Abstract
Background
In patients with heart failure (HF), physical frailty should be assessed to enable risk stratification. No conventional frailty criteria have so far been developed considering HF‐specific outcomes. This study aimed to propose a frailty‐based prognostic score using a nationwide cohort study of Japanese patients with HF.
Methods
We analysed 2721 patients hospitalized for HF and capable of walking at discharge (median age: 76 years [interquartile range 67–83], men: 60.5%). Physical frailty was evaluated at discharge using four quantitative measures: usual walking speed, grip strength, Performance Measure for Activities of Daily Living‐8 (PMADL‐8), and Self‐Efficacy for Walking‐7 (SEW‐7). The primary outcome was a composite of HF rehospitalization and all‐cause mortality within 2 years. A cut‐off point was identified for each measure using receiver operating characteristic analysis in a derivation cohort (n = 1778). Cox proportional hazards model was used to assign a score to each frailty domain according to the correlation with the endpoint. Patients were divided into four categories according to the sum score, and survival was compared by analysing the Kaplan–Meier curve and Cox proportional hazards model. Cumulative incidences of the events according to frailty categories were compared between the derivation cohort and a validation cohort (n = 943).
Results
The cut‐off value and assigned score of each indicator was determined as follows: usual walking speed < 0.98 m/s = 4 points; grip strength < 30.0 kg (men) or 17.5 kg (women) = 5 points; PMADL‐8 ≥ 21 points = 2 points; SEW‐7 ≤ 20 points = 3 points. We stratified patients into four categories according to the sum score: Category I, ≤3 points; Categories II, 4–8 points; Category III, 9–13 points; and Category IV, 14 points. The prevalence and cumulative incidence of the composite outcome for Categories I to IV in the derivation cohort were 27.4%, 25.2%, 26.4%, and 21.0%, and 9.5, 16.3, 26.3, and 36.8/100 person‐years, respectively. Similar results were confirmed in the validation cohort. In Cox proportional hazards model, frailty categories were associated with the composite outcome independent of potential confounders (hazard ratio [95% confidence interval] in reference to Category I: Categories II, 1.51 [0.84–2.72], P = 0.169; Category III, 2.37 [1.32–4.23], P = 0.004; Category IV, 2.66 [1.45–4.89], P = 0.002).
Conclusions
The frailty‐based prognostic score proposed in this study was well associated with prognosis and will serve for risk stratification in patients with HF.
Keywords: Frailty, Heart failure, Prognosis, Cohort study
Introduction
Long‐term management of heart failure (HF) is among the major global issues in healthcare due to a rapidly aging society. Frailty, characterized by reduced functional reserve and impaired adaptive capacity, has been considered a key medical syndrome for risk stratification in patients with wasting disease. 1 Several observational studies have documented the high prevalence of physical frailty and its prognostic effect in patients with HF. 2 , 3 Accordingly, the best clinical practice for long‐term HF management should include assessing physical frailty specific to HF. However, currently available assessment tools for assessing physical frailty were not initially developed based on HF‐specific outcomes.
Another requirement for frailty assessment in patients with HF is objectivity to monitor patients' clinical conditions over time and evaluate the effects of medical interventions. To date, the phenotype model by Fried et al. 4 and the clinical frailty scale by Rockwood et al. 5 have been widely used to assess frailty clinically in patients with HF. 2 These conventional frailty criteria include subjective or qualitative measures and provide insufficient quantitative information. The modified Fried's criteria used in Asia, 6 for example, also assess two domains of exhaustion and physical inactivity by subjective evaluation of ‘yes’ or ‘no’ answers, although both domains may become indicators of common symptoms of patients with HF. The Short Physical Performance Battery is another measure for frailty assessment used to examine patients with HF. 7 , 8 However, a ceiling effect has been often observed for this test. 9 A high proportion of subjects tend to score 11 or 12 points on Short Physical Performance Battery, suggesting that this tool probably has limitations in monitoring changes in frailty among patients with relatively preserved physical function.
Despite the growing evidence on frailty among patients with HF, currently available frailty assessment methods have the aforementioned limitations to be applied in the long‐term management of HF. Therefore, we launched a multicentre prospective cohort study to develop frailty‐based prognostic criteria for heart failure patients (FLAGSHIP). 10 This report documents the primary analysis of the FLAGSHIP study to propose frailty‐based prognostic scores using quantitative and straightforward measures associated with HF‐specific outcomes.
Methods
Study design and subjects
This was a multicentre prospective cohort study. The objectives of the FLAGSHIP cohort study and design have been described elsewhere. 10
The patients included in the FLAGSHIP cohort were as follows: (i) patients hospitalized due to acute HF or worsening chronic HF, capable of walking 20 m at the time of discharge, and (ii) patients aged ≥ 70 years and hospitalized for acute myocardial infarction (AMI) not complicated by HF, and capable of walking 20 m at the time of discharge. The latter population (older AMI patients not complicated by HF) was registered for another substudy to explore the risk factors of HF onset in elderly patients with AMI and not included in the present analysis. In addition, non‐ambulatory patients were not included in our cohort because of an international consensus that frailty is not a disability and that those who are frail and pre‐disabled should be targeted for intervention. 1 Our cohort also includes patients aged < 65 years because even younger patients with HF can be physically feeble due to the reduced cardiac function, chronic inflammatory state, and/or subsequent cachectic state. This decision is supported by the frailty cycle proposed by Fried et al., in which the influence of disease is positioned as a causal factor of sarcopenia, a core component of physical frailty.
The exclusion criteria included the presence of one or more of the following: (i) severe cognitive impairment defined by a score of ≤17 points on the Mini‐Mental State Examination, 11 (ii) severe mental disorder, (iii) difficulty in answering questionnaires, and (iv) an assumed impending mortality. All registered patients were followed up for 2 years after discharge.
In this study, we analysed patients from the FLAGSHIP cohort hospitalized due to HF and enrolled between September 2015 and December 2018. Older patients with AMI not complicated by HF were not included in this analysis as mentioned above. The protocol of the FLAGSHIP cohort study was developed by following the Guidelines for Epidemiological Research proposed by the Japanese Ministry of Health, Labour, and Welfare. Additionally, the study complied with the principles of the Declaration of Helsinki, and the study protocol was approved by the local ethics committee (approval no. 2014–0421). Ethical approval was also obtained from the committees of each participating hospital, and each patient provided written informed consent before being registered in this study.
Measurements of physical frailty
We analysed the metrics of frailty collected just before discharge. Considering the pathophysiology of HF, physical frailty assessment included five domains: slowness, weakness, exhaustion, low physical activity, and weight loss. The required equipment and tools were a 10 m walkway, a stopwatch to assess slowness, a dynamometer to assess weakness, and questionnaires for exhaustion and low physical activity. The required time for the frailty assessment used in this study is similar to that of the conventional methods.
Slowness and weakness were assessed by the 10 m usual walking speed and grip strength, respectively. The measurements were completed within 10 min, and the task did not require professional skills. The 10 m walkway was used in the FLAGSHIP study as it has been a common setting for walking speed measurement in the field of rehabilitation. All grip strength measurements were performed using the Jamar dynamometer (Digital Hand Dynamometer, DHD‐1, SAEHAN Corporation) set at the second handle position. The participants sat with their wrist in a neutral position and elbow flexed at 90°. Before commencing patient enrolment, measurement reliabilities for walking speed and grip strength were confirmed at each hospital. Each collaborating hospital provided good reliability of interclass and intraclass correlation coefficients of >0.9 of testers for both walking speed and grip strength measurements. 10
Exhaustion was assessed using the Performance Measure for Activity of Daily Living‐8 (PMADL‐8) (Supporting Information, Table S1 ). 12 The PMADL‐8 is a standardized questionnaire that uses a four‐category response scale and comprises a list of eight items that potentially require daily physical activity. It was developed to assess functional limitations in patients with chronic HF. The questionnaire for assessing exhaustion in Fried's criteria 4 was validated based on the association with stage of exercise reached in graded exercise testing, as an indicator of oxygen consumption. 13 Declined peak oxygen consumption has also been positioned as a phenotype of fragile older adults. 4 From these reasons, objective assessment of exhaustion should be based on oxygen consumption. The PMADL‐8 is a valid measure of exhaustion based on the high correlation between the PMADL‐8 score and peak VO2 (r = −0.743) in HF patients. 14 The PMADL‐8 is essentially a measure of functional limitations as well as a New York Heart Association Functional Classification. 15
Low physical activity was assessed using a standardized questionnaire named Self‐Efficacy for Walking‐7 (SEW‐7), 16 composed of seven items with a 5‐point Likert scale (Table S2). Walking is a popular, familiar, convenient, and free form of exercise that can be incorporated into everyday life more than any other type of exercise. 17 Self‐efficacy means the belief in one's capabilities to organize and execute the courses of action required to achieve given targets and is regarded as a more important predictor of behaviour than ability. 18 The total score (7–35 points) of the SEW‐7 reportedly has a favourable correlation with step counts (r = 0.596), physical activity‐related energy expenditure (r = 0.615), and moderate to vigorous physical activity (r = 0.581). 16 From the above, the SEW‐7 having the favourable correlation with daily physical activity is valid to be used to assess physical activity.
Weight loss assessed by unintentional weight loss during the past 6 months was considered one of the five domains of the frailty phenotype in the general elderly population. 4 However, due to the lack of habitual body weight measurement in elderly individuals, the objective data of weight loss from the past 6 months at the time of discharge could not be obtained for most patients with HF, especially in the case of first HF onset. Therefore, body mass index (BMI) at discharge was considered an alternative indicator of unintentional weight loss in the FLAGSHIP cohort. Although body weight at discharge is affected by body fluid management medications, patients diagnosed with weight loss based on a low BMI have a high probability of muscle wasting or cachectic condition.
Outcomes
The primary outcome was the composite endpoint of HF rehospitalization and all‐cause death within 2 years after discharge; the secondary outcomes were HF rehospitalization and all‐cause mortality alone. A follow‐up survey for each patient was performed using the medical records of hospitals that took care of the patient, and HF rehospitalization was determined by cardiologists at each site. In patients who were not followed up by enrolling hospitals, prognostic data were obtained using a mail survey sent directly to the patients every 4 months. Each patient was followed up continuously after HF rehospitalization until mortality or until the patient or their family refused to respond. The follow‐up period was defined as the time from discharge until either of the two outcomes occurred.
Statistical analysis
Patients with missing data on physical frailty assessment were excluded from the analysis. Two‐thirds of the patients were randomly assigned to a derivation cohort to develop physical frailty criteria using computer‐generated random numbers, and the other third was reserved as a validation cohort. Continuous variables, with or without normal distribution, were described as either mean ± standard deviation or median [interquartile range]. Categorical variables were expressed as numbers and percentages. Patient characteristics were compared between the two cohorts using the Student's t‐test, Mann–Whitney's U‐test, or χ 2 test, as appropriate.
A cut‐off value for each physical frailty domain was determined using receiver operating characteristic (ROC) curve analysis to predict the primary endpoint in a derivation cohort. The ROC curve was constructed by plotting sensitivity against 1‐specificity. The area under the curve (AUC) was calculated, and the cut‐off value was identified based on Youden's index. In general, the severity of frailty was categorized based on the number of frailty domains that the patient had. However, there is a possibility that the predictive effects on prognosis are different in the various domains. To assess the weightage of each domain, we used a Cox proportional hazards model that included four frailty domains. According to the adjusted coefficients (β) of the Cox proportional hazards model, a score was assigned to each domain, and the sum of the scores was used as the frailty‐based prognostic score.
The severity of physical frailty was categorized into four grades: Category I, Category II, Category III, and Category IV, based on the distribution of the score and the weight of each domain. The cumulative incidence rate of outcomes was calculated according to the frailty categories using the Kaplan–Meier's method, followed by the log‐rank test. A Cox proportional hazards model was then used to examine the association between frailty categories and the study outcomes after adjusting for the potential confounding factors in the derivation cohort. The analysis was adjusted for potential confounders reported by previous studies, 19 , 20 , 21 including age, sex, BMI, left ventricular ejection fraction, history of HF, comorbidities, biochemical data, medications, cognitive function, and depression. Cognitive function was assessed using the Mini‐Mental State Examination, 11 and depression was defined on a Five‐item Geriatric Depression Scale score of ≥2 points. 22 Because few hospitals used NT‐proBNP instead of BNP in their clinical practice, we created a categorical variable, with BNP ≥ 200 pg/mL or NT‐proBNP ≥ 900 pg/mL defined as high BNP according to the Japanese Heart Failure Society statement. 23 In the multivariate analysis, missing values were imputed using multiple imputation by chained equations. 24 Parameter estimates and confidence intervals were obtained by combining 20 imputed data sets, as described by Barnard and Rubin, 25 to account for possible errors in the missing value analysis.
The validity of the frailty category developed in the derivation cohort was examined in the validation cohort. We reproduced the cumulative incidence of the study outcomes according to the severity of frailty and confirmed whether similar results were obtained from the derivation and the validation cohorts.
Subgroup analysis was performed based on left ventricular ejection fraction measured by echocardiography around discharge: HF with reduced ejection fraction (HFrEF, <40%), HF with mid‐range ejection fraction (40–49%), and HF with preserved ejection fraction (≥ 50%). Due to the limited event rate of all‐cause mortality, the composite outcome and HF rehospitalization were compared between the four frailty categories using Kaplan–Meier survival curve according to each HF subgroup.
All statistical analyses were performed with Stata SE 15 (Stata Corporation). A P value of <0.05 was considered statistically significant.
Results
Of the 3272 patients registered in the FLAGSHIP study, we finally analysed 2721 patients hospitalized due to HF after excluding those with missing data on the most variables (n = 8) or at least one frailty domain (n = 155) (Figure 1). Table 1 shows the characteristics of the study participants. Overall, the median age was 76 years, and 60.5% were men. The prevalence of HFrEF (<40%) and preserved ejection fraction (≥50%) were 38.0% and 44.4%, respectively. There were no statistically significant differences among all the variables between the derivation and validation cohorts. The two cohorts showed similar cumulative incidence rates in the study outcomes (log‐rank test: composite outcome, P = 0.850; HF rehospitalization, P = 0.558; all‐cause mortality, P = 0.560) (Figure S1).
Figure 1.
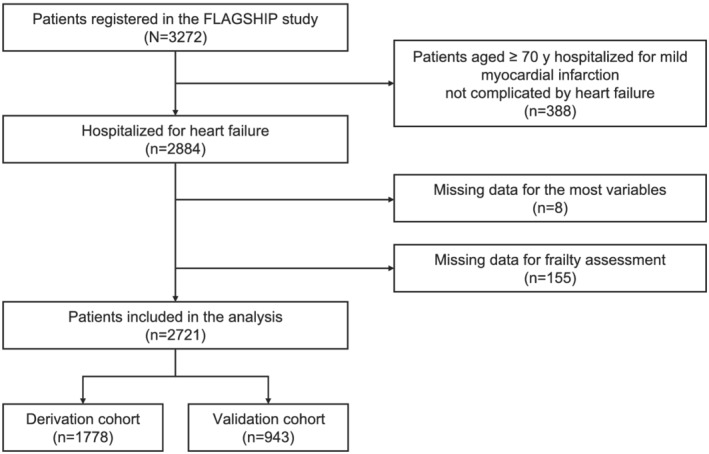
Flowchart of patient selection.
Table 1.
Participant characteristics in the derivation cohort and validation cohort
| Overall (n = 2721) | Derivation cohort (n = 1778) | Validation cohort (n = 943) | |||||
|---|---|---|---|---|---|---|---|
| Missing | Missing | Missing | P | ||||
| Age (years) | 0 | 76 [67–83] | 0 | 76 [67–83] | 0 | 76 [68–83] | 0.870 |
| Men (%) | 0 | 60.5 | 0 | 60.6 | 0 | 60.4 | 0.948 |
| Past HF hospitalization | 0 | 29.7 | 0 | 29.7 | 0 | 29.6 | 0.952 |
| SBP at discharge | 6 | 112 [100–125] | 4 | 111 [100–124] | 2 | 113 [100–127] | 0.086 |
| DBP at discharge | 6 | 64 [55–72] | 4 | 63 [55–72] | 2 | 64 [56–73] | 0.672 |
| HR at discharge | 8 | 73 [64–82] | 5 | 73 [64–83] | 3 | 73 [64–82] | 0.879 |
| Main aetiology | 0 | 0 | 0 | ||||
| Ischemic (%) | 26.9 | 26.4 | 27.7 | 0.825 | |||
| Arrhythmic (%) | 17.5 | 17.6 | 17.3 | ||||
| Valvular (%) | 17.1 | 17.4 | 16.3 | ||||
| Cardiomyopathy (%) | 14.0 | 13.9 | 14.1 | ||||
| Hypertensive (%) | 10.4 | 10.8 | 9.7 | ||||
| Others (%) | 14.1 | 13.9 | 14.9 | ||||
| Af at hospitalization (%) | 0 | 35.2 | 0 | 36.1 | 0 | 33.5 | 0.186 |
| Comorbidities | |||||||
| Diabetes (%) | 0 | 34.9 | 0 | 35.9 | 0 | 33.1 | 0.240 |
| Cardiac surgery (%) | 0 | 12.2 | 0 | 12.3 | 0 | 11.9 | 0.738 |
| Cancer (%) | 0 | 7.2 | 0 | 7.1 | 0 | 7.5 | 0.912 |
| COPD (%) | 0 | 5.7 | 0 | 5.6 | 0 | 6.0 | 0.300 |
| Orthopaedic disease (%) | 0 | 4.7 | 0 | 4.7 | 0 | 4.7 | 0.899 |
| Stroke (%) | 0 | 1.3 | 0 | 1.3 | 0 | 1.3 | 0.756 |
| Biochemical data at discharge | |||||||
| High BNP level (%) | 44 | 61.6 | 26 | 61.5 | 18 | 61.9 | 0.811 |
| Creatinine (mg/dL) | 1 | 1.08 [0.84–1.42] | 1 | 1.07 [0.84–1.40] | 0 | 1.09 [0.85–1.46] | 0.342 |
| eGFR (mL/min/1.73 m2) | 1 | 47 [34–61] | 1 | 47 [34–62] | 0 | 47 [34–61] | 0.414 |
| Sodium (mEq/L) | 0 | 139 [137–141] | 0 | 139 [137–141] | 0 | 140 [138–141] | 0.221 |
| Albumin (g/dL) | 34 | 3.6 [3.3–3.9] | 23 | 3.6 [3.3–3.9] | 11 | 3.6 [3.3–3.9] | 0.291 |
| Anaemia (%) | 6 | 57.7 | 4 | 57.0 | 2 | 59.0 | |
| hs‐CRP (mg/dL) | 74 | 0.28 [0.10–0.80] | 51 | 0.29 [0.10–0.80] | 23 | 0.26 [0.10–0.80] | 0.702 |
| LVEF at discharge (%) | 58 | 39 | 19 | ||||
| <40% | 38.0 | 38.4 | 37.4 | 0.822 | |||
| 40–50% | 17.6 | 17.3 | 18.2 | ||||
| ≥50% | 44.4 | 44.3 | 44.4 | ||||
| Prescription at discharge | |||||||
| Beta blocker (%) | 0 | 74.6 | 0 | 74.2 | 0 | 75.3 | 0.549 |
| HFrEF (<40%) | 88.7 | 88.1 | 89.9 | 0.371 | |||
| HFpEF (≥50%) | 62.5 | 62.5 | 62.4 | 0.979 | |||
| ACEi/ARB (%) | 0 | 63.0 | 0 | 62.4 | 0 | 64.3 | 0.331 |
| HFrEF (<40%) | 71.1 | 70.8 | 71.7 | 0.762 | |||
| HFpEF (≥50%) | 56.7 | 55.3 | 59.5 | 0.160 | |||
| MRA (%) | 0 | 42.1 | 0 | 42.7 | 0 | 40.9 | 0.377 |
| HFrEF (<40%) | 52.6 | 52.5 | 52.9 | 0.900 | |||
| HFpEF (≥50%) | 32.9 | 33.7 | 31.5 | 0.432 | |||
| ≥1 of ACEi, ARB, or MRA (%) | 0 | 76.8 | 0 | 76.6 | 0 | 77.2 | 0.725 |
| HFrEF (<40%) | 85.3 | 84.9 | 86.1 | 0.588 | |||
| HFpEF (≥50%) | 69.3 | 69.0 | 69.8 | 0.789 | |||
| Diuretic (%) | 0 | 81.3 | 0 | 81.5 | 0 | 80.9 | 0.710 |
| Oral inotropic agent (%) | 0 | 11.2 | 0 | 11.1 | 0 | 11.2 | 0.934 |
| Statin (%) | 0 | 37.4 | 0 | 36.2 | 0 | 39.6 | 0.087 |
| Anticoagulant (%) | 0 | 47.8 | 0 | 48.0 | 0 | 47.3 | 0.715 |
| MMSE (points) | 51 | 27 [25–30] | 29 | 27 [25–29] | 22 | 27 [25–30] | 0.594 |
| Depression (%) | 8 | 41.1 | 4 | 40.6 | 4 | 41.9 | 0.542 |
| BMI (kg/m2) | 0 | 21.9 [19.6–24.6] | 0 | 21.9 [19.6–24.7] | 0 | 21.9 [19.7–24.5] | 0.966 |
| Walking speed (m/s) | 0 | 0.97 [0.78–1.15] | 0 | 0.97 [0.78–1.15] | 0 | 0.97 [0.78–1.15] | 0.721 |
| Grip strength | |||||||
| Men (kg) | 0 | 29.6 [23.9–35.9] | 0 | 29.7 [23.8–36.3] | 0 | 29.3 [24.1–35.3] | 0.555 |
| Women (kg) | 0 | 17 [14.2–20.2] | 0 | 16.8 [14.1–19.9] | 0 | 17.2 [14.2–20.6] | 0.100 |
| PMADL‐8 (points) | 0 | 20 [15–24] | 0 | 20 [15–24] | 0 | 20 [15–24] | 0.999 |
| SEW‐7 (points) | 0 | 18 [13–24] | 0 | 18 [13–24] | 0 | 19 [13–24] | 0.831 |
| Length of stay (days) | 0 | 16 [12–24] | 0 | 16 [12–24] | 0 | 16 [12–26] | 0.163 |
ACEi, angiotensin‐converting enzyme; Af, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; HF, heart failure; HR, heart rate; eGFR, estimated glomerular filtration rate; hs‐CRP, high‐sensitive C‐reactive protein; SBP, systolic blood pressure; LVEF, left ventricular ejection fraction; MMSE, Mini‐Mental State Examination; MRA, mineral corticoid antagonist; PMADL‐8, Performance Measure for Activities of Daily Living‐8; SEW‐7, Self‐Efficacy for Walking‐7.
High BNP level was defined as BNP ≥ 200 pg/mL or N‐terminal pro BNP ≥ 900 pg/mL. Anaemia is defined as haemoglobin <13 g/dL for men and <12 g/dL for women. Depression is defined as 5‐item Geriatric Depression Scale ≥ 2 points
Results of the ROC curve analysis conducted in the derivation cohort are summarized in Table 2. The AUCs for predicting the primary endpoint by frailty items were 0.60–0.63 except BMI. The cut‐off point for each physical frailty domain was as follows: usual walking speed, <0.98 m/s; grip strength, <30.0 kg (men) or 17.5 kg (women); PMADL‐8, ≥21 points; SEW‐7, ≤20 points; and BMI, <20.8 kg/m2. Because of the the low predictive accuracy of BMI for the prognosis (AUC: 0.54), we excluded BMI from subsequent analyses for proposing a frailty‐based prognostic score. Results of the Cox proportional hazards model, including these four items, are shown in Table 2. The score corresponding to the adjusted coefficient for each domain was assigned as follows: slowness, 4 points; weakness, 5 points; exhaustion, 2 points; and low physical activity, 3 points. As a result, the total frailty score summing up four items ranged from 0 to 14 points. The distribution of the score is shown in Table S3.
Table 2.
Development of frailty score based on the ROC curve analysis and the Cox proportional hazards model
| Domain | Indicator | ROC curve analysis | Cox proportional hazards model | Score | ||
|---|---|---|---|---|---|---|
| AUC (95% CI) | Cut‐off | Coefficient (95% CI) | P | |||
| Weakness | Grip strength | Men: 0.61 (0.58–0.65) Women: 0.62 (0.57–0.66) | Men: <30.0 kg Women: <17.5 kg | 0.53 (0.34–0.72) | <0.001 | 5 |
| Slowness | Walking speed | 0.63 (0.60–0.65) | <0.98 m/s | 0.39 (0.18–0.59) | <0.001 | 4 |
| Physical inactivity | SEW‐7 | 0.62 (0.59–0.64) | ≤20 points | 0.27 (0.05–0.48) | 0.004 | 3 |
| Exhaustion | PMADL‐8 | 0.60 (0.58–0.63) | ≥21 points | 0.17 (−0.02–0.36) | 0.081 | 2 |
| Weight loss | BMI | 0.54 (0.52–0.57) | <20.8 kg/m2 | — | — | — |
AUC, area under the curve; BMI, body mass index; CI confidence interval; ROC, receiver operating characteristic; PMADL‐8, Performance Measure for Activities of Daily Living‐8; SEW‐7, Self‐Efficacy for Walking‐7.
Based on the distribution of the score and each domain's weight, frailty was classified into four categories as follows: Category I, ≤3 points; Category II, 4–8; Category III, 9–12; and Category IV, 14. The score of 13 points did not exist in calculation. The patients with ≤3 points met only 0–1 domain except for two strong domains, and the patients with 4–8 points met either of the two strong domains, or two of three domains except for weakness. Thus, we classified patients with ≤3 points into Category I, and those with 4–8 points into Category III, which correlated to a state in which an HF‐specific symptom or activity domain coexisted with a strength domain of either slowness or weakness. We defined the patients with ≥9 points as Category III because they met either of the two strength domains, that is, slowness plus weakness, or three of the four domains, that is, slowness plus two other symptom and activity domains. Additionally, to grade further severity, patients who met all four domains (14 points) were defined as category IV. Based on the aforementioned definition, the prevalence of categories I to IV in the derivation cohort was 27.4%, 25.2%, 26.4%, and 21.0%, respectively. The agewise prevalence of each category is presented in Figure 2. Patient characteristics according to frailty status are shown in Table 3.
Figure 2.

Prevalence of each frailty category according to age.
Table 3.
Patient characteristics according to the categorized physical frailty in the derivation cohort
| Category I (n = 487) | Category II (n = 448) | Category III (n = 469) | Category IV (n = 374) | P | |
|---|---|---|---|---|---|
| Age (years) | 66 [57–74] | 73 [67–80] | 80 [73–84] | 83 [77–87] | <0.001 |
| Men (%) | 76.2 | 67.0 | 51.6 | 43.9 | <0.001 |
| Past HF hospitalization | 20.3 | 27.0 | 33.5 | 40.4 | <0.001 |
| SBP at discharge | 110 [99–122] | 111 [99–125] | 112 [101–126] | 112 [101–126] | 0.077 |
| DBP at discharge | 66 [58–75] | 63 [56–72] | 62 [53–71] | 61 [54–70] | <0.001 |
| HR at discharge | 74 [65–84] | 74 [64–84] | 71 [63–80] | 73 [65–81] | 0.036 |
| Main aetiology | |||||
| Ischemic (%) | 25.9 | 26.6 | 25.8 | 27.8 | <0.001 |
| Arrhythmic (%) | 16.6 | 16.3 | 17.1 | 21.1 | |
| Valvular (%) | 11.7 | 17.4 | 20.3 | 21.4 | |
| Cardiomyopathy (%) | 21.6 | 12.9 | 12.4 | 7.0 | |
| Hypertensive (%) | 11.1 | 11.4 | 11.3 | 9.1 | |
| Others (%) | 13.1 | 15.4 | 13.2 | 13.6 | |
| Af at hospitalization (%) | 34.5 | 36.4 | 36.0 | 38.2 | 0.660 |
| Comorbidities | |||||
| Diabetes (%) | 31.8 | 35.3 | 37.5 | 39.8 | 0.084 |
| Cardiac surgery (%) | 8.0 | 11.6 | 15.8 | 14.4 | 0.002 |
| Cancer (%) | 2.3 | 5.4 | 7.0 | 8.0 | 0.002 |
| COPD (%) | 4.5 | 5.6 | 9.6 | 9.4 | <0.001 |
| Orthopaedic disease (%) | 1.4 | 2.7 | 4.1 | 12.3 | <0.001 |
| Stroke (%) | 0 | 0.9 | 2.8 | 1.9 | 0.002 |
| Biochemical data at discharge | |||||
| High BNP level (%) | 51.1 | 60.2 | 68.3 | 67.8 | <0.001 |
| Creatinine (mg/dL) | 0.99 [0.83–1.23] | 1.11 [0.85–1.45] | 1.09 [0.84–1.46] | 1.16 [0.86–1.55] | <0.001 |
| eGFR (mL/min/1.73 m2) | 57 [45–66] | 48 [36–62] | 43 [32–57] | 40 [29–54] | <0.001 |
| Sodium (mEq/L) | 140 [138–141] | 140 [137–141] | 139 [137–141] | 139 [137–141] | 0.080 |
| Anaemia (%) | 36.5 | 54.9 | 65.8 | 75.3 | <0.001 |
| Albumin (g/dL) | 3.8 [3.5–4.0] | 3.6 [3.3–3.9] | 3.5 [3.2–3.8] | 3.4 [3.2–3.7] | <0.001 |
| hs‐CRP (mg/dL) | 0.27 [0.10–0.70] | 0.26 [0.10–0.78] | 0.31 [0.10–0.95] | 0.30 [0.11–0.91] | 0.370 |
| LVEF (%) | |||||
| <40% | 49.8 | 41.4 | 31.2 | 28.8 | <0.001 |
| 40–50 | 19.8 | 16.4 | 16.4 | 17.8 | |
| >50% | 30.4 | 42.3 | 52.4 | 53.4 | |
| Medications | |||||
| Beta blocker (%) | 81.5 | 78.1 | 71.6 | 63.4 | <0.001 |
| HFrEF (<40%) | 92.8 | 92.9 | 87.1 | 69.6 | <0.001 |
| HFpEF (≥50%) | 64.6 | 64.5 | 62.2 | 58.3 | 0.545 |
| ACEi/ARB (%) | 72.5 | 63.4 | 56.3 | 55.6 | <0.001 |
| HFrEF (<40%) | 78.8 | 73.6 | 65.3 | 54.9 | <0.001 |
| HFpEF (≥50%) | 65.3 | 55.9 | 48.6 | 55.7 | 0.016 |
| MRA (%) | 48.3 | 43.3 | 41.8 | 35.8 | 0.004 |
| HFrEF (<40%) | 55.9 | 52.8 | 53.1 | 43.1 | 0.193 |
| HFpEF (≥50%) | 34.7 | 32.3 | 35.6 | 32.0 | 0.822 |
| ≥1 of ACEi, ARB, or MRA (%) | 85.0 | 78.6 | 71.2 | 70.1 | <0.001 |
| HFrEF (<40%) | 91.5 | 87.4 | 79.6 | 72.6 | <0.001 |
| HFpEF (≥50%) | 75.7 | 69.9 | 65.2 | 68.0 | 0.183 |
| Diuretic (%) | 78.2 | 80.1 | 81.4 | 87.4 | 0.005 |
| Oral inotropic agent (%) | 9.2 | 10.0 | 11.1 | 15.0 | 0.049 |
| Statin (%) | 37.8 | 38.4 | 36.0 | 31.8 | 0.174 |
| Anticoagulant (%) | 45.2 | 50.7 | 47.1 | 49.7 | 0.470 |
| MMSE (points) | 29 [27–30] | 28 [25–30] | 26 [24–29] | 26 [24–28] | <0.001 |
| Depression (%) | 27.0 | 32.4 | 45.4 | 62.2 | <0.001 |
| BMI (kg/m2) | 23.1 [20.7–26.0] | 21.9 [19.8–24.7] | 21.48746 | 20.9 [18.5–23.5] | <0.001 |
| Walking speed (m/s) | 1.21 [1.11–1.32] | 1.05 [0.93–1.16] | 0.84 [0.71–0.94] | 0.69 [0.54–0.82] | <0.001 |
| Grip strength | |||||
| Men (kg) | 37.2 [33.5–41.8] | 28.4 [25.1–33.2] | 24.9 [20.3–29.1] | 22.0 [18.2–25.3] | <0.001 |
| Women (kg) | 22.0 [19.5–25.5] | 18.7 [16.8–21.1] | 16.7 [14.1–18.9] | 14.0 [11.3–15.7] | <0.001 |
| PMADL‐8 (points) | 15 [11–18] | 17 [14–20] | 21 [18–24] | 25 [23–28] | <0.001 |
| SEW‐7 (points) | 25 [22–29] | 21 [16–25] | 16 [13–21] | 11 [8–15] | <0.001 |
| Length of stay (days) | 16 [11–22] | 16 [12–26] | 17 [12–25] | 18 [13–28] | <0.001 |
ACEi, angiotensin‐converting enzyme; Af, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; HF, heart failure; HR, heart rate; eGFR, estimated glomerular filtration rate; hs‐CRP, high‐sensitive C‐reactive protein; SBP, systolic blood pressure; LVEF, left ventricular ejection fraction; MMSE, Mini‐Mental State Examination; MRA, mineral corticoid antagonist; PMADL‐8, Performance Measure for Activities of Daily Living‐8; SEW‐7, Self‐Efficacy for Walking‐7.
Anaemia is defined as haemoglobin < 13 g/dL for men and <12 g/dL for women. High BNP level was defined as BNP ≥ 200 pg/mL or N‐terminal pro BNP ≥ 900 pg/mL. Depression is defined as 5‐item Geriatric Depression Scale ≥ 2 points.
Figure 3 shows the survival analysis in the derivation cohort. Event‐free rates continuously decreased according to the severity of frailty in all study outcomes. Results of subgroup analysis based on HF type are presented in Figure S2. Physical frailty was significantly associated with increased risk of the composite outcome among all subgroups. The Cox proportional hazards model was used in the derivation cohort to assess the independent relationship between physical frailty and study outcomes (Table 4). Even after adjusting for potential confounders, frailty Category III and Category IV were statistically significantly associated with the composite outcome (hazard ratio [95% confidence interval] in reference to Category I: Category II, 1.51 [0.84–2.72], P = 0.169; Category III, 2.37 [1.32–4.23], P = 0.004; and Category IV, 2.66 [1.45–4.89], P = 0.002).
Figure 3.
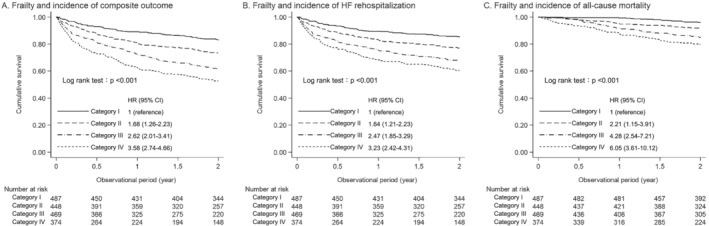
Kaplan–Meier curves for the composite outcome, HF rehospitalization, and all‐cause mortality according to frailty status. HF, heart failure; HR, hazard ratio.
Table 4.
Results of multivariate Cox proportional hazards model in the derivation cohort
| Variables | Hazard ratio | 95% Confidence interval | P |
|---|---|---|---|
| Frailty category I | 1 (reference) | — | — |
| Frailty category II | 1.51 | [0.84–2.72] | 0.169 |
| Frailty category III | 2.37 | [1.32–4.23] | 0.004 |
| Frailty category IV | 2.66 | [1.45–4.89] | 0.002 |
| Age, per 1 SD | 1.04 | [0.83–1.31] | 0.730 |
| Women | 0.84 | [0.61–1.14] | 0.266 |
| Past HF hospitalization | 2.51 | [1.83–3.43] | <0.001 |
| SBP at discharge, per 1 SD | 1.08 | [0.91–1.27] | 0.376 |
| HR at discharge, per 1 SD | 1.08 | [0.94–1.25] | 0.282 |
| Diabetes | 1.24 | [0.91–1.68] | 0.167 |
| Past cardiac surgery | 0.97 | [0.66–1.43] | 0.896 |
| Cancer | 1.21 | [0.76–1.91] | 0.419 |
| COPD | 1.17 | [0.68–2.00] | 0.567 |
| Orthopaedic disease | 0.84 | [0.45–1.57] | 0.585 |
| Stroke | 0.44 | [0.13–1.42] | 0.171 |
| High BNP level | 1.74 | [1.19–2.54] | 0.004 |
| eGFR, per 1 SD | 0.80 | [0.67–0.95] | 0.013 |
| Sodium, per 1 SD | 0.91 | [0.79–1.04] | 0.186 |
| Anaemia | 1.19 | [0.84–1.68] | 0.334 |
| Albumin, per 1 SD | 0.89 | [0.77–1.04] | 0.140 |
| LVEF < 40% | 1.05 | [0.75–1.48] | 0.775 |
| Beta blocker | 0.98 | [0.70–1.37] | 0.907 |
| ACEi/ARB | 0.82 | [0.60–1.10] | 0.181 |
| MRA | 1.15 | [0.85–1.57] | 0.367 |
| Diuretic | 1.42 | [0.89–2.26] | 0.138 |
| Oral inotropic agent | 1.50 | [1.03–2.19] | 0.036 |
| MMSE, per 1 SD | 0.91 | [0.84–0.99] | 0.049 |
| Depression | 1.30 | [0.97–1.76] | 0.081 |
| BMI, per 1 SD | 0.85 | [0.70–1.13] | 0.096 |
ACEi, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, heart rate; LVEF, left ventricular ejection fraction; MMSE, Mini‐Mental State Examination; MRA, mineral corticoid antagonist; SBP, systolic blood pressure.
Anaemia is defined as haemoglobin < 13 g/dL for men and <12 g/dL for women. High BNP level was defined as BNP ≥ 200 pg/mL or N‐terminal pro BNP ≥ 900 pg/mL. Depression is defined as 5‐item Geriatric Depression Scale ≥ 2 points.
The prevalence of Categories I to IV in a validation cohort were 29.5%, 23.2%, 25.8%, and 21.5%, respectively. The validation of our frailty category is summarized in Figure 4, which shows the cumulative incidence rates of the study outcomes according to each cohort. The cumulative incidence in each frailty category was equivalent between the two cohorts, regardless of the type of outcome.
Figure 4.
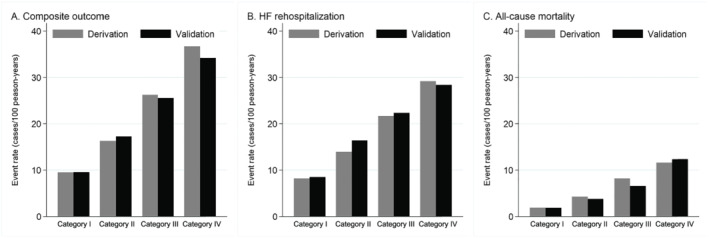
Cumulative incidence of the study outcomes according to frailty status in the deviation cohort and the validation cohort. HF, heart failure.
Discussion
We developed and validated a prognostic score based on physical frailty among Japanese patients with HF using data recorded in a multicentre prospective cohort study. To the best of our knowledge, this is the first large‐scale study to demonstrate the cut‐off points for frailty domains according to the outcome‐based analysis specific to HF. The frailty‐based prognostic score derived from quantitative measures will contribute to the standardization of frailty assessment, the assessment of appropriate clinical intervention, and improved quality of long‐term management for HF.
Physical frailty is estimated to be a comorbidity in 40% (95% confidence interval 31–48%) of patients with HF, 2 leading to poor prognosis. 3 Because the conventional frailty assessment methods were not developed based on clinical outcomes of HF, we aimed to propose prognostic score using frailty domains in patients hospitalized for HF based on statistical estimation. As a result, when patients were categorized into four groups based on the frailty score, severe frailty categories presented a worse prognosis, suggesting the clinical usefulness of our frailty score for risk stratification.
A notable finding of this study was the differences in the predictive effects of frailty domains on prognosis. Conventional frailty assessments determine the severity of frailty using the number of frailty domains. However, there is enough room to discuss the appropriateness of the counting domain method because the weight of each domain has not been examined. Therefore, we used the multivariate Cox proportional hazards model to estimate the relative predictive effect of each domain. We found a different weightage for each domain when predicting prognosis after discharge, and this suggests that the appropriate score should be assigned to each domain for risk stratification. Out of the four domains, weakness and slowness were identified as the two strong factors with 5 and 4 points, respectively. The two‐fold higher weight of the objective items compared with the questionnaire‐measured items indicates the importance of an objective frailty assessment for precise risk stratification. However, exhaustion and low physical activity measured using questionnaires can be used to further categorize physical frailty. Indeed, patients categorized into the severe frailty group who met all the four domains showed worse prognosis than those with a lower grade of frailty. The prognostic capability of the PMADL‐8 in patients with HF was demonstrated in our previous study. 26 Additionally, daily physical activity, assessed using the SEW‐7 in this study, has been reported to be associated with HF prognosis. 27 Therefore, frailty assessment in this study consisted of a comprehensive set of indicators suitable for patients with HF, resulting in the good prognostic relevance.
Responsiveness of our frailty score for intervention and its impact on the prognosis of HF is a topic for future studies. In this cohort study, we used validated questionnaires to evaluate exhaustion and physical inactivity that was assessed using subjective questionnaires of ‘yes’ or ‘no’ answers in previous studies. Accordingly, all the frailty domains were evaluated using objective measures, and each cut‐off point was identified by the outcome‐based analysis specific to HF. Therefore, the frailty score may be modified by clinical intervention, and the decreasing score could be associated with improved prognosis, although this cannot be addressed in the present study. If this hypothesis is clarified, the frailty score in this study may serve as an assessment method to plan clinical interventions when managing HF as well as physical frailty.
Regarding clinical application, although frailty is an independent predictor of worse prognosis, frailty should be assessed along with other known prognostic indicators for appropriate risk stratification. As shown in Figure 2, even patients assigned to the non‐frailty group experienced the composite outcome with a cumulative incidence of 10/100 person‐years, indicating the limited predictive capability of frailty alone. The results of the Cox proportional hazards model showed that several factors were associated with the composite outcome independent of physical frailty, such as history of prior hospitalization due to HF, high level of brain natriuretic peptide, and the use of an inotropic agent. Moreover, a decline in renal function and low cognitive function was associated with the increased outcome. These results indicate that in clinical practice, frailty must be assessed along with HF status and comorbidities associated with the poor prognosis.
Guideline‐directed medical therapy may be another key factor for frailty management of HFrEF. In this study, the prescription rates of standard medications were lower in severely frail patients with HFrEF (Table 3). The lower prescription in severely frail patients may be caused by orthostatic hypotension, a major geriatric syndrome closely associated with frailty. 28 Renal dysfunction is another condition that often presents as comorbidity among frail patients and can prevent clinicians from prescribing standard medications before discharge. Therefore, as the guidelines have recommended, 29 a multidisciplinary care plan needs to function for up‐titration and monitoring of pharmacological therapy for HFrEF after discharge. The relationship between the dosages of standard medications for HFrEF and the change of frailty state will be a topic for future studies.
Low event rates in our population should also be discussed here. The cumulative rate for the composite events during the 2 year observation period was 31.0%, which is less than previous reports. 30 The low event rate may be partly due to the inclusion or exclusion criteria. We included patients capable of walking at discharge according to a state of the less functional reserve but not a state of disability. 1 Additionally, our cohort included patients aged < 65 years and excluded patients with assumed short‐term prognosis or severe cognitive decline. Another possible explanation is the favourable prognosis of patients with HF in Japan. Registry data in Japan have demonstrated that the prognosis of chronic HF has significantly improved since 2000, showing that the 3 year mortality of patients enrolled after 2006 was 15% (5%/year), 31 which seemed much better than that documented in the Western countries. 32 Further, the increase of the prescription rate of standard medications may have contributed to the event rate in this study. For example, in patients with HFrEF, approximately 90% were prescribed beta‐blockers, and 85% were prescribed angiotensin‐converting‐enzyme inhibitors, angiotensin receptor blockers, or mineralocorticoid receptor antagonists at the time of discharge. Due to the reasons presented above, generalization of the findings of this study in Japanese ambulatory patients with HF is acceptable.
Several limitations of this study need to be discussed when interpreting our findings. First, because our frailty category was validated in only the randomly sampled subgroup of the FLAGSHIP cohort, external validity may have to be confirmed. Second, although we adjusted for several confounding factors, the possibility of residual and unmeasured confounding factors could not be completely ruled out because of the nature of the cohort study. Third, the generalizability of the frailty category may be limited to the Asian population of patients because of racial and cultural differences and variations in the prognosis of HF. Furthermore, when applying our definition to other regions with different cultures, the PMADL‐8 may need to be modified to fit their culture‐oriented lifestyle. Functional limitation items used in the HeartQOL questionnaire authorized by the European Society of Cardiology 33 may be a suitable alternative that could be used to replace the PMADL‐8. Finally, although our frailty category is based on a longitudinal analysis, causal relationships between physical frailty and prognosis have yet to be elucidated. Nevertheless, this large‐scale multicentre prospective cohort with few missing data has good qualities for developing HF‐specific frailty category. The causal relationship between physical frailty and prognosis in patients with HF will need to be addressed in future studies.
Conclusions
The findings of this study demonstrated that slowness and weakness were particularly strong factors and that stratification with the two factors and exhaustion and physical inactivity was associated with the prognosis in patients with HF. Thus, the prognostic score proposed in this study will serve as a tool for risk stratification or planning of therapeutic strategies in patients with HF.
Funding
This study is supported by a Grant‐in‐Aid for Scientific Research (A) from the Japan Society for the Promotion of Science (16H01862).
Conflicts of interest
S.Y. has received lecture fees from Tanabe‐Mitsubishi, Abbott‐Japan, Daiichi Sankyo, Toa Eiyo, Otsuka, and Novartis Pharma, as well as collaborative research grants from Epson Kenpokumiai and Minato Medical Science for works outside the submitted manuscript. The other authors have no conflict of interest to disclose.
Author contributions
S.Y., T.A., H.I., T.M., and T.K. contributed to the conception and design of the work. S.Y. and T.A. contributed to the acquisition, analysis, and interpretation of data for the work. T.A. drafted the manuscript. All authors critically revised the manuscript, gave final approval, and agree to be accountable for all aspects of work, ensuring integrity and accuracy.
Supporting information
Figure S1. Kaplan–Meier curves for the composite outcome, HF rehospitalization, and all‐cause mortality in the deviation cohort and the validation cohort.
HF, heart failure.
Figure S2. Kaplan–Meier curves for the composite outcome and HF rehospitalization according to the subgroups of HF.
HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction.
Table S1. Performance Measure for Activities of Daily Living‐8.
Table S2. Self‐Efficacy for Walking‐7.
Table S3. Distribution of frailty scoreS.
Acknowledgements
We are grateful to all of the patients for cooperation with the study and also thank all collaborating investigators for their contributions. The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. 34
Yamada S., Adachi T., Izawa H., Murohara T., Kondo T., and FLAGSHIP collaborators (2021) Prognostic score based on physical frailty in patients with heart failure: a multicenter prospective cohort study (FLAGSHIP), Journal of Cachexia, Sarcopenia and Muscle, 12, 1995–2006, 10.1002/jcsm.12803
References
- 1. Morley JE, Vellas B, van Kan GA, Anker S, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marengoni A, Zucchelli A, Vetrano DL, Aloisi G, Brandi V, Ciutan M, et al. Heart failure, frailty, and pre‐frailty: a systematic review and meta‐analysis of observational studies. Int J Cardiol 2020;316:161–171. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Yuan M, Gong M, Tse G, Li G, Liu T. Frailty and clinical outcomes in heart failure: a systematic review and meta‐analysis. J Am Med Dir Assoc 2018;19:1003–1008.e1. [DOI] [PubMed] [Google Scholar]
- 4. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 5. Rockwood K, Song X, MacKnight C, Bergman H, Hogan D, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shimada H, Makizako H, Doi T, Yoshida D, Tsutsumimoto K, Anan Y, et al. Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J Am Med Dir Assoc 2013;14:518–524. [DOI] [PubMed] [Google Scholar]
- 7. Warraich HJ, Kitzman DW, Whellan DJ, Duncan PW, Mentz RJ, Pastva AM, et al. Physical function, frailty, cognition, depression, and quality of life in hospitalized adults ≥60 years with acute decompensated heart failure with preserved versus reduced ejection fraction. Circ Heart Fail 2018;11:e005254, 10.1161/CIRCHEARTFAILURE.118.005254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hornsby WE, Sareini MA, Golbus JR, Willer CJ, McNamara JL, Konerman MC, et al. Lower extremity function is independently associated with hospitalization burden in heart failure with preserved ejection fraction. J Card Fail 2019;25:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ogawa M, Satomi‐Kobayashi S, Yoshida N, Tsuboi Y, Komaki K, Nanba N, et al. Relationship between oral health and physical frailty in patients with cardiovascular disease. J Cardiol 2019;2020:131–138. [DOI] [PubMed] [Google Scholar]
- 10. Yamada S, Adachi T, Izawa H, Murohara T, Kondo T, FLAGSHIP collaborators . A multicenter prospective cohort study to develop frailty‐based prognostic criteria in heart failure patients (FLAGSHIP): rationale and design. BMC Cardiovasc Disord 2018;18:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 12. Shimizu Y, Yamada S, Suzuki M, Miyoshi H, Kono Y, Izawa H, et al. Development of the performance measure for activities of daily living‐8 for patients with congestive heart failure: a preliminary study. Gerontology 2010;56:459–466. [DOI] [PubMed] [Google Scholar]
- 13. Kop WJ, Appels APWM, De Leon CFM, Bär FW. The relationship between severity of coronary artery disease and vital exhaustion. J Psychosom Res 1996;40:397–405. [DOI] [PubMed] [Google Scholar]
- 14. Kono Y, Yamada S, Iwatsu K, Nitobe S, Tanaka Y, Shimizu Y, et al. Predictive value of functional limitation for disease severity in patients with mild chronic heart failure. J Cardiol 2012;60:411–415. [DOI] [PubMed] [Google Scholar]
- 15. The Criteria Committee of the New York Heart Association . Functional capacity and objective assessment. In Dolgin M, ed. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels, 9th ed; 1994. p 253–256. [Google Scholar]
- 16. Kawajiri H, Adachi T, Kono Y, Yamada S. Development of a self‐efficacy questionnaire for walking in patients with mild ischemic stroke. J Stroke Cerebrovasc Dis 2019;28:317–324. [DOI] [PubMed] [Google Scholar]
- 17. Ogilvie D, Foster CE, Rothnie H, Cavill N, Hamilton V, Fitzsimons CF, et al. Interventions to promote walking: systematic review. Br Med J 2007;334:1204–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bandura A, Freeman WH, Lightsey R. Self‐efficacy: the exercise of control. J Cogn Psychother 1999;13:158–166. [Google Scholar]
- 19. O'Connor CM, Abraham WT, Albert NM, Clare R, Stough WG, Gheorghiade M, et al. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF). Am Heart J 2008;156:662–673. [DOI] [PubMed] [Google Scholar]
- 20. Sokoreli I, de Vries JJG, Pauws SC, Steyerberg EW. Depression and anxiety as predictors of mortality among heart failure patients: systematic review and meta‐analysis. Heart Fail Rev 2016;21:49–63. [DOI] [PubMed] [Google Scholar]
- 21. Ruigómez A, Michel A, Martín‐Pérez M, García Rodríguez LA. Heart failure hospitalization: an important prognostic factor for heart failure re‐admission and mortality. Int J Cardiol 2016;220:855–861. [DOI] [PubMed] [Google Scholar]
- 22. Hoyl MT, Alessi CA, Harker JO, Josephson KR, Pietruszka FM, Koelfgen M, et al. Development and testing of a five‐item version of the Geriatric Depression Scale. J Am Geriatr Soc 1999;47:873–878, http://www.ncbi.nlm.nih.gov/pubmed/10404935 [DOI] [PubMed] [Google Scholar]
- 23. Preventive Committee for Japanese Heart Failure Society 2021 Points to consider when using BNP and NT‐pro BNP levels in the blood for the diagnosis and treatment of heart failure http://www.asas.or.jp/jhfs/topics/bnp201300403.html. Accessed March 17, 2021.
- 24. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 25. Barnard J, Rubin DB. Miscellanea. Small‐sample degrees of freedom with multiple imputation. Biometrika 1999;86:948–955. [Google Scholar]
- 26. Yamada S, Shimizu Y, Suzuki M, Izumi T. Functional limitations predict the risk of rehospitalization among patients with chronic heart failure. Circ J 2012;76:1654–1661. [DOI] [PubMed] [Google Scholar]
- 27. Mediano MFF, Leifer ES, Cooper LS, Keteyian SJ, Kraus WE, Mentz RJ, et al. Influence of baseline physical activity level on exercise training response and clinical outcomes in heart failure: the HF‐ACTION trial. JACC Hear Fail 2018;6:1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liguori I, Russo G, Coscia V, Aran L, Bulli G, Curcio F, et al. Orthostatic hypotension in the elderly: a marker of clinical frailty? J Am Med Dir Assoc 2018;19:779–785. [DOI] [PubMed] [Google Scholar]
- 29. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 30. Konishi M, Ishida J, Springer J, von Haehling S, Akashi YJ, Shimokawa H, et al. Heart failure epidemiology and novel treatments in Japan: facts and numbers. ESC Hear Fail 2016;3:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ushigome R, Karasawa K, Taniguchi K, Ichikawa R, Fukuhara J, Abe O, et al. Temporal trends in clinical characteristics, management and prognosis of patients with symptomatic heart failure in Japan—report from the CHART studies. Circ J 2015;79:2396–2407. [DOI] [PubMed] [Google Scholar]
- 32. Canepa M, Straburzynska‐Migaj E, Drozdz J, Fernandez‐Vivancos C, Pinilla JM, Nyolczas N, et al. Characteristics, treatments and 1‐year prognosis of hospitalized and ambulatory heart failure patients with chronic obstructive pulmonary disease in the European Society of Cardiology Heart Failure Long‐Term Registry. Eur J Heart Fail 2018;20:100–110. [DOI] [PubMed] [Google Scholar]
- 33. Oldridge N, Höfer S, McGee H, Conroy R, Doyle F, Saner H. The HeartQoL: part II. validation of a new core health‐related quality of life questionnaire for patients with ischemic heart disease. Eur J Prev Cardiol 2014;21:98–106. [DOI] [PubMed] [Google Scholar]
- 34. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan–Meier curves for the composite outcome, HF rehospitalization, and all‐cause mortality in the deviation cohort and the validation cohort.
HF, heart failure.
Figure S2. Kaplan–Meier curves for the composite outcome and HF rehospitalization according to the subgroups of HF.
HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction.
Table S1. Performance Measure for Activities of Daily Living‐8.
Table S2. Self‐Efficacy for Walking‐7.
Table S3. Distribution of frailty scoreS.


