Abstract
Background:
The major excitatory and inhibitory neurometabolites in the brain, glutamate and γ-aminobutyric acid (GABA), respectively, are related to the functional MRI signal. Disruption of resting-state functional MRI signals has been reported in psychosis spectrum disorders, but few studies have investigated the role of these metabolites in this context.
Methods:
We included 19 patients with first-episode psychosis and 21 healthy controls in this combined magnetic resonance spectroscopy (MRS) and resting-state functional connectivity study. All imaging was performed on a Siemens Magnetom 7 T MRI scanner. Both the MRS voxel and the seed for functional connectivity analysis were located in the dorsal anterior cingulate cortex (ACC). We used multiple regressions to test for an interaction between ACC brain connectivity, diagnosis and neurometabolites.
Results:
ACC brain connectivity was altered in first-episode psychosis. The relationship between ACC glutamate and ACC functional connectivity differed between patients with first-episode psychosis and healthy controls in the precuneus, retrosplenial cortex, supramarginal gyrus and angular gyrus. As well, the relationship between ACC GABA and ACC functional connectivity differed between groups in the caudate, putamen and supramarginal gyrus.
Limitations:
We used a small sample size. As well, although they were not chronically medicated, all participants were medicated during the study.
Conclusion:
We demonstrated a link between the major excitatory and inhibitory brain metabolites and resting-state functional connectivity in healthy participants, as well as an alteration in this relationship in patients with first-episode psychosis. Combining data from different imaging modalities may help our mechanistic understanding of the relationship between major neurometabolites and brain network dynamics, and shed light on the pathophysiology of first-episode psychosis.
Introduction
Schizophrenia is associated with impairments in executive functioning — particularly cognitive control. These deficits are present before illness onset1 and are stable over the course of the illness,2,3 showing marginal improvement with atypical antipsychotic medication.4,5 Cognitive control deficits have been associated with abnormal activity in the frontal, parietal and subcortical brain regions.6 A core region of the cognitive control network is the anterior cingulate cortex (ACC),7 which has consistently demonstrated altered functional activation during cognitive control tasks in schizophrenia.6,8–12
Functional connectivity measures the temporal coherence of spontaneous neural activity between distinct brain regions. 13 Resting-state functional MRI (fMRI) is a reliable method of assessing functional connectivity,13 and because it does not require participants to perform a task, it is especially useful in patients with schizophrenia, who typically differ in task performance from healthy controls.14–16 Functional connectivity has consistently been used to identify large-scale cognitive networks that interact in a well-characterized manner, including the executive control, salience and default mode networks.17,18 A balance between the activation of task-positive networks (salience, executive control) and deactivation of the default mode network appears to be necessary for cognitive functioning.19 Alterations in large-scale networks and alterations in the relationships between networks have consistently been reported in schizophrenia.20–24
Abnormalities in the excitation/inhibition balance as a result of N-methyl-D-aspartate receptor hypofunction on γ-aminobutyric acidergic (GABA) interneurons are implicated in the pathophysiology of schizophrenia.25–31 Because of their role in neuroenergetics, brain measurements of glutamate and GABA have been related to the fMRI signal.32,33 In healthy participants, GABA concentrations have been correlated with stimulus-induced activity in the measured brain region, and glutamate has been correlated with brain activity at rest and during tasks.34,35
A recent systematic review found converging evidence of negative associations between GABA levels and local brain activity; positive associations between glutamate levels and distal brain activity (brain activity outside the spectroscopy voxel); and less consistent relationships between GABA levels and distal brain activity, as well as between glutamate levels and local brain activity.36 Only a few studies have evaluated the relationships between glutamate11,37,38 or GABA17 and the blood-oxygen-level-dependent (BOLD) response in schizophrenia.39 In patients with first-episode psychosis11 and unmedicated patients with chronic schizophrenia37 compared to healthy controls, we reported an abnormal relationship between ACC glutamate and the BOLD signal in regions of the posterior default mode network during a cognitive inhibitory task. Similar findings have been reported by Falkenberg and colleagues,38 also during an inhibitory task and measurement of glutamate in the ACC. As well, in patients with first-episode psychosis compared to healthy controls, we reported an abnormal relationship between ACC GABA and the BOLD signal in the ACC and the caudate during an inhibitory task.11 Similarly, Limongi and colleagues40,41 reported aberrant resting-state effective connectivity in core nodes of the salience network associated with ACC glutamate in patients with first-episode psychosis and found that inhibitory activity in the ACC decreased with higher glutamate levels in this population. Combining magnetic resonance spectroscopy (MRS) with fMRI to evaluate the extent to which glutamate and GABA measurements predict resting-state functional connectivity might mechanistically shed light onto how cognitive processes are altered in schizophrenia.
In the present study, we combined MRS and fMRI to evaluate the relationship between neurometabolites and ACC functional connectivity in a group of patients with first-episode psychosis and matched healthy controls. Our patient population allowed us to study the neural correlates of psychosis without the confounds of illness chronicity and long-term exposure to antipsychotic medication. Because magnets with higher field strength provide a better signal-to-noise ratio and spectral resolution, we used a 7 T magnet to measure glutamate and GABA from a dorsal ACC voxel, as well as the functional connectivity from the same ACC region of interest to the rest of the brain. In the same participants, we previously reported that glutamate (but not GABA) was significantly lower in patients with first-episode psychosis than healthy controls,42,43 and we have discussed alterations between these neurotransmitters and the BOLD signal during an inhibitory task.11
Methods
Participants
Twenty-three patients with first-episode psychosis, determined by a clinician to be clinically stable, were recruited from the University of Alabama at Birmingham’s outpatient psychiatric clinics (for further clinical information on the sample, see Reid and colleagues42). Twenty-six healthy controls were recruited using flyers. Exclusion criteria were major medical conditions; substance abuse within 6 months of imaging; neurologic disorders; a previous serious head injury with a loss of consciousness for more than 2 minutes; and pregnancy. Diagnoses were established by a psychiatrist and confirmed with a review of patient medical records. We used the Brief Psychiatric Rating Scale to assess symptom severity.44 We characterized cognitive function using the Repeatable Battery for the Assessment of Neuropsychological Status.45 The institutional review boards of the University of Alabama at Birmingham and Auburn University approved this study. All participants gave written informed consent to participate.
We did not obtain both fMRI and MRS data for 4 patients with first-episode psychosis (claustrophobia, participant weight exceeded the maximum for the scanner, study withdrawal, poor image quality) and 5 healthy controls (failed drug screen, magnetic resonance contraindication, loss to follow-up). Therefore, 19 first-episode psychosis and 21 healthy controls were included in the fMRI and MRS analyses.
Image acquisition and preprocessing
Imaging was performed on a whole-body Magnetom 7 T magnetic resonance scanner (Siemens Healthineers) equipped with a 32-channel head coil (Nova Medical) at the Auburn University MRI Research Center. We acquired a high-resolution structural scan for anatomic reference using a 3-dimensional, T1-weighted, magnetization-prepared rapid gradient echo sequence (repetition time 2200 ms, echo time 2.96 ms, inversion time 1050 ms, flip angle 7°, generalized autocalibrating partial parallel acquisition (GRAPPA) acceleration factor 2, field of view 224 × 224 mm, 0.7 mm isotropic voxels; sagittal acquisition). We used the anatomic scan to guide placement of the spectroscopy voxel in the bilateral dorsal ACC (dACC; voxel size 2.7 × 2 × 1 cm3). After shimming with the fast, automatic shim technique using echoplanar signal readout for mapping along projections (FAST-ESTMAP) and optimization of the radiofrequency power, we acquired spectra using a stimulated echo acquisition mode (STEAM) sequence with an ultra-short echo time (repetition time 10 000 ms, echo time 5 ms, mixing time 45 ms, 32 averages, 4 kHz spectral bandwidth, 2048 points), outer volume suppression and variable power radiofrequency pulses and optimized relaxation delays (VAPOUR) water suppression.46 During the MRS scan, participants watched a movie or listened to music. We processed MRS data in LCModel (version 6.3–1)47 using a simulated basis set and default processing parameters (for additional details on methods and MRS metabolite findings, see Reid and colleagues42).
We acquired resting-state fMRI data using a gradient-recalled echo-planar imaging sequence (repetition time 3000 ms, echo time 28 ms, flip angle 70°, field of view 200 × 200 mm, matrix 234 × 234, 1.8 mm slice thickness, voxel size 0.85 × 0.85 × 1.8 mm, 1 mm gap, 37 axial slices, 120 acquisitions per session). We acquired a second anatomic scan before fMRI for coregistration of the functional images (repetition time 2000 ms, echo time 2.89 ms, inversion time 1050 ms, flip angle 7°, GRAPPA acceleration factor 2, field of view 190 × 190 mm, 0.7 mm isotropic voxels).
We performed preprocessing and data analyses in the CONN Toolbox.48 Data preprocessing included realignment, unwarping, slice time correction, coregistration, normalization to Montreal Neurological Institute space, artifact detection, Gaussian smoothing (5 mm full width at half-maximum), denoising, scrubbing and motion regression. Volumes were scrubbed if frame-wise displacement exceeded 0.5 mm or we found a z-score change greater than 3. We did not perform global signal regression.
Statistical analyses
We created a standardized dACC region of interest that corresponded to the MRS voxel location in SPM12 (www.fil.ion.ucl.ac.uk/spm/software/spm12). We calculated the percentage of each participant’s normalized MRS mask that was within the region of interest (mean ± standard deviation = 88.5 ± 10.5%). Using our MRS dACC region of interest and CONN, we performed positive and negative whole-brain dACC connectivity analyses for each group, and between-group analyses in each direction (first-episode psychosis > healthy controls; healthy controls > first-episode psychosis). We used multiple regression analyses to test for voxels with a significant relationship between dACC brain functional connectivity and glutamate and GABA (similar to the BOLD Stroop effect analyses in Overbeek and colleagues11). We conducted whole-brain tests for positive and negative relationships between each neurometabolite and dACC brain connectivity for each group. We used additional multiple regressions to test for an interaction between dACC brain connectivity, diagnosis and each of the neurometabolites. We conducted tests for differences in both directions (first-episode psychosis > healthy controls; healthy controls > first-episode psychosis). We included white matter fraction within the MRS voxel as a covariate of no interest.
We used a cluster-defining voxel-level t threshold of p < 0.01 for within-group dACC brain connectivity analyses. We made cluster-level corrections for multiple comparisons using a false discovery rate (FDR) threshold of pFDR < 0.01. For all other analyses (between-group connectivity analyses; connectivity and neurochemical analyses), both the cluster-defining and FDR thresholds were raised to 0.05.
Results
Demographic information for the participant sample can be found in Table 1.
Table 1.
Participant demographic characteristics
| Measure* | Healthy controls n = 21 |
Patients with first-episode psychosis n = 19 |
Statistic | p value |
|---|---|---|---|---|
| Age, yr | 23.4 ± 4.4 | 22.9 ± 4.4 | t38 = 0.38 | 0.71 |
| Female/male | 5/16 | 4/15 | χ21 = 0.04 | 0.83 |
| Smoker, yes/no | 0/21 | 4/15 | χ21 = 4.91 | 0.027¶ |
| Parental socioeconomic status† | 3.4 ± 3.3 | 4.6 ± 4.5 | t37 = 0.99 | 0.33 |
| RBANS score‡ | 95.1 ± 8.5 | 75.0 ± 15.6 | t33 = 4.84 | < 0.001¶ |
| Treatment duration, d | — | 358 ± 470 | — | — |
| BPRS rating§ | ||||
| Total | 20.8 ± 1.1 | 30.4 ± 8.4 | t33 = 4.79 | < 0.001¶ |
| Positive | 3.0 ± 0.2 | 4.8 ± 2.7 | t33 = 2.65 | 0.012¶ |
| Negative | 3.0 ± 2.4 | 5.6 ± 2.3 | t33 = 4.72 | < 0.001¶ |
BPRS = Brief Psychiatric Rating Scale; RBANS = Repeatable Battery for Assessment of Neuropsychological Status.
Values are presented as mean ± standard deviation or n.
Not available for 1 patient with first-episode psychosis. Ranks determined from the Diagnostic Interview for Genetic Studies (scale of 1–18); higher rank (lower numerical value) corresponds with higher socioeconomic status.
Not available for 1 healthy control and 3 patients with first-episode psychosis.
Not available for 2 healthy controls and 2 patients with first-episode psychosis. Positive subscale: conceptual disorganization, hallucinatory behaviour and unusual thought content. Negative subscale: emotional withdrawal, motor retardation and blunted affect.
Significant at p < 0.05.
ACC brain connectivity
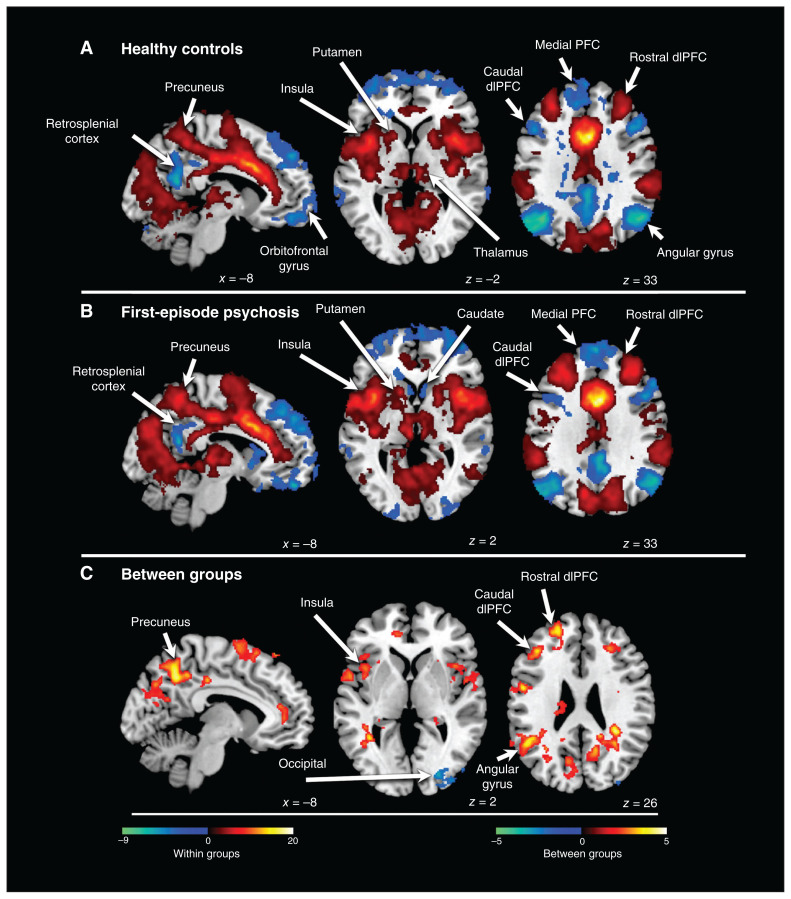
Brain connectivity in the dACC was similar for patients and controls. Positive dACC brain connectivity tracked the salience network (pFDR < 0.01): the dorsolateral prefrontal cortex (dlPFC), supplementary motor area, supramarginal gyrus and insula (Figure 1A and B). Robust positive connectivity with the Rolandic operculum was also evident. Negative connectivity tracked the default mode network (pFDR < 0.01): the medial prefrontal cortex, orbitofrontal cortex, retrosplenial cortex, inferior temporal lobe and angular gyrus. Negative (as opposed to positive) connectivity tracked the central executive network. This was apparent in the dlPFC and superior parietal lobe. In the dlPFC, the rostral portion aligned with the salience network and positively connected to the dACC; the caudal portion aligned with the central executive network and was negatively connected to the dACC. Negative connectivity tracked the default mode network in the inferior parietal lobe (angular gyrus) but extended superiorly into regions associated with the central executive network. We also found disparate connectivity in the precuneus. Superior regions showed positive connectivity, and the retrosplenial cortex showed negative connectivity. We also found significant dACC positive connectivity with the thalamus and putamen. Finally, in patients with first-episode psychosis (but not healthy controls) we found negative connectivity with the ventral caudate.
Figure 1.
Connectivity of the dorsal anterior cingulate cortex. (A) Connectivity patterns in healthy controls. Positive connectivity is depicted with a red colour scale, and negative connectivity is depicted with a blue colour scale. (B) Connectivity patterns in patients with first-episode psychosis. Positive connectivity is depicted with a red colour scale, and negative connectivity is depicted with a blue colour scale. (C) Significant group differences in brain connectivity patterns in the anterior cingulate cortex. Greater connectivity in patients versus controls is depicted with a red colour scale, and greater connectivity in controls versus patients is depicted with a blue colour scale. dlPFC = dorsolateral prefrontal cortex; PFC = prefrontal cortex.
Between-group analysis revealed a pattern of hyperconnectivity in patients with first-episode psychosis (pFDR < 0.05, Figure 1C). Significant regions extended to areas of both positive and negative dACC brain connectivity: the rostral and caudal dlPFC, supplementary motor area, angular gyrus, insula and superior precuneus. However, in the dlPFC, precuneus and inferior parietal lobe, significant regions tended to show a preference for areas between regions of positive and negative dACC brain connectivity. In the analysis of healthy controls > first-episode psychosis, 1 small cluster in the occipital lobe was significant (pFDR < 0.05).
ACC brain connectivity and glutamate
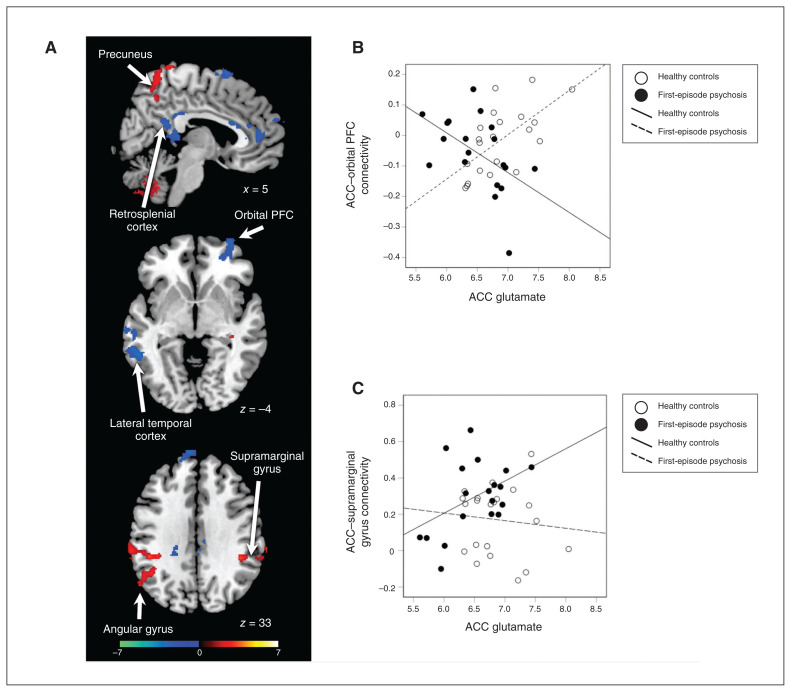
In general, glutamate correlated with ACC brain connectivity strength regardless of whether the connectivity was positive or negative. In healthy controls, significant positive regions included the bilateral supramarginal gyrus (pFDR < 0.05, Appendix 1, Figure S1, available at jpn.ca). Significant negative regions included the left angular gyrus and the left inferior temporal lobe (pFDR < 0.05). In patients with first-episode psychosis, positive regions included the superior precuneus, the bilateral supramarginal gyrus and the bilateral insula (pFDR < 0.05, Appendix 1, Figure S2). Negative regions included the medial prefrontal cortex, the left orbitofrontal cortex, the retrosplenial cortex, the left temporal lobe and the right angular gyrus (pFDR < 0.05).
We observed interaction effects in several areas. In the bilateral supramarginal gyrus, superior precuneus and left angular gyrus, the relationship was significantly more positive in patients with first-episode psychosis (pFDR < 0.05, Figure 2). In the supplemental motor area, medial prefrontal cortex, right orbitofrontal cortex, retrosplenial cortex and temporal cortex, the relationship was more positive in healthy controls (pFDR < 0.05, Figure 2).
Figure 2.
Group differences in the relationship between glutamate levels and dorsal ACC connectivity. (A) Significant group differences in brain connectivity patterns in the ACC. Greater connectivity in patients versus controls is depicted with a red colour scale, and greater connectivity in controls versus patients is depicted with a blue colour scale. (B) Scatterplot of ACC–orbital PFC functional connectivity versus ACC glutamate for individual healthy controls (white circles) and patients with first-episode psychosis (black circles). (C) Scatterplot of ACC–supramarginal gyrus functional connectivity versus ACC glutamate for individual healthy controls (white circles) and patients with first-episode psychosis (black circles). ACC = anterior cingulate cortex; PFC = prefrontal cortex.
ACC brain connectivity and GABA
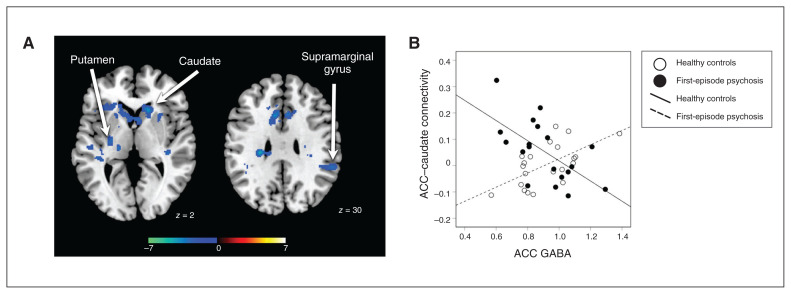
The relationship between GABA and ACC brain connectivity was less consistent between groups than the relationship of glutamate and dACC brain connectivity. In healthy controls, we found a significant positive relationship between local GABA and dACC brain connectivity in the medial and orbital prefrontal cortex and the caudate (pFDR < 0.05, Appendix 1, Figure S3). We found a negative relationship in the left temporal cortex and occipital regions. In patients with first-episode psychosis, we found a significant positive relationship with the retrosplenial cortex and a bilateral negative relationship with the caudate, insula, Rolandic operculum, supramarginal gyrus and calcarine (pFDR < 0.05, Appendix 1, Figure S4). We also found a positive relationship with the right orbital frontal cortex extending into the inferior frontal lobe, a region that was not significant in the dACC brain connectivity analysis of healthy controls but did have significant negative connectivity with the dACC in patients with first-episode psychosis.
Interaction effects revealed that dACC brain connectivity with the caudate, putamen and right supramarginal gyrus was more negative in patients than healthy controls (pFDR < 0.05, Figure 3). We found no significant regions that had a significantly more negative relationship in healthy controls than in patients with first-episode psychosis.
Figure 3.
Group differences in the relationship between GABA levels and dorsal ACC connectivity. (A) Significant group differences in ACC brain connectivity patterns. Greater connectivity in controls versus patients is depicted with a blue colour scale. (B) Scatterplot of ACC–caudate functional connectivity versus ACC GABA for individual healthy controls (white circles) and patients with first-episode psychosis (black circles). ACC = anterior cingulate cortex; GABA = γ-aminobutyric acid.
Discussion
In first-episode psychosis, we found increased resting-state ACC functional connectivity to several brain regions, including the insula, dlPFC, angular gyrus and precuneus. The additional MRS data helped us to mechanistically understand the relationship between excitation/inhibition in the ACC and brain network dynamics. ACC glutamate predicted ACC functional connectivity differently in patients with first-episode psychosis than in healthy controls in the precuneus, retrosplenial cortex, supramarginal gyrus and angular gyrus. In addition, ACC GABA predicted ACC functional connectivity differently in patients with first-episode psychosis compared to healthy controls in the caudate, putamen and supramarginal gyrus.
In both healthy controls and patients with first-episode psychosis, we found patterns of positive functional connectivity with regions belonging to the task-positive networks (such as the insula and dlPFC), as well as patterns of negative functional connectivity with regions belonging to the default mode network (such as the medial PFC, angular gyrus and retrosplenial cortex). These ACC functional connectivity patterns seen in the resting state mapped to well-characterized anticorrelation patterns between the task-positive and default mode networks.49 The results of between-group analysis showed a mix of increased functional connectivity to the task-positive network and decreased negative functional connectivity (appearing as a positive association in the analysis) with regions of the default mode network in patients with first-episode psychosis compared to healthy controls. In the same participants during performance of the Stroop task, we demonstrated increased BOLD signal in patients with first-episode psychosis compared to healthy controls, including in the dlPFC, posterior cingulate cortex and parietal regions, and a lack of deactivation of regions of the default mode network. 11 Together, these results demonstrate that between-group differences in functional connectivity map to known positive and default mode networks and point to an altered balance in network dynamics.
We found that ACC glutamate differentially predicted ACC functional connectivity to regions of the task-positive (bilateral supramarginal gyrus, angular gyrus, supplemental motor area and temporal cortex) and default mode network (precuneus, medial prefrontal, orbitofrontal and retrosplenial cortex) in patients with first-episode psychosis versus healthy controls. Likewise, in the same participants and in replication of results from Falkenberg and colleagues,50 we found that the relationship between ACC glutamate and the BOLD response during the Stroop task in regions of the posterior default mode network was opposite in patients with first-episode psychosis and healthy controls.11 Thus, ACC glutamate appears to modulate both task-positive and default mode networks, but does so in opposite directions in patients with first-episode psychosis and healthy controls. This study adds to our understanding of altered balance between task-positive and default mode networks in schizophrenia by demonstrating that ACC glutamate, presumably through an excitatory mechanism, is affecting the modulation of large neural networks. These alterations were obtained in the context of decreased glutamate levels in patients with first-episode psychosis compared to healthy controls.42
We found that ACC GABA differentially predicted ACC functional connectivity to the caudate, putamen and supramarginal gyrus in patients with first-episode psychosis compared to healthy controls. Likewise, we reported that in the same participants, ACC GABA differentially predicted the BOLD signal in the caudate during the Stroop task; higher GABA was associated with higher caudate activation in patients with first-episode psychosis, but not in healthy controls.11 In the present study, GABA was not significantly different in patients with first-episode psychosis and healthy controls.42 However, at the behavioural level, we found that in patients with first-episode psychosis, higher GABA levels were associated with slower reaction time on the Stroop task11 and worse scores on the Repeatable Battery for the Assessment of Neuropsychological Status,42 suggesting a relationship between GABA and cognition, in agreement with the findings of Marsman and colleagues,51 who identified a negative correlation between medial prefrontal GABA and total IQ in patients with schizophrenia. Dysfunction of frontostriatal networks has been widely reported in schizophrenia and has been linked to cognitive, motor and reward dysfunction.52–54 This study adds to our understanding of frontostriatal dysfunction in schizophrenia by demonstrating that GABA in the ACC, presumably through an inhibitory mechanism, alters the functional relationship between the ACC and the striatum.
Postmortem studies have found evidence of both glutamatergic55 and GABAergic alterations56 in the ACC in schizophrenia. Roberts and colleagues55 found a significant decrease in the number of excitatory synaptic connections, as well as a decrease in the levels of the vesicular glutamate transporter vGLUT1. Because the amount of vGLUT1 is directly related to quantal release of glutamate, it represents a good measure of glutamate levels.57
It is increasingly clear that the functional connectivity of different brain regions is crucial for cognitive function. Presumably, this functional connectivity must be created, maintained and adjusted by active cortical processes, and we should not be surprised that there is a link between the local neurochemistry of a cortical region as determined by MRS spectroscopy and its functional connectivity to other areas, although previous studies have not shown a clear, simple relationship.58
There is no current widely held consensus about the specific neuronal circuits in the cerebral cortex that create and adjust functional connectivity between regions. It should be noted that even something like excitotoxicity59 could in principle create both hypo- and hyperconnectivity, depending on the specifics of the connections between any 2 specific regions. At the simplest level, hypoconnectivity could be the result of generalized decline of activity in 1 region, and hyperconnectivity could be the result of a release phenomenon (i.e., reduction of effective inhibition). It has been proposed that an excitatory/inhibitory imbalance is important for the dysregulation of functional connectivity in schizophrenia,11,25–28 but a definitive answer to the question about what specifically alters patterns of functional connectivity in schizophrenia will require further advances in our basic knowledge of the mechanistic basis of functional connectivity.
Limitations
In this study, we recruited patients with first-episode psychosis to mitigate the confounds of illness chronicity and long-term exposure to antipsychotic medication. However, it needs to be noted that all patients were treated with antipsychotic medications, which may affect metabolite levels60,61 (possibly even in a dose-related fashion62) and resting-state connectivity.20,23,63–65 It will be important for future studies to quantify current and lifetime antipsychotic medication exposure to allow for additional analyses that assess potential antipsychotic confounds. For spectroscopy, we used a STEAM sequence not optimized for GABA measurements, although this sequence was used by Wijtenburg and colleagues66 at 7 T using a larger voxel. Both GABA measurements had a large range; further MRS studies aimed at ascertaining normative metabolite levels and ranges will be needed. As well, the metabolite peaks quantified here do not reflect synaptic glutamate alone but are a combination of neuronal, glial and synaptic glutamate and GABA in the voxel. It is therefore not possible to equate metabolite measurements with neurotransmission. Because of time constraints in the scanning protocol, and because there is no brain region that is unequivocally unaffected in schizophrenia, we did not include a control region MRS voxel. We did not perform global signal regression for resting-state data, because the global signal may not be unrelated to neural activity of interest67 and there is evidence that the global signal may carry diagnostic information.68 In addition, it needs to be noted that our sample size was modest, and replication of our findings in larger samples is warranted, because correlations may only stabilize at larger sample size.69 Further studies combining fMRI with functional MRS might provide a more fine-grained understanding of the link between metabolites and cognitive processes.
Conclusion
The finding here of a different pattern of correlation between local cortical neurochemistry and function connectivity to other regions in patients with first-episode psychosis and healthy controls suggests a fundamental alteration in the process by which functional connectivity is regulated in patients with first-episode psychosis and is a new approach to studying the physical basis of psychosis.
Supplementary Material
Acknowledgements
This work was supported by the UAB School of Medicine Imaging Steering Committee; the UAB Comprehensive Neuroscience Center; the Auburn University MRI Research Center; and the National Institutes of Health (grant number R01MH102951 to A.C.L.).
Footnotes
Competing interests: M. Reid reports support from the National Institute of Mental Health under award number K01MH115272. N. Kraguljac reports membership on a medical advisory board for Neurocrine Biosciences Inc. and grant funding from the National Institute of Mental Health. A. Lahti reports grant funding from National Eye Institute grant RO1MH102951. No other competing interests were declared.
Contributors: G. Overbeek and A. Lahti designed the study. M. Reid and A. Lahti acquired the data, which all authors analyzed. G. Overbeek, T. Gawne, N. Kraguljac and A. Lahti wrote the article, which M. Reid, N. Kraguljac and A. Lahti reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Wood SJ, Pantelis C, Proffitt T, et al. Spatial working memory ability is a marker of risk-for-psychosis. Psychol Med 2003;33:1239–47. [DOI] [PubMed] [Google Scholar]
- 2.Burdick KE, Goldberg JF, Harrow M, et al. Neurocognition as a stable endophenotype in bipolar disorder and schizophrenia. J Nerv Ment Dis 2006;194:255–60. [DOI] [PubMed] [Google Scholar]
- 3.Tyson PJ, Laws KR, Roberts KH, et al. Stability of set-shifting and planning abilities in patients with schizophrenia. Psychiatry Res 2004;129:229–39. [DOI] [PubMed] [Google Scholar]
- 4.Woodward ND, Purdon SE, Meltzer HY, et al. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol 2005;8: 457–72. [DOI] [PubMed] [Google Scholar]
- 5.Keefe RS, Seidman LJ, Christensen BK, et al. Long-term neurocognitive effects of olanzapine or low-dose haloperidol in first-episode psychosis. Biol Psychiatry 2006;59:97–105. [DOI] [PubMed] [Google Scholar]
- 6.Minzenberg MJ, Laird AR, Thelen S, et al. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 2009;66:811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci 2009;12:939–45. [DOI] [PubMed] [Google Scholar]
- 8.Kerns JG, Cohen JD, MacDonald AW, et al. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry 2005;162:1833–9. [DOI] [PubMed] [Google Scholar]
- 9.Weiss EM, Seidentopf C, Golaszewski S, et al. Brain activation patterns during a selective attention test–a functional MRI study in healthy volunteers and unmedicated patients during an acute episode of schizophrenia. Psychiatry Res 2007;154:31–40. [DOI] [PubMed] [Google Scholar]
- 10.Reid MA, Stoeckel LE, White DM, et al. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol Psychiatry 2010;68:625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Overbeek G, Gawne TJ, Reid MA, et al. Relationship between cortical excitation and inhibition and task-induced activation and deactivation: a combined magnetic resonance spectroscopy and functional magnetic resonance imaging study at 7T in first-episode psychosis. Biol Psychiatry Cogn Neurosci Neuroimaging 2019;4:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebisch SJH, Gallese V, Salone A, et al. Disrupted relationship between “resting state” connectivity and task-evoked activity during social perception in schizophrenia. Schizophr Res 2018;193:370–6. [DOI] [PubMed] [Google Scholar]
- 13.Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995;34:537–41. [DOI] [PubMed] [Google Scholar]
- 14.Gurler D, White DM, Kraguljac N, et al. Neural signatures of memory encoding in schizophrenia are modulated by antipsychotic treatment. Neuropsychobiology 2021;80:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraguljac NV, Carle M, et al. Mnemonic discrimination deficits in first-episode psychosis and a ketamine model suggests dentate gyrus pathology linked to N-methyl-D-aspartate receptor hypofunction. Biol Psychiatry Cogn Neurosci Neuroimaging 2018;3:231–8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Hutcheson NL, Reid MA, White DM, et al. Multimodal analysis of the hippocampus in schizophrenia using proton magnetic resonance spectroscopy and functional magnetic resonance imaging. Schizophr Res 2012;140:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27:2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A 2001;98:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weissman DH, Roberts KC, Visscher KM, et al. The neural bases of momentary lapses in attention. Nat Neurosci 2006;9:971–8. [DOI] [PubMed] [Google Scholar]
- 20.Kraguljac NV, White DM, Hadley JA, et al. Abnormalities in large scale functional networks in unmedicated patients with schizophrenia and effects of risperidone. Neuroimage Clin 2015;10:146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maximo JO, Nelson EA, Armstrong WP, et al. Duration of untreated psychosis correlates with brain connectivity and morphology in medication-naive patients with first-episode psychosis. Biol Psychiatry Cogn Neurosci Neuroimaging 2020;5:231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lottman KK, Gawne TJ, Kraguljac NV, et al. Examining resting-state functional connectivity in first-episode schizophrenia with 7 T fMRI and MEG. Neuroimage Clin 2019;24:101959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lottman KK, Kraguljac NV, White DM, et al. Risperidone effects on brain dynamic connectivity—a prospective resting-state fMRI study in Schizophrenia. Front Psychiatry 2017;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 2012;8:49–76. [DOI] [PubMed] [Google Scholar]
- 25.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 2012;37:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 1995;52:998–1007. [DOI] [PubMed] [Google Scholar]
- 27.Javitt DC. Twenty-five years of glutamate in schizophrenia: are we there yet? Schizophr Bull 2012;38:911–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraguljac NV, Frölich MA, Tran S, et al. Ketamine modulates hippocampal neurochemistry and functional connectivity: a combined magnetic resonance spectroscopy and resting-state fMRI study in healthy volunteers. Mol Psychiatry 2017;22:562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wengler K, Goldberg AT, Chahine G, et al. Distinct hierarchical alterations of intrinsic neural timescales account for different manifestations of psychosis. eLife 2020;9::e56151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang GJ, Murray JD, Wang XJ, et al. Functional hierarchy underlies preferential connectivity disturbances in schizophrenia. Proc Natl Acad Sci U S A 2016;113:E219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraguljac NV, McDonald WM, Widge AS, et al. Neuroimaging biomarkers in schizophrenia. Am J Psychiatry 2021;178:509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shulman RG, Hyder F, Rothman DL. Insights from neuroenergetics into the interpretation of functional neuroimaging: an alternative empirical model for studying the brain’s support of behavior. J Cereb Blood Flow Metab 2014;34:1721–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh KD. Which “neural activity” do you mean? fMRI, MEG, oscillations and neurotransmitters. Neuroimage 2012;62:1121–30. [DOI] [PubMed] [Google Scholar]
- 34.Enzi B, Duncan NW, Kaufmann J, et al. Glutamate modulates resting state activity in the perigenual anterior cingulate cortex — a combined fMRI-MRS study. Neuroscience 2012;227:102–9. [DOI] [PubMed] [Google Scholar]
- 35.Duncan NW, Wiebking C, Northoff G. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans—a review of multimodal imaging studies. Neurosci Biobehav Rev 2014;47:36–52. [DOI] [PubMed] [Google Scholar]
- 36.Kiemes A, Davies C, Kempton MJ, et al. GABA, glutamate and neural activity: a systematic review with meta-analysis of multimodal (1)H-MRS-fMRI studies. Front Psychiatry 2021;12:644315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cadena EJ, White DM, Kraguljac NV, et al. A longitudinal multimodal neuroimaging study to examine relationships between resting state glutamate and task related BOLD response in schizophrenia. Front Psychiatry 2018;9:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falkenberg LE, Westerhausen R, Specht K, et al. Resting-state glutamate level in the anterior cingulate predicts blood-oxygen level-dependent response to cognitive control. Proc Natl Acad Sci U S A 2012;109:5069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy-Thootkur M, Kraguljac NV, Lahti AC. The role of glutamate and GABA in cognitive dysfunction in schizophrenia and mood disorders—a systematic review of magnetic resonance spectroscopy studies. Schizophr Res 2020; S0920-9964(20)30077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Limongi R, Jeon P, Mackinley M, et al. Glutamate and dysconnection in the salience network: neurochemical, effective connectivity, and computational evidence in schizophrenia. Biol Psychiatry 2020;88: 273–81. [DOI] [PubMed] [Google Scholar]
- 41.Limongi R, Jeon P, Théberge J, et al. Counteracting effects of glutathione on the glutamate-driven excitation/inhibition imbalance in first-episode schizophrenia: a 7 T MRS and dynamic causal modeling study. Antioxidants 2021;10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reid MA, Salibi N, White DM, et al. 7 T proton magnetic resonance spectroscopy of the anterior cingulate cortex in first-episode schizophrenia. Schizophr Bull 2019;45:180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gawne TJ, Overbeek GJ, Killen JF, et al. A multimodal magneto-encephalography 7 T fMRI and 7 T proton MR spectroscopy study in first episode psychosis. NPJ Schizophr 2020;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep 1962;10:799–812. [Google Scholar]
- 45.Randolph C, Tierney MC, Mohr E, et al. The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 1998;20:310–9. [DOI] [PubMed] [Google Scholar]
- 46.Tkac I, Oz G, Adriany G, et al. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med 2009;62:868–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672–9. [DOI] [PubMed] [Google Scholar]
- 48.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012;2:125–41. [DOI] [PubMed] [Google Scholar]
- 49.Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 2005;102:9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Falkenberg LE, Westerhausen R, Craven AR, et al. Impact of glutamate levels on neural response and cognitive abilities in schizophrenia. Neuroimage Clin 2014;4: 576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marsman A, Mandl RCW, Klomp DWJ, et al. GABA and glutamate in schizophrenia: a 7 T (1)H-MRS study. Neuroimage Clin 2014;6:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cadena EJ, White DM, Kraguljac NV, et al. Evaluation of frontostriatal networks during cognitive control in unmedicated patients with schizophrenia and the effect of antipsychotic medication. NPJ Schizophr 2018;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarpal DK, Argyelen M, Robinson DG, et al. Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am J Psychiatry 2016;173:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarpal DK, Robinson DG, Lencz T, et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry 2015;72:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts RC, McCollum LA, Schoonover KE, et al. Ultrastructural evidence for glutamatergic dysregulation in schizophrenia. Schizophr Res 2020;S0920-9964(20)30032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benes FM. Altered glutamatergic and GABAergic mechanisms in the cingulate cortex of the schizophrenic brain. Arch Gen Psychiatry 1995;52:1015–8; discussion 1019–24. [DOI] [PubMed] [Google Scholar]
- 57.Wilson NR, Kang J, Hueske EV, et al. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci 2005;25:6221–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonen OM, Moffat BA, Kwan P, et al. Resting-state functional connectivity and quantitation of glutamate and GABA of the PCC/precuneus by magnetic resonance spectroscopy at 7T in healthy individuals. PLoS One 2020;15:e0244491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park MTM, Jeon P, Khan AR, et al. Hippocampal neuroanatomy in first episode psychosis: a putative role for glutamate and serotonin receptors. Prog Neuropsychopharmacol Biol Psychiatry 2021;110:110297. [DOI] [PubMed] [Google Scholar]
- 60.de la Fuente-Sandoval C, León-Ortiz P, Azcárraga M, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry 2013;70:1057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kraguljac NV, Morgan CJ, Reid MA, et al. A longitudinal magnetic resonance spectroscopy study investigating effects of risperidone in the anterior cingulate cortex and hippocampus in schizophrenia. Schizophr Res 2019;210:239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Merritt K, McGuire PK, Egerton A, et al. Association of age, antipsychotic medication, and symptom severity in schizophrenia with proton magnetic resonance spectroscopy brain glutamate level: a mega-analysis of individual participant-level data. JAMA Psychiatry 2021;78:667–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hadley JA, Nenert R, Kraguljac NV, et al. Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology 2014;39:1020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kraguljac NV, White DM, Hadley N, et al. Aberrant hippocampal connectivity in unmedicated patients with schizophrenia and effects of antipsychotic medication: a longitudinal resting state functional MRI study. Schizophr Bull 2016;42:1046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hadley JA, Kraguljac NV, White DM, et al. Change in brain network topology as a function of treatment response in schizophrenia: a longitudinal resting-state fMRI study using graph theory. NPJ Schizophr 2016;2:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wijtenburg SA, Rowland LM, Edden RAE, et al. Reproducibility of brain spectroscopy at 7T using conventional localization and spectral editing techniques. J Magn Reson Imaging 2013;38:460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu TT, Nalci A, Falahpour M. The global signal in fMRI: Nuisance or information? Neuroimage 2017;150:213–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang GJ, Murray JD, Repovs G, et al. Altered global brain signal in schizophrenia. Proc Natl Acad Sci U S A 2014;111:7438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schonbrodt FD, Perugini M. At what sample size do correlations stabilize? J Res Pers 2013;47:609–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.