Abstract
Thermoregulation is a homeostatic mechanism that is disrupted in some neurological diseases. Patients with multiple sclerosis (MS) are susceptible to increases in body temperature, especially with more severe neurological signs. This condition can become intolerable when these patients suffer febrile infections such as coronavirus disease-2019 (COVID-19). We review the mechanisms of hyperthermia in patients with MS, and they may encounter when infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Finally, the thermoregulatory role and relevant adaptation to regular physical exercise are summarized.
Keywords: Multiple sclerosis; exercise; thermoregulation; sweat gland; SARS-CoV-2; ANS Autonomic nervous system; APN, Adiponectin; CNS, Central nervous system; COVID-19, Coronavirus disease-2019; EDHF, Endothelial-derived hyperpolarizing factor; eNOS, Endothelial nitric oxide synthase; MS, Multiple sclerosis; NLRP3, NLR family pyrin domain containing 3; NO, Nitric oxide; PACAP, Pituitary adenylate cyclase-activating polypeptide; PAMPs, Pathogen-associated molecular patterns; PGs, Prostaglandins; PGE2, Prostaglandin E2; ROS, Reactive oxygen species; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SNS, Sympathetic nervous system; TRPV-1, Transient receptor potential vanilloid type 1; VDP, Vascular-dilating prostanoids; VEGF, Vascular endothelial growth factor; VIP, Vasoactive intestinal peptide
1. Introduction
Multiple sclerosis (MS) is a neurodegenerative disease characterized by lesions of the central nervous system (CNS). A unique feature of this autoimmune disease is the high prevalence (60–80%) of temperature sensitivity, where neurological signs are exacerbated by increases in environmental or internal body temperatures. The regulation of a near constant body temperature within a physiological range is required for survival and daily function (Corbett et al., 2014). Body temperature is composed of central and skin temperature (Gisolfi et al., 2000; Lim et al., 2008; Lim and Suzuki, 2017). Core temperature is centrally regulated by the brain in response to changes in thermal balance (Gisolfi et al., 2000; Lim, 2020). Venous blood temperature and afferent signals from thermo-sensitive nerves on the body surface act to regulate autonomic nervous and behavioral responses to regulate body temperature (Lim et al., 2008). The control of temperature in the body results from a balance between heat gain and its dissipation (Lim, 2020).
Thermoregulation in humans is accomplished by several independent loops of the thermoregulatory reflex. Peripheral receptors are activated by increases in skin and core temperatures, and activate afferent nerves to signal the preoptic anterior hypothalamus as the integrating center. After integrating the thermal-afferent information, increased efferent nerve outflows lead to activating the thermal-effector responses that result in sweating and cutaneous vasodilation consequent to releasing of acetylcholine and other co-transmitters such as vasoactive intestinal peptide (VIP), pituitary adenylate cyclase-activating polypeptide (PACAP), vascular-dilating prostanoids (VDP), substance P (SP), and channels of transient receptor potential vanilloid type 1 (TRPV-1) (Francisco and Minson, 2018; Gagnon et al., 2016; Taylor, 2011; White et al., 1996). Disturbances of this thermoregulatory reflex occurs in some diseases such as MS, a disease of demyelination, which affects neural conduction.
Thermal fluctuations impact neural conduction (Morrison and Blessing, 2011). Inactivity reduces the reactivity of cutaneous small-vessels, and increases core temperature (Wang, 2005). This could underlie the high sensitivity of patients with MS to increases in core body temperature induced by exogenous agents such as viral infections. Individuals infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) also experience increased core body temperatures, which can exacerbate temperature dysregulation in patients with preexisting dysregulation of body temperature. We review temperature dysregulation in patients with MS and the added burden of the coronavirus disease-19 (COVID-19) pandemic. In addition, we also discuss the adaptions produced by regular physical exercise as an adjunct procedure to mitigate the effects of heat in patients with MS infected the SARS-CoV-2.
2. Thermal dysregulation in MS patients as a challenge during SARS-CoV-2 infection
MS is the primary cause of neuronal dysfunction in juveniles (Edmonds et al., 2010). The autonomic nervous system (ANS) is severely impacted in individuals with MS (Gunal et al., 2002), where dysfunction of sympathetic and parasympathetic nerves is associated with a range of diverse clinical disabilities (Flachenecker et al., 2001; Habek, 2019). Autonomic dysfunction in MS is related to lesions in the periventricular region of the fourth ventricle of the brainstem and medulla (Adamec and Habek, 2013). Autonomic dysfunction results from both demyelination and also axonal loss (de Seze et al., 2001), affecting cardiovascular (66% of patients) (Acevedo et al., 2000; Mincu et al., 2015), bladder (97% of patients) (Haensch and Jörg, 2006), sexual function (80% of patients) (Tepavcevic et al., 2008), bowel (43% of patients) (Hinds et al., 1990), sleep (50% of patients) (Bamer et al., 2008), heat sensitivity (60–90% of patients) (Gallup et al., 2010) and also altered motor symptoms (e.g., fatigue) (thermoregulation).
Hypothalamic lesions can result in both hyperthermia and hypothermia (Linker et al., 2006; White et al., 1996). Increasing core body temperature occurs commonly in patients with MS and can be due to interruptions in central sudomotor pathways which originate from the preoptic hypothalamic area and descend to the intermediolateral of the spinal cord to exit from the central nervous system (CNS) to innervate sweat glands that can lower core temperature (Adamec and Habek, 2013; Saari et al., 2009). Impairment of this pathway worsens neurological signs and symptoms and in severe cases can increase heat sensitivity or Uhthoff phenomenon/syndrome, which describes a transient worsening of neurological symptoms (caused by hot weather, exercise, fever, being in a heated environment etc.) due to demyelination in MS (Jain et al., 2020). Up to 60 to 80% of MS patients experience this phenomenon that can be triggered by increases in core temperature by as little as 0.5°C, resulting in transient symptoms such as visual blurring (Cianfrone et al., 2006; Filingeri et al., 2017; Jain et al., 2020; Syndulko et al., 1996; Wilson et al., 2010). The first report on visual blurring with exercise as a heat stressor was described in 1890 by Uhthoff, who attributed it to increases in core body temperature, leading to the avoidance of exercise by patients with MS (Baker, 2002; Frohman et al., 2013).
Thermal sensitivity is usually measured in patients with MS by responses to heat (e.g., using hot water immersion, heat lamp, and heat cabinet) and evaluating emerging or worsening neurological signs (Leavitt et al., 2014; Nelson et al., 1958; Nelson and McDowell, 1959). Initial studies on healthy individuals, people with neurological disorders and patients with MS reported that two patients with MS died after exposure to increased body temperatures of up to 40 °C for 8–10 h; patients with MS experienced motor weakness, amblyopia, and visual deficits following a heat stress. Such sensitivity in patients with MS even occurred after exposure one limb to temperature increase of up to ∼0.5 ° C (Guthrie, 1950, 1951). A later study of responses to a heat stress (55–60 ° C) in patients with MS reported worsening of signs in 75% patients within 10–15 min (EDMUND and FOG, 1955). Another study indicated that clinical signs appeared in patients with MS after 8 min when body temperatures were increased by 0.8 ° C; clinical signs peaked when body temperature was increased by 1.7 ° C for 28 min (Nelson et al., 1958).
Some studies also suggest that increased core body temperature in patients with MS could result from lowered sweating rates as well as delayed sweat initiation (Krupp et al., 2007; Petajan and White, 1999), or having a higher sweat threshold (Baker, 2002; Huang et al., 2015; Racosta et al., 2015). In addition, the reduction in sweating function in MS patients is largely due to decreased sweat output in individual sweat glands rather than to reduced gland recruitment (Davis et al., 2010a), and that 15 weeks of aerobic endurance was unable to improve sweat function in patients with MS. Thus, heat sensitivity is a characteristic feature of MS (Davis et al., 2005).
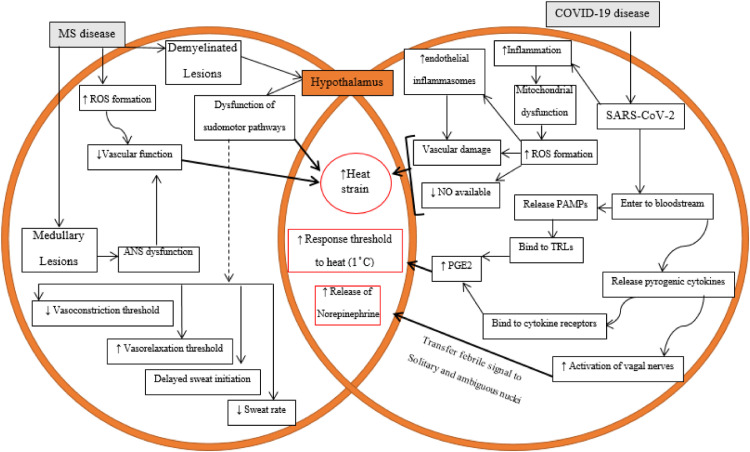
Regardless of being a frequent complaint in patients with MS, our understanding of how increases or decreases in body temperature contribute to worsening of MS symptoms is incomplete. However, as depicted in Fig. 1 , the most likely mechanisms underlying heat sensitivity and the exacerbation of the symptoms of MS can be summarized as follow:
-
A)
Demyelinated axons in brain lesions and peripheral neurons overexpress voltage-gated Na+ (Nav) channels, particularly α-subunits, throughout their length (Smith, 2007; Tartas et al., 2004). There have been generally revealed 9 isoforms of this type of Nav channels (Nav1.1 to 1.9). The isoforms of Nav1.1, Nav1.2, Nav1.3, and Nav1.6 extremely expressed in CNS (Egri and Ruben, 2012). These channels influence enormous characteristics of neurons including refractory phase and excitability of neurons. The contribution of these channels in neuronal electrogenesis is formed based on their different spatial distribution within the axon membrane, with high density in the axon membrane at the node of Ranvier and much lower density in other areas beneath myelin including paranodal and intermodal axon membrane (Peles and Salzer, 2000; Waxman, 1998). Sodium channels illustrate a dynamic changes either in normal CNS (during development) or in pathological CNS. It has been reported that MS patients and its animal model, experimental autoimmune encephalomyelitis (EAE), experience a plasticity in Nav channels in both expression and distribution levels. Notably, Nav1.2 and 1.6 are the most isoforms undergoing increased expression and also they extended to the paranodal and juxtaparanodal areas (Craner et al., 2003, 2004b). Increased Nav channels is associated with some changes in impulse conductance and hyperexcitibility and exacerbating the clinical signs of these patients is partly due to a persistent sodium current which may accompany neural damage and loss of axons that transfer information on central temperature to hypothalamus. Increased intracellular Na+ will cause Ca2+ dysregulation, including by acting on Na+/Ca2+ exchange. Intracellular accrual of Ca2+ initiates neural and axon damages through activating some catabolic enzymes, including proteases and calpains (Agrawal and Fehlings, 1996; Correale et al., 2019; Craner et al., 2004a, 2004b; Stys et al., 1993, 1992). Upon losing the central and peripheral neurons, thus, MS patients in progressive phase of disease do not have an accurate perception of how extent their central body temperature has been increased.
-
B)
Roughly 20% of hypothalamic neurons are stratified as a thermal-sensitive neurons with high rates of action potential in proportional with other thermal-nonsensetive ones (Wechselberger et al., 2006). These neurons not only respond to the temperature of hypothalamus but receive afferent synaptic inputs from skin and spinal thermorecptors; hence, they integrate the central and peripheral temperatures. Thermal sensitivity of these hypothalamic neurons partly results from some selective ionic channels (Boulant and Hardy, 1974; Wechselberger et al., 2006). Normal function of neurons depends on regularly fire action potentials and maintenance resting membrane potentials. In this regard, potassium channels including two-pore domain K + (K2P) channels contribute to the steady outward leak of K + ions and consequent restoring of the rate of action potential (Braun, 2012). Humans express 14 K2P channels which have been categorized into 5 subgroups (Patel and Honoré, 2001). These channels play a leading role in establishing resting membrane potential, regulating potential duration and modulating the response to synaptic inputs (Griffin et al., 1996; Patel and Honoré, 2001; Rush and Rinzel, 1995). Besides, the most majority of potassium channels extensively express in human brain (Maingret et al., 2000; Patel and Honoré, 2001). A scrutiny on every subunit of K2P channels is beyond the scope of this review. Although there is not conclusive document about these channels expression in axons extruded in hypothalamic area and hypothalamic neurons in MS patients, their expression is main factor impacting the thermal sensitivity of neurons. These channels are not voltage-gated, since the absence of canonical voltage sensor domain (Braun, 2012; Medhurst et al., 2001; Wechselberger et al., 2006). In this context, their activity is influenced by physical and chemical stimuli, including mechanical and heat stresses (Braun, 2012). Peripheral and central hyperthermia activate K2P channels, causing the outward flow of potassium ions from neurons and resulting in neuronal hyperpolarization and decreased action potential propagation (Griffin and Boulant, 1995; Wechselberger et al., 2006). Notably, the higher expression of K2P channels on thermal-sensitive neurons, the higher potassium leak current and the lower firing rate of action potentials. In accordance with their extensive expression in brain, potassium channels may be selective candidate in increased thermal sensitivity. In any case, the expression changes of potassium channels in afferent peripheral and central neurons and their role in altering thermal sensitivity of MS patients should be precisely investigated by future studies.
-
C)
Hypothalamic lesion-induced impairments in sympathetic outflows and the destruction of sudomotor pathways reduces sweating and consequently increases body temperature (Haensch and Jörg, 2006; Huang et al., 2015; Keller et al., 2014).
-
D)
There is decreased sweat output per gland (Davis et al., 2005).
-
E)
The reduced current available to excite node of Ranvier results in a blockade in neuronal conduction (Allen, 2018; Davis et al., 2018, 2010b).
-
F)
Other factors that could reduce conduction speed in demyelinated fibers include vasoconstriction and humoral factors (Syndulko et al., 1996). Fatigue, which is associated with heat stress and which occurs in approximately 70% of individuals with MS individuals (Krupp, 2003; Krupp et al., 2007; Marino, 2009; Martin et al., 2005; Nybo and Nielsen, 2001), can result from demyelinated lesions that lower central motor conduction and cortical excitability (Humm et al., 2004; Petajan and White, 2000; Sheean et al., 1997; White et al., 2008). There is evidence that patients with advanced MS pathology likely have more failures in impulse conduction (Smith and McDonald, 1999).
Fig. 1.
Schematic representation of dysfunctional pathways of thermoregulation in individuals with MS disease and the exacerbating role of COVID-19 in increasing heat strain. MS, multiple sclerosis; COVID-19, coronavirus disease-2019; ANS, autonomic nervous system; ROS, reactive oxygen species; NO, nitric oxide; PAMPs, pathogen-associated molecular patterns; TLRs, toll-like receptors; PGE2, prostaglandin E2.
The ability to sense changes in skin temperature drives behavioral responses to heat stress (e.g., decreased physical work, removing clothes, seeking shade, etc.) and can also influence autonomic thermoregulation (Davis et al., 2018, 2010b; Huang et al., 2015, 2014). Studies by Filingere and colleagues (Davis et al., 2005) that used heat sensitivity of skin afferents using exercise as a heat stressor, and also by exposing them to a cold environment, indicated that cold sensitivity is decreased in patients with MS, suggesting that MS pathophysiology independently modulates afferent thermosensory function. Reduced cold-induced conduction speed in nerve fibers is greatest in A fibers, followed by B fibers and less so in C fibers (Syndulko et al., 1996). This divergence in susceptibility to cold block may be related to nerve characteristics such as myelination, diameter, and threshold characteristics. This is supported by findings that nerve fibers, especially A fibers which have lower thresholds, faster conduction, greater myelination and diameters, have greater sensitivity to cold block (Douglas and Malcolm, 1955; Todd, 2002). Several studies suggest that contrary to expectations, the conduction velocity in sensory nerve fibers is increased by higher limb temperatures (Franssen et al., 1999; Franssen and Wieneke, 1994; Tavee, 2019).
Body temperature fluctuations in patients with MS with infectious conditions, such as during the COVID-19 pandemic, could be exacerbated since there is a limited ability to mount counteracting febrile responses in these patients (Davis et al., 2010b). Although the majority of COVID-19 patients are asymptomatic, many others manifest some serious clinical symptoms (Schneider et al., 2021; Wölfel et al., 2020). Fever occurs initially in more than 50% of patients affected with COVID-19 (Hui and Zumla, 2019; Islam et al., 2021; Qiu et al., 2020). Instant diagnosis of infection can prevent its transmission or to initiate countermeasures or successful confinements (Schneider et al., 2021). Although assessing fever may not be efficacious in all COVID-19 patients, it is however critical in individuals with pre-existing thermal dysregulation such as in patients with MS (Racke and Newsome, 2020; Schneider et al., 2021). Individuals with MS are at higher risk during the COVID-19 pandemic, as they often are treated with immunosuppressing drugs (IMDs) (Hughes et al., 2020; Loonstra et al., 2020; Louapre et al., 2020; Maghzi et al., 2020; Willis and Robertson, 2020). Importantly, both diseases (MS and COVID-19) share the ability to induce febrile responses characteristic of cytokine storms (Berger et al., 2020; García, 2020; Sorenson et al., 2017; Zheng et al., 2020).
Fever is part of defensive responses of multicellular organisms (host) to invasion of microorganisms such as viruses, including SARS-CoV-2 (Ogoina, 2011). The febrile response includes endocrine, neurological and immunological mechanisms (Dinarello and Gelfand, 2005; Mackowiak). SARS-CoV-2, as an exogenous pyrogen, can initiate the febrile responses through humoral and neuronal pathways (Ogoina, 2011). Upon entering the circulation, the virus then activates pathogen-associated molecular patterns (PAMPs) or pyrogenic cytokines [pro-inflammatory cytokines, namely, tumor necrosis factor-alpha (TNF-α), IL-1] (Conti et al., 2004; Jiang et al., 1999; Romanovsky et al., 2006; Steiner et al., 2006a; Turrin and Rivest, 2004). The circulatory microorganism's PAMPs and cytokines (released by host) activate toll-like receptors and cytokine receptors, respectively, on capillaries and also on the blood-brain barrier (BBB), leading to the release of prostaglandin E2 (PGE2) (Conti et al., 2004; Romanovsky et al., 2006; Steiner et al., 2006a; Turrin and Rivest, 2004). PGE2 binds to EP2 receptors in the preoptic area to activate thermal neurons in the anterior hypothalamus and reset the thermal balance point to a higher set-point (Mackowiak; Romanovsky et al., 2006; Steiner et al., 2006a; Turrin and Rivest, 2004). It has been argued that the initial phase of the febrile response is dependent on PGE2 synthetized in the liver and lungs prior to migrating to the brain, while the later phase of the febrile response is due to centrally synthetized PGE2 (Gross, 2006; Steiner et al., 2006b). Thus, exogenous pyrogens (such as SARS-CoV-2) are converted to endogenous pyrogens (PAMPs, cytokines) after entering the body. SARS-CoV-2 has a tropism for brainstem centers of the autonomic nervous system, where the virus serves to transfer a fever signal from peripheral nerves (such as the olfactory pathway) transfers to the hypothalamus, thalamus, and brainstem (Blatteis, 2007; Hopkins, 2007; Roth and De Souza, 2001; Xu et al., 2005). Much like fever produced by PGE2, exogenous pyrogen such as SARS-CoV-2 can activate pyrogenic cytokines, which in turn activate the hepatic branch of the vagal nerve and transfer the febrile signal to nerves in solitary and ambiguous nuclei (Blatteis, 2007; Li et al., 2020; Matsuda et al., 2004; Roth and De Souza, 2001; Rummel et al., 2005). The signals from the solitary nucleus are delivered to preoptic and hypothalamic areas through the ventral noradrenergic bundle, resulting in norepinephrine release into these thermoregulatory areas (Blatteis, 2007; Roth and De Souza, 2001). Norepinephrine mediates the vagal pathway by raising core temperature. The resetting of the thermal balance point to a higher set-point through these two humoral and neuronal signals initiates a feedback loop leading to early vasoconstriction and some clinical and behavioral manifestations to maintain body temperature. When there is no longer a fever signal in the CNS, the set-point then returns normal levels to activate heat loss mechanisms such as sweating (Leggett, 2016; Mackowiak).
As discussed above, activation mechanisms to reduce core temperature are impaired in patients with MS. In addition, patients with MS have impairment in vascular function, likely related to reactive oxygen species (ROS)-induced oxidative stress (Dhalla et al., 2000; Fjeldstad et al., 2010; Ohl et al., 2016; Ranadive et al., 2012). Oxidative stress in patients with MS promotes viral replication (Li et al., 2017), while SARS-CoV-2 can impose a thermal burden through ROS production reduce nitric oxide (NO)-mediated vasodilation (Ghosh and Karin, 2002; Xu and Zou, 2009; Zhang, 2008). In addition, MS and COVID-19 leads to inflammation and increased mitochondria-derived ROS production (Alandijany et al., 2013). ROS upregulate endothelial inflammasomes [especially, NLR family pyrin domain containing 3 (NLRP3)] and their signals, including caspase-1 and IL-1β in endothelial cells, thus initiating vascular pathology (Long et al., 2020; Martinon et al., 2002).
High core temperature affects tissue metabolism, and can result in tissue necrosis, especially in neurons, and DNA unwinding (Nagashima et al., 2012). The prevalence of MS can be three times higher than in men, and this can cause more severe symptoms because women have lower blood volumes, smaller heart sizes, a lower free fat mass, a higher percent of subcutaneous and whole fat, a higher body temperature threshold for dilating of cutaneous vessels, circadian changes in sex hormone levels that could lead to differences in body temperature regulation (MITCHELL et al., 1992).
3. Exercise improves thermoregulation
Homeothermic mechanisms cause intrinsic regulation of heat gain (metabolic production) and loss (sweat and evaporation) to maintain core body temperature set point (Gisolfi and Mora, 2016; Madden and Morrison, 2019; Zalewski et al., 2014). Fluctuation from set point of body temperature stimulates hypothalamus to restore core temperature through changes in behaviors and physiological responses induced by the ANS (Morrison and Blessing, 2011). Reductions and increases of core temperature trigger the activation of sympathetic efferent and cholinergic outflows, respectively. Adrenergic efferent release of norepinephrine to constrict cutaneous vessels, while cholinergic efferent secrete acetylcholine (Ach) to lower core temperature by vasodilation (Flouris and Schlader, 2015; Gordon et al., 2019; Morrison and Nakamura, 2019; Tansey and Johnson, 2015). Maintenance of core body temperature is critical for work output and prevents premature fatigue (Adams et al., 2019; Fehling et al., 2015; González-Alonso et al., 1999; Sawka et al., 2001). Physical exercise is a heat stressor that increases core body temperatures through increases in metabolism by exercising muscles (Nadel et al., 1987, 1974). On the other hand, the adaptations induced by exercise training in cardiac output and stroke volume can mitigate this heat stress through facilitating heat transmission to the skin for cooling by evaporation, which is followed by a reduction in early fatigue (Geor and McCutcheon, 1998; Lim, 2020). Thus, improving body temperature regulation attenuates fatigue.
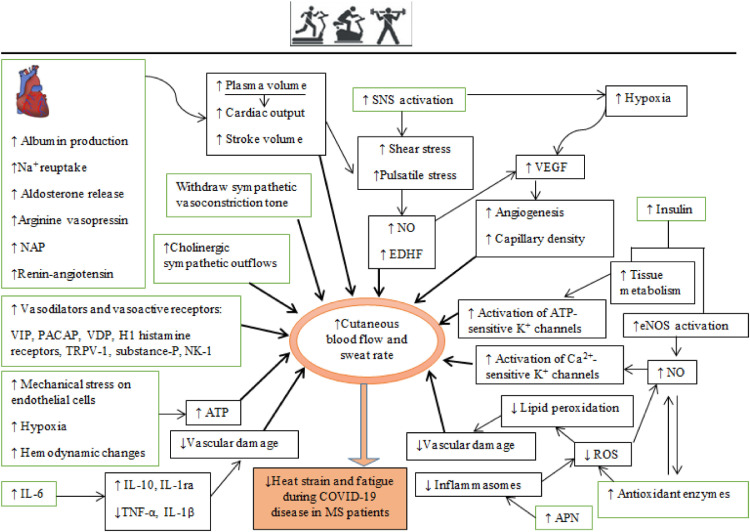
Although physical exercise acts as a heat stressor, regular exposure to this stressor promotes adaptations that limit its detrimental effects (Périard et al., 2016). Although the increase in body temperature is probably a danger in MS patients, especially during or after an infection with corona virus, higher levels of heat stimulation are associated with greater adaptations in heat regulation (Périard et al., 2016). These adaptations to exercise training are akin to the adaptions induced by exposure to heat (Corbett et al., 2014; Nielsen et al., 1993; Weller et al., 2007). Importantly, the probable adaptations acquired from exercise training that help to maintain core body temperature within a narrow range consist of neurophysiological adaptations, including cardiovascular, hematological, and hormonal changes, neural adaptations of the temperature set and thermoregulatory effectors, reduced production of metabolic heat by exercise, improved heat tolerance through the response to heat shock, and improved sweat economy (Fig. 2 ) (Nadel, 1988; Nadel et al., 1980; Takeda and Okazaki, 2018; Werner, 1993; Yamauchi et al., 1997).
Fig. 2.
Schematic diagram of physical exercise-induced adaptations in improving thermal management. The green rectangles represent the main adaptations induced directly by regular physical exercise that are associated with secondary adaptations (in black rectangles). The bold arrows show the main adaptations; oval circles indicate increased cutaneous blood flow resulting from vascular adaptations. Na+, sodium; NAP, natriuretic atrial peptide; VIP, vasoactive intestinal peptide; PACAP, pituitary adenylate cyclase-activating polypeptide; VDP, vascular-dilating prostanoids; TRPV-1, transient receptor potential vanilloid type 1; NK-1, neurokinin-1; NO, nitric oxide; EDHF, endothelial-derived hyperpolarizing factor; VEGF, vascular endothelial growth factor; ROS, reactive oxygen species; APN, adiponectin.
The cardiovascular system regulates blood supply to exercising muscles to support their metabolism and also to the skin for dissipating the heat generated by exercising muscles (Geor and McCutcheon, 1996; Rowell, 2011). Autonomic processes such as skin vasodilation and sweat production are compensatory procedures to maintain body temperature at a normal level (Kenny and McGinn, 2017; Nagashima et al., 2012). Exercise-induced hyperthermia impairs the function of ANS, which is measurable by heart rate variability and lasts > 80 min after the exercise session (Armstrong et al., 2012). Thus, other factors can restore body heat balance. The initial increase in skin blood flow during hyperthermia is primarily due to withdrawal of sympathetic vasoconstriction (McNamara et al., 2014; Wong, 2013). The 85–95% increase in cutaneous blood flow and increased sweating is mediated by cholinergic outflows from sympathetic nerves (Wong, 2013). Vasodilation and sweating is achieved by release of Ach from cholinergic terminals of sympathetic nerves and also by the co-release of other vasodilators (Bennett et al., 2003; Dean et al., 2010; Johnson and Kellogg, 2010; Kellogg et al., 1995; McCord et al., 2006; Wong, 2013; Wong et al., 2004). Vasoactive intestinal peptide (VIP) (Bennett et al., 2003; Dean et al., 2010), pituitary adenylate cyclase-activating peptide (PACAP) (Rambotti et al., 2002; Wallengren, 1997; Warren et al., 1992), vitronectin-derived peptide (VDP) (McCord et al., 2006), histamine (Wong et al., 2004), and substance-P (Wong and Fieger, 2012; Wong and Minson, 2006) are co-released with Ach. These co-transmitters also increase the production of local NO (Kellogg et al., 2012; Klede et al., 2003; McCord et al., 2006; Shibasaki et al., 2002; Wilkins et al., 2004; Wong, 2013; Wong et al., 2005, 2004). For instance, substance-P activates Neurokinin-1 (NK1) receptors to increase NO levels (Wong and Minson, 2006). However, substance-P also causes microvascular dilation by NO independent mechanisms by the degranulation of cutaneous mast cells, causing local increases in histamine levels (Huttunen et al., 1996). Increased cutaneous microvascular reactivity and function are positively related to VO2max (Hodges et al., 2010; Middlebrooke et al., 2005; Roche et al., 2008; Tew et al., 2010).
Improvements in the thermoregulatory components produced by regular physical exercise, especially endurance/aerobic training, are related to increases in cardiac output and antioxidant enzyme levels (Tew et al., 2012, 2010), while increases in skin blood flow are due to increased plasma volume (Ikegawa et al., 2011). Aerobic exercise training for 6–10 days increases the plasma volume (Convertino, 1991; Costill et al., 1976; Fellmann, 1992; Williams et al., 1979), due to increased intravascular protein (albumin) by triggering the production of hepatic albumin and reuptake of electrolytes (sodium) (Convertino et al., 1980; Convertino, 1991; Fellmann, 1992; Nadel, 1996), increases in hormones that regulate fluid volume (e.g., aldosterone, arginine vasopressin, natriuretic atrial peptide (NAP) and renin-angiotensin) (Convertino et al., 1980; Fellmann, 1992; Nagashima et al., 2000; Wade et al., 1985; Yang et al., 1998). Higher blood volume increases blood pressure and this results in increased shear stress in the vasculature (Birk et al., 2013; Laughlin et al., 2008). The increased heart rate during exercise stimulates contractility of heart rate by activation of the sympathetic nervous system, which also increases shear stress (BLAIR et al., 1961). These local hemodynamic changes activate endothelial mechanical-sensitive ion channels such as piezo1 channels and G-proteins, caveolin, and integrins. Mechanical signals lead to calcium entering, activation of endothelial nitric oxide synthase (eNOS) and the production of NO and endothelial-derived hyperpolarizing factor (EDHF) (Awolesi et al., 1994; Caolo et al., 2020; Green et al., 2004; Laughlin et al., 2008; Silva and Zanesco, 2010). NO contributes to approximately 30% increases in responses of cutaneous blood flow to Ach released during physical exercise (Boutsiouki et al., 2004; Holowatz et al., 2005; Kellogg et al., 2005; Laughlin et al., 2008). For better regulation of core temperature, NO and hypoxia resulting from exercise can upregulate vascular endothelial growth factor (VEGF), which promotes microvascular numbers (angiogenesis) and increases capillary density that promotes cutaneous perfusion (Breen et al., 2008; Jensen et al., 2004; Lloyd et al., 2005; Michiels et al., 2000).
The optimization of vascular structure and improved blood flow distribution can attenuate some of the heat strain produced by coronavirus disease in patients with neurological disorders. Increases in antioxidant enzyme levels induced by exercise also assists in promoting skin vasodilation by improving NO bioavailability and reducing damage of lipid peroxidation-mediated damage of vascular endothelial cells (Dawson et al., 2013; Eskurza et al., 2004; Finaud et al., 2006; Holowatz et al., 2006; Leeuwenburgh and Heinecke, 2001; Tew et al., 2012; Violi et al., 1999). Chronic aerobic exercise increases the activity of vasodilatory mediators such as NO, prostacyclin, prostaglandins, bradykinin, and EDHF that promotes cutaneous perfusion and limits exercise-induced increases of core temperature (Colberg et al., 2009, 2002; Gooding et al., 2006; Lenasi and Štrucl, 2008; Sokolnicki et al., 2007; Wang, 2005). In addition, these factors interact to enhance skin perfusion by increasing cutaneous flow produced by Ach (Gaubert et al., 2007; Lenasi and Strucl, 2004; Medow et al., 2008; Padilla et al., 2011; Wang, 2005). Increased insulin-induced tissue metabolism improves endothelial function induced by aerobic exercise by phosphorylating eNOS and increasing NO production (Ghafouri et al., 2011; Harris et al., 2008; Kashyap et al., 2005; Rossi et al., 2005), which activates Ca2+-activated potassium (K +) channels (Bychkov et al., 1998; Kane et al., 2004; Merkus et al., 2006; Misurski et al., 2001) and ATP-sensitive K + channels (McKay and Hester, 1996).
Exercise and endurance training change body composition (reduce subcutaneous body fat), serum lipid and lipoproteins (increases high-density lipoprotein cholesterol [HDL-C]), and attenuates the oxidation of low-density lipoprotein cholesterol (LDL-C) (Gordon et al., 2014; Kodama et al., 2007; Kraus et al., 2002; Neves et al., 2017). Oxidation of LDL-C inhibits the release of NO and mitigates endothelium-dependent vasodilation (Liao et al., 1995). Exercise increases the expression of antioxidant enzymes such as superoxide dismutase (SOD), which increases the bioavailability of NO inhibition of LDL-C oxidation and enhances the vasodilatory sensitivity to chronic activation of β-adrenergic receptors on microvascular walls (Woodman et al., 2005, 2004).
Plasma levels of adiponectin (APN) are increased by regular physical exercise (Saeidi et al., 2020; Zouhal et al. 2021). APN binds to adiponectin receptor 1 (AdipR1) in endothelial cells, mitigating the induction of inflammasomes, especially NLPR3, whose signals include activation of caspase-1 and IL-1β (Du et al., 2016; Hui et al., 2012; Lee et al., 2020, 2018, 2011). Downregulated inflammasomes attenuate ROS production in endothelial cells. Therefore, exercise training increases endothelial APN levels to promote NO production and its bioavailability, and reduces oxidative and apoptotic damage of microvessels (Chen et al., 2003; Gleeson et al., 2011; Moien-Afshari et al., 2008; Plant et al., 2008; Wang et al., 2018; Zhang et al., 2015).
Engaging in regular physical exercise leads to adaptations in the cardiovascular system and skeletal muscles, whereby less metabolic exertion is required to perform the same intensity of exercise (James et al., 2017; Lorenzo et al., 2010; Nadel et al., 1974; Pivarnik et al., 1987; Sawka et al., 1985). Exercise reduces vascular inflammation (Pedersen, 2009; Ribeiro et al., 2010), for example by releasing IL-6 (a myokine released by skeletal muscles into the circulation during physical exercise) (Jankord et al., 2007; Pedersen, 2009; Pedersen and Febbraio, 2008; Pedersen et al., 2004; Suzuki et al., 2020) to increase anti-inflammatory (IL-10, IL-1ra) and lower pro-inflammatory (TNF-α, IL-1β) cytokine levels (Pedersen, 2009; Shephard, 2002; Starkie et al., 2003; Suzuki, 2019).
Other mechanisms related to the beneficial effects of exercise in reducing core temperature includes the release of ATP by endothelial cells and erythrocytes (Ellsworth and Sprague, 2012; Hellsten et al., 1998, 2012; Singel and Stamler, 2005). ATP, which is released during exercise and in response to hypoxia and shear stress, binds to P2Y receptors in micro-vessels to release NO and PGs (prostacyclin, PGE2) (Burnstock et al., 2013; Corr and Burnstock, 1994; Frandsen et al., 2000; Hellsten et al., 1998; Huang et al., 2000; Mortensen et al., 2009a, 2009b; Nyberg et al., 2013) and hyperpolarization of vascular cells (Crecelius et al., 2012; Rosenmeier et al., 2008). Additionally, increased concentrations of plasma ATP during exercise inhibits sympathetic vasoconstriction of α-adrenergic receptors, a phenomenon known as functional sympatholysis (Kirby et al., 2008; Rosenmeier et al., 2004). Exercise, especially endurance training, attenuates the effects of endothelial-dependent vasoconstriction produced by endothelin-1 (Beck et al., 2013; Maeda et al., 2003; Nyberg et al., 2013; Nyberg et al., 2014), thromboxane A2 (Hansen et al., 2011; Stergioulas and Filippou, 2006), and angiotensin II (Rush and Aultman, 2008; Zucker et al., 2015).
Adaptations in sweat rates may be important when monitoring body core temperature. Changes in response to exercise include increases in cholinergic sensitivity, greater rate and efficiency of eccrine glands in sweat production per gland, increased number and sensitivity of muscarinic receptors responsible for sweating, and reduced choline esterase activity (Lorenzo and Minson, 2010; Périard et al., 2016). Thus, exercise training can reduce the threshold for initiating cutaneous blood flow and sweat production in response to increases in core body temperature.
The majority of the vascular benefits produced by exercise training occur via increased NO bioavailability. Exercise-induced increases in NO inhibits virus replication by suppressing ribonucleotide reductase (Ellermann-Eriksen, 2005; Komatsu et al., 1999), and is supported by findings that NOS-deficient mice are more susceptible to viral infections (Gamba et al., 2004; MacLean et al., 1998).
Exercise-induced adaptations in thermoregulation occur within 3–14 days (Garrett et al., 2011), with the initial changes in the cardiovascular system associated with hypervolemia (Lorenzo et al., 2010; Nielsen et al., 1993; Shvartz et al., 1977; Weller et al., 2007) and secondary adaptations related to sweat production (Nielsen et al., 1993; Roberts et al., 1977; Shvartz et al., 1977; Wyndham et al., 1976). Light to moderate-intensity endurance training (≥ 50% VO2max) for at least one week optimal to stimulate these adaptations and restore body temperature to healthy levels (Lorenzo et al., 2010; Périard et al., 2015; Sawka et al., 1985). Furthermore, thermobalance during steady-state exercise occurs within 30–45 min and increases in body temperature occur within 15–20 min of physical exercise (Kenny and Jay, 2011). Thus, exercise of at least 20 min is needed to promote thermoregulatory adaptations. Since MS patients are very sensitive to heat stress, with exacerbating clinical signs during and after infection with SARS-CoV-2 (Willis and Robertson, 2020), management of the risks associated with heat stress should include physical exercise using exercise/rest (intermittent exercise) protocols), with close monitoring of hydration and cool-down strategies.
4. Conclusions
We reviewed thermoregulation management following exercise training in MS patients infected with COVID-19. The mechanisms of exercise-induced vascular benefits involve increased eNOS bioavailability and the anti-inflammatory effects of NO. Regular physical exercise increases vasodilation sensitivity to maintain core body temperature by reducing the sweat threshold and increasing responses of cutaneous vessels. Exercise-induced adaptations in the cardiovascular system mitigates perceived exertion and fatigue during physical exercise through improved thermoregulation, allowing MS patients to better manage neurological signs during and after infection with SARS-CoV-2.
Author contributions
OR, BT, NZ and HZ conceptualized and wrote the first draft. AMT, IL, HZ, and OR developed the study concept. KG, KS, and HZ reviewed and edited the final version of manuscript. All authors contributed to the article and approved the submitted version.
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Acevedo A., Nava C., Arriada N., Violante A., Corona T. Cardiovascular dysfunction inmultiple sclerosis. Acta Neurol. Scand. 2000;101(2):85–88. doi: 10.1034/j.1600-0404.2000.101002085.x. [DOI] [PubMed] [Google Scholar]
- Adamec I., Habek M. Autonomic dysfunction in multiple sclerosis. Clin. Neurol. Neurosurg. 2013;115:S73–S78. doi: 10.1016/j.clineuro.2013.09.026. [DOI] [PubMed] [Google Scholar]
- Adams J., Scott D.M., Brand N.A., Suh H.G., Seal A.D., McDermott B.P., Ganio M.S., Kavouras S.A. Mild hypohydration impairs cycle ergometry performance in the heat: a blinded study. Scand. J. Med. Sci. Sports. 2019;29(5):686–695. doi: 10.1111/sms.13386. [DOI] [PubMed] [Google Scholar]
- Agrawal S.K., Fehlings M.G. Mechanisms of secondary injury to spinal cord axons in vitro: role of Na+, Na (+)-K (+)-ATPase, the Na (+)-H+ exchanger, and the Na (+)-Ca2+ exchanger. J. Neurosci. 1996;16(2):545–552. doi: 10.1523/JNEUROSCI.16-02-00545.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alandijany T., Kammouni W., Chowdhury S.K.R., Fernyhough P., Jackson A.C. Mitochondrial dysfunction in rabies virus infection of neurons. J. Neurovirol. 2013;19(6):537–549. doi: 10.1007/s13365-013-0214-6. [DOI] [PubMed] [Google Scholar]
- Allen D.R. Thermoregulatory dysfunction in multiple sclerosis. Handb. Clin. Neurol. 2018 doi: 10.1016/B978-0-444-64074-1.00042-2. [DOI] [PubMed] [Google Scholar]
- Armstrong R.G., Ahmad S., Seely A.J., Kenny G.P. Heart rate variability and baroreceptor sensitivity following exercise-induced hyperthermia in endurance trained men. Eur. J. Appl. Physiol. 2012;112(2):501–511. doi: 10.1007/s00421-011-1989-x. [DOI] [PubMed] [Google Scholar]
- Awolesi M.A., Widmann M.D., Sessa W.C., Sumpio B.E. Cyclic strain increases endothelial nitric oxide synthase activity. Surgery. 1994;116(2):439–444. discussion 444. [PubMed] [Google Scholar]
- Baker D.G. Multiple sclerosis and thermoregulatory dysfunction. J. Appl. Physiol. 2002;92(5):1779–1780. doi: 10.1152/japplphysiol.01251.2001. [DOI] [PubMed] [Google Scholar]
- Bamer A., Johnson K., Amtmann D., Kraft G. Prevalence of sleep problems in individuals with multiple sclerosis. Multiple Sclerosis J. 2008;14(8):1127–1130. doi: 10.1177/1352458508092807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck D.T., Casey D.P., Martin J.S., Emerson B.D., Braith R.W. Exercise training improves endothelial function in young prehypertensives. Exp. Biol. Med. 2013;238(4):433–441. doi: 10.1177/1535370213477600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett L.A.T., Johnson J.M., Stephens D.P., Saad A.R., Kellogg D.L., Jr Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J. Physiol. 2003;552(1):223–232. doi: 10.1113/jphysiol.2003.042135. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J.R., Brandstadter R., Bar-Or A. COVID-19 and MS disease-modifying therapies. Neurol. Neuroimmunol. Neuroinflammat. 2020;7(4) doi: 10.1212/NXI.0000000000000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk G., Dawson E., Batterham A., Atkinson G., Cable T., Thijssen D., Green D. Effects of exercise intensity on flow mediated dilation in healthy humans. Int. J. Sports Med. 2013;34(05):409–414. doi: 10.1055/s-0032-1323829. [DOI] [PubMed] [Google Scholar]
- BLAIR D.A., GLOVER W.E., RODDIE J.C. Vasomotor responses in the human arm during leg exercise. Circ. Res. 1961;9(2):264–274. [Google Scholar]
- Blatteis C.M. The onset of fever: new insights into its mechanism. Prog. Brain Res. 2007;162:3–14. doi: 10.1016/S0079-6123(06)62001-3. [DOI] [PubMed] [Google Scholar]
- Boulant J.A., Hardy J. The effect of spinal and skin temperatures on the firing rate and thermosensitivity of preoptic neurones. J. Physiol. 1974;240(3):639–660. doi: 10.1113/jphysiol.1974.sp010627. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutsiouki P., Georgiou S., Clough G.F. Recovery of nitric oxide from acetylcholine-mediated vasodilatation in human skin in vivo. Microcirculation. 2004;11(3):249–259. doi: 10.1080/10739680490425958. [DOI] [PubMed] [Google Scholar]
- Braun A.P. Two-pore domain potassium channels: variation on a structural theme. Channels. 2012;6(3):139–140. doi: 10.4161/chan.20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen E., Tang K., Olfert M., Knapp A., Wagner P. Skeletal muscle capillarity during hypoxia: VEGF and its activation. High Alt. Med. Biol. 2008;9(2):158–166. doi: 10.1089/ham.2008.1010. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Arnett T.R., Orriss I.R. Purinergic signalling in the musculoskeletal system. Purinergic Signal. 2013;9(4):541–572. doi: 10.1007/s11302-013-9381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bychkov R., Gollasch M., Steinke T., Ried C., Luft F.C., Haller H. Calcium-activated potassium channels and nitrate-induced vasodilation in human coronary arteries. J. Pharmacol. Exp. Ther. 1998;285(1):293–298. [PubMed] [Google Scholar]
- Caolo V., Debant M., Endesh N., Futers T.S., Lichtenstein L., Bartoli F., Parsonage G., Jones E.A., Beech D.J. Shear stress activates ADAM10 sheddase to regulate Notch1 via the Piezo1 force sensor in endothelial cells. Elife. 2020;9:e50684. doi: 10.7554/eLife.50684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Montagnani M., Funahashi T., Shimomura I., Quon M.J. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 2003;278(45):45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- Cianfrone G., Turchetta R., Mazzei F., Bartolo M., Parisi L. Temperature-dependent auditory neuropathy: is it an acoustic uhthoff-like phenomenon?; A case report. Annals Otol. Rhinol. Laryngol. 2006;115(7):518–527. doi: 10.1177/000348940611500706. [DOI] [PubMed] [Google Scholar]
- Colberg S.R., Azoury K.R., Parson H.K., Vinik A.I. Exercise status affects skin perfusion via prostaglandin, nitric oxide, and EDHF pathways in diabetes. Microvasc. Res. 2009;77(2):120–124. doi: 10.1016/j.mvr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Colberg S.R., Stansberry K.B., McNitt P.M., Vinik A.I. Chronic exercise is associated with enhanced cutaneous blood flow in type 2 diabetes. J. Diabetes Complicat. 2002;16(2):139–145. doi: 10.1016/s1056-8727(01)00222-7. [DOI] [PubMed] [Google Scholar]
- Conti B., Tabarean I., Andrei C., Bartfai T. Cytokines and fever. Front. Biosci. 2004;9(12):1433–1449. doi: 10.2741/1341. [DOI] [PubMed] [Google Scholar]
- Convertino V., Greenleaf J., Bernauer E. Role of thermal and exercise factors in the mechanism of hypervolemia. J. Appl. Physiol. 1980;48(4):657–664. doi: 10.1152/jappl.1980.48.4.657. [DOI] [PubMed] [Google Scholar]
- Convertino V.A. Blood volume: its adaptation to endurance training. Med. Sci. Sports Exerc. 1991;23(12):1338–1348. [PubMed] [Google Scholar]
- Corbett J., Neal R.A., Lunt H.C., Tipton M.J. Adaptation to heat and exercise performance under cooler conditions: a new hot topic. Sports Med. 2014;44(10):1323–1331. doi: 10.1007/s40279-014-0212-8. [DOI] [PubMed] [Google Scholar]
- Corr L., Burnstock G. Analysis of P2-purinoceptor subtypes on the smooth muscle and endothelium of rabbit coronary artery. J. Cardiovasc. Pharmacol. 1994;23(5):709–715. doi: 10.1097/00005344-199405000-00004. [DOI] [PubMed] [Google Scholar]
- Correale J., Marrodan M., Ysrraelit M.C. Mechanisms of neurodegeneration and axonal dysfunction in progressive multiple sclerosis. Biomedicines. 2019;7(1):14. doi: 10.3390/biomedicines7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costill D., Branam G., Fink W., Nelson R. Exercise induced sodium conservation: changes in plasma renin and aldosterone. Med. Sci. Sports. 1976;8(4):209–213. [PubMed] [Google Scholar]
- Craner M.J., Hains B.C., Lo A.C., Black J.A., Waxman S.G. Co-localization of sodium channel Nav1. 6 and the sodium–calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain. 2004;127(2):294–303. doi: 10.1093/brain/awh032. [DOI] [PubMed] [Google Scholar]
- Craner M.J., Lo A.C., Black J.A., Waxman S.G. Abnormal sodium channel distribution in optic nerve axons in a model of inflammatory demyelination. Brain. 2003;126(7):1552–1561. doi: 10.1093/brain/awg153. [DOI] [PubMed] [Google Scholar]
- Craner M.J., Newcombe J., Black J.A., Hartle C., Cuzner M.L., Waxman S.G. Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1. 2 and Nav1. 6 sodium channels and Na+/Ca2+ exchanger. Proc. Natl. Acad. Sci. 2004;101(21):8168–8173. doi: 10.1073/pnas.0402765101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius A.R., Kirby B.S., Luckasen G.J., Larson D.G., Dinenno F.A. ATP-mediated vasodilatation occurs via activation of inwardly rectifying potassium channels in humans. J. Physiol. 2012;590(21):5349–5359. doi: 10.1113/jphysiol.2012.234245. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S.L., Jay O., Wilson T.E. Thermoregulatory dysfunction in multiple sclerosis. Handb. Clin. Neurol. 2018;157:701–714. doi: 10.1016/B978-0-444-64074-1.00042-2. [DOI] [PubMed] [Google Scholar]
- Davis S.L., Korkmas M.A., Crandall C.G., Frohman E.M. Impaired sweating in multiple sclerosis leads to increased reliance on skin blood flow for heat dissipation. FASEB J. 2010;24:991. 925-991.925. [Google Scholar]
- Davis S.L., Wilson T.E., Vener J.M., Crandall C.G., Petajan J.H., White A.T. Pilocarpine-induced sweat gland function in individuals with multiple sclerosis. J. Appl. Physiol. 2005;98(5):1740–1744. doi: 10.1152/japplphysiol.00860.2004. [DOI] [PubMed] [Google Scholar]
- Davis S.L., Wilson T.E., White A.T., Frohman E.M. Thermoregulation in multiple sclerosis. J. Appl. Physiol. 2010;109(5):1531–1537. doi: 10.1152/japplphysiol.00460.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson E.A., Green D.J., Timothy Cable N., Thijssen D.H. Effects of acute exercise on flow-mediated dilatation in healthy humans. J. Appl. Physiol. 2013;115(11):1589–1598. doi: 10.1152/japplphysiol.00450.2013. [DOI] [PubMed] [Google Scholar]
- de Seze J., Stojkovic T., Gauvrit J.-.Y., Devos D., Ayachi M., Cassim F., Saint Michel T., Pruvo J.-.P., Guieu J.-.D., Vermersch P. Autonomic dysfunction in multiple sclerosis: cervical spinal cord atrophy correlates. J. Neurol. 2001;248(4):297–303. doi: 10.1007/s004150170204. [DOI] [PubMed] [Google Scholar]
- Dean L., Kellogg J., Zhao J.L., Wu Y., Johnson J.M. VIP/PACAP receptor mediation of cutaneous active vasodilation during heat stress in humans. J. Appl. Physiol. 2010;109(1):95–100. doi: 10.1152/japplphysiol.01187.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalla N.S., Temsah R.M., Netticadan T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000;18(6):655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- Dinarello C.A., Gelfand J.A. Vol. 16. 2005. Fever and hyperthermia; p. 104. (Harrisons principles of internal medicine). [Google Scholar]
- Douglas W., Malcolm J. The effect of localized cooling on conduction in cat nerves. J. Physiol. 1955;130(1):53–71. doi: 10.1113/jphysiol.1955.sp005392. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Li R., Lau W.B., Zhao J., Lopez B., Christopher T.A., Ma X.-.L., Wang Y. Adiponectin at physiologically relevant concentrations enhances the vasorelaxative effect of acetylcholine via Cav-1/AdipoR-1 signaling. PLoS ONE. 2016;11(3) doi: 10.1371/journal.pone.0152247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds P., Hart S., Gao W., Vivat B., Burman R., Silber E., Higginson I.J. Palliative care for people severely affected by multiple sclerosis: evaluation of a novel palliative care service. Multi. Sclerosis J. 2010;16(5):627–636. doi: 10.1177/1352458510364632. [DOI] [PubMed] [Google Scholar]
- EDMUND J., FOG T. Visual and motor instability in multiple sclerosis. AMA Archives Neurol. Psychiatry. 1955;73(3):316–323. doi: 10.1001/archneurpsyc.1955.02330090062007. [DOI] [PubMed] [Google Scholar]
- Egri C., Ruben P.C. A hot topic: temperature sensitive sodium channelopathies. Channels. 2012;6(2):75–85. doi: 10.4161/chan.19827. [DOI] [PubMed] [Google Scholar]
- Ellermann-Eriksen S. Macrophages and cytokines in the early defence against herpes simplex virus. Virol. J. 2005;2(1):1–30. doi: 10.1186/1743-422X-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth M.L., Sprague R.S. Regulation of blood flow distribution in skeletal muscle: role of erythrocyte-released ATP. J. Physiol. 2012;590(20):4985–4991. doi: 10.1113/jphysiol.2012.233106. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I., Monahan K.D., Robinson J.A., Seals D.R. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J. Physiol. 2004;556(1):315–324. doi: 10.1113/jphysiol.2003.057042. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehling P., Haller J., Lefferts W., Hultquist E., Wharton M., Rowland T., Smith D. Effect of exercise, heat stress and dehydration on myocardial performance. Occup. Med. 2015;65(4):317–323. doi: 10.1093/occmed/kqv015. (Chic Ill) [DOI] [PubMed] [Google Scholar]
- Fellmann N. Hormonal and plasma volume alterations following endurance exercise. Sports Med. 1992;13(1):37–49. doi: 10.2165/00007256-199213010-00004. [DOI] [PubMed] [Google Scholar]
- Filingeri D., Chaseling G., Hoang P., Barnett M., Davis S.L., Jay O. Afferent thermosensory function in relapsing–remitting multiple sclerosis following exercise-induced increases in body temperature. Exp. Physiol. 2017;102(8):887–893. doi: 10.1113/EP086320. [DOI] [PubMed] [Google Scholar]
- Finaud J., Lac G., Filaire E. Oxidative stress. Sports Med. 2006;36(4):327–358. doi: 10.2165/00007256-200636040-00004. [DOI] [PubMed] [Google Scholar]
- Fjeldstad C., Frederiksen C., Fjeldstad A.S., Bemben M., Pardo G. Arterial compliance in multiple sclerosis: a pilot study. Angiology. 2010;61(1):31–36. doi: 10.1177/0003319709334120. [DOI] [PubMed] [Google Scholar]
- Flachenecker P., Reiners K., Krauser M., Wolf A., Toyka K.V. Autonomic dysfunction in multiple sclerosis is related to disease activity and progression of disability. Multi. Sclerosis J. 2001;7(5):327–334. doi: 10.1177/135245850100700509. [DOI] [PubMed] [Google Scholar]
- Flouris A., Schlader Z. Human behavioral thermoregulation during exercise in the heat. Scand. J. Med. Sci. Sports. 2015;25:52–64. doi: 10.1111/sms.12349. [DOI] [PubMed] [Google Scholar]
- Francisco M.A., Minson C.T. Cutaneous active vasodilation as a heat loss thermoeffector. Handb. Clin. Neurol. 2018;156:193–209. doi: 10.1016/B978-0-444-63912-7.00012-6. [DOI] [PubMed] [Google Scholar]
- Frandsen U., Bangsbo J., Langberg H., Saltin B., Hellsten Y. Inhibition of nitric oxide synthesis by systemic NG-monomethyl-l-arginine administration in humans: effects on interstitial adenosine, prostacyclin and potassium concentrations in resting and contracting skeletal muscle. J. Vasc. Res. 2000;37(4):297–302. doi: 10.1159/000025743. [DOI] [PubMed] [Google Scholar]
- Franssen H., Notermans N., Wieneke G. The influence of temperature on nerve conduction in patients with chronic axonal polyneuropathy. Clin. Neurophysiol. 1999;110(5):933–940. doi: 10.1016/s1388-2457(99)00018-8. [DOI] [PubMed] [Google Scholar]
- Franssen H., Wieneke G.H. Nerve conduction and temperature: necessary warming time. Muscle Nerve. 1994;17(3):336–344. doi: 10.1002/mus.880170313. [DOI] [PubMed] [Google Scholar]
- Frohman T.C., Davis S.L., Beh S., Greenberg B.M., Remington G., Frohman E.M. Uhthoff's phenomena in MS—Clinical features and pathophysiology. Nat. Rev. Neurol. 2013;9(9):535–540. doi: 10.1038/nrneurol.2013.98. [DOI] [PubMed] [Google Scholar]
- Gagnon D., Romero S.A., Ngo H., Poh P.Y., Crandall C.G. Plasma hyperosmolality attenuates skin sympathetic nerve activity during passive heat stress in humans. J. Physiol. 2016;594(2):497–506. doi: 10.1113/JP271497. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallup A.C., Gallup G.G., Jr, Feo C. Yawning, sleep, and symptom relief in patients with multiple sclerosis. Sleep Med. 2010;11(3):329–330. doi: 10.1016/j.sleep.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Gamba G., Cavalieri H., Courreges M.C., Massouh E.J., Benencia F. Early inhibition of nitric oxide production increases HSV-1 intranasal infection. J. Med. Virol. 2004;73(2):313–322. doi: 10.1002/jmv.20093. [DOI] [PubMed] [Google Scholar]
- García L.F. Immune response, inflammation, and the clinical spectrum of COVID-19. Front. Immunol. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett A.T., Rehrer N.J., Patterson M.J. Induction and decay of short-term heat acclimation in moderately and highly trained athletes. Sports Med. 2011;41(9):757–771. doi: 10.2165/11587320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Gaubert M.L., Sigaudo-Roussel D., Tartas M., Berrut G., Saumet J.L., Fromy B. Endothelium-derived hyperpolarizing factor as an in vivo back-up mechanism in the cutaneous microcirculation in old mice. J. Physiol. 2007;585(2):617–626. doi: 10.1113/jphysiol.2007.143750. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geor R., McCutcheon L. Influence of training on exercise-associated heat tolerance in Thoroughbred horses. J. Sports Sci. 1996;14(4):349. [Google Scholar]
- Geor R.J., McCutcheon L.J. Thermoregulatory adaptations associated with training and heat acclimation. Veterin. Clin. N Am. 1998;14(1):97–120. doi: 10.1016/s0749-0739(17)30214-6. [DOI] [PubMed] [Google Scholar]
- Ghafouri S., Hajizadeh S., Mani A.R. Enhancement of insulin-induced cutaneous vasorelaxation by exercise in rats: a role for nitric oxide and KCa2+ channels. Eur. J. Pharmacol. 2011;652(1–3):89–95. doi: 10.1016/j.ejphar.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109(2):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Gisolfi C.V., Mora F. MIT Press; 2016. Hot Brain. [Google Scholar]
- Gisolfi C.V., Mora M.T., Mora F., Teruel F.M., Gisolfi L. MIT Press; 2000. The Hot brain: survival, temperature, and the Human Body. [Google Scholar]
- Gleeson M., Bishop N.C., Stensel D.J., Lindley M.R., Mastana S.S., Nimmo M.A. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011;11(9):607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- González-Alonso J., Teller C., Andersen S.L., Jensen F.B., Hyldig T., Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J. Appl. Physiol. 1999;86(3):1032–1039. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- Gooding K.M., Hannemann M.M., Tooke J.E., Clough G.F., Shore A.C. Maximum skin hyperaemia induced by local heating: possible mechanisms. J. Vasc. Res. 2006;43(3):270–277. doi: 10.1159/000091736. [DOI] [PubMed] [Google Scholar]
- Gordon B., Chen S., Durstine J.L. The effects of exercise training on the traditional lipid profile and beyond. Curr. Sports Med. Rep. 2014;13(4):253–259. doi: 10.1249/JSR.0000000000000073. [DOI] [PubMed] [Google Scholar]
- Gordon K., Blondin D.P., Friesen B.J., Tingelstad H.C., Kenny G.P., Haman F. Seven days of cold acclimation substantially reduces shivering intensity and increases nonshivering thermogenesis in adult humans. J. Appl. Physiol. 2019;126(6):1598–1606. doi: 10.1152/japplphysiol.01133.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.J., Maiorana A., O'Driscoll G., Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J. Physiol. 2004;561(1):1–25. doi: 10.1113/jphysiol.2004.068197. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J., Kaple M., Chow A., Boulant J. Cellular mechanisms for neuronal thermosensitivity in the rat hypothalamus. J. Physiol. 1996;492(1):231–242. doi: 10.1113/jphysiol.1996.sp021304. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J.D., Boulant J.A. Temperature effects on membrane potential and input resistance in rat hypothalamic neurones. J. Physiol. 1995;488(2):407–418. doi: 10.1113/jphysiol.1995.sp020975. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross L. Anatomy of a Fever. PLoS Biol. 2006;4(9):e305. doi: 10.1371/journal.pbio.0040305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunal D.I., Afsar N., Tanridag T., Aktan S. Autonomic dysfunction in multiple sclerosis: correlation with disease-related parameters. Eur. Neurol. 2002;48(1):1–5. doi: 10.1159/000064949. [DOI] [PubMed] [Google Scholar]
- Guthrie T. Visual and motor changes in multiple sclerosis as a result of induced temperature changes. Trans. Am. Neurol. Assoc. 1950;51:189–194. [PubMed] [Google Scholar]
- Guthrie T.C. Visual and motor changes in patients with multiple sclerosis: a result of induced changes in environmental temperature. AMA Archives Neurol. Psychiatry. 1951;65(4):437–451. doi: 10.1001/archneurpsyc.1951.02320040027002. [DOI] [PubMed] [Google Scholar]
- Habek M. Immune and autonomic nervous system interactions in multiple sclerosis: clinical implications. Clin. Auton. Res. 2019;29(3):267–275. doi: 10.1007/s10286-019-00605-z. [DOI] [PubMed] [Google Scholar]
- Haensch C.-.A., Jörg J. Autonomic dysfunction in multiple sclerosis. J. Neurol. 2006;253(1):i3–i9. doi: 10.1007/s00415-006-1102-2. [DOI] [PubMed] [Google Scholar]
- Hansen A.H., Nyberg M., Bangsbo J., Saltin B., Hellsten Y. Exercise training alters the balance between vasoactive compounds in skeletal muscle of individuals with essential hypertension. Hypertension. 2011;58(5):943–949. doi: 10.1161/HYPERTENSIONAHA.111.176529. [DOI] [PubMed] [Google Scholar]
- Harris M.B., Mitchell B.M., Sood S.G., Webb R.C., Venema R.C. Increased nitric oxide synthase activity and Hsp90 association in skeletal muscle following chronic exercise. Eur. J. Appl. Physiol. 2008;104(5):795–802. doi: 10.1007/s00421-008-0833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten Y., Maclean D., Rådegran G.r., Saltin B., Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation. 1998;98(1):6–8. doi: 10.1161/01.cir.98.1.6. [DOI] [PubMed] [Google Scholar]
- Hellsten Y., Nyberg M., Jensen L., Mortensen S. Vasodilator interactions in skeletal muscle blood flow regulation. J. Physiol. 2012;590(24):6297–6305. doi: 10.1113/jphysiol.2012.240762. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds J.P., Eidelman B.H., Wald A. Prevalence of bowel dysfunction in multiple sclerosis: a population survey. Gastroenterology. 1990;98(6):1538–1542. doi: 10.1016/0016-5085(90)91087-m. [DOI] [PubMed] [Google Scholar]
- Hodges G.J., Sharp L., Clements R.E., Goldspink D.F., George K.P., Cable N.T. Influence of age, sex, and aerobic capacity on forearm and skin blood flow and vascular conductance. Eur. J. Appl. Physiol. 2010;109(6):1009–1015. doi: 10.1007/s00421-010-1441-7. [DOI] [PubMed] [Google Scholar]
- Holowatz L.A., Thompson C.S., Kenney W.L. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. Am. J. Physiol. Heart Circulat. Physiol. 2006;291(6):H2965–H2970. doi: 10.1152/ajpheart.00648.2006. [DOI] [PubMed] [Google Scholar]
- Holowatz L.A., Thompson C.S., Minson C.T., Kenney W.L. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J. Physiol. 2005;563(3):965–973. doi: 10.1113/jphysiol.2004.080952. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins S.J. Central nervous system recognition of peripheral inflammation: a neural, hormonal collaboration. Acta. Biomed. 2007;78(Suppl 1):231–247. [PubMed] [Google Scholar]
- Huang A., Sun D., Koller A. Shear stress–induced release of prostaglandin H2 in arterioles of hypertensive rats. Hypertension. 2000;35(4):925–930. doi: 10.1161/01.hyp.35.4.925. [DOI] [PubMed] [Google Scholar]
- Huang M., Jay O., Davis S.L. Autonomic dysfunction in multiple sclerosis: implications for exercise. Auton. Neurosci. 2015;188:82–85. doi: 10.1016/j.autneu.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Morris N., Jay O., Davis S. Thermoregulatory dysfunction in multiple sclerosis patients during moderate exercise in a thermoneutral environment (1104.17) FASEB J. 2014;28:1104–1117. [Google Scholar]
- Hughes R., Pedotti R., Koendgen H. COVID-19 in persons with multiple sclerosis treated with ocrelizumab–a pharmacovigilance case series. Mult. Scler. Relat. Disord. 2020;42 doi: 10.1016/j.msard.2020.102192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect. Dis. Clin. 2019;33(4):869–889. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui X., Lam K.S., Vanhoutte P.M., Xu A. Adiponectin and cardiovascular health: an update. Br. J. Pharmacol. 2012;165(3):574–590. doi: 10.1111/j.1476-5381.2011.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humm A., Beer S., Kool J., Magistris M., Kesselring J., Rösler K. Quantification of Uhthoff's phenomenon in multiple sclerosis: a magnetic stimulation study. Clin. Neurophysiol. 2004;115(11):2493–2501. doi: 10.1016/j.clinph.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Huttunen M., Harvima I., Ackermann L., Harvima R., Naukkarinen A., Horsmanheimo M. Neuropeptide-and capsaicin-induced histamine release in skin monitored with the microdialysis technique. Acta Derm. Venereol. 1996;76(3):205–209. doi: 10.2340/0001555576205209. [DOI] [PubMed] [Google Scholar]
- Ikegawa S., Kamijo Y.-i., Okazaki K., Masuki S., Okada Y., Nose H. Effects of hypohydration on thermoregulation during exercise before and after 5-day aerobic training in a warm environment in young men. J. Appl. Physiol. 2011;110(4):972–980. doi: 10.1152/japplphysiol.01193.2010. [DOI] [PubMed] [Google Scholar]
- Islam M.A., Kundu S., Alam S.S., Hossan T., Kamal M.A., Hassan R. Prevalence and characteristics of fever in adult and paediatric patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of 17515 patients. PLoS ONE. 2021;16(4) doi: 10.1371/journal.pone.0249788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Rosso M., Santoro J.D. Wilhelm Uhthoff and Uhthoff's phenomenon. Multiple Sclerosis J. 2020;26(13):1790–1796. doi: 10.1177/1352458519881950. [DOI] [PubMed] [Google Scholar]
- James C.A., Richardson A.J., Watt P.W., Willmott A.G., Gibson O.R., Maxwell N.S. Short-term heat acclimation improves the determinants of endurance performance and 5-km running performance in the heat. Appl. Physiol. Nutrition, Metabolism. 2017;42(3):285–294. doi: 10.1139/apnm-2016-0349. [DOI] [PubMed] [Google Scholar]
- Jankord R., Turk J.R., Schadt J.C., Casati J., Ganjam V.K., Price E.M., Keisler D.H., Laughlin M.H. Sex difference in link between interleukin-6 and stress. Endocrinology. 2007;148(8):3758–3764. doi: 10.1210/en.2006-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen L., Bangsbo J., Hellsten Y. Effect of high intensity training on capillarization and presence of angiogenic factors in human skeletal muscle. J. Physiol. 2004;557(2):571–582. doi: 10.1113/jphysiol.2003.057711. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q., Detolla L., Singh I.S., Gatdula L., Fitzgerald B., van Rooijen N., Cross A.S., Hasday J.D. Exposure to febrile temperature upregulates expression of pyrogenic cytokines in endotoxin-challenged mice. Am. J. Physiol. Regulat., Integrative Comparative Physiol. 1999;276(6):R1653–R1660. doi: 10.1152/ajpregu.1999.276.6.R1653. [DOI] [PubMed] [Google Scholar]
- Johnson J.M., Kellogg D. Thermoregulatory and thermal control in the human cutaneous circulation. Front. Biosci. 2010;2:825–853. doi: 10.2741/s105. [DOI] [PubMed] [Google Scholar]
- Kane G.C., Behfar A., Yamada S., Perez-Terzic C., O'Cochlain F., Reyes S., Dzeja P.P., Miki T., Seino S., Terzic A. ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. Diabetes. 2004;53(suppl 3):S169–S175. doi: 10.2337/diabetes.53.suppl_3.s169. [DOI] [PubMed] [Google Scholar]
- Kashyap S.R., Roman L.J., Lamont J., Masters B.S.S., Bajaj M., Suraamornkul S., Belfort R., Berria R., Kellogg D.L., Jr, Liu Y. Insulin resistance is associated with impaired nitric oxide synthase activity in skeletal muscle of type 2 diabetic subjects. J. Clin. Endocrinol Metabol. 2005;90(2):1100–1105. doi: 10.1210/jc.2004-0745. [DOI] [PubMed] [Google Scholar]
- Keller D.M., Fadel P.J., Harnsberger M.A., Remington G.M., Frohman E.M., Davis S.L. Reduced spontaneous sympathetic nerve activity in multiple sclerosis patients. J. Neurol. Sci. 2014;344(1–2):210–214. doi: 10.1016/j.jns.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D., Jr, Zhao J., Coey U., Green J. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J. Appl. Physiol. 2005;98(2):629–632. doi: 10.1152/japplphysiol.00728.2004. [DOI] [PubMed] [Google Scholar]
- Kellogg D.L., Jr, Pérgola P.E., Piest K.L., Kosiba W.A., Crandall C.G., Grossmann M., Johnson J.M. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ. Res. 1995;77(6):1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- Kellogg D.L., Jr, Zhao J.L., Wu Y., Johnson J.M. Nitric oxide and receptors for VIP and PACAP in cutaneous active vasodilation during heat stress in humans. J. Appl. Physiol. 2012;113(10):1512–1518. doi: 10.1152/japplphysiol.00859.2012. [DOI] [PubMed] [Google Scholar]
- Kenny G.P., Jay O. Thermometry, calorimetry, and mean body temperature during heat stress. Compr. Physiol. 2011;3(4):1689–1719. doi: 10.1002/cphy.c130011. [DOI] [PubMed] [Google Scholar]
- Kenny G.P., McGinn R. Restoration of thermoregulation after exercise. J. Appl. Physiol. 2017;122(4):933–944. doi: 10.1152/japplphysiol.00517.2016. [DOI] [PubMed] [Google Scholar]
- Kirby B.S., Voyles W.F., Carlson R.E., Dinenno F.A. Graded sympatholytic effect of exogenous ATP on postjunctional α-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J. Physiol. 2008;586(17):4305–4316. doi: 10.1113/jphysiol.2008.154252. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klede M., Clough G., Lischetzki G., Schmelz M. The effect of the nitric oxide synthase inhibitor N-nitro-l-arginine-methyl ester on neuropeptide-induced vasodilation and protein extravasation in human skin. J. Vasc. Res. 2003;40(2):105–114. doi: 10.1159/000070707. [DOI] [PubMed] [Google Scholar]
- Kodama S., Tanaka S., Saito K., Shu M., Sone Y., Onitake F., Suzuki E., Shimano H., Yamamoto S., Kondo K. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch. Intern. Med. 2007;167(10):999–1008. doi: 10.1001/archinte.167.10.999. [DOI] [PubMed] [Google Scholar]
- Komatsu T., Srivastava N., Revzin M., Ireland D.D., Chesler D., Reiss C.S. Mechanisms of cytokine-mediated inhibition of viral replication. Virology. 1999;259(2):334–341. doi: 10.1006/viro.1999.9801. [DOI] [PubMed] [Google Scholar]
- Kraus W.E., Houmard J.A., Duscha B.D., Knetzger K.J., Wharton M.B., McCartney J.S., Bales C.W., Henes S., Samsa G.P., Otvos J.D. Effects of the amount and intensity of exercise on plasma lipoproteins. N. Engl. J. Med. 2002;347(19):1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- Krupp L.B. Fatigue in multiple sclerosis. CNS Drugs. 2003;17(4):225–234. doi: 10.2165/00023210-200317040-00002. [DOI] [PubMed] [Google Scholar]
- Krupp L.B., McLinskey N., MacAllister W.S. CRC Press; 2007. Fatigue in Multiple sclerosis, Multiple Sclerosis Therapeutics; pp. 805–818. [Google Scholar]
- Laughlin M.H., Newcomer S.C., Bender S.B. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J. Appl. Physiol. 2008;104(3):588–600. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt V.M., Wylie G., Chiaravalloti N., DeLuca J., Sumowski J.F. Warmer outdoor temperature is associated with task-related increased BOLD activation in patients with multiple sclerosis. Brain Imaging Behav. 2014;8(1):128–132. doi: 10.1007/s11682-013-9267-7. [DOI] [PubMed] [Google Scholar]
- Lee J., Hong J., Umetani M., LaVoy E.C., Kim J., Park Y. Vascular protection by exercise in obesity: inflammasome-associated Mechanisms. Med. Sci. Sports Exercise. 2020;52(12):2538–2545. doi: 10.1249/MSS.0000000000002419. [DOI] [PubMed] [Google Scholar]
- Lee J., Lee Y., LaVoy E.C., Umetani M., Hong J., Park Y. Physical activity protects NLRP 3 inflammasome-associated coronary vascular dysfunction in obese mice. Physiol. Rep. 2018;6(12):e13738. doi: 10.14814/phy2.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Park Y., Dellsperger K.C., Zhang C. Exercise training improves endothelial function via adiponectin-dependent and independent pathways in type 2 diabetic mice. Am. J. Physiol.Heart Circulat. Physiol. 2011;301(2):H306–H314. doi: 10.1152/ajpheart.01306.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuwenburgh C., Heinecke J. Oxidative stress and antioxidants in exercise. Curr. Med. Chem. 2001;8(7):829–838. doi: 10.2174/0929867013372896. [DOI] [PubMed] [Google Scholar]
- Leggett J.E. Goldman-Cecil Medicine. 25th ed. Elsevier Saunders; Philadelphia, PA: 2016. Approach to fever or suspected infection in the normal host; pp. 1849–1854. [Google Scholar]
- Lenasi H., Strucl M. Effect of regular physical training on cutaneous microvascular reactivity. Med. Sci. Sports Exerc. 2004;36(4):606–612. doi: 10.1249/01.mss.0000121948.86377.51. [DOI] [PubMed] [Google Scholar]
- Lenasi H., Štrucl M. The effect of nitric oxide synthase and cyclooxygenase inhibition on cutaneous microvascular reactivity. Eur. J. Appl. Physiol. 2008;103(6):719–726. doi: 10.1007/s00421-008-0769-8. [DOI] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Xu X., Leng X., He M., Wang J., Cheng S., Wu H. Roles of reactive oxygen species in cell signaling pathways and immune responses to viral infections. Arch. Virol. 2017;162(3):603–610. doi: 10.1007/s00705-016-3130-2. [DOI] [PubMed] [Google Scholar]
- Liao J.K., Shin W.S., Lee W.Y., Clark S.L. Oxidized low-density lipoprotein decreases the expression of endothelial nitric oxide synthase. J. Biol. Chem. 1995;270(1):319–324. doi: 10.1074/jbc.270.1.319. [DOI] [PubMed] [Google Scholar]
- Lim C.L. Fundamental concepts of human thermoregulation and adaptation to heat: a review in the context of global warming. Int. J. Environ. Res. Public Health. 2020;17(21):7795. doi: 10.3390/ijerph17217795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C.L., Byrne C., Lee J.K. Human thermoregulation and measurement of body temperature in exercise and clinical settings. Annals Acad. Med. Singapore. 2008;37(4):347. [PubMed] [Google Scholar]
- Lim C.L., Suzuki K. Systemic inflammation mediates the effects of endotoxemia in the mechanisms of heat stroke. Biol. Med. 2017;9(1):1. [Google Scholar]
- Linker R., Mohr A., Cepek L., Gold R., Prange H. Core hypothermia in multiple sclerosis: case report with magnetic resonance imaging localization of a thalamic lesion. Multi. Sclerosis J. 2006;12(1):112–115. doi: 10.1191/135248506ms1268cr. [DOI] [PubMed] [Google Scholar]
- Lloyd P.G., Prior B.M., Li H., Yang H.T., Terjung R.L. VEGF receptor antagonism blocks arteriogenesis, but only partially inhibits angiogenesis, in skeletal muscle of exercise-trained rats. Am. J. Physiol. Heart Circulat. Physiol. 2005;288(2):H759–H768. doi: 10.1152/ajpheart.00786.2004. [DOI] [PubMed] [Google Scholar]
- Long Y., Liu X., Tan X.-z., Jiang C.-x., Chen S.-w., Liang G.-n., He X.-m., Wu J., Chen T., Xu Y. ROS-induced NLRP3 inflammasome priming and activation mediate PCB 118-induced pyroptosis in endothelial cells. Ecotoxicol. Environ. Saf. 2020;189 doi: 10.1016/j.ecoenv.2019.109937. [DOI] [PubMed] [Google Scholar]
- Loonstra F.C., Hoitsma E., van Kempen Z.L., Killestein J., Mostert J.P. COVID-19 in multiple sclerosis: the Dutch experience. Multi. Sclerosis J. 2020;26(10):1256–1260. doi: 10.1177/1352458520942198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo S., Halliwill J.R., Sawka M.N., Minson C.T. Heat acclimation improves exercise performance. J. Appl. Physiol. 2010;109(4):1140–1147. doi: 10.1152/japplphysiol.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo S., Minson C.T. Heat acclimation improves cutaneous vascular function and sweating in trained cyclists. J. Appl. Physiol. 2010;109(6):1736–1743. doi: 10.1152/japplphysiol.00725.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Collongues N., Stankoff B., Giannesini C., Papeix C., Bensa C., Deschamps R., Créange A., Wahab A., Pelletier J. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean A., Wei X.-.Q., Huang F.-.P., Al-Alem U., Chan W.L., Liew F.Y. Mice lacking inducible nitric-oxide synthase are more susceptible to herpes simplex virus infection despite enhanced Th1 cell responses. J. Gen. Virol. 1998;79(4):825–830. doi: 10.1099/0022-1317-79-4-825. [DOI] [PubMed] [Google Scholar]
- Madden C.J., Morrison S.F. Central nervous system circuits that control body temperature. Neurosci. Lett. 2019;696:225–232. doi: 10.1016/j.neulet.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S., Tanabe T., Miyauchi T., Otsuki T., Sugawara J., Iemitsu M., Kuno S., Ajisaka R., Yamaguchi I., Matsuda M. Aerobic exercise training reduces plasma endothelin-1 concentration in older women. J. Appl. Physiol. 2003;95(1):336–341. doi: 10.1152/japplphysiol.01016.2002. [DOI] [PubMed] [Google Scholar]
- Maghzi A.H., Houtchens M.K., Preziosa P., Ionete C., Beretich B.D., Stankiewicz J.M., Tauhid S., Cabot A., Berriosmorales I., Schwartz T.H. COVID-19 in teriflunomide-treated patients with multiple sclerosis. J. Neurol. 2020;267:2790–2796. doi: 10.1007/s00415-020-09944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F., Lauritzen I., Patel A.J., Heurteaux C., Reyes R., Lesage F., Lazdunski M., Honoré E. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000;19(11):2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino F.E. Heat reactions in multiple sclerosis: an overlooked paradigm in the study of comparative fatigue. Int. J. Hyperthermia. 2009;25(1):34–40. doi: 10.1080/02656730802294020. [DOI] [PubMed] [Google Scholar]
- Martin P.G., Marino F.E., Rattey J., Kay D., Cannon J. Reduced voluntary activation of human skeletal muscle during shortening and lengthening contractions in whole body hyperthermia. Exp. Physiol. 2005;90(2):225–236. doi: 10.1113/expphysiol.2004.028977. [DOI] [PubMed] [Google Scholar]
- Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Matsuda K., Park C., Sunden Y., Kimura T., Ochiai K., Kida H., Umemura T. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza a virus in mice. Vet. Pathol. 2004;41(2):101–107. doi: 10.1354/vp.41-2-101. [DOI] [PubMed] [Google Scholar]
- McCord G.R., Cracowski J.-.L., Minson C.T. Prostanoids contribute to cutaneous active vasodilation in humans. Am. J. Physiol.Regulat., Integrative Comparative Physiol. 2006;291(3):R596–R602. doi: 10.1152/ajpregu.00710.2005. [DOI] [PubMed] [Google Scholar]
- McKay M.K., Hester R.L. Role of nitric oxide, adenosine, and ATP-sensitive potassium channels in insulin-induced vasodilation. Hypertension. 1996;28(2):202–208. doi: 10.1161/01.hyp.28.2.202. [DOI] [PubMed] [Google Scholar]
- McNamara T.C., Keen J.T., Simmons G.H., Alexander L.M., Wong B.J. Endothelial nitric oxide synthase mediates the nitric oxide component of reflex cutaneous vasodilatation during dynamic exercise in humans. J. Physiol. 2014;592(23):5317–5326. doi: 10.1113/jphysiol.2014.272898. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhurst A.D., Rennie G., Chapman C.G., Meadows H., Duckworth M.D., Kelsell R.E., Gloger I.I., Pangalos M.N. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Mol. Brain Res. 2001;86(1–2):101–114. doi: 10.1016/s0169-328x(00)00263-1. [DOI] [PubMed] [Google Scholar]
- Medow M.S., Glover J.L., Stewart J.M. Nitric oxide and prostaglandin inhibition during acetylcholine-mediated cutaneous vasodilation in humans. Microcirculation. 2008;15(6):569–579. doi: 10.1080/10739680802091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkus D., Sorop O., Houweling B., Hoogteijling B.A., Duncker D.J. KCa+ channels contribute to exercise-induced coronary vasodilation in swine. Am. J. Physiol Heart Circulat. Physiol. 2006;291(5):H2090–H2097. doi: 10.1152/ajpheart.00315.2006. [DOI] [PubMed] [Google Scholar]
- Michiels C., Arnould T., Remacle J. Endothelial cell responses to hypoxia: initiation of a cascade of cellular interactions. Biochim. Biophys. Acta. 2000;1497(1):1–10. doi: 10.1016/s0167-4889(00)00041-0. [DOI] [PubMed] [Google Scholar]
- Middlebrooke A., Armstrong N., Welsman J., Shore A., Clark P., MacLeod K. Does aerobic fitness influence microvascular function in healthy adults at risk of developing Type 2 diabetes? Diabet. Med. 2005;22(4):483–489. doi: 10.1111/j.1464-5491.2005.01455.x. [DOI] [PubMed] [Google Scholar]
- Mincu R.I., Magda L.S., Florescu M., Velcea A., Mihaila S., Mihalcea D., Popescu B.O., Chiru A., Tiu C., Cinteza M. Cardiovascular dysfunction in multiple sclerosis. Maedica. 2015;10(4):364. (Buchar) [PMC free article] [PubMed] [Google Scholar]
- Misurski D.A., Wu S.-.Q., McNeill J.R., Wilson T.W., Gopalakrishnan V. Insulin-induced biphasic responses in rat mesenteric vascular bed: role of endothelin. Hypertension. 2001;37(5):1298–1302. doi: 10.1161/01.hyp.37.5.1298. [DOI] [PubMed] [Google Scholar]
- MITCHELL J.H., TATE C., RAVEN P., COBB F., KRAUS W. Acute response and chronic adaptation to exercise in women. Med. Sci. Sports Exerc. 1992;24(6):S258–S265. [PubMed] [Google Scholar]
- Moien-Afshari F., Ghosh S., Khazaei M., Kieffer T., Brownsey R., Laher I. Exercise restores endothelial function independently of weight loss or hyperglycaemic status in db/db mice. Diabetologia. 2008;51(7):1327–1337. doi: 10.1007/s00125-008-0996-x. [DOI] [PubMed] [Google Scholar]
- Morrison S., Nakamura K. Central mechanisms for thermoregulation. Annu. Rev. Physiol. 2019;81:285–308. doi: 10.1146/annurev-physiol-020518-114546. [DOI] [PubMed] [Google Scholar]
- Morrison S.F., Blessing W.W. Vol. 2. 2011. Central nervous system regulation of body temperature; pp. 324–344. (Central Regulation of Autonomic Functions). [Google Scholar]
- Mortensen S.P., González-Alonso J., Bune L.T., Saltin B., Pilegaard H., Hellsten Y. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am. J. Physiol. Regulat. Integrat. Comparat. Physiol. 2009;296(4):R1140–R1148. doi: 10.1152/ajpregu.90822.2008. [DOI] [PubMed] [Google Scholar]
- Mortensen S.P., Nyberg M., Thaning P., Saltin B., Hellsten Y. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension. 2009;53(6):993–999. doi: 10.1161/HYPERTENSIONAHA.109.130880. [DOI] [PubMed] [Google Scholar]
- Nadel E. Temperature regulation and prolonged exercise. Prolonged Exerc. 1988 [Google Scholar]
- Nadel E. Importance of albumin in the plasma volume expansion process. Physiologist. 1996;39:A65. [Google Scholar]
- Nadel, E., Mack, G., Nose, H., Tripathi, A., 1987. Tolerance to Severe Heat and exercise: Peripheral vascular Responses to Body Fluid Changes. Heat Stress: Physical Exertion and Environment; Hales, RJS, Richards, DAB, Eds, 117–131.
- Nadel E., Pandolf K., Roberts M., Stolwijk J. Mechanisms of thermal acclimation to exercise and heat. J. Appl. Physiol. 1974;37(4):515–520. doi: 10.1152/jappl.1974.37.4.515. [DOI] [PubMed] [Google Scholar]
- Nadel E.R., Fortney S.M., Wenger C.B. Effect of hydration state of circulatory and thermal regulations. J. Appl. Physiol. 1980;49(4):715–721. doi: 10.1152/jappl.1980.49.4.715. [DOI] [PubMed] [Google Scholar]
- Nagashima K., Cline G.W., Mack G.W., Shulman G.I., Nadel E.R. Intense exercise stimulates albumin synthesis in the upright posture. J. Appl. Physiol. 2000;88(1):41–46. doi: 10.1152/jappl.2000.88.1.41. [DOI] [PubMed] [Google Scholar]
- Nagashima K., Tokizawa K., Uchida Y., Nakamura-Matsuda M., Lin .C.-H. Exercise and thermoregulation. J. Phys. Fitness Sports Med. 2012;1(1):73–82. [Google Scholar]
- NELSON D.A., JEFFREYS W.H., McDOWELL F. Effects of induced hyperthermia on some neurological diseases. AMA Archives Neurol. Psychiatry. 1958;79(1):31–39. doi: 10.1001/archneurpsyc.1958.02340010049003. [DOI] [PubMed] [Google Scholar]
- Nelson D.A., McDowell F. The effects of induced hyperthermia on patients with multiple sclerosis. J. Neurol. Neurosurg. Psychiatr. 1959;22(2):113. doi: 10.1136/jnnp.22.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves E.B., Salamunes A.C.C., de Oliveira R.M., Stadnik A.M.W. Effect of body fat and gender on body temperature distribution. J. Therm. Biol. 2017;70:1–8. doi: 10.1016/j.jtherbio.2017.10.017. [DOI] [PubMed] [Google Scholar]
- Nielsen B., Hales J., Strange S., Christensen N.J., Warberg J., Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J. Physiol. 1993;460(1):467–485. doi: 10.1113/jphysiol.1993.sp019482. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M., Al-Khazraji B.K., Mortensen S.P., Jackson D.N., Ellis C.G., Hellsten Y. Effect of extraluminal ATP application on vascular tone and blood flow in skeletal muscle: implications for exercise hyperemia. Am. J. Physiol. Regulat. Integrat. Comparat. Physiol. 2013;305(3):R281–R290. doi: 10.1152/ajpregu.00189.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M., Mortensen S.P., Hellsten Y. Physical activity opposes the age-related increase in skeletal muscle and plasma endothelin-1 levels and normalizes plasma endothelin-1 levels in individuals with essential hypertension. Acta physiologica. 2013;207(3):524–535. doi: 10.1111/apha.12048. [DOI] [PubMed] [Google Scholar]
- Nyberg M., Seidelin K., Andersen T.R., Overby N.N., Hellsten Y., Bangsbo J. Biomarkers of vascular function in premenopausal and recent postmenopausal women of similar age: effect of exercise training. Am. J. Physiol.Regulat. Integr. Comparat. Physiol. 2014;306(7):R510–R517. doi: 10.1152/ajpregu.00539.2013. [DOI] [PubMed] [Google Scholar]
- Nybo L., Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. J. Appl. Physiol. 2001;91(3):1055–1060. doi: 10.1152/jappl.2001.91.3.1055. [DOI] [PubMed] [Google Scholar]
- Ogoina D. Fever, fever patterns and diseases called ‘fever’–a review. J. Infect. Public Health. 2011;4(3):108–124. doi: 10.1016/j.jiph.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Ohl K., Tenbrock K., Kipp M. Oxidative stress in multiple sclerosis: central and peripheral mode of action. Exp. Neurol. 2016;277:58–67. doi: 10.1016/j.expneurol.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J., Simmons G.H., Bender S.B., Arce-Esquivel A.A., Whyte J.J., Laughlin M.H. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology. 2011;26(3):132–145. doi: 10.1152/physiol.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.J., Honoré E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24(6):339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- Pedersen B.K. The diseasome of physical inactivity–and the role of myokines in muscle–fat cross talk. J. Physiol. 2009;587(23):5559–5568. doi: 10.1113/jphysiol.2009.179515. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen B.K., Febbraio M.A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 2008;88(4):1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Pedersen B.K., Steensberg A., Fischer C., Keller C., Keller P., Plomgaard P., Wolsk-Petersen E., Febbraio M. The metabolic role of IL-6 produced during exercise: is IL-6 an exercise factor? Proc. Nutr. Soc. 2004;63(2):263–267. doi: 10.1079/PNS2004338. [DOI] [PubMed] [Google Scholar]
- Peles E., Salzer J.L. Molecular domains of myelinated axons. Curr. Opin. Neurobiol. 2000;10(5):558–565. doi: 10.1016/s0959-4388(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Périard J., Racinais S., Sawka M.N. Adaptations and mechanisms of human heat acclimation: applications for competitive athletes and sports. Scand. J. Med. Sci. Sports. 2015;25:20–38. doi: 10.1111/sms.12408. [DOI] [PubMed] [Google Scholar]
- Périard J.D., Travers G.J., Racinais S., Sawka M.N. Cardiovascular adaptations supporting human exercise-heat acclimation. Auton. Neurosci. 2016;196:52–62. doi: 10.1016/j.autneu.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Petajan J.H., White A.T. Recommendations for physical activity in patients with multiple sclerosis. Sports Med. 1999;27(3):179–191. doi: 10.2165/00007256-199927030-00004. [DOI] [PubMed] [Google Scholar]
- Petajan J.H., White A.T. Motor-evoked potentials in response to fatiguing grip exercise in multiple sclerosis patients. Clin. Neurophysiol. 2000;111(12):2188–2195. doi: 10.1016/s1388-2457(00)00469-7. [DOI] [PubMed] [Google Scholar]
- Pivarnik J., Goetting M., Senay L., Jr Effect of endurance training and heat acclimation on aerobic capacity, blood volume and plasma testosterone. J. Strength Conditioning Res. 1987;1(2):33–35. [Google Scholar]
- Plant S., Shand B., Elder P., Scott R. Adiponectin attenuates endothelial dysfunction induced by oxidised low-density lipoproteins. Diabetes Vascular Disease Res. 2008;5(2):102–108. doi: 10.3132/dvdr.2008.017. [DOI] [PubMed] [Google Scholar]
- Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect. Dis. 2020;20(6):689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racke M.K., Newsome S.D. Multiple sclerosis and COVID-19: a great opportunity for databases promoting research and collaboration. J. Neuroimmunol. 2020;345 doi: 10.1016/j.jneuroim.2020.577283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racosta J.M., Kimpinski K., Morrow S.A., Kremenchutzky M. Autonomic dysfunction in multiple sclerosis. Auton. Neurosci. 2015;193:1–6. doi: 10.1016/j.autneu.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Rambotti M., Simonetti S., Spreca A. PACAP activated adenylate cyclase in human sweat glands. An ultracytochemical study. Eur. J. Histochem. 2002;46(3):223–228. doi: 10.4081/1683. [DOI] [PubMed] [Google Scholar]
- Ranadive S.M., Yan H., Weikert M., Lane A.D., Linden M.A., Baynard T., Motl R.W., Fernhall B. Vascular dysfunction and physical activity in multiple sclerosis. Med. Sci. Sports Exerc. 2012;44(2):238–243. doi: 10.1249/MSS.0b013e31822d7997. [DOI] [PubMed] [Google Scholar]
- Ribeiro F., Alves A.J., Duarte J.A., Oliveira J. Is exercise training an effective therapy targeting endothelial dysfunction and vascular wall inflammation? Int. J. Cardiol. 2010;141(3):214–221. doi: 10.1016/j.ijcard.2009.09.548. [DOI] [PubMed] [Google Scholar]
- Roberts M.F., Wenger C.B., Stolwijk J., Nadel E.R. Skin blood flow and sweating changes following exercise training and heat acclimation. J. Appl. Physiol. 1977;43(1):133–137. doi: 10.1152/jappl.1977.43.1.133. [DOI] [PubMed] [Google Scholar]
- Roche D.M., Edmunds S., Cable T., Didi M., Stratton G. Skin microvascular reactivity in children and adolescents with type 1 diabetes in relation to levels of physical activity and aerobic fitness. Pediatr. Exerc. Sci. 2008;20(4):426–438. doi: 10.1123/pes.20.4.426. [DOI] [PubMed] [Google Scholar]
- Romanovsky A.A., Steiner A., Matsumura K. Cells that trigger fever. Cell Cycle. 2006;5(19):2195–2197. doi: 10.4161/cc.5.19.3321. [DOI] [PubMed] [Google Scholar]
- Rosenmeier J.B., Hansen J., González-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J. Physiol. 2004;558(1):351–365. doi: 10.1113/jphysiol.2004.063107. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmeier J.B., Yegutkin G.G., González-Alonso J. Activation of ATP/UTP-selective receptors increases blood flow and blunts sympathetic vasoconstriction in human skeletal muscle. J. Physiol. 2008;586(20):4993–5002. doi: 10.1113/jphysiol.2008.155432. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M., Santoro G., Ricco R., Pentimone F., Carpi A. Effect of chronic aerobic exercise on cutaneous microcirculatory flow response to insulin iontophoresis and to ischemia in elderly males. Int. J. Sports Med. 2005;26(07):558–562. doi: 10.1055/s-2004-830333. [DOI] [PubMed] [Google Scholar]
- Roth J., De Souza G. Fever induction pathways: evidence from responses to systemic or local cytokine formation. Braz. J. Med. Biol. Res. 2001;34(3):301–314. doi: 10.1590/s0100-879x2001000300003. [DOI] [PubMed] [Google Scholar]
- Rowell L.B. Cardiovascular adjustments to thermal stress. Compr. Physiol. 2011:967–1023. [Google Scholar]
- Rummel C., Barth S.W., Voss T., Korte S., Gerstberger R., Hubschle T., Roth J. Localized vs. systemic inflammation in guinea pigs: a role for prostaglandins at distinct points of the fever induction pathways? Am. J. Physiol.Regulat. Integrat. Comparat. Physiol. 2005;289(2):R340–R347. doi: 10.1152/ajpregu.00104.2005. [DOI] [PubMed] [Google Scholar]
- Rush J.W., Aultman C.D. Vascular biology of angiotensin and the impact of physical activity. Appl. physiol. Nutri. Metabol. 2008;33(1):162–171. doi: 10.1139/H07-147. [DOI] [PubMed] [Google Scholar]
- Rush M.E., Rinzel J. The potassium A-current, low firing rates and rebound excitation in Hodgkin-Huxley models. Bull. Math. Biol. 1995;57(6):899–929. doi: 10.1007/BF02458299. [DOI] [PubMed] [Google Scholar]
- Saari A., Tolonen U., Pääkkö E., Suominen K., Jauhiainen J., Sotaniemi K., Myllylä V. Sweating impairment in patients with multiple sclerosis. Acta Neurol. Scand. 2009;120(5):358–363. doi: 10.1111/j.1600-0404.2009.01164.x. [DOI] [PubMed] [Google Scholar]
- Sawka M.N., Montain S.J., Latzka W.A. Hydration effects on thermoregulation and performance in the heat. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001;128(4):679–690. doi: 10.1016/s1095-6433(01)00274-4. [DOI] [PubMed] [Google Scholar]
- Sawka M.N., Young A.J., Cadarette B.S., Levine L., Pandolf K.B. Influence of heat stress and acclimation on maximal aerobic power. Eur. J. Appl. Physiol. Occup. Physiol. 1985;53(4):294–298. doi: 10.1007/BF00422841. [DOI] [PubMed] [Google Scholar]
- Schneider A., Kirsten H., Lordick F., Lordick F., Lübbert C., Von Braun A. Covid-19 in outpatients—Is fever a useful indicator for SARS-CoV-2 infection? PLoS ONE. 2021;16(2) doi: 10.1371/journal.pone.0246312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheean G.L., Murray N., Rothwell J.C., Miller D.H., Thompson A.J. An electrophysiological study of the mechanism of fatigue in multiple sclerosis. Brain. 1997;120(2):299–315. doi: 10.1093/brain/120.2.299. [DOI] [PubMed] [Google Scholar]
- Shephard R.J. Cytokine responses to physical activity, with particular reference to IL-6: sources, actions, and clinical implications. Crit. Rev. Immunol. 2002;22(3):165–182. [PubMed] [Google Scholar]
- Shibasaki M., Wilson T.E., Cui J., Crandall C.G. Acetylcholine released from cholinergic nerves contributes to cutaneous vasodilation during heat stress. J. Appl. Physiol. 2002;93(6):1947–1951. doi: 10.1152/japplphysiol.00036.2002. [DOI] [PubMed] [Google Scholar]
- Shvartz E., Shapiro Y., Magazanik A., Meroz A., Birnfeld H., Mechtinger A., Shibolet S. Heat acclimation, physical fitness, and responses to exercise in temperate and hot environments. J. Appl. Physiol. 1977;43(4):678–683. doi: 10.1152/jappl.1977.43.4.678. [DOI] [PubMed] [Google Scholar]
- Silva A.S., Zanesco A. Physical exercise, β-adrenergic receptors, and vascular response. Jornal Vascular Brasileiro. 2010;9:47–56. [Google Scholar]
- Singel D.J., Stamler J.S. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu. Rev. Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- Smith K.J. Sodium channels and multiple sclerosis: roles in symptom production, damage and therapy. Brain Pathol. 2007;17(2):230–242. doi: 10.1111/j.1750-3639.2007.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.J., McDonald W. The pathophysiology of multiple sclerosis⋮ the mechanisms underlying the production of symptoms and the natural history of the disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999;354(1390):1649–1673. doi: 10.1098/rstb.1999.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolnicki L.A., Roberts S.K., Wilkins B.W., Basu A., Charkoudian N. Contribution of nitric oxide to cutaneous microvascular dilation in individuals with type 2 diabetes mellitus. Am. J. Physiol. Endocrinol. Metabol. 2007;292(1):E314–E318. doi: 10.1152/ajpendo.00365.2006. [DOI] [PubMed] [Google Scholar]
- Sorenson M., Furst J., Mathews H., Jason L.A. Dysregulation of cytokine pathways in chronic fatigue syndrome and multiple sclerosis. Fatigue. 2017 [Google Scholar]
- Starkie R., Ostrowski S.R., Jauffred S., Febbraio M., Pedersen B.K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-α production in humans. FASEB J. 2003;17(8):1–10. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- Steiner A.A., Chakravarty S., Rudaya A.Y., Herkenham M., Romanovsky A.A. Bacterial lipopolysaccharide fever is initiated via Toll-like receptor 4 on hematopoietic cells. Blood. 2006;107(10):4000–4002. doi: 10.1182/blood-2005-11-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A.A., Ivanov A.I., Serrats J., Hosokawa H., Phayre A.N., Robbins J.R., Roberts J.L., Kobayashi S., Matsumura K., Sawchenko P.E. Cellular and molecular bases of the initiation of fever. PLoS Biol. 2006;4(9):e284. doi: 10.1371/journal.pbio.0040284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergioulas A.T., Filippou D.K. Effects of physical conditioning on lipids and arachidonic acid metabolites in untrained boys: a longitudinal study. Appl. Physiol. Nutrition Metabol. 2006;31(4):432–441. doi: 10.1139/h06-020. [DOI] [PubMed] [Google Scholar]
- Stys P.K., Sontheimer H., Ransom B.R., Waxman S.G. Noninactivating, tetrodotoxin-sensitive Na+ conductance in rat optic nerve axons. Proc. Natl. Acad. Sci. 1993;90(15):6976–6980. doi: 10.1073/pnas.90.15.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys P.K., Waxman S.G., Ransom B.R. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na (+)-Ca2+ exchanger. J. Neurosci. 1992;12(2):430–439. doi: 10.1523/JNEUROSCI.12-02-00430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K. Characterization of exercise-induced cytokine release, the impacts on the body, the mechanisms and modulations. Int. J. Sports Exerc. Med. 2019;5:1–13. [Google Scholar]
- Suzuki K., Tominaga T., Ruhee R.T., Ma S. Characterization and modulation of systemic inflammatory response to exhaustive exercise in relation to oxidative stress. Antioxidants. 2020;9(5):401. doi: 10.3390/antiox9050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syndulko K., Jafari M., Woldanski A., Baumhefner R.W., Tourtellotte W.W. Effects of temperature in multiple sclerosis: a review of the literature. Neurorehabil. Neural Repair. 1996;10(1):23–34. [Google Scholar]
- Takeda R., Okazaki K. Body temperature regulation during exercise and hyperthermia in diabetics. Diabetes Complicat. 2018:89. [Google Scholar]
- Tansey E.A., Johnson C.D. Recent advances in thermoregulation. Adv. Physiol. Educ. 2015 doi: 10.1152/advan.00126.2014. [DOI] [PubMed] [Google Scholar]
- Tartas M., Durand S., Koitka A., Bouye P., Saumet J., Abraham P. Anodal current intensities above 40 μA interfere with current-induced axon-reflex vasodilatation in human skin. J. Vasc. Res. 2004;41(3):261–267. doi: 10.1159/000078665. [DOI] [PubMed] [Google Scholar]
- Tavee J. Nerve conduction studies: basic concepts. Handb. Clin. Neurol. 2019;160:217–224. doi: 10.1016/B978-0-444-64032-1.00014-X. [DOI] [PubMed] [Google Scholar]
- Taylor N.A. Human heat adaptation. Compr. Physiol. 2011;4(1):325–365. doi: 10.1002/cphy.c130022. [DOI] [PubMed] [Google Scholar]
- Tepavcevic D., Kostic J., Basuroski I., Stojsavljevic N., Pekmezovic T., Drulovic J. The impact of sexual dysfunction on the quality of life measured by MSQoL-54 in patients with multiple sclerosis. Multiple Sclerosis J. 2008;14(8):1131–1136. doi: 10.1177/1352458508093619. [DOI] [PubMed] [Google Scholar]
- Tew G.A., George K.P., Cable N.T., Hodges G.J. Endurance exercise training enhances cutaneous microvascular reactivity in post-menopausal women. Microvasc. Res. 2012;83(2):223–228. doi: 10.1016/j.mvr.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Tew G.A., Klonizakis M., Saxton J.M. Effects of ageing and fitness on skin-microvessel vasodilator function in humans. Eur. J. Appl. Physiol. 2010;109(2):173–181. doi: 10.1007/s00421-009-1342-9. [DOI] [PubMed] [Google Scholar]
- Todd A. Anatomy of primary afferents and projection neurones in the rat spinal dorsal horn with particular emphasis on substance P and the neurokinin 1 receptor. Exp. Physiol. 2002;87(2):245–249. doi: 10.1113/eph8702351. [DOI] [PubMed] [Google Scholar]
- Turrin N.P., Rivest S. Unraveling the molecular details involved in the intimate link between the immune and neuroendocrine systems. Exp. Biol. Med. 2004;229(10):996–1006. doi: 10.1177/153537020422901003. [DOI] [PubMed] [Google Scholar]
- Violi F., Marino R., Milite M., Loffredo L. Nitric oxide and its role in lipid peroxidation. Diabetes Metab. Res. Rev. 1999;15(4):283–288. doi: 10.1002/(sici)1520-7560(199907/08)15:4<283::aid-dmrr42>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Wade C.E., Hill L.C., Hunt M.M., Dressendorfer R.H. Plasma aldosterone and renal function in runners during a 20-day road race. Eur. J. Appl. Physiol. Occup. Physiol. 1985;54(5):456–460. doi: 10.1007/BF00422951. [DOI] [PubMed] [Google Scholar]
- Wallengren J. Vasoactive peptides in the skin. J. Investig. Dermatol. Symp. Proc. 1997;2(1):49–55. doi: 10.1038/jidsymp.1997.11. [DOI] [PubMed] [Google Scholar]
- Wang F., Liu Y., Yang W., Yuan J., Mo Z. Adiponectin inhibits NLRP3 inflammasome by modulating the AMPK-ROS pathway. Int. J. Clin. Exp. Pathol. 2018;11(7):3338. [PMC free article] [PubMed] [Google Scholar]
- Wang J.-.S. Effects of exercise training and detraining on cutaneous microvascular function in man: the regulatory role of endothelium-dependent dilation in skin vasculature. Eur. J. Appl. Physiol. 2005;93(4):429–434. doi: 10.1007/s00421-004-1176-4. [DOI] [PubMed] [Google Scholar]
- Warren J.B., Cockcroft J.R., Larkin S.W., Kajekar R., Macrae A., Ghatei M.A., Bloom S.R. Pituitary Adenylate Cyclase Activating Polypeptide is a Potent Vasodilator in Humans. J. Cardiovasc. Pharmacol. 1992;20(1):83–87. [PubMed] [Google Scholar]
- Waxman S.G. Mass Medical Soc; 1998. Demyelinating Diseases—New Pathological insights, New Therapeutic Targets; pp. 323–325. [DOI] [PubMed] [Google Scholar]
- Wechselberger M., Wright C.L., Bishop G.A., Boulant J.A. Ionic channels and conductance-based models for hypothalamic neuronal thermosensitivity. Am. J. Physiol.Regulat. Integrat. Comparat. Physiol. 2006;291(3):R518–R529. doi: 10.1152/ajpregu.00039.2006. [DOI] [PubMed] [Google Scholar]
- Weller A.S., Linnane D.M., Jonkman A.G., Daanen H.A. Quantification of the decay and re-induction of heat acclimation in dry-heat following 12 and 26 days without exposure to heat stress. Eur. J. Appl. Physiol. 2007;102(1):57–66. doi: 10.1007/s00421-007-0563-z. [DOI] [PubMed] [Google Scholar]
- Werner J. Temperature regulation during exercise: an overview. Exercise, Heat Thermoregulat. 1993:49–84. [Google Scholar]
- White A.T., Davis S.L., Vener J., Wendt L. Effect of Increased core temperature on cortical excitability and fatigue in multiple sclerosis patients: 1786Board# 139 May 29 9: 00 AM-10: 30 AM. Med. Sci. Sports Exerc. 2008;40(5):S300. [Google Scholar]
- White K., Scoones D., Newman P. Hypothermia in multiple sclerosis. J. Neurol., Neurosurg. Psychiatry. 1996;61(4):369–375. doi: 10.1136/jnnp.61.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins B.W., Chung L.H., Tublitz N.J., Wong B.J., Minson C.T. Mechanisms of vasoactive intestinal peptide-mediated vasodilation in human skin. J. Appl. Physiol. 2004;97(4):1291–1298. doi: 10.1152/japplphysiol.00366.2004. [DOI] [PubMed] [Google Scholar]
- Williams E., Ward M., Milledge J., Withey W., Older M., Forsling M. Effect of the exercise of seven consecutive days hill-walking on fluid homeostasis. Clin. Sci. 1979;56(4):305–316. doi: 10.1042/cs0560305. [DOI] [PubMed] [Google Scholar]
- Willis M., Robertson N. Multiple sclerosis and the risk of infection: considerations in the threat of the novel coronavirus, COVID-19/SARS-CoV-2. J. Neurol. 2020;267(5):1567–1569. doi: 10.1007/s00415-020-09822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T.E., Monahan K.D., Fogelman A., Kearney M.L., Sauder C.L., Ray C.A. Aerobic training improves in vivo cholinergic responsiveness but not sensitivity of eccrine sweat glands. J. Invest. Dermatol. 2010;130(9):2328. doi: 10.1038/jid.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wong B.J. Sensory nerves and nitric oxide contribute to reflex cutaneous vasodilation in humans. Am. J. Physiol.Regulat. Integrat. Comparat. Physiol. 2013;304(8):R651–R656. doi: 10.1152/ajpregu.00464.2012. [DOI] [PubMed] [Google Scholar]
- Wong B.J., Fieger S.M. Transient receptor potential vanilloid type 1 channels contribute to reflex cutaneous vasodilation in humans. J. Appl. Physiol. 2012;112(12):2037–2042. doi: 10.1152/japplphysiol.00209.2012. [DOI] [PubMed] [Google Scholar]
- Wong B.J., Minson C.T. Neurokinin-1 receptor desensitization attenuates cutaneous active vasodilatation in humans. J. Physiol. 2006;577(3):1043–1051. doi: 10.1113/jphysiol.2006.112508. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B.J., Tublitz N.J., Minson C.T. Neurokinin-1 receptor desensitization to consecutive microdialysis infusions of substance P in human skin. J. Physiol. 2005;568(3):1047–1056. doi: 10.1113/jphysiol.2005.095372. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B.J., Wilkins B.W., Minson C.T. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J. Physiol. 2004;560(3):941–948. doi: 10.1113/jphysiol.2004.071779. (Lond.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman C.R., Thompson M.A., Turk J.R., Laughlin M.H. Endurance exercise training improves endothelium-dependent relaxation in brachial arteries from hypercholesterolemic male pigs. J. Appl. Physiol. 2005;99(4):1412–1421. doi: 10.1152/japplphysiol.00293.2005. [DOI] [PubMed] [Google Scholar]
- Woodman C.R., Turk J.R., Rush J.W., Laughlin M.H. Exercise attenuates the effects of hypercholesterolemia on endothelium-dependent relaxation in coronary arteries from adult female pigs. J. Appl. Physiol. 2004;96(3):1105–1113. doi: 10.1152/japplphysiol.00767.2003. [DOI] [PubMed] [Google Scholar]
- Wyndham C., Rogers G., Senay L., Mitchell D. Acclimization in a hot, humid environment: cardiovascular adjustments. J. Appl. Physiol. 1976;40(5):779–785. doi: 10.1152/jappl.1976.40.5.779. [DOI] [PubMed] [Google Scholar]
- Xu J., Zhong S., Liu J., Li L., Li Y., Wu X., Li Z., Deng P., Zhang J., Zhong N. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005;41(8):1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zou .M..-H. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120(13):1266–1286. doi: 10.1161/CIRCULATIONAHA.108.835223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M., Matsumoto T., Ohwatari N., Kosaka M. Sweating economy by graded control in well-trained athletes. Pflügers Archiv. 1997;433(6):675–678. doi: 10.1007/s004240050331. [DOI] [PubMed] [Google Scholar]
- Yang R.C., Mack G.W., Wolfe R.R., Nadel E.R. Albumin synthesis after intense intermittent exercise in human subjects. J. Appl. Physiol. 1998;84(2):584–592. doi: 10.1152/jappl.1998.84.2.584. [DOI] [PubMed] [Google Scholar]
- Zalewski P., Bitner A., Słomko J., Szrajda J., Klawe J.J., Tafil-Klawe M., Newton J.L. Whole-body cryostimulation increases parasympathetic outflow and decreases core body temperature. J. Therm. Biol. 2014;45:75–80. doi: 10.1016/j.jtherbio.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res. Cardiol. 2008;103(5):398–406. doi: 10.1007/s00395-008-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li X., Pitzer A.L., Chen Y., Wang L., Li .P..-L. Coronary endothelial dysfunction induced by nucleotide oligomerization domain-like receptor protein with pyrin domain containing 3 inflammasome activation during hypercholesterolemia: beyond inflammation. Antioxid. Redox Signal. 2015;22(13):1084–1096. doi: 10.1089/ars.2014.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C., Kar I., Chen C.K., Sau C., Woodson S., Serra A., Abboud H. Multiple sclerosis disease-modifying therapy and the COVID-19 pandemic: implications on the risk of infection and future vaccination. CNS Drugs. 2020;34(9):879–896. doi: 10.1007/s40263-020-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker I.H., Schultz H.D., Patel K.P., Wang H. Modulation of angiotensin II signaling following exercise training in heart failure. Am. J. Physiol. Heart Circulat. Physiol. 2015;308(8):H781–H791. doi: 10.1152/ajpheart.00026.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]