Abstract
HIV infection requires lifelong antiretroviral therapy (ART) to control disease progression. Although ART has greatly extended the life expectancy of persons living with HIV (PWH), PWH nonetheless suffer from an increase in AIDS-related and non-AIDS related comorbidities resulting from HIV pathogenesis. Thus, an HIV cure is imperative to improve the quality of life of PWH. In this review, we discuss the origins of various SIV strains utilized in cure and comorbidity research as well as their respective animal species used. We briefly detail the life cycle of HIV and describe the pathogenesis of HIV/SIV and the integral role of chronic immune activation and inflammation on disease progression and comorbidities, with comparisons between pathogenic infections and nonpathogenic infections that occur in natural hosts of SIVs. We further discuss the various HIV cure strategies being explored with an emphasis on immunological therapies and “shock and kill”.
Keywords: human immunodeficiency virus (HIV), simian immunodeficiency virus (SIV), latency reversing agents (LRAs), HIV latency, reactivation, pathogenesis, cure, strategies, nonhuman primate models
In 1985, shortly after the first discovery of HIV-1 [1], a group at the New England Regional Primate Research Center (NEPRC) reported the identification of a nonhuman primate (NHP) lentivirus counterpart of HIV-1 (that would later be known as SIVmac), which was responsible for AIDS cases in the rhesus macaques (Macaca mulatta, RMs) colony of the NEPRC [2]. SIVmac, specifically clone SIVmac239 and viral swarm SIVmac251, would become the gold standard for nonhuman primate cure modeling. As discussed later, SIVmac greatly recapitulates many aspects of HIV pathogenesis. Regardless, curing HIV has become a substantial challenge. Antiretroviral therapy (ART) is one of the greatest medical miracles of the last few decades, being able to drastically suppress HIV replication and incredibly extend the life expectancy of persons living with HIV (PWH) [3]. However, ART is virostatic and does not directly eliminate infected cells or proviruses from PWH and the aging PWH population is suffering from increased comorbidities, leading to a decreased quality of life and an increase in healthcare costs [4,5,6]. Additionally, through decades of cure research, only two PWH have been demonstrated to achieve complete HIV remission: the “Berlin patient” and the “London patient”. These two individuals were treated with stem cell transplantations from donors homozygous for the CCR5 ∆32 allele for their cancers [7,8]. However, this strategy is neither scalable nor does it have acceptable toxicity for the vast majority of PWH. In this review, we discuss the origins of SIV strains used in research and the roles of various SIV-nonhuman primate models, as well as the pathogenesis of SIV/HIV and current strategies utilized in HIV cure.
1. You Can Call Me SIV: Introduction and the Origin of SIVmac
In the early 1970s, an outbreak of lymphomas, resembling Burkitt’s lymphoma, was reported in RMs housed at the California National Primate Research Center (CNPRC) [9,10,11,12] and would later be demonstrated to play a role in the SIVmac infections at NEPRC. However, the origin of these pathogenic lentiviruses in the RMs remained unknown then, as studies in the wild macaques from Asia did not identify any circulation of SIV-like viruses in these NHP species [13,14,15]. Meanwhile, a plethora of SIVs was shown to naturally infect multiple species of monkeys and apes in Africa [13]. These viruses are highly divergent from each other and show a diversity profile evocative of host-dependent evolution [16,17], suggesting a very old origin of SIVs, predating the monkey speciation in Africa [18]. Yet, the fact that no New World monkeys carry SIVs, nor are the Old World monkeys in Asia, points to an origin of the AIDS viruses sometime after the speciation of the Asian monkeys [18]. Interestingly, the virus isolated from the macaques in NEPRC and CNPRC was closely related to the SIV naturally infecting sooty mangabeys (Cercocebus atys, SM) [19].
As such, the origin of the SIV infection in captive macaques at the NEPRC was perplexing, particularly when applying the criteria of the cross-species transmission that allowed the identification of the sources of HIVs in chimps and gorillas from Cameroon for HIV-1 [20] and in SMs from West Africa for HIV-2, respectively [21]: (a) genetic, antigenic and phylogenetic similarities between the human and NHP viruses; (b) coincidence between the species habitat and the HIV-1/HIV-2 epicenters; (c) favoring factors of transmission. These requirements were largely not fulfilled for the origin of the SIVmac in the macaque colony at the NEPRC, as the NEPRC did not have any SM. Meanwhile, at the CNPRC, both RMs and SMs were housed at the same time in the 1960s, yet, reports suggested that the two species did not enter in close direct or indirect contact [11]. In a twist of events, however, virus archeology studies performed at the NEPRC clearly demonstrated that the origin of SIVmac was in fact at the CNPRC, from survivors of the original lymphoma outbreak that were shipped to the NEPRC in the 1970s (Figure 1) [22,23]. The virus then went undetected for >10 years in the NEPRC colony. The proofs of the virus transfer are: (i) detection of SIV antibodies in the CRPRC RMs with lymphomas; (ii) pathologies observed were similar to what is now known as pathogenic SIV infection; (iii) detection of SIV antibodies in the SMs in the CRPRC colony prior to the outbreak RM exposure to sooty mangabey tissues; (iv) detection of SIVmac DNA in the spleen and lymph nodes in one of the RMs sent to NERPRC [23]. More recently, extensive phylogenetic analyses of the SIVs naturally infecting SMs from different Primate Centers in the US traced the origin of the SIVmac to SMs in the CNPRC [22]. Moreover, the circumstances of the accidental transmission from SMs to RMs were established to rely on the kuru experiments carried out extensively at the CNPRC and New Iberia Research Center (NIRC) in the 1960s [19]. These experiments by D. Carleton Gadjdusek initially passaged human brain extracts into SMs to try to discover the cause of kuru after he discovered the disease in New Guinea. SM brain extracts were then serially passaged into RMs, allowing for direct transmission of SIV.
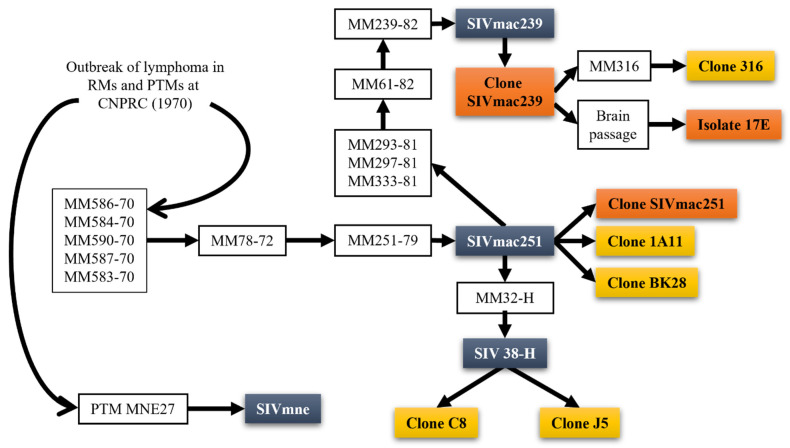
Figure 1.
Origin of SIVmac251, SIVmac239, and derivative clones. SIVmac239 and SIVmac251 originate from rhesus macaques housed at the California National Primate Research Center (CNPRC). The progenitor viruses were from sooty mangabeys at the CNPRC which were used for kuru experiments, allowing for serial passaging and eventual establishment of the SIVmac239 and SIVmac251 isolates. White boxes are animals and passages; bluish grey boxes are the primary strains recovered from passaging; orange boxes are the clones most used in research; yellow boxes are lesser used clones.
Furthermore, studies have shown [22] that the same experiments carried out at the CNPRC were likely responsible for the infection of SIVs of different other species of macaques, such as the pigtailed macaques (Macaca nemestrina) [24] and the stumptailed macaque (Macaca arctoides) [25,26] and crab eating macaque (Macaca fascicularis) [27].
2. Why Don’t You Infect Me? The Animal Model for AIDS Research
There are multiple advantages of the use of the NHP model for AIDS research. The most important of these is that animal studies allows us to perform interventions that would otherwise be impossible to perform in PWH: staged infections, invasive sampling, exploratory interruptions of antiretroviral therapies, testing of new therapeutic approaches and vaccines. The model has been extensively characterized over the last three decades, and a wealth of data is available for comparisons. Moreover, multiple virological, immunological and clinical biomarkers have been extensively tested and developed, conferring the model predictability and consistency. It is therefore not surprising that the NHP models for AIDS research, which recapitulate the key features of HIV infection, provided seminal results for HIV prevention, pathogenesis, and treatment.
The early events of HIV transmission and dissemination in the host, with the potential impact on prevention and treatment were obtained in NHPs and showed a very rapid seeding of the reservoirs [28,29]. Further, the use of NHP models has provided seminal information regarding the persistence of this reservoir and acts as an excellent tool for screening new strategies aimed at inducing cure/functional cure [28,30,31,32,33,34,35,36]. For example, studies showed that the major site of virus replication and CD4+ T-cell depletion is at the mucosal sites, pointing to the mucosa as the major target of vaccine interventions for the prevention of HIV transmission [37,38,39,40] and these studies predated those in PWH study participants by a decade. Further, because of the ease of manipulation in NHPs, it has been shown that diet can have a large effect on disease progression such that high fat diets accelerate progression [41]. Similarly, experimental infections allow for a better understanding of transmission and the differences between routes of infection [42,43,44].
The comparison between natural hosts of various SIV strains, which do not progress to AIDS, have played a big role in understanding pathogenesis. Studies on the cartography of viral dissemination [45] pointed to major differences between pathogenic and nonpathogenic infections at these early stages of infection, that have the potential to drive these different outcomes [46,47,48]. Additionally, studies in natural hosts have also established the key role of the immune activation and inflammation for the progression to AIDS and the development of comorbidities [49,50,51,52,53].
Fifty Ways to Infect a Monkey—SIV/SHIV Strains for Use in Nonhuman Primates
While virtually every SIV strain can be used for studies in NHPs, there are several reference SIV strains that have been extensively used for studies in NHPs. In addition to the SIVmac lineage strains and the other strains accidentally generated through the kuru experiments carried out at the CNPRC (SIVmne, SIVstm and SIVmfa), many other SIV strains have been generated and employed over the years for experiments in macaques. Virtually concomitantly with the discovery of the SIVmac at the CNPRC, SIVsmB670 was isolated from macaques at the Tulane National Primate Research Center (TNPRC) [54]. There, in 1979, a female SM from the Gulf South Research Institute (currently New Iberia Primate Center) suspected of having leprosy was used in an extensive experiment involving serial passages of blood and tissues, with the goal of developing an NHP model for leprosy. Due to the very long incubation of leprosy, these experiments were only partially successful. Nevertheless, the passage of M. leprae to other SMs and RMs resulted in cases of full-blown AIDS in several macaques (particularly in the macaque B670) (Figure 2).
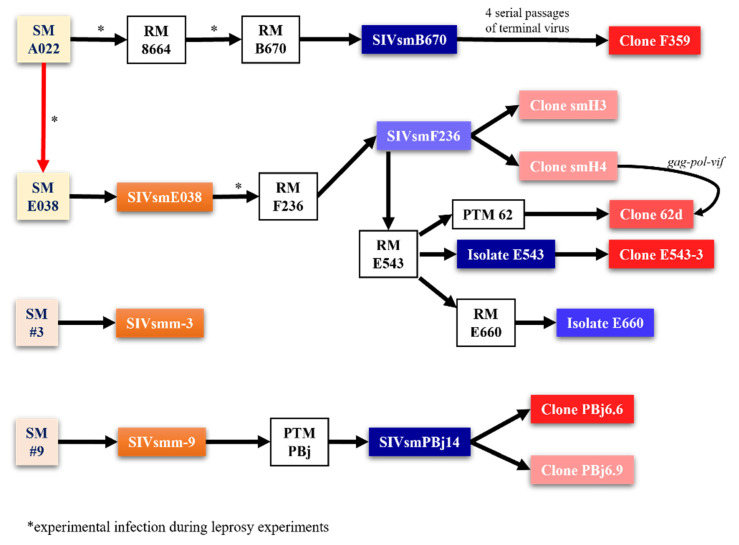
Figure 2.
Isolation of various SIV strains used for NHP models. Different SIV strains vary in their pathogenic features, allowing for different uses by strain and animal species. The origin of different pathogenic isolates from sooty mangabeys at the Tulane National Primate Research Center (TNPRC) and those resulting from leprosy experiments, as designated by asterisk, from a sooty mangabey originally housed at the Gulf South Research Institute (now New Iberia Primate Center). Yellow and tan boxes are progenitor animals; white boxes are animals that were received serial passaging; orange boxes are initial strains isolated from SMs; blue boxes are strains isolated from serial passaging; red boxes are clones; darker tint indicates more prevalence in research.
With each serial passage, the number of AIDS cases increased in the macaque groups and an SIVmac-related, albeit different, SIV could be isolated from both the RMs and SMs (Figure 2) [11].
After the discovery of SIVsmm in the SMs-naturally infected at the TNPRC, a new experiment aimed at rederiving a “clean” viral inoculum for the infection of the RMs was performed. Blood from the SM A022 was passaged into an uninfected SMM (E038), which was used as a source of virus for the infection of a RM (F236) [55]. The isolate SIVsmmF236 was lambda cloned into two relatively low pathogenicity clones (SIVsmH3 and SIVsmH4) [56]. Meanwhile SIVsmmF236 was passaged into a pigtailed macaque (PTM62) and a RM (E543). The isolate SIVsmmE543 was cloned into a highly pathogenic clone (SIVsmmE543-3) [57] and passaged into another naïve macaque (RM E660) [58]. SIVsmmE660 is currently a reference strain. It has a relatively high pathogenicity [59], and it is a tier 2 strain with regards to the neutralization sensitivity [60,61].
One of the issues with the SIVsmm family of reference strains is that they are susceptible to the TRIM5α restriction, unlike SIVmac-derived viruses, resulting in a wide range of viral loads (VLs) based on the TRIM5α genotypes [62]. Conversely, the SIVmac group accumulated mutations that conferred resistance to TRIM5a restriction [62].
More recently, both SIVmac and SIVsmm founder-transmitted infectious molecular clones have been derived for use in vaccine studies [63]. Meanwhile, for the purpose of cure studies, in which reservoir diversity and virus reactivation have to be investigated and which require viral diversity, both tagged [64,65] and barcoded [66,67] SIVmac clones have been produced, that combine the advantages of both infectious molecular clones (IMCs) (uniformity of the pathogenicity of the vial inoculum) and of the viral swarms, thus allowing a proper tracking of the number of viral variants that are reactivated during therapeutic interventions aimed at curbing the reservoir or analytical treatment interruptions (ATIs).
Finally, during a survey of SIVsmm diversity in the Primate Centers in the US, we identified multiple SIVsmm lineages that roughly mirror HIV-1 diversity and selected new potential references strains representative of every lineage [22]. For the vast majority of these new strains transmitted founder IMCs were derived and available.
For the studies of SIV pathogenicity in the African NHP natural hosts, several isolates have been used over the last few decades. Due to the endangered nature of most of the African NHP species, the vast majority of experimental infections in natural hosts are carried in African green monkeys (AGMs). Of these, the sabaeus monkeys are the model of choice due to the availability of a large wild population in the Caribbean. The reference strain for the studies in the sabaeus monkeys is SIVsab92018, which was derived from a chronically infected sabaeus monkey from Senegal [68]. Plasma from this animal was directly inoculated into naïve monkeys and collected during the acute infection for further use, without in vitro passage. A transmitted-founder clone has been derived from the acute plasma [69]. Additionally, SIVsab92018 was also directly passaged into pigtailed macaques and established a model of increased comorbidity prevalence and faster rate of progression while still recapitulating the pathogenic features of HIV infection [70]. Unlike in pigtailed macaques, direct passaging of SIVsab92018 (SIVsab) into RMs results in a state of functional cure, whereby the RMs naturally control the virus replication to below the limits of detection, immune populations are restored during chronic infection, and immune activation and inflammation (IA/INFL) are controlled back to baseline levels [71,72]. We utilize this model for experimental agent testing due to the ability to reactivate virus and bolster viral production by de novo infection off of antiretrovirals, thereby increasing resolution of viral reactivation.
Simian-human immunodeficiency viruses (SHIVs) are chimeric SIV-HIV viruses, which are developed as a method to try and mimic HIV-1 infection as best as possible in NHP models, with a large emphasis on vaccine development with the inclusion of the HIV glycoprotein. This is necessary due to the host restriction factors in NHPs that prevent productive infection of HIV-1 and HIV-2 [73]. The first SHIV developed was in 1992 with an SIVmac239 backbone that had its rev, tat, and env genes replaced with HIV-1 rev, tat, vpu, and env [74]. Replication was lacking in the first SHIV in vivo and thus researchers replaced the env with a dual-tropic CCR5/CXCR4 HIV-1 env, generating SHIV-89.6. SHIV-89.6 replicated to high levels in vivo, but still lacked some pathogenic features, such as sustained CD4+ T-cell depletion [75]. Serial passaging and additional modifications created SHIV-KB9, which recapitulated many features [76]. However, a major problem was that this virus was primarily CXCR4-tropic and resulted in modified (quickened) disease progression and did not properly represent HIV infection [77,78]. Importantly, the CXCR4-tropic SHIVs were overly sensitive to neutralizing antibodies, thus diminishing their usefulness in vaccine studies [78,79]. Thus, CCR5-tropic SHIVs became the focus of developing SHIVs [80,81,82,83,84]. In fact, SHIVSF162P3 has had great success in vaccine and broadly neutralizing antibody studies [82,85,86]. Serial passage of a SHIV using HIV-1Ada env resulted in SHIVAD8 and its derivatives, which have also been used in vaccine, antibody, and therapeutics studies with relative success [87,88,89]. Nonetheless, even the CCR5-tropic SHIVs are not necessarily ideal due to the use of env sequences from chronically infected PWH and their passaging in NHPs results in modified env sequences [82,84,90]. They are therefore not as clinically relevant for vaccine studies as transmitted founder (TF) viruses which have special characteristics that increase fitness, and importantly, will have the relevant Env for targeting in vaccine or antibody studies [91]. Thus, with the new knowledge it became imperative to design transmitted founder SHIVs that do not require passaging [79], such as in vivo competition [92] with rational design and specific residue modifications (Env residue 375) [93,94,95,96] to improve binding and subsequent replication. However, issues still occur with spontaneous control and incomplete CD4+ T-cell depletion [95,96], likely due to insufficient viral replication that then prevents efficient immune escape. This also leads to issues with vaccine and bNAb studies, as the viruses do not properly recapitulate the pathogenicity of HIV, thereby potentially providing exaggerated protection.
3. Everything Put Together Falls Apart. A Brief Introduction to HIV/SIV Pathogenesis
3.1. HIV/SIV Reservoir Is Rapidly Seeded after Transmission
Studies of SIV transmission to RMs allowed us to characterize HIV transmission in great detail, with the goal of identifying windows of opportunity to prevent infection. The mucosal barriers highly hinder infection at the entry site, thereby limiting the infection to a very small, transmitted founder population, which then establishes a productive, disseminating infection in lymphatic tissues [97,98]. In the vast majority of PWH, a single virus initiates systemic infection [99] and the same is true for intravaginal exposure of RMs to low doses of SIVs [44]. While both CXCR4 and CCR5-tropic viruses can be found in sperm and vaginal secretions [100,101,102], the viruses that initiate infection (which are baptized TF viruses) are exclusively CCR5-tropic [99]. TF viruses have a specific fitness due to a lower glycosylation [103,104] and less sensitivity to type I interferons, especially IFN-α [105,106].
During sexual transmission, the virus migrates across the epithelial barrier at the site of entry via M cell transepithelial transport [107,108], dendritic cells (DCs) [109,110], and microtears in the epithelial layer [111]. HIV is then exposed to the local immune cell populations, including lymphocytes and macrophages, and rapidly infects and spreads through the primary target cells: CCR5+ memory CD4+ T cells [112,113]. The virus undergoes rapid dissemination from the site of entry. Thus, two days after intravaginal inoculation, the virus could be detected in the draining and even in the distant lymph nodes, before becoming detectable in circulation (5 days post-inoculation) [109]. Intrarectal transmission results in an even more rapid viral spread throughout the body [114,115], with no window of opportunity for potential interventions being observed upon intrarectal challenge [116]. As such, the study of the early events of HIV/SIV transmission showed that the immune response to infection is a double-edged sword: it helps establish the transmission bottleneck and eliminate virus, but the cellular activation also contributes to infection by increasing the amount of target cells at the site of entry [117].
3.2. Immune Response during Acute Infection Drives Viral Set-Point
During the acute infection, a massive immune response is triggered in response to the viral infection, with two separate waves of cytokines and chemokines. First, IFN-α, IFN-γ, inducible protein 10 (IP-10), interleukin (IL)-12, IL-15, and monocyte chemoattractant protein 1 (MCP-1) all rapidly increase prior to the peak of viremia. This is followed by increases in TNF-α, IL-6, IL-8, IL-18, and IL-12p40 [118,119,120,121]. Production of these molecules is mostly by dendritic cells (DCs), with additional production by monocytes, natural killer (NK) cells, and even T cells. Activation of plasmacytoid DCs is mediated by toll-like receptor 7 (TLR7) after endocytosis of HIV [122]. Myeloid-derived DCs are also responsible for cross presentation of antigens [123] while plasmacytoid DCs produce IFN-α primes T cells [124]. NK cells are direct effectors that are activated during HIV infection and clear infected cells through cytolysis and degranulation. NK cell cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC) result from binding of killer immunoglobulin-like receptors (KIRs), CD16, and the NKG2 protein family [125]. Through degranulation perforin and granzymes are released and induce target cell apoptosis [126]. NK cells produce various cytokines and chemokines, such as IFN-γ and TNFα [127] to limit viral infection and spread and β-chemokines which inhibit HIV entry to CD4+ T cells [128]. The adaptive CD8+ T-cell immune response begins prior to the peak of viremia. CD8+ T cells recognize foreign antigens that are presented on the cell surface by major histocompatibility complex (MHC) class I, stimulating the release of perforin, granzymes, and Fas ligand, leading to target cell apoptosis. CD8+ T cells also release IFN-γ and TNF-α into the microenvironment [129]. CD8+ T cells proliferation peaks around 2 weeks after the viral peak, their activation status being inversely correlated with viral set point. This proves that the post-acute viral control occurs via CD8+ T cells [130]. The emergence of the cellular immune responses exerts pressures on the virus at the transition from acute-to-chronic infection, and mutations are selected in the viral genome for CD8+ T-cell escape, leading to a continuous chess game between the CD8+ T cells that can respond to the new epitopes and subsequent viral escape [131,132]. B cells are also activated during acute infection, generate plasmocytes and initiate antibody production. Initially, the antibody response to HIV is non-neutralizing and does not impact the plasma viremia [133]. However, the antibodies are enriched for IgG3, suggesting that they have not gone through affinity maturation and may be due to the rapid dysregulation of lymphoid tissues where the B cells would interact with T cells for maturation [134]. Indeed, CD4+ T cells are rapidly depleted during acute infection which has deleterious consequences for proper adaptive immune responses [135] due to their role in providing stimulatory cytokines. Beyond that, the elimination of CD4+ T cells helps drive the mucosal dysfunction discussed later and eventually will reduce to levels defining AIDS without treatment.
The viral set point, which occurs around 5–6 weeks post-infection, marks the passage to the chronic infection phase, when the immune system and HIV have reached a pseudo-equilibrium of steady-state viral replication, immune-mediated clearance, viral escape, and T cell adaptation. It is thus unsurprising that the levels of plasma VLs are predictive for the rate of disease progression to AIDS [136,137]. Unlike VLs, the immune activation continues to rise into chronic phase, at which point it eventually hits the immune activation set point, in which CD8+ T-cell activation parallels the rate of CD4+ T-cell loss [138]. The immune activation set-point was also negatively correlated with the viral set point. In conjunction with data demonstrating that immune activation rapidly decreases with ART, it is likely that VLs is one of the drivers of the immune activation set-point [139,140,141].
3.3. Control at Last—Antiretroviral Therapy for HIV
The first antiretroviral (ARV), zidovudine, was approved by the FDA in 1987. Tritherapy, associating nucleozide reverse-transcriptase (RT) inhibitors with either non-nucleoside RT inhibitors or with protease inhibitors was introduced in 1996 and spectacularly impacted the outcome of infection: it completely suppressed viral replication and boosted the CD4+ T-cell counts [142]. Current ARVs target most of the HIV life cycle: entry inhibitors, prevent virus penetration in the target cells, by blocking CCR5 or CXCR4; fusion inhibitors prevent entry; RT inhibitors (nucleoside and non-nucleoside, NRTI and NNRTI, respectively) abort reverse transcription; integrase inhibitors, or integrase strand transfer inhibitors (INSTI) prevent viral integration; protease inhibitors (PI) prevent virion maturation [143]. The current first line of therapy is two NRTIs and either an NNRTI or INSTI, although new data support a two-drug regimen of dolutegravir (INSTI) and lamivudine (NRTI) for initial treatment [144]. ART thus effectively inhibits viral replication and decreases plasma VLs in PWH. In fact, most individuals will achieve viral suppression below the limits of detection, 50 vRNA copies/mL of plasma, as long as they maintain their regimen, and as long as their VLs are undetectable, the paradigm (and media slogan) has become “Undetectable = Untransmittable; U = U” [145].
While ART decreases VLs, it reciprocally restores CD4+ T cell counts, although this is highly variable and dependent upon the stage of disease progression and degree of immunodeficiency at treatment initiation. Studies have shown that the earlier ART initiation the better prognosis, with much better although incomplete restoration of CD4+ T cells [146,147,148], including in the GALT [149]. Studies also show that unsatisfactory CD4+ T cell restoration is correlated with higher mortality [150]. ART administration also contributes to a partial control of the levels of inflammation and immune activation, but cannot restore them to pre-infection levels [151,152] and similar to CD4+ T cell restoration, late ART initiation results in a more limited control of immune activation [153]. With advances in ART and accessibility, ART has drastically increased the life expectancy of PWH. While in the 2000s’ the life expectancy of a 21-year old PWH was 38 years, by 2016 it had increased to 57 years, a nearly 20 year increase [154]. Thus, ART has changed HIV infection from a life-threatening condition to a manageable chronic disease. Yet, life expectancy is still below uninfected persons (64-year life expectancy for 21-year old) [154] and, as discussed later, ART is not curative, nor does it completely prevent AIDS-related comorbidities.
4. At the Zoo. Nonhuman Primate Models for HIV Pathogenesis
4.1. Similarities and Recapitulation of Specific Pathogenicities
Nonhuman primates (NHPs) are excellent models for the study of HIV-1 due to the variety of pathogenic outcomes that can be induced through various combinations of NHP species and SIV strains. Further, due to their size it is possible to take far more consistent sample volumes (blood and tissues) than with other models, e.g., humanized mice, and their use allows for extensive tissue sampling that would otherwise not be possible in humans. Meanwhile, NHPs are outbred and more genetically close to humans than any other model, which allow a more rigorous modeling in NHPs compared to other inbred species. Of the several NHP species that can be utilized, cure research primarily uses RMs infected with SIVmac (either the reference swarm SIVmac251 or the infectious molecular clone SIVmac239) as the reference model. Notably, the SIVsmm family is the only one to induce pathogenic infection to RMs upon direct cross-species transmission of the virus from the natural host (SMs), and SIVsmm infection yields a pathogenic diversity with a wide range of outcomes of the infection (due to a partial TRIM5α restriction) [62], unlike the SIVmac infection [155,156,157,158,159,160]. Therefore, the combination of RM and SIVmac strains is the gold standard for HIV modeling because of it reproduces all the major features of HIV-1 infection in a condensed time frame [31]: (i) integration into host cell genome with similar integration site preference [161,162,163]; (ii) conversion to latency in infected cells; (iii) infected cell distribution to mucosal sites, lymph nodes, and peripheral blood [164,165,166]; (iv) Depletion of memory CD4+ T cells from the mucosal and lymphoid sites [37,38,39,40]; (v) chronic immune activation and inflammation, associating gut dysfunction and microbial translocation [39,113,117,167,168]. Although other species/strain combinations are available, they provide targeted usefulness, such as pigtailed macaques infected with SIVsab, which produces a highly pathogenic infection that is perfectly suited for the study of HIV/SIV-associated comorbidities [51,169,170], but long-term chronic illness is not easily achieved due to the very high pathogenicity of this infection, in which about 40% of SIVsab-infected PTMs progress to AIDS within the 6 months following the SIV challenge [70]. Further, for the study of HIV/SIV effects on the central nervous system (CNS), pigtailed macaques coinfected with SIVDeltaB670 and SIV17E-Fr are used because they quickly progress to immunodeficiency that associates CNS pathologies. This model showed that upon SIV infection, the CNS reservoir is seeded as early as 4 days, and that the macrophages are the major target cells of the virus in the brain [171]. Recently, a new model of RMs infected with a new molecular clone, SIVsmE-CL757, was reported to reproduce the CNS events without the rapid disease induction seen in PTMs [172,173]. At the opposite spectrum of pathogenic diversity from the SIVmac infection, RM exposure to SIVsab leads to a very robust acute SIV infection followed by a spontaneous complete viral suppression below the limits of detection, allowing for investigation of virus reactivation from the latency without the use of ART [72]. This is particularly helpful for understanding the reactivation potential of “shock and kill” latency reversing agents (LRAs), as the lack of ART enables de novo infection and therefore, larger viral bursts, allowing for easier detection of viral reactivation after the administration of latency reversal agents. The caveat is the inability to properly compare the reservoir before and after therapy due to the wide spread of the reactivated virus in the absence of ART.
4.2. Everything about It Is Inflammation and Immune Activation
Chronic T-cell immune activation and systemic inflammation are key pathogenic features of HIV/SIV infection [174,175]. T-cell immune activation and inflammation increase in response to virus early during infection, but they are not resolved after establishment of the viral setpoint, nor after viral suppression with ART [151,152]. In fact, the immune activation set point is one of the strongest predictors of disease progression [138,174,176], better than plasma VLs or CD4+ T-cell counts. This is due to the close association of the immune activation and inflammation with non-AIDS comorbidities and mortality in PWH and SIV-infected NHPs [49,50,51,52,53]. The determinants of chronic immune activation and inflammation in HIV/SIV infection are complex and multiple: (i) activation of the immune response through viral production and replication [139,140,141]; (ii) loss of gastrointestinal tract mucosal barrier integrity through the depletion of Th17 cells, which maintain mucosal barrier integrity [177,178]; (iii) microbial translocation from the lumen into systemic circulation and organs results from the damage to the mucosal barrier and epithelial tight junctions [179,180,181,182]; (iv) coinfections (e.g., hepatitis C virus [183], hepatitis B virus [184], herpes simplex virus type 2 [185], cytomegalovirus [186,187], and Epstein–Barr virus [188]) contribute to antigen-specific immune activation or pattern recognition receptor (PRR) activation and are increasingly active with progressive immunodeficiency [189]; (v) Toxicity of ART and other risk factors [52,190].
The chronic immune activation and inflammation impact disease progression through multiple pathways: (i) activated T cells become HIV/SIV target cells through expressing higher levels of coreceptors CCR5 and CXCR4 [191,192]; (ii) activation of NF-κB results in virus production [193]; (iii) constant activation results in increased T cell turnover and homeostatic proliferation, thereby decreasing the progenitor pool and inducing immune senescence [194,195]; (iv) increased expression of immune checkpoint expression (e.g., PD-1 [196,197,198,199] and CTLA-4 [200]) which results in decreased functionality (T-cell exhaustion) [201]; (v) collagen deposition and fibrosis (via transforming growth factor beta [TGF-β]) damages the fibroblastic reticular cell network in lymph nodes, resulting in aberrant immune reconstitution [202,203,204,205]; (vi) prolonged inflammation facilitates an increased risk of cancers [206,207]; and (vii) chronic inflammation damages vasculature and induces hypercoagulability, resulting in increased risk for cardiovascular diseases (CVD) [49,51,208,209,210]. In the end, these consequences result in both a higher frequency and earlier onset [4,5] of AIDS and non-AIDS comorbidities [211]. Comorbidities also include premature aging [211], sarcopenia [212], nonalcoholic fatty liver disease (NAFLD) [213], and HIV-associated neurocognitive disorder (HAND) [214].
Although there are several mechanisms that contribute to the chronic immune activation and inflammation, gut dysfunction and microbial translocation are arguably the largest contributors. Importantly, the onset of microbial translocation results in a vicious cycle of inflammation, mucosal barrier damage, and more microbial translocation; rinse and repeat [179,215]. Translocated microbial products activate monocytes and macrophages that then produce inflammatory cytokines (IFN-α, TNF-α, IL-1, IL-6, and IL-18), further activating the immune system [170,216,217]. This not only results in chronic immune activation and inflammation, but also drives HIV enteropathy, which was described in the earliest stages of the pandemic, when diarrhea, weight loss, malnutrition, malabsorption and villous atrophy were frequently diagnosed in AIDS patients [218].
4.3. Gut Dysfunction and Microbial Translocation Potentiate Immune Activation and Inflammation
HIV-associated GI pathology is triggered by the early and massive HIV-1 replication, and is characterized by immunological and structural abnormalities, including alterations of both the adaptive and innate mucosal immunity and substantial disruptions of the epithelial barrier [218,219,220]. These changes lead to increased local inflammation, microbial translocation and dysbiosis, and consequently to generalized immune activation and inflammation, and comorbidities [52]. This current pathogenic paradigm of AIDS, for which the impact of HIV infection on gut mucosa is the quintessential determinant of HIV infection pathogenesis, was made possible only through extensive use of NHPs. The animal models allowed invasive serial studies of the gut [219,221], and, as such, the reports on massive rapid depletion of the mucosal CD4+ T cells in NHPs preceded similar observations in humans by a decade [37,38,39,40]. Detailed comparative studies facilitated by invasive sampling at key time points of infection in multiple NHP models with different outcomes of SIV infection furthered this major paradigm shift in AIDS pathogenesis [222].
Intestinal mucosal lesions occur early in HIV infection and are rapidly established as part of a vicious circle in which gut damage, microbial translocation and IA/INFL potentiate each other [219]. Virus suppression with ART improves infection outcome, but frequently does not reverse GI dysfunction [52]. As a result, even in study participants in which the virus is suppressed for prolonged periods of time (some PWH received ART for >20 years), residual levels of IA/INFL nonetheless persist, leading to an only partial immune restoration at the mucosal sites, and an increased frequency accelerated aging and HIV-related comorbidities than in the general population.
Two major mechanisms are responsible for the gut dysfunction observed in HIV infection: (i) First, mucosal CD4+ T cell loss [218,220], the hallmark of HIV/SIV infection [37,223,224,225,226]. The virus infects and kills activated memory and effector CCR5-expressing CD4+ T cells, the major CD4+ T cell subset at the mucosal sites, particularly in the lamina propria of the gut. CD4+ T cell killing occurs in a caspase-1-dependent manner, resulting in a highly inflammatory form of death known as pyroptosis, which drives gut barrier dysfunction through production of inflammatory cytokines [227,228]. Exposure to microbial products may also divert the mechanism of mucosal cell death toward apoptosis [227]. Increased inflammation induced by microbial products is probably also responsible, at least in part, for enhanced bystander lymphoid and epithelial cell death and gut damage [227]. Similar to HIV-1 infection, CD4+ T cell depletion occurs early in SIV-infected macaques, is substantial, and is one of the correlates of the clinical outcome [50,220,229]. Depletion of T-cell subsets that control mucosal defense and homeostasis by limiting bacterial penetration and epithelial barrier integrity and function (i.e., Th-17 and Th-22) has been correlated with the development of intestinal pathogenesis [177,178]. Loss of T helper cells may also facilitate proliferation of opportunistic bacteria and damage to the gut [230]. In support of the direct role played by the CD4+ T cell loss in the gut damage is the observation that in patients with idiopathic CD4 lymphopenia, plasma lipopolysaccharide (LPS) levels are elevated, indicating increased gut permeabilization [231]. (ii) The second mechanism responsible for the gut dysfunction in HIV/SIV infections is through the loss of gut epithelial integrity. In progressive HIV/SIV infection, the excessive gut inflammation induced by virus replication damages the gut epithelium, allowing microbial products to first penetrate the gut mucosa and then translocate into the general circulation [180,219]. Immune cells exposed to these microbial products are subsequently activated through different PRRs, such LPS binding to toll-like receptor 4 (TLR4) [179], and thus lead to further gut damage by either directly fueling virus replication or indirectly through the release of proinflammatory cytokines and excessive cell death [230,232]. Conversely, the natural hosts of SIVs, which do not have progressive infection, have low levels of LPS in the periphery, indicating a lack of microbial translocation throughout infection [179,233]. AGMs were found to rapidly activate and maintain regenerative mechanisms in the gut mucosal tissue, thereby counteracting the vicious cycle [46]. Indeed, intravenous administration of LPS to SIV-infected AGMs resulted in systemic inflammation uncharacteristic of the infection [51,234]. These data were further supported by direct mucosal damage of SIV-infected AGMs through administration of dextran sulphate with similar results: systemic inflammation, T-cell activation, and increased plasma viremia [168]. Conversely, PTMs were treated with sevelamer, which binds LPS, and transiently reduced immune activation, inflammation, and even plasma viremia in the animals [182]. Thus, mucosal barrier damage, microbial translocation, and inflammation/immune activation are irrefutably intertwined.
4.4. Under African Skies—Study of Natural Hosts Demonstrates Important Differences between Pathogenic and Nonpathogenic Infections
The natural reservoir of SIVs is represented by African NHPs. Over 40 species of monkeys in Africa are infected with species-specific SIVs [18]. In their natural hosts, such as AGMs, SMs and mandrills (MNDs), SIV infection appears to be nonpathogenic [18,235,236]. In these species, disease progression is highly uncommon, only occurring in a handful of animals which had greatly outlived their normal life expectancy [237,238].
Extensive studies performed over the last three decades, allowed us to thoroughly characterize the pathogenesis of SIV infections in their natural hosts. Through these comparative pathogenesis studies, we identified similarities and differences between the pathogenic and the nonpathogenic infections, thus establishing features that were specifically associated with the progression to AIDS in the pathogenic infections [239]. The most important shared feature of the pathogenic and nonpathogenic HIV/SIV infections is the robust acute viral replication, followed by high steady-state replication that is higher than in the majority of untreated chronically PWH [68,160,240,241,242,243,244,245]. Meanwhile, African natural hosts similarly undergo a severe CD4+ T-cell depletion at the mucosal sites with the same order of magnitude as that observed in PWH and pathogenic SIV infections, in line with the primary target cell of SIV in African NHPs being the CD4+ T cell [37,39,113,117,233,246,247,248]. Furthermore, the humoral and cellular immune responses are similar between the pathogenic and nonpathogenic SIV infections [13,17,239,249,250,251].
These common features between pathogenic and nonpathogenic infections suggest that the lack of disease progression in natural hosts is not the result of a viral attenuation. Indeed, the rare cases of AIDS documented in African NHPs [237,238] and the observation that direct SIV cross-species transmission from their natural hosts to macaques results in pathogenic infections that progresses to AIDS [70,160,252] confirm that control of disease progression is independent of the virus and instead relies on host adaptations. This likely occurred because of the SIV-African NHP host coevolution occurring over hundreds of millennia [16,22,253,254,255]. This virus-host coevolution allows natural hosts to counteract the deleterious consequences of the SIV infection and resulted in phenotypic features of natural hosts that contribute to the prevention of disease progression to AIDS [16,253,254,256,257,258,259,260,261]. In particular, these would be: few target cells (CCR5+ CD4+ T cells) at mucosal sites [22,247,262,263] and downregulation of CD4 on helper T cells when they transition to memory phenotype [262,264]. The usage of CXCR6 as a coreceptor may also server to further preserve CD4+ cells in AGMs and sooty mangabeys [261,265]. Furthermore, NK cells are found at much higher levels in the lymph node follicles of AGMs than in pathogenic models [266], while also displaying a greater number of terminally differentiated NK cells and increased SIV-specific activity [267]. Thus, this mechanism can help explain the reduced damage occurring in nonpathogenic infection.
The main factor behind the lack of disease progression in the natural hosts of SIVs is their ability to actively control chronic immune activation and inflammation [47,239], the main drivers of disease progression and mortality in PWH [174,175]. Chronic systemic T-cell immune activation and inflammation are kept at bay through an exquisite ability of the natural hosts of SIVs to maintain the integrity of the mucosal barrier throughout the course of SIV infection [48,168], due to specific healing mechanisms recently described [46]. This lack of mucosal dysfunction allows the natural hosts to avert microbial translocation [179,233], in stark contrast to the pathogenic HIV/SIV infections, in which microbial translocation occurs as a result of acute viral replication and proinflammatory responses causing extensive damage to the intestinal mucosa [268].
4.5. How the Heart Approaches What It Yearns—SIV as Models for the Study of HIV-Related Comorbidities
Although ART is able to curb viremia, there is still a disproportionate risk of non-AIDS comorbidities in PWH, with higher rates of CVD, kidney disease, hepatic disease, and other events [269], replacing opportunistic infections as the leading causes of mortality and morbidity. In fact, from 2000 to 2010, AIDS-related deaths in a French study group decreased from 47% to 25% [270], while a multicohort study showed a decrease from 34% to 22% in 1999–2000 to 2009–2011, respectively [271]. The transition from AIDS-related mortality and morbidity to non-AIDS is associated with an increased lifespan for PWH, yet there is still a life expectancy deficit, averaging 8 years less [151,272]. Further, as the PWH population ages, there is an increasing risk of multiple comorbidities arising per individual than in the uninfected population [273].
Due to the differences in natural hosts and pathogenic infections, a method to increasing our understanding of HIV pathogenesis is to compare the two and find differences in host biology. This strategy has allowed for incredible progress in our understanding of HIV transmission, pathogenesis, prevention, and treatment [31,53,274,275]. SIVsab, the SIV that naturally infects AGMs, also infects PTMs. Both infections present with high VLs, but completely opposite disease outcomes. SIVsab-infected AGMs do not progress to AIDS, while SIVsab-infected PTMs present with nearly all pathogenic features of HIV infection and readily progress to simian AIDS [53,169]. As mentioned earlier, comparisons between the two models were integral to understanding immune activation, inflammation, gut dysfunction, and microbial translocation in HIV infection. Indeed, other comorbidities are also investigated with NHP models. PWH are at an undeniably higher risk for CVD [276], which is recapitulated in both SIVsab-infected PTMs and SIVmac-infected RMs. These models present with hypercoagulation, demonstrated by significant increases in D-dimer and thrombin-antithrombin complex. This is especially prevalent in the SIVsab-infected PTMs, where these biomarkers were increased early after infection and associated with cardiovascular lesions and were greatly indicative of progression to AIDS and mortality [51]. Additionally, thrombotic microangiopathy was present in multiple organs, while myocardial hypertrophy, fibrosis, myocarditis and infarction were also observed [51]. This model has also shown that therapeutic interventions for reducing microbial translocation, immune activation, and inflammation resulted in decreased hypercoagulation, further supporting the role of immune activation and inflammation in hypercoagulation [169,182].
Liver dysfunction is frequent in PWH and has multiple sources: (i) infection of the Kupffer and stellate cells in the liver [277,278,279]; (ii) microbial translocation and the chronic inflammation [278,280]; (iii) coagulopathy [52,281]; (iv) cofactors, e.g., hepatitis C virus coinfection [282] and excessive alcohol consumption [283]; and (v) ART toxicity [280]. SIVsab-infected PTMs demonstrated inflammatory infiltrates and hepatic fibrosis, which together resemble chronic active hepatitis [53], and RMs demonstrated that the liver is highly involved in clearing virus from circulation [284]. Further, CD4+ T cells are greatly reduced and CD8+ T cells are highly increased in the liver after SIV infection, indicating the liver as a site of antigenic stimulation and CD4+ T cell depletion [285,286].
Respiratory comorbidities are on the rise with PWH living longer, such as chronic obstructive pulmonary disease (COPD) [287], however the mechanisms are not well elucidated. The SIVsab PTM model was also used to investigate pulmonary lesions that may play a role in the rise of COPD in PWH. In the PTMs, early infection presented with immune infiltrates in the lung parenchyma and near large bronchi. During chronic infection, emphysema and thickened alveolar walls are observed with disruption of the lung architecture and fibrosis, in direct contrast the SIVsab-infected AGMs which presented with no immune infiltration or subsequent lung disruption [53]. The elimination of interstitial macrophages present in the lungs is also a cause for pulmonary disease [288].
Acute renal failure and chronic kidney disease are associated with advanced immunodeficiency and age, therefore greatly increasing the risk in older PWH [289]. HIV-associated nephropathy (HIVAN) can quickly progress to end-stage renal disease and mortality if left untreated [290]. However, like respiratory comorbidities, the mechanisms are not fully known. Although several ART drugs have been associated with kidney damage, they do not explain the full extent of renal disease [291]. It is believed that chronic immune activation and inflammation are likely the main mechanism because early initiation of ART, which allows for better maintenance of immune function, minimizes the risk of kidney disease in PWH [291]. In RMs infected with SHIVKU-1, researchers found the equivalent of HIVAN with glomerulosclerosis and collapsing glomerulopathy [292], and another SHIV-infected RM presented with nephrotic syndrome: peripheral edema, hypoalbuminemia, and proteinuria [293]. In our model of SIVsab-infected PTMs, we have shown similar kidney pathologies to HIVAN, including hyperplasia of the Bowman capsule epithelial lining, glomerulosclerosis and collapsing glomerulopathy, and interstitial nephritis [53].
The rate of HAND in PWH has drastically decreased after the advent of ART, but less severe neurocognitive issues remain and risk increases with age [294]. HAND is a spectrum that includes asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HIV-associated dementia (HAD), with HAD being the most severe form. The spectrum is defined by neuropsychological testing and functional status assessments. The biomarkers accessible by blood for HAND are not very specific: CD4+ T cell count at nadir of depletion, sCD14, sCD163, and viral DNA, all of which can be associated with general progression [294]. Cerebrospinal fluid, however, shows associations with neuronal injury markers, as well as inflammation, demonstrating more specific markers [294]. Further, neuroimaging markers are helpful and functional MRI has demonstrated accelerated aging in the brains of PWH [295]. Animal models allow for invasive approaches and euthanasia further permits investigation into brain pathologies at necropsy with SIV-infected PTMs being the model of choice [296,297].
5. For the Cure, Whenever We May Find Her
5.1. The Need for an HIV Cure
An essential step of the HIV replication cycle is integration into the host genome, whereby it can use host cell machinery to produce its viral mRNA products and RNA genome. Once the viral latency is established, the cells cease to produce viral products, and they can no longer be recognized by the immune system, allowing the provirus to persist in these cells indefinitely [298]. The totality of the integrated proviruses forms the latent reservoir; the HIV-infected CD4+ cells that contain integrated HIV and revert to a resting state with altered gene expression, for example reduced NF-κB, which is normally triggered by T cell activation, results in a pool of hidden, activatable provirus [299]. While ART effectively suppresses the circulating virus [300], the reservoir cells are not impacted by ART, and treatment cessation is always followed by a viral rebound with VL levels similar to those observed pretherapy [301,302,303,304]. The source of this virus rebound is the latent reservoir, which can be reactivated by multiple stimuli inducing T-cell activation and latency reversal. This is the scientific basis of the need for a life-long adherence to ART. ART was one of the greatest achievements of modern medicine, yet long-term toxicity, viral resistance, stigma, and costs, all call for an effective HIV cure aimed at complete HIV eradication from PWH. ART does not completely restore the immune system, nor eradicate HIV. Multiple strategies towards an HIV cure are pursued [305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323], but none effectively curbed the reservoir nor induced robust and durable virus control, except the hematopoietic stem-cell transplantation, which is not scalable and has unreasonable limitations [7,8,324,325]. The major barriers to a successful HIV eradication are: (i) HIV persistence in latently infected cells invisible to immune responses; (ii) inability of a damaged/exhausted immune system to eliminate HIV-infected cells; and (iii) chronic INFL that persists on ART [326,327,328,329,330].
5.2. The Latent Reservoir Currently Prevents HIV Cure
The existence of the HIV latent reservoir is the primary obstacle for cure HIV eradication from the host. The latent reservoir is established immediately following infection, as early as 3 dpi, and prior to detectable viremia [28,29,30,331], in resting CD4+ T cells: [303,304,332,333,334,335] with different immunophenotypes: central memory [312,335,336], transitional memory [312,336], stem cell memory T cells [337], Tregs [338], and follicular T helper CD4+ cells [339]. In addition to the CD4+ T cells, macrophages and monocytes can be latently infected by HIV/SIV [340]. Dendritic cells are suspected to contribute to the reservoir by carrying SIV/HIV virions on their surface [341]. Latently infected cells lack a specific surface marker which would allow specific targeting of the latently infected cells [342] which is one of the major barriers against an HIV cure.
The prospect for an HIV cure became a reality after the success of the “Berlin patient”, a PWH who underwent allogeneic bone marrow stem-cell transplantation to treat acute myeloid leukemia. The donor was chosen specifically for homozygosity for the CCR5 Δ32 allele, and thus without a functional CCR5 coreceptor and resistance to HIV infection; as a result, after two stem cell transplantations, graft-versus-host disease, irradiation, immunosuppressive therapies and whole body irradiation, the Berlin patient presented with a drug-free HIV remission [343] which lasted for 12 years prior to his death. A second patient that underwent a similar procedure with a CCR5 Δ32 allele donor (the “London patient”) is also reported to be in remission [8]. Yet, while cure research got a tremendous boost leading to major improvements in our understanding of the nature of viral reservoirs and of the mechanisms of HIV latency in the decade following this remarkable success story, this procedure is not scalable, and, as such, there were not many subsequent cases of success in this field. The “Boston patients,” which also went through a similar transplantation (yet with stem cells from donors with intact CCR5), rebounded by months 3 and 8 post-ART interruption [344]. As such, these cases demonstrated that standard bone marrow transplantation is not sufficient to cure HIV. Furthermore, while ART can suppress plasma viral RNA to below limits of quantification, cessation of ART results in viral rebound in virtually every situation, including the “Mississippi baby,” who was on ART from 30 h to 18 months of age, and was thought to be functionally cured [345]. In this patient, the virus eventually rebounded 2 years after interruption of ART [346], due to the persistence in the latent reservoir. Additional cases of people believed to have been cured or functionally cured post-cessation of treatment based on conventional measurements of the viral reservoir include the VISCONTI cohort [347] and a South African child [348].
On the other hand, NHP models have demonstrated that early initiation of ART does not prevent the viral rebound post-therapy interruption [28], indicating that the reservoir is established very early in infection, suggesting that interventions aimed at curing HIV infection will need to curb the reservoir rather than prevent its formation. Nevertheless, in the same NHP studies, a delay in virus rebound at the cessation of art was observed in macaques in which therapy was initiated very early, at 3 dpi, prior to detectable viremia. In a case of an PWH treated with allogeneic stem cell transplantation for treatment of acute lymphoblastic leukemia, researchers found that the virus rebounding nearly one year after treatment interruption was phylogenetically distinct from the HIV strain detected in PBMCs prior to transplantation [349]. These rebounds illustrate that not only we do not have an effective cure strategy, but we also have not fully mastered the diagnostic tools necessary for monitoring the effectiveness of various cure strategies, indicating a need for more effective methods and strategies.
5.3. Multi-Trick Pony—Mechanisms of HIV Latency Establishment
HIV latency was first described with in vitro experiments demonstrating that cells that survived infection did not produce virus, but could be induced with 5-iodo-2′-deoxyuridine [350]. Shortly after, studies showed the stimulation of HIV transcription was regulated by the same pathways that induce T-cell activation [351,352,353], which suggested that activated CD4+ T cells were not likely to support latency. However, resting CD4+ T cells poorly support productive infection [354,355]. Thus, the paradigm of reservoir formation became the transition of infected, active CD4+ T cells to a resting state, and it was proven in 1995 that resting CD4+ T cells from PWH can harbor replication competent provirus [356]. In fact, multiple in vitro studies have since supported that infected, activated CD4+ T cells gradually transition back to the resting state and support latent infection [357,358,359,360,361,362,363].
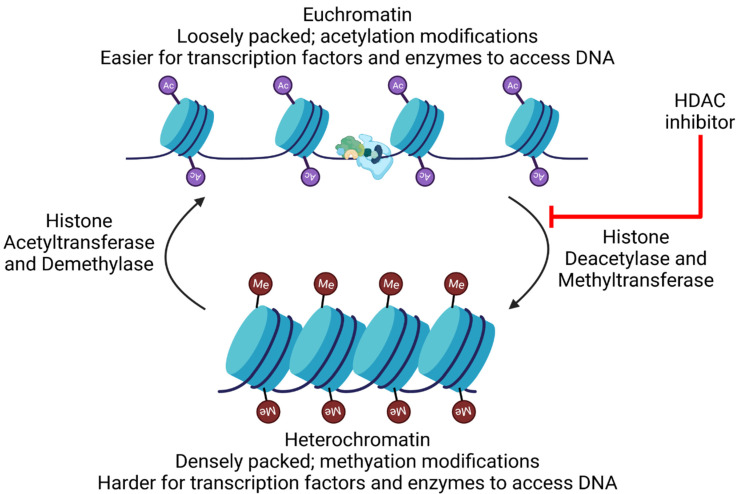
The preferential integration of HIV into transcriptionally active sites [364,365,366] suggests that HIV expression is at least partially independent of the host gene expression. After integration, two nucleosome structures, Nuc-0 and Nuc-1, are formed at the 5′ long terminal repeats (LTR), blocking transcription initiation by RNA polymerase II (RNAP II) [367]. These nucleosomes are associated with epigenetic modifications that contribute to HIV latency: histone deacetylation [368,369,370] and methylation [371,372,373], leading to contraction of the chromatin structure and repression of transcription. Further, not only are the modifications observed, but the histone methyltransferases and deacetylases are also associated with the LTR [368,371,373,374] and recruitment is facilitated by various transacting factors [361,375,376,377]. These data also help explain the strong reactivation potentials of various HDAC inhibitors.
Transcriptional interference is another mechanism driving HIV latency, depending on the relative orientation of the provirus in the host gene. With same sense polarity, the tendency to integrate into active sites can readily cause elongation of the host gene to displace transcription factors at the HIV LTR, thereby preventing transcription initiation [378,379]. When the provirus is integrated in the opposite polarity to the host gene, transcriptional interference manifests with collisions between the elongation complexes of the host gene and HIV transcription [380].
Recruitment of the host factor positive transcription elongation factor b (P-TEFb) from the 7SK small nuclear ribonucleoprotein (snRNP) complex is facilitated by competitive binding of HIV Tat (transactivator of transcription) to HEXIM1, causing the release of P-TEFb [381,382]. P-TEFb then mediates the phosphorylation of RNAP II [383,384] and Spt5 [385], preventing early termination of transcription, which leads to efficient transcription elongation. The bromodomain proteins BRD2 and BRD4 act competitively with HIV Tat for P-TEFb binding, resulting in diminished transcription elongation [386,387]. Thus, it is not surprising that BRD2/4 binding by bromodomain inhibitor JQ1 results in viral reactivation [388,389].
5.4. Reservoir Decay Is Not Curative
Early reservoir decay modeling suggested that maintaining ART for 7.7 years may be able to completely eradicate the latent reservoir [390], yet this has been clearly debunked, with PWH reaching decades without complete clearance on ART. Newer modeling from PWH on ART indicates that the half-life of total HIV DNA is 42 years, whereas the intact provirus half-life is 7 years [391], thereby negating the theory of eradicating HIV-infected cells solely through sustained ART. The data demonstrate that early ART initiation is beneficial for the rate of reservoir decay [391], but still not enough to eliminate the reservoir.
5.5. Somewhere Researchers Cannot Find Me: Technical Obstacles towards Reservoir Quantification
A technical obstacle towards the eradication of the latent reservoir is the lack of a proper quantification of the inducible virus. Initial measurements used cell-associated HIV DNA (caDNA) to quantify the latent reservoir [301,356,392], but it soon became clear that only a fraction of these cells were capable of producing infectious virus [332], thus demonstrating an inherent issue with measuring caDNA: not all cells may be relevant for the recrudescence of infection after ART cessation. Full genome sequencing revealed that the proviruses forming the latent reservoir are both intact and defective [393], further diminishing the significance of caDNA as a measurement of the inducible virus. Reservoir quantification took a step further when a the quantitative viral outgrowth assay (qVOA) was established to be the gold standard for measuring the inducible virus [332]. The qVOA dilutes purified, resting CD4+ T cells from HIV donors and activates them with a stimulant (e.g., phytohemagglutinin [PHA] [332], phorbol 12-myristate 13-acetate [PMA] and ionomycin [394], or anti-CD3/CD28 [303,395]) in the presence of feeder cells (irradiated PBMCs). The original method of activation (PHA) was shown to induce activation in nearly all resting T cells [396], and minimal differences were seen with the other activation methodologies [397]. However, qVOA is time consuming because it requires that stimulated cells are cultured for 14–21 days so that enough p24 can be generated for quantification via ELISA [335]. An alternative method of using PCR as the end quantification [398] decreases time consumption, but has its own issue of viral RNA being produced from a fraction of defective proviruses, thus artificially increasing the size of the replication competent reservoir [399].
An additional problem with the qVOA is that the in vitro stimulation lacked efficacy in reactivating all of the replication-competent viruses from the purified CD4+ T cells, as demonstrated by the observation that multiple rounds of cell activation yielded additional virus [400,401] and sequencing with subsequent infection of cells in vitro confirmed replication capabilities of wells negative for viral outgrowth [400]. Beyond the immediate ramifications towards quantification, this also pointed towards another barrier of HIV cure: HIV-infected T cells can activate and clonally expand without reactivating virus, thereby avoiding the immune response while bolstering the reservoir size [402]. A way to mitigate the problem of incomplete activation, is to perform sequencing for determining the percentage of provirus that has intact provirus.
For full-genome sequencing, researchers extract genomic DNA and use nested PCR with limiting dilutions. The PCR products are then run on agarose gels and extracted for sequencing [403], thus this technique minimizes errors, but it also is highly time consuming and intensive. Unfortunately, reducing the time constraints and labor by using subgenomic sequencing introduces detection and accuracy problems due to either defects in regions outside of the amplified region or deletions overlapping the amplified region [404]. Utilizing next-generation sequencing is one method to increase efficiency and cost effectiveness [405,406,407] and has higher sensitivity than Sanger sequencing, allowing for a better detection of mutations [408]. The recently developed intact proviral DNA assay (IPDA) is based on digital droplet PCR (ddPCR) multiplex technology [409]. It uses primers against conserved regions of env, the packaging signal (PS) and Rev-response element (RRE), to elucidate defective versus intact provirus. The benefit of this assay is that it requires few cells (5 million CD4+ T cells) and does not have the inefficiency of long-distance PCR. The caveat is that by only detecting a small region of the genome (~2%), the IPDA can easily miss other defects that would render the virus replication incompetent [409] and also has issues with polymorphisms affecting detection [410]. To mitigate this issue, a combination of quadruplex qPCR and NGS, termed Q4PCR was developed. Like IPDA, Q4PCR also uses the PS and RRE regions, but also includes primers for pol and gag. Using Q4PCR with NGS showed that IPDA had high variability in detection of true intact provirus due to missing polymorphisms outside of the amplified sequence [411]. Additional head-to-head comparisons between these two methods are warranted.
Nonetheless, the proviral sequencing demonstrated that only a small fraction (~5–7%) of the proviruses are intact, regardless of the timing of ART initiation [393] and accounts for around 60 per million CD4+ T cells [400], a 60-fold increase in the number of replication competent virus estimated by qVOA [412]. These data were initially promising for the eradication of HIV, as it suggested the possibility of eliminating far less infected cells than previously thought. However, studies demonstrated that the ability of defective proviruses to produce viral proteins may be stimulating the immune system, thus contributing to the viral pathogenesis [399,413,414].
5.6. SIVmac-Infected RMs as a Model for Cure Research
In addition to the general roadblocks to cure, there are specific limitations to cure research in humans [31,415]: (a) ART cannot be stopped without the risk of emergence of drug-resistant strains; (b) residual viral replication prevents proper characterization of the reservoir; and (c) invasive sampling of multiple potential reservoir sites is limited. These limitations make use of animal models imperative for the study of the viral reservoir and for testing cure strategies. Although humanized mice have potential for cure research [416,417,418,419,420], size limitations of individual animals prevent detailed reservoir assessment. Therefore, the model of choice is the SIVmac-infected RM on ART.
HIV and SIV share key features of virus persistence: (a) HIV/SIV DNA are similarly integrated in the target cell genome [421,422,423]; (b) response to interferons results in transcriptional control of long terminal repeat sequences (LTRs) through histone acetylation favoring HIV/SIV DNA persistence [424]; (c) costimulatory signals induce latent HIV/SIV without co-engagement of T cell receptors [425]; and (d) distribution of cells containing HIV/SIV DNA and RNA sequences in blood, LNs, and mucosal sites are similar in humans and RMs [164,165,166]. SIVmac infection of RMs reproduces all the stages of HIV infection in a shorter time frame. These characteristics demonstrate similar reservoir dynamics between HIV and SIV infection. Historically, SIVmac was difficult to control with ART, requiring complex and expensive drug combinations [426]. Emergence of new integrase inhibitors and use of coformulated drugs now allow SIVmac suppression with ART regimens that are similar to, or the same as, those used in HIV infection [427], thus further establishing SIVmac-infected RMs as the gold standard model.
6. Still Searching after All These Years: Strategies towards an HIV/SIV Cure
Numerous strategies have been proposed for the elimination of the viral reservoir: (i) ART intensification [428,429,430,431]; (ii) “block and lock” permanent transcriptional silencing [307,432]; (iii) gene editing of CCR5 [433]; (iv) chimeric antigen receptor (CAR) T cells [322,434,435]; (v) apoptosis promotion and viral cytopathic effect enhancement [436,437]; (vi) bone marrow transplantation [344]; (vii) broadly neutralizing antibodies [438,439]; (viii) vaccines and therapeutic vaccines [440]; (ix) regulatory T cell (Treg) manipulation and depletion [338,441,442]; (x) use of checkpoint inhibitors to enhance HIV-specific immune responses [443,444,445,446]; and (xi) “shock and kill” (Figure 3). However, these have been met with limited success.
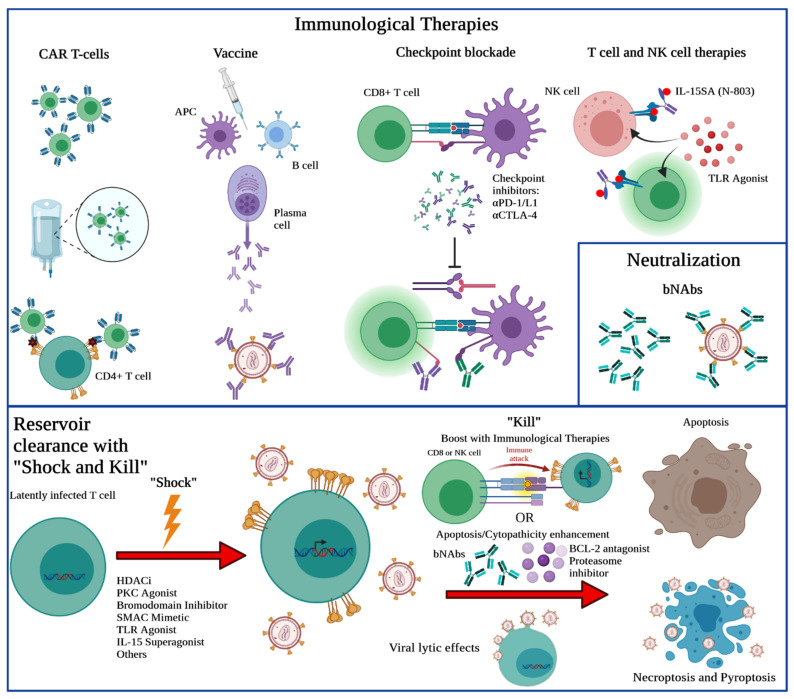
Figure 3.
Cure strategies focusing upon clearance of the latent reservoir. There are many different therapeutics that are being investigated for HIV cure. CAR T-cells are engineered with chimeric receptors to better respond to virally infected cells and eliminate them. Vaccines can induce B cell production of antibodies against virus, but also T-cell responses to help clear the infected cells. PD-1/L1 and CTLA-4 are suppressors of T-cell activation and by blocking these interactions, there can be increased T-cell activation. Il-15 superagonist and TLR agonists can be administered to individuals to bolster the cell-mediated immune response. Broadly neutralizing antibodies (bNAbs) can be directly administered to neutralize virus. Of note, eradication of the latent HIV reservoir and establishment of potent immune responses may result in eventual functional or sterilizing cure and will likely require combination approaches as shown in this schematic with various potential drugs, small molecules, or interleukins as treatment.
In spite of some promising results being reported by previous studies, none of the LRAs tested so far showed enough potency to justify their large-scale use as HIV cure agents [394,447,448]. In addition to this lack of efficacy resulting in insufficient reservoir reactivation [448], some LRAs were reported to induce a massive, indiscriminate T-cell activation that can be detrimental to the host health or even lethal [449,450]. Finally, some LRAs, particularly HDACi, were reported to have a negative impact on the cell-mediated immune response [451,452,453,454].
Other cure strategies also have issues with efficiency, be it the lack of reducing residual viremia or reservoir size with ART intensification [455], insufficient virus reactivation with Treg manipulation [441] and additional toxicity when combined with ART [442], lack of viral clearance in response to checkpoint inhibitors [443,444], inherent resistance and escape mutations against broadly neutralizing antibodies [456], lack of great enough efficiency and long-term stability of gene therapies and CAR T cells [322,457], or safety concerns of bone marrow transplants coupled with the lack of success [344,458]. As such, after more than a decade of intensive research, the end is still not in sight.
6.1. ART Intensification as a Cure Strategy
ART intensification was thought to potentially alleviate some of the residual virus replication in patients on ART [459,460]. However, several studies have demonstrated that ART intensification does not solve the issue of low-level residual replication. In fact, efavirenz, lopinavir/ritonavir, and atazanavir/ritonavir [428], raltegravir [461], dolutegravir [462], and maraviroc [463] were all used to intensify ART, to no avail. Due to the major role of the gut in HIV pathogenesis, another study focused upon the gut when attempting ART intensification with raltegravir and maraviroc. With this combination, there was no benefit to the immune populations of the gut, decreases in inflammatory markers beyond what was seen in the control group, further demonstrating the futility of ART intensification [464].
6.2. “Block and Lock”—Transcriptional Silencing for HIV Functional Cure
HIV transcription involves both viral and cellular machinery. During viral production, HIV initially transcribes short, completely spliced transcripts that create the Tat and the regulator of virion expression (Rev). Tat acts as an autoregulator for HIV and binds to the HIV transactivation response element (TAR) of the HIV promoter. This allows for the recruitment of the RNAP II elongation factor, P-TEFb, that results in transcription initiation and elongation [465,466]. One strategy for an HIV cure is to force the viruses into latency, by blocking viral transcription and locking the viral promoter into a late state, thereby preventing disease progression. This is called the “block-and-lock” strategy, and it utilizes antagonists to viral proteins or host transcription machinery [467]. There are several different targets of “block-and-lock”, but the most investigated is the use of Tat inhibitors, due to Tat’s role in HIV transcription [465,466]. NullBasic was the first Tat-inhibitor developed in 2009 and is comprised of a transdominant Tat mutant which is meant to outcompete wild-type Tat [468]. In vitro, NullBasic-expressing cells produced significantly fewer virus and reduced the efficacy of viral reactivation after PMA stimulation. However, this protein did not completely inhibit production of full-length mRNA and also had to be stably expressed in the cells to silence transcription [469]. Didehydro-cortistatin A (dCA) [470] is currently the most advanced small molecule inhibitor developed. In cell cultures, dCA was shown to inhibit viral activation and even inhibit reactivation after stimulation with prostratin [471]. When administered to HIV-infected, ART-treated, humanized bone marrow/liver/thymus (BLT) mice, treatment of dCA significantly decreased the aggregate number of viral RNA copies vs. controls and increased the time to viral rebound after analytic treatment interruption. There, all mice rebounded by 10 days post-interruption in the control group and 19 days post-interruption in the dCA group [471]. Unfortunately, other block-and-lock small molecule inhibitors, such as HSP90 inhibitors, Jak-STAT inhibitors, and kinase inhibitors, are more prone to side effects, due to their roles in host transcription [472]. In fact, characterization of CDK and the mammalian target of rampamycin (mTOR) inhibitors indicated that due to cellular toxicity, the vast majority these inhibitors had to be discarded [473]. This illustrates the difficulty of developing small molecular inhibitors against host proteins for HIV silencing.
Finally, transcriptional gene silencing was another method of silencing that different groups tested. It is based on the use of short hairpin RNAs and short interfering RNAs that are able to reduce viral burden, but run into issues with delivery methods [467].
6.3. Gene Therapy and Engineered CAR T Cells for HIV Cure
In vitro and ex vivo gene therapy with CRISPR/Cas9 has been able to disrupt proviruses [474,475,476], but the lack of a systemic delivery mechanism and of target effects in humans hinders gene therapy as an HIV intervention [477]. Nonetheless, advancements in gene therapy have allowed for engineered immune cells to be used to combat HIV. For instance, engineered T cells with CAR against HIV have shown promise in eliminating HIV, but are still hindered in vivo by lacking sustained activity of the cells for the time period necessary for eradication, off target effects, the threat of CAR immunogenicity, and the possibility of inducing a cytokine storm in the patients [322]. One of the large issues with CAR T cells is protection of the CAR T cells against HIV infection. Thus, researchers developed conjugated coreceptors, C34 conjugated to CCR5 or CXCR4, and tested them in vitro. These were found to be able to protect against HIV infection, and a conjugate of CXCR4 and 34 peptides from the heptad repeat domain 2 (HR2) of gp41 (C34) demonstrated better protection than the C34-CCR5 conjugate [478]. Following these results, new dual CD4-CAR T cells, expressing both 4-1BB/CD3-ζ and CD28/CD3-ζ ectodomains with a co-expressed C34-CXCR4 fusion inhibitor, to protect against HIV infection, were tested in humanized BLT mice. The results demonstrated elimination of infected cells in vivo, including memory CD4+ T cells, while also reducing the loss of CD4+ T cells during acute phase, and decreasing plasma viremia and cell-associated HIV DNA (from memory CD4+ T cells). However, the protection from HIV was eventually lost over time, demonstrating the need to develop chimeric cells with complete resistance to HIV [479].
6.4. Enhancing Apoptosis and Cytopathic Effects as a Cure Strategy
Due to the production of the HIV protease, infected cells are pushed to a pro-survival state. The HIV protease cleaves procaspase 8 to create Casp8p41, which is capable of binding to BAK and inducing apoptosis [480,481,482,483]. However, in cells producing high levels of the antiapoptotic protein BCL-2, such as central memory CD4+ T cells, which form the bulk of the HIV/SIV reservoir, Casp8p41 binds to BCL-2 instead of BAK, thereby neutralizing the proapoptotic ability of Casp8p41 and inducing the pro-survival phenotype. Transcriptional profiling of CD4+ T cells that survived coculture with HIV-specific CTLs demonstrated that BCL-2 is a major overexpressed marker, further substantiating its role in persistence. Venetoclax is a Bcl2 antagonist, which was shown to induce the death of HIV-infected cells in vitro, with a strong selectivity towards infected cells [436,437]. Another proapoptotic drug is ixazomib, a proteasome inhibitor. Because BCL-2-Casp8p41 complex together and become polyubiquinated and then degraded at the proteasome, the use of a proteasome inhibitor prevented degradation of Casp8p41, allowing for increased activity and binding to BAK. When administered to cells in vitro, ixazomib was able to increase death of HIV-infected cells with the added benefit of reactivating HIV via NF-κB [484]. It thus appeared that the combination of the two drugs is ideal for first increasing viral reactivation, then induce a proapoptotic state, while preventing the decay of the Casp8p41. When this combination was attempted in vitro, the reduction of HIV-infected cells surpassed that of either drug alone. However, when used ex vivo, the nonspecific toxicity was overwhelming [485]. Meanwhile, when venetoclax was administered ex vivo after bryostatin-1 stimulation, there was insignificant clearance of infected cells, but when venetoclax was combined with HIV-specific CTLs, there was a modest decrease in the amount of HIV-infected cells and when anti-CD3/CD28 was used instead of bryostatin-1, there were significant decreases of the infected cell counts [486]. These data thus support the use of BCL-2 antagonists, but also show that more potent, safe LRAs are needed for this strategy, because anti-CD3/CD28 stimulation cannot be used in vivo for safety reasons [487,488].
6.5. Bone Marrow Transplant for HIV Cure
The only two cases of cured PWH have been the “Berlin patient” [343] and “London patient” [8], both of which were treated with allogeneic bone marrow stem-cell transplantation with donors homozygous for the CCR5 Δ32 allele. This resulted in reconstructed immune systems that lacked functional CCR5 coreceptors and thereby conferred resistance to HIV. As much as these two cases bring hope to the world of HIV, they are unfortunately alone. The “Boston patients” tried to recapitulate the results without the CCR5 Δ32 allele and resulted in viral rebound [344]. Additional follow-up attempts have also not been successful [489], especially with the emergence of non-CCR5 tropic virus negating the use of the CCR5 Δ32 [490] and rebound regardless of reservoir quantification [491]. The overall safety of hematopoietic stem cell transplantation is also a heavy burden that restricts this strategy [489].
6.6. Broadly Neutralizing Antibodies for the HIV Cure
The use of broadly neutralizing (bn) antibodies (bnAbs) stem from the study of monoclonal antibodies (mAbs), the first of which being b12, which was isolated from a PWH in the 1990s [492]. bnAbs allow for virus elimination through multiple mechanisms: (i) binding blocks the virus from interacting with host cell receptors and can prevent endocytosis, fusion, or penetration [493]; (ii) aggregation of virions due to antibody binding [493]; (iii) recognition by intracellular TRIM21 and targets to proteosome [494]. Antibodies can also target infected cells for antibody-dependent cellular cytotoxicity, although this is considered a function of non-neutralizing antibodies [495]. About a decade ago, the first bnAbs were isolated from a PWH that displayed potent neutralization specific for the CD4 binding site (CD4bs) of gp120. The primary bnAb studied from this individual, VRC01, neutralized 91% of 190 viral strains representing all major circulating HIV-1 clades; the other two bnAbs from the same individual were not as potent. In comparison, b12 was only able to neutralize 41% of isolates [496]. Other bnAbs have since been identified, with other affinities, e.g., the V3 loop and its glycans, the MPER, and the V1-V2 loop of gp120, and are reviewed in [497]. NHP studies demonstrated efficient protection by various bnAbs against SHIV challenges [498,499,500,501,502,503]. Phase I clinical trials have reported the safety of bnAbs in humans [504,505,506] and follow-up trials have now demonstrated that bnAbs are able to suppress viremia. Additionally, administration of bnAbs 3NBC117 and 10-1074 to PWH during ATI resulted in increased Gag-specific CD8+ and CD4+ T cell responses, as well as lengthened viral suppression [507]. However, there are caveats to the results: (i) suppression is short lived [508,509,510]; (ii) patients developed anti-bnAb responses [508,509], although they were left susceptible to bnAbs targeting different epitopes; (iii) lackluster prevention of virus via cell-to-cell transmission [511,512,513]. Thus, groups are working to develop bnAb cocktails that will cover multiple epitopes for neutralization to potentially enhance efficacy [514,515], including the development of bispecific [516] and trispecific [439] antibodies. bnAbs have been tested in RMs to either cure or induce functional cure. SHIV-infected RMs were treated with bnAbs 3BNC117 and 10-1074 3 days postinfection. In both groups, viremia was controlled while bnAbs were detectable and rebounded after clearance. Postrebound viral control occurred in half of the RMs [517]. However, treatment at 3 dpi lacks clinical relevancy. Thus, repeated experiments were conducted at 14 dpi and ART-treated RMs were included. Postrebound control was partial, and only one animal controlled below the limit of detection. ART did not result in more postrebound controllers [518]. In both studies, CD8+ T-cell depletion resulted in abrogated viral control [517,518], supporting the role of CD8+ T cells in viral control. BnAbs have also been combined with other cure agents. bnAb PGT121 was combined with TLR7 agonist GS-9620 and administered to SHIVSF162P3-infected RMs on ART (initiated 7 dpi). This resulted in delayed viral rebound, with 45% not rebounding and neither adoptive transfer nor CD8+ T-cell depletion demonstrated replication competent virus in those RMs [519]. A follow-up study started ART at 14 dpi to increase clinical relevancy [520], and received a different TLR7 agonist, GS-986, with both bnAbs PGT121 and N6-LS. However, treatments did not result in the same viral control, while still being associated with a delay in viral rebound [520]. Another recent study utilized different combinations of IL-15 superagonist, N-803, with bnAbs 10-1074 and 3BNC117. The combination of N-803 and 10-1074 had an efficacy of postrebound control of 60%, while N-803 + 10-1074 and 3BNC117 had a postrebound efficacy of 75% [521].
6.7. HIV Vaccines for HIV Prevention or Therapeutics
An HIV vaccine is still not in our grasp. Of the main roadblocks against the development of an effective HIV vaccine one is that no individual has been known to have established an HIV infection and spontaneously completely cleared the infection. A small fraction of PWH spontaneously control the plasma viremia to below the limits of detection and are known as elite controllers, but the mechanisms of their viral control have yet to be elucidated and replicated in viremic individuals [522]. Furthermore, the elite controllers are not eradicating the virus, thus being only useful as models of a functional cure. To date, HIV vaccine clinical trials were not successful: the STEP trial had to be discontinued after the first interim review found non-efficacy and, in fact, increased the risk for HIV acquisition in those that were already Ad5 seropositive [523]. Another vaccine strategy based on the use of the canarypox vector (ALVAC) surpassed the threshold necessary for 50% vaccine efficacy in phase 1–2 trials [524] and continual analysis after a 12-month booster showed increased IgG binding antibody response rates and CD4+ T-cell response rates [525]. Unfortunately, after the advancement to a phase 2b/3 trial, HVTN 702, the interim analysis determined that there was no significant difference between infection rates of those vaccinated and thus determined inefficacious [526]. A third vaccine strategy is the use of mosaic vaccines which are focusing on eliciting a CD8+ T-cell-mediated control [527,528]. These vaccines use mosaic proteins, polyvalent antigens formed from peptides of natural sequences determined by computer modeling for the optimal coverage of epitopes [529]. A phase I/IIa clinical trial found that the mosaic Ad26 prime with Ad26 + gp140 boost vaccine regimen was highly immunogenic, eliciting strong binding antibody responses, antibody-dependent cellular phagocytosis responses, and T-cell responses. Further, in RMs they found it produced 66% protection from six SHIV-SF162P3 challenges [528]. This vaccine regimen is now being tested in two clinical trials, HVTN 705 (HPX2008; imbokodo) and HVTN 706 (HPX3002; Mosaico). However, the imbokodo study only provided a 25.2% efficacy estimate against HIV infection and was discontinued after phase 2b [530]. Another vaccine using a recombinant modified vaccinia Ankara-based (MVA-B) vaccine with gp120 and a fused Gag-Pol-Nef polyprotein was found to be well tolerated and increased T cell responses against Gag, but did not change the latent reservoir or time to rebound after ATI [531].
Another vaccine strategy that is based on the use of the RhCMV vector was reported to induce a functional cure in half of the vaccinated monkeys when administered prophylactically; cell-associated DNA was detectable without disease progression [34,532,533]. However, given as a therapeutic, the RhCMV/SIV vaccine did not result in control of infection [30]. To try to improve upon their RhCMV vaccine, Hansen et al. [33] deleted the Rh110 gene to suppress lytic capabilities. This ΔRh110 RhCMV/SIV vaccine enabled viral control and progressive clearance in 59% of vaccinated RMs. Further, 75% of the RMs which cleared viremia were able to control a second challenge 3 years later. An adapted human CMV (HCMV) vaccine was tested in uninfected persons to try to mimic the same unconventional MHC II CD8+ T cell responses seen with the RhCMV vector but were unsuccessful [534]. Peptides-based strategies are also used for HIV vaccines [535,536]. Early peptide vaccines were moderately immunogenic in humans [537,538] and lacked protection in most recipients [539]. Currently, the VAC-3S vaccine utilizes a gp41 motif (3S) with the CRM197 carrier protein to induce responses against HIV and increased CD4 restoration and reduced PD-1 expression, suggesting an improvement of T-cell exhaustion [540]. The Vacc-4x is another peptide-based vaccine that uses p24gag domains. Vacc-4x did not result in protection, but viral set point was reduced after ATI [541]. Vaccinations several years later, in the same participants, resulted in decreased viral DNA and maintained a reduced viral set point at ATI, but protection remained elusive [542]. However, not all peptides are used for vaccines and are reviewed in [543]. Enfuvirtide is a synthetic peptide that binds gp41 and blocks fusion with the host membrane and is a current HIV therapeutic [544] and Maraviroc blocks CCR5 binding [545]. More recently, a short HIV fusion inhibitory peptide with a longer half-life was developed, IBP-CP24, to overcome the half-life and resistance issues of Enfuvirtide. IBP-CP24 shows promising results with a half-life 14-fold greater than enfuvirtide while reducing plasma viremia in HIV-infected humanized mice [546]. At the extreme end of vaccination are two vaccination strategies: (i) an engineered herpesvirus that expresses all nine SIV gene products [547]; (ii) vaccine regimen with DNA, modified vaccinia Ankara, VSV, Ad5, RM rhadinovirus, and DNA a second time, to achieve vaccination containing the entirety of the SIV Env [548]. From the herpesvirus vaccine, 4/6 animals were protected through six intravenous challenges within four months, supporting future investigation [547]. Unfortunately, the sequential proteome-based vaccine method was unable to elicit protective immunity against SIVmac239, demonstrating the difficulty of SIV/HIV vaccines [548].
6.8. Targeting Tregs as a Strategy for Cure Research
The suppressive function of Tregs during HIV infection and the correlations between Treg frequency and HIV/SIV-specific immune responses have led to the strategy of Treg depletion and manipulation. Tregs can be latently infected with HIV and represent a potentially important HIV reservoir: (a) they expand in blood and tissues during chronic HIV and SIV infections [549]; (b) The Treg fraction containing HIV/SIV DNA is higher than in non-Tregs in PWH on ART [550] and RMs [551]; (c) Tregs are less susceptible to cell death than conventional T cells [549]. Meanwhile, during acute infection, Tregs may decisively contribute to the rapid seeding of the HIV reservoir by reversing CD4+ T cell immune activation. Finally, during chronic HIV/SIV infection, multiple lines of evidence support a Treg involvement in suppressing protective effector immune responses against HIV: (a) Treg expansion correlates with loss of CTL function [552,553,554]; (b) ex vivo Treg depletion from blood and LNs enhances T-cell responses to HIV/SIV antigens [549]; (c) HIV nonprogressors have a high perforin/FoxP3 ratio; (d) HLAB27+ and B57+ HIV-specific CD8+ T cells from controllers evade Tregs [555,556]. With the suppression of HIV/SIV-specific immune responses by Tregs, and the potential impact it may have on “shock and kill” efficacy, Treg depletion/manipulation may represent a strong cure strategy. Here, through a single intervention, we could directly reduce the reservoir size (via Treg killing), reactivate the virus, and boost cell-mediated immune responses (Figure 4).
Figure 4.
Treg depletion decreases suppressive signaling and allows for T cell activation and latency reactivation. Through the production of various cytokines and immune checkpoints, Tregs suppress other immune cells towards the resting state. By depleting Tregs with various agents, e.g., IL-2-diphtheria toxin or αCCR4, this should allow for activation of T cells to occur and thereby reactivate latent virus as well as bolster reservoir clearance by no longer impeding the cell-mediated immune responses.
However, Treg depletion has its own issues because Tregs are beneficial through the suppression of general immune activation. Additionally, because the best marker for Tregs, FoxP3, is intracellular, targeting in vivo requires the use of less specific markers. Nonetheless, there are several other targets that have been tested for Treg depletion, such as: targeting CD25, CCR4, and GITR, while low dose cyclophosphamide is used through a different mechanism.
6.8.1. Targeting CD25
Denileukin difitox (ONTAK), IL-2-diphtheria toxin conjugate, has been used to deplete Tregs [557,558]. IL-2 binds to its receptor, CD25 which is enriched on Tregs, allowing the conjugated diphtheria toxin to induce cell death after entry [559]. In cancer patients Ontak showed some efficacy [560,561,562,563,564]. In SIVsab-infected RMs, Ontak depleted ~80% of circulating Tregs and resulted in massive increases in T-cell activation, bolstered SIV-specific immune responses, and viral reactivation [441]. A follow-up study in SIVsab-infected RMs with a new anti-human, bivalent IL-2-DT [565,566] demonstrated similar positive results of depletion of peripheral Tregs, including partial depletion in the lymph nodes (>50%) and intestines (25%), immune activation, viral rebound up-to 103 vRNA copies/mL, and bolstered SIV-specific CD8+ T cell responses [442]. However, when bivalent IL-2-DT was given to functionally cured RMs on ART, there were severe adverse effects necessitating the suspension of treatment [442]. Further, the specificity of Treg depletion was substantially hindered, with CD4+ and CD8+ T cells being greatly depleted, and there was no viral reactivation despite the immune activation [442].
6.8.2. Targeting CCR4
Tregs express high levels of CCR4, especially compared to conventional T cells [567,568,569], and can be used as a coreceptor for HIV [570]. Similar to IL-2-DT, an anti-human CCR4-DT conjugate immunotoxin was tested in RMs. Treatment resulted in limited depletion of Tregs relative to IL-2-DT, with greatly limited depletion in the lymph nodes [571]. The anti-CCR4 monoclonal antibody, mogamulizumab, also showed promise against CCR4+ malignant cells and CCR4+ Tregs [572,573]. Unfortunately, the use of anti-CCR4 immunotoxin in functionally-cured RMs did not result in viral reactivation, regardless of prominent immune activation and proficient Treg depletion [442].
6.8.3. Targeting GITR
Glucocorticoid-induced tumor necrosis factor receptor (GITR) is a member of the tumor necrosis factor receptor family and is expressed on T lymphocytes [574]. The main function of GITR is to protect T lymphocytes from activation-induced cell death [575]. Tregs express more GITR on their surface than non-Tregs and signaling through GITR for Tregs results in suppression of activity [576], different from non-Tregs which results in activation [577,578]. Therefore, anti-GITR antibodies have been explored for the possibility to deplete Tregs and inducing both indirect and direct T-cell activation [579]. In HIV-infected cells, it has been demonstrated that GITR signaling protects infected cells from apoptosis [580]. Thus, although GITR targeting has primarily been established for cancer immunotherapy with some promising results [579,581,582], this can readily be applied to PWH.
6.8.4. Cyclophosphamide (Cy) for Treg Depletion
Cy is a long-standing chemotherapeutic agent, which acts as a nonselective cytoreductive agent in normal chemotherapeutic doses [583,584,585,586]. In low, metronomic dosages, Cy maintains its antitumor properties, but results in decreased adverse effects while improving responses to treatment [587]. Additionally, the low dose treatments result in selective depletion, as well as reduction of suppressive function, of Tregs [588,589,590]. Treg selectivity is attributed to decreased DNA repair and decreased production of glutathione, which eliminates acrolein toxicity [591]. In persons treated with low dose metronomic Cy for four total weeks, Treg depletion occurred and resulted in bolstered cell-mediated cytotoxicity [592]. However, in SIVsab-infected RMs, low dose Cy did not result in acceptable Treg specificity, nor did it greatly induce T-cell activation nor viral reactivation [593]. With additional toxicity occurring when Cy is combined with ART [593], Treg depletion via Cy loses feasibility.
6.9. T-Cell Exhaustion and Targeted Therapies
Tackling the immune system dysregulation in PWH is an important goal of any cure approach. During HIV infection, T cells, especially CD8+ T cells, gradually lose their effector functions, in a state known as T cell exhaustion, which was first described in chronic lymphocytic choriomeningitis virus-infected mice [594]. In fact, in HIV progressors, the basal phosphorylation levels of proteins downstream from T cell receptor signaling were increased and correlated with impaired signaling [201]. These cells are marked by expression of immune checkpoint molecules, such as cytotoxic T lymphocyte-associated molecule-4 (CTLA-4) [200], programmed cell death-1 (PD-1) [196,197,198,199], T-cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT) [595], and lymphocyte activation gene-3 (LAG-3) [596], as well as glycoprotein T-cell immunoglobulin and mucin domain-containing molecule 3 (Tim-3) [315,597]. PD-1 and LAG-3 expression levels are even correlated with time to virus rebound after ART cessation [598].
6.9.1. CTLA-4 Blockade in HIV Cure
CTLA-4+ CD4+ T cells are enriched for replication-competent virus [200]. During HIV infection, CTLA-4 prevents proper stimulation of HIV-specific immune cells, thereby protecting infected cells [599,600]. In PWH treated with Ipilimumab (α-CTLA-4 mAb), there was minimal effects on viremia, although single copy assay demonstrated a trend of decreased viral production [443]. In chronically infected animals treated with CTLA-4 blockade, similar decreases in viremia were noted, but there was also increased SIV-specific immune response [444], indicating therapeutic effects of CTLA-4 blockade. CTLA-4 blockade in ART-treated RMs increased T-cell activation and viremia, but did not augment responses to vaccination, nor increase SIV-specific responses [601]. This is contrary to a mouse study which demonstrated increased HIV-specific B-cell and T follicular helper responses with CTLA-4 blockade combined with HIV virus-like particle vaccination [602]. Thus, the evidence points towards a potential usage for CTLA-4 blockade as an immune boosting agent when combined with an LRA, as standalone CTLA-4 blockade does not potently reactivate virus.
6.9.2. PD-1 Blockade in HIV Cure
PD-1 expressing CD4+ T cells during HIV infection are also enriched for inducible virus and blockade with nivolumab (anti-PD-1) administered to a patient resulted in increased cell-associated unspliced RNA, yet not plasma viremia, consistent with slight latency reversal [199]. A PWH with advanced nonsmall cell lung cancer was given nivolumab and monitored. The patient had insignificant changes in the plasma viral loads, with an increase in cell-associated DNA which normalized one month later. Overall immune activation markers remained stable, although there were increases in IFN-γ+ CD8+ cells and cell counts [603]. More promising data emerged from another nivolumab-treated lung cancer PWH. In this individual, plasma viremia demonstrated latency reactivation beginning at D14, while HIV DNA was decreased, and concomitant immune activation increased along with HIV reverse transcriptase and Nef-specific CD8+ T cells as well, thus pointing towards a shock and kill mechanism [604]. Unfortunately, the effects of PD-1 blockade are simply inconsistent between patients, as another study demonstrated divergent data from the others, with no consistency between changes in cell associated DNA, RNA, or plasma viremia, as well as the HIV-specific immune responses [605]. However, ex vivo treatment of pembrolizumab (monoclonal anti-PD-1 antibody) with the latency reversing agent bryostatin was able to drastically, and significantly increase the amount of virus induction [606]. The ectonucleotidase CD39, which converts ATP to AMP (subsequently converted to the immunosuppressive adenosine by CD73) [607], is used to identify terminally exhausted CD8+ T cells, which are often coexpressing PD-1 [608]. In CD39+ CD8+ T cells, the adenosine receptor, A2aR is expressed at higher levels in PWH, especially in the treatment naïve. Further, in vitro combination inhibition of PD-1 and A2aR was synergistically more effective than either inhibition standalone in rescuing CD8+ T cell function [609]. Thus, CTLA-4 and PD-1 blockades are likely to be most helpful when used in combination with other therapies and/or more potent LRAs.
6.9.3. IL-15 for HIV Cure
IL-15 is associated with the generation and survival of CD8+ T cells [610,611], including HIV-specific CD8+ T cells and is investigated as an ART alternative or enhancement [612]. IL-15 enhances NK cell activation and function [613]. In RMs, IL-15 induced proliferation of SIV-specific CD8+ T cells but did not increase functionality [614,615]. The combination treatment of IL-15 after latency reversal with vorinostat resulted in increased clearance of infected cells [613]. However, free IL-15 administration can be very toxic [616], leading to the development of safer and more effective IL-15 superagonists. The heterodimeric IL-15/IL-15Rα increased CD8+ T cells and NK cells activity and decreased viral RNA in the plasma and LN of SHIV-infected RMs [617]. Different IL-15 modifications have been made, but only N-803 [618] (previously ALT-803), has been tested beyond in vitro due to having the greatest efficacy thus far. N-803 has shown reactivation potential in vitro and primed CD4+ T cells for recognition by immune effectors [619]. N-803 has shown improved NK cell activation and functionality in vitro and in HIV-infected humanized mice and protected against HIV challenge when given up-to 3 days after challenge [319]. In ART-naive, SIV-infected RMs, N-803 transiently decreased VLs by 1–2 logs [620] while in two studies, latency reversal occurred in ART-treated RMs, but only with CD8+ T-cell depletion, which reactivates virus on its own [621,622]. Indeed, in ART-treated SHIV-infected RMs, N-803 did not reactivate latent virus on its own and there was no change in viral DNA, even with immune activation [623]. Thus, N-803 would benefit from combinatorial treatments with a latency reversing agent for reservoir clearance.
6.9.4. IL-21 in HIV Cure
Similar to IL-15, IL-21 expression in SIV/HIV is associated with maintaining the NK, B, and T cell responses [624] and enhances effector functions when given in vitro and ex vivo without large increases in general immune activation [625,626]. Administered to SIV-infected RMs, IL-21 increased CD8+ T cell and NK cell activity and increased SIV-specific antibodies in the serum, without inducing CD4+ T cell activation nor viral reactivation [627]. In SIV-infected RMs on ART, IL-21 improved intestinal CD4+ T cell restoration, with reduced immune activation in both the gut and circulation. After ART cessation, immune activation and plasma VLs remained lower than control animals demonstrating a greatly positive effect of IL-21 treatment [628]. IL-21 in conjunction with IFNα in ART-treated, SIV-infected RMs drastically improved NK cell functionality and Env-specific activity. Further, the treatments resulted in reduced replication competent virus in LNs and increased time to rebound after analytical treatment interruption [629] supporting further investigation of IL-21 treatments.
6.10. “Shock and Kill”—Latency Reactivation for HIV Cure
The “shock and kill” approach has become the most widely explored HIV cure strategy with several different classes of agents being tested as potential LRAs: histone deacetylase inhibitors (HDACis) [630,631,632], protein kinase C (PKC) agonists [633,634], ingenol derivatives [635,636,637,638], bromodomain inhibitors [639], second mitochondrial activator of caspases (SMAC) mimetics [640], stimulator of interferon genes (STING) agonists [641], and Toll-like receptor (TLR) agonists [642,643,644,645,646]. This strategy utilizes LRAs to induce viral transcription and replication, which potentiates antigen recognition by immune surveillance and allows immune effectors to eliminate the productively infected cells, theoretically resulting in the reduction of the viral reservoir.
6.10.1. HDAC Inhibitors for “Shock and Kill”
Theoretically, HDACi are strong candidates for latency reversal. Nucleosomes are a basic structural unit of DNA that contain chromosomal DNA wrapped around two of each core histone, H2A, H2B, H3, and H4, forming an octameric core. As the DNA wraps around the cores, it can be modified with acetylation, methylation, and phosphorylation, which changes the binding tightness through charge, thereby affecting the function [647]. In the case of acetylation, histone acetyl transferases (HATS) acetylate the positively charged lysine residues of the histone N termini. This epigenetic change decreases the electrostatic affinity between the histone proteins and DNA and as a result, the DNA becomes more accessible to transcription factors [648,649,650].
Among genome modifications, deacetylation of the integrated HIV proviral structure around the LTR inhibits transcription of the provirus by tightening the DNA around the histone, thereby preventing proper binding of transcription factors and RNA polymerase II, and therefore preventing viral transcription [361,369,651,652,653] (Figure 5). Disruption of deacetylation has been shown to reactivate latent HIV-1 in vitro [630] and in vivo [632,654,655,656,657,658], however, long lasting changes in the reservoir have yet to be achieved.
Figure 5.
HDAC inhibitors drive chromatin towards a euchromatin state. The HIV LTR is associated with a closed, heterochromatin state suppressing viral transcription by being physically difficult to access. By inhibiting the deacetylation of the histones, this changes the charges associated with the histones and provides a looser conformation such that transcription factors and RNA Polymerase II are better able to access the DNA.
Romidepsin (RMD), a bicyclic class I HDACi (targets HDACs 1, 2, 3, and 8) [659,660,661], produces the most potent HIV reactivation ex vivo when compared to other HDACi [306] even at plasma concentrations that are lower than what is used for chemotherapy. These data were reproducible in cells from ART-treated PWH ex vivo [306]. In RMs, in vivo administration of RMD resulted in a massive increases in T-cell activation and viral rebound in post-treatment controllers [453]. RMD administration to PWH and RMs on ART also demonstrated T-cell activation and viral reactivation [658,662]. Yet, neither of these studies were able to demonstrate a statistically significant decrease in the SIV/HIV reservoir. Ex vivo, RMD (as well as Panobinostat and vorinostat [SAHA]) was shown to have a negative effect on the HIV-specific immune response, i.e., suppression of cytokine production and decreased cellular viability [451], as well as reduced proliferation and viability and also restriction of de novo infections after stimulation with IL-2 and PHA [663]. Unlike Jones et al. [451], Jønsson et al. showed that RMD treatment differentially changed expression patterns of interferon-stimulated genes, such as increases in IFIT1, ISG15, and STAT1, but decreases in APOBEC3G, MX2, and TRIM22 [663]. In RMs, the SIV-specific immune response was not significantly altered [453], nor were the HIV-specific immune responses altered in PWH in vivo [658]. In the BCN02 clinical trial (NCT02616874), PWH received MVA. HIVconsv vaccination and weekly infusions of RMD, and, while RMD administration reduced the total number of vaccine-elicited T cells secreting multiple cytokines, the CD8+ T cells retained their HIV suppressive functionality [664].
6.10.2. Protein Kinase C (PKC) Agonists for “Shock and Kill”
PKC agonists work through the canonical NF-κB pathway to enable HIV reactivation [665,666]. Many of the agents: phorbol esters (prostratin) [633]; bryostatin-1 [667] and its analogs [668,669]; have demonstrated HIV reactivation potential in vitro and ex vivo [670], but the common problem when moving to in vivo models was generalized immune activation and its resulting toxicity/tolerability due to higher doses required for in vivo reactivation [449,671]. To counter this issue, combinatorial LRA treatments, such as bryostatin-1 or prostratin with an HDAC inhibitor (e.g., Romidepsin, SAHA, and largazole) or bromodomain inhibitor JQ1, allow for lower LRA doses with similar or increased potency and decreased toxicity ex vivo [672,673]. Similarly, prodrugs for prostratin, ingenol, and bryostatin-1 were developed to improve tolerability and reduce bolus toxicity, and maintained immune activation and HIV reactivation in vitro and ex vivo [674]. However, these combinations and prodrugs have yet to be tested in vivo and further testing is warranted.
6.10.3. Ingenol Derivatives for “Shock and Kill”
Ingenol 3-angelate is an inflammatory substance extracted from the sap of the Euphorbia peplus plant [675]. Ingenol 3-angelate and its derivatives are structurally analogous to phorbol esters and mechanistically act through the PKC and NF-κB pathway [676,677]. By activating the NF-κB pathway, ingenol derivatives reactivate latent HIV in vitro and ex vivo [636,637,677,678]. Initially, ingenol derivatives were investigated for HIV inhibition and CD4 downregulation through PKC activation and data from the same study showed some derivatives reactivated HIV [679]. With data demonstrating that PKC agonists have anti-latency properties, renewed focus was placed upon new ingenol derivatives that can promote HIV reactivation, while reducing the toxicity associated with early ingenol derivatives, PMA, and prostratin [678,680]. The derivative ingenol-B reactivated HIV in vitro [681], but in ART-treated, SIV-infected RMs, ingenol-B did not reactivate virus in the circulation, but did have a viral blip in the cerebrospinal fluid (CSF). In combination with the HDACi vorinostat, reactivation was achieved in circulation and CSF [173]. In PWH treated with ingenol mebutate gel on the skin, HIV was reactivated locally in skin biopsies without plasma viremia, nor systemic immune activation [682]. The intravenous version of ingenol mebutate, PEP005, was found to have a synergistic reactivation when combined with JQ1, while also downregulating surface receptors CD4, CCR5, and CXCR4 ex vivo [636]. Thus, further research is warranted.
6.10.4. Bromodomain Inhibitors for “Shock and Kill”
JQ1, a small molecule bromodomain inhibitor developed in 2010, was shown to bind to BRD4 [683]. BRD4 competes with Tat for P-TEFb binding, restricting HIV replication [684]. JQ1 treatment of HIV-infected CD4+ T cells modestly reactivated virus, while suppressing T cell proliferation and downregulating CD3, CD28, and CXCR4 in vitro and ex vivo with minimal toxicity [639]. The combination of JQ1 with prostratin produced synergistic increases in reactivation in vitro [388]. Due to the modest reactivation potential of JQ1, new bromodomain inhibitors have been tested, with OTX015 [685], UMB-136 [686], apabetalone [687], and CPI-203 [688] showing greater reactivation potential, while also maintaining minimal toxicity and the synergistic activity with PKC agonists (e.g., prostratin and bryostatin-1) in vitro. Additionally, 8-methoxy-6-methylquinolin-4-ol (MMQO) [689], a quinolone based bromodomain inhibitor, reactivates HIV ex vivo and maintains immunosuppression similar to JQ1, without acting through the Tat transactivator [690]. Thus, these different bromodomain inhibitors are promising LRAs, but require in vivo studies to further elucidate their potential.
6.10.5. Second Mitochondrial Activator of Caspases (SMAC) Mimetics for “Shock and Kill”
SMAC mimetics are of high interest as LRAs because: (i) BIRC2 acts as a repressor of the NF-κB pathway and is antagonized by SMAC mimetics [691,692]; (ii) less generalized immune activation through the noncanonical NF-κB pathway versus canonical pathway [693]; (iii) reactivates HIV in vitro and synergizes with HDACis [694]. The SMAC mimetics AZD5582 [640] and birinapant [695] have both shown reactivation potential in vitro and ex vivo. AZD5582 was also shown to induce viral reactivation in BLT humanized mice and RMs with minimal systemic immune activation, no reduction in CD8+ T-cell immune responses, and low toxicity [696]. AZD5582 also has greater reactivation potency when combined with CD8+ cell depleting antibody M-T807R1 [697]. The SMAC mimetic Ciapavir demonstrated similar functionality in humanized mice and synergized with bromodomain inhibitors JQ1 and I-BET151, but when used with bryostatin-1 or ingenol-3-angelate induced greater toxicity [698]. SMAC mimetics are also showing efficacy against infected macrophages, but this is discussed later. Overall, SMAC mimetics warrant additional exploration in vivo.
6.10.6. Stimulator of Interferon Genes (STING) Agonists for “Shock and Kill”
STING agonists were of initial interest as boosters for the innate immune response and antigen-specific immune responses [699]. Indeed, one STING agonist, 3′3′-cGAMP primed HIV-1-specific CD8+ T cells [700] and cGAMP delivered with nanoparticle PC7A induced protection against HIV through type I IFN and inhibited HIV-1 replication [701]. However, cyclic GMP-AMP (cGAMP) and c-di-AMP are also capable of latency reactivation ex vivo while also increasing the frequency of SIV-specific CD8+ T cells [641]. In an ex vivo combinatorial study, the STING agonist cGAMP and HDACi, resminostat, had additive, but not synergistic reactivation potential [702]. In a pilot study of ART-treated, infected RMs, the STING agonist reactivated SIV in a third of RMs [703]. Thus, STING agonists are promising LRAs, but will be much more effective in combinatorial regimens.
6.10.7. Toll-like Receptor (TLR) Agonists for “Shock and Kill”
TLR agonists are similar to STING agonists in that they were initially looked at for HIV inhibition [704], yet are now investigated as both LRAs and immunomodulatory agents. Because TLRs are pattern recognition receptors they react to signals indicating the necessity for an immune response [705]. TLR2 and TLR9 agonists reactivated HIV from transgenic mouse spleen cells ex vivo [706]. Although that was the first major study to demonstrate and explain the reactivation, a previous clinical trial for the antisense oligodeoxynucleotide phosphorothioate GEM91 [707] had noted increased HIV-1 [708], contradictory to the ex vivo data with GEM91 [704], and it is hypothesized that this was through TLR9 activation [708]. Further investigation into a TLR9 agonist, CPG7909, as a pneumococcal vaccine adjuvant in PWH showed increased immunogenicity, a boost in HIV-specific CD8+ T cells and a reduction in caDNA, but also increased adverse effects [709]. MGN1703 was thus developed to decrease the toxicity of existing TLR9 agonists [710] and was tested ex vivo [711] and then in PWH with 4 weeks [712], followed up with a second study of 24 weeks of treatment [713]. Although MG1703 resulted in increased innate immune responses and HIV reactivation, overall viral burden was not significantly reduced, nor was a there difference in time to rebound [713]. TLR7 agonist GS-9620 reactivated HIV ex vivo and enhanced HIV-specific CD8+ T cells [310]. These results were seconded in SIV-infected RMs, with reduced caDNA and inducible virus after TLR7 agonist GS-986 treatment [35]. However, GS-9620 was unable to reproduce any of the virological effects of GS-986 in RMs [714] nor PWH [715]. Combinatorial TLR2 (Pam2CSK4) and TLR7 (GS-9620) agonists were tested ex vivo and enhanced reactivation potency by acting through separate mechanisms, thus suggesting this strategy should be tested further in vivo to improve outcomes versus single TLR agonist administration [646].
6.11. When Calories Get Serious—Role of Immunometabolism in HIV Pathogenesis and Cure
6.11.1. Immunometabolism and HIV Pathogenesis
Immunometabolism refers to the interface between the previously distinct fields of metabolism and immunology. With time, researchers have clearly shown that these are in fact linked, with specific metabolites being required for a proper function of macrophages, neutrophils and T cells, such as glucose, glutamine, fatty acids, and amino acids [716]. As for the pathways involved, there are six utilized in immune cells: glycolysis (the main metabolic pathway for T cell effector functions [717]), tricarboxylic acid (TCA) cycle, pentose phosphate pathway (PPP), fatty acid oxidation (FAO), fatty acid synthesis (FAS), and amino acid metabolism. Additionally, mTOR is an important regulator in the adaptive immunity, especially for the CD8+ T cell response [718]. However, immunometabolism differs between acute, chronic, and latent viral infections. During acute infection, when the CD8+ T cells are developing they upregulate mTORC1 and aerobic glycolysis to support their energy demands. However, during this time, HIV-specific T cells are beginning their metabolic dysregulation with extensive proliferation and activation, promoting an altered mitochondria that is burnt out to sustain the hyperproliferative state [719]. With chronic infection, T cell exhaustion and T cell metabolism become highly correlated, such as PD-1 ligation which results in diminished glucose and amino acid metabolism and mTOR activity [720,721,722]. Differently from PD-1, CTLA-4 modulates glycolysis and amino acid metabolism, but does not enhance FAO as seen with PD-1 ligation [720], a mechanism which is thought to promote survival during PD-1 ligation when the other metabolites are not being utilized. During chronic viral infections CD8+ T cells were profiled with glycolysis dependency, dysfunctional mitochondria, and abrogated oxidative phosphorylation (OXPHOS), which is involved in the TCA cycle [723]. Interestingly, the same study demonstrated that CMV-specific T cells were more functional and able to utilize OXPHOS, but the glycolysis pathway was inhibited. This points towards differences in the metabolism of not only chronic versus latent infections, but also of functional CD8+ T-cell responses [723]. In HIV controllers, CD8+ T cells were found to be metabolically separate from progressor CD8+ T cells. HIV controller CD8+ T cells were characterized by the upregulation of survival genes pathways and metabolic plasticity, (with functional mitochondria and OXPHOS) and is supported by the mTORC2 pathway [724].
6.11.2. Modulation of Immunometabolic Programming for HIV Cure/Therapeutics
With the complex relationship between HIV/SIV pathogenesis, immunometabolism, and T-cell exhaustion, there is growing interest in targeted metabolic therapies. Another effect of metabolism is the susceptibility to HIV. The accepted paradigm is that CD4+ T-cell susceptibility to HIV increases with differentiation, but what was recently described is the propensity for HIV to selectively infect CD4+ T cells that are utilizing high levels of OXPHOS and glycolysis. Partial in vitro inhibition of glycolysis with 2-deoxy glucose (2-DG) demonstrated that the glycolytic environment is required to complete reverse transcription and had a greater effect in further differentiated cells. Further, limiting glycolysis with 2-DG also showed a selective toxicity towards infected cells and 2-DG was also able to greatly reduce HIV replication after phytohemagglutinin (PHA) stimulation [725], thereby showcasing an additional metabolic regulation of HIV that can potentially be exploited. Regarding dysfunctional mitochondria, IL-12 administration was able to reverse the dependence on glycolysis and restore mitochondrial changes and metabolic pathways [723]. IL-15 is also known to promote FAO and mitochondrial biogenesis [726]. Ex vivo, CD8+ T cells from noncontrollers were shown to be enhanced after IL-15 treatment, with increased fatty acid uptake and enhanced mitochondrial respiratory capacity. Further, IL-15 pretreatment enhanced the SIV-specific CD8+ T-cell response of SIV-infected macaques and restored metabolic plasticity [724]. These two studies thus point towards potential therapeutics for HIV via metabolic restoration.
6.12. True or False—Macrophages and the HIV/SIV Reservoir
6.12.1. Macrophages Contribution to the HIV/SIV reservoir
In addition to the CD4+ T cells, macrophages also harbor provirus and are capable of producing replication competent virus. In macaques infected with SHIVDH12R (highly pathogenic SHIV containing envelope glycoproteins from HIV-1 strain DH12 [727]), following the characteristic extensive depletion of the CD4+ T cells, the remaining virus-producing cells were 95% macrophages with less than 2% expressing the CD4 receptor [728]. Although studies have not been able to concretely agree on the presence of replication competent virus in peripheral blood monocytes, the presence of HIV in tissue macrophages is undeniable [312,729,730,731,732]. HIV studies utilizing humanized myeloid-only-mice (MoM) demonstrated the ability of macrophages to maintain infection without CD4 cells [418]. Further, ART administration to infected MoM after infection resulted in two-thirds of the MoM from developing persistent infection with one third of the treated mice developing persistent infection that allowed for viral rebound after ART cessation, thus demonstrating the ability of HIV to persist in macrophages and reconstitute infection after ART [733]. Unfortunately, HIV infection of macrophages does not lead to viral cytolysis or apoptosis, with macrophages resistant to Vpr-mediated apoptosis [734].
HIV-infected macrophages are particularly present in the brain and central nervous system (CNS) [730]. During acute infection, infiltration of CD4 cells and monocytes from the blood to the brain allows for infection of the microglia and perivascular macrophages, causing neurological disorders, such as asymptomatic neurocognitive impairment, mild neurocognitive disorder, and HIV-associated dementia [735]. Fortunately, ART reduces neurological disorders. In brains of ART-treated and untreated PWH with HIV-associated neurocognitive disorders, genome-wide microarray analysis found that ART reduced the dysregulation of the brain transcriptome relative to untreated individuals, but regardless of ART, there was still a portion of adaptive and innate immune response genes that were upregulated in both treated and untreated.
6.12.2. Crosstalk between Macrophages and Exhausted T Cells
The exhaustion of T lymphocytes during HIV infection may provide further detriment to the host than lack of reservoir clearance. During infection, killing of HIV-infected CD4+ T lymphocytes by CTLs is a major mechanism of viral suppression. However, the elimination of macrophages is a harder task to accomplish. In SIV-infected macaques, CD8+ T cells ex vivo were unable to eliminate infected macrophages to the same extent as they could CD4+ T cells [736]. Although they are not the primary reservoir for HIV/SIV, the interaction between CTLs and infected macrophages yields a new dilemma: the extended formation of the synapse induces further secretion of IFN-γ and other pro-inflammatory cytokines [737], thereby potentially increasing the chronic inflammation during HIV infection. Similarities to this are seen in dendritic cell: T cell interactions [738], and the priming of naïve CD8+ T cells is demonstrated to alleviate the lack of killing [739,740].
6.12.3. Targeting Macrophages with “Shock and Kill”
Although most of the “shock and kill” therapeutics are aimed at CD4+ T cells, recent research shows that SMAC mimetics LCL-161, AT-406 (also known as Debio-1143), and birinapant, can also play a role in the direct elimination of HIV-infected macrophages. With an upregulation of BIRC2 and XIAP in HIVBA-L-infected macrophages, similar to HIV-infected CD4+ T cells, the infected macrophages are 10-100x more susceptible to cell death via SMAC mimetic than uninfected macrophages [741]. Of the three mimetics tested, only AT-406 resulted in viral reactivation in the macrophages [741], supporting previous findings that AT-406 induces viral reactivation in resting CD4+ T cells from PWH and humanized mouse models with ART [742]. Thus, SMAC mimetics are looking to be promising new LRAs.
7. Further to Fly: Future Perspectives for HIV Cure and Avenues to Explore
During progressive HIV infection, a healthy immune response is not present, and agents aimed at improving the immune system of PWH have not recapitulated the viral control observed in our model of RM functional cure [71,72] when used alone. Additionally, LRAs were shown to only reactivate a small portion of reservoir [400,448,743], regardless of cellular activation status [744] and have yet to demonstrate substantial reductions in the latent reservoir. As such, these results further diminish the usefulness of single therapy regimens, as the vast majority of the reservoir will remain untouched because the lack of viral production will prevent recognition by the immune system, let alone the lack of clearance by viral cytopathic effects. Nonetheless, the silver lining in current HIV cure research is that recent combinatorial studies have resulted in much better efficacy than the single treatment studies [521,613,636,702]. However, these studies have been completed either during acute infection or with ART initiation during acute infection, and are thus not as applicable to the majority of PWH. Regardless, those studies point towards the eventual development of a functional cure being possible through bolstering the immune response while limiting extensive viremia. Moving forward, the field will need to continue to rely heavily upon NHP models for HIV cure due to the intricacy of combinatorial studies and, more importantly, the potential for unexpected adverse effects. For example, the use of IL2-DT for Treg depletion had a positive impact on the spontaneously functionally cured SIV infection in RMs [441], but when combined with ART, resulted in unacceptable toxicity [442]. Similarly, Cy used as a cytoreductive agent close to chemotherapeutic dosage with ART resulted in unacceptable toxicity and morbidity [593]. Further, the venetoclax + ixazomib combination had synergistic in vitro efficacy, but did not make it to in vivo testing because ex vivo toxicity with PBMCs was too great [485]. Nevertheless, new combinations must still be tested and given the parallels between cancer and HIV, particularly in relation to the role of immune dysfunction in both disease paradigms, it is imperative that we work closely together to achieve greater results [745]. In addition, the field should take note from its own research on ART and try to find combinations that target different mechanisms of HIV latency persistence or immune dysfunction, similar to how there is an antiretroviral for each step of the HIV life cycle. This is because, as demonstrated in every study, what works for one animal or patient may not work for another and this needs to be taken into consideration. By trying different combinations, we may end up with separate treatments for separate phenotypes or stages of infection, e.g., PWH that initiated ART late during chronic infection may respond better with the inclusion of a PD-1/PD-L1 or CTLA-4 blockade due to the further extent of T-cell exhaustion. Meanwhile, during acute infection, a combination of IL-15/21 or TLR agonist with bnAbs may be more efficient in boosting the innate and adaptive immune responses while decreasing excessive viremia and immune depletion, allowing for the cell-mediated immune response to have a better response to virus. Because researchers control when the animals are infected and begin antiretroviral therapy, the NHP models are a wonderful resource for testing these hypotheses.
One of several strategies worth investigating is combining TLR2 and TLR7 agonists. Dual TLR2/TLR7 agonists resulted in increased efficacy through separate immune activation mechanisms [646], and may work better than single TLR7 agonists for viral reactivation and immune stimulation. Further, combination of TLR2/7 agonists with bnAbs [519,520] may be a valid strategy for clearance after reactivation and maintaining immune control, similar to the GS-9620 + bnAb PGT121 [519] or IL-15 superagonist (N-803) + bnAbs 10-1074 and 3BNC117 [521] studies. Interestingly, a cancer study showed that HDACis increase production of human endogenous retroviral elements in cancer cells and that concomitant treatment of TLR7/8 agonists allows for the cells to induce intrinsic apoptosis when they otherwise would not have enough stimuli to do so, while also at doses that are individually subcytotoxic [746]. This therapy can readily be applied to HIV research, but will first require ex vivo testing to ensure cell viability of uninfected cells is maintained and bystander death is not prolific. Should toxicity prove acceptable, additional inclusion of bnAbs could assist by providing another mechanism of reservoir clearance and would not modulate immune activation, thereby avoiding an increase in the chance of cytokine storm. However, as HDACi do not reactivate a substantial portion of the reservoir [400,448,743], repeated treatments would likely be necessary with advancement to in vivo testing.
Other combinations for improving the immune function should also be investigated. IL-15 and IL-21 have both shown efficacy for improving viral control [319,627,628] but have yet to be tested in combination for HIV cure. In mice, IL-15 and IL-21 both act on B, T, and NK cells, but IL-15 is essential for T cells, whereas IL-21 is more important for NK cells. Indeed, there is still overlap between the two and they act synergistically to boost CD8+ T cells and function [747,748] and, importantly, improve antigen-specific T-cell responses [749]. Thus, a study of the combination of IL-15/21 as an HIV therapeutic is warranted ex vivo and in vivo, although this combination would likely require substantially decreased doses relative to single treatments to prevent cytokine storms. IL-15/21 would likely also not be feasible as a combination with a LRA, due to too much immune stimulation, but could work very well with bnAbs during acute or early chronic infection. One downside to IL-15 therapy is the induction of PD-1 and PD-L1 expression [750] and unsurprisingly, IL-15 superagonist N-803 is currently being tested with PD-1 monoclonal antibodies to reduce the increased PD-1/PD-L1 expression while also inherently decreasing T-cell exhaustion for cancer treatments [751,752,753]. Another method of combination IL-15 or IL-21 and αPD-1 is the use of fusion proteins. By placing IL-15 [754] or IL-21 [755] on a PD-L1 or PD-1 antibody, IL-15/21 are targeted to PD-1 expressing cells, which will be enriched for tumor reactive CD8+ T cells. This can also apply to HIV-specific CD8+ T cells which are likewise enriched for PD-1/PD-L1 [196,197,198,199] and may reduce the risk of inducing systemic immune activation with combination therapy. Both of these concepts deserve further investigation. Further, as neither IL-15, IL-21, nor PD-1 therapies have strong latency reversal potency, in vitro experiments should be carried out with the addition of a stronger LRA. In fact, the combination of a SMAC mimetic (i.e., ciapavir) with a bromodomain inhibitor (i.e., JQ1 or a newer molecule) could be a strong contender, as these two drugs have been shown to be synergistic for latency reversal [698]. This particular combination is preferable for a combination with IL-15 or 21 because both ciapavir and JQ1 induce viral reactivation with minimal immune activation, and thus this should limit the potential adverse effects from systemic immune activation. Nonetheless, NHP modeling should be utilized prior to administration to human study participants to elucidate the extent of adverse effects.
Overall, there are many developing combinatorial strategies that are increasing our hope for an HIV cure or remission. In the meantime, we will continue to rely on ART, one of the most successful therapeutic approach of the 20th century. Similar to Paul and Art, HIV and ART are an exquisite combination and had a tremendous and sustained success. In time, cure research will do exactly what Paul Simon succeeded to do so well—live and succeed without ART.
Acknowledgments
We thank Charles Rinaldo, Bernard Macatangay, Robbie Mailliard, and Theresa Whiteside for helpful discussion and review. Figure 3, Figure 4 and Figure 5 were created with BioRender.com, accessed on 5 November 2021. We also thank Paul Simon and Art Garfunkel for their wonderful music. The following songs titles were used or modified for the title and the section headings: “So Beautiful or so What?”, “You Can Call Me Al”, “Why Don’t You Write Me”, “50 Ways to Leave Your Lover”, Born at the Right Time”, “Everything Put Together Falls Apart”, “Gone at Last”, “At the Zoo”, “Everything About It Is a Love Song”, “Under African Skies”, “How the Heart Approaches What It Yearns”, “For Emily, Whenever I May Find Her”, “One-trick Pony”, “Somewhere They Can’t Find Me”, “Still Crazy After All These Years”, “When Numbers Get Serious”, “True or False”, “Further To Fly”, “Everything About it is a Love Song”, “Graceland”, and “I Know What I Know”.
Author Contributions
Writing—original draft preparation, A.J.K. and C.A.; writing—review and editing, A.J.K., I.P. and C.A. All authors have read and agreed to the published version of the manuscript.
Funding
A.J.K. was supported in part by NIH training grants T32 Immunology of Infectious Diseases (IID) AI060525 and Pittsburgh AIDS Research Training (PART) grant AI065380. IP and CA were supported by grants R01AI119346 (CA), R01DK113919 (IP/CA), R01DK119936 (CA), R01DK131476 (CA), R01DK13481 (IP), RO1 HL117715 (IP), R01 HL123096 (IP) from the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and National Heart, Lung and Blood Institute (NHLBI). Funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barré-Sinoussi F., Chermann J.C., Rey F., Nugeyre M.T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C., et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 2.Daniel M., Letvin N., King N., Kannagi M., Sehgal P., Hunt R., Kanki P., Essex M., Desrosiers R. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985;228:1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- 3.Johnson L.F., Mossong J., Dorrington R.E., Schomaker M., Hoffmann C.J., Keiser O., Fox M.P., Wood R., Prozesky H., Giddy J., et al. Life Expectancies of South African Adults Starting Antiretroviral Treatment: Collaborative Analysis of Cohort Studies. PLoS Med. 2013;10:e1001418. doi: 10.1371/journal.pmed.1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guaraldi G., Orlando G., Zona S., Menozzi M., Carli F., Garlassi E., Berti A., Rossi E., Roverato A., Palella F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011;53:1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 5.Guaraldi G., Zona S., Brothers T.D., Carli F., Stentarelli C., Dolci G., Santoro A., Beghetto B., Menozzi M., Mussini C., et al. Aging with HIV vs. HIV seroconversion at older age: A diverse population with distinct comorbidity profiles. PLoS ONE. 2015;10:e0118531. doi: 10.1371/journal.pone.0118531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallant J., Hsue P., Budd D., Meyer N. Healthcare utilization and direct costs of non-infectious comorbidities in HIV-infected patients in the USA. Curr. Med. Res. Opin. 2018;34:13–23. doi: 10.1080/03007995.2017.1383889. [DOI] [PubMed] [Google Scholar]
- 7.Allers K., Hütter G., Hofmann J., Loddenkemper C., Rieger K., Thiel E., Schneider T. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood. 2011;117:2791–2799. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 8.Gupta R.K., Abdul-Jawad S., McCoy L.E., Mok H.P., Peppa D., Salgado M., Martinez-Picado J., Nijhuis M., Wensing A.M.J., Lee H., et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature. 2019;568:244–248. doi: 10.1038/s41586-019-1027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stowell R.E., Smith E.K., España C., Nelson V.G. Outbreak of malignant lymphoma in rhesus monkeys. Lab. Investig. A J. Tech. Methods Pathol. 1971;25:476–479. [PubMed] [Google Scholar]
- 10.Terrell T.G., Gribble D.H., Osburn B.I. Malignant lymphoma in macaques: A clinicopathologic study of 45 cases. J. Natl. Cancer Inst. 1980;64:561–568. [PubMed] [Google Scholar]
- 11.Gardner M.B. The history of simian AIDS. J. Med. Primatol. 1996;25:148–157. doi: 10.1111/j.1600-0684.1996.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 12.Letvin N.L., Daniel M.D., Sehgal P.K., Desrosiers R.C., Hunt R.D., Waldron L.M., MacKey J.J., Schmidt D.K., Chalifoux L.V., King N.W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 13.Ansari A.A., Silvestri G. Natural Hosts of SIV: Implication in AIDS. Newnes; Oxford, UK: 2014. [Google Scholar]
- 14.Lowenstine L.J., Pedersen N.C., Higgins J., Pallis K.C., Uyeda A., Marx P., Lerche N.W., Munn R.J., Gardner M.B. Seroepidemiologic survey of captive Old-World primates for antibodies to human and simian retroviruses, and isolation of a lentivirus from sooty mangabeys (Cercocebus atys) Int. J. Cancer. 1986;38:563–574. doi: 10.1002/ijc.2910380417. [DOI] [PubMed] [Google Scholar]
- 15.Ohta Y., Masuda T., Tsujimoto H., Ishikawa K., Kodama T., Morikawa S., Nakai M., Honjo S., Hayami M. Isolation of simian immunodeficiency virus from African green monkeys and seroepidemiologic survey of the virus in various non-human primates. Int. J. Cancer. 1988;41:115–122. doi: 10.1002/ijc.2910410121. [DOI] [PubMed] [Google Scholar]
- 16.Ma D., Jasinska A., Kristoff J., Grobler J.P., Turner T., Jung Y., Schmitt C., Raehtz K., Feyertag F., Martinez Sosa N., et al. SIVagm infection in wild African green monkeys from South Africa: Epidemiology, natural history, and evolutionary considerations. PLoS Pathog. 2013;9:e1003011. doi: 10.1371/journal.ppat.1003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raehtz K., Pandrea I., Apetrei C. The well-tempered SIV infection: Pathogenesis of SIV infection in natural hosts in the wild, with emphasis on virus transmission and early events post-infection that may contribute to protection from disease progression. Infect. Genet. Evol. 2016;46:308–323. doi: 10.1016/j.meegid.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VandeWoude S., Apetrei C. Going wild: Lessons from naturally occurring T-lymphotropic lentiviruses. Clin. Microbiol. Rev. 2006;19:728–762. doi: 10.1128/CMR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apetrei C., Lerche N.W., Pandrea I., Gormus B., Silvestri G., Kaur A., Robertson D.L., Hardcastle J., Lackner A.A., Marx P.A. Kuru experiments triggered the emergence of pathogenic SIVmac. AIDS (Lond. Engl.) 2006;20:317–321. doi: 10.1097/01.aids.0000206498.71041.0e. [DOI] [PubMed] [Google Scholar]
- 20.Corbet S., Müller-Trutwin M.C., Versmisse P., Delarue S., Ayouba A., Lewis J., Brunak S., Martin P., Brun-Vezinet F., Simon F., et al. env sequences of simian immunodeficiency viruses from chimpanzees in Cameroon are strongly related to those of human immunodeficiency virus group N from the same geographic area. J. Virol. 2000;74:529–534. doi: 10.1128/JVI.74.1.529-534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Z., Telfier P., Gettie A., Reed P., Zhang L., Ho D.D., Marx P.A. Genetic characterization of new West African simian immunodeficiency virus SIVsm: Geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J. Virol. 1996;70:3617–3627. doi: 10.1128/JVI.70.6.3617-3627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apetrei C., Kaur A., Lerche N.W., Metzger M., Pandrea I., Hardcastle J., Falkenstein S., Bohm R., Koehler J., Traina-Dorge V., et al. Molecular epidemiology of simian immunodeficiency virus SIVsm in U.S. primate centers unravels the origin of SIVmac and SIVstm. J. Virol. 2005;79:8991–9005. doi: 10.1128/JVI.79.14.8991-9005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansfield K.G., Lerch N.W., Gardner M.B., Lackner A.A. Origins of simian immunodeficiency virus infection in macaques at the New England Regional Primate Research Center. J. Med. Primatol. 1995;24:116–122. doi: 10.1111/j.1600-0684.1995.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 24.Benveniste R.E., Morton W.R., Clark E.A., Tsai C.C., Ochs H.D., Ward J.M., Kuller L., Knott W.B., Hill R.W., Gale M.J. Inoculation of baboons and macaques with simian immunodeficiency virus/Mne, a primate lentivirus closely related to human immunodeficiency virus type 2. J. Virol. 1988;62:2091–2101. doi: 10.1128/jvi.62.6.2091-2101.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan A.S., Galvin T.A., Jennings M.B., Gardner M.B., Lowenstine L.J. SIV of stump-tailed macaque (SIVstm) is a divergent Asian isolate. J. Med. Primatol. 1991;20:167–171. doi: 10.1111/j.1600-0684.1991.tb00513.x. [DOI] [PubMed] [Google Scholar]
- 26.Novembre F.J., Hirsch V.M., McClure H.M., Fultz P.N., Johnson P.R. SIV from stump-tailed macaques: Molecular characterization of a highly transmissible primate lentivirus. Virology. 1992;186:783–787. doi: 10.1016/0042-6822(92)90047-S. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy K.R., Johnson W.E., Kirmaier A. Phylogeny and History of the Lost SIV from Crab-Eating Macaques: SIVmfa. PLoS ONE. 2016;11:e0159281. doi: 10.1371/journal.pone.0159281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitney J.B., Hill A.L., Sanisetty S., Penaloza-MacMaster P., Liu J., Shetty M., Parenteau L., Cabral C., Shields J., Blackmore S., et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512:74–77. doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henrich T.J., Hatano H., Bacon O., Hogan L.E., Rutishauser R., Hill A., Kearney M.F., Anderson E.M., Buchbinder S.P., Cohen S.E., et al. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLOS Med. 2017;14:e1002417. doi: 10.1371/journal.pmed.1002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okoye A.A., Hansen S.G., Vaidya M., Fukazawa Y., Park H., Duell D.M., Lum R., Hughes C.M., Ventura A.B., Ainslie E., et al. Early antiretroviral therapy limits SIV reservoir establishment to delay or prevent post-treatment viral rebound. Nat. Med. 2018;24:1430–1440. doi: 10.1038/s41591-018-0130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Policicchio B.B., Pandrea I., Apetrei C. Animal Models for HIV Cure Research. Front. Immunol. 2016;7:12. doi: 10.3389/fimmu.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deleage C., Wietgrefe S.W., Del Prete G., Morcock D.R., Hao X.P., Piatak M., Jr., Bess J., Anderson J.L., Perkey K.E., Reilly C., et al. Defining HIV and SIV Reservoirs in Lymphoid Tissues. Pathog. Immun. 2016;1:68–106. doi: 10.20411/pai.v1i1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen S.G., Marshall E.E., Malouli D., Ventura A.B., Hughes C.M., Ainslie E., Ford J.C., Morrow D., Gilbride R.M., Bae J.Y., et al. A live-attenuated RhCMV/SIV vaccine shows long-term efficacy against heterologous SIV challenge. Sci. Transl. Med. 2019;11:eaaw2607. doi: 10.1126/scitranslmed.aaw2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen S.G., Ford J.C., Lewis M.S., Ventura A.B., Hughes C.M., Coyne-Johnson L., Whizin N., Oswald K., Shoemaker R., Swanson T., et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim S.-Y., Osuna C.E., Hraber P.T., Hesselgesser J., Gerold J.M., Barnes T.L., Sanisetty S., Seaman M.S., Lewis M.G., Geleziunas R., et al. TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Sci. Transl. Med. 2018;10:eaao4521. doi: 10.1126/scitranslmed.aao4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddiqui S., Perez S., Gao Y., Doyle-Meyers L., Foley B.T., Li Q., Ling B. Persistent Viral Reservoirs in Lymphoid Tissues in SIV-Infected Rhesus Macaques of Chinese-Origin on Suppressive Antiretroviral Therapy. Viruses. 2019;11:105. doi: 10.3390/v11020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veazey R.S., DeMaria M., Chalifoux L.V., Shvetz D.E., Pauley D.R., Knight H.L., Rosenzweig M., Johnson R.P., Desrosiers R.C., Lackner A.A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 38.Smit-McBride Z., Mattapallil J.J., McChesney M., Ferrick D., Dandekar S. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4(+) T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J. Virol. 1998;72:6646–6656. doi: 10.1128/JVI.72.8.6646-6656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brenchley J.M., Schacker T.W., Ruff L.E., Price D.A., Taylor J.H., Beilman G.J., Nguyen P.L., Khoruts A., Larson M., Haase A.T., et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehandru S., Poles M.A., Tenner-Racz K., Horowitz A., Hurley A., Hogan C., Boden D., Racz P., Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He T., Xu C., Krampe N., Dillon S.M., Sette P., Falwell E., Haret-Richter G.S., Butterfield T., Dunsmore T.L., McFadden W.M., Jr., et al. High-fat diet exacerbates SIV pathogenesis and accelerates disease progression. J. Clin. Investig. 2019;129:5474–5488. doi: 10.1172/JCI121208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller C.J., Hu J. T Cell—Tropic Simian Immunodeficiency Virus (SIV) and Simian-Human Immunodeficiency Viruses Are Readily Transmitted by Vaginal Inoculation of Rhesus Macaques, and Langerhans’ Cells of the Female Genital Tract Are Infected with SIV. J. Infect. Dis. 1999;179:S413–S417. doi: 10.1086/314795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heeney J.L., Rutjens E., Verschoor E.J., Niphuis H., ten Haaft P., Rouse S., McClure H., Balla-Jhagjhoorsingh S., Bogers W., Salas M., et al. Transmission of simian immunodeficiency virus SIVcpz and the evolution of infection in the presence and absence of concurrent human immunodeficiency virus type 1 infection in chimpanzees. J. Virol. 2006;80:7208–7218. doi: 10.1128/JVI.00382-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stone M., Keele B.F., Ma Z.M., Bailes E., Dutra J., Hahn B.H., Shaw G.M., Miller C.J. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. J. Virol. 2010;84:7083–7095. doi: 10.1128/JVI.00481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deleage C., Immonen T.T., Fennessey C.M., Reynaldi A., Reid C., Newman L., Lipkey L., Schlub T.E., Camus C., O’Brien S., et al. Defining early SIV replication and dissemination dynamics following vaginal transmission. Sci. Adv. 2019;5:eaav7116. doi: 10.1126/sciadv.aav7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrenas F., Raehtz K., Xu C., Law L., Green R.R., Silvestri G., Bosinger S.E., Nishida A., Li Q., Lu W., et al. Macrophage-associated wound healing contributes to African green monkey SIV pathogenesis control. Nat. Commun. 2019;10:5101. doi: 10.1038/s41467-019-12987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kornfeld C., Ploquin M.J., Pandrea I., Faye A., Onanga R., Apetrei C., Poaty-Mavoungou V., Rouquet P., Estaquier J., Mortara L., et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J. Clin. Investig. 2005;115:1082–1091. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raehtz K.D., Barrenäs F., Xu C., Busman-Sahay K., Valentine A., Law L., Ma D., Policicchio B.B., Wijewardana V., Brocca-Cofano E., et al. African green monkeys avoid SIV disease progression by preventing intestinal dysfunction and maintaining mucosal barrier integrity. PLoS Pathog. 2020;16:e1008333. doi: 10.1371/journal.ppat.1008333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuller L.H., Tracy R., Belloso W., De Wit S., Drummond F., Lane H.C., Ledergerber B., Lundgren J., Neuhaus J., Nixon D., et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandrea I., Apetrei C. Where the wild things are: Pathogenesis of SIV infection in African nonhuman primate hosts. Curr. HIV/AIDS Rep. 2010;7:28–36. doi: 10.1007/s11904-009-0034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pandrea I., Cornell E., Wilson C., Ribeiro R.M., Ma D., Kristoff J., Xu C., Haret-Richter G.S., Trichel A., Apetrei C., et al. Coagulation biomarkers predict disease progression in SIV-infected nonhuman primates. Blood. 2012;120:1357–1366. doi: 10.1182/blood-2012-03-414706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deeks S.G., Tracy R., Douek D.C. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39:633–645. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pandrea I., Landay A., Wilson C., Stock J., Tracy R., Apetrei C. Using the pathogenic and nonpathogenic nonhuman primate model for studying non-AIDS comorbidities. Curr. HIV/AIDS Rep. 2015;12:54–67. doi: 10.1007/s11904-014-0245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphey-Corb M., Martin L.N., Rangan S.R., Baskin G.B., Gormus B.J., Wolf R.H., Andes W.A., West M., Montelaro R.C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986;321:435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- 55.Hirsch V.M., Dapolito G., McGann C., Olmsted R.A., Purcell R.H., Johnson P.R. Molecular Cloning of SIV From Sooty Mangabey Monkeys. J. Med. Primatol. 1989;18:279–285. doi: 10.1111/j.1600-0684.1989.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 56.Hirsch V.M., Olmsted R.A., Murphey-Corb M., Purcell R.H., Johnson P.R. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989;339:389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- 57.Hirsch V., Adger-Johnson D., Campbell B., Goldstein S., Brown C., Elkins W.R., Montefiori D.C. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543-3. J. Virol. 1997;71:1608–1620. doi: 10.1128/jvi.71.2.1608-1620.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirsch V.M., Zack P.M., Johnson P.R. Molecular characterization of SIV in tissues from experimentally infected macaques. J. Med. Primatol. 1990;19:287–294. doi: 10.1111/j.1600-0684.1990.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 59.Hirsch V.M., Johnson P.R. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 1994;32:183–203. doi: 10.1016/0168-1702(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 60.Letvin N.L., Rao S.S., Montefiori D.C., Seaman M.S., Sun Y., Lim S.Y., Yeh W.W., Asmal M., Gelman R.S., Shen L., et al. Immune and Genetic Correlates of Vaccine Protection Against Mucosal Infection by SIV in Monkeys. Sci. Transl. Med. 2011;3:81ra36. doi: 10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu F., Ourmanov I., Kuwata T., Goeken R., Brown Charles R., Buckler-White A., Iyengar R., Plishka R., Aoki Scott T., Hirsch Vanessa M. Sequential Evolution and Escape from Neutralization of Simian Immunodeficiency Virus SIVsmE660 Clones in Rhesus Macaques. J. Virol. 2012;86:8835–8847. doi: 10.1128/JVI.00923-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kirmaier A., Wu F., Newman R.M., Hall L.R., Morgan J.S., O’Connor S., Marx P.A., Meythaler M., Goldstein S., Buckler-White A., et al. TRIM5 Suppresses Cross-Species Transmission of a Primate Immunodeficiency Virus and Selects for Emergence of Resistant Variants in the New Species. PLoS Biol. 2010;8:e1000462. doi: 10.1371/journal.pbio.1000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopker M.J., Del Prete G.Q., Estes J.D., Li H., Reid C., Newman L., Lipkey L., Camus C., Easlick J.L., Wang S., et al. Derivation and Characterization of Pathogenic Transmitted/Founder Molecular Clones from Simian Immunodeficiency Virus SIVsmE660 and SIVmac251 following Mucosal Infection. J. Virol. 2016;90:8435–8453. doi: 10.1128/JVI.00718-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brocca-Cofano E., Xu C., Wetzel K.S., Cottrell M.L., Policicchio B.B., Raehtz K.D., Ma D., Dunsmore T., Haret-Richter G.S., Musaitif K., et al. Marginal Effects of Systemic CCR5 Blockade with Maraviroc on Oral Simian Immunodeficiency Virus Transmission to Infant Macaques. J. Virol. 2018;92:e00576-00518. doi: 10.1128/JVI.00576-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Del Prete G.Q., Park H., Fennessey C.M., Reid C., Lipkey L., Newman L., Oswald K., Kahl C., Piatak M., Jr., Quinones O.A., et al. Molecularly tagged simian immunodeficiency virus SIVmac239 synthetic swarm for tracking independent infection events. J. Virol. 2014;88:8077–8090. doi: 10.1128/JVI.01026-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fennessey C.M., Pinkevych M., Immonen T.T., Reynaldi A., Venturi V., Nadella P., Reid C., Newman L., Lipkey L., Oswald K., et al. Genetically-barcoded SIV facilitates enumeration of rebound variants and estimation of reactivation rates in nonhuman primates following interruption of suppressive antiretroviral therapy. PLoS Pathog. 2017;13:e1006359. doi: 10.1371/journal.ppat.1006359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khanal S., Fennessey C.M., O’Brien S.P., Thorpe A., Reid C., Immonen T.T., Smith R., Bess J.W., Jr., Swanstrom A.E., Del Prete G.Q., et al. In Vivo Validation of the Viral Barcoding of Simian Immunodeficiency Virus SIVmac239 and the Development of New Barcoded SIV and Subtype B and C Simian-Human Immunodeficiency Viruses. J. Virol. 2019;94:e01420-01419. doi: 10.1128/JVI.01420-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diop O.M., Gueye A., Dias-Tavares M., Kornfeld C., Faye A., Ave P., Huerre M., Corbet S., Barre-Sinoussi F., Müller-Trutwin M.C. High levels of viral replication during primary simian immunodeficiency virus SIVagm infection are rapidly and strongly controlled in African green monkeys. J. Virol. 2000;74:7538–7547. doi: 10.1128/JVI.74.16.7538-7547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gnanadurai C.W., Pandrea I., Parrish N.F., Kraus M.H., Learn G.H., Salazar M.G., Sauermann U., Töpfer K., Gautam R., Münch J., et al. Genetic identity and biological phenotype of a transmitted/founder virus representative of nonpathogenic simian immunodeficiency virus infection in African green monkeys. J. Virol. 2010;84:12245–12254. doi: 10.1128/JVI.01603-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mandell D.T., Kristoff J., Gaufin T., Gautam R., Ma D., Sandler N., Haret-Richter G., Xu C., Aamer H., Dufour J., et al. Pathogenic features associated with increased virulence upon Simian immunodeficiency virus cross-species transmission from natural hosts. J. Virol. 2014;88:6778–6792. doi: 10.1128/JVI.03785-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pandrea I., Gaufin T., Gautam R., Kristoff J., Mandell D., Montefiori D., Keele B.F., Ribeiro R.M., Veazey R.S., Apetrei C. Functional Cure of SIVagm Infection in Rhesus Macaques Results in Complete Recovery of CD4+ T Cells and Is Reverted by CD8+ Cell Depletion. PLoS Pathog. 2011;7:e1002170. doi: 10.1371/journal.ppat.1002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malikov V., da Silva E.S., Jovasevic V., Bennett G., de Souza Aranha Vieira D.A., Schulte B., Diaz-Griffero F., Walsh D., Naghavi M.H. HIV-1 capsids bind and exploit the kinesin-1 adaptor FEZ1 for inward movement to the nucleus. Nat. Commun. 2015;6:6660. doi: 10.1038/ncomms7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hatziioannou T., Bieniasz P.D. Antiretroviral restriction factors. Curr. Opin. Virol. 2011;1:526–532. doi: 10.1016/j.coviro.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li J., Lord C.I., Haseltine W., Letvin N.L., Sodroski J. Infection of cynomolgus monkeys with a chimeric HIV-1/SIVmac virus that expresses the HIV-1 envelope glycoproteins. J. Acquir. Immune Defic. Syndr. 1992;5:639–646. [PubMed] [Google Scholar]
- 75.Reimann K.A., Li J.T., Voss G., Lekutis C., Tenner-Racz K., Racz P., Lin W., Montefiori D.C., Lee-Parritz D.E., Lu Y., et al. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J. Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karlsson G.B., Halloran M., Li J., Park I.W., Gomila R., Reimann K.A., Axthelm M.K., Iliff S.A., Letvin N.L., Sodroski J. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J. Virol. 1997;71:4218–4225. doi: 10.1128/jvi.71.6.4218-4225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nishimura Y., Igarashi T., Donau O.K., Buckler-White A., Buckler C., Lafont B.A., Goeken R.M., Goldstein S., Hirsch V.M., Martin M.A. Highly pathogenic SHIVs and SIVs target different CD4+ T cell subsets in rhesus monkeys, explaining their divergent clinical courses. Proc. Natl. Acad. Sci. USA. 2004;101:12324–12329. doi: 10.1073/pnas.0404620101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Del Prete G.Q., Lifson J.D., Keele B.F. Nonhuman primate models for the evaluation of HIV-1 preventive vaccine strategies: Model parameter considerations and consequences. Curr. Opin. HIV AIDS. 2016;11 doi: 10.1097/COH.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharma A., Boyd D.F., Overbaugh J. Development of SHIVs with circulating, transmitted HIV-1 variants. J. Med. Primatol. 2015;44:296–300. doi: 10.1111/jmp.12179. [DOI] [PubMed] [Google Scholar]
- 80.Ndung’u T., Lu Y., Renjifo B., Touzjian N., Kushner N., Pena-Cruz V., Novitsky V.A., Lee T.H., Essex M. Infectious simian/human immunodeficiency virus with human immunodeficiency virus type 1 subtype C from an African isolate: Rhesus macaque model. J. Virol. 2001;75:11417–11425. doi: 10.1128/JVI.75.23.11417-11425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pal R., Taylor B., Foulke J.S., Woodward R., Merges M., Praschunus R., Gibson A., Reitz M. Characterization of a simian human immunodeficiency virus encoding the envelope gene from the CCR5-tropic HIV-1 Ba-L. J. Acquir. Immune Defic. Syndr. (1999) 2003;33:300–307. doi: 10.1097/00126334-200307010-00003. [DOI] [PubMed] [Google Scholar]
- 82.Harouse J.M., Gettie A., Eshetu T., Tan R.C., Bohm R., Blanchard J., Baskin G., Cheng-Mayer C. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3) J. Virol. 2001;75:1990–1995. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Z., Huang Y., Zhao X., Skulsky E., Lin D., Ip J., Gettie A., Ho D.D. Enhanced infectivity of an R5-tropic simian/human immunodeficiency virus carrying human immunodeficiency virus type 1 subtype C envelope after serial passages in pig-tailed macaques (Macaca nemestrina) J. Virol. 2000;74:6501–6510. doi: 10.1128/JVI.74.14.6501-6510.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nishimura Y., Shingai M., Willey R., Sadjadpour R., Lee W.R., Brown C.R., Brenchley J.M., Buckler-White A., Petros R., Eckhaus M., et al. Generation of the pathogenic R5-tropic simian/human immunodeficiency virus SHIVAD8 by serial passaging in rhesus macaques. J. Virol. 2010;84:4769–4781. doi: 10.1128/JVI.02279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tartaglia L.J., Gupte S., Pastores K.C., Trott S., Abbink P., Mercado N.B., Li Z., Liu P.T., Borducchi E.N., Chandrashekar A., et al. Differential Outcomes following Optimization of Simian-Human Immunodeficiency Viruses from Clades AE, B, and C. J. Virol. 2020;94 doi: 10.1128/JVI.01860-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bauer A.M., Bar K.J. Advances in simian--human immunodeficiency viruses for nonhuman primate studies of HIV prevention and cure. Curr. Opin. HIV AIDS. 2020;15:275–281. doi: 10.1097/COH.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 87.Gautam R., Nishimura Y., Lee W.R., Donau O., Buckler-White A., Shingai M., Sadjadpour R., Schmidt S.D., LaBranche C.C., Keele B.F., et al. Pathogenicity and mucosal transmissibility of the R5-tropic simian/human immunodeficiency virus SHIV(AD8) in rhesus macaques: Implications for use in vaccine studies. J. Virol. 2012;86:8516–8526. doi: 10.1128/JVI.00644-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shingai M., Donau O.K., Schmidt S.D., Gautam R., Plishka R.J., Buckler-White A., Sadjadpour R., Lee W.R., LaBranche C.C., Montefiori D.C., et al. Most rhesus macaques infected with the CCR5-tropic SHIV(AD8) generate cross-reactive antibodies that neutralize multiple HIV-1 strains. Proc. Natl. Acad. Sci. USA. 2012;109:19769–19774. doi: 10.1073/pnas.1217443109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nishimura Y., Francis J.N., Donau O.K., Jesteadt E., Sadjadpour R., Smith A.R., Seaman M.S., Welch B.D., Martin M.A., Kay M.S. Prevention and treatment of SHIVAD8 infection in rhesus macaques by a potent d-peptide HIV entry inhibitor. Proc. Natl. Acad. Sci. USA. 2020;117:22436–22442. doi: 10.1073/pnas.2009700117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song R.J., Chenine A.L., Rasmussen R.A., Ruprecht C.R., Mirshahidi S., Grisson R.D., Xu W., Whitney J.B., Goins L.M., Ong H., et al. Molecularly cloned SHIV-1157ipd3N4: A highly replication- competent, mucosally transmissible R5 simian-human immunodeficiency virus encoding HIV clade C Env. J. Virol. 2006;80:8729–8738. doi: 10.1128/JVI.00558-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Keele B.F., Derdeyn C.A. Genetic and antigenic features of the transmitted virus. Curr. Opin. HIV AIDS. 2009;4:352–357. doi: 10.1097/COH.0b013e32832d9fef. [DOI] [PubMed] [Google Scholar]
- 92.Del Prete G.Q., Ailers B., Moldt B., Keele B.F., Estes J.D., Rodriguez A., Sampias M., Oswald K., Fast R., Trubey C.M., et al. Selection of unadapted, pathogenic SHIVs encoding newly transmitted HIV-1 envelope proteins. Cell Host Microbe. 2014;16:412–418. doi: 10.1016/j.chom.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li H., Wang S., Kong R., Ding W., Lee F.H., Parker Z., Kim E., Learn G.H., Hahn P., Policicchio B., et al. Envelope residue 375 substitutions in simian-human immunodeficiency viruses enhance CD4 binding and replication in rhesus macaques. Proc. Natl. Acad. Sci. USA. 2016;113:E3413–E3422. doi: 10.1073/pnas.1606636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O’Brien S.P., Swanstrom A.E., Pegu A., Ko S.-Y., Immonen T.T., Del Prete G.Q., Fennessey C.M., Gorman J., Foulds K.E., Schmidt S.D., et al. Rational design and in vivo selection of SHIVs encoding transmitted/founder subtype C HIV-1 envelopes. PLoS Pathog. 2019;15:e1007632. doi: 10.1371/journal.ppat.1007632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bauer A.M., Ziani W., Lindemuth E., Kuri-Cervantes L., Li H., Lee F.H., Watkins M., Ding W., Xu H., Veazey R., et al. Novel Transmitted/Founder Simian-Human Immunodeficiency Viruses for Human Immunodeficiency Virus Latency and Cure Research. J. Virol. 2020;94:e01659-19. doi: 10.1128/JVI.01659-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bar K.J., Coronado E., Hensley-McBain T., O’Connor M.A., Osborn J.M., Miller C., Gott T.M., Wangari S., Iwayama N., Ahrens C.Y., et al. Simian-Human Immunodeficiency Virus SHIV.CH505 Infection of Rhesus Macaques Results in Persistent Viral Replication and Induces Intestinal Immunopathology. J. Virol. 2019;93:e00372-19. doi: 10.1128/JVI.00372-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miller C.J., Li Q., Abel K., Kim E.Y., Ma Z.M., Wietgrefe S., La Franco-Scheuch L., Compton L., Duan L., Shore M.D., et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J. Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sagar M. HIV-1 transmission biology: Selection and characteristics of infecting viruses. J. Infect. Dis. 2010;202((Suppl. 2)):S289–S296. doi: 10.1086/655656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Keele B.F., Giorgi E.E., Salazar-Gonzalez J.F., Decker J.M., Pham K.T., Salazar M.G., Sun C., Grayson T., Wang S., Li H., et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. USA. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haaland R.E., Sullivan S.T., Evans-Strickfaden T., Lennox J.L., Hart C.E. Female genital tract shedding of CXCR4-tropic HIV Type 1 is associated with a majority population of CXCR4-tropic HIV Type 1 in blood and declining CD4(+) cell counts. AIDS Res. Hum. Retrovir. 2012;28:1524–1532. doi: 10.1089/aid.2012.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gianella S., Mehta S.R., Young J.A., Vargas M.V., Little S.J., Richman D.D., Kosakovsky Pond S.L., Smith D.M. Sexual transmission of predicted CXCR4-tropic HIV-1 likely originating from the source partner’s seminal cells. Virology. 2012;434:2–4. doi: 10.1016/j.virol.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muciaccia B., Padula F., Gandini L., Lenzi A., Stefanini M. HIV-1 chemokine co-receptor CCR5 is expressed on the surface of human spermatozoa. AIDS (Lond. Engl.) 2005;19:1424–1426. doi: 10.1097/01.aids.0000180809.04427.04. [DOI] [PubMed] [Google Scholar]
- 103.Chohan B., Lang D., Sagar M., Korber B., Lavreys L., Richardson B., Overbaugh J. Selection for human immunodeficiency virus type 1 envelope glycosylation variants with shorter V1-V2 loop sequences occurs during transmission of certain genetic subtypes and may impact viral RNA levels. J. Virol. 2005;79:6528–6531. doi: 10.1128/JVI.79.10.6528-6531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gnanakaran S., Bhattacharya T., Daniels M., Keele B.F., Hraber P.T., Lapedes A.S., Shen T., Gaschen B., Krishnamoorthy M., Li H., et al. Recurrent signature patterns in HIV-1 B clade envelope glycoproteins associated with either early or chronic infections. PLoS Pathog. 2011;7:e1002209. doi: 10.1371/journal.ppat.1002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parrish N.F., Gao F., Li H., Giorgi E.E., Barbian H.J., Parrish E.H., Zajic L., Iyer S.S., Decker J.M., Kumar A., et al. Phenotypic properties of transmitted founder HIV-1. Proc. Natl. Acad. Sci. USA. 2013;110:6626–6633. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fenton-May A.E., Dibben O., Emmerich T., Ding H., Pfafferott K., Aasa-Chapman M.M., Pellegrino P., Williams I., Cohen M.S., Gao F., et al. Relative resistance of HIV-1 founder viruses to control by interferon-alpha. Retrovirology. 2013;10:146. doi: 10.1186/1742-4690-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Amerongen H.M., Weltzin R., Farnet C.M., Michetti P., Haseltine W.A., Neutra M.R. Transepithelial transport of HIV-1 by intestinal M cells: A mechanism for transmission of AIDS. J. Acquir. Immune Defic. Syndr. 1991;4:760–765. [PubMed] [Google Scholar]
- 108.Fotopoulos G., Harari A., Michetti P., Trono D., Pantaleo G., Kraehenbuhl J.-P. Transepithelial transport of HIV-1 by M cells is receptor-mediated. Proc. Natl. Acad. Sci. USA. 2002;99:9410–9414. doi: 10.1073/pnas.142586899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spira A.I., Marx P.A., Patterson B.K., Mahoney J., Koup R.A., Wolinsky S.M., Ho D.D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu J., Gardner M.B., Miller C.J. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 2000;74:6087–6095. doi: 10.1128/JVI.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kreiss J.K., Hopkins S.G. The association between circumcision status and human immunodeficiency virus infection among homosexual men. J. Infect. Dis. 1993;168:1404–1408. doi: 10.1093/infdis/168.6.1404. [DOI] [PubMed] [Google Scholar]
- 112.Veazey R.S., Tham I.C., Mansfield K.G., DeMaria M., Forand A.E., Shvetz D.E., Chalifoux L.V., Sehgal P.K., Lackner A.A. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: Highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 2000;74:57–64. doi: 10.1128/JVI.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mattapallil J.J., Douek D.C., Hill B., Nishimura Y., Martin M., Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 114.Ribeiro Dos Santos P., Rancez M., Prétet J.-L., Michel-Salzat A., Messent V., Bogdanova A., Couëdel-Courteille A., Souil E., Cheynier R., Butor C. Rapid dissemination of SIV follows multisite entry after rectal inoculation. PLoS ONE. 2011;6:e19493. doi: 10.1371/journal.pone.0019493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miyake A., Ibuki K., Enose Y., Suzuki H., Horiuchi R., Motohara M., Saito N., Nakasone T., Honda M., Watanabe T., et al. Rapid dissemination of a pathogenic simian/human immunodeficiency virus to systemic organs and active replication in lymphoid tissues following intrarectal infection. J. Gen. Virol. 2006;87:1311–1320. doi: 10.1099/vir.0.81307-0. [DOI] [PubMed] [Google Scholar]
- 116.Haase A.T. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu. Rev. Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 117.Li Q., Duan L., Estes J.D., Ma Z.M., Rourke T., Wang Y., Reilly C., Carlis J., Miller C.J., Haase A.T. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 118.Huang X., Liu X., Meyers K., Liu L., Su B., Wang P., Li Z., Li L., Zhang T., Li N., et al. Cytokine cascade and networks among MSM HIV seroconverters: Implications for early immunotherapy. Sci. Rep. 2016;6:36234. doi: 10.1038/srep36234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Muema D.M., Akilimali N.A., Ndumnego O.C., Rasehlo S.S., Durgiah R., Ojwach D.B.A., Ismail N., Dong M., Moodley A., Dong K.L., et al. Association between the cytokine storm, immune cell dynamics, and viral replicative capacity in hyperacute HIV infection. BMC Med. 2020;18:81. doi: 10.1186/s12916-020-01529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roberts L., Passmore J.-A.S., Williamson C., Little F., Bebell L.M., Mlisana K., Burgers W.A., van Loggerenberg F., Walzl G., Djoba Siawaya J.F., et al. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS (Lond. Engl.) 2010;24:819–831. doi: 10.1097/QAD.0b013e3283367836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stacey A.R., Norris P.J., Qin L., Haygreen E.A., Taylor E., Heitman J., Lebedeva M., DeCamp A., Li D., Grove D., et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J. Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beignon A.S., McKenna K., Skoberne M., Manches O., DaSilva I., Kavanagh D.G., Larsson M., Gorelick R.J., Lifson J.D., Bhardwaj N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Investig. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sabado R.L., Babcock E., Kavanagh D.G., Tjomsland V., Walker B.D., Lifson J.D., Bhardwaj N., Larsson M. Pathways utilized by dendritic cells for binding, uptake, processing and presentation of antigens derived from HIV-1. Eur. J. Immunol. 2007;37:1752–1763. doi: 10.1002/eji.200636981. [DOI] [PubMed] [Google Scholar]
- 124.Romagnani C., Della Chiesa M., Kohler S., Moewes B., Radbruch A., Moretta L., Moretta A., Thiel A. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4+ T helper cells and CD4+ CD25hi T regulatory cells. Eur. J. Immunol. 2005;35:2452–2458. doi: 10.1002/eji.200526069. [DOI] [PubMed] [Google Scholar]
- 125.Florez-Alvarez L., Hernandez J.C., Zapata W. NK Cells in HIV-1 Infection: From Basic Science to Vaccine Strategies. Front. Immunol. 2018;9:2290. doi: 10.3389/fimmu.2018.02290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pardo J., Balkow S., Anel A., Simon M.M. Granzymes are essential for natural killer cell-mediated and perf-facilitated tumor control. Eur. J. Immunol. 2002;32:2881–2886. doi: 10.1002/1521-4141(2002010)32:10<2881::AID-IMMU2881>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 127.Scott-Algara D., Truong L.X., Versmisse P., David A., Luong T.T., Nguyen N.V., Theodorou I., Barré-Sinoussi F., Pancino G. Cutting Edge: Increased NK Cell Activity in HIV-1-Exposed but Uninfected Vietnamese Intravascular Drug Users. J. Immunol. 2003;171:5663–5667. doi: 10.4049/jimmunol.171.11.5663. [DOI] [PubMed] [Google Scholar]
- 128.Oliva A., Kinter A.L., Vaccarezza M., Rubbert A., Catanzaro A., Moir S., Monaco J., Ehler L., Mizell S., Jackson R., et al. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J. Clin. Investig. 1998;102:223–231. doi: 10.1172/JCI2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Demers K.R., Reuter M.A., Betts M.R. CD8(+) T-cell effector function and transcriptional regulation during HIV pathogenesis. Immunol Rev. 2013;254:190–206. doi: 10.1111/imr.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ndhlovu Z.M., Kamya P., Mewalal N., Kløverpris H.N., Nkosi T., Pretorius K., Laher F., Ogunshola F., Chopera D., Shekhar K., et al. Magnitude and Kinetics of CD8+ T Cell Activation during Hyperacute HIV Infection Impact Viral Set Point. Immunity. 2015;43:591–604. doi: 10.1016/j.immuni.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bernardin F., Kong D., Peddada L., Baxter-Lowe L.A., Delwart E. Human immunodeficiency virus mutations during the first month of infection are preferentially found in known cytotoxic T-lymphocyte epitopes. J. Virol. 2005;79:11523–11528. doi: 10.1128/JVI.79.17.11523-11528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Goonetilleke N., Liu M.K.P., Salazar-Gonzalez J.F., Ferrari G., Giorgi E., Ganusov V.V., Keele B.F., Learn G.H., Turnbull E.L., Salazar M.G., et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J. Exp. Med. 2009;206:1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tomaras G.D., Yates N.L., Liu P., Qin L., Fouda G.G., Chavez L.L., Decamp A.C., Parks R.J., Ashley V.C., Lucas J.T., et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: Virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 2008;82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Levesque M.C., Moody M.A., Hwang K.K., Marshall D.J., Whitesides J.F., Amos J.D., Gurley T.C., Allgood S., Haynes B.B., Vandergrift N.A., et al. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 2009;6:e1000107. doi: 10.1371/journal.pmed.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Le Hingrat Q., Sereti I., Landay A.L., Pandrea I., Apetrei C. The Hitchhiker Guide to CD4(+) T-Cell Depletion in Lentiviral Infection. A Critical Review of the Dynamics of the CD4(+) T Cells in SIV and HIV Infection. Front. Immunol. 2021;12:695674. doi: 10.3389/fimmu.2021.695674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lavreys L., Baeten J.M., Chohan V., McClelland R.S., Hassan W.M., Richardson B.A., Mandaliya K., Achola J.O.N., Overbaugh J. Higher Set Point Plasma Viral Load and More-Severe Acute HIV Type 1 (HIV-1) Illness Predict Mortality among High-Risk HIV-1–Infected African Women. Clin. Infect. Dis. 2006;42:1333–1339. doi: 10.1086/503258. [DOI] [PubMed] [Google Scholar]
- 137.Mellors J.W., Rinaldo C.R., Gupta P., White R.M., Todd J.A., Kingsley L.A. Prognosis in HIV-1 Infection Predicted by the Quantity of Virus in Plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 138.Deeks S.G., Kitchen C.M.R., Liu L., Guo H., Gascon R., Narváez A.B., Hunt P., Martin J.N., Kahn J.O., Levy J., et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 139.Zaunders J.J., Cunningham P.H., Kelleher A.D., Kaufmann G.R., Jaramillo A.B., Wright R., Smith D., Grey P., Vizzard J., Carr A., et al. Potent antiretroviral therapy of primary human immunodeficiency virus type 1 (HIV-1) infection: Partial normalization of T lymphocyte subsets and limited reduction of HIV-1 DNA despite clearance of plasma viremia. J. Infect. Dis. 1999;180:320–329. doi: 10.1086/314880. [DOI] [PubMed] [Google Scholar]
- 140.Tilling R., Kinloch S., Goh L.E., Cooper D., Perrin L., Lampe F., Zaunders J., Hoen B., Tsoukas C., Andersson J., et al. Parallel decline of CD8+/CD38++ T cells and viraemia in response to quadruple highly active antiretroviral therapy in primary HIV infection. AIDS (Lond. Engl.) 2002;16:589–596. doi: 10.1097/00002030-200203080-00010. [DOI] [PubMed] [Google Scholar]
- 141.Mohri H., Perelson A.S., Tung K., Ribeiro R.M., Ramratnam B., Markowitz M., Kost R., Hurley A., Weinberger L., Cesar D., et al. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J. Exp. Med. 2001;194:1277–1287. doi: 10.1084/jem.194.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Saravolatz L.D., Winslow D.L., Collins G., Hodges J.S., Pettinelli C., Stein D.S., Markowitz N., Reves R., Loveless M.O., Crane L., et al. Zidovudine alone or in combination with didanosine or zalcitabine in HIV-infected patients with the acquired immunodeficiency syndrome or fewer than 200 CD4 cells per cubic millimeter. Investigators for the Terry Beirn Community Programs for Clinical Research on AIDS. N. Engl. J. Med. 1996;335:1099–1106. doi: 10.1056/nejm199610103351503. [DOI] [PubMed] [Google Scholar]
- 143.Deeks S.G., Overbaugh J., Phillips A., Buchbinder S. HIV infection. Nat. Rev. Dis. Primers. 2015;1:15035. doi: 10.1038/nrdp.2015.35. [DOI] [PubMed] [Google Scholar]
- 144.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. [(accessed on 9 September 2021)]; Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Available online: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf.
- 145.Eisinger R.W., Dieffenbach C.W., Fauci A.S. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable. JAMA. 2019;321:451–452. doi: 10.1001/jama.2018.21167. [DOI] [PubMed] [Google Scholar]
- 146.Guadalupe M., Reay E., Sankaran S., Prindiville T., Flamm J., McNeil A., Dandekar S. Severe CD4+ T-Cell Depletion in Gut Lymphoid Tissue during Primary Human Immunodeficiency Virus Type 1 Infection and Substantial Delay in Restoration following Highly Active Antiretroviral Therapy. J. Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Verhoeven D., Sankaran S., Silvey M., Dandekar S. Antiviral Therapy during Primary Simian Immunodeficiency Virus Infection Fails To Prevent Acute Loss of CD4+ T Cells in Gut Mucosa but Enhances Their Rapid Restoration through Central Memory T Cells. J. Virol. 2008;82:4016–4027. doi: 10.1128/JVI.02164-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Macal M., Sankaran S., Chun T.W., Reay E., Flamm J., Prindiville T.J., Dandekar S. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 2008;1:475–488. doi: 10.1038/mi.2008.35. [DOI] [PubMed] [Google Scholar]
- 149.Deleage C., Schuetz A., Alvord W.G., Johnston L., Hao X.-P., Morcock D.R., Rerknimitr R., Fletcher J.L.K., Puttamaswin S., Phanuphak N., et al. Impact of early cART in the gut during acute HIV infection. JCI Insight. 2016;1 doi: 10.1172/jci.insight.87065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kelly C., Gaskell K.M., Richardson M., Klein N., Garner P., MacPherson P. Discordant Immune Response with Antiretroviral Therapy in HIV-1: A Systematic Review of Clinical Outcomes. PLoS ONE. 2016;11:e0156099. doi: 10.1371/journal.pone.0156099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schuetz A., Deleage C., Sereti I., Rerknimitr R., Phanuphak N., Phuang-Ngern Y., Estes J.D., Sandler N.G., Sukhumvittaya S., Marovich M., et al. Initiation of ART during Early Acute HIV Infection Preserves Mucosal Th17 Function and Reverses HIV-Related Immune Activation. PLoS Pathog. 2014;10:e1004543. doi: 10.1371/journal.ppat.1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.de Paula H.H.S., Ferreira A.C.G., Caetano D.G., Delatorre E., Teixeira S.L.M., Coelho L.E., João E.G., de Andrade M.M., Cardoso S.W., Grinsztejn B., et al. Reduction of inflammation and T cell activation after 6 months of cART initiation during acute, but not in early chronic HIV-1 infection. Retrovirology. 2018;15:76. doi: 10.1186/s12977-018-0458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ghislain M., Bastard J.-P., Meyer L., Capeau J., Fellahi S., Gérard L., May T., Simon A., Vigouroux C., Goujard C., et al. Late Antiretroviral Therapy (ART) Initiation Is Associated with Long-Term Persistence of Systemic Inflammation and Metabolic Abnormalities. PLoS ONE. 2015;10:e0144317. doi: 10.1371/journal.pone.0144317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marcus J.L., Leyden W.A., Alexeeff S.E., Anderson A.N., Hechter R.C., Hu H., Lam J.O., Towner W.J., Yuan Q., Horberg M.A., et al. Comparison of Overall and Comorbidity-Free Life Expectancy Between Insured Adults With and Without HIV Infection, 2000–2016. JAMA Netw. Open. 2020;3:e207954. doi: 10.1001/jamanetworkopen.2020.7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hirsch V.M., Dapolito G., Johnson P.R., Elkins W.R., London W.T., Montali R.J., Goldstein S., Brown C. Induction of AIDS by simian immunodeficiency virus from an African green monkey: Species-specific variation in pathogenicity correlates with the extent of in vivo replication. J. Virol. 1995;69:955–967. doi: 10.1128/jvi.69.2.955-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen Z., Telfer P., Reed P., Zhang L., Getti A., Ho D.D., Marx P.A. Isolation and characterization of the first simian immunodeficiency virus from a feral sooty mangabey (Cercocebus atys) in West Africa. J. Med. Primatol. 1995;24:108–115. doi: 10.1111/j.1600-0684.1995.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 157.Smith S.M., Makuwa M., Lee F., Gettie A., Russo C., Marx P.A. SIVrcm infection of macaques. J. Med. Primatol. 1998;27:94–98. doi: 10.1111/j.1600-0684.1998.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 158.Osterhaus A.D., Pedersen N., van Amerongen G., Frankenhuis M.T., Marthas M., Reay E., Rose T.M., Pamungkas J., Bosch M.L. Isolation and partial characterization of a lentivirus from talapoin monkeys (Myopithecus talapoin) Virology. 1999;260:116–124. doi: 10.1006/viro.1999.9794. [DOI] [PubMed] [Google Scholar]
- 159.Takehisa J., Harada Y., Ndembi N., Mboudjeka I., Taniguchi Y., Ngansop C., Kuate S., Zekeng L., Ibuki K., Shimada T., et al. Natural infection of wild-born mandrills (Mandrillus sphinx) with two different types of simian immunodeficiency virus. AIDS Res. Hum. Retrovir. 2001;17:1143–1154. doi: 10.1089/088922201316912754. [DOI] [PubMed] [Google Scholar]
- 160.Silvestri G., Fedanov A., Germon S., Kozyr N., Kaiser W.J., Garber D.A., McClure H., Feinberg M.B., Staprans S.I. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J. Virol. 2005;79:4043–4054. doi: 10.1128/JVI.79.7.4043-4054.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wagner T.A., McLaughlin S., Garg K., Cheung C.Y., Larsen B.B., Styrchak S., Huang H.C., Edlefsen P.T., Mullins J.I., Frenkel L.M. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science. 2014;345:570–573. doi: 10.1126/science.1256304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cohn L.B., Silva I.T., Oliveira T.Y., Rosales R.A., Parrish E.H., Learn G.H., Hahn B.H., Czartoski J.L., McElrath M.J., Lehmann C., et al. HIV-1 integration landscape during latent and active infection. Cell. 2015;160:420–432. doi: 10.1016/j.cell.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ferris A.L., Wells D.W., Guo S., Del Prete G.Q., Swanstrom A.E., Coffin J.M., Wu X., Lifson J.D., Hughes S.H. Clonal expansion of SIV-infected cells in macaques on antiretroviral therapy is similar to that of HIV-infected cells in humans. PLoS Pathog. 2019;15:e1007869. doi: 10.1371/journal.ppat.1007869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sellier P., Mannioui A., Bourry O., Dereuddre-Bosquet N., Delache B., Brochard P., Calvo J., Prévot S., Roques P. Antiretroviral Treatment Start-Time during Primary SIVmac Infection in Macaques Exerts a Different Impact on Early Viral Replication and Dissemination. PLoS ONE. 2010;5:e10570. doi: 10.1371/journal.pone.0010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mannioui A., Bourry O., Sellier P., Delache B., Brochard P., Andrieu T., Vaslin B., Karlsson I., Roques P., Le Grand R. Dynamics of viral replication in blood and lymphoid tissues during SIVmac251 infection of macaques. Retrovirology. 2009;6:106. doi: 10.1186/1742-4690-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bourry O., Mannioui A., Sellier P., Roucairol C., Durand-Gasselin L., Dereuddre-Bosquet N., Benech H., Roques P., Le Grand R. Effect of a short-term HAART on SIV load in macaque tissues is dependent on time of initiation and antiviral diffusion. Retrovirology. 2010;7:78. doi: 10.1186/1742-4690-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Canary L.A., Vinton C.L., Morcock D.R., Pierce J.B., Estes J.D., Brenchley J.M., Klatt N.R. Rate of AIDS Progression Is Associated with Gastrointestinal Dysfunction in Simian Immunodeficiency Virus–Infected Pigtail Macaques. J. Immunol. 2013;190:2959–2965. doi: 10.4049/jimmunol.1202319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hao X.P., Lucero C.M., Turkbey B., Bernardo M.L., Morcock D.R., Deleage C., Trubey C.M., Smedley J., Klatt N.R., Giavedoni L.D., et al. Experimental colitis in SIV-uninfected rhesus macaques recapitulates important features of pathogenic SIV infection. Nat. Commun. 2015;6:8020. doi: 10.1038/ncomms9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pandrea I., Xu C., Stock J.L., Frank D.N., Ma D., Policicchio B.B., He T., Kristoff J., Cornell E., Haret-Richter G.S., et al. Antibiotic and Antiinflammatory Therapy Transiently Reduces Inflammation and Hypercoagulation in Acutely SIV-Infected Pigtailed Macaques. PLoS Pathog. 2016;12:e1005384. doi: 10.1371/journal.ppat.1005384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schechter M.E., Andrade B.B., He T., Richter G.H., Tosh K.W., Policicchio B.B., Singh A., Raehtz K.D., Sheikh V., Ma D., et al. Inflammatory monocytes expressing tissue factor drive SIV and HIV coagulopathy. Sci. Transl. Med. 2017;9:eaam5441. doi: 10.1126/scitranslmed.aam5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zink M.C., Suryanarayana K., Mankowski J.L., Shen A., Piatak M., Jr., Spelman J.P., Carter D.L., Adams R.J., Lifson J.D., Clements J.E. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J. Virol. 1999;73:10480–10488. doi: 10.1128/JVI.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Matsuda K., Riddick N.E., Lee C.A., Puryear S.B., Wu F., Lafont B.A.P., Whitted S., Hirsch V.M. A SIV molecular clone that targets the CNS and induces neuroAIDS in rhesus macaques. PLoS Pathog. 2017;13:e1006538. doi: 10.1371/journal.ppat.1006538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gama L., Abreu C.M., Shirk E.N., Price S.L., Li M., Laird G.M., Pate K.A.M., Wietgrefe S.W., O’Connor S.L., Pianowski L., et al. Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. AIDS (Lond. Engl.) 2017;31:5–14. doi: 10.1097/QAD.0000000000001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Giorgi J.V., Hultin L.E., McKeating J.A., Johnson T.D., Owens B., Jacobson L.P., Shih R., Lewis J., Wiley D.J., Phair J.P., et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 175.Hazenberg M.D., Otto S.A., van Benthem B.H., Roos M.T., Coutinho R.A., Lange J.M., Hamann D., Prins M., Miedema F. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS (Lond. Engl.) 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 176.Chevalier M.F., Petitjean G., Dunyach-Rémy C., Didier C., Girard P.M., Manea M.E., Campa P., Meyer L., Rouzioux C., Lavigne J.P., et al. The Th17/Treg ratio, IL-1RA and sCD14 levels in primary HIV infection predict the T-cell activation set point in the absence of systemic microbial translocation. PLoS Pathog. 2013;9:e1003453. doi: 10.1371/journal.ppat.1003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Brenchley J.M., Paiardini M., Knox K.S., Asher A.I., Cervasi B., Asher T.E., Scheinberg P., Price D.A., Hage C.A., Kholi L.M., et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Favre D., Lederer S., Kanwar B., Ma Z.M., Proll S., Kasakow Z., Mold J., Swainson L., Barbour J.D., Baskin C.R., et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Brenchley J.M., Price D.A., Schacker T.W., Asher T.E., Silvestri G., Rao S., Kazzaz Z., Bornstein E., Lambotte O., Altmann D., et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 180.Estes J.D., Harris L.D., Klatt N.R., Tabb B., Pittaluga S., Paiardini M., Barclay G.R., Smedley J., Pung R., Oliveira K.M., et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010;6:e1001052. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Brenchley J.M., Douek D.C. Microbial translocation across the GI tract. Annu. Rev. Immunol. 2012;30:149–173. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kristoff J., Haret-Richter G., Ma D., Ribeiro R.M., Xu C., Cornell E., Stock J.L., He T., Mobley A.D., Ross S., et al. Early microbial translocation blockade reduces SIV-mediated inflammation and viral replication. J. Clin. Investig. 2014;124:2802–2806. doi: 10.1172/JCI75090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kovacs A., Al-Harthi L., Christensen S., Mack W., Cohen M., Landay A. CD8+ T Cell Activation in Women Coinfected with Human Immunodeficiency Virus Type 1 and Hepatitis C Virus. J. Infect. Dis. 2008;197:1402. doi: 10.1086/587696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bautista-Amorocho H., Castellanos-Domínguez Y.Z., Rodríguez-Villamizar L.A., Velandia-Cruz S.A., Becerra-Peña J.A., Farfán-García A.E. Epidemiology, Risk Factors and Genotypes of HBV in HIV-Infected Patients in the Northeast Region of Colombia: High Prevalence of Occult Hepatitis B and F3 Subgenotype Dominance. PLoS ONE. 2014;9:e114272. doi: 10.1371/journal.pone.0114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sheth P.M., Sunderji S., Shin L.Y.Y., Rebbapragada A., Huibner S., Kimani J., MacDonald K.S., Ngugi E., Bwayo J.J., Moses S., et al. Coinfection with Herpes Simplex Virus Type 2 Is Associated with Reduced HIV-Specific T Cell Responses and Systemic Immune Activation. J. Infect. Dis. 2008;197:1394–1401. doi: 10.1086/587697. [DOI] [PubMed] [Google Scholar]
- 186.Gianella S., Strain M.C., Rought S.E., Vargas M.V., Little S.J., Richman D.D., Spina C.A., Smith D.M. Associations between virologic and immunologic dynamics in blood and in the male genital tract. J. Virol. 2012;86:1307–1315. doi: 10.1128/JVI.06077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Freeman M.L., Mudd J.C., Shive C.L., Younes S.A., Panigrahi S., Sieg S.F., Lee S.A., Hunt P.W., Calabrese L.H., Gianella S., et al. CD8 T-cell expansion and inflammation linked to CMV coinfection in ART-treated HIV infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016;62:392–396. doi: 10.1093/cid/civ840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Petrara M.R., Cattelan A.M., Zanchetta M., Sasset L., Freguja R., Gianesin K., Cecchetto M.G., Carmona F., De Rossi A. Epstein-Barr Virus load and immune activation in Human Immunodeficiency Virus type 1-infected patients. J. Clin. Virol. 2012;53:195–200. doi: 10.1016/j.jcv.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 189.Boulougoura A., Sereti I. HIV infection and immune activation: The role of coinfections. Curr Opin HIV AIDS. 2016;11:191–200. doi: 10.1097/COH.0000000000000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Friis-Møller N., Reiss P., Sabin C.A., Weber R., Monforte A., El-Sadr W., Thiébaut R., De Wit S., Kirk O., Fontas E., et al. Class of antiretroviral drugs and the risk of myocardial infarction. N. Engl. J. Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 191.Contento R.L., Molon B., Boularan C., Pozzan T., Manes S., Marullo S., Viola A. CXCR4–CCR5: A couple modulating T cell functions. Proc. Natl. Acad. Sci.USA. 2008;105:10101–10106. doi: 10.1073/pnas.0804286105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gornalusse G.G., Mummidi S., Gaitan A.A., Jimenez F., Ramsuran V., Picton A., Rogers K., Manoharan M.S., Avadhanam N., Murthy K.K., et al. Epigenetic mechanisms, T-cell activation, and CCR5 genetics interact to regulate T-cell expression of CCR5, the major HIV-1 coreceptor. Proc. Natl. Acad. Sci. USA. 2015;112:E4762–E4771. doi: 10.1073/pnas.1423228112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kawakami K., Scheidereit C., Roeder R.G. Identification and purification of a human immunoglobulin-enhancer-binding protein (NF-kappa B) that activates transcription from a human immunodeficiency virus type 1 promoter in vitro. Proc. Natl. Acad. Sci. USA. 1988;85:4700–4704. doi: 10.1073/pnas.85.13.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Papagno L., Spina C.A., Marchant A., Salio M., Rufer N., Little S., Dong T., Chesney G., Waters A., Easterbrook P., et al. Immune Activation and CD8+ T-Cell Differentiation towards Senescence in HIV-1 Infection. PLOS Biol. 2004;2:e20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cobos Jiménez V., Wit F.W., Joerink M., Maurer I., Harskamp A.M., Schouten J., Prins M., van Leeuwen E.M., Booiman T., Deeks S.G., et al. T-Cell Activation Independently Associates With Immune Senescence in HIV-Infected Recipients of Long-term Antiretroviral Treatment. J. Infect. Dis. 2016;214:216–225. doi: 10.1093/infdis/jiw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Day C.L., Kaufmann D.E., Kiepiela P., Brown J.A., Moodley E.S., Reddy S., Mackey E.W., Miller J.D., Leslie A.J., DePierres C., et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 197.Trautmann L., Janbazian L., Chomont N., Said E.A., Gimmig S., Bessette B., Boulassel M.-R., Delwart E., Sepulveda H., Balderas R.S., et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 198.Zhang J.-Y., Zhang Z., Wang X., Fu J.-L., Yao J., Jiao Y., Chen L., Zhang H., Wei J., Jin L., et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 199.Evans V.A., van der Sluis R.M., Solomon A., Dantanarayana A., McNeil C., Garsia R., Palmer S., Fromentin R., Chomont N., Sékaly R.-P., et al. Programmed cell death-1 contributes to the establishment and maintenance of HIV-1 latency. AIDS (Lond. Engl.) 2018;32:1491–1497. doi: 10.1097/QAD.0000000000001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.McGary C.S., Deleage C., Harper J., Micci L., Ribeiro S.P., Paganini S., Kuri-Cervantes L., Benne C., Ryan E.S., Balderas R., et al. CTLA-4+PD-1- Memory CD4+ T Cells Critically Contribute to Viral Persistence in Antiretroviral Therapy-Suppressed, SIV-Infected Rhesus Macaques. Immunity. 2017;47:776–788.e775. doi: 10.1016/j.immuni.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Schweneker M., Favre D., Martin J.N., Deeks S.G., McCune J.M. HIV-Induced Changes in T Cell Signaling Pathways. J. Immunol. 2008;180:6490–6500. doi: 10.4049/jimmunol.180.10.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Estes J.D., Wietgrefe S., Schacker T., Southern P., Beilman G., Reilly C., Milush J.M., Lifson J.D., Sodora D.L., Carlis J.V., et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J. Infect. Dis. 2007;195:551–561. doi: 10.1086/510852. [DOI] [PubMed] [Google Scholar]
- 203.Estes J.D., Haase A.T., Schacker T.W. The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Semin. Immunol. 2008;20:181–186. doi: 10.1016/j.smim.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zeng M., Smith A.J., Wietgrefe S.W., Southern P.J., Schacker T.W., Reilly C.S., Estes J.D., Burton G.F., Silvestri G., Lifson J.D., et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J. Clin. Investig. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Estes J.D. Pathobiology of HIV/SIV-associated changes in secondary lymphoid tissues. Immunol Rev. 2013;254:65–77. doi: 10.1111/imr.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schottenfeld D., Beebe-Dimmer J. Chronic inflammation: A common and important factor in the pathogenesis of neoplasia. CA: A Cancer J. Clin. 2006;56:69–83. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]
- 207.Dubrow R., Silverberg M.J., Park L.S., Crothers K., Justice A.C. HIV infection, aging, and immune function: Implications for cancer risk and prevention. Curr. Opin. Oncol. 2012;24:506–516. doi: 10.1097/CCO.0b013e328355e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Triant V.A., Lee H., Hadigan C., Grinspoon S.K. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J. Clin. Endocrinol. Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hsue P.Y., Deeks S.G., Hunt P.W. Immunologic basis of cardiovascular disease in HIV-infected adults. J. Infect. Dis. 2012;205((Suppl. 3)):S375–S382. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Levi M., van der Poll T., Büller H.R. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109:2698–2704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 211.He T., Falwell E., Brocca-Cofano E., Pandrea I. Modeling aging in HIV infection in nonhuman primates to address an emerging challenge of the post-ART era. Curr. Opin. Virol. 2017;25:66–75. doi: 10.1016/j.coviro.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dudgeon W.D., Phillips K.D., Carson J.A., Brewer R.B., Durstine J.L., Hand G.A. Counteracting muscle wasting in HIV-infected individuals. HIV Med. 2006;7:299–310. doi: 10.1111/j.1468-1293.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 213.Lemoine M., Serfaty L., Capeau J. From nonalcoholic fatty liver to nonalcoholic steatohepatitis and cirrhosis in HIV-infected patients: Diagnosis and management. Curr. Opin. Infect. Dis. 2012;25:10–16. doi: 10.1097/QCO.0b013e32834ef599. [DOI] [PubMed] [Google Scholar]
- 214.Sanmarti M., Ibáñez L., Huertas S., Badenes D., Dalmau D., Slevin M., Krupinski J., Popa-Wagner A., Jaen A. HIV-associated neurocognitive disorders. J. Mol. Psychiatry. 2014;2:2. doi: 10.1186/2049-9256-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Klatt N.R., Funderburg N.T., Brenchley J.M. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21:6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kim W.K., Sun Y., Do H., Autissier P., Halpern E.F., Piatak M., Jr., Lifson J.D., Burdo T.H., McGrath M.S., Williams K. Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage. J. Leukoc Biol. 2010;87:557–567. doi: 10.1189/jlb.0209082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: Their role in infection and inflammation. J. Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 218.Dandekar S. Pathogenesis of HIV in the gastrointestinal tract. Curr. HIV/AIDS Rep. 2007;4:10–15. doi: 10.1007/s11904-007-0002-0. [DOI] [PubMed] [Google Scholar]
- 219.Brenchley J.M., Price D.A., Douek D.C. HIV disease: Fallout from a mucosal catastrophe? Nat. Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 220.Brenchley J.M., Douek D.C. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Grossman Z., Meier-Schellersheim M., Paul W.E., Picker L.J. Pathogenesis of HIV infection: What the virus spares is as important as what it destroys. Nat. Med. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 222.Douek D.C., Roederer M., Koup R.A. Emerging Concepts in the Immunopathogenesis of AIDS. Annu. Rev. Med. 2008;60:471–484. doi: 10.1146/annurev.med.60.041807.123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Veazey R.S., Mansfield K.G., Tham I.C., Carville A.C., Shvetz D.E., Forand A.E., Lackner A.A. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 2000;74:11001–11007. doi: 10.1128/JVI.74.23.11001-11007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Veazey R.S., Marx P.A., Lackner A.A. The mucosal immune system: Primary target for HIV infection and AIDS. Trends Immunol. 2001;22:626–633. doi: 10.1016/S1471-4906(01)02039-7. [DOI] [PubMed] [Google Scholar]
- 225.Veazey R.S., Lackner A.A. Getting to the guts of HIV pathogenesis. J. Exp. Med. 2004;200:697–700. doi: 10.1084/jem.20041464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Picker L.J., Watkins D.I. HIV pathogenesis: The first cut is the deepest. Nat. Immunol. 2005;6:430–432. doi: 10.1038/ni0505-430. [DOI] [PubMed] [Google Scholar]
- 227.Steele A.K., Lee E.J., Manuzak J.A., Dillon S.M., Beckham J.D., McCarter M.D., Santiago M.L., Wilson C.C. Microbial exposure alters HIV-1-induced mucosal CD4+ T cell death pathways Ex vivo. Retrovirology. 2014;11:14. doi: 10.1186/1742-4690-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Doitsh G., Galloway N.L., Geng X., Yang Z., Monroe K.M., Zepeda O., Hunt P.W., Hatano H., Sowinski S., Muñoz-Arias I., et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Paiardini M., Frank I., Pandrea I., Apetrei C., Silvestri G. Mucosal immune dysfunction in AIDS pathogenesis. AIDS Rev. 2008;10:36–46. [PubMed] [Google Scholar]
- 230.Sandler N.G., Douek D.C. Microbial translocation in HIV infection: Causes, consequences and treatment opportunities. Nat. Reviews. Microbiol. 2012;10:655–666. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- 231.Kovacs S.B., Sheikh V., Thompson W.L., Morcock D.R., Perez-Diez A., Yao M.D., Rupert A.W., Utay N.S., Roby G., Freeman A.F., et al. T-cell depletion in the colonic mucosa of patients with idiopathic CD4 lymphopenia. J. Infect. Dis. 2015 doi: 10.1093/infdis/jiv282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sodora D.L., Silvestri G. Immune activation and AIDS pathogenesis. AIDS (Lond. Engl.) 2008;22:439–446. doi: 10.1097/QAD.0b013e3282f2dbe7. [DOI] [PubMed] [Google Scholar]
- 233.Pandrea I.V., Gautam R., Ribeiro R.M., Brenchley J.M., Butler I.F., Pattison M., Rasmussen T., Marx P.A., Silvestri G., Lackner A.A., et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J. Immunol. 2007;179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pandrea I., Gaufin T., Brenchley J.M., Gautam R., Monjure C., Gautam A., Coleman C., Lackner A.A., Ribeiro R.M., Douek D.C., et al. Cutting edge: Experimentally induced immune activation in natural hosts of simian immunodeficiency virus induces significant increases in viral replication and CD4+ T cell depletion. J. Immunol. 2008;181:6687–6691. doi: 10.4049/jimmunol.181.10.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Silvestri G., Paiardini M., Pandrea I., Lederman M.M., Sodora D.L. Understanding the benign nature of SIV infection in natural hosts. J. Clin. Investig. 2007;117:3148–3154. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Apetrei C., Gormus B., Pandrea I., Metzger M., ten Haaft P., Martin L.N., Bohm R., Alvarez X., Koopman G., Murphey-Corb M., et al. Direct inoculation of simian immunodeficiency virus from sooty mangabeys in black mangabeys (Lophocebus aterrimus): First evidence of AIDS in a heterologous African species and different pathologic outcomes of experimental infection. J. Virol. 2004;78:11506–11518. doi: 10.1128/JVI.78.21.11506-11518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ling B., Apetrei C., Pandrea I., Veazey R.S., Lackner A.A., Gormus B., Marx P.A. Classic AIDS in a sooty mangabey after an 18-year natural infection. J. Virol. 2004;78:8902–8908. doi: 10.1128/JVI.78.16.8902-8908.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pandrea I., Onanga R., Rouquet P., Bourry O., Ngari P., Wickings E.J., Roques P., Apetrei C. Chronic SIV infection ultimately causes immunodeficiency in African non-human primates. AIDS (Lond. Engl.) 2001;15:2461–2462. doi: 10.1097/00002030-200112070-00019. [DOI] [PubMed] [Google Scholar]
- 239.Pandrea I., Sodora D.L., Silvestri G., Apetrei C. Into the wild: Simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol. 2008;29:419–428. doi: 10.1016/j.it.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Apetrei C., Gautam R., Sumpter B., Carter A.C., Gaufin T., Staprans S.I., Else J., Barnes M., Cao R., Jr., Garg S., et al. Virus subtype-specific features of natural simian immunodeficiency virus SIVsmm infection in sooty mangabeys. J. Virol. 2007;81:7913–7923. doi: 10.1128/JVI.00281-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Broussard S.R., Staprans S.I., White R., Whitehead E.M., Feinberg M.B., Allan J.S. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J. Virol. 2001;75:2262–2275. doi: 10.1128/JVI.75.5.2262-2275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gueye A., Diop O.M., Ploquin M.J., Kornfeld C., Faye A., Cumont M.C., Hurtrel B., Barré-Sinoussi F., Müller-Trutwin M.C. Viral load in tissues during the early and chronic phase of non-pathogenic SIVagm infection. J. Med. Primatol. 2004;33:83–97. doi: 10.1111/j.1600-0684.2004.00057.x. [DOI] [PubMed] [Google Scholar]
- 243.Onanga R., Kornfeld C., Pandrea I., Estaquier J., Souquière S., Rouquet P., Mavoungou V.P., Bourry O., M’Boup S., Barré-Sinoussi F., et al. High levels of viral replication contrast with only transient changes in CD4(+) and CD8(+) cell numbers during the early phase of experimental infection with simian immunodeficiency virus SIVmnd-1 in Mandrillus sphinx. J. Virol. 2002;76:10256–10263. doi: 10.1128/JVI.76.20.10256-10263.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Pandrea I., Silvestri G., Onanga R., Veazey R.S., Marx P.A., Hirsch V., Apetrei C. Simian immunodeficiency viruses replication dynamics in African non-human primate hosts: Common patterns and species-specific differences. J. Med. Primatol. 2006;35:194–201. doi: 10.1111/j.1600-0684.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- 245.Souquière S., Onanga R., Makuwa M., Pandrea I., Ngari P., Rouquet P., Bourry O., Kazanji M., Apetrei C., Simon F., et al. Simian immunodeficiency virus types 1 and 2 (SIV mnd 1 and 2) have different pathogenic potentials in rhesus macaques upon experimental cross-species transmission. J. Gen. Virol. 2009;90:488–499. doi: 10.1099/vir.0.005181-0. [DOI] [PubMed] [Google Scholar]
- 246.Pandrea I., Apetrei C., Dufour J., Dillon N., Barbercheck J., Metzger M., Jacquelin B., Bohm R., Marx P.A., Barre-Sinoussi F., et al. Simian immunodeficiency virus SIVagm.sab infection of Caribbean African green monkeys: A new model for the study of SIV pathogenesis in natural hosts. J. Virol. 2006;80:4858–4867. doi: 10.1128/JVI.80.10.4858-4867.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pandrea I., Apetrei C., Gordon S., Barbercheck J., Dufour J., Bohm R., Sumpter B., Roques P., Marx P.A., Hirsch V.M., et al. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2006;109:1069–1076. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pandrea I., Parrish N.F., Raehtz K., Gaufin T., Barbian H.J., Ma D., Kristoff J., Gautam R., Zhong F., Haret-Richter G.S., et al. Mucosal simian immunodeficiency virus transmission in African green monkeys: Susceptibility to infection is proportional to target cell availability at mucosal sites. J. Virol. 2012;86:4158–4168. doi: 10.1128/JVI.07141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Dunham R., Pagliardini P., Gordon S., Sumpter B., Engram J., Moanna A., Paiardini M., Mandl J.N., Lawson B., Garg S., et al. The AIDS resistance of naturally SIV-infected sooty mangabeys is independent of cellular immunity to the virus. Blood. 2006;108:209–217. doi: 10.1182/blood-2005-12-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Schmitz J.E., Zahn R.C., Brown C.R., Rett M.D., Li M., Tang H., Pryputniewicz S., Byrum R.A., Kaur A., Montefiori D.C., et al. Inhibition of Adaptive Immune Responses Leads to a Fatal Clinical Outcome in SIV-Infected Pigtailed Macaques but Not Vervet African Green Monkeys. PLoS Pathog. 2009;5:e1000691. doi: 10.1371/journal.ppat.1000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zahn R.C., Rett M.D., Li M., Tang H., Korioth-Schmitz B., Balachandran H., White R., Pryputniewicz S., Letvin N.L., Kaur A., et al. Suppression of adaptive immune responses during primary SIV infection of sabaeus African green monkeys delays partial containment of viremia but does not induce disease. Blood. 2010;115:3070–3078. doi: 10.1182/blood-2009-10-245225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Klatt N.R., Canary L.A., Vanderford T.H., Vinton C.L., Engram J.C., Dunham R.M., Cronise H.E., Swerczek J.M., Lafont B.A., Picker L.J., et al. Dynamics of simian immunodeficiency virus SIVmac239 infection in pigtail macaques. J. Virol. 2012;86:1203–1213. doi: 10.1128/JVI.06033-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Compton A.A., Emerman M. Convergence and Divergence in the Evolution of the APOBEC3G-Vif Interaction Reveal Ancient Origins of Simian Immunodeficiency Viruses. PLoS Pathog. 2013;9:e1003135. doi: 10.1371/journal.ppat.1003135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Gifford R.J., Katzourakis A., Tristem M., Pybus O.G., Winters M., Shafer R.W. A transitional endogenous lentivirus from the genome of a basal primate and implications for lentivirus evolution. Proc. Natl. Acad. Sci. USA. 2008;105:20362–20367. doi: 10.1073/pnas.0807873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Worobey M., Telfer P., Souquière S., Hunter M., Coleman Clint A., Metzger Michael J., Reed P., Makuwa M., Hearn G., Honarvar S., et al. Island Biogeography Reveals the Deep History of SIV. Science. 2010;329:1487. doi: 10.1126/science.1193550. [DOI] [PubMed] [Google Scholar]
- 256.Apetrei C., Gaufin T., Gautam R., Vinton C., Hirsch V., Lewis M., Brenchley J., Pandrea I. Pattern of SIVagm Infection in Patas Monkeys Suggests that Host Adaptation to Simian Immunodeficiency Virus Infection May Result in Resistance to Infection and Virus Extinction. J. Infect. Dis. 2010;202:S371–S376. doi: 10.1086/655970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Harris L.D., Tabb B., Sodora D.L., Paiardini M., Klatt N.R., Douek D.C., Silvestri G., Muller-Trutwin M., Vasile-Pandrea I., Apetrei C., et al. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J. Virol. 2010;84:7886–7891. doi: 10.1128/JVI.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Palesch D., Bosinger S.E., Tharp G.K., Vanderford T.H., Paiardini M., Chahroudi A., Johnson Z.P., Kirchhoff F., Hahn B.H., Norgren R.B., et al. Sooty mangabey genome sequence provides insight into AIDS resistance in a natural SIV host. Nature. 2018;553:77–81. doi: 10.1038/nature25140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Riddick N.E., Hermann E.A., Loftin L.M., Elliott S.T., Wey W.C., Cervasi B., Taaffe J., Engram J.C., Li B., Else J.G., et al. A Novel CCR5 Mutation Common in Sooty Mangabeys Reveals SIVsmm Infection of CCR5-Null Natural Hosts and Efficient Alternative Coreceptor Use In Vivo. PLoS Pathog. 2010;6:e1001064. doi: 10.1371/journal.ppat.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Vinton C., Klatt N.R., Harris L.D., Briant J.A., Sanders-Beer B.E., Herbert R., Woodward R., Silvestri G., Pandrea I., Apetrei C., et al. CD4-like immunological function by CD4- T cells in multiple natural hosts of simian immunodeficiency virus. J. Virol. 2011;85:8702–8708. doi: 10.1128/JVI.00332-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wetzel K.S., Yi Y., Elliott S.T.C., Romero D., Jacquelin B., Hahn B.H., Muller-Trutwin M., Apetrei C., Pandrea I., Collman R.G. CXCR6-Mediated Simian Immunodeficiency Virus SIVagmSab Entry into Sabaeus African Green Monkey Lymphocytes Implicates Widespread Use of Non-CCR5 Pathways in Natural Host Infections. J. Virol. 2017;91 doi: 10.1128/JVI.01626-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Paiardini M., Cervasi B., Reyes-Aviles E., Micci L., Ortiz A.M., Chahroudi A., Vinton C., Gordon S.N., Bosinger S.E., Francella N., et al. Low levels of SIV infection in sooty mangabey central memory CD4+ T cells are associated with limited CCR5 expression. Nat. Med. 2011;17:830–836. doi: 10.1038/nm.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Pandrea I., Onanga R., Souquiere S., Mouinga-Ondéme A., Bourry O., Makuwa M., Rouquet P., Silvestri G., Simon F., Roques P., et al. Paucity of CD4+ CCR5+ T Cells May Prevent Transmission of Simian Immunodeficiency Virus in Natural Nonhuman Primate Hosts by Breast-Feeding. J. Virol. 2008;82:5501–5509. doi: 10.1128/JVI.02555-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Beaumier C.M., Harris L.D., Goldstein S., Klatt N.R., Whitted S., McGinty J., Apetrei C., Pandrea I., Hirsch V.M., Brenchley J.M. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat. Med. 2009;15:879–885. doi: 10.1038/nm.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Elliott S.T., Wetzel K.S., Francella N., Bryan S., Romero D.C., Riddick N.E., Shaheen F., Vanderford T., Derdeyn C.A., Silvestri G., et al. Dualtropic CXCR6/CCR5 Simian Immunodeficiency Virus (SIV) Infection of Sooty Mangabey Primary Lymphocytes: Distinct Coreceptor Use in Natural versus Pathogenic Hosts of SIV. J. Virol. 2015;89:9252–9261. doi: 10.1128/JVI.01236-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Huot N., Jacquelin B., Garcia-Tellez T., Rascle P., Ploquin M.J., Madec Y., Reeves R.K., Derreudre-Bosquet N., Müller-Trutwin M. Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat. Med. 2017;23:1277–1286. doi: 10.1038/nm.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Huot N., Rascle P., Petitdemange C., Contreras V., Stürzel C.M., Baquero E., Harper J.L., Passaes C., Legendre R., Varet H., et al. SIV-induced terminally differentiated adaptive NK cells in lymph nodes associated with enhanced MHC-E restricted activity. Nat. Commun. 2021;12:1282. doi: 10.1038/s41467-021-21402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Marchetti G., Tincati C., Silvestri G. Microbial Translocation in the Pathogenesis of HIV Infection and AIDS. Clin. Microbiol. Rev. 2013;26:2–18. doi: 10.1128/CMR.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lerner A.M., Eisinger R.W., Fauci A.S. Comorbidities in Persons With HIV: The Lingering Challenge. JAMA. 2020;323:19–20. doi: 10.1001/jama.2019.19775. [DOI] [PubMed] [Google Scholar]
- 270.Morlat P., Roussillon C., Henard S., Salmon D., Bonnet F., Cacoub P., Georget A., Aouba A., Rosenthal E., May T., et al. Causes of death among HIV-infected patients in France in 2010 (national survey): Trends since 2000. AIDS (Lond. Engl.) 2014;28:1181–1191. doi: 10.1097/QAD.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 271.Smith C.J., Ryom L., Weber R., Morlat P., Pradier C., Reiss P., Kowalska J.D., de Wit S., Law M., el Sadr W., et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): A multicohort collaboration. Lancet (Lond. Engl. ) 2014;384:241–248. doi: 10.1016/S0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- 272.Marcus J.L., Chao C.R., Leyden W.A., Xu L., Quesenberry C.P., Jr., Klein D.B., Towner W.J., Horberg M.A., Silverberg M.J. Narrowing the Gap in Life Expectancy Between HIV-Infected and HIV-Uninfected Individuals With Access to Care. J. Acquir. Immune Defic. syndromes. 2016;73:39–46. doi: 10.1097/QAI.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Schouten J., Wit F.W., Stolte I.G., Kootstra N.A., van der Valk M., Geerlings S.E., Prins M., Reiss P., for the A.C.S.G. Cross-sectional Comparison of the Prevalence of Age-Associated Comorbidities and Their Risk Factors Between HIV-Infected and Uninfected Individuals: The AGEhIV Cohort Study. Clin. Infect. Dis. 2014;59:1787–1797. doi: 10.1093/cid/ciu701. [DOI] [PubMed] [Google Scholar]
- 274.Evans D.T., Silvestri G. Nonhuman primate models in AIDS research. Curr Opin HIV AIDS. 2013;8:255–261. doi: 10.1097/COH.0b013e328361cee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lifson J.D., Haigwood N.L. Lessons in nonhuman primate models for AIDS vaccine research: From minefields to milestones. Cold Spring Harb. Perspect. Med. 2012;2:a007310. doi: 10.1101/cshperspect.a007310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Palella F.J., Jr., Phair J.P. Cardiovascular disease in HIV infection. Curr Opin HIV AIDS. 2011;6:266–271. doi: 10.1097/COH.0b013e328347876c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Balagopal A., Ray S.C., De Oca R.M., Sutcliffe C.G., Vivekanandan P., Higgins Y., Mehta S.H., Moore R.D., Sulkowski M.S., Thomas D.L., et al. Kupffer cells are depleted with HIV immunodeficiency and partially recovered with antiretroviral immune reconstitution. AIDS (Lond. Engl.) 2009;23:2397–2404. doi: 10.1097/QAD.0b013e3283324344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tuyama A.C., Hong F., Saiman Y., Wang C., Ozkok D., Mosoian A., Chen P., Chen B.K., Klotman M.E., Bansal M.B. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: Implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatol. (Baltim. Md.) 2010;52:612–622. doi: 10.1002/hep.23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ahsan M.H., Gill A.F., Alvarez X., Lackner A.A., Veazey R.S. Kinetics of liver macrophages (Kupffer cells) in SIV-infected macaques. Virology. 2013;446:77–85. doi: 10.1016/j.virol.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Justice A.C., Freiberg M.S., Tracy R., Kuller L., Tate J.P., Goetz M.B., Fiellin D.A., Vanasse G.J., Butt A.A., Rodriguez-Barradas M.C., et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012;54:984–994. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Violi F., Ferro D., Basili S., Saliola M., Quintarelli C., Alessandri C., Cordova C. Association between low-grade disseminated intravascular coagulation and endotoxemia in patients with liver cirrhosis. Gastroenterology. 1995;109:531–539. doi: 10.1016/0016-5085(95)90342-9. [DOI] [PubMed] [Google Scholar]
- 282.Shmagel K.V., Saidakova E.V., Shmagel N.G., Korolevskaya L.B., Chereshnev V.A., Robinson J., Grivel J.C., Douek D.C., Margolis L., Anthony D.D., et al. Systemic inflammation and liver damage in HIV/hepatitis C virus coinfection. HIV Med. 2016;17:581–589. doi: 10.1111/hiv.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Pandrea I., Happel K.I., Amedee A.M., Bagby G.J., Nelson S. Alcohol’s role in HIV transmission and disease progression. Alcohol Res. Health. 2010;33:203–218. [PMC free article] [PubMed] [Google Scholar]
- 284.Zhang L., Dailey Peter J., Gettie A., Blanchard J., Ho David D. The Liver Is a Major Organ for Clearing Simian Immunodeficiency Virus in Rhesus Monkeys. J. Virol. 2002;76:5271–5273. doi: 10.1128/JVI.76.10.5271-5273.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ahsan M.H., Gill A.F., Lackner A.A., Veazey R.S. Acute and chronic T cell dynamics in the livers of simian immunodeficiency virus-infected macaques. J. Virol. 2012;86:5244–5252. doi: 10.1128/JVI.07080-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Schmitz J.E., Kuroda M.J., Veazey R.S., Seth A., Taylor W.M., Nickerson C.E., Lifton M.A., Dailey P.J., Forman M.A., Racz P., et al. Simian Immunodeficiency Virus (SIV)-Specific CTL Are Present in Large Numbers in Livers of SIV-Infected Rhesus Monkeys. J. Immunol. 2000;164:6015–6019. doi: 10.4049/jimmunol.164.11.6015. [DOI] [PubMed] [Google Scholar]
- 287.Kunisaki K.M. Will expanded ART use reduce the burden of HIV-associated chronic lung disease? Curr Opin HIV AIDS. 2014;9:27–33. doi: 10.1097/COH.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Cai Y., Sugimoto C., Arainga M., Midkiff C.C., Liu D.X., Alvarez X., Lackner A.A., Kim W.-K., Didier E.S., Kuroda M.J. Preferential Destruction of Interstitial Macrophages over Alveolar Macrophages as a Cause of Pulmonary Disease in Simian Immunodeficiency Virus–Infected Rhesus Macaques. J. Immunol. 2015;195:4884–4891. doi: 10.4049/jimmunol.1501194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Campbell L.J., Ibrahim F., Fisher M., Holt S.G., Hendry B.M., Post F.A. Spectrum of chronic kidney disease in HIV-infected patients. HIV Med. 2009;10:329–336. doi: 10.1111/j.1468-1293.2008.00691.x. [DOI] [PubMed] [Google Scholar]
- 290.Kalyesubula R., Wearne N., Semitala F.C., Bowa K. HIV-Associated Renal and Genitourinary Comorbidities in Africa. JAIDS J. Acquir. Immune Defic. Syndr. 2014;67:S68–S78. doi: 10.1097/QAI.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 291.Ryom L., Mocroft A., Lundgren J.D. Antiretroviral therapy, immune suppression and renal impairment in HIV-positive persons. Curr Opin HIV AIDS. 2014;9:41–47. doi: 10.1097/COH.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 292.Stephens E.B., Tian C., Dalton S.B., Gattone V.H., 2nd Simian-human immunodeficiency virus-associated nephropathy in macaques. AIDS Res. Hum. Retrovir. 2000;16:1295–1306. doi: 10.1089/08892220050117050. [DOI] [PubMed] [Google Scholar]
- 293.Clarke C.L., Eckhaus M.A., Zerfas P.M., Elkins W.R. Peripheral edema with hypoalbuminemia in a nonhuman primate infected with simian-human immunodeficiency virus: A case report. J. Am. Assoc. Lab. Anim. Sci. JAALAS. 2008;47:42–48. [PMC free article] [PubMed] [Google Scholar]
- 294.Saylor D., Dickens A.M., Sacktor N., Haughey N., Slusher B., Pletnikov M., Mankowski J.L., Brown A., Volsky D.J., McArthur J.C. HIV-associated neurocognitive disorder—Pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016;12:234–248. doi: 10.1038/nrneurol.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Holt J.L., Kraft-Terry S.D., Chang L. Neuroimaging studies of the aging HIV-1-infected brain. J. Neurovirology. 2012;18:291–302. doi: 10.1007/s13365-012-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Williams R., Bokhari S., Silverstein P., Pinson D., Kumar A., Buch S. Nonhuman primate models of NeuroAIDS. J. Neurovirol. 2008;14:292–300. doi: 10.1080/13550280802074539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Beck S.E., Queen S.E., Metcalf Pate K.A., Mangus L.M., Abreu C.M., Gama L., Witwer K.W., Adams R.J., Zink M.C., Clements J.E., et al. An SIV/macaque model targeted to study HIV-associated neurocognitive disorders. J. Neurovirology. 2018;24:204–212. doi: 10.1007/s13365-017-0582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Finzi D., Blankson J., Siliciano J.D., Margolick J.B., Chadwick K., Pierson T., Smith K., Lisziewicz J., Lori F., Flexner C., et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 299.Pan X., Baldauf H.-M., Keppler O.T., Fackler O.T. Restrictions to HIV-1 replication in resting CD4+ T lymphocytes. Cell Res. 2013;23:876–885. doi: 10.1038/cr.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Chun T.-W., Davey R.T., Engel D., Lane H.C., Fauci A.S. Re-emergence of HIV after stopping therapy. Nature. 1999;401:874–875. doi: 10.1038/44755. [DOI] [PubMed] [Google Scholar]
- 301.Chun T.-W., Stuyver L., Mizell S.B., Ehler L.A., Mican J.A.M., Baseler M., Lloyd A.L., Nowak M.A., Fauci A.S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Perelson A.S., Essunger P., Cao Y., Vesanen M., Hurley A., Saksela K., Markowitz M., Ho D.D. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 303.Wong J.K., Hezareh M., Günthard H.F., Havlir D.V., Ignacio C.C., Spina C.A., Richman D.D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 304.Chun T.W., Engel D., Berrey M.M., Shea T., Corey L., Fauci A.S. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc. Natl. Acad. Sci. USA. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Archin N.M., Kirchherr J.L., Sung J.A., Clutton G., Sholtis K., Xu Y., Allard B., Stuelke E., Kashuba A.D., Kuruc J.D., et al. Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. J. Clin. Investig. 2017;127:3126–3135. doi: 10.1172/JCI92684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wei D.G., Chiang V., Fyne E., Balakrishnan M., Barnes T., Graupe M., Hesselgesser J., Irrinki A., Murry J.P., Stepan G., et al. Histone deacetylase inhibitor romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing. PLoS Pathog. 2014;10:e1004071. doi: 10.1371/journal.ppat.1004071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Mousseau G., Kessing C.F., Fromentin R., Trautmann L., Chomont N., Valente S.T. The Tat Inhibitor Didehydro-Cortistatin A Prevents HIV-1 Reactivation from Latency. mBio. 2015;6 doi: 10.1128/mBio.00465-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Donahue D.A., Kuhl B.D., Sloan R.D., Wainberg M.A. The viral protein Tat can inhibit the establishment of HIV-1 latency. J. Virol. 2012;86:3253–3263. doi: 10.1128/JVI.06648-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Borducchi E.N., Cabral C., Stephenson K.E., Liu J., Abbink P., Ng’ang’a D., Nkolola J.P., Brinkman A.L., Peter L., Lee B.C., et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature. 2016;540:284. doi: 10.1038/nature20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Tsai A., Irrinki A., Kaur J., Cihlar T., Kukolj G., Sloan D.D., Murry J.P. Toll-Like Receptor 7 Agonist GS-9620 Induces HIV Expression and HIV-Specific Immunity in Cells from HIV-Infected Individuals on Suppressive Antiretroviral Therapy. J. Virol. 2017;91:e02166-16. doi: 10.1128/JVI.02166-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Murray S.M., Down C.M., Boulware D.R., Stauffer W.M., Cavert W.P., Schacker T.W., Brenchley J.M., Douek D.C. Reduction of Immune Activation with Chloroquine Therapy during Chronic HIV Infection. J. Virol. 2010;84:12082–12086. doi: 10.1128/JVI.01466-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Josefsson L., von Stockenstrom S., Faria N.R., Sinclair E., Bacchetti P., Killian M., Epling L., Tan A., Ho T., Lemey P., et al. The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. Proc. Natl. Acad. Sci. 2013;110:E4987–E4996. doi: 10.1073/pnas.1308313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Porichis F., Hart M.G., Zupkosky J., Barblu L., Kwon D.S., McMullen A., Brennan T., Ahmed R., Freeman G.J., Kavanagh D.G., et al. Differential impact of PD-1 and/or interleukin-10 blockade on HIV-1-specific CD4 T cell and antigen-presenting cell functions. J. Virol. 2014;88:2508–2518. doi: 10.1128/JVI.02034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Palmer B.E., Neff C.P., LeCureux J., Ehler A., Dsouza M., Remling-Mulder L., Korman A.J., Fontenot A.P., Akkina R. In vivo blockade of the PD-1 receptor suppresses HIV-1 viral loads and improves CD4+ T cell levels in humanized mice. J. Immunol. 2013;190:211–219. doi: 10.4049/jimmunol.1201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Jones R.B., Ndhlovu L.C., Barbour J.D., Sheth P.M., Jha A.R., Long B.R., Wong J.C., Satkunarajah M., Schweneker M., Chapman J.M., et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Zhang Z.-N., Zhu M.-L., Chen Y.-H., Fu Y.-J., Zhang T.-W., Jiang Y.-J., Chu Z.-X., Shang H. Elevation of Tim-3 and PD-1 Expression on T Cells Appears Early in HIV Infection, and Differential Tim-3 and PD-1 Expression Patterns Can Be Induced by Common γ-Chain Cytokines. BioMed Res. Int. 2015;2015:916936. doi: 10.1155/2015/916936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Peretz Y., He Z., Shi Y., Yassine-Diab B., Goulet J.-P., Bordi R., Filali-Mouhim A., Loubert J.-B., El-Far M., Dupuy F.P., et al. CD160 and PD-1 Co-Expression on HIV-Specific CD8 T Cells Defines a Subset with Advanced Dysfunction. PLoS Pathog. 2012;8:e1002840. doi: 10.1371/journal.ppat.1002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Vandergeeten C., Fromentin R., DaFonseca S., Lawani M.B., Sereti I., Lederman M.M., Ramgopal M., Routy J.P., Sekaly R.P., Chomont N. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood. 2013;121:4321–4329. doi: 10.1182/blood-2012-11-465625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Seay K., Church C., Zheng J.H., Deneroff K., Ochsenbauer C., Kappes J.C., Liu B., Jeng E.K., Wong H.C., Goldstein H. In Vivo Activation of Human NK Cells by Treatment with an Interleukin-15 Superagonist Potently Inhibits Acute In Vivo HIV-1 Infection in Humanized Mice. J. Virol. 2015;89:6264–6274. doi: 10.1128/JVI.00563-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Younes S.A., Freeman M.L., Mudd J.C., Shive C.L., Reynaldi A., Panigrahi S., Estes J.D., Deleage C., Lucero C., Anderson J., et al. IL-15 promotes activation and expansion of CD8+ T cells in HIV-1 infection. J. Clin. Investig. 2016;126:2745–2756. doi: 10.1172/JCI85996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Mendez-Lagares G., Lu D., Merriam D., Baker C.A., Villinger F., Van Rompay K.K.A., McCune J.M., Hartigan-O’Connor D.J. IL-21 Therapy Controls Immune Activation and Maintains Antiviral CD8(+) T Cell Responses in Acute Simian Immunodeficiency Virus Infection. AIDS Res. Hum. Retrovir. 2017;33:S81–S92. doi: 10.1089/aid.2017.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Hale M., Mesojednik T., Romano Ibarra G.S., Sahni J., Bernard A., Sommer K., Scharenberg A.M., Rawlings D.J., Wagner T.A. Engineering HIV-Resistant, Anti-HIV Chimeric Antigen Receptor T Cells. Mol. Ther. : J. Am. Soc. Gene Ther. 2017;25:570–579. doi: 10.1016/j.ymthe.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Hütter G., Bodor J., Ledger S., Boyd M., Millington M., Tsie M., Symonds G. CCR5 Targeted Cell Therapy for HIV and Prevention of Viral Escape. Viruses. 2015;7:4186–4203. doi: 10.3390/v7082816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Eberhard J.M., Angin M., Passaes C., Salgado M., Monceaux V., Knops E., Kobbe G., Jensen B., Christopeit M., Kroger N., et al. Vulnerability to reservoir reseeding due to high immune activation after allogeneic hematopoietic stem cell transplantation in individuals with HIV-1. Sci. Transl Med. 2020;12 doi: 10.1126/scitranslmed.aay9355. [DOI] [PubMed] [Google Scholar]
- 325.Saez-Cirion A., Muller-Trutwin M. The Yellow Brick Road towards HIV Eradication. Trends Immunol. 2019;40:465–467. doi: 10.1016/j.it.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 326.Dufour C., Gantner P., Fromentin R., Chomont N. The multifaceted nature of HIV latency. J. Clin. Investig. 2020;130:3381–3390. doi: 10.1172/JCI136227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Margolis D.M., Archin N.M., Cohen M.S., Eron J.J., Ferrari G., Garcia J.V., Gay C.L., Goonetilleke N., Joseph S.B., Swanstrom R., et al. Curing HIV: Seeking to Target and Clear Persistent Infection. Cell. 2020;181:189–206. doi: 10.1016/j.cell.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Massanella M., Fromentin R., Chomont N. Residual inflammation and viral reservoirs: Alliance against an HIV cure. Curr Opin HIV AIDS. 2016;11:234–241. doi: 10.1097/COH.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Passaes C.P., Saez-Cirion A. HIV cure research: Advances and prospects. Virology. 2014;454–455:340–352. doi: 10.1016/j.virol.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 330.Fromentin R., Chomont N. HIV persistence in subsets of CD4+ T cells: 50 shades of reservoirs. Semin. Immunol. 2020:101438. doi: 10.1016/j.smim.2020.101438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Rabezanahary H., Moukambi F., Palesch D., Clain J., Racine G., Andreani G., Benmadid-Laktout G., Zghidi-Abouzid O., Soundaramourty C., Tremblay C., et al. Despite early antiretroviral therapy effector memory and follicular helper CD4 T cells are major reservoirs in visceral lymphoid tissues of SIV-infected macaques. Mucosal Immunol. 2020;13:149–160. doi: 10.1038/s41385-019-0221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Finzi D., Hermankova M., Pierson T., Carruth L.M., Buck C., Chaisson R.E., Quinn T.C., Chadwick K., Margolick J., Brookmeyer R., et al. Identification of a Reservoir for HIV-1 in Patients on Highly Active Antiretroviral Therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 333.Jordan A., Bisgrove D., Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003;22 doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Siliciano J.D., Kajdas J., Finzi D., Quinn T.C., Chadwick K., Margolick J.B., Kovacs C., Gange S.J., Siliciano R.F. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 335.Delobel P., Sandres-Sauné K., Cazabat M., L’Faqihi F.-E., Aquilina C., Obadia M., Pasquier C., Marchou B., Massip P., Izopet J. Persistence of distinct HIV-1 populations in blood monocytes and naive and memory CD4 T cells during prolonged suppressive HAART. AIDS (Lond. Engl.) 2005;19:1739–1750. doi: 10.1097/01.aids.0000183125.93958.26. [DOI] [PubMed] [Google Scholar]
- 336.Chomont N., El-Far M., Ancuta P., Trautmann L., Procopio F.A., Yassine-Diab B., Boucher G., Boulassel M.-R., Ghattas G., Brenchley J.M., et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Buzon M.J., Sun H., Li C., Shaw A., Seiss K., Ouyang Z., Martin-Gayo E., Leng J., Henrich T.J., Li J.Z., et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat. Med. 2014;20:139–142. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Kleinman A.J., Sivanandham R., Pandrea I., Chougnet C.A., Apetrei C. Regulatory T Cells As Potential Targets for HIV Cure Research. Front. Immunol. 2018;9:734. doi: 10.3389/fimmu.2018.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Pallikkuth S., Sharkey M., Babic D.Z., Gupta S., Stone G.W., Fischl M.A., Stevenson M., Pahwa S. Peripheral T Follicular Helper Cells Are the Major HIV Reservoir within Central Memory CD4 T Cells in Peripheral Blood from Chronically HIV-Infected Individuals on Combination Antiretroviral Therapy. J. Virol. 2016;90:2718–2728. doi: 10.1128/JVI.02883-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Wong M.E., Jaworowski A., Hearps A.C. The HIV Reservoir in Monocytes and Macrophages. Front. Immunol. 2019;10:1435. doi: 10.3389/fimmu.2019.01435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Spiegel H., Herbst H., Niedobitek G., Foss H.D., Stein H. Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+ T-helper cells. Am. J. Pathol. 1992;140:15–22. [PMC free article] [PubMed] [Google Scholar]
- 342.Badia R., Ballana E., Castellví M., García-Vidal E., Pujantell M., Clotet B., Prado J.G., Puig J., Martínez M.A., Riveira-Muñoz E., et al. CD32 expression is associated to T-cell activation and is not a marker of the HIV-1 reservoir. Nat. Commun. 2018;9:2739. doi: 10.1038/s41467-018-05157-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Hütter G., Nowak D., Mossner M., Ganepola S., Müßig A., Allers K., Schneider T., Hofmann J., Kücherer C., Blau O., et al. Long-Term Control of HIV by CCR5 Delta32/Delta32 Stem-Cell Transplantation. N. Engl. J. Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 344.Henrich T.J., Hanhauser E., Marty F.M., Sirignano M.N., Keating S., Lee T.H., Robles Y.P., Davis B.T., Li J.Z., Heisey A., et al. Antiretroviral-free hiv-1 remission and viral rebound after allogeneic stem cell transplantation: Report of 2 cases. Ann. Intern. Med. 2014;161:319–327. doi: 10.7326/M14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Persaud D., Gay H., Ziemniak C., Chen Y.H., Piatak M.J., Chun T.-W., Strain M., Richman D., Luzuriaga K. Absence of Detectable HIV-1 Viremia after Treatment Cessation in an Infant. N. Engl. J. Med. 2013;369:1828–1835. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Luzuriaga K., Gay H., Ziemniak C., Sanborn K.B., Somasundaran M., Rainwater-Lovett K., Mellors J.W., Rosenbloom D., Persaud D. Viremic Relapse after HIV-1 Remission in a Perinatally Infected Child. N. Engl. J. Med. 2015;372:786–788. doi: 10.1056/NEJMc1413931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Sáez-Cirión A., Bacchus C., Hocqueloux L., Avettand-Fenoel V., Girault I., Lecuroux C., Potard V., Versmisse P., Melard A., Prazuck T., et al. Post-Treatment HIV-1 Controllers with a Long-Term Virological Remission after the Interruption of Early Initiated Antiretroviral Therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Violari A., Cotton M., Kuhn L., Schramm D., Paximadis M., Shalekoff S., Dias B.d.C., Otwombe K., Liberty A., McIntyre J., et al. Viral and host characterists of a child with perinatal HIV-1 following a prolonged period after ART cessation in the CHER trial; Proceedings of the International AIDS Society 2017; Paris, France. 23–26 July 2017. [Google Scholar]
- 349.Cummins N.W., Rizza S., Litzow M.R., Hua S., Lee G.Q., Einkauf K., Chun T.-W., Rhame F., Baker J.V., Busch M.P., et al. Extensive virologic and immunologic characterization in an HIV-infected individual following allogeneic stem cell transplant and analytic cessation of antiretroviral therapy: A case study. PLoS Med. 2017;14:e1002461. doi: 10.1371/journal.pmed.1002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Folks T., Powell D.M., Lightfoote M.M., Benn S., Martin M.A., Fauci A.S. Induction of HTLV-III/LAV from a nonvirus-producing T-cell line: Implications for latency. Science. 1986;231:600–602. doi: 10.1126/science.3003906. [DOI] [PubMed] [Google Scholar]
- 351.Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 352.Siekevitz M., Josephs S.F., Dukovich M., Peffer N., Wong-Staal F., Greene W.C. Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987;238:1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- 353.Duh E.J., Maury W.J., Folks T.M., Fauci A.S., Rabson A.B. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc. Natl. Acad. Sci. USA. 1989;86:5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Zack J.A., Arrigo S.J., Weitsman S.R., Go A.S., Haislip A., Chen I.S.Y. HIV-1 entry into quiescent primary lymphocytes: Molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-L. [DOI] [PubMed] [Google Scholar]
- 355.Bukrinsky M.I., Stanwick T.L., Dempsey M.P., Stevenson M. Quiescent T Lymphocytes as an Inducible Virus Reservoir in HIV-1 Infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Chun T.-W., Finzi D., Margolick J., Chadwick K., Schwartz D., Siliciano R.F. In vivo fate of HIV-1-infected T cells: Quantitative analysis of the transition to stable latency. Nat. Med. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 357.Sahu G.K., Lee K., Ji J., Braciale V., Baron S., Cloyd M.W. A novel in vitro system to generate and study latently HIV-infected long-lived normal CD4+ T-lymphocytes. Virology. 2006;355:127–137. doi: 10.1016/j.virol.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 358.Bosque A., Planelles V. Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells. Blood. 2009;113:58–65. doi: 10.1182/blood-2008-07-168393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Burke B., Brown H.J., Marsden M.D., Bristol G., Vatakis D.N., Zack J.A. Primary cell model for activation-inducible human immunodeficiency virus. J. Virol. 2007;81:7424–7434. doi: 10.1128/JVI.02838-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Marini A., Harper J.M., Romerio F. An in vitro system to model the establishment and reactivation of HIV-1 latency. J. Immunol. 2008;181:7713–7720. doi: 10.4049/jimmunol.181.11.7713. [DOI] [PubMed] [Google Scholar]
- 361.Tyagi M., Pearson R.J., Karn J. Establishment of HIV Latency in Primary CD4+ Cells Is due to Epigenetic Transcriptional Silencing and P-TEFb Restriction. J. Virol. 2010;84:6425–6437. doi: 10.1128/JVI.01519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Yang H.C., Xing S., Shan L., O’Connell K., Dinoso J., Shen A., Zhou Y., Shrum C.K., Han Y., Liu J.O., et al. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J. Clin. Investig. 2009;119:3473–3486. doi: 10.1172/JCI39199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Yang H.-C. Primary cell models of HIV latency. Curr. Opin. HIV AIDS. 2011;6:62–67. doi: 10.1097/COH.0b013e3283412568. [DOI] [PubMed] [Google Scholar]
- 364.Schröder A.R., Shinn P., Chen H., Berry C., Ecker J.R., Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/S0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 365.Lewinski M.K., Yamashita M., Emerman M., Ciuffi A., Marshall H., Crawford G., Collins F., Shinn P., Leipzig J., Hannenhalli S., et al. Retroviral DNA integration: Viral and cellular determinants of target-site selection. PLoS Pathog. 2006;2:e60. doi: 10.1371/journal.ppat.0020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Han Y., Lassen K., Monie D., Sedaghat A.R., Shimoji S., Liu X., Pierson T.C., Margolick J.B., Siliciano R.F., Siliciano J.D. Resting CD4+ T cells from human immunodeficiency virus type 1 (HIV-1)-infected individuals carry integrated HIV-1 genomes within actively transcribed host genes. J. Virol. 2004;78:6122–6133. doi: 10.1128/JVI.78.12.6122-6133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Verdin E., Paras Jr P., Van Lint C. Chromatin disruption in the promoter of human immunodeficiency virus type 1 during transcriptional activation. EMBO J. 1993;12:3249–3259. doi: 10.1002/j.1460-2075.1993.tb05994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Keedy K.S., Archin N.M., Gates A.T., Espeseth A., Hazuda D.J., Margolis D.M. A limited group of class I histone deacetylases acts to repress human immunodeficiency virus type 1 expression. J. Virol. 2009;83:4749–4756. doi: 10.1128/JVI.02585-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Van Lint C., Emiliani S., Ott M., Verdin E. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO J. 1996;15:1112–1120. doi: 10.1002/j.1460-2075.1996.tb00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Lusic M., Marcello A., Cereseto A., Giacca M. Regulation of HIV-1 gene expression by histone acetylation and factor recruitment at the LTR promoter. EMBO J. 2003;22:6550–6561. doi: 10.1093/emboj/cdg631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Friedman J., Cho W.K., Chu C.K., Keedy K.S., Archin N.M., Margolis D.M., Karn J. Epigenetic silencing of HIV-1 by the histone H3 lysine 27 methyltransferase enhancer of Zeste 2. J. Virol. 2011;85:9078–9089. doi: 10.1128/JVI.00836-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Boehm D., Jeng M., Camus G., Gramatica A., Schwarzer R., Johnson J.R., Hull P.A., Montano M., Sakane N., Pagans S., et al. SMYD2-Mediated Histone Methylation Contributes to HIV-1 Latency. Cell Host Microbe. 2017;21:569–579. doi: 10.1016/j.chom.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Imai K., Togami H., Okamoto T. Involvement of Histone H3 Lysine 9 (H3K9) Methyltransferase G9a in the Maintenance of HIV-1 Latency and Its Reactivation by BIX01294 *. J. Biol. Chem. 2010;285:16538–16545. doi: 10.1074/jbc.M110.103531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Chéné I.d., Basyuk E., Lin Y.-L., Triboulet R., Knezevich A., Chable-Bessia C., Mettling C., Baillat V., Reynes J., Corbeau P., et al. Suv39H1 and HP1γ are responsible for chromatin-mediated HIV-1 transcriptional silencing and post-integration latency. EMBO J. 2007;26:424–435. doi: 10.1038/sj.emboj.7601517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.He G., Margolis D.M. Counterregulation of chromatin deacetylation and histone deacetylase occupancy at the integrated promoter of human immunodeficiency virus type 1 (HIV-1) by the HIV-1 repressor YY1 and HIV-1 activator Tat. Mol. Cell Biol. 2002;22:2965–2973. doi: 10.1128/MCB.22.9.2965-2973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Williams S.A., Chen L.-F., Kwon H., Ruiz-Jarabo C.M., Verdin E., Greene W.C. NF-κB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J. 2006;25:139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Tyagi M., Karn J. CBF-1 promotes transcriptional silencing during the establishment of HIV-1 latency. EMBO J. 2007;26:4985–4995. doi: 10.1038/sj.emboj.7601928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Greger I.H., Demarchi F., Giacca M., Proudfoot N.J. Transcriptional interference perturbs the binding of Sp1 to the HIV-1 promoter. Nucleic Acids Res. 1998;26:1294–1301. doi: 10.1093/nar/26.5.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Lenasi T., Contreras X., Peterlin B.M. Transcriptional Interference Antagonizes Proviral Gene Expression to Promote HIV Latency. Cell Host Microbe. 2008;4:123–133. doi: 10.1016/j.chom.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Han Y., Lin Y.B., An W., Xu J., Yang H.-C., O’Connell K., Dordai D., Boeke J.D., Siliciano J.D., Siliciano R.F. Orientation-Dependent Regulation of Integrated HIV-1 Expression by Host Gene Transcriptional Readthrough. Cell Host Microbe. 2008;4:134–146. doi: 10.1016/j.chom.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Sedore S.C., Byers S.A., Biglione S., Price J.P., Maury W.J., Price D.H. Manipulation of P-TEFb control machinery by HIV: Recruitment of P-TEFb from the large form by Tat and binding of HEXIM1 to TAR. Nucleic Acids Res. 2007;35:4347–4358. doi: 10.1093/nar/gkm443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Muniz L., Egloff S., Ughy B., Jády B.E., Kiss T. Controlling Cellular P-TEFb Activity by the HIV-1 Transcriptional Transactivator Tat. PLoS Pathog. 2010;6:e1001152. doi: 10.1371/journal.ppat.1001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Parada C.A., Roeder R.G. Enhanced processivity of RNA polymerase II triggered by Tat-induced phosphorylation of its carboxy-terminal domain. Nature. 1996;384:375–378. doi: 10.1038/384375a0. [DOI] [PubMed] [Google Scholar]
- 384.Kim Y.K., Bourgeois C.F., Isel C., Churcher M.J., Karn J. Phosphorylation of the RNA polymerase II carboxyl-terminal domain by CDK9 is directly responsible for human immunodeficiency virus type 1 Tat-activated transcriptional elongation. Mol. Cell Biol. 2002;22:4622–4637. doi: 10.1128/MCB.22.13.4622-4637.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Bourgeois C.F., Kim Y.K., Churcher M.J., West M.J., Karn J. Spt5 cooperates with human immunodeficiency virus type 1 Tat by preventing premature RNA release at terminator sequences. Mol. Cell Biol. 2002;22:1079–1093. doi: 10.1128/MCB.22.4.1079-1093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Jang M.K., Mochizuki K., Zhou M., Jeong H.-S., Brady J.N., Ozato K. The Bromodomain Protein Brd4 Is a Positive Regulatory Component of P-TEFb and Stimulates RNA Polymerase II-Dependent Transcription. Mol. Cell. 2005;19:523–534. doi: 10.1016/j.molcel.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 387.Yang Z., Yik J.H.N., Chen R., He N., Jang M.K., Ozato K., Zhou Q. Recruitment of P-TEFb for Stimulation of Transcriptional Elongation by the Bromodomain Protein Brd4. Mol. Cell. 2005;19:535–545. doi: 10.1016/j.molcel.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 388.Li Z., Guo J., Wu Y., Zhou Q. The BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of Tat-transactivation. Nucleic Acids Res. 2013;41:277–287. doi: 10.1093/nar/gks976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Boehm D., Calvanese V., Dar R.D., Xing S., Schroeder S., Martins L., Aull K., Li P.-C., Planelles V., Bradner J.E., et al. BET bromodomain-targeting compounds reactivate HIV from latency via a Tat-independent mechanism. Cell Cycle. 2013;12:452–462. doi: 10.4161/cc.23309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Chun T.-W., Justement J.S., Moir S., Hallahan C.W., Maenza J., Mullins J.I., Collier A.C., Corey L., Fauci A.S. Decay of the HIV Reservoir in Patients Receiving Antiretroviral Therapy for Extended Periods: Implications for Eradication of Virus. J. Infect. Dis. 2007;195:1762–1764. doi: 10.1086/518250. [DOI] [PubMed] [Google Scholar]
- 391.Gandhi R.T., Cyktor J.C., Bosch R.J., Mar H., Laird G.M., Martin A., Collier A.C., Riddler S.A., Macatangay B.J., Rinaldo C.R., et al. Selective Decay of Intact HIV-1 Proviral DNA on Antiretroviral Therapy. J. Infect. Dis. 2021;223:225–233. doi: 10.1093/infdis/jiaa532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Chun T.W., Carruth L., Finzi D., Shen X., DiGiuseppe J.A., Taylor H., Hermankova M., Chadwick K., Margolick J., Quinn T.C., et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 393.Bruner K.M., Murray A.J., Pollack R.A., Soliman M.G., Laskey S.B., Capoferri A.A., Lai J., Strain M.C., Lada S.M., Hoh R., et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat. Med. 2016;22:1043–1049. doi: 10.1038/nm.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Bullen C.K., Laird G.M., Durand C.M., Siliciano J.D., Siliciano R.F. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat. Med. 2014;20:425–429. doi: 10.1038/nm.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Kuzmichev Y.V., Veenhuis R.T., Pohlmeyer C.W., Garliss C.C., Walker-Sperling V.E., Blankson J.N. A CD3/CD28 microbead-based HIV-1 viral outgrowth assay. J. Virus Erad. 2017;3:85–89. doi: 10.1016/S2055-6640(20)30292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Patel S.S., Duby A.D., Thiele D.L., Lipsky P.E. Phenotypic and functional characterization of human T cell clones. J. Immunol. 1988;141:3726–3736. [PubMed] [Google Scholar]
- 397.Beliakova-Bethell N., Hezareh M., Wong J.K., Strain M.C., Lewinski M.K., Richman D.D., Spina C.A. Relative efficacy of T cell stimuli as inducers of productive HIV-1 replication in latently infected CD4 lymphocytes from patients on suppressive cART. Virology. 2017;508:127–133. doi: 10.1016/j.virol.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Laird G.M., Eisele E.E., Rabi S.A., Lai J., Chioma S., Blankson J.N., Siliciano J.D., Siliciano R.F. Rapid quantification of the latent reservoir for HIV-1 using a viral outgrowth assay. PLoS Pathog. 2013;9:e1003398. doi: 10.1371/journal.ppat.1003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Pollack R.A., Jones R.B., Pertea M., Bruner K.M., Martin A.R., Thomas A.S., Capoferri A.A., Beg S.A., Huang S.-H., Karandish S., et al. Defective HIV-1 Proviruses Are Expressed and Can Be Recognized by Cytotoxic T Lymphocytes, which Shape the Proviral Landscape. Cell Host Microbe. 2017;21:494–506. doi: 10.1016/j.chom.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Ho Y.-C., Shan L., Hosmane N.N., Wang J., Laskey S.B., Rosenbloom D.I.S., Lai J., Blankson J.N., Siliciano J.D., Siliciano R.F. Replication-Competent Noninduced Proviruses in the Latent Reservoir Increase Barrier to HIV-1 Cure. Cell. 2013;155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Hosmane N.N., Kwon K.J., Bruner K.M., Capoferri A.A., Beg S., Rosenbloom D.I., Keele B.F., Ho Y.C., Siliciano J.D., Siliciano R.F. Proliferation of latently infected CD4(+) T cells carrying replication-competent HIV-1: Potential role in latent reservoir dynamics. J. Exp. Med. 2017;214:959–972. doi: 10.1084/jem.20170193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Bui J.K., Sobolewski M.D., Keele B.F., Spindler J., Musick A., Wiegand A., Luke B.T., Shao W., Hughes S.H., Coffin J.M., et al. Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir. PLoS Pathog. 2017;13:e1006283. doi: 10.1371/journal.ppat.1006283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Li B., Gladden A.D., Altfeld M., Kaldor J.M., Cooper D.A., Kelleher A.D., Allen T.M. Rapid reversion of sequence polymorphisms dominates early human immunodeficiency virus type 1 evolution. J. Virol. 2007;81:193–201. doi: 10.1128/JVI.01231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Wang Z., Simonetti F.R., Siliciano R.F., Laird G.M. Measuring replication competent HIV-1: Advances and challenges in defining the latent reservoir. Retrovirology. 2018;15:21. doi: 10.1186/s12977-018-0404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Hiener B., Horsburgh B.A., Eden J.S., Barton K., Schlub T.E., Lee E., von Stockenstrom S., Odevall L., Milush J.M., Liegler T., et al. Identification of Genetically Intact HIV-1 Proviruses in Specific CD4(+) T Cells from Effectively Treated Participants. Cell Rep. 2017;21:813–822. doi: 10.1016/j.celrep.2017.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Lee G.Q., Orlova-Fink N., Einkauf K., Chowdhury F.Z., Sun X., Harrington S., Kuo H.H., Hua S., Chen H.R., Ouyang Z., et al. Clonal expansion of genome-intact HIV-1 in functionally polarized Th1 CD4+ T cells. J. Clin. Investig. 2017;127:2689–2696. doi: 10.1172/JCI93289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Kijak G.H., Sanders-Buell E., Pham P., Harbolick E.A., Oropeza C., O’Sullivan A.M., Bose M., Beckett C.G., Milazzo M., Robb M.L., et al. Next-generation sequencing of HIV-1 single genome amplicons. Biomol Detect. Quantif. 2019;17:100080. doi: 10.1016/j.bdq.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Arias A., López P., Sánchez R., Yamamura Y., Rivera-Amill V. Sanger and Next Generation Sequencing Approaches to Evaluate HIV-1 Virus in Blood Compartments. Int. J. Env. Res. Public Health. 2018;15:1697. doi: 10.3390/ijerph15081697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Bruner K.M., Wang Z., Simonetti F.R., Bender A.M., Kwon K.J., Sengupta S., Fray E.J., Beg S.A., Antar A.A.R., Jenike K.M., et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature. 2019;566:120–125. doi: 10.1038/s41586-019-0898-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Kinloch N.N., Ren Y., Conce Alberto W.D., Dong W., Khadka P., Huang S.H., Mota T.M., Wilson A., Shahid A., Kirkby D., et al. HIV-1 diversity considerations in the application of the Intact Proviral DNA Assay (IPDA) Nat. Commun. 2021;12:165. doi: 10.1038/s41467-020-20442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Gaebler C., Lorenzi J.C.C., Oliveira T.Y., Nogueira L., Ramos V., Lu C.-L., Pai J.A., Mendoza P., Jankovic M., Caskey M., et al. Combination of quadruplex qPCR and next-generation sequencing for qualitative and quantitative analysis of the HIV-1 latent reservoir. J. Exp. Med. 2019;216:2253–2264. doi: 10.1084/jem.20190896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Eriksson S., Graf E.H., Dahl V., Strain M.C., Yukl S.A., Lysenko E.S., Bosch R.J., Lai J., Chioma S., Emad F., et al. Comparative Analysis of Measures of Viral Reservoirs in HIV-1 Eradication Studies. PLoS Pathog. 2013;9:e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Imamichi H., Dewar R.L., Adelsberger J.W., Rehm C.A., O’Doherty U., Paxinos E.E., Fauci A.S., Lane H.C. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc. Natl. Acad. Sci. USA. 2016;113:8783–8788. doi: 10.1073/pnas.1609057113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Imamichi H., Smith M., Adelsberger J.W., Izumi T., Scrimieri F., Sherman B.T., Rehm C.A., Imamichi T., Pau A., Catalfamo M., et al. Defective HIV-1 proviruses produce viral proteins. Proc. Natl. Acad. Sci. USA. 2020;117:3704–3710. doi: 10.1073/pnas.1917876117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Apetrei C., Pandrea I., Mellors J.W. Nonhuman primate models for HIV cure research. PLoS Pathog. 2012;8:e1002892. doi: 10.1371/journal.ppat.1002892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Choudhary S.K., Archin N.M., Cheema M., Dahl N.P., Garcia J.V., Margolis D.M. Latent HIV-1 infection of resting CD4(+) T cells in the humanized Rag2(-)/(-) gammac(-)/(-) mouse. J. Virol. 2012;86:114–120. doi: 10.1128/JVI.05590-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Denton P.W., Olesen R., Choudhary S.K., Archin N.M., Wahl A., Swanson M.D., Chateau M., Nochi T., Krisko J.F., Spagnuolo R.A., et al. Generation of HIV latency in humanized BLT mice. J. Virol. 2012;86:630–634. doi: 10.1128/JVI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Honeycutt J.B., Wahl A., Baker C., Spagnuolo R.A., Foster J., Zakharova O., Wietgrefe S., Caro-Vegas C., Madden V., Sharpe G., et al. Macrophages sustain HIV replication in vivo independently of T cells. J. Clin. Investig. 2016;126:1353–1366. doi: 10.1172/JCI84456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Olesen R., Swanson M.D., Kovarova M., Nochi T., Chateau M., Honeycutt J.B., Long J.M., Denton P.W., Hudgens M.G., Richardson A., et al. ART influences HIV persistence in the female reproductive tract and cervicovaginal secretions. J. Clin. Investig. 2016;126:892–904. doi: 10.1172/JCI64212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Garcia J.V. In vivo platforms for analysis of HIV persistence and eradication. J. Clin. Investig. 2016;126:424–431. doi: 10.1172/JCI80562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Nishimura Y., Sadjadpour R., Mattapallil J.J., Igarashi T., Lee W., Buckler-White A., Roederer M., Chun T.W., Martin M.A. High frequencies of resting CD4+ T cells containing integrated viral DNA are found in rhesus macaques during acute lentivirus infections. Proc. Natl. Acad. Sci. USA. 2009;106:8015–8020. doi: 10.1073/pnas.0903022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Shen A., Zink M.C., Mankowski J.L., Chadwick K., Margolick J.B., Carruth L.M., Li M., Clements J.E., Siliciano R.F. Resting CD4+ T lymphocytes but not thymocytes provide a latent viral reservoir in a simian immunodeficiency virus-Macaca nemestrina model of human immunodeficiency virus type 1-infected patients on highly active antiretroviral therapy. J. Virol. 2003;77:4938–4949. doi: 10.1128/JVI.77.8.4938-4949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Crise B., Li Y., Yuan C., Morcock D.R., Whitby D., Munroe D.J., Arthur L.O., Wu X. Simian immunodeficiency virus integration preference is similar to that of human immunodeficiency virus type 1. J. Virol. 2005;79:12199–12204. doi: 10.1128/JVI.79.19.12199-12204.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Barber S.A., Gama L., Dudaronek J.M., Voelker T., Tarwater P.M., Clements J.E. Mechanism for the establishment of transcriptional HIV latency in the brain in a simian immunodeficiency virus-macaque model. J. Infect. Dis. 2006;193:963–970. doi: 10.1086/500983. [DOI] [PubMed] [Google Scholar]
- 425.Shen A., Yang H.C., Zhou Y., Chase A.J., Boyer J.D., Zhang H., Margolick J.B., Zink M.C., Clements J.E., Siliciano R.F. Novel pathway for induction of latent virus from resting CD4(+) T cells in the simian immunodeficiency virus/macaque model of human immunodeficiency virus type 1 latency. J. Virol. 2007;81:1660–1670. doi: 10.1128/JVI.01396-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Shytaj I.L., Norelli S., Chirullo B., Della Corte A., Collins M., Yalley-Ogunro J., Greenhouse J., Iraci N., Acosta E.P., Barreca M.L., et al. A highly intensified ART regimen induces long-term viral suppression and restriction of the viral reservoir in a simian AIDS model. PLoS Pathog. 2012;8:e1002774. doi: 10.1371/journal.ppat.1002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Del Prete G.Q., Smedley J., Macallister R., Jones G.S., Li B., Hattersley J., Zheng J., Piatak M., Jr., Keele B.F., Hesselgesser J., et al. Short Communication: Comparative Evaluation of Coformulated Injectable Combination Antiretroviral Therapy Regimens in Simian Immunodeficiency Virus-Infected Rhesus Macaques. AIDS Res. Hum. Retrovir. 2016;32:163–168. doi: 10.1089/aid.2015.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Dinoso J.B., Kim S.Y., Wiegand A.M., Palmer S.E., Gange S.J., Cranmer L., O’Shea A., Callender M., Spivak A., Brennan T., et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA. 2009;106:9403–9408. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Gandhi R.T., Bosch R.J., Aga E., Albrecht M., Demeter L.M., Dykes C., Bastow B., Para M., Lai J., Siliciano R.F., et al. No Evidence for Decay of the Latent Reservoir in HIV-1–Infected Patients Receiving Intensive Enfuvirtide-Containing Antiretroviral Therapy. J. Infect. Dis. 2010;201:293–296. doi: 10.1086/649569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Gandhi R.T., Zheng L., Bosch R.J., Chan E.S., Margolis D.M., Read S., Kallungal B., Palmer S., Medvik K., Lederman M.M., et al. The Effect of Raltegravir Intensification on Low-level Residual Viremia in HIV-Infected Patients on Antiretroviral Therapy: A Randomized Controlled Trial. PLOS Med. 2010;7:e1000321. doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Hammer S.M., Ribaudo H., Bassett R., Mellors J.W., Demeter L.M., Coombs R.W., Currier J., Morse G.D., Gerber J.G., Martinez A.I., et al. A randomized, placebo-controlled trial of abacavir intensification in HIV-1-infected adults with virologic suppression on a protease inhibitor-containing regimen. HIV Clin. Trials. 2010;11:312–324. doi: 10.1310/hct1105-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Mousseau G., Mediouni S., Valente S.T. Targeting HIV Transcription: The Quest for a Functional Cure. Curr. Top. Microbiol. Immunol. 2015;389:121–145. doi: 10.1007/82_2015_435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Tebas P., Stein D., Tang W.W., Frank I., Wang S.Q., Lee G., Spratt S.K., Surosky R.T., Giedlin M.A., Nichol G., et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 2014;370:901–910. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Deeks S.G., Wagner B., Anton P.A., Mitsuyasu R.T., Scadden D.T., Huang C., Macken C., Richman D.D., Christopherson C., June C.H., et al. A Phase II Randomized Study of HIV-Specific T-Cell Gene Therapy in Subjects with Undetectable Plasma Viremia on Combination Antiretroviral Therapy. Mol. Ther. 2002;5:788–797. doi: 10.1006/mthe.2002.0611. [DOI] [PubMed] [Google Scholar]
- 435.Zhen A., Peterson C.W., Carrillo M.A., Reddy S.S., Youn C.S., Lam B.B., Chang N.Y., Martin H.A., Rick J.W., Kim J., et al. Long-term persistence and function of hematopoietic stem cell-derived chimeric antigen receptor T cells in a nonhuman primate model of HIV/AIDS. PLoS Pathog. 2017;13:e1006753. doi: 10.1371/journal.ppat.1006753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Cummins N.W., Sainski A.M., Dai H., Natesampillai S., Pang Y.P., Bren G.D., de Araujo Correia M.C.M., Sampath R., Rizza S.A., O’Brien D., et al. Prime, Shock, and Kill: Priming CD4 T Cells from HIV Patients with a BCL-2 Antagonist before HIV Reactivation Reduces HIV Reservoir Size. J. Virol. 2016;90:4032–4048. doi: 10.1128/JVI.03179-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Cummins N.W., Sainski-Nguyen A.M., Natesampillai S., Aboulnasr F., Kaufmann S., Badley A.D. Maintenance of the HIV Reservoir Is Antagonized by Selective BCL2 Inhibition. J. Virol. 2017;91 doi: 10.1128/JVI.00012-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Liu J., Ghneim K., Sok D., Bosche W.J., Li Y., Chipriano E., Berkemeier B., Oswald K., Borducchi E., Cabral C., et al. Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science. 2016;353:1045–1049. doi: 10.1126/science.aag0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Xu L., Pegu A., Rao E., Doria-Rose N., Beninga J., McKee K., Lord D.M., Wei R.R., Deng G., Louder M., et al. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science. 2017;358:85–90. doi: 10.1126/science.aan8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Ng’uni T., Chasara C., Ndhlovu Z.M. Major Scientific Hurdles in HIV Vaccine Development: Historical Perspective and Future Directions. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.590780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.He T., Brocca-Cofano E., Policicchio B.B., Sivanandham R., Gautam R., Raehtz K.D., Xu C., Pandrea I., Apetrei C. Cutting Edge: T Regulatory Cell Depletion Reactivates Latent Simian Immunodeficiency Virus (SIV) in Controller Macaques While Boosting SIV-Specific T Lymphocytes. J. Immunol. 2016 doi: 10.4049/jimmunol.1601539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Sivanandham R., Kleinman A.J., Sette P., Brocca-Cofano E., Kilapandal Venkatraman S.M., Policicchio B.B., He T., Xu C., Swarthout J., Wang Z., et al. Nonhuman Primate Testing of the Impact of Different Regulatory T Cell Depletion Strategies on Reactivation and Clearance of Latent Simian Immunodeficiency Virus. J. Virol. 2020;94:e00533-00520. doi: 10.1128/JVI.00533-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Wightman F., Solomon A., Kumar S.S., Urriola N., Gallagher K., Hiener B., Palmer S., McNeil C., Garsia R., Lewin S.R. Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with metastatic melanoma. AIDS (Lond. Engl.) 2015;29:504–506. doi: 10.1097/QAD.0000000000000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Hryniewicz A., Boasso A., Edghill-Smith Y., Vaccari M., Fuchs D., Venzon D., Nacsa J., Betts M.R., Tsai W.-P., Heraud J.-M., et al. CTLA-4 blockade decreases TGF-β, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood. 2006;108:3834–3842. doi: 10.1182/blood-2006-04-010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Petrovas C., Casazza J.P., Brenchley J.M., Price D.A., Gostick E., Adams W.C., Precopio M.L., Schacker T., Roederer M., Douek D.C., et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Velu V., Kannanganat S., Ibegbu C., Chennareddi L., Villinger F., Freeman G.J., Ahmed R., Amara R.R. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J. Virol. 2007;81:5819–5828. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Blazkova J., Chun T.-W., Belay B.W., Murray D., Justement J.S., Funk E.K., Nelson A., Hallahan C.W., Moir S., Wender P.A., et al. Effect of Histone Deacetylase Inhibitors on HIV Production in Latently Infected, Resting CD4+ T Cells From Infected Individuals Receiving Effective Antiretroviral Therapy. J. Infect. Dis. 2012;206:765–769. doi: 10.1093/infdis/jis412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Cillo A.R., Sobolewski M.D., Bosch R.J., Fyne E., Piatak M., Coffin J.M., Mellors J.W. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. USA. 2014;111:7078–7083. doi: 10.1073/pnas.1402873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Gutierrez C., Serrano-Villar S., Madrid-Elena N., Perez-Elias M.J., Martin M.E., Barbas C., Ruiperez J., Munoz E., Munoz-Fernandez M.A., Castor T., et al. Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. AIDS (Lond. Engl.) 2016;30:1385–1392. doi: 10.1097/QAD.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 450.Prins J.M., Jurriaans S., van Praag R.M., Blaak H., van Rij R., Schellekens P.T., ten Berge I.J., Yong S.L., Fox C.H., Roos M.T., et al. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS (Lond. Engl.) 1999;13:2405–2410. doi: 10.1097/00002030-199912030-00012. [DOI] [PubMed] [Google Scholar]
- 451.Jones R.B., O’Connor R., Mueller S., Foley M., Szeto G.L., Karel D., Lichterfeld M., Kovacs C., Ostrowski M.A., Trocha A., et al. Histone Deacetylase Inhibitors Impair the Elimination of HIV-Infected Cells by Cytotoxic T-Lymphocytes. PLoS Pathog. 2014;10:e1004287. doi: 10.1371/journal.ppat.1004287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Shan L., Deng K., Shroff N.S., Durand C.M., Rabi S.A., Yang H.-C., Zhang H., Margolick J.B., Blankson J.N., Siliciano R.F. Stimulation of HIV-1-Specific Cytolytic T Lymphocytes Facilitates Elimination of Latent Viral Reservoir after Virus Reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Policicchio B.B., Xu C., Brocca-Cofano E., Raehtz K.D., He T., Ma D., Li H., Sivanandham R., Haret-Richter G.S., Dunsmore T., et al. Multi-dose Romidepsin Reactivates Replication Competent SIV in Post-antiretroviral Rhesus Macaque Controllers. PLoS Pathog. 2016;12:e1005879. doi: 10.1371/journal.ppat.1005879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Migueles S.A., Weeks K.A., Nou E., Berkley A.M., Rood J.E., Osborne C.M., Hallahan C.W., Cogliano-Shutta N.A., Metcalf J.A., McLaughlin M., et al. Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J. Virol. 2009;83:11876–11889. doi: 10.1128/JVI.01153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Ananworanich J., Chomont N., Fletcher J.L., Pinyakorn S., Schuetz A., Sereti I., Rerknimitr R., Dewar R., Kroon E., Vandergeeten C., et al. Markers of HIV reservoir size and immune activation after treatment in acute HIV infection with and without raltegravir and maraviroc intensification. J. Virus Erad. 2015;1:116–122. doi: 10.1016/S2055-6640(20)30482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Cohen Y.Z., Caskey M. Broadly neutralizing antibodies for treatment and prevention of HIV-1 infection. Curr Opin HIV AIDS. 2018;13:366–373. doi: 10.1097/COH.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 457.Wang C.X., Cannon P.M. The clinical applications of genome editing in HIV. Blood. 2016;127:2546–2552. doi: 10.1182/blood-2016-01-678144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Duarte R., Labopin M., Badoglio M., Cahn J.-Y., Leblond V., Milpied N., Peniket A., Passweg J., Richard C., Russell N., et al. Allogeneic Transplantation in Patients with HIV-Infection: A Pair Matched Cohort Study by the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2020;55:22–23. [Google Scholar]
- 459.Dornadula G., Zhang H., VanUitert B., Stern J., Livornese J.L., Ingerman M.J., Witek J., Kedanis R.J., Natkin J., DeSimone J., et al. Residual HIV-1 RNA in Blood Plasma of Patients Taking Suppressive Highly Active Antiretroviral Therapy. JAMA. 1999;282:1627–1632. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- 460.Palmer S., Maldarelli F., Wiegand A., Bernstein B., Hanna G.J., Brun S.C., Kempf D.J., Mellors J.W., Coffin J.M., King M.S. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. USA. 2008;105:3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Gandhi R.T., Coombs R.W., Chan E.S., Bosch R.J., Zheng L., Margolis D.M., Read S., Kallungal B., Chang M., Goecker E.A., et al. No effect of raltegravir intensification on viral replication markers in the blood of HIV-1-infected patients receiving antiretroviral therapy. J. Acquir. Immune Defic. Syndr. (1999) 2012;59:229–235. doi: 10.1097/QAI.0b013e31823fd1f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Rasmussen T.A., McMahon J.H., Chang J.J., Audsley J., Rhodes A., Tennakoon S., Dantanarayana A., Spelman T., Schmidt T., Kent S.J., et al. The effect of antiretroviral intensification with dolutegravir on residual virus replication in HIV-infected individuals: A randomised, placebo-controlled, double-blind trial. Lancet. HIV. 2018;5:e221–e230. doi: 10.1016/S2352-3018(18)30040-7. [DOI] [PubMed] [Google Scholar]
- 463.Chaillon A., Gianella S., Lada S.M., Perez-Santiago J., Jordan P., Ignacio C., Karris M., Richman D.D., Mehta S.R., Little S.J., et al. Size, Composition, and Evolution of HIV DNA Populations during Early Antiretroviral Therapy and Intensification with Maraviroc. J. Virol. 2018;92:e01589-01517. doi: 10.1128/JVI.01589-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Kim C.J., Rousseau R., Huibner S., Kovacs C., Benko E., Shahabi K., Kandel G., Ostrowski M., Kaul R. Impact of intensified antiretroviral therapy during early HIV infection on gut immunology and inflammatory blood biomarkers. AIDS (Lond. Engl.) 2017;31 doi: 10.1097/QAD.0000000000001515. [DOI] [PubMed] [Google Scholar]
- 465.Kao S.Y., Calman A.F., Luciw P.A., Peterlin B.M. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987;330:489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- 466.Feng S., Holland E.C. HIV-1 tat trans-activation requires the loop sequence within tar. Nature. 1988;334:165–167. doi: 10.1038/334165a0. [DOI] [PubMed] [Google Scholar]
- 467.Ahlenstiel C.L., Symonds G., Kent S.J., Kelleher A.D. Block and Lock HIV Cure Strategies to Control the Latent Reservoir. Front. Cell Infect. Microbiol. 2020;10:424. doi: 10.3389/fcimb.2020.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Meredith L.W., Sivakumaran H., Major L., Suhrbier A., Harrich D. Potent inhibition of HIV-1 replication by a Tat mutant. PLoS ONE. 2009;4:e7769. doi: 10.1371/journal.pone.0007769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Jin H., Li D., Sivakumaran H., Lor M., Rustanti L., Cloonan N., Wani S., Harrich D. Shutdown of HIV-1 Transcription in T Cells by Nullbasic, a Mutant Tat Protein. mBio. 2016;7:e00518-00516. doi: 10.1128/mBio.00518-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Mousseau G., Clementz M.A., Bakeman W.N., Nagarsheth N., Cameron M., Shi J., Baran P., Fromentin R., Chomont N., Valente S.T. An analog of the natural steroidal alkaloid cortistatin A potently suppresses Tat-dependent HIV transcription. Cell Host Microbe. 2012;12:97–108. doi: 10.1016/j.chom.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Kessing C.F., Nixon C.C., Li C., Tsai P., Takata H., Mousseau G., Ho P.T., Honeycutt J.B., Fallahi M., Trautmann L., et al. In Vivo Suppression of HIV Rebound by Didehydro-Cortistatin A, a “Block-and-Lock” Strategy for HIV-1 Treatment. Cell Rep. 2017;21:600–611. doi: 10.1016/j.celrep.2017.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Vansant G., Bruggemans A., Janssens J., Debyser Z. Block-And-Lock Strategies to Cure HIV Infection. Viruses. 2020;12:84. doi: 10.3390/v12010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Vargas B., Giacobbi N.S., Sanyal A., Venkatachari N.J., Han F., Gupta P., Sluis-Cremer N. Inhibitors of Signaling Pathways That Block Reversal of HIV-1 Latency. Antimicrob Agents Chemother. 2019;63:e01744-18. doi: 10.1128/AAC.01744-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Ebina H., Misawa N., Kanemura Y., Koyanagi Y. Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci. Rep. 2013;3:2510. doi: 10.1038/srep02510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Zhu W., Lei R., Le Duff Y., Li J., Guo F., Wainberg M.A., Liang C. The CRISPR/Cas9 system inactivates latent HIV-1 proviral DNA. Retrovirology. 2015;12:22. doi: 10.1186/s12977-015-0150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Yin C., Zhang T., Qu X., Zhang Y., Putatunda R., Xiao X., Li F., Xiao W., Zhao H., Dai S., et al. In Vivo Excision of HIV-1 Provirus by saCas9 and Multiplex Single-Guide RNAs in Animal Models. Mol. Ther. 2017;25:1168–1186. doi: 10.1016/j.ymthe.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Mout R., Ray M., Lee Y.-W., Scaletti F., Rotello V.M. In Vivo Delivery of CRISPR/Cas9 for Therapeutic Gene Editing: Progress and Challenges. Bioconjugate Chem. 2017;28:880–884. doi: 10.1021/acs.bioconjchem.7b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Leslie G.J., Wang J., Richardson M.W., Haggarty B.S., Hua K.L., Duong J., Secreto A.J., Jordon A.P., Romano J., Kumar K.E., et al. Potent and Broad Inhibition of HIV-1 by a Peptide from the gp41 Heptad Repeat-2 Domain Conjugated to the CXCR4 Amino Terminus. PLoS Pathog. 2016;12:e1005983. doi: 10.1371/journal.ppat.1005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Maldini C.R., Claiborne D.T., Okawa K., Chen T., Dopkin D.L., Shan X., Power K.A., Trifonova R.T., Krupp K., Phelps M., et al. Dual CD4-based CAR T cells with distinct costimulatory domains mitigate HIV pathogenesis in vivo. Nat. Med. 2020;26:1776–1787. doi: 10.1038/s41591-020-1039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Ventoso I., Navarro J., Munoz M.A., Carrasco L. Involvement of HIV-1 protease in virus-induced cell killing. Antivir. Res. 2005;66:47–55. doi: 10.1016/j.antiviral.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 481.Algeciras-Schimnich A., Belzacq-Casagrande A.-S., Bren G.D., Nie Z., Taylor J.A., Rizza S.A., Brenner C., Badley A.D. Analysis of HIV Protease Killing Through Caspase 8 Reveals a Novel Interaction Between Caspase 8 and Mitochondria. Open Virol. J. 2007;1:39–46. doi: 10.2174/1874357900701010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Sainski A.M., Natesampillai S., Cummins N.W., Bren G.D., Taylor J., Saenz D.T., Poeschla E.M., Badley A.D. The HIV-1-Specific Protein Casp8p41 Induces Death of Infected Cells through Bax/Bak. J. Virol. 2011;85:7965–7975. doi: 10.1128/JVI.02515-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Sainski A.M., Dai H., Natesampillai S., Pang Y.-P., Bren G.D., Cummins N.W., Correia C., Meng X.W., Tarara J.E., Ramirez-Alvarado M., et al. Casp8p41 generated by HIV protease kills CD4 T cells through direct Bak activation. J. Cell Biol. 2014;206:867–876. doi: 10.1083/jcb.201405051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Natesampillai S., Cummins N.W., Nie Z., Sampath R., Baker J.V., Henry K., Pinzone M., O’Doherty U., Polley E.C., Bren G.D., et al. HIV Protease-Generated Casp8p41, When Bound and Inactivated by Bcl2, Is Degraded by the Proteasome. J. Virol. 2018;92:e00037-18. doi: 10.1128/JVI.00037-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Alto A., Natesampillai S., Chandrasekar A.P., Krogman A., Misra A., Shweta F., VanLith C., Yao J.D., Cummins N.W., Badley A.D. The Combination of Venetoclax and Ixazomib Selectively and Efficiently Kills HIV-Infected Cell Lines but Has Unacceptable Toxicity in Primary Cell Models. J. Virol. 2021;95:e00138-00121. doi: 10.1128/JVI.00138-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Ren Y., Huang S.H., Patel S., Alberto W.D.C., Magat D., Ahimovic D., Macedo A.B., Durga R., Chan D., Zale E., et al. BCL-2 antagonism sensitizes cytotoxic T cell–resistant HIV reservoirs to elimination ex vivo. J. Clin. Investig. 2020;130:2542–2559. doi: 10.1172/JCI132374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Suntharalingam G., Perry M.R., Ward S., Brett S.J., Castello-Cortes A., Brunner M.D., Panoskaltsis N. Cytokine Storm in a Phase 1 Trial of the Anti-CD28 Monoclonal Antibody TGN1412. N. Engl. J. Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 488.Alegre M.L., Vandenabeele P., Depierreux M., Florquin S., Deschodt-Lanckman M., Flamand V., Moser M., Leo O., Urbain J., Fiers W., et al. Cytokine release syndrome induced by the 145-2C11 anti-CD3 monoclonal antibody in mice: Prevention by high doses of methylprednisolone. J. Immunol. 1991;146:1184–1191. [PubMed] [Google Scholar]
- 489.Hütter G. Stem cell transplantation in strategies for curing HIV/AIDS. AIDS Res. Ther. 2016;13:31. doi: 10.1186/s12981-016-0114-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Kordelas L., Verheyen J., Beelen D.W., Horn P.A., Heinold A., Kaiser R., Trenschel R., Schadendorf D., Dittmer U., Esser S. Shift of HIV tropism in stem-cell transplantation with CCR5 Delta32 mutation. N. Engl. J. Med. 2014;371:880–882. doi: 10.1056/NEJMc1405805. [DOI] [PubMed] [Google Scholar]
- 491.Koelsch K.K., Rasmussen T.A., Hey-Nguyen W.J., Pearson C., Xu Y., Bailey M., Marks K.H., Sasson S.C., Taylor M.S., Tantau R., et al. Impact of Allogeneic Hematopoietic Stem Cell Transplantation on the HIV Reservoir and Immune Response in 3 HIV-Infected Individuals. J. Acquir. Immune Defic. Syndr. 2017;75:328–337. doi: 10.1097/QAI.0000000000001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Burton D.R., Barbas C.F., Persson M.A., Koenig S., Chanock R.M., Lerner R.A. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc. Natl. Acad. Sci. USA. 1991;88:10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Klasse P.J. Neutralization of Virus Infectivity by Antibodies: Old Problems in New Perspectives. Adv. Biol. 2014;2014:157895. doi: 10.1155/2014/157895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Mallery D.L., McEwan W.A., Bidgood S.R., Towers G.J., Johnson C.M., James L.C. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21) Proc. Natl. Acad. Sci. USA. 2010;107:19985–19990. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Forthal D., Hope T.J., Alter G. New paradigms for functional HIV-specific nonneutralizing antibodies. Curr. Opin. HIV AIDS. 2013;8:393–401. doi: 10.1097/COH.0b013e328363d486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Wu X., Yang Z.-Y., Li Y., Hogerkorp C.-M., Schief W.R., Seaman M.S., Zhou T., Schmidt S.D., Wu L., Xu L., et al. Rational Design of Envelope Identifies Broadly Neutralizing Human Monoclonal Antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.McCoy L.E. The expanding array of HIV broadly neutralizing antibodies. Retrovirology. 2018;15:70. doi: 10.1186/s12977-018-0453-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Moldt B., Rakasz E.G., Schultz N., Chan-Hui P.-Y., Swiderek K., Weisgrau K.L., Piaskowski S.M., Bergman Z., Watkins D.I., Poignard P., et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc. Natl. Acad. Sci. USA. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Shingai M., Donau O.K., Plishka R.J., Buckler-White A., Mascola J.R., Nabel G.J., Nason M.C., Montefiori D., Moldt B., Poignard P., et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J. Exp. Med. 2014;211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Saunders K.O., Wang L., Joyce M.G., Yang Z.-Y., Balazs A.B., Cheng C., Ko S.-Y., Kong W.-P., Rudicell R.S., Georgiev I.S., et al. Broadly Neutralizing Human Immunodeficiency Virus Type 1 Antibody Gene Transfer Protects Nonhuman Primates from Mucosal Simian-Human Immunodeficiency Virus Infection. J. Virol. 2015;89:8334–8345. doi: 10.1128/JVI.00908-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Gautam R., Nishimura Y., Pegu A., Nason M.C., Klein F., Gazumyan A., Golijanin J., Buckler-White A., Sadjadpour R., Wang K., et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature. 2016;533:105–109. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Julg B., Sok D., Schmidt S.D., Abbink P., Newman R.M., Broge T., Linde C., Nkolola J., Le K., Su D., et al. Protective Efficacy of Broadly Neutralizing Antibodies with Incomplete Neutralization Activity against Simian-Human Immunodeficiency Virus in Rhesus Monkeys. J. Virol. 2017;91:e01187-01117. doi: 10.1128/JVI.01187-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Julg B., Tartaglia L.J., Keele B.F., Wagh K., Pegu A., Sok D., Abbink P., Schmidt S.D., Wang K., Chen X., et al. Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci. Transl. Med. 2017;9:eaal1321. doi: 10.1126/scitranslmed.aal1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Ledgerwood J.E., Coates E.E., Yamshchikov G., Saunders J.G., Holman L., Enama M.E., DeZure A., Lynch R.M., Gordon I., Plummer S., et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin. Exp. Immunol. 2015;182:289–301. doi: 10.1111/cei.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Gaudinski M.R., Coates E.E., Houser K.V., Chen G.L., Yamshchikov G., Saunders J.G., Holman L.A., Gordon I., Plummer S., Hendel C.S., et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults. PLOS Med. 2018;15:e1002493. doi: 10.1371/journal.pmed.1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Huang Y., Zhang L., Ledgerwood J., Grunenberg N., Bailer R., Isaacs A., Seaton K., Mayer K.H., Capparelli E., Corey L., et al. Population pharmacokinetics analysis of VRC01, an HIV-1 broadly neutralizing monoclonal antibody, in healthy adults. mAbs. 2017;9:792–800. doi: 10.1080/19420862.2017.1311435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Niessl J., Baxter A.E., Mendoza P., Jankovic M., Cohen Y.Z., Butler A.L., Lu C.-L., Dubé M., Shimeliovich I., Gruell H., et al. Combination anti-HIV-1 antibody therapy is associated with increased virus-specific T cell immunity. Nat. Med. 2020;26:222–227. doi: 10.1038/s41591-019-0747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Caskey M., Klein F., Lorenzi J.C.C., Seaman M.S., West A.P., Buckley N., Kremer G., Nogueira L., Braunschweig M., Scheid J.F., et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Caskey M., Schoofs T., Gruell H., Settler A., Karagounis T., Kreider E.F., Murrell B., Pfeifer N., Nogueira L., Oliveira T.Y., et al. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat. Med. 2017;23:185–191. doi: 10.1038/nm.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Crowell T.A., Colby D.J., Pinyakorn S., Sacdalan C., Pagliuzza A., Intasan J., Benjapornpong K., Tangnaree K., Chomchey N., Kroon E., et al. Safety and efficacy of VRC01 broadly neutralising antibodies in adults with acutely treated HIV (RV397): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet. HIV. 2019;6:e297–e306. doi: 10.1016/S2352-3018(19)30053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Reh L., Magnus C., Schanz M., Weber J., Uhr T., Rusert P., Trkola A. Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent. PLoS Pathog. 2015;11:e1004966. doi: 10.1371/journal.ppat.1004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Li H., Zony C., Chen P., Chen B.K. Reduced Potency and Incomplete Neutralization of Broadly Neutralizing Antibodies against Cell-to-Cell Transmission of HIV-1 with Transmitted Founder Envs. J. Virol. 2017;91 doi: 10.1128/JVI.02425-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Parsons M.S., Lloyd S.B., Lee W.S., Kristensen A.B., Amarasena T., Center R.J., Keele B.F., Lifson J.D., LaBranche C.C., Montefiori D., et al. Partial efficacy of a broadly neutralizing antibody against cell-associated SHIV infection. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aaf1483. [DOI] [PubMed] [Google Scholar]
- 514.Walker L.M., Huber M., Doores K.J., Falkowska E., Pejchal R., Julien J.-P., Wang S.-K., Ramos A., Chan-Hui P.-Y., Moyle M., et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Kong R., Louder M.K., Wagh K., Bailer R.T., deCamp A., Greene K., Gao H., Taft J.D., Gazumyan A., Liu C., et al. Improving Neutralization Potency and Breadth by Combining Broadly Reactive HIV-1 Antibodies Targeting Major Neutralization Epitopes. J. Virol. 2015;89:2659–2671. doi: 10.1128/JVI.03136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Wagh K., Seaman M.S., Zingg M., Fitzsimons T., Barouch D.H., Burton D.R., Connors M., Ho D.D., Mascola J.R., Nussenzweig M.C., et al. Potential of conventional & bispecific broadly neutralizing antibodies for prevention of HIV-1 subtype A, C & D infections. PLoS Pathog. 2018;14:e1006860. doi: 10.1371/journal.ppat.1006860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Nishimura Y., Gautam R., Chun T.-W., Sadjadpour R., Foulds K.E., Shingai M., Klein F., Gazumyan A., Golijanin J., Donaldson M., et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature. 2017;543:559–563. doi: 10.1038/nature21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Nishimura Y., Donau O.K., Dias J., Ferrando-Martinez S., Jesteadt E., Sadjadpour R., Gautam R., Buckler-White A., Geleziunas R., Koup R.A., et al. Immunotherapy during the acute SHIV infection of macaques confers long-term suppression of viremia. J. Exp. Med. 2020;218 doi: 10.1084/jem.20201214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Borducchi E.N., Liu J., Nkolola J.P., Cadena A.M., Yu W.-H., Fischinger S., Broge T., Abbink P., Mercado N.B., Chandrashekar A., et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature. 2018;563:360–364. doi: 10.1038/s41586-018-0600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Hsu D.C., Schuetz A., Imerbsin R., Silsorn D., Pegu A., Inthawong D., Sopanaporn J., Visudhiphan P., Chuenarom W., Keawboon B., et al. TLR7 agonist, N6-LS and PGT121 delayed viral rebound in SHIV-infected macaques after antiretroviral therapy interruption. PLoS Pathog. 2021;17:e1009339. doi: 10.1371/journal.ppat.1009339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Lim S.-Y., Osuna C.E., Lee J., Silva-Ayala D., Vikhe P., Chen E., Lundgren S., Eliot M., Schalk D., Schultz-Darken N., et al. Combination IL-15 Therapy in a SHIV NHP Model; Proceedings of the Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. 10 December 2021. [Google Scholar]
- 522.Gebara N.Y., El Kamari V., Rizk N. HIV-1 elite controllers: An immunovirological review and clinical perspectives. J. Virus Erad. 2019;5:163–166. doi: 10.1016/S2055-6640(20)30046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Gray G., Buchbinder S., Duerr A. Overview of STEP and Phambili trial results: Two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Curr. Opin. HIV AIDS. 2010;5:357–361. doi: 10.1097/COH.0b013e32833d2d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Bekker L.-G., Moodie Z., Grunenberg N., Laher F., Tomaras G.D., Cohen K.W., Allen M., Malahleha M., Mngadi K., Daniels B., et al. Subtype C ALVAC-HIV and bivalent subtype C gp120/MF59 HIV-1 vaccine in low-risk, HIV-uninfected, South African adults: A phase 1/2 trial. Lancet. HIV. 2018;5:e366–e378. doi: 10.1016/S2352-3018(18)30071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Laher F., Moodie Z., Cohen K.W., Grunenberg N., Bekker L.-G., Allen M., Frahm N., Yates N.L., Morris L., Malahleha M., et al. Safety and immune responses after a 12-month booster in healthy HIV-uninfected adults in HVTN 100 in South Africa: A randomized double-blind placebo-controlled trial of ALVAC-HIV (vCP2438) and bivalent subtype C gp120/MF59 vaccines. PLoS Med. 2020;17:e1003038. doi: 10.1371/journal.pmed.1003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Gray G.E., Bekker L.-G., Laher F., Malahleha M., Allen M., Moodie Z., Grunenberg N., Huang Y., Grove D., Prigmore B., et al. Vaccine Efficacy of ALVAC-HIV and Bivalent Subtype C gp120–MF59 in Adults. N. Engl. J. Med. 2021;384:1089–1100. doi: 10.1056/NEJMoa2031499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Abdul-Jawad S., Ondondo B., van Hateren A., Gardner A., Elliott T., Korber B., Hanke T. Increased Valency of Conserved-mosaic Vaccines Enhances the Breadth and Depth of Epitope Recognition. Mol. Ther. J. Am. Soc. Gene Ther. 2016;24:375–384. doi: 10.1038/mt.2015.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Barouch D.H., Tomaka F.L., Wegmann F., Stieh D.J., Alter G., Robb M.L., Michael N.L., Peter L., Nkolola J.P., Borducchi E.N., et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19) Lancet (Lond. Engl. ) 2018;392:232–243. doi: 10.1016/S0140-6736(18)31364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Fischer W., Perkins S., Theiler J., Bhattacharya T., Yusim K., Funkhouser R., Kuiken C., Haynes B., Letvin N.L., Walker B.D., et al. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat. Med. 2007;13:100–106. doi: 10.1038/nm1461. [DOI] [PubMed] [Google Scholar]
- 530.Johnson & Johnson and Global Partners Announce Results from Phase 2b Imbokodo HIV Vaccine Clinical Trial in Young Women in Sub-Saharan Africa. [(accessed on 13 September 2021)]; Available online: https://www.jnj.com/johnson-johnson-and-global-partners-announce-results-from-phase-2b-imbokodo-hiv-vaccine-clinical-trial-in-young-women-in-sub-saharan-africa.
- 531.Mothe B., Climent N., Plana M., Rosàs M., Jiménez J.L., Muñoz-Fernández M., Puertas M.C., Carrillo J., Gonzalez N., León A., et al. Safety and immunogenicity of a modified vaccinia Ankara-based HIV-1 vaccine (MVA-B) in HIV-1-infected patients alone or in combination with a drug to reactivate latent HIV-1. J. Antimicrob. Chemother. 2015;70:1833–1842. doi: 10.1093/jac/dkv046. [DOI] [PubMed] [Google Scholar]
- 532.Hansen S.G., Vieville C., Whizin N., Coyne-Johnson L., Siess D.C., Drummond D.D., Legasse A.W., Axthelm M.K., Oswald K., Trubey C.M., et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Hansen S.G., Piatak M., Jr., Ventura A.B., Hughes C.M., Gilbride R.M., Ford J.C., Oswald K., Shoemaker R., Li Y., Lewis M.S., et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Murray S.E., Nesterenko P.A., Vanarsdall A.L., Munks M.W., Smart S.M., Veziroglu E.M., Sagario L.C., Lee R., Claas F.H.J., Doxiadis I.I.N., et al. Fibroblast-adapted human CMV vaccines elicit predominantly conventional CD8 T cell responses in humans. J. Exp. Med. 2017;214:1889–1899. doi: 10.1084/jem.20161988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Combadière B., Beaujean M., Chaudesaigues C., Vieillard V. Peptide-Based Vaccination for Antibody Responses Against HIV. Vaccines. 2019;7:105. doi: 10.3390/vaccines7030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Belyakov I.M., Derby M.A., Ahlers J.D., Kelsall B.L., Earl P., Moss B., Strober W., Berzofsky J.A. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA. 1998;95:1709–1714. doi: 10.1073/pnas.95.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Kelleher A.D., Emery S., Cunningham P., Duncombe C., Carr A., Golding H., Forde S., Hudson J., Roggensack M., Forrest B.D., et al. Safety and immunogenicity of UBI HIV-1MN octameric V3 peptide vaccine administered by subcutaneous injection. AIDS Res. Hum. Retrovir. 1997;13:29–32. doi: 10.1089/aid.1997.13.29. [DOI] [PubMed] [Google Scholar]
- 538.Gorse G.J., Keefer M.C., Belshe R.B., Matthews T.J., Forrest B.D., Hsieh R.H., Koff W.C., Hanson C.V., Dolin R., Weinhold K.J., et al. A dose-ranging study of a prototype synthetic HIV-1MN V3 branched peptide vaccine. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J. Infect. Dis. 1996;173:330–339. doi: 10.1093/infdis/173.2.330. [DOI] [PubMed] [Google Scholar]
- 539.Flynn N.M., Forthal D.N., Harro C.D., Judson F.N., Mayer K.H., Para M.F. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 540.Vieillard V., Combadière B., Tubiana R., Launay O., Pialoux G., Cotte L., Girard P.-M., Simon A., Dudoit Y., Reynes J., et al. HIV therapeutic vaccine enhances non-exhausted CD4+ T cells in a randomised phase 2 trial. NPJ Vaccines. 2019;4:25. doi: 10.1038/s41541-019-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Pollard R.B., Rockstroh J.K., Pantaleo G., Asmuth D.M., Peters B., Lazzarin A., Garcia F., Ellefsen K., Podzamczer D., van Lunzen J., et al. Safety and efficacy of the peptide-based therapeutic vaccine for HIV-1, Vacc-4x: A phase 2 randomised, double-blind, placebo-controlled trial. Lancet. Infect. Dis. 2014;14:291–300. doi: 10.1016/S1473-3099(13)70343-8. [DOI] [PubMed] [Google Scholar]
- 542.Rockstroh J.K., Asmuth D., Pantaleo G., Clotet B., Podzamczer D., van Lunzen J., Arastéh K., Mitsuyasu R., Peters B., Silvia N., et al. Re-boost immunizations with the peptide-based therapeutic HIV vaccine, Vacc-4x, restores geometric mean viral load set-point during treatment interruption. PLoS ONE. 2019;14:e0210965. doi: 10.1371/journal.pone.0210965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Chupradit K., Moonmuang S., Nangola S., Kitidee K., Yasamut U., Mougel M., Tayapiwatana C. Current Peptide and Protein Candidates Challenging HIV Therapy beyond the Vaccine Era. Viruses. 2017;9:281. doi: 10.3390/v9100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Dando T.M., Perry C.M. Enfuvirtide. Drugs. 2003;63:2755–2766. doi: 10.2165/00003495-200363240-00005. [DOI] [PubMed] [Google Scholar]
- 545.Dorr P., Westby M., Dobbs S., Griffin P., Irvine B., Macartney M., Mori J., Rickett G., Smith-Burchnell C., Napier C., et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 2005;49:4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Bi W., Xu W., Cheng L., Xue J., Wang Q., Yu F., Xia S., Wang Q., Li G., Qin C., et al. IgG Fc-binding motif-conjugated HIV-1 fusion inhibitor exhibits improved potency and in vivo half-life: Potential application in combination with broad neutralizing antibodies. PLoS Pathog. 2019;15:e1008082. doi: 10.1371/journal.ppat.1008082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Martins M.A., Bischof G.F., Shin Y.C., Lauer W.A., Gonzalez-Nieto L., Watkins D.I., Rakasz E.G., Lifson J.D., Desrosiers R.C. Vaccine protection against SIVmac239 acquisition. Proc. Natl. Acad. Sci. USA. 2019;116:1739–1744. doi: 10.1073/pnas.1814584116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Martins M.A., Gonzalez-Nieto L., Ricciardi M.J., Bailey V.K., Dang C.M., Bischof G.F., Pedreño-Lopez N., Pauthner M.G., Burton D.R., Parks C.L., et al. Rectal Acquisition of Simian Immunodeficiency Virus (SIV) SIVmac239 Infection despite Vaccine-Induced Immune Responses against the Entire SIV Proteome. J. Virol. 2020;94:e00979-00920. doi: 10.1128/JVI.00979-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Moreno-Fernandez M.E., Presicce P., Chougnet C.A. Homeostasis and function of regulatory T cells in HIV/SIV infection. J. Virol. 2012;86:10262–10269. doi: 10.1128/JVI.00993-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Tran T.A., de Goër de Herve M.G., Hendel-Chavez H., Dembele B., Le Névot E., Abbed K., Pallier C., Goujard C., Gasnault J., Delfraissy J.F., et al. Resting regulatory CD4 T cells: A site of HIV persistence in patients on long-term effective antiretroviral therapy. PLoS ONE. 2008;3:e3305. doi: 10.1371/journal.pone.0003305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Allers K., Loddenkemper C., Hofmann J., Unbehaun A., Kunkel D., Moos V., Kaup F.J., Stahl-Hennig C., Sauermann U., Epple H.J., et al. Gut mucosal FOXP3+ regulatory CD4+ T cells and Nonregulatory CD4+ T cells are differentially affected by simian immunodeficiency virus infection in rhesus macaques. J. Virol. 2010;84:3259–3269. doi: 10.1128/JVI.01715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Bi X., Suzuki Y., Gatanaga H., Oka S. High frequency and proliferation of CD4+ FOXP3+ Treg in HIV-1-infected patients with low CD4 counts. Eur. J. Immunol. 2009;39:301–309. doi: 10.1002/eji.200838667. [DOI] [PubMed] [Google Scholar]
- 553.Weiss L., Donkova-Petrini V., Caccavelli L., Balbo M., Carbonneil C., Levy Y. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104:3249–3256. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 554.Nikolova M., Lelievre J.D., Carriere M., Bensussan A., Levy Y. Regulatory T cells differentially modulate the maturation and apoptosis of human CD8+ T-cell subsets. Blood. 2009;113:4556–4565. doi: 10.1182/blood-2008-04-151407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Nilsson J., Boasso A., Velilla P.A., Zhang R., Vaccari M., Franchini G., Shearer G.M., Andersson J., Chougnet C. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Elahi S., Dinges W.L., Lejarcegui N., Laing K.J., Collier A.C., Koelle D.M., McElrath M.J., Horton H. Protective HIV-specific CD8+ T cells evade Treg cell suppression. Nat. Med. 2011;17:989–995. doi: 10.1038/nm.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Queen C., Schneider W.P., Selick H.E., Payne P.W., Landolfi N.F., Duncan J.F., Avdalovic N.M., Levitt M., Junghans R.P., Waldmann T.A. A humanized antibody that binds to the interleukin 2 receptor. Proc. Natl. Acad. Sci. USA. 1989;86:10029–10033. doi: 10.1073/pnas.86.24.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Williams D.P., Parker K., Bacha P., Bishai W., Borowski M., Genbauffe F., Strom T.B., Murphy J.R. Diphtheria toxin receptor binding domain substitution with interleukin-2: Genetic construction and properties of a diphtheria toxin-related interleukin-2 fusion protein. Protein Eng. 1987;1:493–498. doi: 10.1093/protein/1.6.493. [DOI] [PubMed] [Google Scholar]
- 559.Collier R.J. Diphtheria toxin: Mode of action and structure. Bacteriol. Rev. 1975;39:54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Atchison E., Eklund J., Martone B., Wang L., Gidron A., Macvicar G., Rademaker A., Goolsby C., Marszalek L., Kozlowski J., et al. A pilot study of denileukin diftitox (DD) in combination with high-dose interleukin-2 (IL-2) for patients with metastatic renal cell carcinoma (RCC) J. Immuno. (Hagerstown Md. 1997) 2010;33:716–722. doi: 10.1097/CJI.0b013e3181e4752e. [DOI] [PubMed] [Google Scholar]
- 561.Kaminetzky D., Hymes K.B. Denileukin diftitox for the treatment of cutaneous T-cell lymphoma. Biol. Targets Ther. 2008;2:717–724. doi: 10.2147/btt.s3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Baur A.S., Lutz M.B., Schierer S., Beltrame L., Theiner G., Zinser E., Ostalecki C., Heidkamp G., Haendle I., Erdmann M., et al. Denileukin diftitox (ONTAK) induces a tolerogenic phenotype in dendritic cells and stimulates survival of resting Treg. Blood. 2013;122:2185–2194. doi: 10.1182/blood-2012-09-456988. [DOI] [PubMed] [Google Scholar]
- 563.Foss F.M., Sjak-Shie N., Goy A., Jacobsen E., Advani R., Smith M.R., Komrokji R., Pendergrass K., Bolejack V. A multicenter phase II trial to determine the safety and efficacy of combination therapy with denileukin diftitox and cyclophosphamide, doxorubicin, vincristine and prednisone in untreated peripheral T-cell lymphoma: The CONCEPT study. Leuk. Lymphoma. 2013;54:1373–1379. doi: 10.3109/10428194.2012.742521. [DOI] [PubMed] [Google Scholar]
- 564.Telang S., Rasku M.A., Clem A.L., Carter K., Klarer A.C., Badger W.R., Milam R.A., Rai S.N., Pan J., Gragg H., et al. Phase II trial of the regulatory T cell-depleting agent, denileukin diftitox, in patients with unresectable stage IV melanoma. BMC Cancer. 2011;11:515. doi: 10.1186/1471-2407-11-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Wang Z., Zheng Q., Zhang H., Bronson R.T., Madsen J.C., Sachs D.H., Huang C.A., Wang Z. Ontak-like human IL-2 fusion toxin. J. Immunol. Methods. 2017;448:51–58. doi: 10.1016/j.jim.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Peraino J.S., Zhang H., Rajasekera P.V., Wei M., Madsen J.C., Sachs D.H., Huang C.A., Wang Z. Diphtheria toxin-based bivalent human IL-2 fusion toxin with improved efficacy for targeting human CD25(+) cells. J. Immunol. Methods. 2014;405:57–66. doi: 10.1016/j.jim.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Ishida T., Ueda R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci. 2006;97:1139–1146. doi: 10.1111/j.1349-7006.2006.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Nishikawa H., Sakaguchi S. Regulatory T cells in tumor immunity. Int. J. Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 569.Iellem A., Mariani M., Lang R., Recalde H., Panina-Bordignon P., Sinigaglia F., D’Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Agrawal L., Vanhorn-Ali Z., Alkhatib G. Multiple determinants are involved in HIV coreceptor use as demonstrated by CCR4/CCL22 interaction in peripheral blood mononuclear cells (PBMCs) J. Leukoc. Biol. 2002;72:1063–1074. [PubMed] [Google Scholar]
- 571.Wang Z., Pratts S.G., Zhang H., Spencer P.J., Yu R., Tonsho M., Shah J.A., Tanabe T., Powell H.R., Huang C.A., et al. Treg depletion in non-human primates using a novel diphtheria toxin-based anti-human CCR4 immunotoxin. Mol. Oncol. 2016;10:553–565. doi: 10.1016/j.molonc.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Ni X., Jorgensen J.L., Goswami M., Challagundla P., Decker W.K., Kim Y.H., Duvic M.A. Reduction of regulatory T cells by Mogamulizumab, a defucosylated anti-CC chemokine receptor 4 antibody, in patients with aggressive/refractory mycosis fungoides and Sezary syndrome. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015;21:274–285. doi: 10.1158/1078-0432.CCR-14-0830. [DOI] [PubMed] [Google Scholar]
- 573.Ogura M., Ishida T., Hatake K., Taniwaki M., Ando K., Tobinai K., Fujimoto K., Yamamoto K., Miyamoto T., Uike N., et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014;32:1157–1163. doi: 10.1200/JCO.2013.52.0924. [DOI] [PubMed] [Google Scholar]
- 574.Coe D., Begom S., Addey C., White M., Dyson J., Chai J.-G. Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol. Immunother. 2010;59:1367–1377. doi: 10.1007/s00262-010-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Nocentini G., Giunchi L., Ronchetti S., Krausz L.T., Bartoli A., Moraca R., Migliorati G., Riccardi C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc. Natl. Acad. Sci. USA. 1997;94:6216–6221. doi: 10.1073/pnas.94.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Shimizu J., Yamazaki S., Takahashi T., Ishida Y., Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 577.Kanamaru F., Youngnak P., Hashiguchi M., Nishioka T., Takahashi T., Sakaguchi S., Ishikawa I., Azuma M. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J. Immunol. (Baltimore Md. 1950) 2004;172:7306–7314. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- 578.Stephens G.L., McHugh R.S., Whitters M.J., Young D.A., Luxenberg D., Carreno B.M., Collins M., Shevach E.M. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J. Immunol. (Baltimore Md. 1950) 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 579.Mahne A.E., Mauze S., Joyce-Shaikh B., Xia J., Bowman E.P., Beebe A.M., Cua D.J., Jain R. Dual Roles for Regulatory T-cell Depletion and Costimulatory Signaling in Agonistic GITR Targeting for Tumor Immunotherapy. Cancer Res. 2017;77:1108–1118. doi: 10.1158/0008-5472.CAN-16-0797. [DOI] [PubMed] [Google Scholar]
- 580.Lahey T.P., Loisel S.D., Wieland-Alter W. Glucocorticoid-induced tumor necrosis factor receptor family-related protein triggering enhances HIV-specific CD4+ T cell cytokine secretion and protects HIV-specific CD4+ T cells from apoptosis. J. Infect. Dis. 2007;196:43–49. doi: 10.1086/518613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Schoenhals J.E., Cushman T.R., Barsoumian H.B., Li A., Cadena A.P., Niknam S., Younes A.I., Caetano M.D.S., Cortez M.A., Welsh J.W. Anti-glucocorticoid-induced Tumor Necrosis Factor-Related Protein (GITR) Therapy Overcomes Radiation-Induced Treg Immunosuppression and Drives Abscopal Effects. Front. Immunol. 2018;9:2170. doi: 10.3389/fimmu.2018.02170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Shrimali R., Ahmad S., Berrong Z., Okoev G., Matevosyan A., Razavi G.S.E., Petit R., Gupta S., Mkrtichyan M., Khleif S.N. Agonist anti-GITR antibody significantly enhances the therapeutic efficacy of Listeria monocytogenes-based immunotherapy. J. Immunol. Therapy Cancer. 2017;5:64. doi: 10.1186/s40425-017-0266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Appelbaum F.R., Sullivan K.M., Buckner C.D., Clift R.A., Deeg H.J., Fefer A., Hill R., Mortimer J., Neiman P.E., Sanders J.E. Treatment of malignant lymphoma in 100 patients with chemotherapy, total body irradiation, and marrow transplantation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1987;5:1340–1347. doi: 10.1200/JCO.1987.5.9.1340. [DOI] [PubMed] [Google Scholar]
- 584.McCune W.J., Golbus J., Zeldes W., Bohlke P., Dunne R., Fox D.A. Clinical and Immunologic Effects of Monthly Administration of Intravenous Cyclophosphamide in Severe Systemic Lupus Erythematosus. N. Engl. J. Med. 1988;318:1423–1431. doi: 10.1056/NEJM198806023182203. [DOI] [PubMed] [Google Scholar]
- 585.Gladstone D.E., Prestrud A.A., Pradhan A., Styler M.J., Topolsky D.L., Crilley P.A., Hoch S., Huppert A., Brodsky I. High-dose cyclophosphamide for severe systemic lupus erythematosus. Lupus. 2002;11:405–410. doi: 10.1191/0961203302lu229oa. [DOI] [PubMed] [Google Scholar]
- 586.Petri M., Brodsky R.A., Jones R.J., Gladstone D., Fillius M., Magder L.S. High Dose Cyclophosphamide versus Monthly Intravenous Cyclophosphamide for Systemic Lupus Erythematosus. Arthritis Rheum. 2010;62:1487–1493. doi: 10.1002/art.27371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Glode L.M., Barqawi A., Crighton F., Crawford E.D., Kerbel R. Metronomic therapy with cyclophosphamide and dexamethasone for prostate carcinoma. Cancer. 2003;98:1643–1648. doi: 10.1002/cncr.11713. [DOI] [PubMed] [Google Scholar]
- 588.Lutsiak M.E.C., Semnani R.T., De Pascalis R., Kashmiri S.V.S., Schlom J., Sabzevari H. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 589.Ikezawa Y., Nakazawa M., Tamura C., Takahashi K., Minami M., Ikezawa Z. Cyclophosphamide decreases the number, percentage and the function of CD25+ CD4+ regulatory T cells, which suppress induction of contact hypersensitivity. J. Dermatol. Sci. 2005;39:105–112. doi: 10.1016/j.jdermsci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 590.Heylmann D., Bauer M., Becker H., van Gool S., Bacher N., Steinbrink K., Kaina B. Human CD4+CD25+ Regulatory T Cells Are Sensitive to Low Dose Cyclophosphamide: Implications for the Immune Response. PLoS ONE. 2013;8:e83384. doi: 10.1371/journal.pone.0083384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Zhao J., Cao Y., Lei Z., Yang Z., Zhang B., Huang B. Selective Depletion of CD4+CD25+Foxp3+ Regulatory T Cells by Low-Dose Cyclophosphamide Is Explained by Reduced Intracellular ATP Levels. Cancer Res. 2010;70:4850–4858. doi: 10.1158/0008-5472.CAN-10-0283. [DOI] [PubMed] [Google Scholar]
- 592.Ghiringhelli F., Menard C., Puig P.E., Ladoire S., Roux S., Martin F., Solary E., Le Cesne A., Zitvogel L., Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Kleinman A.J., Sivanandham R., Sette P., Brocca-Cofano E., McAndrews C., Keele B.F., Pandrea I., Apetrei C. Lack of Specific Regulatory T Cell Depletion and Cytoreduction Associated with Extensive Toxicity After Administration of Low and High Doses of Cyclophosphamide. AIDS Res. Hum. Retrovir. 2021 doi: 10.1089/aid.2021.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Zajac A.J., Blattman J.N., Murali-Krishna K., Sourdive D.J.D., Suresh M., Altman J.D., Ahmed R. Viral Immune Evasion Due to Persistence of Activated T Cells Without Effector Function. J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Chew G.M., Fujita T., Webb G.M., Burwitz B.J., Wu H.L., Reed J.S., Hammond K.B., Clayton K.L., Ishii N., Abdel-Mohsen M., et al. TIGIT Marks Exhausted T Cells, Correlates with Disease Progression, and Serves as a Target for Immune Restoration in HIV and SIV Infection. PLoS Pathog. 2016;12:e1005349. doi: 10.1371/journal.ppat.1005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Fromentin R., Bakeman W., Lawani M.B., Khoury G., Hartogensis W., DaFonseca S., Killian M., Epling L., Hoh R., Sinclair E., et al. CD4+ T Cells Expressing PD-1, TIGIT and LAG-3 Contribute to HIV Persistence during ART. PLoS Pathog. 2016;12:e1005761. doi: 10.1371/journal.ppat.1005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Vali B., Jones R.B., Sakhdari A., Sheth P.M., Clayton K., Yue F.-Y., Gyenes G., Wong D., Klein M.B., Saeed S., et al. HCV-specific T cells in HCV/HIV co-infection show elevated frequencies of dual Tim-3/PD-1 expression that correlate with liver disease progression. Eur. J. Immunol. 2010;40:2493–2505. doi: 10.1002/eji.201040340. [DOI] [PubMed] [Google Scholar]
- 598.Hurst J., Hoffmann M., Pace M., Williams J.P., Thornhill J., Hamlyn E., Meyerowitz J., Willberg C., Koelsch K.K., Robinson N., et al. Immunological biomarkers predict HIV-1 viral rebound after treatment interruption. Nat. Commun. 2015;6:8495. doi: 10.1038/ncomms9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Kaufmann D.E., Kavanagh D.G., Pereyra F., Zaunders J.J., Mackey E.W., Miura T., Palmer S., Brockman M., Rathod A., Piechocka-Trocha A., et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 600.Elrefaei M., Burke C.M., Baker C.A.R., Jones N.G., Bousheri S., Bangsberg D.R., Cao H. HIV-Specific TGF-β-Positive CD4(+) T Cells Do Not Express Regulatory Surface Markers and Are Regulated by CTLA-4. AIDS Res. Hum. Retrovir. 2010;26:329–337. doi: 10.1089/aid.2009.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Cecchinato V., Tryniszewska E., Ma Z.M., Vaccari M., Boasso A., Tsai W.-P., Petrovas C., Fuchs D., Heraud J.-M., Venzon D., et al. Immune Activation Driven by CTLA-4 Blockade Augments Viral Replication at Mucosal Sites in Simian Immunodeficiency Virus Infection. J. Immunol. 2008;180:5439–5447. doi: 10.4049/jimmunol.180.8.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Lewis P.E., Poteet E.C., Liu D., Chen C., LaBranche C.C., Stanfield-Oakley S.A., Montefiori D.C., Ferrari G., Yao Q. CTLA-4 Blockade, during HIV Virus-Like Particles Immunization, Alters HIV-Specific B-Cell Responses. Vaccines. 2020;8:284. doi: 10.3390/vaccines8020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Le Garff G., Samri A., Lambert-Niclot S., Even S., Lavolé A., Cadranel J., Spano J.-P., Autran B., Marcelin A.-G., Guihot A. Transient HIV-specific T cells increase and inflammation in an HIV-infected patient treated with nivolumab. AIDS (Lond. Engl.) 2017;31 doi: 10.1097/QAD.0000000000001429. [DOI] [PubMed] [Google Scholar]
- 604.Guihot A., Marcelin A.G., Massiani M.A., Samri A., Soulié C., Autran B., Spano J.P. Drastic decrease of the HIV reservoir in a patient treated with nivolumab for lung cancer. Ann. Oncol. 2018;29:517–518. doi: 10.1093/annonc/mdx696. [DOI] [PubMed] [Google Scholar]
- 605.Scully E.P., Rutishauser R.L., Simoneau C.R., Delagrèverie H., Euler Z., Thanh C., Li J.Z., Hartig H., Bakkour S., Busch M., et al. Inconsistent HIV reservoir dynamics and immune responses following anti-PD-1 therapy in cancer patients with HIV infection. Ann. Oncol. 2018;29:2141–2142. doi: 10.1093/annonc/mdy259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Fromentin R., DaFonseca S., Costiniuk C.T., El-Far M., Procopio F.A., Hecht F.M., Hoh R., Deeks S.G., Hazuda D.J., Lewin S.R., et al. PD-1 blockade potentiates HIV latency reversal ex vivo in CD4+ T cells from ART-suppressed individuals. Nat. Commun. 2019;10:814. doi: 10.1038/s41467-019-08798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Antonioli L., Pacher P., Vizi E.S., Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Gupta P.K., Godec J., Wolski D., Adland E., Yates K., Pauken K.E., Cosgrove C., Ledderose C., Junger W.G., Robson S.C., et al. CD39 Expression Identifies Terminally Exhausted CD8+ T Cells. PLoS Pathog. 2015;11:e1005177. doi: 10.1371/journal.ppat.1005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Li J., Huang H.H., Tu B., Zhou M.J., Hu W., Fu Y.L., Li X.Y., Yang T., Song J.W., Fan X., et al. Reversal of the CD8(+) T-Cell Exhaustion Induced by Chronic HIV-1 Infection Through Combined Blockade of the Adenosine and PD-1 Pathways. Front. Immunol. 2021;12:687296. doi: 10.3389/fimmu.2021.687296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Zhang X., Sun S., Hwang I., Tough D.F., Sprent J. Potent and Selective Stimulation of Memory-Phenotype CD8+ T Cells In Vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/S1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 611.Lodolce J.P., Boone D.L., Chai S., Swain R.E., Dassopoulos T., Trettin S., Ma A. IL-15 Receptor Maintains Lymphoid Homeostasis by Supporting Lymphocyte Homing and Proliferation. Immunity. 1998;9:669–676. doi: 10.1016/S1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 612.Mueller Y.M., Bojczuk P.M., Halstead E.S., Kim A.H.J., Witek J., Altman J.D., Katsikis P.D. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood. 2003;101:1024–1029. doi: 10.1182/blood-2002-07-1957. [DOI] [PubMed] [Google Scholar]
- 613.Garrido C., Abad-Fernandez M., Tuyishime M., Pollara J.J., Ferrari G., Soriano-Sarabia N., Margolis D.M. Interleukin-15-Stimulated Natural Killer Cells Clear HIV-1-Infected Cells following Latency Reversal Ex Vivo. J. Virol. 2018;92:e00235-00218. doi: 10.1128/JVI.00235-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Lugli E., Mueller Y.M., Lewis M.G., Villinger F., Katsikis P.D., Roederer M. IL-15 delays suppression and fails to promote immune reconstitution in virally suppressed chronically SIV-infected macaques. Blood. 2011;118:2520–2529. doi: 10.1182/blood-2011-05-351155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Mueller Y.M., Petrovas C., Bojczuk P.M., Dimitriou I.D., Beer B., Silvera P., Villinger F., Cairns J.S., Gracely E.J., Lewis M.G., et al. Interleukin-15 increases effector memory CD8+ t cells and NK Cells in simian immunodeficiency virus-infected macaques. J. Virol. 2005;79:4877–4885. doi: 10.1128/JVI.79.8.4877-4885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Harwood O., O’Connor S. Therapeutic Potential of IL-15 and N-803 in HIV/SIV Infection. Viruses. 2021;13:1750. doi: 10.3390/v13091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Watson D.C., Moysi E., Valentin A., Bergamaschi C., Devasundaram S., Fortis S.P., Bear J., Chertova E., Bess J., Jr., Sowder R., et al. Treatment with native heterodimeric IL-15 increases cytotoxic lymphocytes and reduces SHIV RNA in lymph nodes. PLoS Pathog. 2018;14:e1006902. doi: 10.1371/journal.ppat.1006902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Zhu X., Marcus W.D., Xu W., Lee H.-i., Han K., Egan J.O., Yovandich J.L., Rhode P.R., Wong H.C. Novel Human Interleukin-15 Agonists. J. Immunol. 2009;183:3598–3607. doi: 10.4049/jimmunol.0901244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Jones R.B., Mueller S., O’Connor R., Rimpel K., Sloan D.D., Karel D., Wong H.C., Jeng E.K., Thomas A.S., Whitney J.B., et al. A Subset of Latency-Reversing Agents Expose HIV-Infected Resting CD4+ T-Cells to Recognition by Cytotoxic T-Lymphocytes. PLoS Pathog. 2016;12:e1005545. doi: 10.1371/journal.ppat.1005545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Ellis-Connell A.L., Balgeman A.J., Zarbock K.R., Barry G., Weiler A., Egan J.O., Jeng E.K., Friedrich T., Miller J.S., Haase A.T., et al. ALT-803 Transiently Reduces Simian Immunodeficiency Virus Replication in the Absence of Antiretroviral Treatment. J. Virol. 2018;92:e01748-01717. doi: 10.1128/JVI.01748-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.McBrien J.B., Mavigner M., Franchitti L., Smith S.A., White E., Tharp G.K., Walum H., Busman-Sahay K., Aguilera-Sandoval C.R., Thayer W.O., et al. Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8+ cells. Nature. 2020;578:154–159. doi: 10.1038/s41586-020-1946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.McBrien J.B., Wong A.K.H., White E., Carnathan D.G., Lee J.H., Safrit J.T., Vanderford T.H., Paiardini M., Chahroudi A., Silvestri G. Combination of CD8beta Depletion and Interleukin-15 Superagonist N-803 Induces Virus Reactivation in Simian-Human Immunodeficiency Virus-Infected, Long-Term ART-Treated Rhesus Macaques. J. Virol. 2020;94:e00755-00720. doi: 10.1128/JVI.00755-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Webb G.M., Molden J., Busman-Sahay K., Abdulhaqq S., Wu H.L., Weber W.C., Bateman K.B., Reed J.S., Northrup M., Maier N., et al. The human IL-15 superagonist N-803 promotes migration of virus-specific CD8+ T and NK cells to B cell follicles but does not reverse latency in ART-suppressed, SHIV-infected macaques. PLoS Pathog. 2020;16:e1008339. doi: 10.1371/journal.ppat.1008339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Pallikkuth S., Parmigiani A., Pahwa S. The role of interleukin-21 in HIV infection. Cytokine Growth Factor Rev. 2012;23:173–180. doi: 10.1016/j.cytogfr.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.White L., Krishnan S., Strbo N., Liu H., Kolber M.A., Lichtenheld M.G., Pahwa R.N., Pahwa S. Differential effects of IL-21 and IL-15 on perforin expression, lysosomal degranulation, and proliferation in CD8 T cells of patients with human immunodeficiency virus-1 (HIV) Blood. 2007;109:3873–3880. doi: 10.1182/blood-2006-09-045278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Strbo N., de Armas L., Liu H., Kolber M.A., Lichtenheld M., Pahwa S. IL-21 augments natural killer effector functions in chronically HIV-infected individuals. AIDS (Lond. Engl.) 2008;22:1551–1560. doi: 10.1097/QAD.0b013e3283089367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Pallikkuth S., Rogers K., Villinger F., Dosterii M., Vaccari M., Franchini G., Pahwa R., Pahwa S. Interleukin-21 administration to rhesus macaques chronically infected with simian immunodeficiency virus increases cytotoxic effector molecules in T cells and NK cells and enhances B cell function without increasing immune activation or viral replication. Vaccine. 2011;29:9229–9238. doi: 10.1016/j.vaccine.2011.09.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Micci L., Ryan E.S., Fromentin R., Bosinger S.E., Harper J.L., He T., Paganini S., Easley K.A., Chahroudi A., Benne C., et al. Interleukin-21 combined with ART reduces inflammation and viral reservoir in SIV-infected macaques. J. Clin. Investig. 2015;125:4497–4513. doi: 10.1172/JCI81400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Harper J., Huot N., Micci L., Tharp G., King C., Rascle P., Shenvi N., Wang H., Galardi C., Upadhyay A.A., et al. IL-21 and IFNα therapy rescues terminally differentiated NK cells and limits SIV reservoir in ART-treated macaques. Nat. Commun. 2021;12:2866. doi: 10.1038/s41467-021-23189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Ylisastigui L., Archin N.M., Lehrman G., Bosch R.J., Margolis D.M. Coaxing HIV-1 from resting CD4 T cells: Histone deacetylase inhibition allows latent viral expression. AIDS (Lond. Engl.) 2004;18:1101–1108. doi: 10.1097/00002030-200405210-00003. [DOI] [PubMed] [Google Scholar]
- 631.Archin N.M., Espeseth A., Parker D., Cheema M., Hazuda D., Margolis D.M. Expression of Latent HIV Induced by the Potent HDAC Inhibitor Suberoylanilide Hydroxamic Acid. AIDS Res. Hum. Retrovir. 2009;25:207–212. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Elliott J.H., Wightman F., Solomon A., Ghneim K., Ahlers J., Cameron M.J., Smith M.Z., Spelman T., McMahon J., Velayudham P., et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;10:e1004473. doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Kulkosky J., Culnan D.M., Roman J., Dornadula G., Schnell M., Boyd M.R., Pomerantz R.J. Prostratin: Activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood. 2001;98:3006–3015. doi: 10.1182/blood.V98.10.3006. [DOI] [PubMed] [Google Scholar]
- 634.Korin Y.D., Brooks D.G., Brown S., Korotzer A., Zack J.A. Effects of Prostratin on T-Cell Activation and Human Immunodeficiency Virus Latency. J. Virol. 2002;76:8118–8123. doi: 10.1128/JVI.76.16.8118-8123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Spivak A.M., Bosque A., Balch A.H., Smyth D., Martins L., Planelles V. Ex Vivo Bioactivity and HIV-1 Latency Reversal by Ingenol Dibenzoate and Panobinostat in Resting CD4(+) T Cells from Aviremic Patients. Antimicrob. Agents Chemother. 2015;59:5984–5991. doi: 10.1128/AAC.01077-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Jiang G., Mendes E.A., Kaiser P., Wong D.P., Tang Y., Cai I., Fenton A., Melcher G.P., Hildreth J.E.K., Thompson G.R., et al. Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation. PLoS Pathog. 2015;11:e1005066. doi: 10.1371/journal.ppat.1005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Wang P., Lu P., Qu X., Shen Y., Zeng H., Zhu X., Zhu Y., Li X., Wu H., Xu J., et al. Reactivation of HIV-1 from Latency by an Ingenol Derivative from Euphorbia Kansui. Sci. Rep. 2017;7:9451. doi: 10.1038/s41598-017-07157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Yang H., Li X., Yang X., Lu P., Wang Y., Jiang Z., Pan H., Zhao L., Zhu Y., Khan I.U., et al. Dual effects of the novel ingenol derivatives on the acute and latent HIV-1 infections. Antivir. Res. 2019;169:104555. doi: 10.1016/j.antiviral.2019.104555. [DOI] [PubMed] [Google Scholar]
- 639.Banerjee C., Archin N., Michaels D., Belkina A.C., Denis G.V., Bradner J., Sebastiani P., Margolis D.M., Montano M. BET bromodomain inhibition as a novel strategy for reactivation of HIV-1. J. Leukoc. Biol. 2012;92:1147–1154. doi: 10.1189/jlb.0312165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Sampey G.C., Irlbeck D.M., Browne E.P., Kanke M., McAllister A.B., Ferris R.G., Brehm J.H., Favre D., Routy J.-P., Jones C.D., et al. The SMAC Mimetic AZD5582 is a Potent HIV Latency Reversing Agent. bioRxiv. 2018:312447. doi: 10.1101/312447. [DOI] [Google Scholar]
- 641.Yamamoto T., Kanuma T., Takahama S., Okamura T., Moriishi E., Ishii K.J., Terahara K., Yasutomi Y. STING agonists activate latently infected cells and enhance SIV-specific responses ex vivo in naturally SIV controlled cynomolgus macaques. Sci. Rep. 2019;9:5917. doi: 10.1038/s41598-019-42253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Thibault S., Imbeault M., Tardif M.R., Tremblay M.J. TLR5 stimulation is sufficient to trigger reactivation of latent HIV-1 provirus in T lymphoid cells and activate virus gene expression in central memory CD4+ T cells. Virology. 2009;389:20–25. doi: 10.1016/j.virol.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 643.Novis C.L., Archin N.M., Buzon M.J., Verdin E., Round J.L., Lichterfeld M., Margolis D.M., Planelles V., Bosque A. Reactivation of latent HIV-1 in central memory CD4+T cells through TLR-1/2 stimulation. Retrovirology. 2013;10:119. doi: 10.1186/1742-4690-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Li P., Kaiser P., Lampiris H.W., Kim P., Yukl S.A., Havlir D.V., Greene W.C., Wong J.K. Stimulating the RIG-I pathway to kill cells in the latent HIV reservoir following viral reactivation. Nat. Med. 2016;22:807–811. doi: 10.1038/nm.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Bam R.A., Hansen D., Irrinki A., Mulato A., Jones G.S., Hesselgesser J., Frey C.R., Cihlar T., Yant S.R. TLR7 Agonist GS-9620 Is a Potent Inhibitor of Acute HIV-1 Infection in Human Peripheral Blood Mononuclear Cells. Antimicrob. Agents Chemother. 2017;61:e01369-01316. doi: 10.1128/AAC.01369-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Macedo A.B., Novis C.L., De Assis C.M., Sorensen E.S., Moszczynski P., Huang S.-H., Ren Y., Spivak A.M., Jones R.B., Planelles V., et al. Dual TLR2 and TLR7 agonists as HIV latency-reversing agents. JCI Insight. 2018;3:e122673. doi: 10.1172/jci.insight.122673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 648.McGhee J.D., Felsenfeld G. Nucleosome structure. Annu. Rev. Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- 649.Norton V.G., Marvin K.W., Yau P., Bradbury E.M. Nucleosome linking number change controlled by acetylation of histones H3 and H4. J. Biol. Chem. 1990;265:19848–19852. doi: 10.1016/S0021-9258(17)45450-0. [DOI] [PubMed] [Google Scholar]
- 650.Lee D.Y., Hayes J.J., Pruss D., Wolffe A.P. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993;72:73–84. doi: 10.1016/0092-8674(93)90051-Q. [DOI] [PubMed] [Google Scholar]
- 651.Coull J.J., Romerio F., Sun J.M., Volker J.L., Galvin K.M., Davie J.R., Shi Y., Hansen U., Margolis D.M. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J. Virol. 2000;74:6790–6799. doi: 10.1128/JVI.74.15.6790-6799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Lu H.K., Gray L.R., Wightman F., Ellenberg P., Khoury G., Cheng W.J., Mota T.M., Wesselingh S., Gorry P.R., Cameron P.U., et al. Ex vivo response to histone deacetylase (HDAC) inhibitors of the HIV long terminal repeat (LTR) derived from HIV-infected patients on antiretroviral therapy. PLoS ONE. 2014;9:e113341. doi: 10.1371/journal.pone.0113341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Kim H.-J., Bae S.-C. Histone deacetylase inhibitors: Molecular mechanisms of action and clinical trials as anti-cancer drugs. Am. J. Transl. Res. 2011;3:166–179. [PMC free article] [PubMed] [Google Scholar]
- 654.Archin N.M., Liberty A.L., Kashuba A.D., Choudhary S.K., Kuruc J.D., Crooks A.M., Parker D.C., Anderson E.M., Kearney M.F., Strain M.C., et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Kroon E.D.M.B., Ananworanich J., Pagliuzza A., Rhodes A., Phanuphak N., Trautmann L., Mitchell J.L., Chintanaphol M., Intasan J., Pinyakorn S., et al. A randomized trial of vorinostat with treatment interruption after initiating antiretroviral therapy during acute HIV-1 infection. J. Virus Erad. 2020;6:100004. doi: 10.1016/j.jve.2020.100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Tsai P., Wu G., Baker C.E., Thayer W.O., Spagnuolo R.A., Sanchez R., Barrett S., Howell B., Margolis D., Hazuda D.J., et al. In vivo analysis of the effect of panobinostat on cell-associated HIV RNA and DNA levels and latent HIV infection. Retrovirology. 2016;13:36. doi: 10.1186/s12977-016-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Rasmussen T.A., Tolstrup M., Brinkmann C.R., Olesen R., Erikstrup C., Solomon A., Winckelmann A., Palmer S., Dinarello C., Buzon M., et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: A phase 1/2, single group, clinical trial. Lancet HIV. 2014;1:e13–e21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 658.Søgaard O.S., Graversen M.E., Leth S., Olesen R., Brinkmann C.R., Nissen S.K., Kjaer A.S., Schleimann M.H., Denton P.W., Hey-Cunningham W.J., et al. The Depsipeptide Romidepsin Reverses HIV-1 Latency In Vivo. PLoS Pathog. 2015;11:e1005142. doi: 10.1371/journal.ppat.1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Nakajima H., Kim Y.B., Terano H., Yoshida M., Horinouchi S. FR901228, a Potent Antitumor Antibiotic, Is a Novel Histone Deacetylase Inhibitor. Exp. Cell Res. 1998;241:126–133. doi: 10.1006/excr.1998.4027. [DOI] [PubMed] [Google Scholar]
- 660.Furumai R., Matsuyama A., Kobashi N., Lee K.-H., Nishiyama M., Nakajima H., Tanaka A., Komatsu Y., Nishino N., Yoshida M., et al. FK228 (Depsipeptide) as a Natural Prodrug That Inhibits Class I Histone Deacetylases. Cancer Res. 2002;62:4916–4921. [PubMed] [Google Scholar]
- 661.Grant C., Rahman F., Piekarz R., Peer C., Frye R., Robey R.W., Gardner E.R., Figg W.D., Bates S.E. Romidepsin: A new therapy for cutaneous T-cell lymphoma and a potential therapy for solid tumors. Exp. Rev. Anticancer. 2010;10:997–1008. doi: 10.1586/era.10.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Del Prete G.Q., Oswald K., Lara A., Shoemaker R., Smedley J., Macallister R., Coalter V., Wiles A., Wiles R., Li Y., et al. Elevated plasma viral loads in romidepsin treated SIV-infected rhesus macaques on suppressive combination antiretroviral therapy. Antimicrob. Agents Chemother. 2015 doi: 10.1128/aac.02625-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Jonsson K.L., Tolstrup M., Vad-Nielsen J., Kjaer K., Laustsen A., Andersen M.N., Rasmussen T.A., Sogaard O.S., Ostergaard L., Denton P.W., et al. Histone deacetylase inhibitor romidepsin inhibits de novo HIV-1 infections. Antimicrob. Agents Chemother. 2015;59:3984–3994. doi: 10.1128/AAC.00574-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Rosas-Umbert M., Ruiz-Riol M., Fernandez M.A., Marszalek M., Coll P., Manzardo C., Cedeno S., Miro J.M., Clotet B., Hanke T., et al. In vivo Effects of Romidepsin on T-Cell Activation, Apoptosis and Function in the BCN02 HIV-1 Kick&Kill Clinical Trial. Front Immunol. 2020;11:418. doi: 10.3389/fimmu.2020.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Williams S.A., Chen L.F., Kwon H., Fenard D., Bisgrove D., Verdin E., Greene W.C. Prostratin antagonizes HIV latency by activating NF-kappaB. J. Biol. Chem. 2004;279:42008–42017. doi: 10.1074/jbc.M402124200. [DOI] [PubMed] [Google Scholar]
- 666.Chowdhury I.H., Koyanagi Y., Kobayashi S., Hamamoto Y., Yoshiyama H., Yoshida T., Yamamoto N. The phorbol ester TPA strongly inhibits HIV-1-induced syncytia formation but enhances virus production: Possible involvement of protein kinase C pathway. Virology. 1990;176:126–132. doi: 10.1016/0042-6822(90)90237-L. [DOI] [PubMed] [Google Scholar]
- 667.Bögi K., Lorenzo P.S., Szállási Z., Acs P., Wagner G.S., Blumberg P.M. Differential selectivity of ligands for the C1a and C1b phorbol ester binding domains of protein kinase Cdelta: Possible correlation with tumor-promoting activity. Cancer Res. 1998;58:1423–1428. [PubMed] [Google Scholar]
- 668.DeChristopher B.A., Loy B.A., Marsden M.D., Schrier A.J., Zack J.A., Wender P.A. Designed, synthetically accessible bryostatin analogues potently induce activation of latent HIV reservoirs in vitro. Nat. Chem. 2012;4:705–710. doi: 10.1038/nchem.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Marsden M.D., Wu X., Navab S.M., Loy B.A., Schrier A.J., DeChristopher B.A., Shimizu A.J., Hardman C.T., Ho S., Ramirez C.M., et al. Characterization of designed, synthetically accessible bryostatin analog HIV latency reversing agents. Virology. 2018;520:83–93. doi: 10.1016/j.virol.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Brogdon J., Ziani W., Wang X., Veazey R.S., Xu H. In vitro effects of the small-molecule protein kinase C agonists on HIV latency reactivation. Sci. Rep. 2016;6:39032. doi: 10.1038/srep39032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.McKernan L.N., Momjian D., Kulkosky J. Protein Kinase C: One Pathway towards the Eradication of Latent HIV-1 Reservoirs. Adv. Virol. 2012;2012:805347. doi: 10.1155/2012/805347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Albert B.J., Niu A., Ramani R., Marshall G.R., Wender P.A., Williams R.M., Ratner L., Barnes A.B., Kyei G.B. Combinations of isoform-targeted histone deacetylase inhibitors and bryostatin analogues display remarkable potency to activate latent HIV without global T-cell activation. Sci. Rep. 2017;7:7456. doi: 10.1038/s41598-017-07814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Laird G.M., Bullen C.K., Rosenbloom D.I.S., Martin A.R., Hill A.L., Durand C.M., Siliciano J.D., Siliciano R.F. Ex vivo analysis identifies effective HIV-1 latency–reversing drug combinations. J. Clin. Investig. 2015;125:1901–1912. doi: 10.1172/JCI80142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Sloane J.L., Benner N.L., Keenan K.N., Zang X., Soliman M.S.A., Wu X., Dimapasoc M., Chun T.-W., Marsden M.D., Zack J.A., et al. Prodrugs of PKC modulators show enhanced HIV latency reversal and an expanded therapeutic window. Proc. Natl. Acad. Sci. USA. 2020;117:10688–10698. doi: 10.1073/pnas.1919408117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Adolf W., Chanai S., Hecker E. 3-0-angeloylingenol, the toxic and skin irritant factor from latex of euphorbia anitiquorum l.(euphorbiaceae) and from a derived thai purgative and anthelimintic (vermifuge) drug. Sci. Asia. 1983;9:81–88. doi: 10.2306/scienceasia1513-1874.1983.09.081. [DOI] [Google Scholar]
- 676.Kedei N., Lundberg D.J., Toth A., Welburn P., Garfield S.H., Blumberg P.M. Characterization of the Interaction of Ingenol 3-Angelate with Protein Kinase C. Cancer Res. 2004;64:3243–3255. doi: 10.1158/0008-5472.CAN-03-3403. [DOI] [PubMed] [Google Scholar]
- 677.Jiang G., Mendes E.A., Kaiser P., Sankaran-Walters S., Tang Y., Weber M.G., Melcher G.P., Thompson G.R., III., Tanuri A., Pianowski L.F., et al. Reactivation of HIV latency by a newly modified Ingenol derivative via protein kinase Cδ–NF-κB signaling. AIDS (Lond. Engl.) 2014;28 doi: 10.1097/QAD.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Abreu C.M., Price S.L., Shirk E.N., Cunha R.D., Pianowski L.F., Clements J.E., Tanuri A., Gama L. Dual role of novel ingenol derivatives from Euphorbia tirucalli in HIV replication: Inhibition of de novo infection and activation of viral LTR. PLoS ONE. 2014;9:e97257. doi: 10.1371/journal.pone.0097257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Fujiwara M., Ijichi K., Tokuhisa K., Katsuura K., Wang G.Y.S., Uemura D., Shigeta S., Konno K., Yokota T., Baba M. Ingenol Derivatives are Highly Potent and Selective Inhibitors of HIV Replication in Vitro. Antivir. Chem. Chemother. 1996;7:230–236. doi: 10.1177/095632029600700502. [DOI] [Google Scholar]
- 680.Ersvaer E., Kittang A.O., Hampson P., Sand K., Gjertsen B.T., Lord J.M., Bruserud Ø. The Protein Kinase C Agonist PEP005 (Ingenol 3-Angelate) in the Treatment of Human Cancer: A Balance between Efficacy and Toxicity. Toxins. 2010;2:174–194. doi: 10.3390/toxins2010174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Pandeló José D., Bartholomeeusen K., da Cunha R.D., Abreu C.M., Glinski J., da Costa T.B., Bacchi Rabay A.F., Pianowski Filho L.F., Dudycz L.W., Ranga U., et al. Reactivation of latent HIV-1 by new semi-synthetic ingenol esters. Virology. 2014;462–463:328–339. doi: 10.1016/j.virol.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Jiang G., Maverakis E., Cheng M.Y., Elsheikh M.M., Deleage C., Méndez-Lagares G., Shimoda M., Yukl S.A., Hartigan-O’Connor D.J., Thompson G.R., III, et al. Disruption of latent HIV in vivo during the clearance of actinic keratosis by ingenol mebutate. JCI Insight. 2019;4 doi: 10.1172/jci.insight.126027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Filippakopoulos P., Qi J., Picaud S., Shen Y., Smith W.B., Fedorov O., Morse E.M., Keates T., Hickman T.T., Felletar I., et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Bisgrove D.A., Mahmoudi T., Henklein P., Verdin E. Conserved P-TEFb-interacting domain of BRD4 inhibits HIV transcription. Proc. Natl. Acad. Sci. USA. 2007;104:13690–13695. doi: 10.1073/pnas.0705053104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Lu P., Qu X., Shen Y., Jiang Z., Wang P., Zeng H., Ji H., Deng J., Yang X., Li X., et al. The BET inhibitor OTX015 reactivates latent HIV-1 through P-TEFb. Sci. Rep. 2016;6:24100. doi: 10.1038/srep24100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Huang H., Liu S., Jean M., Simpson S., Huang H., Merkley M., Hayashi T., Kong W., Rodriguez-Sanchez I., Zhang X., et al. A Novel Bromodomain Inhibitor Reverses HIV-1 Latency through Specific Binding with BRD4 to Promote Tat and P-TEFb Association. Front. Microbiol. 2017;8:1035. doi: 10.3389/fmicb.2017.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Zhang X.-x., Lin J., Liang T.-z., Duan H., Tan X.-h., Xi B.-m., Li L., Liu S.-w. The BET bromodomain inhibitor apabetalone induces apoptosis of latent HIV-1 reservoir cells following viral reactivation. Acta Pharmacol. Sin. 2019;40:98–110. doi: 10.1038/s41401-018-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Liang T., Zhang X., Lai F., Lin J., Zhou C., Xu X., Tan X., Liu S., Li L. A novel bromodomain inhibitor, CPI-203, serves as an HIV-1 latency-reversing agent by activating positive transcription elongation factor b. Biochem. Pharmacol. 2019;164:237–251. doi: 10.1016/j.bcp.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 689.Gallastegui E., Marshall B., Vidal D., Sanchez-Duffhues G., Collado J.A., Alvarez-Fernandez C., Luque N., Terme J.M., Gatell J.M., Sanchez-Palomino S., et al. Combination of biological screening in a cellular model of viral latency and virtual screening identifies novel compounds that reactivate HIV-1. J. Virol. 2012;86:3795–3808. doi: 10.1128/JVI.05972-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Abner E., Stoszko M., Zeng L., Chen H.C., Izquierdo-Bouldstridge A., Konuma T., Zorita E., Fanunza E., Zhang Q., Mahmoudi T., et al. A New Quinoline BRD4 Inhibitor Targets a Distinct Latent HIV-1 Reservoir for Reactivation from Other “Shock” Drugs. J. Virol. 2018;92:e02056-17. doi: 10.1128/JVI.02056-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Vamos M., Welsh K., Finlay D., Lee P.S., Mace P.D., Snipas S.J., Gonzalez M.L., Ganji S.R., Ardecky R.J., Riedl S.J., et al. Expedient Synthesis of Highly Potent Antagonists of Inhibitor of Apoptosis Proteins (IAPs) with Unique Selectivity for ML-IAP. ACS Chem. Biol. 2013;8:725–732. doi: 10.1021/cb3005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Finlay D., Vamos M., González-López M., Ardecky R.J., Ganji S.R., Yuan H., Su Y., Cooley T.R., Hauser C.T., Welsh K., et al. Small-Molecule IAP Antagonists Sensitize Cancer Cells to TRAIL-Induced Apoptosis: Roles of XIAP and cIAPs. Mol. Cancer Ther. 2014;13:5–15. doi: 10.1158/1535-7163.MCT-13-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Sun S.-C. The noncanonical NF-κB pathway. Immunol Rev. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Pache L., Dutra S., Spivak M., John M., Murry P., Hwang Y., Maestre M., Manganaro L., Vamos M., Teriete P., et al. BIRC2/cIAP1 Is a Negative Regulator of HIV-1 Transcription and Can Be Targeted by Smac Mimetics to Promote Reversal of Viral Latency. Cell Host Microbe. 2015;18:345–353. doi: 10.1016/j.chom.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Hattori S.I., Matsuda K., Tsuchiya K., Gatanaga H., Oka S., Yoshimura K., Mitsuya H., Maeda K. Combination of a Latency-Reversing Agent With a Smac Mimetic Minimizes Secondary HIV-1 Infection in vitro. Front. Microbiol. 2018;9:2022. doi: 10.3389/fmicb.2018.02022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Nixon C.C., Mavigner M., Sampey G.C., Brooks A.D., Spagnuolo R.A., Irlbeck D.M., Mattingly C., Ho P.T., Schoof N., Cammon C.G., et al. Systemic HIV and SIV latency reversal via non-canonical NF-κB signalling in vivo. Nature. 2020;578:160–165. doi: 10.1038/s41586-020-1951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Mavigner M., Liao L.E., Brooks A.D., Ke R., Mattingly C., Schoof N., McBrien J., Carnathan D., Liang S., Vanderford T.H., et al. CD8 lymphocyte depletion enhances the latency reversal activity of the SMAC mimetic AZD5582 in ART-suppressed SIV-infected rhesus macaques. J. Virol. 2021;95:e01429-01420. doi: 10.1128/JVI.01429-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Pache L., Marsden M.D., Teriete P., Portillo A.J., Heimann D., Kim J.T., Soliman M.S.A., Dimapasoc M., Carmona C., Celeridad M., et al. Pharmacological Activation of Non-canonical NF-κB Signaling Activates Latent HIV-1 Reservoirs In Vivo. Cell Rep. Med. 2020;1:100037. doi: 10.1016/j.xcrm.2020.100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Takahama S., Yamamoto T. Pattern Recognition Receptor Ligands as an Emerging Therapeutic Agent for Latent HIV-1 Infection. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/fcimb.2020.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Kuse N., Sun X., Akahoshi T., Lissina A., Yamamoto T., Appay V., Takiguchi M. Priming of HIV-1-specific CD8(+) T cells with strong functional properties from naive T cells. EBioMedicine. 2019;42:109–119. doi: 10.1016/j.ebiom.2019.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Aroh C., Wang Z., Dobbs N., Luo M., Chen Z., Gao J., Yan N. Innate Immune Activation by cGMP-AMP Nanoparticles Leads to Potent and Long-Acting Antiretroviral Response against HIV-1. J. Immunol. 2017;199:3840–3848. doi: 10.4049/jimmunol.1700972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Palermo E., Acchioni C., Di Carlo D., Zevini A., Muscolini M., Ferrari M., Castiello L., Virtuoso S., Borsetti A., Antonelli G., et al. Activation of Latent HIV-1 T Cell Reservoirs with a Combination of Innate Immune and Epigenetic Regulators. J. Virol. 2019;93:e01194-01119. doi: 10.1128/JVI.01194-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Mavigner M., Brooks A.D., Koblansky A., Galardi C., Madsen H., Margolis D.M., Silvestri G., Chahroudi A. 26 - Sting agonist as a kick and kill agent to target the HIV reservoir. J. Virus Erad. 2019;5:14–15. doi: 10.1016/S2055-6640(20)31049-9. [DOI] [Google Scholar]
- 704.Lisziewicz J., Sun D., Weichold F.F., Thierry A.R., Lusso P., Tang J., Gallo R.C., Agrawal S. Antisense oligodeoxynucleotide phosphorothioate complementary to Gag mRNA blocks replication of human immunodeficiency virus type 1 in human peripheral blood cells. Proc. Natl. Acad. Sci. USA. 1994;91:7942–7946. doi: 10.1073/pnas.91.17.7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Martinsen J.T., Gunst J.D., Højen J.F., Tolstrup M., Søgaard O.S. The Use of Toll-Like Receptor Agonists in HIV-1 Cure Strategies. Front. Immunol. 2020;11:1112. doi: 10.3389/fimmu.2020.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Equils O., Schito M.L., Karahashi H., Madak Z., Yarali A., Michelsen K.S., Sher A., Arditi M. Toll-like receptor 2 (TLR2) and TLR9 signaling results in HIV-long terminal repeat trans-activation and HIV replication in HIV-1 transgenic mouse spleen cells: Implications of simultaneous activation of TLRs on HIV replication. J. Immunol. (Baltimore, Md. 1950) 2003;170:5159–5164. doi: 10.4049/jimmunol.170.10.5159. [DOI] [PubMed] [Google Scholar]
- 707.Séréni D., Tubiana R., Lascoux C., Katlama C., Taulera O., Bourque A., Cohen A., Dvorchik B., Martin R.R., Tournerie C., et al. Pharmacokinetics and Tolerability of Intravenous Trecovirsen (GEM®91), an Antisense Phosphorothioate Oligonucleotide, in HIV-Positive Subjects. J. Clin. Pharmacol. 1999;39:47–54. doi: 10.1177/00912709922007552. [DOI] [PubMed] [Google Scholar]
- 708.Agrawal S., Martin R.R. Was Induction of HIV-1 Through TLR9? J. Immunol. 2003;171:1621–1622. doi: 10.4049/jimmunol.171.4.1621. [DOI] [PubMed] [Google Scholar]
- 709.Winckelmann A.A., Munk-Petersen L.V., Rasmussen T.A., Melchjorsen J., Hjelholt T.J., Montefiori D., Østergaard L., Søgaard O.S., Tolstrup M. Administration of a Toll-Like Receptor 9 Agonist Decreases the Proviral Reservoir in Virologically Suppressed HIV-Infected Patients. PLoS ONE. 2013;8:e62074. doi: 10.1371/journal.pone.0062074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Schmidt M., Hagner N., Marco A., König-Merediz S.A., Schroff M., Wittig B. Design and Structural Requirements of the Potent and Safe TLR-9 Agonistic Immunomodulator MGN1703. Nucleic Acid Ther. 2015;25:130–140. doi: 10.1089/nat.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Offersen R., Nissen S.K., Rasmussen T.A., Ostergaard L., Denton P.W., Sogaard O.S., Tolstrup M. A Novel Toll-Like Receptor 9 Agonist, MGN1703, Enhances HIV-1 Transcription and NK Cell-Mediated Inhibition of HIV-1-Infected Autologous CD4+ T Cells. J. Virol. 2016;90:4441–4453. doi: 10.1128/JVI.00222-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Vibholm L., Schleimann M.H., Højen J.F., Benfield T., Offersen R., Rasmussen K., Olesen R., Dige A., Agnholt J., Grau J., et al. Short-Course Toll-Like Receptor 9 Agonist Treatment Impacts Innate Immunity and Plasma Viremia in Individuals With Human Immunodeficiency Virus Infection. Clin. Infect. Dis. 2017;64:1686–1695. doi: 10.1093/cid/cix201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Vibholm L.K., Konrad C.V., Schleimann M.H., Frattari G., Winckelmann A., Klastrup V., Jensen N.M., Jensen S.S., Schmidt M., Wittig B., et al. Effects of 24-week Toll-like receptor 9 agonist treatment in HIV type 1+ individuals. AIDS (Lond. Engl.) 2019;33 doi: 10.1097/QAD.0000000000002213. [DOI] [PubMed] [Google Scholar]
- 714.Del Prete G.Q., Alvord W.G., Li Y., Deleage C., Nag M., Oswald K., Thomas J.A., Pyle C., Bosche W.J., Coalter V., et al. TLR7 agonist administration to SIV-infected macaques receiving early initiated cART does not induce plasma viremia. JCI Insight. 2019;4 doi: 10.1172/jci.insight.127717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 715.Riddler S., Para M., Benson C., Mills A., Ramgopal M., DeJesus E., Brinson C., Cyktor J., Mellors J., Guo S. Vesatolimod (GS-9620) is safe and pharmacodynamically active in HIV-infected individuals. J. Int. AIDS Soc. 2019;22:43. doi: 10.1016/S2055-6640(20)31079-7. [DOI] [Google Scholar]
- 716.O’Neill L.A.J., Kishton R.J., Rathmell J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016;16:553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.MacIver N.J., Michalek R.D., Rathmell J.C. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Liu Y., Zhang D.-t., Liu X.-g. mTOR Signaling in T Cell Immunity and Autoimmunity. Int. Rev. Immunol. 2015;34:50–66. doi: 10.3109/08830185.2014.933957. [DOI] [PubMed] [Google Scholar]
- 719.Trautmann L., Mbitikon-Kobo F.-M., Goulet J.-P., Peretz Y., Shi Y., Van Grevenynghe J., Procopio F.A., Boulassel M.R., Routy J.-P., Chomont N., et al. Profound metabolic, functional, and cytolytic differences characterize HIV-specific CD8 T cells in primary and chronic HIV infection. Blood. 2012;120:3466–3477. doi: 10.1182/blood-2012-04-422550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Patsoukis N., Bardhan K., Chatterjee P., Sari D., Liu B., Bell L.N., Karoly E.D., Freeman G.J., Petkova V., Seth P., et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Staron M.M., Gray S.M., Marshall H.D., Parish I.A., Chen J.H., Perry C.J., Cui G., Li M.O., Kaech S.M. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8(+) T cells during chronic infection. Immunity. 2014;41:802–814. doi: 10.1016/j.immuni.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Bengsch B., Johnson A.L., Kurachi M., Odorizzi P.M., Pauken K.E., Attanasio J., Stelekati E., McLane L.M., Paley M.A., Delgoffe G.M., et al. Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8(+) T Cell Exhaustion. Immunity. 2016;45:358–373. doi: 10.1016/j.immuni.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Schurich A., Pallett L.J., Jajbhay D., Wijngaarden J., Otano I., Gill U.S., Hansi N., Kennedy P.T., Nastouli E., Gilson R., et al. Distinct Metabolic Requirements of Exhausted and Functional Virus-Specific CD8 T Cells in the Same Host. Cell Rep. 2016;16:1243–1252. doi: 10.1016/j.celrep.2016.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Angin M., Volant S., Passaes C., Lecuroux C., Monceaux V., Dillies M.-A., Valle-Casuso J.C., Pancino G., Vaslin B., Le Grand R., et al. Metabolic plasticity of HIV-specific CD8+ T cells is associated with enhanced antiviral potential and natural control of HIV-1 infection. Nat. Metab. 2019;1:704–716. doi: 10.1038/s42255-019-0081-4. [DOI] [PubMed] [Google Scholar]
- 725.Valle-Casuso J.C., Angin M., Volant S., Passaes C., Monceaux V., Mikhailova A., Bourdic K., Avettand-Fenoel V., Boufassa F., Sitbon M., et al. Cellular Metabolism Is a Major Determinant of HIV-1 Reservoir Seeding in CD4+ T Cells and Offers an Opportunity to Tackle Infection. Cell Metab. 2019;29:611–626.e615. doi: 10.1016/j.cmet.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 726.van der Windt G.J., Pearce E.L. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol Rev. 2012;249:27–42. doi: 10.1111/j.1600-065X.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Igarashi T., Endo Y., Englund G., Sadjadpour R., Matano T., Buckler C., Buckler-White A., Plishka R., Theodore T., Shibata R., et al. Emergence of a highly pathogenic simian/human immunodeficiency virus in a rhesus macaque treated with anti-CD8 mAb during a primary infection with a nonpathogenic virus. Proc. Natl. Acad. Sci. USA. 1999;96:14049–14054. doi: 10.1073/pnas.96.24.14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Igarashi T., Brown C.R., Endo Y., Buckler-White A., Plishka R., Bischofberger N., Hirsch V., Martin M.A. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): Implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. USA. 2001;98:658–663. doi: 10.1073/pnas.98.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Orenstein J.M., Fox C., Wahl S.M. Macrophages as a Source of HIV During Opportunistic Infections. Science (N. Y.) 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 730.Clements J.E., Babas T., Mankowski J.L., Suryanarayana K., Piatak M., Jr., Tarwater P.M., Lifson J.D., Zink M.C. The central nervous system as a reservoir for simian immunodeficiency virus (SIV): Steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J. Infect. Dis. 2002;186:905–913. doi: 10.1086/343768. [DOI] [PubMed] [Google Scholar]
- 731.Zalar A., Figueroa M.I., Ruibal-Ares B., Baré P., Cahn P., de Bracco M.M.d.E., Belmonte L. Macrophage HIV-1 infection in duodenal tissue of patients on long term HAART. Antivir. Res. 2010;87:269–271. doi: 10.1016/j.antiviral.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 732.Yukl S.A., Sinclair E., Somsouk M., Hunt P.W., Epling L., Killian M., Girling V., Li P., Havlir D.V., Deeks S.G., et al. A comparison of methods for measuring rectal HIV levels suggests that HIV DNA resides in cells other than CD4+ T cells, including myeloid cells. AIDS (Lond. Engl.) 2014;28:439–442. doi: 10.1097/QAD.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Honeycutt J.B., Thayer W.O., Baker C.E., Ribeiro R.M., Lada S.M., Cao Y., Cleary R.A., Hudgens M.G., Richman D.D., Garcia J.V. HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat. Med. 2017;23:638–643. doi: 10.1038/nm.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Busca A., Saxena M., Kumar A. Critical role for antiapoptotic Bcl-xL and Mcl-1 in human macrophage survival and cellular IAP1/2 (cIAP1/2) in resistance to HIV-Vpr-induced apoptosis. J. Biol. Chem. 2012;287:15118–15133. doi: 10.1074/jbc.M111.312660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Rappaport J., Volsky D.J. Role of the macrophage in HIV-associated neurocognitive disorders and other comorbidities in patients on effective antiretroviral treatment. J. Neurovirol. 2015;21:235–241. doi: 10.1007/s13365-015-0346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Vojnov L., Martins M.A., Bean A.T., Veloso de Santana M.G., Sacha J.B., Wilson N.A., Bonaldo M.C., Galler R., Stevenson M., Watkins D.I. The majority of freshly sorted simian immunodeficiency virus (SIV)-specific CD8(+) T cells cannot suppress viral replication in SIV-infected macrophages. J. Virol. 2012;86:4682–4687. doi: 10.1128/JVI.06324-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Clayton K.L., Collins D.R., Lengieza J., Ghebremichael M., Dotiwala F., Lieberman J., Walker B.D. Resistance of HIV-infected macrophages to CD8(+) T lymphocyte-mediated killing drives activation of the immune system. Nat. Immunol. 2018;19:475–486. doi: 10.1038/s41590-018-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Mailliard R.B., Smith K.N., Fecek R.J., Rappocciolo G., Nascimento E.J.M., Marques E.T., Watkins S.C., Mullins J.I., Rinaldo C.R. Selective Induction of CTL Helper Rather Than Killer Activity by Natural Epitope Variants Promotes Dendritic Cell–Mediated HIV-1 Dissemination. J. Immunol. 2013;191:2570–2580. doi: 10.4049/jimmunol.1300373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 739.Smith K.N., Mailliard R.B., Piazza P.A., Fischer W., Korber B.T., Fecek R.J., Ratner D., Gupta P., Mullins J.I., Rinaldo C.R. Effective Cytotoxic T Lymphocyte Targeting of Persistent HIV-1 during Antiretroviral Therapy Requires Priming of Naive CD8+ T Cells. mBio. 2016;7:e00473-16. doi: 10.1128/mBio.00473-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Smith K.N., Mailliard R.B., Rinaldo C.R. Programming T cell Killers for an HIV Cure: Teach the New Dogs New Tricks and Let the Sleeping Dogs Lie. Forum Immunopathol. Dis. Ther. 2015;6:67–77. doi: 10.1615/ForumImmunDisTher.2016014160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Campbell G.R., To R.K., Zhang G., Spector S.A. SMAC mimetics induce autophagy-dependent apoptosis of HIV-1-infected macrophages. Cell Death Dis. 2020;11:590. doi: 10.1038/s41419-020-02761-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Bobardt M., Kuo J., Chatterji U., Chanda S., Little S.J., Wiedemann N., Vuagniaux G., Gallay P.A. The inhibitor apoptosis protein antagonist Debio 1143 Is an attractive HIV-1 latency reversal candidate. PLoS ONE. 2019;14:e0211746. doi: 10.1371/journal.pone.0211746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Sanyal A., Mailliard R.B., Rinaldo C.R., Ratner D., Ding M., Chen Y., Zerbato J.M., Giacobbi N.S., Venkatachari N.J., Patterson B.K., et al. Novel assay reveals a large, inducible, replication-competent HIV-1 reservoir in resting CD4(+) T cells. Nat. Med. 2017;23:885–889. doi: 10.1038/nm.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Battivelli E., Dahabieh M.S., Abdel-Mohsen M., Svensson J.P., Tojal Da Silva I., Cohn L.B., Gramatica A., Deeks S., Greene W.C., Pillai S.K., et al. Distinct chromatin functional states correlate with HIV latency reactivation in infected primary CD4(+) T cells. Elife. 2018;7:e34655. doi: 10.7554/eLife.34655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 745.Paiardini M., Dhodapkar K., Harper J., Deeks S.G., Ahmed R. Editorial: HIV and Cancer Immunotherapy: Similar Challenges and Converging Approaches. Front. Immunol. 2020;11:519. doi: 10.3389/fimmu.2020.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Díaz-Carballo D., Saka S., Acikelli A.H., Homp E., Erwes J., Demmig R., Klein J., Schröer K., Malak S., D’Souza F., et al. Enhanced antitumoral activity of TLR7 agonists via activation of human endogenous retroviruses by HDAC inhibitors. Commun. Biol. 2021;4:276. doi: 10.1038/s42003-021-01800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 747.Zeng R., Spolski R., Finkelstein S.E., Oh S., Kovanen P.E., Hinrichs C.S., Pise-Masison C.A., Radonovich M.F., Brady J.N., Restifo N.P., et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Strengell M., Matikainen S., Sirén J., Lehtonen A., Foster D., Julkunen I., Sareneva T. IL-21 in Synergy with IL-15 or IL-18 Enhances IFN-γ Production in Human NK and T Cells. J. Immunol. 2003;170:5464–5469. doi: 10.4049/jimmunol.170.11.5464. [DOI] [PubMed] [Google Scholar]
- 749.Pouw N., Treffers-Westerlaken E., Kraan J., Wittink F., ten Hagen T., Verweij J., Debets R. Combination of IL-21 and IL-15 enhances tumour-specific cytotoxicity and cytokine production of TCR-transduced primary T cells. Cancer Immunol. Immunother. CII. 2010;59:921–931. doi: 10.1007/s00262-010-0818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750.Kinter A.L., Godbout E.J., McNally J.P., Sereti I., Roby G.A., O’Shea M.A., Fauci A.S. The Common γ-Chain Cytokines IL-2, IL-7, IL-15, and IL-21 Induce the Expression of Programmed Death-1 and Its Ligands. J. Immunol. 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 751.Wrangle J.M., Velcheti V., Patel M.R., Garrett-Mayer E., Hill E.G., Ravenel J.G., Miller J.S., Farhad M., Anderton K., Lindsey K., et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: A non-randomised, open-label, phase 1b trial. Lancet. Oncol. 2018;19:694–704. doi: 10.1016/S1470-2045(18)30148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Drusbosky L., Nangia C., Nguyen A., Szeto C., Newton Y., Spilman P., Reddy S.B. Complete response to avelumab and IL-15 superagonist N-803 with Abraxane in Merkel cell carcinoma: A case study. J. ImmunoTherapy Cancer. 2020;8:e001098. doi: 10.1136/jitc-2020-001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753.Knudson K.M., Hicks K.C., Alter S., Schlom J., Gameiro S.R. Mechanisms involved in IL-15 superagonist enhancement of anti-PD-L1 therapy. J. Immunol. Therapy Cancer. 2019;7:82. doi: 10.1186/s40425-019-0551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Jochems C., Tritsch S.R., Knudson K.M., Gameiro S.R., Rumfield C.S., Pellom S.T., Morillon Y.M., Newman R., Marcus W., Szeto C., et al. The multi-functionality of N-809, a novel fusion protein encompassing anti-PD-L1 and the IL-15 superagonist fusion complex. OncoImmunology. 2019;8:e1532764. doi: 10.1080/2162402X.2018.1532764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 755.Li Y., Cong Y., Jia M., He Q., Zhong H., Zhao Y., Li H., Yan M., You J., Liu J., et al. Targeting IL-21 to tumor-reactive T cells enhances memory T cell responses and anti-PD-1 antibody therapy. Nat. Commun. 2021;12:951. doi: 10.1038/s41467-021-21241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]