Abstract
Purpose
High myopia (HM) is one of the leading causes of irreversible vision loss in the world. Many myopia loci have been uncovered with linkage analysis, genome-wide association studies, and sequencing analysis. Numerous pathogenic genes within these loci have been detected in a portion of HM cases. In the present study, we aimed to investigate the genetic basis of 103 patients with nonsyndromic HM, focusing on the reported causal genes.
Methods
A total of 103 affected individuals with nonsyndromic HM were recruited, including 101 patients with unrelated sporadic HM and a mother and son pair. All participants underwent comprehensive ophthalmic examinations, and genomic DNA samples were extracted from the peripheral blood. Whole exome sequencing was performed on the mother and son pair as well as on the unaffected father. Sanger sequencing was used to identify mutations in the remaining 101 patients. Bioinformatics analysis was subsequently applied to verify the mutations.
Results
An extremely rare mutation in AGRN (c.2627A>T, p.K876M) was identified in the mother and son pair but not in the unaffected father. Another two mutations in AGRN (c.4787C>T, p.P1596L/c.5056G>A, p.G1686S) were identified in two unrelated patients. A total of eight heterozygous variants potentially affecting the protein function were detected in eight of the remaining 99 patients, including c.1350delC, p.V451Cfs*76 and c.1023_1024insA, p.P342Tfs*41 in SLC39A5; c.244_246delAAG, p.K82del in SCO2; c.545A>G, p.Y182C in P4HA2; c.415C>T, p.P139S in BSG; c.3266A>G, p.Y1089C in ZNF644; and c.2252C>T, p.S751L and c.1708C>T, p.R570C in CPSF1. Multiple bioinformatics analyses were conducted, and a comparison to a group with geographically matched controls was performed, which supported the potential pathogenicity of these variants.
Conclusions
We provide further evidence for the potential role of AGRN in HM inheritance and enlarged the current genetic spectrum of nonsyndromic HM by comprehensively screening the reported causal genes.
Introduction
High myopia (HM) is defined as a spherical equivalent (SE) refractive error of at least −6 diopters (D), or an axial length (AL) longer than 26 mm, or both [1,2]. The prevalence of myopia in children varies among ethnicities, with 53% in Singapore [3], 47% in China [4], 23% in the United Kingdom (UK) [5], and 31% in Australia [6]. Holden et al. predicted through meta-analyses that almost half of population in the world may become myopic by 2050, and 10% may develop HM [7]. Due to the severe ocular comorbidities, such as retinal detachment, glaucoma, macular degeneration, and cataract [8,9], HM has become the major cause of acquired blindness in East Asia [10]. Once the pathological changes are triggered, there is no effective method to efficiently stop or delay the development of HM.
Genetic factors play pivotal roles in the occurrence and development of high myopia. There is an arising number of studies elucidating the importance of genetic contribution [2]. Hitherto, there have been 25 MYP loci found by linkage analysis and exome sequencing studies [2]. Genome-wide association studies (GWAS) have detected more than 150 gene loci related with myopia [11-15]. Several causative genes have been identified and replicated across different populations, including seven autosomal dominant genes, ZNF644 [16] (Gene ID: 84146, OMIM: 614159), SCO2 [17] (Gene ID: 9997, OMIM: 604272), SLC39A5 [18] (Gene ID: 283375, OMIM: 608730), P4HA2 [19] (Gene ID: 8974, OMIM: 600608), BSG [20] (Gene ID: 682, OMIM: 109480), CCDC111 [21] (Gene ID: 201973, OMIM: 615421), CPSF1 [22] (Gene ID: 29894, OMIM: 606027); two autosomal recessive genes, LEPREL1 [23] (Gene ID: 55241, OMIM: 610341) and LRPAP1 [24] (Gene ID: 4043, OMIM: 104225); and two X-linked gene, OPN1LW [25] (Gene ID: 5956, OMIM: 300822) and ARR3 [26] (Gene ID: 407, OMIM: 301700). However, a large-scale screening manifested that only a handful of patients (<5%) harbored potential causal mutations in these genes [27], suggesting that other unidentified genes are likely to be responsible for HM as well. Kloss et al. analyzed 14 families of high myopia by utilizing whole exome sequencing (WES) and unveiled multiple genetic variants in the known MYP loci (e.g. AGRN (Gene ID: 375790, OMIM: 103320), EME1 (Gene ID: 146956, OMIM: 610885) and HOXA2 (Gene ID: 3199, OMIM: 604685) [28]. However, there is a lack of replicated cases to further prove the potential pathogenicity of these genes. Most recently, the AGRN gene was found to be involved in baseline refractive development through analysis of genetic networks regulating refractive ocular development in collaborative cross progenitor strain mice, further proving its role in myopia formation [29].
In this work, we performed WES and familial cosegregation in a two-generation family composed of an unaffected father and a mother and son pair with HM. An extremely rare mutation in AGRN (c.2627A>T, p.K876M) at MYP14 was identified in this family. Two other mutations in AGRN (c.4787C>T, p.P1596L/c.5056G>A, p.G1686S) were identified in two patients with sporadic HM, respectively. We then performed Sanger sequencing in the remaining patients with sporadic HM and unrelated patients, focusing on six reported causal genes. A total of eight heterozygous variants likely affecting the protein function were detected, enlarging the current genetic spectrum of nonsyndromic HM.
Methods
Sample enrollment
Consecutive patients who came to High Myopia Clinic of the Eye Hospital of Wenzhou Medical University were investigated from the end of 2019 to early 2020. In total, 103 Chinese individuals diagnosed with nonsyndromic HM, as well as 200 healthy controls were recruited for this study. Notably, the affected group included a pair of high myopic mother and son from a Han Chinese family, and 101 sporadic patients; while the unaffected father from the Han Chinese family was chosen as one member of the 200 controls. All participants were without any systemic disease and no age or gender bias was observed in this study group. A panel of ophthalmologists undertook detailed medical assessments on all the participants. The medical records of those who have received surgery were excluded from our study. All patients met the diagnostic criteria of a refractive error of at least -6.0 D, and an ocular axial length of at least 26 mm. The refractive error of controls for both eyes were within the range of -0.50 to +1.0 D. The study was approved by Ethics Committee of the Eye Hospital of Wenzhou Medical University and in accordance with tenets of the Declaration of Helsinki and the ARVO statement on human subjects. Written informed consents were signed by all participants or their statutory guardians. All members enrolled in this study donated about 10 ml of a peripheral blood sample. Peripheral blood was stored in EDTA- containing vacutainer tubes. 5 ml of a peripheral blood sample were kept at -80 °C freezer as a backup. DNA was extracted from leukocytes in peripheral blood by using the Blood DNA Mini Kit (Simgen, Hangzhou, China) [30].
WES and analyses
We performed WES on the mother and son pair as well as the unaffected father. The genomic DNA was sheared into 100 base pairs via the Exome Enrichment V5 Kit (Agilent Technologies, Palo Alto, CA) to generate the whole-exome region libraries, in agreement with the manufacturer’s protocol. A HiSeq 2000 sequencer (Illumina, San Diego, CA) was used to sequence whole exome–enriched DNA libraries. The variants, including single-nucleotide variants and insertion–deletion variants, were identified with GATK. Reads were mapped against UCSC hg19 via Burrows-Wheeler Aligner (BWA).
Sanger sequencing
Sanger sequencing was further used to verify the segregated variants in the family of three. We subsequently performed Sanger sequencing of the AGRN, SLC39A5, SCO2, P4HA2, BSG, ZNF644, and CPSF1 in 101 sporadic high myopia patients. PCR conditions consisted of three steps: an initial 10 min denaturation at 95 °C; 35 cycles at 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 60 s; a final 7 min elongation at 72 °C. PCR primer pairs spanning all coding exons, splicing sites, and untranslated gene region (UTRs) were designed in the online program Exon Primer. Sanger sequencing was used to detect potential causal variants by using the ABI 3730XL automated DNA sequencer (Thermo Fisher Scientific, Carlsbad, CA). In this work, we set up seven panels of ophthalmologists, each responsible for one gene enrolled in the study; 100% coverage was attained.
Pathogenicity evaluation
Mutations with a minor allele frequency (MAF) were assessed in Exome Aggregation Consortium (EXAC) and the Genome Aggregation Database (gnomAD). Pathogenicity was evaluated by SIFT, PolyPhen2 HDIV, PolyPhen2 HVAR, MutationTaster, and PROVEAN. Human Splicing Finder software was used to assess the effect of splicing mutations. Amino acid sequences of multiple species were obtained from the National Center for Biotechnology Information. Multiple sequence alignment and assessment of evolutionary conservation were visualized by Clustal Omega. SMART was used to predict the topological model of the related genes’ polypeptide structure. We considered a variation as potentially affecting the protein function according to the following standards: (1) Splice-site variants fulfilled the GT-AT rules, (2) nonsense or frameshift variations were present, and (3) non-frameshift insertions or deletions or missense mutations were predicted to be pathogenic or probably pathogenic according to the American College of Medical Genetics and Genomics (ACMG) standards and guidelines and comprehensive bioinformatics tools [30]. Phyre2 was used to predict the three-dimensional crystal structures of wild-type and mutant proteins. The predicted crystal structures that had the highest alignment coverage and confidence were chosen and visualized with PyMol software (Version 1.5; DeLano Scientific, San Carlos, CA).
Results
Clinical features
Two hundred and five eyes of 103 consecutive patients with HM were examined (except one eye that lacked refractive diopters). Age of onset for all patients was the preschool years. Of the 103 patients in the study, 49 (47.6%) were male, and 54 (52.4%) were female. Their mean (± standard deviation, SD) examination age was 42.21 (±15.88) years of age. The mean (±SD) spherical refraction was −16.17 D (±5.690 D) for the right eye and −15.82 D (±6.070 D) for the left eye. The mean (±SD) AL was 28.84 mm (± 2.500 mm) for the right eye and 28.89 mm (±2.640 mm) for the left eye. One hundred seventy-six phakic eyes (89.3%) had spherical equivalents greater than −10 D and were identified as extreme HM. No systemic disease was found in all participants.
Mutations in AGRN
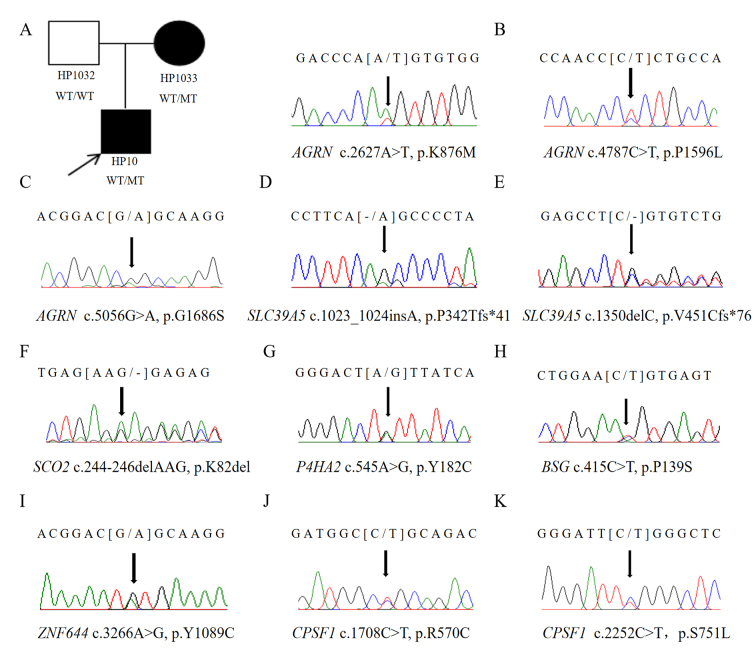
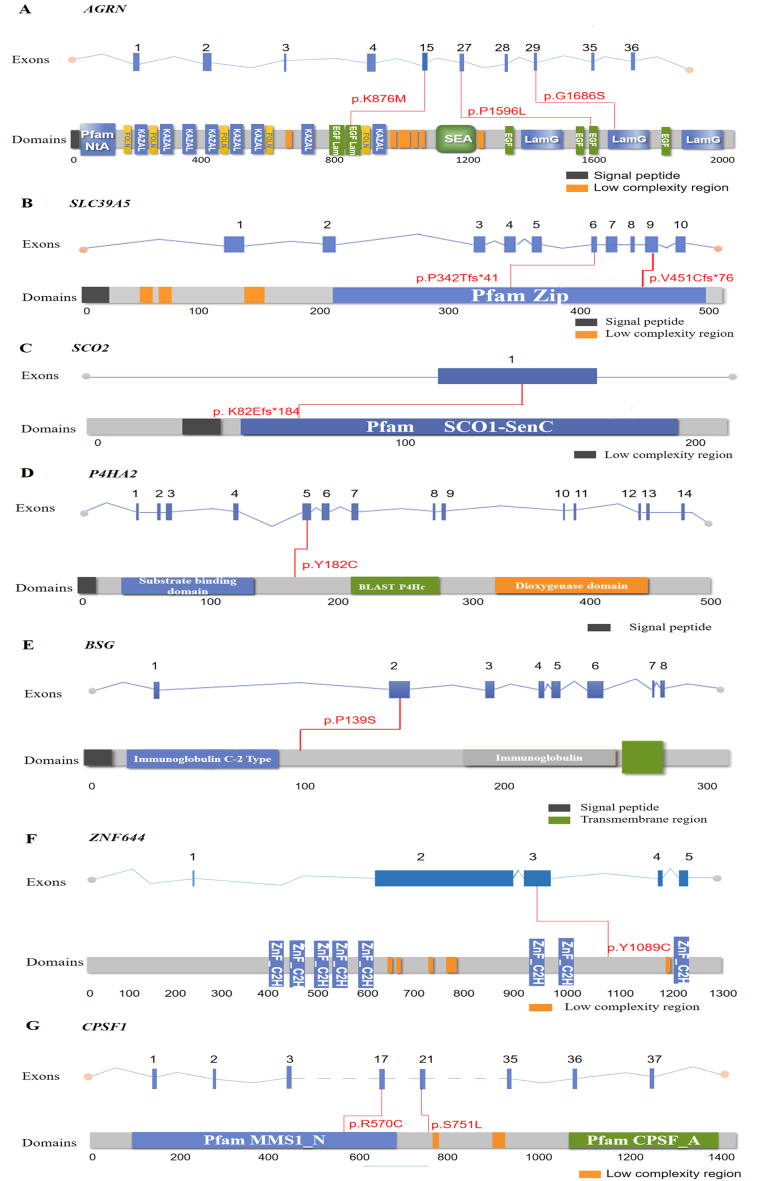
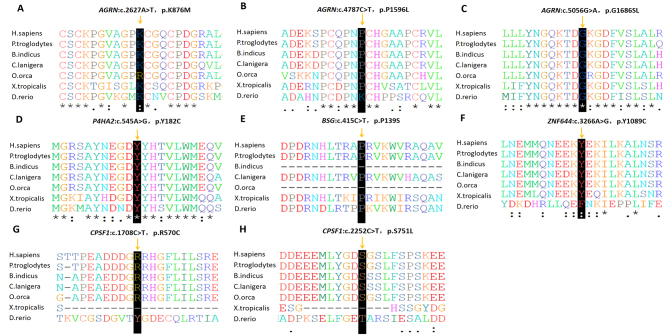
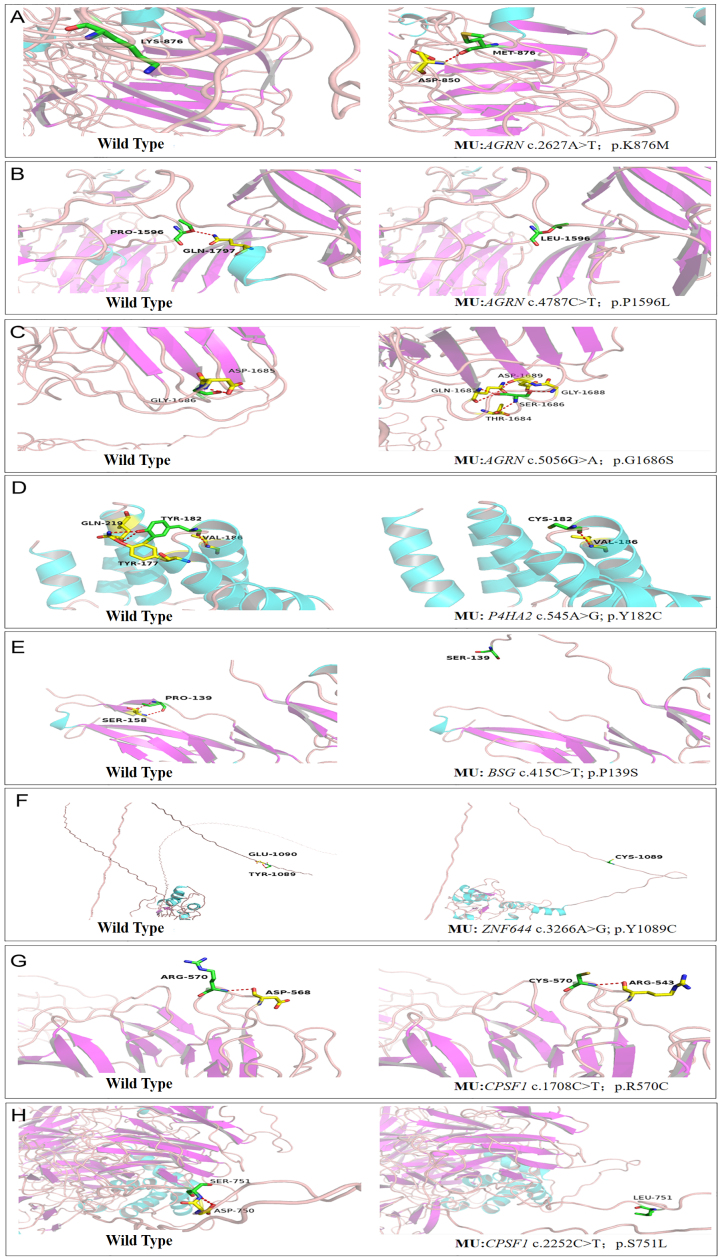
WES was performed on the son (HP10), the mother (HP1033), and the unaffected father (HP1032). The mean read depth for WES was >30X, and the median coverage of the targeted regions reached >95%. The detailed workflow of the variant analyses is shown in Appendix 1. After a comparative analysis of the WES data and familial cosegregation, an extremely rare missense mutation of c.2627A>T (p.K876M) within exon 15 of AGRN was identified at MYP14 (chromosome 1p26; Figure 1A). AGRN encodes agrin, a large proteoglycan that has multiple isoforms which have varied functions. The variant c.2627A>T is within the EGF Lam domain of the AGRN protein (Figure 2A). The pathogenicity of c.2627A>T was predicted by all five bioinformatics tools (Table 1). Multiple orthologous sequence alignment showed that K876M was identified in a highly evolutionarily conserved region across varied species (Figure 3A). We then predicted the topological model via the Phyre2 server in automated mode. The variant K876M was found to generate a newly formed bond between residue 876 and residue 850 (Figure 4A). These findings suggest that the missense mutation c.2627A>T has great potential to influence the protein structure of AGRN. To the best of our knowledge, our work is the first to replicate the role of AGRN as a nonsyndromic myopia-causing gene in Chinese families with HM.
Figure 1.
Potentially causative mutations uncovered in this study. Pedigrees of the family in which high myopia appears to follow an autosomal dominant mode of inheritance. The arrow indicates the location of the mutation (A–K).
Figure 2.
Location of identified variants in the AGRN, SLC39A5, SCO2, ZNF644, BSG, P4HA2, and CPSF1 genes. Exons of human AGRN, SLC39A5, SCO2, ZNF644, BSG, P4HA2, and CPSF1 (upper), and locations of mutated residues with respect to the topological model of the polypeptides (under) are shown. A total of 11 heterozygous variants in red were identified in this study (A–G).
Table 1. Potential causative variants uncovered in 103 Chinese patients with nonsyndromic HM.
| Sample ID | Age | Sex | SEM (diopter) | AL(mm) | Gene | Exon | Mutation | Status | Type | GnomAD | EXAC | SIFT | Mutation Taster | Polyphen2 HDIV | Polyphen2 HVAR | PROVEAN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HP10 | 18 | M | -10.50/-10.25 | 27.30/27.01 | AGRN | 15 | c.2627A>T (p.K876M) | Het | Missense | 4.07983e-06 | 0.000008 | D(0.001) | DC(0.999) | PD(1.000) | PD(1.000) | D(−5.32) |
| HP1033 | 43 | F | −6.00/-5.63 | 24.05/24.19 | AGRN | 15 | c.2627A>T (p.K876M) | Het | Missense | 4.07983e-06 | 0.000008 | D(0.001) | DC(0.999) | PD(1.000) | PD(1.000) | D(−5.32) |
| HP1042 | 41 | M | −6.13/-6.00 | 25.79/25.74 | AGRN | 27 | c.4787C>T (p.P1596L) | Het | Missense | 8.4354e-06 | 0.000009 | D(0.004) | DC(0.999) | PD(0.999) | PD(0.985) | D(−5.97) |
| HP1021 | 17 | M | −8.50/-8.50 | 27.46/27.50 | AGRN | 29 | c.5056G>A (p.G1686S) | Het | Missense | 3.24275e-05 | 0.000017 | D(0.008) | DC(1.000) | PD(1) | PD(0.993) | D(−4.72) |
| WH167 | 32 | F | −29.00/-30.00 | 36.79/35.84 | SLC39A5 | 6 | c.1023_1024insA (p.P342Tfs*41) | Het | Frameshift | Novel | Novel | - | DC(1.000) | - | - | - |
| S111 | 41 | F | −16.50/-19.59 | 27.76/27.95 | SLC39A5 | 9 | c.1350delC (p.V451Cfs*76) | Het | Frameshift | Novel | Novel | - | DC(1.000) | - | - | - |
| H381 | 48 | F | −21.75/-31.24 | 28.68/33.84 | SCO2 | 1 | c.244_246delAAG (p.K82del) | Het | Frameshift | 2.07952e-05 | 0.000017 | - | DC(0.966) | - | - | - |
| M78 | 72 | F | −22.38/NA | 30.36/30.73 | P4HA2 | 5 | c.545A>G (p.Y182C) | Het | Missense | 4.06095e-06 | 0.000008 | D(0.001) | DC(0.999) | PD(1.000) | PD(0.984) | D(−7.23) |
| M15 | 35 | F | −6.75/-17.38 | 25.88/31.25 | BSG | 2 | c.415C>T (p.P139S) | Het | Missense/Splicing | Novel | Novel | D(0.001) | DC(0.999) | P(0.797) | B(0.102) | N(−0.04) |
| HP15 | 23 | F | -10.50/-10.50 | 28.44/28.46 | ZNF644 | 4 | c.3266A>G (p.Y1089C) | Het | Missense | 9.69368e-05 | 0.000255 | D(0.000) | DC(0.999) | PD(0.999) | PD(0.982) | N(−2.24) |
| WH126 | 86 | F | −6.00/-0.75 | 25.36/22.48 | CPSF1 | 21 | c.2252C>T (p.S751L) | Het | Missense | 3.67929e-05 | 0.000027 | D(0.007) | DC(1.000) | P(0.915) | B(0.348) | D(−3.61) |
| WH091 | 68 | F | −27.75/-22.75 | 32.04/30.73 | CPSF1 | 17 | c.1708C>T (p.R570C) | Het | Missense | 8.12222e-06 | 0.000008 | D(0.045) | DC(0.999) | P(0.758) | P(0.601) | N(−1.93) |
Abbreviations: Het, heterozygous; SE, spherical equivalent; AL, axial length; OD, right eye; OS, left eye; M, male; F, female; NA, not available; PD, probably damaging; DC, disease causing; D, deleterious; B, benign; P, possibly damaging; N, neutral
Figure 3.
Evolutionarily conserved analysis manifests evolutionary conservation of the variants. The arrow presents the location of the variants (A–H). Sequencing alignments visualized with Clustal Omega.
Figure 4.
Simulated three-dimensional crystal structures of proteins. Predicted crystal structures of wild-type (left) and mutant (right) proteins. The wild-type (left) and mutant (right) residues are green, while the residues that bind with them are yellow (A–H).
After we verified the variant c.2627A>T of AGRN in the family with Sanger sequencing, we performed Sanger sequencing of the AGRN exons and splicing junction sites in the remaining 101 patients with sporadic HM. Two missense variants (c.4787C>T p.P1596L / c.5056G>A p.G1686S) passed the strict filtering steps (Figure 1B,C). Neither occurred in the 200 controls. Both mutations were present in existing databases (EXAC, GnomAD) with extremely rare frequency. The pathogenicity of the two variants of AGRN were further predicted by different bioinformatics tools (Table 1), and both were presented as evolutionarily conserved (Figure 3B,C). Structural modeling of G1686S and P1596L in AGRN showed significant alterations in the structure, which probably influence the protein function (Figure 4B,C). Due to the lack of genetic information from the family members of these two patients, however, we could not confirm linkage of these variants with HM.
Mutations in SLC39A5, SCO2, P4HA2, and ZNF644
To further investigate the genetic basis of the remaining 99 patients with sporadic HM, we performed Sanger sequencing in all coding exons and splicing sites of four reported autosomal causal genes: SLC39A5, SCO2, P4HA2, and ZNF644. These genes have been ascertained as the causal genes and replicated in multiple studies of populations with HM across the world. Five extremely rare variants potentially affecting the protein function were identified in five patients. Neither the five variants nor variants similar to them (not exactly the same but at the same amino acid sites) occurred in the 200 controls.
Two novel frameshift variants (c.1350delC, p.V451Cfs*76 and c.1023_1024insA, p.P342Tfs*41) in SLC39A5 were detected (Figure 1D,E). The two frameshift variants were within the Zip domain of the SLC39A5 protein (Figure 2B) and predicted to stop the open reading frame at codon 526 and codon 382, respectively, thus generating two different prematurely truncated SLC39A5 proteins. Another frameshift variant (c.244_246delAAG, p. K82del) was found in SCO2 and located in functional domain SCO1-SenC (Figure 1F, Figure 2C), which would markedly influence the function of the SCO2 protein.
Two heterozygous missense mutations were detected in two different genes: c.545A>G (p.Y182C) in P4HA2 and c.3266A>G (p.Y1089C) in ZNF644 (Figure 1G,I). Both variants were located in the coding region (Figure 2D,F) and displayed strong pathogenicity according to the computational analyses (Table 1). The two variants were highly evolutionarily conserved through assessment of multiple sequence alignment of polypeptides of different species (Figure 3D,F). The crystal structure modeling of Y182C in P4HA2 showed the absence of bonds between residue 177 tyrosine and residue 182 tyrosine, and between residue 219 glutamine and residue 182 tyrosine (Figure 4D). The crystal structure modeling of Y1089C in ZNF644 demonstrated the absence of a hydrogen bond between the mutated residue 1089 glutamic and residue 1090 tyrosine (Figure 4F).
Mutations in BSG and CPSF1
Knockdown animal models of BSG and CPSF1 genes have been established and provided convincing evidence of the roles of these genes in the development of myopia [20,22]. Three variants, c.415C>T (p.P139S) in BSG and c.1708C>T (p.R570C) and c.2252C>T (p.S751L) in CPSF1, were identified in this work (Figure 1H,J,K). The c.415C>T variant in the BSG mutation was novel and not present in existing databases (EXAC and gnomAD); the two CPSF1 variants were rare and present in existing databases (EXAC and gnomAD) with low frequency (Table 1). Further analysis through Human Splicing Finder showed that the c.415C>T in the BSG mutation potentially created a new exonic splicing silencer (ESS) site and was predicted to affect the splicing site. Structural modeling of P139S in the BSG gene showed significant alterations in the structure and the absence of the hydrogen bond between the mutated residue 139 proline and residue 158 serine (Figure 4E). The tertiary structures of R570C in the CPSF1 gene showed a new bond between the mutated residue 570 cysteine and residue 543 arginine acid (Figure 4G). Structural modeling of S751L in the CPSF1 gene showed the absence of the bond between the mutated residue 751 serine and residue 750 aspartic acid (Figure 4H).
Discussion
Myopia has become the most common ocular abnormality worldwide. The myopic changes occur in not only the optical media but also the ocular walls [30]. There exists an interplay of genetic factors with environmental stresses in myopia pathogenesis. Studies of two independent population-based cohorts comprising 5,256 and 3,938 individuals of European descent revealed the interactive effect between genetic predisposition and education [31]. The combined effect of these two factors on the risk of developing myopia is much higher than their additive effect. A similar interactive effect was also reported for axial length [13]. Notably, for people with low-frequency variants of a myopia susceptibility gene identified by Tkatchenko et al., time spent reading was associated with differential degree of refractive error [32]. Moreover, a recent study provided evidence of gene–gene interactions and gene–environment interactions for 88% (128) of 146 refractive error-associated variants tested [33]. In one meta-analysis, heritability was estimated at 0.71 for refractive error [34], indicating that myopic changes were due more to genes than to environments.
The AGRN gene was shown to interact with EGR1, which was previously implicated in refractive eye development, and to regulate synaptic physiology in the retina [35,36]. In this study, an extremely rare variant (c.2627A>T, p.K876M) in AGRN was found in a family with HM. We also found two other rare variants (c.4787C>T and c.5056G>A) in the coding sequence of AGRN from two patients with sporadic HM. AGRN was located in the MYP14 locus, which was mapped with HM in Ashkenazi Jewish families [37]. Kloss et al. first reported that a heterozygous mutation (c.1304C>T, p.T435M) in AGRN was detected in a Danish family with HM that appeared to demonstrate autosomal dominant (AD) inheritance transmission [23]. Homozygous or compound heterozygous mutations in AGRN were previously reported to be associated with congenital myasthenia syndrome (CMS), which is characterized as fatigable muscle weakness [38]. AGRN encodes agrin, a large protein that is responsible for the integrity of neuromuscular transmission. The defect of neuromuscular transmission may lead to disorders of the nervous system or muscle, including ciliary muscle and extraocular muscles [39]. A previous study found a 17-month-old boy with CMS who harbored a homozygous mutation in AGRN exhibited ophthalmoplegia and ptosis [40]. Ptosis may develop into myopia [41]. Myopic staphylomata were reported to be related to the path of the lateral rectus and defect of the lateral rectus to the superior rectus band ligament [42]. The ciliary muscle was also thought to be associated with the development of refractive error [43]. The present study is the first to replicate the role of AGRN in HM and suggest AGRN as a possibly HM-causing gene in a Chinese population. The pathogenic mechanism of HM based on the AGRN variation is still obscured, and more efforts are needed to explain the role of AGRN in the pathogenesis of HM.
Potential pathologic mutations were also identified in SLC39A5, SCO2, P4HA2, BSG, ZNF644, and CPSF1. We summarize the reported mutations of the seven autosomal dominant genes from populations with HM worldwide. SLC39A5 and ZNF644 contributed most of the reported mutations (Figure 5A). Previously, mutations in ZNF644 have been found in Chinese, African American, and Caucasian subjects [2]. However, we found only a previously reported mutation in ZNF644. SLC39A5 was involved with the bone morphogenetic protein/transforming growth factor-β (BMP/TGF-β) pathway. This pathway is responsible for modulating the extracellular matrix (ECM) of the sclera, which is thought to be one of the explanations of HM pathomechanism [18,44]. Two frameshift mutations were identified in the SLC39A5 gene in this study. A frameshift mutation was identified in SCO2 (cytochrome c oxidase assembly protein). Previous exome sequencing and Sanger sequencing has uncovered ten heterozygous mutations in SCO2 [2]. A mutation was identified in the BSG gene in this work. Previously, Jin et al. first generated Bsg knockin mice presenting the typical the HM phenotype of AL elongation [20]. A missense mutation was identified in P4HA2, which is critical in the stabilization of collagen formation. Disruption of the function of the P4HA4 protein may generate unstable collagen polypeptide chains, which may lead to sclera inclined to elongation because collagen is a vital part of the ECM of the sclera [45,46]. Two missense mutation were identified in CPSF1, which is highly expressed in human ocular tissues and related to retinal ganglion cell (RGC) axonal growth in zebrafish [22]. Ouyang et al. detected six rare heterozygous loss-of-function variants in CPSF1, and this study further expands the mutational pool of mutations in CPSF1 of HM.
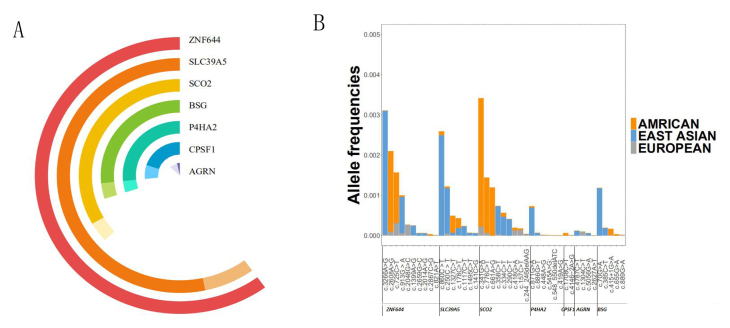
Figure 5.
Mutational spectrum of the seven autosomal dominant genes and allele frequencies of known variants. A: Dark colors represent previously reported mutations, and light colors represent mutations newly discovered in this study. B: Differences in the allele frequency of 43 rare variants between East Asians, Americans, and Europeans.
There is a large spectrum of variants for nonsyndromic HM. Seventy-seven mutations have been identified in AGRN, ZNF644, SCO2, SLC39A5, P4HA2, BSG, and CPSF1, including 34 novel variants and 43 rare variants. Approximately four fifths of patients harbor missense variants. After collecting data from GnomAD database, we depicted a picture to show the differences in allele frequency of 43 rare variants between East Asians, Americans, and Europeans (Figure 5B). Europeans have a lower allele frequency of all mutations. Americans have higher allele frequencies in mutations in SCO2 and ZNF644. East Asians have higher allele frequencies in ZNF644, SLC39A5, and BSG. Given that a few patients with nonsyndromic HM may be mistaken as healthy and enrolled in public databases, the real allele frequencies of known mutations in cohorts with nonsyndromic HM may be higher. ZNF644 contributed the most mutations to the genetic spectrum of nonsyndromic HM. Variants in ZNF644 are frequently found in Europeans, and even more frequently in East Asians and Americans. It is wise to give ZNF644 priority during genetic screening of nonsyndromic HM cases. The prevalence of myopia varies in different areas, and is especially high in East Asians. These studies provide preliminary data implying relatively unique genetic backgrounds of different population and geographic areas. More studies are needed to further elucidate founder effects associated with ethnicity and further dissect the geographic or regional differences. In conclusion, we provided additional evidence for the potential role of AGRN in HM inheritance and enlarged the current genetic spectrum of nonsyndromic HM by comprehensively screening the reported causal genes.
Acknowledgments
We thank the patients and their family members for their participation in this study. This study was supported by the National Natural Science Foundation of China (82125007, 81970838 [Z-BJ]), National Key R&D Program of China (2017YFA0105300 [Z-BJ]), and National Natural Science Foundation of China (82060182 [W-JZ]). Dr. Bing Lin (lbing124@126.com) and Dr. Wen-Juan Zhuang (zh_wenj@163.com) are co-corresponding authors for this paper.
Appendix 1. The workflow diagram of validation.
To access the data, click or select the words “Appendix 1.”
References
- 1.Young TL, Metlapally R, Shay AE. Complex trait genetics of refractive error. Arch Ophthalmol. 2007;125:38–48. doi: 10.1001/archopht.125.1.38. [DOI] [PubMed] [Google Scholar]
- 2.Cai XB, Shen SR, Chen DF, Zhang Q, Jin ZB. An overview of myopia genetics. Exp Eye Res. 2019;188:107778. doi: 10.1016/j.exer.2019.107778. [DOI] [PubMed] [Google Scholar]
- 3.Quek TP, Chua CG, Chong CS, Chong JH, Hey HW, Lee J, Lim YF, Saw SM. Prevalence of refractive errors in teenage high school students in Singapore. Ophthalmic Physiol Opt. 2004;24:47–55. doi: 10.1046/j.1475-1313.2003.00166.x. [DOI] [PubMed] [Google Scholar]
- 4.He M, Zeng J, Liu Y, Xu J, Pokharel GP, Ellwein LB. Refractive error and visual impairment in urban children in southern china. Invest Ophthalmol Vis Sci. 2004;45:793–9. doi: 10.1167/iovs.03-1051. [DOI] [PubMed] [Google Scholar]
- 5.McCullough SJ, O’Donoghue L, Saunders KJ. Six Year Refractive Change among White Children and Young Adults: Evidence for Significant Increase in Myopia among White UK Children. PLoS One. 2016;11:e0146332. doi: 10.1371/journal.pone.0146332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French AN, Morgan IG, Burlutsky G, Mitchell P, Rose KA. Prevalence and 5- to 6-year incidence and progression of myopia and hyperopia in Australian schoolchildren. Ophthalmology. 2013;120:1482–91. doi: 10.1016/j.ophtha.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016;123:1036–42. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379:1739–48. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- 9.Hornbeak DM, Young TL. Myopia genetics: a review of current research and emerging trends. Curr Opin Ophthalmol. 2009;20:356–62. doi: 10.1097/ICU.0b013e32832f8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohno-Matsui K, Lai TY, Lai CC, Cheung CM. Updates of pathologic myopia. Prog Retin Eye Res. 2016;52:156–87. doi: 10.1016/j.preteyeres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Verhoeven VJ, Hysi PG, Wojciechowski R, Fan Q, Guggenheim JA, Hohn R, MacGregor S, Hewitt AW, Nag A, Cheng CY, Yonova-Doing E, Zhou X, Ikram MK, Buitendijk GH, McMahon G, Kemp JP, Pourcain BS, Simpson CL, Makela KM, Lehtimaki T, Kahonen M, Paterson AD, Hosseini SM, Wong HS, Xu L, Jonas JB, Parssinen O, Wedenoja J, Yip SP, Ho DW, Pang CP, Chen LJ, Burdon KP, Craig JE, Klein BE, Klein R, Haller T, Metspalu A, Khor CC, Tai ES, Aung T, Vithana E, Tay WT, Barathi VA, Chen P, Li R, Liao J, Zheng Y, Ong RT, Doring A, Evans DM, Timpson NJ, Verkerk AJ, Meitinger T, Raitakari O, Hawthorne F, Spector TD, Karssen LC, Pirastu M, Murgia F, Ang W, Mishra A, Montgomery GW, Pennell CE, Cumberland PM, Cotlarciuc I, Mitchell P, Wang JJ, Schache M, Janmahasatian S, Igo RP, Jr, Lass JH, Chew E, Iyengar SK, Gorgels TG, Rudan I, Hayward C, Wright AF, Polasek O, Vatavuk Z, Wilson JF, Fleck B, Zeller T, Mirshahi A, Muller C, Uitterlinden AG, Rivadeneira F, Vingerling JR, Hofman A, Oostra BA, Amin N, Bergen AA, Teo YY, Rahi JS, Vitart V, Williams C, Baird PN, Wong TY, Oexle K, Pfeiffer N, Mackey DA, Young TL, van Duijn CM, Saw SM, Bailey-Wilson JE, Stambolian D, Klaver CC, Hammond CJ. Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia. Nat Genet. 2013;45:314–8. doi: 10.1038/ng.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshikawa M, Yamashiro K, Miyake M, Oishi M, Akagi-Kurashige Y, Kumagai K, Nakata I, Nakanishi H, Oishi A, Gotoh N, Yamada R, Matsuda F, Yoshimura N. Comprehensive replication of the relationship between myopia-related genes and refractive errors in a large Japanese cohort. Invest Ophthalmol Vis Sci. 2014;55:7343–54. doi: 10.1167/iovs.14-15105. [DOI] [PubMed] [Google Scholar]
- 13.Fan Q, Wojciechowski R, Kamran Ikram M, Cheng CY, Chen P, Zhou X, Pan CW, Khor CC, Tai ES, Aung T, Wong TY, Teo YY, Saw SM. Education influences the association between genetic variants and refractive error: a meta-analysis of five Singapore studies. Hum Mol Genet. 2014;23:546–54. doi: 10.1093/hmg/ddt431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng CY, Schache M, Ikram MK, Young TL, Guggenheim JA, Vitart V, MacGregor S, Verhoeven VJ, Barathi VA, Liao J, Hysi PG, Bailey-Wilson JE, St Pourcain B, Kemp JP, McMahon G, Timpson NJ, Evans DM, Montgomery GW, Mishra A, Wang YX, Wang JJ, Rochtchina E, Polasek O, Wright AF, Amin N, van Leeuwen EM, Wilson JF, Pennell CE, van Duijn CM, de Jong PT, Vingerling JR, Zhou X, Chen P, Li R, Tay WT, Zheng Y, Chew M, Burdon KP, Craig JE, Iyengar SK, Igo RP, Jr, Lass JH, Jr, Chew EY, Haller T, Mihailov E, Metspalu A, Wedenoja J, Simpson CL, Wojciechowski R, Hohn R, Mirshahi A, Zeller T, Pfeiffer N, Lackner KJ, Bettecken T, Meitinger T, Oexle K, Pirastu M, Portas L, Nag A, Williams KM, Yonova-Doing E, Klein R, Klein BE, Hosseini SM, Paterson AD, Makela KM, Lehtimaki T, Kahonen M, Raitakari O, Yoshimura N, Matsuda F, Chen LJ, Pang CP, Yip SP, Yap MK, Meguro A, Mizuki N, Inoko H, Foster PJ, Zhao JH, Vithana E, Tai ES, Fan Q, Xu L, Campbell H, Fleck B, Rudan I, Aung T, Hofman A, Uitterlinden AG, Bencic G, Khor CC, Forward H, Parssinen O, Mitchell P, Rivadeneira F, Hewitt AW, Williams C, Oostra BA, Teo YY, Hammond CJ, Stambolian D, Mackey DA, Klaver CC, Wong TY, Saw SM, Baird PN. Nine loci for ocular axial length identified through genome-wide association studies, including shared loci with refractive error. Am J Hum Genet. 2013;93:264–77. doi: 10.1016/j.ajhg.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan Q, Verhoeven VJ, Wojciechowski R, Barathi VA, Hysi PG, Guggenheim JA, Hohn R, Vitart V, Khawaja AP, Yamashiro K, Hosseini SM, Lehtimaki T, Lu Y, Haller T, Xie J, Delcourt C, Pirastu M, Wedenoja J, Gharahkhani P, Venturini C, Miyake M, Hewitt AW, Guo X, Mazur J, Huffman JE, Williams KM, Polasek O, Campbell H, Rudan I, Vatavuk Z, Wilson JF, Joshi PK, McMahon G, St Pourcain B, Evans DM, Simpson CL, Schwantes-An TH, Igo RP, Mirshahi A, Cougnard-Gregoire A, Bellenguez C, Blettner M, Raitakari O, Kahonen M, Seppala I, Zeller T, Meitinger T, Ried JS, Gieger C, Portas L, van Leeuwen EM, Amin N, Uitterlinden AG, Rivadeneira F, Hofman A, Vingerling JR, Wang YX, Wang X, Tai-Hui Boh E, Ikram MK, Sabanayagam C, Gupta P, Tan V, Zhou L, Ho CE, Lim W, Beuerman RW, Siantar R, Tai ES, Vithana E, Mihailov E, Khor CC, Hayward C, Luben RN, Foster PJ, Klein BE, Klein R, Wong HS, Mitchell P, Metspalu A, Aung T, Young TL, He M, Parssinen O, van Duijn CM, Jin Wang J, Williams C, Jonas JB, Teo YY, Mackey DA, Oexle K, Yoshimura N, Paterson AD, Pfeiffer N, Wong TY, Baird PN, Stambolian D, Wilson JE, Cheng CY, Hammond CJ, Klaver CC, Saw SM, Rahi JS, Korobelnik JF, Kemp JP, Timpson NJ, Smith GD, Craig JE, Burdon KP, Fogarty RD, Iyengar SK, Chew E, Janmahasatian S, Martin NG, MacGregor S, Xu L, Schache M, Nangia V, Panda-Jonas S, Wright AF, Fondran JR, Lass JH, Feng S, Zhao JH, Khaw KT, Wareham NJ, Rantanen T, Kaprio J, Pang CP, Chen LJ, Tam PO, Jhanji V, Young AL, Doring A, Raffel LJ, Cotch MF, Li X, Yip SP, Yap MK, Biino G, Vaccargiu S, Fossarello M, Fleck B, Yazar S, Tideman JW, Tedja M, Deangelis MM, Morrison M, Farrer L, Zhou X, Chen W, Mizuki N, Meguro A, Makela KM. Meta-analysis of gene-environment-wide association scans accounting for education level identifies additional loci for refractive error. Nat Commun. 2016;7:11008. doi: 10.1038/ncomms11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, Li Y, Zhang D, Zhang H, Li Y, Lu F, Liu X, He F, Gong B, Cai L, Li R, Liao S, Ma S, Lin H, Cheng J, Zheng H, Shan Y, Chen B, Hu J, Jin X, Zhao P, Chen Y, Zhang Y, Lin Y, Li X, Fan Y, Yang H, Wang J, Yang Z. Exome sequencing identifies ZNF644 mutations in high myopia. PLoS Genet. 2011;7:e1002084. doi: 10.1371/journal.pgen.1002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran-Viet KN, Powell C, Barathi VA, Klemm T, Maurer-Stroh S, Limviphuvadh V, Soler V, Ho C, Yanovitch T, Schneider G, Li YJ, Nading E, Metlapally R, Saw SM, Goh L, Rozen S, Young TL. Mutations in SCO2 are associated with autosomal-dominant high-grade myopia. Am J Hum Genet. 2013;92:820–6. doi: 10.1016/j.ajhg.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo H, Jin X, Zhu T, Wang T, Tong P, Tian L, Peng Y, Sun L, Wan A, Chen J, Liu Y, Li Y, Tian Q, Xia L, Zhang L, Pan Y, Lu L, Liu Q, Shen L, Li Y, Xiong W, Li J, Tang B, Feng Y, Zhang X, Zhang Z, Pan Q, Hu Z, Xia K. SLC39A5 mutations interfering with the BMP/TGF-beta pathway in non-syndromic high myopia. J Med Genet. 2014;51:518–25. doi: 10.1136/jmedgenet-2014-102351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo H, Tong P, Liu Y, Xia L, Wang T, Tian Q, Li Y, Hu Y, Zheng Y, Jin X, Li Y, Xiong W, Tang B, Feng Y, Li J, Pan Q, Hu Z, Xia K. Mutations of P4HA2 encoding prolyl 4-hydroxylase 2 are associated with nonsyndromic high myopia. Genet Med. 2015;17:300–6. doi: 10.1038/gim.2015.28. [DOI] [PubMed] [Google Scholar]
- 20.Jin ZB, Wu J, Huang XF, Feng CY, Cai XB, Mao JY, Xiang L, Wu KC, Xiao X, Kloss BA, Li Z, Liu Z, Huang S, Shen M, Cheng FF, Cheng XW, Zheng ZL, Chen X, Zhuang W, Zhang Q, Young TL, Xie T, Lu F, Qu J. Trio-based exome sequencing arrests de novo mutations in early-onset high myopia. Proc Natl Acad Sci USA. 2017;114:4219–24. doi: 10.1073/pnas.1615970114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao F, Wu J, Xue A, Su Y, Wang X, Lu X, Zhou Z, Qu J, Zhou X. Exome sequencing reveals CCDC111 mutation associated with high myopia. Hum Genet. 2013;132:913–21. doi: 10.1007/s00439-013-1303-6. [DOI] [PubMed] [Google Scholar]
- 22.Ouyang J, Sun W, Xiao X, Li S, Jia X, Zhou L, Wang P, Zhang Q. CPSF1 mutations are associated with early-onset high myopia and involved in retinal ganglion cell axon projection. Hum Mol Genet. 2019;28:1959–70. doi: 10.1093/hmg/ddz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo H, Tong P, Peng Y, Wang T, Liu Y, Chen J, Li Y, Tian Q, Hu Y, Zheng Y, Xiao L, Xiong W, Pan Q, Hu Z, Xia K. Homozygous loss-of-function mutation of the LEPREL1 gene causes severe non-syndromic high myopia with early-onset cataract. Clin Genet. 2014;86:575–9. doi: 10.1111/cge.12309. [DOI] [PubMed] [Google Scholar]
- 24.Aldahmesh MA, Khan AO, Alkuraya H, Adly N, Anazi S, Al-Saleh AA, Mohamed JY, Hijazi H, Prabakaran S, Tacke M, Al-Khrashi A, Hashem M, Reinheckel T, Assiri A, Alkuraya FS. Mutations in LRPAP1 are associated with severe myopia in humans. Am J Hum Genet. 2013;93:313–20. doi: 10.1016/j.ajhg.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Gao B, Guan L, Xiao X, Zhang J, Li S, Jiang H, Jia X, Yang J, Guo X, Yin Y, Wang J, Zhang Q. Unique Variants in OPN1LW Cause Both Syndromic and Nonsyndromic X–Linked High Myopia Mapped to MYP1. Invest Ophthalmol Vis Sci. 2015;56:4150–5. doi: 10.1167/iovs.14-16356. [DOI] [PubMed] [Google Scholar]
- 26.Xiao X, Li S, Jia X, Guo X, Zhang Q. X-linked heterozygous mutations in ARR3 cause female-limited early onset high myopia. Mol Vis. 2016;22:1257–66. [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang D, Li J, Xiao X, Li S, Jia X, Sun W, Guo X, Zhang Q. Detection of mutations in LRPAP1, CTSH, LEPREL1, ZNF644, SLC39A5, and SCO2 in 298 families with early-onset high myopia by exome sequencing. Invest Ophthalmol Vis Sci. 2014;56:339–45. doi: 10.1167/iovs.14-14850. [DOI] [PubMed] [Google Scholar]
- 28.Kloss BA, Tompson SW, Whisenhunt KN, Quow KL, Huang SJ, Pavelec DM, Rosenberg T, Young TL. Exome Sequence Analysis of 14 Families With High Myopia. Invest Ophthalmol Vis Sci. 2017;58:1982–90. doi: 10.1167/iovs.16-20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tkatchenko TV, Shah RL, Nagasaki T, Tkatchenko AV. Analysis of genetic networks regulating refractive eye development in collaborative cross progenitor strain mice reveals new genes and pathways underlying human myopia. BMC Med Genomics. 2019;12:113. doi: 10.1186/s12920-019-0560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai XB, Zheng YH, Chen DF, Zhou FY, Xia LQ, Wen XR, Yuan YM, Han F, Piao SY, Zhuang W, Lu F, Qu J, Yu AY, Jin ZB. Expanding the Phenotypic and Genotypic Landscape of Nonsyndromic High Myopia: A Cross-Sectional Study in 731 Chinese Patients. Invest Ophthalmol Vis Sci. 2019;60:4052–62. doi: 10.1167/iovs.19-27921. [DOI] [PubMed] [Google Scholar]
- 31.Verhoeven VJ, Buitendijk GH, Rivadeneira F, Uitterlinden AG, Vingerling JR, Hofman A, Klaver CC. Education influences the role of genetics in myopia. Eur J Epidemiol. 2013;28:973–80. doi: 10.1007/s10654-013-9856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tkatchenko AV, Tkatchenko TV, Guggenheim JA, Verhoeven VJ, Hysi PG, Wojciechowski R, Singh PK, Kumar A, Thinakaran G, Williams C. APLP2 Regulates Refractive Error and Myopia Development in Mice and Humans. PLoS Genet. 2015;11:e1005432. doi: 10.1371/journal.pgen.1005432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pozarickij A, Williams C, Hysi PG, Guggenheim JA. Quantile regression analysis reveals widespread evidence for gene-environment or gene-gene interactions in myopia development. Commun Biol. 2019;2:167. doi: 10.1038/s42003-019-0387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanfilippo PG, Hewitt AW, Hammond CJ, Mackey DA. The heritability of ocular traits. Surv Ophthalmol. 2010;55:561–83. doi: 10.1016/j.survophthal.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald R, Barbat-Artigas S, Cho C, Peng H, Shang J, Moustaine A, Carbonetto S, Robitaille R, Chalifour LE, Paudel H. A Novel Egr-1-Agrin Pathway and Potential Implications for Regulation of Synaptic Physiology and Homeostasis at the Neuromuscular Junction. Front Aging Neurosci. 2017;9:258. doi: 10.3389/fnagi.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mann S, Kröger S. Agrin is synthesized by retinal cells and colocalizes with gephyrin. Mol Cell Neurosci. 1996;8:1–13. doi: 10.1006/mcne.1996.0039. corrected. [DOI] [PubMed] [Google Scholar]
- 37.Wojciechowski R. Nature and nurture: the complex genetics of myopia and refractive error. Clin Genet. 2011;79:301–20. doi: 10.1111/j.1399-0004.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maselli RA, Fernandez JM, Arredondo J, Navarro C, Ngo M, Beeson D, Cagney O, Williams DC, Wollmann RL, Yarov-Yarovoy V, Ferns MJ. LG2 agrin mutation causing severe congenital myasthenic syndrome mimics functional characteristics of non-neural (z-) agrin. Hum Genet. 2012;131:1123–35. doi: 10.1007/s00439-011-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanhaesebrouck AE, Beeson D. The congenital myasthenic syndromes: expanding genetic and phenotypic spectrums and refining treatment strategies. Curr Opin Neurol. 2019;32:696–703. doi: 10.1097/WCO.0000000000000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Dai Y, Han JN, Chen ZH, Ling L, Pu CQ, Cui LY, Huang XS. A Novel AGRN Mutation Leads to Congenital Myasthenic Syndrome Only Affecting Limb-girdle Muscle. Chin Med J (Engl) 2017;130:2279–82. doi: 10.4103/0366-6999.215332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoyt CS, Stone RD, Fromer C, Billson FA. Monocular axial myopia associated with neonatal eyelid closure in human infants. Am J Ophthalmol. 1981;91:197–200. doi: 10.1016/0002-9394(81)90173-2. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Wei Q, Le A, Gawargious BA, Demer JL. Rectus Extraocular Muscle Paths and Staphylomata in High Myopia. Am J Ophthalmol. 2019;201:37–45. doi: 10.1016/j.ajo.2019.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pucker AD, Jackson AR, McHugh KM, Mutti DO. Morphological ciliary muscle changes associated with form deprivation-induced myopia. Exp Eye Res. 2020;193:107963. doi: 10.1016/j.exer.2020.107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng CY, Huang XQ, Cheng XW, Wu RH, Lu F, Jin ZB. Mutational screening of SLC39A5, LEPREL1 and LRPAP1 in a cohort of 187 high myopia patients. Sci Rep. 2017;7:1120. doi: 10.1038/s41598-017-01285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hieta R, Kukkola L, Permi P, Pirila P, Kivirikko KI, Kilpelainen I, Myllyharju J. The peptide-substrate-binding domain of human collagen prolyl 4-hydroxylases. Backbone assignments, secondary structure, and binding of proline-rich peptides. J Biol Chem. 2003;278:34966–74. doi: 10.1074/jbc.M303624200. [DOI] [PubMed] [Google Scholar]
- 46.Myllyharju J. Prolyl 4-hydroxylases, key enzymes in the synthesis of collagens and regulation of the response to hypoxia, and their roles as treatment targets. Ann Med. 2008;40:402–17. doi: 10.1080/07853890801986594. [DOI] [PubMed] [Google Scholar]