Abstract
Building a complex structure such as the cell wall, with many individual parts that need to be assembled correctly from distinct sources within the cell, is a well-orchestrated process. Additional complexity is required to mediate dynamic responses to environmental and developmental cues. Enzymes, sugars, and other cell wall components are constantly and actively transported to and from the plasma membrane during diffuse growth. Cell wall components are transported in vesicles on cytoskeletal tracks composed of microtubules and actin filaments. Many of these components, and additional proteins, vesicles, and lipids are trafficked to and from the cell plate during cytokinesis. In this review, we first discuss how the cytoskeleton is initially organized to add new cell wall material or to build a new cell wall, focusing on similarities during these processes. Next, we discuss how polysaccharides and enzymes that build the cell wall are trafficked to the correct location by motor proteins and through other interactions with the cytoskeleton. Finally, we discuss some of the special features of newly formed cell walls generated during cytokinesis.
The cell wall is assembled via vesicle trafficking along cytoskeletal filaments during growth and division.
Introduction
The study of structure and function of the cell wall began unintentionally in 1665 when Robert Hooke examined oak (Quercus suber) cork under a microscope. He coined the term “cell” to describe the smallest living biological structure based on its similarity to a monk’s room (cell). Since the cellular contents were gone, Hooke actually visualized the cell wall. Since then, modern cell wall research benefited from the characterization of structural components of the extracted cell wall (Harris et al., 2010). Even more recently, cell biology, including live-cell imaging, has been instrumental in advancing our understanding of transport and synthesis of cell wall components and their interaction with the cytoskeleton. Due to space limitations, this review focuses primarily on cytoskeleton-based trafficking of cell wall synthesis and modification enzymes during diffuse growth and cytokinesis. There are excellent reviews of cell wall synthesis and cell growth focused more on tip growing cells (Chebli et al., 2021), cell wall and cytoskeletal modifications during the plant immune response (Bacete et al., 2018; Li and Staiger, 2018), cell wall integrity sensing (Wolf et al., 2012), and secondary cell wall synthesis (Schneider et al., 2016; Meents et al., 2018; Coomey et al., 2020).
Cell division and expansion are two primary generators of plant biomass. As a major component of primary cell walls, cellulose is the most abundant biopolymer on the planet. In addition to global biomass, production and modification of the cell wall is vital to plants. Indeed, around 10% of some plant genomes are invested in cell wall formation and modification (Carpita and McCann, 2008) with an additional hundreds to thousands of genes involved in regulating the cytoskeleton and vesicle trafficking.
Two different stages of cell wall formation are discussed: the de novo construction of the cell wall during cytokinesis and the addition of new cell wall material into already extant cell walls during diffuse growth. In both cases, vesicles containing cell wall materials are trafficked on the cytoskeleton by motor proteins. Vesicles typically originate from the Golgi and are trafficked on the cytoskeleton often through the trans Golgi network (TGN) to and from the plasma membrane and the cell plate. Therefore, properly organized microtubules and microfilaments are critical for the positioning and construction of cell wall components. For example, the rapid and properly positioned addition of cellulose during diffuse growth relies on well-organized microtubules due to direct interactions between microtubules, microtubule linking proteins, and enzymes that generate cell wall polymers called cellulose synthases.
The de novo cell wall is initiated by a conserved land–plant specific structure called the phragmoplast during division (Jürgens, 2005; Buschmann and Zachgo, 2016). The main purpose of the phragmoplast is to generate a new cell wall after separation of duplicated DNA during cytokinesis (Smertenko et al., 2017; Lee and Liu, 2019). An intermediate form of the new cell wall, called the cell plate, is constructed prior to the completion of cytokinesis. Generation and expansion of the cell plate are accompanied by coordinated vesicle trafficking and fusion as well as changes in cell wall composition (Sinclair et al., 2018). The phragmoplast, which contains short actin filaments, forms as an antiparallel array of microtubules, with their plus-ends facing the middle of the cell (Smertenko et al., 2018). The microtubules within the phragmoplast are assembled from spindle microtubules followed by phragmoplast expansion mediated by microtubule-dependent microtubule nucleation (Smertenko et al., 2011; Murata et al., 2013). These microtubules are then disassembled as the cell plate expands (Sasabe and Machida, 2012). The phragmoplast contacts the cortex in a defined location known as the division site or cell plate fusion site (Smertenko et al., 2017; Rasmussen and Bellinger, 2018; Livanos and Müller, 2019). Similar to diffuse growth, cytokinesis depends heavily on proper organization of the cytoskeleton. Therefore, microtubules and microfilaments and proteins that affect cytoskeletal dynamics and organization are discussed in the next section. Specific proteins mentioned in the text can be found in Supplemental Data Set S1.
Factors that organize microtubules and microfilaments at the cell cortex during diffuse growth
Microtubules and actin microfilaments serve as tracks along which motor proteins move cargo, including cell wall components. Factors that mediate microtubule and microfilament positioning play a critical yet sometimes indirect role in how the cell wall is modified, expanded, or generated. Here, we discuss how microtubule and microfilament orientation at the cell cortex are influenced by cell shape and mechanical forces.
Observation of cortical microtubules oriented parallel to the long axis of elliptical or rectangular microchambers supports the hypothesis that cell geometry determines microtubule orientation (Lagomarsino et al., 2007). Microtubule organization was recently empirically tested in protoplasts, which are single cells with enzymatically removed cell walls. When protoplasts are confined in rectangular wells, microtubules align along the long axis, leading to the hypothesis that default microtubule orientation is longitudinal (Durand-Smet et al., 2020). In contrast, in planta, microtubules are often perpendicular to the long axis in many cell types (Hejnowicz, 2000; Hamant et al., 2008; Jacques et al., 2013; Sampathkumar et al., 2014). Therefore, microtubules must be re-oriented: a weak directional cue is sufficient to induce microtubule orientation switching from longitudinal to transverse in silico (Mirabet et al., 2018). Mechanical stress is proposed as one of the directional cues. The simplest model states that microtubules respond to mechanical stress and then guide the orientation of cellulose microfibrils along the direction of maximal tension (Green, 1964). In elongating cells, the growth direction is perpendicular, while microtubules align parallel to the predicted maximal tensile stress direction (Fischer and Schopfer, 1997, 1998; Baskin et al., 1999; Hejnowicz et al., 2000; Sugimoto et al., 2001; Burian et al., 2013). When the stress pattern is changed by laser ablation of nearby cells, microtubules become circumferential in meristems, cotyledons, and hypocotyls (Hamant et al., 2008; Sampathkumar et al., 2014; Robinson and Kuhlemeier, 2018). To further test the microtubule orienting mechanism, spherical protoplasts were confined in rectangular wells in solutions of different osmolarity. The tension induced by imposed reduction in osmolarity bypasses the geometrical cues to orient microtubules along predicted maximal tension after 2 h (Colin et al., 2020). It is unlikely that plant cells undergo similar osmotic changes and it remains to be determined how the change in osmotic potential translates into change in tensile stress. Microtubules may respond to the changes in tensile stress directly, but most likely multiple other proteins are required. Future research is needed to test whether microtubules, their regulators, or other factors are involved in sensing maximal tension.
Actin has vital roles in cell elongation and vesicle trafficking in tip growing cells such as root hairs and pollen tubes, beautifully reviewed here (Szymanski and Staiger, 2018). The importance of actin during diffuse growth and phragmoplast assembly is clear, but the underlying mechanisms are still unknown. Actin filament alignment becomes more parallel and ordered in elongating Arabidopsis (Arabidopsis thaliana) root cells, but the significance is thus far unknown (Dyachok et al., 2011; Arieti and Staiger, 2020). Disruption of the actin cytoskeleton disrupts Golgi movement and influences cellulose synthase complex (CSC) delivery to the plasma membrane (discussed in the “The assembly and trafficking of cellulose synthase complex” section) (Gutierrez et al., 2009; Sampathkumar et al., 2013). Actin may also mediate delivery of noncellulosic polysaccharides due to cell adhesion defects seen in actin and actin regulatory mutants and alteration in wall components by latrunculin B treatment (El-Din El-Assal et al., 2004; Chen et al., 2007; Dyachok et al., 2008). Actin plays different roles during different stages of phragmoplast assembly. Phragmoplast assembly was delayed when the actin-monomer stabilizing protein profilin was microinjected (Valster et al., 1997). In contrast, the transport of NACK1 kinesin (discussed in the “Plus-end-directed kinesins” section) to the phragmoplast midline during early phragmoplast development is sped up by disrupting actin filaments with latrunculin B (Maeda et al., 2020).
Actin motor proteins, myosins, also influence diffuse and polarized growth. There are 13 myosin XIs in Arabidopsis with often redundant functions. Myosin double, triple, and quadruple mutants have reduced cell expansion as do cells treated with a myosin ATPase inhibitor (Samaj et al., 2000; Peremyslov et al., 2008; Peremyslov et al., 2010; Madison et al., 2015). In moss, Myosin XI interacts with RabE and localizes to the phragmoplast midline. The interaction between RabE and myosin XI is critical for polarized growth (Sun et al., 2018; Orr et al., 2020). Reduction in the plant-specific Myosin VIII activity also slows cell expansion in moss (Physcomitrium (Physcomitrella) patens; Wu et al., 2011). Myosin contribution to diffuse growth may be due to its role in Golgi movement within the cell, leading to well-distributed cellulose synthase deposition.
Actin regulatory proteins called formins have demonstrated roles in polarized and diffuse growth (e.g. VidaLi et al., 2009; Cheung et al., 2010; Zhang et al., 2011b; Liu et al., 2018, 2021; Kollárová et al., 2021) but their roles in cytokinesis are less clear. Arabidopsis FORMIN HOMOLOGY5 (FH5) localizes to the cell plate and promotes actin filament formation in vitro. fh5 mutants have reduced endosperm cellularization, suggesting slower cytokinesis (Ingouff et al., 2005). Additionally, FH5 interacts with the membrane-tubulation protein SH3P2 which has an essential role in cell plate formation (Baquero Forero and Cvrčková, 2019). Rice (Oryza sativa) FH5 and Arabidopsis FH4 bind both microtubules and actin, indicating crosstalk between microtubules and the actin cytoskeleton (Deeks et al., 2010; Yang et al., 2011). Lesions in rice FH5 result in compromised longitudinal actin cables but they do not affect the organization of microtubules. However, the short and swollen internode cells of the fh5 (bui1) mutant indicate defects in diffuse growth (Yang et al., 2011; Zhang et al., 2011b). Similar to FH4/FH5, Arabidopsis FORMIN 14 co-localizes with microtubules, binds actin and microtubules in vitro, and is important for cytokinesis during male meiocyte development (Li et al., 2010; Du et al., 2021).
Several valuable approaches targeting groups of formins have also been used to clarify their functions. When all of the class 1 formins were silenced with RNAi in moss, tip growth was unaffected but plants were smaller with fewer cells, suggesting delayed cytokinesis. No stubs or multinucleate cells were observed (VidaLi et al., 2009). Despite often non-obvious roles in cytokinesis, formins sometimes localize to the phragmoplast or phragmoplast midline (e.g. Li et al., 2010; Wu and Bezanilla, 2014; van Gisbergen et al., 2020). The small molecule formin inhibitor SMIFH2, which sidesteps potentially redundant formin function, was used to understand how formins contribute to mitosis and cytokinesis (Zhang et al., 2021). SMIFH2 treatment reduces formin recruitment to the phragmoplast, slows telophase progression, and generates wavy cell plates. Treatment with SMIFH2 also reduces EB1 comet length, and alters turnover of vesicle fusion proteins DYNAMIN RELATED PROTEIN 1A (DRP1A) and KNOLLE (Zhang et al., 2021). This powerful tool might also inhibit myosins in plants as it does in other organisms (Nishimura et al., 2020). Although SMIFH2 concentrations used in tobacco were 10–20-fold lower than those reported in Nishimura et al. (2020), it remains to be conclusively determined whether SMIFH2-induced cytokinesis defects are attributable to disruption solely of formins. Combinations of loss-of-function formin mutants may provide conclusive evidence about their roles in growth and cytokinesis.
Factors that organize microtubules and microfilaments at the cell cortex during telophase in preparation for cytokinesis
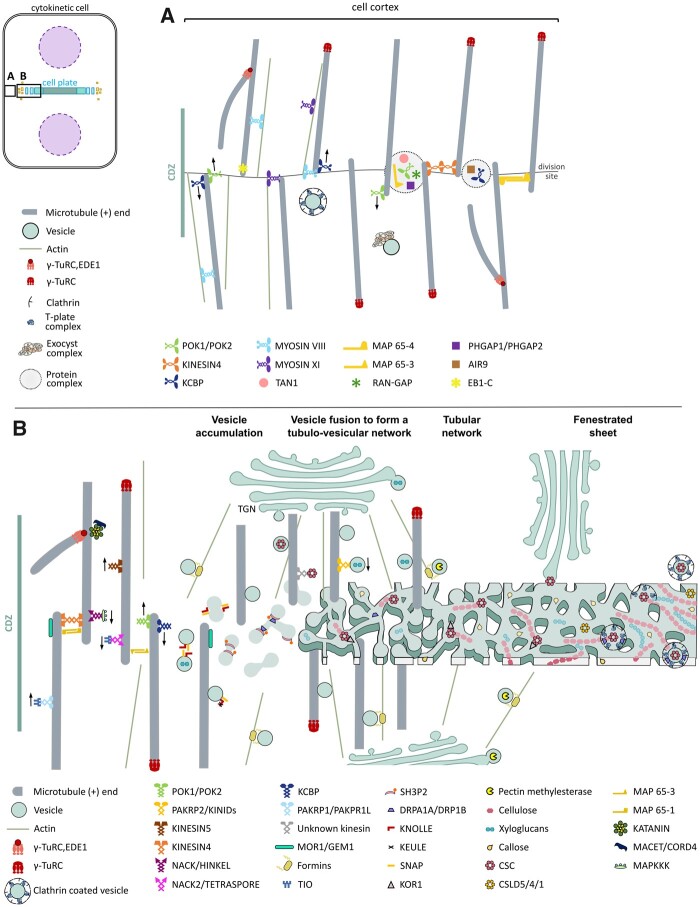
During telophase, but before the phragmoplast reaches the cell cortex, actin filaments and cortical telophase microtubules accumulate at the cell cortex (Figure 1, A; Schmit and Lambert, 1988; Liu and Palevitz, 1992; Panteris et al., 1995; Wu and Bezanilla, 2014). The microtubules nucleate from cortex-localized gamma tubulin and gamma tubulin ring complex structures and/or from the nuclear envelope (McDonald et al., 1993; Panteris et al., 1995; Van Damme, 2009; Kong et al., 2010). What microtubules and microfilaments do at the cell cortex during this stage is not well understood, but recent data suggest that they may be organized by interactions with proteins that localize to the division site (described in the “Proteins that localize to the division site before the phragmoplast reaches the cortex” section). Cortical telophase microtubules interact with TANGLED1 (TAN1) and other division site-localized proteins through transient microtubule plus-end stabilization, leading to their perpendicular orientation, similar to the orientation of the phragmoplast itself (Bellinger et al., 2021). Microtubule plus-end capture is consistent with the in vitro role of TAN1 angle-independent microtubule crosslinking and bundling (Martinez et al., 2020). Cortical telophase microtubules are added into the phragmoplast as it approaches the cell cortex. Further, if many cortical telophase microtubules accumulate on one side of the phragmoplast, they incorporate by parallel bundling into phragmoplast and then phragmoplast expansion along the cortex moves toward the accumulated cortical telophase microtubules. Therefore, cortical telophase microtubules fine tune the position of the phragmoplast to the division site, but it is still unknown how microtubule-binding proteins at the division site other than TAN1 interact with cortical telophase microtubules (Bellinger et al., 2021).
Figure 1.
Model of land-plant cytokinesis. A, The cell cortex underlying the plasma membrane before it comes into contact with the phragmoplast. B, Phragmoplast, membrane, and cell wall structures that form the cell plate from left to right: vesicle accumulation, vesicle fusion to form a tubulo-vesicular network, a tubular network, and fenestrated sheet.
Proteins that localize to the division site before the phragmoplast reaches the cortex
Proteins that accumulate at the division site before the phragmoplast contacts the cell cortex often have essential roles in cytokinesis or phragmoplast positioning. These include the microtubule-crosslinking protein TAN1, the microtubule binding kinesins PHRAGMOPLAST ORIENTING KINESIN (POK1) and POK2 (discussed in the “Plus-end-directed kinesins” section), PLECKSTRIN HOMOLOGY ROP-GAPs (PH-GAPs), the actin and microtubule binding myosin MYOSIN VIII (discussed in the “Phragmoplast cortex interactions” section), MYOSINXI (1, 2, K) (discussed in the next paragraph), and several IQ67 Domain (IQD) proteins. TAN1 is critical for phragmoplast positioning in maize, and bundles microtubules in vitro (Cleary and Smith, 1998; Walker et al., 2007; Martinez et al., 2017, 2020; Smith et al. 1996, 2001). In Arabidopsis, tan1 mutants do not have aberrant phenotypes (Walker et al., 2007), unless combined with a mutant in AUXIN INDUCED IN ROOT CULTURES9 (AIR9), which itself does not have a phenotype deviating from the wild-type situation (Buschmann et al., 2015; Mir et al., 2018). In contrast, tan1 air9 double mutants have division plane defects and short roots (Mir et al., 2018). Mitotic expression of TAN1 restores wild-type growth to the tan1 air9 double mutant (Mills et al., 2021). The AIR9 protein localizes to the phragmoplast and accumulates at the division site after the phragmoplast contacts the cortex (Buschmann et al., 2006, 2015). Two PH-GAPs that interact with POK1 are redundantly required for division plane positioning and accumulate at the division site after metaphase (Stöckle et al., 2016). Finally, three related IQ67 DOMAIN (IQD6, 7, 8) proteins localize to the division site, interact with POK1, and have a role in division plane establishment (Kumari et al., 2021). Assessing localization of these division site proteins in various mutants will provide useful information about how distinct factors establish and maintain the division site until cytokinesis is complete.
Triple myosin XI (xi-1 xi-2 xi-k) mutants have defects in division plane orientation that are rescued by a MYOSIN XI-YFP that localizes to mitotic structures and also the division site. MYOSIN XIs (1, 2, K) promote division plane positioning but when they function during mitosis is currently unknown (Abu-Abied et al., 2018). OPAQUE1, a myosin XI similar to Arabidopsis XI-I, is essential for proper ER organization in maize kernels (Wang et al., 2012) OPAQUE1 is required for phragmoplast guidance in asymmetric divisions and localizes to the phragmoplast midline (Nan et al., 2021). Interestingly, OPAQUE1 interacts with other myosins, actin-binding proteins, and maize POKs. One hypothesis is that OPAQUE1-POK interactions promote phragmoplast guidance to the division site, although this remains to be experimentally tested (Nan et al., 2021).
Several other proteins also localize to the division site and are vital for cytokinesis but their functions in division plane positioning are unclear. The monomeric GTPase RAN GTPase ACTIVATING PROTEINS (RAN-GAPs) localize to the division site and the nuclear envelope, and their knockdown causes severe cytokinesis and growth defects preventing analysis of division plane positioning (Xu et al., 2008). Interestingly, RAN-GAP in onion (Allium cepa) localizes only transiently to the division site, indicating variation in RAN-GAP division site localization may vary across species (Yabuuchi et al., 2015). A microtubule associated protein (MAP) MAP65-4, described more in the “Microtubule bundling proteins with critical roles in diffuse growth and cytokinesis” section, localizes to the division site in addition to the phragmoplast midline, but what it does at the division site is not yet known (Li et al., 2017a). The exocyst complex proteins SECRETORY3 (SEC3), SEC6, SEC5 (and SEC5 paralogs) localize to the cell cortex before the phragmoplast touches the cortex as a wide band that reorients toward the tilting phragmoplast in Physcomitrium patens, but what they do at the cortex remains unknown (Tang et al., 2019).
Phragmoplast cortex interactions
When the phragmoplast reaches the cortex, interactions between the phragmoplast and cortex-localized microtubules and actin filaments guide the phragmoplast precisely to the division site. These interactions are mediated by division site-localized proteins, such as the microtubule and microfilament binding protein MYOSIN VIII in moss (Wu and Bezanilla, 2014). F-actin, Myosin, and cell cortex interactions play an important role in the last slow step of phragmoplast expansion to meet with the mother cell plasma membrane: disruption leads to defects in final phragmoplast-mediated contact with the cell cortex (Chua et al., 2002; Molchan et al., 2002; Gilliland et al., 2003; van Oostende-Triplet et al., 2017). Interestingly, when the phragmoplast is displaced by centrifugation, microns long actin filaments contact the division site. The phragmoplast is subsequently recruited back to the division site, suggesting long distance actindivision site interactions (Arima et al., 2018). Much remains to be learned about how actin and microtubules, and the proteins that crosslink them, function together to guide the phragmoplast to the division site and to complete cytokinesis.
Microtubule regulators such as nucleators, severing proteins, end-binding proteins, and cross-linkers are vital for proper cellular elongation and cytokinesis (Hamada, 2014). Their roles can often be clarified using genetic and biochemical analyses. While many microtubule-associated proteins are essential for both diffuse growth and cytokinesis, others have more specific roles.
Microtubule nucleators
Microtubule nucleators such as gamma tubulin, and the gamma-tubulin ring complex are essential for diffuse growth and cytokinesis (Liu et al., 1993; Shimamura et al., 2004; Murata et al., 2005; Pastuglia et al., 2006; Hotta et al., 2007; Seltzer et al., 2007; Ambrose and Wasteneys, 2014). Unlike animal cells, microtubules nucleate from the plasma membrane, the nucleus, or from preexisting microtubules (Murata et al., 2005; Hotta et al., 2007; Kong et al., 2010; Lee and Liu, 2019). Nucleation from preexisting microtubules depends on the AUGMIN complex and two MOZART1 homolog proteins GAMMA-TUBULIN COMPLEX3 INTERACTING PROTEIN1 (GIP1) and GIP2 to recruit gamma tubulin ring complexes (Ho et al., 2011b; Janski et al., 2012; Hotta et al., 2012; Nakamura et al., 2012; Nakaoka et al., 2012; Murata et al., 2013; Oh et al., 2016; Lee et al., 2017; Lee and Liu, 2019).
During cytokinesis, the phragmoplast expands by addition of new microtubules nucleating on the phragmoplast leading edge (Figure 1, B). Therefore, microtubule-dependent nucleation is critical for phragmoplast expansion (Smertenko et al., 2011; Murata et al., 2013). ENDOSPERM DEFECTIVE1 (EDE1) is a mitosis-specific protein that connects the AUGMIN complex to microtubules during spindle and phragmoplast formation. Phragmoplasts in ede1 mutants are misformed, but no cytokinetic defects are observed (Lee et al., 2017). Surprisingly, the GAMMA-TUBULIN COMPLEX PROTEIN6 (GCP6) is not required for phragmoplast assembly (Miao et al., 2019). Gamma-tubulin ring complexes and AUGMIN complex proteins play both conserved and specific roles in microtubule nucleation during diffuse growth and cytokinesis. It will be interesting to identify how specificity is determined during specific cell-cycle stages.
Microtubule severing proteins
The microtubule severing KATANIN (KTN) complex is composed of a p60 catalytic subunit and p80 microtubule-crossover-targeting subunits: both are required for proper microtubule severing in Arabidopsis (Bouquin et al., 2003; Wang et al., 2017a). Microtubule severing plays critical roles in microtubule array reorientation in response to intrinsic and extrinsic cues, including cell elongation and phragmoplast assembly and dynamics. Although mutants are viable, cell elongation and phragmoplast expansion rates are reduced in ktn1 mutants. KTN1 is required for microtubule reorientation in response to mechanical force, suggesting that severing promotes microtubule reorientation (Bouquin et al., 2003; Wang et al., 2017a). KTN1 is also required for proper phragmoplast expansion, positioning, and morphology (Panteris et al., 2011; Komis et al., 2017; Ovečka et al., 2020; Panteris et al., 2021). ktn1, also called fragile fiber2 (fra2) mutants have reduced callose accumulation and uneven cell plate formation (Panteris et al., 2021). KTN1 is thought to be recruited to the distal phragmoplast through interaction with the microtubule-binding protein MACET4/CORD4 to sever microtubules at the distal phragmoplast (Sasaki et al., 2019). Despite this, overexpression of MACET4/CORD4 in tobacco (Nicotiana tabacum) cultured cells did not lead to increased severing and its in vitro activity suggests it primarily nucleates microtubules (Schmidt and Smertenko, 2019). MACET4/CORD4 and KTN1 are both required for rapid phragmoplast expansion (Sasaki et al., 2019; Schmidt and Smertenko, 2019).
In the ktn1 mutant, cell shape, microtubule orientation, and reorganization in response to mechanical cues are disrupted (Komis et al., 2017). Circumferential microtubule orientation propagated across several cell files around the ablation location in wild-type shoot apical meristem cells while in ktn1 mutants circumferential orientation was limited to a single cell file (Uyttewaal et al., 2012). KTN1 is required for blue light-induced reorientation (Lindeboom et al., 2013). SPIRAL2/TORTIFOLIA1 is a plant-specific microtubule-binding protein required for promoting anisotropic growth and preventing organ twisting (Buschmann et al., 2004; Shoji et al., 2004). SPR2/TOR1 localizes to microtubule cross-overs and may block KTN1-mediated severing there (Wightman et al., 2013). An alternate or additional hypothesis is that SPR2/TOR1 stabilizes minus-ends to promote katanin-mediated severing (Nakamura et al., 2018). Various locations of SPR2 on microtubule-minus ends and microtubule crossovers may allow them to have opposite functions. Interestingly, AUGMIN reduction increases KTN1-mediated severing, suggesting that AUGMIN may also block KTN1 severing (Wang et al., 2018). Similarly, the microtubule bundling performed by MAP65-1 also prevents KTN1-mediated severing in vitro (Burkart and Dixit, 2019), within a time frame similar to induced cortical tension change (Colin et al., 2020). However, the mechanisms for mechanical force-dependent and blue light-induced microtubule reorientation are likely different.
Microtubule bundling proteins with critical roles in diffuse growth and cytokinesis
Microtubule Organization1/Gemini1 (MOR1/GEM1) has an essential role in microtubule organization. mor1 mutants disrupt diffuse growth and cytokinesis (Whittington et al., 2001; Twell et al., 2002; Kawamura et al., 2006). MOR1 contains conserved HEAT-repeat/TOG microtubule-binding domains (Whittington et al., 2001). MOR1 promotes microtubule bundling and polymerization and localizes to cortical microtubules, and mitotic microtubule arrays (Twell et al., 2002; Yasuhara et al., 2002; Hamada et al., 2004; Kawamura et al., 2006; Kawamura and Wasteneys, 2008). The temperature-sensitive mor1 mutants have been used to clarify roles in regulating microtubule dynamics and aberrant cell plate formation (Eleftheriou et al., 2005; Kawamura and Wasteneys, 2008; Steiner et al., 2016). In addition to defects in diffuse growth, mor1/gem1 mutants’ phragmoplasts sometimes split, leading to multiple aberrant new cell walls and irregular membrane accumulation in cell plates (Park and Twell, 2001; Eleftheriou et al., 2005). Interestingly despite the loss of parallel cortical microtubules and reduced microtubule polymer mass in the mor1 mutant at restrictive temperature, cell wall crystallinity remained high, suggesting that microtubule mass is inversely related to cellulose crystallinity (Fujita et al., 2011).
A related TOG-domain-containing protein, CLIP-associating protein (CLASP), has a critical role in organizing interphase microtubules and promoting growth, does not have a conclusive role during cytokinesis (Ambrose et al., 2007; Halat et al., 2020; Kirik et al., 2007). CLASP, which preferentially localizes to cell edges, promotes microtubule passage across cell edges by preventing edge-induced microtubule depolymerization (Ambrose et al., 2011). This edge-induced microtubule depolymerization is similar to collision-induced catastrophe when the microtubule cross-over angles are >40° (Dixit and Cyr, 2004), highlighting the importance of both microtubule–microtubule and microtubule–cell edge interactions in controlling microtubule organization.
Another MAP family of proteins, called MAP65 because its founding member is 65 kilodaltons (Chang-Jie and Sonobe, 1993; Chan et al., 1999; Smertenko et al., 2000), has various roles in diffuse growth and cytokinesis. MAP65 family members typically bundle microtubules (Smertenko et al., 2004; Ho et al., 2012). MAP65-2 is required for axial growth in dark-grown hypocotyls: map65-2 mutants have short hypocotyls. Generation of a double mutant with the related map65-1 strongly reduces both light- and dark-grown hypocotyl elongation. The synthetic phenotype of the map65-1 map65-2 double mutant indicates that these two proteins have overlapping roles in cell elongation (Lucas et al., 2011).
A few MAP65 family members play unique roles in phragmoplast organization during cytokinesis. The map65-3/pleiade mutant has defective cytokinesis because the two phragmoplast disks are spaced further apart, generating a wide phragmoplast midline (Söllner et al., 2002; Müller et al., 2004). MAP65-3 localizes to the phragmoplast midline and crosslinks antiparallel microtubules through its unique C-terminal domain. Other MAP65s do not rescue the map65-3 mutant even when expressed with the MAP65-3 promoter, supporting the unique role of MAP65-3 in phragmoplast organization (Ho et al., 2011a, 2012). Microtubule crosslinking is vital to accumulate microtubule plus-end directed EB1 particles and to restrict multiple plus-end directed kinesins in the midline, including several Kinesin 12 proteins (discussed in the “Plus-end-directed kinesins” section) (Ho et al., 2011a; Herrmann et al., 2018). MAP65-3, through multiple protein interactions, acts as a hub during cytokinesis. MAP65-3 interacts with POK2 and two closely related WD40 proteins, Budding Uninhibited by Benzimidazole (BUB) BUB3;1 and BUB3;2. Double bub3;1 bub3;2 mutants grow normally. However, when bub3;1 bub3;2 mutants are treated with caffeine, a drug known to disrupt phragmoplast attachment and maturation, MAP65-3 localization was abolished at the midline. Subsequent defects include phragmoplast expansion defects, short roots, and altered root cell morphology (Zhang et al., 2018). Interaction between MAP65-3 and its partners is required for their midline accumulation (Herrmann et al., 2018; Zhang et al., 2018). The localization and turnover of MAP65-3 from the phragmoplast midline in phosphoinositide kinase double mutants pi4k-beta1 pi4k-beta2 is altered. This is consistent with the overstabilization of the phragmoplast, possibly due to an endocytosis defect (Lin et al., 2019). Restriction of the phragmoplast midline mediated by MAP65s in moss promotes timely vesicle fusion (Kosetsu et al., 2013; de Keijzer et al., 2017). Indeed, microtubule overlaps recruit the exocyst complex protein SEC6, which in turn recruits the vesicle fusion protein KEULE. SEC6 reduction slowed recruitment of KEULE to the midline (Tang et al., 2019). Overall, MAP65-3 performs critical functions during cytokinesis by crosslinking antiparallel microtubules, recruiting or retaining proteins at the phragmoplast midline often through direct interaction, and promoting vesicle fusion.
In addition to MAP65-3, MAP65-1 and MAP65-2 have partially redundant roles in cytokinesis revealed by mutant interactions with map65-3. map65-1 map65-3 or map65-2 map65-3 double mutants have synthetic cytokinesis defects (Sasabe et al., 2011). map65-1 map65-2 double mutants do not have obvious cytokinesis defects although they produce fewer cells, suggesting they may still be involved in division (Lucas and Shaw, 2012). Interestingly, MAP65-1 localizes to the very leading edge of the phragmoplast, where it crosslinks antiparallel microtubules (Murata et al., 2013). Similarly, single map65-4 mutants do not have an aberrant phenotype, but when combined with map65-3 mutants, significant cytokinesis defects cause gametophyte lethality (Li et al., 2017a). MAP65-4 faintly localizes to the phragmoplast or phragmoplast midline (Smertenko et al., 2008). In the map65-3 mutant, MAP65-4 localizes more prominently in the phragmoplast midline to partially replace MAP65-3 antiparallel bundling function (Li et al., 2017a). MAP65-4 bundles parallel and antiparallel microtubules, and promotes rescue in vitro (Fache et al., 2010). MAP65-4 also localizes to the division site but its function there is unknown (Li et al., 2017a). Further combinatorial mutant analysis of this important family of microtubule-bundling proteins will clarify unique and synergistic functions.
Kinesins
Kinesins are mostly microtubule and sometimes also actin binding motor proteins that transport cargo including vesicles, organelles, and sometimes actin filaments or microtubules. Kinesins in plants either move toward the dynamic plus-end of microtubules or move toward the less dynamic minus-end of microtubules. The minus-end directed kinesins may functionally replace minus-end directed dyneins, which are rare in plants (Nebenführ and Dixit, 2018). Not surprisingly, kinesins are often required for cell expansion and/or cytokinesis.
The phragmoplast directs the movement of Golgi-derived vesicles, likely transported by plus-end directed kinesins, toward the newly developing cell plate. Indeed, even synthetic vesicles are efficiently transported to the newly developing cell plate (Esseling-Ozdoba et al., 2008). Although phragmoplast microtubules with attached vesicles have been observed by electron microscopy (Otegui et al., 2001), the specific kinesins have not been conclusively identified. However, a large number of kinesins play both essential and non-overlapping roles in cytokinesis and phragmoplast positioning, indicating the complexity in coordinating the cell plate formation (Nebenführ and Dixit, 2018; Smertenko et al., 2018). Many plus-end directed kinesins eventually accumulate in the phragmoplast midline, possibly reflecting the sheer abundance of microtubule plus-ends. It is likely that many plus-end directed kinesins transport vesicles containing both cell wall enzymes and precursors as well as proteins essential for regulating microtubule dynamics and vesicle fusion.
Plus-end-directed kinesins
The KINESIN-4 family kinesins play various roles in cell elongation and cytokinesis (Kong et al., 2015), depending on the plant species. FRAGILE FIBER1 (FRA1) was initially identified in a forward genetic screen for deficiency in mechanical strength of inflorescence stems (Zhong et al., 2002). FRA1 is a highly processive plus-end directed kinesin (Zhu and Dixit, 2011). Reduced mechanical strength in fra1 was attributed to defects in cellulose microfibril patterning (Zhong et al., 2002) but later findings demonstrate that fra1-5 does not alter cellulose organization, motility, or density of CSCs at the plasma membrane (Zhu et al., 2015). While FRA1 does not affect cellulose synthesis directly, it associates with cellulose synthase microtubule-uncoupling proteins (CMUs), proteins that bind microtubules, localize to the cell plate, and prevent cell twisting (Zhu et al., 2015; Liu et al., 2016). By modulating the interaction between FRA1 and CMUs, lateral displacement of microtubules is regulated. Microtubule detachment from the cortex may affect the delivery of polysaccharides to the apoplast, contributing to the cell wall defects in fra1-5 (Zhu and Dixit, 2011; Zhu et al., 2015; Ganguly et al., 2017; Ganguly et al., 2020). Indeed, fra1-5 has reduced secretion of fucose-alkyne-labeled pectin (Zhu et al., 2015). However, fra1-5 does not have any cytokinesis defects (Zhu et al., 2015). Similarly, Kinesin-4 in rice, BRITTLE CULM12, is required for proper cell wall composition and organization. Mutants have reduced cell number but lack obvious microtubule structural defects during cytokinesis (Zhang et al., 2010). By contrast, these proteins play a critical role in cytokinesis in moss: two closely related KINESIN-4s (Kin4-1a and Kin4-1c) localize to the phragmoplast midline (Miki et al., 2014) and minimize microtubule overlapping regions there (de Keijzer et al., 2017). KIN4-1c suppresses microtubule plus-end growth in vitro, so KIN4s may restrict microtubule overlap by inhibiting microtubule growth. Increased microtubule overlap regions accumulate more vesicles generating thick cell plates. Fascinatingly, the kin4-1a kin4-1c double mutant phragmoplast does not reorient, indicating division plane defects (de Keijzer et al., 2017).
Plus-end directed Kinesin-12 proteins participate in plant cell division, reviewed beautifully here (Müller and Livanos, 2019). PHRAGMOPLAST-ASSOCIATED KINESIN-RELATED PROTEIN1 (PAKRP1/KIN12A) and closely related PAKRP1-LIKE PROTEIN (PAKRP1L/KIN12B) have redundant roles in phragmoplast assembly in the male microspore. In the male microspore, callose accumulates in failed cell plates in the pakrp1 pakrp1l double mutants. However, vegetative cytokinesis is normal, indicating that there are additional, or different, proteins required for vegetative phragmoplast assembly (Lee et al., 2007). PAKRP1 and PAKRP1L interact with the ARM/HEAT repeats of the kinase TWO IN ONE (TIO), which is essential for cytokinesis in both microspores and during vegetative growth (Oh et al., 2005, 2012; Gillmor et al., 2016). These Kinesin-12 proteins are discussed here because their roles in specific divisions in the microspore and their interaction with TIO suggest that other plus-end directed kinesins may functionally rescue double mutants during vegetative growth, highlighting the need to generate higher-order mutant combinations or otherwise identify proteins that likely traffic vesicles essential for cytokinesis. Two other related Kinesin-12s PHRAGMOPLAST ORIENTING KINESIN1 (POK1) and POK2 also have redundant roles, but in phragmoplast positioning rather than cytokinesis (Müller et al., 2006). Both POK1 and POK2 localize to both the phragmoplast midline and the division site (Lipka et al., 2014; Herrmann et al., 2018; Mills et al., 2021). POK2 is required for fast phragmoplast expansion (Herrmann et al., 2018) and has diffusive plus-end directed movement (Chugh et al., 2018).
Several kinesin-7 plus-end directed kinesins are essential for cytokinesis. Two kinesin-7s, with M-phase specific activator (MSA) sequences, were called NACK1 and NACK2 in tobacco (Ito et al., 1998). Later, mutants in genes encoding NACK11/HINKEL and NACK2/TETRASPORE/STUD in tobacco and Arabidopsis and Nacka-c in moss were shown to have cytokinesis defects (Nishihama et al., 2002; Strompen et al., 2002; Yang et al., 2003; Naito and Goshima, 2015). HINKEL recruitment to the phragmoplast is partially dependent on RUNKEL, a microtubule-binding kinase-like protein essential for phragmoplast expansion (Krupnova et al., 2009, 2013). NACKs are processive plus-end directed kinesins, suggesting that they may transport cell plate building material (Naito and Goshima, 2015). HINKEL/NACK1 recruits Mitogen Activated Protein Kinase Kinase Kinase (MAPKKK) proteins to the phragmoplast midline through its C-terminal stalk domain (Sasabe and Machida, 2012; Sasabe et al., 2015). The Mitogen Activated Protein Kinase (MAPK) phosphorylates MAP65-1, MAP65-3, and EB1 proteins (Sasabe et al., 2006; Smertenko et al., 2006; Kohoutová et al., 2015). MAP65-1 phosphorylation, which also occurs by Aurora Kinase (Boruc et al., 2016), reduces microtubule binding and promotes proper microtubule disassembly at the phragmoplast lagging edge, which is critical for timely phragmoplast expansion (Sasabe et al., 2006; Smertenko et al., 2006). Like PAKRP1 and PAKRP1L, NACK2/TETRASPORE also interacts with the kinase TIO via the yeast-two-hybrid assay (Oh et al., 2014). Although another group of kinesin-7s are not essential for cytokinesis, they form a complex that promotes microtubule polymerization with a caspase-like protein essential for cytokinesis called Extra Spindle Poles1 (Moschou et al., 2013, 2016).
A land-plant specific “orphan” or ungrouped kinesin is sometimes required for cell elongation and is essential for phragmoplast microtubule interdigitation and cytokinesis in Arabidopsis, rice, and moss. The founding member, PHRAGMOPLAST ASSOCIATED KINESIN RELATED PROTEIN2 (PAKRP2), has a long neck and localizes to the phragmoplast midline and in phragmoplast-associated vesicles in Arabidopsis. PAKRP2 trafficking can be blocked by Brefeldin A (BFA) (Lee et al., 2001). PAKRP2 is a processive plus-end-directed kinesin that moves with an unusual combination of small step size and frequent steps to nearby filaments (Gicking et al., 2019). Processive movement and BFA sensitivity suggest it may transport Golgi-derived vesicles on the phragmoplast to the cell plate (Lee et al., 2001). In rice, the PAKRP2 homolog, STEMLESS DWARF1, is essential for phragmoplast morphology and expansion during cytokinesis and also promotes cell elongation. Like PAKRP2, STEMLESS DWARF1 localizes to the phragmoplast midline (Fang et al., 2018). In moss, two PAKRP2-related kinesins called kinesin for interdigitated microtubules 1a (KINID1a) and KINID1b function similarly in both cell elongation and cytokinesis. kinid1a kinid1b double mutants have wide, frayed, and non-interdigitated phragmoplasts and reduced cell elongation due to a lack of microtubule bundling in the phragmoplast and at the growing tip, respectively. The double mutants also have chloroplast positioning defects, which may interfere with phragmoplast expansion and lead to cytokinesis defects (Hiwatashi et al., 2008, 2014).
Minus end-directed kinesins
The minus-end-directed Kinesin-5 family plays a vital role in cytokinesis in moss, tobacco, and Arabidopsis but it may not participate directly in diffuse growth. A tobacco Kinesin-5, Kinesin-Related Protein (NtKRP), localizes to microtubules, and translocates antiparallel phragmoplast microtubules to minimize their overlap (Asada et al., 1997). The related Kinesin-5 AtKRP125c has a temperature-sensitive mutant, radially swollen7 (rsw7), with spindle collapse, defective phragmoplast assembly, and cytokinesis defects. Although rsw7 mutants have swollen cells suggesting defects in diffuse growth, and AtKRP125c localizes to cortical microtubules, the mutant phenotypes observed in mitosis and cytokinesis may account for apparent defects in cell shape (Bannigan et al., 2007). In moss, RNAi knockdown of all Kinesin-5 (a–d) homologs result in similar cytokinesis defects. Counterintuitively, Kinesin-5s are depleted from the phragmoplast midline via minus-end-directed movement (Miki et al., 2014). It is unclear how Kinesin-5 crosslinks or translocates antiparallel microtubules when it is excluded from antiparallel microtubule accumulation at the midline.
Several Kinesin-14 family minus-end-directed kinesins participate in cytokinesis. Kinesin-14s have diverse roles in spindle formation, chloroplast, and nuclear movement (Gicking et al., 2018). VARIED KERNEL SIZE1/ZmKIN11 (VKS1) is required for nuclear movement, spindle, and phragmoplast organization, with the vks1 mutant leading to cytokinesis defects during endosperm cellularization, but not during vegetative development (Huang et al., 2019). This suggests other related proteins may functionally compensate during vegetative cytokinesis. A maize VKS1 paralog, KINDR, transports neocentromeres toward the spindle poles to promote meiotic drive, but has no role in vegetative cytokinesis (Dawe et al., 2018). Another Kinesin-14, kinesin with calponin homology (KCH, calponin homology domains suggest actin binding), binds both actin and microtubules, and localizes to mitotic microtubule structures (Xu et al., 2009; Buschmann et al., 2011; Klotz and Nick, 2012). The KCH protein KinG in Arabidopsis transports the transcription factor SHORTROOT, but kinG mutants have no growth or division defects, suggesting that other kinesins may perform redundant functions (Spiegelman et al., 2018). Mutants have tip growth defects in moss (Yamada and Goshima, 2018) and cell elongation defects in rice (Frey et al., 2010) due to organelle and nuclear movement defects. However, these proteins do have an obvious role in cytokinesis. A kinesin-like calmodulin binding protein (KCBP) (Reddy et al., 1996) localizes to the division site, but loss-of-function mutants do not have cytokinesis or phragmoplast positioning defects (Miki et al., 2014; Buschmann et al., 2015) but do have defects in polarized growth of the trichome (Oppenheimer et al., 1997; Tian et al., 2015). Microinjection of a calmodulin-domain-specific antibody that is thought to constitutively activate KCBP during anaphase causes aberrant phragmoplast positioning and assembly and subsequent cytokinesis defects (Vos et al., 2000). Targeted or conditional mutagenesis may provide more insight into KCBP’s role in cytokinesis. KCBP is a minus-end-directed kinesin, which after artificial oligomerization processively transports acidic phospholipid liposomes, that binds actin as well as microtubules in vitro, and is negatively regulated by calcium/calmodulin with clear roles in chromosome and organelle movement (Kao et al., 2000; Yamada et al., 2017; Yoshida et al., 2019).
Cellulose synthase complexes
Cellulose is a major load-bearing component in the primary cell wall. It is composed of long chains of beta-1,4-linked glucans. Cellulose is made at the plasma membrane by a large complex named the CSC. The CSC was originally observed at the plasma membrane underlying microtubules using freeze fracture as a hexameric rosette structure (Mueller and Brown, 1980). More recently, live imaging was used to track in vivo movement of one of the CSC proteins CELLULOSE SYNTHASE 6 (CESA6) at the plasma membrane, where it often moved along microtubules (Paredez et al., 2006). The exact composition of the CSC in primary cell walls is unknown but likely contains a hexameric, equal stoichiometric combination of CESA1, CESA3 and either CESA6, CESA2, or CESA5 (Daras et al., 2009; Desprez et al., 2007; Persson et al., 2007; Wang et al., 2008; Gonneau et al., 2014; Hill et al., 2014). Mutants in cellulose synthase subunits produce tiny plants with cell elongation and sometimes cytokinesis defects. The cesA1 mutant has defects in cytokinesis (Beeckman et al., 2002), while others such as cesA6 may function only during cell expansion (Fagard et al., 2000), possibly due to CESA6 redundancy with CESA2 and CESA5.
Additional regulation of CESAs via post-translational modification such as phosphorylation may regulate stability or motility of CSCs (Li et al., 2014). Mutating putative phosphorylation sites in CESA1, CESA3, and CESA5 reduced their movement (Chen et al., 2010, 2016; Bischoff et al., 2011). Interestingly, the protein kinase BRASSINOSTEROID INSENSITIVE2 phosphorylates CESA1, highlighting an example of the hormonal regulation of cellulose synthesis via phosphorylation of CESA (Sánchez-Rodríguez et al., 2017).
Isoxaben is an herbicide that disrupts cellulose synthesis. Because many isoxaben-resistant mutants are mapped to CESAs, it is proposed that isoxaben may directly bind CESAs. However, the exact inhibition mechanism is unknown (Scheible et al., 2001; Desprez et al., 2002). Other chemicals, such as the cellulose biosynthesis inhibitor Endosidin 20, are useful tools to understand how CESA reaches the plasma membrane. Multiple ces6 alleles were identified in a screen for mutants resistant to Endosidin20 (Huang et al., 2020). Transgenic lines carrying mutations in catalytic residues of cellulose synthase fail to localize to the plasma membrane, phenocopied by Endosidin20 treatment, highlighting Endosidin20 as a powerful tool to study CESA localization to the plasma membrane. Interestingly, although cellulose-deficient mutants or plants treated with isoxaben have growth defects, removing receptor-like kinases that mediate the cell wall integrity response partially restores growth without affecting cellulose levels (Hématy et al., 2007; Van der Does et al., 2017; Feng et al., 2018; Chaudhary et al., 2020). This suggests an intimate connection between the cell wall and cell-wall integrity pathways to limit growth under conditions disrupting the cell wall (Wolf et al., 2012). Altogether, herbicides that disrupt cellulose synthesis provide valuable tools for understanding assembly and trafficking of CESAs.
Cellulose synthase-associated proteins
It was long proposed that the CSC interacts with microtubules at the plasma membrane by an elusive linker protein (Heath, 1974). The first linker protein identified, CELLULOSE SYNTHASE INTERACTIVE1 (CSI1, Figure 2), forms a physical association between active, plasma membrane-localized CSCs and cortical microtubules to establish and maintain organized and rapid cellulose biosynthesis (Gu et al., 2010; Bringmann et al., 2012; Li et al., 2012; Lei et al., 2013). The direct interaction between the central domain of CESA and CSI1 resides at multiple locations including N-, C-terminal and center regions of CSI1 while the interaction with microtubules are limited to the N- and C-terminal regions of CSI1 (Gu et al., 2010; Li et al., 2012; Lei et al., 2015). CESA particles fail to track with microtubules in the csi1 mutant, and their velocity is reduced (Lei et al., 2012b). Two homologs have different expression levels: CSI3 is expressed in meristematic zones while CSI2 expression is not detected in all tissues. Single csi3 mutants have no obvious aberrant phenotype but csi1 csi3 double mutants have slower CESA particle movement, reduced cellulose content, and smaller dark-grown hypocotyls (Lei et al., 2013). It is currently unclear whether CSI1 or its homologs are essential during cytokinesis. Generating a triple mutant would be required to clarify whether they are required for cytokinesis.
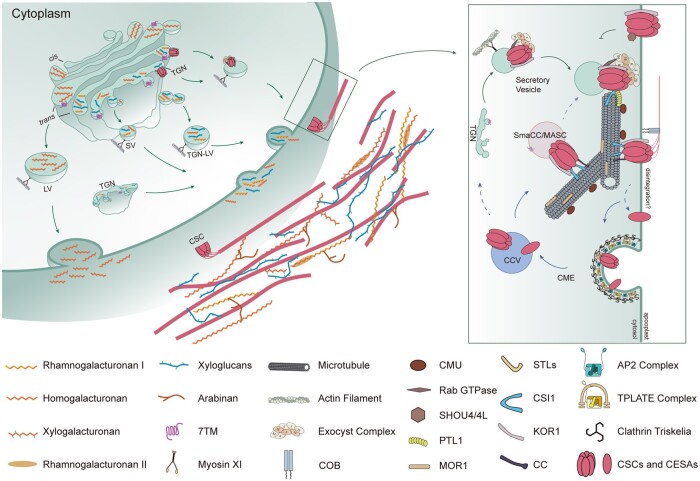
Figure 2.
Model of trafficking of non-cellulosic polysaccharides and cellulose in the primary cell wall. The synthesis of non-cellulosic polysaccharides occurs in the Golgi. Non-cellulosic polysaccharides include xyloglucan and pectins. They are exported along actin filaments or microtubules in vesicles from the Golgi to the plasma membrane and incorporated into the apoplast. Cellulose is synthesized exclusively at the plasma membrane. The components involved in cellulose synthesis and its regulation are highlighted in the box on the right. Proteins associated with CSCs include Cellulose Synthase Interacting Protein 1 (CSI1), Companions of Cellulose synthase (CC), KORRIGAN1 (KOR1), and PATROL1 (PTL1). The accumulation of CSCs at the plasma membrane is influenced by exocytosis and endocytosis. Microtubules and actin filaments play important roles in the trafficking of CSCs to and from the membrane.
There is mounting evidence that the regulation of cell growth is represented by a triangle that connects the cytoskeleton, cell wall, and cell shape. Microtubules guide the deposition of the cell wall and therefore determine the final shape of the cell. Cell geometry feedback influences the orientation of microtubules. CSCs are physically linked to microtubules via CSI1, highlighting the importance of the discovery of the key molecular linker underlying the parallel relationship between cellulose microfibrils and microtubules. However, despite the observation that CSC trajectories were uncoupled from the cortical microtubules in csi1 null mutants (Li et al., 2012), cellulose microfibrils in the csi1 mutants were oriented transversely instead of randomly (Xin et al., 2020). The transverse orientation of cellulose microfibrils is independent of CSC-microtubule linkage. However, the crossed-polylamellate wall architecture was lost in csi1. The loss of crossed-polylamellate wall was phenocopied by removing microtubules with the microtubule-depolymerizing drug oryzalin (Xin et al., 2020). These results are consistent with an earlier study showing that perturbation of microtubule rotation abolished the crossed-polylamellate wall texture (Chan et al., 2010). The crossed-polylamellate wall is important for restricting cell expansion laterally and promoting longitudinal expansion as loss of crossed-polylamellate wall coincides with loss of auxin-induced elongation (Xin et al., 2020).
Cellulase KORRIGAN1 (KOR1/GH9A1/RSW2/JIA) is the only known non-CESA protein that is an integral part of the CSC (Mansoori et al., 2014; Vain et al., 2014). KORRIGAN1 is an integral membrane endoglucanase first identified with an essential role in cell wall formation and cell elongation (Nicol et al., 1998). Later, a stronger mutant allele was identified that affected cytokinesis: KOR1 is also essential for cell plate formation (Lane et al., 2001). KOR1 protein localizes to multiple compartments including the Golgi, the TGN/early endosomes (EEs), and motile puncta at the plasma membrane (Robert et al., 2005; Lei et al., 2014), and the cell plate (Zuo et al., 2000). The transport of KOR1 to the cell plate requires two short amino acid motifs but these motifs are not required for localization at the plasma membrane (Zuo et al., 2000). As an integral part of the CSC, KOR1 may (1) remove disordered glucan chains during cellulose synthesis; (2) terminate cellulose synthesis; and/or (3) produce a short glucan chain “primer” for the initiation of cellulose synthesis. Although the exact role of KOR1 is unknown, glucanase activity is required for efficient cellulose biosynthesis (Nicol et al., 1998; Lane et al., 2001; Mansoori et al., 2014; Vain et al., 2014; Lei et al., 2014).
Non-CESA proteins that transiently associate with the CSC during cellulose biosynthesis include CSI proteins (described in the “Cellulose synthase-associated proteins” section) and COMPANION OF CELLULOSE SYNTHASE (CC) proteins. CC1 and 2 are two related plant-specific transmembrane domain-containing proteins that together prevent growth cessation after exposure to salt stress. cc1 cc2 double mutants have short dark-grown hypocotyls with reduced cellulose content in growth medium supplemented with elevated salt. CC1 colocalizes with and interacts with CESA1, 3, and 6 (Endler et al., 2015, 2016). CC1 binds and bundles microtubules through four hydrophobic motifs that may promote microtubule reorganization after salt-stress (Kesten et al., 2019).
Additional proteins that are less well understood but important for cellulose synthesis and cell elongation include KOBITO, named for a Japanese word meaning small because the mutants are stunted, and COBRA (COB), named because the mutant roots had a snake-like shape (Schindelman et al., 2001; Pagant et al., 2002). kobito mutants have stunted growth, reduced cellulose accumulation, and broken cell walls (Pagant et al., 2002). KOBITO encodes a type-II membrane protein with confirmed localization at the plasma membrane. COB is a glycosyl–phosphatidyl inositol-anchored protein. COB localizes to the Golgi and cell wall (Roudier et al., 2005). cob mutants have disorganized cellulose microfibrils and reduced cellulose content (Roudier et al., 2005). In maize, root-hairless3, a monocot-specific COB homolog, promotes root hair growth and higher grain yield (Hochholdinger et al., 2008). BRITTLE CULM1 (BC1) and BC1-Like4 (BC1L4), COB homologs in rice, also promote cell expansion (Li et al., 2003; Dai et al., 2011). BC1 binds crystalline cellulose in vitro through an N-terminal domain and localizes to vesicles at the plasma membrane and in the cell wall (Liu et al., 2013). It is hypothesized that COB modulates cellulose assembly by its direct interaction with crystalline cellulose (Liu et al., 2013).
The assembly and trafficking of CSC
The rosette CSCs were observed in the Golgi by electron microscopy, suggesting they are assembled there (Haigler and Brown, 1986). Consistently, immunogold labeling also showed CESA3 accumulation in the Golgi during cytokinesis (Zhang et al., 2016), but how CSCs assemble remains unknown. Two related Golgi-localized proteins named from the Greek word stello meaning “to set in order,” STELLO1 and STELLO1, are implicated in the assembly of CSCs because high molecular weight CSC complexes are reduced in stello1 stello2 mutants (Zhang et al., 2016).
After the Golgi, it is unclear if trafficking to the plasma membrane is via Golgi-derived vesicles or the TGN/EE. One difficulty lies in the dual role of the TGN/EE: it contains both secretory and endocytosed material retrieved from the plasma membrane (Viotti et al., 2012; Sinclair et al., 2018; Renna and Brandizzi, 2020). Tracking fluorescent protein-tagged CESA movement revealed that CSC delivery coincides with Golgi pausing directly beneath the insertion site near microtubules (Crowell et al., 2009). However, another study showed partial colocalization between the CSC and TGN/EE markers (Gutierrez et al., 2009). Another type of CSC-containing vesicle, which accumulates under salt stress or isoxaben treatment, is referred to as small CESA compartments (SmaCCs) or microtubule-associated CESA compartments (MASCs). Because SmaCCs/MASCs periodically associate with the Golgi and TGN compartments, SmaCCs/MASCs might also deliver CSCs (Crowell et al., 2009; Gutierrez et al., 2009). However, a recent study showed that SmaCC/MASC formation depends on CSI1 and clathrin-mediated endocytosis (Bashline et al., 2013; Lei et al., 2015). SmaCCs/MASCs act in the rapid recycling of CSCs back to the plasma membrane after being relieved from high salt conditions. Prior to membrane fusion, a third type of CSC-containing vesicle co-localizes with the exocyst complex, an evolutionarily conserved vesicle tethering complex that regulates multiple membrane trafficking processes. CSI1 is a central hub for delivery of CSCs by regulating the tethering of CSC-containing vesicles along microtubules, by defining the domain in the plasma membrane for delivery, possibly by directly interacting with plasma membrane lipids via its C2 domain (Gu and Somerville, 2010), and by bridging multiple cellular components including the exocyst complex, PATROL1 (PTL1) and microtubules. Exocyst subunit mutants are defective in delivering CSCs to the plasma membrane (Zhu et al., 2018b). The regulation of CSC delivery also relies on a plant-specific protein PTL1 (Zhu et al., 2018b). PTL1 is a protein containing a Mammalian Uncoordinated13 Domain (MUN) that promotes exocyst vesicle fusion (Hashimoto-Sugimoto et al., 2013). PTL1 may prime the CSC-containing vesicles for plasma membrane fusion by the exocyst complex (Zhu et al., 2018b). PTL1 also accumulates at the early cell plate, but what it does there is not yet known (Maeda et al., 2020).
CSC transport to the cell plate likely occurs through three different mechanisms. First, CesA proteins (CESA1, CESA3, and CESA6) accumulate faintly on the phragmoplast and coalesce toward the cell plate as early as the tubular vesicular network, when Scarlet Pontamine staining suggested that cellulose accumulates (Miart et al., 2014). Cellulose is preferentially labeled with the Scarlet Pontamine dye (Anderson et al., 2010). Cellulose microfibrils are also observed either during or after cell plate fusion with the plasma membrane using field emission scanning electron microscopy (Fujita and Wasteneys, 2014). Second, when the phragmoplast disassembles in the middle of the cell, motile BFA-sensitive organelles, perhaps Golgi, transport CESA to the cell plate. Finally, when the cell plate fuses with the plasma membrane, CESAs move from the plasma membrane into the cell plate (Miart et al., 2014). Much remains to be discovered about how and when CESAs accumulate at the cell plate, and how or when they might interact with microtubules to initiate cellulose synthesis. Combined light and electron microscopy techniques will be essential for resolving these questions, discussed beautifully here (Chen et al., 2018).
While actin is not essential for formation or insertion of CSCs near microtubules, actin is required for the cell-wide distribution and movement of CSC-containing Golgi bodies and rapid CSC insertion (Gutierrez et al., 2009; Sampathkumar et al., 2013). The actin cytoskeleton provides tracks for myosin-based organelle movement, including the Golgi (Nebenführ and Dixit, 2018). CSC delivery and interaction with the exocyst complex also partially depend on the actin motor protein MYOSIN XI (Zhang et al., 2020). A direct connection between the actin cytoskeleton and exocyst complex was recently identified in moss with the exocyst protein SEC10 containing a formin domain (van Gisbergen et al., 2018).
CesA recruitment to the plasma membrane after isoxaben treatment depends partially on two related 7-transmembrane-domain (7TM) containing proteins with similarity to G-Protein Coupled Receptors (GPCRs) called 7TM1 and 7TM5. CSC secretion is affected in 7tm1 7tm5 double mutants under normal conditions even though mutants do not show any phenotype without isoxaben treatment. 7TM1 and 7TM5 localize to Golgi, TGN, and SmaCCs/MASCs, modulating CSC secretion after stress (McFarlane et al., 2021).
Two related novel transmembrane proteins SHOU4 and SHOU4L that directly interact with CESA1 and CESA3 may negatively regulate exocytosis of CSCs. The increased density of CSCs in shou4 shou4l double mutant epidermal cells presumably reflects increased rates of exocytotic CSC delivery. An elevated level of amorphous cellulose was detected within mutant inflorescence stems (Polko et al., 2018). It remains to be determined whether SHOU4-mediated CSC delivery occurs via a CSI1-dependent route, or represents a novel pathway.
The CSC is a large plasma membrane-localized complex that is predicted to be regulated via endocytosis (Lei et al., 2012a). During cytokinesis, clathrin-mediated endocytosis removes CESA particles both from the middle of the cell plate and the plasma membrane (Miart et al., 2014). The dynamin-like protein, DRP1A is required for clathrin-mediated endocytosis and cytokinesis, and colocalizes with clathrin light chain (Konopka et al., 2008; Konopka and Bednarek, 2008). The defects in radially swollen9 (rsw9/drp1a) result in a cellulose deficiency and defective cell elongation and cytokinesis (Collings et al., 2008). A mutant locus in rice, brittle culm 3, mapped to a dynamin-related gene, OsDRP2B, which is likely required for clathrin-mediated endocytosis. The brittle culm 3 mutant reduces rice cellulose synthase 4 (OsCESA4) protein accumulation at the plasma membrane, whereas overexpression of OsDRP2B has the opposite effect (Xiong et al., 2010). The direct evidence that CESA is a cargo protein in clathrin-mediated endocytosis came from the interaction between CESA and the medium subunit of ADAPTER PROTEIN COMPLEX 2 (AP2M) in vitro and by yeast-two hybrid assays. In ap2m knockout mutants, CESA particle density increases as a result of disrupted clathrin-mediated endocytosis (Bashline et al., 2013). TRANSDUCIN/WD40-2 (TWD40-2) is a member of the TPLATE complex, an evolutionarily ancient adaptor for clathrin-mediated endocytosis (Gadeyne et al., 2014). TPLATE is essential for growth and cytokinesis (Van Damme et al., 2006), while twd40-2 knockdown also increases CESA particle density at the plasma membrane, indicating that the TPLATE complex regulates the endocytosis of CESA (Bashline et al., 2015). Similarly, the TPLATE Complex component MUNISCIN-LIKE (TML) and TPLATE interact with CESA6 and mediate the internalization of CESA6 from the plasma membrane (Sánchez-Rodríguez et al., 2018). Recent generation of a temperature sensitive tplate mutant will be a powerful tool to clarify its role in both diffuse growth and cytokinesis (Wang et al., 2021a).
Non-cellulosic polysaccharide synthesis and delivery
Unlike cellulose, which is synthesized at the plasma membrane and in the late cell plate in the late stages of development, the synthesis of non-cellulosic polysaccharides occurs primarily in the Golgi (Camirand et al., 1987; Brummell et al., 1990; Gibeaut and Carpita, 1994). Major non-cellulosic polysaccharides of the primary cell wall including xyloglucan and pectin are discussed here. One important resource in identifying specific features of carbohydrates comes from a suite of well-characterized monoclonal antibodies (Pattathil et al., 2010). Xyloglucans are beta-1,4-linked glucans, similar to cellulose, with additional sugar modifications on branched side chains. Modification of xyloglucans, based on immunolocalization of terminal fucose antigens, likely occurs within the trans cisternae of the Golgi and the TGN (Zhang and Staehelin, 1992). Consistent with this hypothesis, oligosaccharide-mass-profiling of Golgi-enriched fractions revealed less substituted xyloglucans compared with the apoplastic cell wall fractions (Obel et al., 2009). In phosphatidylinositol 4-kinase b1 and b2 (pi4kb1 pi4kb2) mutants, because the vesicles were unusually large, xyloglucan transport is observed within secretory vesicles from trans Golgi to TGN and to the cell surface (Kang et al., 2011). However, xyloglucan-synthesizing enzymes such as xylosyltransferase (XT1) and galactosyltransferase (MUR3) are detected in cis and medial cisternae of Golgi using immunogold labeling (Chevalier et al., 2010). While it is possible that XT1 and MUR3 are mislocalized in cis and medial Golgi due to overexpression, further investigation will clarify whether the initiation of xyloglucan side chains occurs in early Golgi compartments.
In addition to accumulating at the plasma membrane through the Golgi, xyloglucan accumulates in cell plates. Xyloglucan cell plate accumulation occurs in many species and cell types, including Arabidopsis endosperm, maize root cells, and tobacco cultured cells (Moore and Staehelin, 1988; Otegui and Staehelin, 2000; Sonobe et al., 2000; Baluska et al., 2005). Xyloglucan is trafficked through peripheral Golgi, to vesicles that are likely transported on microtubules to the cell plate (Moore and Staehelin, 1988). Consistently, xyloglucan accumulates faintly on BY-2 phragmoplasts (Sonobe et al., 2000). BFA treatment produces xyloglucan-rich BFA bodies near cell-plate leading-edges in maize root cells (Baluska et al., 2005). The endoxyloglucan transferase protein, which modifies xyloglucans, co-localizes with the ER, the phragmoplast, and the cell plate (Yokoyama and Nishitani, 2001). Recently, an elegant approach combining SYNTAXIN OF PLANTS61 (SYP61)-enriched vesicle isolation and glycome profiling was developed (Wilkop et al., 2019). SYP61 is a syntaxin that co-localizes with a subset of the TGN, and plays a key role in trafficking to the plasma membrane (Sanderfoot et al., 2001; Li et al., 2017b). SYP61 vesicles contain both terminal fucose and galactosylated xyloglucans as well as pectins (described in more detail below). Interestingly, the galactosylated xyloglucan is also present in cell wall fractions, indicating that substituted xyloglucan may already be in its final form in SYP61 TGN-derived vesicles (Wilkop et al., 2019). It is unlikely that SYP61-derived vesicles carry all non-cellulosic polysaccharides from the Golgi to the apoplast. Adaptation of this approach using different GFP-tagged proteins in wild-type and mutant lines will aid the characterization of polysaccharide transport.
Several pectin-synthesizing and modifying enzymes localize in the Golgi, including galacturonosyltransferase (GAUT)1 and GAUT7, HG methyltransferases QUASIMODO2 (QUA2) and QUA3 (Goubet and Mohnen, 1999; Sterling et al., 2001; Mouille et al., 2007; Atmodjo et al., 2011; Miao et al., 2011; Anderson, 2015). Pectin synthesis likely begins in the cis Golgi cisternae. The addition of side chains and subsequent modification occur in late Golgi compartments. Antibodies recognizing pectin-specific epitopes show that rhamnogalacturonan-I (RG-I) modification occurs in trans Golgi and TGN in sycamore maple (Acer pseudoplatanus) cells and alfalfa (Medicago sativa) root border cells (Zhang and Staehelin, 1992; Wang et al., 2017b). Pectin methylesterases (PMEs) are transported to the cell plate directly through Golgi-derived vesicles, rather than through the TGN, because PME accumulation is BFA insensitive in tobacco (Wang et al., 2016). Rhamnogalacturonan-II (RGII) co-accumulates with callose at the cell plate (Zhou et al., 2017).
A comprehensive study using 39 monoclonal antibodies against pectin epitopes revealed that various degrees of substitution of pectin are enriched in SYP61-derived vesicles (Wilkop et al., 2019). SYP61 vesicles contain many proteins involved in cell wall biosynthesis, including ECHIDNA (ECH) and the YPT/RAB GTPase-interacting proteins (YIPs). ECH forms a TGN-localized complex with YIP and is required for pectin and xyloglucan secretion. In yip4a yip4b and ech mutants, pectin and xyloglucan are trapped in the intracellular space, presumably due to ineffective transport to the extracellular space (Boutté et al., 2013; Gendre et al., 2013; Renna et al., 2018). A group of secretory carrier membrane proteins (SCAMPs) localize to the TGN and in secretory vesicle clusters (SVCs) budding from the TGN (Toyooka et al., 2009). Methyl-esterified homogalacturonans, revealed by dual-immunogold labeling, are present in both the TGN and SVCs where SCAMPs were located. Whether SCAMPs are involved in pectin secretion is unknown. We are far from a complete picture of transport of non-cellulosic polysaccharides from specific Golgi compartments to the apoplast. For example, little is known about the localization and assembly of RG-II. Developing techniques that distinguish transport of complex non-cellulosic polysaccharides through specific routes remains a major challenge. Comprehensive localization of >80 glycosyltransferases among which >67 are for pectin and >14 are for xyloglucan biosynthesis and modification will be challenging but immensely informative.
Several orthologous cellulose synthase-like proteins, named CELLULOSE SYNTHASE LIKE5 (CSLD5) in Arabidopsis, CSLD4 in rice, and CSLD1 in maize, are mitotically expressed and required for cytokinesis. Mutants have defects in cell wall expansion and also produce cell wall stubs indicative of cytokinesis defects (Li et al., 2009; Yin et al., 2011; Hunter et al., 2012; Yoo et al., 2012; Yoshikawa et al., 2013; Gu et al., 2016). CSLD5 localizes to punctate structures and accumulates in the cell plate (Gu et al., 2016). The cell wall material generated by this enzyme is unknown, although it may be mannans or xylans (Liepman et al., 2005; Bernal et al., 2007). Interestingly, the Arabidopsis clsd5 cytokinesis defect is enhanced by combining csld5 together with either the csld2 or the clsd3 mutant, even though CSLD2 and CSLD3 typically regulate root-hair growth (Yin et al., 2011; Gu et al., 2016). Unambiguously identifying which cell wall material is generated by these cellulose-synthase like proteins will provide valuable insight into their roles.
Cell plate
Vesicles arrive between interdigitated phragmoplast microtubules (de Keijzer et al., 2017) or a region lacking ribosomes and microtubule overlap called the cell plate assembly matrix that surrounds the cell plate (Seguí-Simarro et al., 2004; McMichael and Bednarek, 2013). Vesicle trafficking during cell plate formation is mediated by vesicle tethering monomeric RAB-GTPases (Minamino and Ueda, 2019). RAB-GTPases including RAB-A2, RAB-A3, and RAB-E1 are essential for cytokinesis and transit through the Golgi and TGN to the cell plate (Chow et al., 2008; Mayers et al., 2017). Another RAB-GTPase, RAB-A5c accumulates in unique vesicles and occasionally with the TGN. RAB-A5c localizes to the cell plate and is essential for cytokinesis and also diffuse growth (Kirchhelle et al., 2016, 2019; Elliott et al., 2020). RAB-A1a-c may function redundantly: triple mutants are hypersensitive to the chemical Endosidin1 during cytokinesis (Qi and Zheng, 2013). Putative guanine exchange factors (GEFs) for RAB GTPase are also required for cell plate formation including STOMATAL CYTOKINESIS DEFECTIVE1 (SCD1), SCD2, and Transport Protein Particle II (TRAPPII) complex components (Falbel et al., 2003; Chow et al., 2008; Jaber et al., 2010; Thellmann et al., 2010; Qi et al., 2011; McMichael et al., 2013; Mayers et al., 2017; Kalde et al., 2019). Recently, TRAPP-Interacting Plant Protein (TRIPP) was identified as a plant-specific member of the highly conserved TRAPPII complex. TRIPP is required for cytokinesis and is trafficked from the TGN to the cell plate (Garcia et al., 2020). Fascinatingly, SCD1, SCD2, and TRAPPII members interact with exocyst proteins (described in the following paragraph), possibly coordinating early and late stages of vesicle trafficking during cell plate formation (Rybak et al., 2014; Mayers et al., 2017). Four related proteins, BIG1-4, that share sequence homology with ADP-ribosylation factor (ARF) GEFs are essential for rerouting vesicles to the cell plate to complete cytokinesis (Richter et al., 2014).
The exocyst complex, a heteromeric complex with eight different subunits (SEC3, SEC5, SEC6, SEC8, SEC10, SEC15, EXO70A1, and EXO84b), is required for vesicle tethering during cytokinesis. Multiple subunits localized to the early and post-cytokinetic cell plate. Exocyst component mutants have defects in initial cell plate assembly (Fendrych et al., 2010; Wu et al., 2013; Rawat et al., 2017; Tang et al., 2019; Brejšková et al., 2021). Once the vesicles reach the midplane, they undergo fusion, mediated by SNARE protein complexes containing the cytokinesis-specific syntaxin KNOLLE (Lauber et al., 1997; Lukowitz et al., 1996; Heese et al., 2001; Zhang et al., 2011a; El Kasmi et al., 2013; Park et al., 2018; Won and Kim, 2020). SNARE complexes are preassembled in the ER, then trafficked through the Golgi and TGN to the cell plate (Chow et al., 2008; Karnahl et al., 2017). KNOLLE is restricted to the cell plate by RAB GTPase-mediated endocytosis (Boutté et al., 2010) and activated by the SEC1/MUNC18 protein KEULE, which also interacts directly with SEC6 (Waizenegger et al., 2000; Assaad et al., 2001; Wu et al., 2013).
The fused vesicles form dumbbell-shaped structures that then coalesce into a tubular–vesicular network (Samuels et al., 1995; Otegui et al., 2001). Dynamin-related proteins (DRPs), large GTPases essential for both cell plate formation and endocytosis, encircle membranes at the cell plate (Gu and Verma, 1996; Kang et al., 2003). In Arabidopsis, overexpression of the essential cytokinetic dynamin, phragmoplastin, causes overaccumulation of callose, possibly due to phragmoplastin-callose synthase (CALS1) interaction (Hong et al., 2001). Many DRPs localize to the cell plate (McMichael and Bednarek, 2013). DRP1A interacts with the membrane tubulation protein SH3P2 (Ahn et al., 2017) to transform vesicles into tubules. Together DRP1A and DRP2B localize to the phragmoplast midline and are essential for endocytosis during cytokinesis (Fujimoto et al., 2008; Fujimoto and Tsutsumi, 2014; Ekanayake et al., 2021).
Mutants that disrupt sterol formation or modification often have defective cytokinesis. DRP1A and sterols co-localize in the cell plate: removal of either disrupts localization and cell plate formation, suggesting their mutual requirement for high-lipid order in the cell plate (Frescatada-Rosa et al., 2014). Two related sterol methyltransferases, SMT2 and SMT3, generate C24-ethyl sterols. The smt2 smt3 double mutants have misoriented phragmoplasts and cell wall stubs, which are partially rescued by exogenous application of sterols (Nakamoto et al., 2015). Other mutants with defects in sterol synthesis or modification also have defects in cytokinesis, likely due to defective endocytosis, independent of a role in steroid hormone brassinosteroid biosynthesis (Willemsen et al., 2003; Schrick et al., 2000; Men et al., 2008; Carland et al., 2010; Wang et al., 2021b). Other lipids are required for proper cytokinesis, including very long chain fatty acids, sphingolipids, diacylglycerol, and phosphoinositides (Bach et al., 2011; Molino et al., 2014; Simon et al., 2016; Vermeer et al., 2017) reviewed beautifully here (Caillaud, 2019).
Callose is mostly found in the cell plate
Callose, a prominent component in the cell plate (Drakakaki, 2015), is rarely represented in primary cell walls, except in plasmodesmata (Wu et al., 2018) or under stress or damage inducing conditions (Cui and Lee, 2016; Záveská Drábková and Honys, 2017). Callose is composed of beta-1,3-linked glucans unlike the beta-1,4-linked glucan chains found in xyloglucans and cellulose. Multiple callose synthases have both discrete and overlapping functions in symplastic transport through plasmodesmata (Wu et al., 2018), wounding and pathogen response (Ellinger and Voigt, 2014; Wang et al., 2021c), phloem development (Slewinski et al., 2012), and pollen surface patterning (Schneider et al., 2016). Mixtures of cellulose with high amounts of callose provide more elasticity in vitro (Abou-Saleh et al., 2018), which may be a critical structural feature during cell plate growth. Biophysical modeling suggests that callose polymerization may provide the spreading force required to promote flattening of the tubulo-vesicular network into the fenestrated sheet (Jawaid et al., 2020).
Callose is synthesized by large protein complexes containing callose synthase (Glucan-synthase-like) integral membrane proteins (Schneider et al., 2016). A callose synthase CalS1/GSL6 localizes to the cell plate (Hong et al., 2001). However, while two other callose synthases, GSL8/MASSUE and GSL10 are essential for cytokinesis, CalS1 is not (Saatian et al., 2018; Töller et al., 2008; Chen et al., 2009; Thiele et al., 2009). A clever approach to circumvent death of callose deposition defective mutants uses a chemical, Endosidin7 (ES7), that prevents callose accumulation (Drakakaki et al., 2011). ES7 prevents callose synthesis in vitro by partially blocking UDP-glucose incorporation into 1,3-linked glucans from Arabidopsis cell membrane extracts. Surprisingly, ES7 does not alter wound- or stress-induced callose accumulation (Park et al., 2014). The specific target of ES7 is unknown, but blocking callose synthesis with ES7 affects cytokinesis in both land plants and algae, suggesting that the target of ES7 is highly conserved (Park et al., 2014; Davis et al., 2020).
Callose synthase activity depends on the presence of calcium (Kauss, 1985; Amor et al., 1995). The ER may be a calcium source for callose synthases during cell plate formation: ER–cell plate association occurs in diverse plant lineages (Porter and Machado, 1960; Schmiedel et al., 1981; Otegui et al., 2001). Intense calcium accumulation in the cell plate has been observed via staining (Wick and Hepler, 1980; Schmiedel et al., 1981) while calcium sequestration disrupts cell plate formation in Tradescantia virginiana (Jürgens et al., 1994). The P. patens sabre mutant has defects in ER connections with the cell plate that are linked to both slow and aberrant callose accumulation, and eventual cytokinesis defects (Cheng and Bezanilla, 2021). One tempting hypothesis is that the slow callose accumulation in the sabre mutant may be due to aberrant calcium gradients near the cell plate due to altered ER connections.
Conclusion
Cell wall modification and construction are dynamic processes during which secretion and endocytosis are tightly controlled. There are many open questions remaining such as how cellulose and non-cellulosic polymer insertion is coordinated, how non-cellulosic polymers are trafficked between the Golgi and plasma membrane and on which cytoskeletal tracks, and how the cytoskeleton and CSC activity are influenced by mechanical and environmental cues.
Accession numbers
Provided in Supplemental Data Set S1.
Supplemental data
Supplemental Data Set S1. Summary of the cytoskeletal and cell wall proteins required for diffuse growth and cytokinesis. Purple text indicates that proteins are involved in both diffuse growth and cytokinesis. Red text indicates that proteins are likely involved in only diffuse growth. Blue text indicates that proteins are likely involved only in cytokinesis.
Supplementary Material
Acknowledgments
Y.G. is supported by the National Science Foundation (NSF) #1951007 and C.G.R. is supported by NSF-CAREER #1942734. We gratefully acknowledge figure design assistance from Donghui Wei and Dr. Morgane Gilliard. We apologize to colleagues whose relevant work was not discussed due to length limitations.
Conflict of interest statement. None declared.
Contributor Information
Ying Gu, Department of Biochemistry and Molecular Biology, Pennsylvania State University, University Park, Pennsylvania 16802.
Carolyn G Rasmussen, Department of Botany and Plant Sciences, Center for Plant Cell Biology, University of California, Riverside, California 92521.
The authors responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) are Ying Gu (yug13@psu.edu) and Carolyn G. Rasmussen (crasmu@ucr.edu)
References
- Abou-Saleh RH, Hernandez-Gomez MC, Amsbury S, Paniagua C, Bourdon M, Miyashima S, Helariutta Y, Fuller M, Budtova T, Connell SD, et al. (2018) Interactions between callose and cellulose revealed through the analysis of biopolymer mixtures. Nat Commun 9: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Abied M, Belausov E, Hagay S, Peremyslov V, Dolja V, Sadot E (2018) Myosin XI-K is involved in root organogenesis, polar auxin transport, and cell division. J Exp Bot 69: 2869–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn G, Kim H, Kim DH, Hanh H, Yoon Y, Singaram I, Wijesinghe KJ, Johnson KA, Zhuang X, Liang Z, et al. (2017) SH3 domain- containing protein 2 plays a crucial role at the step of membrane tubulation during cell plate formation. Plant Cell 29: 1388–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose C, Allard JF, Cytrynbaum EN, Wasteneys GO (2011) A CLASP-modulated cell edge barrier mechanism drives cell-wide cortical microtubule organization in Arabidopsis. Nat Commun 2: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose C, Wasteneys GO (2014) Microtubule initiation from the nuclear surface controls cortical microtubule growth polarity and orientation in Arabidopsis thaliana. Plant Cell Physiol 55: 1636–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose JC, Shoji T, Kotzer AM, Pighin JA, Wasteneys GO (2007) The Arabidopsis CLASP gene encodes a microtubule-associated protein involved in cell expansion and division. Plant Cell 19: 2763–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP (1995) A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA 92: 9353–9357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CT (2015) We be jammin’: an update on pectin biosynthesis, trafficking and dynamics. J Exp Bot 67: 495–502 [DOI] [PubMed] [Google Scholar]
- Anderson CT, Carroll A, Akhmetova L, Somerville C (2010) Real-time imaging of cellulose reorientation during cell wall expansion in Arabidopsis roots. Plant Physiol 152: 787–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arieti RS, Staiger CJ (2020) Auxin-induced actin cytoskeleton rearrangements require AUX1. New Phytol 226: 441–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima K, Tamaoki D, Mineyuki Y, Yasuhara H, Nakai T, Shimmen T, Yoshihisa T, Sonobe S (2018) Displacement of the mitotic apparatuses by centrifugation reveals cortical actin organization during cytokinesis in cultured tobacco BY-2 cells. J Plant Res 131: 803–815 [DOI] [PubMed] [Google Scholar]
- Asada T, Kuriyama R, Shibaoka H (1997) TKRP125, a kinesin-related protein involved in the centrosome-independent organization of the cytokinetic apparatus in tobacco BY-2 cells. J Cell Sci 110(Pt 2): 179–189 [DOI] [PubMed] [Google Scholar]
- Assaad FF, Huet Y, Mayer U, Jürgens G (2001) The cytokinesis gene KEULE encodes a Sec1 protein that binds the syntaxin KNOLLE. J Cell Biol 152: 531–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmodjo MA, Sakuragi Y, Zhu X, Burrell AJ, Mohanty SS, Atwood JA, Orlando R, Scheller HV, Mohnen D (2011) Galacturonosyltransferase (GAUT)1 and GAUT7 are the core of a plant cell wall pectin biosynthetic homogalacturonan:galacturonosyltransferase complex. Proc Natl Acad Sci USA 108: 20225–20230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacete L, Mélida H, Miedes E, Molina A (2018) Plant cell wall-mediated immunity: cell wall changes trigger disease resistance responses. Plant J 93: 614–636 [DOI] [PubMed] [Google Scholar]
- Bach L, Gissot L, Marion J, Tellier F, Moreau P, Satiat-Jeunemaître B, Palauqui J-C, Napier JA, Faure J-D (2011) Very-long-chain fatty acids are required for cell plate formation during cytokinesis in Arabidopsis thaliana. J Cell Sci 124: 3223–3234 [DOI] [PubMed] [Google Scholar]
- Baluska F, Liners F, Hlavacka A, Schlicht M, Van Cutsem P, McCurdy DW, Menzel D (2005) Cell wall pectins and xyloglucans are internalized into dividing root cells and accumulate within cell plates during cytokinesis. Protoplasma 225: 141–155 [DOI] [PubMed] [Google Scholar]
- Bannigan A, Scheible W-R, Lukowitz W, Fagerstrom C, Wadsworth P, Somerville C, Baskin TI (2007) A conserved role for kinesin-5 in plant mitosis. J Cell Sci 120: 2819–2827 [DOI] [PubMed] [Google Scholar]
- Baquero Forero A, Cvrčková F (2019) SH3Ps—evolution and diversity of a family of proteins engaged in plant cytokinesis. Int J Mol Sci 20: 5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashline L, Li S, Anderson CT, Lei L, Gu Y (2013) The endocytosis of cellulose synthase in Arabidopsis is dependent on μ2, a clathrin-mediated endocytosis adaptin. Plant Physiol 163: 150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashline L, Li S, Zhu X, Gu Y (2015) The TWD40-2 protein and the AP2 complex cooperate in the clathrin-mediated endocytosis of cellulose synthase to regulate cellulose biosynthesis. Proc Natl Acad Sci USA 112: 12870–12875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin TI, Meekes HTHM, Liang BM, Sharp RE (1999) Regulation of growth anisotropy in well-watered and water-stressed maize roots. II. Role of cortical microtubules and cellulose microfibrils. Plant Physiol 119: 681–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeckman T, Przemeck GKH, Stamatiou G, Lau R, Terryn N, De Rycke R, Inzé D, Berleth T (2002) Genetic complexity of cellulose synthase a gene function in Arabidopsis embryogenesis. Plant Physiol 130: 1883–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger MA, Uyehara AN, Martinez P, McCarthy MC, Rasmussen CG (2021) Cell cortex microtubules contribute to division plane positioning during telophase in maize. Cold Spring Harbor Laboratory: 2021.01.11.426230. [DOI] [PMC free article] [PubMed]
- Bernal AJ, Jensen JK, Harholt J, Sørensen S, Moller I, Blaukopf C, Johansen B, de Lotto R, Pauly M, Scheller HV, et al. (2007) Disruption of ATCSLD5 results in reduced growth, reduced xylan and homogalacturonan synthase activity and altered xylan occurrence in Arabidopsis. Plant J 52: 791–802 [DOI] [PubMed] [Google Scholar]
- Bischoff V, Desprez T, Mouille G, Vernhettes S, Gonneau M, Höfte H (2011) Phytochrome regulation of cellulose synthesis in Arabidopsis. Curr Biol 21: 1822–1827 [DOI] [PubMed] [Google Scholar]
- Boruc J, Weimer AK, Stoppin-Mellet V, Mylle E, Kosetsu K, Cedeño C, Jaquinod M, Njo M, De Milde L, Tompa P, et al. (2016) Phosphorylation of MAP65-1 by Arabidopsis Aurora kinases is required for efficient cell cycle progression. Plant Physiol 173: 582–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouquin T, Mattsson O, Naested H, Foster R, Mundy J (2003) The Arabidopsis lue1 mutant defines a katanin p60 ortholog involved in hormonal control of microtubule orientation during cell growth. J Cell Sci 116: 791–801 [DOI] [PubMed] [Google Scholar]
- Boutté Y, Frescatada-Rosa M, Men S, Chow C-M, Ebine K, Gustavsson A, Johansson L, Ueda T, Moore I, Jürgens G, et al. (2010) Endocytosis restricts Arabidopsis KNOLLE syntaxin to the cell division plane during late cytokinesis. EMBO J 29: 546–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutté Y, Jonsson K, McFarlane HE, Johnson E, Gendre D, Swarup R, Friml J, Samuels L, Robert S, Bhalerao RP (2013) ECHIDNA-mediated post-Golgi trafficking of auxin carriers for differential cell elongation. Proc Natl Acad Sci USA 110: 16259–16264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejšková L, Hála M, Rawat A, Soukupová H, Cvrčková F, Charlot F, Nogué F, Haluška S, Žárský V (2021) SEC6 exocyst subunit contributes to multiple steps of growth and development of Physcomitrella (Physcomitrium patens). Plant J 106: 831–843. [DOI] [PubMed] [Google Scholar]
- Bringmann M, Li E, Sampathkumar A, Kocabek T, Hauser M-T, Persson S (2012) POM-POM2/cellulose synthase interacting1 is essential for the functional association of cellulose synthase and microtubules in Arabidopsis. Plant Cell 24: 163–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummell DA, Camirande A, Maclachlan GA (1990) Differential distribution of xyloglucan glycosyl transferases in pea Golgi dictyosomes and secretory vesicles. J Cell Sci 96: 705–710 [Google Scholar]
- Burian A, Ludynia M, Uyttewaal M, Traas J, Boudaoud A, Hamant O, Kwiatkowska D (2013) A correlative microscopy approach relates microtubule behaviour, local organ geometry, and cell growth at the Arabidopsis shoot apical meristem. J Exp Bot 64: 5753–5767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkart GM, Dixit R (2019) Microtubule bundling by MAP65-1 protects against severing by inhibiting the binding of katanin. Mol Biol Cell 30: 1587–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann H, Chan J, Sanchez-Pulido L, Andrade-Navarro MA, Doonan JH, Lloyd CW (2006) Microtubule-associated AIR9 recognizes the cortical division site at preprophase and cell-plate insertion. Curr Biol 16: 1938–1943 [DOI] [PubMed] [Google Scholar]
- Buschmann H, Dols J, Kopischke S, Peña EJ, Andrade-Navarro MA, Heinlein M, Szymanski DB, Zachgo S, Doonan JH, Lloyd CW (2015) Arabidopsis KCBP interacts with AIR9 but stays in the cortical division zone throughout mitosis via its MyTH4-FERM domain. J Cell Sci 128: 2033–2046 [DOI] [PubMed] [Google Scholar]
- Buschmann H, Fabri CO, Hauptmann M, Hutzler P, Laux T, Lloyd CW, Schäffner AR (2004) Helical growth of the Arabidopsis mutant tortifolia1 reveals a plant-specific microtubule-associated protein. Curr Biol 14: 1515–1521 [DOI] [PubMed] [Google Scholar]
- Buschmann H, Green P, Sambade A, Doonan JH, Lloyd CW (2011) Cytoskeletal dynamics in interphase, mitosis and cytokinesis analysed through Agrobacterium-mediated transient transformation of tobacco BY-2 cells. New Phytol 190: 258–267 [DOI] [PubMed] [Google Scholar]
- Buschmann H, Zachgo S (2016) The evolution of cell division: from streptophyte algae to land plants. Trends Plant Sci 21: 872–883 [DOI] [PubMed] [Google Scholar]
- Caillaud M-C (2019) Anionic lipids: a pipeline connecting key players of plant cell division. Front Plant Sci 10: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camirand A, Brummell D, Maclachlan G (1987) Fucosylation of xyloglucan: localization of the transferase in dictyosomes of pea stem cells. Plant Physiol 84: 753–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carland F, Fujioka S, Nelson T (2010) The sterol methyltransferases SMT1, SMT2, and SMT3 influence Arabidopsis development through nonbrassinosteroid products. Plant Physiol 153: 741–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC, McCann MC (2008) Maize and sorghum: genetic resources for bioenergy grasses. Trends Plant Sci 13: 415–420 [DOI] [PubMed] [Google Scholar]
- Chang-Jie J, Sonobe S (1993) Identification and preliminary characterization of a 65 kDa higher-plant microtubule-associated protein. J Cell Sci 105(Pt 4): 891–901 [DOI] [PubMed] [Google Scholar]
- Chan J, Crowell E, Eder M, Calder G (2010) The rotation of cellulose synthase trajectories is microtubule dependent and influences the texture of epidermal cell walls in Arabidopsis hypocotyls. J Cell Sci 123: 3490–3495 [DOI] [PubMed] [Google Scholar]
- Chan J, Jensen CG, Jensen LC, Bush M, Lloyd CW (1999) The 65-kDa carrot microtubule-associated protein forms regularly arranged filamentous cross-bridges between microtubules. Proc Natl Acad Sci USA 96: 14931–14936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary A, Chen X, Gao J, Leśniewska B, Hammerl R, Dawid C, Schneitz K (2020) The Arabidopsis receptor kinase STRUBBELIG regulates the response to cellulose deficiency. PLoS Genet 16: e1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebli Y, Bidhendi AJ, Kapoor K, Geitmann A (2021) Cytoskeletal regulation of primary plant cell wall assembly. Curr Biol 31: R681–R695 [DOI] [PubMed] [Google Scholar]
- Cheng X, Bezanilla M (2021) SABRE populates ER domains essential for cell plate maturation and cell expansion influencing cell and tissue patterning. Elife 10: e65166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-W, Persson S, Grebe M, McFarlane HE (2018) Cellulose synthesis during cell plate assembly. Physiol Plant 164: 17–26 [DOI] [PubMed] [Google Scholar]
- Chen S, Ehrhardt DW, Somerville CR (2010) Mutations of cellulose synthase (CESA1) phosphorylation sites modulate anisotropic cell expansion and bidirectional mobility of cellulose synthase. Proc Natl Acad Sci USA 107: 17188–17193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Jia H, Zhao H, Liu D, Liu Y, Liu B, Bauer S, Somerville CR (2016) Anisotropic cell expansion is affected through the bidirectional mobility of cellulose synthase complexes and phosphorylation at two critical residues on CESA3. Plant Physiol 171: 242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Teng N, Wu X, Wang Y, Tang W, Samaj J, Baluska F, Lin J (2007) Disruption of actin filaments by latrunculin B affects cell wall construction in Picea meyeri pollen tube by disturbing vesicle trafficking. Plant Cell Physiol 48: 19–30 [DOI] [PubMed] [Google Scholar]
- Chen X-Y, Liu L, Lee E, Han X, Rim Y, Chu H, Kim S-W, Sack F, Kim J-Y (2009) The Arabidopsis callose synthase gene GSL8 is required for cytokinesis and cell patterning. Plant Physiol 150: 105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Niroomand S, Zou Y, Wu H-M (2010) A transmembrane formin nucleates subapical actin assembly and controls tip-focused growth in pollen tubes. Proc Natl Acad Sci USA 107: 16390–16395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier L, Bernard S, Ramdani Y, Lamour R, Bardor M, Lerouge P, Follet-Gueye M-L, Driouich A (2010) Subcompartment localization of the side chain xyloglucan-synthesizing enzymes within Golgi stacks of tobacco suspension-cultured cells. Plant J 64: 977–989 [DOI] [PubMed] [Google Scholar]
- Chow C-M, Neto H, Foucart C, Moore I (2008) Rab-A2 and Rab-A3 GTPases define a trans-golgi endosomal membrane domain in Arabidopsis that contributes substantially to the cell plate. Plant Cell 20: 101–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N-H, Roberts K, Hepler PK, Valster A, Molchan T, Vos JW (2002) Roles for kinesin and myosin during cytokinesis. Philos Trans R Soc Lond B Biol Sci 357: 761–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh M, Reißner M, Bugiel M, Lipka E, Herrmann A, Roy B, Müller S, Schäffer E (2018) Phragmoplast orienting kinesin 2 is a weak motor switching between processive and diffusive modes. Biophys J 115: 375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary AL, Smith LG (1998) The Tangled1 gene is required for spatial control of cytoskeletal arrays associated with cell division during maize leaf development. Plant Cell 10: 1875–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin L, Chevallier A, Tsugawa S, Gacon F, Godin C, Viasnoff V, Saunders TE, Hamant O (2020) Cortical tension overrides geometrical cues to orient microtubules in confined protoplasts. Proc Natl Acad Sci USA 117: 32731–32738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collings DA, Gebbie LK, Howles PA, Hurley UA, Birch RJ, Cork AH, Hocart CH, Arioli T, Williamson RE (2008) Arabidopsis dynamin-like protein DRP1A: a null mutant with widespread defects in endocytosis, cellulose synthesis, cytokinesis, and cell expansion. J Exp Bot 59: 361–376 [DOI] [PubMed] [Google Scholar]
- Coomey JH, Sibout R, Hazen SP (2020) Grass secondary cell walls, Brachypodium distachyon as a model for discovery. New Phytol 227: 1649–1667 [DOI] [PubMed] [Google Scholar]
- Crowell EF, Bischoff V, Desprez T, Rolland A, Stierhof Y-D, Schumacher K, Gonneau M, Höfte H, Vernhettes S (2009) Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell 21: 1141–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Lee J-Y (2016) Arabidopsis callose synthases CalS1/8 regulate plasmodesmal permeability during stress. Nature Plants 2: 16034. [DOI] [PubMed] [Google Scholar]
- Dai X, You C, Chen G, Li X, Zhang Q, Wu C (2011) OsBC1L4 encodes a COBRA-like protein that affects cellulose synthesis in rice. Plant Mol Biol 75: 333–345 [DOI] [PubMed] [Google Scholar]
- Daras G, Rigas S, Penning B, Milioni D, McCann MC, Carpita NC, Fasseas C, Hatzopoulos P (2009) The thanatosmutation in Arabidopsis thaliana cellulose synthase 3 (AtCesA3) has a dominant-negative effect on cellulose synthesis and plant growth. New Phytol 184: 114–126 [DOI] [PubMed] [Google Scholar]
- Davis DJ, Wang M, Sørensen I, Rose JKC, Domozych DS, Drakakaki G (2020) Callose deposition is essential for the completion of cytokinesis in the unicellular alga, Penium margaritaceum. J Cell Sci 133: jcs249599 [DOI] [PubMed] [Google Scholar]
- Dawe RK, et al. (2018) A kinesin-14 motor activates neocentromeres to promote meiotic drive in maize. Cell 173: 839–850.e18 [DOI] [PubMed] [Google Scholar]
- Deeks MJ, Fendrych M, Smertenko A, Bell KS, Oparka K, Cvrcková F, Zársky V, Hussey PJ (2010) The plant formin AtFH4 interacts with both actin and microtubules, and contains a newly identified microtubule-binding domain. J Cell Sci 123: 1209–1215 [DOI] [PubMed] [Google Scholar]
- Desprez T, Juraniec M, Crowell EF, Jouy H, Pochylova Z, Parcy F, Höfte H, Gonneau M, Vernhettes S (2007) Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 104: 15572–15577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprez T, Vernhettes S, Fagard M, Refrégier G, Desnos T, Aletti E, Py N, Pelletier S, Höfte H (2002) Resistance against herbicide isoxaben and cellulose deficiency caused by distinct mutations in same cellulose synthase isoform CESA6. Plant Physiol 128: 482–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rubbo S, et al. (2013) The clathrin adaptor complex AP-2 mediates endocytosis of brassinosteroid insensitive1 in Arabidopsis. Plant Cell 25: 2986–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Cyr R (2004) Encounters between dynamic cortical microtubules promote ordering of the cortical array through angle-dependent modifications of microtubule behavior. Plant Cell 16: 3274–3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakakaki G, et al. (2011) Clusters of bioactive compounds target dynamic endomembrane networks in vivo. Proc Natl Acad Sci USA 108: 17850–17855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakakaki G (2015) Polysaccharide deposition during cytokinesis: challenges and future perspectives. Plant Sci 236: 177–184 [DOI] [PubMed] [Google Scholar]
- Du P, Wang J, He Y, Zhang S, Hu B, Xue X, Miao L, Ren H (2021) AtFH14 crosslinks actin filaments and microtubules in different manners. Biol Cell 113: 235–249 [DOI] [PubMed] [Google Scholar]
- Durand-Smet P, Spelman TA, Meyerowitz EM, Jönsson H (2020) Cytoskeletal organization in isolated plant cells under geometry control. Proc Natl Acad Sci USA 117: 17399–17408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyachok J, Shao M-R, Vaughn K, Bowling A, Facette M, Djakovic S, Clark L, Smith L (2008) Plasma membrane-associated SCAR complex subunits promote cortical F-actin accumulation and normal growth characteristics in Arabidopsis roots. Mol Plant 1: 990–1006 [DOI] [PubMed] [Google Scholar]
- Dyachok J, Zhu L, Liao F, He J, Huq E, Blancaflor EB (2011) SCAR mediates light-induced root elongation in Arabidopsis through photoreceptors and proteasomes. Plant Cell 23: 3610–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanayake G, Smith JM, Jones KB, Stiers HM, Robinson SJ, LaMontagne ED, Kostos PH, Cornish PV, Bednarek SY, Heese A (2021) DYNAMIN-RELATED PROTEIN DRP1A functions with DRP2B in plant growth, flg22-immune responses, and endocytosis. Plant Physiol 185: 1986–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Din El-Assal S, Le J, Basu D, Mallery EL, Szymanski DB (2004) DISTORTED2 encodes an ARPC2 subunit of the putative Arabidopsis ARP2/3 complex. Plant J 38: 526–538 [DOI] [PubMed] [Google Scholar]
- Eleftheriou EP, Baskin TI, Hepler PK (2005) Aberrant cell plate formation in the Arabidopsis thaliana microtubule organization 1 mutant. Plant Cell Physiol 46: 671–675 [DOI] [PubMed] [Google Scholar]
- El Kasmi F, Krause C, Hiller U, Stierhof Y-D, Mayer U, Conner L, Kong L, Reichardt I, Sanderfoot AA, Jürgens G (2013) SNARE complexes of different composition jointly mediate membrane fusion in Arabidopsis cytokinesis. Mol Biol Cell 24: 1593–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger D, Voigt CA (2014) Callose biosynthesis in arabidopsis with a focus on pathogen response: what we have learned within the last decade. Ann Bot 114: 1349–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott L, Moore I, Kirchhelle C (2020) Spatio-temporal control of post-Golgi exocytic trafficking in plants. J Cell Sci 133: jcs237065 [DOI] [PubMed] [Google Scholar]
- Endler A, Kesten C, Schneider R, Zhang Y, Ivakov A, Froehlich A, Funke N, Persson S (2015) A mechanism for sustained cellulose synthesis during salt stress. Cell 162: 1353–1364 [DOI] [PubMed] [Google Scholar]
- Endler A, Schneider R, Kesten C, Lampugnani ER, Persson S (2016) The cellulose synthase companion proteins act non-redundantly with CELLULOSE SYNTHASE INTERACTING1/POM2 and CELLULOSE SYNTHASE 6. Plant Signal Behav 11: e1135281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esseling-Ozdoba A, Vos JW, van Lammeren AAM, Emons AMC (2008) Synthetic lipid (DOPG) vesicles accumulate in the cell plate region but do not fuse. Plant Physiol 147: 1699–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fache V, Gaillard J, Van Damme D, Geelen D, Neumann E, Stoppin-Mellet V, Vantard M (2010) Arabidopsis kinetochore fiber-associated MAP65-4 cross-links microtubules and promotes microtubule bundle elongation. Plant Cell 22: 3804–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard M, Desnos T, Desprez T, Goubet F, Refregier G, Mouille G, McCann M, Rayon C, Vernhettes S, Höfte H (2000) PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell 12: 2409–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falbel TG, Koch LM, Nadeau JA, Segui-Simarro JM, Sack FD, Bednarek SY (2003) SCD1 is required for cell cytokinesis and polarized cell expansion in Arabidopsis thaliana. Development 130: 4011–4024 [DOI] [PubMed] [Google Scholar]
- Fang J, Yuan S, Li C, Jiang D, Zhao L, Peng L, Zhao J, Zhang W, Li X (2018) Reduction of ATPase activity in the rice kinesin protein stemless Dwarf 1 inhibits cell division and organ development. Plant J 96: 620–634 [DOI] [PubMed] [Google Scholar]
- Fendrych M, Synek L, Pecenková T, Toupalová H, Cole R, Drdová E, Nebesárová J, Sedinová M, Hála M, Fowler JE, et al. (2010) The Arabidopsis exocyst complex is involved in cytokinesis and cell plate maturation. Plant Cell 22: 3053–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, et al. (2018) The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr Biol 28: 666–675.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Schopfer P (1997) Interaction of auxin, light, and mechanical stress in orienting microtubules in relation to tropic curvature in the epidermis of maize coleoptiles. Protoplasma 196: 108–116 [Google Scholar]
- Fischer K, Schopfer P (1998) Physical strain-mediated microtubule reorientation in the epidermis of gravitropically or phototropically stimulated maize coleoptiles. Plant J 15: 119–123 [DOI] [PubMed] [Google Scholar]
- Frescatada-Rosa M, Stanislas T, Backues SK, Reichardt I, Men S, Boutté Y, Jürgens G, Moritz T, Bednarek SY, Grebe M (2014) High lipid order of Arabidopsis cell-plate membranes mediated by sterol and DYNAMIN-RELATED PROTEIN1A function. Plant J 80: 745–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey N, Klotz J, Nick P (2010) A kinesin with calponin-homology domain is involved in premitotic nuclear migration. J Exp Bot 61: 3423–3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Arimura S-I, Nakazono M, Tsutsumi N (2008) Arabidopsis dynamin-related protein DRP2B is co-localized with DRP1A on the leading edge of the forming cell plate. Plant Cell Rep 27: 1581–1586 [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Tsutsumi N (2014) Dynamin-related proteins in plant post-Golgi traffic. Front Plant Sci 5: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Himmelspach R, Hocart CH, Williamson RE, Mansfield SD, Wasteneys GO (2011) Cortical microtubules optimize cell-wall crystallinity to drive unidirectional growth in Arabidopsis. Plant J 66: 915–928 [DOI] [PubMed] [Google Scholar]
- Fujita M, Wasteneys GO (2014) A survey of cellulose microfibril patterns in dividing, expanding, and differentiating cells of Arabidopsis thaliana. Protoplasma 251: 687–698 [DOI] [PubMed] [Google Scholar]
- Gadeyne A, et al. (2014) The TPLATE adaptor complex drives clathrin-mediated endocytosis in plants. Cell 156: 691–704 [DOI] [PubMed] [Google Scholar]
- Ganguly A, DeMott L, Dixit R (2017) The Arabidopsis kinesin-4, FRA1, requires a high level of processive motility to function correctly. J Cell Sci 130: 1232–1238 [DOI] [PubMed] [Google Scholar]
- Ganguly A, Zhu C, Chen W, Dixit R (2020) FRA1 kinesin modulates the lateral stability of cortical microtubules through cellulose synthase-microtubule uncoupling proteins. Plant Cell 32: 2508–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia VJ, et al. (2020) TRIPP is a plant-specific component of the Arabidopsis TRAPPII membrane trafficking complex with important roles in plant development. Plant Cell 32: 2424–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendre D, McFarlane HE, Johnson E, Mouille G, Sjödin A, Oh J, Levesque-Tremblay G, Watanabe Y, Samuels L, Bhalerao RP (2013) Trans-Golgi network localized ECHIDNA/Ypt interacting protein complex is required for the secretion of cell wall polysaccharides in Arabidopsis. Plant Cell 25: 2633–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut DM, Carpita NG (1994) Biosynthesis of plant cell wall polysaccharides. The FASEB J 8: 904–915 [DOI] [PubMed] [Google Scholar]
- Gicking AM, Swentowsky KW, Dawe RK, Qiu W (2018) Functional diversification of the kinesin-14 family in land plants. FEBS Lett 592: 1918–1928 [DOI] [PubMed] [Google Scholar]
- Gicking AM, Wang P, Liu C, Mickolajczyk KJ, Guo L, Hancock WO, Qiu W (2019) The orphan kinesin PAKRP2 achieves processive motility via a noncanonical stepping mechanism. Biophys J 116: 1270–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland LU, Pawloski LC, Kandasamy MK, Meagher RB (2003) Arabidopsis actin gene ACT7 plays an essential role in germination and root growth. Plant J 33: 319–328 [DOI] [PubMed] [Google Scholar]
- Gillmor CS, Roeder AHK, Sieber P, Somerville C, Lukowitz W (2016) A genetic screen for mutations affecting cell division in the Arabidopsis thaliana embryo identifies seven loci required for cytokinesis. PLoS ONE 11: e0146492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gisbergen PAC, Wu S-Z, Chang M, Pattavina KA, Bartlett ME, Bezanilla M (2018) An ancient Sec10-formin fusion provides insights into actin-mediated regulation of exocytosis. J Cell Biol 217: 945–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gisbergen P, Wu S-Z, Cheng X, Pattavina KA, Bezanilla M (2020) In vivo analysis of formin dynamics in the moss P. patens reveals functional class diversification. J Cell Sci 133: jcs.233791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonneau M, Desprez T, Guillot A, Vernhettes S, Höfte H (2014) Catalytic subunit stoichiometry within the cellulose synthase complex. Plant Physiol 166: 1709–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubet F, Mohnen D (1999) Subcellular localization and topology of homogalacturonan methyltransferase in suspension-cultured Nicotiana tabacum cells. Planta 209: 112–117 [DOI] [PubMed] [Google Scholar]
- Green PB (1964) Cell walls and the geometry of plant growth. Brookhaven Symp Biol 16: 203–217 [PubMed] [Google Scholar]
- Gu F, Bringmann M, Combs JR, Yang J, Bergmann DC, Nielsen E (2016) Arabidopsis CSLD5 functions in cell plate formation in a cell cycle-dependent manner. Plant Cell 28: 1722–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseman JM, Lee JS, Bogenschutz NL, Peterson KM, Virata RE, Xie B, Kanaoka MM, Hong Z, Torii KU (2010) Dysregulation of cell-to-cell connectivity and stomatal patterning by loss-of-function mutation in Arabidopsis chorus (glucan synthase-like 8). Development 137: 1731–1741 [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Lindeboom JJ, Paredez AR, Emons AMC, Ehrhardt DW (2009) Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat Cell Biol 11: 797–806 [DOI] [PubMed] [Google Scholar]
- Gu X, Verma DP (1996) Phragmoplastin, a dynamin-like protein associated with cell plate formation in plants. EMBO J 15: 695–704 [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Kaplinsky N, Bringmann M, Cobb A, Carroll A, Sampathkumar A, Baskin TI, Persson S, Somerville CR (2010) Identification of a cellulose synthase-associated protein required for cellulose biosynthesis. Proc Natl Acad Sci USA 107: 12866–12871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Somerville C (2010) Cellulose synthase interacting protein: a new factor in cellulose synthesis. Plant Signal Behav 5: 1571–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler CH, Brown RM Jr (1986) Transport of rosettes from the golgi apparatus to the plasma membrane in isolated mesophyll cells of Zinnia elegans during differentiation to tracheary elements in suspension culture. Protoplasma 134: 111–120 [Google Scholar]
- Halat L, Gyte K, Wasteneys G (2020) The microtubule-associated protein CLASP is translationally regulated in light-dependent root apical meristem growth. Plant Physiol 184: 2154–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T (2014) Chapter 1—Microtubule organization and microtubule-associated proteins in plant cells. In Jeon KW, ed, International Review of Cell and Molecular Biology, Academic Press, pp 1–52 [DOI] [PubMed] [Google Scholar]
- Hamada T, Igarashi H, Itoh TJ, Shimmen T, Sonobe S (2004) Characterization of a 200 kDa microtubule-associated protein of tobacco BY-2 cells, a member of the XMAP215/MOR1 family. Plant Cell Physiol 45: 1233–1242 [DOI] [PubMed] [Google Scholar]
- Hamant O, Heisler MG, Jönsson H, Krupinski P, Uyttewaal M, Bokov P, Corson F, Sahlin P, Boudaoud A, Meyerowitz EM, et al. (2008) Developmental patterning by mechanical signals in Arabidopsis. Science 322: 1650–1655 [DOI] [PubMed] [Google Scholar]
- Harris D, Bulone V, Ding S-Y, DeBolt S (2010) Tools for cellulose analysis in plant cell walls. Plant Physiol 153: 420–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Sugimoto M, Higaki T, Yaeno T, Nagami A, Irie M, Fujimi M, Miyamoto M, Akita K, Negi J, Shirasu K, et al. (2013) A Munc13-like protein in Arabidopsis mediates H+-ATPase translocation that is essential for stomatal responses. Nat Commun 4: 2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath IB (1974) A unified hypothesis for the role of membrane bound enzyme complexes and microtubules in plant cell wall synthesis. J Theor Biol 48: 445–449 [DOI] [PubMed] [Google Scholar]
- Heese M, Gansel X, Sticher L, Wick P, Grebe M, Granier F, Jurgens G (2001) Functional characterization of the KNOLLE-interacting t-SNARE AtSNAP33 and its role in plant cytokinesis. J Cell Biol 155: 239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejnowicz Z (2000) Mechanical stress patterns in plant cell walls and their morphogenetical importance. In A Carbone, M Gromov, P Prusinkiewicz, eds, Pattern Formation in Biology, Vision and Dynamics. pp 240–251 [Google Scholar]
- Hejnowicz Z, Rusin A, Rusin T (2000) Tensile tissue stress affects the orientation of cortical microtubules in the epidermis of sunflower hypocotyl. J Plant Growth Regul 19: 31–44 [DOI] [PubMed] [Google Scholar]
- Hématy K, Sado P-E, Van Tuinen A, Rochange S, Desnos T, Balzergue S, Pelletier S, Renou J-P, Höfte H (2007) A receptor-like kinase mediates the response of arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol 17: 922–931 [DOI] [PubMed] [Google Scholar]
- Herrmann A, Livanos P, Lipka E, Gadeyne A, Hauser M-T, Van Damme D, Müller S (2018) Dual localized kinesin-12 POK2 plays multiple roles during cell division and interacts with MAP65-3. EMBO Rep 19: e46085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JL Jr, Hammudi MB, Tien M (2014) The Arabidopsis cellulose synthase complex: a proposed hexamer of CESA trimers in an equimolar stoichiometry. Plant Cell 26: 4834–4842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiwatashi Y, Obara M, Sato Y, Fujita T, Murata T, Hasebe M (2008) Kinesins are indispensable for interdigitation of phragmoplast microtubules in the moss Physcomitrella patens. Plant Cell 20: 3094–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiwatashi Y, Sato Y, Doonan JH (2014) Kinesins have a dual function in organizing microtubules during both tip growth and cytokinesis in Physcomitrella patens. Plant Cell 26: 1256–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochholdinger F, Wen T-J, Zimmermann R, Chimot-Marolle P, da Costa e, Silva O, Bruce W, Lamkey KR, Wienand U, Schnable PS (2008) The maize (Zea mays L.) roothairless3 gene encodes a putative GPI-anchored, monocot-specific, COBRA-like protein that significantly affects grain yield. Plant J 54: 888–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C-MK, Hotta T, Guo F, Roberson RW, Lee Y-RJ, Liu B (2011a) Interaction of antiparallel microtubules in the phragmoplast is mediated by the microtubule-associated protein MAP65-3 in Arabidopsis. Plant Cell 23: 2909–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C-MK, Hotta T, Kong Z, Zeng CJT, Sun J, Lee Y-RJ, Liu B (2011b) Augmin plays a critical role in organizing the spindle and phragmoplast microtubule arrays in Arabidopsis. Plant Cell 23: 2606–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C-MK, Lee Y-RJ, Kiyama LD, Dinesh-Kumar SP, Liu B (2012) Arabidopsis microtubule-associated protein MAP65-3 cross-links antiparallel microtubules toward their plus ends in the phragmoplast via its distinct C-terminal microtubule binding domain. Plant Cell 24: 2071–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Delauney AJ, Verma DP (2001) A cell plate-specific callose synthase and its interaction with phragmoplastin. Plant Cell 13: 755–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta T, Haraguchi T, Mizuno K (2007) A novel function of plant histone H1: microtubule nucleation and continuous plus end association. Cell Struct Funct 32: 79–87 [DOI] [PubMed] [Google Scholar]
- Hotta T, Kong Z, Ho C-MK, Zeng CJT, Horio T, Fong S, Vuong T, Lee Y-RJ, Liu B (2012) Characterization of the Arabidopsis augmin complex uncovers its critical function in the assembly of the acentrosomal spindle and phragmoplast microtubule arrays. Plant Cell 24: 1494–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, et al. (2020) Endosidin20 targets the cellulose synthase catalytic domain to inhibit cellulose biosynthesis. Plant Cell 32: 2141–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wang H, Huang X, Wang Q, Wang J, An D, Li J, Wang W, Wu Y (2019) Maize VKS1 regulates mitosis and cytokinesis during early endosperm development. Plant Cell 31: 1238–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CT, Kirienko DH, Sylvester AW, Peter GF, McCarty DR, Koch KE (2012) Cellulose synthase-like D1 is integral to normal cell division, expansion, and leaf development in maize. Plant Physiol 158: 708–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingouff M, Gerald JNF, Guérin C, Robert H, Sørensen MB, Van Damme D, Geelen D, Blanchoin L, Berger F (2005) Plant formin AtFH5 is an evolutionarily conserved actin nucleator involved in cytokinesis. Nat Cell Biol 7: 374–380 [DOI] [PubMed] [Google Scholar]
- Ito M, Iwase M, Kodama H, Lavisse P, Komamine A, Nishihama R, Machida Y, Watanabe A (1998) A novel cis-acting element in promoters of plant B-type cyclin genes activates M phase-specific transcription. Plant Cell 10: 331–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber E, Thiele K, Kindzierski V, Loderer C, Rybak K, Jürgens G, Mayer U, Söllner R, Wanner G, Assaad FF (2010) A putative TRAPPII tethering factor is required for cell plate assembly during cytokinesis in Arabidopsis. New Phytol 187: 751–763 [DOI] [PubMed] [Google Scholar]
- Jacques E, Verbelen J-P, Vissenberg K (2013) Mechanical stress in Arabidopsis leaves orients microtubules in a “continuous” supracellular pattern. BMC Plant Biol 13: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janski N, Masoud K, Batzenschlager M, Herzog E, Evrard J-L, Houlné G, Bourge M, Chabouté M-E, Schmit A-C (2012) The GCP3-interacting proteins GIP1 and GIP2 are required for γ-tubulin complex protein localization, spindle integrity, and chromosomal stability. Plant Cell 24: 1171–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawaid MZ, Sinclair R, Cox D, Drakakaki G (2020) A biophysical model for plant cell plate development. bioRxiv: 2020.05.21.109512
- Jürgens G (2005) Cytokinesis in higher plants. Annu Rev Plant Biol 56: 281–299 [DOI] [PubMed] [Google Scholar]
- Jürgens M, Hepler LH, Rivers BA, Hepler PK (1994) BAPTA-calcium buffers modulate cell plate formation in stamen hairs ofTradescantia: evidence for calcium gradients. Protoplasma 183: 86–99 [Google Scholar]
- Kalde M, et al. (2019) Interactions between transport protein particle (TRAPP) complexes and Rab GTPases in Arabidopsis. Plant J 100: 279–297 [DOI] [PubMed] [Google Scholar]
- Kang B-H, Busse JS, Bednarek SY (2003) Members of the Arabidopsis dynamin-like gene family, ADL1, are essential for plant cytokinesis and polarized cell growth. Plant Cell 15: 899–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B-H, Nielsen E, Preuss ML, Mastronarde D, Staehelin LA (2011) Electron tomography of RabA4b- and PI-4Kβ1-labeled trans Golgi network compartments in Arabidopsis. Traffic 12: 313–329 [DOI] [PubMed] [Google Scholar]
- Kao YL, Deavours BE, Phelps KK, Walker RA, Reddy AS (2000) Bundling of microtubules by motor and tail domains of a kinesin-like calmodulin-binding protein from Arabidopsis: regulation by Ca(2+)/Calmodulin. Biochem Biophys Res Commun 267: 201–207 [DOI] [PubMed] [Google Scholar]
- Karnahl M, Park M, Mayer U, Hiller U, Jürgens G (2017) ER assembly of SNARE complexes mediating formation of partitioning membrane in Arabidopsis cytokinesis. Elife 6: e25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H (1985) Callose biosynthesis as a Ca2+-regulated process and possible relations to the induction of other metabolic changes. J Cell Sci Suppl 2: 89–103 [DOI] [PubMed] [Google Scholar]
- Kawamura E, Himmelspach R, Rashbrooke MC, Whittington AT, Gale KR, Collings DA, Wasteneys GO (2006) MICROTUBULE ORGANIZATION 1 regulates structure and function of microtubule arrays during mitosis and cytokinesis in the Arabidopsis root. Plant Physiol 140: 102–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura E, Wasteneys GO (2008) MOR1, the Arabidopsis thaliana homologue of Xenopus MAP215, promotes rapid growth and shrinkage, and suppresses the pausing of microtubules in vivo. J Cell Sci 121: 4114–4123 [DOI] [PubMed] [Google Scholar]
- de Keijzer J, Kieft H, Ketelaar T, Goshima G, Janson ME (2017) Shortening of microtubule overlap regions defines membrane delivery sites during plant cytokinesis. Curr Biol 27: 514–520 [DOI] [PubMed] [Google Scholar]
- Kesten C, et al. (2019) The companion of cellulose synthase 1 confers salt tolerance through a Tau-like mechanism in plants. Nat Commun 10: 857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhelle C, Chow C-M, Foucart C, Neto H, Stierhof Y-D, Kalde M, Walton C, Fricker M, Smith RS, Jérusalem A, et al. (2016) The specification of geometric edges by a plant Rab GTPase is an essential cell-patterning principle during organogenesis in Arabidopsis. Dev Cell 36: 386–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhelle C, Garcia-Gonzalez D, Irani NG, Jérusalem A, Moore I (2019) Two mechanisms regulate directional cell growth in Arabidopsis lateral roots. Elife 8: e47988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik V, Herrmann U, Parupalli C, Sedbrook JC, Ehrhardt DW, Hülskamp M (2007) CLASP localizes in two discrete patterns on cortical microtubules and is required for cell morphogenesis and cell division in Arabidopsis. J Cell Sci 120: 4416–4425 [DOI] [PubMed] [Google Scholar]
- Klotz J, Nick P (2012) A novel actin--microtubule cross-linking kinesin, NtKCH, functions in cell expansion and division. New Phytol 193: 576–589 [DOI] [PubMed] [Google Scholar]
- Kohoutová L, Kourová H, Nagy SK, Volc J, Halada P, Mészáros T, Meskiene I, Bögre L, Binarová P (2015) The Arabidopsis mitogen-activated protein kinase 6 is associated with γ-tubulin on microtubules, phosphorylates EB1c and maintains spindle orientation under nitrosative stress. New Phytol 207: 1061–1074 [DOI] [PubMed] [Google Scholar]
- Kollárová E, Baquero Forero A, Cvrčková F (2021) The Arabidopsis thaliana class II formin FH13 modulates pollen tube growth. Front Plant Sci 12: 599961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komis G, Luptovčiak I, Ovečka M, Samakovli D, Šamajová O, Šamaj J (2017) Katanin effects on dynamics of cortical microtubules and mitotic arrays in Arabidopsis thaliana revealed by advanced live-cell imaging. Front Plant Sci 8: 866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Z, Hotta T, Lee Y-RJ, Horio T, Liu B (2010) The {gamma}-tubulin complex protein GCP4 is required for organizing functional microtubule arrays in Arabidopsis thaliana. Plant Cell 22: 191–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Z, Ioki M, Braybrook S, Li S, Ye Z-H, Julie Lee Y-R, Hotta T, Chang A, Tian J, Wang G, Liu B (2015) Kinesin-4 functions in vesicular transport on cortical microtubules and regulates cell wall mechanics during cell elongation in plants. Mol Plant 8: 1011–1023 [DOI] [PubMed] [Google Scholar]
- Konopka CA, Backues SK, Bednarek SY (2008) Dynamics of Arabidopsis dynamin-related protein 1C and a clathrin light chain at the plasma membrane. Plant Cell 20: 1363–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka CA, Bednarek SY (2008) Comparison of the dynamics and functional redundancy of the Arabidopsis dynamin-related isoforms DRP1A and DRP1C during plant development. Plant Physiol 147: 1590–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosetsu K, de Keijzer J, Janson ME, Goshima G (2013) MICROTUBULE-ASSOCIATED PROTEIN65 is essential for maintenance of phragmoplast bipolarity and formation of the cell plate in Physcomitrella patens. Plant Cell 25: 4479–4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnova T, et al. (2009) Microtubule-associated kinase-like protein RUNKEL needed [corrected] for cell plate expansion in Arabidopsis cytokinesis. Curr Biol 19: 518–523 [DOI] [PubMed] [Google Scholar]
- Krupnova T, Stierhof Y-D, Hiller U, Strompen G, Müller S (2013) The microtubule-associated kinase-like protein RUNKEL functions in somatic and syncytial cytokinesis. Plant J 74: 781–791 [DOI] [PubMed] [Google Scholar]
- Kumari P, Dahiya P, Livanos P, Zergiebel L, Kölling M, Poeschl Y, Stamm G, Hermann A, Abel S, Müller S, et al. (2021) IQ67 DOMAIN proteins facilitate preprophase band formation and division-plane orientation. Nat Plants 7: 739–747 [DOI] [PubMed] [Google Scholar]
- Lagomarsino MC, Tanase C, Vos JW, Emons AMC, Mulder BM, Dogterom M (2007) Microtubule organization in three-dimensional confined geometries: evaluating the role of elasticity through a combined in vitro and modeling approach. Biophys J 92: 1046–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DR, Wiedemeier A, Peng L, Höfte H, Vernhettes S, Desprez T, Hocart CH, Birch RJ, Baskin TI, Burn JE, et al. (2001) Temperature-sensitive alleles of RSW2 link the KORRIGAN endo-1, 4-β-glucanase to cellulose synthesis and cytokinesis in Arabidopsis. Plant Physiol 126: 278–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber MH, Waizenegger I, Steinmann T, Schwarz H, Mayer U, Hwang I, Lukowitz W, Jürgens G (1997) The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J Cell Biol 139: 1485–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YR, Giang HM, Liu B (2001) A novel plant kinesin-related protein specifically associates with the phragmoplast organelles. Plant Cell 13: 2427–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-RJ, Hiwatashi Y, Hotta T, Xie T, Doonan JH, Liu B (2017) The mitotic function of augmin is dependent on its microtubule-associated protein subunit EDE1 in Arabidopsis thaliana. Curr Biol 27: 3891–3897.e4 [DOI] [PubMed] [Google Scholar]
- Lee Y-RJ, Liu B (2019) Microtubule nucleation for the assembly of acentrosomal microtubule arrays in plant cells. New Phytol 222: 1705–1718 [DOI] [PubMed] [Google Scholar]
- Lee Y-RJ, Li Y, Liu B (2007) Two Arabidopsis phragmoplast-associated kinesins play a critical role in cytokinesis during male gametogenesis. Plant Cell 19: 2595–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Li S, Du J, Bashline L, Gu Y (2013) Cellulose synthase INTERACTIVE3 regulates cellulose biosynthesis in both a microtubule-dependent and microtubule-independent manner in Arabidopsis. Plant Cell 25: 4912–4923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Li S, Gu Y (2012a) Cellulose synthase complexes: composition and regulation. Front Plant Sci 3: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Li S, Gu Y (2012b) Cellulose synthase interactive protein 1 (CSI1) mediates the intimate relationship between cellulose microfibrils and cortical microtubules. Plant Signal Behav 7: 714–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Singh A, Bashline L, Li S, Yingling YG, Gu Y (2015) CELLULOSE SYNTHASE INTERACTIVE1 is required for fast recycling of cellulose synthase complexes to the plasma membrane in Arabidopsis. Plant Cell 27: 2926–2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Zhang T, Strasser R, Lee CM, Gonneau M, Mach L, Vernhettes S, Kim SH, Cosgrove DJ, Li S, et al. (2014) The jiaoyao1 mutant is an allele of korrigan1 that abolishes endoglucanase activity and affects the organization of both cellulose microfibrils and microtubules in Arabidopsis. Plant Cell 26: 2601–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepman AH, Wilkerson CG, Keegstra K (2005) Expression of cellulose synthase-like (Csl) genes in insect cells reveals that CslA family members encode mannan synthases. Proc Natl Acad Sci USA 102: 2221–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Sun B, Sasabe M, Deng X, Machida Y, Lin H, Julie Lee Y-R, Liu B (2017a) Arabidopsis MAP65-4 plays a role in phragmoplast microtubule organization and marks the cortical cell division site. New Phytol 215: 187–201 [DOI] [PubMed] [Google Scholar]
- Li J, Staiger CJ (. 2018) Understanding cytoskeletal dynamics during the plant immune response. Annu Rev Phytopathol 56: 513–533 [DOI] [PubMed] [Google Scholar]
- Li M, Xiong G, Li R, Cui J, Tang D, Zhang B, Pauly M, Cheng Z, Zhou Y (2009) Rice cellulose synthase-like D4 is essential for normal cell-wall biosynthesis and plant growth: Rice cellulose synthase-like D4 is essential for normal cell-wall. Plant J 60: 1055–1069 [DOI] [PubMed] [Google Scholar]
- Lindeboom JJ, Lioutas A, Deinum EE, Tindemans SH, Ehrhardt DW, Emons AMC, Vos JW, Mulder BM (2013) Cortical microtubule arrays are initiated from a nonrandom prepattern driven by atypical microtubule initiation. Plant Physiol 161: 1189–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Krishnamoorthy P, Schubert V, Hause G, Heilmann M, Heilmann I (2019) A dual role for cell plate-associated PI4Kβ in endocytosis and phragmoplast dynamics during plant somatic cytokinesis. EMBO J 38: e100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka E, Gadeyne A, Stöckle D, Zimmermann S, De Jaeger G, Ehrhardt DW, Kirik V, Van Damme D, Müller S (2014) The phragmoplast-orienting kinesin-12 class proteins translate the positional information of the preprophase band to establish the cortical division zone in Arabidopsis thaliana. Plant Cell 26: 2617–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Rodriguez-Furlan C, Wang J, van de Ven W, Gao T, Raikhel NV, Hicks GR (2017b) Different endomembrane trafficking pathways establish apical and basal polarities. Plant Cell 29: 90–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Bashline L, Lei L, Gu Y (2014) Cellulose synthesis and its regulation. Arabidopsis Book 12: e0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Lei L, Somerville CR, Gu Y (2012) Cellulose synthase interactive protein 1 (CSI1) links microtubules and cellulose synthase complexes. Proc Natl Acad Sci USA 109: 185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Marc J, Joshi HC, Palevitz BA (1993) A gamma-tubulin-related protein associated with the microtubule arrays of higher plants in a cell cycle-dependent manner. J Cell Sci 104(Pt 4): 1217–1228 [DOI] [PubMed] [Google Scholar]
- Liu B, Palevitz BA (1992) Organization of cortical microfilaments in dividing root cells. Cell Motil Cytoskeleton 23: 252–264 [Google Scholar]
- Liu C, Zhang Y, Ren H (2018) Actin polymerization mediated by AtFH5 directs the polarity establishment and vesicle trafficking for pollen germination in Arabidopsis. Mol Plant 11: 1389–1399 [DOI] [PubMed] [Google Scholar]
- Liu C, Zhang Y, Ren H (2021) Profilin promotes formin-mediated actin filament assembly and vesicle transport during polarity formation in pollen. Plant Cell 33: 1252–1267 [DOI] [PubMed] [Google Scholar]
- Liu L, Shang-Guan K, Zhang B, Liu X, Yan M, Zhang L, Shi Y, Zhang M, Qian Q, Li J, et al. (2013) Brittle Culm1, a COBRA-like protein, functions in cellulose assembly through binding cellulose microfibrils. PLoS Genet 9: e1003704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Schneider R, Kesten C, Zhang Y, Somssich M, Zhang Y, Fernie AR, Persson S (2016) Cellulose-microtubule uncoupling proteins prevent lateral displacement of microtubules during cellulose synthesis in Arabidopsis. Dev Cell 38: 305–315 [DOI] [PubMed] [Google Scholar]
- Livanos P, Müller S (2019) Division plane establishment and cytokinesis. Annu Rev Plant Biol 70: 239–267 [DOI] [PubMed] [Google Scholar]
- Li Y, Qian Q, Zhou Y, Yan M, Sun L, Zhang M, Fu Z, Wang Y, Han B, Pang X, et al. (2003) BRITTLE CULM1, which encodes a COBRA-like protein, affects the mechanical properties of rice plants. Plant Cell 15: 2020–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Shen Y, Cai C, Zhong C, Zhu L, Yuan M, Ren H (2010) The type II Arabidopsis formin14 interacts with microtubules and microfilaments to regulate cell division. Plant Cell 22: 2710–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JR, Courtney S, Hassfurder M, Dhingra S, Bryant A, Shaw SL (2011) Microtubule-associated proteins MAP65-1 and MAP65-2 positively regulate axial cell growth in etiolated Arabidopsis hypocotyls. Plant Cell 23: 1889–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JR, Shaw SL (2012) MAP65-1 and MAP65-2 promote cell proliferation and axial growth in Arabidopsis roots. Plant J 71: 454–463 [DOI] [PubMed] [Google Scholar]
- Lukowitz W, Mayer U, Jürgens G (1996) Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell 84: 61–71 [DOI] [PubMed] [Google Scholar]
- Madison SL, Buchanan ML, Glass JD, McClain TF, Park E, Nebenführ A (2015) Class XI myosins move specific organelles in pollen tubes and are required for normal fertility and pollen tube growth in Arabidopsis. Plant Physiol 169: 1946–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Sasabe M, Hanamata S, Machida Y, Hasezawa S, Higaki T (2020) Actin filament disruption alters phragmoplast microtubule dynamics during the initial phase of plant cytokinesis. Plant Cell Physiol 61: 445–456 [DOI] [PubMed] [Google Scholar]
- Mansoori N, Timmers J, Desprez T, Kamei CLA, Dees DCT, Vincken J-P, Visser RGF, Höfte H, Vernhettes S, Trindade LM (2014) KORRIGAN1 interacts specifically with integral components of the cellulose synthase machinery. PLoS ONE 9: e112387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez P, Dixit R, Balkunde RS, Zhang A, O’Leary SE, Brakke KA, Rasmussen CG (2020) TANGLED1 mediates microtubule interactions that may promote division plane positioning in maize. J Cell Biol 219: e201907184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez P, Luo A, Sylvester A, Rasmussen CG (2017) Proper division plane orientation and mitotic progression together allow normal growth of maize. Proc Natl Acad Sci USA 114: 2759–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayers JR, Hu T, Wang C, Cárdenas JJ, Tan Y, Pan J, Bednarek SY (2017) SCD1 and SCD2 form a complex that functions with the exocyst and RabE1 in exocytosis and cytokinesis. Plant Cell 29: 2610–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AR, Liu B, Joshi HC, Palevitz BA (1993) Gamma-tubulin is associated with a cortical-microtubule-organizing zone in the developing guard cells of Allium cepa L. Planta 191: 357–361 [DOI] [PubMed] [Google Scholar]
- McFarlane HE, Mutwil-Anderwald D, Verbančič J, Picard KL, Gookin TE, Froehlich A, Chakravorty D, Trindade LM, Alonso JM, Assmann SM, et al. (2021) A G protein-coupled receptor-like module regulates cellulose synthase secretion from the endomembrane system in Arabidopsis. Dev Cell 56: 1484–1497.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael CM, Bednarek SY (2013) Cytoskeletal and membrane dynamics during higher plant cytokinesis. New Phytol 197: 1039–1057 [DOI] [PubMed] [Google Scholar]
- McMichael CM, Reynolds GD, Koch LM, Wang C, Jiang N, Nadeau J, Sack FD, Gelderman MB, Pan J, Bednarek SY (2013) Mediation of clathrin-dependent trafficking during cytokinesis and cell expansion by Arabidopsis stomatal cytokinesis defective proteins. Plant Cell 25: 3910–3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meents MJ, Watanabe Y, Samuels AL (2018) The cell biology of secondary cell wall biosynthesis. Ann Bot 121: 1107–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men S, Boutté Y, Ikeda Y, Li X, Palme K, Stierhof Y-D, Hartmann M-A, Moritz T, Grebe M (2008) Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat Cell Biol 10: 237–244 [DOI] [PubMed] [Google Scholar]
- Miao H, Guo R, Chen J, Wang Q, Lee Y-RJ, Liu B (2019) The γ-tubulin complex protein GCP6 is crucial for spindle morphogenesis but not essential for microtubule reorganization in Arabidopsis. Proc Natl Acad Sci USA 116: 27115–27123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Li H-Y, Shen J, Wang J, Jiang L (2011) QUASIMODO 3 (QUA3) is a putative homogalacturonan methyltransferase regulating cell wall biosynthesis in Arabidopsis suspension-cultured cells. J Exp Bot 62: 5063–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miart F, Desprez T, Biot E, Morin H, Belcram K, Höfte H, Gonneau M, Vernhettes S (2014) Spatio-temporal analysis of cellulose synthesis during cell plate formation in Arabidopsis. Plant J 77: 71–84 [DOI] [PubMed] [Google Scholar]
- Miki T, Naito H, Nishina M, Goshima G (2014) Endogenous localizome identifies 43 mitotic kinesins in a plant cell. Proc Natl Acad Sci USA 111: E1053–E1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AM, Morris VH, Rasmussen CG (2021) Functional characterization of TANGLED1 interaction with PHRAGMOPLAST ORIENTING KINESIN1 during mitosis in Arabidopsis. bioRxiv: 2021.04.30.442137
- Minamino N, Ueda T (2019) RAB GTPases and their effectors in plant endosomal transport. Curr Opin Plant Biol 52: 61–68 [DOI] [PubMed] [Google Scholar]
- Mirabet V, Krupinski P, Hamant O, Meyerowitz EM, Jönsson H, Boudaoud A (2018) The self-organization of plant microtubules inside the cell volume yields their cortical localization, stable alignment, and sensitivity to external cues. PLoS Comput Biol 14: e1006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir R, Morris VH, Buschmann H, Rasmussen CG (2018) Division plane orientation defects revealed by a synthetic double mutant phenotype. Plant Physiol 176: 418–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molchan TM, Valster AH, Hepler PK (2002) Actomyosin promotes cell plate alignment and late lateral expansion in Tradescantia stamen hair cells. Planta 214: 683–693 [DOI] [PubMed] [Google Scholar]
- Molino D, Van der Giessen E, Gissot L, Hématy K, Marion J, Barthelemy J, Bellec Y, Vernhettes S, Satiat-Jeunemaître B, Galli T, et al. (2014) Inhibition of very long acyl chain sphingolipid synthesis modifies membrane dynamics during plant cytokinesis. Biochim Biophys Acta 1842: 1422–1430 [DOI] [PubMed] [Google Scholar]
- Moore PJ, Staehelin LA (1988) Immunogold localization of the cell-wall-matrix polysaccharides rhamnogalacturonan I and xyloglucan during cell expansion and cytokinesis in Trifolium pratense L.; implication for secretory pathways. Planta 174: 433–445 [DOI] [PubMed] [Google Scholar]
- Moschou PN, Gutierrez-Beltran E, Bozhkov PV, Smertenko A (2016) Separase promotes microtubule polymerization by activating CENP-E-related kinesin Kin7. Dev Cell 37: 350–361 [DOI] [PubMed] [Google Scholar]
- Moschou PN, Smertenko AP, Minina EA, Fukada K, Savenkov EI, Robert S, Hussey PJ, Bozhkov PV (2013) The caspase-related protease separase (extra spindle poles) regulates cell polarity and cytokinesis in Arabidopsis. Plant Cell 25: 2171–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouille G, Ralet M-C, Cavelier C, Eland C, Effroy D, Hématy K, McCartney L, Truong HN, Gaudon V, Thibault J-F, et al. (2007) Homogalacturonan synthesis in Arabidopsis thaliana requires a Golgi-localized protein with a putative methyltransferase domain. Plant J 50: 605–614 [DOI] [PubMed] [Google Scholar]
- Mueller SC, Brown RM Jr (1980) Evidence for an intramembrane component associated with a cellulose microfibril-synthesizing complex in higher plants. J Cell Biol 84: 315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S, Han S, Smith LG (2006) Two kinesins are involved in the spatial control of cytokinesis in Arabidopsis thaliana. Curr Biol 16: 888–894 [DOI] [PubMed] [Google Scholar]
- Müller S, Livanos P (2019) Plant kinesin-12: Localization heterogeneity and functional implications. Int J Mol Sci 20: 4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S, Smertenko A, Wagner V, Heinrich M, Hussey PJ, Hauser M-T (2004) The plant microtubule-associated protein AtMAP65-3/PLE is essential for cytokinetic phragmoplast function. Curr Biol 14: 412–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Sano T, Sasabe M, Nonaka S, Higashiyama T, Hasezawa S, Machida Y, Hasebe M (2013) Mechanism of microtubule array expansion in the cytokinetic phragmoplast. Nat Commun 4: 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Sonobe S, Baskin TI, Hyodo S, Hasezawa S, Nagata T, Horio T, Hasebe M (2005) Microtubule-dependent microtubule nucleation based on recruitment of gamma-tubulin in higher plants. Nat Cell Biol 7: 961–968 [DOI] [PubMed] [Google Scholar]
- Naito H, Goshima G (2015) NACK kinesin is required for metaphase chromosome alignment and cytokinesis in the moss Physcomitrella patens. Cell Struct Funct 40: 31–41 [DOI] [PubMed] [Google Scholar]
- Nakamoto M, Schmit A-C, Heintz D, Schaller H, Ohta D (2015) Diversification of sterol methyltransferase enzymes in plants and a role for β-sitosterol in oriented cell plate formation and polarized growth. Plant J 84: 860–874 [DOI] [PubMed] [Google Scholar]
- Nakamura M, Lindeboom JJ, Saltini M, Mulder BM, Ehrhardt DW (2018) SPR2 protects minus ends to promote severing and reorientation of plant cortical microtubule arrays. J Cell Biol 217: 915–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Yagi N, Kato T, Fujita S, Kawashima N, Ehrhardt DW, Hashimoto T (2012) Arabidopsis GCP3-interacting protein 1/MOZART 1 is an integral component of the γ-tubulin-containing microtubule nucleating complex. Plant J 71: 216–225 [DOI] [PubMed] [Google Scholar]
- Nakaoka Y, Miki T, Fujioka R, Uehara R, Tomioka A, Obuse C, Kubo M, Hiwatashi Y, Goshima G (2012) An inducible RNA interference system in Physcomitrella patens reveals a dominant role of augmin in phragmoplast microtubule generation. Plant Cell 24: 1478–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan Q, Liang H, Mendoza J, Liu L, Fulzele A, Wright AJ, Bennett EJ, Rasmussen C, Facette MR (2021) The OPAQUE1/DISCORIDA2 myosin XI is required for phragmoplast guidance during asymmetric cell division in maize. bioRxiv: 2021.08.29.458084 [DOI] [PMC free article] [PubMed]
- Nebenführ A, Dixit R (2018) Kinesins and myosins: Molecular motors that coordinate cellular functions in plants. Annu Rev Plant Biol 69: 329–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol F, His I, Jauneau A, Vernhettes S, Canut H, Höfte H (1998) A plasma membrane-bound putative endo-1,4-beta-d-glucanase is required for normal wall assembly and cell elongation in Arabidopsis. EMBO J 17: 5563–5576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihama R, Soyano T, Ishikawa M, Araki S, Tanaka H, Asada T, Irie K, Ito M, Terada M, Banno H, et al. (2002) Expansion of the cell plate in plant cytokinesis requires a kinesin-like protein/MAPKKK complex. Cell 109: 87–99 [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Shi S, Zhang F, Liu R, Takagi Y, Bershadsky AD, Viasnoff V, Sellers JR (2020) The formin inhibitor, SMIFH2, inhibits members of the myosin superfamily. 2020.08.30.274613 [DOI] [PMC free article] [PubMed]
- Obel N, Erben V, Schwarz T, Kühnel S, Fodor A, Pauly M (2009) Microanalysis of plant cell wall polysaccharides. Mol Plant 2: 922–932 [DOI] [PubMed] [Google Scholar]
- Oh SA, Allen T, Kim GJ, Sidorova A, Borg M, Park SK, Twell D (2012) Arabidopsis fused kinase and the kinesin-12 subfamily constitute a signalling module required for phragmoplast expansion. Plant J 72: 308–319 [DOI] [PubMed] [Google Scholar]
- Oh SA, Bourdon V, Dickinson HG, Twell D, Park SK (2014) Arabidopsis fused kinase TWO-IN-ONE dominantly inhibits male meiotic cytokinesis. Plant Reprod 27: 7–17 [DOI] [PubMed] [Google Scholar]
- Oh S-A, Jeon J, Park H-J, Grini PE, Twell D, Park SK (2016) Analysis of gemini pollen 3 mutant suggests a broad function of AUGMIN in microtubule organization during sexual reproduction in Arabidopsis. Plant J 87: 188–201 [DOI] [PubMed] [Google Scholar]
- Oh SA, Johnson A, Smertenko A, Rahman D, Park SK, Hussey PJ, Twell D (2005) A divergent cellular role for the FUSED kinase family in the plant-specific cytokinetic phragmoplast. Curr Biol 15: 2107–2111 [DOI] [PubMed] [Google Scholar]
- van Oostende-Triplet C, Guillet D, Triplet T, Pandzic E, Wiseman PW, Geitmann A (2017) Vesicle dynamics during plant cell cytokinesis reveals distinct developmental phases. Plant Physiol 174: 1544–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer DG, Pollock MA, Vacik J, Szymanski DB, Ericson B, Feldmann K, Marks MD (1997) Essential role of a kinesin-like protein in Arabidopsis trichome morphogenesis. Proc Natl Acad Sci USA 94: 6261–6266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr RG, Furt F, Warner EL, Agar EM, Garbarino JM, Cabral SE, Dubuke ML, Butt AM, Munson M, Vidali L (2020) Rab-E and its interaction with myosin XI are essential for polarized cell growth. New Phytol 229: 1924–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui MS, Mastronarde DN, Kang BH, Bednarek SY, Staehelin LA (2001) Three-dimensional analysis of syncytial-type cell plates during endosperm cellularization visualized by high resolution electron tomography. Plant Cell 13: 2033–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui M, Staehelin LA (2000) Syncytial-type cell plates: a novel kind of cell plate involved in endosperm cellularization of Arabidopsis. Plant Cell 12: 933–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovečka M, Luptovčiak I, Komis G, Šamajová O, Samakovli D, Šamaj J (2020) Spatiotemporal pattern of ectopic cell divisions contribute to mis-shaped phenotype of primary and lateral roots of katanin1 mutant. Front Plant Sci 11: 734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagant S, Bichet A, Sugimoto K, Lerouxel O, Desprez T, McCann M, Lerouge P, Vernhettes S, Höfte H (2002) KOBITO1 encodes a novel plasma membrane protein necessary for normal synthesis of cellulose during cell expansion in Arabidopsis. Plant Cell 14: 2001–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panteris E, Adamakis I-DS, Voulgari G, Papadopoulou G (2011) A role for katanin in plant cell division: microtubule organization in dividing root cells of fra2 and lue1 Arabidopsis thaliana mutants. Cytoskeleton 68: 401–413 [DOI] [PubMed] [Google Scholar]
- Panteris E, Apostolakos P, Galatis B (1995) Telophase–interphase transition in taxol-treated Triticum root cells: cortical microtubules appear without the prior presence of a radial perinuclear array. Protoplasma 188: 78–84 [Google Scholar]
- Panteris E, Kouskouveli A, Pappas D, Adamakis I-DS (2021) Cytokinesis in fra2 Arabidopsis thaliana p60-Katanin mutant: defects in cell plate/daughter wall formation. Int J Mol Sci 22: 1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredez AR, Somerville CR, Ehrhardt DW (2006) Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312: 1491–1495 [DOI] [PubMed] [Google Scholar]
- Park E, Díaz-Moreno SM, Davis DJ, Wilkop TE, Bulone V, Drakakaki G (2014) Endosidin 7 specifically arrests late cytokinesis and inhibits callose biosynthesis, revealing distinct trafficking events during cell plate maturation. Plant Physiol 165: 1019–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, et al. (2018) Concerted action of evolutionarily ancient and novel SNARE complexes in flowering-plant cytokinesis. Dev Cell 44: 500–511.e4 [DOI] [PubMed] [Google Scholar]
- Park SK, Twell D (2001) Novel patterns of ectopic cell plate growth and lipid body distribution in the Arabidopsis gemini pollen1 mutant. Plant Physiol 126: 899–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastuglia M, Azimzadeh J, Goussot M, Camilleri C, Belcram K, Evrard J-L, Schmit A-C, Guerche P, Bouchez D (2006) Gamma-tubulin is essential for microtubule organization and development in Arabidopsis. Plant Cell 18: 1412–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattathil S, et al. (2010) A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol 153: 514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peremyslov VV, Prokhnevsky AI, Avisar D, Dolja VV (2008) Two class XI myosins function in organelle trafficking and root hair development in Arabidopsis. Plant Physiol 146: 1109–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peremyslov VV, Prokhnevsky AI, Dolja VV (2010) Class XI myosins are required for development, cell expansion, and F-Actin organization in Arabidopsis. Plant Cell 22: 1883–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson S, Paredez A, Carroll A, Palsdottir H, Doblin M, Poindexter P, Khitrov N, Auer M, Somerville CR (2007) Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proc Natl Acad Sci USA 104: 15566–15571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polko JK, Barnes WJ, Voiniciuc C, Doctor S, Steinwand B, Hill JL Jr, Tien M, Pauly M, Anderson CT, Kieber JJ (2018) SHOU4 proteins regulate trafficking of cellulose synthase complexes to the plasma membrane. Curr Biol 28: 3174–3182.e6 [DOI] [PubMed] [Google Scholar]
- Porter KR, Machado RD (1960) Studies on the endoplasmic reticulum. IV. Its form and distribution during mitosis in cells of onion root tip. J Biophys Biochem Cytol 7: 167–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Kaneda M, Chen J, Geitmann A, Zheng H (2011) A specific role for Arabidopsis TRAPPII in post-Golgi trafficking that is crucial for cytokinesis and cell polarity: Arabidopsis TRAPPII acts in post-Golgi traffic. Plant J 68: 234–248 [DOI] [PubMed] [Google Scholar]
- Qi X, Zheng H (2013) Rab-A1c GTPase defines a population of the trans-Golgi network that is sensitive to endosidin1 during cytokinesis in Arabidopsis. Mol Plant 6: 847–859 [DOI] [PubMed] [Google Scholar]
- Rasmussen CG, Bellinger M (2018) An overview of plant division‐plane orientation. New Phytol 219: 505–512 [DOI] [PubMed] [Google Scholar]
- Rawat A, Brejšková L, Hála M, Cvrčková F, Žárský V (2017) The Physcomitrella patens exocyst subunit EXO70.3d has distinct roles in growth and development, and is essential for completion of the moss life cycle. New Phytol 216: 438–454 [DOI] [PubMed] [Google Scholar]
- Reddy ASN, Safadi F, Narasimhulu SB, Golovkin M, Hu X (1996) A novel plant calmodulin-binding protein with a kinesin heavy chain motor domain. J Biol Chem 271: 7052–7060 [DOI] [PubMed] [Google Scholar]
- Renna L, Brandizzi F (2020) The mysterious life of the plant trans-Golgi network: advances and tools to understand it better. J Microsc 278: 154–163 [DOI] [PubMed] [Google Scholar]
- Renna L, Stefano G, Slabaugh E, Wormsbaecher C, Sulpizio A, Zienkiewicz K, Brandizzi F (2018) TGNap1 is required for microtubule-dependent homeostasis of a subpopulation of the plant trans-Golgi network. Nat Commun 9: 5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter S, Kientz M, Brumm S, Nielsen ME, Park M, Gavidia R, Krause C, Voss U, Beckmann H, Mayer U, et al. (2014) Delivery of endocytosed proteins to the cell-division plane requires change of pathway from recycling to secretion. Elife 3: e02131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S, Bichet A, Grandjean O, Kierzkowski D, Satiat-Jeunemaître B, Pelletier S, Hauser M-T, Höfte H, Vernhettes S (2005) An Arabidopsis endo-1,4-beta-d-glucanase involved in cellulose synthesis undergoes regulated intracellular cycling. Plant Cell 17: 3378–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Kuhlemeier C (2018) Global compression reorients cortical microtubules in Arabidopsis hypocotyl epidermis and promotes growth. Curr Biol 28: 1794–1802.e2 [DOI] [PubMed] [Google Scholar]
- Roudier F, Fernandez AG, Fujita M, Himmelspach R, Borner GHH, Schindelman G, Song S, Baskin TI, Dupree P, Wasteneys GO, et al. (2005) COBRA, an Arabidopsis extracellular glycosyl–phosphatidyl inositol-anchored protein, specifically controls highly anisotropic expansion through its involvement in cellulose microfibril orientation. Plant Cell 17: 1749–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak K, Steiner A, Synek L, Klaeger S, Kulich I, Facher E, Wanner G, Kuster B, Zarsky V, Persson S, Assaad FF (2014) Plant cytokinesis is orchestrated by the sequential action of the TRAPPII and exocyst tethering complexes. Dev Cell 29: 607–620 [DOI] [PubMed] [Google Scholar]
- Saatian B, Austin RS, Tian G, Chen C, Nguyen V, Kohalmi SE, Geelen D, Cui Y (2018) Analysis of a novel mutant allele of GSL8 reveals its key roles in cytokinesis and symplastic trafficking in Arabidopsis. BMC Plant Biol 18: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaj J, Peters M, Volkmann D, Baluska F (2000) Effects of myosin ATPase inhibitor 2,3-butanedione 2-monoxime on distributions of myosins, F-actin, microtubules, and cortical endoplasmic reticulum in maize root apices. Plant Cell Physiol 41: 571–582 [DOI] [PubMed] [Google Scholar]
- Sampathkumar A, Gutierrez R, McFarlane HE, Bringmann M, Lindeboom J, Emons A-M, Samuels L, Ketelaar T, Ehrhardt DW, Persson S (2013) Patterning and lifetime of plasma membrane-localized cellulose synthase is dependent on actin organization in Arabidopsis interphase cells. Plant Physiol 162: 675–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathkumar A, Krupinski P, Wightman R, Milani P, Berquand A, Boudaoud A, Hamant O, Jönsson H, Meyerowitz EM (2014) Subcellular and supracellular mechanical stress prescribes cytoskeleton behavior in Arabidopsis cotyledon pavement cells. Elife 3: e01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels AL, Giddings TH Jr, Staehelin LA (1995) Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J Cell Biol 130: 1345–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Rodríguez C, et al. (2018) The cellulose synthases are cargo of the TPLATE adaptor complex. Mol Plant 11: 346–349 [DOI] [PubMed] [Google Scholar]
- Sánchez-Rodríguez C, Ketelaar K, Schneider R, Villalobos JA, Somerville CR, Persson S, Wallace IS (2017) BRASSINOSTEROID INSENSITIVE2 negatively regulates cellulose synthesis in Arabidopsis by phosphorylating cellulose synthase 1. Proc Natl Acad Sci USA 114: 3533–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot AA, Kovaleva V, Bassham DC, Raikhel NV (2001) Interactions between syntaxins identify at least five SNARE complexes within the Golgi/prevacuolar system of the Arabidopsis cell. Mol Biol Cell 12: 3733–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe M, Ishibashi N, Haruta T, Minami A, Kurihara D, Higashiyama T, Nishihama R, Ito M, Machida Y (2015) The carboxyl–terminal tail of the stalk of Arabidopsis NACK1/HINKEL kinesin is required for its localization to the cell plate formation site. J Plant Res 128: 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe M, Kosetsu K, Hidaka M, Murase A, Machida Y (2011) Arabidopsis thaliana MAP65-1 and MAP65-2 function redundantly with MAP65-3/PLEIADE in cytokinesis downstream of MPK4. Plant Signal Behav 6: 743–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe M, Machida Y (2012) Regulation of organization and function of microtubules by the mitogen-activated protein kinase cascade during plant cytokinesis. Cytoskeleton 69: 913–918 [DOI] [PubMed] [Google Scholar]
- Sasabe M, Soyano T, Takahashi Y, Sonobe S, Igarashi H, Itoh TJ, Hidaka M, Machida Y (2006) Phosphorylation of NtMAP65-1 by a MAP kinase down-regulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells. Genes Dev 20: 1004–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Tsutsumi M, Otomo K, Murata T, Yagi N, Nakamura M, Nemoto T, Hasebe M, Oda Y (2019) A novel katanin-tethering machinery accelerates cytokinesis. Curr Biol 29: 4060–4070.e3 [DOI] [PubMed] [Google Scholar]
- Scheible WR, Eshed R, Richmond T, Delmer D, Somerville C (2001) Modifications of cellulose synthase confer resistance to isoxaben and thiazolidinone herbicides in Arabidopsis Ixr1 mutants. Proc Natl Acad Sci USA 98: 10079–10084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelman G, Morikami A, Jung J, Baskin TI, Carpita NC, Derbyshire P, McCann MC, Benfey PN (2001) COBRA encodes a putative GPI-anchored protein, which is polarly localized and necessary for oriented cell expansion in Arabidopsis. Genes Dev 15: 1115–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Smertenko A (2019) Identification and characterization of the land-plant-specific microtubule nucleation factor MACET4. J Cell Sci 132: jcs232819 [DOI] [PubMed] [Google Scholar]
- Schmiedel G, Reiss H-D, Schnepf E (1981) Associations between membranes and microtubules during mitosis and cytokinesis in caulonema tip cells of the moss Funaria hygrometrica. Protoplasma 108: 173–190 [Google Scholar]
- Schmit AC, Lambert AM (1988) Plant actin filament and microtubule interactions during anaphase–telophase transition: effects of antagonist drugs. Biol Cell 64: 309–319 [DOI] [PubMed] [Google Scholar]
- Schneider R, Hanak T, Persson S, Voigt CA (2016) Cellulose and callose synthesis and organization in focus, what’s new? Curr Opin Plant Biol 34: 9–16 [DOI] [PubMed] [Google Scholar]
- Schrick K, Mayer U, Horrichs A, Kuhnt C, Bellini C, Dangl J, Schmidt J, Jürgens G (2000) FACKEL is a sterol C-14 reductase required for organized cell division and expansion in Arabidopsis embryogenesis. Genes Dev 14: 1471–1484 [PMC free article] [PubMed] [Google Scholar]
- Seguí-Simarro JM, Austin JR 2nd, White EA, Staehelin LA (2004) Electron tomographic analysis of somatic cell plate formation in meristematic cells of Arabidopsis preserved by high-pressure freezing. Plant Cell 16: 836–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer V, Janski N, Canaday J, Herzog E, Erhardt M, Evrard J-L, Schmit A-C (2007) Arabidopsis GCP2 and GCP3 are part of a soluble gamma-tubulin complex and have nuclear envelope targeting domains. Plant J 52: 322–331 [DOI] [PubMed] [Google Scholar]
- Shimamura M, Brown RC, Lemmon BE, Akashi T, Mizuno K, Nishihara N, Tomizawa K-I, Yoshimoto K, Deguchi H, Hosoya H, et al. (2004) Gamma-tubulin in basal land plants: characterization, localization, and implication in the evolution of acentriolar microtubule organizing centers. Plant Cell 16: 45–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T, Narita NN, Hayashi K, Hayashi K, Asada J, Hamada T, Sonobe S, Nakajima K, Hashimoto T (2004) Plant-specific microtubule-associated protein SPIRAL2 is required for anisotropic growth in Arabidopsis. Plant Physiol 136: 3933–3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MLA, Platre MP, Marquès-Bueno MM, Armengot L, Stanislas T, Bayle V, Caillaud M-C, Jaillais Y (2016) A PtdIns (4) P-driven electrostatic field controls cell membrane identity and signalling in plants. Nat Plants 2: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair R, Rosquete MR, Drakakaki G (2018) Post-Golgi trafficking and transport of cell wall components. Front Plant Sci 9: 1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slewinski TL, Baker RF, Stubert A, Braun DM (2012) Tie-dyed2 encodes a callose synthase that functions in vein development and affects symplastic trafficking within the phloem of maize leaves. Plant Physiol 160: 1540–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smertenko A, et al. (2017) Plant cytokinesis: terminology for structures and processes. Trends Cell Biol 27: 885–894 [DOI] [PubMed] [Google Scholar]
- Smertenko A, Hewitt SL, Jacques CN, Kacprzyk R, Liu Y, Marcec MJ, Moyo L, Ogden A, Oung HM, Schmidt S, et al. (2018) Phragmoplast microtubule dynamics—a game of zones. J Cell Sci 131: jcs203331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smertenko AP, Chang H-Y, Sonobe S, Fenyk SI, Weingartner M, Bögre L, Hussey PJ (2006) Control of the AtMAP65-1 interaction with microtubules through the cell cycle. J Cell Sci 119: 3227–3237 [DOI] [PubMed] [Google Scholar]
- Smertenko AP, Chang H-Y, Wagner V, Kaloriti D, Fenyk S, Sonobe S, Lloyd C, Hauser M-T, Hussey PJ (2004) The Arabidopsis microtubule-associated protein AtMAP65-1: molecular analysis of its microtubule bundling activity. Plant Cell 16: 2035–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smertenko AP, Kaloriti D, Chang H-Y, Fiserova J, Opatrny Z, Hussey PJ (2008) The C-terminal variable region specifies the dynamic properties of Arabidopsis microtubule-associated protein MAP65 isotypes. Plant Cell 20: 3346–3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smertenko AP, Piette B, Hussey PJ (2011) The origin of phragmoplast asymmetry. Curr Biol 21: 1924–1930 [DOI] [PubMed] [Google Scholar]
- Smertenko A, Saleh N, Igarashi H, Mori H, Hauser-Hahn I, Jiang CJ, Sonobe S, Lloyd CW, Hussey PJ (2000) A new class of microtubule-associated proteins in plants. Nat Cell Biol 2: 750–753 [DOI] [PubMed] [Google Scholar]
- Smith LG, Gerttula SM, Han S, Levy J (2001) Tangled1: a microtubule binding protein required for the spatial control of cytokinesis in maize. J Cell Biol 152: 231–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LG, Hake S, Sylvester AW (1996) The tangled-1 mutation alters cell division orientations throughout maize leaf development without altering leaf shape. Development 122: 481–489 [DOI] [PubMed] [Google Scholar]
- Söllner R, Glässer G, Wanner G, Somerville CR, Jürgens G, Assaad FF (2002) Cytokinesis-defective mutants of Arabidopsis. Plant Physiol 129: 678–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonobe S, Nakayama N, Shimmen T, Sone Y (2000) Intracellular distribution of subcellular organelles revealed by antibody against xyloglucan during cell cycle in tobacco BY-2 cells. Protoplasma 213: 218–227 [Google Scholar]
- Spiegelman Z, Lee C-M, Gallagher KL (2018) KinG is a plant-specific kinesin that regulates both intra- and intercellular movement of SHORT-ROOT. Plant Physiol 176: 392–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A, Müller L, Rybak K, Vodermaier V, Facher E, Thellmann M, Ravikumar R, Wanner G, Hauser M-T, Assaad FF (2016) The membrane-associated Sec1/Munc18 KEULE is required for phragmoplast microtubule reorganization during cytokinesis in Arabidopsis. Mol Plant 9: 528–540 [DOI] [PubMed] [Google Scholar]
- Sterling JD, Quigley HF, Orellana A, Mohnen D (2001) The catalytic site of the pectin biosynthetic enzyme α-1, 4-galacturonosyltransferase is located in the lumen of the Golgi. Plant Physiol 127: 360–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöckle D, Herrmann A, Lipka E, Lauster T, Gavidia R, Zimmermann S, Müller S (2016) Putative RopGAPs impact division plane selection and interact with kinesin-12 POK1. Nat Plants 2: 16120. [DOI] [PubMed] [Google Scholar]
- Strompen G, El Kasmi F, Richter S, Lukowitz W, Assaad FF, Jürgens G, Mayer U (2002) The Arabidopsis HINKEL gene encodes a kinesin-related protein involved in cytokinesis and is expressed in a cell cycle-dependent manner. Curr Biol 12: 153–158 [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Williamson RE, Wasteneys GO (2001) Wall architecture in the cellulose-deficientrsw1 mutant of Arabidopsis thaliana: Microfibrils but not microtubules lose their transverse alignment before microfibrils become unrecognizable in the mitotic and elongation zones of roots. Protoplasma 215: 172–183 [DOI] [PubMed] [Google Scholar]
- Sun H, Furt F, Vidali L (2018) Myosin XI localizes at the mitotic spindle and along the cell plate during plant cell division in Physcomitrella patens. Biochem Biophys Res Commun 506: 409–421 [DOI] [PubMed] [Google Scholar]
- Szymanski D, Staiger CJ (2018) The actin cytoskeleton: functional arrays for cytoplasmic organization and cell shape control. Plant Physiol 176: 106–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, de Keijzer J, Overdijk EJR, Sweep E, Steentjes M, Vermeer JEM, Janson ME, Ketelaar T (2019) Exocyst subunit Sec6 is positioned by microtubule overlaps in the moss phragmoplast prior to cell plate membrane arrival. J Cell Sci 132 [DOI] [PubMed] [Google Scholar]
- Thellmann M, Rybak K, Thiele K, Wanner G, Assaad FF (2010) Tethering factors required for cytokinesis in Arabidopsis. Plant Physiol 154: 720–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele K, Wanner G, Kindzierski V, Jürgens G, Mayer U, Pachl F, Assaad FF (2009) The timely deposition of callose is essential for cytokinesis in Arabidopsis. Plant J 58: 13–26 [DOI] [PubMed] [Google Scholar]
- Thoms D, Vineyard L, Elliott A, Shaw SL (2018) CLASP facilitates transitions between cortical microtubule array patterns. Plant Physiol 178: 1551–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Han L, Feng Z, Wang G, Liu W, Ma Y, Yu Y, Kong Z (2015) Orchestration of microtubules and the actin cytoskeleton in trichome cell shape determination by a plant-unique kinesin. Elife 4: e09351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töller A, Brownfield L, Neu C, Twell D, Schulze-Lefert P (2008) Dual function of Arabidopsis glucan synthase-like genes GSL8 and GSL10 in male gametophyte development and plant growth. Plant J 54: 911–923 [DOI] [PubMed] [Google Scholar]
- Toyooka K, Goto Y, Asatsuma S, Koizumi M, Mitsui T, Matsuoka K (2009) A mobile secretory vesicle cluster involved in mass transport from the Golgi to the plant cell exterior. Plant Cell 21: 1212–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twell D, Park SK, Hawkins TJ, Schubert D, Schmidt R, Smertenko A, Hussey PJ (2002) MOR1/GEM1 has an essential role in the plant-specific cytokinetic phragmoplast. Nat Cell Biol 4: 711–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttewaal M, Burian A, Alim K, Landrein B, Borowska-Wykręt D, Dedieu A, Peaucelle A, Ludynia M, Traas J, Boudaoud A, et al. (2012) Mechanical stress acts via katanin to amplify differences in growth rate between adjacent cells in Arabidopsis. Cell 149: 439–451 [DOI] [PubMed] [Google Scholar]
- Vain T, Crowell EF, Timpano H, Biot E, Desprez T, Mansoori N, Trindade LM, Pagant S, Robert S, Höfte H, et al. (2014) The cellulase KORRIGAN is part of the cellulose synthase complex. Plant Physiol 165: 1521–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valster AH, Pierson ES, Valenta R, Hepler PK, Emons A (1997) Probing the Plant Actin Cytoskeleton during Cytokinesis and Interphase by Profilin Microinjection. Plant Cell 9: 1815–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme D (2009) Division plane determination during plant somatic cytokinesis. Curr Opin Plant Biol 12: 745–751 [DOI] [PubMed] [Google Scholar]
- Van Damme D, Coutuer S, De Rycke R, Bouget F-Y, Inzé D, Geelen D (2006) Somatic cytokinetics and pollen maturation in Arabidopsis depend on TPLATE, which has domains similar to coat proteins. Plant Cell 18: 3502–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Does D, et al. (2017) The Arabidopsis leucine-rich repeat receptor kinase MIK2/LRR-KISS connects cell wall integrity sensing, root growth and response to abiotic and biotic stresses. PLoS Genet 13: e1006832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer JEM, van Wijk R, Goedhart J, Geldner N, Chory J, Gadella TWJ Jr, Munnik T (2017) In vivo imaging of diacylglycerol at the cytoplasmic leaflet of plant membranes. Plant Cell Physiol 58: 1196–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, van Gisbergen PAC, Guérin C, Franco P, Li M, Burkart GM, Augustine RC, Blanchoin L, Bezanilla M (2009) Rapid formin-mediated actin-filament elongation is essential for polarized plant cell growth. Proc Natl Acad Sci USA 106: 13341–13346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti C, et al. (2012) Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell 22: 1344–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos JW, Safadi F, Reddy AS, Hepler PK (2000) The kinesin-like calmodulin binding protein is differentially involved in cell division. Plant Cell 12: 979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waizenegger I, Lukowitz W, Assaad F, Schwarz H, Jürgens G, Mayer U (2000) The Arabidopsis KNOLLE and KEULE genes interact to promote vesicle fusion during cytokinesis. Curr Biol 10: 1371–1374 [DOI] [PubMed] [Google Scholar]
- Walker KL, Müller S, Moss D, Ehrhardt DW, Smith LG (2007) Arabidopsis TANGLED identifies the division plane throughout mitosis and cytokinesis. Curr Biol 17: 1827–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Liu W, Wang G, Li J, Dong L, Han L, Wang Q, Tian J, Yu Y, Gao C, et al. (2017a) KTN80 confers precision to microtubule severing by specific targeting of katanin complexes in plant cells. EMBO J 36: 3435–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Wang C, Liu W, Ma Y, Dong L, Tian J, Yu Y, Kong Z (2018) Augmin antagonizes katanin at microtubule crossovers to control the dynamic organization of plant cortical arrays. Curr Biol 28: 1311–1317.e3 [DOI] [PubMed] [Google Scholar]
- Wang G, Wang F, Wang G, Wang F, Zhang X, Zhong M, Zhang J, Lin D, Tang Y, Xu Z, et al. (2012) Opaque1 encodes a myosin XI motor protein that is required for endoplasmic reticulum motility and protein body formation in maize endosperm. Plant Cell 24: 3447–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhuang X, Wang X, Law AHY, Zhao T, Du S, Loy MMT, Jiang L (2016) A distinct pathway for polar exocytosis in plant cell wall formation. Plant Physiol 172: 1003–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. (2021a) Conditional destabilization of the TPLATE complex impairs endocytic internalization. Proc Natl Acad Sci USA 118: e2023456118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Elliott JE, Williamson RE (2008) Features of the primary wall CESA complex in wild type and cellulose-deficient mutants of Arabidopsis thaliana. J Exp Bot 59: 2627–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Li P, Ma Y, Nie X, Grebe M, Men S (2021b) Membrane sterol composition in Arabidopsis thaliana affects root elongation via auxin biosynthesis. Int J Mol Sci 22: 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Chen X, Goldbeck C, Chung E, Kang B-H (2017b) A distinct class of vesicles derived from the trans-Golgi mediates secretion of xylogalacturonan in the root border cell. Plant J 92: 596–610 [DOI] [PubMed] [Google Scholar]
- Wang Y, Li X, Fan B, Zhu C, Chen Z (2021c) Regulation and function of defense-related callose deposition in plants. Int J Mol Sci 22: 2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington AT, Vugrek O, Wei KJ, Hasenbein NG, Sugimoto K, Rashbrooke MC, Wasteneys GO (2001) MOR1 is essential for organizing cortical microtubules in plants. Nature 411: 610–613 [DOI] [PubMed] [Google Scholar]
- Wick SM, Hepler PK (1980) Localization of Ca++-containing antimonate precipitates during mitosis. J Cell Biol 86: 500–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman R, Chomicki G, Kumar M, Carr P, Turner SR (2013) SPIRAL2 determines plant microtubule organization by modulating microtubule severing. Curr Biol 23: 1902–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkop T, Pattathil S, Ren G, Davis DJ, Bao W, Duan D, Peralta AG, Domozych DS, Hahn MG, Drakakaki G (2019) A hybrid approach enabling large-scale glycomic analysis of post-Golgi vesicles reveals a transport route for polysaccharides. Plant Cell 31: 627–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen V, Friml J, Grebe M, van den Toorn A, Palme K, Scheres B (2003) Cell polarity and PIN protein positioning in Arabidopsis require STEROL METHYLTRANSFERASE1 function. Plant Cell 15: 612–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S, Hématy K, Höfte H (2012) Growth control and cell wall signaling in plants. Annu Rev Plant Biol 63: 381–407 [DOI] [PubMed] [Google Scholar]
- Won K-H, Kim H (2020) Functions of the plant Qbc SNARE SNAP25 in cytokinesis and biotic and abiotic stress responses. Mol Cells 43: 313–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Tan X, Wu C, Cao K, Li Y, Bao Y (2013) Regulation of cytokinesis by exocyst subunit SEC6 and KEULE in Arabidopsis thaliana. Mol Plant 6: 1863–1876 [DOI] [PubMed] [Google Scholar]
- Wu S-W, Kumar R, Iswanto ABB, Kim J-Y (2018) Callose balancing at plasmodesmata. J Exp Bot 69: 5325–5339 [DOI] [PubMed] [Google Scholar]
- Wu S-Z, Bezanilla M (2014) Myosin VIII associates with microtubule ends and together with actin plays a role in guiding plant cell division. Elife 3: e03498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S-Z, Ritchie JA, Pan A-H, Quatrano RS, Bezanilla M (2011) Myosin VIII regulates protonemal patterning and developmental timing in the moss Physcomitrella patens. Mol Plant 4: 909–921 [DOI] [PubMed] [Google Scholar]
- Xin X, Lei L, Zheng Y, Zhang T, Pingali SV, O’Neill H, Cosgrove DJ, Li S, Gu Y (2020) Cellulose synthase interactive1- and microtubule-dependent cell wall architecture is required for acid growth in Arabidopsis hypocotyls. J Exp Bot 71: 2982–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong G, Li R, Qian Q, Song X, Liu X, Yu Y, Zeng D, Wan J, Li J, Zhou Y (2010) The rice dynamin-related protein DRP2B mediates membrane trafficking, and thereby plays a critical role in secondary cell wall cellulose biosynthesis. Plant J 64: 56–70 [DOI] [PubMed] [Google Scholar]
- Xu T, Qu Z, Yang X, Qin X, Xiong J, Wang Y, Ren D, Liu G (2009) A cotton kinesin GhKCH2 interacts with both microtubules and microfilaments. Biochem J 421: 171–180 [DOI] [PubMed] [Google Scholar]
- Xu XM, Zhao Q, Rodrigo-Peiris T, Brkljacic J, He CS, Müller S, Meier I (2008) RanGAP1 is a continuous marker of the Arabidopsis cell division plane. Proc Natl Acad Sci USA 105: 18637–18642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuuchi T, Nakai T, Sonobe S, Yamauchi D, Mineyuki Y (2015) Preprophase band formation and cortical division zone establishment: RanGAP behaves differently from microtubules during their band formation. Plant Signal Behav 10: e1060385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Goshima G (2018) The KCH kinesin drives nuclear transport and cytoskeletal coalescence to promote tip cell growth in Physcomitrella patens. Plant Cell 30: 1496–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Tanaka-Takiguchi Y, Hayashi M, Nishina M, Goshima G (2017) Multiple kinesin-14 family members drive microtubule minus end-directed transport in plant cells. J Cell Biol 216: 1705–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka S, Shimono Y, Shirakawa M, Fukao Y, Kawase T, Hatsugai N, Tamura K, Shimada T, Hara-Nishimura I (2013) Identification and dynamics of Arabidopsis adaptor protein-2 complex and its involvement in floral organ development. Plant Cell 25: 2958–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C-Y, Spielman M, Coles JP, Li Y, Ghelani S, Bourdon V, Brown RC, Lemmon BE, Scott RJ, Dickinson HG (2003) TETRASPORE encodes a kinesin required for male meiotic cytokinesis in Arabidopsis. Plant J 34: 229–240 [DOI] [PubMed] [Google Scholar]
- Yang W, Ren S, Zhang X, Gao M, Ye S, Qi Y, Zheng Y, Wang J, Zeng L, Li Q, et al. (2011) BENT UPPERMOST INTERNODE1 encodes the class II formin FH5 crucial for actin organization and rice development. Plant Cell 23: 661–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Schuster C, Beahan CT, Charoensawan V, Peaucelle A, Bacic A, Doblin MS, Wightman R, Meyerowitz EM (2016) Regulation of meristem morphogenesis by cell wall synthases in Arabidopsis. Curr Biol 26: 1404–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara H, Muraoka M, Shogaki H, Mori H, Sonobe S (2002) TMBP200, a microtubule bundling polypeptide isolated from telophase tobacco BY-2 cells is a MOR1 homologue. Plant Cell Physiol 43: 595–603 [DOI] [PubMed] [Google Scholar]
- Yin L, Verhertbruggen Y, Oikawa A, Manisseri C, Knierim B, Prak L, Jensen JK, Knox JP, Auer M, Willats WGT, et al. (2011) The cooperative activities of CSLD2, CSLD3, and CSLD5 are required for normal Arabidopsis development. Mol Plant 4: 1024–1037 [DOI] [PubMed] [Google Scholar]
- Yokoyama R, Nishitani K (2001) Endoxyloglucan transferase is localized both in the cell plate and in the secretory pathway destined for the apoplast in tobacco cells. Plant Cell Physiol 42: 292–300 [DOI] [PubMed] [Google Scholar]
- Yoo C-M, Quan L, Blancaflor EB (2012) Divergence and redundancy in CSLD2 and CSLD3 function during Arabidopsis thaliana root hair and female gametophyte development. Front Plant Sci 3: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida MW, Yamada M, Goshima G (2019) Moss kinesin-14 KCBP accelerates chromatid motility in anaphase. Cell Struct Funct 44: 95–104 [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Eiguchi M, Hibara K-I, Ito J-I, Nagato Y (2013) Rice slender leaf 1 gene encodes cellulose synthase-like D4 and is specifically expressed in M-phase cells to regulate cell proliferation. J Exp Bot 64: 2049–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Záveská Drábková L, Honys D (2017) Evolutionary history of callose synthases in terrestrial plants with emphasis on proteins involved in male gametophyte development. PLoS ONE 12: e0187331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GF, Staehelin LA (1992) Functional compartmentation of the Golgi apparatus of plant cells : immunocytochemical analysis of high-pressure frozen- and freeze-substituted sycamore maple suspension culture cells. Plant Physiol 99: 1070–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Deng X, Sun B, Lee Van S, Kang Z, Lin H, Lee Y-RJ, Liu B (2018) Role of the BUB3 protein in phragmoplast microtubule reorganization during cytokinesis. Nat Plants 4: 485–494 [DOI] [PubMed] [Google Scholar]
- Zhang L, et al. (2021) Analysis of formin functions during cytokinesis using specific inhibitor SMIFH2. Plant Physiol 186: 945–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang H, Liu P, Hao H, Jin JB, Lin J (2011a) Arabidopsis R-SNARE proteins VAMP721 and VAMP722 are required for cell plate formation. PLoS ONE 6: e26129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhang B, Qian Q, Yu Y, Li R, Zhang J, Liu X, Zeng D, Li J, Zhou Y (2010) Brittle Culm 12, a dual-targeting kinesin-4 protein, controls cell-cycle progression and wall properties in rice. Plant J 63: 312–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Huang L, Zhang C, Staiger CJ (2020) Arabidopsis myosin XIK interacts with the exocyst complex to facilitate vesicle tethering during exocytosis. 2020.08.18.255984 [DOI] [PMC free article] [PubMed]
- Zhang Y, et al. (2016) Golgi-localized STELLO proteins regulate the assembly and trafficking of cellulose synthase complexes in Arabidopsis. Nat Commun 7: 11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang Y, Tan H, Wang Y, Li G, Liang W, Yuan Z, Hu J, Ren H, Zhang D (2011b) RICE MORPHOLOGY DETERMINANT encodes the type II formin FH5 and regulates rice morphogenesis. Plant Cell 23: 681–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Burk DH, Morrison WH 3rd, Ye Z-H (2002) A kinesin-like protein is essential for oriented deposition of cellulose microfibrils and cell wall strength. Plant Cell 14: 3101–3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Awano T, Kobayashi M, Matoh T, Takabe K (2017) Immunocytochemical detection of rhamnogalacturonan II on forming cell plates in cultured tobacco cells. Biosci Biotechnol Biochem 81: 899–905 [DOI] [PubMed] [Google Scholar]
- Zhu C, Dixit R (2011) Single molecule analysis of the Arabidopsis FRA1 kinesin shows that it is a functional motor protein with unusually high processivity. Mol Plant 4: 879–885 [DOI] [PubMed] [Google Scholar]
- Zhu C, Ganguly A, Baskin TI, McClosky DD, Anderson CT, Foster C, Meunier KA, Okamoto R, Berg H, Dixit R (2015) The fragile Fiber1 kinesin contributes to cortical microtubule-mediated trafficking of cell wall components. Plant Physiol 167: 780–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S-H, Xue F, Li Y-J, Liu F, Zhang X-Y, Zhao L-J, Sun Y-Q, Zhu Q-H, Sun J (2018a) Identification and functional characterization of a microtubule-associated protein, GhCLASP2, from upland cotton (Gossypium hirsutum L.). Front Plant Sci 9: 882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Li S, Pan S, Xin X, Gu Y (2018b) CSI1, PATROL1, and exocyst complex cooperate in delivery of cellulose synthase complexes to the plasma membrane. Proc Natl Acad Sci USA 115: E3578–E3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Niu Q-W, Nishizawa N, Wu Y, Kost B, Chua N-H (2000) KORRIGAN, an Arabidopsis endo-1,4-β-glucanase, localizes to the cell plate by polarized targeting and is essential for cytokinesis. Plant Cell 12: 1137–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.