Keywords: adolescents, efficacy, Goji berry, Lycium barbarum polysaccharide, randomized clinical trial, safety, subthreshold depression, tolerance
Abstract
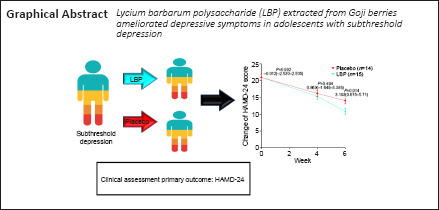
Subthreshold depression is a highly prevalent condition in adolescents who are at high risk for developing major depressive disorder. In preclinical models of neurological and psychiatric diseases, Lycium barbarum polysaccharide (LBP) extracted from Goji berries had anti-depressant effects including but not limited to anti-oxidative and anti-inflammatory properties. However, the effect of LBP on subthreshold depression is unclear. To investigate the clinical efficacy and safety of LBP for treating subthreshold depression in adolescents, we conducted a randomized, double-blind, placebo-controlled trial (RCT) with 29 adolescents with subthreshold depression recruited at The Fifth Affiliated Hospital of Guangzhou Medical University. The participants were randomly assigned to groups where they received either 300 mg LBP (LBP group, n = 15, 3 boys and 12 girls aged 15.13 ± 2.17 years) or a placebo (placebo group, n = 14, 2 boys and 12 girls aged 15 ± 1.71 years) for 6 successive weeks. Interim analyses revealed that the LBP group exhibited a greater change in Hamilton Depression Scale (HAMD-24) scores relative to the baseline and a higher remission rate (HAMD-24 total score ≤ 7) at 6 weeks compared with the placebo group. Scores on the Beck Depression Inventory-II (BDI-II), Pittsburgh Sleep Quality Index (PSQI), Kessler Psychological Distress Scale (Kessler), and Screen for Child Anxiety-Related Emotional Disorders (SCARED) were similar between the LBP and placebo groups. No side effects related to the intervention were reported in either group. These results indicate that LBP administration reduced depressive symptoms in adolescents with subthreshold depression. Furthermore, LBP was well tolerated with no treatment-limiting adverse events. Clinical trials involving a larger sample size are needed to further confirm the anti-depressive effects of LBP in adolescents with subthreshold depression. This study was approved by the Medical Ethics Committee of the Fifth Affiliated Hospital of Guangzhou Medical University (Guangzhou, China; approval No. L2019-08) on April 4, 2019 and was registered on ClinicalTrials.gov (identifier: NCT04032795) on July 25, 2019.
Chinese Library Classification No. R453.9; R749.4+1; R285
Introduction
Subthreshold depression (StD) is characterized by clinically significant depressive symptoms that fall short of the criteria for DSM-defined major depressive disorder (MDD) (Rodríguez et al., 2012). StD is highly prevalent in adolescents, with a lifetime prevalence of approximately 16% (Crockett et al., 2020). It is a leading cause of disability in adolescents, and results in significant functional impairment, compromised quality of life, and increased risk of suicidality (Wesselhoeft et al., 2013; Crockett et al., 2020). Moreover, individuals with StD are at high risk of developing MDD (Lee et al., 2019). Evidence regarding successful treatments for adolescent with StD is limited (Wesselhoeft et al., 2013; Carrellas et al., 2017). Although anti-depressants, psychotherapy, and a combination of both have been suggested to alleviate depressive symptoms, more than 30% of trials failed to achieve a sufficient treatment response (Rush et al., 2006). Furthermore, there is increasing public concern about the side effects and tolerability of anti-depressants (Hetrick et al., 2021). In addition, the cost-effectiveness of psychotherapy may restrict its wide application in adolescents with StD (Cuijpers et al., 2007; Wesselhoeft et al., 2013). Alternative treatments for adolescents with StD are needed to support the mental health of this population and prevent subsequent MDD.
Although the pathogenesis of depressive symptoms has not been characterized, it is known to be multifactorial. For example, disturbances in oxidative stress (OS) have been hypothesized as a potential effector system. Recent studies have repeatedly reported disturbed OS in the prefrontal cortex and hippocampus in individuals with MDD (Bhatt et al., 2020; Nakao et al., 2021; Xu and Xu, 2021), suggesting that depressive symptoms are related to OS in the central nervous system. OS is thought to be caused by an imbalance between the production and accumulation of oxygen reactive species and the anti-oxidant capacity of cells and tissues (Bhatt et al., 2020). Preclinical and clinical studies have further confirmed that depressive symptoms are ameliorated by anti-oxidants (Silva et al., 2016; di Michele et al., 2020). These data imply that anti-oxidants might act as potential anti-depressant agents.
Lycium barbarum polysaccharide (LBP) is a bioactive compound extracted from the Goji berry. It is a form of Chinese traditional herbal medicine with disparate pharmacological properties including immune regulation and anti-aging effects (Xiao et al., 2021). LBP has neuroprotective properties that are likely associated with its anti-oxidative and anti-inflammatory action (Chen et al., 2014; Zhao et al., 2017). For example, LBP was shown to have protective effects in models of partial optic transection injury and focal cerebral ischemic injury (Chu et al., 2013; Zhao et al., 2017). Recently, several research teams including ours showed that LBP might have a therapeutic effect in animal models of depression (Zhao et al., 2019; Fu et al., 2021). This effect appeared to be related to the reduction of neuronal activity in the lateral habenula via a reduced inflammatory reaction (Fu et al., 2021). Given the considerations above, we conducted a 6-week randomized placebo-controlled trial to assess the efficacy of LBP in adolescents with StD. We hypothesized that LBP would reduce depressive symptoms compared to placebo in adolescents with StD.
Participants and Methods
Study design and participants
We conducted a 6-week double-blind randomized placebo-controlled trial to evaluate the efficacy of LBP in adolescents with StD. The study took place at the Fifth Affiliated Hospital of Guangzhou Medical University (Guangzhou, China). The participants were recruited via medical referrals, social media, and media announcements in communities. Ethical approval was obtained from the Medical Ethics Committee of the Fifth Affiliated Hospital of Guangzhou Medical University (Guangzhou, China; approval No. L2019-08) on April 4, 2019 (Additional file 1 (104.4KB, pdf) ). The trial complied with the Declaration of Helsinki and was prospectively registered on ClinicalTrials.gov (Identifier ID: NCT04032795) on July 25, 2019. The participants and their guardians provided written informed consent (Additional file 2 (307.5KB, pdf) ). The writing and editing of this article were performed in accordance with the CONsolidated Standards Of Reporting Trials (CONSORT) Statement (Additional file 3 (486KB, pdf) ).
In this study, we classified individuals as having StD if they scored 15–25 on the Beck Depression Rating Scale (BDI-II) (Beck et al., 1996) but fell short of the criteria for MDD. Youths aged 12 to 18 years were recruited at the Fifth Affiliated Hospital of Guangzhou Medical University (Guangzhou, China). Eligible participants were identified by an experienced psychiatrist (XM) according to our definition of StD. Participants were excluded if they: had been diagnosed with MDD or any other psychiatric disorders according to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-V) criteria (First et al., 2016); had a severe physical disease; had consumed Chinese herbs containing Goji berries in the past 3 months; or were taking hormone or endocrine medications.
Randomization and study procedure
LBP extract was prepared as previously reported (provided by Tianren Ningxia Wolfberry Biotechnology Co., Ltd., Zhongwei, China) (Yang et al., 2012). To Extrapolate the effective dose in previous animal studies (Zhao et al., 2019; Fu et al., 2021), we tentatively used a higher dose of 300 mg/d in human. The participants were randomly assigned in a 1:1 allocation ratio to groups that either took 300 mg LBP (Tablet) or a placebo (a compound of microcrystalline cellulose and starch) once daily for 6 weeks (Both the LBP and placebo were provided by Tianren Ningxia Wolfberry Biotechnology Co., Ltd.). The randomization list was generated by a computer (Excel 2013; Microsoft Office software, Seattle, WA, USA) and held securely by an independent investigator (KL) at the Affiliated Brain Hospital of Guangzhou Medical University. The assessors and participants were all blinded to the treatment allocation. The LBP and placebo tablets were identical in appearance and taste.
Assessments
All assessments were conducted by a trained investigator (XM), who evaluated the participants at baseline, week 4, and week 6. The primary outcome was the change in Hamilton Depression Scale (HAMD-24) score (Bech et al., 1975) from baseline to week 6. The HAMD-24 is used to measure the severity of depression (Amick et al., 2015). It contains 24 items, each of which has five response options (0 to 4). Factors measured by the HAMD-24 scale include anxiety/somatization, weight loss, cognitive impairment, circadian fluctuations, retardation, sleep disturbances, and hopelessness. Secondary outcomes were: the proportion of participants who showed a treatment response (reduction of 50% or more from the baseline) or remission (HAMD-24 total score ≤ 7); changes in BDI-II, Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989), Kessler Psychological Distress Scale (Kessler) (Kessler et al., 2003), and Screen for Child Anxiety-Related Emotional Disorders (SCARED) scores (Birmaher et al., 1997) from baseline to follow-up; tolerability and safety as assessed by the Treatment Emergent Symptom Scale (TESS) (Guy, 1976), which included items measuring behavioral toxicity, laboratory abnormalities, nervous system function, automatic nervous system function, cardiovascular system function, and six other aspects.
Statistical analysis
The sample size for this trial protocol was estimated by GPower 3.1.1 (HHU, Dusseldorf, Germany) and set at 86 per group, assuming a power of 80% and α of 5%, as well as a 20% withdrawal rate for the treatment effect to be robustly detected. Following a procedure coordinated with the Medical Ethics Committee of the Fifth Affiliated Hospital of Guangzhou Medical University, the responsible clinician (XM) requested an interim analysis for clinical consideration. This interim analysis was conducted with a data cutoff on October 30, 2020. The study profile is shown in Figure 1.
Figure 1.
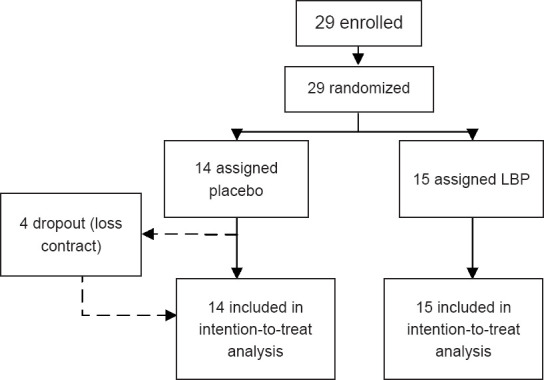
Scheme for group assignment.
LBP: Lycium barbarum polysaccharide.
Demographics and clinical variables were analyzed using a Student's t-test or non-parametric tests (Mann-Whitney test and chi-square test). The primary analysis of the effect of the intervention (LBP or placebo) on the HAMD-24 score was conducted using a linear mixed-effect model, with the HAMD-24 total score as the dependent variable. As fixed effects, we entered the intervention group (LBP or placebo), baseline scores, age, sex, a 3-level categorical variable for time (0, 4, or 6 weeks into the trial), and the interaction between intervention group and time into the model. Between-group comparisons at baseline, week 4, and week 6 for estimated marginal means were reported.
Controlling for baseline score, we used a binary logistic model to analyze the associations between response or remission of HAMD-24 total score and intervention group. The stability of the model was evaluated using a bootstrap method with 1000 resampling steps. We estimated the regression coefficient and 95% confidence intervals (CI). For the HAMD-24 scale, BDI-II, Kessler, and PSQI scores, we used a linear mixed-effect model to analyze the differences between the baseline values and those at week 6. All analyses were conducted following the intent-to-treat principle. Missing data were estimated using a last-observation-carried-forward approach. A two-tailed P value was set at 0.05. Statistical analyses were carried out using SPSS version 24.0 (IBM, Armonk, NY, USA) and graphs were plotted with GraphPad Prism 8.0 software (GraphPad Software Inc., San Diego, CA, USA).
Results
Participant characteristics
Between May 15, 2019 and October 30, 2020, a total of 29 participants were deemed eligible and thus assigned to either the LBP (n = 15) or placebo (n = 14) group. Four participants in the placebo group dropped out during the follow-up period due to loss of contact. As a result, 25 participants completed the study (placebo group: n = 10; LBP group: n = 15).
No significant differences in sociodemographic or clinical characteristics were found between the two groups (P > 0.05) (Table 1).
Table 1.
Demographic and clinical characteristics
| Item | Placebo group (n = 14) | LBP group (n = 15) | t/Z/χ2-value | P-value |
|---|---|---|---|---|
| Age (yr) | 15±1.71 | 15.13±2.17 | –0.183 | 0.856 |
| Gender (female) | 12(85.7) | 12(80) | 0.166 | 0.684 |
| Education (yr) | 9 | 10 | –0.356 | 0.722 |
| HAMD-24 | 20.57±6.81 | 20.87±5.57 | –0.128 | 0.899 |
| BDI-II | 31.29±10.97 | 29.40±9.4 | 0.498 | 0.622 |
| Kessler | 36.57±7.63 | 32.87±6.76 | 1.386 | 0.177 |
| PSQI | 11.00±3.72 | 10.27±3.92 | 0.516 | 0.610 |
| SCARED | 48.43±11.61 | 43.75±13.42 | 1 | 0.637 |
Data are present as mean ± SD (age, HAMD-24, BDI-II, Kessler, PSQI and SCARED) for t-test, number (percentage) (gender) for chi-square test and median (education) for Mann-Whitney test. BDI-II: Beck Depression Inventory-II; HAMD-24: Hamilton Depression Rating Scale; Kessler: The Kessler Psychological Distress Scale; LBP: Lycium barbarum polysaccharide; PSQI: The Pittsburgh Sleep Quality Index; SCARED: Screen for Child Anxiety-Related Emotional Disorders.
Primary outcomes
The total HAMD-24 scores based on estimated marginal means at baseline, week 4, and week 6 are displayed in Figure 2. The difference between the groups gradually increased over the 6-week interval. At the study endpoint (week 6), the estimated group difference in HAMD-24 score between the placebo and the LBP (placebo minus LBP) group was 3.149 (95% CI: 0.675–5.710; F = 6.374; P = 0.014; Cohen's d = 0.860), indicating a greater decrease in depressive symptoms in the LBP group.
Figure 2.
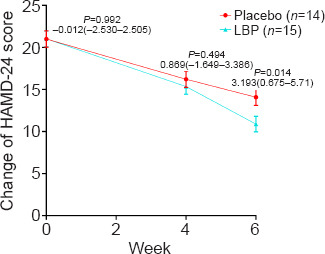
Change in HAMD-24 scores at 0, 4, and 6 weeks in the LBP and placebo groups.
The mean HAMD-24 scores in the intervention groups from the baseline assessment to that at 6 weeks. The reduction in HAMD-24 score was greater in the LBP group than in the placebo group at week 6 (P < 0.05). Error bars represent the 95% CI of the mean. CI: Confidence interval; HAMD-24: Hamilton Depression Rating Scale; LBP: Lycium barbarum polysaccharide.
As for the factors measured by the HAMD-24 (Figure 3A), we found significant changes in cognitive impairment, retardation, and sleep disturbances in both groups between the baseline assessment and week 6. For those in the placebo group, these reductions in score were 1.214 (95% CI: 0.411–2.017; P < 0.01), 1.357 (95% CI: 0.105–2.609; P = 0.029), and 1.286 (95% CI: 0.277–2.295; P < 0.01), respectively. The estimated reductions in the LBP group were 2.2 (95% CI: 1.424–2.976; P < 0.01), 2.333 (95% CI: 1.124–3.543; P < 0.01), and 1.667 (95% CI: 0.692–2.641; P < 0.01), respectively. The factor of hopelessness significantly decreased by 1.6 (95% CI: 0.739–2.461; P < 0.01) from the baseline assessment to week 6 in the LBP group. At week 6, compared with the placebo group, the LBP group exhibited a greater reduction in cognitive impairment (group difference: 0.895; 95% CI: 0.251–1.539; F = 7.663; P = 0.007; Cohen's d = 0.879), retardation (group difference: 1.085; 95% CI: 0.081–2.089; F = 4.628; P = 0.035; Cohen's d = 0.82), and hopelessness (group difference: 0.814; 95% CI: 0.097–1.53; F = 5.111; P = 0.027; Cohen's d = 0.832) (Table 2).
Figure 3.
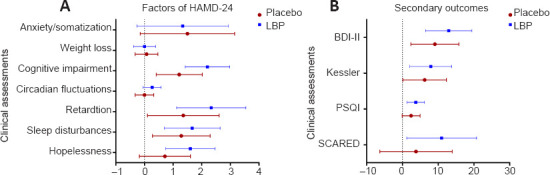
Change in assessment scores from baseline to 6 weeks in the LBP and placebo groups.
(A) Factors measured by the HAMD-24. (B) Secondary outcomes. Results are presented as the change (95% CI) in measurement scores based on the estimated marginal mean from the baseline assessment to that at 6 weeks (baseline score minus that at 6 weeks). Error bars represent the 95% CI of the mean. BDI-II: Beck Depression Inventory-II; CI: confidence interval; HAMD-24: Hamilton Depression Rating Scale; Kessler: The Kessler Psychological Distress Scale; PSQI: The Pittsburgh Sleep Quality Index; SCARED: Screen for Child Anxiety-Related Emotional Disorders.
Table 2.
Differences in clinical characteristics between the treatment groups at 6 weeks
| Clinical assessments | Difference between groups at 6 week (95% CI) | P-value |
|---|---|---|
| HAMD-24 | – | – |
| Anxiety/somatization | –0.156(–1.480–1.167) | 0.815 |
| Weight loss | –0.027(–0.347–0.293) | 0.868 |
| Cognitive impairment | 0.895(0.251–1.539) | 0.007 |
| Circadian fluctuations | 0.06(–0.208–0.328) | 0.657 |
| Retardation | 1.085 (0.081–2.089) | 0.035 |
| Sleep disturbances | 0.592(–0.218–1.401) | 0.15 |
| Hopelessness | 0.814(0.097–1.530) | 0.027 |
| BDI-II | 4.140(–1.247–9.527) | 0.13 |
| Kessler | 2.454(–2.461–7.368) | 0.323 |
| PSQI | 1.496(–0.517–3.51) | 0.143 |
| SCARED | 7.591(–0.556–15.737) | 0.067 |
Results are present as difference (95% CI) of measurement scores based on estimated marginal mean at 6 weeks between intervention groups (Placebo minus LBP). BDI-II: Beck Depression Inventory-II; CI: confidence interval; HAMD-24: Hamilton Depression Rating Scale; Kessler: The Kessler Psychological Distress Scale; PSQI: The Pittsburgh Sleep Quality Index; SCARED: Screen for Child Anxiety-Related Emotional Disorders.
Secondary outcomes
After the 6-week intervention, the response rates for the LBP and placebo groups were 40% and 35.71%, respectively. The logistic model showed that the intervention did not significantly affect the response rate (P = 0.575). The remission rates for the LBP and placebo groups were 33.3% and 7.14%, respectively. We found a significant effect of the intervention on remission (Bootstrap regression coefficient: 2.476; 95% CI: 0.033–50.9; P = 0.03), indicating a greater chance of remission in the LBP group.
In both groups, the BDI-II and Kessler scale scores were significantly reduced from the baseline assessment to that at week 6 (Figure 3B). In the placebo group, these reductions were 9.071 (95% CI: 2.354–15.789; P < 0.01) and 6.214 (95% CI: 0.144–12.285; P = 0.043), respectively. In the LBP group, the estimated reductions were 12.933 (95% CI: 6.443–19.423; P < 0.01) and 7.933 (95% CI: 2.069–13.798; P < 0.01), respectively. No between-group differences were found in the BDI-II and Kessler scores from the baseline assessment to that at 6 weeks (P > 0.05) (Table 2).
In the LBP group, the PSQI and SCARED scores were significantly reduced to 3.733 (95% CI: 1.308–6.159; P < 0.01) and 10.953 (95% CI: 1.186–20.720; P < 0.05), respectively (Figure 3B). No significant changes in PSQI or SCARED scores were observed in the placebo group. There were no between-group differences in PSQI or SCARED scores from the baseline assessment to that at 6 weeks (P > 0.05) (Table 2).
As for safety and tolerability, four participants reported losing weight during the intervention period (one from the LBP group; three from the placebo group). There were no significant differences in adverse events including weigh lost between groups (P > 0.05).
Discussion
To the best of our knowledge, this preliminary randomized placebo-controlled trial is the first to report on the efficacy and tolerability of LBP in adolescents with StD. Despite the small sample size of this interim analysis, our major finding was that 6 weeks of 300 mg/d LBP ameliorated depressive symptoms in adolescents with StD compared with those who took a placebo. Moreover, adolescents with StD in the LBP group were more likely to achieve remission than those in the placebo-controlled group.
Following oral administration of LBP, improvements were observed in core depressive symptoms, including cognitive impairment, retardation, and hopelessness. Thus, the findings of this trial support the anti-depressant effect of LBP with a relatively large effect size.
Previous studies have demonstrated that commonly used anti-depressants, including selective serotonin reuptake inhibitors, affect the regulation of OS and anti-oxidative substances, which partly contributes to the treatment effects (Rebai et al., 2017; Ştefan et al., 2020). We speculate that the anti-depressant effect of LBP is related to its anti-oxidation and anti-inflammatory properties.
Psychotherapy, such as cognitive-behavioral therapy and interpersonal therapy, are primarily recommended for treating StD (Wesselhoeft et al., 2013; Hetrick et al., 2021). The benefit of the LBP intervention in our preliminary trial was substantially greater than the effect size reported in a meta-analysis of trials in which psychotherapy was used to ameliorate depressive symptoms in individuals with StD (Cuijpers et al., 2007). Moreover, the large effect size for the influence of LBP in reducing total HAMD-24 scores (as well as reducing the scores in the sub-factors of cognitive impairment, retardation, and hopelessness) was similar to those in MDD clinical trials with anti-depressant treatments (Lin et al., 2015).
At week 6, we observed significant differences between the LBP and placebo groups in terms of the HAMD-24 total score but not the BDI-II score. The discrepancy may be partly due to the heterogeneity of the measurement tools. Another possibility is that the trial duration was not sufficiently long for differences in BDI-II score to occur between the groups. A beneficial effect of LBP treatment on BDI-II score might be observed if the duration of the intervention is prolonged.
The safety and tolerability of LBP across the 6-week treatment were comparable to those of the placebo. The participants reported no severe adverse events related to the interventions. Although four participants reported losing weight during the intervention period, these reported events were more likely to be related to depressive symptoms than the intervention. To our knowledge, Goji berries have been widely used as food and medicine in China for thousands of years (Tian et al., 2019), and the safety and tolerability profiles of LBP have been confirmed (Cai et al., 2015; Chan et al., 2019; Zhou et al., 2021). However, the long-term safety and tolerability of LBP in adolescents should be further evaluated. It warrants further studies regarding whether LBP has preventive effects on mood disorders such as major depression disorder.
This interim analysis has several limitations. Given the preliminary nature of the current analysis, the generalizability of the findings of this trial are limited by its small size and sample distribution (80% were girls). Although we detected a significant group difference at the study endpoint, the results are susceptible to these biases and thus should be interpreted with caution.
In addition, we observed comparable treatment response rates (≥ 50% HAMD-24 score reduction) in the LBP and placebo groups. It is possible that in this sample of StD adolescents, who had depressive characteristics that were less severe than MDD patients, non-specific therapeutic effects due to the procedure, such as repeated study visits by investigators, may have lead to improved symptoms (Husain et al., 2020). However, as our results showed, the remission rate was almost five times higher in the LBP group than in the placebo group, supporting the treatment efficacy of the 6-week LBP intervention. Another limitation is that the optimal dose of LBP is still unclear. Further clinical trials are needed to establish the effective dose range of LBP.
In conclusion, this clinical trial suggests that 6 weeks of 300 mg/d LBP could ameliorate depressive symptoms in adolescents with StD. The findings reported herein provide preliminary evidence for the use of LBP as an effective treatment option for depressive symptoms in adolescents. Future studies are needed to replicate and validate our findings.
Additional files:
Additional file 1 (104.4KB, pdf) : Hospital ethics approval (Chinese).
Additional file 2 (307.5KB, pdf) : Informed consent form (Chinese).
Additional file 3 (486KB, pdf) : CONSORT checklist.
Acknowledgments
We express our gratitude to all participants and staffs involved in the data collection for this study. We are grateful for the Department of Affective Disorders at the Fifth Affiliated Hospital of Guangzhou Medical University (Guangzhou, China).
Footnotes
Conflicts of interest: The authors declare that they have no conflict of interest.
Editor note: KFS and KL are Editorial Board members of Neural Regeneration Research. They were blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer review handled independently of these Editorial Board member and their research groups.
Financial support: This study was financially supported by The Science and Technology Program of Guangzhou, China, No. 202007030012 (to KFS and KL); and the National Natural Science Foundation of China, No. 81671347 (to KL). The funders of this study played no role in study design, data collection, data analysis, and writing of the manuscript. Corresponding authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
Institutional review board statement: The study was approved by the Medical Ethics Committee of the Fifth Affiliated Hospital of Guangzhou Medical University (Guangzhou, China; approval No. L2019-08) on April 4, 2019 and was registered on ClinicalTrials.gov (Identifier ID: NCT04032795) on July 25, 2019.
Declaration of participant consent: The authors certify that they have obtained the consent forms from participants and their guardians. In the form, participants’ legal guardians have given their consent for participants’ clinical information to be reported in the journal. The participants’ legal guardians understand that patients’ names and initials will not be published.
Reporting statement: The writing and editing of the article were performed in accordance with the CONsolidated Standards Of Reporting Trials (CONSORT) Statement.
Biostatistics statement: The statistical methods of this study were reviewed by the epidemiologist of The Affiliated Brain Hospital of Guangzhou Medical University, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: The study protocol and the statistical analysis plan as well as all data supporting this finding in this interim analysis are available to be shared in 5 years after publication. Qualified researchers should provide proposals to linkangguang@163.com and sign a data access agreement.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was financially supported by the National Natural Science Foundation of China, No. 81671347 (to KL); and The Science and Technology Program of Guangzhou, China, No. 202007030012 (to KFS and KL).
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y.
References
- 1.Amick HR, Gartlehner G, Gaynes BN, Forneris C, Asher GN, Morgan LC, Coker-Schwimmer E, Boland E, Lux LJ, Gaylord S, Bann C, Pierl CB, Lohr KN. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta-analysis. BMJ. 2015;351:h6019. doi: 10.1136/bmj.h6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bech P, Gram LF, Dein E, Jacobsen O, Vitger J, Bolwig TG. Quantitative rating of depressive states. Acta Psychiatr Scand. 1975;51:161–170. doi: 10.1111/j.1600-0447.1975.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 3.Beck AT, Steer RA, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 4.Bhatt S, Nagappa AN, Patil CR. Role of oxidative stress in depression. Drug Discov Today. 2020;25:1270–1276. doi: 10.1016/j.drudis.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 7.Cai H, Liu F, Zuo P, Huang G, Song Z, Wang T, Lu H, Guo F, Han C, Sun G. Practical application of antidiabetic efficacy of Lycium barbarum polysaccharide in patients with type 2 diabetes. Med Chem. 2015;11:383–390. doi: 10.2174/1573406410666141110153858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrellas NW, Biederman J, Uchida M. How prevalent and morbid are subthreshold manifestations of major depression in adolescents? A literature review. J Affect Disord. 2017;210:166–173. doi: 10.1016/j.jad.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 9.Chan HH, Lam HI, Choi KY, Li SZ, Lakshmanan Y, Yu WY, Chang RC, Lai JS, So KF. Delay of cone degeneration in retinitis pigmentosa using a 12-month treatment with Lycium barbarum supplement. J Ethnopharmacol. 2019;236:336–344. doi: 10.1016/j.jep.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Cheng X, Chen J, Yi X, Nie D, Sun X, Qin J, Tian M, Jin G, Zhang X. Lycium barbarum polysaccharides prevent memory and neurogenesis impairments in scopolamine-treated rats. PLoS One. 2014;9:e88076. doi: 10.1371/journal.pone.0088076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu PH, Li HY, Chin MP, So KF, Chan HH. Effect of lycium barbarum (wolfberry) polysaccharides on preserving retinal function after partial optic nerve transection. PLoS One. 2013;8:e81339. doi: 10.1371/journal.pone.0081339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crockett MA, Martínez V, Jiménez-Molina Á. Subthreshold depression in adolescence: gender differences in prevalence, clinical features, and associated factors. J Affect Disord. 2020;272:269–276. doi: 10.1016/j.jad.2020.03.111. [DOI] [PubMed] [Google Scholar]
- 13.Cuijpers P, Smit F, van Straten A. Psychological treatments of subthreshold depression: a meta-analytic review. Acta Psychiatr Scand. 2007;115:434–441. doi: 10.1111/j.1600-0447.2007.00998.x. [DOI] [PubMed] [Google Scholar]
- 14.di Michele F, Talamo A, Niolu C, Siracusano A. Vitamin D and N-acetyl cysteine supplementation in treatment-resistant depressive disorder patients: a general review. Curr Pharm Des. 2020;26:2442–2459. doi: 10.2174/1381612826666200406090051. [DOI] [PubMed] [Google Scholar]
- 15.First MB, Williams JBW, Benjamin LS, Spitzer RL. Clinical Trials Version. Washington, DC: American Psychiatric Association Publishing; 2016. Structured clinical interview for DSM-V. [Google Scholar]
- 16.Fu YW, Peng YF, Huang XD, Yang Y, Huang L, Xi Y, Hu ZF, Lin S, So KF, Ren CR. Lycium barbarum polysaccharide-glycoprotein preventative treatment ameliorates aversive. Neural Regen Res. 2021;16:543–549. doi: 10.4103/1673-5374.293156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guy W. Rockville, MD: National Institute of Mental Health; 1976. ECDEU Assessment Manual for Psychopharmacology, Revised. US Department of Health, Education, and Welfare Publication (ADM) [Google Scholar]
- 18.Hetrick SE, McKenzie JE, Bailey AP, Sharma V, Moller CI, Badcock PB, Cox GR, Merry SN, Meader N. New generation antidepressants for depression in children and adolescents: a network meta-analysis. Cochrane Database Syst Rev. 2021;5:Cd013674. doi: 10.1002/14651858.CD013674.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husain MI, Chaudhry IB, Khoso AB, Husain MO, Hodsoll J, Ansari MA, Naqvi HA, Minhas FA, Carvalho AF, Meyer JH, Deakin B, Mulsant BH, Husain N, Young AH. Minocycline and celecoxib as adjunctive treatments for bipolar depression: a multicentre, factorial design randomised controlled trial. Lancet Psychiatry. 2020;7:515–527. doi: 10.1016/S2215-0366(20)30138-3. [DOI] [PubMed] [Google Scholar]
- 20.Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, Howes MJ, Normand SL, Manderscheid RW, Walters EE, Zaslavsky AM. Screening for serious mental illness in the general population. Arch Gen Psychiatry. 2003;60:184–189. doi: 10.1001/archpsyc.60.2.184. [DOI] [PubMed] [Google Scholar]
- 21.Lee YY, Stockings EA, Harris MG, Doi SAR, Page IS, Davidson SK, Barendregt JJ. The risk of developing major depression among individuals with subthreshold depression: a systematic review and meta-analysis of longitudinal cohort studies. Psychol Med. 2019;49:92–102. doi: 10.1017/S0033291718000557. [DOI] [PubMed] [Google Scholar]
- 22.Lin CH, Chou LS, Chen MC, Chen CC. The relationship between symptom relief and functional improvement during acute fluoxetine treatment for patients with major depressive disorder. J Affect Disord. 2015;182:115–120. doi: 10.1016/j.jad.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Nakao A, Matsunaga Y, Hayashida K, Takahashi N. Role of oxidative stress and Ca(2+) signaling in psychiatric disorders. Front Cell Dev Biol. 2021;9:615569. doi: 10.3389/fcell.2021.615569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rebai R, Jasmin L, Boudah A. The antidepressant effect of melatonin and fluoxetine in diabetic rats is associated with a reduction of the oxidative stress in the prefrontal and hippocampal cortices. Brain Res Bull. 2017;134:142–150. doi: 10.1016/j.brainresbull.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez MR, Nuevo R, Chatterji S, Ayuso-Mateos JL. Definitions and factors associated with subthreshold depressive conditions: a systematic review. BMC Psychiatry. 2012;12:181. doi: 10.1186/1471-244X-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 27.Silva MC, de Sousa CN, Gomes PX, de Oliveira GV, Araújo FY, Ximenes NC, da Silva JC, Vasconcelos GS, Leal LK, Macêdo D, Vasconcelos SM. Evidence for protective effect of lipoic acid and desvenlafaxine on oxidative stress in a model depression in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:142–148. doi: 10.1016/j.pnpbp.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Ştefan MG, Kiss B, Gutleb AC, Loghin F. Redox metabolism modulation as a mechanism in SSRI toxicity and pharmacological effects. Arch Toxicol. 2020;94:1417–1441. doi: 10.1007/s00204-020-02721-6. [DOI] [PubMed] [Google Scholar]
- 29.Tian X, Liang T, Liu Y, Ding G, Zhang F, Ma Z. Extraction, structural characterization, and biological functions of Lycium barbarum polysaccharides: A Review. Biomolecules. 2019;9:389. doi: 10.3390/biom9090389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wesselhoeft R, Sørensen MJ, Heiervang ER, Bilenberg N. Subthreshold depression in children and adolescents - a systematic review. J Affect Disord. 2013;151:7–22. doi: 10.1016/j.jad.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Xiao Z, Deng Q, Zhou W, Zhang Y. Immune activities of polysaccharides isolated from Lycium barbarum L. What do we know so far? Pharmacol Ther. 2021 doi: 10.1016/j.pharmthera.2021.107921. doi: 10.1016/j.pharmthera.2021.107921. [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Xu DS. Prospects for the application of transcranial magnetic stimulation in diabetic neuropathy. Neural Regen Res. 2021;16:955–962. doi: 10.4103/1673-5374.297062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang D, Li SY, Yeung CM, Chang RC, So KF, Wong D, Lo AC. Lycium barbarum extracts protect the brain from blood-brain barrier disruption and cerebral edema in experimental stroke. PLoS One. 2012;7:e33596. doi: 10.1371/journal.pone.0033596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao P, Zhou R, Zhu XY, Liu G, Zhao YP, Ma PS, Wu W, Niu Y, Sun T, Li YX, Yu JQ, Qian ZM. Neuroprotective Effects of Lycium barbarum Polysaccharide on Focal Cerebral Ischemic Injury in Mice. Neurochem Res. 2017;42:2798–2813. doi: 10.1007/s11064-017-2293-x. [DOI] [PubMed] [Google Scholar]
- 35.Zhao R, Master BQ, Master BM, Cai Y. Improving activity of Lycium barbarum. polysaccharide on depressive mice induced by reserpine. Iran J Pharm Res. 2019;18:1556–1565. doi: 10.22037/ijpr.2019.1100763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou J, Li H, Wang F, Wang H, Chai R, Li J, Jia L, Wang K, Zhang P, Zhu L, Yang H. Effects of 2, 4-dichlorophenoxyacetic acid on the expression of NLRP3 inflammasome and autophagy-related proteins as well as the protective effect of Lycium barbarum polysaccharide in neonatal rats. Environ Toxicol. 2021 doi: 10.1002/tox.23358. doi: 10.1002/tox.23358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


