Abstract
Objective
The present systematic review and meta-analysis of randomized clinical trials (RCTs) aimed to investigate the effects of pulmonary rehabilitation in individuals with chronic obstructive pulmonary disease (COPD).
Methods
The RCTs of pulmonary rehabilitation programs published between 1999 and 2021 were retrieved from electronic databases (PubMed, Cochrane Library, and Embase). Two reviewers independently assessed the topical relevance and trial quality and extracted data for meta-analysis using the Stata software version 14.0.
Results
A total of 39 trials involving 2,397 participants with COPD were evaluated. We found that patients who received pulmonary rehabilitation program had significant improvement in the 6-min walk test (6MWT), St. George Respiratory Questionnaire score, and the modified British Medical Research Council score as compared to those who received usual care. Yoga and Tai Chi showed significant improvement in the forced expiratory volume (FEV1)% in 1 s predicted value. However, no significant difference was detected in the modified Borg score, forced vital capacity (FVC), and FEV1/FVC predicted value between the pulmonary rehabilitation and usual care groups.
Conclusion
Yoga and Tai Chi showed a significant improvement in the FEV1% predicted value. Also, pulmonary rehabilitation program improved the exercise capacity, the quality of life, and dyspnoea in patients with COPD.
Key messages
A total of 39 trials involving 2,397 participants with COPD were evaluated.
We found that patients who received pulmonary rehabilitation program had significant improvement in the 6MWT, St. George Respiratory Questionnaire score, and the modified British Medical Research Council score as compared to those who received usual care.
Yoga and Tai Chi showed significant improvement in the FEV1% predicted value.
No significant difference was detected in the modified Borg score, FVC, and FEV1/FVC predicted value between the pulmonary rehabilitation and usual care groups.
Keywords: Pulmonary rehabilitation, chronic obstructive pulmonary disease, randomized controlled trials, systematic review, meta-analysis
Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic disease characterized by persistent respiratory symptoms and airflow limitation [1]. COPD is a progressive and debilitating respiratory disease, leading to a severe burden on the individual and society. It is the only chronic disease with increasing morbidity and mortality [2]. The World Health Organisation (WHO) predicts that by 2030, COPD will become the third leading cause of deaths worldwide [3]. China has the most significant number of COPD patients in the world: about 99 million, with an 8.6% prevalence. The number of deaths each year exceeds 900,000 [4,5].
In COPD therapy, pulmonary rehabilitation is regarded as the hallmark of treatment in all patients [6]. Typically, the pulmonary rehabilitation program is implemented by multidisciplinary teams in the outpatient department, including exercise training, education, nutritional supplement, and psychosocial support. Compared to the traditional community care, pulmonary rehabilitation reduces dyspnoea and fatigue and improves exercise endurance and many areas of health-related quality of life (HRQOL) [7–9].
The main purpose of pulmonary rehabilitation training is to formulate a corresponding pulmonary rehabilitation plan according to the actual situation of the patient, thereby improving the patient’s quality of life and exercise endurance and the symptoms of dyspnoea [10]. In addition, this personalized treatment plan can reduce complications, enhance endurance, social participation, and reduce medical budget [11]. The pulmonary rehabilitation of patients with COPD mainly includes functional exercise, education, oxygen therapy, nutritional support, and psychotherapy [12]. As early as the 1970s, Gimenez et al. [13] conducted a 10-year follow-up study on the pulmonary rehabilitation of patients with COPD. Several randomized controlled trials (RCTs) have investigated the effect of pulmonary rehabilitation for COPD patients, and no consistent outcomes have been reported [14–17]. In addition, a large number of studies [18–31] have been carried out on the influence of mind–body exercise (Tai Chi, Yoga) on COPD, however, the previous meta-analysis [7,32–34] did not include them. Thus, this meta-analysis was conducted to assess the efficacy of pulmonary rehabilitation (including Tai Chi and Yoga) in patients with COPD.
Materials and methods
Search strategy
The databases, including PubMed, Embase, and Cochrane Library, were queried using the keywords “chronic obstructive pulmonary disease/COPD”, “pulmonary rehabilitation”, “exercise training”, “Yoga”, “Tai Chi”, and “randomized controlled trial/RCT”. The search was updated until August 2021. The detailed search strategy is shown in Supplementary Table S1. No restrictions were applied to the language, and cross-references and reviews were assessed to gather all the eligible publications.
Inclusion and exclusion criteria
Studies were included if the following criteria were fulfilled [1]: included patients had a clinical diagnosis of COPD [2]; study design involved an RCT [3]; studies having at least two groups: one group receiving pulmonary rehabilitation and another group receiving usual care [4]; participants received pulmonary rehabilitation programs based on Yoga, Tai Chi, and conventional physical exercises, such as walking, jogging, swimming, and cycling. COPD patients diagnosed according to the Global Obstructive Lung Disease Initiative criteria (Global Strategy for the Diagnosis, Management and Prevention of COPD). The following studies were excluded [1]: reviews, editorials, conference abstracts, letters, and case reports [2]; duplicate publications [3]; basic research or animal studies [4]; studies without sufficient data.
Data extraction and quality assessment
All available data were extracted independently by two researchers according to the inclusion criteria. Any differences were resolved through discussions with the third author. The following data were extracted from each article: first author’s name, year of publication, research design, country, gender, mean age, mean forced expiratory volume in one second (FEV1), sample size, follow-up time, and outcomes assessed. Also, we assessed the risk of bias using the Cochrane Collaboration tool for risk of bias assessment [35].
Data synthesis and analysis
The weighted mean difference (WMD) and 95% confidence interval (CI) were calculated for continuous data. Then, the data were combined according to random-effects (DerSimonian and Laird’s method) or fixed-effects model depending on the significance of the I2 statistic (I2 > 50% was considered statistically significant). If the heterogeneity was significant, the random-effects model was adopted, otherwise fixed-effects model was adopted. The sequential removal of each study for sensitivity analysis was carried out to assess the relative impact of each study comprehensively. Egger’s linear regression test and Begg’s test are used to demonstrate the publication bias; if p < .05, we used the trim and fill method for corrections [36]. The statistical analysis was performed using the Stata software version 14.0 (Stata, TX, USA), and all tests were double-sided.
Results
Study properties
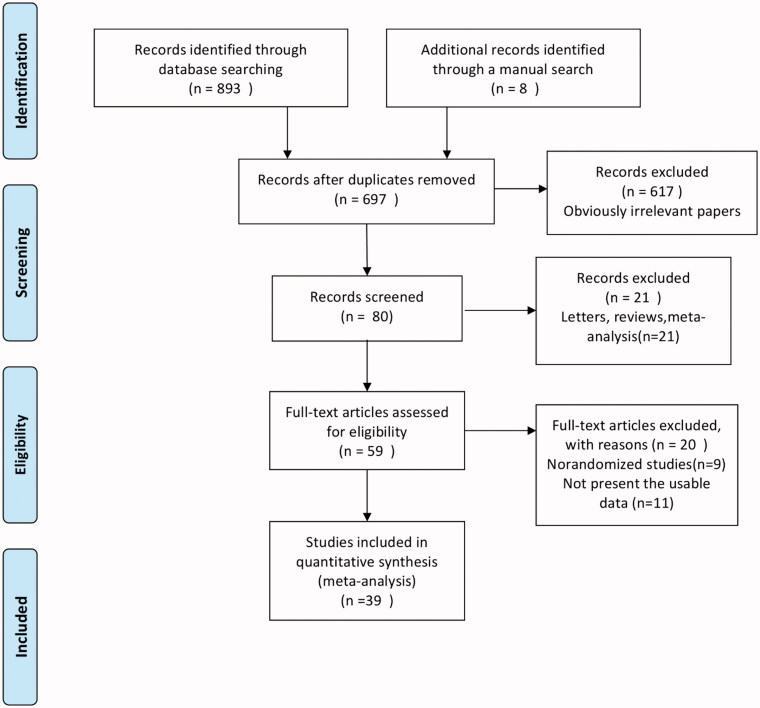
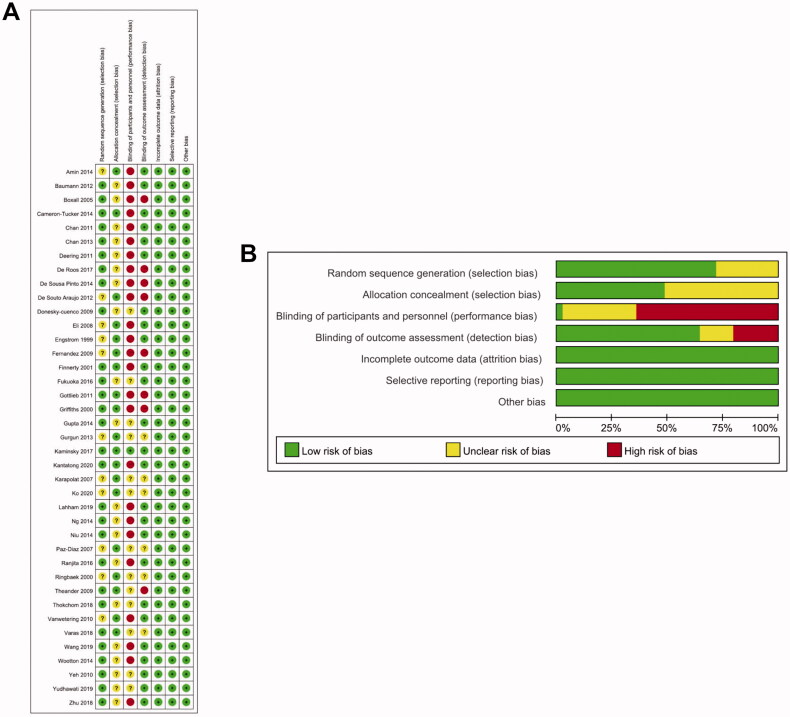
Overall, 893 articles were retrieved from four databases, and 8 articles were identified via manual search. The duplicates were excluded, and after screening the abstracts of the remaining articles, 59 full-text articles were obtained for further review (Figure 1). Based on our selection criteria, 20 studies were further excluded, and finally, 39 studies were included in this meta-analysis [14–31,37–57] (Figure 1). The baseline characteristics of included studies are listed in Table 1. The studies included in the meta-analysis have been conducted in China (19.4%, 7/36), the USA (13.9%, 5/36), Australia (11.1%, 4/36), Turkey (8.3%, 3/36), India (8.3%, 3/36), Sweden (5.6%, 2/36), the UK (5.6%, 2/36), Denmark (5.6%, 2/36), Spain (5.6%, 2/36), Netherlands (5.6%, 2/36), Brazil (5.6%, 2/36), Ireland (2.8%, 1/36), Japan (2.8%, 1/36), Indonesia (5.6%, 1/36), Thailand (2.8%, 1/36), and Germany (2.8%, 1/36). These 36 studies involved a total of 2,397 patients, and the sample size of each study was 8–200 patients. The articles included in the present study were in English and published between 1999 and 2020. The Cochrane Collaboration tool for assessing the risk of bias (Figure 2) showed that poor scores were obtained in the performance and detection bias due to the nature of the intervention as it was not possible to blind the subjects to their allocation.
Figure 1.
Flow diagram for the identification of the studies.
Table 1.
Characteristics of the studies included in this meta-analysis.
| Authors/year of publication | Country | Mean age (years) | Male (%) PR/Con | Mean FEV1 (% or L) PR/Con | Type of study | Intervention |
Follow-up | |
|---|---|---|---|---|---|---|---|---|
| PR | Con | |||||||
| Engstrom/1999 [37] | Sweden | PR: 66 ± 5.4 | 53.8/50 | 30.7/34.1 | RCT | 26 | 24 | 12M |
| Con: 66.8 ± 5.4 | ||||||||
| Griffiths/2000 [14] | UK | PR: 68.2 ± 8.2 | 61.6/58.4 | 39.7/39.4 | RCT | 99 | 101 | 12M |
| Con: 68.3 ± 8.1 | ||||||||
| Ringbaek/2000 [38] | Denmark | PR:61.8 ± 6.8 | 4.17/28.6 | 49.5/44.3 | RCT | 24 | 21 | 2M |
| Con: 64.6 ± 7.7 | ||||||||
| Finnerty/2001 [39] | UK | PR:70.4 ± 8.0 | 69.4/65.5 | 41.2/41.2 | RCT | 36 | 29 | 6M |
| Con: 68.4 ± 10.4 | ||||||||
| Boxall/2005 [15] | Australia | PR:77.6 ± 7.6 | 47.8/65.2 | 40.5/37.7 | RCT | 23 | 23 | 3M |
| Con: 75.8 ± 8.1 | ||||||||
| Karapolat/2007 [40] | Turkey | PR: 65.1 ± 9.4 | 81.5/95.5 | 54.8/55 | RCT | 27 | 22 | 3M |
| Con: 66.6 ± 8.4 | ||||||||
| Paz-Diaz/2007 [41] | USA | PR: 67 ± 5 | 60/85.7 | 34/30 | RCT | 10 | 14 | 2M |
| Con: 62 ± 7 | ||||||||
| Eli/2008 [42] | Turkey | PR: 59.67 ± 8.6 | 15.4/15.4 | 47.77/46.28 | RCT | 39 | 39 | 3M |
| Con: 58.08 ± 11.45 | ||||||||
| Donesky-cuenco/2009 [25] | USA | PR: 72.2 ± 6.5 | 28.6/26.7 | 51.2/44.4 | RCT | 14 | 15 | 3M |
| Con: 67.7 ± 11.5 | ||||||||
| Fernandez/2009 [43] | Spain | PR: 66 ± 8 | NA | 33/38 | RCT | 27 | 14 | 12M |
| Con: 70 ± 5 | ||||||||
| Theander/2009 [44] | Sweden | PR: 66 ± 2 | 25/71.4 | 35.1/32.3 | RCT | 12 | 14 | 3M |
| Con: 64 ± 2 | ||||||||
| Vanwetering/2010 [45] | Netherlands | 64 ± 8.7 | 61.5 | 54.7 | RCT | 16 | 14 | 4M |
| Yeh/2010 [23] | USA | PR: 65 ± 2 | 60/60 | 53/47 | RCT | 5 | 5 | 3M |
| Con: 66 ± 6 | ||||||||
| Chan/2011 [46] | China | PR: 73.6 ± 7.5 | 88/87 | 0.91/0.89 | RCT | 69 | 67 | 3M |
| Con: 73.6 ± 7.4 | ||||||||
| Deering/2011 [47] | Ireland | PR: 67.7 ± 5.3 | NA | 48.5/45.8 | RCT | 25 | 19 | 3M |
| Con: 68.6 ± 5.5 | ||||||||
| Gottlieb/2011 [16] | Denmark | PR: 74.1 | 31.8/35 | 64.27/67.05 | RCT | 22 | 20 | 18M |
| Con: 73.2 | ||||||||
| Baumann/2012 [48] | Germany | PR: 63 ± 11 | 62.2/54.5 | 47/45 | RCT | 37 | 44 | 6M |
| Con: 65 ± 8 | ||||||||
| De Souto Araujo/2012 [49] | Brazil | PR: 56.9 ± 7.9 | 61.5/72.7 | 39.2/45.1 | RCT | 13 | 11 | 2M |
| Con: 71.1 ± 10.1 | ||||||||
| Chan/2013 [18] | China | PR: 71.7 ± 8.2 | 99/87 | 0.89/0.89 | RCT | 70 | 67 | 6M |
| Con: 73.6 ± 7.4 | ||||||||
| Gurgun/2013 [17] | Turkey | PR: 66.8 ± 9.6 | 100/100 | 41.9/39.3 | RCT | 15 | 16 | 2M |
| Con: 67.8 ± 6.6 | ||||||||
| Amin/2014 [50] | USA | PR: 66.8 ± 8.1 | 33/60 | 63.6/60.8 | RCT | 9 | 10 | 3M |
| Con: 72 ± 10.1 | ||||||||
| Cameron-Tucker/2014 [52] | Australia | PR: 64.5 ± 9.13 | 53/54 | NA | RCT | 43 | 41 | 1.5M |
| Con: 67.1 ± 9.41 | ||||||||
| De Sousa Pinto/2014 [54] | Brazil | PR: 68.9 ± 9.2 | 95.7/94.4 | 33.5/34.5 | RCT | 23 | 18 | 3M |
| Con: 71.9 ± 7.6 | ||||||||
| Gupta/2014 [27] | India | PR: 52.5 ± 3.9 | 96/96 | 51.1/49.6 | RCT | 25 | 25 | 3M |
| Con: 52 ± 4.1 | ||||||||
| Ng/2014 [20] | China | PR: 74.16 ± 6.46 | 93.6/88.8 | 1.1/1.23 | RCT | 94 | 98 | 6M |
| Con: 74.13 ± 6.81 | ||||||||
| Niu/2014 [21] | China | PR: 59.7 ± 2.76 | 95/90 | 41.9/43.7 | RCT | 20 | 20 | 6M |
| Con: 61.3 ± 2.89 | ||||||||
| Wootton/2014 [57] | Australia | PR: 69 ± 8 | 58.9/58.3 | 43/43 | RCT | 95 | 48 | 2M |
| Con: 68 ± 9 | ||||||||
| Fukuoka/2016 [26] | Japan | PR: 74.6 ± 6.7 | 100/66.7 | 0.93/1.2 | RCT | 5 | 3 | 0.5M |
| Con: 77 ± 7 | ||||||||
| Ranjita/2016 [29] | India | PR: 53.69 ± 5.66 | NA | NA | RCT | 36 | 36 | 3M |
| Con: 54.41 ± 5.4 | ||||||||
| De Roos/2017 [53] | Netherlands | PR: 69.4 ± 9.7 | 31/38 | 68/65 | RCT | 26 | 26 | 2.5M |
| Con: 71 ± 9.4 | ||||||||
| Kaminsky/2017 [28] | USA | PR: 68 ± 7 | 33/45 | 43/42 | RCT | 21 | 22 | 3M |
| Con: 68 ± 9 | ||||||||
| Thokchom/2018 [30] | India | PR: 57.8 ± 2.68 | 76.2/80 | 1.24/1.22 | RCT | 21 | 20 | 3M |
| Con: 60.65 ± 1.84 | ||||||||
| Varas/2018 [56] | Spain | PR: 69.5 ± 7.4 | 85.7/68.4 | 45.8/52.3 | RCT | 21 | 19 | 12M |
| Con: 64.8 ± 9.1 | ||||||||
| Zhu/2018 [24] | China | PR: 67.87 ± 5.22 | 93/97 | 35.11/40.77 | RCT | 30 | 30 | 9M |
| Con: 68.1 ± 6.57 | ||||||||
| Lahham/2019 [51] | Australia | PR: 68 ± 9 | 58.6/58.6 | 0.9/0.92 | RCT | 29 | 29 | 6M |
| Con: 67 ± 10 | ||||||||
| Wang/2019 [22] | China | PR: 67.83 ± 5.32 | 88.5/87.5 | 55.46/62.55 | RCT | 26 | 24 | 3M |
| Con: 67.86 ± 5.98 | ||||||||
| Yudhawati/2019 [31] | Indonesia | PR: 64.4 ± 10.4 | NA | 43.53/40.87 | RCT | 15 | 15 | 3M |
| Con: 65.33 ± 8.1 | ||||||||
| Kantatong/2020 [19] | Thailand | PR: 69.68 ± 7.67 | 60/76 | 68.21/68.37 | RCT | 25 | 25 | 3M |
| Con: 67.48 ± 10.17 | ||||||||
| Ko/2020 [55] | China | PR:76 ± 8 | 99/96 | 49/46 | RCT | 68 | 68 | 12M |
| Con: 74 ± 7 | ||||||||
PR: pulmonary rehabilitation; Con: control; RCT: randomized controlled trials; FEV1: forced expiratory volume in one second; L: litre; Y: years; M: months; NA: not available.
Figure 2.
Risk of bias assessment for the randomized trials included in the meta-analysis. (A) Risk of bias summary; (B) Risk of bias graph. Symbols. (+): low risk of bias; (?): unclear risk of bias; (–): high risk of bias.
Meta-analysis outcome
6-Min walk test (6MWT)
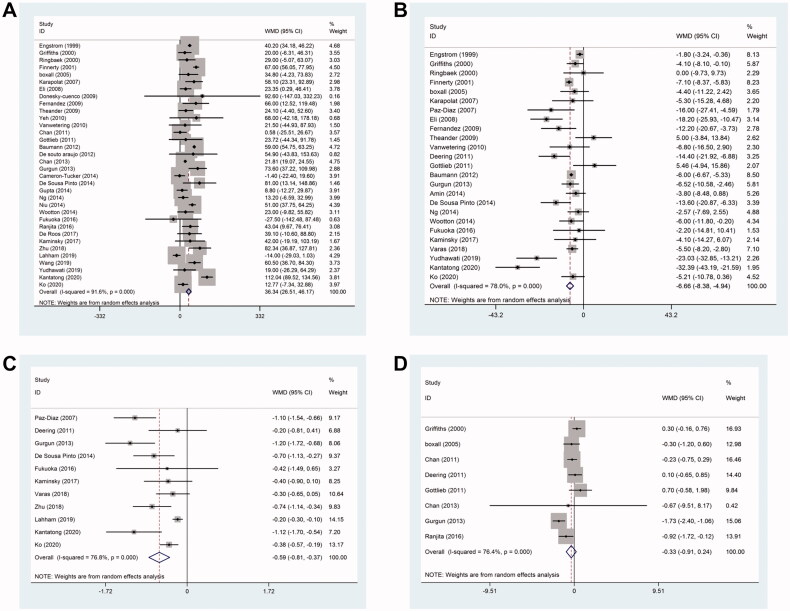
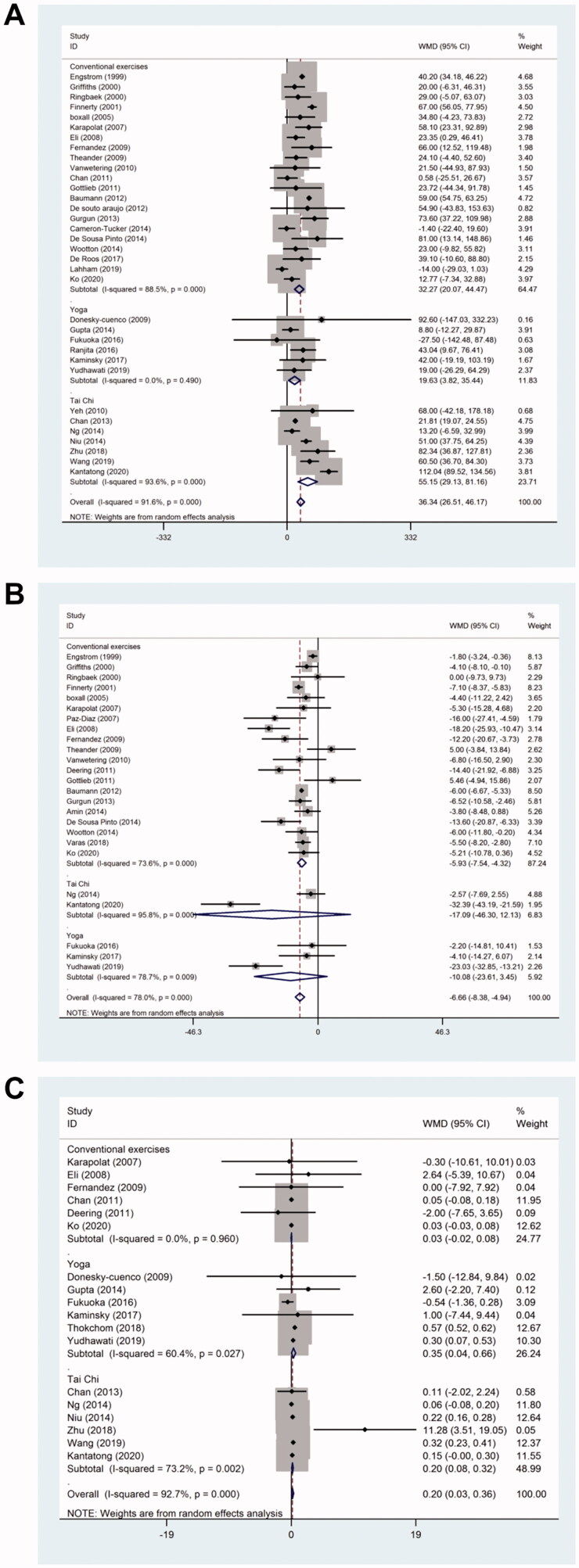
The changes in the exercise capacity from baseline were measured using 6MWT in 34 studies. The 6MWT distance was significantly improved (weighted mean difference (WMD), 36.34; 95% confidence interval (CI): 26.51–46.17; p < .001; I2 = 91.6%) in the pulmonary rehabilitation group compared to the control group (Figure 3(A)).
Figure 3.
Effect of pulmonary rehabilitation in individuals with COPD. (A) 6MWT; (B) SGRQ score; (C) MRC; (D) Borg score.
St george respiratory questionnaire (SGRQ) score
The SGRQ score was reported in 25 studies. Pulmonary rehabilitation showed a significant improvement in the quality of life according to the altered SGRQ total score (WMD, −6.66; 95% CI: −8.38 to −4.94; p < .001; I2 = 78%) (Figure 3(B)).
Modified british medical research council (MRC)
Dyspnoea was measured using the modified British MRC questionnaire in 11 studies. Pulmonary rehabilitation showed significant changes in the MRC (WMD, −0.59; 95% CI: −0.81 to −0.37; p < .001; I2 = 76.8%) (Figure 3(C)).
Modified borg score
Borg score was reported in 8 studies, and no significant difference (WMD, −0.33; 95% CI: −0.91–0.24; p < .001; I2 = 76.4%) was detected between the pulmonary rehabilitation group and the control group (Figure 3(D)).
Forced expiratory volume in one second (FEV1) percentage of predicted normal value
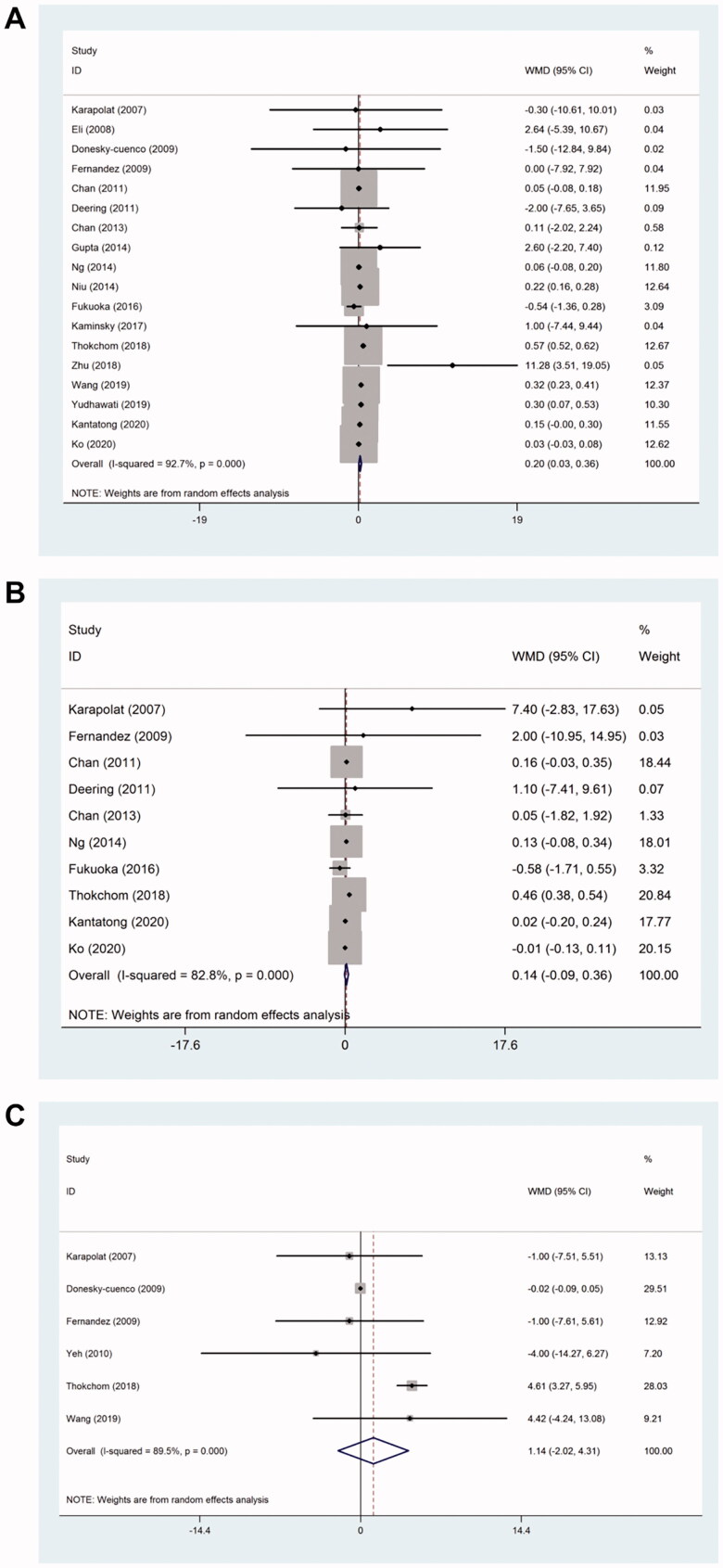
FEV1% predicted value was reported in 18 studies. Pulmonary rehabilitation showed significant changes in the FEV1% predicted value (WMD, 0.20; 95% CI: 0.03–0.36; p < .001; I2 = 92.7%) (Figure 4(A)).
Figure 4.
Effect of pulmonary rehabilitation on lung function in individuals with COPD. (A) FEV1%; (B) FVC%; (C) FEV1/FVC%.
FVC percentage of predicted normal value
FVC% predicted value was reported in 10 studies, and did not differ significantly (WMD, 0.14; 95% CI: −0.09–0.36; p < .001; I2 = 82.8%) between the pulmonary rehabilitation group and the control group (Figure 4(B)).
FEV1/FVC percentage of predicted normal value
FEV1/FVC % predicted value was reported in 6 studies. Interestingly, no significant difference (WMD, 1.14; 95% CI: −2.02–4.31; p < .001; I2 = 89.5%) was observed between the pulmonary rehabilitation and the control groups (Figure 4(C)).
Sensitivity analysis
Sensitivity analysis was performed to examine whether the removal of each study would cause a significant change in the overall trend. However, the results were not altered after the sequential removal of each study, suggesting the reliability and stability of the results in this meta-analysis (Supplementary Figure S1).
Subgroup analysis
The subgroup analysis was performed based on various rehabilitation measures since heterogeneity between the studies was observed in the overall comparisons. For the 6MWT, a significant improvement was observed in the Yoga (WMD, 19.63; 95% CI: 3.82–35.44; p = .49; I2 = 0), Tai Chi (WMD, 55.15; 95% CI: 29.13–81.16; p < .001; I2 = 93.6%), and the conventional exercises (WMD, 32.27; 95% CI: 20.07–44.47; p < .001; I2 = 88.5%) group (Figure 5(A)). For the SGRQ score, a significant improvement was observed in the conventional exercises group (WMD, −5.93; 95% CI: −7.54 to −4.32; p < .001; I2 = 73.6%) (Figure 5(B)) but not in the Yoga and Tai Chi groups. Furthermore, a significant improvement was observed in FEV1 values in the Yoga (WMD, 0.35; 95% CI: 0.04–0.66; p = .027; I2 = 60.4%) and Tai Chi groups (WMD, 0.20; 95% CI: 0.08–0.32; p = 0.002; I2 = 73.2%) (Figure 5(C)) but none was detected in the conventional exercises group.
Figure 5.
Subgroup analysis of the effect of pulmonary rehabilitation in individuals with COPD. (A) 6MWT; (B) SGRQ score; (C) FEV1.
Publication bias
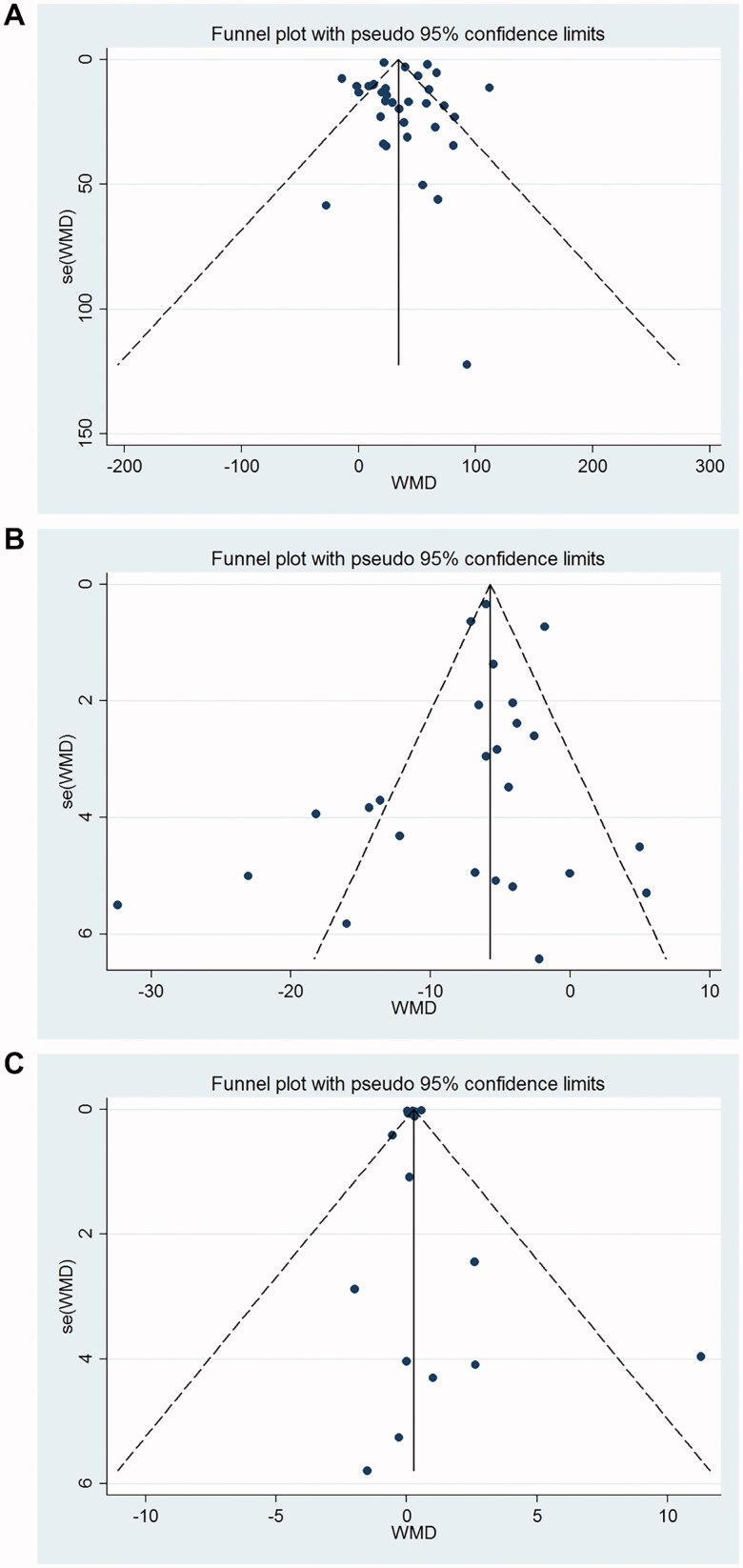
Egger’s linear regression test and Begg’s test showed no publication bias for 6MWD (Begg’s test p = 0.343; Egger’s test p = .63) (Figure 6(A)), SGRQ (Begg’s test p = 0.624; Egger’s test p = .389) (Figure 6(B)), and FEV1 (Begg’s test p = .289; Egger’s test p = .746) (Figure 6(C)).
Figure 6.
Funnel plot for publication bias test. Each point represents a separate study for the indicated association. (A) 6MWT; (B) SGRQ score; (C) FEV1.
Discussion
A comprehensive search was performed for RCTs that evaluated the efficacy of pulmonary rehabilitation in patients with COPD, and finally, 39 trials involving 2,397 COPD patients met our inclusion criteria. This meta-analysis showed that Yoga and Tai Chi significantly improved the FEV1% predicted value. Patients who received the pulmonary rehabilitation program had a marked improvement in the exercise capacity, quality of life, and dyspnoea compared to those who received usual care. Our study verifies known effects of the traditional PR, but that Yoga and Tai Chi can have significant improvement and may have benefit in FEV1 which PR does not do.
Pulmonary rehabilitation was first defined by the American College of Chest Physicians Committee in 1974. It is a proactive method that minimizes COPD symptoms, improves HRQOL, and increases physical and emotional participation in daily life [6,58]. In the latest update, pulmonary rehabilitation is defined as “Comprehensive interventions based on thorough patient assessment, followed by patient-tailored treatment, including but not limited to exercise training, education, and behavioural changes, aimed at improving the physical and psychological well-being of patients with chronic respiratory disease and promoting long-term adherence to health-promoting behaviors” [59]. In order to promote physical and mental health, mind-body exercise has attracted significant attention in the scientific community. The mind-body exercise focuses on mind, body, psychology, and behaviour, including breathing, physical exercise, and meditation [60,61]. It is characterized by gentle and slow movements and body and breathing coordination, represented by Chinese traditional sports Tai Chi and Indian Yoga [62,63]. Mind-body exercise (Tai Chi and Yoga) is easy to learn and practice compared to other forms of exercise, with minimal requirement of equipment and venue [64].
The efficacy of pulmonary rehabilitation in patients with COPD has been investigated by previous meta-analyses. To the best of our knowledge, this is the largest meta-analysis to investigate the efficacy of pulmonary rehabilitation in patients with COPD, encompassing 2,397 patients in 39 RCTs. Recently, Dong et al. [65] conducted a comprehensive meta-analysis about the efficacy of pulmonary rehabilitation in patients with COPD. In the present study, we included newer RCTs, involving more COPD patients than that in the study by Dong et al. and performed a detailed analysis with respect to the exercise capacity, the quality of life, dyspnoea, and lung function.
Pulmonary rehabilitation is a healthy approach for patients with COPD. The main purpose of pulmonary rehabilitation training is to formulate a corresponding program according to the actual situation of the patient to improve the patient’s quality of life and exercise endurance and improve the symptoms of dyspnoea [66]. In clinical practice, 6MWT is often used to assess the changes in the pulmonary functional ability of COPD patients after pulmonary rehabilitation [67]. In this study, pulmonary rehabilitation group showed significantly increased 6MWT distance following intervention when compared individually to the control group, in accordance with the relevant literature [17,40,43]. The evaluation of HRQOL is also a critical issue that needs to be considered while formulating a treatment strategy and evaluating the results. Furthermore, pulmonary rehabilitation has a significant improvement in the quality of life according to the change in SGRQ total score. It is well-documented that individuals with COPD have impaired HRQOL [58,68]. Previous studies demonstrated that SGRQ scores in all areas were improved in COPD patients after pulmonary rehabilitation [69,70].
In COPD Dyspnoea is one of the main respiratory symptoms. At present, several scales can be used to classify and characterize dyspnoea: clinical scale (such as MRC) and psychophysical scale (such as Borg scale) are the most commonly used scales in daily clinical practice [71]. However, from a methodological point of view, special attention should be paid when using Borg scale. Operators must assess the patient's emotional orientation beforehand, make sure they know all the information they need to complete the scale, and that the symptom score is related to feelings, i.e. not judged or corrected. Dyspnoea, as a limiting factor in physical activity, is a chief complaint in patients with COPD. The present study showed that pulmonary rehabilitation significantly altered MRC, as described previously [17,41,54].
Nevertheless, the present meta-analysis has some limitations. First, we did not look for unpublished literature, which is not in line with Cochrane’s method. Second, the heterogeneity of different types of pulmonary rehabilitation programs, their intensity, duration, and quality of learning were prepared for intervention. These factors are incommensurable in the majority of the experiments. Furthermore, the duration of treatment regimens was inconsistent in different studies. Also, although we have summarized the results of all trials, the sample size in the current review might not be sufficient to exclude any significant experimental errors. Finally, although a series of outcome measures are used, the impact of the smallest clinically significant difference based on each indicator may not be reflected.
Conclusions
Despite the limitations, this meta-analysis confirmed that Yoga and Tai Chi have significantly improved the FEV1% predicted value. The pulmonary rehabilitation program improves the exercise capacity, the quality of life, and dyspnoea in patients with COPD. However, additional studies on large datasets and well-designed models are required to substantiate these findings.
Supplementary Material
Author contributions
Liming Lou involved in the conception and design. Liming Lou, Hong Zhang, Dandan Hu and Lixia Wu analysed and interpreted of the data. Yikai Xu drafted of the paper, revised it critically for intellectual content; and the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Incorvaia C, Panella L, Caserta A, et al. . What still prevents to acknowledge a major role for pulmonary rehabilitation in COPD treatment? Acta Biomed. 2019;90(3):218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hillas G, Perlikos F, Tzanakis N.. Acute exacerbation of COPD: is it the "stroke of the lungs"? Int J Chron Obstruct Pulmon Dis. 2016;11:1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathers CD, Loncar D.. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou M, Wang H, Zhu J, et al. . Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the global burden of disease study 2013. The Lancet. 2016;387(10015):251–272. [DOI] [PubMed] [Google Scholar]
- 5.Wang C, Xu J, Yang L, et al. . Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China pulmonary health [CPH] study): a national cross-sectional study. The Lancet. 2018;391(10131):1706–1717. [DOI] [PubMed] [Google Scholar]
- 6.Nici L, Donner C, Wouters E, et al. . American thoracic society/european respiratory society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173(12):1390–1413. [DOI] [PubMed] [Google Scholar]
- 7.Mccarthy B, Casey D, Devane D, et al. . Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coventry PA, Hind D.. Comprehensive pulmonary rehabilitation for anxiety and depression in adults with chronic obstructive pulmonary disease: systematic review and meta-analysis. J Psychosom Res. 2007;63(5):551–565. [DOI] [PubMed] [Google Scholar]
- 9.Steiner MC, Morgan MD.. Enhancing physical performance in chronic obstructive pulmonary disease. Thorax. 2001;56(1):73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lareau SC, Fahy B.. Pulmonary rehabilitation. Am J Respir Crit Care Med. 2018;198(10):P19–P20. [DOI] [PubMed] [Google Scholar]
- 11.Brooke M, Spiliopoulos N, Collins M.. A review of the availability and cost effectiveness of chronic obstructive pulmonary disease (COPD) management interventions in rural Australia and New Zealand. Rural Remote Health. 2017;17(3):4017. [DOI] [PubMed] [Google Scholar]
- 12.Pierobon A, Bottelli ES, Ranzini L, et al. COPD patients’ self-reported adherence, psychosocial factors and mild cognitive impairment in pulmonary rehabilitation. Int J Chron Obstruct Pulmon Dis. 2017;12:2059–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gimenez M, Pham Q, Uffholtz H, et al. . Ten years follow up in patients with chronic obstructive lung disease submitted to a programme of pulmonary rehabilitation. London, England: SAGE Publications Sage UK; 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffiths TL, Burr ML, Campbell IA, et al. . Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet. 2000;355(9201):362–368. [DOI] [PubMed] [Google Scholar]
- 15.Boxall AM, Barclay L, Sayers A, et al. . Managing chronic obstructive pulmonary disease in the community. A randomized controlled trial of home-based pulmonary rehabilitation for elderly housebound patients. J Cardiopulm Rehabil. 2005;25(6):378–385. [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb V, Lyngsø AM, Nybo B, et al. . Pulmonary rehabilitation for moderate COPD (GOLD 2)-does it have an effect? Copd. 2011;8(5):380–386. [DOI] [PubMed] [Google Scholar]
- 17.Gurgun A, Deniz S, Argın M, et al. . Effects of nutritional supplementation combined with conventional pulmonary rehabilitation in muscle-wasted chronic obstructive pulmonary disease: a prospective, randomized and controlled study. Respirology. 2013;18(3):495–500. [DOI] [PubMed] [Google Scholar]
- 18.Chan AW, Lee A, Lee DT, et al. . The sustaining effects of Tai chi Qigong on physiological health for COPD patients: a randomized controlled trial. Complement Ther Med. 2013;21(6):585–594. [DOI] [PubMed] [Google Scholar]
- 19.Kantatong T, Panpanich R, Deesomchok A, et al. . Effects of the tai chi qigong programme on functional capacity, and lung function in chronic obstructive pulmonary disease patients: a ramdomised controlled trial. J Tradit Complement Med. 2020;10(4):354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng L, Chiang LK, Tang R, et al. . Effectiveness of incorporating Tai Chi in a pulmonary rehabilitation program for Chronic Obstructive Pulmonary Disease (COPD) in primary care – a pilot randomized controlled trial. Eur J Integr Med. 2014;6(3):248–258. [Google Scholar]
- 21.Niu R, He R, Luo B-L, et al. . The effect of tai chi on chronic obstructive pulmonary disease: a pilot randomised study of lung function, exercise capacity and diaphragm strength. Heart, Lung and Circulation. 2014;23(4):347–352. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Wu K, Chen X, et al. . The effects of tai chi on lung function, exercise capacity and health related quality of life for patients with chronic obstructive pulmonary disease: a pilot study. Heart Lung Circ. 2019;28(8):1206–1212. [DOI] [PubMed] [Google Scholar]
- 23.Yeh GY, Roberts DH, Wayne PM, et al. . Tai chi exercise for patients with chronic obstructive pulmonary disease: a pilot study. Respir Care. 2010;55(11):1475–1482. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu S, Shi K, Yan J, et al. . A modified 6-form Tai Chi for patients with COPD. Complement Ther Med. 2018;39:36–42. [DOI] [PubMed] [Google Scholar]
- 25.Donesky-Cuenco D, Nguyen HQ, Paul S, et al. . Yoga therapy decreases dyspnea-related distress and improves functional performance in people with chronic obstructive pulmonary disease: a pilot study. J Altern Complement Med. 2009;15(3):225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuoka A, Ueda M, Ariyama Y, et al. . Effect of laughter yoga on pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. 2016. [Google Scholar]
- 27.Gupta A, Gupta R, Sood S, et al. . Pranayam for treatment of chronic obstructive pulmonary disease: results from a randomized, controlled trial. Integr Med A Clin J. 2014;13(1):26. [PMC free article] [PubMed] [Google Scholar]
- 28.Kaminsky DA, Guntupalli KK, Lippmann J, et al. . Effect of yoga breathing (pranayama) on exercise tolerance in patients with chronic obstructive pulmonary disease: a randomized, controlled trial. J Altern Complement Med. 2017;23(9):696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranjita R, Hankey A, Nagendra H, et al. . Yoga-based pulmonary rehabilitation for the management of dyspnea in coal miners with chronic obstructive pulmonary disease: a randomized controlled trial. J Ayurveda Integr Med. 2016;7(3):158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thokchom SK, Gulati K, Ray A, et al. . Effects of yogic intervention on pulmonary functions and health status in patients of COPD and the possible mechanisms. Complement Ther Clin Pract. 2018;33:20–26. [DOI] [PubMed] [Google Scholar]
- 31.Yudhawati R, Hs MR.. Effect of yoga on FEV1, 6-minute walk distance (6-MWD) and quality of life in patients with COPD group B. Adv Respir Med. 2019;87(5):261–268. [DOI] [PubMed] [Google Scholar]
- 32.Puhan MA, Lareau SC.. Evidence-based outcomes from pulmonary rehabilitation in the chronic obstructive pulmonary disease patient. Clin Chest Med. 2014;35(2):295–301. [DOI] [PubMed] [Google Scholar]
- 33.Lacasse Y, Martin S, Lasserson T, et al. . Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. A cochrane systematic review. Eura Medicophys. 2007;43(4):475–485. [PubMed] [Google Scholar]
- 34.Malaguti C, Dal Corso S, Janjua S, et al. . Supervised maintenance programmes following pulmonary rehabilitation compared to usual care for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2021;2021(8):CD013569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins JPT, Altman DG, Gotzsche PC, et al. . The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928–d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duval S, Tweedie RL.. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95(449):89–98. [Google Scholar]
- 37.Engstrom C, Persson LO, Larsson S, et al. . Long-term effects of a pulmonary rehabilitation programme in outpatients with chronic obstructive pulmonary disease: a randomized controlled study. Scand J Rehabil Med. 1999;31(4):207–213. [DOI] [PubMed] [Google Scholar]
- 38.Ringbaek TJ, Broendum E, Hemmingsen L, et al. . Rehabilitation of patients with chronic obstructive pulmonary disease. Exercise twice a week is not sufficient!. Respir Med. 2000;94(2):150–154. [DOI] [PubMed] [Google Scholar]
- 39.Finnerty JP, Keeping I, Bullough I, et al. . The effectiveness of outpatient pulmonary rehabilitation in chronic lung disease* a randomized controlled trial. Chest. 2001;119(6):1705–1710. [DOI] [PubMed] [Google Scholar]
- 40.Karapolat H, Atasever A, Atamaz F, et al. . Do the benefits gained using a Short-Term pulmonary rehabilitation program remain in COPD patients after participation? Lung. 2007;185(4):221–225. [DOI] [PubMed] [Google Scholar]
- 41.Paz-Díaz H, Montes de Oca M, López JM, et al. . Pulmonary rehabilitation improves depression, anxiety, dyspnea and health status in patients with COPD. Am J Phys Med Rehabil. 2007;86(1):30–36. [DOI] [PubMed] [Google Scholar]
- 42.Eli A, Breki e, Ovayolu N, et al. . The efficacy and applicability of a pulmonary rehabilitation programme for patients with COPD in a secondary-care community hospital. Respirology. 2008;13(5):703–707. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez AM, Pascual J, Ferrando C, et al. . Home-based pulmonary rehabilitation in very severe COPD: is it safe and useful? J Cardiopulm Rehabil Prev. 2009;29(5):325–331. [DOI] [PubMed] [Google Scholar]
- 44.Theander K, Jakobsson P, Jorgensen N, et al. . Effects of pulmonary rehabilitation on fatigue, functional status and health perceptions in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Clin Rehabil. 2009;23(2):125–136. [DOI] [PubMed] [Google Scholar]
- 45.van Wetering CR, Hoogendoorn M, Broekhuizen R, et al. . Efficacy and costs of nutritional rehabilitation in muscle-wasted patients with chronic obstructive pulmonary disease in a community-based setting: a prespecified subgroup analysis of the INTERCOM trial. J Am Med Dir Assoc. 2010;11(3):179–187. [DOI] [PubMed] [Google Scholar]
- 46.Chan AW, Lee A, Suen LK, et al. . Tai chi Qigong improves lung functions and activity tolerance in COPD clients: a single blind, randomized controlled trial. Complement Ther Med. 2011;19(1):3–11. [DOI] [PubMed] [Google Scholar]
- 47.Deering BM, Fullen B, Egan C, et al. . Acupuncture as an adjunct to pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2011;31(6):392–399. [DOI] [PubMed] [Google Scholar]
- 48.Baumann HJ, Kluge S, Rummel K, Klose H, et al. . Low intensity, long-term outpatient rehabilitation in COPD: a randomised controlled trial. Respir Res. 2012;13(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Souto Araujo ZT, de Miranda Silva Nogueira PA, Cabral EE, et al. . Effectiveness of low-intensity aquatic exercise on COPD: a randomized clinical trial. Respir Med. 2012;106(11):1535–1543. [DOI] [PubMed] [Google Scholar]
- 50.Amin S, Abrazado M, Quinn M, et al. . A controlled study of community-based exercise training in patients with moderate COPD. BMC Pulm Med. 2014;14(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lahham A, McDonald CF, Moore R, et al. . The impact of home-based pulmonary rehabilitation on people with mild chronic obstructive pulmonary disease: a randomised controlled trial. Clin Respir J. 2020;14(4):335–344. [DOI] [PubMed] [Google Scholar]
- 52.Cameron-Tucker HL, Wood-Baker R, Owen C, et al. . Chronic disease self-management and exercise in COPD as pulmonary rehabilitation: a randomized controlled trial. Int J Chron Obstruct Pulmon Dis. 2014;9:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Roos P, Lucas C, Strijbos JH, et al. . Effectiveness of a combined exercise training and home-based walking programme on physical activity compared with standard medical care in moderate COPD: a randomised controlled trial. Physiotherapy. 2018;104(1):116–121. [DOI] [PubMed] [Google Scholar]
- 54.de Sousa Pinto JM, Martín-Nogueras AM, Calvo-Arenillas JI, et al. . Clinical benefits of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2014;34(5):355–359. [DOI] [PubMed] [Google Scholar]
- 55.Ko FW, Tam W, Siu EHS, et al. . Effect of short-course exercise training on the frequency of exacerbations and physical activity in patients with COPD: a randomized controlled trial. Respirology. 2021;26(1):72–79. [DOI] [PubMed] [Google Scholar]
- 56.Varas AB, Córdoba S, Rodríguez-Andonaegui I, et al. . Effectiveness of a community-based exercise training programme to increase physical activity level in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Physiother Res Int. 2018;23(4):e1740. [DOI] [PubMed] [Google Scholar]
- 57.Wootton SL, Ng LW, McKeough ZJ, et al. . Ground-based walking training improves quality of life and exercise capacity in COPD. Eur Respir J. 2014;44(4):885–894. [DOI] [PubMed] [Google Scholar]
- 58.Ries AL, Bauldoff GS, Carlin B, et al. . Pulmonary rehabilitation: joint ACCP/AACVPR Evidence-Based clinical practice guidelines. Chest. 2007;131(5):4S–42S. [DOI] [PubMed] [Google Scholar]
- 59.Spruit MA, Singh SJ, Garvey C, et al. . An official american thoracic society/European respiratory society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–e64. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Loprinzi PD, Yang L, et al. . The beneficial effects of traditional chinese exercises for adults with low back pain: a meta-analysis of randomized controlled trials. Medicina. 2019;55(5):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zou L, Zhang Y, Yang L, et al. . Are mindful exercises safe and beneficial for treating chronic lower back pain? A systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2019;8(5):628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X, Fu C, Hu W, et al. . The effect of tai chi on the pulmonary rehabilitation of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ann Palliat Med. 2021;10(4):3763–3782. [DOI] [PubMed] [Google Scholar]
- 63.Cramer H, Haller H, Klose P, et al. . The risks and benefits of yoga for patients with chronic obstructive pulmonary disease: a systematic review and Meta-analysis. Clin Rehabil. 2019;33(12):1847–1862. [DOI] [PubMed] [Google Scholar]
- 64.Zou L, Sasaki JE, Wei G-X, et al. . Effects of mind–body exercises (tai chi/yoga) on heart rate variability parameters and perceived stress: a systematic review with meta-analysis of randomized controlled trials. J Clin Med. 2018;7(11):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong J, Li Z, Luo L, et al. . Efficacy of pulmonary rehabilitation in improving the quality of life for patients with chronic obstructive pulmonary disease: evidence based on nineteen randomized controlled trials. Int J Surg. 2020;73:78–86. [DOI] [PubMed] [Google Scholar]
- 66.Vagvolgyi A, Rozgonyi Z, Kerti M, et al. . Effectiveness of pulmonary rehabilitation and correlations in between functional parameters, extent of thoracic surgery and severity of post-operative complications: randomized clinical trial. J Thorac Dis. 2018;10(6):3519–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jenkins S. 6-Minute walk test in patients with COPD: clinical applications in pulmonary rehabilitation. Physiotherapy. 2007;93(3):175–182. [Google Scholar]
- 68.Wadell K, Sundelin G, Henriksson-Larsén K, et al. High intensity physical group training in water-an effective training modality for patients with COPD. Respir Med. 2004;98(5):428–438. [DOI] [PubMed] [Google Scholar]
- 69.Seymour JM, Moore L, Jolley CJ, et al. . Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax. 2010;65(5):423–428. [DOI] [PubMed] [Google Scholar]
- 70.Ninot G, Moullec G, Picot MC, et al. . Cost-saving effect of supervised exercise associated to COPD self-management education program. Respir Med. 2011;105(3):377–385. [DOI] [PubMed] [Google Scholar]
- 71.Crisafulli E, Clini EM.. Measures of dyspnea in pulmonary rehabilitation. Multidiscip Respir Med. 2010;5(3):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.