Abstract
Over the past decade, antimicrobial blue light (aBL) at 400 nm - 470 nm wavelength has demonstrated immense promise as an alternative approach for the treatment of multidrug-resistant microbes. Since our last review was published in 2017, there have been numerous studies that have investigated the efficacy, safety, mechanism, and propensity for resistance development. In addition, researchers have looked at combinatorial approaches that exploit aBL and other traditional and non-traditional therapeutics. To that end, this review aims to update the findings from numerous studies that capitalize on the antimicrobial effects of aBL, with a focus on: efficacy of aBL against different microbes, identifying endogenous chromophores and targets of aBL, Resistance development to aBL, Safety of aBL against host cells, and Synergism of aBL with other antimicrobials. We will also discuss our perspective on the future of aBL.
Keywords: Antimicrobial blue light, infectious diseases, antimicrobial resistance, microbes, photolysis, antimicrobial synergism
1. Introduction
At the beginning of the 20th century, a pioneering physician and Nobel Laureate in the field of chemotherapeutics, Paul Ehrlich, developed the ‘Magic Bullet’ concept. He defined a ‘magic bullet’ as an antimicrobial agent capable of selective destruction of pathogenic microorganisms. Paul Ehrlich hoped to develop a therapeutic strategy that eradicated infectious agents but did not harm the host [1]. In 1910, Ehrlich revolutionized chemotherapeutics, with his work culminating in the development of the first antibiotic ‘magic bullet’ arsphenamine (also known as Salvarsan or compound 606) that was capable of successfully treating both syphilis and African Trypanosomiasis [2,3]. Although the search was exhaustive, for the next 20 years, there would be no further antimicrobial ‘magic bullets’ discovered. Some 20 years later, in a London laboratory belonging to the bacteriologist Alexander Fleming, an accidental discovery was made that would revolutionize medicine for decades to come [4–6].
Fleming had returned from vacation when he noticed an invading mold present on agar plates cultured with Staphylococci. What caught his eye, was that there was a clear zone of inhibition present between the mold (later confirmed to belong to the genus Penicillium) and bacterial culture. This suggested to him that the mold elicited antimicrobial effects against the Staphylococci. Although he published his results in 1929 [7], he was unable to purify the unstable compound, which he named penicillin, but made it available to all who were interested to attempt to isolate penicillin for clinical use. Enthusiasm for the novel antibacterial compound began to wane in the early 1930s, and it would be more than a decade before any clinical application of penicillin would be achieved [4–6].
1.1. Antimicrobial resistance is driving a post-antibiotic era
Since the clinical applicability of penicillin was first validated, numerous classes of highly effective antibiotics have been discovered [8]. While there are many highly effective antibiotics present on the market, they all suffer from one serious drawback, resistance development [9]. Antimicrobial resistance occurs when microbes are no longer susceptible to treatment with an antimicrobial. More specifically, we consider microbes to be resistant to antibiotics when concentrations necessary to inhibit their growth rise above a threshold that would be considered unsafe for clinical application, outside the therapeutic window, and thus beyond the ‘magic bullet’ criteria [10]. It is well established that over-exposure of bacteria to sub-lethal concentrations of antibiotics rapidly induces resistance development [11]. In fact, in early testing over a decade before clinical validation, Alexander Fleming warned of future resistance development to penicillin [12]. The induction of single nucleotide polymorphisms at target sites of antibiotics is a common occurrence, although there are a multitude of antibiotic resistance mechanisms, including expression of antibiotic specific efflux pumps, enzymes etc [13].
This propensity for resistance development is ubiquitous among all classes of antibiotics, and historically, the time taken between the development of novel antibiotics and the generation of antibiotic resistant phenotypes is remarkably short. For example, methicillin, which is a β-lactam antibiotic that belongs to the penicillin class, that is tolerant to penicillinases, was developed in 1959 [14]. In 1960, there were already reports of clinical specimens of methicillin-resistant Staphylococcus aureus, giving rise to the ‘superbug’ known as MRSA [15]. Thus, while it is undeniable that conventional antibiotics fit Ehrlich’s criteria of ‘magic bullets’, it is likely that as we enter the post-antibiotic era, that this characterization may be short lived. It is unquestionable that an innovative ‘magic bullet’ capable of selective antimicrobial effects that does not succumb to resistance generation is essential.
1.2. Antimicrobial blue light: A potential ‘magic bullet’?
Antimicrobial blue light (aBL; 400 – 470 nm wavelength) has been shown to be an effective ‘drug-free’ approach for microbial killing. The accepted hypothesis for aBL mediated killing is through photoexcitation of endogenous porphyrins, which result in the generation of intracellular ROS (see below) that induces membrane damage, DNA damage, lipid peroxidation, etc. (Figure 1) [16]. In addition, our laboratory demonstrated that phototoxic effects of aBL are highly selective against bacteria, with mammalian cells being comparably less susceptible. We attributed this high selectivity to the high concentration of porphyrins within bacteria. By Ehrlich’s definition, aBL indeed fits the criteria of ‘magic bullet’. Since we published our last aBL review in 2017 [16], there have been numerous novel studies that have exploited aBL. This review aims to update the findings from the new studies including Efficacy of aBL against different microbes, Identifying endogenous chromophores and targets of aBL, Resistance development to aBL, Safety of aBL against host cells, and Synergism of aBL with other antimicrobials. Our review is limited to peer-reviewed studies that have exploited aBL as a standalone therapeutic, or in combination with other antimicrobials. Therefore, studies that exclusively exploit aBL in the presence of exogenous photosensitizers are excluded.
Figure 1.
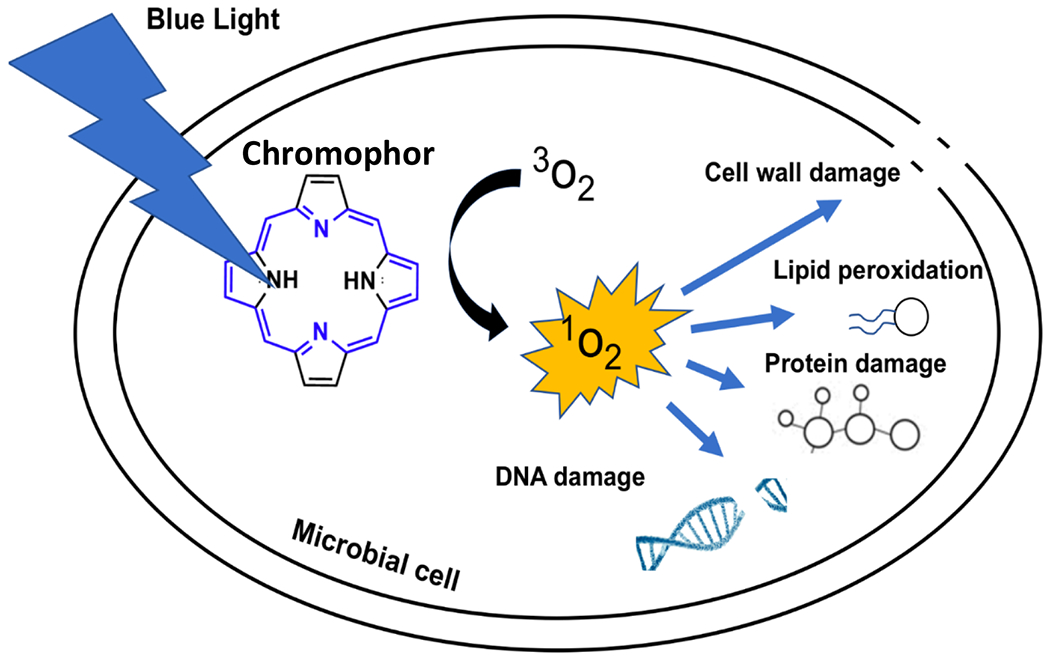
Schematic of the proposed mechanism of aBL for the killing of pathogenic microbes
2. Efficacy of aBL against different microbes
Since our previous review was published in 2017 [16], there have been numerous studies that have demonstrated the efficacy of aBL against many microbes including, bacteria, fungi, parasites, and viruses. The studies have evaluated the importance of light wavelength on antimicrobial effects, aBL as a disinfectant, aBL for the decontamination of blood products, and aBL as a potential therapeutic strategy against infection. A summary of the killing efficacies of aBL against numerous microbes are summarized in Table 1, which is organized by specific microbe to illustrate the range of susceptibilities to aBL (at different wavelengths ranging from 400-470 nm), as determine by numerous studies.
Table 1.
Summary of antimicrobial blue light killing of microbes as determined by numerous studies published 2017 -present
| Microbes | Wavelength (nm) | Radiant exposure (J/cm2) | Viability loss | Reference |
|---|---|---|---|---|
| S. pyogenes | 405 nm | 36 - 50 J/cm2 | 99.99 - 99.999999% | [37,81] |
| N. gonorrhoeae | 405 nm | 45 – 108 J/cm2 | 99.99999% | [49,50] |
| E. coli | 405 - 420 nm | 59 - 603 J/cm2 | 90 - 99.999% | [23,27,29,33,34,60,75] |
| 445 nm | 540 J/cm2 | <90% | [34] | |
| S. aureus | 400 - 420 nm | 50 - 841 J/cm2 | 71 - >99.999% | [17,23,27,37,38,54,68,76,80,81] |
| 450 nm (pulsed) | 4.5 - 7.6 J/cm2 | 80 - 100% | [47] | |
| 470 nm | 603.44 J/cm2 | 80% | [65] | |
| S. carnosus | 405 - 440 nm | 70 – 270 J/cm2 | 90 - 99.9% | [52,62] |
| 450 – 470 nm | 210 -500 J/cm2 | 99 - 99.9% | [53,62] | |
| S. epidermidis | 405 nm | 50 – 122 J/cm2 | 99- 99.999% | [37,81] |
| S. agalactiae | 450 nm (pulsed | 22.8 J/cm2 | 5.5 - 11% | [105] |
| P. aeruginosa | 405 - 415 nm | 10 – 463 J/cm2 | >90 - 100% | [17,23,27,69,75,81,89] |
| 470 nm | 603.44 J/cm2 | 65% | [20] | |
| P. gingivalis | 405 nm | 60 J/cm2 | 64.5% | [2] |
| S. typhimurium | 413 nm | 463.38 J/cm2 | 99.9% | [27] |
| S. enteritidis | 405 nm | 729 J/cm2 | >99.9999 | [28] |
| A. actinomycetemcomitans | 405 nm | 60 J/cm2 | 49.7% | [40] |
| M. fortuitum | 405 – 413 nm | 240 – 583.5 J/cm2 | 93 - 99.999% | [17,27] |
| C. jejuni | 405 nm | 24 J/cm2 | 81.82% | [51] |
| 463 nm | 24 J/cm2 | 99.81% | [51] | |
| P. fluorescens | 400 - 420 nm | 99 - 420 J/cm2 | >99 – 100% | [18,19,62] |
| 450 - 470 nm | 500 - 604.8 J/cm2 | 90 - 99.9% | [62] [63] [19] | |
| M. catarrhalis | 405 nm | 216 J/cm2 | 99.99% | [46,47] |
| P. acnes | 450 nm (pulsed) | 20 – 45 J/cm2 | 100% | [46,47] |
| C. sakazakii | 415 nm | 240 J/cm2 | 99.999999% | [67] |
| S. cerevisiae | 405 nm | 182 J/cm2 | 90% | [22] |
| 450 nm | 526 J/cm2 | 90% | [22] | |
| T. cruzi | 405 nm | 270 J/cm2 | >99% | [39] |
| C. albicans | 405 - 415 nm | 50 – 108 J/cm2 | >80 - 99.9999% | [72,81,90] |
| 450 nm | 360 J/cm2 | >80% | [72] | |
| C. vaginitis | 405 – 415 nm | 225 J/cm2 | >99.9% | [79] |
| 470 nm | 360 J/cm2 | 23.44% | [79] | |
| Prototheca Spp. | 410 nm | 137 – 320 J/cm2 | 99 -99.0% | [25] |
| K. pneumoniae | 410 nm | 108 – 1903 J/cm2 | 66.1 - 100% | [26,81,87] |
| E. moraviensis | 405 nm | 200 J/cm2 | 90% | [58] |
| 450 nm | 750 J/cm2 | 90% | [58] | |
| E. faecium | 405 nm | 540 J/cm2 | 99.99% | [81] |
| L. monocytogenes | 460 – 470 nm | 604.8 J/cm2 | >99.999% | [63] |
| A. baumannii | 405 nm | 144 - 270 J/cm2 | 99.99 - 99.9999 | [75,81,86] |
| A. flavus | 405 nm | 540 J/cm2 | <90% | [91] |
| A. fumigatus | 405 nm | 540 J/cm2 | <90% | [91] |
| F. oxysporum | 405 nm | 540 J/cm2 | <90% | [91] |
| B. cereus | 405 nm | 50 J/cm2 | 99.9% | [37] |
| S. mutans | 405 nm | 340 – 831 J/cm2 | 48 - 54% | [42] |
| L. rubrilucens | 450 – 470 nm | 300 – 500 J/cm2 | >99.999% | [21] |
| Enterococcus Spp. | 405 nm | 360 J/cm2 | 99.999% | [81] |
| SARS-CoV-2 | 450 - 470 nm | 144 J/cm2 | >99% | [56] |
| Phi6 | 405 nm | 1400 J/cm2 | 99.9% | [58] |
2.1. The antimicrobial effect of light is a function of wavelength
In a study by Al-Shammary et al. [17] they investigated the bactericidal effects of different visible light wavelengths, against S. aureus, Pseudomonas aeruginosa and Mycobacterium fortuitum. For all bacteria tested, 405 nm light elicited the most pronounced killing effects following 10 mins of irradiation (total power: 230 mW; reflecting 138 J total radiant exposure) with a ≥96% loss of viability being achieved. Longer wavelengths including 532 nm and 650 nm, resulted in lower rates of killing with equivalent radiant exposures, with ≥51% and 37%, losses of viability being achieved, respectively. In addition, for all bacteria tested, except for M. fortuitum, aBL (405 nm wavelength) induced morphological changes as indicated by scanning electron microscopy. Similarly, in another study by Angarano et al. [18] that tested different wavelengths of visible light (i.e. blue, green, and red light), they too found that it was only the blue spectral region that permitted significant antimicrobial effects. Specifically, the authors found that when P. fluorescens was spread on TSA agar and irradiated with an equivalent radiant exposure (99.7 J/cm2) the killing increased to 3.7-log10 CFU, whereas other wavelengths (470 nm - 644 nm) did not significantly reduce the viability. They repeated these studies on hydrated biofilms, where they found that at a light dose of 99.7 J/cm2, there was no loss of viability at any wavelength that was tested. The authors concluded that because light at 400 nm was the only wavelength that could elicit significant antimicrobial effects, it could potentially be exploited as a disinfectant. The authors, however, stipulated that the absence of significant effects on biofilms, which they hypothesized were protected from light irradiation, may narrow its scope as a decontaminant.
A follow-up study by the same group, Angarano et al. [19] took a deeper look at the antimicrobial effects of different light spectra (400 nm, 420 nm, 570 nm, 584 nm, and 698 nm wavelengths) on the killing of P. fluorescens and Staphylococcus epidermidis biofilms. Irradiances reflecting 2.5% - 100% of the maximum power that was achievable (ranging from 0.043 mW/cm2 to 29.2 mW/cm2) were selected for their study. They found that a ≥ 3-log10 CFU reduction was achieved following 120 min of treatment, irrespective of irradiance. Surprisingly, even the control (unirradiated) was reduced by 3-log10 CFU over 120 min, which the authors attributed to drying. With respect to higher irradiances at 120 min, 100% of the maximum exposure, reflecting 210 J/cm2, resulted in a >6-log10 CFU reduction (3-log10 CFU greater than the unirradiated control). Interestingly, at a 420 nm wavelength, however, these equivalent doses did not reduce the CFU of the P. fluorescens biofilms relative to the untreated control. Increasing the radiant exposure to 420 J/cm2 (240 min exposure) reduced the viability by almost 2 log10 CFU when compared to the negative control. At the other wavelengths tested, there was no influence in viability irrespective of the radiant exposure. With respect to S. epidermidis, 400 nm light was the only wavelength capable of significant bactericidal effects. At 120 min of exposure (210 J/cm2) a >3-log10 CFU reduction was achieved relative to the untreated control. Unlike with P. fluorescens, the authors found there to be no deleterious effects to the biofilm because of drying. Similarly, as with their previous study, aBL was the only spectral region capable of antimicrobial effects, however, it was found that in contrast with P. fluorescens, 420 nm light exposure did not result in any loss of viability
In a related study by Galo et al. [20], the use of aBL (470 nm wavelength) and red light (660 nm wavelength) for the inhibition of S. aureus and P. aeruginosa was investigated. In concordance with the above-mentioned study, aBL resulted in a statistically significant reduction in the number of colonies enumerated with both S. aureus (approximately 80% fewer colonies relative to the untreated control) and P. aeruginosa (approximately 65% fewer colonies relative to the untreated control). Following aBL irradiation, the authors observed changes to colonial sizes which were approximately 45% smaller in S. aureus, and about 38% smaller in P. aeruginosa, relative to their untreated controls. This observation likely indicated attenuated growth rates following irradiation. As with the studies above, there was no inhibitory effects with respect to any bacterium following red-light irradiation (27.93 mW/cm2; 603.44 J/cm2; 6 h of irradiation) when exposed on bacteria inoculated onto solid agar. The authors also evaluated viability of both bacterial species when exposed to red light and aBL in a liquid medium. As with the solid agar experiments, there was no influence on bacterial viability following red light irradiation (30.96 mW/cm2; 334.36 J/cm2; 3 h of irradiation). For S. aureus, they found that aBL was also able to reduce the numbers of bacteria following exposure, with approximately 75% growth inhibition, relative to the untreated control. For P. aeruginosa, the same irradiation reduced the viability by approximately 62%, relative to the untreated control. Again, the authors found there to be significant differences in the colonial sizes following irradiation, however, this was only apparent when the bacteria were left to grow for 24 h. Following 48 h, the sizes of the colonies following aBL were indistinguishable from the untreated control. The authors concluded that aBL could both reduce the viability of bacteria and attenuate their growth.
In line with the above investigations, Schmid et al. [21] evaluated different light wavelengths in the blue spectral region (405, 450, or 470 nm wavelengths) and red light (620 nm wavelength) against a single strain of the respiratory bacterium Legionella rubrilucens. They found 405 nm aBL to be 4-fold more effective at killing when compared to 450 nm aBL, with a 1-log10 CFU reduction requiring 25 J/cm2 and 60 J/cm2, respectively. These findings complement those of Angarano et al. [19] who also found that longer wavelength aBL was less efficacious than aBL at shorter wavelengths. Additionally, they found 470 nm light to be 1.6-fold lower in efficacy when compared to 450 nm light, with 100 J/cm2 being required to kill 1-log10 CFU, suggesting that longer the aBL wavelengths, may be associated with a lower overall efficacy. Unsurprisingly, as with the previous studies, they did not find 620 nm light to elicit any antimicrobial effects, and thus concluded that blue light may be a potential option for the treatment or prevention of L. pneumophila lung infection.
In order to delve deeper into the role of wavelength, Hoenes et al. [22] investigated the capability of aBL at 405 nm or 450 nm to kill the fungal yeast Saccharomyces cerevisiae. They found that with 405 nm aBL the threshold dose for the killing of 1-log10 CFU (90% reduction in viability) a radiant exposure of 182 J/cm2 was required. As with Schmid et al. [21], exposure to 450 nm light wavelength was less effective, requiring the much higher exposure of 526 J/cm2 in order to achieve the same loss of viability. The authors also investigated whether damages to the cell wall may be responsible for this loss of viability using trypan blue staining as an indicator, however, the authors did not see any evidence (at these radiant exposures tested) of damage to the cell wall. In addition, the authors were interested to look at the chromophores present within S. cerevisiae to determine if there are any candidate endogenous photosensitizers that might be responsible for these phototoxic effects. The authors interpretation of the data was that the most important endogenous photosensitizer, that was excitable by 405 nm light, was protoporphyrin IX and potentially zinc protoporphyrin. This was claimed to be disparate from other studies in bacteria that found coproporphyrin to be the most important. The authors suggested that flavins were important for irradiation by 450 nm light, although, it is evident that more work is required to confirm this hypothesis.
Sabino et al. [23] then looked to develop a method to mathematically analyze the kinetics of microbial killing that is induced by aBL (410 nm wavelength) and methylene blue mediated photodynamic therapy (MB-PDT; 660 nm wavelength). In their study, they used microbial strains from ATCC including Escherichia coli, S. aureus, Klebsiella pneumoniae, P. aeruginosa, and Candida albicans. The authors adapted a power law function equation (Weibull equation) that could be applied to the killing kinetics induced by light-based therapeutic approaches; that was distilled into the formula: LDi – LD90 (−log10 (1-1/100))1/t. The LD90: which reflects the radiant exposure required to kill 1-log10 CFU of bacteria (90% loss of viability). It also includes T: tolerance factor, and i: inactivation percentage (or killing percentage). With the use of this formula, the authors were able to identify a tolerance factor for each organism treated with aBL or MB-PDT. They found that if T was >1 it would indicate that bacteria are initially tolerant to killing, whereby a T<1 would be considered initially sensitive, as evidenced by an increase in the concavity of the killing curves. Additionally, they found strong correlations (R2 >0.95) between the experimentally determined efficacy and the mathematically determined efficacy. Using this formula, they also discovered that irrespective of organism, aBL presented with T>1, suggesting that it is met with an initial tolerance to killing. MB-PDT on the other hand, showed variable initial tolerances, with E. coli and S. aureus being more initially sensitive (T<1) and K. pnemoniae more tolerant initially (T>1). P. aeruginosa and C. albicans presented with a constant rate of killing. The authors concluded that application of this mathematical model may aid researchers in standardizing methods for comparing efficacies of light-based treatments between microbial species.
It is clear from the recent literature that have evaluated the role of visible light wavelength on microbicidal activity, that only aBL can elicit significant effects. Even more importantly, the authors that have looked deeper at the role of variable wavelengths within the highly microbicidal blue spectral region. There is a clear consensus amongst the studies that aBL at shorter wavelengths (e.g., 400-410 nm) elicit more significant microbicidal effects than longer wavelengths (e.g., 450-470 nm). These findings are of great importance when determining the optimal aBL wavelength to utilize for disinfection purposes (see below) or for the treatment of infections.
2.2. aBL as a disinfectant
Because burn wards are ideal environments to spread bacteria, these settings can facilitate the acquisition of hospital acquired infections (HAI). Therefore, in a study by Bache et al. [24] they evaluated aBL (405 nm wavelength) as a potential disinfection strategy within hospital patients’ rooms. In their studies, they did not discontinue current cleaning or isolation protocols. They used a high intensity narrow spectrum light environmental decontamination system (HINS-light EDS) to emit aBL that was on continuously with normal room lighting and kept on from 08:00 to 22:00 h for a total of 7 days. They found that there was a consistent reduction in bacterial viability that ranged from 50 - 100%, and there was no correlation identified between loss of bacterial viability and irradiance.
In a separate, but not unrelated study, dos Anjos et al. [25], investigated, for the first time, the potential of aBL (410 nm wavelength) to decontaminate zoonotic algae Prototheca spp. As with Bache et al. they were interested to determine whether aBL has potential as a disinfectant, albeit against a microbe of potential clinical importance, likely to better understand the spectrum of activity of aBL. Specifically, in their study, they evaluated the efficacy of aBL (in two strains of P. zopfi, (genotype 1 and genotype 2) and a single strain of P. blaschkea. The authors found there to be clear algicidal effects amongst all strains tested. The most susceptible strain was found to be P. zopfi (Genotype 1) where a 3-log10 CFU reduction (99.9% reduction) was achieved following 183.32 J/cm2 (38.2 mW/cm2). The P. zopfi (Genotype 2) was more tolerant to aBL with 275 J/cm2 being required to reduce the CFU by 3-log10 CFU. P. blaschkea was even more tolerant to the effects of aBL with only a 2-log10 CFU (99% reduction) reduction being achieved following 320.85 J/cm2. The authors hypothesized that these differential efficacies amongst the tested strains may be based on unique biochemical differences that are specific to each strain. dos Anjos et al. [26] also evaluated aBL (410 nm wavelength; 38.2 mW/cm2) as a strategy to eliminate hypervirulent and hypermucoviscous K. pneumoniae, a bacterial pathogen of great clinical importance, to prevent cross contamination within a clinical paradigm. The authors screened 5 clinical isolates of hypermucoviscous K. pneumoniae all expressing drug resistance (including 2 strains that express extended spectrum β-lactamases [ESBL]). A non-hypermucoviscous ATCC strain was also included in their study. Unlike with Prototeca spp. The authors found that while K. pneumoniae was susceptible to aBL, it was only at exceedingly high radiant exposures. Specifically, they found that exposures of 571 J/cm2 – 965.4 J/cm2 aBL were required to reduce the viability by 99.9%. interestingly, the authors did find the hypermucoviscos strains were more tolerant to aBL which the authors proposed may be (as with Prototheca spp), due to biochemical differences between the strains, although further work is required to corroborate this.
To determine the potential for aBL (413 nm)to disinfect milk for consumption purposes, given the propensity for milk to become contaminated with a variety of pathogenic microbes, dos Anjos et al. [27], evaluated the efficacy of aBL against an array of different microorganisms. Experiments were performed in milk and in PBS, against E. coli, P. aeruginosa, Salmonella typhimurium, S. aureus, and M. fortuitum. They found that 600 J/cm2 (reflecting <100 min of exposure), was enough to kill 5-log10 CFU of all bacterial pathogens (reflecting a 99.999% loss of viability). Interestingly, the authors discovered that for some organisms the killing potential of aBL was higher in milk when compared to PBS. For example, in milk, 33.84 min of exposure (reflecting 203.04 J/cm2) was sufficient to kill 3-log10 CFU. In PBS, however, a >3-fold increase in the aBL radiant exposure (743.04 J/cm2; 123.84 min; 100 mW/cm2 irradiance) was required to reduce the CFU by 3-log10. The authors also analyzed the composition of milk, where they found no differences in most of the milk components, except for riboflavin (that was depleted 1000-fold, following 120 min irradiation), which was not surprising as it was the only component with an absorption at the blue region of visible light. In fact, the authors attributed the enhanced efficacy of aBL against bacteria in milk vs. PBS on the presence of riboflavin. In addition, they hypothesized that the scattering of light by the milk resulted in amplification of light by a process of superposition of backscattered light, thus promoting further phototoxic effects. The authors concluded that with appropriate supplementation of irradiated milk with riboflavin, aBL would be a suitable method for milk decontamination.
In a similar study of relevance, Keyvan et al. [28] looked at the influence of different environmental temperatures, including 4°C, 25°C, and 37°C on the efficacy of aBL (405 nm; 27.7 mW/cm2 irradiance) mediated killing of two strains of Salmonella enteritidis (ATCC 13076 and NCTC 13349). This work was particularly important, as it may determine whether refrigerated food products (such as milk) could be subjected to aBL for effective disinfection at lower temperatures. In their study, bacteria were exposed over long periods of time, including: 1.5, 3, 7.5, and 24 h. They found that for all conditions and strains, 7.5 h (729 J/cm2) was enough for complete eradication. The authors interpretation of the data was that aBL was equally effective at each temperature that was tested. The capabilities of aBL killing at lower temperatures (i.e. 4°C) suggested to the authors that aBL may be used successfully within refrigerators to reduce potential for food contamination.
In line with their previous investigations, dos Anjos et al. [29] the authors assessed killing potential of aBL (410 nm wavelength; 38.2 mW/cm2) against three different strains of multidrug-resistant (MDR) E. coli. Specifically, they were interested to determine whether the efficacy of aBL would be suitable to eradicate E. coli, specifically strains that may be identified in high risk environments. They found that for each strain tested, 180 J/cm2 was the threshold dose that permitted the killing of 3-log10 CFU irrespective of the drug-resistance profiles. The authors concluded that aBL may be a suitable approach for not only preventing cross contamination, but may be a viable option for treating infections caused by MDR E. coli.
It is evident from the above-mentioned studies that aBL (405-413 nm wavelengths) is an effective for disinfecting surfaces (like those present within a clinical environment). In addition, aBL appears to have potential as a decontaminant of decontaminating food-products that may be applied under relevant atmospheric conditions.
aBL for the treatment of microbial biofilms
Biofilms represent an important mediator of recalcitrant infections [30]. Importantly, they have been found to be more tolerant to antibiotics than are their planktonic counterparts. Therefore, a study by Ferrer-Espada et al. [31] sought to determine whether aBL may be a suitable alternative for the treatment of monomicrobial and polymicrobial (dual species) biofilms. In their study, the authors evaluated aBL (405 nm wavelength; 60 mW/cm2 – 92.6 mW/cm2) against mono- or polymicrobial biofilms grown on 96-well microtiter plates or within a CDC biofilm reactor. For the monomicrobial biofilms matured for 24 h, 216 J/cm2 killed: 0.34, 1.20, 3.48-log10 CFU, of C. albicans, MRSA, and P. aeruginosa, respectively. Increasing the radiant exposure to 500 J/cm2 increased the killing to: 2.33, 3.48, and 6.55-log10 CFU, respectively. For 24 h dual species polymicrobial biofilms composed of C. albicans and P. aeruginosa: 216 J/cm2 killed 2.46-log10 CFU of C. albicans, and 5.67-log10 CFU of P. aeruginosa. Increasing the radiant exposure to 500 J/cm2, reduced the log10 CFU further to 3.11 and 6.34 respectively. For the MRSA + P. aeruginosa dual species biofilm: 216 J/cm2 killed 1.42-log10 CFU and 3.94-log10 CFU of MRSA and P. aeruginosa, respectively. Increasing the dose to 500 J/cm2 reduced the CFU by 2.37-log10 CFU and 3.40-log10 CFU, respectively. The authors also looked at 48-hour monomicrobial and polymicrobial biofilms of the same composition. For C. albicans, MRSA and P. aeruginosa, 216 J/cm2 killed: 0.25-log10 CFU, 1.62-log10 CFU, and 3.67-log10 CFU, respectively. Increasing to 500 J/cm2 resulted in a 2.11-log10 CFU, 2.35-log10 CFU, and 6.88-log10 CFU reduction, respectively. For the dual species C. albicans + P. aeruginosa biofilms: 216 J/cm2 resulted in a 2.96-log10 CFU reduction and 5.48-log10 CFU reduction, for C. albicans and P. aeruginosa, respectively. Increasing the radiant exposure to 500 J/cm2 resulted in a: 3.41-log10 CFU and 7.41-log10 CFU reduction, respectively.
For MRSA + P. aeruginosa biofilms, 216 J/cm2 resulted in a: 2.44-log10 CFU and 4.19-log10 CFU reduction, respectively. Increasing the radiant exposure to 500 J/cm2 resulted in a 2.61-log10 CFU reduction and 3.67-log10 CFU reduction, respectively. With respect to the CDC reactor experiments that grew 48 h biofilms, the killing efficacy was found to be lower. For example, monomicrobial P. aeruginosa biofilms exposed to aBL at 216 J/cm2 could only reduce the CFU by 1.48-log10 (compared to 3.67-log10 CFU within 96 well plate 48 h biofilms). For polymicrobial biofilms, the same observation was made. For example, in MRSA + P. aeruginosa biofilms, 216 J/cm2 could only reduce the log10 CFU by 0.8 and 1.80, compared with 2.44 and 4.19 within 96-well plates, respectively. The authors attributed increased thickness of the biofilms which might limit oxygen (which is essential to permit photochemical reactions) from reaching the cells, thus increasing in tolerance within biofilms grown by the CDC reactor vs. the 96-well microtiter plates. However, this hypothesis requires further testing in order to substantiate.
Similarly to Ferrer-Espada et al.[31,32], Halstead et al. [33] too found aBL (405 nm wavelength) to be effective for the decontamination of carbapenemase producing Enterobacteriaceae, specifically on multiple strains of beta-lactamase producing K. pneumoniae and E. coli biofilms. In their study they used an LED flood array that was calibrated to illuminate bacteria at 60 mW/cm2. Unlike the previous studies that have assessed viability of biofilms using the gold standard CFU technique, the authors use the MTT assay to assess viability. They found significant reductions in the viability of all strains, ranging from 66.1% - 97.7% in E. coli and K. pneumoniae following 108 J/cm2. The findings, however, do show more significant killing of both organisms [26,29] and biofilms [32] in general, relative to previous studies. It is however, important to note that the light sources that were applied were very different, and the method of viability determination was similarly disparate, which might explain the differential findings. The authors concluded that their data supported the potential of aBL to treat mature biofilms but conceded that further work looking at polymicrobial biofilms of the Enterobacteriaceae are warranted.
To better understand the optimal light wavelength (379 nm - 454 nm) for elimination of bacterial biofilms, Halstead et al. [34] tested their hypotheses that wavelengths outside of 405 nm would not elicit significant antimicrobial effects. Specifically, they tested wavelengths ranging from 379 nm (UVA) to 452 nm (blue light) using a multiwavelength LED array (MWA) or a single wavelength LED array (SWA) with a peak wavelength of (401 nm). They found that all wavelengths within the blue spectral region of their arrays (400 nm – 454 nm) elicited some antimicrobial effects against all bacteria tested (Acinetobacter baumannii, S. aureus, P. aeruginosa, and E. coli). For the MWA experiments, they found P. aeruginosa biofilms to be most susceptible to aBL (405 – 420 nm) with reductions in biofilms ranging from 73.8% - 88.9% which was dependent on the strain. The reductions in A. baumannii biofilms were comparatively lower, with a maximum loss of 30.7% viability being achieved. Interestingly, for S. aureus, the only wavelength that could reduce the viability (by 5.4%) was 395 nm light, with longer wavelengths actually stimulating growth of viable bacteria. E. coli was most susceptible 395 nm light with a maximum killing of 49.9% being achieved. The authors concluded that the most effective wavelengths that influence biofilm viability were 395 nm, 405 nm, and 420 nm. The authors next investigated the influence of the different light wavelengths on the biofilm biomass on the different bacterial species. Interestingly, they found that biofilm biomass was susceptible to all wavelengths of UVA (395) and aBL (405 nm – 454 nm) with 26.1% - 87.1% losses in viability being observed for all strains tested. For A. baumannii, they observed up to 65.3% reductions and for P. aeruginosa this was as high as 87.1%. E. coli biomass was the least influenced by light irradiation, with negligible effects being observed. The authors surmised that the most effective wavelengths to permit reductions in biofilm were 395 nm and 405 nm.
The authors next looked at the effect of a SWA emitting 400-405 nm light, which was found to be superior in terms of killing efficacy and reductions in biofilm biomass, against most of the isolates tested. For A. baumannii, for example, unlike with the MWA, the authors found that >80 losses in viability were achieved. For all isolates tested, the killing effectiveness of the SWA was significantly improved relative to the MWA. Based on their findings, the authors confirmed that aBL at 405 nm was the most potent bactericide. The authors considered that further work looking at potential thermal effects of aBL to determine appropriate therapeutic parameters before it could be translated to the clinic.
As with Halstead et al [34], Martegani et al. [35] looked at the effectiveness of 2 wavelengths within the blue light spectral region, including 410 nm and 455 nm against P. aeruginosa biofilms. As with the above study, they found that shorter wavelength aBL could effectively kill P. aeruginosa biofilms. For example, they found that 75 J/cm2 of 410 nm aBL was sufficient to inhibit biofilm formation by 2-log10 CFU. In addition, increasing the radiant exposure to 450 J/cm2, further reduced the CFU by 4-log10 (total killing achieved was 6-log10 CFU). Like with previous studies [21,22,35] aBL at 455 nm was less effective. When P. aeruginosa was irradiated with 75 J/cm2 aBL (455 nm) there was no reduction in biofilm production relative to an untreated control. Increasing the radiant exposure to 225 J/cm2 only reduced the viability by approximately 1-log10 CFU. When the authors shone the highest dose of 455 nm aBL (450 J/cm2), however, they did observe more significant inhibitory effects (reflecting an approximately 3-log10 CFU reduction). Similar to Halstead et al. [34], aBL (410 nm) at 75 J/cm2 was sufficient to significantly reduce the biofilm biomass relative to an untreated control, and for 455 nm light, 225 J/cm2 was sufficient to observe very significant reductions in the biofilm biomass relative to the untreated control, which was in concordance with the previous study. With respect to killing of adherent biofilms, the findings were more modest when compared to inhibition. For example, with respect to 410 nm light, 75 J/cm2 only reduced the CFU by approximately 1-log10, and 225 J/cm2 reduced the viability by under 3-log10 CFU, findings that were not unlike those found Halstead et al. [34]. Impressively, however, 450 J/cm2 was enough to reduce the viability by 7-log10 CFU. With respect to 455 nm aBL, there was no significant killing of biofilms until 450 J/cm2 was applied, although this only resulted in a 1-log10 CFU reduction. These findings were not unusual, given the consensus amongst the studies that longer wavelength aBL appears to be less effective than aBL at shorter wavelengths. Given the data, the authors concluded that aBL may be an effective strategy to control biofilms. It is important to note, that P. aeruginosa biofilms have been found to be significantly more susceptible to aBL, than were other species [30,31,32] with observations remaining different depending on wavelength of aBL. Therefore, it is important to appreciate that the appropriate aBL dosimetry necessary to control biofilms is contingent on both wavelength and microbial species; thus further work would be required to determine the flexibility of aBL in controlling biofilms.
In line with the above-mentioned investigations Rupel et al. [36] investigated longer wavelength aBL (445 nm) for Pseudomonas aeruginosa biofilm inhibition in vitro and in vivo. In their study, they only used a single strain ATCC 27853. Initially, they investigated the capability of aBL to attenuate growth of planktonic bacteria. They found that radiant exposures as low as 40 J/cm2 was enough to attenuate growth, with inhibitory effects increasing as light doses increased. They then looked to evaluate the effect of aBL exposure on biofilm biomass, and as with previous studies [31,32] they found significant reductions in the biomass. At the highest irradiances and radiant exposures (600 mW/cm2; 120 J/cm2; 3.33-min exposure) the whole biofilm biomass was almost completely detached, however, although not stated explicitly by the authors, the potential thermal effects that were considered an important factor by Halstead et al. [34] may have conceivably played a role in this detachment. They next wanted to determine whether aBL could inhibit biofilm formation, where they found significant inhibitory effects elicited with all conditions tested. They also performed an in vivo mouse infection experiment, where they infected abrasions with P. aeruginosa. Either at 30 min or 24 h following inoculation of bacteria, treatment was initiated. They found significant reductions in the CFU (approximately 50%) following 60 J/cm2 irradiation (300 mW/cm2; 3.33 min irradiation) and found that inflammation was also significantly reduced. The authors concluded that aBL effectively inhibits and disrupts mature biofilms, as well as inhibits the progression of wound superinfection.
The novel studies that have investigated the potential for aBL as an anti-biofilm agent has illuminated the potential strengths and limitations of aBL as a microbicide. It is without question that biofilms remain an important mediator of infection, and thus a strategy that can effectively mitigate or eliminate them are warranted. The recent literature has indicated that biofilms remain sensitive to aBL, however, this appears to be dependent on the aBL wavelength and dosimetry that is applied.
2.3. aBL for the decontamination of blood products
Platelet transfusion remains an important method to mitigate bleeding in patients, as well as treat bleeding in patients with thrombocytopenia or other diseases that may impede platelet function. Because platelets typically have a short-shelf, due to its propensity for contamination, which is a result of storage at room temperature. Therefore, several studies have explored aBL may increase the shelve-life of platelets and other blood products.
As such, a study by Lu et al. [37] assessed the ability for aBL (405 nm wavelength) to safely decontaminate platelets, to extend their shelf life. Initially, the authors evaluated the susceptibility profiles of aBL in 5 bacteria and a single fungal organism in platelet additive solution including: S. aureus, S. pyogenes, Bacillus cereus, S. epidermidis, C. albicans, and P. aeruginosa. The authors found high antimicrobial effects in all microbes tested with relatively low radiant exposures of 405 nm light, with B. cereus showing almost a 3-log10 CFU reduction following a low dose of 50 J/cm2, and S. pyogenes showing the most potent effects of aBL with a 4-log10 CFU reduction at an equivalent dose. The efficacy of aBL at 470 nm was significantly lower when compared to 405 nm aBL. Remarkably, the effectiveness of aBL when the bacteria were illuminated (over several days) within platelet concentrates were even more impressive with ≥ 8-log10 CFU reductions relative to untreated controls. The authors did not observe any deleterious effects on the platelets following aBL irradiation. Additionally, the platelet activation and aggregation following aBL irradiation was no different to the untreated controls. Furthermore, when mouse whole blood was illuminated with aBL there were no deleterious effects on the platelets, with observations being almost identical to the untreated controls. The authors concluded that their results suggest that aBL may be a suitable approach to kill bacteria within platelet concentrates, and thus extend the shelf life for administration to patients. Importantly the authors understood that clinical validation of this is still required to determine the feasibility.
Additionally, Maclean et al. [38] investigated the potential for aBL (405 nm wavelength) for the decontamination of platelets as well as in platelet recovery in severe combined immunodeficient (SCID) mice. In their study, they only evaluated S. aureus as a model to determine the feasibility of 405 nm aBL to decontaminate platelets. The authors contaminated platelet bags with a low concentration of bacteria (102 CFU/mL). They illuminated the contaminated platelets with low irradiances ranging from 3 mW/cm2 – 10 mW/cm2 for up to 8 h. For example, they found at 10 mW/cm2, it required 4 h (reflecting 288 J/cm2) to eliminate most of S. aureus in the sample (with only 0.3% remaining suggesting just below a 2-log10 CFU reduction). These findings appear to be modest when compared to Lu et al. [37] which found that doses as low at 75 J/cm2 (which would reflect a radiant exposure almost 4-fold lower than what was used within this study), was enough to completely sterilize the bags - showing a difference in microbial contamination upwards of 109 CFU. A potential explanation could be different light sources, irradiances used, and potentially the strain of S. aureus that was used. As with Lu et al [37], the authors did not observe any significant differences in aBL treated vs. untreated mouse blood for the purification of platelets [38].
In line with Lu et al. [37] and Maclean et al. [38], Jankowska et al. [39] investigated, for the first time, the potential to kill the protozoan organism Trypanosoma cruzi within stored human platelets, with the use of 405 nm light. The authors looked at aBL radiant exposures ranging from 36 J/cm2 and 288 J/cm2 (irradiance: 10 mW/cm2 – 15 mW/cm2; approximate exposure of 1 - 8 h, respectively). Following 108 J/cm2, there was close to a 3-log10 reduction of parasites within platelet concentrates. This was lower in plasma with a <1-log10 reduction being achieved. Following 216 J/cm2, there was a much greater reduction in parasite viability with approximately 8-log10 and 10-log10 of parasites being reduced following 4 and 5 h of aBL in platelet concentrates and plasma, respectively. Impressively, the authors also evaluated the potential of parasitemia generation following transfusion of aBL treated platelets into immunocompromised mice. They did not find any evidence of parasitemia in mice even 185 days post inoculation of treated platelets, unlike the untreated control which illustrated significant parasitemia from days 15 post inoculation of mice up until day 185. The authors concluded that given that 270 J/cm2 was enough to reduce the viability of T. cruzi within platelet concentrates with >9-log10 CFU reductions in viability being observed, it may be a suitable approach to decontaminate platelets. The authors understood, however, that in depth studies are still required to fully evaluate this system for platelet decontamination.
Evidence from the studies above demonstrated the feasibility of aBL to decontaminate blood products to permit safe application to a mammalian host. It appears that not only can aBL successfully decontaminate platelets and plasma containing numerous different pathogenic microbes, but it does so with limited adverse effects to the blood products themselves. However, although aBL offers a potentially safe approach to prolonging the shelf-life of plateles/plasma, as corroborated by all the authors, it is essential that further work is performed before translation to human participants.
2.4. aBL as a therapeutic strategy against infections
In previous sections, we evaluated the potential application of aBL as a method for disinfection or decontamination as denoted by the literature. However, numerous studies have similarly found there to be potential for aBL to behave as a therapeutic for the treatment of localized infections, specifically: dental infections, acne, otitis media, intestinal infection, urogenital infection, fungal infection, and COVID-19.
2.4.1. aBL for the treatment of dental infections
In a study by Al Hamzi et al [40], that investigated the potential for broad spectrum blue light (400 nm – 500 nm wavelength) at the high total power of 1W to kill dental pathogenic bacteria derived from patients suffering from chronic gingivitis. They found that 60 J (reflecting 60 seconds of exposure) was sufficient to reduce the viability of Aggregatibacter actinomycetemcomitans by 64.5%. For Porphyramonas gingivalis, the killing was more modest with a total of 49.7% following the same light dose. They concluded that there are clear phototoxic effects elicited against both etiological agents of gingivitis. They proposed that to achieve therapeutic effects within a clinical paradigm, the use of a photosensitizer in addition to aBL may be recommended. Similarly, in a study by Gomez et al. [41] they assessed the potential of aBL at 405 nm to S. mutans biofilm in dentin, in vitro. The authors used a total of 162 dentin specimens for their study, the authors treated them over 5 days, and they found that the groups treated with aBL had a 60.6% reduction in the CFU relative to the untreated groups. However, the authors did not find any statistically significant difference when comparing groups not supplemented with sucrose vs. aBL.
Mohammed et al. [42] also assessed the capability of aBL (405 nm wavelength; irradiances: 470 mW/cm2 – 4054 mW/cm2) for the treatment of dental infections in a similar fashion to Al Hamzi et al. [40] and Gomez et al. [41]. In their study, they used 75 non-carious human permanent molars of patients aged 20-40. To evaluate the efficacy, Streptococcus mutans were grown on agar plates as ‘lawns’ and irradiated with increasing radiant exposures of aBL (110 J/cm2 – 573 J/cm2). The authors subsequently measured the zone of inhibition (ZOI) as an indicator of efficacy. They found that increasing radiant exposures, increased the ZOI with the maximum ZOI being 7 mm following exposure. They additionally applied increasing radiant exposures to planktonic cells and those within biofilms where they saw marginal, but statistically significant reductions in the viability. The authors concluded that 405 nm light may be a good strategy for prophylaxis within clinically applicable timeframes. Additionally, the authors postulated that given the potential photobiomodulatory effects of aBL they may capitalize on immune stimulation to further potentiate clearance of invading microbes. In line with the previous studies, Vaknin et al. [43] sought to determine whether aBL (wide emission spectrum 400 – 500 nm) to control biofilms caused by dental pathogens of importance, including S. mutans and Streptococcus sanguinis. In this study, both organisms were cultured in an oral biofilm on ex vivo bovine enamel blocks for 24 h, either as a monomicrobial biofilm or combined in dual species polymicrobial biofilms. The biofilms were then exposed to 112 J/cm2 aBL. To determine relative survival of each species they performed qPCR targeted at 16s rRNA that is specific to each species from extracted DNA to determine the copy number which may be used to correlate with number of bacteria. They found that the amount of DNA relative to untreated controls were significantly different. Interestingly, within monomicrobial biofilms they found that the 16s rRNA copy number increased by 78% and the S. sanguinis decreased by 85% 24 h after exposure. This phenomenon was also evident 48 h post-aBL exposure, with S. mutans 16s rRNA copy number increasing 47%, and S. sanguinis decreasing by 51%, although the authors did not find this to be statistically significant. Unlike with monomicrobial biofilms, when S. mutans and S. sanguinis were cultured within dual species polymicrobial biofilms, the authors reported a 60% and 61% reduction in the 16s rRNA in the S. mutans and S. sanguinis copy number, respectively, relative to the untreated control 24 h post exposure to aBL. The authors concluded that aBL may be an effective intervention in preventative dentistry and potentially replace the more conventional antibiotic therapy.
A study by Arai et al. [44] also looked at the potential for aBL (405 nm wavelength) to treat multi-species biofilms that are important factors in dental infections. Particularly, they looked at aBL of C. albicans and S. mutans biofilms on denture base resin. In their study, they used 134 square shaped specimens (200 mm3) that were composed of polymethyl methacrylate and biofilm was grown for 24 h on approximately 1 cm sections and irradiated with 280 mW/cm2 aBL. Following 228 J/cm2, the viability of the biofilms was reduced by >3-log10 CFU with respect to C. albicans and S. mutans. Increasing the radiant exposure to 456 J/cm2 killed approximately 6-log10 CFU of C. albicans and almost 5-log10 CFU of S. mutans. The killing efficacy of both C. albicans and S. mutans was further confirmed by live-dead staining and confocal microscopy. The authors next performed scanning electron microscopy, where they found there to be ‘holes’ in the hyphae of C. albicans, and cellular damages were evident in S. mutans, following an aBL exposure of 456 J/cm2. In addition, the authors observed significant increases in ROS production and identified numerous porphyrin species to be present within each organism, with S. mutans containing >100-fold lower porphyrins when compared to C. albicans.
The authors concluded that a dose of 456 J/cm2 was sufficient to enable suitable disinfection of dual species biofilms composed of C. albicans and S. mutans. The authors thus suggested that the use of aBL at 405 nm may be a suitable approach for the disinfection of mature dental plaques.
Interestingly, as a method of capitalizing the effectiveness of aBL and translating as a viable treatment of dental infections Zhang et al. [45] developed a wireless implantable blue micro emitting diode (micro-LED) as a method to permit continuous irradiation by aBL (410 nm; 15 mW total irradiance) within the root canal. In their study, they initially looked at the efficacy of aBL within a micro-LED against Enterococcus faecalis, MRSA, and Prevotella intermedia in the planktonic state, where they observed significant killing following 432 J/cm2, 36 J/cm2, and 1.35 J/cm2. In addition, they also evaluated the plausibility for disinfection within the root canals of extracted human teeth using their micro-LED. Here they used 30 extracted human single-rooted teeth and the root canals were then infected with E. faecalis for a total of 14 days to induce biofilm formation. Specifically, the root canals were segregated into three groups, comprising an n of 10. These included teeth treated with saline, calcium hydroxide, or aBL (via micro-LED). Using confocal microscopy, they then determined the bactericidal depth of each respective treatment. They found that the bactericidal depth of aBL was the most effective with a 100.85 μm At day 3, and 223.97 μm at day 7. This was significantly improved compared to the calcium hydroxide group which had a significantly shallower bactericidal depth of 48.1 μm on day 3 and 86.53 μm on day 7. The saline group had a bactericidal depth of 26.11 and 51.60 μm, on day 3 and 7, respectively. Importantly, they found that temperature remained constant with a range between 28.8°C and 30.4°C. The authors concluded that further optimization of irradiation parameters is necessary as well as develop micro-LEDs that are smaller in size may be necessary. Additionally, the authors plan to adjust the conducting carrier to permit treatment of deep infections.
2.4.2. aBL for the treatment of acne vulgaris
In a study by Bumah et al. [46] investigated the use of pulsed blue light (PBL; 2 mW/cm2 – 3 mW/cm2; 450 nm pulsed at a 33% duty cycle) for the suppression of Propionibacterium acnes. The authors previously discovered that PBL elicited a higher level of antimicrobial effects relative to continuous wave (CW) treatment, and thus they sought to determine whether multiple exposures on agar plate containing P. acnes at ultralow irradiances would permit complete suppression. They found that 20 J/cm2 at intervals of 0, 4, 24, and 48 h was sufficient to reduce the viability by 95% (1.2-log10 CFU reduction). When they exposed 20 J/cm2 at intervals of 0, 3, 6, and 24 h, they were able to achieve total suppression (7-log10 CFU reduction). Their findings thus indicated that 3 h intervals of exposure elicited higher levels of suppression as compared to 4 h intervals. Importantly, the authors realized that a single exposure at their maximum dose (20 J/cm2) was insufficient to fully suppress P. acnes. Since endogenous porphyrins have been implicated as important for the aBL mediated killing of microbes, the authors determined whether fluorescence which is emitted at 635 nm upon excitation with 405 nm light could be explored as an indicator of PBL effectiveness. Indeed, they found that using multiple exposures and irradiances, that fluorescence was found to decrease with increasing irradiation, and increase in the absence of irradiation. The authors concluded that multiple treatments with 3 h intervals were optimal for P. acnes suppression and suggested that the significant increase of fluorescence (thus suggesting an increase in porphyrin abundance) was responsible for this phenomenon.
In a similar study from the same group, Bumah et al. [47] investigated the potential for PBL to suppress planktonic MRSA and P. acnes and those within biofilms using low irradiances of 2-3 mW/cm2. They found that 7.6 J/cm2 (administered over 3 x 30 min intervals), resulted in 100% suppression of MRSA. For P. acnes, they found that treatment with 5 J/cm2 (2 mW/cm2) three times per day over 3 days resulted in 100% suppression of planktonic cultures. They found light doses required for 100% suppression to be significantly less effective against formed biofilms, however, the authors did find the biofilm biomass to be reduced following 4 days of exposure. The authors believe that given the appropriate optimization of light dosimetry it would be feasible to achieve 100% suppression of biofilms.
2.4.3. aBL for the treatment of otitis media
A study by Liu et al. [48], investigated the possibility of using aBL (405 nm wavelength) for the eradication of Moraxella catarrhalis, in vitro, which is a principle cause of otitis media in pediatric patients. The authors screened a total of six different M. catarrhalis strains, five of which were clinical isolates and one was an ATCC 25238 strain. The authors found all M. catarrhalis strains in the planktonic state to be highly susceptible to aBL killing with > 3-log10 CFU reductions being achieved following 172.8 J/cm2 (60 mW/cm2 irradiance; 48 min of irradiation). The authors also performed transmission electron microscopy (TEM) on aBL treated cells and observed ultrastructural changes which were interpreted to suggest cell membrane (and cell wall) as well as damage to intracellular contents. They also evaluated the efficacy of aBL against M. catarrhalis biofilms, where they found 216 J/cm2 (reflecting 1 h of treatment) to be sufficient to kill ≥ 3-log10 CFU. In addition, they performed scanning electron microscopy and observed ultrastructural changes that suggested damage to the biofilm extracellular polymeric substance. The authors concluded that based on the preliminary efficacy studies aBL may be a potential approach for the treatment of otitis media. The authors did however stipulate that it is necessary to evaluate the effectiveness of aBL on other otopathogenic agents such as Streptococcus pneumoniae and Haemophilus influenzae, as well as evaluate the efficacy of aBL against otitis media using appropriate in vivo models.
2.4.4. aBL for the treatment of urogenital infection
In a preliminary study, Wang et al. [49] investigated for the first time, the potential for aBL (405 nm wavelength) to kill Neisseria gonorrhoeae. They found N. gonorrhoeae to be highly susceptible to aBL mediated killing with >5-log10 CFU reductions being achieved with a representative ATCC 700825 strain of N. gonorrhoeae and another clinical isolate. In addition, the authors found that when N. gonorrhoeae was exposed in the presence of a singlet oxygen scavenger, the efficacy was significantly suppressed, suggesting that singlet oxygen was important for full efficacy. The authors concluded that given a more detailed investigation it may be feasible to develop of optical waveguides to treat gonorrhoeae within a clinical model. Subsequently, Wang et al. [50] published a follow up investigation where they examined the antimicrobial effects of aBL on ATCC 700825 and 4 clinical isolates. As in their previous study, they found aBL to be highly effective at killing N. gonorrhoeae with 108 J/cm2 being sufficient to kill > 6-log10 CFU in all strains, with multiple strains demonstrating even higher susceptibilities. For example, in ATCC 700825, only 45 J/cm2 was required to kill > 7-log10 CFU (Figure 2).
Figure 2.
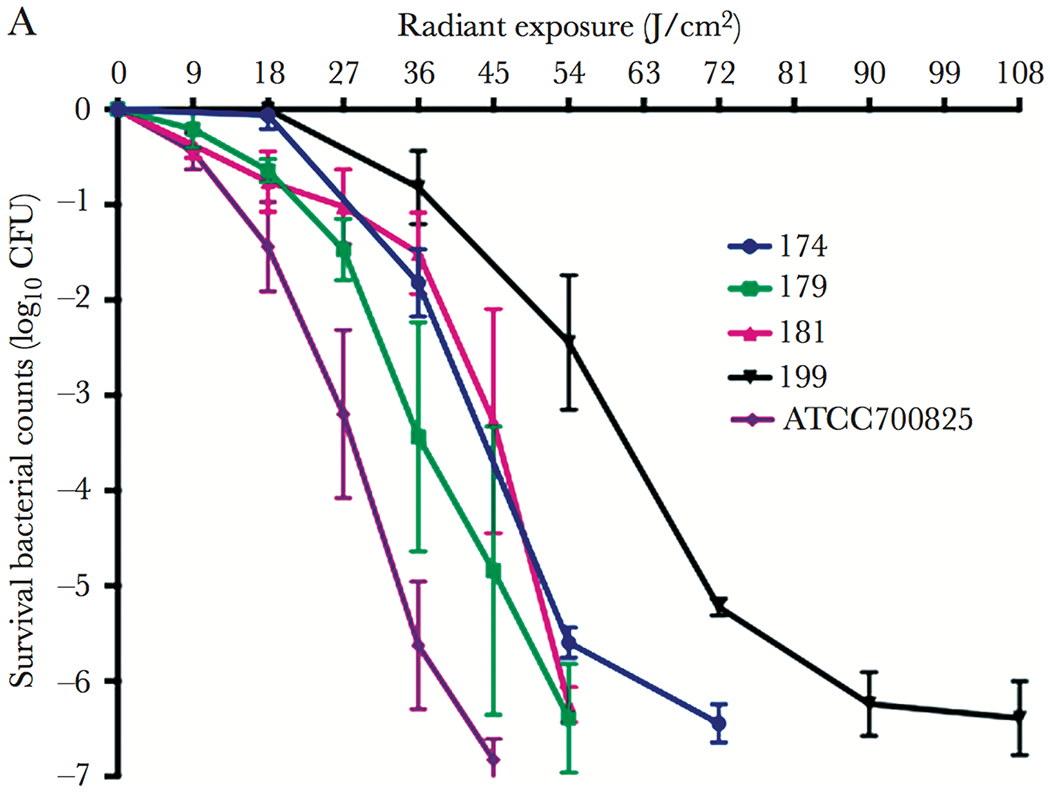
Killing kinetics of different strains of N. gonorrhoeae. Error Bars: standard error of the mean. Taken from Wang et al. [Wang et al.]
2.4.5. aBL for the treatment of intestinal infection
In a preliminary study by Meurer et al. [51] they looked to evaluate the antimicrobial effectiveness of aBL at 405 nm and 464 nm wavelengths against the gastrointestinal bacterium Campylobacter jejuni. They found that with the use of 405 nm aBL 24 J/cm2 was sufficient to eliminate 88.82% of C. jejuni. Interestingly, they found aBL at a wavelength of 464 nm was more effective with a total reduction of 99.81% being achieved, which when compared with previous studies using different organisms, the opposite is typically found. The authors concluded that aBL is an effective treatment strategy against C. jejuni, however, they emphasized that further in vivo studies would be necessary to validate its effectiveness as a therapeutic.
2.4.6. aBL for the prevention of ventilator associated pneumonia
A study by Meurle et al. [52] looked to validate the use of aBL (405 nm wavelength) as a potential strategy for the elimination of Staphylococcus carnosus present within a trachea model contain a saliva substitute. The rationale behind their study was to determine whether they can apply aBL as a method to reduce incidences of ventilator associated pneumonia. Interestingly, they found 9 h of irradiation at 25°C in PBS (reflecting 119.88 J/cm2; irradiance: 3.7 mW/cm2) reduced the CFU by 1.17-log10 CFU (reflecting a 93.4% loss of viability). Interestingly, increasing the temperature to 37°C resulted in a greater viability loss, with a 2.80-log10 CFU reduction (reflecting a 99.84% loss of viability being achieved). Interestingly, when illuminating bacteria within artificial saliva, the killing was even more efficient with a total killing of 3.02-log10 CFU (99.9% reduction) being achieved. The authors concluded that the significant antimicrobial effects elicited under clinically relevant in vitro conditions, would suggest that it would be conceivable to use 405 nm aBL to limit incidences of ventilator associated pneumonia (VAP). Sicks et al.[53] subsequently evaluated the capability of using blue LEDs within endotracheal tubes to prevent VAP. Here, the authors developed a technical tracheal model that permitted testing 48 small blue LEDS (450 nm wavelength) as a means of decontaminating endotracheal tube. The irradiance from these LEDs was up to 13.4 mW/cm2. The authors found that in the exemplar species, S. carnosus, they could kill 3-log10 CFU (99.9%) of bacteria in 6 – 9 h of exposure. The authors concluded that this approach is indeed promising but understood that more work is necessary to validate its potential for the prevention of VAP. They believed that while higher costs may be attributed to placing LEDs on endotracheal tubes, it is unquestionable that the reduced incidences of VAP would relieve the financial burden to healthcare.
In another study, Makdoumi et al. [54] investigated aBL (450 nm wavelength) as a standalone treatment of a single MRSA strain and compared it to the combination of aBL with the photosensitizer riboflavin. They found that aBL could significantly reduce the viability by up to 70% (following 84 J/cm2), however, unsurprisingly, the addition of the photosensitizer riboflavin increased the effectiveness of aBL with almost complete eradication of MRSA being achieved following 30 J/cm2.
2.4.7. aBL as a cutaneous anti-fungal
In a study by Zhao et al. [55] they looked at tissue infected with C. albicans, to determine whether aBL (415 nm) can limit pathogen invasion into the skin of the host. They used a single strain of C. albicans, which was used to infect mouse burns. Initially, they took histology sections 48 h after infection that were stained with periodic acid-shiff (PAS) reagent to determine the localization of C. albicans within skin. In parallel, the authors treated the burn infections with different doses of aBL with radiant exposures of up to 180 J/cm2 being applied. They found that at this radiant exposure, there was a 94.56% reduction in the viability of C. albicans. They next investigated ROS production in the tissue sections, where they found significant increases following 30 mins (90 J/cm2), however, the authors stipulated that the ROS production may have potentially been underestimated given that the signal induced was localized to regions that contained many fungal cells. The authors next looked at invasion depths of C. albicans following different aBL radiant exposures, ranging from 90 J/cm2 – 180 J/cm2. They found that following 90, 135, and 180 J/cm2, invasion depths of 93.36, 86.26, and 39.06 μm, were achieved, suggesting that higher radiant exposures were associated with reduced invasion of C. albicans. The authors concluded that the new knowledge gained from their study into the relationship between aBL radiant exposure and invasion potential could inform treatment strategies of fungal infections.
2.4.8. Blue light as a treatment for COVID-19?
In response to the current COVID-19 pandemic, Zupin et al. [56] carried out a preliminary study to determine the possibility of using aBL (450 nm – 470 nm wavelengths) to induce photobiomodulatory effects within vero cells infected with SARS-CoV-2. In their study, they evaluated: the effects of aBL on SARS-CoV-2 particles outside of the host cell, the effect of pre-aBL irradiation on host cells prior to SARS-CoV-2 infections, and the effectiveness of aBL against host cells infected with SARS-CoV-2. The authors found that the highest anti-viral effects were achieved when aBL was exposed onto Vero cells already infected with SARS-CoV-2. The authors hypothesized that the intact virion particles are resistant to aBL, however, due to the uncoating process that arises during infection of these cells, they become more susceptible. The authors acknowledged that the use of aBL could be a promising strategy for the treatment of SARS-CoV-2, however, further work such as on human cell lines, is required to better predict the translatability of this approach. Vatter et al. [57] too evaluated aBL in a preliminary study, as a potential approach to combat COVID-19, however, due to laboratory restriction, they used the bacteriophage Phi6 infected within a bacterial host Pseudomonas syringae, as a surrogate. The authors found that at the high radiant exposure of 1400 J/cm2, they were able to reduce the plaque forming units (PFU) by 3-log10 (99.9% reduction). The authors were encouraged by these results, however, understood that further work testing 405 nm light on SARS-CoV-2 and other coronaviruses is necessary to validate this a treatment option for aBL.
The recent evidence has suggested the potential for aBL to treat numerous infection types. In addition, the have suggested the potential for aBL to prevent ventilator associated pneumonia, which in the current COVID-19 pandemic is an important application. Interestingly, the several studies that have shown aBL to inactivate SARS-CoV-2 have been encouraging, and have been suggested by the authors to be a potential approach for the treatment of COVID-19. It is important to appreciate, however, that this application is still in its infancy and given the potential logistical complications (i.e. delivery into the respiratory tract) and uncertain efficacy in a pre-clinical or clinical model. Therefore, further work is warranted to investigate whether it may be applied successfully.
3. Identifying endogenous chromophores and targets of aBL
Since aBL has been identified as a viable option for the treatment of infectious agents, it has long been hypothesized that excitation of endogenous chromophores, (porphyrins and/or flavins) that result in reactive oxygen species generation are important factors implicated in microbial killing. This section will review the recent literature that discuss the contribution of endogenous chromophores and ROS production on the effectiveness of aBL.
3.1. Endogenous chromophores implicated in aBL efficacy
In a study by Bumah et al. [46], they found that irradiation of P. acnes using PBL resulted in a decrease in bacterial fluorescence (emission at 620 nm – 670 nm) over a 3-day period of treatment which correlated with the decrease in the CFU of the bacterium. The authors suggested that this correlation between porphyrin fluorescence and the bacterial viability is an indicator that porphyrins are important chromophores during aBL mediated killing. These findings are attributable to a further study by the same group, that sought to quantify the importance of endogenous porphyrins in bacteria. Therefore, Bumah et al. [47] looked specifically at Streptococcus agalactiae (group B Streptococcus; GBS), a bacterium that does not produce porphyrins, to quantifiably determine the importance of porphyrins to aBL killing. In their study, they used GBS COHI (serotype III strain) with exogenously added chromophores of different types including: protoporphyrin IX (PPIX; 0.05 mg/mL – 0.2 mg/mL), coproporphyrin III (CPIII; 0.05 mg/mL – 0.2 mg/mL), nicotinamide adenine dinucleotide (NAD; 2.5 mg/mL – 5 mg/mL), reduced NAD (NADH; 2.5 mg/mL – 5 mg/mL), flavin adenine dinucleotide (FAD; 0.375 mg/mL – 5 mg/mL), or flavin mononucleotide (FMN; 0.375 mg/mL – 5 mg/mL). For all chromophores tested, absorption was found to range between 400 nm – 500 nm with most ranging between 400 – 470 nm. With respect to the non-supplemented bacteria, there was no significant reduction in the bacterial viability following 7.6 J/cm2 PBL that was pulsed 3 times at 30 min intervals over a 24 h period. However, then PPIX was supplemented (0.05 mg/mL) there was a significant loss in viability (91.2% reduction), when illuminated (but no effects were observed in the dark) they found these enhanced effects even when bacteria were washed, which suggested to the authors that PPIX was taken up by the bacteria. When bacteria were incubated with CPIII, at all concentrations tested, there was complete killing of GBS. FMN absorbs light at 405 nm, however, the authors did not observe any significant decreases in the viability of GBS following irradiation. This suggested to the authors that light absorption alone may not be a true indicator of photosensitizing capacity. They did find however, that with increasing concentrations of FMN there was some toxicity that was observed, but only with the highest concentration tested (5 mg/mL). With respect to FAD, the authors found that supplementation of FAD during light irradiation did not have any effect following irradiation. However, they did find that at the highest concentrations tested (2.5 mg/mL and 5 mg/mL) FAD alone (with irradiation) elicited bactericidal effects. The authors suggested that while FAD (like FMN) may not behave like a potent photosensitizer (even though it absorbs light at the appropriate wavelength) there may still be lethal potential. With respect to NAD and NADH, the authors also did not find any significant antimicrobial effects. The authors were not surprised given that these do not strongly absorb blue light. The authors concluded that aBL (450 nm) was unable to kill or suppress growth of GBS, however, the addition of either PPIX or CPIII was enough to enable killing when illuminated with aBL. Application of other strong blue light absorbers (FMN and FAD) or weak absorbers (NAD and NADH) did not elicit significant phototoxic effects when illuminated with 450 nm light. The authors thus concluded that porphyrins are essential for aBL mediated killing and that a strong aBL absorber may not necessarily indicate photosensitizing properties.
Findings by Hessling et al. [58], complement the above study, as the authors investigated the role of porphyrins using another model bacterium, Enterococcus moraviensis, that does not produce porphyrins, to determine whether porphyrins are indeed important for aBL (at 405 nm and 450 nm) mediated killing. In their study, they used a single strain of non-pathogenic E. moraviensis. They irradiated the bacterium for a maximum of 60 h at irradiances of 5 mW/cm2 for 405 nm aBL (1,080 J/cm2 total radiant exposure) or 10 mW/cm2 of 450 nm aBL (2,160 J/cm2). The authors found that 200 J/cm2 of aBL at 405 nm could reduce the viability of E. moraviensis by 1-log10 CFU (90% reduction). Unsurprisingly, at 450 nm aBL, it required 750 J/cm2 to achieve similar levels of efficacy, which is in line with other studies that have found longer wavelength aBL to be less efficacious relative to longer wavelength aBL [18,19,21,22]. Additionally, they suggested that efficacy may be a product of excitation of flavins, lumichrome, or NADH. They found that as radiant exposures of aBL (405 nm) increased, the flavin concentrations decreased. However, they found both lumichrome and NADH to increase, which suggested to the authors that NADH or lumichrome may have been responsible for killing in this bacterium. With respect to NADH, the authors agreed that absorption at 405 nm is weak, although as it has been shown to be an efficient photosensitizer in previous studies, they did not rule out its contribution. Lumichrome was also shown previously to be an efficient photosensitizer by previous studies and has a high absorption at 405 nm, thus suggesting to the authors that it may be responsible for photosensitizing effects. Indeed, the authors understood that lumichrome is not found at high concentrations in unirradiated cells. Their hypothesis that flavin degradation into Lumichrome may offer alternative mechanistic insights into how bacteria are sensitive to 405 nm aBL. The authors thus concluded that if their hypothesis is indeed correct, it may be feasible to kill a multitude of pathogenic microbes irrespective of porphyrin concentration.
In line with Hessling et al. [59], Plavskii et al. [60] also assessed the potential of both porphyrins and flavins to behave as acceptors of blue light, to determine their relative contributions to permit aBL killing of microbes. In their study, they assessed this using ATCC strains of S. aureus, E. coli and C. albicans. In concordance with previous investigations, the authors observed a dose dependent effect on viability when all organisms were exposed to either 405 nm or 445 nm aBL. The authors then identified different intermediate porphyrin absorbances (coproporphyrin, protoporphyin, and uroporphyrin) with emission spectra that appeared unique to each species of porphyrin. The authors illustrated that with respect to 405 nm, this was closest to the peak absorption of all porphyrins tested, whereas 445 was corresponding to a more minimal absorption. The authors subsequently claimed that due to the large differences in absorption of the porphyrins, hypothetically, light at 445 nm should be dozens of times less effective at killing microbes. However, the authors suggested that the differences in effectiveness between the wavelengths was in the range of 3.7 – 6.2 times. This led the authors to hypothesize that there may be other chromophores that are involved with microbial killing by aBL. The authors then suggested that flavins may play a role in the phototoxic effects elicited by aBL, due to their intrinsic photosensitizing properties. The authors found that absorption of flavin mononucleotide absorbed light most at 448 nm, which suggested to the author that at the 445 nm wavelength of aBL, excitation of flavins may be a contributing factor in microbial killing. Although it was not realised by the authors, this disparity in the efficacy may have been a result of the photo-product lumichrome, which was suggested by Hessling et al. [59]. The authors then looked at the spectral-fluorescent characteristics of microbial extracts. Indeed, they found that absorbances and emission spectra were consistent with the presence of porphyrins, with coproporphyrin and uroporphyrin being the most abundant. Additionally, they found that the amount of flavins present within microbial cells depends on the organism, with some of the organisms not showing evidence of flavins (e.g. C. albicans). The authors concluded based on the spectral analyses that porphyrins were implicated in 405 nm aBL and flavins were implicated in 445 nm aBL, due to the dynamics observed with respect to microbial killing. It should be noted, that although this was not stated by the authors, further work is likely necessary to corroborate this further.
It is likely, based on the recent literature, that porphyrins are the principal chromophore that permits the bactericidal effects in bacteria. However, findings from Hessling et al. [59] direct us to a novel chromophore, lumichrome, that may play a contributing role in the killing effects elicited by aBL. Certainly, further work is required to corroborate these hypotheses.
3.2. aBL induced changes in morphology and growth attenuation
In a study by Bowman et al. [61] they looked to evaluate the effects of sub-lethal PBL (450 nm; 3 mW/cm2; 2.7 J/cm2 3 times at 30 min intervals) on the cell membrane of S. aureus. Initially, they used light microscopy to observe damages elicited by the PBL, which did not reveal any evident damages to the cell wall. This indicated to the authors that any damages to the cell wall would be ‘sub-microscopic’. Transmission electron microscopy, however, as determined by the authors, revealed morphological and ultrastructural changes to the cell wall which were consistent with damage. Interesting, findings by Hoenes et al. [62] offer conflicting results, whereby they evaluated how irradiation with 405 nm and 450 nm aBL influenced the cell wall of S. carnosus and P. fluorescens. They did not find any indication of cell wall damage following 90 J/cm2 of 405 nm aBL (70 mW/cm2; enough to reduce the viability by 1-log10 CFU) for S. carnosus and P. fluorescens. With respect to 450 nm aBL they illuminated with 400 J/cm2 and 500 J/cm2 respectively. Given the vast differences between the study, including pulsed treatment with 450 nm (which the authors found to be more effective relative to continuous wave) and the organism that was tested (S. aureus) the difference between the studies may have been expected.
In order to better understand the molecular targets of aBL in bacteria, Hyun and Lee [63] looked into the bactericidal effects of aBL (460 nm – 470 nm) as well as potential targets involved in killing of 10 different pathogenic bacteria including: B. cereus, L. monocytogenes, S. aureus, Enterobacter sakasakii, E. coli O157:H7, P. aeruginosa, S. enterica Typhymurium, Shigella sonnei, Vibrio parahaemolyticus, and Yersinia enterocolitica. Additionally, they looked at a further 12 strains of bacteria that are implicated in food spoilage (e.g. P. fluorescens). Initially they exposed all these bacteria on the surface of tryptic soy agar (TSA) in order to identify the most susceptible to aBL mediated inhibition. As a result, they decided to focus their experiments on P. fluorescens and L. monocytogenes for experimentation on packaged cheese at either 4°C or 25°C. They found that in a planktonic suspension they could eliminate up to 3.6 log10 CFU of either organism after 7 days of treatment (total dose: 604.8 J/cm2). TEM imaging revealed that the cellular structure of each bacteria became significantly disrupted which suggested damage to the cell wall as well as intracellular vacuole formation that may indicate cytoplasmic damages; leading the authors to hypothesize that the cell wall and cytoplasm are targeted by aBL. The cell wall damage is in concordance with Bowman et al. [61], however, it directly conflicts with Hoenes et al. [62]. These differential findings may be a result of light radiant exposures, wavelengths, or strains that were used. The authors then determined, quantitatively, whether the cell wall was indeed damaged by looking at the uptake of propidium iodide (PI) within the cell. They found that uptake was increased during irradiation at both temperatures tested, with 4°C resulting in the highest increases in PI uptake. The authors also looked at the influence of conventional antibiotics including chloramphenicol etc. on the metabolic activity. The authors considered the antibiotics as metabolic inhibitors. With all antibiotics tested, they found decreases in metabolic activity when aBL was introduced concurrently. This led the authors to conclude that this suppression in metabolic activity was in fact an indicator of multiple sites of damage, specifically in cellular component that are related to protein, the cell wall, and protein. Although it was not clearly stated by the authors, it is important to note that these claims still require further work to fully corroborate.
Najjar et al. [64] made a serendipitous discovery that aBL at 405 nm and 445 nm result in growth cessation of B. subtilis when observed under a microscope. Interestingly, the authors noted that only 50% of B. subtilis spores germinated when under the microscope and pulsed with 15 one-second exposures every 1 min, 45 min, or 60 min after induction of germination, as indicated by a lack of changing of the spore coat. When the spores were applied with yellow fluorescent protein excitation of bright field in an analogous manner, no delay in germination was observed, suggesting that indeed blue light inhibits spore germination. To see whether this is specific to B. subtilis, they tested the same parameters on E. coli and Caulobacter crescentus, which they too found to be highly susceptible to growth inhibition. The authors concluded that with respect to live cell imaging that blue light can have negative effects, due to the intrinsic antimicrobial susceptibility. Based on their results, the authors suggested that blue light might have caused cell shrinkage that may have led to this cell cycle arrest. In a similar preliminary study by Galo et al. [65] they found that low radiant exposures of aBL at 470 nm could attenuate growth of S. aureus. They found that at radiant exposures of 54.32 J/cm2 the colonial sizes were significantly smaller (approximately 20%) when compared to the untreated control. The authors concluded that given the modest growth inhibition, low radiant exposures of aBL may not be an appropriate treatment strategy due to limited therapeutic potential.
The capacity for growth inhibition was further studied by Ahmed et al. [66], who applied a femtosecond laser that emitted light wavelengths ranging from 380 – 420 nm, to determine its effect on the growth kinetics of S. aureus. In their study, they used a single strain of S. aureus. They found that when S. aureus was exposed to a total power of 75 mW for 5, 10 and 15 min (79.6, 159, and 239 J/cm2, respectively) using a rage of light wavelengths: 380, 390 or 400 nm that bacterial survival and proliferation rate were influenced; with the latter radiant exposure having the most significant effects. The authors next increased the power to 150 mW or 210 mW, with a maximum radiant exposure of 630 J/cm2 being achieved, following 15 min. As with Najjar et al. [64], They found that samples that were laser exposed grew significantly slower than those that were left unexposed. With similar effects being observed at all wavelengths tested. Interestingly, the authors noticed that growth attenuation was a function of power output, specifically, when bacteria were exposed to higher power such as 150 mW or 210 mW (even at similar radiant exposures) the bacterial growth rate was less affected, than when compared to lower power outputs (i.e. 75 mW). The authors stipulated that the higher power may have resulted into photo-bleaching of endogenous photosensitizing chromophores that may have limited subsequent effects. The authors next wanted to determine the influence of laser wavelength with the growth kinetics. They irradiated samples with the following wavelengths 380, 390, 400, 410, and 420 nm, and different powers: 25, 50, 75, 150, and 210 mW. Unsurprisingly, they found that wavelengths in the range of 390-400 were the most effective at reducing the viability. The authors concluded that at a radiant exposure of 159 J/cm2 with a power of 50 mW, was the most effective at reducing the bacterial viability, which suggested to the authors that it is more effective to increase the exposure time than increase the power.
In line with the above mentioned study, Chu et al. [67] were interested to better understand mechanistic process that are involved with aBL (415 nm wavelength) mediated bacterial killing with a specific important to oxidative stress and damages to lipids and the cell membrane. In their study, they used Cronobacter sakazakii as a representative organism. They initially found that a radiant exposure of 30.06 J/cm2 did not elicit any antimicrobial effects, therefore, the authors aimed to clarify the photochemical processes that were happening within this exposure window. Therefore, they quantified the production of superoxide anion, which they characterized as an important species of ROS, as well as the production of the bi-product of lipid oxidation malondialdehyde (MDA). They found that when C. sakazakii was left in the dark, superoxide anion generation was within a stable range (approximately 0.40 A580/109 cells). However, following irradiation for 30 mins (16.7 mW/cm2; 28.8 J/cm2) there was a 2.3-fold increase in superoxide anion production. Subsequently, they measured MDA production which was approximately 43% higher in the aBL treated group vs. the untreated suggesting that the bacteria were undergoing oxidative stress, as a result of lipid oxidation. The authors then looked at the 9 fatty acid species that are detectable in C. sakazakii and found some of these species to disappear following aBL irradiation. For example, the fatty acid C18:1 which made up 0.87% of the total fatty acid composition vanished following 10.02 J/cm2 aBL. This was the case with multiple other fatty acids, which led the authors to deduce that this was due to fatty acid oxidation. The authors next looked to evaluate how aBL may permeabilize the outer membrane of the cell using 1-N-phenylnaphthylamine as a marker for cell wall permeabilization. They found that permeability of C. sakazakii increased by about 50% following 30.06 J/cm2 relative to the untreated control, suggesting to the authors that this may be an important target of aBL. The authors tested their hypothesis that sub-lethal aBL irradiation results in increases in the total antioxidant activity. Using the Trolox assay, they found that the total antioxidant activity did indeed rise (by 30%) during the sublethal exposure relative to the untreated control. They additionally found transcriptional up-regulation of three genes encoding oxidative-stress resistance, a chaperon, an adhesin, and a capsule biosynthesis gene.
Similarly, Wu et al. [68] investigated the ROS profiles generated during aBL killing of MRSA and assessed the damages elicited onto lipids present in the bacterium. In their study a single strain of MRSA 252 was used as a model. The authors initial experiments revealed that relatively low radiant exposures of aBL (80 J/cm2) were enough to kill > 6-log10 CFU. The authors next observed changes in intracellular porphyrin (coproporphyrin) concentrations as a result of exposure. They did not observe any changes to coproporphyrin when MRSA remained in the dark, however, following aBL irradiation, a significant reduction in coproporphyrin (33.7%) was observed. Next, they wanted to observe changes in generic ROS and singlet oxygen. For the latter, they used singlet oxygen sensor green (SOSG) where they found that non-irradiated bacteria did not increase in fluorescence. However, upon aBL irradiation there was significant increases in fluorescence. Because they hypothesized that singlet oxygen could, in theory, attack other substrates within the bacterium, they also included a generic ROS measurement. They found that in the untreated control there were no changes in generic ROS, however, following only 4 J/cm2 aBL there was a 3.6-fold change in intracellular ROS. They next assessed lipid oxidation, where they used MDA production as a marker. They found that higher radiant exposures of aBL resulted in increases in intracellular MDA content with 5 J/cm2, increasing the MDA by 2.81-fold. Subsequently, using LC/MS they analyzed the composition of 16 fatty acid molecules. They found that with increasing radiant exposures of aBL certain fatty acid molecules (e.g., C20:4) completely disappeared following only 2 J/cm2 aBL. TEM revealed no obvious changes to the cell morphologies except for some ‘roughness’ following aBL irradiation, which were attributed to damages to the cell wall structures. Therefore, the authors looked to evaluate changes in cell wall permeability, by measuring changes to the potassium ion concentration within the extracellular environment. They found that with increasing radiant exposures of aBL (beginning from 3 J/cm2) leaking of K+ coupled with reduced uptake occurred. They attributed this phenomenon to denaturation of outer membrane proteins (such as Na+K+ pumps) which are important for the maintenance of the cell wall. The authors concluded that aBL likely has multiple targets within the cell membrane, such as proteins and lipids. They suggested that the oxidation of membrane proteins may have resulted in denaturation which had a deleterious effect on the integrity of the cell wall.
As with the study above, Fila et al. [69] investigated the targeted effects of aBL on P. aeruginosa biofilm formation and general pathogenicity. As with Wu et al. [68], The authors understood that ROS production is an important mediator of aBL activity, and thus hypothesized that destruction associated with ROS may result in decreases in quorum sensing signaling molecules (QSSMs) as well as a reduced biofilm formation that should quell pathogenicity. In their study, they used 2 reference and 12 clinical isolates of P. aeruginosa which were tested with a single LED with a peak emission of 411 nm and irradiance of 15.7 mW/cm2. The authors found that porphyrins were detectable in extract solutions of P. aeruginosa. The authors found that irradiation of this supernatant or whole cells incubated with appropriate ROS probes (singlet oxygen sensor green (SOSG) or 3’-p-(aminophenyl) fluorescein to detect hydroxyl radicals [•OH]) resulted in significant increases in both singlet oxygen and hydroxyl radicals, suggesting that both type I and type II photochemical reactions were induced by aBL (Figure 3). Additionally, the authors investigated the effects of aBL on the quorum sensing molecule PQS in P. aeruginosa where they did not find any changes in its activity. However, with respect to other QSSMs there were changes to the activity, with Rh1 and Ls showing decreases of up to 63.7% and 82.4%, respectively. Furthermore, P. aeruginosa that was exposed to sub-lethal radiant exposures were found to require longer time periods (up to 220 min) in order to achieve biofilm formation, suggesting that aBL reduces biofilm formation. Next, the authors looked at how aBL attenuated virulence in P. aeruginosa using a Caenorhabditis elegans model of pathogenicity. They found that sub-lethal aBL treated groups significantly prolonged the survival of C. elegans when compared to untreated controls, suggesting that P. aeruginosa indeed became less virulent as a result of aBL exposure. The authors concluded that the robust anti-virulence effects of aBL on P. aeruginosa could permit it to be developed into a viable strategy for the treatment of P. aeruginosa infections.
Figure 3.
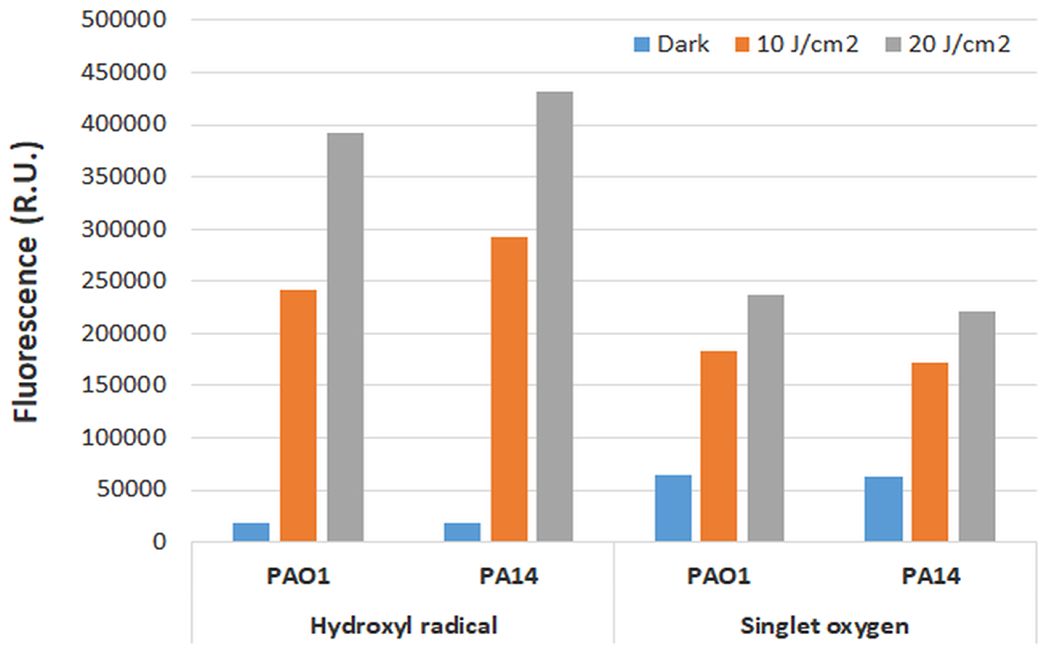
Bar graph showing the changes in relative fluorescence as following 10 J/cm2 or 20 J/cm2. In addition, a dark control was included. Figure taken from Fila et al., [69].
A study by Shany-kdoshim et al. [70] similarly investigated the mechanisms involved when multi-species biofilms formed by oral bacteria are killed by aBL. In their study, they used Fusobacterium nucleatum, P. gingivalis, Streptococcus sanguis, or Actinomyces naesundii. The authors prepared biofilms using all these species and incubated them for 3 days prior to aBL exposure. Following aBL exposure (146 J/cm2; 1.2 W/cm2 irradiance; 2 min of exposure) there was a total of 60% reduction in viability of the total bacteria relative to the untreated control, although the authors did not find this to be statistically significant. However, the authors did notice that the proportions of different bacteria within the biofilms changed. They found that S. sanguinis and A. naeslundii increased from 78% to 87%, whereas P. gingivalis and F. nucleatum dropped from 23% to 13%. The authors then investigated the potential of aBL to kill monospecies biofilms of each that is described above. Interestingly, the only species that was found to be sensitive was P. gingivalis as the loss of viability following aBL was found to be statistically significant. Given the effects observed in multi-species biofilms, the authors hypothesized that the mechanism of aBL activity in multi-species biofilms was based on a light induced paracrine (cell to cell signaling) pathway. To test this hypothesis, the authors transferred supernatants from P. gingivalis (aBL sensitive) or F. nucleatum (aBL tolerant). Crossover of the supernatants that were not previously exposed to aBL did not influence viability of F. nucleatum or P. gingivalis. However, when P. gingivalis biofilm was exposed to aBL treated F. nucleatum supernatant there was a reduction of live P. gingivalis. Interestingly, the addition of P. gingivalis treated supernatant onto F. nucleatum similarly resulted in an increased efficacy of aBL. Furthermore, the effectiveness against F. nucleatum monospecies biofilm was like what was seen in multispecies biofilms. This suggested to the authors that there may be a cross over effect when bacteria are present within multispecies biofilms that contribute to their sensitivities to aBL. Remarkably, they found that when the P. gingivalis supernatant was treated with ROS scavengers prior to application F. nucleatum the effects were inhibited. The authors thus concluded that ROS that is produced may be transferred onto neighboring bacteria that are susceptible to ROS mediated attack (such as F. nucleatum). The authors, however, understood that this is an in vitro study and a further investigation as to the relevance in vivo is warranted.
In addition, as means of understand mechanistic insights (at a molecular level) of how aBL influences Vibrio cholorae, a study by Tardu et al. [71] investigated transcriptional changes that arise from aBL exposure to Vibrio cholerae. The specific rationale behind this study was to evaluate how V. cholerae, which is a water-borne organism, regulates gene expression following aBL. This is given that with respect to visible light, light within the blue spectrum is the only one capable of reaching significant depths within freshwater and marine ecosystems. They initially investigated the genome-wide effect on transcription following aBL exposure, where they identified differential expression in 222 genes (reflecting 6.3% of the genome), with 81 genes being down-regulated and 141 genes being up-regulated. They found 45 gene ontology terms (GO terms) that were found to be enriched, such as those relating to single-organism metabolic process (61 genes), oxidation-reduction processes (32 genes), metabolic process (83 genes) etc. These GO terms were enriched with 14 pathways including: Tri-carboxylic acid pathway, oxidative phosphorylation, fatty acid degradation etc. The rest of the genes were classified as hypothetical. The authors found that this global transcriptional regulation is an effect of oxidative stress. They found that increasing radiant exposures of aBL up-regulated ROS production in a dose-dependent manner, with ROS saturation being achieved following 45 min of irradiation (radiant exposure: unknown). The authors were able to show that expression of genes with increases with rising ROS production, which led to the conclusion that differential gene expression and ROS production were in fact correlated. Interestingly, the data also suggested that this oxidative stress induced by aBL permitted the regulation of genes that are associated with protection and DNA repair, specifically that which may be induced by ultraviolet light.
It was demonstrated by Tardu et al [71] and other studies [67,68] that ROS is an important mediator of aBL activity. This therefore stimulated a study by Dong and Wang [72], reported the construction of a mathematical model based on experimental C. albicans data as a means of determining how the ROS that is generated correlates with photon energy within the blue light spectrum. In their study, they evaluated the following aBL (or UVA) wavelengths including: 385 nm, 405 nm, 415 nm, or 450 nm all of which were irradiated at 50 mW/cm2, for 0-30 min (385 – 415 nm) or 0 – 120 min (450 nm). For all wavelengths tested (except 450 nm) it required at least 30 J/cm2 to result in statistically significant inhibition. For 450 nm, it required 300 J/cm2 to elicit antimicrobial effects. Additionally, increases in radiant exposure resulted in up-regulation of ROS. They mathematically determined that when the radiant exposure is low, the ROS concentration is similarly low, irrespective of the photon energy. Additionally, when the radiant exposure reaches a certain level, this is when the effects of the photon energy begin to become evident. They also found that photon energy within the region of 2.85 –2.95 eV which reflects wavelengths between 420 nm – 435 nm were most efficient at generating ROS.
The studies have shown the importance of ROS in the killing effects of aBL. However, most of the studies have focused on the production of ROS (inclusive of at a genetic level) rather than their direct contribution of the bactericidal effects of aBL. Interestingly, there have been disparate findings of how aBL influences the cell membrane/wall, which calls into question specific targets that are preferentially selected following aBL. Importantly, the authors have also evaluated the role of aBL against biofilms to determine potential mechanisms behind biofilm attenuation or killing of bacteria within polymicrobial environments.
4. Resistance development to aBL
The consensus among those in the field of antimicrobial light (including antimicrobial photodynamic therapy, UVC light etc.) is that resistance development by bacteria to these anti-infection methods is highly unlikely. There are however studies that have brought this into question and have in fact observed the generation of ‘tolerance’[73] Therefore, in this section we will describe all the recent studies that have investigated resistance development to aBL.
In a study by Tomb et al. [74] they investigated the capacity for aBL (405 nm wavelength) to induce resistance development in S. aureus. In their study, they used two strains of S. aureus, one which was a methicillin sensitive (MSSA; strain NCTC 4135) and another was methicillin resistant (MRSA; strain EMRSA-15). Initially, the authors were interested to determine whether culturing MSSA under a low irradiance (0.15, 0.5 and 1 mW/cm2) of aBL would result in resistance development. The authors did not find any significant differences in the killing efficacy when compared with cultures that were grown in the dark, suggesting that there was not increase in tolerance. The authors next wanted to determine whether sequential sub-lethal aBL exposures was associated with increased resistance development. Following 15 cycles of exposure, the authors found that there was no correlation between serial exposure and resistance development. Next, the authors were considering the possibility of altering antibiotic resistance phenotypes as a consequence of aBL exposure. Therefore, using antibiotic impregnated disks, they evaluated ZOI of multiple antibiotics and found that aBL exposure did not negatively influence antibiotic resistance development.
Leanse et al. [75] also investigated the potential for aBL (405 nm wavelength) resistance development, although, in three different species of Gram-negative bacteria including: E. coli (strain UTI89), P. aeruginosa (strain PAO1), and A. baumannii (strain AF0005). In their study, they initially evaluated the killing kinetics of each of these bacteria, where they found that E. coli was more tolerant relative to A. baumannii and P. aeruginosa. With P. aeruginosa and A. baumannii, 144 J/cm2 and 270 J/cm2 were required to kill 5-log10 CFU and 4.69-log10 CFU, respectively. For E. coli, however, 576 J/cm2 was required to kill 4.29-log10 CFU. Following 20 cycles of exposures that were required to kill 99.99% of bacteria (4-log10 CFU reduction) there was no statistically significant correlation between increasing cycles of exposures vs. resistance development. These findings were in concordance with a study performed by Wang et al. [50] and Tomb et al. [74] which also found that sequential exposure of aBL onto N. gonorrhoeae did not result in any significant resistance development. However, Leanse et al. [75] did find that at certain cycles of exposure, there was a statistically significant decrease in the efficacy of aBL. For example, in A. baumannii when the killing efficacy in ‘cycle 1’ was compared with cycles 9, 16, and 17, there was a significant resistance observed. However, this ‘resistance phenotype’ was not stable amongst the cycles of exposure, leading the authors to suggest that this may have been a result of transient transcriptional changes (such as those encoding antioxidants) that influenced resistance development. The authors then proceeded to determine whether serial treatment (using either 108 J/cm2 or 216 J/cm2) over 5 days within an infected abrasion in a mouse model would increase the likelihood of resistance development in the representative species P. aeruginosa. Following treatment of the infection, the authors recovered the bacteria and tested the efficacy of the bacterial isolates in vitro. They did not find any statistically significant differences in the killing efficacy with the serially treated bacteria within mouse wounds vs. unexposed bacteria. The authors concluded that based on their results it is unlikely that serial aBL exposure would result in resistance development. The authors however stipulated that further work testing more species and strains is required to fully validate these findings.
Findings by Rapacka-Zdonczyk et al. [76], challenge those attained by Tomb et al. [74] and Leanse et al. [75]. In their study, they investigated the possibility of tolerance development in S. aureus following both antimicrobial photodynamic inactivation (aPDI) and aBL and found this to be a likely occurrence. They specifically looked at sub-lethal radiant exposures of aBL (considered by the authors as log10 CFU reductions of 1-3 log10 CFU), of aPDI using green light (515 nm; in the presence of Rose Bengal [RB]. For aBL they determined the appropriate log10 CFU reduction would be achieved using 150 J/cm2, for aPDI it was 10 J/cm2 (0.1 μM concentration of RB). The authors found that when S. aureus was exposed for 15 cycles with aBL a significant ‘tolerance’ was observed, although the authors reported that significant ‘tolerance’ was found even following 5 cycles of exposure. Interestingly, the sequentially aBL exposed (at the 10th and 15th cycle) bacteria also presented with ‘tolerance’ to H2O2 which suggested that this may play a role in aBL mediated killing. The authors next wanted to determine if this aBL (and aPDI) ‘tolerant’ phenotype was stable in S. aureus. Therefore, following the 10th consecutive cycle of exposure, bacteria were serially passaged for 5 cycles without light induced selective pressure. The authors did not observe any changes in the aBL-tolerant phenotypes, which suggested that this was stable. This led the authors to conclude that the tolerance phenotype was a result of genetic mutations as a result of DNA damage or through activity of error prone DNA polymerase V. To test this hypothesis, the authors looked at umuC expression which was found to increase following aBL (and aPDI). They also observed increased recA expression which suggested to the authors that an SOS-dependent mechanism was responsible for this observed tolerance. Interestingly, transposon mutants with interruptions within umuC or recA genes were unable to become tolerant to aBL or aPDI following sequential exposure. Remarkably, the authors noted that those sequentially exposed bacteria became more sensitive to antibiotic exposure (with 32 – 128-fold reductions in the minimum inhibitory concentrations [MICs]). The authors claimed that these findings could not be defined as ‘resistance’ development, rather ‘tolerance’ development. Their reasoning was that higher doses of light and/or photosensitizer concentrations (with respect to aPDI) could lead to eradication.
Resistance development to light-based approaches has been evaluated by a multitude of different studies over the years [73], the majority have found there to be little or risk of resistance development following cyclic exposure of microbes to aBL (or aPDT). However, recent studies have questioned this, and it has been suggested that variations in methodology in resistance studies may have contributed to these variable findings [77]. Therefore, to derive a universal conclusion as to the propensity for bacteria to develop resistance to aBL (or aPDT) standardized methods of resistance generation evaluation would be required.
5. Safety of aBL against host cells
The ‘magic bullet’ idea relies on the ability of an agent to elicit strong therapeutic effects against a target (i.e. microbe) without causing significant damage to the host tissue. Therefore, in this section, we will review all the current literature (2017-present) that have experimentally validated any potential safety concerns that may arise from aBL exposure. In addition, based on the recent literature, we have summarized the aBL dosimetry that were found to be associated with limited toxic effects to specific host cells in Table 2.
Table 2.
Summary of validated therapeutic window by antimicrobial blue light as determined by numerous studies published 2017 -present [31,48,82,50,94]
| Cell/tissue type | Wavelength | Experimental paradigm | Validated therapeutic window | Reference |
|---|---|---|---|---|
| Human keratinocytes | 405 nm | In vitro | 216 J/cm2 | [31] |
| Human keratinocytes c | 405 nm | In vitro | 216 J/cm2 | [48] |
| Human colon cells | 405 nm | In vitro | 144 J/cm2 | [81] |
| Human T lymphocytes | 470 nm | In vitro | ≥ 110 J/cm2 | [82] |
| Human monocytes | 470 nm | In vitro | ≥ 110 J/cm2 | [82] |
| Human dermal fibroblasts | 470 nm | In vitro | ≥ 110 J/cm2 | [82] |
| Human embryonic kidney cells | 460 nm | In vitro | ≥60 J/cm2 | [94] |
| Human vaginal epithelial cells | 405 nm | In vitro | 162 J/cm2 | [50] |
| Human keratinocytes | 450 nm | In vitro | ≥84 J/cm2 | [54] |
| Human vaginal epithelial cells | 405 nm | In vitro | 225 J/cm2 | [79] |
| Human skin | 415 + 470 nm | In vitro | ≥3.24 J/cm2 | [85] |
| Mouse skin | 445 nm | In vivo | 60 J/cm2 | [36] |
| Mouse skin | 405 + 460 nm | In vivo | ≥700 J/cm2 | [80] |
| Brain | 405 nm | In vivo | ≥54 J/cm2 | [83] |
| Human vaginal canal | 401 nm | Human clinical trial | ≥30 J/cm2 | [84] |
In the study from our group carried out by Ferrer-Espada et al. [31] that investigated the effects of aBL against monomicrobial and polymicrobial biofilms. They also looked at the potential safety associated with aBL against human keratinocytes at radiant exposures that were associated with significant antimicrobial effects. Safety on the keratinocytes was determined with the use of the MTT assay. Their findings revealed that aBL at a dose of 216 J/cm2 there was no significant loss of viability in human keratinocytes in vitro. However, when keratinocytes were exposed to 500 J/cm2 there was a significant reduction in viability, reflecting approximately 60% (around 0.5-log10 cell viability reduction). Findings were in concordance with another study from our group that was performed by Wang et al. [50] who investigated the effectiveness of aBL on human vaginal epithelial (VK2/E6E7) cells that were co-cultured with N. gonorrhoeae. Killing of intracellular bacteria was assessed by determining the CFU, while the MTT assay was used for determining the viability of VK2/E6E7 cells. It was found that an aBL radiant exposure of 108 J/cm2, (a dose that was sufficient to completely eradicate N. gonorrhoeae within cells) no significant decrease in viability of VK2/E6E7 cells was seen post-exposure. The authors concluded that indeed, the therapeutic effects of aBL against bacteria superseded any deleterious effects on host cells.
A related study by Plattfaut et al. [78] looked to determine if aBL selectively kills wound pathogens over skin cells. Specifically, the authors looked at aBL at 420, 455, or 480 nm on dermal fibroblasts (using CellTiter-Blue which is a resazurin based assay. The authors found that toxicity to cells was contin gent on wavelength that was applied. For example, At the shortest wavelength (420 nm) a of 180 J/cm2 reduced the viability of cells by 52%. These findings are contrary to those found with Ferrer-Espada et al. [31], however, it is important to note that the aBL wavelength, method of viability testing was different (CellTiter-Blue vs. MTT assay) and the cell line tested were different with respect to each study. Interestingly, when the aBL wavelength was increased to 455 nm, 180 J/cm2 was far less deleterious to fibroblasts, with 180 J/cm2 only resulting in a 25% loss of viability. Interestingly, increasing the wavelength to 480 nm did not negatively impact the viability of fibroblasts. Interestingly, that at 180 J/cm2, all these wavelengths resulted in killing of E. coli, S. epidermidis, and P. aeruginosa, with the latter showing the most pronounced effects (>5-log10 CFU reduction). In addition, shorter wavelengths were associated with more pronounced effects as demonstrated previously [22,79]. With respect to S. aureus, however, the authors did not observe any killing with any of the associated wavelengths. Which was suggested by the authors to be a result of the potent antioxidant pigment STX, which was previously shown by our group to be important mediator of aBL tolerance in S. aureus [80]. Unsurprisingly, the effectiveness of all wavelengths against biofilms, was lower than the planktonic counterparts, with only P. aeruginosa showing significant reductions in viability following aBL irradiation. These findings were in line with previous investigations [31,32]. The authors concluded that aBL may be useful for the treatment of wound infections, specifically, those that are caused by P. aeruginosa. However, they stressed the importane of wavelength and bacteria to the potential viability of the therapy. It would likely be importance to balance potential adverse effects to host cells which were found to be associated with shorter aBL wavelengths, with efficacy which was also found to be higher at shorter wavelengths.
Makdoumi et al. [54] similarly evaluated aBL (450 nm wavelength) alone or in combination with riboflavin against human keratinocytes, in vitro. They found that irradiation with aBL alone was effective at killing MRSA while keeping the human keratinocytes unharmed. Specifically, they found that at the highest aBL radiant exposure that was tested (84 J/cm2), that could eliminate 70% of MRSA within a planktonic suspension, no toxicity was observed in human keratinocytes. importantly, the authors found that when aBL (30 J/cm2) was combined with the photosensitizer riboflavin, 99.9% of bacteria were killed. However, the authors found that this dose in conjunction with riboflavin was enough to completely kill the human keratinocytes. The authors concluded that although the addition of riboflavin was superior in terms of MRSA killing, it did result in highly deleterious effects on host cells. The authors concluded that aBL alone could significantly kill MRSA without incurring any significant killing against the host cells. In a corroborating study by Rupel et al. [36], they looked to evaluate the effects of aBL (445 nm) against P. aeruginosa biofilms, also assessed the safety of this against human epithelial cells and against mouse skin. Like with Makdoumi et al. [54], The authors found that at the lower radiant exposures reflecting 40 J/cm2 and 60 J/cm2, there were no significant losses in cell viability, in vitro. However, at a higher radiant exposure of 120 J/cm2 (irradiances: 300 mW/cm2 – 600 mW/cm2), there was a significant loss of cell viability. These findings, however, challenge those achieved by Ferrer-Espada et al. [31], who found that aBL at 216 J/cm2 did not damage keratinicyctes. A potential explanation could be the variable irradiances used by Makdoumi et al. [54] (300 mW/cm2), vs. Ferrer-Espada et al. [31] (60 mW/cm2). Therefore, the generation of thermal effects by Makdoumi et al [54] may have influenced the viability of the epithelial cells. Further work is certainly required to corroborate this hypothesis. Makdoumi et al. [54] , also evaluated the effects of aBL on infected tissue in vivo. They found that application of 600 mW/cm2 onto infected tissue reduced inflammation at the site relative to the untreated control, with no evidence of damage elicited by aBL. The authors concluded that aBL can safely inhibit the progression of wound superinfection.
Wang et al. [79] assessed the viability of both C. vaginitis and human vaginal epithelial cells with the use of the CCK8 assay and CFU counting following aBL (at 405 nm ,415 nm, or 450 nm wavelengths; irradiance of 50 mW/cm2; up to 75 min). Both the model simulation and experimental data indicated that aBL at 405 nm and 415 nm wavelengths were more effective anti-fungal treatments, with complete killing being achieved after 75 mins of irradiation (radiant exposure: 225 J/cm2). In concordance with Ferrer-Espada et al. [31], at this radiant exposure (and wavelengths) the authors did not observe any significant toxicity on the epithelial cells.
In a related study conducted by Shehatou et al. [81] investigated the delivery of aBL with the use of a light diffusing fiber for the treatment of the ESKAPE pathogens (i.e. Enterococcus spp. S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa, and Enterobacter spp.) in addition to other clinically relevant pathogens, including Streptococcus pyogenes and C. albicans. They also investigated whether any toxicity onto human colon 38 cells was observed, using the Almar blue assay. The authors found the most susceptible organism of 405 nm aBL, was S. pyogenes with a 4-log10 CFU reduction being achieved with only 36 J/cm2 and E. faecium was the least susceptible with 540 J/cm2 being required to kill 4-log10 CFU. For K. pneumoniae, S. aureus, and Enterobacter Spp. 144 J/cm2 was sufficient to kill 4-log10 CFU. The authors then measured the toxicity of aBL against human colon cells, where they found no decrease in metabolic activity following 144 J/cm2, which was used as an indicator of cell viability. These findings were in concordance with Wang et al. [50] and Makdoumi et al. [54]. Higher radiant exposures were associated with some losses in viability, and thus the authors considered a dose of 144 J/cm2 to be safe as well as effective against numerous microbial species. The authors concluded that their light diffusing fiber could be safe and effective method to deliver aBL.
In a study by Bumah et al. [82] they investigated the effects of aBL (470 J/cm2) against multiple host cell types including: fibroblasts, Jurkat T-cells, THP-1 monocytes. Cells were irradiated with 3, 55, or 110 J/cm2 aBL. For adult and neonatal fibroblasts, there was no significant loss of viability with any of the radiant exposures that were tested. Additionally, no significant deleterious effects were observed against Jurkat T-cells at all radiant exposures tested. Interestingly, the authors did find the Jurkat T-cells to increase in numbers following irradiation with 3 J/cm2, although it was not found to be statistically significant. Thus, the authors suggested that aBL may have a stimulating (or photobiomodulatory) effect on this cell type. Similar findings were reported with respect to THP-1 monocytes. The authors did stipulate that while there were no statistically significant reductions in the viability of all cell types, 110 J/cm2 exposure did result in some loss of viability. They concluded that as radiant exposures of 110 J/cm2 (or below) had no adverse effects against all cell types, aBL may be applied safely onto host tissue as long as radiant exposures are kept sufficiently low.
In a novel study, carried out by Thurman et al. [83], they evaluated, for the first time, the use of different radiant exposures of aBL (405 nm wavelength) including 36, 45, or 54 J/cm2 against the brains of mice. The authors did not find any behavioral changes in mice upon post-operative examination. In addition, they did not find there to be any pathological changes occurring in the brains of mice, as evaluated by histology, glial fibrillary acidic protein, immunofluorescence, or general markers of apoptosis and necrosis. The authors thus concluded that the use of 405 nm light on the murine brain is safe, suggesting that aBL may be a potential modality against infections occurring within craniotomy sites. The authors understood that this safety assessment was only validated following a single exposure, and it would be important to evaluate the effects of multiple uses on the safety against the murine brain. The authors proposed that it might be good to consider the application of aBL within a chamber of cephalic implants.
In a recent phase 1 clinical trial performed by Pavie et al. [84] they evaluated the safety potential of aBL (401 nm wavelength) against human vaginal mucosa. In their study, they recruited 10 female participants with ages ranging from 18 – 45 that presented with normal cytology. Women who were undergoing antibiotic treatment, corticosteroids, immunosuppressants etc. were excluded as these factors could influence normal vaginal microbiota. aBL was delivered vaginally with the use of a speculum at a total irradiance of approximately 8 mW/cm2 and applied for a total of 30 mins (radiant exposure: 30 J/cm2). Following exposure to aBL, the cytological data did not show any differences in the vaginal microbiota in all participants. Additionally, there was no inflammation observed in any of the participants. In 80% of participants, the vaginal pH was maintained to levels observed prior to irradiation. Only one participant had a reduction in the vaginal pH which was reduced from 5 to 4. No patients reported feeling any pain or thermal effects following or during exposure to aBL nor was there any erythema observed. The authors concluded that aBL did not alter the mucosal tissue and suggested that aBL may be a promising strategy for the treatment of bacterial vaginosis, vulvovaginal candidiasis etc. The authors suggested it was important to investigate further using aBL on genitourinary tracts that have pathologies to validate its effectiveness in which the authors are in the process of evaluating.
In another clinically driven study, Bonnans et al. [85] aimed to understand why both beneficial and potentially damaging effects may arise because of aBL exposure (415 nm or 470 nm wavelength). In their study, they acquired normal human skin (provided from those undergoing plastic surgery or healthy donors) which were prepared via removal of subcutaneous fat and immersion within the appropriate culture medium. They found that when aBL (combined wavelengths: 415 nm + 470 nm) was exposed for 6-18 min (3 mW/cm2; 1 J/cm2 – 3 J/cm2) there was no increase in intracellular ROS. However, when exposed for 36 min (6 J/cm2), there was a significant increase in intracellular ROS (10% increase). Interestingly, when they compared the single wavelengths with combined wavelengths of aBL (415 vs. 415 nm + 470 nm) the single wavelength of 415 nm was more harmful then when it was co-exposed with 470 nm aBL. The authors subsequently investigated the effects of a 415 nm wavelength on the opsin 1 SW photoreceptor of the skin. They found that after 18 min of 415 nm aBL exposure (5 mW/cm2; 5.4 J/cm2) immunofluorescent staining for the opsin 1 SW photoreceptor was decreased suggesting that there was some cell damage observed. Using the same parameters of exposure, the authors observed fragmentation of the papillary dermal fiber, which demonstrated to the authors that aBL was capable of penetrating to this layer of tissue. The authors also observed, following staining of fibrillin-1 (glycoprotein that is part of the extracellular matrix) that a structure in the shape of a candelabra within the upper dermis appeared to be quite damaged. Given the increased ROS production that was found when 415 nm and 470 nm light were combined, the authors investigated the production of cathelicidin antimicrobial peptide (which has been implicated as important in the immune stimulation during bacterial infection). They found that after 1.62 J/cm2 (3 mW/cm2; 9 min of exposure), its production increased. This suggested to the authors that it may permit an increased defense mechanism against bacterial infection. Interestingly, using the same conditions as described above, following application of lipopolysaccharide (LPS), they found that interleukin 8 (IL-8) expression was suppressed compared to unirradiated controls. The authors suggested that this combination had a positive effect on the inflammatory response.
The authors then tested this combination on human subjects that were suffering from acne (moderate – severe). The participants were treated for two weeks (twice/week) for 10 min with the 415 nm + 470 nm combination, followed by one treatment per week for 4 weeks (6 min irradiation). The authors found that the cysts abated in the second month and by the fourth month so did the seborrhea. When the study ended, the authors noted a significant improvement in the microcysts, pustules and inflammatory nodules (figure 4). However, there were only small improvements in the blackheads and non-inflammatory nodules. Because effects were only observed in the second month, it led the authors to conclude that the effects were likely photobiomodulatory in nature.
Figure 4.
Images showing the ‘photolysis’ effect on STX following different illumination times of aBL (460 nm; 20 min or 40 min). Figure taken from Dong et al., [93].
6. Synergism of aBL with other antimicrobials
The potential for aBL to safely and effectively kill pathogenic microbes has been investigated significantly for the past decade. In recent years, methods that employ combination approaches that exploit aBL to enhance the observed therapeutic effects have been investigated. In this section, we will review all the studies that have assessed the antimicrobial potential of aBL in combination with both traditional and non-traditional antimicrobials. In addition, we will discuss the potential for aBL to photolyze endogenous bacterial chromophores to increase their susceptibility to ROS and antimicrobials.
6.1. Combined effects of aBL with conventional antimicrobials
In a study by Wozniak et al. [86] they investigated the potential of using conventional antibiotics as a method of enhancing the effects of aBL (and photodynamic therapy) in extensively drug resistant (XDR) A. baumannii. In their study, they used 2 representative strains (named 127 and 128). The authors found that when exposures of aBL (36.4 J/cm2 to 109.1 J/cm2; 70 mW/cm2; 8.7 min – 26 min of exposure, respectively), it decreased the MICs of several different conventional antibiotics as determined by the disk diffusion assay. For example, the authors found that for doxycycline it reduced the MIC from 128 μg/mL to 64 ¼g/mL in strain 128. The authors identified synergistic interactions between aBL and doxycycline, trimethoprim-sulfamethoxazole, colistin, and imipenem. The authors also looked at the effects of combining aBL + doxycycline on the growth of A. baumannii. They found that this combination resulted in a significant delay in growth of approximately 330 min and 210 min, which suggested to the authors that this was a post-antibiotic effect. Interestingly, the authors found that the combination of aBL and antibiotics resulted in the up regulation of intracellular ROS, which led the authors to suggest that this phenomenon may be responsible for the observed synergies. The authors concluded that given the promising results, combining aBL with conventional antibiotics may be warranted and may slow the development of antimicrobial resistance.
In a related study by dos Anjos et al. [87] they discovered that the application of aBL (410 nm) and photodynamic therapy (PDT) could inhibit numerous clinically relevant β-lactamases and carbapenemases within K. pneumoniae. The authors initially illustrated that K. pneumoniae was more susceptible to MB-PDT when compared to aBL, with 16 J/cm2 and up to 892.28 J/cm2 (depending on the strain), being required to kill K. pneumoniae with MB-PDT and aBL, respectively. The authors used nitrocefin as the detection method of β-lactamases and carbapenemases, where they found that radiant exposures required to kill 3-log10 CFU of K. pneumoniae by both MB-PDT and aBL could significantly destroy both β-lactamases (including extended spectrum β-lactamases) and carbapenemases. The authors thus concluded that exposure to aBL or MB-PDT could potentially reverse resistance to β-lactam antibiotics and thus prolong the use of this class of antimicrobials.
Findings from both studies strongly suggest that aBL may be co-delivered with conventional antibiotics to facilitate treatment of multidrug-resistance. It is clear however, that further work exploring this combination is necessary to determine the full scope of its potential.
6.2. Combined effects of aBL with non-conventional antimicrobials
In a study by Hyun et al. [88] they investigated the potential synergistic effects with aBL (460 - 470 nm) and non-conventional antimicrobial compounds including: ethanol, carvacrol, thymol, acetic acid, caprylic acid, citric acid, malic acid, citrus fruit extract, and NaCl. The authors used a single strain of E. coli O157:H7 as a representative model. Initially, they evaluated the effect of aBL (0.83 mW/cm2; 24 h; 71.7 J/cm2) on the ZOI for all the antimicrobials at sub-MIC concentrations. They found that at the concentration used, (e.g. ethanol [6250 μg/mL; 6.2%]) no inhibition was observed. However, when it was combined with aBL, it resulted in a 10 mm ZOI. The most effective combination was found to be citric acid (1250 μg/mL; ½ MIC) which did not present with any inhibitory effects in the absence of light. However, when combined with aBL, it resulted in a ZOI of 45 mm. They next looked at the bactericidal effects of aBL alone and in combination with different antimicrobials. The authors did not find any significant reduction in the viability of bacteria when exposed to 71.7 J/cm2 (24 h) with a maximum reduction of 0.21 log10 CFU being achieved (at 4°C). However, when combined with the different antimicrobials there was a significant enhancement observed. At 25°C, the authors found that following 4 days of irradiation at 25°C (286 J/cm2), there were significant reduction is the CFU in all combinations tested. They found that combination of aBL with carvacrol and NaCl were particularly effective. The authors next looked at the ROS production during the combination treatments where they found that there was no change following dark incubation of bacteria at 4°C or 25 °C, with only slight ROS production being observed with aBL. The authors found there to be increases in intracellular ROS when aBL (at 4°C) was combined with carvacrol, malic acid, or citric fruit extract with ROS increases up to 57% being observed. Interestingly, when these combinations were exposed at 25°C, no upregulation in ROS was observed. Additionally, the authors found that when aBL was combined the different antimicrobials there was an increase in lipid peroxidation relative to either monotreatment. For example, when carvacrol was combined with aBL, lipid peroxidation increased by 39% at 25°C relative to mono-treatments. Additionally, the authors found that the cell wall integrity was affected by these combinations. Furthermore, the authors did find that while there was damage to DNA, it did not appear to correspond to the effectiveness of a particular combination, with certain combinations (e.g. aBL + carvacrol) resulting in significantly less damage. The authors concluded that these different combinations may be effectively used to improve microbiological safety in public health as well as the food industry, although, they conceded that further optimizations are required.
In a study from our group, Leanse et al. [89] discovered a potent combination approach that exploited aBL (405 nm) and quinine hydrochloride (Q-HCL) for the eradication of Gram-negative bacteria. In their study, they used a representative multidrug-resistant strain of P. aeruginosa (AF0001) and A. baumannii (AF0005). The authors found that when bacteria were exposed to sub-antimicrobial concentrations of Q-HCL (0.125 mg/mL – 1 mg/mL) incapable of eliciting antimicrobial effects, significant enhancement in the efficacy of aBL were observed in both bacteria. For example, they found that 1 mg/mL Q-HCL coupled with 54 J/cm2 (30 mW/cm2; 30 min exposure) was enough to result in at least a 7-log10 CFU reduction. An equivalent dose of aBL only killed 1.05-log10 CFU, illustrating that Q-HCL enhanced the killing efficacy by approximately 105-fold. The authors next evaluated the potential of Q-HCL to enhance the effectiveness of aBL against 24 h old mature biofilms. The authors also found that Q-HCL significantly increased the effectiveness of aBL in both species. For example, with A. baumannii, aBL alone (108 J/cm2) was sufficient to kill 1.11-log10 CFU, however, in combination with Q-HCL, this log10 CFU reduction increased to 5.670-log10 CFU, illustrating a >104-fold enhancement in the antimicrobial effects. When the authors performed scanning electron microscopy, they noticed that when aBL was combined with Q-HCL, the extracellular polymeric substance (EPS) was degraded relative to either monotreatment or negative control. The authors next performed TEM to observe any possible damages arising from this combination approach. They found that exposure to Q-HCL alone resulted in vacuole formation and damage to the cell wall, and aBL alone resulted in spillage or intracellular contents. The combination resulted in more significant damages to the cytoplasm and the cell wall. Next, the authors were interested to determine whether increased uptake of Q-HCL was involved with these effects. In A. baumannii, for example, single cell Raman spectroscopy, found that Q-HCL does enter the bacterial cells (indicated by the Raman intensity at 1362−1) following exposure, however, when aBL was illuminated, there was a significant increase in the Raman intensity, indicating to the authors that aBL indeed increased the uptake of Q-HCL into bacteria. They next looked at the potential translatability of their approach, using an early onset P. aeruginosa abrasion infection model as a representative with bioluminescence monitoring. They found that Q-HCL (2 mg/mL; 100 μL) alone did not influence viability of P. aeruginosa, and following aBL alone only reduced the relative luminescence by 1.30-log10 following 135 J/cm2 exposure. When aBL and Q-HCL were combined at equivalent doses, it resulted in a log10 RLU reduction of 3.08, indicating a greater than 50-fold improvement in the killing effects. Impressively, there was no obvious recurrence of the signal (unlike with the mono-treatments) suggesting that the combination was highly effective. The authors next wanted to determine if the combined aBL and Q-HCL doses would be harmful to mouse skin in vivo. The authors applied the exact doses used to eliminate > 3-log10 RLU (99.9% loss of viability) onto naive mouse skin and found there to be no apoptosis as a result as indicated by the TUNEL assay. The authors concluded that given further studies and optimization that this combination may be an effective approach for the treatment of infections caused by Gram-negative bacteria.
Leanse et al. [90] subsequently performed a follow up study, when they looked at the antifungal effects of combining aBL and Q-HCL against the representative species C. albicans. As with Gram-negative bacteria, the authors determined that the combination was highly effective at killing C. albicans in a planktonic state, with a >7-log10 CFU being killed. The application of aBL alone killed 3.06 log10 CFU, illustrating that the application of Q-HCL increased the effects of aBL by approximately 104-fold. The authors then performed TEM to observe the ultrastructural/morphological changes that resulted from treatment. They found that the combination resulted in changes to the cellular morphology with damages to the cytoplasm, vacuole formation, and detachment of the cytoplasm from the cell wall. aBL alone also induced changes, including elongation of cells, and detachment of the cytoplasm from the cell wall. The application of Q-HCL did not induce many changes when compared to the untreated control, other than some apparent swelling of the cell wall. The authors next evaluated the effectiveness of the combination of aBL and Q-HCL (216 J/cm2 + 1 mg/mL, respectively) against C. albicans 48 h old biofilms, with a 1.73-log10 CFU reduction being achieved. This was found to be 10-fold better than with respect to aBL alone which only killed 0.73 log10 CFU. Q-HCL did have some effect on the viability with a log10 CFU reduction of 0.66 being achieved. Lastly, the authors wanted to determine whether this combined approach could be applied within a mouse model of C. albicans abrasion infection using real-time bioluminescence monitoring. The authors found that the combination of aBL and Q-HCL was approximately 10-fold more effective when compared to aBL alone and found that Q-HCL alone did not influence the viability. The authors next looked at potential toxicity against mouse skin in live mice, where they did find evidence of apoptosis although the authors did not consider this to be highly significant. The authors concluded that the combination of aBL and Q-HCL may have potential applications for the treatment cutaneous candidiasis.
Leanse et al. [91] then investigated the potential for aBL (405 nm) and the combination of aBL and Q-HCL for the treatment of cutaneous mold infections. The authors initially evaluated the effectiveness of aBL against mold conidia of 3 species including: Aspergillus flavus, Fusarium oxysporum, and Aspergillus fumigatus. For A. flavus, F. oxysporum, and A. fumigatus, following irradiation with 540 J/cm2 (they found the effects to be limited with log10 CFU reductions of: 0.86, 0.94, and 0.91 being achieved, respectively. The authors considered that the addition of Q-HCL may potentiate these effects, given that the authors saw significant enhancements of aBL with Gram-negative bacteria and C. albicans, it may to improve the effects against fungal conidia. Indeed, the authors found that for all mold species tested, there was a significant increase the antimicrobial effects. For example, for F. oxysporum they found that co-delivery of aBL and Q-HCL resulted in a 6.52-log10 CFU reduction, indicating a >105-fold improvement in the sporicidal effects. The authors next performed TEM analysis, where they found significant ultrastructural changes occurring as a result of Q-HCL, aBL, and the combination. They found clear evidence of internal damages within the spore as illustrated by the presence of vacuoles. With aBL, there was clear intracellular damages, although the cell wall appeared intact. The combination elicited more significant damage of intracellular material, as well as structural injury to the cell wall. Porphyrins have been implicated as important chromophores necessary for the photochemical reactions that occur during aBL treatment. Therefore, the authors performed ultraperformance liquid chromatography (UPLC) to see if they could identify endogenous porphyrins. The authors did find the presence of endogenous porphyrins with all species tested, with A. fumigatus presenting with the highest concentration of intermediate porphyrins (150 pmol/mg) and A. flavus presenting with the lowest (2.6 pmol/mg). A. flavus contained 19.5 pmol/mg of intermediate porphyrins. Interestingly, most porphyrins in F. oxysporum and A. flavus were coproporphyrin, and A. fumigatus had uroporphyrin as the dominant porphyrin. The authors next assessed the translational capacity of aBL and combined aBL and Q-HCL, for the first time, in a murine model of established A. fumigatus infection using real time bioluminescence monitoring. For aBL, they found that 144 J/cm2 (20 min; 120 mW/cm2) was sufficient to reduce the viability by approximately 1.2-log10 RLU (>90% reduction in viability). When Q-HCL was exposed simultaneously it increased significantly with >2-log10 RLU being reduced (>99%; figure 4). Following the maximum exposure approximately 2.5-log10 RLU were reduced. Importantly, the authors found that there was limited recurrence A. fumigatus 72 h post-exposure, which gave the authors confidence that, if appropriately optimized, it could be translated into a viable clinical approach.
More recently, Leanse et al. [92] discovered an innovative combination approach that pairs aBL (405 nm; 60 mW/cm2) with the P. aeruginosa pigment, pyocyanin, for the eradication of MRSA. In their study, they assessed the effects of this combination on two strains (in a planktonic state) of S. aureus carrying MDR phenotypes against methicillin and vancomycin. In their study, they combined different radiant exposures of aBL, reflecting 0, 54, 108, 162, and 216 J/cm2 with different concentrations of pyocyanin, including: 6.25, 12.5, or 25 μg/mL. For both strains, aBL alone for 216 J/cm2 killed ≤1.5 log10 CFU, when 6.25 μg/mL was applied concurrently the killing effects rose to ≥4-log10 CFU. Increasing the pyocyanin concentration to the maximum 25 μg/mL, resulted in the most pronounced effects with all visible bacteria being eliminated following 162 J/cm2 aBL. The authors serendipitously discovered that when aBL and pyocyanin were co-delivered onto the golden MRSA colonies, that staphyloxanthin (STX) became ‘bleached’ which suggested to the authors that the parallel pathway of ROS production by aBL and pyocyanin, may have synergized, to induce an oxidative burst that degraded the pigment. However, further work would be required to substantiate this hypothesis. The authors next determined the effects of ROS scavenging of singlet oxygen (L-histidine) or hydrogen peroxide (catalase) on the effectiveness of the combination. Although they found there to be some attenuation in efficacy through the addition of catalase, it was not found to be significant, suggesting to the authors that hydrogen peroxide may not have been vital for efficacy. The application of L-histidine, however, was found to impede efficacy of the combination, which suggested to the authors that singlet oxygen was a requirement for full efficacy. Given the important role of biofilms in MRSA infections, the authors next determined whether pyocyanin could synergize with aBL to for improve antimicrobial effects against biofilms. The authors found, that at all concentrations of pyocyanin (reflecting those above), there was a significant improvement in the bactericidal effects. For example, 216 J/cm2 in combination with 25 μg/mL of pyocyanin resulted in close to a 4-log10 CFU reduction of the biofilms, whereas aBL alone killed just over 1-log10 CFU. To validate the clinical translatability of aBL in combination with pyocyanin, the authors applied this combination in an early-onset MRSA skin abrasion infection. The authors found that 216 J/cm2 in combination with a total dose of 2.5 μg (25 μg/mL concentration) was sufficient to reduce the viability of MRSA within the wounds by approximately 99.9% (3-log10 CFU reduction). The application of aBL or pyocyanin only influence the viability marginally. Lastly, the authors evaluated the safety profile of aBL plus pyocyanin on naïve mouse skin, where they did not observe any deleterious effects following hematoxylin and eosin staining, or any evidence of apoptotic cells following the TUNEL assay.
It is clear from the literature that aBL is not only a potent antimicrobial in its own right, but is highly complementary to co-administration with traditional or non-traditional antimicrobials. Therefore, there exists an exciting opportunity for aBL to potentially be integrated into existing clinical workflows to permit rapid and effective treatment of multidrug-resistant pathogens that resist conventional therapeutic approaches.
6.3. Photolysis of endogenous chromophores to potentiate ROS and antimicrobials
Recently, there have been studies that have exploited photolysis by aBL (430 nm – 460 nm) as a method of destroying endogenous antioxidants (such as staphyloxanthin [STX] and graenadene) as a method of sensitizing bacteria (specifically MRSA and GBS) to ROS mediated killing as well as conventional antibiotics.
In a study by Dong et al. [93] it was serendipitously discovered that irradiation of MRSA with 460 nm aBL resulted in photolysis of STX (Figure 4). Given that STX is a carotenoid pigment that is known to aid MRSA in resisting ROS attack (via hydrogen peroxide [H2O2] or singlet oxygen), which allows persistence within host neutrophils, the authors hypothesized that photolysis may render MRSA sensitive to H2O2. Initially, they were interested to determine whether photolysis alone could eliminate MRSA, specifically, as STX is an integral part of the cell wall. The authors found that in fact, the killing effect of 460 nm aBL was limited, and it appeared as though this wavelength of light induced a ‘traumatized’ state, that increased the permeability of S. aureus. However, the killing effects of 460 nm light (120 J/cm2; 90 mW/cm2) on MRSA were highly limited with no significant killing being achieved. The authors subsequently exploited the photolysis effect to potentiate the effects of H2O2 Interestingly, the authors found that when 460 nm aBL exposed MRSA was administered with H2O2 and imaged via confocal laser scanning fluorescence imaging, a much higher fluorescence (indicating increased uptake of H2O2) was observed within MRSA when compared to the unirradiated control. Additionally, when MRSA was exposed (in a planktonic state) to 108 J/cm2 460 nm aBL, significant enhancements in the antimicrobial effects of H2O2 were observed at concentrations as low as 0.0375%, with approximately 7-log10 CFU of MRSA being killed. Because MRSA commonly persists within host macrophages, the authors investigated the potential to exploit the aBL/ H2O2 synergism on intracellular MRSA. They found that macrophages infected with MRSA (1 h infection) that were exposed to 48 J/cm2 aBL (400 mW/cm2; 2 min exposure) twice with a 6 h interval, significantly reduced the burden of MRSA relative to the untreated control. It was noted that even with vancomycin at 5x the MIC was unable to influence the burden of MRSA within these cells. Noteworthily, the authors also found that 460 nm light exposed MRSA were eliminated with the use of whole blood. Next, the authors evaluated the efficacy of this aBL/ H2O2 combination on persister cells and biofilms. For persister cells they were able to reduce the CFU by 2-log10. With biofilms, the effects were more modest, with 360 J/cm2 ‘traumatizing’ the biofilms by 80% and the combination killing 92% of MRSA. With daptomycin 5 x MIC and 24 h incubation, only a 70% reduction was observed.
To validate the translatability of this approach, the Dong et al. [93] attempted a treatment on intradermally infected mice (with 108 CFU) that were left to establish for 60 h prior to treatment. They found that the combination of aBL (24 J/cm2; 200 mW/cm2) and H202 (0.045%) that was administered twice daily for 3 days resulted in a 1.5-log10 CFU reduction relative to the untreated control, which was found to be significantly better when compared to H2O2 alone or 460 nm light alone. The authors also profiled the expression of 200 cytokines and found that in the combined aBL / H2O2 group there was a significant decrease in TNF-α, IL-1α, IL-2, IL-17, MIP-1α, MIP-1β, LIX. Additionally, decreased expression of vascular endothelial growth factor receptor 3 (VEGFR3) was observed, suggesting a reduction in chronic inflammation. The authors also utilized a second model of early onset abrasion infection using a bioluminescent variant of MRSA (strain USA300). The authors too found there to be a significant difference in viabilities as determined by the quantified RLU (approximately 1-log10) between the H2O2 alone and combined aBL/ H2O2 group. The authors concluded that this combination of 460 nm light and H2O2 represents a potential treatment strategy for infections caused by MRSA. In addition, because numerous pathogenic microbes are pigmented, exploiting their photophysical and photochemical properties may present innovative and exciting avenues for the treatment of infectious diseases.
In a similar study that exploits photolysis of STX, Jusuf et al. [94] investigated the effects of silver nanoparticles at low concentrations in combination with ‘pulsed’ 460 nm light against MRSA. The authors stipulated that although silver nanoparticles are highly antimicrobial, at high concentrations, they can be toxic to tissues. Therefore, they initially evaluated the use of pulsed blue light at 460 nm, where they found STX to become 80% bleached following 30 J/cm2 irradiation (100 mW/cm2; 5 min irradiation). The authors then found that the concentration of silver nanoparticles (10 nm) required to inhibit MRSA was greater than 10 μg/mL. Therefore, the authors tested 5 μg/mL in combination with 30 J/cm2 PBL, and they found that following 4 h incubation, a 3-log10 CFU reduction (99.9% loss of viability) was achieved when PBL was combined with silver nanoparticles. At an equivalent incubation time, only a 1-log10 CFU reduction (90% viability loss) was achieved with silver nanoparticles alone, and a negligible loss with PBL alone was achieved. The authors subsequently evaluated uptake of the silver nanoparticles, with the use of transient absorption microscopy, following PBL exposure. They found a significant signal indicative of absorption of the silver nanoparticles in both the treated and untreated groups; however, the treated group had a significantly higher signal (3.33-fold higher). The authors thus attributed the uptake of the silver nanoparticles as a major reason for these enhanced effects. Additionally, the authors recorded significant increases in the intracellular ROS (specifically hydroxyl radicals) with the PBL exposed samples showing a 73% increase relative to the unirradiated sample. Importantly, the authors evaluated the toxicity of silver nanoparticles against mammalian cells, where they found that 10 μg/mL elicited some toxicity (approximately 40% loss of viability), however, as lower working concentrations could be used in combination with PBL, there was no loss of viability at concentrations 5 μg/mL and below. Additionally, 460 nm light at 60 J/cm2 did not influence the viability of cells. Lastly, the authors wanted to determine whether this approach would work against 24 h biofilms. They found significant inhibition in biofilm formation following combined PBL and silver nanoparticles, with 26.7% and 22.9% further decreases being achieved relative to each monotreatment. They also looked at the effectiveness of the combination against mature biofilms, where they found that the combination could reduce the viability by 95% vs. the silver nanoparticles alone which reduced the CFU by 86%. The authors concluded that the promising results could be applied to permit safe concentrations of silver nanoparticles that are below the toxic range. Additionally, the authors suggested that it would be feasible to capitalize on this enhancement with the use of other silver-based products such as silver sulfadiazine or silver based wound dressings.
A follow up study by Hui et al. [95] evaluated whether the uptake effect following STX photolysis may be applied so that they may increase sensitivity of MRSA to conventional antibiotics. Initially, the authors sought to test the hypothesis that photolysis of STX disrupts membrane integrity through an increase in membrane permeability. For example, the authors found that when MRSA was exposed to PBL, the rate and amount of SYTOX green uptake (as a marker for cell membrane permeability) was significantly increased relative to the unirradiated control. The authors then hypothesized that irradiation with PBL would allow for the passive diffusion of small molecule antibiotics that target intracellular components within cells. To test this, the authors used the fluorescently labelled antibiotic gentamicin, where they found that irradiated MRSA took up the antibiotic more efficiently than the unirradiated control. The authors then determined the sizes of molecules that could permeate the functional membrane microdomain (FMM) with the use of fluorescent molecules (fluorescein isothiocyanate dextran [FITC-dextran]) of different molecular weights. For example, they found that FD70 (70 kDa size; 6 nm diameter) could insert into the MRSA cells following 46 J/cm2. Increasing the size to 500 kDa (FD500; 30 nm diameter) did not permit uptake, suggesting to the authors that PBL results in the FMM becoming porous permitting molecules (at the nm level) to enter the cells. The authors subsequently found that PBL also resulted in membrane fluidification which the authors hypothesized would enhance daptomycin insertion. Indeed, they found that in the PBL treated groups there was an increase in daptomycin insertion. The authors next observed how the membrane proteins that are situated near STX (such as PBP2a) were affected by photolysis. They found that a significant proportion of PBP2a became detached from the cell wall, leading the authors to conclude that STX photolysis resulted in both disassembly and detachment of PBP2a from the FMM. Given the significant damage incurred to the cell wall, the authors looked at how they could capitalize on this damage to revive conventional antibiotics. The authors found that PBL resulted in approximately a 5-log10 CFU reduction when 147 J/cm2 were applied. Interestingly, when applied to an STX deficient mutant (ΔCrtM) the effects were negligible, suggesting to the authors that photolysis induced damage to the cell was responsible for the antimicrobial effects. The authors also identified, that 147 J/cm2 would be able to significantly enhance the inhibitory effects of multiple antibiotics. For example, PBL exposure of bacteria reduced the MIC of tetracycline by 16-fold. They then investigated the translatability in a model of MRS A skin infection that matured over 48 h. The authors found that daptomycin alone resulted in an 81% reduction in viability within the wound, while PBL application resulted in a 58% reduction. The combination modestly improved the effects with a 95% reduction in the viability. Hui et al. [95] noted that the wounds of combination treated mice appeared ‘healthier’ with less purulent discharge being observed. Additionally, to assess the safety of laser irradiation the authors looked at the effects on mouse skin (in vivo) and human keratinocytes (in vitro) where they did not observe any associated damages or losses in viability. Based on the promising findings, the authors concluded that phototherapeutic approaches that exploit interactions that are reliant on specific light absorbent chromophores may be warranted.
In a study by our group that was performed by Leanse et al. [80] we exploited the photolytic effects of aBL at 460 nm in order to sensitize MRSA to 405 nm light. The authors found previously that MRSA is comparatively more tolerant to aBL (405 nm) relative to other bacteria. They hypothesized that given the potent antioxidant properties of STX, that this may be responsible for these effects. Therefore, the authors initially tested their hypothesis that STX is responsible for the relative tolerance of MRSA to aBL (405 nm). They initially compared the aBL efficacy against the defined isogenic mutant ΔCrtMthat is deficient in STX production vs. it’s parental wild-type strain (pig1). They found that the ΔCrtM mutant was approximately 1000-fold more sensitive to the effects of aBL relative to the parental wild-type (WT) strain, which suggested to the authors that the presence of STX indeed plays a role in tolerance of MRSA to aBL (405 nm). The authors next wanted to evaluate whether photolysis of STX via 460 nm aBL would ‘sensitize’ MRSA to aBL at 405 nm. It was found that for three different strains of MRSA (USA300, AF0003, and IQ00064), there was a significant improvement in the antimicrobial effects of aBL (405 nm). For example, for MRSA USA300 exposure of 108 J/cm2 of 405 nm light alone, only reduced the log10 CFU by 0.64, however, following a pre-exposure of 180 J/cm2 460 nm aBL (a dose that did not significantly influence the viability) 108 J/cm2 aBL (405 nm) was capable of killing 3.58-log10 CFU, illustrating an approximately 1000-fold potentiation of antimicrobial effects. The authors next wanted to determine whether STX photolysis alone was responsible for this. The authors found that when the ΔCrtM mutant was pre-exposed to 180 J/cm2 460 nm aBL, prior to exposure to 108 J/cm2 405 nm aBL, there were no significant differences when compared to aBL (405 nm) alone. However, with respect to the parental WT strain, pre-exposure to 180 J/cm2 aBL (460 nm) indeed significantly enhanced the effects of 405 nm aBL. The authors additionally found that the photolytic effects of 460 nm aBL were dose-dependent, with higher doses of 460 nm light (90 J/cm2 – 360 J/cm2) being associated with improvements in 405 nm aBL efficacy. The authors next evaluated the combined 460 nm + 405 nm aBL effects on 48 h old biofilms. The authors found following pre-exposure to 180 J/cm2 460 nm aBL, subsequent exposure to 405 nm light resulted in significant antimicrobial effects following 216 J/cm2, and 324 J/cm2, with an approximately 1-log10 CFU potentiation compared with 405 nm alone. To evaluate the translational potential of this combination approach, an early onset MRSA infection model was exposed to the combination of 360 J/cm2 (460 nm aBL) with 342 J/cm2 (405 nm aBL). The authors found a reduction of 1.97-log10 CFU (almost 99% loss of viability) was achieved following the combination (Figure 5). For 405 nm aBL alone, only a 0.28 log10 CFU reduction was achieved, with no loss of viability being observed with 460 nm aBL alone. Impressively, the authors did not find that the radiant exposures required to kill 1.97-log10 CFU of MRSA to be associated with apoptosis on skin cells within live mice. The authors concluded that the combination of 460 nm and 405 nm aBL may be an effective approach against MRSA associated infections, however, they stipulated that further work assessing the effectiveness of the approach against established infections is necessary.
Figure 5.
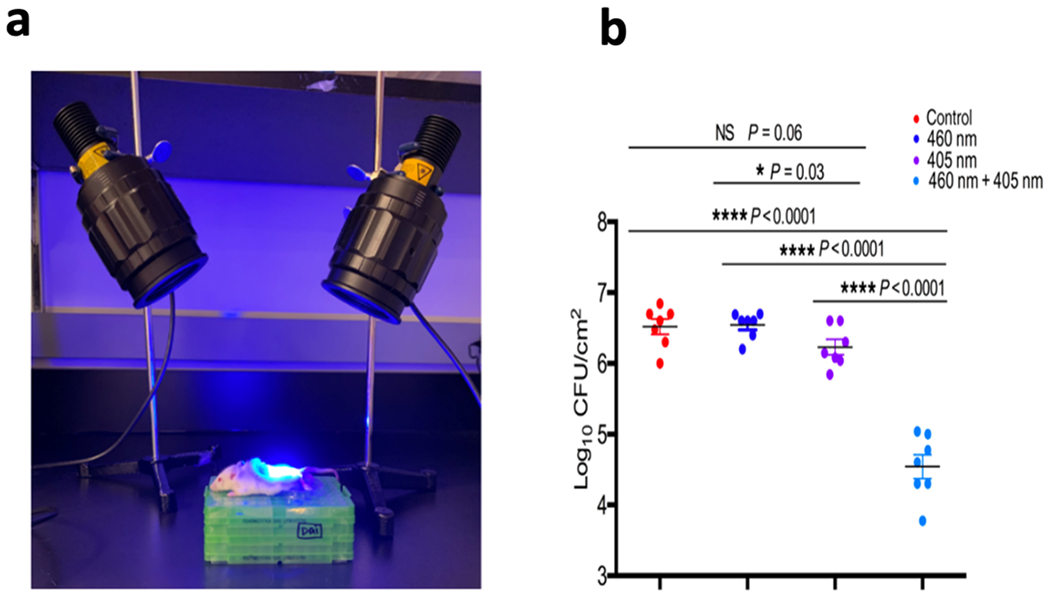
a) Mice image showing treatment of MRSA within an abrasion would in a mouse model using the dual-wavelength (460 nm + 405 nm) approach. b) scatter plot showing the log10 CFU/cm2 (based on the size of wound) following the: control, 460 nm, 405 nm, or combined 460 nm + 405 nm. Statistical analyses were a paired t-test or ANOVA. Error bars: standard error of the mean. Images taken from Leanse et al. [80].
Recently, a study by Xi et al. [96] looked to improve upon the STX photolysis effect first identified by Dong et al. [93] when they used the combination of aBL (460 nm) and Cu-doped carbon spheres (Cu-HCSs). Initially, the authors examined whether the combination of aBL and Cu-HCSs could promote photolysis. They found that while aBL exposure resulted in significant photolysis of STX (59.1% reduction after 3 min [18 J/cm2; 100 mW/cm2]), the combination with Cu-HCSs increased the photolysis to 70.9%. They next looked at the effect on DNA where they found that the integrity of DNA remained stable following aBL or Cu-HCSs alone (even following 30 min of exposure; 100 mW/cm2; 180 J/cm2). However, when they were combined, significant DNA cleavage was achieved. Similar effects were observed with respect to proteins, whereby only the combined treatment resulted in significant cleavage of protein. The authors also found significant enhancement of effects when aBL (360 J/cm2; 60 min; 100 mW/cm2) and Cu-HCSs were combined with a 1.53-log10 CFU reduction being achieved. aBL alone only reduced the CFU by 0.86-log10 CFU. The author clarified that while aBL alone induced an ‘injured state’ similarly to what was found by Dong et al. [93] the concurrent application of aBL and Cu-HCSs resulted in death. Additionally, the authors found that the combination significantly promoted both the production of ROS and the permeability of MRSA relative to the monotreatment controls. The authors next wanted to validate this combination within a mouse model of MRSA infection, in vivo. Following 7 days of combination treatment the mice did not show any significant inflammation or abscesses, that were still evident in all other control groups. Additionally, in the light alone treated group, they achieved approximately a 2-log10 CFU/mL reduction, relative to the untreated control. The Cu-HCSs did not influence viability. However, when combined, the log10 CFU reduction was approximately 4, demonstrating a significant enhancement in the antimicrobial effects. The authors next evaluated the combination against mice that were induced with an MRSA bacteremia. They illuminated the mice for a total of 4 h per day and monitored for 6 days. They found that treated mice had less severe symptoms relative to the control mice. The authors found that the combined treated mice had a significantly lower burden of MRSA (by up to 2 orders of magnitude) within the organs (heart, liver, lung and kidney) relative to each monotreatment. The authors concluded that the combination of aBL and Cu-HCSs may represent a novel method of treating MRSA associated infections.
In line with the above-mentioned studies, Baek et al. [97] investigated the photolysis effect of aBL (466 nm) as a means of promoting the bactericidal activity of plasma activated water (PAW) against S. aureus on stainless steel surfaces. As with the study by Dong et al. [93] they found that exposure to aBL resulted in visible photobleaching of the STX pigment. The authors then found that aBL did not elicit any antimicrobial effects (150 J/cm2; 18.74 mW/cm2; 133 min exposure) and a 1.07-log10 CFU reduction was achieved following 10 min of PAW exposure. When combined with aBL, the total reduction increased to 2.70-log10 CFU. The authors also observed that the combination of PAW and aBL resulted in increases in the leakage of nucleic acids and protein, as well as increases in permeability of the cell wall. The authors concluded that the combination of PAW and aBL were synergistic against S. aureus present on stainless steel.
In a novel study by Jusuf et al. [98] illustrated the potential of granadaene photobleaching was investigated as a method of reducing the virulence of S. agalactiae (NCTC 10/84 strain) and enhancing the effectiveness of conventional antibiotics. The authors hypothesized that given the similarities of granadaene and the carotenoid pigments (i.e. STX) it may be possible to exploit photolysis in S. agalactiae. The authors initially investigated the photobleaching effects of PBL (at different wavelengths: 430 nm – 490 nm) where they found that 60 J/cm2 of 430 nm PBL could photobleach the pigment completely. The authors next evaluated the effect of granadaene photobleaching on its haemolytic activity, given that it has been implicated to be a β-haemolysin/cytolysin. The bacterium was initially cultured on TSA plates to permit the production of granadaene. The bacteria were then exposed to 120 J/cm2, and it was found that photobleaching reduced the haemolytic activity of granadaene by 40.5%. Next, the authors wanted to evaluate whether granadaene photobleaching resulted in sensitization to ROS. They found that the combination 60 J/cm2 of aBL (430 nm) and 12 mM H2O2 was sufficient to completely eradicate S. agalactiae H2O2 alone at 12 mM alone which only reduced the viability by around 1-log10 CFU. With aBL alone (at equivalent doses) did not significantly influence viability. The authors subsequently evaluated the potential sensitization of S. agalactiae to the antibiotic daptomycin. Initially, they found that photobleaching does increase the permeability of S. agalactiae by a maximum of 44.2% (following 60 min of exposure; 100 mW/cm2; 360 J/cm2). It was also found that exposure to 90 J/cm2 aBL could reduce the MIC of daptomycin by 2-fold, compared to daptomycin alone, after a 4 h incubation in TSB solution. Additionally, the authors found there to be an increase in the uptake of daptomycin. Interestingly, when the authors evaluated the effectiveness against other antibiotics (penicillin/ampicillin) they did not observe the same enhancement, which was attributed to the localization of the penicillin binding proteins that are found on the cell membrane. The authors suggested that permeabilization of these antibiotics in S. agalactiae may be less of an issue, than with daptomycin which works by altering the membrane curvature, membrane potential, protein synthesis and inducing ion leaks. The authors concluded that given the reduced haemolysis observed coupled with increases in antimicrobial susceptibility, that graenadene photobleaching may be a potential approach to improve upon the current methods of treatment.
7. Does aBL have a ‘bright’ future?
Since 2017, an extensive evaluation with respect to the efficacy and potential clinical applicability of aBL has been performed. Researchers have broadly evaluated aBL, not only as an antimicrobial, but also as an adjunct therapeutic for potentiated treatment of infectious diseases in vitro and in vivo. There have also been more robust investigations into the potential safety of aBL against host cells and tissues in vivo under numerous paradigms. Additionally, numerous studies have been focused to better understand the endogenous chromophores that facilitate the action of aBL [46,47,58,60]. It has been long hypothesized that aBL requires porphyrins to elicit its effects. There have been studies, however, that have put this requirement into question, and have suggested that flavins or flavin photoproducts (e.g. lumichrome) are sufficient for aBL to elicit phototoxic effects [58], suggesting that the spectra of microorganisms that may be susceptible to aBL may be wider than previously anticipated.
Numerous questions that were previously left unanswered have now been addressed. Importantly, most researchers define aBL as being in the region of 400 - 470 nm, however, it is clear from the numerous studies that were published since 2017, that shorter wavelength aBL (400-410 nm) possess more potent microbicidal properties, when compared to longer wavelength aBL. Most investigators have exploited peak emission wavelengths in the region of 405 nm, 450 nm, and 470 nm. The typical consensus amongst the studies was that 405 nm appeared to be most effective at killing microorganisms [21,42,44]. However, there are several conflicting findings identified amongst different studies that found that aBL at longer wavelengths ranging from 450 nm - 470 nm, effectively inhibit or kill bacteria, where others have not observed particularly potent effects. In addition, because of the potent antimicrobial effects of aBL, numerous researchers have investigated whether other wavelengths that exist within the visible light spectrum may also elicit antimicrobial effects. However, there is a universal agreement in the literature that longer wavelength light existing within the green or red-light spectrum, does not elicit antimicrobial effects. The studies thus implicate 400 nm – 470 nm light as the only wavelengths within the visible light spectrum to truly harbor intrinsic antimicrobial properties.
An important advance since 2017 is based on the serendipitous discovery that aBL can destroy various chromophores, such as STX and graenadene [76,78–80,82], within microbial organisms to render them susceptible to ROS, attenuated for virulence, and facilitate the activity of conventional antimicrobials. Additionally, numerous studies have demonstrated the potential for other compounds to significantly potentiate the effectiveness of aBL both in vivo and in vitro, representing a significant step forward in the potential clinical translatability. For example, it has been documented that fungal organisms (molds in particular) are intrinsically tolerant to aBL mediated killing [99]. In fact, it has been suggested by previous researchers that the high radiant exposures necessary to kill molds, may render them insensitive to treatment in an in vivo paradigm. In our recent studies, we found that the addition of Q-HCL significantly potentiated the effectiveness of aBL against Gram-negative bacteria and C. albicans, both in vitro and in vivo [89,90]. This permitted us to apply lower doses of each respective antimicrobial to elicit highly efficacious microbial killing. More recently, we were able to apply this combination against molds in vivo, which represented the first instance when a cutaneous mold infection has ever been shown to be effectively treated with any light-based approach [90,91].
More recently, in response to the current COVID-19 pandemic, there have been a couple of studies that have provided some evidence that aBL may offer an approach to tackle infections caused by SARS-CoV-2[56,57]. Although further work is required before clinical validation, it is a promising endeavor that merits further consideration. Additionally, given the COVID-19 complications that have been arising in numerous patients who have been placed on ventilators to combat acute respiratory distress syndrome (ARDS), the risk of ventilator associated pneumonia (VAP) is a significant concern. Thus, the studies that have investigated the potential for aBL to prevent VAP are as timely as they are important, and given the findings thus far, there appears to be potential in this method [52].
With the majority of studies that have been published since 2017, there is a general agreement amongst the data that aBL is far less toxic to mammalian host cells when compared to microbial pathogens [31,48,82,50,94]. There do appear to be differences in terms of light radiant exposure, with some studies observing an aBL toxicity threshold of 144 J/cm2 against host cells in vitro [81] and others illustrating that at 216 J/cm2 no harmful side effects are observed [31,48]. However, unsurprisingly all studies have demonstrated that very high doses (e.g. 500 J/cm2) are associated with toxicity against host cells in vitro. It is important to appreciate, however, the differences observed in vitro and in vivo. Past studies have shown that radiant exposures as high as 540 J/cm2 are not associated with host cell toxicity in vivo [100]. Additionally, in our study that combined 2 wavelengths of aBL (405 nm and 460 nm) high radiant exposures (>700 J/cm2 combined) did not result in cellular apoptosis. These data suggest that findings in vitro may not necessarily reflect a clinical situation. A Phase I clinical study demonstrated the intrinsic safety that is associated with aBL (401 nm) against human vaginal mucosa [84], which may increase the confidence that aBL may be safely applied. Of course, this trial only included 10 participants, all of which were healthy, and so it is important to remain cognizant with regards to light dosimetry and human pathology, and thoroughly assess safety in humans before general use.
Importantly, over the past 4 years there have been more studies that have been evaluating resistance development to aBL. In our group, we found that 20 generations of exposure in vitro, and 5 in vivo, did not result in resistance generation. This was in concordance with other investigations [101,102]. However, recently, there have been researchers who have suggested that stable ‘tolerance’ to aBL is a possibility. The authors stipulated that this was ‘tolerance’ and not ‘resistance’ as the bacteria could be killed with higher radiant exposures of aBL. Although, the same phenomenon is observed with antibiotics, where ‘resistance’ occurs when bacteria are susceptible to a concentration is outside the therapeutic window, which as yet, is unknown for aBL.
It is a common mistake to view light-based approaches as restricted to surface treatment, only capable of treating superficial infections. While aBL suffers from an important drawback, in that it can only penetrate tissue modestly (approximately 400 μm) and thus been shown to be effective at treating wound infections; it is important to emphasize that with the use of optical waveguides, aBL could be used to deliver light deep into tissues [103]. Therefore, presently our group is working to develop multiple waveguides capable of delivering light to deeper regions of tissue. Previously, we developed a microneedle array capable of interstitial delivery within tissue, which is in the process of being applied for the treatment of deeper infections [104]. Additionally, we are actively working to exploit aBL for the treatment of deeper localized infections such as urinary tract infections and genital tract infections.
8. Conclusions
In conclusion, it is evident from the literature that aBL possesses potent antimicrobial properties against a vast spectrum of microorganisms. It has the benefit that it is more harmful to bacteria than to host cells, which would fit Ehrlich’s criteria of ‘magic bullet’. Its compatibility with other traditional and non-traditional antimicrobial approaches further suggests its potential utility as an antimicrobial therapeutic. It is clear that aBL indeed has a ‘bright’ future, however, while aBL is safe and effective for the treatment of infections, the therapeutic window still requires further quantification as well as validation in clinical trials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Schwartz RS, Paul Ehrlich’s Magic Bullets, N. Engl. J. Med 350 (2004) 1079–1080. doi: 10.1056/NEJMp048021. [DOI] [PubMed] [Google Scholar]
- [2].Gelpi A, Gilbertson A, Tucker JD, Magic bullet: Paul Ehrlich, Salvarsan and the birth of venereology, Sex. Transm. Infect 91 (2015) 68–69. doi: 10.1136/sextrans-2014-051779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ehrlich P, Address in Pathology ON CHEMIOTHERAPY: Delivered before the Seventeenth International Congress of Medicine, BMJ. 2 (1913) 353–359. doi: 10.1136/bmj.2.2746.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Raju TN, The Nobel Chronicles, Lancet. 353 (1999) 936. doi: 10.1016/S0140-6736(05)75055-8. [DOI] [PubMed] [Google Scholar]
- [5].Ligon BL, Penicillin: Its Discovery and Early Development, Semin. Pediatr. Infect. Dis 15 (2004) 52–57. doi: 10.1053/j.spid.2004.02.001. [DOI] [PubMed] [Google Scholar]
- [6].Kress GH, Francisco S, Sir Alexander Fleming-Discoverer of Penicillin., Cal. West. Med 63 (1945) 153. http://www.ncbi.nlm.nih.gov/pubmed/18747134. [PMC free article] [PubMed] [Google Scholar]
- [7].Fleming A, On the antibacterial action of cultures of a Penicillium, with special reference to their use in the isolation of B. influenza, Br. J. Exp. Pathol 10 (1929) 226–236. doi: 10.1093/clinids/2.1.129. [DOI] [Google Scholar]
- [8].Aminov RI, A brief history of the antibiotic era: Lessons learned and challenges for the future, Front. Microbiol 1 (2010) 1–7. doi: 10.3389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ventola CL, The antibiotic resistance crisis: part 1: causes and threats., P T. 40 (2015) 277–83. [PMC free article] [PubMed] [Google Scholar]
- [10].Rolain JM, Baquero F, The refusal of the Society to accept antibiotic toxicity: Missing opportunities for therapy of severe infections, Clin. Microbiol. Infect 22 (2016) 423–427. doi: 10.1016/j.cmi.2016.03.026. [DOI] [PubMed] [Google Scholar]
- [11].Llor C, Bjerrum L, Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem, Ther. Adv. Drug Saf 5 (2014) 229–241. doi: 10.1177/2042098614554919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rosenblatt-Farrell N, The Landscape of Antibiotic Resistance, Environ. Health Perspect 117 (2009) 30–36. doi: 10.1289/ehp.117-a244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].C Reygaert W, An overview of the antimicrobial resistance mechanisms of bacteria, AIMS Microbiol. 4 (2018) 482–501. doi: 10.3934/microbiol.2018.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stapleton PD, Taylor PW, Methicillin Resistance in Staphylococcus aureus: Mechanisms and Modulation, Sci. Prog 85 (2002) 57–72. doi: 10.3184/003685002783238870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Watkins RR, Holubar M, David MZ, Antimicrobial Resistance in Methicillin-Resistant Staphylococcus aureus to Newer Antimicrobial Agents, Antimicrob. Agents Chemother 63 (2019) 1–14. doi: 10.1128/AAC.01216-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang Y, Wang Y, Wang Y, Murray CK, Hamblin MR, Hooper DC, Dai T, Antimicrobial blue light inactivation of pathogenic microbes: State of the art, Drug Resist. Updat 33–35 (2017) 1–22. doi: 10.1016/j.drup.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Al-Shammary AAK, Mohd Ma’amor NAA, Chen SQ, Lee KS, Mohd Hanafiah K, Bactericidal effects of in vitro 405 nm, 530 nm and 650 nm laser irradiation on methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa and Mycobacterium fortuitum, Lasers Dent. Sci 4 (2020) 111–121. doi: 10.1007/s41547-020-00097-5. [DOI] [Google Scholar]
- [18].Angarano V, Akkermans S, Smet C, Chieffi A, Van Impe JFM, The potential of violet, blue, green and red light for the inactivation of P. fluorescens as planktonic cells, individual cells on a surface and biofilms, Food Bioprod. Process 124 (2020) 184–195. doi: 10.1016/j.fbp.2020.07.019. [DOI] [Google Scholar]
- [19].Angarano V, Smet C, Akkermans S, Watt C, Chieffi A, Van Impe JFM, Visible Light as an Antimicrobial Strategy for Inactivation of Pseudomonas fluorescens and Staphylococcus epidermidis Biofilms, Antibiotics. 9 (2020) 171. doi: 10.3390/antibiotics9040171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Galo IDC, Prado RP, Dos Santos WG, Blue and red light photoemitters as approach to inhibit Staphylococcus aureus and Pseudomonas aeruginosa growth, Brazilian J. Biol 82 (2022) 1–8. doi: 10.1590/1519-6984.231742. [DOI] [PubMed] [Google Scholar]
- [21].Schmid J, Hoenes K, Vatter P, Hessling M, Antimicrobial effect of visible light—photoinactivation of Legionella rubrilucens by irradiation at 450, 470, and 620 nm, Antibiotics. 8 (2019). doi: 10.3390/antibiotics8040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hoenes K, Hess M, Vatter P, Spellerberg B, Hessling M, 405 nm and 450 nm photoinactivation of Saccharomyces cerevisiae, Eur. J. Microbiol. Immunol 8 (2018) 142–148. doi: 10.1556/1886.2018.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sabino CP, Wainwright M, dos Anjos C, Sellera FP, Baptista MS, Lincopan N, Ribeiro MS, Inactivation kinetics and lethal dose analysis of antimicrobial blue light and photodynamic therapy, Photodiagnosis Photodyn. Ther 28 (2019). doi: 10.1016/j.pdpdt.2019.08.022. [DOI] [PubMed] [Google Scholar]
- [24].Bache SE, Maclean M, Gettinby G, Anderson JG, MacGregor SJ, Taggart I, Universal decontamination of hospital surfaces in an occupied inpatient room with a continuous 405 nm light source, J. Hosp. Infect 98 (2018) 67–73. doi: 10.1016/j.jhin.2017.07.010. [DOI] [PubMed] [Google Scholar]
- [25].dos Anjos C, Sellera FP, Gargano RG, Lincopan N, Pogliani FC, Ribeiro MG, Jagielski T, Sabino CP, Algicidal effect of blue light on pathogenic Prototheca species, Photodiagnosis Photodyn. Ther. 26 (2019) 210–213. doi: 10.1016/j.pdpdt.2019.04.009. [DOI] [PubMed] [Google Scholar]
- [26].dos Anjos C, Sabino CP, Sellera FP, Esposito F, Pogliani FC, Lincopan N, Hypervirulent and hypermucoviscous strains of Klebsiella pneumoniae challenged by antimicrobial strategies using visible light, Int. J. Antimicrob. Agents 56 (2020) 106025. doi: 10.1016/j.ijantimicag.2020.106025. [DOI] [PubMed] [Google Scholar]
- [27].dos Anjos C, Sellera FP, de Freitas LM, Gargano RG, Telles EO, Freitas RO, Baptista MS, Ribeiro MS, Lincopan N, Pogliani FC, Sabino CP, Inactivation of milk-borne pathogens by blue light exposure, J. Dairy Sci 103 (2020) 1261–1268. doi: 10.3168/jds.2019-16758. [DOI] [PubMed] [Google Scholar]
- [28].Keyvan E, Kahraman HA, Tunun H, Donmez S, Sen E, Demirtas A, Akyuz AO, Inactivation efficacy of 405 nm light emitting diodes (LEDs) on Salmonella Enteritidis at different illumination temperatures, Food Sci. Technol 2061 (2021). doi: 10.1590/fst.08721. [DOI] [PubMed] [Google Scholar]
- [29].dos Anjos C, Sabino CP, Bueris V, Fernandes MR, Pogliani FC, Lincopan N, Sellera FP, Antimicrobial blue light inactivation of international clones of multidrug-resistant Escherichia coli ST10, ST131 and ST648, Photodiagnosis Photodyn. Ther 27 (2019) 51–53. doi: 10.1016/j.pdpdt.2019.05.014. [DOI] [PubMed] [Google Scholar]
- [30].Costerton JW, Stewart PS, Greenberg EP, Bacterial biofilms: A common cause of persistent infections, Science (80-. ). 284 (1999) 1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- [31].Ferrer-Espada R, Liu X, Goh XS, Dai T, Antimicrobial blue light inactivation of polymicrobial biofilms, Front. Microbiol 10 (2019) 1–10. doi: 10.3389/fmicb.2019.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ferrer-Espada R, Wang Y, Goh XS, Dai T, Antimicrobial Blue Light Inactivation of Microbial Isolates in Biofilms, Lasers Surg. Med (2019) 1–7. doi: 10.1002/lsm.23159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Halstead FD, Ahmed Z, Bishop JRB, Oppenheim BA, The potential of visible blue light (405 nm) as a novel decontamination strategy for carbapenemase-producing enterobacteriaceae (CPE), Antimicrob. Resist. Infect. Control 8 (2019) 1–8. doi: 10.1186/s13756-019-0470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Halstead FD, Hadis MA, Marley N, Brock K, Milward MR, Cooper PR, Oppenheim B, Palinb WM, Violet-blue light arrays at 405 nanometers exert enhanced antimicrobial activity for photodisinfection of monomicrobial nosocomial biofilms, Appl. Environ. Microbiol 85 (2019) 1–16. doi: 10.1128/AEM.01346-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Martegani E, Bolognese F, Trivellin N, Orlandi VT, Effect of blue light at 410 and 455 nm on Pseudomonas aeruginosa biofilm, J. Photochem. Photobiol. B Biol 204 (2020) 111790. doi: 10.1016/j.jphotobiol.2020.111790. [DOI] [PubMed] [Google Scholar]
- [36].Rupel K, Zupin L, Ottaviani G, Bertani I, Martinelli V, Porrelli D, Vodret S, Vuerich R, Passos da Silva D, Bussani R, Crovella S, Parsek M, Venturi V, Di Lenarda R, Biasotto M, Zacchigna S, Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress, Npj Biofilms Microbiomes. 5 (2019) 1–11. doi: 10.1038/s41522-019-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lu M, Dai T, Hu S, Zhang Q, Bhayana B, Wang L, Wu MX, Antimicrobial blue light for decontamination of platelets during storage, J. Biophotonics 13 (2020) 1–19. doi: 10.1002/jbio.201960021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Maclean M, Gelderman MP, Kulkarni S, Tomb RM, Stewart CF, Anderson JG, MacGregor SJ, Atreya CD, Non-ionizing 405 nm Light as a Potential Bactericidal Technology for Platelet Safety: Evaluation of in vitro Bacterial Inactivation and in vivo Platelet Recovery in Severe Combined Immunodeficient Mice, Front. Med 6 (2020) 1–8. doi: 10.3389/fmed.2019.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jankowska KI, Nagarkatti R, Acharyya N, Dahiya N, Stewart CF, Macpherson RW, Wilson MP, Anderson JG, MacGregor SJ, Maclean M, Dey N, Debrabant A, Atreya CD, Complete Inactivation of Blood Borne Pathogen Trypanosoma cruzi in Stored Human Platelet Concentrates and Plasma Treated With 405 nm Violet-Blue Light, Front. Med 7 (2020) 1–11. doi: 10.3389/fmed.2020.617373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Al Hamzi MA, Al Jorany AAA, Al-Shamahy HA, Al Sharani AA, Phototoxic Effect of Visible Blue Light on Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans, Online J. Dent. Oral Heal 2 (2019) 2–5. doi: 10.33552/OJDOH.2019.02.000540. [DOI] [Google Scholar]
- [41].Felix Gomez GG, Lippert F, Ando M, Zandona AF, Eckert GJ, Gregory RL, Photoinhibition of Streptococcus mutans Biofilm-Induced Lesions in Human Dentin by Violet-Blue Light, Dent. J 7 (2019) 113. doi: 10.3390/dj7040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mohamad SA, Milward MR, Kuehne SA, Hadis MA, Palin WM, Cooper PR, Potential for direct application of blue light for photo-disinfection of dentine, J. Photochem. Photobiol. B Biol 215 (2021) 112123. doi: 10.1016/j.jphotobiol.2021.112123. [DOI] [PubMed] [Google Scholar]
- [43].Vaknin M, Steinberg D, Featherstone JD, Feuerstein O, Exposure of Streptococcus mutans and Streptococcus sanguinis to blue light in an oral biofilm model, Lasers Med. Sci 35 (2020) 709–718. doi: 10.1007/s10103-019-02903-4. [DOI] [PubMed] [Google Scholar]
- [44].Tsutsumi-Arai C, Arai Y, Terada-Ito C, Imamura T, Tatehara S, Ide S, Wakabayashi N, Satomura K, Microbicidal effect of 405-nm blue LED light on Candida albicans and Streptococcus mutans dual-species biofilms on denture base resin, Lasers Med. Sci (2021). doi: 10.1007/s10103-021-03323-z. [DOI] [PubMed] [Google Scholar]
- [45].Zhang L, Li Y, Zhang Q, Du N, Li X, Zhang Q, Yuan L, Dong F, Jiang Y, Tang J, Wang Y, Antimicrobial Activity of an Implantable Wireless Blue Light-Emitting Diode against Root Canal Biofilm in Vitro, Photobiomodulation, Photomedicine, Laser Surg. 38 (2020) 694–702. doi: 10.1089/photob.2020.4821. [DOI] [PubMed] [Google Scholar]
- [46].Bumah VV, Masson-Meyers DS, Tong W, Castel C, Enwemeka CS, Optimizing the bactericidal effect of pulsed blue light on Propionibacterium acnes - A correlative fluorescence spectroscopy study, J. Photochem. Photobiol. B Biol 202 (2020) 111701. doi: 10.1016/j.jphotobiol.2019.111701. [DOI] [PubMed] [Google Scholar]
- [47].Bumah VV, Masson-Meyers DS, Enwemeka CS, Pulsed 450 nm blue light suppresses MRSA and Propionibacterium acnes in planktonic cultures and bacterial biofilms, J. Photochem. Photobiol. B Biol 202 (2020) 111702. doi: 10.1016/j.jphotobiol.2019.111702. [DOI] [PubMed] [Google Scholar]
- [48].Liu X, Chang Q, Ferrer-Espada R, Leanse LG, Goh XS, Wang X, Gelfand JA, Dai T, Photoinactivation of Moraxella catarrhalis Using 405-nm Blue Light: Implications for the Treatment of Otitis Media, Photochem. Photobiol 96 (2020) 611–617. doi: 10.1111/php.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang Y, Ferrer-Espada R, Gu Y, Dai T, Antimicrobial blue light: an alternative therapeutic for multidrug-resistant gonococcal infections?, MOJ Sol. Photoenergy Syst 1 (2017) 38–39. doi: 10.15406/mojsp.2017.01.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wang Y, Ferrer-Espada R, Baglo Y, Goh XS, Held KD, Grad YH, Gu Y, Gelfand JA, Dai T, Photoinactivation of Neisseria gonorrhoeae: a paradigm-changing approach for combating antibiotic-resistant gonococcal infection, J. Infect. Dis 220 (2019) 873–881. doi: 10.1093/infdis/jiz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Meurer L, Payne W, Guffey JS, Visible Light as an Inhibitor of Camplyobacter jejuni, Int. J. Antimicrob. Agents 55 (2020) 105818. doi: 10.1016/j.ijantimicag.2019.09.022. [DOI] [PubMed] [Google Scholar]
- [52].Meurle T, Knaus J, Barbano A, Hoenes K, Spellerberg B, Hessling M, Photoinactivation of Staphylococci with 405 nm Light in a Trachea Model with Saliva Substitute at 37 °C, Healthcare. 9 (2021) 310. doi: 10.3390/healthcare9030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sicks B, Hönes K, Spellerberg B, Hessling M, Blue LEDs in endotracheal tubes may prevent ventilator-associated pneumonia, Photobiomodulation, Photomedicine, Laser Surg. 38 (2020) 571–576. doi: 10.1089/photob.2020.4842. [DOI] [Google Scholar]
- [54].Makdoumi K, Hedin M, Bäckman A, Different photodynamic effects of blue light with and without riboflavin on methicillin-resistant Staphylococcus aureus (MRSA) and human keratinocytes in vitro, Lasers Med. Sci. 34 (2019) 1799–1805. doi: 10.1007/s10103-019-02774-9. [DOI] [PubMed] [Google Scholar]
- [55].Zhao Y, Zhang Y, Dong J, Evaluating the efficacy of anti-fungal blue light therapies via analyzing tissue section images, Lasers Med. Sci (2021). doi: 10.1007/s10103-021-03319-9. [DOI] [PubMed] [Google Scholar]
- [56].Zupin L, Gratton R, Fontana F, Clemente L, Pascolo L, Ruscio M, Crovella S, Blue photobiomodulation LED therapy impacts SARS-Cov −2 by limiting its replication in Vero cells, J. Biophotonics (2021) e202000496. doi: 10.1002/jbio.202000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vatter P, Hoenes K, Hessling M, Photoinactivation of the Coronavirus surrogate phi6 by visible light, Photochem. Photobiol 97 (2021) 122–125. doi: 10.1111/php.13352. [DOI] [PubMed] [Google Scholar]
- [58].Hessling M, Wenzel U, Meurle T, Spellerberg B, Hönes K, Photoinactivation results of Enterococcus moraviensis with blue and violet light suggest the involvement of an unconsidered photosensitizer, Biochem. Biophys. Res. Commun 533 (2020) 813–817. doi: 10.1016/j.bbrc.2020.09.091. [DOI] [PubMed] [Google Scholar]
- [59].Hoenes K, Wenzel U, Spellerberg B, Hessling M, Photoinactivation Sensitivity of Staphylococcus carnosus to Visible-light Irradiation as a Function of Wavelength, Photochem. Photobiol 96 (2020) 156–169. doi: 10.1111/php.13168. [DOI] [PubMed] [Google Scholar]
- [60].Plavskii VY, Mikulich AV, Tretyakova AI, Leusenka IA, Plavskaya LG, Kazyuchits OA, I.I. Dobysh, T.P. Krasnenkova, Porphyrins and flavins as endogenous acceptors of optical radiation of blue spectral region determining photoinactivation of microbial cells, J. Photochem. Photobiol. B Biol 183 (2018) 172–183. doi: 10.1016/j.jphotobiol.2018.04.021. [DOI] [PubMed] [Google Scholar]
- [61].Bowman C, Bumah VV, Niesman IR, Cortez P, Enwemeka CS, Structural membrane changes induced by pulsed blue light on methicillin-resistant Staphylococcus aureus (MRSA), J. Photochem. Photobiol. B Biol (2021) 112150. doi: 10.1016/j.jphotobiol.2021.112150. [DOI] [PubMed] [Google Scholar]
- [62].Hoenes K, Bauer R, Spellerberg B, Hessling M, Microbial photoinactivation by visible light results in limited loss of membrane integrity, Antibiotics. 10 (2021) 341. doi: 10.3390/antibiotics10030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hyun JE, Moon SK, Lee SY, Antibacterial activity and mechanism of 460–470 nm light-emitting diodes against pathogenic bacteria and spoilage bacteria at different temperatures, Food Control. 123 (2021) 107721. doi: 10.1016/j.foodcont.2020.107721. [DOI] [Google Scholar]
- [64].El Najjar N, Van Teeseling MCF, Mayer B, Hermann S, Thanbichler M, Graumann PL, Bacterial cell growth is arrested by violet and blue, but not yellow light excitation during fluorescence microscopy, BMC Mol. Cell Biol 21 (2020) 35. doi: 10.1186/s12860-020-00277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Galo IDC, De Lima BE, Santos TG, Braoios A, Prado RP, Dos Santos WG, Staphylococcus aureus growth delay after exposure to low fluencies of blue light (470 nm), Brazilian J. Biol 6984 (2020) 1–7. doi: 10.1590/1519-6984.226473. [DOI] [PubMed] [Google Scholar]
- [66].Ahmed E, El-Gendy AO, Hamblin MR, Mohamed T, The effect of femtosecond laser irradiation on the growth kinetics of Staphylococcus aureus: An in vitro study, J. Photochem. Photobiol. B Biol 221 (2021) 112240. doi: 10.1016/j.jphotobiol.2021.112240. [DOI] [PubMed] [Google Scholar]
- [67].Chu Z, Hu X, Wang X, Wu J, Dai T, Wang X, Inactivation of Cronobacter sakazakii by blue light illumination and the resulting oxidative damage to fatty acids, Can. J. Microbiol 65 (2019) 922–929. doi: 10.1139/cjm-2019-0054. [DOI] [PubMed] [Google Scholar]
- [68].Wu J, Chu Z, Ruan Z, Wang X, Dai T, Hu X, Changes of Intracellular Porphyrin, Reactive Oxygen Species, and Fatty Acids Profiles During Inactivation of Methicillin-Resistant Staphylococcus aureus by Antimicrobial Blue Light, Front. Physiol 9 (2018) 1–9 doi: 10.3389/fphys.2018.01658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fila G, Krychowiak M, Rychlowski M, Bielawski KP, Grinholc M, Antimicrobial blue light photoinactivation of Pseudomonas aeruginosa: Quorum sensing signaling molecules, biofilm formation and pathogenicity, J. Biophotonics 11 (2018) 1–12. doi: 10.1002/jbio.201800079. [DOI] [PubMed] [Google Scholar]
- [70].Shany-Kdoshim S, Polak D, Houri-Haddad Y, Feuerstein O, Killing mechanism of bacteria within multi-species biofilm by blue light, J. Oral Microbiol 11 (2019). doi: 10.1080/20002297.2019.1628577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tardu M, Bulut S, Kavakli IH, MerR and ChrR mediate blue light induced photo-oxidative stress response at the transcriptional level in Vibrio cholerae, Sci. Rep 7 (2017) 1–15. doi: 10.1038/srep40817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Dong J, Wang T, Data driven modeling of the reactive oxygen species stimulated by photon energy in light therapies, IEEE Access. 8 (2020) 18196–18206. doi: 10.1109/ACCESS.2020.2968352. [DOI] [Google Scholar]
- [73].Marasini S, Leanse LG, Dai T, Can microorganisms develop resistance against light based anti-infective agents?, Adv. Drug Deliv. Rev 175 (2021) 113822. doi: 10.1016/j.addr.2021.05.032. [DOI] [PubMed] [Google Scholar]
- [74].Tomb RM, Maclean M, Coia JE, MacGregor SJ, Anderson JG, Assessment of the potential for resistance to antimicrobial violet-blue light in Staphylococcus aureus, Antimicrob. Resist. Infect. Control 6 (2017) 100. doi: 10.1186/s13756-017-0261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Leanse LG, Harrington OD, Fang Y, Ahmed I, Goh XS, Dai T, Evaluating the Potential for Resistance Development to Antimicrobial Blue Light (at 405 nm) in Gram-Negative Bacteria: In vitro and in vivo Studies, Front. Microbiol 9 (2018) 1–7. doi: 10.3389/fmicb.2018.02403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Rapacka-Zdonczyk A, Wozniak A, Pieranski M, Woziwodzka A, Bielawski KP, Grinholc M, Development of Staphylococcus aureus tolerance to antimicrobial photodynamic inactivation and antimicrobial blue light upon sub-lethal treatment, Sci. Rep 9 (2019) 9423. doi: 10.1038/s41598-019-45962-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Rapacka-Zdonczyk A, Wozniak A, Nakonieczna J, Grinholc M, Development of Antimicrobial Phototreatment Tolerance: Why the Methodology Matters, Int. J. Mol. Sci 22 (2021) 2224. doi: 10.3390/ijms22042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Plattfaut I, Demir E, Fuchs PC, Schiefer JL, Stürmer EK, Brüning AKE, Opländer C, Characterization of Blue Light Treatment for Infected Wounds: Antibacterial Efficacy of 420, 455, and 480 nm Light-Emitting Diode Arrays against Common Skin Pathogens Versus Blue Light-Induced Skin Cell Toxicity, Photobiomodulation, Photomedicine, Laser Surg. 39 (2021) 339–348. doi: 10.1089/photob.2020.4932. [DOI] [PubMed] [Google Scholar]
- [79].Wang T, Dong J, Yin H, Zhang G, Blue light therapy to treat Candida vaginitis with comparisons of three wavelengths: an in vitro study, Lasers Med. Sci (2020). doi: 10.1007/s10103-019-02928-9. [DOI] [PubMed] [Google Scholar]
- [80].Leanse LG, Goh XS, Cheng J-X, Hooper DC, Dai T, Dual-wavelength photo-killing of methicillin-resistant Staphylococcus aureus, JCI Insight. 5 (2020). doi: 10.1172/jci.insight.134343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Shehatou C, Logunov SL, Dunman PM, Haidaris CG, Klubben WS, Characterizing the Antimicrobial Properties of 405 nm Light and the Coming® Light-Diffusing Fiber Delivery System, Lasers Surg. Med 51 (2019) 887–896. doi: 10.1002/lsm.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bumah VV, Masson-Meyers DS, Awosika O, Zacharias S, Enwemeka CS, The viability of human cells irradiated with 470-nm light at various radiant energies in vitro, Lasers Med. Sci (2021). doi: 10.1007/s10103-021-03250-z. [DOI] [PubMed] [Google Scholar]
- [83].Thurman CE, Muthuswamy A, Klinger MM, Roble GS, Safety Evaluation of a 405-nm LED Device for Direct Antimicrobial Treatment of the Murine Brain, Comp. Med 69 (2019) 283–290. doi: 10.30802/aalas-cm-18-000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Pavie MC, Robatto M, Bastos M, Tozetto S, Boas AV, Vitale SG, Lordelo P, Blue light-emitting diode in healthy vaginal mucosa—a new therapeutic possibility, Lasers Med. Sci 34 (2019) 921–927. doi: 10.1007/s10103-018-2678-3. [DOI] [PubMed] [Google Scholar]
- [85].Bonnans M, Fouque L, Pelletier M, Chabert R, Pinacolo S, Restellini L, Cucumel K, Blue light: Friend or foe ?, J. Photochem. Photobiol. B Biol 212 (2020) 0–7. doi: 10.1016/j.jphotobiol.2020.112026. [DOI] [PubMed] [Google Scholar]
- [86].Wozniak A, Rapacka-Zdonczyk A, Mutters NT, Grinholc M, Antimicrobials are a photodynamic inactivation adjuvant for the eradication of extensively drug-resistant Acinetobacter baumannii, Front. Microbiol 10 (2019) 1–13. doi: 10.3389/fmicb.2019.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].dos Anjos C, Sellera FP, Ribeiro MS, Baptista MS, Pogliani FC, Lincopan N, Sabino CP, Antimicrobial blue light and photodynamic therapy inhibit clinically relevant β-lactamases with extended-spectrum (ESBL) and carbapenemase activity, Photodiagnosis Photodyn. Ther 32 (2020). doi: 10.1016/j.pdpdt.2020.102086. [DOI] [PubMed] [Google Scholar]
- [88].Hyun JE, Choi C, Lee SY, Synergistic effects of blue light-emitting diodes in combination with antimicrobials against Escherichia coli O157:H7 and their mode of action, J. Photochem. Photobiol. B Biol 213 (2020) 112079. doi: 10.1016/j.jphotobiol.2020.112079. [DOI] [PubMed] [Google Scholar]
- [89].Leanse LG, Dong PT, Goh XS, Lu M, Cheng JX, Hooper DC, Dai T, Quinine enhances photo-inactivation of Gram-negative bacteria, J. Infect. Dis 221 (2020) 618–626. doi: 10.1093/infdis/jiz487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Leanse LG, Goh XS, Dai T, Quinine Improves the Fungicidal Effects of Antimicrobial Blue Light: Implications for the Treatment of Cutaneous Candidiasis, Lasers Surg. Med 52 (2020) 569–575. doi: 10.1002/lsm.23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Leanse LG, dos Anjos C, Wang Y, Murray CK, Hooper DC, Dai T, Effective treatment of cutaneous mold infections by antimicrobial blue light that is potentiated by quinine, J. Infect. Dis 2 (2021) 1–8. doi: 10.1093/infdis/jiab058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Leanse LG, Zeng X, Dai T, Potentiated antimicrobial blue light killing of methicillin resistant Staphylococcus aureus by pyocyanin, J. Photochem. Photobiol. B Biol 215 (2021) 112109. doi: 10.1016/j.jphotobiol.2020.112109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Dong PT, Mohammad H, Hui J, Leanse LG, Li J, Liang L, Dai T, Seleem MN, Cheng JX, Photolysis of Staphyloxanthin in Methicillin-Resistant Staphylococcus aureus Potentiates Killing by Reactive Oxygen Species, Adv. Sci 6 (2019). doi: 10.1002/advs.201900030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Jusuf S, Hui J, Dong PT, Cheng JX, Staphyloxanthin Photolysis Potentiates Low Concentration Silver Nanoparticles in Eradication of Methicillin-Resistant Staphylococcus aureus, J. Phys. Chem. C 124 (2020) 5321–5330. doi: 10.1021/acs.jpcc.9b10209. [DOI] [Google Scholar]
- [95].Hui J, Dong PT, Liang L, Mandal T, Li J, Ulloa ER, Zhan Y, Jusuf S, Zong C, Seleem MN, Liu GY, Cui Q, Cheng JX, Photo-Disassembly of Membrane Microdomains Revives Conventional Antibiotics against MRSA, Adv. Sci 1903117 (2020). doi: 10.1002/advs.201903117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Xi J, An L, Wei G, Huang Y, Li D, Fan L, Gao L, Photolysis of methicillin-resistant Staphylococcus aureus using Cu-doped carbon spheres, Biomater. Sci 8 (2020) 6225–6234. doi: 10.1039/d0bm01239d. [DOI] [PubMed] [Google Scholar]
- [97].Baek KH, Kim H-J, Kang T, Lee YE, Kim D-K, Kang D-H, Jo C, Blue light promotes bactericidal action of plasma-activated water against Staphylococcus aureus on stainless steel surfaces, Innov. Food Sci. Emerg. Technol. 69 (2021) 102663. doi: 10.1016/j.ifset.2021.102663. [DOI] [Google Scholar]
- [98].Jusuf S, Dong P, Hui J, Ulloa ER, Liu GY, Cheng J, Granadaene Photobleaching Reduces the Virulence and Increases Antimicrobial Susceptibility of Streptococcus agalactiae, Photochem. Photobiol (2021) php.13389. doi: 10.1111/php.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Trzaska WJ, Wrigley HE, Thwaite JE, May RC, Species-specific antifungal activity of blue light, Sci. Rep 7 (2017) 1–7. doi: 10.1038/s41598-017-05000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wang Y, Wu X, Chen J, Amin R, Lu M, Bhayana B, Zhao J, Murray CK, Hamblin MR, Hooper DC, Dai T, Antimicrobial Blue Light Inactivation of Gram-Negative Pathogens in Biofilms: In Vitro and in Vivo Studies, J. Infect. Dis 213 (2016) 1380–1387. doi: 10.1093/infdis/jiw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Amin RM, Bhayana B, Hamblin MR, Dai T, Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: in vitro and in vivo studies, Lasers Surg. Med 48 (2016) 562–568. doi: 10.1002/lsm.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zhang Y, Zhu Y, Chen J, Wang Y, Sherwood ME, Murray CK, Vrahas MS, Hooper DC, Hamblin MR, Dai T, Antimicrobial blue light inactivation of Candida albicans: In vitro and in vivo studies, 2016. doi: 10.1080/21505594.2016.1155015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Mallidi S, Anbil S, Bulin AL, Obaid G, Ichikawa M, Hasan T, Beyond the barriers of light penetration: Strategies, perspectives and possibilities for photodynamic therapy, Theranostics. 6 (2016) 2458–2487. doi: 10.7150/thno.16183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kim M, An J, Kim KS, Choi M, Humar M, Kwok SJJ, Dai T, Yun SH, Optical lens-microneedle array for percutaneous light delivery, Biomed. Opt. Express 7 (2016) 4220. doi: 10.1364/boe.7.004220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Bumah VV, Morrow BN, Cortez PM, Bowman CR, Rojas P, Masson-Meyers DS, Suprapto J, Tong WG, Enwemeka CS, The importance of porphyrins in blue light suppression of Streptococcus agalactiae, J. Photochem. Photobiol. B Biol 212 (2020) 111996. doi: 10.1016/j.jphotobiol.2020.111996. [DOI] [PubMed] [Google Scholar]


