Abstract
Myopia is far beyond its inconvenience and represents a true, highly prevalent, sight-threatening ocular condition, especially in Asia. Without adequate interventions, the current epidemic of myopia is projected to affect 50% of the world population by 2050, becoming the leading cause of irreversible blindness. Although blurred vision, the predominant symptom of myopia, can be improved by contact lenses, glasses or refractive surgery, corrected myopia, particularly high myopia, still carries the risk of secondary blinding complications such as glaucoma, myopic maculopathy and retinal detachment, prompting the need for prevention. Epidemiological studies have reported an association between outdoor time and myopia prevention in children. The protective effect of time spent outdoors could be due to the unique characteristics (intensity, spectral distribution, temporal pattern, etc.) of sunlight that are lacking in artificial lighting. Concomitantly, studies in animal models have highlighted the efficacy of light and its components in delaying or even stopping the development of myopia and endeavoured to elucidate possible mechanisms involved in this process. In this narrative review, we (1) summarize the current knowledge concerning light modulation of ocular growth and refractive error development based on studies in human and animal models, (2) summarize potential neurobiological mechanisms involved in the effects of light on ocular growth and emmetropization and (3) highlight a potential pathway for the translational development of noninvasive light-therapy strategies for myopia prevention in children.
Keywords: animal models, dopamine, light, myopia, neurobiology, outdoor activity
Introduction
Myopia results from a mismatch between the axial length of the eye and the power of its refractive components leading images to be focussed in front of the retina and causing blurred vision at distance. 1 The prevalence of myopia exhibits wide geographical variations in the world. In developed nations, the prevalence of the condition among adults ranges from 15% to 49%, 2 and rises up to approximately 69% in 15-year-olds under cycloplegia. 3 In developing countries, the rate of myopia in 15-year-old adolescents is much lower, between 14.7% and 16.2% in Colombia and 5.5% in Africa.3–5 Singapore and East Asian countries like China, Taiwan, Hong Kong and South Korea are the most affected (Figure 1(a)).6–11 While myopia prevalence ranges from 29% in 7-year-olds to 53.1% in 9-year-olds in the school-based population of the Singapore Cohort Study of Risk factors for Myopia (SCORM) (Figure 1(b)), 2 it can reach 69% in 15 years of age with 86% of affected population being Singaporean-Chinese. 3 In young adults, the prevalence of myopia is even higher with 82.3% of Chinese male military conscripts having myopia and 13.1% high myopia. This is particularly worrying as patients with high myopia [more than −5 Dioptres (D)] are at risk of developing pathologic myopia and other associated sight-threatening ocular conditions such as glaucoma, retinal detachment, myopic macular degeneration and choroidal neovascularization.12,13 Pathologic myopia is a major cause of visual impairment and blindness in Asian populations. 14 The risk of developing high myopia increases drastically with the early onset and progression,15,16 especially in Asian populations where myopia progresses faster. 17 Although the precise mechanisms of myopia onset and progression are not completely understood, it is admitted that it involves multiple genetic and environmental factors.
Figure 1.
Average myopia prevalence: (a) in young adults of East Asian Countries during 2012–2020 and (b) in Singapore across different age groups during 1999–2001.
Genetic factors have been mainly related with the finding that children with myopia have myopic parents.7,18,19 Genome-wide association studies (GWAS) and whole-exome sequencing studies on European populations have identified over 200 genetic loci associated with refractive error and myopia.20,21 Genome-wide meta-analysis for refractive error on European and Asian cohorts revealed 24 additional loci (BICC1, BMP2, BMP3, CACNA1D, CD55, CHD7, CHRNG, CNDP2, GRIA4, CYP26A1, GJD2, KCNJ2, KCNQ5, LAMA2, MYO1D, PCCA, PRSS56, RASGRF1, RDH5, RORB, SIX6, TOX, ZIC2 and ZMAT4) associated with myopic features.22 Some of these novel loci are known to be implicated in the development of eye, ion transport, retinoic acid metabolism, neurotransmission and extracellular matrix production. 22 Conversely to the rising myopia prevalence worldwide, genetic predisposition has not significantly changed over the past few decades, which implies that environmental factors, potentially interacting with genetic traits, are mainly at the origin of the ongoing myopia epidemic. 23
Environmental factors influencing myopia onset include, but are not limited to, level of education, near work and time spent outdoors.24–28 Increased time outdoors has been emphasized as an important modifiable environmental factor for myopia control.29,30 Irrespective of physical activity, increased time outdoors is associated with a reduced odds ratio of myopia, even when children perform a high amount of near work.31,32 In addition, Donovan et al. 33 have found myopia progression to be slower during the summer, possibly because of increased outdoor exposure. The exact protective feature(s) of the outdoor environment against myopia are still unclear, but may include variations in accommodation due to uniform dioptric space, increased pupil constriction, increased retinal focus and decreased blur as well as increased spatial frequency and changes in the characteristics of light exposure. 34
While epidemiological studies can only highlight associations between bright light exposure outdoors and myopia prevention,34–38 substantial evidence from animal studies support a protective effect of bright light on experimental myopia development.39–41 In addition, interventional studies in humans have also shown a beneficial effect of both outdoor35,42 and indoor (classroom) exposure 43 to increased but not so intense light levels. According to Rucker, 44 the different patterns in luminance, but also colour contrast, has a significant effect on the refraction and accommodation. In accordance with findings in humans and animal models, a recent meta-analysis of GWAS comprising 160,420 participants of cross ethnicity (European and Asian) revealed 140 genetic associations linked with light-dependent pathways which include genes associated with novel pathways such as anterior-segment morphology (TCF7L2, VIPR2 and MAF) and angiogenesis (FLT1). Furthermore, genes involved in glutamate receptor signalling (GNB3 and CLU) and dopaminergic pathway (DRD1) were identified as key genes in the light-dependent retina-to-sclera signalling cascade potentially controlling ocular growth. 45
In this narrative review, we focus on the literature investigating light-driven modulations of ocular growth and refractive error development in humans and animal models. We will also summarize the current knowledge on neurobiological and photoreceptoral mechanisms involved in the putative effect of light against myopia onset and highlight a potential pathway for the translational development of noninvasive light-therapy strategies to halt or delay myopia onset in children.
Light exposure and myopia in humans
Time outdoors and myopia
Increased time outdoors has been shown to prevent or delay myopia onset in several studies.17,46–50 The protective impact of increased time outdoors against myopia applies even in children performing higher amounts of near work, 17 and is predominantly attributed to intermittent exposure to high levels of sunlight and independent of physical activity.29,30,35,47 Furthermore, increased outdoor time has been shown to have a protective effect on the cumulative incidence rate of myopia in children enrolled in randomized clinical trials in China and Taiwan.29,30,35 On the other hand, increased near-work time and reduced outdoor activities have been suggested to be at the origin of the increased myopia prevalence in older children. 51 In the SCORM study, however, participants who spent more time outdoors were less likely to be myopic while the amount of near work did not predict outdoor activity. Therefore, outdoor activity may be an independent factor and not merely the reciprocal of near work. 46 Interestingly, increases in refractive error progression, axial growth rates and less power loss which occur before myopia onset also seem to be influenced by reduced time spent outdoors. 50 According to Lingham et al., 52 the potential protective effect of outdoor light against myopia is most likely due to one or both of the following factors which are suboptimal in indoor lighting: (1) increased light intensity and (2) favourable spectral composition of light. Although little has been established on the involvement of the spectral composition of light on ocular growth in humans, it is interesting to note that individuals with colour vision red/green colour vision deficiency were reported to be less myopic than individuals with normal colour vision. 53
Bright light and myopia
Epidemiologic research indicates that greater average daily light exposure is associated with a reduced axial elongation during childhood. 38 A study cluster-randomized intervention-controlled trial conducted in Taiwan showed that exposure to outdoor light leads to less myopic shifts, reduced axial elongation and a 54% lower risk of myopia progression. 35 Cross-sectional studies using objective methods (wearable light sensors) to quantify illuminance have shown that Australian myopic children aged 10–15 years had lower average light exposure and lower amount of outdoor time compared with emmetropic children. 54 Furthermore, comparisons between Australian and Singaporean children aged 10–12 years showed that light exposure patterns are of shorter durations and lower intensities in Singaporean children, who tend to have a higher risk of developing myopia. 36 Short exposures of spurts of light in Singaporean children are mostly seen during the periods 9 a.m.–10:30 p.m., 12 p.m.–1 p.m., or 3 p.m.–4 p.m., possibly due to child’s travel time to their school and home in morning and evening. 55 In Australian children, the peaks in outdoor light occur at similar timings, but with greater duration of exposure per hour (10 min or more) when compared with Singaporean Children. 36 Patterns of light exposure are known to be influenced by seasons. Myopia progression and axial length elongation are slower in summer compared with winter.33,56 A study in the United States has shown that children aged 7.6 ± 1.8 years spent more time outdoors during summer time, compared with spring and fall. 57 The light exposure pattern was correlated with the parents’ pattern, suggesting that educational programmes promoting the increase of time outdoors must start with parents.
To date, however, much remains unanswered regarding the characteristics of outdoor light exposure necessary to circumvent myopia in humans. For instance, what is the minimal required outdoor light intensity (threshold) to avoid the myopia onset (e.g. 1000 lux, 10,000 lux)? How long should the exposure to outdoor light be (e.g. 40 min, 2 h per day)? Can the exposure be intermittent or cumulative over time (e.g. 5000 lux for 1 h/day or 1000 lux for 5 h/day)? Addressing these questions in humans, in longitudinal studies using objective wearable light-tracking strategies is essential for the development of effective outdoor programmes against myopia.
Outdoor programmes for myopia prevention
Outdoor preventive measures are vital to control and lower myopia progression in children. For children at risk of developing myopia, preventive interventions should be initiated before the onset of this ocular condition. Trackers that record and quantify light levels 55 and outdoor time have been proposed to encourage outdoor activities among children with daily goal of 2 h per day and at least 14 h per week. 49 According to French et al. 58 Australian children with baseline refraction of + 1D at age 6 years should be targeted as an at-risk group in prevention programmes for myopia, with the goal of maintaining a slightly hyperopic refraction. Intervention in East Asia might need to be earlier than 6 years of age due to the high number of early-onset myopes. According to a meta-analysis performed by Ho et al. 59 on the outdoor research methods in Asian children aged between 4 and 14 years, outdoor exposure slows myopic refraction by 32.9% and axial elongation by 24.9%.
Outdoor programmes in the schools and community can be developed based on the longitudinal data to increase time outdoors. Additional classes involving outdoor activities can be added to each school day or children can be encouraged to go outside for outdoor activities during recess and after school. 59 Organizing community-based outdoor programmes on weekdays may also be beneficial. Importantly, sun-protective strategies such as tree shade, hat and sunglasses can still allow high levels of light to reach the eye and can potentially protect from the myopia development. 60 Nevertheless, given the competitive nature of schooling systems in Asia and sometimes weather and pandemic constraints, increasing time outdoors remains challenging. These restrictions emphasize the need to rethink indoor-based light-therapy strategies for the prevention of myopia. Without a clear understanding of the anatomical, physiological and neurobiological impact of distinct light features (intensity, spectrum, timing, frequency) on ocular growth and myopia development, the development of adequate artificial light-therapy strategies remains challenging.
Experimental research on light and myopia
While epidemiological investigations over many decades highlighted the protective effect of outdoor bright light exposure on myopia, 47 studies in various animal models have scrutinized the impact of various intensities and spectro-temporal modulations of light regimens on ocular growth and refractive error development. 61 These studies, performed in controlled experimental conditions, have tried to elucidate the underlying mechanisms of the protective impact of light against myopia.
Animal models for myopia
The use of animals for studying mechanisms underlying refractive error development dates back to the mid-1970s after Hubel et al., 62 investigating the cortical effects of monocular visual deprivation in young macaques, serendipitously reported that after eyelid suture for many months, the eyes of animals developed high levels of myopia. These findings were afterward established in young tree shrews 63 and chickens. 64 The initial procedures to induce myopia by suturing the eyelids have been replaced with (1) form deprivation myopia (FDM), via reduction of quality (e.g. sharpness and contrast) of retinal image formation using frosted goggles or (2) introducing controlled hyperopic defocus (minus lenses), termed as lens-induced myopia (LIM). Conversely, inducing myopic defocus (plus lenses) leads to lens-induced hyperopia (LIH).
The disruption of visual input, especially retinal sharpness and contrast, is considered to be a significant factor in driving the development of myopia in children, particularly during the early postnatal period.65,66 Within that framework, FDM is presented as an open-loop model, where ocular growth has no defined endpoint. Conversely, LIM relies on feedback control using visual signals and is classified as a closed-loop condition, where aberrant ocular growth ceases when the growth signal has been neutralized. 67 LIM and FDM involve different mechanisms of action where optic nerve section reduces LIM 68 but not FDM,69,70 yet in both processes, the levels of retinal dopamine (DA) or vitreal DA metabolites are reduced,71,72 while DA or its agonists can inhibit myopia induction through stimulation of the D2-receptor.73–76 According to Norton, 77 induced development of myopia, in addition to the normal refractive and ocular development, in most animal species appears to mimic that in human, wherein it is characterized mainly by an abnormal enlargement of the post equatorial segment of the eye with a significant increase in axial length. Irrespective of the animal model, induced refractive error is also characterized by key factors such as ocular vitreous chamber elongation, thinning of the choroid,78,79 and thinning of the fibrous sclera. 80 All these features are also observed in the myopic human eye.81,82
Commonly used animal models in experimental myopia
The most commonly used experimental animal models for myopia research are chickens, guinea pigs, tree shrews, mice and some nonhuman primates (NHP). 83
Chickens The chicken model is the most commonly used model in experimental myopia research, owing to the animals’ rapid eye growth (100 µm per day), diurnal activity, and the reproducibility of experimental paradigms. 83 In addition, the chicken eye is relatively large (8–14 mm), has an excellent optical system and responds quickly to a variety of environmental factors including defocus, blur, and photic stimulations. Despite its unique photoreceptoral complexity, the overall spectral sensitivity to human-visible light in chickens is not very different from humans. 84 Furthermore, differentially expressed genes and proteins involved in either myopia or hyperopia in chickens significantly overlap with those implicated in the pathogenesis of sight-threatening secondary disorders in humans. 85 On the other hand, chickens display many anatomical differences in ocular structures (e.g. cartilaginous and fibrous sclera, lack of fovea, etc.) compared with humans. 86 Furthermore, the well-developed circadian system in chickens is sensitive to constant moderate light intensity and has a significant impact on refractive development. These findings of impact of light on circadian rhythms are not extrapolatable to rhesus monkeys and mice models.87–89 Findings on the impact of light on ocular growth and emmetropization in the chicken model may not be easily/necessarily translatable to humans.
Guinea pigs First presented as a model for experimental myopia by Howlett and McFadden, 90 guinea pigs are diurnal dichromatic mammals with retinas comprising rods, and middle- and short-wavelength cones. The cone proportion in guinea pig retinal photoreceptors is high (8%–17%) in comparison with other species. 91 The guinea pig model has been identified as a convenient model for studying refractive error development, 92 given advantages such as easiness to maintain and breed, in addition to their large eyes (axial length around 8.0 mm) and pupils. Furthermore, these small mammals, respond well to form deprivation 92 and lens-induced defocus. 93 On the other hand, guinea pig retinas lack fovea and the induction of myopia is at times challenging with strain variability. Also, studies requiring lens mounting for long periods of time are challenging as guinea pigs tend to scratch and remove the Velcro base holding the lens.
Tree Shrews Owing to its close association with primates and rodents, tree shrews are widely used for studying refractive error, and understanding neurophysiological mechanisms underlying emmetropization. 78 The ocular morphology of tree shrews is similar to humans; however, these animals lack a fovea, have a thicker lens, and thinner choroid void of choriocapillaries unlike in humans. 77 The tree shrews can develop myopia 94 and can actively compensate for defocus and exhibit a single layer sclera similar to humans. These animals possess dichromatic retinas composed of ~95% of cones. 95
Mouse Given its readily available whole-genome sequence, which is 85% homologous to the human genome, the mouse model has always been a popular model for studying the visual system.96,97 Both FDM and LIM in the mouse can be achieved by mounting diffuser or lens (goggles) to eyes either by means of stitching around the eye and reinforcing with glue or by mounting custom-made assembly to hold the lenses intact. On the other hand, the mouse model lacks a fovea, possesses poor visual aptitudes, and has a small eye (axial length of 3.3 mm) making anatomic assessment troublesome. Nevertheless, under photopic conditions, mice still retain adequate spatial vision to respond to LIM and FDM. 98 Despite the concerns for using the mouse as a model for myopia, it has been established as a useful model for pharmacological and genome manipulation studies in the field of myopia. 99
Rhesus monkeys Among NHPs, rhesus macaques, belonging to the old-world monkeys, constitute one of the most suitable models for refractive error studies. The visual physiology of rhesus monkeys is identical to that of humans with a rod-based retina and a cone-based fovea. 100 Raviola and Wiesel 101 have demonstrated the myopia induction in rhesus macaques. The average axial length of 21-day-old baby rhesus macaques is 14.15 mm, very much close to a human baby which is 17.3 mm. 102 Conversely, ethical concerns, logistics, high operational cost, seasonal breeding, low reproductive rate, difficulties in handling infant monkeys, having a customized myopia-inducing helmets/devices adaptable for monkeys and prolonged experimental procedures to obtain myopic shifts make it more challenging to use rhesus macaques for myopia research.
The impact of light on refractive error development
The protective effect of outdoor light exposure against myopia could be attributed to multiple factors, 17 also including light intensity, pattern, and spectrum but also to reduced peripheral retinal defocus and increased visual spatial frequency. This section summarizes the current knowledge about light modulation and ocular growth based on controlled studies in animal models. Please see Table 1 for more details.
Table 1.
Summary of experimental and observational studies in humans and animal models investigating the impact of light on myopia.
| Findings from key clinical studies and trials | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Methods | Defined light parameters | Key findings | ||||||||
| Study author | Study design | N, age (y) and location | Myopia classification and refraction method | Duration | Intensity | Pattern | Timing | Spectrum/ wavelength | ||
| Interventional studies | Wu et al. 153 | Prospective, interventional study | N = 571, 7–11, Taiwan | ⩽ −0.50D; Cycloplegic autorefraction | 80 min (10 min × 2, 20 min × 2, 10 min × 2) per day | — | Intermittent | Six times divided in the morning and afternoon | — | Time spent outdoors and outdoor activity during school recess is effective in reducing both the onset (p = 0.001) and progression/shift (p = 0.029) of myopia. Nonmyopes with outdoor activity had a greater decrease in myopic shift (OR: 0.18, p = 0.02) than myopes. |
| Hua et al. 43 | Randomized intervention trial, school-based | N = 1713, 6–14, China | ⩽ −0.50D; Cycloplegic autorefraction | — | Median average illuminance of ~558 lux at desks and ~440 lux at blackboards | — | — | Fluorescent (6500K) | Ambient light levels of 558 lux at the desk and 440 lux at the blackboard in classrooms are protective against the onset of myopia compared to 98 lux at the desk and 76 lux at the blackboard levels. A delay in axial growth was observed in both myopic and nonmyopic students under higher light levels. |
|
| He et al. 30 | Cluster-randomized trial, school-based | N = 1848, 6–7, China | ⩽ −0.50D; Cycloplegic autorefraction | 40 min of outdoor activity daily | — | Continuous | End of school day / After school | DL | Intervention group versus control: Cumulative incidence rate of myopia (30.4% versus 39.5%, p < 0.001); 3-year myopia progression (−1.42D versus −1.59D, p = 0 .04); Axial length (0.95 mm versus 0.98 mm; p = 0.07). | |
| Jin et al. 29 | Randomized intervention trial, school-based | N = 3051, 6–14, China | ⩽ −0.50D; Cycloplegic autorefraction | Two additional outdoor recess programmes of 20 min everyday (total 30 min of recess x 2) | — | Intermittent | Morning recess (9:30 a.m.) and afternoon recess (2:30 p.m.) | DL | Intervention group versus control: Myopia incidence (3.70 % versus 8.50 %, p = 0.048); Myopia progression (−0.10 ± 0.65D/year versus −0.27 ± 0.52D/year, p = 0.005); Axial length (0.16 ± 0.30 mm/year versus 0.21 ± 0.21 mm/year, p = 0.034). | |
| Torii et al. 148 | Retrospective, clinic-based | N = 310 myopic children, 10–15, Japan | ⩽ −1.00D; Noncycloplegic autorefraction | — | — | — | — | VL (<400nm) | Non-VL transmitting eyeglasses versus VL transmitting: 1-year axial length elongation (0.25 mm versus 0.17 mm, p < 0.001); Partial VL blocking contact lenses versus VL transmitting:1-year axial length elongation (0.19 mm versus 0.14 mm, p < 0.05). | |
| Wu et al. 35 | Cluster-randomized intervention-controlled trial, school-based | N = 693, 6–7, Taiwan | ⩽ −0.50D; Cycloplegic autorefraction | 40 min (10, 20 and 10 min) recess only in the morning, except Tuesday (40 min additional in the afternoon) | — | Intermittent | Every morning (weekly once both morning and afternoon) | DL | Intervention group versus control: Incidence of new myopia onset (14.47% versus 17.40%) with 35% less risk of myopia in the intervention group (odds ratio, 0.65; 95% CI, 0.42–1.01; p = 0.054); Myopia progression (0.35 D versus 0.47D, p = 0.002); Axial length (0.28 versus 0.33 mm, p = 0.003). Less myopic shift with outdoor time ⩾ 200 min (⩾1000 lux: 0.14D (95% CI: 0.02–0.27, p = 0.02) and ⩾ 3000 lux: 0.16D (95% CI: 0.002–0.32, p = 0.048). |
|
| Observational studies | Read et al. 54 | Cross-sectional, school-based | N = 101, 10–15, Australia | ⩽ −0.50D; Noncycloplegic subjective refraction | — | — | — | — | — | Myopic children versus emmetropic children: Average light exposure (915 ± 519 lux versus 1272 ± 625 lux, p < 0.01); Amount of daily time spent > 1000 lux (91 ± 44 min versus 127 ± 51 min, p < 0.001). |
| Read et al. 38 | Prospective longitudinal study | N = 101,10–15, Australia | ⩽ −0.50D; Noncycloplegic subjective refraction | — | — | — | — | — | Children exposed to low (459 ± 117 lux) daily light exposure showed increased axial length compared to children exposed to moderate (842 ± 109 lux) and high light levels (1455 ± 317 lux) (p = 0.01). The analysis of mean daily durations exposed to various bright light levels revealed that light levels above 3000 lux were associated with less ocular axial growth. |
|
| Landis et al. 154 | Retrospective study | N = 80, 10–15, Australia | ⩽ −0.50D; Noncycloplegic subjective refraction | — | Scotopic < 1–1 lux, Mesopic > 1–30 lux, Indoor photopic > 30–1000 lux, Outdoor photopic > 1000 lux |
— | — | — | Mesopic light (1–30lux) exposure correlated with more myopic refractive error. Compared with myopic children, nonmyopic children were more exposed to both scotopic and photopic light conditions, suggesting an implication of both rod and cone pathways in the development of myopia. |
|
| Ulaganathan et al. 155 | Prospective longitudinal observational study, young adult students | N = 43, 18–30, Australia | ⩽ −0.75D; Noncycloplegic subjective refraction | — | — | — | — | — | Greater time spent in bright light (> 1000 lux) was associated with slower axial eye growth (β = −0.002, p = 0.006). Emmetropes spent more time outdoors than myopes. |
|
| Intensity | Cohen et al. 103 | Chicken | 90 days | — | 12 h/day | 50, 500, 10,000 lux | Continuous | 8 a.m.–8 p.m. | 50 lux: 280–1050; 620 nm 500 lux: 300–1000; 580 nm 10,000 lux: 450–950; 630 nm; Incandescent |
High-intensity light (10,000 lux) induced hyperopia. Low-intensity / dim light (50 lux) resulted in a myopic shift. |
| Ashby et al. 39 | Chicken | Experiment 1: 5 daysExperiment 2: 4 days | FDM | Experiment 1: NL: 12/12 h EL + diffuser removal: 15min/day DL + diffuser removal: 15min/dayExperiment 2: NL: 12/12 hEL: 6 h/day |
Experiment 1: NL: 500 lux EL: 500, 15,000 lux DL: 30,000 lux Experiment 2: NL: 500 luxEL: 50, 15,000 lux |
Continuous | Experiment 1: NL: 7 a.m.–7 p.m. EL: Within 12.30 p.m.–1 p.m.Experiment 2: NL: 7 a.m.–7 p.m.EL: 10 a.m.–4 p.m. |
NL: 400–800 nm, peaking at 530 and 620 nm (Fluorescent) EL/High-intensity light: 300–1000 nm, peaking at 700 nm (Halogen) DL: 360–700 nm |
Exposure to high-intensity daylight (DL: 30,000 lux) or indoor light (EL: 15,000 lux) enhanced the protective effect of a 15-min diffuser removal under 500 lux against FDM. Compared to chickens exposed to NL, exposure to 6 h of 15,000 lux without diffuser removal led to shorter axial length and less myopic refraction in form-deprived eyes. Exposure to 50 lux for 6 h did not increase the magnitude of FDM |
|
| Karouta and Ashby 104 | Chicken | Experiment 1: 7 daysExperiment 2: 11 days | FDM | Experiment 1: NL: 12/12 h EL: 6 h/dayExperiment 2: NL: 12/12 hEL: 5 h/day |
Experiment 1: NL: 500 lux EL: 500, 10,000, 20,000, 30,000, 40,000 luxExperiment 1: NL: 500 luxEL: 500, 10,000, 20,000, 30,000, 40,000 lux |
Continuous | Experiment 1: NL: 7 a.m.–7 p.m. EL: 11 a.m.–5 p.m.Experiment 2: NL: 7 a.m.–7 p.m.EL: 10 a.m.–3 p.m. |
Equal mix of cool (400–650 nm, peaking at 450 nm) and warm (430–700 nm, peaking at 630 nm); LEDs | Increased light intensity led to significantly lesser myopia (p < 0.001) and shorter axial length (p < 0.001). Daily exposure to 40,000 lux almost completely prevented the onset and progression of FDM. |
|
| Ashby and Schaeffel 40 | Chicken | Experiment 1: 5 daysExperiment 2: 4 days | Experiment 1: LIM (−7D or + 7D lenses)Experiment 2: FDM | NL: 12/12 h EL: 5 h/day |
NL: 500 lux EL: 15,000 lux |
Continuous | NL: 7 a.m.–7 p.m. EL: 10 a.m.–3 p.m. |
Experiment 1: NL: 400–800 nm, peaking at 530 and 620 nm (Fluorescent) Experiment 2: EL/High-intensity light: 300–1000 nm, peaking at 700 nm (Halogen) |
High-intensity light (15,000 lux) slowed but did not stop compensation for negative lenses.High-intensity light (15,000 lux) accelerated compensation for positive lenses. High-intensity light reduced FDM by approximately 60%. |
|
| Zhang and Qu 108 | Guinea pigs | 10 weeks | FDM | NL: 12/12 h EL: 12 h/day |
NL: 500 lux EL: 10,000 lux |
Continuous | — | EL/High-intensity light: 365–795 nm, peaking at 450 and 660 nm; LEDs | Animals exposed to high-intensity light (10,000 lux) exhibited more hyperopic refraction (p < 0.001) and shorter axial length (p < 0.001). High-intensity light can retard, but not fully inhibit FDM. |
|
| Siegwart et al. 106 | Tree shrews | 11 days | FDM and LIM (−5D Lenses) | NL: 12/12 h EL: 7.75 h/day |
NL: 500–1000 lux EL: 16,000 lux |
Continuous | NL: 7 a.m.–7 p.m. EL: 9.15 a.m.–5 p.m. |
Fluorescent | Elevated light levels of 16,000 lux reduced FDM and LIM by 44% and 39%, respectively. | |
| Chen et al. 105 | Mice | 4 weeks | FDM | NL: 12/12 h EL: NL for 3 h, High-intensity light for 6 h followed by 3 h of NL |
NL: ~100–200 lux EL: ~2500–5000 lux |
Continuous | 8 a.m.–8 p.m. | Fluorescent | High-intensity light of ~2500–5000 lux significantly suppressed FDM by 46% through reducing ocular axial elongation and shifting refraction towards hyperopia. | |
| Smith et al. 41 | Rhesus monkeys | 23 ± 2 to 132 ± 8 days | FDM | NL: 12/12 h EL: 6 h/day |
NL: 15–630 lux EL: 18,000–28,000 lux |
Continuous | EL: 6 h (in the middle of 12-h light cycle) | NL: fluorescent EL/High-intensity light: metal halide |
High-intensity light of 18,000–28,000 lux for 6 h/day reduced the degree of myopic anisometropia by 87%. Animals raised under high-intensity light exhibited more hyperopic shift in FD eyes when compared with contralateral control eyes and also when compared to FD eyes of animals raised under normal light. |
|
| Smith et al. 156 | Rhesus monkeys | 50–213 days | LIM (−3D lenses) | NL: 12/12 h EL: 6 h/day |
NL: 350 lux EL: 25,000 lux |
Continuous | EL: 6 h (in the middle of 12 h light cycle) | NL: fluorescent EL/High-intensity light: metal halide |
High light intensity did not alter the degree of myopia (p = 0.4) imposed by hyperopic defocus. Recovery from LIM was not affected by light intensity. |
|
| Spectrum | Foulds et al. 130 | Chicken | 14–42 days | — | 12/12 h | Red: 33.37, Blue: 34.44, White: 117.32 cd/m2 |
Continuous | 6 a.m.–6 p.m. | Red: 600–680 nm with a sharp peak at 641 nm Blue: 440–495 nm with a sharp peak at 477 nm White: 420–790 nm with a sharp peak in the blue at 440 nm; LEDs |
Progressive myopia and hyperopia can be induced by red and blue light, respectively. Changes in chromaticity can reverse light-induced myopia or hyperopia in chickens. |
| Najjar et al. 133 | Chicken | 28 days | FDM | 12/12 h | SW: 233.1 lux BEW: 223.8 lux |
Continuous | 7 a.m.–7 p.m. | SW: 3900 K BEW: 9700 K; LEDs |
Moderate intensities of BEW light decreased ocular growth and accelerated recovery from FDM compared with SW light. Retinal and vitreal metabolomic profiles were dependent on spectral content of light. |
|
| Rucker et al. 157 | Chicken | 3 days | ± 6–8 D | 14/10 h | 0.67 Clux for red, blue and white condition | Continuous | 9 a.m.–5 p.m. | Red: 620 nm Blue: 460 nm White light |
Differential effect of blue and red light on choroidal thickness and ocular length was noted, suggesting the involvement of LCA in lens compensation. | |
| Rucker et al. 158 | Chicken | 3 days | — | 12/12 h | Red: 214 Clux Green: 191 Clux Blue: 64 Clux |
Six temporal frequencies: 0, 0.2, 1, 2, 5, and 10 Hz | 9 a.m.–5 p.m. | Red: 619 ± 20 nm Green: 515 ± 35 nm Blue: 460 ± 35 nm Blue/Yellow or Red/Green; LEDs |
Ocular growth is faster under low temporal frequencyies.At low temporal frequencies red/green modulation produced maximal growth. Under high temporal frequency ocular growth is controlled without the involvement of colour stimulus. |
|
| Seidemann and Schaeffel. 131 | Chicken | 2 days | 5 lux | 12/12 h | 5 lux | Continuous | 8 a.m.–8 p.m. | Red: 615 nm Blue: 430 nm; Slide projector with interference filters |
Refraction of chickens measured in complete darkness and under white light with cycloplegia showed a significant difference (p < 0.0012) with chickens exposed to blue light being more hyperopic than the red light. When measured under white light without cycloplegia, no significant difference was observed between blue light and red light reared groups. Imposed chromatic defocus produces a shift in accommodation tonus in chickens. |
|
| Lin et al. 147 | Chicken | Short study: 10 days Long study: 17 days |
— | 12/12 h | 424 lux Red, blue and white (Steady and Flicker) |
Continuous | — | Red: monochromatic red (628 ± 10 nm) Blue: monochromatic blue (464 ± 10 nm) White: broadband white light; LEDs |
Chickens exposed to blue steady or flickering light showed a lesser increase in axial length and vitreous chamber depth than chickens exposed to red or white light. Responses to wavelength defocus in chickens are transient. |
|
| Torii et al. 148 | Chicken | 7 days | FDM | 12/12 h | FL: 1262 ± 502 VL: 1349 ± 462 (UV irradiance 0.413 mW/cm2) Blue light: 1035–1230 lux |
Continuous | — | FL: Fluorescent light VL: 360–400 nm (365 nm peak) + Fluorescent light (UV 290–390 nm) Blue light: 470 nm (LEDs) |
VL suppressed ocular axial elongation and significantly upregulated EGR1 in chorioretinal tissues compared with blue light. | |
| Wang et al. 132 | Chicken | 5 days | FDM | 12/12 h | 500 lux | Continuous | 8 a.m.–8 p.m. | White light: 430–630 nm UV: peak at 375 nm blue:465 nm red: 620 nm; LEDs |
Control eyes of animals exposed to blue and UV light turned out to be 1.0D more hyperopic than control eyes exposed to red and white lights. The change in refraction was not significant between groups exposed to UV and blue light. |
|
| Jiang et al. 139 | Guinea pigs | 4 weeks | LIM and LIH (−4D and + 4D lens) | 12/12 h | 1. White light (350 lux) 2. Red light (300 lux) 3. Blue light (50 lux) |
Continuous | — | White light (Fluorescent) Red 600 ± 5 nm; LEDs Blue light 470 ± 5; LEDs |
Blue light inhibited axial eye growth compared with red and white light Red light induced early thinning of the choroid and relative myopia, compared with white light. |
|
| Liu et al. 137 | Guinea pigs | 12 weeks | — | 12/12 h | Experiment 1: 460 mW/m2 for short-wavelength light and 750 mW/m2 for the middle-wavelength light. Bright light: ~400 mW/m2
Experiment 2: 1770 mW/m2 for short-wavelength, 700 mW/m2 for middle-wavelength and 740 mW/m2 for broadband light. |
Continuous | 8 a.m.–8 p.m. | SL: 430 nm ML: 530 nm Broadband light LEDs |
Middle wavelength group was less hyperopic than the broadband group (p < 0.001) with a faster vitreous extension. Short-wavelength group was more hyperopic with a slower vitreous elongation (p < 0.001) when compared with both ML and broadband light. |
|
| Long et al. 141 | Guinea pigs | 6 weeks | — | 12/12 h | 150 lux | Continuous | — | long wavelength: 760nm mixed wavelength: filtered by opaque glasses without colour Halogen lamps |
Animals exposed to long-wavelength light developed myopia, with significantly longer vitreous chamber depth. | |
| Zou et al. 140 | Guinea pigs | 10 weeks | — | 12/12 h | Irradiance: Blue:1770 mW/m2 Green: 700 mW/m2 White: 740 mW/m2 |
Continuous | 8 a.m.–8 p.m. | SL: 430 nm ML: 530 nm WL: normal lights, 5000 K; LEDs |
Guinea pigs developed relative hyperopia in the SL group and relative myopia in the ML group. The density of S-cones and S-opsins increased while M-cones and M-opsins were decreased (p < 0.05) in SL group. |
|
| Tao et al. 151 | Guinea pigs | 8 weeks | FDM | — | Green flickering light: 800 lux | Flickering at 5 Hz flash rate | — | green: 515–530 nm, peak value 525 nm | Significant reduction in refractive error and increase in axial length after 8 weeks of green flickering light stimulation (p < 0.001). | |
| Ward et al. 143 | Tree shrews | 13 days + 27 days recovery | — | 14/10 h | 1. Red lights 527–749 lux 2. Control group (100–300 lux) |
Continuous | 1, 2, 4, 7 or 14 h | Either 624 ± 10 or 636 ± 10 nm LEDs and fluorescent |
Increase in the hyperopic shift was noted with increasing duration of red light exposure. After red light treatment was discontinued, refractions recovered to baseline |
|
| Gawne et al. 159 | Tree shrews | 13 days | FDM | 14/10 h | 1. Steady Red: 527 lux 2. Flicker red: 329 lux 3. Steady blue: 601 lux 4. Flicker blue: 252 lux 5. Control group under broad spectrum white fluorescent: 100–300 lux |
Continuous | — | red: 628 ± 10 nm blue: 464 ± 10 nm; LEDs and fluorescent |
Animals exposed to red light (both steady and flickering) were significantly hyperopic compared with the control (p < 0.01). Animals exposed to flickering blue light were significantly myopic with longer vitreous chambers. |
|
| Liu et al. 142 | Rhesus monkeys | 51 weeks | — | 12/12 h | Irradiance: Red: 0.043 mW/cm2 Blue: 0.14 mW/cm2 White: 0.024 mW/cm2 |
Continuous | 8 a.m.–8 p.m. | Red: 610 nm Blue: 455 nm LEDs White: 5000 K; LEDs |
No myopia development was noted among monkeys in the blue light group. Monkeys in the red light group remained hyperopic, however showed slightly reduced refraction, when compared with the blue and white light groups, while two monkeys developed myopia. No significant difference in the mean refraction between the blue light group and the white light group was noted. Monkeys sensitive to L-cone stimulation are susceptible to develop myopia when exposed to red light. |
|
| Smith et al. 145 | Rhesus monkeys | 146 ± 7 days | — | 12/12 h | 580 ± 235 lux (range 305–987 lux) | Continuous | — | 1. (red) filter in front of one eye (MRL) > 570 nm 2 (red) filter in front of both eyes (BRL) > 570 nm 3. Binocular 0.1 log NDF 4. Unrestricted vision under typical indoor lighting Fluorescent and incandescent lamps |
The median refractive error for the BRL monkeys was significantly more hyperopic than the NDF and unrestricted monkeys. The MRL monkeys exhibited hyperopic anisometropias that were larger than those in the unrestricted monkeys. |
|
| Pattern | Crewther and Crewther 123 | Chicken | 9 days | LIM (−10D) and LIH (+10D) | 12/12 h | 387 lux | Temporal luminance profiles 1. Stationary 2. Fast-ON 3. Fast-OFF (8% temporal contrast, flicker 4 Hz) |
— | Incandescent lamps | Reduced refractive compensation with + 10D lens Fast-OFF and with −10D lens Fast-ON. Refractive compensation depends on the temporal contrast of the environment. Possible relationship between the type of defocus and the state of adaptation of the retinal ON and OFF system. |
| Yoon et al. 160 | Chicken | 3 days | — | 12/12 h | NL: 300 lux EL 1: 985 lux EL 2: 70 lux, 680 lux and 985 lux EL 3: 985 lux |
Experiment 1: 0.2 Hz Experiment 2: 0.2 Hz Experiment 3: 0 Hz versus 0.2 Hz |
8 a.m.–8 p.m. | Soft (General Electric LEDs, low S-cone, high L-cone stimulation); Daylight (General Electric LEDs, balanced stimulation with L-cone bias); Equal (balanced stimulation with S-cone bias); RGB LEDs High S (high S-cone, low L-cone stimulation); RGB LEDs Within 400 - ~700 nm |
Chickens exposed to equal light conditions of 985 lux at 0.2 Hz showed a significant reduction of axial growth and increased hyperopic shift. Ocular growth is dependent on the interaction between spectral composition, illuminance and temporal modulation of light. Low-frequency modulation of the indoor light source can reduce the ocular growth and refractive error changes. Daylight bulbs with higher S-cone elicitation may protect against axial growth. |
|
| Schwahn and Schaeffel 122 | Chicken | 7 days | FDM, LIM (−8D) and LIH (+8D) | 12/12 h | 150–1500 lux | Continuous and Flicker (6–12 Hz) | 8 a.m.–8 p.m. | Incandescent and xenon lamps | Flickering light of 6 Hz effectively suppressed the development of both FDM and LIM. Flickering light of 12 Hz is effective against LIH changes. |
|
| Lan et al. 124 | Chicken | 5 days | FDM | 10/14 h | NL: 500 lux EL: 15,000 lux |
Experiment 1 1. EL/Constant bright light for 5 h 2. EL/Constant bright light for 10 h.Experiment 2. EL/Intermittent bright light at duty cycle 50% for:a. 60 min cycleb. 30 min cyclec. 15 min cycled. 1 min cycle over a period of 10 h |
10 a.m.–3 p.m. 8 a.m.–6 p.m. |
NL: 400–800 nm (Fluorescent) EL: 300–1000 nm (Halogen) |
The protective effect of bright light depends on the duration of exposure and the frequency cycle of intermittent exposure. Low-frequency cycles of bright light (1:1 min) presented strong inhibition of FDM in chickens. |
|
| Backhouse et al. 114 | Chicken | 3 days | FDM | 12/12 h | NL: 300 lux EL 1: Constant light: 2000 lux EL 2: Bright light: 10,000 lux |
1. Constant light 2. Morning: Normal light + 2 h bright light 3. Midday: Normal light + 2 h bright light 4. Evening: Normal light + 2 h bright light |
6 a.m.–6 p.m. 6 a.m.–8 a.m. 11 a.m.–1 p.m. 4 p.m.–6 p.m. |
Fluorescent and halogen lights | The protective effect of bright light depends on the duration of exposure and the frequency cycle of intermittent exposure. Low-frequency cycles of bright light (1:1 min) presented strong inhibition of FDM in chickens. An increase in daily light exposure continuously during the day is more effective at inhibiting myopia than adding an equivalent dose of bright light over a 2-h period. However, there is significantly less myopia induced in the midday group compared with the evening group (p = 0.018). |
|
| Guo et al. 117 | Chicken | 2 weeks | FDM, LIM (−10D) and LIH (+10D) | 24 h | 70–140 lux | Continuous | — | Fluorescent | FDM, LIM and LIH can still be induced under continuous light.Continuous light, however, affects changes induced by FDM, LIM and LIH. | |
| Padmanabhan et al. 118 | Chicken | 3 weeks | LIM (−10D) and LIH (+10D) | 12/12 h | 331–385 lux | Continuous | — | — | Under constant light eyes fitted with + 10D lenses, became more hyperopic and had shorter vitreous chambers and axial lengths. In eyes fitted with −10D lenses, a small hyperopic shift was observed. LIM and LIH can be reversed under normal light after halting the defocus stimuli. |
|
| Cohen et al. 119 | Chicken | 83 days | — | 24 h | 1. high intensity (~10,000 lux) 2. intermediate intensity (~500 lux) 3. low intensity (~50 lux) |
Continuous | — | Incandescent light | All groups raised under continuous light exposure were hyperopic with the high-intensity group being the most hyperopic. Continuous exposure to low intensity light resulted in emmetropia. High-intensity continuous light resulted in greater corneal flattening. No change in axial length, however vitreous chamber was significantly deeper in the high-intensity group which is independent of corneal flattening and dependent on the light intensity during development. |
|
| Luo et al. 127 | Guinea pigs | 8 weeks | FDM and FLM | 12/12 h | 600 lux | Flicker light: 0.5 Hz flash rate | 6 a.m.–6 p.m. | Flickering light: narrow spectrum, 505 nm LEDs |
Myopia can be induced in guinea pigs with 0.5 Hz flickering light at puberty. FLM group became more myopic: decreased refraction and longer AL compared with the control group (p < 0.05). |
|
| Di et al. 126 | Guinea pigs | 12 weeks | - | 12/12 h | 300 lux 0–600 lux |
1. Flickering light: 0.5 Hz flash rate 2. Flickering light: 5 Hz flash rate |
6 a.m.–6 p.m. | Narrow spectra 505 nm LEDs |
Guinea pigs raised in 0.5 Hz flickering light were more myopic than the group raised in continuous illumination, followed by the group raised at 5 Hz flicker light. | |
| Li et al. 128 | Guinea pigs | 8 weeks | FDM and FLM | 12/12 h | FDM: 300 lux FLM: 0-600 lux |
1. FDM: Continuous 2. FLM: 0.5 Hz flash rate | 6 a.m.–6 p.m. | 600 nm LEDs |
FDM and FLM groups presented a shift to myopic refraction with longer AL when compared with the control group (p < 0.05). | |
| Yu et al. 129 | Mice | 6 weeks | FDM and FLM | 12/12 h | 250 lux | Flickering light group: 2 Hz flash rate | 8 a.m.–8 p.m. | LEDs | Myopia can be induced in mice using flickering lights. Mice raised under flickering light were more myopic and had a longer axial length compared with the control group (p < 0.05). |
|
| Zhou et al. 121 | Mice | 28 days | — | 1. 18/6 h 2. 12/12 h 3. 6/18 h |
300 lux | Continuous | 9 a.m.–3 a.m. 9 a.m.–9 p.m. 9 a.m.–3 p.m. |
400–700 nm Fluorescent light |
Prolonged lighting exposure can induce axial myopia in mice. A trend of myopic development, increasing vitreous chamber depth and thinning of the retina in eyes can be seen from 6/18 to 18/6 groups. |
|
| Smith et al. 88 | Rhesus monkeys | 7 months | LIM (−3D) and LIH (+3D) | — | Top cage: 630 lux Bottom cage: 230 lux |
Continuous | — | Fluorescent light | The average amount of compensating anisometropia, the structural basis for the refractive errors, and the ability to recover from the induced refractive errors were not altered by continuous light exposure. | |
| Timing | Nickla et al. 113 | Chicken | 5 days | LIH (+10D lenses only worn for 2 h/day) | 14/10 h | 500 lux | Intermittent | 5.30 a.m.–7.30 a.m. 12 p.m.–2 p.m. 7.30 p.m.–9.30 p.m. |
— | Myopic defocus in the evening was significantly more effective at inhibiting eye growth than in the morning (p < 0.01). Data for ‘noon’ was similar to that of ‘evening’. |
| Nickla et al. 161 | Chicken | 5 days | Experiment 1: LIM (−10D lenses for 2 h or 6 h/day)Experiment 2: FDM | 14/10 h | 500 lux | Continuous and Intermittent | Experiment 1: Morning (7 a.m.–9 a.m. or 7 a.m.–1 p.m.) Midday (12 p.m.—2 p.m. or 10 a.m.–4 p.m.) Evening (7 p.m.–9 p.m. or 2–8 p.m.) Continuous wear (control)Experiment 2: 2 h between 7 a.m.–9 p.m. |
— | 2 h of defocus stimulated eye growth with morning light exposure. Eyes were more hyperopic when 2-h defocus and light exposure was at noon. Longer exposures at midday inhibited growth and produced hyperopia. FDM for 2 h/day in the morning inhibited ocular growth. |
|
| Nickla et al. 115 | Chicken | 7 days | — | 14/10 h | 700 lux | Intermittent | 7.30 a.m.–7.30 p.m. light, and 12 a.m.–2 a.m. light, 7.30 a.m.–9.30 p.m. light |
— | Light at night disrupts circadian rhythms of axial length and choroidal thickness leading to the development of ametropia. Light caused an acute, transient stimulation in ocular growth rate (p < 0.05) in the subsequent 6-h period (12 a.m.–6 a.m.). |
|
| Sarfare et al. 116 | Chicken | Experiment 1 and 2: 6 daysExperiment 3: 5 days | Experiment 1: FDMExperiment 2: LIM (−10D lenses)Experiment 3: LIM (−10D lenses for 2 h with bright light) | NL: 12/12 h EL: 3 h/day |
25,000–30,000 lux | Continuous | Experiment 1 and 2: Morning (7.30 a.m.–10.30 a.m.) Evening (4.30 p.m.–7.30 p.m.)Experiment 3: Morning (7.30 a.m.–9.30 a.m.)2. Midday (11.30 a.m.–1.30 p.m.)3. Evening (5.30 p.m.–7.30 p.m.) |
NL: 3500K, LED EL: 5700K, LED |
Brief bright light exposure in the evening inhibited ocular growth in both FDM (p = 0.026) and LIM (p = 0.03). Brief bright light and simultaneous hyperopic defocus in the morning significantly inhibited eye growth more than the control (p < 0.01). |
|
BEW, blue-enriched white light; CI, confidence interval; Clux, chicken lux; D, diopter; DL, day light; EL, experimental light; FDM, form deprivation myopia; FLM, flickering light–induced myopia; LCA, longitudinal chromatic aberration; LED, light emitting diodes; LIH, lens-induced hyperopia; LIM, lens-induced myopia; ML, middle-wavelength light; NDF, neutral density filter; NL, normal light; OR, odds ratio; SL, short-wavelength light; SW, soft standard white light; UV, ultra-violet; VL, violet light; WL, white light.
Intensity of light
Findings from animal studies support the notion that higher light levels, similar to those encountered outdoors, are predominant factors for myopia prevention. In chickens, dim ambient lighting of 50 lux delivered as a 12 h/12 h light–dark cycle is deleterious to emmetropization, 103 while exposure to high illuminances of light (15,000 lux) for periods of 5 or 6 h per day delays the development of FDM by 60%. 39 This protective impact of light on FDM is dose-dependent, with exposure to 40,000 lux of light-emitting diode (LED) light for 6 h providing comprehensive protection against the onset of FDM. 104 These protective effects of bright light against myopia have been associated with DA release and the D1 receptor signaling pathway (see ‘Light, dopamine and refractive error regulation’ section for more details).61,105 Similarly, bright light exposure can also reduce, but not overcome, the rate of compensation for monocularly fitted negative lenses (−7D) and enhance the rate of compensation for positive lenses (+7D). 40 Alike chickens, tree shrews exposed to bright light (16,000 lux) for 7.75 h/day for 11 days display a reduced development rate of FDM and LIM, 106 while form-deprived eyes of rhesus monkeys reared under 18,000–28,000 lux of metal halide light (4200K) for 6 h a day over ~150 days are less myopic than those reared in normal light. 41 Interestingly, in rhesus macaques, 25,000 lux of bright light for 6 h per day was not sufficient for stopping LIM, suggesting dissimilarities in mechanisms responsible for FDM and LIM. 107 In guinea pigs, bright light (10,000 lux) reduced the myopic shift induced by form deprivation compared with normal lighting (500 lux). 108 While in mice, bright light exposure (2500–5000 lux) for 6 h/day for 4 weeks prevented FDM and presented a hyperopic shift and reduction in ocular elongation compared with normal lighting (100–200 lux). 105 Analogously to bright light, albeit through different mechanisms, short periods (~3 h/day) of de-focusing lens removal or normal vision per day, even in moderate light levels, can compensate for LIM in chickens. 109 Surprisingly, and contrary to earlier studies in chickens, 103 a recent study in infant rhesus monkeys raised under dim light (~55 lux) showed a hyperopic shift when compared with the monkeys raised under normal light (~504 lux). 110 These differences in response to between dim light chickens and monkeys, may be due to differences in the sensitivity of the circadian system between birds and mammals. 111
Timing and duration of bright light
Prevailing evidence on the impact of light intensities on myopia in animal models has raised the question of whether the intensity of light and timing of exposure are interlinked. This has gained more attention with the notion supporting the role of circadian rhythms in ametropia. 112 Recently, Nickla et al. 113 reported that myopic defocus in chickens raised under light levels of 500 lux was more effective at reducing ocular growth when lenses were worn during the evening compared with when lenses were worn in the morning. These moderations were attributed to alterations in the amplitude of the axial length rhythm. On the other hand, constant daily light exposure (2000 lux) was reported to be more effective at inhibiting myopia than a 2 h dose of bright light (10,000 lux) delivered either in the morning, mid-day or evening. 114 Within that same study, however, 2 h of bright light (10,000 lux) delivered midday was more efficient in inhibiting ocular growth than the same light protocol delivered in the evening. 114 Moreover, chickens exposed to ambient light (700 lux) at night (between 12:00 a.m. and 2:00 a.m.) showed alterations in axial length and choroidal thickness rhythms, which could no longer follow a sinewave function with a 24 h period. This brief light exposure caused a transient stimulation in the ocular growth rate which may have subsequently resulted in myopic refractive error. 115 Interestingly, Sarfare et al. 116 revealed that evening bright light inhibits the effect of continuous hyperopic defocus and form deprivation while morning bright light has a greater inhibitory effect on transient ‘2 h’ hyperopic defocus. These findings suggest a peculiar interaction between the timing and duration of defocus and bright light exposure that the authors attribute to the duration and sign of the defocus signal in operation immediately following the bright light exposures. 116 The abolishment of light/dark cycles has also been studied in animal models and constant light has been shown to disrupt LIM and FDM in chickens.117,118 Corneal flattening was also observed in chickens reared under continuous bright light; however, no distinct observation was made with chickens reared in bright light with a diurnal pattern.119,120 This effect of continuous light on refractive error development and emmetropization appears to be unique to chickens, since rearing infant rhesus macaques in ambient constant light does not affect emmetropization. 88 This interspecies variation was attributed to difference between the avian and mammalian circadian systems. 111 In smaller mammals like mice, prolonged (18 h light/6 h dark) exposure to light does lead to a myopic shift, increased axial length and vitreous chamber depth (VCD), reduction in retinal Egr-1 mRNA transcript level, and decreased scleral fibre diameters in C57BL⁄6 in bred mice. 121
Temporal frequency of light
Emmetropization in chickens is dependent upon the temporal frequency of the light exposure: high temporal frequencies induce hyperopia and low temporal frequencies, myopia.122,123 Lan et al. 124 demonstrated that intermittent exposure to bright light at 15,000 lux for 1:1 and 7:7 min were more effective in controlling FDM when compared with continuous bright light exposure. A possible underlying mechanism for such findings could be that flickering light triggers the retinal ON and OFF pathways, thereby stimulating DA release. 125 Guinea pigs raised in 0.5 Hz flickering light (600 lux) for 12 h/day for 12 weeks presented a greater myopic shift in refraction and a larger increase in axial length ocular length compared with guinea pigs raised in 5 Hz flickering light (600 lux) or a control group which was raised in steady light (300 lux). 126 In another study, guinea pigs exposed to flickering light (505 nm, 600 lux, 0.5 Hz) for 12 h/day for 8 weeks showed a significant decrease in refraction and increase in axial length compared to animals exposed to 12 h/day of steady control light (600 lux). Furthermore, not only the levels of DA, but also of 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) which are primary and secondary metabolites of DA, respectively, were significantly increased in the flickering light group, with DA D1 and D2 receptors upregulated compared with the control. 127 Flickering rate and DA levels may hence play a role in myopia development in guinea pigs. 126 Apart from DA, Li et al. 128 found elevated concentrations of 5-hydroxytryptamine (5-HT) and 5-HT2A receptor expression in guinea pig groups raised under flickering light (600 lux, 0.5 Hz for 12 h/day for 8 weeks), while norepinephrine and epinephrine levels were reduced compared with control groups exposed to 300 lux of light for 12 h/day. C57BL/6 (B6) mice exposed to 6 weeks of flickering light (2 Hz: with 500ms of dark phase per second) for 12 h/day presented with a myopic shift (~ −9D) in refraction and increased axial length compared with the steady light control. 129
Spectral composition of light
The spectral composition of light has also been shown to play a key role in ocular growth and emmetropization. In chickens, exposure to red light (peak wavelength range: 615–641 nm) has been reported to induce myopia while rearing under ultraviolet light (UV) (peak wavelength: 375 nm) or blue light (peak wavelength range: 430–477 nm) induces hyperopia.130–132 Furthermore, ocular DA release and metabolism, as well as vitreal and retinal metabolomic profiles, were highly dependent upon the spectral composition of light.132,133 Among plausible explanations to this wavelength-dependent refractive error regulation, ocular longitudinal chromatic aberration (LCA), which leads to wavelength defocus and higher refraction of short-wavelength light compared with long-wavelength light by ocular optics, was supported by many authors.131,134,135 The hyperopic shift in response to short-wavelength blue light has also been reported in other, but not all, animal species such as Cichlid fish, 136 guinea pigs137–141 and some rhesus monkeys. 142 Comparatively, red light or eye-mounted red filters render tree shrew and rhesus monkey eyes hyperopic, while blue flickering light induces myopia and increases VCD.143–145 Interspecie differences in the spectral responses may to light not only to be due to protocol differences (e.g. duration of light exposure) but also to differences in retinal photoreceptor composition and sensitivity accross species.146,147
The spectral composition of light also has a prominent role in exerting protective effects against FDM and LIM. Torii et al. 148 suggested that exposure to violet light (VL:360–400 nm) can suppress myopia progression in chickens through the upregulation of the “myopia protective gene” EGR1. Similarly, blue and UV light exposure conferred a protective effect against myopia progression with a concomitant increase in the retinal DA levels. 132 However, the applicability of near UV and UV light to humans is limited due to the UV-blocking properties of the crystalline lens149,150 and the nonavailability of near UV receptors unlike in chickens and guinea pigs. In addition, in guinea pigs, short-wavelength blue light of 470 ± 5 nm with an intensity of 50 lux showed inhibition of LIM, while long-wavelength red light of 600 ± 5 nm with an overall luminance of 300 lux presented a myopic shift. The increased sensitivity to blue light by 0.35 log units compared with red light in guinea pigs may have contributed to this short-wavelength mitigation of eye growth. 139
The spectral tuning of refractive error development is also dependent upon the flicker frequency of light. For instance, blue light exposures are protective against myopic eye growth induced by low-frequency flickering light in chickens, while 8 weeks of flickering green light (5 Hz) at 800 lux was found to induce myopia and increase axial lengths in guinea pigs. 151 These findings suggest that high temporal frequencies may reduce the effects of wavelength defocus on ocular refraction, such as at low temporal frequencies, visual inputs are dominated by wavelength defocus signals, inducing hyperopic shifts at short wavelengths and myopic shifts at long wavelengths. While at high temporal frequencies, a myopic shift under blue light and a hyperopic shift under green light is a result of visual inputs being dominated by luminance signals and wavelength defocus signals being weakened. 152
Altogether, observational and experimental studies in humans and animal models suggest that exposure to high-intensity light, both in continuous or intermittent patterns, can slow the development of myopia. However, this impact of high-intensity light against myopia development in animal models is dependent on the means of myopia induction (i.e. more effective in FDM compared with LIM). Furthermore, today there is no clear consensus on a minimum or optimal light intensity to promote emmetropization and prevent or slow myopia development in humans. Such a threshold is variable in animal models, given differences in retinal circuitry and photoreceptoral composition. Conversely, a total of 40 min of outdoor time per day (i.e. a combination of exposure to high-intensity sunlight, increased spatial frequency, increased retinal focus, etc.) seems to be protective against myopia in humans; frequently, animal models for myopia, baring strong myopiagenic stimuli, require longer durations of high-intensity light per day to alleviate the development of this ocular condition. Although the spectral sensitivity to refractive error development in response to light has not yet been fully established, existing studies in animals (chicken and guinea pigs) and humans are in a fragile consensus that short-wavelength light may be protective against axial myopia development. Studies in NHP and tree shrews disagree with the latter statement. Finally, exposure to high-intensity and short-wavelength light needs to be timed carefully to avoid any potential disruptions to the circadian timing system of children and adolescents. Considering that all the parameters of light namely the intensity, duration, spectrum, pattern, and timing of light are synergetic, and given the scarcity of interventional clinical studies using light, tailored light-therapy strategies for myopia prevention are yet to be established.
Physiological mechanisms mediating light-induced myopia prevention
Experimental research has been instrumental for elucidating the anatomo-physiological impact of light on ocular growth refractive error development. Although there are mixed opinions in the myopia research community on the involvement of light in the prevention of myopia, 162 the protective effect of high/higher intensity light against myopia cannot be ruled out, at least in experimental animal models of the condition and interventions in humans. 43 To date, however, the exact spectro-temporal characteristics of protective light regimens remain unclear. Understanding the underlying physiological and molecular mechanisms mediating light-induced myopia prevention is essential for data-driven successful translational interventions (Table 2).
Table 2.
Summary of studies investigating key signalling molecules linked to light-dependent ocular growth and refractive error development.
| Methods and baseline data | Defined light parameters | Key findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neurotransmitters and signalling molecule | Study author | Animal model | Experimental protocol duration | Type of myopia | Intensity | Duration | Pattern | Timing | Spectrum / wavelength/light type | |
| DA | Bartmann et al. 245 | Chicken | Experiment 1: 1–2 weeksExperiment 2: 2–4 days | Experiment 1: FDMExperiment 2: LIM (+4D/−4D) | 1000–3000 lux | 24 h versus 12 h | Continuous | — | 60 W light bulb | Retinal DA and DOPAC levels were reduced in both 24 h and 12 h light exposures in FDM.Levels remained constant under LIM. |
| Cohen et al. 171 | Chicken | 3 days | — | 50, 500 and 10,000 lux | 12 h and 24 h | Continuous | — | 620, 580 and 630 nm 5, 40 and 300 W light bulbs |
Light over a log illuminance range of 1.69–4 is linearly related to vitreous DOPAC level. The intensity of ambient illumination regulated the vitreal DA release rate and refractive development. 24 h light is associated with high vitreal DOPAC and hyperopia development. |
|
| Lan et al. 184 | Chicken | — | FDM | 500 and 15,000 lux | 10 h | Continuous and Intermittent | 8 a.m.–6 p.m. | 530 and 620 nm; fluorescent lamp 700 nm; halogen lamp |
High-intensity light, especially intermittent exposure of 1:1 min, can partially rescue DA levels in the retina and vitreous of FD eyes. | |
| Liu et al. 246 | Chicken | 3 weeks | — | 1500, 0.01–500, 1–1500 µW/cm2 |
12 h light, 12 h light-dim, 24 h light | Continuous | — | 625–650 nm; incandescent bulbs |
Chickens were emmetropic under 12-h light and 12-h light-dim phase, but hyperopic under 24 h light. Retinal and vitreal DA and DOPAC levels were similar among the 3 light groups. Diurnal variation was dependent on the intensity of light with higher intensity associated with higher DA/DOPAC. |
|
| Mathis et al. 201 | Chicken | 8 days | — | 500 and 8500 lux | 1.5 h | Continuous | — | — | Atropine and α2A-ADR antagonists stimulate DA release whereas α2A-ADR agonists suppress its release. Stimulation of DA by atropine was enhanced by bright light, which inhibited axial eye growth. |
|
| Megaw et al. 169 | Chicken | 24 h | FDM | — | 3 h | Continuous | — | — | 3 h of light elevated both retinal- DA, and DOPAC and vitreal- DOPAC levels compared with dark | |
| Parkinson and Rando 247 | Chicken | 48 h | — | 70 foot candela | 48 h | Continuous | — | Fluorescent light | Retinal DA and DOPAC levels were significantly higher under light when compared with total darkness | |
| Stone et al. 167 | Chicken | 4 weeks | FDM | 12 h light versus 2 h of dark | Continuous | — | DA levels were higher in the light-adapted retina compared with dark-adapted ones in control eyes, whereas they were same in the FDM eyes. | |||
| Schwahn and Schaeffel 122 | Chicken | 5–8 days | FDM, LIM and LIH (±8D) | 150–1500 lux | 3 h and 12 h | 6 and 12 Hz flicker | — | 150 W Xenon lamp | FDM, LIM and LIH all were suppressed by flickering light. For FDM and LIM, 12 Hz flicker was more effective than 6 Hz flicker without any correlation between the degree of myopia and retinal DA release. |
|
| Wang et al. 132 | Chicken | 5–7 days | FDM | White 468 lux, red 453 lux, blue 435 lux | 30 min | Continuous | — | White 430–630 nm, red 620 nm, blue 465–470 nm, UV 375 nm; LEDs |
Retinal DA and vitreal DOPAC were higher after exposure to white, red, blue and UV light. Vitreal DOPAC and retinal DA levels were lowest and highest, respectively, under UV light. |
|
| Zawilska et al. 248 | Chicken | 2 days | — | 150 lux | 12 h light, 24 h dark, 24 h light | Continuous | Cool fluorescent lamps |
12 h and 24 h light both produced high levels of DA and DOPAC in the retina compared with the 24 h dark. DA and DOPAC levels oscillated between high during subjective light (high) and dark (low) phases under constant darkness. | ||
| Brainard and Morgan 170 | Rat | 3 weeks | — | 0, 1, 3, 5, 10, 25, 50, 100 and 1000 µW/cm2 | 15 min | Continuous | Daytime | Broad spectrum white light; 500-W tungsten bulb | Exposure to light of ⩾ 5 µW/cm2 leads to an increase in DA synthesis in the retina, reaching saturation by 25 µW/cm2. | |
| Proll et al. 249 | Rat | 1 day | — | 0.1, 0.5, 5, 32.2, 570.3, 1173 lux | 5, 15 and 30 min | Continuous | — | Cool white fluorescent light | Minimum of 5 lux light for ⩾ 5 min stimulates retinal DOPA levels. The stimulation reaches a peak at 32.2 lux. | |
| Chen et al. 105 | Mice | 4 weeks | FDM | NL: 100–200 lux; EL: 2500–5000 lux |
12 h 6 h |
Continuous | — | Fluorescent bulbs | Bright light increases DA receptor 1 activity in the bipolar cells which suppresses FDM. | |
| Landis et al. 250 | Mice | Experiment 1: 2 weeks | LIM (−10D) | Scotopic 1.6 × 10−3 cd/m2, mesopic 1.6 × 101 cd/m2, photopic 4.7 × 103 cd/m2 | Experiment 1: 12 h | Continuous | — | White LEDs | Retinal DOPAC and DOPAC/DA ratio increased with the level of light but not DA. | |
| Experiment 2: 3 h | Experiment 2: 3 h | Photopic light group had the lowest levels of DA and highest levels of DOPAC. Scotopic group had the highest DA levels and lowest DOPAC. Both scotopic and photopic light reduced LIM significantly compared to mesopic light. |
||||||||
| Pardue et al. 189 | Mice | 2–8 weeks | FDM | — | 12 h | Continuous | — | — | Retinal DA levels in dark and light conditions were not different, whereas DOPAC levels were higher under light without any association with FDM. | |
| Strickland et al. 251 | Mice | 4 weeks | LIM (−10D) | 50 cd/m2 | 12 h | Continuous | — | White 420–680 nm, green 525 ± 40 nm, VL400 ± 20 nm; LEDs |
VL induced hyperopia and protected against LIM compared with green or white light without any change in the retinal DA or DOPAC levels. | |
| Zhang et al. 180 | Rhesus monkey | — | — | 1.78 x 105 photons µm−2/s | 5 min | Continuous | — | 500 nm; Halogen light |
Light increases DA release in the primate retina by altering horizontal cell receptive field diameter. | |
| Parkinson and Rando 252 | Rabbit | 48 h | — | 50 foot candela | 48 h | Continuous | — | Fluorescent light | Light activates dopaminergic neurons with increased DA turnover, and synthesis and increased metabolites levels in the retina. | |
| Luo et al. 127 | Guinea pig | 8 weeks | FLM and FDM | 600 lux | 12 h | 0.5 Hz flicker | 6 a.m.–6 p.m. | 505 nm, 2850 K colour temperature; LEDs |
FLM and FDM both induce myopia; however, FLM had increased levels of both retinal and vitreal DA and DOPAC levels, which decreased in the FDM group compared with the control group. | |
| Kirsch and Wagner 211 | Crucian carp | — | — | 0.5 µW/mm2 for 300 ms | 20–40 min | 0.5–3 Hz Flicker | — | 15V halogen lamp | Endogenous DA release in the retina is stimulated by flickering light. GABA inhibits DA release. |
|
| EGR-1 / ZENK/NGFI-A | Fischer et al. 185 | Chicken | 4 days | FDM, LIM (−5, and −7D) and LIH (+7D) | 0.8 cd/m2 | 0.5, 2, 4, 10 or 24 h | Continuous | 7 a.m.–7 p.m. | 100 W incandescent bulb | ZENK synthesis was enhanced with LIH and removal of FD goggles; and suppressed with LIM and FD. ZENK synthesis in bipolar cells is induced by light. |
| Bitzer and Schaeffel 204 | Chicken | 1 day | LIM (−7D) and LIH (+7D) |
300 and 1000 lux | 40 and 120 min | Continuous | — | 555 ± 10 nm and white light | ZENK-expressing cells were increased with positive lenses and reduced with negative lenses after 40 min of ⩾ 300 lux of light. | |
| Agarwal 253 | Mice | 1 day | — | 3–5 foot candles | 12 h | Continuous | 8 a.m.–8 p.m. | — | Higher levels of NGFI-A mRNA were reported in animals kept in light than in the dark. Combination of light and dark cycle exposure also elevates NGFI-A mRNA levels. | |
| Brand et al. 205 | Mice | 8 days | FDM | 120 lux | 15, 30, 60, 90, 120, 360, or 720 min in morning and 30 min in evening | Continuous and Intermittent | 8 a.m.–8 p.m. | 30 W cool white light. | Increases in Egr-1 mRNA expression are associated with both the onset and offset of light.Both Egr-1 mRNA and protein expression levels were reduced in FDM eyes than in the fellow control eyes after 30 and 60 min of light, respectively. Gradual attenuation of retinal image illumination by NDF had no significant effect on Egr-1 mRNA levels. | |
| Zhong et al. 206 | Rhesus monkey | — | LIH (+3D) and FDM | 20% reduced light by diffuser | 30, 60 and 240 min | Continuous | 8 a.m.–8 p.m. | — | 20% reduction in light intensity has no effect on the level of Egr-1, rather optical defocus by plus lens increases Egr-1 levels than plano lenses | |
| NO | Donati et al. 254 | Pig | 1 day | — | 1.73 × 1015 photons mm2 /sec | — | 16 Hz flicker | — | 50 W tungsten halogen lamp | NO in the vitreous of miniature pig eye increased due to flicker light illumination. |
| Hoshi et al. 190 | Rat | 1 day | — | 0.083–1.83 mW/cm2 at the cornea | 3 h | Continuous and 3 Hz Flicker | 11 a.m.–2 p.m. | — | Vitreal NO increased under constant and flickering light, whereas it decreased under dark adaptation | |
| Neal et al. 255 | Rabbit | 1 day | — | 590 lux | 10 min | Continuous and 3 Hz Flicker | — | — | Both continuous and flickering light stimulated the release of retinal NO. | |
| Sekaran et al. 256 | Carp | 1 day | — | 100 µW/cm2 | 10 min | Continuous and 3 Hz Flicker | — | Tungsten lamp | Both continuous and flickering light stimulated the release of retinal NO. | |
| 5-HT | Li et al. 128 | Guinea pig | 6 weeks | FDM and FLM | 600 lux | 12 h | 0.5 Hz flicker | 6 a.m.–6 p.m. | 600 nm- | Flickering light causes progressive myopia and 5-HT and 5-HT2A receptor increased both in FDM and FLM. |
| GABA | Schmid et al. 214 | Chicken | 4 days | FDM | 1500 lux + NV | 2 h | Continuous | — | Fluorescent light | GABA agonists inhibited the protective effect of NV against FDM. Exposure to light for 2 h lowered the inhibitory activity of GABA in FDM eyes. |
| Lam 215 | Goldfish | 1 day | — | Altered with NDF | 4–5 h | 15 flashes per min | — | 60 W tungsten filament bulb | GABA level in the retina was increased with flashing light which was directly proportional to the intensity. Moreover, total GABA in light-adapted retina was more than in the dark-adapted retina. | |
| RA | McCaffery et al. 217 | Mice | 5–30 days | — | — | 10 min and 2 h | Continuous | — | Bright room light | Light causes a direct increase of retinal RA synthesis compared with dark. |
| Yu et al. 257 | Guinea pig | 4 weeks | LIM (−5D) | White 580 lux, blue 500 lux | 12 h | Continuous | 6 a.m.–6 p.m. | White 5000K, blue 440 nm | Animals exposed to blue light were less myopic, had shorter AL and less retinal RA compared with white light. | |
| Melanopsin and ipRGC | Dkhissi-Benyahya et al. 234 | Mice | 7 days | — | 2.8 × 1014 photons/cm2/s) | 15 min | Continuous | — | 480 nm | Melanopsin KO prevented the light-dependent increase in DA, which comparatively increased in dark. |
| Zhang et al. 242 | Mice | — | — | 1.47 x 1013 and 1.9 x 1014 photons cm−2 s−1 | 12 h | 3 sec pulses | — | 470, 525 and 630 nm; LEDs |
Melanopsin photopigment is necessary for light responses in retinal DA neurons. | |
| Wang et al. 138 | Guinea pig | 8 weeks | — | Blue: 0.46 W/cm2
Green: 1.05 W/cm2 White: 0.80 W/cm2 |
12 h | Continuous | 8 a.m.–10 p.m. | Blue:480 nm Green: 530 nm White: 5000 K; LEDS |
Animals exposed to green light had higher pineal gland melatonin, myopic refractive error, longer AL and lower retinal melanopsin in their retinas than the blue light group. Contrarily, MT1 receptor mRNA in retina and sclera were higher in green light group than the blue light group. | |
AL, axial length; DA, dopamine; DOPA, dihydroxyphenylalanine; DOPAC, 3,4-dihydroxyphenylacetic acid; EGR-1, early growth response protein-1; EL, experimental light; FD, form deprived; FDM, form deprivation myopia; FLM, flickering light–induced myopia; GABA, gamma amino butyric acid; 5-HT, 5-hydroxytryptamine; 5-HT2A, 5-HT receptor 2A; ipRGC, intrinsically photosensitive retinal ganglion cells; KO, knockout; LED, light emitting diodes; LIH, lens-induced hyperopia; LIM, lens-induced myopia; MT1, melatonin receptor type 1; NDF, neutral density filter; NL, normal light; NO, nitric oxide; NV, normal vision; RA, retinoic acid; UV, ultra-violet; VL, violet light.
Ocular pathways of myopia control
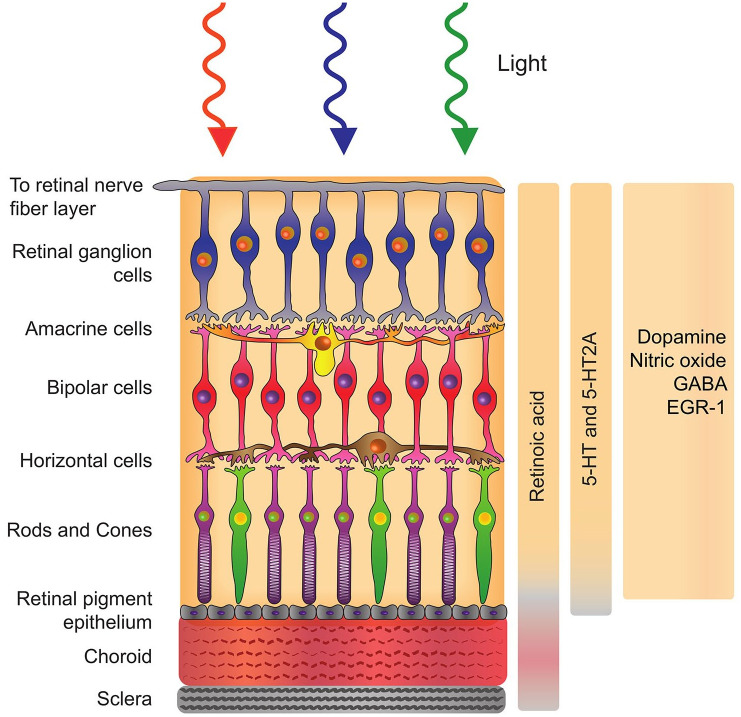
The sclera plays a vital role in determining the shape and size of the eye; consequently, it has long been of high interest for myopia intervention strategies with emphasis given to understanding the mechanism of pharmacological molecules on the scleral metabolism. 163 Nevertheless, subsequent studies have identified that the retina is the key signal regulator in the emmetropization process. Although there exist more convincing reports on the involvement of retinal signalling molecules which include DA and acetylcholine during emmetropization, the particular cell type and associated biochemical pathway involved are yet to be unveiled. 164 It is further hypothesized that these retinal molecules act through a cascade to communicate with retinal pigment epithelium (RPE) and choroid, which in turn releases a series of different molecules that regulate the scleral growth and remodelling 164 (Figure 2).
Figure 2.
Schematic representation of the retina, retinal pigment epithelium, choroid, and sclera with corresponding molecules modulated by light stimulation.
Light, dopamine and refractive error regulation
DA, a neurotransmitter implicated in several physiological, cerebral and retinal functions, has been shown to be involved in the biochemical signalling cascade that controls emmetropization.165–167 In the retina, DA is released by amacrine cells and/or interplexiform cells, depending on the species. 168 A large body of evidence is available to support the hypothesis that DA is implicated in ocular growth 74 and a dose-response relationship has been established between DA synthesis and light intensity.169–171
Light-associated DA activity is mediated possibly through the D2 receptor pathway, thereby altering the signal that triggers axial elongation. Among DA receptor subtypes involved in the signaling, D2-like (D2 and D4) receptor plays a key role in FDM. 172 The ocular refractive development relies mainly on the balance between the activation of D1-like and D2-like receptors. Overactivation of D1-like receptor has been reported to induce hyperopia and vice versa. 173 DA can influence the development of myopia via changes in spatial tuning of cellular responses in the retina. Receptive fields are adjusted based on D1 and D2/D4 receptor activation via varied concentrations of DA. As light influences the release of DA, it is believed that different light conditions play a role in the spatial tuning of retinal cellular responses. 173 In the case of rod–cone gap junctions in bright light conditions, conductance is decreased as DA levels are high. The binding of DA to D2/D4 receptors reduces adenylate cyclase activity, leading to reduced cyclic AMP (cAMP) production and protein kinase A (PKA) inactivity.174,175 Rod–cone gap junctions are thus left uncoupled, resulting in reduced receptive field sizes and better visual acuity. Under low light conditions, DA release is low and rod–cone gap junctions are coupled in order for dim objects to be detected. 176 Apart from D2/D4 receptors, DA can also bind to D1 receptors. Horizontal cell gap junctions and AII amacrine cell gap junctions are regulated via this pathway.177,178 In high light levels, DA binds to D1 receptors and activates adenylate cyclase. Increased cAMP concentrations activate PKA, reducing conductance between horizontal cell-horizontal cell gap junctions. In AII amacrine-AII amacrine gap junctions, the production of protein phosphatase 2A (PP2A) is believed to reduce conductivity and coupling. 179 Horizontal cell coupling is involved in image contrast optimization 180 while AII amacrine cell coupling is involved in the summation of identical signals and removal of noise. 181 This control in rod–cone, horizontal cell, and AII amacrine gap conductance optimizes spatial tuning in the retina and allows for the production of high acuity images with good contrast in different light conditions. Light-regulated DA levels may thus control the development of myopia by modifying the attributes of perceived images.
Retinal DA levels are decreased in chicken eyes subjected to form deprivation, 182 but return to normal levels upon cessation of the latter. 183 Interestingly, Schwahn and Schaeffel 122 were able to prevent FDM but not able to retain DA content and release in the retina with the use of 12 Hz at a duty cycle of 4% flickering lights. The change in retinal DA levels for different durations of flicker lights, however, did not correlate with the level of inhibition of myopia. Conversely, ocular DA levels are also dependent upon the spectral content of light with a trend towards higher retinal DA release under UV light compared with white light. 132 The protective effect of bright light against myopia was also found to be dismissed when chickens wearing diffusers were injected with spiperone, a DA antagonist, before exposure to bright light, 40 indicating DA’s role in the light-driven myopia-control pathway. In chickens that were form deprived, exposure to 15,000 lux of light resulted in partial rescue of retinal DA levels, while it did not alter the levels of ZENK, an immediate early gene in the amacrine cells involved in the regulation of ocular axial length of.184,185 Furthermore, retinal DA may modulate choroidal thickening and a subsequent reduction in axial length by triggering other neuromodulators such as nitric oxide (NO). 186
According to Hartline, 187 luminance-associated visual information is decoded in the brain through parallel pathways, namely, increments (ON) and decrements (OFF). Low luminance and optical blur which are considered as key risk factors of myopia, trigger the ON luminance pathway and thereby lower the release of retinal DA. 188 The role of ON and OFF pathways in refractive error development is extrapolated using a genetically modified mouse model wherein the nob mice were subjected to FDM. The results from the study exhibit that myopia development in mouse eye is stimulated primarily due to defect in ON pathway in addition to the low DA level and blurred vision imposed by form deprivation which is evident from the loss of visual function along the ON pathway. 189
Other neurotransmitters and signaling molecules involved in light-mediated refractive error regulation
Nitric oxide
NO is a neurotransmitter that is involved in the regulation of retinal responses. Vitreous concentrations of NO are dependent on ambient light conditions 190 and may play a role in the protection against developmental myopia. In a study conducted by Carr and Stell, 191 intravitreal injection of NO synthase substrate L-arginine (L-arg) or NO donor sodium nitroprusside was able to dose-dependently inhibit the development of myopic refraction and axial elongation. In addition, NO may also play a role in the regulation of choroidal thickness, as intravitreal injection of NO synthase inhibitor NG-nitro-L-arginine methyl ester rapidly and transiently inhibited choroidal thickening and promoted choroidal thinning in chicken eyes recovering from FDM and in eyes mounted with +15D lenses. 192
Similar to DA, NO affects horizontal cell gap junction conductance and coupling. In the presence of bright light, NO is released in amacrine cells and activates guanylate cyclase after diffusion into retinal neurons. This results in an increase in cyclic guanosine monophosphate (cGMP) levels and the activation of cGMP-dependent protein kinase. Phosphorylation or dephosphorylation of connexin 35 in chickens and connexin 36 in mammals present in the retinal gap junctions alters horizontal and amacrine cell gap junction conductivity via NO, which in turn stimulates retinal cell uncoupling.193–195 Subsequently, this increases the overall optokinetic contrast sensitivity in chicken eyes, especially at high spatial frequencies. 196 NO may thus contribute to the prevention of myopia development by modulating the receptive field sizes and spatial contrast sensitivity.197,198
Atropine
Although low-dose atropine eye drops are used to prevent or slow myopia development in children, 198 the underlying mechanisms of atropine action remain poorly understood. Atropine is a muscarinic antagonist that also acts as a potential alpha 2-adrenergic receptor (α2A-ADR) antagonist. In chickens, that lack muscarinic receptors in the ciliary muscles, atropine can still reduce experimental myopia development, 199 and both atropine and other α2A-ADR antagonists have been shown to stimulate DA release by activating the tyrosine hydroxylase immunoreactive amacrine cells.200,201 Conversely, α2A-ADR agonists strongly suppress the release of DA.200,201 Interestingly, the actions of atropine and bright light of 8500 lux were recently reported as additive, increasing DA release in the vitreous of chickens that received an intravitreal atropine injection and were exposed to bright light for 1.5 h, compared with chickens that received the same treatment but were exposed to 1.5 h of standard light of 500 lux. 201
EGR1 (ZENK)
Early growth response protein-1 (EGR-1) or ZENK is a protein encoded by the EGR-1 gene. Lower levels of EGR-1 or ZENK have been associated with increased axial elongation and vice versa. 202 EGR-1 is considered as a well-established and documented protective gene for myopia.203–205 Upregulation of ZENK is associated with inhibition of ocular elongation linked with hyperopic defocus and the recovery from form deprivation. The modulatory expression of ZENK is clearly evident in amacrine cells containing glucagon. In chickens, EGR-1 suppresses ocular axial elongation and when EGR-1 is knocked down in mice, the eye exhibited distinct axial elongation. 202 It was found that 30 min of exposure to visual stimuli following form deprivation can regulate ocular growth by modulating the expression of ZENK. 185
The intensity of light exposure was reported to be positively correlated with the ZENK expression in chicken retinal amacrine cells; however, this effect is not related to the duration of light exposure. 185 Albeit, Ashby et al. 203 reported bidirectional response of Egr-1 mRNA levels with a 50% decrease and >200% elevation in Egr-1 mRNA levels in lens-induced (−5D) myopic eyes of guinea pigs during the induction (day 7) and the recovery periods, respectively.
ZENK-responsive bipolar cells are usually the cone ON-bipolar cells; hence, the bipolar cells are seen to have ZENK induction as a function of light intensity. 206 Furthermore, EGR-1 mRNA transcript levels are also dependent on the spectral content of light; chickens reared under VL for 7 days (12 h light/dark cycle) showed upregulation of EGR-1 in chorioretinal tissues, compared with blue light exposure. In addition, the eyes exposed to VL were significantly less myopic compared with those exposed to fluorescent light. 148
5-HT and 5-HT2A receptor
Serotonin [i.e. 5-hydroxytryptamine (5-HT)] is a neurotransmitter synthesized in central nervous system. Lens-induced myopic eyes of Guinea pigs have significantly higher levels of 5-HT and 5-HT2A receptor. 207 Constant square-wave 0.5 Hz flickering light can induce myopia of progressive nature in guinea pigs. 128 The 5-HT and 5-HT2A receptors were found to increase in both myopia due to flickering light and form deprivation. This indicates that 5-HT is possibly involved in the induction of myopia and it acts by binding to 5-HT2A receptor. 128 The 5-HT2A receptor expression was found to be increased and the concentrations of norepinephrine and epinephrine were decreased in guinea pigs’ eyes following both the exposure to flickering light and form deprivation. As hypothesized by Li et al. 128 by binding to 5-HT2A receptor, 5-HT may strengthen scleral remodelling and influence ocular axial growth.
Gamma aminobutyric acid
Gamma aminobutyric acid (GABA) is an inhibitory neurotransmitter in the retina and brain. There are three types of GABA receptors, the GABA(A) receptors facilitate the feedback between horizontal cells and cones, GABA(B) receptors regulate intracellular messengers and neuronal function, and GABA(C) receptors are involved in mediating GABAergic synaptic functions in the outer and inner retinas. Eye growth and refractive development in chickens is regulated by these 3 receptors of GABA.208,209 GABA(C) antagonists were found to be most effective at preventing LIM, although other receptors can also prevent myopia. 210 GABA(A) and GABA(C) agonists decreases DA release in the retina, whereas GABA antagonists increase DA release.209,211 Moreover, amacrine cells release GABA molecules which bind to fast-acting ionotropic receptors in the retina.212,213 In FDM, the DA and GABAergic neurotransmitter pathways interact. Exposure to fluorescent lights (1500 lux) for 2 h lowers the inhibitory activity of GABA in form-deprived eyes. 214 This protective effect of bright light against FDM while overcoming the effects of GABA agonists involves an increase in the D2 DA receptor activity. 214 In contrary, goldfish retina demonstrated an increase in GABA level directly proportional to flashing light intensity. 215
Retinoic acid
Retinoic acid (RA) is a lipid-soluble metabolite derived from retinol or vitamin A, which acts as a regulator of growth, differentiation, and development of several cell types, including epithelial and neuronal cells. RA also acts as a neuromodulator that sends information regarding the illumination to the outer plexiform layer of retina. 216 McCaffery et al. 217 first reported the light-mediated increase in RA synthesis in both retina and RPE samples of mice exposed to bright room light for 10 min. This increase was also directly proportional to the age of the mice, wherein the older mice reported increased RA release in response to light. Similarly, the impact of 20 min of bright room light retina and RPE samples revealed a strong RA activity when compared with samples kept in darkness. 218 Dirks et al. 218 also noted that RA synthesis is light-dependent and DA-independent in the carp eye indicating that these two modulatory systems are not inter-dependent but act in parallel. Apart from this, studies in various animal models like chickens,219–221 guinea pigs222,223 and marmosets 224 revealed that changes in RA synthesis are species-dependent, where myopia induction led to a decreased RA level in chickens, and increased RA levels in guinea pigs and marmosets.222,224,225 Choroidal RA biosynthesis is regulated exclusively by retinaldehyde dehydrogenase 2 (RALDH2) 226 and fundal tissue aldehyde dehydrogenase-2 (ADH2). 219 In the sclera, RA and glycosaminoglycan (GAG) levels observe an inverse relationship; with increasing RA levels, the GAG levels decreases and vice versa.220,224,225 The mechanism of action of RA might be through the remodelling of scleral extracellular matrix 224 or the modulation of cell coupling.227,228
Melanopsin and intrinsically photosensitive retinal ganglion cells
Melanopsin is an atypical photopigment expressed in ganglion cells, rendering them intrinsically photosensitive.229,230 These intrinsically photosensitive retinal ganglion cells (ipRGCs) complement the visual photoreceptors and convey photo transduced signals to nonvisual centres in the brain, including the suprachiasmatic nucleus governing most circadian rhythmic expressions in the body (e.g. sleep, alertness, melatonin secretion at night) (for review, see Najjar and Zeitzer 231 ). Melanopsin is predominantly sensitive to bright blue light (~480nm), a wavelength reported to induce hyperopia and reverse experimental myopia in some animal models130–133 and the increase in ocular DA levels upon bright light exposure could potentially be due to the stimulation of melanopsin and the synaptic and functional connection between the ipRGCs and dopaminergic amacrine cells.232,233 In addition, melanopsin knockout mice display a decline in ocular DA levels, 234 and preliminary findings highlight a direct, yet unclear, role of melanopsin in refractive error development. 235 In humans, some authors attributed a reduction in sleep quality observed in highly myopic children to a decreased ipRGC function in myopic eyes, in addition to high demands at school and distress over poor vision. 236 Nevertheless, studies investigating the pupillary light reflex reported no alterations in the response in mild and moderate myopic participants and no associations between refractive error and the ipRGC inputs to the pupil control pathway.237,238
The retinal clock plays an essential role in adapting retinal physiology and visual function to the light/dark changes and holds with its outputs (e.g. melatonin, DA) a major role in the regulation of eye growth and refractive error development in birds and mammals. 239 A additional, potential pathway for photic ocular growth control involves the phase shifting aptitudes of the retinal clock by light 240 through the potential contribution of ipRGCs, neuropsin (OPN5), 241 rods and/or middle-wavelength (MW) cones and excitatory influences upon dopaminergic amacrine cells.242–244
The development of light-therapy strategies for myopia
While increasing exposure to outdoor light levels can successfully be implemented through national outdoor programmes, to prevent the onset of myopia and slow myopia progression in progressing myopes, implementation remains suboptimal in some circumstances. 258 On the other hand, the optimization of architectural lighting or development of light-therapy devices requires a holistic understanding of the benefits and side effects of light characteristics (intensity, timing, pattern and spectrum) on ocular growth and neurophysiology (e.g. circadian rhythms, sleep and alertness). Yet, some studies have shown promising results by either increasing light intensity indoors in school-based interventions 43 or adopting a daily light-therapy approach. 259 According to Hua et al. 43 increasing the ambient light levels to 558 lux at the desk and 440 lux at the blackboard in classrooms can reduce the percentage of new myopia onset. Concomitantly, a pilot study carried out in China has shown that both students and teachers can adapt to a bright classroom having a light intensity between 1,330 and 4,060 lux. 260 These findings suggest that moderately high intensities of light indoors (e.g. classrooms) could yield sufficient protection against myopia in children. 42 In accordance with the latter statement, yet adopting a more individualized light-therapy approach using light delivery glasses, Read et al. 259 showed that exposure to ~500 lux for 30 min in the morning for 1 week increased in choroidal thickness in young adults. Although Read et al. did not investigate the impact of light therapy on refractive error development per se, these results may be promising given the association between choroidal thickness and refractive error development. The spectral modulation of light reaching the retina can also offer promising therapeutics for myopia. A recent study by Ofuji et al. 261 have shown that wearing VL-transmitting glasses for 2 years, and engaging in outdoor activity for 2 h can reduce ocular axial length and increase choroidal thickness in a child with high myopia. As reported by Torii et al., 148 an increased expression of EGR1 gene may have led to this protective effect of VL- transmitting glasses against myopia. In addition, a DA increase in response to the UV light could also be postulated based on data available from the form-deprived chicken model. 132 Neuropsin (OPN5), is a UV-sensitive and bistable (λmax: 380 nm and 470nm) photopigment that is ubiquitously expressed in mammalian ganglion cells.244,262 In a recent study, it was reported that VL stimulation prevented myopia in mice and identified OPN5-expressing retinal ganglion cells as a key for emmetropization in this animal model. 241 On the other side of the spectrum, a randomized controlled trial (ClinicalTrials.gov Identifier: NCT04073238) is ongoing at Zhongshan Ophthalmic Center to test the efficacy of low-level red light therapy (LLLT) to control the progression of myopia, the authors hypothesize that effect of LLLT is potentially through inhibiting scleral hypoxia and thereby improving the choroidal blood perfusion. In a similar study, slowing of myopia progression was shown among children treated with low-level laser therapy for 6 months, probably by inhibiting the NO synthesis and inflammatory cytokines thereby decreasing the severity of oxidative stress.263,264 The effects of the spectral composition of light have to be corroborated carefully with its intensity, pattern, timing, and its effects on circadian system when considering light therapy as a potential treatment for myopia in children.
With the availability of inexpensive lighting systems and electronics nowadays, many nonmedical devices ranging from side lamps to wearables are on the market, with the claim of alleviating the myopia epidemic. Unfortunately, many of such devices overstate their claims and are sometimes not data-driven. Today, to optimize indoor light-therapy strategies for myopia we would suggest the following pathway:
I. Elucidate interspecies differences in the light-driven emmetropization process;
II. Recognize the most suitable animal model(s) for studying the impact of light on emmetropization. This model(s) should closely mimic human ocular physiology and refractive error development;
III. Pinpoint anti-myopiagenic light parameters in the selected animal model and test the synergetic anti-myopiagenic aptitudes of light parameters. For example, test dynamic lighting and tailor the spectral composition and timing of moderate light intensities (indoor) across the day;
IV. Establish reliable short-term biomarkers allowing a fast and reliable, evaluation of the impact of light on refractive error development in humans. Considered one of the earliest observable ocular changes during the development of refractive errors and ocular growth, short-term changes in choroid thickness may represent a reliable biomarker for the signalling cascade that results in longer term changes in ocular growth in response to light. While myopiagenic stimuli (e.g. accommodation, hyperopic defocus) are often associated with a transient thinning of the choroid as compared with anti-myopiagenic stimuli (e.g. anticholinergic agent, myopic defocus) which are associated with a transient choroidal thickening;
V. Evaluate (1) the safety and (2) efficacy of these light parameters in humans by investigating short-term changes in biomarkers;
VI Confirm the preventive efficacy of these light parameters in a randomized longitudinal clinical trial;
VII. Develop data-driven light-therapy strategies/devices for myopia control in children.
Conclusion
In this narrative review, we presented the current knowledge on light-driven modulation of ocular growth and emmetropization based on studies in human and animal models. In addition, we also highlighted potential neurobiological mechanisms involved in the protective effect of light on myopia onset and suggested a potential pathway for the translational development of noninvasive light-therapy strategies for myopia prevention in children. Overall, available data from humans and experimental animal models suggest that high-intensity light even in discontinuous patterns is capable of preventing myopia onset. These findings support the need for well-devised outdoor programmes in children, especially in countries where myopia is prevalent. Nevertheless, less intense light levels (~500 lux) delivered in classrooms or using light delivery wearables may also be protective against myopia. Similarly, the spectro-temporal tuning of such moderate light levels has shown promise for myopia control, especially in animal models. However, the development of tailored light-therapy strategies for myopia control in humans remains challenging given the gaps in understanding the synergetic impact of light parameters within other environmental features. In addition, while working on this review, our team noticed a lack of standardized reporting of experimental light characteristics between studies. The standardization of light reporting through a reporting guideline could allow for a better comparison of findings and protocols between studies, and enable more effective meta-analyses.
During this COVID-19 pandemic, toddlers, children and teenagers alike are exposed to unprecedented amounts of indoor time, sparking concerns over an ever more severe myopia boom.265–268 Notwithstanding these peculiar circumstances, today there is a need for consensus on optimal, feasible and noninvasive light interventions for myopia prevention in children be it through increased time outdoors or adapted architectural lighting or light-therapy devices.
Footnotes
Author contributions: Manuscript writing: ARM, CL, SB, LWYS. Critical revisions: VAB, SSM, DM, RPN. Study design, conceptualization and funding acquirement: RPN.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by A∗STAR-JANSSEN World without disease (A∗JWWD) (18/4/91/00/099), and by Singapore Government through the Industry Alignment Fund – Industry Collaboration Project Grant (I1901E0038) and Johnson and Johnson Vision.
ORCID iDs: Carla Lança  https://orcid.org/0000-0001-9918-787X
https://orcid.org/0000-0001-9918-787X
Raymond P. Najjar  https://orcid.org/0000-0002-3770-2300
https://orcid.org/0000-0002-3770-2300
Contributor Information
Arumugam R. Muralidharan, Singapore Eye Research Institute, Singapore Ophthalmology and Visual Sciences Academic Clinical Programme, Duke-NUS Medical School, Singapore.
Carla Lança, Singapore Eye Research Institute, Singapore; Comprehensive Health Research Center (CHRC), Escola Nacional de Saúde Pública, Universidade Nova de Lisboa, Lisboa, Portugal; Escola Superior de Tecnologia da Saúde de Lisboa (ESTeSL), Instituto Politécnico de Lisboa, Lisboa, Portugal.
Sayantan Biswas, Singapore Eye Research Institute, Singapore.
Veluchamy A. Barathi, Singapore Eye Research Institute, Singapore Ophthalmology and Visual Sciences Academic Clinical Programme, Duke-NUS Medical School, Singapore; Department of Ophthalmology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
Low Wan Yu Shermaine, Singapore Eye Research Institute, Singapore.
Saw Seang-Mei, Singapore Eye Research Institute, Singapore; Ophthalmology and Visual Sciences Academic Clinical Programme, Duke-NUS Medical School, Singapore; Saw Swee Hock School of Public Health, National University of Singapore, Singapore.
Dan Milea, Singapore Eye Research Institute, Singapore; Ophthalmology and Visual Sciences Academic Clinical Programme, Duke-NUS Medical School, Singapore; Singapore National Eye Centre, Singapore.
Raymond P. Najjar, Visual Neurosciences Group, Singapore Eye Research Institute, The Academia, 20 College Road, Discovery Tower Level 6, Singapore 169856; Ophthalmology and Visual Sciences Academic Clinical Programme, Duke-NUS Medical School, Singapore.
References
- 1. Carr BJ, Stell WK. The science behind myopia. In: Kolb H, Fernandez E, Nelson R. (eds) Webvision: the organization of the retina and visual system, 2017. http://www.ncbi.nlm.nih.gov/pubmed/29266913. [PubMed]
- 2. Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt 2012; 32: 3–16. [DOI] [PubMed] [Google Scholar]
- 3. Rudnicka AR, Kapetanakis VV, Wathern AK, et al. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. Br J Ophthalmol 2016; 100: 882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galvis V, Tello A, Otero J, et al. Refractive errors in children and adolescents in Bucaramanga (Colombia). Arq Bras Oftalmol 2017; 80: 359–363. [DOI] [PubMed] [Google Scholar]
- 5. Galvis V, Tello A, Otero J, et al. Prevalence of refractive errors in Colombia: MIOPUR study. Br J Ophthalmol 2018; 102: 1320–1323. [DOI] [PubMed] [Google Scholar]
- 6. Lee YY, Lo CT, Sheu SJ, et al. What factors are associated with myopia in young adults? A survey study in Taiwan Military Conscripts. Invest Ophthalmol Vis Sci 2013; 54: 1026–1033. [DOI] [PubMed] [Google Scholar]
- 7. Yam JC, Tang SM, Kam KW, et al. High prevalence of myopia in children and their parents in Hong Kong Chinese Population: the Hong Kong Children Eye Study. Acta Ophthalmol. Epub ahead of print 24 January 2020. DOI: 10.1111/aos.14350. [DOI] [PubMed] [Google Scholar]
- 8. Jung SK, Lee JH, Kakizaki H, et al. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in Seoul, South Korea. Invest Ophthalmol Vis Sci 2012; 53: 5579–5583. [DOI] [PubMed] [Google Scholar]
- 9. Pan CW, Wu RK, Liu H, et al. Types of lamp for homework and myopia among Chinese school-aged children. Ophthalmic Epidemiol 2018; 25: 250–256. [DOI] [PubMed] [Google Scholar]
- 10. Grzybowski A, Kanclerz P, Tsubota K, et al. A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol 2020; 20: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ding BY, Shih YF, Lin LLK, et al. Myopia among schoolchildren in East Asia and Singapore. Surv Ophthalmol 2017; 62: 677–697. [DOI] [PubMed] [Google Scholar]
- 12. Saw SM, Gazzard G, Shih-Yen EC, et al. Myopia and associated pathological complications. Ophthalmic Physiol Opt 2005; 25: 381–391. [DOI] [PubMed] [Google Scholar]
- 13. Ichibe M, Yoshizawa T, Murakami K, et al. Surgical management of retinal detachment associated with myopic macular hole: anatomic and functional status of the macula. Am J Ophthalmol 2003; 136: 277–284. [DOI] [PubMed] [Google Scholar]
- 14. Wong TY, Ferreira A, Hughes R, et al. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol 2014; 157: 9–25.e12. [DOI] [PubMed] [Google Scholar]
- 15. Chua SYL, Sabanayagam C, Cheung YB, et al. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol Opt 2016; 36: 388–394. [DOI] [PubMed] [Google Scholar]
- 16. Saw SM, Nieto FJ, Katz J, et al. Factors related to the progression of myopia in Singaporean children. Optom Vis Sci 2000; 77: 549–554. [DOI] [PubMed] [Google Scholar]
- 17. French AN, Ashby RS, Morgan IG, et al. Time outdoors and the prevention of myopia. Exp Eye Res 2013; 114: 58–68. [DOI] [PubMed] [Google Scholar]
- 18. Low W, Dirani M, Gazzard G, et al. Family history, near work, outdoor activity, and myopia in Singapore Chinese preschool children. Br J Ophthalmol 2010; 94: 1012–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lim DH, Han J, Chung T-Y, et al. The high prevalence of myopia in Korean children with influence of parental refractive errors: the 2008-2012 Korean National Health and Nutrition Examination Survey. PLoS ONE 2018; 13: e0207690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Solouki AM, Verhoeven VJM, van Duijn CM, et al. A genome-wide association study identifies a susceptibility locus for refractive errors and myopia at 15q14. Nat Genet 2010; 42: 897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hysi PG, Young TL, Mackey DA, et al. A genome-wide association study for myopia and refractive error identifies a susceptibility locus at 15q25. Nat Genet 2010; 42: 902–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verhoeven VJM, Hysi PG, Wojciechowski R, et al. Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia. Nat Genet 2013; 45: 314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tedja MS, Haarman AEG, Meester-Smoor MA, et al. IMI – myopia genetics report. Invest Ophthalmol Vis Sci 2019; 60: M89–M105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li SM, Li H, Li SY, et al. Time outdoors and myopia progression over 2 years in Chinese children: the Anyang Childhood Eye Study. Invest Ophthalmol Vis Sci 2015; 56: 4734–4740. [DOI] [PubMed] [Google Scholar]
- 25. Huang HM, Chang DS, Wu PC. The association between near work activities and myopia in children – a systematic review and meta-analysis. PLoS ONE 2015; 10: e0140419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin LL, Shih YF, Hsiao CK, et al. Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann Acad Med Singap 2004; 33: 27–33. [PubMed] [Google Scholar]
- 27. Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res 2005; 24: 1–38. [DOI] [PubMed] [Google Scholar]
- 28. Lanca C, Yam JC, Jiang W, et al. Near work, screen time, outdoor time and myopia in schoolchildren in the Sunflower Myopia AEEC Consortium. Acta Ophthalmol. Epub ahead of print 17 June 2021. DOI: 10.1111/aos.14942. [DOI] [PubMed] [Google Scholar]
- 29. Jin JX, Hua WJ, Jiang X, et al. Effect of outdoor activity on myopia onset and progression in school-aged children in northeast China: the Sujiatun Eye Care Study. BMC Ophthalmol 2015; 15: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. He M, Xiang F, Zeng Y, et al. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA 2015; 314: 1142–1148. [DOI] [PubMed] [Google Scholar]
- 31. Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology 2008; 115: 1279–1285. [DOI] [PubMed] [Google Scholar]
- 32. Sherwin JC, Reacher MH, Keogh RH, et al. The association between time spent outdoors and myopia in children and adolescents: a systematic review and meta-analysis. Ophthalmology 2012; 119: 2141–2151. [DOI] [PubMed] [Google Scholar]
- 33. Donovan L, Sankaridurg P, Ho A, et al. Myopia progression in Chinese children is slower in summer than in winter. Optom Vis Sci 2012; 89: 1196–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res 2012; 31: 622–660. [DOI] [PubMed] [Google Scholar]
- 35. Wu PC, Chen CT, Lin KK, et al. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology 2018; 125: 1239–1250. [DOI] [PubMed] [Google Scholar]
- 36. Read SA, Vincent SJ, Tan CS, et al. Patterns of daily outdoor light exposure in Australian and Singaporean children. Transl Vis Sci Technol 2018; 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Flitcroft DI, Harb EN, Wildsoet CF. The spatial frequency content of urban and indoor environments as a potential risk factor for myopia development. Invest Ophthalmol Vis Sci 2020; 61: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Read SA, Collins MJ, Vincent SJ. Light exposure and eye growth in childhood. Invest Ophthalmol Vis Sci 2015; 56: 6779–6787. [DOI] [PubMed] [Google Scholar]
- 39. Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci 2009; 50: 5348–5354. [DOI] [PubMed] [Google Scholar]
- 40. Ashby RS, Schaeffel F. The effect of bright light on lens compensation in Chicks. Invest Ophthalmol Vis Sci 2010; 51: 5247–5253. [DOI] [PubMed] [Google Scholar]
- 41. Smith EL, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci 2012; 53: 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morgan IG. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology 2018; 125: 1251–1252. [DOI] [PubMed] [Google Scholar]
- 43. Hua WJ, Jin JX, Wu XY, et al. Elevated light levels in schools have a protective effect on myopia. Ophthalmic Physiol Opt 2015; 35: 252–262. [DOI] [PubMed] [Google Scholar]
- 44. Rucker FJ. The role of luminance and chromatic cues in emmetropisation. Ophthalmic Physiol Opt 2013; 33: 196–214. [DOI] [PubMed] [Google Scholar]
- 45. Tedja MS, Wojciechowski R, Hysi PG, et al. Genome-wide association meta-analysis highlights light-induced signaling as a driver for refractive error. Nat Genet 2018; 50: 834–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dirani M, Tong L, Gazzard G, et al. Outdoor activity and myopia in Singapore teenage children. Br J Ophthalmol 2009; 93: 997–1000. [DOI] [PubMed] [Google Scholar]
- 47. Guggenheim JA, Northstone K, McMahon G, et al. Time outdoors and physical activity as predictors of incident myopia in childhood: a prospective cohort study. Invest Opthalmology Vis Sci 2012; 53: 2856–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guo Y, Liu LJ, Xu L, et al. Outdoor activity and myopia among primary students in rural and urban regions of Beijing. Ophthalmology 2013; 120: 277–283. [DOI] [PubMed] [Google Scholar]
- 49. Jones LA, Sinnott LT, Mutti DO, et al. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci 2007; 48: 3524–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xiang F, He M, Morgan IG. Annual changes in refractive errors and ocular components before and after the onset of myopia in Chinese children. Ophthalmology 2012; 119: 1478–1484. [DOI] [PubMed] [Google Scholar]
- 51. Sun JT, An M, Yan XB, et al. Prevalence and related factors for myopia in school-aged children in Qingdao. J Ophthalmol 2018; 2018: 9781987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lingham G, Mackey DA, Lucas R, et al. How does spending time outdoors protect against myopia? A review. Br J Ophthalmol 2020; 104: 593–599. [DOI] [PubMed] [Google Scholar]
- 53. Qian YS, Chu RY, He JC, et al. Incidence of myopia in high school students with and without red-green color vision deficiency. Invest Ophthalmol Vis Sci 2009; 50: 1598–1605. [DOI] [PubMed] [Google Scholar]
- 54. Read SA, Collins MJ, Vincent SJ. Light exposure and physical activity in myopic and emmetropic children. Optom Vis Sci 2014; 91: 330–341. [DOI] [PubMed] [Google Scholar]
- 55. Verkicharla PK, Ramamurthy D, Nguyen QD, et al. Development of the FitSight fitness tracker to increase time outdoors to prevent myopia. Transl Vis Sci Technol 2017; 6: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gwiazda J, Deng L, Manny R, et al. Seasonal variations in the progression of myopia in children enrolled in the correction of myopia evaluation trial. Invest Ophthalmol Vis Sci 2014; 55: 752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ostrin LA, Sajjadi A, Benoit JS. Objectively measured light exposure during school and summer in children. Optom Vis Sci 2018; 95: 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. French AN, Morgan IG, Mitchell P, et al. Risk factors for incident myopia in Australian schoolchildren. Ophthalmology 2013; 120: 2100–2108. [DOI] [PubMed] [Google Scholar]
- 59. Ho C-L, Wu W-F, Liou YM. Dose-response relationship of outdoor exposure and myopia indicators: a systematic review and meta-analysis of various research methods. Int J Environ Res Public Health 2019; 16: 2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lanca C, Teo A, Vivagandan A, et al. The effects of different outdoor environments, sunglasses and hats on light levels: implications for myopia prevention. Transl Vis Sci Technol 2019; 8: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ashby R. Animal studies and the mechanism of myopia – protection by light? Optom Vis Sci 2016; 93: 1052–1054. [DOI] [PubMed] [Google Scholar]
- 62. Hubel DH, Wiesel TN, LeVay S. Functional architecture of area 17 in normal and monocularly deprived macaque monkeys. Cold Spring Harb Symp Quant Biol 1976; 40: 581–589. [DOI] [PubMed] [Google Scholar]
- 63. Norton TT, Casagrande VA, Sherman SM. Loss of Y-cells in the lateral geniculate nucleus of monocularly deprived tree shrews. Science 1977; 197: 784–786. [DOI] [PubMed] [Google Scholar]
- 64. Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science 1978; 201: 1249–1251. [DOI] [PubMed] [Google Scholar]
- 65. O’Leary DJ, Millodot M. Eyelid closure causes myopia in humans. Experientia 1979; 35: 1478–1479. [DOI] [PubMed] [Google Scholar]
- 66. Robb RM. Refractive errors associated with hemangiomas of the eyelids and orbit in infancy. Am J Ophthalmol 1977; 83: 52–58. [DOI] [PubMed] [Google Scholar]
- 67. Morgan IG, Ashby RS, Nickla DL. Form deprivation and lens-induced myopia: are they different? Ophthalmic Physiol Opt 2013; 33: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res 1995; 35: 1175–1194. [DOI] [PubMed] [Google Scholar]
- 69. Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res 1987; 6: 993–999. [DOI] [PubMed] [Google Scholar]
- 70. Wildsoet CF, Pettigrew JD. Kainic acid-induced eye enlargement in chickens: differential effects on anterior and posterior segments. Invest Ophthalmol Vis Sci 1988; 29: 311–319. [PubMed] [Google Scholar]
- 71. Iuvone PM, Tigges M, Fernandes A, et al. Dopamine synthesis and metabolism in rhesus monkey retina: development, aging, and the effects of monocular visual deprivation. Vis Neurosci 1989; 2: 465–471. [DOI] [PubMed] [Google Scholar]
- 72. Ohngemach S, Hagel G, Schaeffel F. Concentrations of biogenic amines in fundal layers in chickens with normal visual experience, deprivation, and after reserpine application. Vis Neurosci 1997; 14: 493–505. [DOI] [PubMed] [Google Scholar]
- 73. Gao Q, Liu Q, Ma P, et al. Effects of direct intravitreal dopamine injections on the development of lid-suture induced myopia in rabbits. Graefes Arch Clin Exp Ophthalmol 2006; 244: 1329–1335. [DOI] [PubMed] [Google Scholar]
- 74. Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res 2013; 114: 106–119. [DOI] [PubMed] [Google Scholar]
- 75. Nickla DL, Totonelly K. Dopamine antagonists and brief vision distinguish lens-induced- and form-deprivation-induced myopia. Exp Eye Res 2011; 93: 782–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nickla DL, Totonelly K, Dhillon B. Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Exp Eye Res 2010; 91: 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Norton TT. Animal models of myopia: learning how vision controls the size of the eye. ILAR J 1999; 40: 59–77. [DOI] [PubMed] [Google Scholar]
- 78. Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri). Vision Res 1992; 32: 833–842. [DOI] [PubMed] [Google Scholar]
- 79. Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Res 1987; 27: 1139–1163. [DOI] [PubMed] [Google Scholar]
- 80. Gottlieb MD, Joshi HB, Nickla DL. Scleral changes in chicks with form-deprivation myopia. Curr Eye Res 1990; 9: 1157–1165. [DOI] [PubMed] [Google Scholar]
- 81. Curtin BJ, Jampol LM. The myopias: basic science and clinical management. New York: Harper & Row, 1986. [Google Scholar]
- 82. Jonas JB, Wang YX, Dong L, et al. Advances in myopia research anatomical findings in highly myopic eyes. Eye Vis 2020; 7: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schaeffel F, Feldkaemper M. Animal models in myopia research. Clin Exp Optom 2015; 98: 507–517. [DOI] [PubMed] [Google Scholar]
- 84. Rohrer B, Schaeffel F, Zrenner E. Longitudinal chromatic aberration and emmetropization: results from the chicken eye. J Physiol 1992; 449: 363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Riddell N, Crewther SG. Integrated comparison of GWAS, transcriptome, and proteomics studies highlights similarities in the biological basis of animal and human myopia. Invest Opthalmology Vis Sci 2017; 58: 660–669. [DOI] [PubMed] [Google Scholar]
- 86. Arey LB. The vertebrate eye and its adaptive radiation. In: Walls GL. (ed.) The anatomical record. Bloomfield Hills, MI; New York: Cranbrook Press; Wiley, 1944, pp. 411–413. [Google Scholar]
- 87. Smith EL, 3rd, Bradley DV, Fernandes A, et al. Continuous ambient lighting and eye growth in primates. Invest Ophthalmol Vis Sci 2001; 42: 1146–1152. [PubMed] [Google Scholar]
- 88. Smith EL, 3rd, Hung LF, Kee CS, et al. Continuous ambient lighting and lens compensation in infant monkeys. Optom Vis Sci 2003; 80: 374–382. [DOI] [PubMed] [Google Scholar]
- 89. Tkatchenko TV, Shen Y, Braun RD, et al. Photopic visual input is necessary for emmetropization in mice. Exp Eye Res 2013; 115: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Howlett MC, McFadden SA. A fast and effective mammalian model to study the visual regulation of eye growth. Invest Ophthalmol Vis Sci 2002; 43: 2928. [Google Scholar]
- 91. Peichl L, Gonzalez-Soriano J. Morphological types of horizontal cell in rodent retinae: a comparison of rat, mouse, gerbil, and guinea pig. Vis Neurosci 1994; 11: 501–517. [DOI] [PubMed] [Google Scholar]
- 92. Howlett MHC, McFadden SA. Form-deprivation myopia in the guinea pig (Cavia porcellus). Vision Res 2006; 46: 267–283. [DOI] [PubMed] [Google Scholar]
- 93. Howlett MHC, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res 2009; 49: 219–227. [DOI] [PubMed] [Google Scholar]
- 94. Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis). Brain Res 1977; 124: 154–157. [DOI] [PubMed] [Google Scholar]
- 95. Müller B, Peichl L. Topography of cones and rods in the tree shrew retina. J Comp Neurol 1989; 282: 581–594. [DOI] [PubMed] [Google Scholar]
- 96. Tejedor J, de la Villa P. Refractive changes induced by form deprivation in the mouse eye. Invest Ophthalmol Vis Sci 2003; 44: 32–36. [DOI] [PubMed] [Google Scholar]
- 97. Tkatchenko TV, Shen Y, Tkatchenko AV. Mouse experimental myopia has features of primate myopia. Invest Ophthalmol Vis Sci 2010; 51: 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Schmucker C, Schaeffel F. Contrast sensitivity of wildtype mice wearing diffusers or spectacle lenses, and the effect of atropine. Vision Res 2006; 46: 678–687. [DOI] [PubMed] [Google Scholar]
- 99. Zhou X, Huang Q, An J, et al. Genetic deletion of the adenosine A2A receptor confers postnatal development of relative myopia in mice. Invest Ophthalmol Vis Sci 2010; 51: 4362–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bowmaker JK, Dartnall HJ, Lythgoe JN, et al. The visual pigments of rods and cones in the rhesus monkey, Macaca mulatta. J Physiol 1978; 274: 329–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Raviola E, Wiesel TN. Effect of dark-rearing on experimental myopia in monkeys. Invest Ophthalmol Vis Sci 1978; 17: 485–488. [PubMed] [Google Scholar]
- 102. Qiao-Grider Y, Hung LF, Kee CS, et al. Normal ocular development in young rhesus monkeys (Macaca mulatta). Vision Res 2007; 47: 1424–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cohen Y, Belkin M, Yehezkel O, et al. Dependency between light intensity and refractive development under light-dark cycles. Exp Eye Res 2011; 92: 40–46. [DOI] [PubMed] [Google Scholar]
- 104. Karouta C, Ashby RS. Correlation between light levels and the development of deprivation myopia. Invest Ophthalmol Vis Sci 2015; 56: 299–309. [DOI] [PubMed] [Google Scholar]
- 105. Chen S, Zhi Z, Ruan Q, et al. Bright light suppresses form-deprivation myopia development with activation of dopamine d1 receptor signaling in the ON pathway in retina. Invest Ophthalmol Vis Sci 2017; 58: 2306–2316. [DOI] [PubMed] [Google Scholar]
- 106. John T, Siegwart J, Ward AH, et al. Moderately elevated fluorescent light levels slow form deprivation and minus lens-induced myopia development in tree shrews. Invest Ophthalmol Vis Sci 2012; 53: 3457. [Google Scholar]
- 107. Smith EL, Hung LF, Arumugam B, et al. Negative lens-induced myopia in infant monkeys: effects of high ambient lighting. Invest Ophthalmol Vis Sci 2013; 54: 2959–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang L, Qu X. The effects of high lighting on the development of form-deprivation myopia in guinea pigs. Invest Ophthalmol Vis Sci 2019; 60: 4319–4327. [DOI] [PubMed] [Google Scholar]
- 109. Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res 1996; 36: 1023–1036. [DOI] [PubMed] [Google Scholar]
- 110. She Z, Hung LF, Arumugam B, et al. Effects of low intensity ambient lighting on refractive development in infant rhesus monkeys (Macaca mulatta). Vision Res 2020; 176: 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Underwood H, Steele CT, Zivkovic B. Circadian organization and the role of the pineal in birds. Microsc Res Tech 2001; 53: 48–62. [DOI] [PubMed] [Google Scholar]
- 112. Chakraborty R, Ostrin LA, Nickla DL, et al. Circadian rhythms, refractive development, and myopia. Ophthalmic Physiol Opt 2018; 38: 217–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nickla DL, Thai P, Zanzerkia Trahan R, et al. Myopic defocus in the evening is more effective at inhibiting eye growth than defocus in the morning: effects on rhythms in axial length and choroid thickness in chicks. Exp Eye Res 2017; 154: 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Backhouse S, Collins AV, Phillips JR. Influence of periodic vs continuous daily bright light exposure on development of experimental myopia in the chick. Ophthalmic Physiol Opt 2013; 33: 563–572. [DOI] [PubMed] [Google Scholar]
- 115. Nickla DL, Totonelly K. Brief light exposure at night disrupts the circadian rhythms in eye growth and choroidal thickness in chicks. Exp Eye Res 2016; 146: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sarfare S, Yang J, Nickla DL. The effects of brief high intensity light on ocular growth in chick eyes developing myopia vary with time of day. Exp Eye Res 2020; 195: 108039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Guo SS, Sivak JG, Callender MG, et al. Effects of continuous light on experimental refractive errors in chicks. Ophthalmic Physiol Opt 1996; 16: 486–490. [PubMed] [Google Scholar]
- 118. Padmanabhan V, Shih J, Wildsoet CF. Constant light rearing disrupts compensation to imposed- but not induced-hyperopia and facilitates compensation to imposed myopia in chicks. Vision Res 2007; 47: 1855–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cohen Y, Belkin M, Yehezkel O, et al. Light intensity modulates corneal power and refraction in the chick eye exposed to continuous light. Vision Res 2008; 48: 2329–2335. [DOI] [PubMed] [Google Scholar]
- 120. Weiss S, Schaeffel F. Diurnal growth rhythms in the chicken eye: relation to myopia development and retinal dopamine levels. J Comp Physiol A 1993; 172: 263–270. [DOI] [PubMed] [Google Scholar]
- 121. Zhou X, An J, Wu X, et al. Relative axial myopia induced by prolonged light exposure in C57BL/6 mice. Photochem Photobiol 2010; 86: 131–137. [DOI] [PubMed] [Google Scholar]
- 122. Schwahn HN, Schaeffel F. Flicker parameters are different for suppression of myopia and hyperopia. Vision Res 1997; 37: 2661–2673. [DOI] [PubMed] [Google Scholar]
- 123. Crewther DP, Crewther SG. Refractive compensation to optical defocus depends on the temporal profile of luminance modulation of the environment. NeuroReport 2002; 13: 1029–1032. [DOI] [PubMed] [Google Scholar]
- 124. Lan W, Feldkaemper M, Schaeffel F. Intermittent episodes of bright light suppress myopia in the chicken more than continuous bright light. PLoS ONE 2014; 9: e110906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Dong CJ, McReynolds JS. Comparison of the effects of flickering and steady light on dopamine release and horizontal cell coupling in the mudpuppy retina. J Neurophysiol 1992; 67: 364–372. [DOI] [PubMed] [Google Scholar]
- 126. Di Y, Lu N, Li B, et al. Effects of chronic exposure to 0.5Hz and 5Hz flickering illumination on the eye growth of guinea pigs. Curr Eye Res 2013; 38: 1182–1190. [DOI] [PubMed] [Google Scholar]
- 127. Luo X, Li B, Li T, et al. Myopia induced by flickering light in guinea pig eyes is associated with increased rather than decreased dopamine release. Mol Vis 2017; 23: 666–679. [PMC free article] [PubMed] [Google Scholar]
- 128. Li B, Luo X, Li T, et al. Effects of constant flickering light on refractive status, 5-HT and 5-HT2A receptor in guinea pigs. PLoS ONE 2016; 11: e0167902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Yu Y, Chen H, Tuo J, et al. Effects of flickering light on refraction and changes in eye axial length of C57BL/6 mice. Ophthalmic Res 2011; 46: 80–87. [DOI] [PubMed] [Google Scholar]
- 130. Foulds WS, Barathi VA, Luu CD. Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Invest Ophthalmol Vis Sci 2013; 54: 8004–8012. [DOI] [PubMed] [Google Scholar]
- 131. Seidemann A, Schaeffel F. Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Res 2002; 42: 2409–2417. [DOI] [PubMed] [Google Scholar]
- 132. Wang M, Schaeffel F, Jiang B, et al. Effects of light of different spectral composition on refractive development and retinal dopamine in chicks. Invest Ophthalmol Vis Sci 2018; 33: 205–215. [DOI] [PubMed] [Google Scholar]
- 133. Najjar RP, Chao De La Barca JM, Barathi VA, et al. Ocular growth and metabolomics are dependent upon the spectral content of ambient white light. Sci Rep 2021; 11: 7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wald G, Griffin DR. The change in refractive power of the human eye in dim and bright light. J Opt Soc Am 1947; 37: 321–336. [DOI] [PubMed] [Google Scholar]
- 135. Bedford RE, Wyszecki G. Axial chromatic aberration of the human eye. J Opt Soc Am 1957; 47: 5641–565. [DOI] [PubMed] [Google Scholar]
- 136. Kröger RH, Wagner HJ. The eye of the blue acara (Aequidens pulcher, Cichlidae) grows to compensate for defocus due to chromatic aberration. J Comp Physiol A 1996; 179: 837–842. [DOI] [PubMed] [Google Scholar]
- 137. Liu R, Qian YF, He JC, et al. Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Exp Eye Res 2011; 92: 447–453. [DOI] [PubMed] [Google Scholar]
- 138. Wang F, Zhou J, Lu Y, et al. Effects of 530 nm green light on refractive status, melatonin, MT1 receptor, and melanopsin in the guinea pig. Curr Eye Res 2011; 36: 103–111. [DOI] [PubMed] [Google Scholar]
- 139. Jiang L, Zhang S, Schaeffel F, et al. Interactions of chromatic and lens-induced defocus during visual control of eye growth in guinea pigs (Cavia porcellus). Vision Res 2014; 94: 24–32. [DOI] [PubMed] [Google Scholar]
- 140. Zou L, Zhu X, Liu R, et al. Effect of altered retinal cones/opsins on refractive development under monochromatic lights in guinea pigs. J Ophthalmol 2018; 2018: 9197631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Long Q, Chen D, Chu R. Illumination with monochromatic long-wavelength light promotes myopic shift and ocular elongation in newborn pigmented guinea pigs. Cutan Ocul Toxicol 2009; 28: 176–180. [DOI] [PubMed] [Google Scholar]
- 142. Liu R, Hu M, He JC, et al. The effects of monochromatic illumination on early eye development in rhesus monkeys. Invest Ophthalmol Vis Sci 2014; 55: 1901–1909. [DOI] [PubMed] [Google Scholar]
- 143. Ward AH, Norton TT, Huisingh CE, et al. The hyperopic effect of narrow-band long-wavelength light in tree shrews increases non-linearly with duration. Vision Res 2018; 146–147: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Gawne TJ, Ward AH, Norton TT. Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Res 2017; 140: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Smith EL, 3rd, Hung LF, Arumugam B, et al. Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Invest Ophthalmol Vis Sci 2015; 56: 6490–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Rucker F. Monochromatic and white light and the regulation of eye growth. Exp Eye Res 2019; 184: 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Lin G, Taylor C, Rucker F. Effect of duration, and temporal modulation, of monochromatic light on emmetropization in chicks. Vision Res 2020; 166: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Torii H, Kurihara T, Seko Y, et al. Violet light exposure can be a preventive strategy against myopia progression. EBioMedicine 2017; 15: 210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Najjar RP, Teikari P, Cornut PL, et al. Heterochromatic flicker photometry for objective lens density quantification. Invest Ophthalmol Vis Sci 2016; 57: 1063–1071. [DOI] [PubMed] [Google Scholar]
- 150. Teikari P, Najjar RP, Knoblauch K, et al. Refined flicker photometry technique to measure ocular lens density. J Opt Soc Am A 2012; 29: 2469–2478. [DOI] [PubMed] [Google Scholar]
- 151. Tao Y, Li XL, Sun LY, et al. Effect of green flickering light on myopia development and expression of M1 muscarinic acetylcholine receptor in guinea pigs. Int J Ophthalmol 2018; 11: 1755–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Tian T, Zou L, Wu S, et al. Wavelength defocus and temporal sensitivity affect refractive development in guinea pigs. Invest Ophthalmol Vis Sci 2019; 60: 2173–2180. [DOI] [PubMed] [Google Scholar]
- 153. Wu P-C, Tsai C-L, Wu H-L, et al. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology 2013; 120: 1080–1085. [DOI] [PubMed] [Google Scholar]
- 154. Landis EG, Yang V, Brown DM, et al. Dim light exposure and myopia in children. Invest Ophthalmol Vis Sci 2018; 59: 4804–4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ulaganathan S, Read SA, Collins MJ, et al. Influence of seasons upon personal light exposure and longitudinal axial length changes in young adults. Acta Ophthalmol 2019; 97: e256–e265. [DOI] [PubMed] [Google Scholar]
- 156. Smith EL, III, Hung L-F, Arumugam B, et al. Negative lens–induced myopia in infant monkeys: effects of high ambient lighting. Invest Ophthalmol Vis Sci 2013; 54: 2959–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Rucker FJ, Wallman J. Cone signals for spectacle-lens compensation: differential responses to short and long wavelengths. Vision Res 2008; 48: 1980–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Rucker F, Britton S, Taylor C. Color and temporal frequency sensitive eye growth in chick. Invest Ophthalmol Vis Sci 2018; 59: 60003–66013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Gawne TJ, Siegwart JT, Jr, Ward AH, et al. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Exp Eye Res 2017; 155: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Yoon H, Taylor CP, Rucker F. Spectral composition of artificial illuminants and their effect on eye growth in chicks. Exp Eye Res 2021; 207: 108602. [DOI] [PubMed] [Google Scholar]
- 161. Nickla DL, Jordan K, Yang J, et al. Brief hyperopic defocus or form deprivation have varying effects on eye growth and ocular rhythms depending on the time-of-day of exposure. Exp Eye Res 2017; 161: 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ngo C, Saw SM, Dharani R, et al. Does sunlight (bright lights) explain the protective effects of outdoor activity against myopia? Ophthalmic Physiol Opt 2013; 33: 368–372. [DOI] [PubMed] [Google Scholar]
- 163. Lind GJ, Chew SJ, Marzani D, et al. Muscarinic acetylcholine receptor antagonists inhibit chick scleral chondrocytes. Invest Ophthalmol Vis Sci 1998; 39: 2217–2231. [PubMed] [Google Scholar]
- 164. Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron 2004; 43: 447–468. [DOI] [PubMed] [Google Scholar]
- 165. Witkovsky P. Dopamine and retinal function. Doc Ophthalmol 2004; 108: 17–40. [DOI] [PubMed] [Google Scholar]
- 166. McCarthy CS, Megaw P, Devadas M, et al. Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Exp Eye Res 2007; 84: 100–107. [DOI] [PubMed] [Google Scholar]
- 167. Stone RA, Lin T, Laties AM, et al. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci USA 1989; 86: 704–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Djamgoz MB, Wagner HJ. Localization and function of dopamine in the adult vertebrate retina. Neurochem Int 1992; 20: 139–191. [DOI] [PubMed] [Google Scholar]
- 169. Megaw PL, Morgan IG, Boelen MK. Dopaminergic behaviour in chicken retina and the effect of form deprivation. Aust N Z J Ophthalmol 1997; 25(Suppl. 1): S76–S78. [DOI] [PubMed] [Google Scholar]
- 170. Brainard GC, Morgan WW. Light-induced stimulation of retinal dopamine: a dose-response relationship. Brain Res 1987; 424: 199–203. [DOI] [PubMed] [Google Scholar]
- 171. Cohen Y, Peleg E, Belkin M, et al. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res 2012; 103: 33–40. [DOI] [PubMed] [Google Scholar]
- 172. Huang F, Yan T, Shi F, et al. Activation of dopamine D2 receptor is critical for the development of form-deprivation myopia in the C57BL/6 mouse. Invest Opthalmology Vis Sci 2014; 55: 5537–5544. [DOI] [PubMed] [Google Scholar]
- 173. Zhou X, Pardue MT, Iuvone PM, et al. Dopamine signaling and myopia development: what are the key challenges. Prog Retin Eye Res 2017; 61: 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Vaquero CF, Pignatelli A, Partida GJ, et al. A dopamine- and protein kinase A-dependent mechanism for network adaptation in retinal ganglion cells. J Neurosci 2001; 21: 8624–8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Bu JY, Li H, Gong HQ, et al. Gap junction permeability modulated by dopamine exerts effects on spatial and temporal correlation of retinal ganglion cells’ firing activities. J Comput Neurosci 2014; 36: 67–79. [DOI] [PubMed] [Google Scholar]
- 176. Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod-cone coupling. Neuron 2008; 59: 790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Lasater EM, Dowling JE. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci USA 1985; 82: 3025–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Piccolino M, Neyton J, Gerschenfeld HM. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3’:5’-monophosphate in horizontal cells of turtle retina. J Neurosci 1984; 4: 2477–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kothmann WW, Massey SC, O’Brien J. Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. J Neurosci 2009; 29: 14903–14911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Zhang AJ, Jacoby R, Wu SM. Light- and dopamine-regulated receptive field plasticity in primate horizontal cells. J Comp Neurol 2011; 519: 2125–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Smith RG, Vardi N. Simulation of the all amacrine cell of mammalian retina: functional consequences of electrical coupling and regenerative membrane properties. Vis Neurosci 1995; 12: 851–860. [DOI] [PubMed] [Google Scholar]
- 182. Stone RA, Pendrak K, Sugimoto R, et al. Local patterns of image degradation differentially affect refraction and eye shape in chick. Curr Eye Res 2006; 31: 91–105. [DOI] [PubMed] [Google Scholar]
- 183. Pendrak K, Nguyen T, Lin T, et al. Retinal dopamine in the recovery from experimental myopia. Curr Eye Res 1997; 16: 152–157. [DOI] [PubMed] [Google Scholar]
- 184. Lan W, Yang Z, Feldkaemper M, et al. Changes in dopamine and ZENK during suppression of myopia in chicks by intense illuminance. Exp Eye Res 2016; 145: 118–124. [DOI] [PubMed] [Google Scholar]
- 185. Fischer AJ, McGuire JJ, Schaeffel F, et al. Light- and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat Neurosci 1999; 2: 706–712. [DOI] [PubMed] [Google Scholar]
- 186. Nickla DL, Damyanova P, Lytle G. Inhibiting the neuronal isoform of nitric oxide synthase has similar effects on the compensatory choroidal and axial responses to myopic defocus in chicks as does the non-specific inhibitor l-NAME. Exp Eye Res 2009; 88: 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Hartline HK. The response of single optic nerve fibers of the vertebrate eye to illumination of the retina. Am J Physiol 1938; 121: 400–415. [Google Scholar]
- 188. Pons C, Mazade R, Jin J, et al. Neuronal mechanisms underlying differences in spatial resolution between darks and lights in human vision. J Vis 2017; 17: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Pardue MT, Faulkner AE, Fernandes A, et al. High susceptibility to experimental myopia in a mouse model with a retinal ON pathway defect. Invest Ophthalmol Vis Sci 2008; 49: 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Hoshi H, Sato M, Oguri M, et al. In vivo nitric oxide concentration in the vitreous of rat eye. Neurosci Lett 2003; 347: 187–190. [DOI] [PubMed] [Google Scholar]
- 191. Carr BJ, Stell WK. Nitric oxide (NO) mediates the inhibition of form-deprivation myopia by atropine in chicks. Sci Rep 2016; 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Nickla DL, Wildsoet CF. The effect of the nonspecific nitric oxide synthase inhibitor N G-Nitro-L-arginine methyl ester on the choroidal compensatory response to myopic defocus in chickens. Optom Vis Sci 2004; 81: 111–118. [DOI] [PubMed] [Google Scholar]
- 193. Bloomfield SA, Völgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci 2009; 10: 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Xin D, Bloomfield SA. Effects of nitric oxide on horizontal cells in the rabbit retina. Vis Neurosci 2000; 17: 799–811. [DOI] [PubMed] [Google Scholar]
- 195. Shi Q, Teves MM, Lillywhite A, et al. Light adaptation in the chick retina: dopamine, nitric oxide, and gap-junction coupling modulate spatiotemporal contrast sensitivity. Exp Eye Res 2020; 195: 108026. [DOI] [PubMed] [Google Scholar]
- 196. Teves M, Shi Q, Stell WK, et al. The role of cell-cell coupling in myopia development and light adaptation. Invest Ophthalmol Vis Sci 2014; 55: 3036. [Google Scholar]
- 197. Murphy MJ, Crewther DP, Goodyear MJ, et al. Light modulation, not choroidal vasomotor action, is a regulator of refractive compensation to signed optical blur. Br J Pharmacol 2011; 164: 1614–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Chia A, Lu QS, Tan D. Five-year clinical trial on atropine for the treatment of myopia 2 myopia control with atropine 0.01% eyedrops. Ophthalmology 2016; 123: 391–399. [DOI] [PubMed] [Google Scholar]
- 199. McBrien NA, Moghaddam HO, Reeder AP. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Invest Ophthalmol Vis Sci 1993; 34: 205–215. [PubMed] [Google Scholar]
- 200. Iuvone PM, Rauch AL. Alpha2-adrenergic receptors influence tyrosine hydroxylase activity in retinal dopamine neurons. Life Sci 1983; 33: 2455–2463. [DOI] [PubMed] [Google Scholar]
- 201. Mathis U, Feldkaemper M, Wang M, et al. Studies on retinal mechanisms possibly related to myopia inhibition by atropine in the chicken. Graefes Arch Clin Exp Ophthalmol 2020; 258: 319–333. [DOI] [PubMed] [Google Scholar]
- 202. Schippert R, Burkhardt E, Feldkaemper M, et al. Relative axial myopia in Egr-1 (ZENK) knockout mice. Invest Ophthalmol Vis Sci 2007; 48: 11–17. [DOI] [PubMed] [Google Scholar]
- 203. Ashby RS, Zeng G, Leotta AJ, et al. Egr-1 mRNA expression is a marker for the direction of mammalian ocular growth. Invest Ophthalmol Vis Sci 2014; 55: 5911–5921. [DOI] [PubMed] [Google Scholar]
- 204. Bitzer M, Schaeffel F. Defocus-induced changes in ZENK expression in the chicken retina. Invest Ophthalmol Vis Sci 2002; 43: 246–252. [PubMed] [Google Scholar]
- 205. Brand C, Burkhardt E, Schaeffel F, et al. Regulation of Egr-1, VIP, and Shh mRNA and Egr-1 protein in the mouse retina by light and image quality. Mol Vis 2005; 11: 309–320. [PubMed] [Google Scholar]
- 206. Zhong X, Ge J, Smith EL, 3rd, et al. Image defocus modulates activity of bipolar and amacrine cells in macaque retina. Invest Ophthalmol Vis Sci 2004; 45: 2065–2074. [DOI] [PubMed] [Google Scholar]
- 207. Yang JW, Xu YC, Sun L, et al. 5-hydroxytryptamine level and 5-HT2A receptor mRNA expression in the guinea pigs eyes with spectacle lens-induced myopia. Int J Ophthalmol 2010; 3: 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Leung CKS, Yeung CK, Chiang SWY, et al. GABAA and GABAC (GABAA0r) receptors affect ocular growth and form-deprivation myopia. Cutan Ocul Toxicol 2005; 24: 187–196. [Google Scholar]
- 209. Stone RA, Liu J, Sugimoto R, et al. GABA, experimental myopia, and ocular growth in chick. Invest Ophthalmol Vis Sci 2003; 44: 3933–3946. [DOI] [PubMed] [Google Scholar]
- 210. Chebib M, Hinton T, Schmid KL, et al. Novel, potent, and selective GABAC antagonists inhibit myopia development and facilitate learning and memory. J Pharmacol Exp Ther 2009; 328: 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Kirsch M, Wagner HJ. Release pattern of endogenous dopamine in teleost retinae during light adaptation and pharmacological stimulation. Vision Res 1989; 29: 147–154. [DOI] [PubMed] [Google Scholar]
- 212. Contini M, Raviola E. GABAergic synapses made by a retinal dopaminergic neuron. Proc Natl Acad Sci USA 2003; 100: 1358–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Hirasawa H, Puopolo M, Raviola E. Extrasynaptic release of GABA by retinal dopaminergic neurons. J Neurophysiol 2009; 102: 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Schmid KL, Strasberg G, Rayner CL, et al. The effects and interactions of GABAergic and dopaminergic agents in the prevention of form deprivation myopia by brief periods of normal vision. Exp Eye Res 2013; 110: 88–95. [DOI] [PubMed] [Google Scholar]
- 215. Lam DM. The biosynthesis and content of gamma-aminobutyric acid in the goldfish retina. J Cell Biol 1972; 54: 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Pottek M, Weiler R. Light-adaptive effects of retinoic acid on receptive field properties of retinal horizontal cells. Eur J Neurosci 2000; 12: 437–445. [DOI] [PubMed] [Google Scholar]
- 217. McCaffery P, Mey J, Dräger UC. Light-mediated retinoic acid production. Proc Natl Acad Sci USA 1996; 93: 12570–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Dirks P, Tieding S, Schneider I, et al. Characterization of retinoic acid neuromodulation in the carp retina. J Neurosci Res 2004; 78: 177–185. [DOI] [PubMed] [Google Scholar]
- 219. Bitzer M, Feldkaemper M, Schaeffel F. Visually induced changes in components of the retinoic acid system in fundal layers of the chick. Exp Eye Res 2000; 70: 97–106. [DOI] [PubMed] [Google Scholar]
- 220. Seko Y, Shimokawa H, Tokoro T. In vivo and in vitro association of retinoic acid with form-deprivation myopia in the chick. Exp Eye Res 1996; 63: 443–452. [DOI] [PubMed] [Google Scholar]
- 221. Seko Y, Shimizu M, Tokoro T. Retinoic acid increases in the retina of the chick with form deprivation myopia. Ophthalmic Res 1998; 30: 361–367. [DOI] [PubMed] [Google Scholar]
- 222. McFadden SA, Howlett MHC, Mertz JR. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res 2004; 44: 643–653. [DOI] [PubMed] [Google Scholar]
- 223. Mao JF, Liu SZ, Dou XQ. Retinoic acid metabolic change in retina and choroid of the guinea pig with lens-induced myopia. Int J Ophthalmol 2012; 5: 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Troilo D, Nickla DL, Mertz JR, et al. Change in the synthesis rates of ocular retinoic acid and scleral glycosaminoglycan during experimentally altered eye growth in marmosets. Invest Ophthalmol Vis Sci 2006; 47: 1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Mertz JR, Wallman J. Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp Eye Res 2000; 70: 519–527. [DOI] [PubMed] [Google Scholar]
- 226. Rada JAS, Hollaway LR, Lam W, et al. Identification of RALDH2 as a visually regulated retinoic acid synthesizing enzyme in the chick choroid. Invest Ophthalmol Vis Sci 2012; 53: 1649–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Weiler R, Pottek M, He S, et al. Modulation of coupling between retinal horizontal cells by retinoic acid and endogenous dopamine. Brain Res Brain Res Rev 2000; 32: 121–129. [DOI] [PubMed] [Google Scholar]
- 228. Zhang DQ, McMahon DG. Direct gating by retinoic acid of retinal electrical synapses. Proc Natl Acad Sci USA 2000; 97: 14754–14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Provencio I, Rodriguez IR, Jiang G, et al. A novel human opsin in the inner retina. J Neurosci 2000; 20: 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002; 295: 1070–1073. [DOI] [PubMed] [Google Scholar]
- 231. Najjar RP, Zeitzer JM. Anatomy and physiology of the circadian system (chapter 2). In: Miglis MG. (ed.) Sleep and neurologic disease. San Diego, CA: Academic Press, 2017, pp. 29–53. [Google Scholar]
- 232. Viney TJ, Balint K, Hillier D, et al. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol 2007; 17: 981–988. [DOI] [PubMed] [Google Scholar]
- 233. Vugler AA, Redgrave P, Hewson-Stoate NJ, et al. Constant illumination causes spatially discrete dopamine depletion in the normal and degenerate retina. J Chem Neuroanat 2007; 33: 9–22. [DOI] [PubMed] [Google Scholar]
- 234. Dkhissi-Benyahya O, Coutanson C, Knoblauch K, et al. The absence of melanopsin alters retinal clock function and dopamine regulation by light. Cell Mol Life Sci 2013; 70: 3435–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Chakraborty R, Lee DC, Landis EG, et al. Melanopsin knock-out mice have abnormal refractive development and increased susceptibility to form-deprivation myopia. Invest Ophthalmol Vis Sci 2015; 56: 5843. [Google Scholar]
- 236. Ayaki M, Torii H, Tsubota K, et al. Decreased sleep quality in high myopia children. Sci Rep 2016; 6: 33902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Adhikari P, Pearson CA, Anderson AM, et al. Effect of age and refractive error on the melanopsin mediated post-illumination pupil response (PIPR). Sci Rep 2015; 5: 17610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Rukmini AV, Chew MC, Finkelstein MT, et al. Effects of low and moderate refractive errors on chromatic pupillometry. Sci Rep 2019; 9: 4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Besharse JC, McMahon DG. The retina and other light-sensitive ocular clocks. J Biol Rhythms 2016; 31: 223–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Ruan GX, Allen GC, Yamazaki S, et al. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol 2008; 6: e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Jiang X, Pardue MT, Mori K, et al. Violet light suppresses lens-induced myopia via neuropsin (OPN5) in mice. Proc Natl Acad Sci USA 2021; 118: e2018840118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Zhang DQ, Belenky MA, Sollars PJ, et al. Melanopsin mediates retrograde visual signaling in the retina. PLoS ONE 2012; 7: e42647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Buhr ED, Yue WWS, Ren X, et al. Neuropsin (OPN5)-mediated photoentrainment of local circadian oscillators in mammalian retina and cornea. Proc Natl Acad Sci USA 2015; 112: 13093–13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Calligaro H, Coutanson C, Najjar RP, et al. Rods contribute to the light-induced phase shift of the retinal clock in mammals. PLoS Biol 2019; 17: e2006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Bartmann M, Schaeffel F, Hagel G, et al. Constant light affects retinal dopamine levels and blocks deprivation myopia but not lens-induced refractive errors in chickens. Vis Neurosci 1994; 11: 199–208. [DOI] [PubMed] [Google Scholar]
- 246. Liu J, Pendrak K, Capehart C, et al. Emmetropisation under continuous but non-constant light in chicks. Exp Eye Res 2004; 79: 719–728. [DOI] [PubMed] [Google Scholar]
- 247. Parkinson D, Rando RR. Effects of light on dopamine metabolism in the chick retina. J Neurochem 1983; 40: 39–46. [DOI] [PubMed] [Google Scholar]
- 248. Zawilska JB, Bednarek A, Berezin’ska M, et al. Rhythmic changes in metabolism of dopamine in the chick retina: the importance of light versus biological clock. J Neurochem 2003; 84: 717–724. [DOI] [PubMed] [Google Scholar]
- 249. Proll MA, Kamp CW, Morgan WW. Use of liquid chromatography with electrochemistry to measure effects of varying intensities of white light on DOPA accumulation in rat retinas. Life Sci 1982; 30: 11–19. [DOI] [PubMed] [Google Scholar]
- 250. Landis EG, Park HN, Chrenek M, et al. Ambient light regulates retinal dopamine signaling and myopia susceptibility. Invest Ophthalmol Vis Sci 2021; 62: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Strickland R, Landis EG, Pardue MT. Short-wavelength (violet) light protects mice from myopia through cone signaling. Invest Ophthalmol Vis Sci 2020; 61: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Parkinson D, Rando RR. Effect of light on dopamine turnover and metabolism in rabbit retina. Invest Ophthalmol Vis Sci 1983; 24: 384–388. [PubMed] [Google Scholar]
- 253. Agarwal N. Diurnal expression of NGF1-A mRNA in retinal degeneration slow (rds) mutant mouse retina. FEBS Lett 1994; 339: 253–257. [DOI] [PubMed] [Google Scholar]
- 254. Donati G, Pournaras CJ, Munoz JL, et al. Nitric oxide controls arteriolar tone in the retina of the miniature pig. Invest Ophthalmol Vis Sci 1995; 36: 2228–2237. [PubMed] [Google Scholar]
- 255. Neal M, Cunningham J, Matthews K. Selective release of nitric oxide from retinal amacrine and bipolar cells. Invest Ophthalmol Vis Sci 1998; 39: 850–853. [PubMed] [Google Scholar]
- 256. Sekaran S, Cunningham J, Neal MJ, et al. Nitric oxide release is induced by dopamine during illumination of the carp retina: serial neurochemical control of light adaptation. Eur J Neurosci 2005; 21: 2199–2208. [DOI] [PubMed] [Google Scholar]
- 257. Yu M, Liu W, Wang B, et al. Short wavelength (blue) light is protective for lens-induced myopia in guinea pigs potentially through a retinoic acid-related mechanism. Invest Ophthalmol Vis Sci 2021; 62: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Guo Y, Liu L, Lv Y, et al. Outdoor jogging and myopia progression in school children from rural Beijing: the Beijing Children Eye Study. Transl Vis Sci Technol 2019; 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Read SA, Pieterse EC, Alonso-Caneiro D, et al. Daily morning light therapy is associated with an increase in choroidal thickness in healthy young adults. Sci Rep 2018; 8: 8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Zhou Z, Chen T, Wang M, et al. Pilot study of a novel classroom designed to prevent myopia by increasing children’s exposure to outdoor light. PLoS ONE 2017; 12: e0181772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Ofuji Y, Torii H, Yotsukura E, et al. Axial length shortening in a myopic child with anisometropic amblyopia after wearing violet light-transmitting eyeglasses for 2 years. Am J Ophthalmol Case Rep 2020; 20: 101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Kojima D, Mori S, Torii M, et al. UV-sensitive photoreceptor protein OPN5 in humans and mice. PLoS ONE 2011; 6: e26388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Xiong F, Mao T, Liao H, et al. Orthokeratology and low-intensity laser therapy for slowing the progression of myopia in children. Biomed Res Int 2021; 2021: 8915867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Rojas JC, Gonzalez-Lima F. Low-level light therapy of the eye and brain. Eye Brain 2011; 3: 49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Liu J, Li B, Chen Q, et al. Student health implications of school closures during the COVID-19 pandemic: new evidence on the association of e-learning, outdoor exercise, and myopia. Healthcare 2021; 9: 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Wang J, Li Y, Musch DC, et al. Progression of myopia in school-aged children after COVID-19 home confinement. JAMA Ophthalmol 2021; 139: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Chang P, Zhang B, Lin L, et al. Comparison of myopic progression before, during, and after COVID-19 lockdown. Ophthalmology 2021; 128: 1655–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Wong CW, Tsai A, Jonas JB, et al. Digital screen time during the COVID-19 pandemic: risk for a further myopia boom? Am J Ophthalmol 2021; 223: 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]