]
- 317.Seminowicz DA, Moayedi M, The dorsolateral prefrontal cortex in acute and chronic pain. J. Pain 18, 1027–1035 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Brighina F, Piazza A, Vitello G, Aloisio A, Palermo A, Daniele O, Fierro B, rTMS of the prefrontal cortex in the treatment of chronic migraine: A pilot study. J. Neurol. Sci 227, 67–71 (2004). [DOI] [PubMed] [Google Scholar]
- 319.Boccard SGJ, Pereira EAC, Aziz TZ, Deep brain stimulation for chronic pain. J. Clin. Neurosci 22, 1537–1543 (2015). [DOI] [PubMed] [Google Scholar]
- 320.Roy R, de la Vega R, Jensen MP, Miró J, Neurofeedback for pain management: A systematic review. Front. Neurosci 14, 671 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Patel K, Sutherland H, Henshaw J, Taylor JR, Brown CA, Casson AJ, Trujillo-Barreton NJ, Jones AKP, Sivan M, Effects of neurofeedback in the management of chronic pain: A systematic review and meta-analysis of clinical trials. Eur. J. Pain 24, 1440–1457 (2020). [DOI] [PubMed] [Google Scholar]
- 322.Jensen MP, Day MA, Miró J, Neuromodulatory treatments for chronic pain: Efficacy and mechanisms. Nat. Rev. Neurol 10, 167–178 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.decharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JDE, Mackey SC, Control over brain activation and pain learned by using real-time functional MRI. Proc. Natl. Acad. Sci. U.S.A 102, 18626–18631 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Guan M, Ma L, Li L, Yan B, Zhao L, Tong L, Dou S, Xia L, Wang M, Shi D, Self-regulation of brain activity in patients with postherpetic neuralgia: A double-blind randomized study using real-time FMRI neurofeedback. PLOS ONE 10, e0123675 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Goldway N, Ablin J, Lubin O, Zamir Y, Keynan JN, Or-Borichev A, Cavazza M, Charles F, Intrator N, Brill S, Ben-Simon E, Sharon H, Hendler T, Volitional limbic neuromodulation exerts a beneficial clinical effect on Fibromyalgia. NeuroImage 186, 758–770 (2019). [DOI] [PubMed] [Google Scholar]
- 326.Zhang S, Yoshida W, Mano H, Yanagisawa T, Mancini F, Shibata K, Kawato M, Seymour B, Pain control by co-adaptive learning in a brain-machine interface. Curr. Biol 30, 3935–3944.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Stoeckel LE, Garrison KA, Ghosh S, Wighton P, Hanlon CA, Gilman JM, Greer S, Turk-Browne NB, deBettencourt MT, Scheinost D, Craddock C, Thompson T, Calderon V, Bauer CC, George M, Breiter HC, Whitfield-Gabrieli S, Gabrieli JD, LaConte SM, Hirshberg L, Brewer JA, Hampson M, Van Der Kouwe A, Mackey S, Evins AE, Optimizing real time fMRI neurofeedback for therapeutic discovery and development. Neuroimage Clin. 5, 245–255 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Lubianiker N, Goldway N, Fruchtman-Steinbok T, Paret C, Keynan JN, Singer N, Cohen A, Kadosh KC, Linden DEJ, Hendler T, Process-based framework for precise neuromodulation. Nat. Hum. Behav 3, 436–445 (2019). [DOI] [PubMed] [Google Scholar]
- 329.Chapin H, Bagarinao E, Mackey S, Real-time fMRI applied to pain management. Neurosci. Lett 520, 174–181 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Hofmann SG, Asnaani A, Vonk IJJ, Sawyer AT, Fang A, The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cogn. Ther. Res 36, 427–440 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.An H, He R-H, Zheng Y-R, Tao R, Cognitive-behavioral therapy. Adv. Exp. Med. Biol 1010, 321–329 (2017). [DOI] [PubMed] [Google Scholar]
- 332.Rnic K, Dozois DJA, Martin RA, Cognitive distortions, humor styles, and depression. Eur. J. Psychol. Assess 12, 348–362 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Lazarus AA, Abramovitz A, A multimodal behavioral approach to performance anxiety. J. Clin. Psychol 60, 831–840 (2004). [DOI] [PubMed] [Google Scholar]
- 334.Carroll KM, Kiluk BD, Cognitive behavioral interventions for alcohol and drug use disorders: Through the stage model and back again. Psychol. Addict. Behav 31, 847–861 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Verdejo-Garcia A, Garcia-Fernandez G, Dom G, Cognition and addiction. Dialogues Clin. Neurosci 21, 281–290 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Borza L, Cognitive-behavioral therapy for generalized anxiety. Dialogues Clin. Neurosci 19, 203–208 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Clark DA, Beck AT, Cognitive theory and therapy of anxiety and depression: Convergence with neurobiological findings. Trends Cogn. Sci 14, 418–424 (2010). [DOI] [PubMed] [Google Scholar]
- 338.Zhang A, Borhneimer LA, Weaver A, Franklin C, Hai AH, Guz S, Shen L, Cognitive behavioral therapy for primary care depression and anxiety: A secondary meta-analytic review using robust variance estimation in meta-regression. J. Behav. Med 42, 1117–1141 (2019). [DOI] [PubMed] [Google Scholar]
- 339.Webb CA, Rosso IM, Rauch SL, Internet-based cognitive-behavioral therapy for depression: Current progress and future directions. Harv. Rev. Psychiatry 25, 114–122 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Marques S, Barrocas D, Rijo D, Psychological treatments for borderline personality disorder: A review of cognitive-behavioral oriented therapies. Acta Medica Port. 30, 307–319 (2017). [DOI] [PubMed] [Google Scholar]
- 341.Cunningham NR, Kashikar-Zuck S, Coghill RC, Brain mechanisms impacted by psychological therapies for pain: Identifying targets for optimization of treatment effects. Pain Rep. 4, e767 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.McCracken LM, Turk DC, Behavioral and cognitive–behavioral treatment for chronic pain: Outcome, predictors of outcome, and treatment process. Spine 27, 2564–2573 (2002). [DOI] [PubMed] [Google Scholar]
- 343.Jensen MP, Turner JA, Romano JM, Changes in beliefs, catastrophizing, and coping are associated with improvement in multidisciplinary pain treatment. J. Consult. Clin. Psychol 69, 655–662 (2001). [DOI] [PubMed] [Google Scholar]
- 344.Darnall BD, Colloca L, Optimizing placebo and minimizing nocebo to reduce pain, catastrophizing, and opioid use: A review of the science and an evidence-informed clinical toolkit. Int. Rev. Neurobiol 139, 129–157 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Villemure C, Bushnell MC, Mood influences supraspinal pain processing separately from attention. J. Neurosci 29, 705–715 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Longe SE, Wise R, Bantick S, Lloyd D, Johansen-Berg H, McGlone F, Tracey I, Counter-stimulatory effects on pain perception and processing are significantly altered by attention: An fMRI study. Neuroreport 12, 2021–2025 (2001). [DOI] [PubMed] [Google Scholar]
- 347.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I, Flexible cerebral connectivity patterns subserve contextual modulations of pain. Cereb. Cortex 21, 719–726 (2011). [DOI] [PubMed] [Google Scholar]
- 348.Phillips ML, Gregory LJ, Cullen S, Coen S, Ng V, Andrew C, Giampietro V, Bullmore E, Zelaya F, Amaro E, Thompson DG, Hobson AR, Williams SCR, Brammer M, Aziz Q, The effect of negative emotional context on neural and behavioural responses to oesophageal stimulation. Brain 126, 669–684 (2003). [DOI] [PubMed] [Google Scholar]
- 349.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD, Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 303, 1162–1167 (2004). [DOI] [PubMed] [Google Scholar]
- 350.Ellingsen D-M, Wessberg J, Eikemo M, Liljencrantz J, Endestad T, Olausson H, Leknes S, Placebo improves pleasure and pain through opposite modulation of sensory processing. Proc. Natl. Acad. Sci. U.S.A 110, 17993–17998 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C, Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63, 533–543 (2009). [DOI] [PubMed] [Google Scholar]
- 352.Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, Kadetoff D, Ingvar M, Cognitive behavioral therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain 153, 1495–1503 (2012). [DOI] [PubMed] [Google Scholar]
- 353.Yoshino A, Okamoto Y, Okada G, Takamura M, Ichikawa N, Shibasaki C, Yokoyama S, Doi M, Jinnin R, Yamashita H, Horikoshi M, Yamawaki S, Changes in resting-state brain networks after cognitive–behavioral therapy for chronic pain. Psychol. Med 48, 1148–1156 (2018). [DOI] [PubMed] [Google Scholar]
- 354.Lazaridou A, Kim J, Cahalan CM, Loggia ML, Franceschelli O, Berna C, Schur P, Napadow V, Edwards RR, Effects of cognitive-behavioral therapy (CBT) on brain connectivity supporting catastrophizing in fibromyalgia. Clin. J. Pain 33, 215–221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Shpaner M, Kelly C, Lieberman G, Perelman H, Davis M, Keefe FJ, Naylor MR, Unlearning chronic pain: A randomized controlled trial to investigate changes in intrinsic brain connectivity following cognitive behavioral therapy. Neuroimage Clin. 5, 365–376 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Kucyi A, Salomons TV, Davis KD, Cognitive behavioral training reverses the effect of pain exposure on brain network activity. Pain 157, 1895–1904 (2016). [DOI] [PubMed] [Google Scholar]
- 357.Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA, Newhouse PA, Filippi CG, Keefe FJ, Naylor MR, Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J. Pain 14, 1573–1584 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ, Brain activity during biofeedback relaxation: A functional neuroimaging investigation. Brain 124, 1003–1012 (2001). [DOI] [PubMed] [Google Scholar]
- 359.Kobayashi S, Koitabashi K, Effects of progressive muscle relaxation on cerebral activity: An fMRI investigation. Complement. Ther. Med 26, 33–39 (2016). [DOI] [PubMed] [Google Scholar]
- 360.Pessoa L, On the relationship between emotion and cognition. Nat. Rev. Neurosci 9, 148–158 (2008). [DOI] [PubMed] [Google Scholar]
- 361.Lawrence JM, Hoeft F, Sheau KE, Mackey SC, Strategy-dependent dissociation of the neural correlates involved in pain modulation. Anesthesiology 115, 844–851 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Schulz E, Stankewitz A, Witkovský V, Winkler AM, Tracey I, Strategy-dependent modulation of cortical pain circuits for the attenuation of pain. Cortex 113, 255–266 (2019). [DOI] [PubMed] [Google Scholar]
- 363.Foltz EL, White LE, Pain “Relief” by frontal cingulumotomy. J. Neurosurg 19, 89–100 (1962). [DOI] [PubMed] [Google Scholar]
- 364.Sharim J, Pouratian N, Anterior cingulotomy for the treatment of chronic intractable pain: A systematic review. Pain Physician 19, 537–550 (2016). [PubMed] [Google Scholar]
- 365.Dorsi MJ, Lenz FA, Neurosurgical approaches to the treatment of pain, in Wall and Melzack’s Textbook of Pain (Elsevier, 2013). [Google Scholar]
- 366.Poulin J-F, Tasic B, Hjerling-Leffler J, Trimarchi JM, Awatramani R, Disentangling neural cell diversity using single-cell transcriptomics. Nat. Neurosci 19, 1131–1141 (2016). [DOI] [PubMed] [Google Scholar]
- 367.Salter MW, Stevens B, Microglia emerge as central players in brain disease. Nat. Med 23, 1018–1027 (2017). [DOI] [PubMed] [Google Scholar]
- 368.Ji R-R, Chamessian A, Zhang Y-Q, Pain regulation by non-neuronal cells and inflammation. Science 354, 572–577 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Roth BL, DREADDs for neuroscientists. Neuron 89, 683–694 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Hsu NS, Fang HY, David KK, Gnadt JW, Peng GC, Talley EM, Ward JM, Ngai J, Koroshetz WJ, The promise of the BRAIN initiative: NIH strategies for understanding neural circuit function. Curr. Opin. Neurobiol 65, 162–166 (2020). [DOI] [PubMed] [Google Scholar]
- 371.Collins FS, Koroshetz WJ, Volkow ND, Helping to end addiction over the long-term: The research plan for the NIH HEAL initiative. JAMA 320, 129–130 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Volkow ND, Koroshetz WJ, The role of neurologists in tackling the opioid epidemic. Nat. Rev. Neurol 15, 301–305 (2019). [DOI] [PubMed] [Google Scholar]
- 373.Iyengar S, Woller SA, Hommer R, Beierlein J, Wright CB, Tamiz AP, Karp BI, Critical NIH resources to advance therapies for pain: Preclinical screening program and phase II human clinical trial network. Neurotherapeutics 17, 932–934 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Davis KD, Aghaeepour N, Ahn AH, Angst MS, Borsook D, Brenton A, Burczynski ME, Crean C, Edwards R, Gaudilliere B, Hergenroeder GW, Iadarola MJ, Iyengar S, Jiang Y, Kong J-T, Mackey S, Saab CY, Sang CN, Scholz J, Segerdahl M, Tracey I, Veasley C, Wang J, Wager TD, Wasan AD, Pelleymounter MA, Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: Challenges and opportunities. Nat. Rev. Neurol 16, 381–400 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Mackey S, Greely HT, Martucci KT, Neuroimaging-based pain biomarkers: Definitions, clinical and research applications, and evaluation frameworks to achieve personalized pain medicine. Pain Rep. 4, e762 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


