Abstract
Background
Withania somnifera (WS), also known as Ashwagandha, is commonly used in Ayurveda and other traditional medicine systems. WS has seen an increase in worldwide usage due to its reputation as an adaptogen. This popularity has elicited increased scientific study of its biological effects, including a potential application for neuropsychiatric and neurodegenerative disorders.
Objective
This review aims to provide a comprehensive summary of preclinical and clinical studies examining the neuropsychiatric effects of WS, specifically its application in stress, anxiety, depression, and insomnia.
Methods
Reports of human trials and animal studies of WS were collected primarily from the PubMed, Scopus, and Google Scholar databases.
Results
WS root and leaf extracts exhibited noteworthy anti-stress and anti-anxiety activity in animal and human studies. WS also improved symptoms of depression and insomnia, though fewer studies investigated these applications. WS may alleviate these conditions predominantly through modulation of the hypothalamic-pituitary-adrenal and sympathetic-adrenal medullary axes, as well as through GABAergic and serotonergic pathways. While some studies link specific withanolide components to its neuropsychiatric benefits, there is evidence for the presence of additional yet unidentified active compounds in WS.
Conclusion
While benefits were seen in the reviewed studies, significant variability in the WS extracts examined prevents a consensus on the optimum WS preparation or dosage for treating neuropsychiatric conditions. WS generally appears safe for human use; however, it will be important to investigate potential herb-drug interactions involving WS if used alongside pharmaceutical interventions. Further elucidation of active compounds of WS is also needed.
Keywords: Anxiety, depression, insomnia, stress, Ashwagandha, Withania somnifera
1. INTRODUCTION
The botanical Withania somnifera (L.) Dunal, family Solanaceae, is widely known as “Ashwagandha”, a Sanskrit name deriving from its use in traditional medicine in India. Withania somnifera (WS) enjoys a formidable reputation in Ayurvedic medicine as a Rasayana herb, i.e., one that can rejuvenate the body and promote the health of all the tissues [1]. WS is, therefore, also classified as an adaptogen: an agent that promotes homeostasis of the whole body not only by one specific pharmacological mechanism, but by eliciting complex responses as well [2]. The reputed properties of WS include the ability to improve concentration, memory, and mood, as well as providing resilience against pathogens and disease [3].
The WS plant is a 0.5-2m high woody shrub found in the drier parts of tropical and subtropical zones, including the Canary Islands, the Mediterranean, Africa, China, South Asian countries such as India and Sri Lanka, and the Middle East [4]. It is known by several names, including Ashwagandha (Sanskrit), Asgand (Urdu), and Indian Winter Cherry or Poison Gooseberry (English), as well as the term “Indian Ginseng” owing to its adaptogenic effects [4, 5]. In India, WS is extensively cultivated for medicinal purposes, being used in more than 100 formulations in Ayurvedic, Unani, and Siddha medicine [6, 7]. Freshly dried roots are the predominant medicinal component; however, the leaves, flowers, seeds, and fruits of the plant are also used for therapeutic purposes [6].
Traditionally, WS preparations are used for conditions such as arthritis, asthma, goiter, and ulcers, as well as anxiety, insomnia, and neurological disorders. These uses are linked to the botanical’s reputed adaptogenic, anti-stress, and anti-inflammatory properties [6, 7]. Numerous WS commercial products are now readily available to the public in India and other parts of the world. In the United States (US), WS products are classified as “botanical dietary supplements” by the Food and Drug Administration. According to the National Institutes of Health Office of Dietary Supplements, there are currently more than 1,300 products in the US market that contain WS, and its use is becoming increasingly popular [8]. WS was listed as the 6th top-selling herb in US in 2017 [9], rising from the 8th position in 2016 [10]. By 2019, WS was the 5th most popular dietary supplement, with sales exceeding $10 million through mainstream channels (e.g., grocery stores, drug stores) and more than $13 million in terms of natural channels (e.g., supplement and specialty retail outlets) [11].
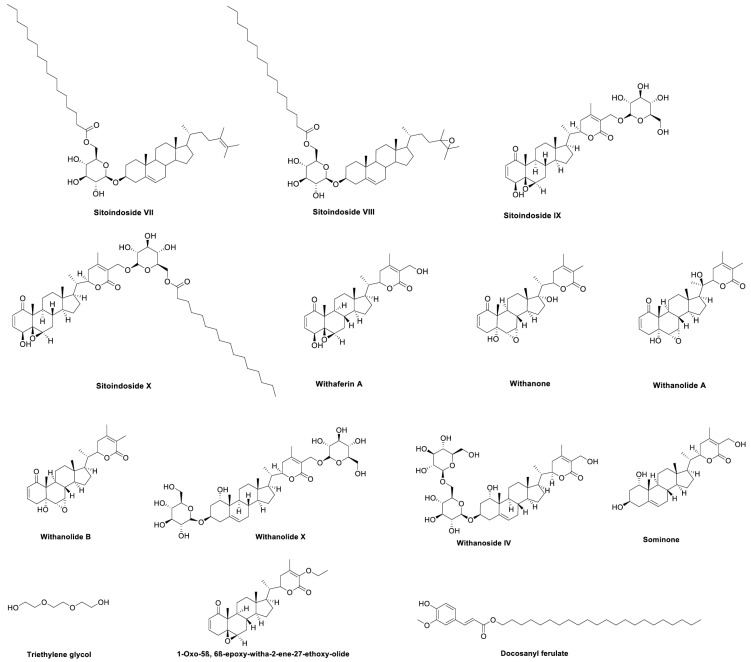
The widespread and growing use of WS by the public highlights the need for a greater understanding of this botanical's biological properties and active phytochemicals to validate and optimize its use. Fortunately, WS is one of the most widely studied medicinal herbs. A recent review by Tetali et al. (2021) summarizes about 140 specialized compounds reported in WS [12]. Among these, the best known are a complex group of steroidal lactones known as withanolides, which also occur as glycosides (withanosides) [12]. Over 70 individual withanolide derivatives have been reported in WS leaf and root [12-14], with higher levels in the leaves than in the roots [15]. WS also contains four sitoindosides, of which sitoindosides IX and X are glycosylated derivatives of the withanolide, withaferin A [16], while sitoindosides VII and VIII are long chain acyl steryl glucosides [17]. Distinct WS chemotypes are recognized based on the withanolide substitution patterns [18, 19]. Multiple alkaloids, phenolic compounds, and organic acids have also been reported in WS [12]. The withanolide derivatives are the most common group to have been examined individually for biological activities [12]. Indeed, some withanolides contain electrophilic sites conferring thiol reactivity, which may play important roles in mediating biological activity associated with antioxidant activity and/or targeting other electrophile sensors that modulate transcriptional or post-transcriptional responses [20]. The structures of specific WS compounds mentioned in this review are shown in Fig. 1.
Fig. (1).
Selected WS compounds tested, or found in WS extracts tested, for effects related to neuropsychiatric disorders.
The varied biological effects of WS have been widely studied and reviewed, including its potential application in brain disorders [21-23]. Neuropharmacological effects of WS root and WS leaf have been studied in preclinical and clinical models [24-26], and two recent reviews have summarized the evidence for the efficacy of WS in neurodegenerative disorders, including Alzheimer’s disease, Huntington’s disease, and Parkinson’s disease [4, 27]. However, one of the most common uses of Ashwagandha products is for stress relief. It is now well accepted that stress can cause functional and structural changes within the brain and has been implicated in the development of most neuropsychiatric disorders, including anxiety, depression, and insomnia [28-30]. The mechanisms by which stress contributes to these disorders include hyperactivity of the Hypothalamic-Pituitary-Adrenal (HPA) axis and dysregulation of the immune system [31, 32]. Given the well-established relationship between stress and neuropsychiatric disorders, it is likely that WS’s anti-stress activity plays a key role in its potential health benefits for depression, anxiety, and insomnia, and vice versa.
Numerous studies on WS have examined its effects on stress, mood, and insomnia in humans (Table 1) and animal models (Tables 2-5). The present article provides a detailed and comprehensive review focusing on these neuropsychiatric effects of WS. Specifically, it presents pre-clinical and clinical evidence of the effects of WS on stress and three of the most common stress-related neuropsychiatric disorders: anxiety, depression, and insomnia. The pathophysiology of these disorders will be discussed as they relate to the effects of WS; however, the reader is referred to previous reviews for more comprehensive discussions of these topics [32-35]. As stress is implicated in all three of the other common disorders, we first review the evidence for the anti-stress effect of WS, and then discuss the evidence for the use of WS in anxiety, depression, and insomnia.
Table 1.
Effects of Ashwagandha (Withania somnifera, WS) on stress, insomnia, anxiety, and depression in human trials.
| WS Material Given | Standardization/ Chemical Composition | Dosage | Control | Subjects | Behavioral Outcomes | Biological Outcomes | Authors’ Conclusions | Refs. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KSM-66; Batch#: KSM/19/S013; Ixoreal Biomed Inc) Capsules of aqueous root extract | >5% withanolide content | 300 mg twice daily with milk or water 8 weeks |
Starch | 80 adults (18-50 yrs) (two-armed trial: 40 healthy and 40 insomnia, male to female ratio NR) | ▼SOL, ▼WASO, ▲TST, ▲TIB, ▲SE, ▼PSQI, ▼HAM-A (insomnia only), ▼MARS rating, ▲sleep quality | Safety: physical exam and lab tests (blood pressure, body weight, routine blood test) | Improved sleep and anxiety | [63] | ||||
| KSM-66 Ashwagandha Root extract (Ixoreal Biomed Inc.) Capsules of aqueous root extract |
NR | 300 mg twice daily with water 12 weeks |
Starch | 50 healthy older adults (60-85 yrs, male to female ratio NR) | ▲WHOQOL-Bref score, ▼sleepiness scale score, ▼MARS rating, ▼sleep quality rating High PGAET rating, high PGATT rating |
Not assessed | ▲QoL, ▲sleep quality, ▲mental alertness | [64] | ||||
| KSM-66 Ashwagandha root extract, (Ixoreal Biomed Inc.) Capsules of aqueous root extract, Batch#: KSM/VG/18/1085 |
NR | 125 mg twice daily OR 300 mg twice daily 8 weeks |
125 mg starch | 60 adults (18-55 yrs, male to female ratio NR) with PSS score ≥20 and no other psychiatric conditions | ▼PSS score, ▼HAM-A score (600 mg dose only), ▼sleep quality rating | ▼serum cortisol | ▼stress, ▼anxiety | [60] | ||||
| KSM-66, (Ixoreal Biomed Inc.) Capsules of aqueous root extract, Batch#: KSM/14/270 |
>5% withanolides | 300 mg twice daily with milk or water 10 weeks |
Starch powder | 60 adults (18-60 yrs; 31:9 male to female for WS group, 16:4 for placebo group) with insomnia | ▼SOL, ▼WASO, ▲TST, ▲TIB, ▲SE, ▼MARS rating, ▲sleep quality rating, ▼PSQI score, ▼HAM-A score | Not assessed | ▼insomnia, ▼anxiety | [65] | ||||
| KSM-66 Ashwagandha; (Ixoreal Biomed) Capsules of root extract | 5% withanolides | 300 mg twice daily with water 8 weeks |
“Inert filler” | 52 overweight to obese adults (18-60 yrs; 38 males, 14 females) with chronic, routine work stress (PSS score ≥20, BMI 25 - 39.9 kg/m2) | ▼PSS score, ▲OHQ score, ▼TFEQ score ▼FCQ-T score |
▼serum cortisol, ▼body weight, ▼BMI | ▼stress, ▲well-being, ▼food cravings | [58] | ||||
| KSM-66 Ashwagandha extract (Ixoreal Biomed) Capsules of root extract prepared “without using alcohol or synthetic solvents” | ≥5% withanolide content | 300 mg twice daily after food with water 8.5 weeks (60 days) |
“Neutral substance” | 64 stressed adults (18-54 yrs; 41 males, 23 females; WHO-5 well-being score <5; PSS score of at least 14) | ▼PSS score, ▼GHQ score, ▼DASS score | ▼serum cortisol | ▼stress | [57] | ||||
| Sensoril®, (Natreon Inc.) Capsules of aqueous extract |
≥8% Withanolide glycosides (withanosides and sitoindosides), ≥32% carrier oligosaccharides, ≤2%Withaferin A | 250 mg twice daily for 1 week, then 500 mg twice daily for 11 weeks 12 weeks |
“Inactive ingredients” | 66 adults (18-75 yrs, 21:13 male to female for WS group, 14:20 for placebo group) with schizophrenia or schizoaffective disorder (MINI and PANSS score ≥60) and recent symptom exacerbation. On stable antipsychotic dose. | ▼PANSS score, ▼PSS score, ▼PANSS single item depression and anxious/depression scores |
▼hsCRP, S100B (nsd), no significant difference in serum IL-6 | ▼stress, depression, and anxiety symptoms in schizophrenia | [66] [59] |
||||
| WS Material Given | Standardization/ Chemical Composition | Dosage | Control | Subjects | Behavioral Outcomes | Biological Outcomes | Authors’ Conclusions | Refs. | ||||
| Sensoril®; (Natreon Inc., New Jersey) Capsules of aqueous extract |
“Minimum concentration of the critical bioactive withanolide glycosides and carrier oligosaccharides, but only traces of Withaferin A” | 250 mg twice daily for 1 week, then 500 mg twice daily for 11 weeks 12 weeks |
“Inactive ingredients” | 66 adults (18-75 yrs, 21:13 male to female for WS group, 14:20 for placebo group) with schizophrenia or schizoaffective disorder (MINI and PANSS score ≥60) | ▼PANSS score, ▼PSS score |
▼hsCRP, S100B (nsd), no significant difference in serum IL-6 | ▼stress in schizophrenia | [59] | ||||
| Sensoril® (Natreon Inc.) or Essentra®; (NutraGenesis) Capsules of aqueous root and leaf extract of a withaferin A and corresponding withanolide glycoside-predominant genetically uniform chemotype |
11.90% withanolide glycosides, 1.05% withaferin A, 40.25% oligosaccharides, 0.05% alkaloids, 3.44% polysaccharides | 125 mg daily OR 125 mg twice daily OR 250 mg twice daily taken before lunch and dinner 8.5 weeks (60 days) |
Excipient placebo | 130 adults (18-60 yrs; 95 males, 38 females) with moderate to severe anxiety (mHAM-A score of 24-42) | ▼mHAM-A score | ▼serum cortisol, ▼CRP, ▼FBG, ▼TC, ▼TG, ▼LDL-C, ▼VLDL-C, ▲HDL-C, ▲Hemoglobin, ▼pulse rate, ▼systolic and diastolic blood pressure | ▼stress, ▼anxiety | [62] | ||||
| Shoden; (Arjuna Natural private Ltd.) Capsules of leaf and root extract |
21 mg withanolide glycosides | 120 mg daily with water in the evening 2 hrs before meal 6 weeks |
Rice powder | 150 adults (18-65 yrs; 72 males, 78 females) with non-restorative sleep (RSQ-W score ≤50) | ▲RSQ-W total score, ▲WHOQOL-Bref score, ▼SOL, ▲SE, ▲TST, ▼WASO, ▼average awakening time | Hematologic safety markers (nsd) | improved sleep quality | [67] | ||||
| Shoden; (Arjuna Natural Ltd.) Capsules of ethanol:water (70:30) extract |
35% withanolide glycosides | 240 mg once daily after dinner with 250 mL of water 8.5 weeks (60 days) |
Roasted rice powder | 60 healthy adults (18-65 yrs; 37 males, 23 females) with HAM-A scores 6-17 | ▼HAM-A score, ▼DASS-21 (near significant) | ▼serum cortisol, ▼serum DHEA-S, ▲serum testosterone (males only) | ▼anxiety, ▼stress | [61] | ||||
| Tablets of ethanolic plant extract | NR / NR | 250 mg twice daily 6 weeks |
Placebo | 39 adults (41.3 + 13.8 yrs; 61.5% male) with GAD, mixed anxiety and depression, panic disorder and adjustment disorder with anxiety | Global Rating Scale score (nsd), ▼HAS score | Not assessed | “Anxiolytic potential”; Safe and well tolerated | [68] | ||||
| Granules of dried root | NR | 4 g thrice daily with milk 8.5 weeks (60 days) |
Granules of wheat flour | 86 participants (16-60 yrs, male to female ratio NR) with GAD | HARS score (▼ anxious mood; other anxiety scores nsd between groups) | Not assessed | ▼anxiety; potential placebo effect | [69] | ||||
| Iranian WS (70% ethanol) Capsules of ethanolic root extract with lactose as excipient |
Not assessed | 1 g daily 6 weeks |
Lactose | 40 adults (39.29 +10.10 yrs; 11:7 male:female for WS group; 40.50 + 7.76; 11:11 male:female for placebo group) with GAD on SSRIs | ▼HAM-A score | Not assessed | ▼anxiety in GAD in combination with SSRIs | [70] | ||||
▼= significant decrease compared to placebo; ▲= significant increase compared to placebo; BAI = Beck Anxiety Inventory; BDI = Beck Depression Inventory; BD-NOS = Bipolar disorder, not otherwise specified; BMI = Body Mass Index; CGI = Clinical Global Impressions scale; CRP = C-reactive protein; DASS = Depression Anxiety Stress Scale; DASS-21 = Depression Anxiety Stress Scale-21; DHEA-S = Dehydroepiandrosterone sulfate; FBG = Fasting Blood Glucose; FCQ-T = Food Cravings Questionnaire – Trait; FQ = Fatigue Questionnaire; GAD = General Anxiety Disorder; GHQ = General Health Questionnaire; HAM-A/HARS = Hamilton Anxiety Rating Scale; HDL-C = High density lipoprotein cholesterol; IL-6 = Interleukin 6; LDL-C = Low density lipoprotein cholesterol; MADRS = Montgomery-Asberg Depression Rating Scale; MARS = Mental Alertness on Rising; mHAM-A = modified Hamilton Anxiety Rating Scale; MINI = Mini-International Neuropsychiatric Interview; MYMOP = Measure Yourself Medical Outcomes Profile; NR = Not reported; NOS = Not otherwise specified; OHQ = Oxford Happiness Questionnaire; nsd = Non-significant decrease; PANSS = Positive and Negative Syndrome Scale; PGAET = Physician’s Global Assessment of Efficacy to Therapy; PGATT = Patient’s Global Assessment of Tolerability to Treatment; PSS = Perceived Stress Scale; PSQI = Pittsburgh Sleep Quality Index; RSQ-W = Restorative Sleep Questionnaire-Weekly; S100B = S100 Calcium-binding protein B; SE = Sleep efficiency; SF-36 = Short Form-36; SOL = Sleep Onset Latency; SSRI = Selective serotonin reuptake inhibitor; SST = Set Shifting Test; STDT = Strategic Target Detection Test; TBT = Total Bed Time; TC = Total Cholesterol; TG = Triglycerides; TIB = Total Time in Bed; TFEQ = Three-Factor Eating Questionnaire; TST = Total Sleep Time; VLDL-C = Very low density lipoprotein cholesterol; WASO = Wake After Sleep Onset; WHOQOL-Bref = WHO Quality of Life-BREF; YMRS = Young Mania Rating Scale; QoL = Quality of Life
1.1. Research Methods
Literature was gathered using the PubMed, Scopus, and Google Scholar databases, as well as from papers cited within the initial articles retrieved. Search terms included combinations of the following words: ashwagandha, ashwaganda, Withania somnifera, Indian ginseng, poison gooseberry, winter cherry, stress, adaptogen, adaptogenic, insomnia, sleep, anxiety, anti-anxiety, depression, anti-depressant, pharmacokinetics, safety, cytochrome P-450, interaction, animal, mouse, rat, rodent, preclinical, clinical, withaferin A, withanolide, cortisol, cytokines, inflammation, inflammatory, serotonin, oxidative stress, antioxidant, GABA, and immune. Only studies where WS and its derivatives were the sole agents being studied were included. Any studies that included WS as part of a multi-herb or multidomain intervention were excluded due to an inability to differentiate the effects of WS from other interventions used.
2. ANTI-STRESS EFFECTS
2.1. Animal Studies
As summarized in Table 2, WS has demonstrated anti-stress activity in several animal models of stress, the most common of which was the Forced Swim Test (FST) [17, 36-42]. The FST was originally developed as a model of depression-like behavior and involved placing a rodent in an inescapable tank filled with water [43]. When used as a model of stress, a drug’s ability to increase the time duration an animal spends actively swimming suggests the ability to cope with stress [44]. In the studies reviewed here, WS consistently increased swim duration in the FST [38-42] (Table 2). Other methods of inducing stress included sleep deprivation [45], exposing animals to cold [40], hypoxia [46], and/or prolonged restraint [17, 38, 40, 47, 48], either alone or in combination [41, 42, 49, 50], or applying electrical shocks to the feet of animals [51-54]. In these studies, WS attenuated a variety of stress-induced changes, including behavioral changes (e.g., memory impairment [45, 46, 52, 53, 55]), biochemical changes (e.g., increased glucocorticoids [38, 39, 41, 42, 46-48, 50, 54, 56]) and physical changes (e.g., gastric ulcers [17, 38, 40, 52]).
Table 2.
Anti-stress effects of Ashwagandha (Withania somnifera, WS) in animal studies.
| Nature of Extract, Standardization | Dosage | Model | Outcomes | Refs. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Root powder (KSM-66), standardized to ≥5% withanolides | 2.5, 5, or 10 g/day p.o. for 21 days | Horse (exercise-, separation-, and noise-induced stress) | ▲stress-induced decrease in peripheral total erythrocyte count, total leukocyte count, hemoglobin, lymphocyte percentage, serotonin, GSH and SOD activity, HDL and LDL cholesterol ▼stress-induced increase in peripheral cortisol, epinephrine, TBARs, blood glucose, ALT, AST, creatinine, triglycerides, IL-6 |
[56] | |||||
| Water-ethanol (7:3) root extract, containing 0.23% (w/w) isowithanone | 500 mg/kg p.o., 6 days per week for 6 weeks | Rat (SPS) | ▲short- and long-term memory (RAWM) ▲hippocampal oxidative stress biomarkers (▼GSSG, ▲GSH/GSSG, ▲GPx activity, catalase, and SOD) |
[55] | |||||
| Withanolide-free root extract | 3.3, 10, 33.3, and 100 mg/kg p.o. for 12 days | Rat (FSS) | ▼stress-induced weight loss ▼stress-induced increase in rectal temperature ▼transient hyperthermic response (FSS) ▼anxiety-like or depression-like behavior (MBT) ▼stress-induced increase in adrenal weight ▼stress-induced increase in plasma cortisol and blood glucose |
[54] | |||||
| Methanolic root extract, standardized to 2.7% (w/w) total withanolides | 10, 20, and 40 mg/kg/day p.o. for 11 days, or as a single dose | Rat (FSS, HPT, TST) | ▼stress-induced weight loss ▼transient hyperthermic response (FSS) ▲reaction time (HPT) ▼depression-like behavior (TST) |
[51] | |||||
| Aqueous methanolic (75:25) root extract, NR | 200 mg/kg p.o. for 21 days before hypoxia and 7 days during hypoxia | Rat (hypobaric hypoxia) | ▼stress-induced increase in corticosterone*^, NO^, AChE activity^, ROS^, lipid peroxidation^, synaptophysin^, nNOS^, L-type calcium channels^, pyknotic cells in CA3 region of hippocampus^, Bax^ ▲stress-induced decrease in ACh^, GSH^, SOD activity^, BDNF^, NCAM^, Bcl-2^ ▼stress-induced memory impairment (MWM) |
[46] | |||||
| Aqueous leaf extract (ASH-WEX), NR | 140 mg/kg/bwt, p.o. for 15 days prior to acute sleep deprivation | Rat (acute sleep deprivation) | ▼stress-induced learning and memory impairments ▼stress-induced motor dysfunction ▼anxiety-like behavior (▼grooming activity) Modulated markers of synaptic plasticity (▼hippocampal PSA-NCAM and NCAM, ▲PSA-NCAM in the pyriform cortex)^ ▼mortalin^, ▲Akt-1 phosphorylation^ |
[45] | |||||
| Ethanolic root extract, NR | 23 mg/kg p.o. for 7 days prior to assessment | Mouse (FST, CRS) | ▲swim duration (FST) ▼stress-induced increase in WBC count, blood glucose, plasma cortisol, and serum triglyceride levels (CRS) |
[41] | |||||
| Alcoholic root extract, NR | FST: 100 mg/kg p.o. for 7 days CRS: 100 mg/kg p.o. for 10 days |
Rat (FST, CRS) | ▲swim duration (FST) ▼stress-induced increase in blood glucose, total cholesterol, triglycerides, serum corticosterone, RBC count, WBC count, neutrophils, lymphocytes, and eosinophils (FST, CRS) ▼stress-induced increase in monocytes, liver weight, and adrenal weight (CRS) ▲stress-induced decrease in spleen weight (CRS) |
[42] | |||||
| Root extract, NR | 40 mg/kg/bwt p.o. for 30 days | Mouse (RST) | ▼stress-induced increase in corticosterone* and hippocampal nNOS-positive neurons ▲stress-induced decrease in ChAT reactivity^ and serotonin^ |
[47] | |||||
| Nature of Extract, Standardization | Dosage | Model | Outcomes | Refs. | |||||
| Water:methanol 40:60 leaf and root extract (“Sensoril®”), standardized to 10% total withanolides | 50 and 100 mg/kg p.o. for 7 or 30 days | Mouse (FST) | ▲stress-induced decrease in ATP, AEC, TAN, and ATP/ADP ratio (7- and 30-day FST) | [36] | |||||
| Root (preparation unclear), NR | 25, 50, 100, and 200 mg/kg p.o. for 14 days | Mouse (RST) | ▲stress-induced decrease in T lymphocyte count (CD3+, CD4+, and CD8+ populations)*, IL-2*, IFN-γ*, polymorphonuclear leucocyte count*, and organ weights (thymus, spleen, axillary lymph nodes) ▼stress-induced increase in serum cortisol |
[48] | |||||
| Glycowithanolide-rich fraction (containing sitoindosides VII-X and withaferin) isolated from aqueous ethanolic (1:1) root extract, standardized to 28-30% withanolide glycosides | 25 and 50 mg/kg p.o. for 21 days, administered 1 hr before assessment | Rat (FSS) | ▼stress-induced hyperglycemia, perturbed glucose tolerance test, and gastric ulcers (FSS) ▼depression-like behavior (FST, LHT) ▼stress-induced memory impairment (EPM, PAT), sexual behavior inhibition (FSS), and immunosuppression (FSS) |
[52] | |||||
| Isolated fraction and pure compound (1-oxo-5β, 6β-epoxy-witha-2-ene-27-ethoxy-olide), extracted from aqueous root extract | Fraction: 20 mg/kg/bwt p.o., administered 30 mins prior to assessment Compound: 2.5 mg/kg/bwt p.o., administered 30 mins prior to assessment |
Rat (CHR) | ▼stress-induced increase in serum corticosterone, CPK, LDH (fraction, compound) ▼stress-induced increase in LPO (compound) ▲time taken to achieve 23˚C rectal temperature (fraction, compound) ▼recovery time taken to achieve 37˚C rectal temperature (fraction, compound) |
[50] | |||||
| Traditional root extract consisting of water:ghee:honey (60:7.5:32.5) and 50% methanolic root extract, NR | Traditional extract: 250 mg/kg Methanolic extract: 250 mg/kg |
Rat (FSS) | ▼depression-like behavior (FST, methanolic extract) ▼stress-induced memory impairment (PAT, both extracts) |
[53] | |||||
| Withanolide-free aqueous fraction (BF), isolated from hydroalcoholic (30:70) root extract and a root extract, NR | Withanolide-free fraction: 12.5, 25, 50, and 100 mg/kg p.o., administered 2 hrs before assessment Root extract: 125 mg/kg p.o., administered 2 hrs before assessment |
Rat (FST) | ▼stress-induced increase in serum GPT, GOT, ALP, triglycerides, and hepatic LPO (fraction, extract) ▲stress-induced decrease in hepatic glycogen (fraction, extract) |
[37] | |||||
| Aqueous root extract and isolated compound (Compound X) from aqueous root extract, NR | Root extract: 360 mg/kg/bwt, administered 30 mins before assessment Compound: 20 mg/kg/bwt, administered 30 mins before assessment |
Rat (CHR) | ▲time taken to achieve 23˚C rectal temperature (extract, compound) ▼recovery time taken to achieve 37˚C rectal temperature (extract, compound) |
[49] | |||||
| Withanolide-free aqueous fraction (BF), isolated from hydroalcoholic (30:70) root extract and a commercial root extract, NR | Fraction: 12.5, 25, 50, 100 mg/kg p.o. for 15 days Root extract: 125 mg/kg p.o. for 15 days |
Rat (HST, FST, RST, RTT) | ▲hypoxia-induced time to convulsion (HST, fraction, extract) ▲swimming duration (FST, fraction, extract) ▼stress-induced gastric ulcers (FST, RST, fraction, extract) ▲antifatigue activity (RTT, fraction, extract) ▼stress-induced hypothermia (FST, fraction, extract) ▼stress-induced autoanalgesia, increase in adrenal weight (RST, fraction, extract) ▲stress-induced decrease in adrenal content of ascorbic acid, cortisol, and cholesterol (RST, fraction, extract) |
[38] | |||||
| Nature of Extract, Standardization | Dosage | Model | Outcomes | Refs. | |||||
| Powdered root, NR | 100 mg/kg p.o. for 7 days | Rat (FST) | ▲swim duration ▼stress-induced increase in plasma corticosterone level, phagocytic index, and avidity index |
[39] | |||||
| Sitoindosides VII and VIII | PTZ: 20 mg/kg i.p., 30 mins prior to assessment FST: 20 and 50 mg/kg i.p., 30 mins prior to assessment OST, TCT: 50 mg/kg p.o. for 4 days RST: 50 and 100 mg/kg p.o. for 4 days; 100 mg/kg i.p., 30 mins before morphine administration Morphine toxicity: 50 mg/kg p.o. for 4 days |
Mouse (FST, OST, TCT) Rat (PTZ, RST) |
▼depression-like behavior (FST) ▼stress-induced autoanalgesia, gastric ulcers (RST) ▼morphine-induced hypothermia (RST) ▼morphine toxicity (OST, TCT) ▼anxiety-like behavior (PTZ-induced) ▼tribulin activity in urine (RST) ▲stress-induced decrease in adrenal content of ascorbic acid, corticosterone |
[17] | |||||
| Alcoholic extract from defatted seeds, NR | 100 mg/kg i.p., administered 30 mins or 1 hr before assessment | Mouse (FST, MIL) Rat (CST, RST, ASA) |
▲swim duration (FST) ▲stress-induced decrease in ascorbic acid and cortisol content in adrenals (FST) ▼stress-induced gastric ulcers (CST, RST, ASA) ▼milk-induced leukocytosis (MIL) |
[40] | |||||
*Peripheral; ^CNS; ▲ – Increased; ▼ – Decreased; ACh = Acetylcholine; AChE = Acetylcholinesterase; ADP = Adenosine diphosphate; AEC = Adenylate energy charge; ALP = Alkaline phosphatase; ALT/GPT = Alanine aminotransaminase; ASA = Aspirin administration; AST/GOT = Aspartate aminotransferase; ATP = Adenosine triphosphate; Bax = Bcl-2-associated X protein; BDNF = Brain-derived neurotrophic factor; ChAT = Choline acetyl transferase; CHR = Cold-Hypoxia-Restraint test; CRS = Cold restraint stress test; CST = Cold stress test; EPM = Elevated plus maze test; FSS = Foot shock stress test; FST = Forced swim test; GPx = Glutathione peroxidase; GSH = Reduced glutathione; GSSG = Oxidized glutathione; HDL = High density lipoprotein; HPT = Hot plate test; HST = Hypoxia stress test; IFN-γ = Interferon gamma; IL-2 = Interleukin 2; IL-6 = Interleukin 6; LDL = Low density lipoprotein; LHT = Learned helplessness test; LPO = Lipid peroxidation; MIL = Milk-induced leukocytosis; MWM = Morris Water Maze test; NCAM = Neural cell adhesion molecule; nNOS = Nitric oxide synthase; NO = Nitric oxide; NR = Not reported; OST = Overcrowding stress test; PAT = Passive avoidance test; PTZ = Pentylenetetrazol administration; RAWM = Radial arm water maze; RBC = Red blood cell; ROS = Reactive oxygen species; RST = Restraint stress test; RTT = Rotarod test; ROS = Reactive oxygen species; SOD = superoxide dismutase; SPS = Single prolonged stress; TAN = Total adenine nucleotide; TBARs = Thiobarbituric acid reactive substances; TCT = Tactile stress test; TST = Tail suspension test; WBC = White blood cell.
Most of the extracts studied were derived from the root of WS [37-39, 41, 42, 46-49, 51, 53-56]. Anti-stress effects were also seen for a leaf extract [45], a leaf and root extract [36] and an extract made from defatted seeds [40]. Extraction methods and test preparations varied, and included alcoholic extracts [40-42, 51], aqueous extracts [45, 49, 50], hydroalcoholic extracts [36-38, 46, 52, 53, 55], a traditional extract made with water, ghee, and honey [53], a withanolide-free fraction [37, 38], a glycowithanolide-rich [52] fraction, and several isolated compounds, including sitoindosides VII and VIII [17], 1-oxo-5β, 6β-epoxy-witha-2-ene-27-ethoxy-olide [64], and a substance named Compound X [49].
2.2. Human Studies
Six studies evaluated the use of WS for anti-stress activity in adults aged 18-75 years [57-62] (Table 1). Study populations included participants who were healthy [61], stressed [57, 60], overweight, or obese, experiencing chronic work stress [58], diagnosed with anxiety [62], and diagnosed with schizophrenia or schizoaffective disorder [59]. The number of participants in each study ranged from 52 to 130 participants.
Each study administered WS as capsules of commercially available preparations (Table 1). Three studies evaluated effects of KSM-66 Ashwagandha®, described as an aqueous WS root extract made without alcohol or synthetic solvents [57, 58, 60]. It is worth noting that the literature description of KSM-66 as an “aqueous” extract differs from the product website [71], describing the use of a traditional process involving milk (except for a vegan version), which may extract more lipophilic molecules due to its fat content. Two other studies used Sensoril®, an aqueous extract of WS leaf and root standardized to ≥10% withanolide glycosides [59, 62]; and one study used Shoden®, a hydroalcoholic (70:30) root and leaf extract of WS standardized to 35% withanolide glycosides [61]. In addition, Auddy et al. (2008) included Essentra®, a version of Sensoril® created for the food and beverages market [62]. The daily dose of WS extract ranged from 240 mg to 1000 mg, with two of the studies using more than one dose in the intervention group [60, 62]. The treatment period was variable, with three studies lasting 8.5 weeks [57, 61, 62], two lasting 8 weeks [58, 60], and one lasting 12 weeks [59].
Stress was assessed via serum cortisol levels and three questionnaires: the Depression Anxiety Stress Scale (DASS), the Perceived Stress Scale (PSS), and the General Health Questionnaire-28 (GHQ-28). The DASS is a self-report questionnaire that measures symptoms of depression, anxiety, and stress and is available in 42- (DASS) and 21-item (DASS-21) versions. The PSS is a self-report questionnaire used to evaluate the level of stress perceived by a respondent in the previous month [58]. The GHQ-28 is a 28-item questionnaire with four item-subsets (somatic, anxiety and insomnia, social dysfunction, and severe depression) that correspond to different categories of stress [57]. Four studies used versions of the PSS [57-60], two studies used versions of the DASS [57, 61], and one study used the GHQ-28 [57]. Five of the six studies measured serum cortisol levels [57, 58, 60-62].
WS supplementation improved stress markers and symptoms in the majority of the human trials as evidenced by statistically significant declines in PSS, DASS, and GHQ-28 scores, as well as decreased serum cortisol levels compared to placebo (Table 1). Lopresti et al. (2019) saw a non-significant decrease in DASS-21 scores [61].
2.3. Proposed Mechanisms for Anti-Stress Effects
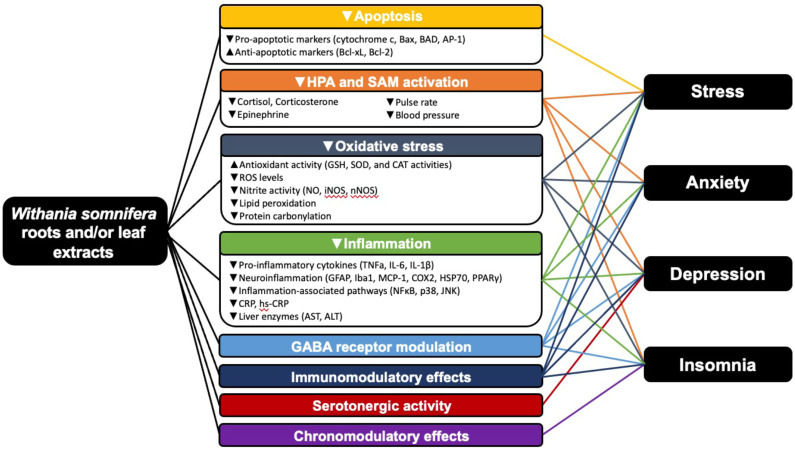
The anti-stress effect of WS has been attributed to several mechanisms, including reduction of glucocorticoids and immune modulation (described below). A more comprehensive overview of mechanisms that may be related to the anti-stress effect of WS is illustrated in Fig. 2.
Fig. (2).
Potential mechanisms by which WS exerts positive benefits on stress, anxiety, depression, and insomnia. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.3.1. Glucocorticoid Reduction
The anti-stress activity of WS is most commonly attributed to its effects on the glucocorticoids cortisol and corticosterone (the major stress hormones in humans and rodents), respectively [72]. Cortisol and corticosterone are elevated during periods of stress and play a central role in stress-induced HPA axis dysfunction. As described in Table 2, various preparations of WS root attenuated the stress-induced increase in peripheral cortisol and corticosterone levels [38, 39, 41, 42, 46-48, 50, 54, 56]. WS was also found to reverse the stress-induced decrease in adrenal cortisol content [38, 40]. In addition to its effects on peripheral cortisol and corticosterone, Baitharu et al. (2013) found that a hydroalcoholic root extract of WS decreased hippocampal levels of corticosterone in a rat model of hypobaric-hypoxia-induced stress [46].
2.3.2. Immune Modulation
WS may also exert its anti-stress effects via modulating the immune system. In the reviewed studies, the effect of WS on immune markers depended on the animal model used. In a horse model of exercise-, separation-, and noise-induced stress, white blood cell count and lymphocyte percentage decreased while the pro-inflammatory cytokine IL-6 increased [56]. A root powder of WS reversed these effects, demonstrating immune-stimulating and anti-inflammatory effects. Similar effects were seen in a rat model of restraint stress, where a root preparation of WS attenuated stress-induced declines in peripheral T-lymphocytes counts (CD3+, CD4+, and CD8+ populations), IL-2, INF-γ, and polymorphonuclear leukocyte counts [48]. However, in rodent models of cold restraint stress and forced swimming-induced stress, stress increased white blood cell counts, neutrophils, lymphocytes, and eosinophils [41, 42]. These effects were reversed by WS, suggesting immune-modulating activity [41, 42]. Fig. 2 describes all demonstrated mechanisms of WS that may be involved with its anti-stress effects.
3. ANTI-ANXIETY EFFECTS
3.1. Animal Studies
To date, animal studies have demonstrated the ability of WS to decrease anxiety-like behavior in several animal models of disease, including stress [17, 54, 73-75], sleep deprivation [45, 76, 77], social isolation [78], and neuroinflammation [79] (Table 3). Anxiety-like behavior was most commonly evaluated using the elevated plus maze [74, 75, 77, 80, 81]: a test in which rodents are placed in an elevated plus-shaped maze with open and closed arms, where increased entries and/or time spent in the open arms reflect anti-anxiety behavior [82]. The elevated plus maze test has been validated to assess pharmaceutical agents for anti-anxiety activity in rodents [82]. The marble burying test, in which increased marble burying is said to indicate anxiety-like behavior, was another commonly used test [54, 73, 83]. However, the validity of this test as a measure of anxiety is questionable [84, 85]. Other measures used to evaluate anxiety-like behavior in rodent studies included the open field test [75], social interaction test [81], novelty-suppressed feeding latency test [81], and pentylenetetrazol-induced defecation and urination [17]. A zebrafish model of benzo[a]pyrene-induced neurotoxicity used the light/dark preference test and the novel tank diving test to assess anxiety-like behavior [86].
Table 3.
Anti-anxiety effects of Ashwagandha (Withania somnifera, WS) in animal studies.
| Nature of Extract, Standardization | Dosage | Model |
Behavioral
Effects |
Biological Effects | Refs. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aqueous leaf extract (ASH-WEX), NR | 140 mg/kg/day p.o. for 8 weeks | Rat (LPS-induced neuroinflammation) | ▼anxiety-like behavior (EPM) |
▼ pro-inflammatory cytokines (TNFα, IL-1β, IL-6)*^ ▼reactive gliosis and neuroinflammation (Iba-1, GFAP, NOX2, iNOS, COX2, MCP-1, HSP70)^ ▼inflammatory pathways (NFκB, p38, JNK)^ |
[79] | |||||||
| Aqueous leaf extract (ASH-WEX), NR | 140 mg/kg/bwt, p.o. for 15 days prior to acute sleep deprivation | Rat (acute sleep deprivation) | ▼anxiety-like behavior (EPM) |
▼reactive gliosis (GFAP), ▼OX-18 ▼pro-inflammatory cytokines (TNFα, IL-6)^ ▼cell survival proteins (NFκB, AP-1) ▼apoptosis (▲Bcl-xL, ▼Cytochrome c) |
[76] | |||||||
| Aqueous leaf extract (ASH-WEX), NR | 140 mg/kg/bwt, p.o. for 15 days prior to acute sleep deprivation | Rat (acute sleep deprivation) | ▼anxiety-like behavior (▼grooming activity) ▼stress-induced learning and memory impairments ▼stress-induced motor dysfunction |
Modulated markers of synaptic plasticity (▼hippocampal PSA-NCAM and NCAM, ▲PSA-NCAM in the pyriform cortex)^ ▼mortalin^, ▲Akt-1 phosphorylation^ |
[45] | |||||||
| Dry leaf powder, NR | 1 mg/g/bwt p.o. for 12 weeks | Rat (high fat diet-induced obesity) | ▼anxiety-like behavior (EPM) | ▼ pro-inflammatory cytokines (TNFα, IL-1β, IL-6)*^ ▼reactive gliosis and neuroinflammation (GFAP, Iba1, PPARγ, iNOS, MCP-1, COX2)^ ▼NFκB pathway^ ▼apoptosis (▼AP-1, ▲Bcl-xL, ▼BAD)^ |
[88] | |||||||
| Aqueous leaf extract, NR | 0.3% concentration (2.8 mg/L water), waterborne administration for 72 hrs | Zebrafish (benzo[a]pyrene-induced neurotoxicity) | ▼anxiety-like behavior (LDPT, NTDT) | ▼pyknotic cells in periventricular gray zone ▼oxidative stress (▼lipid peroxidation ▼protein carbonylation, ▲catalase activity, ▲GSH)^ |
[86] | |||||||
| Withanolide-free root extract | 3.3, 10, 33.3, and 100 mg/kg p.o. for 12 days | Rat (stress) | ▼anxiety-like or depression-like behavior (MBT) | ▼stress-induced weight loss ▼stress-induced increase in rectal temperature ▼transient hyperthermic response ▼stress-induced increase in adrenal weight ▼stress-induced increase in plasma cortisol and blood glucose |
[54] | |||||||
| Root extract, 2.7% (w/w) withanolides | 10, 20, and 40 mg/kg/day p.o. for 12 days, or as a single dose | Mouse (acute stress) | ▼anxiety-like behavior (MBT) | ▼stress-induced changes in weight, basal core temperature, and hyperthermic response | [73] | |||||||
| Hydro-alcoholic root extract, standardized to 2% (w/w) withanolides | 300 mg/kg/bwt p.o. for 30 days | Rat (ischemic stroke) | ▼anxiety-like behavior (EPM) ▲cognitive function (MWM) ▲locomotor function (NDS, NBW, RTT) |
▲acetylcholinesterase activity^ ▼lipid peroxidation^ ▲antioxidant activity^ ▼infarct volume ▼stroke-induced histopathological changes |
[87] | |||||||
| Nature of Extract, Standardization | Dosage | Model |
Behavioral
Effects |
Biological Effects | Refs. | |||||||
| Methanolic (MEWS) and aqueous root extracts (AEWS), NR |
10, 25, 50, and 100 mg/kg i.p., 30 mins prior to assessment | Mouse (obsessive-compulsive behavior) |
▼obsessive compulsive behavior (MBT) ▲anti-OCD action of subtherapeutic dose of fluoxetine at subtherapeutic dose (50 mg/kg) of WS (MBT) |
Not reported | [83] | |||||||
| Several root extracts, using various solvents (alcohol, water, hydro-alcohol (50:50)), ratios (1:6, 1:8, 1:10), and methods (hot continuous percolation (10 hrs) and maceration (10 hrs)), NR |
100 or 200 mg/kg p.o., 30 mins prior to assessment | Mouse (acute stress) | ▼anxiety-like behavior (EPM) for water and hydro-alcoholic extracts prepared by maceration and for hydro-alcoholic extract prepared by hot continuous percolation | Not reported | [74] | |||||||
| Withaferin A and Hydroalcoholic (4:1) root extract, NR | Withaferin A: 10-50 mg/kg i.p., 1-2 hrs prior to assessment Root extract: 100-500 mg/kg p.o., 1-2 hrs prior to assessment |
Rat (acute stress) | ▼anxiety-like behavior (EPM, OFT) | Not reported | [75] | |||||||
| Ethanolic root extract, NR | 50, 100, 200, or 500 mg/kg p.o., one hr prior to assessment | Rat (acute ethanol-induced anxiolysis and withdrawal from chronic ethanol consumption) | ▼anxiety-like behavior (EPM) ▲anti-anxiety action of a subtherapeutic dose of ethanol at subtherapeutic dose (50 mg/kg) of WS (EPM) |
Not reported | [80] | |||||||
| Root extract, NR | 50, 100, 200, or 500 mg/kg p.o. on days 38-42 of social isolation and 1 hr prior to assessment | Rat (social isolation) |
▼anxiety-like behavior (EPM) ▲anti-anxiety action of diazepam at subtherapeutic dose (50 mg/kg) of WS (EPM) ▼depression-like behavior (FST) |
Not assessed | [78] | |||||||
| Root extract, NR | 100 and 200 mg/kg p.o. for 5 days, beginning 3 days prior to sleep deprivation | Mouse (sleep deprivation) |
▼anxiety-like behavior (EPM) ▲locomotor activity |
▼sleep deprivation-induced weight loss ▼oxidative stress (▼lipid peroxidation, ▼nitrite activity, ▲catalase activity, ▲GSH)^ |
[77] | |||||||
| Glycowithanolide-rich fraction (WSG, containing sitoindosides VII-X and withaferin) isolated from aqueous root extract, standardized to 1.13% total steroid content |
20 and 50 mg/kg p.o. for 5 days | Rat (anxiety and depression) | ▼depression-like behavior (LHT, FST) ▼anxiety-like behavior (EPM, SIT, NSFLT) |
▼PTZ-induced increase in rat brain tribulin activity (PTZ) | [81] | |||||||
| Nature of Extract, Standardization | Dosage | Model |
Behavioral
Effects |
Biological Effects | Refs. | |||||||
| Sitoindosides VII and VIII | PTZ: 20 mg/kg i.p., 30 mins prior to assessment FST: 20 and 50 mg/kg i.p., 30 mins prior to assessment OST, TCT: 50 mg/kg p.o. for 4 days prior to assessment RST: 50 and 100 mg/kg p.o. for 4 days prior to restraint; 100 mg/kg i.p., 30 mins before morphine administration Morphine toxicity: 50 mg/kg p.o. for 4 days |
Mouse, rat (stress) | ▼anxiety-like behavior (PTZ-induced) ▼depression-like behavior (FST) |
▼stress-induced autoanalgesia, gastric ulcers (RST) ▼morphine-induced hypothermia (RST) ▼morphine toxicity (OST, TCT) ▼tribulin activity in urine (RST) ▲stress-induced decrease in adrenal content of ascorbic acid, corticosterone |
[17] | |||||||
*Peripheral; ^CNS; ▲ – Increased; ▼ – Decreased; AP-1 = Activator protein 1; Bcl-xL = B-cell lymphoma extra-large; BAD = Bcl-2 associated agonist of cell death; COX-2 = Cyclooxygenase 2; EPM = Elevated plus maze test; FST = Forced swim test; GFAP = Glial fibrillary acidic protein; GSH = Reduced glutathione; HSP70 = Heat shock protein 70; Iba1 = Ionized calcium binding adaptor molecule 1; IL-1β = Interleukin 1 beta; IL-6 = Interleukin 6; iNOS = Inducible nitric oxide synthase; JNK = c-Jun N-terminal kinase; LDPT = Light/dark preference test; LHT = Learned helplessness test; LPS = Lipopolysaccharide; MBT = Marble burying test; MCP-1 = Monocyte chemoattractant protein; MWM = Morris water maze test; NBW = Narrow beam walking test; NDS = Neurological deficit score; NFκB = Nuclear factor kappa B; NOX2 = NADPH oxidase 2; NSFLT = Novelty-suppressed feeding latency test; NTDT = Novel tank diving test; OFT = Open field test; OST = Overcrowding stress test; PPARγ = Peroxisome proliferator-activated receptor gamma; PTZ = pentylenetetrazol administration; RST = Restraint stress test; RTT = Rotarod test; SIT = Social interaction test; TCT = Tactile stress test; TNFα = Tumor necrosis factor alpha.
Anti-anxiety effects were observed for root extracts [48, 54, 73, 74, 77, 78, 80, 81, 83, 87], leaf extracts [76, 79, 86, 88], and isolated compounds from WS, including withaferin A [75] and a mixture of sitoindosides VII-X [17, 81]. WS extracts produced from leaf or root and with various solvents (water, ethanol, methanol, hydroalcoholic), solvent ratios, and extraction methods all produced anti-anxiety effects (Table 3), suggesting the possibility of multiple bioactive compounds. In addition, WS extracts potentiated the effects of well-known anti-anxiety drugs. A subtherapeutic dose of a WS root extract potentiated the anti-anxiety effect of a subtherapeutic dose of diazepam in a rat model of social isolation [78]. In a rat model of alcohol withdrawal, a subtherapeutic dose of an ethanolic root extract of WS potentiated the anti-anxiety action of a subtherapeutic dose of ethanol [80]. Of note, in a mouse model of Obsessive-Compulsive Disorder (OCD), subtherapeutic doses of a methanolic and aqueous root extract potentiated the anti-OCD action of fluoxetine at a subtherapeutic dose, which differentiates WS from an anti-anxiety to an anti-OCD agent, the authors concluded [83].
3.2. Human Studies
The anti-anxiety effect of WS was assessed in ten human trials of adults aged 18-75 years (Table 1). Study populations included participants described as healthy [61], stressed [57, 60], diagnosed with general anxiety disorder or a related condition [62, 68-70], with insomnia [63, 65], or with schizophrenia or schizoaffective disorder [66]. Sample sizes ranged from 39 participants [68] to 130 participants [62], with the majority of studies including between 60 and 80 participants [57, 60, 61, 63, 65, 66].
Most of the trials used commercially available preparations of WS (KSM-66®, Sensoril®, Essentra®, or Shoden®) for a duration of 6-12 weeks (Table 1). These formulations are described in Section 2.2. Three studies used various preparations of WS without reporting any standardization, including a 70% ethanolic root extract [70], an ethanolic extract [68], and the dried root [69]. All studies administered WS as capsules or tablets except for Khyati and Anup (2013), in which dried root granules were used. Daily doses of WS were highly variable, ranging from 125-1000 mg in studies using capsules or tablets up to 12 g of the dried root granules (Table 1). Only two studies compared more than one dose in the intervention group [60, 62].
A majority of studies evaluated anxiety using some variation of the Hamilton Anxiety Rating Scale (HAM-A or HARS), the most commonly used instrument for measuring anxiety in clinical trials [89]. The HAM-A is a self-reported questionnaire used to assess the severity of anxiety as perceived by the respondent. Other instruments included the Depression Anxiety Stress Scale (DASS) described previously [57] and the Positive and Negative Syndrome Scale (PANSS) [66]. The PANSS is a validated tool used to evaluate persons with schizophrenia [90, 91]. Lopresti et al. (2019) used both the HAM-A and the DASS [61].
An improvement in anxiety after supplementation with WS was observed in a majority of studies as measured by changes in anxiety scores (Table 1). In a study of adults who self-reported high stress, Lopresti et al. (2019) found that daily administration of 240 mg Shoden® for 60 days significantly decreased HAM-A (but not DASS) scores compared to placebo [61]. In those with general anxiety disorder, Khyati and Anup (2013) found that administration of either 12 g of a dried WS root preparation or placebo decreased all measured anxiety scores from baseline values [69]. Although larger decreases were noted for all anxiety scores in the intervention group compared to the placebo group, the effect size only differed significantly between groups for anxious mood, as identified by the HARS. Changes in other anxiety score subgroups did not differ significantly between groups, suggestive of a potential placebo effect [69]. Lastly, Salve et al. (2019) found that KSM-66® aqueous root extract produced a significant decrease in HAM-A score with a daily dose of 600 mg but not with 250 mg [60].
Two papers, Cooley et al. (2009) and Chengappa et al. (2013), found during our literature review re excluded from Table 1 [92, 93]. Cooley et al. (2009) evaluated the use of naturopathic care, which included the use of WS for treating anxiety using psychotherapy as the control [92]. This study was excluded because the intervention included many components in addition to WS supplementation, and not all of these components were included in the control, making it difficult to ascertain the effect of WS on anxiety. Chengappa et al. (2013) conducted a randomized, double-blind, placebo-controlled study to evaluate the cognitive effects of a standardized WS extract in bipolar disorder [93]. The investigators noted that symptoms were well-controlled at baseline (i.e., euthymic), and there were no significant differences in anxiety between the treatment groups, as measured by the HAM-A. As a result, no comment can be made regarding the effect of WS on anxiety in this study.
3.3. Proposed Mechanisms for Anti-Anxiety Effects
Research investigating the mechanisms underlying WS’s anti-anxiety effects has primarily focused on the GABA receptor system (described below) and antioxidant and anti-inflammatory activities. Fig. 2 illustrates additional mechanisms of WS that may play a role in its anti-anxiety effects.
3.3.1. Gamma-Aminobutyric Acid (GABA) Related Activity
GABA is the most important inhibitory neurotransmitter in the central nervous system, and GABAergic neurotransmission is thought to play a key role in the regulation of anxiety [94]. GABA type A (GABAA) receptors are the primary site of action for GABA agonist drugs, which stimulate GABAergic activity and are commonly used in the treatment of anxiety disorders [95, 96]. There is substantial pre-clinical evidence suggesting that compounds found in WS interact with and modulate GABAA receptors, which may in part explain the anti-anxiety effect of WS.
Evidence of WS’s direct GABA-mimetic activity was first demonstrated by Mehta et al. (1991), who found that a methanolic root extract of WS increased chloride ion influx in mammalian spinal cord neurons in the absence of GABA and also inhibited GABA binding in a manner similar to GABAA receptor agonists [97]. Receptor-binding assays have shown that components in WS methanolic root extracts display high affinity for GABAA receptors, with significantly less affinity for GABAB, glutamatergic, and opioid receptors [98, 99]. WS’s GABAA receptor-specific activity is supported by several animal studies. The stimulatory actions of morphine and ethanol on ventral tegmental area dopaminergic neurons in rats were suppressed by a methanolic root extract of WS via a GABAA- but not a GABAB-mediated mechanism [100]. In a mouse model of pentylenetetrazol (PTZ)-induced seizures, a sub-effective dose of an undefined WS root extract increased the seizure threshold in mice in combination with sub-therapeutic doses of GABA, a GABA receptor agonist, and diazepam, a GABAA receptor modulator [101]. In an earlier mouse study, Kulkarni et al. (1993) found that a methanolic extract of WS (plant part not specified) in combination with pentobarbital (a GABAA receptor agonist) produced greater protection from PTZ-induced toxicities compared to either agent alone [102]. In addition, the combination of a sub-protective dose of WS with GABA potentiated the protective effects of the extract. In a rat model of sleep disturbance, a selective GABAA agonist (muscimol) potentiated the hypnotic effect of an undefined WS root extract, whereas a GABAA receptor antagonist (picrotoxin) reversed the effect [103]. GABAA receptor antagonists (picrotoxin, bicuculline) blocked the abilities of methanolic and aqueous root extracts of WS to induce depolarization in mice gonadotropin releasing hormone neurons or to increase inward ion currents in mouse substantia gelatinosa neurons and rat brain GABAA channels [96, 104, 105].
Attempts to identify which compound or compounds found in WS are responsible for its GABAA receptor activity have been difficult. Candelario et al. (2015) found that while an aqueous root extract of WS demonstrated GABAA receptor agonist activity in rat brain GABAA channels, neither withaferin A nor withanolide A, two of the major compounds found in WS, had any direct effect on GABAA receptors [104]. Similarly, Schliebs et al. (1997) found that intraperitoneal injection of an equimolar mixture of withaferin A and sitoindosides VII-X had no effect on GABAA receptors in a rat model [106]. Sonar et al. (2019) isolated nine compounds from a methanolic extract of WS, including withanolides and ferulic acid esters. They found no direct GABA-mimetic activity for any of these compounds (at 10 μM) in an ex vivo model [95]. However, two compounds (withanolide B and docosanyl ferulate) did modulate GABAA receptor function by enhancing inhibitory postsynaptic currents, with a similar pharmacological profile to the methanolic extract from which they were isolated and to other known GABAA allosteric modulators [95]. Interestingly, several of the compounds, including withanolide A, had an opposite effect on GABAA receptor function, decreasing its activity [95]. Further research is needed to identify structure-activity relationships of withanolide interactions with GABAA receptors, possible sites of interaction at the GABAA receptor complex, and whether or not specific subpopulations of GABAA receptors are more sensitive to WS compounds. Of note, Candelario et al. (2015) found that WS was also a strong GABAρ1 receptor agonist and that these receptors were 27 times more sensitive to WS than GABAA receptors [104]. GABAρ1 receptors are a subclass of GABAA receptors with unique pharmacological properties, including greater sensitivity to GABA, a lower rate of desensitization, and insensitivity to bicuculline: a GABAA antagonist [104].
3.3.2. Antioxidant and Anti-Inflammatory Activity
The brain is uniquely susceptible to oxidative stress [107], and research suggests that anxiety disorders are marked by decreased antioxidant defenses combined with increased oxidative damage [108]. There are several proposed mechanisms by which oxidative stress contributes to anxiety, including both as a cause and a consequence of neuroinflammation, which similarly has been associated with anxiety disorders [109]. Peripheral inflammation is also thought to directly contribute to neuroinflammation and oxidative stress in the brain [109]. While both oxidative stress and inflammation have been implicated in the pathogenesis of anxiety, a definitive cause-effect relationship has yet to be established for either [108-110].
Animal studies investigating the anti-anxiety effects of WS (Table 3) have demonstrated an association between anxiety-like behavior and the dysregulation of oxidative stress and inflammatory markers, which are ameliorated after administration of WS. An undefined root extract [77, 86] and an aqueous leaf extract [77, 86] of WS both showed an ability to increase catalase activity and levels of reduced glutathione (GSH) in the brain, while reducing lipid peroxidation in a mouse model of acute sleep deprivation and a zebrafish model of benzo[a]pyrene-induced neurotoxicity, respectively. In addition, WS reduced nitrite activity in the mouse model, while reducing protein carbonylation in the zebrafish model [77, 86]. In a rat model of ischemic stroke, a standardized hydroalcoholic root extract of WS also reduced lipid peroxidation and increased antioxidant activity in the brain [87].
In animal models of neuroinflammation and sleep deprivation, an aqueous leaf extract of WS (ASH-WEX) reduced pro-inflammatory cytokines including TNFα and IL-6, both peripherally and centrally [76, 79]. ASH-WEX also reduced markers of reactive gliosis (e.g., GFAP) and neuroinflammation (e.g., NOX2, iNOS, COX2), while modulating several inflammatory pathways and reducing apoptosis [76, 79]. Similarly, in a rat model of high fat diet-induced obesity, a dry leaf powder of WS reduced levels of pro-inflammatory cytokines peripherally and centrally, reduced markers of reactive gliosis and neuroinflammation, modulated the NFκB pathway, and reduced apoptosis [88].
4. ANTI-DEPRESSANT EFFECTS
4.1. Animal Studies
WS has been evaluated for anti-depressant activity in various animal models (Table 4). The most frequently used method of evaluating depression was the Forced Swim Test (FST), a common screening test for agents with potential antidepressant activity; however, the validity of the FST for this use has recently been called into question [111-113]. As described earlier, the FST involves placing a rodent in an inescapable tank filled with water; a drug’s ability to reduce the amount of time an animal is immobile is considered indicative of anti-depressant properties [43, 114]. The FST is also used to assess non-depression endpoints, including coping ability, capacity to learn, endurance, or as a method to induce stress [111]. Another common depression test model was the Learned Helplessness Test (LHT). The LHT involves dividing study animals into three groups with differing exposure to electrical shocks [115, 116]. The first group is not exposed to shocks. A second group is exposed to shocks that the animal can control by a physical mechanism, such as by pressing a lever. A third group is exposed to shocks that are triggered by the activities of the animals in the first group. These shocks are uncontrollable and unavoidable, so the animals develop learned helplessness, which is considered a sign of depression-like behavior. A third method used to model depression was the Tail Suspension Test. Here, mice are suspended from their tail, and researchers measure the time until the mice become immobile, which is interpreted as depression-like behavior [51]. Effects of WS were tested in rodents exposed to these behavioral despair tests alone [81, 117-119], or in animals exposed to stress [17, 51, 52, 54], social isolation [78], or drugs such as clonidine or reserpine [120] prior to testing.
Table 4.
Anti-depressant effects of Ashwagandha (Withania somnifera, WS) in animal studies.
| Nature of Extract, Standardization | Dosage | Model | Behavioral Effects | Biological Effects | Refs. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Aqueous methanolic (40:60) root and leaf extract (AME), withanolide-rich fraction from AME (WF), flavonoid-rich fraction from AME (FF), compound isolated from WF (Withanoside X), Enteric-coated versions (EC-AME, EC-WF, EC-FF, EC-WX) | AME, WF, FF: 60mg/kg p.o. Withanoside X: 10mg/kg p.o. EC-AME: 20mg/kg p.o. EC-WF: 15mg/kg p.o. EC-Withanoside X: 2.5mg/kg p.o. All WS treatments given 4 hrs prior to assessment |
Rat (reserpine) | ▼depression-like behavior (FST) | Not assessed | [120] | ||||
| Withanolide-free root extract | 3.3, 10, 33.3, and 100 mg/kg p.o. for 12 days | Rat (stress) | ▼depression-like or anxiety-like behavior (MBT) | ▼stress-induced weight loss ▼stress-induced increase in rectal temperature ▼transient hyperthermic response ▼stress-induced increase in adrenal weight ▼stress-induced increase in plasma cortisol and blood glucose |
[54] | ||||
| Methanolic root extract, standardized to 2.7% (w/w) total withanolides | 10, 20, and 40 mg/kg/day p.o. for 11 days, or as a single dose | Rat (stress) | ▼depression-like behavior (TST) ▲reaction time (HPT) |
▼stress-induced weight loss ▼transient hyperthermic response (FSS) |
[51] | ||||
| Traditional root extract (Ashwagandha ghrutha) consisting of root:ghee:water (1:4:16) | 20 and 40 mg/kg, given 1 hr prior to assessment (acute) or for 7 days (chronic); Route of administration NR | Mouse | ▼ depression-like behavior (TST, 40 mg/kg acute or 20 and 40 mg/kg chronic WS dosing; FST, 20 and 40 mg/kg chronic WS dosing) ▲ antidepressant activity of low-dose imipramine (FST, TST, 10 mg/kg each, acute or chronic dosing) ▼reserpine-induced catatonia and sedation (ART) alone (20 and 40 mg/kg WS) and in combination with low-dose imipramine 10 mg/kg each) all at chronic dosing |
▼reserpine-induced ptosis (ART), alone (20 and 40 mg/kg) and in combination with low-dose imipramine (10 mg/kg each) all at chronic dosing | [117] | ||||
| Powder, NR | 50, 100 and 150 mg/kg p.o. for 2 weeks | Rat | ▼depression-like behavior (LHT, FST) ▲antidepressant activity of low-dose imipramine (LHT, FST, 16 mg/kg imipramine, 50 mg/kg WS) |
Not assessed | [118] | ||||
| Root extract, NR | 50, 100, 200, or 500 mg/kg p.o. on days 38-42 of social isolation and 1 hr prior to assessment | Rat (social isolation) | ▼depression-like behavior (FST) ▼anxiety-like behavior (EPM) ▲anti-anxiety action of diazepam at subtherapeutic dose (50 mg/kg) of WS (EPM) |
Not assessed | [78] | ||||
| Root extract, NR | 25, 37.5, 50, 100 and 200 mg/kg i.p., 30mins before assessment | Mouse (untreated, clonidine, reserpine) |
▼depression-like behavior (FST; 50 to 200 mg/kg WS) ▲imipramine and fluoxetine antidepressant activity at subtherapeutic dose (37.5 mg/kg) of WS (FST) ▼reserpine- and clonidine-induced depression-like behavior (FST; 100 mg/kg WS) |
Not assessed | [119] | ||||
| Nature of Extract, Standardization | Dosage | Model | Behavioral Effects | Biological Effects | Refs. | ||||
| Glycowithanolide-rich fraction (containing sitoindosides VII-X and withaferin) isolated from aqueous ethanolic (1:1) root extract, standardized to 28-30% withanolide glycosides | 25 and 50 mg/kg p.o. for 21 days, administered 1 hr before assessment | Rat (chronic stress) | ▼depression-like behavior (FST, LHT) ▼stress-induced memory impairment (EPM, PAT), sexual behavior inhibition (FSS) |
▼stress-induced hyperglycemia, perturbed glucose tolerance test, and gastric ulcers (FSS) ▼stress-induced immunosuppression (FSS) |
[52] | ||||
| Glycowithanolide-rich fraction (WSG, containing sitoindosides VII-X and withaferin) isolated from aqueous root extract, standardized to 1.13% total steroid content | 20 and 50 mg/kg p.o. for 5 days | Rat | ▼depression-like behavior (LHT, FST) ▼anxiety-like behavior (EPM, SIT, NSFLT) |
▼PTZ-induced increase in rat brain tribulin activity (PTZ) | [81] | ||||
| Sitoindosides VII and VIII | PTZ, FST: 20 mg/kg i.p., 30 mins prior to assessment FST: 20 and 50 mg/kg i.p., 30 mins prior to assessment OST, TCT: 50 mg/kg p.o. for 4 days RST: 50 and 100 mg/kg p.o. for 4 days; 100 mg/kg i.p., 30 mins before morphine administration Morphine toxicity: 50 mg/kg p.o. for 4 days |
Mouse, rat (stress) | ▼depression-like behavior (FST) ▼anxiety-like behavior (PTZ-induced) |
▼stress-induced autoanalgesia, gastric ulcers (RST) ▼morphine-induced hypothermia (RST) ▼morphine toxicity (OST, TCT) ▼tribulin activity in urine (RST) ▲stress-induced decrease in adrenal content of ascorbic acid, corticosterone |
[17] | ||||
▲ – Increased; ▼ – Decreased; ART = Anti-reserpine test; EPM = Elevated plus maze test; FSS = Foot shock stress test; FST = Forced swim test; HPT = Hot plate test; LHT = Learned helplessness test; NR = Not reported; NSFLT = Novelty-suppressed feeding latency test; OST = Overcrowding stress test; PAT = Passive avoidance test; PTZ = pentylenetetrazol administration; RST = Restraint stress test; SIT = Social interaction test; TCT = Tactile stress test; TST = Tail suspension test.
Anti-depressant effects were observed for WS root extracts [51, 52, 54, 78, 81, 117, 119], leaf and root extracts [120], and isolated compounds from WS, including 27-O-beta-D-glucopyranosylpubesenolide-3-O-beta-D-glucopyranoside (withanoside X) [120] and sitoindosides VII-X [17, 52, 81] (Table 4). Aqueous [81], methanolic [51], and hydroalcoholic extracts [52, 120], along with a traditional root extract made with water and ghee [117] of WS, all demonstrated anti-depressant activity. In addition, WS potentiated the effects of well-known anti-depressant drugs. In mouse and rat models of depression, WS potentiated the antidepressant activity of imipramine (a tricyclic antidepressant) [117-119] and fluoxetine: a selective serotonin reuptake inhibitor [119].
4.2. Human Studies
WS was evaluated for anti-depressant effects in three studies of adults aged 18-75 years who were either stressed [57], healthy [61], or diagnosed with schizophrenia or schizoaffective disorder [66] (Table 1). All studies included between 60 and 66 participants. Each study administered capsules of commercially available preparations of WS: KSM-66 Ashwagandha® [57], Sensoril® [62, 66], and Shoden® [61] (described previously). Daily doses of WS ranged from 240-1000 mg for a period of 8.5 weeks [57, 61] or 12 weeks [66]. Two studies assessed depression using the Depression Anxiety Stress Scale (DASS) [57, 61] (described previously). Chandrasekhar et al. (2012) also used the General Health Questionnaire-28 (GHQ-28), which includes a subset of items corresponding to stress that is categorized as “severe depression” [57]. Gannon et al. (2019) assessed depression using the Positive and Negative Syndrome Scale (PANSS) (described previously) [66].
Chandrasekhar et al. (2012) saw improvement in depression after supplementation with WS, as evidenced by a significant decrease in scores of the DASS and the “severe depression” subset of the GHQ-28 [57]. In Gannon et al. (2019), participants saw significantly increased PANSS scores, indicating an improvement in depression [66]. Lopresti et al. (2019) observed a decrease in DASS-21 score among participants, but the change was not statistically significant [61]. The results of these studies suggest that supplementation with WS may be beneficial for depressive symptoms (Table 1), but there are significant limitations. None of the studies evaluated patients diagnosed with major depressive disorder or seasonal affective disorder, representing a significant gap in the existing human research on WS for depression.
4.3. Proposed Mechanisms for Anti-Depressant Effects
The anti-depressant effect of WS demonstrated in the reviewed studies has been attributed to both its antioxidant and serotonergic activities (detailed below), though other mechanisms are likely involved (Fig. 2).
4.3.1. Antioxidant Activity
The antioxidant activity of WS is one mechanism by which WS may exert its anti-depressant activity. While
the complex pathophysiology of depression is not yet fully understood, oxidative stress has been linked to many psychiatric disorders, including depression [108, 121]. The brain is prone to oxidative stress due to its high oxygen consumption, which can lead to increased production of reactive oxygen species (ROS) and the increased availability of lipids, which are susceptible to ROS-related peroxidation [121]. The oxidative stress hypothesis of depressive disorders proposes that oxidative stress, along with activation of pro-inflammatory and pro-apoptotic mediators, is a critical step in a cascading series of events leading to depressive symptoms [121]. As previously discussed, WS has demonstrated both antioxidant and anti-inflammatory activities in various animal models, making it a good candidate for investigation for anti-depressant activity.
4.3.2. Serotonergic Activity
There is evidence from animal studies that WS may possess serotonergic activity, which could play a role in its anti-depressant effects. In a horse model of stress, in which horses were exposed to exercise-, separation-, and noise-induced stress, Priyanka et al. (2020) found that administration of a root powder of WS for 21 days protected against a stress-induced decrease in serum serotonin levels [56]. Similarly, in a mouse model of restraint stress, an undefined root extract of WS protected against a stress-induced reduction in hippocampal serotonin levels [47]. Withanolide A promoted mRNA expression of serotonin receptors and transporters in wild-type and mutant strains of Chaenorhabditis elegans [122]. Furthermore, molecular docking studies showed that withanolide A bound to human and C. elegans serotonin receptors and serotonin transporters with greater affinity than serotonin, and the Selective Serotonin Reuptake Inhibitor (SSRI) fluoxetine [122]. Unfortunately, the limited number of available human studies have not measured neurotransmitter levels in response to WS administration as a possible mechanism, warranting further investigation.
5. SLEEP-PROMOTING EFFECTS
5.1. Animal Studies
Animal studies have demonstrated sleep-promoting effects of WS, as described in Table 5. Wang et al. (2020) used a Drosophila model of sleep deprivation to screen seven herbal extracts for their sleep-promoting properties, including a hydroalcoholic root extract of WS [123]. WS increased total sleep time and decreased sleep latency while not affecting fly activity during wake time [123]. Kaushik et al. (2017) found that an alcoholic leaf extract of WS high in withanolides, including withaferin A and withanone, was ineffective at inducing sleep in mice [124]. However, aqueous leaf extracts containing triethylene glycol significantly induced non-rapid eye movement sleep in mice at 30 mg/mouse [124]. Furthermore, mice administered isolated triethylene glycol (also 30 mg/mouse) experienced similar effects on non-rapid eye movement sleep, suggesting that this molecule may play a role in the hypnotic effects of WS. Kumar and Kalonia (2007) investigated the sleep-promoting activities of an undefined root extract of WS in sleep-deprived rats, in which rats were given an intraperitoneal injection of WS before 24 hours of sleep deprivation using a grid suspended over water method [77]. WS significantly increased total sleep time and slow-wave sleep, while reducing sleep latency, REM sleep, and total waking time [103].
Table 5.
Sleep-promoting effects of Ashwagandha (Withania somnifera, WS) in animal studies.
| Nature of Extract, Standardization | Dosage | Model | Behavioral Effects | Refs. |
|---|---|---|---|---|
| Hydroalcoholic root extract, ≥2.5% withanolides | 1 mg/ml added to fly food, given from two days after eclosion until the end of the experiment | Drosophila (sleep deprivation) | ▲total sleep ▼sleep latency |
[123] |
| Alcoholic leaf extract and aqueous leaf extracts | Alcoholic extract: 200 mg/kg p.o. prior to sleep Aqueous extract and cyclodextrin-assisted aqueous extract: 30 mg/mouse p.o. prior to sleep Triethylene glycol: 10, 20, and 30 mg/mouse p.o. prior to sleep |
Mouse (sleep induction) | Water extracts and triethylene glycol ▲NREM sleep | [124] |
| Root extract, NR | 100 mg/kg i.p., 30 mins before assessment | Rat (sleep deprivation) | ▼sleep latency ▲duration of slow wave sleep ▲total sleep time ▼total waking |
[103] |
▲ – Increased; ▼ – Decreased; NR = Not reported; NREM = Non-rapid eye movement.
In addition to their sleep-promoting effects, extracts of WS have also been shown to attenuate the adverse effects associated with sleep deprivation. A well-defined water extract of WS (ASH-WEX) was given orally to rats for 15 days, after which they were sleep-deprived for 12 hours [45]. Rats that were administered WS demonstrated improved learning and memory and maintained their motor function compared to untreated sleep-deprived rats [45]. In a mouse model of sleep deprivation, pretreatment with an undefined root extract of WS protected against sleep deprivation-induced weight loss, improved locomotor deficits, and reduced anxiety-like behavior compared to untreated sleep-deprived mice [77].
5.2. Human Studies
Five human studies have investigated WS for its effects on insomnia in adults aged 18-85 years (Table 1). Study populations included participants who were healthy [63, 64], stressed [60], diagnosed with insomnia [65], or had been experiencing non-restorative sleep [67]. Four of those studies included between 50 and 80 participants, while Deshpande et al. (2020) evaluated 150 participants [67].
All the studies used a commercially available preparation of WS, administered in capsule form. Four of the studies used KSM-66 Ashwagandha® [60, 63-65], while Deshpande et al. (2020) used Shoden® [67]. WS was administered at a daily dose of 120-600 mg for 6 weeks [67], 8 weeks [60, 63], 10 weeks [65], or 12 weeks [64]. Salve et al. (2019) evaluated multiple dosages [60].
Several outcome measures were used to assess changes in sleep, and the majority of studies evaluated more than one sleep measure (Table 1). The most commonly used instrument was the sleep quality rating, a seven-point Likert scale with higher ratings indicating worse sleep quality [60, 63-65]. Langade et al. (2019) and Langade et al. (2021) also used the Pittsburgh Sleep Quality Index (PSQI) to assess sleep quality [63, 65]. The mental alertness on rising rating, a three-point scale to assess morning alertness, was used in three studies [63-65]. Three studies used objective sleep actigraphy: a non-invasive, wearable sensor was used to assess various sleep parameters, such as sleep latency, sleep efficiency, total sleep time, and wake after sleep onset [63, 65, 67]. Kelgane et al. (2020) included the Sleepiness Scale, a 4-item questionnaire, to assess the likelihood of falling asleep during daytime activities [64]. Deshpande et al. (2020) included the Restorative Sleep Questionnaire – Weekly (RSQ-W), a 9-item questionnaire, to evaluate whether restful sleep occurred [67].
Overall, supplementation with WS improved various measures of insomnia across a variety of study populations and age groups (Table 1). Due to the small number of human trials involving persons experiencing sleep disturbances and the variability in doses and outcome measures, a minimum dose needed to improve sleep cannot be determined based on these studies alone. Additional studies are needed in the target population to determine the effectiveness of WS on improving insomnia.
5.3. Proposed Mechanisms for Sleep-Promoting Effects
One possible mechanism by which WS may exert its positive effects on sleep is by activating GABAergic neurotransmission. Kumar and Kalonia (2008) found that the sleep-promoting activity of WS in rats was significantly reversed by picrotoxin (a GABA antagonist) and potentiated by muscimol (a GABA agonist), supporting a mechanistic role for GABA [103]. It is also evidenced that WS has chronomodulatory effects on the brain. Jagota and Kowshik (2017) demonstrated that a hydroalcoholic leaf extract of WS restored age-induced changes in several clock genes (e.g., rBmall, rPer1, rCry1, rPer2) in the suprachiasmatic nucleus of middle- and old-aged rats [125]. In a subsequent study, Kukkemane and Jagota (2020) found that in old-aged rats, a hydroalcoholic leaf extract of WS restored the daily rhythms and phases of SIRT1 (a clock modulator) and NRF2, which is a transcription factor controlled by a clock that regulates several endogenous antioxidant enzymes [126]. It is likely that other mechanisms also contribute to the sleep-promoting effects of WS, as shown in Fig. 2.
6. DISCUSSION
WS has been extensively researched due to its prominence as a rasayana or rejuvenating herb in Ayurvedic medicine [1, 19] and its popularity as an adaptogenic botanical supplement in Western countries [11]. The classification of WS as a rasayana or adaptogen is related to its reputed effects on stress. The term “stress” is generally understood as a challenge to the organism that requires a response. Stressors can be physiological (e.g., pathogens or an unfavorable physical environment) or psychological (e.g., fear, anxiety, or social discomfort) [127]. Stress can be “good” (e.g., a demanding situation leading to a beneficial outcome), “tolerable” (e.g., where the individual has sufficient resilience to overcome or adapt to the stressor), or “toxic” (e.g., where the stress response is insufficient, leading to disease) [128]. Rasayanas or adaptogens, such as Ashwagandha, are used to increase resilience to potentially toxic stressors, allowing the stress to become tolerable.
The effects of stress on the body are wide-ranging. While stress may be initiated centrally, the response to psychological stress can include both physiological changes and neuropsychiatric symptoms. Markers related to the sympathetic and parasympathetic nervous systems (neurotransmitters), the HPA axis (stress hormones), inflammation (cytokines), cardiac function, and glucose and lipid metabolism have all been used as objective biomarkers to study the physiological effects of stress [129]. Furthermore, stress has been implicated in the development of many neuropsychiatric disorders, including anxiety [130], depression [131], and insomnia [132]. The mechanisms by which stress may contribute to these disorders include hyperactivity of the HPA and sympathetic-adrenal-medullary (SAM) axes and dysregulation of the immune system [31, 32]. This review aimed to summarize the preclinical and clinical studies investigating the effects of Ashwagandha on stress and its effects on three common stress-related neuropsychiatric disorders: anxiety, depression, and insomnia.
6.1. Evidence for the Ability of WS to Ameliorate Stress and Stress-Related Neuropsychiatric Disorders
6.1.1. Preclinical Evidence
As summarized in Tables 2-5, WS has been widely evaluated in animal models of neuropsychiatric disorders, with most studies focusing on the effects of WS in anxiety and stress. Various WS preparations and compounds have been shown to attenuate stress-related biological and behavioral abnormalities, reduce anxiety- and depression-like behavior, and improve the onset and duration of sleep in these models.
An advantage of animal models is that biological changes in the brain can be measured, providing insights into mechanisms of action not readily measurable in humans. Evidence from pre-clinical studies suggests multiple potential mechanisms for the effects of WS on stress and stress-related neuropsychiatric disorders, including anti-apoptotic activity, reduced activation of HPA and SAM axes, antioxidant and anti-inflammatory effects, GABAergic activity, immune modulation, serotonergic activity, and chronomodulatory effects on sleep (Fig. 2). The effect of WS on GABA and serotonergic pathways, in particular, is noteworthy given that GABA activation has been shown to decrease HPA axis activation [130], while associated serotonin pathways have appeared to reduce in stress [131], contributing to depression. Thus, the modulation of GABAergic and serotonergic pathways by WS may underlie its ability to reduce anxiety, stress, and depression simultaneously. In addition, the ability of WS to reduce NFκB activation and levels of IL-1β is noteworthy since these markers are related to NLRP3 inflammasome activation, which has been associated with stress-induced anxiety and depression in rodents [133]. A study by Xia et al. (2021) found that withaferin A inhibited NLRP3 inflammasome activation in a mouse model of fulminant hepatitis [134]. These potential mechanisms of WS require further exploration in future studies.
6.1.2. Clinical Evidence
The present review focused on 14 clinical studies, examining the neuropsychiatric effects of WS (Table 1). As with the animal studies, anxiety and stress were the most frequently studied conditions, with fewer studies specifically directed toward sleep and depression, though several studies evaluated more than one of these conditions. Effects of WS were, in most cases, evaluated using standardized, validated behavioral questionnaires and objective biological measures, e.g., HPA activation (serum cortisol), SAM activation (pulse rate, blood pressure), or inflammation (CRP, inflammatory cytokines). The results of these clinical studies provide broad support for WS’s ability to reduce stress and anxiety and to improve sleep quality when administered for two or more months. There is limited clinical evidence for the effect of WS on depression. Further studies are needed to determine the possible benefits of WS in individuals diagnosed with specific depressive conditions, such as major depressive disorder or seasonal affective disorder. In addition to the limited number of blood biomarkers evaluated in these clinical studies, a more comprehensive assessment of inflammatory cytokines and neurotransmitter levels would add to our understanding of the mechanisms through which WS achieves the clinical endpoints observed.
Strengths of these clinical studies include that all were reported as double-blind and placebo-controlled. However, the exact nature of the placebo and blinding and randomization methods were not always reported (Table 1). Also, most studies examined WS in participants already experiencing symptoms of stress, anxiety, insomnia, or depression, thereby increasing the clinical relevance of the research. The age and sex of participants were not well controlled in these studies, however. Only three studies examined WS within a relatively narrow age range (25 years) and in middle-aged or older adults [64, 68, 70]. The other studies recruited participants covering a wide age range (e.g., 18 to 75). Given the known effects of age on cognition [135], mood [136], and sleep [137], it would be informative to evaluate WS in more closely defined age groups. In addition, there have been no studies assessing the effects of WS for neuropsychiatric conditions in pediatric populations. Only three studies [59, 66, 67] included similar numbers of male and female participants. Most of the studies had a preponderance of male participants, and four studies [60, 63, 64, 69] did not provide information on participant sex. Since sex is known to influence neuroendocrine stress-responses [138] and that stress-related neuropsychiatric disorders have a higher prevalence in women than men [139], it would be more appropriate to evaluate WS separately in each sex or to ensure even representation of men and women in the placebo and test groups.
While the evidence from pre-clinical and clinical studies generally supports a potential therapeutic role of WS for stress and stress-related neuropsychiatric disorders, significant questions remain unanswered, especially regarding the nature of active compounds found in WS, the variability of WS preparations tested, and the safety of WS products.
6.2. Active Compounds of WS
6.2.1. Identification of Active Compounds in WS
Withanolides are reported to be the main neurologically active compounds of WS [7, 12, 140]; however, only a small number of the withanolides in WS have been evaluated for their neurological effects, and the results are mixed. For example, withanone [141], withanolide A [142], sominone [142], and withanoside IV [142] all improved memory in cognitively impaired rodents. Conversely, withaferin A, withanolide A, three other withanolides, and sitoindosides VII-X did not appear to possess the GABAergic activity seen with extracts of WS [95, 104]. In contrast, a long-chain fatty acid of ferulic acid was active [95].
Only a few of the studies found in the literature tested pure WS compounds for their neuropsychiatric effects. A withanolide derivative (1-oxo-5β, 6β-epoxy-witha-2-ene-27-ethoxy-olide) showed anti-stress effects [50], while withaferin A reduced anxiety-like behavior in a rat stress model [75], and an anti-depressant compound (withanoside X) was identified through bioassay-guided fractionation [120]. An enriched WS extract containing 28-30% withanolide glycosides, sitoindosides VII-X, and withaferin reduced stress related end points, and depression- and anxiety-related behavior in rats [52, 81]. Interestingly, withanolide-free fractions improved endurance and reversed several stress-induced physiological changes [37], and showed anti-anxiety and anti-depressant effects in stressed mice [54], suggesting a role for other groups of compounds. For example, the sitoindosides VII and VIII, which are not withanolides, reduced stress and depressive symptoms in rodents [17]; and a flavonoid-rich fraction was found to reduce depression-like behavior in rats [120]. Triethylene glycol, a compound from WS leaves, was found to induce sleep in mice [124] and reduce anxiety- and depression-like behavior in mice [54], although the concentrations used were high compared to the parent WS extract. Oligosaccharides of WS, despite being abundant in aqueous root extracts [59, 62], have not been evaluated independently for biological effects. Interestingly, the neurotransmitter GABA was produced by in vitro cultures of WS leaves [143], suggesting it may also be present in cultivated WS plants and possibly contributing to the reported GABAergic effects of extracts.
In summary, there have been limited studies linking the withanolides to the neuropsychiatric effects of WS, and other compounds may play a role in these effects. Evidence for specific mechanisms of action of WS compounds relevant to neuropsychiatric disorders is even more limited. Future studies comparing pure compounds, or compound groups, at doses equivalent to their content in active parent extracts would be informative to assess their contribution to WS's functional effects and mechanisms of action. The possibility of synergistic or additive effects [144] between components must also be considered [144] since these may be lost when single compounds are tested, giving a false impression of lack of biological activity.
6.2.2. Test Product Variability
An important feature of the clinical studies and preclinical studies reviewed here is the chemical variation in the type of WS preparation tested. Most of the clinical studies (Table 1) used commercial Ashwagandha products that included an aqueous extract of the root (KSM-66®), an aqueous extract of the root and leaf (Sensoril®, Essentra®), and a hydroethanolic extract of the leaf and root (Shoden®). Two studies used non-commercial ethanolic extracts of WS, and one used granules of dried WS root. For studies that did not report the plant part used, this information was inferred from other studies using the same product where those details were provided. Based on this assumption, all products tested included derivatives of WS root, although some commercial products also had WS leaf extract. The products tested varied in the compound groups measured or used for standardization (withanolides, withanosides, sitoindosides, or oligosaccharides), as well as in the content of these compounds (5% to 35%). Some studies did not report any standardization [60, 64, 68-70]. The products, in some cases, appeared to include excipients that were not fully elucidated, nor was it confirmed in all cases that the placebo used consisted of the inactive components of the test WS product. While the reviewed studies generally found similar benefits of WS on markers of anxiety, depression, and sleep, the variation in test products makes it difficult to compare the studies directly or draw any conclusions regarding the best extraction method, dosage or number of active constituents needed for clinical benefit.
Similarly, a wide variety of WS preparations were tested in preclinical models (Tables 2-5). While most tested extracts were derived from WS root, three studies reported anti-anxiety effects of WS leaf extracts (Table 3). The preparation of the extracts tested in the studies also varied. Extraction solvents included water, methanol, ethanol, or mixtures of these solvents, whereas in two cases, traditional extractions using a mixture of ghee and water [117] or water, ghee and honey were used [53]. Two studies [36, 56] were performed on commercial products (KSM-66®; Sensoril®) used in human trials; however, the description of the products differed. In a horse study, KSM-66® is described as a “root powder” [56], whereas the clinical studies refer to it as capsules of an aqueous root extract (Table 1). Interestingly, the literature description of KSM-66® as an “aqueous” extract differs from the product website describing the use of a traditional process involving milk [71]. Sensoril® is described in mouse study as a methanol:water (40:60) extract [36], whereas the clinical studies (Table 1) all refer to this product as containing an aqueous extract. Communication by the authors with representatives at Natreon Inc. confirmed that the Sensoril® product used in the clinical studies is an aqueous extract. The content of specific compounds or compound groups in the extracts tested was reported in less than half of the preclinical studies found. A few of these studies describe the content of individual compounds (e.g. isowithanone, withaferin and sitoindosides) [55, 81]. In contrast, other studies reported the percentage content of compound groups, such as withanolides, from 0.23% to > 10%, withanolide glycosides 28-30%, and total steroids 1.13% in a glycowithanolide-rich fraction [52, 81]. Further highlighting the variability in reporting for product composition, Chengappa et al. (2018) and Gannon et al. (2019) (two studies that used the same study population to evaluate different outcomes) described the composition of Sensoril® using varying levels of detail [59, 66].
A noteworthy point is that despite the wide variation in the preparation and chemical profiles of the extracts used in the clinical and preclinical studies, most showed biological activity, suggesting that the active compounds are broadly soluble in aqueous and polar organic solvents or that a subset of active compounds are extracted in any given solvent. One exception is that WS root extracts made with 100% ethanol did not show anti-anxiety activity [74], and a 100% alcoholic leaf extract did not induce sleep [124], whereas aqueous extracts from the same plant materials were active. Interestingly, a separate study did show anti-anxiety effects of an ethanolic WS root extract [80], which may have been due to differences in dose or starting WS plant material.
These examples illustrate a challenge commonly encountered in comparing studies performed on a particular botanical, where natural chemical variation in botanical materials is further amplified by the use of multiple extraction methods. The problem can be compounded by the lack of information, or even misinformation, on the test material and the absence of chemical information on the materials tested. As a result, data can at best be regarded as specific to the product tested with extrapolations to other products only possible where there is demonstrated chemical similarity. These issues are being addressed by many natural product journals, which now require chemical analysis information in conjunction with biological data. The application of both targeted and untargeted chemical profiling of botanicals can be used to document the composition of botanical extracts more comprehensively [12, 145].
6.3. Pharmacological Properties
6.3.1. Bioavailability of WS Compounds
It is desirable to evaluate the in vivo oral bioavailability, Plasma Pharmacokinetics (PK) and tissue distribution (particularly to target organs) of putative active compounds, which are often identified through in vitro bioassays or parenteral administration in vivo. There have been relatively few studies on the PK and brain bioavailability of WS compounds. Plasma bioavailability and PK of withaferin A and withanolide A were recorded after intragastric administration of a WS root aqueous extract in mice [146]. Plasma bioavailability of withaferin A was also shown in rats after oral administration of a polyherbal formulation containing WS root [147]. Plasma PK of withanolide A was established in rats following oral administration of the compound, which also showed wide tissue distribution; notably, brain levels were considerably lower than in lung, kidney, liver, heart, and spleen [148]. Four major withanamides were detected in the mouse brain following the intraperitoneal administration of an extract of WS [149]. Oral bioavailability of a root extract of WS standardized to 4.5% withaferin A (AshwaMAX®) was assessed in 13 patients with advanced-stage high-grade osteosarcoma [150]. Patients consumed 1600 mg AshwaMAX®, corresponding to 72 mg of withaferin A. Withaferin A was not detected in plasma samples collected over the following 24 hours [150]. No other bioavailability studies of WS compounds in humans were found.
6.3.2. Safety
The widespread use of WS as a traditional medicine and dietary supplement, as well as current scientific literature, supports its general safety. In a review of clinical trials that used root preparations of WS for a wide variety of conditions, Tandon and Yadav (2020) noted that reasonable safety outcomes were seen with no serious adverse events or changes in vital signs, hematological and biochemical parameters [5]. Mild to moderate transient adverse events, including somnolence, giddiness, vertigo, and drowsiness, were reported in some studies, while other studies involving adults and children did not report any adverse events [5]. Similarly, an earlier review by Alam et al. (2012) reported that WS has been used in all age groups and both sexes, even during pregnancy, without any reported side effects [7]. The safety of KSM-66® Ashwagandha root extract (Ixoreal Biomed, Inc.) was assessed in a randomized, placebo-controlled study [151]. Eighty healthy participants (40 of each sex), aged 18-45 years, were randomized 1:1 to receive 300 mg KSM-66® or a matching placebo twice per day for 8 weeks. To assess safety, researchers assessed vital signs and various hematological and biochemical markers (hemoglobin, neutrophil percentage, platelet count, alkaline phosphatase, Aspartate Aminotransferase (AST), Alanine Aminotransaminase (ALT), thyroid hormone panel). No significant changes were observed in any of the safety parameters compared to placebo, and no adverse events were reported. No serious adverse effects were reported in any of the clinical studies reviewed here. One study [64] formally measured the tolerability of a WS root extract using the Patient's Global Assessment of Tolerability to Therapy and found it to have a high tolerability score.
WS extracts have also been shown to be safe in animal toxicity studies. Acute and sub-acute toxicity of a hydroalcoholic root extract of WS was investigated in female Wistar rats [152]. No behavioral signs of toxicity or gross pathological changes were observed with acute doses up to 2000 mg/kg. Similarly, no signs of subacute toxicity were observed in rats given 500, 1000, or 2000 mg/kg of the extract daily for 28 days. In a separate study, a hydroalcoholic root extract of WS was used to assess prenatal developmental toxicity in rats at doses of 500, 1000, and 2000 mg/kg daily for 28 days [153]. No signs of toxicity, gross pathology changes, or mortality were observed in the pregnant rats or fetuses. Further studies are required to examine the safety of WS extracts prepared using other extraction methods and plant parts.
While these studies suggest that WS does not cause significant toxicity, the possibility of adverse events arising from herb-drug interactions must be considered [154]. Pharmacodynamic interactions between WS and some groups of drugs may exist, as evidenced by additive effects seen in rodent studies between WS and the drugs imipramine [117-119], diazepam [78], and fluoxetine [119]. The GABA-mimetic and serotonergic activities seen with some WS extracts in rodents would suggest caution when co-administering WS with drugs that work by similar mechanisms. Pharmacokinetic interactions may also occur when botanicals alter the activity of drug transporters or drug-metabolizing enzymes [155]. It has been suggested that an IC50 of less than 100 μg/mL for extracts or 100 μM for active constituents should be classified as potent inhibition that could lead to undesirable herb-drug interactions [156, 157]. Various root extracts of WS had IC50 values greater than 100 μg/mL for the cytochrome P450 isoenzymes CYP3A4, CYP2D6, CYP1A2, and CYP2C9 in human liver microsomes (HLM). At the same time, withaferin A and withanolide A did not inhibit these enzymes at doses up to 50 µM [158, 159]. An aqueous extract of WS did not inhibit human recombinant CYP3A4 at doses as high as 1000 µg/mL [160]. Methanol and ethylacetate extracts of WS root had IC50 values of 79 and 58 μg/mL, respectively, for CYP2B6 in HLM, whereas aqueous and ethanolic extracts were not inhibitory at up to 200 μg/mL [161]. None of the extracts inhibited β-esterase-dependent rifampicin metabolism in HLM or induced mRNA of CYP2B6 or CYP3A4 in HepG2 cells. In a detailed biopharmaceutical study of withanone, the compound was found to have IC50 values >100 µM for CYP2C9/11 in rat and human liver microsomes and IC50 values between 28.5 and 80 µM for other CYP isoenzymes (the lowest being 28.5 µM for CYP3A4 in human liver microsomes) [162].
The clinical relevance of these findings will depend on the systemic concentrations of these compounds achieved during regular use of WS. Further studies are required to evaluate this important aspect of the use of WS fully.
6.4. Clinical Considerations
While WS is generally regarded as safe and appears to have anti-anxiety, anti-depressant, and sleep-promoting properties in animal and limited human trials, there was significant heterogeneity of the products used, the level of product standardization, and the doses selected (Table 1). Additionally, it is unknown what herb-herb and herb-drug interactions may exist for WS beyond those previously described for diazepam [78, 101], imipramine [117-119], and fluoxetine [83, 119], and more specifically for the products that have been used in the available human trials. Due to the significant variability in the products used and lack of interaction studies, we are currently unable to make definitive clinical recommendations for the use of WS, both as an independent intervention or as an adjunctive therapy to those already in use for stress-related neuropsychiatric conditions. Additional rigorous studies using standardized products and doses in populations diagnosed with anxiety, depression, and insomnia rather than healthy participants are needed to provide enough definitive evidence to safely integrate WS into clinical management of stress-related neuropsychiatric conditions.
CONCLUSION
Extracts made from Ashwagandha root and/or leaf have shown remarkable anti-stress and anti-anxiety activity in numerous animal models and clinical studies. Fewer studies have examined the effects of WS on depression and sleep, but as before, positive activity was observed here as well. The ability of WS to potentially ameliorate all of these conditions, which often occur as co-morbidities, is of significance. Where a polypharmacy approach may be used in allopathic medicine, WS may offer a single treatment to address each of these conditions simultaneously. WS appears to exert this pan-condition effect by modulating the HPA and SAM axes, as well as GABAergic and serotonergic pathways, all of which are interconnected. Critical gaps in knowledge remain, however. The optimum extraction method and dosage to address any or all of these disorders has yet to be determined, as is the severity of the conditions that are responsive to WS. The association of biological activity to chemical content is a priority area of research for Ashwagandha. While withanolide derivatives are widely stated to be the active compounds of WS, only a few of the many compounds of this group have been specifically tested for their neuropsychiatric effects. Testing of additional withanolides, examination of their additive or synergistic effects, and structure-activity relationships are all warranted. The fact that many of the standardized, active extracts contain 70-95% of uncharacterized materials highlights the possibility of additional, unidentified active compounds. The oligosaccharides found in the roots, for example, have been largely ignored and may modulate the activity of WS, perhaps at the level of the gut. There is also a dearth of studies examining the pharmacokinetic characteristics and biological distribution of WS compounds in animal models and humans. Finally, with the increasing use of WS by the general public, its potential for herb-drug interactions requires attention. Nevertheless, the studies conducted so far suggest that WS by itself has a good safety profile, adding to the potential of this traditional herb to provide resilience against stress and stress-related neuropsychiatric ailments.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- ACh
Acetylcholine
- AChE
Acetylcholinesterase
- ADP
Adenosine diphosphate
- AEC
Adenylate energy charge
- ALP
Alkaline phosphatase
- ALT/GPT
Alanine aminotransaminase
- AP-1
Activator protein 1
- ART
Anti-reserpine test
- ASA
Aspirin administration
- AST/GOT
Aspartate aminotransferase
- ATP
Adenosine triphosphate
- BAD
Bcl-2 associated agonist of cell death
- BAI
Beck Anxiety Inventory
- Bax
Bcl-2-associated X protein
- Bcl-xL
B-cell lymphoma extra-large
- BDI
Beck Depression Inventory
- BDNF
Brain-derived neurotrophic factor
- BD-NOS
Bipolar disorder, not otherwise specified
- BMI
Body Mass Index
- CGI
Clinical Global Impressions scale
- ChAT
Choline acetyl transferase
- CHR
Cold-Hypoxia-Restraint test
- CRP
C-reactive protein
- CRS
Cold restraint stress test
- CST
Cold stress test
- COX-2
Cyclooxygenase 2
- DASS
Depression Anxiety Stress Scale
- DASS-21
Depression Anxiety Stress Scale-21
- DHEA-S
Dehydroepiandrosterone sulfate
- EPM
Elevated plus maze test
- FBG
Fasting Blood Glucose
- FCQ-T
Food Cravings Questionnaire – Trait
- FSS
Foot shock stress test
- FST
Forced swim test
- FQ
Fatigue Questionnaire
- GAD
General Anxiety Disorder
- GFAP
Glial fibrillary acidic protein
- GHQ
General Health Questionnaire
- GPx
Glutathione peroxidase
- GSH
Reduced glutathione
- GSSG
Oxidized glutathione
- HAM-A/HARS
Hamilton Anxiety Rating Scale
- HDL
High density lipoprotein
- HDL-C
High density lipoprotein cholesterol
- HPA
Hypothalamic-pituitary-adrenal
- HPT
Hot plate test
- HSP70
Heat shock protein 70
- HST
Hypoxia stress test
- Iba1
Ionized calcium binding adaptor molecule 1
- IFN-γ
Interferon gamma
- IL-1β
Interleukin 1 beta
- IL-2
Interleukin 2
- IL-6
Interleukin 6
- iNOS
Inducible nitric oxide synthase
- JNK
c-Jun N-terminal kinase
- LDL
Low density lipoprotein
- LDL-C
Low density lipoprotein cholesterol
- LDPT
Light/dark preference test
- LHT
Learned helplessness test
- LPO
Lipid peroxidation
- LPS
Lipopolysaccharide
- MADRS
Montgomery-Asberg Depression Rating Scale
- MARS
Mental Alertness on Rising
- MBT
Marble burying test
- MCP-1
Monocyte chemoattractant protein
- mHAM-A
modified Hamilton Anxiety Rating Scale
- MIL
Milk-induced leukocytosis
- MINI
Mini-International Neuropsychiatric Interview
- MWM
Morris Water Maze test
- MYMOP
Measure Yourself Medical Outcomes Profile
- NBW
Narrow beam walking test
- NCAM
Neural cell adhesion molecule
- NDS
Neurological deficit score
- NFκB
Nuclear factor kappa B
- nNOS
Nitric oxide synthase
- NO
Nitric oxide
- NOS
Not otherwise specified
- NOX2
NADPH oxidase 2
- NR
Not reported
- NREM
Non-rapid eye movement
- nsd
Non-significant decrease
- NSFLT
Novelty-suppressed feeding latency test
- NTDT
Novel tank diving test
- OFT
Open field test
- OHQ
Oxford Happiness Questionnaire
- OST
Overcrowding stress test
- PANSS
Positive and Negative Syndrome Scale
- PAT
Passive avoidance test
- PGAET
Physician’s Global Assessment of Efficacy to Therapy
- PGATT
Patient’s Global Assessment of Tolerability to Treatment
- PPARγ
Peroxisome proliferator-activated receptor gamma
- PSS
Perceived Stress Scale
- PSQI
Pittsburgh Sleep Quality Index
- PTZ
Pentylenetetrazol administration
- RAWM
Radial arm water maze
- RBC
Red blood cell
- ROS
Reactive oxygen species
- RST
Restraint stress test
- RSQ-W
Restorative Sleep Questionnaire-Weekly
- RTT
Rotarod test
- ROS
Reactive oxygen species
- S100B
S100 Calcium-binding protein B
- SAM
Sympathetic-adrenal-medullary
- SE
Sleep efficiency
- SF-36
Short Form-36
- SIT
Social interaction test
- SOD
superoxide dismutase
- SOL
Sleep Onset Latency
- SPS
Single prolonged stress
- SSRI
Selective serotonin reuptake inhibitor
- SST
Set Shifting Test
- STDT
Strategic Target Detection Test
- TAN
Total adenine nucleotide
- TBARs
Thiobarbituric acid reactive substances
- TBT
Total Bed Time
- TC
Total Cholesterol
- TCT
Tactile stress test
- TG
Triglycerides
- TIB
Total Time in Bed
- TFEQ
Three-Factor Eating Questionnaire
- TST
Total Sleep Time
- TNFα
Tumor necrosis factor alpha
- WASO
Wake After Sleep Onset
- WBC
White blood cell
- WHOQOL-Bref
WHO Quality of Life-BREF
- YMRS
Young Mania Rating Scale
- QoL
Quality of Life
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
This work was supported by the National Institutes of Health [T32AT002688; U19AT010829; KL2TR002370; R61AT009628]. The authors have no conflicts of interest. The present manuscript has not been previously published and is not under consideration for publication elsewhere.
REFERENCES
- 1.Balasubramani S.P., Venkatasubramanian P., Kukkupuni S.K., Patwardhan B. Plant-based Rasayana drugs from Ayurveda. Chin. J. Integr. Med. 2011;17(2):88–94. doi: 10.1007/s11655-011-0659-5. [DOI] [PubMed] [Google Scholar]
- 2.Panossian AG, Efferth T, Shikov AN, et al. 2021.
- 3.Doshi G.M., Une H.D., Shanbhag P.P. Rasayans and non-rasayans herbs: Future immunodrug - Targets. Pharmacogn. Rev. 2013;7(14):92–96. doi: 10.4103/0973-7847.120506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dar N.J. MuzamilAhmad. Neurodegenerative diseases and Withania somnifera (L.): An update. J. Ethnopharmacol. 2020;256:112769. doi: 10.1016/j.jep.2020.112769. [DOI] [PubMed] [Google Scholar]
- 5.Tandon N., Yadav S.S. Safety and clinical effectiveness of Withania somnifera (Linn.) Dunal root in human ailments. J. Ethnopharmacol. 2020;255:112768. doi: 10.1016/j.jep.2020.112768. [DOI] [PubMed] [Google Scholar]
- 6.Dar N.J., Hamid A., Ahmad M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell. Mol. Life Sci. 2015;72(23):4445–4460. doi: 10.1007/s00018-015-2012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alam N., Hossain M., Khalil M.I., Moniruzzaman M., Sulaiman S.A., Gan S.H. Recent advances in elucidating the biological properties of Withania somnifera and its potential role in health benefits. Phytochem. Rev. 2012;11(1):97–112. doi: 10.1007/s11101-011-9221-5. [DOI] [Google Scholar]
- 8.Supplements NOoD NIH Office of dietary supplement labels database. 2021.
- 9.Smith S., Kawa K., Eckl V., Morton C., Stredney R. Herbal Supplement Sales in US Increased 8.5% in 2017, Topping $8 Billion: Strongest sales growth in more than 15 years bolstered by continued popularity of Ayurvedic herbs and new formulations of botanicals with general health and nutrition benefits. HerbalGram. 2018;(119):62–71. [Google Scholar]
- 10.Smith T., Kawa K., Eckl V., Morton C., Stredney R. Herbal supplement sales in US increase 7.7% in 2016: consumer preferences shifting toward ingredients with general wellness benefits, driving growth of adaptogens and digestive health products. HerbalGram. 2017;(115):56–65. [Google Scholar]
- 11.Smith T., Georgia M., Eckl V., Morton Reynolds C. US sales of herbal supplements increase by 8.6% in 2019. HerbalGram. 2020;127:54–69. [Google Scholar]
- 12.Tetali S.D., Acharya S., Ankari A.B., Nanakram V., Raghavendra A.S. Metabolomics of Withania somnifera (L.) dunal: Advances and applications. J. Ethnopharmacol. 2021;267:113469. doi: 10.1016/j.jep.2020.113469. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee S., Srivastava S., Khalid A., et al. Comprehensive metabolic fingerprinting of Withania somnifera leaf and root extracts. Phytochemistry. 2010;71(10):1085–1094. doi: 10.1016/j.phytochem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Trivedi M.K., Panda P., Sethi K.K., Jana S. Metabolite Profiling in Withania somnifera Roots Hydroalcoholic Extract Using LC/MS, GC/MS and NMR Spectroscopy. Chem. Biodivers. 2017;14(3) doi: 10.1002/cbdv.201600280. [DOI] [PubMed] [Google Scholar]
- 15.Kaul S.C., Ishida Y., Tamura K., et al. Novel methods to generate active ingredients-enriched ashwagandha leaves and extracts. PLoS One. 2016;11(12):e0166945. doi: 10.1371/journal.pone.0166945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosal S., Lal J., Srivastava R., et al. Immunomodulatory and CNS effects of sitoindosides IX and X, two new glycowithanolides from Withania somnifera. Phytother. Res. 1989;3(5):201–206. doi: 10.1002/ptr.2650030510. [DOI] [Google Scholar]
- 17.Bhattacharya S.K., Goel R.K., Kaur R., Ghosal S. Anti‐stress activity of sitoindosides VII and VIII, new acylsterylglucosides from Withania somnifera. Phytother. Res. 1987;1(1):32–37. doi: 10.1002/ptr.2650010108. [DOI] [Google Scholar]
- 18.Bhatia A., Bharti S.K., Tewari S.K., Sidhu O.P., Roy R. Metabolic profiling for studying chemotype variations in Withania somnifera (L.) Dunal fruits using GC-MS and NMR spectroscopy. Phytochemistry. 2013;93:105–115. doi: 10.1016/j.phytochem.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee P.K., Banerjee S., Biswas S., Das B., Kar A., Katiyar C.K. Withania somnifera (L.) Dunal - Modern perspectives of an ancient Rasayana from Ayurveda. J. Ethnopharmacol. 2021;264:113157. doi: 10.1016/j.jep.2020.113157. [DOI] [PubMed] [Google Scholar]
- 20.Wijeratne E.M., Xu Y.M., Scherz-Shouval R., et al. Structure-activity relationships for withanolides as inducers of the cellular heat-shock response. J. Med. Chem. 2014;57(7):2851–2863. doi: 10.1021/jm401279n. [DOI] [PubMed] [Google Scholar]
- 21.Mandlik D.S., Namdeo A.G. Pharmacological evaluation of Ashwagandha highlighting its healthcare claims, safety, and toxicity aspects. J. Diet. Suppl. 2020 doi: 10.1080/19390211.2020.1741484. [DOI] [PubMed] [Google Scholar]
- 22.Singh N., Bhalla M., de Jager P., Gilca M. An overview on ashwagandha: a Rasayana (rejuvenator) of Ayurveda. Afr. J. Tradit. Complement. Altern. Med. 2011;8(5) Suppl.:208–213. doi: 10.4314/ajtcam.v8i5S.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra L.C., Singh B.B., Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern. Med. Rev. 2000;5(4):334–346. [PubMed] [Google Scholar]
- 24.Yenisetti S.C., Manjunath M.J., Muralidhara C. Neuropharmacological properties of withania somnifera - indian ginseng: an overview on experimental evidence with emphasis on clinical trials and patents. Recent Patents CNS Drug Discov. 2016;10(2):204–215. doi: 10.2174/1574889810666160615014106. [DOI] [PubMed] [Google Scholar]
- 25.Kuboyama T., Tohda C., Komatsu K. Effects of Ashwagandha (roots of Withania somnifera) on neurodegenerative diseases. Biol. Pharm. Bull. 2014;37(6):892–897. doi: 10.1248/bpb.b14-00022. [DOI] [PubMed] [Google Scholar]
- 26.Durg S., Dhadde S.B., Vandal R., Shivakumar B.S., Charan C.S. Withania somnifera (Ashwagandha) in neurobehavioural disorders induced by brain oxidative stress in rodents: a systematic review and meta-analysis. J. Pharm. Pharmacol. 2015;67(7):879–899. doi: 10.1111/jphp.12398. [DOI] [PubMed] [Google Scholar]
- 27.Zahiruddin S., Basist P., Parveen A., et al. Ashwagandha in brain disorders: A review of recent developments. J. Ethnopharmacol. 2020:257. doi: 10.1016/j.jep.2020.112876. [DOI] [PubMed] [Google Scholar]
- 28.Musazzi L., Tornese P., Sala N., Popoli M. What acute stress protocols can tell us about PTSD and stress-related neuropsychiatric disorders. Front. Pharmacol. 2018;9(JUN):758. doi: 10.3389/fphar.2018.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basta M., Chrousos G.P., Vela-Bueno A., Vgontzas A.N. Chronic insomnia and the stress system. Sleep Med. Clin. 2007;2(2):279–291. doi: 10.1016/j.jsmc.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaribeygi H., Panahi Y., Sahraei H., Johnston T.P., Sahebkar A. The impact of stress on body function: A review. EXCLI J. 2017;16:1057–1072. doi: 10.17179/excli2017-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L., Zhao Y., Wang Y., et al. The effects of psychological stress on depression. Curr. Neuropharmacol. 2015;13(4):494–504. doi: 10.2174/1570159X1304150831150507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patriquin M.A., Mathew S.J. The neurobiological mechanisms of generalized anxiety disorder and chronic stress. Chronic Stress (Thousand Oaks) 2017;1:1. doi: 10.1177/2470547017703993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levenson J.C., Kay D.B., Buysse D.J. The pathophysiology of insomnia. Chest. 2015;147(4):1179–1192. doi: 10.1378/chest.14-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ménard C., Hodes G.E., Russo S.J. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience. 2016;321:138–162. doi: 10.1016/j.neuroscience.2015.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsigos C, Kyrou I, Kassi E, Chrousos GP. 2000.
- 36.Bhattacharyya S., Pal D., Banerjee D., Majumder U.K., Ghosal S. Comparative effect of Withania somnifera and Panax ginseng on swim-stress induced impaired energy status of mice. Pharmacologyonline. 2009;2:421–432. [Google Scholar]
- 37.Singh B., Chandan B.K., Gupta D.K. Adaptogenic activity of a novel withanolide-free aqueous fraction from the roots of Withania somnifera Dun. (Part II). Phytother. Res. 2003;17(5):531–536. doi: 10.1002/ptr.1189. [DOI] [PubMed] [Google Scholar]
- 38.Singh B., Saxena A.K., Chandan B.K., Gupta D.K., Bhutani K.K., Anand K.K. Adaptogenic activity of a novel, withanolide-free aqueous fraction from the roots of Withania somnifera Dun. Phytother. Res. 2001;15(4):311–318. doi: 10.1002/ptr.858. [DOI] [PubMed] [Google Scholar]
- 39.Archana R., Namasivayam A. Antistressor effect of Withania somnifera. J. Ethnopharmacol. 1999;64(1):91–93. doi: 10.1016/S0378-8741(98)00107-X. [DOI] [PubMed] [Google Scholar]
- 40.Singh N., Nath R., Lata A., Singh S.P., Kohli R.P., Bhargava K.P. Withania somnifera (ashwagandha), a rejuvenating herbal drug which enhances survival during stress (an adaptogen). Pharm. Biol. 1982;20(1):29–35. [Google Scholar]
- 41.Anju. Adaptogenic and anti-stress activity of Withania somnifera in stress induced mice. Res. J. Pharm. Biol. Chem. Sci. 2011;2(4):676–684. [Google Scholar]
- 42.Puri S., Kumar B., Debnath J., et al. Comparative pharmacological evaluation of adaptogenic activity of Holoptelea integrifolia and Withania somnifera. International Journal of Drug Development and Research. 2011;3(1):84–98. [Google Scholar]
- 43.Can A., Dao D.T., Arad M., Terrillion C.E., Piantadosi S.C., Gould T.D. The mouse forced swim test. J. Vis. Exp. 2012;(59):e3638. doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Commons K.G., Cholanians A.B., Babb J.A., Ehlinger D.G. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem. Neurosci. 2017;8(5):955–960. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manchanda S., Mishra R., Singh R., Kaur T., Kaur G. Aqueous leaf extract of withania somnifera as a potential neuroprotective agent in sleep-deprived rats: a mechanistic study. Mol. Neurobiol. 2017;54(4):3050–3061. doi: 10.1007/s12035-016-9883-5. [DOI] [PubMed] [Google Scholar]
- 46.Baitharu I., Jain V., Deep S.N., et al. Withania somnifera root extract ameliorates hypobaric hypoxia induced memory impairment in rats. J. Ethnopharmacol. 2013;145(2):431–441. doi: 10.1016/j.jep.2012.10.063. [DOI] [PubMed] [Google Scholar]
- 47.Bhatnagar M., Sharma D., Salvi M. Neuroprotective effects of Withania somnifera dunal.: A possible mechanism. Neurochem. Res. 2009;34(11):1975–1983. doi: 10.1007/s11064-009-9987-7. [DOI] [PubMed] [Google Scholar]
- 48.Khan B., Ahmad S.F., Bani S., et al. Augmentation and proliferation of T lymphocytes and Th-1 cytokines by Withania somnifera in stressed mice. Int. Immunopharmacol. 2006;6(9):1394–1403. doi: 10.1016/j.intimp.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Kaur P., Mathur S., Sharma M., Tiwari M., Srivastava K.K., Chandra R. A biologically active constituent of Withania somnifera (ashwagandha) with antistress activity. Indian J. Clin. Biochem. 2001;16(2):195–198. doi: 10.1007/BF02864860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaur P., Sharma M., Mathur S., et al. Effect of 1-oxo-5beta, 6beta-epoxy-witha-2-ene-27-ethoxy-olide isolated from the roots of Withania somnifera on stress indices in Wistar rats. J. Altern. Complement. Med. 2003;9(6):897–907. doi: 10.1089/107555303771952244. [DOI] [PubMed] [Google Scholar]
- 51.Dey A., Chatterjee S.S., Kumar V. Analgesic activity of a Withania somnifera extract in stressed mice. Orient. Pharm. Exp. Med. 2016;16(4):295–302. doi: 10.1007/s13596-016-0245-7. [DOI] [Google Scholar]
- 52.Bhattacharya S.K., Muruganandam A.V. Adaptogenic activity of Withania somnifera: an experimental study using a rat model of chronic stress. Pharmacol. Biochem. Behav. 2003;75(3):547–555. doi: 10.1016/S0091-3057(03)00110-2. [DOI] [PubMed] [Google Scholar]
- 53.Ramanathan M., Srinivasan J., Saravanababu C., Viswanad B., Suresh B. Comparative behavioral activity of methanolic and aqueous Withania somnifera root extracts in stressed rats. Indian J. Pharm. Sci. 2003;65(6):601–604. [Google Scholar]
- 54.Dey A., Chatterjee S.S., Kumar V. Triethylene glycol-like effects of Ashwagandha (Withania somnifera (L.) Dunal) root extract devoid of withanolides in stressed mice. Ayu. 2018;39(4):230–238. doi: 10.4103/ayu.AYU_219_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alzoubi K.H., Al Hilo A.S., Al-Balas Q.A., El-Salem K., El-Elimat T., Alali F.Q. Withania somnifera root powder protects againist post-traumatic stress disorder-induced memory impairment. Mol. Biol. Rep. 2019;46(5):4709–4715. doi: 10.1007/s11033-019-04915-3. [DOI] [PubMed] [Google Scholar]
- 56.Priyanka G., Anil Kumar B., Lakshman M., Manvitha V., Kala Kumar B. Adaptogenic and immunomodulatory activity of ashwagandha root extract: an experimental study in an equine model. Front. Vet. Sci. 2020;7:541112. doi: 10.3389/fvets.2020.541112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chandrasekhar K., Kapoor J., Anishetty S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J. Psychol. Med. 2012;34(3):255–262. doi: 10.4103/0253-7176.106022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choudhary D., Bhattacharyya S., Joshi K. Body weight management in adults under chronic stress through treatment with ashwagandha root extract: a double-blind, randomized, placebo-controlled trial. J. Evid. Based Complementary Altern. Med. 2017;22(1):96–106. doi: 10.1177/2156587216641830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chengappa KNR, Brar JS, Gannon JM, Schlicht PJ. 2018. [DOI] [PubMed]
- 60.Salve J., Pate S., Debnath K., Langade D. Adaptogenic and anxiolytic effects of ashwagandha root extract in healthy adults: a double-blind, randomized, placebo-controlled clinical study. Cureus. 2019;11(12):e6466. doi: 10.7759/cureus.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lopresti A.L., Smith S.J., Malvi H., Kodgule R. An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract: A randomized, double-blind, placebo-controlled study. Medicine (Baltimore) 2019;98(37):e17186. doi: 10.1097/MD.0000000000017186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Auddy B., Hazra J., Mitra A., Abedon B., Ghosal S. A standardized withania somnifera extract significantly reduces stress-related parameters in chronically stressed humans: a double-blind, randomized, placebo-controlled study. Journal of the American Neutraceutical Association. 2008;11(1):50–56. [Google Scholar]
- 63.Langade D., Thakare V., Kanchi S., Kelgane S. Clinical evaluation of the pharmacological impact of ashwagandha root extract on sleep in healthy volunteers and insomnia patients: A double-blind, randomized, parallel-group, placebo-controlled study. J. Ethnopharmacol. 2021;264:113276. doi: 10.1016/j.jep.2020.113276. [DOI] [PubMed] [Google Scholar]
- 64.Kelgane S.B., Salve J., Sampara P., Debnath K. Efficacy and tolerability of ashwagandha root extract in the elderly for improvement of general well-being and sleep: a prospective, randomized, double-blind, placebo-controlled study. Cureus. 2020;12(2):e7083. doi: 10.7759/cureus.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Langade D., Kanchi S., Salve J., Debnath K., Ambegaokar D. Efficacy and safety of ashwagandha (withania somnifera) root extract in insomnia and anxiety: a double-blind, randomized, placebo-controlled study. Cureus. 2019;11(9):e5797. doi: 10.7759/cureus.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gannon J.M., Brar J., Rai A., Chengappa K.N.R. Effects of a standardized extract of Withania somnifera (Ashwagandha) on depression and anxiety symptoms in persons with schizophrenia participating in a randomized, placebo-controlled clinical trial. Ann. Clin. Psychiatry. 2019;31(2):123–129. [PubMed] [Google Scholar]
- 67.Deshpande A., Irani N., Balkrishnan R., Benny I.R. A randomized, double blind, placebo controlled study to evaluate the effects of ashwagandha (Withania somnifera) extract on sleep quality in healthy adults. Sleep Med. 2020;72:28–36. doi: 10.1016/j.sleep.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 68.Andrade C., Aswath A., Chaturvedi S.K., Srinivasa M., Raguram R. A double-blind, placebo-controlled evaluation of the anxiolytic efficacy ff an ethanolic extract of Withania somnifera. Indian J. Psychiatry. 2000;42(3):295–301. [PMC free article] [PubMed] [Google Scholar]
- 69.Khyati S., Anup T. A randomized double blind placebo controlled study of ashwagandha on generalized anxiety disorder. International Ayurvedic Medical Journal. 2013;1(5):1–7. [Google Scholar]
- 70.Fuladi S., Emami S.A., Mohammadpour A.H., Karimani A., Manteghi A.A., Sahebkar A. Assessment of Withania somnifera root extract efficacy in patients with generalized anxiety disorder: A randomized double-blind placebo-controlled trial. Curr. Clin. Pharmacol. 2020 doi: 10.2174/1574884715666200413120413. [DOI] [PubMed] [Google Scholar]
- 71. Ixoreal. KSM-66 Ashwagandha > 2021; 2021.
- 72.Buckingham J.C. Glucocorticoids: exemplars of multi-tasking. Br. J. Pharmacol. 2006;147(Suppl. 1):S258–S268. doi: 10.1038/sj.bjp.0706456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dey A., Chatterjee S.S., Kumar V. Low dose effects of a Withania somnifera extract on altered marble burying behavior in stressed mice. J. Intercult. Ethnopharmacol. 2016;5(3):274–277. doi: 10.5455/jice.20160414104917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jain H., Parial S., Jarald E., Daud A., Ahmad S. Extraction of Ashwagandha by conventional extraction methods and evaluation of its anti-stress activity. International Journal of Green Pharmacy. 2010;4(3):183–185. doi: 10.4103/0973-8258.69178. [DOI] [Google Scholar]
- 75.Khan Z.A., Ghosh A.R. Possible nitric oxide modulation in protective effects of Withaferin A against stress induced neurobehavioural changes. J. Med. Plants Res. 2010;4(6):490–495. [Google Scholar]
- 76.Kaur T., Singh H., Mishra R., et al. Withania somnifera as a potential anxiolytic and immunomodulatory agent in acute sleep deprived female Wistar rats. Mol. Cell. Biochem. 2017;427(1-2):91–101. doi: 10.1007/s11010-016-2900-1. [DOI] [PubMed] [Google Scholar]
- 77.Kumar A., Kalonia H. Protective effect of Withania somnifera Dunal on the behavioral and biochemical alterations in sleep-disturbed mice (Grid over water suspended method). Indian J. Exp. Biol. 2007;45(6):524–528. [PubMed] [Google Scholar]
- 78.Gupta G.L., Rana A.C. Protective effect of Withania somnifera dunal root extract against protracted social isolation induced behavior in rats. Indian J. Physiol. Pharmacol. 2007;51(4):345–353. [PubMed] [Google Scholar]
- 79.Gupta M., Kaur G. Withania somnifera as a Potential Anxiolytic and Anti-inflammatory Candidate Against Systemic Lipopolysaccharide-Induced Neuroinflammation. Neuromolecular Med. 2018;20(3):343–362. doi: 10.1007/s12017-018-8497-7. [DOI] [PubMed] [Google Scholar]
- 80.Gupta G.L., Rana A.C. Effect of Withania somnifera Dunal in ethanol-induced anxiolysis and withdrawal anxiety in rats. Indian J. Exp. Biol. 2008;46(6):470–475. [PubMed] [Google Scholar]
- 81.Bhattacharya S.K., Bhattacharya A., Sairam K., Ghosal S. Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides: an experimental study. Phytomedicine. 2000;7(6):463–469. doi: 10.1016/S0944-7113(00)80030-6. [DOI] [PubMed] [Google Scholar]
- 82.Walf A.A., Frye C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007;2(2):322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaurav B.P.S., Wanjari M.M., Chandekar A., Chauhan N.S., Upmanyu N. Influence of Withania somnifera on obsessive compulsive disorder in mice. Asian Pac. J. Trop. Med. 2012;5(5):380–384. doi: 10.1016/S1995-7645(12)60063-7. [DOI] [PubMed] [Google Scholar]
- 84.de Brouwer G., Fick A., Harvey B.H., Wolmarans W. A critical inquiry into marble-burying as a preclinical screening paradigm of relevance for anxiety and obsessive-compulsive disorder: Mapping the way forward. Cogn. Affect. Behav. Neurosci. 2019;19(1):1–39. doi: 10.3758/s13415-018-00653-4. [DOI] [PubMed] [Google Scholar]
- 85.Thomas A., Burant A., Bui N., Graham D., Yuva-Paylor L.A., Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl.) 2009;204(2):361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mohanty R., Das S.K., Singh N.R., Patri M. Withania somnifera Leaf Extract Ameliorates Benzo[a]pyrene-Induced Behavioral and Neuromorphological Alterations by Improving Brain Antioxidant Status in Zebrafish (Danio rerio). Zebrafish. 2016;13(3):188–196. doi: 10.1089/zeb.2015.1215. [DOI] [PubMed] [Google Scholar]
- 87.Sood A, Kumar A, Dhawan DK, Sandhir R. Propensity of Withania somnifera to attenuate behavioural.Biochemical, and histological alterations in experimental model of stroke. 2016. [DOI] [PMC free article] [PubMed]
- 88.Kaur T., Kaur G. Withania somnifera as a potential candidate to ameliorate high fat diet-induced anxiety and neuroinflammation. J. Neuroinflammation. 2017;14(1):201. doi: 10.1186/s12974-017-0975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matza L.S., Morlock R., Sexton C., Malley K., Feltner D. Identifying HAM-A cutoffs for mild, moderate, and severe generalized anxiety disorder. Int. J. Methods Psychiatr. Res. 2010;19(4):223–232. doi: 10.1002/mpr.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 91.Kay S.R., Opler L.A., Lindenmayer J.P. Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Res. 1988;23(1):99–110. doi: 10.1016/0165-1781(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 92.Cooley K., Szczurko O., Perri D., et al. Naturopathic care for anxiety: a randomized controlled trial ISRCTN78958974. PLoS One. 2009;4(8):e6628. doi: 10.1371/journal.pone.0006628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chengappa K.N., Bowie C.R., Schlicht P.J., Fleet D., Brar J.S., Jindal R. Randomized placebo-controlled adjunctive study of an extract of Withania somnifera for cognitive dysfunction in bipolar disorder. J. Clin. Psychiatry. 2013;74(11):1076–1083. doi: 10.4088/JCP.13m08413. [DOI] [PubMed] [Google Scholar]
- 94.Nuss P. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015;11:165–175. doi: 10.2147/NDT.S58841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sonar V.P., Fois B., Distinto S., et al. Ferulic Acid Esters and Withanolides: In Search of Withania somnifera GABAA Receptor Modulators. J. Nat. Prod. 2019;82(5):1250–1257. doi: 10.1021/acs.jnatprod.8b01023. [DOI] [PubMed] [Google Scholar]
- 96.Bhattarai J.P., Ah Park S., Han S.K. The methanolic extract of Withania somnifera ACTS on GABAA receptors in gonadotropin releasing hormone (GnRH) neurons in mice. Phytother. Res. 2010;24(8):1147–1150. doi: 10.1002/ptr.3088. [DOI] [PubMed] [Google Scholar]
- 97.Mehta A.K., Binkley P., Gandhi S.S., Ticku M.K. Pharmacological effects of Withania somnifera root extract on GABAA receptor complex. Indian J. Med. Res. 1991;94(AUG):312–315. [PubMed] [Google Scholar]
- 98.Orrù A., Marchese G., Casu G., et al. Withania somnifera root extract prolongs analgesia and suppresses hyperalgesia in mice treated with morphine. Phytomedicine. 2014;21(5):745–752. doi: 10.1016/j.phymed.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 99.Ruiu S., Longoni R., Spina L., et al. Withania somnifera prevents acquisition and expression of morphine-elicited conditioned place preference. Behav. Pharmacol. 2013;24(2):133–143. doi: 10.1097/FBP.0b013e32835f3d15. [DOI] [PubMed] [Google Scholar]
- 100.Bassareo V., Talani G., Frau R., et al. Inhibition of morphine-and ethanol-mediated stimulation of mesolimbic dopamine neurons by Withania somnifera. Front. Neurosci. 2019;13(JUN):545. doi: 10.3389/fnins.2019.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kulkarni S.K., Akula K.K., Dhir A. Effect of Withania somnifera Dunal root extract against pentylenetetrazol seizure threshold in mice: possible involvement of GABAergic system. Indian J. Exp. Biol. 2008;46(6):465–469. [PubMed] [Google Scholar]
- 102.Kulkarni S.K., Sharma A., Verma A., Ticku M.K. GABA receptor mediated anticonvulsant action of Withania somnifera root extract. Indian Drugs. 1993;30(7):305–312. [Google Scholar]
- 103.Kumar A., Kalonia H. Effect of Withania somnifera on sleep-wake cycle in sleep-disturbed rats: possible gabaergic mechanism. Indian J. Pharm. Sci. 2008;70(6):806–810. doi: 10.4103/0250-474X.49130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Candelario M., Cuellar E., Reyes-Ruiz J.M., et al. Direct evidence for GABAergic activity of Withania somnifera on mammalian ionotropic GABAA and GABAρ receptors. J. Ethnopharmacol. 2015;171:264–272. doi: 10.1016/j.jep.2015.05.058. [DOI] [PubMed] [Google Scholar]
- 105.Yin H., Cho D.H., Park S.J., Han S.K. GABA-mimetic actions of Withania somnifera on substantia gelatinosa neurons of the trigeminal subnucleus caudalis in mice. Am. J. Chin. Med. 2013;41(5):1043–1051. doi: 10.1142/S0192415X13500705. [DOI] [PubMed] [Google Scholar]
- 106.Schliebs R., Liebmann A., Bhattacharya S.K., Kumar A., Ghosal S., Bigl V. Systemic administration of defined extracts from Withania somnifera (Indian Ginseng) and Shilajit differentially affects cholinergic but not glutamatergic and GABAergic markers in rat brain. Neurochem. Int. 1997;30(2):181–190. doi: 10.1016/S0197-0186(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 107.Cobley J.N., Fiorello M.L., Bailey D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503. doi: 10.1016/j.redox.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fedoce A.D.G., Ferreira F., Bota R.G., Bonet-Costa V., Sun P.Y., Davies K.J.A. The role of oxidative stress in anxiety disorder: cause or consequence? Free Radic. Res. 2018;52(7):737–750. doi: 10.1080/10715762.2018.1475733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salim S., Chugh G., Asghar M. Inflammation in anxiety. Adv. Protein Chem. Struct. Biol. 2012;88:1–25. doi: 10.1016/B978-0-12-398314-5.00001-5. [DOI] [PubMed] [Google Scholar]
- 110.Bouayed J., Rammal H., Soulimani R. Oxidative stress and anxiety: relationship and cellular pathways. Oxid. Med. Cell. Longev. 2009;2(2):63–67. doi: 10.4161/oxim.2.2.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Molendijk M.L., de Kloet E.R. Coping with the forced swim stressor: Current state-of-the-art. Behav. Brain Res. 2019;364:1–10. doi: 10.1016/j.bbr.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 112.Reardon S. Depression researchers rethink popular mouse swim tests. Nature. 2019;571(7766):456–457. doi: 10.1038/d41586-019-02133-2. [DOI] [PubMed] [Google Scholar]
- 113.Shanker J. Stop using mouse swim test as proxy for depression. Nature. 2019;572(7771):586. doi: 10.1038/d41586-019-02548-x. [DOI] [PubMed] [Google Scholar]
- 114.Castagné V., Moser P., Roux S., Porsolt R.D. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. 2010. [DOI] [PubMed] [Google Scholar]
- 115.Vollmayr B., Gass P. Learned helplessness: unique features and translational value of a cognitive depression model. Cell Tissue Res. 2013;354(1):171–178. doi: 10.1007/s00441-013-1654-2. [DOI] [PubMed] [Google Scholar]
- 116.Czéh B., Fuchs E., Wiborg O., Simon M. Animal models of major depression and their clinical implications. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:293–310. doi: 10.1016/j.pnpbp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 117.Jayanthi M.K., Prathima C., Huralikuppi J.C., Suresha R.N., Dhar M. Anti-depressant effects of Withania somnifera fat (Ashwagandha Ghrutha) extract in experimental mice. Int. J. Pharma Bio Sci. 2012;3(1):33–P42. [Google Scholar]
- 118.Maiti T., Adhikari A., Panda A. A study on evaluation of antidepressant effect of Imipramine adjunct with Ashwagandha and Bramhi. International Journal of Ayurvedic Medicine. 2012;3(1):40–47. doi: 10.47552/ijam.v3i1.155. [DOI] [Google Scholar]
- 119.Shah P.C., Trivedi N.A., Bhatt J.D., Hemavathi K.G. Effect of Withania somnifera on forced swimming test induced immobility in mice and its interaction with various drugs. Indian J. Physiol. Pharmacol. 2006;50(4):409–415. [PubMed] [Google Scholar]
- 120.Antony B., Anu Aravind A.P., Benny M., Gupta N.K., Joseph B., Sebastian A. Bioactivity guided fractionation and purification of anti-depressant molecule from ashwagandha (Withania somnifera). Curr. Bioact. Compd. 2020;16(5):681–686. doi: 10.2174/1573407215666190308155305. [DOI] [Google Scholar]
- 121.Bhatt S., Nagappa A.N., Patil C.R. Role of oxidative stress in depression. Drug Discov. Today. 2020;25(7):1270–1276. doi: 10.1016/j.drudis.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 122.Naß J., Abdelfatah S., Efferth T. Induction of stress resistance and extension of lifespan in Chaenorhabditis elegans serotonin-receptor knockout strains by withanolide A. Phytomedicine. 2021;84:153482. doi: 10.1016/j.phymed.2021.153482. [DOI] [PubMed] [Google Scholar]
- 123.Wang Y.Y., Ma W.W., Peng I.F. Screening of sleep assisting drug candidates with a Drosophila model. PLoS One. 2020:15. doi: 10.1371/journal.pone.0236318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kaushik M.K., Kaul S.C., Wadhwa R., Yanagisawa M., Urade Y. Triethylene glycol, an active component of Ashwagandha (Withania somnifera) leaves, is responsible for sleep induction. PLoS One. 2017;12(2):e0172508. doi: 10.1371/journal.pone.0172508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jagota A., Kowshik K. Therapeutic effects of ashwagandha in brain aging and clock dysfunction. 2017. [Google Scholar]
- 126.Kukkemane K., Jagota A. Therapeutic effects of hydro-alcoholic leaf extract of Withania somnifera on age-induced changes in daily rhythms of Sirt1, Nrf2 and Rev-erbα in the SCN of male Wistar rats. Biogerontology. 2020;21(5):593–607. doi: 10.1007/s10522-020-09875-x. [DOI] [PubMed] [Google Scholar]
- 127.McEwen B.S., Stellar E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 1993;153(18):2093–2101. doi: 10.1001/archinte.1993.00410180039004. [DOI] [PubMed] [Google Scholar]
- 128.McEwen B.S. Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress (Thousand Oaks) 2017;1:1. doi: 10.1177/2470547017692328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wiley J.F., Gruenewald T.L., Karlamangla A.S., Seeman T.E. Modeling multisystem physiological dysregulation. Psychosom. Med. 2016;78(3):290–301. doi: 10.1097/PSY.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tafet G.E., Nemeroff C.B. Pharmacological treatment of anxiety disorders: the role of the HPA axis. Front. Psychiatry. 2020;11:443. doi: 10.3389/fpsyt.2020.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tafet G.E., Nemeroff C.B. The links between stress and depression: Psychoneuroendocrinological, genetic, and environmental interactions. J. Neuropsychiatry Clin. Neurosci. 2016;28(2):77–88. doi: 10.1176/appi.neuropsych.15030053. [DOI] [PubMed] [Google Scholar]
- 132.Han K.S., Kim L., Shim I. Stress and sleep disorder. Exp. Neurobiol. 2012;21(4):141–150. doi: 10.5607/en.2012.21.4.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang Y.L., Han Q.Q., Gong W.Q., et al. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J. Neuroinflammation. 2018;15(1):21. doi: 10.1186/s12974-018-1054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xia Y., Wang P., Yan N., Gonzalez F.J., Yan T. Withaferin A alleviates fulminant hepatitis by targeting macrophage and NLRP3. Cell Death Dis. 2021;12(2):174. doi: 10.1038/s41419-020-03243-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Murman D.L. The Impact of Age on Cognition. Semin. Hear. 2015;36(3):111–121. doi: 10.1055/s-0035-1555115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.CDC . NACD. The state of mental health and aging in america: what do the data tell us? The State of Mental Health and Aging in America; 2008. p. 1. [Google Scholar]
- 137.Feinberg I., Carlson V.R. Sleep variables as a function of age in man. Arch. Gen. Psychiatry. 1968;18:239–250. doi: 10.1001/archpsyc.1968.01740020111014. [DOI] [Google Scholar]
- 138.Rincón-Cortés M., Herman J.P., Lupien S., Maguire J., Shansky R.M. Stress: Influence of sex, reproductive status and gender. Neurobiol. Stress. 2019;10:100155. doi: 10.1016/j.ynstr.2019.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35(3):303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Khan S.A., Khan S.B., Shah Z., Asiri A.M. Withanolides: Biologically active constituents in the treatment of Alzheimer’s disease. Med. Chem. 2016;12(3):238–256. doi: 10.2174/1573406411666151030112314. [DOI] [PubMed] [Google Scholar]
- 141.Pandey A., Bani S., Dutt P., Kumar Satti N., Avtar Suri K., Nabi Qazi G. Multifunctional neuroprotective effect of Withanone, a compound from Withania somnifera roots in alleviating cognitive dysfunction. Cytokine. 2018;102:211–221. doi: 10.1016/j.cyto.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 142.Kuboyama T., Tohda C., Komatsu K. Withanoside IV and its active metabolite, sominone, attenuate Abeta(25-35)-induced neurodegeneration. Eur. J. Neurosci. 2006;23(6):1417–1426. doi: 10.1111/j.1460-9568.2006.04664.x. [DOI] [PubMed] [Google Scholar]
- 143.Thirugnanasambantham P., Senthil K., Oh T.J., Choi H.K. Comparative chemometric profiles between leaf tissues of Withania somnifera cultured in vitro and field. Int. J. Pharm. Pharm. Sci. 2015;7(11):66–71. [Google Scholar]
- 144.Caesar L.K., Cech N.B. Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat. Prod. Rep. 2019;36(6):869–888. doi: 10.1039/C9NP00011A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alcazar Magana A., Wright K., Vaswani A., et al. Integration of mass spectral fingerprinting analysis with precursor ion (MS1) quantification for the characterisation of botanical extracts: application to extracts of Centella asiatica (L.). Urban. Phytochem Anal. 2020;31(6):722–738. doi: 10.1002/pca.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Patil D., Gautam M., Mishra S., et al. Determination of withaferin A and withanolide A in mice plasma using high-performance liquid chromatography-tandem mass spectrometry: application to pharmacokinetics after oral administration of Withania somnifera aqueous extract. J. Pharm. Biomed. Anal. 2013;80:203–212. doi: 10.1016/j.jpba.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 147.Muhasaparur Ganesan R., Settu D.K., Murkunde Y., Duraipandian C. Pharmacological and pharmacokinetic effect of a polyherbal combination with Withania somnifera (L.) Dunal for the management of anxiety. J. Ethnopharmacol. 2021;265:113337. doi: 10.1016/j.jep.2020.113337. [DOI] [PubMed] [Google Scholar]
- 148.Singh S.K., Valicherla G.R., Joshi P., et al. Determination of permeability, plasma protein binding, blood partitioning, pharmacokinetics and tissue distribution of Withanolide A in rats: A neuroprotective steroidal lactone. Drug Dev. Res. 2018;79(7):339–351. doi: 10.1002/ddr.21463. [DOI] [PubMed] [Google Scholar]
- 149.Vareed S.K., Bauer A.K., Nair K.M., Liu Y., Jayaprakasam B., Nair M.G. Blood-brain barrier permeability of bioactive withanamides present in Withania somnifera fruit extract. Phytother. Res. 2014;28(8):1260–1264. doi: 10.1002/ptr.5118. [DOI] [PubMed] [Google Scholar]
- 150.Pires N., Gota V., Gulia A., Hingorani L., Agarwal M., Puri A. Safety and pharmacokinetics of Withaferin-A in advanced stage high grade osteosarcoma: A phase I trial. J. Ayurveda Integr. Med. 2020;11(1):68–72. doi: 10.1016/j.jaim.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Verma N., Gupta S.K., Tiwari S., Mishra A.K. Safety of ashwagandha root extract: a randomized, placebo-controlled, study in healthy volunteers. Complement. Ther. Med. 2021;57:102642. doi: 10.1016/j.ctim.2020.102642. [DOI] [PubMed] [Google Scholar]
- 152.Prabu P.C., Panchapakesan S., Raj C.D. Acute and sub-acute oral toxicity assessment of the hydroalcoholic extract of Withania somnifera roots in Wistar rats. Phytother. Res. 2013;27(8):1169–1178. doi: 10.1002/ptr.4854. [DOI] [PubMed] [Google Scholar]
- 153.Prabu P.C., Panchapakesan S. Prenatal developmental toxicity evaluation of Withania somnifera root extract in Wistar rats. Drug Chem. Toxicol. 2015;38(1):50–56. doi: 10.3109/01480545.2014.900073. [DOI] [PubMed] [Google Scholar]
- 154.Borse S.P., Singh D.P., Nivsarkar M. Understanding the relevance of herb-drug interaction studies with special focus on interplays: a prerequisite for integrative medicine. Porto Biomed. J. 2019;4(2):e15. doi: 10.1016/j.pbj.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Feltrin C., Oliveira Simões C.M. Reviewing the mechanisms of natural product-drug interactions involving efflux transporters and metabolic enzymes. Chem. Biol. Interact. 2019;314:108825. doi: 10.1016/j.cbi.2019.108825. [DOI] [PubMed] [Google Scholar]
- 156.Pan Y., Abd-Rashid B.A., Ismail Z., et al. In vitro modulatory effects of Andrographis paniculata, Centella asiatica and Orthosiphon stamineus on cytochrome P450 2C19 (CYP2C19). J. Ethnopharmacol. 2011;133(2):881–887. doi: 10.1016/j.jep.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 157.Obach R.S. Inhibition of human cytochrome P450 enzymes by constituents of St. John’s Wort, an herbal preparation used in the treatment of depression. J. Pharmacol. Exp. Ther. 2000;294(1):88–95. [PubMed] [Google Scholar]
- 158.Savai J., Varghese A., Pandita N., Chintamaneni M. Investigation of CYP3A4 and CYP2D6 Interactions of Withania somnifera and Centella asiatica in Human Liver Microsomes. Phytother. Res. 2015;29(5):785–790. doi: 10.1002/ptr.5308. [DOI] [PubMed] [Google Scholar]
- 159.Savai J., Varghese A., Pandita N., Chintamaneni M. in vitro assessment of CYP1A2 and 2C9 inhibition potential of Withania somnifera and Centella asiatica in human liver microsomes. Drug Metab. Pers. Ther. 2015;30(2):137–141. doi: 10.1515/dmdi-2014-0035. [DOI] [PubMed] [Google Scholar]
- 160.Patil D., Gautam M., Gairola S., Jadhav S., Patwardhan B. Effect of botanical immunomodulators on human CYP3A4 inhibition: implications for concurrent use as adjuvants in cancer therapy. Integr. Cancer Ther. 2014;13(2):167–175. doi: 10.1177/1534735413503551. [DOI] [PubMed] [Google Scholar]
- 161.Kumar S., Bouic P.J., Rosenkranz B. Investigation of CYP2B6, 3A4 and β-esterase interactions of Withania somnifera (L.) dunal in human liver microsomes and HepG2 cells. J. Ethnopharmacol. 2021;270:113766. doi: 10.1016/j.jep.2020.113766. [DOI] [PubMed] [Google Scholar]
- 162.Singh S.K., Valicherla G.R., Bikkasani A.K., et al. Elucidation of plasma protein binding, blood partitioning, permeability, CYP phenotyping and CYP inhibition studies of Withanone using validated UPLC method: An active constituent of neuroprotective herb Ashwagandha. J. Ethnopharmacol. 2021;270:113819. doi: 10.1016/j.jep.2021.113819. [DOI] [PubMed] [Google Scholar]