Abstract
The emergence of antibiotic-resistant bacteria has become a significant and ever-increasing threat to global public health, increasing both morbidity and mortality rates, and the financial burden on health services. Infection by drug-resistant bacteria is anticipated to contribute to the demise of almost 10 million people by the year 2050 unless a competent and effective response is devised to engage with this issue. The emergence and spread of resistance are commonly caused by the excessive or inappropriate use of antibiotics and substandard pharmaceuticals. It arises when pathogens adapt to different conditions and develop self-defence mechanisms. Currently, novel antimicrobial peptides (AMPs) have been reported to be the sole cure for some clinical cases of infectious diseases such as sepsis and skin infections, although these agents may, on occasion, require administration together with an adjunctive low-dose antibiotic. Although AMPs are a promising alternative form of anti-microbial therapy and easily applied in the medical sector, they still have limitations that should not be taken lightly. Hence, this review explores the characteristics, advantages and disadvantages of AMPs for their potential in treating antibiotic-resistant pathogens.
Keywords: Antibiotic resistance, Antimicrobial peptides, AMPs, Bacteriocins, Bacterial resistance, Mechanisms of action, Naturally Occurring AMPs, Synthetic AMPs
Introduction
Antibiotics are anti-bacterial medications that inhibit or kill the growth of bacteria. It is usually competent in the treatment of pathogenic bacteria. However, antibiotics constantly lose their anti-bacterial strength as drug-resistant bacteria emerge with the misuse of the antibiotics (Gould & Bal, 2013; Sengupta, Chattopadhyay & Grossart, 2013; Wright, 2014). The antibiotic-resistant bacteria are evolving worldwide, threatening the effectiveness of antibiotics, which have previously recovered millions of lives from infectious diseases (Golkar, Bagasra & Pace, 2014; Gould & Bal, 2013; Sengupta, Chattopadhyay & Grossart, 2013; Wright, 2014). This scenario is becoming an important public health problem as it will lead to a prolonged hospital stay, increasing the cost of health care and risk of deaths (Golkar, Bagasra & Pace, 2014; Ventola, 2015).
The finding for alternative antimicrobial agents with new mechanisms of action is of urgent need. Although some antibiotics are still effective in killing bacteria, long-term concerns about the good and bad effects of their usage remain to be taken seriously. Worryingly, the number of deaths caused by bacterial infections has risen dramatically, making it one of the leading factors of life-threatening diseases (Morehead & Scarbrough, 2018; World Health Organization, 2020). This problem arises due to the emergence of resistant infectious agents which is a major problem in the treatment of microbial infections (Ventola, 2015). The attempts to discover other substances to replace the function of available antibiotics are still on going and being explored up to this day.
Antimicrobial peptides (AMPs) are one of the alternative components that may inhibit bacterial growth, possibly replacing the function of antibiotics in the future (Pfalzgraff, Brandenburg & Weindl, 2018). These peptides can bind and interact with the negatively charged bacterial cell membranes, resulting in the disruption of the bacterial cell membrane. They cause damage to the cellular membrane, affecting the transportation of the large molecules such as proteins and ruining the morphology of the cells leading to cell death (Lei et al., 2019). Besides, some AMPs such as non-lytic AMPs including buforin II, indolicin and drosocin can also translocate across bacterial membrane to act on intracellular targets, including ribosomes (Cardoso et al., 2019; Le, Fang & Sekaran, 2017). These provide a good rationale for AMPs as a potential alternative for treatment of antibiotic-resistant pathogens. This paper provides an overview on the dilemma and impact of antibiotic resistance, followed by naturally occurring AMPs and synthetic AMPs analogues as a potent antimicrobial agent, in view of finding solutions and improving the quality of health worldwide. The advantages and disadvantages of AMPs are also discussed in relation to those of antibiotics. This paper is intended for all scientists and academicians in related fields to recognize the potential use of AMPs as a therapeutic agent and to serve as a reference for future related studies.
Survey methodology
We conducted a literature search covering publications in 2011 till 2021 in relevant topics. The keywords used in the search included “antimicrobial peptides”, “antibiotic-resistance”, “mechanisms of action”, “AMPs”, “bacteriocins”, “bacterial resistance”, “mode of action”, “multi-drug resistance”, “naturally occurring AMPs” and “synthetic AMPs” through Google Scholar, Web of Science and PubMed Central platform. To ensure a comprehensive and unbiased coverage of the literature, all papers were assessed for information related to the crisis of antibiotic resistance; the history, sources and structure of AMPs; the bacterial resistance mechanisms and mechanisms of action of AMPs; and the benefits or limitations from the use of antibiotics and AMPs. The assessment of the data was performed by multiple individuals for articles in the different sections, followed by compilation and revision by the first and the corresponding author.
The dilemma and impact of antibiotic resistance
Although the world is rapidly moving towards an era of globalization, especially in the field of medical technology, the problem of antibiotic resistance is something that cannot be denied when the number of deaths caused by bacterial infections has risen dramatically, making it as one of the leading factors of life-threatening diseases (Morehead & Scarbrough, 2018). In early 1945, during the era of the discovery of penicillin, Sir Alexander Fleming had proclaimed and warned that antibiotics would one day be a highly demanded drugs and an era of abuse would emerge in the future (Spellberg & Gilbert, 2014). Now, many cases of the microbial resistance against antibiotics are being reported year by year. For example, Gentamicin-Resistance Enterecoccus (GRE) was first reported to be resistant to vancomycin, the drug that is in use to treat Methicillin-resistant Staphylococcus Aureus (MRSA) and Methicillin-resistant coagulase-negative Staphylococci (MR-CoNS) (Sengupta, Chattopadhyay & Grossart, 2013).
The pharmaceutical industries have introduced many new antibiotics in the late 1960s to 2000s which include imipenem, ceftazidime, levofloxacin, linezolid, daptomycin and ceftaroline. However, over time, more and more bacterial resistance appears, and the number of new drug discovery steeply decreases. As a result, bacterial infection becomes a great threat to human health (Ventola, 2015). Worryingly, the genetic traits for antibiotic resistance can be transferred to other bacteria through horizontal gene transfer (HGT). Some resistance can also be caused by mutations at the genetic level of a bacterial cell leading to expression of altered target sites which is no longer recognized by the antibiotics. This has led to the difficulties in controlling bacterial infections, as many antibiotics will not be able to exhibit similar effect over time and in different individuals (Read & Woods, 2014).
To avoid the adverse effects, the use of antibiotics should follow the guidelines in managing infections. Excessive use of antibiotics and misuse can lead to many complications such as diarrhea, indigestion, nausea, yeast infections or digestive problems. Incomplete use of antibiotics will not help killing the germs effectively but will increase the selection of resistance to strive and occupy the niche left by the susceptible strains. Antibiotics can also be toxic if not taken correctly and could turn out to be hazardous if taken more than the recommended doses (Ventola, 2015).
Previously, a high percentage of antibiotic resistance was found in farm animals and reached consumers through meat products (Bartlett, Gilbert & Spellberg, 2013), resulting in the spreading of bacterial resistance to human and adversely affecting human health (Centers for Disease Control and Prevention, 2013). The use of antibiotics in agriculture also affects the microbial balance of the environment (Bartlett, Gilbert & Spellberg, 2013). Certain amount of antibiotics ingested by livestock are excreted in their stools or urine. This causes widespread dissemination to the environment when their stools are used as fertilizers, which can be absorbed into groundwater and even soil (Bartlett, Gilbert & Spellberg, 2013). Moreover, antibiotic namely tetracycline is used as pesticides on plants. This practice causes long-term adverse ecological consequences due to the increase of resistant bacteria contaminating the environment (Golkar, Bagasra & Pace, 2014).
The crisis of antibiotic resistance which is becoming increasingly pervasive today is having a deleterious effect on the disease management. In general, infections caused by resistant bacterial strains are more severe than those by susceptible strains (Cosgrove & Yehuda, 2003). For instance, a significantly higher fatality rate has been reported for MRSA in comparison with that for methicillin-susceptible S. aureus infection (Cosgrove & Yehuda, 2003; Engemann et al., 2003). The adverse effects of antibiotic resistance can be evaluated in accordance with several factors, including an increase in patient mortality, greater resource utilisation, an escalation in the cost of care and reduction in hospital activity (Friedman, Temkin & Carmeli, 2016).
Mortality is the most severe consequence of antibiotic resistance. A report by Centers for Disease Control and Prevention (CDC) in 2019, suggests that more than 2.8 million antibiotic resistance associated infections occur in the United States every year, with over 35 000 fatalities. The annual increase in the number of cases has rendered the use of additional isolation rooms and consumable items necessary, with comparable increases being required in nursing care, support services and associated medical tests (diagnostic test and imaging), and these have placed a greater resource utilisation and the overall cost of care (Friedman, Temkin & Carmeli, 2016). In the wake of this, a relatively substantial amount of hospital spending, in excess of $4.6 billion annual basis, was reported for the treatment of patients infected by antibiotic resistant bacteria (Centers for Disease Control and Prevention, 2019). In addition, this crisis has been observed to rein in everyday hospital activities, with elective operations being cancelled against a background of outbreaks of antibacterial-resistant illnesses (Macraea et al., 2001).
The archival and the diversity of AMPs
AMPs have been in the focus as the alternative in addressing the problem of antibiotic resistance. AMPs have been available naturally and synthetically; the history of naturally occurring AMPs and the evolution of synthetics AMPs are discussed in this topic and the analogies between these two forms of AMPs are compiled in Table 1.
Table 1. The analogies between Naturally Occurring AMPs and Synthetic AMPs.
| Naturally occurring AMPs | Synthetic AMPs | References | |
|---|---|---|---|
| Sources/Origin | -Found in many tissues of many different species -Found in nearly all forms of life, and mostly reported to be isolated from eukaryotes, such as animals, plants and fungi -Found in prokaryotic cells |
-Non-natural sources -Often created by mimicking natural sequences |
(Jiang et al., 2021; Kumar, Kizhakkedathu & Straus, 2018; Nakatsuji & Gallo, 2012) |
| Content | -Comprised of l-amino acids recognizable by proteases | -The rational design of sequences comprising analogous d-amino acids substituted for l-amino acids | (Da Cunha et al., 2017; Zhao et al., 2016) |
| Discovery methods | -Using classic purification and in vitro and in vivo techniques | -Combination of trial-and-error experimentation, screening, or computer-aided design (increasing the peptide post-translational stability without altering biological function). |
(Da Cunha et al., 2017; Jiang et al., 2021) |
| Characteristics | -Frequently susceptible to protease degradation -Low bioavailability (i.e., presence of bioactive molecules at usually low levels). -Low resistance to proteolytic degradation resulting in shorter half-lives |
-High bioavailability -Longer half-lives in vivo, while maintaining a similar activity and selectivity. -Designed to improve their potential without side effects -Incorporation of multiple functions in the same peptide sequence |
(Azmi, Skwarczynski & Toth, 2016; Da Cunha et al., 2017; Jiang et al., 2021; Lei et al., 2019; Lu et al., 2020; Mahlapuu et al., 2016; Wimley, 2019) |
| Examples | -Protegerin -Indolicin -Magainin 2 -Moringa oleifera chitin-binding protein (Mo-CBP) |
-Iseganan (protegerin as template) -Omiganan (developed from indolicin) -Pexiganan (developed from magainin 2) -Mo-CBP3-PepIII (developed from Mo-CBP) |
(Ge et al., 1999; Gottler & Ramamoorthy, 2009; Oliveira et al., 2019; Sader et al., 2004; Trotti et al., 2004) |
The history of naturally occurring AMPs
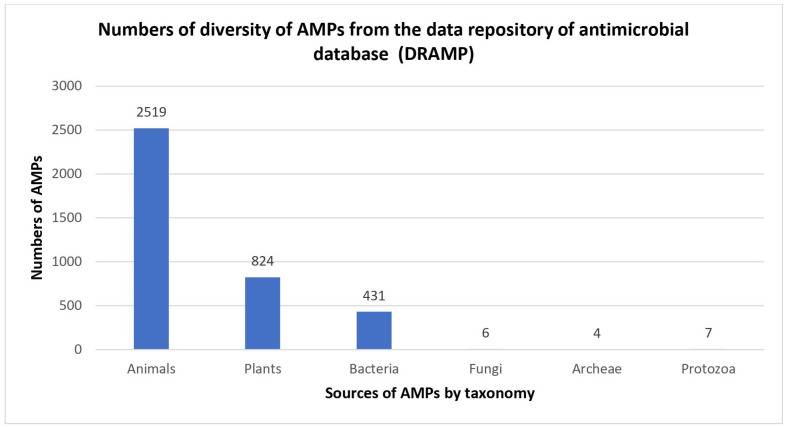
Naturally occurring AMPs that act as host defences are found in nearly all forms of life, and most of them have been reported to be isolated from eukaryotes, such as animals, plants and fungi (Kumar, Kizhakkedathu & Straus, 2018). AMPs were also found in prokaryotic cells when antimicrobial substances known as gramicidins were isolated from Bacillus brevis (Nakatsuji & Gallo, 2012). Historically, bacteria have been among the earliest sources of AMPs, and the percentage of AMPs isolated from bacteria have the potential to increase in the future (Fig. 1). In 1939, Dubos extracted AMPs from Bacillus strain in soil to protect mice from pneumococcal infections (Dubos, 1939). In a previous study, gramicidin showed antibacterial activity against various Gram-positive bacteria (Dubos & Hotchkiss, 1941). Gramicidin is effective in the treatment of infected wounds on guinea pig skin and in the treatment of topical wounds and ulcers (Van Epps, 2006; Gause & Brazhnikova, 1944), thus demonstrating their potential as the first commercially used AMPs in the health industry. After that, in 1941, other AMPs isolated from bacteria, called tyrocidines, were found to be effective against Gram-negative and Gram-positive bacteria (Dubos & Hotchkiss, 1941).
Figure 1. Numbers of diversity of AMPs from the data repository of antimicrobial peptides (DRAMP).
Data obtained from http://dramp.cpu-bioinfor.org/browse/.
In bacteria, AMPs help particular organisms by killing other bacterial species that compete for the same nutrients and ecological niche. Known as bacteriocins, bacterial AMPs can be classified into two classes: lantibiotics and non-lantibiotics. Lantibiotics are AMPs comprising the non-natural amino acid lanthionine. In 1947, a type of lantibiotic AMPs isolated from Lactococcus lactis, known as nisin, was found to be active against a number of Gram-positive bacteria and historically used as a preservative for many years without any noticeable growth of resistance (Mattick & Hirsch, 1947). Meanwhile, non-lantibiotics are AMPs composed of thermostable peptides which do not contain lanthionine and do not undergo post-translational modifications (Heng & Tagg, 2006). Garvicin Q (GarQ) is a type of non-lantibiotic AMPs with a relatively broad antimicrobial spectrum towards Listeria and Lactococcus spp (Tymoszewska et al., 2017).
In plants, AMPs play an important role in their protection against the infection of bacteria or fungi. In 1942, another AMPs, called purothionin, isolated from Triticum aestivum plants (Balls, 1942), was detected to be effective against other bacteria (Ohtani et al., 1977). The thionin family is among the best-studied groups of AMPs isolated from plants, apart from plant defensins and cyclotides.
The highest diversity of AMPs was found in animals. For example, in 1956, AMPs named defensins were discovered from rabbit leukocyte isolation (Hirsch, 1956). Later, in 1960, an AMP called lactoferrin was successfully isolated from cow’s milk (Groves, 1960), followed by the synthesis of the AMPs known as bominins from epithelial cells in 1962 (Kiss & Michl, 1962).
Several discoveries of AMPs from leukocytes have also been documented around 1970s and 1980s. Among these are rabbit-human α-defensins and purothionin (Selsted, Szklarek & Lehrer, 1984; Selsted et al., 1993). In 1980, Hultmark et al. (1980) used silk butterflies as a model system to successfully demonstrate that P9A and P9B could be induced in the hemolymph by co-vaccination with Enterobacter cloacae. Shortly thereafter, these peptides were renamed as cecropin until they became known as the major α-helical AMPs (Hultmark et al., 1980). In 1987, Zasloff (1987) isolated and characterized cationic AMPs from the African toad frog, Xenopus laevis, and named them magainin peptides. A few years later, β-defensin and θ-defensin were characterized after isolation from bovine granulocytes and from leukocytes of the rhesus monkey, respectively (Diamond et al., 1991; Tran et al., 2002).
In the early 1990s, there were several views that lysozyme was one of the first AMPs to exhibit antimicrobial activity involving non-enzymatic mechanisms. Based on these views, AMPs are seen to have a role in the immunity of human that lacks an adaptive immune system (Diamond et al., 2009). In the mid-1990s, several other peptides were also discovered, such as the first anionic AMPs found in X. laevis, while other peptides in the rumen of sheep and cattle were characterized (Brogden, Ackermann & Huttner, 1997). In addition, AMPs have also been found in fruit flies, called Drosophila melanogaster; by losing the genes encoding for AMPs in fruit flies will make them susceptible to fungal infections. This shows the importance of AMPs in protecting flies from microbial invasion (Lemaitre et al., 1996).
There are a lot of studies on AMPs that had been conducted to determine their ability to kill bacteria and fight infections. AMPs exist in almost all multicellular organisms and play roles in the mammalian immune system (Lemaitre et al., 1996). They have been widely identified in many areas of the human body that are usually exposed to germ-like infections. AMPs are important in innate modulation, as they can be produced naturally by various types of blood cells, including neutrophils, eosinophils and platelets, in the event of inflammation or injury, supporting that AMPs are among the agents responsible for fighting infections caused by germs (Diamond et al., 2009).
The evolution of synthetic AMPs
In general, living organisms produce gene-encoded AMPs that provide an immediate defence mechanism upon invasion by pathogens (Annunziato & Costantino, 2020). However, the application of AMPs in the clinical setting has been retsricted due to pharmaceutical limitations such as poor bioavailability, susceptibility to enzymatic degradation and toxicity (Deslouches et al., 2020; Costa et al., 2019). For this reason, synthetic AMPs that can maintain therapeutic effectiveness with higher biological stability and a greater safety profile continue to be developed. Most synthetic AMPs are designed to recapture the amphiphilic properties of natural AMPs which are believed to be the primary determinants of their antibacterial activity. In other words, the natural peptides will be modified to produce de novo scaffolds that resemble the parent peptides (Azmi, Skwarczynski & Toth, 2016).
Historically, various methods were used to optimize AMPs from natural sources, thus generating synthetic variants. In 1881, the azide-coupling method was used by treating the silver salt of glycine with benzoylchloride to create the first N-protected dipeptide, benzoylglycylglycine. Nevertheless, in 1901, Emil Fischer reported the first synthetic dipeptides by hydrolysis of the glycine diketopiperazine, known as glycylglycine (Lichtenthaler, 2002). The development of temporary amino-protecting groups was required to overcome synthetic challenges. In 1931, the carbobenzoxy (Cbz) group was introduced, followed by the ert-butyloxycarbonyl (Boc) group in 1957 (Anderson & McGregor, 1957; Grapsas, Cho & Mobashery, 1994). The discovery of solid phase peptide synthesis (SPSS) was achieved in 1963, when peptide sequences were synthesized on solid support (Merrifield, 1963). However, the major limitations of SPSS included insufficient coupling and degradation reactions, as well as a build-up of by-products (Pedersen et al., 2012; Schnölzer et al., 2009; King, Fields & Fields, 2009). For that reason, several new techniques of protein synthesis have been developed to overcome the limitations of SPPS. For example, chemical ligation and coupling two peptide fragments together were introduced (Kemp & Kerkman, 1981). Other ligation methods such as native chemical ligation (NCL), expressed protein ligation (EPL), and Staudinger ligation were also introduced to overcome the constraint (Dawson et al., 1994).
To date, methods for producing synthetic AMPs are constantly being improved. For example, using cationic peptides based on natural templates is becoming one of the most exciting new strategies for synthesizing AMPs today. These AMPs have complex mechanisms of action and do not readily lead to resistance. With their anti-inflammatory properties, as well as antimicrobial synergy, they hold promises as adjunctive strategies to supplement and enhance current therapies (Fjell et al., 2012). Several computational tools have also evolved in the development of more economical and powerful synthetic AMPs (Cardoso et al., 2019). For instances, the empirical methods, machine learning and de novo computational methods are being used in the optimization of peptides through random processes (Porto, Silva & Franco, 2012). Genetic algorithms also offer an alternative in the development of synthetic AMPs by identifying antibacterial activity-conferring determinants through successive generations of mutations and deletions in the target sequence (Kliger, 2010; Fjell et al., 2011). These candidates are refined over time as lower fitness values are removed from the candidate sequences (Fjell et al., 2011).
Hundreds of synthetic AMPs have been produced with the help of computer-aided design (Wimley, 2019). Previoulsy, naturally occuring AMPs are used as templates to optimize their activity and stability by mutating one or more amino acid residues; this was followed by the de novo design of a variety of synthetic peptides, peptoids, peptidomimetics, oligomers and polymers (Jiang et al., 2021). An example is iseganan, where protegerin is used as the template and one or more amino acid residues has been mutated to other proteinogenic L-amino acids to achieve antimicrobial activity against gram-negative and gram-positive bacteria (Trotti et al., 2004). There are several other examples of synthetic AMPs that are produced using this approach, such as omiganan, which is developed using indolicin; and pexiganan,which in turn, is developed by magainin 2 (Ge et al., 1999; Gottler & Ramamoorthy, 2009; Sader et al., 2004).
Another way of producing synthetic AMPs is utilization of β-amino acids as the building blocks or using non-natural N-substituted amino acids (Jiang et al., 2021). For example, synthesized helical β-peptide that was developed from β-amino acid; and synthesized oligo-N-substituted-glycine-based helical peptoid that was developed by magainin 2 amide. Both of these synthetic AMPs show greater and more stable antibacterial activity compared to naturally occuring AMPs (Chongsiriwatana et al., 2008; Patch & Barron, 2003; Cheng, Gellman & De Grado, 2001). Generally, synthetic AMPs are more stable and possess better activity and selectivity compared to naturally occuring AMPs. However, the limitations of producing synthetic AMPs include the extended time required to do so and the high cost (Jiang et al., 2021).
The structures of AMPs
To clearly identify the potential of AMPs, the structure of AMPs needs to be well elucidated. AMPs are relatively short molecules, containing 12–100 amino acids with an amphipathic structure (Hodges et al., 2011). Several databases exist that manage information and conduct peptide analysis, due to the high numbers of natural, semi-synthetic and synthetic AMPs (Mahlapuu et al., 2016). AMPs can be classified based on their structure, amino acid composition and size. The structural features of AMPs can be divided into four main groups, (a) peptides with amphipathic α-helices (b) β sheets, (c) combined α-helices and β sheet structures (α β) known as a mixed structure and (d) non–α β structure known as extended structure (Fig. 2).
Figure 2. (A–E) Structure of AMPs.

α-helical peptides are the most widely studied types of AMPs to date. The α-helical peptide has two amino acids adjacent to each other with a distance of 0.15 nm between them; the centre is about 100 degrees from the top view. Among the well-known peptides studied in this group are LL-37 and human lactoferricin (Epand & Vogel, 1999; Hunter et al., 2005; Legrand et al., 2005; Pasupuleti, Schmidtchen & Malmsten, 2011). In addition, among the other widely studied AMPs are colistin, melittin, nisin, and Cecropin A-Magainin 2 (CAMA) (Bechinger & Lohner, 2006; Kumar, Kizhakkedathu & Straus, 2018).
β-sheet peptides are composed of at least two β strands with disulfide bonds between these sheets. Interestingly, almost all β-sheet AMPs contain preserved cysteine residues and form disulphide bonds such as gomesin, polyphemusin, protegerin and tachyplesin (Kumar, Kizhakkedathu & Straus, 2018). However, some studies have reported short β-sheet forming AMPs that do not have disulfide bonds (Cândido et al., 2019; Ong et al., 2014; Ong, Gao & Yang, 2013). For example, the synthetic β-sheet AMP known as IK8-all D (irikirik-NH2) which is derived from β-sheet forming peptides (IRIK)2-NH2 (IK8-all L) has shown no formation of disulfide bonds in its design (Ong et al., 2014).
Besides α-helical peptides and β-sheet peptides, there is a kind of AMP structure that had been found with the formation of α-helices and β-sheets (α β) (mixed structure). In this class of AMPs, the two monomers are packed against each other with the β-sheet of one monomer facing the α-helix of another monomer (Kovaleva et al., 2020). Human β-defensin-2 and pine defensin 1 (PsDef1) are among the peptides studied in this group (Jenssen, Hamill & Hancock, 2006; Kovaleva et al., 2020).
Extended/random coil AMPs display another unique structure that has been frequently discussed. This structure consists of two or more proline residues, tryptophan, arginine and histidine which have the capabilities to break the secondary structure elements (Bahar & Ren, 2013). In addition, many peptides such as indolicin and moricin, adopt their active structure only after they interact with the target cell membrane. Indolicin is a hemolytic AMP isolated from bovine neutrophils. It is effective as an antimicrobial agent because it has 13 tridecapeptide amides and an extremely high tryptophan content (Cardoso et al., 2019). Indolicin changes its structural profile to a “boat-like”and transmembrane orientation to translocate the bacterial membrane and act on DNA (Cardoso et al., 2019). Moricin is a random coil AMP that was isolated from Manduca sexta. It consists of one aspartic acid, two arginine and nine lysine residues and features α-helical structures to perform their membrane-associated or intracellular mechanisms of action (Dai et al., 2008)
Apart from those, there have been progressively increasing reports in newly discovered AMPs with cyclic and disulfide-rich AMPs (Figure 2(e)) as well as AMPs with more complex topologies in the past two decades. Some studies reported these as the fifth class of AMPs (Koehbach & Craik, 2019). These peptides have been identified based on the nature of the peptide’s cyclic topology such as “head to tail” or “head to side chain” as well as the nature of the crosslinks such as the presence of disulfide or thioether bridges (Koehbach & Craik, 2019). For example, microcin J25 (lasso peptides) has been found with a head-to-side chain cycle (magenta) threaded by the C-terminal tail and sterically locked in place by bulky residues (cyan) (Rosengren et al., 2003).
The bacterial resistance mechanisms towards antibiotics and the way AMPs can help
Understanding on the mechanisms of antibiotic resistance will allow the relevance of AMPs to be seen as a potential alternative for antibiotics. Antibiotic resistance mechanisms in bacteria and how AMP mechanisms aid in killing bacteria are depicted in Fig. 3. This figure displays four major molecular mechanisms by which bacteria can withstand antibiotic effects. Among these are drug-target modifications, antibiotic-degrading enzymes, antibiotic-altering enzymes and antibiotic efflux pumps (Laws, Shaaban & Rahman, 2019). These resistance mechanisms can occur in one bacterial cell simultaneously, resulting in high levels of resistance to various antibiotic compounds (Peterson & Kaur, 2018). In addition to these four major mechanisms, bacterial biofilm has also attracted a great attention in resistance mechanisms towards antibiotics. Bacteria that attach to the surface and grow as biofilm are protected from killing by antibiotics, thus makes the treatment difficult (Dincer, Uslu & Delik, 2020).
Figure 3. Bacterial resistance mechanisms to antibiotics and the mechanisms of AMPs in bacteria.
Meanwhile, most of the AMPs were found to kill bacterial cells by disrupting the bilayer membrane without the interference of all the available antibiotic resistance characters that might be present in a bacterial cell. However, there are studies indicating that bacteria can resist AMPs treatment at sub-lethal doses and expel them by efficient efflux pumps (Cardoso et al., 2017). Membrane interactions are important in the direct antimicrobial activity of AMPs (Hollmann et al., 2018; Lei et al., 2019). Several models have been introduced to explain the mechanism of disrupting bilayer membranes by AMPs. These models are the barrel-stave model, the toroidal-pore model, and the carpet model. In the barrel-stave model, recruitment of additional peptides placed perpendicularly into the bilayer will lead to the formation of a peptide-lined transmembrane pore. In this pore, the peptides align with the hydrophobic side facing the lipid core of the membrane, while the hydrophilic regions face the interior region of the pore. In the toroidal-pore model, phospholipids bend continuously from one leaflet to another due to the interaction of AMPs. This then results in a pore lined by both peptides and the head groups of phospholipids. For the carpet model, the mechanism is explained by the formation of micelles due to membrane disruptions by the tension in the bilayer as a result of peptide accumulation (Mahlapuu et al., 2016).
Intracellular targeting and inhibition of protein synthesis also act as targets by which some of the AMPs may interfere and express their function and ability to disrupt the cell growth. To reach the cytoplasmic membrane of Gram-negative bacteria, AMPs will translocate through the outer membrane via a self-promoted uptake (Le, Fang & Sekaran, 2017; Mahlapuu et al., 2016). Moreover, activated AMPs cause damages to bacterial cells by attacking an internal target or translocating across the membrane receptors, entering the bacterial cytoplasm and disrupting intracellular targets (Jindal et al., 2015; Malanovic & Lohner, 2016). Bacterial destruction also occurs by the interaction between the electrostatic forces of the positively charged amino acids of the AMPs and the negatively charged cell surface. These create an ion-permeable channel and increase membrane permeability to develop cleavages (Lin & Weibel, 2016).
The translocation of AMPs will not only disrupt the cellular membrane but also target some important processes, such as DNA transcription and replication, RNA synthesis and protein synthesis, enzymatic activity and protein folding or cell wall synthesis (Le, Fang & Sekaran, 2017). For example, indolicin acts by targeting DNA and inhibiting the replication process, indirectly killing the bacteria. Bacterial death caused by AMPs could be the result of multiple and complementary actions. The mode of action of AMPs depends on several factors, including peptide concentrations, the targeted bacterial species, tissue localization and the bacterial growth phase (Kumar, Kizhakkedathu & Straus, 2018; Mahlapuu et al., 2016).
Bacterial cytoplasmic membranes are rich with negatively charged phospholipids, including phosphatidylglycerol, cardiolipin and phosphatidylserine, all of which are highly attracted to the positive charges of AMPs (Ebenhan et al., 2014). Gram-negative bacteria consist of an additional lipopolysaccharide-rich outer membrane that acts as a barrier to the cytoplasmic membranes. The presence of teichoic acids in the cell wall of Gram-positive bacteria also provides an additional electronegative charge to the bacterial surface (Ebenhan et al., 2014). As opposed to bacteria, human cells seem to be rich in neutrally charged phospholipids, such as phosphatidylethanolamine, phosphatidylcholine and sphingomyelin. This fundamental difference between microbial and mammalian membranes has made AMPs a highly selective agent against bacteria (Ebenhan et al., 2014). The presence of cholesterol in humans affects the fluidity of the phospholipid in the membranes via an increased stability of the bilayer, then, reduces the activity of AMPs via stabilization of phospholipids bilayer (Subczynski et al., 2018).
The advantages and disadvantages of AMPs
In developing AMPs as the potential treatment for antibiotic-resistant pathogens, the advantages as well as the limitations of AMPs should be considered and are discussed in this topic.
Advantages of AMPs
The long-term and overly frequent use of conventional antibiotics as antibacterial agents has the potential to cause mutations in the bacterium, thereby increasing resistance to the antibiotics themselves (Bahar & Ren, 2013). This issue has prompted researchers and the pharmaceutical industry to focus on identifying drugs capable of replacing antibiotics. AMPs are a type of cationic peptide, an agent thought to be able to fulfil the role of antibiotics. Unlike antibiotics, AMPs interact with the cell membrane of bacteria by neutralizing the charge and, subsequently, causing bacterial death by penetrating the membrane, thereby reducing the risk of bacterial resistance (Mahlapuu et al., 2016). This ability on the part of AMPs indicates them to be more effective than conventional antibiotics.
AMPs have demonstrated a wide range of capabilities in killing bacteria as well as fungi and viruses (Amso & Hayouka, 2019; Mahlapuu et al., 2016). Interestingly, AMPs have less side effects on the hosts, as their uses cause a very minimal toxicity to the body based on previous studies (Zharkova et al., 2019; Lei et al., 2019; Mahlapuu et al., 2016). For example, a peptide known as citrus-amp1, which is isolated from citrus, exhibited low toxicity effects when tested on Galleria mellonella, a cell line derived from the larval-fat body tissues of the wax moth, and on U87 MG, a human glioblastoma cell line commonly used as a model for cytotoxicity (Kishi et al., 2018). A peptide known as Nisin A also presented low toxicity effects when tested on HT29 and Caco-2 cells by using MTT assay (Maher & McClean, 2006).
AMPs with a simple structure–activity relationship are widely used in the development of medicines. They are particularly useful in this regard because they are associated with excellent water stability and solubility (Dehsorkhi, Castelletto & Hamley, 2014). For example, daptomycin, another type of AMPs, has been used as an anionic antibacterial peptide to treat skin infections stemming from Gram-positive bacteria, thereby showing inhibitory effects on S. aureus and typhoid bacillus Salmonella typhi (Lei et al., 2019).
Additionally, AMPs have also demonstrated a good inhibition of cancer cells (Mahlapuu et al., 2016). In fact, cancer cells are more sensitive to AMPs than normal cells. This is because the cytoskeletons of cancer cells do not grow well when compared with those of normal cells, which allows AMPs to easily enter the lipid membrane and form ion channels or pores. This process eventually destroys the cancer cells by causing the leakage of the cell content (Jäkel et al., 2012; Mahlapuu et al., 2016). More specifically, the content of those cationic AMPs associated with the high acid phospholipids that occupy the outer surface of cancerous cell causes changes in the membrane, extracellular matrix and cytoskeleton (Mahlapuu et al., 2016). The loss of phospholipids asymmetry in cancer cells provides them with more negatively charged residues in their upper leaflet, thus favouring electrostatic attraction of AMPs (Ramos-Martín & D’Amelio, 2021). In terms of acting as antimicrobial agents, AMPs have the potential to fight antibiotic-resistant bacteria. The bactericidal effect of AMPs is generally due to the creation of pores in the bacterial cytoplasmic membrane, which results in a loss of control over the flow of ions through the membrane and, consequently, cell deaths. This renders the use of AMPs a promising strategy for addressing the problem of antibiotic resistance through fulfilling the role of conventional antibiotics (Lei et al., 2019).
Disadvantages of AMPs
Despite the uniqueness and recognised advantages of AMPs, concerns have been raised about certain disadvantages of their excessive use that may eventually lead to the emergence of resistance against AMPs as bacteria will always mutate for survival. Among other disadvantages include several aspects such as toxicity, immunogenicity, haemolytic activity in certain type of human cells, reduced activity based on salt sensitivity, and the high cost of production (Aoki & Ueda, 2013; Moravej et al., 2018). These characteristics render the use of AMPs in the field of medicine more difficult.
There have been challenges in classifying the good AMPs and AMPs that can cause side effects. In some cases, the use of AMPs is associated with a high risk of toxic effects in human cells. For example, certain peptides such as arenicin, LTX-109 and LL-37 have been found to cause side effects (itching, burning and pain) to mammalian cells in vitro and, further, to be toxic with the formation of pore at the membrane, disruption of the membrane and cell lysis, when injected into the bloodstream (Patrulea, Borchard & Jordan, 2020). This problem urges research to look for more new AMPs compounds with less toxicity effects. In addition, although AMPs have been reported not to elicit an immunogenic response (no interference from the action of the host cell), immunogenicity continues to be a concern and even a serious problem in the development of the peptide drugs (Mahlapuu et al., 2016). Based on previous findings, structural properties such as the changes in peptide sequences (modified amino acids), glycosylation changes, the presence of aggregates and other possible factors have been identified as the factors that can lead to immunogenicity of AMPs (da Cunha et al., 2017; Natalia, Brendan & Sam., 2017). These factors may cause the function of AMPs to be disrupted.
A certain number of AMPs have been reported to influence haemolytic activity. Indolicidin, for example, a 13-residue cationic peptide that is rich in tryptophan, has been found to exhibit a broad spectrum of anti-bacterial activity, however it exhibits haemolytic activity that limits their clinical applications (Mirski et al., 2018). Some types of AMPs can interact directly with the host cell and dissolve it, although most AMPs bind to the bacterial opening through electrostatic interactions. The amide peptides exhibit higher antimicrobial activity than natural AMPs, although they are more haemolytic. In addition, the functional analysis of AMPs has demonstrated how their high amphiphilicity and high hydrophobicity contribute to their increased haemolytic capability (Aoki & Ueda, 2013; Bahar & Ren, 2013). However, the haemolytic activity of several AMPs was observed to be different in certain types of different species. For example, based on a previous study, 24 AMPs were evaluated for their haemolytic activity in cells of four different species such as human, dog, rat and bovine. Based on this study, some of the AMPs showed no or less haemolytic activity towards each species, and vice versa (Greco et al., 2020). More thorough studies need to be conducted to identify the most appropriate AMPs that do not cause harm to human.
The fact that some AMPs require electrostatic interactions with microbial membranes to form a skeletal structure has caused them to be more sensitive to salt, which often leads to problems with clinical applications (Andersson, Hughes & Kubicek-Sutherland, 2016; Hollman et al., 2018). Human body fluids that have a high salt concentration disrupt the function of these AMPs and, therefore, deactivate them (Bastos et al., 2018). Thus, the identification of salt-resistant AMPs is essential to improve the effectiveness of AMPs within the human body. AMPs are also rapidly degraded in human body by proteases (Aoki & Ueda, 2013). A feasible production method is required to develop AMPs as drugs. Further studies need to be conducted in this regard, and such efforts will require a lot of investments. For instance, the production of heterologous AMPs within prokaryotic systems is considered to be extremely difficult, as AMPs are associated with the poisoning risk in prokaryotic cells (Aoki & Ueda, 2013).
Conclusion
Physicians and scientists describe the antibiotic resistance crisis as increasingly threatening. This problem may worsen unless the public takes precautionary measures. This issue has prompted research to look for new antimicrobials, leading to ongoing research on the AMPs. However, the development has been slow due to several challenges such as high cost of salvage, potential toxicity and lack of solid guideline for rational design. In addition, it might be possible to modify the characteristics of naturally occurring AMPs to produce synthetic AMPs through certain available methods, but to predict the impact of these modifications in clinical usage is still challenging. Thus, there is a need to observe the effects of structural modifications on their function, activity, and target spectrum. On the other hand, the mechanisms of action of AMPs targeting the cell membrane, make them as a good potential approach in controlling the antibiotic resistant pathogen. A close collaboration between different disciplines and the development of new tools that can decipher the structure-function relationship, as well as efficiently synthesize and modify AMPs molecules will be the key to AMPs related research in the future.
Abbreviations
- AMPs
Antimicrobial peptides
- GRE
Gentamicin-Resistance Enterecoccus
- MRSA
Methicillin-Resistant Staphylococcus Aureus
- MR-CoNS
Methicillin resistant coagulase-negative Staphylococci
- HGT
horizontal gene transfer
- CAMA
Cecropin A-Magainin 2
- DNA
Deoxyribonucleic acid
- RNA
Ribonucleic acid
- U87 MG
Uppsala 87 Malignant Glioma
- LL-37
form of LL-37
- X. laevis
(Xenopus laevis)
- SPSS
solid phase peptide synthesis
- NCL
native chemical ligation
- EPL
expressed protein ligation
Funding Statement
This study was supported by the funds of Ministry of Higher Education, Malaysia and Universiti Putra Malaysia through Fundamental Research Grant Scheme (FRGS/1/2017/SKK11/UPM/01/1) and Putra Grant (GP/2017/9571800). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Nurul Hana Zainal Baharin and Mohd Nasir Mohd Desa conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Nur Fadhilah Khairil Mokhtar conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Banulata Gopalsamy conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Nor Nadiha Mohd Zaki, Mohd Hafis Yuswan, Sahar Abbasiliasi, Amalia Mohd Hashim, Muhamad Shirwan Abdullah Sani and Shuhaimi Mustafa performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
AbdulRahman Muthanna and Nurul Diana Dzaraly conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
This is a literature review study.
References
- Amso & Hayouka (2019).Amso Z, Hayouka Z. Antimicrobial random peptide cocktails: a new approach to fight pathogenic bacteria. Chemical Communication. 2019;55:2007–2014. doi: 10.1039/C8CC09961H.7-14. [DOI] [PubMed] [Google Scholar]
- Anderson & McGregor (1957).Anderson GW, McGregor AC. t-Butyloxycarbonylamino acids and their use in peptide synthesis. Journal of American Chemical Society. 1957;79:6180–6183. doi: 10.1021/ja01580a020. [DOI] [Google Scholar]
- Andersson, Hughes & Kubicek-Sutherland (2016).Andersson DI, Hughes D, Kubicek-Sutherland JZ. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resistance Updates. 2016;26:43–57. doi: 10.1016/j.drup.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Annunziato & Costantino (2020).Annunziato G, Costantino G. Antimicrobial peptides (AMPs): a or a patent review (2015–2020) Expert Opinion on Therapeutic Patents. 2020;30:931–947. doi: 10.1080/13543776.2020.1851679. [DOI] [PubMed] [Google Scholar]
- Aoki & Ueda (2013).Aoki W, Ueda M. Characterization of antimicrobial peptides toward the development of novel antibiotics. Pharmaceuticals. 2013;6:1055–1081. doi: 10.3390/ph6081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmi, Skwarczynski & Toth (2016).Azmi F, Skwarczynski M, Toth I. Towards the development of synthetic antibiotics: designs inspired by natural antimicrobial peptides. Current Medicinal Chemistry. 2016;23:4610–4624. doi: 10.2174/0929867323666160825162435. [DOI] [PubMed] [Google Scholar]
- Bahar & Ren (2013).Bahar AA, Ren D. Antimicrobial peptides. Pharmaceuticals. 2013;6:1543–1575. doi: 10.3390/ph6121543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balls (1942).Balls AK. A crystalline sulphur-protein from wheat. Journal of Washington Academy of Sciences. 1942;32:132–137. [Google Scholar]
- Bartlett, Gilbert & Spellberg (2013).Bartlett JG, Gilbert DN, Spellberg B. Seven ways to preserve the miracle of antibiotics. Clinical Infectious Diseases. 2013;56:1445–1450. doi: 10.1093/cid/cit070. [DOI] [PubMed] [Google Scholar]
- Bastos et al. (2018).Bastos P, Trindade F, da Costa J, Ferreira R, Vitorino R. Human antimicrobial peptides in bodily fluids: current knowledge and therapeutic perspectives in the postantibiotic era. Medicinal Research Reviews. 2018;38:101–146. doi: 10.1002/med.21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechinger & Lohner (2006).Bechinger B, Lohner K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochimica Et Biophysica Acta - Biomembranes. 2006;1758:1529–1539. doi: 10.1016/j.bbamem.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Brogden, Ackermann & Huttner (1997).Brogden KA, Ackermann M, Huttner KM. Small, anionic, and charge-neutralizing propeptide fragments of zymogens are antimicrobial. Antimicrobial Agents and Chemotherapy. 1997;41:1615–1617. doi: 10.1128/AAC.41.7.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cândido et al. (2019).Cândido ES, Cardoso MH, Chan LY, Torres MDT, Oshiro KGN, Porto WF, Ribeiro SM, Haney EF, Hancock REW, Lu TK, Fuente-Nunez Cdela, Craik DJ, Franco OL. Short cationic peptide derived from archaea with dual antibacterial properties and anti-infective potential. ACS Infectious Diseases. 2019;5:1081–1086. doi: 10.1021/acsinfecdis.9b00073. [DOI] [PubMed] [Google Scholar]
- Cardoso et al. (2017).Cardoso MH, De Almeida KC, Candido ES, Murad AM, Dias SC, Franco OL. Comparative nanoUPLC-MSE analysis between magainin I-susceptible and -resistant Escherichia coli strains. Scientific Reports. 2017;7:4197. doi: 10.1038/s41598-017-04181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso et al. (2019).Cardoso MH, Meneguetti BT, Costa BO, Buccini DF, Oshiro KGN, Preza SLE, Carvalho CME, Migliolo L, Franco OL. Non-lytic antibacterial peptides that translocate through bacterial membranes to act on intracellular targets. International Journal of Molecular Sciences. 2019;20(19):4877. doi: 10.3390/ijms20194877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2019).Centers for Disease Control and Prevention Antibiotic Resistance Threats in the United States, 2019. ``http://www.cdc.gov/DrugResistance/Biggest-Threats.html'' www.cdc.gov/DrugResistance/Biggest-Threats.html. 2019 doi: 10.15620/cdc:82532. [DOI] [Google Scholar]
- Centers for Disease Control and Prevention (2013).Centers for Disease Control and Prevention Antibiotic resistance, food, and food animals. 2013. https://www.cdc.gov/foodsafety/challenges/antibiotic-resistance.html. [28 January 2015]. https://www.cdc.gov/foodsafety/challenges/antibiotic-resistance.html
- Cheng, Gellman & De Grado (2001).Cheng RP, Gellman SH, De Grado WF. β-Peptides: from structure to function. Chemical Review. 2001;101:3219–3232. doi: 10.1021/cr000045i. [DOI] [PubMed] [Google Scholar]
- Chongsiriwatana et al. (2008).Chongsiriwatana NP, Patch JA, Czyzewski AM, Dohm MT, Ivankin A, Gidalevits D, Zuckermann RN, Barron AE. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2794–2799. doi: 10.1073/pnas.0708254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove & Yehuda (2003).Cosgrove SE, Yehuda C. The impact of antimicrobial resistance on health and economic outcomes. Clinical Infectious Diseases. 2003;36:1433–1437. doi: 10.1086/375081. [DOI] [PubMed] [Google Scholar]
- Costa et al. (2019).Costa F, Teixeira C, Gomes P, Martins MCL. Clinical application of AMPs, antimicrobial peptides. Advances in Experimental Medicine and Biology. 2019;1117:281–298. doi: 10.1007/978-981-13-3588-4_15. [DOI] [PubMed] [Google Scholar]
- Da Cunha et al. (2017).Da Cunha NB, Cobacho NB, Viana JFC, Lima LA, Sampaio KBO, Dohms SSM, Ferreira ACR, De la Fuente-Núñez C, Costa FF, Franco OL, Dian SC. The next generation of antimicrobial peptides (AMPs) as molecular therapeutic tools for the treatment of diseases with social and economic impacts. Drug Discovery Today. 2017;22:234–248. doi: 10.1016/j.drudis.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai et al. (2008).Dai H, Rayaprolu S, Gong Y, Huang R, Prakasha O, Jiang H. Solution structure, antibacterial activity, and expression profile of Manduca sexta moricin. Journal of Peptide Science. 2008;14:855–863. doi: 10.1002/psc.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson et al. (1994).Dawson PE, Muir TW, Clark-Lewis I, Kent SB. Synthesis of proteins by native chemical ligation. Science. 1994;4;266(5186):776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- Dehsorkhi, Castelletto & Hamley (2014).Dehsorkhi A, Castelletto V, Hamley IW. Self-assembling amphiphilic peptides. Journal of Peptide Science. 2014;20:453–467. doi: 10.1002/psc.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslouches et al. (2020).Deslouches B, Ronald CM, Ken LU, Yuanpu PD. Engineered cationic antimicrobial peptides (ECAPs) to combat multidrug-resistant bacteria. Pharmaceutics. 2020;12(6):501. doi: 10.3390/pharmaceutics12060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond et al. (2009).Diamond G, Beckloff N, Weinberg A, Kisich KO. The roles of antimicrobial peptides in innate host defense. Current Pharmaceutical Design. 2009;15:2377–2392. doi: 10.2147/138161209788682325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond et al. (1991).Diamond G, Zasloff M, Eck H, Brasseur M, Maloy WL, Bevins CL. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincer, Uslu & Delik (2020).Dincer S, Uslu FM, Delik A. Antibiotic resistance in biofilm. IntechOpen. 2020 doi: 10.5772/intechopen.92388. [DOI] [Google Scholar]
- Dubos (1939).Dubos RJ. Studies on a bactericidal agent extracted from a soil Bacillus: I. Preparation of the agent. Its activity in vitro. Journal of Experimental Medicine. 1939;70:1–10. doi: 10.1084/jem.70.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos & Hotchkiss (1941).Dubos RJ, Hotchkiss RD. The production of bactericidal substances by aerobic sporulating bacilli. Journal of Experiment Medicine. 1941;73:629–640. doi: 10.1084/jem.73.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebenhan et al. (2014).Ebenhan T, Gheysens O, Kruger HG, Zeevaart JR, Sathekge MM. Antimicrobial peptides: their role as infection-selective tracers for molecular imaging. BioMed Research International. 2014;2014:867381. doi: 10.1155/2014/867381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engemann et al. (2003).Engemann JJ, Carmeli Y, Cosgrove SE, Fowler VG, Bronstein MZ, Trivette SL, Briggs JP, Sexton DJ, Kaye KS. Adverse clinical and economic outcomes attributable to Methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clinical Infectious Diseases. 2003;36:592–598. doi: 10.1086/367653. [DOI] [PubMed] [Google Scholar]
- Epand & Vogel (1999).Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochimica Et Biophysica Acta - Biomembranes. 1999;1462:11–28. doi: 10.1016/S0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- Fjell et al. (2012).Fjell C, Hiss J, Hancock R. Designing antimicrobial peptides: form follows function. Nature Reviews Drug Discovery. 2012;11:37–51. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- Fjell et al. (2011).Fjell CD, Jenssen H, Cheung WA, Hancock RE, Cherkasov A. Optimization of antibacterial peptides by genetic algorithms and cheminformatics. Chemical Biology and Drug Design. 2011;77:48–56. doi: 10.1111/j.1747-0285.2010.01044.x. [DOI] [PubMed] [Google Scholar]
- Friedman, Temkin & Carmeli (2016).Friedman ND, Temkin E, Carmeli Y. The negative impact of antibiotic resistance. Clinical Microbiology and Infection. 2016;22:416–422. doi: 10.1016/j.cmi.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Gause & Brazhnikova (1944).Gause GF, Brazhnikova MG. Gramicidin S and its use in the treatment of infected wounds. Nature. 1944;154:703. doi: 10.1038/154703A0. [DOI] [Google Scholar]
- Ge et al. (1999).Ge Y, MacDonald DL, Holdray KJ, Thornsberry C, Wexler H, Zasloff M. In vitro antibacterial properties of pexiganan, an analog of magainin. Antimicrobial Agents and Chemotherapy. 1999;43:782–788. doi: 10.1128/AAC.43.4.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkar, Bagasra & Pace (2014).Golkar Z, Bagasra O, Pace DG. Bacteriophage therapy : a potential solution for the antibiotic resistance crisis. Journal of Infection in Developing Countries. 2014;8:129–136. doi: 10.3855/jidc.3573. [DOI] [PubMed] [Google Scholar]
- Gottler & Ramamoorthy (2009).Gottler LM, Ramamoorthy A. Structure, membrane orientation, mechanism, and function of pexiganan—a highly potent antimicrobial peptide designed from magainin. Biochimica Et Biophysica Acta - Biomembranes. 2009;1788:1680–1686. doi: 10.1016/j.bbamem.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould & Bal (2013).Gould IM, Bal AM. New antibiotic agents in the pipeline and how they can help overcome microbial resistance. Virulence. 2013;4:185–191. doi: 10.4161/viru.22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grapsas, Cho & Mobashery (1994).Grapsas I, Cho YJ, Mobashery S. N-(tert-butoxycarbonyloxy)-5-norbornene-endo-2, 3-dicarboximide, a reagent for the regioselective introduction of the tert-butoxycarbonyl (boc) protective group at unhindered amines: application to amino glycoside chemistry. The Journal of Organic Chemistry. 1994;59:1918–1922. doi: 10.1021/JO00086A055. [DOI] [Google Scholar]
- Greco et al. (2020).Greco I, Molchanova N, Holmedal E, Jenssen H, Hummel BD, Watts JL, Håkansson J, Hansen PR, Svenson J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Scientific Reports. 2020;10:13206. doi: 10.1038/s41598-020-69995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves (1960).Groves ML. The isolation of a red protein from milk. Journal of the American Chemical Society. 1960;82:3345–3350. doi: 10.1021/ja01498a029. [DOI] [Google Scholar]
- Heng & Tagg (2006).Heng NCK, Tagg JR. What’s in a name? Class distinction for bacteriocins. Nature Reviews Microbiology. 2006;4:160. doi: 10.1038/nrmicro1273-c. [DOI] [Google Scholar]
- Hirsch (1956).Hirsch JG. Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. Journal of Experimental Medicine. 1956;103:589–611. doi: 10.1084/jem.103.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges et al. (2011).Hodges RS, Jiang Z, Whitehurst J, Mant CT. Development of antimicrobial peptides as therapeutic agents. Pharmaceutical Sciences Encyclopedia: Drug Discovery, Development, and Manufacturing. 2011 doi: 10.1002/9780470571224.pse430. [DOI] [Google Scholar]
- Hollmann et al. (2018).Hollmann A, Martinez M, Maturana P, Semorile LC, Maaffia PC. Antimicrobial peptides: interaction with model and biological membranes and synergism with chemical antibiotics. Frontiers in Chemistry. 2018;6:204. doi: 10.3389/fchem.2018.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark et al. (1980).Hultmark D, Steiner H, Rasmuson T, Boman HG. Insect immunity, Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora Cecropia. European Journal of Biochemistry / FEBS. 1980;16:7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- Hunter et al. (2005).Hunter H, Demcoe A, Jenssen H, Gutteberg T, Vogel H. Human lactoferricin is partially folded in aqueous solution and is better stabilized in a membrane mimetic solvent. Antimicrobial Agents and Chemotherapy. 2005;49:3387–3395. doi: 10.1128/AAC.49.8.3387-3395.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäkel et al. (2012).Jäkel CE, Meschenmoser K, Kim Y, Weiher H, Schmidt-Wolf IGH. Efficacy of a proapoptotic peptide towards cancer cells. In Vivo. 2012;26:419–426. [PubMed] [Google Scholar]
- Jenssen, Hamili & Hancock (2006).Jenssen H, Hamili P, Hancock RE. Peptide antimicrobial agents. Clinical Microbiology Reviews. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang et al. (2021).Jiang Y, Chen Y, Song Z, Tan Z, Cheng J. Recent advances in design of antimicrobial peptides and polypeptides toward clinical translation. Advanced Drug Delivery Reviews. 2021;170:261–280. doi: 10.1016/j.addr.2020.12.016. [DOI] [PubMed] [Google Scholar]
- Jindal et al. (2015).Jindal HM, Le CF, Yusof MYM, Velayuthan RD, Lee VS, Zain SM, Isa DM, Sekaran SD. Antimicrobial activity of novel synthetic peptides derived from indolicidin and ranalexin against Streptococcus pneumoniae. PLOS ONE. 2015;10:e0128532. doi: 10.1371/journal.pone.0128532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp & Kerkman (1981).Kemp DS, Kerkman DJ. Models that demonstrate peptide-bond formation by prior thiol capture 2, Capture by organomercury derivatives. Tetrahedron Letters. 1981;22:185–186. doi: 10.1016/0040-4039(81)80050-0. [DOI] [Google Scholar]
- King, Fields & Fields (2009).King DS, Fields CG, Fields GB. A cleavage method which minimizes side reactions following fmoc solid phase peptide synthesis. International Journal of Peptide and Protein Research. 2009;6(3):255–266. doi: 10.1111/j.1399-3011.1990.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Kishi et al. (2018).Kishi RNI, Stach-Machado D, De Lacorte Singulani J, Dos Santos CT, Fusco-Almeida AM, Cilli EM, Freitas-Astua J, Picchi SC, Machado MA. Evaluation of cytotoxicity features of antimicrobial peptides with potential to control bacterial diseases of citrus. PLOS ONE. 2018;13:e0203451. doi: 10.1371/journal.pone.0203451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss & Michl (1962).Kiss G, Michl H. On the venomous skin secretion of the orange-speckled frog bombina variegate. Toxicon. 1962;1:33–39. doi: 10.1016/0041-0101(62)90006-5. [DOI] [Google Scholar]
- Kliger (2010).Kliger Y. Computational approaches to therapeutic peptide discovery. Peptide Scince. 2010;94:701–710. doi: 10.1002/bip.21458. [DOI] [PubMed] [Google Scholar]
- Koehbach & Craik (2019).Koehbach J, Craik DJ. The vast structural diversity of antimicrobial peptides. Trends in Pharmacological Sciences. 2019;40:517–528. doi: 10.1016/j.tips.2019.04.012. [DOI] [PubMed] [Google Scholar]
- Kovaleva et al. (2020).Kovaleva V, Bukhteeva I, Kit OY, Nesmelova IV. Plant defensins from a structural perspective. International Journal of Molecular Sciences. 2020;21:5307. doi: 10.3390/ijms21155307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, Kizhakkedathu & Straus (2018).Kumar P, Kizhakkedathu JN, Straus SK. Antimicrobial peptides: diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules. 2018;8:4. doi: 10.3390/biom8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws, Shaaban & Rahman (2019).Laws M, Shaaban A, Rahman KM. Antibiotic resistance breakers : current approaches and future directions. FEMS Microbiology Reviews. 2019;43:490–516. doi: 10.1093/femsre/fuz014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, Fang & Sekaran (2017).Le CF, Fang CM, Sekaran SD. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrobial Agents and Chemotherapy. 2017;61:e02340–16. doi: 10.1128/AAC.02340-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand et al. (2005).Legrand D, Elass E, Carpentier M, Mazurier J. Lactoferrin: a modulator of immune and inflammatory responses. Cellular and Molecular Life Sciences. 2005;62:2549–2559. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei et al. (2019).Lei J, Sun L, Huang S, Zhu C, Li P, He J, Mackey V, Coy DH, He Q. The antimicrobial peptides and their potential clinical applications. American Journal Translational Research. 2019;11:3919–3931. [PMC free article] [PubMed] [Google Scholar]
- Lemaitre et al. (1996).Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/toll/cactus controls the potent antifungal response in drosophila adult. Cell. 1996;86:973–983. doi: 10.1016/S0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler (2002).Lichtenthaler FW. Emil fischer, his personality, his achievements, and his scientific progeny. Europian Journal of Organic Chemistry. 2002;24:4095–4122. doi: 10.1002/1099-0690(200212)2002:24>4095. [DOI] [Google Scholar]
- Lin & Weibel (2016).Lin TY, Weibel DB. Organization and function of anionic phospholipids in bacteria. Applied Microbiology and Biotechnology. 2016;100:4255–4267. doi: 10.1007/s00253-016-7468-x. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2020).Lu J, Xu H, Xia J, Ma J, Xu J, Li Y, Feng J. D- and unnatural amino acid substituted antimicrobial peptides with improved proteolytic resistance and their proteolytic degradation characteristics. Frontiers in Microbiology. 2020;11:563030. doi: 10.3389/fmicb.2020.563030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macraea et al. (2001).Macraea MB, Shannon KP, Rayner DM, Kaiser AM, Hoffman PN, French GL. A simultaneous outbreak on a neonatal unit of two strains of multiply antibiotic resistant Klebsiella pneumoniae controllable only by ward closure. Journal of Hospital Infection. 2001;49:183–192. doi: 10.1053/jhin.2001.1066. [DOI] [PubMed] [Google Scholar]
- Maher & McClean (2006).Maher S, McClean S. Investigation of the cytotoxicity of eukaryotic and prokaryotic antimicrobial peptides in intestinal epithelial cells in vitro. Biochemical Pharmacology. 2006;71:1289–1298. doi: 10.1016/j.bcp.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Mahlapuu et al. (2016).Mahlapuu M, Håkansson J, Ringstad L, Björn C. Antimicrobial peptides : an emerging category of therapeutic agents. Frontiers in Cellular and Infection Microbiology. 2016;6:194. doi: 10.3389/fcimb.2016.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanovic & Lohner (2016).Malanovic N, Lohner K. Antimicrobial peptides targeting Gram-Positive bacteria. Pharmaceuticals. 2016;9:59. doi: 10.3390/ph9030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick & Hirsch (1947).Mattick ATR, Hirsch A. Further observations on an inhibitory substance (nisin) from lactic streptococci. Lancet. 1947;250:5–8. doi: 10.1016/s0140-6736(47)90004-4. [DOI] [PubMed] [Google Scholar]
- Merrifield (1963).Merrifield RB. Solid phase peptide synthesis I: synthesis of a tetrapeptide. Journal of the American Chemical Society. 1963;85:2149–2154. doi: 10.1021/ja00897a025. [DOI] [Google Scholar]
- Mirski et al. (2018).Mirski T, Niemcewicz M, Bartoszcze M, Gryko R, Michalski A. Utilisation of peptides against microbial infections –a review. Annals of Agricultural and Environmental Medicine. 2018;25:205–210. doi: 10.26444/aaem/74471. [DOI] [PubMed] [Google Scholar]
- Moravej et al. (2018).Moravej H, Moravej Z, Yazdanparast M, Heiat M, Mirhosseini A, Moghaddam MM, Mirnejad R. Antimicrobial peptides: features, action, and their resistance mechanisms in bacteria. Microbial Drug Resistance. 2018;24:747–767. doi: 10.1089/mdr.2017.0392. [DOI] [PubMed] [Google Scholar]
- Morehead & Scarbrough (2018).Morehead MS, Scarbrough C. Emergence of global antibiotic resistance. Primary Care: Clinic in Office Practices. 2018;45:467–484. doi: 10.1016/j.pop.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Nakatsuji & Gallo (2012).Nakatsuji T, Gallo RL. Antimicrobial peptides: old molecules with new ideas. Journal of Investigative Dermatology. 2012;132:887–895. doi: 10.1038/jid.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natalia, Brendan & Sam (2017).Natalia GB, Brendan WW, Sam JW. The importance of glycosylation of antimicrobial peptides: natural and synthetics approaches. Drug Discovery Today. 2017;22:919–926. doi: 10.1016/j.drudis.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Ohtani et al. (1977).Ohtani K, Okada T, Yoshizumi H, Kagamiyama H. Complete primary structures of two subunits of purothionin A, a lethal protein for brewer’s yeast from wheat flour. Journal of Biochemistry. 1977;82:753–767. doi: 10.1093/oxfordjournals.jbchem.a131752. [DOI] [PubMed] [Google Scholar]
- Oliveira et al. (2019).Oliveira JTA, Souza PFN, Vasconcelos IM, Dias LP, Martins TF, Van Tilburg MF, Guedes MIF, Sousa DOB. Mo-CBP3-PepI, Mo-CBP3-PepII, and Mo-CBP3-PepIII are synthetic antimicrobial peptides active against human pathogens by stimulating ROS generation and increasing plasma membrane permeability. Biochimie. 2019;157:10–21. doi: 10.1016/j.biochi.2018.10.016. [DOI] [PubMed] [Google Scholar]
- Ong et al. (2014).Ong ZY, Cheng J, Huang Y, Xu K, Ji Z, Fan W, Yang YY. Effect of stereochemistry, chain length and sequence pattern on antimicrobial properties of short synthetic β-Sheet forming peptide amphiphiles. Biomaterials. 2014;35:1315–1325. doi: 10.1016/j.biomaterials.2013.10.053. [DOI] [PubMed] [Google Scholar]
- Ong, Gao & Yang (2013).Ong ZY, Gao JS, Yang YY. Short synthetic β-sheet forming peptide amphiphiles as broad spectrum antimicrobials with antibiofilm and endotoxin neutralizing capabilities. Advanced Functional Materials. 2013;23:3682–3692. doi: 10.1002/adfm.201202850. [DOI] [Google Scholar]
- Pasupuleti, Schmidtchen & Malmsten (2011).Pasupuleti M, Schmidtchen A, Malmsten M. Antimicrobial peptides: key components of the innate immune system. Critical Reviews in Biotechnology. 2011;32:143–171. doi: 10.3109/07388551.2011.594423. [DOI] [PubMed] [Google Scholar]
- Patch & Barron (2003).Patch JA, Barron AE. Helical peptoid mimics of magainin-2 amide. Journal of the American Chemical Society. 2003;125:12092–12093. doi: 10.1021/ja037320d. [DOI] [PubMed] [Google Scholar]
- Patrulea, Borchard & Jordan (2020).Patrulea V, Borchard G, Jordan O. An update on antimicrobial peptides (AMPs) and their delivery strategies for wound infections. Pharmaceutics. 2020;12(9):840. doi: 10.3390/pharmaceutics12090840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen et al. (2012).Pedersen SL, Tofteng AP, Malik L, Jensen KJ. Microwave heating in solid-phase peptide synthesis. Chemical Society Review. 2012;41:1826–1844. doi: 10.1039/C1CS15214A. [DOI] [PubMed] [Google Scholar]
- Peterson & Kaur (2018).Peterson E, Kaur P. Antibiotic resistance mechanisms in bacteria: relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Frontiers in Microbiology. 2018;9:2928. doi: 10.3389/fmicb.2018.02928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalzgraff, Brandenburg & Weindl (2018).Pfalzgraff A, Brandenburg K, Weindl G. Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Frontiers in Pharmacology. 2018;9:281. doi: 10.3389/fphar.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto, Silva & Franco (2012).Porto WF, Silva ON, Franco OL. Prediction and rational design of antimicrobial peptides. In: Faraggi E, editor. Protein structure. InTech; London: 2012. pp. 377–396. [Google Scholar]
- Ramos-Martín & D’Amelio (2021).Ramos-Martín F, D’Amelio N. Molecular basis of the anticancer and antibacterial properties of cecropinxj peptide: an in silico study. International Journal of Molecular Sciences. 2021;22(2):691. doi: 10.3390/ijms22020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read & Woods (2014).Read AF, Woods RJ. Antibiotic resistance management. Evolution, Medicine and Public Health. 2014;2014(1):147. doi: 10.1093/emph/eou024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren et al. (2003).Rosengren KJ, Clark RJ, Daly NL, Göransson U, Jones A, Craik DJ. Microcin J25 has a threaded sidechain-to-backbone ring structure and not a head-to-tail cyclized backbone. Journal of the American Chemical Society. 2003;125:12464–12474. doi: 10.1021/ja0367703. [DOI] [PubMed] [Google Scholar]
- Sader et al. (2004).Sader HS, Fedler KA, Rennie RP, Stevens S, Jones RN. Omiganan pentahydrochloride (MBI 226), a topical 12-amino-acid cationic peptide: spectrum of antimicrobial activity and measurements of bactericidal activity. Antimicrobial Agents and Chemotherapy. 2004;48:3112–3118. doi: 10.1128/AAC.48.8.3112-3118.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnölzer et al. (2009).Schnölzer M, Alewood P, Jones A, Alewood D, Kent SBH. In situ neutralization in boc-chemistry solid phase peptide synthesis. International Journal of Peptide and Protein Research. 2009;40:180–193. doi: 10.1111/j.1399-3011.1992.tb00291.x. [DOI] [PubMed] [Google Scholar]
- Selsted, Szklarek & Lehrer (1984).Selsted ME, Szklarek D, Lehrer RI. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infection and Immunity. 1984;45:150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted et al. (1993).Selsted ME, Tang YQ, Morris WL, McGuire PA, Novotny MJ, Smith W, Henschen AH, Cullor JS. Purification, primary structures and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. Journal of Biological Chemistry. 1993;268:6641–6648. doi: 10.1016/S0021-9258(18)53298-1. [DOI] [PubMed] [Google Scholar]
- Sengupta, Chattopadhyay & Grossart (2013).Sengupta S, Chattopadhyay MK, Grossart H. The multifaceted roles of antibiotics and antibiotic resistance in nature. Frontiers in Microbiology. 2013;4:47. doi: 10.3389/fmicb.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg & Gilbert (2014).Spellberg B, Gilbert DN. The future of antibiotics and resistance: a tribute to a career of leadership by John Bartlett. Clinical Infectious Diseases. 2014;59:71–75. doi: 10.1093/cid/ciu392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subczynski et al. (2018).Subczynski WK, Pasenkiewicz-Gierula M, Widomska J, Mainali L, Raguz M. High cholesterol/low cholesterol: effects in biological membranes review. Cell Biochemistry Biophysics. 2018;75:369–385. doi: 10.1007/s12013-017-0792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran et al. (2002).Tran D, Tran PA, Tang YQ, Yuan J, Cole T, Selsted ME. Homodimeric θ-defensins from Rhesus macaque Leukocytes, Isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. Journal of Biological Chemistry. 2002;277:3079–3084. doi: 10.1074/jbc.M109117200. [DOI] [PubMed] [Google Scholar]
- Trotti et al. (2004).Trotti A, Garden A, Wardey P, Symonds P, Langer C, Redman R, Pajak TF, Fleming TR, Henke M, Bourhis J, Rosenthal DI, Junor E, Cmelak A, Sheehan F, Pulliam J, Devitt-Risse P, Fuchs H, Chambers M, O’Sullivan, Ang KK. A multinational, randomized phase iii trial of iseganan hcl oral solution for reducing the severity of oral mucositis in patients receiving radiotherapy for head-and-neck malignancy. International Journal of Radiation Oncology Biology Physics. 2004;58:674–681. doi: 10.1016/S0360-3016(03)01627-4. [DOI] [PubMed] [Google Scholar]
- Tymoszewska et al. (2017).Tymoszewska A, Diep DB, Wirtek P, Aleksandrzak-Piekarczyk T. The non-lantibiotic bacteriocin garvicin q targets man-pts in a broad spectrum of sensitive bacterial genera. Scientific Reports. 2017;7:8359. doi: 10.1038/s41598-017-09102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps (2006).Van Epps HL. René Dubos: unearthing antibiotics. Journal of Experimental Medicine. 2006;203:259. doi: 10.1084/jem.2032fta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola (2015).Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. Pharmacology & Therapeutics. 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- Wimley (2019).Wimley WC. Application of synthetic molecular evolution to the discovery of antimicrobial peptides. Advance in Experimental Medicine and Biology. 2019;1117:241–255. doi: 10.1007/978-981-13-3588-4_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2020).World Health Organization (WHO) Antibiotic resistance. 2020. https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance
- Wright (2014).Wright GD. Something old, something new : revisiting natural products in antibiotic drug discovery 1. Canadian Journal of Microbiology. 2014;60:147–154. doi: 10.1139/cjm-2014-0063. [DOI] [PubMed] [Google Scholar]
- Zasloff (1987).Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin : isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proceeding National Academy Sciences of the United States of America. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao et al. (2016).Zhao Y, Zhang M, Qiu S, Wang J, Peng J, Zhao P, Zhu R, Wang H, Li Y, Wang K, Yan W, Wang R. Antimicrobial activity and stability of the D-amino acid substituted derivatives of antimicrobial peptide polybia-MPI. AMB Express. 2016;6:122. doi: 10.1186/s13568-016-0295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zharkova et al. (2019).Zharkova MS, Orlov DS, Golubeva OY, Chakchir OB, Eliseev IE, Grinchuk TM, Shamova OV. Application of antimicrobial peptides of the innate immune system in combination with conventional antibiotics—a novel way to combat antibiotic resistance? Frontiers in Cellular Infection Microbiology. 2019;9:128. doi: 10.3389/fcimb.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
This is a literature review study.