Abstract
Introduction:
Individuals exposed to interpersonal violence (IPV) commonly develop posttraumatic stress disorder (PTSD) with co-occurring depression and insomnia. Standard PTSD interventions such as cognitive processing therapy (CPT) do not typically lead to remission or to improved insomnia. Cognitive behavioral therapy for insomnia (CBTi) improves insomnia in individuals with PTSD, but PTSD severity remains elevated.
Objective:
Determine whether sequential treatment of insomnia and PTSD is superior to treating only PTSD.
Methods:
In a 20-week trial, 110 participants exposed to IPV who had PTSD, depression and insomnia were randomized to CBTi followed by CPT or to attention control followed by CPT. Primary outcomes following CBTi (or Control) were 6-week change in score on the Insomnia Severity Index (ISI), Clinician-Administered PTSD Scale (CAPS), and Hamilton Rating Scale for Depression (HAM-D). Primary outcomes following CPT were the 20-week change in scores.
Results:
At 6 weeks, the CBTi condition had greater reductions in ISI, HAM-D and CAPS scores than the attention control condition. At 20 weeks, participants in the CBTi+CPT condition had greater reductions in ISI, HAM-D and CAPS scores compared to Control+CPT. Effects were larger for insomnia and for depression than for PTSD. Similar patterns were observed with respect to clinical response and remission. A tipping point sensitivity analyses supported the plausibility of findings.
Conclusions:
The sequential delivery of CBTi and CPT had plausible, significant effects on insomnia, depression and PTSD compared to CPT alone. Effects for PTSD symptoms were moderate and clinically meaningful.
Keywords: insomnia, posttraumatic stress disorder, depression, interpersonal violence, cognitive-behavioral therapy
Introduction
A large majority of the general population will experience a traumatic event in their lifetime and approximately 6% will subsequently meet criteria for posttraumatic stress disorder (PTSD) [1–3]. Individuals exposed to interpersonal violence (IPV)-related traumas are at elevated risk of developing PTSD compared to those exposed to other civilian traumas [4]. In addition, approximately half of individuals with PTSD have a comorbid diagnosis of major depressive disorder (MDD). The co-occurrence of depression with PTSD is also elevated among those exposed to interpersonal traumas [5, 6].
Sleep disturbance is a core feature of both PTSD and depression [7]. Insomnia is the most commonly endorsed PTSD symptom, often persists following targeted PTSD interventions [8], and blunts treatment response [9]. Insomnia interventions delivered to patients with PTSD can dramatically improve sleep, but both PTSD and depressive symptoms remain elevated [9]. Such clinical realities are well-suited for applying the sequential model of treatment [10–12], which consists of delivering two treatments consecutively The sequential model has been applied primarily to depressive disorders, successfully administering various sequential combinations of pharmacotherapy and/or psychotherapy [11]. With respect to insomnia as the co-occurring condition, several trials have evaluated the co-administration of antidepressant pharmacotherapy and either hypnotic pharmacotherapy [13, 14] or cognitive-behavioral therapy for insomnia (CBTi) [15–17]. Sequential or concurrent approaches for PTSD and insomnia have been suggested [18], but few such approaches have been tested.
One such approach was undertaken in a pilot randomized controlled trial (RCT) in which 23 participants first completed prolonged exposure, an evidence-based treatment for PTSD, and were then randomized to receive either supportive care or imagery rehearsal therapy (IRT) and CBTi, which are first line treatments for nightmares and for insomnia, respectively [19]. In this small pilot study, the combined sleep treatments were associated with additional post-trauma treatment improvements in sleep outcomes following the PTSD treatment relative to the control condition that did not reach statistical significance [19].
IRT for nightmares was also assessed in a sequential treatment RCT comparing IRT followed by CBT for PTSD to wait-list plus CBT in a sample of 42 sexual assault survivors [20]. Although IRT was superior to waitlist before the second sequenced treatment was delivered, the improvements in PTSD and nighttime symptoms did not differ significantly between conditions after both groups received CBT. Galvoski and colleagues [21] randomized 108 female interpersonal assault survivors to either sleep-directed hypnosis or a symptom monitoring control condition with all participants then receiving cognitive processing therapy (CPT), which has demonstrated efficacy in reducing PTSD and depressive symptoms [22]. Following CPT, both conditions evidenced improvements in sleep and PTSD measures that did not differ between conditions. Participants in the hypnosis+CPT condition did have greater reductions in depression symptoms. Findings from the two pilot studies that may have been underpowered and the larger trial that did not use a robust or evidence-based insomnia treatment (i.e., hypnosis), are intriguing in that initial therapeutic benefits appear to accrue when targeting sleep first, but inconclusive with respect to the application of the full sequential model to treating comorbid insomnia and PTSD.
We conducted the current RCT to identify the role of insomnia treatment in PTSD and depression treatment outcomes in survivors of IPV as fully detailed in the published study protocol [23]. The focus on IPV survivors was driven by public health significance and ongoing mental health services collaboration with regional community and legal partners who serve IPV survivors. PTSD and depression are highly comorbid among IPV survivors [5, 6, 24], a population underserved in mental health treatment, unlikely to engage in trauma-focused therapies, and with strong preferences for access to sleep-related services [24, 25].
To address IPV survivors’ needs, the current study sequentially delivered, CBTi, a well-supported, highly effective, and first line treatment for insomnia [26], followed by CPT [22]. The decision to lead with an insomnia treatment was based on the high rates of residual insomnia following PTSD treatment [8, 27] and improvements in sleep, mood and PTSD symptoms in trauma samples receiving CBTi [9]. It was also informed by the possibilities that improving sleep could positively alter mechanisms contributing to PTSD symptoms and that the briefer duration of CBTi interventions (typically 5–8 weeks) relative to trauma-focused psychotherapies(typically 12+ weeks) could improve retention. Our primary hypotheses were that, relative to attention control, participants assigned to CBTi would show greater improvements in insomnia, depression, and PTSD severity immediately prior to CPT, and greater improvement in depression and PTSD severity following CPT. Secondary outcomes included effects on diagnosis, remission and response rates for insomnia, depression and PTSD.
Materials and Methods
Trial Design and Oversight
The study was a 20-week, two-arm, single blind, registered RCT (NCT01743339). The trial compared sequential delivery of four weekly sessions of CBTi and 12 weekly sessions of CPT (CBTi+CPT) to sequential delivery of attention control, wherein participants received phone check-ins from staff followed by CPT (Control+CPT). The design, recruitment, and analysis phases of the trial were informed by pre-study planning meetings with IPV survivors and community-based agencies for domestic violence. Based on such meetings, we offered participants the use of a Crisis Nursery for childcare during overnight study visits.
The University of Rochester Medical Center Research Subjects Review Board approved the protocol, which is published [23]. A university data safety monitoring officer oversaw the study, which conducted participant activities between February 2013 and January 2017. Due to the vulnerability of the population, we received a Federal Certificate of Confidentiality (#CC-NR-12-11). All participants provided written informed consent and the study protocol was approved by the institute’s committee on human research. The authors assume responsibility for the accuracy and completeness of the data, analyses, and fidelity to the protocol. There was no industry support or involvement in the trial. Participants were compensated up to a total of $430 for participation, including: $50 and $40 for interviews at Time 1, and Times 2 and 3, respectively; for each of the 3 time points, $50 for an overnight sleep study, $20 for blood draws and $5 for saliva sampling; $25 for participating in CBTi; $50 for participating in CPT.
Participants
We recruited participants at a county domestic violence family court, a local domestic violence survivor emergency shelter via an on-site research assistant, and via phone through community referrals. Participants were between 18–64 years of age and able to speak and read English.
Inclusion criteria was a past year index event resulting in PTSD symptoms endorsed at a level of moderate or higher on the PTSD Symptom Checklist (PCL-S; ≥ 3 on the 1–5 item response range) [28] and meeting the one month duration criteria; full or subthreshold PTSD the latter defined as (i) exposure to a traumatic event, (ii) at least one re-experiencing symptom, and (iii) either three avoidance or two arousal symptoms from the DSM-IV diagnostic criteria [29]; clinical cutoff for moderate depression (> 10 on the Patient Health Questionnaire-9 [30]); and clinically meaningful insomnia meeting research diagnostic criteria for insomnia disorder (≥ 10 on the Insomnia Severity Index (ISI) [31, 32]). Exclusion criteria included evidence of dementia or cognitive impairment (Mini-Mental State Examination [33] score < 24); history of schizophrenia or bipolar I disorder; current suicidality with either plan, intent, or a suicide attempt in the past 6 months; health conditions with immunological components or undergoing or taking immunosuppressive therapies; active alcohol dependence or in remission < 3 months; medication use including antipsychotics, opiate analgesics, and sleep medications (or sedating medications used at night). Other medications were allowable once a stable dose was achieved; participants were requested not to change medications or dosages and/or to inform study staff. Untreated sleep disorders other than insomnia or nightmares, suspected (e.g., any subject with Body Mass Index > 32 and endorsing loud persistent snoring) or observed (an apnea-hypopnea index > 10 or a Periodic Limb Movement Index with arousals >10 assessed via overnight polysomnography (PSG) in a University sleep research laboratory) apnea, were exclusionary. Cohabiting with an IPV perpetrator at the time of recruitment, and for women, being pregnant, were also exclusionary.
Unique characteristics of the population warranted considerations of participant safety. Individuals in an abusive relationship may return to their abusive partners several times before leaving permanently. For those seeking an order of protection, the order itself can reduce intimate partner violence, and not all orders are issued for abuse that is ongoing. Judges periodically change orders from “no contact” to “no offensive contact” which allows the couple to cohabitate for practical reasons (e.g., childcare). If we became aware that a participant had a new abusive relationship, or had reconciled with a former abusive partner, we enacted a safety procedure that began with administration of the Danger Assessment [34], which assesses potential lethality and fatality of abuse. Results were reviewed with the participant, a companion safety plan was developed, and the participant received referral to a Domestic Violence Center. In addition, a case conference (led by W.P. and C.C., authors) was convened to discuss whether continuance in the study constituted an unmitigated risk, or whether the benefits of continued clinical treatment, despite periodic reconciliations, outweighed the participants’ immediate risks. This occurred only once during the trial and the participant was ultimately retained.
Randomization and Masking
Randomization occurred through Wei’s urn model randomization, which provides overall balance at the end of accrual but also gives good, often near-perfect, balance within many strata [35]. Randomization strata included gender, recruitment site, and antidepressant medication class. Assignment to condition was not predetermined, but generated by a computer program that takes into account study balance to date and provided an assignment to condition “A” or Condition “B”. The principal investigator (WP) was not blinded and assigned the participant to either a CBTi therapist or to the CTRL condition staff, who informed the participant of their assignment and scheduled the first CBTi therapy or phone check-in appointment. Throughout the study, recruiters, study staff conducting assessments, CPT therapists, research assistants entering data, and the statistician remained blind to study condition. The CBTi therapists and staff conducting control condition phone calls were necessarily not blinded to condition.
Interventions
Participants in the CBTi+CPT condition attended four, weekly, individual sessions of CBTi, a standardized, multi-component intervention. Although CBTi typically comprises 6–8 sessions, we [36] and others [37] have shown comparable effects on insomnia improvement when standardized intervention components are delivered over 4 sessions. In the current study, we delivered those standard components as follows: stimulus control therapy including establishing a pre-bedtime routine (session 1), sleep psychoeducation (session 1 & 2), sleep restriction therapy (session 2), sleep hygiene (session 2), cognitive therapy (session 3), and self/management/relapse prevention (session 4), which are detailed further elsewhere [23]. Prior to the first session, participants completed a daily sleep diary across a week (reporting time to bed, minutes to fall asleep, number and length of awakenings, time of final awakening, time out of bed for the day), and then maintained daily diaries throughout CBTi. One addition to standard CBTi that we included in this trial was administering the 23-item Fear of Sleep Inventory at baseline, which was developed to elicit sleep–related thoughts and beliefs that are specific to trauma-related insomnia [38]. Items from this inventory endorsed highly were used to inform therapist-participant interactions during sleep psychoeducation and cognitive therapy. Each participant saw the same therapist for the 4 sessions, who were licensed mental health providers or advanced clinical psychology doctoral students trained in CBTi and supervised by author WP. Participant progress and adherence was monitored via daily sleep diaries and weekly homework logs.
Participants in the Control+CPT condition completed sleep diaries and received four weekly phone calls from the clinical coordinator. Content of the calls included brief check-ins and reminders about the protocol, when to start their next one-week sleep diary, and their next appointment.
Following the four-week CBTi or Control period, we offered all participants CPT consisting of a standard, structured, 12-session, weekly protocol. [22, 23]. Although described in more detail elsewhere, this cognitive therapy begins with psychoeducation (session 1) developing a statement of the traumatic event’s impact on each of five key life dimensions: safety, trust, power/control, esteem, and intimacy (session 2). CPT continues with initial steps of cognitive therapy and writing/rewriting accounts of the trauma (sessions 3–5), identifying and challenging beliefs that interfere with recovery (sessions 6–7), exploring how beliefs regarding the five dimensions were altered and challenging maladaptive beliefs associated with each (sessions 8–11) and ending with review and a final impact statement (session 12). Certified CPT therapists were licensed mental health providers, delivered individual weekly sessions, and were blind to study group assignment. Progress and adherence were monitored by weekly homework logs.
Therapist competence and fidelity were assessed following established procedures [39]. Expert clinicians in CBTi and CPT rated audio recordings of sessions, using rating scale instruments developed by two Veterans Health Administration work groups: the ‘Work Group on CBTi Dissemination’ and the ‘Work Group on CPT Dissemination.’ To establish competence, the first two cases conducted by each clinician were rated; minimum competency criteria were met by both CBTi and CPT therapists. To rate adherence to the protocol a random selection of 10% of all sessions for each intervention were rated; both CBTi and CBT therapist sessions were rated at or above the respective Work Group benchmarks for fidelity.
Outcomes and Assessment Points
Primary and secondary outcome measures were obtained from clinician-administered and self-report instruments completed at baseline prior to randomization (Time 1), and approximately 5–6 weeks (post CBTi or Control period; Time 2) and 20 weeks (post CPT for PTSD; Time 3) after baseline. As described previously [23], additional outcomes were obtained from laboratory assessments conducted 1–2 weeks after baseline clinician and self-report instruments were administered and at the same time as time 2 and time 3 instruments, including overnight PSG following established guidelines [40], and evening and morning blood draws and saliva samples at the PSG visit. These laboratory-based outcomes are not reported here.
The primary outcomes included insomnia, depression and PTSD severity at Time 2 (i.e., following the CBTi or Control period) and Time 3. Insomnia severity was measured using the well-validated Insomnia Severity Index (ISI; scores range from 0 to 28; Cronbach’s α = .76) [31]. PTSD severity was assessed with the Clinician Administered PTSD Scale total score (CAPS; range of 0 to 136; Cronbach’s α = .84) [41]. Depression severity was measured with the clinician-administered, 17-item Hamilton Rating Scale for Depression (HAM-D; range of 0 to 52; Cronbach’s α = .70) [42]. For each of these instruments, higher scores indicate greater severity, and scores from insomnia items were subtracted from the total scores for the CAPS and HAM-D.
In addition to the primary focus on symptom severity outcomes, secondary outcomes included evaluation at Time 3 of (1) DSM-IV diagnostic status for PTSD and MDD at baseline (DSM-5 was not yet released at time of trial initiation and insomnia was assessed by severity not diagnosis); (2) clinically meaningful treatment response; and (3) remission. Diagnostic status was assessed using the CAPS criteria for PTSD (criterion A through F), MINI [43] criteria for MDD, and an ISI score < 11 [31], respectively. As used by others, PTSD response was defined as a decrease in total CAPS score of ≥ 10 points [44–46]; depression response was defined as a reduction of ≥ 50% in total HAM-D score [47]; and insomnia response as an ≥ 8 point reduction in total ISI score [31]. PTSD remission was defined as achieving a final total CAPS score below 20 [48]; MDD remission as achieving a total HAM-D score below 8 [49]; and insomnia remission as achieving a total ISI score < 8 [31].
Finally, as recommended to more fully understand the extent to which interventions may be ineffective for some participants [50], we determined the number of participants in each group who had deteriorated at Time 3 as signaled by a reliable increase in symptoms in insomnia, PTSD, or depression. Following Jacobson and Truax [51], the cutoffs for reliable symptom exacerbation in our sample were 5.2 for ISI, 14.0 for CAPS, and 5.2 for HAM-D calculated as ≥ SEdiff × 1.96 where , SDbaseline equals the standard deviation of the sample baseline observations, and r is the test-retest reliability of the ISI, CAPS, and HAM-D (.79, .89, and .87, respectively).
Statistical Analysis
The primary analyses involved mean differences, adjusted for baseline covariate effects [52–54]. For continuous outcomes, we calculated that, with a sample size of 120, the study would have 80% power to detect an effect size ranging between .43, under the reasonable assumption of baseline covariates explaining 30% of the variance in the outcome, to .51 under a more conservative estimate of no variance being explained by covariates. All analyses were undertaken with SAS 9.4 (Cary, NC: SAS Institute Inc.).
Study outcomes were examined using intent-to-treat principles where all those randomized were included. Missing data was imputed using multiple imputation conducted at the scale level under the assumption of missing data being missing at random (MAR). We followed recommendations to include auxiliary variables moderately associated with the primary outcome variables [55, 56] (in our case preliminary correlation analyses warranted the World Health Organization’s Quality of Life subscales (WHOQOL) [57]. In all instances, missingness was related to observed variables, providing some evidence to the plausibility of the MAR assumption. Missing baseline information was imputed first using the full dataset, with imputations then conducted separately by treatment condition [58, 59]. We imputed 200 complete datasets in accordance with recent research on multiple imputation [60–62], which were analyzed and combined [63].
In examining outcomes, we used analysis of covariance to examine mean differences in continuous outcomes between the CBTi and control conditions at both Time 2 (after CBTi or Control and prior to CPT) and at Time 3 (following CPT). A priori, it was decided that covariates would include the stratification variables of gender, site (family court vs. community), and antidepressant medication use (yes/no); age, race/ethnicity (minority vs. majority status), level of education, number of lifetime trauma exposures, and the baseline value of the dependent variable. Any observed baseline differences between conditions on primary outcomes and additional demographic variables were also included as covariates in outcome analyses.
We used Firth’s [64] penalized logistic regression to avoid difficulties in sparseness of cells in comparing diagnostic status, treatment response rates, remission rates, and symptom exacerbation for insomnia, depression and PTSD at Time 3.The same covariates as outlined above were included.
As a form of sensitivity analysis, we used a “tipping point” approach to explore the robustness of the imputation assumptions for the continuous outcomes [65, 66]. Briefly, a tipping point approach progressively adds a constant to the imputed values within the experimental group until the conclusions from the primary analyses are overturned. Each constant represents an increased departure from MAR; to the extent that the constant that overturns the conclusions is implausible, then greater confidence in the primary results is inferred. For our outcomes, we started by adding increments of 2 to each imputed value until results became non-significant. At this threshold, we tested the midpoint of this value to see if the conclusion changed and progressively narrowed down the results in .2 increments (e.g., 0, 2, 4, 6, 8, 7, 6.2, 6.4 offsets).
Results
As shown in Figure 1, we approached 2,414 individuals for the study, screened 797 and randomized 110. Overall, the sample was racially diverse (56% minority), predominantly female (97%), and socio-economically disadvantaged (e.g., 46% unemployed, 66% with annual income under $20,000). At baseline, all participants met inclusion criteria for insomnia. One participant in each condition met criteria for subthreshold PTSD, but did not meet full CAPS-rated diagnostic criteria for PTSD and six participants (four controls and two in the CBTi condition) met inclusion criteria for moderate depression, but did not meet full diagnostic criteria for MDD as assessed by the MINI. As shown in Table 1, there were no statistically significant differences across experimental condition at baseline for categorical demographic variables. Table 2 presents similar information for the continuous measures at baseline. Participants randomized to the CBTi condition had a higher PTSD severity, higher depression severity and lower environmental QOL, which were therefore entered as covariates in outcome analyses.
Figure 1. CONSORT Flow Diagram.
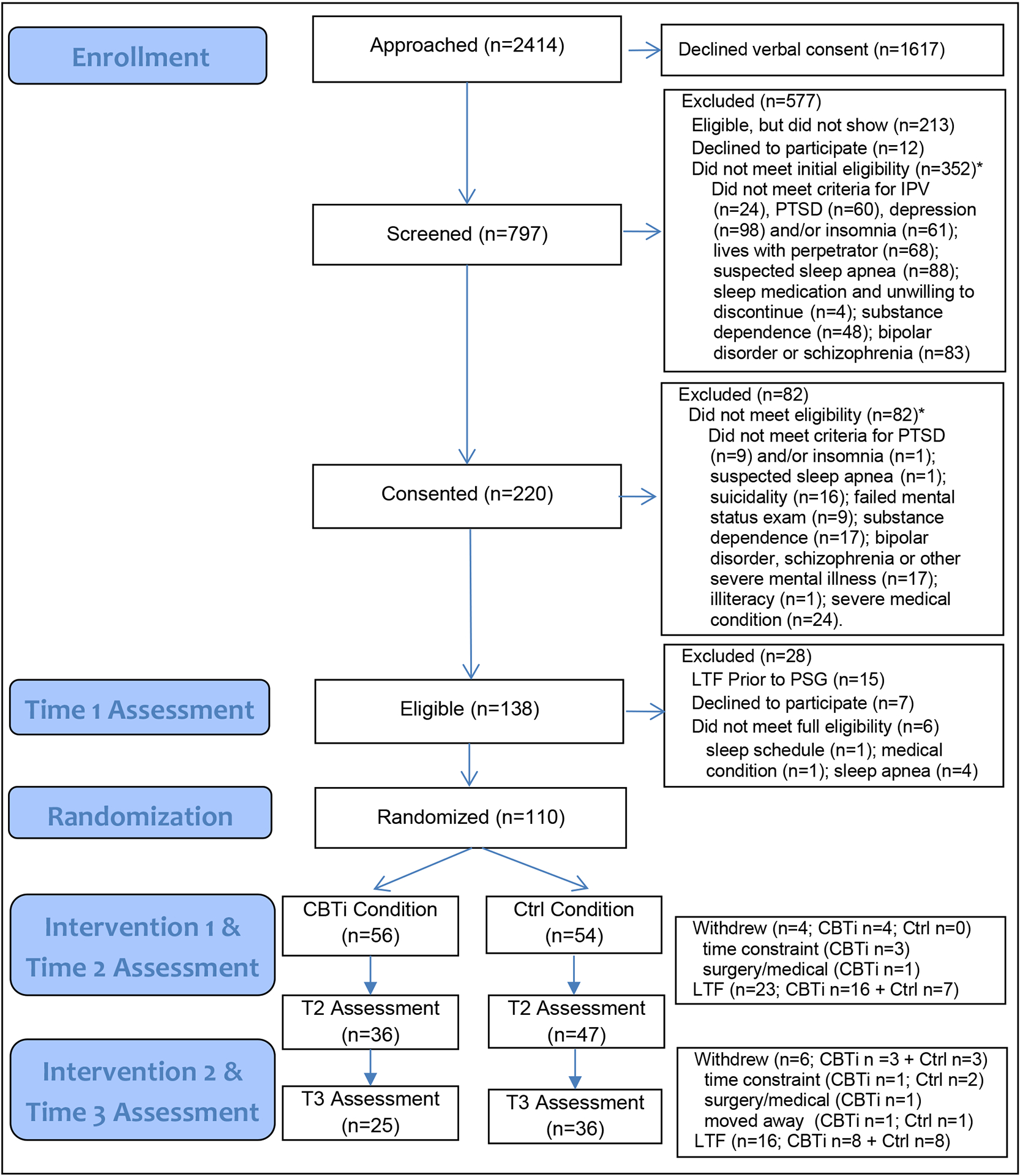
* Ineligibility reasons are not mutually exclusive, so the total reasons for ineligibility do not sum to total ineligible participants.
CBTi = cognitive behavioral therapy for insomnia condition; Ctrl = control condition; LTF = lost to follow-up (i.e., unable to contact); PTSD posttraumatic stress disorder; PSG = polysomnography.
Table 1.
Participant Characteristics
| Characteristic | Full Sample (N = 110) N(%) | Control (n = 54) n(%) | CBT-I (n = 56) n(%) | χ2(df) | p |
|---|---|---|---|---|---|
| Female | 107(97.3) | 53(98.1) | 54(96.4) | 0.31(1) | .580 |
| Recruited at Court Setting | 101(91.8) | 48(88.9) | 53(94.6) | 1.21(1) | .271 |
| Court Order of Protection | 97(88.2) | 47(87.0) | 50(89.3) | 0.13(1) | .714 |
| Antidepressant use | 30(27.3) | 17(31.5) | 13(23.2) | 0.95(1) | .330 |
| Income | |||||
| Under $20,000 | 72(65.5) | 34(63.0) | 38(67.9) | ||
| $20,001 – $40,000 | 17(15.5) | 8(14.8) | 9(16.1) | ||
| $40,001 – $60,000 | 12(10.9) | 7(13.0) | 5(8.9) | ||
| $60,001 – $80,000 | 6(5.5) | 3(5.6) | 3(5.4) | ||
| $80,001 or more | 3(2.7) | 2(3.7) | 1(1.8) | b (4) | .741a |
| Education | |||||
| Some high school | 15(13.6) | 6(11.1) | 9(16.1) | ||
| High school graduate/GED | 21(19.1) | 7(13.0) | 14(25.0) | ||
| Some college | 49(44.5) | 29(53.7) | 20(35.7) | ||
| Bachelor’s degree | 19(17.3) | 8(14.8) | 11(19.6) | ||
| Post-graduate degree | 6(5.5) | 4(7.4) | 2(3.6) | b (4) | .223 |
| Race/Ethnicity | |||||
| Amer Indian/Alaska Native | 2(1.8) | 1(1.9) | 1(1.8) | ||
| Asian | 1(0.9) | 1(1.9) | 0(0.0) | ||
| Black/African American | 43(39.1) | 20(37.0) | 23(41.1) | ||
| White | 54(49.1) | 26(48.1) | 28(50.0) | ||
| More than One Race | 7(6.4) | 5(9.3) | 2(3.6) | ||
| Other | 3(2.7) | 1(1.9) | 2(3.6) | b (5) | .803 |
| Marital Status | |||||
| Single | 53(48.2) | 22(40.7) | 31(55.4) | ||
| Married/Cohabitating | 11(10.0) | 5(9.3) | 6(10.7) | ||
| Separated/divorced | 42(38.2) | 25(46.3) | 17(30.4) | ||
| Widowed | 4(3.6) | 2(3.7) | 2(3.6) | b (3) | .366 |
| Employment Status | |||||
| Not employed | 50(45.5) | 25(46.3) | 25(44.6) | ||
| Employed, part-time | 27(24.5) | 12(22.2) | 15(26.8) | ||
| Employed, full-time | 33(30.0) | 17(31.5) | 16(28.6) | 0.32(2) | .849 |
| Met Criteria for Depressiona | 104(94.6) | 50(92.6) | 54(96.4) | 0.78(1) | .376 |
| Met Criteria for PTSDa | 108(98.2) | 53(98.1)a | 55(98.2) | 0.01(1) | .979 |
Abbreviations: MDD = Major Depressive Disorder; PTSD = Post-Traumatic Stress Disorder.
Two participants met inclusion criteria for subthreshold, but not full diagnostic criteria, for PTSD and six participants met inclusion criteria for moderate, but did not full diagnostic criteria for MDD.
Freeman-Halton exact test.
Table 2.
Continuous Baseline Measures by Experimental Condition.
| CBTi + CPT (n = 56) | Control + CPT (n = 54) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p | ES | |
| Age | 34.21 | 10.81 | 36.80 | 10.92 | −1.25 | .215 | −.24 |
| ISI | 20.91 | 3.80 | 20.70 | 4.45 | .26 | .793 | .05 |
| HAM-D | 25.95 | 4.82 | 23.65 | 5.26 | 2.43 | .017 | .45 |
| HAM-Da (minus sleep items) | 20.59 | 4.39 | 18.42 | 4.96 | 2.39 | .019 | .46 |
| CAPS | 75.50 | 15.40 | 68.22 | 14.35 | 2.56 | .012 | .48 |
| CAPSa (minus sleep items) | 67.91 | 15.22 | 60.76 | 14.07 | 2.56 | .012 | .48 |
| WHOQOL Physical | 46.16 | 14.88 | 47.39 | 14.87 | −.43 | .666 | −.08 |
| WHOQOL Psychological | 38.25 | 16.64 | 40.17 | 17.95 | −.58 | .563 | −.09 |
| WHOQOL Social | 35.59 | 23.36 | 35.02 | 19.63 | .14 | .890 | .03 |
| WHOQOL Environmental | 45.29 | 17.03 | 52.89 | 17.03 | −2.34 | .021 | −.44 |
Abbreviations: CAPS = Clinician Administered PTSD Scale; CBTi = Cognitive Behavioral Therapy for Insomnia; CI = Confidence Interval; CPT = Cognitive processing Therapy ES = Effect Size; HAM-D = Hamilton Depression Rating Scale; ISI = Insomnia Severity Index; WHOQOL = World Health Organization Quality of Life.
total score with sleep items subtracted.
At follow-up assessments, a total of 83 participants (75%) completed the Time 2 assessment and 61 participants (55%) completed the Time 3 assessment, although we included all randomized participants in the analyses following imputation as described above. There was higher attrition in the CBTi condition between Time 1 and Time 2 compared to attention control; attrition between Time 2 and Time 3 was similar between conditions (shown in Fig. 1). There were no baseline differences between attriters and non-attriters at Time 2 or Time 3.
At Time 2, following the first intervention, participants in the CBTi condition had achieved significantly greater reductions in insomnia, depression, and PTSD symptom severity than those in attention control (shown in Table 3). Effect sizes for insomnia and depression outcomes were consistent with a large effect; the effect size for PTSD was consistent with a moderate effect. At Time 3, after which participants in each condition had received the CPT intervention, mean differences persisted. That is, participants in the CBTi+CPT condition continued to have greater improvement in insomnia, depression, and PTSD severity compared to Control+CPT, although effect sizes were generally smaller than at Time 2.
Table 3.
Primary Outcome Measure Following the Insomnia Intervention at Time 2 and Following the Trauma Intervention at Time 3
| CBTi + CPT (n = 56) | Control + CPT (n = 54) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Marginal Mean | SD | Mean | Marginal Mean | SD | t | pb | ESc | |
| ISI | |||||||||
| Time 2 | 12.44 | 12.04 | 6.09 | 17.99 | 18.41 | 5.34 | −6.56 | < .001 | −1.09 |
| Time 3 | 10.22 | 9.96 | 8.11 | 14.14 | 14.41 | 6.66 | −3.28 | .001 | −.59 |
| HAM-D | |||||||||
| Time 2 | 16.32 | 15.11 | 6.44 | 19.92 | 21.17 | 5.95 | −6.27 | < .001 | −.96 |
| Time 3 | 9.75 | 9.25 | 6.70 | 15.32 | 15.83 | 7.88 | −4.58 | < .001 | −.89 |
| HAM-Da (minus sleep items) | |||||||||
| Time 2 | 13.00 | 11.93 | 5.29 | 15.18 | 16.30 | 5.56 | −5.02 | < .001 | −.79 |
| Time 3 | 6.69 | 6.19 | 5.87 | 11.46 | 11.97 | 6.87 | −4.47 | < .001 | −.89 |
| CAPS | |||||||||
| Time 2 | 57.23 | 53.15 | 20.23 | 59.03 | 63.26 | 18.95 | −3.61 | < .001 | −.51 |
| Time 3 | 39.03 | 36.82 | 21.07 | 47.17 | 47.41 | 21.64 | −2.67 | .008 | −.49 |
| CAPSa (minus sleep items) | |||||||||
| Time 2 | 51.10 | 47.12 | 19.32 | 52.25 | 56.38 | 18.56 | −3.43 | .001 | −.48 |
| Time 3 | 34.27 | 31.99 | 19.88 | 39.14 | 41.50 | 20.51 | −2.57 | .010 | −.46 |
Abbreviations: CAPS = Clinician Administered PTSD Scale; CBTi = Cognitive Behavioral Therapy for Insomnia; CI = Confidence Interval; CPT = Cognitive processing Therapy ES = effect size; HAM-D = Hamilton Depression Rating Scale; ISI = Insomnia Severity Index
All analyses are intent-to-treat ANCOVAs with missing values imputed and controlling for gender, age, education, minority status, recruitment site, antidepressant medication use, baseline lifetime traumatic events, baseline PTSD severity, baseline depression, and the dependent variable’s baseline value.
Total scale scores with insomnia items removed are the planned primary outcomes.
The p-values displayed have not been corrected for multiplicity. Significance is therefore set at p < 0.017.
Hedge’s g.
Results of the tipping point analysis support the primary findings. As shown in the Supplemental Table, to overturn Time 2 findings CBTi participants with missing Time 2 data would have needed an ISI score of 11.0, a full HAM-D score of 10.6, and full CAPS score of 12.4 higher than imputed values resulting in respective Time 2 marginal mean scores of 23.0, 26.7, and 65.5, each of which is implausible. As also shown in the Supplemental Table, CBTi+CPT participants with missing Time 3 data would have needed an ISI score of 3.2, a HAM-D score of 6.8, and a CAPS score of 5.2 points higher than the imputed values to overturn Time 3 findings (resulting total scores of 13.1, 16.1, and 42.0). Such ISI and HAM-D scores are implausible, whereas such a CAPS score is plausible but unlikely (as discussed further below).
For the secondary outcomes related to diagnostic status, treatment response, and remission at Time 3 (shown in Table 4), receiving CBTi prior to CPT was associated with greater odds for not meeting CAPS PTSD diagnostic criteria, but was not associated with PTSD remission. The 14% difference in PTSD response rates between conditions was marginally significant (OR = 3.31; 95% CI: .89–12.26, p = .073). In addition, the combination of CBTi+CPT was associated with greater odds of both insomnia and depression remission, insomnia and depression treatment response, and not meeting ISI threshold for insomnia or MINI criteria for MDD diagnosis. Finally, we found little evidence for deterioration at Time 3 and no differences between conditions. Within the raw data, no participants at Time 3 met either the PTSD or depression criterion for deterioration (with the imputed dataset, a few participants would have met deterioration criteria across the 200 imputed datasets, but the lack of variation in most of the datasets prevented statistical analysis). For insomnia, one participant in each condition met deterioration criteria.
Table 4.
Absence of Diagnosis, Remission Rates, and Response Rates for Depression and for PTSD at Time 3.
| Outcome | CBTi + CPT (n = 56)a | Control + CPT (n = 54)a | p | Imputation Data | |
|---|---|---|---|---|---|
| OR | (95% CI) | ||||
| No PTSD Diagnosis | 79.1% | 50.0% | 0.007 | 4.55 | (1.51, 13.75) |
| PTSD Response | 88.0% | 73.9% | 0.073 | 3.31 | (0.89, 12.26) |
| PTSD Remission | 20.0% | 14.7% | 0.267 | 2.18 | (0.55, 8.62) |
| No Depression Diagnosis | 85.4% | 61.1% | 0.003 | 6.52 | (1.91, 22.26) |
| Depression Response | 68.9% | 32.2% | 0.003 | 5.26 | (1.79, 15.44) |
| Depression Remission | 45.8% | 20.2% | 0.009 | 5.01 | (1.50, 16.71) |
| No Insomnia Diagnosisb | 52.2% | 30.1% | 0.033 | 3.47 | (1.10, 10.88) |
| Insomnia Response | 62.7% | 39.7% | 0.031 | 3.36 | (1.12, 10.07) |
| Insomnia Remission | 38.1% | 17.7% | 0.020 | 4.55 | (1.27, 16.31) |
Abbreviations: Cognitive Behavioral Therapy for Insomnia; CI = Confidence Interval; OR = Odds Ratio: CPT = Cognitive processing Therapy.
All analyses are intent-to-treat penalized logistic regressions with missing values imputed and controlling for gender, age, education, minority status, recruitment site, antidepressant medication use, baseline lifetime traumatic events, baseline PTSD severity, and baseline depression.
Original sample at time 3 (CBTi + CPT n = 25; Control + CPT n = 36).
No insomnia diagnosis was based on an Insomnia Severity Index Score of < 8, which is consistent with no insomnia, as unlike for PTSD and depression, no insomnia diagnostic interviews were undertaken in the protocol.
Discussion
In this longitudinal RCT among a socio-economically challenged community sample of individuals exposed to IPV, we observed benefits of sequential CBTi and CPT, compared to receiving CPT only. The large effect of CBTi compared to attention control on insomnia severity prior to CPT expands the generalizability of positive findings on insomnia treatment in the absence of any treatment for the co-occurring condition(s) to the IPV population. As hypothesized, there were also statistically and clinically significant reductions in PTSD and depression severity, supporting CBTi as a beneficial first step in treatingPTSD-related insomnia. Effect sizes at Time 3 were smaller than those observed prior to the initiation of CPT. Overall attrition was large and unbalanced.
To put our Time 2 PTSD severity effects in context, our CBTi pre-post effect size of 0.51 is comparable to the meta-analytic effects of 0.42 for pharmacotherapy treatments calculated by Watts, et al.[67], but considerably smaller than their 1.14 effect size for psychotherapy treatments (and similar findings in another meta-analysis of psychological PTSD treatments [68]). It is not possible to directly compare our Time 3 effect sizes to meta-analytic norms, given that both intervention arms received CPT.
The overall mean change in the full CAPS scores can also be contrasted to findings from RCTs of CPT. In three such trials, the pre-post reduction in CAPS scores in the CPT study arms ranged from 25 to 36 points across studies [69–71]. In the current study, total CAPS severity scores in the Control+CPT condition decreased by ~23 points from baseline to Time 3, compared to a mean reduction of ~36 points in the CBTi+CPT condition, both representing clinically significant changes. The changes were statistically different between groups from baseline to Time 2, as well as from baseline to Time 3. The pattern of changes suggest that CBTi results in relatively quick statistically and clinically meaningful reductions in PTSD symptoms from baseline to Time 2 with an additional clinically meaningful reduction from Time 2 to Time 3. In this sample at risk for poor social determinants of health, personal and community violence, and increased morbidity, the fact that both arms improved in clinically significant ways may be quite meaningful. Whether the study safety strategies contributed to outcomes in some way or whether improved mental health can help individuals better navigate safety remain unanswered questions worthy of further examination.
Our results suggest that the tested sequence leads to enhanced effects. It is certainly possible that providing CPT followed by CBTi could be even more clinically impactful, which should be tested. Similarly, it would also be interesting to assess a combined (as opposed to sequenced) CBTi and CPT intervention. These are empirical questions for which comparative effectiveness study designs are well-suited to address.
It is interesting that the between-group effects on depression severity, which favored the CBTi+CPT condition, were larger than those observed for PTSD severity, but followed a similar pattern from Time 2 to Time 3. This may in part be owing to the initial effect of CBTi on depression being more robust than it is for PTSD. In addition, we used the CAPS instrument for DSM-IV PTSD criteria as the DSM-5 version was not available at the time study enrollment began, That is, since the DSM-5 now includes a Criterion D for negative alterations in mood [72], it is also possible that the DSM-5 version of the CAPS would have captured some reductions in depressive symptoms observed in the study and resulted in a stronger effect on PTSD severity [73]. Well-resourced studies that can engage the population more effectively for a longer follow-up period are also likely needed to discern possible longitudinal effects. This may be particularly important when examining the role of insomnia treatment in PTSD and depression symptom trajectories, as CBTi effects for insomnia symptoms are not only durable but continue to modestly improve following the end of treatment [74]. The utility of “booster” sessions for mental health symptom management might also be explored.
Finally, the three secondary outcomes related to clinical response (no longer meeting diagnosis, treatment response and remission) bear some discussion. Here again, findings were stronger with respect to depression, than to PTSD. Approximately 46% of CBTi+CPT participants achieved depression remission (the most stringent of the three clinical response criteria) compared to 20% in the control condition. In contrast, 20% and 15%, respectively, of participants achieved PTSD remission. The CBTi+CPT condition also had statistically higher odds of meeting the “no diagnosis” and the “treatment response” criteria than the Control+CPT condition. For PTSD response, however, only the least stringent criteria (not meeting PTSD diagnostic criteria) occurred at a higher rate in the CBTi+CPT condition (79% vs. 50%; p = 0.007). In a large trial (n=284) of prolonged exposure for women with PTSD using the same definitions as the current study, 39% of participants in the intervention arm no longer met PTSD diagnostic criteria, 70% had a treatment response and 17% achieved remission at post-treatment [45]. In general, a prior review has found that nonresponse rates across all PTSD treatments are relatively high with low rates of remission [1].
Study limitations require acknowledgement. First, the sample was predominantly women. The CBTi+CPT group had higher PTSD and depression severity scores relative to the Control+CPT group, despite randomized assignment to groups. It is unclear to what extent this baseline difference contributed to observed outcomes; however, treatment effects were adjusted for baseline severity, allowing for greater precision in estimates when baseline differences do exist. Given that the CBTi+CPT group showed on average larger declines and lower scores at Time 2 and Time 3 relative to Control+CPT group, a floor effect in the population or among high severe cases is unlikely. Finally, although statistically significant, depression and PTSD severity differences at baseline were not highly clinically meaningful. A minor limitation is that although participants were required to be stable on medications and antidepressant use was a strata in randomization as recommended [50], we did not assess prior psychotherapy treatment. Neither was there follow-up beyond the Time 3 assessment, which occurred soon after completion of CPT. Importantly, although it is consistent with other trials testing behavioral PTSD interventions, the study attrition rate, was 45% (and higher in the CBTi arm). If effect size assumptions guiding the a priori power analysis were accurate, the limited resources that precluded replacement of ten participants lost to follow-up (to achieve a sample size of 120 participants) may have rendered the study underpowered to detect statistically significant group differences for some outcomes. The pattern of attrition poses a challenge to the interpretation of the findings from the main analyses.
The tipping point sensitivity analyses revealed that CBTi+CPT participants with missing data would have needed a marginal mean ISI score of 13.1 and a HAM-D score 16.1 to overturn their respective Time 3 findings. In both cases, this would have meant Time 3 scores were higher than Time 2 scores, something that did not occur even in the control condition and thus, implausible. For the Time 3 PTSD outcome, the tipping point was at a marginal mean CAPS score of 42.0, which is plausible given that it is an 11.0 point reduction from the Time 2 CAPS score. While certainly debatable, we find it unlikely given that the CTRL+CPT condition (including its 18 missing participants) achieved a mean reduction in CAPS of almost 16 points over the same time period. Nonetheless, the sensitivity analysis underscores that the more robust primary findings are for insomnia and depression.
With respect to the comparability of attrition rates to similar study samples, the RCT of sleep hypnosis plus CPT also had a 45% attrition rate among its ITT sample of 92 [21]. In the smaller trial of prolonged exposure followed by CBTi (n = 41), 61% of participants discontinued [19]. A CPT dismantling study that enrolled female victims of violence with PTSD had an overall attrition rate of 36% [70] and a more pronounced 56% attrition rate among those with past year intimate partner violence, the majority of whom did not initiate treatment at all [75]. The discrepancy in attrition between the CBTi and control conditions during the first treatment phase has few explanations. CBTi condition participants had higher PTSD severity, which may have contributed or, contrary to our rationale, engaging in CBTi prior to completing PTSD treatment may be more difficult than we anticipated.
Despite these limitations, this is an important study for IPV survivors. Many studies use violence involvement as an exclusion criterion. Yet, this difficult to recruit and retain population is perhaps among the most vulnerable individuals susceptible to comorbid PTSD, depression and violence comorbidity. IPV survivors require unique risk protections beyond those specified by research regulatory boards (e.g., a study certificate of confidentiality, alternative means of ongoing contact, ongoing safety assessments). Despite these extensive research protections and resources to support trial participation of IPV survivors, the study results likely generalize to other trauma-exposed populations with similar comorbidities (e.g., [76]). It is also important that future studies explore delivering the interventions using telehealth, flexible scheduling (evenings and weekends), and provide additional resources to accommodate the overnight experiences in the sleep lab.
Overall, the study findings support that CBTi, delivered to IPV survivors, has an immediate and positive effect on sleep, mood, and PTSD symptoms. Compared to delivering the trauma treatment without addressing insomnia, the use of CBTi as an adjuvant or precursor to CPT improves insomnia, depression and PTSD severity compared to CPT alone. The findings expand support for the sequential model of treating co-morbid conditions [10] to a unique population and treatment approach. It would still be useful to test our sequenced treatment approach in other populations with PTSD and in well-powered comparative effectiveness trials to ascertain optimal treatment sequences or combinations for co-occurring insomnia, PTSD and depression.
Supplementary Material
Acknowledgement
The authors wish to thank non-author study team members Ashley Bui, Caitlyn Casey, Matthew Cribbet, Katlyn Evans, Colin Gorman, Beth Ho, LeAnn Nelson, Courteney Olenyk, Martin Seehuus, and Patrick Walsh for collection and preparation of the data presented herein.
Funding Sources
The work presented here was funded by the National Institute of Nursing Research at the National Institutes of Health under award number R01NR013909 (W. Pigeon & K. Heffner, Principal Investigators). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Statement of Ethics
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Study approval statement: This study protocol was reviewed and approved by the University of Rochester Research Subjects Review Board [#00044033].
Consent to participate statement: Written informed consent was obtained from participants to participate in the study.
Conflict of Interest Statement
The authors have no conflicts of interest to declare. Authors W. Pigeon, H. Crean and T. Bishop are employees of the United States (U.S.) Department of Veterans Affairs (VA); the views or opinions expressed herein do not represent those of the VA or the U.S. Government.
Data Availability Statement
The data that support the findings of this study are not publicly available to protect participants who may be at risk for repeated exposure to interpersonal violence, but a limited dataset that excludes some dates of contact and demographic information can be made available by contacting the corresponding author [WP].
References
- 1.Shalev A, Liberzon I, Marmar C. Post-traumatic stress disorder. N Engl J Med. 2017. Jun;376(25):2459–69. [DOI] [PubMed] [Google Scholar]
- 2.Koenen K, Ratanatharathorn A, Ng L, McLaughlin K, Bromet E, Stein D, et al. Posttraumatic stress disorder in the world mental health surveys. Psychol Med. 2017. Oct;47(13):2260–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein RB, Smith SM, Chou SP, Saha TD, Jung J, Zhang H, et al. The epidemiology of dsm-5 posttraumatic stress disorder in the united states: Results from the national epidemiologic survey on alcohol and related conditions-iii. Soc Psychiatry Psychiatr Epidemiol. 2016. Aug;51(8):1137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shalev AY, Gevonden M, Ratanatharathorn A, Laska E, van der Mei WF, Qi W, et al. Estimating the risk of ptsd in recent trauma survivors: Results of the international consortium to predict ptsd (icpp). World Psychiatry. 2019. Feb;18(1):77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rytwinski NK, Scur MD, Feeny NC, Youngstrom EA. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: A meta-analysis. J Trauma Stress. 2013. Jun;26(3):299–309. [DOI] [PubMed] [Google Scholar]
- 6.Stein MB, Kennedy C. Major depressive and post-traumatic stress disorder comorbidity in female victims of intimate partner violence. J Affect Disord. 2001. Oct;66(2–3):133–8. [DOI] [PubMed] [Google Scholar]
- 7.Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989. Jun;146(6):697–707. [DOI] [PubMed] [Google Scholar]
- 8.Gutner CA, Casement MD, Stavitsky Gilbert K, Resick PA. Change in sleep symptoms across cognitive processing therapy and prolonged exposure: A longitudinal perspective. Behav Res Ther. 2013. Dec;51(12):817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pigeon WR, Gallegos AM. Posttraumatic stress disorder and sleep. Sleep Med Clin. 2015. Mar;10(1):41–8. [DOI] [PubMed] [Google Scholar]
- 10.Fava GA. Sequential treatment: A new way of integrating pharmacotherapy and psychotherapy. Psychother Psychosom. 1999. Sep-Oct;68(5):227–9. [DOI] [PubMed] [Google Scholar]
- 11.Guidi J, Fava GA. Sequential combination of pharmacotherapy and psychotherapy in major depressive disorder: A systematic review and meta-analysis. JAMA Psychiatry. 2021. Mar 1;78(3):261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidi J, Tomba E, Fava GA. The sequential integration of pharmacotherapy and psychotherapy in the treatment of major depressive disorder: A meta-analysis of the sequential model and a critical review of the literature. Am J Psychiatry. 2016. Feb;173(2):128–37. [DOI] [PubMed] [Google Scholar]
- 13.Fava M, McCall WV, Krystal A, Wessel T, Rubens R, Caron J, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006. Nov;59(11):1052–60. [DOI] [PubMed] [Google Scholar]
- 14.Fava M, Asnis GM, Shrivastava RK, Lydiard B, Bastani B, Sheehan DV, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: A randomized controlled trial. J Clin Psychiatry. 2011. Jul;72(7):914–28. [DOI] [PubMed] [Google Scholar]
- 15.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008. Apr;31(4):489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carney CE, Edinger JD, Kuchibhatla M, Lachowski AM, Bogouslavsky O, Krystal AD, et al. Cognitive behavioral insomnia therapy for those with insomnia and depression: A randomized controlled clinical trial. Sleep. 2017. Apr;40(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manber R, Buysse DJ, Edinger J, Krystal A, Luther JF, Wisniewski SR, et al. Efficacy of cognitive-behavioral therapy for insomnia combined with antidepressant pharmacotherapy in patients with comorbid depression and insomnia: A randomized controlled trial. J Clin Psychiatry. 2016. Oct;77(10):e1316–e23. [DOI] [PubMed] [Google Scholar]
- 18.Baddeley JL, Gros DF. Cognitive behavioral therapy for insomnia as a preparatory treatment for exposure therapy for posttraumatic stress disorder. Am J Psychother. 2013. Apr;67(2):203–14. [DOI] [PubMed] [Google Scholar]
- 19.Walters EM, Jenkins MM, Nappi CM, Clark J, Lies J, Norman SB, et al. The impact of prolonged exposure on sleep and enhancing treatment outcomes with evidence-based sleep interventions: A pilot study. Psychol Trauma. 2020. Feb;12(2):175–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belleville G, Dube-Frenette M, Rousseau A. Efficacy of imagery rehearsal therapy and cognitive behavioral therapy in sexual assault victims with posttraumatic stress disorder: A randomized controlled trial. J Trauma Stress. 2018. Aug;31(4):591–601. [DOI] [PubMed] [Google Scholar]
- 21.Galovski TE, Harik JM, Blain LM, Elwood L, Gloth C, Fletcher TD. Augmenting cognitive processing therapy to improve sleep impairment in PTSD: A randomized controlled trial. J Consult Clin Psychol. 2016. Feb;84(2):167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Resick PA, Schnicke MP. Cognitive processing therapy for sexual assault victims: A treatment manual. Newbury Park, CA: Sage Publications; 1993. [DOI] [PubMed] [Google Scholar]
- 23.Pigeon WR, Heffner KL, Crean H, Gallegos AM, Walsh P, Seehuus M, et al. Responding to the need for sleep among survivors of interpersonal violence: A randomized controlled trial of a cognitive-behavioral insomnia intervention followed by ptsd treatment. Contemp Clin Trials. 2015. Nov;45(Pt B):252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pigeon WR, Cerulli C, Richards H, He H, Perlis M, Caine E. Sleep disturbances and their association with mental health among women exposed to intimate partner violence. J Womens Health. 2011. Dec;20(12):1923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazzotta CM, Crean HF, Pigeon WR, Cerulli C. Insomnia, posttraumatic stress disorder symptoms, and danger: Their impact on victims’ return to court for orders of protection. J Interpers Violence. 2021. Mar;36(5–6):NP2443–NP63. [DOI] [PubMed] [Google Scholar]
- 26.Winkelman JW. Clinical practice. Insomnia disorder. N Engl J Med. 2015. Oct;373(15):1437–44. [DOI] [PubMed] [Google Scholar]
- 27.Schnurr PP, Lunney CA. Residual symptoms following prolonged exposure and present-centered therapy for ptsd in female veterans and soldiers. Depress Anxiety. 2019. Feb;36(2):162–9. [DOI] [PubMed] [Google Scholar]
- 28.Weathers FW, Ford J. Psychometric review of ptsd checklist (pcl-c, pcl-s, pcl-m, pcl-pr). In: Stamm BH, editor. Measurement of stress, trauma, and adaptation. Lutherville, MD: Sidram; 1996. p. 250–2. [Google Scholar]
- 29.Grubaugh AL, Magruder KM, Waldrop AE, Elhai JD, Knapp RG, Frueh BC. Subthreshold ptsd in primary care: Prevalence, psychiatric disorders, healthcare use, and functional status. J Nerv Ment Dis. 2005. Oct;193(10):658–64. [DOI] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL, Williams JB. The phq-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001. Sep;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morin CM, Belleville G, Belanger L, Ivers H. The insomnia severity index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011. May;34(5):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, et al. Derivation of research diagnostic criteria for insomnia: Report of an American academy of sleep medicine work group. Sleep. 2004. Dec;27(8):1567–96. [DOI] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975. Nov;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 34.Campbell JC, Webster DW, Glass N. The danger assessment: Validation of a lethality risk assessment instrument for intimate partner femicide. J Interpers Violence. 2009. Apr;24(4):653–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Control Clin Trials. 1988. Dec;9(4):345–64. [DOI] [PubMed] [Google Scholar]
- 36.Pigeon WR, Funderburk JS, Cross W, Bishop TM, Crean HF. Brief CBT for insomnia delivered in primary care to patients endorsing suicidal ideation: A proof-of-concept randomized clinical trial. Transl Behav Med. 2019. Jul 4;9(6):9. [DOI] [PubMed] [Google Scholar]
- 37.Edinger JD, Wohlgemuth WK, Radtke RA, Coffman CJ, Carney CE. Dose-response effects of cognitive-behavioral insomnia therapy: A randomized clinical trial. Sleep. 2007. Feb;30(2):203–12. [DOI] [PubMed] [Google Scholar]
- 38.Zayfert C, DeViva J, Pigeon W, Goodson J. Fear of sleep and nighttime vigilance in trauma-related insomnia: A preliminary report on the fear of sleep inventory. In: Zayfert C, editor. The role of fear of sleep in trauma-related insomnia: Biological and psychological perspectives. Symposium conducted at: Annual meeting of the International Society of Traumatic Stress Studies (ISTSS); 2006, November 4-7; Hollywood, CA. [Google Scholar]
- 39.Waltz J, Addis ME, Koerner K, Jacobson NS. Testing the integrity of a psychotherapy protocol: Assessment of adherence and competence. J Consult Clin Psychol. 1993. Aug;61(4):620–30. [DOI] [PubMed] [Google Scholar]
- 40.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The aasm manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Westchester, IL; 2007. [Google Scholar]
- 41.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician-administered ptsd scale. J Trauma Stress. 1995. Jan;8(1):75–90. [DOI] [PubMed] [Google Scholar]
- 42.Hamilton M A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960. Feb;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998. Jan;59(20):22–33. [PubMed] [Google Scholar]
- 44.Monson CM, Fredman SJ, Macdonald A, Pukay-Martin ND, Resick PA, Schnurr PP. Effect of cognitive-behavioral couple therapy for ptsd: A randomized controlled trial. JAMA. 2012. Aug;308(7):700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnurr PP, Friedman MJ, Engel CC, Foa EB, Shea MT, Chow BK, et al. Cognitive behavioral therapy for posttraumatic stress disorder in women: A randomized controlled trial. JAMA. 2007. Feb;297(8):820–30. [DOI] [PubMed] [Google Scholar]
- 46.Schnurr PP, Friedman MJ, Foy DW, Shea MT, Hsieh FY, Lavori PW, et al. Randomized trial of trauma-focused group therapy for posttraumatic stress disorder: Results from a department of veterans affairs cooperative study. Arch Gen Psychiatry. 2003. May;60(5):481–9. [DOI] [PubMed] [Google Scholar]
- 47.Bobo WV, Anglero GC, Jenkins G, Hall-Flavin DK, Weinshilboum R, Biernacka JM. Validation of the 17-item hamilton depression rating scale definition of response for adults with major depressive disorder using equipercentile linking to clinical global impression scale ratings: Analysis of pharmacogenomic research network antidepressant medication pharmacogenomic study data. Hum Psychopharmacol. 2016. May;31(3):185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: A review of the first ten years of research. Depress Anxiety. 2001. May;13(3):132–56. [DOI] [PubMed] [Google Scholar]
- 49.Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the hamilton depression rating scale. J Affect Disord. 2013. Sep;150(2):384–8. [DOI] [PubMed] [Google Scholar]
- 50.Guidi J, Brakemeier EL, Bockting CLH, Cosci F, Cuijpers P, Jarrett RB, et al. Methodological recommendations for trials of psychological interventions. Psychother Psychosom. 2018. Sep;87(5):276–84. [DOI] [PubMed] [Google Scholar]
- 51.Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991. Feb;59(1):12–9. [DOI] [PubMed] [Google Scholar]
- 52.Lipsey MW, Hurley S. Design sensitivity: Statistical power for applied experimental research. In: Bickman L, Rog DJ, editors. The sage handbook of applied social research methods. 2nd ed. Thousand Oaks, California: SAGE Publications, Inc.; 2009. p. 44–77. [Google Scholar]
- 53.Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: Current practice and problems. Stat Med. 2002. Oct;21(19):2917–30. [DOI] [PubMed] [Google Scholar]
- 54.Kahan BC, Jairath V, Dore CJ, Morris TP. The risks and rewards of covariate adjustment in randomized trials: An assessment of 12 outcomes from 8 studies. Trials. 2014. Apr;15:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods. 2001. Dec;6(4):330–51. [PubMed] [Google Scholar]
- 56.Enders CK. Multiple imputation as a flexible tool for missing data handling in clinical research. Behav Res Ther. 2017. Nov;98:4–18. [DOI] [PubMed] [Google Scholar]
- 57.Skevington SM, Lotfy M, O’Connell KA. The world health organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res. 2004. Mar;13(2):299–310. [DOI] [PubMed] [Google Scholar]
- 58.Allison PD. Missing data. Thousand Oaks, California: Sage; 2002. [Google Scholar]
- 59.Sullivan TR, White IR, Salter AB, Ryan P, Lee KJ. Should multiple imputation be the method of choice for handling missing data in randomized trials? Stat Methods Med Res. 2018. Sep;27(9):2610–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bodner TE. What improves with increased missing data imputations? Structural Equation Modeling: A Multidisciplinary Journal. 2008. Oct;15:651–75. [Google Scholar]
- 61.Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007. Sep;8:206–13. [DOI] [PubMed] [Google Scholar]
- 62.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011. Feb 20;30(4):377–99. [DOI] [PubMed] [Google Scholar]
- 63.Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons; 1987. [Google Scholar]
- 64.Firth D Bias reduction of maximum-likelihood-estimates. Biometrika. 1993. Mar;80(1):27–38. [Google Scholar]
- 65.Cro S, Morris TP, Kenward MG, Carpenter JR. Sensitivity analysis for clinical trials with missing continuous outcome data using controlled multiple imputation: A practical guide. Stat Med. 2020. Sep;39(21):2815–42. [DOI] [PubMed] [Google Scholar]
- 66.Yan X, Lee S, Li N. Missing data handling methods in medical device clinical trials. J Biopharm Stat. 2009. Nov;19(6):1085–98. [DOI] [PubMed] [Google Scholar]
- 67.Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 2013. Jun;74(6):e541–50. [DOI] [PubMed] [Google Scholar]
- 68.Cusack K, Jonas DE, Forneris CA, Wines C, Sonis J, Middleton JC, et al. Psychological treatments for adults with posttraumatic stress disorder: A systematic review and meta-analysis. Clin Psychol Rev. 2016. Feb;43:128–41. [DOI] [PubMed] [Google Scholar]
- 69.Monson CM, Schnurr PP, Resick PA, Friedman MJ, Young-Xu Y, Stevens SP. Cognitive processing therapy for veterans with military-related posttraumatic stress disorder. J Consult Clin Psychol. 2006. Oct;74(5):898–907. [DOI] [PubMed] [Google Scholar]
- 70.Resick PA, Galovski TE, Uhlmansiek MO, Scher CD, Clum GA, Young-Xu Y. A randomized clinical trial to dismantle components of cognitive processing therapy for posttraumatic stress disorder in female victims of interpersonal violence. J Consult Clin Psychol. 2008. Apr;76(2):243–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Resick PA, Nishith P, Weaver TL, Astin MC, Feuer CA. A comparison of cognitive-processing therapy with prolonged exposure and a waiting condition for the treatment of chronic posttraumatic stress disorder in female rape victims. J Consult Clin Psychol. 2002. Aug;70(4):867–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diagnostic and statistical manual of mental disorders: DSM 5. 5th ed. American Psychiatric Association, Washington, D.C., 2013. [Google Scholar]
- 73.Flory JD, Yehuda R. Comorbidity between post-traumatic stress disorder and major depressive disorder: Alternative explanations and treatment considerations. Dialogues Clin Neurosci. 2015. Jun;17(2):141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Geiger-Brown JM, Rogers VE, Liu W, Ludeman EM, Downton KD, Diaz-Abad M. Cognitive behavioral therapy in persons with comorbid insomnia: A meta-analysis. Sleep Med Rev. 2015. Oct;23:54–67. [DOI] [PubMed] [Google Scholar]
- 75.Iverson KM, Resick PA, Suvak MK, Walling S, Taft CT. Intimate partner violence exposure predicts ptsd treatment engagement and outcome in cognitive processing therapy. Behav Ther. 2011. Jun;42(2):236–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watkins LE, Sprang KR, Rothbaum BO. Treating ptsd: A review of evidence-based psychotherapy interventions. Front Behav Neurosci. 2018. Nov;12:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not publicly available to protect participants who may be at risk for repeated exposure to interpersonal violence, but a limited dataset that excludes some dates of contact and demographic information can be made available by contacting the corresponding author [WP].


