Abstract
This study was performed to determine (a) the age at which ASD is first diagnosed in Ugandan children receiving mental health services; (b) whether age at diagnosis varies by sex and clinical presentation and (c) the average age of ASD diagnosis in children manifesting comorbid conditions. A retrospective chart review was performed and demographic as well as clinical data were collected from children with ASD diagnoses who attended two mental health clinics in Uganda between 2014 and 2019. Descriptive statistics such as percentages, means and standard deviations were used to summarize the data. Independent t-test was also performed to determine differences in the mean age of diagnosis between males and females. Two hundred and thirty-seven (156 males, 81 females) children with ASD were identified. The average age of ASD diagnosis was (6.9±4.0 years). A statistically significant difference in age of ASD diagnosis was found between males and females [t= −2.106, p=0.036], such that on average females received a diagnosis at least one year later than males. Of the 237 participants, 53.6% were identified with ASD only, 16.0% had ASD and ADHD, 10.5% were diagnosed with ASD and epilepsy and 7.2% had a diagnosis of complex ASD. The results confirm delays in access to ASD diagnosis and suggest that females are more likely to receive a diagnosis of ASD later than males within the Ugandan context. ASD awareness should be intensified to improve public or professional knowledge about ASD in Uganda. Finally, effective strategies should be developed to improve early identification.
Keywords: autism spectrum disorder, sex differences, age of diagnosis, Africa, Uganda
Lay Summary
Data were extracted from the medical folders of children with a diagnosis of ASD receiving mental health services in Central Uganda. We showed that female children receive ASD diagnosis at least one year later than males. Also, in some children, ASD was diagnosed alongside other comorbidities such as ADHD, and epilepsy.
Introduction
Autism spectrum disorder (ASD) refers to a group of neurodevelopmental disorders that are characterized by clinical impairments in social functioning and communication as well as repetitive patterns of behaviours, interests or actions (American Psychiatric Association, 2013). Beyond these core symptoms, many children on the spectrum tend to exhibit symptoms of other neurodevelopmental disorders such as attention deficit hyperactivity disorder (ADHD), learning disabilities and cognitive impairments (Brookman-Frazee et al., 2018; Joshi et al., 2010; Lecavalier et al., 2019; Simonoff et al., 2008). In addition, some children with ASD experience comorbid conditions such as speech impairments and intellectual disabilities (Bernard Paulais et al., 2019; Kjellmer et al., 2018; Petalas et al., 2009; Simonoff et al., 2008). Several studies have suggested that ASD is increasingly becoming more prevalent worldwide (Baxter et al., 2015; Elsabagh et al., 2012; Hansen et al., 2015; Maenner, 2020; Malcolm-Smith et al., 2013; Poovathinal et al, 2018). The increased prevalence may be explained by the expansion of the diagnostic criteria and improved public and professional awareness (Campbell et al., 2011; King and Bearman, 2009). Though there is currently no epidemiological data on the prevalence of ASD in Africa, clinical observations suggest that the proportion of children presenting with clinical impairments in social communication has increased substantially over the past few years (Abubakar et al., 2016; Durkin et al., 2015; Franz et al., 2017).
Research has repeatedly shown that ASD is more common in males than females (Halladay et al., 2015; Lai et al., 2015). Studies involving children with ASD have also demonstrated male predominance ranging from 4:1 in individuals with classic autism and 9:1 in children with Asperger’s syndrome or autism associated with average-to-high intelligence (Begeer et al., 2013; Fombonne, 2003). Some investigators have suggested that compared to males, females may possess intrinsic protective factors (i.e., the Female Protective Effect: Gockley et al., 2015; Robinson et al., 2013; Werling and Geschwind, 2013).
Sex differences have been widely studied in children with and without ASD (Halladay et al., 2015; Wood-Downie et al., 2020). Some earlier studies documented differences in the core characteristics between males and females (Hus et al., 2007; Volkmar et al., 1993) but recent evidence suggests that there are more similarities than differences in social communication, particularly in the early stages of life (Jamison et al., 2017; Kaat et al., 2021). It has also been reported that males show more impairments in repetitive and stereotyped behavior than females in early childhood (Jamison et al., 2017; Van Wijngaarden-Cremers et al., 2014). Compared to males, females begin to experience severe impairments in ASD symptoms during school age and adolescence (Aggarwal and Angus, 2015; Dworzynski et al., 2012; Halladay et al., 2015; Jamison et al., 2017; Kaat et al., 2021). For instance, females are reported to have greater impairments in social communication than males during adolescence (Aggarwal and Angus, 2015; Dworzynski et al., 2012; Kaat et al., 2021). Nonetheless, males are more likely to receive ASD diagnosis earlier than females (Halladay et al., 2015; Jamison et al., 2017). While the cause of this remains unclear (Constantino, 2017), some investigators have argued that molecular pathways that control sexual dimorphic brain development may be important targets for further investigation (Jack et al., 2021). It has also been suggested that the perception and interpretation of female social behavior could partially explain why females are diagnosed later and at lower rates than males (Jamison et al., 2017). Additionally, the fact that society places different expectations in social interactions for males and females across developmental time, combined with females’ putative ability to better camouflage their social impairments could partly explain why females are diagnosed less frequently than males (Aggarwal and Angus, 2015; Dworzynski et al., 2012). Generally, these factors make it harder for inexperienced clinicians to identify ASD symptoms earlier in this demographic group. Therefore, clinicians should be properly oriented about these issues so they can take them into account when conducting diagnostic evaluations. Clearly, sex differences in symptoms of ASD and age of diagnosis has not received much research attention in low-resource communities such as Uganda. It is essential that researchers assess factors surrounding clinical practice and access to care, such as age of diagnosis, in non-Western countries. Findings of such studies have potential broad impact that can help drive policies around public health and access to care in low-resource communities.
There is consensus among the autism research community concerning the value of early diagnosis (Zwaigenbaum et al., 2015). This is because early diagnosis leads to early interventions which lead to improved outcomes and quality of life (Begeer et al., 2013; Landa, 2018; Volkmar, 2014; Zwaigenbuam et al., 2015). Early diagnosis can also reduce uncertainty and concomitant stress experienced by parents and empower them to access information and resources to positively affect outcomes for their children and families (Ouellette-Kuntz et al., 2009). However, discussions around ethical issues of early diagnosis without appropriate intervention services, particularly in LMICs, warrants further research and attention (Abubakar et al., 2016; Durkin et al., 2015; Franz et al., 2017).
Most studies have indicated that ASD can be reliably diagnosed between 18 and 36 months of age (Chawarska et al., 2014; Cox et al., 1999; Turner et al., 2006; Moore and Goodson, 2003; Zwaigenbaum et al., 2015). Yet, recent estimates from the United States suggest that the median age at which ASD is diagnosed in younger children is 33 months (Shaw, 2020). A study involving African children showed that most children receive ASD diagnosis between 9 and 10 years (Bello-Mojeed et al., 2017). These statistics show considerable disparity in the age of ASD diagnosis for children in high and low-income countries. In high-income countries such as the United States of America, clinicians would often administer standardized assessments such as the Autism Diagnostic Observation Schedule, second edition (ADOS-2) as part of routine diagnostic evaluations for ASD (Lord et al., 2012). However, in many African health facilities, the lack of standardized measures represents a significant barrier to ASD assessments (Villagomez et al., 2012). In addition, only a few clinicians (often located in larger or capital cities) are trained to effectively evaluate or diagnose ASD and related disorders (Scherzer et al., 2012; Villagomez et al., 2012). Consequently, the majority of African children are often diagnosed by experienced psychiatrists or pediatricians using clinical assessments (based on DSM-5). The clinical assessments used in these settings would include a comprehensive developmental history obtained from interviews with parents/caregivers and observations of developmental milestones, social and behavioral skills (Fernald et al., 2017; Yousafzai et al., 2012; Volkmar et al., 1993).
The literature on sex differences in age of ASD diagnosis has largely focused on Western populations and results remain conflicting (Begeer et al., 2013; Shattuck et al., 2009; Ouellette-Kuntz et al., 2009). While some reports found no significant differences in age of ASD diagnosis between males and females (Begeer et al., 2013; Mandell et al., 2005; Ouellette-Kuntz et al., 2009; Wiggins et al., 2006), a study conducted in the United States of America documented a later age of diagnosis in females as compared to males with ASD (Shattuck et al., 2009). There is little information about the age at which African children receive professional diagnosis for ASD. Only one recent study estimated the age of diagnosis among a clinical sample of Nigerian children with ASD (Bello-Mojeed et al., 2017), suggesting that the average age of diagnosis is 9 years. To date, no published study has explicitly examined sex differences in age at ASD diagnosis among African children. There is also limited information about the diagnostic characteristics of children with ASD living in African countries such as Uganda. The objectives of this study were to determine: (1) the age at which ASD is first diagnosed in Ugandan children receiving mental health services; (2) whether age at diagnosis varies by sex and clinical presentation and (3) the average age of ASD diagnosis in children manifesting comorbid conditions.
Methods
Study design
We implemented a retrospective chart review of children with ASD diagnosis attending two mental health facilities in Kampala, Uganda. Ethical approval for this study was obtained from the School of Biomedical Sciences Research and Ethics Committee at Makerere University (SBS-REC-716).
Setting
This study was conducted at the Child and Adolescent Mental Health (CAMH) clinic in two national referral hospitals in Kampala, Uganda. The CAMH clinics provide mental health services to the majority of children with developmental disorders including ASD and behavioral problems.
Data source and sample
The data for the present study was obtained from medical folders of children (aged 0–18 years) who were evaluated for possible diagnosis of ASD at the CAMH clinics between June 1, 2014 and July 30, 2019. A five-year period was chosen for this review because it represents the first five years of dedicated, specialized ASD diagnostic and treatment services in the two CAMH facilities. In addition, the period between 2014 and 2019 was the time interval that the research team could access a sizeable number of organized data or records of all the families that had earlier been seen at the two facilities. Th CAMH clinics are run by an interdisciplinary team of clinicians consisting of child and adolescent psychiatrists (with expertise in ASD), clinical psychologists, speech and occupational therapists, and mental health nurses. Five hundred eighty-four (n=584) charts were initially reviewed, and all children who had ASD diagnosis were included in the current manuscript (n=237) (Figure 1).
Figure 1:
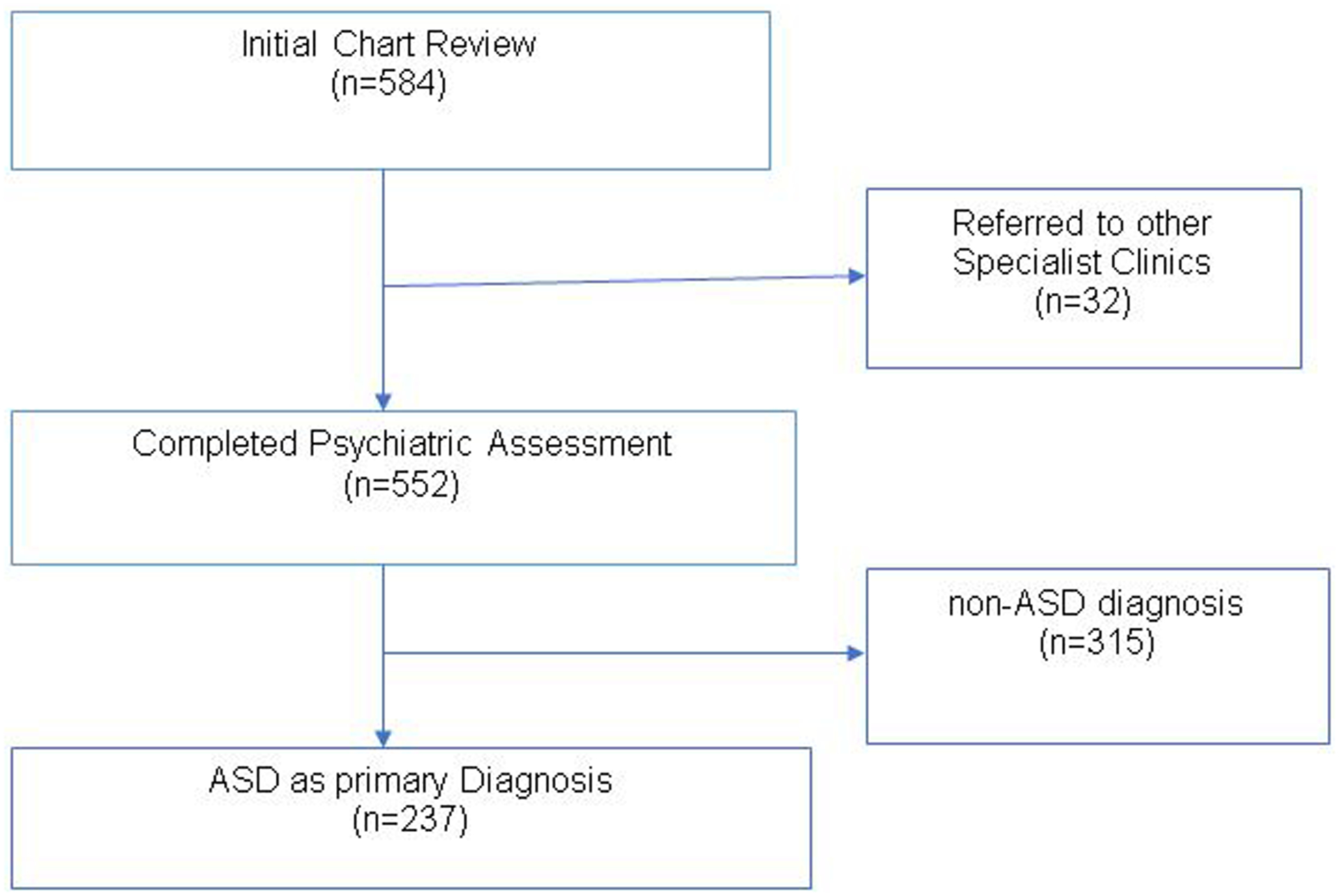
Flow chart of sample identification process
All diagnoses were made by an experienced child and adolescent psychiatrist (the second author, trained in ASD assessments and diagnosis) using the diagnostic criteria recommended by the Diagnostic and Statistical Manual for mental health disorders, fifth edition (DSM-5) (American Psychiatric Association, 2013), informed by a detailed medical history and observation of the child’s skills and behaviors. Direct observation involved social communication and interaction skills, developmental milestones across multiple domains, and play activities. Essentially, the clinician interacted with the child and observed key features of ASD such as difficulty with age-appropriate social interaction, verbal and non-verbal communication/gestures (e.g., pointing or showing objects), circumscribed interests, or repetitive speech or behavior. The child and adolescent psychiatrist who performed the diagnoses has expertise in developmental assessments and ASD diagnosis, and had received extensive training in Autism Diagnostic Observation Schedule (ADOS) while working abroad. In addition, the child psychiatrist has over 15 years of clinical experience in ASD diagnosis and treatment, and had worked in the field in several countries including South Africa and Uganda. Evidence shows that social and communication impairments can optimally be assessed or diagnosed by a clinician experienced in developmental disabilities using a combination of parent interview and direct observation (Zwaigenbaum et al., 2009).
In Uganda, ASD diagnosis is often made by experienced clinicians (trained in ASD or developmental assessments) using clinical assessments and the DSM-5 criteria (American Psychiatric Association, 2013). This is because of the lack of standardized test battery such as Autism Diagnostic Observation Schedule (ADOS). Additionally, within the Ugandan context, only a few clinicians (i.e., child psychiatrists, pediatric neurologists and clinical psychologists) working in Kampala (the capital city) have received training on the ADOS. As most clinicians in this setting cannot afford to buy the ADOS, they rely on their clinical experience and assessment skills using the DSM-5 criteria. It is also important to note that the majority of children with neurodevelopmental disorders including ASD will often be seen by a general practitioner before being referred to specialized clinics for further assessments, diagnosis or management. Mostly, these children and their families will end up in either a pediatric neurology clinic or any of the CAMH clinics in the major referral hospitals where this study was undertaken. These referral hospitals serve adults and children with medical, neurological or psychiatric conditions. Individuals or families are referred to the CAMH clinics from different parts of the country, and represent a range of socioeconomic circumstances.
Procedure
A retrospective chart review of medical records for all children evaluated for possible ASD diagnosis was performed. Four members of the research team (the first and third authors and two research assistants) reviewed the medical charts of all eligible children. Two hundred and thirty-seven children met the inclusion criteria, having received ASD diagnosis. The following data were obtained from each file: name of hospital, sex of child, age at diagnosis, family’s residential address, and diagnostic category assigned to the child at the end of a clinician’s evaluation.
Statistical Analysis
Descriptive statistics such as means, standard deviations, frequencies, and percentages were used to describe the sample. Independent t-test was performed to determine differences in age of diagnosis between boys and girls. SPSS version 26.0 was used for statistical analysis and values of P<0.05 was considered to be significant.
Results
Characteristics of participants
A total of two hundred thirty-seven (n=237) children were included in the final sample, of whom 65.8% (n=156) were males. The majority of the participants (53.6%) had a diagnosis of ASD only while others received a diagnosis of ASD and associated comorbidities including ADHD, epilepsy and speech delays. All children received formal diagnosis of ASD on their initial visit but continued to attend the clinic for follow-up treatments and/or review (Table 1).
Table 1:
Characteristics of Participants (n=237)
| Variables | Total | Male | Female | p-value |
|---|---|---|---|---|
| Number of participants, n (%) | 237 (100) | 156 (65.8) | 81 (34.2) | 0.006 |
| Age at first diagnosis (Mean±SD) | 6.9±4.0 | 6.6±3.9 | 7.8±4.1 | 0.036 |
| Number of clinic visits (Mean±SD) | 2.0±1.4 | 2.1±1.5 | 1.8±0.9 | 0.083 |
| Residential status, n (%) | ||||
| Rural | 25 (16.0) | 13 (16.0) | 0.996 | |
| Urban | 131(84.0) | 68 (84.0) | ||
| CAMH clinics, n (%) | ||||
| Butabika | 102 (65.4) | 38 (46.9) | 0.006 | |
| Mulago | 54 (34.6) | 43 (53.1) |
Sex differences in age of ASD diagnosis
The average age at the time of initial diagnosis was (6.9±4.0) years for the whole sample. However, there was a statistically significant difference in the mean age of diagnosis between males and females (6.60±3.9 vs. 7.75±4.1 years, t=−2.106, p=0.036) (Table 1). In addition, we observed a marginal difference in the number of hospital visits between males and females (t= 1.740, p=0.083). On average, females had a smaller number of visits as compared to males.
The distribution of age of diagnosis by sex is illustrated in Figure 2. The majority of the children (80% of the whole sample; 82.7% of the males, 75.4% of the females) received their first diagnosis before age 10. Of these children, a greater proportion of males received a diagnosis before age four as compared to females [X2 (1,190) = 6.22, p = 0.012].
Figure 2:
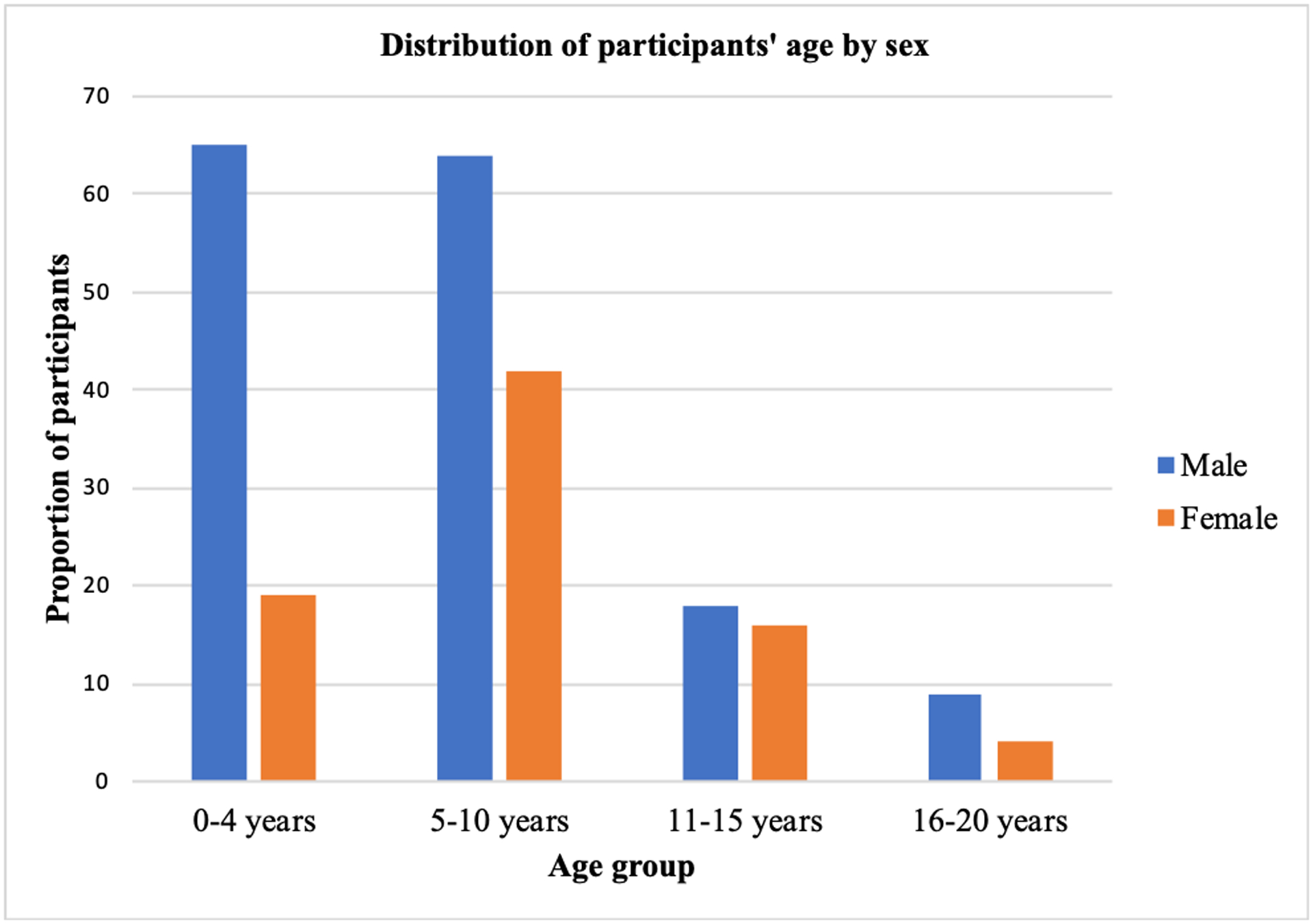
Distribution of participants’ age of diagnosis by sex (males n = 156; females n = 81)
Diagnostic characteristics of participants
Figure 3 illustrates the diagnostic profile of participants. Out of the 237 children who were identified as having ASD, 127 (53.6%, male=83, female=44, mean age= 6.7±3.8) were diagnosed with ASD only, 38 (16.0%, male=25, female=13, mean age =6.2±3.3) had ASD and ADHD, 25 (10.5%, male=19, female=6, mean age =7.8±3.9) were identified as having ASD and Epilepsy, and 17 (7.2%, male=7, female=10, mean age=9.1±5.4) presented with complex ASD. The term complex ASD as used in this paper represents a group of children who received multiple diagnoses including ASD, ADHD, epilepsy and challenging behaviors. Approximately, 2% of children had ASD and speech delays or ASD and challenging behaviours. In general, an equivalent proportion of females and males were represented in each diagnostic profile. Figure 4 represents the average age of diagnosis for each diagnostic profile, disaggregated by sex. Of note, females who received a diagnosis of ASD only (n=44, or 54.3% of females) received this diagnosis on average more than a year after males who received a diagnosis of ASD only (n = 83, or 53.2% of males), t = −1.655, p = 0.100. Additionally, of the children receiving their first ASD diagnosis after the age of 10 (n=47 or 19.8%), 51.1% manifested a comorbid psychiatric, neurological, or behavioural problem.
Figure 3:
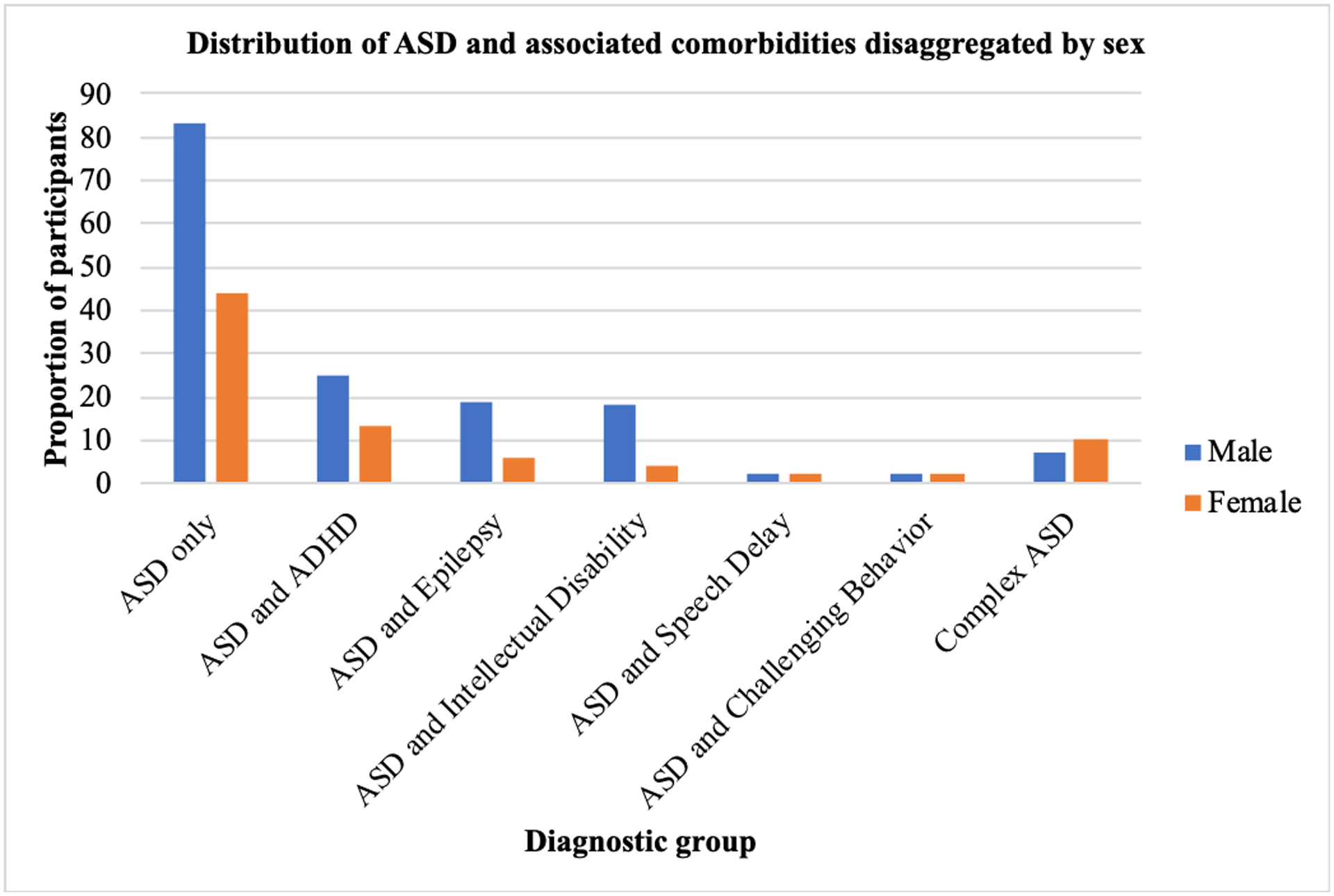
Distribution of ASD and associated comorbidities disaggregated by sex.
Figure 4:
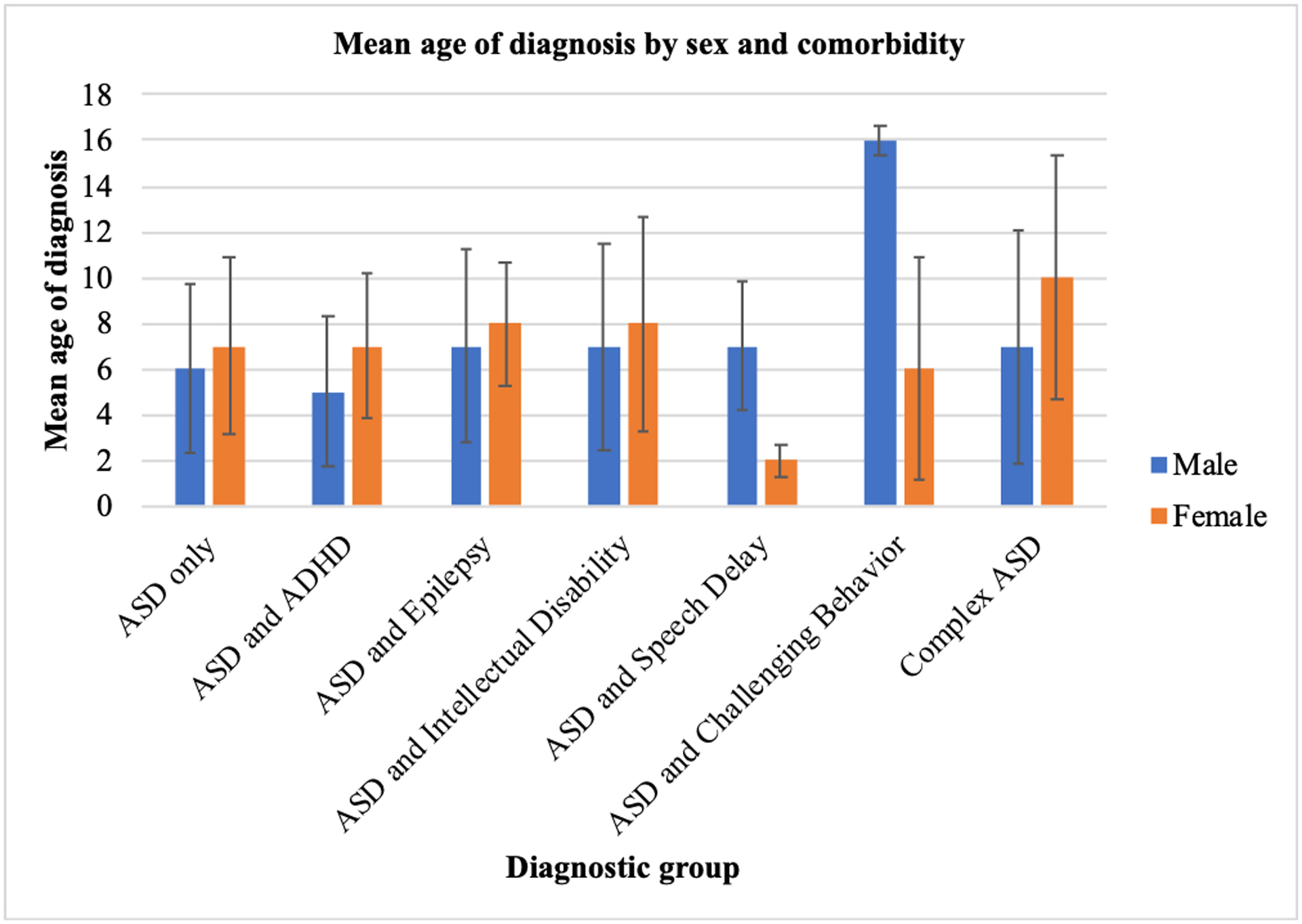
Mean age of diagnosis by sex and comorbidity
4. Discussion
This study examined the impact of sex on the age of ASD diagnosis and identified psychiatric comorbidities associated with autism spectrum disorder among children receiving mental health services in Uganda. We showed that 35% of ASD diagnoses were made in the first 4 years of life, and that an additional 45% of ASD diagnoses were made between ages 5 and 10 years. On average, males received their first diagnosis of ASD earlier than females, and this effect seems to be driven by diagnoses of ASD without comorbid diagnoses. The average age of diagnosis did not appreciably differ by sex in children manifesting ASD and at least one additional co-occurring disorder. The exceptions to this pattern include profiles that constituted a very small proportion of the sample (i.e., ASD + challenging behavior and ASD + ADHD + epilepsy + challenging behaviors). An additional key result is the observation that 46% of the sample presented ASD and at least one additional co-occurring disorder. While phenotypic heterogeneity has been well documented in industrialized countries, this is among the first reports to document this phenomenon in Sub-Saharan Africa.
The age of ASD diagnosis in this sample is similar to what was reported by earlier research that documented the mean age of diagnosis of ASD in a clinical sample of Nigerian children (Bello-Mojeed et al., 2017). The current data builds on previous research that demonstrates late diagnosis of ASD in children living in African countries as compared to industrialized countries. The reasons for delayed diagnosis may include limited public and professional awareness of ASD in Africa and shortage of qualified professionals with expertise in evaluating and diagnosing individuals with ASD. In addition, varying cultural perceptions about developmental disabilities could partly explain why a larger proportion of children receive late diagnoses for ASD and related disorders in these settings. In most African countries including Uganda, some parents and clinicians tend to view problems with or delays in language and social skills as a temporary phenomenon which will be outgrown. As such, even when a given child is manifesting key features of ASD, procuring evaluation and diagnostic services may not be a top priority for parents. In our experience, compared to males, African females with ASD could exhibit a constellation of social skills that mask or camouflage impairing symptoms, making early identification even more challenging. Relatedly, the cultural demand for the expression of social skills in many African cultures is lower in females than males. Thus, males are more likely to be sent to specialized service providers for further evaluation or diagnosis. The combination of this putative camouflage effect and limited demand for the expression of social skills enable females to cope with their social deficits or hide their functional difficulties, resulting in late or no diagnosis (Arinda et al., 2021). Lastly, differences in the interpretation of health, disease and disability across cultures and time might explain delays in diagnosis of ASD and related disabilities. Many African cultures only view disability as an extreme consequence of diseases that renders affected individuals functionally dependent on others and limits their participation in life and socio-economic roles. In view of this, mild neuromotor or social deficits such as ASD is usually not considered as a major problem or health emergency. Thus, most families (particularly those from lower socioeconomic backgrounds), will adopt the “wait and see” approach and hope that their child’s impairments may disappear as they grow older. In summary, a number of factors likely contribute to late diagnoses, and disproportionately those for females, as observed in this study.
Diagnosis of ASD around elementary school age has significant implications for the well-being of affected children. Evidence shows delayed ASD diagnosis reduces access to early educational interventions and treatments, and increases stress in parents. Therefore, it is imperative to create campaigns to increase awareness of ASD and to develop strategies to promote early screening and further evaluation in these settings. As health systems in some African countries such as Uganda are gradually incorporating ASD assessments and treatments into general psychiatric care, it is essential to improve access to early diagnostic evaluations for families in these contexts. This is because, when children receive an early diagnosis, they will be able to receive earlier treatments to improve their health and developmental outcomes. It should however be noted that even though some ASD treatments services are available in Uganda, they are still limited in terms of scope and coverage. Nonetheless, this situation should not be an excuse to delay diagnosis as postponing a diagnosis also may negatively impact the lives of children and families (Zwaigenbaum et al., 2009). Typically, when families get ASD diagnosis for their children in hospitals in Uganda, the clinicians tend to offer therapeutic interventions such as speech or occupational therapy, which help them to better cope with the situation.
Our results also showed statistically significant difference in the mean age of ASD diagnosis and a marginal difference in the number of hospital visits between males and females. This finding supports what was reported by Shattuck et al., (2009) and may relate to sex disparities in parents’ expectations of desirable developmental skills within the African context. In many traditional African cultures, males are expected to be more skillful than females in many domains of behavior including social behavior. This “desirability bias” could mask deficits in social behavior in females and delay recognition and identification of ASD symptoms in this group. Further, anecdotal evidence from the Ugandan context shows that the development of social competence usually emerges later in females than males. Typically, in most African cultures, females are expected to be less sociable compared to males especially before puberty. This expectation may negatively affect social interaction and communication skills in females. The delayed onset of social skills development could be a reason for the late diagnosis of ASD for females in this sample.
While the majority of children were assigned a diagnosis of ASD only, almost half of the sample was identified as having ASD and concomitant psychiatric, neurological, and learning disorders including ADHD, epilepsy and intellectual disability. This finding supports the observed phenotypic heterogeneity among children with ASD that has been widely documented. Clinicians involved in the evaluation and diagnosis of ASD should implement comprehensive assessments that will facilitate identification of co-occurring psychopathology. This will provide useful insights for formulating appropriate treatment plans and interventions.
Several limitations of the current study should be considered when interpreting these results. First, this was a retrospective chart review involving children receiving mental health services from two specific facilities in Uganda. Due to the retrospective nature of the study, no child who is currently receiving treatments at the participating institutions was included. There were missing data such as diagnosis, sex and age at first diagnosis in many of the charts that were excluded. Hence, we are unable to determine if some of those children received ASD diagnoses and the extent to which this might affect our results. Further research is needed to determine whether these findings are representative of the broader Ugandan population. Another major limitation is the lack of cognitive data and information on children’s socioeconomic status. Cognitive assessment is essential to differentiate between idiopathic intellectual disability and autism. In the absence of cognitive profiles for the children, we are unable to evaluate clearly the impact of cognitive function on ASD diagnosis. Given that cognitive data is reported to influence ASD diagnosis, especially in females, we would recommend readers to exercise caution when interpreting our findings as there may be some false positive and false negatives. Further, diagnoses were based on clinical assessments and evaluation of symptoms. Therefore, our estimates may differ if standardized diagnostic tools such as ADOS and ADI-R are employed. Despite the above limitations, the present paper provides insight on the profiles of children receiving early childhood mental health services in East Africa, and can serve as a useful resource for future studies in this area. That said, it would be important for this study to be replicated in large samples across rural and urban health clinics in Uganda using a longitudinal design to reduce systematic bias that may threaten the results. In the near future, we expect to leverage technology (i.e., electronic data management systems) and broaden the period of data collection as well as the variables of interest to provide a more comprehensive picture on these issues to reflect access to diagnostic evaluations and clinical services across the country. Hopefully, our future work would provide additional insights to inform the development of appropriate strategies to improve ASD service delivery within the Ugandan context.
Conclusions and Implications
This study confirms delayed identification of ASD in children living in Sub-Sharan African countries such as Uganda. Importantly, the findings reveal that females are more likely to be diagnosed with ASD later than males. Finally, the results provide further evidence regarding variations in clinical presentation in ASD and associated comorbidities. To improve early identification, there is the need for increased focus on children under 5 years in the region. Our findings have significant value in demonstrating the need to create more awareness about the early symptoms of ASD among parents, health professionals and policy makers in East Africa. Relatedly, clinicians who are qualified to diagnose ASD need to be trained to become more sensitive to recognizing deficits in social skills in females to enhance early detection of ASD in this demographic group. Future research should seek to understand and explain the delays and disparities in age of diagnosis in this population.
Acknowledgements:
We would like to thank Irene Nyantongo for assisting with data collection. We are also grateful to the staff of the participating clinics for supporting us to carry out this study.
Funding
Research reported in this publication was supported by the Fogarty International Centre (FIC) of the National Institutes of Health under grant number D43TW009345 awarded to the Northern Pacific Global Health Fellows Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
The authors have no conflict of interest to declare.
References
- Abubakar A, Ssewanyana D & Newton CR (2016). A systematic review of research on autism spectrum disorders in Sub-Saharan Africa. Behavioural Neurology, 3501910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S, & Angus B (2015). Misdiagnosis versus missed diagnosis: diagnosing autism spectrum disorder in adolescents. Australasian Psychiatry, 23(2), 120–123. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC. [Google Scholar]
- Arinda A, Nakasujja N, & Odokonyero R (2021). Prevalence of autism spectrum disorder symptoms in a paediatric neurology clinic at a tertiary hospital in Uganda. South African Journal of Psychiatry, 27, 1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T & Scott JG (2015). The epidemiology and global burden of autism spectrum disorders. Psychological Medicine, 45(3), 601–613. [DOI] [PubMed] [Google Scholar]
- Begeer S, Mandell D, Wijnker-Holmes B, Venderbosch S, Rem D, Stekelenburg F & Koot HM (2013). Sex differences in the timing of identification among children and adults with autism spectrum disorders. Journal of Autism and Developmental Disorders, 43(5), 1151–1156. [DOI] [PubMed] [Google Scholar]
- Bello-Mojeed MA, Omigbodun OO, Bakare MO & Adewuya AO (2017). Pattern of impairments and late diagnosis of autism spectrum disorder among a sub-Saharan African clinical population of children in Nigeria. Global Mental Health, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard Paulais MA, Mazetto C, Thiébaut E, Nassif MC, Costa Coelho De Souza MT, Stefani AP, … & Adrien JL (2019). Heterogeneities in cognitive and socio-emotional development in children with autism spectrum disorder and severe intellectual disability as a comorbidity. Frontiers in psychiatry, 10, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett D, Warnell F, McConachie H and Parr JR (2016). Factors affecting age at ASD diagnosis in UK: no evidence that diagnosis age has decreased between 2004 and 2014. Journal of Autism and Developmental Disorders, 46(6),1974–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookman-Frazee L, Stadnick N, Chlebowski C, Baker-Ericzén M & Ganger W (2018). Characterizing psychiatric comorbidity in children with autism spectrum disorder receiving publicly funded mental health services. Autism, 22(8),938–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CA, Davarya S, Elsabbagh M, Madden L and Fombonne E (2011). Prevalence and the controversy. In International handbook of autism and pervasive developmental disorders (pp. 25–35). Springer, New York, NY. [Google Scholar]
- Chawarska K, Shic F, Macari S, Campbell DJ, Brian J, Landa R, Hutman T, Nelson CA, Ozonoff S, Tager-Flusberg H & Young GS (2014). 18-month predictors of later outcomes in younger siblings of children with autism spectrum disorder: a baby siblings research consortium study. Journal of the American Academy of Child & Adolescent Psychiatry, 53(12), pp.1317–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN (2017). Taking stock of critical clues to understanding sex differences in the prevalence and recurrence of autism. Autism, 21(6), 769–771. [DOI] [PubMed] [Google Scholar]
- Cox A, Klein K, Charman T, Baird G, Baron-Cohen S, Swettenham J, Drew A & Wheelwright S (1999). Autism spectrum disorders at 20 and 42 months of age: Stability of clinical and ADI-R diagnosis. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 40(5), 719–732. [PubMed] [Google Scholar]
- Durkin MS, Elsabbagh M, Barbaro J, Gladstone M, Happe F, Hoekstra RA, Lee LC, Rattazzi A, Stapel‐Wax J, Stone WL & Tager‐Flusberg H (2015). Autism screening and diagnosis in low resource settings: challenges and opportunities to enhance research and services worldwide. Autism Research, 8(5), 473–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworzynski K, Ronald A, Bolton P, & Happé F (2012). How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders?. Journal of the American Academy of Child & Adolescent Psychiatry, 51(8), 788–797. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, Montiel‐Nava C, Patel V, Paula CS, Wang C & Yasamy MT (2012). Global prevalence of autism and other pervasive developmental disorders. Autism Research, 5(3),160–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald LC, Prado E, Kariger P, & Raikes A (2017). A toolkit for measuring early childhood development in low and middle-income countries.
- Franz L, Chambers N, von Isenburg M & de Vries PJ (2017). Autism spectrum disorder in sub‐saharan africa: A comprehensive scoping review. Autism Research, 10(5), 723–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E (2003). Epidemiological surveys of autism and other pervasive developmental disorders: an update. Journal of Autism and Developmental Disorders, 33(4), 365–382. [DOI] [PubMed] [Google Scholar]
- Fountain C, King MD and Bearman PS (2011). Age of diagnosis for autism: individual and community factors across 10 birth cohorts. Journal of Epidemiology & Community Health, 65(6), 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gockley J, Willsey AJ, Dong S, Dougherty JD, Constantino JN & Sanders SJ (2015). The female protective effect in autism spectrum disorder is not mediated by a single genetic locus. Molecular Autism, 6(1), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halladay AK, Bishop S, Constantino JN, Daniels AM, Koenig K, Palmer K, Messinger D, Pelphrey K, Sanders SJ, Singer AT & Taylor JL (2015). Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Molecular Autism, 6(1), 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SN, Schendel DE & Parner ET (2015). Explaining the increase in the prevalence of autism spectrum disorders: the proportion attributable to changes in reporting practices. JAMA Pediatrics, 169(1), 56–62. [DOI] [PubMed] [Google Scholar]
- Hus V, Pickles A, Cook EH Jr, Risi S, & Lord C (2007). Using the autism diagnostic interview—revised to increase phenotypic homogeneity in genetic studies of autism. Biological psychiatry, 61(4), 438–448. [DOI] [PubMed] [Google Scholar]
- Jack A, Sullivan CAW, Aylward E, Bookheimer SY, Dapretto M, Gaab N, Van Horn JD, Eilbott J, Jacokes Z, Torgerson CM, Bernier RA, Geschwind DH, McPartland JC, Nelson CA, Webb SJ, Pelphrey KA, Gupta AR for the GENDAAR Consortium (2021). A neurogenetic analysis of female autism. Brain, 144(6), 1911–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison R, Bishop SL, Huerta M, & Halladay AK (2017). The clinician perspective on sex differences in autism spectrum disorders. Autism, 21(6), 772–784. [DOI] [PubMed] [Google Scholar]
- Joshi G, Petty C, Wozniak J, Henin A, Fried R, Galdo M, Kotarski M, Walls S & Biederman J (2010). The heavy burden of psychiatric comorbidity in youth with autism spectrum disorders: A large comparative study of a psychiatrically referred population. Journal of Autism and Developmental Disorders, 40(11), 1361–1370. [DOI] [PubMed] [Google Scholar]
- Kaat AJ, Shui AM, Ghods SS, Farmer CA, Esler AN, Thurm A, … & Bishop SL (2021). Sex differences in scores on standardized measures of autism symptoms: a multisite integrative data analysis. Journal of Child Psychology and Psychiatry, 62(1), 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M and Bearman P, 2009. Diagnostic change and the increased prevalence of autism. International Journal of Epidemiology, 38(5),1224–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH & Lord C (2012). Combining information from multiple sources for the diagnosis of autism spectrum disorders for toddlers and young preschoolers from 12 to 47 months of age. Journal of Child Psychology and Psychiatry, 53(2),143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellmer L, Fernell E, Gillberg C, & Norrelgen F (2018). Speech and language profiles in 4-to 6-year-old children with early diagnosis of autism spectrum disorder without intellectual disability. Neuropsychiatric disease and treatment, 14, 2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Auyeung B, Chakrabarti B & Baron-Cohen S, (2015). Sex/gender differences and autism: setting the scene for future research. Journal of the American Academy of Child & Adolescent Psychiatry, 54(1), 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa RJ (2018). Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. International Review of Psychiatry, 30(1), 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecavalier L, McCracken CE, Aman MG, McDougle CJ, McCracken JT, Tierney E, Smith T, Johnson C, King B, Handen B & Swiezy NB, (2019). An exploration of concomitant psychiatric disorders in children with autism spectrum disorder. Comprehensive Psychiatry, 88, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, & Bishop S (2012). Autism diagnostic observation schedule–2nd edition (ADOS-2). Los Angeles, CA: Western Psychological Corporation, 284. [Google Scholar]
- Maenner MJ (2020). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR. Surveillance Summaries, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm-Smith S, Hoogenhout M, Ing N, Thomas KG & de Vries P, (2013). Autism spectrum disorders—Global challenges and local opportunities. Journal of Child & Adolescent Mental Health, 25(1), 1–5. [DOI] [PubMed] [Google Scholar]
- Mandell DS, Novak MM and Zubritsky CD (2005). Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics, 116(6), 1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore V and Goodson S (2003). How well does early diagnosis of autism stand the test of time? Follow-up study of children assessed for autism at age 2 and development of an early diagnostic service. Autism, 7(1), 47–63. [DOI] [PubMed] [Google Scholar]
- Ouellette-Kuntz HM, Coo H, Lam M, Yu CT, Breitenbach MM, Hennessey PE, Holden JJ, Brown HK, Noonan AL, Gauthier RB & Crews LR (2009). Age at diagnosis of autism spectrum disorders in four regions of Canada. Canadian Journal of Public Health, 100(4), 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petalas MA, Hastings RP, Nash S, Lloyd T, & Dowey A (2009). Emotional and behavioural adjustment in siblings of children with intellectual disability with and without autism. Autism, 13(5), 471–483. [DOI] [PubMed] [Google Scholar]
- Poovathinal SA, Anitha A, Thomas R, Kaniamattam M, Melempatt N, Anilkumar A & Meena M (2018). Global Prevalence of Autism: A Mini-Review. SciFed Journal of Autism, 2(1). [Google Scholar]
- Robinson EB, Lichtenstein P, Anckarsäter H, Happé F & Ronald A, (2013). Examining and interpreting the female protective effect against autistic behavior. Proceedings of the National Academy of Sciences, 110(13), 5258–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer AL, Chhagan M, Kauchali S, & Susser E (2012). Global perspective on early diagnosis and intervention for children with developmental delays and disabilities. Developmental Medicine & Child Neurology, 54(12), 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck PT, Durkin M, Maenner M, Newschaffer C, Mandell DS, Wiggins L, Lee LC, Rice C, Giarelli E, Kirby R & Baio J (2009). Timing of identification among children with an autism spectrum disorder: findings from a population-based surveillance study. Journal of the American Academy of Child & Adolescent Psychiatry, 48(5), 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KA (2020). Early identification of autism spectrum disorder among children aged 4 years—Early Autism and Developmental Disabilities Monitoring Network, Six Sites, United States, 2016. MMWR. Surveillance Summaries, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T & Baird G (2008). Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child & Adolescent Psychiatry, 47(8), 921–929. [DOI] [PubMed] [Google Scholar]
- Turner LM, Stone WL, Pozdol SL & Coonrod EE (2006). Follow-up of children with autism spectrum disorders from age 2 to age 9. Autism, 10(3), 243–265. [DOI] [PubMed] [Google Scholar]
- Van Wijngaarden-Cremers PJ, van Eeten E, Groen WB, Van Deurzen PA, Oosterling IJ, & Van der Gaag RJ (2014). Gender and age differences in the core triad of impairments in autism spectrum disorders: a systematic review and meta-analysis. Journal of autism and developmental disorders, 44(3), 627–635. [DOI] [PubMed] [Google Scholar]
- Villagomez AN, Muñoz FM, Peterson RL, Colbert AM, Gladstone M, MacDonald B, … & Brighton Collaboration Neurodevelopmental Delay Working Group. (2019). Neurodevelopmental delay: Case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine, 37(52), 7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Szatmari P, & Sparrow SS (1993). Sex differences in pervasive developmental disorders. Journal of autism and developmental disorders, 23(4), 579–591. [DOI] [PubMed] [Google Scholar]
- Volkmar F, Cook EH, Pomeroy J, Realmuto G, & Tanguay P (1999). Practice parameters for the assessment and treatment of children, adolescents, and adults with autism and other pervasive developmental disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 38(12), 32S–54S [DOI] [PubMed] [Google Scholar]
- Volkmar FR (2014). The importance of early intervention. 2979–2980 [DOI] [PubMed] [Google Scholar]
- Werling DM & Geschwind DH (2013). Understanding sex bias in autism spectrum disorder. Proceedings of the National Academy of Sciences, 110(13), 4868–4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins LD, Baio JON & Rice C (2006). Examination of the time between first evaluation and first autism spectrum diagnosis in a population-based sample. Journal of Developmental & Behavioral Pediatrics, 27(2), S79–S87. [DOI] [PubMed] [Google Scholar]
- Wood‐Downie H, Wong B, Kovshoff H, Cortese S, & Hadwin JA (2020). Research Review: A systematic review and meta‐analysis of sex/gender differences in social interaction and communication in autistic and nonautistic children and adolescents. Journal of Child Psychology and Psychiatry [DOI] [PubMed] [Google Scholar]
- Yousafzai AK, Lynch P, & Gladstone M (2014). Moving beyond prevalence studies: screening and interventions for children with disabilities in low-income and middle-income countries. Archives of disease in childhood, 99(9), 840–848. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bauman ML, Stone WL, Yirmiya N, Estes A, Hansen RL, McPartland JC, Natowicz MR, Choueiri R, Fein D & Kasari C, (2015). Early identification of autism spectrum disorder: recommendations for practice and research. Pediatrics, 136(Supplement 1), pp.S10–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Lord C, Rogers S, Carter A, Carver L, … & Yirmiya N (2009). Clinical assessment and management of toddlers with suspected autism spectrum disorder: insights from studies of high-risk infants. Pediatrics, 123(5), 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]


