Abstract
In 2015, we provided an overview of the use of digital technologies in clinical trials, both as a methodological tool and as a mechanism to deliver interventions. At that time, there was limited guidance and limited use of digital technologies in clinical research. However, since then smartphones have become ubiquitous and digital health technologies have exploded. This paper provides an update to our earlier publication and an overview of how technology has been used in the past five years in clinical trials, providing examples with varying levels of technological integration and across different health conditions. Digital technology integration ranges from the incorporation of artificial intelligence in diagnostic devices to the use of real-world data (e.g., electronic health records) for study recruitment. Clinical trials can now be conducted entirely virtually, eliminating the need for in-person interaction. Much of the published research demonstrates how digital approaches can improve the design and implementation of clinical trials. While challenges remain, progress over the last five years is encouraging, and barriers can be overcome with careful planning.
Keywords: clinical trials, digital technology, smartphones, electronic health records, artificial intelligence
1.0. INTRODUCTION
Who remembers checking the yellow pages to find a local plumber? Or making a collect call home? Today most of us own at least one digital device (desktop computer/laptop, cell phone/smartphone, tablet, smartwatch) and use the Internet to communicate, seek information, and conduct many other activities throughout a typical day. Further, a growing number of individuals are using smartphones to stay online almost constantly, and social media is the means by which many people connect, socialize, and share news (Pew Research Center, 2019b & 2019c). In the last decade, a variety of digital technologies (electronic technologies that utilize mobile devices and/or the Internet) have emerged that facilitate the delivery of healthcare around the world (Bhavnani et al., 2016; Agrawal & Prabakaran, 2020; Trifan et al., 2019). Development and use of digital tools, such as mobile health (mHealth), wearable devices, telehealth, and other information technologies, continues to grow and offer innovative approaches to health care. This has been particularly pronounced during the current COVID-19 pandemic where the use of weekly virtual health care visits for Medicare beneficiaries increased from 13,000 before the COVID-19 pandemic to 1.7 million in April 2020 (Verma, 2020).
Randomized controlled trials (RCTs) are considered the gold standard for providing evidence of the effectiveness of clinical interventions for the prevention and treatment of human conditions and diseases where there is a need for pharmacologic, behavioral, or combination approaches. RCTs using traditional in-person approaches are often highly complex, expensive, time-consuming, and burdensome for both staff and participants (Califf & Rutherford, 2018). Five years ago, we provided an overview of the use of digital technologies in clinical trials, specifically in relation to participant recruitment and retention, delivery of interventions, and data collection (Rosa et al., 2015). We outlined the promise of digital technologies in designing and implementing clinical trials (e.g., to reach a broader population of potential participants and make trials more efficient and less costly) but concluded that most researchers were not incorporating digital technology into clinical trials at that time. Challenges to technology adoption in clinical trials were largely related to concerns about participant privacy/confidentiality, ensuring an adequate infrastructure, confirming participant identity, and assuring the accuracy of data. Despite these challenges, we noted that access to the Internet and mobile technologies was increasing steadily and that most individuals of all ages were using technology in their daily lives, providing strong support for leveraging technology to advance clinical trial conduct.
In the five years since our overview was published, digital health technologies have exploded. The majority of the world now has access to mobile devices, totaling about 8 billion mobile phone subscriptions worldwide (Jonsson et al., 2019), and access is high even among the most traditionally underserved and vulnerable populations (Collins et al., 2016; Naslund et al., 2017). Hence, it is not surprising that digital technologies are increasingly being integrated into clinical trials operations. The integration of digital technologies into clinical trials ranges from the incorporation of artificial intelligence in diagnostic devices (Abramoff et al., 2018) to the use of real-world data (e.g., electronic health records (EHR)) to trial outcomes (Ma, et al 2019). A growing number of clinical trials can also be conducted completely virtually, without any in-person interactions (DeFrancesco, 2013; Turakhia et al., 2019, Steinhubl et al., 2019; Inan et al., 2020). Challenges to technology adoption (such as issues with participant privacy/confidentiality and data accuracy) still remain, however some these challenges have been addressed through the emergence of ethical, regulatory, privacy, and data standards, as well as new technology that addresses data integrity. Many Institutional Review Boards (IRBs) and regulatory agencies have issued guidance on the use of digital tools for clinical studies. Also, guidance is available for investigators interested in, and utilizing data from, existing registries and administrative databases (James et al., 2015; U.S. FDA, 2018; U.S. FDA, 2016).
In this paper, we use the term “clinical trial” as it is defined by clinicaltrials.gov: a study that involves human research participants to add to medical knowledge (including both interventional and observational studies) (U.S. National Library of Medicine, n.d.). We conducted a review of select published clinical trials within the last five years that used and/or discussed some form of digital tool to illustrate progress in incorporating digital technologies, as well as highlighting ongoing challenges. We also considered publications from observational studies, literature reviews, and expert perspectives that we believe will be beneficial to investigators planning to use digital technologies in their trials. The purpose of this paper is to provide a general overview of the use of digital technologies in clinical trials and other clinical research conducted in the past five years and to provide a foundation for the application of these technologies for clinical researchers interested in incorporating them into their work. The objectives are to (1) present a range of examples of how technology is currently being integrated into clinical trials, highlighting innovative digital tools and practices that could advance their utilization in clinical trials processes; (2) provide an overview of ethical considerations and data standards in the conduct of clinical trials using digital therapeutics; and (3) discuss the potential advantages and limitations of digital technologies in clinical trials research.
2.0. INTEGRATING DIGITAL TECHNOLOGIES INTO CLINICAL TRIAL DESIGN AND OPERATIONS
Clinical trials are the cornerstone for generating evidence, however current trials are still overly costly, complex, and lengthy (Lauer et al., 2017; Califf & Rutherford, 2018). Digital technologies offer opportunities and solutions that can lead to reductions in cost, minimize complexity, and reduce burden (NASEM, 2019; Steinhubl et al., 2019; Ali et al. 2020). In this section, we discuss digital technologies that could be used for clinical trial procedures such as recruitment and enrollment, participant consent, delivery of interventions, data collection, and data management. We provide some examples from recent studies of largely phase III clinical trials and observational studies that utilize digital technologies. These examples cover multiple health conditions and include studies that are completely virtual (i.e., no in-person procedures), as well as those that use a combination of digital and traditional approaches for trial operations. Traditional approaches usually require participants to travel to study clinics for recruitment, delivery of study interventions, data collection, and other study procedures.
The authors of these papers provide their experiences, best practices, recommendations, and/or limitations that could be useful to other researchers making decisions about the use of digital tools in their studies. We also reviewed important resources that provide guidance, recommendations, and best practices including Clinical Trials Transformation Initiative (CTTI) Digital Health Trials Program (CTTI, n.d.) with recommendations for the use of mobile technology and electronic records and programs from regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) (U.S. FDA, 2020; U.S. FDA, 2018; U.S. FDA, 2017; U.S. FDA, 2017b; U.S. FDA, 2016; EMA, n.d.).
This literature underscores the need for study teams to plan as carefully and as early as possible, as there is no single best approach that applies to all types of clinical trial design. The goal is not to simply transform processes from paper to digital form, but rather to rethink the overall design and conduct of a trial from a digital perspective and re-engineer aspects of the trial related to participants (Steinhubl et al., 2019). It is important to recognize the need for appropriate infrastructure to support these digital trials and to consider the cost of investing in such infrastructure. We provide considerations and some best practices when planning a clinical trial that uses digital technology in Table I. These considerations are a combination of our own perspectives after reviewing the selected literature and elements adapted from CTTI Digital Health Trials Program white papers and recommendations (CTTI, n.d.).
Table 1.
Best Practice Considerations When Desgining Trials Using Digital Technologies1
| • Select technologies based on study research questions, intervention, endpoints (what is going to be measured), population targeted and overall design features. |
| • Expand trial teams to include technical, regulatory, clinical, operations, and patient expertise. Use their wisdom during study design, as well as implementation and data monitoring. |
| • Consult with leadership officials regarding critical infrastructure to support digital technology needs. |
| ○ Need for hardware, software, equipment, physical space, critical expertise, training, etc. needed to support the digital tools, maintain participant safety and well- being and ensure data privacy and accuracy. |
| • Follow a user-centered approach in study design, considering technology development and refinement before technology tools are selected (include participants and relevant clinical staff). |
| ○ Be mindful of potential participants preferences, as well as issues regarding geographical challenges in accessing Internet. |
| • Consider/anticipate participant privacy/confidentiality issues when selecting digital tools. |
| • Prepare data and safety monitoring plans considering the technology used and potential issues that could impact participant safety, privacy, and wellbeing, as well as risks to data integrity. For example, consider issues like technology updates, server hacking, data sharing, and potential loss of study data. |
| • Sometimes the best approach could be a combination of digital approaches (wearables, smartphones, web portals, virtual reality, mobile apps, telehealth, social media, electronic records, registries, machine learning, etc.) with more traditional activities requiring participants to visit a study site (sample collection, medical procedures, measurements, interventions). |
| • Consider regulatory status and IRB requirements. |
| ○ Review local IRB guidelines regarding e Consent and using digital tools. Consult with IRB early on, describe plans, and discuss potential issues that may impact study timeline. It is important to discuss procedures and concerns when the investigator is seeking waiver of consent |
| ○ Consult drug/devices approval regulations with appropriate regulatory agencies for trials using technology for the purpose of drugs/devices approval. |
| ■ FDA/OHRP/EMA have issued key documents to assist investigators on this area. |
| • Conduct a feasibility study prior to the main trial to understand access to and preferences for technology use in your target population and study staff, as well as troubleshoot infrastructure issues and capacities at study site. Often, the best tool(s) are those that simplify study operations for the user. |
| • Collect only the minimum data necessary for study endpoints; be mindful of big data challenges as well as issues with data quality. |
Adapted from the Clinical Trials Transformation Initiative (CTTI) Digital Health Trials Program (https://www.ctti-clinicaltrials.org/programs/mobile-clinical-trials)
One key aspect to consider when planning a trial is the recruitment and retention of participants. Recruitment is considered the main cause of delays in a clinical trial, and difficulties in recruitment cause about 30% of study failures (Ali et al., 2020; Blatch-Jones, 2020). Fewer than 10% of patients in the U.S. enroll in a clinical trial, and rates of enrollment are even lower among some racial/ethnic communities (Thompson & O’Regan, 2018). A recently published paper (Frampton et al., 2020) provides a systematic map of digital tools used in the last 10 years for the purpose of recruitment and retention. Their review of 105 studies in the U.S and other countries provided characteristics of the digital technologies used and features of the studies that evaluated the effectiveness of these digital tools. Internet sites and social media were the tools most frequently used, largely for the purpose of raising awareness of the trials and assisting investigators in the identification of potential participants. Even though the authors concluded that more rigorous research is needed regarding the efficiency of the tools, their paper provides information regarding the types of technologies and approaches that may be useful to investigators when planning their clinical trials. Another study conducted in UK (Blatch-Jones, 2020) found that database-screening tools were the most widely used and effective for recruitment. Similar tools (social media, text messaging, direct e-mails) were also utilized for recruitment and retention, with various levels of effectiveness.
2.1. Mobile technologies
In our previous publication (Rosa, et al, 2015) we discussed a variety of digital tools that researchers have adopted for recruitment and retention, such as Internet, direct e-mail, texting and social media. Since then, researchers are increasingly using social media in clinical trials to improve outreach, and education (Watson, et al 2018, Poblete & Nieto, 2020; Maggio et al., 2019) as well as delivery of interventions (Park, 2019 & 2020, Radovic et al. (2017).
The general population is increasingly comfortable with engaging in a variety of activities on smartphones, including downloading and using health and wellness mobile apps (Carlo et al., 2019). Mobile technology (smartphone applications, wearable devices, telehealth) offers an opportunity to enhance the efficiency and reach of clinical trials processes, including remote consenting, evaluation of interventions, and collection of outcome data (Perry et al., 2018; Marra et al., 2020).
Electronic Consent.
Mobile technology is a promising tool for the informed consent process. It provides a platform that is easy to use and allows for broad reach of participants while preserving the informed consent principles (Simon, et al, 2018, Lunt, et al, 2019). A 2017 systematic review (Moore et al., 2017) was conducted on the use of electronic consenting (eConsent) for research apps, including information presented and shared, and privacy policies. The review summarizes the eConsent process and procedures to safeguard privacy in 34 app-based studies. Doerr and colleagues (2017) examined participant experience using a self-administered eConsent in the mPower study for Parkinson’s disease and identified useful lessons that could be incorporated as best practices. In a more recent publication, Doerr and colleagues describe creating an eConsent procedure in the All of Us study that takes into consideration participant perspectives as well as federal and local regulations (Doerr et al., 2019).
Intervention.
Several recent publications illustrate the use of mobile technology as a tool to deliver interventions and monitor study and medication adherence (Marsch et al., 2020; Blatch-Jones et al., 2020; Steinhubl et al., 2019; Holtyn et al., 2019). Many researchers are studying mental health, addiction, or other types of health behavior interventions delivered using mobile apps. As one example, Nomura et al. (2019) and Masaki et al. (2020) evaluated the use of an app, paired with an external device, for smoking cessation in a clinical trial. In their study, they compared the CureApp Smoking Cessation (CASC) system, which included the CASC smartphone app, web-based management software, and a mobile carbon monoxide checker, to a control group that received a minimally supportive app. Both groups (584 participants) received standard face-to-face counseling and pharmacological smoking cessation treatment for 12 weeks. The study concluded that the CASC system, combined with standard of care, improved continuous tobacco abstinence rates long-term. Similar promising outcomes from mobile interventions targeting other health domains have been well-documented. For example, Wilhelm and colleagues (2020) describe ways to use the Internet, phones, and apps to provide cognitive-behavioral therapy to those with mental health disorders.
Data Collection.
Mobile technologies offer an opportunity to capture, store, manage, and transmit large volumes of data, as well as for the collection of outcomes measured by mobile devices. However, as illustrated by Perry et al. (2018), investigators are not widely adapting technologies to collect outcome data in clinical trials. In their review, they categorize and consolidate information on using mobile devices to collect and assess outcomes. In addition, Cornet & Holden (2018) conducted a systematic review of smartphone-based passive sensing data (data captured directly from smartphone sensors) and reported benefits as well as challenges of using these tools. They also provide recommendations for investigators planning to use these approaches for data collection. Finally, Russell et al. (2020) discuss statistical and other considerations when selecting mobile technologies to measure outcomes.
Other studies are using innovative combinations of different mobile technologies to conduct trials, or combinations of technology and traditional in-person approaches for more hybrid designs. For example, the Adaptable study (Johnston et al., 2016; Pfaff et al., 2018) compared the effectiveness of two doses of aspirin in preventing ischemic adverse events, combining digital approaches (smartphone, mobile apps, EHR data source) with traditional in-person visits for recruitment and data collection. In contrast, in the Apple Heart study (Perez et al., 2019; Turakhia et al., 2019) used smartphones, mobile apps, sensors, wearable devices, and telehealth to conduct entirely remote operations and data collection with 420,000 individuals with atrial fibrillation.
Similarly, the mPower study (Bot et al., 2016) utilized a study app paired with wearable devices to conduct an observational trial with over 9,000 participants with Parkinson’s disease. The Million Veteran Program (Gaziano et al., 2016) and the All of Us study (Denny et al., 2019) are very large observational cohorts (1 million participants) that are using mobile technology for all study procedures and data collection. Once established, investigators could leverage this same structure and resources for future RCTs to answer critical questions and generate additional evidence. These mobile technology approaches are also being used to collect data for regulatory Phase IV trials (Li et al., 2016; Foroughi et al., 2018).
2.2. Electronic health records (EHR), registries, and administrative databases
The FDA’s definition of real world data (RWD), data relating to patient health status and/or the delivery of health care, notes a range of sources, including electronic health records (EHR), claims and billing databases, product and disease registries, and patient-generated data (U.S. FDA, n.d.). Though they were originally designed to support health care delivery, these sources provide rich opportunities for clinical trials (Cowie et al., 2017). RWD have mostly been used for observational studies, pragmatic comparative effectiveness trials, and surveillance studies (Kibbelaar et al, 2017). An advantage of RWD is that it provides quick and low-cost access to large amounts of clinical data with the potential to complement RCTs and help expedite RCT recruitment (Bartlett et al, 2019, Obeid et al., 2017).
EHRs and other sources of RWD can help improve the efficiency of clinical trials (Cowie et al., 2017; Lauer et al., 2017). Kibbelaar et al. (2017) and Foroughi et al. (2018) discussed the utility of registries (e.g., cancer registries, population-based registries) and advanced EHRs in providing high-quality data for observational oncology trials as well as proposing trials embedded in EHR and randomized registry trials. Studies previously mentioned (Adaptable, All of Us, and the Million Veteran Program) also utilized EHR data (Johnston et al., 2016; Denny et al., 2020; Gaziano et al., 2016). For the Adaptable study, longitudinal EHR data were matched with commercial claims data to improve objective study outcome measurement of service utilization, such as hospitalizations and deaths (Ma et al., 2019). Challenges to using EHR and other RWD may include variable selection, missing data, and/or poor data quality (see Section 4.0 below).
2.3. Artificial Intelligence (AI)
AI refers to the use of computers to simulate human brain processes for acquiring information, using the information, making interpretations or conclusions, and learning from the experience. Mak & Pichika (2019) describe AI as using technology tools to mimic human behavior. Sub-fields of AI are machine learning (ML) and deep learning (DL), where statistical methods are used for learning (Mak & Pichika, 2019). AI methodology has been applied to many areas of health care, including surgery, imaging, and disease diagnosis and treatment, and could be another tool for enhancing personalized medicine. AI can also be used in clinical trial design and patient identification, as well as trial monitoring (Jang, 2019). A few highlighted current applications of AI: (1) Lin et al. (2019) compared AI technology with provider assessment to determine AI’s efficacy in diagnosis and treatment decision-making for children with cataracts; (2) Roggeveen et al. (2019) plan to conduct a superiority trial using an AI system for antibiotic dosing, compared to standard dosing, for critically ill septic patients; and (3) Wijnberge et al. (2020) tested an AI application compared to standard protocols to reduce intraoperative hypotension.
2.4. Blockchain technology
Blockchain technology, originally associated with cryptocurrencies like Bitcoin, uses a decentralized, peer-to-peer computer network that enables databases to store encrypted time-stamped records and documents. Each server, or “node,” in the network processes and verifies each data entry, then archives all transactions, along with the history of every other transaction ever recorded to the network, creating a secure and immutable “chain” of content “blocks.” Applications of this technology in the health sciences are being explored to address some of the common threats to data integrity and security. In a 2018 review of over 300 studies on the use of blockchain technology in healthcare, Mayer and colleagues posit that moving EHRs from independent systems to a blockchain network could make health information sharing more efficient and secure, enabling better collaborative clinical decision-making and more patient control over privacy (Mayer, et al 2018). Blockchain is also being considered as a platform for clinical trials data management for many of the same reasons. Osipenko (2019) describes it “as a way of managing data that prevents its manipulation” and argues that it offers a technical solution that could improve transparency, security, quality, and efficiency of clinical trials. Others have discussed the potential of using this technology to improve clinical trials processes, such as adverse events reporting, trial data management, endpoints adjudication, adherence to reporting requirements, and data sharing (Benchoufi & Ravaud, 2017; Maslove, et al., 2018; Wong, et al., 2019; Zhuang et al., 2018).
3.0. ETHICAL, REGULATORY, PRIVACY, AND DATA STANDARDS CONSIDERATIONS
The primary ethical, human subjects, and legal concerns related to the use of digital tools in clinical research are ensuring proper informed consent procedures with electronic materials and protecting participant privacy/confidentiality when using digital technology (Jacobson et al., 2020). These important challenges and limitations require careful consideration and management. An important guide recently developed by the CTTI provides recommendations for addressing many of these data quality and privacy issues. (Herrington et al., 2018; CTTI, 2018). In addition, McKay and colleagues (2018) produced a systematic review technology effectiveness and quality of mobile technology data and Eagleson and colleagues (2017) describe ethical and privacy concerns.
3.1. Regulatory and ethical considerations
Just five years ago, there were few Institutional Review Board (IRB) guidelines for researchers related to the use of technology in research studies. We are pleased to report that numerous IRBs have now posted procedures and best practices with specific guidance on this subject. For example, Indiana University provides guidance for study investigators on the use of digital tools to facilitate recruitment, informed consent, and data collection (Indiana University, n.d.). Similarly, several other organizations have posted guidelines on the use of social media, mobile devices, and other digital tools. For example, the Center for Technology and Behavioral Health at Dartmouth College offers resources on informed consent (Center for Technology and Behavioral Health, n.d.). As previously noted, the FDA has also provided guidance on the use of electronic consent (U.S. FDA, 2016). To assist both IRBs and researchers, Torous and Nebeker (2017) describe the Connected and Open Research Ethics (CORE) initiative, a group intended to work on best practices and guidelines for the use of technology in clinical research. Further, Bloss and colleagues (2016) described their ideas on redesigning the current human research protection system.
Regulatory and human subject protections agencies in the U.S. and abroad have released guidance(s) on the use of digital technologies in clinical research. The FDA has developed guidance for sponsors, researchers, and IRBs on the use of electronic informed consent (2016) and using EHRs/electronic signatures (2017). The 2016 FDA guidance on using electronic informed consent was developed jointly with the U.S. Office for Human Research Protections (OHRP). OHRP also revised the human subject protection regulations (referred to as the Common Rule) that could be relevant to some areas of secondary research (U.S. OHRP, 2017/2018). In 2018, the FDA created a “real-world evidence” initiative to evaluate the potential of using RWD to support regulatory decisions for drugs and biological products (U.S. FDA, 2018). As part of this initiative, they provided useful definitions and examples of clinical studies and trials using registries and EHRs to generate evidence. The CTTI has also published recommendations on using RWD in clinical trials, especially focused on of study design and recruitment (CTTI, 2019). The FDA has published a Digital Health Innovation Plan for medical devices that outlines their approach to digital health products (U.S. FDA, 2020).
Outside of the U.S., the European Medicines Agency (EMA) released a report with recommendations regarding the use of social media and mobile health data for regulatory purposes (Donegan et al., n.d.). Additionally, in 2015 they established an initiative that provides regulatory recommendations on the use of patient registries in research (European Medicines Agency, n.d.; Olmo et al., 2019). Cave et al. (2019) also published recommendations for using RWD for regulatory purposes in Europe.
3.2. Privacy and data standards
The protection of participants’ data privacy and confidentiality has been an ongoing topic of concern (Thompson & O’Regan, 2018), and recent scandals surrounding the use of social media data are not inspiring confidence among the scientific community on the use of technology in clinical research. Apps do not always include a privacy policy or terms of agreement, and even when they do, the policy language is often at an inappropriate reading level and uses excessive jargon (Robillard et al. 2019). Furthermore, many app privacy policies are not transparent, leaving users unsure of how their data are shared with third parties (Grundy et al. 2019; Huckvalle et al., 2019). When using apps in RCTs, investigators should carefully review the app privacy and data sharing policies and ensure participant understanding during the consent process.
Data protections more broadly have also seen improved guidance in recent years. In 2017, Eagleson et al. provided recommendations on data protections, based on their experience using mobile technology, to include security measures against outside risks. Pagoto and Nebeker (2019) caution that there are few regulations for protecting participants’ data in research using social media, and users are usually unfamiliar with social media privacy policies. The authors propose the engagement and coordination of diverse disciplines/stakeholders to develop ethical standards, best practices, and resources for researchers. One particularly important stakeholder group is potential participants, given that their perspective could be different from that of individuals who conduct research. For example, somewhat surprisingly, a study conducted with social media users found that many do not view the monitoring of their accounts for the purposes of RCT recruitment as a violation of their privacy (Reuter et al., 2019). Finally, as mentioned previously, the CTTI (2018) provides recommendations on the use of mobile technology in clinical trials and includes strategies to ensure data security and data management. Blockchain technology, as noted above, could also be a useful solution to improve data security.
Regarding data standards, the Clinical Data Interchange Standards Consortium (CDISC) created definitions, models, and specifications for data representation in research (CDISC, n.d.). Regulatory agencies, such as the FDA, have adopted these standards, but adoption by the research community has been slower, especially when using data from EHRs or other RWD sources instead of regular electronic data capture systems. EHRs were created for clinical, billing, and operational purposes, not for research, and thus can present challenges when used in clinical trials, including data quality and validation issues, heterogeneity between health systems, data security, and privacy (Cowie et al., 2017). Ethier and colleagues (2017) discussed a solution to capture data from EHRs using CDISC standards to improve data quality for regulatory purposes. Finally, Agrawal & Prabakaran (2020), provide recommendations for analyzing large-scale unstructured data (“Big Data”) generated by digital technologies.
Data interoperability, particularly in countries where health care is privatized, continues to be problematic in multisite RCTs, and adoption of interoperability standards has been slow (Nordo et al., 2018). In the U.S., there have been efforts to encourage the adoption of Fast Healthcare Interoperability Resources (FHIR) standards for exchanging healthcare information electronically (Nordo et al., 2018). Several recent initiatives have focused on FHIR, including a review of the use of FHIR in digital health and identification of the main categories of use (e.g., EHRs, mobile and web applications, data protection) (Lehne et al., 2019) and the development of an approach for creating automated data capture from EHRs using FHIR standards (Zong et al., 2020).
4.0. ADVANTAGES, CHALLENGES, AND LIMITATIONS OF USING DIGITAL TECHNOLOGIES IN CLINICAL TRIALS
As detailed in our previous paper (Rosa et al., 2015), and by many of the authors referenced throughout this paper (e.g., Li et al., 2016; Lauer et al., 2017; Foroughi et al., 2017; Steinhubl et al., 2019), technology offers numerous advantages for clinical trials design and implementation efficiency. Digital technologies are currently used to assess study feasibility, facilitate participant recruitment and retention, enable access to diverse populations, streamline data collection, and facilitate data management. Advantages include lower cost, rapid enrollment, and more complete follow-up data resulting in greater generalizability of results. On the other hand, several challenges previously noted in our earlier publication and others (e.g., Cowie et al., 2017; Eagleson et al., 2017; Huckvalle et al., 2019; Pratap et al., 2020; Frampton et al., 2020) remain. Some of the main challenges include ethical and privacy issues as well as issues related to data quality. A list of the advantages and challenges are summarized below.
4.1. Advantages
reducing costs by reducing the time required for study enrollment,
increasing participation, including that of underrepresented racial/ethnic or socially disadvantaged groups, by increasing trial visibility, targeting online recruitment efforts to specific sub-populations, and reducing burden of travel to sties,
decreasing intervention delivery burden and supervision,
improving intervention fidelity when using smart apps and AI algorithms (i.e., Clinical Decision Support or CDS),
reducing data collection burden (for both participants and study staff) by utilizing data capture models with existing platforms (i.e., EHRs, registries, administrative databases, smartphones),
improving data quality, reproducibility, privacy and sharing when including Blockchain technology algorithms that facilitate data accuracy and security (Benchoufi 2017; Osipenko, 2019; Maslove et al., 2018),
improving analysis of big data when using AI and ML algorithms (Mak & Pichika, 2019).
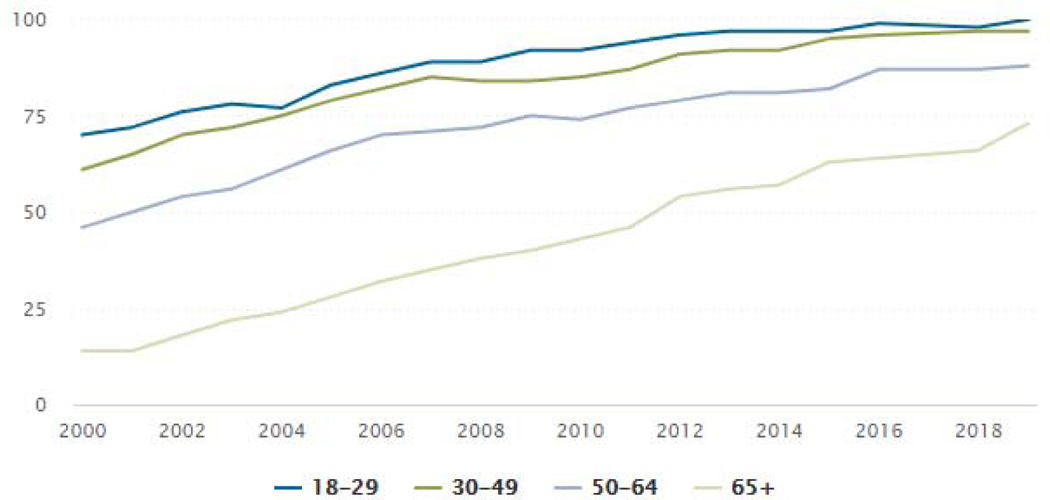
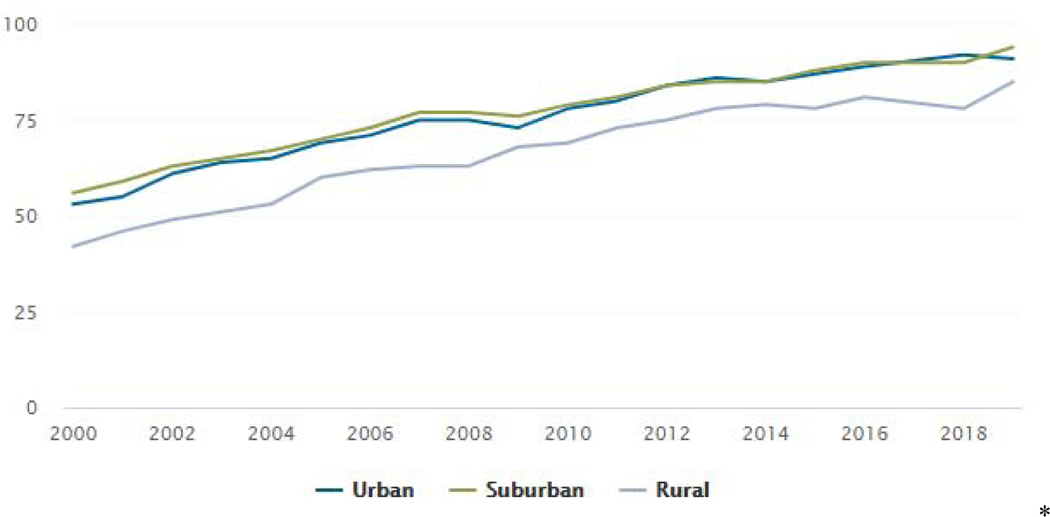
An advantage increasingly being realized is the potential for enrolling a more representative sample for trials, as the “digital divide” continues to decrease (see Figures 1 & 2). Data from 2018 show that more older individuals, as well as more individuals in rural communities, have access to the Internet via broadband or smartphones (Winstanley et al., 2018; Pew Research Center, 2019d). Arguably, the two most beneficial developments since our last report five years ago are the influx of available digital tools (e.g., AI advances and blockchain) that address some of the concerns related to data accuracy and participant privacy, as well as the advancement of clearer guidance from ethics and regulatory groups on incorporating digital technology approaches in the conduct of clinical trials.
Figure 1:
% of U.S. Adults Who Use the Internet, By Age Source: Pew Research Center, 2019d
Figure 2:
% of U.S. Adults Who Use the Internet, By Community Type Source: Pew Research Center, 2019d
4.2. Challenges and Limitations
maintaining privacy/confidentiality of participant data obtained by digital methods, including inadvertently sharing data with unknown third parties,
ensuring that regulatory guidance reflects the latest technological advances,
ensuring access to adequate infrastructure, resources, and staff expertise,
keeping participants engaged and increasing retention,
considering user attitudes, practices, expectations, and preferences, as potential participants may view using their data as a violation of their privacy,
ensuring data quality/accuracy given that, although standards and guidance/best practices have emerged, there are still concerns with data quality, completeness, and accuracy (particularly as digital tools are usually developed for use in other areas and need to be adapted for use in research, and most are not interoperable),
overcoming the complexities surrounding big data (ensuring mobile technologies capture adequate data and selecting specific outcome data from the digital sources).
Another challenge that remains is recruiting adequate, representative samples and avoiding sample bias. As mentioned in section 4.1, the “digital divide” has decreased in the last five years, however individuals from underserved groups and residing in rural areas of the U.S. are still underrepresented in clinical research and less likely to have home broadband access or own a smartphone than those residing in urban or suburban areas (Pew Research Center, 2019 & 2019c; Noonan & Simmons, 2020);
In addition, challenges may emerge as new strategies are implemented to protect data. For example, firewalls to protect data may prohibit Internet-connected statistical software packages from downloading updates and local security policies could conflict with funding agency requirements. As health care systems adopt more rigorous strategies to safeguard protected health information, these same strategies may severely restrict access and analysis for clinical studies.
4.3. Using digital tools during a health crisis
As we finish writing this article, the COVID-19 pandemic has been a catalyst to the expansion of the use of telehealth and other digital technologies (Keesara et al., 2020). Regulations prohibiting or limiting the delivery of in-person health care have forced providers to overcome barriers to technology adoption (Winstanley et al., 2020) and increasingly consider novel digital tools (Iyengar et al., 2020). This crisis has also disrupted clinical trials operations in the U.S and around the world. Most investigators and/or sponsors have temporarily suspended operations or redesigned them to take advantage of available digital tools for recruitment or delivering study procedures, when possible (Noonan & Simmons, 2020). The FDA and the EMA have both issued guidance recommending the use of alternative approaches to in-person visits (including remote and/or virtual tools) to maintain participant safety and to minimize risks to trial integrity (U.S. FDA, 2020; EMA, 2020). Noonan and Simmons (2020) argue that adoption of digital tools could be scaled up to continue research operations during the current crisis, and investigators should consider maintaining these tools and methods once restrictions on trials are lifted. AI approaches are also currently being applied to rapidly identify molecules that are the most promising for COVID-19 drug and vaccine development. In a matter of weeks, a global collaborative team that included engineers and physicists using supercomputers analyzed 1 billion possible molecules and selected the 30 most promising ones for further drug development consideration (University of Texas at Austin, 2020). This public health crisis has further underscored the opportunities for leveraging digital technologies in clinical research and practice.
5.0. CONCLUSIONS
The selected literature cited in this paper illustrates how digital technologies can improve certain aspects of the design and implementation of clinical trials, and we encourage continued innovation in this area to optimize clinical trial efficiency. The rapid integration of digital approaches into clinical care and research during the COVID-19 crisis reflects the progress in this arena and presents an opportunity to better evaluate the value of digital tools used by researchers to enable continuation of study operations during the pandemic. Many challenges remain, including: (1) the impact of the persisting digital divide on recruitment of adequate, representative individuals; (2) privacy and confidentiality of health information shared over the Internet; and (3) technical and cultural barriers regarding data integrity and technology advances. In addition, some clinical trial procedures and operations may not be ready for a digital approach.
We recognize that this paper is not a systematic review of the literature on this topic and that the efficiency, advantages, and limitations of using digital tools should be rigorously studied in order to evaluate their actual performance. We instead provide an update to our publication in 2015 on the value of using digital technologies in clinical research, as well as continued challenges and limitations. The selected research published in the last five years demonstrates the successful application of digital approaches to research, covering a wide spectrum of diseases/conditions and providing useful experiences and best practices, as well as methods that can be used to optimize the design and conduct of clinical trials.
Acknowledgments
Funding
This work was supported by grants from the National Institute on Drug Abuse: UG1DA040309; UG1DA013035 and P30DA029926
Footnotes
Conflicts of interest:
Author LAM is affiliated with Pear Therapeutics, Inc., HealthSim, LLC, and Square2 Systems, Inc. Conflicts of interest are extensively managed by her academic institution, Dartmouth College.
CR, ELW, MB and ANCC report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abramoff MD, Lavin PT, Birch M, Shah N, & Folk JC (2018). Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. npj Digital Medicine, 1, 39. doi: 10.1038/s41746-018-0040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal R. & Prabakaran S. (2020). Big data in digital healthcare: lessons learnt and recommendations for general practice. Heredity, 124, 525–534. doi: 10.1038/s41437-020-0303-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Z, Zibert JR, Thomsen SF (2020) Virtual Clinical Trials: Perspectives in Dermatology. Dermatology 236:375–382. doi: 10.1159/000506418 [DOI] [PubMed] [Google Scholar]
- Bartlett VL, Dhruva SS, Shah ND, Ryan P, & Ross JS (2019). Feasibility of using real-world data to replicate clinical trials evidence. JAMA Network Open, 2(10), e1912869. doi: 10.1001/jamanetworkopen.2019.12869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchoufi M, & Ravaud P. (2017). Blockchain technology for improving clinical research quality. Trials, 18(1), 335. doi: 10.1186/s13063-017-2035-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavnani SP, Narula J, & Sengupta PP (2016). Mobile technology and the digitization of healthcare. European Heart Journal, 37(18), 1428–1438. doi: 10.1093/eurheartj/ehv770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatch-Jones A, Nuttall J, Bull A, Worswick L, Mullee M, Peveler R, Falk S, Tape N, Hinks J, Lane AJ, Wyatt JC, & Griffiths G. (2020). Using digital tools in the recruitment and retention in randomized controlled trials: Survey of UK Clinical Trials Units and a qualitative study. Trials, 21, 304. doi: 10.1186/s13063-020-04234-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss C, Nebeker C, Bietz M, Bae D, Bigby B, Devereaux M, Fowler J, Waldo A, Weibel N, Patrick K, Klemmer S, & Melichar L. (2016). Reimagining human research protections for 21st century science. Journal of Medical Internet Research, 18(12), e329. doi: 10.2196/jmir.6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bot BM, Suver C, Neto EC, Kellen M, Klein A, Bare C, Doerr M, Pratap A, Wilbanks J, Dorsey ER, Friend SH, & Trister AD (2016). The mPower study, Parkinson disease mobile data collected using ResearchKit. Scientific Data, 3, 160011. doi: 10.1038/sdata.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Califf R. & Rutherford JD (2018). Reflections on the clinical research enterprise: past, present and future. Circulation, 138, 1765–1770. doi: 10.1161/CIRCULATIONAHA.118.037900 [DOI] [PubMed] [Google Scholar]
- Carlo AD, Ghomi RH, Renn BN, & Arean PA (2019). By the numbers: ratings and utilization of mobile applications. npj Digital Medicine, 2, 54. doi: 10.1038/s41746-019-0129-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave A, Kurz X, & Arlett P. (2019). Real-world data for regulatory decision making: Challenges and possible solutions for Europe. Clinical Pharmacology & Therapeutics, 106(1), 36–39. doi: 10.1002/cpt.1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Technology and Behavioral Health. (n.d.). Sample consent. Retrieved April 30, 2020. https://www.c4tbh.org/resources/sample-consent-forms/
- Clinical Data Interchange Standards Consortium (CDISC). (n.d.). Academic researcher. Retrieved April 30, 2020. https://www.cdisc.org/new-to-cdisc/academic
- Clinical Trials Transformation Initiative (CTTI). (2019). CTTI Recommendations: Use of Real-World Data to Plan Eligibility Criteria and Enhance Recruitment. Durham, NC: CTTI, 15p. Retrieved April 30, 2020. https://www.ctti-clinicaltrials.org/sites/www.ctti-clinicaltrials.org/files/rwd-recommendamtions_final.pdf [Google Scholar]
- Clinical Trials Transformation Initiative (CTTI). (2018). Recommendations executive summary: Advancing the use of mobile technologies for data capture & improved clinical trials. Retrieved April 30, 2020. https://www.ctti-clinicaltrials.org/sites/www.ctti-clinicaltrials.org/files/mobile-technologies-executive-summary.pdf
- Clinical Trials Transformation Initiative (CTTI). (n.d.). Program: Digital Health Trials. Retrieved April 30, 2020. https://www.ctti-clinicaltrials.org/programs/mobile-clinical-trials
- Collins KM, Armenta RF, Cuevas-Mota J, Liu L, Strathdee SA, & Garfein RS (2016). Factors associated with patterns of mobile technology use among persons who inject drugs. Substance Abuse, 37(4), 606–612. doi: 10.1080/08897077.2016.1176980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet VP, & Holden RJ (2018). Systematic review of smartphone-based passive sensing for health and wellbeing. Journal of Biomedical Informatics, 77, 120–132. doi: 10.1016/j.jbi.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie MR, Blomster JI, Curtis LH, Duclaux S, Ford I, Fritz F, Goldman S, Janmohamed S, Kreuzer J, Leenay M, Michel A, Ong S, Pell JP, Southworth MR, Stough WG, Thoenes M, Zannad F, & Zalewski A. (2017). Electronic health records to facilitate clinical research. Clinical Research in Cardiology, 106, 1–9. doi: 10.1007/s00392-016-1025-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFrancesco L. (2013). FDA okays IT-powered trial. Nature Biotechnology, 31, 184. doi: 10.1038/nbt0313-184a [DOI] [Google Scholar]
- Denny JC, Rutter JL, Goldstein DB, Philippakis A, Smoller JW, Jenkins G, & Dishman E. (2020). The “All of Us” research program. New England Journal of Medicine, 381, 668–676. doi: 10.1056/NEJMsr1809937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr M, Grayson S, Moore S, Suver C, Wilbanks J, & Wagner J. (2019). Implementing a universal informed consent process for the All of Us research program. Pacific Symposium on Biocomputing, 24, 427–438. [PMC free article] [PubMed] [Google Scholar]
- Doerr M, Truong AM, Bot BM, Wilbanks J, Suver C, & Mangravite LM (2017). Formative evaluation of participant experience with mobile econsent in the app-mediated Parkinson mPower study: A mixed methods study. JMIR mHealth and uHealth, 5(2), e14. doi: 10.2196/mhealth.6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan K, Ovelgonne H, Flores G, Fuglerud P, & Georgescu A. (n.d.). Social Media and M-Health Data. Amsterdam, The Netherlands: European Medicines Agency, 29p. Retrieved April 30, 2020. https://www.ema.europa.eu/en/documents/report/social-media-m-health-data-subgroup-report_en.pdf [Google Scholar]
- Eagleson R, Altamirano-Diaz L, McInnis A, Welisch E, De Jesus S, Prapavessis H, … Norozi K. (2017). Implementation of clinical research trials using web-based and mobile devices: challenges and solutions. BMC Medical Research Methodology, 17(1), 43. doi: 10.1186/s12874-017-0324-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier J, Curcin V, McGilchrist MM, Lim Choi Keung LC, Zhao L, Andreasson A, Brodkar P, Michalskir R, Arvanitis TN, Mastellos N, Burgun A, & Delaney BC (2017). eSource for clinical trials: Implementation and evaluation of a standards-based approach in a real world trial. International Journal of Medical Informatics, 106, 17–24. doi: 10.1016/j.ijmedinf.2017.06.006 [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. (n.d.). Patient registries. Retrieved April 30, 2020. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/patient-registries
- European Medicines Agency. (n.d.). Guidance on the management of clinical trials during the covid-19 (coronavirus) pandemic. Retrieved April 30, 2020. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-10/guidanceclinicaltrials_covid19_en.pdf
- Foroughi S, Wong H, Gately L. Lee M, Simons K, Tie J, Burgess AW, & Gibbs P. (2018). Re-inventing the randomized controlled trial in medical oncology: The registry-based trials. Asia-Pacific Journal of Clinical Oncology, 14, 365–373. doi: 10.111/ajco.12992 [DOI] [PubMed] [Google Scholar]
- Frampton GK, Shepherd J, Pickett K, Griffiths G, & Wyatt JC (2020). Digital tools for the recruitment and retention of participants in randomized controlled trials: a systematic map. Trials, 21, 478. doi: 10.1186/s13063-020-04358-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaziano JM, Concatoc J, Brophya M, Fiorea L, Pyarajana S, Breelinga J, Whitbourne S, Deena J, Shannona C, Humphries D, Guarinoc P, Aslanc M, Anderson D, LaFleur R, Hammond T, Schaaf K, Moser J, Huang G, Muralidhar S, & Przygodzkif R. (2016). Million Veteran Program: a mega-biobank to study genetic influences on health and disease. Journal of Clinical Epidemiology, 70, 214–223. doi: 10.1016/j.jclinepi.2015.09.016 [DOI] [PubMed] [Google Scholar]
- Grundy Q, Chiu K, Held F, Continella A, Bero L, & Holz R. (2019). Data sharing practices of medicines related apps and the mobile ecosystem: traffic, content, and network analysis. BMJ, 364, I920. doi: 10.1136/bmj.l920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington WG, Goldsack JC, & Landray MJ (2018). Increasing the use of mobile technology-derived endpoints in clinical trials. Clinical Trials, 15(3), 313–315. doi: 10.1177/1740774518755393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtyn AF, Bosworth E, Marsch LA, McLeman B, Meier A, Saunders EC, Ertin E, Ullah MA, Samiei SA, Hossain M, Kumar S, Preston KL, Vahabzadeh M, Shmueli-Blumberg D, Collins J, McCormack J, & Ghitza UE (2019). Towards detecting cocaine use using smartwatches in the NIDA clinical trials network: Design, rationale, and methodology. Contemporary Clinical Trials, 15, 100392. doi: 10.1016/j.conctc.2019.100392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckvalle K, Torous J, & Larsen ME (2019). Assessment of the data sharing and privacy practices of smartphone apps for depression and smoking cessation. JAMA Network Open, 2(4), 3192542. doi: 10.1001/jamanetworkopen.2019.2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan OT, Tenaerts P, Prindiville SA, Reynolds HR, Dizon DS, Cooper-Arnold K, Turakhia M, Pletcher MJ, Preston KL, Krumholz HM, Marlin BM, Mandl KD, Klasnja P, Spring B, Iturriaga E, Campo R, Desvigne-Nickens P, Rosenberg Y, Steinhubl SR, & Califf RM (2020). Digitizing clinical trials. npj Digital Medicine, 3, 101. doi: 10.1038/s41746-020-0302-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indiana University. (n.d.). Research using online tools & mobile devices. Retrieved April 30, 2020. https://research.iu.edu/compliance/human-subjects/guidance/mobile.html
- Iyengar K, Upadhyaya G, Vaishya R, Jain V, (2020) COVID-19 and applications of smartphone technology in the current pandemic. Diabetes Metab Syndr. Sep-Oct 2020;14(5):733–737. doi: 10.1016/j.dsx.2020.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson NC, Bentley KH, Walton A, Wang SB, Fortgang RG, Millner AJ, Coombs G 3rd, Rodman AM, & Coppersmith DDL (2020). Ethical dilemmas posed by mobile health and machine learning in psychiatry research. Bulletin of the World Health Organization, 98(4), 270–276. doi: 10.2471/BLT.19.237107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang I. (2019). Artificial intelligence in drug development: clinical pharmacologist perspective. Translational and Clinical Pharmacology, 27(3), 87–88. doi: 10.12793/tcp.2019.27.3.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S, Rao SV, & Granger CB (2015). Registry-based randomized clinical trials – a new clinical trial paradigm. Nature Reviews Cardiology, 12, 312–316. doi: 10.1038/nrcadio.2015.33 [DOI] [PubMed] [Google Scholar]
- Johnston A, Jones WS, & Hernandez AF (2016). The ADAPTABLE trial and aspirin dosing in secondary prevention for patients with coronary artery disease. Current Cardiology Reports, 18(8), 81. doi: 10.1007/s11886-016-0749-2 [DOI] [PubMed] [Google Scholar]
- Jonsson P, Carson S, Blennerud G, Shim JK, Arendse B, Husseini A, Lindberg P, Ohman K. Ericsson Mobility Report: November 2019. Stockholm, Sweden: Ericsson, 36p. https://www.ericsson.com/4acd7e/assets/local/mobility-report/documents/2019/emr-november-2019.pdf. [Google Scholar]
- Keesara S, Jonas M, & Schulman K. (2020). Covid-19 and health care’s digital revolution. New England Journal of Medicine, 382, e82. doi: 10.1056/NEJMp2005835 [DOI] [PubMed] [Google Scholar]
- Kibbelaar RE, Oortgiesen BE, van der Wal-Oost AM, Boslooper K, Coebergh JW, Veeger NJGM, Joosten P, Storm H, van Roon EN, & Hoogendoom M. (2017). Bridging the gap between the randomized clinical trial and the real world by combination of population-based registry and electronic health record data: A case study in haemato-oncology. European Journal of Cancer, 86, 178–185. doi: 10.1016/j.ejca.2017.09.007 [DOI] [PubMed] [Google Scholar]
- Lauer MS, Gordon D, Wei G, & Pearson G. (2017). Efficient design of clinical trials and epidemiological research: is it possible? Nature Reviews Cardiology, 14, 493–501. doi: 10.1038/nrcardio.2017.60 [DOI] [PubMed] [Google Scholar]
- Lehne M, Luijten S, Vom Felde Genannt Imbusch P, & Thun S. (2019). The use of FHIR in digital health - a Review of the scientific literature. Studies in Health Technology and Informatics, 267, 52–58. doi: 10.3233/SHTI190805 [DOI] [PubMed] [Google Scholar]
- Li G, Sajobi TT, Menon BK, Korngut L, Lowerison M, James M, Wilton, Stephen B, Williams T, Gill S, Drogos LL, Smith EE, Vohra S, Hill MD, Thabane L, & 2016 Symposium on Registry-Based Randomized Controlled Trials in Calgary. (2016). Registry-based randomized controlled trials: What are the advantages, challenges, and areas for future research? Journal of Clinical Epidemiology, 80, 16–24. doi: 10.1016/j.jclinepi.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Lin H, Li R, Liu Z, Chen J, Yang Y, Chena H, Lin Z, Lai W, Long E, Wu X, Lin D, Zhu Y, Chen C, Wu D, Yu T, Cao Q, Li X, Li J, … Liu Y. (2019). Diagnostic efficacy and therapeutic decision-making capacity of an artificial intelligence platform for childhood cataracts in eye clinics: A multicentre randomized controlled trial. EClinicalMedicine, 9, 52–59. doi: 10.1016/j.eclinm.2019.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt H, Connor S, Skinner H, & Brogden G. (2019). Electronic informed consent: the need to redesign the consent process for the digital age. Internal Medicine Journal, 49(7), 923–929. doi: 10.1111/imj.14339 [DOI] [PubMed] [Google Scholar]
- Ma O, Chung H, Shambhu S, Roe M, Cziraky M, Schuyler Jones W, & Haynes K. (2019). Administrative claims data to support pragmatic clinical trial outcome ascertainment on cardiovascular health. Clinical Trials, 16(4), 419–430. doi: 10.1177/1740774519846853 [DOI] [PubMed] [Google Scholar]
- Maggio LA, Leroux TC, & Artino AR Jr. (2019). To tweet or not to tweet, that is the question: A randomized trial of Twitter effects in medical education. PLoS One, 14(10), e0223992. doi: 10.1371/journal.pone.0223992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak KK & Pichika MR (2019). Artificial intelligence in drug development: present status and future prospects. Drug Discovery Today, 24(3), 773–780. doi: 10.1016/j.drudis.2018.11.014 [DOI] [PubMed] [Google Scholar]
- Marra C, Chen JL, Coravos A, & Stem AD (2020). Quantifying the use of connected digital products in clinical research. npj Digital Medicine, 3, 50. doi: 10.1038/s41746-020-0259-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, Campbell A, Campbell C, Chen CH, Ertin E, Ghitza U, Lambert-Harris C, Hassanpour S, Holtyn AF, Hser YI, Jacobs P, Klausner JD, Lemley S, Kotz D, Meier A, McLeman B, McNeely J, Mishra V, Mooney L. … Young S. (2020). The application of digital health to the assessment and treatment of substance use disorders: The past, current, and future role of the National Drug Abuse Treatment Clinical Trials Network. Journal of Substance Abuse Treatment, 112S, 4–11. doi: 10.1016/j.jsat.2020.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki K, Tateno H, Nomura A, Muto T, Suzuki S, Satake K, Hida E, & Fukunaga K. (2020). A randomized controlled trial of a smoking cessation smartphone application with a carbon monoxide checker. Digital Medicine, 3, 35. doi: 10.1038/s41746-020-0243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslove DM, Klein J, Brohman K, & Martin P. (2018). Using blockchain technology to manage clinical trials data: a proof-of-concept study. JMIR Medical Informatics, 6(4), e11949. doi: 10.2196/11949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AH, da Costa CA, & da Rosa Righi R. (2019). Electronic health records in a blockchain: A systematic review. Health Informatics Journal, 1460458219866350. doi: 10.1177/1460458219866350 [DOI] [PubMed] [Google Scholar]
- McKay FH, Cheng C, Wright A, Shill J, Stephens H, & Uccellini M. (2018). Evaluating mobile phone applications for health behaviour change: A systematic review. Journal of Telemedicine and Telecare, 24(1), 22–30. doi: 10.1177/1357633X16673538 [DOI] [PubMed] [Google Scholar]
- Moore S, Tasse A, Thorogood A, Winship I, Zawati M, & Doerr M. (2017). Consent processes for mobile app mediated research: systematic review. JMIR mHealth and uHealth, 5(8), e126. doi: 10.2196/mhealth.7014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslund JA, Aschbrenner AR, Marsch LA, Unutzer J, Patel V, & Bartels SJ (2017). Digital technology for treating and preventing mental disorders in low-income and middleincome countries: A narrative review of the literature. Lancet Psychiatry, 4(6), 485–500. doi: 10.1016/S2215-0366(17)30096-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (NASEM). (2019). Virtual Clinical Trials: Challenges and Opportunities: Proceedings of a Workshop. Washington, DC: The National Academies Press. doi: 10.17226/25502. [DOI] [PubMed] [Google Scholar]
- Nomura A, Tateno H, Masaki K, Muto T, Suzuki S, Satake K, … Fukunaga K. (2019). A novel smoking cessation smartphone app integrated with a mobile carbon monoxide checker for smoking cessation treatment: Protocol for a randomized controlled Trial. JMIR Research Protocols, 8(2), e12252. doi: 10.2196/12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan D. & Simmons LA (2020). Navigating nonessential research trials during COVID19: The push we needed for using digital technology to increase access for rural participants? Journal of Rural Health (in press). doi: 10.1111/jrh.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordo AH, Levaux HP, Becnel LB, Galvez J, Rao P, Stem K, Prakash E, & Kush RD (2018). Use of EHRs data for clinical research: Historical progress and current applications. Learning Health Systems, 3(1), e10076. doi: 10.1002/lrh2.10076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid JS, Beskow LM, Rape M, Gouripeddi R, Black RA, Cimino JJ, Embi PJ, Weng C, Marnocha R, Buse JB (2017). A survey of practices for the use of electronic health records to support research recruitment. Journal of Clinical and Translational Science, 1(4), 246–252. doi: 10.1017/cts.2017.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmo CA, McGettigan P, & Kurz X. (2019). Barriers and opportunities for use of patient registries in medicines regulation. Clinical Pharmacology & Therapeutics, 106(1), 39–42. doi: 10.1002/cpt.1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipenko L. (2019). Blockchain’s potential to improve clinical trials—an essay. BMJ, 367, I5561. doi: 10.1136/bmj.I5561 [DOI] [PubMed] [Google Scholar]
- Pagoto S. & Nebeker C. (2019). How scientists can take the lead in establishing ethical practices for social media research. Journal of the American Medical Informatics Association, 26(4), 311–313. doi: 10.1093/jamia/ocy174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M. (2019). Information sharing to promote risky health behavior on social media. Journal of Health Communication, 24(4), 359–367. doi: 10.1080/10810730.2019.1604914 [DOI] [PubMed] [Google Scholar]
- Park M. (2020). How smoking advocates are connected online: An examination of online social relationships supporting smoking behaviors. Journal of Health Communication, 25(1), 82–90. doi: 10.1080/10810730.2019.1709924 [DOI] [PubMed] [Google Scholar]
- Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Raimane A, Cheung L, Hung G, Lee J, Kowey P, Talati N, Nag D, Gummidipundi SE, Beatty A, True Hills M, Desai S, … Turakhia MP (2019). Large-scale assessment of a smartwatch to identify atrial fibrillation. New England Journal of Medicine, 381, 20. doi: 10.1056/NEJMoa1901183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry B, Herrington W, Goldsack JC, Grandinetti CA, Vasishy KP, Landray MJ, Bataille L, DiCicco RA, Bradley C, Narayan A, Papadopoulos EJ, Sheth N, Skodacek K, Stem K, Strong TV, Walton MK, & Corneli A. (2018). Use of mobile devices to measure outcomes in clinical research, 2010–2016: A systematic literature review. Digital Biomarkers, 2(1), 11–30. doi: 10.1159/000486347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Research Center. (2019). Mobile technology and home broadband 2019. Retrieved on April 30, 2020. https://www.pewresearch.org/Internet/2019/06/13/mobile-technology-and-home-broadband-2019/
- Pew Research Center. (2019b). Social Media Fact Sheet. Retrieved on September 1, 2020. https://www.pewresearch.org/internet/fact-sheet/social-media/
- Pew Research Center. (2019c). Mobile Fact Sheet. Retrieved on September 1, 2020. https://www.pewresearch.org/internet/fact-sheet/mobile/
- Pew Research Center. (2019d). Internet/Broadband Fact Sheet. Retrieved on September 1, 2020. https://www.pewresearch.org/internet/fact-sheet/internet-broadband/
- Pfaff E, Lee A, Bradford R, Pae J, Potter C, Blue P, Knoepp P, Thompson K, Roumie CL, Crenshaw D, Servis R, & DeWalt DA (2018). Recruiting for a pragmatic trial using the electronic health record and patient portal: successes and lessons learned. Journal of the American Informatics Association, 26(1), 44–49. doi: 10.1093/jamia/ocy138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poblete P. & Nieto E. (2020). Does time matter? WhatsApp vs electronic mail for dental education. A pilot study. European Journal of Dental Education, 24(1), 121–125. doi: 10.1111/eje.12475 [DOI] [PubMed] [Google Scholar]
- Pratap A, Neto EC, Snyder P, Stepnowsky C, Elhadad N, Grant D, Mohebbi MH, Mooney S, Suver C, Wilbanks J, Mangravite L, Heagerty PJ, Arean P, & Omberg L. (2020). Indicators of retention in remote digital health studies: a cross-study evaluation of 100,000 participants. npj Digital Medicine, 3, 21. doi: 10.1038/s41746-020-0224-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovic A, Gmelin T, Stein BD, & Miller E. (2017). Depressed adolescents’ positive and negative use of social media. (2017). Journal of Adolescence, 55, 5–15. doi: 10.1016/j.adolescence.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter K, Zhu Y, Angyan P, Le N, Merchant AA, & Zimmer M. (2019). Public concern about monitoring Twitter users and their conversations to recruit for clinical trials: survey study. Journal of Medical Internet Research, 21(1), e1545. doi: 10.2196/15455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard JM, Feng TL, Sporn AB, Lai J, Lo C, Ta M, & Nadler R. Availability, readability, and content of privacy policies and terms of agreements of mental health apps. Internet Interventions, 17, 100243. doi: 10.1016/j.invent.2019.100243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggeveen LF, Fleuren LM, Guo T, Throall P, Jan de Grooth H, Swart EL, Klausch TLT, van der Voort PHJ, Girbes ARJ, Bosman RJ, & Elbers PWG (2019). Right dose right now: bedside data-driven personalized antibiotic dosing in severe sepsis and septic shock—rationale and design of a multicenter randomized controlled superiority trial. Trials, 20:745. doi: 10.1186/s13063-019-3911-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa C, Campbell AN, Miele GM, Brunner M, Winstanley EL (2015). Using e-technologies in clinical trials. Contemporary Clinical Trials, 45(Pt A), 41–54. doi: 10.1016/j.cct.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C, McCarthy M, Cappelleri J, Wong S. (2020). Choosing a Mobile Sensor Technology for a Clinical Trial: Statistical Considerations, Developments and Learnings, Therapeutic Innovation & Regulatory Science (June). doi. 10.1007/s43441-020-00188-2 [DOI] [PubMed] [Google Scholar]
- Simon C, Schartz H, Rosenthal G, Eisenstein E, & Klein D. (2018). Perspectives on electronic informed consent from patients underrepresented in research in the United States: A focus group study. Journal of Empirical Research on Human Research Ethics, 13(4), 338–348. doi: 10.1177/1556264618773883 [DOI] [PubMed] [Google Scholar]
- Steinhubl SR, Wolff-Hughes DL, Nilsen W, Iturriaga E, & Califf RM (2019). Digital clinical trials: Creating a vision for the future. NPJ Digital Medicine, 2, 126. doi: 10.1038/s41746-019-0203-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MA, & O’Regan R. (2018). Social media and clinical trials: The pros and cons gain context when the patient is at the center. Cancer, 124(24), 4618–4621. doi: 10.1002/cncr.31747 [DOI] [PubMed] [Google Scholar]
- Torous J. & Nebeker C. (2017). Navigating ethics in the digital age: introducing connected and open research ethics (CORE), a tool for researchers and institutional review boards. Journal of Medical Internet Research, 18(12), e29. doi: 10.2196/jmir.6793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifan A, Oliveira M, Wang T, & Kohls E. (2019). Passive sensing of health outcomes through smartphones: Systematic review of current solutions and possible limitations. JMIR Mhealth Uhealth, 7(8), e12649. doi: 10.2196/12649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turakhia MP, Desai M, Hedlin H, Rajmane A, Talati N, Ferris T, Desai S, Nag D, Patel M, Kowey P, Rumsfeld JS, Russo AM, True Hills M, Granger CB, Mahaffey KW, & Perez MV (2019). Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: The Apple Heart study. American Heart Journal, 207, 66–75. doi: 10.1016/j.ahj.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Food and Drug Administration (FDA). (2020). Digital Health Innovation Plan. Silver Spring, MD: U.S. Food and Drug Administration, 8p. https://www.fda.gov/media/106331/download [Google Scholar]
- United States Food and Drug Administration (FDA). (2018). Framework for FDA’s Real-World Evidence Program. Silver Spring, MD: U.S. Food and Drug Administration, 40p. Retrieved April 30, 2020. https://www.fda.gov/media/120060/download [Google Scholar]
- United States Food and Drug Administration (FDA). (2017). Use of Electronic Records and Electronic Signatures in Clinical Investigations Under 21 CFR Part 11 – Questions and Answers, Guidance for Industry (Draft Guidance). Silver Spring, MD: U.S. Food and Drug Administration, 28p. https://www.fda.gov/files/drugs/published/Use-of-Electronic-Records-and-Electronic-Signatures-in-Clinical-Investigations-Under-21-CFR-Part-11-%E2%80%93.pdf [Google Scholar]
- United States Food and Drug Administration (FDA). (2017b). Digital Health Innovation Action Plan. Silver Spring, MD: U.S. Food and Drug Administration, 8p. https://www.fda.gov/media/106331/download [Google Scholar]
- United States Food and Drug Administration (FDA). (2016). Use of Electronic Informed Consent in Clinical Investigations – Questions and Answers. Guidance for Institutional Review Boards, Investigators, and Sponsors. Silver Spring, MD: U.S. Food and Drug Administration, 16p. https://www.fda.gov/media/116850/download [Google Scholar]
- United States Food and Drug Administration (FDA). (n.d.). Real World Evidence. Retrieved April 30, 2020. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence
- U.S. National Library of Medicine. (n.d.) ClinicalTrials.gov Glossary of Common Site Terms. Retrieved September 30, 2020. https://clinicaltrials.gov/ct2/about-studies/glossary
- United States Office for Human Research Protections (OHRP). (2017/2018). Subpart A of 45 CFR Part 46: Basic HHS Policy for Protection of Human Subjects. Rockville, MD: U.S. Office for Human Research Protections, 19p. https://www.hhs.gov/ohrp/sites/default/files/revised-common-rule-reg-text-unofficial-2018-requirements.pdf [Google Scholar]
- University of Texas at Austin, Texas Advanced Computing Center. (April 22, 2020). AI fast-tracks drug discovery to fight COVID-19. EureakAlert! (American Association for the Advancement of Science). https://eurekalert.org/pub_releases/2020-04/uota-afd042220.php
- Verma S. (2020). Early impact of CMS expansion of Medicare telehealth curing COVID-19. Health Affairs Blog. 10.1377/hblog20200715.454789/full/ [DOI] [Google Scholar]
- Watson NL, Mull KE, Heffner JL, McClure JB, & Bricker JB (2018). Participant recruitment and retention in remote ehealth intervention trials: Methods and lessons learned from a large randomized controlled trial of two web-based smoking interventions. Journal of Medical Internet Research 2018, 20(8), e10351. doi: 10.2196/10351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnberge M, Geerts BF, Hol L, Lemmers N, Mulder MP, Berge P, Schenk J, Terwindt LE, Hollmann MW, Vlaar AP, & Veelo DP (2020). Effect of a machine learning-derived early warning system for intraoperative hypotension vs. standard care on depth and duration of intraoperative hypotension during elective noncardiac surgery: The HYPE randomized clinical trial. JAMA, 323(11), 1052–1060. doi: 10.1001/jama.2020.0592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Weingarden H, Ladis I, Braddick V, Shin J, & Jacobson NC (2020). Cognitive-behavioral therapy in the digital age: presidential address. Behavior Therapy, 51(1), 1–14. doi: 10.1016/j.beth.2019.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley EL, Stroup-Menge B, & Snyder K. (2018). The promise of technology-based services for addiction treatment clients residing in non-urban areas. Journal of Studies on Alcohol and Drugs, 79(3), 503–504. doi: 10.15288/jsad.2018.79.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley EL, Lander LR, Zheng W, Law K, Six-Workman A, & Berry JH (2020). Rapid transition of individual and group-based behavioral outpatient visits to telepsychiatry in response to COVID-19. Journal of Addiction Medicine (in press). [DOI] [PubMed] [Google Scholar]
- Wong DR, Bhattacharya S, & Butte AJ (2019). Prototype of running clinical trials in an untrustworthy environment using blockchain. Nature Communications, 10, 917. doi: 10.1038/s41467-019-08874-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y, Sheets L, Shae Z, Tsai JJP, & Shyu C. (2018). Applying blockchain technology for health information exchange and persistent monitoring for clinical trials. AMIA Annual Symposium Proceedings Archive, 2018, 1167–1175. [PMC free article] [PubMed] [Google Scholar]
- Zong N, Wen A, Stone DJ, Sharma DK, Wang C, Yu Y, Liu H, Shi Q, & Jiang G. (2020). Developing an FHIR-based computational pipeline for automatic population of case report forms for colorectal cancer clinical trials using electronic health records. JCO Clinical Cancer Informatics, 4, 201–209. doi: 10.1200/CCI.19.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]