Abstract
Telomerase is a specialized reverse transcriptase (RT) that is minimally composed of a protein catalytic subunit and an RNA component. The RNA subunit contains a short template sequence that directs the synthesis of DNA repeats at the ends of chromosomes. Human telomerase activity can be reconstituted in vitro by the expression of the human telomerase protein catalytic subunit (hTERT) in the presence of recombinant human telomerase RNA (hTR) in a rabbit reticulocyte lysate (RRL) system. We analyzed telomerase activity and binding of hTR to hTERT in RRL by expressing different hTERT and hTR variants. hTRs containing nucleotide substitutions that are predicted to disrupt base pairing in the P3 helix of the pseudoknot weakly reconstituted human telomerase activity yet retained their ability to bind hTERT. Our results also identified two distinct regions of hTR that can independently bind hTERT in vitro. Furthermore, sequences or structures between nucleotides 208 and 330 of hTR (which include the conserved CR4-CR5 domain) were found to be important for hTERT-hTR interactions and for telomerase activity reconstitution. Human TERT carboxy-terminal amino acid deletions extending to motif E or the deletion of the first 280 amino acids abolished human telomerase activity without affecting the ability of hTERT to associate with hTR, suggesting that the RT and RNA binding functions of hTERT are separable. These results indicate that the reconstitution of human telomerase activity in vitro requires regions of hTERT that (i) are distinct from the conserved RT motifs and (ii) bind nucleotides distal to the hTR template sequence.
The physical end of each eukaryotic chromosome consists of a nucleoprotein complex known as the telomere (11). The termini of telomeric DNA cannot be fully replicated by the conventional DNA replication machinery, and consequently, chromosomes shorten at each cell division (33, 34). One solution to this end replication problem is the ribonucleoprotein (RNP) complex, telomerase. Telomerase compensates for telomere erosion by replenishing the sequence repeats (TTAGGG in humans) at the 3′ end of telomeric DNA (16).
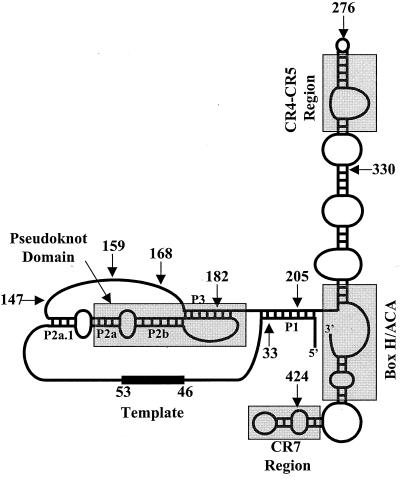
Telomerase activity was initially identified in the ciliated protozoan Tetrahymena thermophila (23). The gene encoding the telomerase protein catalytic subunit was first identified by reverse genetics in the protozoan Euplotes aediculatus (36). Catalytic subunits of telomerase, the telomerase reverse transcriptases (TERTs), contain conserved reverse transcriptase (RT) motifs and a telomerase-specific (T) motif (36, 44). In addition to the RT and T motifs, the amino termini of TERTs from ciliated protozoa share a common motif (CP motif) that is weakly conserved in the catalytic subunits of other organisms (14). TERT has now been identified in several organisms, including budding and fission yeasts, mice, humans, and plants (17, 19, 22, 27, 31, 36, 38, 40, 44). Telomerase catalytic activity is dependent on an intrinsic RNA molecule that contains a short template sequence (24). The gene coding for the RNA subunit of telomerase has also been cloned from several organisms (45). In contrast to the strong homology between the RT-like motifs of the TERTs (46), the telomerase RNA subunit from various organisms differs significantly in terms of size and nucleotide sequence (45). Recently, a secondary structure for the human telomerase RNA (hTR) was proposed based on a phylogenetic comparison of telomerase RNA components identified from a variety of vertebrate species (15) (illustrated in Fig. 1). Four conserved structural elements are universally present in the predicted secondary structure of vertebrate telomerase RNA: these are the pseudoknot domain, the CR4-CR5 domain, the H/ACA box, and the CR7 domain. Interestingly, the predicted vertebrate telomerase RNA secondary structure displays a structural topology similar to the ciliate telomerase RNA (48).
FIG. 1.
Secondary structure of hTR with its telomeric template sequence (nucleotides 46 to 53) and its 5′ and 3′ ends (adapted from the work of Chen et al. [15]). Four universally conserved structural elements among vertebrate telomerase RNAs, including the pseudoknot, the CR4-CR5 region, the H/ACA box, and the CR7 region, are boxed in gray. The P1, P2a.1, P2a, P2b, and P3 helices are indicated. Arrows indicate the nucleotide positions of the 5′ and 3′ ends of the different hTR truncations used in this study.
Human telomerase activity was initially reconstituted in vitro by the addition of recombinant hTR to micrococcal nuclease-treated, partially purified 293 cell extracts (5). Using this reconstitution assay, a minimal functional region of hTR between nucleotides 44 and 205 was identified. Reconstitution of human telomerase by the addition of recombinant hTR to in vitro-translated human TERT (hTERT) in a rabbit reticulocyte lysate (RRL) determined that residues 10 to 159 of hTR are sufficient to generate weak human telomerase activity in vitro (9). More recently, in vitro assembly reactions using different hTR truncations and hTERT synthesized separately in RRL or human cells identified nucleotides 33 and 325 as the 5′ and 3′ functional boundaries of hTR, respectively (52). This latter study also demonstrated that two inactive fragments of hTR (nucleotides 33 to 147 and 164 to 325) can cooperate to reconstitute a human telomerase RNP that is catalytically active in vitro. However, these studies have not investigated the molecular interactions between hTERT and hTR.
Structure and function studies of telomerase components from other organisms have also been performed. Evidence that specific RNA structures and sequences distinct from the template region contribute to the enzymatic action of telomerase has been reported (10, 49). Telomerase RNA mutations predicted to perturb the pseudoknot structure of the Tetrahymena telomerase RNA reconstituted a catalytically active enzyme in vitro (4, 32) but not in vivo (21). RRL reconstitution of the Tetrahymena telomerase using RNA subunit variants identified catalytically inactive mutants that can bind to the protein catalytic component p133 (32) and suggest that telomerase RNA sequences or structures implicated in binding and catalysis are functionally distinct. Establishment of the template boundary by yeast telomerase is determined, at least in part, by a phylogenetically conserved secondary structure within the yeast telomerase RNA subunit (53). A similar function for a conserved sequence upstream of the template region in the telomerase RNA from ciliated protozoa was also reported (3, 35).
Single amino acid substitutions of conserved telomerase motifs identified an RT-like catalytic mechanism for telomerase (9, 12, 17, 25, 27, 36, 56). However, the functional role of TERT domains beyond the RT motifs has not been extensively studied. Recent reports identified functionally important regions within the amino-terminal domain of the protein catalytic subunit of the Tetrahymena and Saccharomyces cerevisiae telomerases (13, 20, 57). The data from these studies suggest a role for the amino terminus of TERT in telomerase RNA binding. An extensive mutational analysis of the Tetrahymena TERT has also demonstrated a specific role for certain residues outside the RT motifs in template definition by Tetrahymena telomerase (41).
Human telomerase activity is detected in more than 85% of transformed and tumor cell lines, yet it is not observed in most normal human diploid cells (1, 47, 50). The inhibition of human telomerase in immortal and cancer cell lines leads to progressive telomere shortening and, in some cell types, cell death (26, 29, 59). A better understanding of the role of telomerase in cancer should validate the use of this enzyme as a target for anticancer therapy (2). Consequently, it is important to clearly understand how telomerase is regulated and to dissect its mechanism of action. One step essential to the mechanism of action of telomerase is the interaction of the telomerase RNA with TERT.
We investigated the functional regions of both hTERT and hTR required for telomerase activity and RNA-protein interactions using human telomerase reconstituted in RRL. Our results suggest the presence in hTR of at least two independent hTERT binding sites, located between nucleotides 33 and 147 and nucleotides 164 and 330. We also identified sequences and possible structures distant from the template region of hTR that are essential for the reconstitution of human telomerase activity in vitro. Expression of amino- and carboxy-terminal hTERT deletions in RRL identified catalytically inactive mutants that retained their ability to associate with hTR, suggesting that the polymerase and RNA binding functions of hTERT are distinct.
MATERIALS AND METHODS
hTERT and hTR plasmid constructs.
Cloning of nucleotides 1 to 451 of hTR into the pUC119 plasmid (phTR + 1) has been described previously (5). Similarly, the construction of the hTR substitution mutants hTR170, hTR180, and hTR190 has also been described (5). Plasmids expressing hTRs, hTR33-147, hTR164-330, hTR164-208, and hTRACA-TGT were generated by PCR from the template vector pGRN33 (18) using Pfu polymerase (Stratagene). To generate phTR33-147, nucleotides 33 to 147 of hTR were amplified by PCR using the 5′ primer 5′-GGGGAAGCTTTAATACGACTCACTATAGGGCCATTTTTTGTCTAACCCTAACTG-3′ and the 3′ primer 5′-CGCGGATCCTCCGGAAGGCGGCAGGCCGAGGC-3′, containing HindIII and BspEI sites, respectively. phTR164-208 and phTR164-330 were constructed using the same 5′ primer. The 3′ primer was 5′-CGCGGATCCCGGGAGGCGAACGGGCCAG-3′ for phTR164-208 and 5′-CGCGGATCCCTCGAGACCCGCGGCTGACAGAGCC-3′ for phTR164-330, both containing an XhoI site. The substitution of the ACA trinucleotide (nucleotide 446 to 448) for TGT in the ACA box of the hTR was introduced by PCR using the 5′ primer hTR + 1 (5′-GGGGAAGCTTTAATACGACTCACTATAGGGTTGCGGAGGGTGGGCCTG-3′) (5) and the 3′ primer 5′-CGCGGATCCTGCGCAACAGTGAGCCGAGTCCTGGGTG-3′, containing HindIII and BamHI sites, respectively. The 5′ primers used for these constructs all contain the phage T7 promoter immediately upstream of hTR sequences. The digested PCR products were cloned into the pUC119 plasmid previously digested with the appropriate restriction enzymes. The sequences of the different hTR plasmid constructs were confirmed by dideoxy sequencing following the manufacturer's instructions (T7 DNA polymerase sequencing kit; Amersham Pharmacia Biotech).
The construction of the pET28b-hTERT expression plasmid has been described previously (7). The ΔCT135 hTERT C-terminal truncation was expressed using pET28b-hTERT digested with ApaLI (cleaves at position 2992 within hTERT cDNA), generating a protein product lacking the last 135 amino acids. The other N- and C-terminal derivatives were generated by PCR amplification using pET28b-hTERT as a template and the appropriate pairs of primers. After digestion of the different PCR products with the appropriate restriction enzymes, the hTERT cDNA derivatives were cloned into the pET28 vectors (Novagen).
Preparation of gel-purified hTRs.
The different RNAs used in the coupled in vitro transcription-translation reactions in RRLs were transcribed in vitro using T7 RNA polymerase (New England Biolabs). Full-length hTR (nucleotides 1 to 451) and hTRs containing nucleotides 1 to 424 (hTR1-424), 1 to 276, 1 to 205, 1 to 182, 1 to 168, and 1 to 159 were in vitro transcribed from the phTR + 1 construct (5) previously digested with FspI, ApaLI, BspEI, SmaI, PvuII, BbvI, and XbaI, respectively. The plasmids phTR170, phTR180, phTR190, and phTR(ACA-TGT) were digested with FspI, whereas phTR33-147, phTR164-208, and phTR164-330 were digested with BspEI, BamHI, and XhoI, respectively. Following in vitro transcription, the reaction products were DNase treated and purified on denaturing acrylamide gels as described previously (6). The concentrations of the gel-purified RNAs were determined by spectrophotometry, and their integrity and size were confirmed by either ethidium bromide staining of denaturing acrylamide gels or Northern blotting.
In vitro transcription and translation.
Wild-type and hTERT derivatives engineered to have a T7 epitope tag at the amino terminus were synthesized in RRLs by incubating pET28b-hTERT or the different pET28-hTERT derivatives in coupled transcription-translation (Promega) reaction mixtures (20 to 25 μl) at a final concentration of 25 ng/μl, in the presence or absence of 2.5 to 5.0 ng of full-length or mutant gel-purified hTR derivatives/μl. Expression in RRL followed the instructions of the manufacturer (Promega) using [35S]methionine (NEN) to radiolabel hTERT during protein synthesis.
Immunoprecipitations.
Immunoprecipitations were performed by incubating 10 to 13.5 μl of reticulocyte lysate with protein A-Sepharose beads that had previously been coated with T7 monoclonal antibody (Novagen) in lysis buffer (10 mM Tris-HCl [pH 7.5], 2.0 mM MgCl2, 1.0 mM EGTA, 5.0 mM β-mercaptoethanol, 20% glycerol, 150 mM NaCl, 1% NP-40, 0.25 mM sodium deoxycholate, 0.2 mM 4-(2-aminoacyl) benzene sulfonyl fluoride hydrochloride (AEBSF), 0.5 μg of leupeptin/ml, 1 g of pepstatin/ml, 38 U of RNAguard [Amersham Pharmacia Biotech]/ml). After a 2- to 3-h incubation at 4°C, protein A-Sepharose-coated beads were washed four times using 1 ml of lysis buffer and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), telomeric repeat amplification protocol (TRAP) assay, or Northern analysis.
Northern analysis.
Total RNA was extracted from RRLs by diluting 5 μl of RRL reaction mixtures with 45 μl of H2O and by the addition of 450 μl of Trizol reagent (GIBCO BRL). To prepare RNA from antibody-coated beads, 75 to 90% of the immunoprecipitates were treated once with phenol and once with chloroform-isoamyl alcohol (24:1) and precipitated with 0.1 volume of 3 M sodium acetate and 2.5 volumes of ethanol in the presence of 10 μg of Escherichia coli tRNA (Sigma). RNAs were separated by electrophoresis on either 4 or 6% acrylamide–7 M urea–0.6× Tris-borate-EDTA gels and then electrophoretically transferred to nylon membranes (Hybond+; Amersham Pharmacia Biotech) in 6 mM trisodium citrate–8 mM dibasic sodium phosphate for 2 h at 350 mA. Blots were probed with random-primed hTR cDNA (8), and hybridizations were performed at 55°C.
Telomerase activity assays.
Telomerase activity was assayed by a two-tube modified TRAP as described previously (7). The positive control used in TRAP assays consisted of partially purified 293 cell extracts prepared as previously described (5).
RESULTS
Nucleotides 1 to 159 of hTR are sufficient for a stable interaction with hTERT in vitro.
Reconstitution of human telomerase activity with recombinant hTR has been accomplished in systems such as RRL (9, 56) and the yeast Saccharomyces cerevisiae (7) and by using purified recombinant hTERT expressed from baculovirus-infected cells (39). These results suggest that the minimal components required to reconstitute human telomerase in vitro are hTERT and hTR. The ability to reconstitute human telomerase with only these two components in vitro and the mechanistic similarity of hTERT to RTs (12, 36, 41, 56) suggest a stable interaction between the protein catalytic subunit and the RNA component of telomerase.
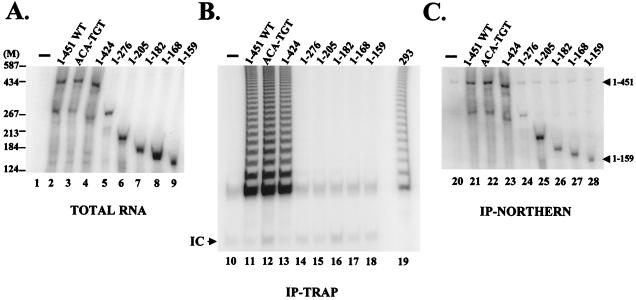
Modified hTRs that had different 3′ truncations or the hTR(ACA-TGT) substitution were added to RRL reaction mixtures during hTERT synthesis to identify hTR sequences or structures that bind the protein catalytic subunit in vitro (Fig. 2). The predicted secondary structures altered in the different hTR variants are described in Table 1 and shown in Fig. 1. Northern blot analysis using an hTR-specific probe was performed on the total RNA extracted from these RRL reactions to demonstrate that the levels and stabilities of the various hTRs in the lysates were not grossly different (Fig. 2A). These lysates were subjected to immunoprecipitation using an antibody to the T7 tag located at the amino terminus of hTERT. The abilities of the different RNAs to reconstitute human telomerase activity (Fig. 2B) and to associate with hTERT (as determined by their coimmunoprecipitation with hTERT) (Fig. 2C) were analyzed. Immunoprecipitates prepared from lysates that expressed hTERT in the presence of wild-type hTR reconstituted human telomerase activity (Fig. 2B, lane 11) and contained hTR1-451 (Fig. 2C, lane 21). Human telomerase activity and hTR were not recovered from protein A-Sepharose if the T7 tag antibody was omitted (data not shown). In addition, an immunoprecipitate from a lysate in which hTERT was synthesized in the absence of hTR (Fig. 2A, lane 1) did not reconstitute human telomerase activity (Fig. 2B, lane 10) nor contain hTR (Fig. 2C, lane 20). All of the tested hTR variants were capable of associating with hTERT (Fig. 2C, lanes 22 to 28). However, only hTR1-451, hTR(ACA-TGT), and hTR1-424 reconstituted a catalytically active human telomerase RNP in vitro (Fig. 2B). These results suggest that the first 159 nucleotides of hTR are sufficient for a stable interaction with hTERT but sequences or structures between nucleotides 276 and 424 of hTR are necessary for the enzymatic function of telomerase in vitro.
FIG. 2.
Sequences or structures located between nucleotides 276 and 424 of hTR are necessary for telomerase activity reconstitution in vitro. (A) Northern blot of total RNA harvested from RRLs in which hTERT was synthesized in the absence of hTR (lane 1) or the presence of wild-type hTR (lane 2), hTR(ACA-TGT) (lane 3), or 3′-truncated hTRs (lanes 4 to 9). The Northern blot was probed for hTR-specific sequences. M, DNA markers (sizes, in base pairs, are on the left). RNAs were separated by electrophoresis on a 4% acrylamide–7 M urea gel. (B and C) Equal volumes of RRL reaction products generated in the absence (−) or presence of the different hTR variants were subjected to immunoprecipitation (IP) with an antibody to the T7 tag. The washed beads were analyzed for telomerase activity (B) and hTERT-hTR coimmunoprecipitation (C). For panel B, telomerase activity was analyzed by the TRAP assay, and 100 ng of partially purified 293 cell extracts was used as a positive control (lane 19). IC, internal PCR control; WT, wild-type. For panel C, hTERT-hTR coimmunoprecipitation was analyzed by Northern blotting using an hTR-specific probe. The arrowheads indicate the positions at which hTR1-451 and hTR1-159 migrate.
TABLE 1.
Summary of hTR mutations analyzed in this study and their telomerase activity and extent of binding to hTERT
| Telomerase RNA | Size (nucleotides) | Sequence and/or structure alterationsa | Telomerase activityb
|
hTERT bindingbd | |
|---|---|---|---|---|---|
| 293c | RRLd | ||||
| hTR1-451 | 451 | None | +++ | +++ | +++ |
| hTR1-424 | 424 | Delete 3′ terminal 27 nt; delete ACA box; partly disrupt the CR7 domain | +++ | +++ | +++ |
| hTR1-276 | 276 | Delete 3′ terminal 175 nt; delete H/ACA box and CR7 domains; disrupt CR4-CR5 domain | +++ | − | ++ |
| hTR1-205 | 205 | Delete 3′ terminal 246 nt; delete H/ACA box, CR7, and CR4-CR5 domains | ++ | − | ++ |
| hTR1-182 | 182 | Delete 3′ terminal 269 nt; delete H/ACA box, CR7, and CR4-CR5 domains; partly disrupt pseudoknot | +/− | − | ++ |
| hTR1-168 | 168 | Delete 3′ terminal 283 nt; delete H/ACA box, CR7, and CR4-CR5 domains, disrupt pseudoknot domain | +/− | − | ++ |
| hTR1-159 | 159 | Delete 3′ terminal 292 nt; delete H/ACA box, CR7, and CR4-CR5 domains, disrupt pseudoknot domain | − | − | ++ |
| hTR170 | 451 | Substitute nt 170–179; disrupt part of P3 helix of pseudoknot | +/− | +/− | +++ |
| hTR180 | 451 | Substitute nt 180–189; disrupt part of P3 helix of pseudoknot | +/− | − | +++ |
| hTR190 | 451 | Substitute nt 190–199; disrupt part of P1 helix | + | + | +++ |
| hTR(ACA-TGT) | 451 | Replace ACA with TGT (nt 446–448) | ND | +++ | +++ |
| hTR33-147 | 114 | Span nt 33–147; lacks P1 helix, complete pseudoknot, CR4-CR5 domain, H/ACA box, and CR7 domains | ND | − | + |
| hTR164-208 | 42 | Span nt 164–208; lacks template sequence, pseudoknot, CR4-CR5, H/ACA, and CR7 domains | ND | − | − |
| hTR164-330 | 166 | Span nt 164–330; lacks template sequence, pseudoknot, H/ACA box, and CR7 domains | ND | − | ++ |
| hTR33-147 + hTR164-208 | 114; 42 | See hTR33-147 and hTR164-208 | ND | − | +; − |
| hTR33-147 + hTR164-330 | 114; 166 | See hTR33-147 and hTR164-330 | ND | +++ | +; ++ |
Structural alterations of hTR variants are based on the predicted secondary structure of hTR described by Chen et al. (15), which is shown in Fig. 1. nt, nucleotides.
The telomerase activity and hTERT binding ability of the different hTR mutations are scored relative to those of wild-type hTR.
The ability of various mutated hTRs to reconstitute telomerase activity from micrococcal nuclease-treated, partially purified 293 extracts was previously determined (5). ND, not determined.
Telomerase activity and hTERT binding of immunoprecipitates from rabbit reticulocyte lysates (RRL) that expressed hTERT in the presence of the different hTR derivatives.
Sequences or structures located between nucleotides 170 and 200 of hTR are required for a catalytic function in the telomerase RNP.
Substitution of nucleotides 170 to 179 (hTR170), 180 to 189 (hTR180), and 190 to 199 (hTR190) of hTR greatly impairs the ability of hTR to reconstitute human telomerase activity in vitro (5) (Table 1). Nucleotides 174 to 183, which are predicted to be part of the P3 helix in the hTR pseudoknot (15) (Fig. 1), are replaced in the hTR170 and hTR180 variants. To investigate whether these mutated hTRs are defective in binding to hTERT, we used the RRL expression system to examine the abilities of hTR170, hTR180, and hTR190 to reconstitute human telomerase activity and to specifically associate with hTERT in vitro.
hTERT was synthesized in RRL in the absence or presence of gel-purified wild-type hTR, hTR170, hTR180, and hTR190 (Fig. 3A, lanes 1 to 5). Using the T7 tag antibody, immunoprecipitates were prepared from these lysates and analyzed for the ability to reconstitute telomerase activity (Fig. 3B) and for hTERT-hTR binding by Northern blot analysis of the coimmunoprecipitated RNAs (Fig. 3C). hTR190 reconstituted low levels of human telomerase activity (Fig. 3B, lane 15). Immunoprecipitates prepared from RRL reactions that expressed hTERT in the presence of either hTR170 or hTR180 contained low (hTR170) (Fig. 3B, lane 13) and undetectable (hTR180) (lane 14) levels of telomerase activity. Northern blot analysis of coimmunoprecipitated RNAs demonstrated that all three mutated hTRs were capable of associating with hTERT as efficiently as wild-type hTR (Fig. 3C, compare lanes 24 to 26 to lane 23). These results indicate that sequences or structures located between nucleotides 170 and 200 of hTR are required for the enzymatic action of the telomerase RNP rather than hTERT binding.
FIG. 3.
Role of the P3 helix of the hTR pseudoknot in telomerase function and two independent hTERT binding sites within hTR. (A) Northern blot of total RNA harvested from RRLs in which hTERT was synthesized in the absence of hTR (lane 1) or presence of wild-type hTR (lane 2) and hTR variants. The Northern blot was probed for hTR-specific sequences. M, DNA markers (sizes, in base pairs, are on the left). RNAs were separated by electrophoresis on a 6% acrylamide–7 M urea gel. (B and C) Equal volumes of RRL reaction products generated in the absence (lanes 11 and 22) or presence of the different hTR variants were subjected to immunoprecipitation (IP) with an antibody to the T7 tag. The washed beads were analyzed for telomerase activity (B) and hTERT-hTR coimmunoprecipitation (C). For panel B, telomerase activity was analyzed by the TRAP assay, and 100 ng of partially purified 293 cell extracts were used as a positive control (lane 21). IC, internal PCR control; WT, wild-type. For panel C, hTERT-hTR coimmunoprecipitation was analyzed by Northern blotting using an hTR-specific probe. The arrowheads indicate the positions at which full-length hTR (FLhTR), hTR164-330, and hTR33-147 migrate.
Two independent hTERT binding sites exist within hTR.
Human telomerase can be reconstituted in RRL by expressing hTERT in the presence of two inactive, nonoverlapping segments of hTR (nucleotides 33 to 147 and 164 to 325) (52). To determine whether hTR fragments spanning nucleotides 33 to 147, 164 to 208, and 164 to 330 bind the protein catalytic subunit, hTERT was expressed in RRL in the presence of distinct hTR segments (hTR33-147, hTR164-208, hTR164-330), individually or pairwise (Fig. 3A, lanes 6 to 10; also Table 1 and Fig. 1). Immunoprecipitates were prepared from these lysates using the T7 tag antibody and analyzed for telomerase activity (Fig. 3B) and hTERT-hTR coimmunoprecipitation (Fig. 3C).
Human telomerase activity was detected from the immunoprecipitate of a lysate that expressed hTERT in the presence of a mixture of hTR33-147 and hTR164-330 (Fig. 3B, lane 20) (52). Northern blot analysis of RNAs extracted from the immunoprecipitates demonstrated that both hTR fragments (nucleotides 33 to 147 and 164 to 330) associated with hTERT (Fig. 3C, lane 31). Immunoprecipitates prepared from RRL extracts in which hTERT was synthesized in the presence of either hTR33-147 or hTR164-330 did not reconstitute human telomerase activity (Fig. 3B, lanes 16 and 18, respectively). This result is consistent with the observation by Tesmer et al. (52) that these two hTR segments, separately, are unable to reconstitute a catalytically active telomerase RNP in vitro. Nonetheless, both hTR33-147 and hTR164-330 were independently capable of binding hTERT, albeit with different efficiencies (Fig. 3C, lanes 27 and 29, respectively). An RNA composed of nucleotides 164 to 330 of hTR was coimmunoprecipitated with hTERT (compare the amount of input RNA [Fig. 3A, lane 8] to bound RNA [Fig. 3C, lane 29]), whereas significantly less hTR33-147 was coimmunoprecipitated (compare Fig. 3A and C for hTR33-147). These results suggest that two different regions, one located between nucleotides 33 and 147 and the other between nucleotides 164 and 330 of hTR, independently interact with the protein catalytic subunit with qualitatively different efficiencies.
Immunopurified hTERT expressed in the presence of hTR164-208 alone or in combination with hTR33-147 did not reconstitute human telomerase activity (Fig. 3B, lanes 17 and 19). Northern blot analysis of coimmunoprecipitated RNAs indicated that the levels of hTR164-208 that associated with hTERT varied from undetectable (Fig. 3C, lanes 28 and 30) to extremely low (data not shown). In contrast, hTR164-330 formed a stable complex with hTERT (Fig. 3C, lanes 29 and 31), suggesting that sequences or structures located between nucleotides 208 and 330 of hTR may be important for hTERT-hTR interactions as well as for the activation of telomerase in vitro (lanes 19 and 20 of Fig. 3B).
Regions within the amino and carboxy termini of hTERT play functionally different roles in telomerase catalysis and hTR binding in vitro
One of the features that distinguish telomerase from conventional RTs is that the telomerase RNA is an intrinsic component of the enzyme (24, 45). Recent evidence suggests a functional role for the amino-terminal domain of the yeast and Tetrahymena TERTs in telomerase RNA binding (13, 20). In order to investigate the function of regions outside the RT motifs of the human telomerase protein catalytic subunit, we generated amino (N)- and carboxy (C)-terminal deletions of hTERT (Fig. 4) and used the RRL expression system to assay the ability of each truncation mutant to reconstitute telomerase activity and bind hTR in vitro. Full-length hTERT and those with N- and C-terminal deletions were synthesized in RRL in the presence of gel-purified wild-type hTR and [35S]methionine (Fig. 5A). Immunoprecipitates were prepared from these lysates using the T7 tag antibody (Fig. 5B) and subjected to telomerase activity assays (Fig. 5C). None of the N- and C-terminal hTERT deletion mutants reconstituted human telomerase activity (Fig. 5C, lanes 14 to 22), whereas full-length hTERT efficiently reconstituted activity (Fig. 5C, lane 13).
FIG. 4.
Summary of hTERT truncations analyzed and their telomerase activity and extent of binding to hTR. Schematic representation of the human telomerase RT with the seven conserved RT-like motifs (1, 2, A, B′, C, D, and E) as well as the T motif (44). Amino- and carboxy-terminal deletions in hTERT (including amino acid positions) are indicated along with the relative telomerase activities and hTR binding abilities these proteins demonstrated after their immunoprecipitation from RRLs in which they were expressed in the presence of recombinant hTR. All these proteins were engineered to express an N-terminal epitope tag (T7 tag).
FIG. 5.
The polymerization and RNA binding functions of hTERT are independent in vitro. (A) Equal volumes of lysate in which the different hTERT proteins (indicated above each lane) were synthesized in the presence of recombinant hTR and [35S]methionine were analyzed for protein expression by 7.5% SDS-PAGE. The gel was dried and exposed to a phosphorimager screen. FLhTERT, full-length hTERT; CT, C-terminal; NT, N-terminal; MOT, motif; M, protein markers (masses, in kilodaltons, are on the right). Equal volumes of the different RRL reaction products were subjected to immunopurification using the T7 tag antibody. The washed beads were analyzed for protein levels using 7.5% SDS-PAGE (B), telomerase activity (C), and hTERT-hTR coimmunoprecipitation (D). (C) Telomerase activity was analyzed by the TRAP assay, and 100 ng of partially purified 293 cell extracts was used as a positive control (lane 23). IC, internal PCR control. (D) hTERT-hTR coimmunoprecipitation was analyzed by Northern blotting using an hTR-specific probe. FLhTR, full-length hTR.
Coimmunoprecipitation of the RNAs was analyzed by Northern blotting using an hTR-specific probe (Fig. 5D) to determine if the N- and C-terminal hTERT truncations affected hTR binding. The two catalytically inactive hTERT constructs with C-terminal deletions bound hTR (Fig. 5D, lanes 26 to 27). An immunoprecipitate from a lysate that expressed the ΔCT186 truncation contained hTR levels similar to those of an immunoprecipitate from a lysate that expressed full-length hTERT (Fig. 5D, compare lanes 27 and 25), yet the amount of hTR coimmunoprecipitated by the ΔCT135 construct with the C-terminal deletion was significantly less (lane 26). However, this result is consistent with the lower levels of the ΔCT135 protein that were expressed and immunoprecipitated from the lysate in this particular experiment (Fig. 5A and B, lanes 3). These results indicate that the defect in the enzymatic activity of the two C-terminal hTERT deletions is not caused by a deficiency in hTR binding.
Immunoprecipitates from RRL extracts that expressed the ΔNT180 and ΔNT280 hTERT constructs with N-terminal truncations contained higher levels of hTR than an immunoprecipitate from a lysate expressing full-length hTERT (Fig. 5D, compare lanes 28 and 29 to lane 25). However, higher levels of these two N-terminally deleted proteins were observed after immunoprecipitation from RRL extracts (Fig. 5B, compare lanes 5 and 6 to lane 2). Deletion of the first 350 amino acids of hTERT significantly compromised its ability to associate with hTR in vitro (Fig. 5D, lane 30). Two larger N-terminal truncations of hTERT (ΔNT542 and ΔNT595) (Fig. 5D, lanes 31 to 32) and hTERT containing the RT motifs in the presence or absence of the T motif (Fig. 5D, lanes 33 to 34) weakly coimmunoprecipitated hTR from RRL extracts. These results indicate that the loss of enzymatic activity of certain hTERT truncated proteins (specifically the two with C-terminal deletions and the ΔNT180 and the ΔNT280 deletions) is not caused by an altered ability of the mutant proteins to associate with hTR in vitro.
DISCUSSION
We demonstrated evidence for the existence of two distinct hTERT binding regions within hTR, located between nucleotides 33 and 147 and nucleotides 164 and 330. Our data also suggest a catalytic role for nucleotides 170 to 190 in the formation of the predicted pseudoknot in hTR, rather than a role in hTERT binding in vitro. The function of regions outside hTERT RT motifs was also examined by expressing a set of amino- and carboxy-terminal deletions in RRL. Our data support a role for N-terminal regions of hTERT in binding hTR. Furthermore, we defined domains of hTERT essential for telomerase activity that are not involved in binding hTR in vitro.
The telomerase RNA variants described in this study were assayed for hTERT binding and reconstitution of human telomerase activity in RRLs. The effects of the RNA mutations on telomerase activity and hTERT binding may be indirect if the mutated RNAs differentially fold and alternatively base pair during in vitro transcription. Secondary structure analysis of these mutated RNAs will be required to assess the different roles of RNA folding, binding, and catalysis. However, all of the hTR variants analyzed in this study (except hTR164-208) retained telomerase activity and/or hTERT-binding ability, suggesting that the mutated RNAs are not grossly misfolded.
The evolutionarily conserved CR7 and H/ACA box domains of hTR are dispensable for the reconstitution of human telomerase activity in vitro
In addition to a highly conserved template region, vertebrate telomerase RNAs contain four predicted structural elements: a pseudoknot, the CR4-CR5 domain, the H/ACA box, and the CR7 domain (15) (Fig. 1). The evolutionary conservation of these domains suggests important roles for these regions in vertebrate telomerase RNA stability, RNP assembly, or function. The abilities of hTR1-424 and hTR(ACA-TGT) to bind hTERT (Fig. 2C) and to reconstitute human telomerase activity (Fig. 2B) suggest that the H/ACA box and the CR7 domain are dispensable in vitro. These observations are consistent with previous reports that examined the functional regions of hTR (5, 9, 43, 52). The CR7 domain is not conserved in small nucleolar RNAs (15), and it may play a specific role in vertebrate telomerase RNA function in vivo rather than in vitro, as demonstrated for the H/ACA box of hTR (42, 43).
Role of the hTR pseudoknot in the enzymatic function of human telomerase.
Ciliate and vertebrate telomerase RNAs possess pseudoknots that are relatively similar in structural topology (15, 48, 51). The hTR pseudoknot is established by helices P2a, P2b, and P3 (Fig. 1). The P3 helix is formed by base pairing between nucleotides 107 and 115 and nucleotides 174 and 183 of hTR (15). An hTR variant with a 17-nucleotide insertion at position 176 of hTR is unable to reconstitute human telomerase activity both in vivo (18) and in vitro (5). This insertion was suggested to disrupt the pseudoknot of hTR (15). Similarly, two of the hTR variants with 10-nucleotide substitutions, hTR170 and hTR180, likely perturb the P3 helix within the hTR pseudoknot (Fig. 1 and Table 1). Our results using the RRL reconstitution system demonstrated that hTR170 and hTR180 bound hTERT (Fig. 3C). However, the hTR170 and hTR180 substitutions significantly altered the ability of hTR to reconstitute a catalytically active telomerase RNP in vitro, suggesting a role for the P3 helix of the hTR pseudoknot in a specific enzymatic action of telomerase but not in hTERT binding. The hTR190 substitution, which is predicted to affect the P1 rather than the P3 helix (15) (Fig. 1), reconstituted human telomerase activity more efficiently than hTR170 and hTR180 (reference 5 and this study) (Table 1). This result suggests that the P1 helix of hTR is not as critical as the P3 helix and is consistent with the observation that nucleotides 5′ of the template that are involved in the formation of the P1 helix are dispensable for telomerase activity in vitro (5, 9, 52). Furthermore, telomerase RNAs from mice and other rodents do not contain the P1 helix (15, 30). In Tetrahymena, the pseudoknot of the telomerase RNA is essential for telomerase RNP assembly and activity in vivo (21). However, mutations predicted to destabilize the pseudoknot structure of the Tetrahymena telomerase RNA do not significantly perturb telomerase activity in vitro (4, 32), indicating that the conditions required for the assembly of a functional telomerase RNP may differ in vitro and in vivo. A more detailed analysis of the hTR pseudoknot will be required to establish the specific role of this structure in the enzymatic function of human telomerase both in vitro and in vivo.
Two independent hTERT binding sites within hTR.
The hTERT-hTR interaction studies suggest that two segments of hTR can independently associate with hTERT in vitro. One hTR fragment, containing the template sequence, spans nucleotides 33 to 147, whereas the other, containing the conserved CR4-CR5 domain, consists of nucleotides 164 to 330 of hTR. Base-pairing interactions through helix P3 may, however, bring these two regions together into one structure in vitro. An RNA composed of nucleotides 33 to 147 of hTR is not predicted to form a complete pseudoknot (Fig. 1). The single-stranded template region of hTR alone is unlikely to provide specific and high-affinity binding to hTERT, since the template must be accessible to hybridize to the substrate DNA during telomere synthesis. In vivo chemical modification of the Tetrahymena telomerase RNA indicates that nucleotides within the template region are not constantly associated with proteins (58). In addition, the template sequences of yeast and Tetrahymena telomerase RNAs have been completely replaced by heterologous sequences without affecting RNP assembly or catalytic activity (28, 55).
Our data and recent work by others (43, 52) suggest that hTERT binding to the evolutionarily conserved CR4-CR5 domain of hTR is necessary for the formation of a fully active human telomerase RNP. First, the hTR coimmunoprecipitation experiments suggest that sequences or structures located between nucleotides 208 and 330 of hTR (which contain the CR4-CR5 domain [Fig. 1]) are critical for the efficient binding of hTR to hTERT (Fig. 3C). Second, hTR1-276 is unable to reconstitute human telomerase activity (Fig. 2), whereas the combination of hTR33-147 and hTR164-330 reconstitutes a catalytically active telomerase (Fig. 3). Therefore, nucleotides 276 to 330 of hTR are required for the reconstitution of a fully active telomerase RNP in vitro. hTR164-330 forms the CR4-CR5 domain in hTR, whereas nucleotides 1 to 276 are not sufficient for the formation of the CR4-CR5 stem-loop structure (Fig. 1). Similarly, a 151-nucleotide deletion at the 3′ end of hTR (1 to 300), which deletes part of the CR4-CR5 domain, drastically alters the ability of hTR to reconstitute human telomerase activity in vitro using hTERT previously synthesized in RRL (52). However, nucleotides 1 to 325 of hTR, which include the conserved CR5 sequences necessary for the formation of the CR4-CR5 stem-loop structure, are sufficient to produce levels of telomerase activity similar to those of wild-type hTR (52).
Our data suggesting that the CR4-CR5 domain of hTR is required for the formation of a fully active human telomerase RNP in vitro is, however, not entirely supported by previous studies. hTR truncations as short as 1 to 205 nucleotides (5) and 10 to 159 nucleotides (9) are sufficient to reconstitute low levels of human telomerase activity in vitro. In this regard, we occasionally detected human telomerase activity from crude RRL extracts that expressed hTERT in the presence of hTR1-276 (data not shown) but not from the immunoprecipitates (Fig. 2). The immunopurification procedure may select telomerase RNPs that can maintain a stable catalytically active conformation in vitro.
Mitchell and Collins (43) recently reported that hTR harbors two independent hTERT binding sites, one located within nucleotides 1 to 209 and the other between nucleotides 241 and 330 of hTR. Nucleotides 241 to 330 of hTR contain the conserved CR4-CR5 domain and are necessary for the reconstitution of telomerase activity in vivo. Furthermore, this latter region is necessary and sufficient for the reconstitution of human telomerase activity in vitro when added in trans with hTR1-209 to hTERT previously synthesized in RRL (43). However, this study and the one by Mitchell and Collins (43) have not distinguished between a binding and a catalytic function for the CR4-CR5 domain. The requirement for the CR4-CR5 domain of hTR to reconstitute a fully active human telomerase RNP may reflect essential physical contacts between this domain and hTERT and/or a direct role in catalytic functions, as was recently demonstrated for a specific structure within the yeast telomerase RNA (53). Similarly, binding to a specific stem-loop structure within the pregenomic RNA of the hepatitis B virus is also critical for the catalytic activation of this viral RT (54).
Domains outside hTERT RT motifs are important for human telomerase activity in vitro.
We investigated the functional role of regions outside the hTERT RT motifs by analyzing the catalytic activity of different amino- and carboxy-terminal hTERT deletions in vitro. Human telomerase activity was completely abolished by deleting 180 and 135 amino acids at the N and C termini, respectively (Fig. 5). The two constructs with C-terminal hTERT deletions, as well as the ΔNT180 and ΔNT280 truncated proteins, bound hTR, though they did not reconstitute a catalytically active enzyme, suggesting that these proteins are not grossly misfolded. hTERT lacking the first 350 amino acids did not associate efficiently with hTR in vitro, whereas hTERT lacking C-terminal amino acids up to motif E bound hTR. These results suggest that a binding domain for hTR may be located within the amino terminus of hTERT. Recent evidence for an RNA binding function in the amino terminus of TERT from S. cerevisiae (20) and Tetrahymena (13) support this proposal. Site-directed mutagenesis of Tetrahymena TERT (tTERT) established a critical role for both the ciliate-specific (CP) and the T motifs in RNA binding, whereas mutations in tTERT RT motifs had little effect on telomerase RNA binding (13). However, the CP motif is not highly conserved in TERTs of nonciliates, and it is likely that the RNA binding domains of TERTs from different organisms vary.
Sequence alignments between TERTs have identified new blocks of conservation in their amino termini in addition to the previously described RT and T motifs (36, 37, 41, 44, 57). One of these regions was independently found by three groups (37, 41, 57) and spans amino acids 134 to 175 of hTERT. This region is absent in the ΔNT180 N-terminal-deletion construct, which was catalytically inactive in vitro (Fig. 4). This result, together with data from the mutational analysis of conserved residues within this new motif (41, 57), suggests an important role for this region in telomerase function.
We have identified functional regions within hTR and the human protein catalytic subunit that are essential for the reconstitution of telomerase activity in vitro. We demonstrated that two nonoverlapping regions of hTR can independently associate with hTERT in vitro and suggest a role for the hTR pseudoknot in the enzymatic action of telomerase. Our data also establish a role for the amino-terminal region of hTERT in binding hTR in vitro and demonstrate that domains outside the conserved RT motifs of hTERT are essential for telomerase reconstitution. A better knowledge of the interactions between the RNA and protein subunits of telomerase, as well as with telomerase-associated proteins, will help to elucidate the molecular mechanisms involved in the assembly, regulation, and mechanism of action of the telomerase RNP.
ACKNOWLEDGMENTS
The hTERT cDNA was provided by Geron Corporation (Menlo Park, Calif.). We thank I. Triki and A. M. Rodriguez for help in the construction of some hTR truncations; G. Kukolj, T. Moriarty, and M. Esmail for comments on the manuscript; and T. Beattie and L. Harrington for sharing their protocol for the Northern analysis of hTR from IP beads.
F.B. is the recipient of a Medical Research Council of Canada (MRCC) Studentship Award. This work was supported by a grant from the MRCC (MT-14026) to C.A.
ADDENDUM IN PROOF
Since the submission of this paper, an analysis of hTERT-hTR interactions was also reported by T. L. Beattie et al. (Mol. Biol. Cell 11:3329–3340, 2000).
REFERENCES
- 1.Artandi S, DePinho R. A critical role for telomeres in suppressing and facilitating carcinogenesis. Curr Opin Genet Dev. 2000;10:39–46. doi: 10.1016/s0959-437x(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 2.Autexier C. Telomerase as a possible target for anticancer therapy. Chem Biol. 1999;6:R299–R303. doi: 10.1016/s1074-5521(99)80122-7. [DOI] [PubMed] [Google Scholar]
- 3.Autexier C, Greider C W. Boundary elements of the Tetrahymena telomerase RNA template and alignment domains. Genes Dev. 1995;15:2227–2239. doi: 10.1101/gad.9.18.2227. [DOI] [PubMed] [Google Scholar]
- 4.Autexier C, Greider C W. Mutational analysis of the Tetrahymena telomerase RNA: identification of residues affecting telomerase activity in vitro. Nucleic Acids Res. 1998;26:787–795. doi: 10.1093/nar/26.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autexier C, Pruzan R, Funk W D, Greider C W. Reconstitution of human telomerase activity and identification of a minimal functional region of the human telomerase RNA. EMBO J. 1996;15:5928–5935. [PMC free article] [PubMed] [Google Scholar]
- 6.Autexier C, Triki I. Tetrahymena telomerase ribonucleoprotein RNA-protein interactions. Nucleic Acids Res. 1999;27:2227–2234. doi: 10.1093/nar/27.10.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachand F, Autexier C. Functional reconstitution of human telomerase expressed in Saccharomyces cerevisiae. J Biol Chem. 1999;274:38027–38031. doi: 10.1074/jbc.274.53.38027. [DOI] [PubMed] [Google Scholar]
- 8.Bachand F, Kukolj G, Autexier C. Expression of hTERT and hTR in cis reconstitutes an active human telomerase ribonucleoprotein. RNA. 2000;6:778–784. doi: 10.1017/s1355838200000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beattie T L, Zhou W, Robinson M O, Harrington L. Reconstitution of human telomerase activity in vitro. Curr Biol. 1998;8:177–180. doi: 10.1016/s0960-9822(98)70067-3. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharyya A, Blackburn E H. A functional telomerase RNA swap in vivo reveals the importance of nontemplate RNA domains. Proc Natl Acad Sci USA. 1997;94:2823–2827. doi: 10.1073/pnas.94.7.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackburn E H, Greider C W, editors. Telomeres. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. [Google Scholar]
- 12.Bryan T, Goodrich K, Cech T. A mutant of Tetrahymena telomerase reverse transcriptase with increased processivity. J Biol Chem. 2000;275:24199–24207. doi: 10.1074/jbc.M003246200. [DOI] [PubMed] [Google Scholar]
- 13.Bryan T, Goodrich K, Cech T. Telomerase RNA bound by protein motifs specific to telomerase reverse transcriptase. Mol Cell. 2000;6:493–499. doi: 10.1016/s1097-2765(00)00048-4. [DOI] [PubMed] [Google Scholar]
- 14.Bryan T M, Sperger J M, Chapman K B, Cech T R. Telomerase reverse transcriptase genes identified in Tetrahymena thermophila and Oxytricha trifallax. Proc Natl Acad Sci USA. 1998;95:8479–8484. doi: 10.1073/pnas.95.15.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J-L, Blasco M A, Greider C W. Secondary structure of vertebrate telomerase RNA. Cell. 2000;100:503–514. doi: 10.1016/s0092-8674(00)80687-x. [DOI] [PubMed] [Google Scholar]
- 16.Collins K. Mammalian telomeres and telomerase. Curr Opin Cell Biol. 2000;12:378–383. doi: 10.1016/s0955-0674(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 17.Counter C M, Meyerson M, Eaton E N, Weinberg R A. The catalytic subunit of yeast telomerase. Proc Natl Acad Sci USA. 1997;94:9202–9207. doi: 10.1073/pnas.94.17.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng J, Funk W D, Wang S-S, Weinrich S L, Avilion A A, Chiu C-P, Adams R R, Chang E, Allsopp R C, Yu J, Le S, West M D, Harley C B, Andrews W H, Greider C W, Villeponteau B. The human telomerase RNA component. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald M, Riha K, Gao F, Ren S, McKnight T, Shippen D. Disruption of the telomerase catalytic subunit gene from Arabidopsis inactivates telomerase and leads to a slow loss of telomeric DNA. Proc Natl Acad Sci USA. 1999;96:14813–14818. doi: 10.1073/pnas.96.26.14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman K L, Cech T R. Essential functions of amino-terminal domains in the yeast telomerase catalytic subunit revealed by selection for viable mutants. Genes Dev. 1999;13:2863–2874. doi: 10.1101/gad.13.21.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilley D, Blackburn E H. The telomerase RNA pseudoknot is critical for the stable assembly of a catalytically active ribonucleoprotein. Proc Natl Acad Sci USA. 1999;96:6621–6625. doi: 10.1073/pnas.96.12.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg R A, Allsopp R C, Chin L, Morin G B, DePinho R A. Expression of mouse telomerase reverse transcriptase during development, differentiation and proliferation. Oncogene. 1998;16:1723–1730. doi: 10.1038/sj.onc.1201933. [DOI] [PubMed] [Google Scholar]
- 23.Greider C W, Blackburn E H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 24.Greider C W, Blackburn E H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 25.Haering C, Nakamura T, Baumann P, Cech T. Analysis of telomerase catalytic subunit mutants in vivo and in vitro in Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 2000;97:6367–6372. doi: 10.1073/pnas.130187397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn W C, Stewart S A, Brooks M W, York S G, Eaton E, Kurachi A, Beijersbergen R L, Knoll J H M, Meyerson M, Weinberg R A. Inhibition of telomerase limits the growth of human cancer cells. Nat Med. 1999;5:1164–1170. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- 27.Harrington L, Zhou W, McPhail T, Oulton R, Yeung D S K, Mar V, Bass M B, Robinson M O. Human telomerase contains evolutionarily conserved catalytic and structural subunits. Genes Dev. 1997;11:3109–3115. doi: 10.1101/gad.11.23.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henning K A, Moskowitz N, Ashlock M A, Liu P P. Humanizing the yeast telomerase template. Proc Natl Acad Sci USA. 1998;95:5667–5671. doi: 10.1073/pnas.95.10.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herbert B-S, Pitts A E, Baker S I, Hamilton S E, Wright W E, Shay J W, Corey D R. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc Natl Acad Sci USA. 1999;96:14276–14281. doi: 10.1073/pnas.96.25.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinkley C, Blasco M, Funk W, Feng J, Villeponteau B, Greider C, Herr W. The mouse telomerase RNA 5′-end lies just upstream of the telomerase template sequence. Nucleic Acids Res. 1998;26:532–536. doi: 10.1093/nar/26.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kilian A, Bowtell D D L, Abud H E, Hime G R, Venter D J, Keese P K, Duncan E L, Reddel R R, Jefferson R A. Isolation of a candidate human telomerase catalytic subunit gene, which reveals complex splicing patterns in different cell types. Hum Mol Genet. 1997;6:2011–2019. doi: 10.1093/hmg/6.12.2011. [DOI] [PubMed] [Google Scholar]
- 32.Licht J D, Collins K. Telomerase RNA function in recombinant Tetrahymena telomerase. Genes Dev. 1999;13:1116–1125. doi: 10.1101/gad.13.9.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lingner J, Cech T. Telomerase and chromosome end maintenance. Curr Opin Genet Dev. 1998;8:226–232. doi: 10.1016/s0959-437x(98)80145-7. [DOI] [PubMed] [Google Scholar]
- 34.Lingner J, Cooper J P, Cech T R. Telomerase and DNA end replication: not a lagging strand problem? Science. 1995;269:1533–1534. doi: 10.1126/science.7545310. [DOI] [PubMed] [Google Scholar]
- 35.Lingner J, Hendrick L L, Cech T R. Telomerase RNAs of different ciliates have a common secondary structure and a permuted template. Genes Dev. 1994;8:1984–1998. doi: 10.1101/gad.8.16.1984. [DOI] [PubMed] [Google Scholar]
- 36.Lingner J, Hughes T R, Shevchenko A, Mann M, Lundblad V, Cech T R. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 37.Malik H, Burke W, Eickbush T. Putative telomerase catalytic subunits from Giardia lamblia and Caenorhabditis elegans. Gene. 2000;251:101–108. doi: 10.1016/s0378-1119(00)00207-9. [DOI] [PubMed] [Google Scholar]
- 38.Martin-Rivera L, Herrera E, Albar J P, Blasco M A. Expression of mouse telomerase catalytic subunit in embryos and adult tissues. Proc Natl Acad Sci USA. 1998;95:10471–10476. doi: 10.1073/pnas.95.18.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masutomi K, Kaneko S, Hayashi N, Yamashita T, Shirota Y, Kobayashi K, Murakami S. Telomerase activity reconstituted in vitro with purified human telomerase reverse transcriptase and human telomerase RNA component. J Biol Chem. 2000;275:22568–22573. doi: 10.1074/jbc.M000622200. [DOI] [PubMed] [Google Scholar]
- 40.Meyerson M, Counter C M, Eaton E N, Ellisen L W, Steiner P, Dickinson S C, Ziaugra L, Beijersbergen R L, Davidoff M J, Liu Q, Bacchetti S, Haber D A, Weinberg R A. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 41.Miller M, Liu J, Collins K. Template definition by Tetrahymena telomerase reverse transcriptase. EMBO J. 2000;19:4412–4422. doi: 10.1093/emboj/19.16.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell J, Cheng J, Collins K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol Cell Biol. 1999;19:567–576. doi: 10.1128/mcb.19.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell J, Collins K. Human telomerase activation requires two independent interactions between telomerase RNA and telomerase reverse transcriptase. Mol Cell. 2000;6:361–371. doi: 10.1016/s1097-2765(00)00036-8. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura T M, Morin G B, Chapman K B, Weinrich S L, Andrews W H, Lingner J, Harley C B, Cech T R. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 45.Nugent C I, Lundblad V. The telomerase reverse transcriptase: components and regulation. Genes Dev. 1998;12:1073–1085. doi: 10.1101/gad.12.8.1073. [DOI] [PubMed] [Google Scholar]
- 46.O'Reilly M, Teichmann S A, Rhodes D. Telomerases. Curr Opin Struct Biol. 1999;9:56–65. doi: 10.1016/s0959-440x(99)80008-6. [DOI] [PubMed] [Google Scholar]
- 47.Oulton R, Harrington L. Telomeres, telomerase, and cancer: life on the edge of genomic stability. Curr Opin Oncol. 2000;12:74–81. doi: 10.1097/00001622-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Romero D P, Blackburn E H. A conserved secondary structure for telomerase RNA. Cell. 1991;67:343–353. doi: 10.1016/0092-8674(91)90186-3. [DOI] [PubMed] [Google Scholar]
- 49.Roy J, Fulton T B, Blackburn E H. Specific telomerase RNA residues distant from the template are essential for telomerase function. Genes Dev. 1998;12:3286–3300. doi: 10.1101/gad.12.20.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shay J W, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 51.ten Dam E, Van Belkum A, Pleij K. A conserved pseudoknot in telomerase RNA. Nucleic Acids Res. 1991;19:6951. doi: 10.1093/nar/19.24.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tesmer V M, Ford L P, Holt S E, Frank B C, Yi X, Aisner D L, Ouellette M, Shay J W, Wright W E. Two inactive fragments of the integral RNA cooperate to assemble active telomerase with the human protein catalytic subunit (hTERT) in vitro. Mol Cell Biol. 1999;19:6207–6216. doi: 10.1128/mcb.19.9.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tzfati Y, Fulton T, Roy J, Blackburn E. Template boundary in a yeast telomerase specified by RNA structure. Science. 2000;288:863–867. doi: 10.1126/science.288.5467.863. [DOI] [PubMed] [Google Scholar]
- 54.Wang H, Zoulim F, Leber E, Kitson J, Seeger C. Role of RNA in enzymatic activity of the reverse transcriptase of hepatitis B virus. J Virol. 1994;68:8437–8442. doi: 10.1128/jvi.68.12.8437-8442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ware T, Wang H, Blackburn E. Three telomerases with completely non-telomeric template replacements are catalytically active. EMBO J. 2000;19:3119–3131. doi: 10.1093/emboj/19.12.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weinrich S L, Pruzan R, Ma L, Ouellette M, Tesmer V M, Holt S E, Bodnar A G, Lichtsteiner S, Kim N W, Trager J B, Taylor R D, Carlos R, Andrews W H, Wright W E, Shay J W, Harley C B, Morin G B. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat Genet. 1997;17:498–502. doi: 10.1038/ng1297-498. [DOI] [PubMed] [Google Scholar]
- 57.Xia J, Peng Y, Mian I, Lue N. Identification of functionally important domains in the N-terminal region of telomerase reverse transcriptase. Mol Cell Biol. 2000;20:5196–5207. doi: 10.1128/mcb.20.14.5196-5207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaug A J, Cech T R. Analysis of the structure of Tetrahymena nuclear RNAs in vivo: telomerase RNA, self-splicing rRNA and U2 snRNA. RNA. 1995;1:363–374. [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang X, Mar V, Harrington L, Robinson M O. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 1999;13:2388–2399. doi: 10.1101/gad.13.18.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]