Abstract
Background:
Mounting evidence has shown that long-term exposure to fine particulate matter [PM in aerodynamic diameter ()] and ozone () can increase mortality. However, the health effects associated with long-term exposure to nitrogen dioxide () are less clear, in particular the evidence is scarce for at low levels that are below the current international guidelines.
Methods:
We constructed a population-based full cohort comprising all Medicare beneficiaries (aged , ) in the southeastern United States from 2000 to 2016, and we then further defined the below-guideline cohort that included only those who were always exposed to low-level , that is, with annual means below the current World Health Organization guidelines (i.e., ). We applied previously estimated spatially and temporally resolved concentrations and assigned annual means to study participants based on their ZIP code of residence. Cox proportional hazards models were used to examine the association between long-term exposure to low-level and all-cause mortality, adjusting for potential confounders.
Results:
About 71.1% of the Medicare beneficiaries in the southeastern United States were always exposed to low-level over the study period. We observed an association between long-term exposure to low-level and all-cause mortality, with a 1.042 (95% CI: 1.040, 1.045) in single-pollutant models and a 1.047 (95% CI: 1.045, 1.049) in multipollutant models (adjusting for and ), per increase in annual concentrations. The penalized spline indicates a linear exposure–response relationship across the entire exposure range. Medicare enrollees who were White, female, and residing in urban areas were more vulnerable to long-term exposure.
Conclusion:
Using a large and representative cohort, we provide epidemiological evidence that long-term exposure to , even below the national and global ambient air quality guidelines, was approximately linearly associated with a higher risk of mortality among older adults, independent of and exposure. Improving air quality by reducing emissions, therefore, may yield significant health benefits. https://doi.org/10.1289/EHP9044
Introduction
Air pollution is among the most critical environmental and public health concerns worldwide (Chen and Kan 2008). The adverse health effects of exposure to ambient fine particulate matter [PM in aerodynamic diameter ()] (Akintoye et al. 2016; Chen and Hoek 2020; Shi et al. 2020) and ozone () (Turner et al. 2016) have been widely reported (Cesaroni et al.2012; Cohen et al. 2017; Danesh Yazdi et al. 2021; Silva et al. 2016; Wei et al. 2020) in previous epidemiological studies; however, the relationship between ambient nitrogen dioxide () exposure and mortality is less understood. Although the risk associated with acute exposure and premature mortality has been studied more extensively (Chen et al. 2012; Chiusolo et al. 2011; Mills et al. 2016; Samoli et al. 2006), the evidence remains limited for long-term exposure. Fewer epidemiological studies have investigated the mortality risks associated with long-term concentrations (Eum et al. 2019; Faustini et al. 2014; Hoek et al. 2013).
gas, as one of the highly reactive nitrogen oxides (), primarily derives from high-temperature combustion processes. In the United States, motor vehicle emissions are the predominant source of , and high levels of are observed along highways and in cities (Di et al. 2020). can be coemitted on roadways with other traffic-related tailpipe and nontailpipe emissions, such as black carbon, organic carbon, and trace metals (WHO 2021). is therefore often considered a surrogate for traffic-related air pollutants (Alotaibi et al. 2019). Other major sources of also include power plants and off-road equipment (U.S. EPA 2011, 2020).
Recent evidence suggests that long-term exposure to may be linked to premature mortality (COMEAP 2018; U.S. EPA 2019). Two recent systematic reviews, Huangfu and Atkinson (2020) and Huang et al. (2021), both reported a positive association between long-term exposure and all-cause mortality, and they noted that more large-scale cohort studies exploring the concentration–response relationship are encouraged (Huang et al. 2021; Huangfu and Atkinson 2020). In addition, several more recent large cohort studies have reported positive associations between and all-cause and cause-specific mortality, expanding the evidence base globally, including studies conducted in Europe (Samoli et al. 2021), Canada (Paul et al. 2020; Zhang et al. 2021), Netherlands (Klompmaker et al. 2020; Klompmaker et al. 2021), Denmark (So et al. 2020), Greece (Kasdagli et al. 2021), Japan (Yorifuji and Kashima 2020), and South Korea (Jung et al. 2020). Yet, among the growing body of literature, a high degree of regional heterogeneity has been observed. A few studies have assessed exposure across a broad geographic area (Heinrich et al. 2013; Jerrett et al. 2013; Maheswaran et al. 2010), albeit at a relatively coarse spatial resolution. Thus, previous studies are limited in their ability to quantify the spatial variability of long-term exposure resulting from local variations in traffic-related emissions, which may impact overall measures of association. Further, although many studies have used multipollutant models to estimate associations with after adjusting for other pollutants, these studies reported mixed results (Faustini et al. 2014; Huang et al. 2021; Stieb et al. 2021), and the independent association between and all-cause mortality remains unclear (COMEAP 2018).
To protect public health from adverse health outcomes induced by air pollution, the World Health Organization (WHO) has set evidence-based guidelines on ambient air pollution to inform environmental policy and national air quality targets (WHO 2006). The target guidelines for is currently set at an annual average of () (WHO 2006). Similarly, in the United States, the National Ambient Air Quality Standards (NAAQS) are set and periodically revised by the U.S. Environmental Protection Agency on the basis of the best available scientific evidence, and the current NAAQS for annual mean is (U.S. EPA 2021). However, it is not clear whether these standards are actually safe enough to protect public health.
We recently estimated temporally and spatially resolved concentrations in the United States through an ensemble model that integrates multiple machine learning algorithms—including neural network, random forest, and gradient boosting—with a variety of predictor variables (e.g., satellite remote sensing and chemical transport models) (Di et al. 2020). This approach allows one to estimate daily at a resolution across the contiguous United States from 2000 to 2016 with an excellent prediction model performance. Therefore, we were able to assess long-term exposures of for population-based cohort studies, with residents living far from monitors, as well as for those potentially exposed to low-level .
To address these critical gaps in knowledge, we conducted a large population-based cohort study encompassing all Medicare beneficiaries ( of age) from 2000 to 2016 in the southeastern United States, using a high-resolution spatiotemporal ensemble model that can better capture air pollution data in rural and suburban areas. Focusing on the independent health effect of long-term exposure to low-level , we performed a multipollutant analysis to estimate the risk of all-cause mortality among the Medicare population associated with exposure (yearly average) to concentrations of below the WHO annual guidelines of () in an effort to better clarify the potential mortality risks attributable to air pollution levels below the current national and global ambient air quality guidelines.
Materials and Methods
Study Population
The study population comprised all Medicare beneficiaries who were of age over from 2000 to 2016 in seven southeastern U.S. states (Alabama, Florida, Georgia, Mississippi, North Carolina, South Carolina, and Tennessee). We constructed an open cohort from 1 January 2000 to 31 December 2016, with all-cause mortality as the outcome. We obtained individual-level characteristics, including the year and age of Medicare enrollment, date of death, current age, sex, race, ZIP code of residence, and Medicaid eligibility [a proxy for socioeconomic status (SES), that is, an individual eligible for both Medicare and Medicaid is likely to be of lower SES], from the Medicare beneficiary denominator file derived from the Centers for Medicare and Medicaid Services (CMS). The ZIP code of residence and calendar year were used for further exposure assignment. We further restricted the population to Medicare beneficiaries who were always exposed to low-level (annual mean ) over the study period (i.e., the low-exposure cohort). This study was approved by the CMS under the data use agreement (RSCH20-55733) and the institutional review board of Emory University (STUDY00000316), and a waiver of informed consent was granted.
Exposure
We applied previously estimated daily concentrations at a resolution in the United States from 2000 to 2016 using an ensemble model that integrated multiple machine learning algorithms and predictor variables (Di et al. 2020). Briefly, we respectively fit a neural network, a random forest, and a gradient boosting model with input predictor variables (satellite remote sensing, chemical transport models, meteorological variables, and multiple land-cover terms) and monitored measurements to generate daily predictions. This ensemble learning approach yielded a cross-validated mean of 0.79 and an average root mean square error of . For each ZIP code, daily concentrations were averaged across all covered grid cells with centroids that fell within the ZIP code boundary. We further calculated annual means (time-varying 1-y averages) and assigned these to Medicare beneficiaries according to their ZIP code of residence.
Covariates
Daily and concentrations were previously estimated at a resolution in the United States from 2000 to 2016 using the same ensemble model (Di et al. 2019; Requia et al. 2020). This trained model produced cross-validated mean values of 0.84 and 0.90 for and , respectively. We then aggregated daily predictions based on all covered grid cells, and further calculated annual averages for and warm-season averages for for each year relative to the ZIP code of residence. The warm season, defined as 1 May to 31 October, is a specific time window for examining the health effects of because the warm climate favors the formation and accumulation of in the atmosphere (Zhang et al. 2019).
We obtained eight ZIP code tabulation areas–level variables from the 2000 U.S. Census (U.S. Census 2002), 2010 U.S. Census (U.S. Census 2011), and the American Community Survey for 2005 to 2012 (U.S. Census 2020), and matched these variables to the ZIP code scale. The eight variables included median home value, percentage of owner-occupied housing units, median household income, population density, percentage of Black population, percentage of Hispanic population, percentage of those with a low education level (i.e., with less than a high school degree), and the percentage of those below the poverty level. Behavioral risk factors, including body mass index (BMI) and percentage of those who have ever smoked, were obtained at the county level from the Behavioral Risk Factor Surveillance System (BRFSS) between 2000 and 2016 (CDC 2020). We assigned county-level variables to a given ZIP code if the centroid was located within the county boundary. We linearly interpolated or extrapolated any missing data based on the available data (Junninen et al. 2004), in other words, all area-level variables were time-varying. These annual average data were assigned to individuals according to the ZIP code of residence.
Daily resolution air temperature data were acquired for the southeastern United States between 2000 and 2016 from a national meteorology data product (Daymet, version 4) (Thornton et al. 2020). Daily temperature data were averaged for each ZIP code, and seasonal averages, including the mean temperature for summer (June–August) and winter (December–February), were calculated for each ZIP code and each year. We then assigned the seasonal mean temperature estimates to each participant according to the ZIP code of residence. Because more evidence has been found that seasonal temperature, particularly summer mean and winter mean temperature, has been associated with both all-cause mortality and air pollution levels (Duan et al. 2019; Park et al. 2011), we adjusted for summer and winter mean temperature in our main analyses.
Statistical Analysis
A counting process survival data set, based on the scheme presented by Andersen and Gill (1982), was constructed using the individual-level data. Namely, each observation represented a single person-year of mortality follow-up, with follow-up taking place at the beginning of the calendar year, whereas deaths were assessed at the end of each calendar year. We fit a series of single-, bi-, and tri-pollutant Cox proportional hazards models, with years of follow-up as the time scale, to estimate hazard ratios (HR) per increase in annual mean exposure associated with all-cause mortality among the elderly population in both cohorts. All models were stratified by 5-y age categories, sex (female, male), and race (White, Black, and other), as well as by Medicaid eligibility, adjusting for indicators of calendar year, summer and winter mean temperature, median home value, median household income, population density, the proportion of owner-occupied housing units, and other demographic and behavioral risk factors, including the percentage of Black and Hispanic populations, education level, population below poverty level, BMI, and the proportion of those who were ever smokers.
To identify the most vulnerable subgroups, we evaluated effect modification by sex (female vs. male), race (White vs. Black vs. other), age ( vs. ), Medicaid eligibility (dual vs. nondual eligibility), urbanicity (quartiles of population density), and area-level SES indicator (a measure showing socioeconomic status; high SES vs. low SES) in tri-pollutant Cox models, by including interaction terms between these potential effect modifiers and . We included race as a covariate and effect modifier in our analysis to reflect the racial disparity. We applied the Wald test (Kaufman and MacLehose 2013) to assess whether one subgroup had a larger effect than another, and the was chosen to suggest statistical significance. Dual eligibility subgroups refer to individuals who were eligible for both Medicare and Medicaid benefits, nondual otherwise. Area-level SES was defined as either below or above the median of the distribution of percentage below the poverty level. To assess the potential nonlinearity of the exposure–response relationship, we fit penalized spline regressions with penalized splines for , adjusting for all covariates and co-pollutants.
We performed the bootstrap method to calculate statistically robust confidence intervals and account for potential spatial dependency in the Cox model. Given that the model treats the observations as independent, it may not adequately capture spatial patterns. bootstrapping was performed by randomly sampling ZIP codes of a total of ZIP codes for each bootstrap replicate ( 2 times the square root of , 500 replicates in total) (Bickel et al.2012). Doing so, breaks down the underlying spatial dependence as randomly sampled ZIP codes were not necessarily adjacent in each bootstrapped sample, yielding more robust standard errors and thus 95% confidence intervals. Therefore, it is least likely that our findings are influenced by spatial correlation.
We conducted several sensitivity analyses to assess the robustness of our results. First, we fit alternative models, excluding different covariates, including co-pollutant, time trends, SES, meteorology variables, behavioral risk factors, and baseline hazard stratification. We also tested how sensitive our models might be to adjust for space with a spatial smoother and with a state-level adjustment. We then compared the results of these models to examine the influence of potential confounders. Second, we evaluated the potential heterogeneity of associations by each U.S. state. Third, we fit single-pollutant penalized spline models, and tested whether the exposure–response relationship held under both scenarios (i.e., single-pollutant vs. multipollutant models).
The Medicare data set was stored and analyzed in the Rollins High-Performance Computing Cluster at Emory University, with Health Insurance Portability and Accountability Act compliance. R software, version 4.0.2 (R Development Core Team) was used in this study. The results were rounded to three decimal places to differentiate the upper and lower bounds of the confidence intervals. The estimated results with were considered statistically significant.
Results
We included a total of 13,590,387 Medicare enrollees residing in 10,193 ZIP codes and 1,701 counties in the southeastern United States, with 107,291,652 person-years of follow-up in our full cohort study between 2000 through 2016. Each ZIP code had a mean [] of Medicare beneficiaries. A total of 4,898,015 (36.0%) participants died between 2000 and 2016. Among the full cohort, 9,669,469 (71.1%) Medicare enrollees living in 7,541 ZIP codes were always exposed to annual mean concentrations below WHO air quality guidelines (21 ppb), with 2,814,617 (29.1%) deaths in 69,077,046 person-years of follow-up. The median follow-up years for the full cohort and the below-WHO guidelines cohort were 8 and 7 y, respectively. Nearly all (99.95%) of the Medicare enrollees were always exposed to annual mean concentrations below the U.S. NAAQS (53 ppb). Detailed characteristics of the study population and summary statistics for all covariates are presented in Table 1 and Table S1.
Table 1.
Descriptive statistics of full cohort () and below-WHO guidelines cohort () created from Medicare beneficiary denominator from 2000 to 2016 in seven southeastern U.S. states.
| Categories | Full cohort | Below-WHO guidelines cohorta | ||
|---|---|---|---|---|
| % | % | |||
| Full cohort | ||||
| Deaths | 4,898,015 | 36.0 | 2,814,617 | 29.1 |
| Total population | 13,590,387 | 100 | 9,669,469 | 100 |
| Total person-years | 107,291,652 | 100 | 69,077,046 | 100 |
| Median follow-up year | 8 | 7 | ||
| Age at entry (y) | ||||
| 65–74 | 13,527,082 | 99.5 | 9,632,655 | 99.6 |
| 75–84 | 53,181 | 0.4 | 30,404 | 0.3 |
| 85–94 | 9,523 | 0.07 | 6,008 | 0.06 |
| 599 | 0.004 | 402 | 0.004 | |
| Sex | ||||
| Male | 5,943,391 | 43.7 | 4,321,795 | 44.7 |
| Female | 7,646,996 | 56.3 | 5,347,674 | 55.3 |
| Race | ||||
| White | 11,217,509 | 82.5 | 8,073,062 | 83.5 |
| Black | 1,745,096 | 12.8 | 1,190,084 | 12.3 |
| Otherb | 627,782 | 4.6 | 406,323 | 4.2 |
| Medicaid eligibility | ||||
| Dual-eligible | 1,718,169 | 12.6 | 1,154,668 | 11.9 |
| Non–dual-eligible | 11,872,218 | 87.4 | 8,514,801 | 88.1 |
Note: The seven states include Alabama, Florida, Georgia, Mississippi, North Carolina, South Carolina, and Tennessee. WHO, World Health Organization.
The cohort was restricted to populations who were always exposed to annual mean levels below the current WHO guidelines, i.e. .
Other included Asian, Hispanic, American Indian or Alaskan Native, and unknown.
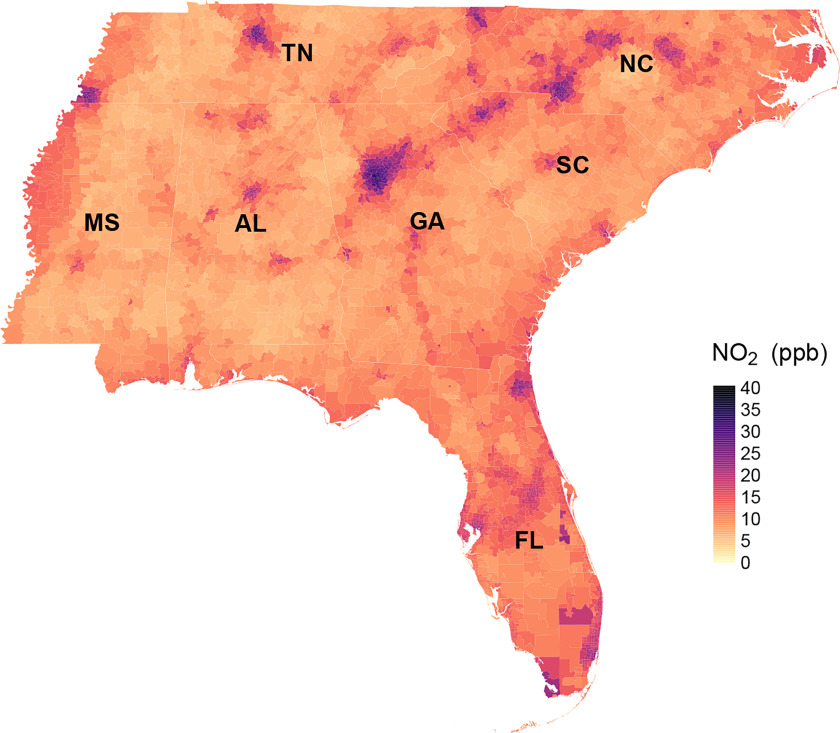
Overall, from 2000 to 2016, the mean annual concentration across the southeastern United States was 13.7 ppb, with an interquartile range (IQR) of 9.3 ppb (Table 2). The spatial distribution of long-term concentrations is presented in Figure 1, which appears to depict patterns consistent with major roadways (Figure S1) and concentrations at resolution (Figure S2). The SDs of the concentrations within ZIP code areas are shown in Figure S3. The temporal trend of long-term concentrations by state is shown in Figure S4. At the beginning of the study period, the lowest annual levels were observed in Mississippi, with the highest annual levels observed in Florida. We observed a declining trend of concentrations over the study period, apart from elevated levels between 2009 and 2011.
Table 2.
Spatial and temporal variability of annual levels (in ppb) in years 2000–2016.
| Categories | Min | Percentile | Max | Mean | ||||
|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | ||||
| Overall | 0.58 | 5.25 | 8.36 | 12.09 | 17.68 | 27.10 | 56.95 | 13.65 |
| By year | ||||||||
| 2000 | 3.32 | 8.26 | 14.11 | 20.17 | 25.62 | 34.34 | 52.47 | 20.30 |
| 2001 | 4.06 | 7.51 | 11.65 | 17.39 | 23.74 | 33.56 | 49.62 | 18.45 |
| 2002 | 2.82 | 6.19 | 9.91 | 15.45 | 21.60 | 30.69 | 42.82 | 16.37 |
| 2003 | 2.71 | 5.28 | 8.97 | 14.06 | 20.60 | 29.67 | 52.11 | 15.32 |
| 2004 | 2.07 | 7.19 | 10.23 | 14.45 | 19.49 | 27.79 | 46.07 | 15.48 |
| 2005 | 3.33 | 6.07 | 9.04 | 13.49 | 19.50 | 27.43 | 44.91 | 14.72 |
| 2006 | 2.11 | 5.44 | 7.90 | 12.13 | 19.28 | 26.50 | 41.54 | 13.97 |
| 2007 | 1.93 | 4.70 | 6.57 | 9.97 | 17.11 | 27.09 | 42.75 | 12.41 |
| 2008 | 2.42 | 6.12 | 8.00 | 11.18 | 16.63 | 25.13 | 35.97 | 12.87 |
| 2009 | 0.93 | 4.74 | 6.33 | 9.30 | 14.56 | 21.00 | 30.62 | 10.78 |
| 2010 | 0.58 | 5.23 | 8.03 | 10.92 | 15.18 | 23.52 | 36.62 | 12.14 |
| 2011 | 3.98 | 7.37 | 10.25 | 12.58 | 15.64 | 22.95 | 41.41 | 13.49 |
| 2012 | 2.94 | 7.77 | 10.00 | 11.85 | 14.59 | 21.15 | 49.47 | 12.86 |
| 2013 | 2.37 | 4.67 | 7.01 | 9.51 | 12.82 | 19.18 | 56.95 | 10.44 |
| 2014 | 2.39 | 4.51 | 6.62 | 9.54 | 14.34 | 21.12 | 39.01 | 10.99 |
| 2015 | 0.97 | 4.78 | 7.78 | 10.48 | 13.83 | 20.05 | 32.08 | 11.16 |
| 2016 | 1.09 | 3.84 | 6.31 | 9.36 | 14.03 | 20.84 | 31.44 | 10.59 |
| By state | ||||||||
| Alabama | 2.21 | 4.71 | 6.96 | 9.77 | 14.45 | 21.50 | 37.55 | 11.17 |
| Florida | 1.98 | 7.28 | 11.35 | 14.87 | 19.32 | 25.50 | 45.19 | 15.54 |
| Georgia | 2.58 | 5.64 | 8.56 | 11.90 | 19.24 | 32.37 | 52.47 | 14.86 |
| Mississippi | 2.24 | 4.63 | 6.78 | 9.52 | 13.87 | 20.19 | 29.85 | 10.70 |
| North Carolina | 0.58 | 5.58 | 8.87 | 12.70 | 19.01 | 29.06 | 46.07 | 14.54 |
| South Carolina | 2.97 | 5.55 | 8.23 | 11.17 | 16.00 | 25.38 | 37.71 | 12.78 |
| Tennessee | 0.94 | 4.55 | 6.87 | 10.08 | 16.90 | 27.71 | 56.95 | 12.58 |
Note: , nitrogen dioxide.
Figure 1.
The spatial distribution of 17-y mean concentrations of annual at ZIP code level in the southeastern United States (2000–2016). Note: , nitrogen dioxide.
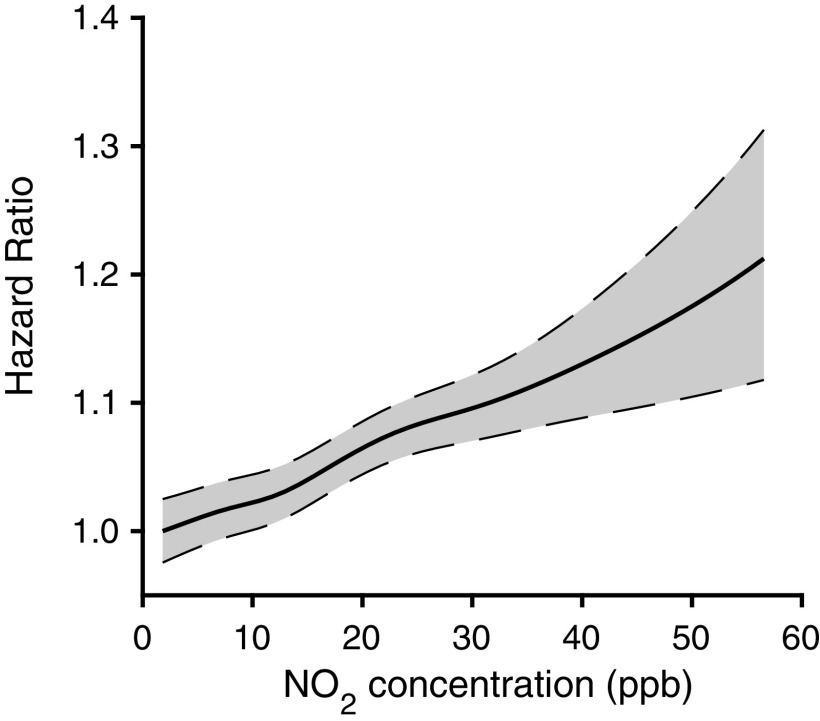
Overall, long-term exposure to , even at low levels, was significantly and positively associated with mortality in all statistical analyses (Table 3). In single-pollutant models, we observed a 1.042 [95% confidence interval (CI): 1.040, 1.045] per increase in concentrations. After adjusting for and , the results for were similar (the estimated results for and are presented in Table S2). The observed relationship between long-term concentrations and mortality appears to be approximately linear across the exposure distribution, because the concentration–response curve does not suggest a threshold for mortality at low concentrations of , the slope of the curve does not level off at high concentrations at least in the range examined, and the nonlinearity derived from the penalized spline fitting is within the model uncertainty (Figure 2).
Table 3.
Estimated hazard ratio of mortality (95% CI) associated with an increase of in concentration using Cox proportional hazards model for both full cohort and below-WHO guidelines cohort.
| Models | Full cohort () | Below-WHO guidelines cohorta () |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Single-pollutantb | 1.042 (1.039, 1.044) | 1.042 (1.040, 1.045) |
| Bi-pollutant ()c | 1.042 (1.040, 1.044) | 1.042 (1.040, 1.045) |
| Bi-pollutant ()d | 1.047 (1.045, 1.049) | 1.047 (1.045, 1.050) |
| Tri-pollutante | 1.047 (1.044, 1.049) | 1.047 (1.045, 1.049) |
Note: Estimates are based on increments for . CI, confidence interval; HR, hazard ratio; , nitrogen dioxide; , ozone; , particulate matter in aerodynamic diameter; WHO, World Health Organization.
The cohort was restricted to populations who were always exposed to annual mean levels below the current WHO guidelines, i.e., .
Single-pollutant model: stratified by age at entry (5-y categories), sex (female, male), race (White, Black, and other), Medicaid eligibility, and adjusted for calendar year, summer and winter mean temperature, median home value, median household income, population density, the proportion of owner-occupied housing units, the percentage of Black and Hispanic populations, education level, population below poverty level, body mass index, and the proportion of those who were ever smokers. The descriptive statistics for these variables are provided in Table 1 and Table S1.
Bi-pollutant (): single-pollutant model further adjusted for annual mean of .
Bi-pollutant (): single-pollutant model further adjusted for annual warm-season average of .
Tri-pollutant: single-pollutant model further adjusted for annual mean of and annual warm-season average of .
Figure 2.
The exposure–response relationship between long-term exposure to and all-cause mortality, derived from tri-pollutant models with adjustment of annual mean of , annual warm-season average of , age at entry (5-y categories), sex (female, male), race (White, Black, and other), Medicaid eligibility, calendar year, summer and winter mean temperature, median home value, median household income, population density, the proportion of owner-occupied housing units, the percentage of Black and Hispanic populations, education level, population below poverty level, body mass index, and the proportion of those who were ever smokers. The descriptive statistics for these variables are provided in Table 1 and Table S1. Shaded areas indicate the 95% confidence bands. Note: , nitrogen dioxide; , particulate matter in aerodynamic diameter; , ozone.
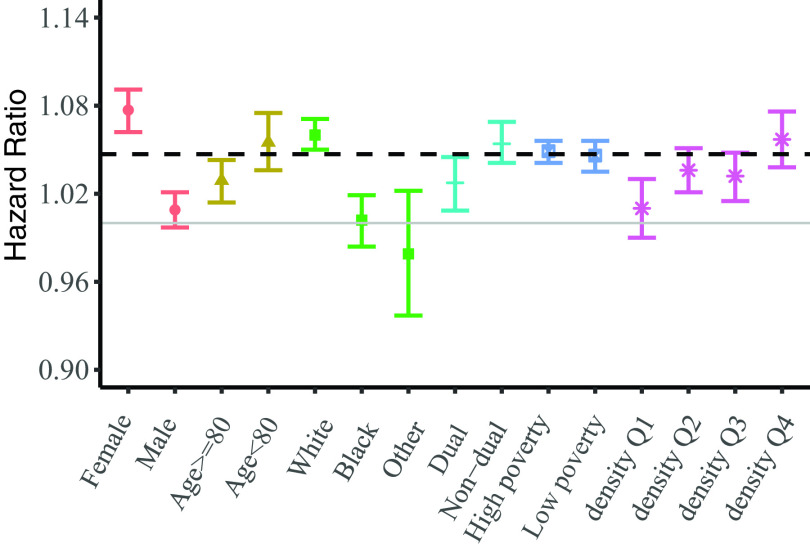
In effect modification analyses, we observed significantly higher risks among White individuals ( 1.060; 95% CI: 1.050, 1.071) (), women ( 1.077; 95% CI: 1.062, 1.091) (), and those residing in urban areas ( 1.057; 95% CI: 1.038, 1.076) (). In addition, measures of association were higher among the relatively younger individuals ( of age), although not significant (). Further details are presented in Figure 3 and Table S3.
Figure 3.
The hazard ratios of mortality associated with a increase in concentrations for study subgroups. Density Q1–Q4 stand for low population density, low-medium population density, medium-high population density, and high population density, respectively. The numeric data for these measures of associations are provided in the Table S3. Note: , nitrogen dioxide; Q, quartile.
In our sensitivity analyses, excluding time trends changed the HR the most compared with the main analysis (1.251; 95% CI: 1.248, 1.254; Table S4). Analyses stratifying by state yielded consistently positive associations between long-term exposure and mortality, with the highest HR observed among Medicare beneficiaries in North Carolina (1.067; 95% CI: 1.060, 1.074; Table S5). Last, the single-pollutant and multipollutant penalized spline models yielded almost identical splines, both suggesting approximately linear exposure–response relationships for annual and all-cause mortality (Figure 2; Figure S5).
Discussion
In this large-scale population-based cohort study, we observed a significant and independent association between long-term exposure to and all-cause mortality among the Medicare population even at levels below global and national guidelines. We further observed a roughly linear trend in mortality risk after adjusting for and , indicating no apparent evidence of a threshold value. We also observed larger measures of association among White populations, women, and urban residents, indicating potential susceptibility among these groups.
Our results of an increased all-cause mortality risk ( 1.047; 95% CI: 1.045, 1.049, per increase in annual average ) are broadly consistent with previous cohort studies (Table S6), particularly among those using spatiotemporal exposure assessments (Cesaroni et al. 2013; Crouse et al. 2015; Eum et al. 2019; Faustini et al. 2014; Fischer et al. 2015; Hart et al. 2011; Hoek et al. 2013; Jerrett et al. 2013; Klompmaker et al. 2021; Nieuwenhuijsen et al. 2018; Paul et al. 2020; Samoli et al. 2021; Turner et al. 2016). In a recent Medicare cohort study, Eum et al. (2019) examined the impact of exposure and mortality by region of the United States between 2000 and 2008 using ground-based monitoring data for measures and a bipollutant Poisson regression model adjusted for (Eum et al. 2019). Overall, they reported an increased all-cause mortality risk with similar measures of association per increase in annual average ( 1.052; 95% CI: 1.051, 1.054).
Other U.S.-based studies have previously reported comparable results, including the American Cancer Society’s Cancer Prevention Study II follow-up study reported by Turner et al. (2016) ( 1.01; 95% CI: 1.00, 1.03 per 10-unit increase) and Jerrett et al. (2013) ( 1.032; 95% CI: 1.008, 1.057 per an IQR increase of 4.1167 ppb) (Jerrett et al. 2013; Turner et al. 2016), whereas larger measures of association have been observed in single-pollutant models reported in the Nurses’ Health Study (Hart et al. 2013) ( 1.10; 95% CI: 1.05, 1.15). Our results are additionally supported by the recent Ontario Population Health and Environment Cohort study (Paul et al. 2020) and a previous Canada-wide study (Crouse et al. 2015). Many previous studies have similarly observed positive associations at low annual average concentrations of (Eum et al. 2019; Hart et al. 2013; Jerrett et al. 2013; Turner et al. 2016), including several recent international studies, such as those in Canada (Crouse et al. 2015; Paul et al. 2020; Zhang et al. 2021), Denmark (Hvidtfeldt et al. 2019; So et al. 2020), Netherlands (Beelen et al. 2008; Klompmaker et al. 2021), United Kingdom (Carey et al. 2013; Tonne and Wilkinson 2013), France (Sanyal et al. 2018), Japan (Yorifuji and Kashima 2020), and South Korea (Jung et al. 2020).
However, because so few previous studies have included estimates for mortality risks using multipollutant models, recent studies with more significantly positive measures of association (Jung et al. 2020; Kasdagli et al. 2021; Yorifuji and Kashima 2020; Zhang et al. 2021) may be less reliable, potentially overestimating associations with given the high correlation between co-pollutants. In a recent critical review and meta-analysis, Huangfu and Atkinson (2020) assessed the results of 24 international cohort studies on mortality risk and reported an overall relative risk of 1.02 (95% CI: 1.01, 1.04) per increase in for all-cause mortality (Huangfu and Atkinson 2020). In addition, Huang et al. (2021) reported a pooled 1.06 (95% CI: 1.04, 1.08) for all-cause mortality per increase in annual exposure (Huang et al. 2021). Likewise, two other meta-analyses, Faustini et al.(2014) and Hoek et al.(2013), respectively reported pooled HRs of 1.04 (95% CI: 1.02, 1.06) and 1.05 (95% CI: 1.03, 1.08) for all-cause mortality per increase in .
Although other factors—such as geographical differences, pollutant composition, and relative urbanicity, in addition to methodological differences that challenge the ability to make comparisons across studies, may impact the variability of measures of association (Hoek et al. 2013), multipollutant models—may provide a more accurate characterization of associated effects. However, at present, it is unclear whether the mortality effects observed in previous studies reflect an independent association (COMEAP 2018), underscoring the potentially important contribution of our analysis to the literature. More work is necessary in this regard to better determine the causality of health effects and whether long-term average concentrations of are adequately representative of complex pollutant mixtures.
Few studies have assessed the shape of the exposure–response relationship between and mortality (Huangfu and Atkinson 2020). Our findings demonstrating evidence of linearity across the exposure distribution are supported by results from other recent studies (Dirgawati et al. 2019; Hanigan et al. 2019), suggesting that long-term exposure to , even at levels below current guidelines, is associated with increased mortality.
Another important finding from our study relates to the differential association observed in subgroup analyses. We found a higher average risk of mortality among White populations when compared with other races. One possible reason is that White populations, although less socially vulnerable and presumably, on average, healthier, might be less resilient to . This is consistent with the finding from other race/ethnicity health disparities studies (Breslau et al. 2006). We also found higher mortality risks among women compared with men, which is at odds with the results reported by Crouse et al. (2015); however, too few studies have investigated differential effects of mortality risk by sex; thus, further investigation is warranted. Last, the effect of modification of age was not apparent, which was similar to the study of and mortality in three Canadian cities (Chen et al. 2013).
Long-term exposure to has been associated with acute and chronic respiratory diseases (Abbey et al. 1993), such as increased bronchial hyperresponsiveness (Jammes et al. 1998), increased respiratory infection (Liang et al. 2020), and decreased lung function (Nori-Sarma et al. 2021). Biological evidence has been reported for plausible mechanisms regarding the health effects of . One critical review suggests that inhalation can induce lung function changes, accelerate pulmonary infections, and aggravate existing lung diseases by triggering a pro-inflammatory response, which is an innate immune response (Hesterberg et al. 2009). Moreover, an in vitro study found that can enhance oxidative stress and lead to the generation of reactive oxygen and nitrogen species (Ayyagari et al. 2007), and another study found could deteriorate the cardiovascular and immune systems in mice (Bevelander et al. 2007).
To the best of our knowledge, few studies have restricted ambient exposure below current annual guidelines to investigate the exposure–response relationship between and mortality in a large-scale population-based study (Chen et al. 2013; Sanyal et al. 2018; Yorifuji and Kashima 2020). Our study includes all Medicare beneficiaries in the southeastern United States, which includes all residents exposed to low-level in both rural and urban areas. Our large, representative sample size provides ample statistical power to characterize complex spatiotemporal patterns among populations exposed to low-level pollution concentrations. Taken together, our results may provide a more confident characterization of the independent mortality effects of through the use of single-, bi-, and multipollutant modeling and a rigorous statistical approach for deriving confidence intervals through an bootstrapping approach (as a comparison, Table S7 shows the standard errors before and after bootstrapping).
Several limitations of this study should be acknowledged. First, as with any exposure assessment at an ecologic scale, the potential for exposure misclassification is of particular concern. The use of ZIP codes to estimate long-term exposure to concentrations may not correlate well with individual-level exposure. Although the comparison of major roadways (Figure S1), concentrations (Figure S2), and ZIP code-scale concentrations (Figure 1) suggests that even though ZIP code-level may serve as a good indicator of traffic pollution at the larger scale, large differences in could still occur within a major source area, for example, at locations near major roadways. As such, a scale of exposure may still be too coarse a resolution given the decay gradient of , which limits the ability to capture local or small-area variations in traffic-related pollution and proximity to roads. Second, the Medicare data do not provide the underlying cause of death necessary for understanding possible causal pathways. Third, given the use of administrative data, we cannot exclude the possibility of outcome misclassification due to coding errors or residual confounding bias on account of individual-level risk factors for mortality, such as smoking, alcohol consumption, and physical activity, which were not ascertained in this study. However, this was a semi-individual study because of the exposure aggregation, and these behaviors have been shown in personal exposure studies (Weisskopf and Webster 2017) to be uncorrelated with outdoor exposure levels; they are correlated only through neighborhood-level SES. Therefore, controlling for neighborhood SES and, secondarily, for neighborhood obesity and smoking, is appropriate for confounding adjustment. That said, we must admit that our neighborhood smoking and obesity information is not ideal, because we have only the information gathered on a county level from the BRFSS, and residual confounding remains a concern. Fourth, our findings may not be generalized to younger age groups or represent the vast differences across the United States, where the pollution composition and demographic characteristics vary significantly. Furthermore, having controlled for and , we cannot rule out the possibility that may be an indicator of other traffic-related air pollutants, such as ultrafine particles, soot, and trace metals or other potential noise-related confounding factors (Beckerman et al. 2008; Moshammer et al. 2020).
In conclusion, we found an association between long-term exposure to and all-cause mortality, independent of and exposure. Our findings contribute to the evidence base of the increased risk of mortality associated with traffic-related air pollution. Nevertheless, our results should be taken as part of a growing, although insufficiently studied, area of air pollution epidemiology. Further research is needed to study the association between long-term exposure and mortality, particularly at low levels, with improved methods and measurements of exposure (e.g., improved with increasing spatial monitoring density). Reconsidering both national and international emissions guidelines may yield significant health benefits.
Supplementary Material
Acknowledgments
We thank the staff of the laboratory of J.S. for the preparation of air pollution data and acknowledge X. Wu for fruitful discussion. L.S. was supported by the National Institutes of Health/National Institute of Environmental Health Sciences (R21 ES032606), Emory HERCULES center (P30 ES019776), and Emory Goizueta Alzheimer’s Disease Research Center (P50 AG025688). J.S. was supported by U.S. Environmental Protection Agency grant RD 83587201.
References
- Abbey DE, Colome SD, Mills PK, Burchette R, Beeson WL, Tian Y. 1993. Chronic disease associated with long-term concentrations of nitrogen dioxide. J Expo Anal Environ Epidemiol 3(2):181–202, PMID: . [PubMed] [Google Scholar]
- Akintoye E, Shi L, Obaitan I, Olusunmade M, Wang Y, Newman JD, et al. . 2016. Association between fine particulate matter exposure and subclinical atherosclerosis: a meta-analysis. Eur J Prev Cardiol 23(6):602–612, PMID: , 10.1177/2047487315588758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alotaibi R, Bechle M, Marshall JD, Ramani T, Zietsman J, Nieuwenhuijsen MJ, et al. . 2019. Traffic related air pollution and the burden of childhood asthma in the contiguous United States in 2000 and 2010. Environ Int 127:858–867, PMID: , 10.1016/j.envint.2019.03.041. [DOI] [PubMed] [Google Scholar]
- Andersen PK, Gill RD. 1982. Cox’s regression model for counting processes: a large sample study. Ann Statist 10(4):1100–1120, 10.1214/aos/1176345976. [DOI] [Google Scholar]
- Ayyagari VN, Januszkiewicz A, Nath J. 2007. Effects of nitrogen dioxide on the expression of intercellular adhesion molecule-1, neutrophil adhesion, and cytotoxicity: studies in human bronchial epithelial cells. Inhal Toxicol 19(2):181–194, PMID: , 10.1080/08958370601052121. [DOI] [PubMed] [Google Scholar]
- Beckerman B, Jerrett M, Brook JR, Verma DK, Arain MA, Finkelstein MM. 2008. Correlation of nitrogen dioxide with other traffic pollutants near a major expressway. Atmos Environ 42(2):275–290, 10.1016/j.atmosenv.2007.09.042. [DOI] [Google Scholar]
- Beelen R, Hoek G, van den Brandt PA, Goldbohm RA, Fischer P, Schouten LJ, et al. . 2008. Long-term effects of traffic-related air pollution on mortality in a Dutch cohort (NLCS-AIR study). Environ Health Perspect 116(2):196–202, PMID: , 10.1289/ehp.10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevelander M, Mayette J, Whittaker LA, Paveglio SA, Jones CC, Robbins J, et al. . 2007. Nitrogen dioxide promotes allergic sensitization to inhaled antigen. J Immunol 179(6):3680–3688, PMID: , 10.4049/jimmunol.179.6.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel PJ, Götze F, van Zwet WR. 2012. Resampling fewer than n observations: gains, losses, and remedies for losses. In: Selected works of Willem van Zwet. Selected Works in Probability and Statistics. van de Geer S, Wegkamp M, eds. New York, NY: Springer, 267–297. [Google Scholar]
- Breslau J, Aguilar-Gaxiola S, Kendler KS, Su M, Williams D, Kessler RC. 2006. Specifying race-ethnic differences in risk for psychiatric disorder in a USA national sample. Psychol Med 36(1):57–68, PMID: , 10.1017/S0033291705006161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey IM, Atkinson RW, Kent AJ, van Staa T, Cook DG, Anderson HR. 2013. Mortality associations with long-term exposure to outdoor air pollution in a national English cohort. Am J Respir Crit Care Med 187(11):1226–1233, PMID: , 10.1164/rccm.201210-1758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2020. Behavioral risk factor surveillance system. https://www.cdc.gov/brfss/annual_data/annual_2020.html [accessed 16 August 2021].
- Cesaroni G, Badaloni C, Gariazzo C, Stafoggia M, Sozzi R, Davoli M, et al. . 2013. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect 121(3):324–331, PMID: , 10.1289/ehp.1205862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesaroni G, Porta D, Badaloni C, Stafoggia M, Eeftens M, Meliefste K, et al. . 2012. Nitrogen dioxide levels estimated from land use regression models several years apart and association with mortality in a large cohort study. Environ Health 11:48, PMID: , 10.1186/1476-069X-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Kan H. 2008. Air pollution and population health: a global challenge. Environ Health Prev Med 13(2):94–101, PMID: , 10.1007/s12199-007-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Goldberg MS, Burnett RT, Jerrett M, Wheeler AJ, Villeneuve PJ. 2013. Long-term exposure to traffic-related air pollution and cardiovascular mortality. Epidemiology 24(1):35–43, PMID: , 10.1097/EDE.0b013e318276c005. [DOI] [PubMed] [Google Scholar]
- Chen J, Hoek G. 2020. Long-term exposure to PM and all-cause and cause-specific mortality: a systematic review and meta-analysis. Environ Int 143:105974, PMID: , 10.1016/j.envint.2020.105974. [DOI] [PubMed] [Google Scholar]
- Chen R, Samoli E, Wong CM, Huang W, Wang Z, Chen B, et al. . 2012. Associations between short-term exposure to nitrogen dioxide and mortality in 17 Chinese cities: the China Air Pollution and Health Effects Study (CAPES). Environ Int 45:32–38, PMID: , 10.1016/j.envint.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Chiusolo M, Cadum E, Stafoggia M, Galassi C, Berti G, Faustini A, et al. . 2011. Short-term effects of nitrogen dioxide on mortality and susceptibility factors in 10 Italian cities: the EpiAir study. Environ Health Perspect 119(9):1233–1238, PMID: , 10.1289/ehp.1002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. . 2017. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389(10082):1907–1918, PMID: , 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMEAP (Committee on the Medical Effects of Air Pollutants). 2018. Nitrogen dioxide: effects on mortality. https://www.gov.uk/government/publications/nitrogen-dioxide-effects-on-mortality [accessed 10 June 2021].
- Crouse DL, Peters PA, Hystad P, Brook JR, van Donkelaar A, Martin RV, et al. . 2015. Ambient PM2.5, O3, and NO2 exposures and associations with mortality over 16 years of follow-up in the Canadian Census Health and Environment Cohort (CanCHEC). Environ Health Perspect 123(11):1180–1186, PMID: , 10.1289/ehp.1409276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh Yazdi M, Wang Y, Di Q, Wei Y, Requia WJ, Shi L, et al. . 2021. Long-term association of air pollution and hospital admissions among Medicare participants using a doubly robust additive model. Circulation 143(16):1584–1596, PMID: , 10.1161/CIRCULATIONAHA.120.050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, et al. . 2019. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ Int 130:104909, PMID: , 10.1016/j.envint.2019.104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, et al. . 2020. Assessing NO2 concentration and model uncertainty with high spatiotemporal resolution across the contiguous United States using ensemble model averaging. Environ Sci Technol 54(3):1372–1384, PMID: , 10.1021/acs.est.9b03358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirgawati M, Hinwood A, Nedkoff L, Hankey GJ, Yeap BB, Flicker L, et al. . 2019. Long-term exposure to low air pollutant concentrations and the relationship with all-cause mortality and stroke in older men. Epidemiology 30(suppl 1):S82–S89, PMID: , 10.1097/EDE.0000000000001034. [DOI] [PubMed] [Google Scholar]
- Duan Y, Liao Y, Li H, Yan S, Zhao Z, Yu S, et al. . 2019. Effect of changes in season and temperature on cardiovascular mortality associated with nitrogen dioxide air pollution in Shenzhen, China. Sci Total Environ 697:134051, PMID: , 10.1016/j.scitotenv.2019.134051. [DOI] [PubMed] [Google Scholar]
- Eum KD, Kazemiparkouhi F, Wang B, Manjourides J, Pun V, Pavlu V, et al. . 2019. Long-term NO2 exposures and cause-specific mortality in American older adults. Environ Int 124:10–15, PMID: , 10.1016/j.envint.2018.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustini A, Rapp R, Forastiere F. 2014. Nitrogen dioxide and mortality: review and meta-analysis of long-term studies. Eur Respir J 44(3):744–753, PMID: , 10.1183/09031936.00114713. [DOI] [PubMed] [Google Scholar]
- Fischer PH, Marra M, Ameling CB, Hoek G, Beelen R, de Hoogh K, et al. . 2015. Air pollution and mortality in seven million adults: the Dutch Environmental Longitudinal Study (DUELS). Environ Health Perspect 123(7):697–704, PMID: , 10.1289/ehp.1408254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanigan IC, Rolfe MI, Knibbs LD, Salimi F, Cowie CT, Heyworth J, et al. . 2019. All-cause mortality and long-term exposure to low level air pollution in the ‘45 and Up Study’cohort, Sydney, Australia, 2006–2015. Environ Int 126:762–770, PMID: , 10.1016/j.envint.2019.02.044. [DOI] [PubMed] [Google Scholar]
- Hart JE, Garshick E, Dockery DW, Smith TJ, Ryan L, Laden F. 2011. Long-term ambient multipollutant exposures and mortality. Am J Respir Crit Care Med 183(1):73–78, PMID: , 10.1164/rccm.200912-1903OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JE, Rimm EB, Rexrode KM, Laden F. 2013. Changes in traffic exposure and the risk of incident myocardial infarction and all-cause mortality. Epidemiology 24(5):734–742, PMID: , 10.1097/EDE.0b013e31829d5dae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich J, Thiering E, Rzehak P, Krämer U, Hochadel M, Rauchfuss KM, et al. . 2013. Long-term exposure to NO2 and PM10 and all-cause and cause-specific mortality in a prospective cohort of women. Occup Environ Med 70(3):179–186, PMID: , 10.1136/oemed-2012-100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesterberg TW, Bunn WB, McClellan RO, Hamade AK, Long CM, Valberg PA. 2009. Critical review of the human data on short-term nitrogen dioxide (NO2) exposures: evidence for NO2 no-effect levels. Crit Rev Toxicol 39(9):743–781, PMID: , 10.3109/10408440903294945. [DOI] [PubMed] [Google Scholar]
- Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, et al. . 2013. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health 12(1):43, PMID: , 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Li H, Wang M, Qian Y, Steenland K, Caudle WM, et al. . 2021. Long-term exposure to nitrogen dioxide and mortality: a systematic review and meta-analysis. Sci Total Environ 776:145968, PMID: , 10.1016/j.scitotenv.2021.145968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu P, Atkinson R. 2020. Long-term exposure to NO2 and O3 and all-cause and respiratory mortality: a systematic review and meta-analysis. Environ Int 144:105998, PMID: , 10.1016/j.envint.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidtfeldt UA, Sørensen M, Geels C, Ketzel M, Khan J, Tjønneland A, et al. . 2019. Long-term residential exposure to PM2.5, PM10, black carbon, NO2, and ozone and mortality in a Danish cohort. Environ Int 123:265–272, PMID: , 10.1016/j.envint.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Jammes Y, Delpierre S, Delvolgo MJ, Humbert-Téna C, Burnet H. 1998. Long-term exposure of adults to outdoor air pollution is associated with increased airway obstruction and higher prevalence of bronchial hyperresponsiveness. Arch Environ Health 53(6):372–377, PMID: , 10.1080/00039899809605723. [DOI] [PubMed] [Google Scholar]
- Jerrett M, Burnett RT, Beckerman BS, Turner MC, Krewski D, Thurston G, et al. . 2013. Spatial analysis of air pollution and mortality in California. Am J Respir Crit Care Med 188(5):593–599, PMID: , 10.1164/rccm.201303-0609OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Park JY, Kim YC, Lee H, Kim E, Kim YL, et al. . 2020. Long-term effects of air pollutants on mortality risk in patients with end-stage renal disease. Int J Environ Public Health 17(2):546, PMID: , 10.3390/ijerph17020546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junninen H, Niska H, Tuppurainen K, Ruuskanen J, Kolehmainen M. 2004. Methods for imputation of missing values in air quality data sets. Atmos Environ 38(18):2895–2907, 10.1016/j.atmosenv.2004.02.026. [DOI] [Google Scholar]
- Kasdagli MI, Katsouyanni K, de Hoogh K, Lagiou P, Samoli E. 2021. Associations of air pollution and greenness with mortality in Greece: an ecological study. Environ Res 196:110348, PMID: , 10.1016/j.envres.2020.110348. [DOI] [PubMed] [Google Scholar]
- Kaufman JS, MacLehose RF. 2013. Which of these things is not like the others? Cancer 119(24):4216–4222, PMID: , 10.1002/cncr.28359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompmaker JO, Hoek G, Bloemsma LD, Marra M, Wijga AH, van den Brink C, et al. . 2020. Surrounding green, air pollution, traffic noise exposure and non-accidental and cause-specific mortality. Environ Int 134:105341, PMID: , 10.1016/j.envint.2019.105341. [DOI] [PubMed] [Google Scholar]
- Klompmaker JO, Janssen N, Andersen ZJ, Atkinson R, Bauwelinck M, Chen J, et al. . 2021. Comparison of associations between mortality and air pollution exposure estimated with a hybrid, a land-use regression and a dispersion model. Environ Int 146:106306, PMID: , 10.1016/j.envint.2020.106306. [DOI] [PubMed] [Google Scholar]
- Liang D, Shi L, Zhao J, Liu P, Schwartz J, Gao S, et al. . 2020. Urban air pollution may enhance COVID-19 case-fatality and mortality rates in the United States. medRxiv. Preprint posted online 7 May7 2020, 10.1101/2020.05.04.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheswaran R, Pearson T, Smeeton NC, Beevers SD, Campbell MJ, Wolfe CD. 2010. Impact of outdoor air pollution on survival after stroke: population-based cohort study. Stroke 41(5):869–877, PMID: , 10.1161/STROKEAHA.109.567743. [DOI] [PubMed] [Google Scholar]
- Mills IC, Atkinson RW, Anderson HR, Maynard RL, Strachan DP. 2016. Distinguishing the associations between daily mortality and hospital admissions and nitrogen dioxide from those of particulate matter: a systematic review and meta-analysis. BMJ Open 6(7):e010751, PMID: , 10.1136/bmjopen-2015-010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshammer H, Poteser M, Kundi M, Lemmerer K, Weitensfelder L, Wallner P, et al. . 2020. Nitrogen-dioxide remains a valid air quality indicator. Int J Environ Res Public Health 17(10):3733, PMID: , 10.3390/ijerph17103733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, Gascon M, Martinez D, Ponjoan A, Blanch J, Garcia-Gil MDM, et al. . 2018. Air pollution, noise, blue space, and green space and premature mortality in Barcelona: a mega cohort. Int J Environ Res Public Health 15(11):2405, PMID: , 10.3390/ijerph15112405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nori-Sarma A, Thimmulappa R, Venkataraman G, Warren JL, Berman JD, Whittaker SD, et al. . 2021. NO2 exposure and lung function decline in a cohort of adults in Mysore, India. Environ Res Commun 3(5):055001, 10.1088/2515-7620/abf2dd. [DOI] [Google Scholar]
- Park AK, Hong YC, Kim H. 2011. Effect of changes in season and temperature on mortality associated with air pollution in Seoul, Korea. J Epidemiol Community Health 65(4):368–375, PMID: , 10.1136/jech.2009.089896. [DOI] [PubMed] [Google Scholar]
- Paul LA, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, Bai L, et al. . 2020. The impact of air pollution on the incidence of diabetes and survival among prevalent diabetes cases. Environ Int 134:105333, PMID: , 10.1016/j.envint.2019.105333. [DOI] [PubMed] [Google Scholar]
- Requia WJ, Di Q, Silvern R, Kelly JT, Koutrakis P, Mickley LJ, et al. . 2020. An ensemble learning approach for estimating high spatiotemporal resolution of ground-level ozone in the contiguous United States. Environ Sci Technol 54(18):11037–11047, PMID: , 10.1021/acs.est.0c01791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoli E, Aga E, Touloumi G, Nisiotis K, Forsberg B, Lefranc A, et al. . 2006. Short-term effects of nitrogen dioxide on mortality: an analysis within the APHEA project. Eur Respir J 27(6):1129–1138, PMID: , 10.1183/09031936.06.00143905. [DOI] [PubMed] [Google Scholar]
- Samoli E, Rodopoulou S, Hvidtfeldt UA, Wolf K, Stafoggia M, Brunekreef B, et al. . 2021. Modeling multi-level survival data in multi-center epidemiological studies: applications from the ELAPSE project. Environ Int 3:347, PMID: , 10.1016/j.envint.2020.106371. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Rochereau T, Maesano CN, Com-Ruelle L, Annesi-Maesano I. 2018. Long-term effect of outdoor air pollution on mortality and morbidity: a 12-year follow-up study for metropolitan France. Int J Environ Res Public Health 15(11):2487, PMID: , 10.3390/ijerph15112487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Wu X, Yazdi MD, Braun D, Awad YA, Wei Y, et al. . 2020. Long-term effects of PM2.5 on neurological disorders in the American Medicare population: a longitudinal cohort study. Lancet Planet Health 4(12):e557–e565, PMID: , 10.1016/S2542-5196(20)30227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RA, West JJ, Lamarque JF, Shindell DT, Collins WJ, Dalsoren S, et al. . 2016. The effect of future ambient air pollution on human premature mortality to 2100 using output from the ACCMIP model ensemble. Atmos Chem Phys 16(15):9847–9862, PMID: , 10.5194/acp-16-9847-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So R, Jørgensen JT, Lim YH, Mehta AJ, Amini H, Mortensen LH, et al. . 2020. Long-term exposure to low levels of air pollution and mortality adjusting for road traffic noise: a Danish nurse cohort study. Environ Int 143:105983, PMID: , 10.1016/j.envint.2020.105983. [DOI] [PubMed] [Google Scholar]
- Stieb DM, Berjawi R, Emode M, Zheng C, Salama D, Hocking R, et al. . 2021. Systematic review and meta-analysis of cohort studies of long term outdoor nitrogen dioxide exposure and mortality. PLoS One 16(2):e0246451, PMID: , 10.1371/journal.pone.0246451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton MM, Shrestha R, Wei Y, Thornton PE, Kao S, Wilson BE. 2020. Daymet: Daily Surface Weather Data on a 1-km Grid for North America, Version 4.Oak Ridge, TN: ORNL Distributed Active Archive Center, 10.3334/ORNLDAAC/1840. [DOI] [Google Scholar]
- Tonne C, Wilkinson P. 2013. Long-term exposure to air pollution is associated with survival following acute coronary syndrome. Eur Heart J 34(17):1306–1311, PMID: , 10.1093/eurheartj/ehs480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MC, Jerrett M, Pope CA III, Krewski D, Gapstur SM, Diver WR, et al. . 2016. Long-term ozone exposure and mortality in a large prospective study. Am J Respir Crit Care Med 193(10):1134–1142, PMID: , 10.1164/rccm.201508-1633OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census. 2002. Summary File 3 Dataset. https://www.census.gov/data/datasets/2000/dec/summary-file-3.html [accessed 16 August 2021].
- U.S. Census. 2011. Summary File 1 Dataset. https://www.census.gov/data/datasets/2010/dec/summary-file-1.html [accessed 16 August 2021].
- U.S. Census. 2020. American Community Survey 1-Year Data (2005–2019). https://www.census.gov/data/developers/data-sets/acs-1year.html [accessed 16 August 2021].
- U.S. EPA (U.S. Environmental Protection Agency). 2011. Air quality guide for nitrogen dioxide. https://www.airnow.gov/sites/default/files/2018-06/no2.pdf [accessed 30 September 2021].
- U.S. EPA. 2019. Integrated Science Assessment (ISA) for Particulate Matter (Final Report, Dec 2019). EPA/600/R-19/188. Washington, DC: U.S. Environmental Protection Agency. [PubMed] [Google Scholar]
- U.S. EPA. 2020. The sources and solutions: fossil fuels. Last updated 11 December 2020. https://www.epa.gov/nutrientpollution/sources-and-solutions-fossil-fuels [accessed 30 September 2021].
- U.S. EPA. 2021. NAAQS table. Last updated 10 February 2021. https://www.epa.gov/criteria-air-pollutants/naaqs-table [accessed 19 August 2021].
- Wei Y, Wang Y, Wu X, Di Q, Shi L, Koutrakis P, et al. . 2020. Causal effects of air pollution on mortality rate in Massachusetts. Am J Epidemiol 189(11):1316–1323, PMID: , 10.1093/aje/kwaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Webster TF. 2017. Trade-offs of personal versus more proxy exposure measures in environmental epidemiology. Epidemiology 28(5):635–643, PMID: , 10.1097/EDE.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2006. Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide. Copenhagen, Denmark: WHO. https://apps.who.int/iris/rest/bitstreams/1344509/retrieve [accessed 3 December 2021]. [PubMed] [Google Scholar]
- WHO. 2021. Review of Evidence on Health Aspects of Air Pollution: Revihaap Project: Technical Report. WHO Regional Office for Europe. https://apps.who.int/iris/bitstream/handle/10665/341712/WHO-EURO-2013-2663-42419-58845-eng.pdf?sequence=1&isAllowed=y [accessed 3 December 2021]. [PubMed] [Google Scholar]
- Yorifuji T, Kashima S. 2020. Long-term exposure to nitrogen dioxide and natural-cause and cause-specific mortality in Japan. Sci Total Environ 741:140465, PMID: , 10.1016/j.scitotenv.2020.140465. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Wei Y, Fang Z. 2019. Ozone pollution: a major health hazard worldwide. Front Immunol 10:2518, PMID: , 10.3389/fimmu.2019.02518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wang J, Kwong JC, Burnett RT, van Donkelaar A, Hystad P, et al. . 2021. Long-term exposure to air pollution and mortality in a prospective cohort: the Ontario Health Study. Environ Int 154:106570, PMID: , 10.1016/j.envint.2021.106570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.