ABSTRACT
The CatSper cation channel is essential for sperm capacitation and male fertility. The multi-subunit CatSper complexes form highly organized calcium signaling nanodomains on flagellar membranes. Here, we report identification of an uncharacterized protein, C2CD6, as a subunit of the mouse CatSper complex. C2CD6 contains a calcium-dependent, membrane-targeting C2 domain. C2CD6 associates with the CatSper calcium-selective, core-forming subunits. Deficiency of C2CD6 depletes the CatSper nanodomains from the flagellum and results in male sterility. C2CD6-deficient sperm are defective in hyperactivation and fail to fertilize oocytes both in vitro and in vivo. CatSper currents are present but at a significantly lower level in C2CD6-deficient sperm. Transient treatments with either Ca2+ ionophore, starvation, or a combination of both restore the fertilization capacity of C2CD6-deficient sperm. C2CD6 interacts with EFCAB9, a pH-dependent calcium sensor in the CatSper complex. We postulate that C2CD6 facilitates incorporation of the CatSper complex into the flagellar plasma membrane and may function as a calcium sensor. The identification of C2CD6 may enable the long-sought reconstitution of the CatSper ion channel complex in a heterologous system for male contraceptive development.
KEY WORDS: C2CD6, CatSper, Capacitation, Fertility, Fertilization, Sperm motility, Mouse
Summary: C2CD6 contains a calcium-dependent membrane targeting C2 domain and is associated with and regulates the localization and organization of the CatSper calcium channel complex in mouse sperm flagella.
INTRODUCTION
Sperm acquires fertilization competence in the female reproductive tract (Chang, 1951). Sperm hyperactivated motility triggered by capacitation is essential for navigation in the oviduct (Suarez, 2016), rheotaxis (Miki and Clapham, 2013) and zona pellucida penetration (Stauss et al., 1995). The uterus and oviduct fluids provide an alkaline environment and a high concentration of bicarbonate, which are essential for capacitation (Vishwakarma, 1962). Sperm motility is regulated by ion channels and ion transporters in the flagellum in response to environmental stimuli (Vyklicka and Lishko, 2020). Calcium influx in sperm is gated by the CatSper ion channel in the flagellum (Ren et al., 2001). The CatSper channel is activated by alkaline pH in rodents (Kirichok et al., 2006) and primates (Lishko et al., 2010), and by progesterone (P4) in primates (Lishko et al., 2011; Strünker et al., 2011). The CatSper channel forms four linear columns of Ca2+ signaling domains along the principal piece of the sperm flagellum (Chung et al., 2014). The CatSper domains organize the spatiotemporal pattern of tyrosine phosphorylation of flagellar proteins, one of the hallmarks of capacitation (Chung et al., 2014; Visconti et al., 1995). Calcium influx caused by CatSper activation results in a powerful, asymmetrical, flagellar-beating movement known as hyperactivation. CatSper is inhibited by efflux of potassium, which is carried out by the Slo3 K+ channel (KCNU1) (Brenker et al., 2014; Chávez et al., 2014; Geng et al., 2017; Santi et al., 2010; Schreiber et al., 1998; Zeng et al., 2011). In human spermatozoa, P4 binds to its sperm membrane receptor ABHD2, which hydrolyzes the endocannabinoid 2-arachidonoylglycerol (2-AG), an inhibitor of the CatSper channel. As a result, P4 activates CatSper by removing 2-AG from the plasma membrane (Miller et al., 2016). Therefore, sperm hyperactivation is regulated by both environmental stimuli in the female reproductive tract and ion channels on sperm flagella.
The CatSper channel is a complex of ten known subunits: CatSper1-4, CatSperβ, γ, δ, ε, ζ, and EFCAB9 (Lin et al., 2021; Vyklicka and Lishko, 2020; Wang et al., 2021). CatSper1-4 subunits form a heteromeric complex with a central Ca2+-selective pore. The remaining six subunits are auxiliary proteins. CatSperβ, γ, δ and ε are putative transmembrane proteins, whereas CatSperζ and EFCAB9 lack transmembrane domains (Chung et al., 2011, 2017; Hwang et al., 2019; Liu et al., 2007; Wang et al., 2009). Genetic ablation in mice and humans have revealed the role of the CatSper subunits in male fertility. Each of the CatSper core subunits (CatSper1-4) is required for the CatSper complex formation, sperm hyperactivation and, thus, for male fertility (Carlson et al., 2003, 2005; Jin et al., 2007; Qi et al., 2007; Quill et al., 2003; Ren et al., 2001). Like CatSper1-4, CatSperδ is essential for CatSper channel complex assembly and male fertility (Chung et al., 2011). In contrast, CatSperζ- or EFCAB9-deficient males exhibit subfertility (Chung et al., 2017; Hwang et al., 2019). In CatSperζ- or EFCAB9-deficient mouse mutants, the CatSper Ca2+ signaling domain organization is affected but the CatSper channel is still functional. EFCAB9 is an EF-hand calcium-binding protein. EFCAB9 interacts with CatSperζ and this interaction requires the binding of Ca2+ to EF-hand domains. Thus, EFCAB9, in partnership with CatSperζ, functions as an intracellular pH-dependent Ca2+ sensor and activator for the CatSper channel (Hwang et al., 2019). Each of the four columns of CatSper domains consists of two rows. However, in CatSperζ- or EFCAB9-deficient mouse sperm, each column contains only one row of CatSper domains instead of two, suggesting a structural role in addition to their Ca2+ sensor function. The CatSper channel is essential for male fertility in humans. Men with loss-of-function mutations in CatSper subunits are infertile as a result of failures in sperm hyperactivation (Avenarius et al., 2009; Brown et al., 2018; Luo et al., 2019; Smith et al., 2013).
CatSper is probably the most complex ion channel known to date. Despite the extensive genetic, super-resolution structural, and electrophysiological studies, a functional CatSper complex has not been reconstituted in a heterologous system. One possible reason for this is that additional subunits are yet to be identified. Serendipitously, we identified a calcium-binding C2 membrane domain protein (C2CD6) as a previously unknown subunit for the CatSper channel complex. Here, we demonstrate that C2CD6 regulates the targeting and organization of the CatSper complex in mouse sperm flagellar membrane and is essential for sperm hyperactivation and male fertility.
RESULTS
Identification of an evolutionarily conserved sperm flagellar protein
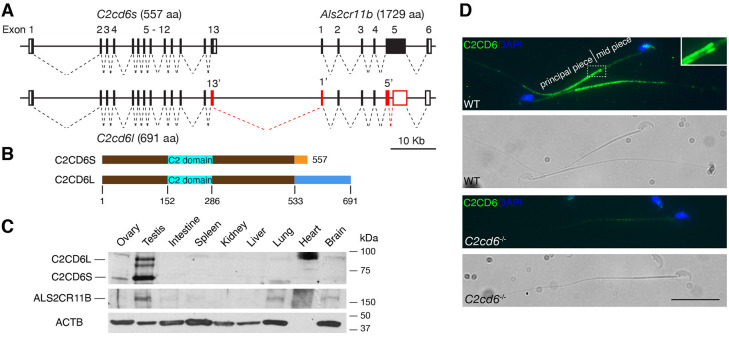
We were interested in interacting proteins of TEX11, a meiosis-specific protein that we previously identified (Yang et al., 2008, 2015). ALS2CR11 was reported as one of the TEX11-interacting proteins in the genome-wide protein-protein interaction study (Rual et al., 2005). However, we find that ALS2CR11 (NP_780409) localizes to sperm flagellum but does not function in meiosis. Because ALS2CR11 contains a calcium-dependent, membrane-targeting C2 domain, it has been renamed as C2CD6 (C2 calcium dependent domain containing 6) (Fig. 1A,B) (Nalefski and Falke, 1996). The C2cd6 gene is conserved in vertebrates. The transcripts from the C2cd6 gene locus are very complex. As we were analyzing the C2cd6 gene structure on chromosome 1, we noticed another uncharacterized gene 3′ downstream, which has a large coding exon (∼5 kb), and named it Als2cr11b (Fig. 1A). Based on expressed sequence tag profiling, both C2cd6 and Als2cr11b transcripts are testis specific.
Fig. 1.
C2CD6 localizes to the principal piece of sperm flagella. (A) Gene structures of C2cd6 and Als2cr11b on chromosome 1. Coding regions of exons are shown as black boxes. 5′ and 3′ untranslated regions are shown as white boxes. Alternative exons of C2cd6l are shown in red. Alternative exons 13′ and 1′ are in-frame. Splicing of an intron within Als2cr11b exon 5 results in an early frame shift in the C2cd6l transcript. GenBank accession numbers for cDNA sequences: C2cd6l, MW717645; C2cd6s, NM_175200; Als2cr11b, XM_011238634. (B) Schematic of the two C2CD6 protein isoforms C2CD6S and C2CD6L. The N-terminal 533 residues are identical. The Ca2+-binding membrane-targeting C2 domain is predicted based on Phyre2. (C) Western blot analysis of C2CD6 and ALS2CR11B in adult mouse tissues. ACTB serves as a loading control. Note that heart lacks ACTB. (D) C2CD6 localizes to the principal piece of wild-type (WT) mouse sperm but not C2cd6-null sperm. Scale bar: 25 µm.
We generated polyclonal antibodies against a recombinant C2CD6 N-terminal 386-aa protein. Western blot analysis showed that C2CD6 was present in adult testis but not in ovary or somatic tissues in mice, demonstrating that C2CD6 is testis specific (Fig. 1C). C2CD6 was not detected in epididymis or vas deferens (Fig. S1A). Interestingly, C2CD6 appeared as two major isoforms: C2CD6S (short, ∼60 kDa) and C2CD6L (long, ∼85 kDa). To determine the nature of these two C2CD6 isoforms, we performed RT-PCR, 3′RACE and sequencing. The presence of two C2CD6 isoforms was due to alternative splicing (Fig. 1A). The C2cd6s transcript (GenBank accession number: NM_175200) consists of 13 exons and encodes a protein of 557 aa. The C2cd6l transcript (GenBank accession number: MW717645) is more complex and harbors an alternative exon 13, an alternative exon 1 of Als2cr11b and splicing of an intron within Als2cr11b exon 5. As a result, the protein (691 aa) encoded by the C2cd6l transcript is larger than C2CD6S (557 aa) but smaller than ALS2CR11B (1729 aa). C2CD6S and C2CD6L share the first 533 aa and the predicted Ca2+-binding membrane targeting domain (Fig. 1B). Therefore, our C2CD6 antibody recognizes both isoforms. We then generated polyclonal antibodies against the C-terminal 200 aa of ALS2CR11B. Western blot analysis identified a protein band of ∼180 kDa in testis but also in lung and brain at a low abundance, suggesting that Als2cr11b encodes a bona fide protein (Fig. 1C). Neither C2CD6 nor ALS2CR11B contains predicted transmembrane domains.
We immunostained mouse caudal epididymal sperm with anti-C2CD6 antibodies. Immunofluorescence analysis revealed that C2CD6 localized specifically to the principal piece of sperm flagellum (Fig. 1D), showing that C2CD6 is a component of sperm flagella. The C2CD6 signal is strong at the beginning of the principal piece (at the annulus region) and tapers off towards the end piece. The C2CD6 signal is specific, as it is absent in C2cd6-deficient sperm (Fig. 1D). Notably, two distinctive columns of C2CD6 can be observed in the principal piece by widefield fluorescence microscopy (Fig. 1D). We further examined the localization of C2CD6 in testicular and caput epididymal sperm (Fig. S1B). Although C2CD6 was not detected on the growing flagella of step 8 spermatids, C2CD6 was present in the principal piece of the flagella in step 10 testicular sperm and beyond and in caput sperm (Fig. S1B). These results demonstrate that C2CD6 is a component of the sperm flagella.
C2CD6 is essential for male fertility and in vivo fertilization
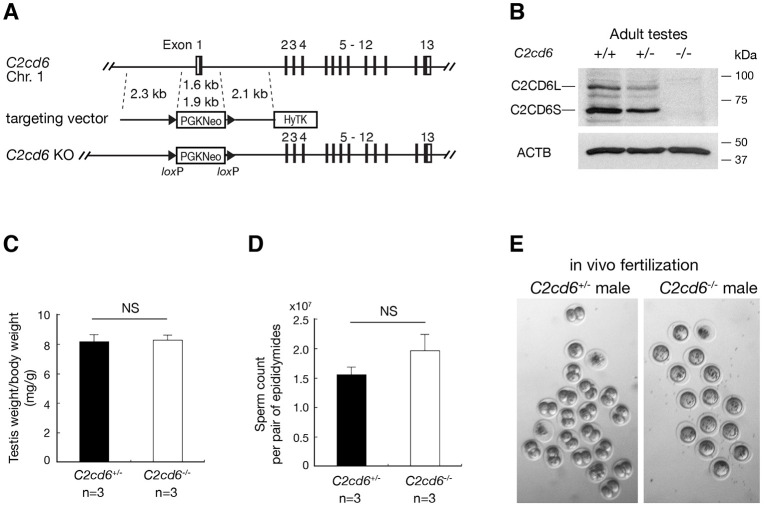
To study the functional requirement of C2cd6, we inactivated C2cd6 by deleting a 1.6-kb genomic region, including exon 1 (containing the initiating codon), through gene targeting in mouse embryonic stem cells (ESCs) (Fig. 2A). Homozygous C2cd6−/− mice were viable and grossly normal. Although C2cd6−/− females had normal fertility, C2cd6−/− males were sterile. C2cd6−/− males produced copulatory plugs, indicating normal mating behavior. Western blotting analysis showed that both C2CD6S and C2CD6L were absent in C2cd6−/− testes, suggesting that the mutant is null (Fig. 2B). Testis weight and sperm count were comparable between C2cd6+/− and C2cd6−/− males (Fig. 2C,D). C2cd6-deficient sperm displayed normal morphology. Histology of C2cd6−/− testes showed apparently normal spermatogenesis (data not shown) and thus C2CD6, unlike TEX11 (Yang et al., 2008), is not essential for meiosis.
Fig. 2.
C2CD6 is essential for in vivo fertilization. (A) Targeted inactivation of the C2cd6 gene. The 1.6-kb deleted region includes exon 1 (containing the initiating start codon) and 600-bp upstream of exon 1 (presumably the promoter region). The neomycin selection marker PGKNeo is flanked by loxP sites. HyTK provides negative selection by ganciclovir in ESCs. (B) Western blot analysis of C2CD6 in adult testes. ACTB serves as a loading control. (C) Testis weight normalized to body weight of males at 3-6 months of age. (D) Sperm count of males at 3-6 months of age. NS, not statistically significant (unpaired Student's t-test). n=3. (E) In vivo fertilization assay. Embryos/unfertilized eggs were flushed from wild-type females mated with either C2cd6+/− or C2cd6−/− males.
C2cd6-deficient sperm were apparently motile; however, C2cd6−/− males were sterile. To probe the cause of male infertility, we performed an in vivo fertilization test. Wild-type C57BL/6 females were injected with pregnant mare's serum gonadotropin (PMSG) followed by human chorionic gonadotropin (hCG) injection, mated with either C2cd6+/− or C2cd6−/− males (three males per genotype), and copulatory plugs were checked. Twenty-four hours after plug check, eggs/embryos were flushed from oviducts of plugged females. The number of two-cell embryos and one-cell embryos/eggs was counted. The majority of embryos (40/59; 68%) from females plugged by C2cd6+/− males were at the two-cell stage; in contrast, only unfertilized eggs (a total of 51) were obtained from females plugged by C2cd6−/− males (Fig. 2E). These data demonstrate that C2CD6 is required for fertilization in vivo.
To determine the capability of C2cd6-deficient sperm in egg activation and embryo development, we performed intracytoplasmic sperm injection. Out of 50 oocytes injected with C2cd6-deficient sperm, 34 embryos reached the two-cell stage and 20 of them further developed to the blastocyst stage. This result shows that C2cd6-deficient sperm can fertilize oocytes by intracytoplasmic sperm injection and support embryo development.
C2CD6 is required for in vitro fertilization and hyperactive motility
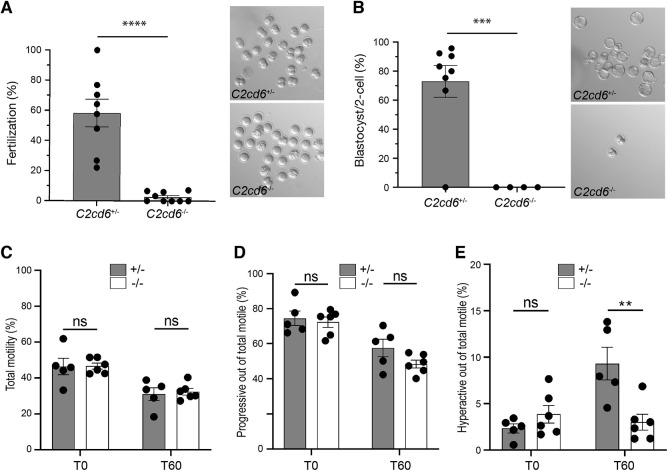
We next investigated whether C2cd6-deficient sperm can fertilize oocytes in vitro. We performed in vitro fertilization (IVF) assays using cumulus-oocyte complexes from wild-type CD1 females (Table 1). An average fertilization rate of 59% versus 2% was obtained when oocytes were incubated with C2cd6+/− versus C2cd6-deficient sperm (Fig. 3A). Only embryos derived from C2cd6+/− sperm developed to blastocysts (76%) after culture in KSOM media (Fig. 3B). The observed 2% fertilization rate obtained with C2cd6−/− sperm was likely due to the low frequency of spontaneous parthenogenetic activation of oocytes (Xu et al., 1997). Therefore, these data indicate that C2CD6 is essential for in vitro fertilization.
Table 1.
Ionophore and SER treatments
Fig. 3.
C2CD6 is required for in vitro fertilization and sperm hyperactivation. (A) In vitro fertilization. CD1 cumulus-oocyte complexes were incubated with C2cd6+/− or C2cd6−/− sperm. Fertilization rate is the percentage of oocytes inseminated that develop into two-cell embryos after 24 h of incubation. A representative image for each treatment is shown. ****P<0.0001. (B) Percentage of two-cell embryos that develop to the blastocyst stage after culture in KSOM media. A representative image for each treatment is shown. ***P<0.001. (C) Percentage of motile sperm immediately after swim-out in TYH medium (T0) and after 60 min of incubation in capacitating conditions in TYH medium (T60). Sperm motility was analyzed by computer-assisted sperm analysis. (D) Percentage of the motile sperm displaying progressive motility at T0 and at T60 of incubation in capacitating conditions. (E) Percentage of the motile sperm displaying hyperactive motility at T0 and at T60 of incubation in capacitating conditions. ns, not statistically significant, **P<0.01. Unpaired Student's t-tests were performed for statistical analysis. n=5 or 6.
To determine whether the in vitro fertilization failure is related to sperm motility, we performed computer-assisted sperm analysis immediately after sperm swim-out from the epididymis (T0) and after 60 min of incubation under capacitating conditions (T60). No differences in total or progressive sperm motility were found between C2cd6+/− and C2cd6−/− males (Fig. 3C,D). Nevertheless, C2cd6-deficient sperm failed to acquire hyperactivated motility after 60 min of incubation under capacitating conditions (Fig. 3E). This could be the cause of the infertility phenotype in C2cd6−/− males, because acquisition of hyperactivated motility is essential for fertilization.
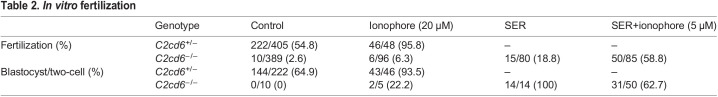
We next evaluated the possibility of restoring fertility of the C2cd6-deficient sperm in vitro. We applied two sperm treatments prior to IVF that have been proven to restore fertility of other infertile and subfertile mouse models (Navarrete et al., 2016, 2019). Transient treatment with a Ca2+ ionophore (ionophore) or sperm energy restriction and recovery (SER) produced a moderate increase in the fertilization rates of C2cd6-deficient sperm: 6.3% and 18.8%, respectively (Table 2). Strikingly, the combination of SER and ionophore treatments of C2cd6-deficient sperm induced an average fertilization rate of 58.8% (Table 2). To analyze embryo development in vitro, the obtained two-cell embryos were further cultured in KSOM media. We observed a blastocyst development rate of 22% in the ionophore treatment-derived embryos, 100% in the SER-derived embryos, and 63% in the SER+ionophore-derived embryos. Although each sperm treatment was able to overcome the infertility phenotype of C2cd6−/− males in vitro, the combination of SER and ionophore treatments was the most effective.
Table 2.
In vitro fertilization
C2CD6-dependent CatSper organization in sperm flagella
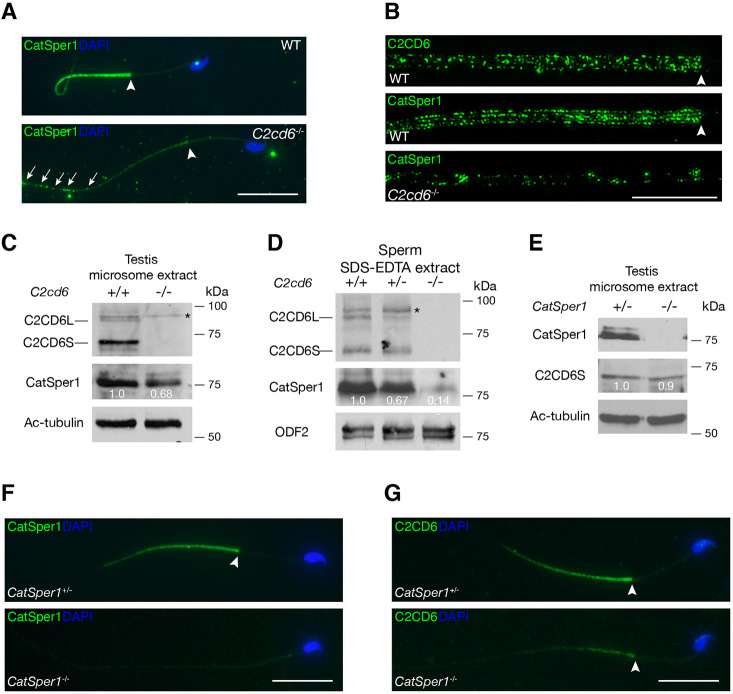
CatSper, the flagellar Ca2+ ion channel, localizes to the principal piece (Fig. 4A) (Chung et al., 2017; Ren et al., 2001). C2CD6 localization to the cauda sperm flagella (Fig. 1D) is strikingly similar to CatSper localization. C2CD6 and CatSper1 exhibited similar localization patterns in testicular and caput epididymal sperm (Fig. S1B,C). Moreover, using comparative proteomics, C2CD6 was shown to be one of the proteins displaying reduced abundance in CatSper1-deficient sperm (Hwang et al., 2019). Therefore, we examined CatSper1 localization in the absence of C2CD6 by immunofluorescence. The CatSper1 signal was severely reduced in C2cd6−/− sperm (Fig. 4A). We also performed super-resolution imaging analysis of CatSper1 and C2CD6 localization in sperm flagellum. As previously reported, CatSper1 formed quadrilateral columns in wild-type flagellum (Fig. 4B). C2CD6 also appeared in columns but was less organized than the CatSper1 columns in wild type (Fig. 4B). In C2cd6−/− sperm, CatSper1 signals were sharply reduced, disorganized, discontinuous, and preferentially distributed to the distal end of the principal piece (Fig. 4A,B).
Fig. 4.
C2CD6 regulates targeting and nanodomain organization of CatSper in sperm flagella. (A) Immunofluorescence analysis of CatSper1 in wild-type (WT) and C2cd6-deficient sperm. Arrowheads indicate the annulus. (B) Super-resolution localization of C2CD6 and CatSper1 in wild-type and C2cd6−/− sperm. The distal region of the flagellar principal piece of the C2cd6−/− sperm is shown. (C) Western blot analysis of C2CD6 and CatSper1 in testis microsome fractions from adult wild-type and C2cd6−/− males. Acetylated tubulin (Ac-tubulin) serves as a loading control. (D) Western blot analysis of C2CD6 and CatSper1 in sperm extracts from adult wild-type, C2cd6+/− and C2cd6−/− males. ODF2, a component of flagellar outer dense fibers, serves as a loading control (Cao et al., 2006). (E) Western blot analysis of CatSper1 and C2CD6 in microsome extracts from adult CatSper1+/− and CatSper1−/− testes. In C-E, western blot band intensity was normalized to Ac-tubulin or ODF2. Relative band intensity is shown. (F) Immunofluorescence analysis of CatSper1 in CatSper1+/− and CatSper1−/− sperm from 3- to 6-month-old males. (G) Immunofluorescence analysis of C2CD6 in CatSper1+/− and CatSper1−/− sperm from 3- to 6-month-old adult males. Additional fluorescence images are provided in Fig. S3. Scale bars: 25 µm (A,F,G); 5 µm (B).
The CatSper complex is partitioned into microsomes (vesicle-like structures formed from pieces of endoplasmic reticulum) in testis extracts (Chung et al., 2017). We prepared microsomes from wild-type and C2cd6−/− testes (Fig. 4C). C2CD6, like CatSper1, was present in testicular microsome fractions. In addition, CatSper1 was abundant in microsome fractions from C2cd6−/− testes (Fig. 4C), suggesting that synthesis of CatSper is not affected in C2cd6−/− testes. We next performed western blotting analysis of sperm extracts. As expected, both C2CD6 and CatSper1 were present in wild-type and C2cd6+/− sperm extracts (Fig. 4D). However, CatSper1 abundance was dramatically reduced in C2cd6−/− sperm extract (Fig. 4D). Taken together, our results demonstrate that CatSper1 is abundant in the testis but fails to become incorporated into sperm flagella fully or efficiently in the absence of C2CD6.
We next sought to address whether CatSper is required for C2CD6 localization. Both CatSper1 and C2CD6 were detected in CatSper1+/− testis microsome extract (Fig. 4E). C2CD6 was present in CatSper1−/− testis microsome extract and its abundance was comparable to that in CatSper1+/− testis (Fig. 4E). As expected, CatSper1 was absent in CatSper1−/− flagellum (Fig. 4F). C2CD6 was sharply reduced in CatSper1−/− flagellum (Fig. 4G). This result is consistent with the reduced abundance of C2CD6 in CatSper1−/− sperm shown by quantitative proteomic analysis (Hwang et al., 2019). These results demonstrate that CatSper is required for C2CD6 localization in sperm flagella. Therefore, the inter-dependent localization of C2CD6 and CatSper1 suggests that C2CD6 might be an important component of the CatSper complex.
CatSper is functional but at a substantially reduced capacity in C2cd6-deficient sperm
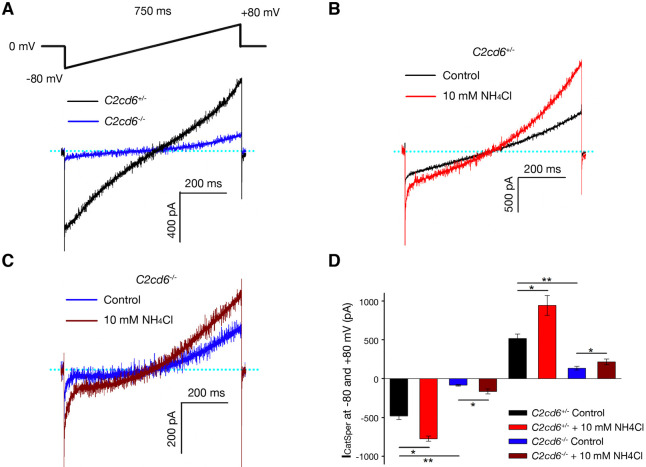
To investigate whether the CatSper channel is functionally assembled in C2cd6-deficient sperm, we recorded the CatSper current (ICatSper) using whole-cell patch-clamp measurements in cauda sperm (Kirichok et al., 2006; Orta et al., 2018). Both inward and outward ICatSper currents recorded from C2cd6−/− sperm were a fraction (17% and 26%, respectively) of the currents from C2cd6+/− sperm (Fig. 5A). The ICatSper in sperm from both C2cd6+/− and C2cd6−/− mice was stimulated by alkalinization induced by addition of 10 mM NH4Cl to the bath solution (Fig. 5B,C). In conclusion, though reduced in magnitude, ICatSper from C2cd6−/− sperm showed voltage- and alkali-induced activation, properties that are characteristic of CatSper.
Fig. 5.
The CatSper channel is functional but at a significantly reduced capacity in C2cd6−/− cauda sperm. (A) Representative CatSper currents in cauda sperm from adult C2cd6+/− and C2cd6−/− mice. (B) CatSper currents in C2cd6+/− cauda sperm before (control) and after alkalization with 10 mM NH4Cl. (C) CatSper currents in C2cd6−/− cauda sperm before (control) and after alkalization with 10 mM NH4Cl. (D) Average CatSper currents in cauda sperm measured at −80 mV and +80 mV. The experiments were performed on cauda sperm from four C2cd6+/− and four C2cd6−/− mice at 3-6 months of age. *P<0.05, **P<0.01 (paired Student's t-test). n=4.
Calcium influx regulates protein kinase A (PKA) phosphorylation and protein tyrosine phosphorylation in sperm upon capacitation. The phosphorylation of PKA substrates was diminished in sperm from C2cd6−/− males in comparison with C2cd6+/− males (Fig. S2A). However, the increase in tyrosine phosphorylation that occurs during sperm capacitation was similar in C2cd6−/− sperm and C2cd6+/− sperm (Fig. S2B). These results are consistent with the biphasic action of Ca2+ in the regulation of cAMP-dependent pathways as previously reported (Navarrete et al., 2015).
Previously, it was reported that addition of bovine serum albumin (BSA) to sperm induced an increase in intracellular Ca2+ concentration, [Ca2+]i (Xia and Ren, 2009). Here, we compared the BSA-induced [Ca2+]i increase in C2cd6+/− and C2cd6−/− sperm. Addition of BSA (5 mg/ml) reproducibly increased [Ca2+]i in the C2cd6+/− sperm; however, this response was substantially smaller in the C2cd6−/− sperm (Fig. S4A,B). Quantification of the normalized BSA responses with respect to ionomycin showed a significantly lower response in C2cd6−/− sperm than in C2cd6+/− sperm (Fig. S4C). Taken together, these results indicate that functional CatSper channels are assembled in the absence of C2CD6 but substantially reduced in capacity (Fig. 5D).
Association of C2CD6 with components of the CatSper complex
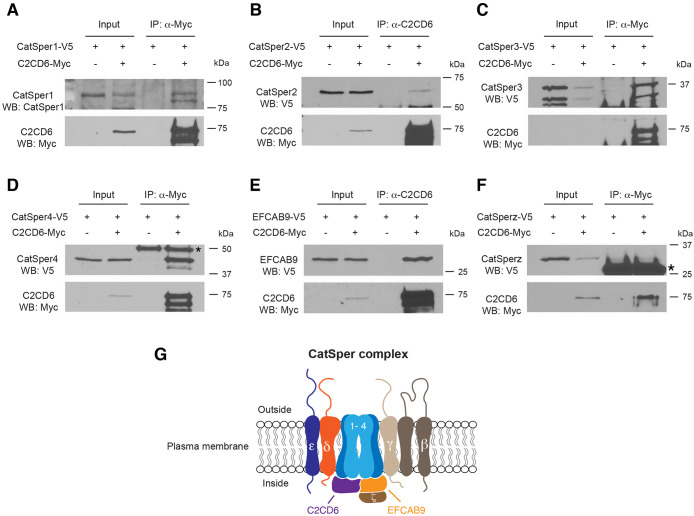
The CatSper complex from sperm protein extracts is not soluble, preventing co-immunoprecipitation analysis of this complex using such protein extracts. To investigate the connection of C2CD6 with the CatSper channel complex, we co-expressed C2CD6 with CatSper complex components in HEK293T cells and tested their interaction by co-immunoprecipitation. C2CD6 and all CatSper components are expressed in testis but are absent in somatic cells such as HEK293T cells. The abundance of C2CD6 in transfected HEK293T cells was low but dramatically enriched by immunoprecipitation (Fig. 6). The full-length C2CD6 migrated at 75 kDa and a slightly smaller isoform was also present in the immunoprecipitated fraction (Fig. 6A). CatSper1 was present in the C2CD6 complex (Fig. 6A). CatSper2 was also in complex with C2CD6 but the association was weak (Fig. 6B). CatSper3 was strongly associated with C2CD6 (Fig. 6C). CatSper4 was readily detected in C2CD6-immunoprecipated proteins, indicating a strong association between CatSper4 and C2CD6 (Fig. 6D). EFCAB9 was abundant in C2CD6-immunoprecipated proteins, showing that EFCAB9 is strongly associated with C2CD6 (Fig. 6E). However, we did not detect association between C2CD6 and CatSperz (TEX40) (Fig. 6F). Collectively, C2CD6 associates with the core components of the CatSper complex (Fig. 6G).
Fig. 6.
C2CD6 associates with subunits of the CatSper channel complex. (A-F) HEK293T cells were transfected with the indicated expression constructs and protein extracts were used for immunoprecipitation (IP) and western blotting (WB) analyses. Input is 1% of the protein extracts used for IP. The antibodies used for IP and WB are shown in each panel. Co-immunoprecipitation of C2CD6 with CatSper1 (A), CatSper2 (B), CatSper3 (C), CatSper4 (D) and EFCAB9 (E) was demonstrated. Asterisk in D indicates Ig heavy chain. (F) C2CD6 is not associated with CatSperz. Asterisk indicates Ig light chain. (G) A working model of the CatSper channel complex. Eleven known subunits of the CatSper channel, including C2CD6, are shown. C2CD6 is associated with CatSper 1-4 and EFCAB9.
DISCUSSION
Here, we demonstrate that C2CD6 associates with the CatSper complex and regulates its localization and function. Our study strongly suggests that in addition to the ten known subunits, C2CD6 is a previously unidentified subunit of the CatSper complex (Fig. 6G). Four core α subunits, CatSper1-4, form the membrane-spanning Ca2+-selective pore. C2CD6 associates with all four α subunits, suggesting that it may directly bind to the CatSper channel core. In addition, C2CD6 associates with EFCAB9 but not with CatSperz, a partner of EFCAB9. We postulate that C2CD6 plays an important role in the CatSper nanodomain organization in sperm flagella. C2CD6 contains a calcium-dependent, membrane-targeting C2 domain. This domain is found in signaling proteins that interact with the cellular membrane (Nalefski and Falke, 1996). This raises the possibility that C2CD6 might facilitate targeting of assembled CatSper complexes to the sperm flagellar plasma membrane or insertion into the membrane. Indeed, CatSper1 is present in testis but its abundance is substantially reduced in sperm from C2cd6−/− males and displays disrupted quadrilateral domain localization in the principal piece. Consistently, the CatSper current is still present in C2cd6-deficient sperm, although it is dramatically reduced. These results indicate that the CatSper complex is not efficiently targeted to the sperm flagella without C2CD6. These C2CD6 results are in stark contrast with the removal of each of the CatSper core subunits. In each of these cases, the remaining subunits are expressed in the testes but are absent in mature sperm (Qi et al., 2007). The presence of the residual CatSper complex in C2CD6-null sperm flagella supports the suggestion that C2CD6 is the main protein for CatSper transport and targeting but other unknown proteins also play a minor role. In addition, C2CD6 might function as a Ca2+ sensor for CatSper independently or as a complex with EFCAB9. Notably, the CatSper channel is compromised but still conducts currents in EFCAB9-deficient sperm (Hwang et al., 2019). In EFCAB9-deficient sperm, C2CD6 might be responsible for Ca2+ sensing. Our results are consistent with a preprint independent study by Hwang et al. (2021).
The cryo-electron microscopic structure of the CatSper channel complex (termed CatSpermasome) has been reported (Lin et al., 2021). Mass spectrometric analysis of the CatSpermasome revealed the presence of C2CD6 in the complex (Lin et al., 2021). However, C2CD6 was not delineated in the structure of this complex. There are several unsolved extra densities in the structure, which likely include C2CD6 and other uncharacterized components. Our in vitro association results (Fig. 6) indicate that C2CD6 may belong to the unsigned cytosolic region map 1 (Lin et al., 2021). CDC42 localizes to four linear domains in sperm flagella and its localization is disrupted in CatSper1-null sperm (Luque et al., 2021). However, CDC42 is absent in the CatSpermasome (Lin et al., 2021), suggesting complexity in the regulation of the CatSper ion channel activity.
C2CD6 exists as at least two isoforms. Both isoforms contain the C2 domain and are present in sperm. It is not known whether these two isoforms are functionally redundant or have isoform-specific functions. Intriguingly, CatSperδ also has two isoforms resulting from alternative splicing (Chung et al., 2011). In addition, C2CD6 is tyrosine phosphorylated upon capacitation (Chung et al., 2014). The physiological consequence of this phosphorylation on C2CD6 is unknown. The Als2cr11b gene encodes a bona fide protein in testis (Fig. 1C). Like C2cd6, Als2cr11b is conserved in vertebrates. Future study is necessary to investigate whether ALS2CR11B is a subunit of the CatSper complex. Genetic ablation of Als2cr11b alone without disruption of C2cd6 is challenging, because Als2cr11b shares exons with C2cd6l (Fig. 1A).
C2cd6−/− sperm do not fertilize oocytes in vivo or in vitro. The sterility phenotype is caused by a failure to induce hyperactivated motility during sperm capacitation. Hyperactivated motility is characterized by a high-amplitude asymmetrical beating of the sperm flagellum (Suárez and Osman, 1987). This flagellar beating pattern is mainly regulated by [Ca2+]i, which is maintained by ion channels and pumps in the plasma membrane (Visconti et al., 2011). Two of the most important sperm [Ca2+]i regulators are the CatSper channel, which is essential for the entrance of Ca2+ into sperm (Ren et al., 2001), and the Ca2+ efflux pump PMCA4 (ATP2B4), which is required for Ca2+ clearance (Wennemuth et al., 2003). The proper assembly and function of the CatSper channel are essential for acquisition of sperm hyperactivated motility and fertility (Chung et al., 2011, 2017; Hwang et al., 2019; Ren et al., 2001). Consistent with our conclusion that C2CD6 is a subunit of the CatSper channel complex, C2cd6-deficient sperm fail to achieve hyperactivated motility after incubation under capacitating conditions.
Ca2+ is a second messenger with pivotal roles in activating (or inhibiting) downstream effectors during sperm capacitation (Navarrete et al., 2015). The transient treatment of sperm with the Ca2+ ionophore A23187 can bypass the activation of the main molecular pathway involved in acquisition of fertilizing capacity during sperm capacitation: the cAMP/PKA pathway (Tateno et al., 2013). This transient sperm ionophore treatment was applied prior to IVF and successfully reversed the male sterility phenotype of CatSper1−/−, sAC−/− and Slo3−/− mice as moderate fertilization was achieved (Navarrete et al., 2016). In line with these previous observations, the ionophore treatment prior to IVF in C2cd6-deficient sperm reversed the sterility phenotype, indicating that an increase of intracellular Ca2+ is sufficient to restore fertility in this mouse model. We have recently developed another sperm treatment that improves fertilization rates and embryo development of sub-fertile mouse models by manipulation of the sperm metabolism (Navarrete et al., 2019). When applied prior to IVF in CatSper1−/− sperm, this SER treatment was not able to restore fertility; however, a combination of the ionophore A23187 and the SER treatments induced a synergistic effect on fertilization rates and embryo development in the CatSper1−/− model (Navarrete et al., 2019). The same synergistic effect was observed on C2cd6-deficient sperm. Interestingly, although the SER treatment did not rescue the sterility phenotype of CatSper1−/− mice (Navarrete et al., 2019), the application of SER treatment alone was able to overcome the sterility phenotype of the C2cd6−/− mice. This could be related to our recent findings that SER treatment induces an elevation of [Ca2+]i. in mouse sperm from wild-type and CatSper1−/− animals (Sánchez–Cárdenas et al., 2021). It could also be due to the presence of residual functional CatSper channels in C2cd6-deficient sperm. Another possible explanation for the difference in the SER treatment response is that C2CD6 could regulate capacitation independently of CatSper.
The CatSper channel is essential for sperm hyperactivation and male fertility in both mice and humans. The CatSper subunits are only expressed in testis and sperm. Traditionally, ion channels are druggable targets. For these reasons, the CatSper channel has been proposed as a target for male contraception with minimal side effects. However, reconstitution of CatSper in a heterologous system has not been achieved, despite the fact that CatSper was discovered two decades ago (Ren et al., 2001). The lack of a heterologous system impedes drug discovery efforts for small molecule inhibitors of CatSper. The challenges for developing a CatSper heterologous system are several fold. First, the CatSper ion channel is extremely complex. It is still possible that not all CatSper-associated proteins are known. Second, the CatSper assembly might require chaperones. The CatSper complex is associated with a testis-specific chaperone, HSPA2 (Chung et al., 2011; Zhu et al., 1997). Third, sperm flagellum is a unique ciliary structure. CatSper forms organized linear domains along the principal piece. Organization of these nanodomains might depend on other flagellar unique structures, such as the fibrous sheath, which is absent in heterologous cells. The identification of C2CD6 might facilitate successful development of a heterologous CatSper system, which is not only crucial for drug development but would also provide an amenable system in which to dissect the mechanistic role of each subunit in CatSper.
MATERIALS AND METHODS
Generation of C2cd6 knockout mice
The targeting strategy was to delete a 1.6-kb genomic region including exon 1 of the C2cd6 gene (Fig. 2A). In the targeting construct, the left (2.3 kb) and right (2.1 kb) homologous arms were PCR amplified from a C2cd6-containing mouse BAC clone (RP24-535E21) with high-fidelity Taq DNA polymerase. A neomycin (PGKNeo) selection marker was inserted between the homologous arms. The HyTK selection marker was cloned adjacent to the right arm. V6.5 ESCs were electroporated with the ClaI-linearized targeting construct and cultured in the presence of 350 µg/ml G418 and 2 µM ganciclovir. ESC clones were screened by long-distance PCR for homologous recombination. Out of 192 ESC clones, nine homologously targeted clones were identified. 2C5 and 1H1 ESC clones were injected into blastocysts and the resulting chimeric mice transmitted the C2cd6 knockout allele through the germline. The C2cd6+/− 2C5 mice were backcrossed to the C57BL/6J strain four times (N4). All the experiments were performed on the C57BL/6J N4 backcrossed mice. Mice were genotyped by PCR of tail genomic DNA with the following primers: wild type (515 bp), ALS-25 (5′-GTATTTCCCATCATGTGGAGGA-3′) and ALS-26 (5′-AGTGGCTTGCCTTCTTCATCAG-3′); C2cd6 knockout allele (341 bp), ALS-9 (5′-TGTGCTATCCACCTTGCCTT-3′) and PGKRNrev2 (5′-CCTACCGGTGGATGTGGAATGTGTG-3′).
CatSper1 knockout mice were previously generated (Ren et al., 2001).
All experiments with mice were performed in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines of the University of Pennsylvania and the University of Massachusetts at Amherst.
Antibody production
The C2cd6 cDNA was amplified from bulk mouse testis cDNA by PCR. The cDNA fragment encoding residues 1-386 was cloned into the pQE-30 vector (QIAGEN). The 6×His-C2CD6 (aa 1-386) fusion protein was expressed in M15 bacteria, affinity purified with Ni-NTA beads, and eluted in 8 M urea. The recombinant fusion protein was used to immunize two rabbits at Cocalico Biologicals. The C2CD6 antiserum (UP2429 and UP2430) was used for western blotting analysis (1:500). Specific antibodies for immunofluorescence were affinity purified with the immunoblot method (Harlow and Lane, 1998). The specificity of the C2CD6 antibody was validated by western blotting and by immunofluorescence analyses in testes and sperm from C2CD6-deficient mice.
The Als2cr11b cDNA fragment encoding the C-terminal 200 residues was cloned into the pQE-30 vector. The 6× His-ALS2CR11B (C-terminal 200 aa) fusion protein was expressed in bacteria, affinity purified, and used to immunize two rabbits at Cocalico Biologicals, resulting in one anti-ALS2CR11B antiserum (UP2443).
In vivo fertilization
Eight-week-old wild-type C57BL/6 females were injected with 7.5 IU of PMSG, then with 7.5 IU of hCG 46 h later, and mated with either C2cd6+/− or C2cd6−/− males. Copulatory plugs were checked 17 h after mating setup. Twenty-four hours after plug check, eggs/embryos were flushed from plugged females. The numbers of two-cell embryos and one-cell embryos/eggs were counted.
Sperm collection
Cauda epididymides from 3-month-old males were dissected and placed in Toyoda–Yokoyama–Hosi (TYH) medium containing 119.37 mM NaCl, 4.7 mM KCl, 1.71 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25.1 mM NaHCO3, 0.51 mM sodium pyruvate, 5.56 mM glucose, 4 mg/ml BSA, 10 µg/ml gentamicin and 0.0006% Phenol Red equilibrated in 5% CO2 at 37°C. After 10 min of sperm swim-out, epididymal tissue was removed and sperm suspensions were further incubated in TYH medium in 5% CO2 at 37°C to allow sperm to capacitate.
Sperm motility analysis
Sperm motility parameters were analyzed immediately after swim-out (T0) and after 60 min (T60) of incubation in capacitating conditions (TYH medium). Sperm suspensions were loaded onto 100-µm-depth chamber slides (Leja, Spectrum Technologies), placed in a slide warmer at 37°C (Minitherm, Hamilton Thorne), and imaged with a 4× dark field objective (Olympus) on a Lab A1 microscope (Zeiss). Ninety frame videos were recorded at 60 Hz and analyzed using a CEROS II computer-assisted sperm analysis system (Hamilton Thorne). The settings for cell recognition included: head size, 5-200; minimum head brightness, 100; and static head elongation, 5-100%. Sperm with average path velocity (VAP)>0 and rectilinear velocity (VSL)>0 were considered motile. Sperm were considered progressive with VAP>50 µm/s and straightness (STR)>50%, and hyperactive with curvilinear velocity (VCL)>271 µm/s, VSL/VCL (LIN)<50%, and amplitude of lateral head displacement (ALH)>3.5 µm. At least five fields per treatment corresponding to a minimum of 200 sperm were analyzed per experiment. Data were presented as the percentage of motile sperm out of the total population, and percentage of progressive or hyperactive sperm out of the motile population.
IVF
Young (7- to 9-week-old) CD-1 females were obtained from Charles River Laboratories. Superovulation was induced by injection first with 7.5 IU of PMSG (493-10, Lee Biosolutions), followed 48 h later with 7.5 IU of hCG (CG5, Sigma-Aldrich). Females were sacrificed 13 h post-hCG injection, the oviducts were dissected, and cumulus-oocyte complexes (COCs) were collected in TL-HEPES medium containing 114 mM NaCl, 3.22 mM KCl, 2.04 mM CaCl2, 0.35 mM NaH2PO4, 0.49 mM MgCl2, 2.02 mM NaHCO3, 10 mM lactic acid (sodium salt) and 10.1 mM HEPES. COCs were thoroughly washed with TYH medium and placed in an insemination drop of TYH medium covered by mineral oil previously equilibrated in an incubator in 5% CO2 at 37°C. COCs (two or three per 90 µl drop) were inseminated with 100,000 sperm that were previously capacitated in TYH medium for 60 min, and then were maintained in an incubator in 5% CO2 at 37°C. After 4 h of insemination, the MII-oocytes were washed, placed in a different drop of TYH media, and incubated overnight in 5% CO2 at 37°C. Dishes were examined 24 h post-insemination, and fertilization was evaluated by the appearance of two-cell-stage embryos. Results were expressed as percentage of two-cell embryos out of the total number of oocytes inseminated.
Enhanced IVF
SER and Ca2+ ionophore treatments prior to IVF improve the rate of fertilization in subfertile and infertile animals (Navarrete et al., 2016; Navarrete et al., 2019). The following sperm treatments prior to IVF were tested: (1) control (TYH); (2) control+Ca2+ ionophore; (3) SER; and (4) SER+Ca2+ ionophore. For each 3-month-old male, one cauda epididymis was collected and placed in TYH medium (tube A) and the other cauda epididymis was collected and placed in TYH medium devoid of glucose and pyruvate (SER-TYH, tube B). After 10 min of incubation in 5% CO2 at 37°C to allow sperm swim-out, cauda tissues were removed. Sperm suspensions were centrifuged twice at 150 g for 5 min and the sperm pellet was washed with 2 ml of TYH for tube A and 2 ml of SER-TYH for tube B. After the final wash, sperm pellets were resuspended in 500 µl of TYH for tube A and 500 µl of SER-TYH for tube B. Each tube was immediately divided into two 250 µl suspensions and incubated in 5% CO2 at 37°C until the sperm motility from treatments 3 and 4 was significantly slow (about 30 min). At that point, the Ca2+ ionophore 4Br-A23187 (C7522, Fisher Scientific) was added to a final concentration of 20 µM for treatment 2 and 5 µM for treatment 4. When used in combination with the SER treatment, 20 µM ionophore was detrimental to the sperm sample and, therefore, the concentration of ionophore was decreased to 5 µM for treatment 4. After a 10-min incubation with Ca2+ ionophore, 1.5 ml of TYH was added to all the treatments followed immediately by centrifugation at 150 g for 5 min. Sperm pellets were then washed again with TYH and centrifuged at 150 g for 5 min. Sperm pellets were resuspended in 500 μl of TYH and used for insemination of COCs. All the procedures after insemination were performed as described above for regular IVF.
Embryo culture
The two-cell embryos from IVF were washed and placed in a dish of equilibrated KSOM media (MR-106-D, Fisher Scientific) covered by light mineral oil (0121-1, Fisher Scientific). Embryo culture dishes were incubated for 3.5 days in 5% CO2, 5% O2, at 37°C. Results are expressed as the percentage of blastocysts out of the total number of two-cell embryos and the percentage of blastocysts out of the total number of oocytes inseminated.
Immunofluorescence and super-resolution imaging
After three incisions, cauda epididymides were incubated in PBS at 37°C for 10 min. For testicular spermatids or caput sperm, seminiferous tubules or caput epididymis were squeezed to obtain sperm suspensions. Swim-out sperm or sperm cell suspensions were placed on slides and fixed in 2% paraformaldehyde in PBS with 0.2% Triton X-100 overnight. The slides were incubated with rabbit anti-C2CD6 (UP2430, 1:100) or rabbit anti-Catsper1 (1:200) antibodies and/or mouse anti-acetylated tubulin (T7451, 1:200, Sigma), then with anti-rabbit FITC-conjugated secondary antibody (1:100, FI-1000, Vector Laboratories) and/or anti-mouse Texas Red-conjugated secondary antibody (1:100, TI-2000, Vector Laboratories), and then mounted with DAPI. Images were captured with an ORCA digital camera (Hamamatsu Photonics) on a Leica DM5500B microscope. For structured illumination microscopy imaging, images were acquired on a GE DeltaVision OMX SR imaging system with PCO sCMOS cameras and were processed using Softworx software.
Testis microsome extraction
One adult testis (∼100 mg) was homogenized on ice in 1 ml 0.32 M sucrose solution with 1× protease inhibitor cocktail (P8340, Sigma-Aldrich). After centrifugation at 300 g at 4°C for 10 min, the supernatant was transferred to an ultra-centrifuge tube and centrifuged at 100,000 g for 1 h. The pellet containing the microsome fraction was resuspended and solubilized in 5 ml PBS with 1% Triton X-100 and 1× protease inhibitor cocktail by rocking at 4°C for 2 h. The suspension was centrifuged at 15,000 g for 30 min and the supernatant was collected for western blot analysis.
Sperm protein extraction
Sperm (∼1.3×107) were collected from one adult mouse by squeezing the cauda epididymides in PBS solution followed by centrifugation at 800 g for 5 min at room temperature. Sperm were homogenized in 100 µl SDS-EDTA solution (1% SDS, 75 mM NaCl, 24 mM EDTA, pH 6.0) and centrifuged at 5000 g for 30 min at room temperature, and then 100 µl 2× SDS-PAGE sample buffer (62.5 mM Tris, pH 6.8, 3% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.02% Bromophenol Blue) was added to 100 µl of supernatant. The samples were heated at 95°C for 10 min and 20 µl of each sample (equivalent to 1.3×106 sperm) was used for western blot analysis.
Electrophysiology
In brief, cauda epididymal sperm were obtained by swim-out in TYH medium (Orta et al., 2018) and stored in HS buffer: 135 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 10 mM lactic acid, 1 mM pyruvic acid, 5 mM glucose, 20 mM HEPES, pH 7.4. For the CatSper current (ICatSper) recording, we used a divalent cation-free solution (DVF) (150 mM Na-gluconate, 2 mM Na2EDTA, 2 mM EGTA, 20 mM HEPES, pH 7.4) (Kirichok et al., 2006; Orta et al., 2018). The intrapipette solution contained 135 mM Cs-MeSO3, 5 mM CsCl, 10 mM EGTA, 20 mM HEPES, pH 7.2. Whole-cell ICatSper was obtained by patch clamping the sperm cytoplasmic droplet (Orta et al., 2018). Seals (>5 GΩ) between the patch pipette and the cytoplasmic droplet in sperm were formed in HS bath solution, and, after achieving whole-cell configuration, the bath solution was changed to DVF solution. ICatSper was induced by applying a voltage-ramp protocol from −80 mV to +80 mV with a 750 ms time duration from a holding potential of 0 mV. For alkinization, 10 mM NH4Cl was added to the bath solution. The ICatSper data were analyzed using Clampfit 10.7 (Molecular Devices) and were plotted with SigmaPlot 10.0 (Systat Software). Data are shown as mean±s.e.m. (Fig. 5D). Differences between treatments (without versus with NH4Cl) were analyzed by paired Student's t-test. Differences between genotypes (C2cd6+/− vs C2cd6−/−) were tested by unpaired Student's t-test. Differences were considered significant when *P<0.05 and **P<0.01.
Western blotting of phospho-PKA substrates and tyrosine phosphorylation
Immediately after swim-out (T0) and after 60 min of incubation in TYH capacitation medium (T60), proteins from 1×106 sperm were extracted for western blot analysis using Laemmli buffer as previously described (Alvau et al., 2016). Protein extracts were analyzed by SDS-PAGE and electro-transferred to PVDF membranes. Membranes were blocked with 5% fat-free milk in T-TBS (TBS with 0.1% Tween 20) and immunoblotting was conducted with phospho-PKA substrate antibody (1:10,000, Cell Signaling Technology, 9624, clone 100G7). Membranes were washed with T-TBS twice and incubated with HRP-conjugated anti-rabbit secondary antibody (1:10,000 dilution; Fisher Scientific, Cat. No. 45-000-682). Enhanced chemiluminescence (ECL) Plus Kit (Fisher Scientific, Cat. No. RPN2132) was used for detection. After development of pPKAs, PVDF membranes were stripped at 65°C for 20 min in 2% SDS, 0.74% β-mercaptoethanol, 62.5 mM Tris, pH 6.5, and then thoroughly washed with T-TBS. PDVF membranes were then blocked with 5% fish gelatin followed by immunoblotting with anti-pY antibody (1:10,000 dilution; Millipore, Cat No. 05-321, clone 4G10). Membranes were washed with T-TBS twice and incubated with HRP-conjugated anti-mouse secondary antibody (1:10,000; Jackson ImmunoResearch Laboratories, 715-035-150). The ECL Plus Kit was used for detection (Fisher Scientific).
Ca2+ imaging
Non-capacitated cauda sperm were used for Ca2+ imaging, loaded with 4 µM of the fluorescent Ca2+ indicator Fluo-3 AM in the presence of 0.05% pluronic acid at 37°C for 40 min, and protected from light. The cells were washed once, pelleted at 3000 rpm (500 g) for 3 min, and resuspended in TYH media. Sperm were attached onto laminin (1 mg/ml; Invitrogen) precoated cover slips. Fluorescence signals were captured as previously described (Nishigaki et al., 2006), using an inverted microscope (Eclipse TE 300 Nikon) with an oil immersion 60× objective (PlanApo N, numerical aperture 1.42). A LED-based pulsed light excitation system and an iXon888 (512×512 pixels) EMCCD camera (Andor Technology) with 1×1 binning using Andor iQ software were employed in these experiments. Samples were kept at 37°C using a PDMI-2 (Harvard Apparatus) temperature controller. Fluorescence images were captured at 1 frame per second with a 2-ms excitation. For recording, Fluo-3-loaded cells were excited with a cyan LED (3.15 A, Luminus Devices), with a bandpass excitation (HQ 480/40X), dichroic mirror (Q505lp), and emission (HQ 535/ 50 M) filters (Chroma Technology).
Cell culture, transfection and immunoprecipitation
The open reading frames of C2cd6s, Efcab9, CatSperζ, CatSper2, CatSper3 and CatSper4 were PCR amplified from bulk mouse testis cDNAs. The CatSper1 open reading frame was amplified from a mouse cDNA clone (MR224271, OriGene). C2cd6s was subcloned into pcDNA3.1/myc-His A vector (V800-20, Invitrogen). The others were TA-cloned into pcDNA3.1/V5-His TOPO TA vectors (K4800-01, Invitrogen). HEK293T cells were cultured in DMEM medium with 10% fetal bovine serum in 5% CO2 at 37°C. Twenty-four to 48 h after transfection with Lipofectamine 2000 (11668-027, Invitrogen), the cells (three wells of a 6-well plate) were lysed in 1 ml IP buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, 0.5% deoxycholate, 1% Triton X-100) with 1× cocktail of protease inhibitors, incubated at 4°C for 1 h, and centrifuged at 12,000 g for 30 min; 10 µl of supernatant (1%) was set aside as input. The bulk of supernatant (∼1 ml) was incubated with antibodies at 4°C for 1 h: 3 µl (0.5 µg/µl) c-Myc monoclonal antibody (631206, Takara Bio), or 1 µl (1.1 µg/µl) anti-V5 antibody (P/N 46-0705, Invitrogen), or 20 µl anti-C2CD6 (UP2429) antibody. For each immunoprecipitation, 10 µl of Dynabeads G or A (Invitrogen) was added followed by overnight incubation at 4°C. Immunoprecipitated proteins were washed five times with the wash buffer (50 mM Tris, pH 7.5, 250 mM NaCl, 0.1% NP-40, 0.05% deoxycholate) and were eluted in 15 µl 2× SDS sample buffer at 95°C. Elutes and inputs were separated by SDS-PAGE and transferred to nitrocellulose membrane. Anti-c-Myc monoclonal, anti-V5 and anti-CatSper1 (gift from Dejian Ren) (Ren et al., 2001) antibodies were used for western blotting.
Supplementary Material
Acknowledgements
We thank Dejian Ren for anti-CatSper1 antibody.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: F.Y., P.J.W.; Methodology: F.Y., M.G.G., N.A.L., G.R.; Validation: F.Y., M.G.G.; Formal analysis: F.Y., M.G.G., D.A.T., P.E.V., P.J.W.; Investigation: F.Y., M.G.G., N.A.L., G.O., D.A.T., J.L.D.l.V.-B., A.D.; Data curation: F.Y., M.G.G., D.A.T., G.R.; Writing - original draft: F.Y., M.G.G., P.E.V., P.J.W.; Writing - review & editing: F.Y., G.O., J.L.D.l.V.-B., G.R., A.D., P.E.V., P.J.W.; Visualization: F.Y., M.G.G., G.R., P.J.W.; Supervision: A.D., P.E.V., P.J.W.; Project administration: P.J.W.; Funding acquisition: P.E.V., P.J.W.
Funding
This study was supported by National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD069592 and HD068157 to P.J.W., HD038082 and HD088571 to P.E.V.) and National Institutes of Health National Research Service Award (T32GM108556 to D.A.T.). Deposited in PMC for release after 12 months.
Peer review history
The peer review history is available online at https://journals.biologists.com/dev/article-lookup/doi/10.1242/dev.199988.
References
- Alvau, A., Battistone, M. A., Gervasi, M. G., Navarrete, F. A., Xu, X., Sánchez-Cárdenas, C., De la Vega-Beltran, J. L., Da Ros, V. G., Greer, P. A., Darszon, A.et al. (2016). The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm. Development 143, 2325-2333. 10.1242/dev.136499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenarius, M. R., Hildebrand, M. S., Zhang, Y., Meyer, N. C., Smith, L. L. H., Kahrizi, K., Najmabadi, H. and Smith, R. J. H. (2009). Human male infertility caused by mutations in the CATSPER1 channel protein. Am. J. Hum. Genet. 84, 505-510. 10.1016/j.ajhg.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenker, C., Zhou, Y., Müller, A., Echeverry, F. A., Trötschel, C., Poetsch, A., Xia, X.-M., Bönigk, W., Lingle, C. J., Kaupp, U. B.et al. (2014). The Ca2+-activated K+ current of human sperm is mediated by Slo3. eLife 3, e01438. 10.7554/eLife.01438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, S. G., Miller, M. R., Lishko, P. V., Lester, D. H., Publicover, S. J., Barratt, C. L. R. and Martins Da Silva, S. (2018). Homozygous in-frame deletion in CATSPERE in a man producing spermatozoa with loss of CatSper function and compromised fertilizing capacity. Hum. Reprod. 33, 1812-1816. 10.1093/humrep/dey278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, W., Gerton, G. L. and Moss, S. B. (2006). Proteomic profiling of accessory structures from the mouse sperm flagellum. Mol. Cell. Proteomics 5, 801-810. 10.1074/mcp.M500322-MCP200 [DOI] [PubMed] [Google Scholar]
- Carlson, A. E., Westenbroek, R. E., Quill, T., Ren, D., Clapham, D. E., Hille, B., Garbers, D. L. and Babcock, D. F. (2003). CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc. Natl. Acad. Sci. USA 100, 14864-14868. 10.1073/pnas.2536658100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, A. E., Quill, T. A., Westenbroek, R. E., Schuh, S. M., Hille, B. and Babcock, D. F. (2005). Identical phenotypes of CatSper1 and CatSper2 null sperm. J. Biol. Chem. 280, 32238-32244. 10.1074/jbc.M501430200 [DOI] [PubMed] [Google Scholar]
- Chang, M. C. (1951). Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 168, 697-698. 10.1038/168697b0 [DOI] [PubMed] [Google Scholar]
- Chávez, J. C., Ferreira, J. J., Butler, A., De La Vega Beltrán, J. L., Treviño, C. L., Darszon, A., Salkoff, L. and Santi, C. M. (2014). SLO3 K+ channels control calcium entry through CATSPER channels in sperm. J. Biol. Chem. 289, 32266-32275. 10.1074/jbc.M114.607556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, J.-J., Navarro, B., Krapivinsky, G., Krapivinsky, L. and Clapham, D. E. (2011). A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat. Commun. 2, 153. 10.1038/ncomms1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, J.-J., Shim, S.-H., Everley, R. A., Gygi, S. P., Zhuang, X. and Clapham, D. E. (2014). Structurally distinct Ca2+ signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell 157, 808-822. 10.1016/j.cell.2014.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, J.-J., Miki, K., Kim, D., Shim, S.-H., Shi, H. F., Hwang, J. Y., Cai, X., Iseri, Y., Zhuang, X. and Clapham, D. E. (2017). CatSperzeta regulates the structural continuity of sperm Ca2+ signaling domains and is required for normal fertility. eLife 6, e23082. 10.7554/eLife.23082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng, Y., Ferreira, J. J., Dzikunu, V., Butler, A., Lybaert, P., Yuan, P., Magleby, K. L., Salkoff, L. and Santi, C. M. (2017). A genetic variant of the sperm-specific SLO3 K+ channel has altered pH and Ca2+ sensitivities. J. Biol. Chem. 292, 8978-8987. 10.1074/jbc.M117.776013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, E. and Lane, D. (1998). Using Antibodies: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Hwang, J. Y., Mannowetz, N., Zhang, Y., Everley, R. A., Gygi, S. P., Bewersdorf, J., Lishko, P. V. and Chung, J.-J. (2019). Dual sensing of physiologic pH and calcium by EFCAB9 regulates sperm motility. Cell 177, 1480-1494.e19. 10.1016/j.cell.2019.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, J. Y., Wang, H., Lu, Y., Ikawa, M. and Chung, J.-J. (2021). C2cd6-encoded CatSperτ targets sperm calcium channel to Ca2+ signaling domains in the flagellar membrane. bioRxiv. 10.1101/2021.08.16.456347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, J., Jin, N., Zheng, H., Ro, S., Tafolla, D., Sanders, K. M. and Yan, W. (2007). Catsper3 and Catsper4 are essential for sperm hyperactivated motility and male fertility in the mouse. Biol. Reprod. 77, 37-44. 10.1095/biolreprod.107.060186 [DOI] [PubMed] [Google Scholar]
- Kirichok, Y., Navarro, B. and Clapham, D. E. (2006). Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 439, 737-740. 10.1038/nature04417 [DOI] [PubMed] [Google Scholar]
- Lin, S., Ke, M., Zhang, Y., Yan, Z. and Wu, J. (2021). Structure of a mammalian sperm cation channel complex. Nature 595, 746-750. 10.1038/s41586-021-03742-6 [DOI] [PubMed] [Google Scholar]
- Lishko, P. V., Botchkina, I. L., Fedorenko, A. and Kirichok, Y. (2010). Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140, 327-337. 10.1016/j.cell.2009.12.053 [DOI] [PubMed] [Google Scholar]
- Lishko, P. V., Botchkina, I. L. and Kirichok, Y. (2011). Progesterone activates the principal Ca2+ channel of human sperm. Nature 471, 387-391. 10.1038/nature09767 [DOI] [PubMed] [Google Scholar]
- Liu, J., Xia, J., Cho, K.-H., Clapham, D. E. and Ren, D. (2007). CatSperβ, a novel transmembrane protein in the CatSper channel complex. J. Biol. Chem. 282, 18945-18952. 10.1074/jbc.M701083200 [DOI] [PubMed] [Google Scholar]
- Luo, T., Chen, H.-Y., Zou, Q.-X., Wang, T., Cheng, Y.-M., Wang, H.-F., Wang, F., Jin, Z.-L., Chen, Y., Weng, S.-Q.et al. (2019). A novel copy number variation in CATSPER2 causes idiopathic male infertility with normal semen parameters. Hum. Reprod. 34, 414-423. 10.1093/humrep/dey377 [DOI] [PubMed] [Google Scholar]
- Luque, G. M., Xu, X., Romarowski, A., Gervasi, M. G., Orta, G., De la Vega-Beltrán, J. L., Stival, C., Gilio, N., Dalotto-Moreno, T., Krapf, D.et al. (2021). Cdc42 localized in the CatSper signaling complex regulates cAMP-dependent pathways in mouse sperm. FASEB J. 35, e21723. 10.1096/fj.202002773RR [DOI] [PubMed] [Google Scholar]
- Miki, K. and Clapham, D. E. (2013). Rheotaxis guides mammalian sperm. Curr. Biol. 23, 443-452. 10.1016/j.cub.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M. R., Mannowetz, N., Iavarone, A. T., Safavi, R., Gracheva, E. O., Smith, J. F., Hill, R. Z., Bautista, D. M., Kirichok, Y. and Lishko, P. V. (2016). Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science 352, 555-559. 10.1126/science.aad6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalefski, E. A. and Falke, J. J. (1996). The C2 domain calcium-binding motif: structural and functional diversity. Protein Sci. 5, 2375-2390. 10.1002/pro.5560051201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete, F. A., García-Vázquez, F. A., Alvau, A., Escoffier, J., Krapf, D., Sánchez-Cárdenas, C., Salicioni, A. M., Darszon, A. and Visconti, P. E. (2015). Biphasic role of calcium in mouse sperm capacitation signaling pathways. J. Cell. Physiol. 230, 1758-1769. 10.1002/jcp.24873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete, F. A., Alvau, A., Lee, H. C., Levin, L. R., Buck, J., Leon, P. M.-D., Santi, C. M., Krapf, D., Mager, J., Fissore, R. A.et al. (2016). Transient exposure to calcium ionophore enables in vitro fertilization in sterile mouse models. Sci. Rep. 6, 33589. 10.1038/srep33589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete, F. A., Aguila, L., Martin-Hidalgo, D., Tourzani, D. A., Luque, G. M., Ardestani, G., Garcia-Vazquez, F. A., Levin, L. R., Buck, J., Darszon, A.et al. (2019). Transient sperm starvation improves the outcome of assisted reproductive technologies. Front. Cell Dev. Biol. 7, 262. 10.3389/fcell.2019.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishigaki, T., Wood, C. D., Shiba, K., Baba, S. A. and Darszon, A. (2006). Stroboscopic illumination using light-emitting diodes reduces phototoxicity in fluorescence cell imaging. BioTechniques 41, 191-197. 10.2144/000112220 [DOI] [PubMed] [Google Scholar]
- Orta, G., de la Vega-Beltran, J. L., Martín-Hidalgo, D., Santi, C. M., Visconti, P. E. and Darszon, A. (2018). CatSper channels are regulated by protein kinase A. J. Biol. Chem. 293, 16830-16841. 10.1074/jbc.RA117.001566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, H., Moran, M. M., Navarro, B., Chong, J. A., Krapivinsky, G., Krapivinsky, L., Kirichok, Y., Ramsey, I. S., Quill, T. A. and Clapham, D. E. (2007). All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl. Acad. Sci. USA 104, 1219-1223. 10.1073/pnas.0610286104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quill, T. A., Sugden, S. A., Rossi, K. L., Doolittle, L. K., Hammer, R. E. and Garbers, D. L. (2003). Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc. Natl. Acad. Sci. USA 100, 14869-14874. 10.1073/pnas.2136654100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, D., Navarro, B., Perez, G., Jackson, A. C., Hsu, S., Shi, Q., Tilly, J. L. and Clapham, D. E. (2001). A sperm ion channel required for sperm motility and male fertility. Nature 413, 603-609. 10.1038/35098027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rual, J.-F., Venkatesan, K., Hao, T., Hirozane-Kishikawa, T., Dricot, A., Li, N., Berriz, G. F., Gibbons, F. D., Dreze, M., Ayivi-Guedehoussou, N.et al. (2005). Towards a proteome-scale map of the human protein-protein interaction network. Nature 437, 1173-1178. 10.1038/nature04209 [DOI] [PubMed] [Google Scholar]
- Sánchez-Cárdenas, C., Romarowski, A., Orta, G., De la Vega-Beltrán, J. L., Martín-Hidalgo, D., Hernández-Cruz, A., Visconti, P. E. and Darszon, A. (2021). Starvation induces an increase in intracellular calcium and potentiates the progesterone-induced mouse sperm acrosome reaction. FASEB J. 35, e21528. 10.1096/fj.202100122R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi, C. M., Martínez-López, P., de la Vega-Beltrán, J. L., Butler, A., Alisio, A., Darszon, A. and Salkoff, L. (2010). The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 584, 1041-1046. 10.1016/j.febslet.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber, M., Wei, A., Yuan, A., Gaut, J., Saito, M. and Salkoff, L. (1998). Slo3, a novel pH-sensitive K+ channel from mammalian spermatocytes. J. Biol. Chem. 273, 3509-3516. 10.1074/jbc.273.6.3509 [DOI] [PubMed] [Google Scholar]
- Smith, J. F., Syritsyna, O., Fellous, M., Serres, C., Mannowetz, N., Kirichok, Y. and Lishko, P. V. (2013). Disruption of the principal, progesterone-activated sperm Ca2+ channel in a CatSper2-deficient infertile patient. Proc. Natl. Acad. Sci. USA 110, 6823-6828. 10.1073/pnas.1216588110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauss, C. R., Votta, T. J. and Suarez, S. S. (1995). Sperm motility hyperactivation facilitates penetration of the Hamster Zona Pellucida. Biol. Reprod. 53, 1280-1285. 10.1095/biolreprod53.6.1280 [DOI] [PubMed] [Google Scholar]
- Strünker, T., Goodwin, N., Brenker, C., Kashikar, N. D., Weyand, I., Seifert, R. and Kaupp, U. B. (2011). The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 471, 382-386. 10.1038/nature09769 [DOI] [PubMed] [Google Scholar]
- Suarez, S. S. (2016). Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res. 363, 185-194. 10.1007/s00441-015-2244-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez, S. S. and Osman, R. A. (1987). Initiation of hyperactivated flagellar bending in mouse sperm within the female reproductive tract. Biol. Reprod. 36, 1191-1198. 10.1095/biolreprod36.5.1191 [DOI] [PubMed] [Google Scholar]
- Tateno, H., Krapf, D., Hino, T., Sanchez-Cardenas, C., Darszon, A., Yanagimachi, R. and Visconti, P. E. (2013). Ca2+ Ionophore A23187 can make mouse spermatozoa capable of fertilizing in vitro without activation of cAMP-dependent phosphorylation pathways. Proc. Natl. Acad. Sci. USA 110, 18543-18548. 10.1073/pnas.1317113110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti, P. E., Bailey, J. L., Moore, G. D., Pan, D., Olds-Clarke, P. and Kopf, G. S. (1995). Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 121, 1129-1137. [DOI] [PubMed] [Google Scholar]
- Visconti, P. E., Krapf, D., de la Vega-Beltrán, J. L., Acevedo, J. J. and Darszon, A. (2011). Ion channels, phosphorylation and mammalian sperm capacitation. Asian J. Androl. 13, 395-405. 10.1038/aja.2010.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwakarma, P. (1962). The pH and bicarbonate-ion content of the oviduct and uterine fluids. Fertil. Steril. 13, 481-485. 10.1016/S0015-0282(16)34633-7 [DOI] [PubMed] [Google Scholar]
- Vyklicka, L. and Lishko, P. V. (2020). Dissecting the signaling pathways involved in the function of sperm flagellum. Curr. Opin. Cell Biol. 63, 154-161. 10.1016/j.ceb.2020.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., Liu, J., Cho, K.-H. and Ren, D. (2009). A novel, single, transmembrane protein CATSPERG is associated with CATSPER1 channel protein. Biol. Reprod. 81, 539-544. 10.1095/biolreprod.109.077107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., McGoldrick, L. L. and Chung, J.-J. (2021). Sperm ion channels and transporters in male fertility and infertility. Nat. Rev. Urol. 18, 46-66. 10.1038/s41585-020-00390-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennemuth, G., Babcock, D. F. and Hille, B. (2003). Calcium clearance mechanisms of mouse sperm. J. Gen. Physiol. 122, 115-128. 10.1085/jgp.200308839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, J. and Ren, D. (2009). The BSA-induced Ca2+ influx during sperm capacitation is CATSPER channel-dependent. Reprod. Biol. Endocrinol. 7, 119. 10.1186/1477-7827-7-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z., Abbott, A., Kopf, G. S., Schultz, R. M. and Ducibella, T. (1997). Spontaneous activation of ovulated mouse eggs: time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol 1,4,5-trisphosphate sensitivity. Biol. Reprod. 57, 743-750. 10.1095/biolreprod57.4.743 [DOI] [PubMed] [Google Scholar]
- Yang, F., Gell, K., van der Heijden, G. W., Eckardt, S., Leu, N. A., Page, D. C., Benavente, R., Her, C., Höög, C., McLaughlin, K. J.et al. (2008). Meiotic failure in male mice lacking an X-linked factor. Genes Dev. 22, 682-691. 10.1101/gad.1613608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F., Silber, S., Leu, N. A., Oates, R. D., Marszalek, J. D., Skaletsky, H., Brown, L. G., Rozen, S., Page, D. C. and Wang, P. J. (2015). TEX11 is mutated in infertile men with azoospermia and regulates genome-wide recombination rates in mouse. EMBO Mol. Med. 7, 1198-1210. 10.15252/emmm.201404967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, X.-H., Yang, C., Kim, S. T., Lingle, C. J. and Xia, X.-M. (2011). Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc. Natl. Acad. Sci. USA 108, 5879-5884. 10.1073/pnas.1100240108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, D., Dix, D. J. and Eddy, E. M. (1997). HSP70-2 is required for CDC2 kinase activity in meiosis I of mouse spermatocytes. Development 124, 3007-3014. 10.1242/dev.124.15.3007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.