Abstract
Zinc, an essential micronutrient in the human body, is a component in over 300 enzymes and participates in regulating enzymatic activity. Zinc metalloenzymes play a crucial role in physiological processes including antioxidant, anti-inflammatory, and immune responses, as well as apoptosis. Aberrant enzyme activity can lead to various human diseases. In this review, we summarize zinc homeostasis, the roles of zinc in zinc metalloenzymes, the physiological processes of zinc metalloenzymes, and aberrant zinc metalloenzymes in human diseases. In addition, potential mechanisms of action are also discussed. This comprehensive understanding of the mechanisms of action of the regulatory functions of zinc in enzyme activity could inform novel zinc-micronutrient-supply strategies for the treatment of diseases.
Keywords: zinc, zinc metalloenzyme, enzyme activity, human disease
1. Introduction
Zinc (Zn2+) is one of the essential trace elements in the human body and plays an extremely important role in physiological processes and pathological states. In the early 20th century, the importance of Zn2+ in human nutrition was controversial. Although some scholars believed that Zn2+ was an indispensable nutritional element for higher animals, there was no experimental proof at that time. In 1934, Todd et al. found that Zn2+ was essential for the development and health of rats [1]. In 1963, Prasad et al. were the first to demonstrate Zn2+ deficiency in the human body and found a relationship between dwarfism and Zn2+ deficiency, which initiated the human study of Zn2+ [2]. Since then, scientists have focused on the role of Zn2+ in human physiology, and various studies on Zn2+ have emerged.
As the second most abundant trace element in the human body, Zn2+ is responsible for the structure and catalytic activity of more than 300 enzymes [3]. The content of Zn2+ in the human body is generally 2–3 grams, less than 50 mg/kg [4]; ninety percent of Zn2+ is found in human muscles and bones [5]. Zn2+ performs essential functions in the human body, mainly by affecting the compositions of enzymes and proteins. First, Zn2+ participates in antioxidant processes to inhibit oxidative stress. Superoxide dismutase (SOD) is the main antioxidant protecting against reactive oxygen species. Zn2+ acts as a cofactor of SOD1, which removes free radicals in both the cytoplasm and extracellular matrix [6]. Zn2+ can reversibly inhibit membrane phosphodiesterase (PDE) and reduce PDE mRNA expression, which decreases the production of the inflammatory cytokines tumor necrosis factor (TNF)-alpha and interleukin (IL)-1 beta, resulting in anti-inflammatory function [7]. Zn2+ is also involved in regulating the immune system. Zn2+ helps to maintain the numbers of lymphocytes, regulatory T cells (Treg), T helper cells (Th), and cytotoxic T cells which function in defense against infection [8]. In addition, Zn2+ participates in cell apoptosis, and an imbalance in Zn2+ homeostasis can cause serious harm to health [9]. Thus, the maintenance of Zn2+ concentrations at normal levels is essential for human health.
Zn2+ metalloenzymes are a series of enzymes regulated by the structure- and activity-maintenance functions of Zn2+ [10]. Since these enzymes are involved in important physiological processes in humans, the disturbance of Zn2+ homeostasis can lead to various serious disorders, such as cardiovascular, immune system, and respiratory diseases. Zn2+ deficiency is a more common cause of health problems than Zn2+ excess. Nearly two billion people are affected by Zn2+ deficiency worldwide, which is more common in developing countries in Africa and Asia [11]. In developed countries, the elderly and people with chronic diseases are more likely to develop Zn2+ deficiency [12].
Considering the importance of Zn2+ in regulating Zn2+ metalloenzyme activity and human health, it is critical to establish the relationships among Zn2+, Zn2+ metalloenzymes and various diseases. In this review, we focus on how Zn2+ participates in regulating Zn2+ metalloenzyme activity and its influence on the physiological processes involved, as well as the enzyme dysregulation caused by Zn2+-homeostasis disorders in different diseases. We summarize the current evidence in the literature and extensively discuss the effect of Zn2+ dysregulation on Zn2+ metalloenzymes and common diseases, which may be beneficial for understanding the severity of Zn2+ homeostasis dysregulation and developing treatment strategies for diseases induced by Zn2+ deficiency.
2. Zn2+ Homeostasis
Since Zn2+ cannot be stored in large amounts in the body, a certain amount of Zn2+ must be consumed daily to maintain Zn2+ homeostasis. The estimated average requirement (EAR) of adults for Zn2+, recommended by the United States and Canada, ranges from 6.8 to 11 mg/day [13]. The main sources of Zn2+ include lean meat, liver, crustaceans, nuts, and eggs [14]. Because phytic acid acts as a chelating agent for Zn2+, vegetarians lack animal tissue-derived Zn2+ and have higher intakes of Zn2+-absorption inhibitors, resulting in a lower bioavailability of Zn2+ than that from normal diets; therefore, they need to consume more Zn2+ to maintain normal Zn2+ concentrations in the body [15].
The entry of dietary Zn2+ from the external environment into the body is mainly controlled by absorption via the gastrointestinal (GI) system. Zn2+ is mainly absorbed on the apical sides of enterocytes and is transported into the cytoplasm via Zn2+ transporters [16]. Ten members of the family of Zn2+ transporters and 14 members of the ZIP (Zrt- and Irt-like protein) family have been identified [17]. ZIP proteins are mainly responsible for transporting Zn2+ from the extracellular space or organelle into the cytoplasm. The ZIP4 protein is mainly located in the apical membrane of the intestinal epithelial cells and related tissues responsible for nutrient absorption and is responsible for transferring extracellular Zn2+ into cells [18]. The amount of ZIP protein is regulated by the Zn2+ concentration. When the Zn2+ concentration is increased, ZIP is removed from the cell surface through endocytosis, protecting the cells from Zn2+ poisoning caused by excessive Zn2+. Most ZIP proteins are located in cell membranes, while most ZNT (Zn2+ transporters) are located in the basolateral membranes of intestinal epithelial cells and are responsible for the transfer of cytoplasmic Zn2+ to the extracellular space; they therefore promote Zn2+ absorption and increase the concentration of Zn2+ in portal venous blood [19]. The expression of ZNT1 increases with increased dietary Zn2+, but Zn2+ deficiency does not have an effect. ZNT2 is involved in the transport of Zn2+ to lysosomes, and heterozygous mutations in the ZNT2 gene lead to temporary Zn2+ deficiency in newborns [20,21,22].
In the basolateral membranes of intestinal cells, Zn2+ enters the circulation mainly through ZNT1 and is then transported to the liver along the portal system. There is currently a hypothesized pathway for the ZNT4 involving vesicle-mediated exocytosis of Zn2+ from intestinal cells [23,24]. In addition to Zn2+ entering the circulatory system in an ionic state, Zn2+ in intestinal cells can bind to metallothionein (MT). Metallothionein, a small and cysteine-rich protein, can interact with oxides to limit Zn2+ absorption in enterocytes [25]. In response to Zn2+, the transcription of MT is rapidly promoted. It has been reported that a Zn2+-induced increase in MT may be involved in protection against intestinal damage caused by anti-inflammatory medicines [25,26].
After entering the circulatory system, Zn2+ mainly binds to albumin and is distributed to Zn2+-rich organs including the liver, muscles, pancreas, and prostate [27]. Zn2+ metabolism in eukaryotic cells is complicated. The metal-responsive element binding transcription factor 1 (MTF-1) acts as a cellular Zn2+ sensor that controls the expression of genes related to Zn2+ homeostasis, including the MT gene and genes involved in intracellular Zn2+ isolation and transportation. A recent study has revealed that MTF-1 reversibly binds Zn2+ through its Zn2+-finger structure and binds the metal-responsive elements in the promoters of these genes, resulting in increased transcription [28].
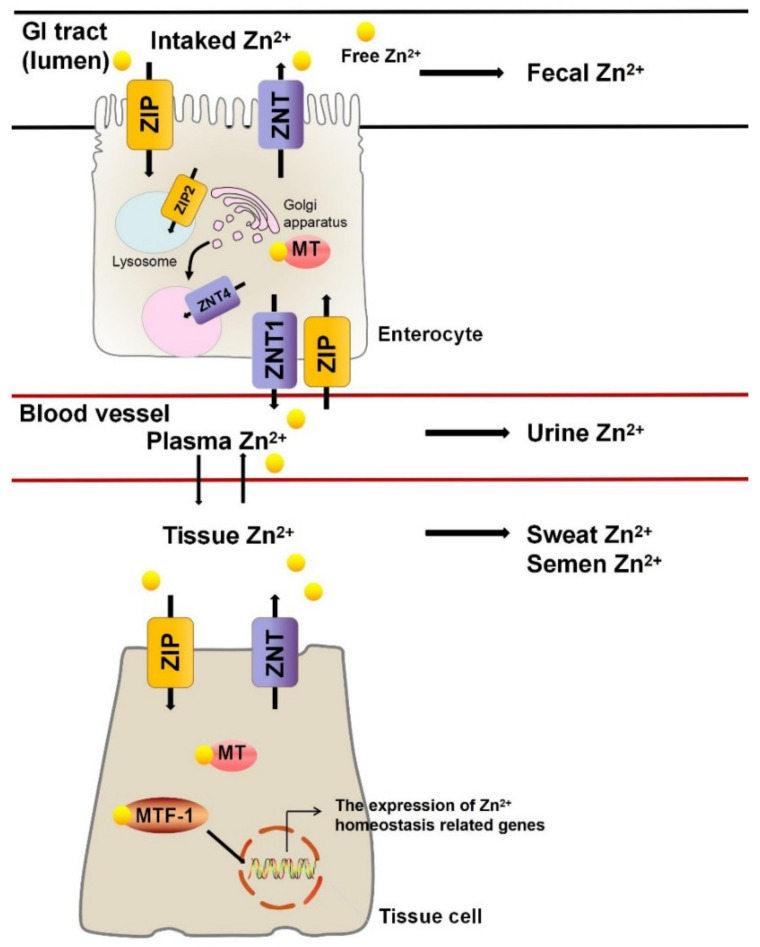
Zn2+ is excreted from the body in the feces through the GI tract. When dietary Zn2+ intake is reduced, the levels of transporters responsible for Zn2+ absorption in the gut are increased and the expression of transporters responsible for Zn2+ excretion are decreased [29]. It has been reported that the pancreas plays an important role in Zn2+ excretion. Pancreatic acinar cells are rich in Zn2+-requiring proenzyme granules. ZNT2, a Zn2+ transporter, is distributed in proenzyme particles and participates in the transport of proenzyme granules in pancreatic acinar cells through the MTF-1 regulatory pathway, leading to the control of the excretion of Zn2+ from the pancreas [30]. Other physiological pathways for the loss of Zn2+ include sweat, urine, and semen [31]. The above findings indicate that ZNT, ZIP, MT, MTF-1, and albumin are involved in regulating the balance of Zn2+ concentrations, the dysfunction of which can lead to Zn2+ homeostasis disorders (Figure 1).
Figure 1.
Regulation of Zn2+ homeostasis. Zn2+ from drinking water and diet is partially absorbed by intestinal cells in the gastrointestinal tract. Zn2+ enters enterocytes via ZIPs and then leaves via ZNTs into the blood circulatory system. Along with the blood, Zn2+ is distributed throughout the tissues. In tissue cells, MTF-1 transcription factor and MT are responsible for regulating Zn2+ concentration. Unused Zn2+ is mainly excreted in the form of feces, urine, sweat, and semen. GI, gastrointestinal; ZIP, Zrt- and Irt-like protein; ZNT, Zn2+ transporters; MT, metallothionein; MTF-1, metal-responsive-element-binding transcription factor-1.
3. Role of Zn2+ and Zn2+ Metalloenzymes in Physiological Processes
Zn2+ is required by more than 300 metalloenzymes for catalytic, structural, and regulatory functions. These metalloenzymes play important roles in the human body, maintaining cell growth and normal function. In order to explore the effects of interactions between Zn2+ metalloenzymes and Zn2+ on human physiological activities, we focus on the functions of Zn2+ and common Zn2+ metalloenzymes regarding the aspects of antioxidant activity, anti-inflammatory effects, immune responses, and apoptosis.
3.1. Zn2+ Metalloenzymes Regulate Antioxidant Activity
Oxidative stress is a state of imbalance between oxidants and antioxidants, which intensifies the rate of oxidative reactions, resulting in the malfunction of redox signaling and molecular damage [32]. Although, as a divalent cation, Zn2+ has no physiological redox activity, it is involved in regulating antioxidant and antioxidant activity as a modulator [32]. The favorable role of Zn2+ in antioxidative reactions has been widely recognized. Previous studies have shown that Zn2+ deficiency is closely associated with increased oxidative damage to lipids, proteins, and DNA [33].
Copper/zinc superoxide dismutase (Cu/Zn-SOD) is one of the Zn2+ metalloenzymes that is an enzymatic antioxidant [34]. It catalyzes the conversion of oxygen free radicals into oxygen and hydrogen peroxide, which is then converted by catalase to water and oxygen. There are mainly two subtypes of Cu/Zn-SOD: one is Cu/Zn-SOD1 (SOD1), in the form of a dimer in the cell, and the other is Cu/Zn-SOD (SOD3), in the form of a tetramer in the extracellular space [35]. By binding to three histidine residues and aspartic acid in Cu/Zn-SOD, Zn2+ connects the structural components and provides the structural framework required for the catalytic function of SOD [36,37,38]. It has been proven that, under Zn2+ deficiency, the activity of SOD1 decreases, leading to an increase in reactive oxygen species (ROS) due to the loss of the cofactor [34]. Apart from acting as a cofactor, Zn2+ can also mask the binding site of derlin-1 on SOD1, preventing endoplasmic-reticulum stress (ERS). Derlin-1 is a member of the endoplasmic-reticulum-associated degradation (ERAD) process, and there is a Zn2+-masked non-exposed derlin-1 binding site in SOD1. In the Zn2+-restricted condition, derlin-1 binds to the binding site exposed on the Zn2+-free SOD1, inducing ERS [39]. Since the ERS-induced unfolded-protein response leads to an increase in ROS, it can result in excessive oxidation and oxidative stress. In addition, prolonged Zn2+ supplementation can enhance the activity of SOD3, preventing ROS damage [40]. Therefore, Zn2+ may play an antioxidant role by participating in the formation of the catalytic structure and function of SOD.
3.2. Zn2+ Metalloenzymes Regulate Inflammation
Zn2+ is well known to participate in anti-inflammatory processes. Inflammation is a defensive response to tissue damage and infection. However, persistent inflammation is a cause of disease. Oxidative stress can trigger an inflammatory response, which then promotes oxidative stress by producing more ROS [41]. Neutrophils are affected by chemokines, move to the inflammatory site, and produce ROS and chemokines, promoting the infiltration of more inflammatory cells. It has been reported that, under Zn2+ deficiency, neutrophils have an increased ability to produce superoxide and a decreased ability to phagocytose at inflammatory sites [42]. Cu/Zn-SOD not only affects oxidative stress but is also crucial in fighting inflammation. When ROS produced by inflammatory cells accumulate, SOD is responsible for eliminating ROS that cause tissue damage. SOD is also involved in regulating neutrophil apoptosis. A previous study showed that an exogenous increase in SOD can promote the apoptosis of neutrophils, preventing the damage caused by chronic inflammation [43]. A clinical study has revealed that bovine SOD has a good effect on the treatment of skin diseases caused by persistent inflammation [44]. As Zn2+ can act as a cofactor to control SOD activity and then regulate the apoptosis of inflammatory cells, it plays an important role in resisting inflammation.
Zn2+ can also regulate inflammation by affecting the activity of matrix metalloproteinases (MMPs). MMPs all have similar structures, and their catalytic domain contains the Zn2+-binding site, which is responsible for maintaining enzymatic activity. In inactive proMMPs, Zn2+ binds to three histidines of the catalytic metalloproteinase domain and one cysteine of the propeptide. When the Zn2+–cysteine bond is disrupted by other proteolytic enzymes, proMMPs becomes active with a combination of water molecules and Zn2+ [45,46]. A recent study has shown that MMPs can establish a chemokine gradient through the degradation and remodeling of the extracellular matrix (ECM), facilitating the movement of inflammatory cells to a damaged site [47]. The inflammatory response caused by MMP disorders can be regulated by the Zn2+ concentration. Zhang et al. demonstrated that the MMP concentrations in Zn2+-deficient mice were decreased, causing the aggregation of ECM, which aggravated the fibrosis of the spleen [48]. On the contrary, it has been reported that a Zn2+-supplemented diet can inhibit MMP2 and MMP9 in a lipid-disorder rabbit model, reduce the inflammatory response, and protect the liver [49]. In patients with atherosclerosis, it has been reported that the serum Zn2+ concentration decreased with an increase in serum MMP9 concentration [50]. A previous study has revealed that Zn2+ supplementation can increase the expression and activity of MMP2 and MMP8, while, under excessive Zn2+ supplementation, the activity of MMP significantly decreases [51]. The effect of Zn2+ on MMP activity may be dose dependent.
The nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) signaling pathway, a major inflammatory signaling pathway, has been shown to be negatively regulated by Zn2+ [41]. Phosphodiesterase (PDE), a critical component upstream of the NF-κB pathway, is responsible for controlling the concentration of cyclic adenosine monophosphate (cAMP) or cyclic guanosine monophosphate (cGMP) in cells, resulting in the effects of these second messengers being reduced. Zn2+ reversibly inhibits the activity of PDE by binding to two histidine and two aspartic acid residues, which leads to an increase in cGMP [52]. cGMP can cross-activate protein kinase A (PKA), resulting in the phosphorylation of Raf-1, decreasing its activity. Through this mechanism, the downstream activation of NF-κB is inhibited and the expression of its target, TNF-α, is also reduced. In addition, PDE4 is the enzyme responsible for cAMP hydrolysis, mainly in inflammatory and immune cells. Zn2+ regulates the inflammatory response by reversibly inhibiting the activity of PDE4 [7,53]. cAMP accumulation induced by PDE4 inhibition activates PKA, further triggering a downstream anti-inflammatory cascade, resulting in a reduced release of inflammatory factors and reduced MMP expression [54]. Thus, Zn2+ may be involved in the induction of anti-inflammatory responses via inhibiting PDE activity.
3.3. Zn2+ Metalloenzymes Regulate the Immune Response
Since Zn2+ deficiency has been found to be significantly associated with immune-system diseases, an increasing number of studies have focused on the importance of Zn2+ in normal immune function [8]. In the face of the invasion of and infection by foreign organisms, the immune system exerts its defense mechanism through the interaction of immune organs, cells, and cytokines.
Various kinases, phosphatases, signaling molecules, and transcription factors constitute the major signaling pathways in the immune system. Protein kinase C (PKC) is a serine/threonine kinase, the regulatory domain of which contains a Zn2+-binding site. Zn2+ is involved in regulating PKC’s structure and facilitating the enzyme’s activity by linking to cysteine residues [55]. The PKC family plays a key role in T-cell-related immune responses. PKC θ has been reported to be involved in T-cell-receptor/CD3 activation and promotes the presentation of antigens from antigen-presenting cells (APCs) to T cells by enhancing adhesion contact between cells [56]. Under a high concentration of Zn2+-chelating agent, PKC activity in immune cells was inhibited [57]. A previous study showed that Zn2+ controlled interferon (IFN)-gamma gene expression in T cells by targeting the calcium-independent PKC activator protein 1 (AP-1) pathway [58]. According to the findings of these studies, Zn2+ may influence the immune system by controlling PKC activation in immune cells.
3.4. Zn2+ Metalloenzymes Regulate Apoptosis
Apoptosis is a highly regulated form of programmed cell death. Normal apoptosis has been proven to be beneficial for maintaining turnover and homeostasis in humans. Caspase, a Zn2+-dependent proteolytic enzyme, plays an important role in apoptosis. In response to both external and internal apoptotic signaling factors, the initiator caspases (e.g., caspase 8 and 9) are activated, which in turn mediate the activation of executioner caspases (e.g., caspase 3). Executioner caspases ultimately cleave key autophagy proteins, leading to cell death [59].
Zn2+ has been reported to control cell apoptosis, which may be through the regulation of caspase activity. Zn2+ can directly inhibit enzyme activation by binding to the cysteine in the active site and the second site of caspase 8, resulting in the inhibition of dimer formation. However, in caspase 6, instead of binding to the active site, Zn2+ binds to an exosite including lysine, glutamic acid, and histidine residues far from the active site, inhibiting enzyme activation by maintaining an inactive conformation [60,61]. By binding to amino-acid residues at key active sites, increased Zn2+ prevents upstream caspase 9 activation, interrupting programmed cell death and triggering the survival and proliferation of damaged cells [60]. In a mouse model of allergic asthma, Zn2+ deficiency reduced the inhibition of caspase 3, leading to increased levels of active caspase 3, causing cell apoptosis. Although Zn2+ supplementation prevents the programmed cell death induced by various stimuli [62,63], based on the above research, a normal Zn2+ status is critical for the regulation of cell apoptosis via the appropriate inhibition of caspase activity.
3.5. Zn2+ Metalloenzymes Regulate Other Physiological Processes
Carbonic anhydrase (CA) is a Zn2+ metalloenzyme that catalyzes the conversion of carbon dioxide and bicarbonate in the presence of Zn2+. When Zn2+ ion binds to the luminal binding site of the active site, carbonic anhydrase can play a catalytic role. In α-, γ-, and δ-CAs, Zn2+ binds to three histidine residues and a hydroxide ion in the active site. In type I β-CAs, there are two cysteine residues; one histidine residue and a hydroxide ion bind with Zn2+, while an aspartate residue replaces the hydroxide ion as the fourth Zn2+ ligand in type II β-CAs [64]. Zn2+ has been used as an inhibitory target for a wide range of carbonic-anhydrase inhibitors [65]. CA participates in physiological processes related to the regulation of pH and carbon-dioxide balance, such as facilitating the decomposition of carbonic acid in the lungs into carbon dioxide and water to aid respiration, coordinating the reabsorption function of the kidney, and maintaining the synthesis and secretion of hydrochloric acid in the stomach. Since CA is widely present in almost all tissues of mammals, the Zn2+ regulation of enzyme activity is crucial. A significantly reduced CA activity in tongue epithelium was identified in rats with a Zn2+-deficient diet, which may be responsible for loss of taste [66]. A clinical study revealed that a low-Zn2+ diet significantly reduced erythrocyte CA activity, which in turn impaired heart and respiratory function during exercise [67].
The alkaline phosphatases (APs) are a group of isoenzymes that catalyze the hydrolysis of phosphate monoesters and promote the synthesis of DNA [68]. The AP in the intestine, placenta, and germinal tissue is tissue specific, while the AP in the circulatory system is not. There are two Zn2+-binding sites and one magnesium-binding site closely spaced at the AP active center. The AP activity reaches its maximum when Zn2+ occupies all three metal-binding sites [69]. Zn2+ concentrations in the normal range may provide protection for AP to function physiologically. Sadighi et al. demonstrated that Zn2+ supplementation caused an increase in AP activity in bone [70]. While there was a significant reduction in the activity and level of AP in the sera of rats fed with a low Zn2+ diet, the growth rate was slower than that of rats fed with a normal Zn2+ diet [71,72]. In addition, Japan’s Practical Guidelines propose that, apart from liver disease, osteoporosis, chronic kidney disease, and diabetes, a reduction in serum AP has been found to be one of the criteria for Zn2+ deficiency [73].
In conclusion, Zn2+ can cause an enzyme to gain or lose catalytic activity by critically influencing its structure in a manner that modulates its catalysis or by preventing the binding of the substrate to the enzyme’s active site. The maintenance of Zn2+ concentrations within a normal range is beneficial for enabling these Zn2+ metalloenzymes to play appropriate catalytic roles in physiological processes.
4. Zn2+ Metalloenzymes and Diseases
Since Zn2+ plays a critical role in regulating the structure and activity of Zn2+ metalloenzymes, Zn2+ deficiency can lead to various diseases by affecting the function of these enzymes. Due to the fact that previous studies have found that Zn2+ deficiency has an impact on human growth and gonadal development, an increasing number of studies have begun to focus on the relationship between Zn2+ deficiency and human disease development and how to use Zn2+ supplementation to treat these diseases. We have summarized the effects of Zn2+ on different diseases through the regulatory effects of Zn2+ metalloenzymes (Table 1).
Table 1.
The list of Zn2+ metalloenzymes and related human diseases.
| Zn2+ Metalloenzyme | Zn2+ Binding Sites | The Effect of Zn2+ on Zn2+ Metalloenzyme | Related Diseases | Ref |
|---|---|---|---|---|
| Copper/Zinc superoxide dismutase | Three histidine residues and one aspartic acid | Activator | CVDs, ALS and AD | [36,74,75,76,77] |
| Matrix metalloproteinase | Three histidine residues of the catalytic domain | Activator | Vascular diseases | [45,46,78] |
| Phosphodiesterase | Two histidine and two aspartic acid residues | Inhibitor | COPD | [52,79] |
| Protein kinase C | Cysteine residues in the regulatory domain | Activator | Immune diseases | [55,80,81] |
| Caspase | Caspase-6: one lysine, one glutamic acid and one histidine residue out of the active site; Caspase-8: one cysteine in the active site and the second binding site is unknown |
Inhibitor | Asthma | [60,61,82] |
| Carbonic anhydrase | α-, γ-, and δ-CAs: three histidine residues and a hydroxide ion Type I β-CAs: two cysteine residues, one histidine residues, and a hydroxide ion Type II β-CAs: two cysteine residues, one histidine, and one aspartate residues |
Activator | Hypogeusia | [64,83] |
| Alkaline phosphatase | Three metal binding sites in active center | Activator | Bone disorder | [69,84,85] |
CVD: cardiovascular disease; ALS: amyotrophic lateral sclerosis; AD: Alzheimer’s disease; COPD: chronic obstructive pulmonary disease.
4.1. SODs in Cardiovascular Diseases, ALS, and AD
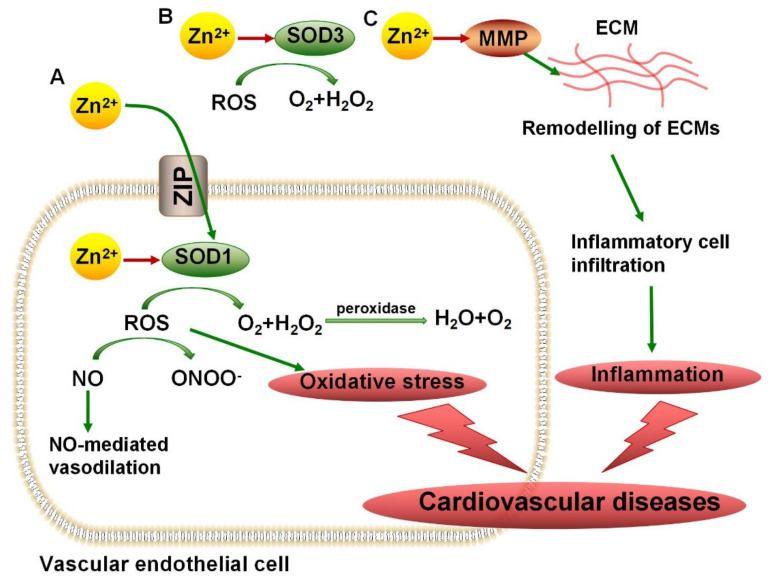
Cardiovascular disease (CVD) is the general term for diseases related to the heart and circulatory system, which have become the main cause of death in the world. An increasing number of studies have identified that chronic inflammation and oxidative stress are risk factors for CVDs. Excessive ROS can damage the structure and function of vascular endothelial cells, affecting the generation and progression of CVDs. Since antioxidant copper/zinc superoxide dismutase (Cu/Zn-SOD) has been well known to balance the ROS inside and outside the cell, various studies have focused on the importance of the SOD-mediated antioxidant system in the antioxidant treatment of CVDs [86]. It has been reported that in SOD1-knockout mice, the level of ROS in blood vessels increased and NO-mediated vasodilation decreased, which made blood vessels more vulnerable to damage [74]. Under the condition of exogenous SOD treatment, hypertension and endothelial relaxation damage can both be alleviated [87]. Zn2+ has been reported to be responsible for maintaining SOD activity. Zn2+-binding sites have been observed in both SOD1 and SOD3. According to an experiment in rats treated with the Zn2+ chelator TPEN, TPEN-induced Zn2+ depletion led to an increase in the size of myocardial infarction by inhibiting SOD activity [88]. Majewski et al. revealed that resveratrol has been shown to improve blood vessel conditions by increasing SOD activities, which may be induced by an increased concentration of Zn2+ [89]. The potential protective mechanism of Zn2+ in cardiovascular diseases through controlling SOD activity is shown in Figure 2A,B.
Figure 2.
The protective and stimulative mechanism of Zn2+ on cardiovascular diseases. (A) Zn2+ enters cells via ZIP, binds with SOD1 to promote ROS breakdown and protects blood vessels from oxidative stress. ROS promotes the conversion of NO to ONOO−. NO is an important component responsible for vasodilation. SOD1 enhances the decomposition of ROS, and the reduced ROS leads to the reduction of NO conversion which facilitates NO-mediated vasodilation. (B) In the extracellular space, Zn2+ can increase the activity of SOD3, thereby promoting the conversion of ROS and alleviating the damage of oxidative stress to blood vessels. (C) The combination of Zn2+ with MMP can enhance the activity of enzymes, promoting the degradation of ECMs and the movement of inflammatory cells to the damaged site, ultimately leading to the damage of vessels due to inflammatory response. ZIP, Zrt- and Irt-like protein; SOD, superoxide dismutase; MMP, matrix metalloproteinase; ECM, extracellular matrix; ROS, reactive oxygen species.
A close relationship between SOD and amyotrophic lateral sclerosis (ALS) has been widely reported. ALS is a type of late-onset degenerative motor neuron disease. Due to its severe lethality, most patients die after about three years because of respiratory failure caused by muscle weakness [90]. It has been reported that about 25% of familial ALS cases are related to a SOD mutation that causes SOD to have a low affinity for Zn2+, reducing antioxidant function, and the accumulation of superoxide damages motor neurons and stimulates a neuronal apoptosis cascade [75]. A previous study has demonstrated that SOD mice with a mutation reducing Zn2+ affinity survived longer under moderate Zn2+ supplementation than those with a Zn2+-deficient diet, while excessive Zn2+ supplementation can inhibit copper absorption, leading to an increased incidence of anemia and early death [91]. The in vitro experiments revealed that wild-type SOD could also induce motor-neuron apoptosis under the condition of Zn2+ deficiency [92]. Since SOD activity plays a key role in the occurrence and progression of ALS, regulating SOD activity using Zn2+ may become a therapeutic target. Recently, McAllum et al. found that treatment with a Zn2+ metal complex increased the motor function and survival rate of SOD1-mutant mice with low Zn2+ affinity, which provided support for the further exploration of the therapeutic effect of Zn2+ supplementation on ALS [93].
Oxidative stress has been shown to be a risk factor for neurodegenerative diseases. In addition to ALS, SOD is also involved in the pathogenesis of Alzheimer’s disease (AD). AD is an age-dependent form of dementia with cognitive decline. The formation of soluble amyloid beta (Aβ) oligomer is critical for the pathogenesis of AD, which impairs synaptic and cognitive function. It has been reported that, in animal models, a reduction in SOD can induce Aβ deposition by enhancing oxidative stress, thus leading to cognitive disorders. In patients with AD, there is a significant decrease in the level and activity of SOD compared to that in healthy people [76,77]. As an increase in the central Zn2+ concentration promotes the deposition of Aβ, while a low serum Zn2+ level may promote the development of AD by triggering depression, the effect of Zn2+ disturbance on AD is complex. However, Greenough et al. revealed that presenilin proteins could maintain the activity of SOD1 by promoting Zn2+ uptake, inhibiting the accumulation of Aβ [94].
4.2. MMPs in Vascular Diseases
Vascular disease is a range of diseases involving vascular dysfunction, including hypertension, atherosclerosis, vascular inflammation, etc. Endothelial dysfunction, aberrant angiogenesis, and abnormal cell survival can lead to vascular diseases [95]. Matrix metalloproteinases (MMPs) have been reported to be responsible for angiogenesis and the reconstruction of vascular tissues. Alterations in MMP activity can lead to uncontrolled inflammatory responses, tissue remodeling, and cell migration [78]. Previous studies have demonstrated that the upregulation of MMP1 and MMP9 activity was significantly positively correlated with the degree of inflammation and organ damage [96,97]. Increased MMP activity can promote the degradation of elastin in the extracellular matrix, resulting in decreased vascular elasticity and thus promoting the progression of hypertension [98]. Since MMPs are Zn2+ metalloproteinases, the degree of Zn2+-ion binding to Zn2+-binding sites determines the activity of MMPs. Since highly active MMPs can remodel ECM and promote the movement of inflammatory cells, Zn2+ may aggravate the inflammatory responses of vascular tissues by increasing the activity of MMPs (Figure 2C). The administration of ellagic acid, a Zn2+ chelator, can inhibit the activity of MMP2, reducing its angiogenic role in the formation and migration of vascular endothelial cells, which is conducive to inhibiting abnormal angiogenesis [99]. Due to the angiogenesis induced by MMP activation, which can promote tumor metastasis, the development and selection of matrix metalloproteinase inhibitors (MMPIs) have attracted increasing attention [100]. Blocking the binding site for Zn2+ or promoting the binding of drugs to free Zn2+ ions may provide two approaches to antagonizing MMP activity.
4.3. Phosphodiesterase in Chronic Obstructive Pulmonary Disease
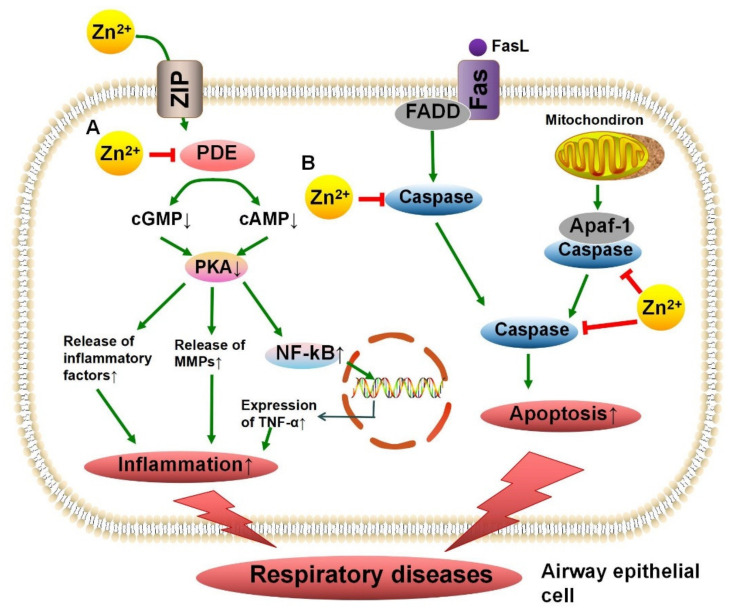
Chronic obstructive pulmonary disease (COPD) is a progressive irreversible disease that involves airway obstruction with severe airway inflammation. The inhibition of the inflammation of airway cells is the key for treatment [101]. Phosphodiesterase (PDE), a Zn2+ metalloenzyme, participates in proinflammatory responses by degrading cAMP, resulting in an increase in TNF-α expression. The inhibition of the activity of PDE4 has been reported to increase the level of cAMP, which in turn inhibits inflammation, relaxes airway smooth muscle, and improves patient symptoms [79]. It has been revealed that decreased serum Zn2+ is significantly associated with COPD, and low Zn2+ levels may lead to the activation of an inflammatory cascade by inhibiting PDE activity [102]. A recent experiment has shown that Zn2+ supplementation can reduce lung macrophages by more than half in mice exposed to cigarette smoke, significantly reducing airway inflammation, which may provide support for micronutrient supplementation for the anti-inflammatory treatment of COPD patients in the future [103]. The mechanism of action of Zn2+ in inhibiting the inflammation in the airways by inhibiting PDE activity is shown in Figure 3A.
Figure 3.
The protective mechanism of Zn2+ on respiratory diseases. Zn2+ can enter airway epithelial cells via ZIP. (A) PDE is responsible for catalyzing the degradation of cGMP and cAMP. Reduced cGMP and cAMP lead to reduced PKA activation, which in turn promotes downstream inflammatory cascades and increased TNF-α expression. Combination of Zn2+ and PDE can inhibit the enzyme activity, which reduces the cell damage caused by inflammation. (B) Exogenous apoptosis is mediated by FasL and Fas, while endogenous apoptosis is mediated by mitochondria. Both pathways activate initiator and executioner caspases to promote apoptosis. Zn2+ protects the airway epithelium by inhibiting caspase activity to prevent excessive cell death. ZIP, Zrt- and Irt-like protein; PDE, phosphodiesterase; FasL, Fas ligand; Fas, tumor necrosis factor receptor superfamily member 6; FADD, Fas-associated death domain; PKA, protein kinase A; NF-Kb, nuclear transcription factor-kappa B.
4.4. Protein Kinase C in Immune Diseases
Immune system diseases are diseases caused by an imbalance in the regulation of the immune system or immune response. Recent studies have shown that protein kinase C (PKC) plays an important role in the occurrence and development of human immune diseases. It has been reported that the knockdown of PKCα and PKCθ is significantly associated with decreased T-cell activity. Genome-wide association studies (GWAS) that analyzed people revealed that there was a significant correlation between mutations at the PKC locus and multiple sclerosis, rheumatoid arthritis, and celiac disease [80]. Zn2+ is important for PKC’s structure and the regulation of its activity; it can enhance enzyme activity by binding to sites in regulatory regions. In the T cells of mice with immunosuppression caused by hypothyroidism, Zn2+ supplementation was beneficial in fighting against a reduction in PKC activity, which is involved in T-cell activation [81]. However, when Zn2+ supplementation increased serum Zn2+ levels far above normal, Zn2+ induced an immunosuppressive effect and inhibited T-lymphocyte activation [104]. In addition, for autoimmune diseases and lymphoma in which increased PKC activity contributes to poor prognosis, the inhibition of Zn2+-binding sites may become a target for PKC inhibitors [105,106].
4.5. Caspase in Asthma
Asthma is a common chronic respiratory disease characterized by chronic eosinophil infiltration into the airway, with various degrees of airflow restriction accompanied by the apoptosis and exfoliation of airway epithelial cells. A previous study has shown that, compared to that in healthy people, the apoptosis of airway epithelial cells in patients with asthma is significantly increased [107]. Caspase is a critical component of the apoptosis process, and changes in its enzymatic activity affect cell survival. Zn2+-binding sites have been discovered in caspase, and Zn2+ binding inhibits its activity. A previous study demonstrated that Zn2+ acted as a cell-protective agent and inhibited the apoptosis of airway epithelial cells [82]. Roscioli et al. reported that Zn2+ deficiency led to increased apoptosis of airway epithelial cells by inhibiting caspase activity [108]. In a mouse model of allergic asthma, a deficiency of Zn2+ in the airway epithelium led to changes in active caspase 3 levels and the promotion of apoptosis [62]. It has been revealed that dietary Zn2+ supplementation can significantly improve the symptoms of asthma patients, providing a new approach to asthma management [109], in which Zn2+ may play an anti-apoptotic role by inhibiting caspase activity (Figure 3B).
4.6. Carbonic Anhydrase in Hypogeusia
The normal activity of CA has been thought to be an important factor in maintaining taste. It has been reported that high levels of CA activity were identified in tongue papillae associated with taste buds [83]. Due to the presence of Zn2+-binding sites in CA and the regulatory effect of Zn2+ on enzyme activity, various studies have focused on the effects of Zn2+ on taste and the treatment of taste disorders [110]. Komai et al. demonstrated that decreased taste sensitivity was associated with decreased CA activity in Zn2+-deficient rats [111]. A clinical trial report revealed that exogenous Zn2+ supplementation had a certain improvement effect on patients with carbonic anhydrase VI deficiency and hypogeusia [112].
4.7. Alkaline Phosphatase in Bone Disorder
In bone, AP can hydrolyze pyrophosphate and increase inorganic phosphate, promoting bone mineralization. Studies have shown that high AP activity is reflected in increased activity of osteoblasts, while low AP activity leads to severe impaired bone mineralization, hypercalcemia, and premature tooth loss [84,85]. The binding of Zn2+ to specific binding sites of enzymes is the key to regulating AP activity. It has been reported that incubation with high concentrations of Zn2+ can promote the proliferation of osteoblasts, promoting bone formation, which may be caused by an increase in AP activity [113]. A clinical study showed that Zn2+ supplementation caused an increase in AP activity and a significant improvement in bone healing [114]. In healthy adult men, Zn2+ supplementation also stimulated bone formation, with a significant increase in AP activity [115].
5. Zn2+ and Zn2+ Metalloenzymes in Cancer
An increasing number of studies have confirmed that Zn2+ deficiency promotes the occurrence and development of tumors [116]. We have reported that Zn2+ deficiency may lead to the dysregulation of important physiological processes in the body, DNA damage, and microRNA expression, resulting in the promotion of cancer [117]. Since the majority of Zn2+ metalloenzymes are involved in regulating these physiological processes, Zn2+ deficiency may affect tumor progression by controlling the activity of enzymes. Previous research has revealed that a decrease in SOD activity caused by Zn2+ deficiency led to oxidative stress, which could induce and maintain the state of cancer cells by impairing DNA stability [118]. Zn2+ supplementation may improve the antitumor therapy of non-small-cell lung cancer by increasing ROS activity [119]. In addition, Zn2+ deficiency can reduce the function of innate and adaptive immune cells, therefore helping tumor cells to evade immune surveillance [116]. In a previous study, we found that Zn2+-deficiency-induced inflammation facilitated the development of esophageal cancer [120]. Taccioli et al. demonstrated that Zn2+ supplementation could ameliorate the inflammation in cancer [121]. Although the promoting effect of Zn2+ deficiency on tumor development has been comprehensively explored, there is a lack of research on the regulatory effect and influence of Zn2+ deficiency on enzymes during tumorigenesis. As Zn2+ supplementation has been gradually applied in nutritional support therapy for cancer patients, further exploration of the regulation of enzyme activity by Zn2+ deficiency in cancer may provide comprehensive guidance for the management of Zn2+ concentrations.
6. Conclusions
In this review, we summarized the pathways of Zn2+ absorption and metabolism, and the roles and potential mechanisms of Zn2+ and Zn2+ metalloenzymes in normal physiology and diseases. Zn2+ can act as a structural component or catalyst to regulate the activity of Zn2+ metalloenzymes, which are involved in antioxidant, anti-inflammatory, immune-response, and apoptotic processes. A deficient or excessive Zn2+ intake leads to changes in enzyme activity and affects the occurrence and development of related diseases, which may provide a novel insight for the regulation of enzyme activity and nutritional treatment of diseases. We hope that the comprehensive understanding and in-depth discussion of the mechanisms by which Zn2+ regulates metalloenzymes, the effects of Zn2+ metalloenzymes on human diseases, and their associated mechanisms will provide new micronutrient supply strategies for the prevention and treatment of human diseases.
Author Contributions
Y.C. wrote the manuscript and designed the figures and the table; H.C. designed, helped to write, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prasad A.S. Discovery of Human Zinc Deficiency: Its Impact on Human Health and Disease. Adv. Nutr. 2013;4:176–190. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prasad A.S., Miale A., Jr., Farid Z., Sandstead H.H., Schulert A.R. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypognadism. J. Lab. Clin. Med. 1963;61:537–549. [PubMed] [Google Scholar]
- 3.Sanna A., Firinu D., Zavattari P., Valera P. Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis. Nutrients. 2018;10:68. doi: 10.3390/nu10010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa Y., Kinoshita M., Shimada S., Kawamura T. Zinc and Skin Disorders. Nutrients. 2018;10:199. doi: 10.3390/nu10020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wastney M.E., Aamodt R.L., Rumble W.F., Henkin R.I. Kinetic analysis of zinc metabolism and its regulation in normal humans. Am. J. Physiol. 1986;251:R398–R408. doi: 10.1152/ajpregu.1986.251.2.R398. [DOI] [PubMed] [Google Scholar]
- 6.Mariani E., Mangialasche F., Feliziani F., Cecchetti R., Malavolta M., Bastiani P., Baglioni M., Dedoussis G., Fulop T., Herbein G., et al. Effects of zinc supplementation on antioxidant enzyme activities in healthy old subjects. Exp. Gerontol. 2008;43:445–451. doi: 10.1016/j.exger.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Von Bülow V., Rink L., Haase H. Zinc-Mediated Inhibition of Cyclic Nucleotide Phosphodiesterase Activity and Expression Suppresses TNF-α and IL-1β Production in Monocytes by Elevation of Guanosine 3′,5′-Cyclic Monophosphate. J. Immunol. 2005;175:4697–4705. doi: 10.4049/jimmunol.175.7.4697. [DOI] [PubMed] [Google Scholar]
- 8.Wessels I., Maywald M., Rink L. Zinc as a Gatekeeper of Immune Function. Nutrients. 2017;9:1286. doi: 10.3390/nu9121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shankar A.H., Prasad A.S. Zinc and immune function: The biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 10.Maret W. Zinc Biochemistry: From a Single Zinc Enzyme to a Key Element of Life. Adv. Nutr. 2013;4:82–91. doi: 10.3945/an.112.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad A.S. Discovery of human zinc deficiency: 50 years later. J. Trace Elem. Med. Biol. 2012;26:66–69. doi: 10.1016/j.jtemb.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Himoto T., Masaki T. Associations between Zinc Deficiency and Metabolic Abnormalities in Patients with Chronic Liver Disease. Nutrients. 2018;10:88. doi: 10.3390/nu10010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim K.H.C., Riddell L.J., Nowson C.A., Booth A.O., Szymlek-Gay E.A. Iron and Zinc Nutrition in the Economically-Developed World: A Review. Nutrients. 2013;5:3184–3211. doi: 10.3390/nu5083184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gać P., Czerwińska K., Macek P., Jaremków A., Mazur G., Pawlas K., Poręba R. The importance of selenium and zinc deficiency in cardiovascular disorders. Environ. Toxicol. Pharmacol. 2021;82:103553. doi: 10.1016/j.etap.2020.103553. [DOI] [PubMed] [Google Scholar]
- 15.Foster M., Samman S. Vegetarian Diets across the Lifecycle: Impact on zinc intake and status. Adv. Food Nutr. Res. 2015;74:93–131. doi: 10.1016/bs.afnr.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Murgia C., Grosser D., Truong-Tran A.Q., Roscioli E., Michalczyk A., Ackland M.L., Stoltenberg M., Danscher G., Lang C., Knight D., et al. Apical Localization of Zinc Transporter ZnT4 in Human Airway Epithelial Cells and Its Loss in a Murine Model of Allergic Airway Inflammation. Nutrients. 2011;3:910–928. doi: 10.3390/nu3110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichten L.A., Cousins R.J. Mammalian Zinc Transporters: Nutritional and Physiologic Regulation. Annu. Rev. Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 18.Dufner-Beattie J., Wang F., Kuo Y.-M., Gitschier J., Eide D., Andrews G.K. The Acrodermatitis Enteropathica Gene ZIP4 Encodes a Tissue-specific, Zinc-regulated Zinc Transporter in Mice. J. Biol. Chem. 2003;278:33474–33481. doi: 10.1074/jbc.M305000200. [DOI] [PubMed] [Google Scholar]
- 19.Kimura T., Kambe T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int. J. Mol. Sci. 2016;17:336. doi: 10.3390/ijms17030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishito Y., Kambe T. Zinc transporter 1 (ZNT1) expression on the cell surface is elaborately controlled by cellular zinc levels. J. Biol. Chem. 2019;294:15686–15697. doi: 10.1074/jbc.RA119.010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liuzzi J.P., Blanchard R.K., Cousins R.J. Differential Regulation of Zinc Transporter 1, 2, and 4 mRNA Expression by Dietary Zinc in Rats. J. Nutr. 2001;131:46–52. doi: 10.1093/jn/131.1.46. [DOI] [PubMed] [Google Scholar]
- 22.Chowanadisai W., Lönnerdal B., Kelleher S.L. Identification of a Mutation in SLC30A2 (ZnT-2) in Women with Low Milk Zinc Concentration That Results in Transient Neonatal Zinc Deficiency. J. Biol. Chem. 2006;281:39699–39707. doi: 10.1074/jbc.M605821200. [DOI] [PubMed] [Google Scholar]
- 23.Wang X., Zhou B. Dietary zinc absorption: A play of Zips and ZnTs in the gut. IUBMB Life. 2010;62:176–182. doi: 10.1002/iub.291. [DOI] [PubMed] [Google Scholar]
- 24.Maret W., Sandstead H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Andrews G.K. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 2000;59:95–104. doi: 10.1016/S0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 26.Sivalingam N., Pichandi S., Chapla A., Dinakaran A., Jacob M. Zinc protects against indomethacin-induced damage in the rat small intestine. Eur. J. Pharmacol. 2011;654:106–116. doi: 10.1016/j.ejphar.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Lu J., Stewart A.J., Sadler P.J., Pinheiro T.J., Blindauer C.A. Albumin as a zinc carrier: Properties of its high-affinity zinc-binding site. Biochem. Soc. Trans. 2008;36:1317–1321. doi: 10.1042/BST0361317. [DOI] [PubMed] [Google Scholar]
- 28.Laity J.H., Andrews G.K. Understanding the mechanisms of zinc-sensing by metal-response element binding transcription factor-1 (MTF-1) Arch. Biochem. Biophys. 2007;463:201–210. doi: 10.1016/j.abb.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Liuzzi J.P., Bobo J.A., Lichten L.A., Samuelson D.A., Cousins R.J. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc. Natl. Acad. Sci. USA. 2004;101:14355–14360. doi: 10.1073/pnas.0406216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo L., Lichten L.A., Ryu M.-S., Liuzzi J.P., Wang F., Cousins R.J. STAT5-glucocorticoid receptor interaction and MTF-1 regulate the expression of ZnT2 (Slc30a2) in pancreatic acinar cells. Proc. Natl. Acad. Sci. USA. 2010;107:2818–2823. doi: 10.1073/pnas.0914941107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kondaiah P., Yaduvanshi P.S., Sharp P.A., Pullakhandam R. Iron and Zinc Homeostasis and Interactions: Does Enteric Zinc Excretion Cross-Talk with Intestinal Iron Absorption? Nutrients. 2019;11:1885. doi: 10.3390/nu11081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prasad A.S. Zinc: Role in immunity, oxidative stress and chronic inflammation. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:646–652. doi: 10.1097/MCO.0b013e3283312956. [DOI] [PubMed] [Google Scholar]
- 34.Wu C.-Y., Steffen J., Eide D.J. Cytosolic Superoxide Dismutase (SOD1) Is Critical for Tolerating the Oxidative Stress of Zinc Deficiency in Yeast. PLoS ONE. 2009;4:e7061. doi: 10.1371/journal.pone.0007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewandowski Ł., Kepinska M., Milnerowicz H. The copper-zinc superoxide dismutase activity in selected diseases. Eur. J. Clin. Investig. 2019;49:e13036. doi: 10.1111/eci.13036. [DOI] [PubMed] [Google Scholar]
- 36.Lewandowski Ł., Kepinska M., Milnerowicz H. Inhibition of copper-zinc superoxide dismutase activity by selected environmental xenobiotics. Environ. Toxicol. Pharmacol. 2018;58:105–113. doi: 10.1016/j.etap.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Perry J., Shin D., Getzoff E., Tainer J. The structural biochemistry of the superoxide dismutases. Biochim. Biophys. Acta BBA—Proteins Proteom. 2010;1804:245–262. doi: 10.1016/j.bbapap.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antonyuk S.V., Strange R.W., Marklund S.L., Hasnain S.S. The Structure of Human Extracellular Copper–Zinc Superoxide Dismutase at 1.7 Å Resolution: Insights into Heparin and Collagen Binding. J. Mol. Biol. 2009;388:310–326. doi: 10.1016/j.jmb.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 39.Homma K., Fujisawa T., Tsuburaya N., Yamaguchi N., Kadowaki H., Takeda K., Nishitoh H., Matsuzawa A., Naguro I., Ichijo H. SOD1 as a Molecular Switch for Initiating the Homeostatic ER Stress Response under Zinc Deficiency. Mol. Cell. 2013;52:75–86. doi: 10.1016/j.molcel.2013.08.038. [DOI] [PubMed] [Google Scholar]
- 40.Kara E., Gunay M., Cicioglu I., Ozal M., Kilic M., Mogulkoc R., Baltaci A.K. Effect of Zinc Supplementation on Antioxidant Activity in Young Wrestlers. Biol. Trace Elem. Res. 2010;134:55–63. doi: 10.1007/s12011-009-8457-z. [DOI] [PubMed] [Google Scholar]
- 41.Jarosz M., Olbert M., Wyszogrodzka G., Mlyniec K., Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25:11–24. doi: 10.1007/s10787-017-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibs K.-H., Rink L. Zinc-Altered Immune function. J. Nutr. 2003;133:1452S–1456S. doi: 10.1093/jn/133.5.1452S. [DOI] [PubMed] [Google Scholar]
- 43.Yasui K., Baba A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm. Res. 2006;55:359–363. doi: 10.1007/s00011-006-5195-y. [DOI] [PubMed] [Google Scholar]
- 44.Niwa Y. Lipid Peroxides and Superoxide Dismutase (SOD) Induction in Skin Inflammatory Diseases, and Treatment with SOD Preparations. Dermatologica. 1989;179:101–106. doi: 10.1159/000248458. [DOI] [PubMed] [Google Scholar]
- 45.Laronha H., Caldeira J. Structure and Function of Human Matrix Metalloproteinases. Cells. 2020;9:1076. doi: 10.3390/cells9051076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagase H., Visse R., Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Krstić J., Santibañez J.F. Transforming Growth Factor-Beta and Matrix Metalloproteinases: Functional Interactions in Tumor Stroma-Infiltrating Myeloid Cells. Sci. World J. 2014;2014:521754. doi: 10.1155/2014/521754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Q., Zhang H.-X., Chen Y., Wang Y., Yang M., Guo M. Zinc Deficiency Induces Oxidative Damage and Causes Spleen Fibrosis. Biol. Trace Elem. Res. 2020;194:203–209. doi: 10.1007/s12011-019-01762-y. [DOI] [PubMed] [Google Scholar]
- 49.Xu C., Huang Z., Liu L., Luo C., Lu G., Li Q., Gao X. Zinc Regulates Lipid Metabolism and MMPs Expression in Lipid Disturbance Rabbits. Biol. Trace Elem. Res. 2015;168:411–420. doi: 10.1007/s12011-015-0367-7. [DOI] [PubMed] [Google Scholar]
- 50.Olechnowicz J., Tinkov A., Skalny A., Suliburska J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018;68:19–31. doi: 10.1007/s12576-017-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grommes J., Binnebösel M., Klink C.D., Von Trotha K.T., Rosch R., Oettinger A.P., Lindlar I., Krones C.J. Balancing zinc deficiency leads to an improved healing of colon anastomosis in rats. Int. J. Color. Dis. 2010;26:295–301. doi: 10.1007/s00384-010-1070-y. [DOI] [PubMed] [Google Scholar]
- 52.Ke H. Implications of PDE4 structure on inhibitor selectivity across PDE families. Int. J. Impot. Res. 2004;16:S24–S27. doi: 10.1038/sj.ijir.3901211. [DOI] [PubMed] [Google Scholar]
- 53.Von Bülow V., Dubben S., Engelhardt G., Hebel S., Plümäkers B., Heine H., Rink L., Haase H. Zinc-dependent suppression of TNF-alpha production is mediated by protein kinase A-induced inhibition of Raf-1, I kappa B kinase beta, and NF-kappa B. J. Immunol. 2007;179:4180–4186. doi: 10.4049/jimmunol.179.6.4180. [DOI] [PubMed] [Google Scholar]
- 54.Ranju V. Scope of adjuvant therapy using roflumilast, a PDE-4 inhibitor against COVID-19. Pulm. Pharmacol. Ther. 2021;66:101978. doi: 10.1016/j.pupt.2020.101978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gopalakrishna R., Jaken S. Protein kinase C signaling and oxidative stress. Free Radic. Biol. Med. 2000;28:1349–1361. doi: 10.1016/S0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 56.Baier G., Wagner J. PKC inhibitors: Potential in T cell-dependent immune diseases. Curr. Opin. Cell Biol. 2009;21:262–267. doi: 10.1016/j.ceb.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 57.Haase H., Hebel S., Engelhardt G., Rink L. Flow cytometric measurement of labile zinc in peripheral blood mononuclear cells. Anal. Biochem. 2006;352:222–230. doi: 10.1016/j.ab.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 58.Hayashi K., Ishizuka S., Yokoyama C., Hatae T. Attenuation of interferon-γ mRNA expression in activated Jurkat T cells by exogenous zinc via down-regulation of the calcium-independent PKC–AP-1 signaling pathway. Life Sci. 2008;83:6–11. doi: 10.1016/j.lfs.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 59.Shalini S., Dorstyn L., Dawar S., Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22:526–539. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eron S.J., MacPherson D.J., Dagbay K.B., Hardy J.A. Multiple Mechanisms of Zinc-Mediated Inhibition for the Apoptotic Caspases-3, -6, -7, and -8. ACS Chem. Biol. 2018;13:1279–1290. doi: 10.1021/acschembio.8b00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Velázquez-Delgado E.M., Hardy J.A. Zinc-mediated Allosteric Inhibition of Caspase-6. J. Biol. Chem. 2012;287:36000–36011. doi: 10.1074/jbc.M112.397752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Truong-Tran A.Q., Grosser D., Ruffin R.E., Murgia C., Zalewski P.D. Apoptosis in the normal and inflamed airway epithelium: Role of zinc in epithelial protection and procaspase-3 regulation. Biochem. Pharmacol. 2003;66:1459–1468. doi: 10.1016/S0006-2952(03)00498-2. [DOI] [PubMed] [Google Scholar]
- 63.Sunderman F.W. The influence of zinc on apoptosis. Ann. Clin. Lab. Sci. 1995;25:134–142. [PubMed] [Google Scholar]
- 64.Supuran C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016;473:2023–2032. doi: 10.1042/BCJ20160115. [DOI] [PubMed] [Google Scholar]
- 65.Montero J.-L., Supuran C., Scozzafava A., Winum J.-Y. Design of Zinc Binding Functions for Carbonic Anhydrase Inhibitors. Curr. Pharm. Des. 2008;14:615–621. doi: 10.2174/138161208783877848. [DOI] [PubMed] [Google Scholar]
- 66.Goto T., Komai M., Bryant B.P., Furukawa Y. Reduction in Carbonic Anhydrase Activity in the Tongue Epithelium and Submandibular Gland in Zinc-Deficient Rats. Int. J. Vitam. Nutr. Res. 2000;70:110–118. doi: 10.1024/0300-9831.70.3.110. [DOI] [PubMed] [Google Scholar]
- 67.Lukaski H.C. Low dietary zinc decreases erythrocyte carbonic anhydrase activities and impairs cardiorespiratory function in men during exercise. Am. J. Clin. Nutr. 2005;81:1045–1051. doi: 10.1093/ajcn/81.5.1045. [DOI] [PubMed] [Google Scholar]
- 68.Zaher D.M., El-Gamal M.I., Omar H.A., Aljareh S.N., Al-Shamma S.A., Ali A.J., Zaib S., Iqbal J. Recent advances with alkaline phosphatase isoenzymes and their inhibitors. Arch. Pharm. 2020;353:e2000011. doi: 10.1002/ardp.202000011. [DOI] [PubMed] [Google Scholar]
- 69.Coleman J.E. Structure and mechanism of alkaline phosphatase. Annu. Rev. Biophys. Biomol. Struct. 1992;21:441–483. doi: 10.1146/annurev.bb.21.060192.002301. [DOI] [PubMed] [Google Scholar]
- 70.Rashidi A.A., Salehi M., Piroozmand A., Sagheb M.M. Effects of Zinc Supplementation on Serum Zinc and C-Reactive Protein Concentrations in Hemodialysis Patients. J. Ren. Nutr. 2009;19:475–478. doi: 10.1053/j.jrn.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 71.Naber T.H., Baadenhuysen H., Jansen J.B., Hamer C.J.V.D., Broek W.V.D. Serum alkaline phosphatase activity during zinc deficiency and long-term inflammatory stress. Clin. Chim. Acta. 1996;249:109–127. doi: 10.1016/0009-8981(96)06281-X. [DOI] [PubMed] [Google Scholar]
- 72.Okegbile E.O., Odunuga O., Oyewo A. Effect of dietary zinc deficiency on alkaline phosphatase and nucleic acids in rats. Afr. J. Med. Med. Sci. 1999;27:189–192. [PubMed] [Google Scholar]
- 73.Kodama H., Tanaka M., Naito Y., Katayama K., Moriyama M. Japan’s Practical Guidelines for Zinc Deficiency with a Particular Focus on Taste Disorders, Inflammatory Bowel Disease, and Liver Cirrhosis. Int. J. Mol. Sci. 2020;21:2941. doi: 10.3390/ijms21082941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Didion S.P., Ryan M.J., Didion L.A., Fegan P.E., Sigmund C.D., Faraci F.M. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ. Res. 2002;91:938–944. doi: 10.1161/01.RES.0000043280.65241.04. [DOI] [PubMed] [Google Scholar]
- 75.Kaur S.J., McKeown S.R., Rashid S. Mutant SOD1 mediated pathogenesis of Amyotrophic Lateral Sclerosis. Gene. 2016;577:109–118. doi: 10.1016/j.gene.2015.11.049. [DOI] [PubMed] [Google Scholar]
- 76.Murakami K., Murata N., Noda Y., Tahara S., Kaneko T., Kinoshita N., Hatsuta H., Murayama S., Barnham K.J., Irie K., et al. SOD1 (Copper/Zinc Superoxide Dismutase) Deficiency Drives Amyloid β Protein Oligomerization and Memory Loss in Mouse Model of Alzheimer Disease. J. Biol. Chem. 2011;286:44557–44568. doi: 10.1074/jbc.M111.279208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nazıroğlu M., Muhamad S., Pecze L. Nanoparticles as potential clinical therapeutic agents in Alzheimer’s disease: Focus on selenium nanoparticles. Expert Rev. Clin. Pharmacol. 2017;10:773–782. doi: 10.1080/17512433.2017.1324781. [DOI] [PubMed] [Google Scholar]
- 78.Wang X., Khalil R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018;81:241–330. doi: 10.1016/bs.apha.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown W.M. Treating COPD with PDE 4 inhibitors. Int. J. Chronic Obstr. Pulm. Dis. 2007;2:517–533. [PMC free article] [PubMed] [Google Scholar]
- 80.Altman A., Kong K.-F. Protein kinase C inhibitors for immune disorders. Drug Discov. Today. 2014;19:1217–1221. doi: 10.1016/j.drudis.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paulazo M.A., Klecha A.J., Sterle H., Valli E., Torti H., Cayrol F., Arcos M.L.B., Cremaschi G.A. Hypothyroidism-related zinc deficiency leads to suppression of T lymphocyte activity. Endocrine. 2019;66:266–277. doi: 10.1007/s12020-019-01936-7. [DOI] [PubMed] [Google Scholar]
- 82.Truong-Tran A.Q., Ruffin R.E., Zalewski P.D. Visualization of labile zinc and its role in apoptosis of primary airway epithelial cells and cell lines. Am. J. Physiol. Cell. Mol. Physiol. 2000;279:L1172–L1183. doi: 10.1152/ajplung.2000.279.6.L1172. [DOI] [PubMed] [Google Scholar]
- 83.Brown D., Garcia-Segura L., Orci L. Carbonic anhydrase is associated with taste buds in rat tongue. Brain Res. 1984;324:346–348. doi: 10.1016/0006-8993(84)90046-5. [DOI] [PubMed] [Google Scholar]
- 84.Nizet A., Cavalier E., Stenvinkel P., Haarhaus M., Magnusson P. Bone alkaline phosphatase: An important biomarker in chronic kidney disease—Mineral and bone disorder. Clin. Chim. Acta. 2020;501:198–206. doi: 10.1016/j.cca.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 85.Linglart A., Duplan M.B. Hypophosphatasia. Curr. Osteoporos. Rep. 2016;14:95–105. doi: 10.1007/s11914-016-0309-0. [DOI] [PubMed] [Google Scholar]
- 86.Fukai T., Ushio-Fukai M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid. Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Laursen J.B., Rajagopalan S., Galis Z., Tarpey M., Freeman B.A., Harrison D.G. Role of Superoxide in Angiotensin II–Induced but Not Catecholamine-Induced Hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.CIR.95.3.588. [DOI] [PubMed] [Google Scholar]
- 88.Skene K., Walsh S.K., Okafor O., Godsman N., Barrows C., Meier P., Gordon M.J., Beattie J.H., Wainwright C.L. Acute dietary zinc deficiency in rats exacerbates myocardial ischaemia–reperfusion injury through depletion of glutathione. Br. J. Nutr. 2019;121:961–973. doi: 10.1017/S0007114519000230. [DOI] [PubMed] [Google Scholar]
- 89.Majewski M., Ognik K., Thoene M., Rawicka A., Juskiewicz J. Resveratrol modulates the blood plasma levels of Cu and Zn, the antioxidant status and the vascular response of thoracic arteries in copper deficient Wistar rats. Toxicol. Appl. Pharmacol. 2020;390:114877. doi: 10.1016/j.taap.2020.114877. [DOI] [PubMed] [Google Scholar]
- 90.Al-Chalabi A., Hardiman O. The epidemiology of ALS: A conspiracy of genes, environment and time. Nat. Rev. Neurol. 2013;9:617–628. doi: 10.1038/nrneurol.2013.203. [DOI] [PubMed] [Google Scholar]
- 91.Ermilova I.P., Ermilov V.B., Levy M., Ho E., Pereira C., Beckman J.S. Protection by dietary zinc in ALS mutant G93A SOD transgenic mice. Neurosci. Lett. 2005;379:42–46. doi: 10.1016/j.neulet.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 92.Estévez A.G., Crow J.P., Sampson J.B., Reiter C., Zhuang Y., Richardson G.J., Tarpey M.M., Barbeito L., Beckman J.S. Induction of Nitric Oxide-Dependent Apoptosis in Motor Neurons by Zinc-Deficient Superoxide Dismutase. Science. 1999;286:2498–2500. doi: 10.1126/science.286.5449.2498. [DOI] [PubMed] [Google Scholar]
- 93.McAllum E.J., Roberts B.R., Hickey J.L., Dang T.N., Grubman A., Donnelly P.S., Liddell J.R., White A.R., Crouch P.J. ZnII(atsm) is protective in amyotrophic lateral sclerosis model mice via a copper delivery mechanism. Neurobiol. Dis. 2015;81:20–24. doi: 10.1016/j.nbd.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 94.Greenough M.A., Volitakis I., Li Q.-X., Laughton K., Evin G., Ho M., Dalziel A.H., Camakaris J., Bush A.I. Presenilins Promote the Cellular Uptake of Copper and Zinc and Maintain Copper Chaperone of SOD1-dependent Copper/Zinc Superoxide Dismutase Activity. J. Biol. Chem. 2011;286:9776–9786. doi: 10.1074/jbc.m110.163964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vanhoutte P.M., Shimokawa H., Feletou M., Tang E.H.C. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017;219:22–96. doi: 10.1111/apha.12646. [DOI] [PubMed] [Google Scholar]
- 96.Teng L., Yu M., Li J.-M., Tang H., Yu J., Mo L.-H., Jin J., Liu X.-Z. Matrix metalloproteinase-9 as new biomarkers of severity in multiple organ dysfunction syndrome caused by trauma and infection. Mol. Cell. Biochem. 2011;360:271–277. doi: 10.1007/s11010-011-1066-0. [DOI] [PubMed] [Google Scholar]
- 97.Mittal R., Patel A.P., Debs L.H., Nguyen D., Patel K., Grati M., Mittal J., Yan D., Chapagain P., Liu X.Z. Intricate Functions of Matrix Metalloproteinases in Physiological and Pathological Conditions. J. Cell. Physiol. 2016;231:2599–2621. doi: 10.1002/jcp.25430. [DOI] [PubMed] [Google Scholar]
- 98.Onal I.K., Altun B., Onal E.D., Kırkpantur A., Oz S.G., Turgan C. Serum levels of MMP-9 and TIMP-1 in primary hypertension and effect of antihypertensive treatment. Eur. J. Intern. Med. 2009;20:369–372. doi: 10.1016/j.ejim.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 99.Huang S.-T., Yang R.-C., Wu H.-T., Wang C.-N., Pang J.-H. Zinc-Chelation Contributes to the Anti-Angiogenic Effect of Ellagic Acid on Inhibiting MMP-2 Activity, Cell Migration and Tube Formation. PLoS ONE. 2011;6:e18986. doi: 10.1371/journal.pone.0018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raffetto J.D., Khalil R.A. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharmacol. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.MacNee W. Pathogenesis of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2005;2:58–266. doi: 10.1513/pats.200504-045SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karadag F., Cildag O., Altinisik M., Kozaci L.D., Kiter G., Altun C. Trace elements as a component of oxidative stress in COPD. Respirology. 2004;9:33–37. doi: 10.1111/j.1440-1843.2003.00534.x. [DOI] [PubMed] [Google Scholar]
- 103.Lang C.J., Hansen M., Roscioli E., Jones J., Murgia C., Ackland M.L., Zalewski P., Anderson G., Ruffin R. Dietary zinc mediates inflammation and protects against wasting and metabolic derangement caused by sustained cigarette smoke exposure in mice. Biometals. 2010;24:23–39. doi: 10.1007/s10534-010-9370-9. [DOI] [PubMed] [Google Scholar]
- 104.Wellinghausen N., Martin M., Rink L. Zinc inhibits interleukin-1-dependent T cell stimulation. Eur. J. Immunol. 1997;27:2529–2535. doi: 10.1002/eji.1830271010. [DOI] [PubMed] [Google Scholar]
- 105.Zhong C., Wu Y., Chang H., Liu C., Zhou L., Zou J., Qi Z. Effect of PKC inhibitor on experimental autoimmune myocarditis in Lewis rats. Oncotarget. 2017;8:54187–54198. doi: 10.18632/oncotarget.17018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chang G., Zheng J., Xiao W., Chang S., Wei Q., Wu H., Tao Y., Yang G., Xie B., Lan X., et al. PKC inhibition of sotrastaurin has antitumor activity in diffuse large B-cell lymphoma via regulating the expression of MCT-1. Acta Biochim. Biophys. Sin. 2018;50:399–407. doi: 10.1093/abbs/gmy021. [DOI] [PubMed] [Google Scholar]
- 107.Bucchieri F., Puddicombe S.M., Lordan J.L., Richter A., Buchanan D., Wilson S.J., Ward J., Zummo G., Howarth P.H., Djukanović R., et al. Asthmatic Bronchial Epithelium Is More Susceptible to Oxidant-Induced Apoptosis. Am. J. Respir. Cell Mol. Biol. 2002;27:179–185. doi: 10.1165/ajrcmb.27.2.4699. [DOI] [PubMed] [Google Scholar]
- 108.Roscioli E., Hamon R., Lester S., Murgia C., Grant J., Zalewski P. Zinc-rich inhibitor of apoptosis proteins (IAPs) as regulatory factors in the epithelium of normal and inflamed airways. Biometals. 2013;26:205–227. doi: 10.1007/s10534-013-9618-2. [DOI] [PubMed] [Google Scholar]
- 109.Riccioni G., D’Orazio N. The role of selenium, zinc and antioxidant vitamin supplementation in the treatment of bronchial asthma: Adjuvant therapy or not? Expert Opin. Investig. Drugs. 2005;14:1145–1155. doi: 10.1517/13543784.14.9.1145. [DOI] [PubMed] [Google Scholar]
- 110.Yagi T., Asakawa A., Ueda H., Ikeda S., Miyawaki S., Inui A. The Role of Zinc in the Treatment of Taste Disorders. Recent Pat. Food Nutr. Agric. 2013;5:44–51. doi: 10.2174/2212798411305010007. [DOI] [PubMed] [Google Scholar]
- 111.Komai M., Goto T., Suzuki H., Takeda T., Furukawa Y. Zinc deficiency and taste dysfunction; Contribution of carbonic anhydrase, a zinc-metalloenzyme, to normal taste sensation. BioFactors. 2000;12:65–70. doi: 10.1002/biof.5520120111. [DOI] [PubMed] [Google Scholar]
- 112.Henkin R.I., Martin B.M., Agarwal R.P. Efficacy of exogenous oral zinc in treatment of patients with carbonic anhydrase VI deficiency. Am. J. Med. Sci. 1999;318:392–405. doi: 10.1097/00000441-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 113.Seo H.-J., Cho Y.-E., Kim T., Shin H.-I., Kwun I.-S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2010;4:356–361. doi: 10.4162/nrp.2010.4.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sadighi A., Mahdavi-Roshan M., Moradi A., Ostadrahimi A. The effects of zinc supplementation on serum zinc, alkaline phosphatase activity and fracture healing of bones. Saudi Med. J. 2008;29:1276–1279. [PubMed] [Google Scholar]
- 115.Peretz A., Papadopoulos T., Willems D., Hotimsky A., Michiels N., Siderova V., Bergmann P., Nève J. Zinc supplementation increases bone alkaline phosphatase in healthy men. J. Trace Elem. Med. Biol. 2001;15:175–178. doi: 10.1016/S0946-672X(01)80063-8. [DOI] [PubMed] [Google Scholar]
- 116.Skrajnowska D., Bobrowska-Korczak B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients. 2019;11:2273. doi: 10.3390/nu11102273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang Y., Tian Y., Zhang H., Xu B., Chen H. Potential pathways of zinc deficiency-promoted tumorigenesis. Biomed. Pharmacother. 2021;133:110983. doi: 10.1016/j.biopha.2020.110983. [DOI] [PubMed] [Google Scholar]
- 118.Eide D.J. The oxidative stress of zinc deficiency. Metallomics. 2011;3:1124–1129. doi: 10.1039/c1mt00064k. [DOI] [PubMed] [Google Scholar]
- 119.Kocdor H., Ates H., Aydin S., Cehreli R., Soyarat F., Kemanli P., Harmanci D., Cengiz H., Kocdor M. Zinc supplementation induces apoptosis and enhances antitumor efficacy of docetaxel in non-small-cell lung cancer. Drug Des. Dev. Ther. 2015;9:3899–3909. doi: 10.2147/DDDT.S87662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wan S., Taccioli C., Jiang Y., Chen H., Smalley K.J., Huang K., Liu X., Farber J.L., Croce C.M., Fong L.Y.Y. Zinc deficiency activates S100A8 inflammation in the absence of COX-2 and promotes murine oral-esophageal tumor progression. Int. J. Cancer. 2011;129:331–345. doi: 10.1002/ijc.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Taccioli C., Chen H., Jiang Y., Liu X.P., Huang K., Smalley K.J., Farber J.L., Croce C.M., Fong L. Dietary zinc deficiency fuels esophageal cancer development by inducing a distinct inflammatory signature. Oncogene. 2011;31:4550–4558. doi: 10.1038/onc.2011.592. [DOI] [PMC free article] [PubMed] [Google Scholar]