Abstract
Methylammonium and ammonium (MEP) permeases of Saccharomyces cerevisiae belong to a ubiquitous family of cytoplasmic membrane proteins that transport only ammonium (NH4+ + NH3). Transport and accumulation of the ammonium analog [14C]methylammonium, a weak base, led to the proposal that members of this family were capable of energy-dependent concentration of the ammonium ion, NH4+. In bacteria, however, ATP-dependent conversion of methylammonium to γ-N-methylglutamine by glutamine synthetase precludes its use in assessing concentrative transport across the cytoplasmic membrane. We have confirmed that methylammonium is not metabolized in the yeast S. cerevisiae and have shown that it is little metabolized in the filamentous fungus Neurospora crassa. However, its accumulation depends on the energy-dependent acidification of vacuoles. A Δvph1 mutant of S. cerevisiae and a Δvma1 mutant, which lack vacuolar H+-ATPase activity, had large (fivefold or greater) defects in the accumulation of methylammonium, with little accompanying defect in the initial rate of transport. A vma-1 mutant of N. crassa largely metabolized methylammonium to methylglutamine. Thus, in fungi as in bacteria, subsequent energy-dependent utilization of methylammonium precludes its use in assessing active transport across the cytoplasmic membrane. The requirement for a proton gradient to sequester the charged species CH3NH3+ in acidic vacuoles provides evidence that the substrate for MEP proteins is the uncharged species CH3NH2. By inference, their natural substrate is NH3, a gas. We postulate that MEP proteins facilitate diffusion of NH3 across the cytoplasmic membrane and speculate that human Rhesus proteins, which lie in the same domain family as MEP proteins, facilitate diffusion of CO2.
Methylammonium and ammonium permeases MEP1, MEP2, and MEP3 of Saccharomyces cerevisiae (35) and the ammonium and methylammonium transport B (AmtB) protein of enteric bacteria (64) are members of a unique family of cytoplasmic membrane transporters that are specific for ammonium (48). (We use ammonium to designate both the charged and uncharged species.) The MEP/Amt family (nomenclature, TC 2.49) occurs ubiquitously in bacteria, archaea, and eukarya (19, 36). Beginning with the pioneering studies of Hackette et al. (16), the activity of MEP/Amt proteins has been assessed by studying transport and accumulation of the ammonium analog methylammonium, which can be 14C labeled. Based on studies with methylammonium it has been proposed that members of the MEP/Amt family transport the charged species NH4+ across the cytoplasmic membrane and concentrate it in an energy-dependent manner (19, 65).
We showed previously that enteric bacteria convert methylammonium to γ-N-methylglutamine in the ATP-dependent reaction catalyzed by glutamine synthetase and hence that methylammonium cannot be used to assess energy-dependent concentrative uptake (62). The same metabolic conversion occurs in other proteobacteria, including methylotrophic pseudomonads (2, 11, 12, 22–24, 51, 58), and in cyanobacteria (41) and plants (13). Contrary to a previous report (60), it also appears to occur in the gram-positive bacterium Corynebacterium glutamicum (39). In contrast, the fungi S. cerevisiae and Penicillium chrysogenum accumulate methylammonium in the absence of metabolism (16, 53).
When provided at high external concentrations, methylammonium accumulates in acidic compartments of fungi and other eukaryotes, thereby neutralizing these compartments and perturbing their function (15, 25, 45, 46, 61, 69). The same is true for ammonium and other weak bases (1, 32, 55, 66). We now present evidence that the charged species CH3NH3+ is accumulated in acidic vacuoles of the yeast S. cerevisiae and the filamentous fungus Neurospora crassa, even when [14C]methylammonium is provided at low external concentrations. Because acidification of vacuoles depends on the activity of the vacuolar H+-ATPase (V-ATPase or V-type H+-ATPase) (3, 4, 9, 27, 50), MEP-dependent sequestration of methylammonium is driven by an energy-requiring secondary process. Therefore, in fungi as in bacteria, the energy-dependent concentration of methylammonium does not provide evidence for its active transport across the cytoplasmic membrane. Interestingly, an N. crassa mutant (vma-1) that cannot acidify its vacuoles couples uptake of methylammonium to the energy-requiring secondary process used in bacteria, i.e., conversion to methylglutamine.
MATERIALS AND METHODS
Strains, media, and growth conditions.
Growth experiments with S. cerevisiae strains 23344c, 31019b, and NCM3243 (Table 1) were performed as previously described (62) at 28°C. The minimal medium was medium 164 (21), which was supplemented appropriately (62). Medium 164 has an initial pH of 6.1 which does not change during growth. The carbon source was glucose, usually at a concentration of 3%, and the nitrogen source was as indicated. YPD medium contained yeast extract (1%), peptone (2%), and glucose (2%). To test the effect of ammonium or methylammonium concentration on the growth of S. cerevisiae, cells were first grown in medium 164 at pH 6.1 with glucose (3%) as the carbon source and 10 mM NH4Cl or glutamate as the nitrogen source. Cells were then inoculated into medium 164 containing different concentrations of NH4Cl or methylammonium and buffered at pH 7.5 or 7.0, respectively, by the addition of 50 mM Tris buffer. The nitrogen source for cells grown in the presence of methylammonium was 10 mM glutamate. Concentrations of NH3 and CH3NH2 were calculated from those of ammonium and methylammonium using pKa values of 9.25 and 10.6, respectively (18).
TABLE 1.
Strains
| Strain | Genotype | Source or reference |
|---|---|---|
| S. cerevisiae | ||
| 23344c | MATα ura3 | 35 |
| 31019b | MATα ura3 Δmep1 Δmep2::LEU2 Δmep3::KanMX2 | 35 |
| NCM3243 | MATα ura3 Δvph1::URA3 | This study |
| Ig43-1 | a/α his4/+ lys/+ met13/+ GLN1/GLN1 | A. P. Mitchell |
| Ig43-3 | a/α his4/+ lys/+ met13/+ gln1-103/ gln1-103 | A. P. Mitchell |
| MM11 | MATα Δstv1::LYS2 ura3-52 | 34 |
| MM50 | MATα leu2-1 ura3-52 | 34 |
| MM108 | MATα his4 tfp1(vma1)Δ101::urA3 ura3-52 | 34 |
| MM112 | MATα his3-Δ200 leu2 lys2 Δstv1::LYS2 Δvph1::LEU2 ura3-52 | 34 |
| N. crassa | ||
| 74A | Wild type | 4 |
| pvn2-53-19A | vma-1RIP2 | 4 |
S. cerevisiae strain NCM3243 (Δvph1::URA3), which carries a deletion of 64% of the VPH1 gene (identical to that in BJ6717 [33]), was constructed from wild-type strain 23344c (aka NCM3018; Leu+ Ura−) (35) as follows. First, the LEU2 marker in plasmid pΔvph1::LEU2 (33), generously provided by E. W. Jones, was replaced with the URA3 marker in plasmid pJES1256 as described below to yield plasmid pJES1276. Then the 4.0-kb fragment of plasmid pJES1276, which carries Δvph1::URA3, was amplified by PCR and introduced into strain 23344c by the lithium acetate method (20). Mutants resulting from recombination of the fragment into the chromosome were selected on minimal medium containing 50 mM NH4Cl as the nitrogen source and lacking uracil. Several Ura+ clones were analyzed by PCR and Southern blot and one that showed the correct replacement of VPH1 was chosen.
Plasmid pJES1276 was constructed in the following several steps. Plasmid pΔvph1::LEU2 was cleaved with XhoI and EcoRI, which yielded two fragments of 2.9 kb, one of 1.2 kb, and one of 0.3 kb. The two 2.9-kb fragments were purified from an agarose gel and ligated. The resulting plasmid, pJES1274, carries the 5′ end of VPH1 followed by a portion of the LEU2 marker. pJES1274 was linearized with EcoRI and made blunt ended with the Klenow fragment of DNA polymerase I. It was then ligated to the 1.2-kb HindIII fragment from plasmid pJES1256, also made blunt ended, which carries the URA3 marker, to yield pJES1275. Finally, the 0.3-kb XhoI-BamHI fragment of pΔvph1::LEU2 made blunt ended, which carries a portion of the 3′ end of VPH1, was ligated into pJES1275 which had been linearized with BamHI and made blunt ended. The resulting plasmid, pJES1276, carries the Δvph1::URA3 deletion and insertion. The correct orientation of cloned fragments was confirmed by sequencing.
S. cerevisiae strains MM50 (wild type), MM108 (Δvma1), MM11 (Δstv1), and MM112 (Δvph1 Δstv1) (34) (Table 1) were kindly provided by M. Manolson. They were grown in YPD medium containing 2% glucose because MM108 and MM112 grow very poorly in minimal medium, even at a low pH. N. crassa strains 74A (wild type) and pvn2-53-19A (vma-1RIP2) (4) (Table 1) were kindly provided by B. J. Bowman. They were maintained on slants in Vogel's minimal medium N (7). They were grown in liquid culture in a modified Vogel's medium in which the usual nitrogen sources (ammonium nitrate at 25 mM) were replaced. Ammonium chloride or proline (10 mM) was the nitrogen source and glucose (2%) was the carbon source. Mycelia were grown from conidia inoculated to an initial density of 2 × 106 conidia/ml into 1-liter flasks containing ∼150 ml of the appropriate medium (optical density at 600 nm [OD600] of ∼0.13). Cultures were grown at 30°C in an orbital shaker (∼175 rpm) and mycelia were harvested by filtration when the OD600 reached 0.5.
Preparation of genomic DNA for analysis of putative Δvph1 strains of S. cerevisiae.
Genomic DNA was prepared by a miniscale extraction procedure as follows. (i) A 2-ml sample of an overnight culture grown in YPD medium at 28°C (OD600 of ≃4) was harvested by centrifugation and washed with 1 ml of H2O. (ii) The cell wall was digested at 37°C for 40 min in 0.1 ml of lysis buffer (1 M sorbitol, 100 mM EDTA, 4 mM β-mercaptoethanol, 0.5 mg of lyticase [Sigma]/ml). (iii) Spheroplasts were lysed by the addition of 0.1 ml of 0.1 M Tris-HCl (pH 8.0)–10 mM EDTA–2% sodium dodecyl sulfate, vigorous vortexing, and incubation at 65°C for 10 min. (iv) Proteins were precipitated by the addition of 0.1 ml of 5 M potassium acetate and incubation for 1 h on ice. They were removed by centrifugation at high speed for 10 min at 4°C. (v) DNA was precipitated by the addition of 1 volume of isopropanol to the supernatant and centrifugation for 20 min. Pellets were washed with 70% ethanol, air dried, and suspended in 0.2 ml of 10 mM Tris-HCl (pH 7.5)–1 U of RNase A. Typically, 2 ml of culture yielded 50 μg of genomic DNA.
Transport assays.
S. cerevisiae strains 23344c, 31019b, and NCM3243 were grown in medium 164 with proline (10 mM) as the nitrogen source, unless otherwise indicated. Strains MM50, MM108, MM11, and MM112 were grown in YPD–2% glucose. Cells were harvested at an OD600 of 0.5. Assays of [14C]methylammonium transport were performed as described previously (62) at 28°C and pH 6.1. The initial concentration of [14C]methylammonium was 6 μM and the assay buffer contained 50 mM HEPES (pH 6.1), 72 mM NaCl, and 2% glucose.
To derepress synthesis of glucose permeases, cells were grown overnight in YPD at 28°C and were then inoculated into modified YPD containing only 0.05% glucose at an OD600 of 0.1 and grown to an OD600 of 0.5. Cells were harvested, washed, and suspended in assay buffer lacking glucose. At this step, cells were kept on ice. Assays of d-[1-14C]glucose transport were performed essentially as for [14C]methylammonium. Cells were warmed for 20 min at 28°C and transport was initiated by adding d-[1-14C]glucose (specific activity, 0.59 Ci/mol) to a final concentration of 170 μM.
For testing the effect of weak bases on transport, S. cerevisiae cells were washed with 50 mM HEPES, pH 7.5, and then incubated at 28°C for 20 min in the same buffer or buffer containing 200 mM NH4Cl or 1 mM chloroquine (pKa values of 8.1 and 10.2) (45), as indicated. Cells were then washed with 50 mM HEPES, pH 7.5, suspended in assay buffer, and used immediately for transport assays.
Harvested mycelia of N. crassa were washed with and suspended in a 1/2 volume of chilled assay buffer. Mycelial suspensions were stored on ice until they were used for transport assays, usually within 3 h. Prior to assaying transport of [14C]methylammonium, mycelial suspensions were warmed to 30°C for 30 min. Assays were initiated by adding [14C]methylammonium (50 or 10 Ci/mol for mycelia grown with ammonium chloride or proline, respectively, as the nitrogen source) to a final concentration of 4 μM. Samples (0.5 ml) were filtered at appropriate intervals and washed. Radioactivity was measured by liquid scintillation counting and transport was normalized to mycelial dry weight, which was 0.7 to 0.9 mg/ml.
Analysis of 14C-labeled products.
14C-labeled products accumulated by S. cerevisiae or N. crassa were analyzed as previously described (62). After the indicated times of exposure to [14C]methylammonium, samples of S. cerevisiae (0.5 ml of cells at an OD600 of 1) were filtered, washed, suspended in 1 ml of water, and boiled for 20 min. Insoluble material was removed by centrifugation. More than 90% of the radiolabel initially present in cells was extracted by this means. Control experiments demonstrated that methylammonium and methylglutamine were stable to the extraction procedure (data not shown).
Mycelia of N. crassa were grown with proline as the nitrogen source. At an OD600 of 0.5 they were concentrated approximately twofold by partial filtration. [14C]methylammonium (50 Ci/mol) was added to 4 ml of concentrated mycelial suspension to a final concentration of 5 μM, and 1-ml samples were filtered at appropriate intervals and washed. Filtered samples were placed in 50% ethanol and boiled for 30 min. The radioactivity extracted from each sample was determined by liquid scintillation counting and the same amount (∼2,400 cpm) was analyzed chromatographically. After chromatography, radioactivity on thin-layer plates was assessed by autoradiography and/or phosphorimaging.
RESULTS
Transport and accumulation of [14C]methylammonium by S. cerevisiae.
As reported previously (8, 35), wild-type S. cerevisiae 23344c grown in synthetic medium with proline as the nitrogen (N) source accumulated [14C]methylammonium (initial external concentration of 6 μM) slowly over a long time (30 to 40 min), whereas strain 31019b, which lacks all three MEP proteins, did not (Fig. 1A). As previously reported (52), concentration by the wild-type strain appeared to be at least 1,000-fold (see legend to Fig. 1). The wild-type strain also accumulated [14C]methylammonium when grown with glutamate, glutamine, or NH4Cl as the N source (activity, 170 to 270 pmol/ml/OD600/min depending on the N source). Extraction and chromatography of 14C-labeled products indicated that ≤2% of the methylammonium was metabolized, apparently to methylglutamine (Fig. 1A inset, lane 3). In agreement with this, a mutant strain, Ig43-3, that lacks glutamine synthetase (gln1-103 lesion) (40; A. Mitchell, personal communication) accumulated [14C]methylammonium as rapidly and to the same extent as its congenic parent strain, Ig43-1 (both grown on glutamine as the N source) (data not shown).
FIG. 1.
Transport of methylammonium (A and C) and glucose (B and D) by S. cerevisiae (see Materials and Methods). Strains for panels A and B were 23344c (wild type; squares), 31019b (Δmep1 Δmep2 Δmep3; circles), and NCM3243 (Δvph1; triangles), and strains for panels C and D were MM50 (wild type; squares), MM11 (Δstv1; open diamonds), MM108 (Δvma1; triangles), and MM112 (Δvph1 Δstv1; closed diamonds). (A) The initial concentration of [14C]methylammonium was 6 μM. Cells were grown in minimal medium with proline (10 mM) as the N source. The degree of concentration of methylammonium by strain 23344c was calculated assuming a cell volume of 70 μm3 and 107 cells/ml/OD600 (59). Inset, 14C-labeled products accumulated intracellularly 25 min after exposure to [14C]methylammonium. Products were separated by thin-layer chromatography and subjected to autoradiography. Lane 1, [14C]methylglutamine; lane 2, [14C]methylammonium; lane 3, wild type; lane 4, Δvph1. (B) The initial concentration of d-[1-14C]glucose was 170 μM. Cells were grown in modified YPD containing 0.05% glucose. (C) As for panel A except cells were grown in YPD–2% glucose. (D) As for panel B.
Effect of weak bases on accumulation of methylammonium.
At higher concentrations than those we employed in transport assays, the weak base methylammonium is commonly used to determine the pH difference across biological membranes (17, 28, 56). Whereas the uncharged species (CH3NH2) can diffuse across membranes in an unmediated manner, the charged species (CH3NH3+) cannot. Hence, partitioning of methylammonium across a membrane allows for an estimation of the pH gradient. We wondered whether accumulation of [14C]methylammonium in our transport experiments might be due to sequestration of the charged species into acidic compartments. To test this, we first determined whether at high concentrations the weak bases ammonium and chloroquine, which are known to accumulate in acidic compartments and increase the luminal pH (6, 44–46), would interfere with the accumulation of [14C]methylammonium. Treatment of wild-type S. cerevisiae strain 23344c at pH 7.5 with ammonium (200 mM) for 20 min prevented accumulation of [14C]methylammonium (Fig. 2A). The same was true for treatment with chloroquine (1 mM), which is structurally unrelated to ammonium and methylammonium. In both cases, accumulation resumed 10 min later and in fact was restored to normal (data not shown). The latter may be accounted for by reacidification of vacuoles and other acidic compartments because the assay buffer contained 2% glucose as the energy source. In agreement with the above interpretations, transport and metabolism of d-[1-14C]glucose were not affected by treatment with ammonium or chloroquine (Fig. 2B).
FIG. 2.
Effect of weak bases on the accumulation of methylammonium (A) and glucose (B) by S. cerevisiae (see Materials and Methods). Transport by strain 23344c (wild type) was assessed after exposure to buffer at pH 7.5 (squares) or to buffer containing 1 mM chloroquine (2 μM unprotonated form; triangles) or 200 mM NH4Cl (3.5 mM NH3; circles). (A) The initial concentration of [14C]methylammonium was 6 μM. Cells were grown in minimal medium with proline (10 mM) as the N source. (B) The initial concentration of d-[1-14C]glucose was 170 μM. Cells were grown in modified YPD containing 0.05% glucose.
Role of V-ATPase in accumulation of [14C]methylammonium.
The pH of the large yeast vacuole is estimated to be between 5.5 and 6.2, which is approximately 0.8 to 1.5 pH units lower than that of the cytosol (49, 50, 68). Acidification of the vacuole is maintained by a specific vacuolar H+-ATPase (V-ATPase), the assembly and function of which involve several proteins (9, 14, 42). Disruption of the VPH1 gene, which codes for a subunit of the V-ATPase required for its assembly, results in the loss of ATPase activity and a defect in acidification of vacuoles (33, 49, 50). Introduction of the Δvph1 lesion into our wild-type strain decreased accumulation of [14C]methylammonium by ∼80%, with little effect on the initial rate of uptake (Fig. 1A). The same was true for disruption of the VMA1 gene (34), which codes for a catalytic subunit of the ATPase (Fig. 1C). Effects of the Δvma1 lesion were studied in a different wild-type background. Like accumulation of [14C]methylammonium in wild-type strains, residual accumulation in the Δvph1 and Δvma1 strains depended on the presence of glucose in the buffer (data not shown). It may be due to accumulation into other acidic compartments, as has been described for entrapment of fluorescent dyes (32) and/or to increased metabolism (see below). As was the case for cells treated with weak bases, the Δvph1 and Δvma1 strains showed no defect in the transport and metabolism of d-[1-14C]glucose (Fig. 1B and D, respectively). A strain that carried both the Δvph1 lesion and a Δstv1 lesion (34), which disrupts a gene homologous to VPH1, also showed a large decrease in accumulation of [14C]methylammonium (Fig. 1C). However, the double mutant strain, which grew poorly even in enriched medium, showed a defect in transport and metabolism of d-[1-14C]glucose (Fig. 1D). The Δstv1 lesion alone had no effect on accumulation of either [14C]methylammonium or d-[1-14C]glucose.
The fact that the initial rate of [14C]methylammonium uptake was little affected by the Δvph1 or Δvma1 alleles is in agreement with the view that these lesions do not alter the expression or activity of MEP proteins, which appear to be localized to the cytoplasmic membrane (38; G. Fink, personal communication). Although the Δvph1 strain grew slower than the wild type or the Δmep triple mutant on various nitrogen sources (Table 2), it showed no particular growth defect at low ammonium concentrations at pH values below 7. This provided an independent line of evidence that the MEP proteins were expressed and localized normally in the Δvph1 strain and had normal physiological function.
TABLE 2.
Growth of S. cerevisiae at pH 6.1 on different nitrogen sources
| Nitrogen sourcea | Concentration (mM) of nitrogen source | Doubling time (min)
|
||
|---|---|---|---|---|
| Wild typeb | Δmep1 Δmep2 Δmep3b | Δvph1 | ||
| Glutamate | 10 | 160 | 160 | 230 |
| Proline | 10 | 200 | 200 | 410 |
| Arginine | 2.5 | 160 | 170 | 180 |
| Urea | 5 | 270 | 270 | 450 |
| NH4Cl | 20 | 160 | 175 | 205 |
| 5 | 160 | 290 | 210 | |
| 1 | 175 | >700 | 215 | |
Cells were grown in medium 164 with glucose (3%) as the carbon source and different nitrogen sources at the concentrations indicated. Strains were 23344c (wild type), 31019b (Δmep1 Δmep2 Δmep3) (35), and NCM3243 (Δvph1).
Final ODs of strains 23344c and 31019b were 13 and 13 on glutamate, 12 and 13 on proline, 11.5 and 11.5 on arginine, 14 and 13.5 on urea, 16 and 16 on 20 mM NH4Cl, 10 and 7 on 5 mM NH4Cl, and 1.3 and 0.4 on 1 mM NH4Cl.
Interestingly, the Δvph1 strain showed increased metabolism of [14C]methylammonium (Fig. 1A). Although methylglutamine accounted for <2% of the 14C-labeled material in wild-type or Δmep strains, methylglutamine and an unidentified new product accounted for ∼20% of the 14C-labeled products in the Δvph1 strain (Fig. 1A inset, lane 4). Similarly, the unidentified product accounted for up to 20% of the 14C-labeled product in the Δvma1 and Δvph1 Δstv1 strains, which were grown in enriched medium (data not shown).
Perturbation of growth of wild-type S. cerevisiae by NH3.
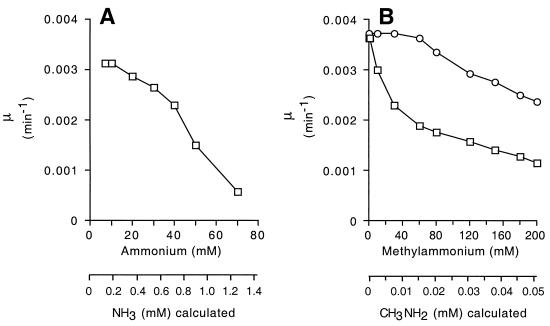
Different synthetic media for S. cerevisiae contain high concentrations of ammonium [usually 35 mM (NH4)2SO4 = 70 mM (NH4+ + NH3)] (Difco manual of dehydrated culture: media and reagents for microbiological and clinical laboratory procedures, Difco Laboratories, Detroit, Mich.). Given that NH3 can perturb the pH of vacuoles, we wondered whether this could contribute to the slow growth of yeast at neutral and higher pH values. As indicated in Table 3, raising the concentration of ammonium from 7 to 70 mM had no effect on growth at pH values of 6.5 or lower (medium 164 with citrate-phosphate buffer and 3% glucose as the carbon source). However, at pH values of 7.5 or higher, it markedly increased the doubling time. For a given concentration of ammonium (NH4+ + NH3) only the concentration of the uncharged species, NH3, increases with increasing pH and hence NH3 must be responsible for growth inhibition. The decrease in growth rate (increase in doubling time) at pH 7.5 was progressive for concentrations of ammonium between 7 and 70 mM (Fig. 3A).
TABLE 3.
Effect of pH on the growth of S. cerevisiae at different ammonium concentrations
| (NH4+ + NH3) concentration (mM) | Doubling time (min) at pH:a
|
|||
|---|---|---|---|---|
| 5.5 | 6.5 | 7.5 | 8.0 | |
| 7 | 165 | 165 | 220 | 420b |
| 70 | 165 | 170 | 1,200b | NGc |
Cells were grown in medium 164 with glucose (3%) as the carbon source and ammonium as the sole nitrogen source. At pH values of 7.5 and 8.0, 50 mM Tris was added to the medium for buffering. The wild-type strain was 23344c.
Initial doubling time.
NG, no growth.
FIG. 3.
Effect of different concentrations of ammonium (A) or methylammonium (B) on the growth rate constant μ of S. cerevisiae. (A) Strain 23344c (wild type) was grown in medium 164 buffered with 50 mM Tris, pH 7.5, with glucose (3%) as the carbon source and NH4Cl as the sole nitrogen source. (B) Strains 23344c (wild type; squares) and 31019b (Δmep1 Δmep2 Δmep3; circles) were grown in medium 164 buffered with 50 mM Tris, pH 7.0, with glucose (3%) as the carbon source and glutamate (10 mM) as the sole nitrogen source.
The synthetic yeast medium commercialized by Difco, yeast nitrogen base without amino acids and ammonium, which is identical to synthetic medium SD, is acidic (pH 4.5) and not buffered (67; Difco manual). In this medium increasing the concentration of ammonium had no effect on growth rate (not shown). At 72 h, when the cells had reached stationary phase, the pH had dropped to 1.5 and no more than 15 mM ammonium had been used. This was sufficient for maximum yield. Because higher concentrations of ammonium are not required, providing them simply causes difficulty if one wishes to buffer the medium to a higher pH.
Perturbation of growth of wild-type S. cerevisiae by methylamine.
Although methylammonium is known to inhibit growth of S. cerevisiae, its effects might be different from those of ammonium for a number of reasons; these include the difference in its pKa value, the difference in its partition coefficient between water and organic solvents, and its failure to be significantly metabolized in the yeast cytoplasm. Methylammonium progressively inhibited growth of wild-type S. cerevisiae at concentrations between 10 and 200 mM (Fig. 3B). Inhibition appeared to be biphasic. Effects at low concentrations were more severe than those of ammonium, whereas effects at high concentrations were less severe. As reported previously (8, 52), growth of the Δmep triple mutant was markedly less sensitive to inhibition by methylammonium than growth of wild-type S. cerevisiae. There was little inhibition up to 60 mM, but above that the decrease in growth rate as a function of concentration (slope of the curve in Fig. 3B) was the same as for the wild type. Growth inhibition of the mutant at high concentrations may be a function of unmediated diffusion of the uncharged species CH3NH2 across the cytoplasmic membrane. Presumably, both CH3NH2 and NH3 diffuse across vacuolar membranes, whose composition differs from that of the cytoplasmic membrane (e.g., differences in lipid composition [5, 57, 63]), in an unmediated manner.
Role of V-ATPase in accumulation of [14C]methylammonium by N. crassa.
Although there have been no biochemical or genetic studies of MEP proteins in N. crassa, its genome carries at least two genes that code for such proteins (Neurospora Genome Project, University of New Mexico [http://biology.unm.edu/biology/ngp/home.html]). As is the case for S. cerevisiae, the vacuole of N. crassa is acidic and its pH has been estimated to be ∼6 (30). However, unlike the case for S. cerevisiae, disruption of the function of the vacuolar ATPase by the vma-1RIP2 mutation apparently caused no decrease in the accumulation of [14C]methylammonium (Fig. 4A). This was true whether mycelia were grown on 10 mM proline or 10 mM ammonium chloride as the N source, although accumulation by mycelia grown on proline was about twice that by mycelia grown on ammonium chloride. The vma-1 mutant of N. crassa showed profoundly increased metabolism of [14C]methylammonium with respect to the congenic wild-type strain (Fig. 4B and Table 4). Whereas the ratio of [14C]methylglutamine to [14C]methylammonium in the wild-type strain increased from 0.1 after 10 min to 0.3 after 25 min of exposure, this ratio in the vma-1 mutant strain increased from 0.7 to 3 over the same interval (Table 4). Presumably, increased metabolism in the mutant strain can be accounted for by a longer residence time of methylammonium in the cytosol, where it can be assimilated by glutamine synthetase. We do not know why a higher proportion of [14C]methylammonium was converted to methylglutamine in the vma-1 mutant strain of Neurospora than in the Δvma1 strain or other vacuolar ATPase mutants of S. cerevisiae. However, we note that vma1 mutant strains of the two organisms differ in a number of respects (4). For example, vacuoles of the Neurospora mutant, which is much more highly branched than its parent, often appear to be distorted and multilamellar, whereas vacuoles of S. cerevisiae appear to be morphologically normal.
FIG. 4.
Transport of methylammonium by N. crassa (A) and characterization of the products accumulated (B) (see Materials and Methods). (A) The initial concentration of [14C]methylammonium was 4 μM. Strains 74A (wild type; squares) and pvn2-53-19A (vma-1; triangles) were grown in modified Vogel's minimal medium with glucose (2%) as the carbon source and 10 mM NH4Cl (open symbols) or 10 mM proline (closed symbols) as the sole nitrogen source. (B) Components of cell extracts (∼2,400 cpm) were separated by thin-layer chromatography and subjected to autoradiography. Lanes 1 to 4, strain 74A (wild type); lanes 5 to 8, strain pvn2-53-19A (vma-1). Extracts were prepared 1 min (lanes 1 and 5), 4 min (lanes 2 and 6), 10 min (lanes 3 and 7), and 25 min (lanes 4 and 8) after exposure to [14C]methylammonium (initial concentration, 5 μM). Cells were grown in Vogel's minimal medium with proline (10 mM) as the N source. Positions of [14C]methylammonium (CH3NH3+) and [14C]methylglutamine (CH3-Gln) are indicated on the left.
TABLE 4.
Quantitative analysis of 14C-labeled products accumulated by N. crassa during transport of [14C]methylammonium
| Time after exposure (min)a | Normalized intensity (%) of indicated product accumulated by:b
|
|||||
|---|---|---|---|---|---|---|
| Wild type
|
vma-1
|
|||||
| CH3NH3+ | CH3-Gln | Origin | CH3NH3+ | CH3-Gln | Origin | |
| 1 | 93 | 2 | 5 | 92 | 4 | 4 |
| 4 | 87 | 4 | 9 | 77 | 16 | 7 |
| 10 | 80 | 8 | 12 | 52 | 37 | 11 |
| 25 | 60 | 17 | 23 | 18 | 57 | 25 |
Thin-layer chromatography plates were analyzed with a Molecular Dynamics PhosphorImager. Samples were as shown in Fig. 4B (see Materials and Methods). Strains were 74A (wild type) and pvn2-53-19A (vma-1) (4).
CH3NH3+, [14C]methylammonium; CH3-Gln, [14C]methylglutamine; origin, 14C-labeled products that were not resolved in our system.
Lack of involvement of the MEP proteins of S. cerevisiae in growth on alternative nitrogen sources.
Marini and colleagues proposed that the MEP proteins of S. cerevisiae were needed to recover ammonium that leaked from cells during growth on nitrogen sources other than ammonium (35). However, we found that the Δmep triple mutant showed no growth defect on a variety of alternative nitrogen sources at pH 6.1 (Table 2), although it had the expected defects at external ammonium concentrations of ≤5 mM. Thus, in our hands growth tests also failed to provide evidence that the MEP proteins concentrate ammonium in an energy-dependent manner.
DISCUSSION
Function of the MEP proteins of fungi.
In S. cerevisiae, transport of [14C]methylammonium across the cytoplasmic membrane depends on the three MEP proteins, and accumulation of [14C]methylammonium provided the strongest evidence that these proteins catalyze energy-dependent concentration of their substrates. However, we have now shown that accumulation appears to depend on energy-dependent sequestration of [14C]methylammonium into vacuoles and other acidic compartments (Fig. 1 and 2) and hence does not provide evidence for energy-dependent concentrative transport across the cytoplasmic membrane. Accumulation of [14C]methylammonium was decreased more than 80% by mutations that decrease or eliminate vacuolar ATPase activity (Fig. 1) and was completely eliminated by weak bases, which are known to neutralize acidic compartments (Fig. 2). Neither affected transport or metabolism of d-[1-14C]glucose.
The second line of evidence that MEP proteins mediated concentrative uptake of their substrates was that mep mutants showed impaired growth on nitrogen sources other than ammonium (reported previously [35] but data were not shown). Growth defects and cross-feeding of ammonium to other strains were attributed to the inability of mep mutants to accumulate the ammonium they leaked during catabolism of alternative nitrogen sources. We were unable to confirm the growth defects previously reported. Rather, we found that the Δmep triple mutant showed no defect in either growth rate or cell yield on several alternative nitrogen sources (Table 2).
Given that the AmtB protein of enteric bacteria, which is homologous to the MEP proteins, fails to concentrate either methylammonium or its natural substrate ammonium (62), the most parsimonious interpretation of the results in fungi is that MEP proteins, like AmtB, facilitate methylammonium and ammonium diffusion. Acidic trapping of the charged species CH3NH3+ in fungal vacuoles implies that the substrate for the MEP proteins is the uncharged species CH3NH2. By analogy, their natural substrate would be NH3. This is in accord with previous findings that MEP/AmtB proteins are required for growth at low ammonium concentrations only at low pH values (62), that is under conditions in which the uncharged species NH3 is limiting. Under other circumstances, i.e., higher ammonium concentrations or higher pH values, NH3 can apparently diffuse across the cytoplasmic membrane in an unmediated manner fast enough to support optimal growth. In fact, if both ammonium concentration and pH are high, NH3 can diffuse across fungal membranes fast enough to inhibit growth, presumably by neutralization of vacuoles and/or other acidic compartments. This may contribute to slow growth of fungi in standard minimal media at pH values of ≥7 (Fig. 3) (49).
Mutant strains of S. cerevisiae and N. crassa that cannot acidify their vacuoles metabolized larger amounts of [14C]methylammonium to γ-N-methylglutamine than their parent strains (Fig. 1 and 4; Table 4). This was particularly true of the N. crassa mutant, which showed no defect in the accumulation of the 14C label (Fig. 4; see Results). Presumably metabolism occurs because methylammonium remains in the cytoplasm long enough to be assimilated in an energy-dependent manner by glutamine synthetase. Thus, the fungal mutant strains behave like bacteria, which lack vacuoles.
Quantitative aspects of ammonium and methylammonium transport.
We noted previously that enteric bacteria require the AmtB protein for optimal growth when the external concentration of the uncharged species NH3 falls to ∼50 nM or below (total concentration of ammonium, 10 μM at pH 7 or 1 mM at pH 5 [62]). S. cerevisiae appears to require the MEP proteins at a concentration of NH3 that is 100-fold higher (5 μM NH3 = 1 mM ammonium at pH 7.1 [62]). The requirement for porters in S. cerevisiae at a higher NH3 concentration is likely due to several factors. (i) The volume of S. cerevisiae is 70 times larger than that of enteric bacteria, whereas its surface area is only 16 times larger (59). Thus, its surface/volume ratio is only 1/5 that of enteric bacteria. (ii) Differences in membrane composition between S. cerevisiae and enteric bacteria (5, 26) may reduce the rate of unmediated diffusion of NH3 in S. cerevisiae. (iii) Sequestration of NH3 into acidic vacuoles in S. cerevisiae may reduce its rate of assimilation into the central intermediates of nitrogen metabolism by cytosolic glutamine synthetase and glutamate dehydrogenase.
Although the pH of the yeast vacuole is at most 0.6 units below that of the buffered medium we used for transport assays (49), [14C]methylammonium was concentrated 1,000-fold rather than the expected 4-fold. Additional concentration, which occurred slowly, may be due to homeostatic mechanisms that allow continued acidification of vacuoles as weak base is accumulated. Whereas concentration of [14C]methylammonium was from 6 μM to 6 mM, vacuoles are known to accumulate basic amino acids and cations to far higher concentrations of several hundred millimolar (29, 43, 47, 54).
Function of the human Rhesus antigen-associated protein RhAG and its homologue from kidney RhCG (RhGK).
Marini and colleagues found that the human Rhesus antigen-associated protein (RhAG) and the Rhesus antigen itself, which are prominent on the surface of red blood cells, show sequence relatedness to MEP/Amt proteins (36). In fact, however, Rh proteins bear sequence relatedness to MEP proteins over only a single domain, whereas MEP/Amt proteins are related to one another across three domains (E. Soupene, unpublished observations). The nematode Caenorhabditis elegans contains members of both the MEP and Rh subfamilies (36).
Marini and colleagues recently proposed that RhAG and its kidney homologue RhCG (31) (RhGK) actively transport NH4+ (37). Their conclusion rests on the ability of the human proteins to complement the growth defects of the Δmep triple mutant of S. cerevisiae at low ammonium concentrations. However, their evidence appears self-contradictory. Cloned RhAG and RhCG allowed the Δmep triple mutant to form small colonies in 5 to 7 days at low ammonium concentrations; they did not allow it to take up [14C]methylammonium, presumably because complementation was partial. Unexpectedly, cloned RhAG and RhCG made the Δmep triple mutant resistant to methylammonium under conditions in which both it and wild-type S. cerevisiae were sensitive. The ad hoc interpretation of the latter finding was that the Rh proteins actively export methylammonium. In summary, the incompatible results were that RhAG and RhCG allow the Δmep triple mutant to grow at low ammonium concentrations but make it resistant to methylammonium.
As discussed in Results, MEP proteins themselves confer sensitivity to methylammonium, and in fact their name derives from this property. The Δmep triple mutant was selected for resistance to growth inhibition by methylammonium (8) and is more resistant than the wild type over a wide range of concentrations (Fig. 3B). In assessing the effect of cloned Rh proteins on the methylammonium resistance of triple mepΔ, Marini et al. used 200 rather than the usual 100 mM methylammonium, an unusual circumstance under which the Δmep triple mutants like the wild type, is sensitive. It is likely that sensitivity is caused by unmediated diffusion of methylammonium (specifically CH3NH2) across the cytoplasmic membrane (see second phase of inhibition in Fig. 3B). Hence the effects of overexpressed RhAG and RhCG may be due to a perturbation of the yeast cytoplasmic membrane that causes a decrease in unmediated CH3NH2 diffusion.
Marini et al. (37) fail to consider our evidence that Amt/MEP proteins are specific for the uncharged species CH3NH2 and NH3 rather than the charged species CH3NH3+ and NH4+ and that they increase the rates of diffusion of the neutral species rather than actively transporting them (62). Although the Rh complex of red blood cells- and other members of the Rh subfamily- may also mediate diffusion of NH3, this seems unlikely physiologically (31). Rather, we speculate that the Rh complex may be the postulated protein facilitator for diffusion of CO2 (10), which like NH3 is a gas that also crosses membranes passively. Further assessment of the substrate specificities of Rh and MEP proteins and of the variety of their physiological roles in different organisms and tissues seems warranted.
ACKNOWLEDGMENTS
We are grateful to B. Bowman, E. Jones, A. Mitchell, and M. Manolson for providing strains and to G. Fink for helpful discussions and encouragement.
This work was supported by National Science Foundation grant MCB-9874443 to S.K. R.M.R. was supported by an NIH MBRS-SCORE grant (SO6 GM52588-04) and an SFSU Faculty Leave with Pay award.
REFERENCES
- 1.Banta L M, Robinson J S, Klionsky D J, Emr S D. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J Cell Biol. 1988;107:1369–1383. doi: 10.1083/jcb.107.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes E M, Zimniak P, Jayakumar A. Role of glutamine synthetase in the uptake and metabolism of methylammonium by Azotobacter vinelandii. J Bacteriol. 1983;156:752–757. doi: 10.1128/jb.156.2.752-757.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman E J, Bowman B J. Identification and properties of an ATPase in vacuolar membranes of Neurospora crassa. J Bacteriol. 1982;151:1326–1337. doi: 10.1128/jb.151.3.1326-1337.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman E J, Kendle R, Bowman B J. Disruption of vma-1, the gene encoding the catalytic subunit of the vacuolar H(+)-ATPase, causes severe morphological changes in Neurospora crassa. J Biol Chem. 2000;275:167–176. doi: 10.1074/jbc.275.1.167. [DOI] [PubMed] [Google Scholar]
- 5.Daum G, Lees N D, Bard M, Dickson R. Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast. 1998;14:1471–1510. doi: 10.1002/(SICI)1097-0061(199812)14:16<1471::AID-YEA353>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 6.Davies L, Satre M, Martin J-B, Gross J D. The target of ammonia action in dictyostelium. Cell. 1993;75:321–327. doi: 10.1016/0092-8674(93)80073-n. [DOI] [PubMed] [Google Scholar]
- 7.Davis R H, de Serres F. Genetic and microbial research techniques for Neurospora crassa. Methods Enzymol. 1970;17A:79–143. [Google Scholar]
- 8.Dubois E, Grenson M. Methylamine/ammonia uptake systems in Saccharomyces cerevisiae: multiplicity and regulation. Mol Gen Genet. 1979;175:67–76. doi: 10.1007/BF00267857. [DOI] [PubMed] [Google Scholar]
- 9.Forgac M. Structure and properties of the vacuolar (H+)-ATPases. J Biol Chem. 1999;274:12951–12954. doi: 10.1074/jbc.274.19.12951. [DOI] [PubMed] [Google Scholar]
- 10.Forster R E, Gros G, Lin L, Ono Y, Wunder M. The effect of 4,4′-diisothiocyanato-stilbene-2,2′-disulfonate on CO2 permeability of the red blood cell membrane. Proc Natl Acad Sci USA. 1998;95:15815–15820. doi: 10.1073/pnas.95.26.15815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genthner B R S, Wall J D. Ammonium uptake in Rhodopseudomonas capsulata. Arch Microbiol. 1985;141:219–224. [Google Scholar]
- 12.Gober J W, Kashket E R. Methylammonium uptake by Rhizobium sp. 32H1. J Bacteriol. 1983;153:1196–1201. doi: 10.1128/jb.153.3.1196-1201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godon C, Krapp A, Leydecker M, Daniel-Vedele F, Caboche M. Methylammonium-resistant mutants of Nicotiana plumbaginifolia are affected in nitrate transport. Mol Gen Genet. 1996;250:357–366. doi: 10.1007/BF02174394. [DOI] [PubMed] [Google Scholar]
- 14.Graham L A, Powell B, Stevens T H. Composition and assembly of the yeast vacuolar H+-ATPase complex. J Exp Biol. 2000;203:61–70. doi: 10.1242/jeb.203.1.61. [DOI] [PubMed] [Google Scholar]
- 15.Greenfield N J, Hussain M, Lenard J. Effects of growth state and amines on cytoplasmic and vacuolar pH, phosphate and polyphosphate levels in Saccharomyces cerevisiae: a 31P-nuclear magnetic resonance study. Biochim Biophys Acta. 1987;926:205–214. doi: 10.1016/0304-4165(87)90205-4. [DOI] [PubMed] [Google Scholar]
- 16.Hackette S L, Skye G E, Burton C, Segel I H. Characterization of an ammonium transport system in filamentous fungi with methylammonium-14C as the substrate. J Biol Chem. 1970;245:4241–4250. [PubMed] [Google Scholar]
- 17.Henning R. pH gradient across the lysosomal membrane generated by selective cation permeability and Donnan equilibrium. Biochim Biophys Acta. 1975;401:307–316. doi: 10.1016/0005-2736(75)90314-4. [DOI] [PubMed] [Google Scholar]
- 18.Hodgman C D, Weast R C, Selby S M, editors. Handbook of chemistry and physics, fortieth ed. Cleveland, Ohio: Chemical Rubber Publishing Co.; 1958. [Google Scholar]
- 19.Howitt S M, Udvardi M K. Structure, function and regulation of ammonium transporters in plants. Biochim Biophys Acta. 2000;1465:152–170. doi: 10.1016/s0005-2736(00)00136-x. [DOI] [PubMed] [Google Scholar]
- 20.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs P, Jauniaux J C, Grenson M. A cis-dominant regulatory mutation linked to the argB-argC gene cluster in Saccharomyces cerevisiae. J Mol Biol. 1980;139:691–704. doi: 10.1016/0022-2836(80)90055-8. [DOI] [PubMed] [Google Scholar]
- 22.Jahns T, Kaltwasser H. Uptake and metabolism of methylammonium by Pseudomonas aeruginosa. FEMS Microbiol Lett. 1990;60:131–135. doi: 10.1016/0378-1097(90)90359-x. [DOI] [PubMed] [Google Scholar]
- 23.Jayakumar A, Schulman I, MacNeil D, Barnes E M. Role of the Escherichia coli glnALG operon in regulation of ammonium transport. J Bacteriol. 1986;166:281–284. doi: 10.1128/jb.166.1.281-284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones J G, Bellion E. In vivo 13C and 15N NMR studies of methylamine metabolism in Pseudomonas species MA. J Biol Chem. 1991;266:11705–11713. [PubMed] [Google Scholar]
- 25.Jones J G, Bellion E. Methylamine metabolism in Hansenula polymorpha: an in vivo 13C and 31P nuclear magnetic resonance study. J Bacteriol. 1991;173:4959–4969. doi: 10.1128/jb.173.16.4959-4969.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadner R J. Cytoplasmic membrane. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 58–87. [Google Scholar]
- 27.Kane P M, Yamashiro C T, Stevens T H. Biochemical characterization of the yeast vacuolar H+-ATPase. J Biol Chem. 1989;264:19236–19244. [PubMed] [Google Scholar]
- 28.Katsu T, Akagi M, Hiramatsu T, Tsuchiya T. Determination of the pH differences across a cell membrane using a methylammonium-selective membrane electrode. Analyst. 1998;123:1369–1372. doi: 10.1039/a801686k. [DOI] [PubMed] [Google Scholar]
- 29.Kitamoto K, Yoshizawa K, Ohsumi Y, Anraku Y. Dynamic aspects of vacuolar and cytosolic amino acid pools of Saccharomyces cerevisiae. J Bacteriol. 1988;170:2683–2686. doi: 10.1128/jb.170.6.2683-2686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Legerton T L, Kanamori K, Weiss R L, Roberts J D. Measurements of cytoplasmic and vacuolar pH in Neurospora using nitrogen-15 nuclear magnetic resonance spectroscopy. Biochemistry. 1983;22:899–903. doi: 10.1021/bi00273a029. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, Chen Y, Mo R, Hui C, Cheng J F, Mohandas N, Huang C H. Characterization of human RhCG and mouse Rhcg as novel nonerythroid Rh glycoprotein homologues predominantly expressed in kidney and testis. J Biol Chem. 2000;275:25641–25651. doi: 10.1074/jbc.M003353200. [DOI] [PubMed] [Google Scholar]
- 32.Makarow M, Nevalainen L T. Transport of a fluorescent macromolecule via endosomes to the vacuole in Saccharomyces cerevisiae. J Cell Biol. 1987;104:67–75. doi: 10.1083/jcb.104.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manolson M F, Proteau D, Preston R A, Stenbit A, Roberts B T, Hoyt M A, Preuss D, Mulholland J, Botstein D, Jones E W. The VPH1 gene encodes a 95-kDa integral membrane polypeptide required for in vivo assembly and activity of the yeast vacuolar H+-ATPase. J Biol Chem. 1992;267:14294–14303. [PubMed] [Google Scholar]
- 34.Manolson M F, Wu B, Proteau D, Taillon B E, Roberts B T, Hoyt M A, Jones E W. STVI gene encodes functional homologue of 95-kDa yeast vacuolar H+-ATPase subunit Vph1p. J Biol Chem. 1994;269:14064–14074. [PubMed] [Google Scholar]
- 35.Marini A-M, Soussi-Boudekou S, Vissers S, André B. A family of ammonium transporters in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:4282–4293. doi: 10.1128/mcb.17.8.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marini A-M, Urrestarazu A, Beauwens R, Bruno A. The Rh (Rhesus) blood group polypeptides are related to NH4+ transporters. Trends Biochem Sci. 1997;22:460–461. doi: 10.1016/s0968-0004(97)01132-8. [DOI] [PubMed] [Google Scholar]
- 37.Marini A-M, Matassi G, Raynal V, André B, Cartron J P, Cherif-Zahar B. The human rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat Genet. 2000;26:341–344. doi: 10.1038/81656. [DOI] [PubMed] [Google Scholar]
- 38.Marini A-M, Springael J Y, Frommer W B, André B. Cross-talk between ammonium transporters in yeast and interference by the soybean SAT1 protein. Mol Microbiol. 2000;35:378–385. doi: 10.1046/j.1365-2958.2000.01704.x. [DOI] [PubMed] [Google Scholar]
- 39.Meier-Wagner J, Nolden L, Jakoby M, Siewe R, Kraemer R, Burkovski A. Multiplicity of ammonium uptake systems in Corynebacterium glutamicum: role of Amt and AmtB. Microbiology (United Kingdom) 2001;147:135–143. doi: 10.1099/00221287-147-1-135. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell A P, Magasanik B. Three regulatory systems control production of glutamine synthetase in Saccharomyces cerevisiae. Mol Cell Biol. 1984;4:2767–2773. doi: 10.1128/mcb.4.12.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montesinos M L, Muro-Pastor A M, Herrero A, Flores E. Ammonium/methylammonium permeases of a Cyanobacterium. J Biol Chem. 1998;273:31463–31470. doi: 10.1074/jbc.273.47.31463. [DOI] [PubMed] [Google Scholar]
- 42.Nelson N, Perzov N, Cohen A, Hagai K, Padler V, Nelson H. The cellular biology of proton-motive force generation by V-ATPases. J Exp Biol. 2000;203:89–95. doi: 10.1242/jeb.203.1.89. [DOI] [PubMed] [Google Scholar]
- 43.Nishimura K, Igarashi K, Kakinuma Y. Proton gradient-driven nickel uptake by vacuolar membrane vesicles of Saccharomyces cerevisiae. J Bacteriol. 1998;180:1962–1964. doi: 10.1128/jb.180.7.1962-1964.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nissani E, Ginsburg H. Protonophoric effects of antimalarial drugs and alkylamines in Escherichia coli membranes. Biochim Biophys Acta. 1989;978:293–298. doi: 10.1016/0005-2736(89)90127-2. [DOI] [PubMed] [Google Scholar]
- 45.Ohkuma S, Poole B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol. 1981;90:656–664. doi: 10.1083/jcb.90.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohsumi Y, Anraku Y. Active transport of basic amino acids driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1981;256:2079–2082. [PubMed] [Google Scholar]
- 48.Pao S S, Paulsen I T, Saier M H., Jr Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plant P J, Manolson M F, Grinstein S, Demaurex N. Alternative mechanisms of vacuolar acidification in H+-ATPase-deficient yeast. J Biol Chem. 1999;274:37270–37279. doi: 10.1074/jbc.274.52.37270. [DOI] [PubMed] [Google Scholar]
- 50.Preston R A, Murphy R F, Jones E W. Assay of vacuolar pH in yeast and identification of acidification-defective mutants. Proc Natl Acad Sci USA. 1989;86:7027–7031. doi: 10.1073/pnas.86.18.7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rapp B J, Landrum D C, Wall J D. Methylammonium uptake by Rhodobacter capsulatus. Arch Microbiol. 1986;146:134–141. [Google Scholar]
- 52.Roon R J, Even H L, Dunlop P, Larimore F L. Methylamine and ammonia transport in Saccharomyces cerevisiae. J Bacteriol. 1975;122:502–509. doi: 10.1128/jb.122.2.502-509.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roon R J, Even H L, Dunlop P, Larimore F L. Negative interactions between amino acid and methylamine/ammonia transport systems of Saccharomyces cerevisiae. J Biol Chem. 1977;252:3599–3604. [PubMed] [Google Scholar]
- 54.Roos W, Schulze R, Steighardt J. Dynamic compartmentation of vacuolar amino acids in Penicillium cyclopium. J Biol Chem. 1997;272:15849–15855. doi: 10.1074/jbc.272.25.15849. [DOI] [PubMed] [Google Scholar]
- 55.Rothman J H, Yamashiro C T, Raymond C K, Kane P M, Stevens T H. Acidification of the lysosome-like vacuole and the vacuolar H+-ATPase are deficient in two yeast mutants that fail to sort vacuolar proteins. J Cell Biol. 1989;109:93–100. doi: 10.1083/jcb.109.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rottenberg H. Proton electrochemical potential gradient in vesicles, organelles, and prokaryotic cells. Methods Enzymol. 1989;172:63–84. doi: 10.1016/s0076-6879(89)72008-5. [DOI] [PubMed] [Google Scholar]
- 57.Schneiter R, Bruegger B, Sandhoff R, Zellnig G, Leber A, Lampl M, Athenstaedt K, Hrastnik C, Eder S, Daum G, Paltauf F, Wieland F T, Kohlwein S D. Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J Cell Biol. 1999;146:741–754. doi: 10.1083/jcb.146.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Servin-Gonzalez L, Ortiz M, Gonzalez A, Bastarrachea F. glnA mutations conferring resistance to methylammonium in Escherichia coli. J Gen Microbiol. 1987;133:1631–1639. doi: 10.1099/00221287-133-6-1631. [DOI] [PubMed] [Google Scholar]
- 59.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 60.Siewe R M, Weil B, Burkovski A, Eikmanns B J, Eikmanns M, Kramer R. Functional and genetic characterization of the (methyl)ammonium uptake carrier of Corynebacterium glutamicum. J Biol Chem. 1996;271:5398–5403. doi: 10.1074/jbc.271.10.5398. [DOI] [PubMed] [Google Scholar]
- 61.Solheim A E, Seglen P O. Cellular and lysosomal uptake of methylamine in isolated rat hepatocytes. Biochem J. 1983;210:929–936. doi: 10.1042/bj2100929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soupene E, He L, Yan D, Kustu S. Ammonia acquisition in enteric bacteria: physiological role of the ammonium/methylammonium transport B (AmtB) protein. Proc Natl Acad Sci USA. 1998;95:7030–7034. doi: 10.1073/pnas.95.12.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tuller G, Nemec T, Hrastnik C, Daum G. Lipid composition of subcellular membranes of an FY1679-derived haploid yeast wild-type strain grown on different carbon sources. Yeast. 1999;15:1555–1564. doi: 10.1002/(SICI)1097-0061(199910)15:14<1555::AID-YEA479>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 64.van Heeswijk W C, Hoving S, Molenaar D, Stegeman B, Kahn D, Westerhoff H V. An alternative PII protein in the regulation of glutamine synthetase in Escherichia coli. Mol Microbiol. 1996;21:133–146. doi: 10.1046/j.1365-2958.1996.6281349.x. [DOI] [PubMed] [Google Scholar]
- 65.von Wiren N, Gazzarrini S, Gojon A, Frommer W B. The molecular physiology of ammonium uptake and retrieval. Curr Opin Plant Biol. 2000;3:254–261. [PubMed] [Google Scholar]
- 66.Weisman L S, Bacallao R, Wickner W. Multiple methods of visualizing the yeast vacuole permit evaluation of its morphology and inheritance during the cell cycle. J Cell Biol. 1987;105:1539–1547. doi: 10.1083/jcb.105.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wiame J M, Grenson M, Arst H N., Jr Nitrogen catabolite repression in yeasts and filamentous fungi. Adv Microb Physiol. 1985;26:1–88. doi: 10.1016/s0065-2911(08)60394-x. [DOI] [PubMed] [Google Scholar]
- 68.Yamashiro C T, Kane P M, Wolczyk D F, Preston R A, Stevens T H. Role of vacuolar acidification in protein sorting and zymogen activation: a genetic analysis of the yeast vacuolar proton-translocating ATPase. Mol Cell Biol. 1990;10:3737–3749. doi: 10.1128/mcb.10.7.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yelin R, Rotem D, Schuldiner S. EmrE, a small Escherichia coli multidrug transporter, protects Saccharomyces cerevisiae from toxins by sequestration in the vacuole. J Bacteriol. 1999;181:949–956. doi: 10.1128/jb.181.3.949-956.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]