Abstract
Synovium-derived mesenchymal stromal cell (Sy-MSC) is a newer member of the mesenchymal stromal cell families. The first successful demonstration of the mesenchymal stromal cell from the human synovial membrane was done in 2001 and since then its potential role for musculoskeletal regeneration has been keenly documented. The regenerative effects of Sy-MSCs are through paracrine signaling, direct cell–cell interactions, and extracellular vehicles. Sy-MSCs possess superior chondrogenicity than other sources of mesenchymal stromal cells. This article aims to outline the advancement of synovium-derived mesenchymal stromal cells along with a specific insight into the application for managing osteoarthritis knee.
Keywords: Synovium, Mesenchymal stromal cell, Chondrogenicity, Osteoarthritis knee
Introduction
Osteoarthritis (OA) of the knee is a chronic degenerative condition of the articular cartilage which is associated with varying degrees of inflammatory synovitis and cartilage destruction of the joint [1]. The articular cartilage is avascular and aneural structure and hence the healing process is poor and results in fibrous tissue. OA knee primarily affects the elderly population which is a major cause of disability in older adults worldwide [2].
In India, the prevalence of osteoarthritis knee ranges from 22 to 39% with female preponderance [3]. The robust increase in the prevalence of OA knee is due to obesity and a sedentary lifestyle. Various modalities of treatment for cartilage rejuvenation are autologous chondrocyte implantation, microfracture, stem cell culture, and implantation as a part of regenerative orthobiologics [4].
Mesenchymal stromal cells (MSCs) are a rich cell source for regenerative medicine particularly in knee osteoarthritis for cartilage regeneration [5, 6]. MSCs are harvested from various tissues such as bone marrow, adipose tissues, skeletal muscles, placenta, umbilical cord, dental pulp, and synovium [7, 8]. Among these, the MSCs harvested from the synovial tissues had the greatest potential for differentiation into chondrogenic cells and their proliferation [9]. Analysis of cells harvested from fibrous and adipose synovium had a similar self-renewal and differentiation capability. Furthermore, in-vivo study demonstrated the differentiation of synovial MSCs into cartilage appropriate to the microenvironment for the repair of cartilage defects in rabbit knees [10].
Mesenchymal stromal cells are multipotent stem cells present in various sites of the body. They have direct and indirect mechanisms in their chondrogenic activity. They act as a stimulus to induce the differentiation of chondroprogenitors to convert into chondrocytes by growth factor secretion such as FGF and TGF-β [11]. They also reduce inflammatory joint disease progression by promoting T-cell class switch from pro to anti-inflammatory Th2 subtype.
A popular treatment option to treat OA is orthobiologic therapies. More trials are needed to determine the best OA treatments among MSCs of bone marrow, adipose tissue, placenta, and synovium, even though the present research investigating their efficacy is informatory. This article throws light on the synovium-derived mesenchymal stromal cells (Sy-MSCs) in the cartilage regeneration in OA knees.
Cellular Therapy in OA Knee
Due to the robust development in the fields of regenerative and translational medicine, the usage of biological products to treat diseases has been of prime importance. Biocellular regenerative medicine aims to regenerate all cells and tissues and exert regenerative homeostasis in the local microenvironment. Cellular therapy is defined as the transplantation of either autologous or allogenic cells or modified cells to replace and regenerate the damaged tissues in a given area of interest. On par with cellular therapy, numerous researchers have demonstrated the usage of cellular elements in osteoarthritis in the past 2 decades.
Due to the intrinsic inability of cartilage to repair, the usage of biological products has become robust among practicing orthopedic surgeons and regenerative experts. Evidence on varied sources of mesenchymal stromal cells (MSC) for the management of osteoarthritis knees is available. The most common explored cellular source is bone marrow-derived MSCs (BM-MSCs) (bone marrow aspirate concentrate) followed by adipose-derived MSCs (ASCs) (adipose stromal cells, stromal vascular fraction, microvascular fragments, microfat, nanofat, and secretomes), and placenta-derived MSCs (P-MSCs) for cartilage regeneration.
Various studies have proved the efficacy, functional outcome, and safety regarding the usage of either BM-MSCs, ASCs, and P-MSCs for the management of osteoarthritis knees. A meta-analysis by Jeyaraman et al. demonstrated the superiority of ASCs in terms of the efficacy and safety profile in the management of osteoarthritis knees than BM-MSCs [12]. Due to the advancing age in the elderly population the yield of harvested MSCs from bone marrow sources is limited [13]. But considering the availability, sources of ASCs are 500 times more than that of BM-MSCs [14]. BM-MSCs have greater cartilage regeneration potential than AD-MSCs but due to the presence of various biological micromolecules and cytokines in the stromal vascular fraction from AD-MSCs, the cartilage regeneration can be accentuated [15]. Soltani et al. demonstrated that a single intra-articular injection of allogenic P-MSCs resulted in a better functional and clinical outcome at the end of the 6 months follow-up period [16]. Hsu et al. reported higher concentrations of glycosaminoglycan secretion with human P-MSCs which appear to be the better agent for chondrogenesis than human BM-MSCs. They concluded that the usage of a 3D culture system for P-MSCs would revolutionize cartilage tissue bioengineering [17].
The unexplored or minimally explored cellular source for cartilage regeneration is synovium-derived mesenchymal stromal cells (Sy-MSCs). In 2001, De Bari et al. demonstrated successful extraction of MSCs from human synovial membrane [18]. The synovial membrane is a specialized connective tissue composed of a double-layered membrane lining the synovial joints and tendon sheaths. The outer layer of the synovial membrane is composed of fibrous, adipose, and areolar components, and the inner layer is composed of sheets of cells (type A macrophage-like synoviocytes and type B fibroblast-like synoviocytes). The components of fibrous and adipose elements give rise to mesenchymal stromal cells named fibrous-synovial MSCs and adipose-synovial MSCs, respectively. Type A cells show positive expression for CD-68 and CD-14. They exhibit a rich expression of collagen III, V, and VI. Type B cells exhibit positive expression for CD-44 and VCAM-1 adhesion molecule [19].
Characterization of Sy-MSCs
Like BM-MSCs and ASCs, Sy-MSCs exhibit multipotent cellular efficacy and regenerative potential in both in-vivo and in-vitro. The benefits of MSCs transplantation depend on the viability and biological properties like controlled proliferation and differentiation, anti-apoptosis, anti-inflammatory, and immunomodulatory effects [20, 21]. The regenerative effects of Sy-MSCs are through paracrine signaling, direct cell–cell interactions, and extracellular vehicles [22]. The properties of various sources of MSCs are compared in Table 1.
Table 1.
Characteristics of the varied sources of MSC therapy for OA knee
| Sources of MSCs | Sources | Potency | Significance | Invasiveness | Ethical consideration |
|---|---|---|---|---|---|
| ESCs | Inner cell mass | Totipotent | Forms an entire organism and irreversible stem cells; ? allogenicity | + | + + + |
| BM-MSCs | Iliac crest | Multipotent |
↑ Potential to regenerate bone and cartilage; Easy to isolate stem cells; No culture required; Auto and allogenicity + + |
− | + |
| AD-MSCs | Abdomen, medial aspect of thigh | Multipotent |
↑ Potential to regenerate cartilage and soft tissues; Complex natured to isolate stem cells; Autologous + + ; ??? Allogenicity |
− | + |
| Sy-MSCs | Synovium around knee joint | Multipotent |
↑ Potential to regenerate cartilage than bone; Culture required for exponentiation; auto and allogenicity + + |
+ | + |
| Pl-MSCs | Amniotic membrane, chorionic plate, chorionic villi, decidua | Pluripotent | Difficult to isolate inner cell mass; ↑ potential to regenerate bone, cartilage and soft tissues; auto and allogenicity + + | + | − |
| Um-MSCs | Umbilical cord, Wharton jelly | Pluripotent | Culture required for exponentiation; auto and allogenicity + + | + | − |
| AF-MSCs | Cytotrophoblast, syncytiotrophoblast | Pluripotent | Culture required for exponentiation; auto and allogenicity + + | + | − |
| PB-MSCs | Circulating mononuclear cells | Multipotent | Enhanced osteogenic and adipogenic potential | + | − |
Sy-MSCs have been revealed to be a multipotent cell source similar to BM-MSCs [23]. Sy-MSCs exhibit osteogenesis, chondrogenesis, and adipogenesis under lineage-specific culture medium [18, 24]. Due to the intrinsic ability for limited senescence, Sy-MSCs can be expanded in greater numbers in monolayer culture in-vitro. Human Sy-MSCs maintain the proliferative ability even after the 10th passage and maintain a linear curve in population doubling capacity. In a pre-clinical study with a rat model, Sy-MSCs exhibit higher CFU, proliferation, and chondrogenic differentiation kinetics and safety than the other sources of MSCs. The higher proliferative potential of Sy-MSCs is due to the telomerase activity which is usually undetectable in the somatic cells [25, 26].
Sy-MSCs co-cultured along with human serum demonstrated enhanced proliferation due to the presence of higher levels of PDGF in human serum which binds to PDGF receptor found in Sy-MSCs. Inversely, the decreased proliferative capacity of Sy-MSCs is noted in the presence of anti-PDGF antibodies [27, 28]. Shirasawa et al. exhibited maximal chondrogenic differentiation by inducing Sy-MSCs with BMP-2, TGF-β, and dexamethasone in pellet culture than BM-MSCs [29].
Various studies observed tenfold rise in synovial fluid-derived MSCs (SF-MSCs) in injured or osteoarthritic knees [30, 31]. These synovial fluid-derived MSCs show similar characterization to Sy-MSCs. SF-MSCs exhibit more clonogenicity and chondrogenicity and lower adipogenicity in-vitro than BM-MSCs. The source for SF-MSCs is from synovial shedding, infrapatellar fat pad, or articular cartilage. The number of SF-MSCs increases as the disease progresses [32].
Genotype and Phenotype
RT-PCR analysis of synovial tissue specimens exhibited the expression of extracellular matrix molecules, adhesion molecules, cytopeptides, and transcription factors in Sy-MSCs [33]. Immunohistochemical analysis of Sy-MSCs showed similar pattern as shown by BM-MSCs [34]. Sy-MSCs show positive expression for CD-10, -13, -44, -49a, -73, -90, -105, -147, and -166, and negative expression for CD-14, -20, -31, -34, -45, -62e, -68, -113, and -117 [35, 36]. Sy-MSCs show negative expression for alkaline phosphatase enzyme and HLA-DR antigens [8].
During the culture of Sy-MSCs, after first passage, immunophenotype exhibits a transformation from CD-34, -45, -62e, and HLA-DR antigens to CD-73, -90, and -105 which are expressed in higher quantities [34]. Such immunophenotypic transformation renders Sy-MSCs as a multipotent cellular population. With the higher expression of CD-90 in Sy-MSCs, the chondrogenic potential of Sy-MSCs are accentuated both in in-vitro and in-vivo [34].
In mice, Futami et al. observed more than 90% positive ratios for CD-29 and -44, less than 10% positive ratios for CD-106, and 50% or more positive ratios for CD-140a among Sy-MSCs and cells derived from bone marrow and muscles [37]. Osteogenesis is evidenced by the higher expression of the mRNA for RNUX2, osteopontin, and type 1 collagen levels in Sy-MSCs whereas adipogenesis is expressed by higher levels of the mRNA for Lpl, PPAR-α, C/EBP-α, and FABP4 levels in Sy-MSCs and the evidence of chondrogenesis is exhibited by the expression of Sox9, type II, and type X collagen in in-vitro Sy-MSCs [37].
Immunomodulation of Sy-MSCs
The immunomodulatory properties of Sy-MSCs enhance their usage in clinical applications [38, 39]. The pathways by which the immunomodulatory mechanisms of Sy-MSCs are regulated through.
T Lymphocyte System
Sy-MSCs mediate immunoregulation, either produced constitutively by MSCs or released following cross-talk with target cells. Nitric oxide and indoleamine 2,3-dioxygenase (IDO), which are only released by Sy-MSCs after triggering by IFNγ produced by target cells [40]. IDO induces the depletion of tryptophan from the local environment, which is an essential amino acid for lymphocyte proliferation. Sy-MSC-derived IDO was reported to be required to inhibit the proliferation of IFNγ-producing TH1 cells and, together with prostaglandin E2 (PGE2), to block NK-cell activity [40].
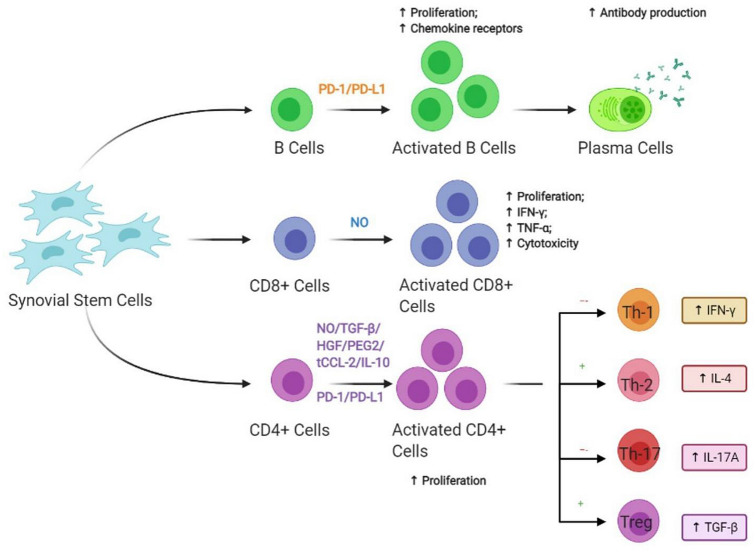
Activated CD8+ T lymphocyte by nitric oxide suppresses the proliferation of cytotoxic T cells, inhibits the production of INF-γ & TNF-α, and attenuates the cytotoxic effects. The activated CD4+ T lymphocyte by TGF-β, nitric oxide, hepatocyte growth factor, and PGE2 secreted by Sy-MSCs enhances lineage-specific differentiation and cellular proliferation [41]. Upon which the immunomodulatory activities are enhanced via T helper 2 cells through increased IL-4 levels and T regulatory cell through increased TGF-β and suppressed via T helper 1 cell through increased INF-γ levels and T helper 17 cells through increased levels of IL-17A as shown in Fig. 1 [42].
Fig. 1.
Immunomodulatory effects of MSCs via T and B lymphocyte system. CD cluster differentiation; IFN-γ interferon-gamma; IL interleukin; TGF-β transforming growth factor-beta
B Lymphocyte System
Sy-MSCs enhance antibody production through the activation of B lymphocytes by soluble factors secreted by them as shown in Fig. 1 [43].
NK Cells
Mesenchymal stromal cells inhibit cytokine production by NK cells when cultured or co-cultured in the media containing IL-2 or -15 [44]. MSCs hamper NK-cell cytolytic effects through soluble factors like HLA-G5, PGE2, IDO system, and also through downregulation of NK receptors like NKp30, NKp44, or NKG2D. Activated NK cells lyse MSCs. Upon exposure with INF-γ increases MHC-I expression on MSCs which conversely decreases the susceptibility to NK-cell-mediated lysis as shown in Fig. 2 [45, 46].
Fig. 2.
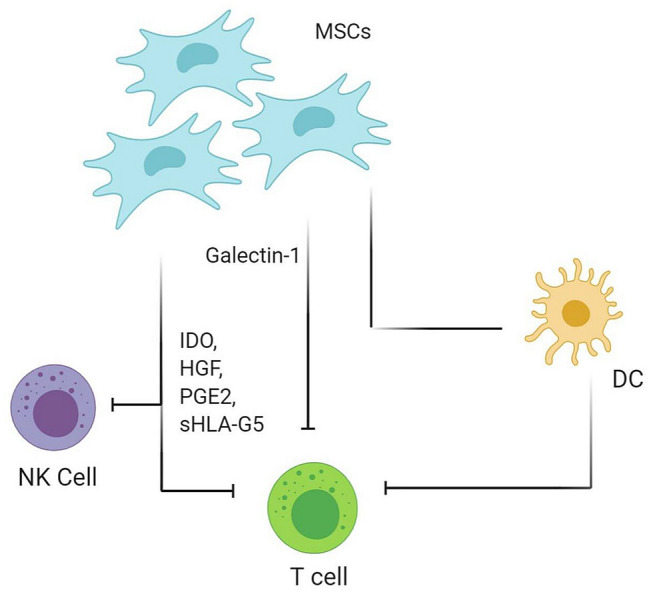
Immunomodulatory effects of MSCs via NK cell and HLA-G5 system. MSCs mesenchymal stem cells; NK cell natural killer cell; DC dendritic cell; IDO indoleamine-pyrrole 2,3-dioxygenase; HGF hepatocyte growth factor; PGE2 prostaglandin E2; sHLA-G5 soluble human leukocyte antigen G5
HLA-G5 System
The production of soluble HLA-G5 by Sy-MSCs has been shown to suppress T-cell proliferation, as well as NK-cell and T-cell cytotoxicity, and to promote the generation of regulatory T cells as shown in Fig. 2 [47, 48].
Harvesting and Delivery Methods of Sy-MSCs
Synovial stromal cells can be harvested from the synovial lining of knee [49], hip [50], or shoulder [51] joints. Fernandes et al. harvested synovial stromal cells arthroscopically from the knee through the anterolateral portal and processed them further to expand the cells for further differentiation and clinical applications [52].
Li et al. [53] studied the feasibility of harvesting synovial stromal cells from arthroscopic flushing fluid from the knee joint for cartilage regeneration. Sy-MSCs were expanded in-vitro and induced for chondrogenic differentiation. These cells were delivered by xenogenic injection of MSC encapsulated by loading them into cross-linking polyPEGDA/HA hydrogel into full-thickness cartilage defects in cartilage groove. They observed a reduction in the defect area at the end of 2 months.
Researchers demonstrated that the repair of torn meniscus upon the suspension of Sy-MSCs on meniscus for 10 min. They further observed that the number of cells adhered to the pathological site underwent dynamic morphological changes over 24 h. These cells showed microspikes and pseudopodia for better adhesion onto the pathological meniscus [54].
Shimomura et al. [55] obtained Sy-MSCs arthroscopically and expanded in-vitro before transplantation to symptomatic chondral knee lesions. The intervention was delivered in two-stages, stage-I arthroscopic evaluation and synovial tissue biopsy from the anterior aspect of the knee followed by administration of the cultured Sy-MSCs after 4 weeks upon making a tissue-engineered construct of the size of the chondral lesion identified initially. All five patients achieved full defect filling at 48 weeks which was demonstrated by MRI during the follow-up. These cases showed no adverse events. Chondrogenesis was demonstrated histologically. Functionally these patients showed full clinical improvement by 24 months.
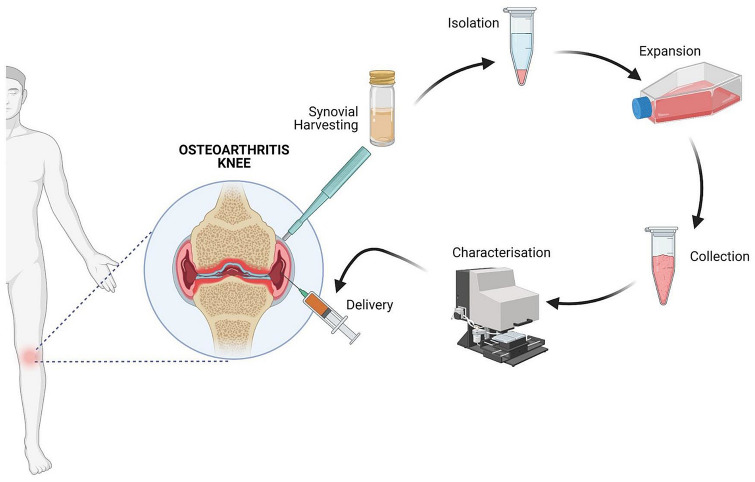
The evidence stated that the infrapatellar fat pad plays a major in the progression of the OA knee [56–58]. Targeting infrapatellar fat pad tissue with synovial stromal cells may reduce inflammation and fibrosis across the knee joint and proceeds with cartilage repair and regeneration [59–61]. The various steps involving Sy-MSC therapy for osteoarthritis knee are shown in Fig. 3.
Fig. 3.
Steps involved in Sy-MSC therapy for osteoarthritis knee
Chondrogenicity of Sy-MSCs
The mesenchymal stromal cells possess the ability to differentiate into trilineage namely osteogenesis, chondrogenesis, and adipogenesis. Sy-MSCs possess superior chondrogenicity than other sources of mesenchymal stromal cells which were evidenced by (1) the origin of synoviocytes and chondrocytes from common progenitor pool [62], (2) the higher expression of CD-44 (hyaluronic acid receptor) and uridine diphosphoglucose dehydrogenase (UDPGD) [62], (3) formation of a continuous layer of the synovial membrane in the area of partial-thickness defects of the cartilage [63] (4) chondrocyte-like cells are present in synovial pannus in rheumatoid arthritis [64], and (5) the expression of type 1, 10, & 11 collagen, cartilage oligomeric matrix protein (COMP), SOX-9, and aggrecan in the synovial tissues [65]. Hence, Sy-MSCs have a greater proliferative effect in cartilage regeneration [19]. Besides cartilage regeneration, various studies demonstrated the regenerative potential of Sy-MSCs in terms of the tendon, ligament, muscle, and bone regeneration [66, 67].
Intracellular Signaling in Chondrogenic Differentiation
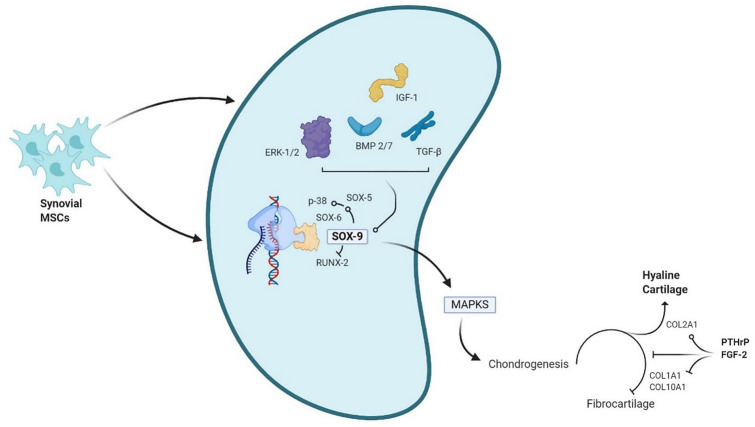
Various researchers have demonstrated the chondrogenic differentiation of MSCs in-vitro with the addition of external biological micromolecules such as growth factors (FGF, PDGF, TGF-β, EGF), bone morphogenetic proteins (BMPs), hedgehog, and Wnt glycoproteins. TGF-β superfamily (TGF-β2 and TGF-β3) has been demonstrated to be the potential inducer of chondrocytes in-vitro [68]. Among BMPs, BMP-2 and -7 are the potential inducers for chondrogenesis and extracellular matrix synthesis, respectively. The molecular interactions between ERK1/2 and SOX-9 stimulate chondrogenic differentiation of MSCs [62, 69]. To avoid the formation of fibrous cartilage, PTHrP or FGF-2 downregulates Col10a1 and Col2a1 during chondrogenesis and increases the deposition of type 2 collagen in the cartilage as shown in Fig. 4 [70].
Fig. 4.
Factors involved in selective chondrogenic differentiation of Sy-MSCs. ERK extracellular signal-regulated kinase; IGF insulin-like growth factor; BMP bone morphogenic protein, TGF-β transforming growth factor-beta; SOX SRY-related HMG box; RUNX runt-related transcription factor; MAPKS mitogen-activated protein kinase; COL collagen; PTHrP parathormone related peptide; FGF fibroblast growth factor
SOX-9 expression helps in the survival and maintenance of chondrocytes in-vitro and in-vivo, expands ECM production and intracellular signaling among chondrocytes [71]. SOX-9 acts as a link protein for L-SOX-5 and SOX-6 transcription factors in maintaining chondrogenesis and also helps in the expression of chondrogenesis regulatory pathways (Wnt, Notch, and hedgehog signaling mechanisms) [72]. The other transcriptional factors that help in maintaining chondrogenesis are Runx2, Barx2, Nkx3.2/Bapx1, Msx1 and 2, β-catenin, Smads, Lef1, AP-1, and AP-2 [68, 73]. Apart from these transcriptional factors, the composition of extracellular matrix maintains chondrocyte morphology, phenotype, and genotype, differentiation, and maturation [74]. Among the various intracellular signals, Ser/Thr protein kinases, and Ser/Thr phosphoprotein phosphatases were the key regulators of chondrogenesis [75]. p38 and ERK1/2 are the key mitogen-activated protein kinases (MAPKs) that regulate chondrocyte signaling involved in the translation of extracellular stimulus into chondrocyte responses and gene expression for chondrocyte differentiation and proliferation [76].
In-Vitro Chondrogenicity by Sy-MSCs
The source of MSCs differs in tissue differentiation and multipotent ability to obtain the tissue of interest [23, 26]. The murine MSCs derived from various sources exhibited that Sy-MSCs demonstrated a greater amount of cartilage matrix production in in-vitro pellet culture [26]. When matched with BM-MSCs, Sy-MSCs derived cartilage pellets were significantly larger [29]. De Bari et al. reported chondrogenic capability of Sy-MSCs was higher than periosteum-derived MSCs in-vitro [77].
The greater regenerative and chondrogenic potential was exhibited by MSCs derived from fibrous and adipose synovium. Though the amount of nucleated cell population was higher in fibrous synovium, MSCs from adipose synovium have more chondrogenic potential and accessibility to extract MSCs [78]. Koga et al. demonstrated an enormous cartilage matrix production after 4 weeks by synovium and bone marrow-derived MSCs when admixed with collagen gel transplanted into rabbit cartilage defects [79].
The fate of cellular therapy depends on specific cell–cell and cell–matrix interactions, which are controlled by extracellular and intracellular signaling molecules [80]. The components of culture media used for in-vitro chondrogenesis by Sy-MSCs include dexamethasone, ascorbic acid, ITS + premix, proline, sodium pyruvate, and TGF-β growth factor [18, 23]. TGF-β superfamily is known to stimulate chondrogenesis differentiation of MSCs. Due to the presence of TGF-β receptors, they undergo dimerization and phosphorylation-dependent signaling events, which are transduced by smad and non-smad pathways to the nucleus. In the nucleus, SOX-9 gets activated to induce the chondrogenic gene expression [80, 81].
Researchers studied the usage of TGF-β superfamily and BMPs in Sy-MSCs induced chondrogenesis. TGF-β1 induced chondrogenesis in the presence of Sy-MSCs pellets obtained from a rabbit model and dexamethasone [82]. Both Sy-MSCs pellets and TGF- β1 induced chondrogenic explant showed positive expression for collagen type II, which is an essential marker for chondrogenesis [82]. Shirasawa et al. demonstrated improved chondrogenesis with the combination of TGF- β3, dexamethasone, and BMP-2 with Sy-MSCs pellets [29].
A superior chondrogenic differentiation of Sy-MSCs has been observed with the simultaneous application of TGF-β1 and IGF-1 [83]. Along with chondrogenic differentiation, the higher amounts of glycosaminoglycan production were observed when Sy-MSCs were seeded along with 3D polyglycolic acid scaffolds and simultaneous application of TGF-β1 and IGF-1 [84]. Shintani et al. demonstrated the superior potential of BMP-2 and -7 in the induction of chondrogenesis than TGF-β1 [85]. A higher dose of BMP-7 in the presence of TGF-β1 demonstrated the enhanced chondrogenesis by Sy-MSCs [81]. Research is still going on to observe the appropriate concentrations of various growth factors for chondrogenesis by Sy-MSCs. The summary of the studies on in-vitro chondrogenicity by Sy-MSCs is given in Table 2.
Table 2.
In-vivo chondrogenicity by Sy-MSCs
| Author | Objective | Animal | Inference |
|---|---|---|---|
| Ozeki et al. [49] | Investigated the effects of single or repetitive intra-articular injections of Sy-MSCs | Rat osteoarthritis model | Periodic injections of Sy-MSCs maintained viable cells without losing their MSC properties in knees and inhibited osteoarthritis progression by secretion of trophic factors |
| Schmal et al. [86] | Compared the regenerative tissue quality following matrix-associated cell implantation using amplified chondrocytes compared to Sy-MSCs for cartilage lesions | Osteochondral lesions in rabbit femur | Cartilage regeneration following matrix-associated implantation using allogenic undifferentiated synovium-derived stem cells in a defect model in rabbits showed similar macroscopic results and collagen composition compared to amplified chondrocytes; however, biomechanical characteristics and histological scoring were inferior |
| Pei et al. [87] | Investigated engineered Sy-MSCs to repair allogeneic full-thickness femoral condyle cartilage | Osteochondral lesions in rabbit knees | Confirmed smooth hyaline cartilage from the regenerate cartilage after following it up for 6 months and hence allogeneic Sy-MSC based premature tissue constructs are a promising stem cell-based approach for cartilage defects |
| Li et al. [88] | To analyze cartilage repair tissue quality following Sy-MSCs transplantation in osteochondral defect | Osteochondral lesions in rabbit knees | Qualified the cartilage quality of the osteochondral lesions repaired through Sy-MSCs in rabbit knees revealed greater tissue quality in the treated animals |
| Lee et al. [89] | To determine the in-vivo effectiveness of Sy-MSCs encapsulated injectable PRP gel in the repair of damaged articular cartilage | Osteochondral lesions in rabbit knees | Platelet-rich plasma to deliver the Sy-MSCs to regenerate full-thickness chondral lesions. The treated group showed significant microscopic and macroscopic scores at 6 months follow-up |
| Shimomura et al. [90] | Combination therapy of Sy-MSCs with HA | Full-thickness cartilage lesions in rabbits | Subjects with Sy-MSCs and HA showed faster integration and improved osteochondral appearance while the controls demonstrated osteoarthritis-like features at 6-month follow-up |
| Li et al. [53] | Utilized Sy-MSCs from arthroscopic washing fluid loaded on to cross-linking hyper-branched polyPEGDA/HA hydrogel | Full-thickness cartilage defects generated in a murine model | Superior results obtained in repairing the chondral lesions compared to the controls or untreated groups. Human arthroscopic flushing fluid-MSCs are a novel and abundant MSC source that have high therapeutic value for cartilage regeneration |
| Shimomura et al. [55] | To assess the safety and efficacy of using a scaffold-free tissue-engineered construct (TEC) derived from autologous Sy-MSCs for effective cartilage repair | Osteochondral lesions of human knees | Autologous scaffold-free TEC derived from synovial MSCs may be used for regenerative cartilage repair via a sutureless and simple implantation procedure in osteochondral lesions of knee |
| Sekiya et al. [92] | To investigate the efficacy of Sy-MSC transplantation in cartilage defects | Femoral condyle chondral lesions of human knees | Transplantation of Sy-MSCs may be less invasive than mosaicplasty and autologous chondrocyte implantation and found superior in treating femoral osteochondral lesions |
In-Vivo Chondrogenicity by Sy-MSCs
Considering the common developmental lineage of the synovial membrane and articular cartilage, Sy-MSCs exhibit a greater capacity to accentuate chondrogenesis when applied to osteoarthritis models in animals. Ozeki et al. [49] in their study showed that Sy-MSCs halted the progression of collagenase-induced osteoarthritis in a rat model. They also evaluated the number of injections of Sy-MSCs needed for the management of osteoarthritis in their murine model. They have shown that the injected Sy-MSCs upregulated the expression of genes related to the chondroprotection such as PRG-4, BMP-2, and BMP-6 over 50-folds. Apart from chondroprotection, they also noted enhanced expression of TSG-6 responsible for immune-modulation and halt the inflammatory cascade [49].
Schmal et al. [86] compared the ability of the allogenic Sy-MSCs to repair the osteochondral lesions in the rabbit femur. They noted improved macroscopic regeneration in the Sy-MSC group compared to the controls. Pei et al. [87] in their study confirmed smooth hyaline cartilage from the regenerated cartilage after following it up for 6 months. Li et al. [88] qualified the cartilage quality of the osteochondral lesions repaired through Sy-MSCs in rabbit knees revealed greater tissue quality in the treated animals. Several studies investigated the effects of scaffolds in mediating the action of Sy-MSCs. Lee et al. [89] in their study investigated platelet-rich plasma to deliver the Sy-MSCs to regenerate full-thickness chondral lesions. The treated group showed significant microscopic and macroscopic scores at 6 months follow-up. Shimomura et al. [90] combined Sy-MSCs with hydroxyapatite (HA) and implanted them into full-thickness cartilage lesions in rabbits. They demonstrated that compared to the control group where only HA was used, subjects with Sy-MSCs and HA showed faster integration and improved osteochondral appearance while the controls demonstrated osteoarthritis-like features at 6-month follow-up. Various studies utilized porcine models to evaluate the porcine Sy-MSCs and found them effective in regenerating partial and full-thickness chondral lesions with improved ICRS score and macroscopic appearance [91].
With regards to human Sy-MSCs, Li et al. [53] utilized Sy-MSCs from arthroscopic washing fluid and studied their effect on murine models, and found superior results in repairing the chondral lesions compared to the controls or untreated groups. Shimomura et al. [55] performed autologous in-vitro cultured Sy-MSC transplantation obtained from an arthroscopic biopsy in 5 patients with a 1.5–3 cm2 chondral lesion. All the patients demonstrated full defect filling at 48 weeks assessed by MRI without any adverse events. Tissue integration and chondrogenesis were also assessed histologically and found to be strongly stained for Sekiya et al. [92] used Sy-MSC transplantation for symptomatic femoral condyle chondral lesions in ten patients and noted significant improvement on MRI scores post-intervention. Histological evaluation showed hyaline cartilage and fibrous cartilage without any deterioration in the Tegner Activity Level Scale [93–96]. Summary of the studies on in-vitro chondrogenicity by Sy-MSCs is given in Table 3.
Table 3.
In-vitro chondrogenicity by Sy-MSCs
| Author | Objective | Animal/medium | Inference |
|---|---|---|---|
| Koga et al. [79] | Compared the in chondrogenic potential of rabbit MSCs | Cartilage defects in rabbits | Demonstrated an enormous cartilage matrix production after 4 weeks by synovium and bone marrow-derived MSCs when admixed with collagen gel transplanted into rabbit cartilage defects |
| Shirasawa et al. [29] | Investigated the optimal conditions for in-vitro chondrogenesis of human Sy-MSCs and compared with BM-MSCs | Six donors during knee operations for ligament injuries | Demonstrated improved chondrogenesis with the combination of TGF-β3, dexamethasone, and BMP-2 with Sy-MSCs pellets. Sy-MSCs have a greater chondrogenesis potential than BM-MSCs |
| Pei et al. [83] | To define cocktails of growth factors that support the growth and chondrogenic differentiation of Sy-MSCs in chemically defined medium | Dulbecco’s modified Eagle’s medium | Proposed a two-step protocol for the derivation of chondrogenic Sy-MSCs: a cocktail of TGF-β1, IGF-I, and FGF-2 is applied first to induce cell growth followed by a cocktail of TGF-β1 and IGF-I applied to induce chondrogenesis |
| Sakimura et al. [84] | To demonstrate the induction of chondrogenesis by TGF-β1 from Sy-MSCs in a 3D polyglycolic acid scaffold | Human synovial membranes from the knees of patients with osteoarthritis or rheumatoid arthritis | Along with chondrogenic differentiation, the higher amounts of glycosaminoglycan production were observed when Sy-MSCs were seeded along with 3D polyglycolic acid scaffolds and simultaneous application of TGF-β1 and IGF-1 |
| Shintani et al. [85] | To compare the potential of BMP-2 and -7 and TGF-β1 to effect the chondrogenic differentiation of synovial explants | Synovial explants from the metacarpal joints of calves | Demonstrated the superior potential of BMP-2 and -7 in the induction of chondrogenesis than TGF-β1 |
| Miyamoto et al. [81] | To investigate the effects of osteogenic protein-1 with or without TGF-β1 on chondrogenesis of human MSCs in-vitro | Synovial membrane of patients with rheumatoid arthritis undergoing TKR | A higher dose of BMP-7 in the presence of TGF-β1 demonstrated the enhanced chondrogenesis by Sy-MSCs |
Engineered Chondrogenesis
The concept of “Engineered Chondrogenesis” came into existence to redifferentiate the de-differentiated chondrocytes in 3D culture systems [97]. Once de-differentiated chondrocytes are cultured in 3D culture systems, it is possible to recover the phenotypic and metabolic properties of chondrocytes. The limitations of 3D culture systems are due to the size of the tissue mass. 3D scaffolds either natural or synthetic that are made up of Sy-MSCs admixed with fibrin gel when cultured with chondrogenic media display a higher expression of cartilaginous characteristics with the expression of Sy-MSCs derived exosomes, proteins for type 2 collagen, aggrecan, and genes for SOX-9 expression [98].
The long-term benefits of 3D scaffolds are questionable, though the results of 3D scaffolds are encouraging. To overcome the potential risks, tissue-engineered constructs (TEC) composed of porcine Sy-MSCs and relevant ECMs generated in-vitro have been developed [99]. TEC cultured in a chondrogenic rich medium exhibits the higher expression of chondrogenic markers and their genes. TEC with human Sy-MSCs along with chondrogenic medium expressed the chondrogenic markers to a similar level as seen in TEC with porcine Sy-MSCs. With the presence of ascorbic acid, significant improvement in the mechanical strength of TEC is noted [100]. The adherence of more than 60% of cells was observed when Sy-MSCs were suspended on a rabbit cartilage defect. This phenomenon explains the direct adherence of Sy-MSCs to cartilage defects with minimal invasion and without the usage of periosteal coverage and scaffolds [101]. When physiological hydrostatic pressure is applied to Sy-MSCs in-vitro, it displays a significant expression of chondrogenic markers [102].
Future Perspectives
Kohno et al. [94] have shown that cellular yield and chondrogenic potential of Sy-MSCs were comparable in patients with rheumatoid arthritis and osteoarthritis and hence the indications for regenerative medicine using primary autologous Sy-MSCs are expanding. Overcoming the horizons of cellular therapy, exosomes derived from the Sy-MSC are being tried for their therapeutic potential in osteoarthritis. Zhu et al. [96] in their study showed the chondrocyte migration and proliferation when stimulated with Sy-MSC-derived exosomes. Being an inexhaustible autologous source, Sy-MSC-derived exosomes represent the future in the management of osteoarthritis and diseases of a similar kind.
Conclusion
Sy-MSCs demonstrates their regenerative mechanisms through paracrine signaling, direct cell–cell interactions, and extracellular vehicles. Sy-MSCs have been shown to possess superior chondrogenicity than other sources of mesenchymal stromal cells. Hence, Sy-MSCs remain a potential source of MSCs in the management of cartilage loss in osteoarthritis.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
For this type of study informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, Im HJ. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Research. 2017;5:16044. doi: 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: Structure, composition, and function. Sports Health. 2009;1(6):461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dieppe, P. (2002). Epidemiology of the rheumatic diseases (2nd ed., p. 377). In: A. J. Silman & M. C. Hochberg (Eds.), Oxford: Oxford University Press, 2001, £95.00. ISBN: 0192631497. International Journal of Epidemiology, 31(5):1079–1080. 10.1093/ije/31.5.1079-a
- 4.Karuppal R. Current concepts in the articular cartilage repair and regeneration. Journal of Orthopaedics. 2017;14(2):A1–A3. doi: 10.1016/j.jor.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang R, Ma J, Han J, Zhang W, Ma J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. American Journal of Translational Research. 2019;11(10):6275–6289. [PMC free article] [PubMed] [Google Scholar]
- 6.Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8):886. doi: 10.3390/cells8080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berebichez-Fridman R, Montero-Olvera PR. Sources and clinical applications of mesenchymal stem cells: State-of-the-art review. Sultan Qaboos University Medical Journal. 2018;18(3):e264–e277. doi: 10.18295/squmj.2018.18.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells—Current trends and future prospective. Bioscience Report. 2015;35(2):e00191. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gale AL, Linardi RL, McClung G, Mammone RM, Ortved KF. Comparison of the chondrogenic differentiation potential of equine synovial membrane-derived and bone marrow-derived mesenchymal stem cells. Frontiers in Veterinary Science. 2019;6:178. doi: 10.3389/fvets.2019.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li N, Gao J, Mi L, Zhang G, Zhang L, Zhang N, Huo R, Hu J, Xu K. Synovial membrane mesenchymal stem cells: Past life, current situation, and application in bone and joint diseases. Stem Cell Research & Therapy. 2020;11(1):381. doi: 10.1186/s13287-020-01885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keating A. Mesenchymal stromal cells. Current Opinion in Hematology. 2006;13(6):419–425. doi: 10.1097/01.moh.0000245697.54887.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeyaraman, M., Muthu, S., & Ganie, P. A. (2020). Does the source of mesenchymal stem cell have an effect in the management of osteoarthritis of the knee? Meta-analysis of randomized controlled trials. Cartilage, 1947603520951623. 10.1177/1947603520951623. [DOI] [PMC free article] [PubMed]
- 13.Wolfstadt JI, Cole BJ, Ogilvie-Harris DJ, Viswanathan S, Chahal J. Current concepts: The role of mesenchymal stem cells in the management of knee osteoarthritis. Sports Health. 2015;7(1):38–44. doi: 10.1177/1941738114529727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Communication and Signaling: CCS. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HJ, Im GI. Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: Greater doses of growth factor are necessary. Journal of Orthopaedic Research. 2009;27(5):612–619. doi: 10.1002/jor.20766. [DOI] [PubMed] [Google Scholar]
- 16.Khalifeh Soltani S, Forogh B, Ahmadbeigi N, Hadizadeh Kharazi H, Fallahzadeh K, Kashani L, Karami M, Kheyrollah Y, Vasei M. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: A pilot study. Cytotherapy. 2019;21(1):54–63. doi: 10.1016/j.jcyt.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Hsu SH, Huang TB, Cheng SJ, Weng SY, Tsai CL, Tseng CS, Chen DC, Liu TY, Fu KY, Yen BL. Chondrogenesis from human placenta-derived mesenchymal stem cells in three-dimensional scaffolds for cartilage tissue engineering. Tissue Engineering Part A. 2011;17(11–12):1549–1560. doi: 10.1089/ten.TEA.2010.0419. [DOI] [PubMed] [Google Scholar]
- 18.De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis and Rheumatism. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Fan J, Varshney RR, Ren L, Cai D, Wang DA. Synovium-derived mesenchymal stem cells: A new cell source for musculoskeletal regeneration. Tissue Engineering. Part B, Reviews. 2009;15(1):75–86. doi: 10.1089/ten.teb.2008.0586. [DOI] [PubMed] [Google Scholar]
- 20.Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World Journal of Stem Cells. 2014;6(5):526–539. doi: 10.4252/wjsc.v6.i5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Research and Therapy. 2016;7(1):125. doi: 10.1186/s13287-016-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caplan AI. Why are MSCs therapeutic? New data: New insight. Journal of Pathology. 2009;217(2):318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis and Rheumatism. 2005;52(8):2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 24.Koga H, Muneta T, Ju YJ, Nagase T, Nimura A, Mochizuki T, Ichinose S, von der Mark K, Sekiya I. Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells. 2007;25(3):689–696. doi: 10.1634/stemcells.2006-0281. [DOI] [PubMed] [Google Scholar]
- 25.De Bari C, Dell'Accio F, Karystinou A, Guillot PV, Fisk NM, Jones EA, McGonagle D, Khan IM, Archer CW, Mitsiadis TA, Donaldson AN, Luyten FP, Pitzalis C. A biomarker-based mathematical model to predict bone-forming potency of human synovial and periosteal mesenchymal stem cells. Arthritis and Rheumatism. 2008;58(1):240–250. doi: 10.1002/art.23143. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell and Tissue Research. 2007;327(3):449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 27.Nimura A, Muneta T, Koga H, Mochizuki T, Suzuki K, Makino H, Umezawa A, Sekiya I. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: Comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis and Rheumatism. 2008;58(2):501–510. doi: 10.1002/art.23219. [DOI] [PubMed] [Google Scholar]
- 28.Tateishi K, Ando W, Higuchi C, Hart DA, Hashimoto J, Nakata K, Yoshikawa H, Nakamura N. Comparison of human serum with fetal bovine serum for expansion and differentiation of human synovial MSC: Potential feasibility for clinical applications. Cell Transplantation. 2008;17(5):549–557. doi: 10.3727/096368908785096024. [DOI] [PubMed] [Google Scholar]
- 29.Shirasawa S, Sekiya I, Sakaguchi Y, Yagishita K, Ichinose S, Muneta T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: Optimal condition and comparison with bone marrow-derived cells. Journal of Cellular Biochemistry. 2006;97(1):84–97. doi: 10.1002/jcb.20546. [DOI] [PubMed] [Google Scholar]
- 30.Jones EA, Crawford A, English A, Henshaw K, Mundy J, Corscadden D, Chapman T, Emery P, Hatton P, McGonagle D. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: Detection and functional evaluation at the single-cell level. Arthritis and Rheumatism. 2008;58(6):1731–1740. doi: 10.1002/art.23485. [DOI] [PubMed] [Google Scholar]
- 31.Sekiya I, Ojima M, Suzuki S, Yamaga M, Horie M, Koga H, Tsuji K, Miyaguchi K, Ogishima S, Tanaka H, Muneta T. Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. Journal of Orthopaedic Research. 2012;30(6):943–949. doi: 10.1002/jor.22029. [DOI] [PubMed] [Google Scholar]
- 32.Jones EA, English A, Henshaw K, Kinsey SE, Markham AF, Emery P, McGonagle D. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis and Rheumatism. 2004;50(3):817–827. doi: 10.1002/art.20203. [DOI] [PubMed] [Google Scholar]
- 33.Revell PA, Al-Saffar N, Fish S, Osei D. Extracellular matrix of the synovial intimal cell layer. Annals of the Rheumatic Diseases. 1995;54(5):404–407. doi: 10.1136/ard.54.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neybecker P, Henrionnet C, Pape E, Grossin L, Mainard D, Galois L, Loeuille D, Gillet P, Pinzano A. Respective stemness and chondrogenic potential of mesenchymal stem cells isolated from human bone marrow, synovial membrane, and synovial fluid. Stem Cell Research and Therapy. 2020;11(1):316. doi: 10.1186/s13287-020-01786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizuno M, Katano H, Mabuchi Y, Ogata Y, Ichinose S, Fujii S, Otabe K, Komori K, Ozeki N, Koga H, Tsuji K, Akazawa C, Muneta T, Sekiya I. Specific markers and properties of synovial mesenchymal stem cells in the surface, stromal, and perivascular regions. Stem Cell Research and Therapy. 2018;9(1):123. doi: 10.1186/s13287-018-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatsushika D, Muneta T, Nakamura T, Horie M, Koga H, Nakagawa Y, et al. Repetitive allogeneic intraarticular injections of synovial mesenchymal stem cells promote meniscus regeneration in a porcine massive meniscus defect model. Osteoarthritis and Cartilage. 2014;22(7):941–950. doi: 10.1016/j.joca.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 37.Futami I, Ishijima M, Kaneko H, Tsuji K, Ichikawa-Tomikawa N, Sadatsuki R, Muneta T, Arikawa-Hirasawa E, Sekiya I, Kaneko K. Isolation and characterization of multipotential mesenchymal cells from the mouse synovium. PLoS ONE. 2012;7(9):e45517. doi: 10.1371/journal.pone.0045517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaplan JM, Youd ME, Lodie TA. Immunomodulatory activity of mesenchymal stem cells. Current Stem Cell Research and Therapy. 2011;6(4):297–316. doi: 10.2174/157488811797904353. [DOI] [PubMed] [Google Scholar]
- 39.Herrero C, Pérez-Simón JA. Immunomodulatory effect of mesenchymal stem cells. Brazilian Journal of Medical and Biological Research. 2010;43(5):425–430. doi: 10.1590/s0100-879x2010007500033. [DOI] [PubMed] [Google Scholar]
- 40.Zhao X, Zhao Y, Sun X, Xing Y, Wang X, Yang Q. Immunomodulation of MSCs and MSC-derived extracellular vesicles in osteoarthritis. Frontiers in Bioengineering and Biotechnology. 2020;8:575057. doi: 10.3389/fbioe.2020.575057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noronha NC, Mizukami A, Caliári-Oliveira C, Cominal JG, Rocha JLM, Covas DT, Swiech K, Malmegrim KCR. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Research and Therapy. 2019;10(1):131. doi: 10.1186/s13287-019-1224-y.Erratum.In:StemCellResTher.2019;10(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang M, Yuan Q, Xie L. Mesenchymal stem cell-based immunomodulation: Properties and clinical application. Stem Cells International. 2018;2018:3057624. doi: 10.1155/2018/3057624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bochev I, Elmadjian G, Kyurkchiev D, Tzvetanov L, Altankova I, Tivchev P, Kyurkchiev S. Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immunoglobulin production in vitro. Cell Biology International. 2008;32(4):384–393. doi: 10.1016/j.cellbi.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 45.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107(4):1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 46.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 47.Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, Tse HF, Fu QL, Lian Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death and Disease. 2016;7(1):e2062. doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bifari F, Lisi V, Mimiola E, Pasini A, Krampera M. Immune modulation by mesenchymal stem cells. Transfusion Medicine and Hemotherapy. 2008;35(3):194–204. doi: 10.1159/000128968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozeki N, Muneta T, Koga H, Nakagawa Y, Mizuno M, Tsuji K, Mabuchi Y, Akazawa C, Kobayashi E, Matsumoto K, Futamura K, Saito T, Sekiya I. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthritis Cartilage. 2016;24(6):1061–1070. doi: 10.1016/j.joca.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 50.Murata Y, Uchida S, Utsunomiya H, Hatakeyama A, Nakashima H, Chang A, Sekiya I, Sakai A. Synovial mesenchymal stem cells derived from the cotyloid fossa synovium have higher self-renewal and differentiation potential than those from the paralabral synovium in the hip joint. American Journal of Sports Medicine. 2018;46(12):2942–2953. doi: 10.1177/0363546518794664. [DOI] [PubMed] [Google Scholar]
- 51.Utsunomiya H, Uchida S, Sekiya I, Sakai A, Moridera K, Nakamura T. Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. American Journal of Sports Medicine. 2013;41(3):657–668. doi: 10.1177/0363546512473269. [DOI] [PubMed] [Google Scholar]
- 52.Fernandes TL, Kimura HA, Pinheiro CCG, Shimomura K, Nakamura N, Ferreira JR, Gomoll AH, Hernandez AJ, Bueno DF. Human synovial mesenchymal stem cells good manufacturing practices for articular cartilage regeneration. Tissue Engineering. Part C, Methods. 2018;24(12):709–716. doi: 10.1089/ten.TEC.2018.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Huang Y, Song J, Li X, Zhang X, Zhou Z, Chen D, Ma PX, Peng W, Wang W, Zhou G. Cartilage regeneration using arthroscopic flushing fluid-derived mesenchymal stem cells encapsulated in a one-step rapid cross-linked hydrogel. Acta Biomaterialia. 2018;79:202–215. doi: 10.1016/j.actbio.2018.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki S, Mizuno M, Sakamaki Y, et al. Morphological changes in synovial mesenchymal stem cells during their adhesion to the meniscus. Laboratory Investigation. 2020;100:916–927. doi: 10.1038/s41374-020-0421-8. [DOI] [PubMed] [Google Scholar]
- 55.Shimomura K, Yasui Y, Koizumi K, Chijimatsu R, Hart DA, Yonetani Y, et al. First-in-human pilot study of implantation of a scaffold-free tissue-engineered construct generated from autologous synovial mesenchymal stem cells for repair of knee chondral lesions. American Journal of Sports Medicine. 2018;46:2384–2393. doi: 10.1177/0363546518781825. [DOI] [PubMed] [Google Scholar]
- 56.Zeng N, Yan ZP, Chen XY, Ni GX. Infrapatellar fat pad and knee osteoarthritis. Aging and Disease. 2020;11(5):1317–1328. doi: 10.14336/AD.2019.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Favero M, El-Hadi H, Belluzzi E, Granzotto M, Porzionato A, Sarasin G, Rambaldo A, Iacobellis C, Cigolotti A, Fontanella CG, Natali A, Ramonda R, Ruggieri P, De Caro R, Vettor R, Rossato M, Macchi V. Infrapatellar fat pad features in osteoarthritis: A histopathological and molecular study. Rheumatology (Oxford) 2017;56(10):1784–1793. doi: 10.1093/rheumatology/kex287. [DOI] [PubMed] [Google Scholar]
- 58.Eymard F, Pigenet A, Citadelle D, Tordjman J, Foucher L, Rose C, Flouzat Lachaniette CH, Rouault C, Clément K, Berenbaum F, Chevalier X, Houard X. Knee and hip intra-articular adipose tissues (IAATs) compared with autologous subcutaneous adipose tissue: A specific phenotype for a central player in osteoarthritis. Annals of the Rheumatic Diseases. 2017;76(6):1142–1148. doi: 10.1136/annrheumdis-2016-210478. [DOI] [PubMed] [Google Scholar]
- 59.Belluzzi E, Stocco E, Pozzuoli A, Granzotto M, Porzionato A, Vettor R, De Caro R, Ruggieri P, Ramonda R, Rossato M, Favero M, Macchi V. Contribution of infrapatellar fat pad and synovial membrane to knee osteoarthritis pain. BioMed Research International. 2019;2019:6390182. doi: 10.1155/2019/6390182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greif DN, Kouroupis D, Murdock CJ, Griswold AJ, Kaplan LD, Best TM, Correa D. Infrapatellar fat pad/synovium complex in early-stage knee osteoarthritis: potential new target and source of therapeutic mesenchymal stem/stromal cells. Frontiers in Bioengineering and Biotechnology. 2020;8:860. doi: 10.3389/fbioe.2020.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang LF, Fang JH, Wu LD. Role of infrapatellar fat pad in pathological process of knee osteoarthritis: Future applications in treatment. World Journal of Clinical Cases. 2019;7(16):2134–2142. doi: 10.12998/wjcc.v7.i16.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Research. Part C, Embryo Today. 2003;69(2):144–155. doi: 10.1002/bdrc.10015. [DOI] [PubMed] [Google Scholar]
- 63.Shintani N, Kurth T, Hunziker EB. Expression of cartilage-related genes in bovine synovial tissue. Journal of Orthopaedic Research. 2007;25(6):813–819. doi: 10.1002/jor.20345. [DOI] [PubMed] [Google Scholar]
- 64.Otero M, Goldring MB. Cells of the synovium in rheumatoid arthritis. Chondrocytes. Arthritis Research Therapy. 2007;9(5):220. doi: 10.1186/ar2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pei M, Luo J, Chen Q. Enhancing and maintaining chondrogenesis of synovial fibroblasts by cartilage extracellular matrix protein matrilins. Osteoarthritis Cartilage. 2008;16(9):1110–1117. doi: 10.1016/j.joca.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kangari P, Talaei-Khozani T, Razeghian-Jahromi I, Razmkhah M. Mesenchymal stem cells: Amazing remedies for bone and cartilage defects. Stem Cell Research and Therapy. 2020;11(1):492. doi: 10.1186/s13287-020-02001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loebel C, Burdick JA. Engineering stem and stromal cell therapies for musculoskeletal tissue repair. Cell Stem Cell. 2018;22(3):325–339. doi: 10.1016/j.stem.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bobick BE, Chen FH, Le AM, Tuan RS. Regulation of the chondrogenic phenotype in culture. Birth Defects Research. Part C, Embryo Today. 2009;87(4):351–371. doi: 10.1002/bdrc.20167. [DOI] [PubMed] [Google Scholar]
- 69.Archer CW, Francis-West P. The chondrocyte. International Journal of Biochemistry and Cell Biology. 2003;35(4):401–404. doi: 10.1016/s1357-2725(02)00301-1. [DOI] [PubMed] [Google Scholar]
- 70.Kim YJ, Kim HJ, Im GI. PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochemical and Biophysical Research Communications. 2008;373(1):104–108. doi: 10.1016/j.bbrc.2008.05.183. [DOI] [PubMed] [Google Scholar]
- 71.Lefebvre V, Dvir-Ginzberg M. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connective Tissue Research. 2017;58(1):2–14. doi: 10.1080/03008207.2016.1183667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kozhemyakina E, Lassar AB, Zelzer E. A pathway to bone: Signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. 2015;142(5):817–831. doi: 10.1242/dev.105536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. Journal of Cellular Biochemistry. 2006;97(1):33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 74.Cancedda R, Castagnola P, Cancedda FD, Dozin B, Quarto R. Developmental control of chondrogenesis and osteogenesis. International Journal of Developmental Biology. 2000;44(6):707–714. [PubMed] [Google Scholar]
- 75.Yoon YM, Oh CD, Kang SS, Chun JS. Protein kinase A regulates chondrogenesis of mesenchymal cells at the post-precartilage condensation stage via protein kinase C-alpha signaling. Journal of Bone and Mineral Research. 2000;15(11):2197–2205. doi: 10.1359/jbmr.2000.15.11.2197. [DOI] [PubMed] [Google Scholar]
- 76.Bobick BE, Kulyk WM. Regulation of cartilage formation and maturation by mitogen-activated protein kinase signaling. Birth Defects Research. Part C, Embryo Today. 2008;84(2):131–154. doi: 10.1002/bdrc.20126. [DOI] [PubMed] [Google Scholar]
- 77.De Bari C, Dell'Accio F, Vandenabeele F, Vermeesch JR, Raymackers JM, Luyten FP. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. Journal of Cell Biology. 2003;160(6):909–918. doi: 10.1083/jcb.200212064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mochizuki T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, Koga H, Sekiya I. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis and Rheumatism. 2006;54(3):843–853. doi: 10.1002/art.21651. [DOI] [PubMed] [Google Scholar]
- 79.Koga H, Muneta T, Nagase T, Nimura A, Ju YJ, Mochizuki T, Sekiya I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell and Tissue Research. 2008;333(2):207–215. doi: 10.1007/s00441-008-0633-5. [DOI] [PubMed] [Google Scholar]
- 80.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 81.Miyamoto C, Matsumoto T, Sakimura K, Shindo H. Osteogenic protein-1 with transforming growth factor-beta1: Potent inducer of chondrogenesis of synovial mesenchymal stem cells in vitro. Journal of Orthopaedic Science. 2007;12(6):555–561. doi: 10.1007/s00776-007-1176-4. [DOI] [PubMed] [Google Scholar]
- 82.Nishimura K, Solchaga LA, Caplan AI, Yoo JU, Goldberg VM, Johnstone B. Chondroprogenitor cells of synovial tissue. Arthritis and Rheumatism. 1999;42(12):2631–2637. doi: 10.1002/1529-0131(199912)42:12<2631::AID-ANR18>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 83.Pei M, He F, Vunjak-Novakovic G. Synovium-derived stem cell-based chondrogenesis. Differentiation. 2008;76(10):1044–1056. doi: 10.1111/j.1432-0436.2008.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sakimura K, Matsumoto T, Miyamoto C, Osaki M, Shindo H. Effects of insulin-like growth factor I on transforming growth factor beta1 induced chondrogenesis of synovium-derived mesenchymal stem cells cultured in a polyglycolic acid scaffold. Cells, Tissues, Organs. 2006;183(2):55–61. doi: 10.1159/000095509. [DOI] [PubMed] [Google Scholar]
- 85.Shintani N, Hunziker EB. Chondrogenic differentiation of bovine synovium: bone morphogenetic proteins 2 and 7 and transforming growth factor beta1 induce the formation of different types of cartilaginous tissue. Arthritis and Rheumatism. 2007;56(6):1869–1879. doi: 10.1002/art.22701. [DOI] [PubMed] [Google Scholar]
- 86.Schmal H, Kowal JM, Kassem M, Seidenstuecker M, Bernstein A, Böttiger K, Xiong T, Südkamp NP, Kubosch EJ. Comparison of regenerative tissue quality following matrix-associated cell implantation using amplified chondrocytes compared to synovium-derived stem cells in a rabbit model for cartilage lesions. Stem Cells International. 2018;2018:4142031. doi: 10.1155/2018/4142031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pei M, He F, Boyce BM, Kish VL. Repair of full-thickness femoral condyle cartilage defects using allogeneic synovial cell-engineered tissue constructs. Osteoarthritis Cartilage. 2009;17(6):714–722. doi: 10.1016/j.joca.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 88.Li H, Qian J, Chen J, Zhong K, Chen S. Osteochondral repair with synovial membrane-derived mesenchymal stem cells. Molecular Medicine Reports. 2016;13(3):2071–2077. doi: 10.3892/mmr.2016.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee JC, Min HJ, Park HJ, Lee S, Seong SC, Lee MC. Synovial membrane-derived mesenchymal stem cells supported by platelet-rich plasma can repair osteochondral defects in a rabbit model. Arthroscopy. 2013;29(6):1034–1046. doi: 10.1016/j.arthro.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 90.Shimomura K, Moriguchi Y, Ando W, Nansai R, Fujie H, Hart DA, Gobbi A, Kita K, Horibe S, Shino K, Yoshikawa H, Nakamura N. Osteochondral repair using a scaffold-free tissue-engineered construct derived from synovial mesenchymal stem cells and a hydroxyapatite-based artificial bone. Tissue Engineering Part A. 2014;20(17–18):2291–2304. doi: 10.1089/ten.tea.2013.0414. [DOI] [PubMed] [Google Scholar]
- 91.To K, Zhang B, Romain K, Mak C, Khan W. Synovium-Derived mesenchymal stem cell transplantation in cartilage regeneration: A PRISMA review of in vivo studies. Frontiers in Bioengineering and Biotechnology. 2019;7:314. doi: 10.3389/fbioe.2019.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sekiya I, Muneta T, Horie M, Koga H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clinical Orthopaedics and Related Research. 2015;473(7):2316–2326. doi: 10.1007/s11999-015-4324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kubosch EJ, Lang G, Furst D, Kubosch D, Izadpanah K, Rolauffs B, Sudkamp NP, Schmal H. The potential for synovium-derived stem cells in cartilage repair. Current Stem Cell Research and Therapy. 2018;13(3):174–184. doi: 10.2174/1574888X12666171002111026. [DOI] [PubMed] [Google Scholar]
- 94.Kohno Y, Mizuno M, Ozeki N, Katano H, Komori K, Fujii S, Otabe K, Horie M, Koga H, Tsuji K, Matsumoto M, Kaneko H, Takazawa Y, Muneta T, Sekiya I. Yields and chondrogenic potential of primary synovial mesenchymal stem cells are comparable between rheumatoid arthritis and osteoarthritis patients. Stem Cell Research and Therapy. 2017;8(1):115. doi: 10.1186/s13287-017-0572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kubosch EJ, Heidt E, Niemeyer P, Bernstein A, Südkamp NP, Schmal H. In-vitro chondrogenic potential of synovial stem cells and chondrocytes allocated for autologous chondrocyte implantation—A comparison: Synovial stem cells as an alternative cell source for autologous chondrocyte implantation. International Orthopaedics. 2017;41(5):991–998. doi: 10.1007/s00264-017-3400-y. [DOI] [PubMed] [Google Scholar]
- 96.Zhu Y, Wang Y, Zhao B, Niu X, Hu B, Li Q, Zhang J, Ding J, Chen Y, Wang Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Research and Therapy. 2017;8(1):64. doi: 10.1186/s13287-017-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chung C, Burdick JA. Engineering cartilage tissue. Advanced Drug Delivery Reviews. 2008;60(2):243–262. doi: 10.1016/j.addr.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pei M, He F, Kish VL, Vunjak-Novakovic G. Engineering of functional cartilage tissue using stem cells from synovial lining: A preliminary study. Clinical Orthopaedics and Related Research. 2008;466(8):1880–1889. doi: 10.1007/s11999-008-0316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ando W, Tateishi K, Hart DA, Katakai D, Tanaka Y, Nakata K, Hashimoto J, Fujie H, Shino K, Yoshikawa H, Nakamura N. Cartilage repair using an in vitro generated scaffold-free tissue-engineered construct derived from porcine synovial mesenchymal stem cells. Biomaterials. 2007;28(36):5462–5470. doi: 10.1016/j.biomaterials.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 100.Ando W, Tateishi K, Katakai D, Hart DA, Higuchi C, Nakata K, Hashimoto J, Fujie H, Shino K, Yoshikawa H, Nakamura N. In vitro generation of a scaffold-free tissue-engineered construct (TEC) derived from human synovial mesenchymal stem cells: Biological and mechanical properties and further chondrogenic potential. Tissue Engineering Part A. 2008;14(12):2041–2049. doi: 10.1089/ten.tea.2008.0015. [DOI] [PubMed] [Google Scholar]
- 101.Koga H, Shimaya M, Muneta T, Nimura A, Morito T, Hayashi M, Suzuki S, Ju YJ, Mochizuki T, Sekiya I. Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Research and Therapy. 2008;10(4):R84. doi: 10.1186/ar2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sakao K, Takahashi KA, Arai Y, Inoue A, Tonomura H, Saito M, Yamamoto T, Kanamura N, Imanishi J, Mazda O, Kubo T. Induction of chondrogenic phenotype in synovium-derived progenitor cells by intermittent hydrostatic pressure. Osteoarthritis Cartilage. 2008;16(7):805–814. doi: 10.1016/j.joca.2007.10.021. [DOI] [PubMed] [Google Scholar]