Abstract
The gut microbiota is a quickly developing bacterial ecosystem with biodiversity. It is an adaptive immunity that varies with food intake, environmental conditions, and human habits, among other factors. Various external stimuli, such as drugs, can influence the gut microbial environment and lead to gut dysbiosis. Recently, gut dysbiosis has been identified as an important factor that leads to several diseases either by the released metabolites or by the gut neuronal connection. In brain disorders, gut dysbiosis is involved in neuropsychiatric manifestations, including autism spectrum disorder, anxiety, and depression by interfering with neurotransmitter homeostasis, and neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease by releasing abnormal metabolites from the gut. Gut dysbiosis has been documented in gut disorders, including inflammatory bowel disease or irritable bowel syndrome. Immune cells in the gut are modulated by external factors such as stress, diet, and drugs to produce inflammatory cytokines, including interleukins (IL-4, IL-6, IL-17, IL-23, etc.). Inflammatory cytokines lead to a cascade of events, which lead to various ailments in the bowel. Beneficial bacteria in the form of probiotics ameliorate the condition and have healthful effects in disease conditions. This warrants further research to identify newer therapeutic strategies for diseases that cannot be cured or are difficult to treat.
Keywords: gut-brain axis, neurodegenerative diseases, inflammatory bowel disease, gut dysbiosis and diseases, gut microbiota
Introduction and background
The human body is inhabited by bacteria, archaea, viruses, and eukaryotic microbes. The organization of microorganisms harboring the gastrointestinal (GI) tract (GIT) is collectively called the “gut microbiome.” The gut microbiome of an individual is specific to that individual, often known as “the microbial signature” [1]. The gut microbiota is diverse, even in healthy individuals [2]. The symbiotic association between microbes and the human body keeps the body healthy and protects against diseases [3,4]. The development of this gut microbiome has been thought to originate only after birth, but recent evidence suggests that the development starts in the fetal stage itself [5,6]. The GIT appears to be involved in many diseases, such as depression, Alzheimer’s disease, and Parkinson’s disease, and a change in the gut microbiome can be noted in various diseases [7-9]. The gut-brain axis (GBA) and its role in feeding and satiety are known facts. Evidence suggests that the neurotransmitters involved in the GBA can be manipulated by the gut microbiome [10,11]. The neurotransmitters, along with the gut microbiome, assist in monitoring and amalgamating gut functions along with emotional and cognitive centers of the brain. They also play a major role in immune system activation, intestinal permeability and motility, and enteroendocrine signaling [10]. The complex dialog between the GBA is mediated by neural, metabolic, endocrine, and immune responses to diverse environmental cues, including diet and components of the intestinal microbiota [12]. Hence, the gut microbiome plays a role in metabolic functions, protection against pathogens, and immune functions; thus, it contributes to normal physiology [2,13,14]. The derangement in the gut microbiome and its harmonical relations with normal physiology is collectively termed “gut dysbiosis.” The concept of gut dysbiosis [15] and its role in diseases/ailments of the brain and intestine are reviewed in this article. This review also aims to explore future research perspectives of the gut microbiome in the management of these diseases.
Review
Gut microbiota
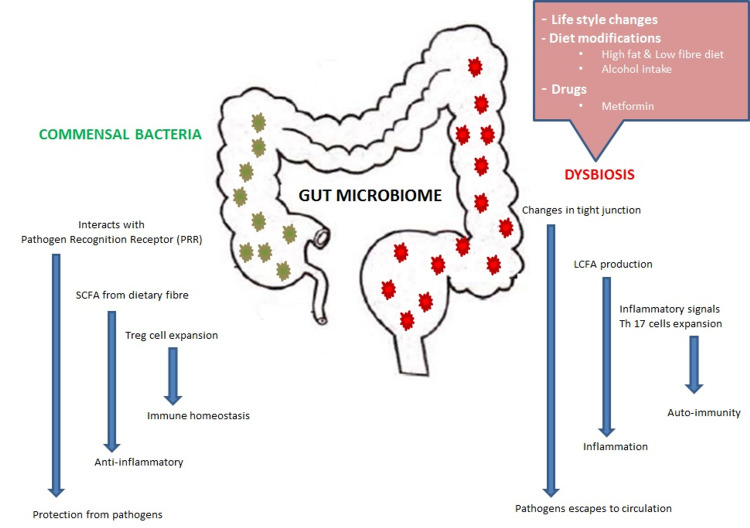
Gut microbial ecology is diverse and complex. The gut microbial system comprises dynamic microbes ranging from beneficial microbiota to opportunistic pathogens. Commensal bacteria colonize the intestine immediately after birth. As humans mature, more bacteria colonize the intestine. On average, a healthy adult GIT harbors approximately 1,000 bacterial species. Firmicutes, Bacteroidaceae, Lachnospiraceae, Actinobacteria, Prevotellaceae, and Ruminococcaceae are the dominant group of bacterial species [7]. Gut microbial species are important for the maintenance of normal human health. For example, they help in the digestion of macromolecules, synthesis of vitamins, metabolism of bile acids, etc. However, the composition of bacterial species varies with lifestyle changes, diet modifications, and medication use [12]. Dysbiosis occurs when there is a functional or compositional imbalance in the gut microbiome. Figure 1 depicts gut microbial ecology and disturbances.
Figure 1. Gut microbial ecology and disturbances.
LCFA: long-chain fatty acid; SCFA: short-chain fatty acid; Th17: helper T cell 17; Treg: regulatory T cell
Physical Inactivity
In obesity, the number of Firmicutes increases compared to abundant Bacteroides. In general, Firmicutes extract more energy from dietary components and lead to more adiposity. This finding was confirmed by Ley et al. who analyzed stool samples from twelve obese and five lean individuals. They also performed genetic sequencing to determine different bacterial strains. Obese individuals had more Firmicutes and fewer Bacteroidetes than lean individuals. Further, when obese volunteers followed a weight-loss diet for months, there was a significant increase in Bacteroides, but not equal to lean individuals [16]. In another study, there was a 20% reduction in Bacteroides in individuals with high-calorie intake, which was directly related to weight gain [17].
Diet Modifications
Recent data from a human study revealed that dietary factors directly influence the composition of the gut microbiota. Particular importance is given to the westernization of diet, which is high in animal fat and proteins and lacks dietary fibers. Saturated fat and animal-derived proteins increase cholesterol levels, increase Firmicutes and Enterobacteriaceae, and reduce Bacteroidetes, such as in an obese gut microbiome environment, which increases the propensity for inflammation, thrombosis, etc. A vegetable-based diet is accompanied by an increase in Prevotella and Firmicutes, which are involved in the degradation of dietary fibers, as well as by an increase in anti-inflammatory and antianxiety activities [18]. Fructose feeding results in a significant reduction in Bacteroidetes and an increase in pathogenic Helicobacteraceae colonies. Alcohol consumption results in increased Bacteroidetes and Firmicutes and decreased Faecalibacterium [19]. This finding correlated well with several other studies. Moreover, alcohol intake increases intestinal permeability through which intestinal metabolites and bacteria enter the systemic circulation, causing organ damage and inflammation.
Dietary intake of herbal products, such as Rhizoma Coptidis, modulates the gut microbiota and reduces cholesterol levels and inflammation. A high-fiber diet increases Prevotella, Bifidobacterium, and Eubacterium spp., which degrade dietary fiber to produce short-chain fatty acids (SCFAs) which are anti-inflammatory. This was confirmed by another study, which showed that SCFA-propionic acid protects the mouse model from autoimmune colitis and increases neuronal plasticity. In the gut, fermented foods, yogurt, and natural marine products increase health-friendly bacteria, such as Lactobacillus and Bifidobacterium [20,21].
Drugs
With the increased usage of drugs, the gut microbiome is impacted by the direct effects of drugs. Different drugs have variable implications on microbial flora and thus play a role in dysbiosis. Nonsteroidal anti‐inflammatory drugs (NSAIDs), antipsychotics, and antidiabetic drugs change the microbial flora [22]. For example, Wang et al. demonstrated increased Proteobacteria and Bacteroides and decreased Firmicutes and Lactobacillus after usage of NSAIDs [23]. This finding was confirmed in a human study by Rogers et al. who found that Prevotella spp. and Bacteroides spp. were abundant in the stools of 155 adults who had taken NSAIDs compared to individuals not taking NSAIDs [24]. Recent animal and human studies have shown that the administration of metformin alters the intestinal microbiome environment. Metformin intake increased Escherichia spp. and Lactobacillus and decreased Intestinibacter [25].
Gut dysbiosis in diseases of the central nervous system
Although gut microbiome ecology and changes have been studied in the past decades, specific changes in the microbiome have been recently implicated in certain diseases. This review attempts to explore the possible mechanism by which changes in the gut microbiota lead to diseases of the central nervous system (CNS).
Gut-Brain Axis
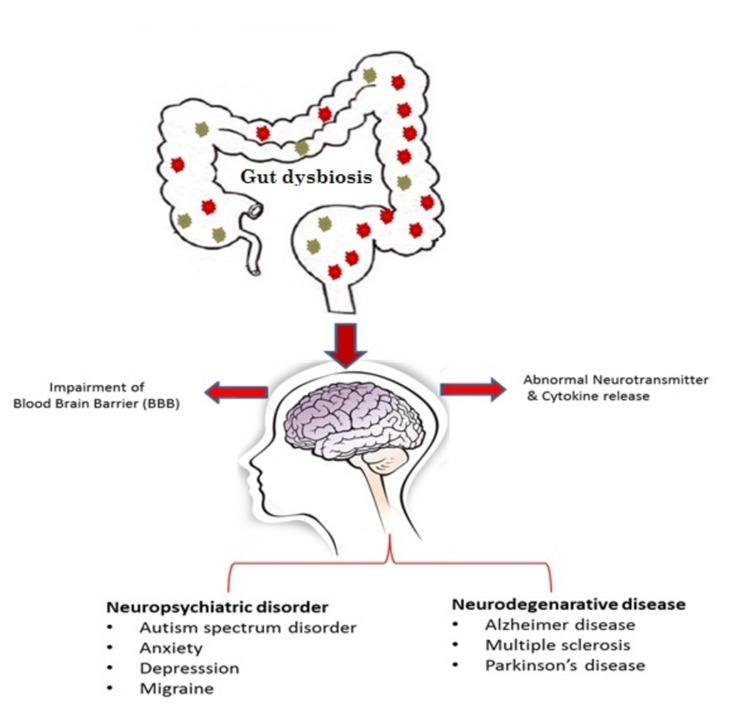
There are no definitive treatments for most neurological diseases. Numerous studies have been performed to understand brain physiology and molecular mechanisms in diseases. Several factors have been found to play a role, ranging from environmental factors to genetic makeup. Most of these factors interface with the changes in the gut microbiome. Gut dysbiosis releases metabolites and cytokines that trigger inflammation, affect the blood-brain barrier (BBB) and brain volume, and can act as false neurotransmitters, resulting in altered brain physiology and neuronal function in neurological disorders, which may be a demyelinating or neuropsychiatric illness. Figure 2 depicts gut dysbiosis in disorders of the brain.
Figure 2. Gut dysbiosis in disorders of the brain.
Neurodegenerative diseases
Parkinson’s Disease
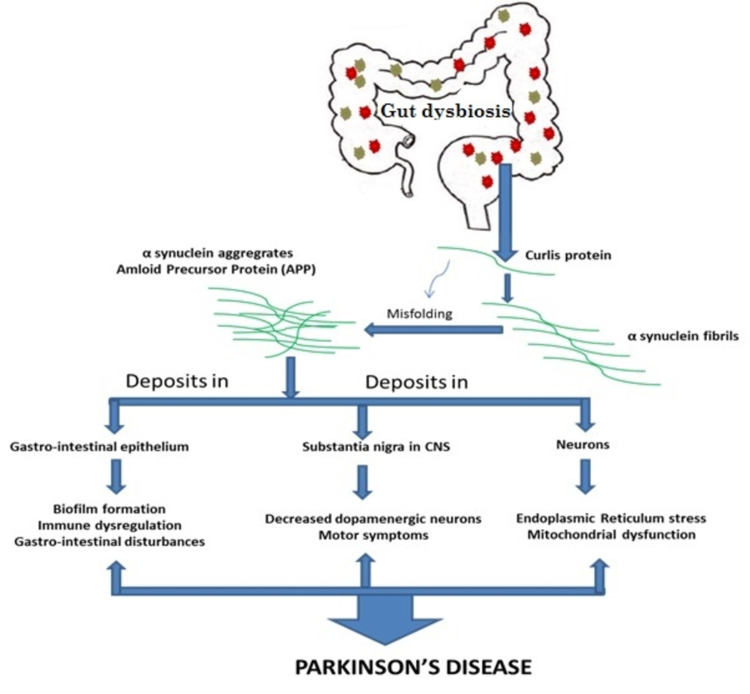
Parkinson’s disease is characterized by muscle weakness and gait abnormalities. Scientists initially thought that it was related to the brain. Patients with Parkinson’s disease often complain of GI symptoms, such as constipation and inflammatory bowel disease (IBD)-like symptoms, which led scientists to explore the GI system in Parkinson’s disease [26,27]. Baark et al. found synuclein deposits in the brain and GI nervous tissue. This confirmed the suspicion, and scientists started to inspect the GI system. They found a bacterial strain in the gut (Escherichia coli) that produces a protein termed curli. Curli proteins coalesce to form synuclein proteins. They also cause synuclein and other proteins to misfold. Synuclein produced from cells acts as a monomer [28]. Multiple monomers join to form a fibril. Fibrils with misfolded protein aggregate to form amyloid fibrils that deposit in the neurons to cause neurodegeneration [29]. Synuclein also deposits in dopaminergic neurons in the brain, resulting in motor symptoms. Curli protein also forms a biofilm in the gastroepithelial tissue, which can prevent human immune defense, resulting in constipation and symptoms of IBD [30]. Figure 3 depicts the effect of gut dysbiosis in Parkinson’s disease.
Figure 3. Role of gut dysbiosis in Parkinson’s disease.
CNS: central nervous system
Alzheimer’s Disease
Alzheimer’s disease is characterized by progressive dementia and cognitive and motor function impairment. The underlying etiology of the disease is debatable. Several theories, such as genetics, neuroinflammation, vascular degeneration, and calcium and energy balance disorder, have been proposed. However, therapies targeting these theories have not been fruitful. Therefore, there is an urgent need for new therapies to prevent and treat this devastating neurological condition [31].
A change in the gut microbiome is one of the crucial underlying factors for developing Alzheimer’s disease [8]. Brandshield et al. reported increased Firmicutes and decreased Bacteroidetes in a mouse model of Alzheimer’s disease [32]. This finding was confirmed by Vogt et al. who showed increased Firmicutes and Bacteroidetes and decreased Bifidobacterium [31]. In another human study of Alzheimer’s disease, Cattaneo et al. showed an increase in the number of Escherichia/Shigella and a decrease in Eubacterium rectale and Bacteroides fragilis [33]. In summary, in Alzheimer’s disease, there is a definite alteration in the gut microbiome, leading to gut dysbiosis. A decrease in anti-inflammatory bacterial species, such as Bifidobacterium, and an increase in proinflammatory species, such as Bacteroidetes and Firmicutes, were seen. This inflammatory microbiome releases cytokines and inflammatory markers into circulation. This disrupts the BBB and leads to an inflammatory state in the CNS, as seen in Alzheimer’s disease.
The hallmark pathogenic feature of Alzheimer’s disease is the accumulation of amyloid-β (Aβ) and tau protein aggregates. The gut microbiome produces various metabolites on food fermentation. One metabolite is SCFA, which can cross the BBB and interfere with Aβ and tau proteins, causing aggregates [34]. This was confirmed in both in-vivo and in-vitro studies. In a large meta-analysis by Xu and Wang, SCFA increased inflammation in the brain and caused aggregation of Aβ and tau proteins [35]. In an in-vivo study by Ho et al., the gut microbiome produced metabolites, such as isovaleric acid, propionic acid, and butyric acid, which caused Aβ polymers in a dose-dependent manner and Aβ aggregates. However, valeric acid administration inhibited Aβ polymer formation [36].
In addition, the gut microbiome could produce neurotransmitters, such as dopamine, acetylcholine, noradrenaline (Escherichia spp. and Bacillus spp.), serotonin, histamine (Enterococcus spp.), and gamma-aminobutyric acid (GABA; Bifidobacterium spp.). These neurotransmitters affect the host’s well-being and maintain homeostasis [11]. Gut dysbiosis causes altered neurotransmitter release, and some can act as false neurotransmitters in the brain, leading to behavioral changes, mood swings, sleep deprivation, depression, and increased anxiety, as seen in Alzheimer’s disease [37].
Neuropsychiatric diseases
Autism Spectrum Disorder
Autism spectrum disorder (ASD) is a developmental disorder characterized by anxiety, depression, forgetfulness, decreased memory, abnormal social behavior, and stress. Complex mixtures of events urge patients to distance themselves from society and decrease their communication behavior. Patients’ cognitive behavior, mood, and memory changes were initially thought to be due to a developmental disorder that affects the brain. However, recent studies have suggested that the gut microbiota can influence mood and behavioral changes starting from infancy to adulthood [38]. The microbiome starts colonizing the gut immediately after birth and starts connecting to the brain in the developmental process. Any inflammation or hindrance during the developmental process results in defective cognition, mood, memory, and abnormal behaviors [39].
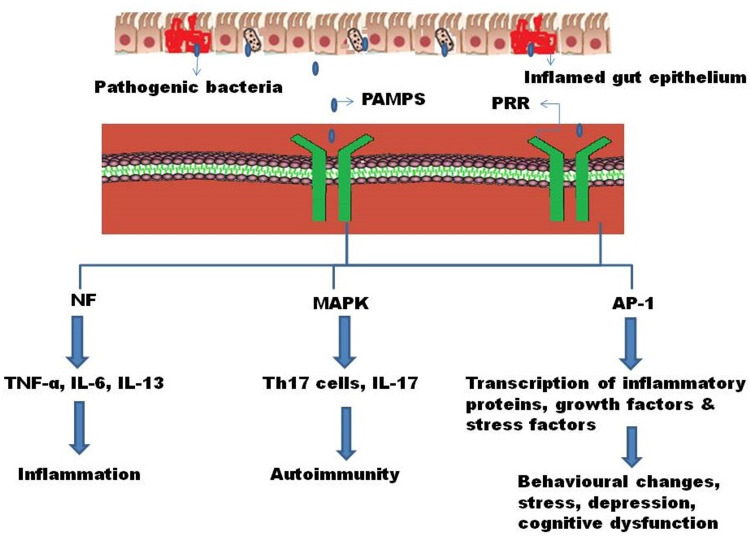
Studies on mice have suggested that an infection during pregnancy results in a cascade of events. Segmented filamentous bacteria from the mother’s gut can induce an inflammatory event that further stimulates inflammatory helper T cells (Th17) cells, which can travel from the mother’s gut to the fetal brain and cause autism-like behavior by interfering with the neurotransmitter function [40]. Human epidemiological studies have suggested that maternal inflammation could be a reason for ASD. This was confirmed by studies of a mouse model, where pregnant and nonpregnant mice were inoculated with segmented filamentous bacteria or human commensal bacteria that can induce Th17 immune cells or cause maternal inflammation. Pregnant mice secreted interleukin (IL)-1, IL-23, and IL-6, which are stimuli for the induction of Th17 cells to produce IL-17; however, this was not seen in nonpregnant mice [41]. Therefore, the maternal gut microbiome could induce inflammatory cytokines that interfere with the neurodevelopmental process and can lead to neurodevelopmental disorders. Gut dysbiosis, infection during pregnancy, or any health issues in early childhood during development can activate deleterious signal transduction pathways. Pathogen-associated molecular proteins (PAMPs), highly specific specialized proteins present in bacterial lipopolysaccharides, lipoteichoic acid, and peptidoglycan layers of pathogens recognized by the pattern recognition receptor (PRR), are present on gut epithelial cells. Moreover, host immune cells produce inflammatory reactions. PAMP association with PRR results in the activation of downstream signal transduction pathways, such as nuclear factor-κB (NF-κB), mitogen-activated protein kinase (MAPK), and activator protein-1 (AP-1). NF-κB activation results in the production of downstream inflammatory mediators, such as tumor necrosis factor-alpha (TNF-α) and ILs (IL-1, IL-6, and IL-13), and can stimulate the AP-1 transduction pathway. Activation of the AP-1 signal transduction pathway results in the transduction of growth factors, inflammatory proteins, and stress factors. Activation of the MAPK signal transduction pathway results in the activation and release of various inflammatory cytokines, notably IL-17 [42,43].
De Angelis et al. demonstrated that anti-inflammatory Faecalibacterium decreased in ASD, whereas increased Enterobacteriaceae and Sutterellaceae were noted [39]. In another study by Hsiao et al., B. fragilis on oral administration improved intestinal integrity, modulated metabolites from the intestine, and improved the symptoms of ASD. Hence, this led to a hypothesis that the gut microbiota can ameliorate the symptoms of ASD. Hence, Hsiao et al. provided oral supplementation of B. fragilis to a mouse model and demonstrated improved gut permeability and reduced behavioral changes, as seen in ASD [44]. Certain species, such as Lactobacillus and Bifidobacterium, and ingestion of probiotics have shown beneficial effects, including improved gut permeability, reduced GI symptoms, and improved cognition and behavior [45]. Figure 4 depicts the effect of gut dysbiosis in ASD.
Figure 4. Effect of gut dysbiosis on ASD.
PAMPS: pathogen-associated molecular proteins; PRR: pattern recognition receptor; NF: necrosis factor; MAPK: mitogen-activated protein kinase; AP-1: activator protein-1; TNF: tumor necrosis factor; IL: interleukin; Th17 cell: helper T cell 17; ASD: autism spectrum disorder
Headache and Migraine
The human gut microbiome is not only associated with neurodegenerative diseases, such as Parkinson’s disease and Alzheimer’s disease, but also associated with neuropsychiatric illnesses, such as depression and autism disorders. Recently, poor gut health has been linked with migraine attacks [46]. The human gut microbiome is constantly connected to the brain either indirectly via secretion of various neurotransmitters (such as serotonin, dopamine, and noradrenaline), various proinflammatory cytokines (such as IL-1, IL-6, and IL-23), and fatty acids or directly via neural brain connection through the vagus nerve. Any disturbance in gut ecology can affect brain homeostasis and mental health [47].
The direct pathway is rare and can be treated surgically, whereas it is difficult to find a causal relationship to the indirect pathway via neurotransmitters, hormones, food fermentation products/metabolites, inflammatory cytokines, etc.; hence, it is difficult to treat. However, all these factors tend to converge at the gut microbiome [48]. Gut dysbiosis provides an inflammatory environment, leads to an imbalance in neurotransmitter release, and causes metabolites to be released into circulation. For decades, certain foods, such as chocolate and wine, have been known to predispose to migraine attacks, but the gut microbiome composition that ferments these foods is unknown. Recently, several studies have shown their microbial composition and relationship with migraine attacks. In a double-blind, randomized controlled trial by Martami et al., in 40 chronic migraine patients, 14 different probiotic strains were given to one group and a placebo was given to another group. They reported a significant reduction in migraine frequency and severity and concluded that probiotic supplementation could effectively prevent migraine attacks [49]. In a large study, Gonazalez et al. analyzed fecal samples from 1,996 participants with or without migraines. They found that individuals suffering from migraines tend to have more nitrate-producing bacteria than those who do not suffer from migraine attacks [50,51].
Proinflammatory cytokines released by the dysbiosis gut cause inflammation and disrupt the integrity of the gut epithelium. Increased glutamate excitatory neurotransmitters released from the dysbiosis gut may enter the circulation and stimulate nociceptive pain receptors in the CNS and trigeminovascular system that predisposes to migraine attacks [52,53]. This has been demonstrated in several studies. Patients with migraines tend to have higher glutamate and GABA levels. Studies have shown that the dysbiosis gut with predominant E. coli and Enterococcus faecalis releases neuropeptide Y, substance P, and calcitonin gene-related peptide (CGRP), which can stimulate and induce nociceptive receptors predisposed to migraine attacks [54]. CGRP administration in a mouse model inhibited gastric acid secretion and could precipitate migraine attacks. Probiotic administration significantly reduced migraine attacks by reducing inflammatory cytokines and neurotransmitter modulation [55]. In a recent study, a significant decrease in migraine attacks was seen with probiotic usage compared to placebo. Studies also found deficient/insufficient vitamin D levels in people with migraine compared to control, showing that vitamin D supplementation in the dysbiosis gut significantly reduces harmful inflammatory bacteria, such as Helicobacter spp. [56].
Gut dysbiosis in diseases of the bowel
Dysbiosis is common in IBD and is characterized by reduced bacterial diversity with a reduction in Bacteroides and an increase in E. coli. Faecalibacterium prausnitzii and Roseburia hominis are reduced in patients with IBD [57-59]. Alteration of the intestinal microflora is associated with abnormal gut immune responses in genetically susceptible individuals. The predisposing genes of IBD are also related to the recognition of pathogenic microbes [60]. The role of probiotics, antibiotics, and microbial implantation in suppressing inflammation in IBD, especially ulcerative colitis, also supports the role of microbiota in IBD [61].
Inflammatory Bowel Disease
IBD results in inflammatory reactions in the brain, activation of behavioral control areas and the hypothalamic-pituitary-adrenal (HPA) axis, and alteration of the BBB. Proinflammatory cytokines of IBD directly affect the brain via BBB or the vagus nerve, altering neuronal plasticity, microglial activation, and dysregulation of the HPA axis, leading to structural and functional changes in the brain [62]. The dysregulated HPA axis can lead to excess cortisol levels, causing depression in IBD patients [63]. Several animal studies demonstrated increased inflammatory cytokine levels in the hippocampal, cerebral, and hypothalamic regions. There is a correlation between CNS imbalance and psychological behaviors in IBD. It also affects the volume of gray matter and the size of the brain. Agostini et al. reported that patients with Crohn’s disease had decreased gray matter volume in the frontal cortex and anterior cingulated gyrus [64].
Stress has deteriorating effects on IBD by mast cell activation, decreasing the anti-inflammatory pathway and increasing the sympathetic tone. Stress also increases intestinal permeability, leading to superinfection and altered neuronal activity in stress-sensitive areas of the brain [65]. Stress also increases inflammation of the gut by reducing the synthesis and metabolism of SCFAs. Behavioral disorders alter cell signaling, which causes oxidative stress and hypoxia, modifying the microbiological environment of the gut [66]. Psychological symptoms, such as depression and anxiety, are not only common in patients with IBD but they also influence the course of the disease [67].
Irritable Bowel Syndrome
IBS is a common functional disorder characterized by recurrent abdominal pain relieved by defecation. It is common in females, with a prevalence ranging from 10% to 15% [68]. Rome-IV criteria are used to diagnose IBS [69]. IBS is classified into four types as IBS-D (diarrhea-predominant), IBS-C (constipation predominant), IBS-M (mixed pattern), and IBS-U (unclassified). IBS is also associated with other functional disorders such as dyspepsia, chronic pelvic pain, and chronic fatigue syndrome. Psychiatric illnesses, such as anxiety and depression, are highly linked with IBS. Although the exact pathogenesis of IBS is poorly understood, genetic predisposition, food intolerance, visceral hypersensitivity, altered GBA, and dysbiosis may play a role [70].
Normally, an intact epithelial barrier enables the microbes to colonize the intestine and perform symbiosis. This is altered by inflammation, infection, and immune dysregulation, leading to the alteration of the gut microbiome predisposing to IBS by altering the gut immunity and modulating the GBA and gut neuromuscular junction [71]. Lactobacillus, Bifidobacterium, and Methanobacteriales are decreased and Bacteroides and E. coli are increased in IBS [72]. Fungal dysbiosis also indicates visceral hypersensitivity of IBS. Chronic low-grade inflammation along with impaired bowel motility also play a role in IBS. This may be due to infection, dysbiosis, and stress. An increased number of immune cells, such as mast cells and lymphocytes, and increased cytokine production are seen in intestinal biopsies of IBS patients [73]. Proinflammatory cytokines, such as IL-6, TNF-α, and IL-1β, are increased in IBS patients. Additionally, these cytokines are associated with depression and anxiety, suggesting the role of GBA [74]. There is also an association between IL-17 and TNF-α with disease symptoms and quality of life among the different IBS types. Patients with IBS often have altered bowel motility influenced by stress via the GBA. This is due to alterations in serotonin metabolism [75]. Diet plays a major role in pathogenesis by altering gut motility, dysbiosis, GBA, and neuroendocrine actions. Visceral hypersensitivity is commonly caused by gut dysbiosis, alteration in the GBA, diet, psychological factors, and genetic predisposition. Hence, targeting GBA and local neural pathways will be beneficial in the management of visceral hypersensitivity. Anxiety and depression are common comorbidities in IBS patients, suggesting that the brain drives these conditions in patients with IBS [76]. Further, stress aggravates the symptoms of IBS, suggesting the role of brain-derived gut pathogenesis. Nevertheless, GI symptoms appear first in IBS patients, followed by anxiety and depression, suggesting the role of GBA [77]. Management of IBS by probiotics, prebiotics, symbiotics, and antibiotics also suggests the major role of dysbiosis in the pathogenesis of IBS [78]. Fecal microbial transplantation (FMT) plays a major role in the treatment of IBS. Several studies have reported favorable outcomes with FMT in IBS. However, few studies were not in favor of FMT [79-81]. Further studies are needed to assess the role of FMT in the management of IBS.
Conclusions
The gut microbiome is an integral part of the human health system, comprising dynamic microbes ranging from beneficial microbiota to opportunistic pathogens, and plays an important role in maintaining the health of human beings. Each individual has a unique microbiota that is very diverse and complex in nature. The microbiome starts colonizing the gut immediately after birth and starts connecting to the brain during the developmental process. Any disequilibrium between the gut microbiome and its harmonical relations with normal physiology leads to gut dysbiosis. Factors such as diet, physical inactivity, and drugs affect the gut microbiome.
Gut dysbiosis results in altered neurotransmitter release and affects the GBA, leading to behavioral changes, mood swings, sleep deprivation, depression, and increased anxiety, as seen in Alzheimer’s disease. Moreover, it leads to the deposition of synuclein proteins, leading to neurodegeneration. The study of the changes in the gut microbiome can help in the early diagnosis of Parkinson’s disease and Alzheimer’s disease, and the prevention of gut dysbiosis plays an important role in the treatment of these neurodegenerative diseases. Current evidence suggests that gut dysbiosis is associated with autism, headache, migraine, IBD, and IBS. Physicians need to focus on gut dysbiosis while treating gut and brain disorders and insist upon a diet that helps maintain the gut microbiome. Lastly, the successful treatment of many neurodegenerative, neuropsychiatric, and gut disorders lies in and around the equilibrium of the gut microbiome.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Introduction to the human gut microbiota. Thursby E, Juge N. Biochem J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Role of the gut microbiota in nutrition and health. Valdes AM, Walter J, Segal E, Spector TD. BMJ. 2018;361:0. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Interaction of the microbiota with the human body in health and diseases. Altveş S, Yildiz HK, Vural HC. Biosci Microbiota Food Health. 2020;39:23–32. doi: 10.12938/bmfh.19-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The human microbiome: from symbiosis to pathogenesis. Eloe-Fadrosh EA, Rasko DA. Annu Rev Med. 2013;64:145–163. doi: 10.1146/annurev-med-010312-133513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The not-so-sterile womb: evidence that the human fetus is exposed to bacteria prior to birth. Stinson LF, Boyce MC, Payne MS, Keelan JA. Front Microbiol. 2019;10:1124. doi: 10.3389/fmicb.2019.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The prenatal gut microbiome: are we colonized with bacteria in utero? Walker RW, Clemente JC, Peter I, Loos RJ. Pediatr Obes. 2017;12 Suppl 1:3–17. doi: 10.1111/ijpo.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The human gut microbiome - a potential controller of wellness and disease. Kho ZY, Lal SK. Front Microbiol. 2018;9:1835. doi: 10.3389/fmicb.2018.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gut microbiota in Alzheimer's disease, depression, and type 2 diabetes mellitus: the role of oxidative stress. Luca M, Di Mauro M, Di Mauro M, Luca A. Oxid Med Cell Longev. 2019;2019:4730539. doi: 10.1155/2019/4730539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The role of the gut microbiota in the pathogenesis of Parkinson's disease. Yang D, Zhao D, Ali Shah SZ, et al. Front Neurol. 2019;10:1155. doi: 10.3389/fneur.2019.01155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuropsychiatric disorders: influence of gut microbe to brain signalling. Scriven M, Dinan TG, Cryan JF, Wall M. Diseases. 2018;6:78. doi: 10.3390/diseases6030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neurotransmitter modulation by the gut microbiota. Strandwitz P. Brain Res. 2018;1693:128–133. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gut microbiota, the immune system, and diet influence the neonatal gut-brain axis. Sherman MP, Zaghouani H, Niklas V. Pediatr Res. 2015;77:127–135. doi: 10.1038/pr.2014.161. [DOI] [PubMed] [Google Scholar]
- 13.Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Lazar V, Ditu LM, Pircalabioru GG, et al. Front Immunol. 2018;9:1830. doi: 10.3389/fimmu.2018.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Interaction between microbiota and immunity in health and disease. Zheng D, Liwinski T, Elinav E. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Part 1: the human gut microbiome in health and disease. Bull MJ, Plummer NT. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4566439/ Integr Med (Encinitas) 2014;13:17–22. [PMC free article] [PubMed] [Google Scholar]
- 16.Obesity alters gut microbial ecology. Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J. Am J Clin Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The effects of vegetarian and vegan diets on gut microbiota. Tomova A, Bukovsky I, Rembert E, Yonas W, Alwarith J, Barnard ND, Kahleova H. Front Nutr. 2019;6:47. doi: 10.3389/fnut.2019.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Impact of drinking alcohol on gut microbiota: recent perspectives on ethanol and alcoholic beverage. Lee E, Lee JE. Curr Opin Food Sci. 2021;37:91–97. [Google Scholar]
- 20.Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Parada Venegas D, De la Fuente MK, Landskron G, et al. Front Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The role of short-chain fatty acids from gut microbiota in gut-brain communication. Silva YP, Bernardi A, Frozza RL. Front Endocrinol (Lausanne) 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pathophysiology of NSAID-associated intestinal lesions in the rat: luminal bacteria and mucosal inflammation as targets for prevention. Colucci R, Pellegrini C, Fornai M, et al. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6281992/ Front Pharmacol. 2018;9:1340. doi: 10.3389/fphar.2018.01340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gut microbiota in NSAID enteropathy: new insights from inside. Wang X, Tang Q, Hou H, et al. Front Cell Infect Microbiol. 2021;11:679396. doi: 10.3389/fcimb.2021.679396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Rogers MA, Aronoff DM. Clin Microbiol Infect. 2016;22:178–179. doi: 10.1016/j.cmi.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metformin: old friend, new ways of action-implication of the gut microbiome? Rodriguez J, Hiel S, Delzenne NM. Curr Opin Clin Nutr Metab Care. 2018;21:294–301. doi: 10.1097/MCO.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 26.Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Chen SG, Stribinskis V, Rane MJ, et al. Sci Rep. 2016;6:34477. doi: 10.1038/srep34477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. Sampson TR, Challis C, Jain N, et al. https://elifesciences.org/articles/53111. Elife. 2020;9:0. doi: 10.7554/eLife.53111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Braak H, de Vos RA, Bohl J, Del Tredici K. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Holmqvist S, Chutna O, Bousset L, et al. Acta Neuropathol. 2014;128:805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- 30.Are amyloid diseases caused by protein aggregates that mimic bacterial pore-forming toxins? Lashuel HA, Lansbury PT Jr. Q Rev Biophys. 2006;39:167–201. doi: 10.1017/S0033583506004422. [DOI] [PubMed] [Google Scholar]
- 31.Gut microbiome alterations in Alzheimer's disease. Vogt NM, Kerby RL, Dill-McFarland KA, et al. Sci Rep. 2017;7:13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altered gut microbiome composition and tryptic activity of the 5xFAD Alzheimer's mouse model. Brandscheid C, Schuck F, Reinhardt S, et al. J Alzheimers Dis. 2017;56:775–788. doi: 10.3233/JAD-160926. [DOI] [PubMed] [Google Scholar]
- 33.Cuervo-Zanatta D, Perez-Grijalva B, González-Magaña E, Hernandez-Acosta J, Murugesan S, García-Mena J, Perez-Cruz C. Studies in Natural Products Chemistry. Philadelphia, PA: Elsevier; 2021. Modulation of the microbiota-gut-brain axis by bioactive food, prebiotics, and probiotics decelerates the course of Alzheimer's disease; pp. 51–86. [Google Scholar]
- 34.Microbiota-derived short chain fatty acids modulate microglia and promote Aβ plaque deposition. Colombo AV, Sadler RK, Llovera G, et al. Elife. 2021;10:0. doi: 10.7554/eLife.59826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gut microbial involvement in Alzheimer's disease pathogenesis. Zhang Y, Geng R, Tu Q. Aging (Albany NY) 2021;13:13359–13371. doi: 10.18632/aging.202994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer's disease-type beta-amyloid neuropathological mechanisms. Ho L, Ono K, Tsuji M, Mazzola P, Singh R, Pasinetti GM. Expert Rev Neurother. 2018;18:83–90. doi: 10.1080/14737175.2018.1400909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Healthy eating, physical activity, and sleep hygiene (HEPAS) as the winning triad for sustaining physical and mental health in patients at risk for or with neuropsychiatric disorders: considerations for clinical practice. Briguglio M, Vitale JA, Galentino R, et al. Neuropsychiatr Dis Treat. 2020;16:55–70. doi: 10.2147/NDT.S229206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pathophysiology of autism spectrum disorders: revisiting gastrointestinal involvement and immune imbalance. Samsam M, Ahangari R, Naser SA. World J Gastroenterol. 2014;20:9942–9951. doi: 10.3748/wjg.v20.i29.9942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. De Angelis M, Piccolo M, Vannini L, et al. PLoS One. 2013;8:0. doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maternal infection and immune involvement in autism. Patterson PH. Trends Mol Med. 2011;17:389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Choi GB, Yim YS, Wong H, et al. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toll-like receptor signaling pathways. Kawasaki T, Kawai T. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Interleukin-17 in chronic inflammatory neurological diseases. Milovanovic J, Arsenijevic A, Stojanovic B, et al. Front Immunol. 2020;11:947. doi: 10.3389/fimmu.2020.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Hsiao EY, McBride SW, Hsien S, et al. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gut microbiota's effect on mental health: the gut-brain axis. Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, Wakefield S. Clin Pract. 2017;7:987. doi: 10.4081/cp.2017.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gut-brain axis and migraine headache: a comprehensive review. Arzani M, Jahromi SR, Ghorbani Z, et al. J Headache Pain. 2020;21:15. doi: 10.1186/s10194-020-1078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. Mol Psychiatry. 2016;21:738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The gut microbiome and the brain. Galland L. J Med Food. 2014;17:1261–1272. doi: 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The effects of a multispecies probiotic supplement on inflammatory markers and episodic and chronic migraine characteristics: a randomized double-blind controlled trial. Martami F, Togha M, Seifishahpar M, Ghorbani Z, Ansari H, Karimi T, Jahromi SR. Cephalalgia. 2019;39:841–853. doi: 10.1177/0333102418820102. [DOI] [PubMed] [Google Scholar]
- 50.Migraines are correlated with higher levels of nitrate-, nitrite-, and nitric oxide-reducing oral microbes in the American Gut Project cohort. Gonzalez A, Hyde E, Sangwan N, Gilbert JA, Viirre E, Knight R. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5080405/ mSystems. 2016;1:0–16. doi: 10.1128/mSystems.00105-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gastrointestinal disorders associated with migraine: a comprehensive review. Cámara-Lemarroy CR, Rodriguez-Gutierrez R, Monreal-Robles R, Marfil-Rivera A. World J Gastroenterol. 2016;22:8149–8160. doi: 10.3748/wjg.v22.i36.8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prolonged trigeminal sensitization induces gut dysbiosis: implications for migraine pathology. Hawkins J, Norton R, Durham P. https://n.neurology.org/content/84/14_Supplement/S51.006 Neurology. 2015;84 (14 Supplement):0. [Google Scholar]
- 53.Cytokines, inflammation, and pain. Zhang JM, An J. Int Anesthesiol Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Microbiota and gut neuropeptides: a dual action of antimicrobial activity and neuroimmune response. Aresti Sanz J, El Aidy S. Psychopharmacology (Berl) 2019;236:1597–1609. doi: 10.1007/s00213-019-05224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calcitonin gene-related peptide (CGRP) and migraine. Durham PL. Headache. 2006;46 Suppl 1:0–8. doi: 10.1111/j.1526-4610.2006.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vitamin D and the host-gut microbiome: a brief overview. Akimbekov NS, Digel I, Sherelkhan DK, Lutfor AB, Razzaque MS. Acta Histochem Cytochem. 2020;53:33–42. doi: 10.1267/ahc.20011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crohn disease--associated adherent-invasive E. coli bacteria target mouse and human Peyer's patches via long polar fimbriae. Chassaing B, Rolhion N, de Vallée A, et al. J Clin Invest. 2011;121:966–975. doi: 10.1172/JCI44632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Sokol H, Pigneur B, Watterlot L, et al. Proc Natl Acad Sci U S A. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Machiels K, Joossens M, Sabino J, et al. Gut. 2014;63:1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 60.Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Jostins L, Ripke S, Weersma RK, et al. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn's disease. Lindsay JO, Whelan K, Stagg AJ, et al. Gut. 2006;55:348–355. doi: 10.1136/gut.2005.074971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. Maes M, Berk M, Goehler L, Song C, Anderson G, Gałecki P, Leonard B. BMC Med. 2012;10:66. doi: 10.1186/1741-7015-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Sofroniew MV. Neuroscientist. 2014;20:160–172. doi: 10.1177/1073858413504466. [DOI] [PubMed] [Google Scholar]
- 64.Functional magnetic resonance imaging study reveals differences in the habituation to psychological stress in patients with Crohn's disease versus healthy controls. Agostini A, Filippini N, Benuzzi F, et al. J Behav Med. 2013;36:477–487. doi: 10.1007/s10865-012-9441-1. [DOI] [PubMed] [Google Scholar]
- 65.Health-related quality of life in inflammatory bowel disease. Functional status and patient worries and concerns. Drossman DA, Patrick DL, Mitchell CM, Zagami EA, Appelbaum MI. Dig Dis Sci. 1989;34:1379–1386. doi: 10.1007/BF01538073. [DOI] [PubMed] [Google Scholar]
- 66.Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Gao X, Cao Q, Cheng Y, et al. Proc Natl Acad Sci U S A. 2018;115:0–9. doi: 10.1073/pnas.1720696115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Depression subtypes in pediatric inflammatory bowel disease. Szigethy EM, Youk AO, Benhayon D, et al. J Pediatr Gastroenterol Nutr. 2014;58:574–581. doi: 10.1097/MPG.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prevalence of irritable bowel syndrome and frequency of symptoms in the general population of Pakistan. Bachani P, Kumar L, Kumar N, et al. https://www.cureus.com/articles/49319-prevalence-of-irritable-bowel-syndrome-and-frequency-of-symptoms-in-the-general-population-of-pakistan. Cureus. 2021;13:0. doi: 10.7759/cureus.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Sperber AD, Dumitrascu D, Fukudo S, et al. Gut. 2017;66:1075–1082. doi: 10.1136/gutjnl-2015-311240. [DOI] [PubMed] [Google Scholar]
- 70.Irritable bowel syndrome: a clinical review. Soares RL. World J Gastroenterol. 2014;20:12144–12160. doi: 10.3748/wjg.v20.i34.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Intestinal microbiota in functional bowel disorders: a Rome foundation report. Simrén M, Barbara G, Flint HJ, et al. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Irritable bowel syndrome: a disease still searching for pathogenesis, diagnosis and therapy. Bellini M, Gambaccini D, Stasi C, Urbano MT, Marchi S, Usai-Satta P. World J Gastroenterol. 2014;20:8807–8820. doi: 10.3748/wjg.v20.i27.8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Postinfection irritable bowel syndrome: the links between gastroenteritis, inflammation, the microbiome, and functional disease. Downs IA, Aroniadis OC, Kelly L, Brandt LJ. J Clin Gastroenterol. 2017;51:869–877. doi: 10.1097/MCG.0000000000000924. [DOI] [PubMed] [Google Scholar]
- 74.Immune activation in patients with irritable bowel syndrome. Liebregts T, Adam B, Bredack C, et al. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 75.Inflammatory cytokines and oxidative stress biomarkers in irritable bowel syndrome: association with digestive symptoms and quality of life. Choghakhori R, Abbasnezhad A, Hasanvand A, Amani R. Cytokine. 2017;93:34–43. doi: 10.1016/j.cyto.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 76.Peripheral and central mechanisms of visceral sensitization in man. Anand P, Aziz Q, Willert R, van Oudenhove L. Neurogastroenterol Motil. 2007;19:29–46. doi: 10.1111/j.1365-2982.2006.00873.x. [DOI] [PubMed] [Google Scholar]
- 77.Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Koloski NA, Jones M, Talley NJ. Aliment Pharmacol Ther. 2016;44:592–600. doi: 10.1111/apt.13738. [DOI] [PubMed] [Google Scholar]
- 78.The Microbiome and Irritable Bowel Syndrome - A Review on the Pathophysiology, Current Research and Future Therapy. Chong PP, Chin VK, Looi CY, Wong WF, Madhavan P, Yong VC. Front Microbiol. 2019;10:1136. doi: 10.3389/fmicb.2019.01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bifidobacterium-rich fecal donor may be a positive predictor for successful fecal microbiota transplantation in patients with irritable bowel syndrome. Mizuno S, Masaoka T, Naganuma M, et al. Digestion. 2017;96:29–38. doi: 10.1159/000471919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Can fecal microbiota transplantation cure irritable bowel syndrome? Halkjær SI, Boolsen AW, Günther S, Christensen AH, Petersen AM. World J Gastroenterol. 2017;23:4112–4120. doi: 10.3748/wjg.v23.i22.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Moayyedi P, Surette MG, Kim PT, et al. Gastroenterology. 2015;149:102–109. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]