Abstract
CD36, also known as the scavenger receptor B2, is a multifunctional receptor widely expressed in various organs. CD36 plays a crucial role in the uptake of long-chain fatty acids, the main metabolic substrate in myocardial tissue. The maturation and transportation of CD36 is regulated by post-translational modifications, including phosphorylation, ubiquitination, glycosylation, and palmitoylation. CD36 is decreased in pathological cardiac hypertrophy caused by ischaemia–reperfusion and pressure overload, and increased in diabetic cardiomyopathy and atherosclerosis. Deficiency of CD36 alleviates diabetic cardiomyopathy and atherosclerosis, while overexpression of CD36 eliminates ischaemia–reperfusion damage, together suggesting that CD36 is closely associated with the progression of cardiovascular diseases and may be a new therapeutic target. This review summarizes the regulation and post-translational modifications of CD36 and evaluates its role in cardiovascular diseases and its potential as a therapeutic target.
Keywords: CD36, Post-translational modification, Diabetic cardiomyopathy, Ischaemia–reperfusion, Cardiac hypertrophy
1. Introduction
CD36 [also known as fatty acid translocase (FAT), glycoprotein IIIb (GPIIIb), or glycoprotein IV], is a member of the class B2 scavenger receptor family, which includes low-density lipoprotein (LDL), high-density lipoprotein (HDL)-bound scavenger receptor B1, and HDL-bound scavenger receptor B3.1–3 The ligands of CD36 are mainly divided into lipid and protein molecules. The former includes oxidized LDL particles,4 long-chain fatty acids (LCFA),5 phospholipids,6 and others, and the latter includes thrombospondin,7 advanced glycation end products (AGEs),8,9 advanced oxidation protein products (AOPPs),10,11 S100 family proteins (S100-A8, S100-A9, S100-A12),12–14 growth hormone-releasing peptide,15 cell-derived microparticles,16 and amyloid proteins.17
CD36 is expressed in various tissues, including endothelial cells,18 cardiac muscle cells,19 renal tubular epithelial cells,20 liver cells,21 adipocytes,22 platelets,23 and macrophages,24 and is involved in many pathophysiological processes, including immune regulation25 and metabolic regulation.26 For example, CD36 on the cytomembrane of endothelial cells optimizes fatty acid uptake in tissues.18 It also facilitates the uptake of AOPPs in renal tubular epithelial cells, which leads to lipotoxicity and renal tubule interstitial fibrosis in diabetic nephropathy.10 In liver cells CD36 is involved in fatty acid metabolism,27 while on platelets it is related to platelet activation.28 In myocardial tissue, CD36 mediates the uptake of long-chain fatty acids.29 Fatty acids provide over 70% of the adenosine triphosphate (ATP)for myocardial tissue and most of the fatty acids enter the cells through protein-mediated diffusion. Importantly, 70% of this intake is mediated by CD36;29 therefore, CD36 plays an essential role in myocardial lipid metabolism.
The synthesis and translocation of CD36 are affected by many stimuli.30 Short-term stimulation of insulin promotes the translocation of CD36 from the endosome to the cell membrane,31 while long-term stimulation induces protein synthesis.32 Hyperglycaemia and hyperlipidaemia also facilitate the translocation of CD36 to the cell membrane.33 It has been proven that impaired synthesis and abnormal distribution of CD36 shorten myocardial energy supply,34 resulting in the impairment of myocardial contractile function.35 Pressure overload decreases the level of one of the controllers of CD36 synthesis, nuclear receptor peroxisome proliferator-activated receptor α (PPARα),36 resulting in insufficient myocardial fatty acid uptake and the accumulation of toxic lipids, ultimately leading to heart failure. However, downregulating CD36 in diabetic cardiomyopathy reduces toxic lipids37 and improves the contractile function of the heart. Therefore, the influence of CD36 on the myocardium may not be consistent and largely depends on the pathological background.
In this review, we summarize gene polymorphism and post-translational modification of CD36 and focus on its role in cardiovascular diseases, particularly ischaemia/reperfusion, diabetic cardiomyopathy, pathological cardiac hypertrophy, physiological cardiac hypertrophy, and atherosclerosis. We also provide some suggestions for basic and clinical research on CD36, to emphasize the significance of CD36 in the cardiovascular system and shed light on its potential as a therapeutic target.
2. Cd36 gene polymorphism
The human CD36 gene is located on 7q21.11, which has 36 kb and consists of 19 exons with multiple upstream regulatory promoters.38 Single nucleotide polymorphisms (SNPs) of CD36 are relatively common and have been shown to be closely related to several cardiovascular diseases.
The rs1761663 of the CD36 gene has independent effects on body mass index and left ventricular mass.39 A study conducted among young adult populations in Australia showed that the rs1527479 and rs1984112 of CD36 are related to exercise, heart rate, and lipid oxidation.40 In the Chinese Han population, the AG genotype of rs1761667 is associated with an increased risk of coronary heart disease (odds ratio 2.337, 95% confidence interval 1.336–4.087; P = 0.003), and the plasma oxLDL level of patients with the AG genotype was significantly higher than that of patients with the GG and AA genotypes.41 For rs1049673, rs7755, and rs321159 sites, patients with premature coronary heart disease have a family genetic predisposition at high LDL-C levels with GA, AA, and TT genotypes.42 Four common CD36 SNPs were found (rs1049673, rs7755, rs3211956, and rs3173798) to be significantly associated with extreme lipid profiles in the male Han population in northern China, but no significance was identified in the female population.43 In addition, rs3211938, which affects susceptibility to malaria, has been shown to resist metabolic syndrome, increase high-density lipoprotein cholesterol, and reduce triglycerides.44 These genetic studies suggest that CD36 gene polymorphism plays a predictive role in distinguishing cardiac disease risk factors. Therefore, early prevention might be carried out for susceptible people, using CD36 genotyping.
3. Cd36 protein
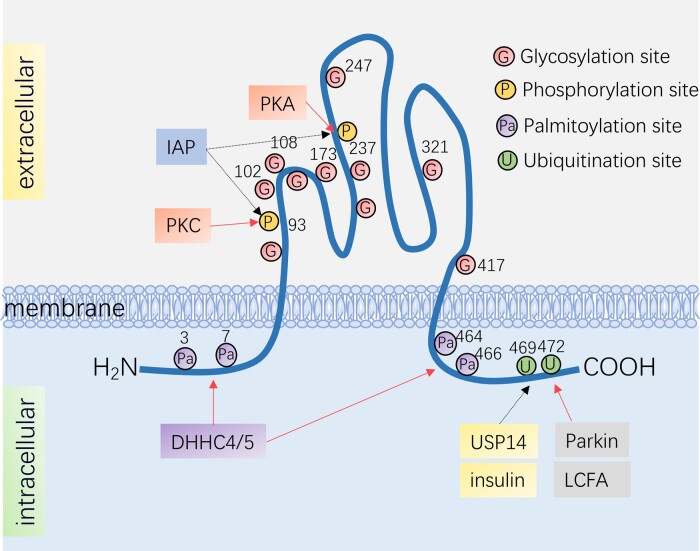
Human CD36 has a total length of 472 amino acids and a predicted molecular weight of 53 kd,45 although due to glycosylation, the actual molecular weight is 88 kd.46 CD36 contains two phosphorylation sites and four palmitoylation sites, which are distributed at the terminals of NH2 and COOH. Two ubiquitination sites are also found at the COOH terminus.
Knowledge of the crystal structure of CD36 remains unclear, but insight into the potential mechanism of FA transport by CD36 can be derived from the recently reported crystal structure of the CD36 family member, lysosomal integral membrane protein-2 (LIMP-2).47 LIMP-2 is a helical bundle where β-glucocerebrosidase binds, and where ligands are most likely to bind to CD36. The crystal structure also shows the existence of a large cavity that serves as a tunnel through which cholesterol (esters) are delivered from the bound lipoprotein to the outer leaflet of the plasma membrane. CD36 is a double transmembrane protein that cannot form a channel by itself to allow for fatty acids to transfer through to the inside.48 Interestingly, the outer ring of CD36 contains a large hydrophobic cavity, which provides a docking site for fatty acids and other hydrophobic ligands,49 facilitating the attraction of hydrophobic ligands to the cell surface, thus promoting the transportation of fatty acids into the cell.
3.1 Cd36 signalling
CD36 is a membrane protein that is synthesized in the polyribosome and then transferred to the endoplasmic reticulum and Golgi apparatus for further processing, and then finally transported by the endosome to the cytomembrane.50 Under the stimulation of insulin51 or contraction,31 it moves to the lipid rafts of the cell membrane to facilitate LCFA uptake, which may be associated with the activation of PI3K-Akt and adenosine monophosphate (AMP) kinase (AMPK). CD36 is not only located in plasma membranes and endosomes but is also distributed on mitochondria,52 although its specific function on mitochondria remains unclear.
The transfer of CD36 from the endosome to the cell membrane surface is mainly regulated by insulin and contraction stimulation,53 which induces CD36 translocation through different signalling pathways, but finally converges into Rab GTPase proteins AS160 and Rab 8a, accelerating CD36 translocation with the (guanosine triphosphate) GTP/ (guanosine diphosphate) GDP cycle.54
PI3K in the insulin signalling pathway has been shown to be critical for CD36 translocation, supported by the fact that specific inhibitors of PI3K (Wortmannin and LY-294002) can eliminate CD36 translocation and downregulate LCFA uptake induced by insulin.55 In addition, among the two downstream effectors of PI3K, PKC-zeta also participates in insulin-induced CD36 translocation, and the other downstream effector, Akt, is involved in the regulation of FOXO1 activity that acts on the CD36 promoter region to regulate CD36 transcription.56 It is worth mentioning that insulin signalling also promotes the translocation of GLUT4. Under the stimulation of insulin, CD36 and GLUT4 are simultaneously transferred to the sarcolemma, resulting in increased fatty acid and glucose uptake. This is contradictory to the Randle cycle phenomenon, which states that a competitive inhibition exists between free fatty acid (FFA) oxidation and glucose utilization.53 Further exploration of the time response of GLUT4 and CD36 to different insulin concentrations, and knowledge of the distinction between the vesicle transport pathway and subcellular trafficking will help in understanding this contradictory phenomenon.57 Recent studies have found that specific family members of vesicle-associated membrane proteins (VAMPs) mediate the intracellular transport of either CD36 or GLUT4. Specifically, VAMP4 translocates CD36 between the mother endosomal compartment and a hypothetical endosomal CD36-specific intermediate compartment,58 while VAMP5 and VAMP7 mediate GLUT4 homing to intracellular compartments.58,59 In particular, the distinct subcellular trafficking of CD36 and GLUT4 has been discussed in detail in a recent review.57
Similar to insulin, contraction stimulation promotes the translocation of GLUT4 and CD36 simultaneously.60 Importantly, contraction stimulation is in an AMPK-dependent manner.61 It has been confirmed that both 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) and oligomycin can induce CD36 translocation by activating AMPK to promote LCFA uptake. Along with various negative feedback mechanisms, AMPK activation-induced CD36 translocation regulates AMPK activity in turn; CD36 forms a protein complex with the AMPK kinase LKB1 and the Src kinase Fyn,62 and this complex promotes Fyn phosphorylation of LKB1 and its nuclear sequestration, hindering LKB1 activation of AMPK. This feedback mechanism facilitates the cells to respond appropriately to the outside fatty acid and balance the fatty acid oxidation and uptake.
3.2 Cd36 post-translational modifications
The synthesis, distribution, and function of CD36 are largely affected by its post-transcriptional modification, including ubiquitination, glycosylation, phosphorylation, and palmitoylation. It is worth noting that although there are many acetylation sites on CD36, further research is required to reveal their effect.
3.2.1 Cd36 ubiquitination
Protein ubiquitination is the coupling of protein and ubiquitin, mediated by a ubiquitin ligase (E3).63 CD36 is the target of the E3 ligase, Parkin64 (Figure 1). Differing from the degradation effect of ubiquitination, the monoubiquitylation of CD36 by Parkin enhances its stability and increases its plasma membrane level. In the presence of Parkin,65 LCFA-induced polyubiquitination and degradation of CD36 is significantly down-regulated, likely because one of the ubiquitination sites of LCFA is also the target of Parkin. A recent study confirmed that USP14 is a deubiquitinating enzyme that mediates CD36 deubiquitylation on macrophages. USP14 cleaves ubiquitin chains from ubiquitinated CD36 proteins, thus avoiding the fate of CD36 being transported into the proteasome for degradation66 (Figure 1). However, whether Parkin and USP14 act as E3 ligases and deubiquitinating enzymes in cardiomyocytes remains unclear.
Figure 1.
Post-translational modifications of CD36. There are two ubiquitination sites on the N-terminus of CD36: Lys472 and Lys469. CD36 ubiquitination levels decrease upon insulin stimulation while increase with the presence of LCFA; Parkin is CD36's E3 ubiquitin ligase, which monoubiquitinates CD36 and enhances its stability, while USP14 mediates CD36 deubiquitylation. There are four palmitoylation sites at the C-terminus and the N-terminus of CD36, Cys3, Cys7, Cys464, Cys466, and DHHC4/5 are palmitoyl transferases that promote palmitoylation of CD36; CD36 also has two phosphorylation sites, Thr93 and Ser237, which are phosphorylated by PKC, PKA, and dephosphorylated by IAP, respectively. The large extracellular loop has 10 observed N-linked glycosylation sites. DHHC, Asp-His-His-Cys; IAP, intestinal alkaline phosphatase; LCFA, long-chain fatty acid; PKA, protein kinase A; PKC, protein kinase C; USP14, ubiquitin specific peptidase 14.
LCFA, a ligand of CD36, significantly enhances the ubiquitination of CD3667 and promotes its degradation after long-term interaction68 (Figure 1). This is a negative factor for the cellular uptake of fatty acids. Recent studies have shown that ubiquitinated CD36 in myocytes stabilizes the structure of insulin receptor substrate 169 and thus maintains insulin signalling. Given that insulin reduces its ubiquitination68 (Figure 1), CD36 may be involved in the self-regulation of the insulin signalling pathway. Moreover, CD36 ubiquitination in macrophages inhibits the formation of atherosclerosis by decreasing fatty acid uptake.70 However, the role of ubiquitinated CD36 in the heart has not yet been elucidated.
3.2.2 Cd36 glycosylation
The glycosylation of CD36 is N-linked at asparagine residues (Asn) mediated by glycosyltransferase.71,72 There are 10 glycosylation modification sites of CD36 in humans, located in the extracellular segment of CD36.73
Apart from increasing the molecular weight of CD36, glycosylation could also stabilize the tertiary folding of polypeptides and is therefore essential for forming the spatial structure of CD36.74 It affects the folding of CD36, thus influencing its correct translocation to the cell membrane. It has been proven that carboxyl-terminal sites Asn247, Asn321, and Asn417 are indispensable for CD36 trafficking.71 Mutations in Asn108 and Asn173 sites result in the abnormal distribution of CD36 on the COS m6 cell membrane.73 Mutations in Asn 102 of CD36 have been found in spontaneously hypertensive rats (SHRs).75 Since Asn102 is located in the fatty acid-binding pocket, mutations at this glycosylation site may have a greater potential to affect fatty acid docking in the CD36 pocket, thereby affecting fatty acid transport. CD36 protein levels in SHR are significantly down-regulated and fatty acid intake is reduced, which may be related to the glycosylation mutation of Asn102. However, the role of Asn102 in SHRs has not yet been confirmed experimentally. Further research is required to clarify the role of Asn102 and other glycosylation sites in cardiovascular diseases.
3.2.3 Cd36 phosphorylation
CD36 has two phosphorylation sites, Thr92 and Ser237, phosphorylated by protein kinase C (PKC)76 and protein kinase A (PKA),77 respectively (Figure 1). CD36 in small intestinal epithelial cells is dephosphorylated by intestinal alkaline phosphatase (IAP)78 (Figure 1). In platelets, phosphorylation of Thr92 mediates the binding of CD36 and thrombospondin.79 Phosphorylation of CD36 at Thr92 is also necessary for the adhesion of plasmodium falciparum-infected erythrocytes to human dermal microvascular endothelial cells under flow condition.80 CD36 phosphorylated at Ser237 downregulates the fatty acid uptake rate of platelets and enterocytes.78,81 However, in vivo studies are insufficient to validate the in vitro findings of CD36 phosphorylation.
3.2.4 Cd36 palmitoylation
Most members of the DHHC (Asp–His–His–Cys) family of proteins have palmitoyl transferase activity, and these members are confirmed to include the main palmitoyl acyl transferases (PATs).82 DHHC4/5 have been shown to be the PATs of CD36 in adipocytes (Figure 1). The absence of either DHHC4 or DHHC5 prevents palmitoylation and the insertion of CD36 on the adipose membrane, thereby destroying the CD36-dependent fatty acid uptake.83 Selenoprotein K (SelK),84 which is neither a PAT nor a palmitoyl-protein thioesterase (PPT), is also required for palmitoylation of CD36 in macrophages, suggesting that other proteins may also be involved in the palmitoylation of CD36.
CD36 has four palmitoylation sites: Cys3, Cys7, Cys464, and Cys466 (Figure 1). Palmitoylation is strengthened by insulin stimulation. Combined mutations of these four palmitoylation sites hinder CD36 translocation to the cell membrane for fatty acid uptake, even in the presence of insulin and AMPK.85 With the inhibition of CD36 palmitoylation by ceruloplasmin,86 a palmitoylation-specific inhibitor, the processing of CD36 at the endoplasmic reticulum and transport through the secretory pathway extends from 90 min to 4 h in melanoma cells, indicating that palmitoylation is essential for the transport and translocation of CD36.
Increased palmitoylation of CD36 is found in liver steatosis and fibrosis.27 The decrease in CD36 palmitoylation downregulates the uptake of fatty acids and helps to balance the fatty acid metabolism in the liver cells, thus eliminating liver steatosis and fibrosis.27 However, no research has revealed the effects of the palmitoylation of CD36 in the cardiovascular system.
4. Cd36 and cardiovascular diseases
4.1 Cd36 and ischaemia/reperfusion
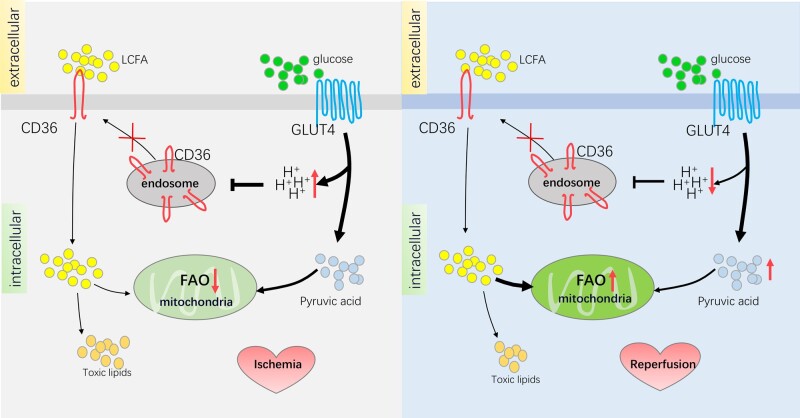
Ischaemia/reperfusion is characterized by the abrupt interruption, followed by the subsequent restoration, of blood flow.87 The cut-off of oxygen and nutrition results in various metabolic changes,88 including alterations in lipid metabolism. The change of CD36 in ischaemia/reperfusion has been shown by using (3) H-labelled metabolic substrates to measure the metabolic changes in Wistar rat hearts at different stages during ischaemia/reperfusion.89 During ischaemia, CD36 of the sarcolemma is down-regulated by 32%, and the fatty acid oxidation rate decreases by 95%. In the reperfusion stage, CD36 levels remained low, but the fatty acid oxidation rate returned to the pre-ischaemic state.89 The increased pH of the endosome may contribute to the low level of CD36 throughout the whole ischaemia/reperfusion period.90 The translocation of CD36 to the sarcolemma is significantly enhanced when the pH of the endosome is high, while a decrease in pH inhibits this translocation. During ischaemia, myocardial glycolysis increased by 86% and lactic acid level increased by seven-fold89 (Figure 2), leading to a low pH state and subsequently suppressed CD36 translocation. When shifting to the reperfusion phase, the lactic acid in cardiomyocytes could not be effectively eliminated in a short period; the cells may still be at a low pH, and CD36 translocation is still suppressed (Figure 2).
Figure 2.
Effects of CD36 in ischaemic/reperfusion. During the ischaemic phase, the distribution of CD36 on the cell membrane decreases, leading to a decline in LCFA uptake and LCFA entering the mitochondria for aerobic oxidation, while GLUT4 increases on the cell membrane, which results in the uptake of more glucose. Because of ischaemia and hypoxia, the anaerobic glycolysis process is enhanced, and produces a large quantity of protons, thus resulting in a low cytoplasmic pH. Hydrion subsequently prevents the transport of CD36 from the endosome to the cell membrane, and further inhibits the distribution of CD36 on the cell membrane. The relatively increased pyruvate acid enters the mitochondria for aerobic oxidation to produce ATP. During this process, the decrease in CD36 prevents the accumulation of toxic lipids and indirectly promotes the aerobic oxidation of glucose, which is beneficial to the survival of myocardial cells during the ischaemic phase. During the reperfusion phase, the anaerobic glycolysis process of glucose decreased and the rate of proton production decreased. However, due to the low pH caused by the large number of protons accumulated in the ischaemic phase, the membrane distribution of CD36 remains at a low level. At the same time, the rate of LCFA entering the mitochondria for aerobic oxidation is enhanced because of the accumulation of triglycerides in the ischaemic phase, which is essential for energy production. During this process, the low level of CD36 helps reduce the accumulation of toxic lipids, and the aerobic oxidation of LCFAs provides most of the energy for the myocardium.
At the same oxygen consumption level, glucose provides more energy than lipids, so it is the preferred choice for maintaining myocardial function.91,92 The reduction of CD36 is beneficial to the conversion of the metabolic substrates from fatty acids to glucose during hypoxia. The consistently low level of CD36 may help cardiomyocytes to maintain energy balance (Figure 2). Moreover, the rate of fatty acid oxidation in cardiomyocytes in ischaemia is reduced to 5% of basal states,89 and a relatively low CD36 prevents the accumulation of triglycerides in the cytosol by reducing the absorption of fatty acids (Figure 2). High concentrations of fatty acids in cardiomyocytes reduce the recovery of ischaemic heart function during the reperfusion stage by triggering insulin resistance and cardiomyocyte apoptosis.93–95 Therefore, the reduction of CD36 during ischaemia benefits the heart by avoiding the excessive accumulation of triglycerides, and a CD36 decrease during ischaemia is a favourable adaptation for cardiomyocytes to survive.
The left ventricle undergoes a wound healing response after myocardial infarction,96 including a strong infiltration of macrophages that promote the removal of dead cells and the renewal of extracellular matrix.97 Contrary to the effects of a decrease in CD36, which is beneficial to the energy production and survival of cardiomyocytes, the reduction of CD36 in macrophages has been proven to be detrimental to the repair stage after myocardial infarction. The lack of CD36 leads to a reduction in the phagocytic receptor (myeloid-epithelial-reproductive-tyrosine kinase, Mertk) and nuclear receptor (nuclear receptor subfamily 4, group A, member 1, Nr4a1),98 which affects the phagocytic function of cardiac macrophages, and exacerbates heart rupture caused by myocardial ischaemia.99 CD36 blockers inhibit phagocytosis of macrophages and restrain cardiac remodelling after myocardial infarction.99 In addition, platelet factor 4 (PF4) reduces the phagocytic function of macrophages and leads to higher mortality from myocardial infarction, which is also attributed to CD36 signalling down-regulation.100 Therefore, the decrease of CD36 or its dysfunction in macrophages deteriorates myocardial infarction. Although CD36 is more abundantly expressed in endothelial cells than in cardiomyocytes,101 the role of endothelial CD36 in ischaemic cardiomyopathy remains unclear.
Although reducing the content of CD36 has contradictory effects on macrophages and cardiomyocytes, current treatments for ischaemia–reperfusion still focus on inhibiting the function of CD36. The likely reason is that there are significantly more cardiomyocytes than macrophages in myocardial tissue,102 and the improvement of cardiomyocyte function by inhibiting CD36 may exceed the harm caused by macrophages. For example, Mansor et al. corrected post-hypoxia/reoxygenation cardiac metabolic disorders by injecting sulfo-N-succinimide oleate (a CD36-specific inhibitor that results in an arrest of the FFA transport103) into the hearts of type 2 diabetic male Wistar rats 4 min before hypoxia.104 Pre-injection of selective CD36 ligand EP 80317 (a synthetic hexapeptide growth hormone-releasing peptide family analogue that is devoid of somatotroph activity105) and azapeptide CP-3(iv) in mice significantly reduced the myocardial infarct size.106,107 Exenatide, a small-molecule drug that regulates glucose metabolism, has also been shown to improve cardiac function after cardiac ischaemia–reperfusion injury by inhibiting the translocation of CD36.108
Drug studies provide strong evidence that inhibiting the function of CD36 contributes to the recovery of heart function, but the most direct evidence comes from heart CD36-knockout (cCD36KO) mice. Inducible cardiomyocyte-specific CD36 ablation does not alter cardiac morphology but improves functional recovery after ischaemia/reperfusion.109 The decrease in fatty acid oxidation rate caused by the decrease in CD36 lays the foundation for increasing the rate of glucose oxidation. It has been shown that this recovery is due to the lower hydrogen ion concentration resulting from the uncoupling of glycolysis from glucose oxidation produced before and after ischaemia.109 However, CD36 systemic knockout mice still suffer from severe ischaemia–reperfusion injury;110 presumably, this relates to the general reduction of CD36. Not restricted to the heart, systemic metabolic changes occur in a vast number of tissues in the systemic knockout mice, and due to various potential adaptive adjustments from the embryonic stage, these mice may be significantly different from heart-specific knockout mice.
However, some studies on ischaemia/reperfusion in spontaneously hypertensive rats have challenged the protective effect of a decline in CD36. Instead of the inhibition of CD36, the overexpression of CD36 in SHRs reduced the infarct size, but the underlying mechanism has not been clarified. Given that the model already has a pathological factor of hypertension, it is possible that the overexpression of CD36 reduces the myocardial injury risk brought by hypertension and indirectly protects the infarcted myocardium caused by ischaemia/reperfusion.111
4.2 Cd36 and diabetic cardiomyopathy
The typical characteristics of diabetic cardiomyopathy are altered lipid metabolism and impaired insulin signalling pathways.112–114 Without any pathological stimulus, CD36 on the cardiac sarcolemma is significantly increased in obese Zucker rats,115 db./db. mice,116 and high-fat-fed rats.117 The up-regulation of CD36 has also been confirmed in the cardiomyocytes of patients with diabetic cardiomyopathy.118
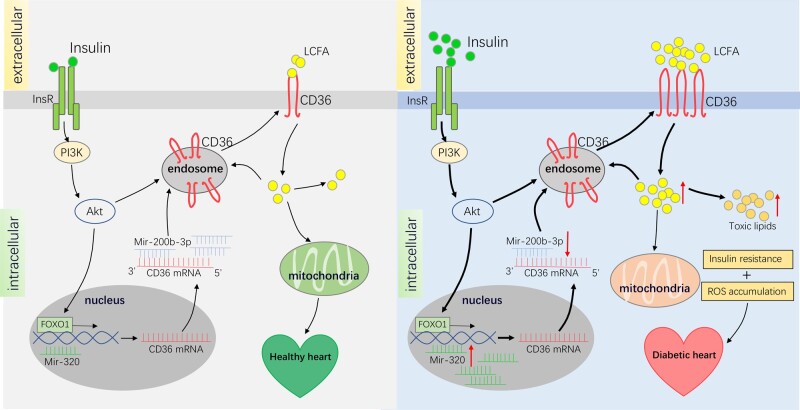
The increase in CD36 expression in the sarcolemma in diabetic cardiomyopathy may be attributed to hyperinsulinaemia, hyperglycaemia, and hyperlipidaemia. Prior to insulin resistance, CD36 translocates from endosomes to the myometrium because of the alkalinization of endosomes, which is caused by lipid-related inhibition of the proton pumping activity of vacuolar-type H+-ATPase (v-ATPase).30,119 In the early stages of diabetes, insulin is at high levels.120 Luiken et al. found that insulin stimulation in isolated myocardial rat cells resulted in a 1.5-fold increase in CD36 on the sarcolemma and a 62% decrease in intracellular CD36, suggesting that insulin could effectively promote the translocation of CD3631 (Figure 3). Chronic insulin stimulation also induces CD36 mRNA translation by activating the transcription factor forkhead box O1 (FOXO1) , which further facilitates CD36 protein synthesis121 (Figure 3). In the late stage of diabetes, the insulin level has decreased significantly, and the cardiomyocytes are resistant to insulin, but CD36 has been permanently transferred to the myometrium during the early stage.117 The decline of insulin does not change the fact that a large number of fatty acids have already been taken into cardiomyocytes. Similar to chronic insulin stimulation, high glucose stimulation increases CD36 mRNA translation,122 followed by increased CD36 expression, and membrane translocation of CD36 by palmitic acid stimulation.33 In the advanced stage of diabetes, hyperglycaemia and hypertriglyceridaemia may occur, thus further promoting CD36 expression and increasing its membrane distribution.123 In addition to external factors such as insulin, glucose, and lipids, the latest research suggests that the increase in CD36 in diabetic cardiomyopathy is also associated with the regulation of microRNA. MiR-320, which acts as a small activating RNA in the nucleus, is highly expressed in mice with diabetic cardiomyopathy and promotes CD36 expression by directly acting on its nuclear transcription124 (Figure 3). In contrast, miR-200b-3p is remarkably reduced in diabetic cardiomyopathy, which is an effective inhibitor of CD36 (Figure 3).
Figure 3.
Effects of CD36 in diabetes cardiomyopathy. In healthy heart, insulin activates the PI3K-Akt pathway, thereby promoting the transportation of CD36 from the endosome to the cell membrane. At the same time, FOXO1 transcription factor promotes the expression of CD36. LCFA absorbed by CD36 acts as a mitochondrial substrate for oxidation and storage as lipids. In diabetes, increased insulin strongly activates the PI3K-Akt pathway, promoting more CD36 from the endosome to the cell membrane, and FOXO1 transcription factor also leads to a robust expression of CD36. In addition, down-regulated mir-200b-3p and upregulated mir-320 also accelerate the transcription and translation of CD36, thus further increasing the distribution of CD36 on the cell membrane, which facilitates the uptake of LCFA in cardiomyocytes. LCFA promotes the transfer of CD36 in the endosome to the cell membrane, further increasing the distribution of CD36 on the cell membrane. Intracellular LCFAs either enter the mitochondria for aerobic oxidation producing energy and the byproducts-ROS or forms triglycerides for energy storage, and the accumulation of triglycerides would trigger insulin resistance. Insulin resistance and ROS assembly deteriorate cardiac function and result in diabetic cardiomyopathy.
CD36 increases during diabetic cardiomyopathy, which in turn worsens heart function.125 The increased distribution of CD36 on the myometrium results in the intake of a large amount of fatty acids. Fatty acids in the cytoplasm could activate peroxisome proliferator-activated receptors (PPAR),26 thereby inducing the up-regulation of enzymes necessary for mitochondrial β-oxidation, leading to a significant increase in fatty acid oxidation rates. However, the rate of fatty acid uptake and storage is higher than the rate of oxidation, resulting in the accumulation of lipids in the cell (Figure 3). Excessive lipid intermediates, including diacylglycerol and ceramide, have been shown to induce insulin resistance126 and trigger myocardial contractile dysfunction.121 At the same time, high levels of β-oxidation of fatty acids produce a large amount of reactive oxygen species (ROS),127 which can also induce inflammation128 and insulin resistance129,130 which aggravates myocardial contractile dysfunction (Figure 3). Lipids could directly lead to contractile dysfunction by promoting myocardial cell apoptosis.131 Therefore, inhibiting the uptake of long-chain fatty acids by targeting CD36 is the preferred strategy to reduce cardiac insulin resistance and ultimately prevent diabetic heart failure.
A fatty acid–glucose balance is essential for maintaining normal cardiac function. The heart operates optimally when it uses a mixture of energy-providing substrates, especially in terms of fatty acids and glucose.132 The preferential use of a single type of substrate impairs the flexibility of metabolism, limits auxiliary metabolic pathways, and affects the correct post-translational modification of putative proteins (particularly the transcriptional factors, such as PGC-1α and PPARα),133 thereby impairing heart function.134,135 In diabetic cardiomyopathy, the metabolic substrate of the myocardium shifts to fatty acids. Cardiomyocytes are insufficiently equipped to store large amounts of lipids with accumulated acylglycerols and ceramides, causing cellular damage by lipotoxicity and insulin resistance.136 Meanwhile, weakened glucose metabolism leads to an insufficient substrate supply of the pentose phosphate pathway and hexosamine biosynthetic pathway, resulting in impaired auxiliary metabolism.137 Therefore, inhibiting myocardial fatty acid metabolism helps to reduce lipotoxicity and maintain the fatty acid–glucose balance, thereby improving diabetic cardiomyopathy.
Several studies have shown that reducing the distribution of CD36 on the sarcolemma, thereby inhibiting the uptake of LCFAs, helps to improve the heart function of diabetic cardiomyopathy.33 For example, mice overexpressing myosin heavy chain (MHC)-PPARα show severe cardiomyocyte lipid accumulation and cardiac dysfunction.138 However, due to the lack of CD36, the offspring produced by crossing MHC-PPARα mice with CD36-deficient mice (MHC-PPARα/CD36-/-mice) had decreased triglyceride accumulation and cardiac dysfunction under basic conditions and on a high-fat diet. Glucagon-like peptide-1 could eliminate the lipotoxicity of diabetic cardiomyopathy by stimulating protein kinase A (PKA) inhibition of the CD36 pathway.139 Exogenous H2S protects diabetic hearts by inhibiting the translocation of CD36.19N-Acetylcysteine also restored Sevo-postC cardioprotection in diabetes by reducing Foxo1 and CD36.140 Fibroblast growth factor 21 (FGF21) deletion aggravates cardiac lipid accumulation by upregulating cardiac Nrf2-driven CD36 expression;141 therefore, FGF21 is a potential agent for the reduction of lipid accumulation as it ameliorates diabetic cardiomyopathy by downregulating the expression of CD36.
4.3. Cd36 and cardiac hypertrophy
4.3.1. Cd36 in hereditary hypertrophic cardiomyopathy
Hereditary hypertrophic cardiomyopathy (HCM) is a disease whose main pathological manifestation is cardiac hypertrophy, and it is caused by a dominant mutation in the gene encoding the cardiac sarcomeric protein.142 A survey of CD36 and hereditary hypertrophic cardiomyopathy patients showed that 37.9% of HCM patients with asymmetric ventricular septal hypertrophy had a loss of CD36 protein,143 which is accompanied by defective myocardial long-chain fatty acid intake, suggesting that the reduced CD36 may play a role in the pathogenesis of hereditary hypertrophy. The relationship between CD36 translocation and left ventricular contractile dysfunction has been verified in HCM mice.143,144 Owing to the decrease in CD36 translocation to the plasma membrane, ATP and triglycerides in the myocardium dramatically decline. Although the mechanism by which CD36 decreases in hereditary hypertrophic cardiomyopathy remains to be investigated, increasing CD36 and improving fatty acid intake may provide a new solution for the treatment of hereditary hypertrophic cardiomyopathy.
4.3.2. Cd36 in pathological/physiological cardiac hypertrophy
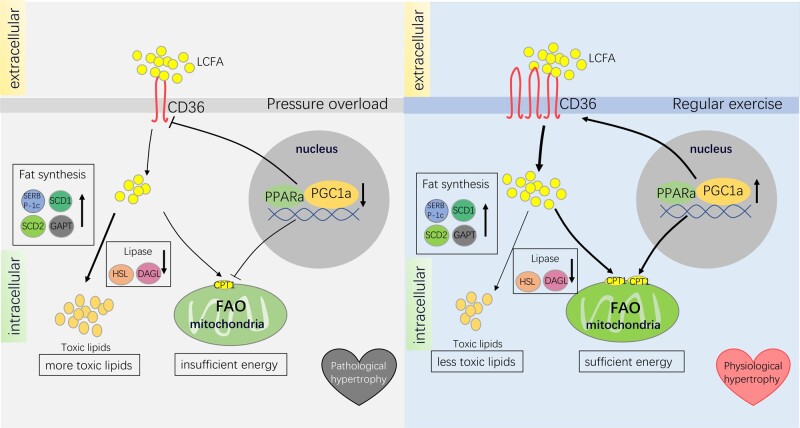
Differing from hereditary hypertrophic cardiomyopathy generated by genetic mutations, secondary cardiac hypertrophy is mainly caused by external stress, including pressure overload or physical exercise. It is naturally divided into physiological cardiac hypertrophy and pathological cardiac hypertrophy according to different stimulus factors and outcomes. Changes in CD36 in two different types of hypertrophic hearts were first demonstrated in 2013.36 Exercise training significantly increased the expression of CD36 in the heart, but pressure overload reduced the expression of CD36.145 The decrease in CD36 in pathological cardiac hypertrophy and the up-regulation in physiological cardiac hypertrophy may be related to PPARα and peroxisome proliferator-activated receptor γ coactivator-1α (PGC1α). Physical exercise increases the expression of PPARα146,147 and PGC1α148,149in the heart, while cardiac pressure overload reduces them150,151 (Figure 4). The promoter region of CD36 contains PPARα response elements.152 It has been shown that the nuclear receptor PPARα and nuclear receptor peroxisome proliferator-activated receptor-γ (PPARγ) regulate CD36 expression in macrophages and cardiac microvascular endothelial cells.153,154
Figure 4.
Effects of CD36 in pathological and physiological cardiac hypertrophy. Under pressure overload, intranuclear PPARα and PGC1a are down-regulated and lead to decreased CD36 expression and LCFA uptake by myocardial cells, resulting in an insufficient energy state. At the same time, the activity of HSL and DAGL that break down fat decreases, while fat synthesis-related enzymes, including SREBP-1c, SCD1, SCD2, and GPAT, are not down-regulated, leading to the accumulation of toxic lipids. Accumulated lipids and insufficient energy support both contribute to the development of pathological cardiac hypertrophy. During regular exercise, intranuclear PPARα and PGC1a increase and upregulate CD36 and fatty acid transfer rate-limiting enzyme CPT1, thus facilitating the uptake and utilization of LCFA. With the increase of CPT1 on the mitochondrial outer membrane, more LCFA undergoes aerobic oxidation in mitochondria. The increase in PPARα and PGC1a would also promote the transcription of mitochondrial oxidative phosphorylation-related proteins, which jointly improves the efficiency of fatty acid oxidation. Moreover, fat synthesis-related enzymes, including SREBP-1c, SCD1, SCD2, and GPAT, and the activity of HSL and DAGL that break down fat are both upregulated, thereby avoiding excessive accumulation of toxic lipids. Sufficient energy and less toxic lipids maintain the proper cardiac function of physiological cardiac hypertrophy. CPT1, carnitine palmitoyl transferase 1; DAGL, diacylglycerol lipase; GPAT, glycerol-3-phosphate acyltransferase; HSL, hormone-sensitive lipase; SCD1, stearoyl-CoA desaturase 1; SCD2, stearoyl-CoA desaturase 2; SREBP-1c, sterol-responsive element-binding protein-1c.
As mentioned before, when myocardial cells are facing a crisis of ischaemia and hypoxia, metabolism is remodelled. As for hypoxic pathological cardiac hypertrophy,155 early and timely reduction of the distribution of CD36 on the cell membrane helps to eliminate the injury caused by a subsequent large intake of fatty acids. The reduction of CD36 not only promotes the substrate transition of fatty acids to glucose92 but also eliminates the accumulation of toxic lipid intermediates. However, the reduction of CD36 may ultimately shorten the energy supply in the chronic stage of pathological cardiac hypertrophy.145 Although the glycolytic pathway of glucose is enhanced in maladaptive pathological cardiac hypertrophy due to the inhibition of fatty acid metabolism, by elevated phosphofructokinase and lactate dehydrogenase levels,145 glycolysis and energy generated from other substrates (lactic acid, branched-chain amino acids, and ketone bodies) could not compensate for the reduction of fatty acid oxidation, putting the heart in an insufficient cardiac energy state (Figure 4). Although the reduction of CD36 leads to a remarkable decline in the overall intake of fatty acids, studies have shown that fat synthesis-related enzymes, including sterol-responsive element-binding protein-1c (SREBP-1c), stearoyl-CoA desaturase 1 (SCD1), stearoyl-CoA desaturase 2 (SCD2), and glycerol-3-phosphate acyltransferase (GPAT), are not down-regulated in pathological cardiac hypertrophic cardiomyocytes (Figure 4). However, the activity and content of hormone-sensitive lipase (HSL) and diacylglycerol lipase (DAGL) that break down fat both decrease, resulting in a 31% increase in triglycerides and a 200% increase in diacylglycerol in myocardial cells143 (Figure 4). Insulin resistance caused by accumulated toxic lipids further exacerbates the lack of energy supply in the heart. In addition, pressure overload-induced cardiac hypertrophy leads to an excessive metabolic shift from fatty acid to glucose.156 As mentioned before, the aberrant preference to one substrate would cripple the metabolic flexibility and cardiac contractility.134,135,157 Fatty acid metabolism needs to be strengthened in order to restore the balance of fatty acid and glucose and improve cardiac metabolism. In other words, heart function will worsen if CD36 is excessively down-regulated.
Recent studies on CD36 cardiac-specific knockout mice and systemic knockout mice have demonstrated the role of CD36 in pathological cardiac hypertrophy. CD36 cardiac-specific knockout mice rapidly transferred from compensatory cardiac hypertrophy to heart failure due to an imbalance of energy. Comparatively, CD36 systemic knockout mice, when compared to wild-type mice, show pronounced myocardial interstitial fibrosis, cardiac enlargement, and contractile dysfunction after transverse aortic constriction (TAC) surgery.158 The myocardium of CD36KO-TAC leads to insufficient energy supply not only due to the decrease of CD36 but also because of the increase of de novo amino acid synthesis from glucose, which further reduces the size of the high-energy phosphate pool.159 However, whether overexpression of CD36 relieves the energy deficiency in pathological cardiac hypertrophy, and thereby stops the heart failure caused by cardiac pressure overload needs further investigation. It is certain that increasing the supply of fatty acids in myocardial cells to expand the high-energy phosphate pool is beneficial for hypertrophic myocardium. Even in the case of a decrease in medium-chain acetyl-CoA dehydrogenase, providing medium-chain fatty acids for cardiomyocytes lacking CD36 and bypassing long-chain fatty acids can still relieve cardiac pressure overload-induced heart failure.34
4.4 Cd36 and atherosclerosis
Atherosclerosis is a progressive chronic inflammation of the arterial wall, manifested by the accumulation of foam cells, retention of macrophages in plaques, and thrombosis.160 CD36 expression in macrophages is significantly increased in human carotid atherosclerotic tissue, particularly in advanced atherosclerosis.161 In diet-induced mouse atherosclerosis models and in vitro atherosclerosis models, CD36 on macrophages was also remarkably elevated.162,163
The main cause of foam cell generation is the excessive influx of modified low-density lipoproteins (LDL), and the accumulation of cholesterol esters in intimal macrophages. As an important scavenger receptor in macrophages, CD36 plays a critical role in cellular oxLDL accumulation and foam cell formation. Macrophages bind with and internalize oxLDL via CD36 due to their high-affinity CD36-oxLDL.23 Along with the internalization of the CD36-oxLDL assembly, oxLDL enters the cell and accumulates, to transfer the macrophages into the foam cells. OxLDL also provides putative oxidized lipids as ligands for the transcription factor PPAR-γ, which is the main transcription factor of CD36, thereby activating CD36 gene expression to further increase the uptake of oxLDL.164 The uptake of oxLDL by CD36 triggers a chain reaction and creates a cycle, putting CD36 in the centre of foam cell formation.
Many pathogenic factors that lead to atherosclerosis are involved in the regulation of CD36. Smoking and hyperglycaemia are risk factors for atherosclerosis;122,165 nicotine promotes the expression of CD36 in macrophages in a PPAR-γ dependent manner, and hyperglycaemia results in high CD36 mRNA translation efficiency, which increases the expression of CD36. Premature atherosclerotic cardiovascular disease is a common destructive complication of systemic lupus erythematosus (SLE), which may be related to elevated CD36 expression, for reasons currently unknown.166 IL-34, a cytokine associated with various inflammations and autoimmune diseases, also increases the expression of CD36 via the p38 MAPK signalling pathway to promote the formation of foam cells, and thus is a potential biomarker of atherosclerosis.167 CCN3 (Cyr61, CTGF, Nov) is an important regulator of vascular homeostasis.168 The lack of CCN3 leads to the increased formation of foam cells, which is attributed to the increased expression of CD36, possibly due to the elevated level of CCN1 protein. Thrombin also promotes the formation of atherosclerosis by regulating protein kinase Cθ dependent activating transcription factor 2-mediated CD36 expression.169 Retinol binding protein 4 (RBP4), an adipokine that plays a decisive role in glucose metabolism and insulin sensitivity, has recently been shown to promote atherosclerosis by upregulating CD36 expression. The method is dependent on the Jun N-terminal kinase signal transducer and activator of transcription 1, and activation of CD36-mediated cholesterol uptake to facilitate the formation of foam cells.170
In addition to the formation of foam cells, CD36 is also involved in the inflammatory response of macrophages. For example, targeting CD36 inhibits the NLRP3 inflammasome, resulting in a decrease in serum IL-1β in atherosclerotic mice.171 CD36-related uptake of oxLDL induces the specific expression profile of macrophage oxidative stress markers (5-epi-5-F2t-IsoP, 15-E1t-IsoP, 8-F3t-IsoP, and 15-keto-15-F2t-IsoP) and inflammation markers (PGDM, 17-trans-PGF3α, and 11β-PGF2α).172 Even relatively low concentrations of oxLDL can induce a durable atherogenic macrophage phenotype through epigenetic histone modification.173 In addition, CD36 was recently found to drive the chronic inflammatory response of macrophages by reprogramming mitochondrial metabolism.174
Generally, the acute inflammatory response gradually subsides, as the infiltrating immune cells gradually migrate to the draining lymph nodes.175 However, inflammation in atherosclerosis does not spontaneously subside. Macrophages, with a pro-inflammatory phenotype, are trapped in the atherosclerotic plaque, causing a continuous inflammatory response.176,177 The oxLDL/CD36 interaction is essential for the retention of macrophages in plaques. The migration chemokines, CCL19 and CCL21, largely decrease in macrophages after oxLDL stimulation,178 with persistent expression of CD146 that promotes macrophage retention in plaques.178 Importantly, the migration of CD36 null macrophages is not inhibited by oxLDL, compared with wild-type macrophages, underscoring the importance of CD36 signalling in macrophage retention.179 Two mechanisms may explain oxLDL/CD36 signal-related macrophage retention. Firstly, oxLDL-mediated CD36 signal transduction results in the continuous activation of focal adhesion kinase and the inactivation of Src homology 2 containing phosphotyrosine phosphatase, which leads to disorders of cytoskeleton assembly and disassembly,179 and the loss of macrophage activity. Secondly, oxLDL can also induce the loss of macrophage polarity by activating the Vav/Rac pathway and inactivating the non-muscle myosin II.176 In addition to oxLDL stimulation, CD36 also mediates the inhibitory effect of advanced glycation end product [Nε-carboxymethyllysine (CML)] on the retention of macrophages.180 CD36 is activated by CML and triggers the production of nicotinamide adenine dinucleotide phosphate oxidase (NOX)-derived ROS, which then promotes F-actin polymerization to inhibit the migration of macrophages and accelerate the development of atherosclerosis in diabetic patients.
CD36 is a glycoprotein with a high expression level in platelets and is closely related to the formation of the prethrombotic phenotype in the high-fat state. Accumulating evidence has shown that there is a high correlation between the platelet activation response to oxLDL and the expression level of CD36.181 CD36 null subjects have slow thrombosis, while elevated platelet CD36 expression may increase the risk of thromboembolism.182,183 Animal experiments showed that the deletion of the CD36 gene protects mice from the increased platelet reactivity associated with hyperlipidaemia and the accompanying thrombotic phenotype.184 OxLDL-mediated CD36 signal transduction can activate platelets in a variety of ways. Firstly, after CD36 is activated, it recruits Src family kinases Fyn and Lyn, and further promotes the activation of non-receptor tyrosine kinases Syk and (c-Jun-N-terminal kinase) JNK, leading to the exposure of P-selectin and activated integrin, thereby enhancing thrombosis.185 Secondly, oxLDL stimulates the continuous generation of NOX2-derived ROS through the CD36-PKC pathway and promotes platelet hyperfunction by regulating cGMP signal transduction.28 Thirdly, in response to the signal of oxLDL/CD36, the activated protein kinase ERK5 promotes the exposure of procoagulant phosphatidylserine (PSer) on the platelet surface,186 thereby causing platelet activation and aggregation.23 Meanwhile, CD36 also activates platelets through the interaction with endothelial cell-derived microparticles oxPL, which makes platelets more sensitive to low concentrations of agonists.16,187 Lastly, advanced glycosylation products (AGEs) in diabetic patients also serve as ligands to bind to platelet CD36, leading to platelet hyperreactivity and inducing a prethrombotic phenotype.8
In view of the important role of CD36 in promoting atherosclerosis, anti-atherosclerotic drugs mainly focus on reducing the expression of CD36. Traditional Chinese medicine, including salvianolic acid B (a hydrophilic component derived from the herbal salvia miltiorrhiza),188 andrographolide (the biologically active component of Andrographis paniculata),189 and scopolamine (plant flavonoids),162 all suppress the development of atherosclerosis by lowering the expression of CD36. Tamoxifen, an anti-breast cancer drug, has also been confirmed to inhibit the expression of CD36 by inhibiting the PPAR-γ signalling pathway, thereby combating atherosclerosis.190 The apoA-I mimetic peptide D4F can also reduce the formation of macrophage-derived foam cells by inhibiting the expression of CD36.191 The new members of the calcitonin gene-related peptide family intermedin and C1q/tumour necrosis factor-related protein 13 (CTRP13) inhibit the formation of macrophage foam cells and reduce atherosclerosis plaques by promoting the degradation of CD36 mRNA and promoting autophagolysosomal-dependent CD36 degradation, respectively.192 Vasostatin-1 is a chromogranin A (CgA)-derived peptide (76 amino acids) that can inhibit vasoconstriction and angiogenesis. Its inhibitory effect on atherosclerosis is also associated with the down-regulation of CD36. Currently, researchers are studying the nanoparticles targeting CD36 specifically to enhance efficacy and reduce side effects.193
5. Conclusion and perspective
Cardiovascular diseases have been seriously endangering the physical and mental health of older adults,194 and they are particularly prominent with the current increase in the aging population. Cardiac lipid metabolism remodelling plays a key role in cardiovascular system diseases such as ischaemia–reperfusion, diabetic cardiomyopathy, and cardiac hypertrophy.195 CD36 is a vital molecule in lipid metabolism.29 Changes in its synthesis, localization, and function directly affect the energy supply and metabolism of the heart. Studies on CD36 in different diseases suggest that CD36 may be potentially useful for treatment. Therefore, transcriptional activation, post-translational modification, and localization changes of CD36 may provide new directions for the treatment of cardiovascular diseases.
Post-translational modifications of CD36 have been studied for nearly 30 years.79 Post-translational modifications, including phosphorylation, ubiquitination, palmitoylation, and glycosylation, accurately regulate the maturation, transport, and positioning of CD36.1In vitro research has revealed the impact of post-translational modification of CD36 on cellular fatty acid uptake in adipocytes83 and muscle cells.69 However, few studies have demonstrated its importance in cardiovascular systems. Whether these modifications of CD36 alter the cellular uptake of fatty acids in the myocardium and further influence cardiac function still remains to be elucidated. Determining which enzymes mediate these modifications in cardiomyocytes and whether these enzymes are useful as new therapeutic targets, requires further investigation.
Fatty acids may also cause excessive lipid accumulation while providing energy.196,197 The increase in fatty acid uptake caused by CD36 can be beneficial or harmful under different pathological conditions. Fatty acids in cells are modulated by CD36 and are also affected by the rates of mitochondrial aerobic oxidation, and triglyceride synthesis and decomposition.198,199 Therefore, to evaluate the role of CD36 in myocardial lipid metabolism, the corresponding mitochondrial aerobic oxidation and triglyceride synthesis also needs to be taken into consideration. Since it is not uncommon that more than two pathological factors are combined at the same time, such as hypertension111 and myocardial infarction200 in diabetes, the impact of CD36 on myocardial lipid metabolism and cardiac function is definitely more complicated when that occurs. Therefore, further research is needed to investigate these situations.
The function of CD36 in regulating lipid metabolism in cardiomyocytes is not limited to lipid uptake. CD36 in mitochondria increases in parallel with the up-regulation of FA oxidation, and recent studies have shown that CD36 mediates a mitochondrial metabolic switch from oxidative phosphorylation to superoxide production in response to its ligand, oxidized LDL.201,202 Moreover, mitochondrial-specific inhibition of superoxide inhibited oxidized LDL-induced nuclear factor-κB (NF-κB) activation and inflammatory cytokine generation.174 In addition, CD36 also forms complexes with certain metabolic molecules and participates in the energy regulation process through direct interactions. For example, CD36 regulates the activity of AMPK by forming a molecular complex with Lyn and LKB1 and inhibits the activation of LKB1,62 which is a known AMPK202 agonist. Further study on the function of CD36 beyond myocardial lipid uptake will help to provide a more comprehensive understanding of myocardial lipid metabolism dysfunction and related cardiovascular complications.
In response to insulin and contraction stimulation, both CD36 and GLUT4 translocate to the sarcolemma to increase fatty acid and glucose uptake, respectively, which is contradictory to the Randle cycle phenomenon.203,204 How to specifically regulate CD36, without affecting the recycling of subcellular GLUT4, is also an area that remains to be determined. Previous studies have focused on the related proteins in the GLUT4 and CD36 vesicle transport pathways. For example, VAMP4 mainly mediates vesicle transport of CD36, while VAMP5 and VAMP7 are involved in GLUT4 vesicle transport.58 These studies on the subcellular transport of CD36 and GLUT4 help explain and distinguish the different energy regulation patterns made by cardiomyocytes in the face of different signal stimuli.
In the cardiovascular system, CD36 is not only expressed on the surface of cardiomyocytes but also exists in other cells, such as endothelial cells.176 Although a large number of studies have reported that the CD36 of endothelial cells is critically involved in the progression of vascular diseases such as atherosclerosis,205,206 little research has been performed to explore its effect on diabetic cardiomyopathy, hypertrophic cardiomyopathy, and other diseases. Therefore, an in-depth study of the effects of CD36 on myocardial metabolism in other types of cells is also an important issue. In addition, in view of the universality of CD36 expression, drugs developed for targeting CD36 should specify cell populations and CD36 sites to limit side effects, avoid the potential for off-target effects, and improve their efficacy.
Data accessibility
Most of the 207 references are accessible in pubmed.
Conflict of interest: none declared.
Funding
This work was supported by the National Natural Science Foundation of China (81570261,82070316), Cardiovascular Health Alliance of China-Advanced Fund (2019-CCA-ACCESS-059).
References
- 1. Luiken JJFP, Chanda D, Nabben M, Neumann D, Glatz JFC.. Post-translational modifications of CD36 (SR-B2): Implications for regulation of myocellular fatty acid uptake. Biochim Biophys Acta 2016;1862:2253–2258. [DOI] [PubMed] [Google Scholar]
- 2. Brinkmann JFF, Abumrad NA, Ibrahimi A, van der Vusse GJ, Glatz JFC.. New insights into long-chain fatty acid uptake by heart muscle: a crucial role for fatty acid translocase/CD36. Biochem J 2002;367:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kronenberg A, Grahl H, Kehrel B.. Human platelet CD36 (GPIIIb, GPIV) binds to cholesteryl-hemisuccinate and can be purified by a simple two-step method making use of this property. Thromb Haemost 1998;79:1021–1024. [PubMed] [Google Scholar]
- 4. Jay AG, Chen AN, Paz MA, Hung JP, Hamilton JA.. CD36 binds oxidized low density lipoprotein (LDL) in a mechanism dependent upon fatty acid binding. J Biol Chem 2015;290:4590–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu J, Yang P, Zuo G, He S, Tan W, Zhang X, Su C, Zhao L, Wei L, Chen Y, Ruan X, Chen Y.. Long-chain fatty acid activates hepatocytes through CD36 mediated oxidative stress. Lipids Health Dis 2018;17:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee S, Eguchi A, Sakamoto K, Matsumura S, Tsuzuki S, Inoue K, Masuda D, Yamashita S, Fushiki T.. A role of CD36 in the perception of an oxidised phospholipid species in mice. Biomed Res 2015;36:303–311. [DOI] [PubMed] [Google Scholar]
- 7. Simantov R, Febbraio M, Silverstein RL.. The antiangiogenic effect of thrombospondin-2 is mediated by CD36 and modulated by histidine-rich glycoprotein. Matrix Biol 2005;24:27–34. [DOI] [PubMed] [Google Scholar]
- 8. Zhu W, Li W, Silverstein RL.. Advanced glycation end products induce a prothrombotic phenotype in mice via interaction with platelet CD36. Blood 2012;119:6136–6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohgami N, Nagai R, Ikemoto M, Arai H, Kuniyasu A, Horiuchi S, Nakayama H.. CD36, a member of class B scavenger receptor family, is a receptor for advanced glycation end products. Ann N Y Acad Sci 2006;947:350–355. [DOI] [PubMed] [Google Scholar]
- 10. Li X, Zhang T, Geng J, Wu Z, Xu L, Liu J, Tian J, Zhou Z, Nie J, Bai X.. Advanced oxidation protein products promote lipotoxicity and tubulointerstitial fibrosis via CD36/β-Catenin pathway in diabetic nephropathy. Antioxid Redox Signal 2019;31:521–538. [DOI] [PubMed] [Google Scholar]
- 11. Iwao Y, Nakajou K, Nagai R, Kitamura K, Anraku M, Maruyama T, Otagiri M.. CD36 is one of important receptors promoting renal tubular injury by advanced oxidation protein products. Am J Physiol Renal Physiol 2008;295:F1871–80. [DOI] [PubMed] [Google Scholar]
- 12. Kerkhoff C, Sorg C, Tandon NN, Nacken W.. Interaction of S100A8/S100A9-arachidonic acid complexes with the scavenger receptor CD36 may facilitate fatty acid uptake by endothelial cells. Biochemistry 2001;40:241–248. [DOI] [PubMed] [Google Scholar]
- 13. Kawano T, Shimamura M, Nakagami H, Iso T, Koriyama H, Takeda S, Baba K, Sasaki T, Sakaguchi M, Morishita R, Mochizuki H.. Therapeutic vaccine against S100A9 (S100 calcium-binding protein A9) inhibits thrombosis without increasing the risk of bleeding in ischemic stroke in mice. Hypertension (Dallas, TX 1979) 2018;72:1355–1364. [DOI] [PubMed] [Google Scholar]
- 14. Farokhzadian J, Mangolian Shahrbabaki P, Bagheri V.. S100A12-CD36 axis: a novel player in the pathogenesis of atherosclerosis? Cytokine 2019;122:154104. [DOI] [PubMed] [Google Scholar]
- 15. Bodart V, Febbraio M, Demers A, McNicoll N, Pohankova P, Perreault A, Sejlitz T, Escher E, Silverstein RL, Lamontagne D, Ong H.. CD36 mediates the cardiovascular action of growth hormone-releasing peptides in the heart. Circ Res 2002;90:844–849. [DOI] [PubMed] [Google Scholar]
- 16. Silverstein RL, Li W, Park YM, Rahaman SO.. Mechanisms of cell signaling by the scavenger receptor CD36: implications in atherosclerosis and thrombosis. Trans Am Clin Climatol Assoc 2010;121:206–220. [PMC free article] [PubMed] [Google Scholar]
- 17. Doens D, Valiente PA, Mfuh AM, Vo AXT, Tristan A, Carreño L, Quijada M, Nguyen VT, Perry G, Larionov OV, Lleonart R, Fernández PL.. Identification of inhibitors of CD36-amyloid beta binding as potential agents for Alzheimer’s disease. ACS Chem Neurosci 2017;8:1232–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Son N-H, Basu D, Samovski D, Pietka TA, Peche VS, Willecke F, Fang X, Yu S-Q, Scerbo D, Chang HR, Sun F, Bagdasarov S, Drosatos K, Yeh ST, Mullick AE, Shoghi KI, Gumaste N, Kim KJin, Huggins L-A, Lhakhang T, Abumrad NA, Goldberg IJ.. Endothelial cell CD36 optimizes tissue fatty acid uptake. J Clin Invest 2018;128:4329–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu M, Du H, Wang B, Chen J, Lu F, Peng S, Sun Y, Liu N, Sun X, Shiyun D, Zhao Y, Wang Y, Zhao D, Lu F, Zhang W.. Exogenous H2S induces Hrd1 s-sulfhydration and prevents CD36 translocation via VAMP3 ubiquitylation in diabetic hearts. Aging Dis 2020;11:286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hou Y, Wu M, Wei J, Ren Y, Du C, Wu H, Li Y, Shi Y.. CD36 is involved in high glucose-induced epithelial to mesenchymal transition in renal tubular epithelial cells. Biochem Biophys Res Commun 2015;468:281–286. [DOI] [PubMed] [Google Scholar]
- 21. Couturier J, Nuotio-Antar AM, Agarwal N, Wilkerson GK, Saha P, Kulkarni V, Lakhashe SK, Esquivel J, Nehete PN, Ruprecht RM, Sastry KJ, Meyer JM, Hill LR, Lake JE, Balasubramanyam A, Lewis DE.. Lymphocytes upregulate CD36 in adipose tissue and liver. Adipocyte 2019;8:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ladanyi A, Mukherjee A, Kenny HA, Johnson A, Mitra AK, Sundaresan S, Nieman KM, Pascual G, Benitah SA, Montag A, Yamada SD, Abumrad NA, Lengyel E.. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene 2018;37:2285–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang M, Kholmukhamedov A, Schulte ML, Cooley BC, Scoggins NO, Wood JP, Cameron SJ, Morrell CN, Jobe SM, Silverstein RL.. Platelet CD36 signaling through ERK5 promotes caspase-dependent procoagulant activity and fibrin deposition in vivo. Blood Adv 2018;2:2848–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frank A-C, Ebersberger S, Fink AF, Lampe S, Weigert A, Schmid T, Ebersberger I, Syed SN, Brune B.. Apoptotic tumor cell-derived microRNA-375 uses CD36 to alter the tumor-associated macrophage phenotype. Nat Commun 2019;10:1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang J, Li Y.. CD36 tango in cancer: signaling pathways and functions. Theranostics 2019;9:4893–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Glatz JFC, Luiken JJFP.. From fat to FAT (CD36/SR-B2): Understanding the regulation of cellular fatty acid uptake. Biochimie 2017;136:21–26. [DOI] [PubMed] [Google Scholar]
- 27. Zhao L, Zhang C, Luo X, Wang P, Zhou W, Zhong S, Xie Y, Jiang Y, Yang P, Tang R, Pan Q, Hall AR, Luong TV, Fan J, Varghese Z, Moorhead JF, Pinzani M, Chen Y, Ruan X.. Z. CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J Hepatol 2018;69:705–717. [DOI] [PubMed] [Google Scholar]
- 28. Magwenzi S, Woodward C, Wraith KS, Aburima A, Raslan Z, Jones H, McNeil C, Wheatcroft S, Yuldasheva N, Febbriao M, Kearney M, Naseem KM.. Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood 2015;125:2693–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim TT, Dyck JRB.. The role of CD36 in the regulation of myocardial lipid metabolism. Biochim Biophys Acta 2016;1861:1450–1460. [DOI] [PubMed] [Google Scholar]
- 30. Glatz JFC, Luiken JJFP.. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J Lipid Res 2018;59:1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luiken JJFP, Koonen DPY, Willems J, Zorzano A, Becker C, Fischer Y, Tandon NN, Van Der Vusse GJ, Bonen A, Glatz JFC.. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes 2002;51:3113–3119. [DOI] [PubMed] [Google Scholar]
- 32. Cheng Y, Liu G, Pan Q, Guo S, Yang X.. Elevated expression of liver X receptor alpha (LXRα) in myocardium of streptozotocin-induced diabetic rats. Inflammation 2011;34:698–706. [DOI] [PubMed] [Google Scholar]
- 33. Angin Y, Steinbusch LKM, Simons PJ, Greulich S, Hoebers NTH, Douma K, van Zandvoort MAMJ, Coumans WA, Wijnen W, Diamant M, Ouwens DM, Glatz JFC, Luiken JJFP.. CD36 inhibition prevents lipid accumulation and contractile dysfunction in rat cardiomyocytes. Biochem J 2012;448:43–53. [DOI] [PubMed] [Google Scholar]
- 34. Sung MM, Byrne NJ, Kim TT, Levasseur J, Masson G, Boisvenue JJ, Febbraio M, Dyck JRB.. Cardiomyocyte-specific ablation of CD36 accelerates the progression from compensated cardiac hypertrophy to heart failure. Am J Physiol Heart Circ Physiol 2017;312:H552–H560. [DOI] [PubMed] [Google Scholar]
- 35. Abumrad NA, Goldberg IJ.. CD36 actions in the heart: lipids, calcium, inflammation, repair and more? Biochim Biophys Acta 2016;1861:1442–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dobrzyn P, Pyrkowska A, Duda MK, Bednarski T, Maczewski M, Langfort J, Dobrzyn A.. Expression of lipogenic genes is upregulated in the heart with exercise training-induced but not pressure overload-induced left ventricular hypertrophy. Am J Physiol Endocrinol Metab 2013;304:E1348–58. [DOI] [PubMed] [Google Scholar]
- 37. Xu L, Chen W, Ma M, Chen A, Tang C, Zhang C, Cai L.. Microarray profiling analysis identifies the mechanism of miR-200b-3p/mRNA-CD36 affecting diabetic cardiomyopathy via peroxisome proliferator activated receptor-gamma signaling pathway. J Cell Biochem 2019;120:5193–5206. [DOI] [PubMed] [Google Scholar]
- 38. Andersen M, Lenhard B, Whatling C, Eriksson P, Odeberg J.. Alternative promoter usage of the membrane glycoprotein CD36. BMC Mol Biol 2006;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hall D, Mayosi BM, Rahman TJ, Avery PJ, Watkins HC, Keavney B.. Common variation in the CD36 (fatty acid translocase) gene is associated with left-ventricular mass. J Hypertens 2011;29:690–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jayewardene AF, Gwinn T, Hancock DP, Mavros Y, Rooney KB.. The associations between polymorphisms in the CD36 gene, fat oxidation and cardiovascular disease risk factors in a young adult Australian population: a pilot study. Obes Res Clin Pract 2014;8:e618-21–e621. [DOI] [PubMed] [Google Scholar]
- 41. Zhang Y, Ling ZY, Deng SB, Du HA, Yin YH, Yuan J, She Q, Chen YQ.. Associations between CD36 gene polymorphisms and susceptibility to coronary artery heart disease. Braz J Med Biol Res 2014;47:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Che JJ, Shao YX, Li GP.. Association between rs1049673 polymorphism in CD36 and premature coronary heart disease. Genet Mol Res 2014;13:7708–7717. [DOI] [PubMed] [Google Scholar]
- 43. Du Y, Chen K, Liu E, Wang X, Li F, Liu T, Zheng X, Li G, Che J.. Gender-specific associations of CD36 polymorphisms with the lipid profile and susceptibility to premature multi-vessel coronary artery heart disease in the Northern Han Chinese. Gene 2020;753:144806. [DOI] [PubMed] [Google Scholar]
- 44. Love-Gregory L, Sherva R, Sun L, Wasson J, Schappe T, Doria A, Rao DC, Hunt SC, Klein S, Neuman RJ, Permutt MA, Abumrad NA.. Variants in the CD36 gene associate with the metabolic syndrome and high-density lipoprotein cholesterol. Hum Mol Genet 2008;17:1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA.. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem 1993;268:17665–17668. [PubMed] [Google Scholar]
- 46. Greenwalt DE, Lipsky RH, Ockenhouse CF, Ikeda H, Tandon NN, Jamieson GA.. Membrane glycoprotein CD36: a review of its roles in adherence, signal transduction, and transfusion medicine. Blood 1992;80:1105–1115. [PubMed] [Google Scholar]
- 47. Neculai D, Schwake M, Ravichandran M, Zunke F, Collins RF, Peters J, Neculai M, Plumb J, Loppnau P, Pizarro JC, Seitova A, Trimble WS, Saftig P, Grinstein S, Dhe-Paganon S.. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature 2013;504:172–176. [DOI] [PubMed] [Google Scholar]
- 48. Pepino MY, Kuda O, Samovski D, Abumrad NA.. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu Rev Nutr 2014;34:281–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gomez-Diaz C, Bargeton B, Abuin L, Bukar N, Reina JH, Bartoi T, Graf M, Ong H, Ulbrich MH, Masson J-F, Benton R.. A CD36 ectodomain mediates insect pheromone detection via a putative tunnelling mechanism. Nat Commun 2016;7:11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Glatz JFC, Nabben M, Heather LC, Bonen A, Luiken JJFP.. Regulation of the subcellular trafficking of CD36, a major determinant of cardiac fatty acid utilization. Biochim Biophys Acta 2016;1861:1461–1471. [DOI] [PubMed] [Google Scholar]
- 51. Samovski D, Dhule P, Pietka T, Jacome-Sosa M, Penrose E, Son N-H, Flynn CR, Shoghi KI, Hyrc KL, Goldberg IJ, Gamazon ER, Abumrad NA.. Regulation of insulin receptor pathway and glucose metabolism by CD36 signaling. Diabetes 2018;67:1272–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Monaco C, Whitfield J, Jain SS, Spriet LL, Bonen A, Holloway GP.. Activation of ampkα2 is not required for mitochondrial fat/cd36 accumulation during exercise. PLoS One 2015;10:e0126122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chanda D, Luiken JJFP, Glatz JFC.. Signaling pathways involved in cardiac energy metabolism. FEBS Lett 2016;590:2364–2374. [DOI] [PubMed] [Google Scholar]
- 54. Samovski D, Su X, Xu Y, Abumrad NA, Stahl PD.. Insulin and AMPK regulate FA translocase/CD36 plasma membrane recruitment in cardiomyocytes via Rab GAP AS160 and Rab8a Rab GTPase. J Lipid Res 2012;53:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Luiken JJFP, Dyck DJ, Han X-X, Tandon NN, Arumugam Y, Glatz JFC, Bonen A.. Insulin induces the translocation of the fatty acid transporter FAT/CD36 to the plasma membrane. Am J Physiol Endocrinol Metab 2002;282:E491–5. [DOI] [PubMed] [Google Scholar]
- 56. Luiken JJFP, Ouwens DM, Habets DDJ, van der Zon GCM, Coumans WA, Schwenk RW, Bonen A, Glatz JFC.. Permissive action of protein kinase C-zeta in insulin-induced CD36- and GLUT4 translocation in cardiac myocytes. J Endocrinol 2009;201:199–209. [DOI] [PubMed] [Google Scholar]
- 57. Luiken JJFP, Nabben M, Neumann D, Glatz JFC.. Understanding the distinct subcellular trafficking of CD36 and GLUT4 during the development of myocardial insulin resistance. Biochim Biophys Acta Mol Basis Dis 2020;1866:165775. [DOI] [PubMed] [Google Scholar]
- 58. Schwenk RW, Dirkx E, Coumans WA, Bonen A, Klip A, Glatz JFC, Luiken JJFP.. Requirement for distinct vesicle-associated membrane proteins in insulin- and AMP-activated protein kinase (AMPK)-induced translocation of GLUT4 and CD36 in cultured cardiomyocytes. Diabetologia 2010;53:2209–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rose AJ, Jeppesen J, Kiens B, Richter EA.. Effects of contraction on localization of GLUT4 and v-SNARE isoforms in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 2009;297:R1228–37. [DOI] [PubMed] [Google Scholar]
- 60. Luiken JJFP, Coort SLM, Koonen DPY, van der Horst DJ, Bonen A, Zorzano A, Glatz JFC.. Regulation of cardiac long-chain fatty acid and glucose uptake by translocation of substrate transporters. Pflugers Arch - Eur J Physiol 2004;448:1–15. [DOI] [PubMed] [Google Scholar]
- 61. Luiken JJFP, Coort SLM, Willems J, Coumans WA, Bonen A, van der Vusse GJ, Glatz JFC.. Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes 2003;52:1627–1634. [DOI] [PubMed] [Google Scholar]
- 62. Samovski D, Sun J, Pietka T, Gross RW, Eckel RH, Su X, Stahl PD, Abumrad NA.. Regulation of AMPK activation by CD36 links fatty acid uptake to β-oxidation. Diabetes 2015;64:353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Popovic D, Vucic D, Dikic I.. Ubiquitination in disease pathogenesis and treatment. Nat Med 2014;20:1242–1253. [DOI] [PubMed] [Google Scholar]
- 64. Abumrad NA, Moore DJ.. Parkin reinvents itself to regulate fatty acid metabolism by tagging CD36. J Clin Invest 2011;121:3389–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim K-Y, Stevens MV, Akter MH, Rusk SE, Huang RJ, Cohen A, Noguchi A, Springer D, Bocharov AV, Eggerman TL, Suen D-F, Youle RJ, Amar M, Remaley AT, Sack MN.. Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. J Clin Invest 2011;121:3701–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang F, Xia X, Chai R, Xu R, Xu Q, Liu M, Chen X, Liu B, Liu S, Liu N.. Inhibition of USP14 suppresses the formation of foam cell by promoting CD36 degradation. J Cell Mol Med 2020;24:3292–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tran TTT, Poirier H, Clement L, Nassir F, Pelsers MMAL, Petit V, Degrace P, Monnot M-C, Glatz JFC, Abumrad NA, Besnard P, Niot I.. Luminal lipid regulates CD36 levels and downstream signaling to stimulate chylomicron synthesis. J Biol Chem 2011;286:25201–25210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Smith J, Su X, El-Maghrabi R, Stahl PD, Abumrad NA.. Opposite regulation of CD36 ubiquitination by fatty acids and insulin: effects on fatty acid uptake. J Biol Chem 2008;283:13578–13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sun S, Tan P, Huang X, Zhang W, Kong C, Ren F, Su X.. Ubiquitinated CD36 sustains insulin-stimulated Akt activation by stabilizing insulin receptor substrate 1 in myotubes. J Biol Chem 2018;293:2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Srikanthan S, Li W, Silverstein RL, McIntyre TM.. Exosome poly-ubiquitin inhibits platelet activation, downregulates CD36 and inhibits pro-atherothombotic cellular functions. J Thromb Haemost 2014;12:1906–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hoosdally SJ, Andress EJ, Wooding C, Martin CA, Linton KJ.. The human scavenger receptor CD36: glycosylation status and its role in trafficking and function. J Biol Chem 2009;284:16277–16288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McDonald AG, Tipton KF, Davey GP.. A mechanism for bistability in glycosylation. PLoS Comput Biol 2018;14:e1006348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vinals M, Xu S, Vasile E, Krieger M.. Identification of the N-linked glycosylation sites on the high density lipoprotein (HDL) receptor SR-BI and assessment of their effects on HDL binding and selective lipid uptake. J Biol Chem 2003;278:5325–5332. [DOI] [PubMed] [Google Scholar]
- 74. Mitra N, Sinha S, Ramya TNC, Surolia A.. N-linked oligosaccharides as outfitters for glycoprotein folding, form and function. Trends Biochem Sci 2006;31:156–163. [DOI] [PubMed] [Google Scholar]
- 75. Lauzier B, Merlen C, Vaillant F, McDuff J, Bouchard B, Beguin PC, Dolinsky VW, Foisy S, Villeneuve LR, Labarthe F, Dyck JRB, Allen BG, Charron G, Des Rosiers C.. Post-translational modifications, a key process in CD36 function: lessons from the spontaneously hypertensive rat heart. J Mol Cell Cardiol 2011;51:99–108. [DOI] [PubMed] [Google Scholar]
- 76. Chu L.-Y., Silverstein R. L.. CD36 ectodomain phosphorylation blocks thrombospondin-1 binding: structure-function relationships and regulation by protein kinase C. Arterioscler Thromb Vasc Biol 2012;32:760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hatmi M, Gavaret JM, Elalamy I, Vargaftig BB, Jacquemin C.. Evidence for cAMP-dependent platelet ectoprotein kinase activity that phosphorylates platelet glycoprotein IV (CD36). J Biol Chem 1996;271:24776–24780. [DOI] [PubMed] [Google Scholar]
- 78. Lynes M, Narisawa S, Millan JL, Widmaier EP.. Interactions between CD36 and global intestinal alkaline phosphatase in mouse small intestine and effects of high-fat diet. Am J Physiol Regul Integr Comp Physiol 2011;301:R1738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Asch AS, Liu I, Briccetti FM, Barnwell JW, Kwakye-Berko F, Dokun A, Goldberger J, Pernambuco M.. Analysis of CD36 binding domains: ligand specificity controlled by dephosphorylation of an ectodomain. Science 1993;262:1436–1440. [DOI] [PubMed] [Google Scholar]
- 80. Ho M, Hoang HL, Lee KM, Liu N, MacRae T, Montes L, Flatt CL, Yipp BG, Berger BJ, Looareesuwan S, Robbins SM.. Ectophosphorylation of CD36 regulates cytoadherence of Plasmodium falciparum to microvascular endothelium under flow conditions. Infect Immun 2005;73:8179–8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Guthmann F, Maehl P, Preiss J, Kolleck I, Rustow B.. Ectoprotein kinase-mediated phosphorylation of FAT/CD36 regulates palmitate uptake by human platelets. Cell Mol Life Sci 2002;59:1999–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. De I, Sadhukhan S.. Emerging roles of DHHC-mediated protein s-palmitoylation in physiological and pathophysiological context. Eur J Cell Biol 2018;97:319–338. [DOI] [PubMed] [Google Scholar]
- 83. Wang J, Hao J-W, Wang X, Guo H, Sun H-H, Lai X-Y, Liu L-Y, Zhu M, Wang H-Y, Li Y-F, Yu L-Y, Xie C, Wang H-R, Mo W, Zhou H-M, Chen S, Liang G, Zhao T-J.. DHHC4 and DHHC5 facilitate fatty acid uptake by palmitoylating and targeting cd36 to the plasma membrane. Cell Rep 2019;26:209–221.e5. [DOI] [PubMed] [Google Scholar]
- 84. Meiler S, Baumer Y, Huang Z, Hoffmann FW, Fredericks GJ, Rose AH, Norton RL, Hoffmann PR, Boisvert WA.. Selenoprotein K is required for palmitoylation of CD36 in macrophages: implications in foam cell formation and atherogenesis. J Leukoc Biol 2013;93:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. van Oort MM, Drost R, Janβen L, Van Doorn JM, Kerver J, Van der Horst DJ, Luiken JJFP, Rodenburg K(CW.. Each of the four intracellular cysteines of CD36 is essential for insulin- or AMP-activated protein kinase-induced CD36 translocation. Arch Physiol Biochem 2014;120:40–49. [DOI] [PubMed] [Google Scholar]
- 86. Thorne RF, Ralston KJ, de Bock CE, Mhaidat NM, Zhang XD, Boyd AW, Burns GF.. Palmitoylation of CD36/FAT regulates the rate of its post-transcriptional processing in the endoplasmic reticulum. Biochim Biophys Acta 2010;1803:1298–1307. [DOI] [PubMed] [Google Scholar]
- 87. Lejay A, Fang F, John R, Van JAD, Barr M, Thaveau F, Chakfe N, Geny B, Scholey JW.. Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus. J Mol Cell Cardiol 2016;91:11–22. [DOI] [PubMed] [Google Scholar]
- 88. Lesnefsky EJ, Chen Q, Tandler B, Hoppel CL.. Mitochondrial dysfunction and myocardial ischemia-reperfusion: implications for novel therapies. Annu Rev Pharmacol Toxicol 2017;57:535–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Heather LC, Pates KM, Atherton HJ, Cole MA, Ball DR, Evans RD, Glatz JF, Luiken JJ, Griffin JL, Clarke K.. Differential translocation of the fatty acid transporter, FAT/CD36, and the glucose transporter, GLUT4, coordinates changes in cardiac substrate metabolism during ischemia and reperfusion. Circ Heart Fail 2013;6:1058–1066. [DOI] [PubMed] [Google Scholar]
- 90. Steinbusch LKM, Wijnen W, Schwenk RW, Coumans WA, Hoebers NTH, Ouwens DM, Coumans WA, Hoebers NTH, Diamant M, Bonen A, Glatz JFC, Luiken JJFP.. Differential regulation of cardiac glucose and fatty acid uptake by endosomal pH and actin filaments. Am J Physiol Cell Physiol 2010;298:C1549–59. [DOI] [PubMed] [Google Scholar]
- 91. Fukushima A, Milner K, Gupta A, Lopaschuk GD.. Myocardial energy substrate metabolism in heart failure : from pathways to therapeutic targets. Curr Pharm Des 2015;21:3654–3664. [DOI] [PubMed] [Google Scholar]
- 92. Umbarawan Y, Syamsunarno MRAA, Koitabashi N, Yamaguchi A, Hanaoka H, Hishiki T, Nagahata-Naito Y, Obinata H, Sano M, Sunaga H, Matsui H, Tsushima Y, Suematsu M, Kurabayashi M, Iso T.. Glucose is preferentially utilized for biomass synthesis in pressure-overloaded hearts: evidence from fatty acid-binding protein-4 and -5 knockout mice. Cardiovasc Res 2018;114:1132–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jelenik T, Flogel U, Alvarez-Hernandez E, Scheiber D, Zweck E, Ding Z, Rothe M, Mastrototaro L, Kohlhaas V, Kotzka J, Knebel B, Muller-Wieland D, Moellendorf S, Godecke A, Kelm M, Westenfeld R, Roden M, Szendroedi J.. Insulin resistance and vulnerability to cardiac ischemia. Diabetes 2018;67:2695–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Liu Y, Neumann D, Glatz JFC, Luiken JJFP.. Molecular mechanism of lipid-induced cardiac insulin resistance and contractile dysfunction. Prostaglandins Leukot Essent Fatty Acids 2018;136:131–141. [DOI] [PubMed] [Google Scholar]
- 95. Adrian L, Lenski M, Tödter K, Heeren J, Böhm M, Laufs U.. AMPK prevents palmitic acid-induced apoptosis and lipid accumulation in cardiomyocytes. Lipids 2017;52:737–750. [DOI] [PubMed] [Google Scholar]
- 96. Gentek R, Hoeffel G.. The innate immune response in myocardial infarction, repair, and regeneration. Adv Exp Med Biol 2017;1003:251–272. [DOI] [PubMed] [Google Scholar]
- 97. Weinberger T, Schulz C.. Myocardial infarction: a critical role of macrophages in cardiac remodeling. Front Physiol 2015;6:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dehn S, Thorp EB.. Myeloid receptor CD36 is required for early phagocytosis of myocardial infarcts and induction of Nr4a1-dependent mechanisms of cardiac repair. FASEB J 2018;32:254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. DeLeon-Pennell KY, Tian Y, Zhang B, Cates CA, Iyer RP, Cannon P, Shah P, Aiyetan P, Halade GV, Ma Y, Flynn E, Zhang Z, Jin Y-F, Zhang H, Lindsey ML.. CD36 is a matrix metalloproteinase-9 substrate that stimulates neutrophil apoptosis and removal during cardiac remodeling. Circ Cardiovasc Genet 2016;9:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lindsey ML, Jung M, Yabluchanskiy A, Cannon PL, Iyer RP, Flynn ER, DeLeon-Pennell KY, Valerio FM, Harrison CL, Ripplinger CM, Hall ME, Ma Y.. Exogenous CXCL4 infusion inhibits macrophage phagocytosis by limiting CD36 signalling to enhance post-myocardial infarction cardiac dilation and mortality. Cardiovasc Res 2019;115:395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Greenwalt DE, Scheck SH, Rhinehart-Jones T.. Heart CD36 expression is increased in murine models of diabetes and in mice fed a high fat diet. J Clin Invest 1995;96:1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD.. Revisiting cardiac cellular composition. Circ Res 2016;118:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Coort SLM, Willems J, Coumans WA, van der Vusse GJ, Bonen A, Glatz JFC, Luiken JJFP.. Sulfo-N-succinimidyl esters of long chain fatty acids specifically inhibit fatty acid translocase (FAT/CD36)-mediated cellular fatty acid uptake. Mol Cell Biochem 2002;239:213–219. [PubMed] [Google Scholar]
- 104. Mansor LS, Sousa Fialho M, da L, Yea G, Coumans WA, West JA, Kerr M, Carr CA, Luiken JJFP, Glatz JFC, Evans RD, Griffin JL, Tyler DJ, Clarke K, Heather LC.. Inhibition of sarcolemmal FAT/CD36 by sulfo-N-succinimidyl oleate rapidly corrects metabolism and restores function in the diabetic heart following hypoxia/reoxygenation. Cardiovasc Res 2017;113:737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Marleau S, Harb D, Bujold K, Avallone R, Iken K, Wang Y, Demers A, Sirois MG, Febbraio M, Silverstein RL, Tremblay A, Ong H.. EP 80317, a ligand of the CD36 scavenger receptor, protects apolipoprotein E-deficient mice from developing atherosclerotic lesions. FASEB J 2005;19:1869–1871. [DOI] [PubMed] [Google Scholar]
- 106. Bessi VL, Labbe SM, Huynh DN, Menard L, Jossart C, Febbraio M, Guerin B, Bentourkia M, Lecomte R, Carpentier AC, Ong H, Marleau S.. EP 80317, a selective CD36 ligand, shows cardioprotective effects against post-ischaemic myocardial damage in mice. Cardiovasc Res 2012;96:99–108. [DOI] [PubMed] [Google Scholar]
- 107. Huynh DN, Bessi VL, Menard L, Piquereau J, Proulx C, Febbraio M, Lubell WD, Carpentier AC, Burelle Y, Ong H, Marleau S.. Adiponectin has a pivotal role in the cardioprotective effect of CP-3(iv), a selective CD36 azapeptide ligand, after transient coronary artery occlusion in mice. FASEB J 2018;32:807–818. [DOI] [PubMed] [Google Scholar]
- 108. Zheng A, Cao L, Qin S, Chen Y, Li Y, Zhang D.. Exenatide regulates substrate preferences through the p38γ mapk pathway after ischaemia/reperfusion injury in a rat heart. Heart Lung Circ 2017;26:404–412. [DOI] [PubMed] [Google Scholar]
- 109. Nagendran J, Pulinilkunnil T, Kienesberger PC, Sung MM, Fung D, Febbraio M, Dyck JRB.. Cardiomyocyte-specific ablation of CD36 improves post-ischemic functional recovery. J Mol Cell Cardiol 2013;63:180–188. [DOI] [PubMed] [Google Scholar]
- 110. Cera M, Salerno A, Fragasso G, Montanaro C, Gardini C, Marinosci G, Arioli F, Spoladore R, Facchini A, Godino C, Margonato A.. Beneficial electrophysiological effects of trimetazidine in patients with postischemic chronic heart failure. J Cardiovasc Pharmacol Ther 2010;15:24–30. [DOI] [PubMed] [Google Scholar]
- 111. Neckář J, Šilhavý J, Zídek V, Landa V, Mlejnek P, Šimáková M, Seidman JG, Seidman C, Kazdová L, Klevstig M, Novák F, Vecka M, Papoušek F, Houštěk J, Drahota Z, Kurtz TW, Kolář F, Pravenec M.. CD36 overexpression predisposes to arrhythmias but reduces infarct size in spontaneously hypertensive rats: gene expression profile analysis. Physiol Genomics 2012;44:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Carpentier AC. Abnormal myocardial dietary fatty acid metabolism and diabetic cardiomyopathy. Can J Cardiol 2018;34:605–614. [DOI] [PubMed] [Google Scholar]
- 113. Jia G, DeMarco VG, Sowers JR.. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol 2016;12:144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Joubert M, Manrique A, Cariou B, Prieur X.. Diabetes-related cardiomyopathy: the sweet story of glucose overload from epidemiology to cellular pathways. Diabetes Metab 2019;45:238–247. [DOI] [PubMed] [Google Scholar]
- 115. Coort SLM, Hasselbaink DM, Koonen DPY, Willems J, Coumans WA, Chabowski A, van der Vusse GJ, Bonen A, Glatz JFC, Luiken JJFP.. Enhanced sarcolemmal FAT/CD36 content and triacylglycerol storage in cardiac myocytes from obese zucker rats. Diabetes 2004;53:1655–1663. [DOI] [PubMed] [Google Scholar]
- 116. Carley AN, Severson DL.. What are the biochemical mechanisms responsible for enhanced fatty acid utilization by perfused hearts from type 2 diabetic db/db mice? Cardiovasc Drugs Ther 2008;22:83–89. [DOI] [PubMed] [Google Scholar]
- 117. Ouwens DM, Diamant M, Fodor M, Habets DDJ, Pelsers MMAL, El Hasnaoui M, Dang ZC, van den Brom CE, Vlasblom R, Rietdijk A, Boer C, Coort SLM, Glatz JFC, Luiken JJFP.. Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia 2007;50:1938–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. García-Rúa V, Otero MF, Lear PV, Rodríguez-Penas D, Feijóo-Bandín S, Noguera-Moreno T, Calaza M, Álvarez-Barredo M, Mosquera-Leal A, Parrington J, Brugada J, Portolés M, Rivera M, González-Juanatey JR, Lago F.. Increased expression of fatty-acid and calcium metabolism genes in failing human heart. PLoS One 2012;7:e37505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Liu Y, Steinbusch LKM, Nabben M, Kapsokalyvas D, van Zandvoort M, Schönleitner P, Antoons G, Simons PJ, Coumans WA, Geomini A, Chanda D, Glatz JFC, Neumann D, Luiken JJFP.. Palmitate-induced vacuolar-type h(+)-atpase inhibition feeds forward into insulin resistance and contractile dysfunction. Diabetes 2017;66:1521–1534. [DOI] [PubMed] [Google Scholar]
- 120. Paolillo S, Marsico F, Prastaro M, Renga F, Esposito L, De Martino F, Di Napoli P, Esposito I, Ambrosio A, Ianniruberto M, Mennella R, Paolillo R, Gargiulo P.. Diabetic cardiomyopathy: definition, diagnosis, and therapeutic implications. Heart Fail Clin 2019;15:341–347. [DOI] [PubMed] [Google Scholar]
- 121. Chistiakov DA, Orekhov AN, Bobryshev YV.. The impact of FOXO-1 to cardiac pathology in diabetes mellitus and diabetes-related metabolic abnormalities. Int J Cardiol 2017;245:236–244. [DOI] [PubMed] [Google Scholar]
- 122. Griffin E, Re A, Hamel N, Fu C, Bush H, McCaffrey T, Asch AS.. A link between diabetes and atherosclerosis: glucose regulates expression of CD36 at the level of translation. Nat Med 2001;7:840–846. [DOI] [PubMed] [Google Scholar]
- 123. Alonso N, Moliner P, Mauricio D.. Pathogenesis, clinical features and treatment of diabetic cardiomyopathy. Adv Exp Med Biol 2018;1067:197–217. [DOI] [PubMed] [Google Scholar]
- 124. Li H, Fan J, Zhao Y, Zhang X, Dai B, Zhan J, Yin Z, Nie X, Fu X-D, Chen C, Wang DW.. Nuclear mir-320 mediates diabetes-induced cardiac dysfunction by activating transcription of fatty acid metabolic genes to cause lipotoxicity in the heart. Circ Res 2019;125:1106–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Luiken JJFP. Sarcolemmal fatty acid uptake vs. mitochondrial beta-oxidation as target to regress cardiac insulin resistance. Appl Physiol Nutr Metab 2009;34:473–480. [DOI] [PubMed] [Google Scholar]
- 126. Petersen MC, Shulman GI.. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol. Sci 2017;38:649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cortassa S, Sollott SJ, Aon MA.. Mitochondrial respiration and ROS emission during β-oxidation in the heart: an experimental-computational study. PLoS Comput Biol 2017;13:e1005588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Agita A, Alsagaff MT.. Inflammation, immunity, and hypertension. Acta Med Indones 2017;49:158–165. [PubMed] [Google Scholar]
- 129. Di Meo S, Iossa S, Venditti P.. Skeletal muscle insulin resistance: role of mitochondria and other ROS sources. J Endocrinol 2017;233:R15–R42. [DOI] [PubMed] [Google Scholar]
- 130. Sung HK, Song E, Jahng JWS, Pantopoulos K, Sweeney G.. Iron induces insulin resistance in cardiomyocytes via regulation of oxidative stress. Sci Rep 2019;9:4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhou X, Chang B, Gu Y.. MicroRNA-21 abrogates palmitate-induced cardiomyocyte apoptosis through caspase-3/NF-κB signal pathways. Anatol J Cardiol 2018;20:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Glatz JFC, Bonen A, Ouwens DM, Luiken JJFP.. Regulation of sarcolemmal transport of substrates in the healthy and diseased heart. Cardiovasc Drugs Ther 2006;20:471–476. [DOI] [PubMed] [Google Scholar]
- 133. Taegtmeyer H, Sen S, Vela D.. Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Ann N Y Acad Sci 2010;1188:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chess DJ, Stanley WC.. Role of diet and fuel overabundance in the development and progression of heart failure. Cardiovasc Res 2008;79:269–278. [DOI] [PubMed] [Google Scholar]
- 135. Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H.. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 2004;18:1692–1700. [DOI] [PubMed] [Google Scholar]
- 136. Glass CK, Olefsky JM.. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab 2012;15:635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Brahma MK, Pepin ME, Wende AR.. My sweetheart is broken: role of glucose in diabetic cardiomyopathy. Diabetes Metab J 2017;41:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yang J, Sambandam N, Han X, Gross RW, Courtois M, Kovacs A, Febbraio M, Finck BN, Kelly DP.. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res 2007;100:1208–1217. [DOI] [PubMed] [Google Scholar]
- 139. Wu L, Wang K, Wang W, Wen Z, Wang P, Liu L, Wang DW.. Glucagon-like peptide-1 ameliorates cardiac lipotoxicity in diabetic cardiomyopathy via the PPARα pathway. Aging Cell 2018;17:e12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lin J, Wang T, Li Y, Wang M, Li H, Irwin MG, Xia Z.. N-Acetylcysteine restores sevoflurane postconditioning cardioprotection against myocardial ischemia-reperfusion injury in diabetic rats. J Diabetes Res 2016;2016:9213034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Yan X, Chen J, Zhang C, Zhou S, Zhang Z, Chen J, Feng W, Li X, Tan Y.. FGF21 deletion exacerbates diabetic cardiomyopathy by aggravating cardiac lipid accumulation. J Cell Mol Med 2015;19:1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sequeira V, Bertero E, Maack C.. Energetic drain driving hypertrophic cardiomyopathy. FEBS Lett 2019;593:1616–1626. [DOI] [PubMed] [Google Scholar]
- 143. Tanaka T, Sohmiya K, Kawamura K.. Is CD36 deficiency an etiology of hereditary hypertrophic cardiomyopathy? J Mol Cell Cardiol 1997;29:121–127. [DOI] [PubMed] [Google Scholar]
- 144. Magida JA, Leinwand LA.. Metabolic crosstalk between the heart and liver impacts familial hypertrophic cardiomyopathy. EMBO Mol Med 2014;6:482–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Iemitsu M, Miyauchi T, Maeda S, Sakai S, Fujii N, Miyazaki H, Kakinuma Y, Matsuda M, Yamaguchi I.. Cardiac hypertrophy by hypertension and exercise training exhibits different gene expression of enzymes in energy metabolism. Hypertens Res 2003;26:829–837. [DOI] [PubMed] [Google Scholar]
- 146. Santos MHH, Higuchi M, de L, Tucci PJF, Garavelo SM, Reis MM, Antonio EL, Serra AJ, Maranhão RC.. Previous exercise training increases levels of PPAR-α in long-term post-myocardial infarction in rats, which is correlated with better inflammatory response. Clinics (Sao Paulo) 2016;71:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Broderick TL, Cusimano FA, Carlson C, Babu JR.. Biosynthesis of the essential fatty acid oxidation cofactor carnitine is stimulated in heart and liver after a single bout of exercise in mice. J Nutr Metab 2018;2018:2785090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Deloux R, Vitiello D, Mougenot N, Noirez P, Li Z, Mericskay M, Ferry A, Agbulut O.. Voluntary exercise improves cardiac function and prevents cardiac remodeling in a mouse model of dilated cardiomyopathy. Front Physiol 2017;8:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Tao L, Bei Y, Lin S, Zhang H, Zhou Y, Jiang J, Chen P, Shen S, Xiao J, Li X.. Exercise training protects against acute myocardial infarction via improving myocardial energy metabolism and mitochondrial biogenesis. Cell Physiol Biochem 2015;37:162–175. [DOI] [PubMed] [Google Scholar]
- 150. Karam CN, Warren CM, Henze M, Banke NH, Lewandowski ED, Solaro RJ.. Peroxisome proliferator-activated receptor-α expression induces alterations in cardiac myofilaments in a pressure-overload model of hypertrophy. Am J Physiol Heart Circ Physiol 2017;312:H681–H690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Oka S, Zhai P, Yamamoto T, Ikeda Y, Byun J, Hsu C-P, Sadoshima J.. Peroxisome proliferator activated receptor-α association with silent information regulator 1 suppresses cardiac fatty acid metabolism in the failing heart. Circ Heart Fail 2015;8:1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. van der Meer DLM, Degenhardt T, Väisänen S, de Groot PJ, Heinäniemi M, de Vries SC, Müller M, Carlberg C, Kersten S.. Profiling of promoter occupancy by PPARalpha in human hepatoma cells via ChIP-chip analysis. Nucleic Acids Res 2010;38:2839–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. He C, Zhang G, Ouyang H, Zhang P, Chen Y, Wang R, Zhou H.. Effects of β2/aβ2 on oxLDL-induced CD36 activation in THP-1 macrophages. Life Sci 2019;239:117000. [DOI] [PubMed] [Google Scholar]
- 154. Madonna R, Salerni S, Schiavone D, Glatz J, Geng Y-J, Caterin R.. Omega-3 fatty acids attenuate constitutive and insulin-induced CD36 expression through a suppression of PPAR α/γ activity in microvascular endothelial cells. Thromb Haemost 2011;106:500–510. [DOI] [PubMed] [Google Scholar]
- 155. Tham YK, Bernardo BC, Ooi JYY, Weeks KL, McMullen JR.. Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch Toxicol 2015;89:1401–1438. [DOI] [PubMed] [Google Scholar]
- 156. Abel ED, Doenst T.. Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc Res 2011;90:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Glatz JFC, Nabben M, Young ME, Schulze PC, Taegtmeyer H, Luiken JJFP.. Re-balancing cellular energy substrate metabolism to mend the failing heart. Biochim Biophys Acta Mol Basis Dis 2020;1866:165579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Nakatani K, Masuda D, Kobayashi T, Sairyo M, Zhu Y, Okada T, Naito AT, Ohama T, Koseki M, Oka T, Akazawa H, Nishida M, Komuro I, Sakata Y, Yamashita S.. Pressure overload impairs cardiac function in long-chain fatty acid transporter CD36-knockout mice. Int Heart J 2019;60:159–167. [DOI] [PubMed] [Google Scholar]
- 159. Umbarawan Y, Syamsunarno MRAA, Koitabashi N, Obinata H, Yamaguchi A, Hanaoka H, Hishiki T, Hayakawa N, Sano M, Sunaga H, Matsui H, Tsushima Y, Suematsu M, Kurabayashi M, Iso T.. Myocardial fatty acid uptake through CD36 is indispensable for sufficient bioenergetic metabolism to prevent progression of pressure overload-induced heart failure. Sci Rep 2018;8:12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han X, Tang D, Chen R.. Research progress on the relationship between atherosclerosis and inflammation. Biomolecules 2018;8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ackers I, Szymanski C, Duckett KJ, Consitt LA, Silver MJ, Malgor R.. Blocking Wnt5a signaling decreases CD36 expression and foam cell formation in atherosclerosis. Cardiovasc Pathol 2018;34:1–8. [DOI] [PubMed] [Google Scholar]
- 162. Li C, Cai C, Zheng X, Sun J, Ye L.. Orientin suppresses oxidized low-density lipoproteins induced inflammation and oxidative stress of macrophages in atherosclerosis. Biosci Biotechnol Biochem 2020;84:774–779. [DOI] [PubMed] [Google Scholar]
- 163. Sato Y, Watanabe R, Uchiyama N, Ozawa N, Takahashi Y, Shirai R, Sato K, Mori Y, Matsuyama T, Ishibashi-Ueda H, Hirano T, Watanabe T.. Inhibitory effects of vasostatin-1 against atherogenesis. Clin Sci (Lond) 2018;132:2493–2507. [DOI] [PubMed] [Google Scholar]
- 164. Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM.. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 1998;93:229–240. [DOI] [PubMed] [Google Scholar]
- 165. Zhou M-S, Chadipiralla K, Mendez AJ, Jaimes EA, Silverstein RL, Webster K, Raij L.. Nicotine potentiates proatherogenic effects of oxLDL by stimulating and upregulating macrophage CD36 signaling. Am J Physiol Heart Circ Physiol 2013;305:H563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Reiss AB, Wan DW, Anwar K, Merrill JT, Wirkowski PA, Shah N, Cronstein BN, Chan ESL, Carsons SE.. Enhanced CD36 scavenger receptor expression in THP-1 human monocytes in the presence of lupus plasma: linking autoimmunity and atherosclerosis. Exp Biol Med (Maywood) 2009;234:354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Liu Q, Fan J, Bai J, Peng L, Zhang T, Deng L, Wang G, Zhao Y, Nong J, Zhang M, Wang Y.. IL-34 promotes foam cell formation by enhancing CD36 expression through p38 MAPK pathway. Sci Rep 2018;8:17347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Shi H, Zhang C, Pasupuleti V, Hu X, Prosdocimo DA, Wu W, Qing Y, Wu S, Mohammad H, Gerson SL, Perbal B, Klenotic PA, Dong N, Lin Z.. CCN3 regulates macrophage foam cell formation and atherosclerosis. Am J Pathol 2017;187:1230–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Raghavan S, Singh NK, Gali S, Mani AM, Rao GN.. Protein kinase Cθ via activating transcription factor 2-mediated CD36 expression and foam cell formation of Ly6C(hi) cells contributes to atherosclerosis. Circulation 2018;138:2395–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Liu Y, Zhong Y, Chen H, Wang D, Wang M, Ou J-S, Xia M.. Retinol-binding protein-dependent cholesterol uptake regulates macrophage foam cell formation and promotes atherosclerosis. Circulation 2017;135:1339–1354. [DOI] [PubMed] [Google Scholar]
- 171. Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ.. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol 2013;14:812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Lara-Guzmán OJ, Gil-Izquierdo Á, Medina S, Osorio E, Álvarez-Quintero R, Zuluaga N, Oger C, Galano J-M, Durand T, Muñoz-Durango K.. Oxidized LDL triggers changes in oxidative stress and inflammatory biomarkers in human macrophages. Redox Biol 2018;15:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Bekkering S, Quintin J, Joosten LAB, van der Meer JWM, Netea MG, Riksen NP.. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol 2014;34:1731–1738. [DOI] [PubMed] [Google Scholar]
- 174. Chen Y, Yang M, Huang W, Chen W, Zhao Y, Schulte ML, Volberding P, Gerbec Z, Zimmermann MT, Zeighami A, Demos W, Zhang J, Knaack DA, Smith BC, Cui W, Malarkannan S, Sodhi K, Shapiro JI, Xie Z, Sahoo D, Silverstein RL.. Mitochondrial metabolic reprogramming by CD36 signaling drives macrophage inflammatory responses. Circ Res 2019;125:1087–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Hamidzadeh K, Christensen SM, Dalby E, Chandrasekaran P, Mosser DM.. Macrophages and the recovery from acute and chronic inflammation. Annu Rev Physiol 2017;79:567–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Park YM. CD36, a scavenger receptor implicated in atherosclerosis. Exp Mol Med 2014;46:e99–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Vieira JM, Norman S, Villa Del Campo C, Cahill TJ, Barnette DN, Gunadasa-Rohling M, Johnson LA, Greaves DR, Carr CA, Jackson DG, Riley PR.. The cardiac lymphatic system stimulates resolution of inflammation following myocardial infarction. J Clin Invest 2018;128:3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Luo Y, Duan H, Qian Y, Feng L, Wu Z, Wang F, Feng J, Yang D, Qin Z, Yan X.. Macrophagic CD146 promotes foam cell formation and retention during atherosclerosis. Cell Res 2017;27:352–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Park YM, Febbraio M, Silverstein RL.. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest 2009;119:136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Xu S, Li L, Yan J, Ye F, Shao C, Sun Z, Bao Z, Dai Z, Zhu J, Jing L, Wang Z.. CML/CD36 accelerates atherosclerotic progression via inhibiting foam cell migration. Biomed Pharmacother 2018;97:1020–1031. [DOI] [PubMed] [Google Scholar]
- 181. Ghosh A, Murugesan G, Chen K, Zhang L, Wang Q, Febbraio M, Anselmo RM, Marchant K, Barnard J, Silverstein RL.. Platelet CD36 surface expression levels affect functional responses to oxidized LDL and are associated with inheritance of specific genetic polymorphisms. Blood 2011;117:6355–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Lee B-C, Lin K-H, Hu C-Y, Lo S-C.. Thromboelastography characterized CD36 null subjects as slow clot formation and indicative of hypocoagulability. Thromb Res 2019;178:79–84. [DOI] [PubMed] [Google Scholar]
- 183. Wilhelmsen P, Kjær J, Thomsen KL, Nielsen CT, Dige A, Maniecki MB, Heegaard N, Grønbæk H, Dahlerup J, Handberg A.. Elevated platelet expression of CD36 may contribute to increased risk of thrombo-embolism in active inflammatory bowel disease. Arch Physiol Biochem 2013;119:202–208. [DOI] [PubMed] [Google Scholar]
- 184. Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, Poliakov E, Sun M, Finton PJ, Curtis BR, Chen J, Zhang R, Silverstein RL, Hazen SL.. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med 2007;13:1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Nergiz-Unal R, Lamers MME, Van Kruchten R, Luiken JJ, Cosemans JMEM, Glatz JFC, Kuijpers MJE, Heemskerk JWM.. Signaling role of CD36 in platelet activation and thrombus formation on immobilized thrombospondin or oxidized low-density lipoprotein. J Thromb Haemost 2011;9:1835–1846. [DOI] [PubMed] [Google Scholar]
- 186. Yang M, Cooley BC, Li W, Chen Y, Vasquez-Vivar J, Scoggins NO, Cameron SJ, Morrell CN, Silverstein RL.. Platelet CD36 promotes thrombosis by activating redox sensor ERK5 in hyperlipidemic conditions. Blood 2017;129:2917–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Ghosh A, Li W, Febbraio M, Espinola RG, McCrae KR, Cockrell E, Silverstein RL.. Platelet CD36 mediates interactions with endothelial cell-derived microparticles and contributes to thrombosis in mice. J Clin Invest 2008;118:1934–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Bao Y, Wang L, Xu Y, Yang Y, Wang L, Si S, Cho S, Hong B.. Salvianolic acid B inhibits macrophage uptake of modified low density lipoprotein (mLDL) in a scavenger receptor CD36-dependent manner. Atherosclerosis 2012;223:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Wu T, Peng Y, Yan S, Li N, Chen Y, Lan T.. Andrographolide ameliorates atherosclerosis by suppressing pro-inflammation and ROS generation-mediated foam cell formation. Inflammation 2018;41:1681–1689. [DOI] [PubMed] [Google Scholar]
- 190. Yu M, Jiang M, Chen Y, Zhang S, Zhang W, Yang X, Li X, Li Y, Duan S, Han J, Duan Y.. Inhibition of macrophage CD36 expression and cellular oxidized low density lipoprotein (oxLDL) accumulation by tamoxifen: a peroxisome proliferator-activated receptor (PPAR)γ-dependent mechanism. J Biol Chem 2016;291:16977–16989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Yao S, Tian H, Miao C, Zhang D-W, Zhao L, Li Y, Yang N, Jiao P, Sang H, Guo S, Wang Y, Qin S.. D4F alleviates macrophage-derived foam cell apoptosis by inhibiting CD36 expression and ER stress-CHOP pathway. J Lipid Res 2015;56:836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Dai X-Y, Cai Y, Sun W, Ding Y, Wang W, Kong W, Tang C, Zhu Y, Xu M-J, Wang X.. Intermedin inhibits macrophage foam-cell formation via tristetraprolin-mediated decay of CD36 mRNA. Cardiovasc Res 2014;101:297–305. [DOI] [PubMed] [Google Scholar]
- 193. Zhang J, Nie S, Martinez-Zaguilan R, Sennoune SR, Wang S.. Formulation, characteristics and antiatherogenic bioactivities of CD36-targeted epigallocatechin gallate (EGCG)-loaded nanoparticles. J Nutr Biochem 2016;30:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, Li X, Wang L, Wang L, Liu Y, Liu J, Zhang M, Qi J, Yu S, Afshin A, Gakidou E, Glenn S, Krish VS, Miller-Petrie MK, Mountjoy-Venning WC, Mullany EC, Redford SB, Liu H, Naghavi M, Hay SI, Wang L, Murray CJL, Liang X.. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet (Lond, Engl )2019;394:1145–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. D'Souza K, Nzirorera C, Kienesberger PC.. Lipid metabolism and signaling in cardiac lipotoxicity. Biochim Biophys Acta 2016;1861:1513–1524. [DOI] [PubMed] [Google Scholar]
- 196. Park T-S, Yamashita H, Blaner WS, Goldberg IJ.. Lipids in the heart: a source of fuel and a source of toxins. Curr Opin Lipidol 2007;18:277–282. [DOI] [PubMed] [Google Scholar]
- 197. Evans RD, Hauton D.. The role of triacylglycerol in cardiac energy provision. Biochim Biophys Acta 2016;1861:1481–1491. [DOI] [PubMed] [Google Scholar]
- 198. Bjorkegren J, Véniant M, Kim SK, Withycombe SK, Wood PA, Hellerstein MK, Neese RA, Young SG.. Lipoprotein secretion and triglyceride stores in the heart. J Biol Chem 2001;276:38511–38517. [DOI] [PubMed] [Google Scholar]
- 199. Heier C, Haemmerle G.. Fat in the heart: the enzymatic machinery regulating cardiac triacylglycerol metabolism. Biochim Biophys Acta 2016;1861:1500–1512. [DOI] [PubMed] [Google Scholar]
- 200. Cheng W, Wu P, Du Y, Wang Y, Zhou N, Ge Y, Yang Z.. Puerarin improves cardiac function through regulation of energy metabolism in Streptozotocin-Nicotinamide induced diabetic mice after myocardial infarction. Biochem Biophys Res Commun 2015;463:1108–1114. [DOI] [PubMed] [Google Scholar]
- 201. Campbell SE, Tandon NN, Woldegiorgis G, Luiken JJFP, Glatz JFC, Bonen A.. A novel function for fatty acid translocase (FAT)/CD36: involvement in long chain fatty acid transfer into the mitochondria. J Biol Chem 2004;279:36235–36241. [DOI] [PubMed] [Google Scholar]
- 202. Bezaire V, Bruce CR, Heigenhauser GJF, Tandon NN, Glatz JFC, Luiken JJJF, Bonen A, Spriet LL.. Identification of fatty acid translocase on human skeletal muscle mitochondrial membranes: essential role in fatty acid oxidation. Am J Physiol Endocrinol Metab 2006;290:E509–15. [DOI] [PubMed] [Google Scholar]
- 203. Steinbusch LKM, Schwenk RW, Ouwens DM, Diamant M, Glatz JFC, Luiken JJFP.. Subcellular trafficking of the substrate transporters GLUT4 and CD36 in cardiomyocytes. Cell Mol Life Sci 2011;68:2525–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Luiken JJFP, Coort SLM, Koonen DPY, Bonen A, Glatz JFC.. Signalling components involved in contraction-inducible substrate uptake into cardiac myocytes. Proc Nutr Soc 2004;63:251–258. [DOI] [PubMed] [Google Scholar]
- 205. Zhao L, Varghese Z, Moorhead JF, Chen Y, Ruan X.. Z. CD36 and lipid metabolism in the evolution of atherosclerosis. Br Med Bull 2018;126:101–112. [DOI] [PubMed] [Google Scholar]
- 206. Choromańska B, Myśliwiec P, Choromańska K, Dadan J, Chabowski A.. The role of CD36 receptor in the pathogenesis of atherosclerosis. Adv Clin Exp Med 2017;26:717–722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Most of the 207 references are accessible in pubmed.
Conflict of interest: none declared.