Abstract
The major transmembrane protein of the red blood cell, known as band 3, AE1, and SLC4A1, has two main functions: 1) catalysis of Cl−/ exchange, one of the steps in CO2 excretion, and 2) anchoring the membrane skeleton. This review summarizes the 150-year history of research on red cell anion transport and band 3 as an experimental system for studying membrane protein structure and ion transport mechanisms. Important early findings were that red cell Cl− transport is a tightly coupled 1:1 exchange and band 3 is labeled by stilbenesulfonate derivatives that inhibit anion transport. Biochemical studies showed that the protein is dimeric or tetrameric (paired dimers) and that there is one stilbenedisulfonate binding site per subunit of the dimer. Transport kinetics and inhibitor characteristics supported the idea that the transporter acts by an alternating access mechanism with intrinsic asymmetry. The sequence of band 3 cDNA provided a framework for detailed study of protein topology and amino acid residues important for transport. The identification of genetic variants produced insights into the roles of band 3 in red cell abnormalities and distal renal tubular acidosis. The publication of the membrane domain crystal structure made it possible to propose concrete molecular models of transport. Future research directions include improving our understanding of the transport mechanism at the molecular level and of the integrative relationships among band 3, hemoglobin, carbonic anhydrase, and gradients (both transmembrane and subcellular) of , Cl−, O2, CO2, pH, and nitric oxide (NO) metabolites during pulmonary and systemic capillary gas exchange.
Keywords: band 3, bicarbonate, chloride, erythrocyte, transport
INTRODUCTION
The most abundant protein in the red blood cell membrane is known as band 3, AE1, capnophorin, and SLC4A1. Band 3 catalyzes transmembrane Cl−/ exchange, one of the steps in CO2 excretion, and is the attachment site for the membrane skeleton. This review is an attempt to summarize ∼150 years of research on red cell inorganic anion transport and the properties of band 3 as an ion transporter. The literature on band 3 and related proteins is vast, and several topics are mentioned only briefly: other transporters in the SLC4 family (1–4); nonmammalian band 3 (5, 6); and the role of band 3 in the red cell membrane skeleton (7, 8), regulation of metabolism (9–11), senescence (12–15), and malaria (8, 16, 17).
A major advance in the field was the determination of the crystal structure of the band 3 membrane domain complexed with Fab fragments (18); membrane domain crystals prepared under microgravity conditions without Fab fragments reveal a similar structure (19). Reithmeier et al. (20) have reviewed many aspects of the band 3 literature in the context of the crystal structure. To minimize repetition of the content of this excellent review, discussion of some topics is limited here, including band 3 synthesis, glycosylation, and targeting. There is also very little reference to the many models of band 3 membrane domain topology and structure that were proposed before the crystal structure was determined.
The review begins with a roughly chronological history of key early findings, from the first evidence for red cell Cl− and transport in the 1800s through the identification of band 3 as the transport protein a century later. The remainder of the article is organized by topic rather than chronology, ending with questions that remain unanswered about the molecular mechanism and cellular physiology of band 3-mediated anion transport.
RED BLOOD CELL ANION TRANSPORT AND THE BAND 3 PROTEIN: 1867–1980
Early Work on the Role of Red Blood Cell Cl− and Transport in CO2 Excretion
Three discoveries in the 1800s showed that Cl− and are transported between red blood cells and plasma. In 1867, Schmidt and Zuntz separately reported that exposure of blood to CO2 causes plasma concentration ([]) to increase much more than exposure of plasma alone to CO2 (21). Exposing blood to CO2 also causes an increase in red cell volume (22) and net movement of Cl− into cells (23). These effects are expected if is formed from CO2 inside the cell and transported outward in exchange for Cl−.
Work in the early 1900s showed that graded increases in cause increases in cellular Cl− (24), and incubation of red cells in isotonic sucrose causes Cl− efflux that is accelerated by small amounts of (25), demonstrating that Cl− can exchange with in both directions. In the 1920s the steady-state distribution of Cl− and between red cells and plasma was found to be that expected for Donnan equilibrium if the membrane is much more permeable to Cl− and than to Na+ and K+ (21, 26, 27):
where [X]i and [X]o are intracellular and extracellular concentrations, r is the Donnan ratio, Vm is the membrane potential, and F, R, and T have their usual meanings. The Donnan ratio r is ∼0.63 at extracellular pH 7.4, 37°C (28, 29) and increases at lower pH because of decreased charge on impermeant intracellular anions (30–32). The red cell volume increase following exposure of blood to CO2 originally observed by Nasse (22) is a consequence of higher intracellular Cl− and , but the cell volume change in the physiological range is small (30).
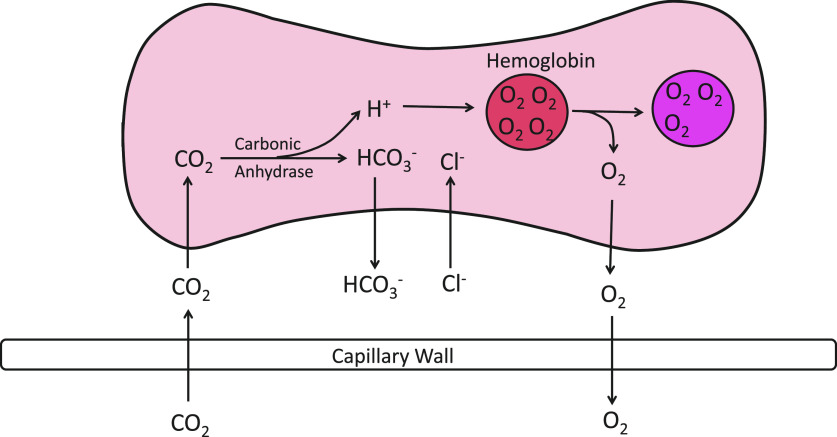
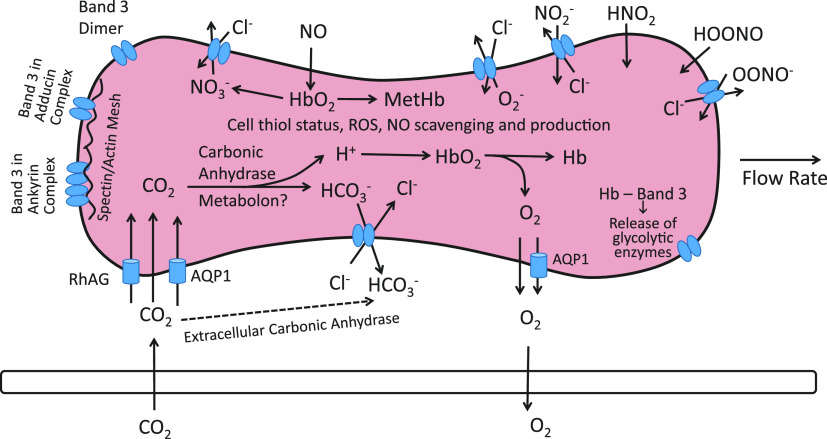
After the discovery of carbonic anhydrase in the early 1930s (33), there was general agreement that the role of red cell Cl− and transport in capillary CO2 exchange is as depicted in Fig. 1 (28, 29, 33–36). In systemic capillaries, incoming CO2 is converted to mainly inside the red cells, and the resulting increase in intracellular [] drives efflux of in exchange for extracellular Cl−. The reverse processes take place in pulmonary capillaries. Under physiological conditions, the half-time for equilibration of and Cl− is <0.1 s (37–39). Red cell Cl−/ exchange is therefore essentially complete during the capillary transit time (40).
Figure 1.
Schematic of the events associated with CO2 and O2 exchange in a systemic capillary as understood after the discovery of carbonic anhydrase but before band 3 was known to be the anion transporter. CO2 diffuses into the cell and is converted to + H+ by carbonic anhydrase. The resultant increase in concentration ([]) drives efflux of , with the charge balanced by influx of Cl−. The H+ generated by incoming CO2 is buffered by hemoglobin, and the slight decrease in pH facilitates O2 release.
Electrically Silent Cl− Transport; Substrate Saturation, 1967–1973
Prior to the mid-1960s, red cell anion permeability was described by the fixed charge hypothesis (41) and was assumed to be conductive. This assumption was shown to be incorrect by the demonstration (42) that ionophore-mediated net cation movement in red cells is limited by a Cl− conductance that is far lower than that expected from tracer Cl− exchange rates (43). A separate study by Scarpa et al. (44) used ionophores and pH equilibration experiments to show that Cl−/ exchange exhibits saturation/competition and is much faster than conductive Cl− transport. Quantitatively, the conductive Cl− permeability PCl of the human red cell membrane is ∼10,000-fold lower than the permeability expected if the tracer Cl− flux were entirely conductive (45). PCl is still larger than PNa or PK, and the red cell membrane potential is near the equilibrium potential for Cl− (46–49).
The very high rate of Cl− and transport at body temperature makes kinetic studies difficult, and most red cell anion transport experiments in the 1960s used or (41, 50). In 1972 Dalmark and Wieth (51) described a method for measuring red cell 36Cl−/Cl− exchange at 0–10°C by manual filtration. Gunn et al. (52) used this method in the first detailed kinetic study of red cell Cl− exchange. By varying the intracellular and extracellular [Cl−] at a fixed concentration of the weak competitor acetate, they showed that the 36Cl−/Cl− exchange flux is a saturable function of [Cl−] and, unlike transport (53), is inhibited by acid pH.
Revolution in Membrane Biology, 1969–1973
At the same time that red cell Cl− transport was shown to consist mainly of saturable obligatory exchange, there were many other advances in membrane biology, in rapid succession:
The long-suspected lipid bilayer structure (54) was confirmed by physical measurements (55, 56).
Electron paramagnetic resonance measurements showed that membrane lipids are more fluid than previously believed (57, 58).
Cell fusion experiments demonstrated that some membrane proteins have lateral mobility (59).
Freeze fracture electron microscopy (60) of red cells revealed intramembranous particles, of which band 3 is a major component.
Chemical labeling experiments demonstrated that a major protein (later determined to be band 3) spans the erythrocyte membrane (61).
A reproducible method for SDS polyacrylamide gel electrophoresis of red cell membrane proteins was developed (62). The third largest Coomassie-stained polypeptide (∼95 kDa) was called band 3.
Singer and Nicolson (63) proposed the fluid mosaic model of membrane structure, which replaced the Davson–Danielli–Robertson unit membrane model (64).
Identification of Band 3 as the Cl−/ Exchanger, 1974–1979
In the mid-1960s, methods were developed for covalent labeling of the red cell membrane (65, 66). One of the amino-reactive labels, 4-acetamido-4′-isothiocyanatostilbene-2,2′-disulfonate (SITS), was shown by Knauf and Rothstein to be an inhibitor of red cell transport (67). Cabantchik and Rothstein (68) extended this work to demonstrate that 4,4′-diisothiocyanatostilbene-2,2′-disulfonate (DIDS) is a very potent transport inhibitor and that [3H]4,4′-diisothiocyanatodihydrostilbene-2,2′-disulfonate (H2DIDS) (central double bond reduced) labels band 3 in proportion to transport inhibition. H2DIDS and DIDS have different reactivities, but these differences did not change the conclusion that band 3 is labeled in proportion to transport inhibition (69, 70). Other amino-reactive agents also inhibit anion transport in proportion to band 3 labeling (71, 72).
The studies connecting band 3 with anion transport used or because red cell divalent anion transport is much easier to measure than Cl− transport. At the time, it was unclear whether transport and Cl−/ exchange were mediated by the same system, because of the opposite pH dependence of monovalent versus divalent anion transport (50, 52, 53). Gunn (73) proposed a unifying model in which a critical titratable group can be protonated to convert band 3 from a monovalent to a divalent anion transporter. As a graduate student, I read about Gunn’s titratable carrier model and realized that the model predicted that during net exchange of Cl− for there should be H+ cotransport with . Decades earlier, Wilbrandt (74) had shown that suspending Cl−-containing red cells in a medium caused the extracellular pH to drop because of Cl− exchange with . I found that if the medium is purged of atmospheric CO2, Cl− efflux into a medium causes the extracellular pH to rise as H+ is cotransported inward very nearly stoichiometrically with (75). This result supported the idea the Cl− and are transported by the same system.
Further evidence of a common system for red cell monovalent and divalent anion transport was provided by Weith (76), who showed that the fractional inhibition of 36Cl−/Cl− and [14C]/ exchange in resealed red cell ghosts is directly proportional to the amount of bound DIDS, as was previously shown for (69, 70). In addition, a wide variety of reversibly acting inhibitors have the same effect on the equilibrium exchange of both Cl− and , measured in the same media and at the same temperature (77). By the end of the 1970s there was general agreement that band 3 is the transporter for Cl−, , , and many other inorganic and organic anions in red cells.
ANION TRANSPORT KINETICS
Alternating Access; Intrinsic Asymmetry; Single-Turnover Experiments
Band 3 was an important experimental system for testing the alternating access mechanism of transport (Fig. 2), which had been proposed for pumps by Jardetzky (78). Using anion gradients or transport inhibitors, the population of transporters could be enriched in either outward-facing or inward-facing states, distinguished by binding of chemical probes (79–81). This recruitment of transporters into one or the other state was some of the best early evidence for alternating access in a transporter.
Figure 2.
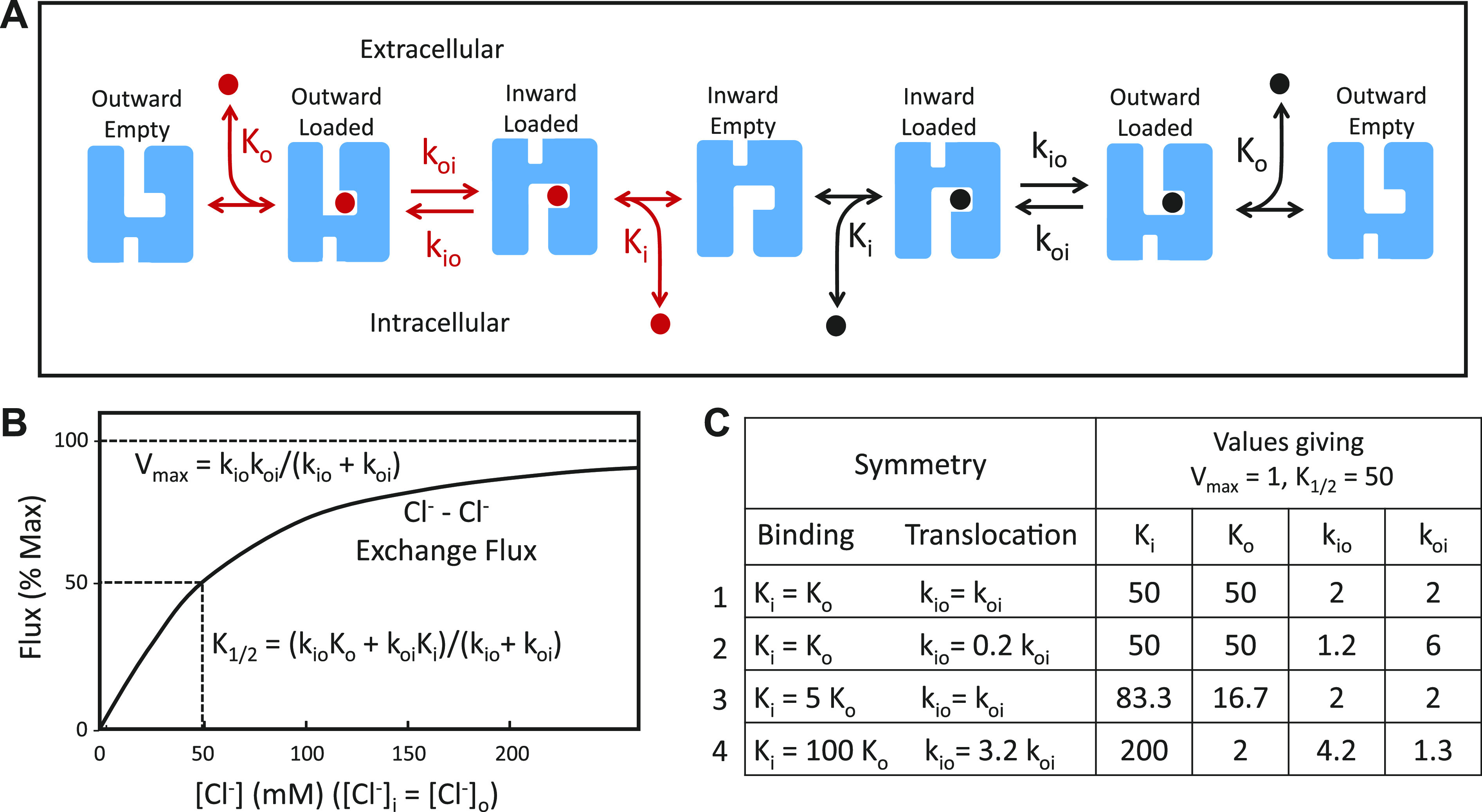
A: catalytic cycle for anion exchange by a ping-pong mechanism. The coupling between influx and efflux results from the extremely slow rate of translocation of the empty transporter. The exchange could be between 2 different ions (red and black), with different dissociation and translocation constants, or it could be tracer exchange of the same anion. B: ping-pong model prediction for the concentration dependence of Cl−/Cl− exchange flux with symmetric Cl− in the absence of competing anions and without any self-inhibition. C: 4 combinations of dissociation constants (Ki, Ko) and translocation rate constants (kio, koi) that all result in the same half-maximal concentration (K1/2) and Vmax for Cl−/Cl− exchange.
One version of alternating access is a “ping-pong” mechanism (82), in which the outgoing anion is released before binding of the incoming anion (Fig. 2). The ping-pong mechanism was supported by Cl−/Cl−, Cl−/Br−, and Br−/Br− exchange experiments showing that the substrate concentration (K1/2) on one side of the membrane resulting in half-maximal flux depends on the concentration of the exchange partner (83). These experiments as well as studies with inhibitors (84–86) also demonstrated intrinsic asymmetry of the transporter, with more empty transporters facing inward than outward.
The abundance of band 3 (1.2 × 106 polypeptides per cell) makes it possible to prepare resealed red cell ghosts containing only slightly more Cl− ions than band 3 polypeptides. Resuspending these ghosts in medium containing extremely low [Cl−] and [] causes rapid efflux of ∼0.7 × 106 Cl− ions resulting from a half-turnover of the catalytic cycle (87). This experiment showed that the Cl− efflux event is possible without influx. The number of Cl− ions released is consistent with the idea that a complete catalytic cycle is one pair of anions exchanging per copy of band 3 polypeptide. Further evidence for a ping-pong mechanism is the transient uphill 36Cl− efflux induced by adding H2DIDS to red cells containing very low [Cl−] (88).
Self-Inhibition
Although there is a large body of evidence for a ping-pong mechanism, the kinetics of band 3-mediated transport are complex. The exchange flux of Cl− and other halides decreases at high concentrations (83, 89, 90), implying that there is an anion binding event that inhibits at least one step in the alternating access catalytic cycle. The self-inhibition site has a higher affinity for I− than for Cl− (89), and the site is inactivated by deprotonation of a group with pK ∼11 at 0°C (90). Self-inhibition can be modeled as a noncompetitive inhibitory site on each band 3 subunit, but as Salhany and coworkers pointed out, other mechanisms are possible, including those with a ternary complex involving both exchanging anions (91) and site-site interactions resulting in negative cooperativity (92).
Estimates of Cl− and Affinities
With symmetric Cl− and concentrations totaling 165 mM, the Cl−/Cl− exchange flux is a concave upward function of [Cl−] and the / exchange flux is a concave downward function of [], indicating that the band 3 affinity is approximately fourfold higher than that of Cl− (76). Self-exchange experiments with symmetric Cl− or as the only permeant anion are also consistent with affinity being higher than that of Cl− (76, 93).
It is inherently difficult to estimate absolute Cl− and affinities from transport measurements. In a self-exchange with symmetric [Cl−], the same measured Vmax and K1/2 can result from an infinite number of combinations of true dissociation constants (Ki and Ko) and translocation rate constants (kio and koi); some examples are in Fig. 2. The same thing is true for exchange experiments with Cl− varied on only one side of the membrane; K1/2 depends on both binding affinity and translocation rate constants. Gunn, Fröhlich, Knauf, and coworkers used a combination of specific transport inhibitors and detailed kinetic analysis to provide strong evidence that Cl− binding to band 3 transport sites is fairly symmetric (Ki ≅ Ko), but koi (inward translocation) is larger than kio (83, 85, 94), similar to line 2 of the table in Fig. 2C. In contrast to Cl−, binding of appears to be asymmetric (Ko << Ki), and koi is smaller than kio (95), similar to line 4 of the table in Fig. 2C. Despite the asymmetries in substrate binding and/or translocation, with the relatively small gradients of Cl− and found in pulmonary and systemic capillaries, the time course of net exchange is predicted to be very similar in each direction (95).
Because of the abundance of band 3, it is possible to use NMR to measure Cl− binding (96–100) and to distinguish among different classes of transport inhibitors (101–103). The NMR results are consistent with a ping-pong model in which translocation rather than binding/release is rate limiting (104), Cl− binding is symmetric (K ∼ 50 mM), and inward Cl− translocation is faster than outward (100). NMR measurements of binding indicate that the dissociation constants for and binding to transport sites on band 3 are 5–10 mM (105), in agreement with transport measurements showing a higher affinity for than for Cl− (76, 93). With an approximately four times higher affinity, the fractional occupancy of transport sites with and Cl− should be similar in vivo, because the Cl− concentration is approximately four times higher.
Affinities and Translocation Rates of Other Band 3 Substrates
Table 1 lists many of the known substrates of mammalian band 3, in very rough order of transport rates. For divalent or pH-titratable anions, the rates are at the pH optimum for transport of the ion (106). There are very large (>1,000-fold) variations in transport rates among different anions, with those similar to Cl− or (e.g., Br−, formate, ) transported most rapidly. Oxyanions with tetrahedral geometry are transported more slowly than planar trigonal anions (107–109).
Table 1.
Approximate band 3-mediated X−/X− or Cl−/X− exchange rates relative to Cl−/Cl− exchange
| Relative Rate | Anion (References) | Additional Information |
|---|---|---|
| 1 | Cl−, (76, 224, 352) | Transported at similar rates; has higher affinity for transport site. |
| 0.3–0.7 | (493) | Fast, similar to . |
| Formate (HCOO−) (108, 225, 227) | Slightly slower than . Also transported as free acid. | |
| (494, 495, 513, 514) | Rate not known exactly because of parallel HNO2 transport. Could be similar to formate. | |
| Br− (83) | Cl−/Br− exchange is faster than Br−/Br− exchange, as predicted by ping-pong mechanism. | |
| HS− (515) | Measured as Jacobs–Stewart cycle of rapid transport of both HS− and H2S. | |
| 0.1–0.3 | Oxalate (−OOCCOO−) (227, 516, 517) | Fastest divalent anion transported by band 3. |
| Superoxide () (518) | Transport rate not clear but probably fast. | |
| Peroxynitrite (OONO−) (375, 496, 497) | Causes oxidative damage of band 3 and reduced transport. Undissociated acid also transported. | |
| F− (43) | Slower than Br−. | |
| OH− (90) | Detectable but hard to quantify because very high pH inhibits monovalent anion transport. | |
| Selenite () (107, 519) | Possible connection with arsenite toxicity. | |
| 0.03–0.1 | Malonate (−OOCCH2COO−) (516, 520) | Almost as fast as oxalate; larger dicarboxylates are slower. |
| I− (493) | Slow, but has high affinity for self-inhibitory site, so some of slow rate could be self-inhibition. | |
| Thiocyanate (SCN−) (51) | Inhibits Cl− transport strongly. | |
| Bisulfite () (521) | Much faster than . | |
| Phosphite () (106, 109) | Much faster than . | |
| Borohydride () (522) | Enters cells in <1 min at 3°C, but rate not quantified. | |
| 0.01–0.03 | Hypophosphite () (106, 109) | Slower than . |
| Glyoxylate (HCOCOO−) (227) | Much slower than . | |
| Glycolate (HOCH2COO−) (227) | Much slower than . | |
| Fluorophosphate () (109) | Slower than planar oxyanions of phosphorus. | |
| Acetate (H3CCOO−) (225, 493) | Hard to quantify because of rapid free acid transport. Used as spectator anion. | |
| 0.003–0.01 | Selenate () (107, 519) | Much slower than selenite. |
| Vanadate (523) | Rate not known precisely; inhibits ATPases and PTPs. | |
| 0.001–0.003 | Dithionite () (91) | Measured as exchange with . |
| Pyruvate (225, 524) | Also transported by monocarboxylate transporter. | |
| Sulfate () (118) | Measured at very low extracellular pH; much slower at neutral pH. | |
| <0.001 | Chromate () (525, 526) | Influx facilitates labeling red cells with 51Cr for red cell lifetime measurements. |
| Glycine anion (H2NCH2COO−) (527) | Slower than glycolate. | |
| / (50, 106, 109, 528, 529) | Also transported by Na+-coupled cotransporter. | |
| Phosphoenolpyruvate (240, 530) | Only known glycolytic intermediate transported across red cell membrane. | |
| Lithium carbonate () (531, 532) | Under physiological conditions represents over half the lithium flux in red cells. | |
| Pyridoxal phosphate (241) | Also reacts with K851. | |
| NBD-taurine (242) | Used to measure transport by fluorescence | |
| Taurine monochloramine (533) | Produced from taurine by neutrophil myeloperoxidase. |
NBD-taurine, 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino] ethanesulfonate; PTP, protein tyrosine phosphatase.
For anions that are transported much more slowly than Cl−, it is possible to estimate binding affinity for outward-facing transport sites by measuring the initial Cl− efflux into media containing only the slowly transported anion X−. The [X−]o that gives half-maximal flux provides an estimate of the dissociation constant of X− for outward-facing sites, because the catalytic cycle is limited by X− influx rather than by Cl− efflux (87). The range of binding affinities determined from Cl−/X− exchange (∼10-fold) is much more limited than the >1,000-fold variations in maximum transport rates (107). The large variations in transport rate are likely a consequence of how each substrate interacts with the transition state between inward-facing and outward-facing states (107, 110, 111).
Is OH− a Substrate for Band 3?
In 1932 Jacobs and Parpart (112) estimated the rates of entry of acid into red cells and concluded, based on a mass action argument, that the data are much more consistent with OH− efflux than with H+ influx. Given the high rates of transport of other monovalent anions, it would not be surprising if OH− is a substrate of band 3. However, as shown by Jacobs and Stewart (113), if small amounts of CO2 are present, a repeated cycle of efflux, extracellular dehydration, CO2 influx, and intracellular hydration will result in net net efflux of OH− equivalents. Therefore, what appears to be Cl−/OH− exchange could actually be the result of atmospheric CO2 acting through the Jacobs–Stewart cycle. Wieth and Bjerrum (90) measured a relatively slow OH− influx in red cells at extracellular pH 12.4, which does not cause irreversible damage to band 3 at 0°C. At this pH the Jacobs–Stewart cycle should not operate because extracellular CO2 is almost entirely in the form of . Therefore, the base influx at very high extracellular pH is likely actual OH− transport by band 3, but it is difficult to make a quantitative comparison between OH− and Cl− fluxes, because raising the extracellular pH to produce an appreciable [OH−] also inhibits monovalent anion exchange (90).
Additional Transport Modes
Although the main transport process carried out by band 3 is 1:1 exchange of monovalent anions, there are other, slower, modes of transport:
Anion conductance. Band 3 mediates most of the red cell conductive Cl− flux (114), which is not a result of reorientation of the empty transporter (115, 116). Band 3 also catalyzes conductive transport of and of OH− (114).
H+- cotransport. As predicted by the titratable carrier model (above), H+ is cotransported with during Cl−/ exchange (75). H+ and can bind to the transporter in either order, with the first bound increasing the affinity for the second by ∼ 10-fold (117, 118).
H+-Cl− cotransport. In the pH range 5.7–7.4 in media purged of atmospheric CO2, red cell pH equilibration rates are much more consistent with H+-Cl− cotransport than with Cl−/OH− exchange; the apparent H+-Cl− cotransport is much slower than Cl−/Cl− exchange but is inhibited by low concentrations of DIDS (119). Kinetic studies with chemically modified band 3 suggest that H+-Cl− cotransport results from a slow event in which 2 Cl− ions are translocated on the protonated (divalent) form of band 3 (120).
K+ transport. At low ionic strength, red cells exhibit a stilbenedisulfonate-sensitive monovalent cation permeability that is very likely mediated by band 3 (121). As discussed below, some variant forms of band 3 can transport cations.
Stilbenedisulfonate transport. At very low pH, band 3 can transport stilbenedisulfonates, which are normally impermeant (122).
BAND 3 STRUCTURE
cDNA Sequence, Expression Pattern, Related Proteins, Nomenclature
Proteolytic fragments of band 3 were sequenced by Edman degradation in the early 1980s (123–125), but it would have taken many more years to sequence the entire protein. Fortunately, cDNA technology was advancing rapidly, and one of the first transport protein cDNAs to be sequenced was mouse band 3 (126). The human band 3 cDNA sequence (127, 128) and the mouse and human gene sequences (129–131) were published shortly afterward.
The main sites of mammalian band 3 gene expression are erythroid cells and the kidney, but it is also expressed in the heart (132), and there is recent evidence that band 3 is involved in sperm capacitation (133). In the kidney, the band 3 gene is transcribed with an alternate promoter (134, 135) resulting in an NH2 terminus that is 65 residues shorter than red cell band 3 (136). Renal band 3 mediates Cl−/ exchange, resulting in efflux across the basolateral membrane of acid-secreting α intercalated cells in cortical and medullary collecting tubules (137, 138).
Soon after band 3 cDNA was sequenced, related transcripts and proteins were identified. To clarify the terminology, Ron Kopito, at a 1989 meeting hosted by Naotaka Hamasaki, proposed the name AE1 (Anion Exchanger 1) for band 3, AE2 for the widely expressed homolog (139–141), and AE3 for the neural homolog (142). AE2 and AE3 are, like AE1, Na+-independent Cl−/ exchangers. The terms “AE1” and “band 3” are used interchangeably; “AE1” is used more often in heterologous expression studies, comparisons among Cl−/ exchangers, and work on the product of the renal transcript kAE1. AE1 is also called capnophorin (143) for its role in transport. Fifty years after Fairbanks et al. (62), the term “band 3” is still commonly used for the red cell protein.
Posttranslational Modifications
Band 3 has one site of N-glycosylation (144) at N642, located in a long extracellular loop between the 7th and 8th transmembrane helices. The carbohydrate is heterogeneous, causing band 3 to run as a broad zone on SDS-PAGE (145–148). Enzymatic deglycosylation does not inhibit anion exchange (149). Band 3 is fatty acylated at C843 (150). Acylation of this residue is not required for trafficking of AE1 to the cell surface (151) or for anion transport (152). Phosphorylation sites are discussed below.
Cytoplasmic and Membrane Domains
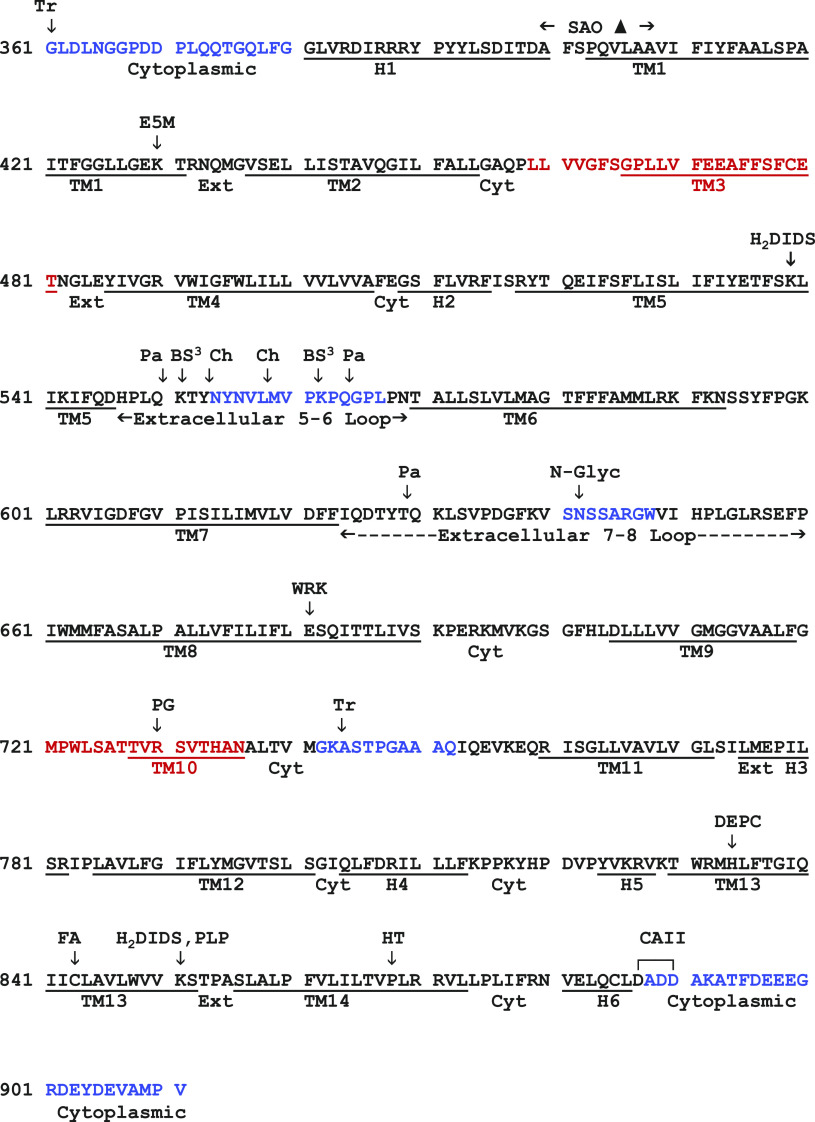
Human band 3 consists of a water-soluble NH2-terminal cytoplasmic domain (360 residues) and a mainly hydrophobic COOH-terminal domain (551 residues) with a short COOH-terminal hydrophilic sequence (127, 128). The cytoplasmic domain is an attachment site for the membrane skeleton via ankyrin (153) and binds hemoglobin and glycolytic enzymes (10). The membrane domain carries out anion exchange; its amino acid sequence is in Fig. 3, annotated with locations of various markers.
Figure 3.
Amino acid sequence of the membrane domain of band 3. Sites of N-glycosylation (N-Glyc) (N642) and fatty acylation (FA) (C843) are indicated. Underlined sequences are membrane α-helices (TM1–TM14) and surface α-helices (H1–H6). Sequences that are not ordered in the crystal structure are shown in blue. The 2 transmembrane sequences (TM3 and TM10) that include both helical and nonhelical segments are in red. Arrows indicate sites of proteolytic cleavage by trypsin (Tr), chymotrypsin (Ch), and papain (Pa); chemical modification by eosin-5-maleimide (E5M), 4,4′-diisothiocyanatodihydrostilbene-2,2′-disulfonate (H2DIDS), bis(sulfosuccinimidyl)suberate (BS3), diethylpyrocarbonate (DEPC), Woodward’s reagent K (WRK), phenylglyoxal (PG), pyridoxal phosphate (PLP), and band 3 HT point mutation. Locations of the Southeast Asian ovalocytosis (SAO) deletion and DADD sequence binding carbonic anhydrase II (CAII) are also indicated. References are in text.
Dimeric Structure
Although band 3 can be monomeric (154–158), it is most likely dimeric in normal intact membranes, with some dimers associating into tetramers. The first evidence for dimers was that Cu2+/o-phenanthroline (CuP) cross-links band 3 to an –S–S– dimer (159). The cross-linked cysteine residues are in the cytoplasmic domain (160–162). The purified cytoplasmic domain is dimeric without cross-linking (163). Crystal (164, 165) and solution (166) structures of the cytoplasmic domain show a tightly associated dimer. The sulfur atoms of the only two cysteines (C201 and C317) are ∼20 Å from each other in adjacent subunits of the crystal structure (164, 165). The cytoplasmic domain undergoes a pH-dependent conformational change (167, 168), which could move the side chains close enough to each other for –S–S– cross-linking.
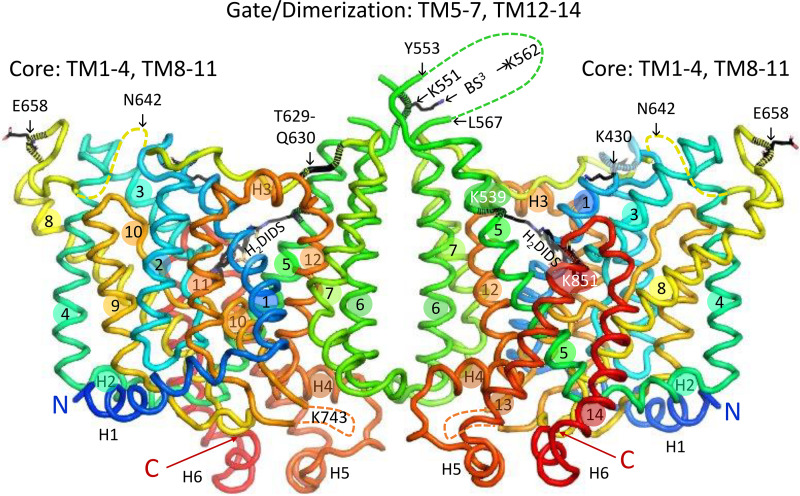
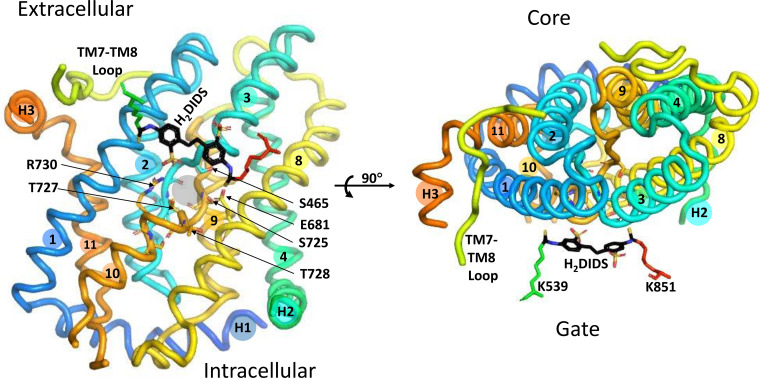
The isolated membrane domain has high α-helix content (169) and is dimeric (170–173). In the crystal structure of the dimer (18), each subunit has 14 membrane-spanning (TM) segments, with subdomains designated core and gate, the terms originally chosen to describe the structurally related bacterial uracil transporter UraA (174). Figure 4 is a ribbon representation (PyMOL) of the membrane domain dimer, viewed from within the membrane. Figure 5 is the same structure viewed from the extracellular side.
Figure 4.
Ribbon representation (PyMOL, pdb 4YZF) of the crystal structure of the membrane domain dimer (18), viewed in the plane of the membrane. Membrane α-helices TM1–TM14 are labeled with numbers in colored circles, and surface helices are labeled H1, etc. The NH2-terminal end of H1 preceded by an unresolved sequence is indicated, as is the COOH-terminal end of H6, which is followed by the unresolved COOH-terminal 24 residues. The 3 internal unresolved sequences are shown as dashed curves. The locations of several extracellular biochemical markers and proteolysis sites are indicated. The glycosylation site N642 is in the unresolved sequence between TM7 and TM8. The helices connecting core and gate are H2 on the cytoplasmic side (foreground on the right-hand subunit) and H3 on the extracellular side (foreground on the left-hand subunit). Residues in the membrane domain that can be cross-linked to the cytoplasmic domain are all in the gate/dimerization domain except for K743 in the disordered sequence between TM10 and TM11, which can be cross-linked to 2 different residues in the cytoplasmic domain (187).
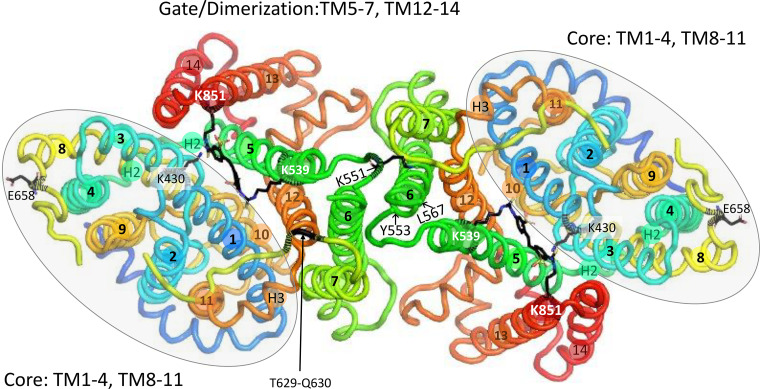
Figure 5.
Ribbon representation of the band 3 dimer crystal structure, viewed from the extracellular side of the membrane to illustrate the relationships among dimer interface, gate, core, and stilbenedisulfonate site. The core domains are in the shaded ovals. Covalently bound 4,4′-diisothiocyanatodihydrostilbene-2,2′-disulfonate (H2DIDS) between core and gate is shown in black sticks for both subunits. One of the connections between core and gate domains is cytoplasmic surface helix H2, which is largely obscured by TM8 in this view; the two ends of H3 are labeled. The second connection between core and gate consists of extracellular helix H3 and the extracellular TM7-TM8 loop. Papain cleavage of the TM7-TM8 loop between T629 and Q630 (shown for the left-hand subunit) appears to stabilize the inward-facing conformation (see text).
The core domain consists of TM1–TM4 and TM8–TM11 arranged in an inverted repeat. TM2, -4,-9, and -11 are nearly perpendicular to the membrane on the side of the core facing away from the gate. TM3 and TM10 both contain helices that go partway through the membrane. The NH2-terminal ends of these helices face each other roughly in the middle of the membrane and are separated by a gap that is the likely substrate anion binding site (see below). TM1 and TM8 are relatively long and are located on each side of the TM3/TM10 helix. The gate domain consists of TM5–TM7 and TM12–TM14, also in an inverted repeat. The gate is connected to the core via cytoplasmic helix H2 between TM4 and TM5 and extracellular helix H3 between TM11 and TM12. The other connection between core and gate is the long extracellular sequence between TM7 and TM8.
The gate domain contains the dimer interface. The TM5-TM6 loops of both subunits are in close proximity to each other, consistent with cross-linking experiments indicating that bis(sulfosuccinimidyl)suberate (BS3), a membrane-impermeant cross-linker (175, 176), forms an intermolecular cross-link involving K551 and/or K562 (177). At the intracellular surface, the TM6-TM7 loop and COOH terminus of H4 of opposite subunits are adjacent to each other. In the membrane interior, there is a cavity between the subunits that molecular dynamics simulations suggest is occupied by lipid, especially cholesterol (178).
Interaction between Cytoplasmic and Membrane Domains
A large body of evidence indicates that the band 3 cytoplasmic domain is connected to the membrane domain by a flexible tether, without stable noncovalent associations between domains (179, 180):
The cytoplasmic domain is released from the membrane by proteolysis under mild conditions (144, 181, 182).
Calorimetry demonstrates independent thermal unfolding of the two domains (183).
Proteolytic removal of the cytoplasmic domain does not have a major effect on transport (184, 185).
The membrane domain, when expressed in either Xenopus oocytes (186) or HEK 293 cells (165), mediates Cl− exchange that is indistinguishable from that of the whole protein.
Mutations that affect the conformation of the cytoplasmic domain do not affect expression of transport activity in HEK cells (165).
However, recent findings have shown that interactions between membrane and cytoplasmic domains are more extensive than previously believed. Rivera-Santiago et al. (187) demonstrated that several zero-length cross-links can be formed between the membrane and cytoplasmic domains of human red cell band 3. The cross-links mainly involve the gate/dimerization region of the membrane domain, and in a structural model of the intact protein the cytoplasmic domain does not appear to interfere with cytoplasmic access of substrate anions (187). The only cross-linked core residue is K743, which can be cross-linked to two different carboxylate side chains in the cytoplasmic domain (187). This residue is in a sequence that is not ordered in the crystal structure but is close to the gate domain (Fig. 4). The fact that some of the cross-links involve two or more partners is evidence that the interactions between the two domains are dynamic. Functional interaction between the two domains is discussed below in reference to tyrosine phosphorylation.
In Situ Proteolysis
Band 3 has been the subject of numerous in situ proteolysis studies. Most of the known proteolysis sites of native band 3 are in sequences that are disordered in the crystal structure (Figs. 3 and 4):
Connection between cytoplasmic and membrane domain: The membrane domain NH2 terminus is G361 (123), and the next 19 residues leading to helix H1 are disordered in the crystal.
TM5-TM6 exofacial loop: Extracellular chymotrypsin or thermolysin produces an NH2-terminal ∼60-kDa fragment with a COOH-terminal tyrosine residue, now known to be Y553 (181, 188, 189), adjacent to a disordered sequence in the crystal structure (dashed green loop in Fig. 4). Proteolysis of the TM5-TM6 loop does not inhibit anion transport (190, 191) or stilbenedisulfonate binding (192). Expression of fragments confirmed that transport does not require this loop to be intact (193).
TM7-TM8 exofacial loop: The sequence (I624–P660) between TM7 and TM8 (yellow in Fig. 4, Fig. 5, and Fig. 6) is ordered in the crystal structure except for a short disordered segment (dashed yellow in Fig. 4) containing the N-glycosylation site. Digestion of cells with papain (194) or isolated membranes with pepsin (124) cleaves between T629 and Q630. This is the only in situ proteolysis site of native band 3 that is resolved in the crystal structure; it is located close to the H3 connection between TM11 (core) and TM12 (gate). Papain cleavage of this site inhibits transport if measured with symmetric anion concentrations (191, 195) but accelerates transport measured under influx-limiting conditions (196). This could be explained if papain cleavage at T629–Q630 destabilizes the outward-facing state.
TM10-TM11 cytoplasmic loop: Trypsin, at low ionic strength, cleaves the cytoplasmic surface of band 3 at K743 (197, 198). Glycosylation scanning in a cell-free translation system (199) indicated that this loop is extracellular, but the loop is not glycosylated when expressed in the plasma membrane of HEK 293 cells (200), suggesting that the TM10-TM11 loop may be transiently exposed to the endoplasmic reticulum (ER) lumen during synthesis/insertion of band 3 (20).
COOH-terminal hydrophilic region: Carboxypeptidase Y digestion experiments demonstrated that the COOH terminus is cytoplasmic (201).
Sites exposed by low or high pH: In situ proteolysis of band 3 exposed to high or low pH has provided information about the stability of transmembrane helices. Pepsin removes most of the surface loops and produces stable membrane-bound fragments (124, 202). Hamasaki and coworkers used in situ proteolysis following exposure of intact membranes to 10–100 mM NaOH to identify band 3 sequences that are more likely to be surrounded by protein than lipid (203–206).
Figure 6.
Left: core domain, viewed from the gate/dimerization domain, showing the relationship between the probable substrate binding pocket (gray circle between the helical portions of TM3 and TM10) and covalently bound 4,4′-diisothiocyanatodihydrostilbene-2,2′-disulfonate (H2DIDS) (black sticks). The side chains of R730 and E681 are on either side of the substrate pocket. One of the H2DIDS sulfonate groups is near the side chain of R730. The positions of other polar side chains (S465, S725, T727, and T728) are indicated. The 3 links between core and gate domains (portion of TM7-TM8 loop and surface helices H2 and H3) are viewed end-on. Right: same structure, rotated to show the view from the extracellular medium. The only gate domain amino acid residues shown are those covalently bound to H2DIDS. The helical portion of TM10 is behind TM1 in this view. H2DIDS is between core and gate, near but not in the substrate binding pocket.
Stilbenedisulfonate Binding Site; Intramolecular Cross-Link
The stilbenedisulfonate binding site is located between the core and gate of each subunit of the dimer (Fig. 5). Prior to covalent reaction, DIDS and H2DIDS bind reversibly with high affinity (69, 70, 207). Many other stilbenedisulfonate derivatives can bind to this site (208–215). The site is accessible only from the extracellular medium and is in a cleft below the membrane surface, consistent with the most recent fluorescence resonance energy transfer (FRET) data (216). The main H2DIDS covalent attachment site is a reactive amino group in the 60-kDa chymotryptic fragment (residues 1–553) (190, 217). This residue (human K539; mouse K558) is not necessary for anion exchange (218–220).
As a postdoctoral fellow with Hermann Passow, I consistently found that if cells were treated with H2DIDS some of band 3 appears to be resistant to chymotrypsin cleavage into the 60-kDa and 35-kDa fragments. It seemed possible that this apparent resistance was the result of H2DIDS, a bifunctional reagent, forming a covalent intramolecular cross-link between the two fragments. To test this idea, I incubated H2DIDS-treated cells at high extracellular pH (9.5) and found that essentially all copies of band 3 migrated at 95 kDa on gels, even if it had previously been cleaved by extracellular chymotrypsin (191).
This result showed that, after covalent attachment of H2DIDS to the 60-kDa fragment, the other –N=C=S group can react with the 35-kDa fragment at high pH to produce an intramolecular cross-link. It also showed that there is one H2DIDS binding site per band 3 polypeptide. Several years later I collaborated (my role was minor) with the group led by Naotaka Hamasaki to identify the site of H2DIDS cross-linking as K851 (221), which is also the site of covalent modification by pyridoxal 5′-phosphate (222).
The H2DIDS cross-linking reaction proved to be a useful tool for studying band 3. The membrane domain crystals (18) were prepared from band 3 that had been internally cross-linked by H2DIDS at high pH. It is known that very high extracellular pH inhibits red cell Cl−/Cl− exchange (90, 223, 224), but even at pH 10 Cl−/Cl− exchange is inhibited only 50% relative to neutral pH. It is of course possible that the H2DIDS-cross-linked structure does not represent a native conformation, but it is more likely to be similar though perhaps not identical to the outward-facing native conformation.
Evidence for Independent Transport by Dimer Subunits
There is a very well-established linear relationship between bound DIDS or H2DIDS and inhibition of red cell anion transport (70, 76, 225–230). Covalent reaction of a portion of band 3 by stilbenedisulfonate lowers the Vmax but does not change the K1/2 for transport (231). The simplest interpretation of these findings is that, in the band 3 dimer, one subunit can transport normally even if the other subunit is completely inhibited by DIDS. It is also possible that that stilbenedisulfonate binding disrupts preexisting subunit interactions (232).
Independent transport by the two subunits of the dimer is consistent with the crystal structure. H2DIDS is bound covalently to residues in the gate domain, but the binding site is not near the dimer interface (Fig. 5). Cross-linking the TM5-TM6 loops of adjacent subunits with BS3 does not inhibit anion transport (233), suggesting that the subunit interface is not closely involved in anion binding or translocation. The subunit interface may represent a relatively fixed scaffold, with the transport-related conformational changes resulting from each core domain moving independently. Additional evidence that the subunit interface is not necessary for transport is that expression of a construct lacking TM6 and TM7 mediates both anion exchange and conductance (234).
Substrate Binding Site
Stilbenedisulfonates bind reversibly with high affinity only to the outward-facing conformation of band 3 (87, 235), and binding is competitive with Cl−, as measured by transport (211, 236) and NMR (97). However, the stilbenedisulfonate site and the Cl− binding site are not identical; Cl− interferes with 4,4′-dibenzamidostilbene-2,2′-disulfonate (DBDS) binding by increasing the DBDS dissociation rate rather than by occupying the same site (237, 238).
The membrane domain crystal structure has no bound substrate anion, but the substrate binding site is likely located between the NH2-terminal ends of the α-helical portions of TM3 and TM10 (18, 20), by analogy with UraA (174). This location is close to but distinct from the site of covalently bound H2DIDS (Fig. 6). The substrate binding pocket is roughly halfway through the membrane, consistent with evidence that the substrate traverses ∼10–15% of the transmembrane electrical field in moving from the extracellular medium to the binding site (239).
As discussed above, variations in binding affinity among different substrates are smaller than the variations in translocation rates. Anions as large as phosphoenolpyruvate (240), pyridoxal phosphate (241), and N-(2-aminoethylsulfonate)-7-nitrobenz-2-oxa-3-diazole (NBD)-taurine (242) are band 3 substrates. Modeling studies will be necessary to gain insight into how anions of various sizes and geometries fit into the binding pocket.
Site of Eosin-5-Maleimide Labeling
Eosin-5-maleimide (E5M) is used clinically to estimate the amount of band 3 per cell (243) and has been used extensively to measure band 3 rotational mobility (see below). E5M reacts covalently with K430 (244, 245) on the extracellular end of TM1 near the DIDS binding pocket but closer to the extracellular surface (Fig. 4 and Fig. 5). The E5M covalent reaction is preceded by reversible binding to a site in the outward-facing conformation that is distinct from the transport site (246, 247).
Role of E681 and R730
The pH dependence of influx during Cl−/ exchange (118) suggested that a carboxyl group on band 3 binds the H+ that is cotransported with . Matt Anderson and I used the arylsulfonic acid Woodward’s reagent K (WRK) to try to identify the carboxyl group. WRK was known to form an enol ester adduct with protein carboxyl groups (248), and we showed that that [3H] can reductively cleave the enol ester to produce a radiolabeled alcohol in place of the original carboxylate (249).
The functional effects of this modification (inhibition of Cl− transport, stimulation of electrogenic Cl−/ exchange) were consistent with the idea that WRK/ converts the glutamate residue associated with H+- cotransport into an alcohol (250, 251). The primary labeled glutamate was identified as E681 in TM8 (252). Alper and coworkers showed that mutagenesis of this residue (E699) in mouse band 3 to glutamine has very similar functional effects (253, 254), providing further evidence that E681, when protonated, converts band 3 from a monovalent to a divalent anion transporter, and the H+ bound to E681 is cotransported with . Cysteine scanning mutagenesis of TM8 showed that the E681C mutant has no transport activity (255); the E681Q mutant has very low transport activity (165).
In the crystal structure (18), the E681 side chain is adjacent to the substrate binding pocket (Fig. 6). On the other side of the substrate binding pocket is the side chain of R730, a probable target of arginine-selective reagents that inhibit red cell anion transport (256–260). The charge configuration at the binding pocket therefore consists of the positive charge on R730 and the NH2-terminal ends of TM3 and TM10 helical dipoles and the negative charge on E681 and substrate anion. Uncharged but polar side chains near the substrate site are S465 in TM3 and S725, T727, and T728 in TM10 (Fig. 6).
The normal anion translocation event is nearly electroneutral (118, 239, 261), but if the charge on E681 is removed by either WRK/ (251) or mutagenesis (253, 254), Cl−/ exchange becomes electrogenic and has altered pH dependence. This is consistent with the idea that, in the normal translocation event, the negative charge on E681 moves with the substrate ion relative to the transmembrane electric field, balanced by the positive charge on R730 and the positive ends of the TM3 and TM10 helix dipoles.
ASSOCIATIONS OF BAND 3 DIMERS
Band 3 Tetramer and Higher Oligomer
A significant fraction of purified band 3 in detergent is tetrameric (171, 262, 263), and the ankyrin-bound form of band 3 is tetrameric (264). Ankyrin contains two folds that could each bind one band 3 dimer (265). Other evidence for tetrameric band 3 is that CuP forms some covalent tetramers in addition to dimers (177, 266). Dimers cross-linked by BS3 can form noncovalent tetramers that are stable in SDS at moderate temperatures, indicating that there are interactions between adjacent dimers (267). Clusters of band 3 larger than tetramer are associated with a variety of pathophysiological conditions, including oxidative stress, hemoglobin denaturation, and cell senescence (268–273).
Glycophorin A
There are many indications that band 3 and glycophorin A (GPA) interact closely (274). Antibodies against GPA reduce band 3 rotational mobility (275). Coexpression with GPA enhances band 3 expression in Xenopus oocytes (276, 277). GPA forms a complex with band 3 in the ER of K562 cells, but knockdown of GPA expression does not decrease surface expression of band 3 in these cells (278). A Wright blood group antigen is formed by GPA and E658 of band 3 (279). Red cells that lack GPA, but not glycophorin B (GPB), have ∼50% lower anion transport rates (228, 229). Different regions of GPA are associated with increased band 3 trafficking and increased anion transport (280, 281). Glycophorin Mur, the product of homologous recombination of GPA and GPB, enhances band 3 expression (282, 283).
Glycolytic Enzymes and Deoxyhemoglobin
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) binds to the band 3 cytoplasmic domain and is inactive when bound (284, 285). Aldolase (286), phosphofructokinase (287), and deoxyhemoglobin (288–290) also bind to the cytoplasmic domain. Deoxyhemoglobin binding interferes with glycolytic enzyme binding (10, 11). The binding of these proteins is reversed by phosphorylation of two tyrosine residues (Y8 and Y21) near the NH2 terminus (291).
The significance of glycolytic enzyme binding to band 3 has been questioned (292) because binding to membranes is not observed at physiological ionic strength. Later work, however, demonstrated GAPDH binding to band 3 in intact cells (293, 294). More recent confocal images show that GAPDH, aldolase, phosphofructokinase, pyruvate kinase, and lactate dehydrogenase are mainly membrane associated in a glycolytic enzyme complex anchored to band 3 in intact cells (295, 296).
Peroxiredoxin
Peroxiredoxin-2 (Prx2), is a very abundant cytosolic protein that scavenges peroxides in red cells (297) and binds to the band 3 cytoplasmic domain (298). It is not known whether band 3 binding is involved in the role of Prx2 (also known as calpromotin) in Ca2+-sensitive K+ transport in red cells (299).
Carbonic Anhydrase II
Vince and Reithmeier (300, 301) used immunofluorescence, immunoprecipitation, and solid-phase binding assays to demonstrate carbonic anhydrase II (CAII) binding to an acidic sequence (D887ADD) near the band 3 COOH terminus. This work led to the idea that bound CAII acts in concert with band 3 to form a transport metabolon that facilitates capillary CO2 exchange (302–307). Transport metabolons have been proposed for other acid-base transporters (308–312) and CO2 channels (283). The band 3-CAII metabolon is discussed further below in connection with ongoing questions.
BAND 3 MOBILITY
Lateral Mobility
Electron microscopy showed that erythrocyte intramembranous particles, including band 3, have lateral mobility (313). In red cells fused with Sendai virus or polyethylene glycol (PEG), surface proteins (mainly band 3) prelabeled with fluorescein can diffuse laterally into the membranes of cells that had not been labeled (314). Fluorescence photobleaching recovery (FPR) experiments demonstrated subpopulations of band 3 with different lateral mobilities (315, 316).
The lateral mobility of band 3 is largely constrained by the spectrin meshwork (7), but recent single-particle experiments have shown that, in relatively rare events (∼3/s), band 3 can “hop” to the adjacent element of the meshwork (317). Very mild trypsin digestion of the band 3 cytoplasmic domain without major disruption of spectrin increases the “hop” rate, consistent with the idea that the cytoplasmic domain, in copies of band 3 not directly bound to the membrane skeleton, hinders movement of band 3 across the lateral cytoskeletal barrier.
The trajectories of single band 3 molecules labeled by quantum dots indicate that in normal red cells there are immobile, constrained, and freely diffusing populations (318). The same methodology applied to mouse erythrocytes showed that ∼40% of band 3 is attached to ankyrin, 33% attached to adducin, and ∼27% not attached, with heterogeneity within each population (319). Surprisingly, the lateral diffusion of GPA measured with quantum dots is distinct from band 3 (320) and indicates that the association between GPA and band 3 in mature red cells may be relatively weak.
Rotational Mobility
Transient dichroism of band 3 labeled with eosin isothiocyanate or iodoacetamido-eosin showed that ∼40% of band 3 has restricted rotational mobility; when either the cytoplasmic domain is cleaved or cytoskeletal proteins are extracted, the mobility of this population increases (321, 322). The rotational mobility of E5M-labeled band 3 reveals heterogeneity, with ∼20% of the population rotating rapidly (correlation time 50 ± 30 µs) and a larger population (variable estimates) with ∼1-ms correlation times (323–327). As is true of lateral diffusion, rotational diffusion is temperature dependent (323) and restricted by binding of ankyrin (326, 327) or hemichromes (324). Band 3 rotation measured with an electron paramagnetic label is consistent with a homogeneous population of band 3 dimers (328, 329).
Do Mobile and Immobile Subpopulations of Band 3 Have Different Transport Properties?
Evidence that mobile and immobile populations of band 3 have the same transport properties comes from work with band 3 Prague, which is misfolded and not present in mature red cells (330). In red cells of heterozygotes for band 3 Prague, the amount of band 3 and the flux are both decreased by 40%. Therefore, each copy of normal band 3 has the same transport activity whether it is in normal red cells or the cells of band 3 Prague heterozygotes. The fraction of laterally mobile band 3 is much lower in the heterozygotes than in normal red cells (330). This suggests that mobile and immobile band 3 have indistinguishable transport properties in normal red cells and that whatever internal molecular motions are associated with transport are independent of lateral diffusion of the whole protein.
SUMMARY OF BAND 3 COMPLEXES
Starting with the classic work of Steck (284), there have been numerous models of the arrangement of red cell membrane proteins (7, 8, 331–334). In mature normal human red cells, band 3 can exist in the following states:
Untethered dimer. Based on the population with the fastest rotational diffusion and lateral diffusion within a cytoskeletal corral, ∼20% of band 3 is in the form of untethered dimer. GPA and Prx2 are sufficiently abundant to be associated with all forms of band 3, including the untethered dimer (7), but GPA does not appear to be associated with the untethered fraction of band 3 (320).
Ankyrin complex, consisting of one band 3 tetramer, one ankyrin molecule, and several other proteins (7). About 40% of the band 3 polypeptides are in this complex. Other integral proteins include GPA, CD47, Rh proteins, GPB, and Landsteiner–Wiener antigens (7, 335, 336). Peripheral proteins include band 4.2 (331, 337) and the glycolytic enzyme complex (295).
Adducin complex (338, 339), at the spectrin-actin junction. The adducin complex is larger than the ankyrin complex and includes band 4.1, dematin, stomatin, and many other integral and peripheral proteins (7, 333, 340), including the glucose transporter GLUT1 (341). Some of the components of the complex are far less abundant than band 3; therefore, the composition of the junctional complex is heterogeneous (see Ref. 7). Red cells that are deficient in stomatin have about half the Cl−/ exchange activity of normal red cells (342); not enough is currently known about the interaction between band 3 and stomatin for a detailed interpretation of this result.
The linear decrease in transport with stilbenedisulfonate binding (70, 76, 225–230) is consistent with the idea that each copy of band 3 has the same transport properties, irrespective of the complex it is in. However, it is still possible that band 3 in, say, the adducin complex has a different transport rate than other copies of band 3. If DIDS binding affinity is the same in all copies of the protein, then there will be a linear decrease in transport with increasing DIDS binding, even if subpopulations have different transport properties. There is no evidence for different transport properties of band 3 in the above states of association, but the possibility cannot be ruled out.
REGULATION OF BAND 3-MEDIATED ANION TRANSPORT
Lack of Physiological Regulation of Red Cell Band 3
The physiological function of transporters is pH homeostasis. Some transporters, including AE2 and AE3, are acid loaders (base extruders), because the inward Cl− gradient exceeds the inward gradient in most cells (1, 2, 4). The regulation of AE2 has been studied extensively by Alper and coworkers. In the physiological pH range, a higher pH activates AE2, resulting in a negative feedback loop that limits cytoplasmic alkalinization (343). Both intracellular and extracellular pH affect transport in the physiological range (344), and both the cytoplasmic and membrane domains are involved in the regulation (345). AE2 is also activated by NH3/ (346) and by hypertonic conditions (347). Although regulatory regions have been identified in AE2 (344, 348–351), the detailed regulatory mechanisms are not yet fully understood.
In contrast to AE2, anion exchange by red cell band 3 is not strongly affected by pH in the physiological range (224, 352, 353). The only physiological regulation of AE1 by pH is a moderate upregulation of kAE1 expression in response to an acid load (354–356). Red cell Cl− transport is very similar in intact red cells and resealed ghosts if Donnan effects on ion distribution are taken into account (93), consistent with the idea that band 3 transport does not require continuous maintenance by cytoplasmic constituents. There is evidence that red cell band 3-mediated oxalate transport is stimulated by increased Ser/Thr phosphorylation following okadaic acid treatment (357, 358), but our laboratory found no effect of okadaic acid on oxalate transport (227). ATP depletion of red cells causes a 30–50% reduction in anion transport (227, 359, 360); the mechanism of this effect is not known, but in any case, the effect is moderate.
The lack of major regulation of red cell band 3 transport during the normal life of the red cell is consistent with its role in capillary CO2 exchange (Fig. 2). When the cell arrives in a pulmonary or systemic capillary, band 3 must exchange Cl− for very rapidly in response to the sudden change in gradient. After the cell leaves the capillary, there is no reason to downregulate transport, because there is very little postcapillary driving force for Cl−/ exchange (361). If transport were downregulated after leaving either a pulmonary or systemic capillary, there would be very little time (<<1 s) to reactivate it when the cell next arrives in a capillary. It is more likely that, for most of the lifetime of the normal red cell, band 3 transport is constitutively active.
Effects of Oxidative Stress, Thiol Status, and Tyrosine Phosphorylation
Although the main transport function of band 3 is not up- and downregulated under physiological conditions, transport activity can be reduced by oxidative stress and other pathophysiological conditions (12, 13, 17, 268, 269, 362–364). The literature on band 3 and red cell oxygen status is too extensive to cover in detail here; the discussion below is focused on effects of oxidative stress on band 3-mediated anion transport.
All 5 cysteine residues in human band 3 can be replaced with serine without major loss of transport function (365). Casey and coworkers (255, 366–368) have used cysteineless band 3 for informative studies of topology and function by cysteine scanning mutagenesis, as reviewed by Reithmeier et al. (20). Despite the fact that band 3 has no cysteine residues that are necessary for transport, treatment with N-ethylmaleimide (NEM) or oxidative stress inhibits red cell anion transport to varying degrees (369–375).
A probable connection between thiol status and transport is tyrosine phosphorylation (363, 376). Sites of tyrosine phosphorylation on band 3 include Y8 and Y21 near the NH2 terminus; Y359 in the linker between membrane and cytoplasmic domains; and Y904 near the COOH terminus (377–380). There is evidence for a role of Y359 and Y904 phosphorylation in kAE1 trafficking (381, 382), but neither is necessary for anion transport; band 3 can be cleaved by trypsin adjacent to Y359 without major effect on transport (184, 185), and transport is normal in band 3 Walton, which is missing residues 901–911 (381).
Phosphorylation of Y8 and Y21 in red cells has been characterized in connection with regulation of metabolism and oxidative damage (291, 380, 383–385). Y8 phosphorylation, most likely by Syk, results in the binding of the phosphorylated cytoplasmic domain to an SH2 sequence (G509–R514) in cytoplasmic helix H2 linking core and gate of the membrane domain (Fig. 7); the binding strongly inhibits anion transport (386). This is the most direct connection to date between the cytoplasmic domain and anion transport and challenges the long-held view that anion transport is independent of the cytoplasmic domain. In addition to inhibiting transport, phosphorylation of Y8 causes displacement of glycolytic enzymes and release of ankyrin (386).
Figure 7.
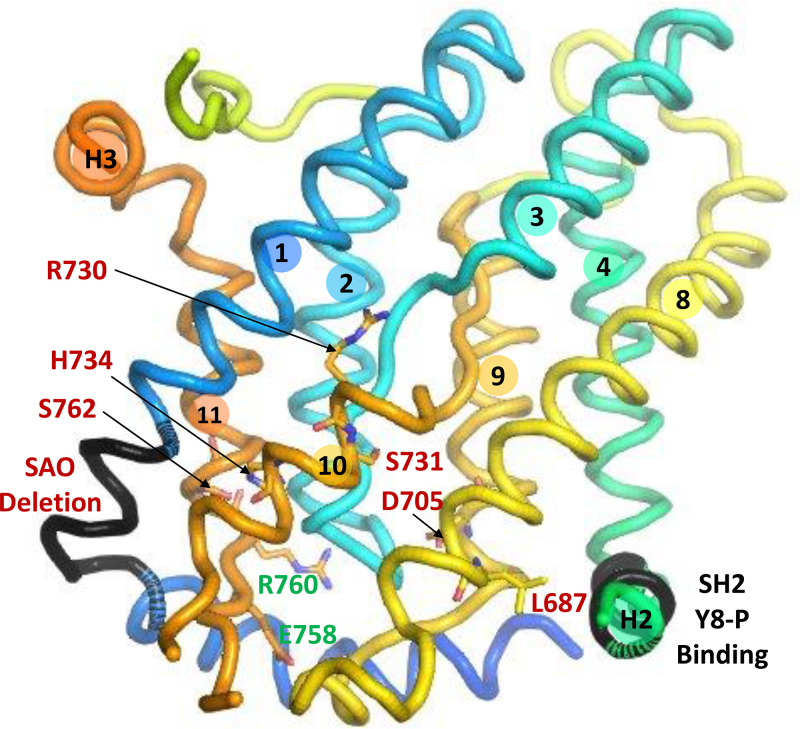
Band 3 core domain, viewed from the gate/dimerization domain, showing the location of the Southeast Asian ovalocytosis (SAO) deletion (411) and the SH2 sequence that binds cytoplasmic domain phosphorylated at Y8 with transport inhibition (386). Sites of human point mutations that cause increased cation leak and major inhibition of anion transport are labeled in red: L687P, D705Y, S731P, H734R (428), R730C (429, 430), and S762R (431). Mutation G796R (not shown), which also causes cation leak and anion transport inhibition (430, 432), is in the TM12 of the gate domain, facing R730. Two sites (E758K and R760Q) of mutations that cause increased cation leak but do not strongly affect anion exchange are labeled in green.
It is not yet clear what physiological or pathophysiological conditions result in Y8 phosphorylation and transport inhibition. Red cell protein tyrosine phosphatase (PTP) activity is normally high (363, 371, 376, 387–389), and it is possible that Y8 is mainly unphosphorylated during most of the normal red cell lifetime (see further discussion below).
ANION TRANSPORT AND NATURALLY OCCURRING HUMAN SLC4A1 MUTATIONS
SLC4A1 genetic variants are associated with hereditary spherocytosis (HS), stomatocytosis (HSt), or distal renal tubular acidosis (dRTA) (16, 20, 390–394). Many dominant SLC4A1 mutations associated with HS cause band 3 deficiency resulting from misfolding and protein instability; band 3 is such a major part of red cell architecture that even a moderate deficiency causes shape changes and/or fragility (16, 390, 395, 396). There is usually no renal phenotype because heterozygotes produce and target sufficient amounts of normal kAE1.
SLC4A1 dRTA variants usually have trafficking defects that result in kAE1 mistargeting (230, 393, 397, 398). In dominant dRTA, the protein is folded and inserted sufficiently to form heterodimers with normal band 3, but the heterodimer has impaired trafficking that reduces the amount of normal functional kAE1 (398, 399). For most dRTA variants, the trafficking defect is rescued in red cells by coexpression of GPA (274, 400), although there are variants that cause both dRTA and HS (230).
It is beyond the scope of this review to discuss the pathophysiology of HS, HSt (401–403), or dRTA (404, 405), all of which can have causes other than SLC4A1 mutations. The focus here is on transport by band 3 variants in red cells or heterologously expressed.
Memphis: K56E
Mueller et al. (406) first described a human band 3 variant with reduced electrophoretic mobility of the 60-kDa chymotryptic fragment as a result of a K56E substitution (407, 408). The mutation has at most minor functional effects; band 3-mediated phosphoenolpyruvate transport in red cells from homozygotes is ∼20% lower than normal (409).
S725R Variant
Yang et al. (410) recently described a band 3 variant, S725R, in which homozygotes have band 3 deficiency, anemia, and dRTA. The S725R protein trafficks to the cell surface when expressed in HEK cells, but it has no transport activity, whereas the S725A protein has normal transport. S725 is close to E681 in the crystal structure (Fig. 6), and the disruption caused by arginine substitution likely results from the arginine side chain interacting with the E681 side chain (410).
Southeast Asian Ovalocytosis
The band 3 Southeast Asian ovalocytosis (SAO) variant has the Memphis mutation and a deletion of residues 400–408 covering the COOH-terminal end of cytoplasmic helix H1 and the NH2-terminal 4 residues of TM1 (411) (Fig. 7). Red cells of SAO heterozygotes are abnormally rigid and resistant to invasion by Plasmodium falciparum (412). The SAO mutation does not cause dRTA, but additional mutations causing dRTA are sometimes found in SAO heterozygotes (394). It was previously believed that homozygosity for the SAO variant is lethal, but SAO homozygotes have survived with careful management (413–416).
The SAO deletion is expected to have a major conformational effect on TM1 (417–419), but the protein is nonetheless stably associated with the membrane. Nearly half the band 3 in heterozygotes is SAO band 3, but SAO band 3 does not bind stilbenedisulfonates and has abnormal mobility (412, 420–422). Normal and SAO band 3 can form heterodimers (423) and higher heterooligomers (419). Heterodimers also form between normal and SAO kAE1 expressed in HEK293 cells (424). Anion transport in red cells of SAO heterozygotes, including those with dRTA mutations, is close to or slightly less than half of normal (226, 423, 425, 426). This is consistent with the idea that the normal copy of band 3 in a SAO heterodimer can transport anions, but possibly more slowly than normal.
HSt Mutations with Very Low Anion Transport and Elevated Cation Transport
Red cells of SAO heterozygotes have elevated Na+ and K+ permeability, especially at low temperatures (427). Several band 3 point mutations associated with HSt also have strongly reduced anion transport and elevated cation permeability: L687P, D705Y, S731P, H734R (428), R730C (429, 430), S762R (431), and G796R (430, 432). Severe anion transport disruption by these mutations is consistent with the crystal structure (Fig. 7). R730 and S731 are very close to the likely substrate binding site. L687 is near the H2 helix connecting core and gate. The S762 and H734 side chains are close to each other in the crystal structure; an arginine substitution for either would disrupt the TM10/TM11 packing near the substrate site. D705 is near the cytoplasmic end of TM9 and is not as close to the substrate site. G796 is in TM12 of the gate (not shown in Fig. 7), facing the NH2-terminal end of the TM10 helix near the substrate binding pocket in the core.
Mutations with Nearly Normal Anion Transport and Elevated Cation Transport
Band 3 with E758K substitution is associated with spherostomatocytosis and somewhat increased cation permeability but has near-normal anion transport when coexpressed with GPA in oocytes (433). The increased cation flux induced by expression of E758K band 3 is not dependent on GPA, raising the possibility that the 86Rb+ flux is by way of a separate pathway. Red cells from a heterozygote for H734R band 3 also have elevated cation transport by pathways other than band 3 (434).
Red cells from heterozygotes for the dominant HS mutation R760Q have moderately increased cation permeability and ∼75% of normal transport activity (428), indicating that the variant protein carries out substantial anion transport. R760Q AE1 expressed in Xenopus oocytes has normal Cl−/ exchange activity (435). The variant does, however, have a trafficking defect (436) and is difficult to detect in red cells of R760Q heterozygotes (437). E758 and R760 are on the cytoplasmic end of TM11 in the core (Fig. 7).
Several kAE1 variants associated with dRTA (R589H/C, G609R, S613F, G701D, D905dup, E906X/K, G609R) have normal or near-normal anion transport activity (425, 438), and some (R589H, G609R, S613F, G701D) have elevated stilbenedisulfonate-sensitive 86Rb permeability at 0°C in Xenopus oocytes (438). The lack of effect of these mutations on anion transport activity is consistent with the crystal structure; the substitutions are not close to the anion binding site. There is no clear pattern to the substitutions that cause increased cation permeability.
It is important to point out that cation transport in band 3 variants is extremely slow compared with normal anion exchange. Ellory et al. (435) calculated that in the cation-transporting band 3 mutants the number of cations transported per copy of band 3 is only ∼1 ion/s. There is no known connection between cation transport in these variants and the K+ transport through red cell band 3 in media of low ionic strength (121).
HS Mutation H834P
The variant H834P results in HS (395) with impaired folding and trafficking to the plasma membrane (436). Chemical modification with diethylpyrocarbonate (439) or site-directed mutagenesis (368, 440, 441) indicates that H834 is important for transport. In the crystal structure it is located near the cytoplasmic end of TM13.
Band 3 HT: P868L
The band 3 variant P868L is associated with acanthocytosis and is known as band 3 HT, because it has a higher maximal anion transport rate than normal (442, 443). The variant is also labeled with H2DIDS less readily. The reason transport is higher in P868L band 3 is not known but it may result from a change in the core-gate connection; P868 is in TM13 of the gate, close to where cytoplasmic helix H3 connects core TM4 to gate TM5.
Mutations Causing Band 3 Null Phenotype: Coimbra, Vienna
The mouse band 3 null phenotype, with absence of band 3 in both red cells and kidney, produces numerous abnormalities, including spherocytosis and severe anemia, GPA deficiency (444), dyserythropoiesis (445), increased Ca2+ leak and cell death (446), but relatively normal membrane skeleton architecture (447). The null phenotype is similar in cattle (448).
There have been a few examples of humans with the band 3 null phenotype, resulting from homozygous band 3 Coimbra (V488M) (449) or Vienna (S477X) (450). The human band 3 null phenotype includes spherocytic anemia, dRTA at 3 mo, and dyserythropoiesis; regular transfusions and oral bicarbonate therapy for dRTA have made it possible for band 3-null patients to survive into childhood (450).
ONGOING QUESTIONS ABOUT BAND 3
Structure and Interaction with Other Proteins
In his excellent review of the red cell membrane skeleton, Lux (7) identified several unanswered questions about band 3, the first of which is “What is the overall structure of band 3?” Although crystal structures of both the membrane and cytoplasmic domains of band 3 are known, there is still uncertainty about the structure of the intact protein. The cross-linking experiments of Rivera-Santiago et al. (187) resulted in a model with extensive contacts between the two domains. However, several residues can be cross-linked to multiple partners, indicating that the association between membrane and cytoplasmic domains has some flexibility. Molecular dynamics simulations by De Vecchis et al. (451) generated alternative models, the most compact of which differs from that of Rivera-Santiago et al. (187). Further modeling as well as direct measurement of binding interactions between the two domains can potentially improve our understanding of the structure of intact band 3.
Another unanswered structural question is the extent of conformational lability of amino acids 812–830, which in the crystal structure (18) include parts of cytoplasmic helices H4 and H5 between TM12 and TM13 (see Fig. 3 and Fig. 4). A peptide consisting of residues 812–827 was originally shown by Kay et al. (452) to inhibit binding of senescent cell IgG to senescent red cells, indicating that the sequence is extracellular. Cysteine scanning mutagenesis and labeling of AE1 expressed in HEK 293 cells also indicated that these residues are extracellular (366, 368). Badior and Casey (453) recently reexamined the topology of this sequence in AE1 expressed in HEK cells and found that this sequence is mainly cytoplasmic, consistent with the crystal structure, but it can reorient transiently to a conformation in which it is exposed to the extracellular medium. Binding of extracellular IgG can potentially trap this rare conformation. Over time, enough band 3 could become bound with IgG to mark the cell as senescent. The reorientation of residues 812–830 could therefore serve as the molecular clock for erythrocyte senescence (453). This new idea will likely be tested in future experiments and simulations.
Transport Mechanism: Rocking versus Elevator
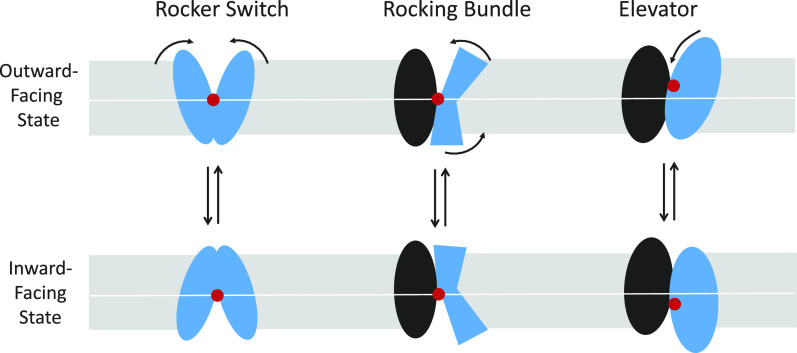
There are many unanswered questions about the molecular mechanism and the integrative physiology of band 3-mediated anion transport. Possible mechanisms for alternate access transporters include rocker switch, rocking bundle, and elevator (454, 455) (Fig. 8). The rocking-type mechanisms have a nearly stationary substrate binding site (red circle in Fig. 8). In elevator-type mechanisms, a domain of the protein, with bound substrate, moves relative to a fixed scaffolding domain (Fig. 8, right). Yeast SLC4 transporter Bor1 appears to have a rocking bundle mechanism (456), but other related transporters, including bacterial UraA (457) and Arabidopsis Bor1 (458), are believed to use an elevator-like mechanism (459).
Figure 8.
Schematic representation of possible mechanisms (rocker switch, rocking bundle, and elevator) for alternating access transport (454, 510, 534). For band 3 rocking bundle and elevator mechanisms, the core domain is blue and the gate domain is black.
For red cell band 3, repeat swap homology modeling (460) predicts an elevator-type mechanism that is consistent with many known experimental properties of red cell anion exchange (461). The elevator mechanism is also consistent with the inhibitory effect of phosphorylated Y8 binding to the SH2 domain in the H2 helix connecting core and gate (386) (Fig. 7); this helix participates in the translocation event in the elevator model.
Transition State
A genuine understanding of the transport mechanism will require knowledge not only of the structure of the inward-facing state but also of the transition state between inward- and outward-facing structures. Insights into the nature of the transition state could help explain some poorly understood properties of band 3-mediated transport:
Variations in transport rates among different substrates. Little is currently known about substrate anion binding to the transition state and whether band 3 forms an occluded state, as is known to exist for UraA (462). Information about anion binding to the transition state (107, 110, 111) could help explain the huge variations in transport rates (Table 1) among anions that appear to have similar affinities for the transport site.
High rate of Cl− and transport. The band 3-mediated Cl−/Cl− exchange flux at body temperature is ∼50,000 ions/s per band 3 polypeptide (224). With symmetric translocation, the forward and reverse unimolecular rate constants would both be 100,000/s (Fig. 2). With asymmetric translocation (94), one of the rate constants is even higher. There is currently no explanation for such high translocation rate constants.
High activation enthalpy and nonlinear Arrhenius plots. The high Cl−/ exchange rate is especially remarkable considering that the activation enthalpy is 20 kcal/mol at 25–38°C and even higher at low temperatures (51, 224). The high rate constant with high activation enthalpy indicates a large positive entropy of activation (463, 464). It is not known how the transition state could have a high entropy and why nonlinear Arrhenius plots are observed for exchange of halides, , and other oxyanions (39, 224, 464, 465).
High activation volume. Band 3-mediated anion exchange has a large dependence on hydrostatic pressure and therefore a high activation volume, indicating that the rate-limiting event results in expansion of the protein-anion complex by 0.15 L/mol (466). There is currently no explanation for the high activation volume.
Low “slippage” rate. The rate constant for translocation without substrate (“slippage”) is extremely low (115, 116). In contrast, for SLC4 Na+- cotransporters (1–3), the catalytic cycle presumably includes reorientation of the protein without substrates. The factors governing the transition state of the empty transporter (forbidden for exchangers; required for cotransporters) is a major unanswered question for SLC4 transporters.
Self-inhibition. It is well established experimentally that high concentrations of halides inhibit halide exchange (83, 89, 90). The molecular basis for self-inhibition is not known and may or may not involve the transition state.
Lipid effects. Molecular dynamics simulations predict an annular lipid layer and cholesterol enrichment at the band 3 dimer interface (178), but there is currently no explanation for how cholesterol enrichment lowers the rate of band 3-mediated anion transport (467–469).
Roles of K539 and K851, if any, in transport. Mutagenesis of the H2DIDS-cross-linked residues K539 and K851 lowers the affinity for reversible binding of stilbenedisulfonates but does not prevent Cl− transport (220). BS3, in addition to the intermolecular cross-link of the TM5-TM6 loop, forms an intramolecular cross-link, probably between K539 and K851 (176, 177). The intramolecular cross-link results in partial transport inhibition with altered pH dependence and apparently lower affinity for the self-inhibitory site (233), but these effects are not understood.
Role of Cytoplasmic Domain and Oxidative Stress in Transport
Oxidative stress produces many changes in subpopulations of band 3, including clustering, release of ankyrin, and diminished anion transport (12, 363, 371, 384, 388, 389, 470–472). The mechanism for these effects is likely to include PTP inhibition, phosphorylation of Y8, and binding of the cytoplasmic domain to an SH2 domain on the membrane domain (386). Further work is needed to understand the many different physiological, pathophysiological, and pharmacological conditions affecting the tyrosine phosphorylation status of band 3 (362, 473–479).
Transport Metabolon
As described above, there are several lines of evidence that CAII binds to an acidic sequence near the band 3 COOH terminus (300, 301). There is also evidence to the contrary; solid-phase binding assays and surface plasmon resonance do not detect CAII binding to band 3 peptides containing the expected CA binding sequence (480, 481), and the two proteins do not detectably associate as measured by FRET or immunoprecipitation (482). It is not clear why binding studies in different laboratories disagree; part of the reason could be the relatively low binding affinity (311).
In addition to binding studies, there is a large body of evidence that CAII association with band 3 has functional consequences in mammalian cells or Xenopus oocytes expressing normal and mutated AE1 and CAII (302–307). These studies showed in several different ways that association of band 3 with catalytically active CAII is required for full transport activity, measured as the rate of intracellular pH change after step increases or decreases in extracellular [Cl−].
Although there is extensive evidence for a functional band 3-CAII metabolon in expression systems, it is also well established that, in red cell band 3, CAII is not necessary for full transport activity, defined as a complete cycle of binding, translocation, and release of in both directions. To measure [14C]/ exchange, the activity of CA must be inhibited to minimize [14C]CO2 flux. Even in the presence of strong CA inhibition, band 3-mediated self-exchange is very rapid, comparable to the Cl− self-exchange (76, 352). Another method for measuring transport in red cells is 18O exchange (482–484). Al-Samir et al. (482) showed that red cells from donors lacking CAII have normal permeability determined by this method, further indication that CAII does not accelerate band 3-mediated transport in red cells. Al-Samir et al. (482) also presented mathematical modeling showing that pulmonary capillary CO2 exchange is more efficient with uniform CA in the cytosol rather than concentrated at the membrane.
In summary, it remains possible that there is a functionally significant red cell band 3-CAII metabolon, but a viable metabolon model needs to demonstrate why concentrating CA at the inner membrane surface is more efficient than distributing CA in close proximity to hemoglobin, the main H+ buffer (482). Future metabolon models also need to explain how bound CAII can preferentially channel to and from band 3 but not interfere with the exchange of Cl− between cytosol and the same inward-facing transport site. The same consideration applies to any proposed metabolon involving a transporter that has more than one substrate, i.e., how to channel one substrate and not get in the way of the other.
Other Unanswered Questions about Capillary Gas Exchange
Figure 9 is an updated version of Fig. 1 showing the events that take place during capillary CO2 exchange. Much more is now known about anion transport through band 3, but there is more to be learned about how band 3-mediated transport contributes to capillary CO2 and O2 exchange, including:
Timing of the effects of deoxygenation on hemoglobin binding to band 3. It is clear that deoxygenation causes increased binding of hemoglobin to band 3 and changes in metabolism (11). It is not known, however, how these changes in metabolism affect the cell during the normal repeated cycling of oxygenation and deoxyhemoglobin. How much deoxygenation, and over what time period, is needed to produce a significant effect on red cell metabolism? This question does not relate directly to anion transport, but it is part of the overall dynamics of the status of band 3 during capillary gas exchange.
Extracellular carbonic anhydrase. Intracellular carbonic anhydrase activity is definitely much higher than that on the surface of endothelial cells in pulmonary and systemic capillaries (485, 486), CAIX bound to the extracellular surface of band 3 (487), or plasma CA resulting from red cell lysis. One role of extracellular CA is to minimize postcapillary pH transients (361). The quantitative contribution of extracellular CA to capillary CO2 exchange may be low but is not known.
Effect of reduced red cell Cl−/ exchange. All vertebrates except lamprey (488) have high rates of red cell Cl−/ exchange, and modeling predicts that inhibition of anion exchange should lower the CO2-carrying capacity of blood (29, 36). In agreement with this prediction, inhibition of band 3 by right atrial infusion of 4,4′-dinitrostilbene-2,2′-disulfonate (DNDS) lowers pulmonary CO2 excretion by ∼15% in dogs (489). Moderate (50%) inhibition of band 3-mediated transport does not appear to have a measurable effect on blood CO2 transport and pulmonary gas exchange, as indicated by SAO heterozygotes (without dRTA) who have normal arterial blood gas values and ∼50% of normal band 3 transport (490). In SAO homozygotes and band 3-null humans, mice, and cattle, the effects of lack of red cell anion exchange are complicated by the coexisting anemia and dRTA. The quantitative effects of graded decreases in red cell anion exchange, without other effects, are not known, either experimentally or theoretically. It is also not known whether a moderate reduction in red cell anion exchange would affect CO2 excretion during exercise.
Possible physiological role of band 3 transport of nitric oxide (NO) metabolites. In the past 20 years there has been an explosion of information on the role of the red cell in NO metabolism. The red blood cell has a role in both the production and scavenging of NO (491, 492), and the NO metabolites nitrate (), nitrite (), and peroxynitrite (OONO−) are all transported by band 3 (Table 1). NO reacts with oxyhemoglobin to produce methemoglobin and , which is transported by band 3 nearly as rapidly as (493); it is not known whether rapid transport is of importance for NO scavenging. Both and OONO− can be transported by diffusion of the cognate weak acids as well as by band 3 (494–497). It is possible that band 3-mediated transport of these solutes is of secondary importance, but an integrative understanding of red cell NO metabolites and their relationship with blood flow regulation (491, 492, 498–500) should include consideration of the possible role of band 3-mediated transport of these metabolites and of .
Intracellular diffusion in red cells during capillary O2 and CO2 exchange. Imaging and mathematical modeling have produced new insights into the processes of intracellular diffusion of CO2, H+, , and non- buffers in red cells (353, 484, 501, 502). Intracellular diffusion of O2 has long been known to be facilitated by hemoglobin (Hb) (503), but recent single-cell imaging studies indicate that intracellular O2 diffusion is slower than previously believed (504). The next level of understanding the intracellular diffusion in red cells during capillary exchange should include the relationships among the intracellular gradients of CO2, H+, buffers, Cl−, dissolved O2, hemoglobin, and deoxyhemoglobin, and how these gradients are affected by HbO2 dissociation rates (505), carbonic anhydrases I and II (506), Cl− binding to hemoglobin (507), and carbamate formation (508). A better understanding of all the diffusion processes would also help resolve the metabolon controversy.
Figure 9.
Events taking place during exchange of CO2 and O2 in a systemic capillary. CO2 enters the cell by a combination of solubility diffusion and transport through aquaporin 1 (AQP1) and Rh associated glycoprotein (RhAG) (535–537). Cytoplasmic CO2 is hydrated by carbonic anhydrase, either bound to band 3 as a metabolon (303) or in the cytosol (482). The formed is transported outward in exchange for Cl− on subunits of the band 3 dimer or tetramer, either as an untethered dimer or in complexes with ankyrin or adducin. The other protein components of these complexes are not shown. The acid formed from CO2 hydration is buffered by hemoglobin, which is simultaneously releasing O2 in response to O2 efflux from the cells by a combination of solubility diffusion and transport through AQP1 (538). In parallel with CO2 uptake and O2 release, there is scavenging and production of nitric oxide (NO) and transport of NO metabolites either inward or outward on band 3 or by diffusion of undissociated acids (see text). Other processes (not shown) are carbamate formation and Cl− binding to hemoglobin. There is also some hydration of CO2 in the plasma.
SUMMARY
Perhaps the most important lesson that has emerged from decades of research on red cell band 3 is that even relatively simple systems are still complex, and not all the answers are in, despite the tremendous progress that has been made in the study of band 3. It is fair to ask whether it is still useful to continue to work on a system that is has been as thoroughly studied as band 3. I think the answer is that band 3 is still a great experimental system for applying new methods, as exemplified by the cross-linking study of Rivera-Santiago et al. (187). Band 3 is also an excellent system for applying computational methods, which have been used to study conformational effects of mutations (417); protein-protein and lipid-protein interactions (178, 451, 509); transport-related conformational changes (461, 510); subcellular diffusion (353, 482, 484); and integrative modeling of the interplay between transport and carbonic anhydrase activity (482, 511, 512). As computational methods improve, it may become possible to model substrate binding to band 3 and gain insights into the nature of the transition state. It should also become possible to simulate, simultaneously, all or most of the processes shown in Fig. 9. The combination of computational approaches and the vast store of existing (and future!) experimental data has the potential for producing a genuine understanding of both the molecular mechanism and the integrative physiology of band 3-mediated anion transport. We are much closer than we were a few years ago, but there is still more to do.
GRANTS
My work on band 3 was supported for many years by NIH Grant R01 GM026861 and institutional support from the University of Iowa, the University of Texas Medical Branch, and the University of Arkansas for Medical Sciences.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
M.L.J. prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
ACKNOWLEDGMENTS
I have spent about half my career studying red cell band 3 and am grateful to many people, including my Ph.D. advisor A. K. Solomon, my postdoctoral advisor Hermann Passow, and the students, technicians, and fellows who have worked in my lab. I am also grateful to all the other colleagues worldwide who made working on band 3 and red cell physiology such a pleasure. Reinhart Reithmeier made helpful suggestions for revision. Lindsay Blake of the University of Arkansas for Medical Sciences (UAMS) Library provided helpful advice on the use of the RefWorks reference management system.
REFERENCES
- 1.Alper SL. Molecular physiology of SLC4 anion exchangers. Exp Physiol 91: 153–161, 2006. [Erratum in Exp Physiol 91: 481, 2006]. doi: 10.1113/expphysiol.2005.031765. [DOI] [PubMed] [Google Scholar]
- 2.Romero MF, Chen AP, Parker MD, Boron WF. The SLC4 family of bicarbonate (HCO3⁻) transporters. Mol Aspects Med 34: 159–182, 2013. doi: 10.1016/j.mam.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker MD, Boron WF. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev 93: 803–959, 2013. doi: 10.1152/physrev.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thornell IM, Bevensee MO. Regulators of Slc4 bicarbonate transporter activity. Front Physiol 6: 166, 2015. doi: 10.3389/fphys.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perlman DF, Musch MW, Goldstein L. Band 3 in cell volume regulation in fish erythrocytes. Cell Mol Biol (Noisy-le-grand) 42: 975–984, 1996. [PubMed] [Google Scholar]
- 6.Garcia-Romeu F, Borgese F, Guizouarn H, Fiévet B, Motais R. A role for the anion exchanger AE1 (band 3 protein) in cell volume regulation. Cell Mol Biol (Noisy-le-grand) 42: 985–994, 1996. [PubMed] [Google Scholar]
- 7.Lux SE 4th. Anatomy of the red cell membrane skeleton: unanswered questions. Blood 127: 187–199, 2016. doi: 10.1182/blood-2014-12-512772. [DOI] [PubMed] [Google Scholar]
- 8.Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood 112: 3939–3948, 2008. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Low PS, Geahlen RL, Mehler E, Harrison ML. Extracellular control of erythrocyte metabolism mediated by a cytoplasmic tyrosine kinase. Biomed Biochim Acta 49: S135–S140, 1990. [PubMed] [Google Scholar]
- 10.Low PS. Structure and function of the cytoplasmic domain of band 3: center of erythrocyte membrane-peripheral protein interactions. Biochim Biophys Acta 864: 145–167, 1986. doi: 10.1016/0304-4157(86)90009-2. [DOI] [PubMed] [Google Scholar]
- 11.Chu H, McKenna MM, Krump NA, Zheng S, Mendelsohn L, Thein SL, Garrett LJ, Bodine DM, Low PS. Reversible binding of hemoglobin to band 3 constitutes the molecular switch that mediates O2 regulation of erythrocyte properties. Blood 128: 2708–2716, 2016. doi: 10.1182/blood-2016-01-692079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arese P, Turrini F, Schwarzer E. Band 3/complement-mediated recognition and removal of normally senescent and pathological human erythrocytes. Cell Physiol Biochem 16: 133–146, 2005. doi: 10.1159/000089839. [DOI] [PubMed] [Google Scholar]
- 13.Lutz HU, Bogdanova A. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front Physiol 4: 387, 2013. doi: 10.3389/fphys.2013.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kay MM, Bosman GJ, Shapiro SS, Bendich A, Bassel PS. Oxidation as a possible mechanism of cellular aging: vitamin E deficiency causes premature aging and IgG binding to erythrocytes. Proc Natl Acad Sci USA 83: 2463–2467, 1986. doi: 10.1073/pnas.83.8.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badior KE, Casey JR. Molecular mechanism for the red blood cell senescence clock. IUBMB Life 70: 32–40, 2018. doi: 10.1002/iub.1703. [DOI] [PubMed] [Google Scholar]
- 16.Bruce LJ, Tanner MJ. Erythroid band 3 variants and disease. Baillieres Best Pract Res Clin Haematol 12: 637–654, 1999. doi: 10.1053/beha.1999.0046. [DOI] [PubMed] [Google Scholar]
- 17.Almukadi H, Schwake C, Kaiser MM, Mayer DC, Schiemer J, Baldwin MR, Hegde S, Lu Y, Hanada T, Chishti AH. Human erythrocyte band 3 is a host receptor for plasmodium falciparum glutamic acid-rich protein. Blood 133: 470–480, 2019. doi: 10.1182/blood-2018-07-865451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arakawa T, Kobayashi-Yurugi T, Alguel Y, Iwanari H, Hatae H, Iwata M, Abe Y, Hino T, Ikeda-Suno C, Kuma H, Kang D, Murata T, Hamakubo T, Cameron AD, Kobayashi T, Hamasaki N, Iwata S. Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science 350: 680–684, 2015. doi: 10.1126/science.aaa4335. [DOI] [PubMed] [Google Scholar]
- 19.Hatae H, Inaka K, Okamura R, Furubayashi N, Kamo M, Kobayashi T, Abe Y, Iwata S, Hamasaki N. Crystallization of human erythrocyte band 3, the anion exchanger, at the international space station “KIBO”. Anal Biochem 559: 91–93, 2018. doi: 10.1016/j.ab.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Reithmeier RA, Casey JR, Kalli AC, Sansom MS, Alguel Y, Iwata S. Band 3, the human red cell chloride/bicarbonate anion exchanger (AE1, SLC4A1), in a structural context. Biochim Biophys Acta 1858: 1507–1532, 2016. doi: 10.1016/j.bbamem.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 21.Henderson LJ. Blood: A Study in General Physiology. New Haven, CT: Yale University Press, 1928. [Google Scholar]
- 22.Nasse H. Untersuchungen über den austritt und eintritt von stoffen (transsudation und diffusion) durch die wand der Haargefässe. Pflugers Arch Gesamte Physiol Menschen Tiere 16: 604–634, 1878. doi: 10.1007/BF01647557. [DOI] [Google Scholar]
- 23.Hamburger HJ. Über den einfluß der atmung auf die permeabilität der blutkörperchen. Z Biol 28: 405–410, 1891. [Google Scholar]
- 24.Van Slyke DD, Cullen GE. Studies of acidosis. I. the bicarbonate concentration of the blood plasma; its significance, and its determination as a measure of acidosis. J Biol Chem 30: 289–346, 1917. doi: 10.1016/S0021-9258(18)86738-2. [DOI] [Google Scholar]
- 25.Fridericia LS. Exchange of chloride ions and of carbon dioxide between blood corpuscles and blood plasma. J Biol Chem 42: 245–257, 1920. doi: 10.1016/S0021-9258(18)87141-1. [DOI] [Google Scholar]
- 26.Van Slyke DD, Wu H, McLean FC. Studies of gas and electrolyte equilibria in the blood: V. factors controlling the electrolyte and water distribution in the blood. J Biol Chem 56: 765–849, 1923. doi: 10.1016/S0021-9258(18)85558-2. [DOI] [Google Scholar]
- 27.Hastings AB, Sendroy J Jr, McIntosh JF, Van Slyke DD. Studies of gas and electrolyte equilibria in the blood. XIII. the distribution of chloride and bicarbonate in the blood of normal and pathological human subjects. J Biol Chem 79: 193–209, 1928. doi: 10.1016/S0021-9258(18)83946-1. [DOI] [Google Scholar]
- 28.Geers C, Gros G. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev 80: 681–715, 2000. doi: 10.1152/physrev.2000.80.2.681. [DOI] [PubMed] [Google Scholar]
- 29.Wieth JO, Andersen OS, Brahm J, Bjerrum PJ, Borders CL Jr.. Chloride-bicarbonate exchange in red blood cells: physiology of transport and chemical modification of binding sites. Philos Trans R Soc Lond B Biol Sci 299: 383–399, 1982. doi: 10.1098/rstb.1982.0139. [DOI] [PubMed] [Google Scholar]
- 30.Jackson DM, Nutt ME. The effect of carbon dioxide on relative red cell volume. J Physiol 123: 367–376, 1954. doi: 10.1113/jphysiol.1954.sp005057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Funder J, Wieth JO. Chloride and hydrogen ion distribution between human red cells and plasma. Acta Physiol Scand 68: 234–245, 1966. doi: 10.1111/j.1748-1716.1966.tb03423.x. [DOI] [Google Scholar]
- 32.Harris EJ, Maizels M. Distribution of ions in suspensions of human erythrocytes. J Physiol 118: 40–53, 1952. doi: 10.1113/jphysiol.1952.sp004771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roughton FJ. Recent work on carbon dioxide transport by the blood. Physiol Rev 15: 241–296, 1935. doi: 10.1152/physrev.1935.15.2.241. [DOI] [Google Scholar]
- 34.Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol 11: 290–302, 1957. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- 35.Crandall ED, Bidani A. Effects of red blood cell HCO3−/Cl− exchange kinetics on lung CO2 transfer: theory. J Appl Physiol Respir Environ Exerc Physiol 50: 265–271, 1981. doi: 10.1152/jappl.1981.50.2.265. [DOI] [PubMed] [Google Scholar]
- 36.Crandall ED, Mathew SJ, Fleischer RS, Winter HI, Bidani A. Effects of inhibition of RBC HCO3−/Cl− exchange on CO2 excretion and downstream pH disequilibrium in isolated rat lungs. J Clin Invest 68: 853–862, 1981. doi: 10.1172/JCI110340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dirken MN, Mook HW. The rate of gas exchange between blood cells and serum. J Physiol 73: 349–360, 1931. doi: 10.1113/jphysiol.1931.sp002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luckner H. Uber die geschwindigkeit des austausches der atemgase im blut. Pflugers Arch 241: 753–778, 1939. doi: 10.1007/BF01766137. [DOI] [Google Scholar]
- 39.Chow EI, Crandall ED, Forster RE. Kinetics of bicarbonate-chloride exchange across the human red blood cell membrane. J Gen Physiol 68: 633–652, 1976. doi: 10.1085/jgp.68.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roughton FJ. The average time spent by the blood in the human lung capillary and its relation to the rates of CO uptake and elimination in man. Am J Physiol 143: 621–633, 1945. doi: 10.1152/ajplegacy.1945.143.4.621. [DOI] [Google Scholar]
- 41.Passow H. Passive ion permeability of the erythrocyte membrane. Prog Biophys Mol Biol 19: 423–467, 1969. [PubMed] [Google Scholar]
- 42.Harris EJ, Pressman BC. Obligate cation exchanges in red cells. Nature 216: 918–920, 1967. doi: 10.1038/216918a0. [DOI] [PubMed] [Google Scholar]
- 43.Tosteson DC. Halide transport in red blood cells. Acta Physiol Scand 46: 19–41, 1959. doi: 10.1111/j.1748-1716.1959.tb01734.x. [DOI] [Google Scholar]
- 44.Scarpa A, Cecchetto A, Azzone GF. The mechanism of anion translocation and pH equilibration in erythrocytes. Biochim Biophys Acta 219: 179–188, 1970. doi: 10.1016/0005-2736(70)90073-8. [DOI] [PubMed] [Google Scholar]
- 45.Hunter MJ. A quantitative estimate of the non-exchange-restricted chloride permeability of the human red cell. J Physiol 218: 49P–50P, 1971. [PubMed] [Google Scholar]
- 46.Balach MM, Casale CH, Campetelli AN. Erythrocyte plasma membrane potential: past and current methods for its measurement. Biophys Rev 11: 995–1005, 2019. doi: 10.1007/s12551-019-00603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jay AW, Burton AC. Direct measurement of potential difference across the human red blood cell membrane. Biophys J 9: 115–121, 1969. doi: 10.1016/S0006-3495(69)86372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lassen UV, Sten-Knudsen O. Direct measurements of membrane potential and membrane resistance of human red cells. J Physiol 195: 681–696, 1968. doi: 10.1113/jphysiol.1968.sp008482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffman JF, Laris PC. Determination of membrane potentials in human and Amphiuma red blood cells by means of fluorescent probe. J Physiol 239: 519–552, 1974. doi: 10.1113/jphysiol.1974.sp010581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deuticke B. Anion permeability of the red blood cell. Naturwissenschaften 57: 172–179, 1970. doi: 10.1007/BF00592968. [DOI] [PubMed] [Google Scholar]
- 51.Dalmark M, Wieth JO. Temperature dependence of chloride, bromide, iodide, thiocyanate and salicylate transport in human red cells. J Physiol 224: 583–610, 1972. doi: 10.1113/jphysiol.1972.sp009914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gunn RB, Dalmark M, Tosteson DC, Wieth JO. Characteristics of chloride transport in human red blood cells. J Gen Physiol 61: 185–206, 1973. doi: 10.1085/jgp.61.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lepke S, Passow H. The permeability of the human red blood cell to sulfate ions. J Membr Biol 6: 158–182, 1971. doi: 10.1007/BF01873461. [DOI] [PubMed] [Google Scholar]
- 54.Gorter E, Grendel F. On bimolecular layers of lipoids on the chromocytes of the blood. J Exp Med 41: 439–443, 1925. doi: 10.1084/jem.41.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engelman DM. Lipid bilayer structure in the membrane of mycoplasma laidlawii. J Mol Biol 58: 153–165, 1971. doi: 10.1016/0022-2836(71)90238-5. [DOI] [PubMed] [Google Scholar]
- 56.Wilkins MH, Blaurock AE, Engelman DM. Bilayer structure in membranes. Nat New Biol 230: 72–76, 1971. doi: 10.1038/newbio230072a0. [DOI] [PubMed] [Google Scholar]
- 57.Hubbell WL, McConnell HM. Motion of steroid spin labels in membranes. Proc Natl Acad Sci USA 63: 16–22, 1969. doi: 10.1073/pnas.63.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hubbell WL, McConnell HM. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc 93: 314–326, 1971. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- 59.Frye LD, Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci 7: 319–335, 1970. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- 60.Pinto da Silva P, Branton D. Membrane splitting in freeze-etching. covalently bound ferritin as a membrane marker. J Cell Biol 45: 598–605, 1970. doi: 10.1083/jcb.45.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bretscher MS. A major protein which spans the human erythrocyte membrane. J Mol Biol 59: 351–357, 1971. doi: 10.1016/0022-2836(71)90055-6. [DOI] [PubMed] [Google Scholar]
- 62.Fairbanks G, Steck TL, Wallach DF. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 10: 2606–2617, 1971. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- 63.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science 175: 720–731, 1972. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 64.Robertson JD. Unit membranes: a review with recent new studies of experimental alterations and a new subunit structure in synaptic membranes. In: Cellular Membranes in Development, edited by Locke M. New York: Academic Press, 1964. p. 1. [Google Scholar]
- 65.Berg HC, Diamond JM, Marfey PS. Erythrocyte membrane: chemical modification. Science 150: 64–67, 1965. doi: 10.1126/science.150.3692.64. [DOI] [PubMed] [Google Scholar]
- 66.Maddy AH. A fluorescent label for the outer components of the plasma membrane. Biochim Biophys Acta 88: 390–399, 1964. [DOI] [PubMed] [Google Scholar]
- 67.Knauf PA, Rothstein A. Chemical modification of membranes. I. effects of sulfhydryl and amino reactive reagents on anion and cation permeability of the human red blood cell. J Gen Physiol 58: 190–210, 1971. doi: 10.1085/jgp.58.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cabantchik ZI, Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol 15: 207–226, 1974. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- 69.Lepke S, Fasold H, Pring M, Passow H. A study of the relationship between inhibition of anion exchange and binding to the red blood cell membrane of 4,4’-diisothiocyano stilbene-2,2’-disulfonic acid (DIDS) and its dihydro derivative (H2DIDS). J Membr Biol 29: 147–177, 1976. doi: 10.1007/BF01868957. [DOI] [PubMed] [Google Scholar]
- 70.Ship S, Shami Y, Breuer W, Rothstein A. Synthesis of tritiated 4,4'-diisothiocyano-2,2'-stilbene disulfonic acid ([3H]DIDS) and its covalent reaction with sites related to anion transport in human red blood cells. J Membr Biol 33: 311–323, 1977. doi: 10.1007/BF01869522. [DOI] [PubMed] [Google Scholar]
- 71.Ho MK, Guidotti G. A membrane protein from human erythrocytes involved in anion exchange. J Biol Chem 250: 675–683, 1975. doi: 10.1016/S0021-9258(19)41949-2. [DOI] [PubMed] [Google Scholar]
- 72.Zaki L, Fasold H, Schuhmann B, Passow H. Chemical modification of membrane proteins in relation to inhibition of anion exchange in human red blood cells. J Cell Physiol 86: 471–494, 1975. doi: 10.1002/jcp.1040860305. [DOI] [PubMed] [Google Scholar]
- 73.Gunn RB. A titratable carrier model for both mono- and di-valent anion transport in human red blood cells. In: Oxygen Affinity of Hemoglobin and Red Cell Acid-Base Status, edited by Rorth M, Astrup P.. Copenhagen: Munksgaard, 1972. p. 823–827. [Google Scholar]
- 74.Wilbrandt W. Untersuchungen über langsamen anionenaustausch durch die erythrocytenmembran. Pflugers Arch 246: 291–306, 1942. doi: 10.1007/BF02562542. [DOI] [Google Scholar]
- 75.Jennings ML. Proton fluxes associated with erythrocyte membrane anion exchange. J Membr Biol 28: 187–205, 1976. doi: 10.1007/BF01869697. [DOI] [PubMed] [Google Scholar]
- 76.Wieth JO. Bicarbonate exchange through the human red cell membrane determined with [14C] bicarbonate. J Physiol 294: 521–539, 1979. doi: 10.1113/jphysiol.1979.sp012944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ku CP, Jennings ML, Passow H. A comparison of the inhibitory potency of reversibly acting inhibitors of anion transport on chloride and sulfate movements across the human red cell membrane. Biochim Biophys Acta 553: 132–141, 1979. doi: 10.1016/0005-2736(79)90035-X. [DOI] [PubMed] [Google Scholar]
- 78.Jardetzky O. Simple allosteric model for membrane pumps. Nature 211: 969–970, 1966. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- 79.Grinstein S, McCulloch L, Rothstein A. Transmembrane effects of irreversible inhibitors of anion transport in red blood cells. Evidence for mobile transport sites. J Gen Physiol 73: 493–514, 1979. doi: 10.1085/jgp.73.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Passow H, Fasold H, Gärtner EM, Legrum B, Ruffing W, Zaki L. Anion transport across the red blood cell membrane and the conformation of the protein in band 3. Ann NY Acad Sci 341: 361–383, 1980. doi: 10.1111/j.1749-6632.1980.tb47184.x. [DOI] [PubMed] [Google Scholar]
- 81.Eidelman O, Cabantchik ZI. The mechanism of anion transport across human red blood cell membranes as revealed with a fluorescent substrate: II. kinetic properties of NBD-taurine transfer in asymmetric conditions. J Membr Biol 71: 149–161, 1983. doi: 10.1007/BF01870683. [DOI] [PubMed] [Google Scholar]
- 82.Cleland WW. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. nomenclature and rate equations. Biochim Biophys Acta 67: 104–137, 1963. doi: 10.1016/0926-6569(63)90211-6. [DOI] [PubMed] [Google Scholar]
- 83.Gunn RB, Fröhlich O. Asymmetry in the mechanism for anion exchange in human red blood cell membranes. evidence for reciprocating sites that react with one transported anion at a time. J Gen Physiol 74: 351–374, 1979. doi: 10.1085/jgp.74.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knauf PA, Spinelli LJ, Mann NA. Flufenamic acid senses conformation and asymmetry of human erythrocyte band 3 anion transport protein. Am J Physiol Cell Physiol 257: C277–C289, 1989. doi: 10.1152/ajpcell.1989.257.2.C277. [DOI] [PubMed] [Google Scholar]
- 85.Knauf PA, Mann NA. Use of niflumic acid to determine the nature of the asymmetry of the human erythrocyte anion exchange system. J Gen Physiol 83: 703–725, 1984. doi: 10.1085/jgp.83.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Knauf PA, Gasbjerg PK, Brahm J. The asymmetry of chloride transport at 38 degrees C in human red blood cell membranes. J Gen Physiol 108: 577–589, 1996. doi: 10.1085/jgp.108.6.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jennings ML. Stoichiometry of a half-turnover of band 3, the chloride transport protein of human erythrocytes. J Gen Physiol 79: 169–185, 1982. doi: 10.1085/jgp.79.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jennings ML, Whitlock J, Shinde A. Pre-steady state transport by erythrocyte band 3 protein: Uphill countertransport induced by the impermeant inhibitor H2DIDS. Biochem Cell Biol 76: 807–813, 1998. doi: 10.1139/bcb-76-5-807. [DOI] [PubMed] [Google Scholar]
- 89.Dalmark M. Effects of halides and bicarbonate on chloride transport in human red blood cells. J Gen Physiol 67: 223–234, 1976. doi: 10.1085/jgp.67.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wieth JO, Bjerrum PJ. Titration of transport and modifier sites in the red cell anion transport system. J Gen Physiol 79: 253–282, 1982. doi: 10.1085/jgp.79.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Salhany JM, Swanson JC. Kinetics of passive anion transport across the human erythrocyte membrane. Biochemistry 17: 3354–3362, 1978. doi: 10.1021/bi00609a028. [DOI] [PubMed] [Google Scholar]
- 92.Salhany JM, Gaines ED. Steady state kinetics of erythrocyte anion exchange. evidence for site-site interactions. J Biol Chem 256: 11080–11085, 1981. doi: 10.1016/S0021-9258(19)68558-3. [DOI] [PubMed] [Google Scholar]
- 93.Funder J, Wieth JO. Chloride transport in human erythrocytes and ghosts: a quantitative comparison. J Physiol 262: 679–698, 1976. doi: 10.1113/jphysiol.1976.sp011615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fröhlich O, Gunn RB. Erythrocyte anion transport: the kinetics of a single-site obligatory exchange system. Biochim Biophys Acta 864: 169–194, 1986. doi: 10.1016/0304-4157(86)90010-9. [DOI] [PubMed] [Google Scholar]
- 95.Knauf PA, Law FY, Leung TW, Gehret AU, Perez ML. Substrate-dependent reversal of anion transport site orientation in the human red blood cell anion-exchange protein, AE1. Proc Natl Acad Sci USA 99: 10861–10864, 2002. doi: 10.1073/pnas.162402399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shami Y, Carver J, Ship S, Rothstein A. Inhibition of C1− binding to anion transport protein of the red blood cell by DIDS (4, 4’-diisothiocyano-2, 2’-stilbene disulfonic acid) measured by [35C1]NMR. Biochem Biophys Res Commun 76: 429–436, 1976. doi: 10.1016/0006-291X(77)90743-4. [DOI] [PubMed] [Google Scholar]
- 97.Falke JJ, Pace RJ, Chan SI. Chloride binding to the anion transport binding sites of band 3. A 35Cl NMR study. J Biol Chem 259: 6472–6480, 1984. doi: 10.1016/S0021-9258(20)82166-8. [DOI] [PubMed] [Google Scholar]
- 98.Falke JJ, Pace RJ, Chan SI. Direct observation of the transmembrane recruitment of band 3 transport sites by competitive inhibitors. A 35Cl NMR study. J Biol Chem 259: 6481–6491, 1984. doi: 10.1016/S0021-9258(20)82167-X. [DOI] [PubMed] [Google Scholar]
- 99.Falke JJ, Chan SI. Evidence that anion transport by band 3 proceeds via a ping-pong mechanism involving a single transport site. A 35Cl NMR study. J Biol Chem 260: 9537–9544, 1985. doi: 10.1016/S0021-9258(17)39268-2. [DOI] [PubMed] [Google Scholar]
- 100.Liu D, Kennedy SD, Knauf PA. Source of transport site asymmetry in the band 3 anion exchange protein determined by NMR measurements of external Cl- affinity. Biochemistry 35: 15228–15235, 1996. doi: 10.1021/bi961443b. [DOI] [PubMed] [Google Scholar]
- 101.Falke JJ, Chan SI. Molecular mechanisms of band 3 inhibitors. 1. Transport site inhibitors. Biochemistry 25: 7888–7894, 1986. doi: 10.1021/bi00372a015. [DOI] [PubMed] [Google Scholar]
- 102.Falke JJ, Chan SI. Molecular mechanisms of band 3 inhibitors. 2. Channel blockers. Biochemistry 25: 7895–7898, 1986. doi: 10.1021/bi00372a016. [DOI] [PubMed] [Google Scholar]
- 103.Falke JJ, Chan SI. Molecular mechanisms of band 3 inhibitors. 3. Translocation inhibitors. Biochemistry 25: 7899–7906, 1986. doi: 10.1021/bi00372a017. [DOI] [PubMed] [Google Scholar]
- 104.Falke JJ, Kanes KJ, Chan SI. The kinetic equation for the chloride transport cycle of band 3. A 35Cl and 37Cl NMR study. J Biol Chem 260: 9545–9551, 1985. doi: 10.1016/S0021-9258(17)39269-4. [DOI] [PubMed] [Google Scholar]
- 105.Galanter WL, Labotka RJ. The binding of nitrate to the human anion exchange protein (AE1) studied with 14N nuclear magnetic resonance. Biochim Biophys Acta 1079: 146–151, 1991. doi: 10.1016/0167-4838(91)90119-K. [DOI] [PubMed] [Google Scholar]
- 106.Labotka RJ, Omachi A. The pH dependence of red cell membrane transport of titratable anions studied by NMR spectroscopy. J Biol Chem 263: 1166–1173, 1988. doi: 10.1016/S0021-9258(19)57281-7. [DOI] [PubMed] [Google Scholar]
- 107.Galanter WL, Hakimian M, Labotka RJ. Structural determinants of substrate specificity of the erythrocyte anion transporter. Am J Physiol Cell Physiol 265: C918–C926, 1993. doi: 10.1152/ajpcell.1993.265.4.C918. [DOI] [PubMed] [Google Scholar]
- 108.Aubert L, Motais R. Molecular features of organic anion permeability in ox red blood cell. J Physiol 246: 159–179, 1975. doi: 10.1113/jphysiol.1975.sp010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Labotka RJ, Omachi A. Erythrocyte anion transport of phosphate analogs. J Biol Chem 262: 305–311, 1987. doi: 10.1016/S0021-9258(19)75927-4. [DOI] [PubMed] [Google Scholar]
- 110.Krupka RM. Role of substrate binding forces in exchange-only transport systems: I. transition-state theory. J Membr Biol 109: 151–158, 1989. doi: 10.1007/BF01870854. [DOI] [PubMed] [Google Scholar]
- 111.Krupka RM. Role of substrate binding forces in exchange-only transport systems: II. implications for the mechanism of the anion exchanger of red cells. J Membr Biol 109: 159–171, 1989. doi: 10.1007/BF01870855. [DOI] [PubMed] [Google Scholar]
- 112.Jacobs MH, Parpart AK. Is the erythrocyte permeable to hydrogen ions? Biol Bull (Woods Hole) 62: 63–76, 1932. doi: 10.2307/1537144. [DOI] [Google Scholar]
- 113.Jacobs MH, Stewart DR. The role of carbonic anhydrase in certain ionic exchanges involving the erythrocyte. J Gen Physiol 25: 539–552, 1942. doi: 10.1085/jgp.25.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Knauf PA, Fuhrmann GF, Rothstein S, Rothstein A. The relationship between anion exchange and net anion flow across the human red blood cell membrane. J Gen Physiol 69: 363–386, 1977. doi: 10.1085/jgp.69.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Knauf PA, Law FY, Marchant PJ. Relationship of net chloride flow across the human erythrocyte membrane to the anion exchange mechanism. J Gen Physiol 81: 95–126, 1983. doi: 10.1085/jgp.81.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fröhlich O, Leibson C, Gunn RB. Chloride net efflux from intact erythrocytes under slippage conditions. evidence for a positive charge on the anion binding/transport site. J Gen Physiol 81: 127–152, 1983. doi: 10.1085/jgp.81.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Milanick MA, Gunn RB. Proton-sulfate co-transport: mechanism of H+ and sulfate addition to the chloride transporter of human red blood cells. J Gen Physiol 79: 87–113, 1982. doi: 10.1085/jgp.79.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Milanick MA, Gunn RB. Proton-sulfate cotransport: external proton activation of sulfate influx into human red blood cells. Am J Physiol Cell Physiol 247: C247–C259, 1984. doi: 10.1152/ajpcell.1984.247.3.C247. [DOI] [PubMed] [Google Scholar]
- 119.Jennings ML. Characteristics of CO2-independent pH equilibration in human red blood cells. J Membr Biol 40: 365–391, 1978. doi: 10.1007/BF01874164. [DOI] [PubMed] [Google Scholar]
- 120.Jennings ML. Evidence for a second binding/transport site for chloride in erythrocyte anion transporter AE1 modified at glutamate 681. Biophys J 88: 2681–2691, 2005. doi: 10.1529/biophysj.104.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jones GS, Knauf PA. Mechanism of the increase in cation permeability of human erythrocytes in low-chloride media. Involvement of the anion transport protein capnophorin. J Gen Physiol 86: 721–738, 1985. doi: 10.1085/jgp.86.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Salhany JM, Cordes KS, Sloan RL. Band 3 (AE1, SLC4A1)-mediated transport of stilbenedisulfonates. I: Functional identification of the proton-activated stilbenedisulfonate influx site. Blood Cells Mol Dis 37: 137–148, 2006. doi: 10.1016/j.bcmd.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 123.Mawby WJ, Findlay JB. Characterization and partial sequence of di-iodosulphophenyl isothiocyanate-binding peptide from human erythrocyte anion-transport protein. Biochem J 205: 465–475, 1982. doi: 10.1042/bj2050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brock CJ, Tanner MJ, Kempf C. The human erythrocyte anion-transport protein. partial amino acid sequence, conformation and a possible molecular mechanism for anion exchange. Biochem J 213: 577–586, 1983. doi: 10.1042/bj2130577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kaul RK, Murthy SN, Reddy AG, Steck TL, Kohler H. Amino acid sequence of the N alpha-terminal 201 residues of human erythrocyte membrane band 3. J Biol Chem 258: 7981–7990, 1983. doi: 10.1016/S0021-9258(20)82016-X. [DOI] [PubMed] [Google Scholar]
- 126.Kopito RR, Lodish HF. Primary structure and transmembrane orientation of the murine anion exchange protein. Nature 316: 234–238, 1985. doi: 10.1038/316234a0. [DOI] [PubMed] [Google Scholar]
- 127.Tanner MJ, Martin PG, High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence. Biochem J 256: 703–712, 1988. doi: 10.1042/bj2560703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lux SE, John KM, Kopito RR, Lodish HF. Cloning and characterization of band 3, the human erythrocyte anion-exchange protein (AE1). Proc Natl Acad Sci USA 86: 9089–9093, 1989. doi: 10.1073/pnas.86.23.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schofield AE, Martin PG, Spillett D, Tanner MJ. The structure of the human red blood cell anion exchanger (EPB3, AE1, band 3) gene. Blood 84: 2000–2012, 1994. doi: 10.1182/blood.V84.6.2000.2000. [DOI] [PubMed] [Google Scholar]
- 130.Sahr KE, Taylor WM, Daniels BP, Rubin HL, Jarolim P. The structure and organization of the human erythroid anion exchanger (AE1) gene. Genomics 24: 491–501, 1994. doi: 10.1006/geno.1994.1658. [DOI] [PubMed] [Google Scholar]
- 131.Kopito RR, Andersson M, Lodish HF. Structure and organization of the murine band 3 gene. J Biol Chem 262: 8035–8040, 1987. doi: 10.1016/S0021-9258(18)47522-9. [DOI] [PubMed] [Google Scholar]
- 132.Alvarez BV, Kieller DM, Quon AL, Robertson M, Casey JR. Cardiac hypertrophy in anion exchanger 1-null mutant mice with severe hemolytic anemia. Am J Physiol Heart Circ Physiol 292: H1301–H1312, 2007. doi: 10.1152/ajpheart.00449.2006. [DOI] [PubMed] [Google Scholar]
- 133.Donà G, Tibaldi E, Andrisani A, Ambrosini G, Sabbadin C, Pagano MA, Brunati AM, Armanini D, Ragazzi E, Bordin L. Human sperm capacitation involves the regulation of the Tyr-phosphorylation level of the anion exchanger 1 (AE1). Int J Mol Sci 21: 4063, 2020. doi: 10.3390/ijms21114063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brosius FC 3rd, Alper SL, Garcia AM, Lodish HF. The major kidney band 3 gene transcript predicts an amino-terminal truncated band 3 polypeptide. J Biol Chem 264: 7784–7787, 1989. doi: 10.1016/S0021-9258(18)83108-8. [DOI] [PubMed] [Google Scholar]
- 135.Kudrycki KE, Shull GE. Primary structure of the rat kidney band 3 anion exchange protein deduced from a cDNA. J Biol Chem 264: 8185–8192, 1989. doi: 10.1016/S0021-9258(18)83167-2. [DOI] [PubMed] [Google Scholar]
- 136.Kollert-Jöns A, Wagner S, Hübner S, Appelhans H, Drenckhahn D. Anion exchanger 1 in human kidney and oncocytoma differs from erythroid AE1 in its NH2 terminus. Am J Physiol Renal Physiol 265: F813–F821, 1993. doi: 10.1152/ajprenal.1993.265.6.F813. [DOI] [PubMed] [Google Scholar]
- 137.Schuster VL, Bonsib SM, Jennings ML. Two types of collecting duct mitochondria-rich (intercalated) cells: lectin and band 3 cytochemistry. Am J Physiol Cell Physiol 251: C347–C355, 1986. doi: 10.1152/ajpcell.1986.251.3.C347. [DOI] [PubMed] [Google Scholar]
- 138.Alper SL, Natale J, Gluck S, Lodish HF, Brown D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci USA 86: 5429–5433, 1989. doi: 10.1073/pnas.86.14.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Demuth DR, Showe LC, Ballantine M, Palumbo A, Fraser PJ, Cioe L, Rovera G, Curtis PJ. Cloning and structural characterization of a human non-erythroid band 3-like protein. EMBO J 5: 1205–1214, 1986. doi: 10.1002/j.1460-2075.1986.tb04348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alper SL, Kopito RR, Libresco SM, Lodish HF. Cloning and characterization of a murine band 3-related cDNA from kidney and from a lymphoid cell line. J Biol Chem 263: 17092–17099, 1988. doi: 10.1016/S0021-9258(18)37502-1. [DOI] [PubMed] [Google Scholar]
- 141.Kudrycki KE, Newman PR, Shull GE. cDNA cloning and tissue distribution of mRNAs for two proteins that are related to the band 3 Cl-/HCO3− exchanger. J Biol Chem 265: 462–471, 1990. doi: 10.1016/S0021-9258(19)40253-6. [DOI] [PubMed] [Google Scholar]
- 142.Kopito RR, Lee BS, Simmons DM, Lindsey AE, Morgans CW, Schneider K. Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell 59: 927–937, 1989. doi: 10.1016/0092-8674(89)90615-6. [DOI] [PubMed] [Google Scholar]
- 143.Wieth JO, Bjerrum PJ. Transport and modifier sites in capnophorin, the anion transport protein of the erythrocyte membrane. In: Structure and Function of Membrane Protein, edited by Quagliariello E, Palmieri F.. Amsterdam: Elsevier/North-Holland, 1983. p. 106. [Google Scholar]
- 144.Drickamer LK. Orientation of the band 3 polypeptide from human erythrocyte membranes. identification of NH2-terminal sequence and site of carbohydrate attachment. J Biol Chem 253: 7242–7248, 1978. doi: 10.1016/S0021-9258(17)34491-5. [DOI] [PubMed] [Google Scholar]
- 145.Yu J, Steck TL. Isolation and characterization of band 3, the predominant polypeptide of the human erythrocyte membrane. J Biol Chem 250: 9170–9175, 1975. doi: 10.1016/S0021-9258(19)40705-9. [DOI] [PubMed] [Google Scholar]
- 146.Mueller TJ, Li YT, Morrison M. Effect of endo-beta-galactosidase on intact human erythrocytes. J Biol Chem 254: 8103–8106, 1979. doi: 10.1016/S0021-9258(19)86856-4. [DOI] [PubMed] [Google Scholar]
- 147.Fukuda MN, Fukuda M, Hakomori S. Cell surface modification by endo-beta-galactosidase. change of blood group activities and release of oligosaccharides from glycoproteins and glycosphingolipids of human erythrocytes. J Biol Chem 254: 5458–5465, 1979. doi: 10.1016/S0021-9258(18)50618-9. [DOI] [PubMed] [Google Scholar]
- 148.Wainwright SD, Tanner MJ, Martin GE, Yendle JE, Holmes C. Monoclonal antibodies to the membrane domain of the human erythrocyte anion transport protein. localization of the C-terminus of the protein to the cytoplasmic side of the red cell membrane and distribution of the protein in some human tissues. Biochem J 258: 211–220, 1989. doi: 10.1042/bj2580211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Casey JR, Pirraglia CA, Reithmeier RA. Enzymatic deglycosylation of human band 3, the anion transport protein of the erythrocyte membrane. effect on protein structure and transport properties. J Biol Chem 267: 11940–11948, 1992. doi: 10.1016/S0021-9258(19)49787-1. [DOI] [PubMed] [Google Scholar]
- 150.Okubo K, Hamasaki N, Hara K, Kageura M. Palmitoylation of cysteine 69 from the COOH-terminal of band 3 protein in the human erythrocyte membrane. acylation occurs in the middle of the consensus sequence of F-I-IICLAVL found in band 3 protein and G2 protein of Rift Valley fever virus. J Biol Chem 266: 16420–16424, 1991. doi: 10.1016/S0021-9258(18)55315-1. [DOI] [PubMed] [Google Scholar]
- 151.Cheung JC, Reithmeier RA. Palmitoylation is not required for trafficking of human anion exchanger 1 to the cell surface. Biochem J 378: 1015–1021, 2004. doi: 10.1042/bj20030847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kang D, Karbach D, Passow H. Anion transport function of mouse erythroid band 3 protein (AE1) does not require acylation of cysteine residue 861. Biochim Biophys Acta 1194: 341–344, 1994. doi: 10.1016/0005-2736(94)90317-4. [DOI] [PubMed] [Google Scholar]
- 153.Bennett V, Stenbuck PJ. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature 280: 468–473, 1979. doi: 10.1038/280468a0. [DOI] [PubMed] [Google Scholar]
- 154.Lukacovic MF, Feinstein MB, Sha’afi RI, Perrie S. Purification of stabilized band 3 protein of the human erythrocyte membrane and its reconstitution into liposomes. Biochemistry 20: 3145–3151, 1981. doi: 10.1021/bi00514a025. [DOI] [PubMed] [Google Scholar]
- 155.Pappert G, Schubert D. The state of association of band 3 protein of the human erythrocyte membrane in solutions of nonionic detergents. Biochim Biophys Acta 730: 32–40, 1983. doi: 10.1016/0005-2736(83)90313-9. [DOI] [PubMed] [Google Scholar]
- 156.Lindenthal S, Schubert D. Monomeric erythrocyte band 3 protein transports anions. Proc Natl Acad Sci USA 88: 6540–6544, 1991. doi: 10.1073/pnas.88.15.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Salhany JM, Cordes KA, Sloan RL. Mechanism of band 3 dimer dissociation during incubation of erythrocyte membranes at 37 degrees C. Biochem J 345: 33–41, 2000. doi: 10.1042/bj3450033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boodhoo A, Reithmeier RA. Characterization of matrix-bound band 3, the anion transport protein from human erythrocyte membranes. J Biol Chem 259: 785–790, 1984. doi: 10.1016/S0021-9258(17)43526-5. [DOI] [PubMed] [Google Scholar]
- 159.Steck TL. Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol 66: 295–305, 1972. doi: 10.1016/0022-2836(72)90481-0. [DOI] [PubMed] [Google Scholar]
- 160.Reithmeier RA, Rao A. Reactive sulfhydryl groups of the band 3 polypeptide from human erythrocyte membranes. identification of the sulfhydryl groups involved in Cu2+-o-phenanthroline cross-linking. J Biol Chem 254: 6151–6155, 1979. doi: 10.1016/S0021-9258(18)50531-7. [DOI] [PubMed] [Google Scholar]
- 161.Rao A, Reithmeier RA. Reactive sulfhydryl groups of the band 3 polypeptide from human erythrocyte membranes. location in the primary structure. J Biol Chem 254: 6144–6150, 1979. doi: 10.1016/S0021-9258(18)50530-5. [DOI] [PubMed] [Google Scholar]
- 162.Thevenin BJ, Willardson BM, Low PS. The redox state of cysteines 201 and 317 of the erythrocyte anion exchanger is critical for ankyrin binding. J Biol Chem 264: 15886–15892, 1989. doi: 10.1016/S0021-9258(18)71561-5. [DOI] [PubMed] [Google Scholar]
- 163.Appell KC, Low PS. Partial structural characterization of the cytoplasmic domain of the erythrocyte membrane protein, band 3. J Biol Chem 256: 11104–11111, 1981. doi: 10.1016/S0021-9258(19)68562-5. [DOI] [PubMed] [Google Scholar]
- 164.Zhang D, Kiyatkin A, Bolin JT, Low PS. Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood 96: 2925–2933, 2000. doi: 10.1182/blood.V96.9.2925. [DOI] [PubMed] [Google Scholar]
- 165.Shnitsar V, Li J, Li X, Calmettes C, Basu A, Casey JR, Moraes TF, Reithmeier RA. A substrate access tunnel in the cytosolic domain is not an essential feature of the solute carrier 4 (SLC4) family of bicarbonate transporters. J Biol Chem 288: 33848–33860, 2013. doi: 10.1074/jbc.M113.511865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhou Z, DeSensi SC, Stein RA, Brandon S, Dixit M, McArdle EJ, Warren EM, Kroh HK, Song L, Cobb CE, Hustedt EJ, Beth AH. Solution structure of the cytoplasmic domain of erythrocyte membrane band 3 determined by site-directed spin labeling. Biochemistry 44: 15115–15128, 2005. doi: 10.1021/bi050931t. [DOI] [PubMed] [Google Scholar]
- 167.Low PS, Westfall MA, Allen DP, Appell KC. Characterization of the reversible conformational equilibrium of the cytoplasmic domain of erythrocyte membrane band 3. J Biol Chem 259: 13070–13076, 1984. doi: 10.1016/S0021-9258(18)90658-7. [DOI] [PubMed] [Google Scholar]
- 168.Zhou J, Low PS. Characterization of the reversible conformational equilibrium in the cytoplasmic domain of human erythrocyte membrane band 3. J Biol Chem 276: 38147–38151, 2001. doi: 10.1074/jbc.M104333200. [DOI] [PubMed] [Google Scholar]
- 169.Oikawa K, Lieberman DM, Reithmeier RA. Conformation and stability of the anion transport protein of human erythrocyte membranes. Biochemistry 24: 2843–2848, 1985. doi: 10.1021/bi00333a005. [DOI] [PubMed] [Google Scholar]
- 170.Reithmeier RA. Fragmentation of the band 3 polypeptide from human erythrocyte membranes. size and detergent binding of the membrane-associated domain. J Biol Chem 254: 3054–3060, 1979. doi: 10.1016/S0021-9258(17)30181-3. [DOI] [PubMed] [Google Scholar]
- 171.Casey JR, Reithmeier RA. Analysis of the oligomeric state of band 3, the anion transport protein of the human erythrocyte membrane, by size exclusion high performance liquid chromatography. oligomeric stability and origin of heterogeneity. J Biol Chem 266: 15726–15737, 1991. doi: 10.1016/S0021-9258(18)98470-X. [DOI] [PubMed] [Google Scholar]
- 172.Wang DN, Kühlbrandt W, Sarabia VE, Reithmeier RA. Two-dimensional structure of the membrane domain of human band 3, the anion transport protein of the erythrocyte membrane. EMBO J 12: 2233–2239, 1993. doi: 10.1002/j.1460-2075.1993.tb05876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang DN, Sarabia VE, Reithmeier RA, Kühlbrandt W. Three-dimensional map of the dimeric membrane domain of the human erythrocyte anion exchanger, band 3. EMBO J 13: 3230–3235, 1994. doi: 10.1002/j.1460-2075.1994.tb06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lu F, Li S, Jiang Y, Jiang J, Fan H, Lu G, Deng D, Dang S, Zhang X, Wang J, Yan N. Structure and mechanism of the uracil transporter UraA. Nature 472: 243–246, 2011. doi: 10.1038/nature09885. [DOI] [PubMed] [Google Scholar]
- 175.Staros JV. N-hydroxysulfosuccinimide active esters: Bis(N-hydroxysulfosuccinimide) esters of two dicarboxylic acids are hydrophilic, membrane-impermeant, protein cross-linkers. Biochemistry 21: 3950–3955, 1982. doi: 10.1021/bi00260a008. [DOI] [PubMed] [Google Scholar]
- 176.Staros JV, Kakkad BP. Cross-linking and chymotryptic digestion of the extracytoplasmic domain of the anion exchange channel in intact human erythrocytes. J Membr Biol 74: 247–254, 1983. doi: 10.1007/BF02332127. [DOI] [PubMed] [Google Scholar]
- 177.Jennings ML, Nicknish JS. Localization of a site of intermolecular cross-linking in human red blood cell band 3 protein. J Biol Chem 260: 5472–5479, 1985. doi: 10.1016/S0021-9258(18)89046-9. [DOI] [PubMed] [Google Scholar]
- 178.Kalli AC, Reithmeier RA. Interaction of the human erythrocyte band 3 anion exchanger 1 (AE1, SLC4A1) with lipids and glycophorin A: molecular organization of the wright (wr) blood group antigen. PLoS Comput Biol 14: e1006284, 2018. doi: 10.1371/journal.pcbi.1006284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang DN. Band 3 protein: Structure, flexibility and function. FEBS Lett 346: 26–31, 1994. doi: 10.1016/0014-5793(94)00468-4. [DOI] [PubMed] [Google Scholar]
- 180.Jiang J, Magilnick N, Tsirulnikov K, Abuladze N, Atanasov I, Ge P, Narla M, Pushkin A, Zhou ZH, Kurtz I. Single particle electron microscopy analysis of the bovine anion exchanger 1 reveals a flexible linker connecting the cytoplasmic and membrane domains. PLoS One 8: e55408, 2013. doi: 10.1371/journal.pone.0055408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Steck TL, Ramos B, Strapazon E. Proteolytic dissection of band 3, the predominant transmembrane polypeptide of the human erythrocyte membrane. Biochemistry 15: 1153–1161, 1976. doi: 10.1021/bi00650a030. [DOI] [PubMed] [Google Scholar]
- 182.Steck TL, Koziarz JJ, Singh MK, Reddy G, Köhler H. Preparation and analysis of seven major, topographically defined fragments of band 3, the predominant transmembrane polypeptide of human erythrocyte membranes. Biochemistry 17: 1216–1222, 1978. doi: 10.1021/bi00600a013. [DOI] [PubMed] [Google Scholar]
- 183.Appell KC, Low PS. Evaluation of structural interdependence of membrane-spanning and cytoplasmic domains of band 3. Biochemistry 21: 2151–2157, 1982. doi: 10.1021/bi00538a026. [DOI] [PubMed] [Google Scholar]
- 184.Lepke S, Passow H. Effects of incorporated trypsin on anion exchange and membrane proteins in human red blood cell ghosts. Biochim Biophys Acta 455: 353–370, 1976. doi: 10.1016/0005-2736(76)90311-4. [DOI] [PubMed] [Google Scholar]
- 185.Grinstein S, Ship S, Rothstein A. Anion transport in relation to proteolytic dissection of band 3 protein. Biochim Biophys Acta 507: 294–304, 1978. doi: 10.1016/0005-2736(78)90424-8. [DOI] [PubMed] [Google Scholar]
- 186.Lepke S, Becker A, Passow H. Mediation of inorganic anion transport by the hydrophobic domain of mouse erythroid band 3 protein expressed in oocytes of Xenopus laevis. Biochim Biophys Acta 1106: 13–16, 1992. doi: 10.1016/0005-2736(92)90215-8. [DOI] [PubMed] [Google Scholar]
- 187.Rivera-Santiago R, Harper SL, Sriswasdi S, Hembach P, Speicher DW. Full-length anion exchanger 1 structure and interactions with ankyrin-1 determined by zero length crosslinking of erythrocyte membranes. Structure 25: 132–145, 2017. doi: 10.1016/j.str.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Drickamer LK. Fragmentation of the 95,000-dalton transmembrane polypeptide in human erythrocyte membranes. J Biol Chem 251: 5115–5123, 1976. doi: 10.1016/S0021-9258(17)33137-X. [DOI] [PubMed] [Google Scholar]
- 189.Jenkins RE, Tanner JA. The structure of the major protein of the human erythrocyte membrane. characterization of the intact protein and major fragments. Biochem J 161: 139–147, 1977. doi: 10.1042/bj1610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cabantchik ZI, Rothstein A. Membrane proteins related to anion permeability of human red blood cells. II. effects of proteolytic enzymes on disulfonic stilbene sites of surface proteins. J Membr Biol 15: 227–248, 1974. doi: 10.1007/BF01870089. [DOI] [PubMed] [Google Scholar]
- 191.Jennings ML, Passow H. Anion transport across the erythrocyte membrane, in situ proteolysis of band 3 protein, and cross-linking of proteolytic fragments by 4,4’-diisothiocyano dihydrostilbene-2,2’-disulfonate. Biochim Biophys Acta 554: 498–519, 1979. doi: 10.1016/0005-2736(79)90387-0. [DOI] [PubMed] [Google Scholar]
- 192.Lieberman DM, Reithmeier RA. Characterization of the stilbenedisulfonate binding site of the band 3 polypeptide of human erythrocyte membranes. Biochemistry 22: 4028–4033, 1983. doi: 10.1021/bi00286a006. [DOI] [PubMed] [Google Scholar]
- 193.Parker MD, Tanner MJ. The disruption of the third extracellular loop of the red cell anion exchanger AE1 does not affect electroneutral Cl-/HCO3- exchange activity. Blood Cells Mol Dis 32: 379–383, 2004. doi: 10.1016/j.bcmd.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 194.Jennings ML, Adams-Lackey M, Denney GH. Peptides of human erythrocyte band 3 protein produced by extracellular papain cleavage. J Biol Chem 259: 4652–4660, 1984. doi: 10.1016/S0021-9258(17)43096-1. [DOI] [PubMed] [Google Scholar]
- 195.Matsuyama H, Kawano Y, Hamasaki N. Anion transport activity in the human erythrocyte membrane modulated by proteolytic digestion of the 38,000-dalton fragment in band 3. J Biol Chem 258: 15376–15381, 1983. doi: 10.1016/S0021-9258(17)43817-8. [DOI] [PubMed] [Google Scholar]
- 196.Jennings ML, Adams MF. Modification by papain of the structure and function of band 3, the erythrocyte anion transport protein. Biochemistry 20: 7118–7123, 1981. doi: 10.1021/bi00528a011. [DOI] [PubMed] [Google Scholar]
- 197.Jennings ML, Anderson MP, Monaghan R. Monoclonal antibodies against human erythrocyte band 3 protein. localization of proteolytic cleavage sites and stilbenedisulfonate-binding lysine residues. J Biol Chem 261: 9002–9010, 1986. doi: 10.1016/S0021-9258(19)84480-0. [DOI] [PubMed] [Google Scholar]
- 198.Kuma H, Shinde AA, Howren TR, Jennings ML. Topology of the anion exchange protein AE1: The controversial sidedness of lysine 743. Biochemistry 41: 3380–3388, 2002. doi: 10.1021/bi015879p. [DOI] [PubMed] [Google Scholar]
- 199.Popov M, Tam LY, Li J, Reithmeier RA. Mapping the ends of transmembrane segments in a polytopic membrane protein. scanning N-glycosylation mutagenesis of extracytosolic loops in the anion exchanger, band 3. J Biol Chem 272: 18325–18332, 1997. doi: 10.1074/jbc.272.29.18325. [DOI] [PubMed] [Google Scholar]
- 200.Popov M, Li J, Reithmeier RA. Transmembrane folding of the human erythrocyte anion exchanger (AE1, band 3) determined by scanning and insertional N-glycosylation mutagenesis. Biochem J 339: 269–279, 1999. doi: 10.1042/bj3390269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lieberman DM, Nattriss M, Reithmeier RA. Carboxypeptidase Y digestion of band 3, the anion transport protein of human erythrocyte membranes. Biochim Biophys Acta 903: 37–47, 1987. doi: 10.1016/0005-2736(87)90153-2. [DOI] [PubMed] [Google Scholar]
- 202.Ramjeesingh M, Gaarn A, Rothstein A. Pepsin cleavage of band 3 produces its membrane-crossing domains. Biochim Biophys Acta 769: 381–389, 1984. doi: 10.1016/0005-2736(84)90321-3. [DOI] [PubMed] [Google Scholar]
- 203.Kang D, Okubo K, Hamasaki N, Kuroda N, Shiraki H. A structural study of the membrane domain of band 3 by tryptic digestion. conformational change of band 3 in situ induced by alkali treatment. J Biol Chem 267: 19211–19217, 1992. doi: 10.1016/S0021-9258(18)41763-2. [DOI] [PubMed] [Google Scholar]
- 204.Hamasaki N, Okubo K, Kuma H, Kang D, Yae Y. Proteolytic cleavage sites of band 3 protein in alkali-treated membranes: fidelity of hydropathy prediction for band 3 protein. J Biochem 122: 577–585, 1997. doi: 10.1093/oxfordjournals.jbchem.a021792. [DOI] [PubMed] [Google Scholar]
- 205.Hamasaki N, Kuma H, Ota K, Sakaguchi M, Mihara K. A new concept in polytopic membrane proteins following from the study of band 3 protein. Biochem Cell Biol 76: 729–733, 1998. doi: 10.1139/o98-085. [DOI] [PubMed] [Google Scholar]
- 206.Hamasaki N, Abe Y, Tanner MJ. Flexible regions within the membrane-embedded portions of polytopic membrane proteins. Biochemistry 41: 3852–3854, 2002. doi: 10.1021/bi011918l. [DOI] [PubMed] [Google Scholar]
- 207.Janas T, Bjerrum PJ, Brahm J, Wieth JO. Kinetics of reversible DIDS inhibition of chloride self exchange in human erythrocytes. Am J Physiol Cell Physiol 257: C601–C606, 1989. doi: 10.1152/ajpcell.1989.257.4.C601. [DOI] [PubMed] [Google Scholar]
- 208.Barzilay M, Cabantchik ZI. Anion transport in red blood cells. III. Sites and sidedness of inhibition by high-affinity reversible binding probes. Membr Biochem 2: 297–322, 1979. doi: 10.3109/09687687909063869. [DOI] [PubMed] [Google Scholar]
- 209.Cabantchik ZI, Knauf PA, Rothstein A. The anion transport system of the red blood cell. the role of membrane protein evaluated by the use of ‘probes’. Biochim Biophys Acta 515: 239–302, 1978. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- 210.Passow H. Molecular aspects of band 3 protein-mediated anion transport across the red blood cell membrane. Rev Physiol Biochem Pharmacol 103: 61–203, 1986. doi: 10.1007/3540153330_2. [DOI] [PubMed] [Google Scholar]
- 211.Fröhlich O. The external anion binding site of the human erythrocyte anion transporter: DNDS binding and competition with chloride. J Membr Biol 65: 111–123, 1982. doi: 10.1007/BF01870474. [DOI] [PubMed] [Google Scholar]
- 212.Rao A, Martin P, Reithmeier RA, Cantley LC. Location of the stilbenedisulfonate binding site of the human erythrocyte anion-exchange system by resonance energy transfer. Biochemistry 18: 4505–4516, 1979. doi: 10.1021/bi00588a008. [DOI] [PubMed] [Google Scholar]
- 213.Schopfer LM, Salhany JM. Characterization of the stilbenedisulfonate binding site on band 3. Biochemistry 34: 8320–8329, 1995. doi: 10.1021/bi00026a013. [DOI] [PubMed] [Google Scholar]
- 214.Scothorn DJ, Wojcicki WE, Hustedt EJ, Beth AH, Cobb CE. Synthesis and characterization of a novel spin-labeled affinity probe of human erythrocyte band 3: characteristics of the stilbenedisulfonate binding site. Biochemistry 35: 6931–6943, 1996. doi: 10.1021/bi960150f. [DOI] [PubMed] [Google Scholar]
- 215.Macara IG, Cantley LC. Interactions between transport inhibitors at the anion binding sites of the band 3 dimer. Biochemistry 20: 5095–5105, 1981. doi: 10.1021/bi00521a001. [DOI] [PubMed] [Google Scholar]
- 216.Knauf PA, Law FY, Leung TW, Atherton SJ. Relocation of the disulfonic stilbene sites of AE1 (band 3) on the basis of fluorescence energy transfer measurements. Biochemistry 43: 11917–11931, 2004. doi: 10.1021/bi048622a. [DOI] [PubMed] [Google Scholar]
- 217.Rudloff V, Lepke S, Passow H. Inhibition of anion transport across the red cell membrane by dinitrophenylation of a specific lysine residue at the H2DIDS binding site of the band 3 protein. FEBS Lett 163: 14–21, 1983. doi: 10.1016/0014-5793(83)81152-1. [DOI] [PubMed] [Google Scholar]
- 218.Garcia AM, Lodish HF. Lysine 539 of human band 3 is not essential for ion transport or inhibition by stilbene disulfonates. J Biol Chem 264: 19607–19613, 1989. . doi: 10.1016/S0021-9258(19)47157-3. [DOI] [PubMed] [Google Scholar]
- 219.Bartel D, Hans H, Passow H. Identification by site-directed mutagenesis of Lys-558 as the covalent attachment site of H2DIDS in the mouse erythroid band 3 protein. Biochim Biophys Acta 985: 355–358, 1989. doi: 10.1016/0005-2736(89)90427-6. [DOI] [PubMed] [Google Scholar]
- 220.Wood PG, Müller H, Sovak M, Passow H. Role of Lys 558 and Lys 869 in substrate and inhibitor binding to the murine band 3 protein: a study of the effects of site-directed mutagenesis of the band 3 protein expressed in the oocytes of Xenopus laevis. J Membr Biol 127: 139–148, 1992. doi: 10.1007/BF00233286. [DOI] [PubMed] [Google Scholar]
- 221.Okubo K, Kang D, Hamasaki N, Jennings ML. Red blood cell band 3. lysine 539 and lysine 851 react with the same H2DIDS (4,4’-diisothiocyanodihydrostilbene-2,2’-disulfonic acid) molecule. J Biol Chem 269: 1918–1926, 1994. doi: 10.1016/S0021-9258(17)42114-4. [DOI] [PubMed] [Google Scholar]
- 222.Kawano Y, Okubo K, Tokunaga F, Miyata T, Iwanaga S, Hamasaki N. Localization of the pyridoxal phosphate binding site at the COOH-terminal region of erythrocyte band 3 protein. J Biol Chem 263: 8232–8238, 1988. doi: 10.1016/S0021-9258(18)68468-6. [DOI] [PubMed] [Google Scholar]
- 223.Bjerrum PJ. The human erythrocyte anion transport protein, band 3. characterization of exofacial alkaline titratable groups involved in anion binding/translocation. J Gen Physiol 100: 301–339, 1992. doi: 10.1085/jgp.100.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Brahm J. Temperature-dependent changes of chloride transport kinetics in human red cells. J Gen Physiol 70: 283–306, 1977. doi: 10.1085/jgp.70.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Halestrap AP. Transport of pyruvate nad lactate into human erythrocytes. evidence for the involvement of the chloride carrier and a chloride-independent carrier. Biochem J 156: 193–207, 1976. doi: 10.1042/bj1560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Schofield AE, Reardon DM, Tanner MJ. Defective anion transport activity of the abnormal band 3 in hereditary ovalocytic red blood cells. Nature 355: 836–838, 1992. doi: 10.1038/355836a0. [DOI] [PubMed] [Google Scholar]
- 227.Jennings ML, Adame MF. Characterization of oxalate transport by the human erythrocyte band 3 protein. J Gen Physiol 107: 145–159, 1996. doi: 10.1085/jgp.107.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bruce LJ, Groves JD, Okubo Y, Thilaganathan B, Tanner MJ. Altered band 3 structure and function in glycophorin A- and B-deficient (MkMk) red blood cells. Blood 84: 916–922, 1994. doi: 10.1182/blood.V84.3.916.916. [DOI] [PubMed] [Google Scholar]
- 229.Bruce LJ, Pan RJ, Cope DL, Uchikawa M, Gunn RB, Cherry RJ, Tanner MJ. Altered structure and anion transport properties of band 3 (AE1, SLC4A1) in human red cells lacking glycophorin A. J Biol Chem 279: 2414–2420, 2004. doi: 10.1074/jbc.M309826200. [DOI] [PubMed] [Google Scholar]
- 230.Toye AM, Williamson RC, Khanfar M, Bader-Meunier B, Cynober T, Thibault M, Tchernia G, Déchaux M, Delaunay J, Bruce LJ. Band 3 Courcouronnes (Ser667Phe): a trafficking mutant differentially rescued by wild-type band 3 and glycophorin A. Blood 111: 5380–5389, 2008. doi: 10.1182/blood-2007-07-099473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Macara IG, Cantley LC. Mechanism of anion exchange across the red cell membrane by band 3: interactions between stilbenedisulfonate and NAP-taurine binding sites. Biochemistry 20: 5695–5701, 1981. doi: 10.1021/bi00523a009. [DOI] [PubMed] [Google Scholar]
- 232.Salhany JM. Allosteric effects in stilbenedisulfonate binding to band 3 protein (AE1). Cell Mol Biol (Noisy-le-grand) 42: 1065–1096, 1996. [PubMed] [Google Scholar]
- 233.Jennings ML, Monaghan R, Douglas SM, Nicknish JS. Functions of extracellular lysine residues in the human erythrocyte anion transport protein. J Gen Physiol 86: 653–669, 1985. doi: 10.1085/jgp.86.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Parker MD, Young MT, Daly CM, Meech RW, Boron WF, Tanner MJ. A conductive pathway generated from fragments of the human red cell anion exchanger AE1. J Physiol 581: 33–50, 2007. doi: 10.1113/jphysiol.2007.128389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Furuya W, Tarshis T, Law FY, Knauf PA. Transmembrane effects of intracellular chloride on the inhibitory potency of extracellular H2DIDS. evidence for two conformations of the transport site of the human erythrocyte anion exchange protein. J Gen Physiol 83: 657–681, 1984. doi: 10.1085/jgp.83.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Shami Y, Rothstein A, Knauf PA. Identification of the Cl- transport site of human red blood cells by a kinetic analysis of the inhibitory effects of a chemical probe. Biochim Biophys Acta 508: 357–363, 1978. doi: 10.1016/0005-2736(78)90337-1. [DOI] [PubMed] [Google Scholar]
- 237.Salhany JM. Anion binding characteristics of the band 3/4,4-dibenzamidostilbene-2,2-disulfonate binary complex: evidence for both steric and allosteric interactions. Biochem Cell Biol 77: 543–549, 1999. doi: 10.1139/o99-061. [DOI] [PubMed] [Google Scholar]
- 238.Salhany JM. Stilbenedisulfonate binding kinetics to band 3 (AE 1): relationship between transport and stilbenedisulfonate binding sites and role of subunit interactions in transport. Blood Cells Mol Dis 27: 127–134, 2001. doi: 10.1006/bcmd.2000.0369. [DOI] [PubMed] [Google Scholar]
- 239.Jennings ML, Schulz RK, Allen M. Effects of membrane potential on electrically silent transport. potential-independent translocation and asymmetric potential-dependent substrate binding to the red blood cell anion exchange protein. J Gen Physiol 96: 991–1012, 1990. doi: 10.1085/jgp.96.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hamasaki N, Matsuyama H, Hirota-Chigita C. Characterization of phosphoenolpyruvate transport across the erythrocyte membrane. evidence for involvement of band 3 in the transport system. Eur J Biochem 132: 531–536, 1983. doi: 10.1111/j.1432-1033.1983.tb07394.x. [DOI] [PubMed] [Google Scholar]
- 241.Nanri H, Hamasaki N, Minakami S. Affinity labeling of erythrocyte band 3 protein with pyridoxal 5-phosphate. Involvement of the 35,000-dalton fragment in anion transport. J Biol Chem 258: 5985–5989, 1983. doi: 10.1016/S0021-9258(20)81993-0. [DOI] [PubMed] [Google Scholar]
- 242.Eidelman O, Cabantchik ZI. A method for measuring anion transfer across red cell membranes by continuous monitoring of fluorescence. Anal Biochem 106: 335–341, 1980. doi: 10.1016/0003-2697(80)90529-1. [DOI] [PubMed] [Google Scholar]
- 243.Farias MG. Advances in laboratory diagnosis of hereditary spherocytosis. Clin Chem Lab Med 55: 944–948, 2017. doi: 10.1515/cclm-2016-0738. [DOI] [PubMed] [Google Scholar]
- 244.Cobb CE, Beth AH. Identification of the eosinyl-5-maleimide reaction site on the human erythrocyte anion-exchange protein: overlap with the reaction sites of other chemical probes. Biochemistry 29: 8283–8290, 1990. doi: 10.1021/bi00488a012. [DOI] [PubMed] [Google Scholar]
- 245.Blackman SM, Cobb CE, Beth AH, Piston DW. The orientation of eosin-5-maleimide on human erythrocyte band 3 measured by fluorescence polarization microscopy. Biophys J 71: 194–208, 1996. doi: 10.1016/S0006-3495(96)79216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Liu SQ, Knauf PA. Lys-430, site of irreversible inhibition of band 3 Cl- flux by eosin-5-maleimide, is not at the transport site. Am J Physiol Cell Physiol 264: C1155–C1164, 1993. doi: 10.1152/ajpcell.1993.264.5.C1155. [DOI] [PubMed] [Google Scholar]
- 247.Knauf PA, Strong NM, Penikas J, Wheeler RB Jr, Liu SQ. Eosin-5-maleimide inhibits red cell Cl- exchange at a noncompetitive site that senses band 3 conformation. Am J Physiol Cell Physiol 264: C1144–C1154, 1993. doi: 10.1152/ajpcell.1993.264.5.C1144. [DOI] [PubMed] [Google Scholar]
- 248.Dunn BM, Anfinsen CB. Kinetics of Woodward’s reagent K hydrolysis and reaction with staphylococcal nuclease. J Biol Chem 249: 3717–3723, 1974. doi: 10.1016/S0021-9258(19)42532-5. [DOI] [PubMed] [Google Scholar]
- 249.Jennings ML, Anderson MP. Chemical modification and labeling of glutamate residues at the stilbenedisulfonate site of human red blood cell band 3 protein. J Biol Chem 262: 1691–1697, 1987. doi: 10.1016/S0021-9258(19)75693-2. [DOI] [PubMed] [Google Scholar]
- 250.Jennings ML, Al-Rhaiyel S. Modification of a carboxyl group that appears to cross the permeability barrier in the red blood cell anion transporter. J Gen Physiol 92: 161–178, 1988. doi: 10.1085/jgp.92.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Jennings ML. Rapid electrogenic sulfate-chloride exchange mediated by chemically modified band 3 in human erythrocytes. J Gen Physiol 105: 21–47, 1995. doi: 10.1085/jgp.105.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Jennings ML, Smith JS. Anion-proton cotransport through the human red blood cell band 3 protein. Role of glutamate 681. J Biol Chem 267: 13964–13971, 1992. doi: 10.1016/S0021-9258(19)49664-6. [DOI] [PubMed] [Google Scholar]
- 253.Chernova MN, Jiang L, Crest M, Hand M, Vandorpe DH, Strange K, Alper SL. Electrogenic sulfate/chloride exchange in xenopus oocytes mediated by murine AE1 E699Q. J Gen Physiol 109: 345–360, 1997. doi: 10.1085/jgp.109.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chernova MN, Stewart AK, Barry PN, Jennings ML, Alper SL. Mouse Ae1 E699Q mediates SO42-i/aniono exchange with [SO42-]i-dependent reversal of wild-type pHo sensitivity. Am J Physiol Cell Physiol 295: C302–C312, 2008. doi: 10.1152/ajpcell.00109.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tang XB, Fujinaga J, Kopito R, Casey JR. Topology of the region surrounding Glu681 of human AE1 protein, the erythrocyte anion exchanger. J Biol Chem 273: 22545–22553, 1998. doi: 10.1074/jbc.273.35.22545. [DOI] [PubMed] [Google Scholar]
- 256.Zaki L, Julien T. Anion transport in red blood cells and arginine-specific reagents. interaction between the substrate-binding site and the binding site of arginine-specific reagents. Biochim Biophys Acta 818: 325–332, 1985. doi: 10.1016/0005-2736(85)90006-9. [DOI] [PubMed] [Google Scholar]
- 257.Zaki L, Böhm R, Merckel M. Chemical labelling of arginyl-residues involved in anion transport mediated by human band 3 protein and some aspects of its location in the peptide chain. Cell Mol Biol (Noisy-le-grand) 42: 1053–1063, 1996. [PubMed] [Google Scholar]
- 258.Wieth JO, Bjerrum PJ, Borders CL Jr.. Irreversible inactivation of red cell chloride exchange with phenylglyoxal, and arginine-specific reagent. J Gen Physiol 79: 283–312, 1982. doi: 10.1085/jgp.79.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Bjerrum PJ, Wieth JO, Borders CL Jr.. Selective phenylglyoxalation of functionally essential arginyl residues in the erythrocyte anion transport protein. J Gen Physiol 81: 453–484, 1983. doi: 10.1085/jgp.81.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Böhm R, Zaki L. Towards the localization of the essential arginine residues in the band 3 protein of human red blood cell membranes. Biochim Biophys Acta 1280: 238–242, 1996. doi: 10.1016/0005-2736(95)00303-7. [DOI] [PubMed] [Google Scholar]
- 261.Grygorczyk R, Schwarz W, Passow H. Potential dependence of the “electrically silent” anion exchange across the plasma membrane of Xenopus oocytes mediated by the band-3 protein of mouse red blood cells. J Membr Biol 99: 127–136, 1987. doi: 10.1007/BF01871232. [DOI] [PubMed] [Google Scholar]
- 262.Nakashima H, Makino S. State of association of band 3 protein from bovine erythrocyte membrane in nonionic detergent. J Biochem 88: 933–937, 1980. doi: 10.1093/oxfordjournals.jbchem.a133081. [DOI] [PubMed] [Google Scholar]
- 263.Nakashima H, Nakagawa Y, Makino S. Detection of the associated state of membrane proteins by polyacrylamide gradient gel electrophoresis with non-denaturing detergents. application to band 3 protein from erythrocyte membranes. Biochim Biophys Acta 643: 509–518, 1981. doi: 10.1016/0005-2736(81)90348-5. [DOI] [PubMed] [Google Scholar]
- 264.Thevenin BJ, Low PS. Kinetics and regulation of the ankyrin-band 3 interaction of the human red blood cell membrane. J Biol Chem 265: 16166–16172, 1990. doi: 10.1016/S0021-9258(17)46203-X. [DOI] [PubMed] [Google Scholar]
- 265.Michaely P, Bennett V. The ANK repeats of erythrocyte ankyrin form two distinct but cooperative binding sites for the erythrocyte anion exchanger. J Biol Chem 270: 22050–22057, 1995. doi: 10.1074/jbc.270.37.22050. [DOI] [PubMed] [Google Scholar]
- 266.Wang K, Richards FM. An approach to nearest neighbor analysis of membrane proteins. application to the human erythrocyte membrane of a method employing cleavable cross-linkages. J Biol Chem 249: 8005–8018, 1974. doi: 10.1016/S0021-9258(19)42065-6. [DOI] [PubMed] [Google Scholar]
- 267.Salhany JM, Sloan RL, Cordes KA. In situ cross-linking of human erythrocyte band 3 by bis(sulfosuccinimidyl)suberate. Evidence for ligand modulation of two alternate quaternary forms: covalent band 3 dimers and noncovalent tetramers formed by the association of two covalent dimers. J Biol Chem 265: 17688–17693, 1990. doi: 10.1016/S0021-9258(18)38218-8. [DOI] [PubMed] [Google Scholar]
- 268.Low PS, Waugh SM, Zinke K, Drenckhahn D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science 227: 531–533, 1985. doi: 10.1126/science.2578228. [DOI] [PubMed] [Google Scholar]
- 269.Waugh SM, Low PS. Hemichrome binding to band 3: nucleation of Heinz bodies on the erythrocyte membrane. Biochemistry 24: 34–39, 1985. doi: 10.1021/bi00322a006. [DOI] [PubMed] [Google Scholar]
- 270.Waugh SM, Willardson BM, Kannan R, Labotka RJ, Low PS. Heinz bodies induce clustering of band 3, glycophorin, and ankyrin in sickle cell erythrocytes. J Clin Invest 78: 1155–1160, 1986. doi: 10.1172/JCI112696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Schlüter K, Drenckhahn D. Co-clustering of denatured hemoglobin with band 3: Its role in binding of autoantibodies against band 3 to abnormal and aged erythrocytes. Proc Natl Acad Sci USA 83: 6137–6141, 1986. doi: 10.1073/pnas.83.16.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Arashiki N, Kimata N, Manno S, Mohandas N, Takakuwa Y. Membrane peroxidation and methemoglobin formation are both necessary for band 3 clustering: mechanistic insights into human erythrocyte senescence. Biochemistry 52: 5760–5769, 2013. doi: 10.1021/bi400405p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.De Franceschi L, Turrini F, del Giudice EM, Perrotta S, Olivieri O, Corrocher R, Mannu F, Iolascon A. Decreased band 3 anion transport activity and band 3 clusterization in congenital dyserythropoietic anemia type II. Exp Hematol 26: 869–873, 1998. [PubMed] [Google Scholar]
- 274.Williamson RC, Toye AM. Glycophorin A. Band 3 aid. Blood Cells Mol Dis 41: 35–43, 2008. doi: 10.1016/j.bcmd.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 275.Nigg EA, Bron C, Girardet M, Cherry RJ. Band 3-glycophorin A association in erythrocyte membrane demonstrated by combining protein diffusion measurements with antibody-induced cross-linking. Biochemistry 19: 1887–1893, 1980. doi: 10.1021/bi00550a024. [DOI] [PubMed] [Google Scholar]
- 276.Groves JD, Tanner MJ. Glycophorin A facilitates the expression of human band 3-mediated anion transport in Xenopus oocytes. J Biol Chem 267: 22163–22170, 1992. doi: 10.1016/S0021-9258(18)41649-3. [DOI] [PubMed] [Google Scholar]
- 277.Groves JD, Tanner MJ. The effects of glycophorin A on the expression of the human red cell anion transporter (band 3) in Xenopus oocytes. J Membr Biol 140: 81–88, 1994. [DOI] [PubMed] [Google Scholar]
- 278.Pang AJ, Reithmeier RA. Interaction of anion exchanger 1 and glycophorin A in human erythroleukaemic K562 cells. Biochem J 421: 345–356, 2009. doi: 10.1042/BJ20090345. [DOI] [PubMed] [Google Scholar]
- 279.Bruce LJ, Ring SM, Anstee DJ, Reid ME, Wilkinson S, Tanner MJ. Changes in the blood group wright antigens are associated with a mutation at amino acid 658 in human erythrocyte band 3: a site of interaction between band 3 and glycophorin A under certain conditions. Blood 85: 541–547, 1995. doi: 10.1182/blood.V85.2.541.541. [DOI] [PubMed] [Google Scholar]
- 280.Young MT, Beckmann R, Toye AM, Tanner MJ. Red-cell glycophorin A-band 3 interactions associated with the movement of band 3 to the cell surface. Biochem J 350: 53–60, 2000. doi: 10.1042/bj3500053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Young MT, Tanner MJ. Distinct regions of human glycophorin A enhance human red cell anion exchanger (band 3; AE1) transport function and surface trafficking. J Biol Chem 278: 32954–32961, 2003. doi: 10.1074/jbc.M302527200. [DOI] [PubMed] [Google Scholar]
- 282.Hsu K, Chi N, Gucek M, Van Eyk JE, Cole RN, Lin M, Foster DB. Miltenberger blood group antigen type III (mi.III) enhances the expression of band 3. Blood 114: 1919–1928, 2009. doi: 10.1182/blood-2008-12-195180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Hsu K. Exploring the potential roles of band 3 and aquaporin-1 in blood CO2 transport-inspired by comparative studies of glycophorin B-A-B hybrid protein GP.mur. Front Physiol 9: 733, 2018. doi: 10.3389/fphys.2018.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Steck TL. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol 62: 1–19, 1974. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Tsai IH, Murthy SN, Steck TL. Effect of red cell membrane binding on the catalytic activity of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem 257: 1438–1442, 1982. doi: 10.1016/S0021-9258(19)68212-8. [DOI] [PubMed] [Google Scholar]
- 286.Murthy SN, Liu T, Kaul RK, Köhler H, Steck TL. The aldolase-binding site of the human erythrocyte membrane is at the NH2 terminus of band 3. J Biol Chem 256: 11203–11208, 1981. doi: 10.1016/S0021-9258(19)68578-9. [DOI] [PubMed] [Google Scholar]
- 287.Jenkins JD, Kezdy FJ, Steck TL. Mode of interaction of phosphofructokinase with the erythrocyte membrane. J Biol Chem 260: 10426–10433, 1985. doi: 10.1016/S0021-9258(19)85100-1. [DOI] [PubMed] [Google Scholar]
- 288.Walder JA, Chatterjee R, Steck TL, Low PS, Musso GF, Kaiser ET, Rogers PH, Arnone A. The interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. J Biol Chem 259: 10238–10246, 1984. doi: 10.1016/S0021-9258(18)90956-7. [DOI] [PubMed] [Google Scholar]
- 289.Murthy SN, Kaul RK, Köhler H. Hemoglobin binds to the amino-terminal 23-residue fragment of human erythrocyte band 3 protein. Hoppe Seylers Z Physiol Chem 365: 9–17, 1984. doi: 10.1515/bchm2.1984.365.1.9. [DOI] [PubMed] [Google Scholar]
- 290.Kaul RK, Köhler H. Interaction of hemoglobin with band 3: a review. Klin Wochenschr 61: 831–837, 1983. doi: 10.1007/BF01537457. [DOI] [PubMed] [Google Scholar]
- 291.Low PS, Allen DP, Zioncheck TF, Chari P, Willardson BM, Geahlen RL, Harrison ML. Tyrosine phosphorylation of band 3 inhibits peripheral protein binding. J Biol Chem 262: 4592–4596, 1987. doi: 10.1016/S0021-9258(18)61234-7. [DOI] [PubMed] [Google Scholar]
- 292.Rich GT, Dawson AP, Pryor JS. Glyceraldehyde-3-phosphate dehydrogenase release from erythrocytes during haemolysis. No evidence for substantial binding of the enzyme to the membrane in the intact cell. Biochem J 221: 197–202, 1984. doi: 10.1042/bj2210197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ercolani L, Brown D, Stuart-Tilley A, Alper SL. Colocalization of GAPDH and band 3 (AE1) proteins in rat erythrocytes and kidney intercalated cell membranes. Am J Physiol Renal Physiol 262: F892–F896, 1992. doi: 10.1152/ajprenal.1992.262.5.F892. [DOI] [PubMed] [Google Scholar]
- 294.Rogalski AA, Steck TL, Waseem A. Association of glyceraldehyde-3-phosphate dehydrogenase with the plasma membrane of the intact human red blood cell. J Biol Chem 264: 6438–6446, 1989. doi: 10.1016/S0021-9258(18)83368-3. [DOI] [PubMed] [Google Scholar]
- 295.Campanella ME, Chu H, Low PS. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc Natl Acad Sci USA 102: 2402–2407, 2005. doi: 10.1073/pnas.0409741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Campanella ME, Chu H, Wandersee NJ, Peters LL, Mohandas N, Gilligan DM, Low PS. Characterization of glycolytic enzyme interactions with murine erythrocyte membranes in wild-type and membrane protein knockout mice. Blood 112: 3900–3906, 2008. doi: 10.1182/blood.V112.11.3900.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Low FM, Hampton MB, Winterbourn CC. Peroxiredoxin 2 and peroxide metabolism in the erythrocyte. Antioxid Redox Signal 10: 1621–1630, 2008. doi: 10.1089/ars.2008.2081. [DOI] [PubMed] [Google Scholar]
- 298.Matte A, Bertoldi M, Mohandas N, An X, Bugatti A, Brunati AM, Rusnati M, Tibaldi E, Siciliano A, Turrini F, Perrotta S, De Franceschi L. Membrane association of peroxiredoxin-2 in red cells is mediated by the N-terminal cytoplasmic domain of band 3. Free Radic Biol Med 55: 27–35, 2013. doi: 10.1016/j.freeradbiomed.2012.10.543. [DOI] [PubMed] [Google Scholar]
- 299.Moore RB, Mankad MV, Shriver SK, Mankad VN, Plishker GA. Reconstitution of Ca2+-dependent K+ transport in erythrocyte membrane vesicles requires a cytoplasmic protein. J Biol Chem 266: 18964–18968, 1991. doi: 10.1016/S0021-9258(18)55157-7. [DOI] [PubMed] [Google Scholar]
- 300.Vince JW, Reithmeier RA. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte C1−/HCO3− exchanger. J Biol Chem 273: 28430–28437, 1998. doi: 10.1074/jbc.273.43.28430. [DOI] [PubMed] [Google Scholar]
- 301.Vince JW, Reithmeier RA. Identification of the carbonic anhydrase II binding site in the Cl−/HCO3− anion exchanger AE1. Biochemistry 39: 5527–5533, 2000. doi: 10.1021/bi992564p. [DOI] [PubMed] [Google Scholar]
- 302.Sterling D, Reithmeier RA, Casey JR. Transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem 276: 47886–47894, 2001. doi: 10.1074/jbc.M105959200. [DOI] [PubMed] [Google Scholar]
- 303.Reithmeier RA. A membrane metabolon linking carbonic anhydrase with chloride/bicarbonate anion exchangers. Blood Cells Mol Dis 27: 85–89, 2001. doi: 10.1006/bcmd.2000.0353. [DOI] [PubMed] [Google Scholar]
- 304.McMurtrie HL, Cleary HJ, Alvarez BV, Loiselle FB, Sterling D, Morgan PE, Johnson DE, Casey JR. The bicarbonate transport metabolon. J Enzyme Inhib Med Chem 19: 231–236, 2004. doi: 10.1080/14756360410001704443. [DOI] [PubMed] [Google Scholar]
- 305.Alvarez BV, Vilas GL, Casey JR. Metabolon disruption: a mechanism that regulates bicarbonate transport. EMBO J 24: 2499–2511, 2005. doi: 10.1038/sj.emboj.7600736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Sowah D, Casey JR. An intramolecular transport metabolon: fusion of carbonic anhydrase II to the COOH terminus of the Cl−/HCO3− exchanger, AE1. Am J Physiol Cell Physiol 301: C336–C346, 2011. doi: 10.1152/ajpcell.00005.2011. [DOI] [PubMed] [Google Scholar]
- 307.Dahl NK, Jiang L, Chernova MN, Stuart-Tilley AK, Shmukler BE, Alper SL. Deficient HCO3− transport in an AE1 mutant with normal Cl- transport can be rescued by carbonic anhydrase II presented on an adjacent AE1 protomer. J Biol Chem 278: 44949–44958, 2003. doi: 10.1074/jbc.M308660200. [DOI] [PubMed] [Google Scholar]
- 308.Casey JR, Sly WS, Shah GN, Alvarez BV. Bicarbonate homeostasis in excitable tissues: role of AE3 Cl−/HCO3− exchanger and carbonic anhydrase XIV interaction. Am J Physiol Cell Physiol 297: C1091–C1102, 2009. doi: 10.1152/ajpcell.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Becker HM, Deitmer JW. Carbonic anhydrase II increases the activity of the human electrogenic Na+/HCO3− cotransporter. J Biol Chem 282: 13508–13521, 2007. doi: 10.1074/jbc.M700066200. [DOI] [PubMed] [Google Scholar]
- 310.Sterling D, Brown NJ, Supuran CT, Casey JR. The functional and physical relationship between the DRA bicarbonate transporter and carbonic anhydrase II. Am J Physiol Cell Physiol 283: C1522–C1529, 2002. doi: 10.1152/ajpcell.00115.2002. [DOI] [PubMed] [Google Scholar]
- 311.Alka K, Casey JR. Bicarbonate transport in health and disease. IUBMB Life 66: 596–615, 2014. doi: 10.1002/iub.1315. [DOI] [PubMed] [Google Scholar]
- 312.Deitmer JW, Becker HM. Transport metabolons with carbonic anhydrases. Front Physiol 4: 291, 2013. doi: 10.3389/fphys.2013.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Pinto da Silva P. Translational mobility of the membrane intercalated particles of human erythrocyte ghosts. pH-dependent, reversible aggregation. J Cell Biol 53: 777–787, 1972. doi: 10.1083/jcb.53.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Fowler V, Branton D. Lateral mobility of human erythrocyte integral membrane proteins. Nature 268: 23–26, 1977. doi: 10.1038/268023a0. [DOI] [PubMed] [Google Scholar]
- 315.Golan DE, Veatch W. Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery: evidence for control by cytoskeletal interactions. Proc Natl Acad Sci USA 77: 2537–2541, 1980. doi: 10.1073/pnas.77.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Tsuji A, Ohnishi S. Restriction of the lateral motion of band 3 in the erythrocyte membrane by the cytoskeletal network: dependence on spectrin association state. Biochemistry 25: 6133–6139, 1986. doi: 10.1021/bi00368a045. [DOI] [PubMed] [Google Scholar]
- 317.Tomishige M, Sako Y, Kusumi A. Regulation mechanism of the lateral diffusion of band 3 in erythrocyte membranes by the membrane skeleton. J Cell Biol 142: 989–1000, 1998. doi: 10.1083/jcb.142.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Kodippili GC, Spector J, Sullivan C, Kuypers FA, Labotka R, Gallagher PG, Ritchie K, Low PS. Imaging of the diffusion of single band 3 molecules on normal and mutant erythrocytes. Blood 113: 6237–6245, 2009. doi: 10.1182/blood-2009-02-205450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kodippili GC, Spector J, Hale J, Giger K, Hughes MR, McNagny KM, Birkenmeier C, Peters L, Ritchie K, Low PS. Analysis of the mobilities of band 3 populations associated with ankyrin protein and junctional complexes in intact murine erythrocytes. J Biol Chem 287: 4129–4138, 2012. doi: 10.1074/jbc.M111.294439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Giger K, Habib I, Ritchie K, Low PS. Diffusion of glycophorin A in human erythrocytes. Biochim Biophys Acta 1858: 2839–2845, 2016. doi: 10.1016/j.bbamem.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Nigg E, Kessler M, Cherry RJ. Labeling of human erythrocyte membranes with eosin probes used for protein diffusion measurements: inhibition of anion transport and photo-oxidative inactivation of acetylcholinesterase. Biochim Biophys Acta 550: 328–340, 1979. doi: 10.1016/0005-2736(79)90219-0. [DOI] [PubMed] [Google Scholar]
- 322.Nigg EA, Cherry RJ. Anchorage of a band 3 population at the erythrocyte cytoplasmic membrane surface: protein rotational diffusion measurements. Proc Natl Acad Sci USA 77: 4702–4706, 1980. doi: 10.1073/pnas.77.8.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Tsuji A, Kawasaki K, Ohnishi S, Merkle H, Kusumi A. Regulation of band 3 mobilities in erythrocyte ghost membranes by protein association and cytoskeletal meshwork. Biochemistry 27: 7447–7452, 1988. doi: 10.1021/bi00419a041. [DOI] [PubMed] [Google Scholar]
- 324.McPherson RA, Sawyer WH, Tilley L. Rotational diffusion of the erythrocyte integral membrane protein band 3: effect of hemichrome binding. Biochemistry 31: 512–518, 1992. doi: 10.1021/bi00117a030. [DOI] [PubMed] [Google Scholar]
- 325.Matayoshi ED, Jovin TM. Rotational diffusion of band 3 in erythrocyte membranes. 1. Comparison of ghosts and intact cells. Biochemistry 30: 3527–3538, 1991. doi: 10.1021/bi00228a025. [DOI] [PubMed] [Google Scholar]
- 326.Che A, Morrison IE, Pan R, Cherry RJ. Restriction by ankyrin of band 3 rotational mobility in human erythrocyte membranes and reconstituted lipid vesicles. Biochemistry 36: 9588–9595, 1997. doi: 10.1021/bi971074z. [DOI] [PubMed] [Google Scholar]
- 327.Cho MR, Eber SW, Liu SC, Lux SE, Golan DE. Regulation of band 3 rotational mobility by ankyrin in intact human red cells. Biochemistry 37: 17828–17835, 1998. doi: 10.1021/bi981825c. [DOI] [PubMed] [Google Scholar]
- 328.Hustedt EJ, Beth AH. Analysis of saturation transfer electron paramagnetic resonance spectra of a spin-labeled integral membrane protein, band 3, in terms of the uniaxial rotational diffusion model. Biophys J 69: 1409–1423, 1995. doi: 10.1016/S0006-3495(95)80010-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Hustedt EJ, Beth AH. Determination of the orientation of a band 3 affinity spin-label relative to the membrane normal axis of the human erythrocyte. Biochemistry 35: 6944–6954, 1996. doi: 10.1021/bi9601518. [DOI] [PubMed] [Google Scholar]
- 330.Jarolim P, Rubin HL, Liu SC, Cho MR, Brabec V, Derick LH, Yi SJ, Saad ST, Alper S, Brugnara C. Duplication of 10 nucleotides in the erythroid band 3 (AE1) gene in a kindred with hereditary spherocytosis and band 3 protein deficiency (band 3PRAGUE). J Clin Invest 93: 121–130, 1994. doi: 10.1172/JCI116935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Korsgren C, Cohen CM. Associations of human erythrocyte band 4.2. Binding to ankyrin and to the cytoplasmic domain of band 3. J Biol Chem 263: 10212–10218, 1988. doi: 10.1016/S0021-9258(19)81500-4. [DOI] [PubMed] [Google Scholar]
- 332.Korsgren C, Peters LL, Lux SE. Protein 4.2 binds to the carboxyl-terminal EF-hands of erythroid alpha-spectrin in a calcium- and calmodulin-dependent manner. J Biol Chem 285: 4757–4770, 2010. doi: 10.1074/jbc.M109.056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Burton NM, Bruce LJ. Modelling the structure of the red cell membrane. Biochem Cell Biol 89: 200–215, 2011. doi: 10.1139/O10-154. [DOI] [PubMed] [Google Scholar]
- 334.Mankelow TJ, Satchwell TJ, Burton NM. Refined views of multi-protein complexes in the erythrocyte membrane. Blood Cells Mol Dis 49: 1–10, 2012. doi: 10.1016/j.bcmd.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Bruce LJ, Ghosh S, King MJ, Layton DM, Mawby WJ, Stewart GW, Oldenborg PA, Delaunay J, Tanner MJ. Absence of CD47 in protein 4.2-deficient hereditary spherocytosis in man: an interaction between the Rh complex and the band 3 complex. Blood 100: 1878–1885, 2002. doi: 10.1182/blood-2002-03-0706. [DOI] [PubMed] [Google Scholar]
- 336.Bruce LJ, Beckmann R, Ribeiro ML, Peters LL, Chasis JA, Delaunay J, Mohandas N, Anstee DJ, Tanner MJ. Band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood 101: 4180–4188, 2003. doi: 10.1182/blood-2002-09-2824. [DOI] [PubMed] [Google Scholar]
- 337.Korsgren C, Cohen CM. Purification and properties of human erythrocyte band 4.2. Association with the cytoplasmic domain of band 3. J Biol Chem 261: 5536–5543, 1986. doi: 10.1016/S0021-9258(19)57248-9. [DOI] [PubMed] [Google Scholar]
- 338.Anong WA, Franco T, Chu H, Weis TL, Devlin EE, Bodine DM, An X, Mohandas N, Low PS. Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton and regulates membrane cohesion. Blood 114: 1904–1912, 2009. doi: 10.1182/blood-2009-02-203216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Franco T, Chu H, Low PS. Identification of adducin-binding residues on the cytoplasmic domain of erythrocyte membrane protein, band 3. Biochem J 473: 3147–3158, 2016. doi: 10.1042/BCJ20160328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.van den Akker E, Satchwell TJ, Williamson RC, Toye AM. Band 3 multiprotein complexes in the red cell membrane; of mice and men. Blood Cells Mol Dis 45: 1–8, 2010. doi: 10.1016/j.bcmd.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 341.Khan AA, Hanada T, Mohseni M, Jeong JJ, Zeng L, Gaetani M, Li D, Reed BC, Speicher DW, Chishti AH. Dematin and adducin provide a novel link between the spectrin cytoskeleton and human erythrocyte membrane by directly interacting with glucose transporter-1. J Biol Chem 283: 14600–14609, 2008. doi: 10.1074/jbc.M707818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Genetet S, Desrames A, Chouali Y, Ripoche P, Lopez C, Mouro-Chanteloup I. Stomatin modulates the activity of the anion exchanger 1 (AE1, SLC4A1). Sci Rep 7: 46170, 2017. doi: 10.1038/srep46170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Alper SL, Chernova MN, Stewart AK. Regulation of Na+-independent Cl−/HCO3− exchangers by pH. JOP 2: 171–175, 2001. [PubMed] [Google Scholar]
- 344.Stewart AK, Chernova MN, Shmukler BE, Wilhelm S, Alper SL. Regulation of AE2-mediated Cl- transport by intracellular or by extracellular pH requires highly conserved amino acid residues of the AE2 NH2-terminal cytoplasmic domain. J Gen Physiol 120: 707–722, 2002. doi: 10.1085/jgp.20028641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Zhang Y, Chernova MN, Stuart-Tilley AK, Jiang L, Alper SL. The cytoplasmic and transmembrane domains of AE2 both contribute to regulation of anion exchange by pH. J Biol Chem 271: 5741–5749, 1996. doi: 10.1074/jbc.271.10.5741. [DOI] [PubMed] [Google Scholar]
- 346.Humphreys BD, Chernova MN, Jiang L, Zhang Y, Alper SL. NH4Cl activates AE2 anion exchanger in Xenopus oocytes at acidic pHi. Am J Physiol Cell Physiol 272: C1232–C1240, 1997. doi: 10.1152/ajpcell.1997.272.4.C1232. [DOI] [PubMed] [Google Scholar]
- 347.Humphreys BD, Jiang L, Chernova MN, Alper SL. Hypertonic activation of AE2 anion exchanger in Xenopus oocytes via NHE-mediated intracellular alkalinization. Am J Physiol Cell Physiol 268: C201–C209, 1995. doi: 10.1152/ajpcell.1995.268.1.C201. [DOI] [PubMed] [Google Scholar]
- 348.Stewart AK, Kerr N, Chernova MN, Alper SL, Vaughan-Jones RD. Acute pH-dependent regulation of AE2-mediated anion exchange involves discrete local surfaces of the NH2-terminal cytoplasmic domain. J Biol Chem 279: 52664–52676, 2004. doi: 10.1074/jbc.M408108200. [DOI] [PubMed] [Google Scholar]
- 349.Kurschat CE, Shmukler BE, Jiang L, Wilhelm S, Kim EH, Chernova MN, Kinne RK, Stewart AK, Alper SL. Alkaline-shifted pHo sensitivity of AE2c1-mediated anion exchange reveals novel regulatory determinants in the AE2 N-terminal cytoplasmic domain. J Biol Chem 281: 1885–1896, 2006. doi: 10.1074/jbc.M509734200. [DOI] [PubMed] [Google Scholar]
- 350.Alper SL, Chernova MN, Stewart AK. How pH regulates a pH regulator: a regulatory hot spot in the N-terminal cytoplasmic domain of the AE2 anion exchanger. Cell Biochem Biophys 36: 123–136, 2002. doi: 10.1385/CBB:36:2-3:123. [DOI] [PubMed] [Google Scholar]
- 351.Stewart AK, Kurschat CE, Vaughan-Jones RD, Alper SL. Putative re-entrant loop 1 of AE2 transmembrane domain has a major role in acute regulation of anion exchange by pH. J Biol Chem 284: 6126–6139, 2009. doi: 10.1074/jbc.M802051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Gasbjerg PK, Knauf PA, Brahm J. Kinetics of bicarbonate transport in human red blood cell membranes at body temperature. J Gen Physiol 108: 565–575, 1996. doi: 10.1085/jgp.108.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Swietach P, Tiffert T, Mauritz JM, Seear R, Esposito A, Kaminski CF, Lew VL, Vaughan-Jones RD. Hydrogen ion dynamics in human red blood cells. J Physiol 588: 4995–5014, 2010. doi: 10.1113/jphysiol.2010.197392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Huber S, Asan E, Jöns T, Kerscher C, Püschel B, Drenckhahn D. Expression of rat kidney anion exchanger 1 in type A intercalated cells in metabolic acidosis and alkalosis. Am J Physiol Renal Physiol 277: F841–F849, 1999. doi: 10.1152/ajprenal.1999.277.6.F841. [DOI] [PubMed] [Google Scholar]
- 355.Mohebbi N, Perna A, van der Wijst J, Becker HM, Capasso G, Wagner CA. Regulation of two renal chloride transporters, AE1 and pendrin, by electrolytes and aldosterone. PLoS One 8: e55286, 2013. doi: 10.1371/journal.pone.0055286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Sabolić I, Brown D, Gluck SL, Alper SL. Regulation of AE1 anion exchanger and H+-ATPase in rat cortex by acute metabolic acidosis and alkalosis. Kidney Int 51: 125–137, 1997. doi: 10.1038/ki.1997.16. [DOI] [PubMed] [Google Scholar]
- 357.Baggio B, Bordin L, Gambaro G, Piccoli A, Marzaro G, Clari G. Evidence of a link between erythrocyte band 3 phosphorylation and anion transport in patients with ‘idiopathic’ calcium oxalate nephrolithiasis. Miner Electrolyte Metab 19: 17–20, 1993. [PubMed] [Google Scholar]
- 358.Baggio B, Bordin L, Clari G, Gambaro G, Moret V. Functional correlation between the Ser/Thr-phosphorylation of band-3 and band-3-mediated transmembrane anion transport in human erythrocytes. Biochim Biophys Acta 1148: 157–160, 1993. doi: 10.1016/0005-2736(93)90173-W. [DOI] [PubMed] [Google Scholar]
- 359.Motais R, Baroin A, Baldy S. Chloride permeability in human red cells: influence of membrane protein rearrangement resulting from ATP depletion and calcium accumulation. J Membr Biol 62: 195–206, 1981. doi: 10.1007/BF01998165. [DOI] [PubMed] [Google Scholar]
- 360.Bursaux E, Hilly M, Bluze A, Poyart C. Organic phosphates modulate anion self-exchange across the human erythrocyte membrane. Biochim Biophys Acta 777: 253–260, 1984. doi: 10.1016/0005-2736(84)90427-9. [DOI] [PubMed] [Google Scholar]
- 361.Bidani A, Crandall ED, Forster RE. Analysis of postcapillary pH changes in blood in vivo after gas exchange. J Appl Physiol Respir Environ Exerc Physiol 44: 770–781, 1978. doi: 10.1152/jappl.1978.44.5.770. [DOI] [PubMed] [Google Scholar]
- 362.Kesely K, Noomuna P, Vieth M, Hipskind P, Haldar K, Pantaleo A, Turrini F, Low PS. Identification of tyrosine kinase inhibitors that halt Plasmodium falciparum parasitemia. PLoS One 15: e0242372, 2020. doi: 10.1371/journal.pone.0242372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Bordin L, Zen F, Ion-Popa F, Barbetta M, Baggio B, Clari G. Band 3 tyr-phosphorylation in normal and glucose-6-phosphate dehydrogenase-deficient human erythrocytes. Mol Membr Biol 22: 411–420, 2005. doi: 10.1080/09687860500233679. [DOI] [PubMed] [Google Scholar]
- 364.Thiagarajan P, Parker CJ, Prchal JT. How do red blood cells die? Front Physiol 12: 655393, 2021. doi: 10.3389/fphys.2021.655393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Casey JR, Ding Y, Kopito RR. The role of cysteine residues in the erythrocyte plasma membrane anion exchange protein, AE1. J Biol Chem 270: 8521–8527, 1995. doi: 10.1074/jbc.270.15.8521. [DOI] [PubMed] [Google Scholar]
- 366.Fujinaga J, Tang XB, Casey JR. Topology of the membrane domain of human erythrocyte anion exchange protein, AE1. J Biol Chem 274: 6626–6633, 1999. doi: 10.1074/jbc.274.10.6626. [DOI] [PubMed] [Google Scholar]
- 367.Tang XB, Kovacs M, Sterling D, Casey JR. Identification of residues lining the translocation pore of human AE1, plasma membrane anion exchange protein. J Biol Chem 274: 3557–3564, 1999. doi: 10.1074/jbc.274.6.3557. [DOI] [PubMed] [Google Scholar]
- 368.Zhu Q, Lee DW, Casey JR. Novel topology in C-terminal region of the human plasma membrane anion exchanger, AE1. J Biol Chem 278: 3112–3120, 2003. doi: 10.1074/jbc.M207797200. [DOI] [PubMed] [Google Scholar]
- 369.Deuticke B. Properties and structural basis of simple diffusion pathways in the erythrocyte membrane. Rev Physiol Biochem Pharmacol 78: 1–97, 1977. doi: 10.1007/BFb0027721. [DOI] [PubMed] [Google Scholar]
- 370.Yamaguchi T, Kimoto E. Inhibition of phosphate transport across the human erythrocyte membrane by chemical modification of sulfhydryl groups. Biochemistry 31: 1968–1973, 1992. doi: 10.1021/bi00122a010. [DOI] [PubMed] [Google Scholar]
- 371.Teti D, Crupi M, Busá M, Valenti A, Loddo S, Mondello M, Romano L. Chemical and pathological oxidative influences on band 3 protein anion-exchanger. Cell Physiol Biochem 16: 77–86, 2005. doi: 10.1159/000087734. [DOI] [PubMed] [Google Scholar]
- 372.Morabito R, Falliti G, Geraci A, Spada GL, Marino A. Curcumin protects -SH groups and sulphate transport after oxidative damage in human erythrocytes. Cell Physiol Biochem 36: 345–357, 2015. doi: 10.1159/000430256. [DOI] [PubMed] [Google Scholar]
- 373.Petty HR, Zhou MJ, Zheng Z. Oxidative damage by phenylhydrazine diminishes erythrocyte anion transport. Biochim Biophys Acta 1064: 308–314, 1991. doi: 10.1016/0005-2736(91)90316-Z. [DOI] [PubMed] [Google Scholar]
- 374.Maria C, Leonardo R, Pietro R, Mario V, Isabella V, Diana T. Erythrocytes anion transport and oxidative change in beta-thalassaemias. Cell Biol Int 34: 655–662, 2010. doi: 10.1042/CBI20090472. [DOI] [PubMed] [Google Scholar]
- 375.Celedón G, González G, Pino J, Lissi EA. Peroxynitrite oxidizes erythrocyte membrane band 3 protein and diminishes its anion transport capacity. Free Radic Res 41: 316–323, 2007. doi: 10.1080/10715760601090305. [DOI] [PubMed] [Google Scholar]
- 376.Zipser Y, Piade A, Kosower NS. Erythrocyte thiol status regulates band 3 phosphotyrosine level via oxidation/reduction of band 3-associated phosphotyrosine phosphatase. FEBS Lett 406: 126–130, 1997. doi: 10.1016/S0014-5793(97)00263-9. [DOI] [PubMed] [Google Scholar]
- 377.Yannoukakos D, Vasseur C, Piau JP, Wajcman H, Bursaux E. Phosphorylation sites in human erythrocyte band 3 protein. Biochim Biophys Acta 1061: 253–266, 1991. doi: 10.1016/0005-2736(91)90291-F. [DOI] [PubMed] [Google Scholar]
- 378.Dekowski SA, Rybicki A, Drickamer K. A tyrosine kinase associated with the red cell membrane phosphorylates band 3. J Biol Chem 258: 2750–2753, 1983. doi: 10.1016/S0021-9258(18)32777-7. [DOI] [PubMed] [Google Scholar]
- 379.Harrison ML, Rathinavelu P, Arese P, Geahlen RL, Low PS. Role of band 3 tyrosine phosphorylation in the regulation of erythrocyte glycolysis. J Biol Chem 266: 4106–4111, 1991. doi: 10.1016/S0021-9258(20)64292-2. [DOI] [PubMed] [Google Scholar]
- 380.Harrison ML, Isaacson CC, Burg DL, Geahlen RL, Low PS. Phosphorylation of human erythrocyte band 3 by endogenous p72syk. J Biol Chem 269: 955–959, 1994. doi: 10.1016/S0021-9258(17)42204-6. [DOI] [PubMed] [Google Scholar]
- 381.Toye AM, Bruce LJ, Unwin RJ, Wrong O, Tanner MJ. Band 3 walton, a C-terminal deletion associated with distal renal tubular acidosis, is expressed in the red cell membrane but retained internally in kidney cells. Blood 99: 342–347, 2002. doi: 10.1182/blood.V99.1.342. [DOI] [PubMed] [Google Scholar]
- 382.Williamson RC, Brown AC, Mawby WJ, Toye AM. Human kidney anion exchanger 1 localisation in MDCK cells is controlled by the phosphorylation status of two critical tyrosines. J Cell Sci 121: 3422–3432, 2008. doi: 10.1242/jcs.035584. [DOI] [PubMed] [Google Scholar]
- 383.Brunati AM, Bordin L, Clari G, James P, Quadroni M, Baritono E, Pinna LA, Donella-Deana A. Sequential phosphorylation of protein band 3 by Syk and Lyn tyrosine kinases in intact human erythrocytes: Identification of primary and secondary phosphorylation sites. Blood 96: 1550–1557, 2000. doi: 10.1182/blood.V96.4.1550. [DOI] [PubMed] [Google Scholar]
- 384.Pantaleo A, Ferru E, Pau MC, Khadjavi A, Mandili G, Mattè A, Spano A, De Franceschi L, Pippia P, Turrini F. Band 3 erythrocyte membrane protein acts as redox stress sensor leading to its phosphorylation by p (72) syk. Oxid Med Cell Longev 2016: 6051093, 2016. doi: 10.1155/2016/6051093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Lewis IA, Campanella ME, Markley JL, Low PS. Role of band 3 in regulating metabolic flux of red blood cells. Proc Natl Acad Sci USA 106: 18515–18520, 2009. doi: 10.1073/pnas.0905999106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Puchulu-Campanella E, Turrini FM, Li YH, Low PS. Global transformation of erythrocyte properties via engagement of an SH2-like sequence in band 3. Proc Natl Acad Sci USA 113: 13732–13737, 2016. doi: 10.1073/pnas.1611904113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Bordin L, Brunati AM, Donella-Deana A, Baggio B, Toninello A, Clari G. Band 3 is an anchor protein and a target for SHP-2 tyrosine phosphatase in human erythrocytes. Blood 100: 276–282, 2002. doi: 10.1182/blood.V100.1.276. [DOI] [PubMed] [Google Scholar]
- 388.Ciana A, Minetti G, Balduini C. Phosphotyrosine phosphatases acting on band 3 in human erythrocytes of different age: PTP1B processing during cell ageing. Bioelectrochemistry 62: 169–173, 2004. doi: 10.1016/j.bioelechem.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 389.Terra HT, Saad MJ, Carvalho CR, Vicentin DL, Costa FF, Saad ST. Increased tyrosine phosphorylation of band 3 in hemoglobinopathies. Am J Hematol 58: 224–230, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 390.Tanner MJ. Band 3 anion exchanger and its involvement in erythrocyte and kidney disorders. Curr Opin Hematol 9: 133–139, 2002. doi: 10.1097/00062752-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 391.Alper SL. Genetic diseases of acid-base transporters. Annu Rev Physiol 64: 899–923, 2002. doi: 10.1146/annurev.physiol.64.092801.141759. [DOI] [PubMed] [Google Scholar]
- 392.Watanabe T. Improving outcomes for patients with distal renal tubular acidosis: recent advances and challenges ahead. Pediatric Health Med Ther 9: 181–190, 2018. doi: 10.2147/PHMT.S174459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Shayakul C, Alper SL. Defects in processing and trafficking of the AE1 Cl−/HCO3− exchanger associated with inherited distal renal tubular acidosis. Clin Exp Nephrol 8: 1–11, 2004. doi: 10.1007/s10157-003-0271-x. [DOI] [PubMed] [Google Scholar]
- 394.Wrong O, Bruce LJ, Unwin RJ, Toye AM, Tanner MJ. Band 3 mutations, distal renal tubular acidosis, and Southeast Asian ovalocytosis. Kidney Int 62: 10–19, 2002. doi: 10.1046/j.1523-1755.2002.00417.x. [DOI] [PubMed] [Google Scholar]
- 395.Jarolim P, Murray JL, Rubin HL, Taylor WM, Prchal JT, Ballas SK, Snyder LM, Chrobak L, Melrose WD, Brabec V, Palek J. Characterization of 13 novel band 3 gene defects in hereditary spherocytosis with band 3 deficiency. Blood 88: 4366–4374, 1996. doi: 10.1182/blood.V88.11.4366.4366. [DOI] [PubMed] [Google Scholar]
- 396.Iwase S, Ideguchi H, Takao M, Horiguchi-Yamada J, Iwasaki M, Takahara S, Sekikawa T, Mochizuki S, Yamada H. Band 3 Tokyo: Thr837-->Ala837 substitution in erythrocyte band 3 protein associated with spherocytic hemolysis. Acta Haematol 100: 200–203, 1998. doi: 10.1159/000040904. [DOI] [PubMed] [Google Scholar]
- 397.Quilty JA, Li J, Reithmeier RA. Impaired trafficking of distal renal tubular acidosis mutants of the human kidney anion exchanger kAE1. Am J Physiol Renal Physiol 282: F810–F820, 2002. doi: 10.1152/ajprenal.00216.2001. [DOI] [PubMed] [Google Scholar]
- 398.Cordat E, Kittanakom S, Yenchitsomanus PT, Li J, Du K, Lukacs GL, Reithmeier RA. Dominant and recessive distal renal tubular acidosis mutations of kidney anion exchanger 1 induce distinct trafficking defects in MDCK cells. Traffic 7: 117–128, 2006. doi: 10.1111/j.1600-0854.2005.00366.x. [DOI] [PubMed] [Google Scholar]
- 399.Toye AM. Defective kidney anion-exchanger 1 (AE1, band 3) trafficking in dominant distal renal tubular acidosis (dRTA). Biochem Soc Symp 72: 47–63, 2005. doi: 10.1042/bss0720047. [DOI] [PubMed] [Google Scholar]
- 400.Tanphaichitr VS, Sumboonnanonda A, Ideguchi H, Shayakul C, Brugnara C, Takao M, Veerakul G, Alper SL. Novel AE1 mutations in recessive distal renal tubular acidosis. loss-of-function is rescued by glycophorin A. J Clin Invest 102: 2173–2179, 1998. doi: 10.1172/JCI4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet 372: 1411–1426, 2008. doi: 10.1016/S0140-6736(08)61588-3. [DOI] [PubMed] [Google Scholar]
- 402.Andolfo I, Russo R, Gambale A, Iolascon A. New insights on hereditary erythrocyte membrane defects. Haematologica 101: 1284–1294, 2016. doi: 10.3324/haematol.2016.142463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Narla J, Mohandas N. Red cell membrane disorders. Int J Lab Hematol 39, Suppl 1: 47–52, 2017. doi: 10.1111/ijlh.12657. [DOI] [PubMed] [Google Scholar]
- 404.Alper SL. Familial renal tubular acidosis. J Nephrol 23: S57–S76, 2010. [PubMed] [Google Scholar]
- 405.Mohebbi N, Wagner CA. Pathophysiology, diagnosis and treatment of inherited distal renal tubular acidosis. J Nephrol 31: 511–522, 2018. doi: 10.1007/s40620-017-0447-1. [DOI] [PubMed] [Google Scholar]
- 406.Mueller TJ, Morrison M. Detection of a variant of protein 3, the major transmembrane protein of the human erythrocyte. J Biol Chem 252: 6573–6576, 1977. doi: 10.1016/S0021-9258(17)39883-6. [DOI] [PubMed] [Google Scholar]
- 407.Yannoukakos D, Vasseur C, Driancourt C, Blouquit Y, Delaunay J, Wajcman H, Bursaux E. Human erythrocyte band 3 polymorphism (band 3 Memphis): characterization of the structural modification (Lys 56—-Glu) by protein chemistry methods. Blood 78: 1117–1120, 1991. doi: 10.1182/blood.V78.4.1117.1117. [DOI] [PubMed] [Google Scholar]
- 408.Jarolim P, Rubin HL, Zhai S, Sahr KE, Liu SC, Mueller TJ, Palek J. Band 3 memphis: a widespread polymorphism with abnormal electrophoretic mobility of erythrocyte band 3 protein caused by substitution AAG—GAG (Lys—Glu) in codon 56. Blood 80: 1592–1598, 1992. doi: 10.1182/blood.V80.6.1592.1592. [DOI] [PubMed] [Google Scholar]
- 409.Ideguchi H, Okubo K, Ishikawa A, Futata Y, Hamasaki N. Band 3-Memphis is associated with a lower transport rate of phosphoenolpyruvate. Br J Haematol 82: 122–125, 1992. doi: 10.1111/j.1365-2141.1992.tb04603.x. [DOI] [PubMed] [Google Scholar]
- 410.Yang E, Seo-Mayer P, Lezon-Geyda K, Badior KE, Li J, Casey JR, Reithmeier RA, Gallagher PG. A Ser725Arg mutation in band 3 abolishes transport function and leads to anemia and renal tubular acidosis. Blood 131: 1759–1763, 2018. doi: 10.1182/blood-2018-01-827725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Jarolim P, Palek J, Amato D, Hassan K, Sapak P, Nurse GT, Rubin HL, Zhai S, Sahr KE, Liu SC. Deletion in erythrocyte band 3 gene in malaria-resistant Southeast Asian ovalocytosis. Proc Natl Acad Sci USA 88: 11022–11026, 1991. doi: 10.1073/pnas.88.24.11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Schofield AE, Tanner MJ, Pinder JC, Clough B, Bayley PM, Nash GB, Dluzewski AR, Reardon DM, Cox TM, Wilson RJ. Basis of unique red cell membrane properties in hereditary ovalocytosis. J Mol Biol 223: 949–958, 1992. doi: 10.1016/0022-2836(92)90254-H. [DOI] [PubMed] [Google Scholar]
- 413.Picard V, Proust A, Eveillard M, Flatt JF, Couec ML, Caillaux G, Fénéant-Thibault M, Finkelstein A, Raphaël M, Delaunay J, Bruce LJ, Pissard S, Thomas C. Homozygous Southeast Asian ovalocytosis is a severe dyserythropoietic anemia associated with distal renal tubular acidosis. Blood 123: 1963–1965, 2014. doi: 10.1182/blood-2014-01-548149. [DOI] [PubMed] [Google Scholar]
- 414.Flatt JF, Stevens-Hernandez CJ, Cogan NM, Eggleston DJ, Haines NM, Heesom KJ, Picard V, Thomas C, Bruce LJ. Expression of SOUTH EAST ASIAN ovalocytic band 3 disrupts erythroblast cytokinesis and reticulocyte maturation. Front Physiol 11: 357, 2020. doi: 10.3389/fphys.2020.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Asnawi AW, Sathar J, Nasir SF, Mohamed R, Jayaprakasam KV, Vellapandian ST. Homozygous Southeast Asian hereditary ovalostomatocytosis: management dilemma. Pediatr Blood Cancer 62: 1872–1873, 2015. doi: 10.1002/pbc.25545. [DOI] [PubMed] [Google Scholar]
- 416.Lavinya AA, Razali RA, Razak MA, Mohamed R, Moses EJ, Soundararajan M, Bruce LJ, Eswaran J, Yusoff NM. Homozygous Southeast Asian ovalocytosis in five live-born neonates. Haematologica 106: 1758–1761, 2021. doi: 10.3324/haematol.2020.268581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Fowler PW, Sansom MS, Reithmeier RA. Effect of the Southeast Asian ovalocytosis deletion on the conformational dynamics of signal-anchor transmembrane segment 1 of red cell anion exchanger 1 (AE1, band 3, or SLC4A1). Biochemistry 56: 712–722, 2017. doi: 10.1021/acs.biochem.6b00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Chambers EJ, Bloomberg GB, Ring SM, Tanner MJ. Structural studies on the effects of the deletion in the red cell anion exchanger (band 3, AE1) associated with South East Asian ovalocytosis. J Mol Biol 285: 1289–1307, 1999. doi: 10.1006/jmbi.1998.2392. [DOI] [PubMed] [Google Scholar]
- 419.Kuma H, Abe Y, Askin D, Bruce LJ, Hamasaki T, Tanner MJ, Hamasaki N. Molecular basis and functional consequences of the dominant effects of the mutant band 3 on the structure of normal band 3 in Southeast Asian ovalocytosis. Biochemistry 41: 3311–3320, 2002. doi: 10.1021/bi011678+. [DOI] [PubMed] [Google Scholar]
- 420.Moriyama R, Ideguchi H, Lombardo CR, Van Dort HM, Low PS. Structural and functional characterization of band 3 from Southeast Asian ovalocytes. J Biol Chem 267: 25792–25797, 1992. doi: 10.1016/S0021-9258(18)35679-5. [DOI] [PubMed] [Google Scholar]
- 421.Sarabia VE, Casey JR, Reithmeier RA. Molecular characterization of the band 3 protein from Southeast Asian ovalocytes. J Biol Chem 268: 10676–10680, 1993. doi: 10.1016/S0021-9258(18)82250-5. [DOI] [PubMed] [Google Scholar]
- 422.Mirchev R, Lam A, Golan DE. Membrane compartmentalization in Southeast Asian ovalocytosis red blood cells. Br J Haematol 155: 111–121, 2011. doi: 10.1111/j.1365-2141.2011.08805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Jennings ML, Gosselink PG. Anion exchange protein in Southeast Asian ovalocytes: heterodimer formation between normal and variant subunits. Biochemistry 34: 3588–3595, 1995. doi: 10.1021/bi00011a013. [DOI] [PubMed] [Google Scholar]
- 424.Cheung JC, Cordat E, Reithmeier RA. Trafficking defects of the Southeast Asian ovalocytosis deletion mutant of anion exchanger 1 membrane proteins. Biochem J 392: 425–434, 2005. doi: 10.1042/BJ20051076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Bertocchio JP, Genetet S, Da Costa L, Walsh SB, Knebelmann B, Galimand J, Bessenay L, Guitton C, De Lafaille R, Vargas-Poussou R, Eladari D, Mouro-Chanteloup I. Red blood cell AE1/band 3 transports in dominant distal renal tubular acidosis patients. Kidney Int Rep 5: 348–357, 2020. doi: 10.1016/j.ekir.2019.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Schischmanoff PO, Cynober T, Miélot F, Leclerc L, Vasseur-Godbillon C, Baudin-Creuza V, Magowan C, Yeung J, Mohandas N, Tchernia G, Delaunay J. Southeast Asian ovalocytosis in white persons. Hemoglobin 23: 47–56, 1999. doi: 10.3109/03630269908996147. [DOI] [PubMed] [Google Scholar]
- 427.Bruce LJ, Ring SM, Ridgwell K, Reardon DM, Seymour CA, Van Dort HM, Low PS, Tanner MJ. South-East Asian ovalocytic (SAO) erythrocytes have a cold sensitive cation leak: implications for in vitro studies on stored SAO red cells. Biochim Biophys Acta 1416: 258–270, 1999. doi: 10.1016/S0005-2736(98)00231-4. [DOI] [PubMed] [Google Scholar]
- 428.Bruce LJ, Robinson HC, Guizouarn H, Borgese F, Harrison P, King MJ, Goede JS, Coles SE, Gore DM, Lutz HU, Ficarella R, Layton DM, Iolascon A, Ellory JC, Stewart GW. Monovalent cation leaks in human red cells caused by single amino-acid substitutions in the transport domain of the band 3 chloride-bicarbonate exchanger, AE1. Nat Genet 37: 1258–1263, 2005. doi: 10.1038/ng1656. [DOI] [PubMed] [Google Scholar]
- 429.Stewart AK, Kedar PS, Shmukler BE, Vandorpe DH, Hsu A, Glader B, Rivera A, Brugnara C, Alper SL. Functional characterization and modified rescue of novel AE1 mutation R730C associated with overhydrated cation leak stomatocytosis. Am J Physiol Cell Physiol 300: C1034–C1046, 2011. doi: 10.1152/ajpcell.00447.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Frumence E, Genetet S, Ripoche P, Iolascon A, Andolfo I, Le Van Kim C, Colin Y, Mouro-Chanteloup I, Lopez C. Rapid Cl−/HCO3− exchange kinetics of AE1 in HEK293 cells and hereditary stomatocytosis red blood cells. Am J Physiol Cell Physiol 305: C654–C662, 2013. doi: 10.1152/ajpcell.00142.2013. [DOI] [PubMed] [Google Scholar]
- 431.Guizouarn H, Borgese F, Gabillat N, Harrison P, Goede JS, McMahon C, Stewart GW, Bruce LJ. South-East Asian ovalocytosis and the cryohydrocytosis form of hereditary stomatocytosis show virtually indistinguishable cation permeability defects. Br J Haematol 152: 655–664, 2011. doi: 10.1111/j.1365-2141.2010.08454.x. [DOI] [PubMed] [Google Scholar]
- 432.Iolascon A, De Falco L, Borgese F, Esposito MR, Avvisati RA, Izzo P, Piscopo C, Guizouarn H, Biondani A, Pantaleo A, De Franceschi L. A novel erythroid anion exchange variant (Gly796Arg) of hereditary stomatocytosis associated with dyserythropoiesis. Haematologica 94: 1049–1059, 2009. doi: 10.3324/haematol.2008.002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Stewart AK, Vandorpe DH, Heneghan JF, Chebib F, Stolpe K, Akhavein A, Edelman EJ, Maksimova Y, Gallagher PG, Alper SL. The GPA-dependent, spherostomatocytosis mutant AE1 E758K induces GPA-independent, endogenous cation transport in amphibian oocytes. Am J Physiol Cell Physiol 298: C283–C297, 2010. doi: 10.1152/ajpcell.00444.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Bogdanova A, Goede JS, Weiss E, Bogdanov N, Bennekou P, Bernhardt I, Lutz HU. Cryohydrocytosis: increased activity of cation carriers in red cells from a patient with a band 3 mutation. Haematologica 95: 189–198, 2010. doi: 10.3324/haematol.2009.010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Ellory JC, Guizouarn H, Borgese F, Bruce LJ, Wilkins RJ, Stewart GW. Review. leaky Cl−-HCO3− exchangers: cation fluxes via modified AE1. Philos Trans R Soc Lond B Biol Sci 364: 189–194, 2009. doi: 10.1098/rstb.2008.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Quilty JA, Reithmeier RA. Trafficking and folding defects in hereditary spherocytosis mutants of the human red cell anion exchanger. Traffic 1: 987–998, 2000. doi: 10.1034/j.1600-0854.2000.011208.x. [DOI] [PubMed] [Google Scholar]
- 437.Jarolim P, Rubin HL, Brabec V, Chrobak L, Zolotarev AS, Alper SL, Brugnara C, Wichterle H, Palek J. Mutations of conserved arginines in the membrane domain of erythroid band 3 lead to a decrease in membrane-associated band 3 and to the phenotype of hereditary spherocytosis. Blood 85: 634–640, 1995. doi: 10.1182/blood.V85.3.634.bloodjournal853634. [DOI] [PubMed] [Google Scholar]
- 438.Walsh S, Borgese F, Gabillat N, Unwin R, Guizouarn H. Cation transport activity of anion exchanger 1 mutations found in inherited distal renal tubular acidosis. Am J Physiol Renal Physiol 295: F343–F350, 2008. doi: 10.1152/ajprenal.00587.2007. [DOI] [PubMed] [Google Scholar]
- 439.Jin XR, Abe Y, Li CY, Hamasaki N. Histidine-834 of human erythrocyte band 3 has an essential role in the conformational changes that occur during the band 3-mediated anion exchange. Biochemistry 42: 12927–12932, 2003. doi: 10.1021/bi0350809. [DOI] [PubMed] [Google Scholar]
- 440.Müller-Berger S, Karbach D, König J, Lepke S, Wood PG, Appelhans H, Passow H. Inhibition of mouse erythroid band 3-mediated chloride transport by site-directed mutagenesis of histidine residues and its reversal by second site mutation of lys 558, the locus of covalent H2DIDS binding. Biochemistry 34: 9315–9324, 1995. doi: 10.1021/bi00029a006. [DOI] [PubMed] [Google Scholar]
- 441.Takazaki S, Abe Y, Yamaguchi T, Yagi M, Ueda T, Kang D, Hamasaki N. Mutation of his 834 in human anion exchanger 1 affects substrate binding. Biochim Biophys Acta 1798: 903–908, 2010. doi: 10.1016/j.bbamem.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 442.Bruce LJ, Kay MM, Lawrence C, Tanner MJ. Band 3 HT, a human red-cell variant associated with acanthocytosis and increased anion transport, carries the mutation pro-868-->leu in the membrane domain of band 3. Biochem J 293: 317–320, 1993. doi: 10.1042/bj2930317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Kay MM, Bosman GJ, Lawrence C. Functional topography of band 3: specific structural alteration linked to functional aberrations in human erythrocytes. Proc Natl Acad Sci USA 85: 492–496, 1988. doi: 10.1073/pnas.85.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Hassoun H, Hanada T, Lutchman M, Sahr KE, Palek J, Hanspal M, Chishti AH. Complete deficiency of glycophorin A in red blood cells from mice with targeted inactivation of the band 3 (AE1) gene. Blood 91: 2146–2151, 1998. doi: 10.1182/blood.V91.6.2146. [DOI] [PubMed] [Google Scholar]
- 445.Paw BH, Davidson AJ, Zhou Y, Li R, Pratt SJ, Lee C, Trede NS, Brownlie A, Donovan A, Liao EC, Ziai JM, Drejer AH, Guo W, Kim CH, Gwynn B, Peters LL, Chernova MN, Alper SL, Zapata A, Wickramasinghe SN, Lee MJ, Lux SE, Fritz A, Postlethwait JH, Zon LI. Cell-specific mitotic defect and dyserythropoiesis associated with erythroid band 3 deficiency. Nat Genet 34: 59–64, 2003. doi: 10.1038/ng1137. [DOI] [PubMed] [Google Scholar]
- 446.Akel A, Wagner CA, Kovacikova J, Kasinathan RS, Kiedaisch V, Koka S, Alper SL, Bernhardt I, Wieder T, Huber SM, Lang F. Enhanced suicidal death of erythrocytes from gene-targeted mice lacking the Cl−/HCO3− exchanger AE1. Am J Physiol Cell Physiol 292: C1759–C1767, 2007. doi: 10.1152/ajpcell.00158.2006. [DOI] [PubMed] [Google Scholar]
- 447.Peters LL, Shivdasani RA, Liu SC, Hanspal M, John KM, Gonzalez JM, Brugnara C, Gwynn B, Mohandas N, Alper SL, Orkin SH, Lux SE. Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell 86: 917–927, 1996. doi: 10.1016/S0092-8674(00)80167-1. [DOI] [PubMed] [Google Scholar]
- 448.Inaba M, Yawata A, Koshino I, Sato K, Takeuchi M, Takakuwa Y, Manno S, Yawata Y, Kanzaki A, Sakai J, Ban A, Ono K, Maede Y. Defective anion transport and marked spherocytosis with membrane instability caused by hereditary total deficiency of red cell band 3 in cattle due to a nonsense mutation. J Clin Invest 97: 1804–1817, 1996. doi: 10.1172/JCI118610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Ribeiro ML, Alloisio N, Almeida H, Gomes C, Texier P, Lemos C, Mimoso G, Morlé L, Bey-Cabet F, Rudigoz RC, Delaunay J, Tamagnini G. Severe hereditary spherocytosis and distal renal tubular acidosis associated with the total absence of band 3. Blood 96: 1602–1604, 2000. [PubMed] [Google Scholar]
- 450.Kager L, Bruce LJ, Zeitlhofer P, Flatt JF, Maia TM, Ribeiro ML, Fahrner B, Fritsch G, Boztug K, Haas OA. Band 3 null(VIENNA), a novel homozygous SLC4A1 p.Ser477X variant causing severe hemolytic anemia, dyserythropoiesis and complete distal renal tubular acidosis. Pediatr Blood Cancer 64: e26227, 2017. doi: 10.1002/pbc.26227. [DOI] [PubMed] [Google Scholar]
- 451.De Vecchis D, Reithmeier RA, Kalli AC. Molecular simulations of intact anion exchanger 1 reveal specific domain and lipid interactions. Biophys J 117: 1364–1379, 2019. doi: 10.1016/j.bpj.2019.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Kay MM, Marchalonis JJ, Hughes J, Watanabe K, Schluter SF. Definition of a physiologic aging autoantigen by using synthetic peptides of membrane protein band 3: localization of the active antigenic sites. Proc Natl Acad Sci USA 87: 5734–5738, 1990. doi: 10.1073/pnas.87.15.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Badior KE, Casey JR. Large conformational dynamics in band 3 protein: significance for erythrocyte senescence signalling. Biochim Biophys Acta Biomembr 1863: 183678, 2021. doi: 10.1016/j.bbamem.2021.183678. [DOI] [PubMed] [Google Scholar]
- 454.Drew D, Boudker O. Shared molecular mechanisms of membrane transporters. Annu Rev Biochem 85: 543–572, 2016. doi: 10.1146/annurev-biochem-060815-014520. [DOI] [PubMed] [Google Scholar]
- 455.Abbas YM, Toye AM, Rubinstein JL, Reithmeier RA. Band 3 function and dysfunction in a structural context. Curr Opin Hematol 25: 163–170, 2018. doi: 10.1097/MOH.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 456.Coudray N, Seyler SL, Lasala R, Zhang Z, Clark KM, Dumont ME, Rohou A, Beckstein O, Stokes DL. Structure of the SLC4 transporter Bor1p in an inward-facing conformation. Protein Sci 26: 130–145, 2017. doi: 10.1002/pro.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Byrne B. It takes two to transport via an elevator. Cell Res 27: 965–966, 2017. doi: 10.1038/cr.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Thurtle-Schmidt BH, Stroud RM. Structure of Bor1 supports an elevator transport mechanism for SLC4 anion exchangers. Proc Natl Acad Sci USA 113: 10542–10546, 2016. doi: 10.1073/pnas.1612603113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Garaeva AA, Slotboom DJ. Elevator-type mechanisms of membrane transport. Biochem Soc Trans 48: 1227–1241, 2020. doi: 10.1042/BST20200290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Vergara-Jaque A, Fenollar-Ferrer C, Kaufmann D, Forrest LR. Repeat-swap homology modeling of secondary active transporters: updated protocol and prediction of elevator-type mechanisms. Front Pharmacol 6: 183, 2015. doi: 10.3389/fphar.2015.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Ficici E, Faraldo-Gómez JD, Jennings ML, Forrest LR. Asymmetry of inverted-topology repeats in the AE1 anion exchanger suggests an elevator-like mechanism. J Gen Physiol 149: 1149–1164, 2017. doi: 10.1085/jgp.201711836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Yu X, Yang G, Yan C, Baylon JL, Jiang J, Fan H, Lu G, Hasegawa K, Okumura H, Wang T, Tajkhorshid E, Li S, Yan N. Dimeric structure of the uracil:proton symporter UraA provides mechanistic insights into the SLC4/23/26 transporters. Cell Res 27: 1020–1033, 2017. doi: 10.1038/cr.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Low PS, Bada JL, Somero GN. Temperature adaptation of enzymes: roles of the free energy, the enthalpy, and the entropy of activation. Proc Natl Acad Sci USA 70: 430–432, 1973. doi: 10.1073/pnas.70.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Glibowicka M, Winckler B, Araníbar N, Schuster M, Hanssum H, Rüterjans H, Passow H. Temperature dependence of anion transport in the human red blood cell. Biochim Biophys Acta 946: 345–358, 1988. doi: 10.1016/0005-2736(88)90410-5. [DOI] [PubMed] [Google Scholar]
- 465.Galanter WL, Labotka RJ. The temperature dependence of human erythrocyte transport of phosphate, phosphite and hypophosphite. Biochim Biophys Acta 1027: 65–71, 1990. doi: 10.1016/0005-2736(90)90049-T. [DOI] [PubMed] [Google Scholar]
- 466.Canfield VA, Macey RI. Anion exchange in human erythrocytes has a large activation volume. Biochim Biophys Acta 778: 379–384, 1984. doi: 10.1016/0005-2736(84)90382-1. [DOI] [PubMed] [Google Scholar]
- 467.Grunze M, Forst B, Deuticke B. Dual effect of membrane cholesterol on simple and mediated transport processes in human erythrocytes. Biochim Biophys Acta 600: 860–869, 1980. doi: 10.1016/0005-2736(80)90489-7. [DOI] [PubMed] [Google Scholar]
- 468.Gregg VA, Reithmeier RA. Effect of cholesterol on phosphate uptake by human red blood cells. FEBS Lett 157: 159–164, 1983. doi: 10.1016/0014-5793(83)81137-5. [DOI] [PubMed] [Google Scholar]
- 469.Jackson P, Morgan DB. The relation between the membrane cholesterol content and anion exchange in the erythrocytes of patients with cholestasis. Biochim Biophys Acta 693: 99–104, 1982. doi: 10.1016/0005-2736(82)90475-8. [DOI] [PubMed] [Google Scholar]
- 470.Turrini F, Mannu F, Cappadoro M, Ulliers D, Giribaldi G, Arese P. Binding of naturally occurring antibodies to oxidatively and nonoxidatively modified erythrocyte band 3. Biochim Biophys Acta 1190: 297–303, 1994. doi: 10.1016/0005-2736(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 471.Pantaleo A, Ferru E, Giribaldi G, Mannu F, Carta F, Matte A, de Franceschi L, Turrini F. Oxidized and poorly glycosylated band 3 is selectively phosphorylated by syk kinase to form large membrane clusters in normal and G6PD-deficient red blood cells. Biochem J 418: 359–367, 2009. doi: 10.1042/BJ20081557. [DOI] [PubMed] [Google Scholar]
- 472.Ferru E, Giger K, Pantaleo A, Campanella E, Grey J, Ritchie K, Vono R, Turrini F, Low PS. Regulation of membrane-cytoskeletal interactions by tyrosine phosphorylation of erythrocyte band 3. Blood 117: 5998–6006, 2011. doi: 10.1182/blood-2010-11-317024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Azouzi S, Romana M, Arashiki N, Takakuwa Y, El Nemer W, Peyrard T, Colin Y, Amireault P, Le Van Kim C. Band 3 phosphorylation induces irreversible alterations of stored red blood cells. Am J Hematol 93: E110–E112, 2018. doi: 10.1002/ajh.25044. [DOI] [PubMed] [Google Scholar]
- 474.Condon MR, Feketova E, Machiedo GW, Deitch EA, Spolarics Z. Augmented erythrocyte band-3 phosphorylation in septic mice. Biochim Biophys Acta 1772: 580–586, 2007. doi: 10.1016/j.bbadis.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Maccaglia A, Mallozzi C, Minetti M. Differential effects of quercetin and resveratrol on band 3 tyrosine phosphorylation signalling of red blood cells. Biochem Biophys Res Commun 305: 541–547, 2003. doi: 10.1016/S0006-291X(03)00762-9. [DOI] [PubMed] [Google Scholar]
- 476.Noomuna P, Risinger M, Zhou S, Seu K, Man Y, An R, Sheik DA, Wan J, Little JA, Gurkan UA, Turrini FM, Kalfa T, Low PS. Inhibition of band 3 tyrosine phosphorylation: a new mechanism for treatment of sickle cell disease. Br J Haematol 190: 599–609, 2020. doi: 10.1111/bjh.16671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Xiong Y, Li Y, Xiong Y, Zhao Y, Tang F, Wang X. Exhaustive running exercise induce tyrosine phosphorylation of band 3 in rat erythrocytes. Cell Physiol Biochem 32: 1060–1071, 2013. doi: 10.1159/000354506. [DOI] [PubMed] [Google Scholar]
- 478.Olivieri O, De Franceschi L, Bordin L, Manfredi M, Miraglia del Giudice E, Perrotta S, De Vivo M, Guarini P, Corrocher R. Increased membrane protein phosphorylation and anion transport activity in chorea-acanthocytosis. Haematologica 82: 648–653, 1997. [PubMed] [Google Scholar]
- 479.Mallozzi C, Di Stasi AM, Minetti M. Peroxynitrite modulates tyrosine-dependent signal transduction pathway of human erythrocyte band 3. FASEB J 11: 1281–1290, 1997. doi: 10.1096/fasebj.11.14.9409547. [DOI] [PubMed] [Google Scholar]
- 480.Boron WF. Evaluating the role of carbonic anhydrases in the transport of HCO3−-related species. Biochim Biophys Acta 1804: 410–421, 2010. doi: 10.1016/j.bbapap.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Piermarini PM, Kim EY, Boron WF. Evidence against a direct interaction between intracellular carbonic anhydrase II and pure C-terminal domains of SLC4 bicarbonate transporters. J Biol Chem 282: 1409–1421, 2007. doi: 10.1074/jbc.M608261200. [DOI] [PubMed] [Google Scholar]
- 482.Al-Samir S, Papadopoulos S, Scheibe RJ, Meißner JD, Cartron JP, Sly WS, Alper SL, Gros G, Endeward V. Activity and distribution of intracellular carbonic anhydrase II and their effects on the transport activity of anion exchanger AE1/SLC4A1. J Physiol 591: 4963–4982, 2013. doi: 10.1113/jphysiol.2013.251181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Itada N, Forster RE. Carbonic anhydrase activity in intact red blood cells measured with 18O exchange. J Biol Chem 252: 3881–3890, 1977. doi: 10.1016/S0021-9258(17)40334-6. [DOI] [PubMed] [Google Scholar]
- 484.Endeward V, Gros G. Extra- and intracellular unstirred layer effects in measurements of CO2 diffusion across membranes—a novel approach applied to the mass spectrometric 18O technique for red blood cells. J Physiol 587: 1153–1167, 2009. doi: 10.1113/jphysiol.2008.165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Crandall ED, O’Brasky JE. Direct evidence of participation of rat lung carbonic anhydrase in CO2 reactions. J Clin Invest 62: 618–622, 1978. doi: 10.1172/JCI109168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Sender S, Gros G, Waheed A, Hageman GS, Sly WS. Immunohistochemical localization of carbonic anhydrase IV in capillaries of rat and human skeletal muscle. J Histochem Cytochem 42: 1229–1236, 1994. doi: 10.1177/42.9.8064130. [DOI] [PubMed] [Google Scholar]
- 487.Orlowski A, De Giusti VC, Morgan PE, Aiello EA, Alvarez BV. Binding of carbonic anhydrase IX to extracellular loop 4 of the NBCe1 Na+/HCO3− cotransporter enhances NBCe1-mediated HCO3− influx in the rat heart. Am J Physiol Cell Physiol 303: C69–C80, 2012. doi: 10.1152/ajpcell.00431.2011. [DOI] [PubMed] [Google Scholar]
- 488.Nikinmaa M, Railo E. Anion movements across lamprey (Lampetra fluviatilis) red cell membrane. Biochim Biophys Acta 899: 134–136, 1987. doi: 10.1016/0005-2736(87)90248-3. [DOI] [PubMed] [Google Scholar]
- 489.Swenson ER, Grønlund J, Ohlsson J, Hlastala MP. In vivo quantitation of carbonic anhydrase and band 3 protein contributions to pulmonary gas exchange. J Appl Physiol (1985) 74: 838–848, 1993. doi: 10.1152/jappl.1993.74.2.838. [DOI] [PubMed] [Google Scholar]
- 490.Seevaamirtham R, Sarathchandra C, Senanayake H, Weerawansa P, Lokunarangoda N, Siribaddana S, Mendis V. Case report on Southeast Asian ovalocytosis: a patient with type 1 renal tubular acidosis and an asymptomatic patient incidentally found. Jaffna Med J 31: 38–41, 2019. doi: 10.4038/jmj.v31i2.79. [DOI] [Google Scholar]
- 491.Helms CC, Gladwin MT, Kim-Shapiro DB. Erythrocytes and vascular function: oxygen and nitric oxide. Front Physiol 9: 125, 2018. doi: 10.3389/fphys.2018.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Vitturi DA, Patel RP. Current perspectives and challenges in understanding the role of nitrite as an integral player in nitric oxide biology and therapy. Free Radic Biol Med 51: 805–812, 2011. doi: 10.1016/j.freeradbiomed.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Gunn RB, Wieth JO, Tosteson DC. Some effects of low pH on chloride exchange in human red blood cells. J Gen Physiol 65: 731–749, 1975. doi: 10.1085/jgp.65.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Jensen FB. Nitrite transport into pig erythrocytes and its potential biological role. Acta Physiol Scand 184: 243–251, 2005. doi: 10.1111/j.1365-201X.2005.01448.x. [DOI] [PubMed] [Google Scholar]
- 495.Jensen FB, Rohde S. Comparative analysis of nitrite uptake and hemoglobin-nitrite reactions in erythrocytes: sorting out uptake mechanisms and oxygenation dependencies. Am J Physiol Regul Integr Comp Physiol 298: R972–R982, 2010. doi: 10.1152/ajpregu.00813.2009. [DOI] [PubMed] [Google Scholar]
- 496.Denicola A, Souza JM, Radi R. Diffusion of peroxynitrite across erythrocyte membranes. Proc Natl Acad Sci USA 95: 3566–3571, 1998. doi: 10.1073/pnas.95.7.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Soszyński M, Bartosz G. Penetration of erythrocyte membrane by peroxynitrite: participation of the anion exchange protein. Biochem Mol Biol Int 43: 319–325, 1997. doi: 10.1080/15216549700204101. [DOI] [PubMed] [Google Scholar]
- 498.Deonikar P, Kavdia M. A computational model for nitric oxide, nitrite and nitrate biotransport in the microcirculation: effect of reduced nitric oxide consumption by red blood cells and blood velocity. Microvasc Res 80: 464–476, 2010. doi: 10.1016/j.mvr.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Kuhn V, Diederich L, Keller TC 4th, Kramer CM, Lückstädt W, Panknin C, Suvorava T, Isakson BE, Kelm M, Cortese-Krott MM. Red blood cell function and dysfunction: redox regulation, nitric oxide metabolism, anemia. Antioxid Redox Signal 26: 718–742, 2017. doi: 10.1089/ars.2016.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol 26: 697–705, 2006. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]
- 501.Richardson SL, Swietach P. Red blood cell thickness is evolutionarily constrained by slow, hemoglobin-restricted diffusion in cytoplasm. Sci Rep 6: 36018, 2016. doi: 10.1038/srep36018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Johnson DE, Casey JR. Cytosolic H+ microdomain developed around AE1 during AE1-mediated Cl−/HCO3− exchange. J Physiol 589: 1551–1569, 2011. doi: 10.1113/jphysiol.2010.201483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Wittenberg JB. The molecular mechanism of hemoglobin-facilitated oxygen diffusion. J Biol Chem 241: 104–114, 1966. doi: 10.1016/S0021-9258(18)96964-4. [DOI] [PubMed] [Google Scholar]
- 504.Richardson SL, Hulikova A, Proven M, Hipkiss R, Akanni M, Roy NB, Swietach P. Single-cell O2 exchange imaging shows that cytoplasmic diffusion is a dominant barrier to efficient gas transport in red blood cells. Proc Natl Acad Sci USA 117: 10067–10078, 2020. doi: 10.1073/pnas.1916641117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Gibson QH, Roughton FJ. The kinetics of dissociation of the first oxygen molecule from fully saturated oxyhaemoglobin in sheep blood solutions. Proc R Soc Lond B Biol Sci 143: 310–334, 1955. doi: 10.1098/rspb.1955.0014. [DOI] [PubMed] [Google Scholar]
- 506.Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem 64: 375–401, 1995. doi: 10.1146/annurev.bi.64.070195.002111. [DOI] [PubMed] [Google Scholar]
- 507.Prange HD, Shoemaker JL Jr, Westen EA, Horstkotte DG, Pinshow B. Physiological consequences of oxygen-dependent chloride binding to hemoglobin. J Appl Physiol (1985) 91: 33–38, 2001. doi: 10.1152/jappl.2001.91.1.33. [DOI] [PubMed] [Google Scholar]
- 508.Gros G, Rollema HS, Forster RE. The carbamate equilibrium of alpha- and epsilon-amino groups of human hemoglobin at 37 degrees C. J Biol Chem 256: 5471–5480, 1981. doi: 10.1016/S0021-9258(19)69225-2. [DOI] [PubMed] [Google Scholar]
- 509.Jin Y, Liang Q, Tieleman DP. Interactions between band 3 anion exchanger and lipid nanodomains in ternary lipid bilayers: atomistic simulations. J Phys Chem B 124: 3054–3064, 2020. doi: 10.1021/acs.jpcb.0c01055. [DOI] [PubMed] [Google Scholar]
- 510.Jiang T, Wen PC, Trebesch N, Zhao Z, Pant S, Kapoor K, Shekhar M, Tajkhorshid E. Computational dissection of membrane transport at a microscopic level. Trends Biochem Sci 45: 202–216, 2020. doi: 10.1016/j.tibs.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Occhipinti R, Boron WF. Mathematical modeling of acid-base physiology. Prog Biophys Mol Biol 117: 43–58, 2015. doi: 10.1016/j.pbiomolbio.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Occhipinti R, Boron WF. Role of carbonic anhydrases and inhibitors in acid-base physiology: insights from mathematical modeling. Int J Mol Sci 20: 3841, 2019. doi: 10.3390/ijms20153841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Shingles R, Roh MH, McCarty RE. Direct measurement of nitrite transport across erythrocyte membrane vesicles using the fluorescent probe, 6-methoxy-N-(3-sulfopropyl) quinolinium. J Bioenerg Biomembr 29: 611–616, 1997. doi: 10.1023/A:1022491220299. [DOI] [PubMed] [Google Scholar]
- 514.Vitturi DA, Teng X, Toledo JC, Matalon S, Lancaster JR Jr, Patel RP. Regulation of nitrite transport in red blood cells by hemoglobin oxygen fractional saturation. Am J Physiol Heart Circ Physiol 296: H1398–H1407, 2009. doi: 10.1152/ajpheart.01303.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Jennings ML. Transport of H2S and HS- across the human red blood cell membrane: rapid H2S diffusion and AE1-mediated Cl−/HS− exchange. Am J Physiol Cell Physiol 305: C941–C950, 2013. doi: 10.1152/ajpcell.00178.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Giebel O, Passow H. The permeability of erythrocyte membranes for organic anions. on the problem of diffusion through the pores. Pflugers Arch Gesamte Physiol Menschen Tiere 271: 378–388, 1960. doi: 10.1007/BF00362917. [DOI] [PubMed] [Google Scholar]
- 517.Saradhadevi V, Sakthivel R, Vedamoorthy S, Selvam R, Parinandi N. Alterations in band 3 protein and anion exchange in red blood cells of renal failure patients. Mol Cell Biochem 273: 11–24, 2005. doi: 10.1007/s11010-005-5904-9. [DOI] [PubMed] [Google Scholar]
- 518.Lynch RE, Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem 253: 4697–4699, 1978. doi: 10.1016/S0021-9258(17)30446-5. [DOI] [PubMed] [Google Scholar]
- 519.Kaur G, Javed W, Ponomarenko O, Shekh K, Swanlund DP, Zhou JR, Summers KL, Casini A, Wenzel MN, Casey JR, Cordat E, Pickering IJ, George GN, Leslie EM. Human red blood cell uptake and sequestration of arsenite and selenite: evidence of seleno-bis(S-glutathionyl) arsinium ion formation in human cells. Biochem Pharmacol 180: 114141, 2020. doi: 10.1016/j.bcp.2020.114141. [DOI] [PubMed] [Google Scholar]
- 520.Hajjawi OS, Hider RC. Malonate transport in human red blood cells. Mol Cell Biochem 75: 43–49, 1987. doi: 10.1007/BF00231607. [DOI] [PubMed] [Google Scholar]
- 521.Labotka RJ, Galanter W, Misiewicz VM. Erythrocyte bisulfite transport. Biochim Biophys Acta 981: 358–362, 1989. doi: 10.1016/0005-2736(89)90048-5. [DOI] [PubMed] [Google Scholar]
- 522.Jennings ML. Reductive methylation of the two 4,4’-diisothiocyanodihydrostilbene-2,2’-disulfonate-binding lysine residues of band 3, the human erythrocyte anion transport protein. J Biol Chem 257: 7554–7559, 1982. doi: 10.1016/S0021-9258(18)34415-6. [DOI] [PubMed] [Google Scholar]
- 523.Cantley LC Jr, Resh MD, Guidotti G. Vanadate inhibits the red cell (Na+, K+) ATPase from the cytoplasmic side. Nature 272: 552–554, 1978. doi: 10.1038/272552a0. [DOI] [PubMed] [Google Scholar]
- 524.Rice WR, Steck TL. Pyruvate flux into resealed ghosts from human erythrocytes. Biochim Biophys Acta 433: 39–53, 1976. doi: 10.1016/0005-2736(76)90176-0. [DOI] [PubMed] [Google Scholar]
- 525.Ormos G, Mányai S. Chromate uptake by human red blood cells: comparison of permeability for different divalent anions. Acta Biochim Biophys Acad Sci Hung 9: 197–207, 1974. [PubMed] [Google Scholar]
- 526.Ebaugh FG Jr, Emerson CP, Ross JF. The use of radioactive chromium 51 as an erythrocyte tagging agent for the determination or red cell survival in vivo. J Clin Invest 32: 1260–1276, 1953. doi: 10.1172/JCI102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.King PA, Gunn RB. Glycine transport by human red blood cells and ghosts: evidence for glycine anion and proton cotransport by band 3. Am J Physiol Cell Physiol 261: C814–C821, 1991. doi: 10.1152/ajpcell.1991.261.5.C814. [DOI] [PubMed] [Google Scholar]
- 528.Timmer RT, Gunn RB. The molecular basis for Na-dependent phosphate transport in human erythrocytes and K562 cells. J Gen Physiol 116: 363–378, 2000. doi: 10.1085/jgp.116.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Shoemaker DG, Bender CA, Gunn RB. Sodium-phosphate cotransport in human red blood cells. kinetics and role in membrane metabolism. J Gen Physiol 92: 449–474, 1988. doi: 10.1085/jgp.92.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Hamasaki N, Hardjono IS, Minakami S. Transport of phosphoenolpyruvate through the erythrocyte membrane. Biochem J 170: 39–46, 1978. doi: 10.1042/bj1700039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Becker BF, Duhm J. Evidence for anionic cation transport of lithium, sodium and potassium across the human erythrocyte membrane induced by divalent anions. J Physiol 282: 149–168, 1978. doi: 10.1113/jphysiol.1978.sp012454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Funder J, Tosteson DC, Wieth JO. Effects of bicarbonate on lithium transport in human red cells. J Gen Physiol 71: 721–746, 1978. doi: 10.1085/jgp.71.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Thomas EL, Grisham MB, Melton DF, Jefferson MM. Evidence for a role of taurine in the in vitro oxidative toxicity of neutrophils toward erythrocytes. J Biol Chem 260: 3321–3329, 1985. doi: 10.1016/S0021-9258(19)83623-2. [DOI] [PubMed] [Google Scholar]
- 534.Forrest LR, Rudnick G. The rocking bundle: a mechanism for ion-coupled solute flux by symmetrical transporters. Physiology (Bethesda) 24: 377–386, 2009. doi: 10.1152/physiol.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Endeward V, Musa-Aziz R, Cooper GJ, Chen LM, Pelletier MF, Virkki LV, Supuran CT, King LS, Boron WF, Gros G. Evidence that aquaporin 1 is a major pathway for CO2 transport across the human erythrocyte membrane. FASEB J 20: 1974–1981, 2006. doi: 10.1096/fj.04-3300com. [DOI] [PubMed] [Google Scholar]
- 536.Endeward V, Cartron JP, Ripoche P, Gros G. RhAG protein of the rhesus complex is a CO2 channel in the human red cell membrane. FASEB J 22: 64–73, 2008. doi: 10.1096/fj.07-9097com. [DOI] [PubMed] [Google Scholar]
- 537.Nakhoul NL, Davis BA, Romero MF, Boron WF. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of xenopus oocytes. Am J Physiol Cell Physiol 274: C543–C548, 1998. doi: 10.1152/ajpcell.1998.274.2.C543. [DOI] [PubMed] [Google Scholar]
- 538.Zwiazek JJ, Xu H, Tan X, Navarro-Ródenas A, Morte A. Significance of oxygen transport through aquaporins. Sci Rep 7: 40411, 2017. doi: 10.1038/srep40411. [DOI] [PMC free article] [PubMed] [Google Scholar]